Abstract
Diffusion tensor imaging is a magnetic resonance imaging technique that is uniquely capable of detecting microstructural tissue damage in mild and moderate traumatic brain injuries (TBIs). To date, it remains unknown if two common analytic techniques, region of interest (ROI) versus voxel-wise (VW) analyses, detect injury in similar locations. The purpose of this systematic review and meta-analysis was to directly compare the regions of abnormality elucidated by each method. Twenty-seven ROI and 11 VW studies met our inclusion criteria. Our ROI meta-analysis identified 11 regions, including the splenium of the corpus callosum, where fractional anisotropy (FA) was significantly decreased in TBI patients, compared with controls. Likewise, we identified higher mean diffusivity/apparent diffusivity constant in the genu, body, and splenium of the corpus callosum. Alternatively, our VW analysis identified one region of high FA in the right superior longitudinal fasciculus and seven regions of low FA, with the two largest located in the corpus callosum. High mean diffusivity and high radial diffusivity, both in the right inferior longitudinal fasciculus, also was revealed by our VW analysis. Moreover, we have shown that the magnitude of damage in the corpus callosum revealed by ROI analysis (z = -3.15) is greater than that demonstrated by VW analysis (z = -1.41). Overall, this study indicates that both ROI and VW analytic methods are sensitive to low FA in the corpus callosum; however, the ROI method has more power to detect the full extent of tissue abnormality in the corpus callosum. More research utilizing standardized methods and reporting is essential to fully characterize the extent to which ROI and VW analyses can concordantly detect other locations of pathology in mild and moderate TBI patients.
Keywords: diffusion tensor imaging, meta-analysis, systematic review, traumatic brain injury
Introduction
Traumatic brain injuries (TBIs) are a major cause of death and disability worldwide, the majority of which are mild or moderate in nature.1 Traumatic axonal injury (TAI), the pathological substrate of mild TBIs is not detectable by conventional computed tomography or magnetic resonance imaging (MRI) modalities. Instead, diffusion tensor imaging (DTI) has emerged as an MRI method able to detect evidence of microscopic pathology not visible on standard anatomical images.2 Despite the growing popularity of DTI over the past two decades, the relative strength of different DTI analytic approaches has not been quantified in a coherent analysis.
DTI analytic approaches that aim to identify regional damage in TBI patients typically employ either a region of interest (ROI) or voxel-wise (VW) method to compare patients with a control group. Detailed descriptions of the two methods, as well as their pros and cons, have been published elsewhere.3 In brief, in ROI analyses, researchers select specific portions of the brain for examination based on a priori hypotheses of injury location. The historic ROI method of the manually tracing region(s) is laborious and rater dependent. While the more modern tractography technique, which utilizes algorithms to segment white matter tracts of interest, overcomes these limitations, it likewise accounts for the spatial heterogeneity of TAI.4,5
Alternatively, in whole–brain VW analyses, every white matter voxel is compared between groups without region specific assumptions. Unlike ROI analyses, this method is not biased towards only detecting pathology in the regions pre-determined by the researchers. VW analyses, however, are limited by the fact that there is comparatively less statistical power when necessary corrections for multiple comparisons are employed. Moreover, the more recent VW method, Tract-Based Spatial Statistics (TBSS), which restricts the analysis to a white matter skeleton to minimize registration errors,6 is limited both by the propensity for type II errors when applying strict thresholds for significance and the fact that areas outside the skeleton cannot be detected.
Few meta-analyses have addressed DTI abnormalities in mild TBI,7,8 namely a report by Aoki and colleagues7 on 13 pooled ROI studies and another report by Aoki and colleagues8 on 17 pooled VW studies. Further, both Aoki studies were restricted to a mild TBI population and did not examine moderate TBI patients who typically demonstrate more extensive TAI.9 To date, no structured meta-analysis using papers published within the same time frame and appraised using the same inclusion/exclusion criteria has examined whether ROI and VW methods identify similar regions of microstructural tissue damage in mild and moderate TBI. Accordingly, the purpose of this systematic review and meta-analysis is to compare the regions of DTI abnormalities in mild and moderate TBI elucidated utilizing a ROI versus a VW analysis to determine if these methods detect the similar locations of injury.
Methods
Literature search strategy
A research librarian identified potential studies by searching the PubMed, EMBASE, PsycINFO, CENTRAL, and Web of Science electronic databases for any entries from inception until June 2017. Search results were limited to English language studies. The full search strategies are available in Appendix A. A combination of Medical Subject Heading (MeSH) terms (or equivalent) and free text were utilized including: brain injuries [MesH], Craniocerebral Trauma [MesH], Head Injuries, Closed, brain trauma, closed head injur*, concuss*, Mild TBI, mild traumatic brain injur*, mtbi AND diffusion tensor imaging [MesH], Diffusion Magnetic Resonance Imaging, diffusion magnetic resonance, diffusion mri, diffusion tensor imag*, diffusion tensor magnetic resonance imaging, diffusion tensor MRI, diffusion tensor tractography, diffusion weighted imag*, DTI, DTT, diffusion tensor, magnetic resonance diffusion tensor imaging. All studies were imported into DistillerSR software (Evidence Partners Incorporated, Ottawa, Ontario) for duplicate removal, screening and data extraction.
Selection criteria
Two independent reviewers (M.S, A.G) conducted an initial title and abstract screening. Studies were included at this stage if both reviewers agreed that the study: 1) was conducted in humans; 2) used DTI; 3) compared a mild and/or moderate TBI population with a control group; 4) did not study children <18 years old; and 5) was an original research paper (e.g., not a case study or review article). The full text of the studies that passed the abstract screening were then reviewed by two independent reviewers (M.S., J.S.) using the aforementioned criteria in addition to the following requirements for inclusion: 1) fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), or the apparent diffusion coefficient (ADC) in white matter was measured in both the TBI and control group; 2) An ROI (including tractography) or VW analysis (including TBSS) was employed; 3) the TBI population did not have a clinically diagnosed psychiatric comorbidity; and 4) the study was published in a peer reviewed journal (e.g., not a meeting abstract). Disagreements in full text screening were resolved by a third reviewer (L.H.). All reviewers were supervised by a board-certified neuroradiologist (M.L.L.)
Data extraction
For each study, the following variables were extracted by two reviewers (N.W., L.H.) and discrepancies were resolved via a collaborative review of the manuscript: number of male and female TBI and control subjects, age of TBI and control subjects, severity of injury, type of TBI population, time since injury, magnet field strength, number of diffusion directions, b value (other than 0), threshold used to test for significance, method of correction for multiple comparisons, and the DTI parameters explored. For ROI papers, we extracted data necessary to calculate a pooled effect size (mean and standard deviation [SD], or p value and sample size or t- statistic and sample size) in each region of interest.10 For VW analysis, we extracted the X, Y, and Z coordinates (in any standard space) and significance level (p or t values) of positive and negative peaks reported in each study. In the event of missing data, the authors were contacted at least twice, to request necessary data.
Statistical analysis
ROI
All analyses were performed using the Comprehensive Meta Analyses Program v.3. We calculated the standardized mean difference (Hedges' g) in DTI parameters between TBI patients and controls in regions of interest reported in ≥2 studies. Given the methodological heterogeneity in DTI studies of TBI,3 we implemented a random effects models when <20 studies were available. The I2 statistic was calculated to determine whether to employ a fixed or random effects model where data from ≥20 studies were avaiable.11 Data from left and right hemispheres were considered as independent regions in order to directly compare ROI with VW analysis. Publication bias was assessed via visual inspection of funnel plots, if ≥10 studies were included in the primary analyses12 and by using the Egger's test. Forest plots were generated using Meta Data Viewer.13
VW
Anisotropic Effect-Size Signed Differential Mapping (AES-SDM)14,15 was used to conduct a meta-analysis across VW studies. We utilized the following pre-processing parameters for all analyses: modality, DTI-FA; randomizations,1; correlation template, FA; anisotropy, 1; isotropic full width at half maximum, 20; and mask, white matter. We applied the default thresholds: peak z > 1, voxel p = 0.005 and cluster size ≥10 voxels.14 To maximize our power, we conducted our AES-SDM analysis combining peak coordinates data from papers that used whole–brain or TBSS voxel-wise methods.8,16 For sensitivity analyses, we considered locations within 10 voxels of the X, Y and Z as consistent with the primary results. Publication bias was assessed using the Egger's test.
Both
If data from TBI subjects were divided into separate groups, we calculated a pooled mean and standard deviation. For longitudinal studies, we utilized the more chronic time-point for analysis. Additionally, we conducted jackknife sensitivity and subgroup analyses (mild TBI patients only, <3 months and ≥3 months since injury) when more than two studies were available.
All procedures for this systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.17 Quality assessment of individual studies was not quantified because suitable metrics are not available for observational DTI studies.
Results
Studies and participants
Eligible studies
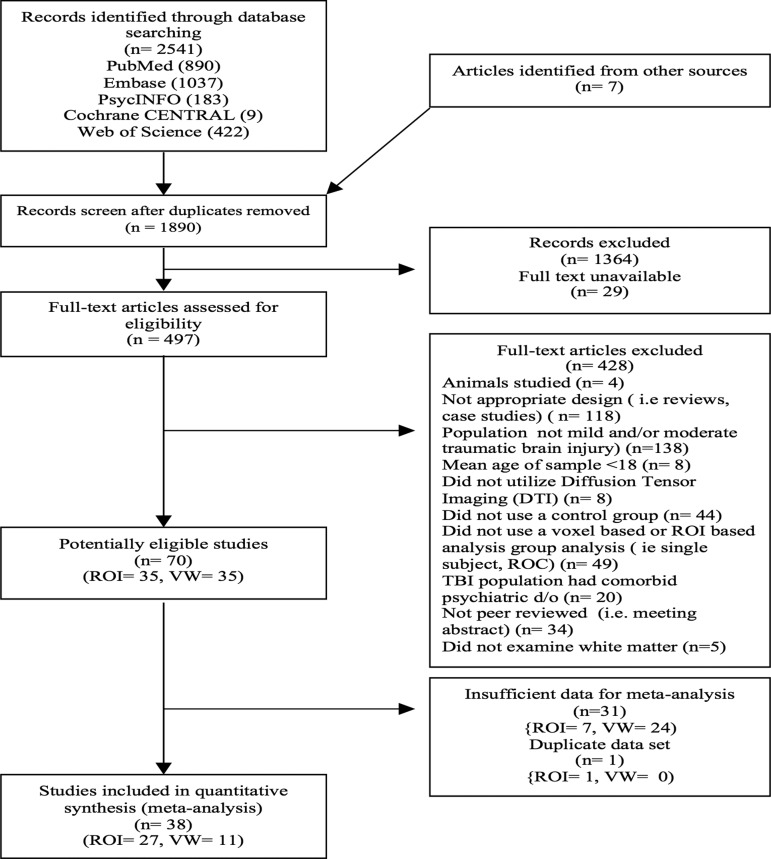
The PRISMA flow diagram (Fig. 1) describes the study selection process for eligibility. We screened 1890 studies of which 24 ROI and six VW studies provided sufficient data for analysis. Three authors18–20 provided the mean (SD) of DTI parameters of all regions explored in their study. Four21–24 authors provided the location of peak coordinates and p values for VW analysis. One author18 provided the original corrected t-Map. This yielded a total of 27 ROI18–20,25–48 and 11 VW18,21–24,49–54 studies suitable for our meta- analysis.
FIG. 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses flow chart of included studies. ROI, region of interest; VW, voxel-wise.
Participants and imaging characteristics
Demographic and imaging information on included studies are described in Supplementary Table 1 (see online supplementary material at http://www.liebertpub.com). A total of 1010 TBI/791 controls were included in meta-analysis of ROI studies and 311 TBI/282 controls were included in VW meta–analysis. The majority of studies (22/27 ROI; 10/11 VW) examined only mild TBI patients. Less than half (12/28 ROI; 5/11 VW) of the studies conducted the DTI scan at an acute or subacute time-point (< 3 months since injury) and the remainder of scans were conducted at a chronic stage (≥ 3 months since injury). Most studies scans were conducted on a 3T scanner (17/27 ROI; 7/11 VW) and used b value of 1000 (18/27 ROI; 8/11 VW). Thirteen of the ROI the studies used manual tracing to delineate regions and the remainder used tractography (8/27), an atlas based (4/27), or an automated segmentation procedure (2/27). Six VW studies used TBSS and five used a non-TBSS whole–brain white matter analytic approach. Eleven ROI studies were corrected for multiple comparison and more than half (6/11) of VW studies applied a correction for multiple comparisons. FA was examined in all ROI and VW studies included in this meta-analysis. MD/ ADC was the next most commonly explored DTI parameter (16 ROI; three VW studies) followed by RD (nine ROI; two VW), and AD (eight ROI; zero VW).
Primary meta-analysis
ROI
We identified 11 regions where FA was lower in TBI subjects compared with controls in the following areas, listed in decreasing magnitude: the posterior internal capsule (right), the posterior internal capsule (bilateral), the centrum semiovale (bilateral), the posterior internal capsule (left), the splenium of the corpus callosum (bilateral), the anterior internal capsule (right), the posterior corona radiata (left), the posterior corona radiata (right), the posterior thalamic radiations (left), the anterior corona radiata (bilateral), and the posterior corona radiata (bilateral).We identified three clusters of high MD/ADC in TBI subjects compared with controls in the splenium, genu, and body of the corpus callosum (Table 1). Effect sizes in all regions are shown in Supplementary Figure 1 and Supplementary Figure 2 (see online supplementary material at http://www.liebertpub.com).
Table 1.
Regions of Significant Difference between TBI Subjects and Controls Identified Pooling ROI Studies
| Primary analysis | Subgroups analysis | |||||
|---|---|---|---|---|---|---|
| Description | Z value | p value | n studies | Mild only | < 3 months since injury only | ≥ 3 months since injury only |
| FA: TBI < control | ||||||
| Posterior internal capsule, right | −3.69 | < 0.01 | 5 | X | X | X |
| Posterior internal capsule, bilateral | −3.41 | < 0.01 | 3 | X | X | |
| Centrum semiovale, bilateral | −3.36 | < 0.01 | 2 | |||
| Posterior internal capsule, Left | −3.17 | < 0.01 | 5 | X | X | |
| Splenium of corpus callosum, bilateral | −3.15 | < 0.01 | 13 | X | ||
| Anterior internal capsule, right | −2.68 | < 0.01 | 5 | X | X | |
| Posterior corona radiata, left | −2.44 | 0.01 | 2 | |||
| Posterior corona radiata, right | −2.42 | 0.02 | 2 | |||
| Posterior thalamic radiations, left | −2.37 | 0.02 | 3 | X | ||
| Anterior corona radiata, bilateral | −2.35 | 0.02 | 2 | |||
| Posterior corona radiata, bilateral | −2.26 | 0.03 | 2 | |||
| Posterior thalamic radiations, right | −2.00 | 0.045 | 2 | |||
| MD/ADC: TBI > control | ||||||
| Splenium of CC, bilateral | 3.35 | < 0.01 | 7 | X | X | X |
| Genu of CC, bilateral | 2.63 | 0.01 | 7 | X | ||
| Body of CC, bilateral | 2.35 | 0.02 | 4 | X | ||
An “X” indicates that the results remained significant when only this subgroup was analyzed. A shaded black box indicates that analysis was not done because <2 studies in subgroup.
TBI, traumatic brain injury; ROI, region of interest; FA, fractional anisotropy; MD, mean diffusivity; ADC, apparent diffusion coefficient; CC, corpus callosum.
VW
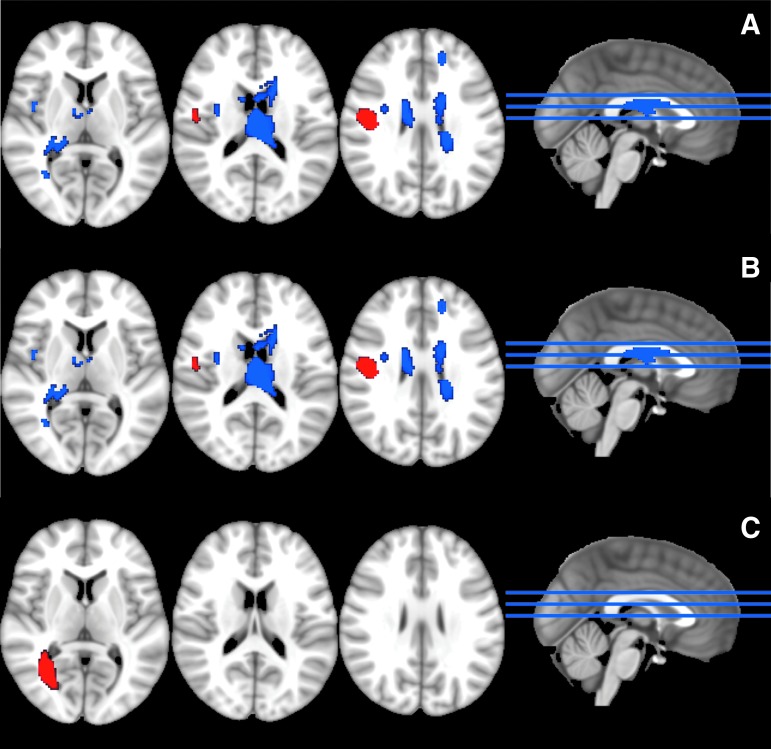
AES-SDM identified one cluster of high FA in TBI subjects, located in the right superior longitudinal fasciculus. There were seven clusters of low FA in TBI subjects, the largest two located in the corpus callosum. Other smaller clusters of low FA included the right superior longitudinal fasciculus, the right inferior fronto-occipital fasciculus, and the right insula (Table 2; Fig. 2A). We found one significant cluster of high MD and one cluster of high RD in TBI subjects, both located in the right inferior longitudinal fasciculus (Table 2; Fig. 2B, 2C).
Table 2.
Regions of Significant Difference between TBI Subjects and Controls Identified Pooling VW Studies
| Primary analysis | Subgroup analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Description | Z value | p value | Cluster size | MNI coordinates | n studies | Mild only | < 3 months since injury only | ≥ 3 months since injury only |
| FA: TBI > control | ||||||||
| Superior longitudinal fasciculus III, right | 1.16 | 0.000006 | 315 | 48, −10, 24 | 10 | X | ||
| FA: TBI < control | ||||||||
| Corpus callosum | −1.77 | 0.000196 | 1164 | −8, 4, 20 | 10 | X | ||
| Corpus callosum | −1.48 | 0.000796 | 148 | 20, −34, 12 | 10 | X | ||
| Superior longitudinal fasciculus III, right | −1.52 | 0.000672 | 56 | 32, −2, 18 | 10 | X | ||
| Corpus callosum | −1.41 | 0.001213 | 40 | −16, 44, 28 | 10 | X | ||
| Corpus callosum | −1.35 | 0.002243 | 30 | 20, −14, 44 | 10 | X | ||
| Inferior fronto-occipital fasciculus, right | −1.34 | 0.002250 | 21 | 34, −60, 6 | 10 | X | X | |
| Insula, right | −1.44 | 0.001219 | 11 | 42, 2, 6 | 10 | X | ||
| MD: TBI > control | ||||||||
| Inferior longitudinal fasciculus, right | 1.39 | 0.000007 | 435 | 30, −58, 2 | 3 | X | X | |
| RD: TBI > control | ||||||||
| Inferior longitudinal fasciculus, right | 1.57 | 0.000033 | 832 | 28, −66, 2 | 2 | |||
Results thresholded at p < 0.005 and cluster size >10 voxels.
An “X” indicates that the results are within 10 voxels in the X, Y, and Z direction of primary findings. Shaded black box indicates that analysis was not done because <2 studies in subgroup.
TBI, traumatic brain injury; VW, voxel-wise; MNI, Montreal Neurological Institute; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
FIG. 2.
Results of voxel-wise meta-analysis. (A) Fractional anisotropy (FA): traumatic brain injury (TBI) subjects demonstrated one cluster of high FA (red) in the right superior longitudinal fasciculus and seven clusters of low FA (blue), the largest two located in the corpus callosum. (B) Mean diffusivity (MD): TBI subjects demonstrated one cluster of high MD (red) in the right inferior longitudinal fasciculus. (C) Radial diffusivity (RD): TBI subjects demonstrated one cluster of high RD (red) located in the right inferior longitudinal fasciculus. Color image is available online.
Sensitivity analyses
ROI
Jackknife (JK) sensitivity analysis of ROI studies demonstrated that findings were retained when all studies were iteratively removed one at a time in all regions except the posterior limb of the internal capsule (bilateral) which was sensitive in two of three of JK analyses. Likewise, High MD/ADC in the genu and splenium of the corpus callosum remained significant when all studies were iteratively removed and was sensitive in two of three JK analyses for the body of the corpus callosum (Supplementary Table 2; see online supplementary material at http://www.liebertpub.com).
VW
Our AES-SDM findings were also highly robust. High FA in the right superior longitudinal fasciculus was sensitive in eight of nine JK analyses and all clusters of low FA remained significant when ≥8 studies were removed. High MD in the right inferior longitudinal fasciculus remained significant in two of three JK analyses (Supplementary Table 3; see online supplementary material at http://www.liebertpub.com).
Subgroup analysis
ROI
All FA ROI findings, except for low FA in the splenium of the corpus callosum (bilateral), remained significant when only mild TBI cases (e.g., excluding moderate TBI) were included in the analysis. Our low FA findings remained consistent in the ≥3 months since injury subgroup in all regions. High MD/ADC in the splenium of the corpus callosum remained significant in all subgroup analyses; however, findings in the genu and body of the corpus callosum only persisted in the <3 months since injury subgroup (Table 1).
VW
VW findings remained in similar regions when only mild TBI cases were included. Although our threshold of 10 voxels in the X, Y and Z direction was not met in the four largest clusters, AES-SDM located these clusters in similar regions as the primary analysis (Table 2 and Supplementary Table 4; see online supplementary material at http://www.liebertpub.com). Our findings of low FA in largest corpus callosum cluster and in the right superior longitudinal fasciculus remained in <3 months since injury subgroup whereas low FA in the second and third largest corpus callosum clusters and in the right inferior fronto-occipital fasciculus only persisted in ≥3 months since injury subgroup. The one cluster of high FA identified in VW analyses was only significant in the ≥3 months since injury subgroup. High MD in the right inferior longitudinal fasciculus persisted in both the mild and ≥3 months since injury subgroups (Table 2; Supplementary Tables 5 and 6; see online supplementary material at http://www.liebertpub.com).
Publication bias
ROI
The Egger's tests identified no evidence of publication bias in any region (Supplementary Table 7; see online supplementary material at http://www.liebertpub.com).
VW
Visual inspection of the funnel plot of low FA in the splenium of the corpus callosum did not reveal publication bias (Supplementary Fig. 3; see online supplementary material at http://www.liebertpub.com). Likewise, the Egger's tests identified no evidence of publication bias in any region (Supplementary Table 8; see online supplementary material at http://www.liebertpub.com).
Discussion
This systematic review and meta-analysis compared DTI findings in mild and moderate TBI patients identified utilizing ROI versus VW analytic approaches. Our findings show that both ROI and VW methods detect low FA in the corpus callosum but the ROI method detects a greater amount of abnormality in this region. Our results were highly robust when applying a jackknife sensitivity analysis and findings remain similar locations when only mild TBI cases were examined.
To date, few studies have meta-analyzed DTI findings in mild TBI. Aoki and colleagues published two studies, which separately assessed ROI and VW studies culled from different timeframes. Their 20127 meta-analysis of ROI studies identified low FA in the splenium and their 20168 meta-analysis, which synthesized FA findings across VW studies, similarly showed the largest cluster of low FA in the splenium. Our meta-analysis, however, more specifically addressed whether ROI and VW revealed similar regions of abnormality across the whole brain by directly comparing studies published within the same time-period and screened using identical criteria. Moreover, we included moderate TBI patients and examined additional DTI parameters to provide a more comprehensive review of the utility of DTI in detecting TAI.
In our direct comparison, we confirmed that both ROI and VW methods are sensitive to low FA in the corpus callosum in mild and moderate TBI. However, other regions of low FA are not concordantly identified across methods. High MD was found in both ROI and VW analyses, but in dissimilar regions. A major caveat in interpreting these findings, however, is the fact that no regions other than the corpus callosum utilized data from more than five studies. This is due to bias imposed by the researcher(s) a priori hypotheses as well as data actually available from the studies for meta-analysis. To sufficiently compare data across regions it is necessary for papers to examine similar ROIs and for authors to report data from all regions explored, regardless of statistical significance.
Only the VW meta-analysis identified a region where FA was higher in TBI patients than in controls. VW meta- analysis by Aoki and colleagues8 did not reveal any regions of high FA; however, our divergent findings may be attributable to the fact that we utilized the raw statistical t-map from the study by Ling and colleagues,18 which primarily reported high FA in their population. This underscores the importance for researchers to make available raw statistical maps so that future meta-analyses can more comprehensively characterize diffusion abnormalities and associated biological changes in mild and moderate TBI.
We have found that the magnitude of the effect size of low FA revealed by our ROI meta- analysis was approximately two times larger than that revealed by our VW meta-analysis. This finding is not unexpected, given that the conservative thresholds and necessary registration procedures applied in VW analyses lead to small cluster sizes that may conceal the full anatomic extent of damage. Additionally, efforts such as clustering, used to minimize the risk of type 1 error in VW analyses, necessarily limit sensitivity. On the other hand, ROI analyses average DTI parameters over the entire prescribed region/tract and hence are inherently more sensitive to detect a greater degree of abnormality.
There are several limitations to this meta-analysis that should be noted. ROI analyses are only reliable insofar as regions are reproducibly localized across subjects and across studies, which is, in turn, conditional upon reliable manual placement of ROIs or robust registration procedures. Moreover, although we aggregated regions based on the naming provided in publications, we cannot exclude the possibility that the white matter in the distinct regions we examined does not completely overlap. For instance, we cannot know whether the internal capsule was delineated as a unique structure or included as part of the larger corticospinal tract. Moreover, despite our diligent efforts, we were only able to retrieve sufficient data for our AES-SDM analysis in 31% of eligible studies. This may have limited our power to detect full extent of pathology revealed by VW methods and highlights the necessity for researchers to make available the coordinates and the precise p values of the abnormalities detected in their studies. Furthermore, AES-SDM results are most reliable, and less prone to Type 1 errors, when statistical parametric maps are included.14 In the present analysis, we were only able to retrieve one original t-map; however, this is more data than has been included in any prior image-based meta-analyses of mild TBI. Finally, unmeasured sources of heterogeneity such as divergent imaging parameters (e.g., field strength; b values), differences in image processing methods and software, and TBI populations (e.g., military vs. civilian) also exist across studies. We did not have sufficient power to conduct subgroup analyses on these or other possible sources of heterogeneity and we, therefore, employed random effects models to account for these potential sources of variance.
Overall, in this systematic review and meta-analysis, we have shown that low FA in the corpus callosum is consistently detected by both ROI and VW methods; however, the effect size of damage revealed by pooling ROI studies was twice as large. These results suggest that VW analyses, which are automated and not subject to rater bias, can be implemented clinically to identify injury in the corpus callosum. On the other hand, ROI analyses, which are bias prone and onerous, may henceforth be exclusively utilized when the magnitude of microstructural tissue damage is an important clinical consideration.
Evidence of tissue damage in regions outside the corpus callosum was incongruently identified across methods; however, this discordance may simply reflect the small number of papers that examined similar regions and the limited data available for our VW meta- analysis. To more accurately synthesize findings across studies and robustly assess the power of ROI versus VW methods for detection of pathology in smaller white matter regions, it is essential for future studies to follow standardized data reporting methods such as those proposed by the National Institute of Neurological Disorders and Stroke's Common Data Elements Project.55
Supplementary Material
Acknowledgments
The authors would like to acknowledge Aliza Goldstein and James Semple, MD, who served as trained reviewers for this project. The authors would also like to graciously thank the authors who provided unpublished data including: Phillip Dean, Paul Echlin, MD, Josef Ling, Christine Mac Donald PhD, Andrew Mayer, PhD, Arnaud Messe, PhD, Adam Octavian, MD, and Vigneswaran Veeramuthu, MD.
This work was supported by the National Institutes of Health (Grants R01NS082432; Principal Investigator: M.L.L and 5F31NS098799-02; Principal Investigator: L.E.H)
Appendix A.
| Database | Search Strategy |
|---|---|
| All databases | Limit applied: English No limitations by date of publication, publication type, or document type |
| PubMed | (((brain injuries) OR (Craniocerebral Trauma) OR (Head Injuries, Closed) OR (brain trauma[ti) OR (closed head injur*[tiab]) OR (concuss*[tiab]) OR (Mild TBI[tiab]) OR (mild traumatic brain injur*[tiab]) OR (mtbi[tiab]) OR (brain trauma) OR (closed head injur*) OR (concuss*) OR (Mild TBI) OR (mild traumatic brain injur*) OR (mtbi))) AND ((diffusion tensor imaging) OR (Diffusion Magnetic Resonance Imaging) OR (diffusion magnetic resonance[tiab]) OR (diffusion mri[tiab]) OR (diffusion tensor imag*[tiab]) OR (diffusion tensor magnetic resonance imaging[tiab]) OR (diffusion tensor MRI[tiab]) OR (diffusion tensor tractography[tiab]) OR (diffusion weighted imag*[tiab]) OR (DTI[tiab]) OR (DTT[tiab]) OR (diffusion[tiab] AND tensor[tiab]) OR (magnetic resonance diffusion tensor imaging[tiab]) OR (diffusion magnetic resonance) OR (diffusion mri) OR (diffusion tensor imag*) OR (diffusion tensor magnetic resonance imaging) OR (diffusion tensor MRI) OR (diffusion tensor tractography) OR (diffusion weighted imag*) OR (DTI) OR (DTT) OR (diffusion AND tensor) OR (magnetic resonance diffusion tensor imaging)) |
| Embase | 'traumatic brain injury'/exp OR 'traumatic brain injury' OR 'brain concussion'/exp OR 'brain concussion' OR ('traumatic brain':ab,ti AND injur*:ab,ti) OR 'tbi':ab,ti OR 'mtbi':ab,ti OR concuss*:ab,ti OR 'brain trauma':ab,ti OR ('closed head':ab,ti AND injur*:ab,ti) AND 'diffusion tensor imaging'/exp OR 'diffusion magnetic resonance':ab,ti OR 'diffusion mri':ab,ti OR 'diffusion tensor':ab,ti OR 'diffusion weighted':ab,ti OR 'dti':ab,ti OR 'dtt':ab,ti |
| Cochrane | 1. exp Diffusion Magnetic Resonance Imaging/ 2. dti.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] 3. diffusion tensor imaging.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] 4. 1 or 2 or 3 5. exp Brain Injuries/ 6. tbi.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] 7. mtbi.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] 8. traumatic brain injur$.mp. [mp = title, original title, abstract, mesh headings, heading words, keyword] 9. 5 or 6 or 7 or 8 10. 4 and 9 |
| PscyINFO | ti = diffusion NEAR/3 tensor OR ab = diffusion NEAR/3 tensor OR kw = diffusion NEAR/3 tensor OR ti = dti OR ab = dti OR kw = dti OR ti = diffusion NEAR/3 mri OR ab = diffusion NEAR/3 mri OR kw = diffusion NEAR/3 mri OR ti = diffusion NEAR/3 “magnetic resonance” OR ab = diffusion NEAR/3 “magnetic resonance” OR kw = diffusion NEAR/3 “magnetic resonance” OR it = ”magnetic resonance imaging” AND it = ”traumatic brain injury” OR ti = TBI OR ab = TBI OR kw = TBI OR ti = mtbi OR ab = mtbi kw = mtbi OR ti = ”mild” NEAR/3 “traumatic” NEAR/3 “Brain” NEAR/3 Injur* OR ab = ”mild” NEAR/3 “traumatic” NEAR/3 “Brain” NEAR/3 Injur* OR kw = ”mild” NEAR/3 “traumatic” NEAR/3 “Brain” NEAR/3 Injur* OR ti = ”moderate” NEAR/3 “traumatic” NEAR/3 “Brain” NEAR/3 Injur* OR ti = ”moderate” NEAR/3 “traumatic” NEAR/3 “Brain” NEAR/3 Injur* OR kw = ”moderate” NEAR/3 “traumatic” NEAR/3 Brain NEAR/3 Injur* OR it = ”brain concussion” OR ti = concuss* OR ab = concuss* OR kw = concuss* |
| Web of Science | 1: TS = (diffusion tensor) OR TS = (dti) OR TS = (diffusion magnetic resonance) 2: TS = (brain n/3 injur*) OR TS = (tbi) OR TS = (mtbi) OR TS = (concuss*) 3: #2 AND #1 |
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Kraus J.F. and Chu L.D. (2005). Epidemiology, in: Textbook of Traumatic Brain Injury, 2nd ed. American Psychiatric Publishing: Arlington, VA [Google Scholar]
- 2. Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., and Lipton M.L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol. 34, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Astrakas L.G. and Argyropoulou M.I. (2010). Shifting from region of interest (ROI) to voxel-based analysis in human brain mapping. Pediatr. Radiol. 40, 1857–1867 [DOI] [PubMed] [Google Scholar]
- 5. Garrison K.A., Rogalsky C., Sheng T., Liu B., Damasio H., Winstein C.J., and Aziz-Zadeh L.S. (2015). Functional MRI preprocessing in lesioned brains: manual versus automated region of interest analysis. Front. Neurol. 6, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 7. Aoki Y., Inokuchi R., Gunshin M., Yahagi N., and Suwa H. (2012). Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 83, 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aoki Y. and Inokuchi R. (2016). A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neurosci. Biobehav. Rev. 66, 119–126 [DOI] [PubMed] [Google Scholar]
- 9. Godoy D.A., Rubiano A., Rabinstein A.A., Bullock R., and Sahuquillo J. (2016). Moderate traumatic brain injury: the grey zone of Neurotrauma. Neurocrit. Care 25, 306–319 [DOI] [PubMed] [Google Scholar]
- 10. Lipsey M.W. and Wilson D.B. (2001). Practical Meta-Analysis. SAGE Publications: Thousand Oaks, CA [Google Scholar]
- 11. Huedo-Medina T.B., Sanchez-Meca J., Marin-Martinez F., and Botella J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11, 193–206 [DOI] [PubMed] [Google Scholar]
- 12. Sterne J.A., Gavaghan D., and Egger M. (2000). Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 53, 1119–1129 [DOI] [PubMed] [Google Scholar]
- 13. Boyles A.L., Harris S.F., A., A., Kristina R., and Thayer A. (2011). Forest plot viewer: a new graphing tool. Epidemiology 22, 746–747 [DOI] [PubMed] [Google Scholar]
- 14. Radua J., Mataix-Cols D., Phillips M.L., El-Hage W., Kronhaus D.M., Cardoner N., and Surguladze S. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611 [DOI] [PubMed] [Google Scholar]
- 15. Radua J., Rubia K., Canales-Rodriguez E.J., Pomarol-Clotet E., Fusar-Poli P., and Mataix-Cols D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nortje G., Stein D.J., Radua J., Mataix-Cols D., and Horn N. (2013). Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 150, 192–200 [DOI] [PubMed] [Google Scholar]
- 17. Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., and Stewart L.A.S. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling J.M., Pena A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C. and Mayer A.R. (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adam O., Donald C.L.M., Rivet D., Ritter J., May T., Barefield M., Duckworth J., LaBarge D., Asher D., Drinkwine B., Woods Y., Connor M., and Brody D.L. (2015). Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology 85, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mac Donald C.L., Barber J., Andre J., Evans N., Panks C., Sun S., Zalewski K., Elizabeth Sanders R., and Temkin N. (2017). 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clin. 14, 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dean P.J., Sato J.R., Vieira G., McNamara A., and Sterr A. (2015). Multimodal imaging of mild traumatic brain injury and persistent postconcussion syndrome. Brain Behav. 5, 45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messe A., Caplain S., Pelegrini-Issac M., Blancho S., Montreuil M., Levy R., Lehericy S., and Benali H. (2012). Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav. 6, 283–292 [DOI] [PubMed] [Google Scholar]
- 23. Sasaki T., Pasternak O., Mayinger M., Muehlmann M., Savadjiev P., Bouix S., Kubicki M., Fredman E., Dahlben B., Helmer K.G., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S., and Koerte I.K. (2014). Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study—clinical article. J. Neurosurg. 120, 882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veeramuthu V., Narayanan V., Kuo T.L., Delano-Wood L., Chinna K., Bondi M.W., Waran V., Ganesan D., and Ramli N. (2015). Diffusion tensor imaging parameters in mild traumatic brain injury and its correlation with early meuropsychological impairment: a longitudinal study. J. Neurotrauma 32, 1497–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., and Meyerand M.E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 23, 794–802 [PMC free article] [PubMed] [Google Scholar]
- 26. Chamard E., Lassonde M., Henry L., Tremblay J., Boulanger Y., Beaumont L.D., and Theoret H. (2013). Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 27, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 27. Chamard E., Lefebvre G., Lassonde M., and Theoret H. (2016). Long-term abnormalities in the corpus callosum of remale concussed athletes. J. Neurotrauma 33, 1220–1226 [DOI] [PubMed] [Google Scholar]
- 28. Clark A.L., Sorg S.F., Schiehser D.M., Bigler E.D., Bondi M.W., Jacobson M.W., Jak A.J., and Delano-Wood L. (2016). White matter associations with performance validity testing in veterans with mild traumatic brain injury: the utility of biomarkers in complicated assessment. J. Head Trauma Rehabil. 31, 346–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delano-Wood L., Bangen K.J., Sorg S.F., Clark A.L., Schiehser D.M., Luc N., Bondi M.W., Werhane M., Kim R.T., and Bigler E.D. (2015). Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav. 9, 500–512 [DOI] [PubMed] [Google Scholar]
- 30. Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O., and Grossman R.I. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 103, 298–303 [DOI] [PubMed] [Google Scholar]
- 31. Grossman E.J., Ge Y., Jensen J.H., Babb J.S., Miles L., Reaume J., Silver J.M., Grossman R.I., and Inglese M. (2012). Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J. Neurotrauma 29, 2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang S.H. and Kim S.Y. (2016). Injury of the corticospinal tract in patients with mild traumatic brain injury: a diffusion tensor tractography study. J. Neurotrauma 33, 1790–1795 [DOI] [PubMed] [Google Scholar]
- 33. Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 34. Kumar R., Gupta R.K., Husain M., Chaudhry C., Srivastava A., Saksena S., and Rathore R.K.S. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric test. Brain Injury 23, 675–685 [DOI] [PubMed] [Google Scholar]
- 35. Lange R.T., Iverson G.L., Brubacher J.R., Madler B., and Heran M.K. (2012). Diffusion tensor imaging findings are not strongly associated with postconcussional disorder 2 months following mild traumatic brain injury. J. Head Trauma Rehabil. 27, 188–198 [DOI] [PubMed] [Google Scholar]
- 36. Levin H.S., Wilde E., Troyanskaya M., Petersen N.J., Scheibel R., Newsome M., Radaideh M., Wu T., Yallampalli R., Chu Z., and Li X. (2010). Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma 27, 683–694 [DOI] [PubMed] [Google Scholar]
- 37. Little D.M., Kraus M.F., Joseph J., Geary E.K., Susmaras T., Zhou X.J., Pliskin N., and Gorelick P.B. (2010). Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 74, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lo C., Shifteh K., Gold T., Bello J.A., and Lipton M.L. (2009). Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J. Comput. Assist. Tomogr. 33, 293–297 [DOI] [PubMed] [Google Scholar]
- 39. Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., and Brody D.L. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 364, 2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maruta J., Palacios E.M., Zimmerman R.D., Ghajar J., and Mukherjee P. (2016). Chronic post-concussion neurocognitive deficits. I. Relationship with white matter integrity. Front. Hum. Neurosci. 10, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsushita M., Hosoda K., Naitoh Y., Yamashita H., and Kohmura E. (2011). Utility of diffusion tensor imaging in the acute stage of mild to moderate traumatic brain injury for detecting white matter lesions and predicting long-term cognitive function in adults: clinical article. J. Neurosurg. 115, 130–139 [DOI] [PubMed] [Google Scholar]
- 42. Meier T.B., Bergamino M., Bellgowan P.S., Teague T.K., Ling J.M., Jeromin A., and Mayer A.R. (2016). Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum. Brain Mapp. 37, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naess-Schmidt E.T., Blicher J.U., Eskildsen S.F., Tietze A., Hansen B., Stubbs P.W., Jespersen S., Ostergaard L., and Nielsen J.F. (2017). Microstructural changes in the thalamus after mild traumatic brain injury: A longitudinal diffusion and mean kurtosis tensor MRI study. Brain Inj. 31, 230–236 [DOI] [PubMed] [Google Scholar]
- 44. Rutgers D.R., Fillard P., Paradot G., Tadie M., Lasjaunias P., and Ducreux D. (2008). Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am. J. Neuroradiol. 29, 1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh K., Trivedi R., D'souza M.M., Chaudhary A., Khushu S., Kumar P., Rathore R.K.S., and Tripathi R.P. (2015). Demonstration of differentially degenerated corpus callosam in patients with moderate traumatic brain injury: with a premise of cortical-callosal relationship. Arch. Neurosci. 2 [Google Scholar]
- 46. Sorg S.F., Delano-Wood L., Luc N., Schiehser D.M., Hanson K.L., Nation D.A., Lanni E., Jak A.J., Lu K., Meloy M.J., Frank L.R., Lohr J.B., and Bondi M.W. (2014). White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J. Head Trauma Rehabil. 29, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilde E.A., Li X., Hunter J.V., Narayana P.A., Hasan K., Biekman B., Swank P., Robertson C., Miller E., McCauley S.R., Chu Z.D., Faber J., McCarthy J., and Levin H.S. (2016). Loss of consciousness is related to white matter injury in mild traumatic brain injury. J. Neurotrauma 33, 2000–2010 [DOI] [PubMed] [Google Scholar]
- 48. Zhang K., Johnson B., Pennell D., Ray W., Sebastianelli W., and Slobounov S. (2010). Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp. Brain Res. 204, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asano Y., Shinoda J., Okumura A., Aki T., Takenaka S., Miwa K., Yamada M., Ito T., and Yokoyama K. (2012). Utility of fractional anisotropy imaging analyzed by statistical parametric mapping for detecting minute brain lesions in chronic-stage patients who had mild or moderate traumatic brain injury. Neurol. Med. Chir. (Tokyo) 52, 31–40 [DOI] [PubMed] [Google Scholar]
- 50. Costanzo M.E., Chou Y.Y., Leaman S., Pham D.L., Keyser D., Nathan D.E., Coughlin M., Rapp P., and Roy M.J. (2014). Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci. Lett. 577, 11–15 [DOI] [PubMed] [Google Scholar]
- 51. Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Bello J.A., and Branch C.A. (2008). Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 52. Smits M., Houston G.C., Dippel D.W., Wielopolski P.A., Vernooij M.W., Koudstaal P.J., Hunink M.G., and van der Lugt A. (2011). Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 53, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wada T., Asano Y., and Shinoda J. (2012). Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR Am. J. Neuroradiol. 33, 2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu Y., Li Z., Bai L., Tao Y., Sun C., Li M., Zheng L., Zhu B., Yao J., Zhou H. and Zhang M. (2014). Loss of microstructural integrity in the limbic-subcortical networks for acute symptomatic traumatic brain injury. Biomed. Res. Int. 2014, 548392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hicks R., Giacino J., Harrison-Felix C., Manley G., Valadka A., and Wilde E.A. (2013). Progress in developing common data elements for traumatic brain injury research: version two–the end of the beginning. J. Neurotrauma 30, 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.