Abstract
Objective
The contribution of brown adipose tissue (BAT) to adult human metabolic control is a topic of ongoing investigation. In context, understanding the cellular events leading to BAT uncoupling, heat production, and energy expenditure is anticipated to produce significant insight into this endeavor. The phosphoinositide interacting regulator of transient receptor potentials (Pirt) was recently put forward as a key protein regulating cold sensing downstream of the transient receptor potential melastatin 8 (TRPM8). Notably, TRPM8 has been identified as a non-canonical regulator of BAT thermogenesis. The aim of this investigation was to delineate the role of Pirt in energy homeostasis and glucose metabolism - and the possible involvement of Pirt in TRPM8-elicited energy expenditure.
Methods
To this end, we metabolically phenotyped male and female Pirt deficient (Pirt−/−) mice exposed to a low-fat chow diet or to a high-fat, high-sugar (HFHS) diet.
Results
We identified that chow-fed female Pirt−/− mice have an increased susceptibility to develop obesity and glucose intolerance. This effect is abrogated when the mice are exposed to a HFHS diet. Conversely, Pirt−/− male mice display no metabolic phenotype on either diet relative to wild-type (WT) control mice. Finally, we observed that Pirt is dispensable for TRPM8-evoked energy expenditure.
Conclusion
We here report subtle metabolic abnormalities in female, but not male, Pirt−/− mice. Future studies are required to tease out if metabolic stressors beyond dietary interventions, e.g. temperature fluctuations, are interacting with Pirt-signaling and metabolic control in a sex-specific fashion.
Keywords: Signaling molecule, Sex differences, Body weight, Energy metabolism, TRPM8, Brown adipose tissue
Highlights
-
•
Pirt is robustly expressed in several nuclei of the hypothalamus.
-
•
Chow-fed female Pirt−/− mice present with increased propensity to gain body weight.
-
•
Pirt is dispensable for icilin-evoked TRPM8-dependent energy expenditure induction.
Abbreviations
- 3V
third ventricle
- ARH
arcuate nucleus
- BAT
brown adipose tissue
- Cidea
cell death-inducing DFFA-like effector A
- CT
cycle of threshold
- DIO
diet-induced obese
- Dio2
iodothyronine deiodinase 2
- eWAT
epididymal white adipose tissue
- GTT
glucose tolerance test
- HFHS
high-fat, high-sugar
- Hprt
hypoxanthine-guanine phosphoribosyltransferase
- IHC
immunohistochemistry
- i.p.
intraperitoneal
- iWAT
inguinal white adipose tissue
- ME
median eminence
- MPO
medial preoptic nucleus
- NE
norepinephrine
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- Pgc-1α
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- PIP2
phosphatidylinositol 4,5-bisphosphate
- Pirt
phosphoinositide interacting regulator of transient receptor potentials
- Pirt−/−
Pirt deficient
- Ppib
peptidylprolyl isomerase B
- Prdm16
PR domain-containing 16
- PVH
paraventricular hypothalamic nucleus
- qPCR
quantitative real-time PCR
- RER
respiratory exchange ratio
- s.c.
subcutaneous
- TBS
tris-buffered saline
- TRPM8
transient receptor potential melastatin 8
- TRPV1
transient receptor potential vanilloid 1
- UCP1
uncoupling protein 1
- VMH
ventromedial hypothalamic nucleus
- WT
wild-type
1. Introduction
The ongoing global obesity epidemic is a consequence of a persistent positive energy balance emerging when food intake chronically exceeds energy expenditure [1]. Since the evidence of active brown adipose tissue (BAT) in humans, targeting of this metabolically active tissue has surfaced as a promising therapeutic intervention to keep energy balance and body weight in check [2], [3], [4], [5]. Physiologically, BAT thermogenesis increases with prolonged exposure to cold, enabling mammals to maintain core body temperature upon fluctuations in environmental temperatures [6]. During this process of adaptive thermogenesis, norepinephrine (NE) released from sympathetic neurons activates β3-adrenoceptors on the cell surface of the brown adipocytes, which initiates a signaling cascade that results in activation of the uncoupling protein 1 (UCP1) and dissipation of heat at the expense of ATP production [6], [7], [8], [9]. In contrast to the indirect activation of UCP1-dependent non-shivering thermogenesis upon cold temperatures, there is evidence to suggest direct cold-sensing by brown adipocytes via transient receptor potential melastatin 8 (TRPM8) [10].
It is well established that TRPM8 channels are expressed in dorsal root ganglia, and they are activated upon temperatures below ∼26 °C [11], [12]. The observation that TRPM8 channels can also be activated by exogenous ligands such as menthol and icilin has ignited interest in exploring a role for TRPM8 targeting in the pharmacological treatment of obesity [11], [12]. Notably, activation of TRPM8 channels with icilin acutely caused an increase in energy expenditure in mice, and long-term treatment with dietary menthol or subcutaneous injections of icilin prevents weight gain or lowers body weight in diet-induced obese (DIO) mice [10], [13]. Moreover, icilin treatment in mice housed at thermoneutrality, and in which BAT thermogenesis is at a minimum, leads to the re-expression of UCP1 protein in the BAT [13]. Although it is not clear whether the effects of TRPM8 activation on whole-body thermogenesis are consequential to direct effects on adipocytes [10] or are mediated indirectly [13], [14], together, these findings suggest that pharmacological targeting of TRPM8 might be a viable anti-obesity strategy and thus underscore the imperative task of parsing out the molecular mechanisms connecting TRPM8 to energy expenditure and BAT thermogenesis.
Phosphoinositide interacting regulator of TRPs (Pirt) was established as an endogenous regulator of TRP channels, including heat-sensing TRP vanilloid 1 (TRPV1) [15] and more recently cold-sensing TRPM8 [16], [17]. Pirt is a membrane protein predominantly expressed in dorsal root ganglia that increases sensitivity to exogenous TRPM8 stimulation in conjunction with the canonical cellular signal molecule phosphatidylinositol 4,5-bisphosphate (PIP2) [16], [18]. Pirt deficient (Pirt−/−) mice have an impaired response to cold, indicating that the protein is involved in temperature sensation/regulation [16]. Moreover, Pirt−/− mice display a blunted behavioral response to icilin [16], suggesting that Pirt is required for both physiological and pharmacological TRPM8-based signaling.
Here we report that Pirt deficient female mice have an increased body weight and impaired glucose tolerance relative to wild-type (WT) mice. This increased susceptibility to develop obesity and glucose intolerance in female Pirt−/− mice is abrogated in the presence of a high-fat, high-sugar (HFHS) diet as well as in male mice irrespective of the dietary regime. Finally, we reveal that Pirt is dispensable for TRPM8-induced BAT thermogenesis in vivo.
2. Material and methods
2.1. Animal housing and phenotyping conditions
The generation of the Pirt−/− mouse is described elsewhere [15]. The cohorts were generated from homozygous breeding. Mice were maintained at 23 ± 1 °C, constant humidity, and on a 12 h light–dark cycle with free access to food and water. Phenotypic analysis of Pirt−/− mice and WT mice was initiated at the age of 8 weeks. A male and female cohort of eight WT and eight Pirt−/− mice was phenotypically monitored on a standard chow diet. Another cohort of eight male and female WT and Pirt−/− mice was switched from chow to HFHS diet (58% kcal from fat; D12331, Research Diets, New Brunswick, NJ, USA) at the age of 8 weeks. Body weight and food intake were assessed on a weekly basis until the age of 25 weeks. All procedures were approved by the Animal Use and Care Committee of Bavaria, Germany.
2.2. Glucose tolerance test
At 26 weeks of age, a glucose tolerance test was performed. Mice were fasted for 6 h and challenged with a bolus injection of glucose (5 μl/g body weight, intraperitoneal (i.p.)). Male mice on a chow and HFHS diet received 1.5 g glucose/kg body weight; female mice on a chow and HFHS diet were injected with 2.0 g glucose/kg body weight. Blood glucose was measured from the tail veins at the indicated timepoints with a handheld glucometer (Abbott GmbH & Co. KG, Wiesbaden, Germany).
2.3. Energy metabolism studies
Energy expenditure, respiratory exchange ratio (RER), and home-cage locomotor activity were assessed in 27-weeks old chow-fed female WT and Pirt−/− mice using a combined indirect calorimetry system (TSE Systems, Bad Homburg, Germany). After a 24 h adaptation phase, oxygen consumption and carbon dioxide production were measured every 10 min for up to 61 h.
2.4. Whole body composition
Whole body composition (fat and lean mass) was measured using nuclear resonance technology (EchoMRI, Houston, TX, USA).
2.5. Acute icilin challenge
Female chow-fed WT and Pirt−/− mice received a single injection of phosphate-buffered saline (PBS) or icilin (2 μmol/kg body weight; 5 μl/g body weight injection volume, subcutaneous (s.c.); Cat.No. 36945-98-9 (ROE01), Bicoll, Planegg, Germany), while oxygen consumption, carbon dioxide production, and locomotor activity were registered with indirect calorimetry.
2.6. Immunohistochemistry
WT and Pirt−/− male mice were anesthetized with carbon dioxide and perfused by intracardiac puncture with saline and fixed with 4% paraformaldehyde (PFA) solution. Brains were harvested, kept in 4% PFA for 24 h at 4 °C, transferred to 30% sucrose, and sliced on a cryostat in the coronal plane at 30 μm. The slices were blocked in 0.25% gelatin and 0.5% Triton X-100 in tris-buffered saline (TBS) for 1 h and incubated with Pirt antibody (1:500, Biorbyt LLC, San Francisco, CA, USA; orb158159) diluted in the blocking solution (TBS containing 0.25% gelatin and 0.5% Triton X-100) at 4 °C, overnight. Sections were washed 3 times in TBS and incubated with Alexa Fluor® 568 (1:500, Thermo Fisher Scientific, Waltham, MA, USA; A-11011) diluted in the blocking solution for 1 h at room temperature and stained with DAPI solution (1:3000, Thermo Fisher Scientific; 62248) for 3 min. Sections were washed in TBS, dried, and mounted with Slowfade Gold mounting medium (Thermo Fisher Scientific). Image stacks (30 μm thick) were collected through the z-axis at an interval of 2 μm using a Leica SP5 scanning confocal microscope equipped with a 20× objective and final images obtained by maximum intensity projection of the z-stack.
2.7. Gene expression analysis
For expression profiling of Pirt, tissues were collected from male C57Bl/6j WT and Pirt−/− mice and immediately frozen on dry ice. RNA was extracted using QIAzol® Lysis Reagent (Qiagen, Hilden, Germany), and cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen). Gene expression was profiled with quantitative real-time PCR (qPCR) using SYBR® Green Real-Time PCR master mix (Life Technologies GmbH, Darmstadt, Germany). The relative expression of the Pirt gene (Forward primer 5′ACCACACCCAAAAGCAACTG′3; Reverse primer 5′GCCCTATCATCCTGAGCACT′3) was normalized to the reference genes hypoxanthine-guanine phosphoribosyltransferase (Hprt) (Forward primer 5′AAGCTTGCTGGTGAAAAGGA′3; Reverse primer 5′TTGCGCTCATCTTAGGCTTT′3) and peptidylprolyl isomerase B (Ppib) (Forward primer 5′GCATCTATGGTGAGCGCTTC′3; Reverse primer 5′CTCCACCTTCCGTACCACAT′3). The threshold cycle method (2−ΔΔCT) of comparative PCR was used to analyze the results.
2.8. Statistical analysis
Differences between genotypes or treatment were assessed by two-way ANOVA followed by Bonferroni's post hoc analysis as appropriate or an unpaired two-tailed Student's t-test. All results are presented as mean ± s.e.m. P < 0.05 was considered statistically significant.
3. Results
3.1. Pirt expression
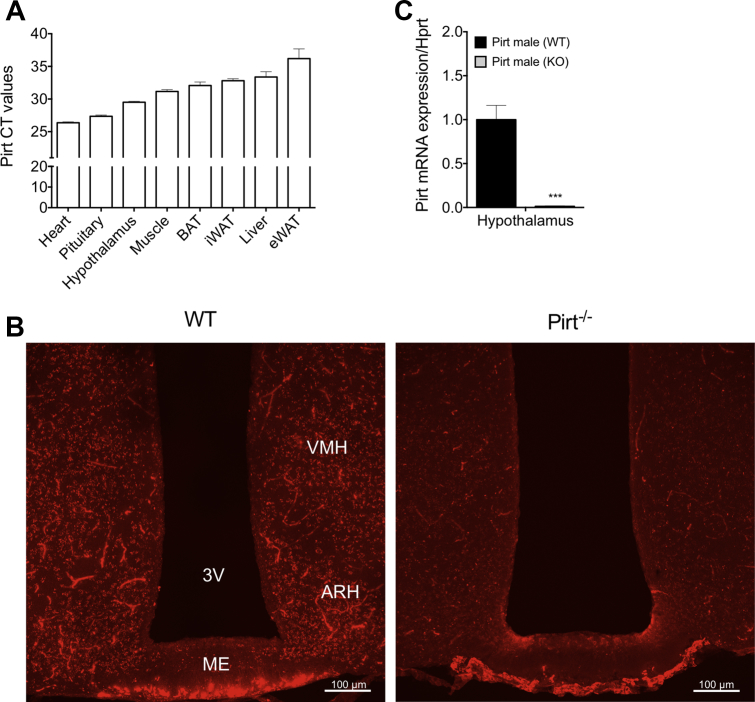
Pirt is reported to be highly expressed in dorsal root ganglia and trigeminal neurons and to a lesser extent in enteric and sympathetic neurons [15]. Aiming to uncover a role for Pirt in energy metabolism, we here investigated Pirt expression in key metabolic tissues including adipose tissue and the hypothalamus. We report that Pirt is highly expressed in the cardiac muscle, the pituitary gland, and the hypothalamus (Figure 1A). Pirt was not detectable in BAT, suggesting that any involvement in energy expenditure is mediated indirectly, likely through the peripheral nervous system (Figure 1A). Conversely, the observation that Pirt is highly expressed in the hypothalamus prompted us to further parse the expression pattern in this region, using immunohistochemistry (IHC) on brain slices from WT and Pirt−/− mice (Figure 1B). Therewith, we identified consistent Pirt protein expression in hypothalamic regions of the arcuate nucleus (ARH), the median eminence (ME), and part of the ventromedial hypothalamic nucleus (VMH). This expression pattern suggests that Pirt might play a role in the central control of energy metabolism – an implication substantiated by the detection of immunoreactivity suggesting Pirt protein expression in other key hypothalamic regions such as the medial preoptic nucleus (MPO) and the paraventricular hypothalamic nucleus (PVH) (Supplemental Figure 1A). Knockout of the Pirt gene and the Pirt protein in the hypothalamus was confirmed by IHC and qPCR (Figure 1B,C).
Figure 1.
Pirt is expressed in hypothalamic nuclei. Pirt gene expression, displayed as cycle of threshold (CT), in the heart muscle, pituitary gland, hypothalamus, quadriceps muscle, brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), liver, and epididymal white adipose tissue (eWAT) of wild-type (WT) mice (n = 6) (A). Immunohistochemistry in the region of the arcuate nucleus (ARH), the median eminence (ME), and part of the ventromedial hypothalamic nucleus (VMH) of brain slices from WT and Pirt−/− (KO) mice (B). Pirt gene expression in the hypothalamus of WT mice compared to Pirt−/− mice (n = 5 per genotype) (C). Scale bars in (B) are 100 μm. Data represent mean ± s.e.m. ***P < 0.001 determined by an unpaired two-tailed Student's t-test comparing WT with Pirt−/− mice. 3V: Third ventricle.
3.2. Global ablation of Pirt has subtle effects on energy homeostasis in chow-fed female mice
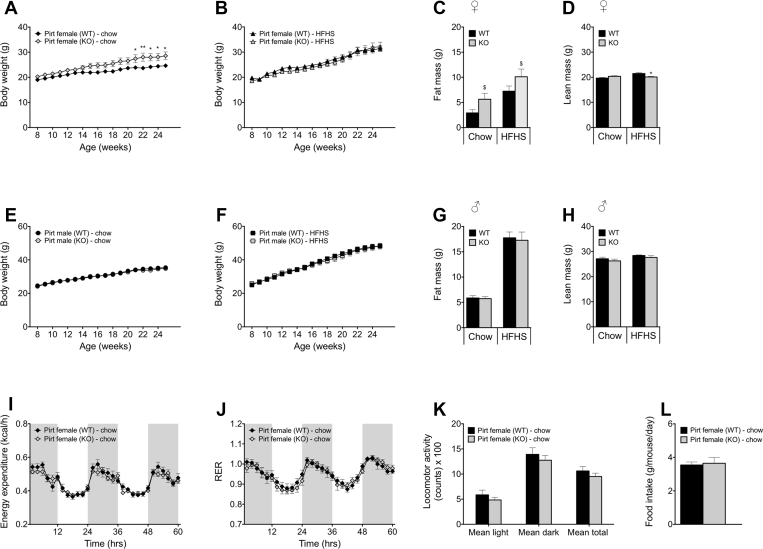
To evaluate the role of Pirt in systemic control of energy metabolism, we exposed both male and female Pirt−/− mice and their WT controls to comprehensive metabolic phenotyping. We introduced a gene-environment metabolic stressor by incorporation of a HFHS diet challenge in parallel cohorts of Pirt−/− and WT male and female mice. Pirt deficiency resulted in a marked increase in body weight in female mice maintained on a chow diet (Figure 2A) without differences in food intake (Figure 2L). Mirroring the difference in body weight, chow-fed female Pirt−/− mice exhibited a trend of more body fat relative to WT controls (Figure 2C, p = 0.064; Supplemental Figure 1B, p = 0.053), while lean mass was comparable between genotypes (Figure 2D). Whereas ablation of Pirt amplified body weight gain in female mice on a normal diet, this effect was annulled when mice were maintained on a HFHS diet (Figure 2B). Yet, body composition analysis revealed a main effect of genotype on fat mass (Figure 2C, p < 0.05; Supplemental Figure 1B, p < 0.05), while lean mass in female Pirt−/− mice on a HFHS diet was significantly reduced (Figure 2D, p < 0.05). Aiming to further understand the enhanced weight gain susceptibility in female Pirt−/− mice, we employed indirect calorimetry to analyze energy expenditure, substrate utilization, and locomotion. We did not observe differences in energy expenditure, RER, or locomotor activity between Pirt−/− and WT female mice (Figure 2I,J,K). Moreover, we failed to measure differences in the expression of thermogenic genes such as Ucp1, iodothyronine deiodinase 2 (Dio2), or PR domain-containing 16 (Prdm16) in the BAT of chow-fed female WT and Pirt−/− mice (Supplemental Figure 2). Pirt knockout did not impact body weight in male mice on a standard chow (Figure 2E) or on a HFHS diet (Figure 2F). Similarly, chow-fed and HFHS-fed male WT and Pirt−/− mice did not differ with respect to food intake (data not shown) or body composition (Figure 2G,H; Supplemental Figure 1C).
Figure 2.
Sex- and diet-specific effects of Pirt deficiency on energy metabolism. Body weight progression of female wild-type (WT) (black) and Pirt−/− (KO) (grey) mice on a standard chow (A) and high-fat, high-sugar (HFHS) diet (B). Fat mass (C) and lean mass (D) of the female cohorts at 27 weeks of age. Body weight progression of male WT and Pirt−/− mice on a standard chow (E) and HFHS diet (F). Fat mass (G) and lean mass (H) of the male cohorts at 27 weeks of age. Energy expenditure (I), respiratory exchange ratio (RER) (J), and mean locomotor activity (K) of female chow-fed WT and Pirt−/− mice at 27 weeks of age. Average daily food intake in female chow-fed WT and Pirt−/− mice from week 8 to week 25 of age (L). Phenotyping cohorts with n = 8 per sex, diet, and genotype. Data represent mean ± s.e.m. Data presented in longitudinal graphs (A,B,E,F,I,J) were analyzed by two-way ANOVA (genotype and time) and data presented in bar graphs (C,D,G,H,K,L) were analyzed by two-way ANOVA (genotype and diet) or an unpaired two-tailed Student's t-test comparing WT with Pirt−/− mice. ANOVA was followed by Bonferroni post hoc multiple comparison analysis to determine statistical significance. *P < 0.05, **P < 0.01 effects of genotype within the diets and $P < 0.05 main effects of genotype irrespective of diet.
3.3. Ablation of Pirt compromises glucose metabolism in female but not male mice
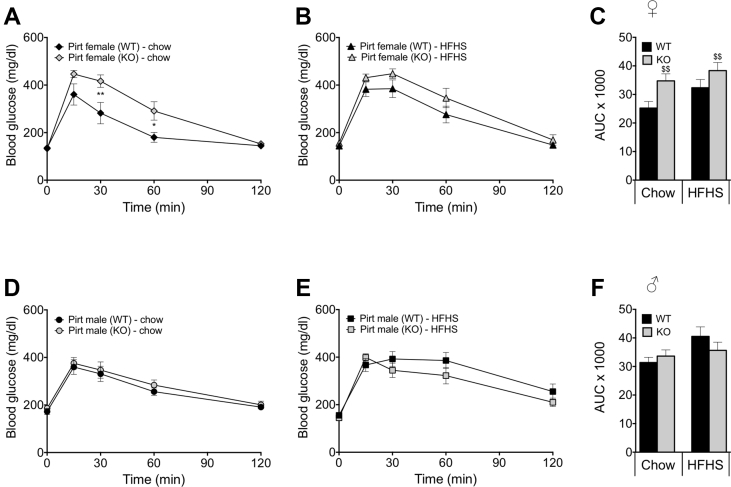
In order to determine the role of Pirt in glucose metabolism, we performed an intraperitoneal glucose tolerance test (GTT) in chow-fed and HFHS-fed (18 weeks of exposure) WT and Pirt−/− mice of both sexes. Female Pirt−/− mice maintained on a chow diet displayed an impaired glucose tolerance relative to WT control mice (Figure 3A,C, p < 0.05 and 0.01 resp.). Corroborating the weight phenotype, the impaired glucose tolerance observed in chow-fed female Pirt−/− mice was abrogated when the female mice were exposed to a HFHS diet; however, the area under the curve of female Pirt−/− mice, irrespective of the feeding regime, was significantly impaired as a main effect of genotype (Figure 3B,C, p < 0.01). No difference in glucose tolerance between male, chow-fed and HFHS-fed WT and Pirt−/− mice was observed (Figure 3D–F).
Figure 3.
Female Pirt deficient mice have an impaired glucose tolerance. Blood glucose traces after an intraperitoneal glucose tolerance test in female chow-fed (A) and high-fat, high-sugar (HFHS) diet-fed (B) and male chow-fed (D) and HFHS diet-fed (E) wild-type (WT) (black) and Pirt−/− (KO) (grey) mice with the corresponding area under the curve (AUC) (C,F) at 26 weeks of age. Phenotyping cohorts with n = 8 per sex, diet, and genotype. Data represent mean ± s.e.m. Data presented in line graphs (A,B,D,E) were analyzed by two-way ANOVA (genotype and time) and data presented in bar graphs (C,F) were analyzed by two-way ANOVA (genotype and diet) comparing WT with Pirt−/− mice. ANOVA was followed by Bonferroni post hoc multiple comparison analysis to determine statistical significance. *P < 0.05, **P < 0.01 effects of genotype within the diets and $$P < 0.01 main effects of genotype irrespective of diet.
3.4. TRPM8-linked amplification in energy expenditure is Pirt-independent
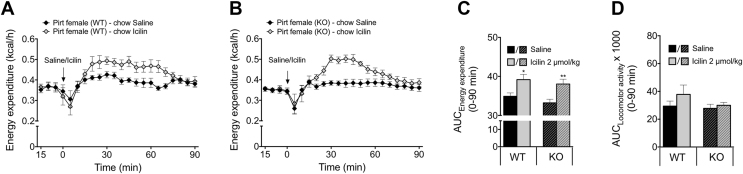
Since Pirt is a regulator of TRPM8 signaling [16], we next explored whether TRPM8-induced thermogenesis is compromised in mice lacking Pirt. A single s.c. injection of the TRPM8 super agonist icilin (2 μmol/kg) significantly increased energy expenditure to a similar extent in WT and Pirt−/− mice relative to saline-treated controls (Figure 4A–C). The amplification in energy expenditure following icilin treatment was independent of locomotor activity (Figure 4D). Thus, pharmacological activation of TRPM8 increases energy expenditure in a Pirt-independent fashion.
Figure 4.
Pharmacological activation of TRPM8 with icilin induces energy expenditure in wild-type and Pirt−/− mice. Energy expenditure of female chow-fed wild-type (WT) (A) and Pirt−/− (KO) mice (B) after subcutaneous injections of icilin (2 μmol/kg) (grey) or saline control (black) (n = 8 per genotype) with respective area under the curve (AUC) after icilin and saline injections (0–90 min) (C) and AUC of locomotor activity in the same period (D). Data represent mean ± s.e.m. *P < 0.05, **P < 0.01 determined by an unpaired two-tailed Student's t-test comparing saline and icilin injections within genotype.
4. Discussion
We here examined the impact of global Pirt gene ablation on energy and glucose metabolism in female and male mice fed either chow or high-fat, high-sugar diet. We identified a subtle increase in the susceptibility to develop obesity and glucose intolerance in Pirt−/− female mice on a normal chow diet. This effect was absent when the animals were maintained on a HFHS diet, although fat mass was significantly increased in both lean and DIO female Pirt−/− mice. Male mice deficient for Pirt displayed no differences in glucose or energy metabolism relative to WT controls.
Recent reports describe a regulatory involvement of Pirt in the TRPM8 signaling pathway [16]. Because pharmacological TRPM8 activation induces UCP1-dependent thermogenesis in BAT [10], [13], we hypothesized that the lack of Pirt would impair energy homeostasis. In concordance with the proposed lack of TRPM8 expression in BAT [13], we did not find Pirt expressed in BAT. Thus, any effect of Pirt on BAT thermogenesis is most likely indirect, through neuronal relays, which is in line with the suggested indirect effect of TRPM8 activation on BAT-dependent thermogenesis [13]. Interestingly, we identified, for the first time, a robust Pirt expression in several hypothalamic nuclei. The hypothalamus serves as the central hub for coordinating energy metabolic cues including sensory signals arising from the peripheral nervous system. Accordingly, the hypothalamic Pirt expression points to a possible central integrative function, which instigated us to continue our investigations on the role of Pirt in energy homeostatic control.
Because Pirt is involved in ambient temperature sensing [16], thermoneutral conditions may blunt the physiological role of Pirt. Consequently, we decided to execute the metabolic phenotyping at temperatures below the thermoneutral zone. Only female Pirt−/− mice that were fed a standard chow diet gained significantly more body weight compared to WT mice without detectable differences in food intake, energy expenditure, or locomotor activity. The fact that the female Pirt−/− mice did not display a higher energy expenditure following the separation in body weight could indicate subtle alterations in energy expenditure. Consequently, we assessed thermogenic gene programs in the BAT of female chow-fed WT and Pirt−/− mice but observed no differences. Thus, the inability of female Pirt−/− mice to adequately activate energy expenditure mechanisms does not seem to result from defective BAT-dependent thermogenesis. Female C57Bl/6 mice are reported to have a higher core body temperature, which is associated with an increased oxygen consumption [19], [20], [21], [22]. Pirt deficient mice exhibit defects in cold sensation [16]; the lack of Pirt in female mice on a standard chow diet could thus influence the capability to adjust metabolism to a mild cold stress. This is speculative, as is the ability of HFHS diet-induced thermogenesis to overwrite this susceptibility. Of note, although DIO female Pirt−/− mice did not differ from WT mice with respect to body weight, deficiency of Pirt did result in an altered body composition and relatively more body fat compared to WT mice, supporting the notion that Pirt deficiency results in subtle metabolic defects in females. Additional work is needed to tease out a possible interaction between Pirt in temperature regulation and Pirt in metabolic control. In context, a limitation of this study is that we did not investigate how Pirt deficient mice defend their body temperature at sub-ambient room temperatures and the potentially associated impact on body weight, which should be a relevant focus for future studies on Pirt in vivo.
Out of the four cohorts of mice studied here, only the female chow-fed Pirt−/− mice had an impaired glucose metabolism compared to WT mice. Because of a tight relationship between obesity, glucose intolerance, and diabetes [23], we cannot rule out that the impaired glucose tolerance in Pirt−/− female mice is secondary to the increased body weight.
Pirt plays a regulatory role in altering the gating properties of TRPM8 following both cold temperature exposure and menthol application as demonstrated through in vitro experiments [16], [18]. We here investigated whether TRPM8 activity also requires Pirt in vivo. Thus, we pharmacologically activated TRPM8 receptors with icilin, a TRPM8 super agonist, and monitored energy expenditure in female WT and Pirt−/− mice. Our results show that the icilin-evoked TRPM8-dependent energy expenditure induction is independent of Pirt. Consistent with this notion, divergent pathways downstream of TRPM8 have been reported [18], and more work is now needed to tease out TRPM8-Pirt signaling under diverse physiological challenges. Finally, since Pirt can regulate the function of other TRP channels, including TRPV1 and heat sensing [15], comprehensive investigations of metabolically relevant TRP channels – in particular those expressed in the hypothalamus – and their possible interconnectedness with Pirt are warranted.
5. Conclusion
In summary, we here report that Pirt contributes subtle, sex-specific effects on energy and glucose regulation. In contrast to emerging evidence based on in vitro studies [16], [18], Pirt does not appear to play a role for TRPM8-stimulated energy expenditure in vivo.
Author contributions
S.J. designed and performed the experiments, analyzed and interpreted data, and drafted the manuscript. G.C. and K.F. helped perform experiments. X.D. kindly provided the Pirt−/− mouse. B.F., M.H.T., T.D.M., and C.C. co-conceptualized the project, analyzed and interpreted data, and co-wrote the manuscript with S.J.
Acknowledgments
We thank Heidi Hofmann, Emilija Malogajski, Luisa Müller, Laura Sehrer, and Jakob Langer for assistance with in vivo and in vitro experiments.
This work was supported by: The Alfred Benzon Foundation, The Lundbeck Foundation Fellowship: R238-2016-2859, The Novo Nordisk Foundation (Grant number NNF17OC0026114), the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program under grant agreement No 694968 (PREMSOT), the Alexander von Humboldt Foundation, the Helmholtz Alliance ICEMED & the Initiative and Networking Fund of the Helmholtz Association, the Helmholtz initiative on Personalized Medicine iMed, the Helmholtz cross-program “Metabolic Dysfunction”, German Research Foundation DFG-TRR152-TP23. Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark and partially funded by an unconditional donation from the Novo Nordisk Foundation (Grant number NNF18CC0034900.)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.02.011.
Contributor Information
Timo D. Müller, Email: timo.mueller@helmholtz-muenchen.de.
Christoffer Clemmensen, Email: chc@sund.ku.dk.
Conflict of interest
M.H.T. serves as a SAB member of ERX Pharmaceuticals, Inc., Cambridge, MA. The Institute for Diabetes and Obesity receives research support from Novo Nordisk and Sanofi-Aventis. B.F. is currently employee of Novo Nordisk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng Y.H., Kokkotou E., Schulz T.J., Huang T.L., Winnay J.N., Taniguchi C.M. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cypess A.M., Kahn C.R. Brown fat as a therapy for obesity and diabetes. Current Opinion in Endocrinology Diabetes and Obesity. 2010;17(2):143–149. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 7.Rehnmark S., Nechad M., Herron D., Cannon B., Nedergaard J. Alpha- and beta-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. Journal of Biological Chemistry. 1990;265(27):16464–16471. [PubMed] [Google Scholar]
- 8.Ikeda K., Maretich P., Kajimura S. The common and distinct features of brown and beige adipocytes. Trends in Endocrinology and Metabolism. 2018;29(3):191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W., Medvedev A.V., Daniel K.W., Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. Journal of Biological Chemistry. 2001;276(29):27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- 10.Ma S., Yu H., Zhao Z., Luo Z., Chen J., Ni Y. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. Journal of Molecular Cell Biology. 2012;4(2):88–96. doi: 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- 11.Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 12.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Clemmensen C., Jall S., Kleinert M., Quarta C., Gruber T., Reber J. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nature Communications. 2018;9(1):4304. doi: 10.1038/s41467-018-06769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahern G.P. Transient receptor potential channels and energy homeostasis. Trends in Endocrinology and Metabolism. 2013;24(11):554–560. doi: 10.1016/j.tem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim A.Y., Tang Z., Liu Q., Patel K.N., Maag D., Geng Y. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133(3):475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z., Kim A., Masuch T., Park K., Weng H., Wetzel C. Pirt functions as an endogenous regulator of TRPM8. Nature Communications. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilton J.K., Salehpour T., Sisco N.J., Rath P., Van Horn W.D. Phosphoinositide-interacting regulator of TRP (PIRT) has opposing effects on human and mouse TRPM8 ion channels. Journal of Biological Chemistry. 2018;293(24):9423–9434. doi: 10.1074/jbc.RA118.003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang M., Wu G.Y., Dong X.Z., Tang Z.X. Phosphoinositide interacting regulator of TRP (Pirt) enhances TRPM8 channel activity in vitro via increasing channel conductance. Acta Pharmacologica Sinica. 2016;37(1):98–104. doi: 10.1038/aps.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Alavez M., Alboni S., Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordrecht, Netherlands) 2011;33(1):89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J.N., Tiselius C., Dare E., Johansson B., Valen G., Fredholm B.B. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiologica. 2007;190(1):63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- 21.Du Bois E.F. The basal metabolism in fever. Journal of the American Medical Association. 1921;77(5):352–355. [Google Scholar]
- 22.Landsberg L., Young J.B., Leonard W.R., Linsenmeier R.A., Turek F.W. Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Transactions of the American Clinical and Climatological Association. 2009;120:287–295. [PMC free article] [PubMed] [Google Scholar]
- 23.Williams L.M., Campbell F.M., Drew J.E., Koch C., Hoggard N., Rees W.D. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS One. 2014;9(8):e106159. doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.