Abstract
Activated protein C (APC) is a vitamin-K dependent plasma serine protease, which functions as a natural anticoagulant to downregulate thrombin generation in the clotting cascade. APC also modulates cellular homeostasis by exhibiting potent cytoprotective and anti-inflammatory signaling activities. The beneficial cytoprotective effects of APC have been extensively studied and confirmed in a number of preclinical disease and injury models including sepsis, type-1 diabetes and various ischemia/reperfusion diseases. It is now well-known that APC modulates downstream cell signaling networks and transcriptome profiles when it binds to the endothelial protein C receptor (EPCR) to activate protease-activated receptor 1 (PAR1) on various cell types. However, despite much progress, details of the downstream signaling mechanism of APC and its crosstalk with other signaling networks are far from being fully understood. In this review, we focus on the cardioprotective properties of APC in ischemic heart disease and heart failure with a special emphasis on recent discoveries related to the modulatory effect of APC on AMP-activated protein kinase (AMPK), PI3K/AKT, and mTORC1 signaling pathways. The cytoprotective properties of APC might provide a novel strategy for future therapies in cardiac diseases.
Keywords: activated protein C, endothelial protein C receptor, heart failure, ischemic heart disease, cardioprotection
1. Protein C System
1.1. Protein C Zymogen and Its Activation Mechanism
Protein C (PC) is a vitamin K-dependent serine protease zymogen that is primarily synthesized by the liver and circulates in plasma with a concentration of 4–5 μg/mL [1]. The gene for PC is located on chromosome 2, encoding 9 exons with 419 amino acids in length [2]. The mature protein contains two chains linked together by a single disulfide bond between Cys141 and Cys277 [2]. The N-terminal light chain contains the gamma-carboxyglutamic acid (Gla) domain and two epidermal growth factor (EGF-1, EGF-2) domains while the C-terminal heavy chain contains the trypsin-like serine protease domain [3]. Protein C through its Gla-domain binds with a high affinity to endothelial protein C receptor (EPCR) and is converted to activated protein C (APC) through a limited proteolytic process by the thrombin-thrombomodulin (TM)- complex on the surface of endothelial cells [4,5,6]. TM is a single chain transmembrane type-1 receptor glycoprotein composed of an N-terminal lectin-like domain, six EGF-like domains, and a short cytoplasmic tail [7,8,9]. The EGF-5 and -6 domains of TM bind to thrombin to change the substrate specificity of thrombin from a procoagulant to an anticoagulant enzyme by rendering it inactive toward fibrinogen but enabling it highly active toward PC [4,10]. The EGF-4 domain of TM binds the PC to facilitate the presentation of the PC zymogen into the catalytic pocket of thrombin [10,11]. The Gla-domain of PC has a high-affinity binding site for EPCR and this interaction on the endothelial cell surface enhances the activation of PC by the thrombin-TM complex approximately 20-fold [6].
1.2. Anticoagulant Mechanism
Upon activation by the thrombin-TM complex, APC can dissociate from its membrane-bound receptor cofactor, EPCR, and bind to its circulating vitamin K-dependent plasma cofactor, protein S, on a negatively charged membrane surface, to initiate its anticoagulant function by proteolytically inactivating factors Va and VIIIa, the essential procoagulant cofactors for thrombin generation in both intrinsic and extrinsic pathways of the blot clotting cascade [12,13,14]. Inactivation of factor Va by APC is mediated through cleavages at three Arg306, Arg506, and Arg679 protease recognition sites [15,16]. The cleavage at the Arg306 site induces the dissociation of factor Va from the negatively charged membrane surface, thereby leading to the complete inactivation of the cofactor [17]. The APC-protein S complex proteolytically inactivates factor VIIIa by a similar manner through the cleavage of the cofactor at Arg336 and Arg562 recognition sites [18]. A defect in the protein C anticoagulant pathway is associated with a hypercoagulable state that increases the clotting propensity of the blood, thereby resulting in pathological vascular thrombosis with variable severity due to uncontrolled thrombin generation [19,20]. The classic example is a clinical-pathological condition referred to it as “APC resistance” which was used to describe the phenomenon of a poor response to the anticoagulant activity of APC [21]. It was later discovered that APC resistance is mainly due to a mutation in the APC’s target gene factor V in which Arg506 of the cofactor is replaced with a Gln [22]. The mutant cofactor was then named Factor V-Leiden which predisposes the carriers to a hypercoagulable phenotype due to the poor recognition and inactivation of the variant cofactor by APC [22]. APC resistance has been demonstrated as a cardiovascular risk factor for atherosclerosis and arterial thrombosis among patients with stroke and venous thrombosis [23,24].
1.3. Anti-Inflammatory and Cytoprotective Mechanism
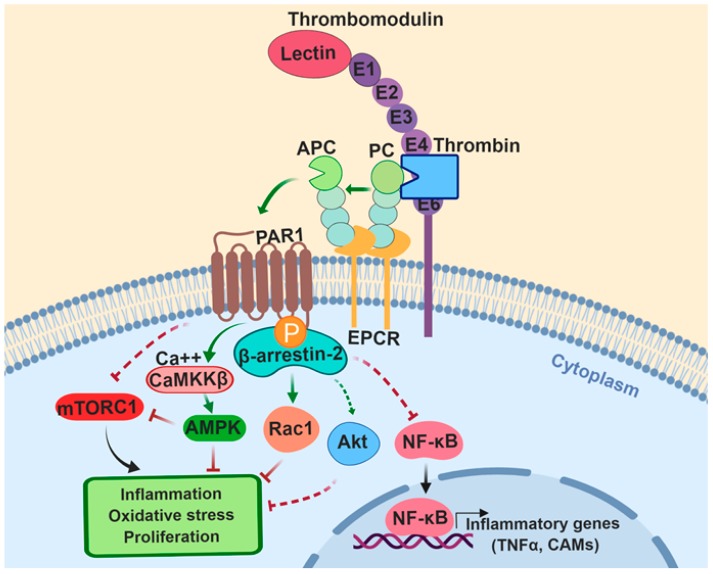
In addition to functioning as an essential anticoagulant protease in the clotting cascade, APC also exhibits potent anti-inflammatory and cytoprotective signaling activities when it remains associated with EPCR on the membrane surface [25]. It has been demonstrated that the Gla-domain of PC and APC binds EPCR with a similar affinity [26]. The protective cellular signaling activity of APC is mediated through the EPCR-bound protease-activating protease-activated receptor 1 (PAR1) on endothelial or other cell types (Figure 1) [27]. The PAR1-dependent anti-inflammatory activity of the APC-EPCR complex mediates the inhibition of expression of inflammatory genes including the inhibition of key transcription factors like the activator protein 1 (AP-1) family c-Fos and FosB [28,29,30]. The protective signaling also results in inhibition of the release of inflammatory cytokines (such as IL-1β, IL-6 and TNF-α), the inhibition of the nuclear translocation of NF-κB and the down-regulation of endothelial cell adhesion molecules such as ICAM-1, VCAM-1 and E-selectin (Figure 1), thereby limiting the leukocyte infiltration through the vascular system [28,29,30]. PAR1 is a G-protein coupled receptor (GPCR) which was first identified as a specific receptor for thrombin on platelets [31]. However, later studies showed that other coagulation proteases can cleave the receptor through different recognition sites on the extracellular domain of the receptor to initiate distinct intracellular signaling responses [32,33,34]. Thrombin cleaves PAR1 at the Arg41 site of the receptor to elicit pro-inflammatory responses in endothelial cells by mediating the phosphorylation of ERK1/2 and activation of the RhoA signaling pathway, thereby leading to disruption of the endothelial barrier function and edema formation [25,35,36]. On the other hand, the APC-EPCR complex cleaves the Arg46 site of PAR1 to initiate anti-inflammatory and cytoprotective signaling responses that culminate in activation of Rac1 signaling that counteracts the barrier disruptive effect of RhoA signaling in vascular endothelial cells [34,36]. Interestingly, it has been found that the occupancy of EPCR by the Gla-domain of PC/APC plays a key role in determining the signaling specificity of PAR1 [34]. Thus, when EPCR is occupied by its natural ligand, the PAR1-dependent signaling specificity of coagulation proteases is cytoprotective independent of the proteases cleaving either Arg41 or Arg46 cleavage sites [37,38]. It has been demonstrated that the receptors of protein C activation (TM) and APC signaling (EPCR and PAR1) are all colocalized in lipid-rafts of endothelial cells, [39] and that the occupancy of EPCR by the Gla-domain of protein C recruits GPCR kinase-5 (GRK-5) to the membrane, thereby phosphorylating the cytoplasmic domain of PAR1 and favoring its interaction with β-arrestin-2 rather than with a G-protein (Figure 1) [38]. Thus, a β-arrestin-2 biased EPCR-dependent PAR1 signaling by either thrombin and APC initiates anti-inflammatory and cytoprotective and anti-apoptotic signaling responses in vascular endothelial cells [34,36,38].
Figure 1.
The protein C activation by the thrombin-thrombomodulin complex and the cytoprotective function of Activated Protein C (APC) in ischemic heart disease and heart failure. The Endothelial Protein C Receptor (EPCR)-dependent cleavage of Protease-Activated Receptor 1 (PAR1) by APC initiates β-arrestin-2 biased signaling that results in the activation of Rac1 GTPase, Akt, and AMPK by the Ca2+/calmodulin-dependent protein kinase-kinase β (CaMKKβ) pathway. The PAR1-biased signaling also inhibits the activation/nuclear translocation of NF-κB and mTORC1 signaling network. E1, E2… represent EGF-like domains. See the text for more details. The figure was prepared by software provided by Biorender.com.
The EPCR- and PAR1-dependent anti-apoptotic activity of APC is partially mediated through the down-regulation of expression of the typical intrinsic apoptosis pathway genes including p53 and Bax and up-regulation of the expression of the oppositely functioning gene Bcl-2 [40]. Additionally, APC has been found to counteract apoptosis by inhibiting caspase-8 activation, which is the typical extrinsic apoptosis pathway gene [41]. Moreover, APC was reported to regulate both the inflammation and apoptosis processes by regulating the endoplasmic reticulum calcium flux and reducing the reactive oxygen species (ROS) accumulation [42]. Even though the anti-apoptotic effects of the APC pathway has been confirmed both in vitro and in vivo systems, additional future studies are required to clarify the mechanism through which APC exerts anti-apoptotic effects under different pathophysiological conditions.
2. Ischemic Heart Disease and APC Cardioprotection
2.1. Ischemia/Reperfusion (I/R) Injury and Cell Death
In the ischemic phase of heart disease, the ischemic injury is primarily caused by the blockage of the oxygen supply to heart tissues [43]. Insufficient oxygen supply results in a decrease in ATP synthesis by the mitochondria through oxidative phosphorylation. The energy and oxygen deficiency enhances the glycolytic rate and elevates H+ production, which eventually decreases the cytosolic pH. Meanwhile, the cytosolic Ca2+ channel is disturbed because the cell compensatory pH regulating system leads to an increase in Ca2+/Na+ exchange [43]. The accumulated Ca2+ would increase the plasma membrane permeability, increase the activity of cell-damaging proteases, and increase mitochondrial permeability by opening the mitochondrial permeability transition pore (mPTP). Such damage occurring in the mitochondrial membrane leads to the leakage of the electron transport chain and generation of ROS, primarily in the superoxide forms [43]. The oxidative stress is the main factor contributing to the intracellular injury that leads to necrotic cell death [43,44].
The re-entry of oxygen supply to myocytes is responsible for altering the intracellular metabolism and environment, thereby inducing further cellular damage called reperfusion injury [45,46]. The damage is mainly caused by the oxygen in the mitochondrial respiratory chain due to the activation and excessive activity of electron transferring enzymes leading to accumulation of ROS, oxidative stress and intracellular damage [47]. ROS is also involved in the activation of several pathogenetic networks including further increasing Ca2+ concentration, activating hydrolases and increasing mPTP that ultimately culminates in cell death [43,48,49]. Various signaling pathways have been reported to be significantly elevated or activated in the reperfusion phase, such as the mitogen-activated protein kinase family (MAPK), c-Jun N-terminal kinase (JNK), NF-κB, and apoptotic pathways [43,46,48,49]. The damaged cells from the I/R injury initiate inflammatory responses that result in the activation of macrophages, endothelial cells, neutrophils, lymphocytes, as well as the complement system, altering the expression levels of pro- and anti-inflammatory cytokines and inducing further cardiac cell death [43,44,45,46,47,48,49,50,51,52,53,54]. Three cell death mechanisms are involved in cardiac I/R injury. Apoptosis, a process of programmed cell death that is induced in I/R injury by the hypoxic stress and excessive oxidative stress from reperfusion [43]. It can be induced through the activation of the pro-apoptosis Bcl-2 family and other apoptotic protease-activating factors that compromise the integrity of the mitochondrial membrane, leading to caspase cascade activation and the degradation of mitochondria which is called mitoptosis [44,55,56]. Another type of programmed cell death, known as necroptosis in I/R injury could induce intensive local inflammation in ischemic tissue [56]. The key regulators of necroptosis are the RIP1 and RIP3 complex and the phosphorylated form of RIP3 can recruit RIP1 to activate the necrosome-induced necroptosis [57]. Additionally, the overexpression of RIP3 has been found to accompany excessive ROS generation and enhanced NF-κB transcription factor accumulation which is involved in the initiation of inflammation [58]. A compensatory cardioprotective signaling mechanism that is initiated in the stressed heart to cope with ischemic stress and ensuing energy depletion is the AMP-activated protein kinase (AMPK) signaling pathway [59,60,61,62]. The AMPK signaling can supply energy to the stressed heart through glycolysis and alternative metabolic pathways including autophagy through the process of degrading intracellular damaged organelles and macromolecules by lysosomal pathways [59,60,61,62,63,64,65]. In addition to providing ATP through alternative metabolic pathways, the AMPK signaling also exerts a cardioprotective effect in the I/R-induced stressed heart through inhibition of inflammatory MAPK and NF-kB signaling pathways [59].
2.2. Cardioprotective Effect of APC against I/R Injury
The observation that APC exhibits potent anticoagulant, anti-inflammatory and cytoprotective properties in conjunction with knowledge of the pathogenic mechanism of I/R injury which is associated with the up-regulation of pro-inflammatory and pro-apoptotic pathways as described above, providing the rationale for evaluating the cardioprotective function of APC in a couple of rat and mouse models of myocardial I/R injury [66,67]. The results indicated that treatment with APC during I/R restores the mean arterial pressure after a short time occlusion and reduces the mortality rate in experimental animals [66,67]. To understand the mechanism by which APC exerts a cardioprotective effect, we investigated this question by using human recombinant APC and its two signaling-selective and anticoagulant-selective derivatives in a mouse model of acute I/R injury [68]. The effect of APC derivatives was assessed on myocardial infarction size, post-ischemic cardiac function recovery and the modulation of inflammatory responses on cardiomyocytes [68]. We discovered that APC attenuates acute ischemic injury in the heart via stimulating the AMPK signaling and the inhibition of NF-κB and JNK signaling pathways by a mechanism(s) that is largely independent of its anticoagulant activity [68]. Thus, the administration of both APC and the signaling-selective APC-2Cys [69], but not the anticoagulant-selective and signaling-defective APC-E170A [70], reduced myocardial infarction and restored cardiac function in the ischemic mouse heart by activating AMPK in both in vivo and ex vivo model systems [68]. Further studies revealed that cardiomyocytes express EPCR and that both APC and APC-2Cys directly trigger AMPK phosphorylation in cardiomyocytes by enhancing the Ca2+/CaMKKβ activity by EPCR- and PAR1-dependent mechanisms (Figure 1) [68].
AMPK is a stress and energy sensitive kinase that can be activated by ATP depletion under ischemic stress [59,71]. AMPK is also an energy sensor and key regulator of metabolism mainly through glucose and fatty acid metabolism to maintain the homeostasis [59]. In myocardium, the AMPK-dependent activity of APC was found to stimulate glucose uptake through increasing the fusion of glucose transporter 4 (GLUT4) to the cell membrane and upregulating the glucose oxidation in the ischemic heart [72]. Interestingly, the upregulation of GLUT4 by the signaling-selective APC-2Cys variant was significantly higher than that of wild-type APC (72). In contrast to the upregulation of glucose uptake, APC-2Cys reduced fatty acid oxidation during I/R injury. This is an interesting observation since it is thought that the I/R-induced acceleration of cardiac fatty acid oxidation creates more ROS as compared to glucose oxidation. These results appear to suggest that APC-mediated reduction in fatty acid oxidation may lead to a decrease in ROS generation, thereby improving the intracellular redox status in the heart during I/R. Further support for this hypothesis was provided by the observation that APC-2Cys significantly increased the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) in an ex vivo working heart perfusion system after 10 min of global ischemia and 20 min of reperfusion [72]. This method has been commonly used as an indicator of the intracellular redox status in the heart tissue.
Interestingly, both wild-type APC and the signaling-selective APC-2Cys also increased the autophagic flux in the heart following I/R [72]. This was demonstrated by measuring the LC3 II/LC3 I ratio as an indicator of autophagy during I/R, which was significantly enhanced with both the wild-type APC and APC-2Cys treatment groups [72]. The increase in the autophagic activity of APC is likely mediated through its activation of AMPK since it has been demonstrated that AMPK is involved in inducing autophagy in the heart during I/R [73]. The activity of APC-2Cys in modulating these metabolic pathways was significantly higher than wild-type APC during I/R since it uniquely enhanced glucose oxidation and attenuated the I/R-initiated fatty acid oxidation by 80% [72]. The mechanism by which the signaling-selective APC-2Cys variants exhibits these unique AMPK-dependent cardioprotective properties is not known and warrants further investigation. It is known that, in addition to EPCR and PAR-1, APC exerts its cytoprotective signaling activities through crosstalk with other G-protein (i.e., PAR-3 [74] and sphingosine 1-phosphate receptor 1 [25]) and a number of non-G-protein coupled (i.e., apolipoprotein E receptor 2 [75], Tie2 [76] and Mac1 [77]) receptors. Thus, it is possible that differences in interaction and/or crosstalk by APC and APC-2Cys with one or more of these receptors participate in their differential modulatory effect on substrate metabolism in the I/R-stressed heart.
APC has been shown to induce the activation of AKT1 in the myocardium, thereby improving the endothelial function of the coronary artery in a global ischemic reperfusion rat model [78]. AKT is a serine/threonine kinase also known as protein kinase B (PKB) which activates mTOR, thereby playing multifaceted roles in the cellular metabolism, proliferation, inflammation, transcription and protein synthesis [79,80,81]. It has been recently found that the cytoprotective effects of APC in I/R injury are associated with the inhibition of mTORC1 signaling network, leading to inhibition of the NLRP3 inflammasome pathway [82]. It was demonstrated that the mTORC1 inhibitory effect of APC was mediated through APC, reducing the expression of the regulatory component of the mTORC1 complex (Raptor) [82], thereby inhibiting the phosphorylation of p70S6K, one of the downstream targets of the mTORC1 signaling pathway [83]. The mTROC1 signaling network is a target for down-regulation by the AMPK signaling pathway as well [59]. Thus, the exact cardioprotective mechanism of APC in the I/R injury and the possible elaborate and complex crosstalk between different signaling networks warrants further investigation.
2.3. Cardioprotective Function of APC in Heart Failure
Mounting evidence in the literature assigns a key cardioprotective role for AMPK in various cardiovascular disease models [84]. It has been established that the activation of AMPK plays a protective role during the initiation and progression of heart failure (HF) by regulating the metabolism and maintaining the homeostasis [84,85,86,87,88]. The activity of AMPK has been found to regulate substrate metabolism/utilization in a rat model of pressure overload-induced cardiac hypertrophy, indicating a critical role for AMPK in cardiac adaptive response under pathological conditions [84]. It has been also found that the AMPKα2 activity protects the heart against pressure overload-induced HF through mediating estrogen-related receptor α (ERRα) using the AMPKα2 deficient mice [89]. In a recent study, both APC and the signaling-selective non-anticoagulant APC-2Cys were shown to protect against pressure overload-induced hypertrophy through the AMPK signaling pathway [90]. AMPK has been found to reduce ROS accumulation by inhibiting NADPH oxidase activation in various cardiac disease models including HF [91,92]. In the pressure overload model, the AMPK-dependent cytoprotective signaling function of APC was found to be critical in the inhibition of ROS accumulation and inflammation through the down-regulation of the activity of p66shc and expression of 4-HNE in the hypertrophic model [90]. Similar cytoprotective effects for APC have been reported in the diabetic nephropathy model through the protease down-regulating p66shc by a PAR1-dependent mechanism [93]. The cardioprotective function and mechanism of APC in HF patients requires further investigation. Whether the protective signaling function of APC is beneficial in HF models with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) remains to be determined.
In addition to its cardioprotective properties, APC exhibits potent cytoprotective and anti-inflammatory effects in a number of other acute and chronic diseases including sepsis [94], ischemic stroke [95], acute kidney injury [96], type-1 diabetes [97], wound healing [98], Plasmodium falciparum malaria [99], post-surgical adhesion band formation [100] and other inflammatory disorders that have been nicely reviewed in recent review articles [101,102]. There are several ongoing pre-clinical and clinical trials evaluating the potential therapeutic utility of APC in animals and humans [101,102]. The future studies with the signaling-selective APC derivatives which do not exhibit significant anticoagulant activities and thus are not associated with an increased risk of bleeding may provide APC-based therapeutic strategies for some of these inflammatory diseases.
3. Conclusions
Ischemic heart disease and heart failure are the leading causes of morbidity and mortality worldwide. There is an urgent need for developing new therapeutic strategies for heart diseases. Results of studies with APC in I/R and HF models are encouraging and warrant further investigation. Because of its potent anticoagulant activity, APC-therapy has been found to also be associated with an increased risk of bleeding. The findings that the signaling-selective APC, lacking anticoagulant function, has a similar cardioprotective function in the I/R injury and HF models may open a new avenue for further investigating the life-saving effects of APC in heart disease without increasing the risk of bleeding.
Acknowledgments
The authors would like to thank Audrey Rezaie for proofreading the article.
Author Contributions
D.R. and J.L. wrote the first draft of the manuscript; H.G. contributed to art and editorial work; and A.R.R. wrote the final draft of the manuscript.
Funding
Financial support for the study was provided by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health HL101917 and HL062565 to ARR; and the National Heart, Lung, and Blood Institute P01HL051971, National Institute on Aging AG049835 and National Institute of General Medicine GM124108 and P20GM104357.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Griffin J.H., Mosher D.F., Zimmerman T.S., Kleiss A.J. Protein C, an antithrombotic protein, is reduced in hospitalized patients with intravascular coagulation. Blood. 1982;60:261–264. [PubMed] [Google Scholar]
- 2.Foster D., Davie E.W. Characterization of a cDNA coding for human protein C. Proc. Natl. Acad. Sci. USA. 1984;81:4766–4770. doi: 10.1073/pnas.81.15.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenflo J. Structure-function relationships of epidermal growth factor modules in vitamin K-dependent clotting factors. Blood. 1991;78:1637–1651. [PubMed] [Google Scholar]
- 4.Esmon C.T. Molecular events that control the protein C anticoagulant pathway. Thromb. Haemost. 1993;70:29–35. doi: 10.1055/s-0038-1646155. [DOI] [PubMed] [Google Scholar]
- 5.Fukudome K., Esmon C.T. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J. Biol. Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 6.Stearns-Kurosawa D.J., Kurosawa S., Mollica J.S., Ferrell G.L., Esmon C.T. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc. Natl. Acad. Sci. USA. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen D.Z., Dittman W.A., Ye R.D., Deaven L.L., Majerus P.W., Sadler J.E. Human thrombomodulin: Complete cDNA sequence and chromosome localization of the gene. Biochemistry. 1987;26:4350–4357. doi: 10.1021/bi00388a025. [DOI] [PubMed] [Google Scholar]
- 8.Weiler H., Isermann B.H. Thrombomodulin. J. Thromb. Haemost. 2003;7:1515–1524. doi: 10.1046/j.1538-7836.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 9.Van de Wouwer M., Collen D., Conway E.M. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 10.Fuentes-Prior P., Iwanaga Y., Huber R., Pagila R., Rumennik G., Seto M., Morser J., Light D.R., Bode W. Structural basis for the anticoagulant activity of the thrombin thrombomodulin complex. Nature. 2000;404:518–525. doi: 10.1038/35006683. [DOI] [PubMed] [Google Scholar]
- 11.Yang L., Rezaie A.R. The fourth epidermal growth factor-like domain of thrombomodulin interacts with the basic exosite of protein C. J. Biol. Chem. 2003;278:10484–10490. doi: 10.1074/jbc.M211797200. [DOI] [PubMed] [Google Scholar]
- 12.Dahlbäck B., Villoutreix B.O. Molecular recognition in the protein C anticoagulant pathway. J. Thromb. Haemost. 2003;1:1525–1534. doi: 10.1046/j.1538-7836.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker F.J., Fay P.J. Regulation of blood coagulation by the protein C system. FASEB J. 1992;6:2561–2567. doi: 10.1096/fasebj.6.8.1317308. [DOI] [PubMed] [Google Scholar]
- 14.Mann K.G., Butenas S., Brummel K. The dynamics of thrombin formation. Arterioscler. Thromb. Vasc. Biol. 2003;23:17–25. doi: 10.1161/01.ATV.0000046238.23903.FC. [DOI] [PubMed] [Google Scholar]
- 15.Kalafatis M., Rand M.D., Mann K.G. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J. Biol. Chem. 1994;269:31869–31880. [PubMed] [Google Scholar]
- 16.Friedrich U., Nicolaes G.A., Villoutreix B.O., Dahlbäck B. Secondary substrate-binding exosite in the serine protease domain of activated protein C important for cleavage at Arg-506 but not at Arg-306 in factor Va. J. Biol. Chem. 2001;276:23105–23108. doi: 10.1074/jbc.M103138200. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaes G.A., Tans G., Thomassen M.C., Hemker H.C., Pabinger I., Varadi K., Schwarz H.P., Rosing J. Peptide bond cleavages and loss of functional activity during inactivation of factor Va and factor VaR506Q by activated protein C. J. Biol. Chem. 1995;270:21158–21166. doi: 10.1074/jbc.270.36.21158. [DOI] [PubMed] [Google Scholar]
- 18.Manithody C., Fay P.J., Rezaie A.R. Exosite-dependent regulation of factor VIIIa by activated protein C. Blood. 2003;101:4802–4807. doi: 10.1182/blood-2003-01-0126. [DOI] [PubMed] [Google Scholar]
- 19.Griffin J.H., Evatt B., Zimmerman T.S., Kleiss A.J., Wideman C. Deficiency of protein C in congenital thrombotic disease. J. Clin. Investig. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reitsma P.H., Bernardi F., Doig R.G., Gandrille S., Greengard J.S., Ireland H., Krawczak M., Lind B., Long G.L., Poort S.R., et al. Protein C deficiency: A database of mutations, 1995 update. On behalf of the subcommittee on plasma coagulation inhibitors of the scientific and standardization committee of the ISTH. Thromb. Haemost. 1995;73:876–889. doi: 10.1055/s-0038-1653885. [DOI] [PubMed] [Google Scholar]
- 21.Svensson P.J., Dahlbäck B. Resistance to activated protein C as a basis for venous thrombosis. N. Engl. J. Med. 1994;330:517–522. doi: 10.1056/NEJM199402243300801. [DOI] [PubMed] [Google Scholar]
- 22.Rosendaal F.R., Koster T., Vandenbroucke J.P., Reitsma P.H. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance) Blood. 1995;85:1504–1508. [PubMed] [Google Scholar]
- 23.Halbmayer W.M., Haushofer A., Schon R., Fischer M. The prevalence of poor anticoagulant response to activated protein C (APC resistance) among patients suffering from stroke or venous thrombosis and among healthy subjects. Blood. Coagul. Fibrinolysis. 1994;5:51–57. doi: 10.1097/00001721-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kiechl S., Muigg A., Santer P., Mitterer M., Egger G., Oberhollenzer M., Oberhollenzer F., Mayr A., Gasperi A., Poewe W., et al. Poor response to activated protein C as a prominent risk predictor of advanced atherosclerosis and arterial disease. Circulation. 1999;99:614–619. doi: 10.1161/01.CIR.99.5.614. [DOI] [PubMed] [Google Scholar]
- 25.Mosnier L.O., Zlokovic B.V., Griffin J.H. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 26.Fukudome K., Kurosawa S., Stearns-Kurosawa D.J., He X., Rezaie A.R., Esmon C.T. The endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptor. J. Biol. Chem. 1996;271:17491–17498. doi: 10.1074/jbc.271.29.17491. [DOI] [PubMed] [Google Scholar]
- 27.Riewald M., Petrovan R.J., Donner A., Mueller B.M., Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 28.Joyce D.E., Gelbert L., Ciaccia A., DeHoff B., Grinnell B.W. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 29.Puthusseri B., Marudamuthu A., Tiwari N., Fu J., Idell S., Shetty S. Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L783–L796. doi: 10.1152/ajplung.00291.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okajima K. Prevention of endothelial cell injury by activated protein C: The molecular mechanism(s) and therapeutic implications. Curr. Vasc. Pharmacol. 2004;2:125–133. doi: 10.2174/1570161043476429. [DOI] [PubMed] [Google Scholar]
- 31.Vu T.K., Hung D.T., Wheaton V.I., Coughlin S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-V. [DOI] [PubMed] [Google Scholar]
- 32.Kondreddy V., Wang J., Keshava S., Esmon C.T., Rao L.V.M., Pendurthi U.R. Factor VIIa induces anti-inflammatory signaling via EPCR and PAR1. Blood. 2018;131:2379–2392. doi: 10.1182/blood-2017-10-813527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin K.M., Covic L., Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121:431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezaie A.R. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112:876–882. doi: 10.1160/th14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coughlin S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 36.Mosnier L.O., Sinha R.K., Burnier L., Bouwens E.A., Griffin J.H. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae J.S., Yang L., Manithody C., Rezaie A.R. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy R.V., Ardeshirylajimi A., Dinarvand P., Yang L., Rezaie A.R. Occupancy of human EPCR by protein C induces β-arrestin-2 biased PAR1 signaling by both APC and thrombin. Blood. 2016;128:1884–1893. doi: 10.1182/blood-2016-06-720581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae J.S., Yang L., Rezaie A.R. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc. Natl. Acad. Sci. USA. 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng T., Liu D., Griffin J.H., Fernandez J.A., Castellino F., Rosen E.D., Fukudome K., Zlokovic B.V. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 41.Liu D., Cheng T., Guo H., Fernández J.A., Griffin J.H., Song X., Zlokovic B.V. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat. Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 42.Toltl L.J., Austin R.C., Liaw P.C. Activated protein C modulates inflammation, apoptosis and tissue factor procoagulant activity by regulating endoplasmic reticulum calcium depletion in blood monocytes. J. Thromb. Haemost. 2011;9:582–592. doi: 10.1111/j.1538-7836.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 43.De Groot H., Rauen U. Ischemia-reperfusion injury: Processes in pathogenetic networks: A review. Transplant. Proc. 2007;39:481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Wu M.Y., Yiang G.T., Liao W.T., Tsai A.P., Cheng Y.L., Cheng P.W., Li C.Y., Li C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol. Biochem. 2018;46:1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 45.Buja L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Crowley L.E., McIntyre C.W. Remote ischaemic conditioning-therapeutic opportunities in renal medicine. Nat. Rev. Nephrol. 2013;9:739–746. doi: 10.1038/nrneph.2013.226. [DOI] [PubMed] [Google Scholar]
- 47.Nichols T.C. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- 48.Yelle D. Ischemic Heart Disease. [(accessed on 15 March 2019)]; Available online: http://www.pathophys.org/acs/
- 49.Chen X., Li X., Zhang W., He J., Xu B., Lei B., Wang Z., Cates C., Rousselle T., Li J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kB pathway. Metabolism. 2018;83:256–270. doi: 10.1016/j.metabol.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills N.L., Tornqvist H., Gonzalez M.C., Vink E., Robinson S.D., Soderberg S., Boon N.A., Donaldson K., Sandstrom T., Blomberg A., et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N. Engl. J. Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 51.Chang N.J., Weng W.H., Chang K.H., Liu E.K., Chuang C.K., Luo C.C., Lin C.H., Wei F.C., Pang S.T. Genome-wide gene expression profiling of ischemia-reperfusion injury in rat kidney, intestine and skeletal muscle implicate a common involvement of MAPK signaling pathway. Mol. Med. Rep. 2015;11:3786–3793. doi: 10.3892/mmr.2015.3235. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman J.W., Jr., Gilbert T.B., Poston R.S., Silldorff E.P. Myocardial reperfusion injury: Etiology, mechanisms, and therapies. J. Extra. Corpor. Technol. 2004;36:391–411. [PubMed] [Google Scholar]
- 53.Hsieh Y.H., Huang S.S., Wei F.C., Hung L.M. Resveratrol attenuates ischemia-reperfusion-induced leukocyte-endothelial cell adhesive interactions and prolongs allograft survival across the MHC barrier. Circ. J. 2007;71:423–428. doi: 10.1253/circj.71.423. [DOI] [PubMed] [Google Scholar]
- 54.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X., Zhang X., Xue L., Hao C., Liao W., Wan Q. Treatment with enriched environment reduces neuronal apoptosis in the periinfarct cortex after cerebral ischemia/reperfusion injury. Cell. Physiol. Biochem. 2017;41:1445–1456. doi: 10.1159/000468368. [DOI] [PubMed] [Google Scholar]
- 56.Yu H., Zhang H., Zhao W., Guo L., Li X., Li Y., Zhang X., Sun Y. Gypenoside protects against myocardial ischemia-reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of CHOP pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell. Physiol. Biochem. 2016;39:123–136. doi: 10.1159/000445611. [DOI] [PubMed] [Google Scholar]
- 57.Vanlangenakker N., Vanden Berghe T., Krysko D.V., Festjens N., Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 58.Vanden Berghe T., Vanlangenakker N., Parthoens E., Deckers W., Devos M., Festjens N., Guerin C.J., Brunk U.T., Declercq W., Vandenabeele P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell. Death. Differ. 2010;17:922–930. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 59.Young L.H., Li J., Baron S.J., Russell R.R. AMP-activated protein kinase: A key stress signaling pathway in the heart. Trends Cardiovasc. Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Quan N., Wang L., Chen X., Luckett C., Cates C., Rousselle T., Zheng Y., Li J. Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1α pathway. J. Mol. Cell. Cardiol. 2018;115:170–178. doi: 10.1016/j.yjmcc.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H., Sun W., Quan N., Wang L., Chu D., Cates C., Liu Q., Zheng Y., Li J. Cardioprotective actions of Notch1 against myocardial infarction via LKB1-dependent AMPK signaling pathway. Biochem. Pharmacol. 2016;108:47–57. doi: 10.1016/j.bcp.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison A., Chen L., Wang J., Zhang M., Yang H., Ma Y., Budanov A., Lee J.H., Karin M., Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015;29:408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma S., Wang Y., Chen Y., Cao F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys. Acta. 2015;1852:271–276. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Gustafsson A.B., Gottlieb R.A. Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416–421. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quan N., Sun W., Wang L., Chen X., Bogan J.S., Zhou X., Cates C., Liu Q., Zheng Y., Li J. Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism. FASEB J. 2017;31:4153–4167. doi: 10.1096/fj.201700063R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pirat B., Muderrisoglu H., Unal M.T., Ozdemir H., Yildirir A., Yucel M., Turkoglu S. Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion. Coron. Artery Dis. 2007;18:61–66. doi: 10.1097/MCA.0b013e328010a44a. [DOI] [PubMed] [Google Scholar]
- 67.Loubele S.T., Spek C.A., Leenders P., van Oerle R., Aberson H.L., Hamulyak K., Ferrell G., Esmon C.T., Spronk H.M., ten Cate H. Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 2009;29:1087–1092. doi: 10.1161/ATVBAHA.109.188656. [DOI] [PubMed] [Google Scholar]
- 68.Wang J., Yang L., Rezaie A.R., Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J. Thromb. Haemost. 2011;9:1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bae J.S., Yang L., Manithody C., Rezaie A.R. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J. Biol. Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 70.Yang L., Bae J.S., Manithody C., Rezaie A.R. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J. Biol. Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 71.Li J., Coven D.L., Miller E.J., Hu X., Young M.E., Carling D., Sinusas A.J., Young L.H. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am. J. Physiol. Heart. Circ. Physiol. 2006;291:H1927–H1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 72.Costa R., Morrison A., Wang J., Manithody C., Li J., Rezaie A.R. Activated protein C modulates cardiac metabolism and augments autophagy in the ischemic heart. J. Thromb. Haemost. 2012;10:1736–1744. doi: 10.1111/j.1538-7836.2012.04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsui Y., Takagi H., Abdellatif M., Sakoda H., Asano T., Levine B., Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and beclin 1 in mediating autophagy. Circ. Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 74.Burnier L., Mosnier L.O. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood. 2013;122:807–816. doi: 10.1182/blood-2013-03-488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X.V., Banerjee Y., Fernández J.A., Deguchi H., Xu X., Mosnier L.O., Urbanus R.T., de Groot P.G., White-Adams T.C., McCarty O.J., et al. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc. Natl. Acad. Sci. USA. 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minhas N., Xue M., Fukudome K., Jackson C.J. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24:873–881. doi: 10.1096/fj.09-134445. [DOI] [PubMed] [Google Scholar]
- 77.Cao C., Gao Y., Li Y., Antalis T.M., Castellino F.J., Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J. Clin. Investig. 2010;120:1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maehata Y., Miyagawa S., Sawa Y. Activated protein C has a protective effect against myocardial I/R injury by improvement of endothelial function and activation of AKT1. PLoS ONE. 2012;7:e38738. doi: 10.1371/journal.pone.0038738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vander Haar E., Lee S.I., Bandhakavi S., Griffin T.J., Kim D.H. Insulin signaling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell. Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 80.Yu J.S., Cui W. Proliferation, survival and metabolism: The role of PI3K/Akt/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 81.Xie Y., Shi X., Sheng K., Han G., Li W., Zhao Q., Jiang B., Feng J., Li J., Gu Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review) Mol. Med. Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nazir S., Gadi I., Al-Dabet M.M., Elwakiel A., Kohli S., Ghosh S., Manoharan J., Ranjan S., Bock F., Braun-Dullaeus R.C., et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood. 2017;130:2664–2677. doi: 10.1182/blood-2017-05-782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 84.Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D.R., Kumada M., Sato K., Schiekofer S., Ohashi K., et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian R., Musi N., D’Agostino J., Hirshman M.F., Goodyear L.J. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 86.Kim M., Shen M., Ngoy S., Karamanlidis G., Liao R., Tian R. AMPK isoform expression in the normal and failing hearts. J. Mol. Cell. Cardiol. 2012;52:1066–1073. doi: 10.1016/j.yjmcc.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu T.M., Tsai J.Y., Chen Y.C., Huang C.Y., Hsu H.L., Weng C.F., Shih C.C., Hsu C.P. Downregulation of Sirt1 as aging change in advanced heart failure. J. Biomed. Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. doi: 10.1161/01.CIR.0000139340.88769.D5. [DOI] [PubMed] [Google Scholar]
- 89.Hu X., Xu X., Lu Z., Zhang P., Fassett J., Zhang Y., Xin Y., Hall J.L., Viollet B., Bache R.J., et al. AMP activated protein kinase-α2 regulates expression of estrogen related receptor- α, a metabolic transcription factor related to heart failure development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cates C., Rousselle T., Wang J., Quan N., Wang L., Chen X., Yang L., Rezaie A.R., Li J. Activated protein C protects against pressure overload-induced hypertrophy through AMPK signaling. Biochem. Biophys. Res. Commun. 2018;495:2584–2594. doi: 10.1016/j.bbrc.2017.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X., Liu J., Lu Q., Ren D., Sun X., Rousselle T., Tan Y., Li J. AMPK: A therapeutic target of heart failure-not only metabolism regulation. Biosci. Rep. 2019;39:BSR20181767. doi: 10.1042/BSR20181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X., Liu J., Hu H., Lu S., Lu Q., Quan N., Rousselle T., Patel M.S., Li J. Dichloroacetate ameliorates cardiac dysfunction caused by ischemic insults through AMPK signal pathway-not only shifts metabolism. Toxicol. Sci. 2019;167:604–617. doi: 10.1093/toxsci/kfy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bock F., Shahzad K., Wang H., Stoyanov S., Wolter J., Dong W., Pelicci P.G., Kashif M., Ranjan S., Schmidt S., et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66shc. Proc. Natl. Acad. Sci. USA. 2013;110:648–653. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toltl L.J., Swystun L.L., Pepler L., Liaw P.C. Protective effects of activated protein C in sepsis. Thromb. Haemost. 2008;100:582–592. doi: 10.1160/TH08-03-0159. [DOI] [PubMed] [Google Scholar]
- 95.Sinha R.K., Wang Y., Zhao Z., Xu X., Burnier L., Gupta N., Fernández J.A., Martin G., Kupriyanov S., Mosnier L.O., et al. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood. 2018;131:1163–1171. doi: 10.1182/blood-2017-10-810895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lattenist L., Jansen M.P., Teske G., Claessen N., Meijers J.C., Rezaie A.R., Esmon C.T., Florquin S., Roelofs J.J. Activated protein C protects against renal ischaemia/reperfusion injury, independent of its anticoagulant properties. Thromb. Haemost. 2016;116:124–133. doi: 10.1160/TH15-07-0584. [DOI] [PubMed] [Google Scholar]
- 97.Xue M., Dervish S., Harrison L.C., Fulcher G., Jackson C.J. Activated protein C inhibits pancreatic islet inflammation, stimulates T regulatory cells, and prevents diabetes in non-obese diabetic (NOD) mice. J. Biol. Chem. 2012;287:16356–16364. doi: 10.1074/jbc.M111.325951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wijewardena A., Lajevardi S.S., Vandervord E., Vandervord J., Lang T.C., Fulcher G., Jackson C.J. Activated protein C to heal pressure ulcers. Int. Wound J. 2016;13:986–991. doi: 10.1111/iwj.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rankin L.G., Austin D.L. The use of activated protein C in severe Plasmodium falciparum malaria. Anaesth. Intensiv. Care. 2007;35:428–432. doi: 10.1177/0310057X0703500320. [DOI] [PubMed] [Google Scholar]
- 100.Dinarvand P., Hassanian S.M., Weiler H., Rezaie A.R. Intraperitoneal administration of activated protein C prevents postsurgical adhesion band formation. Blood. 2015;125:1339–1348. doi: 10.1182/blood-2014-10-609339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao R., Lin H., Bereza-Malcolm L., Clarke E., Jackson C.J., Xue M. Activated Protein C in Cutaneous Wound Healing: From Bench to Bedside. Int. J. Mol. Sci. 2019;19:903. doi: 10.3390/ijms20040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McDonnell C.J., Soule E.E., Walsh P.T., O’Donnell J.S., Preston R.J.S. The Immunoregulatory Activities of Activated Protein C in Inflammatory Disease. Semin. Thromb. Hemost. 2018;44:167–175. doi: 10.1055/s-0037-1608910. [DOI] [PubMed] [Google Scholar]