Abstract
To develop new antibacterial agents, a series of novel triazole-containing pyrazole ester derivatives were designed and synthesized and their biological activities were evaluated as potential topoisomerase II inhibitors. Compound 4d exhibited the most potent antibacterial activity with Minimum inhibitory concentration (MIC) alues of 4 µg/mL, 2 µg/mL, 4 µg/mL, and 0.5 µg/mL against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum, respectively. The in vivo enzyme inhibition assay 4d displayed the most potent topoisomerase II (IC50 = 13.5 µg/mL) and topoisomerase IV (IC50 = 24.2 µg/mL) inhibitory activity. Molecular docking was performed to position compound 4d into the topoisomerase II active site to determine the probable binding conformation. In summary, compound 4d may serve as potential topoisomerase II inhibitor.
Keywords: pyrazole, triazole, ester, antibacterial, topoisomerase II inhibitor
1. Introduction
Bacterial type II topoisomerases (DNA gyrase and topoisomerase IV) are ubiquitous and essential enzymes that have critical roles in the fundamental biological processes of replication, transcription, recombination, repair, and chromatin remodeling [1,2]. The action of topoisomerases is to change the spatial structure of DNA by catenation and decatenation of duplex DNA rings, relaxation of supercoiled DNA, and in the use of DNA topoisomerase II introduction of negative supercoils into DNA in an energy-dependent reaction [3]. Therefore, bacterial DNA topoisomerase II has drawn much attention as a selected target for finding potent antibacterial agents [4,5,6]. For example, a lot of synthetic quinolone antibacterial agents have been marketed and are now widely used for the treatment of bacterial infectious diseases [7,8]. Specifically, sparfloxacin has proven to be a very successful inhibitor of bacterial DNA topoisomerase II [9]. However, the abuse of antibacterial has led to an increase in bacterial resistance, especially in immunocompromised patients [10]. Our current research efforts are to find novel antibacterial agents as DNA topoisomerase II inhibitors.
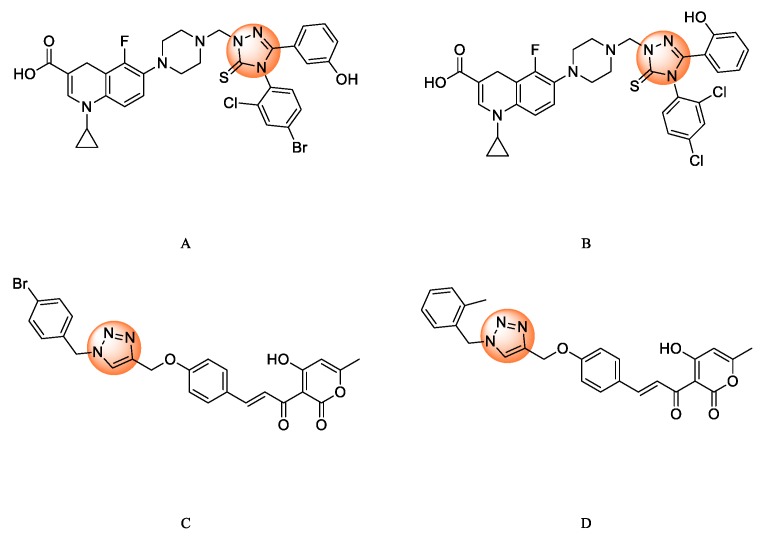
Hybridization was a common method of drug development and design [11]. It was based on combining two or more different biologically active moieties in a single molecule to obtain the corresponding conjugated hybrid molecules [12]. These hybrid molecules may exhibit better activities than their precursors [13]. The novel synthesized heterocyclic molecules can work by the same or different mechanisms of action compared to the precursors [14]. Nitrogen containing heterocyclic may be potential antibacterial drugs which inhibit bacterial topoisomerases [15], such as triazoles [16], quinolones [17], oxazolopyridines, aminopyrazinamides, and pyrazole [18,19,20]. Recently, Plech [21] and Lal [22] reported triazole derivatives as potential topoisomerase II inhibitors, which exhibited greater antibacterial activities than ciprofloxacin (Figure 1).
Figure 1.
Recently discovered triazole derivatives as DNA topoisomerase inhibitors.
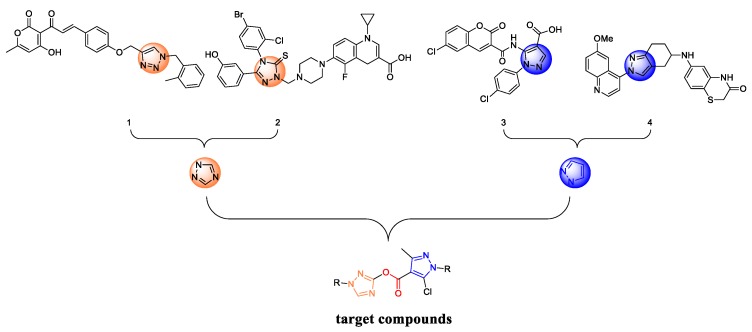
As is well known, prolonged use of antibacterial agents can lead to environmental problems and residual toxicity [23,24]. However, compound containing ester groups, can easily be hydrolyzed to produce low toxicity compounds. In addition, it can increase its soluble fat in vivo and enhance antimicrobial activity. Furthermore, it has multiple charges, enabling them to interact with the bacterial cell surface more strongly than their monomeric counterparts. Recently, Haldar et al. [25] reported that two compounds were linked by an ester group, and the antibacterial activities of the new compounds were improved compared with the previous compounds. In the previous research work [8], we discovered a novel Topoisomerase II (Topo II) inhibitors compound 3, which showed considerable inhibitory activity than ciprofloxacin. In the search for a class of new Topoisomerase II inhibitors, we chose pyrazole as primer molecule, introduced a triazole structure, and used the ester group as a linker to obtain a new scaffold (Figure 2) [26,27]. After that, a series of novel triazole-containing pyrazole ester derivatives were designed and synthesized. Initially, all of the synthesized compounds were used against two fungi, but the results of the antifungal activity were not ideal. Therefore, four bacteria were used to test the antibacterial activity of these compounds, two Gram-positive bacteria, namely Staphylococcus aureus and Listeria monocytogenes, and two Gram-negative bacteria, namely Escherichia coli and Salmonella gallinarum. In addition, molecular docking analysis studies were performed on all derivatives to determine critical structural factors responsible for their antibacterial efficacy.
Figure 2.
The design of the target scaffold.
2. Results and Discussion
2.1. Chemistry
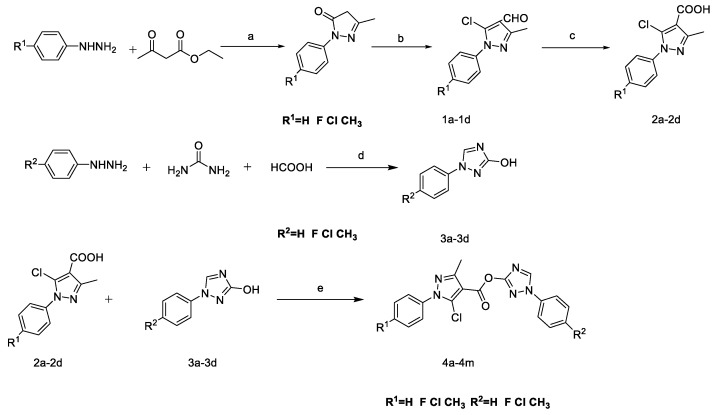
A series of triazole-containing pyrazole ester derivatives were prepared and the route listed in Scheme 1. We dissolved substituted phenylhydrazine with ethanol, then added ethylacetoacetate. Preparation of 1a–d was with Vilsmeier–Haack Reagent (N,N-Dimethylformamide/POCl3). Then, we added KMnO4 as an oxidation reagent to obtain 2a–d. Compounds 3a–d were obtained from phenylhydrazine hydrochloride and urea, we used formic acid under acidic conditions. The preparation of the target compounds was carried out using the reported method [28]. We analyzed all prepared compounds using spectral and elemental methods, which showed that all compounds fully complied with the structure in Table 1.
Scheme 1.
General synthesis of compounds 4a–m. Reagents and conditions: (a) H2O, ethanol, 60 °C; (b) N,N-Dimethylformamide(DMF), POCl3, 90 °C, 5h; (c) KMnO4, 70–80 °C; (d) HCl, 135 °C, H2SO4, 90 °C; (e) Dicyclohexylcarbodiimide(DCC), 4-dimethylaminopyridine(DMAP), DMF, rt.
Table 1.
Minimum inhibitory concentration (MIC) of all compounds against bacteria.
| Compounds | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| R1 | R2 | S.a a | L.m a | E. c a | S.e a | |
| 2a | H | 64 | 32 | 32 | 64 | |
| 2b | F | 64 | >128 | >128 | 64 | |
| 2c | Cl | 64 | 64 | >128 | 64 | |
| 2d | CH3 | 16 | 32 | 16 | 4 | |
| 3a | H | 32 | 32 | 16 | 16 | |
| 3b | F | >128 | 32 | 64 | 64 | |
| 3c | Cl | 64 | >128 | 64 | 64 | |
| 3d | CH3 | 32 | 64 | 16 | 32 | |
| 4a | H | H | 16 | 16 | 8 | 16 |
| 4b | F | H | >128 | >128 | 64 | 64 |
| 4c | Cl | H | 64 | >128 | 64 | 64 |
| 4d | CH3 | H | 4 | 2 | 4 | 0.5 |
| 4e | H | F | 64 | 32 | 32 | 32 |
| 4f | F | F | >128 | >128 | >128 | >128 |
| 4g | Cl | F | 64 | 64 | 64 | 64 |
| 4h | CH3 | F | 8 | 16 | 32 | 32 |
| 4i | F | Cl | >128 | 64 | >128 | 64 |
| 4j | Cl | Cl | 64 | 64 | 64 | 32 |
| 4k | H | CH3 | 4 | 8 | 4 | 4 |
| 4l | F | CH3 | 32 | 64 | 32 | 32 |
|
4m DMSO |
Cl | CH3 | 64 >128 |
32 >128 |
64 >128 |
32 >128 |
| CIP b | 0.125 | 1 | 0.5 | 0.25 | ||
a Abbreviations: Staphylococcus aureus (ATCC-12600); Listeria monocytogenes (ATCC-15313); Escherichia coli (ATCC-25922); Salmonella gallinarum (ATCC-9184). b Ciprofloxacin.
2.2. Antibacterial Activity and Structure-Activity Relationships (SAR) Discussion
In vitro antimicrobial activity of the prepared compounds against four bacteria was evaluated by the conventional agar-dilution method. Ciprofloxacin was selected as a reference standard. The results of the in-vitro antibacterial activity screening of the test compounds are summarized in Table 1.
The results revealed that most of the synthetic compounds exhibited antibacterial activities Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum, demonstrating the rationality of our design strategy. As can be seen from Table 1, among all the intermediate compounds, compound 2d exhibited the most potent antibacterial activity with MIC values of 16 µg/mL against Staphylococcus aureus, but among the tested target compounds, three compounds (i.e., 4d, 4g, and 4k) were found to display improved antibacterial activities against Staphylococcus aureus compared with (16 mg/L), with MIC values ranging from 4 to 8 mg/L. The eye-catching finding was that the target products greater excellent antibacterial activity than intermediate compounds. Among them, compounds 4d and 4k displayed most potent activity with MIC values of 4, 2, 4, and 0.5 µg/mL and 4, 8, 4, and 4 µg/mL against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum, which were similar to the broad-spectrum antibiotic Ciprofloxacin), indicating that they possess antibacterial activity. Compound 4a showed antibacterial activities against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum, Then, we introduced different substituent groups on benzene ring in 4a, the introduction of methyl at R1 displayed significantly enhanced effects compared with that of other compounds. Compound 4d exhibited best antibacterial activity than others. When R2 position was substituted with fluorine, methyl substituted at the R1 site was beneficial to antibacterial activity of the synthesized compounds (e.g., 4h). When the R2 position was substituted with methyl, electron-donating substituents (e.g., 4k) at R1 showed more potent activities than those with electron-withdrawing (e.g., 4l, 4m). When R1 and R2 were simultaneously substituted by electron-withdrawing, their activities were poor compared with other compounds. (e.g., 4j, 4f, 4i). Considering the above SAR results, these findings suggest that the antibacterial potency of designed compounds could be ascribed to a combination of factors, such as the R1 position was substituted with electron-donating groups, which may enhance antibacterial activity, and that the R2 position was substituted with hydrogen and the compound may enhance antibacterial activity.
2.3. Inhibitory Effects against DNA Gyrase and Topoisomerase IV
In order to determine the relationship between compounds and antibacterial activity, the inhibitory activity of selected compounds (4d and 4k) against DNA gyrase and topoisomerase IV isolated from Escherichia coli was examined. As shown in Table 2, 4d showed more potent inhibition than 4k against the two enzymes. The same exhibited the same tendency as the MIC data, suggesting that compound 4d may serve as a potential topoisomerase II inhibitor.
Table 2.
Inhibitory effects of selected compounds against DNA gyrase and topoisomerase IV.
| Compd. | IC50 (µg/mL) | |
|---|---|---|
| Gyrase a | Topo IV b | |
|
4d
4k |
13.5 25.7 |
24.2 33.1 |
| CIP c | 0.25 | 6.9 |
a Topoisomerase II supercoiling activity; b Topoisomerase IV decatenation activity. c Ciprofloxacin.
2.4. Docking Analysis
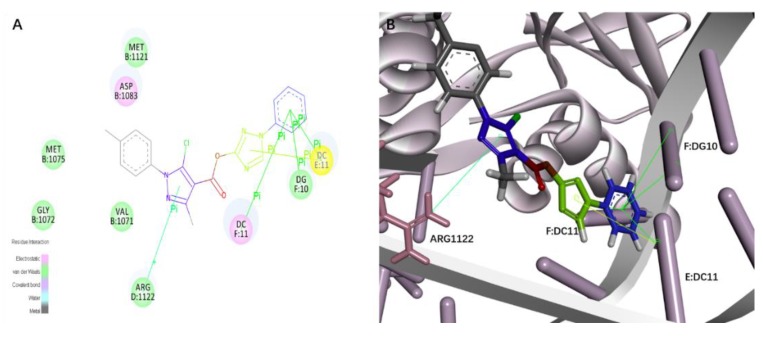
In order to gain a better understanding on the potency of the synthesized compounds and guide further structure, we conducted activity relationships studies. All of the derivatives were docked into the active site of Topo II (PDB entry: 2xcs) in the binding model of compound 4d and Topo II. The skeleton of compound 4d was embedded in the binding pocket, showing that the pose of 4d into the Topo II-binding site had a suitable shape that was complementary to the binding pocket, which means that our design strategy was rational. E:Dc11, F:Dc11, and F:Dg10 established π–π interactions with a benzene ring of compound 4d (distance: 5.01 Å, 4.18 Å, 5.31 Å), E:Dc11 established π–π interactions with triazole ring of compound 4d (distance: 4.60 Å), Arg 1122 established cation–π interactions with pyrazole ring of compound 4d (distance: 5.26 Å) (Figure 3).
Figure 3.
(A) Binding model of 4d (2D diagram). (B) The π–π interactions are displayed as yellow solid lines. The cation–π interactions are displayed as green solid lines.
3. Experimental Section
3.1. Materials and Methods
All chemicals were purchased from Energy, Meryer, and Aladdin Chemicals and were used as received. 1H-NMR spectra analyses were carried out using anAgilent DD2 600 Hz spectrometer with CDCl3 as the solvent and tetramethyllsilane as the internal standard. ESI-MS (Electrospray Ionisation Mass Spectrometry) spectra were carried out on a Mariner System 5304 mass spectrometer. Elemental analyses were performed on a CHN–O–Rapid instrument. Molecular docking was performed with Discovery Studio 3.5 [29]. The 13C-NMR and ESI-MS spectrum of compounds can be found in the Supplementary Materials.
3.1.1. General Procedure for the Synthesis of 5-Chloro-1-aryl-3-methyl-1H-pyrazole-4-carboxylic Acids 2a–d
Intermediates 2a–d were obtained from Reference [30]. Dissolve para-substituted phenyl hydrazine (0.025 mol) with anhydrous ethanol ethyl acetoacetate (0.025 mol) was added portion wise, stirred, and refluxed for 5 h, then the solution was rotary evaporated to form a solid, which was dissolved in DMF (25 mL) and phosphorus oxychloride (20 mL) of cold mixed solution and stirred at 85 °C for 2 h. The resulting product was poured into ice-cold water and the solid was isolated by filtration to give a yellow solid, which was oxidized by KMnO4 solution and stirred at 70–80 °C. After cooling to room temperature, the pH was adjusted to alkaline by the addition of 10% NaOH solution, and the solution was filtered, HCl solution was added to the solution and solid 2a–d eventually separated out. The resulting crude product was recrystallized from anhydrous ethanol to give the pure product.
3.1.2. General Pathway for Prepare of 1-phenyl-1H-1,2,4-triazol-3-ol 3a–d
Intermediates 3a–d were synthesized according to Bin sun et al. [31]. Phenylhydrazine (0.2 mol) and urea (0.25 mol) were dissolved in water (50 mL) and 30% hydrochloric acid (64.8 g) was added for 4 h at 135 °C. Add formic acid (0.3 mol) and 98% concentrated sulfuric acid (4.8 g) at 90 °C for 6 h. Finally, the product was cooled to room temperature, filtered, and washed with water to neutral and dried to give solid 3a–d.
3.1.3. General Procedure for Synthesis of 1-phenyl-1H-1,2,4-triazol-3-yl 5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate 4a–m
Compounds 2a–d (1 mmol) was stirred with triethylamine (1.5 mmol) into DMF (10 mL) medium, then a mixture of DCC (1 mmol) and DMAP (1 mmol) was added in the reaction system, stirred at 25 °C for 1 h. The mixture of intermediate 3 (1.2 mmol) and DMF (6 mL) was added in the reaction, stirred at 25 °C for 3 h. Finally, the product was extracted from chloroform with water, hydrochloric acid (0.2 mol/L), sodium hydroxide (2 mol/L), saturated sodium chloride successively, and then dried, concentrated, and purified by preparative thin layer chromatography (PE: EA = 8:1) [27].
3.1.4. 1-phenyl-1H-1,2,4-triazol-3-yl5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (4a)
White solid, yield 62%; mp 138–140 °C; 1H-NMR (600 MHz, CDCl3) δ 8.00 (s, 1H, Triazole-H), 7.53 (s, 6H, Ph-H), 7.08 (d, J = 8.0 Hz, 1H, Ph-H), 6.98 (t, J = 8.6 Hz, 1H, Ph-H), 6.93 (d, J = 4.4 Hz, 1H, Ph-H), 6.88 (d, J = 8.2 Hz, 1H, Ph-H), 2.55 (s, 3H, CH3). MS (ESI) calculated for C19H15ClN5O2 [M + H]+, 380.1, found 380.1; Anal. Calcd for C19H14ClN5O2: C, 60.09; H, 3.72; N, 18.44%; Found: C, 60.12; H, 3.79; N, 18.33%.
3.1.5. 1-phenyl-1H-1,2,4-triazol-3-yl5-chloro-1-(4-fluorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4b)
White solid, yield 54%; mp166–168 °C; 1H-NMR (600 MHz, CDCl3) δ 7.95 (s, 1H, Triazole-H), 7.51 (dd, J = 8.8, 4.7 Hz, 2H, Ph-H), 7.28 (s, 1H, Ph-H), 7.25 (s, 1H, Ph-H), 7.22 (t, J = 8.4 Hz, 2H, Ph-H), 6.98–6.91 (m, 3H, Ph-H), 2.54 (s, 3H, CH3).MS (ESI) calculated for C19H14ClFN5O2 [M + H]+, 398.1, found 398.1; Anal. Calcd for C19H13ClFN5O2: C, 57.37; H, 3.29; N, 17.61%; Found: C, 57.41; H, 3.31; N, 17.73%.
3.1.6. 1-phenyl-1H-1,2,4-triazol-3-yl5-chloro-1-(4-chlorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4c)
White solid, yield 57%; mp 172–173 °C; 1H-NMR (600 MHz, CDCl3) δ 7.92 (s, 1H, Triazole-H), 7.49 (d, J = 2.3 Hz, 4H, Ph-H), 7.28 (s, 1H, Ph-H), 7.25 (s, 1H, Ph-H), 6.98–6.90 (m, 3H, Ph-H), 2.54 (s, 3H, CH3) MS (ESI) calculated for C19H14Cl2N5O2 [M + H]+,414.1,found 414.1; Anal. Calcd for C19H13Cl2N5O2: C, 55.09; H, 3.16; N, 16.91%; Found: C, 55.13; H, 3.24; N, 17.05%.
3.1.7. 1-phenyl-1H-1,2,4-triazol-3-yl5-chloro-3-methyl-1-(p-tolyl)-1H-pyrazole-4-carboxylate (4d)
White solid, yield 52%; mp 154–156 °C; 1H-NMR (600 MHz, CDCl3) δ8.42 (s, 1H, Triazole-H), 7.57 (d, J = 8.4 Hz, 6H, Ph-H), 7.49 (d, J = 8.3 Hz, 1H, Ph-H), 7.31 (t, J = 8.2 Hz, 2H, Ph-H), 2.62 (s, 3H, CH3), 2.42 (s, 3H, CH3). [M + H]+). MS (ESI) calculated for C20H17ClN5O2 [M + H]+, 394.1, found 394.1; Anal. Calcd for C20H16ClN5O2: C, 61.00; H, 4.10; N, 17.78%; Found: C, 61.05; H, 4.18; N, 17.68%.
3.1.8. 1-(4-fluorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (4e)
White solid, yield 47%; mp 187–189 °C; 1H-NMR (600 MHz, CDCl3) δ7.97 (s, 1H, Triazole-H), 7.58–7.43 (m, 6H, Ph-H), 6.98–6.91 (m, 3H, Ph-H), 2.55 (s, 3H, CH3). MS (ESI) calculated for C19H14ClFN5O2 [M + H]+, 398.1, found 398.1; Anal. Calcd for C19H13ClFN5O2: C, 57.37; H, 3.29; N, 17.61%; Found: C, 57.43; H, 3.30; N, 17.57%.
3.1.9. 1-(4-fluorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-fluorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4f)
White solid, yield 75%; mp 69–71 °C; 1H-NMR (600 MHz, CDCl3) δ 8.03 (s, 1H, Triazole-H), 7.89 (d, J = 8.2 Hz, 2H, Ph-H), 7.51 (dd, J = 8.8, 4.7 Hz, 2H, Ph-H), 7.35 (d, J = 8.1 Hz, 2H, Ph-H), 7.20 (d, J = 8.2 Hz, 2H, Ph-H), 2.54 (s, 3H, CH3). MS (ESI) calculated for C19H13ClF2N5O2 [M + H]+, 416.1, found 416.1; Anal. Calcd for C19H12ClF2N5O2: C, 54.89; H, 2.91; N, 16.84%; Found: C, 54.96; H, 3.10; N, 16.77%.
3.1.10. 1-(4-fluorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-chlorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4g)
White solid, yield 66%; mp 253–254 °C; 1H-NMR (600 MHz, CDCl3) δ 8.43 (s, 1H, Triazole-H), 7.65 (dd, J = 8.2, 4.0 Hz, 2H, Ph-H), 7.54–7.48 (m, 4H, Ph-H), 7.21 (t, J = 8.2 Hz, 2H, Ph-H), 2.61 (s, 3H, CH3). MS (ESI) calculated for C19H13Cl2FN5O2 [M+H]+, 432.0, found 432.0; Anal. Calcd for C19H12Cl2FN5O2: C, 52.80; H, 2.80; N, 16.20%; Found: C, 52.87; H, 2.77; N, 16.28%.
3.1.11. 1-(4-fluorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-3-methyl-1-(p-tolyl)-1H-pyrazole-4-carboxylate (4h)
White solid, yield 71%; mp 99–101 °C; 1H-NMR (600 MHz, CDCl3) δ 8.41 (s, 1H, Triazole-H), 7.66 (dd, J = 8.1, 4.5 Hz, 2H, Ph-H), 7.42 (d, J = 7.6 Hz, 2H, Ph-H), 7.31 (d, J = 7.9 Hz, 2H, Ph-H), 7.21 (t, J = 8.0 Hz, 2H, Ph-H), 2.60 (s, 3H, CH3), 2.43 (s, 3H. CH3). MS (ESI) calculated for C20H16ClFN5O2 [M + H]+, 412.1, found 412.1; Anal. Calcd for C20H15ClFN5O2: C, 58.33; H, 3.67; N, 17.01%; Found: C, 58.41; H, 3.59; N, 17.09%.
3.1.12. 1-(4-chlorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-fluorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4i)
White solid, yield 51%; mp 84–86 °C; 1H-NMR (600 MHz, CDCl3) δ 8.46 (s, 1H, Triazole-H), 7.64 (d, J = 8.6 Hz, 2H, Ph-H), 7.55–7.54 (m, 2H, Ph-H), 7.50 (d, J = 8.6 Hz, 2H, Ph-H), 7.23–7.20 (m, 2H, Ph-H), 2.61 (s, 3H, CH3). MS (ESI) calculated for C19H13Cl2FN5O2 [M + H]+, 432.0, found 432.0; Anal. Calcd for C19H12Cl2FN5O2: C, 52.80; H, 2.80; N, 16.20%; Found: C, 52.86; H, 2.74; N, 16.11%.
3.1.13. 1-(4-chlorophenyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-chlorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4j)
White solid, yield 56%; mp 72–74 °C; 1H-NMR (600 MHz, CDCl3) δ 8.42 (s, 1H, Triazole-H), 7.55–7.53 (d, J = 3.1 Hz, 4H, Ph-H), 7.31 (dd, J = 8.9, 4.7 Hz, 2H, Ph-H), 7.20 (t, J = 8.4 Hz, 2H, Ph-H), 2.42 (s, 3H, CH3). MS (ESI) calculated for C19H13Cl3N5O2 [M + H]+, 448.0, found 448.0; Anal. Calcd for C19H12Cl3N5O2: C, 50.86; H, 2.70; N, 15.61%; Found: C, 50.91; H, 2.64; N, 15.58%.
3.1.14. 1-(p-tolyl)-1H-1,2,4-triazol-3-yl-5-chloro-3-methyl-1-phenyl-1H-pyrazole-4-carboxylate (4k)
White solid, yield 72%; mp 135–138 °C; 1H-NMR (600 MHz, CDCl3) δ 8.43 (s, 1H, Triazole-H), 7.57–7.46 (m, 7H, Ph-H), 7.31-7.40 (d, J = 8.2 Hz, 2H, Ph-H), 2.62 (s, 3H, CH3), 2.42 (s, 3H, CH3).MS (ESI) calculated for C20H17ClN5O2 [M + H]+, 394.1, found 394.1; Anal. Calcd for C20H16ClN5O2: C, 61.00; H, 4.10; N, 17.78%; Found: C, 61.08; H, 4.15; N, 17.84%.
3.1.15. 1-(p-tolyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-fluorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4l)
White solid, yield 46%; mp 141–143 °C; 1H-NMR (600 MHz, CDCl3) δ 8.43 (s, 1H, Triazole-H), 7.55–7.53 (m, 4H, Ph-H), 7.31 (d, J = 8.2 Hz, 2H, Ph-H), 7.23-7.21 (t, J = 8.5 Hz, 2H, Ph-H), 2.61 (s, 3H, CH3), 2.42 (s, 3H, CH3). MS (ESI) calculated for C20H16ClFN5O2 [M + H]+, 412.1, found 412.1; Anal. Calcd for C20H15ClFN5O2: C, 58.33; H, 3.67; N, 17.01%; Found: C, 58.42; H, 3.74; N, 17.07%.
3.1.16. 1-(p-tolyl)-1H-1,2,4-triazol-3-yl5-chloro-1-(4-chlorophenyl)-3-methyl-1H-pyrazole-4-carboxylate (4m)
White solid, yield 49%; mp 174–176 °C; 1H-NMR (600 MHz) δ 7.97 (s, 1H, Triazole-H), 7.53 (d, J = 4.2 Hz, 4H, Ph-H), 7.49 (dd, J = 8.9, 4.5 Hz, 1H, Ph-H), 7.00–6.91 (m, 3H, Ph-H), 2.55 (s, 3H, CH3). MS (ESI) calculated for C20H16Cl2N5O2 [M + H]+, 428.1, found 428.1; Anal. Calcd for C20H15Cl2N5O2: C, 56.09; H, 3.53; N, 16.35%; Found: C, 56.13; H, 3.61; N, 16.44%.
3.2. In Vitro Antibacterial Activity
3.2.1. Medium
The solid media Luria-Bertani (LB)-Broth-Agar-Medium (tryptone 10 g/L, yeast 5 g/L, NaCl 10 g/L, agar 15 g/L, and distilled water 1000 mL, adjusted to pH 7.4 was used for testing the antibacterial activity.
3.2.2. Minimum Inhibitory Concentration (MIC)
The in vitro antibacterial activity for synthesized compounds 4a–m were evaluated using the agar-dilution method [32,33,34]. Two-fold serial dilutions of the compounds and reference drugs (ciprofloxacin) was prepared in LB-Broth-Agar-Medium. Drugs (10.0 mg) were dissolved in DMSO (1 mL) and the solution was diluted with water (9 mL). Further progressive double dilution with melted LB-Broth-Agar-Medium was performed to obtain the required concentrations of 128, 64, 32, 16, 8, 4, 2, 1, 0.5 µg/mL, and the MIC values were calculated separately. The bacterial inocula were prepared by suspending 24 h-old bacterial colonies from LB-Broth-Agar-Medium in 0.85% saline. The inocula were adjusted to 0.5 McFarland Standard (1.56108 CFU/mL). The suspensions were then diluted in 0.85% saline to give 107 CFU/mL. Petri dishes were spot-inoculated with 1 μL of each of the prepared bacterial suspensions (104 CFU/spot) and incubated at 37 °C for 24 h. At the end of the incubation period, the MIC was determined, which is the lowest concentration of the test compound that resulted in no visible growth on the plate. A control test was also performed with test medium supplemented with DMSO at the same dilutions as used in the experiment in order to ensure that the solvent had no influence on bacterial growth.
3.3. Enzyme Inhibition Experimental
The in vitro antibacterial activity of the target compounds was carried out on by the methods of Sato et al. [35] and Peng and Marians [36]. First, the E. coli suspension is extracted to obtain a crude enzyme solution. After the purification step, the selected compounds are determined by gel electrophoresis to obtain data of different gradient concentrations, thereby calculating the IC50 values.
3.4. Molecular Docking
The crystal structures of bacterial DNA topoisomerase II (PDB entry: 2xcs) was downloaded from the RCSB (Research Collaboratory for Structural Bioinformatic) Protein Data Bank. The molecular docking procedure was performed using CDOCKER protocol for receptor–ligand interactions section of DS 3.5 [29].
4. Conclusions
A series of novel triazole-containing pyrazole ester derivatives 4a–m were synthesized and evaluated for their biological activities. Compound 4d showed significant antibacterial activities with MIC values of 4, 2, 4, and 0.5 µg/mL against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella gallinarum. Furthermore, compound 4d displayed the most potent Topo II (IC50 = 13.5 µg/mL) and topoisomerase IV (IC50 = 24.2 µg/mL) inhibitory activity. This study suggests that compound 4d can be used as a potential topoisomerase II inhibitor and this finding will lay the foundation for further structural modification and development of novel topoisomerase II inhibitors.
Supplementary Materials
The following are available online.
Author Contributions
Conceptualization, X.-H.L.; methodology, M.-J.C. and W.W.; software, Z.-L.R. and X.C.; writing—original draft preparation, L.W. and H.L.; writing—review and editing, F.T. and K.M.; funding acquisition, X.-H.L.
Funding
This work was supported by The Basic Science Research Fund Program of ICBR (1632017005), Natural Science Foundation of Education Committee of Anhui Province (KJ2018A0162), and National Key R&D Program of China (Project No. 2017YFD0600805). We are grateful to Jeffrey for checking our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Naeem A., Badshah S.L., Muska M., Ahmad N., Khan K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules. 2016;21:268. doi: 10.3390/molecules21040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteves-Souza A., Rodrigues-Santos C.E., Del Cistia Cde N., Silva D.R., Sant’anna C.M., Echevarria A. Solvent-free synthesis, DNA-topoisomerase II activity and molecular docking study of new asymmetrically N,N’-substituted ureas. Molecules. 2012;17:12882–12894. doi: 10.3390/molecules171112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann W., Sassone-Corsi M., Raffatellu M., Nolan E.M. Esterase-Catalyzed Siderophore Hydrolysis Activates an Enterobactin-Ciprofloxacin Conjugate and Confers Targeted Antibacterial Activity. J. Am. Chem. Soc. 2018;140:5193–5201. doi: 10.1021/jacs.8b01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gellerman G. Recent Developments in the Synthesis and Applications of Anticancer Amonafide Derivatives. A Mini Review. Lett. Drug Des. Discov. 2016;13:47–63. doi: 10.2174/1570180812666150529205049. [DOI] [Google Scholar]
- 5.Mcgarry D.H., Cooper I.R., Walker R., Warrilow C.E., Pichowicz M., Ratcliffe A.J., Salisbury A.M., Savage V.J., Moyo E., Maclean J. Design, synthesis and antibacterial properties of pyrimido[4,5-b]indol-8-amine inhibitors of DNA gyrase. Bioorg. Med. Chem. Lett. 2018;28:2998–3003. doi: 10.1016/j.bmcl.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Kamal A., Satyanarayanaa M., Devaiaha V.V., Yadava J.S., Mullickb B., Nagarajab V. Synthesis and biological evaluation of coumarin linked fluoroquinolones, phthalimides and naphthalimides as potential DNA gyrase inhibitors. Lett. Drug Des. Discov. 2006;3:494–502. doi: 10.2174/157018006778194862. [DOI] [Google Scholar]
- 7.Xie Z., Cheng D., Luo L., Shen G., Pan S., Pan Y., Chen B., Wang X., Liu Z., Zhang Y., et al. Design, synthesis and biological evaluation of 4-bromo-N-(3,5-dimethoxyphenyl)benzamide derivatives as novel FGFR1 inhibitors for treatment of non-small cell lung cancer. J. Enzym. Inhib. Med. Chem. 2018;33:905–919. doi: 10.1080/14756366.2018.1460824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H., Ren Z.L., Wang W., Gong J.X., Chu M.J., Ma Q.W., Wang J.C., Lv X.H. Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase II inhibitors: Design, synthesis and antibacterial activity. Eur. J. Med. Chem. 2018;157:81–87. doi: 10.1016/j.ejmech.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Nastasa C., Vodnar D.C., Ionut I., Stana A., Benedec D., Tamaian R., Oniga O., Tiperciuc B. Antibacterial Evaluation and Virtual Screening of New Thiazolyl-Triazole Schiff Bases as Potential DNA-Gyrase Inhibitors. Int. J. Mol. Sci. 2018;19:222. doi: 10.3390/ijms19010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calver A.D., Falmer A.A., Murray M., Strauss O.J., Streicher E.M., Hanekom M., Liversage T., Masibi M., van Helden P.D., Warren R.M. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg. Infect. Dis. 2010;16:264–271. doi: 10.3201/eid1602.090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh H., Nand B., Sindhu J., Khurana J.M., Sharma C., Aneja K.R. Efficient One Pot Synthesis of Xanthene-Triazole-Quinoline/Phenyl Conjugates and Evaluation of their Antimicrobial Activity. J. Braz. Chem. Soc. 2014;7:1178–1193. doi: 10.5935/0103-5053.20140095. [DOI] [Google Scholar]
- 12.Akhtar M.J., Yar M.S., Khan A.A., Ali Z., Haider M.R. Recent advances in the synthesis and anticancer activity of molecules other than nitrogen containing heterocyclic moeities. Mini-Rev. Med. Chem. 2016;17:1602–1632. doi: 10.2174/1389557516666161031121639. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y., Chen W., Jia Y., Tian Y., Zhao Y., Long F., Rui Y., Jiang X. N-Heterocyclic molecule-capped gold nanoparticles as effective antibiotics against multi-drug resistant bacteria. Nanoscale. 2016;8:13223–13227. doi: 10.1039/C6NR03317B. [DOI] [PubMed] [Google Scholar]
- 14.Fauser B.C., Mannaerts B.M., Devroey P., Leader A., Boime I., Baird D.T. Advances in recombinant DNA technology: Corifollitropin alfa, a hybrid molecule with sustained follicle-stimulating activity and reduced injection frequency. Hum. Reprod. Update. 2009;15:309–321. doi: 10.1093/humupd/dmn065. [DOI] [PubMed] [Google Scholar]
- 15.Tanitame A., Oyamada Y., Ofuji K., Fujimoto M., Suzuki K., Ueda T., Terauchi H., Kawasaki M., Nagai K., Wachi M., et al. Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg. Med. Chem. 2004;12:5515–5524. doi: 10.1016/j.bmc.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Azimvand J. Synthesis of new triazole and oxadiazole containing compound in the azide reaction as an antibacterial drug. Nature. 2012;410:231–235. [Google Scholar]
- 17.Efthimiadou E.K., Thomadaki H., Sanakis Y., Raptopoulou C.P., Katsaros N., Scorilas A., Karaliota A., Psomas G. Structure and biological properties of the copper(II) complex with the quinolone antibacterial drug N-propyl-norfloxacin and 2,2′-bipyridine. J. Inorg. Biochem. 2007;101:64–73. doi: 10.1016/j.jinorgbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 18.El Shehry M.F., Ghorab M.M., Abbas S.Y., Fayed E.A., Shedid S.A., Ammar Y.A. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem. 2017;143:1463–1473. doi: 10.1016/j.ejmech.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 19.Lv X.H., Ren Z.L., Liu H., Li H.D., Li Q.S., Wang L., Zhang L.S., Yao X.K., Cao H.Q. Design, Synthesis and Biological Evaluation of Novel Pyrazole Sulfonamide Derivatives as Potential AHAS Inhibitors. Chem. Pharm. Bull. 2018;66:358–362. doi: 10.1248/cpb.c17-00761. [DOI] [PubMed] [Google Scholar]
- 20.Liu X.H., Zhao W., Shen Z.H., Xing J.H., Xu T.M., Peng W.L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017;125:881–889. doi: 10.1016/j.ejmech.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Plech T., Kaproń B., Paneth A., Kosikowska U., Malm A., Strzelczyk A., Stączek P., Świątek Ś., Rajtar B., Polz-Dacewicz M. Determination of the primary molecular target of 1,2,4-triazole-ciprofloxacin hybrids. Molecules. 2015;20:6254–6272. doi: 10.3390/molecules20046254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lal K., Yadav P., Kumar A., Kumar A., Paul A.K. Design, synthesis, characterization, antimicrobial evaluation and molecular modeling studies of some dehydroacetic acid-chalcone-1,2,3-triazole hybrids. Bioorg. Chem. 2018:236–244. doi: 10.1016/j.bioorg.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Broussard G., Bramanti O., Marchese F.M. [Environmental study on toxicity of water resulting from pesticide and biocide pollution] Giornale Italiano Di Medicina Del Lavoro Ed Ergonomia. 2003;25:149. [PubMed] [Google Scholar]
- 24.Ory J., Bricheux G., Togola A., Bonnet J.L., Donnadieu-Bernard F., Nakusi L., Forestier C., Traore O. Ciprofloxacin residue and antibiotic-resistant biofilm bacteria in hospital effluent. Environ. Pollut. 2016;214:635–645. doi: 10.1016/j.envpol.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Haldar J., Kondaiah P., Bhattacharya S. Synthesis and antibacterial properties of novel hydrolyzable cationic amphiphiles. Incorporation of multiple head groups leads to impressive antibacterial activity. J. Med. Chem. 2005;48:3823–3831. doi: 10.1021/jm049106l. [DOI] [PubMed] [Google Scholar]
- 26.Gomez L., Hack M.D., Wu J., Wiener J.J., Venkatesan H., Santillan A., Jr., Pippel D.J., Mani N., Morrow B.J., Motley S.T., et al. Novel pyrazole derivatives as potent inhibitors of type II topoisomerases. Part 1: Synthesis and preliminary SAR analysis. Bioorg. Med. Chem. Lett. 2007;17:2723–2727. doi: 10.1016/j.bmcl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Lv X.H., Cao H.Q., Ren Z.L., Chu M.J. From Faming Zhuanli Shenqing. 107141283A. Patent CN. 2017 Sep 8;
- 28.Kim D.K., Lee N., Kim Y.W., Chang K., Im G.J., Choi W.S., Kim K.H. Synthesis and evaluation of amino acid esters of 6-deoxypenciclovir as potential prodrugs of penciclovir. Bioorg. Med. Chem. 1999;7:419–424. doi: 10.1016/S0968-0896(98)00235-1. [DOI] [PubMed] [Google Scholar]
- 29.Wu G., Robertson D.H., Brooks R.C., Vieth M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003;24:1549–1562. doi: 10.1002/jcc.10306. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J.J., Liao M., Chu M.J., Ren Z.L., Zhang X., Lv X.H., Cao H.Q. Design, synthesis and anti-tobacco mosaic virus (TMV) activity of 5-chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxamide derivatives. Molecules. 2015;20:807–821. doi: 10.3390/molecules20010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun B., Liu K., Han J., Zhao L.Y., Su X., Lin B., Zhao D.M., Cheng M.S. Design, synthesis, and biological evaluation of amide imidazole derivatives as novel metabolic enzyme CYP26A1 inhibitors. Bioorg. Med. Chem. 2015;23:6763–6773. doi: 10.1016/j.bmc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann P.F., Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H., editors. Manual of Clinical Microbiology. 7th ed. Volume 146. American Society of Microbiology; Washington, DC, USA: 1999. pp. 107–108. [Google Scholar]
- 33.Jorgensen J.H. NCCLS Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard. Infect. Dis. Clin. N. Am. 1993;7:393–409. [PubMed] [Google Scholar]
- 34.Aragade P., Maddi V., Khode S., Palkar M., Ronad P., Mamledesai S., Satyanarayana D. Synthesis and Antibacterial Activity of a New Series of 3-3-(Substituted Phenyl)-1-Isonicotinoyl-1H-Pyrazol-5-yl-2H-Chromen-2-one Derivatives. Arch. Der Pharm. 2009;342:361–366. doi: 10.1002/ardp.200800156. [DOI] [PubMed] [Google Scholar]
- 35.Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob. Agents Chemother. 1986;30:777–780. doi: 10.1128/AAC.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng H., Marians K.J. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 1993;268:24481–24490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.