Abstract
Although previous studies suggest membrane progesterone receptor alpha (mPRα/Paqr7) mediates 17, 20β-dihydroxy-4-pregnen-3-one (DHP) induction of oocyte maturation (OM) in zebrafish, critical information needed to establish mPRα as the receptor mediating OM is lacking. The relative potencies of progestins and specific mPRα agonists in inducing OM matched their relative binding affinities for zebrafish mPRα, supporting its role in OM. Microinjection of pertussis toxin blocked DHP induction of OM and the progestin-induced decrease in cyclic AMP levels, suggesting mPRα activates an inhibitory G protein (Gi). Microinjection of morpholino antisense oligonucleotides to zebrafish pgrmc1 blocked induction of OM by DHP which was accompanied by decreased levels of Pgrmc1 and mPRα on the oocyte plasma membranes. Similarly, treatment of denuded oocytes with a PGRMC1 inhibitor, AG205, blocked the gonadotropin-induced increase in plasma membrane mPRα levels and attenuated DHP induction of OM. Co-incubation with two inhibitors of epidermal growth factor Erbb2, ErbB2 inhibitor II and AG 879, prevented induction of OM by DHP, indicating the likely involvement of Erbb2 in mPRα-mediated signaling. Treatment with AG205 reversed the inhibitory effects of the Erbb2 inhibitors on OM and also inhibited insulin-like growth factor-1 induction of OM. Close associations between Pgrmc1 and mPRα, and between Pgrmc1 and Erbb2 were detected in zebrafish oocytes with in situ proximity ligation assays. The results suggest progestin induction of OM in zebrafish is mediated through an mPRα/Gi/Erbb2 signaling pathway that requires Pgrmc1 for expression of mPRα on oocyte membranes and that Pgrmc1 also is required for induction of OM through Erbb2.
Keywords: Pgrmc1, mPRα, Erbb2, IGF-1, AG205, Adaptor protein, Zebrafish, Oocyte maturation
Summary sentence
Progesterone membrane receptor component 1 is required for plasma membrane expression of membrane progestin receptor alpha mediating progestin induction of oocyte maturation and the completion of germinal vesicle breakdown in zebrafish.
1. Introduction
Fully-grown fish oocytes cannot be fertilized because the first meiotic division is incomplete. A surge in gonadotropin secretion induces the resumption of meiosis through an intermediary, a maturation-inducing steroid (MIS), which is secreted from the ovarian follicle cells of teleost fish and amphibians in response to gonadotropin stimulation (Nagahama and Yamashita, 2008; Yamashita, 1998). The MIS in turn binds to a specific progestin receptor on the oocyte plasma membrane to initiate oocyte maturation (resumption of meiosis, germinal vesicle breakdown) via a nongenomic mechanism, resulting in the formation of a fully mature oocyte capable of being ovulated and fertilized (Nagahama and Yamashita, 2008; Thomas, 2012). The involvement of three distinct classes of receptor proteins, the nuclear progesterone receptor (nPR) (Boonyaratanakornkit et al., 2001), and two types of novel membrane proteins unrelated to nuclear steroid receptors, the seven-transmembrane progesterone membrane receptors (mPRs) (Thomas, 2008), and the single transmembrane progesterone receptor membrane component 1 (PGRMC1) (Peluso et al., 2006; Thomas, 2008), in mediating nongenomic progestin actions have been demonstrated in reproductive tissues, but their roles in MIS induction of OM in amphibians and fish have not been fully resolved (Aizen and Thomas, 2015; Hanna and Zhu, 2011; Mourot et al., 2006).
Silence mRNA experiments in which mPRa antisense oligo nucleotides were microinjected into zebrafish and goldfish oocytes have provided evidence for an involvement of this receptor in 17, 20β-dihydroxy-4-pregnen-3-one (DHP) induction of OM (Tokumoto et al., 2006; Zhu et al., 2003). However, the involvement of nPR in regulating the onset of OM in teleosts remains equivocal. Although overexpression of nPR in zebrafish oocytes was found to accelerate OM in response to DHP (Hanna and Zhu, 2011), knockout of nPR in zebrafish using TALEN technology did not impair OM in this species (Zhu et al., 2015). Studies on the receptors mediating OM in amphibians have yielded conflicting results. Three distinct receptors have been proposed as intermediaries in the stimulatory actions of progestins and androgens on OM, nPR or proteins closely related to the nPR (Bayaa et al., 2000; Martinez et al., 2006), mPRβ (Paqr8) (Josefsberg Ben-Yehoshua et al., 2007), and the nuclear androgen receptor (nAR) (Lutz et al., 2001). For example, the finding that testosterone was more potent than progesterone in inducing OM in Xenopus laevis supports a role for the nAR in OM in this species (Deng et al., 2009). Although androgens also induce OM in teleosts, this only occurs at high concentrations, arguing against an involvement of the nAR in OM in this vertebrate group (Nagahama and Yamashita, 2008; Tokumoto et al., 2011). The finding that testosterone has moderate binding affinity for recombinant zebrafish mPRα and that it can activate MAPK in mPRα-transfected cells (Hanna et al.,2006) suggests androgen induction of OM in fish may be mediated by mPRα. However, information is currently lacking on the correspondence between the binding affinity of testosterone for zebrafish mPRα and its potency in inducing OM. Similarly, there are no reports on the effectiveness of selective nPR and mPR agonists in induction of zebrafish OM which would indicate the relative importance of each receptor in regulation of OM.
Experiments in which pertussis toxin (PTX) was microinjected into rainbow trout and spotted seatrout oocytes have shown that MIS induction of OM in these species is dependent upon activation of an inhibitory G protein (Pace and Thomas, 2005; Yoshikuni and Nagahama, 1994). The finding that DHP treatment causes a decrease in cAMP levels in zebrafish mPRα-transfected cells (Hanna et al., 2006) suggests a similar inhibitory G protein mechanism may mediate DHP-induced OM in zebrafish. However, there are no reports of PTX microinjection studies to confirm this.
On the basis of their observation that pgrmc1 mRNA expression in rainbow trout ovaries increases during oocyte development, Mourot and coworkers suggested that Pgrmc1 may be a participant in progestin-induced OM in fish (Mourot et al., 2006). Overexpression of human PGRMC1 in a cancer cell line was found to increase cell surface expression and receptor functions of mPRα, which suggests PGRMC1 can act as an adaptor protein (Thomas et al., 2014). The pharmacological agent, AG205 (also known as AG-205), has been shown to be a specific PGRMC1 antagonist/inhibitor with no reported off target effects in over a dozen papers, including several recent studies (Terzaghi et al., 2016; Will et al., 2017; Wyse-Jackson et al., 2016). Recent evidence using AG205 suggests PGRMC1 can also act as an adaptor protein for EGFRs in lung cancer cells and zebrafish oocytes (Ahmed et al., 2010; Aizen and Thomas, 2015). Treatment with AG205 blocked the stimulatory effect of two EGFR inhibitors on germinal vesicle breakdown (GVBD) in zebrafish and caused a decrease in Egfr expression on oocyte cell membranes (Aizen and Thomas, 2015). Preliminary evidence was obtained using specific ErbB2 inhibitors (henceforth called Erbb2 inhibitors for their effects on fish Erbb2) for a stimulatory role of Erbb2 in DHP-induced OM in zebrafish (Peyton and Thomas, 2011). Therefore, Pgrmc1 could potentially influence DHP-induced OM in zebrafish through regulating the localization of two receptors, mPRα and Erbb2.
In the present study, the role of mPRα in mediating MIS induction of OM was further evaluated by comparing the binding affinities of selective mPR and nPR progestins and testosterone for zebrafish mPRα to their potencies in inducing OM in this species. The effects of microinjection with PTX on DHP-induced OM was examined to determine whether activation of an inhibitory G protein (Gi) is essential for MIS stimulation of OM in zebrafish. A major goal was to test the hypothesis that Pgrmc1 is involved in mediating rapid progestin signaling and oocyte maturation in zebrafish through mPRα. The effects of down-regulation of Pgrmc1 expression in zebrafish oocytes by microinjection with zebrafish pgrmc1 antisense oligonucleotides on OM and the expression of mPRα on oocyte cell membranes was investigated. A pharmacological approach using AG205, the specific PGRMC1 inhibitor (hereafter named a Pgrmc1 inhibitor for its effects on zebrafish receptors), was used to further examine the involvement of Pgrmc1 in membrane expression of mPRα and in MIS induction of OM. The participation of Erbb2 in MIS induction of OM and the involvement of Pgrmc1 was explored using treatments with AG205 and Erbb2 inhibitor. An in situ proximity ligation assay was used to examine whether Pgrmc1 is very close or coupled to mPRα and Erbb2 in zebrafish oocytes. Insulin-like growth factor-1 (IGF-1) induction of OM was also investigated after treatment with AG205. Collectively, the results suggest MIS induction of OM in zebrafish is mediated through mPRα/Gi/ and Erbb2 signaling and requires Pgrmc1-dependent expression of mPRα on the oocyte plasma membrane.
2. Materials and methods
2.1. Chemicals
Chemicals were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise stated. l7, 20β-dihydroxy-4-pregnen-3-one (DHP) was purchased from Steraloids (Newport, RI) and R5020 (promegestone) from Amersham Pharmacia Biotech (Piscataway, NJ). The selective mPRα agonists, l0-ethenyl-l9-norprogesterone (Org OD 02–0) and l9α-methylprogesterone (Org OD l3–0) (Kelder et al., 20l0) were obtained from N.V. Organon (Oss, The Netherlands). The nuclear progesterone antagonist, RU486 (mifepristone), was purchased from MedChem Express (Monmouth Junction, NJ). [l, 2, 6, 7 3H] l7β-Hydroxyprogesterone (85 Ci/mmol) were purchased from Amersham Pharmacia Biotech and converted to [3H]-l7, 20β-dihydroxy-4-pregnen-3-one ([3H]-DHP) enzymatically as described previously (Scott et al., l982). The ErbB2 inhibitor II (4-(3-phenoxyphenyl)-5-cyano-2H-l, 2, 3-triazole) was purchased from Calicochem (Billerica, MA). PTX was purchased from List Biological Laboratories (Campbell, CA). The IGF-l receptor inhibitor, NVP-ADW742 was purchased from Selleck Chemicals (Houston, TX). Human insulin-like growth factor-l receptor (IGF-lR) (middle region) antibody (Product No. AVARp00004_P050) was purchased from Aviva Systems Biology Corp.
2.2. Animal care
Adult zebrafish were obtained from Segrest Farms (Gibsonton, FL, USA) and maintained in recirculating freshwater tanks at 28 °C on a l4hrL:l0hrD photoperiod at the University of Texas Marine Science Institute as described previously (Aizen and Thomas, 20l5). Fish were deeply anesthetized in 0.0l% tricaine methanesulfonate solution and humanely sacrificed using procedures approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin.
2.3. DHP membrane receptor binding assay
General procedures for assaying membrane binding of radioactive progestins to recombinant mPRα and for membrane extraction were followed as described previously (Hanna et al., 2006; Thomas et al.,2007). Binding of [3H]-DHP to plasma membranes of MDA-MB-23l cells stably transfected with the zebrafish mPRα receptor (Hanna et al., 2006) was determined by filtration assay after incubation at 4 °C for 30 min (Patiño and Thomas, l990). For competitive binding assays plasma membranes were incubated in triplicate with [3H]-DHP (4nM) alone (total binding, TB) and with increasing concentrations of steroid competitors from l0–l0M to l0–5M. The competitors were added to the tubes in l0 μl ethanol. Ten μl ethanol alone did not affect receptor binding. Nonspecific binding (NSB) was measured in a parallel set of tubes in the presence of l00-fold excess non-radiolabeled DHP. Steroid competitor binding was expressed as a percentage of maximum specific binding of [3H]-DHP.
2.4. Zebrafish OM assay
Ovarian follicles were collected and incubated according to procedures described previously for zebrafish (Pang and Thomas, 2009; Pang and Thomas, 2010). Briefly, ovarian follicles were separated with forceps, washed in culture medium, and those with diameters ~ 550 μm were selected for subsequent OM assays. Ovarian follicles were incubated in 60% Leibovitz L-15 culture medium (Sigma-Aldrich) in the presence or absence of steroids or inhibitors. All treatments were conducted in triplicate with 20 or more follicle-enclosed oocytes (FEOs) per incubation well (at least 60 oocytes/treatment). FEOs were used in all OM bioassays in which the potencies of progestins in inducing GVBD were assessed after a 6 h incubation because removal of the follicle layers would release the oocytes from meiotic arrest resulting in spontaneous progestin-independent OM (Pang and Thomas, 2010). For the same reason FEOs were also used for the microinjection studies with PTX and pgrmc1 antisense oligonucleotides in which their inhibitory effects on DHP-induced OM were investigated. GVBD was assessed after 6 or 10 h incubation of the FEOs. At the end of the incubation period the number of oocytes that underwent GVBD (germinal vesicle no longer visible) was counted and expressed as a percentage of the total number of full grown FEOs. Denuded oocytes were prepared by enzymatic digestion as described previously (Pang and Thomas, 2010) and the absence of follicle cells surrounding the oocytes was confirmed by histological examination following nuclei staining with 4’,6’-diamidino-2-phenylindole (DAPI) as shown previously (Pang and Thomas, 2010; Peyton and Thomas, 2011). Denuded oocytes (20–30 oocytes/well, three replicates wells/treatment) were used in OM assays in which the effects of inhibitors of nPR (RU486), Pgrmc1 (AG205), Erbb2 (ErbB2 inhibitor II, AG879) and Igf-1 (NVP) on GVBD were assessed to enable direct actions of these drugs on oocytes to be tested. It should be noted that the rate of spontaneous maturation of denuded oocytes is increased compared to that of FEOs (see Figs. 1C, 3A, and 7B) due to removal of the inhibitory influence of estradiol-17β produced by the follicle cells (Pang and Thomas, 2010). To confirm that the inhibitory effects on OM of AG205 were not due to a non-specific toxic effect of the drug, a DHP rescue experiment was conducted. Oocytes were preincubated with DHP and AG205 for 3 h and the % that underwent GVBD was determined. The medium was then removed and the oocytes were washed prior to a further 3 h incubation with DHP alone after which the GVBD was assessed for a second time.
Fig. 1.
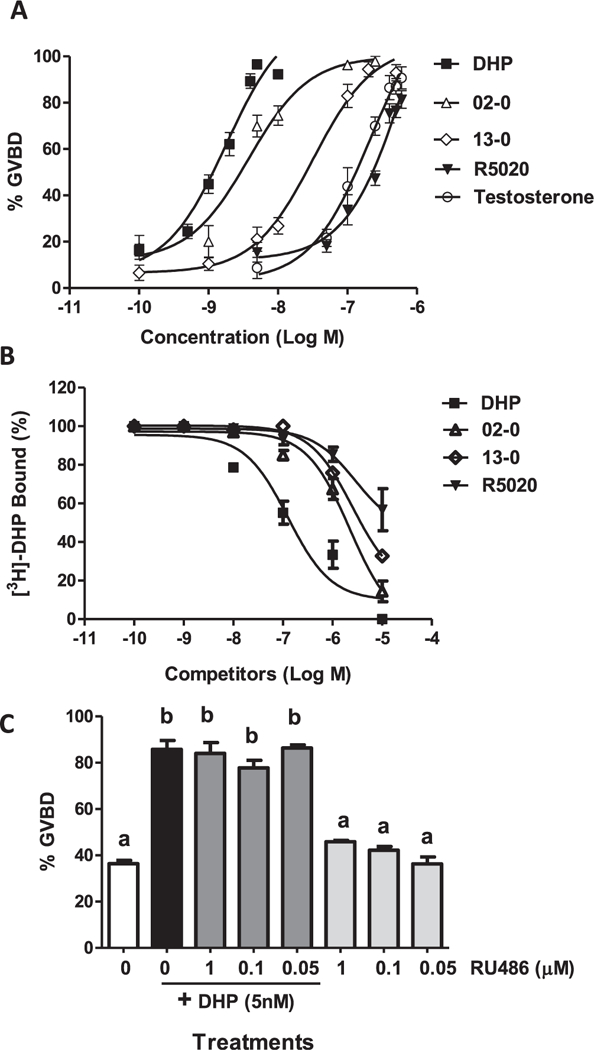
Comparison of the activities of various progestins in the OM bioassay and their binding affinities to zebrafish mPRα. (A) Dose-response relationships of progestins and testosterone for induction of maturation of follicle-enclosed oocytes in the zebrafish OM in vitro bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). DHP, Org OD-02–0 (02–0, mPR agonist), Org OD-13–0 (13–0, mPR agonist), R5020 (nPR agonist), and testosterone, were tested over the concentration range of 10–10–10–6 M. Each treatment group in experiments A and C consisted of three replicate wells of 20 oocytes per well. The entire experiments were repeated three times and similar results were obtained on each occasion. The data represent means ± S.E.M. (B) Competitive binding of DHP, Org OD 02–0, R5020 and Org OD 13–0 over the concentration range of 10–10–10–5 M to plasma membrane preparations of MDA-MB-231 cells stably transfected with zebrafish mPRα, expressed as a percentage of maximum specific DHP binding. Each value is the mean of triplicate estimations (see Supplementary Fig. 2 for statistical comparisons between treatment groups). (C) Effects of co-incubation of 5 nM DHP with RU486 at three different concentrations (1 μM, 0.1 μM, and 0.05 μM) on the percent GVBD of denuded oocytes in the OM bioassay. Results were analyzed by oneway ANOVA followed by the Bonferroni test. Different letters denote significant differences between the treatments groups (p < 0.05) in the post hoc test.
Fig. 3.
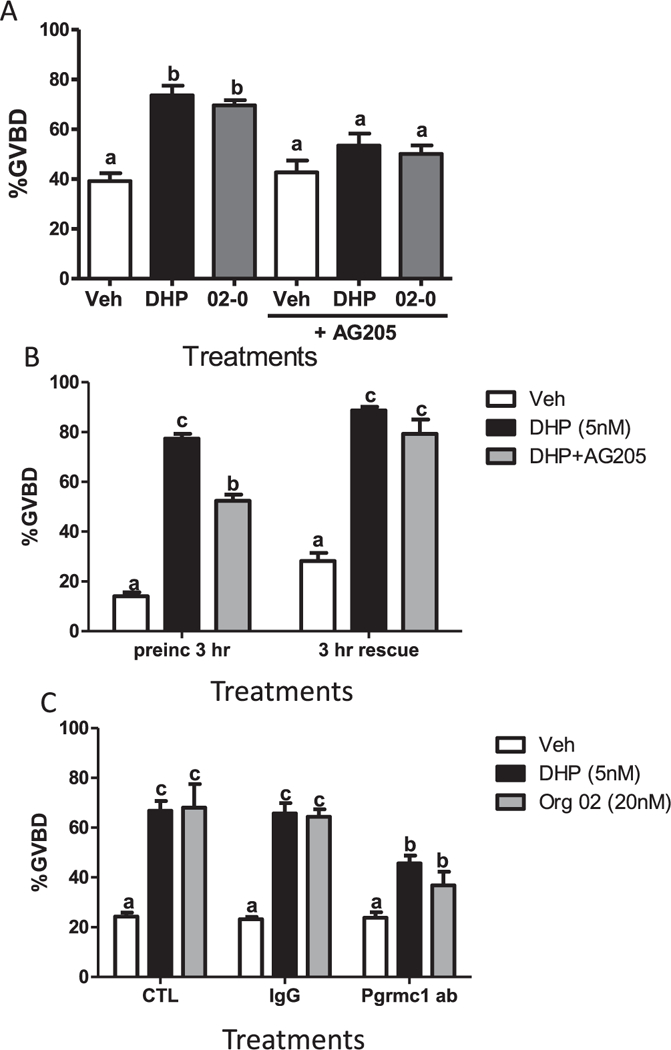
Involvement of Pgrmcl in DHP induction of OM. (A) Effects 3hr preincubation with AG205 on DHP- and Org OD 02–0-induced OM of denuded full-grown oocytes in the in vitro bioassay. (B) Effects of a 3 h co-treatment with DHP and AG205 (preinc 3 h) and subsequent treatment with DHP or vehicle alone for another 3 h (3 h rescue) on OM of denuded full-grown oocytes in the in vitro bioassay. (C) Effects of DHP and Org OD 02–0 on OM of denuded full- grown oocytes co-treated with IgG or the zebrafish Pgrmcl antibody (Pgrmcl ab, 1:300) in the in vitro bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). All data represent means ± S.E.M (n = 9). All the experiments were repeated three times and similar results were obtained on each occasion. (A) Results were analyzed by two-way ANOVA followed by the Bonferroni test. Significant interactive effects of AG205 in the presence of DHP and Org 02 on GVBD were detected in a two-way ANOVA, which was predicted because AG205 decreased the OM response to the progestins. (B and C) Results were analyzed by one-way ANOVA followed by the Bonferroni test. Different letters denote significant differences between the treatments groups (p < 0.05), in the post hoc test.
Fig. 7.
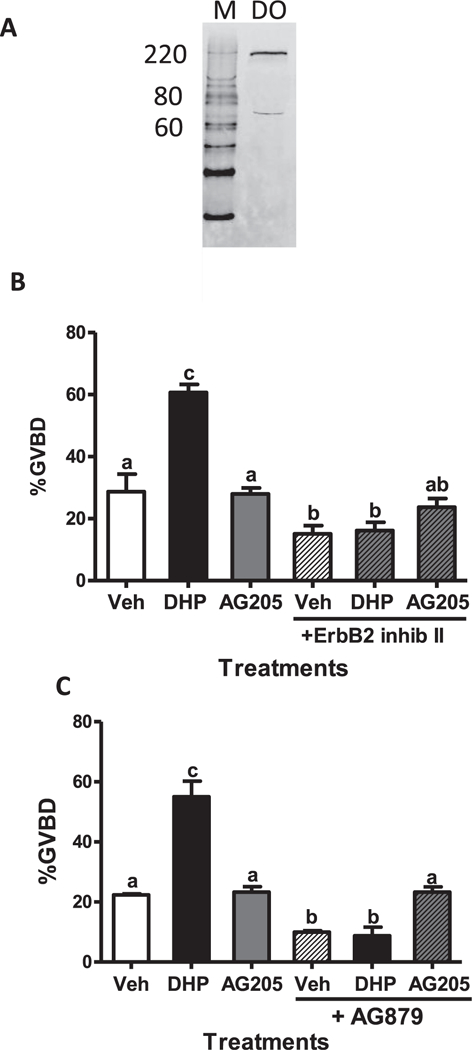
Effects of two different Erbb2 inhibitors, ErbB2 inhibitor II and AG879, on zebrafish OM. Western blot of Erbb2 expression on zebrafish defolliculated (DO) membranes (A). Effects of co-treatment with DHP or AG205 together with Erbb2 inhibitors on OM of denuded oocytes in the in vitro bioassay (B and C). OM was assessed by GVBD (disappearance of germinal vesicle). All data represent means ± S.E.M (n = 3). The entire experiment was repeated three times and similar results were obtained on each occasion. Results were analyzed by one-way ANOVA followed by the Bonferroni test. Different letters denote significant differences between the treatments groups (p < 0.05) in the post hoc test.
2.5. Effects of microinjection of zebrafish oocytes with antisense oligonucleotides to zebrafish pgrmc1 on DHP-induced OM and plasma membrane concentrations of mPRα
Late-stage vitellogenic FEOs (diameter: 450–500 μm), slightly smaller and less mature than those used for the other OM experiments, were used to investigate the effects of Pgrmc1 knockdown on expression of mPRα on oocyte membranes, because upregulation of mPRα occurs at the onset of OM. The FEOs were microinjected with approximately 1–2 nl of morpholino antisense oligonucleotides (1 mM) to zebrafish pgrmc1 (5’GCTCGACTGCTTCTTCAGCCATTTC3’) and standard non-targeting control oligonucleotides (5’CCTCTTACCTCAGTTA CAATTTATA3’) (GeneTools, Philomath, OR) dissolved in 1 × Danieau buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5 mM HEPES pH 7.6) using a Narishige micromanipulator (MM-3) and micro injector (MINJ-4, IM-5B) (Narishige, Japan). The follicles were incubated at 28 °C in the medium containing 10 nM DHP for 10 h. At the end of incubation period oocytes were scored for GVBD. The plasma membranes of the oocytes were extracted for measurement of Pgrmc1 and mPRα protein levels. Membrane proteins (5–8 μg) from oocytes injected with non-targeting or antisense oligonucleotides were run on SDS-PAGE gel, transferred to nitrocellulose membranes and the proteins were detected by Western blot analysis using antibodies for zebrafish Pgrmc1, spotted seatrout mPRα and β-actin (see Western blot analysis for details). Expression of the nuclear progesterone receptor was not measured because it is absent in late stage zebrafish oocytes (Hanna et al., 2010), and the present results with R5020 and RU486 suggested it is not involved in DHP-induced OM in zebrafish.
2.6. Effects of co-treatment with gonadotropin and the PGRMC1 inhibitor, AG205, on plasma membrane levels of the mPRα protein
mPRα protein levels were measured in oocyte membranes after treatment with AG205 in order to determine using an alternative method whether impairment of Pgrmc1 signaling causes decreased plasma membrane expression of mPRα. Late-stage vitellogenic zebrafish FEOs (diameter: 450–500 μm, 30 FEOs/treatment well), were incubated with 10 I.U/ml human chorionic gonadotropin (hCG) alone in DMEM for 6 h to upregulate mPRα protein expression on the oocyte plasma membrane (Zhu et al., 2003), which induces maturational competence (Pang and Ge, 2002). The membrane expression of mPRα in these positive hCG controls was compared to that observed after coincubation with 50 μM AG205 and 10 I.U. hCG. FEOs were used for these studies because it has previously been shown that AG205 is an effective agent in altering the functions of mammalian oocytes when they are surrounded by cumulus cells (Terzaghi et al., 2016). At the end of the incubation period the plasma membranes were extracted for measurement of mPRα by Western Blot analysis using the zebrafish mPRα antibody (1:2000) (see Western blot analysis for general procedures and Supplementary Fig. 1 for validation).
2.7. Pertussis toxin microinjection
The hypothesis that MIS-induced OM in zebrafish depends on the activation of an inhibitory G protein (Gi) was investigated by injecting oocytes capable to undergo OM in response to DHP with PTX which inactivates inhibitory G (Gi/o) proteins. PTX catalyzes ADP-ribosylation of the membrane-associated Gi protein α subunit, causing dissociation of the heterotrimeric G protein and its uncoupling from the receptor, thereby preventing ligand signaling through the receptor (Pace and Thomas, 2005). PTX was activated by incubation with DTT (50 mM) for 30 min at 35 °C (Pace and Thomas, 2005). Heat-inactivated PTX was prepared by incubating the activated PTX for an additional 15 min at 100 °C. FEOs, diameter ~ 550 μm, were injected with 1 nl of activated PTX, heat inactivated PTX, PTX saline buffer, or not injected (controls). Each treatment group consisted of three replicate wells of twenty follicles. The follicles were incubated with 5 nM DHP or vehicle at 28 °C for 6 h after which they were scored for GVBD.
2.8. Western blot analysis
Plasma membranes were prepared from follicle-enclosed and denuded zebrafish oocytes by homogenization in the presence of protease inhibitors followed by centrifugation at 20000× g to pellet the plasma membranes as described previously (Pang and Thomas, 2010). Membrane samples (5–10 μg membrane protein per lane) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Membranes were incubated overnight at 4 °C with either zebrafish Erbb2 antiserum (catalog No. 55748, 1:500; AnaSpec), zebrafish Pgrmc1 antiserum (1:2500) raised in rabbits against a synthetic 14-mer peptide (RGDKPADYGPVEEPC) (Aizen and Thomas, 2015) The zebrafish Pgrmc1 antiserum has previously been validated and the immunoreactive protein detected on zebrafish oocyte membranes (Aizen and Thomas, 2015). The zebrafish mPRα protein was detected using a polyclonal antibody generated against a peptide sequence in the N-terminal region of spotted seatrout mPRα which has 84% amino acid sequence identity with the corresponding region of zebrafish mPRα and has been used to detect mPRα in zebrafish oocyte membranes previously (Pang and Thomas, 2010). The blots were then washed 3 times in PBS and incubated with the HRP – (horseradish peroxidase) conjugated goat anti-rabbit IgG antibody (1:5000) (Cell Signaling, Beverly, MA) for 1 h at room temperature. The blots were washed 3 times in PBS and then treated with enhanced chemiluminescence reagent (SuperSignal West Pico, Pierce) and exposed to X-ray film to visualize the specific protein bands. Image J Software (Bethesda, MD, USA) was used to quantify the relative protein expression level from the ratio of the intensity of each protein band relative to that of the actin loading control.
2.9. cAMP assay
Ovarian follicles from 4 to 6 zebrafish were pooled and those with diameters > 400 μM were used for experimentation. Follicles were mechanically separated with forceps and repeated pipetting, and then incubated with 0.75mg/ml collagenase for 45min to remove the follicle cells. Oocytes were washed 3–5 times in cold buffer with protease inhibitor, and then homogenized using a handheld glass homogenizer. The homogenate was centrifuged at 1000×g for 7 min to pellet the nuclear material, followed by centrifugation at 20,000× g to pellet the membrane fraction. The membrane fraction was diluted in cAMP buffer (20 mM KCl, 12 mM MgCl2, 3mM EDTA, 20 mM Hepes, 10 mM phosphoenolpyruvate, 2mM ATP, 50 nM GTP, 0.2 mM DTT, 1 IU/mL pyruvate kinase, and 1 IU/mL myokinase, pH 7.5) to a concentration of 2–3 mg/ml. The membrane prep was preincubated with activated PTX (aPTX) or inactivated PTX (iPTX, 0.6 μg/ml) for 30 min at 18 °C or no treatment, followed by addition of the DHP or Org OD 02–0 treatments or vehicle (control), incubation at room temp for 15 min, and boiling for 10 min to stop the reaction. The samples were centrifuged to remove insoluble material and the supernatant was analyzed immediately with a cAMP kit (Caymen Chemical, Ann Arbor, MI) following the manufacturer’s instructions. Treatments were run in duplicate and the experiment was repeated 4 times. Data were normalized and presented as relative cAMP concentrations compared to a vehicle control value of one.
2.10. In situ proximity ligation assay
Interactions between Pgrmc1 and mPRα, and between Pgrmc1 and ErBb2 were examined in zebrafish FEOs using an in situ proximity ligation assay (Duolink®, Sigma/Aldrich) and by immunodetection with a rabbit anti-Erbb2 antibody described above under Western blot analysis, and with a rabbit anti-zebrafish mPRα antibody, and with a mouse anti-zebrafish Pgrmc1 antibody (Westerns with the anti-zebrafish mPRα and Pgrmc1 antibodies shown in Supplementary Fig. 1), 1:500, following the manufacturer’s instructions. The Pgrmc1 antibody was generated against a RGDKPADYGPVEEPC peptide sequence in zebrafish Pgrmc1 (GenScript, Piscataway, NJ). The antibody detects an immunoreactive protein band of the correct size and another band double this size likely representing a dimer on Western blots of zebrafish ovarian lysates and membranes (Supplementary Fig. 1). The manufacturer validated the antibody by ELISA using pre-immune serum as a negative control and the result showed that the antibody is highly specific for the zebrafish PGRMC1 peptide with titer of 2.93 vs 0.078 (antiserum vs pre-immune serum, 1:1000), thereby confirming the specificity of the antibody. The assay was conducted using both paraffin embedded tissue sections (Pgmrc1 vs mPRα) and cryo-sections (Pgmrc1 vs Erbb2) with proximity ligation assay probes of anti-rabbit (plus) and anti-mouse (minus) followed by ligase and polymerase treatment and examination under a Nikon fluorescent microscope.
2.11. Statistical analyses
Saturation curves of steroid binding in receptor assays were analyzed by nonlinear regression, using GraphPad Prism Software. All results are shown as the mean ± SEM. One-way or two-way ANOVAs with the Bonferroni multiple-comparison post hoc test or Student’s t test were used to determine statistical differences between control and experimental treatments using GraphPad Prism 5.03 software (La Jolla, CA, USA).
3. Results
3.1. Potencies of selective steroid receptor agonists in inducing OM
The potencies of selective mPR agonists, Org OD 02–0 and Org OD 13–0 (Kelder et al., 2010), the selective nPR agonist R5020 (Thomas et al., 2007), testosterone, which has highest agonist activity for the nuclear androgen receptor, and the zebrafish MIS, DHP, were compared in the in vitro OM bioassay over the concentration range of 10–10–10–6M. Of all the steroids tested DHP was the most potent, causing a concentration-dependent significant increase in GVBD over the concentration range of 1 nM–5nM, with GVBD approaching 100% at the higher concentration (Fig. 1A and Supplementary Fig. 2A). Org OD 02–0 also was a potent inducer of OM, with concentrations of 5 nM and 10 nM inducing significant increases in GVBD (70–75%), and 100 nM of the compound inducing greater than 90% of the oocytes to undergo GVBD (Fig. 1A and Supplementary Fig. 2B). The other selective mPR agonist Org OD-13–0, which has a lower binding affinity for human mPRa (Kelder et al., 2010), also displayed a lower potency than Org OD 02–0 in the zebrafish OM assay. At a concentration of 10 nM the steroid only induced ~ 25% GVBD (significantly different from vehicle controls, P < 0.05) which increased to 80% with 100 nM Org OD-13–0 (Fig. 1A). In contrast, the nPR agonist R5020 and testosterone displayed lower potencies in inducing GVBD. The percent GVBD was not significantly increased by 50 nM concentrations of both compounds, and only reached 30–40% GVBD at a concentration of 100 nM. For both compounds concentrations above 250 nM were required to induce GVBD above 70% (Fig. 1A, Supplementary Fig. 2C, D). Testosterone and R5020 were approximately 100 and 400 times less effective, respectively, than DHP in promoting GVBD.
3.2. Competitive binding of selective steroid receptor agonists to recombinant zebrafish mPRα
In agreement with previous results (Hanna et al., 2006), DHP displayed high binding affinity for membranes prepared from MDA-MB-231 cells transfected with zebrafish mPRα, with an EC50 of approximately 1 × 10–7M (Fig. 1B). The human mPR agonists, Org OD 02–0 and Org OD 13–0, were also effective competitors of [3H]-DHP binding, with EC50s of 2 × 10–6M and 7 × 10–6M, respectively, whereas R5020 had a much lower binding affinity for the receptors and did not displace 50% of bound [3H]-DHP at a concentration of 10–5M (Fig. 1B). Similarly, testosterone has a low RBA for mPRα (~0.8%, Hanna et al., 2006). There was a close correlation between the rank order of the binding affinities of these steroids for zebrafish mPRα and the rank order of their potencies in the OM bioassay (Fig. 1A and B).
3.3. Effects of RU486 on DHP induction of OM
RU486 is a selective nPR antagonist that does not bind the teleost and human mPRas (Thomas et al., 2007). RU486 over the concentration range of 0.05–1.0 μM did not affect GVBD or attenuate the GVBD response to 5 nM DHP in the OM bioassay (Fig. 1C). These results provide further evidence that MIS induction of OM in zebrafish is not mediated through the nPR.
3.4. Effects of pertussis toxin on DHP induction of OM
Microinjection of active PTX (aPTX, 0.6 μg/ml) into mature follicles significantly inhibited oocyte maturation in response to 5 nM DHP (Fig. 2A). Only 24% of aPTX-injected oocytes underwent GVBD in response to DHP whereas the percent GVBD of buffer and heat-inactivated PTX (iPTX) microinjected oocytes and non-injected DHP controls ranged from 85% to 91% and were not significantly different from each other (Fig. 2A). There was no significant difference in the % of oocytes that completed GVBD in vehicle controls, buffer-injected or aPTX-injected oocytes not treated with DHP (< 10% GVBD).
Fig. 2.
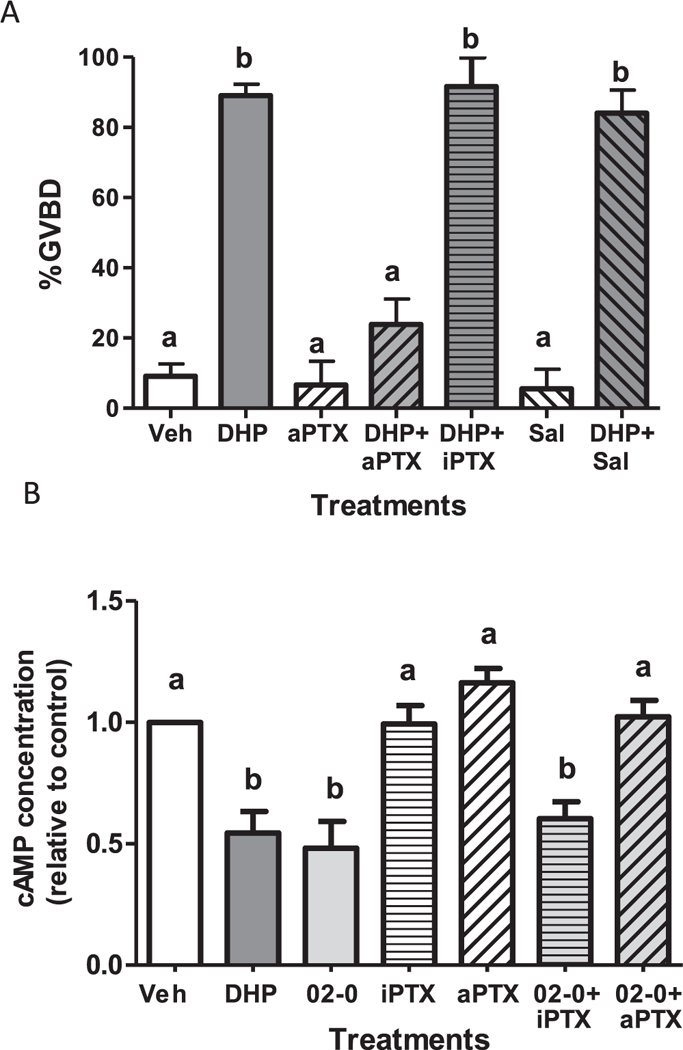
Effects of pertussis toxin treatments on DHP induction of OM (A) and DHP downregulation of cAMP production (B). (A) Effects of microinjection with activated pertussis toxin (aPTX 0.5 μg/μl) or heat-inactivated PTX (iPTX) on the maturation response of zebrafish follicle-enclosed oocytes to 5 nM DHP in the in vitro OM bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). Mature vitellogenic oocytes of diameter ~ 550 μm were injected with 1 nl PTX or saline (Sal) control (< 1% of oocyte volume). An entire experiment was conducted on oocytes from a single donor. The experiment was repeated seven times and the results pooled. The total number of oocytes in the vehicle-, DHP- and aPTX-treated groups was over 100, whereas the number of oocytes in the other treatment groups ranged from 31 to 39. (B). Effects of pre-treatment of oocyte membranes with aPTX or iPTX for 3 h on cAMP production in response to treatment with 10 nM Org OD 02–0. Production of cAMP in response to 5 nM DHP alone was included as a positive control. All data represent means ± S.E.M. Results were analyzed by two-way ANOVA followed by the Bonferroni test. Significant interactive effects of aPTX in the presence of DHP on GVBD and cAMP production were detected in two-way ANOVAs, which was predicted because aPTX blocked OM and the decrease in cAMP levels in response to DHP. Different letters denote significant differences between the treatment groups (p < 0.05) in the post hoc test.
3.5. Effects of DHP and Org OD 02–0 on cAMP concentrations and reversal by PTX treatment
Treatment with 5 nM DHP (MIS) and 10 nM Org OD 02–0 (selective mPR agonist), significantly decreased the production of cAMP by zebrafish oocyte membranes (Fig. 2B). Preincubation with aPTX (0.6 μg/ ml) completely blocked the inhibitory effect of Org OD 02–0 on cAMP production which was similar that of vehicle treated controls, whereas treatment with iPTX was ineffective (Fig. 2B). Collectively, the PTX results indicate that MIS induction of OM is mediated through activation of an inhibitory G protein and down regulation of cAMP production, similar to the findings in spotted seatrout (Pace and Thomas, 2005).
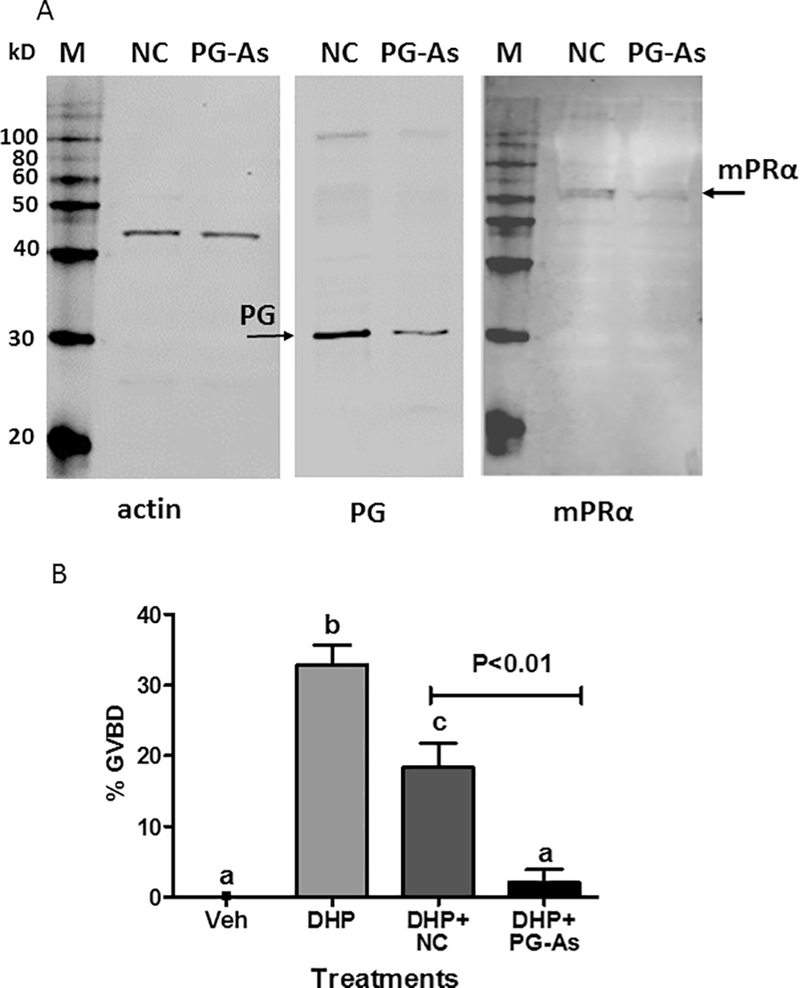
3.6. Involvement of Pgrmc1 in DHP-induced OM
To examine the involvement of Pgrmcl in OM, the effects of the Pgrmcl inhibitor, AG205, on DHP induction of OM were examined. The significant increase in OM induced by 5 nM DHP compared to the vehicle control group was attenuated by co-treatment with the Pgrmcl inhibitor AG205 (20 μM) and was not significantly different from the vehicle controls (Fig. 3A). Similarly, the OM response to 20 nM Org OD 02–0 was significantly reduced by co-treatment with AG205 (Fig. 3A). The % of oocytes completing GVBD was not significantly different from the vehicle treatment group after treatment with AG205 alone (Fig. 3A).
In order to confirm that the inhibitory effect of AG205 on OM was not due to a nonspecific toxic effect of the drug, a DHP rescue experiment was conducted after removal of the drug. As expected, co-treatment of with AG205 for 3 h significantly attenuated the OM response to 5 nM DHP, whereas DHP reversed the inhibitory effects of 20 μM AG205 on OM after washing the pretreated oocytes with L-l5 medium and incubating them with 5 nM DHP for an additional 3 h (Fig. 3B).
To further investigate this proposed role of Pgrmcl in progesterone-dependent induction of OM, the effects of an alternative approach to prevent Pgrmcl signaling, by incubating the denuded oocytes with a specific Pgrmcl antibody, were investigated. Incubation of denuded oocytes with the specific zfPgrmcl polyclonal antibody (Pgrmcl ab) (l:300) significantly attenuated the stimulatory effects of 5 nM DHP and 20 nM Org OD 02–0 on OM, whereas incubation with control rabbit IgG was ineffective (Fig. 3C).
The hypothesis that the involvement of Pgrmc1 in MIS induction of OM is mediated through regulation of plasma membrane expression of mPRα was tested by co-incubating late-stage vitellogenic FEOs with gonadotropin and AG205. Western blot analysis showed that six hours incubation with l0 I.U. hCG alone caused more than a twofold increase in plasma membrane expression of mPRα compared to that of the vehicle treated controls (Fig. 4A and B). Co-incubation with 50 μM AG205 completely blocked the hCG-induced increase in mPRα concentrations and membrane expression of mPRα was not significantly different from vehicle controls (Fig. 4A and B). These results suggest that the Pgrmc1 inhibitor, AG205, attenuates DHP-induced OM through decreasing the expression of mPRα on oocyte plasma membranes.
Fig. 4.
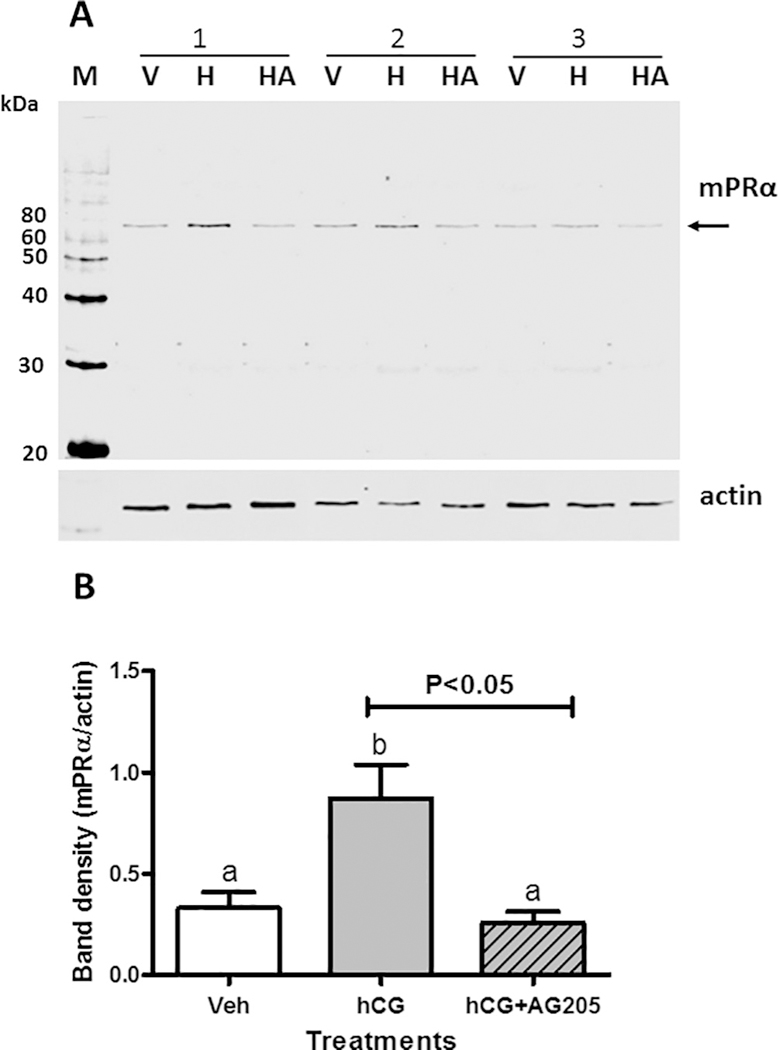
Effects of co-incubation with Pgrmcl inhibitor, AG205, on hCG upregulation of plasma membrane expression of mPRα. (A) Representative Western blot of mPRα expression on ovarian membranes of zebrafish oocytes after incubation for 6h with 10.I.U. hCG/ml (H), and co-incubation with 10 I.U. hCG/ ml and 50 μM AG205 (HA), or no treatment controls (V), (N = 3). (B) The bar graph shows the relative densitometry of the mPRα bands normalized to the actin loading controls. Results were analyzed by one-way ANOVA followed by the Bonferroni test. Significant differences between the paired groups of hCG alone versus hCG plus AG205 were tested by Student’s t-test. Different letters denote significant differences between the treatments groups (p < 0.05) in the post hoc Bonferroni test.
Microinjection experiments with zebrafish pgrmc1 morpholino antisense oligonucleotides were conducted as an unambiguous test of the involvement of Pgrmc1 in DHP-induced OM and regulation of mPRα expression on oocyte plasma membranes. The invasive microinjection procedure appeared to damage some of the zebrafish oocytes because it blunted their responsiveness to DHP. The percent of oocytes injected with morpholino non-targeting controls undergoing GVBD in response to DHP was decreased compared to that of the uninjected DHP controls. The antisense oligonucleotides caused a decrease in Pgrmcl and mPRα protein levels on oocyte membranes (Fig. 5A) and decreased the percent GVBD induced by 5 nM DHP compared to the morpholino non-targeting injected controls (Fig. 5B). These results suggest that Pgrmc1 is involved in the induction of oocyte maturation in zebrafish through altering the oocyte surface expression of mPRα. The finding that the intensity of the immunoreactive Pgrmc1 protein bands was decreased after microinjection of pgrmc1 antisense oligonucleotides further validates the use of this antibody for detection of Pgrmc1.
Fig. 5.
Effects of microinjection with zebrafish pgrmc1 antisense morpholino oligonucleotides on plasma membrane expression of mPRa (A) and DHP induction of OM (B). (A) Western blot analysis of Pgrmc1, mPRα and actin expression on ovarian membranes of zebrafish oocytes after microinjection of zebrafish pgrmc1 antisense morpholino oligonucleotides (PG-As), and non-targeting control morpholino oligonucleotides (NC). (B) Vitellogenic follicle-enclosed oocytes with diameters of 450–500 μm were injected with 1 nl of the oligonucleotides (< 1% of oocyte volume) and incubated with 10nM DHP in the in vitro OM bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). The experiment was repeated two times and the results pooled. The total number of oocytes in each treatment group were: vehicle −30, DHP −14, DHP + PG-As −29, and DHP + NC −16. All data represent means ± S.E.M. Results were analyzed by one-way ANOVA followed by the Bonferroni test. Significant differences between the paired groups of microinjection with Pgrmc1 antisense (PG-As) versus Pgrmc1 non-targeting controls (NC) were tested by Student’s t-test. Different letters denote significant differences between the treatments groups (p < 0.05) in the post hoc Bonferroni test.
3.7. Association of Pgrmc1 with mPRα
The highly sensitive in situ proximity ligation assay was used to investigate Pgrmc1-mPRα protein interactions and their subcellular localization. A close association (distance < 40 nm) between Pgrmcl and mPRα in zebrafish oocytes, shown as bright red dots, was demonstrated in the in situ proximity ligation assay (Fig. 6A). Pgrmc1-mPRα interactions were mainly detected near the oocyte plasma membrane with fewer positive signals in the cytoplasm (Fig. 6A). The follicle cells surrounding the oocytes were visualized by blue DAPI staining of their nuclei. No signals were detected in the negative controls incubated with the Pgrmc1 antibody and IgG (Fig. 6B).
Fig. 6.
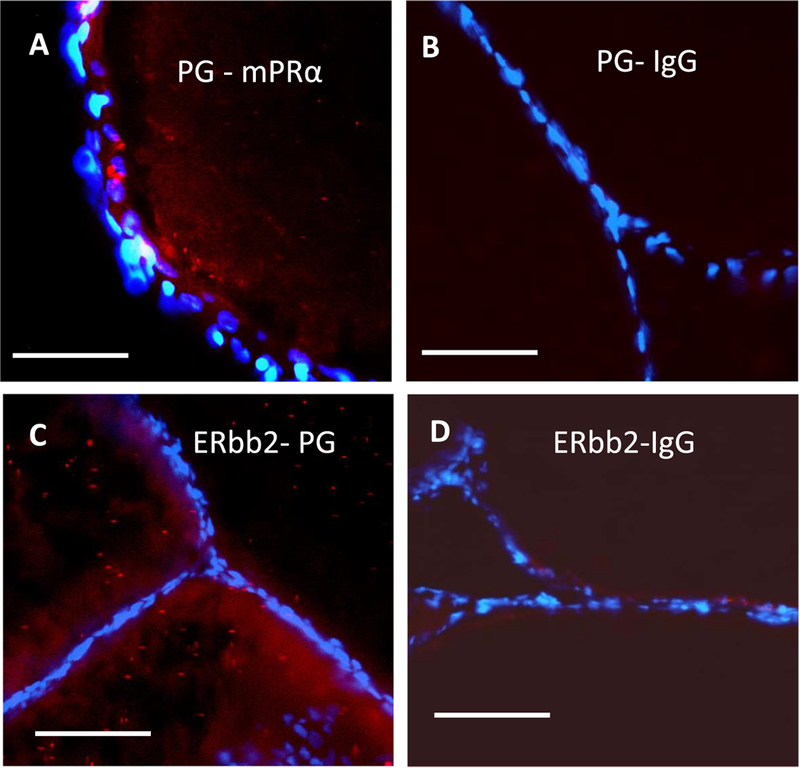
Interactions between Pgrmc1 and mPRα (A, B), and between Pgrmc1 and Erbb2 (C and D) in zebrafish oocytes detected in the in situ proximity ligation assay. Close associations of Pgrmc1 with mPRα are shown as red dots in the image (A). The mPRα antibody was replaced with IgG in the assay as a negative control (B). Close associations of Pgrmc1 with Erbb2 are shown as red dots in the image (C). The Pgrmc1 antibody was replaced with IgG in the assay as a negative control (D). Nuclei of the follicle cells surrounding the oocytes are stained with DAPI (4’, 6-diamidino-2-phenylindole). Scale bars in the images represent 100 μm (μm). The assay was repeated 3 or more times and similar results were obtained on each occasion. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.8. Role ofErbb2 in DHP-induced OM and involvement of Pgrmc1in Erbb2 signaling
Peyton and Thomas (20ll) suggested that a stimulatory influence of Erbb2 on OM is possible, because slight decreases in spontaneous OM were observed after treatment with the Erbb2 inhibitors AG879 or RG13022. Transactivation of Erbb2 in zebrafish oocytes by the MIS, DHP, acting through mPRα/Gi is a plausible mechanism for the induction of OM, because the stimulatory effects of DHP on OM were decreased by treatment with Erbb2 inhibitors (Peyton and Thomas,2011). A major band of the expected size for glycosylated Erbb2 using this antibody (~210kDa) was detected in oocyte membranes by Western blot analysis (Fig. 7A). Incubation of denuded oocytes with two different inhibitors, ErbB2 inhibitor II (50 μM) and AG879 (50 μM), reduced both spontaneous and DHP-induced OM below vehicle control levels, providing further evidence for the involvement of Erbb2 in OM (Fig. 7B and C). Furthermore, when the inhibitors were given together with AG205 spontaneous OM returned to vehicle control levels (Fig. 7B and C).
3.9. Association of Pgrmc1 with Erbb2
A close association between Pgrmc1 and Erbb2 proteins in zebrafish oocytes was demonstrated with the in situ proximity ligation assay (Fig. 6C). Positive signals were primarily detected in the cytoplasm although some Pgrmc1/Erbb2 interactions were also detected near the plasma membrane (Fig. 6C). No signals were detected in the negative controls incubated with Erbb2 antibody and IgG (Fig. 6D).
3.10. Involvement of Igf-1/Pgrmc1 in OM
The potential involvement of Pgrmc1 in induction of OM by Igf-1 was also investigated. Treatment with 100 nM human recombinant IGF-1 for 3 h caused a similar significant increase in OM of denuded oocytes to that induced by 5 nM DHP compared to the vehicle controls (Fig. 8A). Treatment with the specific IGF-1 receptor kinase inhibitor, NPV-ADW742 (1 μM) alone did not affect spontaneous OM. However, NPV-ADW742 completely blocked the stimulation of OM by IGF-1 (Fig. 8A). The percent GVBD induced by IGF-1 was also reduced to control levels by co-treatment with 20 μM AG205 (Fig. 8B), suggesting that Pgrmc1 is involved in Igf-1 signaling mediating OM, possibly through regulating the expression of the Igf-1 receptor (Igf-1r) on the surface of zebrafish oocytes. In support of this idea preliminary evidence was obtained that expression of the Igf-1r on zebrafish ovarian membranes is decreased after treatment with the Pgmrc1 inhibitor, AG205 (Supplementary Fig. 3).
Fig. 8.
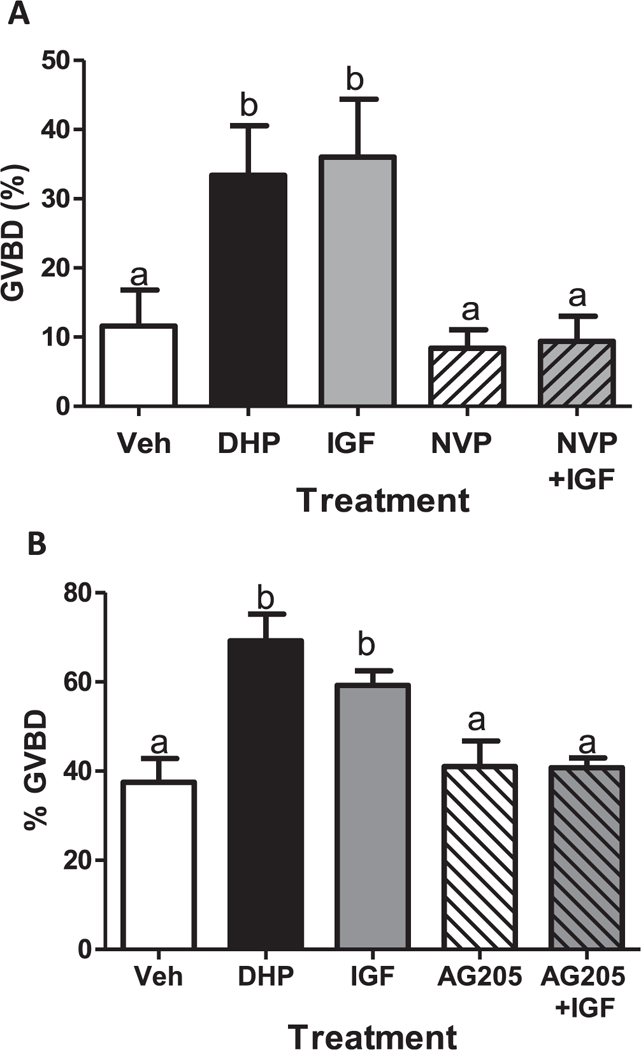
Effects of IGF-1 and the IGF-1 receptor inhibitor (NPV) on OM (A), and the effects of AG205 on OM induced by IGF-1 (B) of denuded oocytes in the in vitro bioassay. OM was assessed by GVBD (disappearance of germinal vesicle). Each treatment was replicated three times in each experiment (20 oocytes/ well). The experiments were repeated three or more times and the results were pooled. All data represent means ± S.E.M. (n = 9). Results were analyzed by one-way ANOVA followed by the Bonferroni test. Different letters denote significant differences between the treatment groups (p < 0.05) in the post hoc test.
4. Discussion
In the present study, we compared the mPRα binding/OM activity relationships of various progestins, including mPR-specific agonists, to determine the role of the receptor in mediating OM. The relative potencies of different progestins in inducing OM were similar to their relative binding affinities for zebrafish mPRα, supporting a role for mPRα in induction of OM. The observation that DHP induction of OM and downregulation of cAMP production were blocked by microinjection of oocytes with aPTX indicates this DHP action mediated through mPRα involves activation of an inhibitory G protein (Gi) and inhibition of adenylyl cyclase (AC) activity. Erbb2 is also a likely intermediary in this pathway because co-incubation with two Erbb2 inhibitors, ErbB2 inhibitor II and AG 879, prevented induction of OM by DHP. A major discovery of the present study is that Pgrmc1 has a critical role in DHP induction of OM in zebrafish through regulating the expression of mPRα on the oocyte plasma membrane. The finding that three experimental approaches, microinjection of pgrmc1 antisense oligonucleotides into oocytes, treatment of denuded oocytes with a Pgrmc1 inhibitor, AG205, and incubation of denuded oocytes with a zebrafish Pgrmc1 antibody, all blocked or attenuated DHP-induced OM, provides strong evidence for an important function of Pgrmc1 in the resumption of meiosis in zebrafish oocytes. Evidence that Pgrmc1 regulates the expression of mPRα and Pgrmc1 on oocyte plasma membranes was obtained from the pgrmc1 antisense microinjection studies and the co-incubation studies with hCG and AG205. Moreover, a close association between the two proteins in or near the oocyte plasma membrane was demonstrated in the in situ proximity ligation assay. Preliminary evidence was also gained using AG205 that Pgrmc1 is also required for Erbb2 and Igf-1 induction of OM. In addition, close interactions between the Pgrmc1 and Erbb2 proteins were detected with the proximity ligation assay. Collectively, these results suggest that Pgrmc1 has important functions as an adaptor protein in OM, regulating the expression of key receptors mediating the resumption of meiosis by the MIS and growth factors on the surface of fish oocytes.
Although the results of previous oocyte microinjection experiments with mPRα and mPRβ antisense oligonucleotides suggested these mPRs are intermediaries in DHP induction of OM in zebrafish (Thomas et al., 2004; Zhu et al., 2003), the potential roles of other receptors, especially nPR, nAR, and Pgrmc1 remained unclear. The present finding that the specific nPR and nAR agonists, R5020 and testosterone, have very low potencies in inducing OM, approximately 1–0.25% that of DHP, and that the nPR antagonist, RU486, did not block DHP-induced OM, suggests that the nPR and nAR are not involved in progestin induction of OM in zebrafish. On the other hand, the fact that the specific mPR agonist, Org OD 02–0, has a high relative potency, approximately 40% that of DHP, supports the hypothesis that mPRs mediate MIS induction of GVBD in this species. Further support for a role for mPRs in MIS induction of OM in this species comes from a comparison of the rank orders of the relative potencies of progestins in inducing OM and their relative binding affinities for recombinant mPRα (Fig. 1A and B). The rank orders of activity are the same in the two assays, DHP, followed by Org OD 02–0, Org OD 13–0, and finally R5020. The low potency of testosterone in inducing OM also correlated with its relatively low binding affinity for recombinant zebrafish mPRα, approximately 1% that of DHP (Hanna et al., 2006). Thus, the activities of all these progestins and testosterone can be explained, at least partially, by their relative binding affinities to mPRα. Although the binding affinities of the specific receptor agonists to the other mPR mediating OM, mPRβ, were not measured, the affinities of other steroids to zebrafish mPRβ and mPRα are very similar (Hanna et al., 2006), suggesting the affinities of the specific agonists are also likely to be similar. Despite recent evidence that high micromolar concentrations of progesterone can bind to recombinant Pgrmc1 in an prokaryotic expression system (Kalukaet al., 2015), direct binding of progesterone at physiological concentrations to Pgrmc1 and its activation of G-protein-dependent pathways have not been clearly demonstrated in eukaryotic cells. Moreover, the finding that the relatively high binding affinities of testosterone and corticosteroids for porcine PGRMC1 (Meyer et al., 1996) do not correlate with the low potencies of these steroids in the zebrafish OM bioassays argues against progestin induction of OM through direct binding to Pgrmc1. The prevailing view is that instead Pgrmc1 acts as an adaptor protein to promote progesterone signaling through trafficking other proteins to the cell membrane that contain the progesterone binding site, such as mPRα (Cahill et al., 2016; Thomas et al.,2014).
Multiple bands with approximate molecular masses of the monomer, dimer, and trimer of the PGRMC1 protein have been shown on Western blots of mammalian cell extracts (Peluso et al., 2012). Oligomerization of PGRMC1 was not prevented by treatment with strong reducing agents that break disulfide bonds and the PGRMC1 monomer was only observed in the presence of detergents (Peluso et al.,2012). Previously, we observed a 60kDa band probably representing the Pgrmc1 dimer on Western blots of zebrafish ovarian fractions using the rabbit anti-zebrafish Pgrmc1 antibody (Aizen and Thomas, 2015). Minor refinements of the protocol in the present study, including the inclusion of protease inhibitors, largely prevented Pgrmc1 oligomerization. The band at approximately 30 kDa on the Western blot using the same zebrafish Pgrmc1 antibody is the expected size of the monomer of this protein. A similar size band is also seen on the Western blot using the mouse polyclonal anti-zebrafish Pgrmc1 antibody. In addition, a weaker band detected with the mouse antibody around 55 kDa most likely represents the dimer of the protein (Supplementary Fig. 1). Collectively these studies suggest that the different molecular mass bands on Western blots of mammalian and fish PGRMC1s represent oligomers of these proteins.
The present results provide clear evidence that DHP induction of OM in zebrafish is mediated through activation of an inhibitory G protein. The demonstration that MIS induction of GVBD in zebrafish, a cyprinid fish, is blocked by microinjection of aPTX, is in agreement with previous findings in a perciform fish, spotted seatrout (Pace and Thomas, 2005), and in a salmonid, rainbow trout (Yoshikuni and Nagahama, 1994), suggesting that MIS activation of a pertussis toxinsensitive inhibitory G (Gi) protein is an ubiquitous mechanism regulating induction of OM in teleosts. DHP activation of a Gi to induce OM is consistent with an involvement of mPRα and mPRβ as intermediaries because they have been shown to signal through activation of a Gi protein in numerous cell types, including reproductive tissues (Dressing et al., 2010; Karteris et al., 2006; Pang et al., 2015; Thomas,2008). MIS activation of a Gi protein to induce OM in fishes is in accord with the well-known requirement for a decline in intra-oocyte cAMP levels in order for meiosis to resume (Nagahama and Yamashita, 2008; Pace and Thomas, 2005; Thomas, 2012), since membrane AC activity is down regulated through activation of the Gi protein as shown in the present study (Fig. 2C).
The present results using two specific Erbb2 inhibitors, AG879 and ErbB2 II, suggest the Erbb2 receptor is also involved in mediating DHP induction of OM, presumably through activation of the βγ subunit of the Gi protein. These results confirm the earlier preliminary findings using AG879 that inhibition of Erbb2 signaling attenuates the OM response to DHP (Peyton and Thomas, 2011). There are only a few other reports to date indicating an involvement of Erbb2 in fish reproduction (Tan and Thomas, 2015; Tingaud-Sequeira et al., 2009). Upregulation of a transducer of Erbb2, tob1a, was observed in flatfish ovaries during the period of oocyte growth (Tingaud-Sequeira et al., 2009). The Erbb2 inhibitor, AG879, was shown to inhibit MIS-induced upregulation of sperm motility through mPRα in Atlantic croaker, indicating mPRs also activate Erbb2 signaling in male gametes (Tan and Thomas, 2015). The mechanism through which Erbb2 induces OM in zebrafish cannot be determined from the results of the present study. ErbB2 activation occurs through dimerization with ligand-bound ErbB receptors such as EGFR, ErbB3, ErbB4, or with itself (Bertelsen and Stang, 2014). Meiotic arrest in zebrafish oocytes is partially mediated through an Egfr/Mapk signaling pathway (Peyton and Thomas, 2011). Therefore, one potential mechanism through which Erbb2 could stimulate OM in zebrafish which warrants investigation is by forming a dimer with Egfr, thereby blocking its signaling. On the other hand, inhibition of Erbb2 and Egfr dimerization could potentially maintain meiotic arrest by promoting Egfr signaling. This would provide a plausible explanation for the present finding, as well as that of an earlier study (Aizen and Thomas,2015), that spontaneous OM is decreased below that of untreated controls when Erbb2 signaling is inhibited, likely preventing its dimerization with Egfr. More detailed studies will be required to determine the precise physiological role of Erbb2 in regulation of OM in fish.
Arguably, the most important discovery of the present study is that Pgrmc1 has a major role in MIS induction of OM in a teleost species that is mediated through its upregulation of mPRα expression on the cell surface. The requirement for adequate PGRMC1 expression for localization of mPRα on the cell membrane and its progestin binding, signaling, and cellular functions was first demonstrated in human cancer cell lines (Thomas et al., 2014), and subsequently in human granulosa/ luteal cells (Sueldo et al., 2015). Moreover, co-immunoprecipitation studies indicated that PGRMC1 is directly coupled to mPRα in human cancer cell membranes (Thomas et al., 2014) and the in situ proximity ligation assay results in the present study suggest that Pgrmc1 is also coupled to mPRα in zebrafish in zebrafish oocyte cell membranes. Interestingly, progesterone-induced prevention of meiotic prophase 1 and primordial follicle formation in murine ovaries has also been shown to be mediated through PGRMC1, regulation of mPRα membrane expression, and declines in cAMP levels (Guo et al., 2016). The microinjection experiments with pgrmc1 antisense oligo nucleotides and the co-incubation studies with gonadotropin and the PGRMC1 inhibitor, AG205, suggest that Pgrmc1 also acts as an adaptor protein in zebrafish oocytes and is required for expression of mPRα on the oocyte membrane and DHP induction of OM. The present results showing that coincubation with the Pgrmc1 inhibitor, AG205, attenuates the inhibitory effects of Erbb2 inhibitors on OM, and that Pgrmc1 is closely associated with Erbb2, are consistent with the hypothesis that Pgrmc1 also act as an adaptor protein for this receptor. Previously our research group has shown that AG205 also decreases Egfr levels on zebrafish membranes, resulting in inhibition of Gper-dependent estrogen signaling that prevents the resumption of meiosis (Aizen and Thomas, 2015). In addition, preliminary evidence was obtained in the present study using AG205 that Pgrmc1 is necessary for Igf-1 stimulation of OM in denuded oocytes, and expression of the Igf-1R on the oocyte membrane (Supplementary Fig. 3). Thus, Pgrmc1 may act as an adaptor protein to regulate the oocyte membrane expression of multiple membrane receptors controlling OM in zebrafish, mPRα, Egfr, Erbb2 and Igf-1R. The fact the PGRMC1 is also a partner with mPRα in progesterone regulation of apoptosis in granulosa cells and in primordial follicle formation (Guo et al., 2016; Sueldo et al., 2015) suggests PGRMC1 has important roles in the regulation of a wide variety of mPRα-dependent functions in vertebrate ovaries and oocytes.
In conclusion, the present results demonstrate that Pgrmc1 has a critical role in DHP stimulation of OM in zebrafish oocytes as an adaptor protein regulating the expression of mPRα on oocyte membranes and thus enabling MIS induction of OM through a DHP/mPRα/Gi signaling pathway. In addition, the first evidence for a role of Erbb2 signaling in spontaneous and MIS-induced OM was obtained in a teleost species. Collectively, these studies compliment previous ones indicating an expanding suite of receptors, protein partners, and signaling pathways regulating the vital process of meiotic maturation in teleost oocytes.
Supplementary Material
Funding
This research was supported by BARD, the United States – Israel Binational Agricultural Research and Development Fund, Vaadia-BARD Postdoctoral Fellowship Award No. FI-448-11(J.A), by the H.E.B. Endowed Chair in Marine Science Institute at the University of Texas at Austin (P.T) and NIH GM100461 (Y.Z.).
Footnotes
Disclosure statement
The authors have nothing to declare.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.10167j.ygcen.2018.04.009.
References
- Ahmed IS, Rohe HJ, Twist KE, Craven RJ, 2010. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J. Biol. Chem. 285, 24775–24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizen J, Thomas P, 2015. Role of Pgrmc1 in estrogen maintenance of meiotic arrest in zebrafish oocytes through Gper/Egfr. J. Endocrinol. 225, 59–68. [DOI] [PubMed] [Google Scholar]
- Bayaa M, Booth RA, Sheng Y, Liu XJ, 2000. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. U.S.A. 97, 12607–12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen V, Stang E, 2014. The mysterious ways of ErbB2/HER2 trafficking. Membranes 4, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP, 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell. 8, 269–280. [DOI] [PubMed] [Google Scholar]
- Cahill MA, Jazayeri JA, Catalano SM, Toyokuni S, Kovacevic Z, Richardson DR, 2016. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochim. Biophys. Acta (BBA) Rev. Cancer 1866, 339–349. [DOI] [PubMed] [Google Scholar]
- Deng J, Carbajal L, Evaul K, Rasar M, Jamnongjit M, Hammes SR, 2009. Nongenomic steroid-triggered oocyte maturation: of mice and frogs. Steroids 74, 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing GE, Pang Y, Dong J, Thomas P, 2010. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell cocultures and its involvement in progestin inhibition of apoptosis. Endocrinology 151, 5916–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Zhang C, Wang Y, Feng L, Wang Z, Niu W, Du X, Tang W, Li Y, Wang C, Chen Z, 2016. Progesterone receptor membrane component 1 mediates progesterone-induced suppression of oocyte meiotic prophase I and primordial folliculogenesis. Sci. Rep. 6, 36869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Zhu Y, 2011. Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes. Mol. Cell. Endocrinol. 337, 80–88. [DOI] [PubMed] [Google Scholar]
- Hanna RN, Daly SCJ, Pang Y, Anglade I, Kah O, Thomas P, Zhu Y, 2010. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain. Biol. Reprod. 82, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y, 2006. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J. Endocrinol. 190, 247–260. [DOI] [PubMed] [Google Scholar]
- Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL, 2007. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol. Endocrinol. 21, 664–673. [DOI] [PubMed] [Google Scholar]
- Kaluka D, Batabyal D, Chiang B-Y, Poulos TL, Yeh S-R, 2015. Spectroscopic and mutagenesis studies of human PGRMC1. Biochemistry 54, 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P,2006. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 20, 1519–1534. [DOI] [PubMed] [Google Scholar]
- Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P, 2010. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids 75, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR, 2001Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc. Natl. Acad. Sci. U.S.A. 98, 13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S, Grandy R, Pasten P, Montecinos H, Montecino M, Olate J, Hinrichs MV, 2006. Plasma membrane destination of the classical Xenopus laevis progesterone receptor accelerates progesterone-induced oocyte maturation. J. Cell. Biochem. 99, 853–859. [DOI] [PubMed] [Google Scholar]
- Meyer C, Schmid R, Scriba PC, Wehling M, 1996. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur. J. Biochem. 239, 726–731. [DOI] [PubMed] [Google Scholar]
- Mourot B, Nguyen T, Fostier A, Bobe J, 2006. Two unrelated putative membrane-bound progestin receptors, progesterone membrane receptor component 1 (PGMRC1) and membrane progestin receptor (mPR) beta, are expressed in the rainbow trout oocyte and exhibit similar ovarian expression patterns. Reprod. Biol. Endocrinol. 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Yamashita M, 2008. Regulation of oocyte maturation in fish. Dev. Growth Diff. 50, 195–219. [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P, 2005. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev. Biol. 285, 70–79. [DOI] [PubMed] [Google Scholar]
- Pang Y, Ge W, 2002. Gonadotropin and activin enhance maturational competence of oocytes in zebrafish (Denio rerio). Biol. Reprod. 66, 259–265. [DOI] [PubMed] [Google Scholar]
- Pang Y, Thomas P, 2009. Involvement of estradiol-17β and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rerio. Gen. Comp. Endocrinol. 161, 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Thomas P, 2010. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev. Biol. 342, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P, 2015. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-α. Am. J. Physiol. Endocrinol. Metab. 308, E899–E911. [DOI] [PubMed] [Google Scholar]
- Patiño R, Thomas P, 1990. Characterization of membrane-receptor activity for 17-alpha,20-beta,21-trihydroxy-4-pregnen-3-one in ovaries of spotted sea-trout (Cynoscion-nebulosus). Gen. Comp. Endocrinol. 78, 204–217. [DOI] [PubMed] [Google Scholar]
- Peluso JK, Lodde V, Liu X, 2012. Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneosly immortalized granulosa cell. Endocrinology 154, 3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M, 2006. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology 147, 3133–3140. [DOI] [PubMed] [Google Scholar]
- Peyton C, Thomas P, 2011. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio). Biol. Reprod. 85, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AP, Sheldrick EL, Flint APF, 1982. Measurement of 17α,20β-dihydroxy-4-pregnen-3-one in plasma of trout (Salmo gairdneri Richardson): seasonal changes and response to salmon pituitary extract. Gen. Comp. Endocrinol. 46, 444–451. [DOI] [PubMed] [Google Scholar]
- Sueldo C, Liu X, Peluso JJ, 2015. Progestin and adipoQ Receptor 7, progesterone membrane receptor component 1 (PGRMC1), and PGRMC2 and their role in regulating progesterone’s ability to suppress human granulosa/luteal cells from entering into the cell cycle. Biol. Reprod. 93 (63) 61 11. [DOI] [PubMed] [Google Scholar]
- Tan W, Thomas P, 2015. Involvement of epidermal growth factor receptors and mitogenactivated protein kinase in progestin-induction of sperm hypermotility in Atlantic croaker through membrane progestin receptor-alpha. Mol. Cell. Endocrinol. 414, 194–201. [DOI] [PubMed] [Google Scholar]
- Terzaghi L, Tessaro I, Raucci F, Merico V, Mazzini G, Garagna S, Zuccotti M, Franciosi F, Lodde V, 2016. PGRMC1 participates in late events of bovine granulosa cells mitosis and oocyte meiosis. Cell Cycle 15, 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, 2008. Characteristics of membrane progestin receptor alpha (mPR alpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 29, 292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, 2012. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 175, 367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Zhu Y, Detweiler C, Doughty K, 2004. Multiple rapid progestin actions and progestin membrane receptor subtypes in fish. Steroids 69, 567–573. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, Groenen P, Kelder J, Vlieg J.d., Zhu Y, Tubbs C, 2007. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor a subtypes and their evolutionary origins. Endocrinology 148, 705–718. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, 2014. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 155, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Chauvigné F, Lozano J, Agulleiro MJ, Asensio E, Cerdá J, 2009. New insights into molecular pathways associated with flatfish ovarian development and atresia revealed by transcriptional analysis. BMC Genomics 10, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Thomas P, Tokumoto T, 2006. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen. Comp. Endocrinol. 145, 101–108. [DOI] [PubMed] [Google Scholar]
- Tokumoto T, Yamaguchi T, Ii S, Tokumoto M, 2011. In vivo induction of oocyte maturation and ovulation in zebrafish. PLoS One 6, e25206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will EA, Liu X, Peluso JJ, 2017. AG 205, a progesterone receptor membrane component 1 antagonist, ablates progesterone’s ability to block oxidative stress-induced apoptosis of human granulosa/luteal cells†. Biol. Reprod. 96, 843–854. [DOI] [PubMed] [Google Scholar]
- Wyse-Jackson AC, Roche SL, Ruiz-Lopez AM, Moloney JN, Byrne AM, Cotter TG, 2016. Progesterone analogue protects stressed photoreceptors via bFGF-mediated calcium influx. Eur. J. Neurosci. 44, 3067–3079. [DOI] [PubMed] [Google Scholar]
- Yamashita M, 1998. Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin. Cell Dev. Biol. 9, 569–579. [DOI] [PubMed] [Google Scholar]
- Yoshikuni M, Nagahama Y, 1994. Involvement of an inhibitory G-protein in the signal transduction pathway of maturation-inducing hormone (17α,20β-dihydroxy-4-pregnen-3-one) action in rainbow trout (Oncorhynchus mykiss) oocytes. Dev. Biol. 166, 615–622. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P, 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. U.S.A. 100, 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu D, Shaner ZC, Chen S, Hong W, Stellwag EJ, 2015. Nuclear progestin receptor (pgr) knockouts in zebrafish demonstrate role for pgr in ovulation but not in rapid non-genomic steroid mediated meiosis resumption. Front. Endocrinol. (Lausanne) 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


