Abstract
Background and Purpose:
Hemispheric stroke studies associating lateropulsion (pusher syndrome) with the location of brain lesions have had mixed results from small, unmatched samples. This study was designed to determine whether lateropulsion localizes to specific brain regions across patients with stroke using a case-control design.
Methods:
Fifty patients with lateropulsion following stroke were matched with 50 stroke patients without lateropulsion using age, time since onset of stroke, admission motor Functional Independence Measure (FIM) score, lesion side and gender. The primary analysis included multivariate lesion symptom mapping using sparse canonical correlations to identify regions most associated with lateropulsion as assessed with the Burke Lateropulsion Scale. Secondary analyses included evaluating paired comparisons for lesion volume, degree of motor impairment, motor and cognitive FIM scores.
Results:
The lesion symptom mapping analysis of all lesions mapped onto a common hemisphere produced an overall significant model (P < 5 × 10−5) with a regional peak at the inferior parietal lobe at the junction of the post-central gyrus (Brodmann Area 2) and Brodmann Area 40 as the lesion location most associated with lateropulsion. Lesion volume was larger for patients with lateropulsion. Despite adequate matching, motor performance and total FIM scores differed at a group level between patients with and without lateropulsion.
Conclusions:
This analysis implicated lesion involvement of the inferior parietal lobe as a key neuroanatomical determinant of developing lateropulsion. A better understanding of the anatomical underpinnings of lateropulsion may improve rehabilitation efforts, including the potential for informing noninvasive neuromodulation approaches.
Keywords: stroke, diagnostic imaging, anatomy, lateropulsion, pusher syndrome
Subject Terms: stroke, magnetic resonance imaging, lateropulsion
Introduction
Lateropulsion (pusher syndrome) is a contralesional bias in the posture of a person with hemispheric stroke and a strong resistance to postural correction to upright vertical.1 Incidence ranges from 10% to 60% of patients with stroke depending on the stroke severity studied.2–5 Lateropulsion can be measured using the Burke Lateropulsion Scale6 and the Scale of Contraversive Pushing.7 Patients with lateropulsion have delayed functional recovery,3,8 especially when right-sided brain lesions are present.9,10 Novel strategies,11,12 such as transcranial neuromodulation techniques, could augment functional recovery, but these approaches are most likely to be effective if guided by neuroanatomical mechanisms underlying lateropulsion.
Lesion studies to date have had limited success in establishing the neuroanatomical basis of lateropulsion, possibly secondary to limited sample sizes,13–16 lack of validated measures of lateropulsion,3,17 and absence of a matched comparison group.3,13,16–18 Prior work has implicated a number of structures including: thalamus,13,15,19,20 parietal-insular vestibular cortex,21,22 temporo-parietal junction,17 and internal capsule.18,20
We used lesion symptom mapping with a case-control design to evaluate the neuro-anatomical basis of lateropulsion among patients with stroke. Our design attempted to extend this prior work by using a valid scale for evaluating lateropulsion, a higher sample size, a matched comparison group, and a more sophisticated multivariate statistical approach for lesion symptom mapping.23,24 The purpose of this study was to compare lesion location across matched pairs of patients with and without lateropulsion after stroke.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Burke Rehabilitation Hospital at sbabyar@burke.org.
Subjects:
Subjects were retrospectively selected from a database of patients with stroke who were admitted to Burke Rehabilitation Hospital (White Plains, NY, USA) from June 1, 2005 through July 30, 2016 (n=1750) who had MRI scans available to the researchers (n=739). These MRI scans were acquired at various hospitals soon after admission for stroke and prior to admission to inpatient rehabilitation, thus scanning parameters varied across subjects. Only scans with sufficient clarity to confidently delineate the anatomical boundaries of the lesion were considered for this study. We sought to maximize the sample size of lateropulsion patients using all data available at our institution. In reviewing all 739 individuals with MRI scans, 86 had lateropulsion, defined as a Burke Lateropulsion Scale (BLS) 6 score of 2 or greater (out of 17) with adequate MRI quality to delineate the lesion. Of these 86, a subject without lateropulsion (BLS score of 0 or 1) that otherwise met our criteria for comparison subjects (described below) was found for 50 participants.
All patients admitted with stroke were screened by physical therapists using the BLS. Subjects were individually matched based on: age (± 6 years); sex; time since onset of stroke (± 10 days); laterality of stroke; and admission motor Functional Independence Measure (FIM) (± 7). These matching criteria were decided in advance by group consensus. Although some patients with lateropulsion could match with more than one patient without it, we selected only a single best-matched comparison. A database of subject characteristics and results of admission and discharge examination procedures by physical therapists, occupational therapists and physicians was generated for comparison of patients with and without lateropulsion. The project was approved by the Institutional Review Board of Burke Rehabilitation Hospital; this board waived the need for patient consent. Procedures followed were in accordance with institutional guidelines.
Lesion Mapping:
The lesion location was manually traced using T2-weighted sequences in native MRI space using ITK-SNAP (http://www.itksnap.org/pmwiki/pmwiki.php) by an examiner blinded to behavioral measures.25 Diffusion weighted imaging sequences were also referenced to confirm lesion boundaries as needed. An experienced neurologist confirmed final decisions about lesion boundaries. MRI data and the associated lesion mask images were then transformed from native space to the Montreal Neurological Institute (MNI) T2 template,26,27 and subsequently to the MNI152 template brain using FSL FLIRT (http://fsl.fmrib.ox.ac.uk/fsldownloads). Neuroanatomical landmarks were used to ensure accurate transfer onto the template brain. Descriptive plots of lesion location were generated for patients with and without lateropulsion. A proportional difference map was also produced for descriptive purposes to compare the relative percentage of lesion overlap between groups. For example, if 35% of patients with lateropulsion had a lesion at a given voxel and 10% of patients without lateropulsion had a lesion at that same voxel, the intensity would be 25%.
For the main lesion symptom mapping analysis, lateropulsion was scored as a continuous variable using the BLS. In order to maximize the statistical power, the lesion masks were mapped to a common hemisphere. This was achieved by flipping the left-sided lesion masks on the X-axis so they were displayed on the right. By mapping to a single hemisphere we have greater statistical power similar to that of sample sizes previously shown to provide accurate lesion symptom mapping using simulated behavioral data.23 Lesion mapping was conducted using the LESYMAP package in R (https://github.com/dorianps/LESYMAP), which uses a multivariate statistical approach called sparse canonical correlations for neuroimaging analyses (SCCAN).23 SCCAN assigns a weight to each voxel to produce a hypothetical score with maximal correlation with the true behavioral score. It uses a 4-fold cross validation correlation, which is the correlation between the predicted score and the true behavioral score. The voxels associated with a deficit are assigned a larger weight and a zero weight is assigned to voxels not related to the deficit.23 The statistical significance of the overall model is determined and the voxels are weighted according to the strength of association with lateropulsion from 0 – 1, as described previously.23 Analyses were conducted in regions with a minimum overlap of 3 subjects.
In addition to the primary analysis, we repeated the analysis with lesions mapped to their native laterality, as opposed to collapsing lesions onto a single hemisphere. This analysis had less statistical power secondary to lower levels of overlapping lesions, but was included to evaluate the possibility of different lesion localization by hemisphere.
Descriptive statistics were generated for the patient characteristics. Matched pairs were compared using paired t-tests for patient characteristics. Nominal data were compared using chi-square analyses and frequency analyses. Lesion volume was analyzed using a mixed-model ANOVA with matched pairs as the within-subject factor and lesion side as the between-subjects factor. Paired t-tests for each lesion side were used post-hoc to compare lesion volume for patients with and without lateropulsion. Pearson product-moment correlation coefficients were generated comparing lesion volume and BLS score. IBM SPSS™ (v24, IBM, Armonk, NY, USA) was used for statistical analysis with statistical significance defined as a P value < 0.05.
Results
Demographics
Fifty matched pairs of MRIs of patients with and without lateropulsion were analyzed. Ages ranged from 44–93, mean of 76.7 (SD = 9.9) years. Twenty-eight pairs had left-sided brain lesions and 22 pairs had right-sided brain lesions.
Examination of data showed that matching was achieved within the inclusion criteria noted above. More females had left hemisphere stroke (n = 17 pairs out of 28) while more men had right hemisphere stroke (n = 14 pairs out of 22) but sex distribution per lesion side was not statistically different (χ2 = 2.92, df = 1, P = 0.087).
Descriptive statistics for characteristics of the matched pairs of patients with and without lateropulsion appear in Table 1. Paired comparisons of patients with and without lateropulsion revealed statistically significant differences in admission and discharge BLS scores and Fugl-Meyer Balance Scores. Despite adequate matching with admission motor FIM scores, admission Motricity Indices and Fugl-Meyer lower extremity motor scores for the contralesional limb were lower for patients with lateropulsion. Total FIM scores were also lower for patients with lateropulsion (Table 1). In comparison to matched pairs with left-sided brain lesions, patients with right-sided brain lesions appear to drive these statistically significant differences (Table I and II in online only Supplemental Material; please see http://stroke.ahajournals.org).
Table 1.
Admission and Discharge Characteristics of Sample and Results of Paired t-Tests Comparing Patients with (Pusher) and without (Non-Pusher) Lateropulsion.
| Admission | Discharge | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Non-Pusher | Pusher | P Value | N | Non-Pusher | Pusher | P Value |
| Age, Mean (SD), y | 50 | 76.7 (9.9) | 76.5 (10.4) | NS | ||||
| Time since Stroke Onset, Mean (SD), d | 50 | 8.5 (5.6) | 9.1 (5.5) | NS | ||||
| Admission Lower Extremity Motricity Index Involved Side, Mean (SD) | 50 | 52.2 (33.1) | 38.6 (32.4) | 0.028 | ||||
| Admission Upper Extremity Motricity Index Involved Side, Mean (SD) | 50 | 44.4 (37.8) | 29.6 (33.8) | 0.026 | ||||
| Burke Lateropulsion Scale Score, Mean (SD) | 44 | 0.2 (0.5) | 4.5 (2.9) | <0.001 | 36 | 0.0 (0.2) | 2.8 (2.9) | <0.001 |
| Upper Extremity Fugl-Meyer Motor Score, Mean (SD) | 18 | 16.7 (19.6) | 18.5 (17.7) | NS | 10 | 22.2 (23.4) | 28.0 (20.8) | NS |
| Lower Extremity Fugl-Meyer Motor Score, Mean (SD) | 42 | 12.5 (9.0) | 9.1 (7.5) | 0.046 | 30 | 18.6 (10.1) | 15.8 (9.0) | NS |
| Cognitive FIM, Mean (SD) | 49 | 18.8 (10.6) | 15.5 (6.2) | 0.038 | 49 | 26.8 (15.7) | 21.6 (8.8) | 0.038 |
| Motor FIM, Mean (SD) | 49 | 19.6 (5.9) | 18.4 (5.6) | NS | 49 | 36.2 (18.0) | 31.1 (14.3) | NS |
| Total FIM, Mean (SD) | 49 | 38.4 (13.3) | 33.9 (10.2) | 0.006 | 49 | 63.0 (24.2) | 52.8 (19.2) | 0.004 |
| Fugl-Meyer Balance Score, Mean (SD) | 42 | 5.1 (2.3) | 3.8 (2.1) | 0.012 | 35 | 7.9 (2.3) | 5.7 (2.4) | <0.01 |
| Line Crossing Total Score, Mean (SD) | 30 | 31.7 (8.4) | 29.8 (11.3) | NS | 12 | 30.9 (9.0) | 34.6 (3.5) | NS |
| Star Cancellation Total Score, Mean (SD) | 12 | 31.3 (20.3) | 41.6 (18.9) | NS | 15 | 44.8 (12.4) | 45.1 (12.9) | NS |
NS = not significant; Time since Onset of Stroke = count of days from stroke onset as recorded in acute care hospital admission record and the day of admission to inpatient rehabilitation.
Lesion Analyses
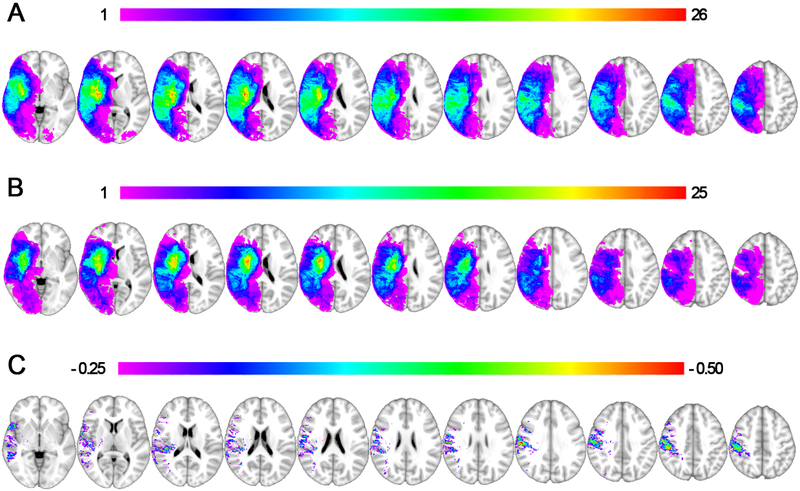
The lesion overlap maps are displayed for patients with and without lateropulsion separately (Figures 1A and1B, respectively) with a subtraction map displayed for descriptive purposes, showing regions with proportionally higher overlap in patients with lateropulsion relative to those without lateropulsion, expressed as a percentage (Figure 1C).
Figure 1. Lesion Overlap and Subtraction Maps.
1A shows a lesion overlap of all subjects with lateropulsion (n = 50, peak overlap of 26/50 at MNI coordinate 31, −13, 11) and 1B shows all subjects in the comparison group without lateropulsion (n = 50, peak overlap of 25/50 at MNI coordinate 30, −14, 5). 1C displays a proportional subtraction, or difference map, between the lateropulsion and comparison group, which demonstrates proportionally higher levels of overlap in patients with lateropulsion at the inferior parietal lobe (peak 55%, MNI coordinate 56, −17, 32). For each image a color palate is displayed with red representing the peak overlap.
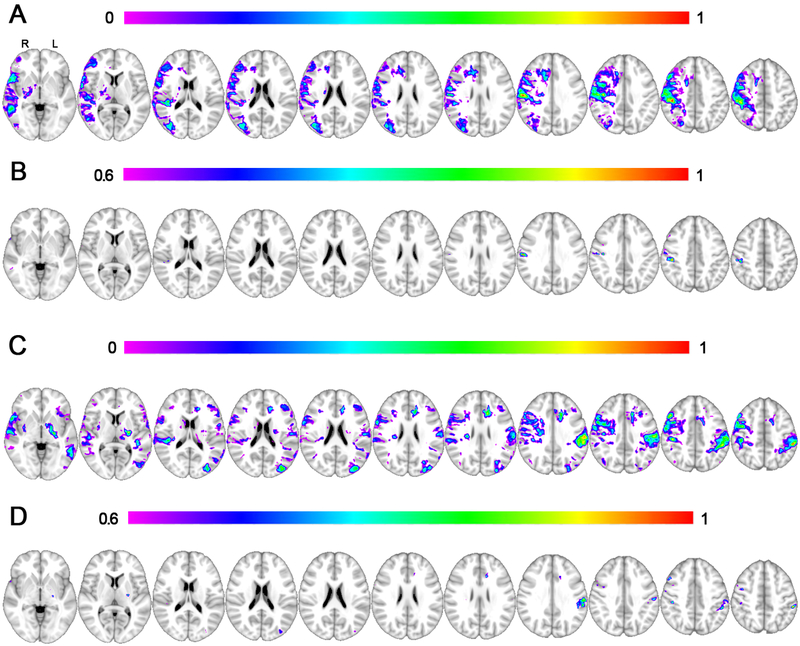
The main lesion symptom mapping analysis was performed using all subjects’ data with all lesions mapped onto the right hemisphere. The overall model was statistically significant with a cross validation correlation r = 0.39, P < 5 × 10−5. The region associated with the highest Burke Lateropulsion Scale scores was the parietal lobule at the junction of the post-central gyrus (Brodmann Area 2) and Brodmann Area 40, peak MNI coordinate 44, −29, 44, see Figure 2A-B. This peak finding from Figure 2B is also shown in Figure 3 at higher magnification in multiple orientations.
Figure 2. Lesion Symptom Mapping Results.
2A shows the results of main lesion symptom mapping analysis with a color-coded scale that varies according to the strength of association with lateropulsion, with the overall model significant at P < 0.001. 2B shows the same results from 2A with a higher threshold, highlighting the most significant region in the inferior parietal lobule with a peak at MNI coordinates 44, −29, 44. Note all lesions were mapped to the right hemisphere for this analysis. In 2C, the same lesion symptom mapping is conducted with lesions mapped true to their laterality, as opposed to collapsing lesions onto a single hemisphere. This overall model was significant (P = 0.001) with the strongest regional findings in the left parietal lobe, which is more clearly evident using a higher statistical threshold (2D) with a peak at MNI coordinates −59, −34, 30.
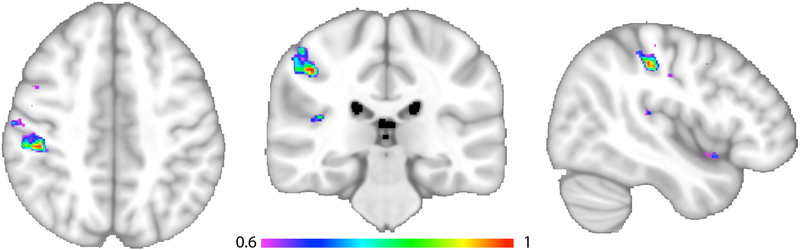
Figure 3. Peak Lesion Symptom Mapping Result.
This figure shows additional views of the peak findings displayed in Figure 2B to better detail the anatomical distribution. The three views are axial, coronal and sagittal, from left to right, with each slice centered on the peak MNI coordinate 44, −29, 44. The region maximally associated with higher levels of lateropulsion was assessed with continuous scores from the Burke Lateropulsion Scale, with all lesions mapped to the right hemisphere. The multivariate statistical approach preferentially weights regions according to their association with lateropulsion on a scale from 0 to 1, with this result at a 0.6 threshold to display the strongest findings. The overall model was significant at p < 0.001.
Secondary lesion symptom mapping analyses were similarly performed for case-control pairs using the native lesion laterality (Figure 2C-D). This analysis was included to evaluate the possibility of differences in localization by hemisphere, with the caveat that the sample size was approximately half that of the main analysis when split into two hemispheres. The overall model was significant with a cross validation r = 0.32, P = 0.001. Results show a similar spatial distribution with the strongest findings in the left parietal lobe with two peaks at MNI coordinate −57, −36, 49 and −59, −34, 30. The strongest findings were in the left hemisphere, which is the side with more case control pairs (n = 28 versus 22).
Mixed-model ANOVA of lesion volume showed a main effect for lateropulsion status (Wilks’ Lambda = .092, F1,48 = 4.15, P = 0.047) but not an interaction between lateropulsion status and side of lesion (Wilks’ Lambda = .997, F1,48 = 0.147, P = 0.703). As noted in Table 2, lesion volume was highly variable but, on average, patients with lateropulsion had greater lesion volume.
Table 2.
Lesion Volume (mm3) for Patients with and without Lateropulsion: Descriptive Statistics and Paired t-test Results.
| n | Mean SD |
P | ||
|---|---|---|---|---|
| All Lesions | Non-Pusher | 50 | 33473.7 (32628.3) |
0.039 |
| Pusher | 50 | 57346.4 (70811.9) |
||
| Left-sided brain lesion | Non-Pusher | 28 | 30468.6 (26659.1) |
NS |
| Pusher | 28 | 58206.0 (82055.6) |
||
| Right-sided brain lesion | Non-Pusher | 22 | 37298.3 (39280.5) |
NS |
| Pusher | 22 | 56252.3 (55145.1) |
||
Non-Pusher = Burke Lateropulsion Scale 0 or 1; Pusher = Burke Lateropulsion Scale 2-17.
Moderate positive correlations existed between BLS scores measured upon admission to inpatient rehabilitation and lesion volume for all patients with lateropulsion (r = 0.636; P < 0.001) and for subjects with left-sided brain lesions and lateropulsion (r = 0.495; P < 0.001). This relationship was not observed for patients with right-sided brain lesions (r = 0.235; P < 0.292).
Discussion
These results suggest that cortical strokes causing lateropulsion localize primarily to the inferior parietal lobe extending from Brodmann Area 2 of the post-central gyrus to Brodmann Area 40 in the inferior parietal lobe. This cortical area may be important for the perception of postural vertical and compiling the egocentric reference system. Three prior groups have highlighted a possible role of the inferior parietal lobe in lateropulsion.17,28,29 Using a lesion subtraction method, Johannsen, et al.29 matched patients with and without lateropulsion (based upon the Scale of Contraversive Pushing) from cortical stroke lesions that did not involve the thalamus. Subtraction image analysis of their CT or MRI images revealed that patients with lateropulsion had unique areas of involvement of the left posterior insula, the left superior temporal gyrus, the left inferior parietal lobule and the right post-central gyrus.29 In the Pérennou, et al.17 study, patients with stroke who failed a seated balance task had greater frequency of lesions in the temporo-parietal junction. These researchers hypothesized that the temporo-parietal junction serves as an integration area where various sensations are evaluated and reconciled to create an egocentric reference system for vertical upright posture. Lesions in this area disrupt this interpretation, yielding an alteration of the subjective postural vertical alignment.17 Santos-Pontelli, et al. noted heterogeneity of lesion sites for groups of patients with and without lateropulsion.28 They specifically evaluated pre-determined areas to evaluate whether they were more involved in patients with lateropulsion. Greater frequency of involvement of the posterior parietal area and the thalamus was noted in the patients with lateropulsion.28 The authors determined that the posterior parietal region might be involved in the graviceptive processing affecting postural upright perception. Some researchers view this egocentric and graviceptive processing as a right hemisphere function.17,30 Our analyses using native laterality of the lesions indicated that lesions of the left inferior parietal lobe had a role in the development of lateropulsion, although we notably had higher statistical power on the left hemisphere in this sample.
Beyond lesion localization studies, other imaging techniques have highlighted the role of the inferior parietal lobule in lateropulsion.14,31 Ticini, et al.14 used perfusion weighted imaging in a small sample to explore the role of the posterior thalamus in lateropulsion as determined by the Scale of Contraversive Pushing. They showed that extra-thalamic stroke created small areas of lower perfusion in the intact “inferior frontal gyrus, middle temporal gyrus, precentral gyrus, inferior parietal lobule and parietal white matter” as well as the “callosal body, temporal white matter, and superior longitudinal fasciculus” (p. e5737) when lateropulsion was present. They postulated that these areas may play an important role in perception of upright and/or postural control and that lesions of the thalamus are not essential for lateropulsion.14
As evidence continues to accrue, researchers should be open to the possibility of a network of structures responsible for perception of vertical posture whereby dysregulation within the network results in behavioral manifestations such as lateropulsion. The current study, viewed in relationship with prior research, indicates that the inferior parietal lobe may be an important node in the network.
Lesion Size
Lesion size was larger for patients with lateropulsion (P = 0.047) compared to those without lateropulsion; this was observed only when pooling data from patients with left and right-sided brain lesions. Prior studies comparing lesion size for patients with and without lateropulsion had included hemorrhagic stroke.15,28 Karnath, et al.15 observed significantly larger lesion sizes for patients with lateropulsion and left thalamic lesions (P = 0.006) compared to patients without lateropulsion. This finding approached significance for patients with right thalamic lesions (P = 0.052). They hypothesized that the greater extremity weakness observed for patients with lateropulsion could be attributed to pressure from large hemorrhagic strokes in the posterior thalamus onto structures like the internal capsule.15 In contrast to the Karnath study, Santos-Pontelli, et al. determined that the ABC/2 method32 for calculating hemorrhagic stroke volume failed to discern patients with and without lateropulsion.28
When examining only patients with lateropulsion, lesion size showed a moderate positive correlation to admission BLS score which was statistically significant for pooled data and for data from patients with left-sided brain lesions and lateropulsion. This relationship did not exist for patients with right-sided brain lesions and lateropulsion (Table 2). No other studies specifically correlated a measure of lateropulsion with lesion size. The closest similar study showed that subjective postural vertical perception (as compared to subjective visual vertical and haptic vertical perceptions) was related to lesion size but only for patients with right-sided brain lesions.33 Advocating for more time in rehabilitation may be indicated for patients with large lesion size, more severe lateropulsion and multiple sensorimotor deficits.9
Lesions of the inferior parietal lobe are associated with the development of lateropulsion in this sample. The inferior parietal cortex appears to hold a central role in the pathway for evaluating and integrating somatosensory, visual, and vestibular inputs. From a clinical perspective, improving the function of this area might improve recovery from lateropulsion which, in turn, would allow patients to focus on relearning activities of daily living during their inpatient rehabilitation. Selectively influencing this area via enhanced proprioceptive input of muscle spindles via exercise may be indicated. Some researchers have incorporated galvanic stimulation for patients with lateropulsion 11,12,34 as a way to modulate input to the network of brain centers responsible for the egocentric postural reference system for vertical upright. Based on the current findings implicating the inferior parietal lobe, one may speculate that modulating the activity of the inferior parietal cortex via transcranial direct current stimulation or transcranial magnetic stimulation may also provide a rational therapeutic strategy to test as a potential intervention for lateropulsion.
Limitations
There are several limitations to the present work. First, by mapping all lesions onto a single hemisphere for the main lesion symptom mapping analysis we are less able to detect unique contributions to lateropulsion from each hemisphere. We attempted to address this by performing analyses using the native laterality of the lesions, but the smaller sample size somewhat limits our confidence in any laterality-specific findings. For example, the higher association of the left inferior parietal lobe may be due to more lesions affecting the left than right hemisphere in this sample. Accruing a sufficiently sized sample of patients with lateropulsion with right-sided brain lesions who could be uniquely matched to patients without lateropulsion is a goal for future analyses. Further study is recommended because, clinically, patients with right-sided brain lesions and lateropulsion have greater motor deficits in matched comparisons, as was found in the current study, and it will be important to further evaluate whether there are unique neuroanatomical substrates that vary by hemisphere.2,10,14,28
Despite adequate matches amongst all included individuals using the admission motor FIM score, as a group, patients with right-sided brain lesion and lateropulsion had slightly more severe motor deficits with an average difference in FIM motor scores of 2 points (Table II, online only Data Supplement; please see http://stroke.ahajournals.org) relative to their matched counterparts. This discrepancy between accurately matched individuals using our pre-set criteria and observing a significant group difference is due to small but consistent differences in FIM motor scores between the cohorts with right-sided brain lesions. Matching was easier for patients with left-sided brain lesions because motor deficits of those with and without lateropulsion were less divergent (Table I, online only Data Supplement). Others have observed this phenomenon.2,15,28 Karnath, et al. presented the only study which showed greater unilateral motor deficits for patients with left extrathalamic lesions and lateropulsion (n = 10) compared to controls; only the upper extremity had greater motor deficits for patients with right extrathalamic lesions and lateropulsion (n = 11). Additional studies with even larger cohorts are warranted to further evaluate the relationship of laterality and its role in lateropulsion.
Finally, it will be important for future studies to match for lesion size between groups, which was not a matching criterion used here. We found that lesions were significantly larger in the lateropulsion group. Finally, it will be useful in future prospectively collected cohorts to include other functional measures beyond FIM motor scores to compare lateropulsion versus comparison groups, such as the NIH Stroke Scale or modified Rankin Score.
Summary
The current study supports and builds upon prior work suggesting that lesions of the inferior parietal lobe are associated with the development of lateropulsion. This region may have a central role in integrating information from somatosensory, visual and vestibular systems. Patients with lateropulsion had larger lesion volume when compared to patients without lateropulsion. In the pooled sample of patients with lateropulsion, lesion volume correlated with severity of lateropulsion; this association appears to be driven by higher correlations for patients with left-sided brain damage. A better understanding of the neuroanatomical basis of lateropulsion may ultimately facilitate novel interventions like transcranial neuromodulation or by augmented sensory input aimed at remediation of lateropulsion.
Supplementary Material
Acknowledgments
We are grateful for technical support from Hana Mahallati, Divija Chopra, Maria Lopez-Cavestany, Sam Snyder, and Noelle Santos. Joel Bruss and Mark Bowren assisted with transforming lesions into MNI space and with lesion mapping.
Sources of Funding
Support for this project was provided by a PSC-CUNY Award, jointly funded by The Professional Staff Congress and The City University of New York. Additional support was from the Department of Physical Therapy, Hunter College of The City University of New York, and Burke Rehabilitation Hospital. Dr. Boes was supported by NIH: K12NS098482 and the Roy J. Carver Trust.
Footnotes
Disclosures: None
References
- 1.Davies PM. Steps to Follow: A Guide to the Treatment of Adult Hemiplegia. Berlin, Germany: Springer; 1985. [Google Scholar]
- 2.Babyar SR, White H, Shafi N, Reding M. Outcomes with stroke and lateropulsion: A case-matched controlled study. Neurorehabil Neural Repair. 2008;22:415–423. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen PM, Wandel A, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Ipsilateral pushing in stroke: Incidence, relation to neuropsychological symptoms, and impact on rehabilitation. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1996;77:25–28. [DOI] [PubMed] [Google Scholar]
- 4.Danells CJ, Black SE, Gladstone DJ, McIlroy WE. Poststroke “pushing:” Natural history and relationship to motor and functional recovery. Stroke. 2004;35:2873–2878. [DOI] [PubMed] [Google Scholar]
- 5.Premoselli S, Cesana L, Cerri C. Pusher syndrome in stroke: Clinical, neuropsychological and neurophysiological investigation. Eura Medicophys. 2001;37:143–151. [Google Scholar]
- 6.D’Aquila MA, Smith T, Organ D, Lichtman S, Reding M. Validation of a lateropulsion scale for patients recovering from stroke. Clin Rehabil. 2004;18:102–9. [DOI] [PubMed] [Google Scholar]
- 7.Karnath HO, Johannsen L, Broetz D, Ferber S, Dichgans J. Prognosis of contraversive pushing. J Neurol. 2002;249:1250–1253. [DOI] [PubMed] [Google Scholar]
- 8.Krewer C, Luther M, Müller F, Koenig E. Time course and influence of pusher behavior on outcome in a rehabilitation setting: A prospective cohort study. Top Stroke Rehabil. 2013;20:331–339. [DOI] [PubMed] [Google Scholar]
- 9.Babyar SR, Peterson MGE, Reding M. Time to recovery from lateropulsion dependent on key stroke deficits: A retrospective analysis. Neurorehabil Neural Repair. 2015;29:207–213. [DOI] [PubMed] [Google Scholar]
- 10.Abe H, Kondo T, Oouchida Y, Suzukamo Y, Fujiwara S, Izumi S. Prevalence and length of recovery of pusher syndrome based on cerebral hemispheric lesion side in patients with acute stroke. Stroke. 2012;43:1654–1656. [DOI] [PubMed] [Google Scholar]
- 11.Krewer C, Rieß K, Bergmann J, Müller F, Jahn K, Koenig E. Immediate effectiveness of single-session therapeutic interventions in pusher behaviour. Gait Posture. 2013;37:246–250. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura J, Kita Y, Yuda T, Ikuno K, Okada Y, Shomoto K. Effects of galvanic vestibular stimulation combined with physical therapy on pusher behavior in stroke patients: A case series. NeuroRehabil. 2014;35:31–37. [DOI] [PubMed] [Google Scholar]
- 13.Karnath HO, Ferber S, Dichgans J. The neural representation of postural control in humans. Proc Natl Acad Sci U S A. 2000;97:13931–13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ticini LF, Klose U, Nägele T, Karnath H. Perfusion imaging in pusher syndrome to investigate the neural substrates involved in controlling upright body position. PLoS One. 2009;4:e5737–e5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnath HO, Johannsen L, Broetz D, Küker W. Posterior thalamic hemorrhage induces “pusher syndrome.” Neurology. 2005;64:1014–1019. [DOI] [PubMed] [Google Scholar]
- 16.Abe H, Kondo T, Kochiyama T, Oouchida Y, Fujiwara S, Izumi S. Delay in pusher syndrome recovery is related to frontal white matter lesions. Int J Neurol Neurother. 2017;4:065. [Google Scholar]
- 17.Pérennou DA, Leblond C, Amblard B, Micallef JP, Rouget E, Pelissier J. The polymodal sensory cortex is crucial for controlling lateral postural stability: Evidence from stroke patients. Brain Res Bull. 2000;53:359–365. [DOI] [PubMed] [Google Scholar]
- 18.Baier B, Janzen J, Müller-Forell W, Fechir M, Müller N, Dieterich M. Pusher syndrome: Its cortical correlate. J Neurol. 2012;259:277–283. [DOI] [PubMed] [Google Scholar]
- 19.Dieterich M, Bartenstein P, Spiegel S, Bense S, Schwaiger M, Brandt T. Thalamic infarctions cause side-specific suppression of vestibular cortex activations. Brain. 2005;128:2052–2067. [DOI] [PubMed] [Google Scholar]
- 20.Baier B, Conrad J, Stephan T, et al. Vestibular thalamus: Two distinct graviceptive pathways. Neurology. 2016;86:134–140. [DOI] [PubMed] [Google Scholar]
- 21.Golden M, D’Aquila MA, Reding M. Clinical and neuroimaging correlates of “pusher phenomenon” following stroke. [Abstract]. J Stroke Cerebrovasc Dis. 2001;10:200. [Google Scholar]
- 22.Reding M, David A, Volpe B. Neuroimaging study of the pusher syndrome post stroke. [Abstract]. J Neurol Sci. 1997;150:S129. [Google Scholar]
- 23.Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB. Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia. 2017;1156:154–166. [DOI] [PubMed] [Google Scholar]
- 24.Mah Y, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137:2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Pontelli T, Pontes-Neto O, de Araujo DB, dos Santos AC, Leite JP. Neuroimaging in stroke and non-stroke pusher patients. Arq Neuropsiquiatr. 2011;69:914–919. [DOI] [PubMed] [Google Scholar]
- 29.Johannsen L, Broetz D, Naegele T, Karnath HO. “Pusher syndrome” following cortical lesions that spare the thalamus. J Neurol. 2006;253:455–63. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW, Cook AC, Larkin PA, et al. The listing phenomenon of hemiplegic patients. Neurol Rep. 1986;10:43–44. [Google Scholar]
- 31.Himmelbach M, Erb M, Karnath HO. Exploring the visual world: The neural substrate of spatial orienting. Neuroimage. 2006;32:1747–1759. [DOI] [PubMed] [Google Scholar]
- 32.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 33.Pérennou DA, Mazibrada G, Chauvineau V, et al. Lateropulsion, pushing and verticality perception in hemisphere stroke: A causal relationship? Brain. 2008;131:2401–2413. [DOI] [PubMed] [Google Scholar]
- 34.Fink GR, Marshall JC, Weiss PH, et al. Compensation for distorted egocentric representation of space implicates right inferior parietal cortex. Cortex. 2002;38:854–859. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.