Abstract
Background
Adolescent overweight and obesity has increased globally, and can be associated with short‐ and long‐term health consequences. Modifying known dietary and behavioural risk factors through behaviour changing interventions (BCI) may help to reduce childhood overweight and obesity. This is an update of a review published in 2009.
Objectives
To assess the effects of diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years.
Search methods
We performed a systematic literature search in: CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, LILACS, and the trial registers ClinicalTrials.gov and ICTRP Search Portal. We checked references of identified studies and systematic reviews. There were no language restrictions. The date of the last search was July 2016 for all databases.
Selection criteria
We selected randomised controlled trials (RCTs) of diet, physical activity and behavioural interventions for treating overweight or obesity in adolescents aged 12 to 17 years.
Data collection and analysis
Two review authors independently assessed risk of bias, evaluated the overall quality of the evidence using the GRADE instrument and extracted data following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions. We contacted trial authors for additional information.
Main results
We included 44 completed RCTs (4781 participants) and 50 ongoing studies. The number of participants in each trial varied (10 to 521) as did the length of follow‐up (6 to 24 months). Participants ages ranged from 12 to 17.5 years in all trials that reported mean age at baseline. Most of the trials used a multidisciplinary intervention with a combination of diet, physical activity and behavioural components. The content and duration of the intervention, its delivery and the comparators varied across trials. The studies contributing most information to outcomes of weight and body mass index (BMI) were from studies at a low risk of bias, but studies with a high risk of bias provided data on adverse events and quality of life.
The mean difference (MD) of the change in BMI at the longest follow‐up period in favour of BCI was ‐1.18 kg/m2 (95% confidence interval (CI) ‐1.67 to ‐0.69); 2774 participants; 28 trials; low quality evidence. BCI lowered the change in BMI z score by ‐0.13 units (95% CI ‐0.21 to ‐0.05); 2399 participants; 20 trials; low quality evidence. BCI lowered body weight by ‐3.67 kg (95% CI ‐5.21 to ‐2.13); 1993 participants; 20 trials; moderate quality evidence. The effect on weight measures persisted in trials with 18 to 24 months' follow‐up for both BMI (MD ‐1.49 kg/m2 (95% CI ‐2.56 to ‐0.41); 760 participants; 6 trials and BMI z score MD ‐0.34 (95% CI ‐0.66 to ‐0.02); 602 participants; 5 trials).
There were subgroup differences showing larger effects for both BMI and BMI z score in studies comparing interventions with no intervention/wait list control or usual care, compared with those testing concomitant interventions delivered to both the intervention and control group. There were no subgroup differences between interventions with and without parental involvement or by intervention type or setting (health care, community, school) or mode of delivery (individual versus group).
The rate of adverse events in intervention and control groups was unclear with only five trials reporting harms, and of these, details were provided in only one (low quality evidence). None of the included studies reported on all‐cause mortality, morbidity or socioeconomic effects.
BCIs at the longest follow‐up moderately improved adolescent's health‐related quality of life (standardised mean difference 0.44 ((95% CI 0.09 to 0.79); P = 0.01; 972 participants; 7 trials; 8 comparisons; low quality of evidence) but not self‐esteem.
Trials were inconsistent in how they measured dietary intake, dietary behaviours, physical activity and behaviour.
Authors' conclusions
We found low quality evidence that multidisciplinary interventions involving a combination of diet, physical activity and behavioural components reduce measures of BMI and moderate quality evidence that they reduce weight in overweight or obese adolescents, mainly when compared with no treatment or waiting list controls. Inconsistent results, risk of bias or indirectness of outcome measures used mean that the evidence should be interpreted with caution. We have identified a large number of ongoing trials (50) which we will include in future updates of this review.
Plain language summary
Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years
Review question
How effective are diet, physical activity and behavioural interventions in reducing the weight of overweight or obese adolescents aged 12 to 17 years?
Background
Across the world, more adolescents are becoming overweight and obese. These adolescents are more likely to suffer from health problems in later life. More information is needed about what works best in treating this problem.
Study characteristics
We found 44 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) comparing diet, physical activity and behavioural (where habits are changed or improved) treatments (interventions) to a variety of control groups delivered to 4781 overweight or obese adolescents aged 12 to 17 years. Our systematic review reports on the effects of multidisciplinary interventions, dietary interventions and physical activity interventions compared with a control group (no intervention, 'usual care,' enhanced usual care or some other therapy if it was also delivered to the intervention group). The adolescents in the included studies were monitored (called follow‐up) for between six months and two years.
Key results
The average age of adolescents ranged from 12 to 17.5 years. Most studies reported the body mass index (BMI). BMI is a measure of body fat and is calculated by dividing weight (in kilograms) by the square of the body height measured in metres (kg/m2). We summarised the results of 28 studies in 2774 adolescents reporting BMI, which on average was 1.18 kg/m2 lower in the intervention groups compared with the control groups. We summarised the results of 20 studies in 1993 adolescents reporting weight, which on average was 3.67 kg lower in the intervention groups compared with the control groups. BMI reduction was maintained at 18 to 24 months of follow‐up (monitoring participants until the end of the study), which on average was 1.49 kg/m2 lower in the intervention groups compared with the control groups. The interventions moderately improved health‐related quality of life (a measure of a person's satisfaction with their life and health) but we did not find firm evidence of an advantage or disadvantage of these interventions for improving self‐esteem, physical activity and food intake. No study reported on death from any cause, morbidity (illnesses) or socioeconomic effects (such as days away from school). Three studies reported no side effects, one reported no serious side effects, one did not provide details of side effects and the rest of the studies did not report whether side effects occurred or not.
We identified 50 ongoing studies which we will include in future updates of our review.
Currentness of evidence
This evidence is up to date as of July 2016.
Quality of the evidence
The overall quality of the evidence was rated as low for most of the outcomes (results) measured, mainly because of limited confidence in how studies were performed, inconsistent results between the studies and the way that some outcomes used do not capture obesity outcomes directly. Also, there were just a few studies for some outcomes, with small numbers of included adolescents.
Summary of findings
Summary of findings for the main comparison. Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12 to 17 years.
| Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12‐17 years | ||||||
|
Patient or population: adolescents (aged 12‐17 years) being overweight or obese Settings: school; community; healthcare Intervention: behaviour changing interventions (behavioural, diet, physical activity (or a combination) components) Comparison: usual care; concomitant therapy; no intervention/wait list | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Usual care, concomitant therapy, no intervention/wait list | Behaviour changing intervention | |||||
|
a) Change in BMI
Follow‐up: 6‐24 months b) Change in BMI z scored Follow‐up: 6‐24 months c) Change in weight Follow‐up: 6‐24 months |
a) The mean BMI change ranged across control groups from ‐1.18 kg/m2 to 2.1 kg/m2 b) The mean BMI z score change ranged across control groups from ‐0.31 units to 0.13 units c) The mean change in weight ranged across control groups from ‐1.8 kg to 8.3 kg |
a) The mean BMI change in the intervention groups was 1.18 kg/m2 lower (1.67 lower to 0.69 lower) b) The mean BMI z score change in the intervention groups was 0.13 units lower (0.21 lower to 0.05 lower) c) The mean change in weight in the intervention groups was ‐3.67 kg lower (‐5.21 lower to ‐2.13 lower) |
‐ | a) 2774 (28) b) 2399 (20) c) 1993 (20) |
a) ⊕⊕⊝⊝
Lowa b) ⊕⊕⊝⊝ Lowb c) ⊕⊕⊕⊝ Moderatec |
a) Lower BMI indicates weight loss b) Lower score indicates weight loss c) Lower weight indicates weight loss |
| Adverse events | See comment | See comment | See comment | See comment | ⊕⊕⊝⊝ Lowe | Only 5 trials reported adverse events and of these details were provided in only 1 showing no substantial differences between intervention and comparator groups |
| Health‐related quality of life Validated self‐reported measures Follow‐up: 6‐24 months | The standardised mean difference for health‐related quality of life ranged across control groups from ‐1.34 to 9.73 | The standardised mean difference for health‐related quality of life in the intervention groups was 0.44 standard deviations higher (0.09 to 0.79 higher) | ‐ | 972 (7) | ⊕⊕⊝⊝ Lowf | A standard deviation of 0.44 represents a moderate difference between groups.g |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Not reported |
| Morbidity | See comment | See comment | See comment | See comment | See comment | Not reported |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) BMI: body mass index: CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded one level due to inconsistency (I2 = 78%), one level due to indirectness (surrogate outcome used); see Appendix 9.
bDowngraded one level due to inconsistency (I2 = 86%), one level due to indirectness (surrogate outcome used); see Appendix 9.
cDowngraded one level due to inconsistency (I2 = 96%); see Appendix 9. d"A BMI z score or standard deviation score indicates how many units (of the standard deviation) a child's BMI is above or below the average BMI value for their age group and sex. For instance, a z score of 1.5 indicates that a child is 1.5 standard deviations above the average value, and a z score of ‐1.5 indicates a child is 1.5 standard deviations below the average value" (NOO NHS 2011). eDowngraded one level due to reporting and other bias and limited information (small number of studies and the majority of trials had less than 80% of participants enrolled included in the analysis); see Appendix 9. fDowngraded one level due to reporting and detection bias (no blinding of participants and personnel) and inconsistency (I2 = 85%); see Appendix 9). gA broadly accurate guide of how to interpret the standard mean difference (SMD) is: less than 0.40 = small, 0.40 to 0.70 = moderate, greater than 0.70 = large (Higgins 2011a).
Background
The prevalence of overweight and obese children and adolescents has increased throughout the world, presenting a global public health crisis (Ng 2014; WHO 2015a). Although once considered to be a condition affecting only high‐income countries, rates of paediatric overweight and obesity have started to rise dramatically in some low‐ to middle‐income countries (Wang 2012). Using the International Obesity Task Force (IOTF) standard definition, the age‐standardised prevalence of overweight and obesity in children and adolescents has increased in both high‐income and low‐ to middle‐income countries since the mid‐1980s (Cole 2000). In 2013, the prevalence of overweight and obese children and adolescents in high‐income countries was estimated at 23.8% (95% confidence interval (CI) 22.9 to 24.7) for boys and 22.6% (95% CI 21.7 to 23.6) for girls. In low‐ to middle‐income countries, the prevalence was estimated as 12.9% (95% CI 12.3 to 13.5) for boys and 13.4% (95% CI 13.0 to 13.9) for girls (Ng 2014). Very young children are also affected. In 2010, de Onis 2010 used the World Health Organization (WHO) growth standards (WHO 2015b) to estimate that over 42 million children under five years of age were overweight or obese, with approximately 35 million of these children living in low‐ to middle‐income countries.
Inequalities in overweight and obesity prevalence have also been documented. Generally, socioeconomically disadvantaged children in high‐income countries (Knai 2012; Shrewsbury 2008), and children of higher socioeconomic status in low‐ to middle‐income countries (Lobstein 2004; Wang 2012) are at greater risk of becoming overweight. However, this relationship may vary by population demographics (e.g. age, gender, ethnicity), and environment (e.g. country, urbanisation) (Wang 2012). The prevalence of obesity varies by ethnicity, with large data sets showing substantial ethnic variation in English (HSCIC 2015), US (Freedman 2006; Skinner 2014), and New Zealand (Rajput 2014) child populations.
While there is some evidence that the rate of increase in paediatric obesity may be slowing in some high‐income countries, current levels remain too high, and continue to rise in many low‐ to middle‐income countries (Olds 2011; Rokholm 2010). However, an additional concern in some high‐income countries such as the USA (Kelly 2013; Skinner 2014), and England (CMO 2015; Ells 2015), is the rise in severe paediatric obesity. While the IOTF published an international definition for severe paediatric (morbid) obesity in 2012 (Cole 2012), often severe obesity prevalence is reported using country‐specific cut‐off points making international comparisons difficult. However, data from the USA (Skinner 2014), and England (Ells 2015), have shown that the prevalence of severe paediatric obesity varies by socioeconomic status and ethnicity, and may result in a greater risk of adverse cardiometabolic events and severe obesity in adulthood (Kelly 2013).
Description of the condition
Childhood overweight and obesity results from an accumulation of excess body fat, and can increase the risk of both short‐ and longer‐term health consequences. Numerous obesity‐related co morbidities can develop during childhood, which include muscular skeletal complaints (Paulis 2014); cardiovascular risk factors such as hypertension, insulin resistance and hyperlipidaemia (Reilly 2003), even in very young children (Bocca 2013); and conditions such as such as sleep apnoea (Narang 2012), asthma (Egan 2013), liver disease, and type 2 diabetes mellitus (Daniels 2009; Lobstein 2004). The condition can also affect psychosocial well‐being, with obese young people susceptible to reduced self‐esteem and health‐related quality of life (Griffiths 2010), and stigmatisation (Puhl 2007; Tang‐Peronard 2008). Evidence also shows that childhood obesity can track into adulthood (Parsons 1999; Singh 2008; Whitaker 1997), and is therefore associated with an increased risk of ill health later in life (Reilly 2003).
Description of the intervention
Given the serious implications associated with childhood and adolescent obesity, effective treatment is imperative. While the fundamental principles of weight management in children and adolescents are the same as in adults (i.e. reduced energy intake and increased energy expenditure), the primary aim of treatment (i.e. weight reduction or deceleration of weight gain) and the most suitable intervention approach vary, and are dependent on the child's age and degree of excess weight, among other considerations. Behaviour changing interventions combining dietary, physical activity and behavioural components are effective and are considered the current best practice in the treatment of childhood obesity in adolescents under 18 years of age (WHO 2015c).
Adverse effects of the intervention
It is not anticipated that diet, physical activity and behavioural interventions will lead to adverse outcomes. However, as with all obesity treatment interventions in children and young people, potential adverse effects should be considered, including effects on linear growth, eating disorders and psychological well‐being.
How the intervention might work
The cause of childhood obesity is multifactorial, including dietary, activity, behavioural and environmental factors (Orsi 2011). Modifying these factors by behaviour changing interventions is considered the line of treatment of childhood overweight and obesity (Spear 2007). Behaviour changing interventions aim to improve dietary intake, increase activity levels, reduce sedentary behaviour, provide techniques to sustain healthy lifestyle and may have parental or family involvement (Kothandan 2014). Interventions may target one behavioural component (diet only or physical activity only) while other interventions integrate several components (diet, physical activity and behavioural modification) that seem to show promising results in decreasing overweight and obesity in adolescents (Jelalian 1999). Behavioural modification is often based on theoretical elements such as cognitive behavioural theory to help adolescents sustain changes and minimise relapse (Doak 2006). Theory‐based interventions address different elements such as healthy food choices, environmental control, positive thinking and goal‐setting that appear to be linked to positive weight outcomes (Dewar 2013; MacDonell 2010; White 2004). Earlier systematic reviews showed that behaviour changing interventions improved weight reduction in children aged 19 and younger (Ho 2012; Wilfley 2007), while one systematic review by Kelly and colleagues showed that interventions addressing nutrition, physical activity and behavioural skills with parental involvement appears to be an effective way to reduce adolescent obesity (Kelly 2008). One systematic review by Ruotsalainen and colleagues showed that supervised physical activity interventions have a favourable effect on adolescent BMI. Ruotsalainen acknowledged the importance of complex interventions that involve behavioural modification and management in the implementation of physical activity in adolescents (Ruotsalainen 2015), while the effect of dietary interventions remains unclear. One systematic review by Collins and colleagues found inconsistent evidence on the role of dietetic interventions in the treatment of childhood obesity (Collins 2006). However, the length, delivery setting and long‐term effect of behaviour changing interventions in the treatment of adolescent obesity remains unclear (Ho 2012; McGovern 2008; Wilfley 2007). The importance of behaviour changing interventions in treating childhood obesity has led to questioning their effectiveness in adolescents and whether different types of behavioural modification are more effective than others.
Why it is important to do this review
The first version of this systematic review was published in 2003 and included analysis of childhood obesity treatment trials published up to July 2001 (Summerbell 2003). The second version was published in 2009 providing an update to the 2003 review (Oude Luttikhuis 2009). To reflect the rapid growth in this field, the third update to this review has been split across six reviews focusing on the following treatment approaches: surgery; drugs; parent‐only interventions; diet, physical activity and behavioural interventions for young children aged 0 to 6 years; school children aged 5 to 11 years and adolescents aged 12 to 17 years. The current review examines the effectiveness of interventions for adolescents aged 12 to 17 years. Previous systematic reviews identified gaps in research assessing interventions specifically for this age group (Doak 2006). Other systematic reviews did not focus on adolescents specifically (Ho 2012; Peirson 2015; Wilfley 2007) or focused on specific interventions (Ruotsalainen 2015). This review has extended the evidence base by including trials of any form of behaviour changing intervention aimed to treat obesity in adolescents aged 12 to 17 years. It also includes the effect of behaviour changing interventions on other adiposity indicators (e.g. waist circumference, body fat), behavioural change, quality of life, self‐esteem and views of the intervention. The results of this current review and other systematic reviews in this series will provide information on which to underpin clinical guidelines and health policy on the treatment of childhood obesity.
Objectives
To assess the effects of diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCT). For cross‐over trials, we only analysed the first phase before cross‐over (if this was six months or more) to avoid the potential of carry over effects. Included studies observed participants for a minimum of six months (this time frame refers to the intervention itself or to a combination of the intervention with a follow‐up phase).
Types of participants
We included studies of overweight or obese adolescents with a mean study age of 12 to 17 years at the commencement of the intervention. We excluded studies of critically ill people, pregnant or breastfeeding women, or adolescents with a syndromic cause for their obesity (e.g. Prader‐Willi syndrome).
Diagnostic criteria
Any method of overweight or obesity classification was acceptable.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Any form of behaviour changing intervention with a primary aim to treat overweight or obesity. Behaviour changing interventions included any form of dietary, physical activity, behavioural therapy, or a combination of these delivered as a single or multicomponent intervention, in any setting, using any delivery method and included studies which examined weight loss or maintenance or both.
Comparator
No treatment/wait list control, usual care or an alternative concomitant therapy providing it is delivered in the intervention arm. Concomitant interventions had to be the same in the intervention and comparator groups to establish fair comparisons.
Types of outcome measures
Primary outcomes
Changes in measured BMI or body weight.
Adverse events.
Secondary outcomes
Health‐related quality of life.
Self‐esteem.
All‐cause mortality.
Morbidity.
Anthropometric measures other than BMI.
Behaviour change.
Participants' views of the intervention.
Socioeconomic effects.
Parenting skill and relationships.
Method and timing of outcome measurement
Changes in BMI (kg/m2) and body weight (kg): measured at baseline and at least at six months.
Adverse events: defined as an adverse outcome that occurred during or after the intervention but was not necessarily caused by it, and measured at baseline and at least at six months.
Health‐related quality of life: evaluated by a validated instrument such as Paediatric Quality of Life Inventory and measured at baseline and at least at six months.
Self‐esteem: evaluated by a validated instrument such as Rosenberg Self‐Esteem Scale and measured at baseline and at least at six months.
All‐cause mortality: defined as any death that occurred during or after the intervention and measured at six months or later. Morbidity: defined as illness or harm associated with the intervention and measured at baseline and six months or later.
Anthropometric measures other than change in BMI: defined by validated tools such as waist circumference, skin‐fold thickness, waist‐to‐hip ratio, dual x‐ray absorptiometry (DXA) or bioelectrical impedance analysis and measured at baseline and at least at six months.
Behaviour change: defined as validated measures of diet and physical activity and measured at baseline and at least at six months. Participants' views of the intervention: defined as documented accounts from participant feedback and measured at baseline and at least at six months.
Parent‐child relationship or assessment of parenting: evaluated by a validated instrument and measured at baseline and at least at six months.
Socioeconomic effects defined as a validated measure of socioeconomic status such as parental income or educational status and measured at baseline and at least at six months.
'Summary of findings' table
We presented a 'Summary of findings' table reporting the following outcomes listed according to priority.
Changes in BMI and body weight.
Adverse events.
Health‐related quality of life.
All‐cause mortality.
Morbidity.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to 14 July 2016 and placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 6).
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (from 1946).
Embase Ovid (1974 to 2016 week 28).
PsycINFO (1806 to July week 1 2016).
CINAHL.
LILACS (Latin American and Caribbean Health Science Information database) (last update 8 July 2016).
ClinicalTrials.gov (www.clinicaltrials.gov).
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch/).
We continuously applied a MEDLINE (via OvidSP) e‐mail alert service established by the Cochrane Metabolic and Endocrine Disorders Group (CMED) to identify newly published studies using the same search strategy as described for MEDLINE (for details on search strategies and search platforms, see Appendix 1). Should we have identified new studies for inclusion, we planned to evaluate these, incorporated findings in our review and resubmitted another review draft (Beller 2013).
If we detected additional relevant key words during any of the electronic or other searches, we modified the electronic search strategies to incorporate these terms and documented the changes.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports. We also contacted study authors of included trials to identify any further studies that we may have missed.
Data collection and analysis
Selection of studies
Two review authors (two of LA‐K, RJ, EL, JC, KR, CO, LA, EM, LE, HF, JO) independently scanned the abstract, title, or both, of every record retrieved, to determine which studies should be assessed further. We investigated all potentially relevant articles as full text. We resolved any discrepancies through consensus or recourse to a third review author (of KR, EL, LA‐K). Where resolution of a disagreement was not possible, we added the article to those 'awaiting assessment' and contacted study authors for clarification. We present an adapted PRISMA flow chart showing the process of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (of LA‐K, EL, JC, RJ, CO, LA, EM, KR, HF, RV, MM, JO) independently abstracted key participant and intervention characteristics and reported data on efficacy outcomes and adverse events using standard data extraction templates as supplied by Cochrane Metabolic and Endocrine Disorders Group, with any disagreements to be resolved by discussion, or, if required, by consultation with a third review author (KR or EL) (for details, see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10).
1. Overview of study populations.
|
Trial ID (trial design) |
Intervention and comparator | Sample sizea | Screened/eligible (n) | Randomised (n) | ITT (n) | Analysed (n) | Finishing study (n) | Randomised finishing study (%) | Follow‐up timeb |
|
Patsopoulou 2017 (parallel RCT) |
I1: activity | ‐ | 2618/‐ | 60 | 55 | 55 | 50 | 83 | 6 months (after 12‐week intervention) |
| I2: activity + diet | 60 | 55 | 55 | 50 | 83 | ||||
| C1: no intervention | 61 | 56 | 56 | 50 | 82 | ||||
| total: | 181 | 166 | 166 | 150 | 82.8 | ||||
|
Jelalian 2016 (parallel RCT) |
I1: CBT‐healthy lifestyle | ‐ | 127 | 24 | ‐ | 17 | 11 | 46.0 | 48 weeks (after 24‐week intervention) |
| C1: CBT | ‐ | 9 | ‐ | 7 | 8 | 88.0 | |||
| total: | 33 | 24 | 19 | ‐ | |||||
|
Norman 2016 (parallel RCT) |
I1: stepped down | 53 | 460/106 | 53 | 53 | 53 | 35 | 66.0 | 12 months (immediately after intervention) |
| C1: enhanced usual care | 53 | 53 | 53 | 53 | 38 | 71.6 | |||
| total: | 106 | 106 | 106 | 73 | 68.8 | ||||
|
Wong 2015 (parallel RCT) |
I1: standard weight loss diet + increase water intake | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 6 months (immediately after intervention) |
| C1: standard weight loss diet | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| total: | 38 | ‐ | ‐ | ‐ | ‐ | ||||
|
Hofsteenge 2014 (parallel RCT) |
I1: group education | 54 | 219/189 | 71 | 71 | 71 | 36 | 50.7 | 18 months (after 36‐week intervention) |
| C1: dietitian only | 54 | 51 | 51 | 51 | 32 | 62.7 | |||
| total: | 122 | 122 | 122 | 68 | 55.7 | ||||
|
Schranz 2014 (parallel RCT) |
I1: resistance training | 17 | 61/56 | 30 | 30 | 30 | 21 | 70.0 | 12 months (6‐month postintervention) |
| C1: no intervention | 17 | 26 | 26 | 26 | 22 | 84.6 | |||
| total: | 56 | 56 | 56 | 43 | 76.8 | ||||
|
Visuthranukul 2015 (parallel RCT) |
I1: low GI diet | 26 | ‐ | 35 | ‐ | 25 | 25 | 71 | 6 months (immediately after intervention) |
| C1: conventional diet | 26 | 35 | ‐ | 27 | 27 | 77 | |||
| total: | 70 | ‐ | 52 | 52 | 74.3 | ||||
|
Pakpour 2015 (parallel RCT) |
I1: motivational interviewing | ‐ | 409/369 | 119 | ‐ | ‐ | 113 | 95.0 | 12 months (42 weeks after 6‐week intervention) |
| I2: motivational interviewing + parental involvement | 119 | ‐ | ‐ | 118 | 99.2 | ||||
| C1: passive control | 119 | ‐ | ‐ | 115 | 96.6 | ||||
| total: | 357 | 346 | 96.9 | ||||||
|
Bean 2014 (parallel RCT) |
I1: motivational interviewing values | 80 | 123/‐ | 58 | ‐ | ‐ | 52 | 89.7 | 6 months (3 months following end of intervention) |
| C1: education control | 80 | 41 | ‐ | ‐ | 35 | 85.4 | |||
| total: | 99 | 87 | 87.9 | ||||||
|
Carraway 2014 (parallel RCT) |
I1: mentor‐led exercise | 11 | ‐ | 11 | ‐ | ‐ | 10 | 91 | 7 months (12‐week intervention) |
| C1: wait list control | 13 | ‐ | 13 | ‐ | ‐ | 11 | 85 | ||
| total: | 24 | ‐ | ‐ | 22 | 91.6 | ||||
|
Sigal 2014 (parallel RCT) |
I1: diet + aerobic training | 62 | 840/358 | 75 | 75 | 75 | 57 | 76 | 6 months (after 22‐week intervention + 4‐week run‐in) |
| I2: diet + resistance training | 62 | 78 | 78 | 78 | 57 | 73 | |||
| I3: diet + aerobic + resistance training | 62 | 75 | 75 | 75 | 58 | 77 | |||
| C1: diet only | 62 | 76 | 76 | 76 | 57 | 75 | |||
| total: | 304 | 304 | 304 | 229 | 75.3 | ||||
|
Love‐Osborne 2014 (parallel RCT) |
I1: motivational interviewing | 80 | ‐ | 82 | 77 | 77 | 94 | 6‐8 months (immediately after intervention) | |
| C1: control | 80 | 83 | 72 | 72 | 87 | ||||
| total: | 165 | 149 | 149 | 90.3 | |||||
|
Kong 2014 (parallel RCT) |
I1: low GI diet | ‐ | ‐ | 52 | ‐ | 34 | 34 | 65.4 | 6 months (immediately following intervention) |
| C1: usual Chinese diet | 52 | ‐ | 27 | 27 | 51.9 | ||||
| total: | 104 | ‐ | 61 | 61 | 58.7 | ||||
|
Luna‐Pech 2014 (parallel RCT) |
I1: normocaloric diet + physical activity | ‐ | ‐ | 29 | ‐ | 26 | 26c | 89.7 | 28 weeks (immediately following intervention) |
| C1: no intervention | 29 | ‐ | 25 | 25d | 86.2 | ||||
| total: | 58 | ‐ | 51 | 51 | 87.9 | ||||
|
Boodai 2014 (parallel RCT) |
I1: multicomponent group sessions | 45 | 224/82 | 41 | 31 | 31 | 31 | 75.6 | 6 months (immediately following intervention) |
| C1: no intervention | 45 | 41 | 32 | 32 | 32 | 78.0 | |||
| total: | 82 | 63 | 63 | 63 | 76.8 | ||||
|
Gourlan 2013 (parallel RCT) |
I1: motivational interviewing + standard weight loss | 30 | ‐/64 | 28 | ‐ | 26 | 26 | 92.9 | 6 months (after 3‐month intervention for standard weight loss group, 6‐month intervention for motivational interviewing group) |
| C1: standard weight loss | 30 | 34 | ‐ | 28 | 28 | 82.4 | |||
| total: | 62 | ‐ | 62 | 54 | 87.1 | ||||
|
Patrick 2013 (parallel RCT) |
I1: website intervention | 26 | 387/101 | 26 | 26 | 26 | 17 | 65.4 | 12 months (immediately after intervention) |
| I2: website + group | 26 | 26 | 26 | 26 | 14 | 53.8 | |||
| I3: website + SMS | 26 | 24 | 24 | 24 | 17 | 70.8 | |||
| C1: usual care | 26 | 25 | 25 | 25 | 16 | 64.0 | |||
| total: | 101 | ‐ | 101 | 64 | 63.4 | ||||
|
Kong 2013 (cluster RCT) |
I1: ACTION | 21 | 101/60 | 31 | ‐ | 28 | 28 | 90.3 | 6 months (intervention for 1 academic year) |
| C1: standard care | 21 | 29 | ‐ | 23 | 23 | 79.3 | |||
| total: | 60 | ‐ | 51 | 51 | 85.0 | ||||
|
Pbert 2013 (cluster RCT) |
I1: "Lookin' Good Feelin' Good" | ‐ | 6/6 schools 176/82 participants |
42 | ‐ | 42 | 42 | 100 | 6 months (16‐week intervention) |
| C1: control | 40 | ‐ | 40 | 40 | 100 | ||||
| total: | 82 | ‐ | 82 | 82 | 100 | ||||
|
Brennan 2013 (cross‐over RCT) |
I1: motivational interviewing | ‐ | 120/‐ | 42 | ‐ | 17 | 17 | 40.5 | 12 months (26‐week intervention) |
| C1: wait list control | 21 | ‐ | 14 | 14 | 66.7 | ||||
| total: | 63 | ‐ | 31 | 31 | 49.2 | ||||
|
Walpole 2013 (parallel RCT) |
I1: motivational interviewing | 16 | 73/52 | 20 | ‐ | 20 | 20 | 100 | 6 months (immediately after intervention) |
| C1: social skills training | 16 | 20 | ‐ | 18 | 18 | 90 | |||
| total: | 40 | ‐ | 38 | 38 | 95 | ||||
|
Toulabi 2012 (parallel RCT) |
I1: behavioural modification | ‐ | 192/152 | 76 | ‐ | ‐ | ‐ | ‐ | 6 months (6‐week intervention) |
| C1: control | 76 | ‐ | ‐ | ‐ | ‐ | ||||
| total: | 152 | ‐ | ‐ | ‐ | ‐ | ||||
|
Debar 2012 (parallel RCT) |
I1: multicomponent intervention | 100 | 2647/2350 | 105 | ‐ | 90 | 90 | 85.7 | 12 months (5‐month intervention) |
| C1: usual care | 100 | 103 | ‐ | 83 | 83 | 80.6 | |||
| total: | 208 | ‐ | 173 | 173 | 83.2 | ||||
|
Ebbeling 2012 (parallel RCT) |
I1: multicomponent intervention | ‐ | 762/374 | 110 | ‐ | 105 | 105 | 95.5 | 24 months (52‐week intervention) |
| C1: control | 114 | ‐ | 104 | 104 | 91.2 | ||||
| total: | 224 | ‐ | 209 | 209 | 93.3 | ||||
|
Vos 2011 (parallel RCT) |
I1: family‐based CBT + nutrition | 35 | 108/81 | 41 | ‐ | 32 | 32 | 78.0 | 24 months (3‐month intervention) |
| C1: wait list control | 35 | 40 | ‐ | 35 | 35 | 87.5 | |||
| total: | 81 | ‐ | 66 | 81.5 | |||||
|
Christie 2011 (parallel RCT) |
I1: HELP weight management | 100 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 12 months (6‐month intervention) |
| C1: enhanced standard care | 100 | ‐ | ‐ | ‐ | ‐ | ‐ | |||
| total: | 174 | ‐ | ‐ | 145 | 83.3 | ||||
|
Wengle 2011 (parallel RCT) |
I1: mentored behaviour changing intervention | ‐ | ‐/38 | 20 | ‐ | 16 | 16 | 80.0 | 6 months (immediately after intervention) |
| C1: unmentored behaviour changing intervention | 18 | ‐ | 16 | 16 | 88.9 | ||||
| total: | 38 | ‐ | 32 | 32 | 84.2 | ||||
|
Ford 2010 (parallel RCT) |
I1: Mandometer | 40 | 115/‐ | 54 | 44 | 44 | 81.5 | 18 months (12‐month intervention) | |
| C1: standard care | 40 | 52 | 43 | 43 | 82.7 | ||||
| total: | 106 | 87 | 87 | 82.1 | |||||
|
Nguyen 2012 (parallel RCT) |
I1: Loozit + additional therapeutic contact | ‐ | 474/249 | 73 | 73 | 73 | 58 | 79.4 | 24 months (intervention continued for 24 months) |
| C1: Loozit | 78 | 78 | 78 | 56 | 71.8 | ||||
| total: | 151 | 151 | 151 | 114 | 78.5 | ||||
|
Grey 2009 (cluster RCT) |
I1: coping skills | ‐ | 426/324 | 112 | 112 | 112 | 87 | 77.7 | 36 weeks (16‐week intervention) |
| C1: general education | 86 | 86 | 86 | 64 | 74.4 | ||||
| total: | 198 | 198 | 198 | 151 | 76.3 | ||||
|
Vissers 2008 (parallel RCT) |
I1: school‐based intervention | ‐ | 506/‐ | 37 | ‐ | 22 | 22 | 59.5 | 6 months (immediately following intervention) |
| C1: control | 39 | ‐ | 31 | 31 | 79.5 | ||||
| total: | 76 | ‐ | 53 | 53 | 69.7 | ||||
|
NCT00132132 (parallel RCT) |
I1: behavioural education | ‐ | ‐ | 15 | ‐ | 8 | 8 | 53.3 | 12‐15 months (12‐month intervention) |
| C1: standard care | 15 | ‐ | 13 | 13 | 86.7 | ||||
| total: | 30 | ‐ | 21 | 21 | 70.0 | ||||
|
Pitetti 2007 (parallel RCT) |
I1: treadmill | ‐ | 42/‐ | 5 | ‐ | 5 | 5 | 100 | 36 weeks (immediately following intervention) |
| C1: control | 5 | ‐ | 5 | 5 | 100 | ||||
| total: | 10 | ‐ | 10 | 10 | 100 | ||||
|
Savoye 2007 (parallel RCT) |
I1: Bright Bodies weight management | 174 (58 per group (originally 3 groups) | 284/271 | 105 | 105 | 105 | 45 | 42.9 | 24 months (52‐week intervention) |
| C1: control | 69 | 69 | 69 | 31 | 44.9 | ||||
| total: | 174 | 174 | 174 | 76 | 43.7 | ||||
|
van Egmond‐Frohlich 2006 (parallel RCT) |
I1: multicomponent intervention | ‐ | 821 | 250 | 250 | ‐ | ‐ | ‐ | 12 months (12‐month intervention)d |
| C1: standard care | 271 | 271 | ‐ | ‐ | ‐ | ||||
| total: | 521 | 521 | ‐ | 423 | 81.2 | ||||
|
Daley 2005 (parallel RCT) |
I1: exercise counselling | 30 | 141/132 | 28 | 28 | 28 | 24 | 85.7 | 28 weeks (8‐week intervention) |
| C1: exercise placebo | 30 | 23 | 23 | 23 | 22 | 95.7 | |||
| C2: control | 30 | 30 | 30 | 30 | 25 | 83.3 | |||
| total: | 81 | 81 | 81 | 71 | 87.7 | ||||
|
Resnicow 2005 (cluster RCT) |
I1: GoGirls high‐intensity behavioural intervention | 75‐120 | ‐ | ‐ | ‐ | 53 | 45 | ‐ | 12 months (6‐month intervention) |
| C1: moderate‐intensity behavioural intervention | 75‐120 | ‐ | ‐ | ‐ | 70 | 62 | ‐ | ||
| total: | ‐ | ‐ | 123 | 107 | ‐ | ||||
|
Jiang 2005 (parallel RCT) |
I1: family‐based intervention | ‐ | 106/75 | 36 | ‐ | 33 | 33 | 91.7 | 24 months (immediately following intervention) |
| C1: control | 39 | ‐ | 35 | 35 | 89.7 | ||||
| total: | 75 | ‐ | 68 | 68 | 90.7 | ||||
|
Carrel 2005 (parallel RCT) |
I1: behaviour changing‐focused gym classes | ‐ | ‐/55 | 27 | ‐ | 27 | 27 | 100 | 9 months (immediately following intervention) |
| C1: standard gym classes | 26 | ‐ | 23 | 23 | 88.5 | ||||
| total: | 53 | ‐ | 50 | 50 | 94.3 | ||||
|
Ebbeling 2003 (parallel RCT) |
I1: low GL diet | ‐ | 30/21 | 8 | 8 | 8 | 7 | 87.5 | 12 months (26‐week intervention) |
| C1: conventional diet | 8 | 8 | 8 | 7 | 87.5 | ||||
| total: | 16 | 16 | 16 | 14 | 87.5 | ||||
|
Saelens 2002 (parallel RCT) |
I1: Healthy Habits | 21 | ‐/59 | 23 | 23 | ‐ | 18 | 78.3 | 28 weeks (12‐week intervention) |
| C1: standard care | 21 | 21 | 21 | ‐ | 19 | 90.5 | |||
| total: | 44 | 44 | ‐ | 37 | 84.1 | ||||
|
Brownell 1983 (parallel RCT) |
I1: mother + child separate | ‐ | ‐ | 14 | ‐ | 12 | 12 | 85.7 | 12 months (16‐week intervention) |
| I2: mother + child together | 15 | ‐ | 12 | 12 | 80 | ||||
| C1: child only | 13 | ‐ | 12 | 12 | 92.3 | ||||
| total: | 42 | ‐ | 36 | 36 | 85.7 | ||||
|
Chandra 1968 (parallel RCT) |
I1: low‐calorie formula Limical | ‐ | 43 | ‐ | ‐ | 18 | 18 | ‐ | 7 months (3‐month intervention) |
| C1: low‐calorie diet | ‐ | ‐ | 17 | 17 | ‐ | ||||
| total: | 43 | ‐ | 35 | 35 | 81.4 | ||||
|
NCT00807560 (parallel RCT) |
I1: family‐based therapy for paediatric overweight | ‐ | ‐ | 39 | ‐ | 13 | 13 | 34.2 | 44 weeks (24‐week intervention) |
| C1: nutrition education control | ‐ | ‐ | 38 | ‐ | 9 | 11 | 38.2 | ||
| total: | 77 | 22 | 24 | 31.1 | |||||
| Grand totale | All interventions | ‐ | 2555 | ‐ | 1801 | ‐ | |||
| All comparators | 1850 | 1255 | |||||||
| All interventions and comparators | 4781 | 3735 | |||||||
‐ denotes not reported
aAccording to power calculation in study publication or report. bDuration of follow‐up/(duration of intervention). c For number of participants finishing the study the publication stated that 26 participants in the normocaloric diet + physical activity group and 25 participants in the no intervention groups finished the study but also reported that there were four dropouts/withdrawals in the normocaloric diet + physical activity group and eight dropouts/withdrawals in the no intervention group. dAt 6 months, only 115 (46%) children in the multicomponent intervention group and 135 (54%) children in the standard care group participated in a follow‐up examination. eNumbers did not add up accurately because of missing data per intervention/comparator groups in some trials.
C: comparator; CBT: cognitive behavioural therapy; FBT‐PO: family‐based therapy for paediatric overweight; GI: glycaemic index; GL: glycaemic load; I; intervention; ITT: intention to treat; n: number of participants; N/A: not applicable; RCT: randomised controlled trial; SMS: short message service.
We provided information including trial identifier about potentially relevant ongoing studies in the Characteristics of ongoing studies table and in Appendix 5 (Matrix of study endpoints (publications and trial documents)). We tried to find the protocol of each included study and reported primary, secondary and other outcomes in comparison with data in publications in Appendix 5.
We e‐mailed all authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 10 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we tried to maximise yield of information by collating all available data and use the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
We used the Cochrane 'risk of bias' assessment tool (Higgins 2011a; Higgins 2011b), and evaluated the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Imbalances in baseline characteristics.
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We judged the above 'Risk of bias' criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We present a 'Risk of bias' graph and a 'Risk of bias summary'. We assessed the impact of individual bias domains on study results at endpoint and study levels. In case of high risk of selection bias, all endpoints investigated in the associated study were marked as 'high risk'.
We evaluated whether imbalances in baseline characteristics existed and how these were addressed (Egbewale 2014).
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessors) we evaluated the risk of bias separately for each outcome type (objective and subjective) (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures.
We considered the implications of missing outcome data from individual participants per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between study arms).
We assessed outcome reporting bias by integrating the results of 'Examination of outcome reporting bias' (Kirkham 2010) (Appendix 6), in the 'Matrix of study endpoints (publications and trial documents)' (Appendix 5), and 'Outcomes (outcomes reported in abstract of publication)' section of the Characteristics of included studies table. This analysis formed the basis for the judgement of selective reporting (reporting bias).
We defined the following endpoints as potentially self‐reported outcomes.
Adverse events.
Health‐related quality of life.
Participant's views of the intervention.
Changes in body weight.
Self‐esteem.
Behaviour change.
We defined the following outcomes as potentially investigator‐assessed outcomes.
Changes in BMI and body weight.
Adverse events.
All‐cause mortality.
Morbidity.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) if they used the same instruments or standardised mean differences (SMD) if they used different instruments with 95% CI. We expressed time‐to‐event data as hazard ratios (HRs) with 95% CIs.
We included studies reporting multiple comparison groups in this review. Where this was the case, we considered whether the aim of the trial was to test for differences between these groups, and whether the study authors found a significant difference. Where there were no demonstrated differences, we merged groups as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Section 7.7.8, Higgins 2011a). In studies that found a difference between groups, we used the data for the control group for each intervention group comparison and reduced the weight assigned to the control group by dividing the number of participants in the control group by the number of intervention groups.
Unit of analysis issues
We used data from the first period of cross‐over trials if available. We collected data from the latest available time point in the follow‐up reported in the studies to avoid double‐counting trials in the same analysis. For cluster RCTs, we used the denominator reported in the trial and considered how the analysis methods used took account of the effect of clustering. Due to the small number of cluster RCTs found, we decided not to adjust the data so we performed sensitivity analyses to ascertain if the results were sensitive to the inclusion of studies with a cluster design.
Dealing with missing data
We obtained relevant missing data from study authors, if feasible, and evaluated important numerical data such as screened, eligible, randomised participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where standard deviations (SD) for outcomes were not reported, and we did not receive information from study authors, we imputed these values by assuming the SD of the missing outcome to be the same as the largest SD from those studies where this information was reported. We investigated the impact of imputation on meta‐analyses by means of sensitivity analyses. Where papers did not report results as change from baseline, we calculated this and for the SD differences followed the methods presented in the Cochrane Handbook for Systematic Reviews of Intervention for imputing these (Section 16.1.3.2, Higgins 2011a), and assumed a correlation of 0.5 between baseline and follow‐up measures as suggested by Follmann 1992.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report study results as meta‐analytically pooled effect estimates. We identified heterogeneity by visual inspection of forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
If we included 10 studies or more for a given outcome, we used funnel plots to assess small‐study effects. Due to several explanations for funnel plot asymmetry, we interpreted results carefully (Sterne 2011).
Data synthesis
Unless there is good evidence for homogeneous effects across studies we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias)and also to external validity, such as directness of results. Two review authors (LA‐K and KR/EL) rated the quality for each outcome. We presented a summary of the evidence in a Table 1, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results on the outcomes as described in Types of outcome measures. If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table.
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of 'Summary of findings' tables (Appendix 9).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out subgroup analyses with investigation of interactions for our primary outcomes.
Duration of follow‐up.
Duration of the intervention.
Duration of postintervention follow‐up.
Type of comparator group.
Mode of delivery of the intervention.
Setting.
Type of intervention.
Theoretical basis to the intervention.
Parental involvement.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
Restricting the analysis to published studies, and by publication language.
Restricting the analysis taking into account risk of bias, as specified in the section Assessment of risk of bias in included studies section.
Restricting the analysis to studies of adolescents without specific health conditions.
Restricting the analysis to studies with no uncertainties (such as study duration, imputed data).
We also tested the robustness of the results by repeating the analysis using different measures of effect size (RRs, ORs, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
This search is up to date as of July 2016 in addition to ongoing e‐mail alerts from MEDLINE. The searches generated 16,106 hits after duplicates were removed. Fifty‐two additional records were identified through non‐database sources. Screening of titles and abstracts identified 736 records to go forward for formal inclusion and exclusion. Forty‐four completed RCTs fulfilled the inclusion criteria and were included in the review. For a detailed description of the included trials, see the Characteristics of included studies table. The search identified 50 ongoing trials, which are reported in the Characteristics of ongoing studies table. The flow of trials through the review is presented in Figure 1.
1.
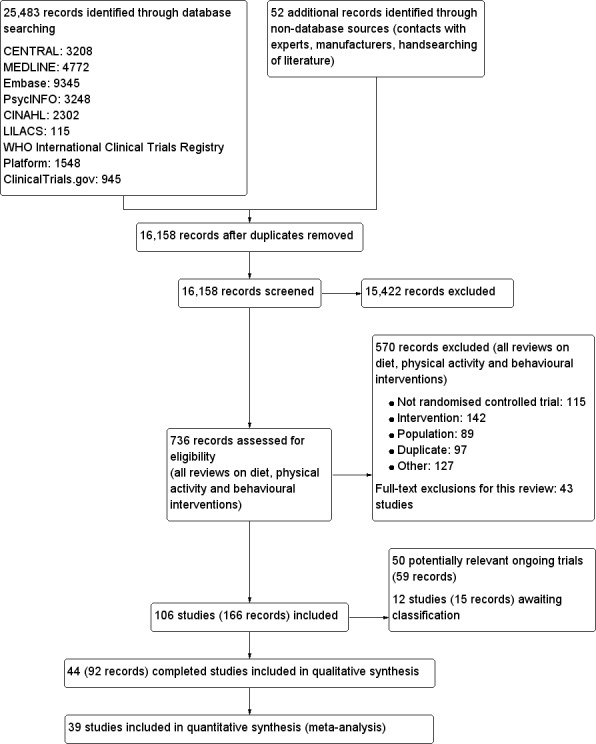
Study flow diagram.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 11). The following is a succinct overview.
Source of data
All data presented in the review were obtained from the published literature. We contacted all study authors for additional information and results are presented in Appendix 10.
Comparisons
To meet the inclusion criteria for the review comparators could be no treatment, usual care or a concomitant therapy providing it was also included in the intervention group. In nine trials the comparator was either no intervention or a wait list control (Brennan 2013; Carraway 2014; Ebbeling 2012; Luna‐Pech 2014; Pakpour 2015; Patsopoulou 2017; Schranz 2014; Toulabi 2012; Vos 2011). Twenty‐three studies used usual care comparators (Bean 2014; Boodai 2014; Carrel 2005; Christie 2011; Daley 2005; Debar 2012; Ebbeling 2003; Ford 2010; Gourlan 2013; Hofsteenge 2014; Jiang 2005; Kong 2013; Love‐Osborne 2014; NCT00132132; NCT00807560; Patrick 2013; Pbert 2013; Pitetti 2007; Saelens 2002; Savoye 2007; Vissers 2008; Visuthranukul 2015; van Egmond‐Frohlich 2006). Twelve trials used a concomitant therapy (Brownell 1983; Chandra 1968; Grey 2009; Jelalian 2016; Kong 2014; Nguyen 2012; Norman 2016; Resnicow 2005; Sigal 2014; Walpole 2013; Wengle 2011; Wong 2015).
Overview of trial populations
The 44 trials included 4781 participants. Approximately 2555 were randomised to an intervention and 1850 to a comparator. The proportion of participants finishing the study, where reported, ranged from 34% to 100% in the intervention groups and from 38% to 100% in the comparator groups. Individual sample size ranged from 10 to 521.
Study design
Thirty‐nine trials were parallel comparisons with individual randomisation. Four trials were cluster RCTs (Grey 2009; Kong 2013; Pbert 2013; Resnicow 2005), and one was a cross‐over trial (Brennan 2013). All 44 trials had a superiority design. Five trials had three arms (Brownell 1983; Daley 2005; Norman 2016; Pakpour 2015; Patsopoulou 2017), and two trials had four arms (Patrick 2013; Sigal 2014). The majority (24) were single centre trials, although the number of centres was unclear or not reported in 10 trials. Two trials had six centres (Grey 2009; Kong 2013), and one study each had two (Brennan 2013), eight (Pbert 2013), 10 (Resnicow 2005), 12 (Toulabi 2012), 15 (Patsopoulou 2017) or 18 (Patrick 2013) centres. Trials were performed from 1968 to 2015. The duration of the intervention ranged from six weeks to two years, and the duration of follow‐up ranged from six months to two years. Eight trials had some form of run‐in period prior to the start of the study (Brownell 1983; Chandra 1968; Luna‐Pech 2014; Norman 2016; Pitetti 2007; Schranz 2014; Sigal 2014; Wengle 2011). None of the trials was terminated early.
Settings
The interventions were carried out in a variety of settings. These included in schools in nine trials (Carrel 2005; Grey 2009; Jiang 2005; Kong 2013; Love‐Osborne 2014; Pbert 2013; Pitetti 2007; Toulabi 2012; Vissers 2008), in the community or home in eight trials (Ebbeling 2012; Nguyen 2012; Patrick 2013; Patsopoulou 2017; Resnicow 2005; Saelens 2002; Schranz 2014; Sigal 2014), and various healthcare settings, including outpatients, primary care, research clinics and other hospital sites in 26 trials (based on author locations where the study did not explicitly report this) (Bean 2014; Boodai 2014; Brennan 2013; Brownell 1983; Carraway 2014; Chandra 1968; Christie 2011; Daley 2005; Debar 2012; Ebbeling 2003; Ford 2010; Gourlan 2013; Hofsteenge 2014; Jelalian 2016; Kong 2014; Luna‐Pech 2014; NCT00132132; NCT00807560; Norman 2016; Pakpour 2015; Savoye 2007; Visuthranukul 2015; Vos 2011; Walpole 2013; Wengle 2011; Wong 2015).
Participants
The diagnostic criteria for overweight and obesity differed between the trials. Twelve trials included participants with BMI on or above the 85th percentile for age and height (Bean 2014; Carraway 2014; Ebbeling 2012; Grey 2009; Jelalian 2016; Kong 2013; Love‐Osborne 2014; NCT00132132; NCT00807560; Pbert 2013; Walpole 2013; Wengle 2011), nine trials used a cut‐off of on or above the 95th percentile (Boodai 2014; Carrel 2005; Ebbeling 2003; Ford 2010; Kong 2014; Luna‐Pech 2014; Norman 2016; Pakpour 2015; Savoye 2007), three trials used a cut‐off of on or above the 90th percentile (Debar 2012; Gourlan 2013; Resnicow 2005), and two trials used a cut‐off of on or above the 98th percentile (Christie 2011; Daley 2005). The remaining trials used a range of different criteria to define overweight or obesity for inclusion in the trials (Characteristics of included studies table; Appendix 3).
Thirty‐four trials reported the mean BMI at baseline (Brennan 2013; Brownell 1983; Carrel 2005; Christie 2011; Debar 2012; Ebbeling 2003; Ebbeling 2012; Ford 2010; Gourlan 2013; Grey 2009; Hofsteenge 2014; Jelalian 2016; Jiang 2005; Kong 2014; Love‐Osborne 2014; Luna‐Pech 2014; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patsopoulou 2017; Pbert 2013; Pitetti 2007; Resnicow 2005; Saelens 2002; Savoye 2007; Schranz 2014; Sigal 2014; Toulabi 2012; Vissers 2008; Visuthranukul 2015; Vos 2011; Walpole 2013; Wengle 2011) and ranged from 26 kg/m2 to 37 kg/m2 in all but one study (Brownell 1983), which was an outlier with BMIs in the three study groups ranging from 42 kg/m2 to 45.5 kg/m2. Sixteen trials reported BMI z scores (Bean 2014; Boodai 2014; Brennan 2013; Carraway 2014; Daley 2005; Love‐Osborne 2014; Luna‐Pech 2014; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Pbert 2013; Vos 2011; Walpole 2013; Wengle 2011), and these ranged across all trials between 1.8 and 3.2 with the exception of one study (Vos 2011), which had a mean BMI Standard Deviation Score (SDS) of 4.2. One study reported BMI percentile only at baseline (at 94.5%) (Kong 2013), and two trials did not report BMI measures at baseline (Chandra 1968; Gourlan 2013). Twenty trials reported on change in weight.
Thirty‐seven trials reported the gender of the study participants (Bean 2014; Boodai 2014; Brennan 2013; Brownell 1983; Carrel 2005; Chandra 1968; Christie 2011; Daley 2005; Debar 2012; Ebbeling 2003; Ebbeling 2012; Ford 2010; Gourlan 2013; Grey 2009; Hofsteenge 2014; Jelalian 2016; Jiang 2005; Kong 2013; Kong 2014; Love‐Osborne 2014; Luna‐Pech 2014; NCT00132132; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Pbert 2013; Pitetti 2007; Resnicow 2005; Savoye 2007; Schranz 2014; Sigal 2014; Vissers 2008; Visuthranukul 2015; Vos 2011; Walpole 2013, and the proportion of participants who were female ranged from 33% to 77% in all cases except the trials by Debar 2012 and Resnicow 2005 where all participants were female, and Schranz 2014 where all participants were male. Four trials did not report the gender of participants at baseline (Saelens 2002; Toulabi 2012; Wengle 2011; Wong 2015). Participants ages ranged from 12 to 17.5 years in all trials that reported mean age at baseline, 11 trials did not report mean age at baseline (Brennan 2013; Brownell 1983; Chandra 1968; Christie 2011; Daley 2005; Gourlan 2013; NCT00132132; Resnicow 2005; Saelens 2002; Toulabi 2012; Wong 2015).
Nineteen trials reported ethnicity (Bean 2014; Brownell 1983; Carraway 2014; Daley 2005; Debar 2012; Ebbeling 2012; Ford 2010; Grey 2009; Hofsteenge 2014; Jelalian 2016; Kong 2013; Love‐Osborne 2014; Norman 2016; Patrick 2013; Pbert 2013; Resnicow 2005; Savoye 2007; Sigal 2014; Vos 2011). Most trials reported a mixture of ethnic backgrounds for their participants as can be seen in Appendix 3, two trials reported exclusively on African‐Americans (Resnicow 2005) and participants described as 'white' (Brownell 1983).
Three trials focused on participants with specific conditions, Jelalian 2016 included people with depression, Luna‐Pech 2014 included people with asthma and Pitetti 2007 included people with autism living in a residential school.
Interventions
Thirty‐four trials included interventions that were multidisciplinary (Bean 2014; Boodai 2014; Brennan 2013; Brownell 1983; Christie 2011; Daley 2005; Debar 2012; Gourlan 2013; Grey 2009; Hofsteenge 2014; Jelalian 2016; Jiang 2005; Kong 2013; Kong 2014; Love‐Osborne 2014; NCT00132132; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Patsopoulou 2017; Pbert 2013; Resnicow 2005; Saelens 2002; Savoye 2007; Sigal 2014; Toulabi 2012; van Egmond‐Frohlich 2006; Vissers 2008; Vos 2011; Walpole 2013; Wengle 2011; Wong 2015), where the focus was on at least two components of diet, physical activity and behavioural approaches to weight management. Five trials were focused solely on dietary interventions (Ebbeling 2003; Ebbeling 2012; Ford 2010; Luna‐Pech 2014; Visuthranukul 2015), and five focused solely on physical activity interventions (Carraway 2014; Carrel 2005; Chandra 1968; Pitetti 2007; Schranz 2014). Twenty‐nine trials had a theoretical basis to their interventions, these included six which had a cognitive behavioural or social cognitive theoretical basis (Hofsteenge 2014; Jelalian 2016; Nguyen 2012; Norman 2016; Pbert 2013; Vos 2011), eight which used motivational interviewing approaches (Bean 2014; Brennan 2013; Christie 2011; Gourlan 2013; Love‐Osborne 2014; Pakpour 2015; Resnicow 2005; Walpole 2013), and 15 that used a variety of other psychological approaches (Boodai 2014; Brownell 1983; Daley 2005; Debar 2012; Grey 2009; Jiang 2005; Kong 2013; Kong 2014; NCT00132132; Patrick 2013; Saelens 2002; Savoye 2007; Toulabi 2012; Vissers 2008; Wengle 2011). Thirteen trials did not use a behavioural approach or did not report the theoretical basis to any behavioural component of their intervention (Carrel 2005; Chandra 1968; Ebbeling 2003; Ebbeling 2012; Ford 2010; Luna‐Pech 2014; NCT00807560; Patsopoulou 2017; Pitetti 2007; Schranz 2014; Sigal 2014; Visuthranukul 2015; Wong 2015). Twenty‐six trials involved parents in the intervention (Bean 2014; Boodai 2014; Brennan 2013; Brownell 1983; Carraway 2014; Christie 2011; Daley 2005; Debar 2012; Ebbeling 2012; Grey 2009; Hofsteenge 2014; Jelalian 2016; Jiang 2005; Kong 2013; Kong 2014; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Resnicow 2005; Savoye 2007; Toulabi 2012; Visuthranukul 2015; Vos 2011; Wengle 2011). In 15 trials, there was no parental involvement (Carrel 2005; Chandra 1968; Ebbeling 2003; Ford 2010; Gourlan 2013; Love‐Osborne 2014; Luna‐Pech 2014; NCT00132132; Pbert 2013; Pitetti 2007; Saelens 2002; Schranz 2014; Sigal 2014; Vissers 2008; Walpole 2013), and in three trials there were two intervention groups, one which included the parent and one which did not (Brownell 1983; Pakpour 2015; Patsopoulou 2017).
Nineteen trials delivered the intervention in a group format (Boodai 2014; Brownell 1983; Carrel 2005; Christie 2011; Daley 2005; Debar 2012; Grey 2009; Hofsteenge 2014; NCT00132132; Nguyen 2012; Pakpour 2015; Patsopoulou 2017; Pitetti 2007; Resnicow 2005; Savoye 2007; Schranz 2014; Toulabi 2012; Visuthranukul 2015; Vos 2011), for specific details see Characteristics of included studies table. In 14 studies, the intervention was individually based (Bean 2014; Brennan 2013; Ebbeling 2012; Ford 2010; Gourlan 2013; Jiang 2005; Kong 2013; Kong 2014; Luna‐Pech 2014; Norman 2016; Pbert 2013; Saelens 2002; Sigal 2014; Walpole 2013), and in 10 studies, there was a mixture of some group and some individual sessions, or the trial did not report the mode of delivery (Carraway 2014; Chandra 1968; Ebbeling 2003; Jelalian 2016; Love‐Osborne 2014; NCT00807560; Patrick 2013; Vissers 2008; Wengle 2011; Wong 2015).
The duration of the intervention was six months or less in 30 trials (Bean 2014; Boodai 2014; Brennan 2013; Brownell 1983; Carraway 2014; Chandra 1968; Christie 2011; Daley 2005; Debar 2012; Ebbeling 2003; Gourlan 2013; Grey 2009; Jelalian 2016; Kong 2013; Kong 2014; NCT00807560; Pakpour 2015; Patsopoulou 2017; Pbert 2013; Resnicow 2005; Saelens 2002; Schranz 2014; Sigal 2014; Toulabi 2012; Vissers 2008; Visuthranukul 2015; Vos 2011; Walpole 2013; Wengle 2011; Wong 2015), and greater than six months in 14 trials (Carrel 2005; Ebbeling 2012; Ford 2010; Hofsteenge 2014; Jiang 2005; Love‐Osborne 2014; Luna‐Pech 2014; NCT00132132; Nguyen 2012; Norman 2016; Patrick 2013; Pitetti 2007; Savoye 2007; van Egmond‐Frohlich 2006).
Outcomes
Twenty‐three trials explicitly stated a primary endpoint in the publication (Appendix 5); in 20 of these, this was a measure of body composition such as weight or BMI. In the trial by Schranz 2014, the primary outcomes were exercise self‐efficacy, physical self‐worth and self‐esteem. In the trial by Walpole 2013, the primary outcome was self‐efficacy and in Daley 2005 the primary outcome was physical self‐perceptions. All except one of the included trials (Chandra 1968) reported a BMI variable as an outcome; this was BMI in 29 trials, BMI z score in 20 trials, BMI percentile in five trials (15 trials reported more than one BMI variable, Brennan 2013; Debar 2012; Hofsteenge 2014; Jiang 2005; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Pbert 2013; Saelens 2002; Savoye 2007; Visuthranukul 2015; Walpole 2013; Wengle 2011). Twenty trials reported weight and five trials reported rates of adverse events as described in the Effects of interventions section. Twenty‐nine trials reported other anthropometric measures (Boodai 2014; Brennan 2013; Brownell 1983; Carraway 2014; Carrel 2005; Chandra 1968; Ebbeling 2003; Ebbeling 2012; Ford 2010; Grey 2009; Hofsteenge 2014; Kong 2013; Kong 2014; NCT00132132; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Patsopoulou 2017; Pbert 2013; Saelens 2002; Savoye 2007; Schranz 2014; Sigal 2014; Toulabi 2012; Vissers 2008; Vos 2011; Walpole 2013; Wengle 2011); this was percent body fat in 14 trials, trunk fat percentage in two trials, waist circumference in 16 trials, waist‐to‐height ratio in three trials, waist‐to‐hip ratio in two trials, fat mass in five trials, trunk fat mass in two trials, lean mass in three trials and percentage of overweight in two trials. Nine trials reported health‐related quality of life (Debar 2012; Ford 2010; Hofsteenge 2014; Nguyen 2012; Luna‐Pech 2014; Pakpour 2015; Patrick 2013; van Egmond‐Frohlich 2006; Vos 2011). Nine trials reported self‐esteem (Brennan 2013; Carraway 2014; Daley 2005; Debar 2012; Hofsteenge 2014; Nguyen 2012; Pakpour 2015; Patrick 2013; Schranz 2014). Eleven trials reported behavioural change in terms of dietary intake (Brennan 2013; Ebbeling 2003; Ebbeling 2012; Kong 2013; Kong 2014; Pakpour 2015; Patrick 2013; Pbert 2013; Saelens 2002; Visuthranukul 2015; Wengle 2011), while four trials reported dietary behaviour (Brennan 2013; Ford 2010; Grey 2009; Pakpour 2015). Twelve trials reported behavioural change in terms of physical activity (Debar 2012; Ebbeling 2012; Gourlan 2013; Grey 2009; Jelalian 2016; Kong 2013; Kong 2014; Pakpour 2015; Pbert 2013; Saelens 2002; Sigal 2014; Wengle 2011), while three trials reported physical activity behaviour (Grey 2009; Kong 2013; Pakpour 2015). One trials reported parenting and relationships (Brennan 2013). Eight trials reported views of the intervention (Brennan 2013; Christie 2011; Debar 2012; Jelalian 2016; Nguyen 2012; Pbert 2013; Saelens 2002; Wengle 2011).
Ten trials reported outcomes at more than one time point (Debar 2012; Ebbeling 2012; Hofsteenge 2014; Jiang 2005; Nguyen 2012; Norman 2016; Patrick 2013; Resnicow 2005; Savoye 2007; Schranz 2014).
Excluded studies
We excluded 570 of 736 articles after evaluation of the publication. The main reasons for exclusion were the intervention (aim not focused on treating overweight/obesity and duration of intervention and follow‐up less than six months) and study design (not an RCT). Many trials had other reasons for exclusion such as lack of relevant outcomes or the control was not no intervention/usual care or concomitant intervention as long as this was also provided in the intervention arm (for further details see Characteristics of excluded studies table, which lists the 44 studies that closely missed the inclusion criteria for this review).
Studies awaiting classification
Twelve studies are awaiting classification as we await clarification from the study authors. These may be included when this review is updated if they meet the inclusion criteria.
Ongoing studies
Fifty studies are ongoing or have completed but results are not available. These may be included when this review is updated if they meet the inclusion criteria.
Risk of bias in included studies
For details on risk of bias of included studies, see the Characteristics of included studies table. For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies, see Figure 2 and Figure 3. Many trials did not report adequate information to assess the risk of bias and we assessed 30 trials at high risk of bias on at least one domain. We assessed 10 trials at high risk of bias on three or more domains (Daley 2005; Hofsteenge 2014; Kong 2014; Luna‐Pech 2014; NCT00807560; Patsopoulou 2017; Pbert 2013; Schranz 2014; Sigal 2014; van Egmond‐Frohlich 2006).
2.
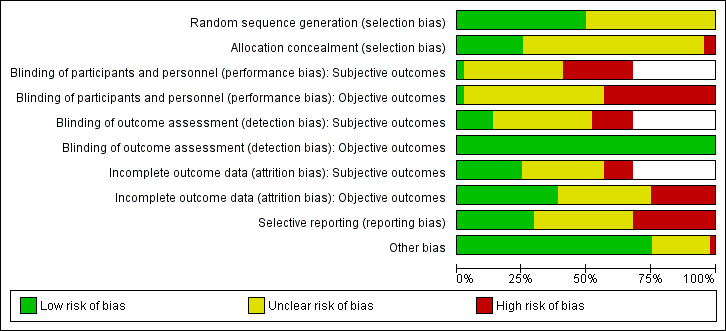
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (blank cells indicate that the particular outcome was not investigated in some studies).
3.
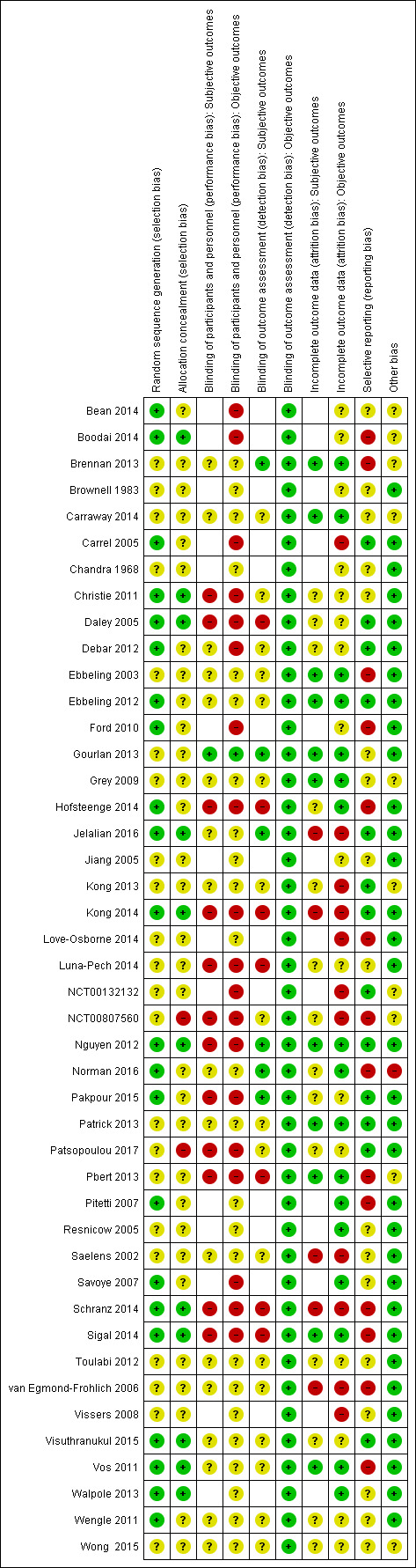
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not report that particular outcome).
Allocation
The majority of trials provided sufficient information on random sequence generation and were judged at low risk of bias. However, most trials provided inadequate information on concealment of allocation. Twenty‐two studies reported adequate random sequence generation, but only 11 of these also described adequate concealment of allocation (Boodai 2014; Christie 2011; Daley 2005; Jelalian 2016; Kong 2014; Nguyen 2012; Schranz 2014; Sigal 2014; Visuthranukul 2015; Vos 2011; Walpole 2013), and therefore have a low risk of selection bias. One trial described allocation concealment and was at high risk of bias (Patsopoulou 2017). One trial was also high risk of bias for allocation concealment (NCT00807560). The risk of selection bias was uncertain in the remaining 31 included studies.
Blinding
Only one study had a low risk of performance bias (subjective and objective outcomes) as participants and personnel were blinded to treatment allocation (Gourlan 2013). Nineteen studies had a high risk of performance bias for subjective and objective outcomes (Christie 2011; Daley 2005; Hofsteenge 2014; Kong 2014; Luna‐Pech 2014; NCT00807560; Nguyen 2012; Pakpour 2015; Patsopoulou 2017; Pbert 2013; Schranz 2014; Sigal 2014) or objective outcomes only (subjective outcomes not reported) (Bean 2014; Boodai 2014; Carrel 2005; Debar 2012; Ford 2010; NCT00132132; Savoye 2007). The risk of performance bias was unclear in the remaining 24 studies.
The risk of detection bias was low for objective outcomes (regardless of whether outcome assessors were blinded to treatment allocation) in 44 studies. For subjective outcomes, six studies blinded outcome assessors (Brennan 2013; Gourlan 2013; Jelalian 2016; Nguyen 2012; Norman 2016; Pakpour 2015), and this was unclear in 17 studies (Carraway 2014; Christie 2011; Debar 2012; Ebbeling 2003; Ebbeling 2012; Grey 2009; Kong 2013; NCT00807560; Patrick 2013; Patsopoulou 2017; Saelens 2002; Toulabi 2012; van Egmond‐Frohlich 2006; Visuthranukul 2015; Vos 2011; Wengle 2011; Wong 2015). The risk of detection bias was judged to be high in the remaining seven studies reporting subjective ouLena1234tcomes (Daley 2005; Hofsteenge 2014; Kong 2014; Luna‐Pech 2014; Pbert 2013; Schranz 2014; Sigal 2014).
Incomplete outcome data
The risk of attrition bias was low in 17 studies (Brennan 2013; Carraway 2014; Ebbeling 2003; Ebbeling 2012; Gourlan 2013; Grey 2009; Hofsteenge 2014; Nguyen 2012; Norman 2016; Patrick 2013; Pbert 2013; Pitetti 2007; Resnicow 2005; Savoye 2007; Sigal 2014; Vos 2011; Walpole 2013). Eleven studies had a high risk of attrition bias on objective and subjective (where reported) outcome measures due to an imbalance in attrition between study groups (Carrel 2005; Jelalian 2016; Kong 2013; Kong 2014; Love‐Osborne 2014; NCT00132132; NCT00807560; Saelens 2002; Schranz 2014; van Egmond‐Frohlich 2006; Vissers 2008). The remaining 16 studies were at unclear risk of bias due to insufficient reporting of attrition, such as not providing reasons for missing data.
Selective reporting
Fourteen studies were at high risk of reporting bias (Boodai 2014; Brennan 2013; Ebbeling 2003; Ford 2010; Hofsteenge 2014; Love‐Osborne 2014; NCT00807560; Norman 2016; Pbert 2013; Pitetti 2007; Schranz 2014; Sigal 2014; van Egmond‐Frohlich 2006; Vos 2011). In 17 studies, this was unclear, as the study protocol or trial record were unavailable, or the trial had yet to be fully published. The remaining 13 studies reported all outcomes as stated.
Other potential sources of bias
There was high risk of bias in one study as the run‐in programme was conducted to minimise participant attrition, but may have resulted in a more motivated sample of participants and parents compared with non‐run‐in trial cohorts (Norman 2016). There was an unclear risk of other sources of bias in 10 studies (Bean 2014; Boodai 2014; Brennan 2013; Carraway 2014; Grey 2009; Kong 2013; NCT00132132; NCT00807560; Pbert 2013; Wong 2015). In one of these studies, the participating churches (which were the unit of randomisation) requested that the comparison condition received a meaningful intervention, which may have had an impact on results (Resnicow 2005). The remaining 32 studies were at low risk of other sources of bias.
Effects of interventions
See: Table 1
None of the included studies reported all‐cause mortality, morbidity and socioeconomic effects.
All diet, physical activity and behavioural interventions versus controls
Primary outcomes
Forty‐four trials compared a diet, physical activity, behavioural intervention or a combination of these with usual care, no active intervention or wait list control or a concomitant therapy. Five studies assessed more than one intervention (Brownell 1983; Pakpour 2015; Patrick 2013; Patsopoulou 2017; Sigal 2014). In one three arm trial, Brownell 1983 assessed a multicomponent intervention with three different approaches; mothers attended sessions with the adolescents, mothers attended separate sessions to the adolescent or only the adolescents received the intervention. The study by Pakpour 2015 compared motivational interviewing with a passive control (no details); there were two intervention groups, one with parental involvement and one without. In the study by Patsopoulou 2017, there were two intervention groups compared to no intervention, an activity group and an activity plus diet group with parental involvement. Sigal 2014 allocated participants to four arms comparing different physical activity interventions with a diet‐only control group. All groups received the dietary intervention and this was compared to a diet plus aerobic exercise intervention, a diet plus resistance training intervention and a diet plus aerobic plus resistance training intervention. The study by Patrick 2013 compared three different website interventions (website alone, website and group sessions, website and text messages) with a usual care control. There were no substantial differences in the study between the interventions and these were therefore merged into one group as described in the Measures of treatment effect section. One study provided gender stratified analyses (Norman 2016). One study had two control groups, a usual care control and an exercise placebo of body conditioning (an attention control) (Daley 2005). As there was no aim in the study to test the difference between these two control groups, and the attention control was not intended to promote weight control, we merged these two groups into one control arm for the purpose of the review. The other 37 trials each assessed different interventions as described above (Bean 2014; Boodai 2014; Brennan 2013; Carrel 2005; Chandra 1968; Christie 2011; Debar 2012; Ebbeling 2003; Ebbeling 2012; Ford 2010; Gourlan 2013; Grey 2009; Hofsteenge 2014; Jelalian 2016; Jiang 2005; Kong 2013; Kong 2014; NCT00132132; NCT00807560; Nguyen 2012; Love‐Osborne 2014; Luna‐Pech 2014; Norman 2016; Pbert 2013; Pitetti 2007; Resnicow 2005; Saelens 2002; Savoye 2007; Schranz 2014; Sigal 2014; Toulabi 2012; van Egmond‐Frohlich 2006; Vissers 2008; Visuthranukul 2015; Vos 2011; Walpole 2013; Wengle 2011).
Outcomes are considered here at the longest point of follow‐up for each study. Sensitivity analyses and subgroup analyses around these outcomes are reported below.
Changes in body mass index or body weight
All but one study (Chandra 1968) reported BMI variables as outcomes. Twenty‐nine studies reported BMI and 20 studies reported BMI z score. Twenty‐eight trials (33 comparisons) reported BMI change data that were suitable for meta‐analysis. Pooling the studies in a random‐effects meta‐analysis demonstrated a reduction in BMI in the intervention groups compared with control groups at the longest point of follow‐up (MD ‐1.18 kg/m2 (95% CI ‐1.67 to ‐0.69); P < 0.00001; 2774 participants; 28 studies; 34 comparisons; low quality evidence; Analysis 1.1).
1.1. Analysis.
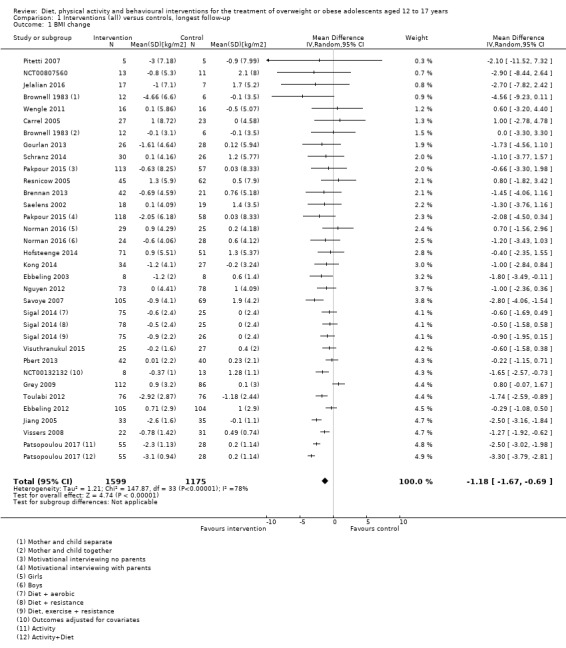
Comparison 1 Interventions (all) versus controls, longest follow‐up, Outcome 1 BMI change.
Meta‐analysis of BMI z score at the longest point of follow‐up showed a reduction in the intervention groups compared with the control groups (MD ‐0.13 units (95% CI ‐0.21 to ‐0.05); P = 0.002; 2399 participants; 20 studies; low quality evidence; Analysis 1.2).
1.2. Analysis.
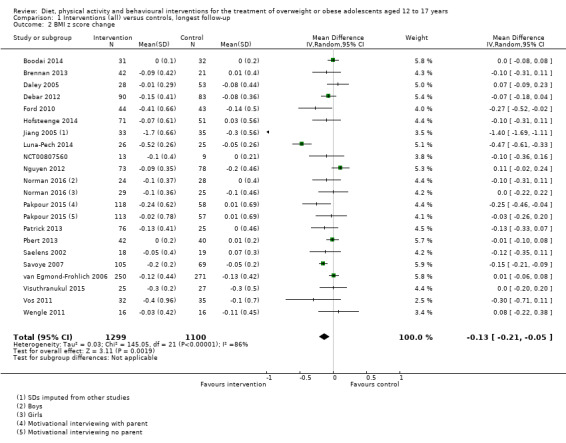
Comparison 1 Interventions (all) versus controls, longest follow‐up, Outcome 2 BMI z score change.
Five studies did not report BMI or BMI z score outcomes in a format that was suitable for meta‐analysis (Carraway 2014; Christie 2011; Love‐Osborne 2014; Walpole 2013; Wong 2015). The study by Walpole 2013 reported median BMI and BMI z scores and the study reported no substantial differences between groups. In the study by Wong 2015, the mean BMI z score did not substantially differ between groups. Christie 2011 reported the effect estimate for BMI at six months, which was not substantially different between groups. The study by Love‐Osborne 2014 reported the proportion of participants with increases or decreases in BMI z scores at different thresholds. The only relevant result was that a higher proportion of participants in the control group decreased their BMI z score by at least 0.1 compared with the intervention group. One study has not currently reported BMI outcomes having only recently published secondary outcomes (Bean 2014). The study by Carraway 2014 did not present BMI data for a mentor‐led exercise programme compared with wait list control.
Five studies reported BMI percentiles (Bean 2014; Brennan 2013; Debar 2012; Kong 2013; Patrick 2013). Analysis 1.3 shows results from four studies (Brennan 2013; Debar 2012; NCT00807560; Patrick 2013). The results favoured the interventions in two studies. In one study, where three interventions were combined, there was a reduction in the BMI percentile in the control group (Patrick 2013). Kong 2013 reported the median BMI percentile. This reduced in the intervention group and increased in the control group (P = 0.04). Bean 2014 did not report data.
1.3. Analysis.
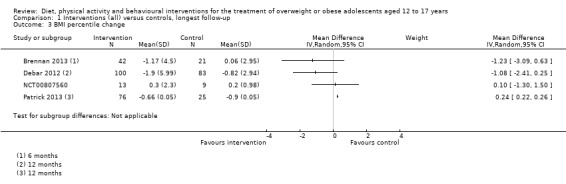
Comparison 1 Interventions (all) versus controls, longest follow‐up, Outcome 3 BMI percentile change.
Twenty studies (24 comparisons) reported weight change and were pooled in a random‐effects meta‐analysis which demonstrated a reduction in weight in the intervention groups compared with controls at the longest point of follow‐up (MD ‐3.67 kg (95% CI ‐5.21 to ‐2.13); P < 0.00001; 1993 participants; 20 studies; moderate quality evidence; Analysis 1.4).
1.4. Analysis.
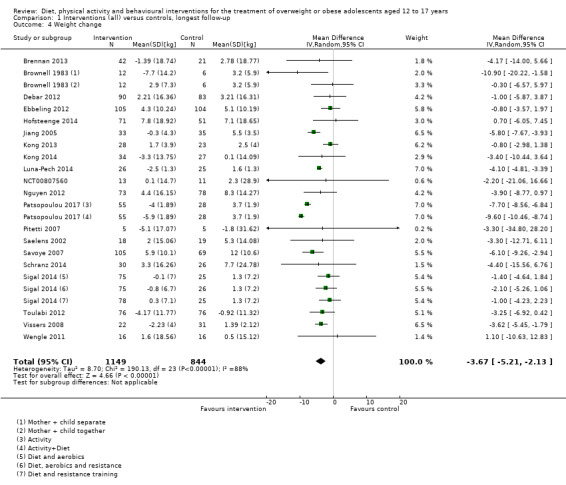
Comparison 1 Interventions (all) versus controls, longest follow‐up, Outcome 4 Weight change.
Adverse events
Five trials reported rates of adverse events (Ebbeling 2012; Ford 2010; NCT00132132; NCT00807560; Sigal 2014). There were no adverse events reported in either group in the studies by Ford 2010; NCT00132132; and NCT00807560. In the study by Ebbeling 2012, 6.4% of participants experienced an adverse event in the intervention group (no details); it was not reported whether adverse events occurred in the comparator group. Sigal 2014 reported the proportions of participants experiencing adverse events, the proportion withdrawing owing to adverse events and detailed the specific adverse events in each of their four groups (see Appendix 8 for details). Adverse events were experienced in 25% of participants in the diet plus aerobic exercise group; 19% in the diet plus resistance training group; 21% in the diet plus aerobic plus resistance training group and 24% in the diet‐only group.
Secondary outcomes
Anthropometric measures other than body mass index
Twenty‐eight trials reported anthropometric measures other than BMI using objective measures (Boodai 2014; Brownell 1983; Brennan 2013; Carrel 2005; Ebbeling 2003; Ebbeling 2012; Ford 2010; Grey 2009; Hofsteenge 2014; Kong 2013; Kong 2014; NCT00132132; NCT00807560; Nguyen 2012; Norman 2016; Pakpour 2015; Patrick 2013; Patsopoulou 2017; Pbert 2013; Saelens 2002; Savoye 2007; Schranz 2014; Sigal 2014; Toulabi 2012; Vissers 2008; Vos 2011; Walpole 2013; Wengle 2011). Fourteen studies reported percentage of body fat change that was suitable for meta‐analysis. Meta‐analysis demonstrated a reduction in percentage of body fat in the intervention group compared with the control group at the longest follow‐up points (MD ‐1.08% (95% CI ‐1.69 to ‐0.46); P = 0.0006; 1886 participants; 14 studies; Analysis 3.1). Three studies reported other measures of body fat that included percentage of fat (Carraway 2014; Pakpour 2015), and percentage of body fat‐SDS (Ford 2010). All three studies found a reduction in body fat in the intervention group compared to the control group (see Table 3 for details).
3.1. Analysis.
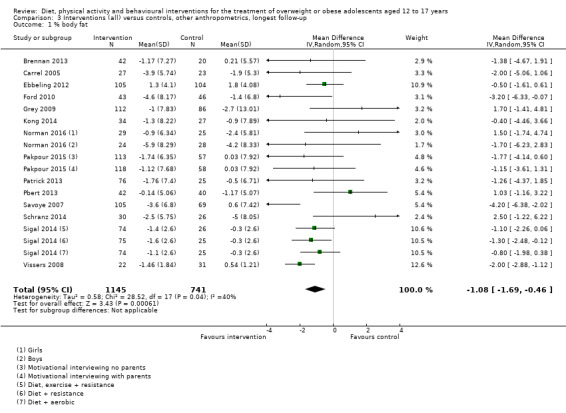
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 1 % body fat.
2. Anthropometric data.
| Study | Tool | Outcome | Findings |
| Norman 2016 | Child's BMI ‐ median BMI for age and gender)/median BMI for age and gender × 100 | % over median BMI | At 12 months, a significant treatment effect was observed in boys in the stepped care group compared to the enhanced usual care group (P = 0.002) |
| Schranz 2014 | Scales | Body mass (kg), mean ± SD | At 12 months, there was no significant difference (P= 0.69) between the intervention (99.3 ± 16.6; 30 participants) and control group (104 ± 27.1; 26 participants) |
| Calliper | Sum of skinfolds (mm), mean ± SD | At 12 months, there was no significant difference (P= 0.86) between the intervention (249.4 ± 72.9; 30 participants) and control group (248.1 ± 96.9; 26 participants) | |
| Carraway 2014 | By comparing actual BMI to BMI at the 50th percentile | % overweight) | Participants in both groups were able to reduce % overweight from baseline to post‐test by 1.98% and from baseline to follow‐up by 1.48% |
| DXA | % fat | Reduction from baseline to postintervention tended towards significance. However, this reduction was reversed across follow‐up | |
| Pakpour 2015 | Bioelectrical impedance analysis | % fat, mean ± SD | At 12 months, the motivational interview + parental involvement group had significantly (P = 0.001) lower body fat (42.32 ± 3.56%; 118 participants) compared to the control (45.33 ± 4.86%; 115 participants) but was not superior (P= 0.38) to the motivational interviewing group (45.62 ± 4.32%; 113 participants) |
| Kong 2014 | Bioimpedence | % visceral fat, median (interquartile range) | At 6 months, no significant reduction of visceral fat in either the low GI group (from 14.2 (10.8 to 19.8) to 12.0 (10.0 to 19.0); 27 participants) or the control group (from 12.5 (9.0 to 19.0) to 13.5 (7.5 to 20.5); 34 participants) |
| Brennan 2013 | Body scan | Trunk lean mass (kg), mean ± SD | At 6 months, the intervention group had lower truncal lean mass (21.67 ± 4.72; 42 participants) compared to the control group (21.99 ± 3.96; 20 participants) |
| Toulabi 2012 | Anthropometric tape | Hip circumference (cm), mean ± SD | At 6 months, the intervention group had a significantly lower (P = 0.001) circumference (105.18 ± 7.38; 76 participants) compared to the control (109.80 ± 6; 76 participants) |
| Vos 2011 | Anthropometric tape | Waist circumference‐SDS, MD, 95% CI | At 12 months, the mean change in the intervention group (‐0.6, ‐1.2 to ‐0.00; 32 participants) was significantly lower (P = 0.03) compared to the control group (‐0.2, ‐0.7 to 0.5; 34 participants) |
| Stadiometer and anthropometric tape | Waist circumference/height, MD, 95% CI | At 12 months, the mean change in the intervention group (‐0.03, ‐0.06 to 0.00; 32 participants) was significantly lower (P = 0.03) compared to the control group (‐0.01, ‐0.03 to 0.00; 34 participant) | |
| Ford 2010 | Bioimpedence | % body fat‐SDS, MD, 95% CI | At 12 months, the Mandometer group had a greater fall in body fat (‐0.32, 0.22 to 0.41; 43 participants) compared to the control group (‐0.07, ‐0.04 to 0.18; 45 participants) |
| Chandra 1968 | Beam balance | Average weight loss (% of expected) | At 17 months, the difference between the intervention group (Limical) and the control tended to level off (112% in the intervention in comparison to 114% in the control) |
BMI: body mass index; CI: confidence interval; DXA: dual‐energy X‐ray absorptiometry; GI: glycaemic index; kg: kilogram; MD: mean difference; mm; millimetre; SD: standard deviation; SDS: standard deviation score.
Two trials reported data for percentage of trunk fat that was suitable for meta‐analysis. Random‐effects meta‐analysis showed an MD of ‐0.84% ((95% CI ‐3.10 to 1.43); P = 0.47; 123 participants; 2 trials; Analysis 3.2).
3.2. Analysis.
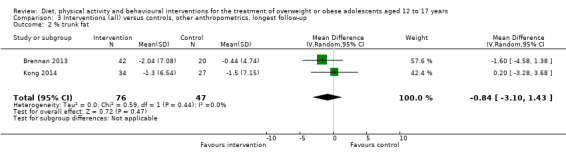
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 2 % trunk fat.
Seventeen trials reported data for waist circumference that was suitable for meta‐analysis. Random‐effects meta‐analysis demonstrated a reduction in waist circumference in the intervention group compared with the control group at the longest follow‐up points (MD ‐2.26 cm (95% CI ‐3.80 to ‐0.72); P = 0.004; 1997 participants; 17 trials; Analysis 3.3). Two studies reported other measures of abdominal adiposity (Kong 2014; Vos 2011). Percentage of visceral fat did not change in either the intervention or control group (Kong 2013). One study found that both waist circumference minus SDS and waist‐to‐height ratio significantly decreased in the intervention arm compared to the control arm (Vos 2011) (see Table 3 for details).
3.3. Analysis.
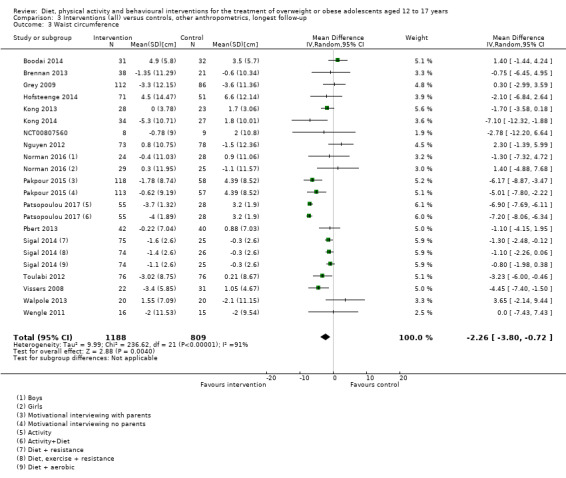
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 3 Waist circumference.
Pooling three trials in a random‐effects meta‐analysis showed no substantial reduction in waist‐to‐height ratio in the intervention group compared to control group at the longest follow‐up points (MD 0 (95% CI ‐0.02 to 0.02); P = 0.98; 276 participants; 3 trials; Analysis 3.4). Waist‐to‐hip ratio did not substantially reduce in the intervention group compared to the control group (MD 0.01 (95% CI ‐0.01 to 0.03); P = 0.22; 211 participants; 2 trials; Analysis 3.5). In one study, hip circumference seemed to decrease in the intervention group compared to the control group (Toulabi 2012; see Table 3).
3.4. Analysis.
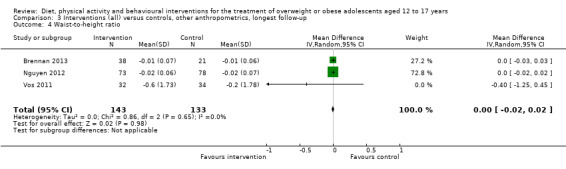
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 4 Waist‐to‐height ratio.
3.5. Analysis.
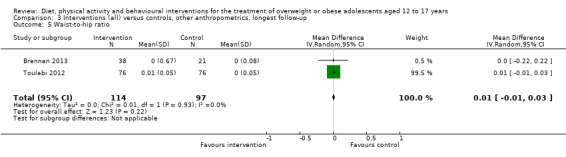
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 5 Waist‐to‐hip ratio.
Five trials reported data for fat mass that was suitable for meta‐analysis. Pooling the studies in a random‐effects meta‐analysis demonstrated a reduction in fat mass in the intervention group compared to the control group at the longest follow‐up points (MD ‐3.13 kg (95% CI ‐4.70 to ‐1.56); P < 0.0001; 673 participants; 5 trials; Analysis 3.6). The study by Schranz 2014 found no substantial difference for the reduction in body mass between the intervention and control groups (see Table 3 for details).
3.6. Analysis.
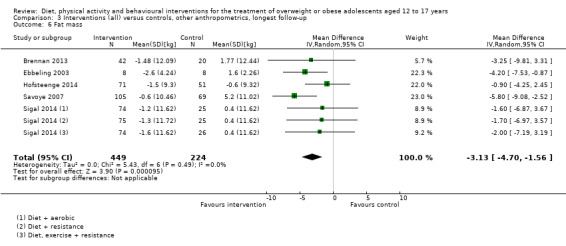
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 6 Fat mass.
Two trials reported data for trunk fat mass that was suitable for meta‐analysis. Random‐effects meta‐analyses showed no substantial reduction in trunk fat mass in the intervention group compared to the control group (MD ‐0.94 kg (95% CI ‐2.49 to 0.61); P = 0.24; 184 participants; 2 trials; Analysis 3.7).
3.7. Analysis.
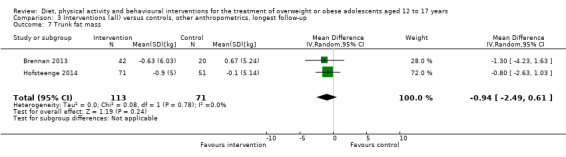
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 7 Trunk fat mass.
Three trials reported data for lean mass that was suitable for meta‐analysis. Random‐effects meta‐analysis showed no substantial increase in body lean mass in the intervention group compared to the control group (MD ‐0.21 kg (95% CI ‐0.88 to 0.47); P = 0.55; 417 participants; 3 trials; Analysis 3.8). The Brennan 2013 trial found that participants in the intervention group had lower truncal lean mass compared to the control group, however, statistical significance was not reported (see Table 3 for details).
3.8. Analysis.
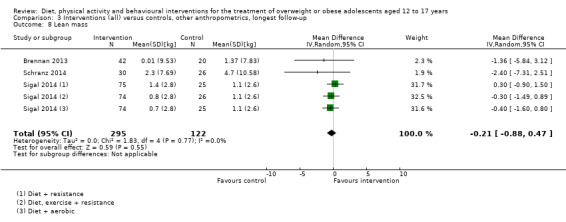
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 8 Lean mass.
Two trials reported data for percentage of overweight that was suitable for meta‐analysis. Random‐effects meta‐analysis showed no substantial difference in the percentage of overweight between the intervention group and control group (MD ‐5.55% (95% CI ‐13.67 to 2.57); P = 0.18; 73 participants; 2 trials; Analysis 3.9). One study reported percentage of BMI reduction and found that 75% of participants in the intervention group reduced their BMI compared to 23% of participants in the control group (NCT00132132). In one trial, percentage of overweight reduced in both intervention and control at seven months' follow‐up (Carraway 2014; see Table 3 for details). In one study, percentage of boys over the median BMI was lower in the intervention group compared to boys in the control group (Norman 2016). In an earlier study, average weight loss was not lower in the intervention group compared to the control (Chandra 1968; see Table 3).
3.9. Analysis.
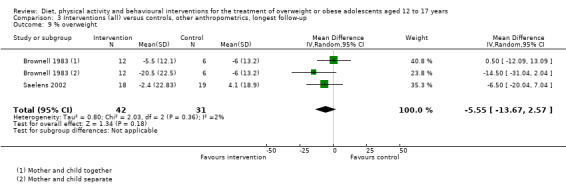
Comparison 3 Interventions (all) versus controls, other anthropometrics, longest follow‐up, Outcome 9 % overweight.
Health‐related quality of life and self‐esteem
Thirteen trials reported health‐related quality of life and self‐esteem using validated self‐reported tools (Brennan 2013; Carraway 2014; Daley 2005; Debar 2012; Ford 2010; Hofsteenge 2014; Luna‐Pech 2014; Nguyen 2012; Patrick 2013; Pakpour 2015; Schranz 2014; van Egmond‐Frohlich 2006; Vos 2011). Seven trials reported data for health‐related quality of life change that was suitable for meta‐analysis. Random‐effects meta‐analysis demonstrated an improvement in health‐related quality of life in the intervention group compared with the control group at the longest follow‐up points ((SMD 0.44 (95% CI 0.09 to 0.79); P = 0.01; 972 participants; 7 studies; low quality evidence Analysis 4.1). An SD of 0.44 represents a moderate difference between groups (Higgins 2011a). The study by Ford 2010 reported narrative findings of health‐related quality of life that improved in both the intervention and control arms but did not reach statistical significance (see Table 4 for further details).
4.1. Analysis.
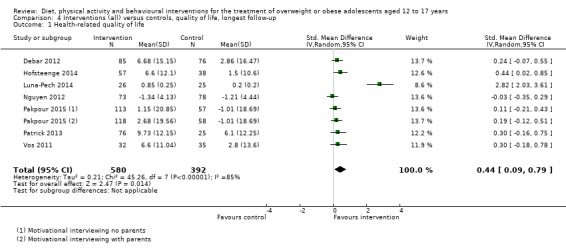
Comparison 4 Interventions (all) versus controls, quality of life, longest follow‐up, Outcome 1 Health‐related quality of life.
3. Behavioural change ‐ health‐related quality of life and self‐esteem.
| Study | Tool | Outcome | Findings |
| Hofsteenge 2014 | Body Esteem Scale | Body appearance, mean ± SD | At 18 months, body appearance scores did not significantly improve in either the intervention (from 1.8 ± 0.7 to 2.2 ± 0.7; 57 participants) or the control group (from 1.9 ± 0.8 to 0.3 ± 0.75; 38 participants) |
| Weight satisfaction, mean ± SD | At 18 months, weight satisfaction scores did not significantly improve in either the intervention (from 1.6 ± 0.7 to 1.7 ± 0.7; 57 participants) or the control group (from 1.7 ± 0.6 to 1.8 ± 0.7; 38 participants) | ||
| Body attribution, mean ± SD | At 18 months, body attribution scores did not significantly improve in either the intervention (from 1.8 ± 0.8 to 2.3 ± 0.8; 57 participants) or the control group (from 1.9 ± 1.0 to 2.0 ± 0.6; 38 participants) | ||
| Schranz 2014 | Physical self‐worth scale | Global physical self‐worth, mean ± SD | At 12 months, there was no significant difference (P= 0.52) for physical self‐worth between the intervention (2.40 ± 0.54; 30 participant) and control group (2.44 ± 0.57; 26 participants) |
| Carraway 2014 | 40‐item Children and Youth Physical Self‐Perception Profile measure | Global self‐esteem | At 7 months, significant change in global self‐esteem by time for both groups |
| Pakpour 2015 | 20‐item weight efficacy lifestyle questionnaire | Weight efficacy, mean ± SD | At 12 months, the motivational interview + parental involvement had significantly higher confidence scores for their ability to lose weight (108.05 ± 24.28; 118 participants) compared to the control (93.21 ± 29.60; 115 participants; P = 0.002) and the motivational interview group (101.27 ± 27.23; 113 participants; P = 0.04) |
| Ford 2010 | Paediatric quality of life inventory | Health‐related quality of life | Measures of quality of life improved in both the Mandometer and control group with no significant change at 12 months |
| van Egmond‐Frohlich 2006 | KINDL‐K (self‐report) and KINDL‐E (parents), 24 items with subscales family, friends, school, self‐worth, mental and physical well‐being (4 items per domain) | Health‐related quality of life | At 12 months, (total group) statistically significant improvements in obesity‐related quality of life and subdomains family, friends, school, physical and mental well‐being |
KINDL: Fragebogen für KINDer und Jugendliche zur Erfassung der gesundheitsbezogenen Lebensqualität (questionnaire for children and adolescents to record health‐related quality of life); SD: standard deviation.
Six trials reported data for self‐esteem change that was suitable for meta‐analysis. Random‐effects meta‐analysis demonstrated no substantial improvement in self‐esteem for either group at the longest follow‐up points (SMD 0.09 (95% CI ‐0.08 to 0.27; P = 0.30, 613 participants; 6 trials; Analysis 4.3). Four trials reported other measures of self‐esteem and found no substantial improvement in either group for body appearance, weight satisfaction, body attribution (Hofsteenge 2014), weight efficacy (Pakpour 2015), and physical self‐worth (Schranz 2014; van Egmond‐Frohlich 2006). One trial reported improvement in global self‐esteem post intervention and at seven months' follow‐up for both intervention and control (Carraway 2014) (see Table 4 for further details).
4.3. Analysis.
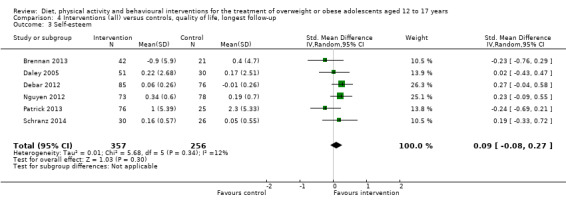
Comparison 4 Interventions (all) versus controls, quality of life, longest follow‐up, Outcome 3 Self‐esteem.
All‐cause mortality
None of the studies reported all‐cause mortality.
Morbidity
None of the studies reported morbidity.
Behavioural change
Dietary intake
Six trials (454 participants) reported macronutrient and glycaemic consumption assessed by either food record or 24‐hour dietary recall (Ebbeling 2003; Ebbeling 2012; Kong 2014; Pbert 2013; Saelens 2002; Visuthranukul 2015). Monitored intakes included fat, protein, carbohydrates, sugar, fibre, glycaemic load and glycaemic index.
Three trials reported fat intake as percentage of total energy intake for 132 participants (Ebbeling 2003; Pbert 2013; Saelens 2002). There was no substantial reduction in fat intake in either group in the studies by Pbert 2013 and Saelens 2002. In the study by Ebbeling 2003, fat intake significantly decreased in the control group but increased in the intervention group at 12 months (see Table 5 for details).
4. Behavioural change ‐ dietary intake.
| Study | Tool | Outcome | Findings |
| Pbert 2013 | 24‐hour dietary recall | Fat (% of energy), MD, 95% CI | At 6 months, no significant change in the intervention arm (1.21, ‐1.99 to 4.41;2 participants) and the control arm (MD 1.19, 95% CI −1.92 to 4.30; 40 participants) for fat intake |
| Ebbeling 2003 | 7‐day food record | Fat (% of energy), mean ± SD | At 12 months, a significant decrease (P= 0.03) in the conventional reduced fat arm (from 33 ± 1 to 29 ± 3; 7 participants) but tended to increase in the glycaemic load arm (27 ± 2 to 29 ± 3; 7 participants) |
| Saelens 2002 | 2‐day dietary recall | Fat (% of energy), mean ± SD | At 7 months, no significant change in the Healthy Habits intervention arm (from 33.9 ± 8.8 to 32.9 ± 9.4; 18 participants) and the typical care arm (from 34.3 ± 4.9 to 35.6 ± 6.4; 18 participants) |
| Kong 2014 | 3‐day dietary record | Protein (% of energy), mean ± SD | At 6 months, the low GI group had a significantly (P = 0.002) higher protein intake (17.9 ± 3.8; 34 participants) compared to the control (15.9 ± 2.7; 27 participants) |
| Ebbeling 2003 | 7‐day food record | Protein (% of energy), mean ± SD | At 12 months, there was no significant change in protein intake in the glycaemic load arm (from 17 ± 2 to 20 ± 1; 7 participants) or the conventional reduced fat group (from 16 ± 1 to 18 ± 2; 7 participants) |
| Kong 2014 | 3‐day diet record | Carbohydrate (% of energy), mean ± SD | At 6 months, there was no significant difference for carbohydrate mean intake between the low GI group (50.8 ± 8.1; 34 participants) and the control group (49.5 ± 6.6; 27 participants) |
| Ebbeling 2003 | 7‐day food record | Carbohydrate (% of energy), mean ± SD | At 12 months, there was a non‐significant decrease (P = 0.07) in the reduced glycaemic load arm (from 58 ± 3, to 52 ± 4; 7 participants) and no significant change in the conventional reduced fat arm |
| Kong 2014 | 3‐day diet record | Glycaemic load (per 100 kcal), mean ± SD | At 6 months, there was no significant (P = 0.175) difference for glycaemic load mean intake between the low GI group (117.7 ± 42.5; 34 participants) and the control group (106.3 ± 42.7; 27 participants) |
| Pbert 2013 | 24‐hour dietary recall | Glycaemic load, MD, 95% CI | At 6 months, the was a reduction in the intervention arm (‐22.93, ‐39.68 to ‐6.18) but not in the control arm (‐11.08, ‐27.36 to 5.20) |
| Ebbeling 2003 | 7‐day food record | Glycaemic load (g/100 kcal), mean ± SD | At 12 months, there was a significant (P = 0.007) decrease in the reduced glycaemic load arm (from 86 ± 5 to 69 ± 6; 7 participants) but not in the conventional reduced fat arm (from 79 ± 2 to 79 ± 7; 7 participants) |
| Visuthranukul 2015 | 3‐day dietary record | Low glycaemic index diet (items/day), MD ± SD | At 6 months, the consumption of low glycaemic food items increased in the intervention group (3.6 ± 1.6; 25 participants) while the control group consumed fewer items (‐0.4 ± 1.5; 27 participants) |
| Kong 2014 | 3‐day dietary record | Glycaemic index, mean ± SD | At 6 months, there was no significant (P = 0.175) difference for glycaemic index mean intake between the low GI group (74.4 ± 8.7; 34 participants) and the control group (76.8 ± 10.2; 27 participants) |
| Ebbeling 2003 | 7‐day food record | Glycaemic index, mean ± SD | At 12 months, there was no significant reduction in the reduced glycaemic load arm (from 58 ± 2 to 53 ± 3; 7 participants) or the conventional reduced fat arm (from 59 ± 1 to 56 ± 2; 7 participants) |
| Pbert 2013 | 24‐hour dietary recall | Total sugar (g/day), MD, 95% CI | At 6 months, total sugar intake reduced in the intervention arm (‐29.15, ‐49.08 to ‐9.22) but not in the control arm (‐11.37, ‐30.71 to 7.96) |
| Ebbeling 2012 | 24‐hour dietary recall (3 recalls) | Sugar (g/day), MD ± SD | At 2 years, the intervention group consumed less sugar compared to the control (‐19 ± 7, P = 0.005; 209 participants) |
| Kong 2014 | 3‐day dietary record | Fibre (g/1000 kcal), mean ± SD | At 6 months, the low GI group had a higher (P = 0.041) fibre intake (6.5 ± 3.3; 34 participants) compared to the control arm (5.3 ± 2.5; 27 participants) |
| Ebbeling 2003 | 7‐day food record | Fibre (g/1000 kcal), mean ± SD | At 12 months, fibre intake did not increase significantly in the reduced glycaemic load arm (from 8 ± 1 to 10 ± 1; 7 participants) or the conventional reduced fat arm (from 8 ± 1 to 10 ± 2; 7 participants) |
| Pakpour 2015 | FFQ | Fruits and juice (servings/day), mean ± SD | At 12 months, the motivational interview + parental involvement group had a higher (P = 0.030) intake of fruit servings (1.33 ± 0.93; 118 participants) compared to the control group (1.23 ± 0.97; 115 participants), but did not significantly (P = 0.17) differ compared to the motivational interview group (1.31 ± 0.96; 113 participants) |
| Patrick 2013 | FFQ | Fruit and vegetable (servings per 1000 calories), mean ± SE | At 12 months, fruit and vegetable consumption did not change the web‐only group (from 1.9 ± 0.01 to 2.9 ± 0.01; P= 0.685; 26 participants), web + sessions group (from 2.3 ± 0.01 to 2.9 ± 0.01; P = 0.398; 26 participants) and web + SMS group (from 2.0 ± 0.01 to 2.6 ± 0.01; P = 0.369; 24 participants) compared to the usual care group (from 1.9 ± 0.01 to 2.0 ± 0.01; 25 participants) |
| Kong 2013 | FFQ | Fruits and vegetables (servings/day), median, 95% CI | At 6 months, there was no change in the intervention arm (‐0.22, ‐0.72 to 0.41; 28 participants) or the control arm (‐1.16, ‐0.56 to 0.02; 23 participants) for fruit and vegetables intake |
| Wengle 2011 | 4‐day record | Fruits and vegetables (serving/day), mean ± SD | At 6 months, fruits and vegetables consumption did not significantly increase in either the non‐mentored group (from 1.9 ± 1.5 to 1.8 ± 1.7; P = 0.79; 14 participants) or the mentored group (from 2.9 ± 2.7 to 2 ± 2; P= 0.63; 14 participants) |
| Pakpour 2015 | FFQ | Vegetables (servings/day) mean ± SD | At 12 months, there was no significant difference for vegetable consumption across the motivational interview + parental involvement group (1.77 ± 0.76; 118 participants), motivational interview group (1.71 ± 0.80; 113 participants), and the control group (1.64 ± 0.80; 115 participants) |
| Brennan 2013 | Dietary questions from the YBRS | Vegetables (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.901) for vegetables intake between the intervention (2.98 ± 1.13; 41 participants) and the control group (3.00 ± 0.71; 21 participants) |
| Brennan 2013 | Dietary questions from the YBRS | Juice (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.056) for juice consumption between the intervention (2.07 ± 1.18; 40 participants) and control group (2.71 ± 1.31; 21 participants) |
| Ebbeling 2012 | 24‐hour dietary recall (3 recalls) | Fruit juices beverages (serving/day), MD ± SD | At 2 years, there was no difference in the reduction of fruit juice consumption between the intervention and control group (‐3 ± 17; P = 0.44; 209 participants) |
| Pakpour 2015 | FFQ | Milk (servings/day), mean ± SD | At 12 months, there was no significant difference for milk intake across the motivational interview + parental involvement group (0.96 ± 0.43; 118 participants), motivational group (0.95 ± 0.29; 113 participants) and the control group (0.97 ± 0.37; 115 participants) |
| Brennan 2013 | Dietary questions from the YBRS | Milk (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.99) for milk consumption between the intervention (3.63 ± 1.61; 41 participants) and the control group (3.59 ± 1.56; 21 participants) |
| Pakpour 2015 | FFQ | Non‐diet soda (servings/day), mean ± SD | At 12 months, the motivational interview + parental involvement group consumed fewer sugary drinks (0.48 ± 0.35; 118 participants) compared to the control group (0.95 ± 0.33; P = 0.001; 115 participants) and the motivational interview group (0.77 ± 0.38; P = 0.01; 113 participants) |
| Kong 2013 | FFQ | Sweetened drinks (glasses/day), median, 95% CI | At 6 months, there was a significant reduction in sweetened drinks consumption in the intervention arm (‐0.12, ‐0.47 to ‐0.08; 28 participants) compared to the control arm (‐0.16, ‐0.57 to 0.22; 23 participants) |
| Ebbeling 2012 | 24‐hour dietary recall (3 recalls) | Sugar‐sweetened beverages (serving/day), MD ± SD | At 2 years, the intervention group consumed fewer sugar‐sweetened beverages compared to the control group (‐58 ± 21; P = 0.007; 209 participants) |
| Pakpour 2015 | FFQ | Snacks/desserts (servings/day), mean ± SD | At 12 months, the motivational interview + parental involvement group consumed fewer snacks (3.89 ± 1.65; 118 participants) compared to the control group (4.55 ± 1.71; P < 0.001; 115 participants) and the motivational interview group (4.12 ± 1.43; P = 0.04; 113 participants) |
| Wengle 2011 | 4‐day dietary record | Total snack food (servings/week), mean ± SD | At 6 months, the non‐mentored group had a non‐significant decrease (P = 0.06) in snacks consumption (from 10.3 ± 6.9 to 6.3 ± 4.2; 14 participants). Similarly, the mentored group had a non‐significant (P = 0.07) reduction in snacks consumption (from 8.9 ± 3.3 to 7.4 ± 3.8; 14 participants) |
| Pakpour 2015 | FFQ | Total dietary fat (g), mean ± SD | At 12 months, the motivational interview + parental involvement group had a lower (P = 0.012) fat consumption (74.40 ± 42.39; 118 participants) compared to the control group (95.73 ± 40.10; 115 participants). There was a reduction (P = 0.041) when comparing the motivational interview + parental involvement group to the motivational interview group (98.42 ± 49.83; 113 participants) |
| Saturated fat (g), mean ± SD | At 12 months, the control group had a lower (P < 0.001) consumption of saturated fat (30.03 ± 20.84; 115 participants) compared to the motivational interview + parental involvement group (35.52 ± 21.48; 118 participants). Similarly, the motivational interview group had a lower consumption (32.54 ± 22.86; P = 0.029; 113 participants) compared to the motivational interview + parental involvement group | ||
| Fried foods (servings/day), mean ± SD | At 12 months, the motivational interview + parental involvement group consumed fewer (P= 0.01) servings of fried foods (0.63 ± 0.33; 118 participants) compared to the control group (0.90 ± 0.44; 115 participants). Similarly, the motivational interview + parental involvement group consumed fewer fried foods compared to the motivational group (0.82 ± 0.30, P = 0.042; 113 participants) | ||
| Pbert 2013 | 24‐hour dietary recall | % calories saturated fat, MD, 95% CI | At 6 months, there was no change % calories of saturated fat in the intervention arm (1.21, ‐1.99 to 4.41) or the control arm (1.19, 95% CI ‐1.92 to 4.30) |
| Added sugar (g/day), MD, 95% CI | At 6 months, sugar intake reduced in the intervention arm (‐18.87, ‐40.17 to 2.43) but not in the control arm (‐7.20, ‐27.59 to 13.19) | ||
| Brennan 2013 | Dietary questions from the YBRS | Fruit (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.518) between the intervention (3.49 ± 0.32; 39 participants) and the control group (3.48 ± 1.17; 21 participants) for fruit consumption |
| Potatoes (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.141) for potatoes intake between the intervention group (2.25 ± 0.78; 40 participants) and the control group (2.67 ± 0.86; 21 participants) | ||
| Carrots (serving/day), mean ± SD | At 6 months, there was no significant difference (P = 0.948) for carrots intake between the intervention group (2.15 ± 0.52; 41 participants) and the control group (2.38 ± 0.92; 21 participants) | ||
| Salad (serving/day), mean ± SD | At 6 months, there was no difference (P = 0.515) for salad consumption between the intervention group (2.38 ± 1.21; 40 participants) and the control group (2.57 ± 1.50; 21 participants) | ||
| Ebbeling 2012 | 24‐hour dietary recall (3 recalls) | Artificially sweetened beverages (servings/day), MD ± SD | At 2 years, there was no difference in the consumption of artificially sweetened beverages between the intervention and control group (0.1 ± 0.1, P = 0.32; 209 participants) |
| Unsweetened beverages (servings/day), MD ± SD | At 2 years, the consumption of unsweetened beverages remained higher in the intervention group compared to the control group (0.6 ± 0.2, P < 0.001; 224 participants) | ||
| Wengle 2011 | 4‐day dietary record | High fat/sugar (servings/week), mean ± SD | At 6 months, the non‐mentored group demonstrated a significant decrease (P = 0.02) in high fat/sugar consumption (from 3.6 ± 0.8 to 2.2 ± 0.9; 14 participants) while the mentored group had no significant (P = 0.19) nutritional changes (from 4.2 ± 2.7 to 3.7 ± 1.5; 14 participants) |
| Fast food (servings/week), mean ± SD | At 6 months, the non‐mentored group demonstrated significant decrease (P = 0.02) in fast food consumption (from 1.6 ± 1.5 to 0.8 ± 1.0; 14 participants) while the mentored group had no significant (P = 0.36) nutritional changes (from 1.1 ± 1.1 to 0.7 ± 0.8; 14 participants) | ||
| Whole‐grain foods (servings/day), mean ± SD | At 6 months, the non‐mentored group demonstrated significant increase (P = 0.02) in whole‐grains consumption (from 0.8 ± 0.9 to 2.0 ± 1.4; 14 participants) while the mentored group had no significant (P = 0.73) nutritional changes (from 1.2 ± 0.8 to 1.0 ± 1.0; 14 participants) |
CI: confidence interval; FFQ: Food Frequency Questionnaire; MD: mean difference; SD: standard deviation; SE: standard error; YBRS: Youth Behavioural Risk Survey.
Two trials reported protein intake as percentage of total energy intake for 75 participants (Ebbeling 2003; Kong 2014). At 12 months, there was no substantial increase in protein intake in the either group in the study by Ebbeling 2003. In the study by Kong 2014 the intervention group increased protein consumption compared to the control group at six months (see Table 5 for details). The same trials (Ebbeling 2003; Kong 2014) reported carbohydrate intake. There was no substantial change in carbohydrate consumption in either the intervention or control group at six and 12 months (see Table 5 for details).
Two trials reported total sugar intake for 291 participants (Ebbeling 2012; Pbert 2013). Participants in the intervention group reported lower intakes compared to the control group in both studies (Ebbeling 2012; Pbert 2013). See Table 5 for details.
Two trials reported fibre intake for 75 participants (Ebbeling 2003; Kong 2014). At 12 months, fibre intake did not change substantially in either the intervention or control group in one study (Ebbeling 2003). The second study reported an increase in fibre consumption in the intervention group compared to the control group (Kong 2014). See Table 5 for details.
Three trials reported glycaemic load intake for 157 participants (Ebbeling 2003; Kong 2014; Pbert 2013). Glycaemic load decreased in the intervention group in two studies (Ebbeling 2003; Pbert 2013), but there was no substantial difference between the intervention and control groups in one study (Kong 2014). See Table 5 for details.
Three trials reported glycaemic index intake for 127 participants (Ebbeling 2003; Kong 2014; Visuthranukul 2015). Glycaemic index did not change in either group in the studies by Ebbeling 2003 and Kong 2014. At six months, the intervention group (25 participants) reported a higher consumption of low glycaemic index foods compared to the control group (27 participants) in one study (Visuthranukul 2015). See Table 5 for details.
Five trials (650 participants) reported fruit and vegetable intake (Brennan 2013; Kong 2013; Pakpour 2015; Patrick 2013; Wengle 2011), while two trials (408 participants) reported vegetable intake (Brennan 2013; Pakpour 2015). Dietary consumption was assessed using the Fat, Fruit and Vegetable Diet Questionnaire (FFVDQ), diet record or dietary questions from the Center for Disease Control Youth Risk Behavior System (YRBS). In all studies, except for one (Pakpour 2015), fruit and vegetable consumption did not substantially increase. In the study by Pakpour 2015, the motivational interviewing and parental involvement group had a significantly higher intake of fruit servings compared to the control group. See Table 5 for details.
Four trials (668 participants) reported beverage consumption (juice, milk and sugar‐sweetened drinks) assessed by either dietary questions YRBS, 24‐hour dietary recall or Food Frequency Questionnaire (FFQ) (Brennan 2013; Ebbeling 2012; Kong 2013; Pakpour 2015). Juice and milk consumption did not change substantially in either the intervention and control group in three trials (Brennan 2013; Ebbeling 2012; Pakpour 2015). Reports on sugar‐sweetened drinks (606 participants) were significantly lower in the intervention group compared to the control group (Ebbeling 2012; Kong 2013; Pakpour 2015). See Table 5 for details.
Five trials (742 participants) reported several dietary findings assessed by the FFQ, 24‐hour dietary recall or the food record (Brennan 2013; Ebbeling 2012; Pakpour 2015; Pbert 2013; Wengle 2011) (see Table 5 for details). The consumption of fatty food items generally decreased in the intervention group compared to the control group (Pakpour 2015; Wengle 2011). There was no substantial difference between the intervention and control groups in healthier dietary consumption such as increasing salad intake and reducing added sugar (Brennan 2013; Pbert 2013). However, whole‐grain intake (Wengle 2011) and unsweetened beverage consumption (Ebbeling 2012) were higher in the intervention group compared to the control group.
Dietary behaviour
Four trials (674 participants) reported changes in dietary behaviours assessed by either objective (e.g. Mandometer) or validated self‐reported questionnaires (e.g. FFVDQ) (Brennan 2013; Ford 2010; Grey 2009; Pakpour 2015). Eating behaviours such as eating speed and usual food choices did not change substantially in either group in studies by Grey 2009 and Ford 2010. Dietary self‐efficacy improved in the intervention group (118 participants) but not in the control group in one study (Pakpour 2015), while there was no change in either group (198 participants) in another study (Grey 2009). Dietary behaviour in relation to fat intake did not improve in either the intervention or control group (Brennan 2013). See Table 6 for details.
5. Behavioural change ‐ dietary behaviour.
| Study | Tool | Outcome | Findings |
| Pakpour 2015 | 15‐ item Child Dietary Self‐Efficacy Scale | Dietary self‐efficacy, mean ± SD | At 12 months, the motivational interview + parental involvement group scored significantly higher (7.11 ± 2.62; 118 participants) compared to the control group (5.71 ± 2.82; 115 participants; P < 0.001) and the motivational interview group (6.88 ± 2.72; 113 participants; P = 0.01) |
| Brennan 2013 | FFVDQ | Fat substitution | At 6 months, there was no difference (P= 0.093) in fat substitution between the intervention group (2.42 ± 0.93; 42 participants) and the control group (2.51 ± 0.82; 21 participants) |
| Modify meat | At 6 months, there was no difference (P = 0.063) for the removal of fat from meat and choosing low fat cuts of meat between the intervention group (1.93 ± 0.81; 40 participants) and control group (2.43 ± 0.99; 19 participants) | ||
| Avoid frying | At 6 months, there was no difference (P = 0.289) in terms of avoiding fried food such as fried chicken, fish and chips between the intervention group (1.44 ± 0.46; 40 participants) and the control group (1.64 ± 0.57; 19 participants) | ||
| Fat replacement | At 6 months, there was no difference (P = 0.289) for the use of fruit and vegetables as an alternative to high‐fat ingredients such as meat and desserts in the intervention group (2.97 ± 0.65; 40 participants) and the control group (2.71 ± 0.73; 19 participants) | ||
| Avoid fat | At 6 months, there was no difference (P = 0.142) in avoiding fat as flavouring in terms of adding high‐fat condiments such as butter, cream and cheese sauces to meals in the intervention group (2.50 ± 0.54; 40 participants) and the control group (2.40 ± 0.56; 19 participant) | ||
| Fruit and vegetable | At 6 months, there was no difference (P = 0.992) between the intervention (2.98 ± 0.67; 40 participants) and the control group (2.92 ± 0.71; 19 participants) for fruit and vegetable consumption | ||
| Ford 2010 | Mandometer | Eating speed, mean ratio, 95% CI | At 12 months, eating speed did not change for either the Mandometer group (mean ratio 0.89, 95% CI 0.77 to 1.02; 44 participants) or the standard care group (1.04, 0.86 to 1.25; 23 participants) |
| Satiety at end of meal (arbitrary units 0‐100), MD, 95% CI | At 18 months, satiety at the end of a meal did not change for either the Mandometer group (MD 3.7, 95% ‐11.2 to 3.8; 44 participants) or the standard care group (‐4.1, ‐18.6 to 9.5; 23 participants) | ||
| Portion size (g), mean decrease, 95% CI | At 18 months, portion size did not change for either the Mandometer group (‐31, 95% CI ‐2 to 64; 43 participants) or the standard care group (‐3, ‐54 to 60; 21 participants) | ||
| Grey 2009 | Health behaviour questionnaire (14 items student's usual food selections) | Usual food choices, MD, 95% CI | At 12 months, health behaviour in terms of food choices did not improve in either the coping skill training group (1.2, ‐0.4 to 2.7; 112 participants) or the general education group (1.0, ‐0.7 to 2.6; 86 participants) |
| Health behaviour questionnaire (15 items Dietary Self‐Efficacy) | Dietary self‐efficacy, MD, 95% CI | At 12 months, health behaviour in terms of dietary self‐efficacy did not change in either the coping skills training group (1.0, ‐0.7 to 2.6, 112 participants) or the general education group (‐0.5, ‐2.2 to 1.3, in 86 participants) |
CI: confidence interval; FFVDQ: Fat, Fruit and Vegetables Diet Questionnaire; MD: mean difference; SD: standard deviation.
Physical activity
Thirteen trials (1376 participants) reported several measures of physical activity assessed using objective tools (Jelalian 2016; Kong 2013; Pbert 2013; Sigal 2014; Wengle 2011), or validated self‐reported tools (Debar 2012; Ebbeling 2012; Grey 2009; Gourlan 2013; Kong 2014; Pakpour 2015; Saelens 2002; Sigal 2014). Three trials reported data for mild‐to‐vigorous physical activity that was suitable for meta‐analysis (Kong 2013; Pbert 2013; Wengle 2011), while the study by Jelalian 2016 could not be meta‐analysed (Table 7). Random‐effects meta‐analysis demonstrated no substantial improvement in mild‐to‐vigorous physical activity (MD 0.39 (95% CI ‐15.07 to 15.85); P = 0.96; 129 participants; 3 trials; Analysis 5.1). Two trials reported data for activity length (hours per day) that was suitable for meta‐analysis (Gourlan 2013; Pakpour 2015). Random‐effects meta‐analysis showed no substantial improvement in activity length (MD ‐0.11 hours/day (95% CI ‐0.98 to 0.75); P = 0.80; 129 participants; 2 trials; Analysis 5.2). Only two trials reported screen time (hours per day) that was suitable for meta‐analysis (Ebbeling 2012; Wengle 2011). Random‐effects meta‐analysis showed a favourable effect for the intervention group compared with the control group at the longest follow‐up points (MD ‐0.60 hours/day (95% CI ‐0.65 to ‐0.55); P < 0.00001; 241 participants; 2 trials; Analysis 5.3).
6. Behavioural change ‐ physical activity.
| Study | Tool | Outcome | Findings |
| Jelalian 2016 | Sense Wear Mini monitor | MVPA (% time spent), mean ± SD | There was no significant increase in % time spent in MVPA in either the intervention group (7.5 ± 4.0 at baseline to 8.5 ± 3.9 at 48 weeks) and control group (7.9 ± 2.2 at baseline to 8.9 ± 4.4) |
| Pakpour 2015 | 7‐day physical activity recall interview | Self‐reported physical activity duration (hours/day), mean ± SD | At 12 months, the motivational interview + parental involvement reported a significantly higher duration of physical activity (1.30 ± 0.66; 118 participants) compared to the control group (0.66 ± 0.32; 115 participants; P= 0.004) and the motivational interview group (1.12 ± 0.61; 113 participants; P = 0.01) |
| Sigal 2014 | Pedometer | Steps (counts/day), MD, 95% CI | At 6 months, the number of steps did not significantly increase in the aerobic training group (303, ‐2039 to 2644; 38 participants), resistance training group (1821, ‐1097 to 4739; 53 participants), combined group (959, 9‐1975 to 3893; 38 participants) and the control group (2224, ‐1122 to 5569; 36 participants) |
| Gourlan 2013 | 7‐day physical activity recall | Physical activity length (hours/day), mean ± SD | At 6 months, the standard weight loss programme + motivational interviewing significantly (P < 0.01) increased self‐reported physical activity (1.30 ± 0.82; 26 participants) compared to the standard weight loss programme group (0.99 ± 0.62; 28 participants) |
| Kong 2013 | 3‐day physical activity recall | MVPA (30‐minute blocks/day), median, 95% CI | At 6 months, there was no change in the intervention arm (0.0, ‐2.0 to 0.7; 27 participants) or the control group (‐0.9, ‐1.3 to 0.4; 20 participants) |
| Debar 2012 | 24‐hour physical activity recall (adapted from the 7‐day validated recall) | Physical activity (minutes/day), mean ± SD | At 6 months, physical activity did not significantly improve for either the intervention group (from 55.35 ± 51.81 to 64.77 ± 67.60; 104 participants) or the control group (from 49.68 ± 39.47 to 56.39 ± 53.12; 102 participants) |
| Wengle 2011 | Accelerometer | Steps (1000/day), mean ± SD | At 6 months, there was no difference (P = 0.27) in the number of steps between the intervention group (9.1 ± 3.5; 14 participants) and the control group (7.6 ± 2.9; 11 participants) |
| Saelens 2002 | 7‐day physical activity recall interview | Physical activity (kcal/kg/day), mean ± SD | At 7 months, no significant change in the Healthy Habits intervention arm (6.7 ± 5.6 to 6.3 ± 3.5; 18 participants) and the typical care arm (34.3 ± 4.9 to 35.6 ± 6.4; 18 participants) |
| Kong 2014 | International Physical Activity Questionnaire (IPAQ), 7‐day recall | Physical activity level (MET‐ minutes/week), median (interquartile range) | At 6 months, there was no significant (P = 0.235) difference for activity levels between the low GI group (1453, 740 ‐ 2780; 28 participants) and the control group (2266, 960 to 4318; 18 participants) |
| Debar 2012 | 24‐hour physical activity recall (adapted from the 7‐day validated recall) | Physical activity level (MET/day), mean ± SD | At 6 months, the MET for self‐reported physical activity did not significantly improve for either the intervention group (from 4.28 ± 3.97 to 4.84 ± 5.11; 104 participants) or the control group (from 3.80 ± 3.13 to 4.47 ± .82; 102 participants) |
| Ebbeling 2012 | 24‐hour physical activity recall (3 recalls) | Daily physical activity level (MET/day), MD ± SD | At 2 years, there was no difference in activity levels between the intervention group and control group (0.01 ± 0.04; P = 0.86; 224 participants) |
| Grey 2009 | Goldin Shephard activity survey | Physical activity (METs/week), MD, 95% CI | At 12 months, the MET for self‐reported physical activity did not change in either the coping skill training group (2.6, ‐4.1 to 9.4; 112 participants) and the general education group (6.5, ‐0.7 to 13.7; 86 participants) |
CI: confidence interval; GI: glycaemic index; MD: mean difference; MET: metabolic equivalent of task; MVPA: moderate to vigorous physical activity; SD: standard deviation.
5.1. Analysis.
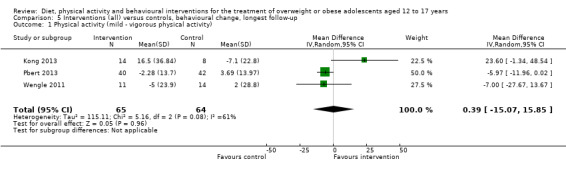
Comparison 5 Interventions (all) versus controls, behavioural change, longest follow‐up, Outcome 1 Physical activity (mild ‐ vigorous physical activity).
5.2. Analysis.
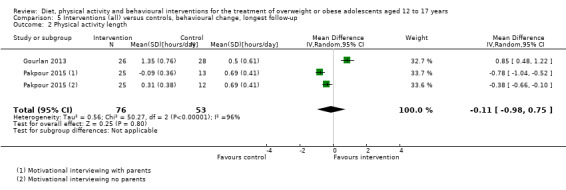
Comparison 5 Interventions (all) versus controls, behavioural change, longest follow‐up, Outcome 2 Physical activity length.
5.3. Analysis.
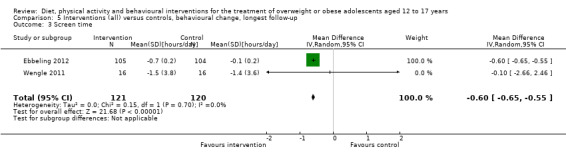
Comparison 5 Interventions (all) versus controls, behavioural change, longest follow‐up, Outcome 3 Screen time.
Overall, physical activity from validated self‐reported measures did not substantially improve in either the intervention or control groups for increasing number of steps per day (Sigal 2014; Wengle 2011), amount per day (Debar 2012; Saelens 2002), intensity (Kong 2013), and metabolic equivalent for self‐reported physical activity (Debar 2012; Ebbeling 2012; Grey 2009; Kong 2014). See Table 7 for details.
Physical activity behaviour
Three trials (566 participants) reported behavioural change in relation to physical activity assessed by self‐reported measures (Grey 2009; Kong 2013; Pakpour 2015), or objective measures (Jelalian 2016). There was no substantial reduction in the hours spent viewing television for either the intervention or control groups in one trial (Kong 2013), while one trial showed a decrease in hours spent in sedentary behaviour in the intervention arm (Jelalian 2016). Two studies reported improvement in physical activity self‐efficacy in the intervention group at 12 months (Grey 2009; Pakpour 2015). See Table 8 for details.
7. Behavioural change ‐ physical activity behaviour.
| Study | Tool | Outcome | Findings |
| Jelalian 2016 | Sense Wear Mini monitor | Sedentary behaviour (percentage of time spent), mean ± SD | The CBT‐healthy lifestyle decreased percentage of time spent in sedentary behaviour (from 71.2 ± 11.5 at baseline to 65.0 ± 12.0 at 48 weeks). There was no change in the control group (from 69.1 ± 9.0 at baseline to 69.0 ± 9.04 at 48 weeks) |
| Pakpour 2015 | 5‐item Physical Exercise Self‐efficacy Scale | Physical exercise self‐efficacy | At 12 months, the motivational interview + parental involvement group had significantly (P = 0.003) higher confidence scores (6.68 ± 1.97; 118 participants) compared to the control group (5.39 ± 2.18; 115 participants). However, the motivational interview group scored significantly higher (6.75 ± 1.15; P = 0.01 113 participants) compared to the motivational interview + parental involvement group |
| Kong 2013 | 11‐item Planet Health Study Questionnaire | Television weekday viewing (hours/day), median, 95% CI | At 6 months, there was no change in the intervention arm (‐0.4, ‐1.0 to 0.2; 14 participants) or the control group (0.2, ‐0.3 to 0.6; 8 participants) |
| Television weekend viewing (hours/day) | At 6 months, there was no change in the intervention arm (‐0.1, ‐0.8 to 0.0; 14 participants) or the control (0.5, ‐2.0 to 1.5, 8 participants) | ||
| Grey 2009 | Health Behaviour Questionnaire (5‐item Physical Activity Self‐Efficacy Scale) | Physical activity self‐efficacy | At 12 months, health behaviour in terms of physical activity self‐efficacy did not change in either the coping skills training group (0.6, 0.0 to 1.3; 112 participants) or the general education group (0.3, ‐0.3 to 1.0; 86 participants) |
CBT: cognitive behavioural therapy; CI: confidence intervals; MD: mean difference; SD: standard deviation
Participants' views of the intervention
Eight trials reported participants' views of the multidisciplinary intervention (Brennan 2013; Christie 2011; Debar 2012; Jelalian 2016; Nguyen 2012; Pbert 2013; Saelens 2002; Wengle 2011). Participants reported high levels of satisfaction for the components (Jelalian 2016; Nguyen 2012; Saelens 2002), services (Debar 2012), and overall quality (Brennan 2013) of multidisciplinary interventions. Similarly, participants were satisfied with the person delivering the intervention whether it was a nurse (Debar 2012), mentor (Pbert 2013; Wengle 2011), or clinician (Brennan 2013). Overall, participants thought that a multidisciplinary weight‐related programme met their needs (Debar 2012), and in terms of comfort in discussing their behaviour (Pbert 2013), and ability to focus and deal with their concerns (Brennan 2013), and helped them to make behavioural changes (Christie 2011; Nguyen 2012). See Table 9 for details.
8. Views of the intervention.
| Study | Tool | Outcome | Findings |
| Jelalian 2016 | Session Evaluation Form ‐ 4‐point Likert scale from 1 = strongly agree to 4 = strongly disagree | Application (I will be able to apply what I learned from this session in my life), % | CBT‐healthy lifestyle 75% (18 participants) agreed or strongly agreed Control 77.8% (7 participants) agreed or strongly agreed |
| Relevant (topic of this session was relevant to my life), % | CBT‐healthy lifestyle 83.3% (20 participants) agreed or strongly agreed Control 100% (9 participants) agreed or strongly agreed |
||
| Comfortable (I felt comfortable participating in this session), % | CBT‐healthy lifestyle 62.5% (15 participants) agreed or strongly agreed Control 88.9% (8 participants) agreed or strongly agreed |
||
| Helpful (session was helpful to me), % | CBT‐healthy lifestyle 70.8% (17 participants) agreed or strongly agreed Control 88.9% (8 participants) agreed or strongly agreed |
||
| Pbert 2013 | Patient Exit Interview Survey and nurse checklist | % of visits in which the students thought that the school nurse was very helpful in their learning how to eat healthy and be physically active, (%) | At 6 months, a higher proportion of participants in the intervention group (98%) thought the school nurse was very helpful compared to the control group (71%) (P= 0.003) |
| % of visits in which the students thought that they feel very comfortable in discussing their weight‐related behaviours with the nurse, (%) | At 6 months, a higher proportion of participants in the intervention group (88%) felt more comfortable with the nurse compared to the control group (62%) (P = 0.01) | ||
| Brennan 2013 | CHOOSE HEALTH Consumer satisfaction survey ‐ 5‐point Likert scale ranging from 1 = strongly disagree to 5 = strongly agree, with a higher score indicating higher treatment acceptability | Satisfaction with Quality of Service (mean ± SD) | 4.71 ± 0.47 for 70% of adolescents in the intervention arm |
| Programme met needs (mean ± SD) | 4.06 ± 0.75 for 70% of adolescents in the intervention arm | ||
| Recommend to others (mean ± SD) | 4.35 ± 0.79 for 70% of adolescents in the intervention arm | ||
| Return to the programme if needed (mean ± SD) | 3.81 ± 1.05 for 70% of adolescents in the intervention arm | ||
| Now able to deal more effectively with concerns (mean ± SD) | 4.06 ± 0.56 for 70% of adolescents in the intervention arm | ||
| Able to focus on my concerns (mean ± SD) | 4.00 ± 0.35 for 70% of adolescents in the intervention arm | ||
| Clinician listened to me (mean ± SD) | 4.24 ± 0.56 for 70% of adolescents in the intervention arm | ||
| Involved in treatment planning/decision making (mean ± SD) | 4.29 ± 0.47 for 70% of adolescents in the intervention arm | ||
| Clinician provided adequate explanations (mean ± SD) | 4.41 ± 0.62 for 70% of adolescents in the intervention arm | ||
| Clinician was not negative towards us (mean ± SD) | 4.59 ± 1.00 for 70% of adolescents in the intervention arm | ||
| Clinician knew what she was talking about (mean ± SD) | 4.71 ± 0.47 for 70% of adolescents in the intervention arm | ||
| Clinician was friendly and warm (mean ± SD) | 4.64 ± 0.49 for 70% of adolescents in the intervention arm | ||
| Felt free to express myself (mean ± SD) | 4.41 ± 0.80 for 70% of adolescents in the intervention arm | ||
| Clinician understood my thoughts and feelings (mean ± SD) | 4.25 ± 0.86 for 70% of adolescents in the intervention arm | ||
| Debar 2012 | 1 to 5 Likert scale, 5 = excellent) | Rating of intervention services (mean ± SD) | 4.4 ± 0.8 |
| 1 to 5 Likert scale, 5 = defiantly met their needs) | Programme met their needs (mean ± SD) | 4.0 ± 1.0 | |
| Nguyen 2012 | 7‐point Likert scales, 1 = poor; 3 = fair; 5 = good; 7 = excellent | Programme quality rating (median, IQR) | At 24 months, 5, 5 to 6; 93 participants |
| 7‐point Likert scales, 1 = not at all; 4 = moderately; 7 = extremely | Satisfaction with the amount of help received during the programme (median, IQR) | At 24 months, 6, 5 to 6; 93 participants | |
| Yes or no (%) | Did the Loozit programme help you make changes to your eating habits? (% yes) | At 24 months, yes: 88%; 90 participants | |
| Yes or no (%) | Did the Loozit programme help you make changes to your physical activity? (% yes) | At 24 months, yes: 82%; 92 participants | |
| Yes or no (%) | Did the Loozit programme help you make changes to other areas of your life? (% yes) | At 24 months, yes: 47%; 92 participants | |
| A 7‐point Likert scales, 1 = not at all; 4 = moderately; 7 = extremely | Additional therapeutic contact: SMS text messages (median, IQR) | At 24 months, 4, 4 to 5; 18 participants | |
| A 7‐point Likert scales, 1 = not at all; 4 = moderately; 7 = extremely | Additional therapeutic contact: e‐mails (median, IQR) | At 24 months, 5, 5 to 6; 11 participants | |
| A 7‐point Likert scales, 1 = not at all; 4 = moderately; 7 = extremely | Additional therapeutic contact: telephone coaching sessions (median, IQR) | At 24 months, 6, 4 to 6; 24 participants | |
| Yes or no (%) | Did the telephone coaching sessions help you: achieve your goals? (% yes) | Yes: 91%; 33 participants | |
| Yes or no (%) | Did the telephone coaching sessions help you: set goals for healthy living? (% yes) | Yes: 94%; 33 participants | |
| Yes or no (%) | Did the telephone coaching sessions help you: set goals for physical activity? (% yes) | Yes: 85%; 33 participants | |
| Yes or no (%) | Did the telephone coaching sessions help you: in other ways? (% yes) | Yes: 39%; 33 participants | |
| Christie 2011 | Structured and semi‐structured interviews | Ease of delivery, participation and influence on weight, quality of life, self‐management, emotional, behavioural and family functioning | Participants and their families found the intervention highly engaging, respectful and helpful in making behavioural changes |
| Wengle 2011 | Helpfulness of having a mentor on a scale of 1‐10 | Helpfulness of having a mentor | 13 participants reported ≥ 7/10 |
| Getting along with the mentor on a scale of 1‐10 | How well they got along with their mentor | 15 participants reported ≥ 8/10 | |
| Saelens 2002 | 5‐point Likert scale, 1 = not at all; 5 = very much | Satisfaction for intervention components (mean ± SD) | Reported significantly (P < 0.01) greater satisfaction for mailed materials/manual than the computer interaction. Reported similar levels of satisfaction between the mailed materials/manual (3.57 ± 1.13) and the physician counselling (3.39 ± 0.92), and between physician counselling and the computer interaction (2.98 ± 1.06) |
CBT: cognitive behavioural therapy; IQR: interquartile range; SD: standard deviation
Socioeconomic effects
None of the studies reported socioeconomic effects.
Parenting and relationships
Only one trial reported parent‐adolescent communication (Brennan 2013). There was no substantial difference between adolescents in the intervention and control groups for parent‐adolescent negative, problematic and supportive communication measures (see Table 10). However, participants in the intervention group reported higher positive communication with parents compared to the control group (Brennan 2013).
9. Parenting and relationships.
| Study | Tool | Outcome | Findings |
| Brennan 2013 | Parent Adolescent Communication Scale designed to assess communication between parent and adolescents. Open communication provides an indication of the freedom of expression, understanding and satisfaction during family communications with high scores indicating positive communication | Open communication | At 6 months, intervention group had significantly increased scores compared to control group |
| Parent Adolescent Communication Scale designed to assess communication between parent and adolescents. Problem communication focuses on negative interaction styles and constraints in communication with high scores indicating negative communication | Problems in communication | At 6 months, there was no difference between the intervention group and the control group | |
| The Family Problem Solving Communication Index to measure problem solving and coping during family interactions. Incendiary indicates high levels of problematic communication that inflame a difficult interaction with high scores indicating high levels of problematic communication | Incendiary communication | At 6 months, there was no difference between the intervention group and control group | |
| The Family Problem Solving Communication Index to measure problem solving and coping during family interactions. Affirming communication indicates high levels of supportive communication styles that calm a difficult interaction with high scores indicating supportive communication | Affirming communication | At 6 months, there was no difference between the intervention group and control group |
Subgroup analyses
We performed a number of subgroup analyses to test the effects of different durations of follow‐up, duration of the interventions, different types of comparators, mode of delivery of the intervention, setting, type of intervention, psychological basis for the interventions and involvement of parents on the outcomes of BMI and BMI z score.
For duration of follow‐up, the studies were divided into three subgroups, six months' follow‐up, 12 months' follow‐up and 18 months' follow‐up or more. Some studies reported follow‐up at more than one time point and are included in the analyses. There were no subgroup differences regarding duration of follow‐up (Analysis 2.1; Analysis 6.1; Analysis 6.2). For change in BMI score (Analysis 2.2), combining studies with a duration of the intervention of six months or less and studies with a duration of the intervention of greater than six months indicated a statistically significant subgroup difference (P = 0.02).
2.1. Analysis.
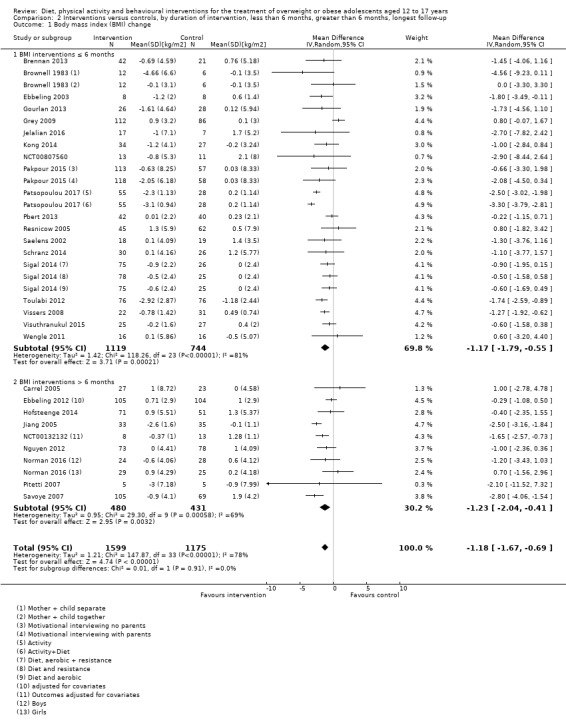
Comparison 2 Interventions versus controls, by duration of intervention, less than 6 months, greater than 6 months, longest follow‐up, Outcome 1 Body mass index (BMI) change.
6.1. Analysis.
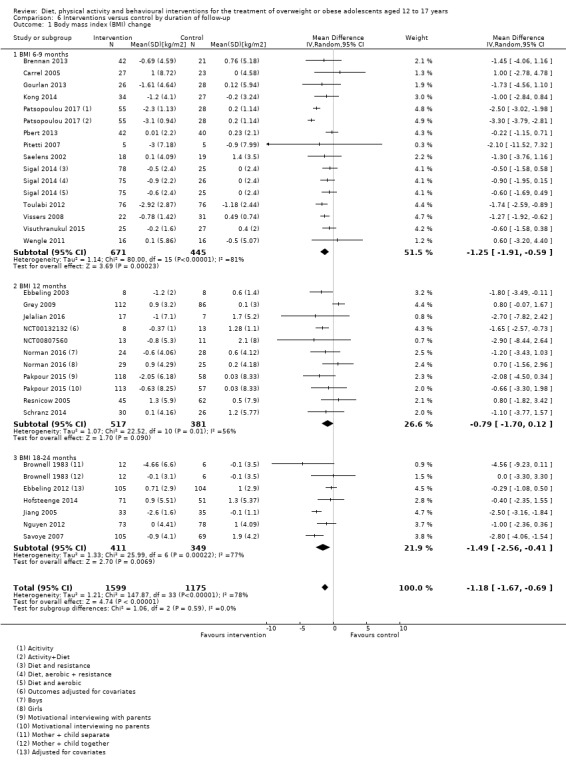
Comparison 6 Interventions versus control by duration of follow‐up, Outcome 1 Body mass index (BMI) change.
6.2. Analysis.
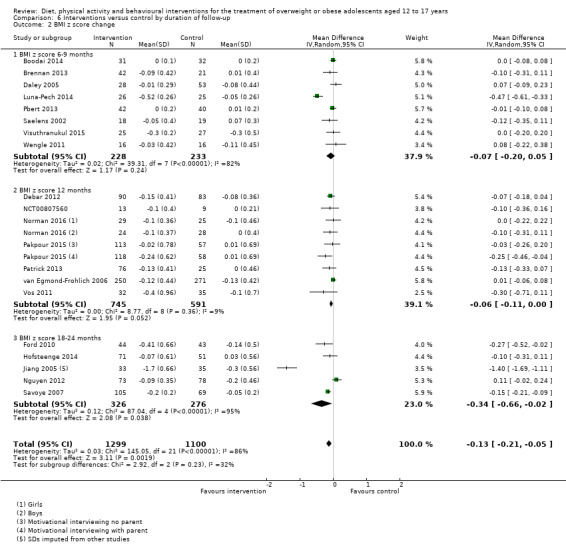
Comparison 6 Interventions versus control by duration of follow‐up, Outcome 2 BMI z score change.
2.2. Analysis.
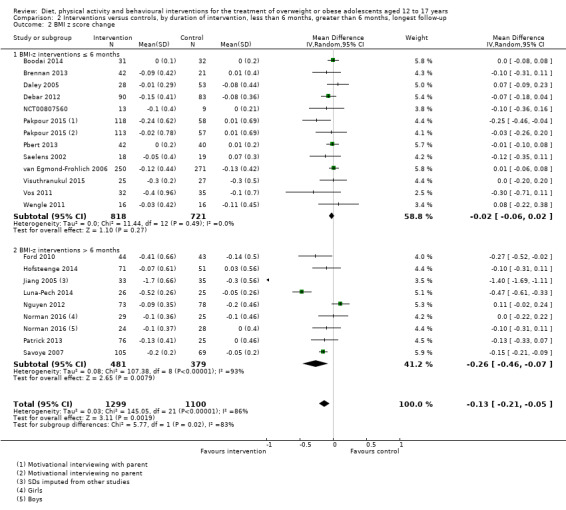
Comparison 2 Interventions versus controls, by duration of intervention, less than 6 months, greater than 6 months, longest follow‐up, Outcome 2 BMI z score change.
Studies were furthermore divided by whether they had a period of postintervention follow‐up (defined as the period after the active intervention and up to the final measurement) and the duration of that period: no postintervention follow‐up, less than six months, six months to less than 12 months and postintervention follow‐up lasting 12 months or longer. The duration of no postintervention follow‐up was calculated by subtracting the active intervention period from the total duration of the study (i.e. intervention and all follow‐up duration). There were no subgroup differences for BMI (Analysis 7.1). For BMI z score, there was a statistically significant subgroup difference (P = 0.007). The effect size for BMI z score appeared greater in studies with a postintervention follow‐up lasting 12 months or longer (Analysis 7.2).
7.1. Analysis.
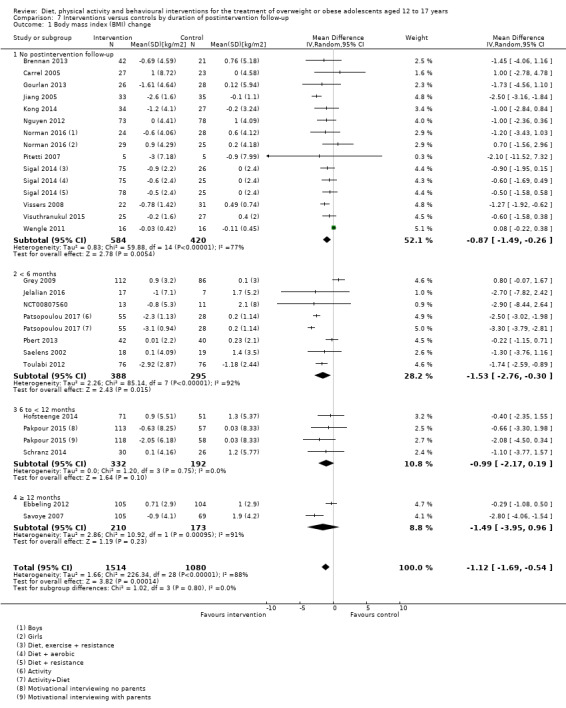
Comparison 7 Interventions versus controls by duration of postintervention follow‐up, Outcome 1 Body mass index (BMI) change.
7.2. Analysis.
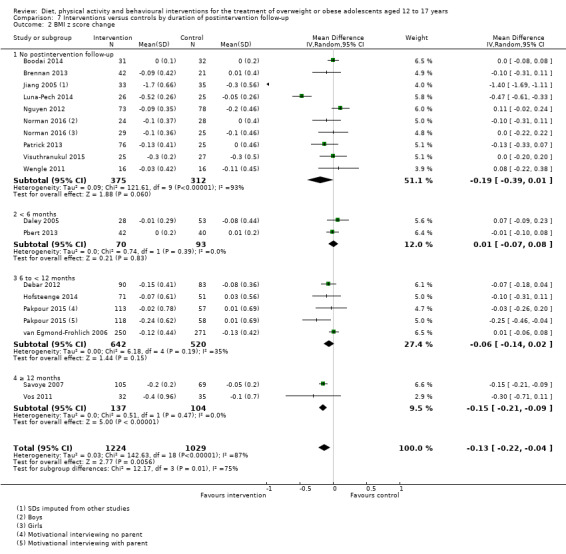
Comparison 7 Interventions versus controls by duration of postintervention follow‐up, Outcome 2 BMI z score change.
When outcomes from studies with comparators described as no intervention or wait list control, usual care or a concomitant therapy were combined, the effect sizes for BMI change appeared larger for studies with no intervention or wait list control comparators and for studies with usual care controls (P = 0.008; Analysis 8.1).
8.1. Analysis.
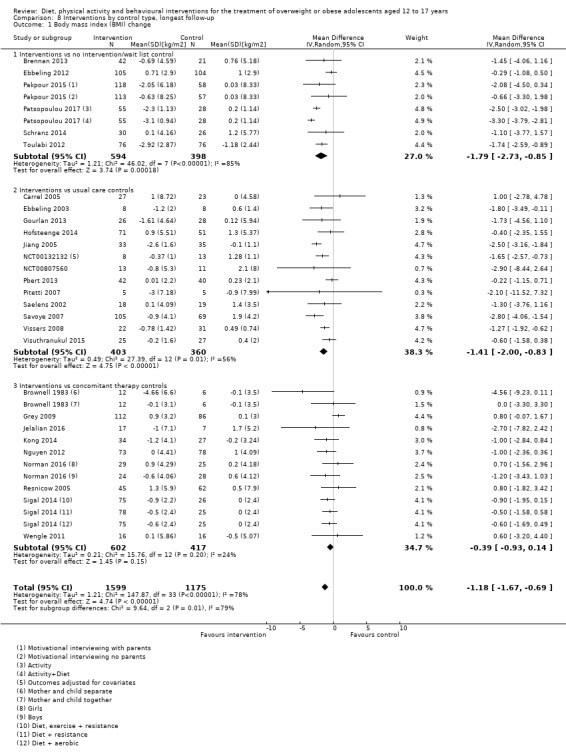
Comparison 8 Interventions by control type, longest follow‐up, Outcome 1 Body mass index (BMI) change.
Studies were divided into three groups to test the effect of the mode of delivery of the intervention: group, individual and group plus individual delivery. There were no subgroup differences (Analysis 9.1; Analysis 9.2).
9.1. Analysis.
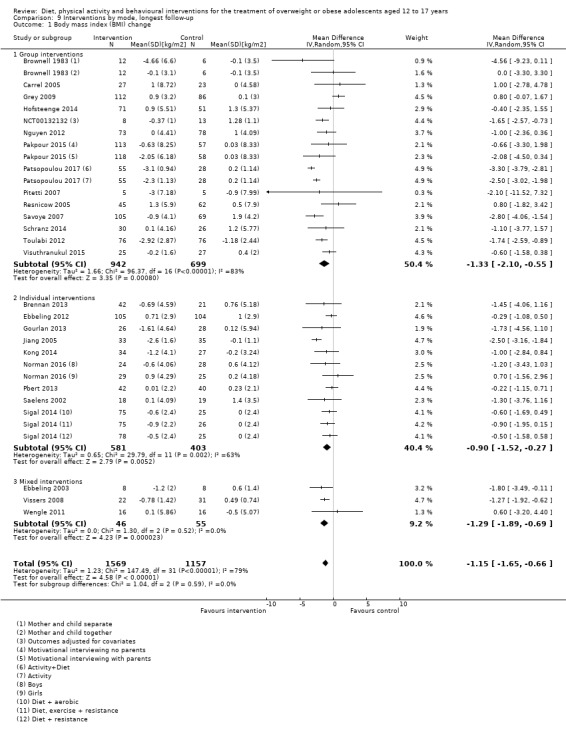
Comparison 9 Interventions by mode, longest follow‐up, Outcome 1 Body mass index (BMI) change.
9.2. Analysis.
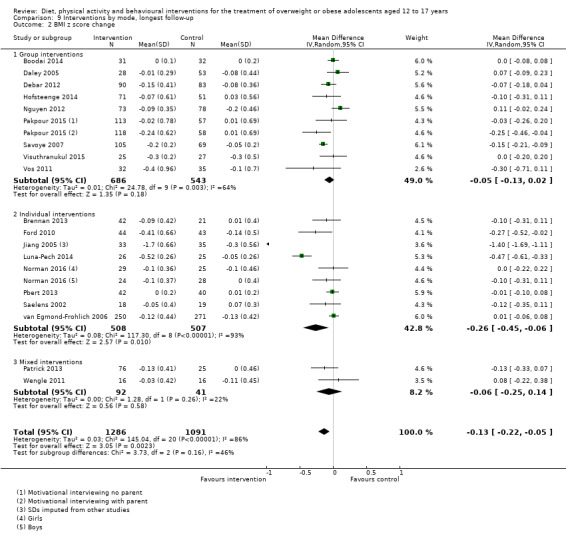
Comparison 9 Interventions by mode, longest follow‐up, Outcome 2 BMI z score change.
For setting, the studies were divided into three subgroups, school, community and healthcare settings. There were no subgroup differences (Analysis 10.1; Analysis 10.2).
10.1. Analysis.
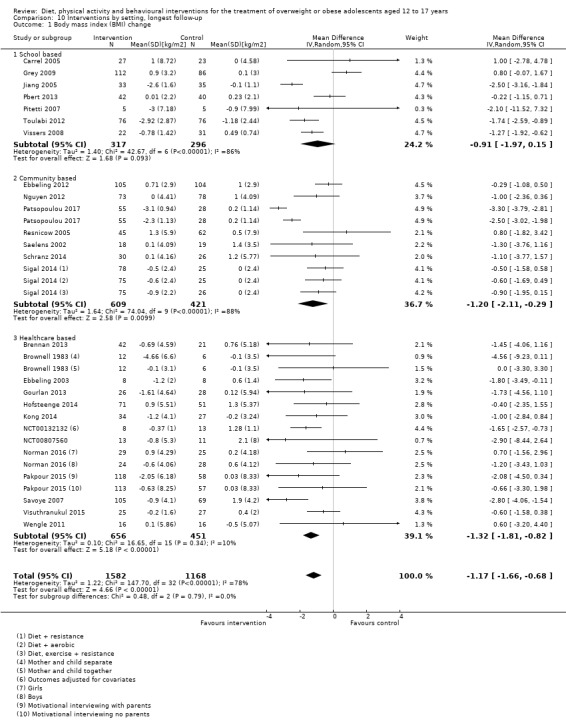
Comparison 10 Interventions by setting, longest follow‐up, Outcome 1 Body mass index (BMI) change.
10.2. Analysis.
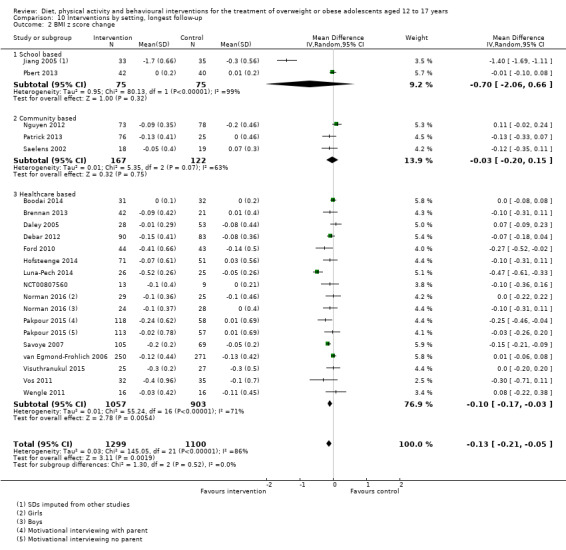
Comparison 10 Interventions by setting, longest follow‐up, Outcome 2 BMI z score change.
The majority of studies were multidisciplinary interventions; however, some studies focused on diet alone or physical activity interventions alone. There were no subgroup differences (Analysis 11.1; Analysis 11.2).
11.1. Analysis.
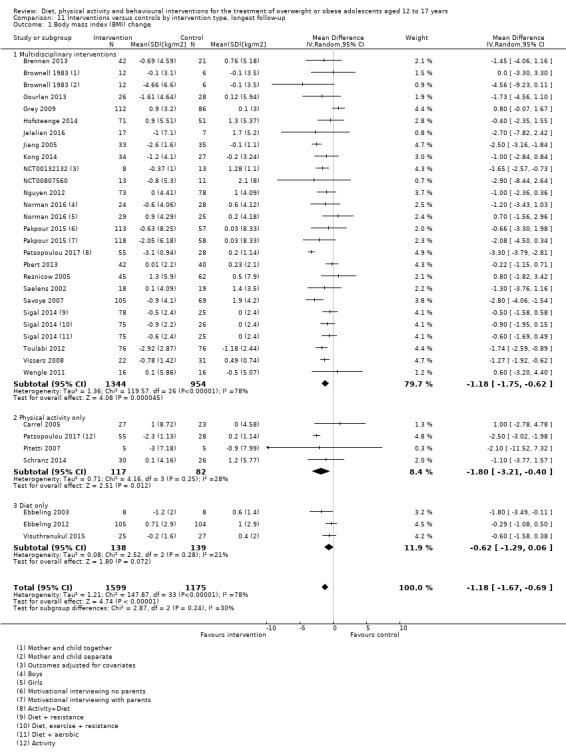
Comparison 11 Interventions versus controls by intervention type, longest follow‐up, Outcome 1 Body mass index (BMI) change.
11.2. Analysis.
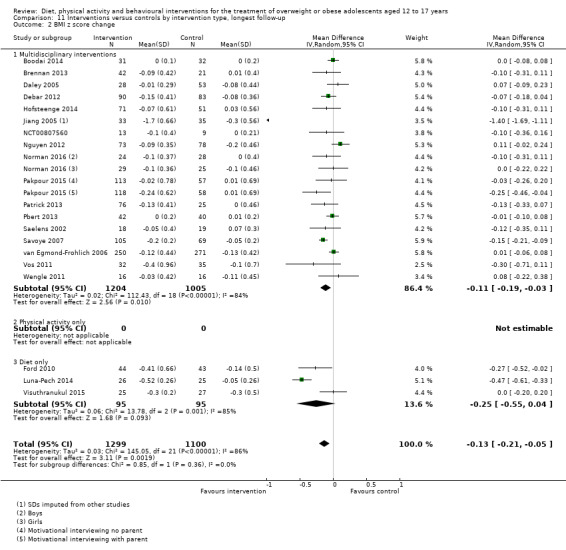
Comparison 11 Interventions versus controls by intervention type, longest follow‐up, Outcome 2 BMI z score change.
Studies were categorised into four groups according to any theoretical basis for the intervention. These were those applying cognitive behavioural techniques, those applying motivational interviewing techniques, those reporting any other psychological technique and those not reporting any theoretical basis to the intervention or no psychological component to the intervention (BMI: Analysis 12.1; BMI z score: Analysis 12.2). For BMI change, there was a subgroup difference (P = 0.09).
12.1. Analysis.
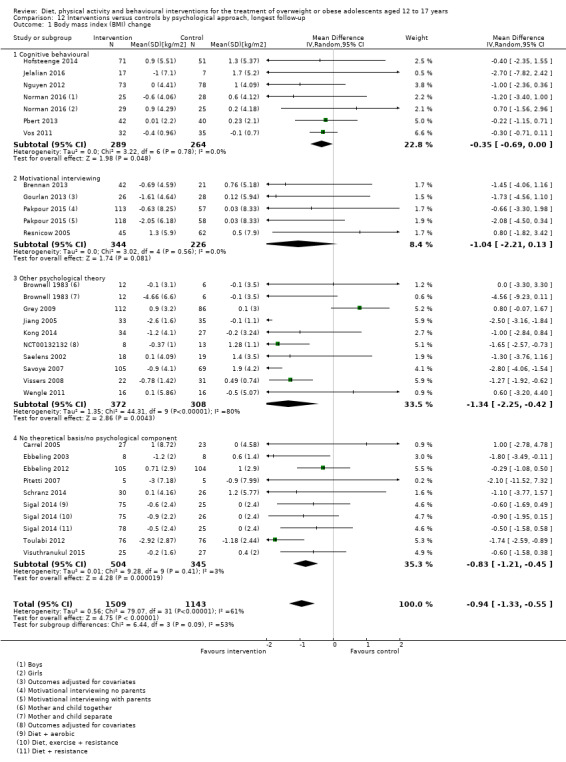
Comparison 12 Interventions versus controls by psychological approach, longest follow‐up, Outcome 1 Body mass index (BMI) change.
12.2. Analysis.
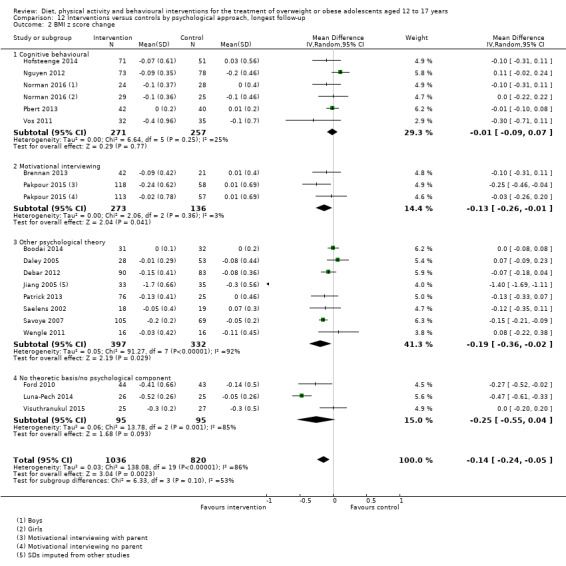
Comparison 12 Interventions versus controls by psychological approach, longest follow‐up, Outcome 2 BMI z score change.
A number of studies involved parents in their interventions while others did not explicitly provide any intervention to parents. There were no subgroup differences (Analysis 13.1; Analysis 13.2).
13.1. Analysis.
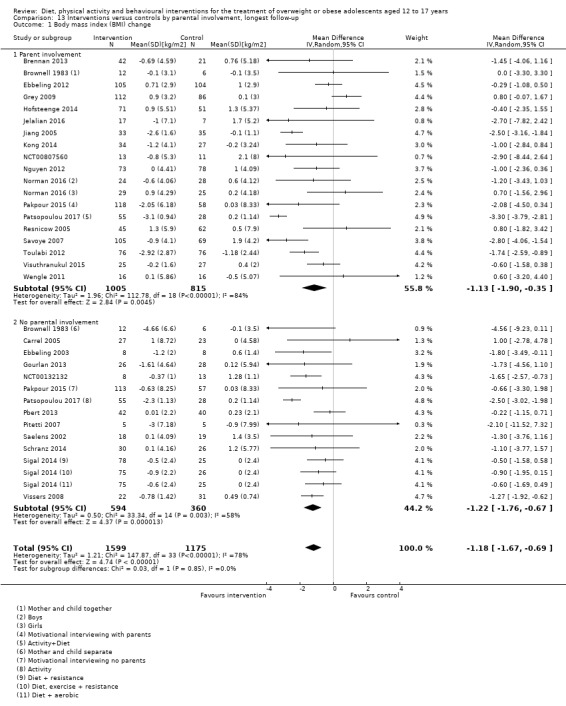
Comparison 13 Interventions versus controls by parental involvement, longest follow‐up, Outcome 1 Body mass index (BMI) change.
13.2. Analysis.
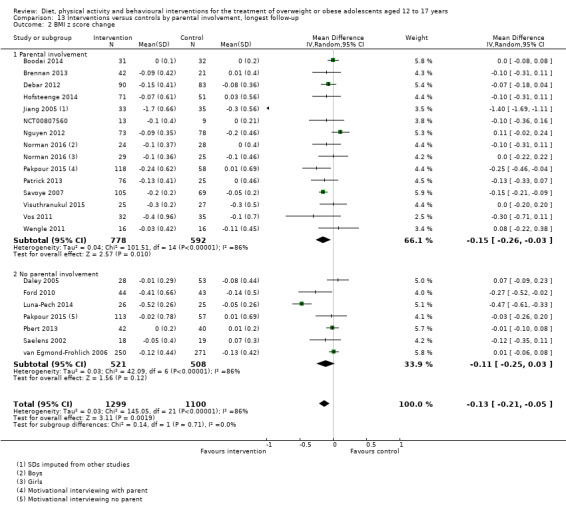
Comparison 13 Interventions versus controls by parental involvement, longest follow‐up, Outcome 2 BMI z score change.
We performed subgroups analyses to test the effects of different tools of self‐reported measures and different outcomes reported. For health‐related quality of life, studies were divided into two subgroups, studies using the Pediatric Quality of Life (PedsQL) tool and studies using other self‐reported tools. There were no subgroup differences (Analysis 4.4).
4.4. Analysis.
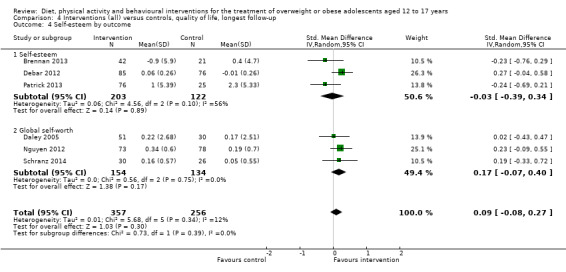
Comparison 4 Interventions (all) versus controls, quality of life, longest follow‐up, Outcome 4 Self‐esteem by outcome.
Studies measuring self‐esteem were divided into two subgroups, studies measuring total self‐esteem and studies measuring global self‐worth. There were no subgroup differences (Analysis 4.4; Analysis 4.5).
4.5. Analysis.
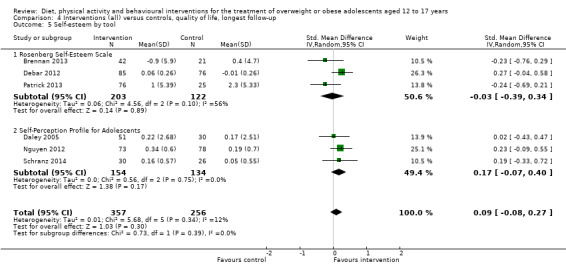
Comparison 4 Interventions (all) versus controls, quality of life, longest follow‐up, Outcome 5 Self‐esteem by tool.
Results of the subgroup analyses should be interpreted with caution given the large number of variable that we assessed.
Sensitivity analyses
We did not perform sensitivity analysis restricting to published studies as all included studies were published. We did not perform sensitivity analysis restricting studies to type of funding as there was no industrial funding influence. We performed sensitivity analyses for the following factors: longest follow‐up with 20% or less attrition; longest follow‐up with 30% or less attrition; language of publication; study design; removal of one trial specifically aimed at adolescents with severe autism (Pitetti 2007); removal of one trial specifically aimed at overweight adolescents with asthma (Luna‐Pech 2014); removal of one trial specifically aimed at overweight or obese adolescents with depression (Jelalian 2016); removal of one trial where there was some uncertainty over the duration of follow‐up and no response was received to a request for clarification from the authors (Toulabi 2012), and removal of one study where SDs were imputed from other studies (Pitetti 2007). These sensitivity analyses were undertaken on both the BMI and BMI z score meta‐analyses as appropriate to each study.
For the sensitivity analysis testing the effect of attrition, results did not differ between comparisons for either sensitivity analyses when compared with the main summary effects for studies with 20% or less attrition at the longest follow‐up: BMI (MD ‐1.34 kg/m2 (95% CI ‐2.05 to ‐0.63); P = 0.0002; 1486 participants; 15 studies; 18 comparisons); BMI z score (MD ‐0.22 (95% CI ‐0.39 to ‐0.05); P = 0.01; 951 participants; 11 studies; 11 comparisons) and for studies with 30% or less attrition at the longest follow‐up: BMI (MD ‐1.07 kg/m2 (95% CI ‐1.62 to ‐0.51); P = 0.0002; 2135 participants; 20 studies; 25 comparisons); BMI z score (MD ‐0.18 (95% CI ‐0.32 to ‐0.05); P = 0.008; 1066 participants; 13 studies; 13 comparisons). Heterogeneity for these sensitivity analyses did not vary consistently when compared with the heterogeneity seen in the main analyses (20% or less attrition:BMI: I2 = 72%; BMI z score: I2 = 91%; 30% or less attrition: BMI: I2 = 77%; BMI z score: I2 = 90%). Therefore, we included all studies regardless of rates of attrition in the analyses and subgroup.
Sensitivity analyses on the primary analyses for BMI and BMI z score removing individual trials did not have a considerable impact on the results and these trials were retained in the analyses. The removal of the study by van Egmond‐Frohlich 2006 (published in German) slightly increased the BMI z score (MD ‐0.14 (95% CI ‐0.23 to ‐0.05); P = 0.002; 1878 participants; 19 studies; 21 comparisons), but heterogeneity remained the same (I2 = 86%). The removal of the study by Pitetti 2007 from the BMI analysis did not change the MD or the CIs (MD ‐1.18 (95% CI ‐1.67 to ‐0.69); P < 0.00001; 2764 participants; 27 studies; 33 comparisons). The removal of the study by Jelalian 2016 changed BMI MD and CIs by one second decimal point only (MD ‐1.17 kg/m2 (95% CI ‐1.66 to ‐0.68); P < 0.00001; 2750 participants; 27 studies; 33 studies). The removal of the study by Toulabi 2012 reduced the MD in BMI slightly and narrowed the CIs slightly (MD ‐1.15 kg/m2 (95% CI ‐1.66 to ‐0.64); P < 0.0001; 2622 participants; 27 studies; 33 comparisons) but the result remained statistically significant with heterogeneity of I2 = 78%. Removing the study by Luna‐Pech 2014 from the BMI z score meta‐analysis reduced the MD slightly and narrowed the CIs slightly (MD ‐0.11 (95% CI ‐0.19 to ‐0.03); P = 0.006; 2348 participants; 19 studies; 21 comparisons) but the result remained statistically significant and only reduced the heterogeneity slightly to I2 = 83%. One study did not report measures of variance for the BMI z score (Jiang 2005), and we imputed the SDs from another study. Removing this study from the meta‐analysis reduced the effect size and reduced the 95% CIs; however, the direction of effect remained the same (MD ‐0.08 (95% CI ‐0.14 to ‐0.02); P = 0.007; 2331 participants; 19 studies; 21 comparisons) and reduced heterogeneity but remained considerable (I2 = 70%).
The removal of cluster RCTs where it was unclear if the effect of clustering had been taken account of in the analysis (Grey 2009; Pbert 2013) from the BMI analysis increased the MD (MD ‐1.34 (95% CI ‐1.80 to ‐0.89); P < 0.00001; 26 studies; 32 comparisons; 2494 participants) but heterogeneity remained highly significant (I2 = 71%).
Assessment of reporting bias
We generated a funnel plot for the primary outcome of BMI as this analysis included the highest number of studies on which to assess publication bias. Inspection of the funnel plot suggested the possibility of a small‐study bias (Figure 4).
4.
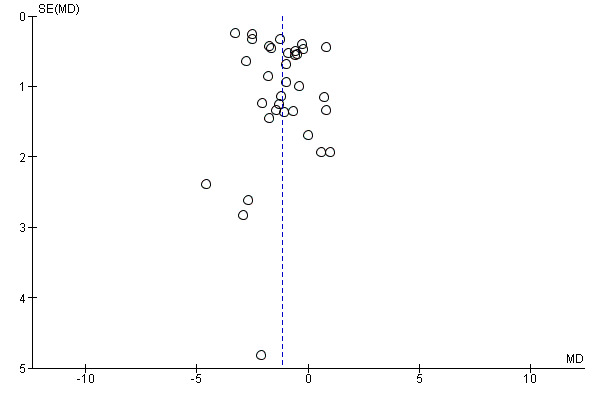
Funnel plot of comparison: 1 Interventions (all) versus controls, longest follow‐up, outcome: 1.1 BMI change (kg/m2)
Ongoing studies
We found 50 ongoing RCTs (see Characteristics of ongoing studies table). Forty‐four of these RCTs are parallel trials, two are cluster trials, two are cross‐over trials, one is a factorial trial and in one the design is unclear. Eight trials are three‐arm trials and one is a four‐arm trial. The ages of the participants in these trials incorporate the range of five to 18 years in 43 trials and are not clearly reported in seven trials. In one trial, the target population is classed as overweight according to Cole international cut‐offs for child obesity, in 22 trials the target population are children classed as obese (using different methods), in 23 trials the population is described as overweight or obese and in four trials the classification is unclear.
Six trials include a physical activity intervention, four trials include a nutritional intervention, three trials include dietary supplements, 19 trials include a behavioural intervention and 21 trials include a multicomponent intervention.
Thirty‐four trials compare the intervention to usual care or no intervention, 12 compare to a concomitant therapy also delivered in the intervention arm, one trial compares to wait list control, two trials compare the intervention to placebo and in one trial the control is not clearly defined. BMI was the primary outcome (BMI, BMI z score, BMI‐SDS, BMI percentile) in 36 trials and secondary outcome in five trials. Weight change ass the primary outcome in four trials and the secondary outcome in one trial. Two trials did not state if weight change was the primary or secondary outcome and body composition (unclear what parameters) are the secondary outcome in one trial and other outcome in one trial. The estimated study completion dates, where reported, range from March 2011 to December 2021.
Discussion
Summary of main results
This systematic review summarised 44 RCTs examining the effect of behaviour changing interventions for treating overweight and obesity in adolescents aged 12 to 17 years. We only included trials with at least a six‐month outcome assessment with the aim of assessing the longer‐term effects of these types of interventions. Interventions and comparators varied between the included trials. Outcomes assessed also varied between groups; although all trials reported a measure of body weight, the most commonly reported measures were BMI and BMI z score. To allow comparisons across trials, we analysed outcome data at the longest follow‐up period. Many trials did not report adequate information to assess the risk of bias and 27 trials were at high risk of bias on at least one domain.
Overall, behaviour changing interventions were more successful than the comparators in reducing BMI (low quality evidence), BMI z score (low quality evidence) and weight (moderate quality evidence) in overweight and or obese adolescents. The effects of behaviour changing interventions were maintained at 18 to 24 months' follow‐up for both BMI and BMI z score.
There were subgroup differences showing larger effects for both BMI and BMI z score where comparison groups were described as no intervention/wait list control or usual care compared to those testing concomitant interventions delivered to both the intervention and control group (P = 0.008). There were no subgroup differences between interventions with and without parental involvement or by intervention type or setting (health care, community, school) or mode of delivery (individual versus group).
Adverse events were reported in only five trials (low quality evidence). Minor adverse events occurred in one four‐arm study, but there were no details in the others.
Secondary outcomes were reported less consistently across trials. Overall, behaviour changing interventions were more successful than the comparators in reducing percentage of body fat, waist circumference and total fat mass. Fewer trials measured quality of life and self‐esteem. Behaviour changing interventions improved quality of life at the longest follow‐up period (low quality evidence) but not self‐esteem.
Overall, trials measuring physical activity showed no improvement for objective and self‐reported activity although evidence was limited. However, two trials reported improvement in physical activity self‐efficacy at 12 months. Dietary intakes were inconsistently measured and reported across trials, and trials used different tools to assess dietary intake and reported outcomes using different units of measure. The majority of trials reported no substantial effect of behaviour changing interventions on dietary intake although again evidence was limited.
Overall completeness and applicability of evidence
This review identified 44 completed trials and 50 ongoing trials assessing the effect of diet, physical activity and behavioural interventions at six months or longer. The review included studies conducted in the US, Europe, Asia and the Middle East including participants of different ethnicity. Most trials included both genders except for two trials that were restricted to females and one trial was restricted to males. Over half of the trials followed the percentile range to diagnose overweight or obesity. The majority of interventions had a multidisciplinary approach which included a combination of behavioural, dietary and physical activity components. Most trials reported details of the intervention that were mainly carried out for six months or less. The theoretical approach behind the intervention varied across studies with five studies using only the cognitive behavioural approach and four studies using only motivational interviewing. The majority of studies used either a mixture or other psychological theories (e.g. social cognitive theory, social learning theory, theory of planned behaviour) or did not have a theoretical approach behind the intervention. Only five trials used a mixed delivery approach (both individual and group mode) while the majority used either an individual or group delivery mode of the intervention. Studies were mainly conducted in healthcare settings while fewer trials were conducted in schools.
All 44 trials reported measures of body weight but BMI and BMI z score were the commonly assessed outcomes. Adverse events were only reported in five trials and only one trial provided sufficient details of adverse events. Reports on quality of life, self‐esteem, diet, physical activity, views of the intervention, and parenting and relationships were less consistent across trials. There was heterogeneity in measurement tools, assessed outcomes and unit of outcome measures. The duration of follow‐up varied across trials with most trials reporting outcomes at six to nine months and fewer trials reporting outcomes at two years. Overall, behaviour changing interventions have a favourable effect on measures of body weight including BMI, BMI z score and weight change in adolescents. We found no results for our outcomes of morbidity, mortality or socioeconomic effects of intervention.
Quality of the evidence
We rated the quality of evidence for the outcomes of interest in this review as low with the exception of weight which was moderate (Table 1). We judged the risk of bias for many individual studies to be unclear or high for a number of domains, but we found the impact of bias on the results for BMI and weight outcomes to be limited due to the influence of low risk of bias studies in the analysis. For some outcomes where studies had high loss to follow‐up, we saw a bigger effect size in sensitivity analyses. Anthropometric measures (BMI, BMI z score, weight) were downgraded for inconsistency (some variation in point estimates, poor overlap of CIs, significant statistical heterogeneity). Both BMI outcomes were downgraded further for indirectness due to the surrogacy of outcome measures (BMI and BMI z score). We maintained our decision to downgrade BMI outcomes for inconsistency even though we had some evidence that variation in effect could be explained by use of active controls in some studies. Within subgroup analysis heterogeneity remained high, indicating that there may be factors in addition to type of control intervention which give a credible explanation for inconsistency of effects across these outcomes.
Adverse events were downgraded due to risk of bias (inconsistency in reporting of data, reporting bias and attrition bias). Health‐related quality of life was downgraded for risk of bias (lack of blinding of outcome assessors) and inconsistency (variability in point estimates, overlap in CI and significant statistical heterogeneity). Therefore, results should be interpreted with caution.
Potential biases in the review process
We included four cluster randomised trials in this review but did not adjusted the data to account for the unit of randomisation due to their limited influence on the review findings. While our approach risks introducing a unit of analysis error, the impact of this decision was questionable for the outcomes affected. Most information in our review came from studies which randomised participants rather than clusters. Sensitivity analyses restricted to individually randomised trials provided some indirect evidence that supported our approach. Given that the removal of cluster trials did not change the results, calculating effective sample sizes would reduce their weight in the analysis. Therefore, it is unlikely that the nature of the results would alter with less weight accorded to the cluster trials.
The large number of subgroup analyses in our review carries inherent risks of multiplicity and the statistically significant results for subgroup analyses we have found could simply reflect a chance finding. This should be borne in mind when considering the applicability of the results to decision‐making. We conducted several other sensitivity analyses and found no significant differences. Where data of relevance were missing, either to allow assessment of eligibility, or at the data extraction stage and assessment of bias, we contacted the study authors for further information. Twelve trials are awaiting classification as information is currently unavailable to the review.
Agreements and disagreements with other studies or reviews
The UK National Institute for Health and Care Excellence (NICE) guidance emphasises the importance of behaviour changing weight management services in the treatment of overweight and obesity in young people under the age of 18 years (NICE 2013). However, the guidelines show that there is inconsistent evidence on the long‐term effect of such interventions. The short‐term effect of behaviour changing interventions on weight loss has been reported in a previous review (Wilfley 2007). Our results add to the evidence base and demonstrate that in trials with longer follow‐up at 18 to 24 months, weight loss is maintained. Group‐based interventions have been recommended by NICE and our review is consistent in that there were no differences between group‐based and individually delivered interventions.
Overall the findings of our review are consistent with previous systematic reviews examining the effect of behaviour changing interventions for the treatment of childhood obesity (Kelly 2008; Ruotsalainen 2015; Whitlock 2010). Several reviews have questioned the optimal length of behaviour changing interventions (Ho 2012; Kelly 2008; Wilfley 2007), we found that both short‐term interventions (less than six months) and long‐term interventions (greater than six months) have a favourable effect on BMI, although in subgroup analyses longer interventions seemed to produce a greater effect on BMI. We found inconsistent evidence on the effect of behaviour changing interventions on dietary intake as reported by one previous systematic review (Collins 2006). One previous review reported the beneficial effect of physical activity on quality of life (Penedo 2005), we found similar favourable effect of behaviour changing interventions on quality of life but not self‐esteem. The current review synthesises the most up‐to‐date and highest‐quality research available on the effectiveness of different types of behaviour changing interventions for treating overweight or obesity in adolescents aged 12 to 17 years on a large range of outcomes. Future updates will incorporate the large number of ongoing trials which may strengthen this evidence base.
Authors' conclusions
Implications for practice.
We explored the impact of different types of behaviour changing interventions on adolescents being overweight and obese. Overall, there is low‐to‐moderate quality evidence that behaviour changing interventions reduce measures of body weight including BMI, BMI z score and weight change in adolescents. Additionally, lifestyle interventions seem to reduce indicators of adiposity such as percentage of body fat, waist circumference and total fat mass. There is low quality evidence that behaviour changing interventions have a moderate effect on health‐related quality of life. There is inconclusive evidence on the effects of behaviour changing interventions on self‐esteem, dietary intake and physical activity in adolescents. Most of the evidence is from multicomponent interventions which include a combination of behavioural, dietary and physical activity interventions. We found no subgroup differences for type of intervention, behavioural approach, mode of delivery or setting. Similar levels of weight reduction are seen in trials with longer follow‐up periods of up to two years suggesting that weight loss is maintained in these studies. However, the results of this review should be interpreted with caution as most of the evidence was rated as low quality due to inconsistency, indirectness or risk of bias for many of the outcomes measured. The risk of adverse events could not be assessed reliably as they were not measured by most of the trials.
Implications for research.
This systematic review identified 50 ongoing trials of behaviour changing interventions targeting adolescent overweight and obesity and incorporation of the results of these when they become available may strengthen the evidence base. Further research is required to determine whether the interventions have any adverse effects. Economic data are needed and trials reporting the costs of behaviour changing interventions should be measured and reported. Consistent measures of health‐related quality of life, self‐esteem, dietary and physical activity outcomes using validated tools should be used in future research and reports should provide adequate and transparent methodological details such as allocation concealment, blinding, attrition, selective reporting and validity of tools. Higher quality trials are required as the current evidence is of low methodological quality.
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2017 | New search has been performed | This is an update of the former Cochrane Review 'Interventions for treating obesity in children and adolescents.' |
| 26 April 2017 | New citation required and conclusions have changed | Given the rapid growth in the treatment of child and adolescent obesity, we have split the original review ('Interventions for treating obesity in children and adolescents') into six separate reviews, with a specific intervention and age focus
|
History
Review first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 11 October 2008 | New citation required and conclusions have changed | This review concludes that combined behavioural lifestyle interventions compared to standard care or self‐help can produce a significant and clinically meaningful reduction in overweight in children and adolescents. The search was updated to May 2008. Some amendments were made to update the search strategies. No changes have been made to other aspects of the methodology. Forty‐six new studies have been included. These included information on drug interventions for treating obesity in adolescents. The added evidence suggests that lifestyle interventions appear to have positive effects in the treatment of child and adolescent obesity. Furthermore, orlistat and sibutramine were found to have beneficial effects on adiposity in obese adolescents. However, a range of adverse effects was noted. |
| 3 July 2008 | Amended | Converted to new review format. Authorship changed with new authors and new contact person. |
Notes
Part of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
We thank Drs Carrel, Christie, DeBar, Ebbeling, Ford, Grey, Ives, Krude, Makkes, Norman, Pbert, Pitetti, Savoye, Wengle and Wiegan, for providing additional information about their trials. We thank Ms Jennifer Cooper for her help with the screening. We thank the Cochrane Editorial Unit (Toby Lasserson) for reviewing this work. We thank the Metabolic and Endocrine Disorders Group editorial team (Bernd Richter) for extracting the German study. We thank Mr Graham Hewitt for translating a Spanish paper to confirm its status as excluded, Dr Karoline Freeman for translating a German publication to confirm its status as excluded, Dr Huayi Huang for translating a Chinese publication to confirm its status as excluded and Dr Soroush Abulfatehi for translating an Iranian publication to confirm its status as excluded. We acknowledge the editorial contributions by Gudrun Paletta and Bernd Richter (Cochrane Metabolic and Endocrine Disorders Group).
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
|
Part I: Obesity 1. [mh ^Obesity] 2. [mh ^"Obesity, Morbid"] 3. [mh ^"Obesity, Abdominal"] 4. [mh ^"Pediatric Obesity"] 5. [mh ^Overweight] 6. [mh ^"Weight Loss"] 7. (adipos* or obes*):ti,ab 8. (overweight* or ("over" next weight*)):ti,ab 9. ("weight" near/1 (reduc* or los* or control* or manage*)):ti,ab 10. {or #1‐#9} Part II: Intervention 11. [mh "Behavior Therapy"] 12. [mh "Counseling"] 13. [mh ^"Family Therapy"] 14. [mh ^"Social Support"] 15. [mh ^"Program Evaluation"] 16. [mh "Exercise"] 17. [mh "Exercise Therapy"] 18. [mh "Physical Education and Training"] 19. [mh "Exercise Movement Techniques"] 20. [mh ^"Motor Activity"] 21. [mh Diet] 22. [mh "Diet Therapy"] 23. [mh ^"Patient Education as Topic"] 24. [mh ^"Health Education"] 25. [mh "Health Behavior"] 26. [mh "Health Promotion"] 27. [mh ^"School Health Services"] 28. [mh ^"School Nursing"] 29. [mh ^"Life style"] 30. (("obesity" near/4 "intervention") or "program" or "programme" or "camp" or "camps"):ti,ab 31. ("lifestyle" or "life style"):ti,ab 32. exercis*:ti,ab 33. (physic* next (activ* or fit*)):ti,ab 34. (walk* or jog* or swim* or ("weight" next lift*) or danc* or "aerobics"):ti,ab 35. ((physic* or strength* or resist* or "circuit" or "weight" or aerob* or "cross" or "endurance" or structur*) near/4 train*):ti,ab 36. ("behavioral" or "behavioural" or (("behavior" or "behaviour") next "modification") or psychoth* or "psychosocial"):ti,ab 37. (("group" or "family" or cognit* or behav*) next therap*):ti,ab 38. (counseling or counselling):ti,ab 39. educat*:ti,ab 40. (("parent" or "parents" or "family") next ("based" or "focused" or "directed" or "centered" or "only" or "led")):ti,ab 41. (diet* or "healthy nutrition" or (nutrition* next ("knowledge" or educat* or therap* or program* or intervention*))):ti,ab 42. {or #11‐#41} Part III: Part I + Part II and additional MeSH/subheading combination 43. #10 and #42 44. [mh ^Obesity] or [mh ^"Obesity, Morbid"] or [mh ^Overweight] 45. [mh /DH,PC,RH,TH,PX][diet therapy or prevention & control or rehabilitation or therapy or psychology] 46. #44 and #45 47. #43 or #46 Part IV: Population (adapted fromLeclercq 2013) 48. [mh ^Adolescent] 49. [mh Child] 50. [mh ^Infant] 51. [mh ^Pediatrics] 52. "minors":ti,ab 53. ("boy" or "boys" or "boyhood"):ti,ab 54. girl*:ti,ab 55. ("kid" or "kids"):ti,ab 56. infant*:ti,ab 57. ("baby" or "babies"):ti,ab 58. ("toddler" or "toddlers"):ti,ab 59. ("child" or "childs" or children* or childhood* or childcare* or schoolchild*):ti,ab 60. adolescen*:ti,ab 61. juvenil*:ti,ab 62. youth*:ti,ab 63. (teen* or preteen*):ti,ab 64. (underage* or ("under" next age*)):ti,ab 65. pubescen*:ti,ab 66. (paediatric* or pediatric*):ti,ab 67. {or #48‐#66} Part V: Part III AND IV and additional MeSH/subheading combination 68. #47 and #67 69. [mh ^"Pediatric Obesity"] 70. [mh /DH,PC,RH,TH,PX] 71. #69 and #70 72. #68 or #71 |
| MEDLINE (OvidSP) |
|
Part I: Obesity 1. Obesity/ 2. Obesity, Morbid/ 3. Obesity, Abdominal/ 4. Pediatric Obesity/ 5. Overweight/ 6. Weight Loss/ 7. (adipos* or obes*).tw. 8. (overweight* or over weight*).tw. 9. (weight adj1 (reduc* or los* or control* or manage*)).tw. 10. or/1‐9 Part II: Intervention 11. exp Behavior Therapy/ 12. exp Counseling/ 13. Family Therapy/ 14. Social Support/ 15. Program Evaluation/ 16. exp Exercise/ 17. exp Exercise Therapy/ 18. exp "Physical Education and Training"/ 19. exp Exercise Movement Techniques/ 20. Motor Activity/ 21. exp Diet/ 22. exp Diet Therapy/ 23. Patient Education as Topic/ 24. Health Education/ 25. exp Health Behavior/ 26. exp Health Promotion/ 27. School Health Services/ 28. School Nursing/ 29. Life style/ 30. ((obesity adj3 intervention) or program or programme or camp?).tw. 31. (lifestyle or life style).tw. 32. exercis*.tw. 33. (physic* adj (activ* or fit*)).tw. 34. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 35. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 36. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 37. ((group or family or cognit* or behav*) adj therap*).tw. 38. counsel?ing.tw. 39. educat*.tw. 40. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 41. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 42. or/11‐41 Part III: Part I + Part II and additional MeSH/subheading combination 43. 10 and 42 44. Obesity/ or Obesity, Morbid/ or Overweight/ or Weight Loss/ 45. diet therapy.fs. or prevention & control.fs. or rehabilitation.fs. or therapy.fs. or psychology.fs. 46. 44 and 45 47. 43 or 46 Part IV: Population (adapted fromLeclercq 2013) 48. Adolescent/ 49. exp Child/ 50. Infant/ 51. Pediatrics/ 52. minors.tw. 53. (boy or boys or boyhood).tw. 54. girl*.tw. 55. infant*.tw. 56. (baby or babies).tw. 57. toddler?.tw. 58. (kid or kids).tw. 59. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 60. adolescen*.tw. 61. juvenil*.tw. 62. youth*.tw. 63. (teen* or preteen*).tw. 64. (underage* or under age*).tw. 65. pubescen*.tw. 66. p?ediatric*.tw. 67. or/48‐66 Part V: Part III AND IV and additional MeSH/subheading combination 68. 47 and 67 69. Pediatric Obesity/ 70. diet therapy.fs. or prevention & control.fs. or rehabilitation.fs. or therapy.fs. or psychology.fs. 71. 69 and 70 72. 68 or 71 Part VI: Study filter (Cochrane Handbook 2008 RCT filter ‐ sensitivity and precision maximizing version) 73. randomized controlled trial.pt. 74. controlled clinical trial.pt. 75. randomi?ed.ab. 76. placebo.ab. 77. clinical trials as topic/ 78. randomly.ab. 79. trial.ti. 80. or/73‐79 81. exp animals/ not humans/ 82. 80 not 81 Part VII: Part V + Part VI 83. 72 and 82 |
| Embase (OvidSP) |
|
Part I: Obesity 1. obesity/ 2. morbid obesity/ 3. abdominal obesity/ 4. childhood obesity/ 5. weight reduction/ 6. weight control/ 7. (adipos* or obes*).tw. 8. (overweight* or over weight*).tw. 9. (weight adj1 (reduc* or los* or control* or manage*)).tw. 10. or/1‐9 Part II: Intervention 11. behavior therapy/ 12. cognitive therapy/ 13. exp counseling/ 14. family therapy/ 15. social support/ 16. exp program evaluation/ 17. exp exercise/ 18. exp physical education/ 19. exp physical activity/ 20. exp motor activity/ 21. training/ 22. exp diet/ 23. exp diet therapy/ 24. nutritional health/ 25. child nutrition/ 26. feeding behavior/ 27. patient education/ 28. health promotion/ 29. health literacy/ 30. nutrition education/ 31. health education/ 32. school health education/ 33. school health service/ 34. lifestyle/ 35. lifestyle modification/ 36. ((obesity adj3 intervention) or program or programme or camp?).tw. 37. (lifestyle or life style).tw. 38. exercis*.tw. 39. (physic* adj (activ* or fit*)).tw. 40. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 41. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 42. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 43. ((group or family or cognit* or behav*) adj therap*).tw. 44. counsel?ing.tw. 45. educat*.tw. 46. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 47. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 48. or/11‐47 Part III: Part I + Part II and additional MeSH/subheading combination 49. 10 and 48 50. obesity/ or morbid obesity/ 51. pc.fs or rh.fs or th.fs. [prevention.fs. or rehabilitation.fs. or therapy.fs.] 52. 50 and 51 53. 49 or 52 Part IV: Population (adapted fromLeclercq 2013) 54. juvenile/ 55. adolescent/ 56. child/ 57. infant/ 58. baby/ 59. toddler/ 60. preschool child/ 61. school child/ 62. pediatrics/ 63. minors.tw. 64. (boy or boys or boyhood).tw. 65. girl*.tw. 66. infant*.tw. 67. (baby or babies).tw. 68. toddler?.tw. 69. (kid or kids).tw. 70. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 71. adolescen*.tw. 72. juvenil*.tw. 73. youth*.tw. 74. (teen* or preteen*).tw. 75. (underage* or under age*).tw. 76. pubescen*.tw. 77. p?ediatric*.tw. 78. or/54‐77 Part V: Part III AND IV and additional MeSH/subheading combination 79. 53 and 78 80. childhood obesity/ 81. pc.fs or rh.fs or th.fs. [prevention.fs. or rehabilitation.fs. or therapy.fs.] 82. 80 and 81 83. 79 or 82 Part VI: Study filter (Wong 2006afilter ‐ SDSSGS version) 84. random*.tw. or clinical trial*.mp. or exp treatment outcome/ Part VII: Part V + Part VI 85. 83 and 84 |
| PsycINFO (OvidSP) |
|
Part I: Obesity 1. exp Overweight 2. (adipos* or obes*).tw. 3. (overweight* or over weight*).tw. 4. or/1‐3 Part II: Intervention 5. Weight Control/ 6. Weight Loss/ 7. Aerobic Exercise/ 8. Diets/ 9. exp Exercise/ 10. Movement Therapy/ 11. Dance Therapy/ 12. exp Physical Activity/ 13. Physical Fitness/ 14. Health Behavior/ 15. Health Promotion/ 16. Health Knowledge/ 17. Health Literacy/ 18. Health Education/ 19. Client Education/ 20. Lifestyle/ 21. Physical Education/ 22. exp Program Evaluation/ 23. Educational Programs/ 24. Educational Therapy/ 25. exp Program Development/ 26. School Based Intervention/ 27. School Counseling/ 28. Counseling/ 29. Group Counseling/ 30. Family Therapy/ 31. Support Groups/ 32. Social Support/ 33. School Counselors/ 34. exp Behavior Modification/ 35. Cognitive Behavior Therapy/ 36. Cognitive Therapy/ 37. ((obesity adj3 intervention) or program or programme or camp?).tw. 38. (lifestyle or life style).tw. 39. exercis*.tw. 40. (physic* adj (activ* or fit*)).tw. 41. (walk* or jog* or swim* or weight lift* or danc* or aerobics).tw. 42. ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) adj3 train*).tw. 43. (behavio?ral or behavio?r modification or psychoth* or psychosocial).tw. 44. ((group or family or cognit* or behav*) adj therap*).tw. 45. counsel?ing.tw. 46. educat*.tw. 47. ((parent? or family) adj (based or focused or directed or centered or only or led)).tw. 48. (diet* or healthy nutrition or (nutrition* adj (knowledge or educat* or therap* or program* or intervention*))).tw. 49. or/5‐48 Part III: Part I + Part II 50. 4 and 49 Part IV: Population (adapted fromLeclercq 2013) 51. minors.tw. 52. (boy or boys or boyhood).tw. 53. girl*.tw. 54. infant*.tw. 55. (baby or babies).tw. 56. toddler?.tw. 57. (kid or kids).tw. 58. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 59. adolescen*.tw. 60. juvenil*.tw. 61. youth*.tw. 62. (teen* or preteen*).tw. 63. (underage* or under age*).tw. 64. pubescen*.tw. 65. p?ediatric*.tw. 66. or/51‐65 Part V: Part III AND IV and additional MeSH/subheading combination 67. 50 and 66 Part VI: Study filter (Eady 2008filter ‐ BS version) 68. control*.tw. OR random*.tw. OR exp Treatment/ Part VII: Part V + Part VI 69. 67 and 68 |
| CINAHL (EBSCOhost) |
|
Part I: Obesity S1. MH "Obesity+" S2. TX (adipos* or obes*) S3. TX (overweight* or "over weight*") S4. S1 OR S2 OR S3 Part II: Intervention S5. MH "Weight Loss" S6. MH "Behavior Modification+" S7. MH "Counseling" S8. MH "Family Therapy" S9. MH "Support, Psychosocial" S10.MH "Support Groups" S11.MH "Program Evaluation" S12.MH "Program Implementation" S13.MH "Exercise+" S14.MH "Sports+" S15.MH "Therapeutic Exercise+" S16.MH "Physical Fitness" S17.MH "Physical Education and Training+" S18.MH "Health Education+" S19.MH "Diet+" S20.MH "Diet Therapy+" S21.MH "Health Behavior" S22.MH "Eating Behavior" S23.MH "Health Promotion" S24.MH "School Health Services+" S25.MH "Life style changes" S26.MH "Life style" S27.TX (weight N1 (reduc* or los* or control* or manage*)) S28.TX ((obesity N3 intervention) OR program OR programme OR camp#) S29.TX (lifestyle or "life style") S30.TX exercis* S31.TX (physic* N1 (activ* or fit*)) S32.TX (walk* or jog* or swim* or weight lift* or danc* or aerobics) S33.TX ((physic* or strength* or resist* or circuit or weight or aerob* or cross or endurance or structur*) N3 train*) S34.TX (behavio#ral or behavio#r modification or psychoth* or psychosocial) S35.TX ((group or family or cognit* or behav*) N1 therap*) S36.TX counsel#ing S37.TX educat* S38.TX ((parent# or family) N1 (based or focused or directed or centered or only or led)) S39.TX (diet* or "healthy nutrition" or (nutrition* N1 (knowledge or educat* or therap* or program* or intervention*))) S40.S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 Part III: Part I + Part II and additional MeSH/subheading combination S41.S4 AND S40 S42.(MH "Obesity+/DH/ED/PC/PF/RH/TH") [diet therapy or education or prevention & control or psychosocial factors or rehabilitation or therapy] S43.S41 OR S42 Part IV: Population (based onLeclercq 2013I S44.MH "Adolescence" S45.MH "Child+" S46.MH "Infant" S47.MH "Pediatrics" S48.TX minors S49.TX (boy OR boys OR boyhood) S50.TX girl* S51.TX infant* S52.TX (baby OR babies) S53.TX toddler# S54.TX (kid OR kids) S55.TX (child OR childs OR children* OR childhood* OR childcare* OR schoolchild*) S56.TX adolescen* S57.TX juvenil* S58.TX youth* S59.TX (teen* or preteen*) S60.TX (underage* or under age*) S61.TX pubescen* S62.TX (paediatric* OR pediatric*) S63.S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S62 Part V: Part III AND IV and additional MeSH/subheading combination S64.S43 AND S63 S65.(MH "Pediatric Obesity/DH/ED/PC/PF/RH/TH") [diet therapy or education or prevention & control or psychosocial factors or rehabilitation or therapy] S66.S64 OR S65 Part VI: Study filter (Wong 2006bfilter ‐ SDSSGS version) S67.MH "treatment outcomes+" OR MH "experimental studies+" or random* Part VII: Part V + Part VI S68.S66 AND S67 |
| LILACS (IAHx) |
| ((((MH:"Obesity" OR MH:"Obesity, Morbid" OR MH:"Obesity, Abdominal" OR MH:"Pediatric Obesity" OR MH:"Overweight" OR adipos$ OR obes$ OR overweight$ OR "over weight" OR sobrepes$ OR "exceso de peso" OR "excesso de peso") AND (MH:"Weight Loss" OR MH:"Exercise" OR MH:"Exercise Therapy" OR MH:"Physical Education and Training" OR MH:"Exercise Movement Techniques" OR MH:"Weight Reduction Programs" OR MH:"Motor Activity" OR MH:"Behavior Therapy" OR MH:"Counseling" OR MH:"Family Therapy" OR MH:"Social Support" OR MH:"Program Evaluation" OR MH:"Diet" OR MH:"Diet Therapy" OR MH:"Patient Education as Topic" OR MH:"Health Education" OR MH:"Health Behavior" OR MH:"Health Promotion" OR MH:"Weight Reduction Programs" OR MH:"School Health Services" OR MH:"Life style" OR exerci$ OR ejerci$ OR ((physic$ OR fisic$) AND (activ$ OR ativid$ OR fit$ OR educac$ OR entrenam$ OR treinam$)) OR ((physic$ OR fisic$ OR strength$ OR forca OR fuerza OR resist$ OR circuit$ OR weight OR aerob$ OR endurance OR structur$ OR estructur$) AND train$ OR treina$ OR entrena$) OR program$ OR "estilo de vida" OR padres OR pais OR familia OR familias OR familiar OR terapia OR orienta$ OR educa$ OR diet$ OR nutric$ OR "weight reduction" OR "weight loss" OR "weight control" OR "control de peso")) OR (MH:"Obesity/diet therapy" OR MH:"Obesity, Morbid/diet therapy" OR MH:"Overweight/diet therapy" OR MH:"Obesity/prevention & control" OR MH:"Obesity, Morbid/prevention & control " OR MH:"Overweight/prevention & control" OR MH:"Obesity/rehabilitation" OR MH:"Obesity, Morbid/rehabilitation" OR MH:"Overweight/rehabilitation" OR MH:"Obesity/therapy" OR MH:"Obesity, Morbid/therapy" OR MH:"Overweight/therapy" OR MH:"Obesity/psychology" OR MH:"Obesity, Morbid/psychology" OR MH:"Overweight/psychology")) AND (MH:"Adolescent" OR MH:"Child" OR MH:"Pediatrics" OR MH:"Infant" OR minors OR boy OR boys OR girl$ OR kid OR kids OR child OR childs OR children$ OR childhood$ OR childcare$ OR schoolchild$ OR escolar$ OR adolescen$ OR preadolescen$ OR juvenil$ OR juventud$ OR youth$ OR teen$ OR preteen$ OR underage$ OR pubescen$ OR paediatri$ OR pediatri$ OR joven$ OR jovem$ OR niños OR niñas OR crianca$ OR menin$ OR "menor de edad" OR "menores de edad" OR "menor de idade" OR "menores de idade")) OR MH:"Pediatric Obesity/diet therapy" OR MH:"Pediatric Obesity/prevention & control" OR MH:"Pediatric Obesity/rehabilitation" OR MH:"Pediatric Obesity/therapy" OR MH:"Pediatric Obesity/psychology" [activated filter "Controlled Clinical Trial"] |
| ICTRP Search Portal (Advanced search) |
|
[activated "Search for clinical trials in children"]: in Title: obes* OR overweight* OR in Condition: obes* OR overweight* Recruitment Status: ALL |
| ClinicalTrials.gov (Advanced search) |
|
Conditions: obese OR overweight OR obesity Study type: Interventional Studies Age Group: Child (birth‐17) |
Appendix 2. Description of interventions
| Trial ID | Interventions | Comparators |
| Patsopoulou 2017 | I1: activity programme 3‐day per week training programme (45 minutes per training session) for 12 weeks. Instructed to add an extra 30‐45 minutes of walking or other sport activity of their preference at least once per week and to reduce inactivity | No intervention |
| I2: as for activity group + nutritional education introductory meeting (45‐60 minutes). Parents were invited to attend sessions | ||
| Jelalian 2016 | CBT for depression and healthy lifestyle plus exercise: CBT treatment adapted into 1 integrated protocol that addresses depression, an exercise component, weight and advice regarding healthy eating. Weekly 60‐minute group aerobic exercise sessions were required and facilitated by a physiotherapist | CBT for depression treatment only |
| Norman 2016 | Stepped down care based on cognitive behavioural modification, individual sessions of counselling health education and telephone calls. Consisted of 3 × 4‐month steps but the number and frequency of each intervention step varied. Aimed for adolescents to lose 4 lb every 4 months and if the 4‐lb weight loss was achieved, the participant was 'stepped‐down' to the next level of reduced intensity | Enhanced usual care. 1 counselling visit, 1 health education visit, materials and monthly mailings of weight‐related materials |
| Hofsteenge 2014 | Group education on dietary behaviour, physical activity and energy balance | Referral to a dietitian |
| Schranz 2014 | Resistance training | No intervention |
| Visuthranukul 2015 | Low GI diet. Monthly visits for 6 months. Each visit consisted of a 2‐hour, small class teaching session with parental participation (4‐5 families per class) | Conventional diet. Monthly visits for 6 months (1 per month for 6 months) |
| Wong 2015 | Monthly dietary counselling with a registered dietitian and daily text messaging | Monthly dietary counselling with a registered dietitian and daily text messaging |
| Pakpour 2015 | I1: MI. Physical activity and dietary intervention, 40‐minute session weekly for 6 weeks | Passive control: no details |
| I2: MI + parental involvement. As for MI group with 1‐hour parental session after 6 weeks | ||
| Bean 2014 | I1: MI values. 2 sessions, values clarification, re‐examined value/behaviour congruency and ideas for change + TEENS family‐based intervention | Education control. Watched 2 educational videos, week 1 and week 10 + TEENS family‐based intervention |
| Carraway 2014 | 12‐week mentor‐led exercise programme. Each adolescent paired with a college student mentor who was majoring in exercise physiology or related field. Mentor/mentee pairs met for 3 exercise sessions per week, each lasting 1 hour. Activity type varied by session and was decided collaboratively and included exercise machines (treadmill, stationary bike, elliptical), racquetball, tennis, group outdoor activities (flag football, tag). Mentees wore heart rate monitors to monitor exercise intensity. In addition to exercise, the 12‐week programme included individual weekly cognitive behavioural‐based challenges, or lessons. Mentors received 12 hours of training in MI and behavioural strategies to increase physical activity and modify eating. Mentors also received weekly supervision on goal setting and related topics. Parents of adolescents attended monthly parent meetings to discuss progress and build support through education | Wait list control |
| Sigal 2014 | I1: diet + aerobic training, reduced calorie diet and 4 weekly visits to gym for aerobic training over 22 weeks | Diet only: reduced calorie diet and usual activity |
| I2: diet + resistance training: reduced calorie diet and 4 weekly visits to gym for resistance training over 22 weeks | ||
| I3: diet + aerobic + resistance training: reduced calorie diet and 4 weekly visits to gym for aerobic and resistance training over 22 weeks | ||
| Love‐Osborne 2014 | MI: diet and exercise goal setting, additional text messages for half the group | Control group, no details |
| Kong 2014 | Low GI diet individual counselling diet, physical activity and behavioural components. 7 sessions over 6 months | Usual Chinese diet: individual counselling diet, physical activity and behavioural components. 7 sessions over 6 months |
| Luna‐Pech 2014 | Normocaloric diet and physical activity, seen every 2 weeks for 28 weeks | No intervention, free diet, seen every 2 weeks for 28 weeks |
| Boodai 2014 | Group sessions focusing on reduction in sedentary behaviour, diet, promotional of physical activity | No intervention, advice to attend primary care |
| Gourlan 2013 | MI + standard weight loss. Behaviour modification as well as standard weight loss intervention. No details of number sessions | Standard weight loss: balanced diet, a healthy lifestyle and physical activity |
| Patrick 2013 | I1: website intervention. To promote weight loss and healthy behaviours. 51‐week intervention, website for adolescents and for parents | Usual care. Given printed materials |
| I2: website + group. To promote weight loss and healthy behaviours. 51‐week intervention, website for adolescents and for parents. Also group support sessions (number not stated) | ||
| I3: website + SMS. To promote weight loss and healthy behaviours. 51‐week intervention, website for adolescents and for parents. Text messages sent weekly | ||
| Kong 2013 | ACTION. MI, visit to clinician every 2‐3 weeks for a total of 8 visits over 1 academic year, DVD toolkit | Standard care. 1 clinician visit and printed information |
| Pbert 2013 | "Lookin' Good Feelin' Good." 6 × 1‐on‐1 counselling sessions conducted over 2 months focused on behavioural change, diet and physical activity goals | 6 × 1‐on‐1 visits with the school nurse, information pamphlets |
| Brennan 2013 | MI. 13 sessions over 4‐6 months with or without parent. Then maintenance phase of 2 × 1‐hour clinic sessions and 7 × 15‐minute maintenance telephone call session and a final face‐to‐face session 6 months after the last treatment session | Wait list control |
| Walpole 2013 | MI: focus on unhealthy behaviours, 6 sessions, 30‐minutes long | Social skills training: finding ways to navigate social situations, 6 sessions, 30‐minutes long |
| Toulabi 2012 | Behavioural modification. Dietary modification and techniques for increasing physical activity. 8 × 45‐minute sessions, held twice per week and group physical activity 1 hour per day, 3 days per week, for 6 weeks | Educational booklets |
| Debar 2012 | Multicomponent intervention. Group meetings, 16 times (90 minutes each) over 5 months for adolescents and 12 sessions for parents | Usual care. Clinic visit, information and resources |
| Ebbeling 2012 | Multicomponent intervention. Emphasis on the consumption of sugar‐sweetened beverages, provided with written information, telephone calls and 3 check‐in visits (20 minutes per visit). Monthly motivational telephone calls with parents (30 minutes per call) | Control: no details |
| Vos 2011 | Family‐based CBT + nutrition. Educational sessions, computer package, 7 children group meetings ‐ 2.5 hours' duration biweekly. 5 parent meetings, 1 parent and child meeting | Wait list control |
| Christie 2011 | Multicomponent motivational and solution‐focused family‐based weight management programme. 12 HELP sessions (45 minutes each) across 6 months | Enhanced standard care, 1 educational session of 40 minutes addressing eating behaviours, healthy activity, uses standard national guidance |
| Wengle 2011 | I1: mentored lifestyle intervention. Nutrition and activity counselling at baseline, 1, 2, 3 and 6 months. 1‐2 hour once per week mentoring sessions to achieve activity goals, participate in physical activity, and discuss and set nutritional goals | Unmentored lifestyle intervention. Nutrition and activity counselling at baseline, 1, 2, 3 and 6 months |
| Ford 2010 | Mandometer. Real‐time feedback during meals to slow down speed of eating and reduce total intake. Weekly visits for 6 weeks, then every 2 weeks for 6 weeks then monthly. Telephone support | Standard care. 3 monthly visits, emphasis on diet and physical activity goal setting |
| Nguyen 2012 | I1: Loozit + ATC. Healthy lifestyle intervention based on cognitive behavioural approach with an intensive treatment phase followed by a longer maintenance phase. 7 × 75‐minute weekly group sessions then 7 × 60‐minute sessions over 24 months. Telephone coaching, e‐mails, text messages (32 electronic and 14 telephone coaching sessions). Adolescent and parent | Loozit as for intervention except no ATC |
| Grey 2009 | Coping skills. 13 sessions over 16 weeks: 8 nutrition and activity classes + 5 coping skill training. Telephone counselling for 9 months | General education. 8 nutrition and activity classes over 16 weeks |
| Vissers 2008 | School‐based intervention. Physical activity and nutrition sessions and gym membership. 6 nutrition sessions: individual or group (maximum of 2 group sessions) once per month | Standard gym classes |
| NCT00132132 | Behavioural education programme. Monthly 4‐hour session which incorporated: exercise, empowerment, education and incentives | Standard of care control: education on physical activity and nutrition |
| Pitetti 2007 | Treadmill. Use of treadmill in usual school activity sessions (3 per week) and also taken to the gym to use the treadmill on days and evenings when not participating in usual school activity sessions | Usual school activity sessions and Leisure activity of choice |
| Savoye 2007 | Bright Bodies weight management. Nutrition/behaviour modification once (40 minutes each) per week. Parental involvement in the nutrition related topics. Behaviour modification sessions were held separately for parents and children. Attended twice per week for 6 months and then every other week for an additional 6 months | Diet and exercise counselling and brief psychosocial counselling. 30 minute session every 6 months |
| van Egmond‐Frohlich 2006 | Multicomponent intervention: after a rehabilitation programme participating physicians of the intervention group received a practice guideline: this addressed the guilt or fate problem, treatment aims (health promotion activities), guidance on fat‐limiting mixed diet, promotion of a physical active lifestyle, local support resources, promotion of flexible control of eating and patient guidance according to the public health counselling and self‐management model. The outpatient programme was intended to have health check‐ups every 4 weeks during the first 12 months (10‐12 appointments) |
Standard care |
| Daley 2005 | Exercise counselling. 8 weekly behavioural sessions focused on attitudes to exercise. Offered aerobic exercise modalities, asked to exercise intermittently for 30 minutes, 3 times per week for 8 weeks | C1: exercise placebo. 24 sessions over 8 weeks; performed light body‐conditioning/stretching exercises |
| C2: usual care | ||
| Resnicow 2005 | Go Girls. High intensity. Weekly (20‐26) behavioural sessions. 1 meeting/week for 6 months (parents attending 10‐13 times), 1 day retreat, 6 MI telephone calls. 2‐way paging messages to support behavioural change. Retreats (unclear how many) | Moderate intensity 6 sessions over 6 months (1 per month), parents attending 3. Sessions were selected from the larger pool of sessions delivered to the intervention group |
| Jiang 2005 | Family‐based intervention. 104 weeks (2 years). Unclear number of sessions | Usual care |
| Carrel 2005 | Lifestyle‐focused gym classes. Fitness‐oriented group gym classes, movement time was 42 minutes of a 45‐minute class period. 36‐ week (9 month) intervention, 90 sessions | Standard gym classes. 36‐week (9‐month) intervention, 90 sessions (5 times every 2 weeks for a 45‐minute class period), Movement time 25 minutes of the 45‐minute period |
| Ebbeling 2003 | Low GL diet. 12 sessions over 6 months (12 dietary counselling sessions) and a 6‐month follow‐up (2 dietary counselling sessions) | Conventional low‐fat diet. 12 sessions over 6‐months (12 dietary counselling sessions) and a 6‐month follow‐up (2 dietary counselling sessions) |
| Saelens 2002 | Healthy Habits. Computer‐ and telephone‐delivered intervention, focus on diet and physical activity goals, 1 tailored physician‐counselling session. 12 telephone call sessions with counsellor (10‐20 minutes). Overall duration was 16 weeks with 13 sessions | Standard care. Non‐tailored physician‐counselling session |
| Brownell 1983 | I1: mother‐child separate sessions of behaviour modification, social support, nutrition and exercise | Child‐only sessions of behaviour modification, social support, nutrition and exercise |
| I2: mother‐child together sessions of behaviour modification, social support, nutrition and exercise | ||
| Chandra 1968 | Low‐calorie formula using Limical (Sarabhai Chemicals) for 1 day (4 servings) containing proteins 70 g, fat 20 g, carbohydrates 110 g | Low‐calorie diet without the aid of Limical |
| NCT00807560 | Family‐based therapy for paediatric overweight to resolve the eating disorder and return the patient to healthy psychosocial and physiological developmental trajectories through active family involvement across 3 treatment phases | Nutritional educational control condition will receive a minimal nutrition and physical activity education curriculum across 16 sessions over 24 weeks |
|
aThe term 'adequate' refers to sufficient use of the intervention/comparator with regard to dose, dose escalation, dosing scheme, provision for contraindications and other features necessary to establish a fair contrast between intervention and comparator. ATC: additional therapeutic contact; C: comparator; CBT: cognitive behavioural therapy; DVD: digital versatile disc; GI: glycaemic index; GL: glycaemic load; HELP: Healthy Eating and Lifestyle Programme; I: intervention; MI: motivational interviewing; SMS: short message service. | ||
Appendix 3. Baseline characteristics (I)
| Trial ID | Intervention and comparator | Duration of intervention (duration of follow‐up) | Description of participants | Study period (year to year) | Country | Setting | Ethnic groups (%) | Socioeconomic status |
| Patsopoulou 2017 | I1: activity | 12 weeks (at 6 months) | Overweight and obese adolescents, aged 13‐15 years, no organic cause for their obesity and on no medications | 2011‐2014 | Greece | Public training centre | ‐ | ‐ |
| I2: activity + diet | ‐ | ‐ | ||||||
| C1: no intervention | ‐ | ‐ | ||||||
| Jelalian 2016 | I1: CBT + healthy lifestyle | 6 months (at 12 months) | Aged 12‐18 years, depressed, overweight or obese (BMI > 25 kg/m2 or BMI percentile > 85th for gender and age) | ‐ | USA | University (based on author location) | Child Latino: 33.3 Child minority race: 58.3 | Parent education: < high school: 17.4% high school: 47.8% > high school: 34.8% Household income (USD): < 5000: 4.2% 5000‐9999: 16.7% 10,000‐14,999: 4.2% 15,000‐25,999: 16.7% 26,000‐49,999: 20.8% 50,000‐74,999: 20.8% 75,000‐99,999: 8.3% 100,000‐149,000: 4.2% |
| C1: CBT | Child Latino: 33.3 Child minority race: 44.4 | Parent education:
< high school 0%;
high school 22.2%
> high school 77.8% Household income (USD): < 5000: 11.1% 5000‐9999: 11.1% 10,000‐14,999: 0% 15,000‐25,999: 22.2% 26,000‐49,999: 11.1% 50,000‐74,999: 33.3% 75,000‐99,999: 0% 100,000‐149,000: 11.1% |
||||||
| Norman 2016 | I1: stepped down care | 12 months + 2 weeks run‐in (at 12 months) | Aged 11‐13 years, obese (≥ 95th percentile for age and gender) | ‐ | USA | Primary care | Girls
African‐American: 7
Asian/Pacific Islander: 0
Hispanic: 83
White non‐Hispanic: 7
Multiethnic or other: 3 Boys African‐American: 4 Asian/Pacific Islander: 4 Hispanic: 71 White non‐Hispanic: 17 Multiethnic or other: 4 |
Parent highest education (%): Girls: ≤ high school degree 44 some college/associates degree 28 ≥ Bachelor's degree 28 Boys: ≤ high school degree 29 some college/associates degree 42 ≥ Bachelor's degree 29 Parent annual income (USD) : Girls: < 35,000: 39% 35,000‐49,900: 30% 50,000‐74,900: 8% ≥ 75,000: 23% Boys: < 35,000: 31% 35,000‐49,900: 23% 50,000‐74,900: 19% ≥ 75,000: 27% |
| C1: enhanced usual care | Girls
African ‐American: 0
Asian/Pacific Islander: 0
Hispanic: 92
White non‐Hispanic: 0
Multiethnic or other: 8 Boys African‐American: 4 Asian/Pacific Islander: 4 Hispanic: 81 White non‐Hispanic: 7 Multiethnic or other: 4 |
Parent highest education (%): Girls: ≤ high school degree: 28 some college/associates degree: 56 ≥ Bachelor's degree: 16 Boys: ≤ high school degree: 37 some college/associates degree: 26 ≥ Bachelor's degree: 37 Parent annual income (USD): Girls: < 35,000: 36% 35,000‐49,900: 32% 50,000‐74,900: 24% ≥ 75,000: 8% Boys: < 35,000: 43% 35,000‐49,900: 14% 50,000‐74,900: 25% ≥ 75,000: 18% |
||||||
| Hofsteenge 2014 | I1: group education | 36 weeks (at 18 months) | Aged 12‐18 years with overweight or obesity according to Cole criteria | 2006‐2009 | The Netherlands | Outpatient | Western: 50.7 Non‐Western: 49.3 | ‐ |
| C1: dietitian only | Western: 35.3 Non‐Western: 64.7 | ‐ | ||||||
| Schranz 2014 | I1: resistance training | 6 months (at 12 months) | Aged 13‐17 years, very overweight or obese (Cole criteria) | 2010‐2011 | Australia | Community (gym) | ‐ | ‐ |
| C1: no intervention | ‐ | ‐ | ||||||
| Visuthranukul 2015 | I1: low GI diet | 6 months (at 6 months) | Obese Thai children aged 9‐16 years | 2010‐2013 | Thailand | Hospital | ‐ | ‐ |
| C1: conventional diet | ‐ | ‐ | ||||||
| Wong 2015 | I1: standard weight loss diet + increase water intake | 6 months (immediately following intervention) | Overweight and obese adolescents who consumed 4 cups of water per day at screening | ‐ | USA | Hospital (based on authors location) | ‐ | ‐ |
| C1: standard weight loss diet | ‐ | ‐ | ||||||
| Pakpour 2015 | I1: motivational interviewing | 6 weeks (at 12 months) | 13‐18 years, obese (≥ 95th percentile for age and gender) | ‐ | Iran | Outpatient | ‐ | Mother education, years 6.26 (3.42) father education, years 7.43 (3.86) household income (1000 rials) 7434.63 (1891.39) |
| I2: motivational interviewing + parental involvement | ‐ | Mother education, years 6.19 (3.54) father education, years 7.28 (3.91) household income (1000 trials) 7304.10 (2136.48) | ||||||
| C1: passive control | ‐ | Mother education, years 5.92 (3.89) father education, years 8.26 (4.21) household income (1000 rials) | ||||||
| Bean 2014 | I1: motivational interviewing values | 3 months (at 6 months) | Overweight aged 11‐18 years, BMI ≥ 85th percentile for age and gender | 2009‐2011 | USA | Healthcare (based on author location) | Black: 75.4 White: 19.3 Other: 5.3 | Family income (USD): < 40,000: 56% ≥ 40,000: 44% Parent education: ≤ high school graduate: 19.6 some college: 43.1 college degree or beyond: 37.3 |
| C1: education control | Black: 68.3 White: 19.5 Other: 12.2 | Family income (USD): < 40,000 (48.6%) ≥ 40,000 (51.4%) Parent education: ≤ high school graduate: 34.3 some college: 20.0 college degree or beyond: 45.7 | ||||||
| Carraway 2014 | I1: mentor‐led exercise | 12 weeks (at 7 months) | Overweight aged 12‐17 years, BMI ≥ 85th percentile for age and gender | ‐ | USA | Healthcare (based on author location) | African‐American: 27.3 White: 63.6 Other: 9.1 | ‐ |
| C1: wait list control | African‐American: 61.5 White: 30.8 Other: 7.7 | ‐ | ||||||
| Sigal 2014 | I1: diet + aerobic training | 22 weeks + 4‐week run‐in (at 6 months) | Overweight or obese adolescents, aged 14‐18 years, BMI ≥ 95th percentile or ≥ 85th percentile + diabetes or cardiovascular risk factor | 2005‐2011 | Canada | Community | White: 72.0 Black: 8.0 Mixed: 6.7 Arabic: 5.3 Asian: 1.3 Hispanic: 5.3 Other: 1.3 Native Canadian: 0 | ‐ |
| I2: diet + resistance training | White: 70.5 Black: 14.1 Mixed: 2.6 Arabic: 1.3 Asian: 6.4 Hispanic: 2.6 Other: 2.6 Native Canadian: 0 | ‐ | ||||||
| I3: diet + aerobic + resistance training | White: 62.7 Black: 16.0 Mixed: 4.0 Arabic: 5.3 Asian: 2.7 Hispanic: 4.0 Other: 2.7 Native Canadian: 2.7 | ‐ | ||||||
| C1: diet only | White: 82.9 Black: 2.6 Mixed: 5.3 Arabic: 2.6 Asian: 2.6 Hispanic: 0 Other: 1.3 Native Canadian: 2.6 | ‐ | ||||||
| Love‐Osborne 2014 | I1: motivational interviewing | 6‐8 months (at 6‐8 months) | BMI ≥ 85% | 2010‐ | USA | School‐based health centres | Hispanic: 88 | ‐ |
| C1: control | Hispanic: 89 | ‐ | ||||||
| Kong 2014 | I1: low GI diet | 6 months (interim data from a 12‐month intervention) | Adolescents, BMI ≥ 95th percentile | 2010‐2012 | Hong Kong | University | ‐ | ‐ |
| C1: usual Chinese diet | ‐ | ‐ | ||||||
| Luna‐Pech 2014 | I1: normocaloric diet + physical activity | 28 weeks (at 28 weeks) | Aged 12‐16 years, asthma, BMI ≥ 95th percentile of the CDC BMI‐for‐age growth charts | ‐ | Mexico | Tertiary care | ‐ | ‐ |
| C1: no intervention | ‐ | ‐ | ||||||
| Boodai 2014 | I1: multicomponent group sessions | 6 months (at 6 months) | Aged 10‐14 years, BMI > 95th percentile | 2009 | Kuwait | Primary care | ‐ | ‐ |
| C1: no intervention | ‐ | ‐ | ||||||
| Gourlan 2013 | I1: motivational interviewing + standard weight loss | 3 months' standard weight loss group + 6 months' motivational interview group (at 6 months). | Aged 11‐18 years, BMI > 90th age‐ and gender‐specific percentiles | ‐ | France | Hospital | ‐ | ‐ |
| C1: standard weight loss | ‐ | ‐ | ||||||
| Patrick 2013 | I1: website intervention | 12 months (at 12 months) | Aged 12‐16 years, 'high risk' for diabetes, overweight BMI > 85th percentile for age and gender, weight and height > 85th percentile or weight > 120% of ideal for height | ‐ | USA | Home | White: 26.9 African‐American: 15.4 Native American: 0 Asian or Pacific Islander: 3.8 Multiethnic or other: 3.8 Preferred not to state: 23.1 Did not state: 26.9 | ‐ |
| I2: website + group | White: 23.1 African‐American: 7.7 Native American: 0 Asian or Pacific Islander: 7.7 Multiethnic or other: 3.8 Preferred not to state: 15.4 Did not state: 42.3 | ‐ | ||||||
| I3: website + SMS | White: 8.3 African‐American: 12.5 Native American: 4.2 Asian or Pacific Islander: 0 Multiethnic or other: 0 Preferred not to state: 16.7 Did not state: 58.3 | ‐ | ||||||
| C1: usual care | White: 12.0 African‐American: 28.0 Native American: 0 Asian or Pacific Islander: 4 Multiethnic or other: 4 Preferred not to state: 16 Did not state: 36.0 | ‐ | ||||||
| Kong 2013 | I1: ACTION | 1 academic year (at 6 months) | 9th‐11th grade, BMI ≥ 85th percentile | 2009‐2010 | USA | School‐based health centres | Asian: 14 Hispanic: 75 Native American: 0 Multiple: 11 | Carer years of education: 0‐6: 11% 7‐11: 29% 12 (high school graduate): 21% 13‐15: 25% ≥ 16: 14% |
| C1: standard care | Asian: 4 Hispanic: 61 Native American: 13 Multiple: 22 | Carer years of education: 0‐6: 9% 7‐11: 35% 12 (high school graduate): 22% 13‐15: 26% ≥ 16: 9% | ||||||
| Pbert 2013 | I1: "Lookin' Good Feelin' Good" | 16 weeks (at 6 months) | Grade 9‐11, BMI ≥ 85th percentile for age and gender | 2008‐2009 | USA | School | White: 73.8 Black: 14.3 Hispanic: 14.3 | ‐ |
| C1: control | White: 80.0 Black: 5.0 Hispanic: 15.0 | ‐ | ||||||
| Brennan 2013 | I1: motivational interviewing | 26 weeks (at 12 months) | Aged 11‐19 years, overweight or obese according to the international cut‐off points for BMI | 2003‐ | Australia | University clinic | ‐ | ‐ |
| C1: wait list control | ‐ | ‐ | ||||||
| Walpole 2013 | I1: motivational interviewing | 6 months (immediately following intervention) | Overweight and obese (BMI ≥ 85th percentile), aged 10‐18 years | 2010‐2012 | Canada | Outpatient clinic | ‐ | Household income (CAD) (n = 18): 0‐20,000: 21% 20,000‐40,000: 0% 40,000‐50,000 7% 50,000‐60,000: 14% 60,000‐80,000: 21% > 80,000: 37% |
| I2: social skills training | ‐ | Household income (CAD) (n = 14): 0‐20,000: 6% 20,000‐40,000: 11% 40,000‐50,000: 6% 50,000‐60,00: 11% 60,000‐80,000: 17% 80,000: 49% | ||||||
| Toulabi 2012 | I1: behavioural modification | 6 weeks (at 6 months) | BMI > 28 kg/m2 in 15 year olds, BMI ≥29 kg/m2 in 16 and 17 year olds | 2004‐2006 | Iran | School | ‐ | ‐ |
| C1: control | ‐ | ‐ | ||||||
| Debar 2012 | I1: multicomponent intervention | 5 months (at 12 months) | Aged 2‐17 years, female, age‐ and gender‐adjusted BMI ≥ 90th percentile | ‐ | USA | Primary care | White: 75 | Family income: > USD75,000: 40.0% Grade in school: 6th‐8th: 52.8% 9th‐12th: 47.1% |
| C1: usual care | White: 75 | Family income: > USD75,000: 36.5% Grade in school: 6th‐8th: 52.4% 9th‐12th: 47.6% |
||||||
| Ebbeling 2012 | I1: multicomponent intervention | 52 weeks (at 24 months) | BMI ≥ 85th percentile for gender and age, aged 13‐18 years | 2007‐2011 | USA | Home | White: 55
Black: 24
Asian: 4
Multiple or other: 18 Ethnic group: Hispanic: 25 Non‐Hispanic: 75 |
Annual household income (USD) (%):
< 30,000: 27
30,000‐59,999: 35 ≥ $60,000: 38 Parental educational level (%): Some high school: 2 High‐school diploma or General Education Development certificate: 21 Some college or vocational school: 25 Associate's degree: 6 Bachelor's degree: 30 Some graduate school or graduate degree: 15 |
| C1: control | White: 56 Black: 24 Asian: 4 Multiple or other: 17 Ethnic group: Hispanic: 17 Non‐Hispanic: 83 | Annual household income (USD) (%): < 30,000: 27 30,000‐59,999: 30 ≥ 60,000: 43 Parental educational level (%): Some high school: 4 High‐school diploma or General Education Development certificate: 18 Some college or vocational school: 21 Associate's degree: 12 Bachelor's degree: 29 Some graduate school or graduate degree: 16 | ||||||
| Vos 2011 | I1: family‐based CBT + nutrition | 3 months (at 24 months) | Aged 8‐17 years, overweight or obesity, increased risk of comorbidity | ‐ | Netherlands | Healthcare (based on author location) | North European: 35 Other: 65 |
‐ |
| C1: wait list control | North European: 28 Other: 72 |
‐ | ||||||
| Christie 2011 | I1: HELP weight management | 6 months (at 12 months) | Aged 13‐17 years, obese defined as BMI > 98th centile | 2011‐2013 | UK | Primary care | ‐ | ‐ |
| C1: enhanced standard care | ‐ | ‐ | ||||||
| Wengle 2011 | I1: mentored lifestyle intervention | 24 weeks (at 24 weeks) | Aged 12‐16 years, BMI > 85th percentile | 2006‐2007 | Canada | Outpatient | ‐ | ‐ |
| I2: unmentored lifestyle intervention | ‐ | ‐ | ||||||
| Ford 2010 | I1: Mandometer | 12 months (at 18 months) | Obese adolescents, BMI > 95th centile | 2004‐2007 | UK | Hospital‐based obesity clinic | Non‐white: 9 | ‐ |
| C1: standard care | Non‐white: 15 | ‐ | ||||||
| Nguyen 2012 | I1: Loozit + ATC | 24 months (at 24 months) | Aged 13‐16 years, overweight to moderately obese (BMI z score range 1.0‐2.5) | 2006‐2009 | Australia | Community health centres | ‐ | ‐ |
| I2: Loozit | ‐ | ‐ | ||||||
| Grey 2009 | I1: coping skills | 16 weeks (at 36 weeks) | In 7th grade, BMI > 85th percentile, family history of type 2 diabetes mellitus | ‐ | USA | School | White: 0.9 White Hispanic: 42.0 African‐American: 55.4 Bi/Other: 1.8 |
Carer education, %: < high school 24.1 high school 39.3 Trade school or college 36.6 Carer income (USD), %: < 5000 15.2 5000‐9999 17.0 10,000‐14,999 15.2 15,000‐19,999 9.8 20,000‐29,000 14.3 30,000‐39,000 6.3 ≥ 40,000 10.7 Missing 11.6 |
| C1: general education | White: 4.7 White Hispanic: 48.8 African‐American: 40.7 Bi/Other: 5.8 |
Carer education, %: < high school 16.3 high school 33.7 trade school or college 50.0 Carer income (USD), %: < 5000 12.8 5000‐9999 15.1 10,000‐14,999 16.3 15,000‐19,999 10.5 20,000‐29,000 7.0 30,000‐39,000 15.1 ≥ 40,000 11.6 Missing 11.6 | ||||||
| Vissers 2008 | I1: school‐based intervention | 24 weeks (at 24 weeks) | Overweight | ‐ | Belgium | School | ‐ | ‐ |
| C1: control | ‐ | ‐ | ||||||
| NCT00132132 | I1: behavioural education | 12 months (12‐15 months) | Overweight, BMI > 85%, aged 10‐20 years | ‐ | USA | Healthcare (based on author location) | ‐ | ‐ |
| C1: standard care | ‐ | ‐ | ||||||
| Pitetti 2007 | I1: treadmill | 36 weeks (at 36 weeks) | Aged 14‐19 years, autism, BMI > 30 kg/m2 | ‐ | USA | Residential school | ‐ | ‐ |
| C1: control | ‐ | ‐ | ||||||
| Savoye 2007 | I1: Bright Bodies weight management | 12 months (at 24 months) | Aged 8‐16 years, BMI > 95th percentile | ‐ | USA | Obesity clinic | Non‐Hispanic white: 38.1 Non‐Hispanic black: 38.1 Hispanic: 23.8 |
‐ |
| C1: control | Non‐Hispanic white: 34.8 Non‐Hispanic black: 39.1 Hispanic: 26.1 |
‐ | ||||||
| van Egmond‐Frohlich 2006 | I1: multicomponent intervention | 12 months (12 months) | Obese children and adolescents, aged 9‐16 years | 2002/2003? | Germany | Outpatient | ‐ | High school: 15% |
| C1: standard care | ‐ | High school: 12% | ||||||
| Daley 2005 | I1: exercise counselling | 8 weeks (at 28 weeks) | Clinically obese, 11‐16 years | 2002‐2006 | UK | Outpatient | White: 82.7 Black: 9.9 South Asian: 7.4 | Index of multiple deprivation rank scores: quartile 1 (least deprived): 16% quartile 2: 14.8% quartile 3: 17.3% quartile 4 (most deprived): 51.9% |
| C1: exercise placebo | ||||||||
| C2: control | ||||||||
| Resnicow 2005 | I1: Go Girls. high‐intensity behavioural intervention | 6 months (at 12 months) | Aged 12‐16 years, girls, BMI > 90th percentile for age and gender | ‐ | USA | Community ‐ churches | African‐American: 100 | Household income > USD40,000 |
| C1: moderate‐intensity behavioural intervention | ||||||||
| Jiang 2005 | I1: family‐based intervention | 24 months (at 24 months) | Grade 7‐9, obese | ‐ | China | School | ‐ | ‐ |
| C1: control | ‐ | ‐ | ||||||
| Carrel 2005 | I1: lifestyle‐focused gym classes | 9 months (at 9 months) | BMI > 95th percentile for age | ‐ | USA | School and outpatients | ‐ | ‐ |
| C1: standard gym classes | ‐ | ‐ | ||||||
| Ebbeling 2003 | I1: low GL diet | 26 weeks (at 12 months) | Aged 13‐21 years, BMI > 95th percentiles | ‐ | USA | Research clinic | ‐ | ‐ |
| C1: conventional diet | ‐ | ‐ | ||||||
| Saelens 2002 | I1: Healthy Habits | 12 weeks (at 28 weeks) | Aged 12‐16 years, 20‐100% above the median (50th percentile) for BMI | ‐ | USA | Clinic/home | ‐ | ‐ |
| C1: standard care | ‐ | ‐ | ||||||
| Brownell 1983 | I1: mother + child separate | 16 weeks (1 year later) | Aged 12‐16, ≥ 20% mean weight for age, gender and height | ‐ | USA | Healthcare (based on author location) | White: 100 | Predominately lower‐middle class families |
| I2: mother + child together | White: 100 | Predominately lower‐middle class families | ||||||
| C1: child only | White: 100 | Predominately lower‐middle class families | ||||||
| Chandra 1968 | I1: low‐calorie formula Limical | 3 months (at 7 months ‐ for this report but the author stated that the follow‐up period extended to 2 years) | Moderately obese boys and girls, aged 9‐17 years. | ‐ | ‐ | Outpatient | ‐ | ‐ |
| C1: low‐calorie diet | ‐ | ‐ | ||||||
| NCT00807560 | I1: family‐based therapy for paediatric overweight | 24 weeks (at 12 months) | Aged 13‐17 years, BMI percentile > 85% for gender and age | ‐ | USA | Outpatient eating and weight disorders clinic | ‐ | ‐ |
| C1: nutritional‐ educational control condition | ‐ | ‐ | ‐ | |||||
| ‐ denotes not reported ATC: additional therapeutic contact; BMI: body mass index; C: comparator; CAD: Canadian dollars; CBT: cognitive behavioural therapy; CDC: Centers for Disease Control and Prevention; GI: glycaemic index; GL: glycaemic load; HELP: Healthy Eating and Lifestyle Programme; I: intervention; SD: standard deviation; SDS: standard deviation score; USD: US dollars | ||||||||
Appendix 4. Baseline characteristics (II)
| Trial ID | Intervention and comparator | Sex (female %) | Age (mean (SD)) | BMI/BMI percentile/BMI z score (mean kg/m² (SD)) | Body weight (mean kg (SD)) | Parental weight/BMI | Comedications/ cointerventions | Comorbidities/conditions |
| Patsopoulou 2017 | I1: activity | 48.3 | 14.04 (0.8) | 32.6 (3.50) | 81.0 (8.9) | ‐ | ‐ | ‐ |
| 12: activity + diet | 53.3 | 14.01 (0.8) | 32.3 (3.0) | 80.7 (7.8) | ‐ | ‐ | ‐ | |
| C1: no intervention | 52.5 | 14.04 (0.8) | 33.4 (4.0) | 85.0 (8.0) | ‐ | ‐ | ‐ | |
| Jelalian 2016 | I1: CBT + healthy lifestyle | 70.8 | 15.25 (1.51) | 36.8 (7.9) | 101.43 (24.82) | ‐ | ‐ | ‐ |
| C1: CBT | 77.8 | 14.44 (1.67) | 37.6 (4.3) | 100.08 (12.79) | ‐ | ‐ | ‐ | |
| Norman 2016 | I1: stepped down care | 27 | Girls 12 (0.9) Boys 12 (0.8) |
BMI percentile: Girls 97.3 (2.5) Boys 98.1 (1.3) |
‐ | ‐ | ‐ | ‐ |
| C1: enhanced usual care | 24 | Girls 11.8 (1.0) Boys 11.7 (0.9) |
BMI percentile: Girls 97.3 (2.4) Boys 97.8 (1.8) |
‐ | ‐ | ‐ | ‐ | |
| Hofsteenge 2014 | I1: group education | 53.5 | 14.5 (1.7) | 33.3 (4.6) | 94.7 (18.4) | ‐ | ‐ | Impaired fasting glucose: 8.5% Impaired glucose intolerance: 5.6% |
| C1: dietitian only | 58.8 | 14.4 (1.8) | 33.6 (5.1) | 92.2 (18.5) | ‐ | ‐ | Impaired fasting glucose: 5.9% Impaired glucose intolerance: 3.9% | |
| Schranz 2014 | I1: resistance training | 0 | 14.9 (1.4) | 32.2 (4.3) | 97.7 (18.3) | ‐ | ‐ | ‐ |
| C1: no intervention | 0 | 15.1 (1.6) | 32.6 (5.0) | 99.1 (23.7) | ‐ | ‐ | ‐ | |
| Visuthranukul 2015 | I1: low GI diet | 36 | 11.9 (1.9) | 34.2 (5.8) | 83.8 (16.0) | ‐ | ‐ | ‐ |
| C1: conventional diet | 29.7 | 12.0 (2.1) | 33.1 (6.6) | 84.5 (23.2) | ‐ | ‐ | ‐ | |
| Wong 2015 | I1: standard weight loss diet + increase water intake | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard weight loss diet | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pakpour 2015 | I1: motivational interviewing | 53.8 | 15.59 (1.31) | 33.07 (8.87) BMI z score: 2.83 (0.79) BMI percentile: 95.19 (4.72) |
‐ | Mother BMI: 39.46 (9.81) Father BMI: 36.22 (9.89) |
‐ | ‐ |
| I2: motivational interviewing + parental involvement | 35.9 | 15.57 (1.38) | 33.09 ( 5.86) BMI z score: 2.82 (0.62) BMI percentile: 94.63 (5.01) |
‐ | Mother BMI: 39.48 (8.87) Father BMI: 36.32 (9.83) |
‐ | ‐ | |
| C1: passive control | 45.4 | 15.78 (1.19) | 32.92 (7.79) BMI z score: 2.75 (0.67) BMI percentile: 95.56 (4.63) |
‐ | Mother BMI: 39.31 (8.64) Father BMI: 36.02 (8.78) |
‐ | ‐ | |
| Bean 2014 | I1: motivational interviewing values | 75.9 | 13.6 (1.8) | BMI percentile: 98.9 (1.0) BMI z score: 2.4 (0.3) |
‐ | ‐ | ‐ | ‐ |
| C1: education control | 70.7 | 14.1 (1.7) | BMI percentile: 98.9 (1.3) BMI z score: 2.4 (0.3) |
‐ | ‐ | ‐ | ‐ | |
| Carraway 2014 | I1: mentor‐led exercise | 54.5 | 13.64 (1.75) | BMI z score: 2.01 (0.44) | ‐ | ‐ | ‐ | ‐ |
| C1: wait list control | 61.5 | 13.69 (1.6) | BMI z score: 2.2 (0.42) | ‐ | ‐ | ‐ | ‐ | |
| Sigal 2014 | I1: diet + aerobic training | 70.7 | 15.5 (1.4) | 34.7 (4.3) | 97.1 (1.8)a | ‐ | Metformin: 2 (2.7) Oral contraceptives: 12 (16) Stimulants: 1 (1.3) |
Normal glucose tolerance (%): 62 (82.7) |
| I2: diet + resistance training | 70.5 | 15.9 (1.5) | 35.1 (4.4) | 100.1 (1.7)a | ‐ | Metformin 1 (1.3) Oral contraceptives: 13 (17) Stimulants: 1 (1.3) |
Normal glucose tolerance (%): 66 (84.6) | |
| I3: diet + aerobic + resistance training | 70.7 | 15.5 (1.3) | 34.7 (4.3) | 97.8 (1.8)a | ‐ | Metformin 1 (1.3) Oral contraceptives: 5 (6.7) Stimulants: 3 (4) |
Normal glucose tolerance (%): 67 (89.3) | |
| C1: diet only | 68.4 | 15.6 (1.3) | 34.2 (4.4) | 97.9 (1.8)a | ‐ | Metformin 1 (1.3) Oral contraceptives: 16 (21.1) Stimulants: 3 (4) |
Normal glucose tolerance (%): 68 (89.5) | |
| Love‐Osborne 2014 | I1: motivational interviewing | 58 | 15.7 (1.5) | 31.9 (6.2) BMI z score: 1.92 (0.46) |
‐ | ‐ | ‐ | Severe dyslipidaemia: 1 |
| C1: control | 46 | 16.0 (1.5) | 31.6 (6.5) BMI z score: 1.89 (0.52) |
‐ | ‐ | ‐ | Type 2 diabetes: 2 Severe dyslipidaemia: 1 | |
| Kong 2014 | I1: low GI diet | 59.6 | 16.8 (1.0) | 31.6 (4.2) | 87.6 (13.0) | ‐ | ‐ | Impaired glucose tolerance: 11.5% |
| C1: usual Chinese diet | 53.8 | 16.7 (1.0) | 30.2 (3.5) | 82.9 (14.9) | ‐ | ‐ | Impaired glucose tolerance: 15.4% | |
| Luna‐Pech 2014 | I1: normocaloric diet + physical activity | 53.8 | 14 (0.7) | 28.3 (0.9) BMI z score: 2.18 (0.3) |
57.9 (8.0) | ‐ | ‐ | Asthma: 100% |
| C1: no intervention | 44 | 14 (0.3) | 27.1 (0.9) BMI z score: 2.17 (0.2) |
53.8 (7.1) | ‐ | ‐ | Asthma: 100% | |
| Boodai 2014 | I1: multicomponent group sessions | 48.8 | 12.4 (1.2) | BMI z score 2.2 (0.3) | ‐ | ‐ | ‐ | ‐ |
| C1: no intervention | 48.8 | 12.4 (1.2) | BMI z score 2.2 (0.3) | ‐ | ‐ | ‐ | ‐ | |
| Gourlan 2013 | I1: motivational Interviewing + standard weight loss | 41 | ‐ | 29.56 (4.75) | ‐ | ‐ | ‐ | ‐ |
| C1: standard weight loss | ‐ | 29.59 (5.92) | ‐ | ‐ | ‐ | ‐ | ||
| Patrick 2013 | I1: website intervention | 61.5 | 14.1 (1.4) | BMI z score: 2.2 (0.4) BMI percentile: 98.1 (0.1) | ‐ | ‐ | ‐ | ‐ |
| I2: website + group | 69.2 | 14.3 (1.5) | BMI z score: 2.2 (0.4) BMI percentile: 97.8 (0.1) | ‐ | ‐ | ‐ | ‐ | |
| I3: website + SMS | 50.0 | 14.3 (1.8) | BMI z score: 2.2 (0.3) BMI percentile: 97.9 (0.1) | ‐ | ‐ | ‐ | ‐ | |
| C1: usual care | 72.0 | 14.5 (1.5) | BMI z score: 2.2 (0.4). BMI percentile: 98.1 (0.1) | ‐ | ‐ | ‐ | ‐ | |
| Kong 2013 | I1: ACTION | 61.0 | 15.0 (1.0) | BMI percentile 94.5 (4.1) | 78.5 (12.5) | ‐ | ‐ | ‐ |
| C1: standard care | 57.0 | 14.6 (0.7) | BMI percentile 94.4 (4.6) | 78.1 (18.1) | ‐ | ‐ | ‐ | |
| Pbert 2013 | I1: "Lookin' Good Feelin' Good" | 64.3 | 15.9 (1.03) | 32.78 (5.91) BMI z score 1.95 (0.44) | ‐ | ‐ | ‐ | ‐ |
| C1: Control | 75.0 | 15.7 (1.01) | 31.24 (5.33) BMI z score: 1.81 (0.41) | ‐ | ‐ | ‐ | ‐ | |
| Brennan 2013 | I1: motivational interviewing | 54.0 | ‐ | 31.84 (4.52) BMI z score: 2.08 (0.37) BMI percentile: 97.40 (2.72) |
88.91 (18.57) | ‐ | ‐ | ‐ |
| C1: wait list control | ‐ | 31.67 (4.76) BMI z score: 2.08 (0.40) BMI percentile: 97.29 (2.94) |
87.65 (17.98) | ‐ | ‐ | ‐ | ||
| Walpole 2013 | I1: motivational Interviewing | 70 | 14.1 (1.8) | 30.2 (2.8) BMI z score: 2.51 (0.47) |
‐ | Mother: 30.8 (7.6) Father: 26.8 (4.5) |
‐ | ‐ |
| C1: social skills training | 45 | 13.7 (1.7) | 30.4 (5.0) BMI z score: 2.64 (0.73) |
‐ | Mother: 29.1 (6.1) Father: 28.6 (5.1) |
‐ | ‐ | |
| Toulabi 2012 | I1: behavioural modification | ‐ | ‐ | 30.43 (2.39) | 81.67 (10.94) | ‐ | ‐ | ‐ |
| C1: control | ‐ | ‐ | 30.33 (1.93) | 84.43 (10.79) | ‐ | ‐ | ‐ | |
| Debar 2012 | I1: multicomponent intervention | 100 | 14.1 (1.5) | 32.03 (4.79) BMI percentile: 97.09 (2.27) |
86.0 (15.2) | ‐ | ‐ | ‐ |
| C1: usual care | 100 | 14.3 (1.5) | 31.84 (4.63) BMI percentile: 97.10 (2.29) |
84.6 (15.6) | ‐ | ‐ | ‐ | |
| Ebbeling 2012 | I1: multicomponent intervention | 47 | 15.3 (0.7) | 30.4 (5.2) | 85.2 (16.8) | ‐ | ‐ | ‐ |
| C1: control | 42 | 15.2 (0.7) | 30.1 (4.7) | 86.1 (17) | ‐ | ‐ | ‐ | |
| Vos 2011 | I1: family‐based CBT + nutrition | 55 | 13.3 (2.0) | 32.4 (4.7) BMI‐SDS 4.2 (0.7) | 85.7 (18.4) | ‐ | ‐ | ‐ |
| C1: wait list control | 51.3 | 13.1 (1.9) | 32.5 (3.9) BMI‐SDS 4.3 (0.6) | 85.7 (17.98) | ‐ | ‐ | ‐ | |
| Christie 2011 | I1: HELP weight management | 62.6 | ‐ | 32.3 (4.4) | ‐ | ‐ | ‐ | ‐ |
| C1: enhanced standard care | ‐ | ‐ | ‐ | ‐ | ‐ | |||
| Wengle 2011 | I1: mentored lifestyle intervention | ‐ | 14.4 (1.5) | 31.8 (5.7) BMI z: 2.00 (0.41) | ‐ | ‐ | ‐ | ‐ |
| C1: unmentored lifestyle intervention | ‐ | 14.5 (1.4) | 32.8 (4.5) BMI z score: 2.16 (0.35) | ‐ | ‐ | ‐ | ‐ | |
| Ford 2010 | I1: Mandometer | 56 | 12.7 (2.2) | 34.4 | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 56 | 12.5 (2.3) | 33.1 | ‐ | ‐ | ‐ | ‐ | |
| Nguyen 2012 | I1: Loozit + ATC | 53.8 | 14.2 (1) | 30.8 (3.5) BMI z score: 2.02 (0.29) |
82.4 (12.4) | ‐ | ‐ | ‐ |
| C1: Loozit | 49.3 | 14 (0.9) | 30.8 (4.2) BMI z score: 2.03 (0.37) |
82.4 (12.4) | ‐ | ‐ | ‐ | |
| Grey 2009 | I1: coping skills | 62.5 | 12.8 (0.7) | 30.5 (7.2) | ‐ | ‐ | ‐ | ‐ |
| C1: general education | 41.6 | 12.6 (0.7) | 30.3 (6.0) | ‐ | ‐ | ‐ | ‐ | |
| Vissers 2008 | I1: school‐based intervention | 67.6 | 17.5 (1.3) | Girls: 29.3 (2.9) Boys: 28.9 (2.0) | Girls: 79.9 (10.7) Boys: 89.8 (9.6) |
‐ | ‐ | ‐ |
| C1: control | 69.2 | 17.5 (1.3) | Girls: 29.3 (4.1) Boys: 28.7 (3.6) | Girls 81.1 (13.8) Boys 92.3 (19.1) |
‐ | ‐ | ‐ | |
| NCT00132132 | I1: behavioural education | 46.7 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 73.3 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pitetti 2007 | I1: treadmill | 40 | 16.6 (1.9) | 33.2 (7.8) | 98 (18.3) | ‐ | Autism‐related medications reported | Autism: 100% |
| C1: control | 40 | 17.4 (1.1) | 30.9 (8.5) | 93 (32.) | ‐ | Autism‐related medications reported | Autism: 100% | |
| Savoye 2007 | I1: Bright Bodies weight management | 55.2 | 11.9 (2.5) | 35.8 (7.6) | 87 (25.1) | ‐ | ‐ | ‐ |
| C1: control | 68.1 | 12.4 (2.3) | 36.2 (6.2) | 91.2 (23.3) | ‐ | ‐ | ‐ | |
| van Egmond‐Frohlich 2006 | I1: multicomponent intervention | 58 | 13.2 (1.8) | BMI‐SDS 2.3 (0.4) | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 55 | 13.5 (1.7) | BMI‐SDS 2.3 (0.5) | ‐ | ‐ | ‐ | ‐ | |
| Daley 2005 | I1: exercise counselling | 55.6 | ‐ | BMI‐SDS 3.17 (0.33) | ‐ | ‐ | ‐ | ‐ |
| C1: exercise placebo | ‐ | BMI‐SDS 3.22 (0.61) | ‐ | ‐ | ‐ | ‐ | ||
| C2: control | ‐ | BMI‐SDS 3.32 (0.37) | ‐ | ‐ | ‐ | ‐ | ||
| Resnicow 2005 | I1: GoGirls high‐intensity behavioural intervention | 100 | ‐ | 32 (5.8) | 87.9 (17.73) | ‐ | ‐ | ‐ |
| C1: moderate‐intensity behavioural intervention | 100 | ‐ | 33.2 (7.3) | 84.1 (20.84) | ‐ | ‐ | ‐ | |
| Jiang 2005 | I1: family‐based intervention | 39.4 | 13.3 (0.6) | 26.6 (1.7) | 70.1 (5.7) | ‐ | ‐ | ‐ |
| C1: control | 40.0 | 13.2 (0.7) | 26.1 (1.5) | 71.2 (6.4) | ‐ | ‐ | ‐ | |
| Carrel 2005 | I1: lifestyle‐focused gym classes | 52 | 12.5 (0.5) | 32 (6) | ‐ | ‐ | ‐ | ‐ |
| C1: standard gym classes | 43 | 12.5 (0.7) | 30 (4) | ‐ | ‐ | ‐ | ‐ | |
| Ebbeling 2003 | I1: low GL diet | 68.8 | 16.9 (1.3) | 34.9 (1.0) | 103.5 (6.0) | ‐ | ‐ | ‐ |
| C1: conventional diet | 15.3 (0.9) | 34.9 (1.0) | 104.7 (4.8) | ‐ | ‐ | ‐ | ||
| Saelens 2002 | I1: Healthy Habits | ‐ | ‐ | 31.0 (3.5) | 85.5 (13.9) | ‐ | ‐ | ‐ |
| C1: standard care | ‐ | ‐ | 30.7 (3.1) | 80.5 (13.5) | ‐ | ‐ | ‐ | |
| Brownell 1983 | I1: mother + child separate | 78.6 | ‐ | 45.5, (7.1) | 83.6 (16.8) | 43.4% 'overweight' | ‐ | ‐ |
| I2: mother + child together | ‐ | 42.4 (12.0) | 80.5 (28.3) | ‐ | ‐ | |||
| C1: child only | ‐ | 42.0 (6.5) | 81.1 (18.7) | ‐ | ‐ | |||
| Chandra 1968 | I1: low‐calorie formula Limical | 70 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: low‐calorie diet | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| NCT00807560 | I1: family‐based therapy for paediatric overweight | 65 | 15.0 (1.52) | 35.3 (5.5) | 96.6 (14.4) | ‐ | ‐ | ‐ |
| C1: nutritional‐educational control condition | 69 | 15.2 (1.6) | 36.6 (6.6) | 103.3 (28.9) | ‐ | ‐ | ‐ | |
| ‐ denotes not reported ATC: additional therapeutic contact; BMI: body mass index; CBT: cognitive behavioural therapy; C: comparator; GI: glycaemic index; GL: glycaemic load; HELP: Healthy Eating and Lifestyle Programme; I: intervention; SD: standard deviation; SDS: standard deviation score; SMS: short message service. ainconsistencies between tables in the publication | ||||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Trial ID | Endpoints quoted in trial document (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Study results posted in trial register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c |
| Patsopoulou 2017 |
Source: NCT02653508 Primary outcome measures: BMI; weight; height; heart rate; blood pressure; waist circumference; 50‐m sprint run test; family eating and activity habits |
No Last verified: January 2016 History of changes: 0 documented changes |
Primary outcome measure: ‐ | Primary outcome measure: ‐ |
| Secondary outcome measures: BMI; weight; height; heart rate; blood pressure; waist circumference; 50‐m sprint run test; family eating and activity habits | Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measures: anthropometric measurements (BMI, weight, height, waist circumference) fitness assessment (50‐m sprint run test), Family Eating and Activity Habits Questionnaire, blood pressure | Other outcome measure: anthropometric measurements (BMI, weight, height, waist circumference), Fitness assessment (50‐m sprint run test), Family Eating and Activity Habits Questionnaire, blood pressure | ||
| Jelalian 2016 |
Source: NCT01128764 Primary outcome measure: depressed mood |
No Last verified: July 2014 History of changes: 3 documented changes |
Primary outcome measures: depressed mood and BMI | Primary outcome measure: ‐ |
| Secondary outcome measure: weight | Secondary outcome measures: % time spent in MVPA and sedentary behaviour | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: treatment feasibility/acceptability | Other outcome measure: depressed mood, BMI, MVPA, acceptability of intervention | ||
| Norman 2016 | Source: N/T | Other outcome measures: BMI, waist circumference, body fat, fasting blood lipids, blood pressure | Other outcome measures: BMI, adiposity and biometric measures | |
| Hofsteenge 2014 |
Source: NTR691, ISRCTN27626398 Primary outcome measures: BMI, body composition, glucose‐intolerance, insulin resistance |
No Last verified: August 2015 |
Primary outcome measure: BMI‐SDS | Primary outcome measure: ‐ |
| Secondary outcome measures: dietary behaviour, physical activity, sedentary behaviour, quality of life, self‐esteem, cost‐effectiveness | Secondary outcome measures: glucose tolerance, dietary behaviour, physical activity, sedentary behaviour and self‐esteem | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI‐SDS, body composition, metabolic components, effect modifiers, blood pressure, HDL cholesterol, PedsQL, Body Esteem Scale | ||
| Schranz 2014 |
Source: ACTRN12609001078246 Primary outcome measure: self‐concept (self‐esteem) |
No Last verified: August 2015 |
Primary outcome measures: exercise self‐efficacy, physical self‐worth and self‐esteem | Primary outcome measure: ‐ |
| Secondary outcome measures: body composition, strength | Secondary outcome measures: body composition, strength | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: exercise self‐efficacy, resistance training confidence, self‐esteem, body composition | ||
| Visuthranukul 2015 |
Source: NCT02049788 Primary outcome measures: change in body composition measured by BIA and DXA |
No Last verified: January 2014 |
Primary outcome measure: body composition changes (refers to fat mass and fat‐free mass) | Primary outcome measure: ‐ |
| Secondary outcome measure: change in metabolic syndrome risks | Secondary outcome measures: metabolic syndrome risk changes which were blood pressure, fasting plasma glucose, plasma insulin and serum lipid profiles | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI z score, fat and fat‐free mass, fasting plasma insulin, HOMA‐IR | ||
| Wong 2015 | Source: N/T | Primary outcome measure: ‐ | Primary outcome measure: ‐ | |
| Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: water intake, urine specific gravity, BMI z score, cardiometabolic risk factors | |||
| Pakpour 2015 |
Source: NCT02180802 Primary outcome measures: BMI, dietary self‐efficacy, weight self‐efficacy, physical exercise self‐efficacy, physical activity |
No Last verified: August 2014 History of changes: 1 documented changes |
Primary outcome measures: changes in BMI, the Child Dietary Self‐Efficacy Scale (CDSS), the Weight Efficacy Lifestyle questionnaire (WEL), the Physical Exercise Self‐Efficacy Scale (PES) and self‐reported physical activity and diet | Primary outcome measures: BMI z score, anthropometric, biochemical, psychometric and behavioural outcome variables |
| Secondary outcome measures: cholesterol, triglycerides, % body fat | Secondary outcome measures: changes in anthropometric measures, cholesterol, triglycerides and body fat | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: ‐ | ||
| Bean 2014 |
Source: published protocol and trial record: NCT00167830 Primary outcome measure ‐ source protocol: BMI percentile Primary outcome measures ‐ source trial record: BMI, metabolic indicators, fitness measures |
No Last verified: January 2014 History of changes: 8 documented changes |
Primary outcome measures: BMI percentile in methods publication, retention and adherence in results publication | Primary outcome measure: ‐ |
|
Secondary outcome measures ‐ source protocol: dietary intake, physical activity, attrition, and compliance Secondary outcome measures ‐ source trial record: participant's compliance with exercise and diet, parental compliance and support, knowledge of nutritional principles, attitude toward healthy behaviours, self‐esteem, motivation, negativity and family cohesiveness |
Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | ||
|
Other outcome measure ‐ source protocol: ‐ Other outcome measure ‐ source trial record: ‐ |
Other outcome measure: ‐ | Other outcome measures: adherence (overall, dietitian visits, behavioural support visits) | ||
| Carraway 2014 | Source: N/T | Primary outcome measure: ‐ | Other outcome measures: perceived athletic competence, physical activity, social anxiety, social support | |
| Secondary outcome measure: ‐ | ‐ | |||
| Other outcome measures: height, weight, body composition, BMI, % overweight, VO2max, physical appearance, Children and Youth Physical Self‐Perception Profile, accelerometry, perceived social support, social anxiety | ‐ | |||
| Sigal 2014 |
Source: NCT00195858 Primary outcome measure: change in % body fat (MRI scan) |
No Last verified: April 2015 History of changes: 8 documented changes |
Primary outcome measure: % body fat by MRI | Primary outcome measure: % body fat |
| Secondary outcome measures: resting energy expenditure (indirect calorimetry); lean body mass; abdominal visceral and subcutaneous fat; waist and hip circumference; apolipoprotein A1; plasma insulin; HOMA‐IR; apoprotein B; CRP; HDL cholesterol; LDL cholesterol; triglycerides; total:HDL cholesterol ratio; fasting and 2‐hour postload glucose; HbA1c; blood pressure; health‐related quality of life | Secondary outcome measures: body weight, BMI, waist circumference, systolic and diastolic blood pressure, energy intake, steps per day, muscle strength, glucose levels, lipid levels, hip circumference, resting energy expenditure, cardiorespiratory fitness, musculoskeletal fitness, insulin, HbA1c, apoliproteins, high‐sensitivity CRP, non‐esterified fatty acids, quality of life, body image, mood, self‐esteem, adverse events | Secondary outcome measure: waist circumference | ||
| Love‐Osborne 2014 | Source: N/T | Primary outcome measure: BMI z score (basis of sample size calculation) | Primary outcome measure: ‐ | |
| Secondary outcome measure: fitness testing | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: BMI z score, sports participation | |||
| Kong 2014 |
Source: NCT01278563 Primary outcome measure: BMI |
No Last verified: January 2011 History of changes: 0 documented changes |
Primary outcome measure: BMI | Primary outcome measure: ‐ |
| Secondary outcome measures: waist circumference, % body fat | Secondary outcome measure: obesity indices | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other: cardiometabolic risk factors | Other outcome measures: BMI, body weight and waist circumference | ||
| Luna‐Pech 2014 | Source: N/T | Other outcome measures: asthma‐related quality of life, BMI z score, weight, macronutrient intake, acute asthma attacks, night‐time awakenings, use of inhaled corticosteroids, pulmonary function | Other outcome measures: asthma‐related quality of life, BMI z score, acute asthma attacks, night‐time awakenings, use of inhaled corticosteroids, pulmonary function | |
| Boodai 2014 |
Source: ISRCTN37457227 Primary outcome measure: change in BMI z score |
No Last verified: August 2015 |
Primary outcome measure: change in BMI z score | Primary outcome measure: BMI z score |
| Secondary outcome measures: change in quality of life, change in blood pressure and blood‐based cardiometabolic risk factors (fasting lipids, triglycerides, insulin, glucose), changes in estimated fat and fat‐free mass (using BIA) | Secondary outcome measures: blood pressure, waist circumference | Secondary outcome measure: ‐ | ||
| Gourlan 2013 | Source: N/T | Other outcome measures: motivation for physical activity, perceived competence, perceived autonomy support, physical activity, BMI | Other outcome measures: BMI, autonomy support, integrated and identified regulations, amotivation | |
| Patrick 2013 |
Source: NCT00412165 Primary outcome measure: BMI |
No Last verified: August 2012 History of changes: 5 documented changes |
Primary outcome measure: BMI z score | Primary outcome measure: BMI |
| Secondary outcome measures: metabolic and physiological manifestations of insulin resistance, BMI, waist‐to‐hip ratio, % body fat, behavioural measures of diet and physical activity | Secondary outcome measures: % body fat, health‐related quality of life, behaviour change physical activity, diet | Secondary outcome measures: adiposity, physical activity, diet, sedentary behaviour | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: ‐ | ||
| Kong 2013 |
Source: NCT00841334 Primary outcome measure: BMI percentile |
No Last verified: January 2013 History of changes: 2 documented changes |
Primary outcome measure: ‐ | Primary outcome measure: ‐ |
| Secondary outcome measures: insulin resistance, lipids, dietary intake, blood pressure, physical activity | Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measures: height, weight, BMI percentile, waist circumference, blood pressure, dietary intake, physical activity, television viewing, participant satisfaction, HDL cholesterol, triglycerides, fasting plasma glucose, fasting insulin, insulin resistance | Other outcome measures: BMI percentile and waist circumference, blood pressure, HOMA‐IR, triglycerides and HDL cholesterol | ||
| Pbert 2013 | Source: N/T | Other outcome measures: height, weight, BMI, BMI z score, blood pressure, waist circumference, dietary intake, physical activity, sedentary behaviour, acceptability | Other outcome measures: ate breakfast, intake of total sugar and added sugar, drink soda ≤ once per day, eat at fast food restaurants ≤ once per week, BMI, activity, caloric intake | |
| Brennan 2013 |
Source: ACTRN12610000111077 Primary outcome measures: body composition, BMI, cardiovascular fitness |
No Last verified: February 2010 History of changes: no documented changes |
Primary outcome measure: body composition | Primary outcome measure: ‐ |
| Secondary outcome measures: energy intake and diet quality, energy expenditure and physical activity, self‐reported eating habits, self‐reported daily physical activity and sedentary behaviour, resting metabolic rate, cardiovascular fitness, eating and weight‐related psychopathology, psychopathology, self‐esteem, social support, family interaction, parenting approach, social skills, negative cognitions, knowledge of factors related to overweight and obesity, involvement of family and friends in adolescents' adoption of health behaviour, hip and waist circumference | Secondary outcome measures: height, weight, BMI, BMI z score, waist and hip circumference, cardiovascular fitness, resting metabolic rate, pubertal status, eating and physical activity behaviours, energy intake and nutritional intake, energy expenditure and physical activity, psychosocial assessments (psychosocial functioning, psychopathology, family functioning, motivation for change), satisfaction | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: weight control behaviour, impulse regulation, social support from family and parent‐adolescent problem communication, treatment acceptability, body composition (body fat, % body fat, lean mass) and anthropometric measures (weight, BMI, BMI‐for‐age z score and percentiles), cardiovascular fitness | ||
| Walpole 2013 |
Source: NCT01246349 Primary outcome measures: weight Efficacy Life‐style Questionnaire, Child Dietary Self‐Efficacy Scale |
Yes (study results), first received: 17 January 2014 Last verified: March 2014 History of changes: 26 documented changes |
Primary outcome measure: self‐efficacy | Primary outcome measure: ‐ |
| Secondary outcome measures: BMI, waist circumference, psychological well‐being, self‐esteem, quality of life, depression, coping | Secondary outcome measures: BMI z score, waist circumference | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: self‐efficacy, BMI z scores, number of sessions attended | ||
| Toulabi 2012 | Source: N/T | Other outcome measures: BMI, body weight, height, waist circumference, hip circumference, and waist‐to‐hip ratio, depression scores, students' and parents' nutrition knowledge | Other outcome measures: weight, BMI, and waist and hip circumferences, students' and parents' nutrition knowledge, symptoms of depression | |
| Debar 2012 |
Source: NCT01068236 Primary outcome measure: BMI z score |
No Last verified: March 2010 History of changes: 1 documented change |
Primary outcome measure: BMI z score | Primary outcome measure: ‐ |
| Secondary outcome measures: blood pressure, fasting lipid profile, fasting glucose | Secondary outcome measures: dietary intake, physical activity, health behaviours, eating and mood disorder symptoms, body satisfaction, internalisation of sociocultural attitudes toward appearance, self‐esteem, quality of life | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: BMI z score | ||
| Ebbeling 2012 |
Source: NCT00381160 Primary outcome measure: BMI |
No Last verified: August 2012 History of changes: 12 documented changes |
Primary outcome measure: BMI | Primary outcome measure: BMI |
| Secondary outcome measure: ‐ | Secondary outcome measures: body fat as % total body weight, dietary intake, physical activity | Secondary outcome measures: consumption of sugar‐sweetened beverages, weight, change in body fat as a % body weight | ||
| Vos 2011 |
Source: ISRCTN36146436 Primary outcome measure: BMI |
No Last verified: September 2011 |
Primary outcome measure: BMI‐SDS | Primary outcome measure: ‐ |
| Secondary outcome measures: waist circumference, insulin sensitivity, secretion of gastrointestinal hormones, cardiovascular fitness, quality of life | Secondary outcome measures: weight, waist circumference, waist‐to‐height ratio, blood pressure, glucose, insulin, C‐peptide, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, free fatty acids, free T4, TSH, inflammation parameters (CRP, adiponectin), mixed meal tolerance, insulin resistance, insulin sensitivity, physical fitness, health‐related quality of life | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI‐SDS, health‐related quality of life, waist circumference SDS, physical fitness, insulin resistance, lipid profile, high‐sensitive CRP or for adiponectin | ||
| Christie 2011 |
Source: ISRCTN99840111 Primary outcome measure: BMI |
No Last verified: August 2015 |
Primary outcome measure: BMI at 6 months (end of intervention) | Primary outcome measure: ‐ |
| Secondary outcome measures: quality of life, waist circumference, cardiovascular risk factors, psychological function, lifestyle; cardiometabolic risk factors; health economic data | Secondary outcome measures: HRQoL, BMI at 12 months, waist circumference, fat mass/fat percentage, Eating Attitudes Test, self‐esteem, psychological health, lifestyle, fasting insulin and glucose, fasting lipids (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, total cholesterol:HDL cholesterol ratio, peripheral blood pressure, cost‐effectiveness | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI, BMI z score, fat mass, self‐esteem, eating behaviours, quality of life, process evaluation, association of pulse wave velocity (proxy for arterial stiffness) and breathlessness score poststep test, self‐reported exertion | ||
| Wengle 2011 | Source: N/T | Primary outcome measures: BMI and BMI z score | Primary outcome measure: ‐ | |
| Secondary outcome measures: changes in metabolic profile, nutrition, physical activity | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: BMI z score, waist circumference, HDL cholesterol, LDL:HDL ratio, consumption high‐calorie foods and snacks, fast food restaurant visits, screen time, feasibility of intervention | |||
| Ford 2010 |
Source: NCT00407420 Primary outcome measure: change in BMI‐SDS |
No Last verified: December 2006 History of changes: 0 documented changes |
Primary outcome measure: change in BMI‐SDS | Primary outcome measure: BMI‐SDS |
| Secondary outcome measures: biochemical parameters including insulin sensitivity using glucose and insulin measures, physical activity measured by CSA, state of well‐being, rate of eating and grams of food consumed in mandometer arm, fat‐free mass (bioimpedance) | Secondary outcome measures: % body fat/body fat SDS, grams of food consumed, speed of eating, development of satiety (Mandometer and subgroup of control arm only), fasting glucose and insulin concentrations, lipid profile, high‐sensitivity CRP, insulin resistance, paediatric quality of life, blood pressure | Secondary outcome measures: body fat SDS, change in portion size, HDL cholesterol | ||
| Nguyen 2012 |
Source: ACTRN12606000175572 Primary outcome measures: BMI z score, waist circumference z score |
No Last verified: May 2006 |
Primary outcome measures: BMI z score and waist‐to‐height ratio (protocol paper states primary outcomes are BMI z score and waist circumference z score) | Primary outcome measure: ‐ |
| Secondary outcome measures: fasting insulin, glucose total cholesterol, HDL cholesterol, LDL cholesterol, blood pressure, physical activity, food intake and eating patterns; psychosocial assessment | Secondary outcome measures: parallel changes in metabolic and self‐reported psychosocial and behavioural variables, physical activity, adverse events | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI z score, waist‐to‐height ratio, total cholesterol level, triglycerides level, global self‐worth, dietary, physical activity, sedentary behaviour | ||
| Grey 2009 | Source: N/T | Other outcome measures: weight, % body fat, height, BMI, waist circumference, insulin, insulin resistance, impaired glucose tolerance, lipids, child depressive symptoms, health behaviours and attitudes, physical activity | Other outcome measures: anthropometric measures, lipids, depressive symptoms, BMI | |
| Vissers 2008 | Source: N/T | Primary outcome measure: weight | Primary outcome measure: ‐ | |
| Secondary outcome measures: BMI, body fat, skinfold thickness, hip and waist circumference, waist‐to‐hip ratio, fasting glucose, cholesterol and triglycerides | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: weight, BMI, waist circumference, fasting glucose | |||
| NCT00132132 |
Source: NCT00132132 Primary outcome measures: change in BMI, % participants with BMI reduction |
Yes (study results) Last verified: May 2015 History of changes: 9 documented changes |
Primary outcome measure: ‐ | Primary outcome measure: ‐ |
| Pitetti 2007 | Source: N/T | Primary outcome measures: body weight, BMI | Primary outcome measure: ‐ | |
| Secondary outcome measures: energy expenditure, exercise capacity (treadmill walking frequency, speed, elevation) | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: BMI, treadmill walking | |||
| Savoye 2007 |
Source: NCT00409422 Primary outcome measures: weight, BMI, percentage body fat, lipids, blood pressure, glucose, insulin, insulin resistance |
No Last verified: June 2008 History of changes: 2 documented changes |
Primary outcome measure: change in BMI | Primary outcome measure: ‐ |
| Secondary outcome measure: ‐ | Secondary outcome measures: weight, % body fat, total body fat, blood pressure, plasma glucose, insulin, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, insulin resistance | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: BMI z score, BMI, % body fat, total body fat mass, total cholesterol, density lipoprotein cholesterol, insulin resistance | ||
| van Egmond‐Frohlich 2006 | Source: N/T | Primary outcome measure: ‐ | Primary outcome measure: ‐ | |
| Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | |||
| Other outcome measures: BMI‐SDS, physical activity, eating behaviour, quality of life | Other outcome measures: anthropometric measures, questionnaires on eating behaviour, physical activity, quality of life, self‐efficacy, subjective rating of the intervention | |||
| Daley 2005 |
Source: ISRCTN83888112 Primary outcome measures: physical self‐perceptions, depression, fitness, BMI |
No Last verified: September 2009 History of changes: no documented changes |
Primary outcome measure: physical self‐perceptions | Primary outcome measure: ‐ |
| Secondary outcome measure: ‐ | Secondary outcome measures: depression, self‐perceptions, affect, aerobic fitness, physical activity, height, weight, BMI | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measures: children's Depression Inventory score, physical self‐worth, self‐esteem, physical activity over time, BMI | ||
| Resnicow 2005 | Source: N/T | Primary outcome measure: BMI | Primary outcome measure: ‐ | |
| Secondary outcome measures: % body fat; waist and hip circumferences; blood pressure; serum measures of lipids, insulin, glucose; cardiovascular fitness | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: BMI, % body | |||
| Jiang 2005 |
Source: N/T ‐ |
Other outcome measures: weight, height, BMI, cholesterol, triglycerides, blood pressure | Other outcome measures: BMI, cholesterol, triglycerides, blood pressure | |
| Carrel 2005 | Source: N/T | Other outcome measures: BMI, % body fat, cardiovascular fitness, insulin sensitivity | Other outcome measures: BMI, % body fat, VO2max, insulin level | |
| Ebbeling 2003 | Source: N/T | Other outcome measures: dietary outcomes including glycaemic load, BMI, body mass, fat mass, weight, insulin resistance | Other outcome measures: glycaemic load, BMI, fat mass, insulin resistance | |
| Saelens 2002 | Source: N/T | Primary outcome measure: BMI z score | Primary outcome measure(s): ‐ | |
| Secondary outcome measures: weight, height, dietary intake, physical activity, sedentary behaviour, problematic eating and weight‐related behaviours and beliefs, physician counseling, behavioural skills use, participant satisfaction | Secondary outcome measure: ‐ | |||
| Other outcome measure: ‐ | Other outcome measures: BMI z scores, behavioural skills use, energy intake, % calories from fat, physical activity, sedentary behaviour, problematic weight‐related or eating behaviours/beliefs, feasibility, participant satisfaction | |||
| Brownell 1983 | Source: N/T | Other outcome measures: weight, % above mean weight, BMI, Developmental Index | Other outcome measures: weight loss, blood pressure | |
| Chandra 1968 | Source: N/T | Other outcome measure: weight (% expected) | Other outcome measure: ‐ | |
| NCT00807560 |
Source: NCT00807560 Primary outcome measure: BMI z score |
Yes (study results) Last verified: December 2015 History of changes: 9 documented changes |
Primary outcome measure: ‐ | Primary outcome measure: ‐ |
| Secondary outcome measures: % completion, waist measurement, hip measurement, height, weight, BMI, BMI percentile | Secondary outcome measure: ‐ | Secondary outcome measure: ‐ | ||
| Other outcome measure: ‐ | Other outcome measure: ‐ | Other outcome measure: ‐ | ||
| "‐" denotes not reported. aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study) BIA: bioelectrical impedence analysis; BMI: body mass index; CRP: C‐reactive protein; CSA: computer science applications; DXA: dual‐energy x‐ray absorptiometry; EMA: European Medicines Agency; FDA: Food and Drug Administration; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA‐IR: homeostatic model assessment, insulin resistance; HRQoL: health‐related quality of life; LDL: low‐density lipoprotein; MRI: magnetic resonance imaging; MVPA: moderate‐to‐vigorous physical activity; N/T: no trial document available; PedsQL: Pediatric Quality of Life; SDS: standard deviation score; TSH: thyroid stimulating hormone; VO2max: maximum volume of oxygen. | ||||
Appendix 6. Examination of outcome reporting bias according to ORBIT classification
| Trial ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Patsopoulou 2017 | N/A | ‐ | ‐ | ‐ | ‐ |
| Jelalian 2016 | N/A | ‐ | ‐ | ‐ | ‐ |
| Norman 2016 | N/A | ‐ | ‐ | ‐ | ‐ |
| Hofsteenge 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Schranz 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Visuthranukul 2015 | N/A | ‐ | ‐ | ‐ | ‐ |
| Wong 2015 | N/A | ‐ | ‐ | ‐ | ‐ |
| Pakpour 2015 | N/A | ‐ | ‐ | ‐ | ‐ |
| Bean 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Carraway 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Sigal 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Love‐Osborne 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Kong 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Luna‐Pech 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Boodai 2014 | N/A | ‐ | ‐ | ‐ | ‐ |
| Gourlan 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Patrick 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Kong 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Pbert 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Brennan 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Walpole 2013 | N/A | ‐ | ‐ | ‐ | ‐ |
| Toulabi 2012 | N/A | ‐ | ‐ | ‐ | ‐ |
| Debar 2012 | N/A | ‐ | ‐ | ‐ | ‐ |
| Ebbeling 2012 | N/A | ‐ | ‐ | ‐ | ‐ |
| Vos 2011 | N/A | ‐ | ‐ | ‐ | ‐ |
| Christie 2011 | N/A | ‐ | ‐ | ‐ | ‐ |
| Wengle 2011 | N/A | ‐ | ‐ | ‐ | ‐ |
| Ford 2010 | N/A | ‐ | ‐ | ‐ | ‐ |
| Nguyen 2012 | N/A | ‐ | ‐ | ‐ | ‐ |
| Grey 2009 | N/A | ‐ | ‐ | ‐ | ‐ |
| Vissers 2008 | N/A | ‐ | ‐ | ‐ | ‐ |
| NCT00132132 | N/A | ‐ | ‐ | ‐ | ‐ |
| Pitetti 2007 | N/A | ‐ | ‐ | ‐ | ‐ |
| Savoye 2007 | N/A | ‐ | ‐ | ‐ | ‐ |
| van Egmond‐Frohlich 2006 | N/A | ‐ | ‐ | ‐ | ‐ |
| Daley 2005 | N/A | ‐ | ‐ | ‐ | ‐ |
| Resnicow 2005 | N/A | ‐ | ‐ | ‐ | ‐ |
| Jiang 2005 | N/A | ‐ | ‐ | ‐ | ‐ |
| Carrel 2005 | N/A | ‐ | ‐ | ‐ | ‐ |
| Ebbeling 2003 | N/A | ‐ | ‐ | ‐ | ‐ |
| Saelens 2002 | N/A | ‐ | ‐ | ‐ | ‐ |
| Brownell 1983 | N/A | ‐ | ‐ | ‐ | ‐ |
| Chandra 1968 | N/A | ‐ | ‐ | ‐ | ‐ |
| NCT00807560 | N/A | ‐ | ‐ | ‐ | ‐ |
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed but report no results
( Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 7. Definition of endpoint measurement
| Trial ID | Behaviour change | Changes in BMI and body weight | Height | Health‐related quality of life or self‐esteem | All‐cause mortality/morbidity | Socioeconomic effects | Parent‐child relationship or assessment of parenting | Participants' views of the intervention | Severe/serious adverse events |
| Patsopoulou 2017 | ‐ | Weight measured using Tanita HD646 scales (reference provided) to 0.1 kg. Participants removed shoes and jackets. BMI determined according to: BMI = weight/height2 | Height measured to 0.1 cm using stretch stature method and PE87 portable stadiometers (reference provided). Participants removed shoes and jackets | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Jelalian 2016 | Physical activity assessed using Sense Wear Mini monitor | Weight measured on digital scale with participants wearing light clothing and no shoes. BMI was calculated as kg/m2 | Measured on wall mounted stadiometer | ‐ | ‐ | ‐ | ‐ | Session Evaluation Form given to adolescents at end of each therapy session with questions about usefulness of session rated on a 4‐point Likert scale (1 = strongly agree to 4 = strongly disagree | ‐ |
| Norman 2016 | ‐ | Weight measured using calibrated digital scale while participant was
wearing light clothing.
BMI calculated as kg/m2. BMI z scores calculated using CDC Vital and Health Statistics. BMI percentile calculated using age‐ and gender‐specific median, SD and power of the Box‐Cox transformation (reference provided). % over median BMI calculated as adolescent's percentage over median BMI for age and gender (formulae provided). % body fat determined from DXA (model details provided). Waist circumference based on the mean of 2 measurements following standardised procedures |
Height (without shoes) measured using stadiometer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hofsteenge 2014 | Dutch Eating Behaviour Questionnaire | Body weight measured within 0.1 kg with calibrated electronic flat scale. For calculation of BMI‐SDS or z scores, using Dutch reference database. Waist circumference measured and recorded with flexible band to an accuracy of 0.1 cm. Body composition assessed with DXA | Height measured with an accuracy of 0.1 cm with an electronic stadiometer | PedsQL CHQ BES ‐ validated questionnaire on general feelings about appearance, weight satisfaction and evaluations of attributions to others about one's body and appearance. Mean scores range from 0 (worst possible score) to 4 (best possible score), with higher scores representing a better body esteem |
‐ | ‐ | ‐ | ‐ | ‐ |
| Schranz 2014 | ‐ | Body mass using digital scales (model details provided). Skinfolds measured (biceps, triceps, subscapular, iliac crest, supraspinale, abdominal, front thigh, medial calf) to calculate a sum of skinfolds measure, using Harpenden callipers. Stature, mass and skinfold measurements taken using ISAK protocols. Body composition (% body fat, lean mass and bone mineral density) assessed using whole body DXA scanning (details provided) | Height measured with stadiometer (model details provided) | Physical Self‐Worth Scale ‐ 4‐choice structured alternative format. Self‐Perception Profile for Adolescents ‐ 4‐choice structured alternative format. For all measures, a higher score (or positive effect size change) indicated higher self‐efficacy/confidence or beliefs/self‐perception |
‐ | ‐ | ‐ | ‐ | ‐ |
| Visuthranukul 2015 | Dietary: 3‐day dietary records, not extracted as per review protocol. Physical activity questionnaire: unclear if validated |
Weight measured without shoes and with light clothing using a stadiometer to nearest 0.1 kg. BMI z score calculated based on (WHO 2009) growth reference using WHO AnthroPlus programme. Waist circumference measured at umbilicus level after normal exhalation with participants in standing position. Hip circumference measured at maximum circumference of hips. Mid‐upper arm circumference measured the circumference at middle point between olecranon process of ulna and acromion process of scapula | Measured without shoes using a stadiometer to nearest 0.1 cm | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wong 2015 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pakpour 2015 | Youth Adolescent Food Frequency Questionnaire: valid and reliable against the 24‐hour dietary recall adolescent populations. The Persian version of the questionnaire was found to be valid and reliable for use in Iranian adolescents. Objective Physical Activity using a GT3 X monitor. Child Dietary Self‐Efficacy Scale 15‐item tool assessing dietary self‐efficacy gathered on a 3‐point Likert scale (from "not sure" to "very sure"). Responses ranged from ‐15 to 15, with higher scores indicating higher dietary self‐efficacy. Weight Efficacy Lifestyle Questionnaire, a 20‐item tool assessing adolescents' confidence in their ability to lose weight. Items scored on a 10‐point Likert scale (from 0 = not confident to 9 = very confident), and items scores were averaged. Physical Exercise Self‐Efficacy Scale, adolescents' confidence in their ability to perform physical activity a 5‐item tool with responses scored on a 4‐point Likert scale (0 = uncertain to 9 = very certain) |
Weight for adolescents and parents measured to nearest 0.1 kg on calibrated digital scales. BMI calculated in kg/m2. BMI z score or SDS recommended by WHO. BMI percentile calculated according to the CDC's age‐ and gender‐specific reference norms. Waist circumference measured midway between lowest rib and superior border of iliac crest with inelastic measuring tape at end of normal expiration to nearest 0.1 cm. % body fat measured by DXA, a valid measure that provided a more accurate assessment of body composition than body weight alone. BIA conducted |
Height for adolescents and parents measured to nearest 0.1 cm after removing shoes. Height measured using a stadiometer (model details provided) | PedsQL | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bean 2014 | ‐ | Weight measured in light clothing without shoes to nearest 0.1 kg using an electric scale. (reference provided). BMI z scores and age‐ and gender‐specific BMI percentiles determined using Epi Info software. |
Height measured to nearest 0.1 cm using a stadiometer (reference provided) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Carraway 2014 | Physical activity levels assessed by accelerometer (model details provided). Children and Youth Physical Self‐Perception Profile, self‐administered, 40‐item measure assessing self‐perception in 6 domains (validated) |
BMI calculated using measured height and weight. Weight measured to nearest 0.1 lb using a Digital Cardinal Scale (reference provided). % overweight calculated by comparing actual BMI to BMI at the 50th percentile for participant's age and gender. Body composition assessed by DXA |
Height measured to nearest 0.1 inches using a stadiometer (details provided) | ‐ | ‐ | ‐ | Multidimensional Scale of Perceived Social Support a 12‐item measure that assessed perceptions of social support in several domains, including family (validated) | ‐ | ‐ |
| Sigal 2014 | Pedometers (model details provided) to assess physical activity, asked to maintain step‐count logs for 7 days at baseline and 6 months | Body composition assessed by MRI with a 1.5‐T system (details of system provided). Weight in kg measured using a Health O Meter manual scale (details provided). Waist circumference measured at middle distance between last floating rib and iliac crest using a retractable ergonomic measuring tape (details provided). Hip circumference measured at widest point, over buttocks |
Height in cm measured using a Health O Meter manual scale (details provided) | PedsQL‐Adolescent version) Harter Physical Self‐Perceptions Profile for Children adapted physical self‐perception profile questionnaire, 36‐item self‐reporting scale developed to determine domain‐specific judgements of competence, and a global perception of their worth or esteem as a person. It contains 6 separate subscales consisting of 5 specific domains: sport competence (athletic ability, ability to learn sports); perception of physical condition and fitness; perception of an attractive body (confidence in personal appearance); perception of physical strength; and physical self‐worth, as well as a general domain of global self‐worth |
‐ | ‐ | ‐ | ‐ | Recorded all directly observed adverse events and those spontaneously reported by participants. Participants also questioned about adverse events at each study visit. Serious adverse events and hospitalisations, not defined further |
| Love‐Osborne 2014 | ‐ | Weight measured without shoes with same calibrated scale. BMI recorded as various categories of BMI and subgroups |
Height measured without shoes with same stadiometer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Luna‐Pech 2014 | ‐ | Digital scale (model details provided), BMI z score | Stadiometer | Standardized Pediatric Asthma Quality of Life Questionnaire Spanish Version | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kong 2014 | Physical activity levels: self‐administered, validated, Chinese version of International Physical Activity Questionnaires | BMI ≥ 95th percentile of local age‐ and gender‐specific references. % body fat by bioimpedence (Tanita physician digital scale) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Boodai 2014 | ‐ | Weight measured to 0.1 kg in light indoor clothing, not wearing shoes. BMI z scores calculated based on (CDC 2000) reference data. Waist circumference: details not reported |
Height measured to 0.1 cm with a portable stadiometer (model details provided), not wearing shoes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gourlan 2013 | Perceived competence assessed by 14‐item questionnaire ‐ a scale based on recommendations by Bandura 1997 (not validated). Motivation for physical activity assessed via French version of the Behavioral Regulation Exercise Questionnaire (validated). Physical activity measures assessed using the 7‐day Physical Activity Recall Interview (validated). Total energy expenditure assessed by accelerometer by multiplying each intensity level by an intensity factor (1.5 for light intensity, 4 for moderate intensity, 6 for hard intensity, 10 for very hard intensity (validated)) |
Weight recorded in light clothes and without shoes (digital balance scale; model details provided) to nearest 1 kg | Measured to nearest 0.5 mm using a wall‐mounted stadiometer (model details provided) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Patrick 2013 | Personal views and questionnaires (unclear if questionnaires were completed by participants/member of team) ‐ not validated. Dietary intake assessed using the Youth/Adolescent Questionnaire, a validated self‐administered food frequency questionnaire for adolescents (not relevant to review protocol). Physical activity assessed using 7‐day Physical Activity Recall Interview, developed for the Stanford Five‐City Project. Sedentary behaviour assessed using 8‐item survey based on survey that measured hours spent doing various sedentary behaviours during school and non‐school days |
Weight measured using a calibrated digital scale. % body fat assessed by DXA |
Height (without shoes) measured using stadiometer with participant standing erect against a wall with heels close to wall | Health‐related quality of life. Rosenberg Self‐Esteem Scale used to assess self‐esteem, a 10‐item survey where each item had a 4‐point ordinal response scale and a score range of 10‐40, with higher scores indicating greater self‐esteem |
‐ | ‐ | ‐ | ‐ | ‐ |
| Kong 2013 | Dietary intake assessed using the YAQ. Not relevant to review protocol. Physical activity assessed using 3‐Day Physical Activity Recall and an accelerometer (model details provided) (validated) |
Weight measured twice without shoes and mean for analysis,
measured to nearest 0.1 kg on a strain‐gauge digital scale (model details provided). Waist circumference measured twice to nearest 1 mm with a steel tape and averaged |
Height measured twice without shoes and averaged for analysis. Height measured to nearest 1 mm using a Schorr vertical measuring board |
‐ | ‐ | ‐ | ‐ | Process evaluation conducted to monitor how well study was implemented. In addition to monitoring fidelity of motivational interviewing used by intervention clinician, participant attendance, length of clinic visit, participant satisfaction | ‐ |
| Pbert 2013 | Physical activity assessed by accelerometer (model details provided) for 7‐day period. Mean daily minutes of light, moderate and vigorous activity calculated using published cut‐off points (validated). Sedentary behaviour, TV watching and playing computer or video games on a school day measured using 2 items from the Youth Risk Behavior Survey. Dietary intake assessed with 24‐hour Dietary Recall Interview (not extracted as per review protocol). 8‐item instrument used to assess healthful and unhealthful dietary behaviours targeted by intervention. Instrument was designed to be completed independently by adolescents and was based on literature review, expert feedback, and feasibility testing, which found it to be feasible in public health and primary care settings, similar to the performance of the longer Food Habits Questionnaire and Rate Your Plate (not validated) |
Weight in kg, measured in light clothing by a research assistant. Waist circumference measured as mean of 2 measurements midway between rib cage and superior border of iliac crest. Bodyweight and body fat measured using leg‐to‐leg BIA system (details provided) |
Height measured using standard methodology, wearing light clothing, and no shoes | ‐ | ‐ | ‐ | ‐ | Perceived helpfulness of nurse intervention and level of comfort in discussing weight with school nurse. % visits in which the students thought that school nurse was very helpful in their learning how to eat healthy and be physically active. % visits in which students thought that they feel very comfortable in discussing their weight‐related behaviours with the nurse |
‐ |
| Brennan 2013 | Resting metabolic rate determined through indirect calorimetry at the laboratory. Diet assessed by 7‐day weighed food diary (not validated). Energy expenditure and physical activity measured using accelerometers for a 7‐day monitoring period. Participants wore the actigraph on their right hip attached using a firm‐fitting elastic belt strapped around their hips as per operator's instructions. Data included if participants had worn the actigraph for ≥ 10 hours each day on ≥ 5 days including 1 weekend day. Physical activity and sedentary behaviour using Self‐Administered Physical Activity Checklist (validated) |
body weight measured to nearest 10 g on a calibrated set of digital scales by trained independent assessors in presence of a trained assistant. Body composition determined through whole body DXA (model details provided). A total body scan was conducted to providing estimates of fat mass and lean tissue mass for the entire body and 4 subregions. Body circumference measurements taken from the right side of body at hip, waist, upper arm and forearm using a steel tape measure to nearest 1 mm |
Standing height measured with a calibrated stadiometer to nearest 0.5 cm | Rosenberg Self‐Esteem Scale used as measure of self‐esteem for adolescents and parents | ‐ | ‐ | Parent Adolescent Communication Scale and the Family Problem Solving Communication Index used to assess family functioning | ‐ | ‐ |
| Walpole 2013 | ‐ | BMI z score and waist circumference | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Toulabi 2012 | Students and parents' nutritional knowledge. 12 questions (with 1.5 points given to each correct answer) before and after implementing interventional programme (not extracted as per review protocol) | Weight (without wearing shoes or excess clothes) measured by same nurse using a digital scale (model details provided) to nearest 0.1 kg. Waist and hip circumferences measured to nearest 0.1 cm using a plastic measuring tape |
Nurse who provided intervention ‐ measured to nearest 0.1 cm using a plastic measuring tape (while standing against wall, without wearing shoes, and their occiputs and heels touching the wall); a level was placed on top of head, parallel with floor, to indicate height on measuring tape | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Debar 2012 | Certified dietary interviewers conducted 3 unannounced 24‐hour telephone dietary recalls (not reported as per review protocol). Adapted 24‐hour telephone physical activity recall from the 7‐day physical activity recall (not validated). Validated questionnaire measures included: hours per week of screen time and mean days per week of breakfast eaten (both adapted from the Youth Risk Behavior Survey). Mean times per week a "family" meal eaten together, and mean times per week fast food and sweetened beverages/sodas consumed (adapted from the Project EAT Student Survey) |
Weight measured with participants lightly clothed and without shoes and taken 3 times for quality assurance | Height measured with participants lightly clothed and without shoes and taken 3 times for quality assurance, measured to nearest 0.5 cm by using a Harpenden portable stadiometer (model details provided) monthly | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ebbeling 2012 | 2 × 24‐hour dietary and physical activity recall interviews conducted by telephone at baseline and another 2 at the end of intervention period (not validated). Dietary intake (not validated). Energy intake from sugar‐sweetened beverages (not validated). Volumetric consumption of all non‐caloric beverages (not validated). Recall of physical activity and inactivity, including sleep, using a protocol modelled after validated methodology. Participant asked to recall activity performed most during respective 15‐minute time blocks throughout the preceding day (12:00 AM to 11:59 PM) and then to rate the relative intensity of each reported activity as light, moderate, hard or very hard MET level assigned to each activity to calculate a physical activity factor (kcal/kg/hour). As points of reference, resting = 1.0, and brisk walking = 5.0. Asked participants to estimate usual number of hours per day spent watching television, using a computer (for purposes other than doing homework), and playing video games |
Trained personnel measured weight
using calibrated scales. BIA used to calculate body fat as a % total body weight (reference for calculation provided) |
Trained personnel measured height using calibrated stadiometers | ‐ | ‐ | ‐ | ‐ | Adherence to Instructions, beverage delivery logistics and overall enjoyment of participation. 1) How well did you follow the study instructions to drink the BASH beverages delivered to your home? Range: 0 = not at all (0) to 10 = very well. mean 8.4 (SD 1.7). 2) How well did you follow the study instructions to not buy or drink sugar‐sweetened beverages? Range: 0 = not at all (0) to 10 = very well, mean ‐8.1 (SD 2.1). 3) How was the number of beverages that you received each week? Range: 0 = too few to 10 = too many, mean ‐6.4 (SD 1.9). 4) How was the frequency (once per week) of beverage deliveries? Range: 0 = not often enough to 10 = too often, mean ‐5.4 (SD 1.5). 5) Did you enjoy participating in the BASH study? Range: 0 = not at all (0) to 10 = very much, mean ‐8.6 (SD 1.9) |
‐ |
| Vos 2011 | ‐ | Assistant measured weight to nearest 0.1 kg using electronic scale (model details provided) in underwear and barefoot. BMI calculated as kg/m2. Participants classified as obese using BMI gender‐ and age‐specific international cut‐off levels developed by Cole et al. BMI expressed as SDS for Dutch references. Waist measured with an anthropometric tape midway between lower rib margin and iliac crest at end of gentle expiration and expressed as SDS (waist circumference‐SDS) |
Height to nearest of 0.1 cm with a stadiometer (model details provided) | Disease‐generic measure for children with chronic diseases (DISABKIDS) and KIDSCREEN | ‐ | ‐ | ‐ | ‐ | ‐ |
| Christie 2011 | Questions on smoking activities, meal skipping, sociable eating and frequency of 5 fruit per day intake (did not appear to use validated tools). Actigraph accelerometer 7‐day measurement once in each participant to calibrate activity diary |
BMI (kg/m2) at end of intervention. Waist circumference using standardised protocol. Non‐invasive measurement of fat mass/% fat by bioimpedance scales (model details provided) |
‐ | PedsQL, Impact of Weight on Quality of Life‐Kids, see below. Rosenberg Self‐Esteem Scale: global self‐esteem scale valid and reliable for adolescents |
‐ | ‐ | ‐ | Structured and semi‐structured interviews administered to providers, young people and their parents to assess how acceptable the programme was, ease of delivery, participation and influence on weight, quality of life, self‐management, emotional, behavioural and family functioning | ‐ |
| Wengle 2011 | Nutritional tools not validated. Activity: all participants wore uniaxial accelerometer (model details provided) at mid‐axillary line using an elastic waist strap, except during sleep and water activities, for 7 days. Provided reliable and valid measurements of physical activity levels during walking, running and free‐living activities. Data from accelerometer downloaded to a computer, and software provided with accelerometer used to calculate number of steps and time spent in moderate‐to‐vigorous physical activity based on age‐dependent validated criteria. Activity (activity log) not validated |
Trained study staff.
Lightweight clothing with shoes removed. Weight measured to nearest 0.1 kg using combined standing stadiometer scale (model details provided). Waist circumference measured just above iliac crest |
Trained study staff.
Lightweight clothing with shoes removed. Height measured to nearest 1 cm using combined standing stadiometer and scale (model details provided) and measuring tape |
‐ | ‐ | ‐ | ‐ | Views measured by asking participants. Helpfulness of having a mentor score and get along with their mentor score | ‐ |
| Ford 2010 | ‐ | Body weight in kg measured with SECA scales to 1 decimal point. Waist circumference measured in cm to 1 decimal point with a standard anthropometric tape at the maximal circumference. MI adjusted for age and gender to give a BMI‐SDS with British 1990 growth reference data from the Child Growth Foundation. % body fat/body fat SDS by bioimpedence (reference provided) |
Height measured to nearest 0.1 cm with stadiometer | PedsQL | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nguyen 2012 | Self‐reported ‐ dietary intake assessed from 15‐item food frequency questionnaire. Unclear if validated, not data abstracted Physical activity and sedentary behaviours measured with Children's Leisure Activities Study Survey |
Anthropometry using standard procedures and calibrated instruments. Weight measured with portable scales (model details provided) to nearest 0.1 kg, with shoes and heavy clothing removed. By trained staff members, who are blinded to treatment allocation. BMI z scores calculated based on age‐ and gender‐specific reference values |
Height measured to nearest 0.1 cm using fixed stadiometer at The Children's Hospital at Westmead (Holtain Limited; Crymych, Dyfed, UK) or portable stadiometer (model details provided) when measurements were conducted at community health centres | SF‐36. 45‐item Harter Self‐Perception Profile for Adolescents provided measure of global self‐worth (1 = low and 4 = high) and perceived mean competence in 8 domains (scholastic, social acceptance, athletic, physical appearance, job, romantic appeal, close friendship, behavioural conduct). Importance attributed to each domain also measured using 16‐item scale |
‐ | ‐ | ‐ | Questions about satisfaction with the Loozit programme | ‐ |
| Grey 2009 | Questionnaire data collected by trained research staff. Health Behavior Questionnaire used to measure health behaviours and attitudes. Usual Food Choices (14‐items, measured student's usual food selections), Dietary Self‐Efficacy (15 items, e.g. "How sure are you that you can eat a baked potato instead of French fries?"), and Physical Activity Self‐Efficacy (5 items, e.g. "How sure are you that you can choose to jog during recess?") scales used. Validity and consistency measured. Revised Godin‐Shephard Activity Survey, a self‐administered instrument in which participants report number of times in an average week that they spent > 15 minutes in activities classified as mild (3 METs), moderate (5 METs) or strenuous (9 METs) |
Measured with scale (model details provided). Measured in light indoor clothing with bare feet | All measurements taken by research staff/nurses. Wall‐mounted stadiometer, calibrated in 1/8 cm intervals. Measured in light indoor clothing with bare feet | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vissers 2008 | ‐ | Weight measured using digital column scale (model details provided). % body fat assessed using BIA. Skinfold thickness measured using a calliper at 4 sites: biceps, triceps, subscapular, suprailiac. Hip and waist circumference measured to nearest 1 mm using non‐elastic measuring tape. Waist circumference measured placing tape in horizontal plane around abdomen midway between iliac crest and floating ribs at end of a normal expiration. WHR calculated |
Height measured to nearest cm using stadiometer (model details provided) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| NCT00132132 | ‐ | Change in BMI and proportion of participants with reduction in BMI not defined | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pitetti 2007 | ‐ | Weight measured to nearest 0.11 kg (or ¼ lb) measured on a standard physician's scale (model details provided) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Savoye 2007 | ‐ | Weight measured (with participant in socks with no shoes and wearing a light gown) in kg to
nearest 0.1 kg using medical weight scale (model details provided), zeroed and calibrated before each weight. % body fat determined by body fat analyser (model details provided). Total body fat calculated by multiplying % body fat by weight in kg |
Height measured with stadiometer (model details provided), calibrated in 0.1‐cm intervals | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| van Egmond‐Frohlich 2006 | Physical activity questionnaire | ‐ | ‐ | KINDL questionnaire | ‐ | ‐ | ‐ | ‐ | ‐ |
| Daley 2005 | Physical Activity Questionnaire for Adolescents used to collect participants' involvement in different physical activities. Each physical activity component scored between 1 = not involved to 5 = involved 5‐7 times per week | Weight measured to nearest 0.1 kg using balance scale. From these values, BMI values calculated. All values expressed as SDS (z scores), relative to current UK standards | Height measured to nearest completed 0.1 cm using wall‐mounted stadiometer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Resnicow 2005 | ‐ | Weight: Tanita scale (model details provided). Shoes, socks and outer clothing removed. % body fat: same scale as used for weight. Waist and hip circumference: tape measurements obtained twice per site. Waist measured at navel, and hip measurement taken at broadest points on hips and buttocks. Third measure obtained if first 2 measurements varied by > 2 cm |
Measured with a stadiometer | ‐ | ‐ | ‐ | ‐ | Intervention: brief questionnaire that queried their perceptions regarding overall programme and individual intervention elements. Comparator: similar questionnaire excluding items addressing elements not included in their condition, e.g. pagers |
‐ |
| Jiang 2005 | ‐ | Weight measured without outer clothing and calibrated to 0.1 kg | Height measured without shoes and calibrated to 0.1 cm | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Carrel 2005 | ‐ | Weight measured on calibrated beam balance platform scale to nearest 0.1 kg. % body fat and % fat‐free body mass measured by DXA |
Height measured on wall‐mounted stadiometer to nearest 0.5 cm. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ebbeling 2003 | Dietary intake: 7 days food diary (not validated) | BMI in kg/m2 Fat mass measured by DXA using Hologic instrumentation (model details provided) | Height measured using wall‐mounted stadiometer (model details provided) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Saelens 2002 | 2‐day dietary recall interview (not extracted as per review protocol). Seven‐Day Physical Activity Recall interview (validated). Sedentary behaviour through self‐report questionnaire (not validated). Cognitive dietary restraint and eating disinhibition through the Three‐Factor Eating Questionnaire (validated) |
Weight measured at baseline in the paediatric clinic using a calibrated standard digital scale. Weight measured at post‐treatment and follow‐up on calibrated balance beam scale | Stated height measured by stadiometer, no other details | ‐ | ‐ | ‐ | ‐ | Physician counselling, behavioural skills use and participant satisfaction questions | ‐ |
| Brownell 1983 | ‐ | Weight; % above mean weight. BMI, Developmental Index (based on normative changes in height and weight). Weight measured with balance‐beam scale in street clothes with no shoes | Measured with balance‐beam scale | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chandra 1968 | ‐ | Mean weight loss in kg. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| NCT00807560 | Moderate to Vigorous Physical Activity Measure. Sedentary Activity Checklist. Youth and Parent/Guardian Eating Questionnaire. PACE + Fruit Vegetable Screening Measure. PACE+ Dietary Fat Screening Measure |
Z score calculated using Baylor College of Medicine Children's Nutrition Research Center's online BMI calculator | ‐ | PedsQL | ‐ | ‐ | ‐ | ‐ | ‐ |
| BASH: Beverages and Student Health; BES: Body Esteem Scale; BIA: bioelectrical impedence analysis; BMI: body mass index; CDC: Centers for Disease Control and Prevention; CHQ: Child Health Questionnaire; DXA: dual‐energy x‐ray absorptiometry; ISAK: International Society for the Advancement of Kinathropometry; KINDL: Fragebogen für KINDer und Jugendliche zur Erfassung der gesundheitsbezogenen Lebensqualität (questionnaire for children and adolescents to record health‐related quality of life); MET: metabolic equivalent; MRI: magnetic resonance imaging; N/D: not defined; N/I: not investigated; PACE: Patient‐Centered Assessment and Counseling for Exercise; PedsQL: Paediatric Quality of Life; SD: standard deviation; SDS: standard deviation score; SF‐36: 36‐item Short Form; TV: television; WHO: World Health Organization; WHR: waist‐to‐hip ratio. | |||||||||
Appendix 8. Adverse events
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (n) | Deaths (n (%)) | Participants with adverse events (n (%)) | Participants with severe/serious adverse events (n (%)) | Participants discontinuing study due to adverse events (n (%)) | Participants hospitalised (n (%)) | Participants with outpatient treatment (n (%)) | Participants with specific adverse events (description) (n (%)) |
| Patsopoulou 2017 | I1: activity | 55 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: activity + diet | 55 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C1: no intervention | 56 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Jelalian 2016 | I1: CBT + healthy lifestyle | 17 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: CBT | 7 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Norman 2016 | I1: step down care | 53 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: enhanced usual care | 53 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hofsteenge 2014 | I1: group education | 71 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: dietitian only | 51 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Schranz 2014 | I1: resistance training | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: no intervention | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Visuthranukul 2015 | I1: low GI diet | 25 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: conventional diet | 27 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Wong 2015 | I1: standard weight loss diet + increase water intake | ‐ | |||||||
| C1: standard weight loss diet | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pakpour 2015 | I1: motivational interviewing | 80 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: motivational interviewing + parental involvement | 119 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C1: passive control | 119 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Bean 2014 | I1: motivational interviewing | 58 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: education control | 41 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Carraway 2014 | I1: mentor‐led exercise | 11 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: wait list control | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Sigal 2014 | I1: diet + aerobic training | 75 | ‐ | 19 (25) | 0 | 2 (3) | 0 | ‐ | Upper body: 3 (4) Lower body: 9 (12) Musculoskeletal injury: 0 Anxiety or depression: 1 (1) Headache: 0 Fainting: 0 Respiratory infection: 0 Other: 1 (1) |
| I2: diet + resistance training | 78 | ‐ | 14 (19) | 0 | 0 | 0 | ‐ | Upper body: 4 (5) Lower body: 10 (13) Musculoskeletal injury: 1 (1) Anxiety or depression: 0 Headache: 0 Fainting: 0 Respiratory infection: 1 (1) Other: 0 | |
| I3: diet + aerobic+ resistance training | 75 | ‐ | 16 (21) | 0 | 0 | 0 | ‐ | Upper body: 7 (9) Lower body: 8 (11) Musculoskeletal injury: 2 (3) Anxiety or depression: 0 Headache: 0 Fainting: 0 Respiratory infection: 0 Other: 2 (3) | |
| C1: diet only | 76 | ‐ | 18 (24)a | 0 | 0 | 0 | ‐ | Upper body: 6 (8) Lower body: 4 (5) Musculoskeletal injury: 0 Anxiety or depression: 3 (4) Headache: 1 (1) Fainting: 1 (1) Respiratory infection: 1 (1) Other: 2 (3) | |
| Love‐Osborne 2014 | I1: motivational interviewing | 82 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 83 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kong 2014 | I1: low GI diet | 52 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: usual Chinese diet | 52 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Luna‐Pech 2014 | I1: normocaloric diet + physical activity | 29 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: no intervention | 29 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Boodai 2014 | I1: multicomponent group sessions | 41 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: no intervention | 41 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Gourlan 2013 | I1: motivational Interviewing + standard weight loss | 28 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard weight loss | 34 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Patrick 2013 | I1: website intervention | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: website + group | 26 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| I3: website + SMS | 24 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C1: usual care | 25 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kong 2013 | I1: ACTION | 31 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 29 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pbert 2013 | I1: "Lookin' Good Feelin' Good" | 42 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 40 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Brennan 2013 | I1: motivational interviewing | 42 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: wait list control | 21 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Walpole 2013 | I1: motivational Interviewing | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: social skills training | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Toulabi 2012 | I1: behavioural modification | 76 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 76 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Debar 2012 | I1: multicomponent intervention | 105 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: usual care | 103 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Ebbeling 2012 | I1: multicomponent intervention | 110 | ‐ | 7 (6.4) | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 114 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vos 2011 | I1: family‐based CBT + nutrition | 41 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: wait list control | 40 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| I1: HELP weight management | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C1: enhanced standard care | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Wengle 2011 | I1: mentored lifestyle intervention | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: unmentored lifestyle intervention | 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Ford 2010 | I1: Mandometer | 44 | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 43 | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nguyen 2012 | I1: Loozit + ATC | 78 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: Loozit | 73 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Grey 2009 | I1: coping skills | 112 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: general education | 86 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vissers 2008 | I1: school‐based intervention | 37 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 39 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| NCT00132132 | I1: behavioural education | 15 | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 15 | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Pitetti 2007 | I1: treadmill | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Savoye 2007 | I1: Bright Bodies weight management | 105 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 69 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| van Egmond‐Frohlich 2006 | I1: multicomponent intervention | 250 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 271 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Daley 2005 | I1: exercise counselling | 28 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: exercise placebo | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: control | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Resnicow 2005 | I1: GoGirls high‐intensity behavioural intervention | 53 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: moderate‐intensity behavioural intervention | 70 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Jiang 2005 | I1: family‐based intervention | 36 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: control | 39 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Carrel 2005 | I1: lifestyle‐focused gym classes | 27 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard gym classes | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Ebbeling 2003 | I1: low GL diet | 8 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: conventional diet | 8 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Saelens 2002 | I1: healthy habits | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: standard care | 21 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Brownell 1983 | I1: mother + child separate | 14 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: mother + child together | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C1: child only | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chandra 1968 | I1: low‐calorie formula Limical | 18 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: low‐calorie diet | 17 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| NCT00807560 | I1: family‐based therapy for paediatric overweight | 38 | |||||||
| C1: nutritional educational control condition | 39 | ‐ | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported. ATC: additional therapeutic contact; C: comparator; CBT: cognitive behavioural therapy; GI: glycaemic index; GL: glycaemic load; HELP: Healthy Eating and Lifestyle Programme; I: intervention; n: number of participants; SMS: short message service. a injuries in the control group related to intervention occurred during run‐in (prior to randomisation). | |||||||||
Appendix 9. Checklist to aid consistency and reproducibility of GRADE assessments
| Changes in BMI, BMI z score, body weight | Adverse events | Health‐related quality of life | ||
| Study limitations (risk of bias)a | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | |
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | No | Unclear | No (↓) | |
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | No | Unclear | No | |
| 5. Was an objective outcome used? | Yes | Unclear | No | |
| 6. Were x 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | No (↓) | Yes | |
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | No (↓) | No (some studies did not report health‐related quality of life despite mentioning) | |
| 8. No other biases reported (i.e. no potential of other bias)? | Yes | No (↓) | Yes | |
| 9. Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | |
| Inconsistencyb | 1. Point estimates did not vary widely? | No (some variability) | N/A | Yes |
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Some | N/A | Some | |
| 3. Was the direction of effect consistent? | Yes | N/A | Yes | |
| 4. What was the magnitude of statistical heterogeneity (as measured by I2) ‐ low (I2 < 40%), moderate (I2 40%‐60%), high I2 > 60%)? | High (↓) | N/A | High (↓) | |
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | Statistically significant (↓) | N/A | Statistically significant (↓) | |
| Indirectnessa | 1. Were the populations in included studies applicable to the decision context? | Highly applicable | Applicable | Highly applicable |
| 2. Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |
| 3. Was the included outcome not a surrogate outcome? | No (↓) for BMI and BMI z score | Yes | Yes | |
| 4. Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | |
| 5. Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |
| Imprecisionc | 1. Was the confidence interval for the pooled estimate not consistent with benefit? | No | N/A | No |
| 2. What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?e | High | High | High | |
| 3. What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?e | Large | Moderate | Moderate | |
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | Unclear | N/A | |
| Publication biasd | 1. Was a comprehensive search conducted? | Yes | Yes | Yes |
| 2. Was grey literature searched? | Yes | Yes | Yes | |
| 3. Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |
| 4. There was no industry influence on studies included in the review? | Yes | Yes | Yes | |
| 5. There was no evidence of funnel plot asymmetry? | Unclear | N/A | Unclear | |
| 6. There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | |
|
aQuestions on risk of bias were answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency were primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I2. cWhen judging the width of the confidence interval it was recommended to use a clinical decision threshold to assess whether the imprecision was clinically meaningful. dQuestions addressed comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepended on the context of the systematic review area. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table. BMI: body mass index; N/A: not applicable. | ||||
Appendix 10. Survey of study investigators providing information on included trials
| Trial ID | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Cocca 2016 | 12 January 2017 | No | 12 January 2017 Aim of the study, participants weight status at baseline, weight outcomes |
N/A |
| Jelalian 2016 | 17 October 2016 | 18 October 2016 | 17 October 2016 Details of the intervention, randomisation and blinding |
18 October 2016 Parents were involved in both conditions, research assistance were blinded, computer randomisation. |
| Norman 2016 | 3 February 2016 | 4 February 2016 | 05 February 2016 Allocation concealment, details of blinding, selective reporting of outcomes and control outcomes |
11 March 2016 Participants were randomised. Randomisation was performed by permutated block algorithm. Measurement staff were blinded. There were other outcome measures that were not included in the paper. These included the following: physical activity based on self‐report, quality of life, calories, food and nutrient intake based on self‐report, sedentary behaviour based on self‐report. |
| Hofsteenge 2014 | 21 October 2015 | 22 October 2015 | No information required | N/A |
| Schranz 2014 | 22 October 2015 | 22 October 2015 | No information required | N/A |
| Visuthranukul 2015 | 29 October 2015 | 30 October 2015 | 19 November 2015 Details of blinding, selective reporting of outcomes |
‐ |
| Christie 2011 | 22 October 2015 | 23 October 2015 | 22 October 2015 Request for BMI and further details on risk of bias (randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes) |
23 October 2015 Cannot release data before publication |
| Wong 2015 | 17 January 2017 | 18 January 2017 | 18 January 2017 Further data |
18 January 2017 Unable to share data at this stage |
| Carraway 2014 | 17 January 2017 | N/A | Stratified results, allocation concealment, details of blinding, selective reporting of outcomes | N/A |
| Pakpour 2015 | 22 October 2015 | 23 October 2015 | No information required | N/A |
| Sigal 2014 | 22 October 2015 | 22 October 2015 | No information required | N/A |
| Love‐Osborne 2014 | 22 October 2015 | No | N/A | N/A |
| Kong 2014 | 30 October 2015 | 30 October 2015 | No information required | N/A |
| Luna‐Pech 2014 | 22 October 2015 | No | N/A | N/A |
| Gourlan 2013 | 23 October 2015 | 26 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
‐ |
| Patrick 2013 | 23 October 2015 | 23 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
‐ |
| Kong 2013 | 22 October 2015 | No | N/A | N/A |
| Pbert 2013 | 23 October 2015 | 23 October 2015 | 26 October 2015 Request randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
28 October 2015 Computer random number generator. Random allocation was conducted by the study statistician and investigators were blinded to allocation. Impractical to blind participants and personnel. Reported on all physiological and eating and activity behavioural outcomes. We did not report on psychosocial outcomes that could be considered intermediary variables. |
| Brennan 2013 | 23 October 2015 | 5 November 2015 | 19 November 2015 Allocation concealment and selective reporting of outcomes |
‐ |
| Walpole 2013 | 23 October 2015 | No | N/A | N/A |
| Toulabi 2012 | 13 January 2015 | No | N/A | N/A |
| Ebbeling 2012 | 23 October 2015 | 23 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
12 November 2015 Data management software allocated treatment assignment. Participants and staff implementing the beverage delivery were not masked to group assignment. All personnel who assessed study outcomes were masked to group assignment. All outcomes measured were reported |
| Vos 2011 | 23 October 2015 | 23 October 2015 | No information required | N/A |
| Nguyen 2012 | 21 October 2015 | No | N/A | N/A |
| Wengle 2011 | 23 October 2015 | 23 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
N/A |
| Bean 2014 | 22 October 2015 | 22 October 2015 | 26 October 2015 Published data, anticipated date of completion |
N/A |
| Ford 2010 | 23 October 2015 | 23 October 2015 | 26 October 2015 Allocation concealment, blinding, and attrition at 18 months |
27 October 2015 Allocation concealed. Not possible to blind. Lost to follow‐up at 18 months (stopped attending obesity clinic) |
| Grey 2009 | 23 October 2015 | 26 October 2015 | 29 October 2015 Request randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
29 October 2015 Randomisation was electronic with permuted blocks. Allocation was conducted by the study statistician to assure concealment. Blinding was not carried out. Reported all the outcomes measured except for psychosocial outcomes which we are writing a manuscript for and anticipated submission date January 2016 |
| Vissers 2008 | 29/09/2015 | No | N/A | N/A |
| NCT00132132 | 22 October 2015 | No | N/A | N/A |
| Pitetti 2007 | 29 October 2013 23 October 2015 |
29 October 2013 23 October 2015 |
29 October 2013 23 October 2015 Request randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
23 October 2013 and 29 October 2015 Randomised allocation using random assignment or random placement. We flipped a coin and "heads" assigned the participant to the treadmill group and "tails" assigned the participants to the control group. Participants were severely developmentally disabled therefore cannot identify group allocation. Outcome assessors were not part of the research team. All outcomes were reported |
| Savoye 2007 | 23 October 2015 | 26 October 2015 | 29 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
29 October 2015 Randomisation was electronic with permuted blocks. Randomisation sequence was maintained by the study statistician to assure concealment. Participants were not blinded to treatment group. Personnel were not blinded during treatment phase either because they knew if subject was going to the programme. All outcomes measured except psychosocial outcomes are reported, these are being drafted for publication (self‐concept of children and family dynamics of family using Piers‐Harris Self Concept Scale and Family Assessment Device, respectively). Submission aimed January 2016 |
| Boodai 2014 | 22 October 2015 | 25 October 2015 | No information required | N/A |
| Debar 2012 | 6 August 2015 | 7 August 2015 | 6 August 2015 Published data |
7 August 2015 A pilot study of a larger trial (already included in this review) |
| Resnicow 2005 | 23 October 2015 | No | N/A | N/A |
| Daley 2005 | 23 October 2015 | No | N/A | N/A |
| Jiang 2005 | 23 October 2015 | No | N/A | N/A |
| Carrel 2005 | 23 October 2015 | 23 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
26 October 2015 Random number generator from our statistician to be assigned to intervention or control group. participants were not blinded to their intervention (they knew if they were in the intervention or control class). We reported all outcomes |
| Ebbeling 2003 | 23 October 2015 | 23 October 2015 | 26 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
12 November 2015 Allocation in sealed envelopes, opened by dietitian in sequence. No details of whether envelopes were opaque. Participants and staff implementing the dietary interventions were not masked to group assignment. All personnel who assessed study outcomes were masked to group assignment. Not all outcomes measured were reported |
| Saelens 2002 | 23 October 2015 | 30 October 2015 | 30 October 2015 Randomisation procedures, allocation concealment, details of blinding, selective reporting of outcomes |
|
| Brownell 1983 | No e‐mail available | N/A | N/A | N/A |
| Chandra 1968 | No e‐mail available | N/A | N/A | N/A |
| NCT00807560 | 5 August 2015 | N/A | 5 August 2015 Published data |
N/A |
| ACTRN12607000632493 | 22 October 2015 | N/A | 22 October 2015 Published data |
N/A |
| ACTRN12611000862943 | 18 January 2017 | Out of office | 18 January 2017 Trial status |
N/A |
| Chew 2016 | 12 January 2017 | N/A | 12 January 2017 Six months data |
N/A |
| TCTR20130515001 | 02 September 2016 | 5 September 2016 | 2 September 2016 Published data |
5 September 2016 Manuscript amendments, no published data |
| EUCTR2009‐016921‐32‐ES | 6 August 2015 6 October 2016 |
N/A | 6 August 2015 6 October 2016 Published data |
N/A |
| IRCT201012235440N1 | 21 October 2016 6 October 2016 |
N/A | 21 October 2016 6 October 2016 Published data |
N/A |
| ISRCTN04152711 | 22 October 2015 | N/A | 22 October 215 Published data |
N/A |
| NCT00562263 | 1 September 2016 | N/A | 1 September 2016 Published data |
N/A |
| NCT00940966 | 5 August 2015 22 October 2016 |
5 August 2015 22 October 2016 N/A |
Published data | NA |
| NCT01677923 | 2 September 2016 | N/A | 2 September 2016 Published data |
N/A |
| NCT00940966 | 5 August 2015 06 October 2016 |
N/A | 5 August 2015 06 October 2016 Published data |
N/A |
| NCT01764113 | 5 August 2015 | 5 August 2015 | 5 August 2015 Published data |
5 August 2015 Ongoing and will notify once complete |
| NCT01794546 | 22 October 2015 | 23 November 2015 | 22 October 2015 Published data |
23 November 2015 Ongoing and cannot share |
| NCT02086851 | 21 October 2015 | N/A | 21 October 2015 Published data |
N/A |
| NCT02228278 | 2 September 2016 | 06 September 2016 | 2 September2016 Published data |
6 September 2016 Currently drafting a manuscript |
| NCT00998413 | 22 October 2015 22 October 2016 |
E‐mail error | 22 October 215 22 October 2016 Published data |
N/A |
| Patsopoulou 2017 | 16 January 2017 | 18 January 2017 | 16 January2017 Further data/anticipated date of publication |
18 January 2017 Shared published manuscript |
| NCT02745795 | 12 January 2017 | N/A | 12 January 2017 Anticipated completion date |
N/A |
| NCT02794090 | 13 January 2017 | N/A | 13 January 2017 Further data/anticipated date of publication |
N/A |
| Ramalho 2016 | 16 January 2017 | 16 January 2017 | 16 January2017 Further data/anticipated date of publication |
16 January 2017 Initial phase of the project |
| N/A: not applicable | ||||
Appendix 11. Health‐related quality of life: instruments
| Trial ID | Name (type of measurement) | Dimensions (subscales) (number of items) | Validated instrument | Answer options | Scores | Direction of scales | Minimal important difference |
| Hofsteenge 2014 | PedsQL 4.0 | Physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), school functioning (5 items) | Yes | 5‐point Likert scale (0 = never a problem; 4 = almost always a problem) | Items reversed scored and linearly transformed to 0‐100 scale. A total scale score and physical and psychosocial health summary scores also calculated | Higher scores indicate better HRQoL | ‐ |
| CHQ Child Form | 12 domains assess physical, behavioural, mental, social functioning | Yes | Each item contains 4, 5 or 6 response alternatives | Items summed up (some recoded/recalibrated) and transformed to 0‐100 scale. A physical summary scale computed, mean of CHQ‐subscales physical functioning, role/social limitations‐physical, general health perceptions, bodily pain. Also a psychosocial summary scale, mean of CHQ‐subscales of role/social limitations emotional, role/social limitations‐behavioural, self‐esteem, mental health and general behaviour | Higher scores indicate better HRQoL | ‐ | |
| Pakpour 2015 | PedsQL 4.0 SF‐15 | 15 items covering 4 dimensions: physical functioning scale (5 items), emotional functioning scale (4 items), social functioning scale (3 items), school functioning scale (3 items) | Yes | ‐ | Items reverse scored and linearly transformed to scale of 0‐100. | Higher scores indicate better QoL | ‐ |
| Sigal 2014 | PedsQL ‐ Adolescent version | Physical functioning (8‐items), emotional functioning (5‐items), social functioning (5‐items), school functioning (5‐items) | Yes | ‐ | Total scale score derived by mean of all 23 items, as is a psychosocial health summary scored (derived from mean of items in the emotional, social, school functioning subscales) | ‐ | ‐ |
| Luna‐Pech 2014 | PAQLQ, Spanish Version | Symptoms, activity limitation and emotional function | Yes | 7‐point interval scale, from 1 = severe impairment to 7 = no impairment | ‐ | Higher scores indicate better outcomes | 0.5 |
| Patrick 2013 | Ped QL | 23‐items | Yes | ‐ | 0‐100 scale | Higher scores indicate better QoL | ‐ |
| Debar 2012 | PedsQL | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Nguyen 2012 | Mental Health Inventory 5, a mental health assessment component of the Medical Outcomes Study SF‐36 | 5 questions | Yes | ‐ | ‐ | 5 indicating most favourable health and 30 indicating least favourable health |
‐ |
| Christie 2011 | PedsQL | Physical, emotional, social, school; 2 domain scores (physical and psychosocial functioning); total score | Yes | ‐ | ‐ | ‐ | ‐ |
| IWQOL‐ Kids | 27 items, 4 scales: physical comfort (6 items), body esteem (9 items), social life (6 items), family relations (6 items) | Yes | ‐ | ‐ | ‐ | ‐ | |
| Vos 2011 | DISABKIDS and KIDSCREEN | DISABKIDS: 37 questions divided over 6 subscales of 6‐7 items each. Only the first 5 subscales (31 items) used to calculate HRQoL, because the last subscale focuses on medication use related to a disease. QOL‐physical, QOL‐independence, QOL‐emotion, QOL‐social exclusion, QOL‐social inclusion. KIDSCREEN: 52 questions divided over 10 subscales of 3‐7 items each |
Yes | 5‐point scale: 0 = never , 1 = almost never/seldom, 2 = average/sometimes, 3 = quite often, 4 = always | Total and domains scores of questionnaire expressed as a % between 0 and 100 | Higher scores reflect better HRQoL | ‐ |
| Ford 2010 | PedsQL 4.0 | ‐ | Yes | ‐ | ‐ | ‐ | ‐ |
| van Egmond‐Frohlich 2006 | KINDL‐K (self‐report) and KINDL‐E (parents) | 24 items with subscales family, friends, school, self‐worth, mental and physical well‐being (4 items per domain) | Yes | Children: 3‐point scale: never, sometimes, very often. Parents: 5‐point scale: never, seldom. sometimes. often always |
Total score, mean score, transformed score (0‐100) | Higher scores reflect better HRQoL (10 or 11 items have to be reversed) | ‐ |
| CHQ: Child Health Questionnaire; DISABKIDS: disease‐generic measure for children with chronic diseases; HRQoL: health‐related quality of life; IWQOL: Impact of Weight on Quality of Life; PAQLQ: Standardized Paediatric Asthma Quality of Life Questionnaire; PedsQL: Paediatric Quality of Life; QoL: quality of life; SF‐15: 15‐item Short Form; SF‐36: 36‐item short‐form health survey. | |||||||
Appendix 12. Self‐esteem: instruments
| Trial ID | Name (type of measurement) | Dimensions (subscales) (number of items) | Validated instrument | Answer options | Scores | Direction of scales | Minimal important difference |
| Hofsteenge 2014 | BES | General feelings about appearance, weight satisfaction and evaluations of attributions to others about one's body and appearance | Yes | Range of scores from 0 = worst possible score to 4 = best possible score | Total mean scores calculated | Higher scores represent a better body esteem | ‐ |
| Schranz 2014 | PSW scale | Measures global self‐worth | Yes | 4‐choice structured alternative format | To minimise socially desirable responses, the PSW scale uses a 4‐choice structured alternative format (e.g. 1 scenario presented to participants is as follows: 'Some kids are proud of themselves physically', but 'Other kids don't have much to be proud of physically') | Higher score (or positive effect size change) indicates higher self‐efficacy/confidence or beliefs/self‐perception | ‐ |
| SPPA | Measures youth's perceived competence in academics and other areas (e.g. athletics) as well as their sense of general self‐worth | Yes | 4‐choice structured alternative format | Using the same 4‐choice structured alternative format and scoring scale as the PSW scale | Higher score (or positive effect size change) indicates higher self‐efficacy/confidence or beliefs/self‐perception | ‐ | |
| Pakpour 2015 | WEL Questionnaire | 20‐item tool assessing adolescents' confidence in their ability to lose weight | Yes | 10‐point Likert scale, ranging from 0 = not confident to 9 = very confident | Items scored on a 10‐point Likert scale and items scores were averaged | Higher scores indicate better self‐esteem | ‐ |
| Patrick 2013 | Rosenberg Self‐Esteem Scale | 10‐item survey that measures self‐esteem | Yes | Each item has 4‐point Likert scale ranging from 1 = strongly agree to 4 = strongly disagree | Includes 10 items and respondents indicate their level of agreement to each item, score range 10‐40 | Higher scores indicate a positive self‐attitude while a low score indicates a negative attitude towards the self | ‐ |
| Brennan 2013 | Rosenberg Self‐Esteem Scale |
‐ | Yes | ‐ | ‐ | ‐ | ‐ |
| Debar 2012 | Rosenberg Self‐Esteem Scale |
‐ | Yes | ‐ | ‐ | ‐ | ‐ |
| Nguyen 2012 | Harter Self‐Perception Profile for Adolescents | 45‐item provided a measure of global self‐worth | Yes | 1 = low and 4 = high | ‐ | Higher scores indicate better self‐esteem | |
| Daley 2005 | Harter's Self‐Perception Profile for Adolescents | Global self‐worth subscale assesses extent to which participants like themselves as a person and the way they are living their lives | Yes | 6 items devised in a structured alternative format on a scale between 1 = low competence and 4 = high competence | Score responses 1‐4 | Higher scores indicate better global self‐worth | ‐ |
| BES: Body Esteem Scale; PSW: Physical Self‐Worth; SPPA: Self‐Perception Profile for Adolescents; WEL: Weight Efficacy Lifestyle. | |||||||
Data and analyses
Comparison 1. Interventions (all) versus controls, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BMI change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 3 BMI percentile change | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Weight change | 20 | 1993 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐5.21, ‐2.13] |
Comparison 2. Interventions versus controls, by duration of intervention, less than 6 months, greater than 6 months, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 1.1 BMI interventions ≤ 6 months | 19 | 1863 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.79, ‐0.55] |
| 1.2 BMI interventions > 6 months | 9 | 911 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.04, ‐0.41] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 2.1 BMI‐z interventions ≤ 6 months | 12 | 1539 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 2.2 BMI‐z interventions > 6 months | 8 | 860 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.46, ‐0.07] |
Comparison 3. Interventions (all) versus controls, other anthropometrics, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % body fat | 14 | 1886 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.69, ‐0.46] |
| 2 % trunk fat | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐3.10, 1.43] |
| 3 Waist circumference | 17 | 1997 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐3.80, ‐0.72] |
| 4 Waist‐to‐height ratio | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 5 Waist‐to‐hip ratio | 2 | 211 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 6 Fat mass | 5 | 673 | Mean Difference (IV, Random, 95% CI) | ‐3.13 [‐4.70, ‐1.56] |
| 7 Trunk fat mass | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.49, 0.61] |
| 8 Lean mass | 3 | 417 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.88, 0.47] |
| 9 % overweight | 2 | 73 | Mean Difference (IV, Random, 95% CI) | ‐5.55 [‐13.67, 2.57] |
Comparison 4. Interventions (all) versus controls, quality of life, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health‐related quality of life | 7 | 972 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.09, 0.79] |
| 2 Health‐related quality of life by tool | 7 | 972 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.09, 0.79] |
| 2.1 Pediatric Quality of Life Inventory | 4 | 703 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.07, 0.39] |
| 2.2 Other self‐reported tools | 3 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.98 [‐0.35, 2.31] |
| 3 Self‐esteem | 6 | 613 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.27] |
| 4 Self‐esteem by outcome | 6 | 613 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.27] |
| 4.1 Self‐esteem | 3 | 325 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.34] |
| 4.2 Global self‐worth | 3 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.40] |
| 5 Self‐esteem by tool | 6 | 613 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.08, 0.27] |
| 5.1 Rosenberg Self‐Esteem Scale | 3 | 325 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.39, 0.34] |
| 5.2 Self‐Perception Profile for Adolescents | 3 | 288 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.40] |
4.2. Analysis.
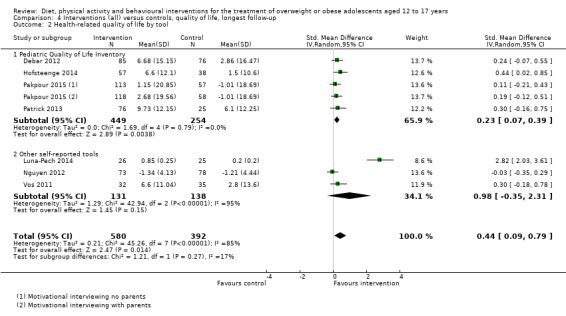
Comparison 4 Interventions (all) versus controls, quality of life, longest follow‐up, Outcome 2 Health‐related quality of life by tool.
Comparison 5. Interventions (all) versus controls, behavioural change, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical activity (mild ‐ vigorous physical activity) | 3 | 129 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐15.07, 15.85] |
| 2 Physical activity length | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.98, 0.75] |
| 3 Screen time | 2 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.65, ‐0.55] |
Comparison 6. Interventions versus control by duration of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 1.1 BMI 6‐9 months | 13 | 1116 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.91, ‐0.59] |
| 1.2 BMI 12 months | 9 | 898 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.70, 0.12] |
| 1.3 BMI 18‐24 months | 6 | 760 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐2.56, ‐0.41] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 2.1 BMI z score 6‐9 months | 8 | 461 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.20, 0.05] |
| 2.2 BMI z score 12 months | 7 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.11, 0.00] |
| 2.3 BMI z score 18‐24 months | 5 | 602 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.66, ‐0.02] |
Comparison 7. Interventions versus controls by duration of postintervention follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 24 | 2594 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.69, ‐0.54] |
| 1.1 No postintervention follow‐up | 12 | 1004 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.49, ‐0.26] |
| 1.2 < 6 months | 7 | 683 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.76, ‐0.30] |
| 1.3 6 to < 12 months | 3 | 524 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐2.17, 0.19] |
| 1.4 ≥ 12 months | 2 | 383 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐3.95, 0.96] |
| 2 BMI z score change | 17 | 2253 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| 2.1 No postintervention follow‐up | 9 | 687 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.39, 0.01] |
| 2.2 < 6 months | 2 | 163 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.08] |
| 2.3 6 to < 12 months | 4 | 1162 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.14, 0.02] |
| 2.4 ≥ 12 months | 2 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.21, ‐0.09] |
Comparison 8. Interventions by control type, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 1.1 Interventions vs no intervention/wait list control | 6 | 992 | Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐2.73, ‐0.85] |
| 1.2 Interventions vs usual care controls | 13 | 763 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [0.00, ‐0.83] |
| 1.3 Interventions vs concomitant therapy controls | 9 | 1019 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.93, 0.14] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.22, ‐0.05] |
| 2.1 Interventions vs no intervention/wait list control | 4 | 527 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.42, ‐0.05] |
| 2.2 Interventions vs usual care controls | 13 | 1583 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.04] |
| 2.3 Interventions vs concomitant therapy controls | 3 | 289 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.05, 0.16] |
8.2. Analysis.
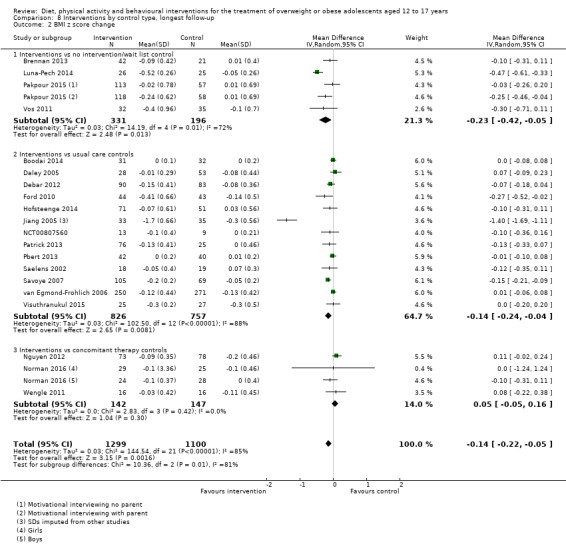
Comparison 8 Interventions by control type, longest follow‐up, Outcome 2 BMI z score change.
Comparison 9. Interventions by mode, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 26 | 2726 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.65, ‐0.66] |
| 1.1 Group interventions | 14 | 1641 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.10, ‐0.55] |
| 1.2 Individual interventions | 9 | 984 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.52, ‐0.27] |
| 1.3 Mixed interventions | 3 | 101 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.89, ‐0.69] |
| 2 BMI z score change | 19 | 2377 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.05] |
| 2.1 Group interventions | 9 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.02] |
| 2.2 Individual interventions | 8 | 1015 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.45, ‐0.06] |
| 2.3 Mixed interventions | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.14] |
Comparison 10. Interventions by setting, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 27 | 2750 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.66, ‐0.68] |
| 1.1 School based | 7 | 613 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.97, 0.15] |
| 1.2 Community based | 7 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.11, ‐0.29] |
| 1.3 Healthcare based | 13 | 1107 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐1.81, ‐0.82] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 2.1 School based | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.06, 0.66] |
| 2.2 Community based | 3 | 289 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.15] |
| 2.3 Healthcare based | 15 | 1960 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.17, ‐0.03] |
Comparison 11. Interventions versus controls by intervention type, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 1.1 Multidisciplinary interventions | 22 | 2298 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.75, ‐0.62] |
| 1.2 Physical activity only | 4 | 199 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.21, ‐0.40] |
| 1.3 Diet only | 3 | 277 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.29, 0.06] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 2.1 Multidisciplinary interventions | 17 | 2209 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.03] |
| 2.2 Physical activity only | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Diet only | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.55, 0.04] |
Comparison 12. Interventions versus controls by psychological approach, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 27 | 2652 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.33, ‐0.55] |
| 1.1 Cognitive behavioural | 6 | 553 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.69, ‐0.00] |
| 1.2 Motivational interviewing | 4 | 570 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.21, 0.13] |
| 1.3 Other psychological theory | 9 | 680 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.25, ‐0.42] |
| 1.4 No theoretical basis/no psychological component | 8 | 849 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.21, ‐0.45] |
| 2 BMI z score change | 18 | 1856 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.05] |
| 2.1 Cognitive behavioural | 5 | 528 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 2.2 Motivational interviewing | 2 | 409 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.01] |
| 2.3 Other psychological theory | 8 | 729 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.36, ‐0.02] |
| 2.4 No theoretic basis/no psychological component | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.55, 0.04] |
Comparison 13. Interventions versus controls by parental involvement, longest follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body mass index (BMI) change | 28 | 2774 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.67, ‐0.69] |
| 1.1 Parent involvement | 18 | 1820 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.90, ‐0.35] |
| 1.2 No parental involvement | 13 | 954 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.76, ‐0.67] |
| 2 BMI z score change | 20 | 2399 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 2.1 Parental involvement | 14 | 1370 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.03] |
| 2.2 No parental involvement | 7 | 1029 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.25, 0.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bean 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 11‐18 years; BMI ≥ 85th percentile for age and gender (CDC 2000); parent willing to participate; an identified primary care physician; no underlying medical condition which would preclude weight loss through behavioural intervention Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: one Treatment before study: all participants had been enrolled in the TEENS RCT. Parents attended biweekly groups. Participants met on alternating weeks with a dietitian and behavioural support specialist for 6 months. Supervised physical activity at least 3 times per week Description of interventions MI Values : 3 main components of TEENS family‐based intervention are physical activity, dietary intervention and behaviour support. MI Values targets adolescents alone to enhance autonomy for change. 2 × 30‐minute sessions at weeks 1 and 10. Trained interventionists were independent of TEENS. Participants selected target behaviours and included TEENS participation, diet, exercise or a combination. Session 1 included a values clarification exercise, session 2, explored progress in TEENS, re‐examined value/behaviour congruency and elicited participant ideas for change Education control : 30‐minute videos focused on healthy eating and exercise for adolescents at weeks 1 and 10. Participants complete a knowledge quiz to ensure treatment adherence All participants proceeded with TEENS multidisciplinary obesity treatment programme as usual |
|
| Outcomes | Outcomes reported in abstract of publication: adherence (overall, dietitian visits, behavioural support visits) | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT00167830 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to investigate if brief (two‐session) MI can enhance treatment effects among adolescents with obesity enrolled in a multidisciplinary treatment [the T.E.E.N.S. (Teaching Encouragement Exercise Nutrition Support) Program]." | |
| Notes | Implemented within the TEENS programme. Publication reported no eligible outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "participants are randomized to treatment condition using a random number generator" Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "T.E.E.N.S. interventionists (dietitian and behavioral support specialists) are blind to participant treatment condition" Comment: suggests that the MI interventionists were unblinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details but low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "Ninety‐nine participants were randomized and completed baseline assessments…" Comment: no details of numbers randomised who did not complete baseline assessments |
| Selective reporting (reporting bias) | Unclear risk | Comment: limited outcomes published to date, study reports that BMI‐percentile to be published |
| Other bias | Unclear risk | Comment: trial reported no data and it was unclear if they tested for interaction |
Boodai 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 10‐14 years, obese (BMI) > 95th percentile, have at least 1 parent who expressed a willingness to attend the intervention, no serious underlying medical condition that might be either a cause or consequence of their obesity Exclusion criteria: non‐obese, major disease, underlying pathological cause of obesity, aged < 10 years or > 14 years at study inception, not attending a mainstream school in the public sector, unable or unwilling to attend treatment sessions if randomised to intervention group Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: one Treatment before study: none Description of interventions Low‐intensity group programme : 6 sessions of 1 hour. Delivered by a physician with specialist training in nutrition and a dietitian. Programme adapted from the Scottish Childhood Obesity Treatment Trial. Treatment materials were provided. Sessions focused on changing behaviours such as reduction in sedentary behaviour (screen‐media use); diet (used a modified 'traffic light diet' reference given); promotion of physical activity Theoretically based on behaviour change techniques to all 3 of the targeted behaviours (including self‐monitoring of sedentary behaviour; identifying the main barriers to behaviour change; problem solving; goal setting; relapse prevention). Ideally at least 1 parent should have been present The intervention group split into boys (n = 21) and girls (n = 20) in accordance with cultural norms of Kuwait. Sessions were delivered on 2 consecutive days Control : informed that they were obese and advised to attend primary care |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score | |
| Study details |
Run‐in period: none Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: ISRCTN37457227 |
|
| Stated aim for study | Quote from publication: "to test the hypothesis that a 'good practice' intervention for the treatment of adolescent obesity in Kuwait would have a greater effect on primary and secondary outcomes than allocation to a control group. The secondary aims were to test the feasibility of conducting such a trial in Kuwait and to test the feasibility of using a good practice intervention and referral to primary care as a control condition." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "computer generated randomisation list that allocated participants to intervention or control group, with participants balanced for gender in blocks of 10." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "participants assigned a unique study code prior to random allocation … To ensure concealment of allocation, codes were sent electronically to a statistician (JHM) who produced a computer generated randomisation list." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "The statistician informed the researcher responsible for delivering the intervention (SAB) of the allocation, and families were invited to intervention or control groups as appropriate." Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "Outcome measures were made by the same trained research assistants who were blinded to group allocation and were not involved in delivery of the treatment intervention." Comment: investigator‐assessed |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "The analysis was intention‐to‐treat, where we used data from all adolescents for whom data were available on the basis of the group to which they were allocated, regardless of their adherence to the protocol (attendance)." Comment: reported and reasons explained ITT is a modified ITT as only those who had data were included |
| Selective reporting (reporting bias) | High risk | Comment: quality of life, blood lipids, fat‐mass and fat‐free mass not reported |
| Other bias | Unclear risk | Comment: no participants in the control arm attended primary care and received the intervention |
Brennan 2013.
| Methods | Cross‐over RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 11‐19 years, overweight or obese according to the international cut‐off points for BMI in children, living with a parent or adult carer who was prepared to be involved in treatment Exclusion criteria: had an intellectual or physical disability that prevented them from participating in the programme Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 2 Treatment before study: none Description of interventions M : individual sessions aimed at improving body composition, cardiovascular fitness, improve eating habits, physical activity habits and promote positive psychosocial functioning by moving participants closer to the Australian recommendations. The programme aimed to instigate small maintainable improvements. Theoretical basis. Sessions included a review of the previous session, discussion of homework, goal achievement and monitoring; discussion, questions and practice of strategies; setting of exercises for the session and assisted with setting goals; summary of the session material and setting of home practice tasks. Adolescents were then given the choice of attending the remaining sessions alone, or with the support of a parent. 12 sessions over 4‐6 months (psycho‐education; eating behaviour; physical activity; healthy food choices; exercise; behaviour charts and barriers; recognising thoughts and emotions; helpful thoughts and emotions; assertive communication; problem solving and planning; staying on track; maintenance and closure). Between session 11 and 12 had a telephone call, maintaining change. Weekly sessions for the first 10 weeks. Parents and adolescents received a programme workbook. Between sessions, adolescents were encouraged to monitor their behaviour change goals and record their eating and physical activity habits. The physical activity component aimed to promote physical activity habits consistent with guidelines recommending at least 60 minutes of moderate‐to‐vigorous physical activity per day and no more than 2 hours per day in non‐educational screen activities. Rather than specifying goals in terms of specific caloric intake or expenditure targets, aimed to move participants closer to recommendations for eating such as consumption of a variety of foods from each of the 5 food groups (cereals, vegetables, fruit, diary, and meat products and alternatives), the selection of low‐fat alternatives, and the consumption of water. Parental support: provided with written session materials for sessions 7 (strengthening parent adolescent relationship), 8 (encouraging appropriate behaviour) and 9 (discouraging inappropriate behaviour). Maintenance phase: while the control group given the intervention telephone call sessions were completed every second week and a face‐to‐face session at 3 months which was followed by monthly telephone call session and a final face‐to‐face session conducted at 6 months. Researcher received training in MI Control : wait list control ‐ offered the intervention after 6 months (postintervention) |
|
| Outcomes | Outcomes reported in abstract of publication: weight control behaviour, impulse regulation, social support from family and parent‐adolescent problem communication, treatment acceptability, body composition (body fat, % body fat, lean mass) and anthropometric measures (weight, BMI, BMI‐for‐age z score and percentiles), cardiovascular fitness | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ACTRN12610000111077 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "evaluate the efficacy of the CHOOSE HEALTH program, a family based cognitive behavioural lifestyle program targeting improved eating and activity habits, in improving body composition, cardiovascular fitness, eating and activity behaviours in overweight and obese adolescents; 2) to explore the impact of The CHOOSE HEALTH Program on psychosocial wellbeing in overweight and obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "After completing all assessments, participants were asked to select a number between 1 and 3 and the selected number was compared to an investigator randomly selected number, which was then used to allocate the participant to the treatment or control condition." Comment: unclear how the investigator number was randomly selected or how the combination of these 2 elements produced an allocation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details. The reliance on self‐report data to measure psychosocial factors, and the use of indirect self‐report measures of physical activity and energy intake, may result in self‐report biases |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "Assessors and observers were blind to participant's group allocation, treatment adherence, and stage of intervention. The treating clinician was not involved in physical assessment sessions. The analysis of all monitoring data was completed by an independent research assistant who was blind to treatment stage and condition." Comment: appropriate |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "Assessors and observers were blind to participant's group allocation, treatment adherence, and stage of intervention. The treating clinician was not involved in physical assessment sessions. The analysis of all monitoring data was completed by an independent research assistant who was blind to treatment stage and condition." Comment: appropriate |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: reasons for dropout unclear. Intervention group was larger and had a higher % of dropouts compared to the control group. Complete valid data were not available for all measures or participants. It is possible that the non‐completers differed from the completers in important ways and consequently results were not representative of those obtained had the entire sample completed all assessments. ITT was used with first value carried forward method |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: reasons for dropout unclear. Intervention group was larger and had a higher % of dropouts compared to the control group. Complete valid data were not available for all measures or participants. It is possible that the non‐completers differed from the completers in important ways and consequently results were not representative of those obtained had the entire sample completed all assessments. ITT was used with first value carried forward method |
| Selective reporting (reporting bias) | High risk | Comment: psychosocial outcomes not published |
| Other bias | Unclear risk | Comment: the lead author was both the clinician and evaluator which may result in an evaluator bias |
Brownell 1983.
| Methods | Parallel RCT, randomisation ratio: 1:1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐16, ≥ 20% mean weight for age, gender and height; free of medical conditions or medications that would influence body weight, not involved in other formal weight loss programmes, able to attend all sessions with their mothers. Mothers could be any weight Exclusion criteria: ‐ Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions Programme of behaviour modification, nutrition education, exercise instruction and social support, described in a treatment manual. 16 weekly sessions 45‐ to 60‐minutes' duration, groups of 5‐8. Discussed the week's didactic material and matters such as feelings about being overweight, family difficulties and food preparation. Follow‐up sessions every 2 months during 1‐year maintenance period. Each parent deposited USD15: USD10 refunded if all treatment sessions were attended, and USD5 refunded for attendance at follow‐up meetings. Mothers also paid USD3 fee before each meeting. As an incentive, each child deposited USD8 at the start of each month during treatment; USD2 was returned to the child each week ≥ 1 lb was lost. Trainer was a Masters level psychologist and a Doctoral level psychologist Mother‐child separately : mothers and children met concurrently in separate groups Mother‐child together : attended all treatment sessions and met together in the same group Child alone : children met in groups, but the mothers did not take part in the formal treatment programme |
|
| Outcomes | Outcomes reported in abstract of publication: weight loss, blood pressure | |
| Study details |
Run‐in period: orientation session prior to programme Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to test three methods of parent involvement in the treatment of obese adolescents aged 12 to 16 years. Short‐term and long‐term changes in weight and blood pressure were assessed.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "assigned randomly from stratified blocks." Comment: method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not reported but low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: missing data reported but reasons not explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes reported as in methods, but protocol not available |
| Other bias | Low risk | Comment: no other bias |
Carraway 2014.
| Methods | Parallel RCT, randomisation ratio: ‐, superiority design | |
| Participants |
Inclusion criteria: male and female adolescents aged 12‐17 years, who were at or above the 85th percentile for BMI based on age and gender, willing and able to complete 3 sets of physical and questionnaire assessments (at baseline, postintervention and follow‐up), come to the gym 3 times per week to exercise with their assigned mentor for the duration of 3 months. Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Mentor : mentor‐led exercise programme Control : wait list control |
|
| Outcomes | Outcomes reported in abstract of publication: perceived athletic competence, physical activity, social anxiety, social support | |
| Study details |
Run‐in period: none Study terminated early: no |
|
| Publication details |
Language of publication: English Unclear funding Publication status: unpublished thesis Trial identifier: ‐ |
|
| Stated aim for study | Quote from publication: "the purpose of the current pilot study was to assess whether adding a group‐based component to increase problem‐solving skills, communication skills, parental social support, and problem‐focused coping with teasing as a part of a mentor‐led exercise intervention (MENTOR+CBT) would decrease social avoidance, improve social support, and increase perceived competence for physical activity as well as physical activity level in obese adolescents beyond the effects of mentor‐led exercise alone (MENTOR only)." | |
| Notes | Relevant for this review is MENTOR only vs wait list control. The second cohort examining MENTOR + CBT are not randomised. Contacted author (17 January 2017) for stratified outcomes and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: dropouts described. Attrition low for both groups but different between groups with 9% in the intervention group and 15% in the control group |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: dropouts described. Attrition low for both groups but different between groups with 9% in the intervention group and 15% in the control group |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge |
| Other bias | Unclear risk | Comment: insufficient information to judge |
Carrel 2005.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: BMI > 95th percentile for age Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: unclear Treatment before study: none Description of interventions Behaviour changing‐focused, fitness‐oriented gym classes : class size limited to 14 students to allow for increased instructor attention, increased opportunity for motivation and less time standing in line. The curriculum was personalised to match the student's skill levels and encourage student participation. Competitive games were de‐emphasised, and behaviour changing focused activities (walking, cycling and snowshoeing) were encouraged. A consistent warm‐up plan brought students into movement participation as quickly as possible soon after they entered the gym. Typical movement time was 42 minutes of a 45‐minute class period, as children did not change clothes for this class to increase activity time. Skills were taught with the class broken down into groups of 2 for promoting more movement and less time watching. The ethos of the class encouraged physical fitness, less self‐conscious focus on appearance and full group participation. Training for instructors not stated. Standard gym classes : movement time averaged 25 minutes of the 45‐minute period. Participated in the traditional physical education classes of 35‐40 students. The same class topics (e.g. football, mile run/walk, kickball) were taught as in the intervention group, but in a different format, as typical issues in the traditional class were a greater range of skill levels, longer lines during skill development drills and larger numbers of students on teams when games were played. These issues tended to result in less movement and a tendency for students to hold back and not enter into play. For both groups: 36‐week (9‐month) intervention, 90 sessions. The frequency of classes was 5 times every 2 weeks for a 45‐minute class period. All received a small nutrition education component. This consisted of educational handouts to participants to develop healthier eating habits. The nutrition portion focuses on the Food Guide Pyramid, recommended servings of food, appropriate portion sizes, healthier food choices |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, % body fat, VO2max, insulin level | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine whether a school‐based fitness program can improve body composition, cardiovascular fitness level, and insulin sensitivity in overweight children…" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "randomly assigned," "using a random number generator from our statistician." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "participants were not blinded to their intervention (they knew if they were in the intervention or control class)." Comment: high risk of performance bias |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: dropouts only in control arm (differential attrition between groups), reasons provided, baseline characteristics only reported on completers |
| Selective reporting (reporting bias) | Low risk | Comment: no CT record; however, author confirmed "reported all outcomes." |
| Other bias | Low risk | Comment: no other bias |
Chandra 1968.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: not clearly mentioned: 43 children with simple nutritional obesity were enrolled. All had a weight excess of ≥ 20% more than the 50th percentile for American boys and girls (Stuart and Stevenson 1959) Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none, observed for 1 month prior to trial Description of interventions Intervention (low‐calories formula) : low‐calorie formula using Limical (Sarabhai Chemicals) for 1 day (4 servings) contains proteins 70 g, fat 20 g, Carbohydrate 110 g Control : given a low‐calorie diet without the aid of Limical All participants received general instructions such as abstention from sweets, chocolates, cakes, ice‐cream and potatoes. Advised not to take any snacks in between meals but the number of meals was not reduced |
|
| Outcomes | Outcomes reported in abstract of publication: no abstract | |
| Study details |
Run‐in period: children were observed for 1 month before being included in trial Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Other funding: "Limical was supplied in generous quantities through the courtesy of Dr. Barbhaiya, Medical Director, Sarabhai Chemicals, for which the author is grateful." Publication status: peer‐reviewed journal |
|
| Stated aim for study | Comment: no stated aims | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "By a random allocation, they were assigned to the two groups." Comment: no further details |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "By a random allocation, they were assigned to the two groups." Comment: no further details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: nothing stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: nothing stated, objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: unclear why the sample fell from 43 to 35 at 3 and 7 months |
| Selective reporting (reporting bias) | Unclear risk | Comment: only mean weight loss reported |
| Other bias | Low risk | Comment: no other bias |
Christie 2011.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 13‐17 years living in Greater London, UK, Obese defined as BMI > 98th centile for BMI using the UK 1990 growth reference Exclusion criteria: significant mental health problems; chronic illness (asthma permitted as long has not more than 1 course of oral steroids in preceding 3 months or on 2nd dose or more of inhaled steroids); known secondary obesity, monogenic obesity syndrome or use of medications known to promote obesity; significant learning disability; insufficient command of English language; previous participation in behavioural weight management programmes in the past 12 months (excluding commercial programmes such as Weight Watchers); BMI ≥ 40 kg/m2 Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions HELP : a solution‐focused and motivational weight management programme based on national guidelines. Young people and families attended 12 × 40‐ to 45‐minute sessions over 6 months. 4 components:
Providers (trained Graduate Health Workers) received training in specific MI techniques and use of solution‐focused questioning. Used manual to enable delivery in a standardised manner by all the providers. Control : enhanced standard care, 1 prewritten standardised educational session delivered to young people within 3 months of recruitment by a practice nurse. The session lasted 40 minutes (although the abstract stated 2 hours) and incorporated standard national guidance and published information on obesity, addressing eating behaviours, healthy activity levels and healthy eating patterns. No training in delivery style offered. Recruitment compensation: all participants received a voucher (GBP20) and travel costs |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, BMI z score, fat mass, self‐esteem, eating behaviours, quality of life, process evaluation, association of pulse wave velocity (proxy for arterial stiffness) and breathlessness score post‐step test, self‐reported exertion | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ISRCTN99840111 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: journal supplement |
|
| Stated aim for study | Quote from publication: "To assess whether a motivational multi‐component lifestyle intervention delivered in the community was effective in reducing body mass index (BMI) and improving related health outcomes in obese adolescents." | |
| Notes | Data reported in 2 abstracts only, therefore limited information available. Main source of information was a published protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomisation to the HELP Trial is performed using a secure website." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomisation will be undertaken independently of the investigators." Comment: third party allocation to groups undertaken |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "assessments will be undertaken blind to allocation status." Comment: investigator‐assessed |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: missing data not reported for each group separately, stated used ITT principles |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: missing data not reported for each group separately, stated used ITT principles |
| Selective reporting (reporting bias) | Unclear risk | Comment: data not fully published, available in 2 abstracts only |
| Other bias | Low risk | Comment: no other bias |
Daley 2005.
| Methods | Parallel RCT, randomisation ratio: 1:1:1, superiority design | |
| Participants |
Inclusion criteria: clinically obese and aged 11‐16 years (BMI centile > 98th UK standard), no medical condition that would restrict ability to be active 3 times per week for 8 weeks, not diagnosed with insulin‐dependent diabetes or receiving oral steroids Exclusion criteria: medical conditions that would restrict the ability to be active 3 times per week for 8 weeks, unwillingness to attend supervised exercise sessions 3 times per week for 8 weeks, major cognitive or psychiatric impairments, and diagnosis of insulin‐dependent diabetes mellitus or oral steroid treatment Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Exercise counselling : knowledge and psychological skills and tools to sustain changes in their exercise behaviour (Transtheoretical Model) to promote positive exercise attitudes and experiences. 8 weeks, hourly sessions (1‐4: focus on cognitive‐based intervention strategies such as cognitive reappraisal and consciousness raising; 5‐8, more behavioural‐based interventions were introduced, e.g. goal setting, self‐monitoring and finding social support). Participants followed a broad structured curriculum of topics over the course of the intervention (provided in the protocol). Encouraged participants to discuss their thoughts and feelings about exercise, to assist with problem‐solving. Weight loss per se was not an intervention goal and no weight loss targets were set, although sensible eating habits were encouraged as part of the exercise therapy intervention. Offered a range of aerobic exercise modalities, such as stepping, cycling, seated rowing, dance mat and walking, and asked to exercise intermittently for 30 minutes (4‐minute warm up, 4 minutes' moderate intensity at 40‐59% heart rate reserve, 2 minutes' rest between each bout), 3 times per week for 8 weeks. Mini games also included. A GBP25 sports store voucher was given to participants at the end of the intervention phase, and a contribution of GBP2.50 toward travel expenses to attend intervention sessions and assessments was made for each visit to the trial centre. An additional GBP10 sports store voucher was given to participants when they completed their final follow‐up assessment. Training of interventionists not reported Exercise placebo : 24 sessions over 8 weeks; performed light body‐conditioning/stretching exercises, during which heart rate was maintained at 40% of heart rate reserve, and no exercise counselling or behavioural change advice was given. Also, participated in other sedentary activities, such as balance and catching tasks, pool, darts and table football Control : usual care group continued with their lives as usual; were given the opportunity to complete exercise sessions at the centre once they had completed the study |
|
| Outcomes | Outcomes reported in abstract of publication: Children's Depression Inventory score, physical self‐worth, self‐esteem, and physical activity over time, BMI. | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ISRCTN83888112 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The primary aim of SHOT [SHeffield Obesity Trial] is to examine the effects of a supervised exercise therapy intervention in young people who are obese." | |
| Notes | For the purpose of the review, we combined the exercise placebo group and control group as the exercise placebo was not intended to promote weight loss and was therefore considered to be a no treatment comparator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A researcher from an independent University will perform the randomisation procedures by allocating participants to groups according to a computer generated random list." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "A researcher from an independent University will perform the randomisation procedures by allocating participants to groups according to a computer generated random list." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "The second author provided the intervention and collected measures. The trial statistician was blinded to group codes." Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "The second author provided the intervention and collected measures. The trial statistician was blinded to group codes." Comment: no blinding but objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: numbers reported but no reasons provided, used ITT analysis |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: numbers reported but no reasons provided, used ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Low risk | Comment: no other bias |
Debar 2012.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐17 years, female health plan members with an age‐ and gender‐adjusted BMI ≥ 90th percentile Exclusion criteria: significant cognitive impairment or psychosis, severe obesity (BMI > 45), use of medications known to affect body weight and pregnancy Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Description of interventions Primary care‐based multicomponent behaviour changing intervention : 90‐minute group sessions over 5 months. Addressed issues associated with obesity in adolescent girls (e.g. depression, disordered eating patterns, poor body image). Session included reviewing goals and problem solving to overcome barriers and challenges to increased activity, discussion of topics particularly pertinent to adolescent girls and specific behavioural and cognitive tools for coping, including: self‐monitoring of dietary intake, physical activity and screen time; stimulus control and environmental changes, stepwise goal‐setting and problem solving; setting goals for increasing pleasant activities; cognitive restructuring techniques to combat negative self‐talk. Groups met 16 times, weekly for 3 months and biweekly during months 4 and 5. Increasing physical activity was promoted by using tailored forms of exercise (e.g. exergaming), goals included 30‐60 minutes of physical activity at least 5 days per week; 15 minutes of daily yoga (provided with training in safe and basic yoga practices, equipment, an instructional booklet and CD), limiting screen time to 2 hours per day; and increasing "found exercise" opportunities whenever possible. Core activities chosen to overcome obstacles such as embarrassment. Participants provided with exergaming equipment (details provided). Also, strategies for increasing physical activity (e.g. pedometers, resistance bands) promoted. Dietary intake and eating patterns followed caloric guidelines (1600‐ 1800 kcal daily), and 3 main areas for dietary change emphasised: decreasing portion sizes, limiting consumption of energy dense foods and increasing consumption of lower energy‐dense foods. Other dietary strategies also covered. Parents had 12 separate weekly group sessions in the first 3 months. Nutritional and physical activity principles the teens would learn discussed, encouraged to increase or maintain the frequency of family meals. Paediatric providers received study‐sponsored training in motivational enhancement techniques for health behaviour change. Interventionist included Master's level nutritionists and health educators and Doctoral level clinical psychologists, nutritional and physical activity principals Usual care control : participants met with their paediatric primary care providers to encourage healthy behaviour changing changes. Received a packet of materials, including outlines of evidence‐based approaches to weight management for youths and adults, a parents' guide to help adolescents make healthy lifestyle changes, local resources for weight management and healthy activity, and suggested books and online materials on healthy lifestyle change |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT01068236 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "This study evaluated a primary care‐based, multicomponent lifestyle intervention specifically tailored for overweight adolescent females." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Eligible adolescents were randomized to the intervention or control condition by a computer program using a well validated procedure to balance age and obesity severity. Project interventionists informed participants of treatment assignment to keep assessors masked." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk |
Quote from publication: "Project interventionists informed participants of treatment assignment to keep assessors masked." Comment: participants not blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "Project interventionists informed participants of treatment assignment to keep assessors masked." Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: self‐reported outcomes collected by staff for some subjective outcomes (diet and physical activity) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: measures were collected by staff blinded to participant treatment assignment |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: numbers but not reasons for drop‐outs were reported, ITT analysis was used |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: numbers but not reasons for drop‐outs were reported, ITT analysis was used |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Low risk | Comment: no other bias |
Ebbeling 2003.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 13‐21 years Exclusion criteria: ‐ Diagnostic criteria: BMI that exceeded gender‐ and age‐specific 95th percentiles |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Both groups received similar behavioural therapy: social cognitive theory provided a conceptual framework for the educational and behavioural components of treatment that was consistent between intervention groups. Counselling focused on enhancing self‐efficacy for dietary change using the concepts of behavioural capability (knowledge and skill) and self‐control. Participant expectations (anticipated outcomes), expectancies (values ascribed to outcomes) and perceptions of environmental influences were discussed during treatment sessions. The topic modules were the primary mechanism for facilitating self‐assessment, goal setting and problem solving. These modules were designed to promote dialogue between the participant and study dietician. 1 topic module was devoted to physical activity, with participants in both groups receiving information based on current recommendations. 12 sessions over 6 months (12 dietary counselling sessions) and a 6‐month follow‐up (2 dietary counselling sessions) Reduced GL diet : written materials included topic modules, food choice lists and a select‐a‐meal menu. The topic modules were the primary mechanism for presenting nutrition intervention messages. These modules were designed to promote dialogue between the participant and study dietician. Food choice lists were used to enhance practical application of intervention messages presented in the topic modules. For the intervention group, lists corresponded to food groups delineated by a reduced GL food pyramid. The select‐a‐meal menu contained recipes and ideas for meal planning to complement the food choice lists. Nutrition bars were offered to participants in the experimental (Balance Bar; provided by Kraft Foods, Inc, Northfield, IL) Reduced fat diet, described as a conventional diet : written materials included topic modules, food choice lists and a select‐a‐meal menu. The topic modules were the primary mechanism for presenting nutrition intervention messages. These modules were designed to promote dialogue between the participant and study dietician. Food choice lists were used to enhance practical application of intervention messages presented in the topic modules. For the conventional group, the lists corresponded to the diabetes food pyramid and were presented as an exchange system. Nutrition bars were offered to conventional groups (Nature Valley Granola Bar; General Mills, Inc, Minneapolis, MN) for occasional use as snacks. The interventionist was a dietician trained in the study protocol |
|
| Outcomes | Outcomes reported in abstract of publication: GL, BMI, fat mass, insulin resistance | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "(1) to develop a reduced‐ GL diet for use in an adolescent population; (2) to determine whether adolescents following this diet will successfully achieve long‐term reduction of GL; (3) to compare the long‐term effects of a reduced‐GL diet with those of a conventional reduced‐fat diet in a pilot study involving obese adolescents." | |
| Notes | Participants aged 13‐21 years, unclear mean age. Control described as conventional diet based on recommendations in 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "participants were randomly assigned to experimental (reduced GL) or conventional (reduced fat) dietary treatment." Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no mention of blinding, but objective outcome |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons provided |
| Selective reporting (reporting bias) | High risk | Comment: body mass was calculated but not reported in follow‐up measures |
| Other bias | Low risk | Comment: no other bias |
Ebbeling 2012.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: grade 9 or 10 and BMI ≥ 85th percentile for gender and age, aged 13‐18 years, reported consuming ≥ 1 serving (i.e. 360 mL or 12 fluid ounces) per day of sugar‐sweetened beverages (i.e. soft drinks, juice drinks containing < 100% juice, punches, lemonades, iced teas and sports drinks). Each participant lived predominantly in 1 household (i.e. no more than 1 weekend every 2 weeks in a secondary household) Exclusion criteria: dieting for the purpose of weight loss or taking prescription medications that might affect body weight, reported smoking ≥ 1 cigarette in the past week or diagnosed as having a major medical illness or eating disorder, BMI < 25th percentile Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Description of interventions Multicomponent intervention : designed to reduce consumption of sugar‐sweetened beverages. Emphasis on displacing sugar‐sweetened beverages with non‐caloric beverages in the home as a strategy to decrease consumption. Home delivery of non‐caloric beverages (e.g. bottled water and 'diet' beverages) every 2 weeks. Written intervention messages with instructions to drink the delivered beverages and not to buy or drink sugar‐sweetened beverages were mailed to participants. Unsweetened water was recommended over artificially sweetened beverages. Discussions during telephone calls and check‐in visits focused exclusively on beverage consumption, with no attention to other dietary behaviours or to physical activity. 3 check‐in visits with participants (20 minutes per visit). Monthly motivational telephone calls with parents (30 minutes per call). Home delivery of non‐caloric beverages (e.g. bottled water and 'diet' beverages) every 2 weeks. At 6 months (pilot study), each participant received a USD100 gift certificate to a local shopping mall at the end of the study. Control group received weekly home deliveries of non‐caloric beverages for 4 weeks after completion of follow‐up measurements. At 1 year, control group received USD50 supermarket gift cards at 4 and 8 months but did not receive instructions on what to purchase with the cards. Groups were led by dieticians, Master's level therapists, psychologists or psychiatrists. Staff were trained Control : no details |
|
| Outcomes | Outcomes reported in abstract of publication: consumption of sugar‐sweetened beverages, BMI, weight, change in body fat as % body weight | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT00381160 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "assess the effect on weight gain of an intervention that included the provision of non‐caloric beverages at home for overweight and obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Eligible participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, stratified by gender and BMI (< 85th percentile for gender and age, ≥ 85th percentile). The sequence of random assignments was permuted within stratum in blocks of 2, 4, and 6." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "To avoid any bias in the enrolment procedure, personnel conducting recruitment were masked to sequence." Comment: no details of how allocation was concealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details on blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details on blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: "All personnel who assessed study outcomes were unaware of the group assignments. The interviewer was masked to group assignment." Comment: self‐reported data |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "All personnel who assessed study outcomes were unaware of the group assignments. The interviewer was masked to group assignment." |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons provided, used ITT for analysis |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons provided, used ITT for analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Low risk | Comment: no other bias |
Ford 2010.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 9 to < 18 years, BMI > 95th centile, minimal or no learning difficulties, no underlying medical problem, no medication for insulin resistance Exclusion criteria: ‐ Diagnostic criteria: BMI > 95th centile |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Titration period: ‐ Description of interventions Mandometer group : computerised device providing real‐time feedback during meals to slow down speed of eating and reduce total intake. Saw a research nurse trained in use of Mandometer once per week for 6 weeks, every second week for a further 6 weeks, and once every 6th week thereafter. Research nurse telephoned support and encouragement every second week from week 12 onwards. Participants were trained in the use of Mandometer including test meals, setting of training lines and eating speed. Dietary advice by a paediatric dietitian 4 times over 12 months based on the UK Food Standards Agency 'eatwell plate' educational tool. Clinician met the participants every 4 months, emphasising the need to change eating habits and improve physical activity as advocated in the standard clinic. Standard care controls : initial 1‐hour discussion about reasons for, and implications of, obesity and behaviour changing measures that may improve BMI. Multidisciplinary team composed of a clinician, paediatric dietitian and exercise specialist. Emphasis placed on implementing changes to increase levels of enjoyable physical activity to national recommended levels (60 minutes of exercise per day) alongside a balanced diet, based on the 'eatwell plate.' Set their own dietary goals and targets, with practical advice and guidance from the dietician, facilitation to exercise rather than prescription. MI techniques used to engage participants and families in the decision‐making process for changes in behaviour. Appointments at 3‐monthly intervals |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, body fat SDS, change in portion size, HDL cholesterol | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT00407420 |
|
| Publication details |
Language of publication: English Other funding (a charity funded by a commercial organisation) Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To determine whether modifying eating behaviour with use of a feedback device facilitates weight loss in obese adolescents." | |
| Notes | Galhardo 2011 is a linked publication but this is a substudy of 27 participants only with no relevant outcomes. Not extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "An independent statistician unconnected with clinical practice used computer generated random numbers… to prepare randomisation lists…" Comment: block randomisation. Methods appear appropriate |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "assigned patients sequentially according to the generated lists…" Comment: unclear if concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "we could not blind participants." Comment: no blinding, investigator‐assessed outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not described, investigator‐assessed, outcomes objective unlikely to be affected by outcome assessor blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: reported and reasons explained for drop‐outs but further exclusions from analyses at 18 months were not detailed |
| Selective reporting (reporting bias) | High risk | Comment: HRQoL, lipids, insulin and blood pressure data not reported, narrative comment only |
| Other bias | Low risk | Comment: no other bias |
Gourlan 2013.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 11‐18 years, BMI > 90th age‐ and gender‐specific percentiles, no unstable or uncontrollable diseases Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions MI + standard weight loss : MI‐based intervention and self‐determination theory to elicit and reinforce the adolescent's change talk to minimise resistance and resolve ambivalence to change (reference provided). 2 face‐to‐face, semi‐structured interviews for 30 minutes over 3 months where 4 MI principles (making the participant's acquaintance and building awareness, alternatives and problem solving, goal setting and agenda setting, behaviour modification consequences and perspectives) were used to encourage adolescents to articulate their concerns and goals, and develop their autonomy. 6 × 20‐minute telephone sessions over 6 months. Standard weight loss programme aimed to promote physical activity, provide information and tips to encourage/help with physical activity and the benefits of performing physical activity, promote a balance diet and provide information/tips/self‐monitoring on how to achieve healthy behaviour. A sport and exercise sciences Doctoral student delivered MI sessions following MI training including 40 hours of reading and 32 hours of training formation Standard weight loss programme (SWLP) : as described above. Delivered by a Doctor of Medicine specialised in paediatric obesity and certified in behavioural and cognitive therapies |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, autonomy support, integrated and identified regulations, motivation | |
| Study details |
Run‐in period: none Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: ‐ |
|
| Stated aim for study | Quote from publication: "The primary purpose of this study was to assess the effectiveness of an MI‐based intervention in addition to a standard weight loss programme (SWLP) on PA [physical activity] and body mass index (BMI) of obese adolescents. The second purpose was to explore some of the underlying motivational processes accompanying these effects." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The project manager assigned each adolescent randomly and independently to the SWLP [standard weight loss programme] group (n = 34) or the SWLP + MI group (n = 28)." Comment: no further details |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "The project manager assigned each adolescent randomly and independently to the SWLP group (n = 34) or the SWLP + MI group (n = 28)." Comment: no further details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote from publication: "Participants, the health care provider and data collectors were blinded to the group (SWLP or SWLP + MI) the adolescents were assigned to." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote from publication: "Participants, the health care provider and data collectors were blinded to the group (SWLP or SWLP + MI) the adolescents were assigned to." Comment: appropriate |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: "Participants, the health care provider and data collectors were blinded to the group (SWLP or SWLP + MI) the adolescents were assigned to." Comment: appropriate |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Participants, the health care provider and data collectors were blinded to the group (SWLP or SWLP + MI) the adolescents were assigned to." Comment: appropriate |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to judge, no protocol or trial registry data available |
| Other bias | Low risk | Comment: no other bias |
Grey 2009.
| Methods | Cluster RCT, randomisation ratio: 2:1, superiority design | |
| Participants |
Inclusion criteria: in 7th grade, BMI > 85th percentile, family history of type 2 diabetes mellitus, parents who were English or Spanish speaking, no other major health problems Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 6 Treatment before study: none Description of interventions CST : nutrition and exercise educational content enhanced by the inclusion of CST, based on Bandura's social learning theory). To increase students' sense of competence and mastery, open discussion and questions and answers about events occurring in the past week with which the youth had difficulty, youth role‐playing common situations, practicing new coping skills. Encouragement and reinforcement provided by other students and teacher. Materials developed by a clinical psychologist who was an expert in CST. 13 sessions over 16 weeks. No mention of length of sessions: 8 nutrition and activity classes + 5 coping skill training (Getting to know you; Change in actions; Get up and move; Stress identification and reduction; Aim for life skills; Healthy lifestyle tic‐tac‐toe; Resolving conflict and effective communication; What's on your plate?; Size up your portions; What's in a food label?; Eating in a fast food world; How to "go out" without guilt; Recipe makeover). Physical activity component focused on reducing leisure time sedentary behaviours that may compete with activity, learned creative ways to increase physical activity in a non‐structured exercise programme, including culturally relevant approaches, such as aerobic dancing. Materials developed by a registered dietitian. A non‐diet, family centred approach used as nutrition education component. Major dietary goal was to provide highest nutritional quality for lowest caloric intake. Emphasis placed on lowering dietary fat intake to < 30%, improving food and drink choices, and decreasing total calories by focusing on appropriate portion sizes, while including culturally specific foods and being sensitive to the costs of foods. Health coaching provided only to participants in intervention group, commencing at end of 16‐week intervention programme, and lasting until the 12‐month data collection ‐ 9 months of weekly 5‐ to 10‐minute telephone health coaching provided by an advanced practice nurse, nutritionist, family therapist or psychologist, on a rotating basis. Teachers in schools trained by study staff to deliver intervention. Students compensated for their time with a small token (toy worth USD5‐10) following data collection General education : 8 sessions in 16 weeks. Intervention component: Get up and move; Healthy lifestyle tic‐tac‐toe; What's on your plate?; Size up your portions; What's in a food label?; Eating in a fast food world; How to "go out" without guilt; Recipe makeover. Physical activity and nutritional component as above |
|
| Outcomes | Outcomes reported in abstract of publication: anthropometric measures, lipids, depressive symptoms, BMI | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To evaluate the impact of a multifaceted, school‐based intervention on inner city youth at high risk for type 2 diabetes mellitus (T2DM) and to determine whether the addition of coping skills training (CST) and health coaching improves outcomes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Schools were randomized." Comment: no description of randomisation process but there were some baseline differences (not anthropometric measures) likely due to the cluster randomisation ‐ potential bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no mention of blinding, objective measures |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons stated, ITT analysis used |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons stated, ITT analysis used |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes reported as stated but no protocol to confirm |
| Other bias | Unclear risk | Unclear if they accounted for clustering in analysis. Attendance at sessions/telephone calls received very low. Dropout rate moderate |
Hofsteenge 2014.
| Methods | Parallel RCT, randomisation ratio: 3:2, superiority design | |
| Participants |
Inclusion criteria: adolescents with overweight or obesity (defined according to the Cole criteria) aged 11‐18 years who were referred to the outpatient paediatric obesity clinic of the VU University Medical Center Exclusion criteria: not Dutch‐speaking, obesity as a result of a known syndrome or organic cause (hypothyroidism), mental retardation, physical limitations and diagnosed type 2 diabetes mellitus Diagnostic criteria: Cole criteria |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Go4it multidisciplinary group treatment : based on evidence and published educational materials (references given). 7 group sessions (90‐minute duration) with interval of 2‐3 weeks: education on dietary behaviour, physical activity and energy balance. Group size 8‐12 adolescents. Also received CBT regarding how to improve lifestyle regarding a healthy weight and how to maintain an adequate energy balance. Dietician, paediatrician/endocrinologist and psychologist involved. Booster groups sessions scheduled after 6, 14, 26 and 36 weeks. 2 parental sessions consisted of education concerning the health risks of overweight, healthy physical activity and dietary behaviour and how to support their children. Special materials were developed for this programme; an information book, workbook, and dietary and activity diary, and specific worksheets for every session Current regular care : consisting of referral to a dietitian in the home care setting |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, body composition, metabolic components, effect modifiers, blood pressure, HDL cholesterol, PedsQL, Body Esteem Scale | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NTR691, ISRCTN27626398 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to investigate the effectiveness and cost‐effectiveness of this multidisciplinary group treatment for obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "block randomisation…using SPSS for random selection." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of concealment of allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "The randomisation could not be blinded to the researcher and participants." Comment: self‐reported outcome measurement could be at risk of bias owing to lack of blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "The randomisation could not be blinded to the researcher and participants." Comment: adjudicated/investigator‐assessed outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "The randomisation could not be blinded to the researcher and participants." Comment: outcome assessor not blind |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "The randomisation could not be blinded to the researcher and participants." Comment: adjudicated/investigator‐assessed, outcome assessor not blinded but unlikely to be at risk of bias |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk |
Quote from publication: "numbers and reasons explained. No ITT analysis for subjective outcomes…" Comment: unclear |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk |
Quote from publication: "all participants were analyzed in the group to which they were randomly assigned…" Comment: reported and reasons explained |
| Selective reporting (reporting bias) | High risk | Comment: physical activity and sedentary behaviour not reported |
| Other bias | Low risk | Comment: no other bias noted |
Jelalian 2016.
| Methods | Parallel RCT, randomisation ratio: 2:1, superiority design | |
| Participants |
Inclusion criteria: 12‐ to 18‐years‐old, Diagnostic and Statistical Manual of Mental Disorders criteria for current major depressive episode or dysthymia; Clinical Depression Severity Rating Scale (CDRS) score ≥ 65; BMI > 25 kg/m2 or BMI percentile ≥ 85th percentile for gender and age; and parent or carer willing to participate Exclusion criteria: without reliable transportation; taking weight‐altering medications within 6 months prior to study initiation; unable to do moderate‐to‐vigorous physical activity; weighing > 300 lb; in foster care; receiving special needs education; previous participant in study authors' weight loss studies; currently enrolled in a weight loss programme; diagnosed with obesity‐related disorders; requiring immediate weight loss management or diseases affecting absorption or processing of nutrients Diagnostic criteria: as above |
|
| Interventions |
Description of interventions CBT for depression and healthy lifestyle + exercise : individual CBT treatment adapted into 1 integrated protocol that addressed depression, exercise component, weight and advice regarding healthy eating. Weekly 60‐minute group aerobic exercise sessions were required and facilitated by a physiotherapist. Individual meetings with a nutritionist individually up to 4 times. Parents involved CBT for depression : standard CBT treatment only. Parents involved |
|
| Outcomes |
Primary outcomes: depressed mood, BMI Secondary outcomes: moderate‐to‐vigorous physical activity, sedentary behaviour Other outcome: ‐ |
|
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT01128764 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Test the feasibility of a novel intervention that integrated healthy lifestyle enhancement and CBT in a clinical sample of depressed, overweight/obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author: "stratified randomization was conducted with an urn randomization computer program using a 2:1 randomization schedule in favor of CBT‐HL [cognitive behavioural therapy‐healthy lifestyle]." |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Study staff was masked to allocation sequence until interventions were assigned. The project coordinator was responsible for enrolling participants and notifying families of treatment assignment." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from author: "research assistants were blinded to condition." Comment: not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from author: "research assistants were blinded to condition." Comment: not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: only report number of participants reporting positive views of the intervention |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: numbers reported and reasons explained. Authors mentioned ITT but presented completers outcomes only. High attrition rate; however, this was a pilot study |
| Selective reporting (reporting bias) | Low risk | Comment: reported all outcomes |
| Other bias | Low risk | Comment: no other bias noted |
Jiang 2005.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: grades, defined as weight‐for‐height ≥ 120% of the Chinese reference Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Family‐based behavioural treatment : 104 weeks (2 years). Unclear how many sessions. Focused on dietary behaviour modification, 1 or 2 main behaviours which were related to obesity were chosen for each child based on an assessment of relevant dietary and exercise patterns at baseline. Then a new goal behaviour and interval behaviours were defined. Each goal and interval behaviour was discussed with the child and the parents and was agreed to by the child. A diary was kept by the children on their behaviour to monitor adherence to the recommended lifestyle changes. The parents monitored the diary and their child's progress in achieving the new behaviours. Paediatricians (researchers) visited the families once per month to observe the family environment, look for where foods were stored, cooking styles and what types of foods were used commonly in the family. The behavioural diary was checked and gaps in the recordings were discussed. Potential methods of reinforcement and penalty were also discussed with the parents and children during home visits. A 'traffic light' food item list was provided to help decrease energy intake and promote a balanced diet: 'red light' foods were those high in fat or calories; 'green light' foods were low in fat and calories; and 'yellow light' foods were intermediate. Children encouraged to eat fewer red light foods and more green light foods and parents encouraged to buy more green light foods instead of red light foods. Daily calorie requirements, based on the Chinese recommended daily allowance, were discussed and Chinese food composition tables given to each family. Dietary behaviours were suggested to the family, including eating slowly, having soup before meals, eating green light foods first, brushing teeth immediately after each meal and having meals without staple foods for supper. Exercise for 20‐30 minutes per day for 4 days per week (3 weekdays and 1 day on weekends) was advised. Children were asked to choose from running, playing football, climbing stairs and using a skipping rope. Children also urged to decrease sedentary time, e.g. watching television, and to go for a walk after supper instead Control : normal school and family life and did not receive any special intervention, the school had a similar curriculum, including physical education, as most other middle schools in Beijing |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, cholesterol, triglycerides, blood pressure | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "A family based behavioural treatment was developed and tested, to see if its use was feasible in China and to evaluate its impact on obese schoolchildren." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "children were then divided randomly…" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: no discussion of drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcomes were all reported, but no clinical trial record of protocol |
| Other bias | Low risk | Comment: no other bias |
Kong 2013.
| Methods | Cluster RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: 9th‐11th grade, BMI ≥ 85th percentile Exclusion criteria: BMI ≥ 40 kg/m2, previous diagnosis of diabetes, blood pressure in the range of stage 2 hypertension, antipsychotic or corticosteroid medications, not ambulatory, anorexia nervosa, bulimia nervosa, psychosis, suicidal ideation, hospitalisation, pregnancy Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 6 Treatment before study: none Description of interventions ACTION : based on the Transtheoretical Model, 3 primary components:
At the first visit, participants received the DVD, a DVD player and a summary of medical results, along with American Academy of Pediatrics (AAP) obesity prevention/treatment recommendations. Feedback was provided to the adolescent. Participants were asked to review the DVD and to follow‐up in 2‐3 weeks with topics they would like to discuss. Subsequent visits were individually tailored to the adolescent's stage of change with the intention of moving towards goal setting for healthier eating and physical activity. Also had a newsletter for carers (obesity risk reduction strategies) and after each visit, telephone updates were given to the carer using MI techniques. The intervention provider received a 2‐day training workshop in MI Standard care : trained clinician with trial protocol. Received 1 clinic visit similar in content to the first visit of the intervention group given at baseline, except not given the DVD or DVD player. Published recommendations were provided |
|
| Outcomes | Outcomes reported in abstract of publication: BMI percentile and waist circumference, blood pressure, HOMA‐IR, triglycerides and HDL cholesterol | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT00841334. |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Was undertaken to determine feasibility of a school‐based health center (SBHC) weight management program." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "were randomised." Comment: randomisation process was not described but there were no baseline differences |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no mention of blinding; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: numbers and reasons reported |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: numbers and reasons reported, but differential attrition between groups. Missing outcomes for individuals within each cluster |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Unclear risk | Comment: unclear if they have accounted for clustering in the analysis |
Kong 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 15‐18, male or female of Chinese ethnicity, obesity BMI ≥ 95th percentile of local age‐ and gender‐specific references no major medical illnesses, no chronic medications Exclusion criteria: concurrent participation in any clinical trial, dietary intervention or weight loss programme, concomitant intake of weight‐reducing agent, active and uncontrolled endocrine diseases, significant renal impairment or liver impairment, gastrointestinal problems that would prevent them from following the test diets, active malignant disease, pregnant or lactating, any medical illness or condition including known non‐compliance, as judged by the investigators Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions Low GI diet : counselled by dietitian at weeks 0, 2, 4, 6 and 8 and at 8‐week intervals, total 7 × 30‐minute sessions. Parents encouraged to participate. Strategy of increasing energy expenditure and reducing caloric intake using lifestyle behavioural change. Individualised menu plan with 20% caloric restriction based on his/her current diet. Practical tips were given based on behavioural principles including goal setting, knowledge acquisition, problem solving, feedback and reinforcement. Consumption of low GI food, use of healthy fat and avoidance of high GI food based on the low GI pyramid emphasised. Targeted proportion of energy from carbohydrate and fat: 45‐50% and 30‐35%, remainder from protein. 2 booklets were provided, 1 with food portion size exchange and tips, the other listing low GI foods and meal plans. Encouraged to perform aerobic exercise ≥ 3 days of 30 minutes per week. Ongoing support and encouragement was provided, target goals were redefined based on the participant's feelings and progress, efforts and achievements were acknowledged to enhance self‐efficacy. Motivational telephone calls (10‐15 minutes) were made 5 times Control : conventional Chinese diet, based on the standard food pyramid promoted by Hong Kong Department of Health with advice on daily proportion of carbohydrate, fat and protein without information about low GI diet. Emphasis on reducing energy intake by limiting dietary fat intake and high caloric foods. Targeted proportion of energy from carbohydrate and fat: 55‐60% and 25‐30%, remainder from protein. The conventional Chinese diet was presumed to be a high GI diet (≥ 70). Received counselling from a research nurse. The contact times, both individual counselling and telephone reinforcement, and the dietary assessment methods were the same in each group |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, body weight and waist circumference | |
| Study details |
Run‐in period: ‐ Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: NCT01278563 |
|
| Stated aim for study | Quote from publication: "to evaluate the impact of low GI diet versus a conventional Chinese diet on the body mass index (BMI) and other obesity indices of obese adolescents." | |
| Notes | Interim analysis of 6‐month data (18‐month trial) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization were carried out using computer‐generated random numbers." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "sealed in opaque envelopes and in blocks of 6 further stratified by gender. Treatment assignment was done by an independent personnel who opened the envelopes with consecutive numbers." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: self‐reported outcome measurement. No details on blinding |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: investigator‐assessed, no details on blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: self‐reported outcome measurement, no details on outcome assessor blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: investigator‐assessed, no details on outcome assessor blinding but objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk |
Quote from publication: "All the outcome variables were analysed on the basis of the intention‐to‐treat (ITT) principle…" Comment: missing data but reasons not reported, imbalance between groups, numbers suggest no ITT |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote from publication: "All the outcome variables were analysed on the basis of the intention‐to‐treat (ITT) principle…" Comment: missing data but reasons not reported, imbalance between groups, numbers suggest no ITT |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported as stated |
| Other bias | Low risk | Comment: nothing of note |
Love‐Osborne 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: adolescents with BMI ≥ 85% Exclusion criteria: ‐ Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 (2 schools) Treatment before study: ‐ Description of interventions Health educator involvement : health educator visits in school, participants completed an assessment tool on dietary and exercise habits and the educator used feedback from this tool within a motivational framework to support change and start goal‐setting discussions. Goals were reviewed and modified at each visit. Participants encouraged to choose 1 nutrition goal and 1 physical activity goal. Recommended targets for physical activity (1 hour per day) were discussed but participants could choose their own goal. The frequency of visits was participant led, they could choose from 2‐week, 1‐month, or 2‐month return visits. Mean 5 visits (range 1 to 8). Existing resources for physical activity and healthy eating within the school or community were linked to. Participants asked to complete a weekly log (self‐monitoring of weight weekly and lifestyle behaviours daily) and return this, when 5 were returned a USD10 gift card was received, and a second gift card for an additional 10 log sheets. Intervention group randomised to receive 2 weekly text messages (1 individualised to reinforce goals and 1 log sheet reminder) or no text messages for the first semester. All received text messages during second semester. Study staff received training in MI Control : no details Both groups received preventive services, including physical examinations and laboratory screening in the school‐based health centre. |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, sports participation | |
| Study details |
Run‐in period: none Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: ‐ |
|
| Stated aim for study | Quote from publication: "to evaluate whether a health educator (HE) providing additional contact time with students and helping them to set personal goals to improve lifestyle would lead to improved BMI outcomes in overweight or obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "students were randomised." Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: investigator‐assessed. Blinding not reported but low risk of bias from objective outcome |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: reasons for withdrawals only partially explained. Imbalance in dropouts |
| Selective reporting (reporting bias) | High risk | Comment: outcomes only reported in categories and subgroups, no overall results by study arm. The intervention group were also randomised into 2 groups, but no results were reported for these 2 groups |
| Other bias | Low risk | Comment: no other bias |
Luna‐Pech 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐16 years; stable asthma (diagnostic criteria stated); obesity (BMI ≥ 95th percentile of the CDC BMI‐for‐age growth charts); Tanner scale stage 2‐3; skin prick test positive for ≥ 1 allergen, forced expiratory volume in 1 second (FEV1) > 80% predicted value for age and height. Medically treated for asthma for at least 6 months Exclusion criteria: other prescribed dietary programme, undergoing allergen immunotherapy or other chronic diseases or comorbidities Diagnostic criteria: as above |
|
| Interventions |
Number of study centres:1 Treatment before study: ‐ Description of interventions Normocaloric diet : programme adjusted at follow‐up visits by nutritionist according to individual feeding habits and preferences based on an equivalent exchange system using food lists with a group of measured or weighed foods of approximately the same nutritional value. Individual energy needs calculated according to resting energy expenditure using standard guidance (references given), applying mild or moderate physical activity criteria and individually adjusted. 10‐15% proteins, 50‐60% carbohydrates, 25‐30% fat. Daily meal pattern: breakfast 25%, lunch 30%, snack 15‐20%, dinner 25‐30%. Free diet : no details Both groups instructed to fill in a daily 24‐hour dietary recall at home, and attend follow‐up visits every 2 weeks for 28 weeks. All attended the follow‐up sessions of 45 minutes to assess aspects of asthma control (i.e. need of rescue and basal medications, review the dietary recall, perform peak expiratory flow and establish an action plan for worsening of asthma symptoms |
|
| Outcomes | Outcomes reported in abstract of publication: ARQoL, BMI z score, acute asthma attacks, night‐time awakenings, use of inhaled corticosteroids, pulmonary function | |
| Study details |
Run‐in period: 2‐weeks (written dietary and respiratory symptoms recall to corroborate stability of asthma and adherence to researcher's instructions Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: ‐ |
|
| Stated aim for study | Quote from publication: "to evaluate whether a program of supervised ND [normocaloric diet] would improve asthma‐related quality of life (AR‐QOL), specifically in obese pubertal adolescents with asthma. Secondarily, we assessed the effects of the dietary program on some clinical indicators of asthma control." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "were randomly allocated to." Comment: details not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: details not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote from publication: "Due the nature of the intervention, the study could not be blinded, and this could have induced investigator bias." |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "Due the nature of the intervention, the study could not be blinded, and this could have induced investigator bias." |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote from publication: "Due the nature of the intervention, the study could not be blinded, and this could have induced investigator bias." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Ordinary scheduled medical follow‐up was assigned to a single pediatric allergist, who was blinded to group allocation." "Due the nature of the intervention, the study could not be blinded, and this could have induced investigator bias." Comment: investigator‐assessed, low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: not clearly reported, missing data reported, did not correspond to the numbers used for the final analysis and no discussion of this made |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: not clearly reported, missing data reported, did not correspond to the numbers used for the final analysis and no discussion of this made |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported as stated in methods, but protocol not available |
| Other bias | Low risk | Comment: nothing of note |
NCT00132132.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: 10‐20 years; BMI > 85% Exclusion criteria: endocrine disorder; on psychotropic medications Diagnostic criteria: BMI > 85% |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions Behavioural education programme : monthly meetings for 4 hours; included exercise, education, empowerment and incentives: registration‐monitoring of choices of liquid intake, monitoring of sedentary behaviours (hours watching television, computer, video games), monitoring of heart rate, monitoring of metabolic equivalents, MI, monitoring of exercise abilities; 1 hour of exercise (including strength training); educational lectures on nutrition and medical aspects of obesity and Type 2 diabetes; projects/games; empowerment tools such as leading exercises and presenting food labels for discussion. Referral to a dietitian (minimum 3 visits) Standard of care control : education on physical activity and nutrition in a primary care clinic setting. Referral to a dietitian (minimum 3 visits) |
|
| Outcomes | Outcomes reported in abstract of publication: no abstract | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT00132132 |
|
| Publication details |
Language of publication: English Funding not reported Publication status: results published in ClinicalTrials.gov record: NCT00132132 |
|
| Stated aim for study | Quote from publication: "evaluate the impact a behavioural intervention can have on BMI." | |
| Notes | Unpublished study, results posted in ClinicalTrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on concealment of allocation |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: designated as an open‐label study. Outcomes investigator‐assessed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from trial record: "open label." Comment: objective outcomes unlikely to be affected by detection bias |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote from trial record: "Per protocol analysis: participants who attended at least one intervention session in addition to their final assessment…" Comment: missing data with minimal explanation, differential loss between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes stated reported |
| Other bias | Unclear risk | Comment: no baseline measurements of key outcomes reported. Limited details reported in ClinicalTrials.gov record |
NCT00807560.
| Methods | Parallel, open‐label RCT | |
| Participants |
Inclusion criteria: aged 13‐17 years; living with at least 1 parent or guardian who was willing to participate in treatment; BMI percentile > 85% for gender and age Exclusion criteria: current psychotic illness; current alcohol/drug dependence; active suicidality; eating disorders; history of bariatric surgery; medication associated with significant weight changes (e.g. antipsychotic drugs); serious medical or physical conditions resulting in significant weight changes (e.g. pregnancy, genetic disorders); complications of obesity that contraindicate moderate physical activity (e.g. orthopaedic disorders) Diagnostic criteria:‐ |
|
| Interventions |
Number of study centres:‐ Treatment before study:‐ Description of interventions Family‐based therapy for paediatric overweight : to resolve the eating disorder and return the participant to healthy psychosocial and physiological developmental trajectories through active family involvement across 3 treatment phases. No further details provided Nutritional educational control condition : minimal nutrition and physical activity education curriculum across 16 sessions over 24 weeks |
|
| Outcomes |
Primary outcomes: height and weight for BMI z score Secondary outcomes: Youth and Parent/Guardian Eating Questionnaire; Child Depression Inventory; PedsQL; Moderate to Vigorous Physical Activity Measure; Sedentary Activity Checklist; PACE + Fruit Vegetable Screening Measure; PACE+ Dietary Fat Screening Measure; parent 24‐hour dietary recall; parent overweight status, height and weight (converted into BMI z score) |
|
| Study details |
Run‐in period: ‐ Study terminated early:‐ Trial identifier: NCT00807560 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: results published in ClinicalTrials.gov record: NCT00807560 |
|
| Stated aim for study | Quote: "to determine whether a parent/guardian intervention for adolescent overweight/obesity more effective than a nutritional counselling education curriculum for reducing body mass index z‐score (BMI Z‐score) and related outcomes." | |
| Notes | Unpublished study, results posted in ClinicalTrials.gov | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: nothing stated |
| Allocation concealment (selection bias) | High risk | Comment: open label |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: open label |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: open label |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: nothing stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: data for subjective outcomes not provided |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: high number of dropouts, 66% for intervention group and 71‐77% for control group and no reasons provided. Study flow chart indicated all participants completed but data at follow‐up were provided for only approximately one‐third of participants |
| Selective reporting (reporting bias) | High risk | Comment: did not report secondary outcomes |
| Other bias | Unclear risk | Comment: not enough information to judge |
Nguyen 2012.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 13‐16 years; overweight to moderately obese (i.e. BMI z score range 1.0‐2.5); home access to a landline telephone; home access to the Internet to receive e‐mails or access to a mobile telephone to receive SMS messages; ability to attend the group programme for 7 weeks in the first instance on the specified days; at least 1 of the adolescent's parents/carers willing to participate in the initial 7 parent group sessions Exclusion criteria: severely obese (i.e. BMI z score > 2.5) or if there was a secondary cause for overweight/obesity; intellectual disability, significant medical illness, psychiatric disturbance; taking medications that affect weight status; inability to take part in physical activity sessions; poor level of spoken English (adolescent or parent/carer) Diagnostic criteria: BMI z score range 1.0‐2.5 |
|
| Interventions |
Number of study centres: unclear Treatment before study: none Description of interventions Loozit intervention : 2 phases
Intervention 2 : same interventions as above with additional therapeutic contact of 14 telephone coaching sessions +32 SMS/e‐mail messages aimed to enhance adolescents' knowledge, skills and confidence to initiate and maintain required changes in dietary and activity behaviours. If there was a parking fee when attending to the community health centres participants were reimbursed |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score; waist‐to‐height ratio; total cholesterol level; triglycerides; global self‐worth; dietary, physical activity or sedentary behaviour | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ACTRN12606000175572 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to determine the effect of additional therapeutic contact to usual treatment on body mass index (BMI) z score and waist circumference z‐score in overweight and obese adolescents aged 13‐16 years (at baseline) who participate in a community‐based weight management program (the Loozit group program)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A computer generated randomisation sequence…" Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "a set of consecutively numbered opaque envelopes containing the group allocation …the next numbered envelope is opened by a research assistant… revealing the group allocation and this is recorded along with the individuals pre‐assigned identification number." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: self‐reported outcome measurement, no blinding of participants or personnel |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: outcome assessors blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: outcome assessors blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote from publication: "An estimation of intervention effect on outcome measures will be obtained at each follow‐up observation on an intention to treat basis." |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "An estimation of intervention effect on outcome measures will be obtained at each follow‐up observation on an intention to treat basis." |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Low risk | Comment: no other risks of bias |
Norman 2016.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 11‐13 years, BMI ≥ 95th age‐ and gender‐specific percentiles; literate in English; planned to be a San Diego County resident for the next year; willing to return to the physician's clinic for counselling sessions; could attend measurement visits; had a parent or guardian willing to participate who were literate in English or Spanish. Exclusion criteria: without reliable transportation; taking weight‐altering medications within 6 months prior to study initiation; unable to do moderate‐to‐vigorous physical activity; weight > 300 lb; in foster care; receiving special needs education; previous participant in study authors' weight loss studies; currently enrolled in a weight loss programme; diagnosed with obesity‐related disorders; requiring immediate weight loss management or diseases affecting absorption or processing of nutrients Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: not clear Treatment before study: none Description of interventions Stepped‐down care : based on a combination of Chronic Care Model and social cognitive theory. Chronic Care Model provided a conceptual framework for the healthcare delivery for adolescents with obesity of chronic illness management in a primary care setting. Chronic Care Model emphasises interdisciplinary team input. Within the Chronic Care Model framework, social cognitive theory constructs of behaviour self‐management were applied and included: building self‐efficacy, goal setting, feedback, identifying barriers and social support. The intervention followed modified recommendations from the American Academy of Pediatrics for treatment of childhood obesity and consisted of 3 × 4‐month steps. The goal was for adolescents to lose at least 4 lb every 4 months. If the participant did not meet the goal, then the step was repeated. If a 4‐lb weight loss was achieved, the participant was 'stepped‐down' to the next level of reduced intensity. The number and frequency of treatment elements varied for each intervention step. At the start, the physician provided brief counselling on healthy behaviours. If progress was not made, then follow‐up occurred and focused on weight management strategies. Face‐to‐face health educator visits occurred monthly in step 1 and bi‐monthly in step 2 and were available to the child and parent, but the parent was not required to attend. Biweekly telephone calls were used to review progress and help adolescents set new goals and discuss barriers and solutions, and speak to parent to reinforce parental involvement and emphasise importance of healthy changes in the home environment to encourage goal attainment. Diet and physical activity education materials were distributed to adolescents and their parents at each visit. Pedometers were distributed at the initial health educator visit to monitor physical activity and help participants set appropriate physical activity goals. Enhanced usual care : received an initial counselling session, 1 health education visit, materials on how to improve weight‐related behaviours, monthly follow‐up mailings on weight‐related issues and a pedometer. At baseline, and 4‐ and 8‐month assessments, adolescents in both study groups received USD15, and at 12 months they received a USD25 incentive for completing measurements. Parents received a USD15 incentive for completing measures at each assessment and USD20 at each measurement point to compensate for transportation costs. Physicians, nurse practitioners and health educators delivered the therapy |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, adiposity, biometric outcomes | |
| Study details |
Run‐in period: 2‐week run‐in screening programme was conducted. Adolescent‐parent dyads were asked to perform some of the activities that would be required of them if they were to be enrolled in the intervention trial. These tasks included attending a measurement visit; scheduling and completing a telephone call with a study staff member; locating a food item at home, reading the food label and describing the nutrition content; tracking basic food intake and physical activity in a written diary over 4 days; and scheduling and attending a follow‐up appointment Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Funding not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "evaluating the stepped‐down approach to weight loss, targeting changes in body mass index (BMI), adiposity, blood pressure, fasting blood glucose and lipids among adolescents with obesity." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Simple randomization to study arm was determined by a computer using a permutated block algorithm and was stratified within the primary care provider site." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of concealment of allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: not mentioned |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from author: "Measurement staff were blinded, self‐reported." Comment: outcome assessors blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from author: "Measurement staff were blinded." Comment: outcome assessors blinded |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: not applicable as data not reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk |
Quote from publication: "Intent‐to‐treat analyses were conducted using available data and assuming data were missing at random." Comment: stated ITT and reports all numbers randomised |
| Selective reporting (reporting bias) | High risk |
Quote from author: "There were other outcome measures that were not included in the paper. These included the following: Physical Activity based on self‐report, Quality of Life, Calories, food and nutrient intake based on self‐report, sedentary behavior based on self‐report." Comment: reported by the author but reasons not explained |
| Other bias | High risk |
Quote from publication: "The run‐in programme was conducted to minimize participant attrition, but may have resulted in a more motivated sample of participants and parents compared with non‐run in trial cohorts." Comment: study participants may have had higher motivation and much more likely to follow the intervention |
Pakpour 2015.
| Methods | Parallel RCT, randomisation ratio: 1:1:1, superiority design | |
| Participants |
Inclusion criteria: aged 13‐18 years, obese (≥ 95th percentile for age and gender), adolescents lived with a parent or adult carer who was prepared to be involved in treatment Exclusion criteria: taking weight‐related medication, having a diagnosis of an eating disorder, being pregnant, having clinical mental health conditions or psychosis Diagnostic criteria: BMI > 95th percentile for age and gender |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Weekly sessions: participants in both intervention groups received 6 × 40‐minute individual counselling sessions on diet and exercise MI : targeted improved eating and physical activity behaviour. All adolescents were encouraged to express their personal motivation to change their physical activity and dietary behaviour. Adolescents assisted with reducing resistance and overcoming ambivalence about behaviour change. MI techniques such as reflective listening, open‐ended questions and eliciting self‐motivational statements. The intervention was initiated based on the underlying spirit of the MI. Session included: in the first phase, the counsellors aimed to foster a confident relationship with the adolescents, address the positive and negative effects of obesity, and increase the adolescents' awareness of their current weight status. In the second phase, alternative courses of action were taken into account, costs and benefits of actions were discussed, and adolescents' readiness to change was evaluated. In the third phase, adolescents were invited to engage in goal setting and planning for change. Adolescents were encouraged to create an action plan. Also encouraged to achieve at least 60 minutes of moderate‐to‐vigorous intensity physical activity daily as recommended by the World Health Organization. Encouraged to eat a variety of foods from each of the 4 major food groups and low‐fat alternatives. The facilitators (dietitian or exercise specialist) completed 20 hours of MI training from a certified and experienced MI trainer MI + parental Involvement : identical MI sessions to above. In addition, 1 in‐person parent MI session identical in MI style as used for adolescents, focusing on the adolescents' weight, parents' attitudes and behaviours regarding children's physical activity and dietary habits, parent monitoring and supervision; the goal was to promote progress toward the child's intervention goals and attitudes by the parents. The role of parenting in preparing healthy foods for their child and encouraging/monitoring them for being physically active were discussed. Delivered at the end of the 6th session and lasted for > 60 minutes. Passive control group : no details |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, anthropometric, biochemical, psychometric and behavioural outcome variables | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT02180802 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to evaluate and compare the role of parental involvement in MI interventions for obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "To ensure adequate concealment of allocation, a research coordinator performed the randomization procedure by using a computer generated randomization schedule/sequence." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of how allocation was concealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "Adolescents could not be blinded to intervention allocation." Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "Adolescents could not be blinded to intervention allocation." Comment: no blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: "All outcomes were assessed by 2 blinded and trained physicians. The blinded assessors passed a series of training courses for testing and interviewing according to the standard program protocol." Comment: investigator assessed |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Anthropometric measures at baseline and 12 months after randomization were taken by 2 assessors blinded to group allocation." Comment: investigator assessed |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk |
Quote from publication: "Due to financial restrictions, only 25 randomly selected adolescents in each group (n = 75) provided accelerometer data." Comment: states ITT but only reported completers in the table |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk |
Quote from publication: "Due to financial restrictions, only 25 randomly selected adolescents in each group (n = 75) provided accelerometer data." Comment: states ITT but only reported completers in the table |
| Selective reporting (reporting bias) | Low risk | Comment: reported what was initially stated except for BMI percentile which was not reported at 12 months |
| Other bias | Low risk | Comment: no other bias |
Patrick 2013.
| Methods | Parallel RCT, randomisation ratio: 1:1:1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐16 years, at "high risk" for diabetes, as defined by the American Diabetes Association expert consensus panel, overweight BMI > 85th percentile for age and gender, weight and height > 85th percentile, or weight > 120% of ideal for height) + any 2 of the following risk factors: family history of Type 2 diabetes mellitus in a first‐ or second‐degree relative, race/ethnicity (American Indian, African‐American, Hispanic, Asian/Pacific Islander), or signs of insulin resistance (acanthosis nigricans, hypertension, dyslipidaemia, polycystic ovary syndrome) Exclusion criteria: diagnosis of diabetes, pregnant, not planning to be in the San Diego area over the entire study period, had any medical condition that would prevent them from participating in the intervention Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 18 Treatment before study: none Description of interventions 4 comparator groups, 3 included a website intervention: website only, website and group and website and SMS, and 1 usual care. Website only : website and tutorials designed to promote weight loss and healthy behaviours related to obesity such as educational topics and challenges based on skill building exercises, a reward system to encourage success, evaluation for assessment of progress and feedback on progress. Web tutorials on several behaviour change strategies, such as goal setting, seeking social support and positive self‐statements. Theoretical based on the behavioural determinants model and the Transtheoretical Model of behaviour change. 3 phases, phase 1 (weeks 1‐17) entailing education on healthy behaviours, phase 2 (weeks 18‐34) interactive games and quizzes for the participant to select challenges and goals, phase 3 (weeks 35‐51) interactive and also encouraged working on multiple behaviours at the same time. Also had weekly "check‐in" e‐mails, monthly mailed tip sheets and, if necessary, a telephone call from a health counsellor. Based on the "stoplight approach," participants were encouraged to limit red‐light activities (unproductive, low energy), increase green‐light activities (high energy) and do yellow‐light activities in moderation. The website also included information on recommended food portion sizes, categorisation of foods into the stoplight approach, and a resource library that included tip sheets and recipes. Parent completed an adult version of the programme website and received monthly group sessions. Website and group : attendance and participation in group sessions in addition to the website (treatment as 'website only'). Rewarded with mileage incentives and a lottery for prizes such as cookbooks or other materials to assist with healthy behaviour change. Providers underwent a 2‐hour counselling training. Website and SMS : minimum of 3 text messages per week that related to weekly challenges and intervention goals as well as the website (treatment as 'website only'). Reminder text messages sent if the participant did not log on to the website by the 4th day of the intervention. Participants could also communicate via text messages with a health counsellor if they had questions. Participants were provided with mobile phones and prepaid text message plans that allowed research staff to monitor SMS use Control : given printed materials produced by the American Diabetes Association and the American Heart Association |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, adiposity, physical activity, diet, sedentary behaviour | |
| Study details |
Run‐in period: none Study terminated early: no |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal Trial identifier: NCT00412165 |
|
| Stated aim for study | Quote from publication: "to evaluate the effectiveness of an intervention targeting this population that was offered to participants recruited through clinical sites but primarily delivered through combinations of three modalities: the web, group sessions for adolescents and parents, and short message service (SMS). Hypothesized that, compared with usual care, all active treatment conditions would produce better behavioral, weight, and quality‐of‐life and psychological outcomes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Participants were randomized…" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons provided, used ITT analysis |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons provided, used ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as stated |
| Other bias | Low risk | Comment: no other bias |
Patsopoulou 2017.
| Methods | Parallel RCT, randomisation ratio: 1:1:1, superiority design | |
| Participants |
Inclusion criteria: overweight or obese students Exclusion criteria: ‐ Diagnostic criteria: Cole criteria |
|
| Interventions |
Number of study centres: 15 Treatment before study: ‐ Description of interventions Activity : training was directed by a professional teacher of physical education. The training programme was designed according to the type and intensity of exercise that school children normally performed. Many activities were delivered as games to encourage enthusiasm and participation. Endurance type activities accounted for most of the time spent in training (about 50% team sports and 50% running games), with attention to co‐ordination and flexibility skills Diet + activity : training programme + the same activity trainer information was presented about the reasons behind childhood obesity, dietary and cooking habits, and the motivation for weight loss to involve the whole family in the "battle" against obesity. Discussion with participants on the food pyramid, food choices, food labels, food preparation and cooking, eating habits, regular meals and controlling environments that stimulate overeating. The topics discussed were given to the adolescents in the form of a printed notebook, while parents were also invited to attend these sessions Control : no intervention |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, waist circumference, blood pressure, Family Eating and Activity Habits Questionnaire | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT02653508 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "test the efficacy of two intervention groups, physical activity in isolation and combination of physical activity with provision of dietary information, in improving overweight and obesity in adolescents and also to compare each of them to a control group." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "All public middle and high schools (26 in the total) in the city of Larissa in Greece were informed about the purposes of the study. Seventeen secondary and high schools took part in the study…" |
| Allocation concealment (selection bias) | High risk | Quote from publication: "One hundred eighty one adolescents were enrolled and randomized in the three groups of the study, by the same professional teacher of physical education who conducted the program." |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "randomized in the three groups of the study, by the same professional teacher of physical education who conducted the program." Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "randomized in the three groups of the study, by the same professional teacher of physical education who conducted the program." Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: "completed by parents and adolescents. randomized adolescents in each group were not aware of the existence of the other two study groups." Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "measurement by a single trained nurse, member of the research team." Comment: investigator‐assessed, not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Low drop‐outs and a modified ITT was carried out Comment: reported but not all reasons explained |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Low drop‐outs and a modified ITT was carried out Comment: reported but not all reasons explained |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported as mentioned in the clinical trial record |
| Other bias | Low risk | Comment: no other bias |
Pbert 2013.
| Methods | Cluster RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: participants in grade 9‐11, BMI ≥ 85th percentile for age and gender. Provided assent and had parental consent. Had at least 1 English‐speaking parent Exclusion criteria: planning to move out of the area, had a medical condition that precluded adherence to the intervention, diagnosis of a serious psychiatric illness, genetic or endocrine cause of obesity, taking a medication associated with weight gain or weighing ≥ 300 lbs Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 8 Treatment before study: none Description of interventions "Lookin' Good Feelin' Good" intervention : incorporated recommendations for the prevention and treatment of child and adolescent overweight and obesity and based on social cognitive theory. 6 × 1‐to‐1 counselling sessions of 18‐29 minutes over 2 months, during the school day in non‐academic classes in the school nurse's office. Used the 5‐3‐2‐1‐0 approach to support making 5 daily key behaviour changes: ≥ 5 servings of fruit and vegetables; 3 structured meals; ≤ 2 hours of television, computer, electronic games; ≥ 1 hour moderate physical activity, 0 limit soda and sugar‐sweetened drinks. A participant‐centred counselling approach allowed school nurses to tailor the intervention to the student's needs. At each visit there was a weigh‐in and feedback, review of diet and physical activity logs, assessing progress to behaviour change goals, reviewing successes, problem solving, setting new goals, assessing behaviour and identifying barriers. Provided and instructed in the use of a pedometer. A USD25 gift certificate was provided at each assessment. School nurses were trained through a daylong group training session Control : 6 × 1‐to‐1 visits with the school nurse for 8.5‐9 minutes over 2 months to review behaviour changes, read 6 informational pamphlets on weight management and had questions answered |
|
| Outcomes | Outcomes reported in abstract of publication: ate breakfast, intake of total sugar and added sugar, drink soda ≤ 1 time/day, ate at fast‐food restaurants ≤ 1 time/week, BMI, activity, caloric intake | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Funding not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The purpose of this study was to test the feasibility and efficacy of a school nurse delivered weight management intervention on BMI, diet, physical activity, and sedentary behavior among overweight and obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Schools were pair matched on total enrolment, gender, race and ethnicity, and percent receiving reduced or free lunch; 1 from each pair was then randomly assigned to either the school nurse counselling intervention group or the control group". "Random assignment was conducted by the study statistician." Comment: majority of baseline characteristics associated with obesity were similar |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "Participants were not blinded to condition as they received the intervention allocated to their school." Comment: high risk of performance bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "Participants were not blinded to condition as they received the intervention allocated to their school." Comment: high risk of performance bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "Assessments were completed by a research assistant who was not blinded to school condition at baseline and 2 and 6 months following baseline in the school nurse office." Comment: not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Assessments were completed by a research assistant who was not blinded to school condition at baseline and 2 and 6 months following baseline in the school nurse office." Comment: not blinded; however, objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: low drop‐outs, reasons given, ITT analysis |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: low drop‐outs, reasons given, ITT analysis |
| Selective reporting (reporting bias) | High risk |
Quote from publication: "We reported on all physiologic and eating and activity behavioral outcomes. We did not report on psychosocial outcomes that could be considered intermediary variables." Comment: not reported baseline weight controls or intervention or height data |
| Other bias | Unclear risk |
Quote from publication: "Models were also adjusted for baseline level of the outcome and for any baseline characteristics that differed significantly by group at baseline." Comment: unclear if they have accounted for clustering in the analysis |
Pitetti 2007.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 14‐19 years; a physician and a psychologist, both experienced in autism, confirmed that diagnosis met the DSM‐IV‐Text Revised; manifested moderate‐to‐profound mental retardation as assessed by standardised tests of intelligence, including the Leiter International Performance Scale, Slosson Intelligence Test, Wechsler Adult Intelligence Scale, or a combination of these; all had histories of lengthy and restrictive placement resulting from their severe maladaptive behaviours; identified by staff as able to follow instructions to perform treadmill walking Exclusion criteria: had no medical contraindications (i.e. cardiovascular/respiratory anomalies) or significant primary sensory or motor impairments that restricted them from treadmill walking Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Treadmill walking group : weekly activity, regular activity classes (i.e. 15‐30 minutes, Monday‐Wednesday‐Friday) consisted of treadmill walking, when regular activity classes were not planned (i.e. Tuesday and Thursday), taken to the gym and given the opportunity to walk on the treadmill, given the opportunity to walk on the treadmill at their residence in the evenings. All 10 participants trained to walk unassisted on the treadmill during their regular class time. Initial frequency of twice per week, progression of 1 day every 2 weeks to a peak frequency of 5 times per week. Duration was for 8 minutes per session, with a progression of 1‐2 minutes every 2‐3 weeks to a peak duration of 20 minutes per session. Initial treadmill speed 2.4‐3.5 mph, with a progression of 0.1‐0.3 mph every 2‐3 weeks to a peak speed of 3.7‐4.1 mph. Initial grade of 0%, with progressive increases of 0.5%. The activities took place under the guidance of a staff member with a Master's degree in adapted physical activity Control group : leisure activity of choice 3 times per week. Not a strict schedule. Student Support Plan designed and implemented by the residential treatment facility staff members, allowed for 30 minutes of 'leisure activity', 3 times per week, at the campus gym. These activities took place under the guidance of a staff member with a Master's degree in adapted physical activity. Classes permitted participants to engage in the following activities; basketball (shooting, dribbling, passing), jumping rope, roller skating, scooter‐board activities, object control skills (i.e. throwing and catching the ball), striking a ball (i.e. tennis or nerf) with a tennis racket or plastic bat and cycling skills (e.g. adult bicycle or tricycle) |
|
| Outcomes | Outcomes reported in abstract of publication: BMI, treadmill walking | |
| Study details |
Run‐in period: prior to initiating the study, all 10 participants had been trained to walk unassisted (i.e. not holding on to railing) on the treadmill during their regular class time (e.g. 1‐3 times per week, duration of 5‐10 minutes) and, therefore, were acclimated to the use of the treadmill Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Funding not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The purpose of this study was to determine the efficacy of incorporating a 9‐month treadmill walking program into the weekly academic curriculum of youth with severe developmental disabilities including autism." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: randomisation was unclear from the manuscript. After contact with the authors to check on method of randomisation information was provided: "it was a randomized allocation using random assignment or random placement. We flipped a coin and "heads" assigned the participant to the treadmill group and "tails" assigned the participants to the control group." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: randomisation by coin toss |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: authors stated, "Participants were severely developmentally disabled, so they had limited to no ability to be cognizant of whether they were in the supplemental treadmill walking or control group." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details blinding outcomes assessors. No member of the research team or outcome assessors participated during or was present in the exercise portion, respectively. Objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no attrition |
| Selective reporting (reporting bias) | High risk | Comment: exercise capacity (treadmill walking frequency, speed, elevation) not reported. Authors stated that all measured outcomes were reported |
| Other bias | Low risk | Comment: no other bias |
Resnicow 2005.
| Methods | Cluster RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: African‐American girls aged 12‐16 years and BMI > 90th percentile for age and gender, churches where the majority of members' household income was above USD40,000. Exclusion criteria: unclear Diagnostic criteria: used the 90th percentile as inclusion cut‐off point rather than the 85th percentile based on formative research and pilot studies. Girls on the lower end of the obesity continuum, i.e. 85th to 90th percentile, were different behaviourally and psychologically, and combining girls from the low and high ends of the obesity continuum would adversely affect group cohesion |
|
| Interventions |
Number of study centres: 10 Treatment before study: none Description of interventions High intensity (24‐26 sessions) : girls attended weekly group behavioural sessions. Parents were encouraged to attend sessions (around 12 sessions). Each group session included an experiential, interactive behavioural activity, at least 30 minutes of moderate‐to‐vigorous physical exercise, and preparation or consumption (or both) of low‐fat, portion‐controlled meals or snacks. Target behaviours included: increased fruit and vegetable intake, decreased fat intake, decreased fast‐food intake, decreased sedentary behaviour and increased physical activity. Girls were taught to reshape their target behaviours using the principles of substitution, moderation and abstinence. Girls focused their behaviour change on target foods or priority behaviours identified or selected by the girls. At the beginning of each intervention cycle, girls only attended a 1‐day retreat where participants completed a low ropes course and team building activities, ate healthy portion‐controlled meals and attended a group session on hunger and satiety. All girls received a 2‐way paging device. Messages, developed by the girls based on their target foods and activity patterns, were sent to them throughout the day and in key times when they needed reminders about their eating or physical activity. Received 4‐6 MI calls by telephone over the 6 months of intervention to support and help in behavioural change Moderate intensity (6 sessions) control : sessions were selected from the larger pool of sessions delivered to the high‐intensity group. Parents were encouraged to attend sessions (3 sessions). Topics included fat facts, barriers to physical activity, fad diets, neophobia (i.e. fear of new foods) and benefits of physical activity. Girls did not receive the 2‐way pagers, MI telephone calls or kick‐off retreat |
|
| Outcomes | Outcomes reported in abstract of publication: BMI units and % body fat | |
| Study details |
Run‐in period: churches were contacted either by telephone or in‐person and administered a brief screening instrument that queried their membership numbers and socioeconomic status Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Other funding: "given that the sponsoring agency was unable to provide any additional funding, we were able to complete the study in only 10 of the projected 12 churches." Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The primary aim of the project, called Go Girls, was to develop and test a culturally tailored intervention program for overweight 12‐ to 16‐year‐old AA [African‐American] adolescents and their parents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Before randomization, a pool of middle and upper income churches was identified by project staff based on prior projects as well as telephone directory and internet searches, Churches were then contacted either by telephone or in person. Only churches that reported that the majority of members' household income was above $40,000 [USD40,000] were included." "A total of 10 churches (including the aggregate church) were randomized to condition, five treatment (high intensity) and five comparison (moderate intensity)." Comment: there was no difference in baseline parameters between groups |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "A total of 10 churches (including the aggregate church) were randomized to condition, five treatment (high intensity) and five comparison (moderate intensity)." Comment: no further details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no mention of blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no mention of blinding, objective outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk |
Quote from publication: "no evidence of differential attrition, because girls in the moderate‐intensity group who dropped out did not significantly differ from drop‐outs in the high‐intensity group." "In intention‐to‐treat analysis, there was no significant group difference for BMI, the trial's main outcome, nor were there significant group effects for any of the secondary outcome." Comment: reported numbers lost to follow‐up but reasons not provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol unavailable |
| Other bias | Low risk |
Comment: before randomisation, a pool of middle‐ and upper‐income churches was identified by project staff based on prior projects as well as telephone directory and Internet searches. Churches were then contacted either by telephone or in‐person and administered a brief screening instrument that queried their membership numbers and socioeconomic status. Only churches that reported that the majority of members household income was above USD40,000 were included. Churches requested that the comparison condition receive a meaningful intervention. It was agreed on by the church representatives and study staff that 6 sessions represented a meaningful intervention of sufficient benefit that would not jeopardise between‐group differences. However, using different timings in the comparator (once per month instead of once per week) and this may have had an impact. For cluster analysis ‐ quote: "Outcomes were analyzed with a mixed model repeated measures ANOVA program, SAS PROC MIXED (SAS Institute, Cary, NC), that allows for adjustment of subject non‐independence within churches." |
Saelens 2002.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐16 years, 20‐100% above the median (50th percentile) for BMI for age and gender. Interested in weight control, not currently engaged in another weight control programme, and otherwise healthy as determined by a paediatrician Exclusion criteria: ‐ Diagnostic criteria: as above. |
|
| Interventions |
Number of study centres: unclear Treatment before study: none Description of interventions Healthy Habits : computer and telephone intervention. Adolescents engaged in a computer program after baseline assessment, the program was adapted from PACE+ software designed for adolescents and modified for overweight adolescents. It assessed eating, physical activity and sedentary behaviour and guided adolescents through individualised plans generated to increase physical activity or decrease sedentary behaviour and decrease dietary fat or increase fruits/vegetables or decrease overeating/snacking. Plan generation included identifying benefits, barriers and specific strategies to achieve goals. Action plan summaries were generated, and a provider summary was produced for the behaviours targeted by the adolescent. Met with a paediatrician to discuss action plans (tailored physician counselling), and approximately 1 week after the clinic visit met the study author to discuss upcoming mail and telephone contacts and to learn food self‐monitoring. Calls from a telephone counsellor 1 week later detailed telephone scripts to address adolescents' weight change since the last call, the link between weight change and eating and physical activity behaviours, instruction and feedback on self‐monitoring, goals and behavioural skills. Provided with a manual and information sheets. Self‐monitoring of food and beverage intake and calories consumed. Foods were categorised into green, red or no colour foods. Green defined as ≤ 1 g of fat per serving, 150 calories per serving and providing a good source of ≥ 1 valuable dietary components (e.g. calcium, fibre or protein), red foods were defined as having ≥ 5 g of fat per serving or were diet versions of high‐fat foods. The eventual green food goal was 40 green food servings (based on standard serving sizes) or more per week, and the red food goal was 15 red food servings per week. Encouraged to self‐monitor physical activity daily with a goal of a minimum of 60 minutes of at least moderate‐intensity physical activity on 5 days per week with gradual increases from baseline. Overall duration 16 weeks with 13 sessions. Paediatricians trained with the study protocol and telephone counsellors (with at least a Bachelor's degree in psychology or nutrition) received weekly supervision Standard care control : non‐tailored physician‐counselling session. 1 session in the 16‐week treatment period by the same paediatrician as the intervention group. Assessed/encouraged the adolescent's motivation for weight‐related behaviour change, provided information about short‐ and long‐term health consequences of high weight status and benefits of better weight control, made recommendations for healthful eating consistent with the Food Guide Pyramid, reviewed physical activity recommendations for adolescents (60 minutes per day of at least moderate‐intensity physical activity), and encouraged consistency and persistence with health behaviour changes. Used a worksheet to facilitate the discussions. Adolescents encouraged to implement recommended behaviour changes on their own and with the help of their family. Adolescents received USD25 for post‐treatment and USD25 for follow‐up assessment. In the intervention group, a lottery occurred after all adolescents had completed post‐treatment assessment for USD50. Adolescents were awarded 1 point each for meeting self‐monitoring, physical activity, calorie, green food goals and red food goals each week based on the counsellor's review of self‐monitoring booklets. Points were accumulated for tickets for a study‐based lottery (1 point = 1 ticket) |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z scores, behavioural skills use, energy intake, % calories from fat, physical activity, sedentary behaviour and problematic weight‐related or eating behaviours/beliefs, feasibility and participant satisfaction | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "This study evaluates the post‐treatment and short‐term follow‐up efficacy of, as well as participant satisfaction for, a 4‐month behavioral weight control program for overweight adolescents initiated in a primary care setting and extended through telephone and mail contact." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Randomization occurred by selection among opaque envelopes labelled with levels of percent overweight, each envelope containing an HH [Healthy Habits] or TC [typical care] card." Comment: unclear how envelopes were selected or how the labelling affected selection |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Randomization occurred by selection among opaque envelopes labelled with levels of percent overweight, each envelope containing an HH or TC card." Comment: unclear if assignment envelopes were used with appropriate safeguards (e.g. if envelopes were sealed or sequentially numbered) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no blinding of carers, unclear if participants blinded |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no blinding of carers, unclear if participants blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not specified |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified, outcomes unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: reasons not provided, imbalance in dropouts, ITT used |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: reasons not provided, imbalance in dropouts, ITT used |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to judge |
| Other bias | Low risk | Comment: no other bias |
Savoye 2007.
| Methods | Parallel RCT, randomisation ratio: 2:1, superiority design | |
| Participants |
Inclusion criteria: BMI > 95th percentile based on the CDC growth chart, aged 8‐16 years, English‐speaking ability, shown an interest in the weight management programme, have a carer (e.g. father, mother or grandparent) who was willing to participate in the educational component of the programme. Exclusion criteria: had diabetes, psychiatric disorder (e.g. schizophrenia, severe autism or mental retardation, or psychosis) or other serious medical condition that would preclude participation in the programme, taking medications that potentially cause significant weight gain (e.g. risperidone, olanzapine, clozapine) as well as using medications for weight loss or involved in a coexisting weight management programme. Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: none Description of interventions Bright Bodies weight management : nutrition/behaviour modification once (40 minutes each) per week: behaviour modification classes for participants and carers were held separately. Topics were provided from the Smart Moves Workbook, a curriculum developed for overweight children and used in the authors' pilot study. Sample topics included Ready, Set, Goal!, Risky Business: Identifying High‐risk Situations, Environmental Engineering, Mirror, Mirror on the Wall, Bullies, Teasers, and Other Annoying People and Oops I Slipped ‐ Understanding a Relapse. Attended the programme twice per week for 6 months and then every other week for an additional 6 months. Techniques included self‐awareness, goal setting, stimulus control, CST, cognitive behaviour strategies and contingency management. Behaviour modification classes for carers included topics that reflected the challenges that parents verbalised. Classes emphasised the importance of the parents' roles in modelling healthy behaviour change. Motivational tools were used to encourage regular attendance. Each class of high‐intensity exercise to sustain 65‐80% of the age‐adjusted maximal heart rate held twice (50 minutes each) and nutrition/behaviour modification once (40 minutes each) per week. Participants were also encouraged to exercise 3 additional days at home per week and to decrease sedentary behaviours. The minimum activity that each participant completed was 100 minutes per week (2 × 50‐minute sessions) for the first 6 months and 100 minutes twice per month for the last 6 months. The nutrition education component of the weight management programme used a non‐diet approach that emphasised low‐fat, nutrient‐dense foods of moderate portion sizes. No details of trainer provided, registered dietitians supervised nutritional component Control : received diet and exercise counselling by registered dietitians and physicians along with brief psychosocial counselling by a social worker. The participant and carer were both involved in setting the nutrition and activity goals to ensure that the clinic plan was realistic and accepted by both. Exercise counselling included decreasing sedentary activities (computer and video games) and finding an activity the participant enjoyed enough to engage in on a regular basis. Nutrition counselling included decreasing intake of juice, switching to diet beverages, switching from whole‐fat to low‐fat milk and bringing lunch to school vs choosing hot lunch. 30‐minute session every 6 months |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, BMI, % body fat, total body fat mass, total cholesterol, HDL and LDL cholesterol, insulin resistance | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: NCT00409422 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to compare changes in BMI, body composition, insulin sensitivity, blood pressure, and lipid profiles, a secondary aim was to compare nutrition components by randomizing families in the weight management group to either the better food choices or structured meal plan approaches." | |
| Notes | The study initially re‐randomised those in the weight management group to either a meal plan group or a better food choices group. After high levels of attrition in the meal plan group (at 6 months, 83%) this arm was discontinued. No results for this group were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Participants were randomly assigned 2:1 by using a permuted block design to the weight management or clinic control group by the same co‐investigators who recruited the participants. Random assignments were generated by computer and concealed by the study statistician (Dr Dziura)." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: stated allocation was maintained by the study statistician and was concealed but no details reported. |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "Participants were not blinded to treatment group. They knew if they were returning back to clinic only (control) or Program (experimental) at the school. Personnel were not blinded during treatment phase either because they knew if subject was going to the Program." Comment: high risk of performance bias |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details on blinding; however, low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons provided, used ITT analysis |
| Selective reporting (reporting bias) | Unclear risk |
Quote from publication: "We have reported all the outcomes measured except for psychosocial outcomes which we are writing a manuscript for as we speak. We assessed self‐concept of children and family dynamics of family using Piers‐Harris Self Concept Scale and Family Assessment Device, respectively. We plan to submit this manuscript by January 1." Comment: the results at 6 and 12 months were different in each publication |
| Other bias | Low risk | Comment: no other bias |
Schranz 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: males aged 13‐17 years; considered to be very overweight or obese (very overweight defined as being above the mid‐point between the age‐ and gender‐specific BMI cut‐offs for overweight and obese, e.g. if the age‐ and gender‐specific BMI cut‐offs for overweight and obesity were 23 kg/m2 and 25 kg/m2, respectively, then the BMI cut‐off for very overweight was calculated to be 24.0 kg/m2; Cole criteria); Tanner stage ≥ 2; categorised as a low or moderate risk by the Sports Medicine Australia screening questionnaire or, if categorised as high risk, obtained a medical clearance from their general practitioner and had no history of injuries or musculoskeletal conditions Exclusion criteria: < Tanner stage 2; categorised as a high risk by the Sports Medicine Australia pre‐exercise screening questionnaire and did not obtain a medical clearance; current or previous injuries which would prevent them from participating in resistance training; exhibit musculoskeletal conditions which would place them 'at risk' as a result resistance training Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 or 2 gymnasia Treatment before study: ‐ Description of interventions 6‐month resistance training programme : 3 × 75‐minute sessions per week on non‐consecutive days. Each session included a 10‐minute warm‐up, 60 minutes of resistance training, and a 5‐minute static stretching cool down. A total of 10 separate multijoint exercises and single‐joint exercises for major muscle groups were trained during each session. Weight‐stacked machines and free‐weight exercises were used Control : no intervention: instructed to continue with normal everyday activities. After completion of the 12‐month assessment, offered a complimentary 3‐month gym membership |
|
| Outcomes | Outcomes reported in abstract of publication: exercise self‐efficacy, resistance training confidence, self‐esteem, body composition | |
| Study details |
Run‐in period: orientation to the resistance training equipment run by 1 of the trainers to familiarise with machines, estimate 1‐repetition maxima to guide strength testing, to determine a starting load Study terminated early: no Trial identifier: ACTRN12609001078246 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to determine the effect of a 6‐month resistance training intervention on the self‐concept strength and body composition of overweight and obese adolescent males." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "random number generator." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Before recruitment began an impartial individual, using a random number generator, produced a random list of group allocation for a possible 70 participants… Each group allocation was written on a piece of paper and concealed inside a non‐transparent envelope which was sealed and the corresponding participant number written on the outside." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "group allocation which was revealed to the participant (and the Principal investigator) after baseline testing was complete." Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "group allocation which was revealed to the participant (and the Principal investigator) after baseline testing was complete." Comment: investigator‐assessed |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "All outcome variables (with the exception of DEXA [dual‐energy X‐ray absorptiometry] which the lead author conducted) were conducted by trained research assistants who were blinded to group allocation." Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "All outcome variables (with the exception of DEXA which the lead author conducted) were conducted by trained research assistants who were blinded to group allocation." Comment: investigator‐assessed |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk |
Quote from publication: "they were included in the analysis at all subsequent assessment sessions." Comment: no missing data reported and reasons explained, but handling of missing data not reported, differential dropout between groups; however, states all were included in the analysis at all assessment sessions which suggests an ITT analysis (although no details) and baseline BMI is differently reported which may suggest a different number was used |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk |
Quote from publication: "they were included in the analysis at all subsequent assessment sessions." Comment: no missing data reported and reasons explained, but handling of missing data not reported, differential dropout between groups, however, states all were included in the analysis at all assessment sessions which suggests an ITT analysis (although no details) and baseline BMI is differently reported which may suggest a different number was used |
| Selective reporting (reporting bias) | High risk | Comment: bone mineral density not reported |
| Other bias | Low risk | Comment: no other bias |
Sigal 2014.
| Methods | Parallel RCT, randomisation ratio: 1:1:1:1, superiority design | |
| Participants |
Inclusion criteria: BMI ≥ 95th percentile for age and gender or ≥ 85th percentile for age/gender with an additional diabetes risk factor (criteria stated), or both; waist circumference ≥ 75th percentile for age and gender; aged 14‐18 years; post‐pubertal (Tanner stage IV or V); sedentary for ≥ 4 months prior to enrolment. Had to attend ≥ 13 out of 16 prescribed sessions (> 80% adherence) during run‐in Exclusion criteria: participation in previous 4 months in a regular programme of exercise or aerobic sports ≥ 2 times per week for at least 20 minutes per session; diabetes; body weight > 159 kg or BMI > 45 kg/m2, or both; use of any performance‐enhancing medication; use of medication/herbal supplement likely to affect body composition, lipids or glucose metabolism (metformin permitted); significant weight change; uncontrolled hypertension; activity restrictions due to disease; other illness; unwillingness/lack of availability to attend exercise and/or nutrition sessions; significant cognitive deficit; pregnancy or intention; inability to communicate in English or French Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions All groups had 3 counselling sessions (baseline, 3 months and 6 months) by a registered dietitian to promote healthy eating, with a daily energy deficit of 250 kcal (see details of run‐in session). Also had telephone support at 6 weeks and 4 months. The 3 exercising groups attended gyms 4 times weekly. Duration 22 weeks. Exercise was supervised by personal trainers weekly to 3 months, and biweekly from 3‐6 months. Personal trainers monitored attendance and exercise progression by reviewing sign‐in sheets and exercise logs. To encourage adherence and retention, participants completing all measurement assessments received a USD50 gift certificate. In addition, participants who maintained good compliance (≥ 70% of sessions attended) obtained a free gym membership renewal for a subsequent 6‐month period (postintervention) Diet + aerobic exercise : followed diet plan + exercised on treadmills, elliptical machines or bicycle ergometers. Heart rate monitors used to adjust workloads to achieve target heart rates. Gradual progressed in exercise duration (20‐45 minutes per aerobic exercise session) and intensity (65‐85% of maximum heart rate) Diet + resistance exercise : followed diet plan + performed 7 exercises using weight machines or free weights, progressing from 2 sets of 15 repetitions at moderate intensity to 3 sets of 8 repetitions at the maximum resistance that could be moved 8 times (8‐RM), duration progressed to a maximum of 45 minutes Diet + aerobic + resistance exercise : followed diet plan + full aerobic training programme + resistance training programme during each session Diet‐only control : followed the same eating plan as those in the exercise groups, wait list control as offered the option to begin an exercise programme (with a free gym membership) for the subsequent 6 months |
|
| Outcomes | Outcomes reported in abstract of publication: % body fat, waist circumference | |
| Study details |
Run‐in period: 4‐week run‐in period to assess compliance with low‐intensity aerobic and resistance exercise for 4 sessions per week. Individually supervised by a personal trainer 2 sessions per week. Also small group sessions (n = 12) covering various topics: barriers in achieving healthful eating, solutions to overcome them, taste panels. The recommended macronutrient energy distribution was 15‐20% protein, 50‐55% carbohydrates and 30% fat. To qualify for randomisation, participants had to attend at least 13 out of 16 prescribed sessions (> 80% adherence) during run‐in Study terminated early: no Trial identifier: NCT00195858 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to determine the effects of aerobic training, resistance training, or their combination on percentage body fat and cardiometabolic risk markers in previously inactive postpubertal overweight and obese adolescents." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization was stratified by degree of overweight (85th‐95th BMI percentile or ≥95th BMI percentile) and sex and done in blocks randomly varying between 4 or 8 participants. Randomization sequences were prepared by an independent statistician and entered into a telephone based central randomization program." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk | Comment: as above |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "The exercise specialist … informed participants of their group assignments allowing the research coordinator to remain blinded." Comment: no blinding of participants or personnel |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk |
Quote from publication: "The exercise specialist … informed participants of their group assignments allowing the research coordinator to remain blinded." Comment: no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Comment: self‐reported outcome measurement, high risk of bias for self‐reported outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Outcome assessors…were blinded to the study group of participants…" Comment: investigator‐assessed outcomes. Unlikely that blinding was maintained, but low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: missing numbers reported and reasons explained |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: missing numbers reported and reasons explained |
| Selective reporting (reporting bias) | High risk | Comment: self‐esteem, HRQoL and Past Day Physical Activity Recall not reported |
| Other bias | Low risk | Comment: no other bias |
Toulabi 2012.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: absence of morbid obesity (hormonal disorders such as hypothyroidism, Cushing's syndrome, etc.), absence of weight‐reducing diets or drugs affecting body weight, participation of 1 parent (with a minimum educational level of 9th grade) in the study, tendency of the students and parents to lose weight, BMI > 28 kg/m2 in the 1st grade students (15 years old), and BMI ≥ 29 kg/m2 in the 2nd grade and 3rd grade students (16 and 17 years old) Exclusion criteria: consuming special diets, taking drugs affecting body weight, or not complying with the intended conditions or criteria (due to the holidays or examination periods) Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 12 schools Treatment before study: none Description of interventions Behaviour modification programme : implemented by a nursing expert and a physical education expert for the participants (based on the compiled training booklets). 24‐hour diet record for participants and parents; face‐to‐face nutritional instructions for the parents supported by an educational booklet (during 4 × 1‐hour weekly sessions); face‐to‐face nutritional instructions for the participants on dietary modification and techniques for increasing physical activity, supported by an educational booklet (during 8 × 45‐minute sessions, held twice per week); exercises demonstrated by the physical education expert at school in a group, 1 hour per day, 3 days per week, for 6 weeks. The exercise programme consisted of warming up for 10 minutes, performing aerobic exercises for 40‐45 minutes and cooling down for 5‐10 minutes, and included rapid and vigorous walking, running, rope jumping, zigzag movements, high‐knees, butterfly movements, stepping exercises and exercises for strengthening important muscles (e.g. rectus abdominal, quadriceps, trapezius and latissimus dorsi) Control : provided with educational booklets after data collection |
|
| Outcomes | Outcomes reported in abstract of publication: weight, BMI, and waist and hip circumferences; students' and parents' nutrition knowledge; symptoms of depression | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: trial was registered at Metabolism Research Center, Tehran University of Medical Sciences, Tehran under the number 578 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to determine the influence of a "behavior modification" program on body mass index (BMI) in obese public high school students in Iran." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Participants were randomly assigned to either intervention or control." Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: the same nurse who provided the intervention measured outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: the same nurse who provided the intervention measured outcomes; however, low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: no discussion of attrition |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: no discussion of attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clinical trial record or protocol identified |
| Other bias | Low risk | Comment: no other bias |
van Egmond‐Frohlich 2006.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: participating in an inpatient rehabilitation programme with obesity as the primary indication (BMI > 97th percentile), aged 9‐16 years, informed consent. Exclusion criteria: secondary obesity, language barriers, duration of rehabilitation < 28 days, type 1 diabetes, psychiatric disease. Diagnostic criteria: BMI > 97th percentile. |
|
| Interventions |
Number of study centres: 320 physicians Treatment before study: rehabilitation programme Description of interventions Behavioural education programme : after the rehabilitation programme, participating physicians of the intervention group received a practice guideline. Telephone consultation was offered. Key items of the guideline were: addressing the guilt or fate problem; treatment aims (health promotion activities); guidance on fat‐limiting mixed diet; promotion of a physical active lifestyle; local support resources; promotion of flexible control of eating; patient guidance according to the public health counselling and self‐management model; the outpatient programme was intended to have health check‐ups every 4 weeks during the first 12 months (10‐12 appointments); the intervention was financed by the health insurer AOK Sachsen‐Anhalt in the context of research funding Standard of care control : ‐ |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, HRQoL, behaviour, self‐efficacy | |
| Study details |
Run‐in period: rehabilitation programme (unknown duration) Study terminated early: no Trial identifier: none |
|
| Publication details |
Language of publication: German Non‐commercial funding: BMBR, AOK Sachsen‐Anhalt, LVA Sachsen‐Anhalt Publication status: peer‐reviewed journal |
|
| Stated aim for study | "To study the effects of structured outpatient care on the long‐term rehab success in children and adolescents" [translation] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication (translation): " … according to randomisation criteria … allocation … according to sex and age …" Comment: no further details |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication (translation): "… external methods centre of the Martin‐Luther‐University Halls …" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: nothing stated |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: nothing stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: nothing stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: intervention was used by < 50% of participants |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: intervention was used by < 50% of participants |
| Selective reporting (reporting bias) | High risk | Comment: data only partially reported with usually no differentiation between intervention and control group |
| Other bias | Low risk | Comment: no other bias |
Vissers 2008.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: overweight volunteers in 3rd grade secondary vocational education schools who donated a blood sample. Were in 3rd grade secondary vocational education schools in the Flemish province of Antwerp, Belgium Exclusion criteria: ‐ Diagnostic criteria: no details |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Description of interventions School‐based behaviour changing intervention : intervention was based on concepts from health behaviour change models such as the Social Cognitive Theory, Theory of Planned Behavior and Transtheoretical Model and Stages of Change. A monthly counselling session with a physiotherapist to coach them to increase their daily physical activity. They also received a free subscription to a nearby fitness club and were instructed to work out at least 3 times per week. These workout sessions consisted of a mix of aerobic and strength exercises. In general, a session this would typically include: short warming up and stretching; 30 minutes of aerobic exercises such as treadmill running, cycling, rowing, stepping; strength exercises focused on large muscle groups such as pectoral, upper arm, abdominal and leg muscles; and a cooling down period. The practical implementation of these general guidelines was left to the respective fitness centres. Participants in the intervention group were offered nutritional counselling by a dietitian. Topic of these counselling sessions was healthy food choices and maintaining a proper energy balance. Every session there was a specific theme, e.g. breakfast, snacks, drinks, dairy products, fast food, portion sizes, nutritional labelling, motivation and coping strategies, based on the Flemish model of 'the active food pyramid', as proposed by the Flemish institute of Health Promotion, an inventory of specific food product and popular brands in adolescents, categorised by health effect, was developed as an attractive hang‐up model. Main goals were improving food knowledge, attitude and behaviour Total sessions: 6 nutrition sessions: individual or group (maximum of 2 group sessions) once per month. Took place in school after or between school hours. 6 counselling sessions once per month. No mention of total length of session but they included 30‐minute aerobic exercises. Training for instructors not stated Control : continued to participate in standard gym classes |
|
| Outcomes | Outcomes reported in abstract of publication: weight, BMI, waist circumference, fasting glucose | |
| Study details |
Run‐in period: none Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To study the effect of a multidisciplinary school‐based lifestyle intervention for overweight and obese students attending vocational secondary school (VSE). VSE provides practice‐oriented education in which young people learn a specific occupation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "were randomised…" Comment: no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "The researchers were not blinded for the randomization when assessing parameters such as weight and waist circumference due to limited means." Comment: outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: numbers and reasons reported, differential dropouts between study groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no clinical trial record or protocol |
| Other bias | Low risk | Comment: no other bias |
Visuthranukul 2015.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 9‐16 years; BMI > International Obesity Task Force cut‐off corresponding to BMI of 30 kg/m2 in adulthood. Exclusion criteria: behavioural and intellectual problems that might be an obstacle to follow the diet instruction, underlying diseases that might affect a weight management programme used drugs associated with weight increment or reduction attended other weight management programmes. Diagnostic criteria: as above. |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions Low‐GI diet : individual goals for weight management were set. Emphasis on low‐GI foods was provided. A dietitian emphasised the selection of low‐GI carbohydrates that fit Thai culture and their routine lives. The energy distribution was carbohydrate 50‐55%, protein 15‐20% and fat 30‐35%. Each visit consisted of a 2‐hour, small class teaching session with parental participation (4‐5 families/class). The contents varied from the 1st to 6th visit, starting from portion size and food exchange, modest energy restriction, principle of GI, sources of low‐GI diet, cooking demonstration of low‐GI dishes, guidance about food labelling and some games about GI of common food and beverages Control group : received conventional instructions at the nutrition clinic about low energy (approximately 1200‐1300 kcal/day), low‐fat (25% of total energy from fat) and high‐fibre diet by another dietitian who gave the dietary advices with special emphasis on the energy restriction such as energy count and how to avoid high‐fat Thai dishes as well as sources of high‐fibre diet. Both groups received the same instruction about physical activity, by increasing non‐weight bearing exercise 30 minutes per day at least 3 times per week, increasing physical activity in their routine lives and decreasing sedentary activity |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, fat, fat‐free mass, fasting plasma insulin, HOMA‐IR | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT02049788 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The objective of this study was to compare the effectiveness of a low‐GI diet program and a standard counselling program in the treatment of obese Thai children." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Participants were randomly allocated (by computer generated randomization blocks of 10) to receive either conventional obesity clinic advice or an intervention of a low‐GI diet." Comment: appropriate |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "The researcher who did not relate to data collection and data analysis used computer to generate the random allocation sequence. Other researchers enrolled participants and assigned them to interventions." Comment: appropriate |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: not mentioned |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not mentioned |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not reported, but low risk of bias from objective outcomes. Investigator‐assessed |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk |
Quote from publication: "8 out of 35 participants (22.9%) lost to follow‐up 8 out of 35 participants (22.9%) lost to follow‐up and 2 participants out of 35 participants (5.7%) withdrew their consents because of the travelling problems." Comment: only reported baselines and results data for participants completing the study. Reasons for dropouts not provided. States ITT but completers only are reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk |
Quote from publication: "8 out of 35 participants (22.9%) lost to follow‐up 8 out of 35 participants (22.9%) lost to follow‐up and 2 participants out of 35 participants (5.7%) withdrew their consents because of the travelling problems." Comment: only reported baselines and results data for participants completing the study. Reasons for dropouts not provided. States ITT but completers only are reported |
| Selective reporting (reporting bias) | Low risk | Comment: report all outcomes intended to measure in trial documents and main publication |
| Other bias | Low risk | Comment: no other bias |
Vos 2011.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: obesity, aged 8‐17 years, living in the Hague and in the area around the Hague and referred to a paediatrician for overweight or obesity, and increased risk of comorbidity (e.g. hypertension, family history of diabetes mellitus, hypercholesterolaemia, cardiovascular disease (or a combination of these) before the age of 55 years, Hindustani ethnicity). Exclusion criteria: knowledge of the Dutch language, intelligence or social skills insufficient to participate in the group, use of medication that might have an effect on weight loss, medical comorbidities that could affect participation, or previous enrolment in another cognitive behavioural treatment programme with the focus on reducing obesity. Diagnostic criteria: not stated. |
|
| Interventions |
Number of study centres: 1. Treatment before study: none. Description of interventions Family‐based intervention : an individual consultation with the child psychologist, group intervention with 8‐10 participants in each group. Received a treatment manual with the objectives and goals of the treatment and, per session, information about the topics discussed. The focus was on the effort to change habits more than weight reduction goals. Adolescents were made aware of their own actions and their way of living that has led to their obesity. Several cognitive behavioural techniques were learned (knowledge, skills and attitude) during the intensive phase of the treatment during 6 bi‐weekly sessions of 2.5 hours per session. Each session contained: determination of body weight, homework discussion and evaluation, education, physical activity, role playing, discussing homework for next meeting and set goals linked to educational topics of the session. The educational topics included group bond to encourage peer support, individual motivation for participating, nutritional information and the balance between energy intake and energy expenditure, healthy nutrition, self‐control techniques, coping with teasing and being teased, self‐image, cognitive strategies and relapse techniques. Current physical activity level and sedentary behaviour of the child also reviewed, energy intake versus energy expenditure was evaluated and visualised for the adolescent by a computer program. Options to change or optimise physical and sedentary activities were debated and the participants were advised how to find suitable exercise programmes. Taught how to read product labels and obtain information on misleading advertisement and discuss how to deal with meals (breakfast, lunch, diner, 2 healthy snacks), and how to make healthy choices and develop healthy eating habits (small bits, slow eating, eating at the family table, no other activities during eating). Parents had separate parallel parent group sessions (5 evenings) by a dietitian and a social worker. Included information on healthy nutrition, product information, quantities, eating moments, eating locations, how to help their children and parenting styles. Final session was a joint session to celebrate the end of the programme, where participants were encouraged to bring healthy snacks, games and activities were also planned instead of the intake of food alone. Delivered by dietitian, physiotherapist and psychologist, unclear if same for each meeting. No information on training of those delivering the programme. Wait list control : initial advice on physical activity and nutrition. During the 12‐month study period, the children were seen at start, after 3 months and at end of the period just before they start the treatment. Wait list control offered the treatment after 12 months. |
|
| Outcomes | Outcomes reported in abstract of publication: BMI‐SDS, HRQoL, waist circumference SDS, physical fitness, insulin resistance, lipid profile, high‐sensitive C‐reactive protein or for adiponectin. | |
| Study details |
Run‐in period: none. Study terminated early: no. Trial identifier: ISRCTN36146436. |
|
| Publication details |
Language of publication: English. Non‐commercial funding Publication status: peer‐reviewed journal. |
|
| Stated aim for study | Quote from publication: "the effect evaluation of a family‐based cognitive behavioral multidisciplinary lifestyle treatment. The intervention aims to establish long‐term weight reduction and stabilization, reduction of obesity related health consequences and improvement of self‐image by change of lifestyle and learning cognitive behavioral techniques." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Following informed consent of all participating children and parents the children are stratified by gender and ethnicity ('North European' and 'Other') and randomized to the intervention or control group according to coin‐tossing. In order to obtain a similar size of the intervention and control groups, blocked randomization is applied with an allocation ratio of 1:1. Randomization is carried out by a member of the team who does not take part in the treatment…" Comment: randomisation by coin tossing |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Participants are allocated randomisation codes known by the researcher and research coordinator." Comment: randomisation by coin toss |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "An experienced assistant blinded for the study design measures weight and height." Comment: appropriate |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: numbers and reasons provided, ITT analysis used |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: numbers and reasons provided, ITT analysis used |
| Selective reporting (reporting bias) | High risk | Comment: some collected data not reported e.g. free fatty acid, gut hormones (all time points) lipids and inflammatory markers (postintervention) |
| Other bias | Low risk | Comment: no other bias |
Walpole 2013.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: overweight and obese, BMI ≥ 85th percentile for age and gender (WHO 2002), 10‐18 years, attending a local paediatric outpatient clinic Exclusion criteria: current use of medication with possible adverse effects of weight gain/loss, non‐English speaking, developmental delay, pregnant or diagnosed with an active eating disorder, or both Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ Description of interventions Motivational interviewing intervention : counselling sessions were approximately 30 minutes long, 6 sessions held at the time of regularly scheduled healthy lifestyles appointments. Interventionist was a clinical psychology doctoral student trained in MI. Guided toward increasing awareness of unhealthy behaviours, consideration of whether current behaviour was consistent with personal values and envision how change may be helpful. Where ambivalence or resistance to change, agenda setting, decisional balances and scale questions with empathy and supporting autonomy were used. The interviewer was trained in MI and received professional supervision Social skills training: counselling sessions were approximately 30 minutes long, 6 sessions held at the time of regularly scheduled healthy lifestyles appointments. Offered advice rather than attempting to elicit ideas, prescribed goals to work on without specific regard for the client's readiness to change. Used a standardised treatment manual, developed and validated for children and adolescents. Sessions were based around finding appropriate ways to navigate typical social situations. Different interventionist to the MI group who was provided with feedback at monthly intervals |
|
| Outcomes | Outcomes reported in abstract of publication: self‐efficacy, BMI z scores, number of sessions attended | |
| Study details |
Run‐in period: ‐ Study terminated early: no Trial identifier: NCT01246349 |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to determine whether MI, in addition to a standard care program, would significantly increase self‐efficacy (a parameter thought to be integral in making and sustaining behavior changes) and promote BMI reduction in children aged 10‐18 years compared to a control intervention (social skills training)." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "allocation to one of the four strata was made subsequent to computer‐generated block permutations, which determined the intervention assignment." Comment: block randomisation to ensure balanced groups |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "study interventionists were blind to the randomization process, which was carried out by a research coordinator…" Comment: adequate |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote from publication: "participants were unaware of the specific intervention group to which they were assigned." Comment: investigator‐assessed. Unlikely participants could be blinded to allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "measurements were assessed at the clinic site by staff who were blinded to the intervention assignment…" Comment: investigator‐assessed |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: missing data reported and reasons explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: HRQoL and self‐esteem not yet published |
| Other bias | Low risk | Comment: no other bias |
Wengle 2011.
| Methods | Parallel RCT, randomisation ratio: 1:1, superiority design | |
| Participants |
Inclusion criteria: aged 12‐16 years, BMI > 85th percentile for age and gender, free of morbidities that might have impaired or contraindicated their safe participation in the study Exclusion criteria: physical/intellectual limitations (e.g. developmental delay, psychiatric illness, significant non‐obesity‐related medical conditions, orthopaedic problems, recent surgery performed or planned), treatment with medications that might interfere with weight or growth control (e.g. corticosteroids or thyroid hormone), and psychological illness (e.g. major depression or an eating disorder) Diagnostic criteria: as above |
|
| Interventions |
Number of study centres: ‐ Treatment before study: none Description of interventions Mentoredbehaviour changingintervention : adolescents and family members attended a 1‐day group educational workshop at the beginning of the study, given specific instructions and written materials regarding how to self‐assess behaviour and environment, and successively implement small changes by setting goals toward measurable and attainable outcomes. Learned about the study recommendations for physical activity and nutrition. Visits at baseline, 1, 2, 3 and 6 months included nutrition and activity counselling. Met with a mentor in person for 1‐2 hours once per week to achieve activity goals, participate in physical activity, and discuss and set nutritional goals. Additionally, they agreed to communicate (either through telephone or e‐mail) twice per week for support. Mentoring was provided by mentor volunteers were recruited from the University of Toronto (Ontario). Unclear who delivered the remaining behaviour changing interventions. The mentor had regular contact with study personnel, and monthly group meetings and were trained to standardise the intervention Control : as above without the additional mentor support |
|
| Outcomes | Outcomes reported in abstract of publication: BMI z score, waist circumference, HDL, LDL:HDL ratio, consumption high‐calorie foods and snacks, fast food restaurant visits, screen time, feasibility of intervention | |
| Study details |
Run‐in period: all attended 1‐day workshop prerandomisation Study terminated early: no Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To conduct a pilot study designed to measure the impact of a healthy lifestyle intervention with or without individualized mentorship on adiposity, metabolic profile, nutrition and physical activity in overweight teens." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "blocked randomization with random number generator, 1:1 treatment allocation, mentor‐mentee pair matched according to sex." Comment: appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: unclear if blinding was carried out |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: unclear if blinding was carried out |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: unclear if blinding was carried out |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: unclear if blinding was carried out but low risk of bias from objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: 6 adolescents withdrew shortly after the behaviour changing intervention started (reasons given) but the study did not provide any baseline measures for them |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: 6 adolescents withdrew shortly after the behaviour changing intervention started (reasons given) but the study did not provide any baseline measures for them |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes reported as stated but no protocol to confirm |
| Other bias | Low risk | Comment: no other bias |
Wong 2015.
| Methods | Parallel RCT, randomisation ratio: ‐, superiority design | |
| Participants |
Inclusion criteria: ‐ Exclusion criteria: ‐ Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: ‐ Treatment before study: ‐ Description of interventions Standard weight loss + advice + behavioural support : standard weight loss diet + advice + behavioural support (counselling, cookbook of recipes and health guides) to increase habitual water intake to 8 cups/day Control : standard weight loss diet + advice and behavioural support (counselling, cookbook of recipes and health guides) |
|
| Outcomes | Outcomes reported in abstract of publication: water intake, urine specific gravity, BMI z score, cardiometabolic risk factors | |
| Study details |
Run‐in period: ‐ Study terminated before regular end (for benefit/because of adverse events): ‐ Trial identifier: ‐ |
|
| Publication details |
Language of publication: English Non‐commercial funding Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "to conduct a pilot study comparing two standard weight loss diets, either with (Experimental) or without (Control) additional advice and behavioral support to increase habitual water intake to 8 cups per day." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not specified; however, low risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Other bias | Unclear risk | No details |
ARQoL: asthma‐related quality of life; BMI: body mass index; CBT: cognitive behavioural therapy; CDC: Centers for Disease Control and Prevention; CST: coping skills training; CT: computer tomography; DSM‐IV‐Text Revised: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition ‐ Text Revised; GI: glycaemic index; GL: glycaemic load; HDL: high‐density lipoprotein; HELP: Healthy Eating and Lifestyle Programme; HOMA‐IR: homeostatic model assessment, insulin resistance; HRQoL: health‐related quality of life; ITT: intention to treat; LDL: low‐density lipoprotein; MI: motivational interviewing; mph: miles per hour; n: number of participants; PACE+: Patient‐Centered Assessment and Counseling for Exercise plus Nutrition; PedsQL: Pediatric Quality of Life; RCT: randomised controlled trial; SDS: standard deviation score; TEENS: Teaching Encouragement Exercise Nutrition Support
‐ denotes not reported
Note: where the judgement is 'Unclear' and the description is blank, the trial did not report that particular outcome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balagopal 2005 | Study < 6 months. |
| Belenchia 2013 | Primary and secondary outcomes not reported (measures glucose control). Did not meet the aim of the review. |
| Braet 2004 | Intervention study, not an RCT. |
| Carrel 2007 | Secondary analysis, not an RCT. |
| Crandall 2006 | Drug intervention. |
| Daly 2013 | Study < 6 months. |
| Dancy 2006 | Intervention study, not an RCT. |
| De Jesus 2013 | Study < 6 months. |
| DeVore 2013 | Prospective follow‐up, not an RCT. |
| Doyle 2008 | Study < 6 months. |
| Dreyer‐Gillette 2014 | Compared 2 different interventions. |
| Faulkner 2013 | Intervention study, not an RCT. |
| Fonseca 2016 | Study < 6 months. |
| Freire 2013 | Intervention study, not an RCT. |
| Garnett 2014 | Compared 2 different interventions. |
| Giel 2013 | Not an RCT, compared to an aged‐matched control. |
| Hay 2016 | Comparing 2 different interventions. |
| Jelalian 1999 | Systematic review. |
| Jelalian 2010 | Non‐relevant comparator (comparing 2 different interventions). |
| Jones 2009 | Intervention not aiming to treat overweight or obesity. |
| Jones 2008 | Non‐relevant intervention (intervention not aiming to treat obesity). |
| Lee 2013a | Study < 6 months. |
| Lee 2013b | Study < 6 months. |
| Lee 2013c | Study < 6 months. |
| Lovely 2013 | Study < 6 months. |
| Lubans 2008 | Quasi‐experimental design. |
| NCT00863083 | Study terminated due to poor enrolment. |
| NCT01044134 | Study terminated (achieved n = 38, due to slow recruitment, which was 2/3 of target. |
| NCT02011646 | Study terminated (the principal investigator changed institutions). |
| NCT02295761 | Study < 6 months. |
| Nunes 2016 | Not relevant intervention (the effect of interdisciplinary therapy in the parameters of the oxidative stress and the anti‐inflammatory responses). |
| Parks 2014 | Drug intervention. |
| Racil 2013 | Study < 6 months. |
| Sabzghabaee 2013 | Study < 6 months. |
| Sarvestani 2009 | Experimental quasi design. |
| Shrewsbury 2011 | Study < 6 months. |
| Sussman 2013 | Qualitative study. |
| Van 2013 | Not relevant age (< 12 years). |
| Ventura 2009 | Study < 6 months. |
| Wadden 1990 | Study < 6 months. |
| Wiegand 2014 | Participants not overweight or obese. |
| Wilson 2012 | 2 different interventions. |
| Xanthopoulos 2013 | Only % weight change in association with carers' weight loss reported. |
n: number of participants; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Angerer 2015.
| Methods |
Type of study: controlled clinical trial Allocation: ‐ Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: Enrollment: Inclusion criteria: Exclusion criteria: |
| Interventions |
Interventions: nutrition counselling, sport and life‐skill training Comparator: ‐ |
| Outcomes |
Primary outcome: ‐ Secondary outcome: ‐ Other outcome: medical and psychological outcomes |
| Study identifier | ‐ |
| Official title | Fit4You ‐ a programme for prevention and reduction of overweight in apprentices in the workplace setting |
| Stated purpose of study | "Examined the effect of a multimodal programme including nutrition counselling, sport, and life‐skill training on medical and psychological outcomes." |
| Notes | Unclear if it is an RCT |
Barbeau 2003.
| Methods |
Type of study: ‐ Allocation: randomised Intervention model: Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: obesity Enrollment: 55 Inclusion criteria: triceps skinfold > 85th percentile for gender, race and age; not involved in any other weight control or exercise programme; could not have limitations to physical activity Exclusion criteria: ‐ |
| Interventions |
Intervention: high‐intensity physical training and lifestyle education Interventions: moderate‐intensity physical training and lifestyle education Comparators: lifestyle education |
| Outcomes |
Primary outcome: fasting leptin Secondary outcome: Other outcomes: body composition, visceral adipose tissue, subcutaneous abdominal adipose tissue, cardiovascular fitness, heart rate, daily physical activity, time spent sleeping, dietary intake |
| Study identifier | ‐ |
| Official title | Influence of physical training on plasma leptin in obese youths |
| Stated purpose of study | Effect of 2 intensities of physical training on leptin in obese teenagers |
| Notes | Anthropometric outcomes not reported |
Bohlin 2012.
| Methods |
Type of study: non‐inferiority study Allocation: randomised Intervention model: ‐ Masking: open Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 35 Inclusion criteria: children aged 5‐16 years Exclusion criteria: ‐ |
| Interventions |
Intervention: multidisciplinary treatment provided by the obesity treatment clinic followed by frequent telephone consultations with parents Comparator: the usual multidisciplinary treatment visits delivered provided by the obesity treatment clinic |
| Outcomes |
Primary outcome: BMI‐SDS Secondary outcome: ‐ Other outcome: ‐ |
| Study identifier | ‐ |
| Official title | Can telephone consultations substitute visits in treatment of childhood obesity? Results from a randomized trial |
| Stated purpose of study | "To explore the possibility to substitute nurse‐visits at the clinic with more frequent telephone‐consultations without degrading treatment outcome measured in BMI‐SDS." |
| Notes | Unclear if the mean age of participants was 12‐16 years old. Contacted author (19 October 2015) requesting mean age and risk of bias |
Campos 2017.
| Methods |
Type of study: ‐ Allocation: ‐ Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: ‐ Enrollment: 148 Inclusion criteria: ‐ Exclusion criteria: ‐ |
| Interventions |
Interventions: aerobic training + resistance training Comparator: aerobic training |
| Outcomes |
Primary outcome: Secondary outcome: Other outcomes: body mass, BMI, fat mass, visceral and subcutaneous fat, insulin resistance |
| Study identifier | ‐ |
| Official title | HOMA‐AD: the role of different types of physical exercise in obese adolescents |
| Stated purpose of study | "The purpose of this study was to investigate the effects of different kinds of exercise in the sensitive index predictor of insulin resistance." |
| Notes | Library cannot provide reference |
Cocca 2016.
| Methods |
Type of study: ‐ Allocation: randomised. Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: ‐ Enrollment: 41. Inclusion criteria: ‐ Exclusion criteria: ‐ |
| Interventions |
Intervention: school physical activity based on tactical ball games (4 × 60‐minute sessions/week), and attended meetings of nutritional counselling with their families (1 hour/week) Comparator: regular school physical education |
| Outcomes |
Outcomes: cholesterol, HDL cholesterol, triglycerides, waist circumference, blood pressure and glycaemia values Primary outcome: Secondary outcome: Other outcome: |
| Study identifier | ‐ |
| Official title | Effects of a school‐based intervention program on metabolic syndrome parameters in school‐aged youth |
| Stated purpose of study | "The aim of this study was to assess the impact of a school intervention program on the parameters of MetS [metabolic syndrome] in Mexican secondary school students." |
| Notes | Unclear whether all participants were overweight or obese at baseline and focus of study is on metabolic syndrome rather than weight loss. No data, only P values and most of the outcomes are cardiovascular disease risk factors rather than weight. Contacted author (12 January 2017) to clarify inclusion criteria of participants, aim of the study and weight outcomes |
Garrett 1979.
| Methods |
Type of study: ‐ Allocation: ‐ Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: obesity Enrollment: ‐ Inclusion criteria: aged 11‐16 years, females. Exclusion criteria: ‐ |
| Interventions |
Intervention: ‐ Comparator: ‐ |
| Outcomes |
Primary outcome: ‐ Secondary outcome: ‐ Other outcome: ‐ |
| Study identifier | ‐ |
| Official title | Group behavioural therapy versus family behavioural therapy in treatment of obese adolescent females |
| Stated purpose of study | ‐ |
| Notes | Library cannot locate full‐text |
Gracianette 1982.
| Methods |
Type of study: ‐ Allocation: ‐ Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: obesity Enrollment: ‐ Inclusion criteria: obese adolescents Exclusion criteria: ‐ |
| Interventions |
Intervention: ‐ Comparator: ‐ |
| Outcomes |
Primary outcome: ‐ Secondary outcome: ‐ Other outcome: ‐ |
| Study identifier | ‐ |
| Official title | The application of behavioural techniques to change eating habits and/or exercise habits to effect weight reduction and maintenance in adolescents: a year‐long study |
| Stated purpose of study | |
| Notes | Library cannot locate full‐text |
Li 2006.
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: overweight or obesity Enrollment: 120 Inclusion criteria: girl students with simple obesity and overweight aged (mean ± SD) 15.5 ± 3.7 years Exclusion criteria: |
| Interventions |
Interventions: reducing weight therapy composed of aerobic exercise (body exercise, power, jump and flexible sports, body and mental health sports, relax sports), reasonable diet (protein:carbohydrate:fat = 5:4:1) and mental modification Comparator: ‐ |
| Outcomes |
Primary outcome: Secondary outcome: Other outcomes: BMI, body mass, chest circumference, waistline, hip circumference, power of gripping, 800 m running, standing long jump, sit‐up, quiet heart rate, blood glucose, blood fat, cholesterol, insulin and leptin |
| Study identifier | ‐ |
| Official title | Anti‐obesity effect of comprehensive diet and sports in girl students with simple obesity or overweight |
| Stated purpose of study | To investigate the effective antiobesity therapy with comprehensive diet prescription and sports for girl students with simple obesity and overweight |
| Notes | Article in Chinese, lack of resources to translate. Contacted author (11 August 2015) for an English version |
Makkes 2015.
| Methods |
HELIOS study Type of study: efficacy Allocation: randomised Intervention model: parallel assignment Masking: none Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 80 Inclusion criteria: children and adolescents aged 8‐18 years referred to Heideheuvel by their own paediatrician. They must have BMI‐SDS ≥ 2.3 according to the growth curves based on the 4th Dutch National Growth Study of 1997 (this corresponds to the 99th percentile) and comorbidity related to obesity (e.g. obstructive sleep apnoea syndrome, raised insulin, diabetes type 2, liver function disorders, dyslipidaemia, worn out joints) or a BMI‐SDS ≥ 3.0 (this corresponds to the 99.9th percentile) Exclusion criteria: having syndromal or chromosomal determined obesity, obesity caused by endocrine disorders (hypothyroidism, Cushing's syndrome, primary hyperinsulinaemia, pseudohypoparathyroidism, acquired (structural) hypothalamic damage) or medicine use (e.g. oral steroids, antiepileptic drugs, antidepressants), severe psychiatric problems, an IQ < 75 or similar school level or if their parents are not willing to participate in the treatment |
| Interventions |
Intervention A: intensive combined behaviour changing inpatient treatment for 6 months during weekdays, followed by biweekly hospital admissions of 2 days for 4 months Intervention B: intensive combined behaviour changing inpatient treatment for 2 months during weekdays, followed by biweekly return visits of 2 days during the next 4 months, then followed by 6 monthly return visits of 2 days Comparator: usual care: will receive usual care for 1 year, after which the participants will be randomly allocated to the groups A and B |
| Outcomes |
Primary outcome: BMI‐SDS (costs of treatment per change in relative BMI) Secondary outcomes: psychological and psychosocial data on issues such as motivation, competence, self‐esteem, anxiety and mental stress; cardiovascular risk factors (blood pressure, serum lipids, liver function tests, glucose and insulin); waist circumference; dietary behaviour; eating behaviour, physical activity (sedentary behaviour); quality of life (generic, weigh‐specific and health‐related quality of life) Other outcome: ‐ |
| Study identifier | NTR1678. |
| Official title | Health effects of behaviour changing interventions in obese children and adolescents study (Helios) ‐ a randomised controlled trial |
| Stated purpose of study | Quote: "to compare the cost‐effectiveness of these two intensive one‐year inpatient treatments to each other and to usual care for severely obese children and adolescents." |
| Notes | Study only intended to compare the 2 active interventions. The thesis states that for 'logistical reasons' they randomised to a wait list control group, review authors assumed as they were only able to take a small number of participants through the interventions at any 1 time. They also randomise to the groups in 2 batches so that every 6 months for 2 years they start a new intervention 1 and 2 with 10 participants each. The flow chart and number of participants do not show the wait list control group but include them when they are included in intervention A and B. There is 1 analysis for the 3 groups (6 and 12 months only as appropriate) which was described as an "additional analysis." For the purpose of the review, the comparison with the wait list control would be relevant; however, there are no numbers included in the analysis, no baseline characteristics and the only data for BMI‐SDS is from a figure. Awaiting further clarification from author or publication of the data to know if this group will be analysable |
NCT00462267.
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: obesity/overweight Enrollment: 215 Inclusion criteria: aged 13‐15 years, BMI ≥ 90%, 1 or both parent(s) willing to participate in study assessments and parent sessions Exclusion criteria: BMI ≥ 99%, significant cognitive impairment, pregnant, congenital heart disease that limits activity, serious asthma requiring oral prednisone, taking medications that increase appetite |
| Interventions |
Intervention: enriched behaviour changing intervention, multicomponent group teen and parent sessions, individual telephone‐based coaching contact and a distinct collaborative care component with follow‐up visits to the youth's primary care provider Comparator: usual care |
| Outcomes |
Primary outcome: BMI z score Secondary outcomes: quality of life, self‐esteem, depression, unhealthy eating practices, weight and shape concerns, sociocultural attitudes toward appearance, satisfaction, dietary intake, personal and family eating patterns, physical activity, sedentary behaviours, personal and family physical activity patterns Other outcome: ‐ |
| Study identifier | NCT00462267 |
| Official title | Examining the feasibility of collaborative care treatment for overweight adolescents |
| Stated purpose of study | "Examine the effectiveness of a primary care based intervention to help overweight teen girls adopt healthy lifestyle practices." |
| Notes | Contacted author (6 August 2015), author reply: "The study you identified was a small pilot that supported the larger trial published in Pediatrics (included in this review). Unfortunately, we don't have analytic resources to provide more detail than what is available in the paper itself." |
NCT00475163.
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: prevention |
| Participants |
Condition: obesity Enrollment: 34 Inclusion criteria: aged 13‐17 years, able to participate in physical activity Exclusion criteria: on medications that could affect weight |
| Interventions |
Intervention: weekly contacts with mentors to improve physical activity pattern, self‐esteem and weight loss in obese teenagers over a 6‐month period Comparator: ‐ |
| Outcomes |
Primary outcomes: weight gain, fitness, self‐esteem Secondary outcome: Other outcome: |
| Study identifier | NCT00475163. |
| Official title | Mentors in motion: a physical activity intervention for obese adolescents |
| Stated purpose of study | Assessing the impact of mentoring on the behaviour changing choices of adolescents who are overweight |
| Notes | Study listed as completed on ClinicalTrials.gov, but no study results available (last checked 16 August 2016). Contacted authors (7 August 2015) for published/unpublished data, author response (15 September 2015): data had not been published and the work is still pending (no results available, checked 21 November 2016) |
Shapiro 1976.
| Methods |
Type of study: ‐ Allocation: ‐ Intervention model: ‐ Masking: ‐ Primary purpose: ‐ |
| Participants |
Condition: ‐ Enrollment: ‐ Inclusion criteria: ‐ Exclusion criteria: ‐ |
| Interventions |
Intervention: ‐ Comparator: ‐ |
| Outcomes |
Primary outcome: Secondary outcome: Other outcome: |
| Study identifier | ‐ |
| Official title | A comparison of various reward and monitoring procedures in the behavioral treatment of overweight children |
| Stated purpose of study | ‐ |
| Notes | Library cannot locate full‐text |
BMI: body mass index; HDL: high‐density lipoprotein; HOMA‐AD: homeostatic model assessment ‐ adiponectin; IQ: intelligence quotient; RCT: randomised controlled trial; SD: standard deviation; SDS: standard deviation score.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12607000632493.
| Trial name or title | Acronym: eGAME (Electronic Games to Aid Motivation to Exercise) |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight Enrollment: estimated 330 Inclusion criteria: aged 10‐14 years, living in the greater metropolitan Auckland area, overweight (according to the Cole international cut‐offs for child obesity); play ≥ 2 hours of video games per week; have no contraindications to perform physical activity; required to own a PlayStation® 2 or 3 gaming console but not the EyeToy™ or Dance Mat technology Exclusion criteria: if they already own active video games or have a medical condition that would prohibit exercise |
| Interventions |
Intervention: eGAME: a USB motion‐capture camera to place a picture of the gamer onscreen. The gamer then interacts with the images on screen. The upgrade consisted of an EyeToy™ camera, dance mat and a selection of active video games. Children encouraged to meet physical activity recommendations to perform 60 minutes of moderate‐to‐vigorous physical activity on most days of the week by supplementing periods of inactivity with active video game play and substituting periods of inactive video game play with the active version Comparator: control group: continue normal video game playing and physical activity behaviour. At the end of the study, received the active video games upgrade package |
| Outcomes |
Primary outcomes: change in BMI z score and centile Secondary outcomes: % body fat, waist circumference, physical fitness, PAQ‐C score, mean daily time spent in light‐to‐vigorous activities (minutes), mean daily time spent in active video games (minutes) and mean daily time spent in non‐active video games (minutes) Other outcomes: mean daily time spent in light activities (minutes), moderate activities (minutes) and vigorous activities (minutes); psychological variables intention, self‐efficacy, barrier efficacy, perceived competence, perceived enjoyment; and mean daily total energy consumed from snacks (kJ) |
| Starting date |
Study start date: 2008 Study completion date: unclear |
| Contact information | Responsible party/principal investigator: University of Auckland/Ralph Maddison |
| Study identifier | ACTRN12607000632493 |
| Official title | Active video games to improve body composition and physical activity in children |
| Stated purpose of study | Quote: "to determine the effects of an active video game intervention over 6 months on: body mass index (BMI), percent body fat, waist circumference, cardio‐respiratory fitness, and physical activity levels in overweight children." |
| Notes | Contacted author (22 October 2015) for anticipated study completion data |
ACTRN12611000139976.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: blinded, masking used. |
| Participants |
Condition: overweight and obesity (≥ 85th centile) Enrollment: 570 Inclusion criteria: aged 12‐17 years; overweight or obese (≥ 85th centile) according to the age‐ and gender‐specific BMI data from the International Obesity Task Force; Internet access at home or at a location where regular use is possible (i.e. school, library). Recruitment will be restricted to those living in the Melbourne metropolitan area (to facilitate complete follow‐up assessments by maximising opportunities to collect height and weight data, and to contain study costs) Exclusion criteria: young people with a known endocrine or chromosomal cause for their obesity; taking prescription medication resulting in weight changes (e.g. prednisolone); complications of overweight that contraindicate moderate physical activity (e.g. orthopaedic disorders); past or current medical diagnosis of a clinical eating disorder (i.e. anorexia or bulimia nervosa, binge eating disorder) or significant health disability or condition which prevents participation |
| Interventions |
Intervention: Internet‐based programme for 12 weeks, 1 session per week for self‐directed 30‐60 minutes. Goals will be set by the participant from a framework recommended within the programme (e.g. to increase moderate activity from 0 to 30 minutes on most days) and reviewed every week, activities will be specific to intervention group but not to each individual. Participants will receive a pedometer and a walking programme to help achieve goal of 60 minutes of moderate‐to‐vigorous activity per day. Cognitive behaviour aspect of the programme will address self‐monitoring, goal setting, stimulus control, social eating, etc. Participants will receive feedback at least weekly. Parents of participants will be sent monthly newsletters for the 3 months of the intervention programme with tips on creating a supportive environment. After the 12‐week programme is finished, participants will receive monthly follow‐up via text or e‐mail asking them to review their eating and physical activity habits, and provide feedback. They can also review the materials from the programme Comparator: publicly available generic health information about healthy eating and physical activity at start of 12 weeks treatment period. Information will be presented in both paper and online format (e.g. participant will receive a pack with flyers about the physical activity requirements, healthy eating and planning healthy meals and a sheet with links to websites about the age appropriate healthy eating, physical activity and mental health). No cognitive behaviour element |
| Outcomes |
Primary outcome: reduction in BMI z score Secondary outcomes: waist circumference, % body fat, blood pressure, psychological distress, quality of life, eating disorder, programme content satisfaction questionnaire Other outcome: adherence |
| Starting date |
Study start date: July 2011 Study completion date: actual date last participant enrolled: 1 October 2013 |
| Contact information | Responsible party/principal investigator: Joanne Williams, The Royal Children's Hospital, Victoria, Australia |
| Study identifier | ACTRN12611000139976 |
| Official title | Staying fit adolescent weight management study |
| Stated purpose of study | Quote: "Staying Fit has been designed as an Internet‐based adolescent weight management program for overweight or mildly obese Australian young people. Goals of the program include: targeting weight loss, promoting weight maintenance, decreasing weight and shape concerns, healthy eating and increasing physical activity." |
| Notes | Trial website (www.rch.org.au/cah/research.cfm?doc_id=14887) states that they are currently preparing publications (accessed 16 August 2016) |
ACTRN12611000862943.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open (masking not used) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 107 Inclusion criteria: males and females aged 5‐16 years; BMI ≥ 98th centile, or > 91st centile with weight‐related comorbidities. Ready for change as assessed by questionnaire and overall assessment of level of motivation by Healthy Lifestyles Co‐ordinator on interview. Committed family member Exclusion criteria: significant comorbidities (i.e. any medical condition serious enough to make it impossible for a child/adolescent to embark on a programme of increasing physical activity) |
| Interventions |
Intervention: physical activity ‐ fitness assessments and knowledge of importance of physical fitness at 0, 6, 12, 18 and 24 months. Initial home visits by Active Families co‐ordinator (1 hour), then weekly activity sessions for 40 weeks during the year (1.5 hours per session). Dietary education ‐ dietitian input and initial home visits (1 hour). Psychology input ‐ input as group at commencement of intervention (2 × 1‐hour sessions), and then at family/individual level as indicated Comparator: brief dietary education by means of pamphlet |
| Outcomes |
Primary outcomes: BMI‐SDS; quality of life; physical activity Secondary outcomes: dietary behaviour; sedentary activity; glycaemic control Other outcome: ‐ |
| Starting date |
Study start date: 9 January 2012 Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: Yvonne Anderson; Child and Adolescent Centre, Taranaki Base Hospital |
| Study identifier | ACTRN12611000862943. |
| Official title | The effect of a multi‐disciplinary obesity intervention compared to usual practice in those ready to make lifestyle changes: design and rationale of Whanau Pakari |
| Stated purpose of study | Quote: "to improve local obesity services for 5‐16 year olds in the Taranaki region, and assess whether the devised intervention programme shows benefits in those participants that are assessed as ready to make healthy lifestyle changes." |
| Notes | Recruitment status: active, not recruiting. Contacted author (17 January 2017) for trial status |
ACTRN12613001037796.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy study Allocation: randomised block design will be used. Obese participants will be randomised to 1 of 3 groups and will be stratified according to age and gender Intervention model: single blind (outcome assessors) Primary purpose: effect on myocardial function |
| Participants |
Condition: obesity Enrollment: 100 Inclusion criteria: BMI > 95th percentile (age and gender specific), children and adolescents aged 7‐16 years Exclusion criteria: hypertension (defined as blood pressure > 95th centile for systolic or diastolic values), any history or evidence of heart disease or an abnormal resting or stress echocardiography which indicates it would be unsafe to participate (or both), any chronic disease (e.g. chronic asthma, kidney disease, diabetes, current smoking habits or an orthopaedic/neurological disorder that may limit ability to exercise), diagnosed attention deficit hypersensitivity disorder and use of steroid medications |
| Interventions |
Intervention 1: high‐intensity interval training. Walk, run or cycle at 85‐95% of their maximal heart rate at intervals of 4 × 4 minutes, with 3‐minute active breaks (˜ 60% of maximal heart rate) between intervals. Total exercise time 40 minutes Intervention 2: moderate‐intensity exercise. Walk, run or cycle continuously at 70% maximal heart rate for approximately 44 minutes to equalise the energy expenditure performed by high‐intensity interval training group. Total exercise time 50 minutes Common across interventions: dietary education sessions that will parallel sessions provided to those assigned to the control group. 30‐minute session every second week for 3 months, followed by 1 session every 2 months for the next 9 months. Exercise training 3 times per week for 12 months. Months 1‐3, 2 supervised sessions in the gym and 1 session at home. Months 4‐12, all training at home. Consists of walking or running on a treadmill, or on a bike for the older children Comparator: control: dietary education sessions, 8‐10 × 20‐minute individual diet intervention sessions over 12 months. A session every second week for 3 months, followed by 1 session every 2 months for the next 9 months |
| Outcomes |
Primary outcome: myocardial function (peak systolic tissue velocity) Secondary outcomes: vascular function (flow‐mediated dilation assessment), quantity of visceral and subcutaneous adipose tissue, myocardial structure and function, body composition, cardiorespiratory fitness, autonomic function, blood biochemistry, physical activity and nutrition Other outcome: ‐ |
| Starting date |
Study start date: October 2013 Study completion date: January 2015 (last participant enrolled) |
| Contact information | Responsible party/principal investigator: The University of Queensland/Norwegian University of Science and Technology |
| Study identifier | ACTRN12613001037796 and NCT01991106 |
| Official title | Effects of exercise intensity and nutrition advice on myocardial function in obese children and adolescents: a multicentre randomised controlled trial study protocol. |
| Stated purpose of study | Quote: "The primary aim of this randomised controlled trial is to compare the effectiveness of three 12‐month interventions: HIIT [high‐intensity interval training] and nutrition advice, MICT [moderate‐intensity exercise] and nutrition advice or nutrition advice alone on myocardial function in obese children and adolescents." |
| Notes | Study is ongoing, but not recruiting participants (last updated: 4 January 2017) |
ACTRN12615000558527.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: cluster randomised controlled trial Masking: blinded (masking used) outcome assessors Primary purpose: prevention |
| Participants |
Condition: overweight, obese Enrollment: 570 Inclusion criteria: sites: participating in standard Go4Fun programme in 2015; 2014 attendance mean of at least 20 children per programme per term. Participants: males and females aged 7‐13 years with no comorbidities; BMI > 85th percentile for their age and gender (according to Australian Institute of Health and Welfare classification of overweight/obesity in children); be enrolled in and meet the general criteria to participate in the Go4Fun programme at 1 of the sites participating in this study which includes having a parent or adult carer able to accompany them to each session Exclusion criteria: sites: not willing to participate in a trial and adhere to standardised procedures for duration of the trial. Participants: parent/guardian not willing to provide written and informed consent |
| Interventions |
Intervention: Go4Fun programme is a community‐based, multidisciplinary family‐focused programme and will also be eligible to receive incentives for reaching certain levels of attendance and for goal attainment. Children will receive a skipping rope for attending 80% of programme sessions (8 sessions) and a frisbee for 100% attendance (10 sessions). Once the goals are achieved children are eligible to receive agreed incentives as follows; a vegetable slicer once 2 goals are achieved, a USD10 Rebel voucher once 4 goals are achieved and a height adjustable tennis set (Totem Tennis) once 6 goals are achieved Comparator: standard community weight management programme (namely Go4Fun) without the structured behaviour incentives |
| Outcomes |
Primary outcome: BMI z score Secondary outcomes: waist circumference z score; BMI; difference in rate of attendance at programme sessions between intervention and control sites; difference in mean rate of nutrition goal attainment between intervention and control groups; difference in mean rate of physical activity goal attainment between intervention and control groups Other outcome: ‐ |
| Starting date |
Study start date: 2 February 2015 Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: Julie Redfern; Cardiovascular Division, The George Institute for Global Health, Level 10, King George V Building, Missenden Road, Camperdown NSW 2050 |
| Study identifier | ACTRN12615000558527 |
| Official title | The effectiveness of a community weight management program with a behavioural incentive scheme compared to participation in a standard community weight management program in overweight children on relative weight loss and behaviour change |
| Stated purpose of study | Quote: "to determine the effectiveness of enhanced goal setting linked to a structured incentive scheme designed to improve the sustained health and wellbeing of overweight/obese children within the context of an existing community‐based program." |
| Notes | As of 1 July 2015, recruitment has commenced and follow‐up assessments are ongoing. No data cleaning or analysis of results had begun at the time of this submission (protocol publication 2016) |
Chew 2016.
| Trial name or title | Acronym: LITE |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: ‐ Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 60 Inclusion criteria: adolescents 10‐16 years referred to the Weight Management Clinic Exclusion criteria: ‐ |
| Interventions |
Intervention: the LITE (Lifestyle Intervention for obese teenagers) group programme is a 6‐month, family‐based behavioural lifestyle intervention, specifically designed to treat obesity in adolescents 10‐16 years Comparator: usual care |
| Outcomes |
Outcomes: outcome measurement are assessed at 3 and 6 months postbaseline and include anthropometric measurements, physical activity, dietary intake, metabolic profile, improvement in positive parenting behaviour and measurement of family support Primary outcome: not clearly stated Secondary outcome: not clearly stated Other outcome: ‐ |
| Starting date |
Study start date: ‐ Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: ‐ |
| Study identifier | ‐ |
| Official title | The LITE randomised controlled trial |
| Stated purpose of study | Quote: "This study was designed to address the gap in service provision of a family based weight management program for overweight and obese adolescents. The LITE (Lifestyle Intervention for obese teenagers) group program is a 6‐month, family‐based behavioural lifestyle intervention, specifically designed to treat obesity in adolescents 10‐16 years referred to the Weight Management Clinic." |
| Notes | Journal abstract. Data presented for BMI z score at 3 months but not 6 months. High attrition from both groups. Contacted author (12 January 2016) for 6 months' data |
ChiCTR‐TRC‐13003501.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel Masking: ‐ Primary purpose: treatment |
| Participants |
Condition: obesity with non‐alcoholic fatty liver disease Enrollment: 56 Inclusion criteria: postpubertal Chinese adolescents aged 14‐18 years with primary obesity attending the Obesity and Lipid Disorder Clinic in Prince of Wales Hospital Exclusion criteria: hepatic viral infection, daily usage of alcohol, from parental or self‐report BMI < 95th centile of local reference, using steatogenic or anti‐diabetic drugs (or both), concurrent participation in any clinical trial, dietary intervention or weight loss programme, concomitant intake of a weight‐reducing agent, any chronic medical illness, unwillingness to attend regular follow‐up appointments |
| Interventions |
Intervention: behaviour changing intervention with modification of diet, physical activity and behaviour patterns, consisting of counselling sessions weekly and then bi‐monthly for 1 year. Sessions 15‐20 minutes long, taken by dietitians/nutritionists. Also motivational interviewing, such as discussing barriers to lifestyle change and child's and parents' feelings about the progress. Provided with a booklet for portion size exchange and tips for eating out Comparator: conventional care with follow‐up in the Obesity and Lipid Disorder Clinic every 16 weeks |
| Outcomes |
Primary outcomes: not stated as primary/secondary: the degree of change of intrahepatic triglyceride content of non‐alcoholic fatty liver disease after intervention; anthropometric measurements: height, weight, body fat and waist circumference; blood tests: plasma fasting glucose, lipid profile, plasma alanine aminotransferase, aspirate aminotransferase and serum insulin Secondary outcome: ‐ Other outcome: ‐ |
| Starting date |
Study start date: August 2013 Study completion date: unclear |
| Contact information | Responsible party/principal investigator: Dorothy Chan, The Chinese University of Hong Kong |
| Study identifier | ChiCTR‐TRC‐13003501 |
| Official title | Behaviour changing intervention in obese Chinese adolescents with nonalcoholic fatty liver disease: a randomized controlled study |
| Stated purpose of study | Quote: "A lifestyle modification programme (LMP) developed by the Centre for Nutritional Studies of The Chinese University of Hong Kong for the treatment of obesity and obesity‐related diseases. It is clinically proven and developed based on motivational interviewing and behavioural modification, to accompany improving knowledge regarding diet and exercise. Lifestyle intervention with modification of diet, physical activity and behaviour patterns that have been shown to be effective among adults could reduce the severity and prevalence of nonalcoholic fatty liver disease (NAFLD) in obese children and adolescents." |
| Notes |
DRKS00004583.
| Trial name or title | Acronym: TeAM (Telephone counselling as Adiposity Management) |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel assignment Masking: ‐ Primary purpose: treatment (maintenance) |
| Participants |
Condition: obesity Enrollment: 150 Inclusion criteria: aged 14‐18 years, completion of a structured inpatient obesity therapy programme (4‐6 weeks of inpatient treatment) Exclusion criteria: current involvement in weight loss treatment, psychiatric conditions interfering with participation (e.g. eating disorder, psychosis), medication interfering with participation or weight maintenance, underlying chronic disease interfering with weight maintenance |
| Interventions |
Intervention 1: telephone counselling and tailored SMS messages Intervention 2: telephone counselling, tailored SMS messages and in addition access to a password‐protected web‐forum for interaction with other participants Comparator: no intervention, medical care as usual |
| Outcomes |
Primary outcome: BMI‐SDS Secondary outcomes: sociodemographics, psychosocial status (KIDSCREEN, self‐image scale), daily physical activity, leisure time habits (MoMo Questionnaire) and eating behaviour (DEBQ‐C, FFQ) usage of health services (EQ‐5D) Other outcome: ‐ |
| Starting date |
Study start date: 2012 Study completion date: April 2015 |
| Contact information | Responsible party/principal investigator: Jana Markert, Universität Leipzig |
| Study identifier | DRKS00004583 |
| Official title | Feasibility and efficacy of a weight maintenance treatment approach for adolescent obesity via telephone counselling following an obesity treatment program: a randomized controlled trial |
| Stated purpose of study | Quote: "to evaluate a) the feasibility and b) the efficacy of a six month aftercare weight maintenance treatment (maintaining BMI‐SDS, i.e. standard deviation score of body mass index) following reconvalescent care for adolescent obesity based on telephone counselling, with a follow up period of two years." |
| Notes | Undertook a feasibility study which is the main publication. Baseline characteristics but no results presented. Contact with author suggests submission to journal publication due late September 2015. No publications available yet (last checked trial record on 21 November 2016) |
DRKS00005299.
| Trial name or title | Acronym: KLAKS‐Study |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity, other disorders of pancreatic internal secretion Enrollment: actual 60 Inclusion criteria: aged 8‐18 years, BMI > 97th percentile Exclusion criteria: syndromal obesity, pregnancy |
| Interventions |
Intervention: endurance exercise for 60 minute/week on top of a standardised obesity therapy programme; resistance exercise for 60 minute/week on top of a standardised obesity therapy programme. Comparator: unsupervised physical activity for 60 minute/week on top of a standardised obesity therapy programme |
| Outcomes |
Primary outcome: change in BMI‐SDS Secondary outcomes: change in waist circumference, body fat distribution and body fat content (skinfolds: triceps, biceps, subscapular, suprailiacal); change in metabolic profile; assessment of motor skills; psychological parameters: eating disorder symptoms (Child Eating Disorder Examination, ChEDE‐Q); depressive symptoms (Depressions‐Inventar für Kinder und Jugendliche, DIKJ); weight bias internalisation (Weight Bias Internalization Scale, WBIS) Other outcome: ‐ |
| Starting date |
Study start date: September 2013 Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: Susann Blüher, Universität Leipzig |
| Study identifier | DRKS00005299 |
| Official title | Impact of resistance versus endurance exercise on anthropometric and metabolic parameters within a standardized one year obesity treatment program for obese children and adolescents |
| Stated purpose of study | Quote: "evaluate which exercise modality is more favourable in influencing weight status, body composition and associated metabolic and cardiovascular risk factors." |
| Notes | Recruiting ongoing (according to trial record, accessed 16 August 2016) |
DRKS00006781.
| Trial name or title | Acronym: moveHIT |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: blinded (participant, investigator/therapist) Primary purpose: prevention |
| Participants |
Condition: obesity, metabolic syndrome Enrollment: actual 70 Inclusion criteria: male or female, 13‐18 years, overweight and obesity (BMI‐SDS > 90th percentile) Exclusion criteria: pregnancy, severe orthopaedic disabilities |
| Interventions |
Intervention: high‐intensity interval training (twice per week for 60 minutes) and reception of weekly text message reminders and e‐mails for 6 months. Website use based on principles of the social cognitive theory (social support and self‐efficacy training regarding initiation and maintenance of physical activity) through posts and individualised feedback Comparator: high‐intensity interval training (twice per week for 60 minutes) without media support for 6 months |
| Outcomes |
Primary outcome: participation rate (number of days training) Secondary outcomes: anthropometric parameters, including body weight, BMI, BMI‐SDS; waist, hip, neck circumferences, waist‐to‐hip ratio, waist‐to‐height ratio; blood pressure; daily physical exercise and daily sedentary behaviour; health‐related quality of life; self‐efficacy, social support and outcome expectation over physical activity; internalisation of stigmatisation Other outcome: ‐ |
| Starting date |
Study start date: April 2014 Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: IFB Adipositas Erkrankungen, Universität Leipzig/Sabine Herget |
| Study identifier | DRKS00006781. |
| Official title | Feasibility of a media‐supported high‐intensity interval training program for overweight and obese adolescents |
| Stated purpose of study | Quote: "The present study aims to evaluate a) The feasibility of a six months high intensity interval‐training program for overweight or obese adolescents and b) Whether motivation to participate in and to adhere to a six months high‐intensity interval‐training program can be increased by regular, tailored text messages and the support of a password‐protected webpage with participant profile for individualized feedback." |
| Notes | Follow‐up ongoing (according to trial record, accessed 16 August 2016) |
DRKS00007879.
| Trial name or title | Acronym: SRT‐Joy |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: blinded (participants, study personnel, outcome assessors) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 226 Inclusion criteria: males and females, aged 8‐16; BMI > 97th percentile; informed consent by parents Exclusion criteria: secondary obesity, hyperkinetic disorder with medication, mental retardation |
| Interventions |
Intervention: self‐regulation training with the developed computer program (Approach‐Avoidance‐Training). The training will be an add‐on to the treatment as usual (inpatient rehabilitation treatment). The training will take place on 6 sessions for 10‐15 minutes each, spread over 2 consecutive weeks Comparator: will receive a placebo training. This is a similar computer program as the intervention programme but with the difference that no learning effect will occur. The training will be an add‐on to the treatment as usual (inpatient rehabilitation treatment). The training will be held on 6 sessions for 10‐15 minutes each, spread over 2 consecutive weeks |
| Outcomes |
Primary outcome: BMI‐SDS Secondary outcomes: self‐regulation skills of the children, weight‐specific self‐efficacy, dietary intake Other outcome: |
| Starting date |
Study start date: treatment allocation 7 April 2015 to 30 September 2015 Study completion date: 12‐month analysis estimated to be complete 31 December 2016 |
| Contact information | Responsible party/principal investigator: Universität Potsdam Lehrstuhl für Beratungspsychologie, Germany |
| Study identifier | DRKS00007879 |
| Official title | Development and evaluation of a computer‐based self‐regulation training for obese children and adolescents |
| Stated purpose of study | Quote: "The PC [personal computer]‐based self‐regulation training "SRT‐Joy" will be implemented in a randomized‐controlled multi‐center clinical study with obese children and adolescents aged from 8 to 16 years. It is expected that the experimental group will show an increase in self‐regulating capacity and a better course of BMI‐SDS, compared to the control group." |
| Notes | www.psych.uni‐potsdam.de/counseling/research/adipositas‐training‐d.html |
EUCTR2009‐016921‐32‐ES.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: double blind Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 60 Inclusion criteria: attending the Pediatric Endocrinology Service on an out‐patient basis; 12‐17 years; BMI ≥ 2 SD and ≤ 4 SD for age and gender; educational level that permits adequate communication and agree to co‐operate in all the tests and examinations; use of an effective contraceptive method, negative pregnancy test where appropriate; informed consent of the parents Exclusion criteria: obesity secondary to an endocrine disease or the use of medications such as cortisol; concomitant administration of other psychotropic medication; taking any vitamins or nutritional supplements or any antiobesity preparations; known psychiatric disorder; treated with any type of structured psychotherapy regimen; type 2 diabetes mellitus, arterial hypertension (blood pressure > 95th percentile for gender and height) or steatotic liver; any severe food intolerance, or with a known allergy to substances used in the study; treatment with oral hypoglycaemic agents; pregnant or breastfeeding |
| Interventions |
Intervention: dietary supplementation with an oral capsule of tryptophan 140 mg for 6 months Comparator: oral placebo for 6 months. |
| Outcomes |
Primary outcomes: weight, height, BMI, weight/height z scores, BMI z score, and height/blood pressure and waist/hip circumference Z scores. Secondary outcomes: dietary intake, anxiety, depression and other symptoms associated with eating disorders; plasma tryptophan levels; tryptophan/large neutral amino acids ratio, and plasma serotonin. Other outcome: ‐ |
| Starting date |
Study start date: unclear Study completion date: unclear |
| Contact information | Responsible party/principal investigator: Fundació Sant Joan de Déu Spain/Morales R |
| Study identifier | EUCTR2009‐016921‐32‐ES |
| Official title | A phase II, randomized, double‐blind, placebo‐controlled, in parallel groups clinical trial to assess the safety and efficacy of dietary supplementation with tryptophan to achieve weight loss, and its neuropsychological effects in adolescents with obesity |
| Stated purpose of study | Quote: "To assess the efficacy of a treatment of dietary supplementation with tryptophan to achieve weight loss, and the improvement of clinical parameters such as reduced body mass index and waist/hip ratio." |
| Notes | Trial status completed (according to trial record, accessed 16 August 2016). Contacted author (6 August 2015) for study completion date and there is an e‐mail error (cannot reach author). Recontacted author (6 October 2016) for results/published work |
IRCT201012235440N1.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: ‐ Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight and obesity Enrollment: target 115 Inclusion criteria: aged 12‐17 years; overweight or obese according to WHO cut‐off; living with a parent or adult carer prepared to participate in the study Exclusion criteria: physical disability that prevents participation |
| Interventions |
Intervention: intensive phase: 17 focus group discussions, twice per week and a 45‐minute face‐to‐face session of nutritional counselling at the end of programme Maintenance programme: 7 focus group discussions for 6 months Intervention involved improvement in diet, increase in the level of physical activity and improvement of stress management which was implemented through motivational interviews, group discussions and educational publications Comparator: conventional diet counselling |
| Outcomes |
Primary outcomes: obesity‐related behaviours, laboratory indices, BMI, body composition Secondary outcome: health‐related quality of life Other outcome: ‐ |
| Starting date |
Study start date: May 2011 Study completion date: September 2012 |
| Contact information | Responsible party/principal investigator: Research Institute for Endocrine Sciences, Tehran/Parisa Amiri |
| Study identifier | IRCT201012235440N1 |
| Official title | Effectiveness of a theory‐based intervention on obesity‐related behaviours, laboratory indices, body composition, body mass index and health‐related quality of life in overweight adolescents |
| Stated purpose of study | Quote: "aims at evaluating the effectiveness of a TORBA [theory of obesity‐related behaviors in adolescents]‐based intervention on obese adolescents' weight reduction and its related factors." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (21 October 2015) for study completion date. Re‐contacted (6 October 2016) for results/publication |
IRCT2013103115211N1.
| Trial name or title | Acronym: SCT: social cognitive theory |
| Methods |
Type of study: ‐ Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: supportive care |
| Participants |
Condition: obesity Enrollment: target 150 Inclusion criteria: second‐ and third‐grade female students in middle schools who according to the WHO 2007 Tables have BMI ≥ 85th percentile; aged 12‐16 years Exclusion criteria: using drugs associated with overweight and obesity; received and followed any diet by students for losing weight before the study; any metabolic disease |
| Interventions |
Intervention: students and parents taught about nutrition and physical activity with participating in theory and practical seminars, conferences and workshops (12 sessions and each session 60 minutes). In addition, 6 sessions held for individual counselling about nutrition and physical activity (each session 60 minutes). Sent 48 messages, given an educational CD, attended practical exercise classes (40 sessions and each session 90 minutes). Interventions duration: 6 months Comparator: no intervention, after which 4 education sessions for students and their parents (each session 90 minutes) |
| Outcomes |
Primary outcomes: weight, height, BMI, waist circumference, nutritional behaviour, physical activity behaviour, nutrition self‐efficacy, physical activity self‐efficacy, perceived social support in nutrition, perceived social support in physical activity, outcome expectation in nutrition, outcome expectation in physical activity Secondary outcome: ‐ Other outcome: ‐ |
| Starting date |
Study start date: December 2013 Study completion date: May 2014 |
| Contact information | Responsible party/principal investigator: Tehran University of Medical Sciences/Mohamad Bagherniya |
| Study identifier | IRCT2013103115211N1. |
| Official title | Study on the effect of nutrition education and physical activity intervention using social cognitive theory for overweight and obese student girls of middle school of Shahinshahr |
| Stated purpose of study | Quote: "to correct two important criteria of lifestyle, nutrition and physical activity, and ultimately reduce the prevalence of overweight and obesity and promoting level of health among adolescents." |
| Notes |
ISRCTN04152711.
| Trial name or title | Acronym: SWITCH (SmartWeight in Teenagers Choosing Health) |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: cluster Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight or obese Enrollment: 140 Inclusion criteria: aged 11‐16 years, attending participating dental practices, overweight or obese (BMI ≥ 85th centile) and consuming ≥ 1 can (or equivalent) of soft drink per day Exclusion criteria: serious underlying medical condition or eating disorder, being unable to communicate effectively in English or being on a special prescribed diet |
| Interventions |
Intervention: 3‐4 motivational interviewing sessions, to reduce consumption of soft drinks, in addition to usual dental healthcare advice from the dentist. Sessions were followed up with a maintenance phase which included text, e‐mail and telephone follow‐up Comparator: no intervention: usual dental healthcare advice from their dentist and received a healthy eating leaflet at the 6‐month follow‐up |
| Outcomes |
Primary outcomes: BMI; waist circumference Secondary outcome: mean daily consumption of soft drinks Other outcomes: physical activity levels, sedentary behaviour, social support, process evaluation |
| Starting date |
Study start date: July 2011 Study completion date: March 2013 |
| Contact information | Responsible party/principal investigator: University College London/Richard G Watt, Marie Murphy |
| Study identifier | ISRCTN04152711. |
| Official title | Preventing obesity in young people attending primary dental care settings: an exploratory randomised controlled trial |
| Stated purpose of study | Quote: "to test the feasibility of a MI intervention aimed at reducing soft drink consumption in adolescents attending dental surgeries." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (22 October 2015) for publication |
NCT00562263.
| Trial name or title | Acronym: TEENS |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight Enrollment: 257 Inclusion criteria: aged 11‐18 years, BMI ≥ 85th percentile for age and gender, ≥ 1 adult in household committed to programme meetings Exclusion criteria: previous enrolment; underlying genetic, neurological, endocrine or metabolic conditions that preclude weight loss; weight > 400 lb, pregnancy; inability to understand programme instructions due to language or mental disability; primary residence > 30‐mile radius; primary participating parent pregnant during parent's intervention |
| Interventions |
Intervention: behaviour changing modification with parents attending 12 educational sessions covering strategies to manage children's health behaviours Comparator: behaviour changing modification (parent does not attend parent education sessions) |
| Outcomes |
Primary outcome: changes in BMI z score Secondary outcomes: changes in body composition, metabolic and anthropometric measures, fitness measures, dietary intake and quality of life scores. Other outcome: ‐ |
| Starting date |
Study start date: October 2007 Study completion date: November 2013 |
| Contact information | Responsible party/principal investigator: Gary L. Francis, Virginia Commonwealth University |
| Study identifier | NCT00562263 |
| Official title | Barriers to effective weight loss in overweight adolescents enrolled in an intensive, team‐based, family‐centered lifestyle modification program |
| Stated purpose of study | Quote: "to investigate the impact of a comprehensive, team‐based, family‐centered, lifestyle modification program on body weight, metabolic abnormalities, fitness measures, and self‐esteem in overweight adolescents beginning the study at ages 11‐18 years." |
| Notes | The reference provided in the clinical trial record ("automatically linked") is not the same study, and has already been excluded as it is not a randomised controlled trial: Ning Y, Yang S, Evans RK, Stern M, Sun S, Francis GL, Wickham EP 3rd. Changes in body anthropometry and composition in obese adolescents in a behaviour changing intervention program. European Journal of Nutrition 2014;53(4):1093‐102. doi: 10.1007/s00394‐013‐0612‐9. Contacted author on 1 September 2016 for published data |
NCT00850629.
| Trial name or title | Acronym: MAINTAIN |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: weight regain Enrollment: 77 Inclusion criteria: aged 10‐17 years; primary adiposity at recruitment with a BMI > 97th percentile; willingness of candidates and their families to actively participate in the 3 parts of the study: residential weight reduction programme (participants who stay about 24 days, on average, at least 4‐week treatment programme focusing in behaviour changing change, physical activity and healthful eating) Exclusion criteria: participation in another clinical trial or intake of experimental medication within 30 days before the inclusion date, personal relationships or dependencies between participants and study team, severe chronic diseases that were incompatible with the planned intervention (i.e. severe damage of liver or kidney, clotting disorder, psychological or psychiatric disorders, systemic infections, endocrine diseases as well as malabsorption, food allergies or special diets, pregnancy) |
| Interventions |
Intervention: initial weight loss at a residential weight reduction programme. 1‐year group multiprofessional behaviour changing intervention with monthly meetings at the paediatric outpatient obesity clinic following BABELUGA (Berlin Adiposity Therapy Program for children and adolescents and their families) behaviour changing monitoring map (behaviour changing counselling) Comparator: initial weight loss at a residential weight reduction programme. Received usual medical care, but no particular programme (as the intervention group throughout the whole year) |
| Outcomes |
Primary outcome: change in age‐ and gender‐adjusted BMI z score Secondary outcomes: change in age‐ and gender‐adjusted waist circumference percentile, change in age‐ and gender‐adjusted blood pressure percentile, change in fasting lipid profile, change in fasting insulin and fasting glucose, change in plasma visfatin level, change in plasma adiponectin level, proportion of children achieving a BMI < 85th percentile for age and gender Other outcome: ‐ |
| Starting date |
Study start date: October 2009 Study completion date: August 2012 |
| Contact information | Responsible party/principal investigator: Joachim Spranger, Professor, Charite University, Berlin, Germany |
| Study identifier | NCT00850629 |
| Official title | Hormonal regulation of body weight maintenance |
| Stated purpose of study | Quote: "It is the aim of the therapy to lose weight and maintenance; improvement of physical fitness and endurance capacity; implementation of active lifestyle in daily living; building social networks, making new friend; changing eating patterns at home (with support of the family); strengthening and activating the family as social resource; treatment of co‐morbidities." |
| Notes | Study still ongoing; the investigators are still analysing data. Therefore, they did not wish to share their data publicly (2016, study protocol) |
NCT00881478.
| Trial name or title | Acronym: FOCUS |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind, outcomes assessor Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 102 Inclusion criteria: aged 8‐16 years, BMI ≥ 85th percentile for age and gender Exclusion criteria: currently taking a weight loss medication, gastrointestinal disorder, psychiatric illness under the care of a psychiatrist, Cushing's syndrome, hypothalamic or genetic aetiology of obesity, uncontrolled or untreated thyroid disease, current diagnosis of cancer, history of an eating disorder such as bulimia or anorexia nervosa, surgery in the past 3 months, surgery planned in the ensuing 6 months, any chronic illness that could affect weight status |
| Interventions |
Intervention: nutrition counselling + portion control. Nutrition counselling with registered dietitian in addition to teaching about use of a portion control tool Comparator: nutrition counselling alone with registered dietitian |
| Outcomes |
Primary outcome: change in age‐ and gender‐adjusted BMI z score Secondary outcomes: change in age‐ and gender‐adjusted waist circumference percentile, change in age‐ and gender‐adjusted blood pressure percentile, change in fasting lipid profile, change in fasting insulin and fasting glucose, change in plasma visfatin level, change in plasma adiponectin level, proportion of children achieving a BMI < 85th percentile for age and gender Other outcome: ‐ |
| Starting date |
Study start date: August 2009 Study completion date: August 2014 |
| Contact information | Responsible party/principal investigator: Josephine Ho, Alberta Children's Hospital, Calgary |
| Study identifier | NCT00881478 |
| Official title | Family intervention for obese children using portion control strategy (F.O.C.U.S.) for weight control ‐ a randomized controlled trial |
| Stated purpose of study | Quote: "The purpose of this study is to assess the efficacy of a family intervention using a portion control tool to help control weight in obese children. The investigators hypothesize that the use of portion control tools by the parents and child will result in a greater decrease in the child's BMI over a 6 month period compared with the control group." |
| Notes | Last update: 29 August 2014 |
NCT00940966.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: elevated triglycerides, systolic hypertension, insulin resistance, abdominal obesity Enrollment: 40 Inclusion criteria: aged 13‐18 years, BMI > 95% for age or > 30 kg/m2 for young adults, with pre‐existing metabolic syndrome Exclusion criteria: on any chronic medication other than antihistamines, asthma medications, oral contraceptives or diabetes medications; smoke > 5 cigarettes/day; alcoholism or drug abuse; significant abnormality not associated with metabolic syndrome; currently taking Byetta; familial hypercholesteremia if the investigator considers the history to be severe; pregnant or desiring pregnancy |
| Interventions |
Intervention: non‐energy restricted controlled carbohydrate programmes: energy‐restricted very‐low carbohydrate diet; low glycaemic index diet; restricted ketogenic diet Comparator: standard American Diabetes Association diet |
| Outcomes |
Primary outcome: weight loss Secondary outcome: ‐ Other outcome: ‐ |
| Starting date |
Study start date: July 2006 Study completion date: May 2012 |
| Contact information | Responsible party/principal investigator: WVU department of paediatrics/Steven Sondike MD |
| Study identifier | NCT00940966. |
| Official title | A pilot study to determine the efficacy of a low carbohydrate diet in treatment of adolescents with metabolic syndrome |
| Stated purpose of study | Quote: "to determine the effectiveness of two different non‐energy restricted controlled carbohydrate programs with the American Diabetes Associations' diet on glycosylated hemoglobin and other diabetes risk factors in obese adolescents with metabolic syndrome, a constellation of symptoms associated with the development of type 2 diabetes and cardiovascular disease." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (5 August 2015) for study completion date. Recontacted (6 October 2016) for results/publication |
NCT00998413.
| Trial name or title | Acronym: Mikado (Multifactorial intervention for children with asthma and overweight) |
| Methods |
Type of study:interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: prevention |
| Participants |
Condition: asthma, overweight, obesity Enrollment: actual 104 Inclusion criteria: asthma and overweight (BMI‐SDS > 1), aged 6‐16 years, living in Southern Limburg Exclusion criteria: congenital malformations of the airways or other chronic lung diseases like cystic fibrosis, mental retardation or syndromes, heart disease |
| Interventions |
Intervention: multifaceted family‐based, physical exercise, nutrition, cognitive behavioural therapy, parental sessions and individualised counselling for 18 months. Small groups of 8‐12 children. Based on health counselling model. Group exercises (twice per week during initial phase, 3 times per month during the follow‐up phase), with a duration of 60 minutes per session guided by an experienced paediatric physiotherapist or a paediatric sport instructor. A dietitian and psychologist guide 18 behaviour changing sessions with a duration of 75‐90 minutes. Use a workbook with additional information for each behaviour changing session, homework and space for individualised goal setting. Small presents as incentives for participation and achievements. 3 basic dietary guidelines: healthy food choice, regular eating pattern, and normalised portion sizes. Children receive individualised counselling sessions. Parents follow 10 parental sessions of 60 minutes guided by the dietitian and psychologist Comparator: standard usual care according to the standards of the Dutch Society of General Practitioners and the Paediatric Pulmonology section of the Dutch Society of Paediatrics |
| Outcomes |
Primary outcomes: forced expiratory volume in 1 second (FEV1 %) predicted value, BMI‐SDS Secondary outcomes: lung function parameters, inflammatory parameters, asthma control, medication use, asthma symptoms, health‐related quality of life, asthma‐related quality of life, sleep‐related breathing disorders, gastro‐oesophageal reflux disease, psychosocial problems, eating behaviour, weight, height, BMI), waist and hip circumference, 4‐fold skinfold measurement, food record, accelerometer output Other outcome: ‐ |
| Starting date |
Study start date: January 2010 Study completion date: September 2013 |
| Contact information | Responsible party/principal investigator: Maastricht University Medical Center/Edward Dompeling |
| Study identifier | NCT00998413. |
| Official title | Secondary prevention of asthma in overweight/obese children by a combined dietary‐behavioural‐physical activity intervention |
| Stated purpose of study | Quote: "to determine the efficacy of a multifactorial intervention with weight reduction, behavioural therapy, and physical exercise on the severity and control of asthma in obese children." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (22 October 2015) for study completion date but e‐mail error. Recontacted (6 October 2016) and e‐mail error |
NCT01023139.
| Trial name or title | Acronym: AOS |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight Enrollment: estimated 100 Inclusion criteria: BMI ≥ U. weighted mean of the 95th percentile based on age and gender; willing to lose weight to meet and continue study medication for the 12‐month treatment period and meet personal weight loss goal; willing to not start any new weight loss products; males or non‐pregnant females (pregnancy determined by self‐report); females of childbearing potential if practicing acceptable method of contraception Exclusion criteria: weight loss ≥ 10 lb in previous 3 months; active gastrointestinal disorders (except gastro‐oesophageal reflux disease); at least 2 out of 3 blood pressure readings either systolic or diastolic ≥ 95 percentile for height and age or pulse ≥ 95 beats per minute at initial visit; drug‐treated diabetes mellitus or hypertension; drugs or supplements (or both) administered for the first time or withdrawn during the past 6 months which have a significant impact on body weight or digestion; inability or unwillingness to comply with protocol requirements; unwilling to avoid consumption of alcoholic beverages; smoking or has started a smoking cessation programme within the past 6 months; previous treatment with prescription sibutramine; history of recurrent nephrolithiasis; major psychiatric or eating disorders; kidney, liver or thyroid disorder; drugs that are contraindicated; cardiovascular disease; history of bleeding problems, migraine headaches or seizures; stroke; history of pulmonary hypertension, osteopenia or osteoporosis; current recreational drug use or overused prescription medications; history of glaucoma; pregnancy |
| Interventions |
Intervention: continuing behavioural therapy. Study uses a multidisciplinary approach along with pharmacotherapy (use of Meridia) to motivate and establish behaviour changes in adolescents (aged 12‐18 years) during the first phase of the study. The group will continue on monthly behaviour modifications and be evaluated at 3 and 6 months. This arm follows the end of the phase 1 which incorporates behavioural therapy, nutrition counselling and pharmacotherapy with sibutramine while medically supervised. Participants randomised to this arm no longer receive medication and will receive behavioural therapy once per month and then evaluated at 3 and 6 months for weight loss maintenance Comparator: no intervention: standard of care. No intervention following phase 1 of the study done during this 2nd phase. Participants have their height and weights examined at 3 and 6 months following end of phase 1. During these 2 visits, they receive counselling from the physician regarding food choices and exercise maintenance |
| Outcomes |
Primary outcome: % change in BMI z score Secondary outcomes: absolute weight change, waist circumference change Other outcome: ‐ |
| Starting date |
Study start date: April 2009 Study completion date: March 2011 |
| Contact information | Responsible party/principal investigator: Brooke Army Medical Center/Jorge L Cabrera |
| Study identifier | NCT01023139. |
| Official title | Efficacy in adolescents of continued behavior modification following a six month sibutramine‐based weight management intervention |
| Stated purpose of study | Quote: "This study uses a multidisciplinary approach along with pharmacotherapy (use of Meridia) to motivate and establish behavior changes in adolescents (12‐18yo) during the first phase of the study." |
| Notes | Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently." |
NCT01221220.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel Masking: single blind (outcome assessor) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 160 Inclusion criteria: aged 8‐15 years, obese (BMI > 95th percentile on the 2000 CDC BMI reference) on the date of randomisation. Standard Stanford Pediatric Weight Control Program eligibility criteria apply: both child and parent/guardian must want to join, both child and at least 1 parent/guardian must agree to attend sessions and must agree to not miss > 2 consecutive sessions Exclusion criteria: diagnosed with a medical condition affecting growth (a genetic or metabolic disease/syndrome associated obesity, type 1 diabetes, type 2 diabetes taking medication, chronic gastrointestinal diseases, chronic renal diseases, uncorrected structural heart disease, heart failure, heart transplant, anorexia nervosa or bulimia nervosa or binge eating disorder (present or past), AIDS or HIV infection, pregnancy); taking medications affecting growth (systemic corticosteroids > 2 weeks in the past year, insulin, oral hypoglycaemic drugs, thyroid hormone, growth hormone); have a condition limiting their participation in the interventions (e.g. unable to participate in routine physical education classes at school, requiring oxygen supplementation for exertion, developmental or physical disability preventing participation in interventions, children or parents/guardians who cannot medically participate in mild dietary restrictions, increased physical activity for any reason, or a combination of these); have a condition limiting participation in the assessments (child or primary carer unable to read surveys in English or Spanish, child ≥ 2 grade levels delayed in school for reading and writing in his/her native language); unable to read, understand or complete informed consent in English or Spanish; plan to move from the San Francisco Bay Area within the next 18 months |
| Interventions |
Intervention: behavioural treatment + environmental strategies. 6‐month, family‐based, group, behavioural weight control programme + home‐based environmental intervention Comparator: active comparator: behavioural treatment only. 6‐month, family‐based, group, behavioural weight control programme |
| Outcomes |
Primary outcome: BMI Secondary outcomes: waist circumference, triceps skinfold, resting heart rate, dietary intake/meals eaten with television, weight concerns, depressive symptoms, daily energy intake, physical activity, systolic and diastolic blood pressure, fasting blood lipids, insulin/glucose metabolism Other outcome: ‐ |
| Starting date |
Study start date: September 2010 Study completion date: February 2015 (estimated) |
| Contact information | Responsible party/principal investigator: Thomas Robinson Stanford University |
| Study identifier | NCT01221220. |
| Official title | Environmental strategies & behavior change to reduce overeating in obese children |
| Stated purpose of study | Quote: "There is a need for effective weight control methods for obese children. Environmental strategies such as reducing the size of dishware and serving utensils, storing food out of view and reducing food consumption while watching television may reduce food intake without requiring conscious, cognitive self‐control. The investigators propose to test these methods when added to a current state‐of‐the‐art behavioral program." |
| Notes | Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently" (accessed 17 August 2016) |
NCT01350531.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: estimated 200 Inclusion criteria: African‐American adolescents, aged 12‐16 years and 11 months; obesity (BMI ≥ 95th percentile or BMI > 30 kg/m2); may have primary obesity or obesity in combination with other medical comorbidities; youth with mild mental retardation may be included if they are capable of reading and understanding the study measures Exclusion criteria: obesity secondary to medication use for another disorder; obesity in a youth with medical condition that prevents their participation in normal exercise; thought disorders; serious cognitive impairments; pregnant or have a medical condition where weight loss is contraindicated; do not live with their primary carer |
| Interventions |
Intervention: skills training: includes skills most proximal to adhering to the eating and weight loss plan (e.g. calorie counting, making healthy food choices, measuring food portions, scheduling snacks and meals, meal planning, completing food logs daily, following an exercise plan) Comparator: contingency management: behavioural principles to counteract the reinforcing mechanisms of food and inactivity |
| Outcomes |
Primary outcomes: change in weight‐related outcomes (height, weight and % body fat) Secondary outcomes: change in adherence to weight loss recommendations (objective measures of cardiovascular fitness, BLOCK Kids FFQ, and the Frequency of Fast Food Use Questionnaire); change in physiological functioning (plasma glucose, insulin, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol and triglyceride levels); change in motivation (Importance Ruler) Other outcome: ‐ |
| Starting date |
Study start date: September 2009 Study completion date: June 2014 |
| Contact information | Responsible party/principal investigator: Wayne State University/Sylvie Naar‐King, PhD and K‐L Cathy Jen, PhD |
| Study identifier | NCT01350531. |
| Official title | Interventionist procedures for adherence to weight loss recommendations in black adolescents, phase two |
| Stated purpose of study | Quote: "To refine intervention protocols from our preliminary studies that maximize adolescent and parent skills, informed by learning theory, through the use of home and community‐based interventions in which in‐vivo opportunities are used to promote practice in making changes in dietary, exercise and sedentary behaviors in AAAO [African‐American adolescents with obesity] and their families (PHASE I); To develop intervention protocols that utilize findings from basic science regarding intrinsic and extrinsic motivation to maximize adolescent and family adherence to recommendations for obesity‐related behavior change in AAAO and their families (PHASE I); To develop an adaptive intervention using a sequential multiple randomized assignment trial (SMART design) (PHASE II); To refine the intervention including qualitative analysis of interviews from participant families and to develop further community participation in preparation for a confirmatory randomized clinical trial (PHASE III)." |
| Notes | Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently" (accessed 17 August 2016) |
NCT01374386.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: childhood obesity Enrollment: estimated 100 Inclusion criteria: aged 12‐14 years; BMI > 25 kg/m2; gender‐ and age‐specific 85th percentile cut‐off points from the CDC growth chart; parental consent and subject assent Exclusion criteria: physician‐determined musculoskeletal, cardiopulmonary, metabolic, psychological, neurodevelopmental or behavioural conditions that make mild‐to‐moderate physical activity potentially hazardous |
| Interventions |
Intervention: Exergaming: access to usual physical activity at the gym as well as access to Exergaming equipment (video games that require physical activity). Exergaming equipment will be available for use at the YMCA (Young Men's Christian Association) daily. Exergames include a mixture of aerobic and resistance exercise activities Comparator: usual physical activity at the gym, no access to Exergames |
| Outcomes |
Primary outcome: change in mean daily physical activity Secondary outcomes: change in caloric expenditure, body composition (total body estimates of fat mass, fat‐free mass, and lean body mass) and BMI (BMI z score) Other outcome: ‐ |
| Starting date |
Study start date: October 2010 Study completion date: October 2012 |
| Contact information | Responsible party/principal investigator: Charles Drew University of Medicine and Science/David Martins |
| Study identifier | NCT01374386 |
| Official title | RCMI clinical research infrastructure initiative: exergaming |
| Stated purpose of study | Quote: "to gather information on how much exercising with video games (ExerGaming) can increase the physical activity among overweight and youth. This study will try to see if participating in physical activity and exercising with video games at the same time can make overweight children move around more to better their own health." |
| Notes | Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently" (accessed 17 August 2016) |
NCT01448551.
| Trial name or title | Acronym: MPOWERed |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: single blind (outcome assessor) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 60 Inclusion criteria: BMI ≥ 95th percentile for gender and age according to CDC growth charts; enrolment in the MPOWER programme; at least 1 parent willing and able to participate in the MPOWER programme with the adolescent; absence of any major medical illness, disability or moderate/severe mental disorder (e.g. liver disease, renal failure, cancer, bipolar disorder) Exclusion criteria: presence of any major medical illness, disability or moderate/severe mental disorder; physical, mental or cognitive handicaps that prevent participation; chronic use of medications that may affect study outcomes; pregnancy or planning pregnancy in the next 6 months; lactating; within 6 months postpartum |
| Interventions |
Intervention: tailored mobile messages to enhance weight loss for teens. Participants receive daily messages over 20 weeks. The content of the messages is tailored on participants' responses to the MPOWER enrolment survey and the initial tailoring questionnaire. The tailored messages utilise various degrees of personalisation, adaptation and feedback ‐ including momentary assessments with feedback requiring a text reply from the adolescents Comparator: participation in the MPOWER programme without receiving tailored text messages |
| Outcomes |
Primary outcomes: session attendance; adherence to MPOWER programme homework assignments; satisfaction with the MPOWER programme; drop‐out Secondary outcomes: change in BMI; objective measurement of daily participation in moderate‐to‐vigorous activity; self‐efficacy for achieving MPOWER recommendations for the 6 target behaviours; intrinsic motivation to increase intake of fruits and vegetables and to increase physical activity; change in self‐report of the 6 behaviours targeted in the intervention Other outcome: ‐ |
| Starting date |
Study start date: October 2011 Study completion date: February 2015 |
| Contact information | Responsible party/principal investigator: University of Michigan/Susan J Woolford, MD, MPH |
| Study identifier | NCT01448551 |
| Official title | Novel individually tailored mobile messages to enhance weight loss for teens |
| Stated purpose of study | Quote: "The investigators hypothesize that program participants who receive the tailored text messages will experience lower attrition rates, increased treatment adherence, and greater weight loss compared to those program participants who do not receive the messages." |
| Notes | Last updated: 30 November 2015 |
NCT01456221.
| Trial name or title | Acronym: O3WLIRADOL (Omega‐3 Fatty Acids for Weight Loss and Insulin Resistance in Adolescents) |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: double blind (participant, investigator) Primary purpose: supportive care |
| Participants |
Condition: obesity, insulin resistance Enrollment: estimated 300 Inclusion criteria: aged 12‐18 years; male and female; BMI > 95th percentile of the National Center for Health Statistics reference; informed consent Exclusion criteria: diagnosed with type 2 diabetes mellitus, cardiovascular disease or kidney disease; allergic to fish |
| Interventions |
Intervention: omega 3 supplement (1.1 g of docosahexaenoic acid and eicosapentaenoic acid) containing omega 3: docosahexaenoic acid and eicosapentaenoic acid fatty acids and a hypocaloric diet. 30 capsules provided every month for 3 months. Personalised diet including: reduction of 700 kcal from the usual diet considering lipids and carbohydrates, increasing fruits and vegetables intake up to 6 portions daily each and incrementing the intake of fibre to 30 g per day through the inclusion of whole grains Comparator: sunflower oil supplement and a hypocaloric diet. Personalised diet including: reduction of 700 kcal from the usual diet considering lipids and carbohydrates, increasing fruits and vegetables intake up to 6 portions daily each and incrementing the intake of fibre to 30 g per day through the inclusion of whole grains |
| Outcomes |
Primary outcome: change in insulin resistance Secondary outcome: nutritional status (measured by weight, stature, BMI, waist) Other outcome: ‐ |
| Starting date |
Study start date: July 2012 Study completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Pediatric Hospital CMN "Siglo XXI/Mardia Lopez‐Alarcon, PhD |
| Study identifier | NCT01456221 |
| Official title | The impact of using omega3 long‐chain polyunsaturated fatty acids in weight loss and insulin resistance in obese adolescents |
| Stated purpose of study | Quote: "to evaluate if a supplement containing omega‐3 long chain polyunsaturated fatty acids for three months reduce obesity and insulin resistance to obese adolescents if administered together with a hypocaloric diet." |
| Notes | Last updated: 3 February 2016 |
NCT01514279.
| Trial name or title | Acronym: IMPACT (Ideas Moving Parents and Adolescents to Change Together) |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: single blind Masking: parallel Primary purpose: treatment |
| Participants |
Condition: overweight, obese Enrollment: estimated 360 Inclusion criteria: aged 11‐15 years, found at standard school screenings to be overweight (BMI 85‐94th percentile for age/gender) or obese (> 95th percentile for age/gender) Exclusion criteria: medications that alter appetite or weight; inability to understand English; stage 2 hypertension or stage 1 hypertension with end‐organ damage (left ventricular hypertrophy, microalbuminuria); severe behavioural problems that preclude group participation (as reported by parent/guardian); involvement in another weight management programme; family expectation to move from the region within 1 year; presence of a known medical condition that itself causes obesity (e.g. Prader‐Willi syndrome) or interfere with glycated haemoglobin (sickle cell disease) |
| Interventions |
Intervention 1; HealthyCHANGE: cognitive behavioural strategies to address diet, physical activity, sedentary behaviour and sleep for children. Intensive series of group sessions, followed by rotating monthly face‐to‐face meetings or telephone calls Intervention 2: SystemCHANGE: system improvement and choice architecture theories seek to teach a set of skills using family self‐designed experiments to redesign daily routines regarding eating, activity and sleep. Intensive series of group sessions, followed by rotating monthly face‐to‐face meetings or telephone calls Comparator: Tools4CHANGE: 1 × 60‐minute face‐to‐face meeting at initiation of the study with a dietitian who is also trained in recommendations for exercise and sedentary behaviour (representing usual care) |
| Outcomes |
Primary outcome: slope of BMI Secondary outcomes: dietary intake, blood pressure, physical activity, sleep, cardiovascular risk factors, body composition, fitness, quality of life, DNA Other outcome: ‐ |
| Starting date |
Study start date: February 2011 Study completion date: December 2017 |
| Contact information | Responsible party/principal investigator: Elaine Borawski, Case Western Reserve University/Leona Cuttler, MD |
| Study identifier | NCT01514279 |
| Official title | Targeting obesity and blood pressure in urban youth (consortium title: Childhood Obesity Prevention and Treatment Research [COPTR] and site project name IMPACT (Ideas Moving Parents and Adolescents to Change Together) |
| Stated purpose of study | Quote: "The project assesses the effects of three interventions on Body Mass Index(BMI) in overweight and obese urban 5th‐8th grade youth: a cognitive‐behavioral intervention (HealthyChange), a systems improvement intervention (SystemsChange), and an education‐only intervention (Tools4Change). In addition the study assesses the potential additional impact of a school‐community based intervention on outcomes." |
| Notes | Last updated: 26 January 2016 |
NCT01677923.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: estimated 400 Inclusion criteria: aged 10‐17 years; weight > 85th percentile for age and gender (by International Obesity Task Force); living in Kaunas and its region; no obvious chronic diseases; not on steroid or other long‐term treatment; informed consent of the participant and parents Exclusion criteria: diagnosis of type 1 diabetes mellitus; chronic illness that may affect physical activity and metabolic profile; insulin treatment; steroid treatment; planning to move from Kaunas or its region in the period of 1 year |
| Interventions |
Intervention: intervention duration: 12 months Intervention 1: intensive diet + physical activity group: seen by a dietitian once per month for diet re‐evaluation; physical therapist, who will give physical activity course twice per week (1 hour each); paediatric endocrinologist every 3 months Intervention 2: intensive diet + physical activity group + insulin sensitisation: metformin 1000 mg/day. Children seen by a dietitian once per month for diet re‐evaluation; physiotherapist, who will give physical activity course twice per week (1 hour each); paediatric endocrinologist every 3 month Comparator 1: insulin sensitisation without intensive diet and physical activity. Metformin 1000 mg/day after standardised information on healthy lifestyle, diet and exercise during the first visit only. Seen by paediatric endocrinologist every 3 months Comparator 2: during the first visit participants get standardised information on healthy lifestyle, diet and exercise. Followed‐up at 12 months |
| Outcomes |
Primary outcome: BMI changes Secondary outcomes: glucose homeostasis; lipid profile; metabolic syndrome; hepatosteatosis; polycystic ovary syndrome and hyperandrogenism in females Other outcome: safety |
| Starting date |
Study start date: May 2013 Study completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Lithuanian University of Health Sciences/Rasa Verkauskiene |
| Study identifier | NCT01677923 |
| Official title | Phase 3: effect of diet, physical activity and insulin sensitizer metformin on obesity and associated risks in children and adolescents |
| Stated purpose of study | Quote: "to get better effect of weight decrease and metabolic processes repair in the intensive treatment group with intervention of physical activity, diet correction and Metformin use." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (2 September 2016) for results/publication |
NCT01688453.
| Trial name or title | Acronym: PRALIMAP‐INES |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: prevention |
| Participants |
Condition: overweight Enrollment: 1250 Inclusion criteria: attending grade 10 in participating schools; aged ≤ 18 years; able to complete a questionnaire; overweight or obese according to the International Obesity Task Force criteria (for BMI) and the McCarthy criteria (for waist circumference), have a high eating disorder score, ,express the need for management of excess weight, or a combination of these; confirmed as corresponding to the inclusion criteria by the physician; agree to an overweight and obesity care management programme Exclusion criteria: ‐ |
| Interventions |
Intervention: strengthened care management: 5 collective sessions with the same standard operating procedure as the standard‐care management with supplementary interventions between each session: strengthened solicitation with the adolescent and the family, peer‐led educational sessions, motivational interviews, financial support for physical activity practice, cooking classes and multidisciplinary consultation meetings Comparator: standard‐care management: 5 collective sessions of 2 hour each about nutritional practices (food and physical activity) and on changes of nutritional behaviours. The sessions are organised in high schools by the healthcare team in collaboration with paediatricians, dietitians and the Sickness Insurance Primary Fund |
| Outcomes |
Primary outcomes: change in BMI; change in overweight and obesity status; change in BMI Z score Secondary outcomes: change in waist circumference; change in eating attitude score; change in anxiety and depression scores Other outcome: change in nutritional behaviour |
| Starting date |
Study start date: September 2012 Study completion date: September 2015 |
| Contact information | Responsible party/principal investigator: Institut National de la Santé Et de la Recherche Médicale, France/Serge Briançon, Pr |
| Study identifier | NCT01688453 |
| Official title | Reduction of inequalities in overweight and obesity management care access among high school adolescents |
| Stated purpose of study | Quote: "to investigate whether a strengthened care management (CM) for socially less advantaged adolescents in school in the short and long term has an equivalent effect as a standard‐CM on decreasing the prevalence of overweight and obesity among socially advantaged adolescents." |
| Notes | Last updated: 12 February 2016. Also has a non‐randomised arm of socially advantaged adolescents following the standard care management intervention |
NCT01764113.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: adolescent obesity Enrollment: 21 Inclusion criteria: aged ≥ 14 years; BMI ≥ 95th percentile for age and gender Exclusion criteria: ‐ |
| Interventions |
Intervention: mindful eating: adolescents and parents receive mindful eating‐based behavioural modification programme over multiple sessions. At least 1 parent would be expected to attend the counselling sessions. Duration: 6 months Comparator: standard nutritional counselling provided by a registered dietitian. At least 1 parent would be expected to attend the counselling sessions. Duration: 6 months |
| Outcomes |
Primary outcome: BMI Secondary outcome: quality of life Other outcome: fasting glucose |
| Starting date |
Study start date: December 2012 Study completion date: December 2013 |
| Contact information | Responsible party/principal investigator: Mayo Clinic/Seema Kumar |
| Study identifier | NCT01764113 |
| Official title | Effect of mindful eating on body mass index and cardiovascular risk markers in obese adolescents: a pilot randomized clinical trial |
| Stated purpose of study | Quote: "to study the effect of a family based mindfulness training program with special focus on diet and nutrition on weight and cardiovascular risk markers in obese adolescents." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (5 August 2015) for expected publication date, author response: data ongoing and will notify once accepted |
NCT01794546.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: cross‐over Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: paediatric obesity Enrollment: 40 Inclusion criteria: aged 10‐17 years; BMI ≥ 95th percentile for age and gender; no known significant obesity comorbidity or cause requiring urgent medical evaluation or treatment in a subspecialty programme other than an obesity programme; no known physical limitations to changes in diet or activity level (i.e. concern for cardiac disease, primary gastrointestinal disease or orthopaedic concerns); patient at Wareham Pediatrics practice Exclusion criteria: unstable home environment; inability to actively participate in treatment; physician diagnosis of a major medical illness or eating disorder; chronic use of any medication or supplement that may affect study outcomes; another member of the family participating in the study; planning to relocate from current area of residence during the proposed time frame for study participation |
| Interventions |
Intervention: telehealth, alternate visits between a dietitian and behavioural medicine provider for either 30 minutes or 1 hour. 12 sessions over 6 months. The dietitian provides dietary and physical activity recommendations, and the behavioural medicine provider counsels on strategies for achieving specific goals. Applying a Chronic Care Model, self‐management support will be augmented by linkages to community resources Comparator: wait list control group |
| Outcomes |
Primary outcome: BMI Secondary outcomes: satisfaction, compliance Other outcome: ‐ |
| Starting date |
Study start date: February 2013 Study completion date: January 2015 |
| Contact information | Responsible party/principal investigator: Children's Hospital Boston/Cara B Ebbeling, PhD |
| Study identifier | NCT01794546 |
| Official title | Integrated care for pediatric obesity using telehealth |
| Stated purpose of study | Quote: "to evaluate telehealth for treating pediatric obesity in collaboration with a community practice (Wareham Pediatrics)." |
| Notes | Trial status completed (according to trial record, accessed 17 August 2016). Contacted author (23 November 2015) for expected publication date, author response: data are ongoing and cannot share unless published |
NCT01796067.
| Trial name or title | Acronym: FIT: Families Improving Together |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: factorial Masking: open label Primary purpose: treatment |
| Participants |
Condition: overweight, obese Enrollment: estimated 520 Inclusion criteria: parent or primary carer who lives in the same house; live within 60 miles of the programme's office; ≥ 3 grandparents who are African‐American; access to the Internet; aged 11‐16 years; BMI ≥ 85th to < 99th percentile; no medical condition that would limit participation in moderate‐intensity exercise including life‐threatening illness (e.g. immobile, severely disabled or bed ridden); available and able to participate in measures and intervention activities over the next year Exclusion criteria: chronic illness; require a specialised diet (may not be eligible); developmental delay; partaking currently in another weight loss programme |
| Interventions |
Intervention: 8‐week face‐to‐face group programme comparing motivational plus family weight loss to a comprehensive health education programme. Then participants undertake either an 8‐week online tailored intervention or online control and then participants have online intervention or control booster sessions for 6 months. 4 groups: Group 1: motivational and family weight loss + online intervention Group 2: motivation and family weight loss + online control Group 3: basic health education and online intervention Group 4: basic health education and online control Comparator: see above |
| Outcomes |
Primary outcome: change in BMI (BMI z score) Secondary outcomes: change in moderate‐to‐vigorous physical activity in parents and adolescents; change of 24‐hour dietary recall in parents and adolescents; change in psychosocial variables (autonomy‐support, monitoring, communication); change in moderate‐to‐vigorous physical activity; change of 24‐hour dietary recall; change in BMI z score in parents Other outcome: ‐ |
| Starting date |
Study start date: July 2012 Study completion date: June 2017 |
| Contact information | Responsible party/principal investigator: University of South Carolina/Dawn K Wilson, PhD |
| Study identifier | NCT01796067. |
| Official title | Families Improving Together (FIT) for weight loss |
| Stated purpose of study | Quote: "to test the effects of an integrated intervention curriculum and the added effects of a tailored web‐based intervention on reducing z‐BMI in overweight African American adolescents." |
| Notes | Last updated: 19 July 2016 |
NCT01804855.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: non‐inferiority study Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcome assessor) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 134 (126 in trial record) Inclusion criteria: aged 12‐17 years, BMI > 98th percentile, fluent in English, parent(s) willing to participate in the programme, written informed consent and assent prior to any study‐specific procedures. Exclusion criteria: severe intellectual difficulties which would limit the child's ability to engage in group activity, obesity secondary to genetic condition, limitations to engaging in physical activity or use of medication known to effect body weight, limitations to using a smart phone device and known family issues that would affect general compliance and attendance at follow‐up visits |
| Interventions |
Intervention: W82GO (underpinned by behavioural change theory (Transtheoretical Model and social cognitive theory) uses strategies including stimulus control, self‐monitoring, positive reinforcement, goal setting and problem solving to facilitate lifestyle change), 6 × 2‐hour weekly sessions for adolescents and parents followed by randomisation to a maintenance phase, of 3 × 3‐month booster maintenance sessions over 46 weeks and using a smart phone application: incorporates evidence‐based behavioural change tools such as self‐monitoring, goal setting and peer support. Evidence‐based tips as a text tip, a video tip or an image tip. Aim to increase knowledge with regard to healthy eating, physical activity, physical fitness and sleep. Encouraged to engage in daily goal setting and monitor progress. Monthly telephone calls Comparator: W82GO followed by randomisation to usual care maintenance phase: 3 booster sessions at 3, 6 and 9 months and no smart phone intervention |
| Outcomes |
Primary outcome: BMI z score Secondary outcomes: weight, height, waist circumference, fat mass, physical activity, lipids, glucose, insulin, glycated haemoglobin, psychosocial health, adverse events, health‐related quality of life Other outcome: ‐ |
| Starting date |
Study start date: 2013 Study completion date: 2014 |
| Contact information | Responsible party/principal investigator: Grace O'Malley, Temple Street Children's University Hospital. Dublin, Ireland |
| Study identifier | NCT01804855 |
| Official title | Effectiveness of a smartphone app for adolescent obesity management |
| Stated purpose of study | Quote: "This study will test whether treatment can be delivered via an Android app and whether such treatment reduces obesity." |
| Notes | Some conflicting information between the protocol and clinical trials record, e.g. sample size and outcomes. Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently" (accessed 18 August 2016) |
NCT01840631.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: intervention Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 80 Inclusion criteria: aged 9‐17 years, BMI percentile for age of ≥ 95, able to attend monthly sessions with a parent or guardian (or both) Exclusion criteria: diabetes at baseline, mental or psychological disease that would interfere with understanding, disease or medication causing obesity or weight loss, and participants in an alternative weight management programme, impaired glucose tolerance |
| Interventions |
Intervention: group nutritional counselling for a total of 6 classes. Classes meet 1 time per month for 60 minutes (30 minutes for dietetic session and 30 minutes for discussion/questions). Classes cover topics including nutrition, exercise and behaviour to promote healthy eating. All content developed prior to starting the intervention. Each group session have a maximum of 7 children with 1 parent/carer per child and a minimum of 5 children with a carer. All groups receive standard of care for physical fitness counselling which includes recommending 1 hour of physical activity per day and limiting screen time to < 2 hours per day. All groups evaluated for depression and appropriately referred if found to be depressed. Behavioural strategies, such as mindful eating, included in the nutrition education (participants receive the same information in both interventions) Comparator: individual nutritional counselling (usual care): nutritionist conducts the nutritional counselling with 1 family at a time. Individual sessions once per month for 30 minutes for a total of 6 sessions. All groups receive standard of care for physical fitness counselling which includes recommending 1 hour of physical activity per day and limiting screen time to < 2 hours per day. All groups evaluated for depression and appropriately referred if found to be depressed. Behavioural strategies, such as mindful included in the nutrition (participants receive the same information in both interventions) |
| Outcomes |
Primary outcome: mean BMI change Secondary outcomes: correlation between change in BMI and dietary composition change, correlation between change in BMI and increased physical activity, effect of change in BMI on metabolic profile Other outcome: ‐ |
| Starting date |
Study start date: April 2013 Study completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Rubina Heptulla, Albert Einstein College of Medicine of Yeshiva University, New York |
| Study identifier | NCT01840631. |
| Official title | A randomized controlled trial to Study the effects of group versus individual dietary counseling in pediatric obesity |
| Stated purpose of study | Quote: "The primary aim of our study is to assess whether group counselling is a non‐inferior intervention compared to the usual care of individual counselling in the management of childhood obesity. In order to achieve this aim, the investigators will compare the mean change in BMI after 6 months of intervention in the two study arms." |
| Notes | Last updated: 24 June 2015 |
NCT02034994.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: prevention |
| Participants |
Condition: obesity but only in second part of the study Enrollment: 1200 Inclusion criteria: secondary prevention programme (relevant to our review): overweight and obese children, 6th and 7th grades students, consent form signed by parents/tutors Exclusion criteria: pregnant girls |
| Interventions |
Intervention: behavioural: eating behaviour and physical activity change: a combination of 2 prevention school‐based programmes addressed to schoolchildren over 1 year. Primary prevention developed monthly to reduce cookies and sugar‐sweetened beverage consumption, reduction of sedentary activities, increase meal consumption frequency and quality, etc, in all children from 6th and 7th grades. Overweight and obese children invited to participate on a secondary prevention programme in which daily physical activity classes performed by physical education teachers in school facilities Comparator: no intervention |
| Outcomes |
Primary outcome: BMI Secondary outcomes: mean lean and fatty body mass proportions Other outcomes: sedentary activities, sugar‐sweetened beverages intake, cookies intake, fruits intake, physical activity |
| Starting date |
Study start date: February 2014 Study completion date: December 2014 |
| Contact information | Responsible party/principal investigator: Rosely Sichieri, Rio de Janeiro State University |
| Study identifier | NCT02034994 |
| Official title | Combining primary and secondary prevention for reduction of excessive weight gain in school |
| Stated purpose of study | Quote: "The main objective is to evaluate the effects of a multicomponent, school‐based intervention combining change in nutritional behaviors with after school physical activity activities in reducing the excessive weight gain in schoolchildren." |
| Notes | Only part 2 of the study is relevant. Trial record stated, "The recruitment status of this study is unknown because the information has not been verified recently" (accessed 18 August 2016) |
NCT02086851.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: paediatric obesity Enrollment: 110 Inclusion criteria: aged 11‐16 years; BMI > 85th percentile; 1 parent or guardian committed to participate in protocol Exclusion criteria: previous enrolment in IRB3354, IRB3008 or HM11113; underlying genetic, neurological, endocrine or metabolic condition that precludes weight loss with conventional diet and exercise programmes; weight > 400 lb, pregnancy; inability to understand study instructions due to language barrier or mental disability; primary residence outside a 30‐mile radius of study location |
| Interventions |
Intervention: behaviour changing intervention + parent motivational interviewing: 6‐month intensive behaviour changing modification, includes a structured exercise programme, nutrition education and dietary modification and behavioural support. Followed by a 6‐month maintenance phase with monthly booster sessions. Parents participate in 4 dedicated "pre‐treatment" parent psychoeducational sessions Comparator: behaviour changing intervention alone (no parent psychoeducational sessions) |
| Outcomes |
Primary outcome: change in BMI z scores Secondary outcomes: changes in body composition, BMI, insulin sensitivity, blood pressure, serum lipids, fitness measures, electrocardiographic parameters, dietary intake, quality of life scores, BMI of participating parents or body composition of participating parents Other outcome: ‐ |
| Starting date |
Study start date: September 2011 Study completion date: August 2015 |
| Contact information | Responsible party/principal investigator: Virginia Commonwealth University/Edmond P Wickham, MD, MPH |
| Study identifier | NCT02086851 |
| Official title | Impact of a structured parent intervention on weight loss and behavioral change in overweight adolescents enrolled in a lifestyle modification program |
| Stated purpose of study | Quote: "The study will enroll 110 overweight and obese adolescents ages 11‐16 in a lifestyle modification program focusing on dietary modification and exercise. Parents will be randomized into control and motivational interviewing‐based intervention groups. The primary hypothesis is that adolescents whose parents are in the intervention group will have improved compliance, weight loss and health outcomes compared with adolescents whose parents do not receive the intervention." |
| Notes | Trial status completed (according to trial record, accessed 18 August 2016). Contacted author (21 October 2015) for expected publication date |
NCT02228278.
| Trial name or title | Acronym: Text4Fit |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: treatment |
| Participants |
Condition: paediatric obesity Enrollment: 25 Inclusion criteria: aged 13‐17 years; overweight or obese (BMI > 85th percentile); attend or will start attending UF Pediatric Lipid Clinic during the study period; own a mobile telephone that can receive text messages Exclusion criteria: current diagnosis of a psychiatric eating disorder; pregnancy; medical disease that would contraindicate moderate physical activity, as determined by clinician from the Lipid Clinic |
| Interventions |
Intervention: typical clinic visits plus text messages: daily texts for 12 weeks, with fitness and nutrition messages to support their health goals Comparator: current standard care: clinic visits every 3 months with anthropometric assessments and counselling on physical activity and nutrition goals by a healthcare provider, typically with no patient‐provider communication between visits |
| Outcomes |
Primary outcomes: feasibility and acceptability of text message intervention Secondary outcomes: changes from baseline proportion of healthy food choices versus unhealthy choices; changes from baseline time spent doing physical activity; changes from baseline BMI z score Other outcome: ‐ |
| Starting date |
Study start date: April 2015 Study completion date: February 2016 |
| Contact information | Responsible party/principal investigator: University of Florida/Lindsay Thompson, MD |
| Study identifier | NCT02228278 |
| Official title | Text 4 Fit Project: healthcare text messaging as an adjunct to routine care for overweight and obese adolescents enrolled in a pediatric lipid clinic |
| Stated purpose of study | Quote: "to determine if health‐related text messages sent from healthcare providers to overweight and obese adolescents enrolled at a paediatric lipid clinic will result in increased adherence to their nutrition and physical activity goals and improve their weight loss. The study will also assess if the volume of texts per week impacts outcomes." |
| Notes | Trial status completed (according to trial record, accessed 18 August 2016). Contacted author (2 September 2016) for expected publication date, author response: currently drafting a manuscript |
NCT02280772.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel Masking: double blind (participant, carer, investigator, outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: overweight and obesity Enrollment: 96 Inclusion criteria: aged 6‐17 years; overweight (> +1 SD) or obesity (> +2 SD) based on the WHO growth charts/references Exclusion criteria: drug therapy for a chronic disease (including drugs that influence appetite or body weight); type 1 or 2 diabetes mellitus; history of surgical treatment of obesity; participation in another programme for treating obesity during the project or 3 months prior to recruitment, or both; secondary causes of obesity; pregnancy |
| Interventions |
Intervention: glucomannan orally, 3 g/day (in 3 divided doses) for 12 weeks, 3 months' follow‐up. Prior to the intervention, all children will receive dietetic advice and be encouraged to engage in physical activity Comparator: placebo. Maltodextrin orally, 3 g/day (in 3 divided doses) for 12 weeks, 3 months' follow‐up. Prior to the intervention, all children will receive dietetic advice and they will be encouraged to engage in physical activity |
| Outcomes |
Primary outcome: BMI‐for‐age z score difference Secondary outcomes: body composition; BMI‐for‐age z score difference; proportion of participants with dyslipidaemia; proportion of participants with impaired fasting plasma glucose; physical activity; adverse events; blood pressure (systolic and diastolic) Other outcome: ‐ |
| Starting date |
Study start date: April 2015 Study completion date: February 2017 |
| Contact information | Responsible party/principal investigator: Bartlomiej M Zalewski |
| Study identifier | NCT02280772 |
| Official title | Effect of glucomannan supplementation on body weight in overweight and obese children: protocol of a randomised controlled trial |
| Stated purpose of study | Quote: "We aim to systematically evaluate the efficacy of GNN consumption for the management of overweight and obesity in children." |
| Notes | Last updated: 7 April 2015 |
NCT02353637.
| Trial name or title | Acronym: Peds |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: open label Primary purpose: basic science |
| Participants |
Condition: paediatric obesity Enrollment: estimated 45 Inclusion criteria: severely obese adolescents; 13‐17‐year‐old males, 21‐17‐year‐old postmenarcheal females; primary obesity; BMI ≥ 99th percentile for age. Exclusion criteria: current diagnosis of type 2 diabetes mellitus; gall bladder, renal or liver disorders; known eating disorders; known endocrine disorders (e.g. hyperthyroidism or polycystic ovary syndrome); pregnancy; genetic disorder (e.g. Prader‐Willi Syndrome); mental retardation; severe depression; use of any chronic medicine which could impact appetite |
| Interventions |
Intervention: high protein, restricted carbohydrates utilising partial meal replacements diet, behavioural counselling (combination of immediate, short‐term and long‐term individual and family psychotherapy sessions to increase motivation to change diet and physical activity level), dietitian meetings weekly for the first 2 months, then every other week for additional 4 months, followed by monthly meeting for additional 6 months for each patient/family Comparator: ad lib, low glycaemic load diet, behavioural counselling (combination of immediate, short‐term and long‐term individual and family psychotherapy sessions to increase motivation to change diet and physical activity level), dietitian meetings weekly for the first 2 months, then every other week for additional 4 months, followed by monthly meeting for additional 6 months for each patient/family |
| Outcomes |
Primary outcome: improvement in BMI z scores; weight loss; metabolic abnormalities Secondary outcome: ‐ Other outcome: ‐ |
| Starting date |
Study start date: November 2014 Study completion date: June 2016 |
| Contact information | Responsible party/principal investigator: University of Florida/Madeline Joseph, MD |
| Study identifier | NCT02353637 |
| Official title | Partial meal replacements providing high protein, restricted carbohydrates in the treatment of adolescents with severe obesity: a randomized controlled trial |
| Stated purpose of study | Quote: "to investigate, in severely obese adolescents, the effects of a high protein, restricted carbohydrates utilizing partial meal replacements diet (HPRC‐PMR) and compare it to an ad lib, low‐glycemic load (LGL) diet on weight loss, body composition, and bio‐chemical markers of lipid metabolism, insulin resistance, and inflammation over a 12 months period." |
| Notes | Last updated: 24 June 2015 |
NCT02444689.
| Trial name or title | Acronym: EMPower |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity, hypertension Enrollment: 90 Inclusion criteria: elevated blood pressure defined as ≥ 90th percentile or 120/80 mmHg, whichever is lower; overweight or obese; speaks English Exclusion criteria: no smart phone or smart phone data plan; unwilling to send/receive SMS messages or download and use the study applications; neurological impairment/developmental delay; taking anti‐hypertensive medication or medication known to affect blood pressure; prior diagnosis of congenital heart disease or cancer; pregnancy; taking medication with weight gain as adverse effect; taking medications for weight loss/participation in another weight loss programme |
| Interventions |
Intervention: electronic media application: motivation, education and coaching regarding therapeutic lifestyle changes via a smart phone electronic media application Comparator: standard care |
| Outcomes |
Primary outcome: BMI z score Secondary outcomes: daytime ambulatory systolic blood pressure; left ventricular mass index Other outcome: ‐ |
| Starting date |
Study start date: July 2015 Study completion date: July 2018 |
| Contact information | Responsible party/principal investigator: Tammy M Brady, Assistant Professor Pediatric Nephrology, Johns Hopkins University |
| Study identifier | NCT02444689 |
| Official title | EMPower: electronic media powering positive health changes in youth |
| Stated purpose of study | Quote: "The purpose of this study is to evaluate the effectiveness of a technology‐based behavioral Healthy Lifestyle intervention on adiposity (body mass index z‐score), blood pressure (mean daytime ambulatory systolic BP [blood pressure]), and heart size (LVM [left ventricular mass]) in comparison to standard care." |
| Notes |
NCT02615353.
| Trial name or title | Acronym: Preventing Diabetes in Latino Youth |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: prevention of type 2 diabetes mellitus |
| Participants |
Condition: obesity Enrollment: estimated 120 Inclusion criteria: Latino (self‐report); aged 12‐16 years; obese (BMI percentile ≥ 95th percentile for age and gender or BMI ≥ 30 kg/m2); prediabetic (fasting glucose ≥ 100 mg/dL or 2‐hour post‐oral glucose tolerance test glucose ≥ 120 mg/dL, or both) Exclusion criteria: taking medication(s) or diagnosed with a condition that influences carbohydrate metabolism, physical activity, cognition, or a combination; type 2 diabetes (fasting glucose ≥ 126 mg/dL or 2‐hour glucose ≥ 200 mg/dL; recent hospitalisation (previous 2 months); currently enrolled in (or within previous 6 months) a formal weight loss programme; diagnosed depression or other condition that may impact quality of life |
| Interventions |
Intervention: culturally grounded intensive behaviour changing intervention guided by social cognitive theory over 6 months Comparator: usual care control |
| Outcomes |
Primary outcome: Secondary outcomes: improvements in glucose tolerance; reductions in 2‐hour glucose following an oral glucose tolerance test Other outcomes: quality of life; body composition; decrease in fat mass and increase in lean tissue mass by dual‐energy X‐ray absorptiometry |
| Starting date |
Study start date: January 2016 Study completion date: December 2021 |
| Contact information | Responsible party/principal investigator: Arizona State University |
| Study identifier | NCT02615353 |
| Official title | Preventing diabetes in Latino youth |
| Stated purpose of study | Quote: "To date, no diabetes prevention studies have been conducted in obese Latino youth with prediabetes, a highly vulnerable and underserved group. Therefore, investigators propose a randomized‐controlled trial to test the short‐term (6‐month) and long‐term (12‐month) efficacy of a culturally‐grounded, lifestyle intervention, as compared to usual care, for improving glucose tolerance and reducing diabetes risk in 120 obese Latino adolescents with prediabetes." |
| Notes | Last updated: 17 April 2016 |
NCT02687516.
| Trial name or title | Acronym: FABO |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: cross‐over assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: ‐ Enrollment: 120 Inclusion criteria: families of children aged 8‐16 years attending an outpatient's obesity clinic; BMI ≥ 35 kg/m2 or BMI ≥ 30 kg/m2 with obesity‐related comorbidity; both the child and at least 1 of the parents agrees to actively participate in the treatment Exclusion criteria: severe somatic or psychiatric illness that makes adherence to the treatment programme impossible; somatic conditions, syndromes or medications that lead to pathological weight gain; participation in other obesity treatment programmes |
| Interventions |
Intervention: 17 weekly manualised sessions with subsequent follow‐up every 3 month for 2 years Comparator: treatment as usual, default treatment at the obesity clinic |
| Outcomes |
Primary outcomes: BMI; waist circumference; body composition Secondary outcomes: blood samples; blood pressure; activity level/inactivity; sleep pattern; psychological measures; food records Other outcomes: parenting scale; barriers to treatment participation scale; treatment acceptability |
| Starting date |
Study start date: February 2014 Study completion date: December 2018 |
| Contact information | Responsible party/principal investigator: Petur B Juliusson, MD/PhD, University of Bergen |
| Study identifier | NCT02687516 |
| Official title | Treatment of severely obese children and adolescents in common health care settings: an effectiveness study employing "Family‐based Behavioral Social Facilitation Treatment." |
| Stated purpose of study | Quote: "The purpose of the FABO study is to evaluate the effect of family‐based behavioral weight loss treatment (FBBT) compared with the effect of today's standard treatment given to children and adolescents suffering from obesity at the Obesity Outpatient Clinic (OOC), Haukeland University Hospital." |
| Notes | Last updated: 19 February 2016 |
NCT02711488.
| Trial name or title | Acronym: PAAPAS‐DC |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcome assessor) Primary purpose: primary and secondary prevention (secondary prevention programme relevant here) |
| Participants |
Condition: obesity Enrollment: 3000 Inclusion criteria: both prevention programmes: 5th and 6th grades students; consent form signed by parents/tutors; secondary prevention programme: overweight and obese children. Exclusion criteria: pregnancy |
| Interventions |
Intervention: encouraging students to change their eating habits and food consumption over 9 months (from March to November). Monthly 1‐hour sessions in the classroom will be given by the class teacher, and includes playing games, staging theatre sketches, watching movies and puppet shows, and writing and drawing contests. Activities designed to discourage students from consuming sugar‐sweetened beverages as well as getting them to replace snacks, particularly processed foods (especially cookies) with fresh fruit or healthy homemade food. To reinforce the messages of the in‐class nutritional sessions, a set of messages will be sent to the families in the form of illustrated booklets and recipes. The secondary prevention strategy at households will be developed for those with excessive weight. The family will receive additional motivation to change these behaviours, using the community health agents as the encourager Comparator: no intervention |
| Outcomes |
Primary outcome: BMI Secondary outcome: body composition Other outcomes: adherence to the protocol at household level; physical activity; food intake |
| Starting date |
Study start date: March 2016 Study completion date: December 2016 |
| Contact information | Responsible party/principal investigator: Rosely Sichieri, MD, PhD. Full Professor of Epidemiology, Rio de Janeiro State University |
| Study identifier | NCT02711488 |
| Official title | Managing adolescent obesity at local level by combining primary and secondary intervention |
| Stated purpose of study | Quote: "to develop, implement and evaluate a prevention program for obesity among adolescents in Brazil combining the primary care health system implemented in the country in recent decades with primary prevention at schools." |
| Notes | Only the secondary prevention programme relevant for this review. Study is not yet open for participant recruitment (last updated: 11 March 2016) |
NCT02745795.
| Trial name or title | Acronym: IMAGINE |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: double blind (subject, outcome assessor) Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 83 Inclusion criteria: aged 14‐19 years; BMI ≥ 85th percentile according to WHO charts (de Onis 2010). Exclusion criteria: recent weight loss ≥ 10% of body weight; pregnancy; breastfeeding; endocrine disease; present therapy with antidepressant or hypoglycaemic drugs; present treatment for food behaviour disease or depression; cognitive impairment of the student or his/her legal tutor |
| Interventions |
Intervention: motivational interview, group submitted to 3 face‐to‐face interviews using motivational interview techniques to elicit motivation for adhesion to a diet and physical activity plan intended to lose weight. The interviews will be done at schools with 3‐month intervals Comparator: conventional interview, group submitted to 3 face‐to‐face interviews without using motivational interview techniques to elicit motivation for adhesion to a diet and physical activity plan intended to lose weight. The interviews will be done at schools with 3‐month intervals |
| Outcomes |
Primary outcomes: motivation scores to adhere to a diet and physical activity plan; motivation scores assessed by 2 self‐report confidential paper questionnaires (self‐regulation and perceived competence questionnaire to begin or maintain a healthy diet and self‐regulation and perceived competence questionnaire to begin or maintain regular physical exercise) Secondary outcomes: self‐concept score; quality of life score; depressive symptoms score; anxiety symptoms; abdominal circumference; blood pressure; weight; height Other outcome: |
| Starting date |
Study start date: October 2015 Study completion date: May 2016 |
| Contact information | Responsible party/principal investigator: Maria do Céu Machad, Director of the Department of Pediatry of Centro Hospitalar Lisboa Norte, Chair of Pediatry at Medicine School of the University of Lisbon, Centro Hospitalar Lisboa Norte |
| Study identifier | NCT02745795 |
| Official title | Efficacy of motivational interview in the treatment of obesity and overweight in adolescents (IMAGINE) |
| Stated purpose of study | Quote: "The objective of the study is to investigate the efficacy of motivational interview intervention with adolescent students at a school environment on the adhesion to a therapeutic plan to lose weight." |
| Notes | Contacted author (12 January 2017) for data/anticipated study completion data |
NCT02794090.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 37 Inclusion criteria: aged 5‐14 years; patients at outpatient paediatric centre; parents attended at least 4 of 7 meetings in a parental/education group Exclusion criteria: obesity‐related syndromes; non‐Swedish speaking families |
| Interventions |
Intervention: telephone consultation each month except for summer holidays for 18 months. The treating nurse communicated with 1 of the parents Comparator: usual care according to regular treatment routines at the clinic for 18 months. The child and parent(s) at regular visits to the nurse at the clinic |
| Outcomes |
Primary outcome: BMI‐SDS Secondary outcomes: working time required for the healthcare personnel; families experience of the treatment Other outcome: ‐ |
| Starting date |
Study start date: May 2007 Study completion date: January 2013 |
| Contact information | Responsible party/principal investigator: Pernilla Danielsson, PhD paediatric nurse, Karolinska Institutet |
| Study identifier | NCT02794090 |
| Official title | Exclusive telephone coaching in maintaining weight loss ‐ an randomized controlled trial of childhood obesity treatment |
| Stated purpose of study | Quote: "This study evaluates if usual physical care visits to an outpatient pediatric clinic can be replaced with more frequent and shorter Telephone coaching Contacts during 18 months." |
| Notes | Last updated: 8 June 2016. Contacted author (13 January 2017) for further data |
NTR5676.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel Masking: none Primary purpose: ‐ |
| Participants |
Condition: overweight, obesity Enrollment: 1000 Inclusion criteria: aged 11‐13 years (1st grade high school students) in Dutch high schools Exclusion criteria: unable to engage in physical activity |
| Interventions |
Intervention: 30% increase in absolute strength exercises in physical education classes, once per month a motivational lesson given by a mentor and for the first 5 months an additional online motivational lesson (once per month) Comparator: ‐ |
| Outcomes |
Primary outcome: body composition Secondary outcomes: daily physical activity, social cognitive determinants; BMI z score, strength Other outcome: ‐ |
| Starting date |
Study start date: January 2015 Study completion date: January 2017 |
| Contact information | Responsible party/principal investigator: Gill Ten Hoor, Maastricht University Medical Centre (MUMC+) |
| Study identifier | NTR5676 |
| Official title | The focus on strength programme: an innovative strength‐based physical activity intervention to improve body composition and to stimulate physical activity in adolescents (12‐18 years) with overweight or obesity. |
| Stated purpose of study | Quote: "a one year combined strength‐ and motivational program for high schools improve body composition and motivation in overweight and obese youngsters." |
| Notes | Status: open, patient inclusion (date registered 8 February 2016) |
Ramalho 2016.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: effectiveness and cost‐effectiveness trial Allocation: randomised Intervention model: parallel assignment Masking: ‐ Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: 120 Inclusion criteria: aged 13‐18 years; BMI ≥ 25 kg/m2 Exclusion criteria: ‐ |
| Interventions |
Intervention: Internet‐based programme intervention + treatment as usual. Programme based on cognitive behavioural therapy. 9‐month, 2‐phase programme for weight loss and maintenance. Phase 1: weight loss through weight control, healthy eating and life‐style strategies. Phase 2: weight maintenance through weight maintenance skills Comparator: treatment as usual |
| Outcomes |
Outcomes: weight; eating related variables; physical activity; costs Primary outcome: unclear Secondary outcome: unclear Other outcomes: disorder eating behaviour; intuitive eating; physical activity; body image; impulsivity |
| Starting date |
Study start date: Study completion date: |
| Contact information | Responsible party/principal investigator: |
| Study identifier | ‐ |
| Official title | Taking the route back: new technologies for overweight and obesity treatment in childhood and adolescence ‐ study protocol |
| Stated purpose of study | Quote: "a randomized controlled trial to examine the effectiveness and cost‐effectiveness of an internet‐based program intervention as supplementary tool for weight loss treatment in overweight and obese adolescents." |
| Notes | Contacted author (16 January 2017) for results/anticipated publication date |
RBR‐38p23s.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel (3 arms) Masking: open label Primary purpose: treatment |
| Participants |
Condition: obesity Enrollment: target 39 Inclusion criteria: aged 10‐19 years; willingness to participate in all programme activities BMI for age and gender > 95th percentile (CDC 2000) Exclusion criteria: psychological disorders; use of medications that could interfere in the variables; physical difficulties that impeded the development of all activities |
| Interventions |
Interventions: Complex intervention: 20 meetings with the adolescents (16 psychological intervention twice per week, 4 monthly nutritional orientations). 36 sessions of physical exercises 3 times per week. 9 meetings with parents that include nutritional meetings and physical education Simple intervention: 4 monthly meetings with adolescents for nutritional orientation and 36 sessions of physical exercises. 9 meetings with the parents with a psychologist, nutritionist and a physical educator. Comparator: meetings with a nutritionist and a physical educator for the adolescents and parents and physical exercises sessions for adolescents |
| Outcomes |
Primary outcomes: BMI, social competence, behavioural change, academic performance Secondary outcomes: intrinsic motivation and health‐related outcomes. Other outcome: |
| Starting date |
Study start date: March 2010 Study completion date: March 2011 |
| Contact information | Responsible party/principal investigator: University Federal de São Paulo, Brazil/Graziela Sapienza |
| Study identifier | RBR‐38p23s |
| Official title | Multifocal intervention in obese adolescents: social competence, behavior problems, academic performance and weight reduction |
| Stated purpose of study | |
| Notes |
Spieker 2015.
| Trial name or title | Acronym: POMC |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel assignment Masking: single blind (assessors at baseline) Primary purpose: prevention |
| Participants |
Condition: obesity and overweight Enrollment: 48 Inclusion criteria: female military dependents; aged 12‐17 years; at high risk for excess weight gain (BMI ≥ 85th percentile) who report lifetime loss of control eating or ≥ 2 current indicators of loss of control eating (e.g. eating in response to negative affect, feelings of guilt or shame around eating) Exclusion criteria: presence of a chronic major medical illness (e.g. renal, hepatic, gastrointestinal, endocrinological); documented, obesity‐related medical complication requiring a more intensive intervention approach; psychiatric conditions (e.g. psychosis, suicidality) that would impede programme participation; current weight loss treatment; current weight‐affecting medication usage; pregnancy (current or recent); current breastfeeding |
| Interventions |
Intervention:interpersonal psychotherapy‐based intervention: 1 initial 1.5‐hour individual session, and 12 weekly 90‐minute group sessions that take place after school hours. The interpersonal inventory interview to identify the strengths, problems and goals of the adolescent. Information used to link problems to loss of control eating and other disturbed eating attitudes or behaviours. Sessions delivered over different phases. A psychoeducation phase (sessions 1‐3), such as education about the predictors of excess weight gain, outlining problem areas and discussing the link between feelings and interpersonal interactions. Intermediate phase (sessions 4‐9) where group members practice skills such as hypothetical scenarios and role‐plays. Training on how interpersonal strategies can be applied to different, specific relationships in the adolescents' lives, particularly relationships that are linked to loss of control and emotionally induced eating. A termination phase (sessions 10‐12) the group reviews improvements in loss of control eating and related symptoms, with a focus on identifying possible warning signs of a return or increase in problematic eating and knowing when to seek help. Finally, group members are encouraged to continue the interpersonal work on their own Comparator:health education control: receives a workbook on how to live a healthier life, discussing topics unrelated to eating. Participants complete 4 monthly 90‐minute group meetings during after school hours to discuss the information presented in the workbook. The workbook will serve as a guide for teens to improve their self‐image, build friendships, resist peer pressure and engage in goal‐setting |
| Outcomes |
Primary outcomes: % participants who do not gain weight during follow‐up; total weight change as markers of outcomes Secondary outcome: not clearly stated Other outcomes: eating disorder examination; Schedule for Affective Disorders and Schizophrenia for School‐Age Children; Social Adjustment Scale ‐ Self‐Report; Family Adaptation and Cohesion, Evaluation Scales Inventory; Beck Depression Inventory State‐Trait Anxiety Inventory for Children ‐ a trait scale; Life Events and Coping Inventory; Eating Disorder Diagnostic Scale; Treatment Acceptability Questionnaire; Inventory of Parent and Peer Attachment; Family Resilience Assessment Scale; Perceived Stress Scale; The Emotional Eating Scale adapted for Children. Physical assessments: height and weight; blood pressure; waist circumference; blood pressure; high‐density lipoprotein cholesterol; triglycerides; glucose; insulin; glycated haemoglobin. Parent questionnaires: Parent Stress Index ‐ Short Form; Child Behavior Checklist |
| Starting date |
Study start date: ‐ Study completion date: ‐ |
| Contact information | Responsible party/principal investigator: research support provided by a grant from the Uniformed Services University (USUHS72NC‐01) to Tracy Sbrocco and intramural support from NICHD (Z1aHD00641) to Jack A Yanovski, a Commissioned Officer in the United States Public Health Service |
| Study identifier | ‐ |
| Official title | Preventing Obesity in the Military Community (POMC): the development of a clinical trials research network |
| Stated purpose of study | Quote: "IPT will decrease loss of control eating and reduce these adverse outcomes. A secondary hypothesis of this study is that girls in the IPT group who maintain their weight or experience weight loss will demonstrate an improvement in components of the metabolic syndrome at follow‐up visits." |
| Notes | Quote: "We use percentage of participants who do not gain weight during follow‐up and total weight change as markers of our study outcomes. We hypothesize that POMC [Preventing Obesity in Military Communities] intervention programs will reduce weight gain beyond the control comparison programs in the period following study participation." |
TCTR20130515001.
| Trial name or title | Acronym: ‐ |
| Methods |
Type of study: ‐ Allocation: randomised Intervention model: parallel Masking: single blind (participants) Primary purpose: prevention |
| Participants |
Condition: overweight Enrollment: target 320 Inclusion criteria: aged 13‐18 years; secondary school students; BMI for age and gender 85th percentile (WHO 2007) Exclusion criteria: musculoskeletal disorder; cardiovascular disease |
| Interventions |
Intervention: behavioural weight reduction programme (no details) Comparator: no intervention |
| Outcomes |
Primary outcome: BMI Secondary outcomes: intrinsic motivation; health‐related outcomes Other outcome: ‐ |
| Starting date |
Study start date: July 2013 Study completion date: December 2013 |
| Contact information | Responsible party/principal investigator: Preventive Medicine and Social Department Faculty of medicine Chulalongkorn Thailand/Kanlayanee No‐in |
| Study identifier | TCTR20130515001 |
| Official title | Effectiveness of weight reduction program on intrinsic motivation and health related outcomes in overweight secondary school students |
| Stated purpose of study | |
| Notes | Trial status completed (according to trial record, accessed 18 August 2016). Contacted author (2 September 2016) for expected publication date, author response: manuscript amendments and still not published |
BMI: body mass index; CDC: Centers for Disease Control and Prevention; DEBQ‐C: Dutch Eating Behaviour Questionnaire for Children; FFQ: Food Frequency Questionnaire; PAQ‐C: Physical Activity Questionnaire for Older Children; SD: standard deviation; SDS: standard deviation score; WHO: World Health Organization.
Differences between protocol and review
Given the rapid growth in the treatment of child and adolescent obesity, the original review was split into six separate reviews, with a specific intervention and age focus.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in adolescents aged 12 to 17 years.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in children aged 5 to 11 years.
Diet, physical activity and behavioural interventions for the treatment of overweight or obesity in infants aged 0 to 4 years.
Drug interventions for the treatment of obesity in children and adolescents.
Parent‐only interventions for childhood overweight or obesity.
Surgery for the treatment of obesity in children and adolescents.
For behaviour changing interventions, we included only randomised controlled trials that were specifically designed to treat obesity in children and observed participants for a minimum of six months. The rationale for introducing this criterion arose from the belief that many interventions appear to be effective in the short term (up to three months), but not in the long term (Glenny 1997). It seemed to be more important to evaluate the longer‐term effects of treatments, as this would provide a more valuable indication of effectiveness, given the chronic nature of obesity.
We did not present prediction intervals as originally described for the suite of reviews for consistency as the other reviews did not do this either in consultation with the review group.
We did not perform the following sensitivity analyses.
Restricting the analysis taking into account risk of bias, as specified in the section Assessment of risk of bias in included studies section, but we did look at the effects of attrition bias and other potential bias introduced by analysis of cluster randomised controlled trials.
Restricting the analysis to very long or large studies to establish how much they dominated the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, funding source and country.
This is in line with other reviews in this series.
Contributions of authors
Lena Al‐Khudairy (LA‐K): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future updates.
Emma Loveman (EL): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future updates.
Jill Colquitt (JC): acquiring trial reports, trial selection and data extraction.
Emma Mead (EM): acquiring trial reports, trial selection, data extraction and screening trial alert.
Rebecca E Johnson (RJ): trial selection and data extraction.
Hannah Fraser (HF): trial selection and data extraction.
Omotayo Joan Olajide (JO): trial selection and data extraction.
Marie Murphy (MM): data extraction.
Rochelle Velho (RV): data extraction.
Claire O'Malley (CO): trial selection.
Liane Azevedo (LA): trial selection.
Louisa J Ells (LE): trial selection, review of drafts and update draft.
Maria‐Inti Metzendorf (MIM): search strategy development and review of drafts.
Karen Rees (KR): trial selection, data extraction, data analysis, data interpretation, review of drafts and future updates.
Sources of support
Internal sources
University Medical Center, Groningen, Netherlands.
The Children's Hospital at Westmead, Sydney, Australia.
Centre for Food Physical Activity and Obesity Research, University of Teesside, UK.
The Wolfson Research Institute, University of Durham, UK.
-
Australian National Health & Medical Research Council, Australia.
Postgraduate Research Scholarship for Ms Shrewsbury
External sources
No sources of support supplied
Declarations of interest
LA‐K: none known.
EL: none known.
JC: none known.
EM: none known.
RJ: none known.
HF: none known.
JO: none known.
MM: none known.
RV: none known.
CO: none known.
LA: none known.
LE: Louisa Ells is seconded to Public Health England two days per week but undertook this review within her role as Reader in Public Health and Obesity at Teesside University.
MIM: none known.
KR: none known.
New
References
References to studies included in this review
Bean 2014 {published data only}
- Bean M, Mazzeo S, Stern M, Bowen D, Ingersoll K. A values‐based motivational interviewing (MI) intervention for pediatric obesity: study design and methods for MI values. Contemporary Clinical Trials 2011;32:667‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean M, Powell P, Quinoy A, Ingersoll K, Wickham E, Mazzeo S. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: results from the MI Values randomized controlled trial. Pediatric Obesity 2015;10(2):118‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00167830. Barriers to treatment in obese adolescents. clinicaltrials.gov/ct2/show/NCT00167830 Date first received: 9 September 2005.
Boodai 2014 {published data only}
- Boodai S, McColl J, Reilly J. National Adolescent Treatment Trial for Obesity in Kuwait (NATTO): project design and results of a randomised controlled trial of a good practice approach to treatment of adolescent obesity in Kuwait. Trials 2014;15:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37457227. National adolescent treatment trial for obesity in Kuwait. www.isrctn.com/ISRCTN37457227 Date first assigned: 1 December 2009.
Brennan 2013 {published data only}
- ACTRN12610000111077. The efficacy of a cognitive behavioural lifestyle intervention in improving body composition and cardiovascular fitness in overweight and obese adolescents. A wait‐listed randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000111077 Date first registered 3 February 2010.
- Brennan L, Walkley J, Fraser S, Greenway K, Wilks R. Motivational interviewing and cognitive behaviour therapy in the treatment of adolescent overweight and obesity: study design and methodology. Contemporary Clinical Trials 2008;29(3):359‐75. [DOI] [PubMed] [Google Scholar]
- Brennan L, Walkley J, Wilks R, Fraser S, Greenway K. Physiological and behavioural outcomes of a randomised controlled trial of a cognitive behavioural lifestyle intervention for overweight and obese adolescents. Obesity Research & Clinical Practice 2013;7(1):e23‐e41. [DOI] [PubMed] [Google Scholar]
- Brennan L, Wilks R, Walkley J, Fraser S, Greenway K. Treatment acceptability and psychosocial outcomes of a randomised controlled trial of a cognitive behavioural lifestyle intervention for overweight and obese adolescents. Behaviour Change 2012;29(01):36‐62. [DOI] [PubMed] [Google Scholar]
Brownell 1983 {published data only}
- Brownell K, Kelman J, Stunkard A. Treatment of obese children with and without their mothers: changes in weight and blood pressure. Pediatrics 1983;71(4):515‐23. [PubMed] [Google Scholar]
Carraway 2014 {published data only}
- Carraway M. Project MENTOR+: Mentor‐led Exercise with Cognitive‐behavioral Therapy to Improve Perceived Competence, Reduce Social Anxiety, and Increase Physical Activity in Overweight Adolescents [Doctorate of Philosophy in Clinical Psychology Thesis]. Greenville (NC): East Carolina University, 2014. [Google Scholar]
Carrel 2005 {published data only}
- Carrel A, Clark R, Peterson S, Nemeth B, Sullivan J, Allen D. Improvement of fitness, body composition, and insulin sensitivity in overweight children in a school‐based exercise program: a randomized, controlled study. Archives of Pediatrics & Adolescent Medicine 2005;159(10):963‐8. [DOI] [PubMed] [Google Scholar]
Chandra 1968 {published data only}
- Chandra R. Obesity in childhood ‐ a clinical trial of low‐calorie "limical". Indian Journal of Pediatrics 1968;35(24):23‐6. [DOI] [PubMed] [Google Scholar]
Christie 2011 {published data only}
- Christie D, Hudson L, Costa S, Mathiot A, Holt R Kinra S, Kessel A, Wong I, et al. Effects of a motivational lifestyle intervention (the Healthy Eating and Lifestyle Programme (HELP)) on metabolic outcomes in obese adolescents: findings from a randomized controlled trial. Pediatric Diabetes 2015;16:45. [Google Scholar]
- Christie D, Hudson L, Costa S, Mathiot A, Holt R, Kinra S, et al. RCT of a motivational lifestyle intervention (the Healthy Eating and Lifestyle Programme (HELP)) for obese young people. Archives of Disease in Childhood 2015;100:A2‐A2. [Google Scholar]
- Christie D, Hudson L, Kinra S, Nazareth I, Holt R, Kessel A, et al. Does a motivational lifestyle intervention (the Healthy Eating and Lifestyle Programme (HELP) work for obese young people?. Journal of Adolescent Health 2015;2:S19. [Google Scholar]
- Christie D, Hudson L, Mathiot A, Cole T, Karlsen S, Kessel A, et al. Assessing the efficacy of the Healthy Eating and Lifestyle Programme (HELP) compared with enhanced standard care of the obese adolescent in the community: study protocol for a randomized controlled trial. Trials 2011;12:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S, Hudson L, Christie D, Kinra S, Mathiot A, Wong I, et al. Fitness may be more important for cardiovascular health than adiposity in adolescent girls. Obesity Reviews 2014;15:108. [Google Scholar]
- ISRCTN99840111. HELP trial: healthy eating lifestyle programme for adolescents. www.isrctn.com/ISRCTN99840111 Date first assigned: 21 January 2011.
Daley 2005 {published data only}
- Daley A, Copeland R, Wright N, Roalfe A, Wales J. Exercise therapy as a treatment for psychopathologic conditions in obese and morbidly obese adolescents: a randomized, controlled trial. Pediatrics 2006;118(5):2126‐34. [DOI] [PubMed] [Google Scholar]
- Daley A, Copeland R, Wright N, Wales J. Protocol for: Sheffield Obesity Trial (SHOT): a randomised controlled trial of exercise therapy and mental health outcomes in obese adolescents [ISRCNT83888112]. BMC Public Health 2005;5(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCNT83888112. The psychological effects of exercise therapy in clinically obese children. www.isrctn.com/ISRCTN83888112 Date first assigned: 17 August 2005.
Debar 2012 {published data only}
- DeBar L, Stevens V, Perrin N, Wu P, Pearson J, Yarborough B, et al. A primary care‐based, multicomponent lifestyle intervention for overweight adolescent females. Pediatrics 2012;129(3):e611‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01068236. Primary care treatment for overweight adolescent females (SHINE). clinicaltrials.gov/ct2/show/NCT01068236 Date first received: 11 February 2010.
Ebbeling 2003 {published data only}
- Ebbeling C, Leidig M, Sinclair K, Hangen J, Ludwig D. A reduced‐glycemic load diet in the treatment of adolescent obesity. Archives of Pediatrics & Adolescent Medicine 2003;157(8):773‐9. [DOI] [PubMed] [Google Scholar]
Ebbeling 2012 {published data only}
- Ebbeling C, Feldman H, Chomitz V, Antonelli T, Gortmaker S, Osganian S, et al. A randomized trial of sugar‐sweetened beverages and adolescent body weight. New England Journal of Medicine 2012;367(15):1407‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00381160. Reducing sugar‐sweetened beverage consumption in overweight adolescents (BASH). clinicaltrials.gov/show/NCT00381160 Date first received: 25 September 2006.
Ford 2010 {published data only}
- Ford A, Bergh C, Södersten P, Sabin M, Hollinghurst S, Hunt L, et al. Treatment of childhood obesity by retraining eating behaviour: randomised controlled trial. BMJ 2010;340:b5388. [DOI] [PubMed] [Google Scholar]
- Galhardo J, Hunt L, Lightman S, Sabin M, Bergh C, Sodersten P, et al. Normalizing eating behavior reduces body weight and improves gastrointestinal hormonal secretion in obese adolescents. Journal of Clinical Endocrinology & Metabolism 2011;97(2):E193‐201. [DOI] [PubMed] [Google Scholar]
- NCT00407420. Can a novel treatment using "Mandometer®" technology improve weight loss in a childhood obesity clinic?. clinicaltrials.gov/ct2/show/NCT00407420 Date first received: 1 December 2006.
Gourlan 2013 {published data only}
- Gourlan M, Sarrazin P, Trouilloud D. Motivational interviewing as a way to promote physical activity in obese adolescents: a randomised‐controlled trial using self‐determination theory as an explanatory framework. Psychology & Health 2013;28(11):1265‐86. [DOI] [PubMed] [Google Scholar]
Grey 2009 {published data only}
- Grey M, Berry D, Davidson M, Galasso P, Gustafson E, Melkus G. Preliminary testing of a program to prevent type 2 diabetes among high‐risk youth. Journal of School Health 2004;74(1):10‐5. [DOI] [PubMed] [Google Scholar]
- Grey M, Jaser S, Holl M, Jefferson V, Dziura J, Northrup V. A multifaceted school‐based intervention to reduce risk for type 2 diabetes in at‐risk youth. Preventive Medicine 2009;49(2):122‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofsteenge 2014 {published data only}
- Hofsteenge G, Chinapaw M, Delemarre‐van de Waal H, Weijs P. Long‐term effect of the Go4it group treatment for obese adolescents: a randomised controlled trial. Clinical Nutrition 2014;33(3):385‐91. [DOI] [PubMed] [Google Scholar]
- Hofsteenge G, Chinapaw M, Weijs P, Tulder M, Delemarre‐van de Waal H. Go4it; study design of a randomised controlled trial and economic evaluation of a multidisciplinary group intervention for obese adolescents for prevention of diabetes mellitus type 2. BMC Public Health 2008;8:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge G, Weijs P, Delemarre‐van de Waal H, Wit M, Chinapaw M. Effect of the Go4it multidisciplinary group treatment for obese adolescents on health related quality of life: a randomised controlled trial. BMC Public Health 2013;13:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR691. GO4IT ‐ the effectiveness of a group intervention programme on the lifestyle of adolescents with obesity. www.trialregister.nl/trialreg/admin/rctview.asp?TC=691 Date first registered: 19 July 2006.
- Weijs P, Hofsteenge A, Chinapaw M, Delemarre‐van de Waal H. Long‐term effect of the Go4it group treatment for obese adolescents: a randomised controlled trial. Clinical Nutrition, Supplement 2012;7:130. [DOI] [PubMed] [Google Scholar]
Jelalian 2016 {published data only}
- Jelalian E, Jandasek B, Wolff J, Seaboyer L, Jones R, Spirito A. Cognitive‐behavioral therapy plus healthy lifestyle enhancement for depressed, overweight/obese adolescents: results of a pilot trial. Journal of Clinical Child & Adolescent Psychology 2016 Jun 16 [Epub ahead of print]:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01128764. Integrated treatment for comorbid depression and obesity in adolescents. clinicaltrials.gov/ct2/show/NCT01128764 Date first received: 21 May 2010.
Jiang 2005 {published data only}
- Jiang J, Xia X, Greiner T, Lian G, Rosenqvist U. A two year family based behaviour treatment for obese children. Archives of Disease in Childhood 2005;90(12):1235‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2013 {published data only}
- Kong A, Sussman A, Yahne C, Skipper B, Burge M, Davis S. School‐based health center intervention improves body mass index in overweight and obese adolescents. Journal of Obesity 2013;2013:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2014 {published data only}
- Kong A, Choi K, Chan R, Lok K, Ozaki R, Li A, et al. A randomized controlled trial to investigate the impact of a low glycemic index (GI) diet on body mass index in obese adolescents. BMC Public Health 2014;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01278563. A trial to investigate the impact of a low glycemic index (GI) diet on body mass index and obesity related cardiovascular and hormonal factors in Chinese adolescents. clinicaltrials.gov/ct2/show/NCT01278563 Date first received: 18 January 2011.
Love‐Osborne 2014 {published data only}
- Love‐Osborne K, Fortune R, Sheeder J, Federico S, Haemer M. School‐based health center‐based treatment for obese adolescents: feasibility and body mass index effects. Childhood Obesity 2014;10:424‐31. [DOI] [PubMed] [Google Scholar]
Luna‐Pech 2014 {published data only}
- Luna‐Pech J, Torres‐Mendoza B, Luna‐Pech J, Garcia‐Cobas C, Navarrete‐Navarro S, Elizalde‐Lozano A. Normocaloric diet improves asthma‐related quality of life in obese pubertal adolescents. International Archives of Allergy & Immunology 2014;163:252‐8. [DOI] [PubMed] [Google Scholar]
NCT00132132 {published data only}
- NCT00132132. Study of impact of behavioral intervention ‐ exercise, nutrition, education ‐ on body mass index (BMI). clinicaltrials.gov/ct2/show/study/NCT00132132 Date first received: 17 August 2005.
NCT00807560 {published data only}
- NCT00807560. Parent‐based treatment for pediatric overweight (PO). clinicaltrials.gov/ct2/show/NCT00807560 Date first received: 11 December 2008.
Nguyen 2012 {published data only}
- ACTRN12606000175572. Community‐based weight management of overweight and obese adolescents: a randomised controlled trial of extended therapeutic contact vs recommended care with primary outcomes body mass index standard deviation score and waist circumference standard deviation score. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12606000175572 Date first registered: 15 May 2006.
- Kornman K, Shrewsbury V, Chou A, Nguyen B, Lee A, O'Connor J, et al. Electronic therapeutic contact for adolescent weight management: the Loozit study. Telemedicine Journal & E‐Health 2010;16:678‐85. [DOI] [PubMed] [Google Scholar]
- Nguyen B, Shrewsbury V, Lau C, O'Connor J, Steinbeck K, Hill A, et al. Adolescent and parent views of an adolescent weight management program: lessons from the Loozit randomised controlled trial. Obesity Research and Clinical Practice 2012;6:56. [Google Scholar]
- Nguyen B, Shrewsbury V, O'Connor J, Lau C, Steinbeck K, Hill A, Baur L. A process evaluation of an adolescent weight management intervention: findings and recommendations. Health Promotion International 2015;30(2):201‐12. [DOI] [PubMed] [Google Scholar]
- Nguyen B, Shrewsbury V, O'Connor J, Steinbeck K, Hill A, Shah S, et al. Two‐year outcomes of an adjunctive telephone coaching and electronic contact intervention for adolescent weight‐loss maintenance: the Loozit randomized controlled trial. International Journal of Obesity 2013;37(3):468‐72. [DOI] [PubMed] [Google Scholar]
- Nguyen B, Shrewsbury V, O'Connor J, Steinbeck K, Lee A, Hill A, et al. Twelve‐month outcomes of the Loozit randomized controlled trial: a community‐based healthy lifestyle program for overweight and obese adolescents. Archives of Pediatrics & Adolescent Medicine 2012;166(2):170‐7. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, O'Connor J, Steinbeck K, Stevenson K, Lee A, Hill A, et al. A randomised controlled trial of a community‐based healthy lifestyle program for overweight and obese adolescents: the Loozit® study protocol. BMC Public Health 2009;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2016 {published data only}
- Norman G, Huang J, Davila E, Kolodziejczyk J, Carlson J, Covin J, et al. Outcomes of a 1‐year randomized controlled trial to evaluate a behavioral 'stepped‐down' weight loss intervention for adolescent patients with obesity. Pediatric Obesity 2016;11(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakpour 2015 {published data only}
- NCT02180802. Parental involvement improves the effect of motivational interviewing on weight loss in obese adolescents: a randomized controlled trial study. clinicaltrials.gov/ct2/show/NCT02180802 Date first received: 1 July 2014.
- Pakpour A, Gellert P, Dombrowski S, Fridlund B. Motivational interviewing with parents for obesity: an RCT. Pediatrics 2015;135(3):e644‐52. [DOI] [PubMed] [Google Scholar]
Patrick 2013 {published data only}
- NCT00412165. PACE‐iDP: an intervention for youth at risk for diabetes. clinicaltrials.gov/ct2/show/NCT00412165 Date first received: 13 December 2006.
- Patrick K, Norman G, Davila E, Calfas K, Raab F, Gottschalk M, et al. Outcomes of a 12‐month technology‐based intervention to promote weight loss in adolescents at risk for type 2 diabetes. Journal of Diabetes Science and Technology 2013;7(3):759‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patsopoulou 2017 {published data only}
- NCT02653508. Evaluating the efficacy of the Feeding Exercise Trial in Adolescents (FETA Project) in Central Greece (FETA). clinicaltrials.gov/show/NCT02653508 Date first received: 17 December 2015.
- Patsopoulou A, Tsimtsiou Z, Katsioulis A, Malissiova E, Rachiotis G, Hadjichristodoulou C. Evaluating the efficacy of the Feeding Exercise Randomized Trial in overweight and obese adolescents. Childhood Obesity 2017;13(2):128‐37. [DOI] [PubMed] [Google Scholar]
- Patsopoulou A, Tsimtsiou Z, Katsioulis A, Rachiotis G, Malissiova E, Hadjichristodoulou C. Prevalence and risk factors of overweight and obesity among adolescents and their parents in Central Greece (FETA Project). International Journal of Environmental Research and Public Health 2015;13(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pbert 2013 {published data only}
- Pbert L, Druker S, Gapinski M, Gellar L, Magner R, Reed G, et al. A school nurse‐delivered intervention for overweight and obese adolescents. Journal of School Health 2013;83(3):182‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitetti 2007 {published data only}
- Pitetti K, Rendoff A, Grover T, Beets M. The efficacy of a 9‐month treadmill walking program on the exercise capacity and weight reduction for adolescents with severe autism. Journal of Autism and Developmental Disorders 2007;37(6):997‐1006. [DOI] [PubMed] [Google Scholar]
Resnicow 2005 {published data only}
- Resnicow K, Taylor R, Baskin M, McCarty F. Results of Go Girls: a weight control program for overweight African‐American adolescent females. Obesity Research 2005;13(10):1739‐48. [DOI] [PubMed] [Google Scholar]
Saelens 2002 {published data only}
- Saelens B, Sallis J, Wilfley D, Patrick K, Cella J, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obesity Research 2002;10(1):22‐32. [DOI] [PubMed] [Google Scholar]
Savoye 2007 {published data only}
- NCT00409422. Effects of a comprehensive weight management program on obese adolescents and children. www.clinicaltrials.gov/ct2/show/NCT00409422 Date first received: 6 December 2006.
- Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014;37(2):317‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long‐term results of an obesity program in an ethnically diverse pediatric population. Pediatrics 2011;127(3):peds‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoye M, Shaw M, Dziura J, Tamborlane W, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA 2007;297(24):2697‐704. [DOI] [PubMed] [Google Scholar]
Schranz 2014 {published data only}
- ACTRN12609001078246. Can resistance training change the strength, body composition and self‐concept of overweight and obese adolescent males? A randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609001078246 Date first registered: 16 December 2009. [DOI] [PubMed]
- Schranz N, Olds T, Tomkinson G. Can resistance training change the strength, body composition and self‐concept of overweight and obese adolescent males? A randomised controlled trial. Journal of Science and Medicine in Sport 2012;15:S358. [DOI] [PubMed] [Google Scholar]
- Schranz N, Tomkinson G, Parletta N, Petkov J, Olds T. Can resistance training change the strength, body composition and self‐concept of overweight and obese adolescent males? A randomised controlled trial. British Journal of Sports Medicine 2014;48:1482‐8. [DOI] [PubMed] [Google Scholar]
Sigal 2014 {published data only}
- Alberga A, Goldfield G, Kenny G, Hadjiyannakis S, Phillips P, Prud'homme D, et al. Healthy Eating, Aerobic and Resistance Training in Youth (HEARTY): study rationale, design and methods. Contemporary Clinical Trials 2012;33:839‐47. [DOI] [PubMed] [Google Scholar]
- Goldfield G, Kenny G, Alberga A, Prud'homme D, Hadjiyannakis S, Gougeon R, et al. Effects of aerobic training, resistance training, or both on psychological health in adolescents with obesity: the HEARTY Rrandomized controlled trial. Journal of Consulting and Clinical Psychology 2015;83(6):1123. [DOI] [PubMed] [Google Scholar]
- NCT00195858. Healthy Eating Aerobic and Resistance Training in Youth (HEARTY) trial. clinicaltrials.gov/show/NCT00195858 Date first received: 12 September 2005.
- Sigal R, Alberga A, Goldfield G, Prud'homme D, Hadjiyannakis S, Gougeon R, et al. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: the Healthy Eating Aerobic and Resistance Training in Youth randomized clinical trial. JAMA Pediatrics 2014;168:1006‐14. [DOI] [PubMed] [Google Scholar]
Toulabi 2012 {published data only}
- Toulabi T, Nikoo M, Amini F, Nazari H, Mardani M. The influence of a behavior modification interventional program on body mass index in obese adolescents. Journal of the Formosan Medical Association / Taiwan Yi Zhi 2012;111(3):153‐9. [DOI] [PubMed] [Google Scholar]
van Egmond‐Frohlich 2006 {published data only}
- Egmond‐Frohlich A, Brauer W, Goldschmidt H, Hoff‐Emden H, Oepen J, Zimmermann E. Effects of a programme for structured outpatient follow‐up care after inpatient rehabilitation of obese children and adolescents ‐ a multicentre, randomized study. Rehabilitation 2006;45(1):40‐51. [DOI] [PubMed] [Google Scholar]
Vissers 2008 {published data only}
- Vissers D, Meulenaere A, Vanroy C, Vanherle K, Sompel A, Truijen S, et al. Effect of a multidisciplinary school‐based lifestyle intervention on body weight and metabolic variables in overweight and obese youth. E‐SPEN, the European E‐journal of Clinical Nutrition and Metabolism 2008;3(5):e196‐202. [Google Scholar]
Visuthranukul 2015 {published data only}
- NCT02049788. Effects of a low glycemic index in obese children. clinicaltrials.gov/show/NCT02049788 Date first received: 28 January 2014.
- Visuthranukul C, Sirimongkol P, Prachansuwan A, Pruksananonda C, Chomtho S. Low‐glycemic index diet may improve insulin sensitivity in obese children. Pediatric Research 2015;78(5):567‐73. [DOI] [PubMed] [Google Scholar]
Vos 2011 {published data only}
- ISRCTN36146436. The effect of lifestyle treatment on childhood obesity. www.isrctn.com/ISRCTN36146436 Date first assigned: 13 July 2010.
- Vos R, Huisman S, Houdijk E, Pijl H, Wit J. The effect of family‐based multidisciplinary cognitive behavioral treatment on health‐related quality of life in childhood obesity. Quality of Life Research 2012;21(9):1587‐94. [DOI] [PubMed] [Google Scholar]
- Vos R, Wit J, Pijl H, Houdijk E. Long‐term effect of lifestyle intervention on adiposity, metabolic parameters, inflammation and physical fitness in obese children: a randomized controlled trial. Nutrition & Diabetes 2011;1(10):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos R, Wit J, Pijl H, Kruyff C, Houdijk, E. The effect of family‐based multidisciplinary cognitive behavioral treatment in children with obesity: study protocol for a randomized controlled trial. Trials 2011;12(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walpole 2013 {published data only}
- NCT01246349. Motivational interviewing for weight loss. clinicaltrials.gov/ct2/show/study/NCT01246349 Date first received: 11 November 2010.
- Walpole B, Dettmer E, Morrongiello B, McCrindle B, Hamilton J. Motivational interviewing as an intervention to increase adolescent self‐efficacy and promote weight loss: methodology and design. BMC Public Health 2011;11(1):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole B, Dettmer E, Morrongiello B, McCrindle B, Hamilton J. Motivational interviewing to enhance self‐efficacy and promote weight loss in overweight and obese adolescents: a randomized controlled trial. Journal of Pediatric Psychology 2013;39(9):944‐53. [DOI] [PubMed] [Google Scholar]
Wengle 2011 {published data only}
- Wengle J, Hamilton J, Manlhiot C, Bradley T, Katzman D, Sananes R, et al. The 'Golden Keys' to health ‐ a healthy lifestyle intervention with randomized individual mentorship for overweight and obesity in adolescents. Paediatrics & Child Health 2011;16(18):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2015 {published data only}
- Wong J, Ebbeling C, Robinson L, Feldman H, Ludwig D. Does the recommendation to drink 8 cups of water per day promote weight loss?. FASEB Journal 2015;29:747‐18. [Google Scholar]
References to studies excluded from this review
Balagopal 2005 {published data only}
- Balagopal P, George D, Patton N, Yarandi H, Roberts W, Bayne E, et al. Lifestyle‐only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. Journal of Pediatrics 2005;146(3):342‐8. [DOI] [PubMed] [Google Scholar]
Belenchia 2013 {published data only}
- Belenchia A, Tosh A, Hillman L, Peterson C. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. American Journal of Clinical Nutrition 2013;97(4):774‐81. [DOI] [PubMed] [Google Scholar]
Braet 2004 {published data only}
- Braet C, Tanghe A, Decaluwé V, Moens E, Rosseel Y. Inpatient treatment for children with obesity: weight loss, psychological well‐being, and eating behavior. Journal of Pediatric Psychology 2004;29(7):519‐29. [DOI] [PubMed] [Google Scholar]
Carrel 2007 {published data only}
- Carrel A, Clark R, Peterson S, Eickhoff J, Allen D. School‐based fitness changes are lost during the summer vacation. Archives of Pediatrics & Adolescent Medicine 2007;161(6):561‐4. [DOI] [PubMed] [Google Scholar]
Crandall 2006 {published data only}
- Crandall M. The effectiveness of a program of comprehensive behavior modification therapy and sibutramine for obese adolescents. Thesis 2006; Vol. 69:3841.
Daly 2013 {published data only}
- Daly P. Obese Adolescent Females and Actual Behavioral Responses to a Mindful Eating Intervention [Doctoral dissertation]. Tucson (AZ): University of Arizona, 2013. [Google Scholar]
Dancy 2006 {published data only}
- Dancy B, Crittenden K, Talashek M. Effectiveness of the mother/daughter health promotion intervention in enhancing daughters' healthy eating behavior. APHA 134th Annual Meeting and Exposition; 2006 Nov 4‐8; Boston (MA). 2006.
De Jesus 2013 {published data only}
- Jesus S, Prapavessis H. The effects of a peer modeling intervention on cardiorespiratory fitness parameters and self‐efficacy in obese adolescents. Behavioral Medicine 2013;39(4):129‐37. [DOI] [PubMed] [Google Scholar]
DeVore 2013 {published data only}
- DeVore S, Kohli R, Lake K, Nicholas L, Dietrich K, Balistreri W, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. Journal of Pediatric Gastroenterology and Nutrition 2013;57(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2008 {published data only}
- Doyle A, Goldschmidt A, Huang C, Winzelberg A, Taylor C, Wilfley D. Reduction of overweight and eating disorder symptoms via the internet in adolescents: a randomized controlled trial. Journal of Adolescent Health 2008;43(2):172‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreyer‐Gillette 2014 {published data only}
- Dreyer‐Gillette M, Odar Stough C, Best C, Beck A, Hampl S. Comparison of a condensed 12‐week version and a 24‐week version of a family‐based pediatric weight management program. Childhood Obesity 2014;10(5):375‐82. [DOI] [PubMed] [Google Scholar]
Faulkner 2013 {published data only}
- Faulkner M, Michaliszyn S, Hepworth J, Wheeler M. Personalized exercise for adolescents with diabetes or obesity. Biological Research for Nursing 2013;20:1099800413500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fonseca 2016 {published data only}
- Fonseca H, Prioste A, Sousa P, Gaspar P, do Céu Machado M. Effectiveness analysis of an internet‐based intervention for overweight adolescents: next steps for researchers and clinicians. BMC Obesity 2016;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01904474. E‐therapeutic program for obese adolescents. clinicaltrials.gov/ct2/show/NCT01904474 Date first received: 9 July 2013.
Freire 2013 {published data only}
- Freire S, Fisberg M, Cozzolino S. Dietary intervention causes redistribution of zinc in obese adolescents. Biological Trace Element Research 2013;154(2):168‐77. [DOI] [PubMed] [Google Scholar]
Garnett 2014 {published data only}
- Garnett S, Gow M, Ho M, Baur L, Noakes M, Woodhead H, et al. Improved insulin sensitivity and body composition, irrespective of macronutrient intake, after a 12 month intervention in adolescents with pre‐diabetes; RESIST a randomised control trial. BMC Pediatrics 2014;14(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giel 2013 {published data only}
- Giel K, Zipfel S, Schweizer R, Braun R, Ranke M, Binder G, et al. Eating disorder pathology in adolescents participating in a lifestyle intervention for obesity: associations with weight change, general psychopathology and health‐related quality of life. Obesity Facts 2013;6(4):307‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2016 {published data only}
- Hay J, Wittmeier K, MacIntosh A, Wicklow B, Duhamel T, Sellers E, et al. Physical activity intensity and type 2 diabetes risk in overweight youth: a randomized trial. International Journal of Obesity 2016;40:607‐14. [DOI] [PubMed] [Google Scholar]
Jelalian 1999 {published data only}
- Jelalian E, Saelens B. Empirically supported treatments in pediatric psychology: pediatric obesity. Journal of Pediatric Psychology 1999;24(3):223‐48. [DOI] [PubMed] [Google Scholar]
Jelalian 2010 {published data only}
- Jelalian E, Lloyd‐Richardson EE, Mehlenbeck RS, Hart CN, Flynn‐O'Brien K, Kaplan J, et al. Behavioral weight control treatment with supervised exercise or peer‐enhanced adventure for overweight adolescents. Journal of Pediatrics 2010;157(6):923‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2008 {published data only}
- Jones M, Luce K, Osborne M, Taylor K, Cunning D, Doyle A, et al. Randomized, controlled trial of an internet‐facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics 2008;121(3):453‐62. [DOI] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones M. Reducing Binge Eating and Overweight in Adolescents via the Internet [Doctoral thesis]. Palo Alto (CA): Palo Alto University, 2009. [Google Scholar]
Lee 2013a {published data only}
- Lee S, Kuk J. Changes in fat and skeletal muscle with exercise training in obese adolescents: comparison of whole‐body MRI and dual energy X‐ray absorptiometry. Obesity 2013;21(10):2063‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013b {published data only}
- Lee S, Deldin A, White D, Kim Y, Libman I, Rivera‐Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. American Journal of Physiology. Endocrinology and Metabolism 2013;305(10):E1222‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013c {published data only}
- Lee S, Burns S, White D, Kuk J, Arslanian S. Effects of acute exercise on postprandial triglyceride response after a high‐fat meal in overweight black and white adolescents. International Journal of Obesity 2013;37(7):966‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovely 2013 {published data only}
- Lovely R, Hossain J, Ramsey J, Komakula V, George D, Farrell D, et al. Obesity‐related increased γ' fibrinogen concentration in children and its reduction by a physical activity‐based lifestyle intervention: a randomized controlled study. Journal of Pediatrics 2013;163(2):333‐8. [DOI] [PubMed] [Google Scholar]
Lubans 2008 {published data only}
- Lubans D, Morgan P. Evaluation of an extra‐curricular school sport programme promoting lifestyle and lifetime activity for adolescents. Journal of Sports Sciences 2008;26(5):519‐29. [DOI] [PubMed] [Google Scholar]
NCT00863083 {published data only}
- NCT00863083. Reducing barriers to behavior change among youth with pediatric overweight and obesity. clinicaltrials.gov/ct2/show/NCT00863083 Date first received: 16 March 2009.
NCT01044134 {published data only}
- NCT01044134. Think AHEAD (a healthy eating and drinking) study. clinicaltrials.gov/ct2/show/NCT01044134 Date first received: 5 January 2010.
NCT02011646 {published data only}
- NCT02011646. Healthy body study. clinicaltrials.gov/ct2/show/NCT02011646 Date first received: 10 December 2013.
NCT02295761 {published data only}
- NCT02295761. Promoting overweight adolescents physical activity. clinicaltrials.gov/ct2/show/NCT02295761 Date first received: 18 February 2014.
Nunes 2016 {published data only}
- Nunes JE, Cunha HS, Freitas ZR, Nogueira AM, Dâmaso AR, Espindola FS, et al. Interdisciplinary therapy changes superoxide dismutase activity and adiponectin in obese adolescents: a randomised controlled trial. Journal of Sports Sciences 2016;34(10):945‐50. [DOI] [PubMed] [Google Scholar]
Parks 2014 {published data only}
- Parks E, Zemel B, Moore R, Berkowitz R. Change in body composition during a weight loss trial in obese adolescents. Pediatric Obesity 2014;9(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Racil 2013 {published data only}
- Racil G, Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. European Journal of Applied Physiology 2013;113(10):2531‐40. [DOI] [PubMed] [Google Scholar]
Sabzghabaee 2013 {published data only}
- Sabzghabaee AM, Khayam I, Kelishadi R, Ghannadi A, Soltani R, Badri S, et al. Effect of Zizyphus jujuba fruits on dyslipidemia in obese adolescents: a triple‐masked randomized controlled clinical trial. Medical Archives 2013;67(3):156. [PubMed] [Google Scholar]
Sarvestani 2009 {published data only}
- Sarvestani R, Jamalfard M, Kargar M, Kaveh M, Tabatabaee H. Effect of dietary behaviour modification on anthropometric indices and eating behaviour in obese adolescent girls. Journal of Advanced Nursing 2009;65(8):1670‐5. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2011 {published data only}
- Shrewsbury V, Nguyen B, O'Connor J, Steinbeck K, Lee A, Hill A, et al. Short‐term outcomes of community‐based adolescent weight management: the Loozit® Study. BMC Pediatrics 2011;11(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 2013 {published data only}
- Sussman A, Montoya C, Werder O, Davis S, Wallerstein N, Kong A. An adaptive CBPR approach to create weight management materials for a school‐based health center intervention. Journal of Obesity 2013;2013(978482):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van 2013 {published data only}
- Vann L, Stanford F, Durkin M, Hanna A, Knight L, Stallworth J. "Moving and losing": a pilot study incorporating physical activity to decrease obesity in the pediatric population. Journal of the South Carolina Medical Association (1975) 2013;109(4):116‐20. [PubMed] [Google Scholar]
Ventura 2009 {published data only}
- Ventura E, Davis J, Byrd‐Williams C, Toledo‐Corral C, Spruijt‐Metz D, Weigensberg M, et al. The effects of a randomized, controlled, modified‐carbohydrate nutrition intervention on the metabolic syndrome in overweight Latino adolescents. Obesity 2009;17:S183‐4. [Google Scholar]
Wadden 1990 {published data only}
- Wadden T, Stunkard A, Rich L, Rubin C, Sweidel G, McKinney S. Obesity in black adolescent girls: a controlled clinical trial of treatment by diet, behavior modification, and parental support. Pediatrics 1990;85(3):345‐52. [PubMed] [Google Scholar]
Wiegand 2014 {published data only}
- Wiegand S, Bau A, Ernert A, Krude H. MAINTAIN: an intervention study of weight regain after weight loss in adolescents and children reveals an only minor role of leptin in weight regain. Hormone Research in Paediatrics 2014;82:1‐507. [Google Scholar]
Wilson 2012 {published data only}
- Wilson A, Jung M, Cramp A, Simatovic J, Prapavessis H, Clarson C. Effects of a group‐based exercise and self‐regulatory intervention on obese adolescents' physical activity, social cognitions, body composition and strength: a randomized feasibility study. Journal of Health Psychology 2012;17:1223‐37. [DOI] [PubMed] [Google Scholar]
Xanthopoulos 2013 {published data only}
- Xanthopoulos M, Moore R, Wadden T, Bishop‐Gilyard C, Gehrman C, Berkowitz R. The association between weight loss in caregivers and adolescents in a treatment trial of adolescents with obesity. Journal of Pediatric Psychology 2013;38(7):766‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Angerer 2015 {published data only}
- Angerer P, Niedermeier H, Graf T, Manthey A, Marten‐Mittag B, Schmidt H, et al. Fit4You ‐ a programme for prevention and reduction of overweight in apprentices in the workplace setting. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany) 2015;77:S95‐6. [PUBMED: 23970389] [DOI] [PubMed] [Google Scholar]
Barbeau 2003 {published data only}
- Barbeau P, Gutin B, Litaker M, Ramsey L, Cannady W, Allison J, et al. Influence of physical training on plasma leptin in obese youths. Canadian Journal of Applied Physiology 2003;28(3):382‐96. [DOI] [PubMed] [Google Scholar]
Bohlin 2012 {published data only}
- Bohlin A, Klaesson S, Kowalski J. Can telephone consultations substitute visits in treatment of childhood obesity? Results from a randomized trial. Obesity Facts 2012;5(Suppl 1):237. [Google Scholar]
Campos 2017 {published data only}
Cocca 2016 {published data only}
- Cocca A, Lomas R, Enríquez M, Cocca M, Ceballos O. Effects of a school‐based intervention program on metabolic syndrome parameters in school‐aged youth. Obesity Reviews 2016;17:131‐2. [Google Scholar]
Garrett 1979 {published data only}
- Garrett L. Group behavioral therapy versus family behavioral therapy in treatment of obese adolescent females [Doctor of Philosophy thesis]. University of Pennsylvania, 1979:1364‐5. [Google Scholar]
Gracianette 1982 {published data only}
- Gracianette C. The application of behavioral techniques to change eating habits and/or exercise habits to effect weight reduction and maintenance in adolescents: a year‐long study [Doctor of Philosophy thesis]. Baton Rouge (LA): Louisiana State University and Agricultural & Mechanical College, 1982. [Google Scholar]
Li 2006 {published data only}
- Li M. Anti‐obesity effect of comprehensive diet and sports in girl students with simple obesity or overweight. Chinese Journal of Clinical Rehabilitation 2006;10(32):44‐6. [Google Scholar]
Makkes 2015 {unpublished data only}
- Makkes S. Intensive inpatient treatment for severely obese children and adolescents: costs and effects [Doctoral Thesis]. Amsterdam (Netherlands): VU University Amsterdam, 2015. [Google Scholar]
- Makkes S, Halberstadt J, Renders C, Bosmans J, Baan‐Slootweg O, Seidell C. Health Effects of Lifestyle Interventions in Obese children and adolescents Study (HELIOS) ‐ a randomised controlled trial. Obesity Reviews 2011;12:58. [Google Scholar]
- Makkes S, Halberstadt J, Renders C, Bosmans J, Baan‐Slootweg O, Seidell J. Cost‐effectiveness of intensive inpatient treatments for severely obese children and adolescents in the Netherlands; a randomized controlled trial (HELIOS). BMC Public Health 2011;11(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR1678. Het HELIOS onderzoek naar behandeling van kinderen en adolescenten met ernstige obesitas. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1678 Date first registered: 20 February 2009.
NCT00462267 {published data only}
- NCT00462267. Examining the feasibility of collaborative care treatment for overweight adolescents (SHINE‐Garfield). clinicaltrials.gov/ct2/show/NCT00462267 Date first received: 17 April 2007.
NCT00475163 {published data only}
- NCT00475163. Mentors in motion: a physical activity intervention for obese adolescents (MIM). clinicaltrials.gov/show/NCT00475163 Date first received: 17 May 2007.
Shapiro 1976 {published data only}
- Shapiro J. A comparison of various reward and monitoring procedures in the behavioral treatment of overweight children. Dissertation 1976; Vol. 36, issue 11‐B:5816‐7.
References to ongoing studies
ACTRN12607000632493 {published data only}
- ACTRN12607000632493. Active video games to improve body composition and physical activity in children. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12607000632493 Date first registered: 12 December 2007.
- Maddison R, Foley L, Mhurchu CN, Jull A, Jiang Y, Prapavessis H, et al. Feasibility, design and conduct of a pragmatic randomized controlled trial to reduce overweight and obesity in children: the electronic games to aid motivation to exercise (eGAME) study. BMC Public Health 2009;9:146. [DOI: 10.1186/1471-2458-9-146] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12611000139976 {published data only}
- ACTRN12611000139976. Staying fit adolescent weight management study. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000139976 Date first registered: 7 February 2011.
ACTRN12611000862943 {published data only}
- ACTRN12611000862943. Whanau Pakari: a multidisciplinary intervention for child and adolescent obesity. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343300 Date first registered: 15 August 2011.
ACTRN12613001037796 {published data only}
- ACTRN12613001037796. Effect of exercise intensity on cardiac and vascular function, and intra‐abdominal fat in obese children and adolescents. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613001037796 Date first registered: 18 September 2013.
ACTRN12615000558527 {published data only}
- ACTRN12615000558527. The effectiveness of a community weight management program with a behavioural incentive scheme compared to participation in a standard community weight management program in overweight children on relative weight loss and behaviour change. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368491 Date first registered: 29 May 2015.
Chew 2016 {published data only}
- Chew C, Kelly SM, Rajasegaran K, Oh JY, Lim M, Lim SC, et al. Three month outcome of a family based intervention program for adolescent obesity: the Lite randomised controlled trial. Obesity Reviews 2016;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR‐TRC‐13003501 {published data only}
- ChiCTR‐TRC‐13003501. Lifestyle intervention in obese Chinese adolescents with nonalcoholic fatty liver disease: a randomized controlled study. www.chictr.org.cn/showprojen.aspx?proj=6059 Date first registered: 21 August 2013.
DRKS00004583 {published data only}
- DRKS00004583. Feasibility and efficacy of a weight maintenance treatment approach for adolescent obesity via telephone counseling following an obesity treatment program: a randomized controlled trial. drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00004583 Date first registered: 30 November 2012.
- Marked J, Herget S, Marschke S, Vogel M, Hilbert A, Bluher S. Weight maintenance treatment approach for adolescent obesity via telephone counseling following an inpatient obesity treatment program ‐ study concept. Obesity Facts Vol. 5:30.
- Markert J, Herget S, Marschke S, Lehnert T, Falkenberg C, Blüher S. Case management via telephone counseling and SMS for weight maintenance in adolescent obesity: study concept of the TeAM program. BMC Obesity 2014; Vol. 1, issue 1:8. [DOI] [PMC free article] [PubMed]
- Markert J, Herget S, Vogel M, Gausche R, Hilbert A, Bluher S. After care treatment approach for adolescent obesity via telephone counseling following an obesity treatment program‐study concept. Hormone Research in Paediatrics Vol. 78:279.
DRKS00005299 {published data only}
- DRKS00005299. Impact of resistance versus endurance exercise on anthropometric and metabolic parameters within a standardized one year obesity treatment program for obese children and adolescents. drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005299 Date first registered: 13 September 2013.
DRKS00006781 {published data only}
- DRKS00006781. Feasibility of a media‐supported high‐intensity interval training program for overweight and obese adolescents. drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00006781 Date first registered: 17 September 2014.
DRKS00007879 {published data only}
- DRKS00007879. Development and evaluation of a computer‐based self‐regulation training for obese children and adolescents. drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00007879 Date first registered: 13 July 2015.
EUCTR2009‐016921‐32‐ES {published data only}
- EUCTR2009‐016921‐32‐ES. A phase II, randomized, double‐blind, placebo‐controlled, in parallel groups clinical trial to assess the safety and efficacy of dietary supplementation with tryptophan to achieve weight loss, and its neuropsychological effects in adolescents with obesity. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐016921‐32 Date first registered: not available.
IRCT201012235440N1 {published data only}
- IRCT201012235440N1. Effectiveness of a theory‐based intervention on obesity‐related behaviors, laboratory indices, body composition, body mass index and health‐related quality of life in overweight adolescents. www.irct.ir/searchresult.php?id=5440&number=1 Date first registered: 18 February 2011.
IRCT2013103115211N1 {published data only}
- IRCT2013103115211N1. Study on the effect of nutrition education and physical activity intervention using social cognitive theory for overweight and obese student girls of middle school of Shahinshahr. www.irct.ir/searchresult.php?id=15211&number=1 Date first registered: 27 November 2013.
ISRCTN04152711 {published data only}
- ISRCTN04152711. Preventing obesity in young people attending primary dental care settings: an exploratory randomised controlled trial. www.isrctn.com/ISRCTN04152711 Date first assigned: 24 May 2012.
- Murphy M, Porter J, Yusuf H, Ntouva A, Newton T, Kolliakou A, et al. Considerations and lessons learned from designing a motivational interviewing obesity intervention for young people attending dental practices: a study protocol paper. Contemporary Clinical Trials 2013;36(1):126‐34. [DOI] [PubMed] [Google Scholar]
NCT00562263 {published data only}
- NCT00562263. Barriers to effective weight loss in overweight adolescents (TEENS). clinicaltrials.gov/ct2/show/NCT00562263 Date first received: 20 November 2007.
NCT00850629 {published data only}
- NCT00850629. Hormonal regulation of body weight maintenance. clinicaltrials.gov/ct2/show/NCT00850629 Date first received: 17 February 2009.
NCT00881478 {published data only}
- NCT00881478. Family intervention for obese children using portion control strategy for weight control (FOCUS). clinicaltrials.gov/ct2/show/NCT00881478 Date first received: 13 April 2009.
NCT00940966 {published data only}
- NCT00940966. A pilot study to determine the efficacy of a low carbohydrate diet in treatment of adolescents with metabolic syndrome. clinicaltrials.gov/ct2/show/NCT00940966 Date first received: 15 July 2009.
NCT00998413 {published data only}
- NCT00998413. Weight‐reduction intervention in asthmatic children with overweight/obesity. clinicaltrials.gov/ct2/show/NCT00998413 Date first received: 19 October 2009.
- Willeboordse M, Kant KD, Laat MN, Schayck OC, Mulkens S, Dompeling E. Multifactorial intervention for children with asthma and overweight (Mikado): study design of a randomised controlled trial. BMC Public Health 2013;13:494. [DOI: 10.1186/1471-2458-13-494] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01023139 {published data only}
- NCT01023139. Efficacy in adolescents of continued behavior modification following a six month sibutramine‐based weight management intervention (AOS). clinicaltrials.gov/ct2/show/NCT01023139 Date first received: 1 December 2009.
NCT01221220 {published data only}
- NCT01221220. Environmental strategies & behavior change to reduce overeating in obese children. clinicaltrials.gov/ct2/show/NCT01221220 Date first received: 13 October 2010.
NCT01350531 {published data only}
- NCT01350531. Interventionist procedures for adherence to weight loss recommendations in black adolescents. clinicaltrials.gov/ct2/show/NCT01350531 Date first received: 21 April 2011.
NCT01374386 {published data only}
- NCT01374386. Impact of Exergaming on adolescent youth. clinicaltrials.gov/ct2/show/NCT01374386 Date first received: 14 June 2011.
NCT01448551 {published data only}
- NCT01448551. MPOWERed messages: tailored mobile messages to enhance weight loss for teens. clinicaltrials.gov/ct2/show/NCT01448551 Date first received: 5 October 2011.
NCT01456221 {published data only}
- NCT01456221. Intervention study with omega‐3 fatty acids for weight loss and insulin resistance in adolescents (O3WLIRADOL). clinicaltrials.gov/ct2/show/NCT01456221 Date first received: 12 October 2011.
NCT01514279 {published data only}
- NCT01514279. Ideas moving parents and adolescents to change together (IMPACT). clinicaltrials.gov/ct2/show/NCT01514279 Date first received: 20 December 2011.
NCT01677923 {published data only}
- NCT01677923. Obesity in children and adolescents: associated risks and early intervention (OCA). clinicaltrials.gov/ct2/show/NCT01677923 Date first received: 30 July 2012.
NCT01688453 {published data only}
- NCT01688453. Overweight management and social inequalities (PRALIMAP‐INES). clinicaltrials.gov/ct2/show/NCT01688453 Date first received: 7 September 2012.
NCT01764113 {published data only}
- NCT01764113. Effect of mindful eating on body mass index in obese adolescents. clinicaltrials.gov/ct2/show/NCT01764113 Date first received: 7 January 2013.
NCT01794546 {published data only}
- NCT01794546. Integrated care for pediatric obesity using telehealth. clinicaltrials.gov/ct2/show/NCT01794546 Date first received: 15 February 2013.
NCT01796067 {published data only}
- NCT01796067. Families improving together (FIT) for weight loss. clinicaltrials.gov/ct2/show/NCT01796067 Date first received: 22 January 2013.
- Wilson D, Kitzman‐Ulrich H, Resnicow K, Horn M, George S, Siceloff E, et al. An overview of the Families Improving Together (FIT) for weight loss randomized controlled trial in African American families. Contemporary Clinical Trials 2015;31(42):145‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01804855 {published data only}
- NCT01804855. Effectiveness of a smartphone app for adolescent obesity management. clinicaltrials.gov/ct2/show/NCT01804855 Date first received: 4 March 2013.
- O'Malley G, Clarke M, Burls A, Murphy S, Murphy N, Perry I. A smartphone intervention for adolescent obesity: study protocol for a randomised controlled non‐inferiority trial. Trials 2014;15(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01840631 {published data only}
- NCT01840631. A trial to study the effects of group versus individual dietary counseling in pediatric obesity. clinicaltrials.gov/ct2/show/NCT01840631 Date first received: 8 March 2013.
NCT02034994 {published data only}
- NCT02034994. Combining primary and secondary prevention for reduction of excessive weight gain in school. clinicaltrials.gov/ct2/show/NCT02034994 Date first received: 10 January 2014.
NCT02086851 {published data only}
- NCT02086851. Study of a structured parent intervention on adolescent weight loss modification program. clinicaltrials.gov/ct2/show/NCT02086851 Date first received: 7 February 2014.
NCT02228278 {published data only}
- NCT02228278. Healthcare text messaging to improve diet, physical activity and weight loss in a pediatric lipid clinic (Text4Fit). clinicaltrials.gov/ct2/show/NCT02228278 Date first received: 25 August 2014.
NCT02280772 {published data only}
- NCT02280772. Effect of glucomannan supplementation on body weight in overweight and obese children. clinicaltrials.gov/ct2/show/NCT02280772 Date first received: 29 October 2014.
- Zalewski B, Szajewska H. Effect of glucomannan supplementation on body weight in overweight and obese children: protocol of a randomised controlled trial. BMJ Open 2015;5(4):e007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02353637 {published data only}
- NCT02353637. The Peds Obesity study. clinicaltrials.gov/ct2/show/NCT02353637 Date first received: 21 January 2015.
NCT02444689 {published data only}
- NCT02444689. EMPower: electronic media powering positive health changes in youth. clinicaltrials.gov/ct2/show/NCT02444689 Date first received: 12 May 2015.
NCT02615353 {published data only}
- NCT02615353. Preventing diabetes in Latino youth. clinicaltrials.gov/ct2/show/NCT02615353 Date first received: 4 November 2015.
NCT02687516 {published data only}
- NCT02687516. Treatment of severely obese children and adolescents employing "Family‐based Behavioral Social Facilitation Treatment". clinicaltrials.gov/ct2/show/NCT02687516 Date first received: 21 February 2014.
NCT02711488 {published data only}
- NCT02711488. Managing adolescent obesity at local level by combining primary and secondary intervention (PAAPAS‐DC). clinicaltrials.gov/ct2/show/NCT02711488 Date first received: 15 February 2016.
NCT02745795 {published data only}
- NCT02745795. Efficacy of motivational interview in the treatment of obesity and overweight in adolescents (IMAGINE). clinicaltrials.gov/ct2/show/NCT02745795 Date first received: 5 April 2016.
NCT02794090 {published data only}
- NCT02794090. Childhood obesity treatment ‐ telephone coaching vc usual care. clinicaltrials.gov/ct2/show/NCT02794090 Date first received: 31 May 2016.
NTR5676 {published data only}
- NTR5676. The focus on strength programme: an innovative strength‐based physical activity intervention to improve body composition and to stimulate physical activity in adolescents (12‐18years) with overweight or obesity. www.trialregister.nl/trialreg/admin/rctview.asp?TC=5676 Date first registered: 8 February 2016.
Ramalho 2016 {published data only}
- Ramalho SM, Conceiçao E, Saint Maurice P, Machado P. Taking the route back: new technologies for overweight and obesity treatment in childhood and adolescence ‐ study protocol. Obesity Facts 2016;9:368‐108. [Google Scholar]
RBR‐38p23s {published data only}
- RBR‐38p23s. Multifocal intervention in obese adolescents: social competence, behavior problems, academic performance and weight reduction. www.ensaiosclinicos.gov.br/rg/RBR‐38p23s/ Date first registered: 23 April 2013.
Spieker 2015 {published data only}
- Spieker EA, Sbrocco T, Theim KR, Maurer D, Johnson D, Bryant E, et al. Preventing obesity in the military community (POMC): the development of a clinical trials research network. International Journal of Environmental Research and Public Health 2015;12(2):1174‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20130515001 {published data only}
- TCTR20130515001. Effectiveness of weight reduction program on intrinsic motivation and health related outcomes in overweight secondary school students. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20130515001 Date first registered: 15 May 2013.
Additional references
Bandura 1997
- Bandura, A. Self‐efficacy: The exercise of control. New York: Freeman & Co, 1997. [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2:36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocca 2013
- Bocca G, Ongering EC, Stolk RP, Sauer PJ. Insulin resistance and cardiovascular risk factors in 3‐ to 5‐year old overweight or obese children. Hormone Research in Paediatrics 2013;80(3):201‐6. [DOI] [PubMed] [Google Scholar]
CDC 2000
- Centers for Disease Control Growth Charts. Centers for Disease Control Growth Charts. www.cdc.gov/growthcharts 2000.
CMO 2015
- Chief Medical Officer. Annual report of the Chief Medical Officer: surveillance volume, 2012: on the state of the public's health. www.gov.uk/government/publications/chief‐medical‐officer‐annualreport‐surveillance‐volume‐2012 (accessed 30 March 2015).
Cole 2000
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320(7244):1240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2012
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatric Obesity 2012;7(4):284‐94. [DOI] [PubMed] [Google Scholar]
Collins 2006
- Collins C, Warren J, Neve M, McCoy P, Stokes B. Measuring effectiveness of dietetic interventions in child obesity: a systematic review of randomized trials. Archives of Pediatrics & Adolescent Medicine 2006;160(9):906‐22. [DOI] [PubMed] [Google Scholar]
Daniels 2009
- Daniels SR. Complications of obesity in children and adolescents. International Journal of Obesity 2009;33(Suppl 1):S60‐5. [DOI] [PubMed] [Google Scholar]
de Onis 2010
- Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition 2010;92(5):1257‐64. [DOI] [PubMed] [Google Scholar]
Dewar 2013
- Dewar D, Morgan P, Plotnikoff R, Okely A, Collins C, Batterham M, et al. The nutrition and enjoyable activity for teen girls study: a cluster randomized controlled trial. American Journal of Preventive Medicine 2013;45(3):313‐7. [DOI] [PubMed] [Google Scholar]
Doak 2006
- Doak C, Visscher T, Renders C, Seidell J. The prevention of overweight and obesity in children and adolescents: a review of interventions and programmes. Obesity Reviews 2006;7(1):111‐36. [DOI] [PubMed] [Google Scholar]
Eady 2008
- Eady AM, Wilczynski NL, Haynes RB. PsycINFO search strategies identified methodologically sound therapy studies and review articles for use by clinicians and researchers. Journal of Clinical Epidemiology 2008;61(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egan 2013
- Egan K, Ettinger A, Bracken M. Childhood body mass index and subsequent physician‐diagnosed asthma: a systematic review and meta‐analysis of prospective cohort studies. BMC Pediatrics 2013;13(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egbewale 2014
- Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Medical Research Methodology 2014;14:49:49. [DOI: 10.1186/1471-2288-14-49] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ells 2015
- Ells LJ, Hancock C, Copley VR, Mead E, Dinsdale H, Kinra S, et al. Prevalence of severe childhood obesity in England: 2006‐2013. Archives of Disease in Childhood 2015;100(7):631‐6. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Freedman 2006
- Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring, Md.) 2006;14(2):301‐8. [DOI] [PubMed] [Google Scholar]
Glenny 1997
- Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International Journal of Obesity and Related Metabolic Disorders 1997;21(9):715‐37. [DOI] [PubMed] [Google Scholar]
Griffiths 2010
- Griffiths LJ, Parsons TJ, Hill AJ. Self‐esteem and quality of life in obese children and adolescents: a systematic review. International Journal of Pediatric Obesity 2010;5(4):282‐304. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2012
- Ho M, Garnett S, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta‐analysis. Pediatrics 2012;130:e1647‐71. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
HSCIC 2015
- Health and Social Care Information Centre. National Child Measurement Programme ‐ England, 2012‐13 school year. http://content.digital.nhs.uk/catalogue/PUB13115 (accessed 30 March 2015).
Kelly 2008
- Kelly S, Melnyk B. Systematic review of multicomponent interventions with overweight middle adolescents: implications for clinical practice and research. Worldviews on Evidence‐Based Nursing 2008;5(2):113‐35. [DOI] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128(15):1689‐712. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Knai 2012
- Knai C, Lobstein T, Darmon N, Rutter H, McKee M. Socioeconomic patterning of childhood overweight status in Europe. International Journal of Environmental Research and Public Health 2012;9(4):1472‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kothandan 2014
- Kothandan S. School based interventions versus family based interventions in the treatment of childhood obesity ‐ a systematic review. Archives of Public Health 2014;72:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobstein 2004
- Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Reviews 2004;5(Suppl 1):4‐104. [DOI] [PubMed] [Google Scholar]
MacDonell 2010
- MacDonell K, Ellis D, Naar‐King S, Cunningham P. Predictors of home‐based obesity treatment efficacy for African American youth. Children's Health Care 2010;39(1):1‐4. [Google Scholar]
McGovern 2008
- McGovern L, Johnson J, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Treatment of pediatric obesity: a systematic review and meta‐analysis of randomized trials. Journal of Clinical Endocrinology & Metabolism 2008;93(12):4600‐5. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narang 2012
- Narang I, Mathew JL. Childhood obesity and obstructive sleep apnea. Journal of Nutrition and Metabolism 2012;2012:134202. [DOI: 10.1155/2012/134202; PUBMED: 22957216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2014
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis of the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Weight management: lifestyle services for overweight or obese children and young people. NICE Guidelines PH47, 2013. www.nice.org.uk/guidance/ph47 (accessed 24 May 2017).
NOO NHS 2011
- National Obesity Observatory on behalf of the Public Health Observatories in England. A simple guide to classifying body mass index in children, 2011. www.noo.org.uk/uploads/doc/vid_11601_A_simple_guide_to_classifying_BMI_in_children.pdf (accessed 3 November 2015).
Olds 2011
- Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International Journal of Pediatric Obesity 2011;6(5‐6):342‐60. [DOI] [PubMed] [Google Scholar]
Orsi 2011
- Orsi C, Hale D, Lynch, J. Pediatric obesity epidemiology. Current Opinion in Endocrinology, Diabetes and Obesity 2011;18(1):14‐22. [DOI] [PubMed] [Google Scholar]
Parsons 1999
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity 1999;23(Suppl 8):S1‐S107. [PubMed] [Google Scholar]
Paulis 2014
- Paulis WD, Silva S, Koes BW, Middelkoop M. Overweight and obesity are associated with musculoskeletal complaints as early as childhood: a systematic review. Obesity Reviews 2014;15(1):52‐67. [DOI] [PubMed] [Google Scholar]
Peirson 2015
- Peirson L, Fitzpatrick‐Lewis D, Morrison K, Warren R, Ali M, Raina P. Treatment of overweight and obesity in children and youth: a systematic review and meta‐analysis. CMAJ Open 2015;3(1):E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penedo 2005
- Penedo F, Dahn J. Exercise and well‐being: a review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry 2005;18(2):189‐93. [DOI] [PubMed] [Google Scholar]
Puhl 2007
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation's children. Psychology Bulletin 2007;133(4):557‐80. [DOI] [PubMed] [Google Scholar]
Rajput 2014
- Rajput N, Tuohy P, Mishra S, Smith A, Taylor B. Overweight and obesity in 4‐5‐year‐old children in New Zealand: results from the first 4 years (2009‐2012) of the B4School Check programme. Journal of Paediatrics and Child Health 2014;51(3):334‐43. [DOI] [PubMed] [Google Scholar]
Reilly 2003
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Archives of Diseases in Childhood 2003;88(9):748‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rokholm 2010
- Rokholm B, Baker JL, Sørenson TI. The levelling off of the obesity epidemic since the year 1999 ‐ a review of evidence and perspectives. Obesity Reviews 2010;11(12):835‐46. [DOI] [PubMed] [Google Scholar]
Ruotsalainen 2015
- Ruotsalainen H, Kyngäs H, Tammelin T, Kääriäinen M. Systematic review of physical activity and exercise interventions on body mass indices, subsequent physical activity and psychological symptoms in overweight and obese adolescents. Journal of Advanced Nursing 2015;1(11):2461‐77. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2008
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross‐sectional studies 1990‐2005. Obesity (Silver Spring, Md.) 2008;16(2):275‐84. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh AS, Mulder C, Twisk JW, Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews 2008;9(5):474‐88. [DOI] [PubMed] [Google Scholar]
Skinner 2014
- Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999‐2012. JAMA Pediatrics 2014;168(6):561‐6. [DOI] [PubMed] [Google Scholar]
Spear 2007
- Spear B, Barlow S, Ervin C, Ludwig D, Saelens B, Schetzina K, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120(4):S254‐88. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stuart and Stevenson 1959
- Stuart HC, Stevenson SS, Nelson WE. Textbook of pediatrics. Philadelphia: Saunders, 1959. [Google Scholar]
Tang‐Peronard 2008
- Tang‐Peronard JL, Heitmann BL. Stigmatization of obese children and adolescents, the importance of gender. Obesity Reviews 2008;9(6):522‐34. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang Y, Lim H. The global childhood obesity epidemic and the association between socio‐economic status and childhood obesity. International Review of Psychiatry 2012;24(3):176‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitaker 1997
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine 1997;337(13):869‐73. [DOI] [PubMed] [Google Scholar]
White 2004
- White M, Martin P, Newton R, Walden H, York‐Crowe E, Gordon S, et al. Mediators of weight loss in a family‐based intervention presented over the Internet. Obesity Research 2004;12(7):1050‐9. [DOI] [PubMed] [Google Scholar]
Whitlock 2010
- Whitlock E, O'Connor E, Williams S, Beil T, Lutz K. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics 2010;125:396‐418. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Report of a Joint WHO/FAO Expert Consultation. Diet nutrition and the prevention of chronic diseases. http://www.who.int/dietphysicalactivity/publications/trs916/en/. Geneva, Switzerland, 2002.
WHO 2009
- WHO. WHO AnthroPlus for personal computers manual: software for assessing growth of the world’s children and adolescents. http://www.who.int/growthref/tools/who_anthroplus_manual.pdf 2009.
WHO 2015a
- World Health Organization. Fact sheet on overweight and obesity. www.who.int/mediacentre/factsheets/fs311/en (accessed 30 March 2015).
WHO 2015b
- World Health Organization. The WHO child growth standards. www.who.int/childgrowth/publications/technical˙report˙pub/en/ (accessed 30 March 2015).
WHO 2015c
- World Health Organization. http://www.who.int/end‐childhood‐obesity/interim‐report‐for‐comment/en/. (accessed 5 June 2017).
Wilfley 2007
- Wilfley D, Tibbs T, Buren D, Reach K, Walker M, Epstein L. Lifestyle interventions in the treatment of childhood overweight: a meta‐analytic review of randomized controlled trials. Health Psychology 2007;26(5):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2006a
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wong 2006b
- Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. Journal of Nursing Scholarship 2006;38(2):194‐9. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Oude Luttikhuis 2009
- Oude Luttikhuis H, Baur L, Jansen H, Shrewbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001872.pub2] [DOI] [PubMed] [Google Scholar]
Summerbell 2003
- Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001872] [DOI] [PubMed] [Google Scholar]


