Abstract
Background
Lactoferrin, a normal component of human colostrum and milk, can enhance host defenses and may be effective for prevention of sepsis and necrotizing enterocolitis (NEC) in preterm neonates.
Objectives
Primary objective 1. To assess the safety and effectiveness of lactoferrin supplementation to enteral feeds for prevention of sepsis and NEC in preterm neonates
Secondary objectives
1. To determine the effects of lactoferrin supplementation to enteral feeds to prevent neonatal sepsis and/or NEC on duration of positive‐pressure ventilation, development of chronic lung disease (CLD) or periventricular leukomalacia (PVL), length of hospital stay to discharge among survivors, and adverse neurological outcomes at two years of age or later
2. To determine the adverse effects of lactoferrin supplementation for prophylaxis of neonatal sepsis and/or NEC
When data were available, we analyzed the following subgroups.
1. Gestational age < 32 weeks and 32 to 36 weeks
2. Birth weight < 1000 g (extremely low birth weight (ELBW) infants) and birth weight < 1500 g (very low birth weight (VLBW) infants)
3. Type of feeding: breast milk versus formula milk
Search methods
We used the search strategy of the Cochrane Neonatal Review Group (CNRG) to update our search in December 2016. We searched the databases Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PREMEDLINE, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), as well as trial registries and conference proceedings.
Selection criteria
Randomized controlled trials (RCTs) evaluating oral lactoferrin at any dose or duration to prevent sepsis or NEC in preterm neonates.
Data collection and analysis
Review authors used standard methods of the CNRG.
Main results
This review includes six RCTs. Trial results show that lactoferrin supplementation to enteral feeds decreased late‐onset sepsis (typical risk ratio (RR) 0.59, 95% confidence interval (CI) 0.40 to 0.87; typical risk difference (RD) ‐0.06, 95% CI ‐0.10 to ‐0.02; number needed to treat for an additional beneficial outcome (NNTB) 17, 95% CI 10 to 50; six trials, 886 participants; low‐quality evidence) and NEC stage II or III (typical RR 0.40, 95% CI 0.18 to 0.86; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.01; NNTB 25, 95% CI 17 to 100; four studies, 750 participants; low‐quality evidence). Lactoferrin supplementation did not have an effect on "all‐cause mortality" (typical RR 0.65, 95% CI 0.37 to 1.11; typical RD ‐0.02, 95% CI ‐0.05 to 0; six studies, 1041 participants; low‐quality evidence).
Lactoferrin supplementation to enteral feeds with probiotics decreased late‐onset sepsis (RR 0.27, 95% CI 0.12 to 0.60; RD ‐0.13, 95% CI ‐0.19 to ‐0.06; NNTB 8, 95% CI 5 to 17; one study, 321 participants; low‐quality evidence) and NEC stage II or III (RR 0.04, 95% CI 0.00 to 0.62; RD ‐0.05, 95% CI ‐0.08 to ‐0.03; NNTB 20, 95% CI 12.5 to 33.3; one study, 496 participants; low‐quality evidence), but not "all‐cause mortality" (low‐quality evidence).
Lactoferrin supplementation to enteral feeds with or without probiotics decreased bacterial and fungal sepsis but not CLD or length of hospital stay (low‐quality evidence). Investigators reported no adverse effects and did not evaluate long‐term neurological outcomes and PVL.
Authors' conclusions
Evidence of low quality suggests that lactoferrin supplementation to enteral feeds with or without probiotics decreases late‐onset sepsis and NEC stage II or III in preterm infants without adverse effects. Completed ongoing trials will provide data from more than 6000 preterm neonates, which may enhance the quality of the evidence. Clarification regarding optimal dosing regimens, types of lactoferrin (human or bovine), and long‐term outcomes is needed.
Keywords: Humans; Infant, Newborn; Enteral Nutrition; Administration, Oral; Bacterial Infections; Bacterial Infections/epidemiology; Bacterial Infections/prevention & control; Cause of Death; Chronic Disease; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/epidemiology; Enterocolitis, Necrotizing/prevention & control; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/prevention & control; Lactobacillus rhamnosus; Lactoferrin; Lactoferrin/administration & dosage; Lung Diseases; Lung Diseases/epidemiology; Mycoses; Mycoses/epidemiology; Mycoses/prevention & control; Numbers Needed To Treat; Probiotics; Probiotics/administration & dosage; Randomized Controlled Trials as Topic; Retinopathy of Prematurity; Retinopathy of Prematurity/epidemiology; Sepsis; Sepsis/prevention & control
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants
Review question: Does administering lactoferrin with feeds decrease the risk of sepsis or necrotizing enterocolitis in preterm babies?
Background: Preterm babies are at risk for blood infection (sepsis) and/or gastrointestinal injury (necrotizing enterocolitis, or NEC). Many babies with sepsis or NEC die or develop long‐term brain and lung injury despite treatment with antibiotics. Lactoferrin, a protein that is present in human milk, has been shown to be effective against infection when tested in animals and in the laboratory. Lactoferrin also enhances the ability of babies to fight infection.
Study characteristics: Through literature searches updated to December 2016, we found six studies that enrolled 1041 preterm babies and tested the role of lactoferrin along with feeds. We also found large ongoing studies that may increase the strength of our findings when their results become available.
Key results: Evidence of low quality suggests that oral lactoferrin with or without a probiotic decreases blood infection and NEC in preterm infants with no adverse effects. Clarification regarding dosing, duration, type of lactoferrin (human or bovine), and development of preterm babies is still needed.
Quality of evidence: Evidence is of low quality.
Summary of findings
Summary of findings for the main comparison.
Oral lactoferrin compared with placebo for prevention of sepsis and necrotizing enterocolitis in preterm infants
| Oral lactoferrin compared with placebo for prevention of sepsis and necrotizing enterocolitis in preterm infants | ||||||
| Patient or population: preterm infants Settings: neonatal intensive care units Intervention: oral lactoferrin alone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lactoferrin alone | |||||
| Any late‐onset sepsis ‐ all infants | Low‐risk populationa | RR 0.59 (0.40 to 0.87) | 886 (6 studies) | ⊕⊕⊝⊝ Low1,2,3 | Blinding of healthcare provider and blinding of outcome assessment unclear Moderate or severe heterogeneity (> 50% heterogeneity) |
|
|
82 per 1000 (42 to 132 per 1000) |
37 per 1000 (32 to 42 per 1000) | |||||
| High‐risk populationb | ||||||
|
270 per 1000 (170 to 333 per 1000) |
146 per 1000 (59 to 182 per 1000) | |||||
| Bacterial sepsis | Low‐risk populationa | RR 0.46 (0.29 to 0.74) | 760 (4 studies) | ⊕⊕⊕⊝ Low1,3 | Moderate or severe heterogeneity (> 50% heterogeneity) |
|
|
81 per 1000 (42 to 119 per 1000) |
37 per 1000 (32 to 42 per 1000) | |||||
| High‐risk populationb | ||||||
|
226 per 1000 (119 to 333 per 1000) |
98 per 1000 (59 to 136) | |||||
| All‐cause mortality | Low‐risk populationa | RR 0.65 (0.37 to 1.11) | 1041 (6 studies) | ⊕⊕⊕⊝ Low1,3 | Moderate or severe heterogeneity (> 50% heterogeneity) |
|
|
54 per 1000 (32 to 75 per 1000) |
37 per 1000 (0 to 100) | |||||
| High‐risk populationb | ||||||
|
54 per 1000 (0 to 103 per 1000) |
42 per 1000 (0 to 100) | |||||
| NEC ≥ stage II | Study populationb | RR 0.4 (0.18 to 0.86) | 750 (4 studies) | ⊕⊕⊕⊝ Low1,3 | Moderate or severe heterogeneity (> 50% heterogeneity) |
|
|
80 per 1000 (17 to 200 per 1000) |
20 per 1000 (0 to 34 per 1000) | |||||
| Chronic lung disease | Study populationb | RR 0.86 (0.52 to 1.42) | 520 (3 studies) | ⊕⊕⊝⊝ Low1 | ||
|
162 per 1000 (36 to 282 per 1000) |
148 per 1000 (26 to 300) | |||||
| Threshold retinopathy of prematurity | Study populationb | RR 0.50 (0.27 to 0.97) | 400 (2 studies) | ⊕⊕⊝⊝ Low4 | ||
|
159 per 1000 (113 to 205 per 1000) |
107 per 1000 (39 to 175 per 1000) | |||||
| Length of stay among survivors | Mean length of stay among survivors in the intervention groups was 1.8 higher (2.23 lower to 5.83 higher) | 505 (1 study) | ⊕⊕⊝⊝ Low5 | |||
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio aStudies included infants ≥ 1500 g bStudies included infants < 1500 g | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1Methods of randomization and allocation concealment are not available for 1 study 2Blinding of the healthcare provider and blinding of outcome assessment unclear 3Moderate or severe heterogeneity (> 50% heterogeneity) 4Only 2 studies 5Only 1 study
Summary of findings 2.
Oral lactoferrin + probiotics compared with placebo for prevention of sepsis and necrotizing enterocolitis in preterm infants
| Oral lactoferrin + probiotics compared with placebo for prevention of sepsis and necrotizing enterocolitis in preterm infants | ||||||
| Patient or population: preterm infants Settings: neonatal intensive care units Intervention: lactoferrin + probiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lactoferrin + probiotics | |||||
| Any late‐onset sepsis ‐ all infants | Study population | RR 0.27 (0.12 to 0.6) | 319 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 143 per 1000 | 46 per 1000 | |||||
| Bacterial sepsis | Study population | RR 0.28 (0.11 to 0.72) | 319 (1 study) | ⊕⊕⊕⊝ Moderate a,b |
||
| 119 per 1000 | 33 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.54 (0.25 to 1.18) | 496 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 70 per 1000 | 38 per 1000 | |||||
| NEC ≥ stage II | Study population | RR 0.04 (0 to 0.62) | 496 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 54 per 1000 | 0 per 1000 | |||||
| Chronic lung disease | Study population | RR 0.67 (0.25 to 1.79) | 319 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 60 per 1000 | 38 per 1000 | |||||
| Threshold retinopathy of prematurity | Study population | RR 0.76 (0.39 to 1.49) | 319 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 113 per 1000 | 86 per 1000 | |||||
| Length of stay among survivors | Mean length of stay among survivors in the intervention groups was 2 higher (1.88 lower to 5.88 higher) | 496 (1 study) | ⊕⊕⊝⊝ Lowa,b | |||
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aBlinding of the healthcare provider and blinding of outcome assessment unclear bData from a single study
Background
Description of the condition
Neonatal sepsis is the most common cause of neonatal death worldwide (Lawn 2006). The incidence of neonatal sepsis in the developed world is reported to be between one and four cases per 1000 live births (Stoll 2004b). In the developing world, the rate of neonatal sepsis is significantly higher (6.5 to 38 per 1000 live hospital births) (Zaidi 2005). Sepsis is a particular problem in very low birth weight (VLBW) infants (birth weight < 1500 g); early‐onset sepsis (sepsis in infants at < 72 hours of life) occurs in about 1.5% and late‐onset sepsis in about 21% of VLBW infants (Stoll 2002; Stoll 2005). Most infections are caused by Staphylococcus and Candida species. Mortality and morbidity (including patent ductus arteriosus, prolonged ventilation, prolonged need for intravascular access, bronchopulmonary dysplasia, necrotizing enterocolitis, and increased length of hospital stay) are significantly increased among infected infants. In a large cohort study of infants born weighing less than 1000 g, infected infants had a significantly higher incidence of adverse neurodevelopmental outcomes at follow‐up when compared with uninfected infants (Stoll 2004a).
Necrotizing enterocolitis (NEC) occurs in 1% to 5% of admissions to the neonatal intensive care unit (NICU) (Lin 2006). The most consistent risk factors are prematurity and low birth weight. Gastrointestinal immaturity, enteral feeding (especially formula feeding), presence of bacteria, and inflammation in the gastrointestinal (GI) tract may all contribute to the development of NEC (Lin 2006). Host‐pathogen interactions trigger inflammation in the gut, which may contribute to the pathogenesis of NEC and septic shock (Blackwell 1997; Neish 2004). NEC significantly increases mortality (attributable mortality of 15% to 30%) and morbidity (including surgery in 20% to 40% of infants and delayed neurodevelopment) (Bell 1978; Lin 2006; Stoll 2004a).
Mortality and morbidity due to sepsis and NEC remain high despite the use of potent antimicrobial agents (Stoll 2002; Stoll 2005). Increased use of antimicrobials has led to the emergence of antibiotic‐resistant strains of bacteria (Levy 1998). Adverse pulmonary and neurodevelopmental outcomes after sepsis or NEC may be due to inflammatory injury (Adams‐Chapman 2006; Speer 1999). Agents that modulate inflammation and enhance host defenses may improve the outcomes of infants with neonatal sepsis or NEC.
Description of the intervention
The glycoprotein lactoferrin is a component of the innate immune response and a potent immunomodulator (Legrand 2016). It is found in significant concentrations in human colostrum and in lower concentrations in human milk, tears, saliva, and seminal fluid, and in secondary granules of neutrophils. Lactoferrin has broad‐spectrum antimicrobial activity against bacteria, fungi, viruses, and protozoa, which may result from its ability to sequester iron, or may occur as a direct lytic effect on microbial cell membranes (Valenti 2005). Proteolysis of lactoferrin under acidic conditions (as would occur in the stomach or in the phagolysosomes of neutrophils) yields peptides called lactoferricins, which have enhanced antimicrobial activity (Gifford 2005).
Current increased interest in lactoferrin stems not only from improved understanding of its functions, but also from its increased availability in various forms and sources. Lactoferrin processed from bovine and human milk is available commercially as a food supplement (Swedish Dairies Association, Tatua Co‐operative Dairy Company in New Zealand, Lacto Bretagne Associes' in Belgium, Milei in Germany, Morinaga Industries in Japan, DoMO Food Ingredients, a subsidiary of Friesland Dairy Foods, in the Netherlands, etc). In the United States, human recombinant lactoferrin (talactoferrin from Agennix, Inc., Houston, Texas, USA) has an investigational new drug status for clinical research purposes. Lactoferrin expression in transgenic rice (Ventrus Biosciences, New York City, New York, USA) and in transgenic maize (Meristem Therapeutics, Clermont‐Ferrand, France) is being researched. Bovine lactoferrin is less expensive than human lactoferrin and is affordable even in developing countries.
How the intervention might work
Lactoferrin inhibits the growth of Staphylococcus epidermidis and Candida albicans in vitro (Valenti 2005). It reduces the minimum inhibitory concentrations of vancomycin against S epidermidis and of antifungal agents such as azoles and amphotericin against Candida (Kuipers 1999; Leitch 1999). Lactoferrin and lactoferrin‐derived peptides are highly effective in vitro against antibiotic‐resistant Klebseilla and Staphylococcus aureus (Nibbering 2001).
Lactoferrin prophylaxis is effective in animal models of systemic and intestinal infection. In mice infected with Escherichia coli, pretreatment with lactoferrin improved survival from 4% to 70% (Zagulski 1989). In neonatal rats, lactoferrin reduced the severity of blood and liver infection after enteral infection with E coli (Edde 2001). Parenteral prophylaxis with lactoferrin enhanced survival in a neonatal rat model of polymicrobial infection with C albicans and S epidermidis (Venkatesh 2007). In a germ‐free, colostrum‐deprived piglet model challenged with E coli lipopolysaccharide, oral pretreatment with lactoferrin reduced mortality from 74% to 17% after challenge with E coli lipopolysaccharide (Artym 2004). In animal colitis, lactoferrin reduced intestinal injury and inflammation (Togawa 2002). The systemic effects of oral lactoferrin generally are thought to be indirect and probably are initiated by contact with intestinal epithelial cells and gut‐associated lymphoid tissue (GALT). Lactoferrin modulates cytokine and/or chemokine production by GALT cells, which then enter the systemic circulation and influence circulating leukocytes (Bellamy 1992; Tomita 2002). Lactoferrin and other similar products in milk (probiotics) create an environment for growth of beneficial bacteria within the gut, reducing colonization with pathogenic bacteria. Demonstrated intestinal receptors for lactoferrin and its ability to modulate intestinal cell differentiation and proliferation (Buccigrossi 2007) make lactoferrin a promising agent for prevention or treatment of NEC.
In adult humans, oral recombinant human lactoferrin has been found to be safe and well tolerated. Oral lactoferrin has shown promise as an antitumor agent (Hayes 2006) and has been shown to reduce viremia in chronic hepatitis C infection (Iwasa 2002; Tanaka 1999). In patients with acute myeloid leukemia and neutropenia, lactoferrin reduced the incidence, duration, and severity of bacteremia due to enteric pathogens (Trumpler 1989). To date, animal and human studies have reported no significant adverse effects.
Lactoferrin provides significant potential benefit for premature infants including antimicrobial and immunomodulatory effects and promotion of neurodevelopment (Manzoni 2016; Ochoa 2017). Systematic reviews on probiotics in preterm infants have reported decreased NEC and mortality (Alfaleh 2014; Dermyshi 2017). Lactoferrin has been reported to act synergistically with probiotic strains of bacteria, enhancing their growth and inhibiting intestinal pathogens (Chen 2017; Tian 2010).
Why it is important to do this review
The potential beneficial effects of lactoferrin make it a promising agent for prevention of neonatal sepsis and NEC. This review evaluated the role of lactoferrin supplementation of enteral feeds in prevention of neonatal sepsis or NEC in preterm neonates.
Objectives
Primary objective
To assess the safety and effectiveness of lactoferrin supplementation to enteral feeds for prevention of sepsis and NEC in preterm neonates
Secondary objectives
To determine the effects of lactoferrin supplementation to enteral feeds to prevent neonatal sepsis and/or NEC on duration of positive‐pressure ventilation, development of chronic lung disease (CLD) or periventricular leukomalacia (PVL), length of hospital stay to discharge among survivors, and adverse neurological outcomes at two years of age or later
To determine the adverse effects of enteral lactoferrin supplementation in the prophylaxis of neonatal sepsis and/or NEC
When data were available, we analyzed the following subgroups.
Gestational age < 32 weeks and 32 to 36 weeks
Birth weight < 1000 g (extremely low birth weight (ELBW) infants) and birth weight < 1500 g (very low birth weight (VLBW) infants)
Type of feeding: breast milk versus formula milk
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials that have been completed (published or unpublished).
Types of participants
Preterm (< 37 completed weeks of gestation) neonates (< 28 days).
Types of interventions
Lactoferrin supplementation of enteral feeds at any dosage or duration used to prevent neonatal sepsis or NEC compared with placebo or no intervention. Separate analyses were performed for oral lactoferrin given with or without additional probiotics.
Types of outcome measures
Primary outcomes
-
Confirmed or suspected sepsis during hospital stay
Confirmed sepsis is defined as clinical signs and symptoms consistent with infection and microbiologically proven with a positive blood culture, cerebrospinal fluid (CSF) culture, urine culture (obtained by a suprapubic tap), or culture from a normally sterile site (eg, pleural fluid, peritoneal fluid, autopsy specimens) for bacteria or fungi
Suspected sepsis is defined as clinical signs and symptoms consistent with sepsis without isolation of a causative organism
-
NEC Bell's stage II or III during hospital stay
Necrotizing enterocolitis (NEC) (definitive NEC and perforated NEC, Bell's stage II or III) (Bell 1978) during hospital stay
"All‐cause mortality" during hospital stay
Secondary outcomes
Neurological outcome at two years of age or later (neurodevelopmental outcome as assessed by a validated test)
Chronic lung disease (CLD) in survivors (CLD defined as oxygen requirement at 36 weeks' postmenstrual age (PMA))
Adverse outcomes directly attributable to oral lactoferrin: increased gastric residuals (gastric aspirate > 10% of oral feed), vomiting, and other GI disturbances during hospital stay
Periventricular leukomalacia (PVL) (defined as necrosis of brain white matter in a characteristic distribution, ie, in the white matter dorsal and lateral to the external angles of lateral ventricles involving particularly the centrum semi‐ovale and optic and acoustic radiations and diagnosed by magnetic resonance imaging (MRI), or as periventricular cystic lesions seen on cranial ultrasonography (Volpe 1995) at discharge or at neurodevelopmental follow‐up)
Duration of assisted ventilation through an endotracheal tube measured in days during hospital stay
Length of hospital stay measured in days to discharge for survivors
Post hoc analyses of bacterial infection, fungal infection, threshold retinopathy of prematurity, and urinary tract infection
Search methods for identification of studies
We used the standard search methods of the Cochrane Neonatal Review Group and updated the search in December 2016.
Electronic searches
We identified relevant trials by searching the following.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library.
Electronic journal reference databases: MEDLINE (1966 to present) and PREMEDLINE, Embase (1980 to present), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to present).
Websites for ongoing trials: www.clinicaltrials.gov, www.controlled‐trials.com, Australian and New Zealand Clinical Trials Registry (http://www.anzctr.org.au), and the World Health Organization (WHO) International Clinical Trials Registry and Platform (http://www.who.int/ictrp/search/en/).
Abstracts of conferences from proceedings of Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research, and European Society for Pediatric Research) from 1990 to the present in the journal Pediatric Research and in Abstracts Online
We used this search strategy for MEDLINE and PREMEDLINE (adapted strategy as needed to suit Embase, CINAHL, and CENTRAL).
explode "sepsis" [all subheadings in MIME, MJME].
sepsis or septicemia.
septic.
NEC.
"necrotizing enterocolitis".
# 1 or # 2 or # 3 or # 4 or # 5.
explode "infant ‐ newborn" [all subheadings in MIME, MJME].
Neonat*.
Newborn*.
# 7 or # 8 or # 9.
# 6 and # 10.
"lactoferrin" [all subheadings on MIME, MJME].
talactoferrin.
# 10 or # 11.
# 9 and # 12.
We applied no language restrictions. We searched randomized and quasi‐randomized trials identified by review of study abstracts.
Searching other resources
We contacted study authors who published in this field to ask about unpublished articles.
We performed additional searches of the reference lists of identified clinical trials and of review authors' personal files.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group for conducting a systematic review (http://neonatal.cochrane.org/en/index.html).
Selection of studies
Two review authors assessed the titles and abstracts of studies identified by the search strategy to determine eligibility for inclusion in this review. If this could not be done reliably by title and abstract review, we obtained full‐text versions for assessment. We resolved differences by mutual discussion and obtained full‐text versions of all eligible studies for quality assessment.
Data extraction and management
We designed forms for documenting trial inclusion/exclusion, for extracting data, and for requesting additional published information from authors of the original reports. We independently extracted data using specially designed paper forms.
Assessment of risk of bias in included studies
Two review authors (MP, GS) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane "Risk of bias" tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 2 for a detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses according to recommendations of the Cochrane Neonatal Review Group. We analyzed all randomly assigned infants on an "intention‐to‐treat basis," irrespective of whether they received their allocated treatment. We analyzed treatment effects in individual trials. We used the statistical package (RevMan 5.3) provided by the Cochrane Collaboration. We reported risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs) for dichotomous outcomes, and weighted mean differences for continuous outcomes. We calculated and reported the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) for statistically significant reductions in RD.
Unit of analysis issues
We included randomized and quasi‐randomized trials and used each participant as the unit of analysis. We did not encounter repeated measurements, and we excluded cluster‐randomized and cross‐over trials.
Dealing with missing data
We contacted Manzoni and colleagues to obtain missing data on infection from the complete cohort and are awaiting a response.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using the I2 statistic to check the appropriateness of pooling data and performing meta‐analyses. We deferred meta‐analysis if heterogeneity was high (≥ 75%). We used the following cut‐offs to report the degree of heterogeneity: < 25% no heterogeneity; 25% to 49% low heterogeneity; 50% to 74% moderate heterogeneity; and ≥ 75% high heterogeneity. If we detected statistical heterogeneity, we explored possible causes (eg, differences in study quality, participants, intervention regimens, or outcome assessments) by performing post hoc subgroup analyses.
Assessment of reporting biases
We included fewer than 10 trials in our meta‐analysis and hence did not create a funnel plot for reporting bias.
Data synthesis
We used a fixed‐effect model for meta‐analysis when appropriate, with Review Manager software (RevMan 2014) supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: any late‐onset sepsis ‐ all infants, bacterial sepsis, all‐cause mortality, NEC ≥ stage II, chronic lung disease, threshold retinopathy of prematurity, length of stay among survivors.
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based on the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a "Summary of findings" table to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to one of four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Key subgroups were based on the following.
-
Gestational age.
Preterm infants (32 to 36 weeks' gestational age).
Preterm infants (< 32 weeks' gestational age).
-
Birth weight.
VLBW infants (birth weight < 1500 g).
ELBW infants (birth weight < 1000 g).
-
Feedings.
Breast milk feeding.
Formula feeding.
Sensitivity analysis
We did not identify a need for and did not perform a sensitivity analysis.
Results
Description of studies
Results of the search
Our search strategy yielded six randomized controlled trials (published in eight reports) that were eligible for inclusion. Three published reports described one multicenter trial of oral lactoferrin prophylaxis in premature neonates (Manzoni 2014), and the other five included studies enrolled preterm neonates from the United States (Sherman 2016), Turkey (Akin 2014), Canada (Barrington 2016), India (Kaur 2015), and Peru (Ochoa 2015). Refer to the Characteristics of included studies table for details.
Included studies
This continuation of a randomized trial (Manzoni 2009) was conducted to enhance power for assessing effects of oral bovine lactoferrin in prevention of NEC. Thirteen neonatal intensive care units (NICUs) in Italy and New Zealand participated and enrolled neonates from October 1, 2007, through July 31, 2010. Interventions and patient populations and outcomes were similar to those included in the Manzoni 2009 study.
Manzoni 2009: Manzoni and coworkers randomly assigned VLBW infants (birth weight < 1500 g) in 11 Italian NICUs to oral bovine lactoferrin alone or in combination with a probiotic (Lactobacillus rhamnosus GG) or to placebo. Late‐onset sepsis, defined as isolation of a pathogen in the blood, peritoneal fluid, or CSF after three days of life, was the primary outcome of interest. Secondary outcomes assessed included gram‐positive, gram‐negative, or fungal sepsis; mortality before hospital discharge; urinary tract infection; fungal colonization; progression from fungal colonization to invasive fungal infection; bronchopulmonary dysplasia (BPD); severe intraventricular hemorrhage (grade III or IV); threshold retinopathy of prematurity (ROP); NEC ≥ stage II; alteration of liver functions; and adverse effects.
Manzoni 2012: This report presents the secondary analysis of data from Manzoni 2009 pertaining to fungal colonization and invasive fungal infections. Interventions and patient populations were similar to those in the Manzoni 2009 study. Prophylaxis with antifungal drugs was an exclusion criterion and was not permitted by the study protocol. Primary outcomes assessed were incidence rates of fungal colonization and invasive fungal infection. Secondary outcomes included intensity of fungal colonization, rate of progression to infection in colonized infants, frequencies of single fungal species in all groups, and mortality related to invasive fungal infections.
This prospective, single‐center, double‐blind, randomized controlled trial was performed at Ankara University, Turkey, between December 2009 and January 2011. Investigators randomly assigned inborn neonates born at < 1500 g or at gestational age < 32 weeks to bovine lactoferrin (200 mg/d) or placebo (2 mL of saline), once a day until discharge. Exclusion criteria were lack of parental consent, severe congenital malformations, severe hypoxic ischemic encephalopathy (HIE), and death before 72 hours of life. Primary outcomes assessed were nosocomial sepsis as defined by criteria of the Centers for Disease Control and Prevention and NEC stage II. Secondary outcomes included safety (feeding tolerance, abdominal distention, emesis, and gastric residuals), length of hospital stay, and maturation of regulatory T‐cell (Treg) levels.
Ochoa and coworkers enrolled 190 premature infants < 2500 g in five neonatal intermediate and intensive care units in Lima, Peru, who were admitted to the NICU during the first 72 hours of life. Researchers randomly assigned neonates to oral bovine lactoferrin (200 mg/kg/d divided into three doses) or to oral maltodextrin (200 mg/kg/d in three divided doses) for four weeks; they dissolved both in human milk or formula or in 5% glucose solution. The primary outcome assessed was the number of confirmed episodes of late‐onset sepsis in the first month of life; secondary outcomes assessed were incidence of gram‐positive and gram‐negative bacterial sepsis, fungal sepsis, pneumonia, diarrhea, and mortality in the first month of life.
This randomized clinical trial of human recombinant lactoferrin (talactoferrin (TLF)) conducted in the United States enrolled a total of 120 neonates (60 in each group). Investigators randomly assigned preterm infants with birth weight of 750 to 1500 g to enteral TLF or to placebo from 1 to 29 days of life at a dose of 150 mg/kg every 12 hours. (TLF was provided by Agennix, Inc.) Primary outcomes assessed were bacteremia, meningitis, pneumonia, urinary tract infection, and necrotizing enterocolitis; secondary outcomes were sepsis syndrome and suspected NEC.
This randomized controlled trial of oral bovine lactoferrin in Montreal, Canada, enrolled 79 neonates between January 2011 and April 2013. Investigators randomly assigned preterm infants in the NICU at CHU Sainte Justine, with a gestational age at birth between 23 0/7 and 30 6/7 weeks, who were less than 48 hours of age, to oral lactoferrin or placebo. The exclusion criterion was the presence of intestinal abnormalities that would prevent enteral feeding, such as gastroschisis. The intervention group received 100 mg per day of bovine lactoferrin, divided into two doses per day, starting on the first day of enteral feeding (day of enrollment) or at the latest at 48 hours of age and until 36 weeks' PMA or discharge home. The control group received milk without lactoferrin. The primary outcome assessed was feeding tolerance, defined as the length of time required to achieve 140 mL/kg/d; secondary outcomes were death, late‐onset sepsis, combined variable of death or late‐onset sepsis, NEC stage II or III, duration of total parenteral nutrition (TPN), number of times made nil by mouth, growth variables at discharge, ROP, and BPD.
This trial randomized inborn neonates with birth weight less than 2000 g, who had no maternal risk factors for sepsis, to bovine lactoferrin or to placebo from day 1 to day 28 of life. The dose of lactoferrin ranged from 100 to 250 mg and was based on birth weight. Criteria for exclusion were congenital anomalies, severe birth asphyxia, history of maternal chorioamnionitis, suspected congenital infection, and family history of cow's milk allergy. Neonates with culture‐proven early‐onset sepsis were also excluded. The primary outcome was culture‐proven late‐onset sepsis. Secondary outcome measures were probable late‐onset sepsis, any late‐onset sepsis, and sepsis‐attributed mortality.
Excluded studies
Investigators enrolled healthy, formula‐fed infants at 34 weeks' gestation or later and at four weeks of age or younger from a pediatric clinic. Infants received formula supplemented with lactoferrin (850 mg/L) or commercial cow's milk‐based formula (102 mg/L) for 12 months. Researchers collected growth parameters and information on gastrointestinal, respiratory, and colic illnesses for the infants' first year. Review authors excluded this study, as most enrolled infants were beyond the neonatal period and trial authors did not assess our prespecified neonatal outcomes.
This community‐based, randomized, double‐blind, placebo‐controlled trial compared supplementation with bovine lactoferrin versus placebo. Investigators randomly assigned 577 weaned children at 12 to 18 months and followed them for six months with daily home visits. Treatment was given to prevent diarrhea, and outcomes assessed included number of diarrheal episodes, longitudinal prevalence of diarrhea, and severity of diarrhea and dehydration. Review authors excluded this study, as participants were not neonates.
This non‐randomized, retrospective, observational study compared the lactoferrin prophylaxis cohort (2004‐2011) with an historical cohort without lactoferrin prophylaxis (2001‐2004). The prophylaxis cohort received 100 mg of bovine lactoferrin and a probiotic. This conference abstract reported rates of NEC, late‐onset sepsis, and ROP treatment. Review authors excluded this study because it was a non‐randomized study.
Studies awaiting classification
This randomized controlled trial of lactoferrin supplementation included preterm infants with birth weight ≤ 1500 g and/or gestational age ≤ 32 weeks. The study excluded neonates if fetal‐onset disorders were recognizable at birth, and if milk intolerance, family history of allergy, and use of infant formula supplemented with lactoferrin were reported. The intervention group (n = 650) received a daily dose of 100 mg of lactoferrin, and the control group (n = 650) received only standard therapy. Primary outcomes to be assessed were antioxidant effects of lactoferrin and its ability to reduce free radical‐related diseases in the newborn; these were assessed through neurodevelopmental follow‐up. The secondary outcome was identification of a panel of markers for assessment of oxidative stress and for correlation with the lactoferrin antioxidant effect. This study planned to enroll 1300 neonates starting January 2011. We have re‐requested details of the study from the principal investigator.
This was a prospective, double‐blind, randomized, placebo‐controlled study of preterm infants (n = 60) with gestational age 26 ± 0 to 35 ± 6 weeks. Researchers excluded neonates if born weighing < 600 g, or if they had life‐threatening congenital malformations, non‐Dutch or English‐speaking parents, or a history of allergy among parents or siblings. Trial investigators randomly assigned infants to standard preterm formula, standard preterm formula with probiotics (galacto‐oligosaccharides 28.5%, lactose 9.5%, galactose 0.5%, minerals 3.5%, fat 1.5%, and water 3%), or standard preterm formula with dairy lactoferrin 1 mg/100 mL (n = 20 in each group). The primary outcome assessed was composition of the gut flora at six weeks of full enteral feeds, incidence of infection, oxidative stress, and iron status. Secondary outcomes assessed were growth (weight, length, and head circumference), feeding intolerance, and psychomotor development at one year of age. This unpublished study was completed in 2009. We have re‐requested details of the study from the principal investigator.
This recently completed randomized controlled study enrolled 180 preterm neonates (< 37 weeks' gestation counting from the first day of the last menstrual period and confirmed by Ballard score) admitted to the NICUs of Ain Shams University Hospitals during the period from August 2014 to December 2015. Researchers further randomly subdivided enrolled participants into three groups according to the dose regimen of lactoferrin supplementation: Group A (60 preterm neonates) received oral lactoferrin supplementation at a dose of 100 mg/d starting on day 1 and continuing for four to six weeks; Group B (60 preterm neonates) received oral lactoferrin supplementation at a dose of 100 mg/d starting on day 3 (48 to 72 hours) of life and continuing for four to six weeks; and Group C (60 preterm neonates) matched subjected neonates and received placebo in the form of distilled water. Primary outcomes included evaluation of the effectiveness of oral lactoferrin in preventing neonatal sepsis according to Tollner score, hematological scoring system (HSS), and positive blood culture.over four to six weeks of life. Secondary outcomes included evaluation of the effects of lactoferrin supplementation on long‐term complications of BPD (defined by clinical symptoms and signs and chest X‐ray findings), ROP (as defined by the International Classification of Retinopathy of Prematurity (ICROP)), NEC (defined by Modified Bell's criteria), and any reported side effects for bovine lactoferrin. The trial was completed in October 2016.
Ongoing studies
This double‐blind, randomized, controlled trial included neonates weighing between 500 g and 2500 g and at ≤ 36 weeks' gestation, who were born in or were referred to the NICU of one of the participating hospitals during the first 48 hours of life. Investigators randomly assigned preterm neonates to one of three groups: low‐dose lactoferrin (100 mg/d), high‐dose lactoferrin (150 mg/kg/twice daily), or placebo (distilled water). The primary outcome assessed was blood culture positivity; secondary outcomes were complete blood count with differential leukocyte count and C‐reactive protein quantitative assay. This study was scheduled to start in June 2013 and planned to enroll 180 preterm neonates through January 2016. We have re‐requested details of the study from the principal investigator.
This multicenter, randomized, placebo‐controlled trial conducted in the United Kingdom is examining prophylactic enteral lactoferrin supplementation to prevent late‐onset invasive infection in very preterm infants. Infants are eligible to participate if gestational age at birth is < 32 weeks, if they are < 72 hours old, and if written informed parental consent is obtained. Researchers randomly assign infants to receive lactoferrin (150 mg/kg/d to a maximum of 300 mg) or placebo. Primary outcomes assessed include the incidence of microbiologically confirmed or clinically suspected late‐onset infection from trial entry until hospital discharge. Secondary outcomes include "all‐cause mortality" before hospital discharge; necrotizing enterocolitis (NEC) Bell’s stage II or III; severe ROP treated medically or surgically; BPD; a composite of invasive infection, major morbidity (NEC, ROP, or BPD), and mortality; number of days of administration of antibiotics per infant from 72 hours until death or discharge from hospital; number of days of administration of antifungal agents per infant; and length of hospital stay. This study is coordinated by the National Perinatal Epidemiology Unit Clinical Trials Unit, at the University of Oxford, UK; it was scheduled to start in September 2013 and planned to enroll 2200 preterm neonates.
This phase 3 randomized controlled trial of oral lactoferrin for prevention of sepsis in infants (NEOLACTO study) is being conducted in Peru. Neonates with birth weight between 500 g and 2000 g and born in or referred to the neonatal unit of one of the participating hospitals during the first 72 hours of life are eligible. Investigators randomly assign preterm neonates to oral bovine lactoferrin (200 mg/kg/d divided in three doses) or oral maltodextrin (200 mg/kg/d in three divided doses) for eight weeks. The primary outcome assessed is a composite outcome of first episode of late‐onset sepsis or sepsis‐associated death. The secondary outcome is neurodevelopment at 24 months' corrected age assessed by the Mullen Scale for Early Learning. This trial started enrolling in May 2012 and targeted to enroll 414 neonates through January 2016. We have requested details of the study from the principal investigator.
The Lactoferrin Infant Feeding Trial (LIFT) to prevent sepsis and death in preterm infants is a double‐blind, randomized, controlled trial that is being conducted in Australia and New Zealand. Eligibility for inclusion is based on the following: (1) doctor and parents are substantially uncertain whether bovine lactoferrin (BLF) is indicated or contraindicated, (2) < 1500 g birth weight, (3) < 7 days old, and (4) written informed consent from the parent. Researchers will randomly assign neonates to BLF at 200 mg/kg/d dissolved in breast milk or formula until 34 weeks' corrected gestational age or hospital discharge or to placebo (breast milk or formula (without BLF)). The primary outcome that will be assessed is mortality or major morbidity before hospital discharge. Morbidity is defined as the diagnosis of sepsis, brain injury, chronic lung disease, necrotizing enterocolitis, or severe retinopathy. Secondary outcomes that will be assessed include mortality related to sepsis (as assessed by positive blood culture). This study started enrollment in January 2014 and planned to enroll 1100 infants.
Risk of bias in included studies
Allocation
In the multicenter trial of Manzoni 2009, investigators stratified randomization by center and generated randomization sequences by using computer software. The pharmacy at each center prepared the interventions and diluted them in milk feeds on the basis of random sequences. Allocation concealment is unclear, as it is difficult to predict whether the pharmacy was aware of future allocations. Akin 2014 did not report random sequence generation nor allocation concealment. Sherman 2016 randomly assigned enrolled neonates centrally using a permuted block method. Ochoa 2015, Barrington 2016, and Kaur 2015 had low risk of selection bias, as researchers reported adequate randomization and allocation concealment methods.
Blinding
Manzoni 2009 investigators diluted interventions in feeds and blinded clinical and research staff to the intervention. When the infant was not fed and interventions were administered by orogastric tube without milk, it is not clear whether blinding was adequate. Other included studies did not show performance bias.
None of the included studies explicitly reported blinding of outcome assessors.
Incomplete outcome data
Researchers in included studies assessed outcomes at hospital discharge and adequately accounted for incomplete data.
Selective reporting
Included studies did not reveal selective outcome reporting or other biases.
Effects of interventions
Six randomized controlled studies (published as eight reports) were eligible for inclusion; three reports described one multicenter trial of oral lactoferrin prophylaxis in premature neonates performed in Italy and New Zealand (Manzoni 2014). The other five included studies were conducted in the United States (Sherman 2016), Turkey (Akin 2014), India (Kaur 2015), Canada (Barrington 2016), and Peru (Ochoa 2015).
Lactoferrin supplementation of enteral feeds versus placebo (comparison 1)
All six included trials provided outcome data for this comparison (Akin 2014; Barrington 2016; Kaur 2015; Manzoni 2014; Ochoa 2015; Sherman 2016).
Late‐onset sepsis (outcome 1.1)
All infants (outcome 1.1.1)
Lactoferrin supplementation of enteral feeds in preterm infants decreased late‐onset sepsis (typical RR 0.59, 95% CI 0.40 to 0.87; typical RD ‐0.06, 95% CI ‐0.10 to ‐0.02; NNTB 17, 95% CI 10 to 50; six studies, 886 participants) (Figure 1). Results show moderate heterogeneity (I2 = 55%) among the six trials for this outcome. We downgraded evidence to low quality because of potential risk of selection and performance bias in the included studies and moderate heterogeneity.
Figure 1.
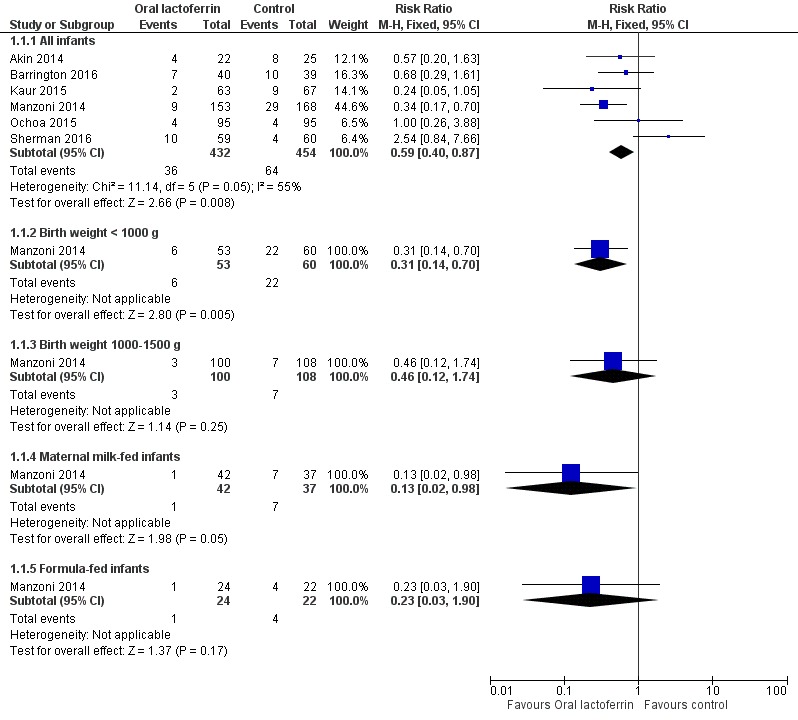
Forest plot of comparison: 1 Lactoferrin supplementation with enteral feeds versus placebo, outcome: 1.1 Any late‐onset sepsis.
Subgroup analyses for the outcome of late‐onset sepsis
Birth weight < 1000 g (outcome 1.1.2)
The estimated risk ratio for the outcome of late‐onset sepsis in ELBW infants was 0.31 (95% CI 0.14 to 0.70; RD ‐0.25, 95% CI ‐0.40 to ‐0.10; NNTB 4, 95% CI 2.5 to 25; one study, 113 participants) (Figure 1). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only one study.
Birth weight 1000 to 1500 g (outcome 1.1.3)
The estimated risk ratio for the outcome of late‐onset sepsis in this subgroup was 0.46 (95% CI 0.12 to 1.74; RD ‐0.03, 95% CI ‐0.09 to 0.020; one study, 208 participants) (Figure 1). We downgraded the quality of evidence to low because of unclear risk of selection and performance bias, and because data were derived from only one study.
Exclusively maternal milk‐fed infants (outcome 1.1.4)
The estimated risk ratio for the outcome of late‐onset sepsis in exclusively maternal milk fed infants was 0.13 (95% CI 0.02 to 0.98; RD ‐0.17, 95% CI ‐0.30 to ‐0.03; NNTB 5.8, 95% CI 3.3 to 33; one study, 79 participants) (Figure 1). This suggests a decrease in late‐onset sepsis among preterm infants exclusively receiving maternal milk supplemented with lactoferrin. We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only one study.
Formula‐fed infants (outcome 1.1.5)
The estimated risk ratio for the outcome of late‐onset sepsis in formula‐fed infants was 0.23 (95% CI 0.03 to 1.90; RD ‐0.14, 95% CI ‐0.32 to 0.04; one study, 46 participants) (Figure 1). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only one study.
NEC ≥ stage II (outcome 1.2)
Oral lactoferrin supplementation in preterm infants decreases NEC ≥ stage II (typical RR 0.40, 95% CI 0.18 to 0.86; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.01; NNTB 25, 95% CI 17 to 100; four studies, 750 participants) (Figure 2). We observed no heterogeneity (I2 = 0%) among the four trials for this outcome. We downgraded the quality of evidence to low because of risk of bias in the included trials and moderate heterogeneity.
Figure 2.
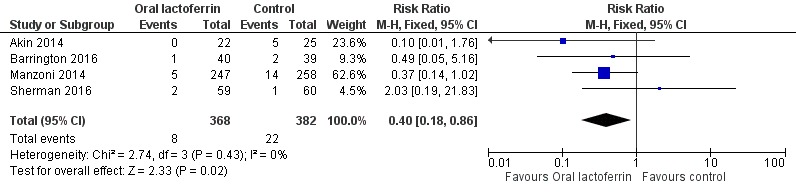
Forest plot of comparison: 1 Lactoferrin supplementation with enteral feeds versus placebo, outcome: 1.2 NEC ≥ stage II.
All‐cause mortality (outcome 1.3)
Lactoferrin supplementation of enteral feeds in preterm infants did not affect "all‐cause mortality" (typical RR 0.65, 95% CI 0.37 to 1.11; typical RD ‐0.02, 95% CI ‐0.05 to 0.00; six studies, 1071 participants) (Figure 3). We noted moderate heterogeneity (I2 = 54%) among the six included trials for this outcome. We downgraded the quality of evidence to low because of risk of bias in the included studies and moderate heterogeneity.
Figure 3.
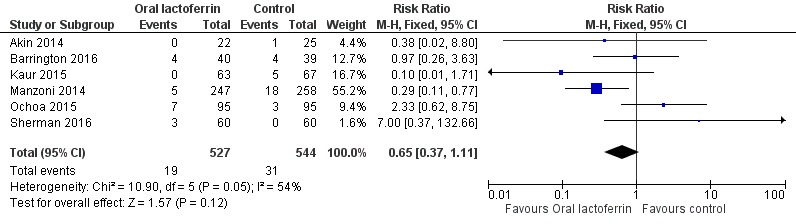
Forest plot of comparison: 1 Lactoferrin supplementation with enteral feeds versus placebo, outcome: 1.3 All‐cause mortality.
Bacterial sepsis (outcome 1.4)
The estimated risk ratio for the outcome of bacterial sepsis in preterm infants was 0.46 (95% CI 0.29 to 0.74; RD ‐0.07, 95% CI ‐0.11 to ‐0.03; NNTB 14, 95% CI 9 to 33; four studies, 760 participants) (Figure 1). We downgraded the quality of evidence to moderate because of unclear risk of detection bias in the included trials and unclear risk of selection bias in one trial.
Fungal sepsis (outcome 1.5)
The estimated risk ratio for the outcome of fungal sepsis in preterm infants was 0.11 (95% CI 0.02 to 0.60; RD ‐0.05, 95% CI ‐0.09 to ‐0.02; NNTB 20, 95% CI 11 to 50; two studies, 451 participants). This suggests a decrease in fungal sepsis among preterm infants whose feedings were supplemented with lactoferrin. We downgraded the quality of evidence to moderate because of unclear risk of bias.
Chronic lung disease (outcome 1.6)
The estimated typical risk ratio for the outcome of chronic lung disease was 0.86 (95% CI 0.52 to 1.42) and typical RD was ‐0.02 (95% CI ‐0.06 to 0.02) (three studies, 520 participants) (Figure 4). We observed no heterogeneity (I2 = 0%) among the two trials for this outcome. We downgraded the quality of evidence to low because of unclear risk of bias in the two included studies, and because data were derived from only two studies.
Figure 4.
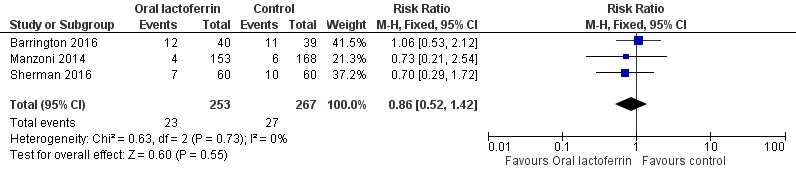
Forest plot of comparison: 1 Lactoferrin supplementation with enteral feeds versus placebo, outcome: 1.6 Chronic lung disease.
Duration of mechanical ventilation (outcome 1.7)
The estimated mean difference for the outcome of duration of mechanical ventilation in preterm infants was ‐0.53 (95% CI ‐1.47 to 0.41; two studies, 511 participants). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only two studies.
Length of hospital stay among survivors (outcome 1.8)
The estimated mean difference for the outcome of length of hospital stay among survivors in preterm infants was 1.80 (95% CI ‐2.23 to 5.83; one study, 505 participants). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only one study.
Threshold retinopathy of prematurity (outcome 1.9)
The estimated risk ratio for the outcome of threshold ROP in preterm infants was 0.50 (95% CI 0.27 to 0.94; RD ‐0.07, 95% CI ‐0.12 to ‐0.01; NNTB 14, 95% CI 8 to 100; two studies, 400 participants). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only two studies.
Urinary tract infection (outcome 1.10)
The estimated risk ratio for the outcome of urinary tract infection in preterm infants was 0.31 (95% CI 0.11 to 0.88; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; NNTB 20, 95% CI 11 to 100; two studies, 440 participants). We downgraded the quality of evidence to low because of unclear risk of bias, and because data were derived from only two studies.
Other outcomes
No study reported adverse effects for this comparison.
Included studies did not assess the following outcomes: neurological outcome at two years of age or older, and PVL.
Lactoferrin supplementation of enteral feeds in combination with probiotics versus placebo (comparison 2)
We derived outcome data for analyses for this comparison from one trial (Manzoni 2009), in which investigators randomly assigned preterm infants to oral bovine lactoferrin or oral bovine lactoferrin in combination with the probiotic Lactobacillus rhamnosus GG or placebo. We conducted subgroup analyses using birth weight and types of milk subgroups for late‐onset sepsis for the outcome of "late‐onset sepsis." Data for subgroup analyses for other outcomes were not available.
Late‐onset sepsis (outcome 2.1)
All infants (outcome 2.1.1)
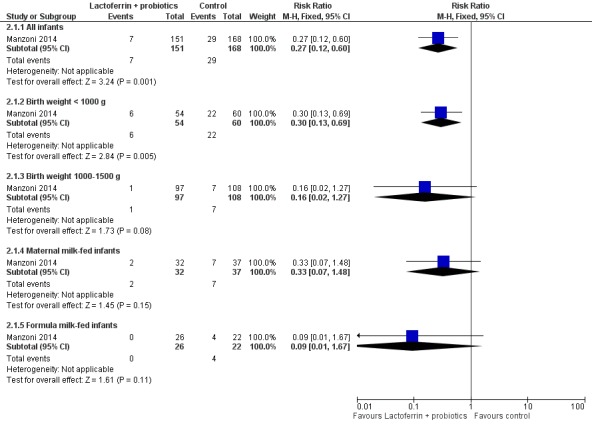
Lactoferrin supplementation of enteral feeds in combination with probiotics in preterm infants decreases late‐onset sepsis (RR 0.27, 95% CI 0.12 to 0.60; RD ‐0.13, 95% CI ‐0.19 to ‐0.06; NNTB 8, 95% CI 5 to 17; one study, 321 participants) (Figure 5). We downgraded the quality of evidence to low because data were obtained from only one study.
Figure 5.
Forest plot of comparison: 2 Lactoferrin + LGG versus placebo, outcome: 2.1 Any late‐onset sepsis.
Subgroup analyses for the outcome of late‐onset sepsis
Birth weight < 1000 g (outcome 2.1.2)
The estimated risk ratio for the outcome of late‐onset sepsis in ELBW infants was 0.30 (95% CI 0.13 to 0.69; RD ‐0.26, 95% CI ‐0.40 to ‐0.11; NNTB 5, 95% CI 2 to 9; one study, 114 participants) (Figure 5). This suggests a decrease in late‐onset sepsis among ELBW infants who were supplemented with lactoferrin in combination with probiotics. We downgraded the quality of evidence to low because data were obtained from only one study.
Birth weight 1000 to 1500 g (outcome 2.1.3)
The estimated risk ratio for the outcome of late‐onset sepsis in preterm infants with birth weight from 1000 to 1500 g was 0.16 (95% CI 0.02 to 1.27; RD ‐0.05, 95% CI ‐0.11 to 0.0; one study, 205 participants) (Figure 5). We downgraded the quality of evidence to low because data were obtained from only one study.
Exclusively maternal milk‐fed infants (outcome 2.1.4)
The estimated risk ratio for the outcome of late‐onset sepsis in preterm infants fed exclusively on maternal milk was 0.33 (95% CI 0.07 to 1.48; RD ‐0.13, 95% CI ‐0.28 to 0.02; one study, 69 participants) (Figure 5). We downgraded the quality of evidence to low because data were obtained from only one study.
Exclusively formula‐fed infants (outcome 2.1.5)
The estimated risk ratio for the outcome of late‐onset sepsis in preterm infants fed formula milk was 0.09 (95% CI 0.01 to 1.67; RD ‐0.18, 95% CI ‐0.35 to ‐0.01; one study, 48 participants) (Figure 5). We downgraded the quality of evidence to low because data were obtained from only one study.
NEC ≥ stage II (outcome 2.2)
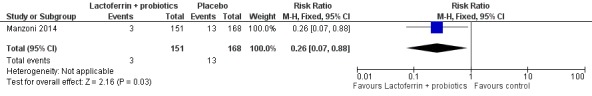
Lactoferrin supplementation of enteral feeds in combination with probiotics in preterm infants decreased NEC ≥ stage II in preterm infants (RR 0.04, 95% CI 0.00 to 0.62; RD ‐0.05, 95% CI ‐0.08 to ‐0.03; NNTB 20, 95% CI 12.5 to 33.3; one study, 496 participants) (Figure 6). We downgraded the quality of evidence to low because data were obtained from only one study.
Figure 6.
Forest plot of comparison: 2 Lactoferrin + LGG versus placebo, outcome: 2.5 NEC ≥ stage II.
All‐cause mortality (outcome 2.3)
The estimated risk ratio for the outcome of "all‐cause mortality" in preterm infants was 0.54 (95% CI 0.25 to 1.18; RD ‐0.03, 95% CI ‐0.07 to 0.01; one study, 496 participants) (Figure 7). We downgraded the quality of evidence to low because data were obtained from only one study.
Figure 7.

Forest plot of comparison: 2 Lactoferrin + LGG versus placebo, outcome: 2.4 All‐cause mortality.
Bacterial sepsis (outcome 2.4)
The estimated risk ratio for the outcome of bacterial sepsis in preterm infants was 0.28 (95% CI 0.11 to 0.72; RD ‐0.09, 95% CI ‐0.14 to ‐0.03; NNTB 11, 95% CI 7 to 33; one study, 319 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Fungal sepsis (outcome 2.5)
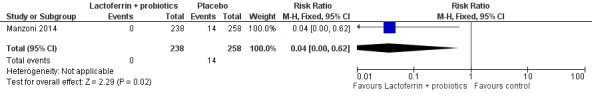
The estimated risk ratio for the outcome of fungal sepsis in preterm infants was 0.26 (95% CI 0.07 to 0.88; RD ‐0.06, 95% CI ‐0.10 to ‐0.01; NNTB 16.6, 95% CI 10 to 100; one study, 319 participants) (Figure 8). This suggests a decrease in fungal sepsis among preterm infants whose feedings were supplemented with lactoferrin in combination with probiotics. We downgraded the quality of evidence to low because of unclear risk of bias, and because data were obtained from only one study.
Figure 8.
Forest plot of comparison: 2 Lactoferrin + LGG versus placebo, outcome: 2.3 Fungal infection.
Chronic lung disease (outcome 2.6)
The study definition of chronic lung disease was oxygen requirement greater than 30% for 28 days, positive‐pressure ventilation at 36 weeks, or both. We have requested data from the study authors on infants who required oxygen at 36 weeks' corrected age.
The estimated risk ratio for the outcome of chronic lung disease in preterm infants was 0.67 (95% CI 0.25 to 1.79; RD ‐0.02, 95% CI ‐0.07 to 0.03; one study, 319 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Duration of mechanical ventilation (outcome 2.7)
The estimated mean difference for the outcome of "duration of mechanical ventilation" in preterm infants was ‐1.10 (95% CI ‐3.04 to 0.84; one study, 321 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Length of hospital stay among survivors (outcome 2.8)
The estimated mean difference for the outcome of "length of hospital stay among survivors" in preterm infants was 2.00 (95% CI ‐1.88 to 5.88; one study, 496 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Threshold retinopathy of prematurity (outcome 2.9)
The estimated risk ratio for the outcome of threshold ROP in preterm infants was 0.76 (95% CI 0.39 to 1.49; RD ‐0.03, 95% CI ‐0.09 to 0.04; one study, 319 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Urinary tract infection (outcome 2.10)
The estimated risk ratio for the outcome of urinary tract infection in preterm infants was 0.67 (95% CI 0.25 to 1.79; RD ‐0.02, 95% CI ‐0.07 to 0.03; one study, 319 participants). We downgraded the quality of evidence to low because data were obtained from only one study.
Other outcomes
The study included in this comparison (Manzoni 2009) reported no adverse effects due to lactoferrin supplementation of enteral feeds in combination with probiotics.
This study did not assess the following outcomes: neurological outcome at two years of age or older, and PVL.
Discussion
Summary of main results
We identified six randomized controlled trials that enrolled 1041 preterm infants and evaluated lactoferrin supplementation of enteral feeds with or without probiotics (Lactobacillus rhamnosus GG) compared with placebo. Lactoferrin supplementation of enteral feeds compared with placebo led to decreased late‐onset sepsis (typical risk ratio (RR) 0.59, 95% confidence interval (CI) 0.40 to 0.87; typical risk difference (RD) ‐0.06, 95% CI ‐0.10 to ‐0.02; number needed to treat for an additional beneficial outcome (NNTB) 17, 95% CI 10 to 50; six trials, 886 participants; low‐quality evidence) and necrotizing enterocolitis (NEC) stage II or III (typical RR 0.40, 95% CI 0.18 to 0.86; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.01; NNTB 25, 95% CI 17 to 100; four studies, 750 participants; low‐quality evidence). Lactoferrin supplementation of enteral feeds did not have an effect on "all‐cause mortality" (typical RR 0.65, 95% CI 0.37 to 1.11; typical RD ‐0.02, 95% CI ‐0.05 to 0; six studies, 1041 participants; low‐quality evidence). Lactoferrin supplementation of enteral feeds also decreased bacterial sepsis (RR 0.46, 95% CI 0.29 to 0.74; RD ‐0.07, 95% CI ‐0.11 to ‐0.03; NNTB 14, 95% CI 9 to 33; four studies, 760 participants), fungal sepsis (RR 0.06, 95% CI 0.00 to 0.98; RD ‐0.05, 95% CI ‐0.09 to ‐0.02; NNTB 20, 95% CI 11 to 50; one study, 321 participants; low‐quality evidence), threshold retinopathy of prematurity (ROP) (RR 0.50, 95% CI 0.27 to 0.94; RD ‐0.07, 95% CI ‐0.12 to ‐0.01; NNTB 14, 95% CI 8 to 100; two studies, 400 participants), and urinary tract infection (RR 0.31, 95% CI 0.11 to 0.88; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; NNTB 20, 95% CI 11 to 100; two studies, 440 participants). In subgroup analyses, extremely low birth weight (ELBW) infants and those fed exclusively maternal milk showed a reduction in late‐onset sepsis after oral lactoferrin supplementation (one study; low‐quality evidence). Investigators reported no differences in chronic lung disease, duration of mechanical ventilation, or length of hospital stay.
Lactoferrin supplementation of enteral feeds with a probiotic compared with placebo decreased late‐onset sepsis (RR 0.27, 95% CI 0.12 to 0.60; RD ‐0.13, 95% CI ‐0.19 to ‐0.06; NNTB 8, 95% CI 5 to 17; one study, 321 participants; low‐quality evidence), NEC ≥ stage II (RR 0.04, 95% CI 0.00 to 0.62; RD ‐0.05, 95% CI ‐0.08 to ‐0.03; NNTB 20, 95% CI 12.5 to 33.3; one study, 496 participants; low‐quality evidence), and fungal sepsis (RR 0.26, 95% CI 0.07 to 0.88; RD ‐0.06, 95% CI ‐0.10 to ‐0.01; NNTB 16.6, 95% CI 10 to 100; one study, 319 participants; low‐quality evidence). Researchers reported no differences in "all‐cause mortality," chronic lung disease, urinary tract infection, duration of mechanical ventilation, or length of hospital stay.
Investigators noted no adverse effects related to lactoferrin supplementation nor to the probiotic. None of the included studies assessed long‐term neurological outcomes or periventricular leukomalacia (PVL).
Overall completeness and applicability of evidence
The six randomized controlled trials were performed in neonatal intensive care units in Italy, New Zealand, United States, Peru, Turkey, Canada, and India. Trials are currently ongoing in Australia and New Zealand, Egypt, United Kingdom, Peru, and the Netherlands. Studies have evaluated oral lactoferrin in both the developing and the developed world. Completion of all ongoing and registered trials will yield data from more than 6000 preterm neonates from across the globe and may enhance the quality and applicability of evidence related to use of oral lactoferrin in preterm neonates.
A major concern of investigators in the initial trials was safety of oral lactoferrin in premature neonates, especially ELBW infants, who are at high risk of developing sepsis and NEC. In this review involving more than 1000 preterm neonates, researchers observed no adverse effects due to oral lactoferrin. One trial evaluated human recombinant lactoferrin; all other trials used bovine lactoferrin. Bovine lactoferrin has a 69% DNA sequence homology to human lactoferrin (Pierce 1991). Differences in glycosylation patterns of human recombinant and bovine lactoferrins may be responsible for differences in susceptibility to proteolysis and pathogen adhesion (Barboza 2012; Bellamy 1992). Whether human lactoferrin is as effective in vivo as bovine lactoferrin, or whether higher doses of human lactoferrin can be tolerated, needs to be confirmed in future trials.
The optimal timing of prophylaxis appears to be within the first three days of life, according to findings of Manzoni and coworkers (Manzoni 2009). The duration of prophylaxis with oral lactoferrin that provides optimal benefit without adverse effects for preterm neonates remains unclear. Trials used oral lactoferrin for 28 to 45 days of life, and it is not clear whether prophylaxis of increased duration was more effective in preventing late‐onset sepsis or NEC.
Quality of the evidence
We assessed the quality of evidence using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) method (Guyatt 2008); we downgraded the quality of evidence to low or very low on the basis of potential risk of bias, availability of data from only one or two studies, and the presence of moderate to severe heterogeneity. We could not assess publication bias, as we included only six studies in the review. Five out of six included studies reported explicit randomization and allocation concealment without risk of bias. In Akin 2014, investigators could not assess generation of randomization sequences and allocation concealment, and the risk of selection bias was unclear. We noted that included studies were at low or unclear risk for performance bias. In Manzoni 2009, researchers diluted interventions in feeds, and clinical and research staff were blinded to the intervention. When the infant was not fed and interventions were administered by orogastric tube without milk, it is not clear whether blinding was adequate. Other included studies did not show risk of performance bias. None of the included studies explicitly reported blinding of outcome assessors (detection bias). Investigators in included studies noted no attrition bias, performed all outcome assessments before hospital discharge, and adequately accounted for incomplete data.
Potential biases in the review process
We strove to decrease bias in the review process. Both review authors performed the literature search using an inclusive search strategy and combined search results. Our search strategy revealed eight reports on prespecified neonatal outcomes from six randomized clinical trials. Our post hoc analysis of evaluation of fungal sepsis, bacterial sepsis, threshold retinopathy of prematurity, or urinary tract infection did not change the conclusions of the review. We contacted investigators of published randomized controlled trials and searched conference proceedings for data and missing information with limited success.
Agreements and disagreements with other studies or reviews
We identified no other reviews that synthesized data from trials of lactoferrin supplementation of enteral feeds in preterm neonates by meta‐analysis. Turin 2014 and Ochoa 2017 reviewed the details of published and ongoing clinical trials on oral lactoferrin prophylaxis in preterm neonates. Lingappan 2013 reviewed and expanded on the biology, antimicrobial effects, and immunomodulatory effects of lactoferrin and commented on efficacy and safety related to its use in the newborn.
Authors' conclusions
We found low‐quality evidence to suggest that lactoferrin supplementation of enteral feeds decreases late‐onset sepsis and NEC ≥ stage II in preterm infants without adverse effects. Low‐quality evidence also indicates that lactoferrin supplementation of enteral feeds in combination with probiotics decreases late‐onset sepsis and NEC ≥ stage II in preterm infants without adverse effects. Although enteral lactoferrin holds great promise for prevention of neonatal sepsis and NEC, questions regarding optimal dosage and type (bovine or human recombinant lactoferrin), or whether lactoferrin should be regulated as a food additive or as a medication, remain unanswered.
Completed ongoing and registered trials will provide data from more than 6000 preterm neonates, and this will enhance the quality and applicability of evidence for oral lactoferrin prophylaxis in preterm infants. Study findings should also clarify effects of exclusive maternal milk feeding and addition of probiotics to lactoferrin supplementation. Clinical randomized trials evaluating lactoferrin prophylaxis should assess not only short‐term beneficial effects, but also long‐term neurodevelopmental and pulmonary outcomes.
Acknowledgements
We sincerely acknowledge the help of Yolanda Montagne Brosseau, Trials Search Coordinator, Cochrane Neonatal Review Group, in performing the literature search in Embase.
We acknowledge the contributions of Dr Steve Abrams, previous co‐author of this review.
The Cochrane Neonatal Review Group has been funded in part by federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
We used the standard methods of the Cochrane Collaboration and the Cochrane Neonatal Review Group to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation and blinding and reporting of all outcomes of all infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved disagreements by discussion. We added this information to the Characteristics of included studies table. We evaluated the following issues and entered the findings into the risk of bias table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
a. Low risk (any truly random process, eg, random number table; computer random number generator);
b. High risk (any non‐random process, eg, odd or even date of birth; hospital or clinic record number); or
c. Unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
a. Low risk (eg, telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
c. Unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
a. Low risk, high risk, or unclear risk for participants; or
b. Low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
a. Low risk for outcome assessors;
b. High risk for outcome assessors; or
c. Unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with total randomized participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorized the methods as:
a. Low risk (< 20% missing data);
b. High risk (≥ 20% missing data); or
c. Unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as:
a. Low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
b. High risk (when not all the study's prespecified outcomes have been reported; when one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; when study fails to include results of a key outcome that would have been expected to have been reported); or
c. Unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns that we had about other possible sources of bias (eg, whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. Low risk;
b. High risk; or
c. Unclear risk.
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1.
Lactoferrin supplementation with enteral feeds versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any late‐onset sepsis | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All infants | 6 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.40, 0.87] |
| 1.2 Birth weight < 1000 g | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.70] |
| 1.3 Birth weight 1000‐1500 g | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.12, 1.74] |
| 1.4 Maternal milk‐fed infants | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.98] |
| 1.5 Formula‐fed infants | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.90] |
| 2 NEC ≥ stage II | 4 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.18, 0.86] |
| 3 All‐cause mortality | 6 | 1071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.11] |
| 4 Bacterial sepsis | 4 | 760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.29, 0.74] |
| 5 Fungal infection | 2 | 451 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.09, ‐0.02] |
| 6 Chronic lung disease | 3 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.52, 1.42] |
| 7 Duration of mechanical ventilation | 2 | 511 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.47, 0.41] |
| 8 Length of stay among survivors | 1 | 505 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐2.23, 5.83] |
| 9 Threshold retinopathy of prematurity | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.27, 0.94] |
| 10 Urinary tract Infection | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
Analysis 1.1.
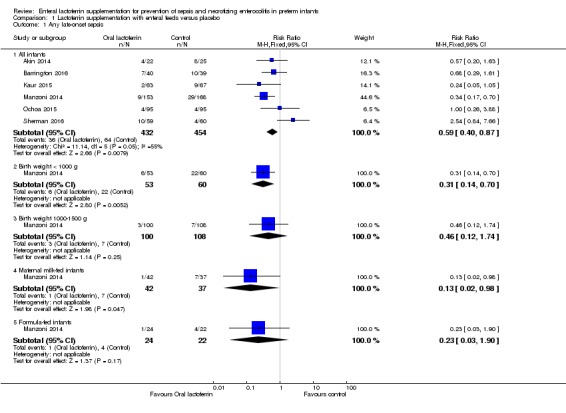
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 1 Any late‐onset sepsis.
Analysis 1.2.

Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 2 NEC ≥ stage II.
Analysis 1.3.
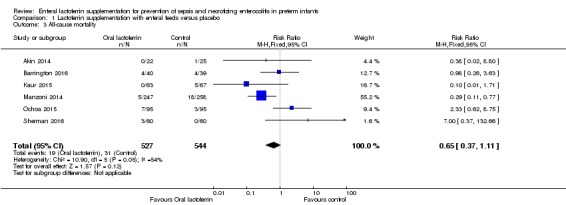
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 3 All‐cause mortality.
Analysis 1.4.
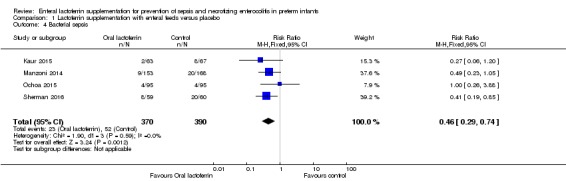
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 4 Bacterial sepsis.
Analysis 1.5.
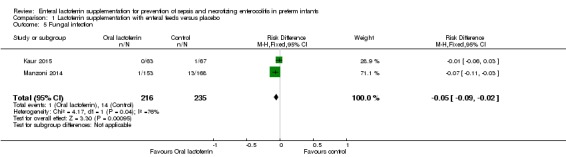
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 5 Fungal infection.
Analysis 1.6.
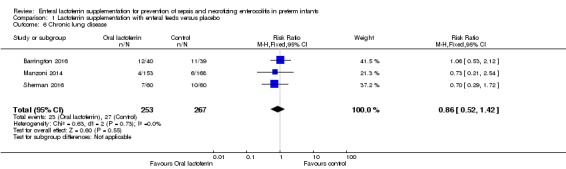
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 6 Chronic lung disease.
Analysis 1.7.
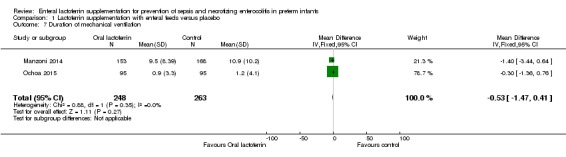
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 7 Duration of mechanical ventilation.
Analysis 1.8.
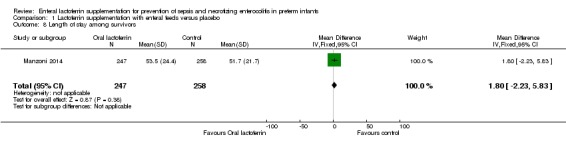
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 8 Length of stay among survivors.
Analysis 1.9.

Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 9 Threshold retinopathy of prematurity.
Analysis 1.10.
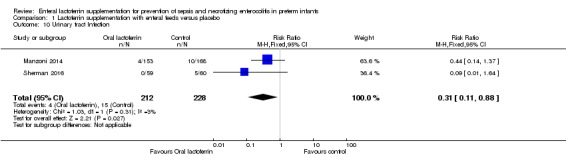
Comparison 1 Lactoferrin supplementation with enteral feeds versus placebo, Outcome 10 Urinary tract Infection.
Comparison 2.
Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any late‐onset sepsis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All infants | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.60] |
| 1.2 Birth weight < 1000 g | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.69] |
| 1.3 Birth weight 1000‐1500 g | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.27] |
| 1.4 Maternal milk‐fed infants | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.48] |
| 1.5 Formula milk‐fed infants | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.67] |
| 2 NEC ≥ stage II | 1 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.62] |
| 3 All‐cause mortality | 1 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.18] |
| 4 Bacterial sepsis | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.72] |
| 5 Fungal Infection | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.88] |
| 6 Chronic lung disease | 1 | 319 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 7 Duration of mechanical ventilation | 1 | 319 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐3.04, 0.84] |
| 8 Length of stay among survivors | 1 | 496 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.88, 5.88] |
| 9 Threshold retinopathy of prematurity | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 10 Urinary tract infection | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.79] |
Analysis 2.1.
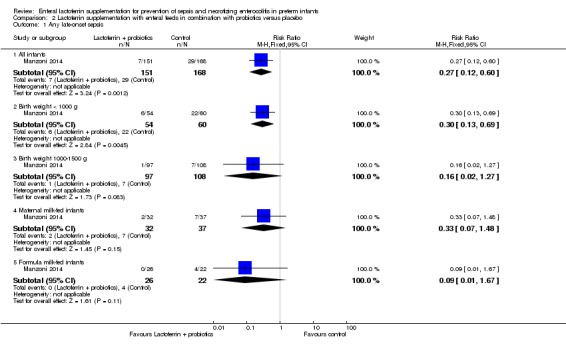
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 1 Any late‐onset sepsis.
Analysis 2.2.
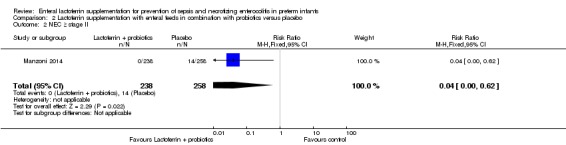
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 2 NEC ≥ stage II.
Analysis 2.3.
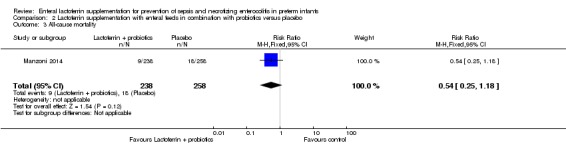
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 3 All‐cause mortality.
Analysis 2.4.
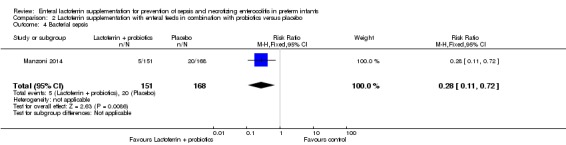
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 4 Bacterial sepsis.
Analysis 2.5.
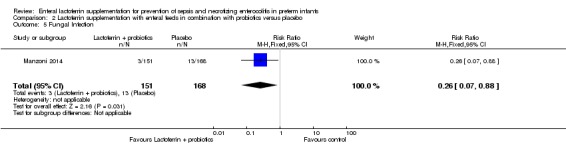
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 5 Fungal Infection.
Analysis 2.6.
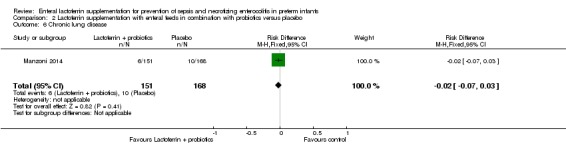
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 6 Chronic lung disease.
Analysis 2.7.
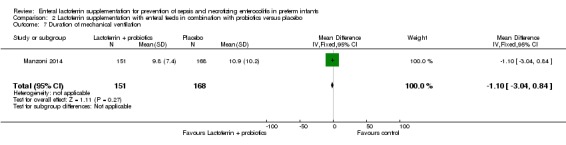
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 7 Duration of mechanical ventilation.
Analysis 2.8.

Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 8 Length of stay among survivors.
Analysis 2.9.
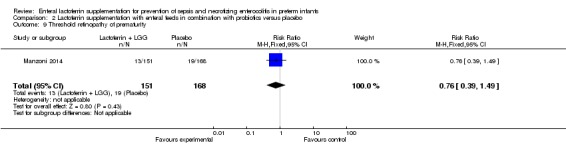
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 9 Threshold retinopathy of prematurity.
Analysis 2.10.
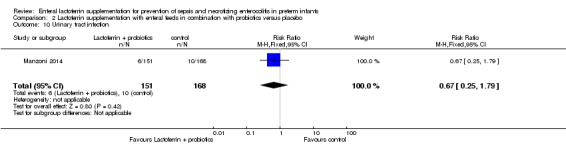
Comparison 2 Lactoferrin supplementation with enteral feeds in combination with probiotics versus placebo, Outcome 10 Urinary tract infection.
What's new
Last assessed as up‐to‐date: 31 December 2016.
| Date | Event | Description |
|---|---|---|
| 3 May 2017 | New citation required but conclusions have not changed | Conclusions are unchanged |
| 11 February 2017 | New search has been performed | We updated the literature search in December 2016, added 2 new studies, updated data for previously included studies, and added 1 excluded study and 1 ongoing study. This review updates the review, "Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants" (Pammi 2015) |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 9 September 2014 | New citation required but conclusions have not changed | We updated the search in July 2014. We revised the review by adding 4 new included studies, 4 new ongoing studies, and 2 "studies awaiting classification." We revised the text and conclusions of the review. Additionally, we used the GRADE method to rate the quality of evidence |
| 22 August 2014 | New search has been performed | This review updates the review, "Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants" (Pammi 2011) |
| 9 November 2011 | Amended | Abstract, Results: We corrected Manzoni reference from Manzoni 2008 to Manzoni 2009 |
| 11 July 2011 | New citation required but conclusions have not changed | We made no changes to the conclusions |
| 11 July 2011 | New search has been performed | This review updates the review, "Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants," which was published in the Cochrane Database of Systematic Reviews (Pammi 2010) We performed an updated search in July 2011 and identified 2 additional ongoing studies (Akin 2009 and Ochoa 2011a) |
| 7 December 2010 | Amended | We updated review author contact details |
| 11 May 2010 | Amended | Copyeditor made minor text edits |
| 7 July 2008 | Amended | We were able to review the study protocol |
Differences between protocol and review
We have assessed the quality of evidence using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) method post hoc. We have included the outcomes of threshold of retinopathy and urinary tract infection and have performed subgroup analyses of fungal and bacterial sepsis as post hoc analyses.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective, single‐center, double‐blind, randomized controlled trial | |
| Participants | Inborn neonates, birthweight < 1500 g or gestational age < 32 weeks. Exclusion criteria were lack of parental consent, severe congenital malformations, and severe HIE or death before 72 hours of life | |
| Interventions | Bovine lactoferrin (200 mg/d) or placebo (2 mL of saline) once a day until discharge | |
| Outcomes | Primary outcomes: nosocomial sepsis as defined by CDC criteria, NEC stage II. Secondary outcomes: safety (feeding tolerance, abdominal distention, emesis, and gastric residuals), length of hospital stay, maturation of Treg levels | |
| Notes | Ankara University, Turkey; conducted between December 2009 and January 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinicians and outcome assessors were unaware of study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed data from 47/50 enrolled neonates |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Completeness of follow up | Low risk | Followed up 47/50 enrolled neonates |
| Blinding of outcome assessment | Low risk | Coordinator who was blinded to the study group assessed the outcome |
| Methods | Single‐center, blinded, randomized trial | |
| Participants | Inborn infants at < 31 weeks' gestation, enrolled in the first 48 hours of life. Exclusion criteria were proven or suspected gastrointestinal anomalies, serious cardiac anomalies, moribund and not expected to survive | |
| Interventions | Milk supplemented with 100 mg of bovine lactoferrin OR milk with no lactoferrin supplementation. All infants received probiotics as per unit policy | |
| Outcomes | Primary outcome: feed tolerance defined as time taken to achieve feeds to 140 mL/kg/d. Secondary outcomes: late‐onset sepsis, death, NEC, duration of TPN, growth variables, BPD, ROP | |
| Notes | Study conducted in Montreal, Canada, from December 2012 to September 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the technician in the kitchen who prepared the milk knew the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None noted |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Completeness of follow up | Low risk | No attrition noted |
| Blinding of outcome assessment | Unclear risk | Blinding of outcome assessors was not explicit |
| Methods | Single‐center, randomized, placebo‐controlled trial | |
| Participants | Inborn neonates admitted in the first 12 hours of birth with no maternal risk factors for sepsis were enrolled. Exclusion criteria were congenital anomalies, severe birth asphyxia, history of maternal chorioamnionitis, suspected congenital infection, family history of cow's milk allergy. Neonates with culture‐proven early‐onset sepsis were also excluded | |
| Interventions | Infants were randomized to bovine lactoferrin (100‐250 mg/d based on birth weight) or placebo once daily for the first 28 days of life | |
| Outcomes | Primary outcome: culture‐proven late‐onset sepsis. Secondary outcomes: probable late‐onset sepsis, any late‐onset sepsis, sepsis‐attributed mortality | |
| Notes | Conducted in northern India between May 2012 and June 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Ranomization by a computer‐generated random table |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physician and parents were blinded. Study drug and placebo sachets with lactoferrin were similar in appearance and were prepared by the hospital pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate < 10% accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Completeness of follow up | Low risk | Loss to follow‐up < 10% |
| Blinding of outcome assessment | Unclear risk | Blinding of outcome assessors was not explicit |
| Methods | Prospective, multicenter, double‐blind, placebo‐controlled, randomized trial | |
| Participants | Premature neonates with birth weight < 1500 g within the first 3 days of life, enrolled from 13 neonatal intensive care units in Italy and New Zealand, from October 1, 2007, through July 31, 2010 | |
| Interventions | Bovine lactoferrin (100 mg/d) alone or bovine lactoferrin (100 mg/d) with Lactobacillus rhamnosus LGG (6 × 109 CFU/mL) or placebo Interventions were diluted in milk feeds. If infants were not being fed, interventions were administered through an orogastric tube |
|
| Outcomes | Primary outcomes: NEC ≥ stage II, death and/or ≥ stage II NEC before discharge Secondary outcomes: mortality attributable to NEC, mortality not associated with NEC before discharge |
|
| Notes | Continuation of the Manzoni 2009 study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to 1 of 3 groups by computer‐generated allocation sequences |
| Allocation concealment (selection bias) | Low risk | Randomly assigned in pharmacy; allocation concealment adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study authors reported that clinical and research staff were unaware of study group, as interventions and placebo were diluted in milk. In a group of infants who were not fed, investigators administered interventions by an orogastric tube, but blinding in that situation is not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/485 in the intervention arm and 5/258 in the placebo arm had missing or incomplete data. Intention‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | High risk | No report on neonatal infection for the cohort |
| Other bias | Unclear risk | None noted |
| Completeness of follow up | Unclear risk | Assessed in the hospital before discharge |
| Blinding of outcome assessment | Unclear risk | Blinding of outcome assessors not explicit (for NEC stage II or III) |
| Methods | Randomised, placebo‐controlled, double‐blind study of 190 premature infants < 2500 g in 5 neonatal Intermediate and intensive care units in Lima, Peru | |
| Participants | Birth weight between 500 and 2500 g; admitted to NICU in the first 72 hours of life | |
| Interventions | Oral bovine lactoferrin (200 mg/kg/d divided into 3 doses) for 4 weeks OR oral maltodextrin (200 mg/kg/d in 3 divided doses) for 4 weeks Both dissolved in human milk or formula or 5% glucose solution |
|
| Outcomes | Primary outcome: number of confirmed episodes of late‐onset sepsis in the first month of life Secondary outcomes: incidence of gram‐positive and gram‐negative bacterial sepsis, fungal sepsis, pneumonia, diarrhea, mortality in the first month of life |
|
| Notes | Trial ID: www.clinicaltrials.gov: NCT01264536 Peruvian study in 5 neonatal units in Lima, enrolled between January 31 and August 6, 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐randomized, stratified by weight by a third party |
| Allocation concealment (selection bias) | Low risk | Randomization performed before enrollment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for and included in the analysis |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Completeness of follow up | Low risk | Loss to follow‐up < 10% |
| Blinding of outcome assessment | Unclear risk | Not explicit |
| Methods | Phase 1 and Phase 2 randomized clinical trial | |
| Participants | Preterm infants with birth weight of 750 to 1500 g in participating units in the United States, enrolled within 24 hours of birth | |
| Interventions | Infants were given enteral human recombinant lactoferrin (talactoferrin, TLF) or placebo from day 1 to 29 days of life at a dose of 300 mg/kg/d | |
| Outcomes | Primary outcomes: reduction in hospital‐acquired infection; bacteremia, meningitis, pneumonia, urinary tract infection, necrotizing enterocolitis Secondary outcomes: mortality, duration of hospitalization, time to regain birth weight, time to reach full enteral feeds |
|
| Notes | Trial ID: ClinicalTrials.gov Identifier: NCT00854633 Trial conducted in participating units in the United States, between July 1, 2009, and March 17, 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by a central computer system |
| Allocation concealment (selection bias) | Low risk | Centrally randomized |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | None noted |
| Completeness of follow up | Low risk | All primary and secondary outcomes assessed during hospital stay |
| Blinding of outcome assessment | Unclear risk | Not explicit |
BPD: bronchopulmonary dysplasia CDC: Centers for Disease Control and Prevention CFU: colony‐forming units CSF: cerebrospinal fluid HIE: hypoxic ischemic encephalopathy NEC: necrotizing enterocolitis NICU: neonatal intensive care unit ROP: retinopathy of prematurity TLF: talactoferrin TPN: total parenteral nutrition Treg: regulator T‐cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| King 2007 | We excluded this study, as participants were not neonates. Enrolled healthy, formula‐fed infants at 34 weeks' gestation or later and at 4 weeks of age or younger. Infants received formula supplemented with lactoferrin (850 mg/L) or commercial cow's milk‐based formula (102 mg/L) for 12 months. Investigators collected growth parameters and information on gastrointestinal, respiratory, and colic illnesses for the infants' first year |
| Meyer 2016 | This is not a randomized study. It is a retrospective, observational study comparing the lactoferrin prophylaxis cohort (2004‐2011) with an historical cohort without lactoferrin prophylaxis (2001‐2004). The prophylaxis cohort received 100 mg of bovine lactoferrin and a probiotic |
| Ochoa 2013 | We excluded this study, as participants were not neonates. This community‐based, randomized, double‐blind, placebo‐controlled trial compared supplementation with bovine lactoferrin vs placebo. Researchers randomly assigned 577 weaned children at 12 to 18 months and followed them for 6 months with daily home visits. The aim was prevention of diarrhea; outcomes assessed were number of diarrhea episodes, longitudinal prevalence of diarrhea, severity of diarrhea, and dehydration |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective, double‐blind, randomized, placebo‐controlled study |
| Participants | Preterm infants with gestational age 26 to 36 weeks |
| Interventions | Infants will be randomly assigned to: 1. standard preterm formula; 2. standard preterm formula with probiotics (galacto‐oligosaccharides 28.5%, lactose 9.5%, galactose 0.5%, minerals 3.5%, fat 1.5%, water 3%); or 3. standard preterm formula with dairy lactoferrin 1 mg/100 mL |
| Outcomes | Primary outcomes: composition of gut flora at 6 weeks of full enteral feeds, incidence of infection, oxidative stress, iron status Secondary outcomes: growth (weight, length, and head circumference), feeding intolerance, psychomotor development at 1 year of age |
| Notes | Unpublished study completed in 2009. Study author contacted for data Trial ID: ISRCTN71737811 |
| Methods | Controlled phase 4 trial of lactoferrin supplementation in preterm infants |
| Participants | Newborn infants with birth weight ≤ 1500 g and/or gestational age ≤ 32 weeks Exclusion criteria: fetal‐onset disorders and/or recognizable at birth, milk intolerance, family history of allergy, use of infant formula supplemented with lactoferrin |
| Interventions | Intervention group (n = 650) received a daily dose of 100 mg of lactoferrin + standard therapy; control group (n = 650) received only standard therapy |
| Outcomes | Primary outcome: evaluation of the antioxidant effect of lactoferrin and its ability to reduce free radical‐related disease in the newborn through assessment of neurodevelopmental follow‐up Secondary outcome: identification of a panel of markers for assessing oxidative stress and for correlating with the lactoferrin antioxidant effect |
| Notes | We have requested details of the study from the principal investigator Trial ID: ClinicalTrials.gov identifier: NCT01172236 |
| Methods | Prospective randomized controlled study |
| Participants | Preterm infants (< 37 weeks' gestation) |
| Interventions | Enteral lactoferrin supplementation at 100 mg/d starting on day 1 or day 3 (early vs late) |
| Outcomes | Neonatal sepsis by Tollner score, hematological scoring system, and positive blood culture |
| Notes | Study completed enrollment of 180 preterm neonates admitted to NICU at Ain Shams University Hospitals, from August 2014 to December 2015; clinicaltrials.gov NCT02959229 |
NICU: neonatal intensive care unit.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Lactoferrin Infant Feeding Trial (LIFT) to Prevent Sepsis and Death in Preterm Infants |
| Methods | Double‐blind, randomized, controlled trial in Australia and New Zealand |
| Participants | Babies are eligible if (1) doctor and parents are substantially uncertain whether BLF is indicated or contraindicated, (2) birth weight < 1500 g, (3) < 7 days old, and (4) parent gives written informed consent |
| Interventions | Bovine lactoferrin (BLF): dosage 200 mg/kg/d dissolved in breast milk or formula, until 34 weeks' corrected gestational age or hospital discharge, whichever is sooner Placebo: breast milk or formula (without BLF), until 34 weeks' corrected gestational age or hospital discharge, whichever is sooner |
| Outcomes | Primary outcome: mortality or major morbidity before hospital discharge. Morbidity is defined as incidence of sepsis or brain injury or chronic lung disease or necrotizing enterocolitis or severe retinopathy Secondary outcome: mortality related to culture‐proven sepsis |
| Starting date | January 15, 2014; currently enrolling toward a target of 1100 infants |
| Contact information | Professor William Tarnow‐Mordi: williamtm@med.usyd.edu.au; coordinator: Alpana Ghadge: alpana.ghadge@ctc.usyd.edu.au |
| Notes | Trial ID: ANZCTR: ACTRN12611000247976 |
| Trial name or title | A Multi‐centre, Randomized, Placebo‐Controlled Trial of Prophylactic Enteral Lactoferrin Supplementation to Prevent Late‐Onset Invasive Infection in Very Preterm Infants (ELFIN) |
| Methods | Phase 3, multicenter, placebo‐controlled, randomized controlled trial in the United Kingdom |
| Participants | Infants will be eligible to participate if: 1. gestational age at birth < 32 weeks; 2. < 72 hours old; and 3. written informed parental consent is obtained. If infants are receiving antibiotic treatment for suspected or confirmed infection, they are still eligible for recruitment Exclusion criteria include: 1. infants with severe congenital anomalies; 2. anticipated enteral fasting longer than 14 days; and 3. infants who, in the opinion of the treating clinician, have no realistic prospect of survival. |
| Interventions | Infants will be randomly allocated to receive lactoferrin (150 mg/kg/d to a maximum of 300 mg) or placebo. Until discharge, they will be monitored for late‐onset invasive infection, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity, length of hospital stay, and length of time in intensive care |
| Outcomes | Primary outcome: incidence of microbiologically confirmed or clinically suspected late‐onset infection from trial entry until hospital discharge Secondary outcomes: 1. All‐cause mortality before hospital discharge. 2. Necrotizing enterocolitis (NEC): Bell’s stage II or III. 3. Severe retinopathy of prematurity (ROP) treated medically or surgically. 4. Bronchopulmonary dysplasia (BPD): Infant is still receiving mechanical ventilator support or supplemental oxygen at 36 weeks' postmenstrual age. 5. A composite of invasive infection, major morbidity (NEC, ROP, or BPD as defined above), and mortality. 6. Total number of days of administration of antibiotics per infant from 72 hours until death or discharge from hospital. 7. Total number of days of administration of antifungal agents per infant. 8. Total length of stay until discharge home. 9. Length of stay in (1) intensive care, (2) high dependency care, (3) special care. |
| Starting date | September 2013 with plans to enroll 2200 neonates |
| Contact information | Chief Investigator: Professor William McGuire: William.McGuire@hyms.ac.uk Trial Coordinator, James Griffiths: james.griffiths@npeu.ox.ac.uk |
| Notes | This study is coordinated by the National Perinatal Epidemiology Unit Clinical Trials Unit, at the University of Oxford, UK: ISRCTN88261002 |
| Trial name or title | Lactoferrin for Prevention of Sepsis in Infants (NEOLACTO) |
| Methods | Phase 3 randomized controlled trial in Peru |
| Participants | Neonates with birth weight between 500 g and 2000 g and born in or referred to the neonatal unit of one of the participating hospitals in the first 72 hours of life Exclusion criteria: neonates with underlying gastrointestinal problems that prevent oral intake, predisposing conditions that profoundly affect growth and development (chromosomal abnormalities, structural brain anomalies, severe congenital abnormalities), family history of cow's milk allergy; also, those deemed not to have the chance to complete subsequent study visits (patients who before 1 month of age would not be living in Lima) and neonates whose parents decline to participate |
| Interventions | Intervention group will receive oral bovine lactoferrin (200 mg/kg/d divided into 3 doses) for 8 weeks. Control group will receive oral maltodextrin (200 mg/kg/d in 3 divided doses) for 8 weeks |
| Outcomes | Primary outcome: composite outcome of first episode of late‐onset sepsis or sepsis‐associated death Secondary outcome: neurodevelopment at 24 months' corrected age, as assessed by the Mullen Scale for Early Learning |
| Starting date | May 2012 with plans to enroll 414 neonates |
| Contact information | Theresa J Ochoa, MD Universidad Peruana Cayetano Heredia: theresa.j.ochoa@uth.tmc.edu |
| Notes | Trial ID: ClinicalTrials.gov Identifier: NCT01525316 |
| Trial name or title | Oral Lactoferrin Supplementation for Prevention of Sepsis in Preterm Neonates |
| Methods | Double‐blind, randomized, controlled trial |
| Participants | Preterm neonates with birth weight between 500 g and 2500 g and ≤ 36 weeks' gestation, born in or referred to the neonatal intensive care unit of one of the participating hospitals in the first 48 hours of life Exclusion criteria: neonates with underlying gastrointestinal problems that prevent oral intake, neonates with predisposing conditions that profoundly affect growth and development (chromosomal abnormalities, structural brain anomalies, severe congenital abnormalities), neonates with a family background of cow's milk allergy, neonates who will not have the chance to complete the study time (who will be referred to another hospital), neonates whose parents decline to participate, neonates with early‐onset sepsis |
| Interventions | Preterm neonates will be randomly assigned to 1 of 3 groups: low‐dose lactoferrin (100 mg/d), high‐dose lactoferrin (150 mg/kg/twice daily), or placebo (distilled water) |
| Outcomes | Primary outcome: blood culture positivity Secondary outcome: complete blood count with differential leukocyte count and C‐reactive protein quantitative assay |
| Starting date | June 2013 and plans to enroll 180 neonates |
| Contact information | Mostafa AM Elmokadem: drmooselmokadem@hotmail.com; Egypt: Ain Shams University |
| Notes | Trial ID: ClinicalTrials.gov identifier: NCT01821989 |
BLF: bovine lactoferrin BPD: bronchopulmonary dysplasia NEC: necrotizing enterocolitis ROP: retinopathy of prematurity
Contributions of authors
Mohan Pammi wrote the text of the protocol and the review, formulated the search strategy, performed the literature search, and is the corresponding author.
Gautham Suresh assisted in checking the accuracy of the data, assessing risk of bias of included studies, and writing the final version of this review.
Sources of support
Internal sources
None, Other.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded by federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
-
National Institute for Health Research, UK.
UK editorial support for Cochrane Neonatal has been funded by funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the review authors and are not necessarily those of the NHS, the NIHR, or the UK Department of Health
Declarations of interest
Agennix, Inc., donated human recombinant lactoferrin for Dr Pammi's laboratory research from 2006 through 2009.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Akin IM, Atasay B, Dogu F, Okulu E, Arsan S, Karatas HD, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T‐regulatory cells. American Journal of Perinatology 2014;31(12):1111‐20. [DOI: 10.1055/s-0034-1371704; PUBMED: 24839144] [DOI] [PubMed] [Google Scholar]
- Barrington KJ, Assaad MA, Janvier A. The Lacuna Trial: a double‐blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. Journal of Perinatology 2016;36(8):666‐9. [DOI: 10.1038/jp.2016.24; PUBMED: 26938920] [DOI] [PubMed] [Google Scholar]
- Kaur G, Gathwala G. Efficacy of bovine lactoferrin supplementation in preventing late‐onset sepsis in low birth weight neonates: a randomized placebo‐controlled clinical trial. Journal of Tropical Pediatrics 2015;61(5):370‐6. [DOI: 10.1093/tropej/fmv044; PUBMED: 26224129] [DOI] [PubMed] [Google Scholar]
- Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very‐low‐birth‐weight neonates: a randomized clinical trial. Early Human Development 2014;90 Suppl 1:S60‐5. [DOI: 10.1016/S0378-3782(14)70020-9; PUBMED: 24709463] [DOI] [PubMed] [Google Scholar]; Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, The Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late‐onset sepsis in very low‐birth‐weight neonates: a randomized trial. JAMA 2009;302(13):1421‐8. [PUBMED: DOI: 10.1001/jama.2009.1403; PubMed: 19809023]] [DOI] [PubMed] [Google Scholar]; Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, The Italian Society of Neonatology. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 2012;129(1):116‐23. [PUBMED: DOI: 10.1542/peds.2011‐0279; PubMed: 22184648]] [DOI] [PubMed] [Google Scholar]
- Ochoa TJ, Zegarra J, Cam L, Llanos R, Pezo A, Cruz K, et al. NEOLACTO Research Group. Randomized controlled trial of lactoferrin for prevention of sepsis in Peruvian neonates less than 2500 g. Pediatric Infectious Disease Journal 2015;34(6):571‐6. [DOI: 10.1097/INF.0000000000000593; NCT01264536] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MP, Adamkin DH, Niklas V, Radmacher P, Sherman J, Wertheimer F, et al. Randomized controlled trial of talactoferrin oral solution in preterm infants. Journal of Pediatrics 2016;175:68‐73.e3. [DOI: 10.1016/j.jpeds.2016.04.084; 3360.7] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- King JC Jr, Cummings GE, Guo N, Trivedi L, Readmond BX, Keane V, et al. A double‐blind, placebo‐controlled, pilot study of bovine lactoferrin supplementation in bottle‐fed infants. Journal of Pediatric Gastroenterology and Nutrition 2007;44(2):245‐51. [DOI: 10.1097/01.mpg.0000243435.54958.68; PUBMED: 17255839] [DOI] [PubMed] [Google Scholar]
- Meyer MP, Alexander T. Reduced rates of necrotizing enterocolitis following routine supplementation with Lactobacillus GG and lactoferrin: an observational study. Poster Session: Neonatology: Neonatal GI Physiology & NEC: Probiotics, Antibiotics and Microbiome. Pediatric Academic Societies Meeting, May 16, 2016, Baltimore Convention Center. Baltimore, Maryland, 2016. [E‐PAS2016:4159.485] [Google Scholar]
- Ochoa T, Chea‐Woo E, Baiocchi N, Pecho I, Campos M, Prada A, et al. Randomized double‐blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. Journal of Pediatrics 2013;162(2):349–56. [DOI: 10.1016/j.jpeds.2012.07.043; PUBMED: 22939927] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- ISRCTN71737811. Effect of prebiotic or lactoferrin supplementation in formula on the gut flora of preterm infants. www.controlled‐trials.com/ISCRTN71737811 (first received 05 September 2008). [DOI: 10.1186/ISRCTN71737811] [DOI]
- NCT01172236. Supplementation with lactoferrin in preterm newborns (lactoprenew). clinicaltrials.gov/show/NCT01172236 (first received 28 July 2010).
- NCT02959229. Early versus late lactoferrin in prevention of neonatal sepsis [Systematic randomized, single blinded, placebo‐controlled trial of early versus late lactoferrin in prevention of neonatal sepsis]. clinicaltrials.gov/show/NCT02959229 (first received 27 October 2016).
References to ongoing studies
- ACTRN12611000247976. Lactoferrin infant feeding trial (LIFT) to prevent sepsis and death in preterm infants [Does lactoferrin improve survival free from morbidity in very low birth weight infants? Lactoferrin Infant Feeding Trial: a randomised controlled trial]. www.anzctr.org.au/ACTRN12611000247976 (first received 03 March 2011).
- ISRCTN88261002. Enteral lactoferrin In neonates. www.controlled‐trials.com/ISRCTN88261002 (first received 05 June 2013). [DOI: 10.1186/ISRCTN88261002] [DOI]
- NCT01525316. Lactoferrin for prevention of sepsis in infants (NEOLACTO). clinicaltrials.gov/show/NCT01525316 (first received 30 January 2012).
- NCT01821989. Oral lactoferrin supplementation for prevention of sepsis in preterm neonate. clinicaltrials.gov/show/NCT01821989 (first received 27 March 2013).
Additional references
- Adams‐Chapman I, Stoll BJ. Neonatal infection and long‐term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290‐7. [DOI: 10.1097/01.qco.0000224825.57976.87; PUBMED: 16645492] [DOI] [PubMed] [Google Scholar]
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD005496.pub4] [DOI] [PubMed] [Google Scholar]
- Artym J, Zimecki M, Kruzel ML. Enhanced clearance of Escherichia coli and Staphylococcus aureus in mice treated with cyclophosphamide and lactoferrin. International Immunopharmacology 2004;4(9):1149‐57. [DOI: 10.1016/j.intimp.2004.05.002; PUBMED: 15251111] [DOI] [PubMed] [Google Scholar]
- Barboza M, Pinzon J, Wickramasinghe S, Froehlich J, Moeller I, Smilowitz J, et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria‐host interactions. Molecular Cell Proteomics 2012;11(6):M111.015248. [DOI: 10.1074/mcp.M111.015248; PUBMED: 22261723] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N‐terminal region of bovine lactoferrin. Journal of Applied Bacteriology 1992;73(6):472‐9. [PUBMED: 1490908] [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor‐kappa B in cytokine gene regulation. American Journal of Respiratory Cell and Molecular Biology 1997;17(1):3‐9. [PUBMED: 9224203] [DOI] [PubMed] [Google Scholar]
- Buccigrossi V, Marco G, Bruzzese E, Ombrato L, Bracale I, Polito G, et al. Lactoferrin induces concentration‐dependent functional modulation of intestinal proliferation and differentiation. Pediatric Research 2007;61(4):410‐4. [DOI: 10.1203/pdr.0b013e3180332c8d; PUBMED: 17515863] [DOI] [PubMed] [Google Scholar]
- Chen PW, Liu ZS, Kuo TC, Hsieh MC, Li ZW. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017;30(2):237‐48. [DOI] [PubMed] [Google Scholar]
- Dermyshi E, Wang Y, Yan C, Hong W, Qiu G, Gong X, Zhang T. The "golden age" of probiotics: a systematic review and meta‐analysis of randomized and observational studies in preterm infants. Neonatology 2017;112(1):9‐23. [DOI] [PubMed] [Google Scholar]
- Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP. Lactoferrin protects neonatal rats from gut‐related systemic infection. American Journal of Physiology. Gastrointestinal and Liver Physiology 2001;281(5):G1140‐50. [PUBMED: 11668022] [DOI] [PubMed] [Google Scholar]
- Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin‐derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cellular and Molecular Life Sciences 2005;62(22):2588‐98. [DOI: 10.1007/s00018-005-5373-z; PUBMED: 16261252] [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 09 March 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TG, Falchook GF, Varadhachary GR, Smith DP, Davis LD, Dhingra HM, et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Investigational New Drugs 2006;24(3):233‐40. [DOI: 10.1007/s10637-005-3690-6; PUBMED: 16193240] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org..
- Iwasa M, Kaito M, Ikoma J, Takeo M, Imoto I, Yamauchi K, et al. Lactoferrin inhibits hepatitis C virus viremia in chronic hepatitis C patients with high viral loads and HCV genotype 1b. American Journal of Gastroenterology 2002;97(3):766‐7. [DOI: 10.1111/j.1572-0241.2002.05573.x; PUBMED: 11922584] [DOI] [PubMed] [Google Scholar]
- Kuipers ME, Vries HG, Eikelboom MC, Meijer DK, Swart PJ. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrobial Agents and Chemotherapy 1999;43(11):2635‐41. [PUBMED: 10543740] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Wilczynska‐Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. International Journal of Epidemiology 2006;35(3):706‐18. [DOI: 10.1093/ije/dyl043; PUBMED: 16556647] [DOI] [PubMed] [Google Scholar]
- Legrand D. Overview of lactoferrin as a natural immune modulator. Journal of Pediatrics 2016;173(Suppl):S10‐5. [DOI: 10.1016/j.jpeds.2016.02.071] [DOI] [PubMed] [Google Scholar]
- Leitch EC, Willcox MD. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Current Eye Research 1999;19(1):12‐9. [PUBMED: 10415452] [DOI] [PubMed] [Google Scholar]
- Levy SB. Antimicrobial resistance: bacteria on the defence. Resistance stems from misguided efforts to try to sterilize our environment. BMJ 1998;317(7159):612‐3. [PUBMED: 9727983] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PW, Stoll BJ. Necrotizing enterocolitis. Lancet 2006;368(9543):1271‐83. [DOI: 10.1016/S0140-6736(06)69525-1; PUBMED: 17027734] [DOI] [PubMed] [Google Scholar]
- Lingappan K, Arunachalam A, Pammi M. Lactoferrin and the newborn: current perspectives. Expert Reviews of Anti‐Infective Therapy 2013;11(7):695‐707. [DOI: 10.1586/14787210.2013.811927; PUBMED: 23879609] [DOI] [PubMed] [Google Scholar]
- Manzoni P. Clinical benefits of lactoferrin for infants and children. Journal of Pediatrics 2016;173(Suppl):S43‐52. [DOI: 10.1016/j.jpeds.2016.02.075; PUBMED: 27234411] [DOI] [PubMed] [Google Scholar]
- Neish AS. Molecular aspects of intestinal epithelial cell‐bacterial interactions that determine the development of intestinal inflammation. Inflammatory Bowel Diseases 2004;10(2):159‐68. [PUBMED: 15168817] [DOI] [PubMed] [Google Scholar]
- Nibbering PH, Ravensbergen E, Welling MM, Berkel LA, Berkel PH, Pauwels EK, et al. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic‐resistant bacteria. Infection and Immunity 2001;69(3):1469‐76. [DOI: 10.1128/IAI.69.3.1469-1476.2001; PUBMED: 11179314] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa TJ, Sizonenko SV. Lactoferrin and prematurity: a promising milk protein?. Biochemistry and Cell Biology 2017;95(1):22‐30. [DOI: 10.1139/bcb-2016-0066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Colavizza D, Benaissa M, Maes P, Tartar A, Montreuil J, et al. Molecular cloning and sequence analysis of bovine lactotransferrin. European Journal of Biochemistry 1991;196(1):177‐84. [PUBMED: 2001696] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. https://gdt.gradepro.org/app/handbook/handbook.html [Updated October 2013].
- Speer CP. Inflammatory mechanisms in neonatal chronic lung disease. European Journal of Pediatrics 1999;158(Suppl 1):S18‐22. [PUBMED: 10592094] [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285‐91. [PUBMED: 12165580] [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292(19):2357‐65. [DOI: 10.1001/jama.292.19.2357; PUBMED: 15547163] [DOI] [PubMed] [Google Scholar]
- Stoll BJ. Infections of the neonatal infant. In: Behrman RE, Kliegman R, Jenson HB editor(s). Nelson's Textbook of Pediatrics. 17th Edition. Philadelphia: Saunders, 2004:623‐40. [Google Scholar]
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. National Institute of Child Health and Human Development. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram‐negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002‐2003. Pediatric Infectious Disease Journal 2005;24(7):635‐9. [PUBMED: 15999007] [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ikeda M, Nozaki A, Kato N, Tsuda H, Saito S, et al. Lactoferrin inhibits hepatitis C virus viremia in patients with chronic hepatitis C: a pilot study. Japanese Journal of Cancer Research 1999;90(4):367‐71. [PUBMED: 10363572] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Maddox IS, Ferguson LR, Shu Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 2010;23(3):593‐6. [DOI] [PubMed] [Google Scholar]
- Togawa J, Nagase H, Tanaka K, Inamori M, Umezawa T, Nakajima A, et al. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. American Journal of Physiology. Gastrointestinal and Liver Physiology 2002;283(1):G187‐95. [DOI: 10.1152/ajpgi.00331.2001; PUBMED: 12065306] [DOI] [PubMed] [Google Scholar]
- Tomita M, Wakabayashi H, Yamauchi K, Teraguchi S, Hayasawa H. Bovine lactoferrin and lactoferricin derived from milk: production and applications. Biochemistry and Cell Biology 2002;80(1):109‐12. [PUBMED: 11908633] [DOI] [PubMed] [Google Scholar]
- Trumpler U, Straub PW, Rosenmund A. Antibacterial prophylaxis with lactoferrin in neutropenic patients. European Journal of Clinical Microbiology and Infectious Diseases 1989;8(4):310‐3. [PUBMED: 2497005] [DOI] [PubMed] [Google Scholar]
- Turin CG, Zea‐Vera A, Pezo A, Cruz K, Zegarra J, Bellomo S, et al. NEOLACTO Research Group. Lactoferrin for prevention of neonatal sepsis. Biometals 2014;27(5):1007‐16. [DOI: 10.1007/s10534-014-9754-3; PUBMED: 24935001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti P, Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cellular and Molecular Life Sciences 2005;62(22):2576‐87. [DOI: 10.1007/s00018-005-5372-0; PUBMED: 16261253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Pham D, Kong L, Weisman L. Prophylaxis with lactoferrin, a novel antimicrobial agent, enhances survival in a neonatal rat model of co‐infection. Advances in Therapy 2007;24(5):941‐54. [PUBMED: 18029319] [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. 3rd Edition. Philadelphia, London: WB Saunders, 1995. [Google Scholar]
- Zagulski T, Lipinski P, Zagulska A, Broniek S, Jarzabek Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. British Journal of Experimental Pathology 1989;70(6):697‐704. [PUBMED: 2690922] [PMC free article] [PubMed] [Google Scholar]
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital‐acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175‐88. [DOI: 10.1016/S0140-6736(05)71881-X; PUBMED: 15794973] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007137.pub2] [DOI] [PubMed] [Google Scholar]
- Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007137.pub3] [DOI] [PubMed] [Google Scholar]
- Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD007137.pub4] [DOI] [PubMed] [Google Scholar]


