Abstract
Background
Rosacea is a common chronic skin condition affecting the face, characterised by flushing, redness, pimples, pustules and dilated blood vessels. The eyes are often involved and thickening of the skin with enlargement (phymas), especially of the nose, can occur in some people. A range of treatment options are available but it is unclear which are most effective.
Objectives
To assess the efficacy and safety of treatments for rosacea.
Search methods
We updated our searches, to July 2014, of: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2014, Issue 6), MEDLINE (from 1946), EMBASE (from 1974) and Science Citation Index (from 1988). We searched five trials registers and checked reference lists for further relevant studies.
Selection criteria
Randomised controlled trials in people with moderate to severe rosacea.
Data collection and analysis
Study selection, data extraction, risk of bias assessment and analyses were carried out independently by two authors.
Main results
We included 106 studies, comprising 13,631 participants. Sample sizes of 30‐100 and study duration of two to three months were most common. More women than men were included, mean age of 48.6 years, and the majority had papulopustular rosacea, followed by erythematotelangiectatic rosacea.
A wide range of comparisons (67) were evaluated. Topical interventions: metronidazole, azelaic acid, ivermectin, brimonidine or other topical treatments. Systemic interventions: oral antibiotics, combinations with topical treatments or other systemic treatments, i.e. isotretinoin. Several studies evaluated laser or light‐based treatment.
The majority of studies (57/106) were assessed as 'unclear risk of bias', 37 'high risk ' and 12 'low risk'. Twenty‐two studies provided no usable or retrievable data i.e. none of our outcomes were addressed, no separate data reported for rosacea or limited data in abstracts.
Eleven studies assessed our primary outcome 'change in quality of life', 52 studies participant‐assessed changes in rosacea severity and almost all studies addressed adverse events, although often only limited data were provided. In most comparisons there were no statistically significant differences in number of adverse events, most were mild and transient. Physician assessments including investigators' global assessments, lesion counts and erythema were evaluated in three‐quarters of the studies, but time needed for improvement and duration of remission were incompletely or not reported.
The quality of the body of evidence was rated moderate to high for most outcomes, but for some outcomes low to very low.
Data for several outcomes could only be pooled for topical metronidazole and azelaic acid. Both were shown to be more effective than placebo in papulopustular rosacea (moderate quality evidence for metronidazole and high for azelaic acid). Pooled data from physician assessments in three trials demonstrated that metronidazole was more effective compared to placebo (risk ratio (RR) 1.98, 95% confidence interval (CI) 1.29 to 3.02). Four trials provided data on participants' assessments, illustrating that azelaic acid was more effective than placebo (RR 1.46, 95% CI 1.30 to 1.63). The results from three studies were contradictory on which of these two treatments was most effective.
Two studies showed a statistically significant and clinically important improvement in favour of topical ivermectin when compared to placebo (high quality evidence). Participants' assessments in these studies showed a RR of 1.78 (95% CI 1.50 to 2.11) and RR of 1.92 (95% CI 1.59 to 2.32),which were supported by physicians' assessments. Topical ivermectin appeared to be slightly more effective than topical metronidazole for papulopustular rosacea, based on one study, for improving quality of life and participant and physician assessed outcomes (high quality evidence for these outcomes).
Topical brimonidine in two studies was more effective than vehicle in reducing erythema in rosacea at all time points over 12 hours (high quality evidence). At three hours the participants' assessments had a RR of 2.21 (95% CI 1.52 to 3.22) and RR of 2.00 (95% CI 1.33 to 3.01) in favour of brimonidine. Physicians' assessments confirmed these data. There was no rebound or worsening of erythema after treatment cessation.
Topical clindamycin phosphate combined with tretinoin was not considered to be effective compared to placebo (moderate quality evidence).
Topical ciclosporin ophthalmic emulsion demonstrated effectiveness and improved quality of life for people with ocular rosacea (low quality evidence).
Of the comparisons assessing oral treatments for papulopustular rosacea there was moderate quality evidence that tetracycline was effective but this was based on two old studies of short duration. Physician‐based assessments in two trials indicated that doxycycline appeared to be significantly more effective than placebo (RR 1.59, 95% CI 1.02 to 2.47 and RR 2.37, 95% CI 1.12 to 4.99) (high quality evidence). There was no statistically significant difference in effectiveness between 100 mg and 40 mg doxycycline, but there was evidence of fewer adverse effects with the lower dose (RR 0.25, 95% CI 0.11 to 0.54) (low quality evidence). There was very low quality evidence from one study (assessed at high risk of bias) that doxycycline 100 mg was as effective as azithromycin. Low dose minocycline (45 mg) was effective for papulopustular rosacea (low quality evidence).
Oral tetracycline was compared with topical metronidazole in four studies and showed no statistically significant difference between the two treatments for any outcome (low to moderate quality evidence).
Low dose isotretinoin was considered by both the participants (RR 1.23, 95% CI 1.05 to 1.43) and physicians (RR 1.18, 95% CI 1.03 to 1.36) to be slightly more effective than doxycycline 50‐100 mg (high quality evidence).
Pulsed dye laser was more effective than yttrium‐aluminium‐garnet (Nd:YAG) laser based on one study, and it appeared to be as effective as intense pulsed light therapy (both low quality evidence).
Authors' conclusions
There was high quality evidence to support the effectiveness of topical azelaic acid, topical ivermectin, brimonidine, doxycycline and isotretinoin for rosacea. Moderate quality evidence was available for topical metronidazole and oral tetracycline. There was low quality evidence for low dose minocycline, laser and intense pulsed light therapy and ciclosporin ophthalmic emulsion for ocular rosacea. Time needed to response and response duration should be addressed more completely, with more rigorous reporting of adverse events. Further studies on treatment of ocular rosacea are warranted.
Plain language summary
Treatments for rosacea
Review question
Which treatments are effective for rosacea?
Background
Rosacea is a common skin condition causing flushing, redness, red pimples and pustules on the face, and should not be confused with acne. Dilated blood vessels may appear near the surface of the skin (telangiectasia). It can also cause inflammation of the eyes or eyelids, or both (ocular rosacea). Some people can develop a thickening of the skin, especially of the nose (rhinophyma). Although the cause of rosacea remains unclear, a wide variety of treatments are available for this persistent (chronic) and recurring and often distressing disease. These include medications applied directly to the skin (topical), oral medications and light‐based therapies. We wanted to discover how people assessed their treatments: if the treatments changed their quality of life, if they saw changes in their condition and if there were side effects. From the doctors, we wanted to discover whether treatments changed the severity of rosacea, as well as how long it took before symptoms reduced and reappeared.
Study characteristics
We reviewed 106 studies (up to July 2014) which included 13,631 people with moderate to severe rosacea. Most were between 40 and 50 years old, with more than twice as many women as men. Most studies lasted between eight to 12 weeks, with the longest lasting 40 weeks. The majority of people in these studies suffered from two rosacea subtypes, the subtype with pimples and pustules, or the subtype that causes flushing and persistent redness.
Of the 106 studies, 66 reported that they received funding, mainly by pharmaceutical companies. We were confident funding did not affect the results in 56 of these studies but had concerns about the remaining 10.
Key results
Most of the treatments appeared to be effective in treating rosacea. Almost half of the studies reported how people assessed their treatments. Only 11 assessed changes to quality of life. Almost all studies reported side effects, although this information was often limited. Studies mostly evaluated changes in the number of pimples and pustules, and redness. Only five studies included ocular rosacea. None included the rare variant called 'granulomatous rosacea'.
Topical treatments
Two separate treatments, metronidazole and azelaic acid, were effective and safe in reducing rosacea symptoms. Improvements tended to appear after three to six weeks. With metronidazole, very few people experienced mild itching, skin irritation and dry skin. For some, azelaic acid caused mild burning, stinging or irritation. More research is needed to conclude which of these two treatments is best.
Ivermectin, a new treatment, was more effective than placebo and slightly more effective than metronidazole. Another newly registered treatment called brimonidine, especially for reducing redness, was shown to work up to 12 hours after being applied.
Oral treatments
Antibiotics such as tetracycline, a low dose of doxycycline or a low dose of minocycline reduced the number of pimples and pustules. Low dose doxycycline (40 mg) was likely as effective as 100 mg, but with much fewer side effects of diarrhoea and nausea. Azithromycin may be as effective as 100 mg doxycycline, but only one study addressed this treatment and better quality studies are needed to confirm this.
A low dose of isotretinoin (0.3 mg/kg), a vitamin A‐related drug, appeared to be slightly more effective than 50‐100 mg doxycycline for treating pimples and pustules. However, extra precautions need to be taken regarding contraception in women of childbearing age as this drug is known to cause malformations in the foetus.
Light‐based therapies
Laser therapy and intense pulsed light therapy were both effective for the treatment of telangiectasia, but the studies examining these treatments only reported limited data.
Rosacea of the eyes or eyelids, or both (ocular rosacea)
Better quality studies are required on ocular rosacea, though ciclosporin 0.05% ophthalmic emulsion appeared to be more effective than artificial tears.
Quality of the evidence
We rated the quality of the evidence for several outcomes as very low to high. There was high quality evidence for azelaic acid, topical ivermectin, brimonidine, doxycycline and isotretinoin. The lower quality evidence for other treatments was mostly because there were few people in the studies, making the results less precise, and the lack of blinding (people knew which treatments they were receiving).
Summary of findings
Summary of findings for the main comparison. Metronidazole compared to placebo for rosacea.
| Metronidazole compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Metronidazole Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Metronidazole | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 252 (3 studies1) | ⊕⊕⊕⊝ moderate2 | Bjerke 1989 RR 1.68, 95% CI 1.25 to 2.28; P = 0.0007, Nielsen 1983a RR 3.05, 95% CI 1.57 to 5.94; P = 0.001, Bleicher 1987 (within‐participant study) RR 7. These are clinically important improvements |
| Proportion of participants with adverse event | 161 per 1000 | 191 per 1000 (151 to 243) | RR 1.19 (0.94 to 1.51) | 1773 (6 studies3) | ⊕⊕⊕⊕ high | Most instances of these adverse events were mild and consisted of pruritus, skin irritation and dry skin |
| Physician‐assessed improvement in rosacea severity | 288 per 1000 | 570 per 1000 (371 to 869) | RR 1.98 (1.29 to 3.02) | 334 (3 studies4) | ⊕⊕⊕⊝ moderate2,5 | The results are both statistically significant and clinically important |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 602 (7 studies6) | ⊕⊕⊕⊝ moderate5,7 | In the separate studies (but not in Bitar 1990) there was a greater reduction of erythema in the groups treated with metronidazole, but data were inadequately reported. Except in Koçak 2002 data were adequately reported with a MD of ‐1.40 (95% CI ‐2.47 to ‐0.33; P = 0.01) in favour of metronidazole |
| Lesion count | See comment | See comment | Not estimable | 1964 (8 studies8) | ⊕⊕⊕⊝ moderate7 | No SDs reported, data were skewed but appeared to support data of physician‐assessed improvement |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 514 (5 studies9) | ⊕⊕⊕⊕ high | Based on interim data improvement started around four weeks |
| Duration of remission | 409 per 1000 | 205 per 1000 (102 to 405) | RR 0.50 (0.25 to 0.99) | 88 (1 study10) | ⊕⊕⊕⊝ moderate11,12 | 9/44 in metronidazole group relapsed, versus 18/44 in vehicle group during six months follow‐up |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Bjerke 1989, Nielsen 1983a, Bleicher 1987 2 Downgraded one level due to serious imprecision (wide confidence intervals) 3Beutner 2005, Bitar 1990, Bjerke 1989, Breneman 1998, Koçak 2002, Nielsen 1983a 4Bjerke 1989, Breneman 1998, Nielsen 1983a 5 Although for two studies the sequence generation and allocation concealment was unclear (Bjerke 1989 and Nielsen 1983a), the blinding was ensured for both Bleicher 1987 and Nielsen 1983a, and stated as double‐blind for Bjerke 1989 and therefore we considered it unlikely that this would have an impact on this outcome assessment and decided only to downgrade for imprecision 6 Bitar 1990, Bjerke 1989, Bleicher 1987, Breneman 1998, Dahl 1998, Koçak 2002,Nielsen 1983a 7 Downgraded one level due to serious imprecision (small sample sizes in the individual studies, pooling not possible due to missing SDs) 8Beutner 2005, Bitar 1990, Bjerke 1989, Bleicher 1987, Breneman 1998, Dahl 1998, Koçak 2002, Nielsen 1983a 9Bitar 1990, Bjerke 1989, Bleicher 1987, Breneman 1998, Nielsen 1983a 10Dahl 1998
11 Although we judged the domains for sequence generation, allocation concealment as unclear and the method of blinding of participants and physicians was not reported, there was no attrition bias nor selective reporting and therefore we concluded there was no serious risk of bias for this outcome assessment
12 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met)
Summary of findings 2. Azelaic acid compared to placebo for rosacea.
| Azelaic acid compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Azelaic acid Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Azelaic acid | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity Marked improvement to complete remission on Likert scale | 421 per 1000 | 636 per 1000 (552 to 733) | RR 1.46 (1.30 to 1.63) | 1179 (4 studies1) | ⊕⊕⊕⊕ high | This is a clinically important improvement in favour of azelaic acid |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 1245 (5 studies2) | ⊕⊕⊕⊕ high | Bjerke 1999 RR 1.00, 95% CI 0.62 to 1.62; P = 0.02, Carmichael 1993 (within‐participant) 24/33 on the azelaic acid side and 19/33 on placebo side, Draelos 2013a RR 2.39, 95% CI 1.12 to 5.09; P = 0.02, Thiboutot 2003a and Thiboutot 2003b 18% and 8% respectively for azelaic acid treated groups and limited to no data for the placebo groups |
| Physician‐assessed improvement in rosacea severity | 497 per 1000 | 655 per 1000 (586 to 730) | RR 1.32 (1.18 to 1.47) | 1179 (4 studies1) | ⊕⊕⊕⊕ high | Data for these assessments from four studies illustrated that azelaic acid was more effective than placebo |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 1245 (5 studies2) | ⊕⊕⊕⊕ high | Decrease in erythema in groups treated with azelaic acid ranged from 44% to 47.9% and for placebo from 28% to 37.9%, telangiectasia minimal changes. SDs missing |
| Lesion count | The mean lesion count in the control group was ‐9.5 inflammatory lesions | The mean lesion count in the control group was 3.90 lower (5.87 to 1.93 lower) | 401 (1 study3) | ⊕⊕⊕⊝ moderate4 | No SDs were reported in (Bjerke 1999; Thiboutot 2003a; Thiboutot 2003b) and data were skewed in Carmichael 1993. All four studies showed a greater reduction in lesions in azelaic acid treated groups (see Analysis 2.3) | |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 1245 (5 studies2) | ⊕⊕⊕⊕ high | This was not a pre‐specified outcome, but all studies showed clear improvement after three to six weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Bjerke 1999, Draelos 2013a, Thiboutot 2003a, Thiboutot 2003b 2Bjerke 1999, Carmichael 1993, Draelos 2013a, Thiboutot 2003a, Thiboutot 2003b 3Draelos 2013a 4 Downgraded one level due to serious imprecision (wide confidence interval)
2.3. Analysis.
Comparison 2 Topical azelaic acid versus placebo, Outcome 3 Incomplete data on which further analysis is not possible.
| Incomplete data on which further analysis is not possible | ||||
|---|---|---|---|---|
| Study | Intervention | Summary Outcomes | Comment | Notes |
| Bjerke 1999 | 76 were treated with azelaic cream 20% BID versus 38 with placebo BID. | Decrease in erythema 47.9% versus 37.9%, in telangiectasia 22.3% versus 23.5%. Decrease in lesions 73.4% versus 50.6%. |
No SDs were reported. | BID = twice a day SD = standard deviation |
| Carmichael 1993 | Azelaic cream 20% BID versus placebo BID. Within‐patient comparison in 33 patients. |
VAS scale of improvement 6.9 (1.15) to 2.6 (1.72) for azelaic acid treated side versus 7.0 (1.15) to 4.5 (2.30) for placebo treated side
Erythema index decreased from 539.6 (76.98) to 500.6 (84.45) at the azelaic acid treated side and from 533.5 (82.15) to 518.3 (95.36) at the placebo treated side
Telangiectasia (VAS scores) decreased from 4.3 (2.30) to 4.2 (1.71) at the azelaic acid treated side and from 4.4 (2.30) to 4.5 (2.30) at the placebo side Papule count 2.5 (2.87) versus 6.3 (4.6), pustule count 0.0 (0.17) versus 0.4 (0.57). |
Data are skewed. | BID = twice a day |
| Draelos 2013a | 198 were treated with azelaic acid 15% foam BID versus 203 vehicle foam BID | There were no statistically significant differences between the 2 groups in end‐of‐treatment or end‐of‐study erythema and telangiectasia | BID = twice a day | |
| Thiboutot 2003a | 164 were treated with azelaic acid 15% BID versus 165 with vehicle BID. | Marked improvement or complete remission according to investigator: 51% versus 27% (investigators reported P < 0.001). Overall improvement in erythema : 44% versus 29% (investigators reported P = 0.0017). Overall improvement in telangiectasia: Unchanged in 77% versus 80% (investigators reported 'not statistically significant'). Change in number of inflammatory lesions from 17.5 to 6.8 versus 17.6 to 10.5. | No SDs were reported, can only be estimated from figures | BID = twice a day SD = standard deviation |
| Thiboutot 2003b | 169 were treated with azelaic acid 15% BID versus 166 with vehicle BID Same reference describes 2 studies. |
Marked improvement or complete remission according to investigator: 46% versus 31% (investigators reported P < 0.0048). Overall improvement in erythema : 46% versus 28% (investigators reported P = 0.0005). Overall improvement in telangiectasia: Unchanged in 73% versus 78% (investigators reported 'not statistically significant'). Change in number of inflammatory lesions from 17.8 to 8.9 versus 18.5 to 12.1. |
No SDs were reported, can only be estimated from figures | BID = twice a day SD = standard deviation |
Summary of findings 3. Topical ivermectin compared to placebo for rosacea.
| Topical ivermectin compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Topical ivermectin Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical ivermectin | |||||
| HRQOL DLQI and RosaQoL | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | Although data were statistically significant in favour of ivermectin, the clinical importance is unclear as MID in reduction of DLQI score was not reached and the MID is not yet established for RosaQoL2 |
| Participant‐assessed improvement in rosacea severity Likert scale, good to excellent improvement | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | RR 1.78, 95% CI 1.50 to 2.11 (Stein 2014a), RR 1.92, 95% CI 1.59 to 2.32 (Stein 2014b). Both studies showed a statistically significant and clinically important improvement in favour of topical ivermectin |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | RR 0.54, 95% CI 0.29 to 1.01 (Stein 2014a), RR 1.00, 95% CI 0.55 to 1.82 (Stein 2014b) |
| Physician‐assessed improvement in rosacea severity Investigator's Global Assessment of clear or almost clear | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | RR 3.30, 95% CI 2.27 to 4.79 (Stein 2014a), RR 2.10, 95% CI 1.57 to 2.81 (Stein 2014b). The results of both studies are in concordance with the assessments of the participants |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | MD ‐8.40, 95% CI ‐9.93 to ‐6.87 (Stein 2014a), MD ‐8.90, 95% CI ‐10.45 to ‐7.35 (Stein 2014b). Both of these differences are statistically significant and clinically important |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 1371 (2 studies1) | ⊕⊕⊕⊕ high | Improvement in both studies was seen after four weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Stein 2014a, Stein 2014b 2 MID = minimal important difference
Summary of findings 4. Topical brimonidine compared to vehicle for rosacea.
| Topical brimonidine compared to vehicle for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Topical brimonidine Comparison: Vehicle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical brimonidine | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity Patient Satisfaction Assessment ‐ grade 2 improvement | See comment | See comment | Not estimable | 553 (2 studies1) | ⊕⊕⊕⊕ high | At 3 hours RR 2.21, 95% CI 1.52 to 3.22 (Fowler 2013a) and RR 2.00, 95% CI 1.33 to 3.01 (Fowler 2013b). At each time point in both studies brimonidine was shown to be more effective than vehicle in an improvement which was statistically significant |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 553 (2 studies1) | ⊕⊕⊕⊕ high | RR 1.17, 95% CI 0.79 to 1.74 (Fowler 2013a), RR 1.40, 95% CI 0.97 to 2.02 (Fowler 2013b). Adverse events were mild and transient |
| Physician‐assessed improvement in rosacea severity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No reporting of data other than "No aggravations in the severity of IGA were observed" |
| Assessment of erythema or telangiectasia Clinician Erythema Assessment ‐ grade 2 improvement | See comment | See comment | Not estimable | 553 (2 studies1) | ⊕⊕⊕⊕ high | At 3 hours RR 2.82, 95% CI 1.85 to 4.30 (Fowler 2013a), RR 1.78, 95% CI 1.25 to 2.55 (Fowler 2013b) |
| Lesion count ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No reporting of data other than "No aggravations in the severity of lesion counts were observed" |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 553 (2 studies1) | ⊕⊕⊕⊕ high | Improvement was seen within 30 min |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | There was no rebound or worsening of erythema after treatment cessation in comparison to baseline assessments |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 5. Topical azelaic acid compared to topical metronidazole for rosacea.
| Topical azelaic acid compared to topical metronidazole for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Topical azelaic acid Comparison: Topical metronidazole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical metronidazole | Topical azelaic acid | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 491 (3 studies1) | ⊕⊕⊝⊝ low2,3 | RR 1.23, CI 95% 1.04 to 1.44; P = 0.01 (Elewski 2003), RR 1.00, 95% CI 0.83 to 1.21 (Wolf 2006), Maddin 1999 (within‐participant) authors report P = 0.02 in favour of azelaic acid |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 491 (3 studies1) | ⊕⊕⊝⊝ low2,4 | RR 3.64, 95% CI 1.81 to 7.31; P = 0.0003 (Elewski 2003), RR 0.74, 95% CI 0.52 to 1.07 (Wolf 2006). In Maddin 1999 1 participant reported stinging on azelaic acid treated site |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 491 (3 studies1) | ⊕⊕⊝⊝ low2,5 | RR 1.26, 95% CI 1.03 to 1.53; P = 0.02 (Elewski 2003), RR 1.05, 95% CI 0.79 to 1.39 (Wolf 2006), Maddin 1999 score 2.7 (SD 1.0) versus 3.1 (SD 1.0) (higher is worse) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 491 (3 studies) | ⊕⊕⊝⊝ low2,6 | RR 1.35, 95% CI 1.05 to 1.75; P = 0.02 (Elewski 2003), RR 0.99, 95% CI 0.69 to 1.42 (Wolf 2006), in Maddin 1999 the participants and physicians had contradictory judgements |
| Lesion counts | See comment | See comment | Not estimable | 491 (3 studies1) | ⊕⊕⊕⊝ moderate2 | No SDs were reported, all three studies demonstrated a clinically important reduction in lesion count in both treatment arms |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 491 (3 studies1) | See comment | Improvement for both arms was seen after four to six weeks in all three studies |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Elewski 2003, Maddin 1999, Wolf 2006 2 Downgraded one level due to serious risk of bias (all three studies stated to be double‐blind, but method of blinding was not described) 3 Downgraded one level due to serious inconsistency (Elewski 2003 and Wolf 2006 no statistically significant difference (severe heterogeneity unexplained (I2 >60%), and the 95% CIs do overlap but lead to different interpretation of the effect estimate, but in Maddin 1999 azelaic was more effective) 4 Downgraded one level due to serious inconsistency (statistically significant difference in participants reporting adverse events in Elewski 2003 (in favour of metronidazole), not confirmed in Wolf 2006 (severe heterogeneity unexplained (I2>60% and the 95% CIs did not overlap)) 5 Downgraded one level due to serious inconsistency (no statistically significant difference in Wolf 2006, but in Elewski 2003 and Maddin 1999 azelaic acid is more effective, severe heterogeneity unexplained and the 95% CI do overlap but lead to different interpretation of the effect estimate) 6 Downgraded one level due to inconsistency (no statistically significant difference in Wolf 2006, but in Elewski 2003 and Maddin 1999 azelaic acid is more effective according to physicians (but metronidazole is more effective according to participants in Maddin 1999)
Summary of findings 6. Topical ivermectin compared to topical metronidazole for rosacea.
| Topical ivermectin compared to topical metronidazole for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Topical ivermectin Comparison: Topical metronidazole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical metronidazole | Topical ivermectin | |||||
| HRQOL DLQI, proportion of participants that reported at end of study that rosacea had no impact on QoL | 640 per 1000 | 711 per 1000 (647 to 775) | RR 1.11 (1.01 to 1.21) | 962 (1 study1) | ⊕⊕⊕⊕ high | Reduction in DLQI was 5.18 in ivermectin group and 3.92 in metronidazole group (both meeting minimal important difference) |
| Participant‐assessed improvement in rosacea severity Likert scale ‐ good to excellent improvement | 748 per 1000 | 853 per 1000 (800 to 912) | RR 1.14 (1.07 to 1.22) | 962 (1 study1) | ⊕⊕⊕⊕ high | This is a statistically significant difference and in concordance with the results on number of participants that experienced no deleterious effect on their quality of life |
| Proportion of participants with adverse event | 8 per 1000 | 19 per 1000 (6 to 61) | RR 2.28 (0.71 to 7.35) | 962 (1 study1) | ⊕⊕⊕⊝ moderate2 | |
| Physician‐assessed improvement in rosacea severity | 754 per 1000 | 852 per 1000 (799 to 905) | RR 1.13 (1.06 to 1.20) | 962 (1 study1) | ⊕⊕⊕⊕ high | These assessments are consistent with the assessments of the participants |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | The mean lesion count in the control groups was ‐23.60 inflammatory lesions | The mean lesion count in the intervention groups was 4.10 lower (5.18 to 3.02 lower) | 962 (1 study1) | ⊕⊕⊕⊕ high | Both treatments showed clinically important reductions in lesion counts | |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 962 (1 study1) | ⊕⊕⊕⊕ high | This was not a predefined outcome, but clear improvement could be seen for both treatment arms around six weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Taieb 2015 2 Downgraded one level due to serious imprecision (wide confidence interval due to low occurrence of events)
Summary of findings 7. Ciclosporin ophthalmic emulsion 0.05% compared to artificial tears for ocular rosacea.
| Ciclosporin ophthalmic emulsion 0.05% compared to artificial tears for ocular rosacea | ||||||
| Patient or population: Participants with ocular rosacea Intervention: Ciclosporin ophthalmic emulsion 0.05% Comparison: Artificial tears | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Artificial tears | Ciclosporinophthalmic emulsion 0.05% | |||||
| HRQOL Ocular Surface Disease Index (scale 0 to 100, 100 worst) | The mean OSDI in the control group was 16.9 | The mean OSDI in the intervention group was 8.6 lower (15.42 to 1.78 lower) | 37 (1 study1) | ⊕⊕⊝⊝ low2 | The difference between change scores at end of study equates to a moderate improvement in quality of life in favour of ciclosporin ophthalmic emulsion | |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | RR 2.32 (0.10 to 53.42) | 37 (1 study1) | ⊕⊕⊝⊝ low2 | |||
| Physician‐assessed improvement in rosacea severity Schirmer score | The mean physician‐assessed improvement in rosacea severity in the control group was ‐1.4 | The mean physician‐assessed improvement in rosacea severity in the intervention group was 4.1 higher (1.66 to 6.54 higher) | 37 (1 study1) | ⊕⊕⊝⊝ low2 | ||
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Schechter 2009 2 Downgraded two levels due to very serious imprecision (very wide confidence interval due to low sample size, optimal information size is not met)
Summary of findings 8. Clindamycin phosphate 1.2% + tretinoin 0.025% gel compared to placebo for rosacea.
| Clindamycin phosphate 1.2% + tretinoin 0.025% gel compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Clindamycin phosphate 1.2% + tretinoin 0.025% gel Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Clindamycin phosphate 1.2% + tretinoin 0.025% gel | |||||
| HRQOL RosaQoL | See comment | See comment | Not estimable | 83 (1 study1) | ⊕⊕⊕⊝ moderate2 | No mean scores were provided, only percentages of participants that had improved per item on the 21 survey items, no statistically significant difference for any item |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 275 per 1000 | 674 per 1000 (390 to 1000) | RR 2.45 (1.42 to 4.23) | 83 (1 study1) | ⊕⊕⊕⊝ moderate3 | Worsening of rosacea, facial scaling, as well as dry skin were reported most often in the active treatment group |
| Physician‐assessed improvement in rosacea severity PGA as defined by Wilkin 2004 | See comment | See comment | Not estimable | 83 (1 study1) | ⊕⊕⊕⊝ moderate2 | None of the primary features of the PGA showed statistically significant differences between the treatment groups except for oedema in favour of placebo |
| Assessment of erythema or telangiectasia | 150 per 1000 | 257 per 1000 (105 to 627) | RR 1.71 (0.70 to 4.18) | 83 (1 study1) | ⊕⊕⊕⊝ moderate3 | RR 1.71 (95% CI 0.70 to 4.18) refers to erythema. Telangiectasia RR 2.42, 95% CI 0.95 to 6.17 |
| Lesion count | The mean lesion count in the control group was ‐3.13 inflammatory lesions | The mean lesion count in the intervention group was 3.96 higher (1.28 lower to 9.20 higher) | 83 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | There was no improvement |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Chang 2012 2 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met) 3 Downgraded one level due to serious imprecision (wide confidence interval due to low sample size, optimal sample size is not met)
Summary of findings 9. Tetracycline compared to placebo for rosacea.
| Tetracycline compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Tetracycline Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tetracycline | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 474 per 1000 | 701 per 1000 (403 to 1000) | RR 1.48 (0.85 to 2.57) | 39 (1 study1) | ⊕⊕⊕⊝ moderate2 | |
| Proportion of participants with adverse event | 53 per 1000 | 50 per 1000 (3 to 744) | RR 0.95 (0.06 to 14.13) | 39 (1 study1) | ⊕⊕⊕⊝ moderate2 | Only one adverse event was reported in each group, diarrhoea in the tetracycline group, maculopapular rash in the placebo group |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 107 (2 studies3) | ⊕⊕⊕⊝ moderate2 | RR 4.04, 95% CI 1.66 to 9.83; P = 0.002 (Marks 1971) and RR 1.72, 95% CI 1.18 to 2.50; P = 0.005 (Sneddon 1966) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 39 (1 study1) | ⊕⊕⊕⊝ moderate4 | There were no significant changes in erythema (Marks 1971) |
| Lesion count | The mean lesion count in the control group was 1.41 inflammatory lesions | The mean lesion count in the intervention group was 14.64 lower | 39 (1 study1) | ⊕⊕⊕⊝ moderate5 | Crude MD ‐14.64 but skewed data (Marks 1971) | |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Marks 1971 2 Downgraded one level due to serious imprecision (wide confidence interval due to low sample size, optimal sample size is not met) 3Marks 1971 and Sneddon 1966 4 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met) 5 Downgraded one level due to serious imprecision (skewed data and low sample size, optimal sample size is not met)
Summary of findings 10. Doxycycline 40 mg compared to placebo for rosacea.
| Doxycycline 40 mg compared to placebo for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Doxycycline 40 mg Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Doxycycline 40 mg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 537 (2 studies1) | ⊕⊕⊕⊕ high | RR 1.14, 95% CI 0.85 to 1.53 (Del Rosso 2007a) and RR 1.27, 95% CI 1.04 to 1.55 (Del Rosso 2007b) |
| Physician‐assessed improvement in rosacea severity Investigator's Global Assessment, two point improvement | See comment | See comment | Not estimable | 537 (2 studies1) | ⊕⊕⊕⊕ high | RR 1.77, 95% CI 1.24 to 2.52; P = 0.002 (Del Rosso 2007a) and RR 1.41, 95% CI 0.87 to 2.29 (Del Rosso 2007b) and IGA score of 0 or 1 RR 1.59, 95% CI 1.02 to 2.47; P = 0.04 (Del Rosso 2007a) and RR 2.37, 95% CI 1.12 to 4.99; P = 0.02 (Del Rosso 2007b) |
| Assessment of erythema or telangiectasia Clinician's Erythema Assessments scale 0 to 4 | See comment | See comment | Not estimable | 537 (2 studies1) | ⊕⊕⊕⊕ high | Mean change in CEA ‐2.7 (doxycycline group) versus ‐1.8 (placebo group), investigators report P = 0.017 (Del Rosso 2007a); and ‐1.4 and ‐1.2 respectively (Del Rosso 2007b) |
| Lesion counts Scale from: ‐4.3 to ‐11.8 | See comment | See comment | Not estimable | 537 (2 studies1) | ⊕⊕⊕⊝ moderate2 | MD ‐5.90, 95% CI ‐9.37 to ‐2.43; P = 0.0009 (Del Rosso 2007a) and MD ‐5.20, 95% CI ‐8.27 to ‐2.13; P = 0.0009 (Del Rosso 2007b) |
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 537 (2 studies1) | ⊕⊕⊕⊕ high | The steepest changes in graph plots occurred within three weeks in the doxycycline group |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Del Rosso 2007a and Del Rosso 2007b 2 Downgraded one level due to serious imprecision (wide confidence interval)
Summary of findings 11. Azithromycin compared to doxycycline 100 mg for rosacea.
| Azithromycin compared to doxycycline 100 mg for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Azithromycin Comparison: Doxycycline 100 mg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg | Azithromycin | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | 800 per 1000 | 784 per 1000 (616 to 1000) | RR 0.98 (0.77 to 1.25) | 67 (1 study1) | ⊕⊝⊝⊝ very low2,3 | There was no statistically significant difference between the groups, but in both treatment arms the majority of participants considered themselves improved |
| Proportion of participants with adverse event | 67 per 1000 | 108 per 1000 (21 to 551) | RR 1.62 (0.32 to 8.26) | 67 (1 study1) | ⊕⊝⊝⊝ very low2,4 | |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion counts | The mean lesions count in the control group was 2.34 inflammatory lesions | The mean lesions count in the intervention group was 0 higher | 67 (1 study1) | ⊕⊝⊝⊝ very low2,5 | Lesion count decreased in azithromycin group from 19.24 (SD 9.67) to 1.90 (SD 3.28) at 3 months and for doxycycline from 18.86 (SD 8.95) to 2.34 (SD 3.47). Skewed data | |
| Time needed until improvement of the skin lesions ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission | See comment | See comment | Not estimable | 67 (1 study1) | ⊕⊝⊝⊝ very low2,3 | No data on duration of remission, but both groups showed no statistically significant change between the third month of treatment and the second month post‐treatment in the mean inflammatory lesion counts |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Akhyani 2008 2 Downgraded two levels due to very serious risk of bias (allocation concealment was at high risk of bias, no blinding) 3 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met, optimal sample size is not met) 4 Downgraded one level due to serious imprecision (wide confidence interval due to low sample size, optimal sample size is not met) 5 Downgraded one level due to serious imprecision (large SDs and skewed data, low sample size, optimal sample size is not met)
Summary of findings 12. Doxycycline 40 mg + metronidazole 1% gel compared to doxycycline 100 mg + metronidazole 1% gel for rosacea.
| Doxycycline 40 mg + metronidazole 1% gel compared to doxycycline 100 mg + metronidazole 1% gel for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Doxycycline 40 mg + metronidazole 1% gel Comparison: Doxycycline 100 mg + metronidazole 1% gel | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg + metronidazole 1% gel | Doxycycline 40 mg + metronidazole 1% gel | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 553 per 1000 | 138 per 1000 (61 to 299) | RR 0.25 (0.11 to 0.54) | 91 (1 study1) | ⊕⊕⊝⊝ low2,3 | The majority of these adverse events were gastrointestinal complaints |
| Physician‐assessed improvement in rosacea severity Reduction in Investigator's Global Assessment | The mean physician‐assessed improvement in rosacea severity in the control group was ‐1.6 | The mean physician‐assessed improvement in rosacea severity in the intervention group was 0.00 higher (0.11 lower to 0.11 higher) | 91 (1 study1) | ⊕⊕⊝⊝ low2,4 | ||
| Assessment of erythema or telangiectasia Clinician's Erythema Assessment | The mean assessment of erythema or telangiectasia in the control group was ‐4.0 | The mean assessment of erythema or telangiectasia in the intervention group was 0 higher | 91 (1 study) | ⊕⊕⊝⊝ low2,4 | Reduction in CEA 4.2 in doxycycline 40 mg and 4.0 in doxycycline 100 mg group, investigator's state P = 0.50 | |
| Lesion count | The mean lesion count in the control group was ‐12.2 inflammatory lesions | The mean lesion count in the intervention group was 0.30 lower (3.03 lower to 2.43 higher) | 91 (1 study1) | ⊕⊕⊝⊝ low2,3 | ||
| Time needed until improvement of the skin lesions | See comment | See comment | Not estimable | 91 (1 study1) | ⊕⊕⊝⊝ low2,4 | A clear improvement was seen from week four for both groups. |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Del Rosso 2008 2 Downgraded one level due to serious risk of selection bias and attrition bias (sequence generation and allocation concealment at unclear risk of bias, high drop‐out rate and although ITT analysis judged at unclear risk of bias) 3 Downgraded one level due to serious imprecision (wide confidence interval due to low sample size, optimal sample size is not met) 4 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met)
Summary of findings 13. Doxycycline 40 mg + azelaic acid gel compared to doxycycline 40 mg + metronidazole gel for rosacea.
| Doxycycline 40 mg + azelaic acid gel compared to doxycycline 40 mg + metronidazole gel for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Doxycycline 40 mg + azelaic acid gel Comparison: Doxycycline 40 mg + metronidazole gel | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 40 mg + metronidazole gel | Doxycycline 40 mg + azelaic acid gel | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity Excellent improvement on a 4‐point Likert scale | 465 per 1000 | 489 per 1000 (368 to 651) | RR 1.05 (0.79 to 1.40) | 207 (1 study1) | ⊕⊕⊕⊕ high | Excellent improvement was reported in approximately half of each intervention group |
| Proportion of participants with adverse event | 69 per 1000 | 19 per 1000 (4 to 89) | RR 0.27 (0.06 to 1.28) | 207 (1 study1) | ⊕⊕⊕⊕ high | |
| Physician‐assessed improvement in rosacea severity Investigator's Global Assessment of 0, 1 or 2 (clear to mild) | 723 per 1000 | 781 per 1000 (672 to 918) | RR 1.08 (0.93 to 1.27) | 207 (1 study1) | ⊕⊕⊕⊕ high | |
| Clinician's Erythema Assessment ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Lesion count | The mean lesion count in the control group was ‐9.4 inflammatory lesions | The mean lesion count in the intervention group was 1.10 lower (4.91 lower to 2.71 higher) | 207 (1 study1) | ⊕⊕⊕⊝ moderate2 | ||
| Time needed until improvement | See comment | See comment | 207 (1 study1) | ⊕⊕⊕⊕ high | From four weeks on improvement could be seen for both treatment arms | |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Del Rosso 2010 2 Downgraded one level due to serious imprecision (wide confidence interval)
Summary of findings 14. Minocycline 45 mg compared to minocycline 45 mg + azelaic acid gel for rosacea.
| Minocycline 45 mg compared to minocycline 45 mg + azelaic acid gel for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Minocycline 45 mg Comparison: Minocycline 45 mg + azelaic acid gel | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Minocycline 45 mg + azelaic acid gel | Minocycline 45 mg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Proportion of participants with adverse event | 533 per 1000 | 368 per 1000 (208 to 651) | RR 0.69 (0.39 to 1.22) | 60 (1 study1) | ⊕⊕⊝⊝ low2,3 |
|
| Physician‐assessed improvement in rosacea severity Mean change in Investigator's Global Assessment (Likert scale 0 to 5). Scale from: 0 to 4. | The mean physician‐assessed improvement in rosacea severity in the control groups was ‐2.0 on IGA | The mean physician‐assessed improvement in rosacea severity in the intervention groups was 0.00 higher (0.32 lower to 0.32 higher) | 60 (1 study1) | ⊕⊕⊝⊝ low2,3 |
||
| Assessment of erythema or telangiectasia Mean change in CEA scale (Likert scale 0 to 4). Scale from: 0 to 4. | The mean assessment of erythema or telangiectasia in the control group was ‐4 on CEA | The mean assessment of erythema or telangiectasia in the intervention group was 1.00 higher (0.18 lower to 2.18 higher) | 60 (1 study1) | ⊕⊕⊝⊝ low2,3 |
||
| Lesion count | The mean lesion count in the control group was ‐12 inflammatory lesions | The mean lesion count in the intervention group was 1.00 higher (0.93 lower to 2.93 higher) | 60 (1 study1) | ⊕⊕⊝⊝ low2,3 |
In both groups there was a clinically important reduction in lesion counts of 11.00 (SD 4.49) in the minocycline group and 12.00 (SD 3.00) in the comparator group | |
| Time needed until improvement | See comment | See comment | Not estimable | 60 (1 study1) | ⊕⊕⊝⊝ low2,3 |
Improvement was seen in both arms at four weeks |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Jackson 2013 2 Downgraded one level due to serious risk of performance and detection bias (blinding was assessed as at unclear risk of bias) 3 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met)
Summary of findings 15. Topical metronidazole compared to oral (oxy)tetracycline for rosacea.
| Topical metronidazole compared to oral (oxy)tetracycline for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Topical metronidazole Comparison: Oral (oxy)tetracycline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral (oxy) tetracycline | Topical metronidazole | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 182 (3 studies1) | ⊕⊕⊕⊝ moderate2 | RR 0.71, 95% CI 0.40 to 1.26 (Monk 1991), RR 0.96, 95% CI 0.80 to 1.17 (Nielsen 1983b) and in Schachter 1991 no exact data were provided other than that "both groups considered their condition much improved" |
| Proportion of participants with adverse event | See comment | See comment | Not estimable | 258 (4 studies3) | ⊕⊕⊕⊝ moderate4 | No adverse event (Nielsen 1983b), RR 1.06, 95% CI 0.32 to 3.55 (Monk 1991), 12 adverse events reported in metronidazole group and 9 in tetracycline group (Schachter 1991), RR 0.70, 95% CI 0.30 to 1.65 (Veien 1986) |
| Physician‐assessed improvement in rosacea severity | See comment | See comment | Not estimable | 81 (2 studies5) | ⊕⊕⊕⊝ moderate2 | RR 0.80, 95% CI 0.47 to 1.35 (Monk 1991), RR 1.00, 95% 0.89 to 1.13 (Nielsen 1983b) |
| Assessment of erythema or telangiectasia | See comment | See comment | Not estimable | 258 (4 studies3) | ⊕⊕⊝⊝ low2,6 | Erythema score ‐1.4 versus ‐1.3 (Monk 1991), "the reduction of erythema was the same in both groups, and the number and extent of telangiectases were unchanged" (Nielsen 1983b), in Schachter 1991 no differences in erythema nor telangiectasia were seen in either group. In Veien 1986 the percentage of no improvement was 11.1 in the metronidazole group versus 12.5 in the tetracycline group |
| Lesion count | See comment | See comment | 258 (4 studies3) | ⊕⊕⊕⊝ moderate2 | Complete clearance in 75% versus 66% of participants (Monk 1991), "the reduction of papules and pustules was the same in both groups" (Nielsen 1983b), decrease of 68% versus 77% in papule count and of 53% and 61% in pustule count (Schachter 1991). In Veien 1986 only medians were provided with 11.1 lesions in the metronidazole group and 0 in the tetracycline group | |
| Time needed until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Monk 1991, Nielsen 1983b, Schachter 1991 (number of participants randomised in Schachter 1991 was unclear) 2 Downgraded one level due to serious imprecision (low sample sizes) 3Monk 1991, Nielsen 1983b, Schachter 1991, Veien 1986 (number of participants randomised in Schachter 1991 was unclear) 4 Downgraded one level due to serious imprecision (wide confidence intervals due to low sample sizes) 5Monk 1991, Nielsen 1983b 6 Downgraded one level due to serious heterogeneity (in contrast to the other three studies, Schachter 1991 did not show any improvement in erythema and telangiectasia
Summary of findings 16. Low dose isotretinoin 0.3 mg/kg compared to doxycycline 50‐100 mg for rosacea.
| Low dose isotretinoin 0.3 mg/kg compared to doxycycline 100 mg for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Low dose isotretinoin 0.3 mg/kg Comparison: Doxycycline 100 mg after 14 days tapered to 50 mg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline 100 mg | Low dose isotretinoin 0.3 mg/kg | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 Good to excellent improvement on 5‐point Likert scale | 644 per 1000 | 792 per 1000 (676 to 921) | RR 1.23 (1.05 to 1.43) | 261 (1 study2) | ⊕⊕⊕⊕ high | Low dose isotretinoin is considered by the participants to be slightly more effective than doxycycline 100 mg |
| Proportion of participants with adverse event | 171 per 1000 | 204 per 1000 (127 to 328) | RR 1.19 (0.74 to 1.92) | 299 (1 study2) | ⊕⊕⊕⊕ high | |
| Physician‐assessed improvement in rosacea severity1 Complete remission or marked improvement on a 6‐point Likert scale) | 689 per 1000 | 813 per 1000 (710 to 938) | RR 1.18 (1.03 to 1.36) | 261 (1 study2) | ⊕⊕⊕⊕ high | In agreement with the participant‐assessed changes |
| Assessment of erythema or telangiectasia Improved or healed | 783 per 1000 | 736 per 1000 (650 to 846) | RR 0.94 (0.83 to 1.08) | 285 (1 study2) | ⊕⊕⊕⊕ high | Telangiectasia were improved or "healed" RR 1.03, 95% CI 0.77 to 1.37 |
| Lesion count1 | The mean lesion count in the control group was ‐13 inflammatory lesions | The mean lesion count in the intervention group was 3 lower | 261 (1 study2) | ⊕⊕⊕⊕ high | ||
| Time needed until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Per‐protocol analysis 2Gollnick 2010
Summary of findings 17. Pulsed dye laser compared to Nd:YAG laser for rosacea.
| Pulsed dye laser compared to Nd:YAG laser for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Pulsed dye laser Comparison: Nd:YAG laser | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nd: YAG laser | Pulsed dye laser | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 | The mean participant‐assessed improvement in rosacea severity in the control group was 34 percent | The mean participant‐assessed improvement in rosacea severity in the intervention group was 16.33 higher (1.94 to 34.6 higher) | 14 (1 study2) | ⊕⊕⊝⊝ low3 |
||
| Proportion of participants with adverse event1 Pain as assessed by VAS (0 to 10; higher score is worse) | See comment | See comment | Not estimable | 14 (1 study2) | ⊕⊕⊝⊝ low4 |
Pain was assessed on the PDL treated side 3.87 and 3.07 on the Nd:YAG side, the investigators state P = 0.0028 |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia1 Spectrophotometer to assess facial redness | The mean assessment of erythema or telangiectasia in the control group was ‐2.5 percent | The mean assessment of erythema or telangiectasia in the intervention group was 6.4 lower (11.6 to 1.2 lower) | 14 (1 study2) | ⊕⊕⊝⊝ low3 |
||
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Within‐participant 2Alam 2013 3 Downgraded two levels due to very serious imprecision (very wide confidence interval due to low sample size, optimal sample size is not met) 4 Downgraded two levels due to very serious imprecision (very low sample size, optimal sample size is not met)
Summary of findings 18. Pulsed dye laser compared to intense pulsed light therapy for rosacea.
| Pulsed dye laser compared to intense pulsed light therapy for rosacea | ||||||
| Patient or population: Participants with rosacea Intervention: Pulsed dye laser (PDL) Comparison: Intense pulsed light therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intense Pulsed Light Therapy | Pulsed Dye Laser | |||||
| HRQOL ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Participant‐assessed improvement in rosacea severity1 VAS. Scale from: 0 to 10 (0 being a poor and 10 an excellent result) | The mean participant‐assessed improvement in rosacea severity in the control group was 7 | The mean participant‐assessed improvement in rosacea severity in the intervention group was 1 higher | 40 (1 study2) | ⊕⊕⊝⊝ low3,4 |
Median was 8 (range 2 to 10) for PDL group and 7 (range 2 to 10) for IPL group (10% and 90% percentiles) | |
| Proportion of participants with adverse event Pain as assessed with a VAS scale. Scale from: 0 to 10 | Pain assessed on a VAS scale in the control group was 7 | Pain assessed on a VAS scale in the intervention group was 3 lower | 40 (1 study2) | ⊕⊕⊝⊝ low3,4 |
Median was 4 (range 2 to 6) for PDL group and 7 (range 2 to 10) for IPL group (10% and 90% percentiles) | |
| Physician‐assessed improvement in rosacea severity ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Assessment of erythema or telangiectasia 5‐point Likert scale | See comment | See comment | 40 (1 study2) | ⊕⊕⊕⊝ moderate4,5 |
On the PDL treated side 18 had an excellent (75% to 100% vessel clearance) response and 12 a good response (50% to 74% clearance) and on the IPL treated sides 11 had an excellent response and 19 a good response | |
| Lesion count ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Time until improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| Duration of remission ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study addressed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Within‐participant design 2Nymann 2010 3 Downgraded one level due to serious performance and detection bias (investigators and participants were not blinded) 4 Downgraded one level due to serious imprecision (low sample size, optimal sample size is not met) 5 "Clinical efficacy was evaluated by one blinded trained physician"
Background
We have listed unfamiliar terms in the glossary of terms in Table 19.
1. Glossary of unfamiliar terms.
| Term | Definition |
| Acne | A skin condition characterised by the inflammation or infection of sebaceous glands (usually attached to hair follicles) resulting in comedones (whiteheads and blackheads) and inflammatory lesions such as papules (pimples), pustules, and nodules) |
| Bacillus oleronius | A bacteria found in Demodex mites |
| Bacterial resistance | Resistance of a micro‐organism to an antimicrobial drug that was originally effective for treatment of infections caused by this micro‐organism |
| Body dysmorphic disorder | An anxiety disorder surrounding perceived flaws in one's own appearance |
| Cytokines | A small protein released by cells, and having a specific effect on the behavior of other cells, or on the interactions or communications between cells |
| Demodex folliculorum | A species of face mite found in human hair follicles |
| Down‐regulation | Process of reducing or suppressing a response to a stimulus |
| Epidermal barrier | The skin's front line of defence in the upper layer of the skin (the epidermis) against environmental factors such as UV light, chemicals, bacteria and other organisms and limits water loss from the body |
| Innate immune response | The first line generic defence of the immune system against infection and other organisms |
| Keratinocytes | A predominant cell type in the outermost layer of skin (epidermis), and when found in the basal layer, are referred to as 'basal cells' or 'basal keratinocytes'. Their main function is the formation of a barrier against environmental damage |
| Matrix‐Metalloproteinases | Zinc dependent enzymes that promote break down of proteins like collagen. They regulate various inflammatory and repair processes |
| Neurovascular dysregulation | A failure of the vascular response, vasodilation, and neurosensory symptoms to regulate properly Dysfunction of both nerves and vascular elements, controlling the calibre of blood vessels |
| Nodule | Solid, raised area in or under the skin |
| Nodularities | An increased density of tissues |
| Pathophysiology | The functional changes that accompany a particular syndrome or disease (combined terms of ‘patho’ (path, related to disease) and ‘physiology’ (a branch of biology that specialises in the study of the functions of living organisms and their parts) |
| Phototype | A classification of skin type based on a person's sensitivity to sunlight |
| Pustule | A small bump on the skin containing purulent material (pus) in the top layer (epidermis) or beneath it (dermis) |
| Reactive oxygen species (ROS) | Chemically reactive molecules containing oxygen, or oxygen‐derived radicals, having important roles in cell signalling (communication and interaction) and homeostasis (the maintenance of a steady state) |
| Retinoids | Chemical compounds related chemically to Vitamin A |
| Stratum corneum | The outermost layer of the epidermis |
| Stye | A bacterial infection of a gland at the base of any eyelash, causing painful swelling on the inner or outer eyelid |
| Toll‐like receptors | A class of proteins that play a key role in the innate immune system, activating immune cell responses |
Description of the condition
Definition and clinical features
Rosacea is a chronic skin disease that can affect the cheeks, nose, eyes, chin and forehead. It is characterised by recurrent episodes of flushing, erythema (redness), papules (pimples), pustules and telangiectasia (permanent distended blood capillary vessels with a spidery appearance) (Elewski 2011; Korting 2009; Marks 2007; Powell 2005). Although there is no standard clinical definition of the condition, rosacea is generally classified into four subtypes and one variant (Wilkin 2002; Wilkin 2004).
Subtype 1: erythematotelangiectatic rosacea, where the clinical features include flushing and persistent central facial erythema (redness) with or without telangiectasia.
Subtype 2: papulopustular rosacea, characterised by persistent central facial erythema with transient, central face papules or pustules, or both.
Subtype 3: phymatous rosacea, where thickening of the skin is seen with irregular surface nodularities, and enlargement. This may occur on the nose (rhinophyma), chin, forehead, cheeks or ears.
Subtype 4: ocular rosacea, characterised by ocular involvement, including inflammation of different parts of the eye and eyelid. It may be found in up to 58% of cases but it is frequently undiagnosed.
Variant: granulomatous rosacea, which is non‐inflammatory and characterised by hard, brown, yellow or red cutaneous papules, or nodules of uniform size.
Progression from one subtype to another is possible, patients may express signs and symptoms of more than one subtype, and each individual characteristic may change from absent to severe (Powell 2005; Tan 2013; Wilkin 2004).
Symptoms
Rosacea primarily affects the face and is accompanied by the physical discomfort of flushes, persistent erythema and the effects of eye lesions and inflammatory lesions, and it can also lead to psychological problems over and above these physical symptoms.
The disease can cause embarrassment, anxiety, low self‐esteem and lack of confidence, and may even lead to depression, social anxiety disorder or body dysmorphic disorder (Abram 2009; Elewski 2011; Landow 2005; Powell 2005). If there is eye involvement, it may result in varying and sometimes severe manifestations which may produce significant additional discomfort (Lazaridou 2011; Oltz 2011; Vieira 2013).
There are no standard validated tools for assessing the severity of rosacea or its signs and symptoms (Gessert 2003). Thus clinicians tend to assess the severity of rosacea by focusing on the papules and pustules, whereas people with rosacea place greater emphasis on the erythema. There would appear to be poor consistency in assessments between individuals when detailed scoring scales are used (Bamford 2004; Gessert 2003). Moreover, several studies have demonstrated that objective clinical parameters of skin disease are often poorly correlated with quality of life, and that physicians tend to underestimate the impact of skin disease on an individual (Chren 1996; Nicholson 2007). Rosacea has a significant negative impact on quality of life (Aksoy 2010; Cresce 2014; Moustafa 2014; van der Linden 2014). A validated disease‐specific quality of life instrument (RosaQoL) has been developed, and RosaQoL scores have been used in several studies as one of the outcome parameters (Baldwin 2010; Bamford 2012; Fleischer 2005; Kini 2010; Nicholson 2007). Much work remains to be done to improve the quality of reporting of patient reported outcomes (PRO) in studies on rosacea (van Zuuren 2013).
Epidemiology and causes
Rosacea usually presents in the second or third decade of life, with up to 10% of the population affected (Berg 1989). It is reportedly more common in fair‐skinned people of Celtic and northern European heritage (Culp 2009; Korting 2009; Powell 2005) and women appear to be more often affected than men (Culp 2009; Powell 2005). However, a proportionately larger number of men develop phymatous changes (thickening skin, irregular surface nodularities, and enlargement), sometimes as a result of progression from subtype 1 or subtype 2 to subtype 3 (Powell 2005; Tan 2013; Wilkin 2004). The prevalence of rosacea varies from less than 1% to more than 20%, indicating a range which is most likely attributable to differences in the populations studied and the methodological approach used (Tan 2013b). Prevalence studies of rosacea in darker skin phototypes are sparse.
The pathophysiology of rosacea is very complex and the exact cause remains unclear. A number of hypotheses have been proposed. Both genetic and mostly environmental stimuli and triggers, for example heat, sunlight, stress, certain foodstuffs and Demodex mites stimulate an augmented innate immune response and neurovascular dysregulation (Del Rosso 2012; Elewski 2011; Steinhoff 2011; Steinhoff 2013). In rosacea affected skin, elevated abnormal cathelicidin (an antimicrobial peptide) and elevated serine protease (kallikrein‐5) induce increased LL‐37, which results in inflammation, neurovascular effects and vascular changes (Del Rosso 2012; Yamasaki 2007;Yamasaki 2011). More recently mechanisms and components of rosacea pathophysiology have been categorised into (a) increase of Toll‐like receptors on keratinocytes, (b) augmented innate immunity, (c) neurovascular dysregulation (d) vascular changes, (e) reactive oxygen species (ROS), (f) stratum corneum permeability barrier dysfunction, (g) ultraviolet (UV) radiation and (h) microbes, e.g. Demodex,Bacillus oleronius (Del Rosso 2012; Del Rosso 2013a; Steinhoff 2011; Tisma 2009). The current hypothesis is that rosacea is an inflammatory disorder that may develop in individuals with rosacea‐prone skin, initiated by several triggers (Steinhoff 2011). Possible triggers that have been investigated are gastrointestinal (digestive) tract diseases, infestation with Demodex folliculorum, Bacillus oleronius, epidermal barrier defect, and childhood stye (Bamford 2006; Elewski 2009; Forton 2007; Forton 2012; Lacey 2007).
Description of the intervention
As with most chronic skin diseases, rosacea requires long‐term treatment, therapies are numerous, and their use is frequently based on anecdotal evidence (Elewski 2011; Layton 2013; Powell 2005). Management strategies for people with rosacea can often be tailored to the specific subtype of rosacea (Baldwin 2006; Jansen 1997; Powell 2005) but, because rosacea can have a significant impact on quality of life, these strategies should also be directed towards achieving improvements in general well‐being (Bikowski 2004; Elewski 2011). In certain individuals successful management of rosacea is possible through avoidance of some of the triggers, in particular those which cause flushing, that is certain foods and beverages, sunlight and some types of cosmetics (Elewski 2011).
Topical interventions
If a small number of papules and pustules are present, topical rather than systemic therapy is considered to be the first‐line of treatment (Del Rosso 2013b; Elewski 2011). This includes a range of formulations of metronidazole, azelaic acid, clindamycin lotion, permethrin 5% cream, tretinoin cream, 10% sulphacetamide with sulphur (5%) and benzoyl peroxide alone or in combination with erythromycin or clindamycin (Culp 2009; Del Rosso 2013b; Elewski 2011; Korting 2009). The Food and Drug Administration (FDA) approved topical ivermectin 1% for papulopustular rosacea and European Medicines Agency (EMA) approval is expected in the near future (Layton 2013; Stein 2014a). Brimonidine tartrate gel 5%, a topical selective α‐adrenergic receptor agonist with vasoconstrictive activity, was recently approved for the treatment of persistent erythema in rosacea (Del Rosso 2013c; Fowler 2012a; Fowler 2013a).
Systemic interventions
If the skin lesions are more extensive, oral antibiotics such as tetracyclines or azithromycin are usually recommended (Alikhan 2010; Bakar 2009; Culp 2009; Elewski 2011). Second‐generation tetracyclines, for example doxycycline and minocycline, have been used more recently with reportedly fewer side effects (Bikowski 2003; Korting 2009; Sloan 2008). To reduce not only the side effects but also the risk of bacterial resistance, a novel approach has been advocated that involves the use of a subantimicrobial dosage of doxycycline, which separates the anti‐inflammatory effect of doxycycline from its antimicrobial properties by limiting the plasma concentration to a range below the minimum inhibitory concentrations for susceptible bacteria (Baldwin 2010; Del Rosso 2007a; Sapadin 2006; Walker 2006). Not infrequently, oral treatment may be combined initially with topical treatment so that as the rosacea improves the systemic treatment can be discontinued and improvement can be maintained by continuation with the topical treatment alone (Elewski 2011).
Other interventions
The vascular manifestations of rosacea appear to respond fairly effectively to light‐based therapies such as pulsed dye laser or intense pulsed light (Culp 2009; Kawana 2007; Korting 2009; Tanghetti 2014). In the more severe or persistent cases of papulopustular and phymatous rosacea, oral 13‐cis‐retinoic acid (isotretinoin) therapy may be required. Isotretinoin has a well‐recognised safety profile but should only be given to women under close supervision and should include an appropriate contraception strategy (Korting 2009; Nickle 2014).
Traditional therapies for ocular rosacea include oral tetracyclines, eyelid hygiene and warm compresses (Stone 2004; Oltz 2011; Vieira 2013). Topical metronidazole gel and fusidic acid gel are also reportedly successful (Barnhorst 1996; Seal 1995; Vieira 2013). Rhinophyma may require surgical intervention, but low dose isotretinoin and laser therapy have also been used (Powell 2005; Taghizadeh 2008; Tanghetti 2014).
How the intervention might work
Although a lack of understanding of the pathophysiology of rosacea continues to hamper therapeutic efforts (Baldwin 2006; Elewski 2011), metronidazole and azelaic acid are generally considered as the first‐line in topical medicaments. It is also now widely recognised that the therapeutic efficacy of both can be attributed to their anti‐inflammatory and antioxidant effects (Bhatia 2012; Elewski 2011; Feldman 2014; Naranayan 2007). Ivermectin has demonstrated activity against Demodex in addition to possible inflammatory properties (Layton 2013; Stein 2014a). Brimonidine targets the α‐adrenergic receptors in the smooth muscle sheath located around the vessel wall of the superficial blood vessels of the skin and coupled with its potent vasoconstrictive activity it can provide a reduction of facial erythema after application (Del Rosso 2013c).
Tetracyclines down‐regulate the production of inflammatory cytokines, inhibit matrix‐metalloproteinases (MMP), and down‐regulate the inflammatory response in papulopustular rosacea (Baldwin 2006; Del Rosso 2007a). Doxycycline has been shown to inhibit neutrophil activity and several pro‐inflammatory reactions including those associated with phospholipase A2, endogenous nitric oxide and interleukin‐6 (Baldwin 2006; Bikowski 2003). Subantimicrobial doses of doxycycline can be important in minimising the development of microbial resistance (Bikowski 2003; Korting 2009; Sloan 2008). Isotretinoin has anti‐inflammatory properties, it diminishes sebaceous gland size and number and can help retard or halt development of rhinophyma (Baldwin 2006; Erdogan 1998; Gollnick 2010; Uslu 2012).
Laser therapy is capable of reducing both erythema and telangiectasia (Shim 2013). The pulsed dye laser (PDL) with the 595 nm wavelength targets haemoglobin and delivers all of the administered energy in a wavelength that is actively taken up by the haemoglobin in blood vessels causing vessel destruction (Bernstein 2008; Butterwick 2006; Kim 2011; Shim 2013). The 532 nm frequency‐doubled, potassium‐titanyl‐phosphate (KTP) and the neodymium‐doped, yttrium‐aluminium‐garnet (Nd:YAG) laser can also deliver wavelengths which are readily absorbed by haemoglobin (Bernstein 2008; Butterwick 2006; Karsai 2008). Intense pulsed light with a wavelength between 550 nm and 670 nm is readily absorbed by both melanin and oxyhaemoglobin, and has also been used in the treatment of telangiectasia (Butterwick 2006; Kawana 2007; Nymann 2010).
Why it is important to do this review
Although rosacea is a common and distressing disorder, there is continuing debate over which therapy, or which combination of therapies, is most likely to offer benefits to patients. This systematic review was conducted to examine the different management options and to try and determine the most effective strategy in the treatment of rosacea.
Objectives
To assess the efficacy and safety of treatments for rosacea.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
People older than 19 years with moderate to severe rosacea (diagnosed clinically).
Types of interventions
Any type of intervention used, either alone or in combination, to treat rosacea versus placebo, no treatment or active treatment. We also considered the effects of avoidance of some foodstuffs, for example spicy food, as well as the use of certain cosmetics and sunscreens.
Types of outcome measures
Primary outcomes
Change in health‐related quality of life (HRQOL) at end of study
Participant‐assessed changes in rosacea severity at end of study
Proportion of participants who reported an adverse event throughout the study period
Secondary outcomes
Physician‐assessed changes in rosacea severity. These included the following:
physician's global assessment of rosacea severity at end of study;
assessment of erythema or telangiectasia, or both, at end of study;
reduction in lesion counts (treatment success defined as greater than 50% reduction in lesion counts);
time needed until improvement of the skin lesions;
duration of remission.
We produced 'Summary of findings' tables of the following outcomes listed according to priority:
change in HRQOL;
participant‐reported improvement of rosacea;
proportion of participants who reported an adverse event;
physician's global assessment of improvement of rosacea;
assessment of erythema or telangiectasia, or both;
reduction in lesion counts;
time needed until improvement of the skin lesions;
duration of remission.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press or in progress).
Electronic searches
For this update, we revised the search strategies for all our databases (see the section on Differences between protocol and review for details). We searched the following databases up to 1 July 2014:
Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 6) in The Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
EMBASE via Ovid (from 1974) using the strategy in Appendix 4;
LILACS (Latin American and Caribbean Health Science Information database) (from 1982) using the strategy in Appendix 5;
Science Citation Index (from 1988) (see Appendix 6); and
BIOSIS (previously searched from 1970 to March 2002) (see Appendix 7).
Trials registers
We (EvZ and MvdL) searched the following trials registers on 20 July 2014 with the search terms 'rosacea' and 'rhinophyma':
metaRegister of Controlled Trials (www.controlled‐trials.com);
US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov);
Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch);
the Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
References from published studies
The reference lists of all identified RCTs and key review articles were checked for further references to relevant trials (EvZ and ZF).
Unpublished literature
Attempts were made (EvZ and ZF) to locate unpublished and ongoing trials through correspondence with authors and pharmaceutical companies (see Table 20 and Table 21).
2. Pharmaceutical companies contacted.
| Name | Response | Additional | Comment |
| Bayer | Yes | Yes | Added information on Del Rosso 2010 Christopher Billis <christopher.billis@bayer.com> and that ongoing studies were not yet published |
| Roche | Yes | No | ‐ |
| ASTA Medica | Yes | No | ‐ |
| Merck | Yes | No | ‐ |
| Dumex‐Alpharma | Yes | No | ‐ |
| Galderma | Yes | Yes | Patricia.VanLith@galderma.com, michael.graeber@galderma.com August 2014 several times contact with Galderma NL, France and US, provided lots of extra information regarding brimonidine and ivermectin |
| AHP Pharma | No | No | ‐ |
| Yamanouchi | No | No | ‐ |
| Dermik Laboratories | No | No | ‐ |
| CollaGenex | No | No | Taken over by Galderma |
3. Investigators contacted.
| Name | Response | Additional | Comment |
| Akhyani 2008 | Yes | Yes | mghiasi@sina.tums.ac.ir. (sequence generation and allocation concealment) "In efficacy of azithromycin vs. doxycycline in the treatment of rosacea: a randomised open clinical trial" Patients were allocated to the trial using a randomised numbers table. Unfortunately this trial was not blinded" "The randomised number table generated by computer. The list was only in access of physician, and patients could not see that |
| Altinyazar 2005 | Yes | Yes | After email contact with the primary investigator and following on from discussion between the review authors, this was judged to be quasi‐randomised, i.e. a CCT |
| Benkali 2014 | Yes | Yes | nathalie.wagner@galderma.com, 1‐8‐2014 (sequence generation and allocation concealment)
1) Regarding the allocation sequence generated for the 4 subsequent groups consisting of different doses or regimen for topical applications, the randomisation list was created before the study started, with a 1:1:1:1 ratio and block size of 4. This randomisation list was generated by a designated biostatistician and was distributed to the clinical supply team in a sealed envelope (see the attached pdf file for the randomisation memo) 2) As explained above, only the 4 arms treated with topical products were to be randomised. The block size of 4 was not known by the sites, so foreseeing the next allocation was possible but unlikely. Since the study had 2 treatment groups for QD regimen and 2 treatment groups for BID regimen, subjects and the personnel who distributed the medication necessarily knew this information Of note, the primary objective of this study was PK assessment (and not efficacy), an objective measure, and the primary comparison was topical versus eye drop which was in no way planned to be randomised or blinded 3) This study was not posted on CT.gov since it was classified as a phase 1 study |
| Berardesca 2008 | Yes | Yes | Berardesca@berardesca.it. After email communication with the investigators to clarify aspects of trial conduct, the criterion for sequence generation was changed from UNCLEAR to High risk, i.e. the study was not a RCT and participants appear to have been allocated to the intervention by alternation |
| Berardesca 2012 | Yes | Yes | 18‐8‐2014 maurizio.caserini@polichem.com (sequence generation and allocation concealment and blinding) 29‐9‐2013 replies 1. This was a randomised, double‐blind, parallel‐group, placebo‐controlled study Patients, having signed their informed consent and who satisfied all inclusion and exclusion criteria at inclusion visit were randomly assigned to one of two treatment groups (P‐3075 cream, placebo), according to a computer‐generated randomisation list Patients were sequentially assigned to the next available randomisation number, starting from the lowest number provided to each investigational site Furthermore, for ethical reason, in order to minimise the exposure to placebo, randomisation was unbalanced between the P‐3075 and placebo groups with a 2:1 ratio using blocks of 3 treatments 2/3. The double blind study design was guaranteed by the use of placebo cream units, which were identical to the active product in terms of size, shape, volume, colour. The tubes (P‐3075 and placebo) were identically labelled for clinical use as it is in a double‐blind procedure 4. Thank you for this observation (you are the first). We confirm that the correct value is ‐167.00 and not 167.00, as reported in our database. It was a typing error that was not detected when the manuscript was transformed in draft paper by the editor 5. As described in the paper, a 4‐point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) was used by the investigators at each visit for the clinical evaluation of erythema The results at Day 28 (end of treatment) showed that, in the P‐3075 group, erythema was absent in 27 patients (96.4%) and mild in 1 (3.6%), while in the placebo group, erythema was absent in 9 patients (64.3%) and mild in 5 (35.7%). There were no cases of moderate or severe intensity at Day 28 in both groups. The statistically significance values were reported in the paper as you underlined. For completeness the baseline clinical assessment for erythema was as follows: for P‐3075 absent in 7 patients, mild in 14, moderate in 6 and severe in 1 and for placebo absent in 3 patients, mild in 6 and moderate in 5 |
| Beutner 2005 | Yes | Yes | kbeutner@anacor.com and bcalvarese@dowpharmsci.com, LAmdahl@dowpharmsci.com. Useful additional information provided by primary investigator, on randomisation, allocation concealment and characteristics of patients |
| Bribeche 2015 | Yes | Yes | 'ridha.bribech@gmail.com' 30‐8‐2014
Dear professor Bribeche (allocation concealment and blinding)
reply 7‐9‐2014: 1‐ During enrolment we used an allocation randomiser programme: http://www.randomizer.org/
2‐ Only the participants were blinded to treatment, praziquantel ointment and the placebo had the same colour (white), and ointment were given to participants in identical boxes for both groups (white box with a blue cover)
Next mail 7‐9‐2014: The reply to our first question is more on sequence generation, and not concealment of the allocation. Who was responsible for using that programme and who had access to the generated list? Reply 10‐9‐2014: Me and professor Fedotov VP, were responsible for using this programme, both of us had access to the generated list and a doctor from our department (Dr Makurina); who was fully unaware of the aims of the study and overseen the enrolment |
| Buendia‐Bordera 2013 | Can't find mail address, sent invite on LinkedIn | ||
| Chosidow 2014 NCT00882531 |
Yes | olivier.chosidow@hmn.aphp.fr and emilie.sbidian@hmn.aphp.fr,18‐7‐2014
Is the NCT00882531 published and if so give us a pdf of the publication? Reply 18‐7‐2014 "Hi Esther, we are still in processing the manuscript and hope submitting the paper before the end of 2014" and "We could send your our submitted manuscript?" |
|
| Cunliffe 1977 | No | No | ‐ |
| Dahl 2001 | Yes | Yes | Dahl.MarkV@mayo.edu."Subjects will be randomised to 1 of the 2 treatment groups at a ratio of 1:1. The randomisation process will be done in blocks of 4, stratified by investigators. The randomisation will be carried out using SAS PROC PLAN" |
| Del Rosso 2010 | Yes | Yes | jqdelrosso@yahoo.com, 1‐8‐2014 (sequence generation, allocation concealment) Chris Billis [Christopher.billis@bayer.com]; Keith Flanders [keith.flanders@bayer.com] 15‐8‐2014 Randomisation was done centrally by the generation of a randomisation list using the randomisation program RANCODE (version 3.6). Randomisation used blocks. Whole randomisation blocks were allocated to each site. In each study site, each newly enrolled patient was allocated to study medication with the lowest randomisation number available in that particular site at the subjects baseline visit. The patient randomisation number was entered into the CRF immediately after allocation. Each patient retained the randomisation number originally allocated at Baseline for the duration of the study Six drug tubes (tubes with a blinded label to cover the trademarks) and 3 bottles were packaged by a CMO in individual numbered kit boxes. Each patient was issued an individual numbered kit box containing 6 tubes and 3 bottles of study materials. The study drug was not to be dispensed by the investigator, but was dispensed by and returned to qualified study personnel (e.g., practice or clinic nurses) not involved with the selection and the assessment of the patients. At the control visits after Weeks 4, 8 and 12, patients returned empty, partially used, and unused containers to qualified study personnel before being examined by the investigator. Study drug compliance was assessed by the qualified study personnel. The patient was advised not to discuss the treatment schedule with the investigator 19‐8‐2014 sent additional mail regarding SD of lesions 3‐9‐2014, resent, received 4‐9‐2014 |
| Dreno 1998 | Yes | No | Old study, no further data available |
| Draelos 2005b; Draelos 2006 | Yes | Yes | zdraelos@northstate.net. On 2006. Sequence generation? "Subjects were randomised based on the order in which they presented to the office". Allocation concealment? "The research coordinator maintained the blind which was not shared with anyone, including the investigator." 5 dropouts but in which group? The dropouts were for personal reasons, not related to product. They were random between the groups On 2005 Sequence generation? "Subjects were randomised based on severity of disease and the order in which they presented to the office". Allocation concealment? 'The research coordinator maintained the double blind." Dropouts? "The drop outs were one in each group." |
| Draelos 2009 | No | No | zdraelos@northstate.net, 2‐8‐2014 (sequence generation and allocation concealment and blinding, how many randomised to each group, separate data for participants with rosacea? losses to follow‐up?) 9‐8‐2014 sent again. No reaction |
| Draelos 2013a | Yes | Yes | zdraelos@northstate.net, 2‐8‐2014 (blinding and details on RosaQoL data) Reply 2‐8‐2014 This was the pivotal trial for FDA approval. The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers. I do not have more detail on the QOL scores |
| Draelos 2013b | Yes | Yes | zdraelos@northstate.net (sequence generation, stratification and allocation concealment and blinding) Reply 2‐8‐2014 I will answer your questions below: 1. the method used to generate the allocation sequence as “were divided equally into two groups” does not seem at random. Subjects were randomised in two balanced populations based on a computer generated randomisation sequence 2. How was stratification done during the sequence generation? That is, can you describe the method used to generate the allocation sequence in sufficient detail to allow us an assessment of whether it should produce comparable groups stratified for demographics and presence and severity of acne, eczema, rosacea and atopic dermatitis? The data for each person was entered into a database and then the computer randomisation balanced the two groups for all of the characteristics you have mentioned 3. the method used to conceal the allocation sequence to ensure that intervention allocations could not have been foreseen in advance of, or during, enrolment i.e. participants and investigators enrolling participants could not foresee the upcoming assignment (this is not the same as blinding!!). I realize that randomisation and blinding are not the same. The products were identically packaged 4. How were the investigators and participants blinded to the treatment the participants received? Yes, both the investigator and the participant did not know the product identity which was concealed through identically appearing products packaged identically 5. Are there separate data for women on rosacea? No, the data was not analysed in this fashion. follow‐up mail that allocation concealment is not yet satisfactorily answered 9‐8: sent again, no reply |
| Ertl 1994 | Yes | No | Dr Levine, study 17 years old, no further data available |
| Fabi 2011 | No | No | sfabi@gbkderm.com 2‐8‐2014 (sequence generation and allocation concealment and dropouts?) Follow‐up mail 11‐8‐2014 and 17‐8‐2014, no reply |
| Fowler 2007 | Yes | Yes | fowlerjoe@msn.com and christian.loesche@galderma.com. "Randomisation was done by using a computer generated table provided by the sponsor. Neither subjects nor investigators and study staff had any control over this" |
| Fowler 2012a; Fowler 2012b; Fowler 2013a; Fowler 2013b | Yes | Yes | fowlerjoe@msn.com and Jean Jacovella (Jean.JACOVELLA@galderma.com) 22‐8‐2014, 27‐8 Asked for separate exact data of PSA and CEA at different time points, wash‐out period and details on AE Received replies 25‐9‐2014 |
| Fowler 2013a | Yes | Yes | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith) is this Fowler 2013? 28‐7‐2014 confirmed (NCT01355471 and NCT01789775 are the same studies) Received replies 25‐9‐2014 |
| Freeman 2012 | Yes | Yes | 'summer.moon@med.lecom.edu' 3‐8‐2014 (sequence generation and allocation concealment and blinding)
Follow‐up mail 11‐8‐2014
reply 12‐8‐2014 1) Random selection by study coordinator
2) Medication and placebo allocation was the responsibility of the study coordinator who randomly selected which product to provide each subject (2:1 ratio). The investigators were unaware of the selection process and the study coordinator was not privy, prior to selection of product, of the type or severity of disease of any subject 3) Investigators were not privy to the medication/placebo selection process. No medication tubes were shown to the investigators. No questions were asked about the topical product (i.e. Odor, color or feel). There was no communication between the study coordinator and the investigators regarding the medication/placebo selection follow‐up mail 12‐8‐2014 Regarding 1) this answer still does not inform us the method, so what method did the study coordinator used? Regarding 2) You describe that the investigators were unaware of selection process, but if the study coordinator was aware who received what, then the allocation was NOT concealed, even if he was not privy Regarding 3) if the patients knew what they received they could tell the investigators, as slip of the tongue. So it might be that study coordinator did not say anything, the patients could say something to the investigator. So was there any possibility that patients knew what they received? And if not why not? Why did they not know what they received (as stated as double‐blinded) Response: 12‐8‐2014: Randomisation: every third patient was given placebo to create a 2:1 ratio Follow‐up mail: then it is not truly randomised but quasi‐randomised as you know every third patient gets placebo it is no longer at random and we have to exclude the study |
| Frucht‐Pery 1993 | No | No | ‐ |
| Gollnick 2010 | Yes | Yes | 3‐8‐2014 harald.gollnick@med.ovgu.de christoph.willers@almirall.com (sequence generation and allocation concealment clarification of N) 5‐9‐2014 follow‐up, and received replies with clear information |
| Huang 2012 | No | No | 18‐7‐2014 lglzsj@163.com (sequence generation and allocation concealment) 9‐8‐2014 follow‐up mail and 17‐8‐2014, no reply |
| Jackson 2007 | No | No | ‐ |
| Jackson 2013 | Yes | Yes | jacksonjmark@gmail.com 9‐8‐2014 1. The dropouts are noted below: 5 total Two adverse events were classified as possibly related to the study medication – an upset stomach and generalized urticaria in separate patients both receiving ER minocycline + azelaic acid 15%. Four adverse events in three patients (all receiving ER minocycline + azelaic acid 15%) were severe but not suspected to be related to the study medication (bilateral oophorectomy with dermoid cyst removal, gastric erosion after lap band surgery, a severe respiratory infection, and cholecystitis) 2. The CEA was a scale of 0 to 4 so there was a typo on 295. The IGA went from 0 to 5. As for the total CEA it was a combined number of the CEA for each location of the face as per the table below APPENDIX B Clinician’s Erythema Assessment Scale ERYTHEMA Definition 0 None No redness present 1 Mild Slight pinkness 2 Moderate Definite redness 3 Significant Marked erythema 4 Severe Fiery redness ERYTHEMA Score • Check one box for each area of the face based upon the definitions given above • Enter the Erythema Score for each area of the face • Sum all of the individual Erythema Scores to obtain the Total Erythema Score Erythema Score Forehead Chin Nose Right Cheek Left Cheek none (0) none (0) none (0) none (0) none (0) mild (1) mild (1) mild (1) mild (1) mild (1) moderate (2) moderate (2) moderate (2) moderate (2) moderate (2) significant (3) significant (3) significant (3) significant (3) significant (3) severe (4) severe (4) severe (4) severe (4) severe (4) |
| Jansen 1997 | No | No | ‐ |
| Jorizzo 1998 | Yes | No | ‐ |
| Karabulut 2008 | Yes | Yes | dr.aa.karabulut@gmail.com. After email contact with the primary investigator this study was excluded |
| Karsai 2008 | No | No | ‐ |
| Kendall 2014 | Yes | Yes | james.kendall@galderma.com 6‐8‐2014 (sequence generation and allocation concealment and blinding and dropouts) 9‐9‐2014 reply: I did not receive your previous e‐mails as my address is jim.kendall@galderma.com 70 patients were enrolled in the study. Two subjects withdrew from the study and they were both in the brimonidine tartrate gel 0.5% treatment group in phase 1 and did not enter phase 2. One for an adverse event and one for a protocol deviation |
| Koch 1999 | Yes | No | Appeared to be wrong R Koch |
| Koçak‐Altintas 2005 | Yes | Yes | After extensive email contact, clarified as a CCT |
| Laquieze 2007 | No | No | ‐ |
| Lebwohl 1995 | Yes | No | Old study, no further data available |
| Leyden 2011 | Yes | No | jjleyden@mindspring.com 7‐8‐2014 (sequence generation and allocation concealment) Reply 12‐8‐2014: I am now Emeritus and mostly out of the loop and my clinical research nurse has retired. Nobody in the clinical trials unit was there when that study was done and I can't get the details you are asking for Sorry! Jim Leyden |
| Leyden 2014 | Yes | Yes | 19‐7‐2014, info@sol‐gel.com Ofer.Toledano@sol‐gel.com My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT00940992 “A Study of DER 45‐EV Gel to Treat Rosacea (SGTDER45EV)”. Can you tell us if the NCT00940992 is published and if so give us a pdf of the publication? Reply 19‐7‐2014 Ofer.Toledano@sol‐gel.com, Gaby.Peleg@sol‐gel.com . The study results were published by James Leyden in the JDD (journal of drugs in dermatology) in June 2014, volume 13, issue 6, p.685 |
| Luger 2015 | Yes | Yes | Mderma@uni‐muenster.de (allocation concealment and blinding) Reply 18‐8‐2014 The generation of the random code list was performed in a validated environment by an independent CRO not involved in study conduct and monitoring using the software RANCODE Version 3.6. Central randomisation was performed by this CRO. For eligible subjects, investigators called the randomisation centre and provided the patient’s identification number and gender. Patients were subsequently randomised and the study centre was notified of the treatment number of the patient via telefax by the randomisation centre. Treatment allocation provided by the central randomisation service was documented in the CRF and monitored. ad 2) The investigational product and its matching vehicle had a similar appearance and all subject kits were packaged in the same way. The randomisation list was kept strictly confidential. It was accessible only to authorized persons who were not involved in the conduct, monitoring and analysis of the study, until time of unblinding. Based on the randomisation list, sets of sealed individual code envelopes were prepared for emergency procedures. No emergency unblinding occurred during the study |
| Lupin 2014 | No | No | Ulthera, Inc. Mark Lupin, M.D 23‐7‐2014 info @cosmedica.ca office@cosmedica.com, sent several mails no reply (sequence generation and allocation concealment, dropouts) |
| Mostafa 2009 | No | No | S Mokadem no response |
| National Rosacea Society | No | No | ‐ |
| Neuhaus 2009 | No | No | ‐ |
| G Plewig | No | No | ‐ |
| Powell 2005 | Yes | No | ‐ |
| A Rebora | No | No | ‐ |
| Rigopoulos 2005 | No | No | ‐ |
| Sainthillier 2005 | No | No | ‐ |
| Salem 2013 | No | No | dr_doaasalem@yahoo.com 10‐08‐2014 Resent 17‐8‐2014 and 3‐9‐2014 (sequence generation and allocation concealment and blinding), no replies |
| Seité 2013 | Yes | Yes | sophie.seite@loreal.com 16‐7‐2014 (sequence generation and allocation concealment and blinding, dropouts)
Reply 12‐8‐2014: 1. The allocation sequence was generated by a statistician using a specific software 2. As soon as they have been recruited (because they answered to the inclusion criteria) by the investigating dermatologist (only one = Dr Zelenkova) a number given chronologically, as indicated in the allocation sequence purchase to the investigator, was attributed to the patient (the first was the N°1, the 2nd the N° 2…) 3. After enrolment and at the end of the 1st visit, a nurse (in the absence of the investigating dermatologist) give the products allocated to the patient’s number. Both products was in the same packaging (blind white packaging) without any indication about formula reference (only reference of study and number of patient) and some information about use (topical use only…) 4. None dropped out between the stop of metronidazole treatment (Week 8) and the end of the study (week 16). 67 patients were included before metronidazole treatment, 1 dropped out due to irritative dermatitis at day 53 (before the end of the 8‐week Metronidazole treatment); So 66 patients remained after 8 weeks, 32 received the test formula and 34 the vehicle 5. More detail about the 66 patients included in this study are available (see below (printscreens)) 25‐8‐2014, received additional info on Investigator's assessments |
| Sharquie 2006 | No | No | ‐ |
| Stein 2014b | Yes | Yes | 19‐7‐2014 asked Galderma if NCT01493687 it is published and looks the same as NCT01494467 (Patricia van Lith), confirmed 28‐7 are the same and are Stein Gold
MLSTEIN1@hfhs.org and Jean.JACOVELLA@galderma.com on details DLQI and SDs 3‐9‐2014, received data |
| Taieb 2015 | Yes | Yes | 19‐7‐2014 asked Galderma if NCT01493947 is published (Patricia van Lith), confirmation 28‐7‐2014, EADV abstract 2014 alain.taieb@chu‐bordeaux.fr (sequence generation and allocation concealment and blinding, dropouts) Response 19‐8‐2014 The study was a parallel group study of 960 subjects; however 1800 kit numbers are randomised in blocks of 6. The RANUNI routine of the SAS system was used to randomly assign, in balanced blocks, kit to a treatment (Ivermectin 1% cream, Metronidazole 0.75% cream). Prior to the start of the study, a randomisation list was generated by the statistician and was secured with restricted access. Treatment assignment was balanced into consecutive blocks in a 1:1 ratio and kit numbers were assigned sequentially in chronological order. The study design was investigator‐blinded. The integrity of the blinding was ensured by packaging the products in identical tubes, not allowing the investigator and subject to discuss study treatments, and requiring a third party other than the investigator to dispense the medication. Study population and causes for withdrawal are summarised in the figure below |
| Thiboutot 2003a; Thiboutot 2003b; Thiboutot 2008; Thiboutot 2009 | No | No | ‐ |
| Tirnaksiz 2012 | No | No | figentirnaksiz@gmail.com 17‐8‐2014 ((sequence generation and allocation concealment) resent 3‐9‐2014 no reply |
| Torok 2005 | Yes | Yes | helenmtorok@aol.com. "The patients were not cognizant nor were they aware of the different formulations Nor their unique characteristics so they were easily utilized and dispensed in unmarked tubes". "Central randomisation that was computer generated" |
| Two 2014 | Yes | Yes | rgallo@ucsd.edu 17‐8‐2014 (sequence generation and allocation concealment and blinding)
resent 3‐9‐2014, received reply 4‐9‐2014 1. The allocation sequence was generated by an unblinded member of the study team who worked off‐site in a separate laboratory to group in a 2‐to‐1 fashion, so that 8 of those numbers were assigned to the treatment group, and 4 to the control group. As subjects were enrolled in the study, they were sequentially assigned a unique study identification number from 1‐12 by the blinded study coordinator, with the first subject to enrol in the study being assigned the study identification number of 1. The list matching study identification numbers to their corresponding treatment group was only accessible by this unblinded member of the study team 2. The allocation sequence was created prior to enrolling any subjects in the study, therefore ensuring that intervention allocations could not be foreseen in advance of, or during, enrolment 3. This study was conducted in a double‐blind fashion so that both participants and investigators were blinded as to which intervention group participants were assigned. As stated previously, randomisation was completed by an unblinded member of the study team who worked off‐site and had no contact with enrolled subjects. The list of treatment group assignments was stored on a password‐protected computer accessible only to this unblinded study team member. This same unblinded member of the study team was also responsible for preparing all study medication. Once prepared, the study medication was placed into a bottle labelled with the participant’s unique study identification number that was assigned to the participant at the time of enrolment in the trial. The unblinded study team member dispensed the bottles of prepared medication to the study’s clinical coordinator, who was also blinded, for distribution to subjects. Both the treatment and the control creams were identical in appearance and viscosity so that the two drugs could not be distinguished by look or feel Resent regarding exact data IGE and CEA 12‐9‐2013 Received 12‐9‐2013 exact data + SD |
| Wilkin 1989; Wilkin 1993 | No | No | ‐ |
| Wittpenn 2005 | Yes | Yes | Additional information could not be used |
| Wolf 2006 | No | No | ‐ |
| Yoo 2011 | No | No | ellen.marmur@mountsinai.org 18‐8‐2014 (sequence generation and allocation concealment and blinding) Resent 3‐9 |
| ACTRN12614000004662 | Yes | No | Medical Research Institute of New Zealand, Anna Hunt anna.hunt@mrinz.ac.nz. sent 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? ACTRN12614000004662 “A single‐blind randomised controlled trial of topical Kanuka honey for the treatment of rosacea”. Can you tell us if ACTRN12614000004662 is published and if so give us a pdf of the publication? email reply 29‐7‐2014 Dear Dr van Zuuren, Thank you for your email and interest in our study. We are currently analysing this 138 participant Phase 3 study. It is not yet published, but we would be happy to send you the publication when that time comes |
| EUCTR2006‐001999‐20‐HU | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2006‐003707‐40‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2009‐013111‐35‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2010‐018319‐13‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2010‐021150‐19‐NL | Yes | No | Study of Mireille, is still ongoing |
| EUCTR2010‐023566‐43‐DE | Yes | No | 23‐9‐2014 Dr. Bertil Wachall, studien@infectopharm.com 2‐10‐2014 Thank you for your request concerning our permethrin rosacea trial (permethrin 5% and 2.5% vs metronidazole cream). Unfortunately, the data are not published or submitted up to now. We hope this will be done in the next months, but the principal investigator who is responsible for the publication seems to be very busy. In addition, please be informed that we are currently conducting another permethrin trial (permethrin 5% vs placebo cream) in PPR‐patients. We expect the results of this trial in spring 2015 (we discussed with PI, study does NOT appear in EUCTR (EUDRACT‐Nr. 2013‐000979‐32) |
| EUCTR2011‐002057‐65‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2011‐002058‐30‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2011‐004791‐11‐CZ | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2012‐001044‐22‐SE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| EUCTR2013‐005083‐26‐DE | Yes | No | 23‐9‐2014, sent e‐mail to Galderma NL and several more to Galderma International, no reply |
| IRCT2014010516079N1 | No | No | 23‐9‐2014 G_faghihi@med.mui.ac.ir, p_khosravani@resident.mui.ac.ir My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already completed or submitted for publication. IRCT2014010516079N1 “Comparison of dapsone 5% Topical gel with metronidazole 0.75% efficacy in combination with oral doxycycline in papulopustular rosacea”. Can you tell us if IRCT2014010516079N1 is already completed? Or submitted for publication? |
| IRCT2014010516079N1 | Yes | No | 23‐9‐2014 kosaraoofi@yahoo.com; kosaraoofi@gmail.com; mehdirj@aol.co.uk My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already submitted for publication. IRCT2014030416837N1 “Effects of permethrin 5% topical gel in comparison with placebo on Demodex density in rosacea patients: a double‐blind, randomised clinical trial”. Can you tell us if IRCT2014030416837N1 is already submitted for publication? Reply: 23‐9‐2014 Dear Dr Zuuren Many thanks for your query, We are in the process of completing and submitting the article. Regards, Mehdi |
| JPRN‐UMIN000008315 | Mari Wataya‐Kaneda mkaneda@derma.med.osaka‐u.ac.jp not sent mail as they are still recruiting | ||
| NCT00041977 | Yes | No | Info@pariserderm.com 16‐7‐2014
My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion (NCT00041977 “A Multicentre, randomised, Double‐Blind, Placebo‐Controlled, Clinical Trial to Determine the Effects of Doxycycline Hyclate 20 Mg Tablets [Periostat(R)] Administered Twice Daily for the Treatment of Acne Rosacea”)
Has this study ever been published as I could not find it? If not, do you have a contact at CollaGenex Pharmaceuticals, as on the web site of clinicaltrials.gov this is not provided, but we found your name on it, also asked Galderma (Patricia van Lith) Follow up mail to Dr Pariser 11‐8‐2014 Reply 13‐8: They sent me a study, but is not correct one, but on acne, so sent again request |
| NCT00249782 | No | No | Allergan, results published on the Internet, as word doc and pdf we made several, but unsuccessful, attempts to contact Allergan |
| NCT00348335 | Yes | No | jrwittpenn@aol.com, jwittpenn@ocli.net 16‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion (NCT00348335 “Efficacy of topical cyclosporin 0.05% for the treatment of ocular rosacea”) Has this study ever been published as I could not find it? (I do have the 2005 one) Follow‐up mail 11‐8‐2014 Reply: 12‐8‐2014 The study was discontinued when early results showed that we did not have a reproducible method of quantifying injection. It was far too variable and did not appear to correspond at all to patients reporting symptomatic improvement. John Wittpenn |
| NCT00417937 | Yes | Yes | 19‐7‐2014 Alan Fleischer <afleisch@wakehealth.edu>My colleagues and I are updating our Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already included in our review?
NCT00417937 “A Multicenter Trial of a Topical Medication for Papulopustular Rosacea Applied Twice Daily Versus Once Daily”. Is this the same study as published in Thiboutot DM, Fleisher AB, Del Rosso JQ, Graupe K. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. Journal of Drugs in Dermatology 2008;7(6):541‐6.? Or is it another one? Reply 19‐7‐2014: I do believe that this is the exact same study. Sorry that my name appears in lots of clinical trials settings (Thiboutot 2008) |
| NCT00436527 | Yes | No | 19‐7‐2014, asked Galderma if it is published (Patricia van Lith), several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella |
| NCT00483145 | Yes | No | mhaedersdal@dadlnet.dk 16‐7‐2014
My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion (NCT00483145 Laser‐mediated photodynamic therapy of acne vulgaris and rosacea). It has been completed in 2007.
Has this study ever been published as I could not find it? Reply 16‐7‐2014: We were unsuccessful in recruiting rosacea patients and as thus, published a case report, which is attached. Results from treating acne patients were published as a RCT, we have removed this one from ongoing studies |
| NCT00495313 | Yes | No | Sent 16‐7‐2014 message via LinkedIn, and Galderma (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella |
| NCT00560703 | Yes | No | 16‐7‐2014, asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella |
| NCT00617903 | Yes | No | 18‐7‐2014 clinical‐trials‐contact@bayerhealthcare.com My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT00617903 “Exploration of Safety and Efficacy of AzA 15% Foam Twice a Day in Rosacea”. I found a study of Draelos published in 2013 in CUTIS, but that one included far more participants than the 84 mentioned in the NCT00617903 study. Can you tell us if the NCT00617903 is published and if so give us a pdf of the publication? 11‐8‐2014 follow‐up mail 15‐8‐2014: Christopher Billis <christopher.billis@bayer.com>, resent 15‐8‐2014, not published and no additional info |
| NCT00621218 | No | No | 18‐7‐2014 via website Valeant Pharmaceuticals who took over Coria Laboratories, asked if it is published |
| NCT00667173 | No | No | 18‐7‐2014 via website Valeant Pharmaceuticals who took over Dow Pharmaceutical Sciences, Inc, asked if it is published |
| NCT00697541 | Yes | 18‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella | |
| NCT01016782 | No | No | 19‐7‐2014, mail though website Sandoz. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01016782 “Study of 0444 Gel in the Treatment of Inflammatory Lesions of Rosacea)”. Can you tell us if the NCT01016782 is published and if so give us a pdf of the publication? |
| NCT01125930 | Yes | No | 19‐7‐2014, maierl@med.umich.edu. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published?
NCT01125930 “Atralin Gel for the Treatment of Rosacea”. Can you tell us if NCT01125930 is published and if so give us a pdf of the publication?
Reply 29‐7‐2014: Dear Dr. van Zuuren, The study has not yet been published yet. When it has been accepted for publication, I can notify you. Thank you, Lisa Maier |
| NCT01134991 | Yes | No | 19‐7‐2014 dov@foamix.co.il. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01134991 “Study to Evaluate the Safety and Efficacy of Topical Minocycline FXFM244 in Rosacea Patients”. Can you tell us if NCT01134991 is published and if so give us a pdf of the publication? Reply 21‐7 Dov Tamarkin, Ph.D. dov.tamarkin@foamixpharma.com, study is still ongoing |
| NCT01186068 | Yes | No | 19‐7‐2014 fowlerjoe@msn.com My colleagues and I are updating our Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published?
NCT01186068 “A randomised, double‐blind, vehicle‐controlled, parallel‐group study of the dose‐response profile of V‐101 cream in subjects with erythematous rosacea”
Can you tell us if the NCT01186068 is published and if so give us a pdf of the publication? (By the way we will include your dose‐finding studies and phase III studies on brimonidine, and might need to contact you later about these. Follow‐up 11‐8‐2014 beyer@sambrown.com Reply 12‐8‐2014 Dr Fowler: not published |
| NCT01257919 | Yes | No | 22‐7‐2014. Bayer sent though website, Novum, info@novumprs.com My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? The study was supported by Bayer and Novum are listed as "locations" on clinicaltrials.gov NCT01257919 “ Safety and Pharmacokinetics of Azelaic Acid Foam, 15% in Papulopustular Rosacea Can you tell us if the study has been published if so could I request a pdf of the publication? If not could we please access the data? 15‐8‐2014: Christopher Billis <christopher.billis@bayer.com>, resent 15‐8‐2014, not published and no additional info |
| NCT01308619 | Yes | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella | |
| NCT01398280 | Yes | Yes | 22‐7‐2014 Tissa Hata, MD, University of California, San Diego, thata@ucsd.edu My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01398280 Effects of Aminocaproic Acid (ACA) on Rosacea‐specific Inflammation If this has been published could I kindly ask for the citation and/or if you have a pdf available? If it has not been published are the data available? Follow‐up 11‐8‐2014 and 17‐8‐2014 17‐8‐2014: EvZ: This is Two 2014!!! Reply: 18‐8‐2014: Thank you for your interest in our research. Attached is the paper which was published in the JID. Thanks! Tissa |
| NCT01426269 | Yes | 21‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014
Follow‐up mails 14 August with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella 5‐9‐2014: Warren.WINKELMAN@galderma.com. 22‐9‐2014, received all we needed |
|
| NCT01449591 | Yes | Yes | Novartis Pharmaceuticals, no contact details, sent mail through Dutch website
12‐8‐2014 reply pieter.ekkel@novartis.com U vraagt om informatie over de studie NCT01449591 (CBFH772A2203) met als compound BFH772. De enige informatie die we nu kunnen delen over deze studie zijn gepubliceerd op ‘Novartis clinical trial database’, onder ‘Novartis Institute for Biomedical Research’, Dermatology/Skin, CBFH772 http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public 22‐8‐2014: What was the rationale behind study, are they going to proceed with further studies? will it be published? Reply 9‐9‐2014: BFH772 is currently under investigation and has not been approved for use other than for use as part of a clinical trial. Therefore, at this present time, no further information can be provided other than what is publicly available at the previously indicated location (http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public ) Whether or not results of trial NCT01449591 "Safety, Tolerability and Efficacy of BFH77s in Rosacea Patients" will be published in medical journals in the future cannot be anticipated at this stage. Please do not hesitate to reach out to us again in six months' time to inquire about potential updates, if of interest.” |
| NCT01451619 | Yes | No | 22‐7‐2014. Merck Sharp & Dohme Corp, steven.cragle@merck.com, heather.stamatacos@merck.com, kelley.dougherty@merck.com Reply 23‐7‐2014pamela_eisele@merck.com I checked with our researchers and have been told that the study results have been submitted for publication and are currently under review for consideration by the Journal of Clinical Pharmacology. It is unclear at this point when the data may be available, as they are being considered by the publication |
| NCT01513863 | Yes | No | 19‐7‐2014 GDGongas@novumprs.com. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT01513863 “A Therapeutic Equivalence Study of Two Metronidazole 1%Topical Gel Treatments for Patients With Rosacea (MTZG)”. Can you tell us if NCT01513863 is published and if so give us a pdf of the publication? 21‐7‐2014 reply Aimee Brown, ABrown@novumprs.com. "Thank you Dr. Zuuren for your inquiry, however this study has not yet been published." |
| NCT01555463 | No | No | Bayer, mailed via website 22‐7‐2014 15‐8‐2014: Christopher Billis <christopher.billis@bayer.com>, resent 15‐8‐2014, not published and no additional info |
| NCT01579084 | No | No | Allergan, sent mail 22‐07‐2014 through website no response |
| NCT01614743 | Yes | No | 22‐7‐2014 Steven H. Dayan, Medical Director, DeNova Research, MyClinicalTeam@drdayan.com My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, could you kindly confirm the status? It is reported on clinicaltrials.gov as This study is ongoing, but not recruiting participants When do you expect to complete it or if it is completed ... publish the data? Reply 22‐7‐2014. We appreciate your inquiry, and look forward to speaking with you. We are currently forwarding your message to Annie, our patient coordinator, and she will respond soon. emailed again 29‐7 2014. reply 29‐7‐2014 of Selika@denovaresearch.com I appreciate you reaching out to us. This study is in the process of being written up, unfortunately due to a signed confidentiality agreement, we cannot provide you with any of the data at this time |
| NCT01631656 | Yes | Yes | Amy McMichael, Wake Forest School of Medicine 23‐7‐2014 amcmicha@wakehealth.edu, amcmicha@wfubmc.edu (sequence generation, allocation concealment), several e‐mail exchanges, no replies to this |
| NCT01659853 | Yes | No | 20‐7‐2014 asked Galderma if it is published (Patricia van Lith), 28‐7‐2014, not yet published, but I thought already submitted so sent another mail. several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014 Follow‐up mails 14 August with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella |
| NCT01735201 | No | No | Allergan, results posted on clinicaltrials.gov clinicaltrials@allergan.com, 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published, we saw results published on clinicaltrials.gov? NCT01735201 “AGN‐199201 for the Treatment of Erythema With Rosacea”. Can you tell us if NCT01735201 is published and if so give us a pdf of the publication? Follow‐up 11‐8‐2014 and 17‐8‐2014 no replies |
| NCT01740934 | Yes | No | Rock Creek Pharmaceuticals, Inc. M Varga, 23‐7‐2014 health@rockcreekpharma.com response 1‐8‐2014: Thanks very much for your interest in our just concluded clinical trial, we are in the process of writing the clinical study report. We will make it available to you. If you have additional question, please do not hesitate to contact me.Dr Ernest Okorie,MD gddssconsultant@gmail.com |
| NCT01784133 | No | No | Cutanea Life Sciences, Inc 23‐7‐2014, info@cutanealife.com This study has been completed. My colleagues and I are conducting a Cochrane review (Interventions for rosacea) this study has been identified as potentially eligible for inclusion. Can you please indicate if the study has been published and if so could I request a pdf or the citation? If not are the data available? 10‐8‐2014 Resent e‐mail |
| NCT01828177 | PreCision Dermatology, Inc.Syd Dromgoole, as study is still ongoing not sent mail | ||
| NCT01885000 | Yes | Yes | 21‐7‐2014 asked Galderma if it is published (Patricia van Lith) several follow‐up mails also to Maria‐Jose Rueda marie‐jose.rueda@galderma.com last 11‐8‐2014
Follow‐up mails 14 august with several people of Galderma including Maria‐Jose Rueda and Jean Jacovella 19‐8‐2014 received poster abstracts Layton 2014 |
| NCT01917539 | Angela Chang, University of Miami AChang2@med.miami.edu or Bradford Lee blee@post.harvard.edu Still recruiting patients at the moment, not sent mail |
||
| NCT01933464 | Anna Di Nardo, MD, PhD, University of California, San Diego. As study is still recruiting not sent mail | ||
| NCT01993446 | No | No | Dermira, Inc. Beth Zib, info@dermira.com 23‐7‐2014 Follow‐up 11‐8‐2014 no replies |
| NCT02036229 | Rina Segal, Rabin Medical Center, rinas3@clalit.org.il not sent mail as not yet open to recruitment | ||
| NCT02052999 | No | No | Amorepacific Corporation BeomJoon Kim, Professor Department of Dermatology, Chungang University Hospital, sent mail through website 23‐7‐2014 My colleagues and I are conducting a Cochrane review (Interventions for rosacea) and one of your studies have been identified as potentially eligible for inclusion, but not sure if it is already published? NCT02052999 “Study to evaluate the efficacy and safety of PAC‐14028 cream in rosacea patients”. Can you tell us if NCT02052999 is published and if so give us a pdf of the publication? Study is performed by BeomJoon Kim |
| NCT02075671 | George Washington University, Jack Short, jshort@mfa.gwu.edu as they are still recruiting, not sent mail | ||
| NCT02120924 | Actavis Inc. John Capicchioni Akesis, LLC As they are still recruiting, not sent mail | ||
| NCT02132117 | No | No | Allergan clinicaltrials@allergan.com seems the same as NCT02131636 which I did NOT add? NCT02131636 Efficacy and Safety of AGN‐199201 in Patients With Persistent Erythema Associated With Rosacea NCT02132117 Safety and Efficacy of AGN‐199201 in Patients With Persistent Erythema Associated With Rosacea Also NCT02095158 Longterm and efficacy and safety clinicaltrials@allergan.com sent several mails, to ask if these are same studies, no replies |
|
NCT02144181 Evaluation of the Safety and Efficacy of the Ulthera® System for the Treatment of Signs and Symptoms of Erythematotelangiectatic Rosacea |
No | No | Ulthera, Inc. Mark Lupin, MD is LUPIN 2014 part of this? As they are still recruiting, not sent mail e‐mail sent 29‐7‐2014 to confirm, office@cosmedica.com Dear Colleagues I have received no further response could you please confirm with Dr Lupin?There appears to be a poster in JAAD 2014 vol17 Iss 5 referring to this trial? NCT01756027 Evaluation of the safety and effectiveness of microfocused ultrasound with visualization (MFU‐V) for the treatment of erythematotelangiectatic rosacea Mark Lupin, MD, The Department of Dermatology and Skin Science, University of British Columbia, Vancouver, Canada I also have this trial NCT02144181 which appears to be still recruiting and the contact person is Dr Mark Lupin Resent 22‐8‐2014 no replies |
| NCT02147691 | Leon Kircik, M.D., Derm Research, PLLC wedoderm@yahoo.com As they are still recruiting, not sent mail | ||
| NCT02204254 | Florence Le Duff, leduff.f2@chu‐nice.fr, not sent e‐mail as they are still recruiting |
RCT = randomised controlled trial CCT = controlled clinical trial (quasi‐randomised)
Translation
We did not apply any language restrictions and several studies published in the French, Spanish, Italian, Norwegian and Danish languages were translated by one author (EvZ). One article in the Chinese language was translated by Ching‐Chi Chi (see Acknowledgements).
Data collection and analysis
We followed the previously published protocol (van Zuuren 2000) for this review. As this is the third update of the original publication (van Zuuren 2004), changes have been made over time in accordance with the current requirements of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These changes are clarified in 'Differences between protocol and review'. Some parts of the methods section of this review use text that was originally published in other Cochrane reviews co‐authored by EVZ, ZF and BC (predominantly El‐Gohary 2014 and van Zuuren 2012).
Selection of studies
Two review authors (EvZ and ZF) independently assessed the abstracts of studies identified from the searches. We obtained full‐text copies of all relevant and potentially relevant studies, those appearing to meet the inclusion criteria, and those for which there were insufficient data in the title and abstract to make a clear decision. The two authors then independently assessed the full‐text papers and resolved any disagreement on the eligibility of included studies through discussion and consensus, or through a third party (MvdL). All irrelevant studies were excluded and their details and reasons for exclusion were noted in the 'Characteristics of excluded studies' table in RevMan (Revman 2014).
Data extraction and management
Details of eligible trials were extracted and summarised using structured data extraction forms (EvZ, ZF). Disagreements were resolved by discussion. Study details were entered into the 'Characteristics of included studies' table in RevMan (Revman 2014) by two authors (EvZ, ZF). The review authors only included data if there was an independently reached consensus, and any disagreements were resolved by discussion between the authors.
The following details were extracted:
trial methods, method of allocation, masking of participants and outcomes assessors, and date and setting of study;
participants, sample size, age, sex, inclusion and exclusion criteria, if there was ocular involvement, exclusion of participants after randomisation, and proportion of losses at follow up;
intervention and comparison, length of study, type and dosage;
outcomes, primary and secondary outcomes reported in the study;
sources of funding and support if reported.
Assessment of risk of bias in included studies
Two review authors (EvZ and ZF) independently assessed risk of bias using the Cochrane Collaboration tool for assessing risk of bias as described in Chapter 8, section 8.5 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The following domains were rated for each of the included studies as 'low risk of bias', 'high risk of bias', and 'unclear risk of bias' if the risk of bias was uncertain or unknown:
(a) the allocation sequence was adequately generated ('sequence generation'); (b) the allocation was adequately concealed ('allocation concealment'); (c) knowledge of the allocated interventions was adequately prevented during the study ('blinding'); (d) incomplete outcome data were adequately addressed; (e) reports of the study were free of suggestion of selective outcome reporting; and (f) the study was apparently free of other sources of bias that could put it at high risk of bias. This would include adequate study duration, i.e. a minimum of four weeks, and that previous oral and topical rosacea therapy was discontinued for a minimum of four weeks prior to the initial assessment. If the investigators declared any support or funding of the study by the pharmaceutical industry this was noted and assessed to determine if it represented a potential risk of bias in the conduct or reporting of the study (Bero 2013).
These assessments were reported in the 'Risk of bias' table for each individual study. See 'Characteristics of included studies'.
We also categorised and reported the overall risk of bias of each of the included studies according to the following:
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
Measures of treatment effect
Two treatment comparisons
We presented continuous outcomes, where possible, on the original scale as reported in each individual study with a mean change from baseline with its associated standard deviation in parentheses. Risk ratios (RR) were calculated for dichotomous outcomes and if statistically significant were presented with either: the number needed to treat for one additional beneficial outcome (NNTB); or number needed to treat for one additional harmful outcome (NNTH).
Any outcome data which reported physician‐assessments of the time needed until improvement of the skin lesions were presented as a descriptive narrative of the general trend within the groups at the first time point where an improvement was seen. In future updates, and if studies report adequate time‐to‐event outcomes data, we will follow the recommendations for analysing this type of outcome as described in Chapter 9, section 9.2.6 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
All outcome data were reported with their associated 95% confidence interval (CI).
Skewed data
Outcome data reported for asymmetrical distributions as counts, for example papules or pustules, were often skewed and frequently inappropriately analysed. We did not enter these types of outcome data into a meta‐analysis but reported them separately for individual comparisons, where this was possible (section 9.4.5.3) (Higgins 2011).
Unit of analysis issues
Cross‐over studies
Unit of analysis issues can arise in studies where participants have been randomised to multiple treatments in multiple periods, or where there has been an inadequate wash‐out period. In general, for cross‐over studies we only used data from the first treatment period, unless otherwise stated.
Within‐patient studies
In studies that reported paired data but where these were not adjusted for the within‐participant variability, a McNemar's test was applied and presented with the corresponding P value. If only the crude RR or raw data were presented and we were not able to adjust for the within‐participant variability, the RR was reported without a P value or 95% CI. In future updates, paired data from studies with no suspicion of contamination across intervention sites will be analysed separately using the generic inverse‐variance method in RevMan after accounting for the within‐participant variability (see Chapter 16, section 16.4.4: Methods of analysis for cross‐over trials) (Higgins 2011). If this is not possible but adequate data are available, the McNemar's test will be applied. For future updates and in those instances where data from within‐participant studies may be pooled together with data from between‐participant studies, the RR from the between‐participant studies will be calculated and combined in a meta‐analysis using the generic inverse‐variance method.
More than two treatment comparisons
Multi‐arm trials were included in the review if at least one arm constituted a relevant intervention for rosacea, and separate data extraction was carried out for each pair‐wise comparison. These studies were included as pair‐wise comparisons. For future updates, to prevent double‐counts of participants if treatment arms from multi‐arm studies are to be pooled more than once, these will be partitioned according to the number of comparisons carried out and the analysis will follow the recommendations in Chapter 16, section 16.5.4 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
If data were missing from trials which were less than 10 years old, reasonable attempts were made to contact the investigators or sponsors of these studies (see Table 20; Table 21). We re‐analysed data according to the intention‐to‐treat (ITT) principle whenever possible. For dichotomous outcomes, if authors had conducted a per‐protocol analysis we carried out an ITT analysis with imputation setting the missing data to their baseline values, after checking the degree of imbalance in the dropouts between the arms to determine the potential impact of bias (section 16.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). For continuous outcomes a per‐protocol analysis was carried out in place of an ITT analysis.
Assessment of heterogeneity
Clinical heterogeneity was assessed by examining the characteristics of the studies, the similarity between the types of participants, the interventions, the comparisons and the outcomes as were specified in the criteria for included studies. Although there is inevitably a degree of heterogeneity between the studies included in a review, if this could be explained by clinical reasoning and a coherent argument could be made for combining the studies, these were entered into a meta‐analysis.
The clinical diversity between many of the studies in this review as well as the limited number of studies that could be combined for each intervention only allowed us to make assessments of heterogeneity between the studies in just two of the comparisons. We assessed heterogeneity based on thresholds for the interpretation of I² where < 40% might not be important, 30% to 60% represents moderate and 50% to 90% substantial heterogeneity (Higgins 2011). If the I² statistic was more than 60% (Higgins 2011) and could not be explained by clinical reasoning we did not enter these data into a meta‐analysis.
Assessment of reporting biases
The low number of studies evaluating similar interventions and comparisons did not permit an assessment of publication bias. In future updates, if a sufficient number of trials assessing similar effects are identified for inclusion in this review, publication bias will be assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997) as described in section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry is identified we will try to assess other possible causes and these will be explored in the discussion if appropriate.
Data synthesis
Two review authors (EvZ, ZF) analysed the data in RevMan (Revman 2014) and reported them as specified in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data synthesis was only carried out if we were able to identify a sufficient number of studies (n ≥ 3) investigating similar treatments and which reported data that could be pooled (Treadwell 2006). We used a random‐effects model to combine the results of individual studies in this review. For comparisons where data synthesis was not feasible, the data has been reported separately in tables as 'Incomplete data on which further analysis is not possible' and presented in the review as a narrative summary, where appropriate. If applicable in future updates, synthesis of data and reporting of analyses from multiple studies evaluating similar interventions will take into consideration individual studies categorised with a summary high or variable risk of bias. If a sufficient number of such studies are identified, we will present analyses stratified according to overall risk or alternatively restrict the analyses to studies at low risk of bias and this will be reported accordingly. The GRADE approach was applied to interpret the results for the main comparisons, and GRADE profiler (GRADEpro) was used to create 'Summary of findings' tables (Schünemann 2009). Outcome‐specific information concerning the quality of evidence from studies per comparison was addressed and the magnitude of effect of the interventions was examined and presented.
Subgroup analysis and investigation of heterogeneity
In view of the paucity of included studies covering any one specific intervention, we did not carry out any subgroup analyses. In future updates, we plan to carry out subgroup analyses if we identify at least moderate to substantial heterogeneity (as defined above) and if we are able to include at least 10 studies. The subgroups we will consider include: differences in treatment effect by differing baseline risk, and possible differences in effect caused by the range of modes of administration of the interventions used, that is topical, systemic and different dosing regimens.
Sensitivity analysis
We did not conduct any sensitivity analyses in this review. If a sufficient number of studies (n = 10) investigating similar interventions had been included, we planned to conduct sensitivity analyses to assess the robustness of our review results.
Results
Description of studies
See 'Characteristics of included studies' and 'Characteristics of excluded studies'.
Results of the search
The updated searches for this review identified an additional 823 citations of potentially eligible studies. Searching the trial registers identified 54 ongoing studies, and three additional references were found through other resources giving a total of 880 references. There were 23 duplicates, and a further 751 references were excluded from further evaluation after examination of the titles and abstracts. The remaining 106 studies were further assessed for eligibility. Of these, 40 studies (reported in 37 references as three references reported on two studies) were included. Twelve studies appeared to be duplicate publications and are listed under the primary references, three were excluded with reasons (see 'Characteristics of excluded studies'), 12 studies are awaiting further assessment (see 'Characteristics of studies awaiting classification'), and 42 are ongoing trials (see 'Characteristics of ongoing studies' section) (see Figure 1). We also undertook a re‐assessment of the studies which had been excluded in the earlier version of this review, and eight of these are now included in this update (see also 'Differences between protocol and review') (see Figure 1).
1.
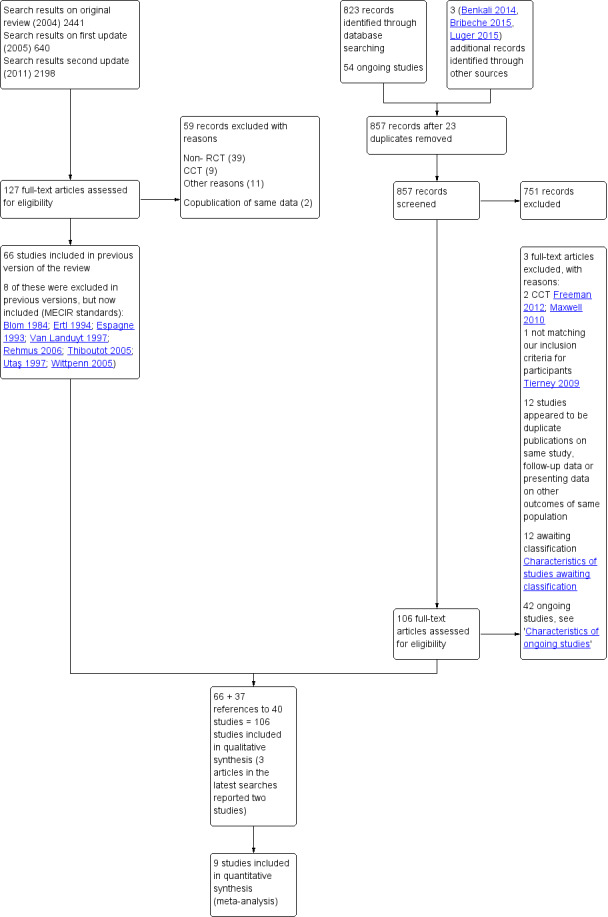
Study flow diagram.
Included studies
The current version of this review has 106 included studies. In addition to the 58 trials already in this review there are 40 newly included studies. There are also eight studies which had been excluded in earlier versions of this review but have been re‐evaluated for eligibility and included (see Table 22). A total of 13,631 participants were studied (see 'Characteristics of included studies').
4. Newly included studies for this update.
| Newly included studies | |
| 1 | Alam 2013 |
| 2 | Bamford 2012 |
| 3 | Benkali 2014 |
| 4 | Berardesca 2012 |
| 5 | Bribeche 2015 |
| 6 | Buendia‐Bordera 2013 |
| 7 | Chang 2012 |
| 8 | Del Rosso 2010 |
| 9 | Draelos 2009 |
| 10 | Draelos 2013a |
| 11 | Draelos 2013b |
| 12 | Fabi 2011 |
| 13 | Fowler 2012a |
| 14 | Fowler 2012b |
| 15 | Fowler 2013a |
| 16 | Fowler 2013b |
| 17 | Gollnick 2010 |
| 18 | Huang 2012 |
| 19 | Huang 2014 |
| 20 | Jackson 2013 |
| 21 | Kendall 2014 |
| 22 | Kim 2011 |
| 23 | Leyden 2011 |
| 24 | Leyden 2014 |
| 25 | Luger 2015 |
| 26 | Lupin 2014 |
| 27 | NCT00249782 |
| 28 | NCT01426269 |
| 29 | NCT01449591 |
| 30 | NCT01885000 |
| 31 | Nymann 2010 |
| 32 | Rodríguez 2003 |
| 33 | Salem 2013 |
| 34 | Seité 2013 |
| 35 | Stein 2014a |
| 36 | Stein 2014b |
| 37 | Taieb 2015 |
| 38 | Tirnaksiz 2012 |
| 39 | Two 2014 |
| 40 | Yoo 2011 |
| Formerly excluded studies | |
| 1 | Blom 1984 |
| 2 | Ertl 1994 |
| 3 | Espagne 1993 |
| 4 | Rehmus 2006 |
| 5 | Thiboutot 2005 |
| 6 | Utaş 1997 |
| 7 | Van Landuyt 1997 |
| 8 | Wittpenn 2005 |
Characteristics of the participants
Most of the participants in the included studies had papulopustular rosacea, however in 16 of the studies most or all of the participants had erythematotelangiectatic rosacea. The participants were between 40 and 50 years of age, with a mean of 48.6 years; there were more women (8332) than men (3718) and the gender was unreported for 1581 participants. Thirty‐five of the studies were carried out before the year 2000, the remainder (71) were conducted after 2000. The number of participants in the individual studies varied widely from 6 to 1299 and sample sizes of between 30 and 100 participants were the most common.
It was agreed between the review authors that two studies, NCT01426269 and Thiboutot 2008, should be included but that these were considered as maintenance studies. In NCT01426269, 130 participants took part in the second phase of the study and were randomised to doxycycline 40 mg or placebo after having obtained an Investigator's Global Assessment (IGA) of clear or near clear during the open‐label first phase of treatment with doxycycline 40 mg combined with metronidazole gel, both once daily. In Thiboutot 2008, the investigators enrolled 172 participants in the pilot phase of the study out of which only 136 continued into the second phase (maintenance phase), but these constituted the participants who had already achieved an improvement of > 75% reduction in inflammatory lesions.
Characteristics of the interventions
The trials were grouped into 11 categories of interventions: topical metronidazole (15); topical azelaic acid (7); topical brimonidine (6); topical ivermectin (2); topical metronidazole, azelaic acid or other topical treatments, or both (35); oral antibiotics (10); oral antibiotics combined with topical treatments (6); oral antibiotics compared with topical antibiotics (5); other systemic treatments (10); laser and light‐based therapies (7); and other treatments or combined treatments (3).
In 13 of the studies the individuals served as their own controls, with active treatment and placebo assigned to either the left or right side of the face (Alam 2013; Barnhorst 1996; Bleicher 1987; Buendia‐Bordera 2013; Carmichael 1993; Fabi 2011; Karsai 2008; Maddin 1999; Mostafa 2009; Neuhaus 2009; Nymann 2010; Tirnaksiz 2012; Yoo 2011). The duration of treatment ranged between two and three months with a mean of 9.7 weeks, and only five studies addressed interventions for ocular rosacea (Barnhorst 1996; Salem 2013; Schechter 2009; Sharquie 2006; Wittpenn 2005).
Heterogeneity in study design, skewed data, missing standard deviations, and a mix of different comparators and dosing regimens did not, in general, permit pooling of the data or allow the authors to make accurate and direct comparisons of a substantial number of the interventions.
Characteristics of the outcomes
Only 11 out of the 106 included studies (Bamford 2012; Bribeche 2015; Chang 2012; Draelos 2013a; Luger 2015; NCT01426269; Schechter 2009; Stein 2014a; Stein 2014b; Taieb 2015; Weissenbacher 2007) reported assessments of change in 'quality of life' as a result of the interventions. However, this number has risen by nine compared to the former update, which would appear to illustrate the steadily increasing recognition of quality of life as a key outcome by investigators in rosacea studies. Nearly half (52) of the remaining studies evaluated participant‐assessed changes in rosacea severity. The patient‐reported outcomes (PROs) which were reported in the 52 studies included not only assessments of changes in severity but also, in most instances, patient satisfaction associated with these changes.
We evaluated these PROs against the checklist for describing and assessing patient‐reported outcomes in clinical trials (see Table 23), which is described in Chapter 17.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We found that hardly any of them matched the recommended criteria. In the vast majority of studies the self‐assessments were made by way of questionnaires and instruments which evaluated the resolution of symptoms either jointly or separately with patient satisfaction related to the treatment. While most of these instruments were based on Likert‐type scales, a very small number of the studies utilised visual analogue scales (VAS) in their assessments (Neuhaus 2009; Nymann 2010; Weissenbacher 2007).
5. Checklist for describing and assessing patient‐reported outcomes (PROs) in clinical trials.
| 1. What were PROs measuring? a. What concepts were the PROs used in the study measuring? b. What rationale (if any) for selection of concepts or constructs did the authors provide? c. Were patients involved in the selection of outcomes measured by the PROs? |
| 2. Omissions a. Were there any important aspects of health (e.g. symptoms, function, perceptions) or quality of life (e.g. overall evaluation, satisfaction with life) that were omitted in this study from the perspectives of the patient, clinician, significant others, payers, or other administrators and decision‐makers? |
| 3. If randomised trials and other studies measured PROs, what were the instruments' measurement strategies?
a. Did investigators use instruments that yield a single indicator or index number, a profile, or a battery of instruments?
b. If investigators measure PROs, did they use specific or generic measures, or both? c. Who exactly completed the instruments? |
| 4. Did the instruments work in the way they were supposed to work ‐ validity? a. Had the instruments used been validated previously (provide reference)? Was evidence of prior validation for use in this population presented? b. Were the instruments re‐validated in this study? |
| 5. Did the instruments work in the way they were supposed to work ‐ ability to measure change? a. Are the PROs able to detect change in patient status, even if those changes are small? |
| 6. Can you make the magnitude of effect (if any) understandable to readers? a. Can you provide an estimate of the difference in patients achieving a threshold of function or improvement, and the associated number needed to treat (NNT)? |
|
Table 17.6.a Patrick D, Guyatt GH, Acquadro C. Chapter 17: Patient‐reported outcomes. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. |
There was wide diversity in the format of the questionnaires; many appeared to be unvalidated, used a range of scaling which offered a choice of from three to seven points on a Likert scale covering similar outcomes across the different questionnaires, and in several of them the physician and participant assessments were combined and expressed as composite scores. In the majority of the questionnaires it was not clear how the ratings correlated with the scaling of the items nor how reliable the interval‐level measurements were between the individual items (see also van Zuuren 2013). Additionally, in a number of the patient satisfaction questionnaires the judgements appeared to have been framed in such a way that only positive responses were possible, which would most likely lead to biased assessments being made (Bjerke 1999; Breneman 1998; Lebwohl 1995; Maddin 1999; Sauder 1997).
The quality of life assessment tools which were utilised in nine of the studies had been validated and were internationally recognised. Three studies used more than one instrument (Stein 2014a; Stein 2014b; Taieb 2015). The disease‐specific RosaQoL was used in eight studies (Bamford 2012; Chang 2012; Draelos 2013a; Luger 2015; NCT01426269; Stein 2014a; Stein 2014b; Taieb 2015). Another disease‐specific instrument, the Ocular Surface Disease Index (OSDI), was used in Schechter 2009 and the dermatology‐specific instrument Dermatology Life Quality Index (DLQI) in the other studies (Bribeche 2015; Stein 2014a; Stein 2014b; Taieb 2015; Weissenbacher 2007). In all of these studies the investigators provided citations to reports indicating that the tools had been previously validated, as was specified in the PRO checklist (Table 23).
Adverse events were addressed in the majority of the included studies, although often limited data were provided.
Most of the studies (81) assessed erythema or telangiectasia, or both, although the presence of erythema or telangiectasia was an inclusion criterion in just 63 of the 106 studies (16 of these were conducted in participants with subtype 1 rosacea). In this update there were clearly more studies focusing on erythema, which was assessed utilising mostly four to five‐point Likert scales. The Clinician's Erythema Assessment with a grading scale from 0 (clear skin, no signs of erythema) to 4 (severe erythema, fiery redness) was used in 16 of the studies (Bribeche 2015; Del Rosso 2007a; Del Rosso 2007b; Del Rosso 2008; Fowler 2007; Fowler 2012a; Fowler 2012b; Fowler 2013a; Fowler 2013b; Jackson 2013; Kendall 2014; Leyden 2011; NCT01426269; NCT01885000; Two 2014; Wolf 2006). The inter‐rater and intra‐rater reliability of this scale have recently been evaluated, and were demonstrated to be reliable when used by trained investigators (Tan 2014). In 68 studies clinician‐assessed numbers of papules or pustules were used as an outcome in preference to a more patient‐relevant measure such as participant assessment of appearance.
Outcomes assessments of ocular disease were only carried out in five of the included studies (Barnhorst 1996; Salem 2013; Schechter 2009; Sharquie 2006; Wittpenn 2005).
Funding
In 66 of the 106 included studies the investigators reported they had received funding, mostly from pharmaceutical companies, and declarations of competing interest were provided by the investigators in 46 of the total number. In 10 instances we were not reassured that the funding support, or employment, of any of the investigators by the pharmaceutical company would not represent a potential source of bias. However, in most cases when studies were double or even triple‐blinded and there was no evidence of selective reporting we did not consider funding an additional source of bias.
Excluded studies
Sixty‐two studies, three of which were newly identified, were excluded from this review. Thirty‐nine out of the total number of studies were excluded only after evaluation of their full‐text copies and this was largely on the basis that they were non‐randomised trials. Eleven studies were designated controlled clinical trials after contact with the investigators or following examination of the full‐text of the reports, and the remaining 12 studies were excluded for other reasons (see 'Characteristics of excluded studies' and Figure 1).
Risk of bias in included studies
Only 12 of the studies (Bleicher 1987; Chang 2012; Del Rosso 2007a; Del Rosso 2007b; Fowler 2012a; Fowler 2012b; Fowler 2013a; Fowler 2013b; Gollnick 2010; Luger 2015; Stein 2014a; Stein 2014b) met all of the criteria across all of the domains in the Cochrane Collaboration's tool for assessing the risk of bias, and therefore these studies were considered to be at 'low risk of bias' (plausible bias unlikely to seriously alter the results). Almost half of the studies (57) were categorised as 'unclear risk of bias' (plausible bias that raised some doubt about the results) because one or more criteria were assessed as unclear, and the remaining 37 studies were assessed as 'high risk of bias' (plausible bias that seriously weakened confidence in the results) because one or more of the criteria were not met. Further details of these assessments are available in the 'Risk of bias' table corresponding to each study in the 'Characteristics of included studies', and are also presented in the 'Risk of Bias' graph in Figure 2 and the 'Risk of Bias' summary in Figure 3.
2.
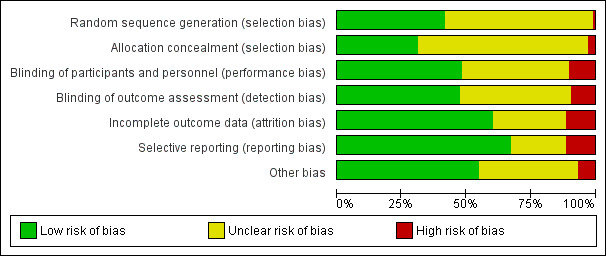
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
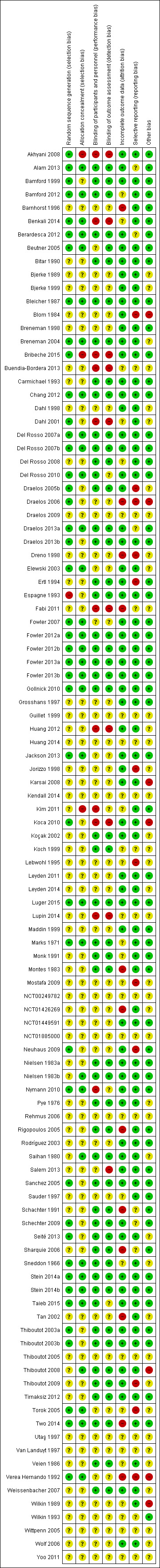
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Some of these assessments were to a certain extent based on the inadequate reporting of the criteria that are a prerequisite in the evaluation of methodological rigour in terms of trial design and conduct. Concealment of the allocation sequence and blinding are key domains in the assessment of risk of bias and most of the studies in this review provided insufficient detail to enable accurate judgements to be made. Protocol deviations, losses to follow‐up with incomplete data, and subsequent per‐protocol analyses, were other important sources of potential bias in a number of the included studies (see 'Risk of bias' table in 'Characteristics of included studies').
Allocation
The methods used to generate the allocation sequence and how the sequence was concealed, such that participants and investigators enrolling participants could not foresee the upcoming assignment, are the most important and sensitive indicators that bias has been minimised in a clinical trial (Schulz 1995).
Sequence generation
In 59 out of the 106 trials in this review the method of sequence generation was not described at all, or was at best unclear. In one study (Espagne 1993) allocation to the intervention was based on up to four participants in each of 18 clinics, but not all clinics enrolled four participants. The report did not provide any reassurance that the allocation sequence was adequately generated and there was lack of evidence that any form of central randomisation had been employed for the 18 clinics involved in this study, and therefore we judged this domain as high risk of bias. In the remaining studies (46) the method used to generate the allocation sequence was described in sufficient detail; therefore this domain was judged as low risk of bias for these studies.
Allocation concealment
Concealment of the allocation sequence was reported adequately in only 33 of the trials and involved either a form of central allocation, was pharmacy‐controlled or was through the use of serially numbered opaque envelopes (see 'Risk of bias' tables in 'Characteristics of included studies'). The majority of studies received a judgement of unclear risk of bias for this domain and the investigators in three studies (Akhyani 2008; Bribeche 2015; Kim 2011) informed us that the providers of care had access to the computer‐generated list, which we judged as high risk of bias.
Blinding
Effective blinding was achieved in half of the studies by the use of unmarked or identically appearing tubes, capsules or tablets. Some of the interventions were coded left or right for the within‐patient studies. Blinding of outcome assessment was reported clearly in only 50 of the 106 included studies.
Incomplete outcome data
In slightly more than half of the studies (64/106) incomplete outcome data appeared to have been adequately addressed and any missing outcome data were reasonably well‐balanced across intervention groups with similar reasons for missing data across the groups. However, in 42 of the 106 studies the reporting of missing outcome data was largely inadequate. Attrition was one of the main causes of incomplete outcome data. The reasons for attrition varied and these were often dependent on the assignment of the participant to one or other particular group; thus, for example, more dropouts tended to occur in groups receiving the active intervention secondary to any side effects, as opposed to dropouts due to lack of efficacy in the corresponding placebo group. In 30 studies we judged this domain as at unclear risk of bias. When there were more than 20% of dropouts and no ITT analysis was applied, or when dropouts in one arm exceeded 20%, we judged this domain as at high risk of bias (12 studies).
Selective reporting
The reporting quality in most of the older studies was consistent with the editorial style and standards existing at the time of publication. Although the protocols for most of the included studies were not available, based on the information in the methods section of the reports 73 out of the 106 studies appeared to have reported all pre‐specified outcomes and were therefore judged to be free of selective reporting. In the remaining studies, rarely was more than one outcome inadequately addressed, but in some instances these outcomes were reported only as a graph plot without any clearly discernible data. For 21 studies this domain was therefore judged as at unclear risk of bias. In those instances where one or more pre‐specified primary or secondary outcomes were not addressed, or if the data analysis appeared to be flawed after it was re‐analysed, we judged this domain as at a high risk of bias (12 studies).
Other potential sources of bias
Fifty‐eight of the studies appeared to be free of other forms of bias, whereas in 41 studies this domain was judged to be unclear. This judgement was based in part on an assessment of the extent to which funding by the sponsors may have had an impact on the results of a study. When there was no evidence of selection bias, nor performance or detection bias as double‐blinding was ensured, we did not consider sponsoring or financial compensation a threat for other bias. However, if we were uncertain about selection bias and if the method of blinding was not described in sufficient detail, we concluded that there was insufficient information to permit a clear judgement. Further reasons for possible other bias were: if groups were treated unequally or, in some of the older studies, if there was an inadequate wash‐out period before the start of the study. Seven of the included studies were largely not free of other forms of bias. In most of these studies there was baseline imbalance between the groups, but in one study participants switched to the other treatment arm if they failed to respond to the allocated treatment, and one study was designed as a superiority trial but reported as a non‐inferiority trial.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18
Twenty‐two studies provided no usable or retrievable data and did not contribute further to the results of this review (see Table 24). The main reasons why data could not be used were: none of our outcomes were addressed, no separate data were reported for participants with rosacea, very limited data were available in abstracts to conference proceedings, and it was unclear how many participants were randomised to each treatment arm. Pooling of outcome data across studies to provide a summary estimate of effect was only possible for two interventions and comparisons; these investigated the effects of topical metronidazole and topical azelaic acid against placebo.
6. Included studies with no usable or irretrievable data.
| Study ID | Interventions and comparisons | N | Comments |
| Benkali 2014 | Four different concentrations brimonidine tartrate gel | 102 | None of our outcomes were addressed |
| Blom 1984 | Sulphur 10% cream versus lymecycline | 40 | Unclear how many were randomised to each group, minimal reporting of outcomes. Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many |
| Buendia‐Bordera 2013 | PDL + post‐laser serum versus PDL + placebo gel | 31 | Poster, very limited data reported, not able to contact PI |
| Draelos 2006 | Azelaic acid 15% gel + habitual self‐selected skin cleanser and moisturizer versus azelaic acid 15% gel + standardised PHA (polyhydroxy acid) containing cleanser, and anti‐aging moisturizer |
67 | None of our primary outcomes were addressed combined with that it was unclear how many participants were randomised to each intervention. Because very limited outcomes data were reported no reliable conclusions could be drawn |
| Draelos 2009 | Facial foundation with niacinamide and N‐acetylglucosamine, cleanser and moisturizer versus marketed foundation + cleanser and moisturizer | 146 | Poster, lot of data missing, PI did not reply to e‐mail. Also included patients with sensitive skin, no separate data reported for participants with rosacea |
| Draelos 2013b | Gentle foaming cleanser containing hydrophobically modified polymers versus commercial gentle liquid non‐foaming facial cleanser | 40 | Participants with other skin diseases (atopic dermatitis, eczema, acne) were included and no separate data reported for participants with rosacea |
| Ertl 1994 | Isotretinoin + topical tretinoin versus topical tretinoin versus isotretinoin | 22 | Data unreliable, its re‐analysis using the individual participant data confirmed its flawed analysis by the investigators |
| Espagne 1993 | Metronidazole gel versus placebo gel | 51 | Allocation to intervention was based on up to four participants in each of 18 clinics but not all clinics enrolled four participants. The report did not provide any reassurance that the allocation sequence was adequately generated and no evidence that any form of central randomisation had been employed for the 18 clinics involved in this study |
| Fabi 2011 | IPL + azelaic acid versus IPL | 20 | Poster, very limited data reported, PI failed to respond to several e‐mails |
| Guillet 1999 | Metronidazole 75% gel versus metronidazole 0.75% lotion | 114 | Poster, very limited data reported, old study, not able to contact PI |
| Huang 2014 | Doxycycline 40 mg versus placebo | 170 | Poster abstract, limited data, unclear how many were randomised to each group, PI failed to respond to several e‐mails |
| Jorizzo 1998 | Metronidazole versus placebo | 277 | Unclear how many participants were initially recruited. Unclear how many participants started in each group, no SDs, dropout rate unclear. Data seem very skewed |
| Lupin 2014 | MFU‐V one treatment versus MFU‐V two treatments | 12 | Poster abstract, limited data, unclear how many were randomised to each group, PI failed to respond to several e‐mails |
| NCT00249782 | Dapsone 5% gel QD vs dapsone 5% BID, versus metronidazole gel versus dapsone + metronidazole gel versus vehicle | 400 | Unclear how many were randomised to each group, Allergan failed to respond to several e‐mails requesting further data |
| Rehmus 2006 | Antiinflammatory cream versus placebo | 40 | Poster, no results provided, very limited data reported |
| Thiboutot 2005 | Doxycycline versus placebo | 134 | Poster, lot of data are missing, PI did not reply to e‐mail |
| Utaş 1997 | Ketoconazole oral versus ketoconazole cream versus ketoconazole oral + cream versus placebo cream versus placebo pills | 53 | Letter, limited and no exact data |
| Van Landuyt 1997 | Clonidine versus placebo | 60 | Interim report only on first 30 participants, incomplete and very limited data |
| Wilkin 1989 | Nadolol versus placebo, four arms, crossover, 3 periods | 15 | Small groups, unclear what dropout rate was. No separate data for period A |
| Wilkin 1993 | Topical clindamycin versus tetracycline | 43 | Unclear how many participants were assigned to each group, dropouts not mentioned, no exact data provided |
| Wittpenn 2005 | Topical ciclosporin A versus artificial tears | 20 | Unclear how many randomised to each group, poster with very limited data see also Table 21 |
| Yoo 2011 | PDL + calcium dobesilate versus PDL | 6 | Poster, with incomplete and missing data |
A substantial number of the studies included in this review were categorised as 'unclear' or 'high' risk of bias (see Figure 2 and Figure 3) and therefore caution is advised in the interpretation of their results and in the extrapolation of the effects of the interventions.
We have addressed our pre‐specified outcomes under the following intervention headings.
Topical interventions: studies with only topical metronidazole (comparisons 1 to 5).
Topical interventions: studies with only topical azelaic acid (comparisons 6 to 8).
Topical interventions: studies with only topical ivermectin (comparison 9).
Topical interventions: studies with only topical brimonidine (comparisons 10 to 13).
Topical interventions: studies with topical metronidazole, azelaic acid, and/or other topical treatments (comparisons 14 to 41).
Systemic interventions: studies with oral antibiotics (comparisons 42 to 49).
Systemic interventions: studies with oral antibiotics combined with topical treatments (comparisons 50 to 55).
Systemic interventions: studies with oral antibiotics compared with topical antibiotics (comparison 56).
Studies with other systemic treatments (comparisons 57 to 61).
Other interventions: studies with laser‐/light‐based treatment (comparisons 62 to 67).
Topical interventions: studies with only topical metronidazole
(1) Topical metronidazole versus placebo
Nine trials at low to unclear risk of bias provided data for this comparison (Barnhorst 1996; Beutner 2005; Bitar 1990; Bjerke 1989; Bleicher 1987; Breneman 1998; Dahl 1998; Koçak 2002; Nielsen 1983a), see also Table 1.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Only three studies reported relevant data and although these could not be pooled for this outcome they provided some evidence that metronidazole was more effective than placebo.
In Bjerke 1989 43 out of 50 participants in the metronidazole group considered themselves improved compared with 24 out of 47 in the placebo group (RR 1.68, 95% CI 1.25 to 2.28; P = 0.0007; NNTB = 3, 95% CI 2 to 6); and similarly in Nielsen 1983a 25 out of 41 (metronidazole group) versus 8 out of 40 (placebo) (RR 3.05, 95% CI 1.57 to 5.94; P = 0.001; NNTB = 3, 95% CI 2 to 5). These were clinically important improvements. A within‐participant design was used in Bleicher 1987, which did not report the analysis adjusted appropriately for this design, therefore pooling of data with the other two studies was not possible. In this study the majority (28/37) of participants reported a greater improvement on the metronidazole treated side than on the placebo side (4/37), RR of 7.
Proportion of participants who reported an adverse event throughout the study period
Six of the studies (Beutner 2005; Bitar 1990; Bjerke 1989; Breneman 1998; Koçak 2002; Nielsen 1983a) provided adequate data for this outcome. In the three‐armed study of Beutner 2005 the proportion of participants reporting adverse events in the two active treatment arms were similar (32% to 33%) and, therefore, following statistical advice these totals were combined and entered into the analysis. The number of participants in the metronidazole group compared to the placebo group who experienced adverse events (RR 1.19, 95% CI 0.94 to 1.51; I² = 0%) was not significantly different across the six studies and in most instances these adverse events were mild and consisted of pruritus, skin irritation and dry skin. See Analysis 1.1.
1.1. Analysis.
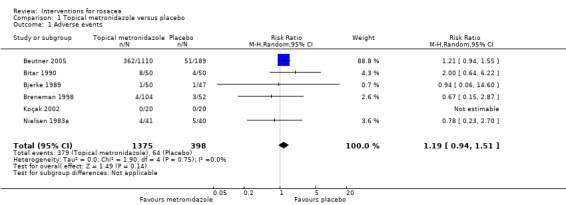
Comparison 1 Topical metronidazole versus placebo, Outcome 1 Adverse events.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The pooled data from three studies (Bjerke 1989; Breneman 1998; Nielsen 1983a) for this outcome pointed to an improvement in rosacea severity in the active intervention group, which was largely in agreement with the participant‐assessed outcomes for this comparison. Topical metronidazole was more effective than placebo and the results were both statistically significant and clinically important (RR 1.98, 95% CI 1.29 to 3.02; P = 0.002; NNTB 4, 95% CI 3 to 10). Heterogeneity between the studies was assessed with I² = 44%. See Analysis 1.2.
1.2. Analysis.
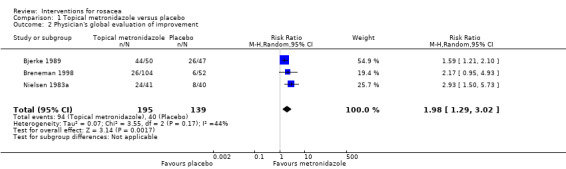
Comparison 1 Topical metronidazole versus placebo, Outcome 2 Physician's global evaluation of improvement.
Although a different rating scale (1 to 7, worst = 7) was used in Bitar 1990, the results were not dissimilar to those in the other three studies. In this study the mean rating in severity in the metronidazole group (n = 50) was 2.80 (SD 1.41) and 3.30 (SD 1.41) in the placebo group (n = 50) with a mean difference (MD) of ‐0.50 (95% CI ‐1.05 to 0.05; P = 0.08). In the split‐face study (Bleicher 1987) 29/37 participants were assessed as improved on the metronidazole treated side compared with 1/37 on the placebo side (RR = 29).
Only one study assessed ocular rosacea (Barnhorst 1996) but the data as reported were unusable and not amenable to re‐analysis. See Analysis 1.3.
1.3. Analysis.
Comparison 1 Topical metronidazole versus placebo, Outcome 3 Incomplete data on which further analysis is not possible.
| Incomplete data on which further analysis is not possible | ||||
|---|---|---|---|---|
| Study | Interventions | Summary Outcomes | Comment | Notes |
| Barnhorst 1996 | 13 participants were treated with lid hygiene plus warm compresses plus metronidazole 0.75% gel in one eye BID, versus lid hygiene plus warm compresses in the other eye. Within‐patient comparison. |
No adverse events reported. Eye and eyelid grading pre‐post mean (SD) ‐1.5 (1.7) versus ‐1.0 (1.7). Authors report significant improvement in treatment group but not in control group, P = 0.022 versus P = 0.10 [inappropriate analysis]. No direct comparison reported. Eye pre‐post mean (SD) ‐0.4 (1.0) versus ‐0.3 (0.9). Eyelid pre‐post mean (SD) ‐1.1 (0.9) versus ‐0.7 (0.8) |
Small group (13 participants),
within‐patient comparison. Not much data. Participant not blinded. Data skewed. |
BID = twice a day SD = standard deviation |
| Beutner 2005 | 557 were treated with metronidazole gel 1% QD versus 553 with metronidazole 1% cream QD versus 189 with metronidazole gel vehicle. | Adverse events 186/557 versus 176/553 versus 51/189.
Subjects rated as success according to physicians 38.4% versus 35.4% versus 27.5%. Reduction in lesion count 66.7% versus 58.3% vs. 46.2% |
Large vehicle effect. | QD = once daily |
| Bitar 1990 | 50 were treated with metronidazole cream 1% BID versus 50 with placebo cream BID. | Erythema and telangiectasia, no statistical difference. Number of papules after a month 4.5 (4.24) versus 6.5 (4.96). Number of pustules 1.5 (1.41) versus 3.4 (4.94). | Data on papules and pustules are skewed. | BID = twice a day |
| Bjerke 1989 | 50 were treated with metronidazole cream 1% BID versus 47 with placebo cream BID. | Erythema: 3 score reduction 2% versus 5%, 2 score reduction 26% versus 5% and 1 score reduction 46% versus 45%, unchanged 26% versus 41%, worse 0% versus 5%. Lesion count reduction 78% versus 48%, reduction of papules 75% versus 43%, reduction of pustules 100% versus 81%. | No SDs were reported. | BID = twice a day N = number SD = standard deviation |
| Bleicher 1987 | 40 were treated with metronidazole 0.75% BID versus 40 with placebo BID. | Adverse events, one complained of tearing when gel came to close to the eyes.
Reduction in erythema, 0.8 versus 0.3 (erythema rating 0 to 3, higher is worse).
Increase in telangiectasia of 0.3 at both sides (rating 0‐3) Decrease in lesion counts, 65.1% versus 14.9%. |
No SDs were reported. Within‐patient comparison. | BID = twice a day SD = standard deviation |
| Breneman 1998 | 104 were treated with metronidazole 1% QD versus 52 with placebo QD. | Mean decrease in erythema score of 0.9 in metronidazole group versus 0.5 in placebo group. Decrease in lesion count 8 versus 3. |
No SDs were reported. | SD = standard deviation QD = once daily |
| Dahl 1998 | 44 were treated with metronidazole 0.75% BID versus 44 with placebo BID. | At baseline 35/44 had no or mild erythema versus 32/44. At end of study this was 32/43 versus 24/44. Telangiectasia (no significant difference or effect) Lesion count 3.3 versus 5.8, relapse rate 23% versus 42%, free of lesions 53% versus 32%. | No SDs reported. N of adverse events unclear. | BID = twice a day SD = standard deviation |
| Koçak 2002 | 20 patients were treated with metronidazole 0.75% gel BID versus 20 with placebo BID. | No local adverse events in any group Mean change from baseline in papules ‐5.10 (23.36) versus 0.25 (11.25) with a MD of ‐5.35 (95% CI ‐16.71 to 6.01). Mean change from baseline in pustules ‐2.50 (13.65) versus ‐0.20 (9.20) with a MD of ‐2.30 (95% CI ‐9.51 to 4.91). No effects on rhinophyma and telangiectasia. |
Most data are skewed. | BID = twice a day SD = standard deviation |
| Nielsen 1983a | 41 were treated with metronidazole 1% QD versus 40 with placebo QD. | Reduction on erythema from 3.8 to 2.5 for metronidazole group and from 3.7 to 3.1 in placebo group. Authors state P < 0.05. There were no effects on telangiectasia. Papules count 8.6 versus 16.6, and pustules count 0.3 versus 0.8. |
No SDs were reported. | SD = standard deviation QD = once daily |
Assessment of erythema or telangiectasia, or both, at end of study
Erythema was assessed in seven studies (Bitar 1990; Bjerke 1989; Bleicher 1987; Breneman 1998; Dahl 1998; Koçak 2002; Nielsen 1983a). However, in all of these studies this outcome was inadequately reported that is standard deviations were missing or data were given without baseline values except for the three‐armed study of Koçak 2002. In this study the mean change from baseline in erythema score (0 to 3) was ‐1.45 (SD 2.00) in the metronidazole group compared to ‐0.05 (SD 1.39) in the placebo group with a MD of ‐1.40 (95% CI ‐2.47 to ‐0.33; P = 0.01). Bjerke 1989; Bleicher 1987; Breneman 1998; Dahl 1998 and Nielsen 1983a also showed a greater reduction of erythema with metronidazole treatment (see Analysis 1.3).
Lesion counts
In eight of the studies these outcomes were reported as continuous data but without the corresponding SDs and the data were skewed, that is not normally distributed. Although the data analysis in these studies was potentially flawed, it did nevertheless provide some supporting evidence of a positive treatment effect of metronidazole over placebo (see Analysis 1.3).
Time needed until improvement of the skin lesions
This was not a pre‐specified outcome for any of the studies but based on interim data from five of the studies (Bitar 1990; Bjerke 1989; Bleicher 1987; Breneman 1998; Nielsen 1983a) a noticeable improvement was seen at around four weeks.
Duration of remission
Only one trial (Dahl 1998) addressed this outcome and demonstrated that continued treatment with metronidazole gel alone could maintain remission (initiated by tetracycline and topical metronidazole) of moderate to severe rosacea.
(2) Metronidazole and sunscreen sun protection factor (SPF) 15 versus placebo
Only one study at high risk of bias with a 26% dropout rate and skewed data provided data for this comparison (Tan 2002).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Although the data for this outcome were presented as graph plots and were largely indiscernible, the investigators reported that there was a more noticeable improvement in rosacea severity in the metronidazole combined with sunscreen SPF 15 group than in the placebo group (P = 0.0002).
Proportion of participants who reported an adverse event throughout the study period
A small number of participants reported adverse events and these were similar in both groups: 1/61 in the metronidazole group and 3/59 in the placebo (RR 0.32, 95% CI 0.03 to 3.01). There was no statistically significant difference in local tolerance of the intervention between the two groups.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In the metronidazole group 17/61 had clearing or marked improvement compared to 2/59 in the placebo group (RR 8.22, 95% CI 1.99 to 34.04; P = 0.004; NNTB = 5, 95% CI 3 to 9).
Assessment of erythema or telangiectasia, or both, at end of study
The mean reduction in erythema at the end of the study, measured on a 4‐point scale (4 = severe) was 0.89 (SD 0.6) in the treatment group and 0.58 (SD 0.13) in the placebo group (P = 0.001), however these data were skewed. Telangiectasia (on a 4‐point scale) were reduced by 0.3 (SD 0.53) in the metronidazole + sunscreen SPF 15 group compared to 0.07 (SD 0.47) in the placebo group (P = 0.03).
Lesion counts
There was a reduction in the mean number of lesions, 13.6 (SD 17.25) in the active intervention group compared with placebo, 4.6 (SD 12.28) (MD ‐9.00, 95% CI ‐15.23 to ‐2.77). However the data were incomplete and skewed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(3) Metronidazole 0.75% cream versus metronidazole 1% cream
Only one study assessed as at high risk of bias compared these interventions and provided relevant outcome data (Dahl 2001).
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Adverse events were mild and comparable in both groups, 14/36 compared to 15/36 (RR 0.93, 95% CI 0.53 to 1.64).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
There was no statistically significant difference in assessments between the two groups at the end of the study. Twenty of the 36 participants using the 0.75% metronidazole cream were clear or nearly clear at the end of the study compared with 13 out of 36 in the 1% cream group (RR 1.54, 95% CI 0.91 to 2.60).
Assessment of erythema or telangiectasia, or both, at end of study
The percentage change in the total erythema severity score from baseline to endpoint was comparable (range 25% to 30%) with a difference that was not statistically significant between the two groups.
Lesion counts
The overall reductions in lesion counts were similar in both groups at the end of the study (62% versus 60%).
Time needed until improvement of the skin lesions
After six weeks both groups showed a reduction in inflammatory lesion counts of around 50%.
Duration of remission
Not assessed.
(4) Metronidazole 0.75% cream versus 0.75% gel
The investigators in a single study assessed as at a high risk of bias compared these two interventions and were unable to provide any additional data over and above what had been reported in the poster (Dreno 1998).
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
No serious adverse events were reported, with no details about the number of participants reporting side effects.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Although this was a pre‐specified outcome it was not addressed (see 'Risk of Bias' under Characteristics of included studies for this study).
Assessment of erythema or telangiectasia, or both, at end of study
The investigators reported "both erythema and telangiectasia scores were not significantly different at evaluation time".
Lesion count
The reduction in lesion count was similar in both the cream and gel groups (61.3% in the cream group versus 63.5% in the gel group).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(5) Metronidazole 0.75% in microemulsion versus metronidazole 0.75% in commercial gel
One within‐participant study assessed as at unclear risk of bias, evaluated this comparison (Tirnaksiz 2012).
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
There were no side effects on either treated side of the face.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
The mean change from baseline in erythema (0 to 3, with 3 being worse) was ‐1.75 (SD 0.49) for the microemulsion group compared to ‐0.91 (SD 0.60) in the commercial gel group with a MD of ‐0.84, however no 95% CI or P value could be calculated as we were not able to adjust for within‐participant variability. Telangiectasia were also scored on a scale from 0 to 3 and the mean change from baseline was ‐1.28 (SD 0.37) for the microemulsion group versus ‐0.41 (SD 0.65) in the commercial gel group, with a MD of ‐0.87; like in the assessment of erythema no 95% CI nor P value could be calculated.
Lesion counts
The mean change from baseline in lesion counts was ‐2.18 (SD 2.02) in the microemulsion group compared to ‐1.18 (SD 1.24) in the commercial gel group, with a MD of ‐1.0, but we were not able to adjust for within‐participant variability and therefore no 95% CI and P value could be calculated.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Topical interventions: studies with only topical azelaic acid
(6) Azelaic acid versus placebo
This comparison was evaluated by five trials assessed as at unclear risk of bias (Bjerke 1999; Carmichael 1993; Draelos 2013a; Thiboutot 2003a; Thiboutot 2003b), see also Table 2.
Primary outcomes
Change in HRQOL at end of study
This outcome was addressed in Draelos 2013a, but only limited data were provided; the investigators reported "there were no statistically significant differences between the two groups in end‐of‐treatment or end‐of‐study erythema, telangiectasia, or QOL scores". E‐mail correspondence with the trialists provided no additional details.
Participant‐assessed changes in rosacea severity at end of study
Four studies reported data for this outcome. Pooled outcome data from these studies indicated an improvement in rosacea severity and that the rates of complete remission or marked improvement, as assessed by the participants, were 60% to 80% in the azelaic acid group as compared with 45% to 55% in the placebo group (RR 1.46, 95% CI 1.30 to 1.63; P < 0.00001; NNTB = 5, 95% CI 4 to 8). This was a clinically important improvement in favour of azelaic acid. Heterogeneity between the studies was assessed with I² = 0%. See Analysis 2.1.
2.1. Analysis.
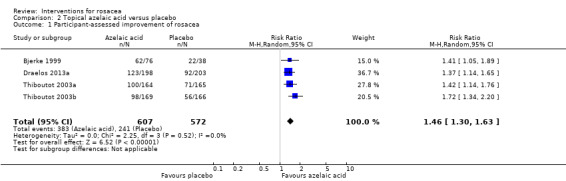
Comparison 2 Topical azelaic acid versus placebo, Outcome 1 Participant‐assessed improvement of rosacea.
Proportion of participants who reported an adverse event throughout the study period
There was no statistically significant difference in the number of adverse events reported by the participants in Bjerke 1999: in the azelaic acid group 30/76 compared with 15/38 in the placebo group (RR 1.00, 95% CI 0.62 to 1.62). In the Carmichael 1993 study, 24/33 participants reported adverse events on the side treated with azelaic acid and 19/33 on the side treated with placebo. In Draelos 2013a 21 adverse events were reported in 198 participants in the azelaic acid foam group and nine adverse events in the vehicle foam group (203 participants) (RR 2.39, 95% CI 1.12 to 5.09; P = 0.02; NNTH = 17; 95% CI 10 to 100). These were considered to be transient and of mild to moderate intensity, with burning, stinging or irritation being the most commonly reported.
The data for adverse events in Thiboutot 2003a and Thiboutot 2003b were combined and inadequately reported with minimal data available for the adverse events in the placebo group. Adverse events related to azelaic acid were reported in 18% of the participants in Thiboutot 2003a and in 8.4% in Thiboutot 2003b. Burning, stinging and itching were more frequent in the azelaic acid treated group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Data for these assessments from four studies illustrated that azelaic acid was more effective than placebo. The rates of treatment success in Draelos 2013a were 69.2% in the azelaic acid group and 57.6% in the vehicle group. In both the Thiboutot 2003a and Thiboutot 2003b studies the percentages were 61% and 62% in the azelaic acid groups and 40% and 48% in the placebo groups, and correspondingly 80% and 60% in Bjerke 1999 (RR 1.32, 95% CI 1.18 to 1.47; P < 0.00001; NNTB = 7, 95% CI 5 to 10). There was low heterogeneity between the studies, which was unlikely to be important as I² = 16%. See Analysis 2.2.
2.2. Analysis.
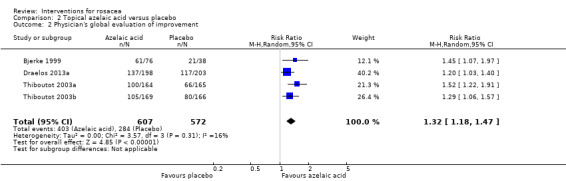
Comparison 2 Topical azelaic acid versus placebo, Outcome 2 Physician's global evaluation of improvement.
In the single within‐patient study (Carmichael 1993), 16/33 of the participants showed an improvement, based on a Likert scale rating, on the azelaic acid treated side compared with 1/33 on the placebo treated side, and there was no visible improvement in the remaining 16 participants (P < 0.001, McNemar's test). There was an overall improvement with complete remission or marked improvement in 30/33 sides treated with azelaic acid compared to 11/33 of the sides treated with placebo; crude RR of 2.72. The report did not provide SDs for any of the outcomes data and it was not possible to calculate the RR, therefore the data were not pooled with the other studies evaluating this comparison.
Assessment of erythema or telangiectasia, or both, at end of study
There was minimal to no effect in improvement of erythema and telangiectasia in all five of the studies (see Analysis 2.3).
Lesion counts
The mean number of lesions in Draelos 2013a reduced by 13.4 (SD 10.4) in the azelaic acid group compared to 9.5 (SD 9.73) in the vehicle foam group (MD ‐3.90, 95% CI ‐5.87 to ‐1.93; P = 0.0001). No SDs were reported for these outcomes in Bjerke 1999; Thiboutot 2003a and Thiboutot 2003b, and in Carmichael 1993 the data were skewed. See Analysis 2.3.
Time needed until improvement of the skin lesions
This was not a pre‐specified outcome in any of the studies but all studies showed clear improvement after three to six weeks.
Duration of remission
Not assessed.
(7) Azelaic acid 15% gel once daily versus azelaic acid 15% gel twice daily
A single study at high risk of bias compared the safety and effectiveness of azelaic acid 15% gel applied once daily versus twice daily (Thiboutot 2008). No statistically significant differences were reported in any of the efficacy endpoints between the two regimens.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
At the end of the study 29/45 participants on the once daily regimen considered themselves improved, which they rated as marked to excellent, compared to 27/47 on the twice daily regimen (RR 1.12, 95% CI 0.81 to 1.56).
Proportion of participants who reported an adverse event throughout the study period
The number of participants experiencing adverse events was comparable in both groups, that is 18 out of 45 participants in the once daily group versus 17 out of 47 in the twice daily group (RR 1.11, 95% CI 0.66 to 1.86), with pain, pruritus and burning sensations being the most frequently reported.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
There was no statistically significant difference between the two treatment regimens: 20 of the 45 participants in the single daily application improved versus 22 of 47 in the twice daily group (RR 0.95, 95% CI 0.61 to 1.48). Treatment success, defined as clear or minimal lesions, was achieved in 13 of 45 in the once daily group versus 15 of 47 in the twice daily group (RR 0.91, 95% CI 0.49 to 1.68).
Assessment of erythema or telangiectasia, or both, at end of study
No exact data were provided but the investigators stated that "treatment with AzA 15% gel led to a decrease in the intensity of erythema over the course of the study with no statistically significant difference between the QD group and BID group", where QD is treatment once daily and BID twice daily. Six participants in both groups showed an improvement in telangiectasia (RR 1.04, 95% CI 0.36 to 3.00).
Lesion counts
The mean change from baseline in the once daily group in lesion counts was ‐11.60 (SD 4.98) compared to ‐13.80 (SD 4.65) for the twice daily group (MD 2.20, 95% CI 0.23 to 4.17; P = 0.03). This difference was statistically significant but not clinically important.
Time needed until improvement of the skin lesions
Improvement was seen from week four in both groups.
Duration of remission
Not assessed.
(8) Azelaic acid 15% gel twice daily as maintenance therapy versus vehicle twice daily
Thiboutot 2009 was a two‐phase study in which participants, demonstrating a level of treatment effectiveness at week 12, were randomised to receive either azelaic acid gel or its vehicle twice daily as maintenance therapy. We have only included data from the maintenance phase (second phase) of this study. The study was assessed at high risk of bias due to selective reporting.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
This was a predefined outcome but was not addressed and we therefore judged the domain for selective reporting as at a high risk of bias (see 'Risk of Bias' under Characteristics of included studies for this study).
Proportion of participants who reported an adverse event throughout the study period
Adverse events were reported in 22 of the 67 participants using azelaic acid and in 20 of 69 in the vehicle‐only group (RR 1.13, 95% CI 0.68 to 1.87).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Success, as determined by an IGA and defined as clear, minimal or mild, was reported for 39 out of 67 participants in the azelaic acid group and for 31 out of 69 in the vehicle‐only group (RR 1.30, 95% CI 0.93 to 1.80). There was no statistically significant difference between the groups for this outcome.
Assessment of erythema or telangiectasia, or both, at end of study
No exact data were provided, but the investigators stated that no change in erythema or in telangiectasia was observed in either group.
Lesion counts
The increase in mean inflammatory lesion count in the maintenance phase was 5.5 in the azelaic acid group and 7.5 (data estimated from figure) in the vehicle group, investigators stated that P = 0.03. However, this difference of two lesions between groups was not considered to be clinically important.
Time needed until improvement of the skin lesions
Not applicable.
Duration of remission
Seventeen out of the 67 participants in the azelaic acid group relapsed compared with 24 of 69 in the vehicle‐only group (RR 0.73, 95% CI 0.43 to 1.23) with no statistically significant difference between the two groups.
Topical interventions: studies with only topical ivermectin
(9) Topical ivermectin versus placebo
Two studies at low risk of bias addressed this comparison (Stein 2014a; Stein 2014b), see also Table 3.
Primary outcomes
Change in HRQOL at end of study
More participants in the ivermectin group experienced improvements in HRQOL at the end of the study than in the control groups. Based on the DLQI at the end of the Stein 2014a study, 231/451 participants in the ivermectin group compared to 77/232 in the vehicle group considered that the disease had "no effect on their overall quality of life" (RR 1.54, 95% CI 1.26 to 1.89; P < 0.0001; NNTB = 6, 95% CI 4 to 10). In Stein 2014b these were 236/459 in the ivermectin group versus 76/229 in the vehicle group (RR 1.55, 95% CI 1.26 to 1.90; P < 0.0001; NNTB = 6, 95% CI 4 to 10).
Scores in the DLQI range from 0 to 30: a DLQI score of 0 to 1 is considered to have almost no impact on HRQOL, whereas 2 to 5 is considered to have a small effect, 6 to 10 a moderate effect, 11 to 20 a very large effect and 21 to 30 an extremely large effect on HRQOL. Therefore a reduction in score can be seen as an improvement in HRQOL. The mean change from baseline in DLQI for the ivermectin group (n = 436, per‐protocol population as provided for this outcome) in Stein 2014a was ‐3.50 (SD 2.77) compared to ‐2.30 (SD 2.71) in the vehicle group (n = 221) with a MD of ‐1.20 (95% CI ‐1.64 to ‐0.76; P < 0.00001). In Stein 2014b the reductions in DLQI were similar: ‐3.20 (SD 2.54) in the ivermectin group (n = 445, per‐protocol population as provided for this outcome) versus ‐2.10 (SD 2.48) in the vehicle group (n = 218) with a MD of ‐1.10 (95% CI ‐1.51 to ‐0.69; P < 0.00001). Although the minimal important difference (MID) for the DLQI is yet to be established for the different skin diseases there is general acceptance that this ranges between 2.5 and 5, and therefore the impact of both treatments provided a small improvement in HRQOL but the difference, although statistically significant, was not clinically important (Basra 2008).
In re‐analysing the data for this outcome we used the identical N per protocol populations for the groups as for the DLQI outcome. The disease‐specific RosaQoL (range 1 to 5) assessments in Stein 2014a showed reductions of 0.64 (SD 0.7) in the ivermectin group and 0.35 (SD 0.5) in the vehicle group (MD ‐0.29, 95% CI ‐0.38 to ‐0.20; P < 0.00001). In Stein 2014b the reductions were 0.60 (SD 0.6) in the ivermectin group versus a reduction of 0.35 (SD 0.5) in the vehicle group (MD ‐0.25, 95% CI ‐0.34 to ‐0.16; P < 0.00001). Although the differences were statistically significant, the clinical importance was unclear as the MID for RosaQoL still needs to be established.
Participant‐assessed changes in rosacea severity at end of study
Data for this outcome were reported in an ITT analysis (last observation carried forward (LOCF)). Participants' assessments at the end of the study (Stein 2014a) showed that there was a good to excellent improvement in 311/451 in the ivermectin group compared to 90/232 in the vehicle group (RR 1.78, 95% CI 1.50 to 2.11; P < 0.00001; NNTB = 4, 95% CI 3 to 5). In Stein 2014b these numbers were 304/459 in the ivermectin group versus 79/229 in the vehicle group (RR 1.92, 95% CI 1.59 to 2.32; P < 0.00001; NNTB = 4, 95% CI 3 to 5). Both studies showed a statistically significant and clinically important improvement in favour of topical ivermectin.
Proportion of participants who reported an adverse event throughout the study period
In Stein 2014a 19/451 reported side effects in the ivermectin group compared to 18/232 in the vehicle group (RR 0.54, 95% CI 0.29 to 1.01), skin burning was reported in eight and six cases respectively. In Stein 2014b 30/459 participants reported an adverse event in the ivermectin group compared to 15/229 in the vehicle group (RR 1.00, 95% CI 0.55 to 1.82).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
An Investigator's Global Assessment of clear or almost clear (Stein 2014a) was attained by 173/451 in the ivermectin group and 27/232 in the vehicle group (RR 3.30, 95% CI 2.27 to 4.79; P < 0.00001; NNTB = 4, 95% CI 4 to 5). In Stein 2014b similar global assessments were reported in 181/459 of the ivermectin group and 43/229 of the vehicle group (RR 2.10, 95% CI 1.57 to 2.81; P < 0.00001; NNTB = 5, 95% CI 4 to 8). The results of both studies were in concordance with the assessments of the participants.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
A mean change from baseline of ‐20.40 (SD 8.72) was seen in the ivermectin group (n = 451) at the end of the study compared to ‐12.00 (SD 10.10) in the vehicle group (n = 232) with a MD of ‐8.40 (95% CI ‐9.93 to ‐6.87; P < 0.00001) (Stein 2014a). The reductions in lesion counts in Stein 2014b were ‐22.3 (SD 8.21) in the ivermectin group (n = 459) and ‐13.40 (SD 10.50) in the vehicle group (n = 229) with a MD of ‐8.90 (95% CI ‐10.45 to ‐7.35; P < 0.00001). Both of these differences were statistically significant and clinically important.
Time needed until improvement of the skin lesions
This was not a predefined outcome, but improvement in both studies was seen after four weeks.
Duration of remission
Not assessed.
Topical interventions: studies with topical brimonidine
(10) Various concentrations of topical brimonidine gel once daily versus vehicle once daily after a single application
In a single study assessed at low risk of bias various concentrations of brimonidine gel (0.07%, 0.18% and 0.5%) were compared versus vehicle to determine which concentration was most effective for reducing erythema in rosacea after a single application (Fowler 2012a).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
This outcome was evaluated with the Patient's Self Assessment (PSA) tool with scores from 0 to 4 (clear to severe). A cumulative grade 1 improvement was experienced over 12 hours by 25/28 (89.3%) participants in the 0.07% group, 27/31 (87.1%) in the 0.18% group, 28/31 (90.3%) in the 0.5% group, and in 18/32 (56.3%) in the vehicle group. The highest concentration (0.5%) of brimonidine was more effective than vehicle in reducing erythema based on participants' assessments (RR 1.61, 95% CI 1.16 to 2.23; P = 0.004; NNTB = 3, 95% CI 3 to 8).
A cumulative grade 2 improvement over 12 hours was noticed in 12/28 (42.9%) participants in the 0.07% group, 14/31 (45.9%) in the 0.18% group, 19/31 (61.3%) in the 0.5% group, and 7/32 in the vehicle group. The 0.5% brimonidine gel was more effective than vehicle (RR 2.80, 95% CI 1.37 to 5.71; P = 0.005; NNTB = 3, 95% CI 2 to 6).
Proportion of participants who reported an adverse event throughout the study period
The majority of the adverse events were transient and mild in intensity and consisted of skin irritation, erythema, skin burning and dry skin. In the 0.07% group 5/28 participants reported an adverse event, 4/31 in the 0.18% group, 6/31 in the 0.5% group, and 6/32 in the vehicle group. Comparing the highest brimonidine concentration with vehicle there was no statistically significant difference in participants experiencing an adverse event between the two groups (RR 1.03, 95% 0.37 to 2.86).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
Physicians used the Clinician's Erythema Assessment (CEA) scale (0 to 4, clear to severe) to assess this outcome. The cumulative grade 1 improvement over 12 hours was reached in 24/28 participants in the group treated with 0.07%, in 28/31 in the group treated with 0.18%, in 30/31 treated with 0.5%, and in 21/32 of the participants treated with vehicle. The physician‐assessed changes in the comparison of the highest concentration (0.5%) with vehicle showed that brimonidine 0.5% was more effective (RR 1.47, 95% CI 1.14 to 1.91; P = 0.003; NNTB = 4, 95% CI 3 to 8).
A cumulative grade 2 improvement was seen in 14/28 of the participants in the 0.07% gel group, in 24/31 in the 0.18% gel group, in 24/31 in the 0.5% gel group, and in 9/32 in the vehicle gel group. Brimonidine 0.5% demonstrated greater efficacy than vehicle, represented by an effect which was statistically significant (RR 2.75, 95% CI 1.53 to 4.94; P = 0.0007; NNTB = 3, 95% CI 2 to 4). These physician‐assessed changes were in concordance with the assessments made by the participants.
Erythema was also assessed with a Chroma Meter, and the investigators reported that the values for brimonidine 0.5% were lower (investigators report P < 0.001) and that the onset of effect was within 30 minutes, reaching a maximum effect with a duration of between four to six hours, followed by a reappearance of the redness after eight hours.
Lesion counts
No data were provided but investigators reported that "no aggravations in the severity of inflammatory lesions were observed".
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(11) Various concentrations of topical brimonidine gel versus vehicle, with different dosing regimens over four weeks
A dose‐ranging study assessed as at low risk of bias to evaluate optimal concentration and dose regimen of brimonidine tartrate (BT) (0.18% once a day (QD), 0.18% twice a day (BID), 0.5% QD versus vehicle (QD and BID) (Fowler 2012b). We have only reported end‐of‐study data, that is at day 29.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
A grade 2 improvement on the Patient's Self Assessment (PSA) scale was assessed every three hours up to 12 hours for participants treated with brimonidine 0.18% once daily, 0.18% twice daily, 0.5% once daily and vehicle once or twice daily. Of note, the participants in the vehicle twice daily group scored themselves better on the PSA scale than in the vehicle once daily group.
| PSA grade 2 improvement | BT 0.18% QD | BT 0.18% BID | BT 0.5% QD | Vehicle QD | Vehicle BID |
| 3 hours after application | 17/54 | 20/54 | 25/53 | 7/55 | 11/53 |
| 6 hours after application | 13/54 | 15/54 | 26/53 | 8/55 | 11/53 |
| 9 hours after application | 9/54 | 19/54 | 22/53 | 5/55 | 13/53 |
| 12 hours after application | 9/54 | 16/54 | 20/53 | 5/55 | 10/53 |
At three hours after application brimonidine 0.5% was shown to be more effective than once daily vehicle (RR 3.71, 95% CI 1.75 to 7.83; P = 0.0006; NNTB = 4, 95% CI 2 to 6) and also when compared to vehicle twice daily (RR 2.27, 95% CI 1.25 to 4.13; P = 0.007; NNTB = 4, 95% CI 3 to 10). Brimonidine 0.5% was more effective than vehicle once or twice daily at every time point, even at 12 hours, compared to vehicle twice daily (RR 2.00, 95% 1.04 to 3.86; P = 0.04; NNTB = 6, 95% CI 3 to 50).
Proportion of participants who reported an adverse event throughout the study period
The adverse events were mild and transient, with fewer participants reporting adverse events in the vehicle twice daily group. There were no meaningful changes in intraocular pressure, blood pressure or heart rate in any of the treatments. In the 0.18% once daily group 22/54 participants reported an adverse event, in the 0.18% twice daily group 25/54, in the 0.5% once daily group 24/53, in the vehicle once daily group 25/55, and in the vehicle twice daily group 17/53. There was no statistically significant difference in the proportion of participants that experienced an adverse event in the 0.5% brimonidine group versus vehicle twice daily group (RR 1.41, 95% CI 0.86 to 2.31).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
This outcome was assessed with the CEA scale, and a grade 2 improvement at the different time points was compared.
| CEA grade 2 improvement | BT 0.18% QD | BT 0.18% BID | BT 0.5% QD | Vehicle QD | Vehicle BID |
| 3 hours after application | 21/54 | 22/54 | 27/53 | 12/55 | 9/53 |
| 6 hours after application | 21/54 | 18/54 | 23/53 | 12/55 | 12/53 |
| 9 hours after application | 20/54 | 23/54 | 26/53 | 12/55 | 15/53 |
| 12 hours after application | 17/54 | 18/54 | 20/53 | 15/55 | 16/53 |
Three hours after application the number of participants in the brimonidine 0.5% group achieving a grade 2 improvement on the CEA scale was statistically significant higher than in the vehicle twice daily group (RR 3.00, 95% CI 1.56 to 5.75; P = 0.0009; NNTB = 3, 95% CI 2 to 6). Participants in the brimonidine 0.5% group showed greater improvement than those in the vehicle twice daily group at all time points with the exception of 12 hours after application (RR 1.25, 95% CI 0.73 to 2.14).
Lesion counts
No data were provided but investigators reported that "no aggravations in the severity of inflammatory lesions were observed".
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
In the four week follow‐up no important aggravation in facial erythema was seen in any of the groups. However, isolated cases that had a worsening in PSA or CEA were seen but were not tied to a specific treatment group.
(12) Topical brimonidine 0.5% versus vehicle over 4 weeks
Two studies with similar study design assessed as at low risk of bias addressed this comparison (Fowler 2013a; Fowler 2013b) (see Table 4).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Assessments with the PSA scale were performed at day 1, 15 and 29, but we have chosen to report only the day 29 data.
|
PSA grade 1 improvement Fowler 2013a |
BT 0.5% | Vehicle |
PSA grade 2 improvement Fowler 2013a |
BT 0.5% | Vehicle |
| 30 minutes after application | 92/129 | 65/131 | 30 minutes after application | 39/129 | 19/131 |
| 3 hours after application | 99/129 | 61/131 | 3 hours after application | 61/129 | 28/131 |
| 6 hours after application | 96/129 | 63/131 | 6 hours after application | 54/129 | 23/131 |
| 9 hours after application | 93/129 | 59/131 | 9 hours after application | 50/129 | 26/131 |
| 12 hours after application | 85/129 | 59/131 | 12 hours after application | 48/129 | 25/131 |
|
PSA grade 1 improvement Fowler 2013b |
BT 0.5% | Vehicle |
PSA grade 2 improvement Fowler 2013b |
BT 0.5% | Vehicle |
| 30 minutes after application | 93/148 | 73/145 | 30 minutes after application | 36/148 | 25/145 |
| 3 hours after application | 112/148 | 77/145 | 3 hours after application | 53/148 | 26/145 |
| 6 hours after application | 106/148 | 72/145 | 6 hours after application | 56/148 | 26/145 |
| 9 hours after application | 106/148 | 71/145 | 9 hours after application | 52/148 | 25/145 |
| 12 hours after application | 94/148 | 78/145 | 12 hours after application | 48/148 | 26/145 |
After 30 minutes a grade 1 improvement on the PSA scale (Fowler 2013a) was seen in 92/129 participants in the brimonidine group compared to 65/131 in the vehicle group (RR 1.44, 95% CI 1.17 to 1.76; P = 0.0005; NNTB = 5, 95% CI 3 to 10). In Fowler 2013b similar results were seen in 93/148 in the brimonidine group versus 73/145 in the vehicle group (RR 1.25, 95% CI 1.02 to 1.53; P = 0.03; NNTB = 9, 95% CI 5 to 100). A grade 2 improvement on the PSA scale was observed in Fowler 2013a in 39/129 participants treated with brimonidine 0.5% and 19/131 in the vehicle group (RR 2.08, 95% CI 1.28 to 3.41; P = 0.003; NNTB = 7, 95% CI 4 to 17), which was statistically significant in favour of brimonidine, but was not confirmed in Fowler 2013b (RR 1.41, 95% CI 0.89 to 2.23).
We have chosen to not report RR at every time point, and have only reported the data three hours after application as it is fairly clear that the effect of brimonidine diminishes progressively over the 12 hour period. Three hours after application in the Fowler 2013a study a grade 1 improvement on the PSA scale was reported in 99/129 treated with brimonidine and in 61/131 treated with vehicle (RR 1.65, 95% CI 1.34 to 2.03; P < 0.00001; NNTB = 4, 95% CI 3 to 6). This was confirmed in Fowler 2013b (RR 1.43, 95% CI 1.19 to 1.70; P < 0.00001; NNTB = 5, 95% CI 4 to 9). Brimonidine was more effective than vehicle at each time point in both studies except at 12 hours in Fowler 2013b.
In Fowler 2013a a statistically significant 2 grade improvement in PSA was noticed in the brimonidine group three hours after application (RR 2.21, 95% CI 1.52 to 3.22; P < 0.0001; NNTB = 4, 95% CI 3 to 7) and in Fowler 2013b (RR 2.00, 95% CI 1.33 to 3.01; P = 0.0009; NNTB = 6, 95% CI 4 to 13). At each time point in both studies brimonidine was shown to be more effective than vehicle with an improvement which was statistically significant.
Proportion of participants who reported an adverse event throughout the study period
In the brimonidine group in Fowler 2013a 38/129 participants reported an adverse event compared to 33/131 in the vehicle group (RR 1.17, 95% CI 0.79 to 1.74), and in Fowler 2013b 50/148 in the brimonidine group versus 35/145 in the vehicle group (RR 1.40, 95% CI 0.97 to 2.02). In both studies (Fowler 2012a; Fowler 2012b) adverse events were mild and transient, and the most frequently reported were worsening of erythema, flushing, pruritus and skin irritation.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
No data were reported for both studies other than "no aggravations in the severity of telangiectasia, IGA or inflammatory lesion counts were observed during either the treatment or follow‐up phase of either study".
Assessment of erythema or telangiectasia, or both, at end of study
As with the participants' assessments we chose to report the end of study (day 29) data.
|
CEA grade 1 improvement Fowler 2013a |
BT 0.5% | Vehicle |
CEA grade 2 improvement Fowler 2013a |
BT 0.5% | Vehicle |
| 30 minutes after application | 87/129 | 57/131 | 30 minutes after application | 31/129 | 11/131 |
| 3 hours after application | 105/129 | 64/131 | 3 hours after application | 61/129 | 22/131 |
| 6 hours after application | 107/129 | 70/131 | 6 hours after application | 54/129 | 23/131 |
| 9 hours after application | 98/129 | 58/131 | 9 hours after application | 46/129 | 22/131 |
| 12 hours after application | 94/129 | 63/131 | 12 hours after application | 36/129 | 15/131 |
|
CEA grade 1 improvement Fowler 2013b |
BT 0.5% | Vehicle |
CEA grade 2 improvement Fowler 2013b |
BT 0.5% | Vehicle |
| 30 minutes after application | 96/148 | 70/145 | 30 minutes after application | 36/148 | 25/145 |
| 3 hours after application | 119/148 | 78/145 | 3 hours after application | 60/148 | 33/145 |
| 6 hours after application | 111/148 | 85/145 | 6 hours after application | 55/148 | 28/145 |
| 9 hours after application | 112/148 | 81/145 | 9 hours after application | 44/148 | 29/145 |
| 12 hours after application | 95/148 | 72/145 | 12 hours after application | 48/148 | 33/145 |
In (Fowler 2013a) physicians' assessments at 30 minutes after application recorded a grade 1 improvement in the CEA scale in 87/129 participants in the brimonidine group versus 57/131 in the vehicle group, which was statistically significant in favour of brimonidine, and in concordance with the assessments made by the participants (RR 1.55, 95% CI 1.23 to 1.95; P = 0.0002; NNTB = 5, 95% CI 3 to 9); and further confirmed in Fowler 2013b (RR 1.34, 95% CI 1.09 to 1.65; P = 0.005; NNTB = 6, 95% CI 4 to 20). In Fowler 2013a a grade 2 improvement 30 minutes after application was seen in 31/129 participants treated with brimonidine versus 11/131 treated with vehicle (RR 2.86, 95% CI 1.50 to 5.45; P = 0.001; NNTB = 7, 95% CI 5 to 15). This was not confirmed in Fowler 2013b (RR 1.41, 95% CI 0.89 to 2.23), but both the participants and the physicians were in agreement in Fowler 2013b about the grade 2 improvements in erythema.
In Fowler 2013a at three hours after application a grade 1 improvement in CEA was seen in 105/129 in the brimonidine group versus 64/131 in the vehicle group (RR 1.67, 95% CI 1.37 to 2.02; P < 0.00001; NNTB = 4, 95% CI 3 to 5). Similar results were seen in Fowler 2013b (RR 1.49, 95% CI 1.26 to 1.77; P < 0.00001; NNTB = 4, 95% CI 3 to 7). At all time points in both studies brimonidine was more effective than vehicle in reaching a grade 1 improvement on the CEA scale.
In Fowler 2013a at three hours after application, a grade 2 improvement in CEA was observed in 61/129 in the brimonidine group versus 22/131 in the vehicle group (RR 2.82, 95% CI 1.85 to 4.30; P < 0.00001; NNTB = 4, 95% CI 3 to 5), which was statistically significant in favour of brimonidine; and in Fowler 2013b (RR 1.78, 95% CI 1.25 to 2.55; P = 0.002; NNTB = 6, 95% CI 4 to 15). In both studies there was a statistically significant difference favouring brimonidine at all time points except at 30 minutes, 9 and 12 hours in Fowler 2013b,
Lesion counts
See above, no aggravations.
Time needed until improvement of the skin lesions
Improvement was seen within 30 minutes.
Duration of remission
There was no rebound or worsening of erythema after treatment cessation in comparison to baseline assessments.
(13) Brimonidine 0.33% versus vehicle
One study assessed as at unclear risk of bias examined the effect of brimonidine 0.33% gel versus vehicle on erythema and considered only patient‐reported outcomes (NCT01885000).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Patient‐reported outcomes were assessed using the following instruments: a 'facial redness questionnaire' that addressed satisfaction, embarrassment and self‐consciousness; a 'subject satisfaction questionnaire' which addressed satisfaction with overall treatment, improvement of facial redness and time it took to work; and a 'Subject Diary' (treatment compliance and redness control). PSA was also addressed, but no exact data were provided and it was reported that the mean scores were statistically significantly lower in the brimonidine group.
The results of the facial redness questionnaire at baseline showed that a small number of participants, 2/48 in the brimonidine group and 0/44 in the vehicle group, were satisfied to very satisfied with their appearance. At day 8 (end of study) 17/48 (36.9%) in the brimonidine group were satisfied or very satisfied compared to 9/44 (21.5%) in the vehicle group (RR 1.73, 95% CI 0.86 to 3.48; P = 0.12, however the investigators reported P < 0.05). Participant assessments included perceptions of embarrassment, such that at baseline 44/48 (91.7%) in the brimonidine group felt embarrassed and in the vehicle group 42/44 (95.5%). At day 8 the figures were 34/48 (71.7%) compared to 40/44 (90.4%) respectively (RR 0.78, 95% CI 0.64 to 0.96; P = 0.02; NNTB = 5, 95% CI 3 to 20). Feeling self conscious was also evaluated by the participants, and at baseline 40/48 (83.3%) in the brimonidine group felt self conscious and 41/44 (93.1%) in the vehicle group. At day 8 35/48 (73.4%) in the brimonidine group and 39/44 (88%) in the vehicle group felt self conscious (RR 0.82, 95% CI 0.67 to 1.01; P = 0.06).
The results of the Subject Satisfaction Questionnaire (SSQ) (feedback on treatment regimen) revealed that 25/48 (52.2%) in the brimonidine group were either satisfied or very satisfied with the overall treatment compared to 14/44 (30.9%) in the vehicle group (RR 1.64, 95% CI 0.98 to 2.73; P = 0.06). Improvement in facial redness was scored satisfied or very satisfied in 21/48 (43.5%) in the active treatment group versus 8/44 (19%) in the vehicle group (RR 2.41, 95% CI 1.19 to 4.87; P = 0.01; NNTB = 4, 95% CI 3 to 15). The time taken to reach an effect was assessed by the participants and 22/48 (45.6%) in the brimonidine group were satisfied to very satisfied compared to 9/44 (21.4%) in the vehicle group (RR 2.24, 95% CI 1.16 to 4.33; P = 0.02; NNTB = 4, 95% CI 3 to 15).
The participant diaries revealed that 39/48 (81.1%) of the participants in the brimonidine group were able to control their facial redness that day compared to 18/44 (40.6%) participants in the control group (RR 1.99, 95% CI 1.36 to 2.90; P = 0.0004; NNTB = 3, 95% CI 2 to 5).
Proportion of participants who reported an adverse event throughout the study period
The adverse events that were reported were mild and transient worsening of erythema or worsening of rosacea, more of which were reported in the brimonidine group, with 14/48 participants in the brimonidine group reporting an adverse event versus 7/44 in the vehicle group (RR 1.83, 95% CI 0.82 to 4.12).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
No data were provided, but investigators reported that mean scores in CEA were statistically significantly lower in the brimonidine groups at all time points (P < 0.001).
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Topical interventions: studies with topical metronidazole, azelaic acid, and/or other topical treatments
(14) Topical azelaic acid versus topical metronidazole
Three studies assessed as at unclear risk of bias provided data for this comparison (Elewski 2003; Maddin 1999; Wolf 2006), which had a within‐participant study design but the trialists did not account for this within their analyses, therefore only summary statistics are presented. See also Table 5.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
In Elewski 2003 97/124 participants in the azelaic acid gel group considered themselves to have a good to excellent improvement versus 81/127 in the metronidazole gel group (RR 1.23, CI 95% 1.04 to 1.44; P = 0.01; NNTB = 8, 95% CI 4 to 34), and in Wolf 2006 57 out of 78 versus 60 of 82, respectively (RR 1.00, 95% CI 0.83 to 1.21).
In the Maddin 1999 study participants considered the 20% azelaic acid cream more effective than the metronidazole 0.75% cream. Severity was rated on a 5‐point scale (0 to 4, higher = worse), the mean score on the azelaic acid treated side was 1.87 (SD 0.76) compared with 2.33 (SD 0.95) on the metronidazole treated side (investigators reported P = 0.02).
Proportion of participants who reported an adverse event throughout the study period
The number of participants in Elewski 2003 experiencing adverse events was higher and statistically significant in the azelaic acid group with 32 of 124 as compared to 9 of 127 in the metronidazole group (RR 3.64, 95% CI 1.81 to 7.31; P = 0.0003; NNTH = 6, 95% CI 4 to 10). There was no statistically significant difference in adverse events between the groups in Wolf 2006: 29 of 78 versus 41 of 82 (RR 0.74, 95% CI 0.52 to 1.07). The adverse events reported in both Elewski 2003 and Wolf 2006 were mild to moderate and mostly transient, with skin dryness, scaling, stinging and burning being the most frequent. In Maddin 1999 only one participant reported an adverse event, that is stinging, on the side of the face which had been treated with azelaic acid cream.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In agreement with the participants' assessments, the physicians in the Elewski 2003 study considered the azelaic acid group significantly more improved than the metronidazole group. In the azelaic acid group 86/124 participants were considered to have an IGA score of clear, minimal or mild versus 70/127 in the placebo group (RR 1.26, 95% CI 1.03 to 1.53; P = 0.02; NNTB = 8, 95% CI 4 to 50). In Wolf 2006 44 out of the 78 participants in the azelaic acid group were considered to be cleared or nearly cleared versus 44 of the 82 in the metronidazole group, with no statistically significant difference between the groups (RR 1.05, 95% CI 0.79 to 1.39).
The investigators in the Maddin 1999 study evaluated 'Global Improvement in severity of rosacea' (1 = complete clearance to 6 = exacerbation). At 15 weeks the score for the azelaic acid treated side was 2.7 (SD 1.0) compared with 3.1 (SD 1.0) on the metronidazole treated side; the investigators suggested limited superiority of azelaic acid over metronidazole but they failed to adjust for the within‐participant design of their study.
Assessment of erythema or telangiectasia, or both, at end of study
In Elewski 2003 70/124 showed improvement in erythema in the azelaic acid group compared to 53/127 in the metronidazole group (RR 1.35, 95% CI 1.05 to 1.75; P = 0.02; NNTB = 7, 95% CI 4 to 50). A decrease of one point on the four‐point Likert scale (none to severe) was considered to be an improvement. In Wolf 2006 33/78 participants in the azelaic acid group attained an erythema score of 0 or 1 (same scale) compared to 35/82 in the metronidazole gel group (RR 0.99, 95% CI 0.69 to 1.42). In Maddin 1999 both participants and investigators assessed erythema. The investigators scored a reduction of 0.83 on the azelaic acid treated side compared to a reduction of 0.51 on the metronidazole side, whilst the participants scored a greater reduction on the metronidazole side (reduction of 0.23 (SD 0.58) on the azelaic acid side and a reduction of 0.74 (SD 0.57) on the metronidazole treated side).
Lesion counts
The decrease in inflammatory lesion counts reported in Elewski 2003 was 12.9 in the azelaic acid group versus 10.7 in the metronidazole group. No SDs were provided, but the investigators reported a P value of 0.003 and although this was a statistically significant difference it was not clinically important. In Maddin 1999 the decrease in lesion count was expressed as a percentage, 78.5% in the azelaic acid group versus 69.4% in the metronidazole group. In Wolf 2006 this was reported as median change reductions of 80% and 77% respectively.
Time needed until improvement of the skin lesions
An improvement for both arms was seen after four to six weeks in all three studies.
Duration of remission
Not assessed.
(15) Azelaic acid 20% versus metronidazole 0.75% versus permethrin 5%
Only one study at high risk of bias with a within‐participant design compared these interventions (Mostafa 2009). Investigators' conclusions were based on the analysis of skewed and unreliable data analysis.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Side effects included itching, burning sensation, oedema and scales, and were mostly transient. The investigators reported that "there were no statistically significant differences among the three groups and almost decreased at the end visit".
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
The reductions in mean erythema scores (scale unclear) was 0.60 (SD 0.66) for the 16 sites treated with azelaic acid, 0.30 (SD 0.48) for the 16 sites treated with metronidazole, and 0.25 (SD 0.51) for the sites treated with permethrin. The investigators stated that the changes from baseline were statistically significant (P < 0.05), but that there was no statistically significant difference between the three treatments.
Lesion counts
Although the analysis was based on skewed data and unreliable data analysis, all three treatments reduced the mean number of lesion counts, with 3.60 (SD 2.33) for azelaic acid, 3.70 (SD 2.92) for metronidazole and 2.60 (SD 3.24) for permethrin cream.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Lesion counts and erythema assessments were provided six months after the end of treatment, but the report provided no indication of how long the participants were in remission before they relapsed.
(16) Topical permethrin versus topical metronidazole
One three‐armed study (Koçak 2002) assessed as at unclear risk of bias reported data for this comparison. Most of the data that were reported were skewed. The authors, however, concluded that permethrin 5% cream showed comparable effectiveness to metronidazole on both erythema and papules, but indicated that this did not apply to pustules.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in either intervention group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
The mean change in erythema score (scale 0 to 3, 3 = severe) from baseline to day 60 was ‐1.26 (SD 2.09) in the permethrin group versus 1.45 (SD 2.00) in the metronidazole 0.75% group. Data were skewed. Neither treatment was shown to be more effective than the other for rhinophyma or telangiectasia.
Lesion counts
The MD in number of papules was ‐4.33 (SD 28.72) in the permethrin group versus ‐5.10 (SD 23.36) in the metronidazole group. The MD in pustules was ‐1.74 (SD 13.52) for the permethrin group and ‐2.5 (SD 13.65) in the metronidazole group. Data were skewed, but not for pustules.
Most of the data that were reported were skewed but the authors concluded that permethrin 5% cream showed comparable effectiveness to metronidazole on both erythema and papules, but indicated this did not apply to pustules.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(17) Topical permethrin versus placebo
This was the last comparison of this three‐armed study (Koçak 2002). Most data were skewed.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in either intervention group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
The mean change in erythema score (scale 0 to 3, 3 = severe) from baseline to day 60 was ‐1.26 (SD 2.09) in the permethrin group versus ‐0.05 (SD 1.39) in the placebo group. Data were skewed. Neither treatment was shown to be more effective than the other for rhinophyma or telangiectasia.
Lesion counts
The MD in number of papules was ‐4.33 (SD 28.72) in the permethrin group versus +0.25 (SD 11.25) in the placebo group. The MD in pustules was ‐1.74 (SD 13.52) for the permethrin group and ‐0.20 (SD 9.20) in the placebo group. Data were skewed, but not for pustules.
Most of the data that were reported were skewed, but the authors concluded that permethrin 5% cream was more effective than placebo.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(18) Benzoyl peroxide acetone versus vehicle
Two studies provided data for this comparison. Leyden 2014 was a three‐armed study comparing benzoyl peroxide 1% and 5% against vehicle and was assessed as at unclear risk of bias. Only data for our secondary outcomes were provided. Montes 1983 was assessed as at high risk of bias and complete data were only reported for the first four weeks. The lack of baseline values hampered our ability to interpret the data.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
There was no statistically significant difference between the two groups in the number of participants reporting adverse events (Montes 1983): 26/33 in the benzoyl peroxide group versus 18/31 in the vehicle group (RR 1.36, 95% CI 0.96 to 1.92). Irritation and burning were the most frequently reported side effects in both groups. The rate of adverse events was high in both groups, which the authors indicated could be attributed to the vehicle in that the benzoyl peroxide gel may have a greater dehydrating effect than the newer aqueous gels. This outcome was not assessed in Leyden 2014.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In the study of Leyden 2014 the physicians rated 12/32 participants as a treatment success in the benzoyl peroxide 1% group compared to 6/30 in the vehicle group, which was not statistically significant (RR 1.88, 95% CI 0.81 to 4.36). However, the higher concentration did show a statistically significant difference as 16/30 in the benzoyl peroxide 5% group were considered to have a treatment success (RR 2.67, 95% CI 1.21 to 5.88; P = 0.01). There was no statistically significant difference between the 5% and the 1% group (RR 0.70, 95% CI 0.40 to 1.23). In Montes 1983 the overall response score, rated on a scale of 0 to 4 (4 = worst), at the end of four weeks was 2.69 (benzoyl peroxide) versus 3.71 (vehicle). However, no baseline values were reported.
Assessment of erythema or telangiectasia or both at end of study
In the study of Leyden 2014 the investigators reported there were no changes in persistent erythema or telangiectasia in any of the groups. In Montes 1983 the investigators also reported that there were no statistically significant differences seen in the severity of erythema and telangiectasia.
Lesion counts
In Leyden 2014 lesion counts reduced by 21.6 (SD 23.31) in the benzoyl 1% group versus 7.4 (SD 17.24) in the vehicle group (MD ‐14.20, 95% CI ‐24.36 to ‐4.04; P = 0.006), which was a statistically significant difference in favour of benzoyl peroxide 1%. The reduction in the 5% group was smaller (14.1 (SD 8.78)) and compared to the vehicle the MD was ‐6.70 (95% CI ‐13.62 to 0.22; P = 0.06). There was no statistically significant difference between the 1% and 5% group (MD ‐7.50, 95% CI ‐16.17 to 1.17). These were rated on a scale of 0 to 3 (3 = worst) in Montes 1983 and the improvement in scores appeared to favour benzoyl peroxide. The papule scores at four weeks were 0.89 (benzoyl peroxide) compared with 1.91 (vehicle), and pustules scores 0.46 (benzoyl peroxide) versus 1.31 in the placebo group (investigators reported P < 0.05). No baseline values were reported.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(19) Benzoyl peroxide 5% with clindamycin 1% gel versus placebo
There were two individual reports at unclear risk of bias (Breneman 2004; Leyden 2004) involving the same study participants but focusing on different outcomes measures. Some SDs were lacking, and most data were skewed.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
The mean scores, rated as 0 to 4 (4 = worst), at the end of the study were 1.54 (much to slightly better) in the benzoyl peroxide with clindamycin group versus 2.50 (slightly better) in the placebo group (investigators reported P = 0.0002). This outcome was only assessed at 12 weeks.
Proportion of participants who reported an adverse event throughout the study period
There was no statistically significant difference in the number of participants between the groups reporting adverse events. There were 7 out of 27 participants in the benzoyl peroxide with clindamycin group who reported adverse events versus 4/26 in the placebo group (RR 1.69, 95% CI 0.56 to 5.08). Treatment‐related adverse events included localised burning and itching, which are both well‐known side effects of benzoyl peroxide.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The mean scores, rated 0 to 5 (5 = worst), at the end of the study were 1.85 (which was equivalent to a marked improvement) in the active treatment group versus 2.96, indicating minimal improvement in the placebo group. In the benzoyl peroxide with clindamycin group 11 of the 27 participants compared with 4/26 in the placebo group were considered to have a marked improvement or complete clearance (RR 2.65, 95% CI 0.96 to 7.25). The mean percentage change from baseline in overall rosacea severity assessment was ‐29.3% for benzoyl peroxide with clindamycin and ‐10.6 for the vehicle group (investigators reported P = 0.01).
Global photographic improvement was assessed on a 7‐point scale (−2 to +4, 4 = best) in Leyden 2004. The investigators reported a mean Global photographic comparison rating of 1.6 in the active intervention group versus 0.7 in the placebo group (P < 0.001, investigator reported).
Assessment of erythema or telangiectasia, or both, at end of study
Mean erythema score decreased, 0.63 in the benzoyl peroxide with clindamycin group and 0.33 in the vehicle group (investigators reported P = 0.07). There were also no statistically significant differences between the two groups in telangiectasia.
Lesion counts
The reduction in mean lesion counts in the treatment group was 71.3% (SD 25.3) versus 19.3% (SD 89.6) in the placebo group (Breneman 2004). Mean papule counts decreased from 15.6 (SD 7.8) to 3.9 (SD 3.6) in the benzoyl peroxide with clindamycin group versus a decrease from 16.8 (SD 10) to 13.4 (SD 14.6) in the placebo group, and the pustule counts decreased from 2.5 (SD 3.8) to 0.8 (SD 2.4) versus from 2.5 (SD 4.0) to 2.0 (SD 4.5) respectively. The investigators in this study also concluded that a treatment effect, that is a reduction in the number of lesions, was demonstrated in the benzoyl peroxide and clindamycin group, which we were unable to confirm because the data as reported were skewed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(20) Sodium sulphacetamide 10% and sulphur 5% versus placebo
Only one study evaluated these interventions but the overall reporting quality was inadequate: the number of participants in each treatment arm was not reported, improvement as an outcome was ill‐defined, and the data reported as continuous outcomes were skewed and largely unusable (Sauder 1997). This study was categorised as at unclear risk of bias. For further details see the 'Risk of bias' tables in 'Characteristics of included studies'.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
A larger percentage of participants (90%) in the active treatment group considered themselves improved as compared with the placebo group (58%) (investigators reported P < 0.001).
Proportion of participants who reported an adverse event throughout the study period
Adverse events were reported as 38% in the active group versus 29% in the placebo group. Application site reactions such as dryness, erythema and pruritus were the most commonly reported adverse events.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Based on these assessments, 98% in the active treatment group versus 68% of the participants in the placebo group demonstrated an improvement (investigators reported P < 0.001).
Assessment of erythema or telangiectasia, or both, at end of study
Improvement in erythema was seen in 83% of the active treatment group compared to 31% in the vehicle group (investigators reported P < 0.001).
Lesion counts
The mean lesion count reductions were reported as 78% versus 36% for the active treatment group and vehicle group respectively, with a corresponding reduction of 30.5 lesions and 9.4 lesions (investigators reported P < 0.001).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(21) Sodium sulphacetamide 10% and sulphur 5% versus metronidazole 0.75%
Two studies which we assessed as unclear to high risk of bias reported data for this comparison (Lebwohl 1995; Torok 2005).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
No exact data were provided in Lebwohl 1995, but the investigators reported there were no statistically significant treatment differences in any of the participant assessments. Although this was a pre‐specified outcome in Torok 2005, this was not addressed (see 'Risk of bias' under Characteristics of included studies for this study).
Proportion of participants who reported an adverse event throughout the study period
Fewer participants experienced adverse events in the metronidazole group but the difference between groups was not statistically significant. In Lebwohl 1995 5/31 versus 3/32 (RR 1.72, 95% CI 0.45 to 6.59) and in Torok 2005 48/75 versus 41/77 (RR 1.20, 95% CI 0.92 to 1.57) reported adverse events. Most adverse events were mild and transient and consisted of dryness, pruritus, burning and stinging.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
These assessments indicated that there was some evidence that sodium sulphacetamide 10% with sulphur 5% was more effective than metronidazole 0.75% gel. Although no SDs were provided in Lebwohl 1995 the overall severity reduced from 2.2 to 1.1 on a scale of 0 to 3 (none to severe) in the sodium sulphacetamide 10% with sulphur 5% (investigators reported P < 0.01) and from 2.1 to 0.6 in the metronidazole group (investigators reported P < 0.01). The difference was, according to the investigators, statistically significant (P = 0.002). No baseline values were reported on Physician's Global Assessment but the investigators concluded that "treatment mean contrasts show statistically significant differences favouring sodium sulphacetamide/sulphur at all times points (week eight P = 0.001)".
In Torok 2005 51 of the 75 participants were considered to have been cleared, or to have shown good to excellent improvement, in the sulphacetamide plus sulphur group versus 43 of the 77 in the metronidazole gel group (RR 1.22, 95% CI 0.95 to 1.57).
Assessment of erythema or telangiectasia, or both, at end of study
In Lebwohl 1995 the mean erythema scores decreased from 2.3 to 1.2 after eight weeks (SDs were missing, investigators reported P < 0.05) in the sulphacetamide plus sulphur group and from 2.2 to 0.7 in the metronidazole group (investigators reported P < 0.05). The difference was reported to be statistically significant in favour the sulphacetamide plus sulphur group (P = 0.017). This difference was not confirmed in Torok 2005 where 45/75 treated with sulphacetamide plus sulphur showed at least one grade improvement on a scale from 0 to 3 compared to 43/77 on metronidazole (RR 1.07, 95% CI 0.82 to 1.41).
Lesion counts
No SDs were provided for baseline values. There was no statistically significant difference in decrease of the papule count for these interventions in Lebwohl 1995 as reported by the authors. However, there was a statistically significant difference in decrease in the pustule count in favour of sodium sulphacetamide plus sulphur (investigators reported P = 0.006). For Torok 2005 the mean reductions in lesion counts were 80% in the sulphacetamide plus sulphur group and 72% in the metronidazole group.
Time needed until improvement of the skin lesions
This was not a predefined outcome but improvement was noted after four to six weeks in both studies.
Duration of remission
Not assessed.
(22) Pimecrolimus 1% versus placebo
Twice daily applications of pimecrolimus 1% were compared with placebo (vehicle) in Weissenbacher 2007. The study was assessed as at unclear risk of bias.
Primary outcomes
Change in HRQOL at end of study
The 'quality of life impairment' (Dermatology Life Quality Index, score 0 to 30, higher score = more impairment) showed a reduction of the mean absolute value from 5.50 to 3.10 in the pimecrolimus group versus 6.70 to 3.70 in the vehicle group, which were both small reductions (investigators reported P = 0.75).
Participant‐assessed changes in rosacea severity at end of study
The subjective severity score (VAS 0 to 100 mm, higher = worse) indicated an improvement of the mean absolute value from 53.45 to 48.95 in the pimecrolimus group and from 64.75 to 43.35 in the vehicle group (investigator reported P = 0.48).
Proportion of participants who reported an adverse event throughout the study period
Two adverse events were reported, but it was unclear in which group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Mean absolute values for the total rosacea severity score reduced from 6.88 to 4.68 in four weeks in the pimecrolimus group versus 7.00 to 4.33 in the vehicle group. The difference was not statistically significant (investigators stated P = 0.59).
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(23) Metronidazole 1% cream versus pimecrolimus cream
One study at high risk of bias compared these interventions (Koca 2010) but there was an appreciable baseline imbalance at enrolment, that is an increased duration and severity of disease in the pimecrolimus arm as compared with the metronidazole arm. The conclusions reached by the investigators did not appear to plausibly reflect the data that were reported as the data were massively skewed.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Four of the 24 participants in the metronidazole group reported adverse events (burning and stinging) compared to 2/25 in the pimecrolimus group (itching) (RR 2.08, 95% CI 0.42 to 10.34).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
There was no statistically significant difference in global improvement between the two groups. In the metronidazole group all (24/24) of the participants showed a measure of improvement as compared with 22 out of 25 participants in the pimecrolimus group (RR 1.13, 95% CI 0.96 to 1.33).
Assessment of erythema or telangiectasia, or both, at end of study
On a scale from 0 to 3 (higher = worse), erythema scores reduced by 0.92 (SD 0.24) in the metronidazole group and 0.92 (SD 0.35) in the pimecrolimus group (MD 0.0, 95% CI 0.17 to 0.17). Both treatments failed to show any improvement in telangiectasia.
Lesion counts
The mean changes in number of lesion counts were from 16.0 (SD 4.6) to 0.6 (SD 1.5) in the metronidazole group and from 26 (SD 14.4) to 3.7 (SD 6.8) in the pimecrolimus group. These data were skewed, and there was a clinically important baseline imbalance in the number of lesions.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(24) Brimonidine 0.05% gel versus azelaic acid 15% gel
Limited data from a poster abstract were reported in the single study at unclear risk of bias comparing these interventions in 70 participants (Kendall 2014).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
After 2 weeks 9/35 of the participants reported a two grade improvement on the PSA on a scale from 0 to 4 (higher indicating worse) in the brimonidine group compared to 7/35 in the azelaic acid group (RR 1.29, 95% CI 0.54 to 3.07).
Proportion of participants who reported an adverse event throughout the study period
Not assessed.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
No global assessments were assessed.
Assessment of erythema or telangiectasia, or both, at end of study
The judgements of the investigators were not in concordance with the judgements of the participants. A two grade improvement on the CEA (scale 0 to 4, higher indicating worse) was seen in 12/35 of the participants in the brimonidine group versus 4/35 in the azelaic acid group (RR 3.00, 95% CI 1.07 to 8.40; P = 0.04), which was a statistically significant difference in favour of brimonidine. Chroma Meter readings decreased by 9.64% and 2.35% respectively.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(25) Ivermectin 1% cream versus metronidazole 0.75% cream
This comparison was evaluated in a single study at low risk of bias (Taieb 2015), see Table 6.
Primary outcomes
Change in HRQOL at end of study
The DLQI score at baseline was 6.93 in the ivermectin group and 6.05 in the metronidazole group. A reduction of 5.18 on the DLQI was seen in the ivermectin group compared to a reduction of 3.92 in the metronidazole group but no SDs were provided. A DLQI score of 0 to 1 equates to no effect on HRQOL, a score of 2 to 5 a small effect, and a score of 6 to 10 represents a moderate effect. Although the minimal important difference (MID) for the DLQI is yet to be established for the different skin diseases there is general acceptance that this ranges between 2.5 and 5, and therefore the impact of both these treatments was a small improvement in HRQOL but the difference between the groups in terms of reduction of the DLQI scores was not clinically important (Basra 2008). At the end of the 16 weeks 339/478 in the ivermectin group compared to 310/484 in the metronidazole group reported that the disease had no deleterious effect on their quality of life (RR 1.11, 95% CI 1.01 to 1.21; P = 0.02; NNTB = 15, 95% CI 8 to 100), which was statistically significant in favour of ivermectin.
Participant‐assessed changes in rosacea severity at end of study
In the ivermectin group 409/478 participants rated their improvement as good or excellent compared to 362/484 in the metronidazole group (RR 1.14, 95% CI 1.07 to 1.22; P < 0.0001; NNTB = 10, 95% CI 7 to 17), which was a statistically significant difference and in concordance with the results on the number of participants that experienced no deleterious effect on their quality of life.
Proportion of participants who reported an adverse event throughout the study period
In the ivermectin group 9/478 participants experienced an adverse event compared to 4/484 in the metronidazole group (RR 2.28, 95% CI 0.71 to 7.35). The reactions were mild and consisted of skin irritation, dryness and hypersensitivity.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Based on an IGA scale 406/478 were clear or almost clear in the ivermectin group compared to 365/484 in the metronidazole group (RR 1.13, 95% CI 1.06 to 1.20; P = 0.0002; NNTB = 10, 95% CI 7 to 20), which was consistent with the assessments of the participants.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
The mean change from baseline in lesion count was ‐27.70 (SD 8.85) in the ivermectin group compared to ‐23.60 (SD 8.23), which were both clinically important reductions (MD ‐4.10, 95% CI ‐5.18 to ‐3.02; P < 0.00001).
Time needed until improvement of the skin lesions
This was not a predefined outcome but clear improvement could be seen for both treatment arms around six weeks.
Duration of remission
Not assessed.
(26) Erythromycin 2% gel versus metronidazole 0.75% gel
Only one study with a small sample size compared these two interventions (Verea Hernando 1992). A baseline imbalance in severity of the disease at enrolment placed the study at a serious risk of bias.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
There was no statistically significant difference between the two groups for this outcome: 16 of the 22 participants considered themselves improved with erythromycin gel versus 17 of 18 in the metronidazole gel group (RR 0.77, 95% CI 0.58 to 1.02).
Proportion of participants who reported an adverse event throughout the study period
These were inadequately reported in the study and therefore we have not included any of the data in this review.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Although this was a pre‐specified outcome it was not addressed (see 'Risk of bias' under Characteristics of included studies for this study).
Assessment of erythema or telangiectasia, or both, at end of study
Only one participant in the erythromycin group had an improvement in erythema compared with two in the metronidazole group.
Lesion counts
Baseline imbalance between the groups with respect to the number of papules and pustules was quite marked and placed the study at a serious risk of bias. The total number of papules in the erythromycin group was 571 at baseline, which reduced to 250 after three months, while the number at baseline in the metronidazole group was 476, which reduced to 317. The baseline number of pustules was 160 for the erythromycin group and reduced to 126 after three months, and for the metronidazole group the baseline number of pustules was 63 and the number at the end of the study was 33.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(27) Topical ciclosporin ophthalmic emulsion 0.05% versus artificial tears for the treatment of ocular rosacea
One study at unclear risk of bias examined this comparison (Schechter 2009), see Table 7.
Primary outcomes
Change in HRQOL at end of study
Assessment of changes in quality of life were carried out with the Ocular Surface Disease Index (OSDI) (scale 0 to 100, 100 = worst). Baseline scores were 19.1 (SD 13.9) in the topical ciclosporin group and 16.9 (SD 15.8) in the artificial tears group. The difference between the change scores at completion of the study was ‐8.6 in favour of topical ciclosporin (95% CI ‐15.42 to ‐1.78; P = 0.01), which equated to a moderate improvement in quality of life.
Participant‐assessed changes in rosacea severity at end of study
Not assessed.
Proportion of participants who reported an adverse event throughout the study period
Only one participant in the topical ciclosporin group (n = 21) reported an adverse event and 0/16 in the artificial tears group, which consisted of stinging (RR 2.32, 95% CI 0.10 to 53.42).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The data from these assessments provided evidence for the effectiveness of topical ciclosporin in the treatment of ocular rosacea. The Schirmer's test determines whether the eye produces enough tears to keep it moist. Paper strips are inserted into the eye for several minutes to measure the production of tears, and then the paper is removed and the amount of moisture measured. At baseline the mean Schirmer scores were 9.7 mm (SD 5.1) in the ciclosporin group compared with 10.2 mm (SD 5.8) in the artificial tears group. The difference in change scores between the groups at the end of the study was 4.1 (95% CI 1.66 to 6.54; P = 0.001), which points to a significant improvement in the ciclosporin group. Furthermore, the change score of 3.6 in the tear break‐up time (95% CI 2.59 to 4.61; P < 0.00001) provided an indication of the role played by topical ciclosporin in increasing tear production.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(28) Clindamycin phosphate 1.2% + tretinoin 0.025% gel versus placebo
The efficacy of this topical treatment was examined in one study at low risk of bias (Chang 2012), see Table 8.
Primary outcomes
Change in HRQOL at end of study
Quality of life was assessed with the disease‐specific RosaQoL. However, no means of scores were provided, only percentages of participants that had improved per item on the 21 survey items, making these data less usable. The investigators reported that there were no statistically significant differences for any item.
Participant‐assessed changes in rosacea severity at end of study
Not assessed.
Proportion of participants who reported an adverse event throughout the study period
Twenty‐nine adverse events were reported in 43 participants on the combination treatment of clindamycin and tretinoin, compared to 11 adverse events in 40 participants in the placebo group (RR 2.45, 95% CI 1.42 to 4.23; P = 0.001, NNTH = 3, 95% CI 2 to 5). Worsening of rosacea, facial scaling as well as dry skin were reported most often in the active treatment group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
None of the primary features of the Physician's Global Assessment as defined by Wilkin 2004 showed statistically significant differences between the treatment groups except for oedema in favour of placebo.
Assessment of erythema or telangiectasia, or both, at end of study
Regarding improvement of subtype only the erythematotelangiectatic subtype showed a statistically significant difference in favour of the combination treatment of clindamycin and tretinoin as 12/43 were improved compared to 4/40 in the placebo group (RR 2.79, 95% CI 0.98 to 7.95; P = 0.05; NNTB = 6, 95% CI 3 to 50). Erythema improved in 11/43 on the active treatment versus 6/40 on placebo (RR 1.71, 95% CI 0.70 to 4.18), and telangiectasia in 13/43 compared to 5/40 (RR 2.42, 95% CI 0.95 to 6.17).
Lesion counts
Both treatments had no or minimal effect on inflammatory lesions. The mean change from baseline in lesion count was 0.83 (SD 10.84) in the group treated with clindamycin and tretinoin and ‐3.13 (SD 13.28) in the group treated with placebo, with a MD of 3.96 (95% CI ‐1.28 to 9.20).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(29) Rosacea treatment system (RTS) (gentle cleanser, metronidazole 0.75% gel, hydrating complexion corrector and skin balancing sunscreen SPF 30) versus RTS without metronidazole
One three‐armed study with a small sample size (30 participants) assessed as at unclear risk of bias addressed this comparison (Leyden 2011).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Participant assessments were made with a 5‐point Likert scale (0 = none, 4 = severe). No SDs were provided and the investigator was unable to provide these. The mean score in the RTS + metronidazole group went from 2.6 to 2.0, whilst the group on RTS without metronidazole had a smaller reduction from 2.5 to 2.2.
In the RTS + metronidazole group 50% were very satisfied and 20% satisfied compared to 30% very satisfied and 40% satisfied in the comparator group.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in either group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
None had more than moderate improvement on a 7‐point Likert scale. In the RTS + metronidazole group 4/10 achieved moderate improvement versus 1/10 in the comparator group (RR 4.00, 95% CI 0.54 to 29.80).
Assessment of erythema or telangiectasia, or both, at end of study
Erythema decreased on a 5‐point Likert scale from 2.8 to 2.4 in the RTS + metronidazole group and from 2.5 to 2.3 in the RTS without metronidazole group. No SDs were provided.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(30) Rosacea treatment system (RTS) (gentle cleanser, metronidazole 0.75% gel, hydrating complexion corrector and skin balancing sunscreen SPF 30) versus metronidazole 0.75% and standard skin care regimen
This was the second comparison from the three‐armed study of Leyden 2011.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Assessments were made on a 5‐point Likert scale. In the RTS + metronidazole group the scores went from 2.6 to 2.0, and in the metronidazole group + standard skin care regimen the score remained unchanged at 2.0.
Percentages regarding satisfaction were for the RTS + metronidazole group 50% very satisfied and 20% satisfied, and for the metronidazole group + standard skin care regimen 78% was satisfied.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in the group on RTS + metronidazole (n = 10), and two participants reported adverse events in the group treated with metronidazole + standard skin care regimen (n = 10) (RR 0.20, 95% CI 0.01 to 3.70).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In the RTS + metronidazole group 4/10 showed moderate improvement versus 1/10 in the metronidazole + standard skin care regimen group (RR 4.00, 95% CI 0.54 to 29.80).
Assessment of erythema or telangiectasia, or both, at end of study
There was a decrease in erythema from 2.8 to 2.4 in the RTS + metronidazole group, it remained at 2.3 in the metronidazole + standard care regimen group.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(31) Rosacea treatment system (RTS) without metronidazole versus metronidazole 0.75% and standard skin care regimen
This was the third comparison in Leyden 2011.
Primary outcomes
Change in health‐related quality of life (HRQOL) at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
In the RTS group without metronidazole the score went from 2.5 to 2.2, and in the metronidazole group + standard skin care regimen it remained at 2.0.
In the RTS group without metronidazole 30% were very satisfied and 40% satisfied, whilst in the group on the metronidazole and standard skin care regimen 78% were satisfied.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in the RTS without metronidazole group (n = 10), and two participants reported adverse events in the group treated with the metronidazole + standard skin care regimen (n = 10) (RR 0.20, 95% CI 0.01 to 3.70).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Only 1/10 showed moderate improvement in both groups (RR 1.00, 95% CI 0.07 to 13.87).
Assessment of erythema or telangiectasia, or both, at end of study
There was a slight reduction from 2.5 to 2.3 in the RTS without metronidazole group and it remained at 2.3 in the metronidazole + standard skin care regimen group.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(32) 4‐Ethoxybenzaldehyde 1% versus placebo
The anti‐inflammatory effect of this intervention in reducing facial erythema was evaluated in only one study (Draelos 2005b), assessed as at high risk of bias. No SDs were reported.
Primary outcomes
Change in health‐related quality of life (HRQOL) at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
There were no adverse events reported in either group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Ten out of 20 participants in the active group had a marked improvement from baseline compared to 0/10 in the vehicle group (RR 11.00, 95% CI 0.71 to 170.64), which was not statistically significant.
Assessment of erythema or telangiectasia, or both, at end of study
In the active treatment group an improvement in erythema was seen in 43.7% in the active treatment group, and a 16.7% improvement in the vehicle group. No exact baseline values or study endpoint values were reported.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(33) Cream containing 1% extract of a flavonoid‐rich plant Chrysanthellum indicum versus placebo
One trial (Rigopoulos 2005) assessed as at high risk of bias reported data for this comparison.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
A larger number of participants in the active intervention than in the placebo cream group reported improvement in rosacea severity, 60 of 125 participants with the flavonoid cream and 36 of 121 in the placebo arm (RR 1.61, 95% CI 1.16 to 2.24; P = 0.004; NNTB = 6, 95% CI 4 to 17).
Proportion of participants who reported an adverse event throughout the study period
There was no statistically significant difference in the number of participants experiencing adverse events: 13 of the 125 in the flavonoid cream group experienced adverse events and eight out of 121 in the placebo group (RR 1.57, 95% CI 0.68 to 3.66).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Based on the final investigators' assessment 64 of 125 participants in the active treatment group showed improvement versus 52 out of 121 in the placebo group (RR 1.19, 95% CI 0.91 to 1.56). Clearing or marked improvement on the rosacea overall assessment (seven grade scale) was scored as 78/125 in the active treatment group compared to 61/121 on placebo (RR 1.24, 95% CI 0.99 to 1.55).
Assessment of erythema or telangiectasia, or both, at end of study
Reduction in erythema was 53.65% in the flavonoid rich cream group versus 44.23% for the placebo group.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(34) Praziquantel 3% ointment versus vehicle ointment
This comparison was evaluated in a single study at high risk of bias (Bribeche 2015).
Primary outcomes
Change in HRQOL at end of study
The DLQI decreased in the praziquantel group from 15.8 (very large effect on quality of life) to 4.6 (small effect on quality of life), which was a clinically important reduction. The reduction in the placebo group was smaller, from 14.6 to 7.9 (moderate effect on quality of life).
Participant‐assessed changes in rosacea severity at end of study
Not assessed.
Proportion of participants who reported an adverse event throughout the study period
In the praziquantel group 1/43 reported an adverse event versus 2/22 in the vehicle group (RR 0.26, 95% CI 0.02 to 2.67). Dryness was mild in intensity and resolved after using a moisturizer.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Based on the IGA (5‐point Likert scale) at baseline 39/43 had a score of mild to moderate in the praziquantel group and at the end of study 35/43 had a score of minimal or clear. For the vehicle group at baseline 21/22 scored mild to moderate and after 16 weeks 5/22 scored minimal or clear.
Assessment of erythema or telangiectasia, or both, at end of study
At the start of the study 38/43 had a score of moderate to significant erythema on the 5‐point CEA scale, and at the end of the study 38/43 had no or mild erythema. In the vehicle group 19/22 had moderate to significant erythema at the start of study and after 16 weeks 9/22 had no or mild erythema.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(35) BFH772 1% (betamethasone, calcipotriol) ointment versus metronidazole 1% cream
One small sample size (N = 36) three‐armed study of participants with erythema consistent with erythematotelangiectatic rosacea reported data for this comparison (NCT01449591). This study was assessed as at unclear risk of bias.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
The mean participant's assessment of flushing frequency was ‐0.2 in the BFH772 1% group compared to ‐0.3 in the metronidazole group.
The mean change in self assessments of erythema, after 12 weeks, in the BFH772 1% group showed no change: 0 (SD 0.7) compared to ‐0.5 (SD 0.8) in the metronidazole group with a MD of 0.5 (95% CI ‐0.10 to 1.10).
Proportion of participants who reported an adverse event throughout the study period
Six out of the 12 participants in the BFH722 1% group reported adverse events compared to 4/12 in the metronidazole group (RR 1.50, 95% CI 0.56 to 4.00). Most adverse events were not drug related (gastrointestinal disorders, psychiatric disorders etc).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The mean change in IGA of rosacea was ‐0.4 (SD 0.5) in the BFH722 1% group versus ‐0.5 (SD 0.5) in the metronidazole group (MD 0.10, 95% CI ‐0.30 to ‐0.50).
Assessment of erythema or telangiectasia, or both, at end of study
IGA of telangiectasia showed a change from baseline of ‐0.2 (SD 0.4) in the BFH722 1% group compared to 0.4 (SD 1.2) in the metronidazole group with a MD of ‐0.60 (95% CI ‐1.32 to 0.12).
Lesion counts
There was a small increase in mean number of lesion counts of 0.5 (SD 0.1) in the BFH722 1% group compared to a small decrease of 0.5 (SD 1.4) in the metronidazole group (MD 1.00, 95% CI 0.21 to 1.79; P = 0.01).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(36) BFH772 1% (betamethasone, calcipotriol) ointment versus vehicle ointment
This comparison was evaluated in NCT01449591. None of the data showed that BFH772 1% was any better than vehicle ointment.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
In the group treated with BFH722 1% ointment the mean participant‐assessed change in flushing frequency was ‐0.2 compared to ‐0.7 in the vehicle ointment group.
The change in facial redness was 0 (SD 0.7) for the BFH772 group compared to ‐0.5 (SD 0.9) in the vehicle ointment group (MD 0.50, 95% CI ‐0.15 to 1.15).
Proportion of participants who reported an adverse event throughout the study period
In the BFH722 1% group 6/12 reported an adverse event compared to 4/12 in the vehicle group (RR 1.50, 95% CI 0.56 to 4.00).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The reductions on the IGA scale were 0.4 (SD 0.5) for the BFH772 1% group and 0.5 (SD 0.7) for the vehicle ointment group (MD 0.10, 95% CI ‐0.39 to 0.59).
Assessment of erythema or telangiectasia, or both, at end of study
IGA of telangiectasia showed a change from baseline of ‐0.2 (SD 0.4) in the BFH722 1% group compared to 0.1 (SD 0.6) in the vehicle ointment group with a MD of ‐0.30 (95% CI ‐0.71 to 0.11).
Lesion counts
There were minimal changes in lesion counts: 0.5 (SD 1.0) in the BFH772 1% group and 0.1 (SD 0.4) in the vehicle ointment group (MD 0.40, 95% CI ‐0.21 to 1.01).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(37) TDT 068 gel versus vehicle gel
TDT 068, a topical drug‐free gel containing ultra‐deformable SequessomeTM vesicles, was evaluated in a single study (Luger 2015) assessed as at low risk of bias. None of the outcomes suggested that TDT 068 gel was more effective than vehicle gel.
Primary outcomes
Change in HRQOL at end of study
The RosaQoL (range 1 to 5) reduced by 0.08 (SD 0.38) in the TDT 068 gel group and by 0.08 (SD 0.37) in the vehicle group (MD 0.00, 95% CI ‐0.20 to 0.20).
Participant‐assessed changes in rosacea severity at end of study
Not assessed.
Proportion of participants who reported an adverse event throughout the study period
Adverse events were reported in 5/40 in the TDT 068 gel group versus 4/21 in the vehicle group and consisted mostly of skin irritation and pruritus (RR 0.66, 95% CI 0.20 to 2.19).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
This outcome was assessed with the Rosacea Standard Grading System (RSGS) (Wilkin 2004). Investigators stated that there was no statistically significant difference in reduction in the total score at the end of the four week study (investigators reported a difference at 4 weeks of 0.94 (SD 2.03), 95% CI –0.20 to 2.08; P value of 0.11).
Assessment of erythema or telangiectasia, or both, at end of study
Non‐transient erythema decreased from baseline by 0.34 (SD 0.63) in the TDT 068 gel group (n = 38) and 0.05 (SD 0.51) in the vehicle group (n = 20) (MD ‐0.29, 95% CI ‐0.59 to 0.01). Transient erythema reduced from baseline by 0.55 (SD 0.66) and 0.35 (SD 0.50) respectively (MD ‐0.20, 95% CI ‐0.50 to 0.10). Telangiectasia reduced by 0.26 (SD 0.55) and 0.15 (SD 0.50) respectively (MD ‐0.11, 95% CI ‐0.39 to 0.17). All of these differences were not statistically significant.
Lesion counts
No exact data were provided other than as a graphical representation, which suggested no change.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(38) Crotamiton versus benzyl benzoate
This comparison was examined in a single study that only addressed one of our outcomes, that is adverse events (Rodríguez 2003). The study was assessed as at unclear risk of bias.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
There were no adverse events in either group.
Secondary outcomes
None of our secondary outcomes were assessed.
(39) Skin care product containing ambophenol, neurosensine and La Roche‐Posay thermal spring water versus vehicle
This cosmetic was evaluated in one study that provided limited data (Seité 2013). The study was assessed as at unclear risk of bias.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Global efficacy of rosacea was assessed by the participants to be good or excellent in 10/32 in the test formula group compared to 5/34 in the vehicle group (RR 2.13, 95% CI 0.81 to 5.54).
Proportion of participants who reported an adverse event throughout the study period
Not assessed.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Rosacea improved or was cured in 20/32 in the test formula group compared to 11/34 in the vehicle group (RR 1.93, 95% CI 1.11 to 3.37; P = 0.02). A rating of global efficacy of good to excellent was seen in 10/32 in the test formula group compared to 1/34 in the vehicle group (RR 10.63, 95% CI 1.44 to 78.36; P = 0.02). Both these assessments were not in concordance with the participants' judgements, where no statistically significant difference was seen.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(40) SEI003 cream versus vehicle
One study with a small sample size (Two 2014), assessed as at high risk of bias, evaluated the efficacy of this topical serine protease inhibitor.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in either group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The IGA decreased by 1.00 (SD 0.57) in the SEI003 group (n = 11) and 0.7 (SD 0.5) in the vehicle group (n = 4), MD of 0.30, however these data were skewed, analysed inappropriately and have not been summarised or reported here.
Assessment of erythema or telangiectasia, or both, at end of study
The CEA reduced by 4.10 (SD 1.62) in the SEI003 group and by 3.5 (SD 1.43) in the vehicle group (MD ‐0.60). However, as with the IGA outcome, these data were skewed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(41) P‐3075 cream (based on hydroxypropyl chitosan and potassium azeloyl diglycinate) versus placebo cream
This comparison was evaluated in a single study at unclear risk of bias (Berardesca 2012) and which provided limited data, mainly on erythema.
Primary outcomes
None of our primary outcomes were assessed.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
No global efficacy assessment was done.
Assessment of erythema or telangiectasia, or both, at end of study
No exact data per group were provided on the data assessed with the Mexameter, but the authors stated that at the end of treatment "the composite erythema index representing the sum of the 4 site‐specific erythema indices showed a statistically significant decrease at day 28 of 167.00; P < 0.001)", in favour of P‐3075.
Based on a 4‐point Likert scale (0 = none, 3 = severe) the investigators concluded "that at day 28 in the P‐3075 group, the clinical assessment of erythema showed a statistically significant decrease (P = 0.005 in the chi‐square test and P = 0.011 in the Fisher exact test)". At day 28, 27/28 of the participants treated with P‐3075 had no erythema, 1/28 had mild erythema, and in the placebo group 9/14 had no erythema and 5 had mild erythema.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Systemic interventions: studies with oral antibiotics
(42) Tetracycline versus placebo
Two trials at unclear risk of bias were included (Marks 1971; Sneddon 1966), see Table 9.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Only one of the studies provided data for this outcome (Marks 1971). Based on these participant‐assessed outcomes there was insufficient evidence to demonstrate that tetracycline was more effective than placebo. In the tetracycline group 14/20 participants considered they were better to much better versus 9/19 in the placebo group (RR 1.48, 95% CI 0.85 to 2.57).
Proportion of participants who reported an adverse event throughout the study period
In Marks 1971 only one adverse event was reported in each group, diarrhoea in the tetracycline group and maculopapular erythema in the placebo group (RR 0.95, 95% CI 0.06 to 14.13). This outcome was not assessed in Sneddon 1966.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In contrast with the participant‐assessed changes these assessments indicated that tetracyclines appeared to be significantly more effective than placebo in the treatment of rosacea. In the Marks 1971 study 17 out of 20 participants in the tetracycline group were considered to be improved versus 4 of 19 in the placebo group (RR 4.04, 95% CI 1.66 to 9.83; P = 0.002; NNTB = 2, 95% CI 2 to 3). In Sneddon 1966 28 of 36 participants in the tetracycline group improved versus 19 of 42 in placebo (RR 1.72, 95% CI 1.18 to 2.50; P = 0.005; NNTB = 4, 95% CI 2 to 9).
Assessment of erythema or telangiectasia, or both, at end of study
This outcome was not assessed in Sneddon 1966, and there were no significant changes in erythema in Marks 1971.
Lesion counts
The mean reduction in number of lesions in Marks 1971 was 16.05 (SD 13.45) in the tetracycline group (n = 17) compared to 1.41 (SD 9.52) in the placebo group (n = 17) but these data were skewed (MD ‐14.64).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(43) Anti‐inflammatory dose doxycycline 40 mg versus placebo
Two studies assessed as at low risk of bias evaluated this comparison. Study duration in Del Rosso 2007a and Del Rosso 2007b was 16 weeks, but in Del Rosso 2007b the participants were re‐evaluated at 20 weeks, and therefore only the data from the 16 week assessment was analysed for this review. See Table 10.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
The number of participants reporting adverse events was 56/127 in the doxycycline group versus 48/124 in the placebo group (RR 1.14, 95% CI 0.85 to 1.53) in Del Rosso 2007a, and 93/142 versus 74/144 respectively in Del Rosso 2007b (RR 1.27, 95% CI 1.04 to 1.55; P = 0.02; NNTH = 8, 95% CI 4 to 34). The majority of these adverse events were considered to be mild or moderate in severity.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The data from the IGA in Del Rosso 2007a indicated that doxycycline 40 mg was more effective than placebo. Fifty‐eight participants out of the 127 in the doxycycline 40 mg group achieved a 2‐point or greater improvement in IGA score compared with 32 out of 124 in the placebo group (RR 1.77, 95% CI 1.24 to 2.52; P = 0.002; NNTB = 5, 95% CI 4 to 13). Thirty‐nine participants in the doxycycline group achieved an IGA score of 0 (clear) or 1 (near clear) versus 24 in the placebo group (RR 1.59, 95% CI 1.02 to 2.47; P = 0.04; NNTB = 10, 95% CI 5 to 100).
In Del Rosso 2007b there was no statistically significant difference in IGA: 32 participants of the 142 in the doxycycline 40 mg group had achieved a 2‐point or greater improvement in IGA score compared with 23 of 144 in the placebo group (RR 1.41, 95% CI 0.87 to 2.29). However, more than twice as many participants achieved an IGA score of 0 (clear) or 1 (near clear) in the doxycycline group: 21 participants in the doxycycline group achieved an IGA score of 0 or 1 versus 9 in the placebo group (RR 2.37, 95% CI 1.12 to 4.99; P = 0.02; NNTB = 12, 95% CI 7 to 100).
Assessment of erythema or telangiectasia, or both, at end of study
In Del Rosso 2007a the mean change in CEA scale (range 0 to 4, 0 = none and 4 is severe redness) was ‐2.7 in the doxycycline group versus ‐1.8 in the placebo group (investigators stated P = 0.017). In Del Rosso 2007b there was no statistically significant difference between the groups with a reduction of 1.4 in the doxycycline group and 1.2 in the placebo group.
Lesion counts
The mean reduction in lesion count in Del Rosso 2007a was 11.8 (SD 14.04) in the doxycycline group and 5.9 (SD 14.04) in the placebo group (MD ‐5.90, 95% CI ‐9.37 to ‐2.43; P = 0.0009). In Del Rosso 2007b these reductions were 9.50 (SD 13.23) and 4.3 (SD 13.23) for the doxycycline group and placebo group respectively (MD ‐5.20, 95% CI ‐8.27 to ‐2.13; P = 0.0009).
Time needed until improvement of the skin lesions
The data were presented in the reports as graph plots, which did not permit accurate data to be extracted. However, the steepest changes in the graph plots occurred within the first three weeks in the doxycycline group, which provided an indication of the time needed for improvement of inflammatory lesions relative to placebo.
Duration of remission
Not assessed.
(44) Anti‐inflammatory dose doxycycline 40 mg versus placebo as maintenance therapy during 40 weeks
This study at high risk of bias evaluated the efficacy in preventing relapse and safety of long‐term treatment with doxycycline after a 12 week treatment with doxycycline 40 mg and topical metronidazole. Only participants that achieved an IGA of clear or near clear entered the second, randomised phase (NCT01426269).
Primary outcomes
Change in HRQOL at end of study
The RosaQoL scores (1 to 5) were 3.3 for both groups at the end of the first open phase of the study and decreased to 2.8 in the participants that continued with doxycycline (n = 65), while the participants that switched to placebo (n = 65) had a smaller reduction to 3.1.
Participant‐assessed changes in rosacea severity at end of study
This outcome was assessed with a satisfaction questionnaire which reported the percentages of participants that were not bothered by or did not experience symptoms. The percentages for tightness of the skin were 64.6% for the doxycycline group and 60% for the placebo group, sensitivity of the skin 64.6% versus 55.4%, stinging and burning 66.1% versus 47.7%, roughness 55.4% versus 49.3%, and itchy skin sensation 58.4% versus 52.3%, indicating that higher percentages not experiencing symptoms were in the doxycycline group.
Proportion of participants who reported an adverse event throughout the study period
Adverse events were reported in 8/65 of the participants in the doxycycline group and 9/65 in the placebo group (RR 0.89, 95% CI 0.37 to 2.16).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
At the start of the randomised second phase all 65 participants in both groups had an IGA of clear or near clear. At the end of 40 weeks 41/65 were still clear to near clear in the doxycycline group, while in the placebo group this number had dropped to 33/65.
Assessment of erythema or telangiectasia, or both, at end of study
The mean score change in CEA score was ‐0.50 (SD 2.14) for the doxycycline group and 0.40 (SD 2.36) for the placebo group (MD ‐0.90, 95% CI ‐1.67 to ‐0.13; P = 0.02), which was a statistically significant difference in favour of doxycycline.
Lesion counts
The mean number of lesion counts increased by 0.90 (SD 1.61) in the doxycycline group and 0.30 (SD 1.20) in the placebo group (MD 0.60, 95% CI 0.11 to 1.09; P = 0.02), which was a statistically significant difference in favour of placebo; but such a small difference in lesion count was unlikely to be clinically important. However, it suggested that when treatment success had been achieved with doxycycline there was a prolonged, sustained and relevant effect which was shown to continue up to 40 weeks in the placebo group.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
During the 40 weeks, 9/65 relapsed in the doxycycline group compared to 18/65 in the placebo group (RR 0.50, 95% CI 0.24 to 1.03).
(45) Azithromycin versus doxycycline
Only one study assessed as at high risk of bias addressed this comparison (Akhyani 2008). See Table 11.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Although there was no measurable difference in change in severity between the two treatment groups, 29 out of 37 participants in the azithromycin group considered themselves improved versus 24 of 30 in the doxycycline group (RR 0.98, 95% CI 0.77 to 1.25).
Proportion of participants who reported an adverse event throughout the study period
Diarrhoea was reported in four of the 37 participants in the azithromycin group, and two out of 30 in the doxycycline group experienced epigastric burning (RR 1.62, 95% CI 0.32 to 8.26).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
At baseline these were 19.24 (SD 9.67) in the azithromycin group and 1.90 (SD 3.28) at 3 months, and similarly in the doxycycline group 18.86 (8.95) and 2.34 (3.47) at three months. However, in addition to having large SDs these data were skewed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
No data were available for the duration of remission, but both groups showed no statistically significant change between the third month of treatment and the second month post‐treatment in the mean inflammatory lesion counts.
(46) Ampicillin versus placebo
One study at unclear risk of bias provided data for this comparison (Marks 1971). The dosage of ampicillin was not reported.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
These assessments demonstrated significant improvements, which were in favour of ampicillin over placebo, such that 14 out of 17 participants treated with ampicillin versus 9/19 in the placebo group (RR 1.74, 95% CI 1.03 to 2.93; P = 0.04; NNTB = 3, 95% CI 2 to 17) considered themselves improved.
Proportion of participants who reported an adverse event throughout the study period
Three of the 17 participants treated with ampicillin reported adverse events versus 1/19 in the placebo group (RR 3.35, 95% CI 0.38 to 29.26). The adverse events were mild and transient, and one participant in the ampicillin group experienced diarrhoea.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
These were generally in line with the participant‐assessed changes but there was no statistically significant difference between the groups. Nine of 17 participants treated with ampicillin reported improvement compared with 4/19 in the placebo group (RR 2.51, 95% CI 0.94 to 6.70).
Assessment of erythema or telangiectasia, or both, at end of study
There were no significant changes in erythema.
Lesion counts
The mean change from baseline was ‐11.2 (SD 19.23) in the ampicillin group (n = 15) and 1.41 (SD 9.52) in the placebo group, but these had large SDs and skewed data (MD ‐9.79, SD 14.86; P = 0823).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(47) Oral tetracycline versus ampicillin
Only one study at unclear risk of bias provided data for this comparison (Marks 1971).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
These assessments did not indicate any difference in efficacy between the two interventions: 14 of 20 participants treated with tetracycline considered themselves improved versus 14 of 17 in the ampicillin group (RR 0.85, 95% CI 0.59 to 1.22).
Proportion of participants who reported an adverse event throughout the study period
Most side effects were mild and transient, 3/17 participants in the ampicillin group reported adverse events compared with 1/20 in the tetracycline group (RR 0.28, 95% CI 0.03 to 2.48).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
These were in line with the participant‐assessed changes, 17 of 20 in the tetracycline group reported they had improved versus 9/17 in the ampicillin group (RR 1.61, 95% CI 0.99 to 2.61).
Assessment of erythema or telangiectasia, or both, at end of study
There were no significant changes in erythema.
Lesion counts
The mean change from baseline was ‐16.45 (SD 8.83) in the tetracycline group and ‐11.53 (SD 15.96) in the placebo group, but these had large SDs and skewed data (MD ‐4.86 (SD 16.4); P = 0.4249).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(48) Oral oxytetracycline versus oral metronidazole
Only one study assessed as at unclear risk of bias provided data for this comparison (Saihan 1980).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
These were combined with the physician assessments and reported as unified scores.
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in either group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The combined scores of the participants and physicians demonstrated that there was no statistically significant difference between the two groups in rosacea severity at the completion of the study. The mean severity scores (scale ‐1 to 3, with 3 = much improved) were 2.60 (SD 0.70) in the tetracycline group (n = 20) versus 2.30 (SD 1.00) in the metronidazole group (n = 18) with a MD of 0.30 (95% CI ‐0.25 to 0.85; P = 0.29).
Assessment of erythema or telangiectasia or both at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(49) Clarithromycin and omeprazole versus placebo in Helicobacter pylori positive patients with rosacea
The data from the single study at unclear risk of bias evaluating these interventions were skewed, had large SDs and were considered to be unusable (Bamford 1999). There were 22 participants in the clarithromycin and omeprazole group and 22 in the placebo group.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
One participant in the treatment group reported headaches during treatment, but no adverse events were reported in the placebo group (RR 3.00, 95% CI 0.13 to 69.87).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The mean change in total rosacea severity score was ‐4.50 (SD 2.12) in the active treatment group compared to ‐3.20 (SD 2.95) in the placebo group, but these data were very skewed. It should be noted that 25% of the participants in the active treatment group were still positive for Helicobacter pylori despite having reported completing the antibiotic therapy.
Assessment of erythema or telangiectasia, or both, at end of study
The mean reductions in erythema intensity were 2.00 (SD 1.55) in the active treatment group and 1.80 (SD 1.71) in the placebo group.
Lesion counts
The mean reduction in pustule count was 15.30 (SD 9.56) in the active treatment group and 9.30 (SD 12.03) in the placebo group, and the data were very skewed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Systemic interventions: studies with oral antibiotics combined with topical treatments
(50) Anti‐inflammatory dose doxycycline 40 mg and metronidazole gel 1% versus doxycycline 100 mg and metronidazole gel 1%
Only one study assessed as at unclear risk of bias evaluated these interventions (Del Rosso 2008). SDs were missing, some of which we were able to calculate. See Table 12.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Four times as many adverse events were reported in the higher dose group compared with the 40 mg dose group. Six of the 44 participants treated with the anti‐inflammatory dose of 40 mg had adverse events versus 26 of 47 participants in the 100 mg group (RR 0.25, 95% CI 0.11 to 0.54; P = 0.0005; NNTH = 3, 95% CI 2 to 5). The majority of these adverse events were gastrointestinal complaints.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The mean reduction in IGA was 1.6 (SD 0.27) in the 40 mg doxycycline group (n = 44) and also in the 100 mg doxycycline group (n = 47) (MD 0.00, 95% CI ‐0.11 to 0.11).
Assessment of erythema or telangiectasia, or both, at end of study
Change in CEA from baseline (0 to 4, 0 = no redness present, 4 = severe redness) was ‐4.2 for the 40 mg group and ‐4.0 for the 100 mg group (investigators stated P = 0.50).
Lesion counts
The mean change from baseline in lesion count was ‐12.5 (SD 6.64) for the 40 mg group versus ‐12.2 (SD 6.64) for the 100 mg group (MD ‐0.30, 95% CI ‐3.03 to 2.43).
Time needed until improvement of the skin lesions
Although this was not a pre‐specified outcome a clear improvement was seen from week four in both groups.
Duration of remission
Not assessed.
(51) Anti‐inflammatory dose doxycycline 40 mg and azelaic acid 15% gel versus anti‐inflammatory dose doxycycline 40 mg and metronidazole gel 1%
These treatments were evaluated in one study at unclear risk of bias (Del Rosso 2010). See Table 13.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Excellent improvement was reported by 52/106 of the participants in the doxycycline + azelaic acid group compared to 47/101 in the doxycycline + metronidazole group (RR 1.05, 95% CI 0.79 to 1.40). The improvement score (1 = excellent, 4 = worse) was 1.6 in the doxycycline + azelaic acid group and 1.7 in the comparator group.
Proportion of participants who reported an adverse event throughout the study period
Very few participants reported adverse events: 2/106 in the doxycycline + azelaic acid group and 7/101 in the doxycycline + metronidazole group (RR 0.27, 95% CI 0.06 to 1.28).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Treatment responses based on an IGA score of 0, 1 or 2 (clear, minimal or mild) were seen in 83/106 in the doxycycline + azelaic acid group and in 73/101 in the doxycycline + metronidazole group (RR 1.08, 95% CI 0.93 to 1.27). Assessments of treatment success (IGA 0 or 1) were 66/106 versus 53/101 participants respectively (RR 1.19, 95% CI 0.94 to 1.50). Investigators' overall rating of improvement was 1.8 for both groups.
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
Mean reductions in lesion counts were 10.5 (SD 9.14) for the doxycycline + azelaic acid group and 9.4 (SD 9.38) in the comparator group (MD ‐1.10, 95% CI ‐4.91 to 2.71).
Time needed until improvement of the skin lesions
Although this was not a pre‐specified outcome, improvement could be seen for both treatment arms after four weeks.
Duration of remission
Not assessed.
(52) Anti‐inflammatory dose doxycycline 40 mg combined with topical metronidazole 1% gel twice daily versus placebo capsules combined with topical metronidazole 1% gel twice daily
A single study assessed as at unclear risk of bias was included but provided very limited outcome data for this comparison, and SDs were missing (Fowler 2007). After week 12 metronidazole was discontinued, therefore we have reported data at 12 weeks.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Participants in the doxycycline with metronidazole group reported 39 adverse events compared with 23 in the placebo with metronidazole group, however the report was unclear on how many of the participants experienced these adverse events.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The IGA score reduced by 1.3 in the doxycycline + metronidazole group (n = 30) compared to 0.8 in the placebo + metronidazole group (n = 32) (investigators reported P = 0.01).
Assessment of erythema or telangiectasia, or both, at end of study
The mean reduction in erythema in the doxycycline + metronidazole group was 0.91 and in the comparator group 0.66 (investigators reported P = 0.01).
Lesion counts
At 12 weeks the mean reduction in inflammatory lesion counts was 13.86 in the doxycycline + metronidazole group and 8.47 in the comparator group (investigators reported P = 0.002).
Time needed until improvement of the skin lesions
Improvement in the lesion counts was seen within four weeks in the doxycycline + metronidazole group as compared to within eight weeks for the placebo + metronidazole group.
Duration of remission
Not assessed.
(53) Combined effect of anti‐inflammatory dose doxycycline with metronidazole gel versus metronidazole gel alone
Only one study (Sanchez 2005) at unclear risk of bias provided data for this comparison.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Thirty‐three adverse events were reported: 14 in the doxycycline plus metronidazole group versus 19 in the metronidazole gel alone group, but it was unclear in how many participants these occurred.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Numeric data were not provided and both of these outcome measures had to be estimated from figures in the report, and SDs were calculated. The mean change from baseline in Global Severity score was ‐1.43 (SD 1.6) for the doxycycline combined with metronidazole group (n = 20) compared to ‐0.42 (SD 1.6) for the metronidazole gel only group (n = 20) (MD ‐1.01, 95% CI ‐2.00 to ‐0.02; P = 0.05).
Assessment of erythema or telangiectasia, or both, at end of study
Mean changes from baseline also had to be estimated from figures, but the investigators reported that they "failed to demonstrate a change in Clinician's Global erythema scale due to disparity in location number of affected facial sites".
Lesion counts
Changes from baseline in lesion counts at week 12 were ‐15.6 (SD 9.5) for the doxycycline with metronidazole group and ‐7.9 (SD 9.5) for the metronidazole gel only group with a MD of ‐7.70 (95% CI ‐13.59 to ‐1.81; P = 0.01), which was a clinically important difference.
Time needed until improvement of the skin lesions
Improvements were seen between four and eight weeks.
Duration of remission
Not assessed.
(54) Minocycline 45 mg versus minocycline 45 mg and topical azelaic acid 15% gel
This comparison was evaluated in a single study assessed as at unclear risk of bias (Jackson 2013). There was no statistically significant difference for any outcome between the treatment arms. See Table 14.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Although only two adverse events were related to the study medication (upset stomach and urticaria), 11/30 in the minocycline only group reported an adverse event compared to 16/30 in the combined treatment group (RR 0.69, 95% CI 0.39 to 1.22).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The mean change from baseline in the IGA was ‐2.00 (SD 0.63) for both groups (MD 0.00, 95% CI ‐0.32 to 0.32).
Assessment of erythema or telangiectasia, or both, at end of study
Erythema was evaluated with the CEA scale and scored ‐3.00 (SD 2.68) in the minocycline only group compared to ‐4.00 (SD 1.90) in the minocycline with azelaic acid group (MD 1.00, 95% CI ‐0.18 to 2.18).
Lesion counts
In both groups there was a clinically important reduction in lesion counts of 11.00 (SD 4.49) in the minocycline group and 12.00 (SD 3.00) in the comparator group (MD 1.00, 95% CI ‐0.93 to 2.93).
Time needed until improvement of the skin lesions
Although this was not a pre‐specified outcome, improvement was seen in both arms at four weeks.
Duration of remission
Not assessed.
(55) Oral metronidazole and topical hydrocortisone 1% cream versus oral placebo and topical hydrocortisone 1% cream
Only one study (Pye 1976) assessed as at unclear risk of bias provided outcome data for these interventions.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Adverse events were confined to two participants in the metronidazole plus hydrocortisone group and one participant in the placebo group (RR 1.87, 95% CI 0.19 to 18.38).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Although the study was inadequately reported, the data available for this outcome indicated that oral metronidazole appeared to be almost four times more effective than placebo. Ten of the 15 participants treated with oral metronidazole plus hydrocortisone showed an improvement in severity scores compared with only two of the 14 participants in the placebo plus hydrocortisone group (RR 4.64, 95% CI 1.23 to 17.68; P = 0.02; NNTB = 2, 95% CI 2 to 5).
Assessment of erythema or telangiectasia, or both, at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Systemic interventions: studies with oral antibiotics compared with topical antibiotics
(56) Topical metronidazole versus oral (oxy)tetracycline
Four studies at unclear risk of bias were included in this comparison. Nielsen 1983b investigated the effects of metronidazole 1% cream versus oral oxytetracycline for the treatment of rosacea. The other two studies, Veien 1986 and Schachter 1991, utilised tetracycline instead of oxytetracycline. In Monk 1991 metronidazole gel 0.75% was compared with oxytetracycline.
Although the quality of reporting of these studies was generally poor, they indicated that there was no statistically significant difference in effectiveness between metronidazole cream and oxytetracycline. The Schachter 1991 study was assessed as being at high risk of bias and provided only very limited data. See Table 15.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Based on these assessments no statistically significant difference in efficacy could be demonstrated. In Monk 1991 eight of the 16 participants in the topical metronidazole group considered themselves improved versus 12 of 17 in the oxytetracycline group (RR 0.71, 95% CI 0.40 to 1.26). Correspondingly, in Nielsen 1983b there was no statistically significant difference in the assessments between the interventions in that 22/25 participants in the metronidazole group considered themselves improved versus 21/23 in the oxytetracycline group (RR 0.96, 95% CI 0.80 to 1.17). In Schachter 1991 no exact data were provided other than that "both groups considered their condition much improved".
Proportion of participants who reported an adverse event throughout the study period
No adverse events were reported in Nielsen 1983b. In both groups in Monk 1991 two participants reported flaking of the skin and two participants experienced gastrointestinal problems (RR 1.06, 95% CI 0.32 to 3.55). It was unclear how many participants were randomised in Schachter 1991 to each group, but 12 participants reported an adverse event in the metronidazole group and nine in the tetracycline group. In Veien 1986 7/38 participants in the metronidazole group (skin irritation (4), skin dryness (1), stinging (2)) and 10/38 in the tetracycline group (skin irritation (4), skin dryness (4), stinging (2)) reported adverse events (RR 0.70, 95% CI 0.30 to 1.65).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
These were in agreement with the participants' assessments and showed no statistically significant difference between the two interventions. In Monk 1991 9 out of 16 in the metronidazole group were improved versus 12 of 17 in the oxytetracycline group (RR 0.80, 95% CI 0.47 to 1.35); and 24 of 25 versus 22 of 23 respectively in Nielsen 1983b (RR 1.00, 95% CI 0.89 to 1.13). No baseline data were provided in Schachter 1991, making the data unusable.
Assessment of erythema or telangiectasia, or both, at end of study
In Monk 1991 all participants (16) showed improvement in erythema in the metronidazole group compared to 9/17 in the oxytetracycline group. The mean erythema score reduced from 2.5 to 1.1 in the metronidazole group and from 2.4 to 1.1 in the oxytetracycline group. No exact data were provided in Nielsen 1983b but it was stated that "the reduction of erythema was the same in both groups, and the number and extent of telangiectases were unchanged". In Schachter 1991 no differences in erythema nor telangiectasia were seen in either group. In Veien 1986 the percentages of no improvement of erythema after 8 weeks were 11.1% in the metronidazole group versus 12.5% in the tetracycline group.
Lesion counts
In Monk 1991 at baseline the metronidazole group had a mean papule and pustule count of 25 (mean grade 3.7) versus a mean count of 20 (mean grade 2.9) in the oxytetracycline group. By week nine both treatment groups had shown a reduction of more than 50%, with 100% clearing in 75% versus 66% respectively; while the mean papule and pustule grade had fallen to 1.3 versus 1.1. In Nielsen 1983b the investigators stated that "the reduction of papules and pustules was the same in both groups". In Schachter 1991 a decrease of 68% in papule count was seen in the metronidazole group and of 77% in the tetracycline group; for pustules the percentage decrease was 53% and 61% respectively. In Veien 1986 only medians were provided, and at week eight the median for inflammatory lesions was 11.1 for metronidazole versus 0 in the tetracycline group.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Studies with other systemic treatments
(57) Zinc sulphate versus placebo
Two studies provided data for this comparison (Bamford 2012; Sharquie 2006). No SDs or exact data were reported in follow‐up assessments in the study of Sharquie 2006 (study assessed as at high risk of bias). In Bamford 2012 (assessed as at unclear risk of bias) there were no statistically significant differences for any outcome between the groups, whilst in Sharquie 2006 the authors reported that zinc sulphate was effective for rosacea.
Primary outcomes
Change in HRQOL at end of study
This was assessed with the RosaQoL (rated 1 to 5) in Bamford 2012. The baseline value for the group treated with zinc sulphate (n = 22) was 3.10 (95% CI 2.88 to 3.50) and for the placebo group (n = 22) 3.29 (95% CI 3.06 to 3.53). At the end of three months the score reduced to 2.90 (95% CI 2.67 to 3.12) in the zinc sulphate group and to 2.99 (95% CI 2.73 to 3.26) in the placebo group. The adjusted MD between the groups was 0.07 (95% CI ‐0.14 to 0.27; P = 0.53).
Participant‐assessed changes in rosacea severity at end of study
Not assessed.
Proportion of participants who reported an adverse event throughout the study period
The number of participants experiencing adverse events in Bamford 2012 was 17/27 in the group treated with zinc sulphate and 14/26 in the placebo group (RR 1.17, 95% CI 0.74 to 1.85). In Sharquie 2006 the number of participants who reported an adverse event in the zinc sulphate group was 3/13 compared to 0/12 for the placebo group (RR 6.50, 95% CI 0.37 to 114.12). Most of the adverse events reported in the zinc sulphate group were gastric upset, nausea, discomfort and diarrhoea.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
In Bamford 2012 this was assessed with the Standard Grading System for Rosacea, with a total severity score ranging from 0 to 12. At baseline the score in the zinc sulphate group was 6.32 (95% CI 5.76 to 6.87), and reduced to 5.09 (95% CI 4.18 to 6.00). For the placebo group the baseline score was 6.91 (95% CI 6.31 to 7.50), and at 3 months 4.06 (95% CI 4.07 to 5.65) with an adjusted MD between the groups of 0.57 (95% CI 0.47 to 1.62; P = 0.28).
In Sharquie 2006 the Physician Global Evaluation score in the zinc sulphate group decreased from 8 (SD 2.0) at baseline to 1.6 (no SD provided) and in the placebo group an increase from 7 (SD 1.3) at baseline to 7.6 (no SD provided) was reported. Although no details were provided the investigators reported that for the nine participants with ocular rosacea "all eye involvement disappeared after 3 months' treatment with zinc sulphate".
Assessment of erythema or telangiectasia, or both, at end of study
This was not assessed in Bamford 2012. In Sharquie 2006 the authors reported an improvement in the zinc sulphate group but no exact data were provided.
Lesion counts
This outcome was not assessed in Bamford 2012. The numbers of papules and pustules were not reported in Sharquie 2006 and although the investigators reported improvements in the zinc sulphate group this was not supported by the data in the figures.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(58) Isotretinoin versus doxycycline
One study at low risk of bias assessed this comparison (Gollnick 2010), see Table 16. This study consisted of two time periods, the first was a dose finding study for isotretinoin, and the second phase compared 0.3 mg isotretinoin with doxycycline 100 mg 14 days and then tapered to 50 mg per day. We have noted and reported that there was inconsistency in the denominators used by the investigators in the per‐protocol analyses for the different outcomes. See 'Characteristics of included studies' and 'Table 21'.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Data were presented, as reported, in a per‐protocol analysis. In the group treated with isotretinoin 0.3 mg/kg daily 102/129 participants considered themselves to have achieved a good to excellent improvement compared to 85/132 in the doxycycline group (RR 1.23, 95% CI 1.05 to 1.43; P = 0.009; NNTB 7, 95% CI 4 to 25), which was a statistically significant difference in favour of isotretinoin.
Proportion of participants who reported an adverse event throughout the study period
In the isotretinoin group 30/147 participants reported adverse events compared to 26/152 in the doxycycline group (RR 1.19, 95% CI 0.74 to 1.92). There were more gastrointestinal and respiratory complaints reported in the doxycycline group; and cheilitis, dry mouth and lips were more frequent occurrences in the isotretinoin group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Data were presented based on a per‐protocol analysis. A complete remission or marked improvement was observed in 105/129 participants in the isotretinoin group compared to 91/132 in the doxycycline group (RR 1.18, 95% CI 1.03 to 1.36; P = 0.02; NNTB = 9, 95% CI 5 to 50), which was in concordance with the participant‐assessed changes.
Assessment of erythema or telangiectasia, or both, at end of study
Erythema was improved or "healed" in 105/142 participants in the isotretinoin group compared to 112/143 in the doxycycline group (RR 0.94, 95% CI 0.83 to 1.08).
Telangiectasia improved or were "healed" in 56/142 of the participants isotretinoin group versus 55/143 in the doxycycline group (RR 1.03, 95% CI 0.77 to 1.37).
Lesion counts
There was an overall reduction of 16 lesions in the isotretinoin group compared to a reduction of 13 in the doxycycline group.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(59) Oral ivermectin with oral metronidazole versus oral ivermectin
These treatments were assessed in Salem 2013 but the report only provided limited data and was assessed as at high risk of bias.
Primary outcomes
None of our primary outcomes were assessed.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
A marked improvement or complete remission was seen in 47/60 participants in the oral ivermectin only group compared to 59/60 in the combined treatment group (RR 0.80, 95% CI 0.69 to 0.91; P = 0.001; NNTB = 5, 95% CI 4 to 12), an effect which was statistically significant in favour of the combined treatment. Although no details were provided regarding signs and symptoms of ocular rosacea, the investigators reported that "combined therapy was superior in decreasing the D. folliculorum count in all groups and in reducing the mite count to the normal level in rosacea and in anterior blepharitis".
Assessment of erythema or telangiectasia or both at end of study
Not assessed.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(60) Rilmenidine versus placebo
Only one study assessed as at unclear risk of bias examined this comparison (Grosshans 1997).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Six out of 15 participants in the rilmenidine group considered their rosacea improved compared with 6/19 in the placebo group (RR 1.27, 95% CI 0.51 to 3.14). Based on these data rilmenidine appeared to be of limited effectiveness when compared to placebo.
Proportion of participants who reported an adverse event throughout the study period
Although only mild adverse events were reported, there was no statistically significant difference in the number of participants experiencing adverse events, that is 8/15 (rilmenidine) versus 8/19 (placebo) (RR 1.27, 95% CI 0.62 to 2.57).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
The physicians' assessments indicated that 5/15 participants in the rilmenidine group versus 1/19 in the placebo group showed improvement (RR 6.33, 95% CI 0.83 to 48.59), which was in line with the participants' assessments that rilmenidine was not considered to be effective. There was a tendency towards fewer flushing episodes in the rilmenidine group. The mean decrease in number of flushes was 13 versus 5 (rilmenidine and placebo respectively). No SDs were reported in this study.
Assessment of erythema or telangiectasia, or both, at end of study
There was no apparent difference in facial redness between the groups but no exact data were reported.
Lesion counts
The number of participants with at least a 50% reduction in lesion count was 10/15 in the rilmenidine group versus 11/19 with placebo (decrease in lesion count 1 versus 2 and no SDs were provided).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(61) Dark sulphonated shale oil versus placebo
One study at unclear risk of bias evaluated the effectiveness of this intervention but it was only available as an abstract, which provided very limited usable data (Koch 1999).
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
No side events were reported in any group.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
No data were provided but the authors reported that there was a statistically significant difference in favour of dark sulphonated oil.
Assessment of erythema or telangiectasia, or both, at end of study
It was reported by the investigators that there was a statistically significant difference in reduction of erythema in favour of the active treatment group.
Lesion counts
Lesion counts reduced from 15.9 to 4.3 in the treatment group and 16.1 to 14.1 in the placebo group (investigators reported P < 0.0001).
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Other interventions: studies with laser or light‐based treatment
(62) Dual wavelength laser system (595 + 1064 nm) versus 595 nm pulsed dye laser (PDL) or Nd:YAG laser
One study (Karsai 2008) assessed as at unclear risk of bias evaluated the efficacy of these treatments for telangiectasia on the nose. Dual wavelength laser was allocated to one side of the nose, and PDL or Nd:YAG on the other side. As only limited data were available we have not reported the data for these three treatments based on the individual comparisons.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
Adverse events included transient purpura and immediate post‐treatment erythema. The investigators stated "there was no significant between‐group difference in the incidence of treatment related adverse effects".
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
Dual wavelength laser resulted in an improvement in 18 of the 20 sides of the nose versus two of the 10 sides treated with PDL and two of the 10 sides treated with Nd:YAG, an RR of 4.5 in favour of the dual wavelength treatment over both single wavelength therapies.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(63) Pulsed dye laser (PDL) versus Nd:YAG laser
In contrast with the previous comparison, in this study assessed at unclear risk of bias the cheek on one side of the face was treated with PDL and the other side with Nd:YAG (Alam 2013). See Table 17.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
According to the participants, redness improved by a mean of 52% on the PDL treated site and 34% on the Nd:YAG treated site with a MD of ‐16.33 (95% CI ‐34.6 to ‐1.94; P = 0.03).
Proportion of participants who reported an adverse event throughout the study period
Two participants experienced post‐treatment swelling and dropped out of the trial. A VAS was used to assess pain, and a score of 3.87 was recorded on the PDL treated side and 3.07 on the Nd:YAG side, which according to the investigators was statistically significant in favour of Nd:YAG (P = 0.0028).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
Erythema was assessed with a spectrophotometer and there was a reduction of 8.9% on the PDL treated side compared to a lower reduction of 2.5% on the Nd:YAG treated side, with a MD of ‐6.4 (95% CI ‐11.6 to ‐1.2; P = 0.02).
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(64) Pulsed dye laser (PDL) versus intense pulsed light therapy (IPL) versus no treatment
Very limited and largely unusable data were reported in this single within‐participant study which addressed these interventions (Neuhaus 2009). The investigators concluded that both PDL and IPL were equally effective for erythematotelangiectatic rosacea. The study was assessed as at high risk of bias.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
The efficacy of treatment and improvement in symptoms was assessed on a VAS. The participants rated a reduction of 3.2 for erythema on the side treated with PDL, and a reduction of 3.6 on the IPL treated side. The investigators reported that this was statistically significant compared to no treatment (P < 0.05), however no data were provided for the untreated group. They also concluded that there was no statistically significant difference between PDL and IPL.
Proportion of participants who reported an adverse event throughout the study period
Not assessed.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
No statistically significant reduction in erythema, compared to no treatment, was seen in the spectrophotometer assessments for PDL and IPL, except for IPL on the cheek (investigators reported P = 0.04).
The investigators also graded telangiectasia and erythema on a 4‐point Likert scale and, although they did not provide specific data, stated that compared to the untreated control there were statistically significant differences in favour of PDL and IPL of the overall telangiectasia score and erythema score (P < 0.01), but not between PDL and IPL.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(65) Pulsed dye laser (PDL) versus intense pulsed light (IPL) therapy
Whilst in the previous comparison the emphasis was on comparing PDL or IPL to no treatment, in this within‐participant study with 40 participants these treatments were compared against each other (Nymann 2010). See also Table 18. The study was assessed as at high risk of bias.
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Satisfaction with treatment was scored on a VAS with 0 being a poor and 10 an excellent result. The median score (with 10% and 90% percentiles) at end of treatment was 8 (2. 10) for PDL treatment and 7 (2, 10) for the IPL treated side (investigators reported P = 0.05).
Proportion of participants who reported an adverse event throughout the study period
Pain was also assessed on a VAS with 0 being no pain and 10 worst imaginable pain. The median scores and their 10% and 90% percentiles were 4 (2, 6) for PDL and 7 (2, 10), indicating that PDL was less painful (investigators reported P < 0.001).
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
For the PDL treated side 18 had an excellent response (75% to 100% vessel clearance) and 12 a good response (50% to 74% clearance), and for the IPL treated side 11 had an excellent response and 19 a good response.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Other treatments or treatment combinations
(66) Pulsed dye laser (PDL) combined with tacrolimus ointment versus tacrolimus ointment
These treatment modalities were evaluated in one study (Huang 2012) assessed as at high risk of bias.
Primary outcomes
Change in HRQOL at end of study
Participant‐assessed changes in rosacea severity at end of study
Neither of the above outcomes were assessed.
Proportion of participants who reported an adverse event throughout the study period
The data for this outcome were inadequately reported. Four participants reported local reactions to tacrolimus, and in the combined treatment group erythema and purpura, which are well known side effects of PDL therapy.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Treatment was rated to be very effective (effective rate 60% to 89%) or cured (effective rate ≥ 90%) in 24/30 participants treated with PDL combined with tacrolimus and in 18/30 of the participants treated with tacrolimus only (RR 1.33, 95% CI 0.95 to 1.88).
Assessment of erythema or telangiectasia or both at end of study
Not assessed separately.
Lesion counts
Not assessed separately.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
(67) Pulsed dye laser (PDL) combined with pretreatment of niacin cream versus PDL
A single within‐participant study assessed as at high risk of bias provided data for this comparison (Kim 2011).
Primary outcomes
Change in HRQOL at end of study
Not assessed.
Participant‐assessed changes in rosacea severity at end of study
Satisfaction with treatment was scored on a VAS (10 highest satisfaction). At the end of treatment the VAS score was 5.06 (SD 2.73) for the combined treatment on the halves of 18 faces compared to 3.67 (SD 2.06) on the PDL only treated other halves of 18 faces. The data were aggregated and analysed as PDL with combined treatment versus PDL alone, but because no adjustments were made to account for the within‐participant variation we have only presented the summary statistics.
Proportion of participants who reported an adverse event throughout the study period
All participants experienced transient erythema and oedema after exposure to laser, but without scarring, infections, crusting or hyperpigmentation in the treated areas.
Secondary outcomes
Physician‐assessed changes in rosacea severity at end of study
Not assessed.
Assessment of erythema or telangiectasia, or both, at end of study
Objective assessments of erythema were made using polarization colour imaging, rated on an erythema scale (100 to 1000), in addition to subjective improvement of erythema which was assessed on a 4‐point Likert scale.
The reduction on the objective erythema scale was 29.2 for the PDL + niacin cream and 18.4 for the PDL only group, which were both clinically important; but according to the investigators the difference was not statistically significant. In the subjective assessments where improvement was scored on a Likert scale from 0 to 3 (with 3 being an excellent improvement) 76% to 100% showed a score of 1.65 (SD 1.01) for the combined treatment group versus 0.87 (SD 0.76) for the PDL only group.
On the combined treatment side, 10 sides showed an improvement of more than 50% and three showed a > 75% improvement whilst on the side treated with only PDL just three showed an improvement of more than 50% and none an improvement of more than 75%.
Lesion counts
Not assessed.
Time needed until improvement of the skin lesions
Not assessed.
Duration of remission
Not assessed.
Discussion
Summary of main results
One hundred and six studies were included in the updated version of this review. In this update there was a small increase in the number of studies which reported assessments of our first primary outcome 'change in quality of life', but there was little change in the number of studies (approximately half) which addressed participants' assessments of improvement in rosacea severity, which was one of the other primary outcomes in this review. Adverse events were reported in most of the studies, although the data were often very limited and frequently incomplete.
The majority of studies focused on papule and pustule counts which, although they may provide a quantifiable, objective and more readily intelligible outcome, are generally considered to be clinician‐centred rather than patient‐preferred. Rosacea is a chronic skin disease and the importance of self‐assessments by the participants of the effectiveness of the interventions should not be underestimated. Slightly more than half of the studies evaluated erythema, using a variety of scales, principally Likert scales, but there was a lack of uniformity in their ratings making interpretations in any of the relevant comparisons more difficult.
In day‐to‐day clinical practice, clinicians and patients need to know how rapidly lesions will respond to treatment and, once an optimal response has been achieved, how long this will last. Although these would appear to be key issues in clinical decision making, the time to response, which was one of our secondary outcomes, was not a pre‐specified outcome in any study. In a few instances it could be roughly estimated from some of the interim data that were reported. Duration of remission was only assessed and reported in four of the studies (Akhyani 2008; Dahl 1998; NCT01426269; Thiboutot 2009).
Pooling of data was not feasible for most of the treatment options, and was only possible for several outcomes in the trials which evaluated topical metronidazole and azelaic acid (see Table 1; Table 2). Both these treatments were shown to be more effective than placebo, with the quality of evidence rated as moderate to high for several outcomes for metronidazole, and high for azelaic acid. Which of these two treatments is most effective still needs to be established because the results from three of the studies (Elewski 2003; Maddin 1999; Wolf 2006) were contradictory (quality of the evidence moderate to low, see Table 5).
Although most comparisons were evaluated in single studies, we have provided 'Summary of findings' tables for the most current and more frequently prescribed therapies as we considered these would prove to be the most useful for clinical decision making. We did not provide 'Summary of findings' tables for treatments that are no longer favoured or had been evaluated in studies at high risk of bias, factors which we consider would make their data less usable.
Topical ivermectin 1% is registered for papulopustular rosacea by the FDA and registration in Europe is expected in the near future. Two studies showed a statistically significant and clinically important improvement in favour of topical ivermectin when compared to placebo, with the quality of evidence rated as high (Table 3). Furthermore, based on one recently published study (Taieb 2015) topical ivermectin appeared to be slightly more effective than topical metronidazole for subtype 2 rosacea (Table 6). Another new compound, brimonidine, which was recently approved by both the FDA and EMA for treatment of erythema in rosacea, was shown to be more effective than vehicle in reducing erythema at all time points over 12 hours, with the quality of evidence rated as high (see Table 4). Topical clindamycin phosphate combined with tretinoin was not considered to be effective compared to placebo (moderate quality of the evidence) (see Table 8). For ocular rosacea topical ciclosporin ophthalmic emulsion demonstrated effectiveness with the quality of the evidence rated as low (see Table 7).
Of the comparisons assessing oral treatments, there was moderate quality evidence that tetracycline is effective but this was based on two rather old studies which were of short duration (Table 9). The newer tetracyclines such as doxycycline and minocycline were evaluated in several comparisons but mainly at the lower dose. The anti‐inflammatory dose of 40 mg doxycycline was shown to be effective for papulopustular rosacea (moderate to high quality evidence for the outcomes listed) (see Table 10) and as effective as 100 mg doxycycline but with one quarter of the side effects (low quality evidence) (see Table 12). There was no statistically significant difference in effectiveness or safety when low dose doxycycline was combined with either topical metronidazole or azelaic acid (Table 13). There was very low quality evidence from one study assessed as at high risk of bias that doxycycline 100 mg was as effective as azithromycin (Table 11). Unfortunately we were unable to include further studies evaluating the effectiveness of azithromycin, even though this is a frequently prescribed drug for rosacea. Low dose minocycline 45 mg either combined with topical azelaic acid gel or as stand‐alone therapy was effective for papulopustular rosacea (low quality evidence) (see Table 14).
Oral (oxy)tetracycline was compared with topical metronidazole in four studies (Monk 1991; Nielsen 1983b; Schachter 1991; Veien 1986) and showed no statistically significant difference between the two treatment modalities for any outcome, with the quality of evidence rated as low to moderate for the outcomes listed (see Table 15).
Isotretinoin is frequently prescribed 'off‐label' for rosacea, albeit in the absence of any evidence underpinned by RCTs, therefore it was reassuring to be able to include one study comparing the effectiveness of isotretinoin 0.3 mg/kg with doxycycline 100 mg. Low dose isotretinoin was considered by both the participants and the physicians to be slightly more effective than doxycycline 50 to 100 mg (high quality of the evidence) (see Table 16). Although there was no statistically significant difference in the number of adverse events between the two treatment groups, isotretinoin has a well known safety profile and can only be prescribed in women of child bearing age following the Risk Management Programme of the FDA and American Medical Association (AMA).
Pulsed dye laser (PDL) was more effective than Nd:YAG laser based on one study (quality of evidence rated low) (see Table 17), and it appeared to be as effective as intense pulsed light therapy (quality of the evidence rated low to moderate) (see Table 18).
No studies could be included that addressed the variant granulomatous rosacea.
Overall completeness and applicability of evidence
Study duration was less than eight weeks in 32/106 studies, which is an inadequate period of time to demonstrate an optimal treatment effect for some of the interventions. Because rosacea is a chronic disease there is a pressing need for more studies that evaluate strategies focused on therapies that are capable of maintaining remission. Consequently, the evidence was noticeably incomplete for some of these interventions such as, for example, patient education and avoidance measures for trigger factors, that is certain foods and exposure to heat and sunlight, or the use of non‐irritating cosmetics. The review also failed to identify any eligible studies addressing dietary manipulation or sun protective measures for the treatment of rosacea. However, the majority of included studies provided enough evidence to draw conclusions on effectiveness of the various treatment options for the different subtypes, notwithstanding the fact that most people with rosacea suffer from more than one subtype and often a combination of treatments will be needed to reach a satisfactory result. Moreover, in clinical practice it is not always possible to definitively differentiate between subtype 1 and subtype 2 rosacea. Indeed, people may have more signs and symptoms of subtype 1 at a point in time and at another point in time more features of subtype 2.
Treatments for subtype 1, erythematotelangiectatic rosacea
Erythema or telangiectasia, or both, were inclusion criteria in 63 studies, however only 16 studies addressed subtype 1, erythematotelangiectatic rosacea. In 47 studies erythema and telangiectasia were part of the diagnosis of subtype 2, papulopustular rosacea. Whilst erythema or telangiectasia were not pre‐specified inclusion criteria for 18 studies, these were assessed as an outcome at baseline and follow‐up in a study only in participants with papulopustular rosacea.
Around 40% (43) of the total number of included studies demonstrated a reduction in erythema but in general not of telangiectasia. In view of the potential impact of these reductions on the quality of life for people with rosacea, future updates of this review should place increased emphasis on the effects of interventions on both erythema and telangiectasia. The use of different scoring systems to assess improvements of erythema and telangiectasia, and the paucity and variability of evidence on the effects of interventions on this subtype of rosacea, did not in most cases permit firm conclusions to be made. The Clinician's Erythema Assessment (CEA) tool which rates erythema on a 5‐point Likert scale (from 0 = clear to 4 = severe erythema, fiery redness) was used in 16 of the 106 studies included in this review. The CEA has been validated and is reported to have a high inter‐rater and good intra‐rater reliability when used by experienced and trained raters (Tan 2014). Applying the same scale in future studies will enable more accurate and directly quantifiable comparisons of erythema between the different interventions.
Topical treatments
Robust evidence based on data gathered using the CEA scale came from several studies evaluating brimonidine (Fowler 2012a; Fowler 2012b; Fowler 2013a; Fowler 2013b; Kendall 2014; NCT01885000). There was high quality evidence that brimonidine was effective for erythema over a 12 hour period, with a peak effect occurring between three and six hours. As erythema also occurs in other subtypes of rosacea, its use is not restricted to subtype 1. None of the data showed that BFH772 1% (betamethasone and calcipotriol) was any better than vehicle ointment (NCT01449591), and the same holds true for TDT 068 gel (ultra‐deformable SequessomeTM vesicles) (Luger 2015).
Laser and light therapies
Lasers and light therapies would appear to have a major clinical role to play in the treatment of erythematotelangiectatic rosacea, but these treatment modalities are still largely under researched in RCTs. There was some evidence that PDL, Nd:YAG laser and intense pulsed light therapy are capable of reducing erythema and telangiectasia on the face (Alam 2013; Karsai 2008; Kim 2011; Neuhaus 2009; Nymann 2010). Because clearance of the redness and telangiectasia occurring on the face is highly desirable, and can be a source of personal embarrassment and lead to low self esteem, further studies of laser and light‐based therapies should be considered a priority (Menezes 2009).
Treatments for subtype 2, papulopustular rosacea
Topical treatments for papules and pustules
Pooled data for topical metronidazole (Bjerke 1989; Breneman 1998; Nielsen 1983a) and azelaic acid (Bjerke 1999; Draelos 2013a; Thiboutot 2003a;Thiboutot 2003b) indicate that both are effective treatments for rosacea. However, based on the assessments in Elewski 2003 and Maddin 1999, azelaic acid would appear to be more effective than metronidazole albeit with more side effects. The more recent study of Wolf 2006 failed to demonstrate improved effectiveness of one of these two treatments over the other and, therefore, further supporting evidence is still required.
No statistically significant difference in effect was reported between the two concentrations of topical metronidazole or when different vehicles were compared in three studies (Beutner 2005; Dahl 2001; Dreno 1998). Topical metronidazole was also shown to be effective in maintaining remission (Dahl 1998).
A single daily dose of azelaic acid appears to be as effective as the twice daily dose, and is also likely to result in improved compliance (Thiboutot 2008). This comparison warrants further investigation.
The results in Thiboutot 2009 illustrate that there is insufficient evidence to conclude that azelaic acid is either effective or ineffective for maintenance treatment. Rosacea is a chronic disease and therefore RCTs investigating the effectiveness of azelaic acid and metronidazole used in maintenance therapy are still required.
Topical ivermectin was shown to be more effective than placebo (high quality evidence) (Stein 2014a; Stein 2014b) and slightly more effective than metronidazole (moderate to high quality evidence) (Taieb 2015).
The effectiveness or otherwise of benzoyl peroxide in the treatment of papulopustular rosacea remains unclear. The conflicting results in Leyden 2011 and the inadequate study design coupled with a short study duration of four weeks did not enable any definitive conclusions to be drawn from Montes 1983. Benzoyl peroxide combined with clindamycin was investigated in Breneman 2004 but the data were incomplete; no standard deviations were reported and the data were skewed, which did not permit firm conclusions to be made about the efficacy of this combined intervention.
Sodium sulphacetamide 10% in combination with sulphur 5% would appear to be more effective than metronidazole but further research is warranted, especially because two of the studies for this intervention were assessed as being at high risk of bias (Lebwohl 1995; Torok 2005) and one study (Sauder 1997) at unclear risk of bias.
The evidence for the effectiveness of permethrin for rosacea was inconclusive, and therefore further trials with a rigorous study design are still required (Koçak 2002; Mostafa 2009).
There was no evidence to support the effectiveness of pimecrolimus, however this was based on very limited and largely unusable data presented in two studies (Koca 2010; Weissenbacher 2007).
Several studies which examined topical calcineurin antagonists could not be included as they were not RCTs or did not match the pre‐specified inclusion criteria for this review (Chu 2005; Chu 2007; Crawford 2005; Garg 2008; Lee 2008). Further well‐designed, double‐blind RCTs which examine the potential benefits of calcineurin antagonists as a treatment option for rosacea are required.
No eligible studies were identified for dapsone or topical tretinoin, although these treatments are still in fairly common use in the treatment of rosacea (Jansen 1997; Thiboutot 2000; Wilkin 1994). Clindamycin combined with topical tretinoin was not shown to be effective compared to placebo in treating papulopustular rosacea (Chang 2012).
Oral treatments for papules and pustules
Two studies (Marks 1971; Sneddon 1966) evaluated the effects of tetracycline. In both of these studies the physicians' assessments indicated an improvement in severity, but only Marks 1971 provided data on the participants' assessments of treatment. In contrast, although the six week study duration may appear to have been too short, the assessments of the participants nevertheless failed to provide any evidence of a difference in effectiveness between tetracycline and placebo. The data from these two studies are further supported by Monk 1991; Nielsen 1983b; Schachter 1991 and Veien 1986, which compared (oxy)tetracycline with topical metronidazole. Tetracyclines are used extensively for the treatment of rosacea and, although their efficacy may be widely accepted by clinicians, this is currently not substantiated by high level evidence from robust and methodologically sound clinical trials.
Whilst a number of studies included in this review (Del Rosso 2007a; Del Rosso 2007b; Del Rosso 2010; Fowler 2007; Sanchez 2005) demonstrated the efficacy of an anti‐inflammatory dose of doxycycline as a reduction in physician‐assessed lesion counts, quite significantly the participants' views and satisfaction with the effects of this intervention were not assessed. Furthermore, while there was a measurable decrease in lesion counts as a result of the intervention, it was unclear if these counts were continuing to decrease or had stabilised by the time the studies were completed.
Although a number of studies have shown that anti‐inflammatory doses of doxycycline do not have an antimicrobial effect on skin flora, nor do they lead to an increase in the number or severity of resistant organisms, studies with a longer duration and which are capable of providing conclusive evidence of the efficacy of an anti‐inflammatory dose of doxycycline are still required (Bikowski 2007; Fowler 2007; Korting 2009; Sloan 2008). There is evidence from these trials that the 40 mg dose is at least as effective as the 100 mg dose and has a correspondingly lower risk of adverse effects (Del Rosso 2008); although these events may be mild to moderate, more were reported with the 100 mg of doxycycline than the 40 mg dose. Therefore, because anti‐inflammatory doses of antibiotics represent a novel approach in the management of rosacea, further trials evaluating the effects of such dosing regimens of other antibiotics should be encouraged (Bikowski 2007; Fowler 2007). Low dose minocycline is yet another tetracycline with demonstrable effectiveness in papulopustular rosacea. The data for the various outcomes of all investigated tetracyclines were assessed as low to high quality evidence.
Although a number of studies which examined the effects of azithromycin were retrieved in our searches, they were excluded from this review because they were not RCTs (Bakar 2004; Bakar 2006; Bakar 2009; Dereli 2005). Only one study which compared azithromycin with doxycycline (Akhyani 2008), assessed as at high risk of bias, was included but the data were skewed and consequently more research is required on the effects of this intervention.
Low dose isotretinoin 0.3 kg/kg was slightly more effective for papulopustular rosacea than doxycycline 50 to 100 mg (high quality evidence) (Gollnick 2010).
Several studies examined other interventions such as rilmenidine and ampicillin (Grosshans 1997; Marks 1971) and, although the latter showed some evidence of effectiveness, neither are now considered as treatment options by clinicians. There were contradictory results for oral zinc (Bamford 2012; Sharquie 2006) and limited data on oral ivermectin (Salem 2013).
Topical treatments for erythema and telangiectasia in papulopustular rosacea
More than half of the included studies assessed the effects of interventions on erythema or telangiectasia, or both, in participants with papulopustular rosacea. These are important participant‐preferred outcomes and are also integral to the physician‐assessed changes in rosacea severity. These outcomes have been reported in the Effects of interventions section of this review. As stated above under 'Topical treatments for erythema is subtype 1', brimonidine can also be used for treatment of erythema in other subtypes.
Based on the data reported in Bjerke 1989; Bleicher 1987; Breneman 1998, Dahl 1998, Elewski 2003, Koçak 2002, Monk 1991, Nielsen 1983a, Tan 2002, Tirnaksiz 2012 and Wolf 2006, topical metronidazole appears to be effective in reducing erythema. Azelaic acid (Elewski 2003; Thiboutot 2003a; Thiboutot 2003b) and sulphacetamide combined with sulphur (Lebwohl 1995; Sauder 1997; Torok 2005) are equally effective in reducing erythema. Two studies provided some evidence for the effectiveness of permethrin on erythema (Koçak 2002; Mostafa 2009), however further research is required. The scales used in the assessments of these treatments varied widely, the reporting was mostly incomplete, and it remained unclear if these treatments merely had an effect on perilesional redness or additionally improved background or persistent redness. More evidence is needed on the effectiveness or otherwise of five other topical interventions, P‐3075 cream (Berardesca 2012); 4‐ethoxybenzaldehyde (Draelos 2005b); praziquantel (Bribeche 2015); a skin care product containing ambophenol, neurosensine and La Roche‐Posay thermal spring water (Seité 2013); and SEI003 cream (serine protease inhibitor) (Two 2014). These interventions were addressed in small sample size studies. A rosacea treatment system consisting of gentle cleanser, metronidazole 0.75% gel, hydrating complexion corrector and skin balancing sunscreen SPF 30 was not more effective than metronidazole + the standard care regimen of the rosacea treatment system without metronidazole; data reporting was however incomplete and SDs were missing (Leyden 2011). Cream containing a 1% extract of a flavonoid‐rich plant Chrysanthellum indicum was also not more effective than placebo in reducing erythema (Rigopoulos 2005).
Oral treatment for erythema and telangiectasia in papulopustular rosacea
Oral doxycycline (Del Rosso 2007a; Del Rosso 2008; Fowler 2007; NCT01426269), (oxy)tetracycline (Monk 1991; Nielsen 1983b; Veien 1986), minocycline (Jackson 2013) and isotretinoin (Gollnick 2010) appeared to be effective for reducing erythema, but further research is needed to confirm these findings. These outcomes were reported incompletely, on different scales, and it was unclear if erythema only diminished around the lesions because of a satisfactory response to treatment or if this was attributable to fading of the background or persistent erythema.
Treatments for subtype 3, phymatous rosacea
Surgical therapies as well as ablative laser therapies have been used with reportedly good results for this subtype of rosacea, but no eligible RCTs were identified for this systematic review.
Treatments for subtype 4, ocular rosacea
The symptoms of ocular rosacea are often mild but can also be severe and debilitating, and although ocular involvement occurs in 60% of people with rosacea, only five trials included in this review examined the treatment of ocular rosacea (Barnhorst 1996; Salem 2013; Schechter 2009; Sharquie 2006; Wittpenn 2005). Only the studies of Barnhorst 1996 and Schechter 2009 provided usable data.
Topical treatments
Although there was insufficient evidence to support the efficacy of topical metronidazole for ocular rosacea (Barnhorst 1996), there was some evidence of a consistent improvement in all outcomes and that ciclosporin a 0.05% ophthalmic emulsion was more effective than artificial tears in the treatment of ocular rosacea (Schechter 2009).
Treatments for the variant granulomatous rosacea
No studies were identified that assessed the variant granulomatous rosacea.
Adverse events
The adverse events reported were mostly mild and transient and were comprised of skin irritation, pruritus, tinging or burning or dry skin. In most of the studies the number and types of adverse events did not differ significantly between active treatment and the placebo group, however these were not always reported adequately or completely.
Quality of the evidence
Limitations in study design and implementation
Although the overall clinical design of the included studies appeared to be adequate, our assessments of risk of bias revealed some of the limitations in the quality of the studies covering many of the interventions.
There was considerable variation in how well the studies were reported and in particular the methods used to generate the randomisation sequence, to conceal the allocation, and the measures taken to blind investigators and participants. These factors, compounded with unsuccessful attempts to contact many of the investigators for additional information, created difficulties in making accurate assessments of the risk of bias in some of the included studies.
A significant proportion of the outcome data was not normally distributed (skewed). SDs were frequently missing from study reports, which meant that in many instances continuous outcome data could not be entered in a meta‐analysis. For most treatment comparators it was not possible to pool data relating to the various studies, and it was only possible to pool the data for a limited number of outcomes in the trials evaluating topical metronidazole and azelaic acid.
However, whilst recognising these limitations the authors consider that the body of evidence summarised in this review is sufficient to allow certain conclusions to be drawn about the effectiveness of several of the interventions used in the treatment of rosacea.
Indirectness of the evidence
The participants included in the studies were to a large extent representative of the population as pre‐specified in 'Types of participants'. Almost half of the studies (49) included in this review were placebo‐controlled trials, which may only provide limited evidence on the advantages or disadvantages of new relative to existing interventions. However, 46 active‐controlled studies were also included and these gave access to data not only on the risks and benefits of individual interventions but also the comparative efficacy of these interventions; thus these head‐to‐head trials are more likely to have provided evidence that is both relevant and direct. Eleven studies included a placebo in addition to an active treatment arm.
Patient‐relevant primary outcomes are a pre‐requisite for informing evidence‐based clinical decision making but the importance of patient‐reported outcomes (PRO), and specifically those used in evaluating the impact of interventions on quality of life, appears to have been underestimated by the investigators in most of the included studies. Improvement in symptoms may not necessarily equate with or translate into measurably significant changes in quality of life for the individual; and therefore whilst a moderate change in some of the physical symptoms, that is erythema, may be interpreted by clinicians as evidence of effective treatment it does little to address the wider psychological distress or physical disfigurement that may occur in those with chronic rosacea. Thus, although the majority of reports appeared to recognise the importance of patient‐assessment of symptoms, in reality greater emphasis was placed on the reporting of lesion counts as primary outcomes whereas self‐assessments were almost always considered as secondary outcomes.
Inconsistency of results
Although a diverse range of interventions were considered in this systematic review, the majority of participants in the included studies had subtype 1 and 2 rosacea. The results for specific outcomes were fairly consistent across the very limited number of studies and interventions where pooling of data was feasible.
Imprecision of results
The rather limited number of studies that were included in this review examining similar interventions did not permit any substantive assessment of the degree of precision of effect. Small sample sizes were responsible for most of the imprecision.
Publication bias
A large number of abstracts to conference proceedings were identified. Some were published in full but a number were not otherwise available. There is a possibility that a number of reports, in particular those which conclude negative outcomes, involve serious adverse effects or a lack of effect, may have been sponsored by parties with potentially vested interests, remain unpublished.
Potential biases in the review process
Serious attempts were made to limit bias in the review process by ensuring a comprehensive search for potentially eligible studies. The authors' independent assessments of eligibility of studies for inclusion in this review and the extraction of data minimised the potential for additional bias beyond that detailed in the 'Risk of bias' tables. The incomplete reporting of trial details, results, or both in some of the included studies, and our inability to obtain satisfactory clarification from trial investigators, may have contributed to some biased assessment in the review process. Where these conditions applied this was explicitly stated in the text of our review.
Agreements and disagreements with other studies or reviews
A number of studies, reviews and guidelines have been published since we last updated this review, a fuller listing of these can be found in DynaMed (https://dynamed.ebscohost.com/). Although we are in broad agreement that the conclusions reached in these other studies reflect the present findings, we express a level of disagreement with the methods used to reach these conclusions, more specifically in the assessments of the quality of the evidence and how these might correlate with the individual recommendations. All of the studies that were identified appear to have comprehensively and succinctly covered this clinical topic and, based on the references cited, providing a degree of reassurance that a competent literature search had been undertaken. These studies included an update of guidelines produced by the American Acne and Rosacea Society (AARS) (Del Rosso 2013b; Del Rosso 2014a; Del Rosso 2014b; Del Rosso 2014b; Tanghetti 2014), the S1 Guideline developed by the German Society of Dermatology (Reinholz 2013), a consensus document proposing an evidence‐based treatment approach by the Rosacea International Expert Group (ROSIE) (Elewski 2011) and two literature reviews (Korting 2009; Moustafa 2014b).
No details were reported on how studies were selected for inclusion, however the S1 Guideline developers stated that the consensus recommendations of the AARS were based on a "thorough literature review and observations from clinical experience", and also indicated that the process involved an "informal expert panel for consensus". The literature cited in both of these guidelines was complete but there was no indication of how the primary research had been critically appraised by the expert panels, and it appeared that the recommendations, although consensus based, were underpinned by study level assessments of evidence from relevant clinical trials. Similarly, neither of the two other reviews or the consensus document referred to any aspects of the methodological quality of the studies supporting the specific interventions, which was distinct from the more detailed exploration of the quality of the evidence undertaken in this review. We have emphasized, where appropriate in this review, the limitations in study design of the included studies and the potential impact of these limitations on the quality of the evidence. Although we have identified these limitations in our review, they may be of less relevance to well‐established therapies, but may provide cautionary information for those interventions where benefits may marginally exceed potential harms or, indeed, may be critical for the further assessment of newer therapies.
Effect measures can enable clinically meaningful comparisons of efficacy to be made between trials. These were reported, mostly in general terms, in only one of the guidelines (Reinholz 2013), and only for the emerging therapies in Moustafa 2014b; whereas we have reported these magnitudes of effect in more detail, as either RR or MD, for many of the outcomes in single studies as well as in the meta‐analyses across several studies.
Many of the studies evaluating the effects of interventions on rosacea emphasize the significant impact of this condition on the psychosocial well‐being and quality of life of the individual, thus it was somewhat surprising to see that neither of the guidelines involved patients or patient advocacy groups but appeared to be more reliant on the contribution of expert panels. In contrast, and in terms of recognising the significant impact of this condition on patients, we have tried to ensure that we received timely, patient‐relevant input at all stages of conducting and reporting in this review and have also included a consumer as a co‐author.
Guidelines provide balanced information on the benefits and limitations of the therapeutic interventions being evaluated. Their process of development should be transparent, robust and reproducible, and should clearly demonstrate that the supporting evidence was systematically reviewed. The strength of clinical recommendations in the two guidelines did not correspond to the widely‐recognised GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach to developing and presenting recommendations for the appropriate health care for specific clinical conditions or circumstances (Guyatt 2008). In contrast, we used the GRADE method in this review to examine and categorise the quality level of a body of evidence and to explain our confidence in the effect estimates for several of the interventions, which we have presented in the 'Summary of findings' tables.
Authors' conclusions
Implications for practice.
Based on only those studies which are most likely to have provided reliable results, that is those that are reproducible, repeatable and therefore valid, and selecting the most rigorously described and conducted studies, we have made several conclusions. Evidence of treatment effect could be demonstrated for several of the interventions studied. In daily practice often a combination of treatments should be chosen based on the activity of signs and symptoms of the specific subtype, or combination of subtypes.
There is high quality evidence to support the effectiveness and safety of brimonidine topical gel for reducing erythema over 12 hours after application in the management of persistent erythema in rosacea. There was low to moderate quality evidence of the effectiveness of pulsed dye laser and intense pulsed light therapy for erythematotelangiectatic rosacea.
For subtype 2, papulopustular rosacea, topical metronidazole, azelaic acid, topical ivermectin, anti‐inflammatory dose doxycycline (40 mg), tetracycline and isotretinoin 0.3 mg/kg appear to be effective and safe for short‐term use (moderate to high quality of evidence). It still needs to be established whether azelaic acid is more effective than topical metronidazole, but topical ivermectin appeared to be slightly more effective than topical metronidazole. There is evidence that 40 mg doxycycline is at least as effective as 100 mg, with evidence of fewer adverse effects (low quality of evidence). There is low quality evidence for the effectiveness and safety of low dose minocycline 45 mg and very low quality evidence of azithromycin for this subtype. There is no clear evidence that any one of these treatments, or any combination of treatments, has a particular advantage in terms of higher remission rates or fewer adverse effects.
No studies could be included that addressed treatment of phymatous rosacea (subtype 3).
For ocular rosacea (subtype 4), ciclosporin 0.05% ophthalmic emulsion was shown to be more beneficial than artificial tears (low quality evidence).
Clinical decision making on the choice of intervention for rosacea should be based on high‐level evidence if it is available, but in the absence of such evidence for any specific intervention these decisions should continue to be guided by clinical experience and patients' individual characteristics and preferences until further evidence becomes available.
Implications for research.
The impact of available treatment on ocular rosacea warrants further examination, and this might include the removal of Meibomian cysts. Less direct interventions, such as dietary adjustments, avoidance measures for trigger factors, the use of sunscreens and patient education, in addition to trials investigating which is the most effective treatment for phymatous rosacea and granulomatous rosacea, are further areas of much needed research. Conceivably some of the studies listed in the 'Characteristics of ongoing studies' section of this review will be able to provide answers to these remaining questions.
There was wide variability in not only the conduct but also the quality of reporting of many of the trials. A major area for improvement would be in the standardisation of outcome reporting in any future research, as suggested by the COMET (Core Outcome Measures in Effectiveness Trials) Initiative (http://www.comet‐initiative.org/). The use of proprietary severity scales and non‐standardised erythema scales significantly hampered our ability to combine study results for meta‐analysis. Outcomes collected in future trials should be primarily based on a standardised scale of the participant's assessment of the treatment efficacy, and also have a greater emphasis on changes in quality of life as a result of the interventions. Standardised and uniform scales should be developed and used for physicians' assessments, and these should reliably reflect global evaluation, lesion counts, and assessment of erythema and telangiectasia.
Time needed for a response and response duration should be addressed more completely, and adverse events reported more rigorously. Furthermore, to ensure improved clinical decision making, future research should place a greater emphasis on the management and treatment of rosacea based on the staging pattern of the disease.
Future randomised controlled trials must be well‐designed, well‐conducted, and adequately delivered with subsequent reporting, including high‐quality descriptions of all aspects of methodology. Rigorous reporting needs to conform to the Consolidated Standards of Reporting Trials (CONSORT) statement, and this will enable appraisal and interpretation of results and accurate judgements to be made about the risk of bias and the quality of the evidence of the selected outcomes. Although it is uncertain whether the reported quality mirrors actual study conduct, it is noteworthy that studies with unclear methodology have been shown to produce biased estimates of treatment effects (Schulz 1995). Adherence to guidelines, such as the CONSORT statement, would help ensure complete reporting.
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2017 | Amended | Author information (affiliation) updated |
| 15 July 2015 | Amended | Correct label of 'Update' added |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 15 July 2015 | New search has been performed | The review has been updated. |
| 28 April 2015 | New citation required and conclusions have changed | Update, 58 new studies added, review updated according to MECIR, revised background, new comparisons, new conclusions, 18 summary of findings tables |
| 12 April 2011 | Amended | minor errors amended |
| 16 February 2011 | New search has been performed | New search for studies, extensive update of background section, and new results and additional conclusions added. |
| 16 February 2011 | New citation required and conclusions have changed | Extra conclusions on new studies. Change in authoring team. |
| 7 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank the librarian Jan Schoones, Waleus Library of Leiden University Medical Centre for his help with the searches for trials in Web of Science for this update. We would like to thank Ching‐Chi Chi from the Department of Dermatology & Centre for EBM, Chang Gung Memorial Hospital, Chiayi, Taiwan for his translation of Huang 2012 from Chinese into English.
We would like to acknowledge Sanna Prinsen from VU Medical Centre, Amsterdam for her expert input on the reliability of the CEA scale as described in Tan 2014.
The support of Finola Delamere, Laura Prescott and Liz Doney was great. They always responded promptly, and guided us through the sometimes frustrating moments of the process of updating the review.
People who were of great value in earlier versions of this review include Tina Leonard, Dedee Murrell, Mrs DL Brand de Heer, and Hywel Williams. Of course nothing could have been realised without our former co‐authors. Thanks for your hard work.
The Cochrane Skin Group editorial base would like to thank the following people who were authors on previous versions of this review: Mark Graber and Sharon Kramer.
Appendices
Appendix 1. Skin Group Specialised Register/CRS search strategy
"pyoderma faciale" or rosacea or rhinophyma
Appendix 2. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Rosacea] explode all trees #2 rosacea:ti,ab #3 rhinophyma:ti,ab #4 "pyoderma faciale":ti,ab #5 {or #1‐#4}
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Rosacea/ 2. rosacea.ti,ab. 3. Rhinophyma.ti,ab. 4. pyoderma faciale.ti,ab. 5. or/1‐4 6. randomized controlled trial.pt. 7. controlled clinical trial.pt. 8. randomized.ab. 9. placebo.ab. 10. clinical trials as topic.sh. 11. randomly.ab. 12. trial.ti. 13. 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp animals/ not humans.sh. 15. 13 not 14 16. 5 and 15
Appendix 4. EMBASE (Ovid) search strategy
1. rosacea/ 2. rosacea.ti,ab. 3. rhinophyma.ti,ab. 4. pyoderma faciale.ti,ab. 5. or/1‐4 6. crossover procedure.sh. 7. double‐blind procedure.sh. 8. single‐blind procedure.sh. 9. (crossover$ or cross over$).tw. 10. placebo$.tw. 11. (doubl$ adj blind$).tw. 12. allocat$.tw. 13. trial.ti. 14. randomized controlled trial.sh. 15. random$.tw. 16. or/6‐15 17. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 18. human/ or normal human/ 19. 17 and 18 20. 17 not 19 21. 16 not 20 22. 5 and 21
Appendix 5. LILACS search strategy
rosacea or rhinophyma
Appendix 6. Science Citation Index search strategy
1. TS=("rosacea" OR "rhinophyma" OR "rozacea" OR "rosacea*" OR "rhinophyma*" OR "rozacea*" OR "flushing" 2. TS="facial" AND ("telangiectasis" OR "telangiectasia" OR "erythema" OR "edema" OR "oedema" OR "edema*" OR "oedema*") 3. 1 or 2 4. TS=("therap*" OR "therapy" OR "treat*" OR "treatment" OR "surgery" OR "surger*" OR "surgic*" OR "antibiotic" OR "anti‐biotic*" OR "antibiotics" OR "antibiotic*" OR "tetracycline" OR "doxycycline" OR "minocycline" OR "permethrine" OR "benzoyl peroxide" OR "oral contraceptive" OR "oral contraceptives" OR "tetracyclin*" OR "doxycyclin*" OR "minocyclin*" OR "permethrin*" OR "benzoyl peroxid*" OR "oral contracept*" OR "diane 35" OR "diane35" OR "erythromycin" OR "sulphur" OR "sulfur" OR "erythromycin*" OR "sulphur*" OR "sulfur*" OR "azelaic acid" OR "tretinoin" OR "isotretinoin" OR "laser" OR "spironolactone" OR "tretinoin*" OR "isotretinoin*" OR "laser*" OR "spironolacton*" OR "adrenal cortex hormone" OR "adrenal cortex hormones" OR "adrenal cortex hormone*" OR "corticosteroid*" OR "corticosteroids" OR "corticosteroid" OR "metronidazole" OR "metronidazol*" OR "zinc" OR "massage*" OR "massage" OR "massages" OR "ivermectin" OR "brimonidine" OR "azithromycin" OR "doxycyclin" OR "intense pulsed light" OR "metronidazol" OR "ivermectin*" OR "brimonidin*" OR "azithromycin*" OR "doxycyclin*" OR "intense pulsed light*" OR "metronidazol*") 5. TS=(Randomized Controlled Trial OR Controlled Clinical Trial OR randomized controlled trials OR random allocation OR double‐blind method OR single‐blind method OR randomized controlled trial OR controlled clinical trial OR randomized controlled trials OR random allocation OR double‐blind method OR single‐blind method OR clinical trial OR clinical trials OR "clinical trial" OR ((singl* OR doubl* OR trebl* OR tripl* ) AND (mask* OR blind* )) OR "latin square" OR placebos OR placebo* OR random* OR research design [mh:noexp] OR comparative study OR evaluation studies OR follow‐up studies OR prospective studies OR cross‐over studies OR control* OR prospective* OR volunteer* OR randomised controlled trial OR randomised controlled trials OR randomized active control trials OR randomized active control trial OR RaCT OR RaCTs) 6. 3 and 4 and 5
Appendix 7. BIOSIS search strategy
1(rosacea or rozacea).mp.[mp=title, keywords, heading words, registry words, abstracts, biosystematic codes/super taxa, title, book title, original language book title, title, original language book title, biosystematic codes/super taxa, subject headings, heading words] 2 clinical trial.mp. [mp=title, keywords, heading words, registry words, abstracts, biosystematic codes/super taxa, title, book title, original language book title, title, original language book title, biosystematic codes/super taxa, subject headings, heading words] 3 randomi$.mp.[mp=title, keywords, heading words, registry words, abstracts, biosystematic codes/super taxa, title, book title, original language book title, title, original language book title, biosystematic codes/super taxa, subject headings, heading words] 4 1 and 2 and 3 5 double blind.mp.[mp=title, keywords, heading words, registry words, abstracts, biosystematic codes/super taxa, title, book title, original language book title, title, original language book title, biosystematic codes/super taxa, subject headings, heading words] 6 1 and 5 7 6 not 4 8 from 7 keep 5,8,10‐13, 15,17‐19 9 from 8 keep 1
Data and analyses
Comparison 1. Topical metronidazole versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 6 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.94, 1.51] |
| 2 Physician's global evaluation of improvement | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.29, 3.02] |
| 3 Incomplete data on which further analysis is not possible | Other data | No numeric data |
Comparison 2. Topical azelaic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐assessed improvement of rosacea | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.30, 1.63] |
| 2 Physician's global evaluation of improvement | 4 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.18, 1.47] |
| 3 Incomplete data on which further analysis is not possible | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akhyani 2008.
| Methods | RCT, prospective, active‐controlled, open‐label Date of study Unreported Setting Department of Dermatology, Razi Hospital; Department of Ophthalmology, Farabi Hospital, Teheran, Iran |
|
| Participants |
Randomised: 67 participants (mean age 47.93 years (SD 14.18), 37 male, 30 female) Inclusion criteria
Exclusion criteria
Neither ocular involvement nor phymas Dropouts and withdrawals
Baseline data mean (SD) Lesion counts; azithromycin group 19.24 (9.67), doxycycline group 18.86 (8.95) |
|
| Interventions | Three months Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, month 1, 2, 3, and 5 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 288): "The authors wish to acknowledge Pakhshe Razi Co. (Tehran, Iran) for providing azithromycin (azithromycin, 250 mg capsule, Chemiedaru)." | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) Skewed data for lesion counts See comparison 45 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 285): "Patients were allocated to the trial using a randomized numbers table in a one‐to‐one fashion" Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Following extensive e‐mail contact with the investigators we were informed that the providers of care had access to the computer‐generated list Comment: We judged this as at high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 284): "....an open clinical trial." The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (page 284): "....an open clinical trial." Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/67 (13.4%); 5 in azithromycin group, 4 in doxycycline group. Analysis followed ITT principle, withdrawals were balanced across groups, reasons were reported, all participants were accounted for and included in the analysis Comment: We considered this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration and wash‐out period adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Alam 2013.
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study January to July 2012 Setting Department of Dermatology, Northwestern University, Chicago, IL, US |
|
| Participants |
Randomised: 16 participants (mean age 42 years (range 24 to 52), 8 male, 8 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Six months Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, month 7 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 438): "Funded by the Northwestern University Department of Dermatology" | |
| Declaration of interest | Quote (page 438): "None declared" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 63 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 439): "This was a randomized controlled split‐face study with allocation ratio 1:1, using random block size of 2" and "A random number generator was used to generate 0s and 1s, which were designated as left or right" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 440): "Each random assignment was sealed individually in an opaque, sequentially numbered envelope (M.A.). Assignments were made consecutively, with subjects receiving PDL to the left or right side of the face, and Nd:YAG laser to the contralateral side" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 440): "Subjects were blinded as to which facial side received which laser treatment. They were laser naive before the study, and both laser treatments were performed (N.V.) in the same room after subjects donned occlusive eye‐protective goggles. The investigator obtaining spectroscopy measurements (M.W.) was not present during treatments and blinded regarding allocation" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/16 (12.5%) dropped out reporting post‐treatment swelling. Per‐protocol analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was available on clinicaltrials.gov (NCT01529996) and the pre‐specified primary outcome "rating on global improvement scale" has not been assessed, nor mentioned anymore in the methods section of present publication Comment: We judged this as at unclear risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Bamford 1999.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Screening and enrolment between February 1996 and June 1997 Setting Dermatology Section, St Mary's ‐ Duluth Clinic Health System, Duluth, Minnesota, US |
|
| Participants |
Randomised: 44 participants (mean age 56.9 years (SD 12.9) in treatment group, 58.9 years (SD 11.9) in control group, gender unreported) Inclusion criteria:
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Duluth Rosacea score; clarithromycin group 10.8 (3.5), placebo group 11.1 (4.2) |
|
| Interventions | Two weeks Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, day 60 Outcomes of the trial (as reported) Primary outcomes
Method: Duluth Rosacea Scoring Instrument Secondary outcomes None ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 663): "Astra Merck, Wayne, PA, provided the major funding for the study as well as omeprazole (Prilosec) and matching placebos. Abbott laboratories, North Chicago, Ill, supplied the clarithromycin. Cortecs Diagnostics Ltd, London, England, donated the Helisal Rapid Whole Blood Test. Meretek Diagnostics, Inc, Houston, Tex, donated the 13C urea breath tests." | |
| Declaration of interest | None reported | |
| Notes | None of our primary outcomes were addressed. Follow‐up 2 months; 25% in the treatment group tested positive still for Helicobacter pylori after treatment. For the N of pustules the data are quite skewed and for the total score very skewed See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 660): "Patients were randomly assigned to groups receiving active treatment or placebo. Dispensing of study medications according to a randomised registry list provided by the project programmer." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (660): "Treatment status was not disclosed to investigators, coordinators or patients throughout study." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 660): "Double‐blind, placebo medication resembled active treatment." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator‐assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/44 (4.5%); 2 withdrawals in clarithromycin group, reasons reported Comment: Low number of dropouts at follow‐up, and although per‐protocol analysis considered to be at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period described, groups treated equally Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
Bamford 2012.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study August 2006 to July 2008 Setting Essentia Health Duluth Clinic, MN, US |
|
| Participants |
Randomised: 53 participants (mean age 47.3 years, 14 male, 39 female) Inclusion criteria
Exclusion criteria
Ocular involvement: Unclear Dropouts and withdrawals
Baseline data mean Rosacea severity; zinc group 6.30 (95% CI 5.83 to 6.76), placebo group 6.77 (95% CI 6.22 to 7.32) |
|
| Interventions | Three months Intervention
Comparator
Subjects were required to refrain from using oral or topical treatments for rosacea while participating in the trial |
|
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 462): "thank the Duluth Clinic Foundation for grant support that made this study possible" | |
| Declaration of interest | Quote (page 459): "None declared" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 57 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 460): "Randomization was carried out following a sequence of random numbers using random block size created by a biostatistician and maintained at the research pharmacy of the healthcare organization" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 460): "Randomization was carried out following a sequence of random numbers using random block size created by a biostatistician and maintained at the research pharmacy of the healthcare organization" Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 459‐60): "double‐blind" and "Treatment was masked from participants, investigators, and study staff". Capsules probably of identical appearance Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/53 (17%); zinc group (5), placebo group (4), reasons reported. Per‐protocol analysis Comment: We judged this as at an unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00395226). Only the primary outcome was listed in the protocol. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Barnhorst 1996.
| Methods | RCT, prospective, placebo‐controlled, investigator‐blinded, within‐patient comparison Date of study Unreported Setting Department of Ophthalmology, Cleveland Clinic Foundation, Cleveland, US |
|
| Participants |
Randomised: 13 participants (mean age 72.8 years (range 40 to 90), 7 male, 6 female) Inclusion criteria
Exclusion criteria
Dropouts and withdrawal
Baseline data mean (SD) Eye and eyelid grading: metronidazole site 4.5 (1.1), control site 4.5 (1.0) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Method: grading sheet (1 to 5) (higher score is worse) Pre‐treatment scores were compared with post‐treatment scores with respect to ocular surface, eyelid margin, and combined eyelid plus ocular surface Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Withdrawals were not included in the analysis by the review authors. Because it is a within‐patient study, patients can make errors with which eye to treat or treat both eyes. One of our primary outcomes was addressed (adverse events) See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1881): "One eye was assigned randomly to receive lid hygiene and warm compresses twice daily, while the other eye received lid hygiene and compresses twice daily." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 1881): "An observer who was masked to the treated and control eye completed a physician data sheet." Participants were not blinded Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were investigator as well as participant‐assessed Quote (page 1881): "An observer who was masked to the treated and control eye completed a physician data sheet." Comment: We judged this at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/13 (23%), reasons reported
Quote (page 1881): "Those patients reporting noncompliance were removed from the study." Comment: We considered this as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate Comment: The study appeared to be free of other forms of bias |
Benkali 2014.
| Methods | Randomised, prospective, within‐patient comparison Date of study Unreported Setting Multicentre in US |
|
| Participants |
Randomised: 102 participants (mean age 41.6 years, 40 male, 62 female) Inclusion criteria
Ocular involvement: Unclear, probably not Exclusion criteria
Dropouts and withdrawals
Baseline data (number) CEA score 3 (moderate); 0.07% group 22, 0.18% QD group 22, 0.18% BID group 21, 0.5% group 24 CEA score 4 (severe); 0.07% group 5, 0.18% QD group 3, 0.18% BID group 5, 0.5% group 0 |
|
| Interventions | Four weeks
Intervention
Comparator 1
Comparator 2
Comparator 3
Each subject received one drop of brimonidine tartrate 0.2% ophthalmic solution in each eye every 8 hours over a 24 hour period, as proposed in the US prescribing information. After a 2 day wash‐out period they received the dermal applications as described above |
|
| Outcomes | Assessments (47): day 1 (10x), after 2 days wash‐out day 4 (10x), 5, 10, 18 (10x), 19, 24 and 32 (13x) Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 162): "Funding for this study was provided by Galderma R&D, SNC. Funding for writing assistance was provided by Galderma Laboratories, L.P." | |
| Declaration of interest | Quote (page 162): "K. Benkali, F. Rony, R. Bouer, and N. Wagner are employees of Galderma R&D, Sophia Antipolis, France. M. Leoni, A. Fernando, and M. Graeber are employees of Galderma R&D, Princeton, NJ, USA" | |
| Notes | None of our primary nor secondary outcomes were addressed (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 163): "One hundred and two (102) subjects were randomly assigned to 1 of the 4 brimonidine gel regimens" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "Regarding the allocation sequence generated for the 4 subsequent groups consisting of different doses or regimen for topical applications, the randomization list was created before the study started, with a 1:1:1:1 ratio and block size of 4. This randomization list was generated by a designated biostatistician and was distributed to the clinical supply team in a sealed envelope" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "This randomization list was generated by a designated biostatistician and was distributed to the clinical supply team in a sealed envelope" Comment: Adequate, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14/102 (13.7%); 6/102 during ophthalmic dosing, and an additional 8/102 during dermal dosing, unclear from which group, reasons unreported. Per‐protocol analysis Comment: We judged this as at an unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate Comment: The study appeared to be free of other forms of bias |
Berardesca 2012.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Between April and June 2009 Setting Multicentre in Europe (Italy, Switzerland and Belgium) |
|
| Participants |
Randomised: 42 participants (mean age 39.8 years (range 20 to 60), 11 male, 31 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data mean Nothing reported |
|
| Interventions | Four weeks
Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, day 7, 14, 28 and 42 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 41 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 38): " were randomized"
Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "according to a computer generated randomization list".. "with a 2:1 ratio using blocks of 3" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: Form of central allocation, "the randomization list was generated by the statistician and kept under lock and key until the data base lock, as usual" and "Patients were sequentially assigned to the next available randomization number, starting from the lowest number provided to each investigational site" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 37): "double‐blind"
Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "placebo cream units, which were identical to the active product in terms of size, shape, volume, color. The tubes (P‐3075 and placebo) were identically labeled for clinical use as it is in a double‐blind procedure." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 37): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement After e‐mail communication: "placebo cream units, which were identical to the active product in terms of size, shape, volume, color. The tubes (P‐3075 and placebo) were identically labeled for clinical use as it is in a double‐blind procedure." Outcomes were investigator‐assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow up Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No exact data were provided regarding assessment of sign and symptoms of rosacea, only generic comment Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period described, groups treated equally Comment: The study appeared to be free of other forms of bias |
Beutner 2005.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study March 2003 to January 2004 Setting Multicentre study in the US |
|
| Participants |
Randomised: 1299 participants (557 in metronidazole gel group, 553 in metronidazole cream group, and 189 in vehicle gel group) (mean age 48.4 ± 13.02 years, range 18 to 92 for metronidazole gel group; 48.3 ± 13.04 years, range 18 to 88 for metronidazole cream group; 47.8 ± 12.05 years, range 22 to 81 for vehicle gel group; sex 149 male, 408 female for metronidazole gel group; 143 male, 410 female in metronidazole cream group; and 48 male, 141 female in vehicle gel group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data (mean) Lesion count: metronidazole gel group (18.3), metronidazole cream group (18.1) vehicle group (18.4) |
|
| Interventions | 10 weeks Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 10): "Supported by Galderma R&D Inc." | |
| Declaration of interest | Page 10; Dr Beutner and Mr Calvarese are employees of Dow Pharmaceutical Sciences. Dr Graeber is an employee of Galderma R&D Inc | |
| Notes | One of our primary outcomes is addressed (adverse events) Poster presentation, after e‐mail contact extensive information has been provided by authors See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 10): "This was a multicenter, randomized, investigator‐blind, active and vehicle‐controlled, parallel comparison." After e‐mail contact with investigators we received additional information which enabled us to change the grading for this criterion from 'Unclear' to 'Yes' Quote: "Prior to the start of the study, a randomization list was supplied by the Sponsor. Drug supplies for the entire trial were numbered sequentially. The drug supplies for Metronidazole Gel 1%, Noritate Cream 1%, and Vehicle Gel were packaged according to the randomization list in blocks of 7 using a ratio of 3:3:1. Study drug supplies were distributed to each of the investigational sites in complete blocks in order to maintain the randomization ratio within an investigational site. A unique drug kit number was associated with each drug supply kit, and this corresponded to the subject number. These numbers were assigned sequentially as subjects entering the study at each investigational site." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence was not described in sufficient detail in the report E‐mail contact with the investigator confirmed "the randomization schedule remained blinded from those involved in the clinical conduct of the study until the database lock memo was issued" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. This was probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 10): "...investigator blind." E‐mail contact with the investigator confirmed "the study drugs were different in appearance. To protect the blinding, a study staff designee, other than the Investigator making evaluations, dispensed and collected study drug from subjects. Additionally, both the person in charge of study drug dispensation and the subject were instructed not to discuss the study treatment with the Investigator or other evaluator(s)". Participants were not blinded Comment: We judged this as at unclear risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 10): "...investigator blind." Comment: As the investigators were the outcome assessors the report was unclear how they were blinded E‐mail contact with the investigator confirmed "the study drugs were different in appearance. To protect the blinding, a study staff designee, other than the Investigator making evaluations, dispensed and collected study drug from subjects. Additionally, both the person in charge of study drug dispensation and the subject were instructed not to discuss the study treatment with the Investigator or other evaluator(s)" Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants unclear, dropouts not reported E‐mail contact with the investigator confirmed "57 (10.2%) discontinued in metronidazole gel group, 72 (13.0%) in metronidazole cream and 27 (14.3%) in vehicle gel group". Reasons for dropouts stated and ITT analysis LOCF Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, but unclear if there was a 'wash‐out' period, unclear if groups were treated equally E‐mail contact with the investigator confirmed "no financial arrangements have been made with any of the investigators. Each listed investigator was required to disclose to the sponsor whether the investigator had a proprietary interest in this product or a significant equity in the sponsor and none disclosed any such interests" Comment: We judged this as at a low risk of bias |
Bitar 1990.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Hotel‐Dieu Hospital; University of Montreal, Montréal, Québec, Canada |
|
| Participants |
Randomised: 100 participants (mean age 50.3 years (SD 1.6) in treatment group, 50.8 years (1.9) in control group, 41 male, 59 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Number of papules; metronidazole group 8.1 (0.7), control group 8.9 (0.7) Number of pustules; metronidazole group 3.2 (0.4), control group 4.3 (0.6) |
|
| Interventions | Two months Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 248): "This study was supported by Rhône‐Poulenc Pharma Inc, Montréal, Canada" | |
| Declaration of interest | None declared | |
| Notes | We only included first 4 weeks (quality of the study declined after 4 weeks) One of our primary outcomes was addressed (adverse events) See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 243): "50 patients were randomly assigned to treatment with metronidazole 1% cream, while the other 50 patients received placebo cream." "Metronidazole 1% cream and placebo cream were randomly distributed." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 242): "...double‐blind." Quote (page 243): "Tubes were identical in appearance and creams were of same colour and consistency." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 242‐3): "...double‐blind." "Tubes were identical in appearance and creams were of same colour and consistency." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/100 (18%); metronidazole group (8), control group (10) in second month, similar reasons reported and balanced across both groups. ITT analysis only first month Comment: No dropouts in first month and we only included data for the first month, therefore considered as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration was adequate, and participants on antibiotics or vasodilators were excluded. Compliance was assessed Quote (page 243): Concomitant medications which were "considered to be vital to the general health of the patients were permitted and noted", i.e. nonsteroidal anti‐inflammatory and antihypertensive agents. The dropout rate was high in the second month in both groups, and in the absence of an ITT analysis only data from the first month was entered into the RevMan analysis Comment: We considered this as at low risk of bias |
Bjerke 1989.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, Department of Dermatology, Haukeland Hospital, Bergen; Rikshopitalet, Oslo; Florø Hospital, Florø of Ullevål Hospital Oslo, Regionsykehuset, Trondheim, Norway |
|
| Participants |
Randomised: 97 participants (mean age 47 years (range 18 to 77), 44 male, 53 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) No details reported |
|
| Interventions | Two months Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Page 187, one of the investigators is employed by Dumex, the manufacturer of metronidazole. No conflict of interest declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 188): "The trial was a randomized...." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 188): "The trial was double‐blind." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 188): "The trial was double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/97 (4.1%), ITT analysis. Reasons for withdrawals reported Comment: We considered this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period before study started unclear, no other local or oral treatment was allowed, study duration adequate, no sponsoring mentioned, however, study details are incomplete Comment: Insufficient information to assess whether important risk of bias exists |
Bjerke 1999.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, Dermatology Department, Haukeland Hospital, of Ullevål, and National Hosital (Rikshospitalet), Oslo, Norway |
|
| Participants |
Randomised: 116 participants (mean age 48.4 years in treatment group, 50.3 years in control group, 57 male, 59 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of inflammatory lesions; azelaic acid group 30.8, placebo group 31.7 |
|
| Interventions | Three months Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, month 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | One of the investigators was employed by Schering AG Berlin, Germany, the manufacturer of the azelaic acid cream. However, none declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 6 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 456): "The assignment of study medication was random." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 456): "double‐blind, parallel group comparison between azelaic acid 20% cream and its vehicle." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 456): "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/116 (6.9%); azelaic acid group (5), placebo group (1), unclear from which group (2)
Quote (page 456): "All available patients (completed and withdrawals) were included in a confirmatory Intention‐to‐treat analysis of treatment differences with the results achieved at their last observation carried forward (LOCF)." Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate. Unclear if there was a wash‐out period before study, unclear if groups were treated equally, no sponsoring mentioned Comment: Insufficient information to assess whether important risk of bias exists |
Bleicher 1987.
| Methods | RCT, prospective, placebo‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Two centres, Department of Dermatology, Harvard Medical School; Department of Dermatology, Massachusetts General Hospital, Boston, US |
|
| Participants |
Randomised: 40 participants (mean age 48.7 years, 16 male, 24 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of lesions counts; 30.8 |
|
| Interventions | Nine weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 614): "Study was funded, in part, by Curatek Pharmaceuticals, Elk Grove Village, Ill. The metronidazole gel and vehicle placebo used were also provided by Curatek Pharmaceuticals. Statistical analysis was performed by an independent statistical consultant." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 610): "Patients were randomly assigned to receive either 0.75% metronidazole in a water based gel or the gel‐base alone to each half of the face." "Randomization by Curatek Pharmaceuticals, Elk grove Village, Ill." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 610): "Randomization by Curatek Pharmaceuticals, Elk grove Village, Ill." Comment: Appears to be a form of central randomisation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 609 and 610): "double‐blind" and "Identical appearing tubes, colour coded and labelled right and left containing active treatment or placebo." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 609‐10): "...double‐blind." and "Identical appearing tubes, colour coded and labelled right and left containing active treatment or placebo." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 611 and 612): "Two patients did not complete the study. One discontinued after 2 days due to a flare‐up in rosacea related to withdrawal from his systemic antibiotic therapy. Data on this patient were not included in the results. A second patient withdrew at five weeks because of a severe unilateral flare‐up on the placebo‐treated side." Comment: Second patient was included in the analysis. We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate. Wash‐out period before study at least 3 weeks. Other treatments that might affect rosacea were required to be discontinued The study appears to be free of other forms of bias |
Blom 1984.
| Methods | Randomised, prospective, active‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, regional Hospital Örebro, Sweden |
|
| Participants |
Randomised: 40 (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Total number of lesions; sulfur group 213, lymecycline group 143 |
|
| Interventions | Four weeks Intervention
Comparator
Unreported how many participants were randomised into each group |
|
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 359): "This work was supported by Essex Läkemedel AB" | |
| Declaration of interest | None declared | |
| Notes | Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many. Lack of usable data and inability to trace the investigators (see Table 24) One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 358): "Patients were allocated to either regimen 1 or 2 according to a randomization code" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 358): "double‐blind study" Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 358): "double‐blind study" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/40 dropouts, probably in lymecycline group, no reasons mentioned. Per‐protocol analysis Comment: Low number of dropouts and although per protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Only number of papules and pustules is addressed and not the other primary efficacy outcome measures Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Participants who failed to respond or got worse were switched to the alternative treatment, unclear who and how many Comment: We judged this as at a high risk of bias |
Breneman 1998.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, setting not specified other than in Cincinnati, Ohio, US |
|
| Participants |
Randomised: 156 participants (mean age 48.5 years (SD 12.6) in treatment group and 46.9 years (SD 11.9) in control group, 51 male, 105 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of papules; metronidazole group 13, placebo group 15 Number of pustules; metronidazole group 2, placebo group 3 Baseline rosacea severity score; metronidazole group 2.10 (0.24), placebo group 2.16 (0.33) |
|
| Interventions | 10 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Unclear, reprint requests "Dermik Laboratories Inc,", the manufacturer of metronidazole, but no source of funding reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 44): "This was a double‐blind, randomized, parallel group clinical trial..." "Patients were randomly assigned..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 44): "This was a double‐blind, randomized, parallel group clinical trial comparing the efficacy of metronidazole 1% cream to vehicle." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 44) : "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17/156 (10.8%); metronidazole group (15), placebo group (2). Reasons reported. Per‐protocol analysis Comment: Double the number (104) patients were enrolled in the active treatment group compared to 52 in the vehicle group. The percentage of excluded patients in the treatment group was higher than in the vehicle group. Because far more people in this group took prohibited medication that could have influenced in a positive way the outcomes on rosacea, the review authors consider that this does not pose any threat to the validity of the results in this study Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study. Adequate study duration. No medication allowed that might influence outcome Comment: The study appears to be free of other forms of bias |
Breneman 2004.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, University Dermatology Consultants, Cincinnati; The Savin Centre, New Haven; Department of Dermatology, University of Pennsylvania School of Medicine, US |
|
| Participants |
Randomised: 53 participants (mean age 43.1 years (SD 11.7) in treatment group and 45.7 years (12.9) in control group, 8 male and 18 female in treatment group, 9 male and 17 female in control group) Inclusion criteria
No ocular rosacea Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of papules and pustules; treatment group 17.7 (9.7), vehicle group 19.3 (11.4) |
|
| Interventions | Twelve weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
Leyden 2004 ‐ same study, different outcome measures. Overall global improvement as rated by 3 independent investigators using photographs ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 381): "This study was supported by Dermik Laboratories, a division of Aventis Pharmaceuticals Inc, Berwyn, PA", Dermik Laboratories is the manufacturer of BenzaClin® | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) Some SDs are lacking, and most data are skewed. This also applies to Leyden 2004 See comparison 19 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 382): "Patients were randomly assigned in a 1:1 ratio...... Randomization was performed according to a computer generated random code." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 382): "Treatments were identified by a code number, which was assigned in chronological order at each site." Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 382): "BP/C gel and vehicle only gel were supplied in identical jars and were indistinguishable in color, texture, and smell. Both were packaged in identical patient kits with indistinguishable labelling." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17/156 (10.8%); metronidazole group (15), placebo group (2). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | No information about sponsorship or support was reported. Wash‐out period before study unreported, nor if other medications were recorded or allowed that might influence the outcomes Comment: Insufficient information to assess whether important risk of bias exists |
Bribeche 2015.
| Methods | RCT, prospective, placebo‐controlled, single‐blind Date of study November 2012 to August 2013 Setting Dermatology Clinic of Zaporozhye, University Hospital, Zaporozhye, Ukraine |
|
| Participants |
Randomised: 65 participants (age 25 to 67 years, 32 male, 33 female) Inclusion criteria
Ocular rosacea: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data (N or mean (range)) IGA score minimal; praziquantel (4), vehicle (1) IGA score mild; praziquantel (11), vehicle (9) IGA score moderate; praziquantel (28), vehicle (12) CEAS score mild; praziquantel (5), vehicle (3) CEAS score moderate; praziquantel (12), vehicle (8) CEAS score significant; praziquantel (26), vehicle (11) DLQI; praziquantel 15.8 (4 to 23), vehicle 14.6 (5 to 21) |
|
| Interventions | 12 weeks with 4 weeks follow‐up Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 1 Epub): "Funding: None" | |
| Declaration of interest | Quote (page 1 Epub): "Conflicts of interest: None" | |
| Notes | Two of our primary outcomes was addressed (quality of life and adverse events) See comparison 34 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2 Epub): "were randomly assigned" and "using a computer‐generated randomization schedule" Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Not sure if allocation concealment and blinding are confused. There was insufficient information to permit a clear judgement After e‐mail communication it became clear that two investigators had access to the list Comment: We judged this as at a high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" in which the subjects were blinded to the treatment affectation" Comment: Investigators not blinded. The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (page 2 Epub): "The assignment was performed in a single‐blinded manner (for safety reasons" in which the subjects were blinded to the treatment affectation" After e‐mail‐communication: "praziquantel ointment and the placebo had the same colour (white), and ointment were given to participants in identical boxes for both groups" Comment: Outcomes were participant and investigator assessed. As the investigators were not blinded the outcome measurement of IGA and CEA are likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/65 (3%), ITT analysis. Reasons for withdrawals reported Comment: We considered this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period prior to study entry adequate, no other treatments allowed, no sponsoring Comment: The study appears to be free of other forms of bias |
Buendia‐Bordera 2013.
| Methods | Randomised, prospective, placebo‐controlled, within‐patient comparison
Date of study Unreported Setting Instituto de Fotomedicina, Centro Medico Teknon, Barcelona, Spain |
|
| Participants |
Randomised: 31 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Not reported Baseline data (mean) Nothing reported |
|
| Interventions | One treatment, follow‐up 30 days Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, day 1, 9, 21 and 30 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Nothing reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed Abstract, few data presented. Unable to contact principal investigator, no exact data are provided (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 43): "applied on a randomized side of the face" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts and withdrawals Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Carmichael 1993.
| Methods | RCT, prospective, placebo‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Department of Dermatology, University Wales College of Medicine, Cardiff, UK |
|
| Participants |
Randomised: 33 participants (mean age 56.9 years for males and 52.8 years for females, 15 male, 18 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data mean (SEM) Number of papules; azelaic acid site 13.0 (1.5), vehicle site 13.3 (1.6) Number of pustules; azelaic acid site 1.2 (0.4), vehicle site 1.6 (0.5) |
|
| Interventions | 13 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared. Two investigators were employed by Schering AG, Berlin, Germany, the manufacturer of azelaic acid cream | |
| Notes | One of our primary outcomes was addressed (adverse events) Subjective severity scale and overall rating by physicians is not consistent and data on inflammatory lesions were skewed See comparison 6 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S19): "Allocation of the preparations to the facial side was randomized." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page S19): "Comparison between 20% azelaic acid and its identical‐appearing vehicle." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts (page S21). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period adequate before start, study duration adequate. No topical or systemic medications that could influence outcomes were allowed Comment: The study appears to be free of other forms of bias |
Chang 2012.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Two centres, Massachusetts General Hospital, Boston and Stanford Hospital and Clinic, Redwood City, US |
|
| Participants |
Randomised: 83 participants (mean age 52.2 years, 23 male, 57 female and 3 gender unreported) Inclusion criteria
Exclusion criteria
Ocular involvement: Unclear Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; clindamycin + tretinoin group 14.3 (9.5), placebo group 18.7 (14.1) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 2, 6 and 12 Outcomes of the trial (as reported) Primary outcomes:
Secondary outcomes:
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (338): "This study was funded by a grant from Medicis" | |
| Declaration of interest | Quote (338):"The authors have no conflict of interest to disclose" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 28 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 334): "Qualifying subjects were randomized via a computerized random number generator" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 334): "The research staff member who randomized the study population was not involved in any study assessments." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 334): "CT gel and placebo gel were indistinguishable on visual inspection with respect to color, consistency and odor" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/83 were not included in the analyses. Per‐protocol analysis Comment: Low number of participants excluded from analysis and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00823901). In the protocol reduction of transient erythema was the single secondary outcome and was specified in Methods section of the report but embedded in improvement of clinical features of rosacea. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally The study appeared to be free of other forms of bias |
Dahl 1998.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (6 centres) in US We only included second phase (first phase was open and not controlled) |
|
| Participants |
Randomised: 88 participants (mean age 48.6 years in treatment group versus 43.7 years in control group, 32 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; metronidazole group 0.9 (2.2) and vehicle group 0.5 (1.0) |
|
| Interventions | Six months Intervention
Comparator
|
|
| Outcomes | Assessments (7): baseline, week 4, 8, 12, 16, 20 and 24 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 683): "The study was funded by a grant from Galderma Laboratories Inc, Fort Worth, Tex." | |
| Declaration of interest | Quote (page 683): "Dr Herndon is a paid consultant for Galderma Laboratories Inc. Drs Tuley and Czernielewski and Mr Baker are employees of Galderma laboratories Inc." | |
| Notes | None of our primary outcomes were addressed. We only included the double‐blind randomised second phase See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 680): "...were randomized into 2 treatment groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 680): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 680): "..double‐blind.." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/88 (37.5%); metronidazole group (14) and vehicle group (19). 9/44 in metronidazole group relapsed, versus 18/44 in vehicle group. No subjects discontinued because of adverse events
Quote (page 680): "An intention‐to‐treat analysis was conducted for relapse rates, lesion counts and erythema. For subjects who experienced relapse or discontinued for other reasons, lesions counts and erythema were carried forward as data for all subsequent visits to prevent drop‐out bias" Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Adequate study duration, sponsorship and declaration of interest stated. No wash‐out period (first phase was active treatment), unclear if groups were treated equally aside from intervention Comment: Insufficient information to assess whether an important risk of bias exists |
Dahl 2001.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Department of Dermatology, Mayo Medical School, Scottsdale; Department of Dermatology, Baylor College of Medicine, Houston; Department of Dermatology, University of Missouri, Kansas City School of Medicine, US |
|
| Participants |
Randomised: 72 participants (mean age 45 years (range 22 to 78) in 0.75% cream group versus 47 years (range 28 to 75) in metronidazole 1% group, 10 male and 26 female in metronidazole 0.75% group versus 11 male and 25 female in metronidazole 1% group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of inflammatory lesions; metronidazole 0.75% group 19, metronidazole 1% group 25 |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 738): "Supported by Galderma Laboratories, Inc." | |
| Declaration of interest | Quote (page 738): "Dr Tuley and Mr Baker are employees of Galderma Laboratories. Drs Dahl, Jarratt, and Kaplan all received financial compensation from Galderma Laboratories, Inc for performing this study" | |
| Notes | None of our primary outcomes were addressed See comparison 3 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 725): "Patients were randomly assigned to receive 0.75% metronidazole cream or 1.0% metronidazole cream." E‐mail contact with the investigator confirmed "subjects were randomised to 1 of the 2 treatment groups at a ratio of 1:1. The randomisation process was done in blocks of 4, stratified by investigators. The randomisation was carried out using SAS PROC PLAN." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 724): "A double‐blind format was not used because the study drugs were label‐blinded commercial products contained in tubes of different sizes and shapes." Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (page 724) : "A double‐blind format was not used because the study drugs were label‐blinded commercial products contained in tubes of different sizes and shapes." Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/72 (15.3%); metronidazole 0.75% group (4), metronidazole 1% group (7). ITT analysis, based on LOCF However, "Intention to treat population ranged from 30 to 35 subjects in 0.75% metronidazole group and from 29 to 34 in 1.0% metronidazole group." Page 725 Comment: ITT population did not appear to include all randomised participants. Unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period adequate, study duration adequate, groups treated equally. Sponsoring by Galderma Laboratories, Inc. 2 authors are employees of Galderma Quote (page 723): "The authors received financial compensation from Galderma Laboratories, Inc for performing this study." Comment: The study was not double‐blind combined with the financial support may pose a potential risk of bias |
Del Rosso 2007a.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study June 2004 to April 2005 Setting Multicentre, 14 sites in US |
|
| Participants |
Randomised: 251 participants (age 46.8 (SD 13.2) in treatment group and 47.6 (SD 11.5) in placebo group, 91% (SD 71.7) female in treatment group, and 95% (SD 76.6) female in placebo group) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Lesion counts (papules, pustules, nodules); doxycycline group 19.5 (8.8), placebo group 20.3 (10.4) Clinical erythema assessment; doxycycline group 9.7 (3.0), placebo group 9.5 (2.7) |
|
| Interventions | 16 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | All authors have received grants from Collagenex or worked as consultants for Collagenex (page 791) | |
| Notes | One of our primary outcomes was addressed (adverse events) Some SD were missing and these were calculated by the review authors See comparison 43 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 794): "For each study site, a master randomisation list in blocks of 4 was prepared by the sponsor for all study sites. With the use of a computer‐generated randomisation scheme, patients were assigned in equal proportions (1:1) to receive drug or placebo." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 794): "Master randomisation list in blocks of 4 was prepared by the sponsor for all study sites." Comment: A form of central randomisation was used. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to the identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to the identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study, adequate study duration, clinically significant concomitant drug therapy was forbidden Study supported by Collagenex Pharmaceuticals. All authors have received grants from Collagenex or worked as consultants for Collagenex Comment: As the study appeared to be triple‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
Del Rosso 2007b.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study June 2004 to April 2005 Setting Multicentre, 14 sites in US |
|
| Participants |
Randomised: 286 participants (age 46.3 (SD 12.7) in treatment group and 47.6 in placebo group, 94% (SD 66.2) female in treatment group, and 95% (SD 66.0) female in placebo group) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Lesion count; doxycycline group 20.5 (11.7), placebo group 21.23 (12.5) Clinical erythema assessment; doxycycline group 9.5 (2.9), placebo group 9.1 (2.5) |
|
| Interventions | 16 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | All authors have received grants from Collagenex or worked as consultants for Collagenex (page 791) | |
| Notes | One of our primary outcomes was addressed (adverse events) Some SD were missing and these were calculated by the review authors See comparison 43 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 794): "For each study site, a master randomisation list in blocks of 4 was prepared by the sponsor for all study sites. With the use of a computer‐generated randomisation scheme, patients were assigned in equal proportions (1:1) to receive drug or placebo." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 794): "Master randomisation list in blocks of 4 was prepared by the sponsor for all study site." Comment: A form of central randomisation was used. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 794): "Investigators, study site personnel, and patients were blinded with respect to identity of the study medication being taken. All the employees of the sponsor and its affiliates who were involved in data monitoring, data entry, or data analysis were blinded as well." "Study drug and placebo capsules were identical in size, shape, and colour." Blinding of the outcomes assessors, key personnel, was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before the study, adequate study duration, clinically significant concomitant drug therapy was forbidden Study supported by Collagenex Pharmaceuticals. All authors have received grants from Collagenex or worked as consultants for Collagenex Comment: As the study appeared to be triple‐blinded and there was no selective reporting we do not consider that the sponsorship and support represented any additional bias |
Del Rosso 2008.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Valley Hospital Medical Center, Las Vegas; Department of Dermatology, Advanced Skin Research Center, Omaha, University of Washington, Washington, US |
|
| Participants |
Randomised: 91 participants (age 44.3 years in 40 mg group and 45.2 in 100 mg group, 29 females and 15 males in 40 mg group and 35 females and 12 males in 100 mg group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and wWithdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | 16 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 576): "The study was supported through educational grants from Collagenex Corporation" | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) All SD are missing and these were calculated by the review authors See comparison 50 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 574): "Subjects were randomized to receive daily administration of drugs." Comment: Insufficient information about the method used to generate the allocation sequence to allow an assessment of whether it should produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 574): "Both the doxycycline 100 mg capsules and the 40 mg capsules were over encapsulated to ensure the capsules were indistinguishable during administration and to maintain a double‐blind study." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 574): "Both the doxycycline 100 mg capsules and the 40 mg capsules were over encapsulated to ensure the capsules were indistinguishable during administration and to maintain a double‐blind study." Blinding of the outcomes assessors, key personnel, and participants was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data were adequately addressed, reasons for withdrawal reported, no differences between the 2 groups. ITT analysis Comment: High but balanced dropout rate and although combined with ITT analysis (LOCF) judged as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate wash‐out period before study started, adequate study duration, clinically significant concomitant drug therapy was not permitted Comment: The study appears to be free of other forms of bias |
Del Rosso 2010.
| Methods | Randomised, prospective, active‐control, investigator‐blind Date of study February to July 2009 Setting Multicentre US |
|
| Participants |
Randomised: 207 participants (mean age 49 years, 71 male, 136 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean) Number of inflammatory lesions; azelaic acid group 20.6, metronidazole group 21.9 |
|
| Interventions | 12 weeks Intervention
Comparator
Patients were instructed how to clean their face and what to use to clean their face and what moisturizer to use. No other soaps, cleansers and moisturizers were allowed |
|
| Outcomes | Assessments (6): baseline week 2, 4, 6, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 612): "This study was supported by Intendis" | |
| Declaration of interest | Quote (page 612): "Dr Del Rosso is a consultant to and serves as a speaker for ...Galderma...Intendis...Dr Bruce has served as an investigator (grants) for Actavis......Dr Jaratt has served as consultant for Stiefel...He has received honoraria from ...Galderma, ...He has been principal investigator for...Galderma..Intendis...Dr Menter is a consultant, speaker, and is on the advisory board for Abbott....He is a consultant and speaker for Eli Lilly and Stiefel. He is an investigator for .....He has received grants and honoraria from ....etc "He received honoraria from Galderma...." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 51 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 608‐9): "were randomized at a ratio of 1:1.." and "randomly assigned" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "Randomization was done centrally by the generation of a randomization list using the randomization program RANCODE (version 3.6). Randomization used blocks." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "..each newly enrolled patient was allocated to study medication with the lowest randomization number available in that particular site at the subjects baseline visit." Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 608): "investigator‐blinded" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "Six drug tubes (tubes with a blinded label to cover the trademarks) and 3 bottles were packaged by a CMO in individual numbered kit boxes. ...The patient was advised not to discuss the treatment schedule with the investigator." Comment: Blinding of investigators effective, however participants were not blinded but unlikely to represent a threat to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 608): "investigator‐blinded". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement After e‐mail communication: Blinding of investigators effective, but in view of the different treatment regime once versus twice daily, blinding of participants was not ensured and therefore we judged this as at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/207 (6.3%); azelaic acid group (6), metronidazole group (7), reasons in part reported. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov NCT00855595, and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, clinically significant concomitant drug therapy was not permitted, groups treated equally Comment: The study appears to be free of other forms of bias |
Draelos 2005b.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Wake Forest University School of Medicine, Winston‐Salem, North Carolina, US |
|
| Participants |
Randomised: 30 participants (ages between 20 and 65, gender unreported, both sexes) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Four weeks
Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 881): "This study was funded by an educational grant from Cutanix Corporation" | |
| Declaration of interest | Quote (page 881): "Zoe Draelos, MD, has indicated no significant interest with commercial supporters, Bryan Fuller, PhD, is the inventor of the active, which was licensed through the Oklahoma Health Sciences Center to Cutanix" | |
| Notes | One of our primary outcomes was addressed (adverse events) SDs are missing from the report See comparison 32 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 882): "The 30 subjects were randomized at a 2:1 ratio." Comment: Unclear E‐mail contact with the investigator confirmed a random number generator was used Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 882): "All products were dispensed in identical bottles with identical labelling. Neither the dermatologist investigator nor the subjects knew the contents of the bottle." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 882): "All products were dispensed in identical bottles with identical labelling. Neither the dermatologist investigator nor the subjects knew the contents of the bottle." Blinding of the outcomes assessors, key personnel, and participants was ensured, and it was unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for the 2 withdrawals were reported, but unclear in which group. After clarification with the author this was confirmed as 1 in each group. Per‐protocol analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | High risk | Percentage improvement in dermatitis was not addressed, no exact data were reported for the self‐assessments carried out by the participants Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | One of the investigators is the inventor of the formula, which may represent a potential conflict of interests. No baseline balance descriptives. Treatment duration adequate, no wash‐out prior to study described Comment: We judged this as at unclear risk of bias |
Draelos 2006.
| Methods | RCT, prospective, "placebo"‐controlled, investigator‐blinded Date of study Unreported Setting Department of Dermatology; Wake Forest University School of Medicine, Winston‐Salem, North Carolina, US |
|
| Participants |
Randomised: 67 participants (age between 19 to 66, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not specified Dropouts and withdrawals
Baseline data mean (SD) Lesion counts; group non‐standardised care 10, group PHA skin care 7 (estimated from a graph) |
|
| Interventions | 12 weeks Intervention
Comparator
Unclear to which groups the other five participants were allocated |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Two investigators were employed by NeoStrata Company, Inc., Princeton, NJ, however, no conflict of interest declared | |
| Notes | None of our primary outcomes were addressed The combination of incomplete and selective reporting of outcome data did not permit entry of any data into a meta‐analysis. It was unclear how many participants were randomised to each intervention and because very limited outcomes data were reported no reliable conclusions could be drawn (Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 23): "The investigation was designed as a 12‐week investigator blinded, randomized study of parallel groups." Comment: Unclear E‐mail contact with the investigator confirmed "a randomisation schedule with a random number generator was developed" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 23): "...investigator‐blinded." Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 23): "...investigator‐blinded." Comment: Both the participant and the investigator were outcomes assessors and the report was unclear what measures were used, if any, to blind study personnel from knowledge of which intervention a participant received Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five participants were lost to follow‐up for "personal reasons", and it was unclear how many occurred in each group, at which stage of the study, and whether data were available for any of the other assessment time points. Per‐protocol analysis. After e‐mail contact with the author: "the dropouts were for personal reasons, not related to product. They were random between the groups" Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | Not all predefined outcomes were addressed or reported clearly, i.e. Investigator's Global Assessment of rosacea, observations of tingling and tightness by participants. No precise data were reported, data had to be estimated from figures Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Wash‐out period adequate, study duration adequate. No baseline descriptives Study sponsorship was not reported, but 2 authors were from Neostrata Company the manufacturer of the PHA cleanser and moisturizer. Unclear how many participants started in each group. Possible imbalance in the baseline scores of lesion count in the 2 groups. The actual comparison was non‐standardised skin care versus PHA moisturizer Comment: We judged this as at a high risk of bias |
Draelos 2009.
| Methods | Randomised, prospective, active‐controlled, double‐blind
Date of study Unreported Setting Unspecified, US |
|
| Participants |
Randomised: 146 women, age not reported Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Six weeks (first 2 weeks wash‐out period) Intervention
Comparator
Unclear how many were randomised to each group |
|
| Outcomes | Assessments (2): baseline, week 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared but four investigators are employed by The Proctor and Gamble Company, Cincinnati, OH, US | |
| Notes | Poster abstract, limited data None of our primary outcomes was addressed, no response from PI to fill in gaps (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB82): "subjects were randomized to" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page AB82): "double‐blind" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page AB82): "double‐blind". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on drop‐outs and withdrawals Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Draelos 2013a.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (20) in US |
|
| Participants |
Randomised: 401 participants (mean age 48.5 years (range 19 to 83 years), 103 male, 298 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data N (%) Moderate rosacea; azelaic acid group 172 (86.9), vehicle group 189 (93.1) Severe rosacea; azelaic acid group 26 (13.1), vehicle group 14 (6.9) |
|
| Interventions | 12 weeks
Intervention
Comparator
|
|
| Outcomes | Assessments (5); baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None declared. Quote (page 315): "Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals" | |
| Declaration of interest | Quote (page 306): "Dr. Draelos is a researcher for Bayer HealthCare Pharmaceuticals. Dr. Elewski has conducted clinical research for Bayer HealthCare Pharmaceuticals and Galderma Laboratories, LP. Mr. Staedtler and Dr. Havlickova are employees of Bayer HealthCare Pharmaceuticals" | |
| Notes | All our primary outcomes are addressed See comparison 6 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 308): "The computer‐generated randomization procedure used blocks. Whole randomization blocks were allocated to the study centers, ensuring that the comparison groups maintained the planned allocation ratio for the treatment groups overall and within each center" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Form of central allocation Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 307): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 307): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement After e‐mail communication: "The blind was maintained by dispensing the vehicle and the vehicle plus the active in identical containers" Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41/401 (10.2%); azelaic acid group (21), vehicle group (20), reasons reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was available on clinicaltrials.gov (NCT01025635). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. However, exact data on QoL scores were missing which is a primary outcome in our review Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally The study appeared to be free of other forms of bias |
Draelos 2013b.
| Methods | Randomised, prospective, active‐controlled, double‐blind
Date of study Unreported Setting Dermatology clinic and the routine setting of a woman's home, US |
|
| Participants |
Randomised: 40 women (age unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data mean Nothing reported |
|
| Interventions | Three weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3); baseline, week 1 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared. Three investigators are employed by Johnson & Johnson Consumer Companies, Inc, Skillman, NU, US | |
| Notes | None of our primary outcomes were addressed There are no separate data on women with rosacea (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 314‐6): "randomized".."were divided equally into two groups" and "Study participants were stratified and balanced for demographics and presence and severity of acne, eczema, rosacea and atopic dermatitis"
Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "Subjects were randomized in two balanced populations based on a computer generated randomization sequence" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: No further additional information to change our judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 314‐5): "double‐blind" Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "..identically appearing products packaged identically" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 314‐5): "double‐blind". Outcomes were investigator as well participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement After e‐mail communication: "..identically appearing products packaged identically" Comment: Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow up Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, clinically significant concomitant drug therapy was not permitted, groups treated equally Comment: The study appears to be free of other forms of bias |
Dreno 1998.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unspecified Setting Multicentre, several centres in France |
|
| Participants |
Randomised: 100 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Unclear Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared. One investigator was employed by Galderma, manufacturer of at least one of the investigated drugs | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 4 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (S272): "This multicenter, controlled, randomized, investigator‐masked study..." Comment: Insufficient information about the method used to generate the allocation sequence to allow an assessment of whether it should produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page S272): "...investigator‐masked study." Not clear what measures were used to blind study participants and personnel from knowledge of which intervention a participant received Outcomes assessments: Principally by the investigators Comment: The report did not provide sufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page S272): "...investigator‐masked study." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (S272): "100 patients enrolled and analysed for ITT..." (21 withdrew/12 losses to follow up). Per‐protocol analysis at week 12 ‐ 67/100 Comment: Losses were accounted for but the data analysis as reported appeared to be per‐protocol with exclusion of outcome data for 33/100 participants. We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | One pre‐specified outcome was inadequately addressed and reported: Investigator's Global Assessment of improvement Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Wash‐out period not stated, study duration adequate, unclear if groups were treated equally. Poster abstract Comment: Insufficient information to permit a clear judgement |
Elewski 2003.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Multicentre, 13 centres in US |
|
| Participants |
Randomised: 251 participants (mean age 49 years in treatment group versus 46 years in control group, 32 male and 92 female versus 34 male and 93 female) Inclusion criteria
Ocular involved: Participants with marked involvement were excluded Exclusion criteria
Dropouts and withdrawals
Baseline data mean Lesion counts; azelaic group 18, metronidazole group 19 |
|
| Interventions | 15 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page 1444): "The authors received financial compensation from Berlex Laboratories Inc, Montville, NJ, for serving as principal investigators for this study" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1145): "Computer‐generated block wise randomisation method was used to ensure balance between the groups..." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 1445): "Assignment occurred by the physician in ascending order with newly accepted patient receiving study medication with the lowest randomisation number available in the center." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 1445): "To preserve blinding, study medication was dispensed and collected only by a study nurse or assistant not involved with selection and assessment of patients." Comment: The report was also unclear what measures were used to blind study participants from knowledge of which intervention they received or any information relating to whether the intended blinding was effective Comment: Insufficient information to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 1445): "To preserve blinding, study medication was dispensed and collected only by a study nurse or assistant not involved with selection and assessment of patients." Comment: Assignment to intervention was by the investigators who were also the outcomes assessors. No satisfactory evidence of blinding. Outcomes were investigator and participant assessed Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. All participants were accounted for Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Wash‐out period and study duration adequate, not permitted to receive any concurrent therapy. Authors received financial compensation from Berlex Laboratories, Inc, Montville, NJ, for serving as principal investigators for this study Comment: Insufficient information to assess whether important risk of bias exists |
Ertl 1994.
| Methods | Randomised, prospective, placebo‐controlled (both groups have same topical treatment but different systemic treatments), double‐blind, cross‐over Date of study March to May 1991 Setting Department of Dermatology University of Arizona, and University of Pennsylvania School of Medicine, US |
|
| Participants |
Randomised: 22 participants (mean age 59 years, 12 male, 10 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria:
Dropouts and withdrawals
Baseline data mean Individual participant data are provided for lesion counts, comparable |
|
| Interventions | 16 weeks to cross‐over but oral isotretinoin withheld Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (2): baseline and week 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | After 16 weeks cross‐over but oral isotretinoin withheld; second phase unbalanced comparison. We only included first phase One of our primary outcomes was addressed (adverse events) Data unreliable, its re‐analysis using the individual participant data confirmed its flawed analysis by the investigators (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 320): "three separate treatment groups were randomly assigned" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 320) : "Subjects were given coded bottles containing either isotretinoin or placebo capsules. The creams were dispensed in tubes containing either 0.025% tretinoin cream or the vehicle" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/22 lost to follow‐up; data presented as individual participant data Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. Data unreliable, its re‐analysis using the individual participant data confirmed its flawed analysis by the investigators Comment: We judged this as at a high risk of bias |
| Other bias | Low risk | Wash‐out phase before study started adequate, study duration adequate, groups treated equally, in first 16 weeks, no sponsoring Comment: The study appeared to be free of other forms of bias |
Espagne 1993.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study April to October 1990 Setting Multicentre (18), France |
|
| Participants |
Randomised: 51 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Inflammatory lesions; metronidazole group 10.7 (7.9), placebo group 15.4 (12.5) |
|
| Interventions | Six weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline week 3 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Two investigators were employees of Schering Plough | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) Allocation to intervention was based on up to 4 participants in each of 18 clinics but not all clinics enrolled 4 participants. The report did not provide any reassurance that the allocation sequence was adequately generated (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote (page 129): "la randomisation a porté sur des groups de 4, chaque médicin constituent un centre et devant inclure 4 malades" Comment: Allocation to intervention was based on up to 4 participants in each of 18 clinics but not all clinics enrolled 4 participants. The report did not provide any reassurance that the allocation sequence was adequately generated and there was lack of evidence that any form of central randomisation had been employed for the 18 clinics involved in this study |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 130): "Les emballages, les tubes, la coloration des gels étaient strictement comparables et indiscernables par les malades ou les expérimateureurs" (packaging, tubes, colour of gels were indistinguishable for participants and investigators). Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/51 (11.7%); metronidazole group (2), placebo group (4), ITT (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out phase before study started adequate, study duration adequate, groups treated equally Comment: This study appears to be free of other forms of bias |
Fabi 2011.
| Methods | Randomised, prospective, controlled, within‐patient comparison Date of study Unreported Setting Laser clinic, San Diego, US |
|
| Participants |
Randomised: 20 participants (mean age 46.5 years, 2 male, 9 female, 9 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Six weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3); baseline, week 2 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Poster abstract, limited data, unable to contact investigators One of our primary outcomes was addressed (participant assessed changes in rosacea severity). No exact data were provided (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 969): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported. Outcomes were investigator as well participant‐assessed Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/20 (45%); reasons unreported. Per‐protocol analysis Comment: High dropout rate assessed as at a high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Fowler 2007.
| Methods | RCT, prospective 'placebo'‐controlled (both treatment arms had same topical treatment; one arm systemic active treatment versus placebo), double‐blind Date of study Unreported Setting Multicentre ‐ unclear which ones but at least Department of Dermatology, University of Louisville, Louisville, US |
|
| Participants |
Randomised: 72 participants (age unclear, 16 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of lesions; doxycycline group 21.3 and placebo group 18.7 Basal erythema score; doxycycline group 8.6 and placebo group 9.2 |
|
| Interventions | 16 weeks Intervention
Comparator
After 12 weeks, metronidazole gel stopped, but oral medication or placebo continued until week 16 |
|
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed We only included data from the first 12 weeks of the study See comparison 52 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 642): "This was a randomized, multi‐center, outpatient, double‐blind placebo‐controlled trial." E‐mail contact with the investigator confirmed randomisation was carried out using a computer‐generated table provided by the sponsor Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence was not reported E‐mail contact with the investigator confirmed "pharmacy‐controlled central allocation and neither investigators or study staff were involved in the generation of the sequence" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 642): "This was a randomized...double‐blind..." Comment: The report did not describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Insufficient information to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 642): "This was a randomized...double‐blind..." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/72 (11.1%); doxycycline group (6) and placebo group (2). Per‐protocol analysis Comment: Low number of dropouts, and although slightly unbalanced, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate. Wash‐out phase before study not reported, groups treated equally Comment: We judged this as at low risk of bias |
Fowler 2012a.
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind
Date of study
Unreported Setting Multicentre (5) in US |
|
| Participants |
Randomised: 122 participants (mean age 45.7 years (SD 12.1), 30 male, 92 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None Baseline data N (%) CEA moderate; BT 0.07% 22 (78.6), BT 0.18% 23 (74.2), BT 0.5% 23 (74.2), vehicle 25 (78.1) CEA severe; BT 0.07% 6 (21.4), BT 0.18% 8 (25.8), BT 0.5% 8 (25.8), vehicle 7 (21.9) PSA mild; BT 0.07% 1 (3.6), BT 0.18% 1 (3.2), BT 0.5% 0 (0), vehicle 2 (6.3) PSA moderate; BT 0.07% 12 (42.9), BT 0.18% 24 (77.4), BT 0.5% 26 (83.9), vehicle 26 (81.3) PSA severe; BT 0.07% 15 (53.6), BT 0.18% 6 (19.4), BT 0.5% 5 (16.1), vehicle 4 (12.5) |
|
| Interventions | One application, follow‐up 12 hours
Intervention
Comparator 1
Comparator 2
Comparator 3
|
|
| Outcomes | Assessments (14): baseline, 30 min, 1 hour and then each hour until 12 hours Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 633): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 633): "The investigators received grants for conducting the studies. YL and ML are employees of Galderma R&D" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 10 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 634): "Subjects were randomized in a 1:1:1:1 ratio to receive" and "randomization lists were generated prior to study initiation by an independent statistician using SAS hoc Plan procedure (SAS Institute, Cary, NC, U.S.A.)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 634): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labelling and packaging had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 634): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT00989014). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | This is a phase II study, duration for this design adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
Fowler 2012b.
| Methods | Randomised, prospective, active‐ and placebo‐controlled, double‐blind
Date of study
Unreported Setting Multicentre (17) in US |
|
| Participants |
Randomised: 269 participants (mean age 44.3 years, 52 male, 217 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data N (%) CEA moderate; BT 0.18% QD 44 (81.5), BT 0.18% BID 42 (77.8), BT 0.5% 47 (88.7), vehicle QD 48 (87.3), vehicle BID 44 (83) CEA severe; BT 0.18% QD 10 (18.5), BT 0.18% BID 12 (22.2), BT 0.5% 6 (11.3), vehicle QD 7 (12.7), vehicle BID 9 (17) PSA moderate; BT 0.18% QD 45 (83.3), BT 0.18% BID 45 (83.3), BT 0.5% 44 (83), vehicle QD 46 (83.6), vehicle BID 45 (84.9) PSA severe; BT 0.18% QD 9 (16.7), BT 0.18% BID 9 (16.7), BT 0.5% 9 (17), vehicle QD 9 (16.4), vehicle BID 8 (5.1) |
|
| Interventions | Four weeks, and four weeks follow‐up
Intervention
Comparator 1
Comparator 2
Comparator 3
Comparator 4
|
|
| Outcomes | Assessments (23): baseline (5x), day 1 (5x), 15 (5x), 29 (5x), week 5, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 633): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 633): "The investigators received grants for conducting the studies. YL and ML are employees of Galderma R&D" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 11 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 634): "Subjects were randomized in a 1:1:1:1:1 ratio to the groups" and "randomization lists were generated prior to study initiation by an independent statistician using SAS hoc Plan procedure (SAS Institute, Cary, NC, U.S.A.)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 634): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labelling and packaging had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 634): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/269 (3.3%); BT 0.18% QD (2), BT 0.18% BID (2), BT 0.5% (2), vehicle QD (2), vehicle BID (1), reasons reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01174030). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | This is a phase II study, duration for this design adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
Fowler 2013a.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind
Date of study
May 2011 to September 2011 Setting Multicentre in US and Canada |
|
| Participants |
Randomised: 260 participants (mean age 48.8 years, 54 male, 206 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data N (%) CEA moderate; brimonidine tartrate 0.5% gel group 111 (86), vehicle gel group 113 (86.3) CEA severe; brimonidine tartrate 0.5% gel group 18 (14), vehicle gel group 18 (13.7) PSA mild; brimonidine tartrate 0.5% gel group 0 (0), vehicle gel group 1 (0.8) PSA moderate; brimonidine tartrate 0.5% gel group 107 (82.9), vehicle gel group 114 (87) PSA severe; brimonidine tartrate 0.5% gel group 22 (17.1), vehicle gel group 16 (12.2) |
|
| Interventions | Four weeks with four weeks follow up
Intervention
Comparator
A wash‐out period was mandatory for subjects receiving prescription medications for inflammatory conditions, rosacea, or acne (for most treatments 4 weeks, isotretinoin 6 months) |
|
| Outcomes | Assessments (6): baseline, day 1, 15, 29, week 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 656): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 656): "The investigators received grants for conducting the studies. Ms. Rudisill and Dr. Leoni are employees of Galderma R&D." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 12 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 651): "Subjects were randomized in a 1:1 ratio to the groups of BT gel 0.5% and vehicle gel" and "Randomization lists were generated prior to study initiation by an independent statistician using SAS Proc Plan procedure" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 651): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labeling and packaging had access" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 651): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/260 (2.3%); brimonidine tartrate 0.5% gel group (2), vehicle gel group (4). ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01355458). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
Fowler 2013b.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind
Date of study
May 2011 to November 2011 Setting Multicentre in US and Canada |
|
| Participants |
Randomised: 293 participants (mean age 47.5 years, 80 male, 213 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data N (%) CEA moderate; brimonidine tartrate 0.5% gel group 108 (73), vehicle gel group 115 (79.3) CEA severe; brimonidine tartrate 0.5% gel group 40 (27), vehicle gel group 30 (20.7) PSA mild; brimonidine tartrate 0.5% gel group 0 (0), vehicle gel group 2 (6.3) PSA moderate; brimonidine tartrate 0.5% gel group 129 (87.2), vehicle gel group 122 (84.1) PSA severe; brimonidine tartrate 0.5% gel group 19 (12.8), vehicle gel group 23 (15.9) |
|
| Interventions | Four weeks with four weeks follow‐up
Intervention
Comparator
A wash‐out period was mandatory for subjects receiving prescription medications for inflammatory conditions, rosacea, or acne (for most treatments 4 weeks, isotretinoin 6 months) |
|
| Outcomes | Assessments (6): baseline, day 1, 15, 29, week 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 656): "The two studies were funded by Galderma R&D" | |
| Declaration of interest | Quote (page 656): "The investigators received grants for conducting the studies. Ms. Rudisill and Dr. Leoni are employees of Galderma R&D." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 12 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 651): "Subjects were randomized in a 1:1 ratio to the groups of BT gel 0.5% and vehicle gel" and "Randomization lists were generated prior to study initiation by an independent statistician using SAS Proc Plan procedure" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 651): "The randomization lists were then sent to the clinical supply group, and only the personnel directly involved with labeling and packaging had access" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 651): "The integrity of the blinding was ensured by packaging the topical gels in identical tubes and requiring a third party other than the investigator/evaluator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/293 (3.4%); brimonidine tartrate 0.5% gel group (7), vehicle gel group (3). Reasons not reported. ITT analysis. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on clinicaltrials.gov (NCT01355471). The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
Gollnick 2010.
| Methods | Randomised, prospective, active‐ and placebo‐control, double‐blind Date of study Unreported Setting Multicentre (35) in Germany |
|
| Participants |
Randomised: 573 participants (mean age 53.3 years (SD 14.0), 259 male, 290 female, 24 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data median Number of inflammatory lesions; isotretinoin 0.1 group 17, isotretinoin 0.3 group 18, isotretinoin 0.5 group 16, doxy 18, placebo 19 Physician's Global Assessment; all groups 5 |
|
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Comparator 4
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 6, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 514): "The study was supported by Almirall Hermal GmbH" | |
| Declaration of interest | Quote (page 514): "Professor Gollnick received lecturer fees for the subject rosacea from various firms: Almirall Hermal GmbH, Galderma, Schering/Intendis" | |
| Notes | Two of our primary outcome were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 58 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 506): "were allocated to 5 different treatment groups in a randomized and blinded manner" and "For random assignment to the different treatment groups patients were stratified according to weight (50–70, 71–90, 91–110 and 111–130 kg). After a request by fax through the treating physician a central stratified randomization and mailing of the medication occurred." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 506): "The study medications were blinded according to § 10 of the German Drug Law (Arzneimittelgesetz, AMG) and provided by Almirall Hermal GmbH, Reinbek, Germany. Isotretinoin was employed as capsules with 10 mg isotretinoin and doxycycline as tablets with 50 mg doxycycline each. Due to the double dummy study design each patients had to take both isotretinoin/placebo capsules or doxycycline/placebo tablets." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72/573 (12.6%); Isotretinoin 0.1 mg/kg (10/111), Isotretinoin 0.3 mg/kg (18/147), Isotretinoin 0.5 mg/kg (16/116), doxycycline (20/152), placebo (8/47). reasons reported, Per‐protocol analysis Comment: Low and balanced number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available on https://www.clinicaltrialsregister.eu/ctr‐search/search as EudraCT‐Nr 2006‐002410‐35. The pre‐specified outcomes and those mentioned in the methods section appeared to have been reported. Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, groups treated equally. However, cohorts, and flow diagram are rather unclear. Study supported by Almirall Hermal GmbH and the Principal Investigator received fees Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship or support represented any additional bias |
Grosshans 1997.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Clinique Dermatologique des Hospiteaux Universitaires de Strasbourg, France |
|
| Participants |
Randomised: 34 participants (mean age 44 years (SD 13) in treatment group versus 49 years (14) in control group, 6 male, 28 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Four months Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) Males tend to have more severe rosacea and all the males were in the control group See comparison 60 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 688): "Il' s' aggisait d'un essai randomisé en double insu." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 687): "....en double insu." [translated as 'double‐blind'] Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 687): "....en double insu." [translated as 'double‐blind'] Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/34 (14.7%); rilmenidine group (2) and placebo group (3). ITT analysis. All participants appear to have been accounted for (pages 688, 689) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period long enough before the study, no concomitant therapy for rosacea was allowed, additional medication recorded, sponsorship or support not reported Comment: We judged this as at a low risk of bias |
Guillet 1999.
| Methods | RCT, prospective, active‐controlled, investigator‐masked Date of study Unreported Setting Multicentre, 9 centres in Europe (France, Ireland, Spain, and Belgium) |
|
| Participants |
Randomised: 114 participants (age 22 to 82 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts/Withdrawals: Unclear Baseline data mean (SD) Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, week 12, and maybe more Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | A poster of an old study, much information is either poorly reported or missing, e.g. number of dropouts None of our primary outcomes were addressed (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S145): "The randomised, investigator‐blinded study lasted twelve weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page S145): "...investigator masked." The report did not clarify what measures were used to blind study participants and personnel from knowledge of which intervention a participant received Comment: Insufficient information to make a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page S145): "...investigator masked." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Inadequate reporting of rates of attrition and exclusions to permit clear judgement of (e.g. number randomised not stated, no reasons for missing data provided) |
| Selective reporting (reporting bias) | Unclear risk | Methods section not specific about which outcomes were being sought Quote (page S145): "To compare the efficacy and safety as well as the cosmetic acceptability?" Comment: Insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Study duration adequate, wash‐out phase before study started adequate, groups treated equally, no information about sponsorship or support. Inadequate detail about the baseline characteristics of the participants, the interventions delivered, and methods of standardisation of outcomes assessment across the 9 international centres Comment: Insufficient information to assess whether important risk of bias exists |
Huang 2012.
| Methods | Randomised, prospective, active‐controlled Date of study Unreported Setting Department of Dermatology, the People's Hospital, Zhengzhou, China |
|
| Participants |
Randomised: 60 participants (mean age 31.63 years (SD 9.16), 36 male, 24 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts/Withdrawals: None Baseline data mean Nothing reported |
|
| Interventions | Three months
Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 4 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Translated from Chinese, see Acknowledgements See comparison 66 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 308): "divided randomly into two groups" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported and no sham laser treatment Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported and no sham laser treatment. Investigator and participant assessed outcomes Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study started too short Comment: Insufficient information to assess whether important risk of bias exists |
Huang 2014.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind
Date of study
Unreported Setting Multicentre US |
|
| Participants |
Randomised: 170 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Not reported Baseline data mean Nothing reported |
|
| Interventions | 12 weeks
Intervention
Comparator
Unclear how many were randomised to each group |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page AB9): "Funded by Galderma Laboratories LP" | |
| Declaration of interest | None declared. Several investigators are employed by Galderma Laboratories LP | |
| Notes | One of our primary outcomes was addressed (adverse events) Limited data from poster abstract (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB9): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page AB9): "double‐blind" Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page AB9): "double‐blind" Comment: Outcomes were investigator and participant assessed. Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Poster abstract, with limited information Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided. Pubished as protocol NCT01308619 in clinicaltrials.gov Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Jackson 2013.
| Methods | Randomised, prospective, active‐controlled, double‐blind
Date of study
Unreported Setting Two centres in US |
|
| Participants |
Randomised: 60 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Total lesion count: minocycline 15 (7), minocycline + azelaic acid 15 (5) IGA: minocycline 3 (1), minocycline + azelaic acid 3 (1) CEA: minocycline 9 (2), minocycline + azelaic acid 9 (3) |
|
| Interventions | 12 weeks with four week follow up
Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 4, 8, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 298): "Funding for the study was provided by Medicis" | |
| Declaration of interest | Quote (page 298): "Dr Jackson has served as a speaker, consultant, and investigator for Medicis" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 54 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 294/295): "Treatment was randomly allocated in blocks of 2. Blocks were centrally assigned to investigators as needed and based on enrollment" "The randomization process assigned equal numbers of patients to each treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 294): "Treatment was randomly allocated in blocks of 2. Blocks were centrally assigned to investigators as needed and based on enrollment" Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 295): "Blinded study medication was identified using the patient randomization number" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 295): "Blinded study medication was identified using the patient randomization number" Comment: Outcomes were investigator and participant assessed Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/60 (8.3%); all in minocycline + azelaic acid group, reasons reported Comment: Low number of dropouts and ITT analysis (LOCF) judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out before the study started adequate, no concomitant therapy for rosacea was allowed Comment: We judged this as at low risk of bias |
Jorizzo 1998.
| Methods | RCT, prospective, placebo‐controlled and active‐controlled (4 treatment arms), double‐blind Date of study Unreported Setting Multicentre, Department of Dermatology, Bowman Gray School of Medicine, Wake Forest University, Winston Salem; Department of Dermatology, Mount Sinai Medical School, New York, US |
|
| Participants |
Randomised: 277 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Unclear Baseline data mean (SD) Nothing reported |
|
| Interventions | 10 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Unclear how many participants started in each group |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 7 and 10 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 502): "Supported by Dermik Laboratories, Inc., 500 Arcola Rd, Collegeville, PA 19426." | |
| Declaration of interest | Quote (page 502): "Dr Tobey formerly was formerly Vice President of Research and Development, Dermik Laboratories, Inc." | |
| Notes | One of our primary outcomes was addressed (adverse events) Unclear how many participants started in each group (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 502) : ".... randomized, double‐blind, multicenter trial." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 502): "Patients were blinded as to treatment, and evaluators were blinded as to treatment and application regimen." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 502): "Patients were blinded as to treatment, and evaluators were blinded as to treatment and application regimen." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported. ITT analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Unclear how many participants were in each intervention group. Withdrawals were unreported Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period prior to study entry adequate. Study was supported by Dermik Laboratories, Inc, 500 Arcola Rd, Collegeville, PA 19426. One co‐investigator was formerly vice‐president of research and development, Dermik Laboratories Comment: Realistic and potential risk of bias |
Karsai 2008.
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Participants were recruited from September to November 2006 Setting Laserklinik Karlsruhe, Karlsruhe, Germany |
|
| Participants |
Randomised: 20 participants (age 62 years ± 12.3, 14 male, 6 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria:
Dropouts and withdrawals: None Baseline data mean (SD) Nothing reported |
|
| Interventions | One treatment Intervention
Comparator 1
Comparator 2
If no effect, treatment was repeated up to 3 times in same session Evaluation after 4 weeks |
|
| Outcomes | Assessments (2): baseline and week 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page 702): "The authors have indicated no significant interest with commercial supporters" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 62 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 703): "Patients were randomized to receive one of four treatment regimens." "Twenty patients were studied using the sequence delivery of PDL and NdYAG wavelets combined on one side of their nose This could be right or left side. The other side received either PDL, or NdYAG." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 704): "blinded assessment of photographs taken before and after final evaluation". Investigators were blinded with respect to treatment modality, it is unclear if participants knew what treatment they were receiving on each side of the nose. Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts reported. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias. |
| Other bias | High risk | The investigator used a Chi2 statistic on cell values less than 5, invalidating the analysis Also, reports "possible confounding with ages that was not accounted for" Comment: We judged this as at a high risk of bias |
Kendall 2014.
| Methods | Randomised, prospective, active‐controlled, double‐blind, cross‐over
Date of study
Unreported Setting Multicentre US |
|
| Participants |
Randomised: 70 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 2/70 (2.9%) in brimonidine group; adverse event (1) and protocol deviation (1) Baseline data mean Nothing reported |
|
| Interventions | 15 days
Intervention
Comparator
Wash‐out period (unspecified) and cross‐over |
|
| Outcomes | Assessments (2): baseline and day 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared, investigators employed by Galderma Laboratories, L.P., Fort Worth, TX | |
| Notes | Poster, limited data
Quote: "The results of the second period were discarded as there was significant treatment carryover from the first period" One of our primary outcomes was addressed (participants‐assessed changes in rosacea severity (PSA)) See comparison 24 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page A182): "Subjects were randomized 1:1 to.." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page A181): "double‐masked" Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page A181): "double‐masked". Investigator and participant assessed outcomes Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/70 (2.9%) in brimonidine group; adverse event (1) and protocol deviation (1). Poster abstract, limited information Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data. Comment: There was insufficient information to permit a clear judgement |
Kim 2011.
| Methods | Randomised, prospective, active‐controlled, open label, within‐patient comparison Date of study August 2009 to March 2010 Setting Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea |
|
| Participants |
Randomised: 18 participants (mean age 31.1 years, 5 male, 13 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Three treatments at three weekly intervals Intervention
Comparator
|
|
| Outcomes | Assessments (5), baseline, week 3, 6, 9 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 67 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 575): "According to a computer‐generated randomization, each cheek was randomly assigned to.." Comment: Probably done |
| Allocation concealment (selection bias) | High risk | Quote (page 575): "The randomization schedule was not concealed from physicians who carried out the treatment" Comment: We judged this as at a high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 574): "randomized, open, split‐face.." Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 574): randomized, open, split‐face.." and "Photographs were taken by the same blinded physician at baseline".. "on the blinded investigators’ and patients’ own evaluation. Three blinded dermatologists assessed.." Comment: These statements are contradictory. Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/18 (16.6%); due to difficulty in attending follow‐up because of distance. Per‐protocol analysis Comment: The moderate dropout rate with per‐protocol analysis represents a potential risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Pre‐study wash‐out period adequate, study duration adequate Comment: The study appeared to be free of other forms of bias |
Koca 2010.
| Methods | RCT, prospective, active‐controlled, open‐label Date of study Unreported Setting Dermatology department, Zonguldak Karaelmas University, Turkey |
|
| Participants |
Randomised: 49 participants (age 50.7 ± 9.1 years in metronidazole group versus 48.4 ± 9.4 years in pimecrolimus group, 16 male and 8 female in metronidazole group and 13 male and 12 female in pimecrolimus group) Inclusion criteria
No ocular rosacea Exclusion criteria
Dropouts and withdrawals: 1 in pimecrolimus group (deterioration of disease) Baseline data mean (SD) Inflammatory lesions: metronidazole group 16.0 (4.6), pimecrolimus group 26.0 (14.4) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) Conclusions do not reflect data reported, therefore the data could not be included in the meta‐analysis See comparison 23 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2): "Patients were randomly assigned to receive either pimecrolimus 1% cream or metronidazole 1% cream twice daily for 12 weeks." "Randomization was carried out using random‐number generation from standard tables." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (251): "Open‐label" Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (251): "Open‐label" Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, all participants were accounted for. One lost to follow up in pimecrolimus group (deterioration of disease) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | Substantial baseline imbalance between groups: mean of inflammatory lesion count at baseline was higher in pimecrolimus group, 26.0 ± 11.7, versus 16.0 ± 4.6 in metronidazole group and disease duration was also longer in pimecrolimus group, 33.7 ± 33.4 months versus 16.8 ± 18.3 months in metronidazole group Study duration adequate, wash‐out period before study adequate, groups treated equally, sponsorship or support and other potential conflicts of interest not reported Comment: Baseline imbalance may be a result of 'failed' randomisation. We judged this as at a high risk of bias |
Koch 1999.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Dermatological Practice Kassel, Germany |
|
| Participants |
Randomised: 30 participants (age unclear, 11 male, 19 female) Inclusion criteria
Exclusion criteria: Not stated Dropouts and withdrawals: Not stated Baseline data mean (SD) Nothing reported |
|
| Interventions | Six weeks Intervention
Comparator
Unclear how many in each group |
|
| Outcomes | Assessments (3): baseline, week 3 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | Poster, very limited reporting of trial details and outcomes data One of our primary outcomes was addressed (adverse events) See comparison 61 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 143‐4): "A double‐blind, randomised, placebo controlled clinical study..." and "Patients randomly received either..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 143‐4): "Double‐blind." and "...coated tablets with 200 mg sodium salt of dark sulfonated shale oil, died substance per tablet, or optically identical coated tablets without any active ingredient." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 143‐4): "Double‐blind." and "...coated tablets with 200 mg sodium salt of dark sulfonated shale oil, died substance per tablet, or optically identical coated tablets without any active ingredient." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Poster with a lot of missing data Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 143): "To evaluate the efficacy and tolerance..." Primary and secondary outcomes unclear, difficult to judge if all outcomes were addressed. Subjective reporting of several outcomes unsupported by data Comment: Insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Study duration adequate, wash‐out period unclear, unclear if groups were treated equally, sponsorship, support unreported Comment: Inadequate trial details to enable a clear judgement |
Koçak 2002.
| Methods | RCT, prospective, active‐ and placebo‐controlled (3‐armed study), double‐blind Date of study 1999 to 2000 Setting Outpatient Clinic of Dermatology at Ankara Education and Research Hospital, Turkey |
|
| Participants |
Randomised: 63 participants (mean age 51 years (range 20 to 80), 15 male, 48 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 0 Baseline data mean (SEM) Erythema score; permethrin group 2.60 (0.48), metronidazole group 2.85 (0.36), placebo group 2.65 (0.48) Papules; permethrin group 6.04 (7.60), metronidazole group 8.00 (6.70), placebo group 4.85 (4.10) Pustules; permethrin group 2.30 (3.73), metronidazole group 4.90 (4.78), placebo group 2.60 (3.36) Demodex folliculorum; permethrin group 2.20 (1.04), metronidazole group 2.60 (0.74), placebo group 2.70 (0.80) |
|
| Interventions | Two months Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (5): baseline, day 15, 30, 45 and 60 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported. However, investigators thanked Glaxo‐Wellcome, quote (page 269): "The authors thank Glaxo‐Wellcome for their contributions to packaging the two drugs and the placebo in identical boxes." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) Data on number of papules, pustules and Demodex folliculorum were skewed See comparison 1, 16 and 17 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 266): "They were randomly assigned to three groups to receive permethrin (n = 23), metronidazole (n = 20) and placebo (n = 20)." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 266): "Patients were given permethrin 5% cream, metronidazole 0.75% gel, placebo cream in packages looking identical." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 266): "Patients were given permethrin 5% cream, metronidazole 0.75% gel, placebo cream in packages looking identical." Outcomes were investigator and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. ITT analysis Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Appears to have been in part sponsored by Glaxo Wellcome (page 269). Wash‐out period unreported. Unclear if concomitant therapy that might influence rosacea was allowed Comment: Insufficient information to assess whether important risk of bias exists |
Lebwohl 1995.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Department of Dermatology, Mount Sinai Medical Center, New York and Chicago, Illinois, US |
|
| Participants |
Randomised: 63 participants (age range 25 to 80, 21 male, 42 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number of papules; sulphacetamide and sulphur group 12.1 and metronidazole group 13.5 Number of pustules; sulphacetamide and sulphur group 4.6 and metronidazole group 3.3 |
|
| Interventions | Eight weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 191): "This study was supported by a grant of Dermik laboratories." | |
| Declaration of interest | Two of the investigators are employed by Dermik Laboratories, however none declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events), however data were not reported, only that there was no statistical difference between the two groups See comparison 21 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (192): "...were randomly assigned to the two treatment groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 191): "...investigator blinded." The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 191): "...investigator blinded." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants withdrawn, 5 in the sulphacetamide and sulphur group, 1 in metronidazole group Comment: Unclear whether dropouts were included in analysis. Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | High risk | No data were available for "participants evaluation of overall response", "cosmetic acceptability as considered by participant", and "willingness to use again by participant". Only information reported that "there was no statistical difference between the 2 groups" Comment: We judged this as at a high risk of bias. Participant's evaluation is one of the principal outcome measures |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study adequate, groups were treated equally. The sodium sulphacetamide group tended to have greater overall severity scores and greater number of pustules but this was not statistically significant. Supported by a grant from Dermik Laboratories, 2 investigators were employees of Dermik Laboratories Comment: Insufficient information to assess whether important risk of bias exists |
Leyden 2011.
| Methods | Randomised, prospective, active‐controlled, investigator‐blinded
Date of study
Unreported Setting Unspecified, US |
|
| Participants |
Randomised: 30 participants (mean age 45 years, all female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Four weeks
Intervention
Comparator 1
Comparator 2
The women were instructed to apply the supplied sunscreen daily and to wear protective clothing when exposed to sun |
|
| Outcomes | Assessments (3): baseline, week 2 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 1185): "The study was funded by OMP, Inc" | |
| Declaration of interest | Quote (page 1185): "Dr Leyden has been an investigator and consultant for OMP, Inc" | |
| Notes | One of our primary outcomes was addressed (participant assessed changes in rosacea severity) See comparison 29, 30 and 31 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1180): "patients were randomly assigned (in a 1:1:1 ratio)" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 1179): "...investigator blinded." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 1179): "...investigator blinded."
Comment: Investigator and participant assessed outcomes Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/30 (3.3%); metronidazole plus standard skin care group, reason reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period before study started adequate Comment: The study appeared to be free of other forms of bias |
Leyden 2014.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre in US |
|
| Participants |
Randomised: 92 participants (mean age 51.2 years, 25 male, 67 female) Inclusion criteria:
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None reported Baseline data mean Inflammatory lesions: vehicle 19.9, BPO 1% 28.6, BPO 5% 22.9 |
|
| Interventions | 12 weeks
Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page 688): "The author has not disclosed any relevant conflicts" | |
| Notes | None of our primary outcomes was addressed See comparison 18 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 685): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 685): "...double‐blind" Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 685): "...double‐blind"
Comment: Only investigator assessed outcomes Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available (NCT00940992), and the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, no wash‐out period before study started described. Limited information Comment: There was insufficient information to permit a clear judgement |
Luger 2015.
| Methods | Randomised, prospective, placebo‐controlled Date of study Unreported Setting Multicentre (4) Germany |
|
| Participants |
Randomised: 61 participants (mean age 51.7 years, 13 male, 48 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Total RosaQoL score (Nicholson 2007); TDT 068 2.9 (0.71), vehicle 2.9 (0.67) Total rosacea standard grading system (Wilkin 2004); TDT 068 7.8 (1.66), vehicle 8.1 (1.73) |
|
| Interventions | Four weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 2 and 4 (and 2 phone calls, one at week 1 and one at week 5) Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 1): Editorial assistance with the preparation of the manuscript was provided by Bollin Strategies Ltd., UK, and was funded by Pro Bono Bio Entrepreneur Ltd., UK | |
| Declaration of interest | Quote (page 1): "T. Luger and N. Peukert have no conflict of interest to declare. M. Rother is a paid consultant of Pro Bono Bio Entrepreneur Ltd" | |
| Notes | Two of our primary outcomes were addressed (quality of life and adverse events) See comparison 37 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2): "..were stratified in a 4:1 female/male ratio and randomized according to a random permuted block scheme in a 2:1 ratio .." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: Patients were subsequently randomised and the study centre was notified of the treatment number of the patient via telefax by the randomisation center. Sets of sealed individual code envelopes were prepared for emergency procedures Comment: Adequate, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 2): "The investigator and other study team members involved in the evaluation of the safety and efficacy end‐points, the patients, the monitors, the sponsor and clinical research organization staff remained blinded to treatment until database lock." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: The investigational product and its matching vehicle had a similar appearance and all subject kits were packaged in the same way Comment: Blinding ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 2): "The investigator and other study team members involved in the evaluation of the safety and efficacy end‐points, the patients, the monitors, the sponsor and clinical research organization staff remained blinded to treatment until database lock." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study. Insufficient information to permit a clear judgement After e‐mail communication: The investigational product and its matching vehicle had a similar appearance and all subject kits were packaged in the same way Comment: Blinding ensured, risk of detection bias low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/61 (9.8%); TDT 068 (3), vehicle (3), reasons reported. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available (NCT01666509), and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Lupin 2014.
| Methods | Randomised, prospective, active‐controlled, open‐label Date of study Unreported Setting The Department of Dermatology and Skin Science, University of British Columbia, Vancouver, Canada |
|
| Participants |
Randomised: 12 participants (mean age 49.8 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: None reported Baseline data mean Nothing reported |
|
| Interventions | One or two treatments
Intervention
Comparator
Unclear how many were randomised to each group |
|
| Outcomes | Assessments (4): baseline, week 2, 4 and week 12/13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page AB43): "Supported by Ulthera" | |
| Declaration of interest | None declared | |
| Notes | Two of our outcomes were addressed (participant‐assessed changes in rosacea severity, and adverse events). After 3 attempts failed to contact PI for further details (see Table 21 and Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB 43): "were randomized.." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported. Investigator and participant assessed outcomes Comment: The outcome measurement was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts reported There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided. Protocol available at clinicaltrials.gov NCT01756027 Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Maddin 1999.
| Methods | RCT, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Division of Dermatology Skin Care Centre at University of British Columbia, Canada |
|
| Participants |
Randomised: 40 participants (mean age 52.2 years for males and 49.6 years for females, 11 male, 29 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 3/40 (7.5%)
Baseline data mean (SEM) Number of inflammatory lesions; azelaic acid treated site 11.3 (0.88), metronidazole treated site 11.40 (1.03) |
|
| Interventions | 15 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 8 and 9 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 961): "Supported by a grant provided by Allergan, Inc." | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 962): "A single‐center, randomized, double‐blind, contralateral, split‐face..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 962): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 962): "...double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 3 withdrawals were accounted for and reasons for withdrawal reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study adequate, additional medications that might influence outcome were not allowed Comment: We judged this as at a low risk of bias |
Marks 1971.
| Methods | RCT, prospective, active‐controlled and placebo‐controlled (3‐armed study), double‐blind Date of study Unreported Setting Institute of Dermatology, St John's Hospital for Diseases of the Skin, London, UK |
|
| Participants |
Randomised: 64 participants (mean age 47.8 years, 27 male, 29 female and 8 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of lesions; tetracycline group 21.05 (12.79), ampicillin group 21.06 (20.48), placebo group 18.47 (13.14) |
|
| Interventions | Six weeks Intervention
Comparator 1
Comparator 2
Number of participants reported as having completed the trial, but unclear how many were initially randomised to each group |
|
| Outcomes | Assessments (8): baseline, week 1, 2, 3, 4, 5, 6 and 7 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 1051): "We are grateful to Pfizer Ltd. for supplying the tetracycline, ampicillin, and placebo packed in identical capsules; and to Miss C. Pullin, of the Wellcome Research Laboratories, for statistical analysis of the results. R. M. is in receipt of a grant from the Medical Research Council." | |
| Declaration of interest | None declared | |
| Notes | Total number randomised not explicitly stated. 56 participants completed the trial, but at least 64 participant numbers were allocated so it is possible that eight or more participants dropped out Dosage of ampicillin not reported Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Data on lesions counts were quite skewed See comparison 42, 46 and 47 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1049): "Dispensing of study medications randomly allocated to one of 3 coded treatment groups by hospital dispensary." Comment: Appears to have been done centrally by the dispensary. Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: Central allocation by the dispensary Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 1049): "The placebo, tetracycline, and ampicillin were supplied in identical capsules." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 1049): "The placebo, tetracycline, and ampicillin were supplied in identical capsules." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number randomised not explicitly stated. 56 participants completed the trial, but at least 64 were allocated, so eight or more participants dropped out. Unclear how many participants were initially randomised in each group. Further one withdrawal in the placebo group Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration short (6 weeks), wash‐out period at start of the study adequate, no concomitant medication that might influence rosacea were allowed Quote (page 1051): "We are grateful to Pfizer Ltd. for supplying the tetracycline, ampicillin, and placebo packed in identical capsules;.... R. M. is in receipt of a grant from the Medical Research Council." Comment: We judged this as at a low risk of bias |
Monk 1991.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Participants from 4 different centres, Department of Dermatology, Bedford General Hospital, Bedford; Department of Dermatology, Bridgend General Hospital, Bridgend; Department of Dermatology, Queen Alexandra Hospital, Cosham; Department of Dermatology, Royal South Hants Hospital, Southampton, UK |
|
| Participants |
Randomised: 33 participants (mean age 46.9 years in metronidazole 0.75% gel + placebo capsules group, and 50.7 years in placebo gel + oxytetracycline group, 8 male and 8 female versus 9 male and 8 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Number papules and pustules; metronidazole group 25 and oxytetracycline group 20 Erythema grade; metronidazole group 2.5 and oxytetracycline group 2.4 |
|
| Interventions | Nine weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 3, 6 and 9 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 91): "We wish to thank Bioglan Laboratories for kindly providing the materials for this study" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 91): "The patients were randomly allocated in a double‐blind fashion of treatment." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 91): "...placebo gel (having the same base as the active preparation)." Comment: Assuming the placebo capsules were similar. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 91): "Double‐blind" "...placebo gel (having the same base as the active preparation)."
Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/33 (18.2%); metronidazole group (4) and oxytetracycline group (2). Incomplete outcome data were adequately addressed, reasons for withdrawal were reported. Per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period before start of the study adequate, no concomitant rosacea therapy was allowed. Additional therapy was noted Comment: We judged this as at low risk of bias |
Montes 1983.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study "Winter months", Dermatology Department in Buenos Aires, Argentina Setting Only data from the first 4 weeks included. Study biased after 4 weeks |
|
| Participants |
Randomised: 64 participants (age unclear, 19 male, 39 female, 6 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Four weeks, then a further four weeks for participants who showed improvement Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed. Only first 4 weeks included. Study biased after 4 weeks as people who did not respond to treatment "were dropped from the study" (page 187) See comparison 18 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 186): "This randomized, double‐blind...". Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 186) : "...matching placebo gel." "double‐blind" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 186) : "...matching placebo gel." "double‐blind" Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who showed no improvement at end of the first 4 weeks were dropped from the study. Per‐protocol analysis (page 186‐7). Only data up to week 4 are considered Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate (bit short), sponsorship or support unreported. Other topical or systemic treatment were not allowed Comment: The study appeared to be free of other forms of bias |
Mostafa 2009.
| Methods | RCT, prospective, active‐controlled, 3‐armed, double‐blind, within‐patient comparison Date of study Unreported Setting Department of Dermatology and Venereology, Faculty of Medicine, Zagazig University, Zagazig, Egypt |
|
| Participants |
Randomised: 24 participants (mean age 51.08 ± 5.9 years (range 42 to 61), 1 male, 23 female) Inclusion criteria
Ocular Involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Nothing reported Baseline data mean (SD) Inflammatory lesion count; azelaic acid group 4.2 (2.7), metronidazole 5.4 (3.3), permethrin group 4.5 (3.7) |
|
| Interventions | 15 weeks Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (11): baseline, week 3, 6, 9 and 15, and then monthly for another 6 months Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page 22): "None declared" | |
| Notes | One of our primary outcomes was addressed (adverse events). Participant' assessment was evaluated but not regarding rosacea severity. Investigators conclusions were based on the analysis of skewed and unreliable data analysis See comparison 15 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 23): "The 24 patients were randomly allocated to three groups." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 23): "...double‐blind comparison of azelaic acid 20% cream, metronidazole 0.75% cream and permethrin 20% cream." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 23): "...double‐blind comparison of azelaic acid 20% cream, metronidazole 0.75% cream and permethrin 20% cream." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts unclear Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified outcomes are addressed adequately or reported completely, e.g. photographic assessment and Physician's Gobal Assessment Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period unclear, and unclear if groups were treated equally Comment: Insufficient information to assess whether an important risk of bias exists |
NCT00249782.
| Methods | Randomised, prospective, active and placebo‐controlled, single‐blind Date of study November 2005 to May 2006 Setting Multicentre (27), US |
|
| Participants |
Randomised: 400 participants (age and gender unreported) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts and withdrawals: One participant randomised in error but did not receive treatment, for rest nothing reported Baseline data mean (SD) Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Comparator 3
Comparator 4
Unclear how many were randomised to each group |
|
| Outcomes | Assessments (6): baseline, week 2, 4, 8, 12 and 13 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Allergan sponsored the study | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | Study has been completed May 2006. Website accessed 19‐7‐2014 additional information on http://www.allerganclinicaltrials.com/results/medical_aesthetics.htm One of our primary outcomes was addressed (adverse events). Unclear how many were randomised to each group (see Table 24), no reply from Allergan after several email attempts (see Table 21) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (published as pdf on Allergan website ): "Randomization: Subjects were assigned in a 1:1:1:1:1 ratio to the five treatment groups" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "...single‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "...single‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropout reported. ITT. Limited data available Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | No exact data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Limited data are provided Comment: There was insufficient information to permit a clear judgement |
NCT01426269.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (US) |
|
| Participants |
Randomised: 130 participants (mean age 49.4 years, 44 male, 86 female) Inclusion criteria
Ocular Involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported other than "(IGA) score of clear or near clear" for all that entered the second phase |
|
| Interventions | All participants receive doxycycline 40 mg and metronidazole gel 1% once daily during phase 1 (baseline to week 12)
Second phase 40 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (11): baseline, every 4 weeks up to 40 weeks Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (clinicaltrials.gov) "Sponsor: Galderma Laboratories, L.P." | |
| Declaration of interest | Quote (clinicaltrials.gov) "Principal Investigators are not employed by the organization sponsoring the study" | |
| Notes | Study includes two phases; first phase (12 weeks) all participants (230) received doxycycline 40 mg combined with topical metronidazole gel. We only included the randomised second phase. All our primary outcomes were addressed Information found on clinicaltrial.gov and of a poster provided by Galderma See comparison 44 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (clinicaltrials.gov): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "...double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 85/130 (65%); 38/65 in doxycycline group, 47/65 in placebo group, reasons reported and 27 were due to relapse (primary endpoint for this study) Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol was available at clinicaltrials.gov. The pre‐specified outcomes appeared to have been reported in addition to RosaQol scores and a patient satisfaction questionnaire Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Treatment duration adequate, groups treated equally. Not all study data are available as study is not published yet Comment: There was insufficient information to permit a clear judgement |
NCT01449591.
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind Date of study September 2011 to February 2012 Setting Multicentre (5), US |
|
| Participants |
Randomised: 36 participants (mean age 47.4 years, 12 male, 24 female) Inclusion criteria
No ocular involvement Exclusion criteria
Dropouts withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator 1
Comparator 2
Application frequency not reported |
|
| Outcomes | Assessments (8): baseline, week 1, 2, 4, 8, 10, 12 and 14 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes prespecified for this review |
|
| Funding source | Sponsor: Novartis Pharmaceuticals | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | Study was completed December 2012. Website accessed 19‐7‐2014. Data reported on: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public
One of our primary outcomes was addressed (participant‐assessed changes of rosacea severity) See comparison 36 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (on Novartis website): "This was a multicenter, randomized, blinded, comparator‐ and vehicle‐controlled study". Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/36 (11.1%); BFH772 (1), vehicle (1), metronidazole (2), reasons reported. Per‐protocol analysis. Comment: Low and balanced number of dropouts and although per‐protocol analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, no wash‐out period before start of study reported, groups treated equally Comment: The study appeared to be free of other forms of bias |
NCT01885000.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (14), Europe |
|
| Participants |
Randomised: 92 participants (mean age 54.1 years (SD 12.8), 36 male, 56 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 4/92 (4.3%), unclear from which group Baseline data N (%) CEA moderate; brimonidine group 20 (41.7), vehicle group 25 (56.8) CEA severe; brimonidine group 28 (58.3), vehicle group 19 (43.2) |
|
| Interventions | Eight days
Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, day 2, and day 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Sponsor: Galderma | |
| Declaration of interest | No information on clinicaltrials.gov | |
| Notes | This study was completed November 2013. Website accessed 21‐7‐2013. Galderma provided additional data
Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 13 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (clinicaltrials.gov): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (clinicaltrials.gov): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/92 (4.3%), unclear from which group, analysis not clear Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Data incomplete poster/conference abstract (EADV 2014) full study not yet published Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Limited data are provided Comment: There was insufficient information to permit a clear judgement |
Neuhaus 2009.
| Methods | RCT, prospective, active‐controlled and controlled with "no treatment, investigator‐blinded, within‐patient comparison Date of study Unspecified Setting Dermatologic Surgery and Laser Center, Department of Dermatology, University of California, San Francisco, US |
|
| Participants |
Randomised: 30 participants (mean age 45.8 ± 10.6 years, 9 male, 20 female and 1 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Three treatment sessions, each month Intervention
Comparator 1
Comparator 2
|
|
| Outcomes | Assessments (4): baseline, month 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 927): "This study was funded by the American Society for Dermatologic Surgery Cutting Edge Research Grant." | |
| Declaration of interest | Quote (page 920): "The authors have indicated no significant interest with commercial supporters" | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 64 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 921): "Treatment randomization was performed using a random number generator." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 921): "Only the patient and the investigator performing the therapies were aware of their treatment allocation." "A blinded investigator gave.." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 921): "Only the patient and the investigator performing the therapies were aware of their treatment allocation." "A blinded investigator gave.."
Outcomes were investigator‐ and participant assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 922): "One patient in the IPL/control group dropped out after first treatment because of excessive swelling reaction. This patient did not return for follow‐up. Remaining 29 patients completed all three treatment sessions." Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | High risk | No exact data are provided, only P values. All outcome measures are addressed but without exact data Comment: We judged this as at a high risk of bias |
| Other bias | Low risk | No wash‐out period before study, study duration adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Nielsen 1983a.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study January to February 1982 Setting Department of Dermatology, Central Hospital, Boden, Sweden |
|
| Participants |
Randomised: 81 participants (mean age 47 years, 32 male, 49 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean Number of papules; metronidazole group 23.8 and placebo group 27.5 Number of pustules; metronidazole group 0.6 and placebo group 1.0 |
|
| Interventions | Two months Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 332): "The assays of plasma metronidazole were kindly performed by A/S Dumex Laboratories, Copenhagen, who also manufactured the test creams." | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 1 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 328): "....in accordance with a randomized administration scheme." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 327): "...double‐blind." Comment: Although not explicitly stated it would appear that the active intervention and placebo cream were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 327): "...double‐blind." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/81 (4.9%); metronidazole group (1) and placebo group (3) due to absence of improvement. Withdrawals/dropouts were accounted for but not included in the analysis. Per‐protocol analysis Comment: Low number of drop‐outs and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | A/S Dumex manufactured the test creams. Study duration adequate, no additional rosacea treatment allowed, adequate wash‐out period before study started Comment: The study appeared to be free of other forms of bias |
Nielsen 1983b.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study March to May 1982 Setting Department of Dermatology, Central Hospital, Boden, Sweden |
|
| Participants |
Randomised: 51 participants (mean age 44 years, 17 male, 34 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Two months Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline and month 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 65): "Coded tablets and test creams were kindly provided by A/S Dumex, Copenhagen" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes was addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 63): "Fifty‐one randomly selected patients etc..." "Patients were assigned at random to one of the two courses of treatment." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | Quote (page 65): 'Coded tablets and test creams were kindly provided by A/S Dumex." Comment: Form of central allocation. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 63): "...double‐blind." Comment: Although not explicitly stated in the report it would appear that the active interventions were matched with similar and indistinguishable placebos |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 63): "...double‐blind." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropouts are accounted for and included in ITT analysis Comment: Low number of dropouts combined with ITT analysis, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Adequate study duration, adequate wash‐out period before study started. No additional rosacea therapy allowed Comment: The study appeared to be free of other forms of bias |
Nymann 2010.
| Methods | Randomised, active‐controlled, investigator‐blind, within‐patient comparison Date of study Unreported Setting Dermatology Department of Bispebjerg Hospital, Copenhagen, Denmark |
|
| Participants |
Randomised: 40 participants (mean age 54 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Three treatments at six week intervals Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline and month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes:
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 143): "Dermatologic Development, Hørsholm, Denmark lent the Ellipse Flex. Role of Companies: Danish Dermatologic Development,Hørsholm, Denmark, and Candela Corporation,Wayland, Massachusetts, USA, approved the treatment settings before study initiation. The companies had no role in design and conduct of the study, neither in the collection, analysis, and interpretation of data, nor in the preparation of the manuscript, review, or approval of the manuscript." | |
| Declaration of interest | Quote (page 143): "None declared" | |
| Notes | 31 had telangiectasia related to rosacea, one had telangiectasia due to treatment with corticosteroids, and seven had idiopathic telangiectasia (and one died) Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 65 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 144): "Patients were randomly allocated.." and "Randomization was carried out by patients drawing lots between opaque sealed envelopes, containing cards with subject number and split side treatment code" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 144): "Randomization was carried out by patients drawing lots between opaque sealed envelopes, containing cards with subject number and split side treatment code" Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators and participants were not blinded during the treatment phase Comment: The outcome was likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 145): "Clinical efficacy was evaluated by one blinded trained physician" Outcomes were investigator and participant‐assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/40 (2.5%); died not related to treatment. Per‐protocol analysis Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period adequate before study started Comment: The study appeared to be free of other forms of bias |
Pye 1976.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unspecified Setting Department of Dermatology, Bristol Royal Infirmary, Bristol, UK |
|
| Participants |
Randomised: 29 participants (age 24 to 86 years, gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 1 in each group because of headache Baseline data mean (SD) Nothing reported |
|
| Interventions | Six weeks Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline and week 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Nothing reported | |
| Declaration of interest | Nothing declared | |
| Notes | None of our primary outcomes were addressed See comparison 55 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1212): "The treatment was allocated at random..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 1212): "The treatment was allocated at random without the knowledge of the doctor or the patient." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 1211): "...double‐blind" "...metronidazole 200 mg twice daily or a lactose placebo tablet.." Comment: Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 1211): "...double‐blind." "...metronidazole 200 mg twice daily or a lactose placebo tablet.." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals in each group were accounted for. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration short, unclear if groups were treated equally and if additional rosacea therapy was allowed, adequate wash‐out period before study Comment: Insufficient information to assess whether an important risk of bias exists |
Rehmus 2006.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, US |
|
| Participants |
Randomised: 40 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator
No other skin care products or emollients were allowed for the duration of the study |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page AB64): "100% supported by Nu Skin Enterprises" | |
| Declaration of interest | Quote (page AB64): "Salary support through clinical trials sponsored by Nu Skin Enterprises" | |
| Notes | Poster abstract, no results presented, limited data (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page AB64): "Each subject was randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page AB64): "double‐blind." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page AB64): "double‐blind." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study. Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data provided Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unclear and no data provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Rigopoulos 2005.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre. Department of Dermatology, University of Athens, Andreas Aygros Hospital, Athens, Greece; IRIS, Institute de Recherches et d'Innovations Scientifiques, Paris, France; 4 Private Practices, Germany |
|
| Participants |
Randomised: 246 participants (mean age 48.9 years (range 18 to 80), 34 male and 91 female in treatment group, 36 male and 85 female in placebo group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Erythema severity; treatment group 2.71 (0.07) and placebo group 2.86 (0.07) Rosacea overall severity; treatment group 3.21 (0.1) and placebo group 3.3 (0.08) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 568): "This study was supported by a grant from the research Division of the European Council – 5th plan." | |
| Declaration of interest | Quote (page 568): "None of the authors has any conflict of interest to declare including financial arrangements, interest or share holding options with the company manufacturing the product." | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). The review authors imputed SDs for mean reduction from baseline in rosacea severity score using 3 correlations between the baseline and final measurements See comparison 33 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 565): "This multicentre, randomized, double‐blind, parallel group, placebo‐controlled, study..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 564‐5): "Double‐blind" and "As the active ingredient resulted in a slightly coloured final product, colour of placebo (vehicle) was adjusted accordingly." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 564‐5): "Double‐blind" and "As the active ingredient resulted in a slightly coloured final product, colour of placebo (vehicle) was adjusted accordingly."
Outcomes were investigator‐ and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 96 participants in the treatment group and 100 in the placebo group appear to be included in analysis. The analysis excluded the remaining participants (20%) because of "missing grade values for any examination" (quote page 566). Per‐protocol analysis Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally. Study was supported by a grant from the research Division of The European Council ‐ fifth plan None of the authors have any conflicts of interest to declare, including financial arrangements Comment: The study appeared to be free of other forms of bias |
Rodríguez 2003.
| Methods | Randomised, prospective, active‐controlled, double‐blind
Date of study
Unreported Setting Centro Dermatológico Pascua, Ciudad de Mexico, Mexico |
|
| Participants |
Randomised: 34 participants (mean age unreported, 11 male, 20 female, 3 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | 45 days Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 38 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 128): "se dividieron al azar en dos grupos" (were divided at random in two groups) Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 126): "estudio doble ciego" (double‐blind) Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 126): "estudio doble ciego" (double‐blind) Comment: Outcomes were investigator and participant‐assessed. Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/34 (8.8%) of benzyl benzoate group, lost to follow‐up (2), pregnancy (1). Per‐protocol analysis Comment: Unbalanced, but low number of follow up, judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no wash‐out period before study described, groups treated equally Comment: The study appeared to be free of other forms of bias |
Saihan 1980.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Bristol Royal Infirmary, Bristol, UK |
|
| Participants |
Randomised: 40 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not specified Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 6 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Nothing reported | |
| Declaration of interest | Nothing declared | |
| Notes | Although independent assessments were made by the participant and 2 doctors these were combined and presented as a composite score See comparison 48 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 443): "...treated...on a random double‐blind basis." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | Quote (page 443): "...coded tablets being issued by the pharmacist." Comment: Pharmacy‐controlled, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 443): "Double‐blind basis...coded tablets." Comment: Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 443): "Double‐blind basis...coded tablets." Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two dropouts were accounted for but not included in analyses. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study adequate. No sponsorship or conflict of interest reported Comment: Insufficient information to assess whether an important risk of bias exists |
Salem 2013.
| Methods | Randomised, prospective, active‐controlled, single‐blind Date of study June 2011 to February 2012 Setting Dermatology and Ophthalmology Clinic of the Mansoura University Hospitals, Mansoura City, Egypt |
|
| Participants |
Randomised: 120 participants (mean age 36.1 years (SD 12.4), 56 male, 64 female) Inclusion criteria
Ocular involvement: Participants with ocular manifestations were included Exclusion criteria
Additionally in patients with anterior blepharitis
Dropouts and withdrawals
Baseline data mean (SD) Demodex density acne group (30); ivermectin group 12.3 (3.2), combined group 12.9 (6.1) Demodex density rosacea group (30); ivermectin group 51.7 (20.8), combined group 51.5 (26.3) Demodex density peri‐oral dermatitis group (30); ivermectin group 21.3 (7.5), combined group 21.9 (6.8) Demodex density blepharitis group (30); ivermectin group 12.8 (6.8), combined group 15 (5.7) |
|
| Interventions | Two weeks
Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 1, 2, 3 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page e347): "Conflict of Interest: None" | |
| Notes | None of our primary outcomes was addressed See comparison 59 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page e344): "randomly assigned to either combined therapy or ivermectin treatment at a ratio of 1:1 (15 patients for each treatment regimen from each group) using a computer‐generated randomization schedule." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page e344): "The assignment was done in a single‐blinded manner, in which the subjects were blinded to the treatment assignment." Comment: It looks like allocation concealment is confused with blinding, we did not receive additional information of the principal investigators. The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page e344): "The assignment was done in a single‐blinded manner, in which the subjects were blinded to the treatment assignment." Comment: Investigators were not blinded. The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (page e344): "Assessment of the outcome samples was done by two unblinded parasitologists and then reviewed by another independent blinded professor of parasitology to avoid bias" Comment: The other outcomes were assessed by unblinded investigators and the measurement of those outcomes was likely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before start of study adequate, groups treated equally Comment: The study appeared to be free of other forms of bias |
Sanchez 2005.
| Methods | RCT, prospective, "placebo"‐controlled (placebo tablets, but also topical metronidazole), double‐blind Date of study Unreported Setting Unspecified, US |
|
| Participants |
Randomised: 40 participants (mean age 41.6 years in metronidazole + doxycycline group versus 38.8 years in metronidazole + placebo group, 3 male and 17 female versus 5 male and 15 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Total inflammatory lesions; metronidazole group 25.9 (3.7), doxycycline group 27.3 (3.6) Clinician's Global severity score; metronidazole group 2.6 (0.17), doxycycline group 2.7 (0.17) Clinician's Global Erythema assessment; metronidazole group 9.8 (0.71), doxycycline group 9.5 (0.69) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, week 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 791): "Supported by CollaGenex Pharmaceuticals, Inc." | |
| Declaration of interest | Quote (page 791): "Conflicts of interest: None identified". One of the investigators was employed by CollaGenex | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 53 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 792): "Randomization was accomplished by assigning numbers to the sub antimicrobial dose doxycycline and placebo bottles based on the SAS statistical software randomization procedure. Each patient entering the study received the next sequentially numbered bottle." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Unclear if this was done 'centrally'. There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 792): "...double‐blind." and "All study tablets were identical in size, shape, and colour (white)." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 792): "...double‐blind." and "All study tablets were identical in size, shape, and colour (white)." Outcomes were investigator and participant‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal were reported. ITT analysis (LOCF) was carried out Comment: Low number of dropouts, ITT analysis, judged as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period adequate. Concomitant medications that could influence rosacea were prohibited Comment: The study appeared to be free of other forms of bias |
Sauder 1997.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, Dermatology department of different centres in Canada and UK |
|
| Participants |
Randomised: 103 participants (mean age 50 years, 40 male, 63 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals 9/103 (8.7%); treatment group (2) and placebo group (1), due to adverse events, for the remaining 6 it is unclear from which group, but were also excluded from the analysis Baseline data mean (SD) Inflammatory lesion count; treatment group 35.6 and placebo group 28.0 |
|
| Interventions | Eight weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 1, 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 85): "This study was funded in part by GenDerm Corporation, Lincolnshire, Illinois" | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Unclear how many participants in each group, although it was reported "comparable numbers". Skewed data See comparison 20 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 80): "...dispensed to patients in a randomized, double‐blind fashion." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 80): "...double‐blind fashion..." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 80): "...double‐blind fashion..." Outcomes were investigator‐ and participant assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers/participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/103 (8.7%); treatment group (2) and placebo group (1), due to adverse events, for the remaining 6 it is unclear from which group, but were also excluded from the analysis Comment: Low number of dropouts, but unclear how many started in each group and how many dropped out in each group, judged as unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration rather short, wash‐out period before study adequate, no concomitant medication allowed Comment: The study appeared to be free of other forms of bias |
Schachter 1991.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Multicentre, Women's College Hospital, Toronto, Ontario; General Practice in Ontario, and Rhône Poulenc Rorer Canada, Montreal, Quebec, Canada |
|
| Participants |
Randomised: 125 participants (mean age 45.4 ± 1.3 (SEM), 40 male, 61 female, 24 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: 24/125 (19.2 %) withdrew for reasons not related to treatment, unclear from which group Baseline data mean (SEM) Number of papules; metronidazole group 18.35 (1.9), tetracycline group 21.04 (1.9) Number of pustules; metronidazole group 4.67 (0.7), tetracycline group 4.40 (0.7) |
|
| Interventions | Two months Intervention
Comparator
Only number of participants that completed the study |
|
| Outcomes | Assessments (3): baseline, month 1 and 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes None ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported, but one investigator was employed by Rhone Poulenc Rorer Canada, manufacturer of metronidazole | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were assessed (participant‐assessed changes in rosacea severity and adverse events) See comparison 56 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 221): "Patients were randomly administered either metronidazole cream and placebo capsules or placebo cream and tetracycline capsules." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 221): "Placebo and active cream and capsules were matched as appropriate." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 221): "Placebo and active cream and capsules were matched as appropriate." Outcomes were investigator‐ and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many participants were actually allocated to each group. After randomisation 24/125 (19%) withdrew from the study for reasons unrelated to treatment. Additional withdrawals occurred during the course of the study (9)
Metronidazole group 2/49 discontinued treatment because of lack of efficacy, or adverse events (5) Tetracycline group 2/52 discontinued because of adverse events The dropouts and withdrawals were not included in the analysis Comment: The high dropout rate and the per‐protocol analysis represents a potentially high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Data presented in graphs had to be extracted from figures All pre‐specified outcomes appear to have been addressed Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally, sponsorship or support not reported Comment: The study appeared to be free of other forms of bias |
Schechter 2009.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Florida Eye, Microsurgical Institute, Florida, US |
|
| Participants |
Randomised: 37 participants (age 75.6 years in cyclosporine group and 69.6 years in artificial tears group, 15 males and 6 females in cyclosporine group and 9 males and 7 females in artificial tears group) Inclusion criteria
Exclusion criteria Eyelid defects, lagophthalmos, sensitivity to study medication Dropouts and withdrawals
Baseline data mean (SD) Schirmer score; cyclosporine group 9.7 mm (5.1) and artificial tear group 10.2 mm (5.8) |
|
| Interventions | Three months Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 658): "This study was funded by an unrestricted educational grant from Allergan, Inc." | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (quality of life) See comparison 27 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 653): "Patients were randomised by computer." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 653): "...double‐masked clinical trial." "The vials for each product were identical, ensuring patient and clinicians masking." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 653): "...double‐masked clinical trial." "The vials for each product were identical, ensuring patient and clinicians masking." Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken. Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/37 (8.1%) Dropouts and withdrawals reasons were clarified. Per‐protocol analysis Comment: Low number of dropouts and although per‐protocol analysis judged as at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | The pre‐specified primary outcomes were addressed, other than one of the secondary outcome measures, the quality of excreta of the Meibomian glands Comment: We judged this as at an unclear risk of bias |
| Other bias | Low risk | Participants were in the older age group and almost exclusively Caucasians. Wash‐out period before study and duration adequate, no additional medication allowed that might influence outcome. The study was funded by an unrestricted educational grant from Allergan, Inc (page 659) Comment: The study appeared to be free of other forms of bias |
Seité 2013.
| Methods | Randomised, prospective, vehicle‐controlled, double‐blind Date of study Unreported Setting Single‐centre Europe |
|
| Participants |
Randomised: 66 participants (mean age 52 years (SD 11), 19 males, 47 females) Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: None Baseline data mean (SD) Intensity of the rosacea; light (26), moderate (31), severe (9) |
|
| Interventions | Eight weeks Intervention
Comparator
All participants were treated the 8 weeks before with topical metronidazole |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 6 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 924): "The studies were funded by la Roche‐Posay Pharmaceutica Laboratories France" | |
| Declaration of interest | Quote (page 924): "All the authors except M Skalikova, L. Gibejova and H. Zelenkova, are employees of L'Oréal.' | |
| Notes | The article covers 3 studies, only study 3 is a RCT and is included in this review
One of our primary outcomes was addressed (participant assessed changes in rosacea severity). Skewed data See comparison 39 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 922): " randomized....." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "The allocation sequence was generated by a statistician using a specific software" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement No further information after e‐mail communication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 922): "double blind...." After e‐mail communication: "Both products was in the same packaging (blind white packaging) without any indication about formula reference" Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 922): "double blind...." After e‐mail communication: "Both products was in the same packaging (blind white packaging) without any indication about formula reference" Comment: Outcomes were investigator and participant‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | The article provided only limited data Comment: There was insufficient information to permit a clear judgement |
Sharquie 2006.
| Methods | RCT, prospective, placebo‐controlled, double‐blind, cross‐over Date of study Recruitment between October 2002 and August 2004 Setting Department of Dermatology, College of Medicine, University of Baghdad, Iraq |
|
| Participants |
Randomised: 25 participants (age 48.2 ± 9.3 years (range 21 to 64), 9 male, 16 female) Inclusion criteria
Ocular involvement: Yes in nine participants Exclusion criteria
Dropouts and withdrawals: 6/25 (24%) for unknown reasons, 5 from placebo group and 1 from treatment group Baseline data mean Sharquie rosacea severity score; zinc group 8, placebo group 7 |
|
| Interventions | Three months (thereafter cross‐over) Intervention
Comparator
|
|
| Outcomes | Assessments (7): baseline, each month up to month 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | We only included data for the first 3 months of the study. One of our primary outcomes was addressed (adverse events) See comparison 57 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 858): "Patients were randomly allocated." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 858): "Zinc sulphate or the identical placebo capsules were given in a double‐blind manner." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 858): "Zinc sulphate or the identical placebo capsules were given in a double‐blind manner." Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/25 (24%) participants dropped out for unknown reasons and were not included in the analysis Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | For intermediate outcomes only means reported, no SDs. Inadequate reporting of lesion counts Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, groups treated equally, sponsorship or support unreported Comment: The study appeared to be free of other forms of bias |
Sneddon 1966.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study December 1964 for 1 year Setting Department of Dermatology of Royal Infirmary, Sheffield and Doncater Gate Hospital, Rotherham, UK |
|
| Participants |
Randomised: 85 participants (mean age 47 years, 26 male, 52 female, 7 gender not reported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean (SD) Nothing reported |
|
| Interventions | Four weeks Intervention
Comparator
Number of participants that completed the study (78) |
|
| Outcomes | Assessments (3): baseline, week 2 and 4 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 42 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 649): "...dispensed by the pharmacist according to a random table." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: Form of central allocation, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (649): "...tetracycline 250 mg twice daily or a dummy placebo indistinguishable in appearance." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (649): "...tetracycline 250 mg twice daily or a dummy placebo indistinguishable in appearance." Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants were actually randomised to each group. Withdrawals were accounted for for first month. Per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Older study of short duration. Unreported if wash‐out period before study, if concomitant medication was allowed, sponsorship or support Comment: Insufficient information to assess whether an important risk of bias exists |
Stein 2014a.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study December 2011 to July 2013 Setting Multicentre, US and Canada |
|
| Participants |
Randomised: 683 participants (mean age 50.4 years (SD 12.09) 217 male, 466 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; 30.9 (14.33) IGA moderate; 560 (82%) participants IGA severe; 123 (18%) participants |
|
| Interventions | 12 weeks Intervention
Comparator
Subjects were instructed to avoid rosacea triggers such as sudden exposure to heat, certain foods and excessive sun exposure |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 323): "The study was funded by Galderma R&D" | |
| Declaration of interest | Quote (page 323): "The investigators received grants for conducting the studies. Ms Liu and Dr Jacovella are employees of Galderma R&D" | |
| Notes | All our primary outcomes were addressed See comparison 9 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: Central allocation, probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 317): "The integrity of the blinding was ensured by packaging the topical creams in identical tubes with no visible difference between the creams, and requiring a third party other than the investigator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 59/683 (8.6%); ivermectin group (37), vehicle group (22), reasons reported. Per‐protocol analysis and ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493687) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started not reported, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship/support represented any additional bias |
Stein 2014b.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study December 2011 to August 2013 Setting Multicentre, US and Canada |
|
| Participants |
Randomised: 688 participants (mean age 50.2 years (SD 12.29) 229 male, 459 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Number of inflammatory lesions; 32.9 (13.70) IGA moderate; 522 (76%) participants IGA severe; 166 (24%) participants |
|
| Interventions | 12 weeks Intervention
Comparator
Subjects were instructed to avoid rosacea triggers such as sudden exposure to heat, certain foods and excessive sun exposure |
|
| Outcomes | Assessments (5): baseline, week 2, 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 323): "The study was funded by Galderma R&D" | |
| Declaration of interest | Quote (page 323): "The investigators received grants for conducting the studies. Ms Liu and Dr Jacovella are employees of Galderma R&D" | |
| Notes | All our primary outcomes were addressed See comparison 9 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: Central allocation, probably done. |
| Allocation concealment (selection bias) | Low risk | Quote (page 317): "Randomization lists were generated prior to study initiation by a statistician, and were then sent to a clinical supply group, and only personnel directly involved with labeling and packaging (not site personnel) had access." Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 317): "The integrity of the blinding was ensured by packaging the topical creams in identical tubes with no visible difference between the creams, and requiring a third party other than the investigator to dispense the medication." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were investigator‐assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 59/683 (8.6%); ivermectin group (37), vehicle group (22), reasons reported. Per‐protocol analysis and ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493687) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, wash‐out period before study started not reported, groups treated equally. Study supported by Galderma R&D. All investigators have received grants from Galderma R&D or were employees of Galderma R&D Comment: As the study appeared to be double‐blinded and there was no selective reporting we do not consider that the sponsorship/support represented any additional bias |
Taieb 2015.
| Methods | Randomised, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Multicentre (64), 10 European countries |
|
| Participants |
Randomised: 962 (mean age 51.5 years (SD 13.3), 335 male, 627 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SD) Mean inflammatory lesion count; ivermectin group 32.87 (13.95), metronidazole 32.07 (12.75) |
|
| Interventions | 16 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (6): baseline, week 3, 6, 9, 12 and 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Epub page 1 "This study was funded by Galderma R&D." | |
| Declaration of interest | Epub page 1 "The investigators received grants for conducting the studies. Mrs. Peirone and Mr. Jacovella are employees of Galderma R&D." | |
| Notes | All our primary outcomes were addressed See comparison 25 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (Epub): "randomized" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups After e‐mail communication: "are randomized in blocks of 6. The RANUNI routine of the SAS system was used to randomly assign, in balanced blocks, kit to a treatment (Ivermectin 1% cream, Metronidazole 0.75% cream)." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "a randomization list was generated by the statistician and was secured with restricted access. .. and kit numbers were assigned sequentially in chronological order." Comment: Adequate, probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (Epub): "...investigator‐blinded." Comment: The report provided insufficient detail about the measures used to blind study personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "The integrity of the blinding was ensured by packaging the products in identical tubes, not allowing the investigator and subject to discuss study treatments, and requiring a third party other than the investigator to dispense the medication" Comment: Blinding investigators ensured, low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (Epub): "...investigator‐blinded." Comment: Investigator and participant assessed outcomes. Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement After e‐mail communication: Blinding investigators ensured, but due to the different treatment regime once versus twice daily and participants were outcome assessors as well, we judged this as at an unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/962 (5.2%); ivermectin group (32), metronidazole group (28), reasons reported. Authors state to have performed an ITT analysis (LOCF) Comment: Low and balanced number of dropouts, combined with ITT analysis judged as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available at clinicaltrials.gov (NCT01493947) and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period not described, groups treated equally Comment: the study appears to be free of other forms of bias |
Tan 2002.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre (6), Canada (Windsor, Ontario; Montreal, Quebec; Alberta, Quebec; Waterloo, Ontario; Sainte‐Foy, Ontario; Winnipeg, Manitoba) |
|
| Participants |
Randomised: 120 participants (mean age 51 years (treatment group) versus 47.7 years (placebo group), 31 male, 89 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Inflammatory lesion count; metronidazole group 18.5 (2.0) and placebo group 20.4 (1.7) Erythema score; metronidazole group 2.13 (0.04) and placebo group 2.10 (0.04) Telangiectasia score; metronidazole group 1.70 (0.08) and placebo group 1.73 (0.08) Rosacea severity score; metronidazole group 2.13 (0.05) and placebo group 2.20 (0.05) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 529): "Sponsored by Stiefel Canada Inc", one of the investigators was employed by Stiefel | |
| Declaration of interest | None declared | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events). Data are skewed See comparison 2 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 530): "This randomized, double‐blind...study..." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment:There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (529): "...double‐blind." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (529): "...double‐blind." Outcomes were investigator and participant assessed Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers, participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31/120 (25.8%); metronidazole group (17) and placebo group (14). All participants were accounted for (including the dropouts and withdrawals). Per‐protocol analysis Comment: High dropout rate combined with a per‐protocol analysis judged as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period before study adequate, no concomitant medication that could influence rosacea permitted. Sponsored by Stiefel Canada Inc, manufacturer of the active intervention. One investigator was employed by Stiefel Comment: Insufficient information to assess whether an important risk of bias exists |
Thiboutot 2003a.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, 13 centres in the US |
|
| Participants |
Randomised: 329 participants (mean age 48 years (treatment group) versus 49 years (placebo group), 39 male and 125 female versus 45 male and 120 female) Inclusion criteria
Ocular involvement: Unclear, no participants with marked ocular involvement were included Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic group 17.5 and vehicle group 17.6 |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 836): "Supported by Berlex Laboratories" | |
| Declaration of interest | Quote (page 836): "Dr Thieroff‐Ekerdt is an employee of Berlex Laboratories. Dr Graupe is an employee of Schering AG. Dr Thiboutot received financial compensation from Berlex Laboratories for her role as a principal investigator in these studies" | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 6 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 837): "Patients were randomly assigned to treatment with either AzA gel or vehicle gel. The randomization list was prepared by a computer program ensuring equal numbers of patients per treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 837): "Patients were allocated to treatment in the sequence of entry into the studies, i.e., in each center each newly admitted patient received the study medication with the lowest randomization number available." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46/329 (13.9%); azelaic group (31) and vehicle group (15), dropouts and withdrawals were accounted for. ITT analysis (LOCF) Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration and wash‐out period before study adequate, no concomitant medication that could influence rosacea allowed. Other medication recorded Sponsoring: Berlex Laboratories, second investigator was an employee of Berlex laboratories, third author is an employee of Schering, and first author received financial compensation from Berlex laboratories for her role as principal investigator (page 836) Comment: The review authors do not consider that the commercial sponsorship introduced any additional bias the study was double‐blind, and all pre‐specified outcome measures were addressed and analysis of data was according to ITT principle |
Thiboutot 2003b.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Ureported Setting Multicentre, 14 centres in the US |
|
| Participants |
Randomised: 335 participants (mean age 48 years (treatment group) versus 47 years (placebo group), 47 male and 122 female versus 46 male and 120 female) Inclusion criteria
Ocular involvement: Unclear, no participants with marked ocular involvement were included Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic group 17.8 and vehicle group 18.5 |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 836): "Supported by Berlex Laboratories" | |
| Declaration of interest | Quote (page 836): "Dr Thieroff‐Ekerdt is an employee of Berlex Laboratories. Dr Graupe is an employee of Schering AG. Dr Thiboutot received financial compensation from Berlex Laboratories for her role as a principal investigator in these studies" | |
| Notes | Same reference as Thiboutot 2003a (report of 2 studies). Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 6 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 837): "Patients were randomly assigned to treatment with either AzA gel or vehicle gel. The randomization was prepared by a computer program ensuring equal numbers of patients per treatment group." Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 837): "Patients were allocated to treatment in the sequence of entry into the studies, ie, in each center each newly admitted patient received the study medication with the lowest randomization number available." The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Although not explicitly stated it would appear that the active intervention and placebo tablets were similar and most probably indistinguishable by participants and investigators. The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 836): "double‐blind" Comment: Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39/335 (11.6%); azelaic group (19) and vehicle group (20). Dropouts and withdrawals were accounted for and included in the analysis. ITT analysis (LOCF) Comment: We judged this as at low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported. Comment: We judged this as at a low risk of bias. |
| Other bias | Low risk | Wash‐out period adequate, study duration adequate, no concomitant medication that could influence rosacea allowed. Other medication recorded Sponsoring: Berlex Laboratories, second investigator was an employee of Berlex laboratories, third investigator was an employee of Schering and first investigator received financial compensation from Berlex laboratories for her role as principal investigator (page 836) Comment: The review authors do not consider that the commercial sponsorship introduced any additional bias the study was double‐blind, and all pre‐specified outcome measures were addressed and analysis of data was according to ITT principle |
Thiboutot 2005.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Multicentre, US |
|
| Participants |
Randomised: 134 participants (mean age 44.5 years in doxycycline group and 48.9 years in placebo group, 40 male, 94 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | 16 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 12 and week 16 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 17): "100% supported by CollaGenex" | |
| Declaration of interest | Quote (page 17): "Drs. Thiboutot, Beer, and Skidmore have received consulting and speaking fees from CollaGenex. Dr. Berman has received research grant support from CollaGenex. Drs. Leyden and Fowler have received consulting fees from CollaGenex." | |
| Notes | Poster presentation, a lot of information is lacking (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 17): "A multi‐center, double‐blind, randomized, placebo‐controlled trial was undertaken" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 17): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 17): "double‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25/134 (18.7%); unclear from which group. Analysis unclear Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Thiboutot 2008.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Multicentre, 7 centres in US |
|
| Participants |
Randomised: 92 participants (mean age 48.5 years in QD group versus 49.6 years in BID group, 11 male and 34 female versus 17 male and 30 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 4, 8 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | One of the investigators was employed by Intendis | |
| Declaration of interest | Quote (page 545): "Dr Thiboutot has participated in clinical trials and has been a consultant/advisor for Intendis Inc,....Dr Fleischer has participated in clinical trials and has been a consultant/advisor for Intendis Inc,....Dr Del Rosso has received grant/research support/honoraria from and has been a consultant for etc and....Intendis Inc...Dr Graupe is employed by Intendis GmbH, Berlin, Germany" | |
| Notes | 1 centre (20 participants) was excluded as assessments were not in conformity with protocol One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 7 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 542): "Patients were randomized to receive either AzA 15% gel once daily or AzA 15% gel twice daily." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Low risk | As both groups received 2 tubes for each study day it is unlikely that allocation could have been foreseen Comment: The report provides sufficient detail and reassurance that participants and investigators enrolling participants could not foresee the upcoming assignment. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 541): "double‐blind". Both groups received a morning and evening tube for each study day. The subjects in the QD group received 1 application with vehicle gel each day of the study (page 542) Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 541): "double‐blind". Both groups received a morning and evening tube for each study day. The subjects in the QD group received 1 application with vehicle gel each day of the study (page 542). Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/92 (4.3%); 2 in each group, 1 centre was excluded (20 participants, as IGA assessments were not in conformity with study protocol). Reasons for withdrawal are reported. ITT analysis (LOCF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the prespecified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | All of the investigators in this study have received sponsorship from pharmaceutical industry. The study was designed as a superiority study, but was inappropriately reported as a successful non‐inferiority study, without any reference to the number of participants recruited, the planned design, or sample size. Study duration adequate, unclear if there was a wash‐out period before study or if other medications were allowed Comment: A potential risk of bias cannot be excluded |
Thiboutot 2009.
| Methods | RCT (only second phase), prospective, placebo‐controlled (only second phase), double‐blind (only second phase), cross‐over study (we only included second phase) Date of study Unreported Setting Multicentre in US |
|
| Participants |
Randomised: 136 participants (mean age 46.4 years in azelaic acid gel group versus 47.5 years in vehicle group, 18 male and 49 female in azelaic acid group versus 17 male and 52 female in vehicle group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Inflammatory lesion count; azelaic acid group 1.39, vehicle group 1.55 |
|
| Interventions | 24 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (7): baseline, every for weeks up to 24 weeks Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 647): "Research funding was provided by Indendis Inc" | |
| Declaration of interest | Quote (page 647): "Dr Fleischer.... he served as a consultant for...Intendis..He has served as an investigator for...Intendis...He also served on the speaker bureaus for...Intendis..Dr Del Rosso has served as a consultant, speaker and researcher for...Intendis...Dr Thiboutot has served as an investigator and consultant for Intendis Inc..." | |
| Notes | We only included second phase as first phase (up to 12 weeks). All participants received doxycycline 100 mg BID + azelaic acid 15% gel BID in first phase. However, participants who did not respond in first phase were not included in second phase, this means these data cannot be generalised for all participants with papulopustular rosacea. Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 9 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 640): "Subjects eligible for the maintenance phase of the study were randomized to apply either AzA 15% gel or its vehicle twice daily for an additional 24 weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page (640); "double‐blind" Comment. Vehicle gel was used. Probably identical appearance The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page (640); "double‐blind" Comment. Vehicle gel was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We only included second phase of the study. 14/136 (10.3%), 7 in both groups. ITT analysis carried out (LCOF) Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | High risk | Not all pre‐specified outcomes were addressed and reported. Data missing in the maintenance phase, self‐assessment by participants Comment: We judged this as at a high risk of bias |
| Other bias | Unclear risk | No wash‐out phase, only participants were included who had attained at least a 75% reduction in number of inflammatory lesions in the first phase on a combination of doxycycline 100 mg to 200 mg plus azelaic acid 15% gel twice daily. Study duration adequate, not allowed to use other medications that might influence rosacea. Research funding was provided by Intendis, Inc. First author served as an investigator and consultant for Intendis, as did second and third author Comment: Phase 1 of this study is a run‐in period (equivalent to wash‐out). However, insufficient information to assess whether an important risk of bias exists |
Tirnaksiz 2012.
| Methods | Randomised, prospective, active‐controlled, double‐blind, within‐patient comparison Date of study Unreported Setting Department of Dermatology, School of Medicine, Gazi University, Ankara, Turkey |
|
| Participants |
Randomised: 12 participants (mean age 39.8 years, 5 male, 7 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Inflammatory lesion count; micro emulsion group 3.75 (0.74), commercial gel group 3.01 (0.59) Erythema score; micro emulsion group 2.50 (0.22), commercial gel group 2.08 (0.26) Telangiectasia; micro emulsion group 1.50 (0.17), commercial gel group 1.08 (0.31) |
|
| Interventions | Six weeks
Intervention
Comparator
To prevent cross‐over, hands were washed between the right and left applications. None of the patients used any other kind of treatment during the study period. No restrictions were placed on diet or the use of cosmetics |
|
| Outcomes | Assessments (4); baseline, week 2, 4 and 6 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 591): "This study was partly supported by a Grant from Gazi University, Turkey (SBE‐11‐2001/10)" | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 5 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 585): "This study was designed as a randomized, double‐blind, bilateral split‐face paired comparison" and "Patients were assigned to receive the commercial gel and microemulsion formulation to each half of the face." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 585): "Each patient received a pair of identical‐appearing vials, labeled right and left, one containing commercial gel and the other microemulsion formulation." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 585): "Each patient received a pair of identical‐appearing vials, labeled right and left, one containing commercial gel and the other microemulsion formulation
Comment: Outcomes were investigator‐ and participant assessed Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Treatment duration adequate, wash‐out period before the study adequate Comment: The study appeared to be free of other forms of bias |
Torok 2005.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Multicentre (6) in US. Trillium Creek Dermatology Center, Medina, Ohio; Department of Dermatology, Thomas Jefferson University, Pennsylvania; Radiant Research, Tucson, Arizona; International Research Services, Inc, Rockland, Maine; Derm Research, Inc, Austin, Texas |
|
| Participants |
Randomised: 152 participants (mean age 47 years (range 19 to 77), 43 male, 109 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean (SEM) Inflammatory lesion count; sulphacetamide group 18 (1), metronidazole group 17 (1) |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (5): baseline, week 3, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 357): "This study was supported by Stiefel Laboratories, Inc" | |
| Declaration of interest | Quote (page 357): "Dr Torok is a consultant and advisory board member for, is on speaker's bureau and received research grants from Galderma Laboratories, LP, Stiefel Laboratories Inc....Dr Webster is a consultant and speaker for and has received a grant from ..Galderma Laboratories, LP, Stiefel Laboratories Inc..Dr Egan is a consultant for Stiefel Laboratories Inc". Others no conflict of interest | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 21 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 358): "Subjects were randomly assigned to treatment with either sodium sulphacetamide 10% and sulfur 5% with sunscreens or metronidazole 0.75% cream." E‐mail contact with the investigator confirmed computer‐generated and central allocation Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment not reported E‐mail contact with the investigator confirmed central allocation Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 357): "...investigator‐blinded." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 357): "...investigator‐blinded." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were accounted for and included in analysis, but unclear how missing data was imputed Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | High risk | Participant's assessment of global improvement was not addressed Comment: As this was one of our principal outcomes, this was considered as at a high risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period adequate, groups treated equally aside from intervention. Study sponsored by Stiefel Laboratories, Inc. The first two investigators have received grants from Stiefel Laboratories Comment: Sponsorship and the fact that one investigator is a consultant for the sponsor raises concerns about the potential for bias |
Two 2014.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study July 2011 to December 2012 Setting University of California, San Diego (UCSD) Dermatology Clinic, US |
|
| Participants |
Randomised: 15 participants (mean age 60 years, 3 male, 7 female, 5 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean IGA score; SEI003 group 1.8, vehicle group 2.0 CEA score; SEI003 group 9, vehicle group 7 |
|
| Interventions | 12 weeks Intervention
Comparator
Application frequency unclear |
|
| Outcomes | Assessments (5): baseline, week 2, 6, 9 and 12 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 1145): "In vitro analysis described in this work was supported in part by the United States National Institutes of Health (NIH) grant R01‐AR052728 to RLG." | |
| Declaration of interest | Quote (page 1145): "Neither Therapeutics nor Skin Epibiotics provided any financial compensation for the study or to any members of the study team with the exception of EH, who left his position at UCSD for employment opportunities at these companies part‐way through the study" | |
| Notes | One of our primary outcomes was addressed (adverse events) See comparison 40 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1143): " randomized, double‐blind, placebo controlled study".."randomized 2:1...."
Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups
After e‐mail communication: "The allocation sequence was generated by an unblinded member of the study team who worked off‐site in a separate laboratory" Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement After e‐mail communication: "The allocation sequence was created prior to enrolling any subjects in the study" by a third party Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 1143): "Subjects, study coordinators, and those performing clinical assessments were blinded"
Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement After e‐mail communication: "study medication was placed into a bottle labeled with the participant’s unique study identification number that was assigned to the participant at the time of enrolment in the trial" by a third party, and "Both the treatment and the control creams were identical in appearance and viscosity so that the two drugs could not be distinguished by look or feel." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 1143): "Subjects, study coordinators, and those performing clinical assessments were blinded"
Comment: Outcomes were investigator and participant assessed. Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study
Insufficient information to permit a clear judgement
After e‐mail communication: "study medication was placed into a bottle labeled with the participant’s unique study identification number that was assigned to the participant at the time of enrolment in the trial" by a third party, and "Both the treatment and the control creams were identical in appearance and viscosity so that the two drugs could not be distinguished by look or feel." Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/15 (26.6%) in the SEI003 group and per‐protocol analysis Comment: High and unbalanced dropout rate combined with per‐protocol analysis judged as at high risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for this study NCT01398280 was available at https://www.clinicaltrialsregister.eu/ctr‐search/search and the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Low risk | Study duration adequate, no information regarding wash‐out period Comment: The study appeared to be free of other forms of bias |
Utaş 1997.
| Methods | Randomised, prospective, active and placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology, Erciyes University Medical School, Kayseri, Turkey |
|
| Participants |
Randomised: 53 participants (mean age 46.9 years, 7 male, 46 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Two weeks Intervention
Comparator 1
Comparator 2
Comparator 1
Comparator 1
|
|
| Outcomes | Assessments (2): baseline and week 2 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed Older study, described in letter, a lot of information is lacking (see Table 24) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 69): "Quote: "The patients were randomized into 5 groups" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 69): "double‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 69): "double‐blind". Only investigator assessed outcomes Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants/healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported, no exact data were provided Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unclear and no data provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Letter provided only limited data Comment: There was insufficient information to permit a clear judgement |
Van Landuyt 1997.
| Methods | Randomised, prospective, active‐controlled, double‐blind Date of study Unreported Setting Service de Dermatologie, Hôpital Saint Jacques |
|
| Participants |
Randomised: 60 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Not reported |
|
| Interventions | 30 days Intervention
Comparator
|
|
| Outcomes | Assessments (at least 3): baseline, 15 days and 30 days Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | This is a very brief interim report, full study has never been published, data only reported for 30 participants and largely unusable (see Table 24) None of our primary outcomes were addressed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 729): "randomisée" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 729): "double insu." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 729): "double insu" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Interim report on 30 participants Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
Veien 1986.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Multicentre, Department of Dermatology, Marselisborg Hospital, Arhus; Department of Dermatology, Genthofte Hospital, Copenhagen; Odense University Hospital, Odense; and Dermatology Clinic, Aalborg, Denmark |
|
| Participants |
Randomised: 76 participants (mean age 52.4 years, 36 male/39 female and 1 gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts/Withdrawals: 6/76 (7.9%); unclear how many from each group Baseline data mean (SD) Means for lesions or erythema were not reported |
|
| Interventions | Eight weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 2, 4 and 8 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 210); "The metronidazole cream and tetracycline tablets were supplied by the Danish drug company, Dumex Ltd." | |
| Declaration of interest | None declared | |
| Notes | None of our primary outcomes were addressed See comparison 56 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 209): "The study was performed in 4 centers as a double‐blind, randomized trial." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 209): "double‐blind" "...placebo tablets identical in appearance to the tetracycline tablets." "...placebo cream was cream base of metronidazole cream." Comment: The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 209): "double‐blind" "...placebo tablets identical in appearance to the tetracycline tablets." "...placebo cream was cream base of metronidazole cream." Outcomes were investigator assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/76 (7.9%); unclear how many participants from each group, per‐protocol analysis Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration adequate, wash‐out period before study rather short for oral therapy (2 weeks), unclear if other medications were allowed Comment: Insufficient information to assess whether an important risk of bias exists |
Verea Hernando 1992.
| Methods | RCT, prospective, active‐controlled, double‐blind Date of study Unreported Setting Dermatology department, Juan Canalejo Hospital, La Coruña, Spain |
|
| Participants |
Randomised: 40 participants (mean age 57.8 (14) years in the erythromycin group and 62.2 (12) years in metronidazole group, 13 male, 27 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data total Number of papules; erythromycin group 571 and metronidazole group 476 Number of pustules; erythromycin group 160 and metronidazole group 63 |
|
| Interventions | Three months Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) See comparison 26 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 474) (translation): "Patients included in the study were assigned a key number by the pharmacy service that assigned them to one of the two groups through a table of random numbers generated by computer." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 474) (translation): "They were randomised by a computer generated distribution numbered list by the pharmacy." Comment: Pharmacy‐controlled randomisation. Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 474): "...doble ciego." [Translated as double‐blind] Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 474): "...doble ciego." [Translated as double‐blind] Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts and withdrawals (> 20% in erythromycin group) were reported but unclear at which time points and no evidence of ITT analysis (page 475) Comment: We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | High risk | Physician's Global Assessment was not addressed or reported Comment: We judged this as at a high risk of bias |
| Other bias | High risk | Wash‐out period unclear, study duration adequate, groups probably treated equally. Baseline imbalance between the groups in number of pustules and papules Comment: The baseline imbalance in the groups puts the study at serious risk of bias |
Weissenbacher 2007.
| Methods | RCT, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Department of Dermatology and Allergy Biederstein, Munich, Germany |
|
| Participants |
Randomised: 40 participants (mean age 58 years, 25 male, 15 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria: Not stated Dropouts and withdrawals: None Baseline data mean Rosacea severity score; pimecrolimus group 6.88, vehicle group 7 |
|
| Interventions | Four weeks Intervention
Comparator
|
|
| Outcomes | Assessments (4): baseline, week 1, 2 and 3 Outcomes of the trial (as reported) Primary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | Quote (page 728): "M.B. is employed by Novartis Pharma, the manufacturer of Elidel (pimecrolimus)." | |
| Notes | We only included data from the first 4 weeks, second part of study was open‐phase. Two of our primary outcomes were addressed (quality of life and participant‐assessed changes in rosacea severity) See comparison 22 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 729): "Forty patients with papulopustular rosacea were investigated in a single‐centre, randomized, double‐blind vehicle‐controlled study." Comment:Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 728): "double‐blind", only first 4 weeks Comment: Vehicle cream was used. Probably identical appearance The report provided sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 728): "double‐blind", only first 4 weeks
Vehicle cream was used. Probably identical appearance. Outcomes were investigator and participant assessed. Blinding of participants and key study personnel was ensured, and it is unlikely that the blinding could have been broken Comment: We judged this as at a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals reported Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Unclear if there was a wash‐out period, duration of double‐blinded part too short (4 weeks), unclear if additional medications were allowed and recorded. One of the investigators was employed by the manufacturer of Elidel (Novartis Pharma) Comment: Insufficient information to assess whether an important risk of bias exists |
Wilkin 1989.
| Methods | RCT, prospective, active‐controlled, placebo‐controlled, double‐blind, cross‐over Date of study Unreported Setting McGuire Veterans Administration Medical Center, Richmond, US |
|
| Participants |
Randomised: 15 participants (age range 41 to 60 years, 4 male, 11 female) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Not stated, unclear Baseline data mean (SD) Nothing reported |
|
| Interventions | 53 days Intervention
Comparator 1
Comparator 2
Comparator 3
|
|
| Outcomes | Assessments for period A (2): baseline, day 18 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 202): "Supported by a grant from E.R. Squibb & Sons, Inc, New Brunswick, New Jersey" | |
| Declaration of interest | None declared | |
| Notes | We included only period A, first study period, however, no separate data for this period (see Table 24) None of our primary outcomes were addressed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 202): "All persons were randomly assigned to one of four 2‐way cross‐over treatment groups in a double‐blind manner." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if tablets were comparable/similar in appearance Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 203): "were analyzed in a blinded manner" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported, other than 1 participant who dropped out reasons unclear Comment: We judged this as at unclear risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | High risk | Wash‐out period was included in study design, other rosacea treatment did not have to be stopped, study duration too short (period of 17 to 18 days), groups treated equally. Small sample size Comment: We judged this as at a high risk of bias |
Wilkin 1993.
| Methods | RCT, prospective, active‐controlled, investigator‐blinded Date of study Unreported Setting Two centres in US |
|
| Participants |
Randomised: 43 participants (age range 25 to 70 years, both male and female, numbers not specified) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals: Unclear Baseline data mean (SD) Signs and symptoms of rosacea were comparable for both groups |
|
| Interventions | 12 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (2): baseline, week 12 Outcomes of the trial (as reported) Primary outcomes:✴
Secondary outcomes Not stated ✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 65): "Supported by a grant of Upjohn Company, Kalamazoo, Michigan" | |
| Declaration of interest | None declared | |
| Notes | Unclear how many participants were assigned to each group, dropouts not mentioned, no exact data provided (see Table 24) One of our primary outcomes was addressed (participant‐assessed changes in rosacea severity) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 66): "Patients were randomly assigned to one of two regimens." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 65): "...investigator‐blinded." "...double‐blinded." Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 65): "...investigator‐blinded." "...double‐blinded." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal outcomes data reported, dropouts and withdrawals unreported Comment: Insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Unclear what study outcomes were, not stated in Methods section Comment: Insufficient information to permit a clear judgement |
| Other bias | Low risk | Study duration and wash‐out period adequate, groups appear to have been treated equally Comment: The study appears to be free of other forms of bias |
Wittpenn 2005.
| Methods | Randomised, prospective, placebo‐controlled, double‐blind Date of study Unreported Setting Private Practice, Stony Brook, NY, Rand Eye Institute, Pompano Beach, Florida, US |
|
| Participants |
Randomised: 20 (age and gender unreported) Inclusion criteria
Ocular involvement: Yes Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | Three months Intervention
Comparator
Unclear how many were randomised to each group, application frequency unclear |
|
| Outcomes | Assessments (at least 2): baseline and month 3 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (in abstract): "None" | |
| Declaration of interest | Quote (in abstract): "JR Wittpenn, Allergan, B Schechter, Allergan" | |
| Notes | None of our outcomes were addressed. This study was part of NCT00348335 (see Table 21). Poster with very limited data (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (on poster): "patients were randomized to cyclosporine A or artificial tears for 3 months." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (on poster): "double‐masked." Comment: The report did not provide sufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (on poster): "double‐masked." Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only limited data were provided, no report on dropouts Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Abstract provided only limited data Comment: There was insufficient information to permit a clear judgement |
Wolf 2006.
| Methods | RCT, prospective, active‐controlled, investigator‐blind Date of study Unreported Setting Multicentre (15) in US |
|
| Participants |
Randomised: 160 participants (mean age 51.1 ± 10.7 years (range 32 to 78) in metronidazole group and 51.1 ± 11.3 years (range 31 to 77) in azelaic acid group, 26 male and 56 female in metronidazole group, and 18 male and 60 female in azelaic group) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data median Inflammatory lesions; metronidazole group 17 and azelaic acid group 14.5 |
|
| Interventions | 15 weeks Intervention
Comparator
|
|
| Outcomes | Assessments (6): baseline, week 3, 6, 9, 12 and 15 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | Quote (page 3): "This study was supported by a grant from Galderma Laboratories, LP" | |
| Declaration of interest | Quote (page 3): "Mr Kerrouche and Ms Arsonnaud are from Galderma Laboratories, LP, Sophi‐Antipolis, France. Dr Wolf is an advisory board member, consultant, researcher and speaker for Galderma Laboratories | |
| Notes | Two of our primary outcomes were addressed (participant‐assessed changes in rosacea severity and adverse events) See comparison 14 in Effects of interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 4): "Patients were randomized in a 1:1 fashion to treatment with metronidazole 1% gel once daily or azelaic acid 15% gel twice daily for a period of 15 weeks." Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during enrolment, was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 4): 'investigator‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 4): 'investigator‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (healthcare providers and participants) during the study Insufficient information to permit a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/160 (15%); metronidazole group (14) and azelaic acid group (10). Both ITT and per‐protocol analyses reported performed Comment: We judged this as at a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was not available, but the pre‐specified outcomes and those mentioned in the methods section appeared to have been reported Comment: We judged this as at a low risk of bias |
| Other bias | Unclear risk | Study duration and wash‐out period adequate, groups treated equally. Supported by a grant from Galderma Laboratories. First investigator was an advisory board member, consultant, researcher, and speaker for Galderma Laboratories, two other investigators are from Galderma Comment: We judged this as at unclear risk of bias |
Yoo 2011.
| Methods | Randomised, prospective, active‐controlled, single‐blind, within‐patient comparison
Date of study
Unreported Setting Mount Sinai Medical Center, New York, NY, US |
|
| Participants |
Randomised: 6 participants (age and gender unreported) Inclusion criteria
Ocular involvement: Unclear Exclusion criteria
Dropouts and withdrawals
Baseline data mean Nothing reported |
|
| Interventions | 12 weeks (4 sessions of laser with 2 week intervals) Intervention
Comparator
|
|
| Outcomes | Assessments (3): baseline, 16 and 20 Outcomes of the trial (as reported) Primary outcomes
Secondary outcomes
✴Denotes outcomes pre‐specified for this review |
|
| Funding source | None reported | |
| Declaration of interest | None declared | |
| Notes | One of our primary outcomes was addressed (adverse events) Poster abstract, limited information is provided (see Table 24) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 918): "and concurrently received PDL treatment to one randomized side" Comment: Insufficient detail was reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | The method used to conceal the allocation sequence, that is to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment was not reported Comment: There was insufficient information to permit a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 918): "single‐blind" Comment: The report provided insufficient detail about the measures used to blind study participants and personnel from knowledge of which intervention a participant received, to permit a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 918): "single‐blind" Comment: Uncertainty with the effectiveness of blinding of outcomes assessors (participants, healthcare providers) during the study Insufficient information to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1/6 for personal reasons lost to follow‐up. Comment: There was insufficient information to permit a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Only limited data were provided (protocol available at clinical trials.gov NCT00945373) Comment: There was insufficient information to permit a clear judgement |
| Other bias | Unclear risk | Only limited data were provided Comment: There was insufficient information to permit a clear judgement |
BID = twice a day, BZP = benzoyl peroxide, ITT = intention‐to‐treat analysis, N = number, n/a = not applicable, ns = not significant, no further data available, QD = once daily, RCT = randomised controlled trial, RWBT = rapid whole blood test, SD = standard deviation, SEM = standard error of the mean, TID = three times a day, UBT = urea breath test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aitken 1983 | Not a randomised controlled trial (RCT). No description of rosacea, unclear if additional medication was allowed, no site of evaluation is recorded, no intention‐to‐treat analysis (ITT). Lots of information is lacking |
| Aizawa 1992 | Not a RCT |
| Altinyazar 2005 | Quote: "Patients were randomly assigned..." Page 253 Comment: The investigator confirmed by email that this consisted of "60 sealed envelopes including names of treatments, half to half. Patients selected an envelope" This is a form of quasi‐randomisation. CCT |
| Aronson 1987 | Open allocation, "based on arrival", quasi‐randomised. CCT |
| Bakar 2006 | Not a RCT |
| Bang Soon 2007 | Not a RCT |
| Bartholomew 1982 | CCT, no evidence of randomisation |
| Berardesca 2008 | After e‐mail communication with the investigators to clarify aspects of trial conduct the judgement for sequence generation was changed from 'unclear' to 'high risk of bias'. The participants were allocated to the intervention by alternation. CCT |
| Beridze 2005 | CCT |
| Bernstein 1982 | Not a RCT |
| Bjerke 1989a | Not a RCT. Narrative report about treatment |
| Bukvic‐Mokos 1998 | CCT |
| Chu 2005 | Not a RCT. Case report |
| Colón 2007 | Study to assess cumulative irritation potential and not treatment effect on rosacea |
| Cunliffe 1977 | CCT |
| Del Rosso 2004 | Not a RCT. Narrative report about 2 studies |
| Dereli 2005 | Not a RCT. Open‐label study |
| Draelos 2005 | Not a RCT of effects of interventions on rosacea. Unit of randomisation = barrier tests on the arms |
| Erdogan 1998 | Not a RCT |
| Fernandez‐Obregon 2004 | Not a RCT |
| Fleischer 2005 | Open‐label, observational study |
| Freeman 2012 | After e‐mail contact appeared to be quasi‐randomised |
| Frigerio 1969 | Not a RCT |
| Frucht‐Pery 1993 | Quote: "Treatment (either doxycycline protocol or tetracycline hydrochloride protocol) was suggested to each patient at random. Those who refused the suggested protocol were offered the treatment with the other protocol." Page 89 Comment: Method used to randomise participants to the interventions was inadequate. Not a RCT |
| Garg 2008 | Not a RCT. Open‐label study |
| Gedik 2005 | Not a RCT. Rosacea patients were given triple therapy consisting of amoxicillin, clarithromycin and lansoprazole |
| Go 1976 | Not a RCT |
| Goldsmith 1989 | Not a RCT. Narrative review |
| Hofer 2004 | Not a RCT, no blinding, all participants were treated with isotretinoin |
| Irvine 1988 | Not a RCT |
| Jackson 2007 | Poster, without data. Unsuccessful attempts at contacting authors |
| Karabulut 2008 | Contact with investigators via electronic mail, responses clear that the allocation sequence was inadequately generated |
| Koçak‐Altintas 2005 | Quote: "...randomly divided into two groups." Comment: Following extensive email communication with the principal investigator we were unable to receive reassurances that the allocation sequence was adequately generated and therefore this study has been classified as a CCT |
| Laquieze 2007 | This study did not match the inclusion criteria for this review |
| Lee 2008 | Participants with steroid‐induced rosacea, and rosacea patients were excluded |
| Liu 2006 | Systematic review of 5 studies, all included in present review |
| Loo 2004 | Not a RCT |
| Maxwell 2010 | After reading full text appears to be CCT |
| Meekin 2008 | No participants with rosacea |
| Mraz 2008 | Not a RCT |
| Määttä 2006 | Not a RCT |
| Nasir 1985 | Not a RCT |
| Nielsen 1983 | Not a RCT |
| Ortiz 2009 | Not a RCT. Open‐label study |
| Parodi 2008 | Not a RCT |
| Ruggero 2005 | Not a RCT. Open, observational study |
| Sainthillier 2005 | This study did not match the inclusion criteria for this review |
| Seal 1995 | Participants with chronic blepharitis, few had associated rosacea. No separate data available for participants with rosacea. Many criteria were assessed as unclear or inadequate. No ITT |
| Sehgal 2008 | Not a RCT. Case report |
| Shanler 2007 | Not a RCT. Case report |
| Signore 1995 | Open‐label pilot study with 6 participants, of which 1 dropped out Quote: "Patients were selected randomly and consecutively." "Patients were instructed to apply 0.75% metronidazole gel to the right side of the face...and 5% permethrin to the left side." Page 177 Comment: Quasi‐randomised. CCT |
| Stoudemayer 2006 | Poster, limited data available. Not a RCT |
| Tierney 2009 | Ten participants with telangiectasia, no mention of rosacea at all |
| Togsverd‐Bo 2009 | Not a RCT. Case report of 4 treated participants |
| Torresani 1997 | Not a RCT |
| Trumbore 2009 | Not a RCT, no control. Open‐label |
| Uebelhoer 2007 | Population did not fit the inclusion criteria. Participants with photodamage without rosacea were also included. Unclear which participants had rosacea and no separate data available for participants with rosacea |
| Veien 1988 | Not a clinical trial |
| Veraldi 1996 | Not a RCT |
| Viera 2007 | Not a RCT. A narrative review on incyclinide |
| Yu 2006 | Not a RCT, open‐label study |
| Öztürkcan 2004 | Not a RCT |
RCT = randomised controlled trial CCT = controlled clinical trial (quasi‐randomised)
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12614000004662.
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | 138 participants with baseline facial Rosacea Severity Score (RSS) of 2 or greater |
| Interventions | Topical medical grade Kanuka honey versus cetomacrogol cream |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study has been completed. Website accessed 21‐7‐2014. Study is currently being written‐up, will be included when published |
IRCT2014030416837N1.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 40 participants with papulopustular rosacea |
| Interventions | Permethrin 5% gel versus placebo gel for 12 weeks |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Website accessed 23‐9‐2014, study is submitted for publication. Will be included when full data is reported |
NCT00560703.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 72 participants with facial rosacea and blepharitis |
| Interventions | Doxycycline 40 mg versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Completed July 2009, no study results reported. Attempts to contact CollaGenex Pharmaceuticals unsuccessful Website accessed 16‐7‐2014, sent mail to Galderma NL and international. There are some outcomes data reported on clinicaltrials.gov. Will be included when full data is reported |
NCT00617903.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 84 participants with papulopustular rosacea |
| Interventions | Azelaic acid 15% foam twice daily versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Completed June 2008, no study results reported yet. Website accessed 18‐7‐2014, sent e‐mail, is now changed into Bayer, some outcome data reported on clinicaltrials.gov. Information Bayer that study is not published yet. Will be included when full data is reported |
NCT00882531.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 156 participants with papulopustular rosacea |
| Interventions | Isotretinoin 0.25 mg/kg, 1 per day, 4 months of treatment versus placebo |
| Outcomes |
Primary Outcome measures
Secondary outcome measures
|
| Notes | Study completed, but not yet published Website accessed 18‐7‐2014, sent e‐mail to O Chosidow, they will send submitted abstract (will be later this year). Will be included when published |
NCT01125930.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 68 participants with erythematotelangiectatic rosacea |
| Interventions | 46 weeks, Atralin gel 0.05% versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed January 2013. Website accessed 19‐7‐2014 (some outcome data on clinicaltrials.gov), sent mail 19‐7‐2014 reply 29‐7‐2014. The study has not been published yet, but they will notify us. Will be included when published |
NCT01451619.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 60 participants with rosacea |
| Interventions | Laropiprant versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed April 2012. Website accessed 19‐7‐2014, submitted for publication. Will be included when published |
NCT01579084.
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 64 participants with moderate to severe facial erythema associated with rosacea |
| Interventions | AGN‐199201 Dose A versus AGN‐199201 Dose B versus AGN‐199201 Dose C versus vehicle (once and twice daily dosages) |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study completed June 2013. Website accessed 21‐7‐2014. E‐mail sent 22‐7‐2014 through website. Some data published on ClinicalTrials.gov. Will be included when published |
NCT01614743.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 10 participants with rosacea |
| Interventions | Incobotulinumtoxin A versus saline injections |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study was completed August 2014. Website accessed 19‐7‐2014. Paper is written‐up will be included in the review when published |
NCT01631656.
| Methods | Randomised, single‐blind, active‐controlled, within participant |
| Participants | 10 subjects with mild to moderate rosacea |
| Interventions | Azelaic acid 15% gel + Nd: YAG laser versus azelaic acid 15% gel |
| Outcomes |
Primary outcome measures
|
| Notes | Study was completed February 2011. Website accessed 20‐7‐2014. Article written‐up not yet published, unclear if it is truly randomised or CCT, no further reply received |
NCT01735201.
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 357 participants with moderate to severe facial erythema associated with rosacea |
| Interventions | AGN‐199201 Dose A versus AGN‐199201 Dose B versus AGN‐199201 Dose C versus vehicle (once and twice daily dosages) |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | The study has been completed June 2013. Website accessed 21‐7‐2014. Part of data published on ClinicalTrials.gov. Will be included when published |
NCT01740934.
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 117 participants with mild to moderate rosacea |
| Interventions | Anatabloc cream versus vehicle cream |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Notes | Study has been completed August 2013. Website accessed 20‐7‐2014. They are writing study down. Will be included when published |
Characteristics of ongoing studies [ordered by study ID]
EUCTR2006‐001999‐20‐HU.
| Trial name or title | Assessment of the efficacy and safety of three concentration: 1%, 0.3%, 0.1% of CD5024 cream once daily and CD5024 1% cream twice daily, versus its vehicle and versus metronidazole cream (Rozex®) in patients with papulopustular rosacea over 12 weeks |
| Methods | Randomised, single‐blind, active and placebo‐controlled |
| Participants | 270 |
| Interventions | CD5024 1% once daily, CD5024 0.3% once daily, CD5024 0.1% once daily, CD5024 1% twice daily, versus vehicle versus metronidazole 0.75% |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 10‐10‐2006, completed 2‐8‐2007 |
| Contact information | |
| Notes | Dose finding study for ivermectin, website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2006‐003707‐40‐DE.
| Trial name or title | Activity of twice daily per os administration of CD06713 at 8 mg versus its placebo during 4 weeks treatment, in patients with erythemato‐telangiectatic rosacea |
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | 48 participants with erythemato‐telangiectatic rosacea |
| Interventions | Ondansetron 8 mg versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 9‐1‐2007, completed 23‐5‐2007 |
| Contact information | |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2006‐007029‐29‐EE.
| Trial name or title | Non inferiority study of metronidazole 0.75% cream versus reference therapy in the local treatment of papulopustular rosacea |
| Methods | Randomised, single‐blind, placebo and active‐controlled |
| Participants | 300 participants with papulopustular rosacea |
| Interventions | Metronidazole 0.75% cream versus metronidazole 0.75% gel versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 08‐05‐2007 |
| Contact information | Not provided, but sponsored by Pierre Fabre Dermatologie |
| Notes | Website accessed 23‐9‐2014, still ongoing in France |
EUCTR2008‐003854‐13‐FR.
| Trial name or title | An investigator blind parallel group vehicle control study comparing the efficacy and safety of CD 5024 1% cream with metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment |
| Methods | Randomised, active and vehicle‐controlled, investigator‐blinded |
| Participants | 600 participants with papulopustular rosacea |
| Interventions | Ivermectin 1% versus placebo versus metronidazole 0.75% |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 15‐01‐2009, still ongoing |
| Contact information | Not provided but sponsored by GALDERMA R&D SNC |
| Notes | Website accessed 23‐9‐2014 |
EUCTR2009‐013111‐35‐DE.
| Trial name or title | Effect of CD08514 versus placebo, in patients presenting with type 1 rosacea, over an 8‐week treatment |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | Number unclear, participants with moderate to severe erythemato‐telangiectactic rosacea |
| Interventions | Famotidin‐ratiopharm® 40 and 10 mg BID versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 9‐10‐2009, completed 7‐6‐2010 |
| Contact information | Galderma R&D SNC |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2010‐018319‐13‐DE.
| Trial name or title | A double‐blind, vehicle controlled, parallel group study assessing the activity of CD5024 1% cream in subjects with papulopustular rosacea over 12 weeks treatment |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 317 participants with papulopustular rosacea |
| Interventions | CD5024 1% cream (ivermectin) versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 17‐08‐2010, study completed 02‐05‐2011 |
| Contact information | GALDERMA R&D SNC, France |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2010‐021150‐19‐NL.
| Trial name or title | Doxycycline versus minocycline in the treatment of rosacea: a randomised controlled trial ‐ DoMino‐study |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | Number not stated, participants with papulopustular rosacea |
| Interventions | Doxycycline 40 mg versus minocycline 100 mg |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 5‐10‐2010 |
| Contact information | Study is ongoing |
| Notes | Website accessed 23‐9‐2014, study of Mireille MD van der Linden |
EUCTR2010‐023566‐43‐DE.
| Trial name or title | Multizentrische, randomisierte, doppelblinde, kontrollierte Phase III‐Studie zur Behandlung der papulopustulären Rosazea mit Permethrin Creme 5 % (InfectoScab®) versus Permethrin Creme 2,5 % versus Metronidazol Creme 0,75 % (Rozex®) ‐ Papulopustuläre Rosazea‐Behandlung mit Permethrin Creme versus Metronidazol Creme |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | Number of participants unclear, participants with papulopustular rosacea |
| Interventions | Permethrin 5% cream versus permethrin 2.5% cream versus metronidazole 0.75% cream |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | Study is completed 27‐02‐2013 |
| Contact information | INFECTOPHARM Arzneimittel GmbH Dr. Bertil Wachall, studien@infectopharm.com |
| Notes | Website accessed 23‐9‐2014, sent e‐mail |
EUCTR2011‐002057‐65‐DE.
| Trial name or title | Effect of CD08100/02 3% gel versus placebo in subjects presenting with erythematotelangiectatic rosacea over a 4 week treatment period |
| Methods | Randomised, single‐blind, placebo‐controlled, within‐participant |
| Participants | Number unclear, participants with erythematotelangiectatic rosacea |
| Interventions | Diclofenac sodium 3% gel versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 23‐12‐2013 study completed |
| Contact information | Galderma R&D SNC, France. cta.coordinator@galderma.com |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2011‐002058‐30‐DE.
| Trial name or title | Effect of CD08100/02 3% gel versus placebo gel in subjects presenting with papulopustular rosacea over a 6‐week treatment period |
| Methods | Randomised, single‐blind, placebo‐controlled, within‐participant |
| Participants | Number unclear, participants with papulopustular rosacea |
| Interventions | Diclofenac sodium 3% gel versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | Study is completed 23‐03‐2012 |
| Contact information | Galderma R&D SNC, France. cta.coordinator@galderma.com |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL |
EUCTR2011‐004791‐11‐CZ.
| Trial name or title | Efficacy and safety of CD5024 1% cream versus metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment, followed by a 36‐week extension period |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 960 participants with papulopustular rosacea |
| Interventions | CD5024 1% cream (ivermectin) versus metronidazole 0.75% cream |
| Outcomes |
Primary outcome measures
Secondary outcome measures
For second part:
|
| Starting date | 10‐1‐2012, study has been completed 19‐12‐2013 |
| Contact information | Galderma R&D SNC, France cta.coordinator@galderma.com |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL. Same number of participants as Taieb 2015 (part A), however, that study did not have an extension of 36 weeks (part B) |
EUCTR2012‐001044‐22‐SE.
| Trial name or title | A multicentre, randomised, double‐blind, vehicle‐controlled, parallel group study to demonstrate the efficacy and assess the safety of CD07805/47 gel 0.5% applied topically once daily in subjects with moderate to severe facial erythema of rosacea |
| Methods | Randomised, double‐blind, vehicle‐controlled |
| Participants | 140 participants with facial rosacea |
| Interventions | Briminodine tartrate 0.5% versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 15‐08‐2012 |
| Contact information | cta.coordinator@galderma.com |
| Notes | Website accessed 23‐9‐2014, sent mail to Galderma NL. Looks like design of Fowler 2013a but other number of participants and Jackson 2013 |
EUCTR2013‐005083‐26‐DE.
| Trial name or title | Effect of CD07805/47 gel in subjects presenting with flushing related to erythematotelangiectatic or papulopustular rosacea ‐ Effect of CD07805/47 gel in rosacea flushing |
| Methods | Randomised, single‐blind, placebo‐controlled |
| Participants | Number unreported, mild to moderate erythematotelangiectatic rosacea (ETR) or mild to moderate papulopustular rosacea (PPR) |
| Interventions | Brimonidine 0.5% gel versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures Period 1
Period 2
Others:
|
| Starting date | 28‐2‐2014, study completed June 2014 |
| Contact information | cta.coordinator@galderma.com |
| Notes | Website accessed 23‐9‐2014, email sent 23‐9‐2014 to Galderma NL |
IRCT2014010516079N1.
| Trial name or title | Comparison of dapsone 5% topical gel with metronidazole 0.75% efficacy in combination with oral doxycycline In papulopustular rosacea |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | 56 participants with papulopustular rosacea |
| Interventions | Dapsone 5% gel b.i.d. + doxycycline 100 mg versus metronidazole 0.75% gel + doxycycline 100 mg for 12 weeks |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 10‐4‐2013 study is completed |
| Contact information | Dr. Gita Faghihi G_faghihi@med.mui.ac.ir or Dr. Parastoo Khosravani p_khosravani@resident.mui.ac.ir |
| Notes | Website accessed 23‐9‐2014, email sent 23‐9‐2014 |
JPRN‐UMIN000008315.
| Trial name or title | Clinical trial for development of topical rapamycin treatment for rosacea |
| Methods | Randomised, placebo‐controlled, cross‐over |
| Participants | 5 participants with rosacea |
| Interventions | 0.2% rapamycin ointment versus vehicle |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | Still recruiting |
| Contact information | Mari Wataya‐Kaneda, mkaneda@derma.med.osaka‐u.ac.jp |
| Notes | Website accessed 23‐9‐2014, not sent mail as they are still recruiting |
NCT00041977.
| Trial name or title | A multicentre, randomised, double‐blind, placebo‐controlled, clinical trial to determine the effects of doxycycline hyclate 20 mg tablets (Periostat(R)) administered twice daily for the treatment of acne rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 150 men and women with rosacea, erythema, papules/pustules, and telangiectasia |
| Interventions | Doxycycline hyclate 20 mg tablets (Periostat(R)) administered twice daily versus placebo |
| Outcomes | Not specified |
| Starting date | June 2002 |
| Contact information | Not provided |
| Notes | Study has been completed. Tried to contact CollaGenex Pharmaceuticals without success Website accessed 16‐7‐2014, sent e‐mail to D. Pariser for more information, and asked Galderma NL and international |
NCT00436527.
| Trial name or title | MetroGel 1% hydration study: a kinetic regression study |
| Methods | Randomised, single blind, no treatment control, within‐participant |
| Participants | 26 participants with rosacea |
| Interventions | Metronidazole gel 1% versus no treatment |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2006 |
| Contact information | Galderma |
| Notes | Study has been completed August 2006. Website accessed 19‐7‐2014, sent e‐mail to Galderma NL and international |
NCT00495313.
| Trial name or title | Determine the effects of COL‐101 administered once daily with metronidazole topical gel, 1% versus doxycycline hyclate 100 mg administered once daily with metronidazole topical gel, 1% in patients with moderate to severe rosacea |
| Methods | Randomised, double‐blind, active‐controlled |
| Participants | 91 participants with papulopustular rosacea, with erythema and telangiectasia |
| Interventions | Vibramycin plus metronidazole versus Oracea® delayed‐release plus metronidazole |
| Outcomes | Not specified |
| Starting date | March 2007 |
| Contact information | CollaGenex Pharmaceuticals (C Powala, VP, Drug Development & Regulatory Affairs) |
| Notes | Study completed December 2007. Tried to contact CollaGenex Pharmaceuticals without success Website accessed 16‐7‐2014, sent message via LinkedIn, and asked Galderma NL and international |
NCT00621218.
| Trial name or title | A pilot study to compare tretinoin gel, 0.05% to tretinoin gel vehicle when dosed once or twice daily in female subjects with classical rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 26 with erythrophagocytotic rosacea |
| Interventions | Tretinoin gel 0.05% bid versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2008 |
| Contact information | Coria Laboratories, Ltd (D. Innes Cargill, PhD) |
| Notes | Study completed December 2008, no study results reported yet Website accessed 18‐7‐2014, sent e‐mail, company is acquired by Valeant Pharmaceuticals |
NCT00667173.
| Trial name or title | A phase 2, multi‐centre, evaluator‐blind, randomised, vehicle‐controlled clinical study to assess the safety and efficacy of IDP‐115 in the treatment of rosacea |
| Methods | Randomised, single‐blind, 3 arms, placebo‐controlled |
| Participants | 140 with facial rosacea and inflammatory lesions |
| Interventions | Drug: IDP‐115 topical application for 12 weeks versus vehicle versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | November 2007 |
| Contact information | Dow Pharmaceutical Sciences, Inc |
| Notes | Study has been completed July 2008, no published data yet, seeking initial approval September 2010 Website accessed 18‐7‐2014, Dow Pharmaceuticals Sciences is acquired by Valeant Pharmaceuticals, sent e‐mail |
NCT00697541.
| Trial name or title | A phase II, single‐centre, two‐way crossover relative systemic bioavailability study of Col‐118 administered topically as a 0.18 % facial gel and brimonidine ophthalmic solution 0.2% administered to the eye in subjects with moderate to severe erythematous rosacea |
| Methods | Randomised, double‐blind, active‐control |
| Participants | 20 male and female subjects with moderate to severe erythematous rosacea will be randomised into 2 groups of 10 subjects |
| Interventions | 0.18% Col‐118 facial gel (1.8 mg brimonidine) versus 0.2% brimonidine ophthalmic solution (0.1 mg brimonidine tartrate drop) versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2008 |
| Contact information | Galderma (Michael Graeber, MD, Head of US Development) |
| Notes | Study completed June 2008, study results not reported Website assessed 18‐7‐2014, sent e‐mail to Galderma NL and international |
NCT01016782.
| Trial name or title | Multi‐centre, double‐blind, randomised, vehicle‐controlled, parallel group study of 0444 gel |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 867 participants with rosacea |
| Interventions | 70 days, 0444 gel versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2008 |
| Contact information | Fougera Pharmaceuticals Inc |
| Notes | Study completed 2009, results not reported Website accessed 19‐7‐2014, company acquired by Sandoz in 2012, sent e‐mail through website Sandoz |
NCT01134991.
| Trial name or title | Pilot, randomised, double blind, placebo controlled, parallel group, dose range finding study, to evaluate the tolerability and safety of FXFM244 antibiotic foam and to monitor its clinical effect in moderate to severe rosacea patients |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 21 moderate to severe rosacea patients |
| Interventions | 12 weeks, 1% FXFM244 versus 4% FXFM244 versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2010 |
| Contact information | Foamix Ltd |
| Notes | Study terminated (difficulties in recruitment). Website accessed 19‐7‐2014, sent e‐mail. Reply 21‐7‐2014, according to Dov Tamarkin, PhD the study is still ongoing |
NCT01186068.
| Trial name or title | A randomised, double‐blind, vehicle‐controlled, parallel‐group study of the dose‐response profile of V‐101 cream in subjects with erythematous rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 175 participants with erythematous rosacea |
| Interventions | V‐101 versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2010 |
| Contact information | Vicept Therapeutics, Inc. (Chief Operating Officer) |
| Notes | Study has been completed (no data reported), but after e‐mail contact not yet published |
NCT01257919.
| Trial name or title | Investigator‐blinded, randomised, cross‐over, multiple dose phase I study on safety and pharmacokinetics of topically applied azelaic acid foam, 15% compared to azelaic acid gel, 15% in subjects with papulopustular rosacea |
| Methods | Randomised, investigator‐blind, cross‐over, multiple dose phase I study |
| Participants | 21 participants with papulopustular rosacea |
| Interventions | Azelaic acid foam versus azelaic acid gel |
| Outcomes |
Primary outcome measures
|
| Starting date | January 2011 |
| Contact information | Bayer, no further contact details are provided, sent mail through website Bayer, Novum info@novumprs.com, e‐mails sent, but not yet published |
| Notes | Study has been completed March 2011. Website accessed 19‐7‐2014 |
NCT01308619.
| Trial name or title | A multicentre, randomised, double‐blind, placebo‐controlled evaluation of rosacea‐related inflammatory biochemical markers in the skin of adults with papulopustular rosacea treated with daily doxycycline 40 mg (30 mg immediate release / 10 mg delayed release beads) capsules |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 170 participants with papulopustular rosacea |
| Interventions | Doxycycline 40 mg versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | April 2011 |
| Contact information | Galderma Laboratories, no further contact information is provided |
| Notes | Study has been completed August 2012. Website accessed 19‐7‐2014, sent mail to Galderma NL and international |
NCT01513863.
| Trial name or title | A therapeutic equivalence study of two metronidazole 1% topical gel treatments for patients with rosacea (MTZG). A randomised, double‐blind, placebo controlled, parallel design, multi‐site clinical study to compare the bioequivalence of two metronidazole 1% topical gel formulations in patients with moderate to severe rosacea |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 602 participants with moderate to severe rosacea |
| Interventions | Metronidazole topical gel 1% versus metronidazole topical gel 1% (Metrogel) versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2011 |
| Contact information | Taro Pharmaceuticals USA GDGongas@novumprs.com. Study has not been published yet, e‐mail 21‐7‐2014 ABrown@novumprs.com, confirmed, not yet submitted |
| Notes | Study has been completed September 2012. Website accessed 19‐7‐2014 |
NCT01555463.
| Trial name or title | A randomised, double‐blind, vehicle‐controlled, multicentre, parallel‐group clinical trial to assess the safety and efficacy of azelaic acid foam, 15% topically applied twice daily for 12 weeks in subjects with papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 961 participants with papulopustular rosacea |
| Interventions | Azelaic acid foam 15% versus vehicle |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2012 |
| Contact information | Bayer, no further contact details provided, e‐mail contact with Bayer not yet submitted for publication |
| Notes | Study has been completed January 2014. Website accessed 20‐7‐2014 |
NCT01659853.
| Trial name or title | A multicentre, randomised, controlled, double‐masked, crossover design study to compare efficacy and assess safety of CD07805/47 gel 0.5% applied once daily vs azelaic acid gel 15% applied twice daily in subjects with erythema of rosacea |
| Methods | Randomised, double‐blind, cross‐over, active and placebo‐controlled |
| Participants | 70 participants with erythema of rosacea |
| Interventions | CD07805/47 gel 0.5% versus azelaic acid 15% gel versus vehicle |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | September 2012 |
| Contact information | Galderma Laboratories, L.P. No further contact details provided |
| Notes | Study has been completed December 2012. Website accessed 20‐7‐2014, sent e‐mail (outcomes reported on clinicaltrials.gov) not yet published according to Galderma, but I thought already submitted, sent another e‐mail |
NCT01784133.
| Trial name or title | A phase 2, randomised, vehicle‐controlled, double‐blind, multicentre study to evaluate the safety and efficacy of three once‐daily CLS001 topical gels versus vehicle administered for 12 weeks to subjects with papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 240 participants with papulopustular rosacea |
| Interventions | Omiganan versus placebo |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2013 |
| Contact information | Cutanea Life Sciences, Inc. No further contact details provided.info@cutanealife.com, sent mail 23‐7‐2014 and 10‐8‐2014 |
| Notes | Study has been completed March 2014. Website accessed 20‐7‐2014 |
NCT01828177.
| Trial name or title | A multicentre randomised evaluator‐blinded vehicle‐controlled parallel group evaluation of twice daily PDI‐320 in comparison to its monads in adults with rosacea |
| Methods | Randomised, single‐blind, active and placebo‐controlled |
| Participants | 200 participants with rosacea |
| Interventions | PDI‐320 versus PDI‐320 monad #1 versus PDI‐320 monad #2 versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2013 |
| Contact information | PreCision Dermatology, Inc.Syd Dromgoole, PhD. No further contact details provided, as study is still ongoing, not sent mail |
| Notes | Study is ongoing. Website accessed 20‐7‐2014 |
NCT01917539.
| Trial name or title | Efficacy of Pulsed Light Therapy for Meibomian gland dysfunction and dry eye syndrome |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 140 participants with facial rosacea and diagnosis of mild to moderate dry eye syndrome with meibomian gland dysfunction and ocular rosacea |
| Interventions | Pre‐existing dry eye treatment + sham treatment versus pre‐existing dry eye treatment + Pulsed Light Therapy |
| Outcomes |
Primary outcome measures
|
| Starting date | June 2013 |
| Contact information | Angela Chang, University of Miami AChang2@med.miami.edu, Bradford Lee blee@post.harvard.edu |
| Notes | This study is currently recruiting participants. Website accessed 21 July 2014. As study is still recruiting not sent mail |
NCT01933464.
| Trial name or title | An analysis of the effect of topical cromolyn sodium on rosacea‐associated erythema |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 10 participants with rosacea associated erythema |
| Interventions | Cromolyn sodium versus normal saline |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | August 2013 |
| Contact information | Anna Di Nardo, MD, PhD, University of California, San Diego. As study is still recruiting not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
NCT01993446.
| Trial name or title | A randomised, double‐blind, vehicle‐controlled study of the safety and efficacy of topical DRM02 in subjects with rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 30 participants with rosacea |
| Interventions | DRM02 versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | October 2013 |
| Contact information | Dermira, Inc. Beth Zib no further contact details provided, info@dermira.com sent mail 23‐7‐2014 and 11‐8‐2014 |
| Notes | Study has been completed March 2014. Website accessed 20‐7‐2014 |
NCT02036229.
| Trial name or title | A randomised, double blind, placebo‐controlled, half‐face study to evaluate the effect of topical ivermectin cream 0.5% on demodicidosis |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 50 subjects with clinical and laboratory diagnosis of demodicidosis with symmetrical facial eruption (including papulopustular rosacea) |
| Interventions | Ivermectin 0.5% cream versus vehicle cream |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2014 |
| Contact information | Rina Segal, Rabin Medical Center, rinas3@clalit.org.il, not sent mail as not yet open to recruitment |
| Notes | This study is not yet open for participant recruitment. Website accessed 20‐7‐2014 |
NCT02052999.
| Trial name or title | An open label pilot study to evaluate the efficacy of PAC‐14028 in the treatment of erythematotelangiectatic rosacea and papulopustular rosacea |
| Methods | Randomised, open‐label, active and placebo‐controlled |
| Participants | 80 participants with erythema‐telangiectatic or papulopustular rosacea |
| Interventions | PAC‐14028 cream 1% versus metronidazole gel 0.75% versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | February 2013 |
| Contact information | Amorepacific Corporation BeomJoon Kim, Professor Department of Dermatology, Chungang University Hospital 23‐7‐2014, sent mail through website |
| Notes | Study has been completed August 2013. Website accessed 20‐7‐2014 |
NCT02075671.
| Trial name or title | Photodynamic therapy for papulopustular rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 30 participants with papulopustular rosacea |
| Interventions | Aminolevulinic acid topical solution 20% + Blu‐U Light versus vehicle + Blu‐U Light |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | April 2014 |
| Contact information | George Washington University, Jack Short, jshort@mfa.gwu.edu, as they are still recruiting, not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
NCT02120924.
| Trial name or title | A multicentre, double‐blind, randomised, parallel‐group, vehicle‐controlled study to evaluate the safety and clinical equivalence of a generic azelaic acid gel, 15% and the reference listed Finacea® (azelaic acid) gel, 15% in patients with moderate facial rosacea |
| Methods | Randomised, double‐blind, active and placebo‐controlled |
| Participants | 1100 participants wild moderate rosacea |
| Interventions | Generic azelaic acid gel, 15% versus Finacea® (azelaic acid) gel, 15% versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | July 2013 |
| Contact information | Actavis Inc. No further contact details provided |
| Notes | Still recruiting. Website accessed 20‐7‐2014. As they are still recruiting, not sent mail |
NCT02132117.
| Trial name or title | Safety and efficacy of AGN‐199201 in patients with persistent erythema associated with rosacea |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | 440 participants with persistent erythema associated with rosacea |
| Interventions | AGN‐199201 versus vehicle |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2014 |
| Contact information | Allergan, clinicaltrials@allergan.com seems the same as NCT02131636 which I did not add, sent mail, see Table 21 |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
NCT02144181.
| Trial name or title | of the safety and efficacy of the Ulthera® System for the treatment of signs and symptoms of erythematotelangiectatic rosacea |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 88 participants with erythematotelangiectatic rosacea |
| Interventions | Two low‐density Ulthera System treatments versus three low‐density Ulthera System treatments versus two high‐density Ulthera System treatments versus three high‐density Ulthera System treatments |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2014 |
| Contact information | Ulthera, Inc. Mark Lupin, MD. No further contact details provided. (is Lupin 2014, now an include, part of this study?). Sent mail 29‐7‐2014 |
| Notes | Still recruiting. Website accessed 20‐7‐2014 |
NCT02147691.
| Trial name or title | Finacea 15% and brimonidine 0.33% gel in the treatment of rosacea ‐ a pilot study |
| Methods | Randomised, single‐blind, active‐controlled |
| Participants | 20 participants with moderate to severe rosacea |
| Interventions | Azelaic acid 15% gel + brimonidine 0.33% gel versus brimonidine 0.33% gel |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2014 |
| Contact information | Leon Kircik, M.D., Derm Research, PLLC, as they are still recruiting, not sent mail |
| Notes | This study is currently recruiting participants. Website accessed 20‐7‐2014 |
NCT02204254.
| Trial name or title | Prospective, open label, randomised study comparing bipolar radiofrequency potentiated by infrared light to doxycycline in patient with papulopustular rosacea |
| Methods | Randomised, open label, active‐controlled |
| Participants | 40 participants with papulopustular rosacea |
| Interventions | Bipolar radiofrequency potentiated by infrared light versus doxycycline |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | Recruiting |
| Contact information | Florence le Duff, leduff.f2@chu‐nice.fr |
| Notes | Website accessed 23‐9‐2014, as they are still recruiting, not sent e‐mail |
Differences between protocol and review
For this update we have revised all of the search strategies and added a search of the LILACS database. LILACS is now a standard resource used in Cochrane Skin Group reviews. The Cochrane randomised control trial filters for MEDLINE and EMBASE have been refined since the previous version of this review, and the latest versions have been used for this update. We also removed many terms that were symptoms of rosacea rather than being synonyms for the disease itself. We checked the new strategies against the previous ones to ensure they were robust enough to capture relevant RCTs.
For this update we added an additional physician‐assessed outcome 'assessment of erythema or telangiectasia, or both, at end of study'. In previous versions these data were in part reflected in 'Other data' tables, but we thought it would be more appropriate to have this outcome as a separate listed outcome, as erythema is very bothersome to people with rosacea. We have removed 'dropout rates' as an outcome as this is already listed in the 'Characteristics of included studies' tables, as well as under attrition bias in the risk of bias tables. Adverse events moved to primary outcomes as these should include events that are of potential harm (MECIR C14).
We also revised the 'Methods' section to meet the latest requirements of the Cochrane Handbook for Systematic Reviews of Interventions as well as the MECIR reporting standards. As it is unlikely we will encounter cluster trials we removed this item under 'units of analysis issues'.
For this update the additional comparisons of interventions of within‐participant studies have been included after extracting the RR or MD and SE for those that appropriately accounted for the variability. These studies were then included in meta‐analyses (where appropriate) with the other studies using a generic inverse‐method of analysis in Revman.
After consulting Toby Lasserson, Alain Mayhew and the Editorial board of the Cochrane Skin Group we agreed that funding would be added in 'Characteristics of included studies' tables. However, in cases where we considered that the financial support, or the employment of the investigators by the pharmaceutical company, might have posed a risk of bias we would report this, just like in former versions, in the domain 'Other bias' of the risk of bias tables.
We have added 'Summary of findings' tables according to the latest requirements of the Cochrane Handbook for Systematic Reviews of Interventions and MECIR.
All fixed‐effect model calculations for data synthesis have been recalculated to the random‐effects model in line with the Cochrane Handbook for Systematic Reviews of Interventions.
In the former 2011 update, at the request of the Skin Group odds ratios have been changed into risk ratios. Because risk and odds are different when events are common, the risk ratio and the odds ratio also differ when events are common. The Skin Group recommends that because many of the outcomes of trials of skin conditions are common events risk ratios should be used.
In the protocol (published in 2001) we had planned, under the section Types of studies, to include randomised controlled trials in people with moderate to severe rosacea. By the time the review was first published 2004 'Types of studies' had been amended to randomised controlled trials (RCTs) that met the methodological criteria. This remained the same for the substantial update that was published in 2005. We had previously excluded trials that were RCTs and otherwise matched our inclusion criteria if they were assessed to be of low methodological quality. Following the advice of the Skin Group's editors, we re‐assessed all of the excluded RCTs and those which matched the inclusion criteria were included in the former update of this review (2011) and the participant data were analysed (if appropriate).
For the former update we rewrote the Methods section, especially the 'Data collection and analysis' section after comments from our referees. We added a part on 'Assessment of heterogeneity of studies' and only pooled outcomes if we had at least three studies to provide an acceptable body of evidence to examine the intervention. In earlier versions we pooled the data of two studies.
Having abandoned the use of quality judgments to exclude some trials, we assessed all included studies using the Cochrane Collaboration's tool for assessing bias.
Contributions of authors
EvZ was the contact person with the editorial base. EvZ co‐ordinated the contributions from the co‐authors and together with ZF wrote the final draft of the protocol. EvZ and ZF screened papers against eligibility criteria. EvZ and ZF obtained data on ongoing and unpublished studies. EvZ, ZF, and BC updated the Methods sections. EvZ and ZF extracted data for the review and sought additional information about papers. EvZ and ZF entered data into RevMan and in the Result section EvZ, ZF and BC analysed and interpreted data. EvZ, ZF and MvdL drafted the clinical sections of the Background, Discussion and Conclusions and responded to the clinical comments of the referees. EvZ, ZF, BC responded to the methodology and statistics comments of the referees. LC was the consumer co‐author and checked the review for readability and clarity. EvZ is the guarantor of the final review.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
No sources of support found, Netherlands.
External sources
Acne and Rosacea Society of Canada, Canada.
-
The National Insititute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
The authors have no declarations of interest.
Joel Bamford who is a clinical referee on this updated review said, 'I am one author of two studies included: Bamford 1999 and Bamford 2012 and two studies referenced: Bamford 2004 and Bamford 2006'.
Edited (no change to conclusions)
References
References to studies included in this review
Akhyani 2008 {published data only}
- Akhyani M, Ehsani AH, Ghiasi M, Jafari AK. Comparison of efficacy of azithromycin vs. doxycycline in the treatment of rosacea: a randomized open clinical trial. International Journal of Dermatology 2008;47(3):284‐8. [PUBMED: 18289334] [DOI] [PubMed] [Google Scholar]
Alam 2013 {published data only}
- Alam M, Voravutinon N, Warycha M, Whiting D, Nodzenski M, Yoo S, et al. Comparative effectiveness of nonpurpuragenic 595‐nm pulsed dye laser and microsecond 1064‐nm neodymium:yttrium‐aluminum‐garnet laser for treatment of diffuse facial erythema: A double‐blind randomized controlled trial. Journal of the American Academy of Dermatology 2013;69(3):438‐43. [PUBMED: 23688651] [DOI] [PubMed] [Google Scholar]
Bamford 1999 {published data only}
- Bamford JT, Tilden RL, Blankush JL, Gangeness DE. Effect of treatment of Helicobacter pylori infection on rosacea. Archives of Dermatology 1999;135(6):659‐63. [UI: 99303186; PUBMED: 10376693] [DOI] [PubMed] [Google Scholar]
Bamford 2012 {published data only}
- Bamford JT, Gessert CE, Haller IV, Kruger K. Rosacea unresponsive to oral zinc sulfate, a randomized controlled clinical trial. Journal of Investigative Dermatology 2009;129 Suppl 1:S41. [EMBASE: 70385883] [Google Scholar]
- Bamford JT, Gessert CE, Haller IV, Kruger K, Johnson BP. Randomized, double‐blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. International Journal of Dermatology 2012;51(4):459‐62. [PUBMED: 22435439] [DOI] [PubMed] [Google Scholar]
Barnhorst 1996 {published data only}
- Barnhorst DA, Foster JA, Chern KC, Meisler DM. The efficacy of topical metronidazole in the treatment of ocular rosacea. Ophthalmology 1996;103(11):1880‐3. [UI:97098344; PUBMED: 8942885] [DOI] [PubMed] [Google Scholar]
Benkali 2014 {published data only}
- Benkali K, Leoni M, Rony F, Bouer R, Fernando A, Graeber M, et al. Comparative pharmacokinetics and bioavailability of brimonidine following ocular and dermal administration of brimonidine tartrate ophthalmic solution and gel in patients with moderate‐to‐severe facial erythema associated with rosacea. British Journal of Dermatology 2014;171(1):162‐9. [PUBMED: 24506775] [DOI] [PubMed] [Google Scholar]
Berardesca 2012 {published data only}
- Berardesca E, Iorizzo M, Abril E, Guglielmini G, Caserini M, Palmieri R, et al. Clinical and instrumental assessment of the effects of a new product based on hydroxypropyl chitosan and potassium azeloyl diglycinate in the management of rosacea. Journal of Cosmetic Dermatology 2012;11(1):37‐41. [PUBMED: 22360333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beutner 2005 {published data only}
- Beutner K, Calvarese B. A multi‐center, investigator‐blind clinical trial to assess the safety and efficacy of metronidazole gel 1% as compared to metronidazole gel vehicle and metronidazole cream 1% in the treatment of rosacea. Journal of the American Academy of Dermatology 2005;52(3 Suppl):P10. [Google Scholar]
Bitar 1990 {published data only}
- Bitar A, Bourgouin J, Doré N, Dubuc R, Giroux JM, Madeleine‐Landry EA. A double‐blind randomised study of metronidazole (Flagyl) 1% cream in the treatment of acne rosacea, a placebo controlled study. Drug Investigation 1990;2(4):242‐8. [EMBASE: 1991066113] [Google Scholar]
Bjerke 1989 {published data only}
- Bjerke JR, Nyfors A, Austad J, Rajka G, Gjertsen BT, Haavelsrud O, et al. Metronidazole (Elyzol) 1% cream v. placebo cream in the treatment of rosacea. Clinical Trials Journal 1989;26(3):187‐94. [EMBASE: 1989151247] [Google Scholar]
Bjerke 1999 {published data only}
- Bjerke R, Fyrand O, Graupe K. Double‐blind comparison of azelaic acid 20% cream and its vehicle in treatment of papulo‐pustular rosacea. Acta Dermato‐Venerologica 1999;79(6):456‐9. [UI: 20065819; PUBMED: 10598760] [DOI] [PubMed] [Google Scholar]
Bleicher 1987 {published data only}
- Bleicher PA, Charles JH, Sober AJ. Topical metronidazole therapy for rosacea. Archives of Dermatology 1987;123(5):609‐14. [UI: 8721097; PUBMED: 2953312] [PubMed] [Google Scholar]
Blom 1984 {published data only}
- Blom I, Hornmark AM. Topical treatment with sulfur 10 per cent for rosacea. Acta Dermato‐Venereologica 1984;64(4):358‐9. [EMBASE: 1984179892] [PubMed] [Google Scholar]
Breneman 1998 {published data only}
- Breneman DL, Stewart D, Hevia O, Hino PD, Drake LA. A double‐blind, multicenter clinical trial comparing efficacy of once‐daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis 1998;61(1):44‐7. [UI:98127231; PUBMED: 9466083] [PubMed] [Google Scholar]
Breneman 2004 {published data only}
- Breneman D, Savin R, VandePol C, Vamvakias G, Levy S, Leyden J. Double‐blind, randomized, vehicle‐controlled clinical trial of once‐daily benzoyl peroxide/clindamycin topical gel in the treatment of moderate to severe rosacea. International Journal of Dermatology 2004;43(5):381‐7. [PUBMED: 15117375] [DOI] [PubMed] [Google Scholar]
- Leyden LL, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/Clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis 2004;73(6 Suppl):11‐7. [PUBMED: 15228129] [PubMed] [Google Scholar]
Bribeche 2015 {published data only}
- Bribeche MR, Fedotov VP, Gladichev VV, Pukhalskaya DM, Kolitcheva NL. Clinical and experimental assessment of the effects of a new topical treatment with praziquantel in the management of rosacea. International Journal of Dermatology 2015; Vol. 54, issue 4:481‐7. [DOI: 10.1111/ijd.12552; PUBMED: 25040098] [DOI] [PubMed]
Buendia‐Bordera 2013 {published data only}
- Buendia‐Bordera G, Ciscar E. Skin barrier function assessment by in vivo confocal microscopy and other non‐invasive optical measurements on patients suffering from rosacea to evaluate the efficacy of a post‐laser serum. Lasers in Surgery and Medicine 2013;45 Suppl 25:43. [EMBASE: 71034059] [Google Scholar]
Carmichael 1993 {published data only}
- Carmichael A, Marks R, Graupe KA, Zaumseil RP. Topical azelaic acid in the treatment of rosacea. Journal of Dermatological Treatment 1993;4(1 Suppl):S19‐22. [EMBASE: 1993216511] [Google Scholar]
Chang 2012 {published data only}
- Chang AL, Alora‐Palli M, Lima XT, Chang TC, Cheng C, Chung CM, et al. A randomized, double‐blind, placebo‐controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. Journal of Drugs in Dermatology 2012;11(3):333‐9. [PUBMED: 22395584] [PubMed] [Google Scholar]
Dahl 1998 {published data only}
- Dahl MV, Katz HI, Krueger GG, Millikan LE, Odom RB, Parker F, et al. Topical metronidazole maintains remissions of rosacea. Archives of Dermatology 1998;134(6):679‐83. [UI: 98307542; PUBMED: 9645635] [DOI] [PubMed] [Google Scholar]
Dahl 2001 {published data only}
- Dahl MV, Jarratt MJ, Kaplan D, Tuley MR, Baker MD. Once‐daily topical metronidazole cream formulations in the treatment of papules and pustules of rosacea. Journal of the American Academy of Dermatology 2001;45(5):723‐30. [PUBMED: 11606923] [DOI] [PubMed] [Google Scholar]
Del Rosso 2007a {published data only}
- Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, et al. Two randomized phase III clinical trials evaluating anti‐inflammatory dose doxycycline (40‐mg doxycycline, USP capsules) administered once daily for treatment of rosacea. Journal of the American Academy of Dermatology 2007;56(5):791‐802. [PUBMED: 17367893] [DOI] [PubMed] [Google Scholar]
Del Rosso 2007b {published data only}
- Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, et al. Two randomized phase III clinical trials evaluating anti‐inflammatory dose doxycycline (40‐mg doxycycline, USP capsules) administered once daily for treatment of rosacea. Journal of the American Academy of Dermatology 2007;56(5):791‐802. [PUBMED: 17367893] [DOI] [PubMed] [Google Scholar]
Del Rosso 2008 {published data only}
- Rosso JQ, Caveney S. Comparison of anti‐inflammatory dose doxycycline (30‐mg immediate‐release beads, 10 mg delayed‐release beads)vs antibiotic dose doxycycline (100mg) in the treatment of rosacea. 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States, 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine and Surgery 2012;31(1):A7‐8. [EMBASE: 70706922] [Google Scholar]
- Rosso JQ, Schlessinger J, Werschler P. Comparison of anti‐inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. Journal of Drugs in Dermatology 2008;7(6):573‐6. [PUBMED: 18561589] [PubMed] [Google Scholar]
Del Rosso 2010 {published data only}
- Rosso JQ, Bruce S, Jarratt M, Menter A, Staedtler G. Efficacy of topical azelaic acid (AzA) gel 15% plus oral doxycycline 40 mg versus metronidazole gel 1% plus oral doxycycline 40 mg in mild‐to‐moderate papulopustular rosacea. Journal of Drugs in Dermatology 2010;9(6):607‐13. [PUBMED: 20645521] [PubMed] [Google Scholar]
Draelos 2005b {published data only}
- Draelos ZD, Fuller BB. Efficacy of 1% 4‐ethoxybenzaldehyde in reducing facial erythema. Dermatologic Surgery 2005;31(7 Pt 2):881‐5. [PUBMED: 16029682] [DOI] [PubMed] [Google Scholar]
Draelos 2006 {published data only}
- Draelos ZD, Green BA, Edison BL. An evaluation of a polyhydroxy acid skin care regimen in combination with azelaic acid 15% gel in rosacea patients. Journal of Cosmetic Dermatology 2006;5(1):23‐9. [PUBMED: 17173568] [DOI] [PubMed] [Google Scholar]
Draelos 2009 {published data only}
- Draelos Z, Erthel K, Schnicker M, Bacon R, Vickery S. Facial foundation with niacinamide and N‐acetylglucosamine improves skin condition in women with sensitive skin. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB82. [EMBASE: 70142048] [Google Scholar]
Draelos 2013a {published data only}
- Draelos ZD, Elewski B, Staedtler G, Havlickova B. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double‐blind, vehicle‐controlled study. Cutis; Cutaneous Medicine for the Practitioner 2013;92(6):306‐17. [PUBMED: 24416747] [PubMed] [Google Scholar]
Draelos 2013b {published data only}
- Draelos Z, Hornby S, Walters RM, Appa Y. Hydrophobically modified polymers can minimize skin irritation potential caused by surfactant‐based cleansers. Journal of Cosmetic Dermatology 2013;12(4):314‐21. [PUBMED: 24305430] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby S, Fowler J, Walters RM, Appa Y, Draelos Z. Tolerance of facial cleansers on adults with clinically sensitive skin. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB66. [EMBASE: 70997350] [Google Scholar]
Dreno 1998 {published data only}
- Dreno B, Dubertret L, Naeyaert JM, Brassine M, Marks R, Powell F, et al. Comparison of the clinical efficacy and safety of metronidazole 0.75% cream with metronidazole 0.75% gel in the treatment of rosacea. Journal of the European Academy of Dermatology & Venereology 1998;11(2 Suppl):S272‐3. [Google Scholar]
Elewski 2003 {published data only}
- Elewski BE, Fleisher AB, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Archives of Dermatology 2003;139(11):1444‐50. [PUBMED: 14623704] [DOI] [PubMed] [Google Scholar]
Ertl 1994 {published data only}
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low‐dose oral isotretinoin in rosacea. Archives of Dermatology 1994;130(3):319‐24. [UI: 94175557; PUBMED: 8129410] [PubMed] [Google Scholar]
Espagne 1993 {published data only}
- Espagne E, Guillaume JC, Archimbaud A, Baspeyras M, Boitier F, Bussière M, et al. Double‐blind study versus excipient of 0.75% metronidazole gel in the treatment of rosacea [Étude en double insu contre excipient du métronidazole gel a 0,75 p.100 dans le traitement de la rosacée]. Annales de Dermatologie et de Vénéréologie 1993;120(2):129‐33. [UI: 93370955; PUBMED: 8240534] [PubMed] [Google Scholar]
Fabi 2011 {published data only}
- Fabi S, Peterson J, Goldman M. Combination 15% azelaic acid gel and intense pulse light therapy for mild to moderate rosacea. 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States. Conference Start: 20110330 Conference End: 20110403. Lasers in Surgery & Medicine 2011;43 Suppl 23:968‐9. [EMBASE: 70640329] [Google Scholar]
Fowler 2007 {published data only}
- Fowler JFJr. Combined effect of anti‐inflammatory dose doxycycline (40‐mg doxycycline, usp monohydrate controlled‐release capsules) and metronidazole topical gel 1% in the treatment of rosacea. Journal of Drugs in Dermatology 2007;6(6):641‐5. [PUBMED: 17668530] [PubMed] [Google Scholar]
Fowler 2012a {published data only}
- Fowler J, Jarratt M, Moore A, Meadows K, Pollack A, Steinhoff M, et al. Once‐daily topical brimonidine tartrate gel 0·5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle‐controlled studies. British Journal of Dermatology 2012;166(3):633‐41. [PUBMED: 22050040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows K, Jarratt M, Jones T, Pollack A, Stough D, Leoni M. A single application of brimonidine tartrate gel significantly reduces moderate to severe facial erythema of rosacea. 46th Annual Scientific Meeting of the Australasian College of Dermatologists Sydney, NSW Australia. Conference Start: 20130519 Conference End: 20130522. Australasian Journal of Dermatology 2013;54 Suppl 2:48. [EMBASE: 71067783] [Google Scholar]
- Meadows K, Pollack A, Jarratt M, Jones T. A single application of brimonidine tartrate gel significantly reduces moderate to severe facial erythema associated with rosacea. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB41. [EMBASE: 70704014] [Google Scholar]
Fowler 2012b {published data only}
- Fowler J, Jarratt M, Moore A, Meadows K, Pollack A, Steinhoff M, et al. Once‐daily topical brimonidine tartrate gel 0·5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle‐controlled studies. British Journal of Dermatology 2012;166(3):633‐41. [PUBMED: 22050040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J, Moore A, Meadows K, Grande K. Once‐daily topical brimonidine tartrate gel 0.5% is safe and efficacious in the treatment of moderate to severe persistent facial erythema associated with rosacea. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB17. [EMBASE: 70703920] [Google Scholar]
- Fowler J, Moore A, Meadows K, Grande K, Steinhoff M, Leoni M. Once‐daily topical brimonidine tartrate gel 0.5% is safe and efficacious in the treatment of moderate to severe persistent facial erythema of rosacea. 46th Annual Scientific Meeting of the Australasian College of Dermatologists Sydney, NSW Australia. Conference Start: 20130519 Conference End: 20130522. Australasian Journal of Dermatology 2013;54 Suppl 2:48‐9. [EMBASE: 71067784] [Google Scholar]
Fowler 2013a {published data only}
- Fowler J Jr, Jackson M, Moore A, Jarratt M, Jones T, Meadows K, et al. Efficacy and safety of once‐daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double‐blind, and vehicle‐controlled pivotal studies. Journal of Drugs in Dermatology 2013;12(6):650‐6. [PUBMED: 23839181] [PubMed] [Google Scholar]
- Fowler J, Jackson M, Steinhoff M, Jarratt M, Jones T, Meadows K, et al. Efficacy and safety of once‐daily brimonidine tartrate gel 0.5% for moderate to severe facial erythema of rosacea – Two randomized, double‐blind and vehicle controlled Phase III studies. 46th Annual Scientific Meeting of the Australasian College of Dermatologists Sydney, NSW Australia. Conference Start: 20130519 Conference End: 20130522. Australasian Journal of Dermatology 2013;54 Suppl 2:49. [EMBASE: 71067785] [Google Scholar]
- Fowler JF, Moore A, Meadows K, Jackson M, Leoni M, Jarratt MT, at al. Efficacy and safety of once‐daily brimonidine tartrate gel 0.5% for moderate to severe facial erythema of rosacea: Two randomized, double‐blind,and vehicle‐controlled phase III studies. 71st Annual Meeting of the American Academy of Dermatology Miami Beach, FL United States. Conference Start: 20130301 Conference End: 20130305. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB15. [EMBASE: 70997145] [Google Scholar]
- Jackson JM, Fowler J, Moore A, Jarratt M, Jones T, Meadows K, et al. Improvement in facial erythema within 30 minutes of initial application of brimonidine tartrate in patients with rosacea. Journal of Drugs in Dermatology 2014;13(6):699‐704. [PUBMED: 24918560] [PubMed] [Google Scholar]
Fowler 2013b {published data only}
- Fowler J Jr, Jackson M, Moore A, Jarratt M, Jones T, Meadows K, et al. Efficacy and safety of once‐daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double‐blind, and vehicle‐controlled pivotal studies. Journal of Drugs in Dermatology 2013;12(6):650‐6. [PUBMED: 23839181] [PubMed] [Google Scholar]
- Jackson JM, Fowler J, Moore A, Jarratt M, Jones T, Meadows K, et al. Improvement in facial erythema within 30 minutes of initial application of brimonidine tartrate in patients with rosacea. Journal of Drugs in Dermatology 2014;13(6):699‐704. [PUBMED: 24918560] [PubMed] [Google Scholar]
Gollnick 2010 {published data only}
- Gollnick H, Blume‐Peytavi U, Szabó EL, Meyer KG, Hauptmann P, Popp G, et al. Systemic isotretinoin in the treatment of rosacea ‐ doxycycline ‐ and placebo‐controlled, randomized clinical study. Journal der Deutschen Dermatologischen Gesellschaft 2010;8(7):505‐15. [DOI: 10.1111/j.1610-0387.2010.07345.x; PUBMED: 20337772] [DOI] [PubMed] [Google Scholar]
- Gollnick H, Matthies C, Werth R. Double‐blind, double‐dummy, randomized, placebo‐controlled, five armed,multicenter phase II/III study to evaluate the efficacy and safety of different concentrations of isotretinoin versus doxycycline in the treatment of rosacea, subtype II and III. 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Conference Start: 20100305 Conference End: 20100309. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB41. [EMBASE: 70142682] [Google Scholar]
Grosshans 1997 {published data only}
- Grosshans E, Michel C, Arcade B, Cribier B. Rilmenidine in rosacea: a double blind study versus placebo [Rilménidine dans la rosacée: étude en double insu contre placebo]. Annales de Dermatologie et Venereologie 1997;124(10):687‐91. [UI: 98413298; PUBMED: 9740864] [PubMed] [Google Scholar]
Guillet 1999 {published data only}
- Guillet B, Rostain E, Powell F, Gimenez Camarasa C, Dahan E, Piérard, et al. Metronidazole 0.75% gel and lotion are both effective in the treatment of rosacea. Journal of the European Academy of Dermatology and Venereology 1999;12(2 Suppl):S145. [Google Scholar]
Huang 2012 {published data only}
- Huang YE, Li XL, Li TJ. Clinical research of topical tacrolimus ointment combined with 585 nm pulsed dye laser in the treatment of rosacea [Chinese]. Journal of Clinical Dermatology 2012;41(5):308‐9. [EMBASE: 2012449799] [Google Scholar]
Huang 2014 {published data only}
- Huang E, Nardo A, Gallo R, Caveney S, Gottschalk RW. Surface cathelicidin expression is a predictor of treatment success in papulopustular rosacea. 2013 International Investigative Dermatology Meeting Edinburgh United Kingdom. Conference Start: 20130508 Conference End: 20130511. Journal of Investigative Dermatology 2013;133 Suppl 1:S181. [EMBASE: 71083535] [Google Scholar]
- Huang EY, Nardo A, Preson NJ, Gallo RL, Gottschalk RW. Multicenter, randomized, double‐blind, placebo‐controlled evaluation of rosacea related inflammatory biomarkers in papulopustular rosacea adults treated with doxycycline 40 mg modified release. 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Conference Start: 20140321 Conference End: 20140325. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB9. [EMBASE: 71390129] [Google Scholar]
Jackson 2013 {published data only}
- Jackson JM, Kircik LH, Lorenz DJ. Efficacy of extended‐release 45 mg oral minocycline and extended‐release 45 mg oral minocycline plus 15% azelaic acid in the treatment of acne rosacea. Journal of Drugs in Dermatology 2013;12(3):292‐8. [PUBMED: 23545911] [PubMed] [Google Scholar]
Jorizzo 1998 {published data only}
- Jorizzo JL, Lebwohl M, Tobey RE. The efficacy of metronidazole 1% cream once daily compared with metronidazole 1% cream twice daily and their vehicles in rosacea: a double blind clinical trial. Journal of the American Academy of Dermatology 1998;39(3):502‐4. [UI: 98409174; PUBMED: 9738794] [DOI] [PubMed] [Google Scholar]
Karsai 2008 {published data only}
- Karsai S, Roos S, Raulin C. Treatment of facial telangiectasia using a dual‐wavelength laser system (595 and 1,064 nm): a randomized controlled trial with blinded response evaluation. Dermatologic Surgery 2008;34(5):702‐8. [PUBMED: 18318728] [DOI] [PubMed] [Google Scholar]
Kendall 2014 {published data only}
- Kendall J, Winkelman W. A comparison of 3 assessments in the treatment of rosacea in the context of a comparative effectiveness study. ISPOR 19th Annual International Meeting Montreal, QC Canada. Conference Start: 20140531 Conference End: 20140604. Value in Health 2014;17(3):A181‐2. [EMBASE: 71488504] [Google Scholar]
Kim 2011 {published data only}
- Kim TG, Roh HJ, Cho SB, Lee JH, Lee SJ, Oh SH. Enhancing effect of pretreatment with topical niacin in the treatment of rosacea‐associated erythema by 585‐nm pulsed dye laser in Koreans: A randomized, prospective, split‐face trial. British Journal of Dermatology 2011; Vol. 38, issue 5:510‐3. [DOI: 10.1111/j.1365-2133.2010.10174.x.; PUBMED: 21143465] [DOI] [PubMed]
Koca 2010 {published data only}
- Koca R, Altinyazar HC, Ankarali H, Muhtar S, Tekin NS, Cinar S. A comparison of metronidazole 1% cream and pimecrolimus 1% cream in the treatment of patients with papulopustular rosacea: a randomized open‐label clinical trial. Clinical and Experimental Dermatology 2010;35(3):251‐6. [PUBMED: 19594764] [DOI] [PubMed] [Google Scholar]
Koçak 2002 {published data only}
- Koçak M, Yagli S, Vahapoglu G, Eksioglu M. Permethrin 5% cream versus metronidazole 0.75% gel for the treatment of papulopustular rosacea. Dermatology 2002;205(3):265‐70. [PUBMED: 12399675] [DOI] [PubMed] [Google Scholar]
Koch 1999 {published data only}
- Koch R, Wilbrand G. Dark sulfonated shale oil versus placebo in the systemic treatment of rosacea [P‐011]. Journal of the European Academy of Dermatology and Venerology 1999;12(32 Suppl):S143‐4. [Google Scholar]
Lebwohl 1995 {published data only}
- Lebwohl MG, Medansky RS, Russo CL, Plott RT. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% (Sulfacet‐R) lotion and metronidazole 0.75% (Metrogel) in the treatment of rosacea. Journal of Geriatric Dermatology 1995;3(5):183‐5. [Google Scholar]
Leyden 2011 {published data only}
- Leyden JJ. Efficacy of a novel rosacea treatment system: an investigator‐blind, randomized, parallel‐group study. Journal of Drugs in Dermatology 2011;10(10):1179‐85. [PUBMED: 21968669] [PubMed] [Google Scholar]
Leyden 2014 {published data only}
- Leyden JJ. Randomized, phase 2, dose‐ranging study in the treatment of rosacea with encapsulated benzoyl peroxide gel. Journal of Drugs in Dermatology 2014;13(6):685‐8. [PUBMED: 24918558] [PubMed] [Google Scholar]
Luger 2015 {published data only}
- Luger T, Peukert N, Rother M. A multicentre, randomized, placebo‐controlled trial establishing the treatment effect of TDT 068, a topical formulation containing drug‐free ultra‐deformable phospholipid vesicles, on the primary features of erythematotelangiectatic rosacea. Journal of the European Academy of Dermatology and Venereology 2015; Vol. 29, issue 2:283‐90. [DOI: 10.1111/jdv.12520; PUBMED: 24754379] [DOI] [PubMed]
Lupin 2014 {published data only}
- Lupin M. Evaluation of the safety and effectiveness of microfocused ultrasound with visualization (MFU‐V) for the treatment of erythematotelangiectatic rosacea. 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Conference Start: 20140321 Conference End: 20140325. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB43. [EMBASE: 71390263] [Google Scholar]
Maddin 1999 {published data only}
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. Journal of the American Academy of Dermatology 1999;40(6 Pt 1):961‐5. [UI: 99292050; PUBMED: 10365928] [DOI] [PubMed] [Google Scholar]
Marks 1971 {published data only}
- Marks R, Ellis J. Comparative effectiveness of tetracycline and ampicillin in rosacea: a controlled trial. Lancet 1971;2(7733):1049‐52. [UI: 72022755; PUBMED: 4106909] [DOI] [PubMed] [Google Scholar]
Monk 1991 {published data only}
- Monk BE, Logan RA, Cook J, White JE, Mason RBS. Topical metronidazole in the treatment of rosacea. Journal of Dermatological Treatment 1991;2(3):91‐3. [EMBASE: 1991353230] [Google Scholar]
Montes 1983 {published data only}
- Montes LF, Cordero AA, Kriner J, Loder J, Flanagan AD. Topical treatment of acne rosacea with benzoyl peroxide acetone gel. Cutis 1983;32(2):185‐90. [PUBMED: 6225627] [PubMed] [Google Scholar]
Mostafa 2009 {published data only}
- Mostafa FF, Harras MA, Gomaa SM, Al Mokadem S, Nassar AA, Abdel Gawad EH. Comparative study of some treatment modalities of rosacea. Journal of the European Academy of Dermatology and Venereology 2009;23(1):22‐8. [PUBMED: 18705632] [DOI] [PubMed] [Google Scholar]
NCT00249782 {unpublished data only}
- NCT00249782. A phase II, randomized, partial‐blind, parallel‐group, active‐ and vehicle‐controlled, multicenter study of the safety and efficacy of ACZONE™ (dapsone) gel, 5% in subjects with papulopustular rosacea. http://clinicaltrials.gov/ct/show/NCT00249782 (accessed 19 July 2014).
NCT01426269 {unpublished data only}
- NCT01426269. Evaluation of relapse, efficacy and safety of long‐term treatment with Oracea® capsules compared to placebo after an initial 12 week treatment regimen with Oracea® and MetroGel® 1% in adults with rosacea. clinicaltrials.gov/show/NCT01426269 (accessed 21 July 2014).
NCT01449591 {unpublished data only}
- NCT01449591. A proof of concept (PoC) study to evaluate the safety, tolerability, and efficacy of 12 week administration of BFH772 ointment in rosacea patients. clinicaltrials.gov/show/NCT01449591 (accessed 19 July 2014).
NCT01885000 {unpublished data only}
- NCT01885000. Patient‐Reported Outcome of facial erythema (PROOF). clinicaltrials.gov/show/NCT01885000 (accessed 21 July 2014).
Neuhaus 2009 {published data only}
- Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatologic Surgery 2009;35(6):920‐8. [PUBMED: 19397667] [DOI] [PubMed] [Google Scholar]
Nielsen 1983a {published data only}
- Nielsen PG. Treatment of rosacea with 1% metronidazole cream. A double‐blind study. British Journal of Dermatology 1983;108(3):327‐32. [PUBMED: 6219689] [DOI] [PubMed] [Google Scholar]
Nielsen 1983b {published data only}
- Nielsen PG. A double‐blind study of 1% metronidazole cream versus systemic oxytetracycline therapy for rosacea. British Journal of Dermatology 1983;109(1):63‐5. [UI: 83231308; PUBMED: 6222756] [DOI] [PubMed] [Google Scholar]
Nymann 2010 {published data only}
- Nymann P, Hedelund L, Haedersdal M. Long‐pulsed dye laser vs. intense pulsed light for the treatment of facial telangiectasias: A randomized controlled trial. Journal of the European Academy of Dermatology and Venereology 2010;24(2):143‐6. [PUBMED: 20205349] [DOI] [PubMed] [Google Scholar]
Pye 1976 {published data only}
- Pye RJ, Burton JL. Treatment of rosacea by metronidazole. Lancet 1976;1(7971):1211‐2. [UI: 76195079; PUBMED: 58258] [DOI] [PubMed] [Google Scholar]
Rehmus 2006 {published data only}
- Rehmus W, Kim J. A double‐blind, placebo‐controlled study of a natural anti‐inflammatory for treatment of rosacea. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB64. [Google Scholar]
Rigopoulos 2005 {published data only}
- Rigopoulos D, Kalogeromitros D, Gregoriou S, Pacouret JM, Koch C, Fisher N, et al. Randomized placebo‐controlled trial of a flavonoid‐rich plant extract‐based cream in the treatment of rosacea. Journal of the European Academy of Dermatology and Venereology 2005;19(5):564‐8. [PUBMED: 16164709] [DOI] [PubMed] [Google Scholar]
Rodríguez 2003 {published data only}
- Rodríguez Acar M, Medina Hernández E. A comparative, double‐blind study about efficacy and safety of crotamiton vs benzyl benzoate in the treatment of rosacea with demodecidosis [Estudio doble ciego, comparativo, sobre la eficacia y seguridad del crotamitón versus benzoato de bencilo en el tratamiento de la rosácea con demodecidosis]. Dermatología Revista Mexicana 2003;47(3):126‐30. [EMBASE: 2007502703] [Google Scholar]
Saihan 1980 {published data only}
- Saihan EM, Burton JL. A double‐blind trial of metronidazole versus oxytetracycline therapy for rosacea. British Journal of Dermatology 1980;102(4):443‐5. [PUBMED: 6446314] [DOI] [PubMed] [Google Scholar]
Salem 2013 {published data only}
- Salem DA, El‐Shazly A, Nabih N, El‐Bayoumy Y, Saleh S. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin‐metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. International Journal of Infectious Diseases 2013;17(5):343‐7. [PUBMED: 23294870] [DOI] [PubMed] [Google Scholar]
Sanchez 2005 {published data only}
- Sanchez J, Somolinos AL, Almodóvar PI, Webster G, Bradshaw M, Powala C. A randomized, double‐blind, placebo‐controlled trial of the combined effect of doxycycline hyclate 20‐mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. Journal of the American Academy of Dermatology 2005;53(5):791‐7. [PUBMED: 16243127] [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Somolinos A, Webster G, Bradshaw M. Combined effect of doxycycline hyclate 20 mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. Journal of the American Academy of Dermatology 2004;50(3):48. [DOI] [PubMed] [Google Scholar]
Sauder 1997 {published data only}
- Sauder DN, Miller R, Gratton D, Danby W, Griffiths C, Philips SB. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double‐blind study. Journal of Dermatological Treatment 1997;8(2):79‐85. [EMBASE: 1997242784] [Google Scholar]
Schachter 1991 {published data only}
- Schachter D, Schachter RK, Long B, Shiffman N, Lester R, Miller S, et al. Comparison of metronidazole 1% cream versus oral tetracycline in patients with rosacea. Drug Investigation 1991;3(4):220‐4. [EMBASE: 1991314872] [Google Scholar]
Schechter 2009 {published data only}
- Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Advances in Therapy 2009;26(6):651‐9. [PUBMED: 19551353] [DOI] [PubMed] [Google Scholar]
Seité 2013 {published data only}
- Seité S, Benech F, Berdah S, Bayer M, Veyrat S, Segot E, et al. Management of rosacea‐prone skin: evaluation of a skincare product containing Ambophenol, Neurosensine, and La Roche‐Posay Thermal spring water as monotherapy or adjunctive therapy. Journal of Drugs in Dermatology 2013;12(8):920‐4. [EMBASE: 2013521246] [PubMed] [Google Scholar]
Sharquie 2006 {published data only}
- Sharquie KE, Najim RA, Al‐Salman HN. Oral zinc sulfate in the treatment of rosacea: a double‐blind, placebo‐controlled study. International Journal of Dermatology 2006;45(7):857‐61. [PUBMED: 16863527] [DOI] [PubMed] [Google Scholar]
Sneddon 1966 {published data only}
- Sneddon IB. A clinical trial of tetracycline in rosacea. British Journal of Dermatology 1966;78(12):649‐52. [PUBMED: 4224811] [DOI] [PubMed] [Google Scholar]
Stein 2014a {published data only}
- Stein L, Kircik L, Fowler J, Tan J, Draelos Z, Fleischer A, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double‐blind, vehicle‐controlled pivotal studies. Journal of Drugs in Dermatology 2014;13(3):316‐23. [PUBMED: 24595578] [PubMed] [Google Scholar]
Stein 2014b {published data only}
- Stein L, Kircik L, Fowler J, Tan J, Draelos Z, Fleischer A, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double‐blind, vehicle‐controlled pivotal studies. Journal of Drugs in Dermatology 2014;13(3):316‐23. [PUBMED: 24595578] [PubMed] [Google Scholar]
Taieb 2015 {published data only}
- Taieb A, Ortonne JP, Ruzicka T, Roszkiewicz J, Berth‐Jones J, Peirone MH, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator‐blinded trial. British Journal of Dermatology 2015; Vol. 172, issue 4:1103‐10. [PUBMED: 25228137] [DOI] [PubMed]
Tan 2002 {published data only}
- Tan JKL, Girard C, Krol A, Murray HE, Papp KA, Poulin Y, et al. Randomized placebo‐controlled trial of metronidazole 1% cream with sunscreen SPF 15 in treatment of rosacea. Journal of Cutaneous Medicine and Surgery 2002;6(6):529‐34. [PUBMED: 12001006] [DOI] [PubMed] [Google Scholar]
Thiboutot 2003a {published data only}
- Thiboutot D, Thieroff‐Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: Results from 2 vehicle‐controlled, randomized phase III studies. Journal of the American Academy of Dermatology 2003;48(6):836‐45. [PUBMED: 12789172] [DOI] [PubMed] [Google Scholar]
Thiboutot 2003b {published data only}
- Thiboutot D, Thieroff‐Ekerdt R, Graupe K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: Results from 2 vehicle‐controlled, randomized phase III studies. Journal of the American Academy of Dermatology 2003;48(6):836‐45. [PUBMED: 12789172] [DOI] [PubMed] [Google Scholar]
Thiboutot 2005 {published data only}
- Thiboutot D. Efficacy and safety of subantimicrobial‐dose doxycycline for the treatment of rosacea. Journal of the American Academy of Dermatology 2005;52(3 Suppl 1):17. [Google Scholar]
Thiboutot 2008 {published data only}
- Thiboutot DM, Fleisher AB, Rosso JQ, Graupe K. Azelaic acid 15% gel once daily versus twice daily in papulopustular rosacea. Journal of Drugs in Dermatology 2008;7(6):541‐6. [PUBMED: 18561584 ] [PubMed] [Google Scholar]
Thiboutot 2009 {published data only}
- Thiboutot DM, Fleischer AB, Rosso JQ, Rich PH. A Multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance therapy. Journal of Drugs in Dermatology 2009;8(7):639‐48. [PUBMED: 19588640] [PubMed] [Google Scholar]
Tirnaksiz 2012 {published data only}
- Tirnaksiz F, Kayiş A, Çelebi N, Adişen E, Erel A. Preparation and evaluation of topical microemulsion system containing metronidazole for remission in rosacea. Chemical & Pharmaceutical Bulletin 2012;60(5):583‐92. [PUBMED: 22689395] [DOI] [PubMed] [Google Scholar]
Torok 2005 {published data only}
- Torok HM, Webster G, Dunlap FE, Egan N, Jarratt M, Stewart D. Combination sodium sulfacetamide 10% and sulfur 5% cream with sunscreens versus metronidazole 0.75% cream for rosacea. Cutis 2005;75(6):357‐63. [PUBMED: 16047874] [PubMed] [Google Scholar]
Two 2014 {published data only}
- Two AM, Hata TR, Nakatsuji T, Coda AB, Kotol PF, Wu W, et al. Reduction in serine protease activity correlates with improved rosacea severity in a small, randomized pilot study of a topical serine protease inhibitor. Journal of Investigative Dermatology 2014;134(4):1143‐5. [PUBMED: 24213369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Utaş 1997 {published data only}
- Utaş S, Ünver Ü. Treatment of rosacea with ketoconazole. Journal of the European Academy of Dermatology and Venereology 1997;8(1):69‐70. [EMBASE: 1997013740] [Google Scholar]
Van Landuyt 1997 {published data only}
- Landuyt H, Joubert‐Lequain I, Humbert P, Lucas A, Drobacheff C, Mercier M, et al. Treatment of rosacea. Clonidine (0.075 mg per day) versus placebo (initial results) [Traitement de la rosacée]. Annales de Dermatologie et de Vénéréologie 1997;124(10):729. [PUBMED: 9740876] [PubMed] [Google Scholar]
Veien 1986 {published data only}
- Veien NK, Christiansen JV, Hjorth N, Schmidt H. Topical metronidazole in the treatment of rosacea. Cutis 1986;38:209‐10. [PUBMED: 2945705] [PubMed] [Google Scholar]
Verea Hernando 1992 {published data only}
- Verea Hernando M, Margusino Framiñán L, Seco Vilariño C, Feal Cortizas B, Cuña Estévez B. Comparative study of topical erythromycin and topical metronidazole in the treatment of rosacea [Estudio comparativo entre eritromicina tópica y metronidazol tópico en el tratamiento de la rosácea]. Farmacia Clinica 1992;9(6):472‐9. [EMBASE: 1992280716] [Google Scholar]
Weissenbacher 2007 {published data only}
- Weissenbacher S, Merkl J, Hildebrandt B, Wollenberg A, Braeutigam M, Ring J, et al. Pimecrolimus cream 1% for papulopustular rosacea: a randomized vehicle‐controlled double‐blind trial. British Journal of Dermatology 2007;156(4):728‐32. [PUBMED: 17493072] [DOI] [PubMed] [Google Scholar]
Wilkin 1989 {published data only}
- Wilkin JK. Effect of nadolol on flushing reactions in rosacea. Journal of the American Academy of Dermatology 1989;20(2 Pt 1):202‐5. [PUBMED: 2521641] [DOI] [PubMed] [Google Scholar]
Wilkin 1993 {published data only}
- Witt S, Wilkin JK. Double blind, parallel study of efficacy and safety of clindamycin lotion in the treatment of rosacea. Clinical Pharmacology and Therapeutics 1987;41(2):176. [Google Scholar]
- Wilkin JK, Witt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. International Journal of Dermatology 1993;32(1):65‐7. [UI: 93146772; PUBMED: 8425809] [DOI] [PubMed] [Google Scholar]
Wittpenn 2005 {published and unpublished data}
- Wittpenn JR, Schechter B. Efficacy of cyclosporine a for the treatment of ocular rosacea. Investigative Ophthalmology & Visual Science 2005;46:E‐Abstract 2846. [Google Scholar]
Wolf 2006 {published data only}
- Wolf JE Jr, Kerrouche N, Arsonnaud S. Efficacy and safety of once‐daily metronidazole 1% gel compared with twice‐daily azelaic acid 15% gel in the treatment of rosacea. Cutis 2006;77(4 Suppl):3‐11. [PUBMED: 16706244] [PubMed] [Google Scholar]
Yoo 2011 {published data only}
- Yoo J, Marmur E, Frankel AL, Chaarani J, Turner R, Singer G. Combination therapy for the treatment of erythematotelangiectatic rosacea. Lasers in Surgery & Medicine 2011;43 Suppl 23:918. [Google Scholar]
References to studies excluded from this review
Aitken 1983 {published data only}
- Aitken G. Acne rosacea: Efficacy of a metronidazole cream [Efficacité d' une crème au métronidazole]. Presse Médicale 1983;12(23):1490‐1. [EMBASE: 6222348] [PubMed] [Google Scholar]
Aizawa 1992 {published data only}
- Aizawa H, Niimura M. Oral spironolactone therapy in male patients with rosacea. Journal of Dermatology 1992;19(5):293‐7. [EMBASE: 1992221634] [DOI] [PubMed] [Google Scholar]
Altinyazar 2005 {published data only}
- Altinyazar HC, Koca R, Tekin NS, Eştürk E. Adalapene vs. metronidazole gel for the treatment of rosacea. International Journal of Dermatology 2005;44(3):252‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aronson 1987 {published data only}
- Aronson IK, Rumsfield JA, West DP, Alexander J, Fisher JH, Paloucek FP. Evaluation of topical metronidazole gel in acne rosacea. Drug Intelligence & Clinical Pharmacy 1987;21(4):346‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakar 2006 {published data only}
- Bakar B. Acne rosacea: an open comparative trial of azithromycin versus oxytetracycline therapy: study of 50 patients [P139]. Journal of the American Academy of Dermatology 2006;54(3 Suppl 1):AB24. [Google Scholar]
Bang Soon 2007 {published data only}
- Bang Soon K, Seung Ho Ch, Mi Kyung Ch. Treatment of rosacea with photopnematic therapy [212]. Lasers in Surgery & Medicine 2007;39(19 Suppl):65. [Google Scholar]
Bartholomew 1982 {published data only}
- Bartholomew RS, Reid BJ, Cheesbrough MJ, MacDonald M, Galloway NR. Oxytetracycline in the treatment of ocular rosacea: a double‐blind trial. British Journal of Ophthalmology 1982;66(6):386‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berardesca 2008 {published data only}
- Berardesca E, Cameli N, Cavallotti C, Levy JL, Piérard GE, Paoli Ambrosi G. Combined effects of silymarin and methylsulfonylmethane in the management of rosacea: clinical and instrumental evaluation. Journal of Cosmetic Dermatology 2008;7(1):8‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beridze 2005 {published data only}
- Beridze LR, Mikaia LA, Bakuridze AD. Perolen cream for therapy of rosacea. Georgian Medical News 2005;120(3):55‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Bernstein 1982 {published data only}
- Bernstein JE, Soltani K. Alcohol‐induced rosacea flushing blocked by naloxone. British Journal of Dermatology 1982;107(1):59‐61. [EMBASE: 1982170962] [DOI] [PubMed] [Google Scholar]
Bjerke 1989a {published data only}
- Bjerke JR. Rosacea. Clinical features and treatment [Rosacea. Klinikk og behandling]. Tidsskrift for den Norske Laegeforening 1989;109(23):2295‐7. [PubMed] [Google Scholar]
Bukvic‐Mokos 1998 {published data only}
- Bukvic‐Mokos Z, Basta‐Juzbasic A, Barasic‐Drusko V. Treatment of Helicobacter pylori infection in the management of rosacea. Acta Dermatovenerologica Croatica 1998;6(4):185‐8. [EMBASE: 1999113348] [Google Scholar]
Chu 2005 {published data only}
- Chu CY. The use of 1% pimecrolimus cream for the treatment of steroid‐induced rosacea. British Journal of Dermatology 2005;152(2):396‐9. [PUBMED: 15727676] [DOI] [PubMed] [Google Scholar]
Colón 2007 {published data only}
- Colón LE, Johnson LA, Gottschalk RW. Cumulative irritation potential among metronidazole gel 1%, metronidazole gel 0.75%, and azelaic acid gel 15%. Cutis 2007;79(4):317‐21. [PUBMED: 17500380] [PubMed] [Google Scholar]
Cunliffe 1977 {published data only}
- Cunliffe WJ, Dodman B, Binner JG. Clonidine and facial flushing in rosacea. British Medical Journal 1977;1(6053):105. [EMBASE: 0978017820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Rosso 2004 {published data only}
- Rosso JQ. The use of topical azelaic acid 15% gel or metronidazole 0.75% gel for the treatment of rosacea: Evaluation of a single‐site comparative trial subset. Journal of the American Academy of Dermatology 2004;50(3):174. [Google Scholar]
Dereli 2005 {published data only}
- Dereli T, Inanir I, Kilinç I, Gençoğlan G. Azithromycin in the treatment of papulopustular rosacea. The Journal of Dermatology 2005;32(11):926‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Draelos 2005 {published data only}
- Draelos ZD, Ertel K, Berge C. Niacinamide‐containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis 2005;76(2):135‐41. [PUBMED: 16209160] [PubMed] [Google Scholar]
Erdogan 1998 {published data only}
- Erdogan FG, Yurtsever P, Aksoy D, Eskioglu F. Efficacy of low‐dose isotretinoin in patients with treatment‐resistant rosacea. Archives of Dermatology 1998;134(7):884‐5. [EMBASE: 1998253772] [DOI] [PubMed] [Google Scholar]
Fernandez‐Obregon 2004 {published data only}
- Fernandez‐Obregon A. Oral use of azithromycin for the treatment of acne rosacea. Archives of Dermatology 2004;140(4):489‐90. [EMBASE: 2004163208] [DOI] [PubMed] [Google Scholar]
Fleischer 2005 {published data only}
- Fleischer A, Suephy C. The face and mind evaluation study: An examination of the efficacy of rosacea treatment using physician ratings and patients' self‐reported quality of life. Journal of Drugs in Dermatology 2005;4(5):585‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Freeman 2012 {published data only}
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo‐controlled, double‐blind trial. Journal of Drugs in Dermatology 2012;11(12):1410‐4. [PUBMED: 23377509] [PubMed] [Google Scholar]
Frigerio 1969 {published data only}
- Frigerio G, Mazzoni P, Ferrari V. Dimensions of the sample and power of clinical experimentation in a study of antibiotic therapy [Dimensioni del camione e potenza dell' esperimento clinico in una ricerca di terapia antibiotica]. Bollettino Chimico Farmaceutico 1969;108(8):506‐14. [PubMed] [Google Scholar]
Frucht‐Pery 1993 {published data only}
- Frucht‐Pery J, Sagi E, Hemo I, Ever‐Hadani P. Efficacy of doxycycline and tetracycline in ocular rosacea. American Journal of Ophthalmology 1993;116(1):88‐92. [UI: 93318953; PUBMED: 8328549] [DOI] [PubMed] [Google Scholar]
Garg 2008 {published data only}
- Garg G, Thami GP. Clinical efficacy of tacrolimus in rosacea. Journal of the European Academy of Dermatology and Venereology 2008;23(2):239‐40. [PUBMED: 18498336] [DOI] [PubMed] [Google Scholar]
Gedik 2005 {published data only}
- Gedik GK, Karaduman A, Sivri B, Caner B. Has Helicobacter pylori eradication therapy any effect on severity of rosacea symptoms?. Journal of the European Academy of Dermatology and Venereology 2005;19(3):398‐9. [PUBMED: 15857486] [DOI] [PubMed] [Google Scholar]
Go 1976 {published data only}
- Go MJ, Wuite J. Comparative study of triamcinolone acetonide and hydrocortisone 17‐butyrate in rosacea with special regard to rebound phenomenon. Dermatologica 1976;152(1 Suppl):239‐46. [PUBMED: 133841] [DOI] [PubMed] [Google Scholar]
Goldsmith 1989 {published data only}
- Goldsmith MF. New topical therapy for acne rosacea offers conspicuous improvement, no systemic effects. JAMA 1989;261(14):2014‐5. [PUBMED: 2522563] [PubMed] [Google Scholar]
Hofer 2004 {published data only}
- Hofer T. Continuous 'microdose' isotretinoin in adult recalcitrant rosacea. Clinical & Experimental Dermatology 2004;29(2):204‐5. [PUBMED: 14987287] [DOI] [PubMed] [Google Scholar]
Irvine 1988 {published data only}
- Irvine C, Kumar P, Marks R. Isotretinoin in the treatment of rosacea and rhinophyma. Acne and related disorders ‐ Proceedings of an international symposium. London, 1988:301‐5.
Jackson 2007 {published data only}
- Jackson M. Combined effect of anti‐inflammatory dose doxycycline and azelaic acid gel 15% in the treatment of rosacea. Journal of the American Academy of Dermatology 2007;56(2 Suppl 2):AB19. [DOI] [PubMed] [Google Scholar]
Karabulut 2008 {published data only}
- Karabulut AA, Izol Serel B, Eksioglu HM. A randomized, single‐blind, placebo‐controlled, split‐face study with pimecrolimus cream 1% for papulopustular rosacea. Journal of the European Academy of Dermatology and Venereology 2008;22(6):729‐34. [PUBMED: 18328059] [DOI] [PubMed] [Google Scholar]
Koçak‐Altintas 2005 {published data only}
- Koçak‐Altintas AG, Koçak‐Midillioglu I, Gul U, Bilezickci B, Isiksacan O, Duman S. Effects of topical metranidazole in ocular rosacea. Annals of Ophthalmology 2005;37(2):77‐84. [EMBASE: 2005358977] [Google Scholar]
Laquieze 2007 {published data only}
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. Journal of Dermatological Treatment 2007;18(3):158‐62. [PUBMED: 17538804] [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee DH, Li K, Suh DH. Pimecrolimus 1% cream for the treatment of steroid‐induced rosacea: An 8‐week split‐face clinical trial. British Journal of Dermatology 2008;158(5):1069‐76. [PUBMED: 18363758] [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu RH, Smith MK, Basta SA, Farmer ER. Azelaic acid in the treatment of papulopustular rosacea: A systematic review of randomized controlled trials. Archives of Dermatology 2006;142(8):1047‐52. [PUBMED: 16924055] [DOI] [PubMed] [Google Scholar]
Loo 2004 {published data only}
- Loo WJ, Ayyalaraju A, Chawla M, Finlay AY, Coles EC, Marks R. Ivermectin cream in rosacea: Comparison with metronidazole gel. British Journal of Dermatology 2004;151(68):61. [Google Scholar]
Määttä 2006 {published data only}
- Määttä M, Kari O, Tervahartiala T, Wahlgren J, Peltonen S, Kari M, et al. Elevated expression and activation of matrix metalloproteinase 8 in tear fluid in atopic blepharoconjunctivitis. Cornea 2008;27(3):297‐301. [PUBMED: 18362656 ] [DOI] [PubMed] [Google Scholar]
Maxwell 2010 {published data only}
- Maxwell EL, Ellis DA, Manis H. Acne rosacea: Effectiveness of 532 nm laser on the cosmetic appearance of the skin. Journal of Otolaryngology ‐ Head & Neck Surgery 2010;39(3):292‐6. [PUBMED: 20470675] [PubMed] [Google Scholar]
Meekin 2008 {published data only}
- Meekin TO, Lertzman BH, Hahn HH, Arcara K. Randomized study of intense pulsed light and pulsed dye laser in the treatment of facial telangiectasia. Journal of the American Academy of Dermatology 2008;40 Suppl 20:25. [Google Scholar]
Mraz 2008 {published data only}
- Mraz S, Beutner K. The combination of metronidazole gel and sodium sulfacetamide cleanser is efficacious and well‐tolerated by rosacea patients [P114]. Journal of the American Academy of Dermatology 2008;58(2 Suppl 2):AB14. [Google Scholar]
Nasir 1985 {published data only}
- Nasir MA. Treatment of rosacea with tetracycline and metronidazole ‐ A comparative study. Journal of the Pakistan Medical Association 1985;35(5):148‐9. [PUBMED: 3160868] [PubMed] [Google Scholar]
Nielsen 1983 {published data only}
- Nielsen PG. The relapse rate for rosacea after treatment with either oral tetracycline or metronidazole cream. British Journal of Dermatology 1983;109(1):122. [PUBMED: 6222753] [DOI] [PubMed] [Google Scholar]
Ortiz 2009 {published data only}
- Ortiz A, Elkeeb L, Truitt A, Hindiyeh R, Aquino L, Tran M, Weinstein G. Topical PRK 124 (0.125%) lotion for improving the signs and symptoms of rosacea. Journal of Drugs in Dermatology 2009;8(5):459‐62. [PUBMED: 19537369] [PubMed] [Google Scholar]
Öztürkcan 2004 {published data only}
- Oztürkcan S, Ermertcan AT, Sahin MT, Afşar FS. Efficiency of benzoyl peroxide‐erythromycin gel in comparison with metronidazole gel in the treatment of acne rosacea. Journal of Dermatology 2004;31(8):610‐7. [PUBMED: 15492433] [DOI] [PubMed] [Google Scholar]
Parodi 2008 {published data only}
- Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, et al. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clinical Gastroenterology and Hepatology 2008;6(7):759‐64. [PUBMED: 18456568 ] [DOI] [PubMed] [Google Scholar]
Ruggero 2005 {published data only}
- Ruggero C, Barbareschi M, Veraldi S. Pulse‐therapy with azithromycin in acne rosacea and peri‐oral dermatitis: an open study. Journal of the American Academy of Dermatology 2005;52(3 Suppl 1):P4. [Google Scholar]
Sainthillier 2005 {published data only}
- Sainthillier J‐M, Mac‐Mary S, Creidi P, Msika P, Chadoutaud B, Humbert P. Comparative evaluation by colorimetry and videocapillaroscopy of the efficacy of a cream composed of peptide of lupin and soya isoflavones on erythrocouperose [Évaluation comparative par colorimétrie et vidéocapillaroscopie de l'efficacité d'une crème composée de peptide de lupin et d'isoflavones de soja sur l'érythrocouperose]. Nouvelles Dermatologiques 2005;24(2):99‐104. [EMBASE: 2005110281] [Google Scholar]
Seal 1995 {published data only}
- Seal DV, Wright P, Ficker L, Hagan K, Troski M, Menday P. Placebo controlled trial of fusidic acid gel and oxytetracycline for recurrent blepharitis and rosacea. British Journal of Ophthalmology 1995;79(1):42‐5. [UI: 95186449; PUBMED: 7880791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sehgal 2008 {published data only}
- Sehgal VN, Sharma S, Sardana K. Rosacea/acne rosacea: Efficacy of combination therapy of azithromycin and topical 0.1% tacrolimus ointment. Journal of the European Academy of Dermatology and Venereology 2008;22(11):1366‐8. [PUBMED: 18435734] [DOI] [PubMed] [Google Scholar]
Shanler 2007 {published data only}
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1‐adrenergic receptor agonist, oxymetazoline. Archives of Dermatology 2007;143(11):1369‐71. [PUBMED: 18025359] [DOI] [PubMed] [Google Scholar]
Signore 1995 {published data only}
- Signore RJ. A pilot study of 5 percent permethrin cream versus 0.75 percent metronidazole gel in acne rosacea. Cutis 1995;56(3):177‐9. [UI 96098375; PUBMED: 8565604] [PubMed] [Google Scholar]
Stoudemayer 2006 {published data only}
- Stoudemayer M, Zhen Y, Stoudemayer T, Kligman A. Evaluation of a topical treatment containing 8% sulfur and 2% resorcinol in the treatment of centrofacial rosacea. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB17. [Google Scholar]
Tierney 2009 {published data only}
- Tierney E, Hanke CW. Randomized controlled trial: Comparative efficacy for the treatment of facial telangiectasias with 532 nm versus 940 nm diode laser. Lasers in Surgery & Medicine 2009;41(8):555‐62. [PUBMED: 19746429] [DOI] [PubMed] [Google Scholar]
Togsverd‐Bo 2009 {published data only}
- Togsverd‐Bo K, Wiegell SR, Wulf HC, Haedersdal M. Short and limited effect of long‐pulsed dye laser alone and in combination with photodynamic therapy for inflammatory rosacea. Journal of the European Academy of Dermatology and Venereology 2009;23(2):200‐1. [PUBMED: 18452529] [DOI] [PubMed] [Google Scholar]
Torresani 1997 {published data only}
- Torresani C, Pavesi A, Manara GC. Clarithromycin versus doxycycline in the treatment of rosacea. International Journal of Dermatology 1997;36(12):942‐6. [PUBMED: 9466207] [DOI] [PubMed] [Google Scholar]
Trumbore 2009 {published data only}
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. Journal of Drugs in Dermatology 2009;8(3):299‐304. [PUBMED: 19271381] [PubMed] [Google Scholar]
Uebelhoer 2007 {published data only}
- Uebelhoer NS, Bogle MA, Stewart B, Arndt KA, Dover JS. A split‐face comparison study of pulsed 532‐nm KTP laser and 595‐nm pulsed dye laser in the treatment of facial telangiectasias and diffuse telangiectatic facial erythema. Dermatologic Surgery 2007;33(4):441‐8. [PUBMED: 17430378] [DOI] [PubMed] [Google Scholar]
Veien 1988 {published data only}
- Veien NK. Metronidazole cream for the local treatment of rosacea [Metronidazolcreme til lokalbehandling af rosacea]. Ugeskrift for Laeger 1988;150(6):381‐2. [EMBASE: 2968013] [PubMed] [Google Scholar]
Veraldi 1996 {published data only}
- Veraldi S, Scarabelli G, Rizzitelli G, Caputo R. Treatment of rosacea fulminans with isotretinoin and topical alclometasone dipropionate. European Journal of Dermatology 1996;6(2):94‐6. [EMBASE: 1996085850] [Google Scholar]
Viera 2007 {published data only}
- Viera MH, Perez OA, Berman B. Incyclinide. Dual MMP‐2/MMP‐9 inhibitor treatment of acne treatment of rosacea. Drugs of the Future 2007;32(3):209‐14. [DOI: 10.1358/dof.2007.032.03.1083308; EMBASE: 2007289664] [DOI] [Google Scholar]
Yu 2006 {published data only}
- Yu TG, Zheng YZ, Zhu JT, Guo W. Effect of treatment of rosacea in females by Chibixiao Recipe in combination with minocycline and spironolactone. Chinese Journal of Integrative Medicine 2006;12(4):277‐80. [PUBMED: 17361524] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12614000004662 {unpublished data only}
- ACTRN12614000004662. A single‐blind randomised controlled trial of topical Kanuka honey for the treatment of rosacea. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365179 (accessed 21 July 2014).
IRCT2014030416837N1 {unpublished data only}
- IRCT2014030416837N1. Effects of permethrin 5% topical gel in comparison with placebo on Demodex density in rosacea patients: a double‐blind, randomized clinical trial. www.irct.ir/searchresult.php?keyword=&id=16837&number=1&prt=6008&total=10&m=1 (accessed 23 September 2014).
NCT00560703 {unpublished data only}
- NCT00560703. Efficacy and safety of COL‐101 for the treatment of blepharitis in patients with facial rosacea. clinicaltrials.gov/ct2/show/NCT00560703 (accessed 16 July 2014).
NCT00617903 {unpublished data only}
- NCT00617903. A 12‐week exploratory, multicenter, double‐blind, vehicle‐controlled study to investigate the efficacy and safety of topical azelaic acid 15% foam twice daily in patients with papulopustular rosacea. clinicaltrials.gov/ct2/show/NCT00617903 (accessed 18 July 2014).
NCT00882531 {unpublished data only}
- NCT00882531. Evaluation of the efficacy of isotretinoin versus placebo in terms of response rate among patients presenting papular‐pustular rosacea resistant to standard therapy. clinicaltrials.gov/ct2/show/NCT00882531 (accessed 18 July 2014).
NCT01125930 {unpublished data only}
- NCT01125930. Investigation of the topical retinoid, Atralin gel 0.05% for the treatment of erythematotelangiectatic rosacea. clinicaltrials.gov/ct2/show/NCT01125930 (accessed 19 July 2014).
NCT01451619 {unpublished data only}
- NCT01451619. A randomized, double‐blind, placebo‐controlled, multi‐center, parallel group study to assess the pharmacodynamics of MK‐0524 in subjects with moderate to severe erythematotelangiectatic rosacea. (protocol No. 155). clinicaltrials.gov/show/NCT01451619 (accessed 19 July 2014).
NCT01579084 {unpublished data only}
- NCT01579084. Safety and tolerability of AGN‐199201 in patients with erythema associated with rosacea. clinicaltrials.gov/show/NCT01579084 (accessed 21 July 2014).
NCT01614743 {unpublished data only}
- NCT01614743. A double‐blinded, randomized placebo controlled pilot study comparing the efficacy and safety of Incobotulinumtoxin A versus saline injections to the cheek region in patients with rosacea. clinicaltrials.gov/show/NCT01614743 (accessed 19 July 2014).
NCT01631656 {unpublished data only}
- NCT01631656. Combination Finacea gel and vascular Nd:Yag laser therapy for mild to moderate rosacea. clinicaltrials.gov/show/NCT01631656 (accessed 20 July 2014).
NCT01735201 {unpublished data only}
- NCT01735201. AGN‐199201 for the treatment of erythema with rosacea. clinicaltrials.gov/show/NCT01735201 (accessed 21 July 2014).
NCT01740934 {unpublished data only}
- NCT01740934. An eight‐week, multi‐site, double‐blind, randomized, vehicle‐controlled, parallel‐group trial to evaluate the safety, tolerability, and effects of Anatabloc® crème in subjects with rosacea followed by an open‐label extension. clinicaltrials.gov/show/NCT01740934 (accessed 20 July 2014).
References to ongoing studies
EUCTR2006‐001999‐20‐HU {unpublished data only}
- EUCTR2006‐001999‐20‐HU. Assessment of the efficacy and safety of three concentration:1%, 0.3%, 0.1% of CD5024 cream once daily and CD5024 1% cream twice daily, versus its vehicle and versus metronidazole cream (Rozex®) in patients with papulopustular rosacea over 12 weeks. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2006‐003707‐40‐DE {unpublished data only}
- EUCTR2006‐003707‐40‐DE. Activity of twice daily per os administration of CD06713 at 8mg versus its placebo during 4 weeks treatment, in patients with erythemato‐telangiectatic rosacea. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2006‐007029‐29‐EE {unpublished data only}
- EUCTR2006‐007029‐29‐EE. Non inferiority study of metronidazole 0.75% cream versus reference therapy in the local treatment of papulopustular rosacea. apps.who.int/trialsearch/ (accessed 23‐9‐2014).
EUCTR2008‐003854‐13‐FR {unpublished data only}
- EUCTR2008‐003854‐13‐FR. An investigator blind parallel group vehicle control study comparing the efficacy ad safety of CD 5024 1% cream with metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2009‐013111‐35‐DE {unpublished data only}
- EUCTR2009‐013111‐35‐DE. Effect of CD08514 versus placebo, in patients presenting with type 1 rosacea, over an 8‐week treatment. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2010‐018319‐13‐DE {unpublished data only}
- EUCTR2010‐018319‐13‐DE. A double‐blind, vehicle controlled, parallel group study assessing the activity of CD5024 1% cream in subjects with papulopustular rosacea over 12 weeks treatment. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2010‐021150‐19‐NL {unpublished data only}
- EUCTR2010‐021150‐19‐NL. Doxycycline versus minocycline in the treatment of rosacea: a randomised controlled trial. ‐ DoMino‐study. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2010‐023566‐43‐DE {unpublished data only}
- EUCTR2010‐023566‐43‐DE. No English title [Multizentrische, randomisierte, doppelblinde, kontrollierte Phase III‐Studie zur Behandlung der papulopustulären Rosazea mit Permethrin Creme 5 % (InfectoScab®) versus Permethrin Creme 2,5 % versus Metronidazol Creme 0,75 % (Rozex®) ‐ Papulopustuläre Rosazea‐Behandlung mit Permethrin Creme versus Metronidazol Creme]. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2011‐002057‐65‐DE {unpublished data only}
- EUCTR2011‐002057‐65‐DE. Effect of CD08100/02 3% gel versus placebo in subjects presenting with erythematotelangiectatic rosacea over a 4 week treatment period. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2011‐002058‐30‐DE {unpublished data only}
- EUCTR2011‐002058‐30‐DE. Effect of CD08100/02 3% gel versus placebo gel in subjects presenting with papulopustular rosacea over a 6‐week treatment period. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2011‐004791‐11‐CZ {unpublished data only}
- EUCTR2011‐004791‐11‐CZ. Efficacy and safety of CD5024 1% cream versus metronidazole 0.75% cream in subjects with papulopustular rosacea over 16 weeks treatment, followed by a 36‐week extension period. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2012‐001044‐22‐SE {unpublished data only}
- EUCTR2012‐001044‐22‐SE. A multicenter, randomized, double‐blind, vehicle‐controlled, parallel group study to demonstrate the efficacy and assess the safety of CD07805/47 gel 0.5%applied topically once daily in subjects with moderate to severe facial erythema of rosacea. apps.who.int/trialsearch/ (accessed 23 September 2014).
EUCTR2013‐005083‐26‐DE {unpublished data only}
- EUCTR2013‐005083‐26‐DE. Effect of CD07805/47 gel in subjects presenting with flushing related to erythematotelangiectatic or papulopustular rosacea ‐ Effect of CD07805/47 gel in rosacea flushing. apps.who.int/trialsearch/ (accessed 23 September 2014).
IRCT2014010516079N1 {unpublished data only}
- IRCT2014010516079N1. Comparison of dapsone 5% topical gel with metronidazole 0.75% efficacy in combination with oral doxycycline in papulopustular rosacea. apps.who.int/trialsearch/ (accessed 23 September 2014).
JPRN‐UMIN000008315 {unpublished data only}
- JPRN‐UMIN000008315. Clinical trial for development of topical rapamycin treatment for rosacea. apps.who.int/trialsearch/ (accessed 23 September 2014).
NCT00041977 {published data only}
- NCT00041977. A multicenter, randomized, double‐blind, placebo‐controlled, clinical trial to determine the effects of doxycycline hyclate 20 mg tablets [Periostat(R)] administered twice daily for the treatment of acne rosacea. clinicaltrials.gov/ct2/show/NCT00041977 (accessed 21 July 2014).
NCT00436527 {unpublished data only}
- NCT00436527. MetroGel 1% hydration study: a kinetic regression study. clinicaltrials.gov/show/NCT00436527 (accessed 19 July 2014).
NCT00495313 {unpublished data only}
- NCT00495313. Determine the effects of COL‐101 administered once daily with metronidazole topical gel, 1% versus doxycycline hyclate 100 mg administered once daily with metronidazole topical gel, 1% in patients with moderate to severe rosacea. clinicaltrials.gov/ct2/show/NCT00495313 (accessed 16 July 2014).
NCT00621218 {unpublished data only}
- NCT00621218. A pilot study to compare tretinoin gel, 0.05% to tretinoin gel vehicle when dosed once or twice daily in female subjects with classical rosacea. clinicaltrials.gov/ct2/show/NCT00621218 (accessed 18 July 2014).
NCT00667173 {unpublished data only}
- NCT00667173. A phase 2, multi‐center, evaluator‐blind, randomized, vehicle‐controlled clinical study to assess the safety and efficacy of IDP‐115 in the treatment of rosacea. clinicaltrials.gov/ct2/show/NCT00667173 (accessed 18 July 2014).
NCT00697541 {unpublished data only}
- NCT00697541. A phase II, single‐center, two‐way crossover relative systemic bioavailability study of Col‐118 administered topically as a 0.18 % facial gel and brimonidine ophthalmic solution 0.2% administered to the eye in subjects with moderate to severe erythematous rosacea. clinicaltrials.gov/ct2/show/NCT00697541 (accessed 18 July 2014).
NCT01016782 {unpublished data only}
- NCT01016782. Multi‐center, double‐blind, randomized, vehicle‐controlled, parallel group study of 0444 gel. clinicaltrials.gov/ct2/show/NCT01016782 (accessed 19 July 2014).
NCT01134991 {unpublished data only}
- NCT01134991. Pilot, randomized, double blind, placebo controlled, parallel group, dose range finding study, to evaluate the tolerability and safety of FXFM244 antibiotic foam and to monitor its clinical effect in moderate to severe rosacea patients. clinicaltrials.gov/ct2/show/NCT01134991 (accessed 19 July 2014).
NCT01186068 {unpublished data only}
- NCT01186068. A randomized, double‐blind, vehicle‐controlled, parallel‐group study of the dose‐response profile of V‐101 cream in subjects with erythematous rosacea. clinicaltrials.gov/ct2/show/NCT01186068 (accessed 19 July 2014).
NCT01257919 {unpublished data only}
- NCT01257919. Investigator‐blinded, randomized, cross‐over, multiple dose phase I study on safety and pharmacokinetics of topically applied azelaic acid foam, 15% compared to azelaic acid gel, 15% in subjects with papulopustular rosacea. clinicaltrials.gov/show/NCT01257919 (accessed 19 July 2014).
NCT01308619 {unpublished data only}
- NCT01308619. A multicenter, randomized, double‐blind, placebo‐controlled evaluation of rosacea‐related inflammatory biochemical markers in the skin of adults with papulopustular rosacea treated with daily doxycycline 40 mg (30 mg immediate release / 10 mg delayed release beads) capsules. clinicaltrials.gov/show/NCT01308619 (accessed 19 July 2014).
NCT01513863 {unpublished data only}
- NCT01513863. A randomized, double‐blind, placebo controlled, parallel design, multi‐site clinical study to compare the bioequivalence of two metronidazole 1% topical gel formulations in patients with moderate to severe rosacea. clinicaltrials.gov/show/NCT01513863 (accessed 19 July 2014).
NCT01555463 {unpublished data only}
- NCT01555463. A randomized, double‐blind, vehicle‐controlled, multicenter, parallel‐group clinical trial to assess the safety and efficacy of azelaic acid foam, 15% topically applied twice daily for 12 weeks in subjects with papulopustular rosacea. clinicaltrials.gov/show/NCT01555463 (accessed 20 Juy 2014).
NCT01659853 {unpublished data only}
- NCT01659853. A multicenter, randomized, controlled, double‐masked, crossover design study to compare efficacy and assess safety of CD07805/47 gel 0.5% applied once daily vs azelaic acid gel 15% applied twice daily in subjects with erythema of rosacea. clinicaltrials.gov/show/NCT01659853 (accessed 20 July 2014).
NCT01784133 {unpublished data only}
- NCT01784133. A phase 2, randomized, vehicle‐controlled, double‐blind, multicenter study to evaluate the safety and efficacy of three once‐daily CLS001 topical gels versus vehicle administered for 12 weeks to subjects with papulopustular rosacea. clinicaltrials.gov/show/NCT01784133 (accessed 20 July 2014).
NCT01828177 {unpublished data only}
- NCT01828177. A multicenter randomized evaluator‐blinded vehicle‐controlled parallel group evaluation of twice daily PDI‐320 in comparison to its monads in adults with rosacea. clinicaltrials.gov/show/NCT01828177 (accessed 20 July 2014).
NCT01917539 {unpublished data only}
- NCT01917539. Efficacy of Pulsed Light Therapy for Meibomian gland dysfunction and dry eye syndrome. clinicaltrials.gov/show/NCT01917539 (accessed 21 July 2014).
NCT01933464 {unpublished data only}
- NCT01933464. An analysis of the effect of topical cromolyn sodium on rosacea‐associated erythema. clinicaltrials.gov/show/NCT01933464 (accessed 20 July 2014).
NCT01993446 {unpublished data only}
- NCT01993446. A randomized, double‐blind, vehicle controlled study of the safety and efficacy of topical DRM02 in subjects with rosacea. clinicaltrials.gov/show/NCT01993446 (accessed 20 July 2014).
NCT02036229 {unpublished data only}
- NCT02036229. A randomised, double blind, placebo controlled, half‐face study to evaluate the effect of topical ivermectin cream 0.5% on demodicidosis. clinicaltrials.gov/ct2/show/NCT02036229 (accessed 20 July 2014).
NCT02052999 {unpublished data only}
- NCT02052999. An open label pilot study to evaluate the efficacy of PAC‐14028 in the treatment of erythematotelangiectatic rosacea and papulopustular rosacea. clinicaltrials.gov/show/NCT02052999 (accessed 20 July 2014).
NCT02075671 {unpublished data only}
- NCT02075671. Photodynamic therapy for papulopustular rosacea. clinicaltrials.gov/show/NCT02075671 (accessed 20 July 2014).
NCT02120924 {unpublished data only}
- NCT02120924. A multicenter, double‐blind, randomized, parallel‐group, vehicle‐controlled study to evaluate the safety and clinical equivalence of a generic azelaic acid gel, 15% and the reference listed Finacea® (azelaic acid) gel, 15% in patients with moderate facial rosacea. clinicaltrials.gov/show/NCT02120924 (accessed 20 July 2014).
NCT02132117 {unpublished data only}
- NCT02132117. Safety and efficacy of AGN‐199201 in patients with persistent erythema associated with rosacea. clinicaltrials.gov/show/NCT02132117 (website accessed 20 July 2014).
NCT02144181 {unpublished data only}
- NCT02144181. Evaluation of the safety and efficacy of the Ulthera® System for the treatment of signs and symptoms of erythematotelangiectatic rosacea. clinicaltrials.gov/show/NCT02144181 (accessed 20 July 2014).
NCT02147691 {unpublished data only}
- NCT02147691. Finacea 15% and brimonidine 0.33% gel in the treatment of rosacea ‐ a pilot study. clinicaltrials.gov/show/NCT02147691 (accessed 20 July 2014).
NCT02204254 {unpublished data only}
- NCT02204254. Prospective, open label, randomized study comparing bipolar radiofrequency potentiated by infrared light to doxycycline in patient with papulopustular rosacea. clinicaltrials.gov/show/NCT02204254 (accessed 23 September 2014).
Additional references
Abram 2009
- Abram K, Silm H, Maaroos HI, Oona M. Subjective disease perception and symptoms of depression in relation to healthcare‐seeking behaviour in patients with rosacea. Acta Dermato‐Venereologica 2009;89(5):488‐91. [PUBMED: 19734974] [DOI] [PubMed] [Google Scholar]
Aksoy 2010
- Aksoy B, Altaykan‐Hapa A, Egemen D, Karagöz F, Atakan N. The impact of rosacea on quality of life: effects of demographic and clinical characteristics and various treatment modalities. British Journal of Dermatology 2010;163(4):719‐25. [PUBMED: 20545683] [DOI] [PubMed] [Google Scholar]
Alikhan 2010
- Alikhan A, Kurek L, Feldman SR. The role of tetracyclines in rosacea. American Journal of Clinical Dermatology 2010;11(2):79‐87. [PUBMED: 20141228] [DOI] [PubMed] [Google Scholar]
Bakar 2004
- Bakar O, Demirçay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. International Journal of Dermatology 2004;43(2):151‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakar 2009
- Bakar O, Demircay Z, Toker E, Cakir S. Ocular signs, symptoms and tear function tests of papulopustular rosacea patients receiving azithromycin. Journal of the European Academy of Dermatology and Venereology 2009;23(5):544‐9. [PUBMED: 19250326] [DOI] [PubMed] [Google Scholar]
Baldwin 2006
- Baldwin HE. Oral therapy for rosacea. Journal of Drugs in Dermatology 2006;5(1):16‐21. [PUBMED: 16468287] [PubMed] [Google Scholar]
Baldwin 2010
- Baldwin HE. A community‐based study of the effectiveness of doxycycline 40 mg (30‐mg immediate‐release and 10‐mg delayed‐release beads) on quality of life and satisfaction with treatment in participants with rosacea. Cutis 2010;86(5 Suppl):26‐36. [PUBMED: 21229828] [PubMed] [Google Scholar]
Bamford 2004
- Bamford JTM, Gessert CE, Renier CM. Measurement of the severity of rosacea. Journal of the American Academy of Dermatology 2004;51(5):697‐703. [EMBASE: 2004494171] [DOI] [PubMed] [Google Scholar]
Bamford 2006
- Bamford JT, Gessert CE, Renier CM, Jackson MM, Laabs SB, Dahl MV, et al. Childhood stye and adult rosacea. Journal of the American Academy of Dermatology 2006;55(6):951‐5. [PUBMED: 17097390] [DOI] [PubMed] [Google Scholar]
Basra 2008
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994‐2007: a comprehensive review of validation data and clinical results. British Journal of Dermatology 2008;159(5):997‐1035. [PUBMED: 18795920] [DOI] [PubMed] [Google Scholar]
Berg 1989
- Berg M, Lidén S. An epidemiological study of rosacea. Acta Dermato‐Venereologica 1989;69(5):419‐23. [PUBMED: 2572109] [PubMed] [Google Scholar]
Bernstein 2008
- Bernstein EF, Kligman A. Rosacea treatment using the new‐generation, high‐energy, 595 nm, long pulse‐duration pulsed‐dye laser. Lasers in Surgery & Medicine 2008;40(4):233‐9. [PUBMED: 18412227] [DOI] [PubMed] [Google Scholar]
Bero 2013
- Bero L. Why the Cochrane risk of bias tool should include funding source as a standard item 2013. www.thecochranelibrary.com/details/editorial/5655431/Why‐the‐Cochrane‐risk‐of‐bias‐tool‐should‐include‐funding‐source‐as‐a‐standard‐item.html (accessed 14 January 2015). [DOI] [PMC free article] [PubMed]
Bhatia 2012
- Bhatia ND, Rosso JQ. Optimal management of papulopustular rosacea: rationale for combination therapy. Journal of Drugs in Dermatology 2012;11(7):838‐44. [PUBMED: 22777226] [PubMed] [Google Scholar]
Bikowski 2003
- Bikowski JB. Subantimicrobial dose doxycycline for acne and rosacea. Skinmed 2003;2(4):234‐45. [PUBMED: 14673277] [DOI] [PubMed] [Google Scholar]
Bikowski 2004
- Bikowski JB, Goldman MP. Rosacea: Where are we now?. Journal of Drugs in Dermatology 2004;3(3):251‐61. [PUBMED: 15176158] [PubMed] [Google Scholar]
Bikowski 2007
- Bikowski J. Demystifying anti‐inflammatory dose antibiotics for management of rosacea. Practical Dermatology 2007;April:22‐4. [Google Scholar]
Butterwick 2006
- Butterwick KJ, Butterwick LS, Han A. Laser and light therapies for acne rosacea. Journal of Drugs in Dermatology 2006;5(1):35‐9. [PUBMED: 16468290] [PubMed] [Google Scholar]
Chren 1996
- Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality‐of‐life measure for patients with skin disease: reliability, validity, and responsiveness. Journal of Investigative Dermatology 1996;107(5):707‐13. [PUBMED: 8875954] [DOI] [PubMed] [Google Scholar]
Chu 2007
- Chu CY. An open‐label pilot study to evaluate the safety and efficacy of topically applied pimecrolimus cream for the treatment of steroid‐induced rosacea‐like eruption. Journal of the European Academy of Dermatology and Venereology 2007;21(4):484‐90. [PUBMED: 17373975] [DOI] [PubMed] [Google Scholar]
Crawford 2005
- Crawford KM, Russ B, Bostrom P. Pimecrolimus for treatment of acne rosacea. Skinmed 2005;4(3):147‐50. [PUBMED: 15891250] [DOI] [PubMed] [Google Scholar]
Cresce 2014
- Cresce ND, Davis SA, Huang WW, Feldman SR. The quality of life impact of acne and rosacea compared to other major medical conditions. Journal of Drugs in Dermatology 2014;13(6):692‐7. [PUBMED: 24918559] [PubMed] [Google Scholar]
Culp 2009
- Culp B, Scheinfeld N. Rosacea: A Review. P & T 2009;34(1):38‐45. [PUBMED: 19562004] [PMC free article] [PubMed] [Google Scholar]
Del Rosso 2012
- Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. Journal of Clinical and Aesthetic Dermatology 2012;5(3):16‐25. [PUBMED: 22468176] [PMC free article] [PubMed] [Google Scholar]
Del Rosso 2013a
- Rosso JQ, Gallo RL, Tanghetti E, Webster G, Thiboutot D. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis 2013;91(3 Suppl):1‐8. [PUBMED: 23833998] [PubMed] [Google Scholar]
Del Rosso 2013b
- Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield L, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis 2013;92(6):277‐84. [PUBMED: 24416742] [PubMed] [Google Scholar]
Del Rosso 2013c
- Rosso JQ. Management of facial erythema of rosacea: what is the role of topical α‐adrenergic receptor agonist therapy?. Journal of the American Academy of Dermatology 2013;69(6 Suppl 1):S44‐56. [PUBMED: 24229637] [DOI] [PubMed] [Google Scholar]
Del Rosso 2014a
- Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield L, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, Part 3: A status report on systemic therapies. Cutis 2014;93(1):18‐28. [PUBMED: 24505581] [PubMed] [Google Scholar]
Del Rosso 2014b
- Rosso JQ, Thiboutot D, Gallo R, Webster G, Tanghetti E, Eichenfield LF, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis 2014;93(3):134‐8. [PUBMED: 24738094] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Gohary 2014
- El‐Gohary M, Zuuren EJ, Fedorowicz Z, Burgess H, Doney L, Stuart B, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elewski 2009
- Elewski BE. Results of a national rosacea patient survey: Common issues that concern rosacea sufferers. Journal of Drugs in Dermatology 2009;8(2):120‐3. [PUBMED: 19213226] [PubMed] [Google Scholar]
Elewski 2011
- Elewski BE, Draelos Z, Dréno B, Jansen T, Layton A, Picardo M. Rosacea ‐ global diversity and optimized outcome: Proposed international consensus from the Rosacea International Expert Group. Journal of the European Academy of Dermatology and Venereology 2011;25(2):188‐200. [PUBMED: 20586834] [DOI] [PubMed] [Google Scholar]
Feldman 2014
- Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. Journal of Managed Care Pharmacy 2014;20(6):623‐9. [PUBMED: 24856600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forton 2007
- Forton F. Standardized skin surface biopsy: method to estimate the Demodex folliculorum density, not to study the Demodex folliculorum prevalence. Journal of the European Academy of Dermatology and Venereology 2007;21(9):1301‐2. [PUBMED: 17894752] [DOI] [PubMed] [Google Scholar]
Forton 2012
- Forton FM. Papulopustular rosacea, skin immunity and Demodex: pityriasis folliculorum as a missing link. Journal of the European Academy of Dermatology and Venereology 2012;26(1):19‐28. [PUBMED: 22017468] [DOI] [PubMed] [Google Scholar]
Gessert 2003
- Gessert CE, Bamford JT. Measuring the severity of rosacea: A review. International Journal of Dermatology 2003;42(6):444‐8. [PUBMED: 12786870] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jansen 1997
- Jansen T, Plewig G. Rosacea: Classification and treatment. Journal of the Royal Society of Medicine 1997;90(3):144‐50. [PUBMED: 9135612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawana 2007
- Kawana S, Ochiai H, Tachihara R. Objective evaluation of the effect of intense pulsed light on rosacea and solar lentigines by spectrophotometric analysis of skin color. Dermatologic Surgery 2007;33(4):449‐54. [PUBMED: 17430379] [DOI] [PubMed] [Google Scholar]
Kini 2010
- Kini SP, Nicholson K, DeLong LK, Dannemann T, Estaris J, Foster J, et al. A pilot study in discrepancies in quality of life among three cutaneous types of rosacea. Journal of the American Academy of Dermatology 2010;62(6):1069‐71. [PUBMED: 20466185] [DOI] [PubMed] [Google Scholar]
Korting 2009
- Korting HC, Schöllmann C. Current topical and systemic approaches to treatment of rosacea. Journal of the European Academy of Dermatology and Venereology 2009;23(8):876‐82. [PUBMED: 19508315] [DOI] [PubMed] [Google Scholar]
Lacey 2007
- Lacey N, Delaney S, Kavanagh K, Powell FC. Mite‐related bacterial antigens stimulate inflammatory cells in rosacea. British Journal of Dermatology 2007;157(3):474‐81. [PUBMED: 17596156] [DOI] [PubMed] [Google Scholar]
Landow 2005
- Landow K. Rosacea: The battle goes on. Comprehensive Therapy 2005;31(2):145‐58. [PUBMED: 15901945] [DOI] [PubMed] [Google Scholar]
Layton 2013
- Layton A, Thiboutot D. Emerging therapies in rosacea. Journal of the American Academy of Dermatology 2013;69(6 Suppl 1):S57‐65. [PUBMED: 24229638] [DOI] [PubMed] [Google Scholar]
Lazaridou 2011
- Lazaridou E, Fotiadou C, Ziakas NG, Giannopoulou C, Apalla Z, Ioannides D. Clinical and laboratory study of ocular rosacea in northern Greece. Journal of the European Academy of Dermatology and Venereology 2011;25(12):1428‐31. [PUBMED: 21366706] [DOI] [PubMed] [Google Scholar]
Leyden 2004
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis 2004;73(6 Suppl):11‐7. [PUBMED: 15228129] [PubMed] [Google Scholar]
Marks 2007
- Marks R. The enigma of rosacea. Journal of Dermatological Treatment 2007;18(6):326‐8. [PUBMED: 18058493] [DOI] [PubMed] [Google Scholar]
Menezes 2009
- Menezes N, Moreira A, Mota G, Baptista A. Quality of life and rosacea: Pulsed dye laser impact. Journal of Cosmetic & Laser Therapy 2009;11(3):139‐41. [DOI: 10.1080/14764170902741311; PUBMED: 19462330] [DOI] [PubMed] [Google Scholar]
Moustafa 2014
Moustafa 2014b
- Moustafa FA, Sandoval LF, Feldman SR. Rosacea: new and emerging treatments. Drugs 2014;74(13):1457‐65. [PUBMED: 25154627] [DOI] [PubMed] [Google Scholar]
Naranayan 2007
- Narayanan S, Hünerbein A, Getie M, Jäckel A, Neubert RH. Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system. Journal of Pharmacy and Pharmacology 2007;59(8):1125‐30. [PUBMED: 17725855] [DOI] [PubMed] [Google Scholar]
Nicholson 2007
- Nicholson K, Abramova L, Chren MM, Yeung J, Chon SY, Chen SC. A pilot quality‐of‐life instrument for acne rosacea. Journal of the American Academy of Dermatology 2007;57(2):213‐21. [PUBMED: 17445948] [DOI] [PubMed] [Google Scholar]
Nickle 2014
- Nickle SB, Peterson N, Peterson M. Updated Physician's Guide to the Off‐label Uses of Oral Isotretinoin. Journal of Clinical and Aesthetic Dermatology 2014;7(4):22‐34. [PUBMED: 24765227] [PMC free article] [PubMed] [Google Scholar]
Oltz 2011
- Oltz M, Check J. Rosacea and its ocular manifestations. Optometry 2011;82(2):92‐103. [PUBMED: 21276570] [DOI] [PubMed] [Google Scholar]
Powell 2005
- Powell FC. Clinical practice. Rosacea. New England Journal of Medicine 2005;352(8):793‐803. [PUBMED: 15728812] [DOI] [PubMed] [Google Scholar]
Reinholz 2013
- Reinholz M, Tietze JK, Kilian K, Schaller M, Schöfer H, Lehmann P, et al. Rosacea ‐ S1 guideline. Journal der Deutschen Dermatologischen Gesellschaft 2013;11(8):768‐90. [PUBMED: 23647643] [DOI] [PubMed] [Google Scholar]
Revman 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sapadin 2006
- Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. Journal of the American Academy of Dermatology 2006;54(2):258‐65. [PUBMED: 16443056] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. www.cc‐ims.net/gradepro. Version 3.2 [updated March 2009] 2009.
Shim 2013
- Shim TN, Abdullah A. The effect of pulsed dye laser on the dermatology life quality index in erythematotelangiectatic rosacea patients: an assessment. Journal of Clinical and Aesthetic Dermatology 2013;6(4):30‐2. [PUBMED: 23630639] [PMC free article] [PubMed] [Google Scholar]
Sloan 2008
- Sloan B, Scheinfeld N. The use and safety of doxycycline hyclate and other second‐generation tetracyclines. Expert Opinion on Drug Safety 2008;7(5):571‐7. [PUBMED: 18759709] [DOI] [PubMed] [Google Scholar]
Steinhoff 2011
- Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. Journal of Investigative Dermatology 2011;15(1):2‐11. [PUBMED: 22076321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinhoff 2013
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. Journal of the American Academy of Dermatology 2013;69(6 Suppl 1):S15‐26. [PUBMED: 24229632] [DOI] [PubMed] [Google Scholar]
Stone 2004
- Stone DU, Chodosh J. Oral tetracyclines for ocular rosacea: An evidence‐based review of the literature. Cornea 2004;23(1):106‐9. [PUBMED: 14701969] [DOI] [PubMed] [Google Scholar]
Taghizadeh 2008
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. Journal of Plastic, Reconstructive & Aesthetic Surgery 2008;61(3):330‐3. [PUBMED: 18267312] [DOI] [PubMed] [Google Scholar]
Tan 2013
- Tan J, Blume‐Peytavi U, Ortonne JP, Wilhelm K, Marticou L, Baltas E, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. British Journal of Dermatology 2013;169(3):555‐62. [PUBMED: 23600367] [DOI] [PubMed] [Google Scholar]
Tan 2013b
- Tan J, Berg M. Rosacea: current state of epidemiology. Journal of the American Academy of Dermatology 2013;69(6 Suppl 1):S27‐35. [PUBMED: 24229634] [DOI] [PubMed] [Google Scholar]
Tan 2014
Tanghetti 2014
- Tanghetti E, Rosso JQ, Thiboutot D, Gallo R, Webster G, Eichenfield LF, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 4: a status report on physical modalities and devices. Cutis 2014;93(2):71‐6. [PUBMED: 24605343] [PubMed] [Google Scholar]
Thiboutot 2000
- Thiboutot DM. Acne and rosacea. New and emerging therapies. Dermatologic Clinics 2000;18(1):63‐71. [PUBMED: 10626112] [DOI] [PubMed] [Google Scholar]
Tisma 2009
- Tisma VS, Basta‐Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. Journal of the American Academy of Dermatology 2009;60(2):270‐6. [PUBMED: 19028405] [DOI] [PubMed] [Google Scholar]
Treadwell 2006
- Treadwell, JT, Tregear SJ, Reston JT, Turkelson CM. A system for rating the stability and strength of medical evidence. BMC Medical Research Methodology 2006;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uslu 2012
- Uslu M, Şavk E, Karaman G, Şendur N. Rosacea treatment with intermediate‐dose isotretinoin: follow‐up with erythema and sebum measurements. Acta Dermato‐Venereologica 2012;92(1):73‐7. [PUBMED: 21952746] [DOI] [PubMed] [Google Scholar]
van der Linden 2014
van Zuuren 2012
- Zuuren EJ, Fedorowicz Z, Carter B, Andriolo RB, Schoones J. Interventions for female pattern hair loss. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD007628.pub3] [DOI] [PubMed] [Google Scholar]
van Zuuren 2013
- Zuuren EJ, Fedorowicz Z. Lack of 'appropriately assessed' patient‐reported outcomes in randomized controlled trials assessing the effectiveness of interventions for rosacea. British Journal of Dermatology 2013;168(2):442‐4. [PUBMED: 22803770] [DOI] [PubMed] [Google Scholar]
Vieira 2013
- Vieira AC, Mannis MJ. Ocular rosacea: common and commonly missed. Journal of the American Academy of Dermatology 2013;69(6 Suppl 1):S36‐41. [PUBMED: 24229635] [DOI] [PubMed] [Google Scholar]
Walker 2006
- Walker C, Webster G. A multicenter, double‐blind, randomized trial to evaluate long‐term anti‐inflammatory dose doxycycline (40 mg) therapy: Results of the lack of effect on bacterial flora. Presented at: Fall Clinical Dermatology Conference. Las Vegas, NV, USA, 7‐11 October 2006. 2006.
Wilkin 1994
- Wilkin JK. Rosacea. Pathophysiology and treatment. Archives of Dermatology 1994;130(3):359‐62. [EMBASE: 1994109375] [DOI] [PubMed] [Google Scholar]
Wilkin 2002
- Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Journal of the American Academy of Dermatology 2002;46(4):584‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilkin 2004
- Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, et al. Standard classification of rosacea: Report of the national rosacea society expert committee on the classification and staging of rosacea. Journal of the American Academy of Dermatology 2004;50(6):907‐12. [PUBMED: 15153893] [DOI] [PubMed] [Google Scholar]
Yamasaki 2007
- Yamasaki K, Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nature Medicine 2007;13(8):975‐80. [PUBMED: 17676051] [DOI] [PubMed] [Google Scholar]
Yamasaki 2011
- Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. Journal of Investigative Dermatology. Symposium Proceedings / the Society for Investigative Dermatology, Inc. European Society for Dermatological Research 2011;15(1):12‐5. [PUBMED: 22076322] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Zuuren 2000
- Zuuren EJ, Powell FC, Graber M. Interventions for rosacea. Cochrane Database of Systematic Reviews 2000, Issue 12. [DOI: 10.1002/14651858.CD003262] [DOI] [Google Scholar]
van Zuuren 2004
- Zuuren EJ, Graber MA, Hollis S, Chaudhry M, Gupta AK. Interventions for rosacea. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003262.pub2; PUBMED: 14974010] [DOI] [PubMed] [Google Scholar]
van Zuuren 2005
- Zuuren EJ, Graber MA, Hollis S, Chaudhry M, Gupta AK, Gover M. Interventions for rosacea. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003262.pub3; PUBMED: 16034895] [DOI] [PubMed] [Google Scholar]
van Zuuren 2007
- Zuuren EJ, Gupta AK, Gover MD, Graber M, Hollis S. Systematic review of rosacea treatments. Journal of the American Academy of Dermatology 2007;56(1):107‐15. [PUBMED: 17190628] [DOI] [PubMed] [Google Scholar]
van Zuuren 2011
- Zuuren EJ, Kramer S, Carter B, Graber MA, Fedorowicz Z. Interventions for rosacea. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD003262.pub4] [DOI] [PubMed] [Google Scholar]


