Abstract
Background
Pain is one of the most common symptoms in children and young people (CYP) with life‐limiting conditions (LLCs) which include a wide range of diagnoses including cancer. The current literature indicates that pain is not well managed, however the evidence base to guide clinicians is limited. There is a clear need for evidence from a systematic review to inform prescribing.
Objectives
To evaluate the evidence on the effectiveness of different pharmacological interventions used for pain in CYP with LLCs.
Search methods
The following electronic databases were searched up to December 2014: CENTRAL (in the Cochrane Library), MEDLINE, EMBASE, PsycINFO and CINAHL. In addition, we searched conference proceedings and reference lists of included studies. For completeness, we also contacted experts in the field. No language restrictions were applied.
Selection criteria
Randomised controlled trials (RCTs), quasi‐randomised studies and other studies that included a clearly defined comparator group were included. The studies investigated pharmacological treatments for pain associated with LLCs in CYP. The treatment included those specifically developed to treat pain and those that acted as an adjuvant, where the treatment was not primarily developed to treat pain but has pain relieving properties. The LLC was identified by its inclusion in the Richard Hain Directory of LLCs.
Data collection and analysis
Citations were screened by five review authors. Data were extracted by one review author and checked by a second. Two review authors assessed the risk of bias of included studies. A sufficient number of studies using homogeneous outcomes was not identified so a meta‐analysis was not possible.
Main results
We identified 24,704 citations from our database search. Nine trials with 379 participants fulfilled our inclusion criteria. Participants had cerebral palsy (CP) in five of the studies and osteogenesis imperfecta (OI) in the other four. Participants across the trials ranged in age from 2 to 19 years. All studies, apart from one cross‐over trial, were parallel designed RCTs. Three of the trials on CP evaluated intrathecal baclofen (ITB) and two botulinum toxin A (BoNT‐A). All of the OI trials evaluated the use of bisphosphonates (two alendronate and one pamidronate). No trials were identified that evaluated a commonly used analgesic in this patient group. Pain was a secondary outcome in five of the eight identified studies. Overall the quality of the trials was mixed. Only one study involved over 100 participants.
For the two ITB studies for pain in CP, in the same study population but assessed at different time points in their disease, both found an effect on pain favouring the intervention compared to the control group (standard care or placebo) (mean difference (MD) 4.20, 95% confidence interval (CI) 2.15 to 6.25; MD 26.60, 95% CI 2.61 to 50.59, respectively). In these studies most of the adverse events related to the procedure or device for administration rather than the drug, such as swelling at the pump site. In one trial there were also eight serious adverse effects; these included difficulty swallowing and an epileptic seizure. The trial did not state if these occurred in the intervention group. At follow‐up in both BoNT‐A trials there was no evidence of a difference in pain between the trial arms among CP participants. The adverse events in the BoNT‐A trials mostly involved those who received the intervention drug and involved seizures. Gastrointestinal problems were the most frequent adverse event in those who received alendronate. The trial investigating pamidronate found no evidence of a difference in pain compared to the control group. No adverse events were reported in this trial.
Authors' conclusions
Published, controlled evidence on the pharmacological interventions for pain in CYP with LLCs is limited. The evidence that is currently available evaluated pain largely as a secondary outcome and the drugs used were all adjuvants and not always commonly used in general paediatric palliative care for pain. Based on current data this systematic review is unable to determine the effects of pharmacological interventions for pain for CYP with LLCs. Future trials with larger populations should examine the effects of the drugs commonly used as analgesics; with the rising prevalence of many LLCs this becomes more necessary.
Plain language summary
Drug treatments for pain in children and young people with life‐limiting conditions
Pain is commonly experienced in children and young people with diseases that are not curable and which may shorten their lives. These may be cancers or other diseases. Sometimes the pain is under‐treated, particularly for those nearing the end of their lives. There are many different types of drugs that have been developed to treat pain. There are also drugs that were not developed primarily to treat pain but which have an action that may provide pain relief. However, clinical guidelines to support doctors in their choice of treatment for pain are limited. This is because there are few trials specifically in children and young people that have tested the benefits and safety of such drugs.
In this review we sought to find out precisely what the evidence is on drug treatments for pain in children and young people with diseases that are not curable and that may shorten their lives.
We searched five large databases of published research projects. We found nine relevant randomised controlled trials. Five were for children and young people with cerebral palsy and four for those with a degenerative bone disease called osteogenesis imperfecta.
Overall, these trials did not find clear evidence of a benefit of the drugs tested in the treatment of pain. This was apart from the two on cerebral palsy where pain relief occurred with the use of baclofen delivered via a catheter into the spinal cord. However the procedure to deliver this medication resulted in most side effect reported in these trials; this was swelling at the site of the catheter, and in one study it reported that this occurred in around half of the children (8/17). Five children also leaked spinal fluid from the catheter resulting in headache and nausea and, for two children, a prolonged hospital stay.
The trials were limited by the quality of their methods and most did not set out to measure the benefit of the drug in reducing pain as a main focus. In conclusion, the evidence on pain treatment in children and young people with life‐limiting health conditions is very limited, and only evaluated in participants with certain diseases and not for drug treatments primarily used to treat pain. The trials that were identified evaluated the drugs in small samples of children. There remains a need for more research to help guide doctors in their decisions on how to treat pain in these children and young people.
Background
Description of the condition
Pain is one of the most common symptoms in children and young people (CYP) with life‐limiting conditions (LLCs) (Beretta 2010;Feudtner 2011; Wolfe 2000). In this review, LLC refers to ‘any condition for which there is no reasonable hope of cure and from which the child or young adult will die prematurely’. LLCs are also defined as ‘those for which curative treatment may be feasible but can fail’ (ACT 2009). LLCs are seen to be rising in the UK (Fraser 2012), with 32 per 10,000 CYP having an LLC. Sources of pain in this population include ongoing tissue damage due to pathological processes, recurrent injury, therapy and invasive diagnostic or therapeutic procedures.
Increasing evidence suggests that pain is not well managed in such CYP, especially towards the end of life. In one large cross‐sectional study of CYP with cancer deemed ‘palliative’, Goldman 2006 found that 91.5% of the 164 CYP in the study experienced pain in the month before death. In another study, 87% of 47 CYP with cancer experienced pain during the ‘end stage’ (Beretta 2010); and in an earlier study 53% of 30 ‘dying’ CYP experienced pain in the last week of their lives (Drake 2003).
Types of pain
Pain can be characterised in several ways, of which none is deemed a gold standard. Pain can, for example, be characterised by mechanism or pathophysiology, intensity, temporality or by location.
1. Pain mechanisms
Two basic pain mechanisms are known, nociceptive and neuropathic. Nociceptive pain occurs as the result of tissue damage or inflammation due to physical, chemical or thermal injury (for example traumatic or ischaemic pain, arthritis, muscle spasm, mucositis, gastritis, or other visceral inflammatory processes). Neuropathic pain occurs when a lesion of the central or peripheral nervous system causes nociceptive dysfunction (IASP 2012) (for example from direct tumour invasion or neural toxicity from chemotherapy or infection). Nociceptive and neuropathic pain can occur separately or together, in the same individual. The importance of distinguishing between these two mechanisms is that analgesics are developed for action on specific mechanisms, and so an outcome can vary depending on the type of pain.
2. Intensity
Pain intensity is usually measured on a scale of 0 to 10, or 0 to 100, using a linear visual analogue scale (VAS). It may also be measured by another pain intensity measurement tool such as the Wong‐Baker Faces Scale (Wong 1988). Intensity can be described using a four‐point categorical pain intensity scale with corresponding wording, none, mild, moderate, severe. Intensity may also be characterised in the World Health Organization (WHO) two‐step pain management algorithm, mild (VAS 4 to 6), moderate (VAS 7 to 8) or severe (VAS 9 to 10). This algorithm recommends pharmacological interventions of increasing potency to be used for mild and moderate to severe pain (WHO 2012). The 2012 version of the WHO document differs from the original three‐step ladder, which included a middle step of using a ‘weaker’ opioid for moderate pain before a ‘stronger’ opioid in the third step (WHO 1996). Pain intensity measurement is potentially more complex in CYP who are too young, preverbal or non‐verbal, and are unable to describe or quantify their pain. The literature on this subject is enormous, and a large number of pain measurement tools suitable for CYP of different ages in a variety of settings have been devised (Stinson 2006; Von Baeyer 2009). However, no definitive tools adequately measure persistent pain in CYP with palliative care needs. It is important to recognise that in clinical studies, the accuracy and reliability of such tools depend on their validity for use in the situation described (see also Secondary outcomes).
3. Temporality
Pain can be described by its temporality although, as is emphasised in the WHO guidelines, temporality does not define treatment strategies. Such pain can be described as:
acute pain (< 30 days),
chronic pain (> 3 months, with behaviours in response to pain that does not remit (Hain 2011),
persistent pain (covers long‐term pain related to medical illness),
episodic or recurrent pain (occurs intermittently over a long period of time and the child can be pain free in between each painful episode),
breakthrough pain (temporary increase in the severity of pain over and above the pre‐existing baseline pain level, and can be predictable or unpredictable with or without an identifiable cause),
incident pain (from an identifiable cause),
end of dose pain (occurs before a scheduled dose of an around‐the‐clock analgesic) (WHO 2012).
4. Location
Pain is sometimes characterised by its location in the body, such as bone pain, headache, abdominal pain or musculoskeletal pain.
Description of the intervention
For this review, we have focused on pharmacological interventions for the relief of pain causally related to the LLC (disease‐related pain). Pain due to diagnostic and therapeutic procedures and postoperative pain have been excluded from this review.
We have assessed the evidence on the effectiveness of pharmacological interventions using a framework adapted from the WHO guidelines for pain management in CYP with medical conditions (WHO 2012). These include the following.
1. Non‐opioid analgesics such as paracetamol and non‐steroidal anti‐inflammatory drugs (NSAIDs).
2. Opioids such as morphine, methadone, hydromorphone, buprenorphine, codeine, fentanyl and oxycodone.
3. Local anaesthetics such as lidocaine, bupivacaine and levo‐bupivacaine.
4. Adjuvant analgesics. This group includes all drugs given for pain but their primary indication is not analgesia. For example, most drugs commonly used for neuropathic pain can act or be specifically used as an adjunct analgesic, such as tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs), and anticonvulsants such as gabapentin and carbamazepine. Muscle relaxants and antispasmodics such as baclofen and hyoscine, steroids, the adrenergic analgesic clonidine and the N‐methyl‐D‐aspartate (NMDA) antagonists ketamine and dexmedetomidine are included in this category. Another is botulinum toxin A (BoNT‐A), which is primarily used in CYP with cerebral palsy (CP) as an adjunct to other therapeutic techniques (such as physiotherapy). It is used as a means of reducing muscle tone and spasticity.
How the intervention might work
Pharmacological interventions used to treat pain in CYP with LLCs are numerous and varied; they work in different and complex ways, with some mechanisms of action still poorly understood. We have briefly considered the mechanisms of action according to the above groupings, giving examples from each group. We recognise that these interventions may be used for pain from a variety of causes, occurring in a variety of temporalities and in a range of clinical conditions as defined by the International Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10) codes.
1. Non‐opioids
Non‐opioid analgesics traditionally include paracetamol and the NSAIDs (for example ibuprofen, diclofenac, ketorolac). Some of these analgesics, such as ketorolac and diclofenac, are still of uncertain potency. Paracetamol is an analgesic and antipyretic and is probably the most popular simple analgesic used in CYP for pain of mild to moderate intensity (Anderson 2008). Paracetamol has numerous putative mechanisms of analgesia, such as inhibiting prostaglandin synthesis within the CNS (cyclo‐oxygenase (COX)‐3, COX‐2b), blocking impulse generation within the bradykinin‐sensitive chemoreceptors responsible for the generation of nociceptive impulses, and antagonising NMDA (Jacqz‐Aigrain 2006). The recommended oral dosage starts at 20 mg/kg as a single dose, then 10 to 15 mg/kg every 8 to 12 hours for neonates up to 500 mg; 1 g every 4 to 6 hours for 16‐ to 18‐year olds (BNF 2012).
NSAIDs are a diverse group of drugs that share similar antipyretic, analgesic and anti‐inflammatory effects but may show different response characteristics (Jacqz‐Aigrain 2006). Ibuprofen, for example, is a propionic acid derivative and a non‐selective COX inhibitor, and its recommended dosage ranges from 5 mg/kg for infants aged 1 to 3 months up to 300 to 400 mg for CYP 12 to 18 years old, 3 to 4 times daily by mouth (BNF 2012).
2. Opioids
Numerous opioids are used to relieve pain in CYP with LLCs, including (but not limited to) morphine, codeine, buprenorphine, fentanyl, methadone and oxycodone. Opioids bind to specific receptors found principally in the central nervous system and the gastrointestinal tract. Morphine is widely regarded as the first‐line major opioid in CYP with LLCs who are experiencing severe pain. Morphine acts directly on opioid receptors; and a principal metabolite morphine‐6‐glucuronide (M6G) also has analgesic activity. Opioids can cause constipation and itch, as well as serious adverse effects such as extreme somnolence and depression of respiration, particularly when used in excess in opioid‐naive individuals and young infants. Age‐related changes in the pharmacokinetics of opioids are still not well understood. However, it is known that most age‐related changes are more apparent in the first year of life (as the result of pharmacokinetic differences, particularly reduced renal clearance in the first few months of life) and from then on the ability of CYP to metabolise opioids seems similar to that of adults (Ballentine 2012). Total body morphine clearance is 80% of adult values by six months of age (Bouwmeester 2004). However, it has been shown that M6G may have faster renal clearance in CYP, and therefore they may actually need higher doses given at shorter intervals than in adults (Mashayekhi 2009). Data on the use of opioids in CYP with LLCs are lacking (Zernikow 2009) and ongoing debate requires further study to provide conclusive evidence.
3. Local anaesthetics
Local anaesthetics are ion channel (Na+) blocking drugs that can treat and prevent all types of pain by blocking nociceptive pathways and suppressing nociceptor excitability. They are normally given by injection close to nerves peripherally or centrally (intrathecal or epidural) but topical preparations, including a low‐dose transdermal patch formulation that is effective for some types of neuropathic pain, are also available. Local anaesthetics in clinical use include the amides lidocaine, bupivacaine and levo‐bupivacaine, and the esters benzocaine, tetracaine and chloroprocaine.
4. Adjuvants
Adjuvants of interest in this review are drugs with a primary indication that is not for pain but that nevertheless have analgesic properties. Examples of adjuvants for neuropathic pain include some anticonvulsants, antidepressants, steroids and the NMDA antagonist ketamine. Skeletal muscle relaxants such as baclofen and antispasmodics such as hyoscine are sometimes given for pain relief. Another example is botulinum toxin A (BoNT‐A), which is used in cerebral palsy as a means of reducing muscle tone and spasticity. Bisphosponates are used to slow bone loss and, in turn, have an analgesic effect. Adjuvants make up a varied group and work in many different ways. In this review we have considered only adjuvants that are explicitly administered for pain relief.
Routes of administration
In CYP the preferred route, where possible, is oral because it is the simplest, most effective and least painful (WHO 2012). However, other routes are frequently necessary because of varying clinical needs. Examples include buccal, rectal, transdermal, intramuscular, subcutaneous, intravenous, epidural and intrathecal routes.
Why it is important to do this review
The evidence base that is currently available to guide clinical practice in this area of pain management in CYP with LLCs is limited and, whilst some clinical reviews have been published, no systematic review of the international literature has been performed to date. It is recognised, as evidenced in a recent survey conducted by the Association for Paediatric Palliative Medicine (APPM), that clinicians have an urgent need for systematic review evidence to support their prescribing (Brook 2012).
Objectives
To evaluate the evidence on the effectiveness of different pharmacological interventions used for pain in CYP with LLCs.
Methods
Criteria for considering studies for this review
Types of studies
We have included randomised controlled trials (RCTs) (including cluster RCTs and cross‐over trials), quasi‐randomised studies, n of 1 studies, studies that are not randomised but include a clearly defined comparator group, and time series analyses that have investigated pharmacological treatments for pain associated with LLCs in CYP.
Types of participants
Trials with participants who were CYP aged 0 to 18 years of either sex and with pain related to their LLCs were included. We determined whether a condition is life‐limiting by using the Richard Hain Directory (Hain 2013) of ICD‐10 diagnoses that have been judged by professionals working in palliative care of CYP to be life‐limiting, and that were recently used in a paper plotting the national prevalence of LLCs in this population (Fraser 2012). The directory is neither determinative nor definitive but provides a list of conditions that can possibly limit life in CYP during their childhood or as a young person. As some conditions can present with a range of severity (such as cerebral palsy) the authors recognise that this will result in inclusion of some CYP who may not meet the ACT 2009 definition of life‐limiting condition. In addition, following discussion by members of this review group, one review author (EB) contacted the lead author of the directory to enquire about the classification of osteogenesis imperfecta (OI) as a LLC, as this diagnosis was not listed in the original directory. OI has since been added to the directory. The conditions listed in the directory can be broken down into the following groups: infections; leukaemia; other malignant neoplasms; other neoplasms; other diseases of blood and blood‐forming organs; cystic fibrosis; other endocrine, nutritional and metabolic disorders; epilepsy; cerebral palsy and other paralytic syndromes; other disorders of the nervous system; diseases of the circulatory system; diseases of the respiratory system; diseases of the musculoskeletal system and connective tissue; diseases of the genitourinary system; conditions originating in the perinatal period; congenital anomalies and other causes; and non‐malignant haematological disorders (Cochrane 2007).
Types of interventions
Interventions included any pharmacological intervention given at any dose for any time period, on its own or in combination and with a control or comparator group (see below). The treatment included those specifically developed to treat pain and those that acted as an adjuvant where the treatment was not primarily developed to treat pain but which has pain relieving properties. We did not include studies on non‐pharmacological interventions or where the treatment for pain was as a result of an investigation or treatment.
Control or comparator groups included any other pharmacological interventions; psychological interventions such as relaxation, hypnosis and cognitive behavioural therapy; placebo; or alternative dosing regimens or routes of administration.
The intervention could be undertaken in any setting, including home, hospital, hospice and residential school.
Types of outcome measures
For all outcome measures, we have reported on the mechanisms of reporting pain in this population, which commonly features preverbal and non‐verbal children, and have taken into consideration in our own results the types of outcome measures used (for example observational, proxy, self‐report).
Primary outcomes
The primary outcomes were pain control and adverse events. Pain control is measured by changes in pain intensity scales; other indicators such as changes in physiological parameters are used (baseline or final value scores at end of follow‐up) and include both continuous and dichotomous pain outcomes. We have reported what each paper suggests as an adequate reduction of pain or period of maintenance of pain reduction. We planned, as advised in the 'Authoring or Assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group' guidance, to only include studies that used moderate or greater pain as the baseline in any meta‐analysis (Cochrane 2011); however, this was not applicable as not enough homogeneous data were available to combine in a meta‐analysis. To facilitate the review process, all forms of pain measurement in CYP, both validated and non‐validated, were considered during the review process. We reported data on all adverse events identified.
Secondary outcomes
As the effectiveness of analgesia is also measured in terms of changes in physical and psychological functioning and well‐being (McGrath 2008), we have included assessments using validated instruments, psychological or social measures such as mental health status and functioning scales, quality of life, well‐being and quality of care scales for CYP, such as the Pediatric Quality of Life InventoryTM (PedsQL) (Varni 1999) and European Quality of Life 5‐Dimensions (EQ‐5D) (Ravens‐Sieberer 2010) for their family. Health service use, including length of stay and number of hospital admissions, were considered for review. At review stage it was found that trials may include multiple outcomes, in one case the trials reported results for over 20 measures. For ease of readability and interpretation for our review we report 'other outcomes' to encompass the many outcomes reported by the studies, we only present those that were of primary interest in the trials.
Search methods for identification of studies
We used a combination of indexed and free‐text terms to reflect the concepts of ‘pharmacological intervention’, ‘CYP’ and 'pain’. The LLC element was identified during screening of papers. We modified the search terms according to the constraints of each database.
Electronic searches
The following electronic databases were searched.
CENTRAL (2014, Issue 11 of 12) (in The Cochrane Library).
MEDLINE (1946 to week 3 November 2014) (via OvidSP).
EMBASE (1974 to 16 December 2014) (via OvidSP).
PsycINFO (1806 to week 2 December 2014) (via OvidSP).
CINAHL (1980 to December 2014) (via EBSCOhost).
No language restrictions were applied. Please see Appendix 1 for the search strategies used.
Searching other resources
We undertook the following additional search strategies.
Conversations with colleagues or key authors, or a review of papers that they recommended.
Contact with key authors who have published in this field.
Conference proceedings, where available, for the International Symposium on Paediatric Pain and the European Association of Palliative Care.
Forward and backward citation searches of included studies.
Handsearching of key journals (Journal of Pain and Symptom Management and Palliative Medicine).
Data collection and analysis
Selection of studies
Five review authors (EB, JL, BC, LJ and HR) screened abstracts of all identified studies against the inclusion criteria. A second review author (EB) screened a sample of the same abstracts to validate the process (10% of the cohort). We retrieved all possibly relevant articles in full text for assessment against the inclusion criteria. We have links to researchers with many different languages skills within University College London (UCL) and so were able to translate any non‐English studies; however, none were applicable for translation. We included a PRISMA study flow diagram (Liberati 2009) to document the screening process, as recommended in Part 2, section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Data extraction and management
One review author (EB) extracted the data using a standardised data extraction form developed by the review authors and a second review author checked the data extraction (BC or LJ). Where possible, the following information was obtained for each study.
The number of patients eligible, the number of participants randomly assigned, and reasons why patients were not included in the trial.
The number of participants evaluated at follow‐up(s) and what the follow‐up time points were.
Participant demographics including age, sex, diagnosis, ICD‐10 code, and type of healthcare setting (hospital, hospice, home, residential school).
Trial design features on masking, whether parallel group or cross‐over, features of randomisation, and sample size calculation.
Any necessary additional data on trial design and outcomes to allow completion of the Cochrane Collaboration’s tool for assessing risk of bias.
Comparison interventions, including duration and mode.
Outcome data on pain reduction at all time points, including how an outcome was measured, and mean or categorical scores of the main outcome and other outcomes.
Adverse effects.
Blinding of researchers and participants to the allocation of those participants receiving the intervention or the control.
Dropout rates and reasons why.
Concurrent use of other drugs including analgesics, and any drug exclusions.
Quality of life of CYP and family, and how this was measured.
Other behavioural and psycho‐social measures, and the scales used to measure them.
In cases where information is lacking, we attempted to make contact with trial authors or trial sponsors.
Assessment of risk of bias in included studies
We assessed and reported on the risk of bias of included RCTs using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2011a). This recommends explicit reporting of the following quality elements for RCTs: sequence generation; allocation concealment; blinding; completeness of outcome data; and selective outcome reporting. For each quality domain, we assessed whether the risk of bias was low (if the study matched the criteria), high (if the study did not match the criteria) or unclear (if under‐reporting was noted). We defined trials as having an overall low risk of bias if they scored a low risk of bias on four of the five domains in the risk of bias table. We labelled a trial as having an unclear risk of bias if the trial provided too few details to allow a judgement of 'high’ or 'low’ risk of bias. Two review authors (EB and VV) assessed the risk of bias of the included studies; disagreements were resolved by discussion. Where needed, we contacted study authors to ask for additional information. We incorporated the results of the risk of bias assessment into the review through systematic narrative description and commentary about each item. This led to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of the results of the review.
Measures of treatment effect
The null hypothesis tested is that, for the primary outcomes examined, the pharmacological interventions have no effect compared with placebo or other interventions. Where there were appropriate data, for dichotomous outcomes we calculated the risk ratio (RR) with 95% confidence interval (CI), and for continuous data we estimated the mean difference (MD) with 95% CI.
Unit of analysis issues
We planned to seek statistical advice if we identified trials using a cluster design (in which participants were randomly assigned at group level).
Dealing with missing data
If doubts had arisen about missing data (participant dropouts, etc.) we would have sought to contact the study authors to obtain further information and if we are unable to obtain data we would have stated that. We planned, if needed, to address the potential impact of missing data on our findings in the 'Discussion’ section of the review.
Assessment of heterogeneity
A meta‐analysis was not conducted and so evaluation of heterogeneity between trials (Higgins 2011b) was not necessary.
Assessment of reporting biases
A sufficient number of studies were not identified and so a meta‐analysis was not possible.
Data synthesis
For this review we first sought to categorise the studies according to whether they considered nociceptive pain, neuropathic pain, or both. We then grouped the identified evidence by the LLC ICD‐10 (disease) classification and then by the different pharmacological interventions used (that is non‐opioids, opioids, local anaesthetics, and adjuvants). We planned that if there were sufficient trials by class of treatment, and they were sufficiently similar in measurement and population and of sufficient quality, we would combine their individual data in a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Meta‐analysis was not conducted and so no subgroup analysis was undertaken.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore (by excluding trials) the influence of the following factors.
Unpublished trials.
Trials at high risk of bias.
Summary of findings tables
We had planned to use the GRADE system (Schunemann 2008) to assess the quality of the evidence associated with specific outcomes (for example pain reduction, quality of life improvement, adverse effects) and construct a ‘Summary of findings’ table using the GRADE software. However, because of the small cohort of heterogenous trials that were found, comprising different conditions, drugs and outcomes, a summary of findings table was not constructed. It would not have added any meaning for the reader although the authors note that it is possible to create a summary of findings table despite the lack of meta‐analysis.
Results
Description of studies
Results of the search
See Characteristics of included studies; Characteristics of excluded studies.
We identified 24,704 citations from our electronic database search. Thirty‐nine potential studies were identified from the citations. We also handsearched all included studies' bibliographies, searched other relevant Cochrane reviews (such as reviews on interventions for pain in other CYP populations) and contacted authors in the field to enquire for advice on other relevant trials for our review. This yielded another eight studies for full‐text retrieval. See Figure 1.
1.
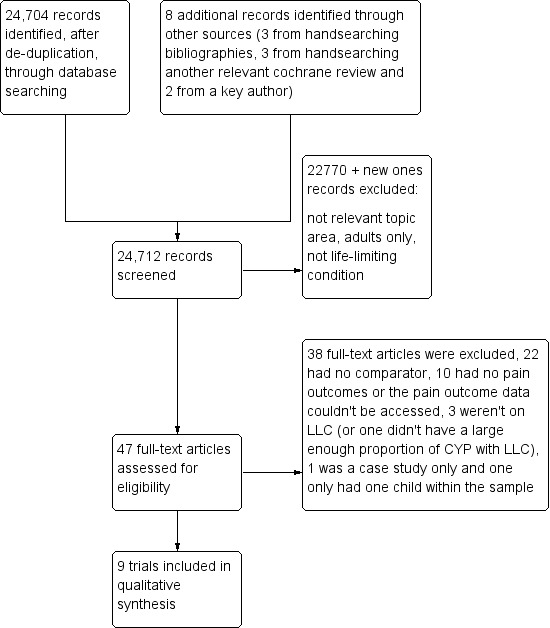
Review screening, selection and assessment steps, and numbers at each stage.
Included studies
On full‐text retrieval, nine trials in 10 papers matched our inclusion criteria (Bishop 2013; Bonouvrie 2011; Copeland 2014; Hoving 2007; Hoving 2009 (published in two papers, see reference); Letocha 2005; Russo 2007; Seikaly 2005; Ward 2011).The Hoving 2007 and Hoving 2009 trials used the same population but they tested different interventions; the 2007 trial was a dose finding study and the 2009 trial reported the longer‐term trial. In addition, we identified one ongoing trial (Bonouvrie 2013).
The nine completed studies involved 379 participants in total. Three studies were undertaken in North America (Letocha 2005; Seikaly 2005; Ward 2011), three in the Netherlands (Bonouvrie 2011; Hoving 2007; Hoving 2009), two in Australia (Copeland 2014; Russo 2007) and one in the UK (Bishop 2013). All were parallel RCTs apart from one cross‐over RCT (Seikaly 2005). Five trials investigated participants with cerebral palsy (CP) (Bonouvrie 2011; Copeland 2014; Hoving 2007; Hoving 2009; Russo 2007). CP is a disorder of movement or posture, or both, as a result of non‐progressive but permanent damage to the developing brain before, during or immediately after delivery. Pain in CP is likely to be of mixed nociceptive and neuropathic origin. In the trials that were included the participants were being treated for spasticity, which is a muscle control disorder characterised by tight and stiff muscles and an inability to control the muscles. Three of the trials on CP were investigating intrathecal baclofen (Bonouvrie 2011; Hoving 2007; Hoving 2009). Baclofen is a muscle relaxant and acts at the spinal cord level to inhibit the release of excitatory neurotransmitters. Intrathecal baclofen is administered directly into the spinal fluid and, because oral baclofen is poorly transferred through the blood‐brain barrier, direct use of the intrathecal route allows lower doses to be administered. The two other trials on CP investigated the use of botulinum toxin A (BoNT‐A) (Copeland 2014; Russo 2007). BoNT‐A is primarily used, as described earlier, in CYP with CP as an adjunct to other therapeutic techniques as a means of reducing muscle tone and spasticity. It does this by blocking the release of acetylcholine from the neuromuscular junction and so weakening the muscle. The other four trials involved participants with osteogenesis imperfecta (OI) (Bishop 2013; Letocha 2005; Seikaly 2005; Ward 2011). OI is an inherited, primarily autosomal dominant disorder of type I collagen that is characterised by bone fragility and leads to a range of clinical expressions varying in severity. All three trials evaluated the use of bisphosphonates (two looked at oral alendronate (Seikaly 2005; Ward 2011), one looked at oral risedronate (Bishop 2013) and the other at intravenous pamidronate (Letocha 2005)). Alendronate, pamidronate and risedronate are types of bisphosphonate, a class of drugs that prevent the loss of bone mass. Bisphosphonates inhibit the digestion of bone by inactivating osteoclasts, the cells that break down bone tissue, thereby slowing bone loss.
The ongoing trial was from the Netherlands. It is evaluating three months of continuous intrathecal baclofen treatment in CP participants compared with a placebo control (Bonouvrie 2013).
Cerebral palsy (CP)
Participants
The five studies on CP involved 105 participants. The types of CP varied.
One study (a pilot study) involved CYP aged 8 to 17 years with dystonic CP, ICD‐10 code: G80.3, and Gross Motor Function Classification System (GMFCS) level V (Bonouvrie 2011).
One study referred to the sample as just 'CP', labelled for this review as 'CP, unspecified', ICD‐10 code: G80.9; GMFCS level V in 38 cases and IV in 3 cases (Copeland 2014).
Two studies (using the same population) (Hoving 2007; Hoving 2009) involved CYP with spastic tetraplegic cerebral palsy, ICD‐10 code: G80.0, Spastic diplegia cerebral palsy, labelled in these studies as 'other CP', G80.8 and dyskinetic cerebral palsy, G80.3. One child was classified on the Gross Motor Function Classification System at level III, two at level IV, and 14 at level V.
One study involved CYP with hemiplegic cerebral palsy, labelled in this review as 'other CP', ICD‐10 code: G80.8, with no mention of GMFCS level (Russo 2007).
The age range varied. In one study participants were 8 to 17 years old (Bonouvrie 2011), in one study 2 to 16 years old (Copeland 2014), in two studies 7 to 16 years old (the same population) (Hoving 2007; Hoving 2009) and in the other study 3 to 16 years old (Russo 2007).
Setting
All studies were hospital based, three of which were multi‐site (Hoving 2007; Hoving 2009; Russo 2007) and two single‐site (Bonouvrie 2011; Copeland 2014).
Osteogenesis imperfecta (OI)
Participants
The four studies on OI involved 274 participants in total. There are eight types of OI, I to VIII, all of varying severities. Pain in OI may be due to bone destruction or associated deformity and therefore is likely to be of a mixed nature. Three studies (Bishop 2013; Seikaly 2005; Ward 2011) included CYP with a range of OI types (I, II, III and IV) and one (Letocha 2005) included CYP with types III and IV. The age range in the studies varied: 4 to 15 years old (Bishop 2013), 4 to 13 years old (Letocha 2005), 3 to 15 years old (Seikaly 2005) and 4 to 19 years old (Ward 2011).
Setting
All studies were hospital based, with two being multi‐site (Bishop 2013; Ward 2011) and the others single‐site (Letocha 2005; Seikaly 2005).
Excluded studies
Most studies were excluded due to the lack of a comparator group or not having pain outcomes, see the Characteristics of excluded studies table.
Risk of bias in included studies
All trials were at some risk of bias, see Figure 2 and Figure 3. Three were assessed as high risk in relation to blinding (Hoving 2009; Letocha 2005; Russo 2007) and one in relation to attrition, 27% (29/109) in the intervention group compared with 13% (4/26) in the comparison group (Ward 2011).
2.
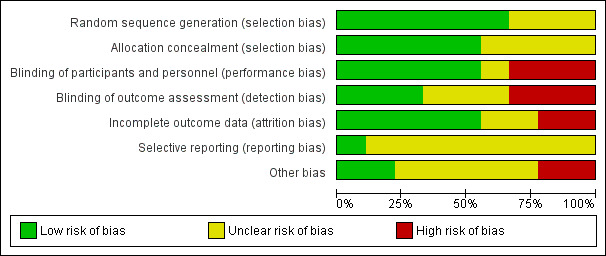
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
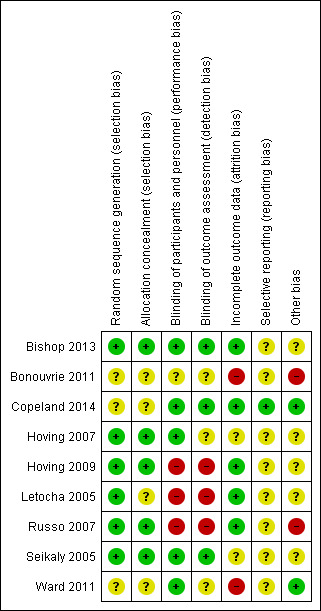
Risk of bias summary: review authors' judgements about each risk of bias item for each included study; + = study matched criteria, ‐ = study did not match criteria, ? = unclear if study matched or did not match the criteria.
Allocation
Six trials adequately described randomisation sequence generation (Bishop 2013; Hoving 2007; Hoving 2009; Letocha 2005; Russo 2007; Seikaly 2005) and five of these adequately described allocation concealment (Bishop 2013; Hoving 2007; Hoving 2009; Russo 2007; Seikaly 2005).
Blinding
Five trials were described as double‐blind (Bishop 2013; Copeland 2014; Hoving 2007; Seikaly 2005; Ward 2011). Three of these trials appeared to be at low risk of both performance and detection bias (Bishop 2013; Copeland 2014; Seikaly 2005), in the other two they did not state if the outcome assessor was blinded. One trial was described as single‐blind (Russo 2007). It used blinded assessors for a subset of outcomes, but all other assessments performed by the paediatric rehabilitation specialist were unblinded. No placebo injections were administered in the control group due to the requirement of general anaesthesia and so CYP and parents were unblinded to their assignment group and could have revealed this, making the trial high risk. One trial was labelled as unclear as it did not mention blinding (Bonouvrie 2011), and two high risk (Letocha 2005; Hoving 2009). In the Letocha 2005 trial investigators were blinded for vertebral area and compression measures only. Blinding of patients and their families was not possible in the Hoving 2009 trial as CYP either received the intervention delivered via a pump and standard care (of physiotherapy, speech therapy and occupational therapy) or in the control group received standard care only.
Incomplete outcome data
Four trials were low risk, either because few participants (Bishop 2013) were lost from the trial or they clearly stated the methods of dealing with missing data (Copeland 2014; Letocha 2005; Russo 2007). Copeland used a two‐group comparison on all participants in an intention‐to‐treat analysis. The trial by Russo 2007 also used an intention‐to‐treat analysis, the missing data were reported and were balanced across both arms of the trial; with the intention‐to‐treat analysis the missing data were unlikely to have affected the results. Letocha used a per protocol and repeated‐measures model of analysis. One trial was deemed high risk (Ward 2011) as although they used intention‐to‐treat analysis, the previous on‐treatment observation was carried forward for the missing data and the number lost to follow‐up (and number of adverse events) was much higher in the treatment group (27% (29/109) versus 13% (4/26)). The reasons for missing outcome data may be related to true outcome and the pain may be higher in those who did not return. Four trials were labelled as unclear (Bonouvrie 2011; Hoving 2007; Hoving 2009; Seikaly 2005). In the trial by Bonouvrie 2011 analysis or incomplete data were unclear and data were only completed for two of the four participants. The missing data in the Hoving 2007 trial were unclear with no reasons provided except the fact that they excluded one male due to use of open label medications and the authors reported deviating from the protocol by starting with a lower dose for one participant. There were no details in the Hoving 2009 trial about missing data or how these would be dealt with. In the Seikaly 2005 trial only 17 participants completed the two year study and it was not clear why three participants dropped out.
Selective reporting
Eight of the trials did not cite or refer to a protocol for the reader to assess what the planned outcomes were intended to be, and so the risk of selective reporting was unclear (Bishop 2013; Bonouvrie 2011; Hoving 2007; Hoving 2009; Letocha 2005; Russo 2007; Seikaly 2005; Ward 2011). Only one trial referred to a protocol for the trial which clearly laid out planned outcomes and so was low risk (Copeland 2014).
Other potential sources of bias
Two trials were deemed high risk (Bonouvrie 2011; Russo 2007). In the study in which only two participants completed the study, two ended the study prematurely and the trial period was hampered by serious complications making it high risk for other sources of bias (Bonouvrie 2011). In the other study there was no placebo, only standard therapy, and so other biases could have affected this trial (Russo 2007). Two trials were low risk (Copeland 2014; Ward 2011). In both trials the baseline characteristics appeared balanced and the trials did not stop early. Four trials were labelled unclear (Hoving 2007; Hoving 2009; Letocha 2005; Seikaly 2005). In the Hoving trials they reported that results were confined to relatively older CYP with intractable spastic CP who relied on wheeled mobility (Hoving 2009). In the Letocha 2005 trial no information was provided on the gender of CYP or exactly what the control group received (no placebo), and so unclear sample bias was present. Lastly, in the Seikaly 2005 trial some outcomes excluded CYP with type I OI and so the trial only reflected types III and IV, but the authors did not explain why.
Effects of interventions
Cerebral palsy (CP)
Intrathecal baclofen versus placebo or therapy as normal
Three small trials (of two populations) of CYP (aged 7 to 17 years) with CP evaluated intrathecal baclofen (ITB) versus intrathecal placebo (Bonouvrie 2011; Hoving 2007) or standard therapy (including any physiotherapy, speech therapy and occupational therapy) (Hoving 2009).
Pain
In two studies (Hoving 2007; Hoving 2009) pain was evaluated as a primary outcome emerging from the individually formulated problems and being separately analysed by a visual analogue scale (VAS) (0 to 10) rated either by the patients themselves, if they had sufficient ability to do so, or by their parents.
In the other trial pain was evaluated as a secondary outcome and measured by a VAS (Bonouvrie 2011).
In one trial of 17 CYP (mean age 13 years, range 7 to 16) they found that pain measured using a VAS improved significantly after administration of the drug in the intervention group compared to standard therapy in the control group (MD 4.20, 95% CI 2.15 to 6.25) (Hoving 2009). In the same study population, at 6 months bodily pain or discomfort measured using the domain score of the Child Health Questionnaire‐Parent Form 50 (CHQ‐PF50) improved in the intervention group (MD 26.60, 95% CI 2.61 to 50.59). Likewise, at this time point there was a significant difference comparing pain scores using the VAS in the ITB group when compared with placebo (MD 4.20, 95% CI 2.15 to 6.26) (Hoving 2007).
The other trial on ITB involved four CYP (aged 8, 9, 14 and 17 years). Compared with blinded placebo treatment, pain scores increased 0.5 points during blinded ITB treatment and pain scores were 2.6 points lower than at baseline (Bonouvrie 2011).
Safety
Trials reported safety measures, these were: number of adverse effects experienced, what the effects were, and the number of patients who dropped out due to adverse effects. Some of the adverse effects were related to the procedure or device for administration, and none were related to the intervention drug administered. Whilst all trials reported safety measures, Hoving 2009 reported these six months after the end of the trial. At this time point those in the control group would have also received the intervention (as a wait‐list control).
Nine adverse effects of ITB were registered in eight participants in the Hoving 2007 trial of 17 CYP, and they mostly related to lowered cerebrospinal fluid (CSF) pressure for example lethargy. Fourteen of the 17 participants in Hoving 2009 experienced a total of 51 non‐procedure or device related adverse effects; again, the most frequently observed event was lethargy. Fourteen of the 17 participants also experienced a total of 29 procedure or device related adverse events, most were related to swelling at the pump site (Hoving 2009).
The most common adverse effect in two trials, irrespective of trial arm, was CSF leakage (Bonouvrie 2011; Hoving 2007). In Bonouvrie 2011 this occurred in two patients in the ITB group, which resulted in headache, nausea, the blinded trial phase to be discontinued in one patient and in an extended hospital admission in the other patient. CSF leakage from the catheter connection occurred in three participants in Hoving 2007. In one of these, the catheter connection was defective and a new catheter was inserted; in the other two the cap was reconnected. Five of the 51 non‐procedure or device related adverse events in Hoving 2009 were considered as serious as they resulted in significant disability. These were: difficulty swallowing, dysarthria, excessive hypotonia in two cases and epileptic seizure. It was not clear whether the non‐procedure or device related serious adverse events were in those who received the intervention or in those in the control group. Three of the 29 procedure related events were considered serious, these were: incomplete operation, abrupt lack of ITB effect and pain at the pump site; all resulted in a prolonged hospital stay (Hoving 2009). None of the participants in either of the Hoving trials dropped out because of adverse effects (Hoving 2007; Hoving 2009).
Other outcomes
Other main outcomes in the trials were:
three main problems in daily care, dressing and speaking (using VAS), changes in dystonia (using the Barry‐Albright Dystonia (BAD) scale) and comfort and happiness (both using VAS) (Bonouvrie 2011);
individually formulated problems (comprising of pain (see results above) and ease of care such as operating a wheelchair) (Hoving 2007; Hoving 2009);
health related quality of life on self‐care capability (using the Caregiver Assistance Scale of the Pediatric Evaluation of Disability Inventory self‐care domain (PEDI)) (Hoving 2009);
spasticity (using the Ashworth scale) (Hoving 2007).
Hoving 2007 found ease of care significantly improved in the ITB group compared with the placebo group (MD.10, 95% CI 2.52 to 5.68). In the same trial spasticity was found to improve at 2, 4 and 6 hours after administration of the intervention drug for all muscle groups apart from hip flexors at one time point. After administration of the placebo there were no significant changes from baseline reported. The VAS for individual problems improved significantly for the ITB group compared with the control arm (MD 4.10, 95% CI 2.67 to 5.53) (Hoving 2009). The six‐month change score for caregiver assistance did not significantly differ between the trial arms. In their trial of four participants Bonouvrie 2011 found no improvement in problems related to daily care or in dystonia during the blinded placebo and ITB treatment. They found comfort increased in both groups. Happiness scores decreased slightly during blinded placebo although they were maintained in those who received ITB treatment. Dystonia did not improve in either trial arm.
Botulinium toxin A or botulinium toxin A and occupational therapy versus placebo or occupational therapy alone
Two trials of CYP with CP investigated botulinium toxin A (BoNT‐A). One trial compared the effect of intravenous BoNT‐A with a placebo in 41 CYP with a mean age of 7 years (range 2 to 16 years) (Copeland 2014). The other trial in 43 children with a mean age of 8 years (range 7 to 9 years) compared localised injections of BoNT‐A undertaken under anaesthetic plus occupational therapy versus occupational therapy alone (Russo 2007).
Pain
Pain was evaluated in both trials as a secondary outcome: it was measured by the Pediatric Pain Profile (PPP) in one study (Copeland 2014) and the other used a VAS (0 to 5) (unclear if parent or patient rated the pain) (Russo 2007). In one trial there were no significant between group differences at 4 or 16 weeks (MD ‐2.67, 95% CI ‐10.18 to 4.84; MD 2.59, 95% CI ‐3.75 to 8.93, respectively) (Copeland 2014). In the other trial no differences were found between the treatment arms in reporting pain at 3 and 6 months (2 participants in each group, OR 1.05, 95% CI 0.13 to 8.24; 1 participant in each group, OR 1.05, 95% CI 0.06 to 17.95, respectively) (Russo 2007).
Safety
In one trial of 43 participants two of the children in the control group and one in the intervention group experienced an adverse event (Russo 2007). In the control group this involved two hospital admissions for seizures in one child with epilepsy and three hospital admissions for medical reasons in another child. In the intervention group, one adverse event was reported in a child with epilepsy (this resulted in admission to hospital for seizure management shortly after injection). In the intervention group there were a total of 22 adverse effects, the most frequent were feeling unwell after the anaesthetic and excessive weakness in the injected limb. No adverse effects were reported in the control group.
In the other trial on BoNT‐A, of 41 participants three participants in the intervention group and one in the placebo group experienced an adverse event (Copeland 2014). In the intervention group, one adverse event was systemic involving increased drooling or decreased vocalization and was possibly related to the injection. Another was neurologic, involving prolonged seizure resulting in a five day hospital admission, and was unlikely to be related to the injection. In the control group two adverse effects were respiratory related (pneumonia 15 weeks post‐injection, resulting in a one day hospital admission, unlikely to be related to the injection; and croup resulting in a one day hospital admission) and one was gastroenterological (vomiting and diarrhoea, resulting in a six day hospital admission, unlikely to be related to the injection). There were also other effects reported for 23 participants that were described as either moderate or mild, and significantly more participants in the intervention group experienced these (OR 9.36, 95% CI 2.24 to 39.12). Moderate adverse events reported in both trial arms included seizures and respiratory symptoms. In regards to mild adverse events in the intervention group, most related to bruising at the injection site.
Other outcomes
Other outcomes which were declared as primary outcomes in the trials were: performance and satisfaction in areas of concern for care and comfort using the Canadian Occupational Performance Measure (COPM) (Copeland 2014) and activity participation (using the Assessment of Motor and Process Skills (AMPS) and the Goal Attainment Scaling (GAS)) (Russo 2007).
The Copeland 2014 trial reported significant between group differences favouring the BoNT‐A‐treated group on COPM performance at 4 weeks (MD 2.2, 95% CI 0.8 to 3.5) and for COPM satisfaction (MD 2.2, 95% CI 0.5 to 3.9). These effects were retained at 16 weeks for COPM satisfaction (MD 1.8, 95% CI 0.1 to 3.5) but not for performance.
In Russo 2007 the intervention group improved significantly on the GAS score for goal attainment at 3 months but not at 6 months (MD 3.00, 95% CI 5.31 to 20.69; MD 3.90, 95% CI ‐6.68 to 14.48, respectively). The difference between the groups on the AMPS was not significant at 3 and 6 months (motor skills MD ‐0.22, 95% CI ‐0.67 to 0.23; MD ‐0.15, 95% CI ‐0.65 to 0.35; and process skills MD ‐0.14, 95% CI ‐0.63 to 0.35; MD ‐0.18, 95% CI ‐0.68 to 0.32, respectively).
Osteogenesis imperfecta (OI)
Oral alendronate versus placebo
Two trials investigated oral alendronate versus placebo (Seikaly 2005; Ward 2011).
Pain
Pain was evaluated in the Seikaly 2005 cross‐over trial of 20 CYP (mean age 9 years, range 3 to 15 years) as one of the primary outcomes; it was measured by number of pain‐free days per month, and the number of days that analgesia was administered for skeletal pains. In the Ward 2011 trial of 139 CYP (mean age 11 years, range 4 to 19 years) it was a secondary outcome; measured by the number of patients with bone pain and the number of days per week that the patients experienced bone pain. In the cross‐over trial a significant decrease favouring the intervention treatment was found in pain scores and analgesic use at 12 months at the end of the cross‐over two‐treatment periods (MD ‐3.63, 95% CI ‐5.17 to ‐2.09; MD ‐2.00, 95% CI ‐3.57 to ‐0.43, respectively) (Seikaly 2005). In the other trial fewer patients receiving alendronate compared to placebo (37% (38/102) versus 57% (17/30)) experienced bone pain at 24 months but this was not statistically significant (OR 0.45, 95% CI 0.20 to 1.04); there was no significant difference in the alendronate arm between baseline and follow‐up in the number of days per week during which patients suffered bone pain (MD ‐0.73, 95% CI ‐4.69 to 3.23) (Ward 2011).
Safety
Two of the 20 participants had adverse effects in the trial by Seikaly 2005, in the intervention group. The two participants developed abdominal discomfort which was relieved after the child followed instructions to stay upright for two hours after administration of alendronate. No adverse events were reported.
In the other trial of 139 participants gastrointestinal symptoms were also the most commonly experienced adverse event, occurring in just over half of all participants in both trial arms (OR 1.23, 95% CI 0.47 to 3.23) (Ward 2011). For two alendronate patients (1.8%) and one placebo patient (3.3%) a serious adverse event resulted in withdrawal from the study; none were deemed to be drug related by the study investigators (it was not described what the serious adverse events were). Six patients in the alendronate group withdrew from the study due to adverse events including abdominal pain, vomiting, extraskeletal ossification, leukopenia, agitation and syringomyelia or platybasia. Only the abdominal pain and vomiting were attributed to the study drug. The trial did not state whether any participants withdrew from the placebo group.
Other outcomes
Other outcomes reported in both trials included bone mineral density (BMD) (measured by BMD DEXA Z scores) (Seikaly 2005; Ward 2011). The primary outcome in the Ward 2011 trial was changes in lumber vertebrae BMD. The Seikaly 2005 trial also investigated quality of life: self‐care (measured using the WeeFIM system 18‐item tool that measures performance in essential daily activities), mobility (using the modified Pediatric Evaluation of Disability Inventor (PEDI)) and well‐being (using self‐reported scores 1 to 10). Secondary outcomes included: physical evaluation, food records, blood and urine analysis, stool guaiac, renal ultrasound, skeletal survey (including rate of fractures).
The Seikaly 2005 trial reported a significant increase in BMD Z score, 0.89 with alendronate compared to ‐0.12 with placebo (MD 1.01, 95% CI 0.55 to 1.47). Significant improvement was observed in well‐being scores (MD 3.19, 95% CI 2.25 to 4.13) and an increase in self‐care with alendronate versus placebo (MD 3.58, 95% CI 1.06 to 6.10), but no improvements in mobility were observed. No changes were observed in the secondary outcomes apart from cross‐linked N‐telopeptide of type 1 collagen divided by urinary creatinine (uNTX/uCr) which decreased by 56% after 1 year of alendronate therapy, but the decrease in the frequency of bone fractures was not significant. In the other trial the alendronate group had a significant increase in lumbar spine BMD Z score at 24 months (MD 1.18, 95% CI 0.90 to 1.46) (Ward 2011).
Oral risedronate versus placebo
One trial of CYP investigated oral risedronate versus a placebo (Bishop 2013).
Pain
Pain evaluation was not the main focus of this trial. It was evaluated using pain scales (type not noted) and as an adverse event. The trial reported in its discussion section that there was no difference in pain scales between the trial arms.
Safety
There was no difference between the trial arms in the safety profile, including the number of children experiencing adverse events (OR 0.46, 95% CI 0.09 to 2.24). When pain was reported as an adverse event there was also no significant difference between the trial arms in the number of participants experiencing pain (OR 1.54, 95% CI 0.52 to 4.56). There were two serious adverse events that were possibly or probably study related. Both occurred in the intervention group but the authors did not describe what the events were. One patient on risedronate withdrew because of an adverse event; this was Crohn's disease, which was believed by the investigators to be possibly related to the study drug.
Other outcomes
Other outcomes reported were lumber spine and total body areal BMD Z scores and clinical fractures. Using an ANCOVA model with fixed effects for age, treatment and centre they found a significant difference in lumber spine BMD Z score that favoured those taking risedronate (at 6 and 12 months, P < 0.0001). There were fewer non‐vertebral fractures reported at one year follow‐up in those in the intervention group compared to control (OR 0.46, 95% CI 0.23 to 0.95). No children had a vertebral fracture. No difference was found between the trial arms in total body BMD Z score.
Intravenous pamidronate versus no treatment control group
One trial of 18 CYP investigated intravenous pamidronate versus a no treatment control group (Letocha 2005).
Pain
Pain was evaluated as a secondary outcome, measured by a 4 point self‐reported pain scale (from 4 = no pain to 1 = intractable pain). No changes in self‐reported bone pain were found (MD ‐0.11, 95% CI ‐0.83 to 0.61).
Safety
All participants experienced acute phase reactions upon the first infusion cycle of pamidronate. What these reactions were are not described; no other complications were noted.
Other outcomes
The primary outcome in this trial was changes in bone density (vertebral DXA Z score, height, and area) (Letocha 2005). Significant increases were observed in DXA Z score with pamidronate at 6 months (MD 21.59, 95% CI 5.79 to 37.39) and at 12 months (MD 25.60, 95% CI 11.48 to 39.72). Increases in L1 to L4 midvertebral height (P = 0.014) and total vertebral area (P = 0.003) were found compared with the controls. However, during extended treatment (after the trial), DXA Z scores and vertebral heights and areas did not increase significantly.
Discussion
Summary of main results
This review set out to consider the pharmacological interventions evaluated for pain in CYP with LLCs. Whilst we identified nine relevant studies, the primary objective of the pharmacological intervention was not the treatment of pain in all studies (Bishop 2013; Bonouvrie 2011; Copeland 2014; Hoving 2007; Hoving 2009; Letocha 2005; Russo 2007; Seikaly 2005; Ward 2011). Neither was it in the ongoing trial that was identified (Bonouvrie 2013). Trial participants' ages ranged from 3 to 19 years, with the mean ages ranging from 8 to 11 years). Participants in trials had been diagnosed with cerebral palsy (CP) in five of the studies and osteogenesis imperfecta (OI) in the other four studies. All studies, apart from one cross‐over trial, were parallel group designed RCTs. Eligible studies evaluating pharmacological interventions for other LLCs in CYP were not found. There was heterogeneity in the eight included trials and it was therefore not possible to combine their outcome data in a meta‐analysis. Pain measures were reported as secondary outcomes in six studies (Bishop 2013; Bonouvrie 2011; Copeland 2014; Letocha 2005; Russo 2007; Ward 2011). The effects on pain for the different drugs in the differing populations varied amongst the studies and are summarised below.
Intrathecal baclofen for pain in cerebral palsy (CP)
Two studies (using the same population, n = 17 participants) evaluated the use of intrathecal baclofen (ITB) for pain in CYP with CP (Hoving 2007; Hoving 2009). One found no significant difference in pain between ITB and placebo (Hoving 2007). Both found pain scores to significantly improve in the ITB arm compared with the placebo group.
Intramuscular botulinum toxin A for pain in cerebral palsy (CP)
In both trials pain was found to be not significantly different in the botulinum toxin A (BoNT‐A) group as compared to placebo (Copeland 2014) or occupational therapy alone (Russo 2007).
Oral alendronate or risedronate for pain in osteogenesis imperfecta (OI)
Three studies investigated the use of a bisphosphonate drug administered orally; in two this was alendronate and in one risedronate. One cross‐over trial found a significant decrease in pain in the alendronate group compared to placebo (Seikaly 2005). There were no significantly different results for pain in the RCT investigating alendronate compared to placebo (Ward 2011) or in the RCT investigating risedronate compared to placebo (Bishop 2013).
Intravenous pamidronate for pain in osteogenesis imperfecta (OI)
The RCT on pamidronate found no changes in bone pain in the pamidronate group as compared to the no treatment control group (Letocha 2005).
Safety and adverse events
In the ITB studies most of the adverse events were related to the procedure or device for administration, these included swelling at the pump site and CSF leakage from the catheter (Bonouvrie 2011; Hoving 2007; Hoving 2009). Whilst these events are not related to the intervention drug itself, it is recognised that the administration is a risky procedure as it involves the central nervous system. The events are important to highlight here because of their negative and potentially serious impact on the patient. The adverse events in the BoNT‐A trials were sparse and mostly involved those participants who received the intervention drug, where increased seizures were reported in two participants with pre‐existing epilepsy. Regarding the bisphosphonates for OI, no adverse events were mentioned in the pamidronate trial but in the alendronate trials gastrointestinal problems seemed to be the most frequent adverse event in those who received alendronate. No differences in adverse event profiles were found in the trial arms in the risedronate study. There were serious adverse events in both arms, some of which may have been study related. The authors provided no details on what they were, apart from one person in the intervention arm leaving the study because of Cohn's disease.
Overall completeness and applicability of evidence
In this review we found that published, controlled evidence about the pharmacological interventions for pain in CYP with LLCs is very limited. The evidence that is currently available from RCTs has evaluated pain largely as a secondary outcome and the drugs used were all adjuvants, not drugs which are used primarily for pain. The drugs explored in the included studies may not be commonly used in general paediatric populations in palliative care primarily for the management of pain (Himelstein 2006). Baclofen is regularly used in paediatric practice to relieve muscle spasm and associated pain, and may also be used as an adjuvant to other therapies. Bisphosphonates are used frequently for managing pain in specific circumstances such as conditions that cause osteoporosis and painful bony metastasis. In general paediatric practice these events are not common.
The generalisability of the review is extremely limited. Moreover, the conditions explored in the included studies may not always be considered life‐limiting. OI is classified into a number of categories that vary in severity and prognosis. CP is a general term used to describe a number of neurological conditions that affect movement and co‐ordination, and covers 10 different codes in the Hain directory (Hain 2013). It is difficult to interpret the results of the CP studies as the condition varies so widely in severity. CP with uncontrolled and painful spasticity and OI of all types are often treated in specialist tertiary clinical centres.
There is an extensive number of conditions included in the Hain directory which are considered as life‐limiting but for which we found no evidence with respect to pain management, these include leukaemia, cystic fibrosis and congenital abnormalities. It was striking to note the lack of studies of any methodological type (RCT or other quantitative design) evaluating treatments for pain in CYP for LLCs, such as NSAIDS and opioids. This is despite the increasing prevalence of CYP with LLCs (Fraser 2012) and pain being reported as a key symptom in a large proportion of conditions (Beretta 2010;Bradshaw 2005; Feudtner 2011; Goldman 2006; Hechler 2008;Jalmsell 2006; Wolfe 2000). In particular, we found no evidence on commonly used pain relief medications recommended by WHO, such as opioids when pain is severe, nor on the relative efficacy of differing routes of administration that might meet differing clinical needs, for example in neonates, children and adolescents with their varying capacities (WHO 2012).
Quality of the evidence
This review faced a number of difficulties in the quality of the evidence identified. Apart from one larger trial which had 109 participants in the treatment arm and 30 in the placebo arm (Ward 2011), all studies were small, with a maximum of 23 participants in any treatment arm. Small sample size is a recurring issue in the palliative care of CYP, partly due to the relative rarity of many LLCs and also due to ethical and practical challenges to conducting research in this patient group. Use of cross‐over trial methodology may enhance the investigation of the use of newly developed drugs, where it may be deemed unethical to expose one group to a potentially beneficial treatment and withhold it from another.
There are difficulties in defining LLCs in the palliative care of CYP, and in this review we used the Hain directory of life‐limiting illnesses. The Hain directory provides a comprehensive list of conditions, developed by experts in palliative care of CYP, and has been used in a series of recent prevalence studies (Fraser 2012; Fraser 2014). The Hain directory is subject to regular appraisal and new additions are likely to be made in future updates. Of note is that OI has only recently been added. There is also the problem in palliative care of CYP of comparisons made across many diagnostic groupings (LLCs are heterogenous and so issues arise when attempting to group them together). As recently found, not only are LLCs increasing in incidence but non‐malignant conditions are more common than malignant conditions, and so research needs to reflect this (Fraser 2014).
There are also difficulties in relation to the pharmacology of medications for pain as dosage and indication will vary across age ranges of CYP. In the eight included studies in this review, no two studies reported results for the same age range. Studies on the whole did not use validated pain outcome measures. The use of outcomes such as proxy VAS rather than a validated structured questionnaire is likely to introduce bias or heterogeneity and may preclude potential future meta‐analysis.
Only one trial (Copeland 2014) provided a protocol. Included trials did not clearly report how missing data were handled, and two were at high risk of biased results due to the dropout rate of participants in one trial (Bonouvrie 2011) and the lack of a placebo in another (Russo 2007). Four trials were labelled as unclear for other potential biases; these were generally due to a lack of information about the placebo, exclusion criteria or sample bias (Hoving 2007; Hoving 2009; Letocha 2005; Seikaly 2005).
Potential biases in the review process
The search strategy has strengths and limitations which impact the review. We conducted a comprehensive database search, handsearched the bibliographies of key papers and contacted authors when appropriate. Due to the long list of LLCs (as illustrated in the Hain directory), it was decided to omit the condition element from the terms used in our search strategy. This resulted in a higher number of citations than many systematic reviews would screen. Another limitation is that we excluded studies on perioperative pain and procedural pain, however this may have been linked to a LLC in some CYP. Finally, in the review process we had two authors completing the risk of bias tables (EB, VV), however for the data extraction the first few trials were independently extracted by two review authors and when a consensus was reached one author (EB) continued to extract the trials and a second author verified the data collection. This was rather than, as the protocol stated, a second author duplicating the entire data collection. Because a meta‐analysis wasn't conducted we decided that a second author (LJ or BC) extracting the first few trials but verifying all the data would be sufficient for the level of data extracted. In future versions of this review, should any more studies be identified we will adopt duplicate independent data extraction.
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic review including only RCTs and controlled studies has been carried out solely to examine the effects of pharmacological interventions for pain in CYP with LLCs. The WHO has conducted a systematic review on the pharmacological treatment of persisting pain in CYP with medical illnesses which overlap with the current review (WHO 2012). However, the WHO review focuses on pain in CYP with many types of medical conditions not exclusively LLCs. It also uses evidence not only restricted to RCTs. RCTs were reported from adult studies and on postoperative pain but not in pharmacological interventions for pain specifically in CYP with LLCs. In this review trials were small and heterogeneous. We found substantial heterogeneity between trials for pain outcomes, conditions and the drugs used. Other Cochrane reviews have been conducted on some overlapping topics. One review looked at BoNT‐A and CP, which found high level evidence supporting the use of BoNT‐A as an adjunct to managing the upper limb in CYP with spastic CP but did not look specifically at pain as an outcome (Hoare 2010). Another Cochrane review looked at OI and bisphosphonates and their effectiveness in increasing BMD; it found that oral or intravenous bisphosphonates increase BMD in CYP and in adults with OI but, like the current review, did not reveal any conclusions about the effect the drugs had on pain (Phillipi 2008). A third explored the effectiveness of transdermal fentanyl for cancer pain (Hadley 2013). The review authors did not identify any studies in children. From the evidence from nine studies they concluded, in their implications for practice, that pain appeared to be improved and the majority of patients would have no worse than mild pain. However, these findings are subject to methodological weaknesses in the original studies, including small sample sizes.
Research data on pain in children with chronic pain conditions might be generalisable in considering specific pain mechanisms or analgesic effects that are common to chronic pain and life‐limiting conditions. However, the research on pharmacological interventions for children with chronic pain conditions is sparse (Mathew 2014).
Authors' conclusions
Implications for practice.
Based on current available data this systematic review is unable to determine the effects of pharmacological interventions for pain for CYP with LLCs. Our review provides limited evidence to guide policy makers on the pharmacological interventions to use for reducing pain in LLCs in CYP or to further inform clinicians on their practice. Health providers and clinicians may consult the WHO guidelines on Pharmacological Treatment of Persisting Pain in Children With Medical Illnesses (WHO 2012) and the Association for Paediatric Palliative medicine Master Formulary (APPM 2015). In the case of the WHO guidelines, for example, there are 19 clinical recommendations; these include when to use analgesic treatment according to the child’s level of pain severity, and what the medicines of choice are. In the absence of CYP studies these have been based on expert guidance or clinician consensus and clinical trial evidence from adult RCTs and studies in conditions that are not life‐limiting. Until more studies in CYP with LLCs have been undertaken, this remains the best basis for pain management in this population.
Implications for research.
Currently, there is a lack of evidence from RCTs that solely examine the effects of pharmacological interventions for pain for CYP with LLCs. Clinical trials need to be conducted to inform clinicians' practice. Trials need to specify clearly: 1) the target condition(s), 2) the age range(s) of CYP, 3) the type of pain covered (nociceptive, neuropathic, or both), 4) the best route of administration, and 5) the most effective dosing schedule for different ages and weights. Future trials should aim to examine the effects of the drugs with larger populations and also be clear on methods for evaluating pain (McGrath 2008).
As mentioned earlier, it would be interesting to know the different types of routes of administration of drugs in CYP and which are best to use in different age groups and varying conditions. Future trials should also report methods of dealing with incomplete and missing data, details on the placebo, and provide a detailed protocol to ensure selective reporting is not present. These details were scarcely reported in the trials included in this review. Because of the reasons mentioned in the quality of the evidence section, these trials do have obstacles to overcome but with the rising prevalence of many LLCs this becomes only more necessary.
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 9, 2013 Review first published: Issue 3, 2015
| Date | Event | Description |
|---|---|---|
| 12 April 2016 | Amended | Contact Person amended. |
Notes
We performed a restricted search (MEDLINE (OVID)) from December 2014 to March 2017 and retrieved 1498 records. We identified one potentially relevant study that had been completed but no results were available. We contacted Purdue Pharma about this study, who confirmed in June 2017 that the population did not have life‐limiting conditions. We identified one study in Chinese (Chen 2015) and contacted the authors for more information, but at June 2017 had not received a response. As we did not identify any potentially relevant studies likely to change the conclusions, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published.
Chen 2015: Chen, S.‐S., L. Liu, et al. (2015). "Analgesic effect of fentanyl in neonates during mechanical ventilation." Zhongguo Dangdai Erke Zazhi 17(10): 1045‐1050
Acknowledgements
The authors would like to thank Jo Abbot for conducting the searches for this review and also Anna Hobson for her continued support as Managing Editor of the Cochrane Pain, Palliative and Supportive Care Group. We would like to thank authors from trials in this review for helping us with our enquiries: Laura Bonouvrié, and Ray Russo for sending through the raw results from their trials. We thank Megan Thorley for alerting us to when the trial was published and to Marjanke Hoving for sending through papers that were relevant to our review. We would also like to thank all the authors from other trials that weren't included in this review for their assistance in answering our queries and to the reviewers for their helpful comments on the protocol and the review. We thank Marie Curie Cancer Care for core funding of some of the review authors (EB, LJ, BC).
CRG funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Pain] explode all trees
#2 MeSH descriptor: [Pain Management] this term only
#3 (pain* or headache* or migraine* or neuralgia or neuropathic):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Analgesics] explode all trees
#6 MeSH descriptor: [Anesthesia, Local] this term only
#7 MeSH descriptor: [Anticonvulsants] explode all trees
#8 MeSH descriptor: [Antidepressive Agents] explode all trees
#9 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees
#10 MeSH descriptor: [Muscle Relaxants, Central] explode all trees
#11 MeSH descriptor: [Parasympatholytics] explode all trees
#12 MeSH descriptor: [Serotonin Uptake Inhibitors] explode all trees
#13 MeSH descriptor: [Steroids] explode all trees
#14 (acetaminophen or "acetylsalicylic acid" or "alendronic acid" or alfentanil or amitriptyline or aspirin or baclofen or benzocaine or bupivacaine or buprenorphine or butorphanol or carbamazepine or chloroprocaine or "choline magnesium trisalicylate" or clonazepam or clonidine or codeine or dexamethasone or dexmetetomidine or dextroamphetamine or dextropropoxyphene or diamorphine or diazepam or diclofenac or dihydrocodeine or domperidone or fentanyl or fluoxetine or gabapentin or hydrocodone or hydromorphone or "hyoscine hydrobromide" or ibuprofen or ketamine or ketoprofen or ketorolac or "levo bupivacaine" or lidocaine or loperamide or lorazepam or mefenamic acid or meperidine or methadone or methylphenidate or midazolam or morphine or naproxen or nitrous oxide or nortriptyline or oxycodone or pamidronate or paracetamol or paroxetine or pentazocine or pethidine or phenobarbital or "phenytoin" or piroxicam or pregabalin or propoxyphene or "risedronate sodium" or "sodium clodronate" or tetracaine or tramadol or "valproic acid"):ti,ab,kw (Word variations have been searched)
#15 #5 or #6 or #7 or #8 or #9 or #10 or #11 or 12 or #13 or #14
#16 MeSH descriptor: [Infant] explode all trees
#17 MeSH descriptor: [Child] explode all trees
#18 MeSH descriptor: [Adolescent] this term only
#19 (neonate* or newborn or infant* or child* or adolescen* or paediatric* or pediatric* or baby or babies or toddler* or teen* or juvenile* or boy* or girl*):ti,ab,kw (Word variations have been searched)
#20 #16 or #17 or #18 or #19
#21 #4 and #15 and #20
MEDLINE (Ovid)
1. exp Pain/ 2. Pain Management/ 3. (pain* or headache* or migraine* or neuralgia or neuropathic).mp. 4. or/1‐3 5. exp Analgesics/ 6. Anesthesia, Local/ 7. exp Anticonvulsants/ 8. exp Antidepressive Agents/ 9. exp Anti‐Inflammatory Agents, Non‐Steroidal/ 10. exp Muscle Relaxants, Central/ 11. exp Parasympatholytics/ 12. exp Serotonin Uptake Inhibitors/ 13. exp Steroids/ 14. (acetaminophen or "acetylsalicylic acid" or "alendronic acid" or alfentanil or amitriptyline or aspirin or baclofen or benzocaine or bupivacaine or buprenorphine or butorphanol or carbamazepine or chloroprocaine or "choline magnesium trisalicylate" or clonazepam or clonidine or codeine or dexamethasone or dexmetetomidine or dextroamphetamine or dextropropoxyphene or diamorphine or diazepam or diclofenac or dihydrocodeine or domperidone or fentanyl or fluoxetine or gabapentin or hydrocodone or hydromorphone or "hyoscine hydrobromide" or ibuprofen or ketamine or ketoprofen or ketorolac or "levo bupivacaine" or lidocaine or loperamide or lorazepam or mefenamic acid or meperidine or methadone or methylphenidate or midazolam or morphine or naproxen or nitrous oxide or nortriptyline or oxycodone or pamidronate or paracetamol or paroxetine or pentazocine or pethidine or phenobarbital or "phenytoin" or piroxicam or pregabalin or propoxyphene or "risedronate sodium" or "sodium clodronate" or tetracaine or tramadol or "valproic acid").mp. 15. or/5‐14 16. exp Infant/ 17. exp Child/ 18. Adolescent/ 19. (neonate* or newborn or infant* or child* or adolescen* or paediatric* or pediatric* or baby or babies or toddler* or teen* or juvenile* or boy* or girl*).mp. 20. 16 or 17 or 18 or 19 21. 4 and 15 and 20 22. clinical trial/ 23. n of 1 stud*.mp. 24. ((single case or single‐case) adj (report* or stud*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 25. ("comparator group*" or "comparision group*").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 26. or/22‐25 27. randomized controlled trial.pt. 28. controlled clinical trial.pt. 29. randomized.ab. 30. placebo.ab. 31. drug therapy.fs. 32. randomly.ab. 33. trial.ab. 34. or/27‐33 35. exp animals/ not humans.sh. 36. 34 not 35 37. 26 or 36 38. (animal/ or nonhuman/) not human/ 39. 37 not 38 40. 21 and 39 41. limit 40 to ("newborn infant (birth to 1 month)" or "infant (1 to 23 months)" or "preschool child (2 to 5 years)" or "child (6 to 12 years)" or "adolescent (13 to 18 years)")
EMBASE (Ovid)
1. exp Pain/
2. Pain Management/
3. (pain* or headache* or migraine* or neuralgia or neuropathic).mp.
4. or/1‐3
5. exp Analgesics/
6. Anesthesia, Local/
7. exp Anticonvulsants/
8. exp Antidepressive Agents/
9. exp Anti‐Inflammatory Agents, Non‐Steroidal/
10. exp Muscle Relaxants, Central/
11. exp Parasympatholytics/
12. exp Serotonin Uptake Inhibitors/
13. exp Steroids/
14. or/5‐14
15. exp Pain/
16. Pain Management/
17. (pain* or headache* or migraine* or neuralgia or neuropathic).mp.
18. or/15‐17
19. exp Analgesics/
20. Anesthesia, Local/
21. exp Anticonvulsants/
22. exp Antidepressive Agents/
23. exp Anti‐Inflammatory Agents, Non‐Steroidal/
24. exp Muscle Relaxants, Central/
25. exp Parasympatholytics/
26. exp Serotonin Uptake Inhibitors/
27. exp Steroids/
28. (acetaminophen or "acetylsalicylic acid" or "alendronic acid" or alfentanil or amitriptyline or aspirin or baclofen or benzocaine or bupivacaine or buprenorphine or butorphanol or carbamazepine or chloroprocaine or "choline magnesium trisalicylate" or clonazepam or clonidine or codeine or dexamethasone or dexmetetomidine or dextroamphetamine or dextropropoxyphene or diamorphine or diazepam or diclofenac or dihydrocodeine or domperidone or fentanyl or fluoxetine or gabapentin or hydrocodone or hydromorphone or "hyoscine hydrobromide" or ibuprofen or ketamine or ketoprofen or ketorolac or "levo bupivacaine" or lidocaine or loperamide or lorazepam or mefenamic acid or meperidine or methadone or methylphenidate or midazolam or morphine or naproxen or nitrous oxide or nortriptyline or oxycodone or pamidronate or paracetamol or paroxetine or pentazocine or pethidine or phenobarbital or "phenytoin" or piroxicam or pregabalin or propoxyphene or "risedronate sodium" or "sodium clodronate" or tetracaine or tramadol or "valproic acid").mp.
29. or/19‐28
30. exp Infant/
31. exp Child/
32. Adolescent/
33. (neonate* or newborn or infant* or child* or adolescen* or paediatric* or pediatric* or baby or babies or toddler* or teen* or juvenile* or boy* or girl*).mp.
34. 30 or 31 or 32 or 33
35. 18 and 29 and 34
36. random$.tw.
37. factorial$.tw.
38. crossover$.tw.
39. cross over$.tw.
40. cross‐over$.tw.
41. placebo$.tw.
42. (doubl$ adj blind$).tw.
43. (singl$ adj blind$).tw.
44. assign$.tw.
45. allocat$.tw.
46. volunteer$.tw.
47. Crossover Procedure/
48. double‐blind procedure.tw.
49. Randomized Controlled Trial/
50. Single Blind Procedure/
51. or/36‐50
52. clinical trial/
53. time series analysis/
54. n of 1 stud*.mp.
55. ((single case or single‐case) adj (report* or stud*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
56. ("comparator group*" or "comparision group*").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
57. 52 or 53 or 54 or 55 or 56
58. 51 or 57
59. (animal/ or nonhuman/) not human/
60. 58 not 59
61. 35 and 60
PsycINFO (Ovid)
1. exp Pain/ 2. Pain Management/ 3. (pain* or headache* or migraine* or neuralgia or neuropathic).mp. 4. or/1‐3 5. exp analgesic drugs/ 6. Local anesthetics/ 7. exp Anticonvulsive drugs/ 8. exp Antidepressant drugs/ 9. exp Anti inflammatory drugs/ 10. exp Muscle Relaxing drugs/ 11. exp Serotonin Uptake Inhibitors/ 12. exp Steroids/ 13. (acetaminophen or "acetylsalicylic acid" or "alendronic acid" or alfentanil or amitriptyline or aspirin or baclofen or benzocaine or bupivacaine or buprenorphine or butorphanol or carbamazepine or chloroprocaine or "choline magnesium trisalicylate" or clonazepam or clonidine or codeine or dexamethasone or dexmetetomidine or dextroamphetamine or dextropropoxyphene or diamorphine or diazepam or diclofenac or dihydrocodeine or domperidone or fentanyl or fluoxetine or gabapentin or hydrocodone or hydromorphone or "hyoscine hydrobromide" or ibuprofen or ketamine or ketoprofen or ketorolac or "levo bupivacaine" or lidocaine or loperamide or lorazepam or mefenamic acid or meperidine or methadone or methylphenidate or midazolam or morphine or naproxen or nitrous oxide or nortriptyline or oxycodone or pamidronate or paracetamol or paroxetine or pentazocine or pethidine or phenobarbital or "phenytoin" or piroxicam or pregabalin or propoxyphene or "risedronate sodium" or "sodium clodronate" or tetracaine or tramadol or "valproic acid").mp. 14. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15. (neonate* or newborn or infant* or child* or adolescen* or paediatric* or pediatric* or baby or babies or toddler* or teen* or juvenile* or boy* or girl*).mp. 16. 4 and 14 and 15 17. clinical trials/ 18. (randomis* or randomiz*).tw. 19. (random$ adj3 (allocat$ or assign$)).tw. 20. ((clinic$ or control$) adj trial$).tw. 21. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 22. (crossover$ or "cross over$").tw. 23. random sampling/ 24. Experiment Controls/ 25. Placebo/ 26. placebo$.tw. 27. exp program evaluation/ 28. treatment effectiveness evaluation/ 29. ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. 30. or/17‐29 31. 16 and 30 32. exp Time Series/ 33. n of 1 stud*.mp. 34. ((single case or single‐case) adj (report* or stud*)).mp. 35. ("comparator group*" or "comparision group*").mp. 36. or/32‐35 37. 36 and 16 38. 37 or 31
CINAHL (EBSCO)
S39 S38 or S31 S38 (S21 AND S37) S37 S32 OR S33 OR S34 OR S35 OR S36 S36 ("comparator group*" or "comparision group*") S35 ((single case or single‐case) N1 (report* or stud*)) S34 n of 1 stud* S33 (MH "Time Series") S32 (MH "Clinical Trials") S31 S21 AND S30 S30 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 S29 (allocat* random*) S28 (MH "Quantitative Studies") S27 (MH "Placebos") S26 placebo* S25 (random* allocat*) S24 (MH "Random Assignment") S23 (Randomi?ed control* trial*) S22 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) S21 S4 AND S15 AND S20 S20 S16 OR S17 OR S18 OR S19 S19 (neonate* or newborn or infant* or child* or adolescen* or paediatric* or pediatric* or baby or babies or toddler* or teen* or juvenile* or boy* or girl*) S18 (MH "Adolescence+") S17 (MH "Child+") S16 (MH "Infant+") S15 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 S14 (acetaminophen or "acetylsalicylic acid" or "alendronic acid" or alfentanil or amitriptyline or aspirin or baclofen or benzocaine or bupivacaine or buprenorphine or butorphanol or carbamazepine or chloroprocaine or "choline magnesium trisalicylate" or clonazepam or clonidine or codeine or dexamethasone or dexmetetomidine or dextroamphetamine or dextropropoxyphene or diamorphine or diazepam or diclofenac or dihydrocodeine or domperidone or fentanyl or fluoxetine or gabapentin or hydrocodone or hydromorphone or "hyoscine hydrobromide" or ibuprofen or ketamine or ketoprofen or ketorolac or "levo bupivacaine" or lidocaine or loperamide or lorazepam or mefenamic acid or meperidine or methadone or methylphenidate or midazolam or morphine or naproxen or nitrous oxide or nortriptyline or oxycodone or pamidronate or paracetamol or paroxetine or pentazocine or pethidine or phenobarbital or "phenytoin" or piroxicam or pregabalin or propoxyphene or "risedronate sodium" or "sodium clodronate" or tetracaine or tramadol or "valproic acid") S13 (MH "Steroids+") S12 (MH "Serotonin Uptake Inhibitors+") S11 (MH "Parasympatholytics+") S10 (MH "Muscle Relaxants, Central+") S9 (MH "Antiinflammatory Agents, Non‐Steroidal+") S8 (MH "Antidepressive Agents+") S7 (MH "Anticonvulsants+") S6 (MH "Anesthesia, Local") S5 (MH "Analgesics+") S4 S1 OR S2 OR S3 S3 (pain* or headache* or migraine* or neuralgia or neuropathic) S2 (MH "Pain Management (Iowa NIC)") S1 (MH "Pain+")
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bishop 2013.
| Methods | Randomised placebo controlled parallel trial, multi‐centre | |
| Participants | Children with osteogenesis imperfecta (OI) from 13 countries across North and South America, Europe, Africa and Australia. Children had either a history of at east one non‐traumatic or low‐impact fracture and an age‐adjusted and sex adjusted areal BMD Z score of ‐1.0 or less for either total body or lumber spine sites, or an adjusted areal BMD Z score of ‐2.0 or less irrespective of a history of fractures | |
| Interventions | Route oral risedronate or placebo for 1 year. Children who weighed 10‐30 kg received 2.5 mg risedronate or placebo daily, those who weighed more received 5 mg daily | |
| Outcomes | Height, absorptiometry scans of lumber spine and total body, clinical fractures were reported as an adverse event and secondary outcome. Serum and urine samples for bone turn over markers. Reports pain outcomes as adverse event | |
| Notes | Study funded by the pharmaceutical industry, the funders helped to obtain, analyse and interpret the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age (4 to 9 and 10 to 15 years) children were randomised in a 2:1 ratio by telephone‐based interactive voice response system in several permutated blocks of 10 to 12 |
| Allocation concealment (selection bias) | Low risk | Stratified by age (4 to 9 and 10 to 15 years) children were randomised in a 2:1 ratio by telephone‐based interactive voice response system in several permutated blocks of 10 to 12 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study treatment was masked from patients, investigators and study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study treatment was masked from patients, investigators and study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 87/94 in the intervention group and 49/49 in placebo group completed trial |
| Selective reporting (reporting bias) | Unclear risk | Although not stated as a primary outcome some of the findings on pain are reported only in the discussion section |
| Other bias | Unclear risk | Conflicts of interest are declared in regards to pharmaceutical funding |
Bonouvrie 2011.
| Methods | Single‐centre double blind randomised case controlled trial | |
| Participants | 4 CYP were randomised. Age range: 8 to 17 years old, 3 male, 1 female. Cerebral palsy, ICD‐10: G80.3 (all Gross Motor Function Classification System level V) | |
| Interventions | A baseline observation period and a dose‐finding period. After the baseline observation period, an intrathecal catheter was introduced. Route: Intrathecal baclofen either continuously via the external micro‐infusion pump or in a daily bolus dosage in case of the external catheter and intrathecal placebo as control Dose: The dosage was increased with approximately 25 micrograms per day until an optimal dosage was found. The maximal dosage was 200 micrograms per day Study period: All patients started with a trial period, in which they received ITB treatment and had a dose finding period Then they were randomised into two groups, the intervention or control group.The optimal dosage was maintained for at least three days. After this period, the patients were randomised in two groups for blinded treatment and received either ITB or intrathecal placebo treatment for four days. Hereafter, the intrathecal catheter was removed. After the trial treatment, the patients, caregivers and doctors decided, for or against implantation of a definite programmable pump (Medtronic) for continuous ITB treatment |
|
| Outcomes | Primary: Visual Analogue Scale (VAS) of 'three main problems of daily care' (ascertained before study via interview) “0” representing “no problems” and “10” representing “impossible to do”. Barry‐Albright Dystonia (BAD) scale for dystonia Secondary: Pain (of mixed origin) was assessed with a VAS, with “0” representing “no pain” and “10” representing “very severe pain”, Comfort was assessed using a VAS (score 0 to 10), with a higher score representing more comfort) happiness was also assessed; very sad looking face (score 1) to very happy looking face (score 5). Gross Motor Function Classification System (GMFCS) and Pediatric Evaluation of Disability Inventory (PEDI) were only tested prior to starting the screening treatment |
|
| Notes | Pilot study, only 2 participants completed the study Financial assistance: Dr Phelps "stichting voor spastici” (Project number: 99.047) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/4 CYP completed trial |
| Selective reporting (reporting bias) | Unclear risk | The abstract talks of results for all 4 participants when only 2 completed the study. No protocol so unclear what outcomes were intended |
| Other bias | High risk | Only 2 participants completed the study, 2 ended the study prematurely. Trial period hampered by serious complications |
Copeland 2014.
| Methods | Single‐centre double‐blind, randomised controlled trial with sham control | |
| Participants | 41 CYP randomised. Age range 2.3 to 16 years old, 27 male, 14 female. Cerebral palsy, ICD‐10: G80.8 (Gross Motor Function Classification System level IV = 3, level V = 38) | |
| Interventions | Route: Intravenous botulinum toxin A (BoNT‐A) and placebo (saline injection then ultrasound and blunt needle that doesn't penetrate the skin) Dose: 0.5 to 4 units Botox/kg/muscle group (Allergan PLC, Dublin, Ireland) as clinically indicated to maximum dose of 12 U Botox/kg body weight (or total 400 units) Study period: 16 weeks. Follow‐ups were at baseline, 4 and 16 weeks Muscle groups selected for injection were determined prior to randomisation and based on parent’s or caregiver’s goals for improving ease of care and comfort in conjunction with musculoskeletal assessment findings of hypertonicity |
|
| Outcomes | Primary: Parent ratings in identified areas of concerns for their child’s care and comfort, using the Canadian Occupational Performance Measure (COPM). Secondary: Pain (of mixed origin) (using the paediatric pain profile), ease of care (Care and Comfort Hypertonicity Questionnaire (CCHQ)), health status (measured using the Caregiver Priorities and Child Health Index of Life with Disabilities [CPCHILD]) Questionnaire and quality of life (measured using the Cerebral Palsy Quality of Life Questionnaire for Children (CPQOL‐child)) |
|
| Notes | Protocol published (Thorley 2012) Funding: Allergan Australia PLC to the Royal Children’s Hospital Foundation (Brisbane) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that prior to randomisations, participants will be stratified according to primary goal areas (upper limb or lower limb) in order to allow block randomisation with the intention that similar numbers of CYP with predominantly upper and lower limbs injected will be in each arm of the study. No details on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, different clinicians conducted procedures to the clinicians who recorded results and participants were blind to the procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A different physician conducted the procedures (treatment injections and sham blunt needle procedure) to the nurse that collected the observations and was blinded to the procedures so detection unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Uses two‐group comparisons on all participants on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting present in the study from assessing the outcomes intended in the protocol |
| Other bias | Low risk | Baseline characteristics are quite balanced, didn't stop early, however the study may be underpowered to detect some secondary outcomes |
Hoving 2007.
| Methods | Multi‐centre double‐blind, randomised, placebo controlled trial | |
| Participants | 17 CYP randomised. Age range: 7 to 16, 9 females, 8 males. Cerebral palsy, G80.0, G80.8, G80.3. One child was classified on the Gross Motor Function Classification System at Level III, two at Level IV, and 14 at Level V | |
| Interventions | Route: Intrathecally via the catheter baclofen or placebo was administered Dose: During the first two test days the bolus randomly contained baclofen 25 μg or placebo. On each of the subsequent six test days the bolus contained baclofen 50 μg or placebo, then baclofen 75 μg or placebo, and, finally, baclofen 100 μg or placebo Study period: 2 first test days then 6 subsequent test days (see above) |
|
| Outcomes | Primary: Spasticity (measured by the original Ashworth scale and individually formulated problems measured by visual analogue scale (VAS) (pain (of mixed origin) and ease of care emerged from the individually formulated problems and were analysed separately using VAS) | |
| Notes | Same participants as Hoving 2009. This is dose finding part of the trial Three CYP in the CITB group and four in the Control group used oral baclofen Funding: Research Fund of the University Hospital Maastricht and Medtronic Inc., Heerlen, the Netherlands. Medtronic Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician generated randomisation lists of patients order |
| Allocation concealment (selection bias) | Low risk | An independent statistician generated the allocation and independent pharmacist prepared the medication so the allocation was concealed to the clinicians providing the medication |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, clinicians involved in administering the medication were blinded to the contents of the study medication boluses |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Pharmacist prepared placebo and treatment boluses – everyone involved in the study received numbered medication. However not stated that analyst was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The missing data is unclear with no reasons provided except the fact they excluded one male as performed open label on him (it was not blinded) |
| Selective reporting (reporting bias) | Unclear risk | No information provided on protocol so unclear |
| Other bias | Unclear risk | Results are 'confined to relatively older CYP with intractable spastic CP who rely on wheeled mobility' (Hoving 2009) |
Hoving 2009.
| Methods | Multi‐centre non‐blinded, randomised, placebo controlled trial | |
| Participants | 17 CYP randomised. Age range: 7 to 16 years, 9 females, 8 males. Cerebral palsy, G80.0, G80.8, G80.3. One child was classified on the Gross Motor Function Classification System at Level III, two at Level IV, and 14 at Level V | |
| Interventions | Route: Randomised to receive a programmable synchromed infusion pump (Medtronic, Inc., Minneapolis, MN) of continuous intrathecal baclofen (CITB) after either 1 month (CITB group) or 6 months (Control group who initially received intrathecal placebo), standard therapy continued in both groups Dose: Dose finding study previous study (Hoving 2007). Mean daily CITB dose was 67 µg (SD 25) right after pump implantation and 176 µg (SD 118) 6 months later Study period: 6 months. Tested at baseline and then 6 months |
|
| Outcomes | Primary ‐ Caregiver assistance scale of the self care domain of the Pediatric Evaluation of Disability Inventory (PEDI) and the visual analogue scale (VAS) for individually formulated problems (ease of care and pain [of mixed origin] came out of the individually formulated problems and were analysed separately using VAS) Secondary ‐ Body function and structure (measured by the original Ashworth scale), capability (measured by the functional skills scale in the self‐care domain of the PEDI) and gross motor function (measured by the Gross Motor Function Measure (GMFM)), health related quality of life (measured by the Child Health Questionnaire‐Parent Form 50 (CHQ‐PF50)) |
|
| Notes | Same participants as Hoving 2007 Three participants in the CITB group and four in the control group used oral baclofen Funding: Research Fund of the University Hospital Maastricht and Medtronic Inc., Heerlen, the Netherlands. Medtronic Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician generated randomisation lists |
| Allocation concealment (selection bias) | Low risk | The investigator who enrolled the CYP had no entry to this list and was, at the time of each enrolment, not aware of the next assignment in the sequence. For assignment, the investigator called the independent statistician who consulted the allocation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible as the intervention was a pump inserted either one month or 6 months after test treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not possible (see above) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data (no lost to follow‐up or discontinued intervention) |
| Selective reporting (reporting bias) | Unclear risk | No information provided on protocol so unclear |
| Other bias | Unclear risk | Results are 'confined to relatively older CYP with intractable spastic CP who rely on wheeled mobility' |
Letocha 2005.
| Methods | Single‐centre, non‐blinded, randomised control trial. No placebo control | |
| Participants | 18 CYP were randomised. Age range: 4 to 13 years. Osteogenesis imperfecta type III and IV included, ICD‐10: Q78.0 | |
| Interventions | Route: Intravenous pamidronate versus control (no treatment) Dosage: 10 mg/m2/day for 3 days every 3 months Study period: 12 months initially, then 7 participants in the treatment group were given an additional 6 to 21 months of IV pamidronate All participants were seen quarterly at the National Institutes of Health Clinical Center. Serum markers of bone formation and growth parameters were measured at each visit. Antero‐posterior (AP) and lateral radiographs of the spine and lower extremity long bones and DXA at vertebrae L1 to L4 were obtained at baseline and every 6 months. QCT scans of the spine were performed at the National Institutes of Health Clinical Center at 0 and 12 months |
|
| Outcomes | Primary: Vertebral DXA Z score, height, and area Secondary: Serum markers of bone formation, growth (length and sitting height to the nearest 0.01 cm), Antero‐posterior (AP) and lateral radiographs of the spine and lower extremity long bones and DXA at vertebrae L1–L4, Upper and lower extremity fracture rates, gross motor function (Brief Assessment of Motor Function (BAMF) scale) and NIH functional assessment pain score (assessing pain likely to be of mixed origin) |
|
| Notes | 4 participants in each group were receiving recombinant growth hormone (0.06 mg/kg/day for 6 days/week) Unclear what control group received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly generated numbers' |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded (blinded for vertebral area and compression measures) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not completely blinded, investigators were blinded for vertebral area and compression measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A per protocol and repeated‐measures model of analysis was used, no incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No information provided on then protocol so unclear |
| Other bias | Unclear risk | No information on gender of participants or what the control group received (no placebo), unclear sample bias |
Russo 2007.
| Methods | A multi centre, single‐blind randomised controlled trial. Standard therapy control | |
| Participants | 43 CYP randomised. Age range: 3 to 16 years, 23 male, 20 female. Hemiplegic cerebral palsy, ICD‐10: G80.8 | |
| Interventions | Route: Treatment Group (botulinum toxin A (BoNT‐A) localised injections and occupational therapy) and control ‐ received the standardised occupational therapy program only (no placebo injections) Dose: The maximal dose of BTX‐A per muscle according to Russman et al 1997 was followed; however, all of the muscles across the upper limb were injected if tone was affected. Total injected dose did not exceed 12 U/kg of body weight, to a maximum dose of 300 U of Botox (Allergan, Australia Pty Ltd). The intervention was given under anaesthetic. Study period: 6 months. Localised injections of BTX‐A into the affected upper limb and weekly occupational therapy for 4 weeks, follow‐ups at 1 month (Assessment of Motor and Process Skills, Goal Attainment Scaling, pain scale), 3 and 6 months |
|
| Outcomes | Primary: Activity participation (measured by Assessment of Motor and Process Skills [AMPS], and Goal Attainment Scaling (GAS)) Secondary: Body structure (measured using modified Ashworth Scale and the modified Tardieu Scale), self‐perception (using the Self Perception Profile for Children (older children) and the Pictorial Scale of Perceived Competence and Social Acceptance for Young Children), activity participation (using the Pediatric Evaluation of Disability Inventory (PEDI) ‐ Self care domain), quality of life (using the Pediatric Quality of Life Scale), pain (likely to be of mixed origin) (using a visual analogue scale (VAS)) and subjective function and cosmesis rating (same, better or worse since the intervention) |
|
| Notes | Funding: Financial Markets Foundation for Children and Allergan Australia Pty Ltd The authors kindly provided raw data from their study on pain outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Random assignment schedule and envelopes (concealed, opaque, and foil lined) were prepared by an independent statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind, principle outcome measures were functional measures and assessments were blinded, but all of the assessments performed by the paediatric rehabilitation specialist were unblinded and no placebo injections were administered in the control group due to the requirement of general anaesthesia. CYP and parents were unblinded and knew their assignment group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Used blinded assessors for a subset of outcomes (all outcomes except for MTS and MAS). But participants and parents knew their assignment group and could have revealed this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were on an intention‐to‐treat basis. 3 refused intervention: 2 treatment, 1 control but took part in follow up, low risk due to missing data balanced on both arms of the trial and with intention to treat analysis, missing data is unlikely to affect results |
| Selective reporting (reporting bias) | Unclear risk | No information provided on protocol so unclear |
| Other bias | High risk | No placebo, only standard therapy (no participants received no therapy) |
Seikaly 2005.
| Methods | Single‐centre double‐blinded, placebo controlled cross‐over trial | |
| Participants | 20 CYP randomised. Age range: 3 to 15 years, 11 males. Osteogenesis imperfecta types I, III and IV. ICD‐10: Q78.0 | |
| Interventions | Route: Oral alendronate versus placebo Dose: 5 mg/day (participants who weighed < 30 kg); or 10 mg/day (participants who weighed > 30 kg) administered orally with at least 8 ounces of water, 30 minutes before food intake; patients were also advised to maintain the upright position for at least 30 minutes after ingestion of the medication to reduce the chance of oesophageal irritation. All subjects were maintained on a diet with adequate daily calcium (1000 to 1300 mg/d), phosphorus (800 to 1200 mg/d), and vitamin D (400 IU/d) intake, at least 100% daily referenced intake (DRI). Study period: Participants were evaluated at baseline, then every 3 months (except when otherwise indicated), and at the conclusion of the study (2 years) |
|
| Outcomes | Primary: bone mineral density (BMD) (measured by BMD DEXA Z scores) and QoL‐functional abilities, mobility (using the modified Pediatric Evaluation of Disability Inventor (PEDI)), self‐care score (using the WeeFIM system), well‐being scores (1 to 10) and pain (likely to be nociceptive rather than neurogenic but possibly of mixed origin) including number of pain free days per month and number of days that analgesia was administered for skeletal pains (parents were asked these two questions too) Secondary: Physical evaluation, food records, blood and urine analysis, stool guaiac, renal ultrasound, skeletal survey |
|
| Notes | 17 participants completed the study 2 CYP with type I OI were excluded from the QoL analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated (SAS 6.12). Included two period cross‐over |
| Allocation concealment (selection bias) | Low risk | Pharmacist at institution assigned groups so allocation was concealed from those involved |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: other members of the research team were blinded to treatment until data analysis, and participants blinded too |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unlikely to be detected as research team assessing outcomes were blinded and radiologists detecting the fractures were also blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Repeated measures analysis of variance. 17/20 participants completed the 2 year study (unclear why dropped out) |
| Selective reporting (reporting bias) | Unclear risk | No information provided on the protocol so unclear |
| Other bias | Unclear risk | Some outcomes excluded CYP with type I OI and so only reflect types III and IV, unclear why |
Ward 2011.
| Methods | Multi‐centre randomised, double blind, parallel‐group, placebo‐controlled study | |
| Participants | 139 CYP were randomised. Age range: 4 to 19 years, 78 male, 61 female.Type I, III, or IV osteogenesis imperfecta, ICD‐10: Q78.0 | |
| Interventions | Route: Oral alendronate versus placebo Dose: Alendronate doses were 5 mg/day in participants weighing less than 40 kg and 10 mg/day for those weighing 40 kg and greater. In younger participants weighing less than 40 kg the dose was halved Study period: 24 months (follow ups every 3 months) |
|
| Outcomes | Primary: The change in LS (lumbar vertebrae 1 to 4) area lBMD z‐score. Secondary: Pain (likely to be of mixed origin) measured by number of patients with bone pain and number of days per week patients experience bone pain, cortical width determined radiographically at the midpoint of the second metacarpal, the number of radiologically confirmed fractures, the number of investigator‐reported fractures, and the change in cortical width of iliac bone determined by transiliac biopsy. Change from baseline in body mass index z‐score, and change from baseline in the relative amount of unmineralized osteoid in trabecular bone. In addition, a variety of clinical, radiological, and biochemical outcome variables were assessed. |
|
| Notes | Financial assistance: Merck Research Laboratories, Whitehouse Station (NJ). LMW is supported by a New Investigator Award through the Canadian Institutes for Health Research and a Career Development Award by the Canadian Child Health Clinician Scientist Program. Disclosure Statement: LMW has received more than $10,000 as a consultant to Novartis; F.H.G. has received more than $10,000 as Merck principal investigator; MPW owns stocks/stock options less than $10,000 in Merck; NV, NH, and AL are employees of Merck, Co. Inc. and own stocks valued at $10,000 or more in Merck | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was 3:1 ALM to placebo and stratified according to weight at baseline. No other information stated |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind‐ evaluate by radiologists who were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For missing data, the previous on‐treatment observation was carried forward”. Used “intention‐to‐treat” analysis. The number lost to follow‐up (and number of adverse events) is higher in the ALN group, 27% (29/109) versus 13% (4/26) Reason for missing outcome data may be related to true outcome e.g. pain score. The pain may be higher in those who did not return |
| Selective reporting (reporting bias) | Unclear risk | No information provided on protocol so unclear |
| Other bias | Low risk | Baseline characteristics appear balanced; didn't stop early |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adiyaman 2004 | The study had no comparator |
| Alvarez Lopez 2002 | Case study only |
| Andiran 2008 | The study had no comparator |
| Astrom 1998 | The study had no comparator |
| Astrom 2002 | The study had no comparator |
| Astrom 2010 | The study had no comparator |
| Banerjee 2002 | The study had no comparator |
| Barros 2012 | The study had no pain outcomes |
| Bhadada 2009 | The study had no comparator |
| Darnell 2008 | The author could not access the data |
| DiMeglio 2005 | The study had no pain outcomes |
| DiMeglio 2006 | The study had no pain outcomes |
| Dominguez‐Bartmess 2012 | Not a life‐limiting condition |
| Falk 2003 | The study had no comparator |
| Finkel 2005 | The study had no comparator |
| Forin 2005 | The study had no comparator |
| Gatti 2005 | The study had no pain outcomes |
| Glorieux 1998 | The study had no comparator |
| Gonzalez 2001 | The study had no comparator |
| Hoving 2006 | The study had no pain outcomes |
| Hoving 2008 | The study had no comparator |
| Jakubowska 2008 | The study had no comparator |
| Kokavec 2008 | The study had no comparator |
| Land 2007 | The study had no comparator |
| Morris 2013 | Whilst disease is life‐limiting, it is not necessary life limited in childhood or as a young person |
| Plotkin 2000 | No data on pain outcomes (tried contacting authors) |
| Pressac 2002 | The study had no comparator |
| Rauch 2009 | OI type I (mildest form of OI) so not LLC |
| Ruggiero 2007 | The study had no comparator |
| Ruggiero 2013 | The study had no comparator |
| Saarenmaa 1999 | Not enough of sample had a LLC, data not broken down, tried to contact author for raw data |
| Sakkers 2004 | The study had no pain outcomes |
| Salehpour 2010 | The study had no comparator |
| Sanger 2007 | The study had no comparator |
| Van Schaeybroeck 2000 | Sample includes only one child |
| Ward 2005 | The study had no pain outcomes |
| Zeitlin 2003 | The study had no comparator |
Characteristics of ongoing studies [ordered by study ID]
Bonouvrie 2013.
| Trial name or title | The IDYS trial |
| Methods | RCT |
| Participants | Patients aged 4 to 25 years with confirmed diagnosis of dystonic cerebral palsy |
| Interventions | Group 1: 3 months of continuous intrathecal baclofen treatment, Group 2: placebo |
| Outcomes | Primary: activities of daily living. Secondary: body functions, spasticity, pain, comfort and sleep‐related breathing disorders |
| Starting date | Not stated |
| Contact information | NTR3642 |
| Notes |
Differences between protocol and review
The screening process changed from the protocol to the review. More studies emerged from the search strategy than anticipated and so five review authors shared the screening, rather than two as stated in the protocol, with 10% of the abstracts being screened by a second review author. Also, the data were extracted by one author and all were checked by a second author (rather than as per the protocol with only the first few trials being extracted by two review authors and, when a consensus was reached, just one author continuing to extract the rest). The type of intervention was widened to include not only a treatment that was intended primarily to treat pain but treatments that acted as adjuvants, relieving pain as a secondary property. We were unable to report all secondary outcomes that were not measuring pain or safety for all studies due to the large number of unrelated outcomes reported by some studies.
The title was amended from 'Pharmacological interventions for pain for life‐limiting conditions in children and adolescents'.
Contributions of authors
| Draft the protocol | All |
| Develop a search strategy | All |
| Search for studies (usually 2 review authors) | EB, JL, HR, BC, LJ |
| Obtain copies of studies | EB |
| Select which studies to include (2 + 1 arbiter) | EB, BC, LJ |
| Extract data from studies (2 review authors) | EB, VV |
| Enter data into RevMan | EB |
| Carry out the analysis | EB |
| Interpret the analysis | All |
| Draft the final write‐up of the review | EB |
| Update the review | EB, LJ, MB‐L |
| Serve as content expert | RH, JL, RM |
| Take responsibility for grammar and language | LJ |
| Serve as methodologist | BC, VV |
| Serve as statistician | VV |
Sources of support
Internal sources
-
Marie Curie Cancer Care core grant funding, UK.
Emma Beecham's post is funded by Marie Curie Cancer Care's core grant MCCC‐FCO‐11‐U
External sources
No sources of support supplied
Declarations of interest
EB has no relevant conflicts of interest to declare. BC has no relevant conflicts of interest to declare. RH has no relevant conflicts of interest to declare; RH is currently, and has been in the past, principal investigator in a number studies of analgesic compounds but has never personally received payment or compensation for this work, or participated in promotional or other activities on behalf of manufacturers or their representatives. RM has no relevant conflicts of interest to declare. JL has no relevant conflicts of interest to declare. HR has no relevant conflicts of interest to declare. VV has no relevant conflicts of interest to declare. MBL has no relevant conflicts of interest to declare. LJ has no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bishop 2013 {published data only}
- Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;382:1424‐32. [DOI] [PubMed] [Google Scholar]
Bonouvrie 2011 {published data only}
- Bonouvrie LA, Schie PEM, Becher JG, Ouwerkerk WJR, Reeuwijk A, Jeroen Vermeulen R. Effects of intrathecal baclofen on daily care in children with secondary generalized dystonia: a pilot study. European Journal of Paediatric Neurology 2011;15(6):539‐43. [DOI] [PubMed] [Google Scholar]
Copeland 2014 {published data only}
- Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne‐Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. The Journal of Pediatrics 2014;165(1):140‐6. [DOI] [PubMed] [Google Scholar]
Hoving 2007 {published data only}
- Hoving MA, Raak EP, Spincemaille GH, Palmans LJ, Sleypen FA, Vles JS. Intrathecal baclofen in children with spastic cerebral palsy: a double‐blind, randomized, placebo‐controlled, dose‐finding study. Developmental Medicine & Child Neurology 2007;49(9):654‐9. [DOI] [PubMed] [Google Scholar]
Hoving 2009 {published data only}
- Hoving MA, Raak EP, Spincemaille GH, Palmans LJ, Becher JG, Vles JS. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: A randomised controlled trial. European Journal of Paediatric Neurology 2009;13(3):240‐6. [DOI] [PubMed] [Google Scholar]
- Hoving MA, Raak EP, Spincemaille GH, Kranen‐Mastenbroek VH, Kleef M, Gorter JW, Vles JS, Dutch Study Group on Child Spasticity. Safety and one‐year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. European Journal of Paediatric Neurology 2009;13(3):247‐56. [DOI] [PubMed] [Google Scholar]
Letocha 2005 {published data only}
- Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short‐term functional improvement. Journal of Bone and Mineral Research 2005;20:977–86. [DOI] [PubMed] [Google Scholar]
Russo 2007 {published data only}
- Russo RN, Crotty M, Miller MD, Murchland S, Flett P, Haan E. Upper‐limb botulinum toxin A injection and occupational therapy in children with hemiplegic cerebral palsy identified from a population register: A single‐blind, randomized, controlled trial. Pediatrics 2007;119(5):e1149‐e58. [DOI] [PubMed] [Google Scholar]
Seikaly 2005 {published data only}
- Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, Herring JA. Impact of alendronate on quality of life in children with osteogenesis imperfecta. Journal of Pediatric Orthopedics 2005;25(6):786‐91. [DOI] [PubMed] [Google Scholar]
Ward 2011 {published data only}
- Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. The Journal of Clinical Endocrinology and Metabolism 2011;96(2):355‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adiyaman 2004 {published data only}
- Adiyaman P, Ocal G, Berberoglu M, Evliyaoglu O, Aycan Z, Cetinkaya E. The clinical and radiological assessment of cyclic intravenous pamidronate administration in children with osteogenesis imperfecta. Turkish Journal of Pediatrics 2004;46(4):322‐8. [PubMed] [Google Scholar]
Alvarez Lopez 2002 {published data only}
- Alvarez Lopez JC, Gonzalez De Zarate J, Herrero Gento E. Treatment of pain in a case of osteogenesis imperfecta type III. Anales Españoles de Pediatría 2002;57(3):277‐8. [PubMed] [Google Scholar]
Andiran 2008 {published data only}
- Andiran N, Alikasifoglu A, Gonc N, Ozon A, Kandemir N, Yordam N. Cyclic pamidronate therapy in children with osteogenesis imperfecta: results of treatment and follow‐up after discontinuation. Journal of Pediatric Endocrinology and Metabolism 2008;21(1):63‐72. [DOI] [PubMed] [Google Scholar]
Astrom 1998 {published data only}
- Astrom, E, Soderhall S. Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatrica 1998;87(1):64‐8. [DOI] [PubMed] [Google Scholar]
Astrom 2002 {published data only}
- Astrom E, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Archives of Disease in Childhood 2002;86(5):356‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Astrom 2010 {published data only}
- Astrom E, Magnusson P, Eksborg S, Söderhäll S. Biochemical bone markers in the assessment and pamidronate treatment of children and adolescents with osteogenesis imperfecta. Acta Paediatrica 2010;99(12):1834‐40. [DOI] [PubMed] [Google Scholar]
Banerjee 2002 {published data only}
- Banerjee I, Shortland GJ, Evans WD, Gregory JW. Osteogenesis imperfecta and intravenous pamidronate. Archives of Disease in Childhood 2002;87(6):562–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barros 2012 {published data only}
- Barros ER, Saraiva GL, Oliveira TP, Lazaretti‐Castro M. Safety and efficacy of a 1‐year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2012;25(5‐6):485‐91. [DOI] [PubMed] [Google Scholar]
Bhadada 2009 {published data only}
- Bhadada SK, Santosh R, Bhansali A, Upreti V, Dutta P. Osteogenesis imperfecta. Journal of the Association of Physicians of India 2009;57:33‐6. [PubMed] [Google Scholar]
Darnell 2008 {published data only}
- Darnell CM, Thompson J, Stromberg D, Roy L, Sheeran P. Effect of low‐dose naloxone infusion on fentanyl requirements in critically ill children. Pediatrics 2008;121(5):e1363‐71. [DOI] [PubMed] [Google Scholar]
DiMeglio 2005 {published data only}
- Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2005;18:43‐53. [DOI] [PubMed] [Google Scholar]
DiMeglio 2006 {published data only}
- DiMeglio LA, Peacock M. Two‐year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. Journal of Bone and Mineral Research 2006;21(1):132‐40. [DOI] [PubMed] [Google Scholar]
Dominguez‐Bartmess 2012 {published data only}
- Dominguez‐Bartmess SN, Tandberg D, Cheema AM, Szalay EA. Efficacy of alendronate in the treatment of low bone density in the pediatric and young adult population. The Journal of Bone and Joint Surgery. American volume 2012;94(10):e62. [DOI] [PubMed] [Google Scholar]
Falk 2003 {published data only}
- Falk MJ, Heeger S, Lynch KA, DeCaro KR, Bohach D, Gibson KS. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics 2003;111(3):573‐8. [DOI] [PubMed] [Google Scholar]
Finkel 2005 {published data only}
- Finkel JC, Finley A, Greco C, Weisman SJ, Zeltzer L. Transdermal fentanyl in the management of children with chronic severe pain. Cancer 2005;104(12):2847‐57. [DOI] [PubMed] [Google Scholar]
Forin 2005 {published data only}
- Forin V, Arabi A, Guigonis V, Filipe G, Bensman A, Roux C. Benefits of pamidronate in children with osteogenesis imperfecta: an open prospective study. Joint, Bone, Spine: Revue du Rhumatisme 2005;72(4):313‐8. [DOI] [PubMed] [Google Scholar]
Gatti 2005 {published data only}
- Gatti D, Viapiana O, Idolazzi L, Fracassi E, Adami S. Neridronic acid for the treatment of bone metabolic diseases. Expert Opinion on Drug Metabolism and Toxicology 2009;5(10):1305‐11. [DOI] [PubMed] [Google Scholar]
Glorieux 1998 {published data only}
- Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. New England Journal of Medicine 1998;339(14):947‐52. [DOI] [PubMed] [Google Scholar]
Gonzalez 2001 {published data only}
- Gonzalez E, Pavia C, Ros J, Villaronga M, Valls C, Escola J. Efficacy of low dose schedule pamidronate infusion in children with osteogenesis imperfecta. Journal of Pediatric Endocrinology 2001;14(5):529‐33. [DOI] [PubMed] [Google Scholar]
Hoving 2006 {published data only}
- Hoving MA, Kranen‐Mastenbroek VHJM, Raak EPM, Spincemaille GHJJ, Hardy ELM, Vles JSH. Placebo controlled utility and feasibility study of the H‐reflex and flexor reflex in spastic children treated with intrathecal baclofen. Clinical Neurophysiology 2006;117(7):1508‐17. [DOI] [PubMed] [Google Scholar]
Hoving 2008 {published data only}
- Hoving MA, Raak EP, Spincemaille GH, Kranen‐Mastenbroek VH, Kleef M, Gorter JW, Vles JS. Safety and one‐year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. European Journal of Paediatric Neurology 2009;13(3):247‐56. [DOI] [PubMed] [Google Scholar]
Jakubowska 2008 {published data only}
- Jakubowska‐Pietkiewicz E, Chlebna‐Sokol D. New trends in the treatment of osteogenesis imperfecta type III ‐ own experience. Ortopedia Traumatologia Rehabilitacja 2008;10(6):593‐601. [PubMed] [Google Scholar]
Kokavec 2008 {published data only}
- Kokavec M, Novorolsky K, Pribilincova Z. Role of an interdisciplinary approach in the healing of long bone fractures in patients with osteogenesis imperfecta. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2008;75(3):185‐9. [PubMed] [Google Scholar]
Land 2007 {published data only}
- Land C, Rauch F, Travers R, Glorieux FH. Osteogenesis imperfecta type VI in childhood and adolescence: Effects of cyclical intravenous pamidronate treatment. Bone 2007;40(3):638‐44. [DOI] [PubMed] [Google Scholar]
Morris 2013 {published data only}
- Morris CR, Kuypers FA, Ansari M, Sweeters N, Stewart M, Gildengorin G, et al. A randomized, placebo‐controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso‐occlusive pain episodes. Red Cell Disorders 2013;98:1375‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotkin 2000 {published data only}
- Plotkin H, Rauch F, Bishop NJ, Monpetit K, Ruck‐Gibis J, Travers R, Glorieux FH. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. The Journal of Clinical Endocrinology and Metabolism 2000;85(5):1846–50. [DOI] [PubMed] [Google Scholar]
Pressac 2002 {published data only}
- Pressac M, Boutten A, Couderc R, Forin V. Biological monitoring of osteogenesis imperfecta. [French] Suivi de l'osteogenese imparfaite traitee. Immuno‐Analyse et Biologie Specialisee 2002;17(3):160‐4. [Google Scholar]
Rauch 2009 {published data only}
- Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. Journal of Bone and Mineral Research 2009;24(7):1282‐9. [DOI] [PubMed] [Google Scholar]
Ruggiero 2007 {published data only}
- Ruggiero A, Barone G, Liotti L, Chiaretti A, Lazzareschi I, Riccardi R. Safety and efficacy of fentanyl administered by patient controlled analgesia in children with cancer pain. Supportive Care in Cancer 2007;15(5):569‐73. [DOI] [PubMed] [Google Scholar]
Ruggiero 2013 {published data only}
- Ruggiero A, Coccia P, Arena R, Maurizi P, Battista A, Ridola V, et al. Efficacy and safety of transdermal buprenorphine in the management of children with cancer‐related pain. Pediatric Blood & Cancer 2013;60(3):433‐7. [DOI] [PubMed] [Google Scholar]
Saarenmaa 1999 {published data only}
- Saarenmaa E, Huttunen P, Leppäluoto J, Meretoja O, Fellman V. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: A randomized trial. The Journal of Pediatrics 1999;134(2):144–50. [DOI] [PubMed] [Google Scholar]
Sakkers 2004 {published data only}
- Sakkers R, Kok D, Engelbert R, Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functiona outcome with olpadronate in children with osteogenesis imperfecta: a 2‐year randomised placebo‐controlled study. Lancet 2004;363(9419):1427‐31. [DOI] [PubMed] [Google Scholar]
Salehpour 2010 {published data only}
- Salehpour S, Tavakkoli S. Cyclic pamidronate therapy in children with osteogenesis imperfecta. Journal of Pediatric Endocrinology 2010;23(1‐2):73‐80. [DOI] [PubMed] [Google Scholar]
Sanger 2007 {published data only}
- Sanger TD, Bastian A, Brunstrom J, Damiano D, Delgado M, Dure L, et al. the Child Motor Study Group. Prospective open‐label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. Journal of Child Neurology 2007;22(5):530‐7. [DOI] [PubMed] [Google Scholar]
Van Schaeybroeck 2000 {published data only}
- Schaeybroeck P, Nuttin B, Lagae L, Schrijvers E, Borghgraef C, Feys P. Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo‐controlled, double‐blind study. Neurosurgery 2000;46(3):603‐12. [DOI] [PubMed] [Google Scholar]
Ward 2005 {published data only}
- Ward LM, Denker AE, Porras A, Shugarts S, Kline W, Travers R, et al. Single‐dose pharmacokinetics and tolerability of alendronate 35‐ and 70‐milligram tablets in children and adolescents with osteogenesis imperfecta type I. The Journal of Clinical Endocrinology and Metabolism 2005;90(7):4051‐6. [DOI] [PubMed] [Google Scholar]
Zeitlin 2003 {published data only}
- Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics 2003;111(51):1030‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bonouvrie 2013 {published data only}
- Bonouvrié LA, Becher JG, Vles JSH, Boeschoten K, Soudant D, Groot V, et al. Intrathecal baclofen treatment in dystonic cerebral palsy: a randomized clinical trial: the IDYS trial. BMC Pediatrics 2013;13:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACT 2009
- Association for Children’s Palliative Care. Children’s palliative care definitions. http://www.togetherforshortlives.org.uk/assets/0000/1638/CPC_definitions.pdf (accessed 20 June 2012).
Anderson 2008
- Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Pediatric Anesthesia 2008;18(10):915‐21. [DOI] [PubMed] [Google Scholar]
APPM 2015
- Association for Paediatric Palliative medicine (APPM) Master Formulary 2015. Available at: http://www.appm.org.uk/10.html. 3rd.
Ballentine 2012
- Ballantine N, Daglish EB. Chapter 17: Using medications in children. In: Goldman A, Hain R, Liben S editor(s). Oxford Textbook of Palliative Care for Children. 2nd Edition. Oxford: Oxford University Press, 2012:234‐46. [Google Scholar]
Beretta 2010
- Beretta S, Polastri D, Clerici CA, Casanova M, Cefalo G, Ferrari A, et al. End of life in children with cancer: experience at the Pediatric Oncology Department of the Istituto Nazionale Tumori in Milan. Pediatric Blood & Cancer 2010;54(1):88‐91. [DOI] [PubMed] [Google Scholar]
BNF 2012
- Paediatric Formulary Committee. British National Formulary for Children 2012‐2013. London: British Medical Association, the Royal Pharmaceutical Society of Great Britain, the Royal College of Paediatrics and Child Health, and the Neonatal and Paediatric Pharmacists Group, July 2012. [Google Scholar]
Bouwmeester 2004
- Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NHG. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. British Journal of Anaesthesia 2004;92:208‐17. [DOI] [PubMed] [Google Scholar]
Bradshaw 2005
- Bradshaw G, Hinds PS, Lensing S, Gattuso JS, Razzouk BI. Cancer‐related deaths in children and adolescents. Journal of Palliative Medicine 2005;8(1):86‐95. [DOI] [PubMed] [Google Scholar]
Brook 2012
- Brook L, Aindow A, Jassel S. Prescribing in paediatric palliative care: an association for paediatric palliative medicine survey. BMJ Supportive & Palliative Care 2012; Vol. 2 Suppl 1:A24.
Cochrane 2007
- Cochrane H, Liyanage S, Nantambi R. Palliative Care Statistics for Children and Young Adults. Health and Care Partnerships Analysis. London: Department of Health, 2007.
Cochrane 2011
- Cochrane Pain, Palliative and Supportive Care Review Group. Authoring or Assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group [updated September 2011]. http://papas.cochrane.org/sites/papas.cochrane.org/files/uploads/L%20‐%20PaPaSAuthor%26RefereeGuidance.pdf (accessed 19 July 2013).
Drake 2003
- Drake R, Frost J, Collins JJ. The symptoms of dying children. Journal of Pain and Symptom Management 2003;26(1):594‐603. [DOI] [PubMed] [Google Scholar]
Feudtner 2011
- Feudtner C, Kang TI, Hexem KR, Friedrichsdorf SJ, Osenga K, Siden H, et al. Pediatric palliative care patients: a prospective multicenter cohort study. Pediatrics. 2011;127(6):1094‐101. [DOI] [PubMed] [Google Scholar]
Fraser 2012
- Fraser LK, Miller M, Hain R, Norman P, Aldridge J, McKinney PA, et al. Rising national prevalence of life‐limiting conditions in children in England. Pediatrics 2012;129(4):e923‐e9. [DOI] [PubMed] [Google Scholar]
Fraser 2014
- Fraser LK, Lidstone V, Miller M, Aldridge J, Norman P, McKinney PA, Parslow RC. Patterns of diagnoses among children and young adults with life‐limiting conditions: A secondary analysis of a national dataset. Palliative Medicine 2014;28(6):513–20. [DOI] [PubMed] [Google Scholar]
Goldman 2006
- Goldman A, Hewitt M, Collins GS, Childs M, Hain R. Symptoms in children/young people with progressive malignant disease: United Kingdom Children's Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics 2006;117(6):e1179‐e86. [DOI] [PubMed] [Google Scholar]
Hadley 2013
- Hadley G, Derry S, Moore RA, Wiffen PJ. Transdermal fentanyl for cancer pain. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010270.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hain 2011
- Hain R, Ballantine N. Children: different-but the same species. European Journal of Palliative Care 2011;18(6):288‐92. [Google Scholar]
Hain 2013
- Hain R, Devins M, Hastings R, Noyes J. Paediatric palliative care: development and pilot study of a 'Directory' of life‐limiting conditions. BMC Palliative Care 2013;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hechler 2008
- Hechler T, Blankenburg M, Friedrichsdorf SJ, Garske D, Hubner B, Menke A, et al. Parents' perspective on symptoms, quality of life, characteristics of death and end‐of‐life decisions for children dying from cancer. Klinische Padiatrie 2008;220(3):166‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16. Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Himelstein 2006
- Himelstein BP. Palliative care for infants, children, adolescents, and their families. Journal of Palliative Medicine 2006;9(1):163‐81. [DOI] [PubMed] [Google Scholar]
Hoare 2010
- Hoare BJ, Wallen MA, Imms C, Villanueva E, Rawicki HB, Carey L. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE). Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003469.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
IASP 2012
- International Association for the Study of Pain. Taxonomy. http://www.iasp‐pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm (accessed 15 September 2012).
Jacqz‐Aigrain 2006
- Jacqz‐Aigrain E, Anderson BJ. Pain control: non‐steroidal anti‐inflammatory agents. Seminars in Fetal and Neonatal Medicine 2006;11(4):251‐9. [DOI] [PubMed] [Google Scholar]
Jalmsell 2006
- Jalmsell L, Kreicbergs U, Onelöv E, Steineck G, Henter J‐I. Symptoms affecting children with malignancies during the last month of life: a nationwide follow‐up. Pediatrics 2006;117(4):1314‐20. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidiset JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151:4. [DOI] [PubMed] [Google Scholar]
Mashayekhi 2009
- Mashayekhi SO, Ghandforoush‐Sattari M, Routledge PA, Hain RD. Pharmacokinetic and pharmacodynamic study of morphine and morphine 6‐glucuronide after oral and intravenous administration of morphine in children with cancer. Biopharmaceutics & Drug Disposition 2009;30(3):99‐106. [DOI] [PubMed] [Google Scholar]
Mathew 2014
- Mathew E, Kim E, Goldschneider KR. Pharmacological treatment of chronic non‐cancer pain in pediatric patients. Paediatric Drugs 2014;16(6):457‐71. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath PJ, Walco G, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain 2008;9:771‐83. [DOI] [PubMed] [Google Scholar]
Phillipi 2008
- Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005088.pub2] [DOI] [PubMed] [Google Scholar]
Ravens‐Sieberer 2010
- Ravens‐Sieberer U, Wille N, Badia X, Bonsel G, Burström, K, Cavrini G, et al. Feasibility, reliability, and validity of the EQ‐5D‐Y: results from a multinational study. Quality of Life Research 2010;19(6):887‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2008
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence‐Based Medicine 2008;13(6):162‐3. [DOI] [PubMed] [Google Scholar]
Stinson 2006
- Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self‐report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1‐2):143‐57. [DOI] [PubMed] [Google Scholar]
Varni 1999
- Varni JW, Seid M, Rode CA. The PedsQL (TM): measurement model for the pediatric quality of life inventory. Medical Care 1999;37(2):126‐39. [DOI] [PubMed] [Google Scholar]
Von Baeyer 2009
- Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS‐11) for children's self‐reports of pain intensity. Pain 2009;143:223‐7. [DOI] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization. Cancer Pain Relief, Second Edition, With a Guide to Opioid Availability. Geneva: World Health Organisation, 1996.
WHO 2012
- World Health Organisation. WHO Guidelines on Pharmacological Treatment of Persisting Pain in Children With Medical Illnesses. Geneva: World Health Organisation, 2012. [PubMed]
Wolfe 2000
- Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem‐Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. New England Journal of Medicine 2000;342(5):326‐33. [DOI] [PubMed] [Google Scholar]
Wong 1988
- Wong D. Baker C. Pain in children: comparison of assessment scales. Pediatric Nursing 1988;14(1):9‐17. [PubMed] [Google Scholar]
Zernikow 2009
- Zernikow B, Michel E, Craig F, Anderson BJ. Pediatric palliative care: use of opioids for the management of pain. Pediatric Drugs 2009;11(2):129‐51. [DOI] [PubMed] [Google Scholar]


