Introduction
Peripheral neuropathy is caused by damage to peripheral nerves and is an important cause of neuropathic pain including hyperalgesia, allodynia, and dysesthesias[76]. Peripheral neuropathy is the most common neurodegenerative disorder and is increasing in prevalence due to the epidemic of diabetes, which leads to peripheral neuropathy in most patients, as well as the increase cancer survivorship among patients treated with neurotoxic chemotherapy agents. Unfortunately, there are not good therapies for neuropathic pain, which is a complex syndrome that is often multidrug-resistant[10,12]. As such, there is great interest in preventing the development of neuropathic pain by protecting at risk patients from developing peripheral neuropathy. Many peripheral neuropathies are due to loss of the long axons, and so defining the mechanism of axon degeneration may identify therapeutic targets for preserving vulnerable axons and preventing the development of peripheral neuropathy[21,56].
Axon degeneration is a self-destructive process that is activated in peripheral neuropathies[14,22]. Conceptually this degeneration program is akin to the apoptotic pathway—it is a biochemical pathway driving the dismantling of injured axons in much the same way that the apoptotic pathway orchestrates the programmed death of dysfunctional cells, although the molecular mechanisms are distinct. Recent genetic and biochemical studies have identified the central components of this axon degeneration program[26]. This review will focus on SARM1, the central executioner of the axon degeneration pathway, highlighting evidence that SARM1 is essential for the development of peripheral neuropathy in mouse models of disease, the function of SARM1 as an injury-activated NADase enzyme, and the regulatory network of axon survival and axon degeneration proteins that control SARM1 activation and hence the balance between axon maintenance and axon loss. These mechanistic insights reveal clear strategies for the development of therapies to block axon degeneration and prevent the development of peripheral neuropathy.
Throughout this review we use the term “axon degeneration” to refer exclusively to the SARM1-dependent axon self-destruction pathway that promotes pathological axon loss rather than the distinct Bax- and caspase -dependent pathway that promotes developmental axon pruning. However, there is some molecular overlap between these pathways, and so there may be an interesting interplay between the mechanisms of axon loss in disease and development that will be an important topic for future study[46,47,51,55,62].
Discussion
SARM1 is the central executioner of axon degeneration following nerve injury and in models of peripheral neuropathy
Genetic screens in Drosophila and primary mouse neurons identified SARM1 (Sterile alpha and TIR motif–containing 1) as an essential component of the axon degeneration program. Axon degeneration is dramatically delayed after nerve transection in the absence of SARM1 in both flies and mice, demonstrating an evolutionary conservation of this pro-degenerative function across 400 million years of evolution[27,44]. In cultured neurons, SARM1 is also required for axon loss in response to neurotoxic chemotherapeutics and mitochondrial toxins[27,57]. Not only is SARM1 required for axon degeneration, but activation of SARM1 is sufficient to trigger axon degeneration in the absence of injury consistent with the hypothesis that SARM1 is the executioner of degeneration program[25,73]. In addition to triggering axon loss, SARM1 also promotes a non-apoptotic form of neuronal cell death known as Sarmoptosis in response to hypoxia[33], infection with neurotropic viruses[41], mitrochondrial dysfunction[57], and in forms of programmed non-apoptotic cell death in C. elegans[9].
While the link between SARM1 and axon degeneration was originally identified in response to axotomy, recent studies show that SARM1 also mediates axon loss in mouse models of peripheral neuropathy in response to the chemotherapy agents vincristine and paclitaxel and in a model metabolic syndrome[23,60]. Geisler et al developed an in vivo model of chemotherapy-induced peripheral neuropathy (CIPN) in response to vincristine treatment that models moderately severe CIPN in human patients. SARM1 knockout mice are completely protected from developing this neuropathy[23]. In wild-type mice, four weeks of vincristine treatment induces pronounced mechanical allodynia and thermal hyperalgesia, a significant decrease in tail compound nerve action potential amplitude, loss of intraepidermal nerve fibers and significant degeneration of myelinated axons in the distal sural and toe nerves. These findings are consistent with the development of a sensory predominant distal axonal neuropathy. In SARM1 knockout mice, the development of mechanical allodynia and heat hypersensitivity is blocked and the loss in tail CNAP amplitude is prevented. Moreover, SARM1 knockout mice do not lose unmyelinated fibers in the skin or myelinated axons in the sural or toe nerves after vincristine[23]. This effect is not limited to vincristine, as the absence of SARM1 also blocks the development of neuropathy in response to paclitaxel and high fat diet[60]. These results reveal that subacute/chronic axon loss occurs via a SARM1-mediated axonal destruction pathway. Hence, SARM1 not only mediates classical Wallerian degeneration but also the dying-back axonopathy, which is the form of axon loss characteristic of peripheral neuropathy and other neurodegenerative diseases such as ALS and Parkinson’s. In addition, the SARM1 knockout mice are viable, have a normal lifespan, and show no obvious phenotype in the absence of injury, suggesting that inhibiting SARM1 may be safe[27,30,44]. These findings strongly support the premise that targeting the SARM1 pathway is an exciting therapeutic option to prevent CIPN, other peripheral neuropathies, and potentially other neurodegenerative diseases of axon loss[32,78]. The central role of SARM1 in promoting degeneration has motivated detailed studies of its mechanism of action.
SARM1 is an injury-activated NAD+ consuming enzyme
SARM1 is an intracellular protein with an N-terminal region with multiple armadillo repeat motifs (ARMs), two tandem sterile alpha motif (SAM) domains, and a C-terminal toll-interleukin receptor (TIR) domain. Detailed structure function analysis has defined the roles of each domain for the activity of SARM1[27]. Among these domains, only TIR domains have been previously implicated in signaling, present in Toll-like receptors and adaptors where they serve as scaffolds to recruit proteins that activate innate immune signaling[42]. As with innate immune receptors, the SARM1 TIR domain is the pro-degenerative signaling region of the SARM1 molecule. The SARM1 SAM domains mediate multimerization of SARM1, and this multimerization is essential for SARM1 activity. Finally, the N-terminal ARM region of SARM1 is autoinhibitory, binding to the SARM1 TIR domain and blocking its function[27,58]. Upon injury, the N-terminal autoinhibition is relieved, allowing TIR-TIR domain activation and promotion of degeneration. These studies defined the key domains of SARM1, but left open the central question—how does the SARM1 TIR domain promote axon degeneration?
A recent breakthrough in the field identified the SARM1 TIR domain as the founding member of a new class of NAD+ consuming enzymes, and demonstrated that this activity is required for SARM1-dependent axon degeneration[17,18]. NAD+ is a metabolite that is an essential cofactor for many oxidation/reduction reactions in the cell. More recently, it was discovered that NAD+ can also serve as a substrate for NAD+ cleaving enzymes (NADases) such as PARPs and Sirtuins. Following axotomy, NAD+ levels drop well before there are morphological changes to the axon[66], and SARM1 is required for this loss of NAD+ both in vitro and in vivo[25]. Moreover, SARM1 activation via chemically induced TIR dimerization triggers depletion of neuronal NAD+ within minutes, followed by ATP loss and later by morphological destruction of the axon. This SARM1-induced NAD+ depletion occurs via chemical breakdown of NAD+ rather than synthetic blockade or efflux[25]. TIR domains serve as scaffolding proteins in innate immune signaling, and so the demonstration that dimerized SARM1 TIR domains trigger NAD+ loss suggested that they bind and activate an associated NADase enzyme. Surprisingly, Essuman et al. demonstrated that rather than SARM1 TIR binding a known NADase, the SARM1 TIR domain is the enzyme that cleaves NAD+, generating nicotinamide and the calcium-mobilizing products ADPR or cADPR[17]. While this was the first demonstration that a TIR domain can have enzymatic activity, subsequent studies demonstrated that TIR domains from bacteria and archaebacteria are active NADases, demonstrating that this is the primordial function of this ancient protein domain[18]. In SARM1, the glutamic acid at position 642 of SARM1 is required for its enzymatic activity in vitro. When a catalytically-dead SARM1 is reintroduced into SARM1 KO neurons, this mutant protein cannot mediate injury-dependent NAD+ loss or axon degeneration. Hence, the catalytic activity of SARM1 is required for axon degeneration, consistent with the model that degeneration is triggered either by the loss of NAD+ or by the generation of the bioactive products ADPR and cADPR[17]. This is an exciting finding, as it implies that a chemical inhibitor of the SARM1 enzyme should be an effective inhibitor of axon degeneration.
A model for regulation of the SARM1 axon degeneration pathway
The countervailing actions of axonal survival and axonal degeneration factors determines whether an axon will be maintained or destroyed. Axon survival factors promote axonal maintenance and so inhibition or genetic loss of such survival factors promotes axon degeneration. In contrast, axon degeneration factors promote axonal loss and so inhibition or genetic loss of such degeneration factors promotes axonal survival. SARM1 is the central axon degeneration factor. A number of other axon survival and axon degeneration proteins have been identified, and recently interactions between SARM1 and these other proteins has defined a unified axon degeneration pathway.
The first identified axon survival factor is the Wlds protein, which was identified as the product of the causative mutation in mice bearing the autosomal dominant “Wallerian Degeneration Slow” (Wlds) that dramatically delays axonal degeneration[37]. Wlds is a chimeric fusion protein comprised of the NAD biosynthetic enzyme nicotinamide mononucleotide adenyltransferase (NMNAT1) and a fragment of the ubiquitination factor UBE4B[15]. While there was initially controversy as to the functional domains of the Wlds protein, it is now clear that NMNAT1 is the axoprotective component[2] and that mislocalization of NMNAT1 into the axon is profoundly axoprotective[5,49]. While Wlds is not a natural protein, Gilley et al. demonstrated that it substitutes for NMNAT2, an endogenous axon survival factor with the same enzymatic function as NMNAT1[28]. NMNAT2 is delivered to the axon by fast axonal transport and is a labile protein with a very short half-life. Upon axotomy or other insults that inhibit axonal transport, delivery of NMNAT2 to the axon is impaired, preexisting NMNAT2 is degraded, and axon degeneration begins. Because Wlds and axonally-targeted NMNAT1 are much more stable than NMNAT2, their expression substitutes for the loss of NMNAT2. In subsequent genetic studies, Gilley and colleagues showed that loss of NMNAT2 likely activates SARM1[29,30]. NMNAT2 knockout mice are embryonic lethal with dramatic axonal defects. However, NMNAT2, SARM1 double knockout mice are viable, have a normal lifespan, and maintain healthy axons and synapses. Similarly, genetic knockout of NMNAT2 in cultured neurons triggers axon degeneration, but only in the presence of SARM1. These findings show that NMNAT2 is only necessary when SARM1 is present, suggesting that either a) NMNAT2-mediated NAD+ biosynthesis compensates for basal SARM1 NADase activity or b) NMNAT2 inhibits injury-dependent activation of the SARM1 NADase. Complementary biochemical studies from Sasaki et al. distinguished between these possibilities by developing an NAD+ flux assay allowing them to assay separately NAD+ biosynthesis and NAD+ consumption in both healthy and injured axons. They demonstrate that injury activates the SARM1 NADase, and that NMNAT enzymes block this injury-induced activation of the SARM1 [48]. Together, these findings identify NMNAT2 as an axon survival factor that blocks activation of SARM1, although the molecular mechanism of inhibition is unknown. This model implies that injury and disease can induce degeneration by blocking the delivery of NMNAT2 to the axon, and so may explain why the distal most portion of axons are the first to degenerate in dying-back axonopathies.
Having defined the relationship between NMNAT2 and SARM1, it is now possible to understand the mechanism-of-action of the MAP3 kinase DLK (dual leucine zipper kinase) and the ubiquitin ligase Phr1, two pro-degenerative factors. DLK is an important neuronal stress kinase[4,20], is a key regulator of the axon injury response program[31,53,61,70,72], and was the first gene identified that promotes axon degeneration[39]. DLK and the closely related MAP3K LZK[67] activate a JNK signaling pathway that promotes axon degeneration by speeding the turnover of axonal survival factors[54,59,64]. Inhibition of DLK/LZK either genetically or pharmacologically boosts the level of axonal NMNAT2 which in turn inhibits SARM1. Consistent with this model, the protective effect of inhibiting this MAP kinase pathway is lost in the absence of NMNAT2[59,64]. The atypical SCF E3 ligase complex Phr1/Fbxo45/Skp1a, originally identified as a key regulator of synapse development[13,50,65,68,77], also promotes axon degeneration by speeding the turnover of NMNAT2[6,11,16,69,71]. Inhibiting this ligase boosts the levels of NMNAT2 and its fly ortholog and leads to long-lasting protection of injured axons in both flies and mice[6,69]. As with the MAP kinase pathway, this protection is lost in the absence of NMNAT2. The finding that both DLK/LZK MAP Kinase signaling and the Phr1 ligase promote axon degeneration by speeding the turnover of NMNAT2 would be consistent with these proteins working together to regulate NMNAT2 levels. Surprisingly, this is not the case. Instead, the MAPK pathway and the Phr1 ligase independently target distinct pools of NMNAT2. NMNAT2 can be palmitoylated and this is a key regulator of its axonal transport and turnover[38]. The DLK/LZK MAP kinase pathway selectively promotes the turnover of palmitoylated NMNAT2, while the Phr1 ligase promotes the turnover of non-palmitoylated NMNAT2. Dual inhibition of the MAPK pathway and the Phr1 ligase leads to a very large increase in NMNAT2 levels and dramatically enhanced axonal protection[59].
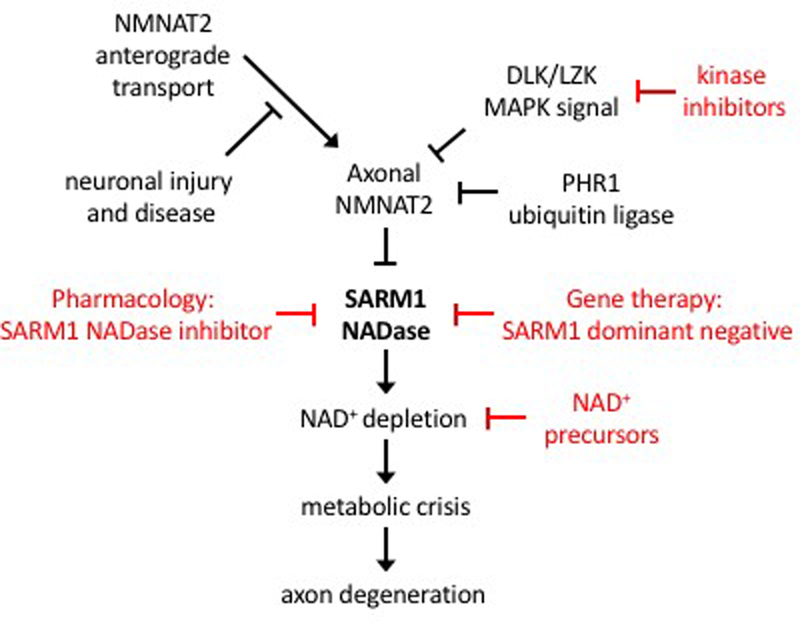
These mechanistic insights into the function of axon survival and axon degeneration proteins support a unified model for a core axon degeneration program (Figure 1). SARM1 is the central executioner of the axon degeneration program whose activation triggers NAD+ cleavage and a subsequent metabolic catastrophe. SARM1 is inhibited by the delivery of NMNAT2 via axon transport. Injury or disease that impairs axon transport will reduce the levels of NMNAT2 and promote degeneration. The neuronal stress kinase DLK/LZK pathway and the ubiquitin ligase Phr1 promote the turnover of NMNAT2 and so tune the susceptibility of axons to degenerate. Other proteins have been identified that regulate axon degeneration [7,8,19,40,43,47,63]. It will be interesting to determine whether these additional factors interact with this core degeneration program, as suggested for the recently described Axundead protein[43], or act via independent mechanisms.
Figure 1. A Unified Model of the Axon Degeneration Pathway and Sites for Therapeutic Intervention.
The SARM1 NADase is the central executioner of the axon degeneration pathway. Upon activation, SARM1 triggers NAD+ depletion, which elicits a metabolic crisis in the axon and subsequent axon degeneration. SARM1 activation is blocked in the presence of axonal NMNAT2, which is a labile protein that must be constantly delivered via fast axonal transport from the cell body. Neuronal injury and disease can interrupt delivery of NMNAT2 to the axon, allowing for SARM1 activation and induction of axon degeneration. The turnover of axonal NMNAT2 is promoted by the activity of the neuronal stress kinases DLK and LZK as well as the PHR1 ubiquitin ligase complex. There are a number of potential sites of therapeutic intervention in this pathway (red). These include inhibitors of the SARM1 NADase, dominant negative versions of SARM1 that block its activation, kinase inhibitors of DLK/LZK, and NAD+ precursors to help maintain NAD+ levels.
Therapeutic Targets in the Axon Degeneration Pathway
Having defined a core axon degeneration pathway, we will now consider scenarios in which it could be useful to inhibit this pathway, and potential methods for developing therapies targeting the pathway. The requirement of SARM1 for the development of chemotherapy-induced peripheral neuropathy in mouse models highlights CIPN as an exciting clinical target. Moreover, blocking axonal degeneration is a particularly attractive treatment strategy for CIPN, because the axonal insult is limited to the period of treatment and axoprotective strategies can be initiated prior to this insult. Unlike other side effects of chemotherapy, CIPN often persists for the life of the patient, and so preventing the development of CIPN should significantly improve the quality of life for cancer survivors[3,52]. In addition, CIPN is the dose limiting side effect for many chemotherapeutics, so the development of neuropathy often forces a decrease in the dose or even complete cessation of treatment with the offending agent. Such changes in dosing regimen can dramatically decrease the effectiveness of cancer therapy. Therefore, methods to prevent CIPN should allow for the full dose of chemotherapy and, hence, improved cancer survivorship.
While CIPN is an ideal target for axoprotective therapy, such an approach could also be useful for the prevention or treatment of other neuropathies. Diabetic and genetic neuropathies tend to be slowly progressive, and patients can be identified early in the course of the disease. We speculate that upon diagnosis a relatively small number of axons are affected. If so, then treatment with an axoprotective agent could block the degeneration of surviving axons and halt the progression of the neuropathy. Since the peripheral nervous system axons can regenerate, it is even possible that inhibiting further degeneration may allow for damaged axons to regenerate and thereby lead to improvements in the symptoms of a preexisting neuropathy. While it is attractive to speculate about the potential benefits of axoprotection for the treatment of peripheral neuropathy, these are complex diseases that lead to many aberrations in neuronal function[76]. The role of axon degeneration in the human disorder will not be clear until effective treatments to block such degeneration are developed.
Mechanistic insights into the axon degeneration program highlight a number of potential therapeutic targets (Figure 1). As the central executioner of axon degeneration, SARM1 is a particularly attractive target. The identification of SARM1 as an NADase enzyme suggests that inhibitors of enzymatic function could block axon degeneration. Selective inhibitors have been developed for other families of NADases[36,74], and so SARM1 is likely a druggable target. In addition to small molecule inhibitors, Geisler et al. recently developed a very potent dominant negative version of SARM1 that blocks the activation of wild type SARM1 and so protects axons[24]. Gene therapy using AAV-mediated delivery of this SARM1 dominant negative in the mouse provided long-lasting axonal protection following axotomy, the strongest known trigger of axon degeneration. An alternative to blocking SARM1-mediated NAD+ destruction is to compensate for the loss of NAD+ by boosting its biosynthesis. In cultured neuron models, NAD+ precursors can provide some axon protection[35,48]. These NAD+ precursors are natural products that are considered safe by the FDA, and there is great interest in their potential value for treating or preventing a variety of diseases[75]. In addition to directly targeting SARM1, therapies could also target upstream pathways regulating SARM1. There are efforts to develop drugs that can boost the function or expression of NMNAT2[1]. Targeting the degradation of NMNAT2 is another alternative. Potent inhibitors of DLK/LZK have been developed that block MAPK-dependent neuronal cell death. These inhibitors are being investigated as treatments for neurodegenerative disease of the central nervous system[34,45]. The effect of DLK/LZK inhibitors on NMNAT2 levels suggests that they could be useful for inhibiting the axon loss in peripheral neuropathies. Finally, inhibiting the Phr1 ligase would in theory also boost NMNAT2 levels, however ubiquitin ligases are poor drug targets. While there are great challenges ahead before treatments to block axon degeneration are a reality, the tremendous progress in understanding the fundamental mechanism of axon degeneration has identified a series of exciting druggable targets.
Conclusion
Peripheral neuropathies are the most common form of neurodegenerative disease and are an important cause of chronic pain. Axon degeneration is a central component of many peripheral neuropathies, and studies in animal models demonstrate that blocking axon degeneration can prevent the development of peripheral neuropathy. Recent studies have identified the molecular mechanism driving axon loss, highlighting the central role for SARM1 as an injury-inducible NADase that triggers axon loss. Dissection of the mechanism-of-action of SARM1 and its upstream regulators have identified a number of druggable targets in the pathway. This tremendous mechanistic progress raises hopes that therapies will be developed to halt axon degeneration for the prevention and treatment of peripheral neuropathy and other diseases of axon loss.
Acknowledgements
This work was supported by funds from the National Institutes of Health RO1-CA219866 and RO1-NS087632. Conflict of interest: A.D. is a co-founder, member of the scientific advisory board, and stockholder of and receives financial compensation from Disarm Therapeutics.
References
- [1].Ali YO, Bradley G, Lu H-C. Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Sci Rep 2017;7:43846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004;305:1010–1013. [DOI] [PubMed] [Google Scholar]
- [3].Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res 2014;6:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Asghari Adib E, Smithson LJ, Collins CA. An axonal stress response pathway: degenerative and regenerative signaling by DLK. Curr Opin Neurobiol 2018;53:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci 2010;30:13291–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep 2013;3:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, Twiss JL, Alvarez J, Court FA. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci 2011;31:966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bhattacharya MRC, Geisler S, Pittman SK, Doan RA, Weihl CC, Milbrandt J, DiAntonio A. TMEM184b Promotes Axon Degeneration and Neuromuscular Junction Maintenance. J Neurosci 2016;36:4681–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blum ES, Abraham MC, Yoshimura S, Lu Y, Shaham S. Control of nonapoptotic developmental cell death in Caenorhabditis elegans by a polyglutamine-repeat protein. Science 2012;335:970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boyette-Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag 2018;8:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brace EJ, Wu C, Valakh V, DiAntonio A. SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury. J Neurosci 2014;34:8398–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014;348:f7656. [DOI] [PubMed] [Google Scholar]
- [13].Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 2006;51:57–69. [DOI] [PubMed] [Google Scholar]
- [14].Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 2014;15:394–409. [DOI] [PubMed] [Google Scholar]
- [15].Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci USA 2000;97:11377–11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desbois M, Crawley O, Evans PR, Baker ST, Masuho I, Yasuda R, Grill B. PAM forms an atypical SCF ubiquitin ligase complex that ubiquitinates and degrades NMNAT2. J Biol Chem 2018;293:13897–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 2017;93:1334–1343.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Essuman K, Summers DW, Sasaki Y, Mao X, Yim AKY, DiAntonio A, Milbrandt J. TIR Domain Proteins Are an Ancient Family of NAD+-Consuming Enzymes. Curr Biol 2018;28:421–430.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Farley JE, Burdett TC, Barria R, Neukomm LJ, Kenna KP, Landers JE, Freeman MR. Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc Natl Acad Sci USA 2018;115:1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Farley MM, Watkins TA. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu Rev Pathol 2018;13:93–116. [DOI] [PubMed] [Google Scholar]
- [21].Fukuda Y, Li Y, Segal RA. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front Neurosci 2017;11:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geden MJ, Deshmukh M. Axon degeneration: context defines distinct pathways. Curr Opin Neurobiol 2016;39:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geisler S, Doan RA, Strickland A, Huang X, Milbrandt J, DiAntonio A. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 2016;139:3092–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Geisler S, Huang SX, Strickland A, Doan RA, Summers DW, Mao X, Park J, DiAntonio A, Milbrandt J. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J Exp Med 2019;216:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 2015;348:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 2016;89:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci 2013;33:13569–13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol 2010;8:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep 2015;10:1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gilley J, Ribchester RR, Coleman MP. Sarm1 Deletion, but Not WldS, Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Rep 2017;21:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science 2009;323:802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, Bowser R, Freeman MR, Brown RH. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 2016;139:1094–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim Y, Zhou P, Qian L, Chuang J-Z, Lee J, Li C, Iadecola C, Nathan C, Ding A. MyD88–5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med 2007;204:2063–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Le Pichon CE, Meilandt WJ, Dominguez S, Solanoy H, Lin H, Ngu H, Gogineni A, Sengupta Ghosh A, Jiang Z, Lee S-H, Maloney J, Gandham VD, Pozniak CD, Wang B, Lee S, Siu M, Patel S, Modrusan Z, Liu X, Rudhard Y, Baca M, Gustafson A, Kaminker J, Carano RAD, Huang EJ, Foreman O, Weimer R, Scearce-Levie K, Lewcock JW. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- [35].Liu H-W, Smith CB, Schmidt MS, Cambronne XA, Cohen MS, Migaud ME, Brenner C, Goodman RH. Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc Natl Acad Sci USA 2018;115:10654–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017;355:1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci 1989;1:27–33. [DOI] [PubMed] [Google Scholar]
- [38].Milde S, Gilley J, Coleman MP. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol 2013;11:e1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 2009;12:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mishra B, Carson R, Hume RI, Collins CA. Sodium and potassium currents influence Wallerian degeneration of injured Drosophila axons. J Neurosci 2013;33:18728–18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mukherjee P, Woods TA, Moore RA, Peterson KE. Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity 2013;38:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nanson JD, Kobe B, Ve T. Death, TIR, and RHIM: Self-assembling domains involved in innate immunity and cell-death signaling. J Leukoc Biol 2018. [DOI] [PubMed] [Google Scholar]
- [43].Neukomm LJ, Burdett TC, Seeds AM, Hampel S, Coutinho-Budd JC, Farley JE, Wong J, Karadeniz YB, Osterloh JM, Sheehan AE, Freeman MR. Axon Death Pathways Converge on Axundead to Promote Functional and Structural Axon Disassembly. Neuron 2017;95:78–91.e5. [DOI] [PubMed] [Google Scholar]
- [44].Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou Y-J, Nathan C, Ding A, Brown RH, Conforti L, Coleman M, Tessier-Lavigne M, Züchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 2012;337:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Patel S, Harris SF, Gibbons P, Deshmukh G, Gustafson A, Kellar T, Lin H, Liu X, Liu Y, Liu Y, Ma C, Scearce-Levie K, Ghosh AS, Shin YG, Solanoy H, Wang J, Wang B, Yin J, Siu M, Lewcock JW. Scaffold-Hopping and Structure-Based Discovery of Potent, Selective, And Brain Penetrant N-(1H-Pyrazol-3-yl)pyridin-2-amine Inhibitors of Dual Leucine Zipper Kinase (DLK, MAP3K12). J Med Chem 2015;58:8182–8199. [DOI] [PubMed] [Google Scholar]
- [46].Pease SE, Segal RA. Preserve and protect: maintaining axons within functional circuits. Trends Neurosci 2014;37:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pease-Raissi SE, Pazyra-Murphy MF, Li Y, Wachter F, Fukuda Y, Fenstermacher SJ, Barclay LA, Bird GH, Walensky LD, Segal RA. Paclitaxel Reduces Axonal Bclw to Initiate IP3R1-Dependent Axon Degeneration. Neuron 2017;96:373–386.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sasaki Y, Vohra BPS, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci 2009;29:6526–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 2000;26:345–356. [DOI] [PubMed] [Google Scholar]
- [51].Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci 2010;30:6375–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shah A, Hoffman EM, Mauermann ML, Loprinzi CL, Windebank AJ, Klein CJ, Staff NP. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry 2018;89:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012;74:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci USA 2012;109:E3696–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Tešić Mark M, Molina H, Tessier-Lavigne M. Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell 2016;164:1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Simon DJ, Watkins TA. Therapeutic opportunities and pitfalls in the treatment of axon degeneration. Curr Opin Neurol 2018;31:693–701. [DOI] [PubMed] [Google Scholar]
- [57].Summers DW, DiAntonio A, Milbrandt J. Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J Neurosci 2014;34:9338–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Summers DW, Gibson DA, DiAntonio A, Milbrandt J. SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc Natl Acad Sci USA 2016;113:E6271–E6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Summers DW, Milbrandt J, DiAntonio A. Palmitoylation enables MAPK-dependent proteostasis of axon survival factors. Proc Natl Acad Sci USA 2018;115:E8746–E8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Turkiew E, Falconer D, Reed N, Höke A. Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J Peripher Nerv Syst 2017;22:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 2015;77:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vohra BPS, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci 2010;30:13729–13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wakatsuki S, Tokunaga S, Shibata M, Araki T. GSK3B-mediated phosphorylation of MCL1 regulates axonal autophagy to promote Wallerian degeneration. J Cell Biol 2017;216:477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A. MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron 2000;26:313–329. [DOI] [PubMed] [Google Scholar]
- [66].Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol 2005;170:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Welsbie DS, Mitchell KL, Jaskula-Ranga V, Sluch VM, Yang Z, Kim J, Buehler E, Patel A, Martin SE, Zhang P-W, Ge Y, Duan Y, Fuller J, Kim B-J, Hamed E, Chamling X, Lei L, Fraser IDC, Ronai ZA, Berlinicke CA, Zack DJ. Enhanced Functional Genomic Screening Identifies Novel Mediators of Dual Leucine Zipper Kinase-Dependent Injury Signaling in Neurons. Neuron 2017;94:1142–1154.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wu C, Wairkar YP, Collins CA, DiAntonio A. Highwire function at the Drosophila neuromuscular junction: spatial, structural, and temporal requirements. J Neurosci 2005;25:9557–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol 2012;10:e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 2010;191:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yamagishi Y, Tessier-Lavigne M. An Atypical SCF-like Ubiquitin Ligase Complex Promotes Wallerian Degeneration through Regulation of Axonal Nmnat2. Cell Rep 2016;17:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 2009;138:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 2015;160:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yoon YK, Oon CE. Sirtuin Inhibitors: An Overview from Medicinal Chemistry Perspective. Anticancer Agents Med Chem 2016;16:1003–1016. [DOI] [PubMed] [Google Scholar]
- [75].Yoshino J, Baur JA, Imai S-I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab 2018;27:513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017;10:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 2000;26:331–343. [DOI] [PubMed] [Google Scholar]
- [78].Ziogas NK, Koliatsos VE. Primary Traumatic Axonopathy in Mice Subjected to Impact Acceleration: A Reappraisal of Pathology and Mechanisms with High-Resolution Anatomical Methods. J Neurosci 2018;38:4031–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]