Abstract
Background
Cerebral palsy (CP) is a neurodevelopmental disorder resulting from an injury to the developing brain. It is the most common form of childhood disability with prevalence rates of between 1.5 and 3.8 per 1000 births reported worldwide. The primary impairments associated with CP include reduced muscle strength and reduced cardiorespiratory fitness, resulting in difficulties performing activities such as dressing, walking and negotiating stairs.
Exercise is defined as a planned, structured and repetitive activity that aims to improve fitness, and it is a commonly used intervention for people with CP. Aerobic and resistance training may improve activity (i.e. the ability to execute a task) and participation (i.e. involvement in a life situation) through their impact on the primary impairments of CP. However, to date, there has been no comprehensive review of exercise interventions for people with CP.
Objectives
To assess the effects of exercise interventions in people with CP, primarily in terms of activity, participation and quality of life. Secondary outcomes assessed body functions and body structures. Comparators of interest were no treatment, usual care or an alternative type of exercise intervention.
Search methods
In June 2016 we searched CENTRAL, MEDLINE, Embase, nine other databases and four trials registers.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs of children, adolescents and adults with CP. We included studies of aerobic exercise, resistance training, and 'mixed training' (a combination of at least two of aerobic exercise, resistance training and anaerobic training).
Data collection and analysis
Two review authors independently screened titles, abstracts and potentially relevant full‐text reports for eligibility; extracted all relevant data and conducted 'Risk of bias' and GRADE assessments.
Main results
We included 29 trials (926 participants); 27 included children and adolescents up to the age of 19 years, three included adolescents and young adults (10 to 22 years), and one included adults over 20 years. Males constituted 53% of the sample. Five trials were conducted in the USA; four in Australia; two in Egypt, Korea, Saudi Arabia, Taiwan, the Netherlands, and the UK; three in Greece; and one apiece in India, Italy, Norway, and South Africa.
Twenty‐six trials included people with spastic CP only; three trials included children and adolescents with spastic and other types of CP. Twenty‐one trials included people who were able to walk with or without assistive devices, four trials also included people who used wheeled mobility devices in most settings, and one trial included people who used wheeled mobility devices only. Three trials did not report the functional ability of participants. Only two trials reported participants' manual ability. Eight studies compared aerobic exercise to usual care, while 15 compared resistance training and 4 compared mixed training to usual care or no treatment. Two trials compared aerobic exercise to resistance training. We judged all trials to be at high risk of bias overall.
We found low‐quality evidence that aerobic exercise improves gross motor function in the short term (standardised mean difference (SMD) 0.53, 95% confidence interval (CI) 0.02 to 1.04, N = 65, 3 studies) and intermediate term (mean difference (MD) 12.96%, 95% CI 0.52% to 25.40%, N = 12, 1 study). Aerobic exercise does not improve gait speed in the short term (MD 0.09 m/s, 95% CI −0.11 m/s to 0.28 m/s, N = 82, 4 studies, very low‐quality evidence) or intermediate term (MD −0.17 m/s, 95% CI −0.59 m/s to 0.24 m/s, N = 12, 1 study, low‐quality evidence). No trial assessed participation or quality of life following aerobic exercise.
We found low‐quality evidence that resistance training does not improve gross motor function (SMD 0.12, 95% CI −0.19 to 0.43, N = 164, 7 studies), gait speed (MD 0.03 m/s, 95% CI −0.02 m/s to 0.07 m/s, N = 185, 8 studies), participation (SMD 0.34, 95% CI −0.01 to 0.70, N = 127, 2 studies) or parent‐reported quality of life (MD 12.70, 95% CI −5.63 to 31.03, n = 12, 1 study) in the short term. There is also low‐quality evidence that resistance training does not improve gait speed (MD −0.03 m/s, 95% CI −0.17 m/s to 0.11 m/s, N = 84, 3 studies), gross motor function (SMD 0.13, 95% CI −0.30 to 0.55, N = 85, 3 studies) or participation (MD 0.37, 95% CI −6.61 to 7.35, N = 36, 1 study) in the intermediate term.
We found low‐quality evidence that mixed training does not improve gross motor function (SMD 0.02, 95% CI −0.29 to 0.33, N = 163, 4 studies) or gait speed (MD 0.10 m/s, −0.07 m/s to 0.27 m/s, N = 58, 1 study) but does improve participation (MD 0.40, 95% CI 0.13 to 0.67, N = 65, 1 study) in the short‐term.
There is no difference between resistance training and aerobic exercise in terms of the effect on gross motor function in the short term (SMD 0.02, 95% CI −0.50 to 0.55, N = 56, 2 studies, low‐quality evidence).
Thirteen trials did not report adverse events, seven reported no adverse events, and nine reported non‐serious adverse events.
Authors' conclusions
The quality of evidence for all conclusions is low to very low. As included trials have small sample sizes, heterogeneity may be underestimated, resulting in considerable uncertainty relating to effect estimates. For children with CP, there is evidence that aerobic exercise may result in a small improvement in gross motor function, though it does not improve gait speed. There is evidence that resistance training does not improve gait speed, gross motor function, participation or quality of life among children with CP.
Based on the evidence available, exercise appears to be safe for people with CP; only 55% of trials, however, reported adverse events or stated that they monitored adverse events. There is a need for large, high‐quality, well‐reported RCTs that assess the effectiveness of exercise in terms of activity and participation, before drawing any firm conclusions on the effectiveness of exercise for people with CP. Research is also required to determine if current exercise guidelines for the general population are effective and feasible for people with CP.
Plain language summary
Exercise interventions for improving activity, participation and quality of life in people with cerebral palsy
Review question
Does exercise improve activity, participation in life situations and quality of life in people with cerebral palsy (CP)?
Background
Cerebral palsy (CP) is caused by an injury to an infant's brain that interrupts normal development. People with CP have reduced muscle strength and aerobic fitness, which may impact their ability to perform activities such as standing, walking, running and to participate in everyday life. Exercise is defined as a planned, structured and repetitive activity that aims to improve fitness. Aerobic exercise aims to improve aerobic fitness, while strength training aims to improve muscle strength. Health professionals often prescribe exercise to people with CP, primarily to improve function, but there has been no comprehensive evaluation of the evidence for the effectiveness of these interventions in people with CP.
Study characteristics
In June 2016 we searched for all studies that investigated the effectiveness of exercise for people with CP. We included 29 trials with a total of 926 participants with CP, 53% of whom were male. Five trials were conducted in the USA; four in Australia; two in Egypt, Korea, Saudi Arabia, Taiwan, the Netherlands, and the UK; three in Greece; and one apiece in India, Italy, Norway, South Africa.
One trial included only adults with CP and three trials included adolescents and young adults. Most trials included children with CP who could walk independently, with or without a walking aid. Four trials also included people who used wheeled mobility devices (e.g. wheelchairs) in most settings and one trial included people who used wheeled mobility devices only. Three trials did not clearly report participants' functional ability and only two trials reported participants' manual ability (use of hands when handling objects). Eight trials compared aerobic exercise to usual care (i.e. the care a patient usually receives in practice), 15 trials compared resistance training (a type of exercise to improve muscular strength) to either usual care or no treatment, 4 trials compared mixed training (aerobic exercise and resistance training) to usual care or no treatment, and 2 trials compared aerobic exercise to resistance training.
Key results
Aerobic exercise may improve activity as indicated by motor function but does not appear to improve gait speed, walking endurance, participation or aerobic fitness among children with CP in the short or intermediate term. There is no research regarding the effect of aerobic exercise on participation or quality of life.
Resistance training does not appear to improve motor function, gait speed or participation in the short or intermediate term, or quality of life in the short term, in children and adolescents with CP but may improve muscle strength.
Mixed training does not improve motor function or gait speed but does improve participation in children and adolescents with CP in the short term.
We found no difference between aerobic and resistance training on motor function but a difference in muscle strength in the short term.
Although the evidence suggests that exercise might be safe for people with CP, only 16 trials (55%) included information on adverse events; these trials reported no serious adverse events. All of the studies we found had small numbers of participants, meaning that we cannot be sure the results are accurate.
Quality of the evidence
We judged the quality of evidence for all comparisons to be low or very low. All of the studies had small sample sizes. There were very few trials involving adults with CP or people with CP who could not walk, so our results may not apply to these groups of people. Few trials provided clear detail about the frequency, intensity and duration of exercise prescribed. Further research assessing the effectiveness of exercise for activity and participation is needed. Such research should determine if the amount and intensity of exercise prescribed to people with CP has an impact on its effectiveness, and whether current guidelines on exercise for the general population apply to people with CP.
Summary of findings
Background
Description of the condition
Cerebral palsy (CP) is defined as "a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non‐progressive disturbances that occurred in the developing fetal or infant brain" (Rosenbaum 2007, p 11). Children with CP may also present with cognitive impairments, hearing and visual impairments, communication difficulties and epilepsy (Rosenbaum 2007). Most children with CP are diagnosed at around one to two years of age (Ashwal 2004; Herskind 2015), following a medical history and physical examination that identify a non‐progressive motor deficit (Ashwal 2004; Rosenbaum 2007). Neuroimaging techniques, preferably magnetic resonance imaging (MRI), may be used in conjunction with the history and physical examination to establish aetiology and prognosis (Ashwal 2004).
CP is the most common form of childhood disability, with reported prevalence rates of between 1.5 and 3.8 per 1000 live births in different areas of Europe and the USA (SCOPE 2002; Kirby 2011). The prevalence of CP varies not only by geographical location but also by birth weights and gestational age, with higher prevalence rates reported in children born preterm or at low birth weight (Platt 2007; Sellier 2010; Andersen 2011). Other factors associated with CP include multiple births, maternal infection during pregnancy, having a relative with CP, breech position and placental abruption (O'Callaghan 2011; Tollånes 2014; Trønnes 2014). The prevalence of severe CP in Europe, defined by an inability to walk and a severe intellectual disability, is approximately 0.43 per 1000 live births (SCOPE 2002). Children without severe impairments are expected to live well into adulthood (Strauss 1998a; Blair 2001; Brooks 2014). Although less is known about the life expectancy of adults with CP, evidence suggests that adults with CP who maintain a high level of function have a slightly lower life expectancy than the general population (Strauss 1998b; Brooks 2014).
The primary impairments associated with CP include reduced muscle strength (Riad 2012; Nooijen 2014), reduced cardiorespiratory fitness (Verschuren 2010; Nieuwenhuijsen 2011; Nooijen 2014), and poor selective motor control (Østensjø 2004). As a result of these impairments, people with CP may have difficulty performing everyday activities such as eating, dressing, walking, running, jumping and negotiating stairs (Østensjø 2004; Ross 2007; Opheim 2009; Klingels 2012). Intensive rehabilitation is often provided in childhood to improve gross motor function. Indeed, many children who are non‐ambulatory at age two to three years will be ambulatory by the time they reach adolescence (Wu 2004). About 54% of five‐year‐old children in Europe and 56% of eight‐year‐old children in the USA are independently ambulatory despite having CP (Beckung 2008; Kirby 2011). However, a subsequent decline in gross motor function often occurs in adolescence and young adulthood (Bottos 2001; Sandström 2004; Hanna 2009; Kerr 2011). Up to 50% of adults with CP report experiencing deterioration in walking function from young adulthood (Bottos 2001; Opheim 2009). Adults with CP attribute deterioration in walking function to reduced muscle strength, reduced cardiorespiratory fitness, fatigue and pain (Jahnsen 2004; Opheim 2009). Conversely, adults who experience improvements or no change in walking function over time credit this to improvements in balance, muscle strength and cardiorespiratory fitness (Opheim 2009). Poor gross motor function may also contribute to reduced quality of life and unemployment, which is high among young adults with CP (Soyupek 2010; Verhoef 2014).
Although CP is defined by the presence of motor disorders, the clinical presentation of CP can vary considerably, making it difficult to compare individuals at one point in time or to evaluate changes in an individual's condition over time. Traditionally, CP has been classified according to the type of motor abnormality (for example, spasticity, dystonia, choreoathetosis, ataxia) and anatomical distribution of CP (for example, bilateral, unilateral) (Rosenbaum 2007). More recently, classification systems that allow categorisation of people with CP according to their level of functional impairment have been developed; the Gross Motor Function Classification System (GMFCS) and Manual Ability Classification System (MACS) are two such systems. The GMFCS is a five‐point scale that distinguishes between levels of motor function based on functional mobility and the need for assistive technology, particularly mobility aids (Palisano 1997; Palisano 2008). A full description of the GMFCS is presented in Appendix 1. To summarise, from six years of age children in level I of the GMFCS are able to walk indoors and outdoors without assistance and can perform gross motor skills such as running and jumping; children in level II can also walk indoors and outdoors without assistance but have only minimal ability to perform gross motor skills like running and jumping; children in level III require a mobility device to walk indoors and outdoors and may require wheeled mobility for travelling long distances; children in level IV use wheeled mobility in most settings; children in level V are limited in their ability to maintain antigravity head and trunk postures and to control arm and leg movements, and they are transported in a manual wheelchair in all settings. Although developed for children with CP, the GMFCS has been used successfully to classify motor function in adults with CP (Sandström 2004). For its part, the MACS is a five‐point scale that classifies how children aged four years or older with CP use their hands when handling objects in daily activities (Eliasson 2006). A full description of the MACS is in Appendix 2. Children in level I of the MACS handle objects easily and successfully. They may have limitations in the ease of performing tasks that require speed and accuracy. Children in level II handle most objects but with reduced quality, speed or both. Children in level III have difficulty handling objects and need help to prepare or modify activities. Children in level IV can only handle a limited selection of easily managed objects in adapted situations and require continuous support and assistance.
Description of the intervention
Exercise is defined as "physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of one or more components of physical fitness is an objective" (Caspersen 1985, p 128). The components of physical fitness that exercise may improve include muscle strength, muscle endurance and cardiorespiratory fitness. The focus of this review will be on exercise interventions categorised as resistance training or aerobic training. Resistance training involves the body's muscles working or holding against an applied force. Body weight, free weights, machine weights, and elastic bands are often used to apply force (USDHHS 2008). Current guidelines for resistance training to improve muscle strength for youth suggest that one to three sets of 6 to 15 repetitions of a muscle strengthening exercise should be performed at an intensity of 50% to 85% of one repetition maximum (RM) (i.e. the maximum weight a person can lift with one repetition) (Faigenbaum 2009). Alternatively, if people do not perform one RM tests, therapists can establish the intensity by prescribing a repetition range and determining the maximum load that people can lift for the prescribed range. Current guidelines for adults suggest that in order to improve muscle strength, inexperienced people should perform one to three sets of 8 to 12 repetitions of a muscle strengthening exercise at loads corresponding to 60% to 70% of one RM (American College of Sports Medicine 2009). People should engage in resistance training on two to three days per week (American College of Sports Medicine 2009; Faigenbaum 2009), and at least eight weeks of training are required to observe an increase in muscle strength (Faigenbaum 2009). Aerobic training involves moving the body's large muscles in a rhythmic manner for a sustained period of time (USDHHS 2008). Walking, running, cycling and arm ergometry are examples of aerobic exercise. Current guidelines for aerobic exercise to improve cardiorespiratory fitness suggest that people with CP should engage in aerobic exercise two to three times per week at an intensity of 60% to 95% of peak heart rate, between 40% to 80% of heart‐rate reserve (HRR) or between 50% and 65% of VO2 peak (i.e. maximum oxygen consumption), for at least 20 minutes per session (Verschuren 2016). Further, a training programme should continue for at least 8 consecutive weeks when training three times a week or for 16 consecutive weeks when training twice a week (Verschuren 2016). Many exercise programmes target muscle strength, anaerobic fitness, cardiorespiratory fitness or a combination of these components. We will refer to such programmes as 'mixed training'.
How the intervention might work
The goal of treatment for people with CP has shifted from targeting impairments of the motor system to targeting activity limitations and participation restriction, where activity is defined as a person's ability to execute a task, and participation is defined as a person's involvement in a life situation (WHO 2001). Indeed, people with CP have identified improving restricted mobility and poor upper limb function as primary therapeutic goals (Vargus‐Adams 2011). However, many experts believe there is an association between motor impairments, activity limitation and participation restriction, so targeting one may well affect another. There is evidence that impairments, particularly muscle strength, are associated with activity in children with CP (Østensjø 2004; Ross 2007; Voorman 2007; Verschuren 2009; Klingels 2012; Park 2013). Although less information is available about the association between cardiorespiratory fitness and activity, aerobic training, resistance training and mixed training have proven efficacy on activity in older adults (Liu 2009; Giné‐Garriga 2014), a population who experience similar declines in physical functioning as young adults with CP (Nusselder 2005; Day 2007). Improvements in activity provided by exercise may translate to improved participation in mobility‐based behaviours for people with CP (Park 2013; Bjornson 2014).
Exercise may also have benefits in terms of pain relief and quality of life for people with CP. Some adults report using exercise as a treatment for pain and find it moderately effective (Engel 2002; Hirsh 2011), which may positively impact on quality of life. Further, a positive association between physical activity and physical, behavioural, emotional and social quality of life has been reported in children with CP (Bjornson 2008; Maher 2016). As exercise is structured physical activity, the implementation of an exercise programme may result in improvements in quality of life for people with CP.
The aim of this review, to assess the effects of exercise interventions on activity, participation and quality of life in people with CP, reflects the goals of people with CP and their clinicians and therefore is of most interest to users of this review. While the association between physical fitness and activity suggests that improving physical fitness may improve activity, the physiological, biomechanical, and neuromuscular adaptations that may occur as a result of exercise training in people with CP are not understood. It is also possible that the effect of exercise on activity performance may vary according to the person's baseline level of functional ability. For example, improving muscle strength in children in GMFCS level III, who have reduced muscle strength compared to children in GMFCS level I (Eek 2008), may result in greater improvements in activity because of their greater potential for improvement. Conversely, improvements in muscle strength may be small in people with a greater degree of functional impairment because of their inability to exercise at an adequate intensity.
Why it is important to do this review
Although CP begins in childhood, it impacts the individual's whole life course as well as the healthcare system. Identifying appropriate interventions to alleviate disability throughout the life of a person with CP is urgent. Health professionals often recommend exercise for people with CP, partly because of its known importance for improving physical functioning in other populations. This is reflected in the growing number of publications on the topic of exercise in CP.
Ten reviews have investigated the effectiveness of exercise interventions in children with CP (Dodd 2002; Taylor 2005; Anttila 2008; Mockford 2008; Rogers 2008; Verschuren 2008; Scianni 2009; Butler 2010; Novak 2013; Rameckers 2014). Eight of these included articles published up to July 2008 (Dodd 2002; Taylor 2005; Anttila 2008; Mockford 2008; Rogers 2008; Verschuren 2008; Scianni 2009; Butler 2010); one included articles up to December 2012 (Novak 2013), and one included articles published up to August 2014 (Rameckers 2014). Four reviews focused solely on randomised controlled trials (RCTs) (Anttila 2008; Scianni 2009; Butler 2010; Rameckers 2014); the remaining six included experimental or quasi‐experimental studies. Nine reviews provided a narrative summary of the evidence (Dodd 2002; Taylor 2005; Anttila 2008; Mockford 2008; Rogers 2008; Verschuren 2008; Butler 2010; Novak 2013; Rameckers 2014). Only one review conducted a meta‐analysis of RCTs (Scianni 2009). However, this review specifically examined the effectiveness of muscle strengthening, rather than all exercise interventions, in children with CP.
Two reviews have investigated the effectiveness of exercise interventions in adults with CP (Dodd 2002; Jeglinsky 2010). These reviews included observational studies published up to March 2002 and 2009, respectively. Both reviews conducted descriptive analyses. One meta‐analysis specifically investigated the effect of strength training in children and adults with CP (Park 2014b).
An up‐to‐date and comprehensive assessment of the evidence surrounding exercise interventions in adults and children with CP is required to guide consumers, health professionals and policymakers.
Objectives
To assess the effects of exercise interventions in people with CP, primarily in terms of activity, participation and quality of life. Secondary outcomes assessed body functions and body structures. Comparators of interest were no treatment, usual care or an alternative type of exercise intervention.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (where sequence generation is systematically determined but not truly random, for example, based on order of entry or date of birth).
Types of participants
Children, adolescents and adults of any age with a diagnosis of CP, irrespective of level of functional ability (i.e. Gross Motor Function Classification System (GMFCS) levels I to V and the Manual Ability Classification System (MACS) levels I to IV).
Types of interventions
We included studies of exercise that met the definition in Caspersen 1985 (see Description of the intervention). We included studies of aerobic and resistance training and studies that used a combination of exercises, where at least one exercise was categorised as resistance training, aerobic training or anaerobic training (that is, 'mixed training'). We included interventions that targeted both the upper and lower limbs. We did not include studies of stretching interventions. We did not include studies of interventions, such as constraint‐induced movement therapy or bimanual therapy, where the intervention did not specifically target one or more components of physical fitness (i.e. muscle strength, muscle endurance and cardiorespiratory fitness).
Comparisons of interest were exercise versus no treatment, usual care or an alternative exercise intervention (e.g. a comparison of resistance training and aerobic exercise).
Types of outcome measures
Primary outcomes
-
Activity, defined as a person's ability to execute a task (WHO 2001). Examples of outcome measures for activity include the Gross Motor Function Measure 66‐ or 88‐item (GMFM‐66 or GMFM‐88; Russell 1989), Assisted Hand Assessment (AHA) (Krumlinde‐Sundholm 2003), timed walk tests, Melbourne Assessment of Unilateral Upper Limb Function (MAUULF; Randall 1999), ABILHAND‐Kids questionnaire (Arnould 2004), Activities Scale for Kids (ASK; Young 2000), International Physical Activity Questionnaire (IPAQ; Craig 2003), accelerometers, and pedometers. Subdomains of activity are:
activity capacity (i.e. a person's ability to execute a task in a standardised environment);
activity capability (i.e. a person's ability to execute a task in his or her daily environment); and
activity performance (i.e. what a person actually does in his or her environment) (Holsbeeke 2009).
Participation, defined as a person's involvement in a life situation. This may include participation in domestic life (e.g. acquiring a place to live or managing a household); employment or education; and community, social, and civic life (WHO 2001). Examples of outcome measures for participation include the Paediatric Evaluation of Disability Inventory (PEDI; Haley 1992), the Waisman Activities of Daily Living Scale (W‐ADL; Maenner 2013), and Assessment of Life Habits questionnaire (LIFE‐H; Fougeyrollas 1998).
Quality of life, defined as the impact of disease and treatment on physical, psychological and social functioning (Schipper 1996; Solans 2008), as measured by, for example, the Short Form‐36 (SF‐36) health survey (Ware 1993) and the Child Health Questionnaire (CHQ; Landgraf 1998).
Incidence and nature of adverse events such as injury, cardiac events, stiffness and delayed onset muscle soreness, where reported.
Secondary outcomes
-
Body functions and body structures, defined as changes in physiological systems or in anatomical structures (WHO 2001). These include:
muscle strength and endurance, as measured by, for example, dynamometry;
cardiorespiratory fitness, as measured by, for example, the Shuttle Run Test (SRT; Verschuren 2006);
pain, as measured by, for example, a visual analogue scale (VAS) (McCormack 1988);
fatigue, as measured by, for example, the Fatigue Severity Scale (Krupp 1989); and
depression, as measured by, for example, the Center for Epidemiological Studies ‐ Depression Scale (CES‐D; Radloff 1977).
As studies assessed change in a wide range of body structures and functions following exercise, we limited the included outcomes to those targeted by a specific exercise intervention. For example, for studies of resistance training, we reported the effect on muscle strength; for studies of aerobic exercise we reported the effect on aerobic fitness; for studies of mixed training, we reported the effect on muscle strength and aerobic and anaerobic fitness.
We planned to include studies that used any validated scale that measures these primary and secondary outcomes. However, as trials used a range of outcome measures to assess these outcomes, we included any measure that purported to assess them, regardless of whether or not it was validated specifically in people with CP. See Differences between protocol and review. We collected outcomes for the following time points: short term (zero to one month postintervention), intermediate term (more than one month and up to six months' postintervention), and long term (more than six months' postintervention). We presented all available results for the primary outcomes in 'Summary of findings' tables.
Search methods for identification of studies
Electronic searches
We searched all available years of the following databases in June 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5), in the Cochrane Library, which contains the Cochrane Developmental, Psychosocial and Learning Problem Specialised Register (searched 14 June 2016).
Ovid MEDLINE (1946 to June Week 1 2016).
Embase Ovid (1980 to 2016 Week 24).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 June 2016).
Cochrane Database of Systematic Reviews (CDSR; 2016, Issue 6), part of the Cochrane Library.
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2 ), part of the Cochrane Library (searched 15 May 2015; DARE was not updated after this date).
Science Citation Index Web of Science (1970 to 9 June 2016).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 9 June 2016).
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; searched 14 June 2016).
Health Services Research Projects in Progress (HSRPRoj; wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm; searched 23 June 2016).
OpenGrey (www.opengrey.eu; searched 16 June 2016).
National Rehabilitation Information Center (www.naric.com; searched 23 June 2016).
PEDro ( Physiotherapy Evidence Database; www.pedro.org.au; searched 23 June 2016).
UKCRN Study Portfolio (public.ukcrn.org.uk/search; searched 16 June 2016).
ClinicalTrials.gov (clinicaltrials.gov; searched 16 June 2016).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en; searched 20 June 2016).
We used the search strategy for MEDLINE Ovid, which incorporates the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2011), and we adapted this strategy, as appropriate, for other sources. We did not limit searches by language, date or publication status. The strategy for each source is reported in Appendix 3. For a detailed record of the searches (including search dates and the number of records found in each source), see Appendix 4.
Searching other resources
We handsearched the reference lists of eligible trials and relevant systematic reviews identified from the search to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors (JMR and EEC) independently checked the titles and abstracts of the search results and excluded studies that did not meet the inclusion criteria outlined above (Criteria for considering studies for this review). In cases that appeared to meet the inclusion criteria, or where there was any doubt as to whether we should have excluded the report, we retrieved the full text of the report. Two review authors (JMR and EEC) independently reviewed these papers against the inclusion criteria (Criteria for considering studies for this review), resolving any disagreements regarding the exclusion of a report at any stage through discussion, and where necessary, through consultation with a third review author (SN). We recorded our decisions in a PRISMA diagram (Moher 2009).
Data extraction and management
Two review authors (JMR and EEC) independently extracted data using a standardised form developed for the purpose. We resolved disagreements regarding the extraction of data by discussion. If we could not reach a resolution, we consulted a third review author (NEO'C). The form included the following information, where available.
Country of origin.
Study design.
Sample size: treatment and control groups.
Study population (treatment and control groups): sex, age, ethnicity, distribution of CP, type of motor abnormality and gross motor function. Where sufficient information was provided, we classified children and adults according to GMFCS level and MACS level, as these scales provide a comprehensive indication of functional ability above that provided by classifying individuals according to type of motor abnormality and anatomical distribution of CP. Although we proposed to classify general gross motor function as unaided walking, walking with aids or unable to walk (Beckung 2008), most studies reported the GMFCS level of participants. Therefore, we reported the GMFCS level where available and use of mobility aids when the GMFCS level was not available.
Intervention: aim of the intervention, type of exercise programme (e.g. aerobic exercise), mode of delivery (e.g. home programme), type(s) of location(s) where the intervention occurred (including any necessary infrastructure or relevant features), supervised or unsupervised programme, exercise mode (e.g. cycle ergometry, treadmill), exercise dose (i.e. duration, intensity, and frequency of exercise), tailoring of intervention to individual, modification of intervention (what, why, when, how), duration of programme. Following data extraction, we combined information on modification of the intervention with information on tailoring of the intervention to individual to create one category.
Intervention provider: profession, expertise, background, specific training received.
Fidelity or adherence to programme: how or by whom this was assessed.
Outcome measures (Types of outcome measures).
Results: short‐term (zero to one month postintervention), intermediate‐term (greater than one month to six months' postintervention), and long‐term (more than six months' postintervention) follow‐up.
Measures of adherence to the exercise programme.
Adverse effects.
Conflicts of interest.
Declarations of conflicts of interest.
Sources of funding.
Assessment of risk of bias in included studies
Two review authors (JMR and EEC) independently assessed risk of bias using Cochrane's tool for assessing risk of bias (Higgins 2011a). A third review author (SN) resolved any persistent disagreements between them. We assessed the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. We scored all domains as being at low, high or unclear risk of bias. We present operational definitions for making judgements on each domain in Appendix 5.
We assigned included studies an overall rating of high, low or unclear risk of bias. Where we rated one or more domains at high risk of bias, we rated the study at high risk of bias overall. Where we did not rate a study at high risk of bias for any domain but rated it at unclear risk of bias for one or more domains, we rated that study at unclear risk of bias overall. We rated a study at low risk of bias overall if we rated it as low risk of bias for all domains.
Measures of treatment effect
Dichotomous data
No study used dichotomous outcomes. Table 5 outlines our plans for dealing with such data and other methodological decisions that were not possible or appropriate to deploy, should it be necessary to use these methods in future updates of this review. Please also see our protocol (Ryan 2015).
1. Additional methods table.
| Binary data | We planned to present the relative risk (or risk ratio) with a 95% confidence interval, and calculate the number needed to treat for an additional beneficial outcome as an absolute measure of treatment effect. We will report the odds ratio (OR) with a 95% confidence interval in future updates of this review, as most studies with a dichotomous outcome report the OR. |
| Cluster trials | We planned to seek direct estimates of the effect from an analysis that accounted for cluster design. Where the analysis in a cluster trial did not account for the cluster design, we planned to use the approximately correct analysis approach, presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). |
| Crossover trials | Where studies presented repeated measurements over time, we planned to only include data from 1 time point from an individual study in any single meta‐analysis. If inadequate data were available to conduct this analysis, we planned to only include data from the first phase of the cross‐over trial, as if it were from a parallel trial design. We planned to combine the results of cross‐over studies with those of parallel studies by imputing the post‐treatment, between‐condition correlation coefficient from an included study that presents individual participant data, and use this to calculate the standard error of the SMD, using the generic inverse‐variance method. |
| Assessment of reporting biases | Where we identified evidence of publication bias, we planned to consider its likely influence on the observed effect sizes in our interpretation of the results. However, as common tests of publication bias lack sensitivity, we planned to consider the possible influence that a dominance of small trials might have on pooled effect sizes in our interpretation. |
| Subgroup analysis and identification of heterogeneity | We planned to further explore possible clinical heterogeneity through preplanned subgroup analysis based on important clinical features. We predicted that some trials would include ambulatory participants only (i.e. people who could walk with or without a mobility aid; GMFCS level I, II, and III), and some studies would include participants who could walk without a mobility aid only (i.e. GMFCS level I and II). Where adequate data allowed, we planned to undertake 2 subgroup analyses for studies that included ambulatory people only (i.e. GMFCS level I, II, and III), and for studies that included ambulatory people who walk without a mobility aid only (i.e. GMFCS level I and II). |
| Sensitivity analysis | We planned to explore the impact of studies at high risk of bias by reanalysis after excluding studies rated at overall high risk of bias. We also planned to explore the impact of excluding studies at high risk of bias for missing data through reanalysis. We planned to explore the influence of using imputed correlation coefficients in our approach to including cross‐over and cluster trials by reanalysing these data with adjusted (higher and lower) coefficient values. |
GMFCS: Gross Motor Function Classification System; SMD: standardised mean difference.
Continuous data
Where pooled studies used the same scale on a continuous outcome measure, we presented the effect size as a mean difference (MD) with 95% confidence intervals (CI). Where studies used different scales to measure the same construct or used different versions of an outcome measure that scored the outcome differently, we presented the standardised mean difference (SMD) with 95% CI. We used a rule of thumb to interpret the magnitude of effect for the SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988).
Clinically important differences
Clinically important differences have been developed for a number of outcome measures (for example, GMFM, weeFIM, one‐minute walk test) for ambulatory children and adolescents (GMFCS levels I to III) aged 4 to 19 years old (Oeffinger 2008; Hassani 2014). However, there are no well‐established and accepted thresholds for clinically important differences across the range of possible outcome measures and possible participants. Where possible, our discussion of the results considered the size of effects for our primary outcomes in light of contemporary research literature on clinically important differences.
Unit of analysis issues
Cluster‐randomised trials
We did not include any cluster‐randomised trials in this review. See our protocol, Ryan 2015, and Table 5 for details of methods archived for use in future updates of this review.
Cross‐over trials
We identified only one cross‐over trial from which we were unable to include any data. See Ryan 2015 and Table 5 for details of methods archived for use in future updates of this review.
Studies with multiple treatment groups
Where studies included multiple treatment groups, we combined results across all eligible intervention groups and compared them with the combined results across all eligible control groups, making single pairwise comparisons.
Dealing with missing data
Where the report of an included study presented insufficient data to enter into the meta‐analysis, we requested access to missing data from the authors with two reminder requests sent at monthly intervals in the event of non‐response. We specifically requested data relating to the effect of the intervention (e.g. means and standard deviations (SDs)) on any of the outcomes of interest (e.g. adverse events) and details of dropouts. We did not routinely request other methodological details or information relating to the 'Risk of bias' assessments. See Ryan 2015 and Table 5 for details of methods archived for use in future updates of this review. We conducted analyses using only the available data; we did not impute missing data.
Assessment of heterogeneity
We assessed clinical variation across studies by comparing the distribution of important factors among trials (for example, participant age, sex, and functional ability (GMFCS level), characteristics of the interventions). We assessed statistical heterogeneity and its impact using the Chi2 test and the I2 statistic (Deeks 2011). We used the Chi2 test to determine whether differences in effects across studies are compatible with chance alone and the I2 statistic to describe the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance).
Assessment of reporting biases
We considered the possible influence of publication and small‐study biases on review findings. Where we identified sufficient data (equal to or greater than 10 studies in a meta‐analysis), we examined funnel plots and used the test proposed by Egger 1997 to test for funnel plot asymmetry.
Data synthesis
We pooled the results from included studies using Review Manager 5 (RevMan 5) software (Review Manager 2014). Comparisons of interest were exercise versus no treatment or usual care, and comparisons of one type of exercise intervention versus another. We did not pool data from these two comparisons together in a single meta‐analysis. We believe that the effect sizes for each of these comparisons are likely to vary considerably and that it is not theoretically justifiable to include exercise and usual care in one comparison group. Where studies compared two types of exercise interventions, we interpreted and discussed the results in the context of the evidence, or lack of evidence, of the effectiveness of each exercise intervention compared to usual care or no treatment.
We attempted to deal with clinical heterogeneity by performing separate meta‐analyses for each category of exercise intervention (i.e. resistance training, aerobic training and mixed training). We believe that the type of exercise performed could impact the effect size and that combining these interventions could mask the true effect of each individual intervention. We performed separate meta‐analyses for studies in children and adolescents versus adults. We defined children as aged 19 years and below, adolescents as individuals aged 10 to 19 years inclusive, and adults as aged 20 years and older (WHO 2013). We report the results for adults and for children and adolescents as a group as the majority of studies included children and adolescents, as opposed to children or adolescents only.
We used a random‐effects model to combine studies since we expected studies to vary somewhat in terms of the interventions, comparisons and populations. We considered separate meta‐analyses for different types of exercise intervention and for short‐term (zero to one month postintervention), intermediate‐term (more than one month to six months' postintervention), and long‐term (more than six months' postintervention) outcomes. Where meta‐analyses were not possible, we conducted a narrative synthesis of the data. We also considered contextual data in our interpretation of the evidence.
There was large variation between studies in terms of the muscle groups targeted by interventions and the muscle groups whose strength investigators assessed as an outcome measure. We therefore applied the following rules for extracting outcome data to be included in the pooled analysis. Where resistance training targeted one muscle group in the lower limbs, we extracted data on a continuous scale for the targeted muscle group. Where resistance training interventions targeted multiple muscle groups in the lower limbs and assessed the strength of multiple muscle groups, we extracted data on a continuous scale for the knee extensors. We chose to extract data on the knee extensors because the knee extensors were the most commonly trained and assessed muscle group. Where exercises targeted right and left limbs and presented data on each limb individually, we took the data from the right limb. Where concentric and eccentric muscle strength was trained and assessed, we took data on concentric muscle strength, as this was more common. Where data on muscle strength at multiple speeds was presented, we took data for muscle strength assessed at 60°/second as this was the most consistently assessed speed across trials. Where trials of lower limb resistance training, aerobic training or mixed training (that included lower limb resistance training) assessed activity using the GMFM‐88 or GMFM‐66, we extracted the combined score (%) for dimensions D and E (i.e. standing, walking, running and jumping), as studies often only assessed activities in dimensions D and E. If this was not available, we extracted the individual score (%) for dimension E (i.e. walking, running and jumping), and if this was not available, we extracted the total score (%) for the GMFM‐88 or GMFM‐66.
Subgroup analysis and investigation of heterogeneity
Due to the small number of trials that could be included in each meta‐analysis, we did not conduct subgroup analysis. See Ryan 2015 and Table 5 for our published strategy for subgroup analysis and investigation of heterogeneity.
Sensitivity analysis
We assessed the influence of our analysis model by reanalysing data using a fixed‐effect model instead of a random‐effects model. Other planned sensitivity analyses were not possible. Please see Ryan 2015 and Table 5 for our published strategy for exploring the impact of studies at high risk of bias due to missing data, and the influence of using imputed correlation coefficients in meta‐analyses including cross‐over and cluster trials.
Summary of findings table
Two authors (JMR, EEC) used the GRADE approach to assess the quality of the body of evidence (Guyatt 2008). To ensure consistency of GRADE judgements, we applied the criteria below to each domain equally for all key comparisons.
Limitations of studies: downgrade once if less than 75% of included studies are at low risk of bias across all 'Risk of bias' domains.
Inconsistency: downgrade once if heterogeneity is statistically significant (P < 0.10) and I2 > 40%.
Indirectness: downgrade once if more than 50% of the participants are outside the target group.
Imprecision: downgrade once if fewer than 400 participants for continuous data and fewer than 300 events for dichotomous data (Guyatt 2011).
Publication bias: downgrade where there is direct evidence of publication bias.
We presented the GRADE judgements for all outcomes for comparisons of aerobic exercise versus usual care, resistance training versus usual care, mixed training versus usual care, and aerobic exercise versus resistance training, in the Effects of interventions section. We also presented GRADE ratings for outcomes where there were sufficient data to conduct meta‐analyses for comparisons of aerobic exercise versus usual care, resistance training versus usual care, mixed training versus usual care, and aerobic exercise versus resistance training in 'Summary of findings' tables, which we constructed using GRADEpro GDT 2014.
See Table 1; Table 2; Table 3; Table 4.
Summary of findings for the main comparison. Aerobic exercise versus usual care.
| Aerobic exercise versus usual care | ||||||
|
Patient or population: children and adolescents with cerebral palsy
Intervention: aerobic exercise Setting: mixed (community, outpatients, home) Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with aerobic exercise | |||||
|
Activity Gross motor function assessed with the Gross Motor Function Measure (follow‐up 0 to 1 month) |
The mean gross motor function ranged across control groups from 0.20% to 65.13% | The standardised mean gross motor function in the intervention group was 0.53 higher (0.02 higher to 1.04 higher) | — | 65 (3) | ⊕⊕⊝⊝ Lowa,c,d | Higher score indicates improved activity A rule of thumb for interpreting SMD is that 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) |
|
Activity Gait speed assessed with a timed walk test (follow‐up 0 to 1 month) |
The mean gait speed ranged across control groups from 0.63 m/s to 2.40 m/s | The mean gait speed in the intervention groups was0.09 m/s faster (0.11 m/s slower to 0.28 m/s faster) | — | 82 (4) | ⊕⊝⊝⊝ Very lowa,b,c,d | Higher speed indicates improved activity |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAll studies are at high risk of bias because it is not possible to blind personnel or participants to group allocation. bHeterogeneity statistically significant: P < 0.1, I2 > 40%. cNumber of participants < 400. dWe did not downgrade on the basis of publication bias, as there can be no direct evidence with so few trials for any given intervention.
Summary of findings 2. Resistance training versus usual care.
| Resistance training versus usual care | ||||||
| Patient or population: children and adolescents with cerebral palsy Setting: mixed (home, physiotherapy clinic, school, community gym) Intervention: resistance training Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with resistance training | |||||
|
Activity Gross motor function assessed with the Gross Motor Function Measure (follow‐up 0 to 1 month) |
The mean gross motor function ranged across control groups from 60.80% to 81.30% | The standardised mean gross motor function in the intervention groups was 0.12 higher (0.19 lower to 0.43 higher) | — | 164 (7) | ⊕⊕⊝⊝ Lowa,b,c | A rule of thumb for interpreting the SMD is that 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) Higher score indicates improved activity |
|
Activity Gross motor function assessed with the Gross Motor Function Measure (follow‐up > 1 month to 6 months) |
The mean gross motor function ranged across control groups from 61.80% to 74.30% | The standardised mean gross motor function in the intervention groups was 0.13 higher (‐0.30 lower to 0.55 higher) | — | 85 (3) | ⊕⊕⊝⊝ Lowa,b,c | A rule of thumb for interpreting SMD is that 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) Higher score indicates improved activity |
|
Activity Gait speed assessed with a timed walk test (follow‐up 0 to 1 month) |
The mean gait speed ranged across control groups from0.30 m/s to 1.17 m/s | The mean gait speed in the intervention groups was 0.03 m/s faster (0.02 m/s slower to 0.07 m/s faster) | — | 185 (8) | ⊕⊕⊝⊝ Lowa,b,c | Higher speed indicates improved activity |
|
Activity Gait speed assessed with a timed walk test (follow‐up > 1 month to 6 months) |
The mean gait speed ranged across control groups from 0.68 m/s to 1.06 m/s | The mean gait speed in the intervention groups was 0.03 m/s slower (0.17 m/s slower to 0.11 m/s faster) | — | 84 (3) | ⊕⊕⊝⊝ Lowa,b,c | Higher speed indicates improved activity |
|
Participation Assessed with various measures (follow‐up 0 to 1 month) |
The mean participation in the control group ranged from 7.40 to 31.14 | The standardised mean participation in the intervention groups was 0.34 higher (0.01 lower to 0.70 higher) | — | 127 (2) |
⊕⊕⊝⊝ Lowa,b,c |
A rule of thumb for interpreting SMD is that 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) Higher score indicates improved participation |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAll trials are at high risk of bias because it is not possible to blind personnel or participants to group allocation. bNumber of participants < 400. cWe did not downgrade on the basis of publication bias, as there can be no direct evidence with so few trials for any given intervention.
Summary of findings 3. Mixed training versus usual care.
| Mixed training versus usual care | ||||||
| Patient or population: children and adolescents with cerebral palsy Setting: mixed (school, home) Intervention: mixed training Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with mixed training | |||||
|
Activity Gross motor function assessed with the Gross Motor Function Measure (follow‐up 0 to 1 month) |
The mean gross motor function in the control groups ranged from 30.76% to 90.11% | The standardised mean gross motor function in the intervention groups was 0.02 higher (0.29 lower to 0.33 higher) | — | 163 (4) | ⊕⊕⊝⊝ Lowa,b,c | A rule of thumb for interpreting SMD is that 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) Higher score indicates improved activity |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAll trials are at high risk of bias because it is not possible to blind personnel or participants to group allocation. bNumber of participants < 400. c We did not downgrade on the basis of publication bias, as there can be no direct evidence with so few trials for any given intervention.
Summary of findings 4. Resistance training versus aerobic exercise.
| Resistance training versus aerobic exercise | ||||||
| Patient or population: children with cerebral palsy Setting: home or not reported Intervention: resistance training Comparison: aerobic exercise | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with aerobic exercise | Risk with resistance training | |||||
|
Activity Gross motor function assessed with various measures (follow‐up 0 to 1 month) |
The mean gross motor function in the aerobic exercise groups ranged from 44.09% to 63.30% | The standardised mean gross motor function in the intervention groups was 0.02 higher (0.50 lower to 0.55 higher) | — | 56 (2) | ⊕⊕⊝⊝ Lowa,b,c | Higher score indicates improved activity |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAll trials are at high risk of bias because it is not possible to blind participants or personnel to group allocation. bNumber of participants < 400. cWe did not downgrade on the basis of publication bias, as there can be no direct evidence with so few trials for any given intervention.
Results
Description of studies
Results of the search
We ran our searches in May 2015 and updated them in June 2016. Our searches yielded 11,100 original research studies, reviews and abstracts from databases and a further three reports from additional sources (Seniorou 2007; Nsenga Leunkeu 2013; Mitchell 2016). After removing duplicates, we screened the titles and abstracts of the remaining 8071 records against our inclusion criteria (Criteria for considering studies for this review) and retrieved 145 full‐text reports for further assessment. We used translators to evaluate two reports published in languages other than English. We excluded 99 studies (102 reports) that did not meet the inclusion criteria (see Excluded studies; Characteristics of excluded studies tables) and included 29 studies (from 37 reports) in the review (see Included studies; Characteristics of included studies tables). One additional trial, Carlon 2014, was only available as a conference abstract and is described in the Characteristics of studies awaiting classification tables. We also identified five ongoing trials (Gillett 2015; ISRCTN90378161; NCT02754128; NCT02766491; RBR‐5rh6cg), which we describe in Characteristics of ongoing studies tables. Figure 1 presents a summary flow diagram.
1.
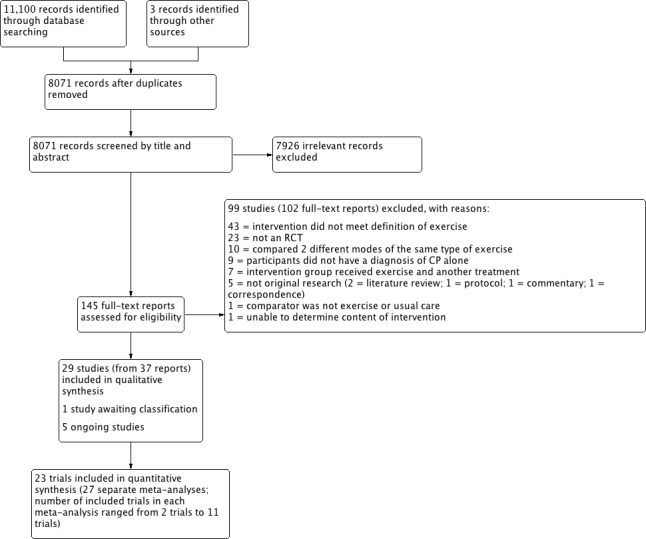
8071‐7926‐Study flow diagram.
Included studies
This review includes 29 studies from 37 reports (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Verschuren 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Bryant 2013; Chen 2012; Chrysagis 2012; Mattern‐Baxter 2013; Taylor 2013; Tedla 2014; Emara 2015; Lee 2015; Mitchell 2016). We provided a detailed description of each included study in the Characteristics of included studies tables.
Design
All included trials were RCTs. One trial used a randomised cross‐over design (Reid 2010). One trial allowed participants to continue or cross‐over into the exercise training programme after nine months of training (Van den Berg‐Emons 1998); we analysed this trial up to the point at which the participants were allowed to cross over. Two trials were quasi‐randomised (Unnithan 2007; Mattern‐Baxter 2013). Twenty‐six trials contained two arms (Van den Berg‐Emons 1998; Dodd 2003; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Verschuren 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Mattern‐Baxter 2013; Taylor 2013; Tedla 2014; Emara 2015; Lee 2015; Mitchell 2016), two trials contained three arms (McCubbin 1985; Bryant 2013), and one trial contained four arms (Engsberg 2006).
Participants
The 29 trials involved a total of 926 participants. The number of participants per trial ranged from 12 in Chrysagis 2009 to 102 in Mitchell 2016. We judged 24 trials to be small (N < 50) (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Reid 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013; Taylor 2013; Emara 2015; Lee 2015), four trials to be medium sized (between 50 and 100 participants) (Verschuren 2007; Fowler 2010; Scholtes 2010; Tedla 2014), and one trial to be large (≥ 100 participants) (Mitchell 2016). The exact number of participants in Olama 2011 was unclear, as authors provided contradictory information. All participants had a diagnosis of CP. Two trials included children only (aged less than 10 years) (Mattern‐Baxter 2013; Emara 2015), six trials included adolescents only (aged 10 to 19 years) (Unger 2006; Unnithan 2007; Gharib 2011; Olama 2011; Smania 2011; Chrysagis 2012), 17 trials included children and adolescents up to the age of 20 years (Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Fowler 2010; Reid 2010; Scholtes 2010; Johnston 2011; Pandey 2011; Chen 2012; Bryant 2013; Tedla 2014; Lee 2015; Mitchell 2016; Verschuren 2007), three trials included adolescents and young adults from the age of 14 to 22 years (McCubbin 1985; Chrysagis 2009; Taylor 2013), and one trial included adults over the age of 20 years (Maeland 2009). All trials included males and females. The mean percentage of males and females in the included studies was 53% (SD 10%; range 31% to 67%) and 47% (SD 10%; range 33% to 69%), respectively.
Twenty‐six trials included people with spastic CP (Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Verschuren 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Taylor 2013; Tedla 2014; Emara 2015; Lee 2015; Mitchell 2016). Of these, 10 specifically included people with spastic diplegia (Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Maeland 2009; Fowler 2010; Taylor 2013; Tedla 2014; Emara 2015), one included children with unilateral CP only (Mitchell 2016), two did not state if participants had unilateral or bilateral CP (Pandey 2011; Lee 2015), and the remainder included participants with unilateral and bilateral spastic CP (Van den Berg‐Emons 1998; Unger 2006; Verschuren 2007; Lee 2008; Chrysagis 2009; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Smania 2011; Chen 2012; Chrysagis 2012). One trial included children with dyskinetic and spastic (unilateral and bilateral) CP (Bryant 2013), one trial included children with spastic and hypotonic CP (Mattern‐Baxter 2013), and one trial included children with spastic, athetoid, ataxic and mixed CP (McCubbin 1985).
Eight trials included people classified in GMFCS levels I, II and III (Dodd 2003; Engsberg 2006; Seniorou 2007; Chrysagis 2009; Fowler 2010; Scholtes 2010; Chrysagis 2012; Lee 2015). Six trials included people classified in GMFCS levels I and II (Liao 2007; Verschuren 2007; Lee 2008; Chen 2012; Mattern‐Baxter 2013; Mitchell 2016). Two trials included people in GMFCS levels I, II, III and IV (Smania 2011; Tedla 2014). One trial included people in GMFCS level II only (Gharib 2011), two included people in GMFCS levels II and III (Maeland 2009; Taylor 2013), one included people in GMFCS levels II, III and IV (Johnston 2011), and one included people in GMFCS levels IV and V (Bryant 2013). Eight trials did not state participants' GMFCS level (McCubbin 1985; Van den Berg‐Emons 1998; Unger 2006; Unnithan 2007; Olama 2011; Pandey 2011; Emara 2015; Reid 2010). Of these, three trials stated that participants were able to walk with or without aids or that they occasionally used a wheelchair (Unger 2006; Unnithan 2007; Pandey 2011), one trial stated that participants were able to walk independently (Olama 2011), and one trial reported including people who were both ambulant and wheelchair bound (Van den Berg‐Emons 1998). Two trials reported the number of participants classified in each MACS level; participants were in MACS levels I, II and III (Reid 2010; Mitchell 2016).
Only one trial reported the ethnicity of participants, which included African Americans, whites, Asians and others (Fowler 2010).
Settings
Trials took place in a number of geographical locations: Australia (Dodd 2003; Reid 2010; Taylor 2013; Mitchell 2016), Egypt (Gharib 2011; Olama 2011), Greece (Unnithan 2007; Chrysagis 2009; Chrysagis 2012), India (Pandey 2011), Italy (Smania 2011), Korea (Lee 2008; Lee 2015), Norway (Maeland 2009), Saudi Arabia (Tedla 2014; Emara 2015), South Africa (Unger 2006), Taiwan (Liao 2007; Chen 2012), the Netherlands (Van den Berg‐Emons 1998; Verschuren 2007; Scholtes 2010), the UK (Seniorou 2007; Bryant 2013), and the USA (McCubbin 1985; Engsberg 2006; Fowler 2010; Johnston 2011; Mattern‐Baxter 2013).
Interventions
Aerobic exercise
Six trials compared aerobic exercise to usual care (Van den Berg‐Emons 1998; Chrysagis 2009; Gharib 2011; Smania 2011; Chrysagis 2012; Emara 2015). One trial compared aerobic exercise to a physical therapy session, the content of which was not clear (Mattern‐Baxter 2013). One trial with three arms compared aerobic exercise on a static bike, aerobic exercise on a treadmill and usual care (Bryant 2013). Modes of aerobic exercise included swimming; cycling on a stationary bike; wheelchair driving; negotiating stairs; and walking or running on a gait trainer, treadmill or overground.
Resistance training
Ten trials compared lower limb resistance training to usual care, active movements without resistance or no physiotherapy (Dodd 2003; Liao 2007; Seniorou 2007; Lee 2008; Maeland 2009; Scholtes 2010; Pandey 2011; Taylor 2013; Lee 2015; Mitchell 2016). Two trials compared upper limb, lower limb, and trunk resistance training to usual care (Unger 2006; Tedla 2014).
One trial with four arms compared resistance training of the dorsiflexors, plantarflexors, plantar and dorsiflexors verus no resistance training (Engsberg 2006). Two trials compared upper limb strength training to normal activity or active movements without resistance (McCubbin 1985; Reid 2010).
Mixed training
Four trials compared mixed training to usual care (Unnithan 2007; Verschuren 2007; Fowler 2010; Chen 2012). Mixed training consisted of aerobic and lower limb resistance training (Fowler 2010; Chen 2012); upper and lower limb and trunk resistance training and aerobic exercise (Unnithan 2007); and aerobic, anaerobic and general strength training (Verschuren 2007).
Two trials compared aerobic exercise to resistance training, either of the trunk, upper and lower limbs (Johnston 2011), or of the lower limbs only (Olama 2011).
Duration, frequency and intensity of interventions
The duration of aerobic exercise programmes ranged from two weeks (Smania 2011) to nine months (Van den Berg‐Emons 1998). The duration of resistance training programmes ranged from 4 weeks (Pandey 2011) to 20 weeks (Mitchell 2016). The duration of mixed training programmes ranged from 12 weeks (Unnithan 2007; Fowler 2010; Chen 2012) to 8 months (Verschuren 2007). The duration of interventions in one trial comparing aerobic exercise to lower limb resistance training was 12 weeks (Johnston 2011). It was not clear how long the intervention was in a second trial comparing aerobic exercise to resistance training, as authors provided contradictory information (Olama 2011).
Aerobic exercise
For aerobic interventions, participants were prescribed the intervention for two days per week (Verschuren 2007; Chrysagis 2009), three days per week (Unnithan 2007; Fowler 2010; Gharib 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Emara 2015), four days per week (Van den Berg‐Emons 1998), five days per week (Johnston 2011; Smania 2011), six days per week (Mattern‐Baxter 2013), or seven days per week (Olama 2011). The prescribed duration of exercise per session ranged from 12 min to 60 min. One trial did not report duration of exercise (Olama 2011). The intensity of aerobic exercise was unclear or not reported in eight trials (Verschuren 2007; Chrysagis 2009; Gharib 2011; Johnston 2011; Olama 2011; Chen 2012; Bryant 2013; Mattern‐Baxter 2013). One trial reported that participants were prescribed walking "at a comfortable speed" (Chrysagis 2012), and one trial reported that participants were prescribed walking at 75% of their comfortable walking speed (Emara 2015). Two trials did not report the intensity prescribed but reported that walking speed gradually increased throughout the programme (Smania 2011), and that the mean time spent at > 70% of heart rate reserve was 49% (SD 17%) when heart rate was randomly measured in participants during the intervention (Van den Berg‐Emons 1998). Two trials prescribed an intensity of 65% to 75% of heart rate maximum and 70% to 80% of heart rate reserve, respectively (Unnithan 2007; Fowler 2010).
Seven trials did not report fidelity to the intervention (Unnithan 2007; Chrysagis 2009; Gharib 2011; Olama 2011; Smania 2011; Chen 2012; Emara 2015), although one reported excluding participants from the analysis if they missed more than three of the prescribed sessions (Gharib 2011). Six trials reported attendance at sessions (Van den Berg‐Emons 1998; Verschuren 2007; Fowler 2010; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013); the average adherence to the prescribed number of sessions across trials ranged from 77.0% to 93.0%. One trial also reported the average time walked per session (Mattern‐Baxter 2013), which was 28.2 min a day (range 9.6 min a day to 39.3 min a day) out of a prescribed 20.0 to 40.0 min a day. Only one trial reported fidelity to the intensity of the intervention, reporting that the mean percentage of heart‐rate maximum attained during sessions was 52.2% (SD 12.2%; range 8% to 77%) (Fowler 2010).
Resistance training
For resistance training interventions, participants were prescribed the intervention for one to three days a week (Unger 2006), two days per week (Verschuren 2007; Pandey 2011; Taylor 2013), three days a week (McCubbin 1985; Dodd 2003; Liao 2007; Seniorou 2007; Unnithan 2007; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Olama 2011; Chen 2012; Tedla 2014; Lee 2015), five days a week (Johnston 2011), or six days a week (Mitchell 2016). Therapists prescribed the following sets, repetitions and intensity: two sets of 10 repetitions to fatigue (although only 0.25 kg, 0.45 kg or 0.90 kg weights were used) (Lee 2008); two sets of 10 repetitions at 75% of one RM (Chen 2012); three sets of eight repetitions at 0% to 100% of eight RM (Scholtes 2010); three sets of 10 repetitions at an intensity that was not reported, 50% to 70% of maximum torque, or 10 RM, respectively (McCubbin 1985; Seniorou 2007; Reid 2010); three sets of 8 to 10 repetitions at 8 to 12 RM (Dodd 2003); three sets of 10 to 12 repetitions at 60% to 80% of one RM (Taylor 2013); three sets of 6 to 10 repetitions at 80% of one RM (Tedla 2014); four sets of 12 to 15 repetitions at 60% to 75% of one RM and four to six repetitions at 85% of one RM (Maeland 2009); one to three sets of 6 to 12 repetitions to fatigue (Unger 2006); six sets of five repetitions at 80% or more of one RM (Engsberg 2006); 7 to 11 activities with 5 to 10 repetitions or 20 repetitions, depending on the exercise, at 75% of one RM (Mitchell 2016); two sets of 10 repetitions at 20% of one RM and one set of as many repetitions as possible at 50% of one RM (Liao 2007); one set of 10 repetitions at 50% of 10 RM gradually increased to 10 repetitions at 100% of 10 RM (Olama 2011); three to five sets of 8 to 15 repetitions depending on the exercise (intensity not reported) (Unnithan 2007). Three trials did not report the number of sets, repetitions or intensity prescribed (Verschuren 2007; Johnston 2011; Pandey 2011). Two trials did not report the sets or repetitions prescribed but reported that exercises were performed at eight RM and 10 RM in Lee 2015 and Fowler 2010, respectively.
Eleven studies did not provide information on fidelity to the intervention (McCubbin 1985; Engsberg 2006; Unger 2006; Seniorou 2007; Unnithan 2007; Lee 2008; Olama 2011; Pandey 2011; Chen 2012; Tedla 2014; Lee 2015). Of the studies that reported adherence to the prescribed number of sessions (Dodd 2003; Verschuren 2007; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Taylor 2013), the average adherence ranged from 88.9% (Reid 2010) to 93.3% (Dodd 2003). One study reported that participants completed, on average, 32.4 hours of potential 60 hours of training (Mitchell 2016). One trial reported that the mean sets performed were 147.7 (SD 23.4) out of a possible 162 (Dodd 2003). Only two trials provided information on fidelity to the intensity of the intervention. One trial reported that the mean rating of exertion at the end of each session was 6.9 (SD 1.1) out of 10, and that participants increased their training load from session 3 to 24 by a mean of 183% (SD 23%) (Taylor 2013). The second trial reported that participants increased their training load by a mean of 17.5 kg (SD 11.7 kg; range 0 kg to 40.8 kg) (Fowler 2010).
Comparator
Nineteen trials reported that the control group received usual physiotherapy. Eleven trials reported the general content of usual physiotherapy (Liao 2007; Unnithan 2007; Lee 2008; Maeland 2009; Gharib 2011; Smania 2011; Chrysagis 2012; Bryant 2013; Tedla 2014; Emara 2015; Lee 2015), although this varied across individuals, and it was difficult to determine what usual care each participant received. Ten trials stated the dose of usual physiotherapy that was prescribed (Van den Berg‐Emons 1998; Liao 2007; Unnithan 2007; Scholtes 2010; Gharib 2011; Smania 2011; Chrysagis 2012; Tedla 2014; Emara 2015; Lee 2015). Four trials indicated that participants in the control group continued with usual activities, but not specifically usual physiotherapy (Chrysagis 2009; Fowler 2010; Reid 2010; Chen 2012). For two trials, it was unclear what the control group did during the intervention period; one trial reported that the control group received no strengthening (Engsberg 2006) and the authors of the second trial provided no information about the comparator (Unger 2006). Two trials investigating the effect of resistance training prescribed active movements without resistance to the control group (McCubbin 1985; Seniorou 2007). Pandey 2011 was the only trial to specifically state that all participants were not allowed to receive usual physiotherapy for the duration of the trial. Two trials tracked physiotherapy received by all participants and reported that physiotherapy did not differ between the control and intervention group (Verschuren 2007; Mitchell 2016), although neither trial reported the content of physiotherapy, and one trial did not report the dose (Verschuren 2007).
Ongoing studies
All ongoing studies were RCTs conducted in Australia (Gillett 2015), Canada (NCT02754128), the UK (ISRCTN90378161; NCT02766491), and Brazil (RBR‐5rh6cg). The number of participants that studies recruited ranged from 22 in NCT02754128 to 60 in ISRCTN90378161. Participants were aged 15 to 30 years (Gillett 2015), 7 to 17 years (NCT02754128), 7 to 14 years (NCT02766491), 4 to 11 years (RBR‐5rh6cg), and 10 to 19 years (ISRCTN90378161). Three studies specified that they included children with spastic CP (RBR‐5rh6cg; Gillett 2015; NCT02766491). Two studies specified that participants had hemiplegia and diplegia (Gillett 2015; NCT02754128), one specified that participants had diplegia (RBR‐5rh6cg), and two did not specify the anatomical distribution of CP participants (ISRCTN90378161; NCT02766491). Two studies included participants in GMFCS level I and II (Gillett 2015; NCT02754128), two studies included participants in GMFCS levels I, II and III (ISRCTN90378161; NCT02766491), and one study included participants in GMFCS level IV (RBR‐5rh6cg). Three studies were investigating the effect of resistance training compared to usual care (ISRCTN90378161), no training (Gillett 2015), or conventional stretching and upper limb exercises (NCT02766491). One study was comparing resistance training to aerobic exercise (NCT02754128), and one study was comparing trunk control exercises to conventional aquatic therapy (RBR‐5rh6cg).
Studies awaiting classification
The single study awaiting classification was a RCT comparing aerobic training delivered three times a week for nine weeks, to an arts programme of the same duration, delivered in a school in Australia (Carlon 2014). Participants were 19 children with a mean age of three years classified in GMFCS levels I, II and III. We contacted the authors when we identified the study to determine if the results had been published as a full report and were informed that a full report had not been published at the time. We were unable to determine from the abstract if the intervention met our definition of aerobic exercise.
Excluded studies
We excluded 99 studies (102 reports) following full‐text screening and provide details in the Characteristics of excluded studies table. The main reasons for exclusion were as follows: in 43 studies the intervention did not meet the definition of aerobic or resistance training, as defined in the Description of the intervention section; 23 studies were not RCTs; 10 studies compared two different modes of the same type of exercise (Stackhouse 2007; Willoughby 2010; Kim 2012; Olama 2012; Grecco 2013a; Grecco 2013b; Moreau 2013; Su 2013; Hussein 2014; Swe 2015); nine studies did not involve participants with a diagnosis of CP alone (Katz‐Leurer 2009; Speyer 2010; Salem 2012; Angulo‐Barroso 2013; Ayhan 2014; Hammond 2014; Williams 2014; Lowe 2015; Hsieh 2016); in seven studies exercise was provided as intervention in conjunction with another treatment, and it was not possible to determine the independent effect of exercise on the outcome (Patikas 2006; Bandholm 2012; Williams 2013; Slaman 2014; Van Wely 2014; Preston 2015; Sherief AEAA 2015); five studies were not original research (Hornyak 2008; Taylor 2009; Boyd 2010; Roberti 2011; Verschuren 2014); one study did not use exercise or usual care as the comparator (Ahlborg 2006); and in one study it was not possible to determine the content of the intervention (Kumar 2013).
Risk of bias in included studies
Figure 2 and Figure 3 summarise the 'Risk of bias' assessments for all included trials. We judged all trials as being at high risk of bias overall because we rated at least one domain as being at high risk of bias.
2.
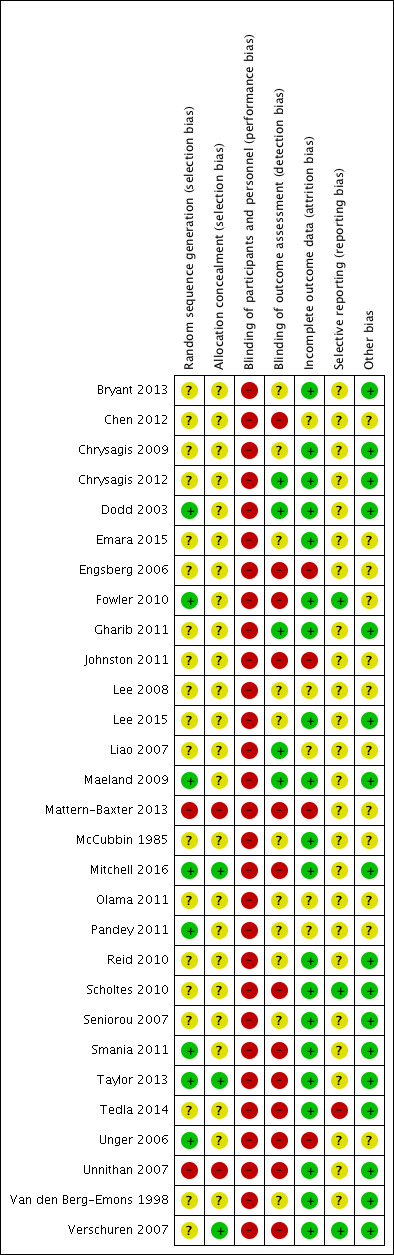
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
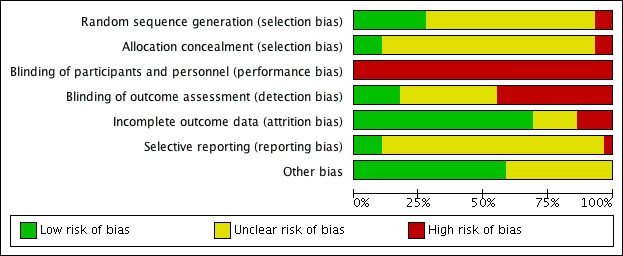
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We judged eight trials to be at low risk of selection bias, as two used adequate methods of sequence generation (Taylor 2013; Mitchell 2016), and six provided sufficient information to determine the sequence generation was random (Dodd 2003; Unger 2006; Maeland 2009; Fowler 2010; Pandey 2011; Smania 2011).
We judged 19 trials to be at unclear risk of selection bias: 18 because they failed to adequately report the methods used to generate a random sequence (McCubbin 1985; Van den Berg‐Emons 1998; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Chrysagis 2009; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Tedla 2014; Emara 2015; Lee 2015), and one because, although the authors stated that participants were randomly assigned to two groups using a four‐block randomisation process, they did not describe the randomisation process (Verschuren 2007).
We rated two trials at high risk of selection bias as they used a quasi‐randomisation method (Unnithan 2007; Mattern‐Baxter 2013).
Allocation concealment
We judged three trials to be at low risk of selection bias as they used adequate methods of allocation concealment (Verschuren 2007; Taylor 2013; Mitchell 2016).
We judged 24 trials that failed to adequately report the methods used to conceal allocation at unclear risk of selection bias (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Tedla 2014; Emara 2015; Lee 2015).
We judged two trials at high risk of selection bias as they used a quasi‐randomisation method (Unnithan 2007; Mattern‐Baxter 2013).
Blinding
Blinding of participants and personnel (performance bias)
We judged all 29 trials to be at high risk of performance bias as participants or personnel were not blinded to group allocation (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Verschuren 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013; Taylor 2013; Tedla 2014; Emara 2015; Lee 2015; Mitchell 2016).
Blinding of outcome assessment (detection bias)
We rated 13 trials at high risk of detection bias (Engsberg 2006; Unger 2006; Unnithan 2007; Verschuren 2007; Fowler 2010; Scholtes 2010; Johnston 2011; Smania 2011; Chen 2012; Mattern‐Baxter 2013; Taylor 2013; Tedla 2014; Mitchell 2016). Objective outcomes were assessed by assessors blinded to group allocation in six of these trials, but participants or their parents were responsible for self‐report measures, and they were not blinded to group allocation, suggesting that a lack of blinding was likely to have affected a number of trial outcomes (Unger 2006; Verschuren 2007; Fowler 2010; Scholtes 2010; Smania 2011; Taylor 2013). The remaining studies stated that at least some of the assessments were conducted by a person who was not blind to group allocation (Engsberg 2006; Unnithan 2007; Johnston 2011; Chen 2012; Mattern‐Baxter 2013; Tedla 2014; Mitchell 2016).
Eleven trials did not report adequate information to determine if outcome assessors were blinded to group allocation, so we judged these trials to be at unclear risk of detection bias (McCubbin 1985; Van den Berg‐Emons 1998; Seniorou 2007; Lee 2008; Chrysagis 2009; Reid 2010; Olama 2011; Pandey 2011; Bryant 2013; Emara 2015; Lee 2015).
We rated five trials at low risk of detection bias because assessors were blinded to group allocation (Dodd 2003; Liao 2007; Maeland 2009; Gharib 2011; Chrysagis 2012).
Incomplete outcome data
We considered 20 trials to be at low risk of attrition bias, as they had no missing data, low rates of missing data (10% or less) that were evenly distributed across groups, or the authors performed an intention‐to‐treat analysis (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Seniorou 2007; Unnithan 2007; Verschuren 2007; Chrysagis 2009; Maeland 2009; Fowler 2010; Reid 2010; Scholtes 2010; Gharib 2011; Smania 2011; Chrysagis 2012; Bryant 2013; Taylor 2013; Tedla 2014; Emara 2015; Lee 2015; Mitchell 2016).
We judged five trials, which did not provide sufficient information on the number of participants who withdrew from the study or the reasons for withdrawal, at unclear risk of bias (Liao 2007; Lee 2008; Olama 2011; Pandey 2011; Chen 2012).
Four trials had high rates of missing data (three had 20% or more, and one had more than 30%), missing data were not evenly distributed across groups, or reasons for missing data were likely to be related to the trial outcome and were therefore judged as being at high risk of bias (Engsberg 2006; Unger 2006; Johnston 2011; Mattern‐Baxter 2013).
Selective reporting
We considered three trials to be at low risk of reporting bias, as they adequately reported outcome data described in the trial protocol (Verschuren 2007; Fowler 2010; Scholtes 2010).
We judged 25 trials to be at unclear risk of bias: one because a trial protocol was available and outcomes were reported across two reports, but some outcomes were not reported to date (Mitchell 2016), and 24 because a trial protocol was not available and therefore it was not possible to determine if all expected outcomes were reported (McCubbin 1985; Van den Berg‐Emons 1998; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Lee 2008; Chrysagis 2009; Maeland 2009; Reid 2010; Gharib 2011; Johnston 2011; Olama 2011; Pandey 2011; Smania 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013; Taylor 2013; Emara 2015; Lee 2015).
We rated one trial at high risk of bias because data on strength and gross motor function were reported incompletely and could not be entered into a meta‐analysis (Tedla 2014).
Other potential sources of bias
We considered 12 trials to be at unclear risk of other potential bias: 2 for not providing any demographic data (McCubbin 1985; Olama 2011), 3 for providing demographic and baseline outcome data for those who completed the study only (Liao 2007; Chen 2012; Mattern‐Baxter 2013), 3 for being unclear about whether all demographic or baseline data were reported (Lee 2008; Pandey 2011; Emara 2015); 1 for providing outcome data only for those who completed the study (Unger 2006), 1 for providing demographic and baseline data only for those who completed and for not reporting all outcome data for those who completed the study (Johnston 2011), 1 for reporting demographic data only for those who completed the study and outcome data only for those participants who improved (Engsberg 2006), and 1 for unaccounted missing data (Fowler 2010).
We considered 17 trials to be at low risk of bias for other potential bias (Van den Berg‐Emons 1998; Dodd 2003; Seniorou 2007; Unnithan 2007; Verschuren 2007; Chrysagis 2009; Maeland 2009; Reid 2010; Scholtes 2010; Gharib 2011; Smania 2011; Chrysagis 2012; Bryant 2013; Taylor 2013; Tedla 2014; Lee 2015; Mitchell 2016).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
All 29 trials reported outcomes at short‐term time points (i.e. zero to one month postintervention). Nine trials reported outcomes at intermediate time points (i.e. more than one month to six months' postintervention) (Dodd 2003; Seniorou 2007; Verschuren 2007; Lee 2008; Scholtes 2010; Johnston 2011; Bryant 2013; Mattern‐Baxter 2013; Taylor 2013). One trial reported that assessments were made preintervention, postintervention and at 'follow‐up' but did not state when the follow‐up assessment occurred (Pandey 2011). We therefore did not report this follow‐up time point. No trials reported outcomes at time points beyond six months' postintervention.
As described under Data synthesis, we performed separate meta‐analyses for studies in adults versus children and adolescents. We reported the results for three trials involing adolescents and adults (aged 10 to 22 years) (McCubbin 1985; Chrysagis 2009; Taylor 2013). We identified sufficient data (at least 10 studies in a meta‐analysis) to examine funnel plots for two meta‐analyses and found no indication of publication bias (see Figure 4; Figure 5).
4.
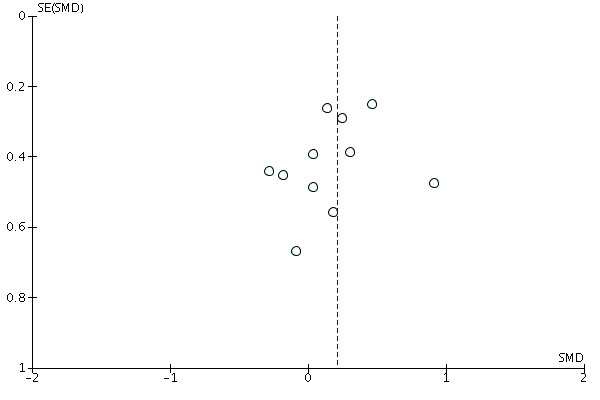
Funnel plot of comparison: 6 Resistance training and mixed training versus usual care, outcome: 6.1 Activity: gross motor function; short term.
5.
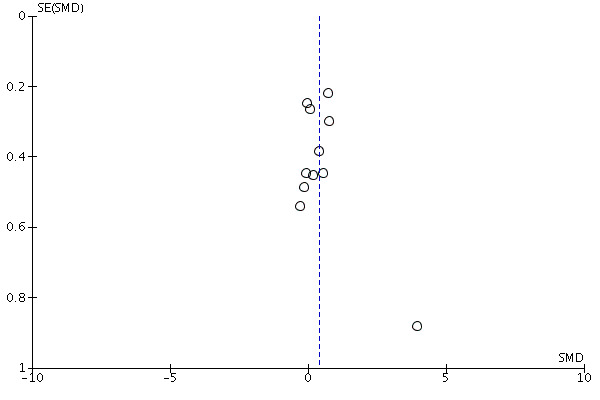
Funnel plot of comparison: 6 Resistance training and mixed training versus usual care, outcome: 6.6 Muscle strength; short term.
Comparison 1: aerobic exercise versus usual care
Seven trials comparing aerobic exercise to usual care reported at least one primary or secondary outcome of interest (Van den Berg‐Emons 1998; Chrysagis 2009; Gharib 2011; Smania 2011; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013). One trial did not report any outcomes of interest (Emara 2015), so we do not report its results.
Activity
Pooled results
Three trials assessed gross motor function in children and adolescents following aerobic exercise (Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013). For one three‐armed trial, we combined data from two aerobic exercise groups (bike and treadmill exercise) to compare all modes of aerobic exercise to usual care (Bryant 2013).
The pooled analysis of three trials in 65 children and adolescents indicated that aerobic training improved gross motor function compared to usual care in the short term (SMD 0.53, 95% CI 0.02 to 1.04, P = 0.04, I2 = 0%, Analysis 1.1; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013).
1.1. Analysis.
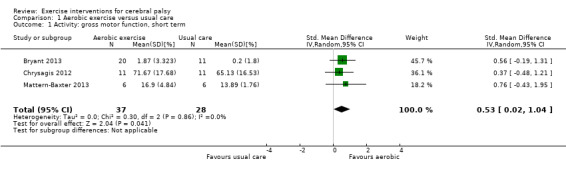
Comparison 1 Aerobic exercise versus usual care, Outcome 1 Activity: gross motor function, short term.
Pooled analysis of four trials with high heterogeneity involving 82 children and adolescents (GMFCS levels I to IV), demonstrated that aerobic exercise did not result in an improvement in gait speed in the short term (MD 0.09 m/s, 95% CI −0.11 m/s to 0.28 m/s, P = 0.38, I2 = 78%, Analysis 1.2; Gharib 2011; Smania 2011; Chrysagis 2012; Mattern‐Baxter 2013).
1.2. Analysis.
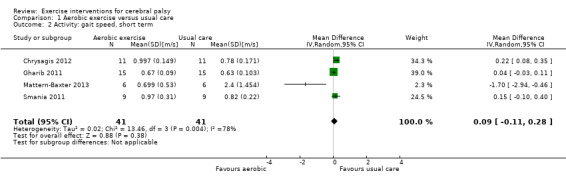
Comparison 1 Aerobic exercise versus usual care, Outcome 2 Activity: gait speed, short term.
Single study results
One trial of 12 adolescents and young adults (GMFCS levels I to III; Chrysagis 2009) reported that there was no between‐group difference in gross motor function over time (P = 0.11). Although the authors reported that the mean score on dimension E of the GMFM increased following aerobic exercise from 59.02% to 65.04% in the short‐term, and that there was little short‐term change in the comparison group (mean increased from 59.02% to 59.95%), they did not provide information to allow us to calculate the mean difference between groups.
One trial, Smania 2011 (18 children and adolescents, GMFCS levels I to IV), reported a greater short‐term improvement in walking endurance, measured as the distance walked (meters) at self‐selected walking speed for six minutes, following aerobic exercise (Cohen's d = 0.44, P = 0.02). Using the postintervention means and SDs provided for each group, we calculated an MD of 41.00 m per 6 minutes walked (95% CI −46.98 m to 128.98 m, Analysis 1.3).
1.3. Analysis.
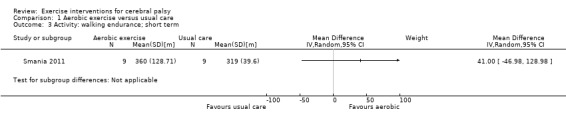
Comparison 1 Aerobic exercise versus usual care, Outcome 3 Activity: walking endurance; short term.
Only one trial, Mattern‐Baxter 2013 (12 children), compared aerobic exercise versus usual care for gait speed in the intermediate term, reporting no between‐group difference in gait speed (MD −0.17 m/s, 95% CI −0.59 m/s to 0.24 m/s, Analysis 1.4).
1.4. Analysis.
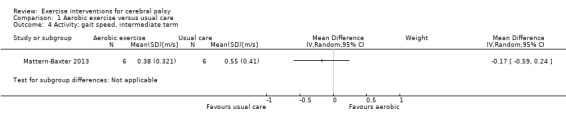
Comparison 1 Aerobic exercise versus usual care, Outcome 4 Activity: gait speed, intermediate term.
Although gross motor function was measured in children and adolescents with CP at an intermediate time point in two trials, the authors of one trial did not provide a P value to support this or data to allow us to calculate it (Bryant 2013). The second trial, Mattern‐Baxter 2013, found that gross motor function improved in the intermediate term (MD 12.96%, 95% CI 0.52% to 25.40%, 12 children, Analysis 1.5).
1.5. Analysis.
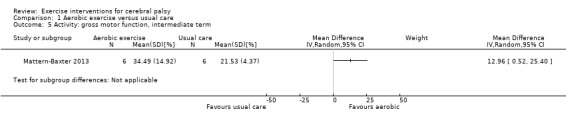
Comparison 1 Aerobic exercise versus usual care, Outcome 5 Activity: gross motor function, intermediate term.
One trial, Van den Berg‐Emons 1998 (20 children and adolescents, ambulatory and non‐ambulatory), reported that there was no evidence of a difference in the change in daily physical activity (calculated as the ratio of total energy expenditure to sleeping or resting metabolic rate between participants in the intervention group versus usual care after nine months of aerobic exercise (P value not reported). Using the postintervention means and SDs provided, we calculated an MD of 0.21 (95% CI 0.04 to 0.38, Analysis 1.6) in daily physical activity.
1.6. Analysis.
Comparison 1 Aerobic exercise versus usual care, Outcome 6 Activity: daily physical activity; short term.
Participation
No trial assessed the effect of aerobic exercise in comparison to usual care on participation.
Quality of life
No trial comparing aerobic exercise to usual care assessed quality of life.
Adverse events
Pooled results
No meta‐analysis was possible.
Single study results
Four out of eight trials did not report monitoring adverse events (Van den Berg‐Emons 1998; Chrysagis 2009; Gharib 2011; Emara 2015). Four trials reported that participants did not experience any adverse events (Smania 2011; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013).
Body functions and body structures
Pooled results
No meta‐analysis was possible.
Single study results
Only one trial in 20 ambulatory and non‐ambulatory children and adolescents assessed aerobic fitness following an aerobic exercise intervention (Van den Berg‐Emons 1998). This trial reported that after nine months of aerobic exercise there was a greater increase in aerobic fitness in participants with CP in the intervention group compared to the usual care group in the short term (P < 0.05; effect size and exact P value not reported). Our analysis of postintervention means and SDs provided an MD of 0.06 W/kg FFM (95% CI −0.71 W/kg FFM to 0.83 W/kg FFM, Analysis 1.7).
1.7. Analysis.
Comparison 1 Aerobic exercise versus usual care, Outcome 7 Aerobic fitness; short term.
Sensitivity analysis
To assess the influence of our analysis model on the results, we repeated the pooled analyses using a fixed‐effect model instead of a random‐effects model. Using a fixed‐effect model, there was some evidence of an improvement in gait speed following aerobic exercise compared to usual care in 82 children and adolescents in the short term, with no change in heterogeneity (MD 0.08 m/s, 95% CI 0.02 m/s to 0.14 m/s, P = 0.01, I2 = 78%, 4 studies; Gharib 2011; Smania 2011; Chrysagis 2012; Mattern‐Baxter 2013; analysis not shown). Using a fixed‐effect model instead of a random‐effects model had no impact on gross motor function in children and adolescents in the short term (analysis not shown).
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that aerobic exercise improves gross motor function and physical activity but does not improve walking endurance or aerobic fitness in children and adolescents with CP in the short term. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision, once for inconsistency) that aerobic exercise does not improve gait speed in children and adolescents with CP in the short term.
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that aerobic exercise improves gross motor function but does not improve gait speed in children and adolescents with CP in the intermediate term.
See Table 1.
Comparison 2: resistance training versus usual care
Fourteen trials comparing resistance training to usual care reported outcomes of interest; 11 included children and adolescents (Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Reid 2010; Scholtes 2010; Pandey 2011; Tedla 2014; Lee 2015; Mitchell 2016), one included adolescents only (Unger 2006), one included adolescents and young adults aged 14 to 22 years (Taylor 2013), and one included adults (Maeland 2009). One trial investigating the effect of resistance training in adolescents and young adults with CP did not report any outcomes of interest, so we do not report the results of this trial (McCubbin 1985).
Activity
Pooled results
Eight trials assessed gross motor function in children and adolescents using the GMFM (Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Scholtes 2010; Tedla 2014; Lee 2015). Tedla 2014 did not report between‐group differences, so we could not extract data. For the remaining seven studies, resistance training did not improve short‐term gross motor function more than usual care (SMD 0.12, 95% CI −0.19 to 0.43, P = 0.45, I2 = 0%, 164 children and adolescents, Analysis 2.1; Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Scholtes 2010; Lee 2015). Gross motor function also did not improve following resistance training in 85 children and adolescents (GMFCS levels I to III) in the intermediate term (SMD 0.13, 95% CI −0.30 to 0.55, P = 0.57, I2 = 0%, 3 studies, Analysis 2.2; Dodd 2003; Lee 2008; Scholtes 2010).
2.1. Analysis.
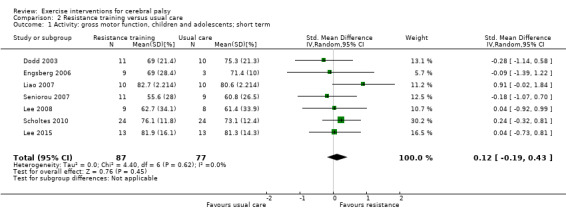
Comparison 2 Resistance training versus usual care, Outcome 1 Activity: gross motor function, children and adolescents; short term.
2.2. Analysis.
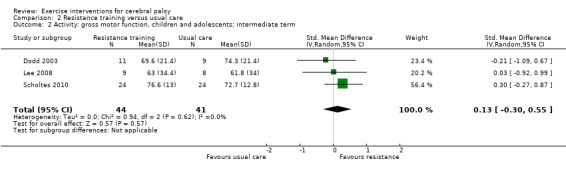
Comparison 2 Resistance training versus usual care, Outcome 2 Activity: gross motor function, children and adolescents; intermediate term.
Short‐term gait speed did not improve in 185 children and adolescents with CP (GMFCS levels I to III) following resistance training compared to usual care (MD 0.03 m/s, 95% CI −0.02 m/s to 0.07 m/s, P = 0.20, I2 = 0%, 8 studies, Analysis 2.3; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Lee 2008; Scholtes 2010; Pandey 2011). In addition, resistance training did not improve intermediate‐term gait speed in comparison to usual care (MD −0.03 m/s, 95% CI −0.17 m/s to 0.11 m/s, P = 0.65, I2 = 0%, 84 children and adolescents, 3 studies, Analysis 2.4; Dodd 2003; Lee 2008; Scholtes 2010).
2.3. Analysis.
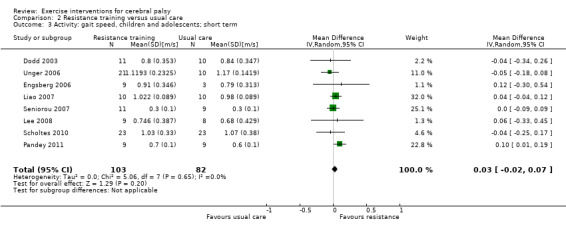
Comparison 2 Resistance training versus usual care, Outcome 3 Activity: gait speed, children and adolescents; short term.
2.4. Analysis.
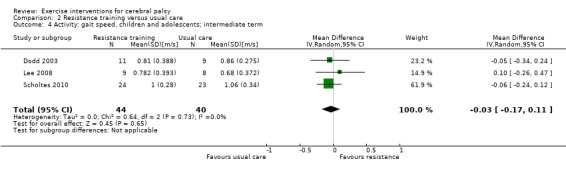
Comparison 2 Resistance training versus usual care, Outcome 4 Activity: gait speed, children and adolescents; intermediate term.
Single study results
Maeland 2009 reported that resistance training did not improve short‐term gait speed in 12 adults in GMFCS levels II and III (MD 0.30 m/s, 95% CI −0.28 m/s to 0.88 m/s, Analysis 2.5); short‐term gross motor function, assessed with a timed stair climb (MD 10.00 stairs, 95% CI −13.55 stairs to 33.55 stairs, Analysis 2.6); or short‐term walking endurance (MD 127.00 m, 95% CI −95.08 m to 349.08 m, Analysis 2.7).
2.5. Analysis.
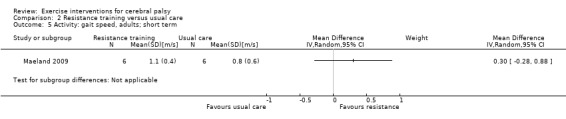
Comparison 2 Resistance training versus usual care, Outcome 5 Activity: gait speed, adults; short term.
2.6. Analysis.
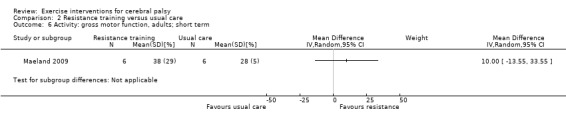
Comparison 2 Resistance training versus usual care, Outcome 6 Activity: gross motor function, adults; short term.
2.7. Analysis.
Comparison 2 Resistance training versus usual care, Outcome 7 Activity: walking endurance, adults; short term.
One trial in 48 adolescents and young adults in GMFCS levels II and III reported that, in the short term, resistance training did not improve gait speed (MD 0.01 m/s, 95% CI −0.06 m/s to 0.07 m/s), gross motor function (MD 0.9%, 95% CI −3.0% to 4.7%), walking endurance (MD 0.1 m, 95% CI −20.6 m to 20.9 m), or steps per day (MD −28 steps/day, 95% CI −1373 steps/day to 1317 steps/day) (Taylor 2013). The same trial reported that resistance training did not improve these outcomes in the intermediate term: gait speed (MD – 0.05 m/s, 95% CI – 0.11 m/s to 0.02 m/s), gross motor function (MD 1.0%, 95% CI –2.6% to 4.5%), walking endurance (MD −12.3 m, 95% CI −34.8 m to 10.2 m) and steps per day (MD −1093 steps/day, 95% CI −2316 steps/day to 130 steps/day).
Only one trial, Mitchell 2016, assessed walking endurance in 101 children and adolescents (GMFCS level I and II), reporting that walking endurance improved following resistance training in comparison to usual care (MD 38.9 m, 95% CI 12.3 m to 64.5 m). Mitchell 2016 also reported that resistance training did not result in an improvement in steps per day among children in the short term (MD 563.7 steps/day, 95% CI −706.5 steps/day to 1833.8 steps/day).
Participation
Pooled results
Pooled analysis of two trials, Mitchell 2016 and Scholtes 2010, suggested that short‐term participation did not improve in participants in GMFCS levels I to III receiving resistance training versus usual care (SMD 0.34, 95% CI −0.01 to 0.70, P = 0.06, I2 = 0%, 127 children and adolescents, Analysis 2.8).
2.8. Analysis.
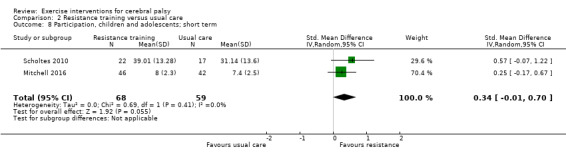
Comparison 2 Resistance training versus usual care, Outcome 8 Participation, children and adolescents; short term.
Single study results
One trial, Scholtes 2010, also reported that participation did not improve in the intermediate term (P = 0.12). We calculated an MD of 0.37 (95% CI −6.61 to 7.35, 36 children and adolescents, Analysis 2.9) based on the data provided.
2.9. Analysis.
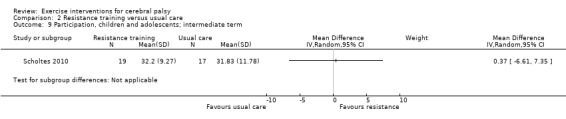
Comparison 2 Resistance training versus usual care, Outcome 9 Participation, children and adolescents; intermediate term.
Quality of life
Pooled results
No meta‐analysis was possible.
Single study results
One trial, Engsberg 2006, reported that parent‐reported quality of life improved in 12 children and adolescents with CP following resistance training in the short term, but participant‐reported quality of life did not. However, there was no evidence of a between‐group difference for parent‐ or child‐reported quality of life based on the data provided (parent reported: MD 12.70, 95% CI −5.63 to 31.03, Analysis 2.10; child reported: MD 11.70, 95% CI −8.32 to 31.72, Analysis 2.11).
2.10. Analysis.
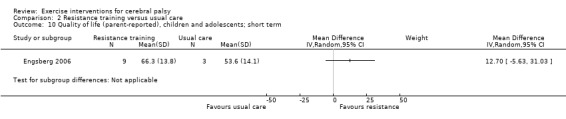
Comparison 2 Resistance training versus usual care, Outcome 10 Quality of life (parent‐reported), children and adolescents; short term.
2.11. Analysis.
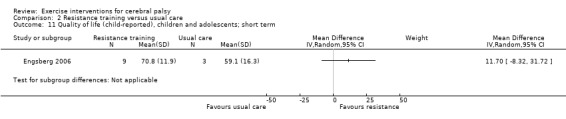
Comparison 2 Resistance training versus usual care, Outcome 11 Quality of life (child‐reported), children and adolescents; short term.
Adverse events
Pooled results
No meta‐analysis was possible.
Single study results
Seven trials did not state whether they recorded any adverse events (McCubbin 1985; Unger 2006; Seniorou 2007; Lee 2008; Reid 2010; Pandey 2011; Lee 2015). Two trials stated that neither participants nor physiotherapists reported or registered any adverse events (Engsberg 2006; Maeland 2009). One trial stated that there were no adverse events that led to missing training sessions (Dodd 2003). However, one participant reported pressure on the shoulders from a loaded backpack, and two participants reported mild foot and ankle discomfort during heel raises (Dodd 2003). Exercises were modified and enabled participants to continue without incident (Dodd 2003). Four trials stated that participants reported muscle soreness, which subsided with rest (Scholtes 2010; Taylor 2013; Tedla 2014; Mitchell 2016). One trial reported that a participant in the intervention group had seizures during the study period, but this was deemed unrelated to the training (Mitchell 2016). In addition, two participants in one trial reported minor calf strain and discomfort to the plantar fascia, respectively, resulting in the programme being adjusted but no missed sessions (Taylor 2013). One trial reported that participants experienced discomfort due to wearing a weighted vest (Liao 2007).
Body structure and function
Twelve trials assessed muscle strength following resistance training (Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Lee 2008; Maeland 2009; Reid 2010; Scholtes 2010; Pandey 2011; Taylor 2013; Tedla 2014; Mitchell 2016). It was not possible to extract data from Tedla 2014, which did not present the necessary numerical data. We did not report muscle strength from a second trial because the authors only included data on participants whose muscle strength improved in the analysis (Engsberg 2006).
Pooled results
Muscle strength improved more with resistance training compared to usual care in children and adolescents in GMFCS levels I to III, both in the short term (SMD 0.53, 95% CI 0.00 to 1.06, P = 0.05, I2 = 70%, 247 children and adolescents, 8 studies, Analysis 2.12; Dodd 2003; Liao 2007; Seniorou 2007; Lee 2008; Reid 2010; Scholtes 2010; Pandey 2011; Mitchell 2016) and in the intermediate term (SMD 0.50, 95% CI 0.06 to 0.94, P = 0.03, I2 = 1%, 84 children and adolescents, 3 studies, Analysis 2.13; Dodd 2003; Lee 2008; Scholtes 2010).
2.12. Analysis.
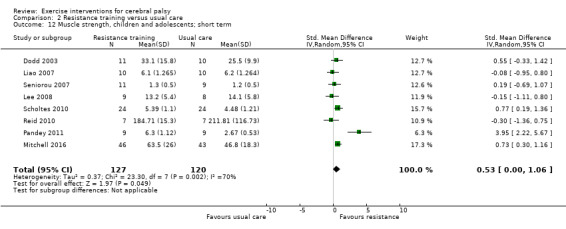
Comparison 2 Resistance training versus usual care, Outcome 12 Muscle strength, children and adolescents; short term.
2.13. Analysis.
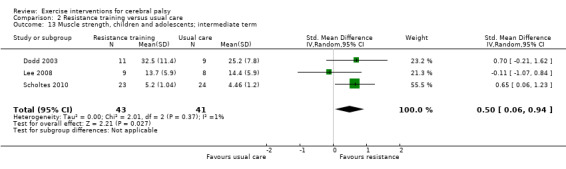
Comparison 2 Resistance training versus usual care, Outcome 13 Muscle strength, children and adolescents; intermediate term.
Single study results
The results of a single trial, Taylor 2013, suggested that resistance training resulted in a short‐term improvement in muscle strength in 48 adolescents and young adults in GMFCS levels II and III (MD 26.7% increase in strength, 95% CI 7.9% to 45.5%) but not in the intermediate term (MD 21.7%, 95% CI –17.3% to 61.7%).
One trial, Maeland 2009, which included 12 adults in GMFCS levels II and III, reported that resistance training did not result in short‐term improvements in muscle strength compared to usual care (P = 0.78). We calculated an MD of −7.00 Nm/s (95% CI −58.74 Nm/s to 44.74 Nm/s, Analysis 2.14) using data provided.
2.14. Analysis.
Comparison 2 Resistance training versus usual care, Outcome 14 Muscle strength, adults; short term.
Sensitivity analysis
To assess the influence of our analysis model on the results, we repeated the pooled analyses using a fixed‐effect model instead of a random‐effects model. Using a fixed‐effect model resulted in a change in the effect size for the effect of short‐term muscle strength in 247 children and adolescents, with no change in heterogeneity (SMD 0.55, 95% CI 0.28 to 0.81, P < 0.001, I2 = 70%, 8 studies; Dodd 2003; Liao 2007; Seniorou 2007; Lee 2008; Reid 2010; Scholtes 2010; Pandey 2011; Mitchell 2016; analysis not shown). Using a fixed‐effect model instead of a random‐effects model had no impact on intermediate‐term muscle strength in children and adolescents, or on gait speed, gross motor function, or participation in children and adolescents at any time point.
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that resistance training improves walking endurance but does not improve short‐term gross motor function, gait speed, physical activity, participation or quality of life in children and adolescents with CP. There is very low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision, once for consistency) that resistance training does improve short‐term muscle strength in children and adolescents with CP. There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that resistance training improves muscle strength but does not improve short‐term gait speed, gross motor function, walking endurance or physical activity in adolescents and young adults with CP. There is also low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that resistance training does not improve gait speed, gross motor function, walking endurance or muscle strength in adults with CP in the short term.
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that resistance training does not improve gait speed, gross motor function, walking endurance, physical activity or muscle strength in adolescents and young adults with CP in the intermediate term. There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that resistance training improves intermediate‐term muscle strength but not gross motor function, gait speed or participation in children and adolescents with CP.
See Table 2.
Comparison 3: mixed training versus usual care
Four trials compared mixed training to usual care in children and adolescents with CP (Verschuren 2007; Fowler 2010; Chen 2012) or adolescents only (Unnithan 2007).
Activity
Pooled results
There was no statistically significant between‐group difference in short‐term gross motor function in 163 participants in GMFCS levels I to III receiving mixed training versus usual care (SMD 0.02, 95% CI −0.29 to 0.33, P = 0.90, I2 = 0%, 4 studies, Analysis 3.1; Unnithan 2007; Verschuren 2007; Fowler 2010; Chen 2012).
3.1. Analysis.
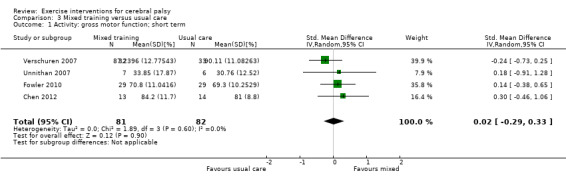
Comparison 3 Mixed training versus usual care, Outcome 1 Activity: gross motor function; short term.
Single study results
One trial, Fowler 2010, reported that mixed training did not improve self‐selected short‐term gait speed over 30 seconds (MD 0.10 m/s, 95% CI −0.07 m/s to 0.27 m/s, 58 children and adolescents, Analysis 3.2) or walking endurance, measured as the speed per distance completed on the 600‐yard (548.6 m) walk test (MD 6.50 m/min, 95% CI −14.91 m/min to 27.91 m/min, 55 children and adolescents, Analysis 3.3) compared to usual care, in participants in GMFCS levels I to III.
3.2. Analysis.
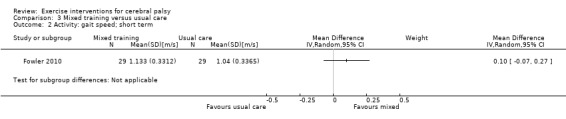
Comparison 3 Mixed training versus usual care, Outcome 2 Activity: gait speed; short term.
3.3. Analysis.
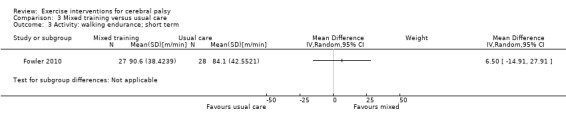
Comparison 3 Mixed training versus usual care, Outcome 3 Activity: walking endurance; short term.
One study, Verschuren 2007, reported that mixed training improved gross motor function, as measured by dimension D but not dimension E of the GMFM, in 65 children and adolescents (GMFCS levels I and II) in the intermediate term. When we calculated effect sizes using postintervention means and SDs, the effect size for dimension D was MD −2.62% (95% CI −6.26% to 1.03%) and dimension E was MD −4.90% (95% CI −11.58% to 1.78%). No trials reported intermediate changes in gait speed or walking endurance.
Participation
Pooled results
No meta‐analysis was possible.
Single study results
One trial, Verschuren 2007, reported that short‐term participation (as indicated by 'overall activities' scores for participation on the CAPE; King 2004) improved in 65 children and adolescents (GMFCS levels I and II) following mixed training compared to usual care (P = 0.002). We calculated an MD of 0.40 (95% CI 0.13 to 0.67, Analysis 3.4) using the data provided. There was no between‐group difference in participation in the intermediate term (MD 0.15, 95% CI −0.25 to 0.54, Analysis 3.5).
3.4. Analysis.
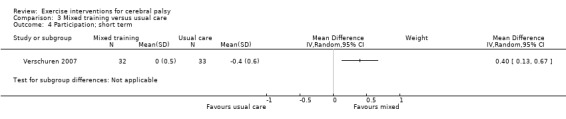
Comparison 3 Mixed training versus usual care, Outcome 4 Participation; short term.
3.5. Analysis.
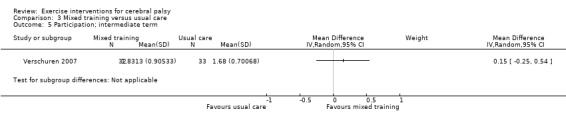
Comparison 3 Mixed training versus usual care, Outcome 5 Participation; intermediate term.
Quality of life
Pooled results
No meta‐analysis was possible.
Single study results
Two trials, Fowler 2010 and Verschuren 2007, reported quality of life following mixed training. One trial, Verschuren 2007, recorded quality of life in children and adolescents using the TNO‐AZL Questionnaire for Children's Health‐Related Quality of Life Parent Form (TACQOL‐PF; Vogels 2000). Scale scores are obtained by adding items scores (between three and seven per scale) within scales and transforming crude scale scores to a 0‐to‐100 scale. Quality of life was scored on seven scales (pain and symptoms, basic motor functioning, autonomy, cognitive functioning, social functioning, global positive emotions, and global negative emotions). The authors reported a statistically significant between‐group difference in scores on the 'basic motor functioning' (P = 0.001) and 'cognitive functioning' (P = 0.04) subscales in the short term but did not report effect sizes. We calculated short‐term effect sizes for 'basic motor functioning' (MD 3.80, 95% CI 1.71 to 5.89) and 'cognitive functioning' (MD 1.10, 95% CI −1.02 to 3.22); analyses not shown. The authors reported that there were no between‐group differences on any scale in the intermediate term (Verschuren 2007).
Another trial, Fowler 2010, assessed quality of life in 58 children and adolescents (GMFCS levels I to III) using participant responses for the Pediatric Quality of Life Inventory SF15 (PedsQL; Chan 2005) and parent responses for the Pediatric Outcomes Data Collection Instrument (PODCI; Daltroy 1998). We extracted the total score from the PedsQL (scale 0 to 100, higher score indicates more positive quality of life) and the score from each of the four sections in the PODCI (global functioning and symptoms, happiness, treatment expectations, and satisfaction with symptoms). There was no between‐group difference in participant‐reported quality of life in the short term (MD 3.5, 95% CI −2.0 to 8.8). There was a between‐group difference in score on one of five subscales (treatment expectations) on the PODCI in the short‐term (MD 17.7, 95% CI 5.2 to 30.0).
Adverse events
Pooled results
No meta‐analysis was possible.
Single study results
One trial, Unnithan 2007, did not report adverse events. One trial, Chen 2012, reported observing no adverse effects during the intervention for either group. One trial, Verschuren 2007, reported that one child fell and fractured her radius during an exercise session. One trial, Fowler 2010, reported observing 28 mild events (in 18 participants) potentially related to the study: 6 observed falls; 17 complaints of soreness, muscle cramping or mild pain; 4 reports of fatigue; and 1 skin rash related to wearing a heart‐rate monitor. Fowler 2010 also noted 30 events unrelated to study procedures: illness, tooth loss, headache, stomachache, tonsillectomy and skin irritation from orthotic use.
Body structure and function
Pooled results
Mixed training did not result in improved short‐term aerobic fitness in 78 children and adolescents (GMFCS levels I, II and II), compared to usual care (SMD 0.05, 95% CI −0.39 to 0.50, P = 0.81, I2 = 0%, 2 studies, Analysis 3.6; Unnithan 2007; Verschuren 2007).
3.6. Analysis.
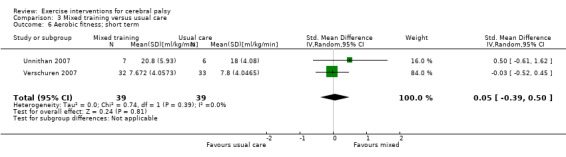
Comparison 3 Mixed training versus usual care, Outcome 6 Aerobic fitness; short term.
Mixed training also did not improve short‐term muscle strength in 150 children and adolescents with CP compared to usual care (SMD 0.08, 95% CI −0.24 to 0.40, P = 0.63, I2 = 0%, 3 studies, Analysis 3.7; Verschuren 2007; Fowler 2010; Chen 2012).
3.7. Analysis.
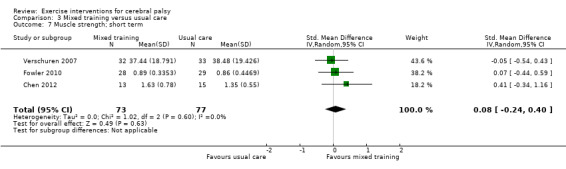
Comparison 3 Mixed training versus usual care, Outcome 7 Muscle strength; short term.
Single study results
One trial, Verschuren 2007 (65 children and adolescents), reported that anaerobic fitness improved in the short term (MD 25.20 W, 95% CI 8.89 W to 41.51 W, Analysis 3.8). The same trial reported that mixed training did not result in improvements in intermediate‐term aerobic fitness (MD −0.13 min achieved on shuttle run/walk test, 95% CI −2.10 min to 1.84 min, Analysis 3.9) or anaerobic fitness (MD −31.28 W, 95% CI −71.89 W to 9.33 W, Analysis 3.10), but it did result in improved muscle strength. We calculated an effect size using postintervention means and SDs: MD −1.04 repetitions (95% CI −10.33 repetitions to 8.25 repetitions, Analysis 3.11).
3.8. Analysis.
Comparison 3 Mixed training versus usual care, Outcome 8 Anaerobic fitness; short term.
3.9. Analysis.
Comparison 3 Mixed training versus usual care, Outcome 9 Aerobic fitness; intermediate term.
3.10. Analysis.
Comparison 3 Mixed training versus usual care, Outcome 10 Anaerobic fitness; intermediate term.
3.11. Analysis.
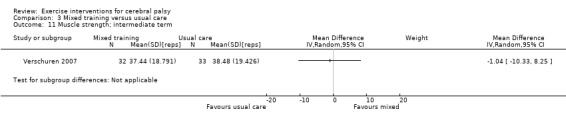
Comparison 3 Mixed training versus usual care, Outcome 11 Muscle strength; intermediate term.
Sensitivity analysis
Using a fixed‐effect model rather than a random‐effects model had no impact on the effect size for muscle strength or gross motor function among children (Verschuren 2007; Fowler 2010; Chen 2012). Analyses not shown.
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that mixed training does not improve gross motor function, gait speed, walking endurance, aerobic fitness or muscle strength in children and adolescents with CP in the short term. There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that mixed training improves short‐term participation and anaerobic fitness in children and adolescents with CP.
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that mixed training does not improve intermediate‐term gross motor function, participation, aerobic fitness, anaerobic fitness or muscle strength in children and adolescents with CP.
See Table 3.
Comparison 4: resistance training versus aerobic exercise
Two trials, Johnston 2011 and Olama 2011, compared resistance training to aerobic exercise.
Activity
Pooled results
In two studies, the pooled results indicated there was no statistically significant between‐group change in short‐term gross motor function among 56 children and adolecents (SMD 0.02, 95% CI −0.50 to 0.55, P = 0.94, I2 = 0%; Johnston 2011; Olama 2011).
Single study results
In one study in 26 children and adolescents (GMFCS levels II to IV), there was no statistically significant between‐group change in short‐term gait speed (MD 0.12 m/s, 95% CI −0.15 m/s to 0.39 m/s, Analysis 4.2; Johnston 2011), intermediate‐term gait speed (MD 0.19 m/s, 95% CI −0.05 m/s to 0.43 m/s, Analysis 4.3) or intermediate‐term gross motor function (MD 4.70%, 95% CI −12.70% to 22.10%, Analysis 4.4).
4.2. Analysis.
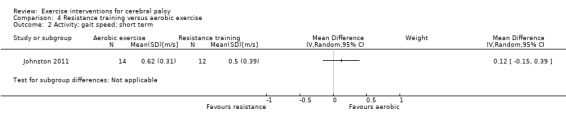
Comparison 4 Resistance training versus aerobic exercise, Outcome 2 Activity: gait speed; short term.
4.3. Analysis.
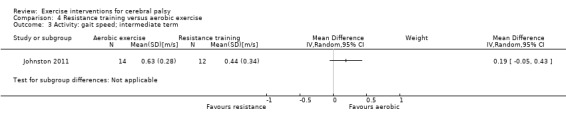
Comparison 4 Resistance training versus aerobic exercise, Outcome 3 Activity: gait speed; intermediate term.
4.4. Analysis.
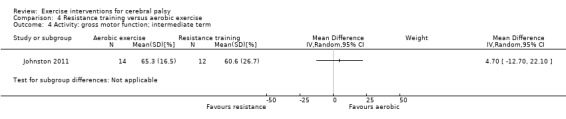
Comparison 4 Resistance training versus aerobic exercise, Outcome 4 Activity: gross motor function; intermediate term.
Participation
Pooled results
No meta‐analysis was possible.
Single study results
One study in 26 children and adolescents, Johnston 2011, reported a between‐group difference in participation as measured by the 'with whom' subscale of the CAPE (King 2004) in the short term (P = 0.05). As the authors did not provide an effect size, we calculated this using the data provided and found a between‐group difference for the 'diversity' (MD 6.60, 95% CI 0.35 to 12.85) and 'with whom' (MD 0.48, 95% CI 0.08 to 0.88) subscales of the CAPE, but not for the subscales of 'intensity' (MD 0.48, 95% CI −0.22 to 1.18), 'where' (MD 0.28, 95% CI −0.07 to 0.63) or 'enjoyment' (MD −0.06, 95% CI −0.48 to 0.36). The authors did not report an effect size for between‐group differences in participation in the intermediate term (Johnston 2011). Using the data provided, we calculated effect sizes for the following subscales: 'diversity' (MD 5.20, 95% CI −2.53 to 12.93), 'intensity' (MD 0.59, 95% CI −0.34 to 1.52), 'with whom' (MD 0.18, 95% CI −0.35 to 0.71), 'where' (MD 0.45, 95% CI −0.01 to 0.91) and 'enjoyment' (MD −0.02, 95% CI −0.55 to 0.51). Analyses not shown.
Quality of life
Pooled results
No meta‐analysis was possible.
Single study results
One study of 26 children and adolescents, Johnston 2011, reported a group‐by‐time interaction for child‐reported quality of life (P = 0.02) but did not state at what time point between‐group differences occurred. Our analysis of the reported data indicated that there was no between‐group difference in child‐reported quality of life or adult‐reported quality of life in the short term (child reported: MD 9.50, 95% CI −4.59 to 23.59; adult reported: MD 6.00, 95% CI −11.29 to 23.29) or intermediate term (child reported: MD −1.00, 95% CI −14.61 to 12.61; adult reported: MD 5.10, 95% CI −8.63 to 18.83). Analyses not shown.
Adverse events
Pooled results
No meta‐analysis was possible.
Single study results
One trial did not state that adverse events were recorded (Olama 2011). Some children reported experiencing leg pain in one trial (Johnston 2011).
Body structure and function
Pooled results
There was no statistically significant between‐group change in muscle strength in the short term (SMD −0.11, 95% CI −0.64 to 0.41, P = 0.67, I2 = 0%, 56 children and adolescents, 2 studies, Analysis 4.5; Johnston 2011; Olama 2011).
4.5. Analysis.
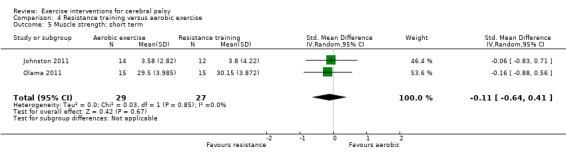
Comparison 4 Resistance training versus aerobic exercise, Outcome 5 Muscle strength; short term.
No trial assessed aerobic fitness following the intervention.
Single study results
There was no statistically significant between‐group change in muscle strength in the intermediate term (MD −0.03 N/kg, 95% CI −2.71 to 2.65 N/kg, 26 children and adolescents, 1 study, Analysis 4.6; Johnston 2011).
4.6. Analysis.
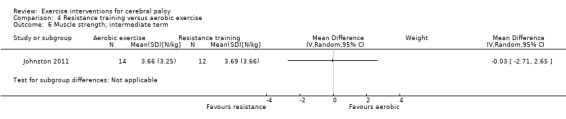
Comparison 4 Resistance training versus aerobic exercise, Outcome 6 Muscle strength; intermediate term.
Sensitivity analysis
Repeating the analysis using a fixed‐effect model instead of a random‐effects model had no impact on the effect size or statistical significance of any outcome (analyses not shown).
Quality of evidence
There is low‐quality evidence (RCT evidence: high, downgraded once for limitations, once for imprecision) that there is no difference between the effect of aerobic exercise and resistance training on gait speed, gross motor function, quality of life, or muscle strength in children and adolescents with CP in the short or intermediate term.
See Table 4.
Post hoc analyses
Folllowing identification of included studies, we noted a large overlap between the content of aerobic exercise interventions and mixed training interventions, and a large overlap between the content of resistance training interventions and mixed training interventions, respectively. We therefore conducted the following post hoc analyses: pooled analyses of aerobic exercise and mixed training versus usual care, and pooled analyses of resistance training and mixed training versus usual care. These analyses only include trials in children and adolescents, as no mixed training trials included adults with CP. Given the post hoc nature of these analyses, readers should interpret results with caution.
Comparison 1: aerobic exercise (incorporating mixed training interventions) versus usual care
Pooled results
In a meta‐analysis of seven studies, aerobic exercise improved short‐term gross motor function more than usual care in 228 children and adolescents (SMD 0.36, 95% CI 0.09 to 0.62, P = 0.008, I2 = 0%, Analysis 5.1; Unnithan 2007; Verschuren 2007; Fowler 2010; Chen 2012; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013). However, a meta‐analysis of two studies (77 children and adolescents) showed that aerobic exercise did not improve gross motor function in the intermediate term (SMD 0.25, 95% CI −1.15 to 1.64, P = 0.73, I2 = 77%, Analysis 5.2; Verschuren 2007; Mattern‐Baxter 2013).
5.1. Analysis.
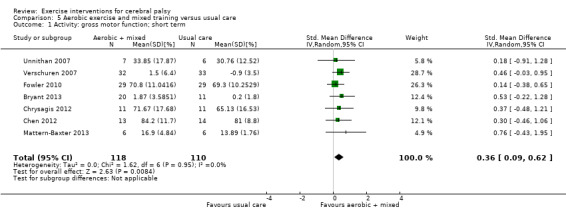
Comparison 5 Aerobic exercise and mixed training versus usual care, Outcome 1 Activity: gross motor function; short term.
5.2. Analysis.
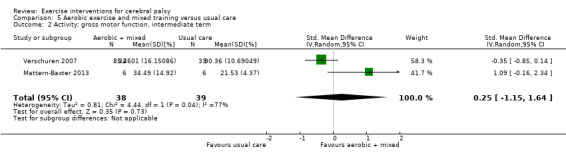
Comparison 5 Aerobic exercise and mixed training versus usual care, Outcome 2 Activity: gross motor function, intermediate term.
In a meta‐analysis of five studies (140 children and adolescents), aerobic exercise did not result in an improvement in gait speed (MD 0.10 m/s, 95% CI −0.05 m/s to 0.24 m/s, P = 0.18, I2 = 70%, Analysis 5.3; Fowler 2010; Gharib 2011; Smania 2011; Chrysagis 2012; Mattern‐Baxter 2013). In addition, aerobic exercise did not improve short‐term walking endurance in two studies of 73 children and adolescents (MD 8.43 m, 95% CI −12.38 m to 29.23 m, P = 0.43, I2 = 0%, Analysis 5.4; Fowler 2010; Smania 2011). There was also no between‐group difference in short‐term aerobic fitness following aerobic exercise in three studies of 98 children and adolescents (SMD 0.06, 95% CI −0.34 to 0.45, P = 0.78, I2 = 0%, Analysis 5.5; Van den Berg‐Emons 1998; Unnithan 2007; Verschuren 2007).
5.3. Analysis.
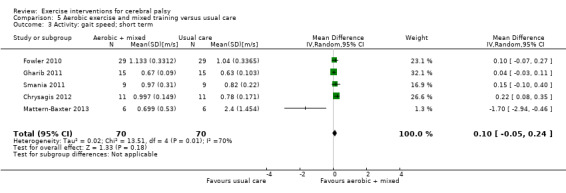
Comparison 5 Aerobic exercise and mixed training versus usual care, Outcome 3 Activity: gait speed; short term.
5.4. Analysis.
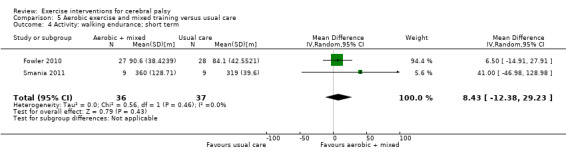
Comparison 5 Aerobic exercise and mixed training versus usual care, Outcome 4 Activity: walking endurance; short term.
5.5. Analysis.
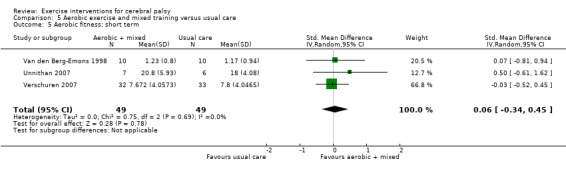
Comparison 5 Aerobic exercise and mixed training versus usual care, Outcome 5 Aerobic fitness; short term.
Sensitivity analysis
Using a fixed‐effect model rather than a random‐effects model had no impact on the short‐term results for aerobic fitness, gross motor function or walking endurance. There was, however, a change in the short‐term effect size for gait speed (MD 0.08 m/s, 95% CI 0.02 m/s to 0.14 m/s, P = 0.006, I2 = 70%, 140 participants). Using a fixed‐effect model also had a small impact on the intermediate effect size for gross motor function (SMD −0.16, 95% CI −0.62 to 0.29, P = 0.49, I2 = 77%, 228 participants). Analyses not shown.
Comparison 2: resistance training (incorporating mixed training interventions) versus usual care
Pooled results
A meta‐analysis found that resistance training did not improve gross motor function in the short term (SMD 0.21, 95% CI −0.01 to 0.43, P = 0.06, I2 = 0%, 327 children and adolescents, 11 studies, Analysis 6.1; Dodd 2003; Engsberg 2006; Liao 2007; Seniorou 2007; Unnithan 2007; Verschuren 2007; Lee 2008; Fowler 2010; Scholtes 2010; Chen 2012; Lee 2015) nor in the intermediate term (SMD −0.08, 95% CI −0.41 to 0.24, P = 0.62, I2 = 1%, 150 children and adolescents, 4 studies Analysis 6.2; Dodd 2003; Verschuren 2007; Lee 2008; Scholtes 2010).
6.1. Analysis.
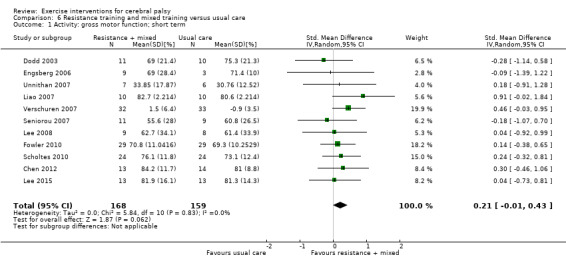
Comparison 6 Resistance training and mixed training versus usual care, Outcome 1 Activity: gross motor function; short term.
6.2. Analysis.
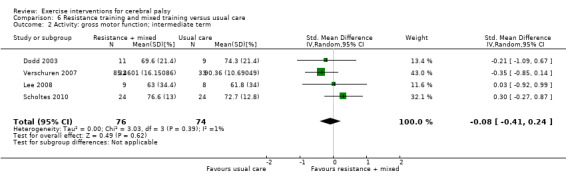
Comparison 6 Resistance training and mixed training versus usual care, Outcome 2 Activity: gross motor function; intermediate term.
Similarly, a meta‐analysis of nine studies involving 243 children and adolescents found that resistance training did not improve short‐term gait speed (MD 0.03 m/s, 95% CI −0.01 m/s to 0.08 m/s, P = 0.13, I2 = 0%, Analysis 6.3; Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Seniorou 2007; Lee 2008; Fowler 2010; Scholtes 2010; Pandey 2011).
6.3. Analysis.
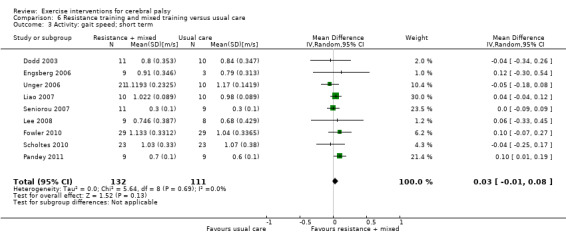
Comparison 6 Resistance training and mixed training versus usual care, Outcome 3 Activity: gait speed; short term.
Resistance training improved participation in the short term (SMD 0.35, 95% CI 0.07 to 0.64, P = 0.02, I2 = 0%, 192 children and adolescents, 3 studies, Analysis 6.4; Verschuren 2007; Scholtes 2010; Mitchell 2016) but not the intermediate term (MD 0.15, 95% CI −0.24 to 0.54, P = 0.46, I2 = 0%, 101 children and adolescents, 2 studies, Analysis 6.5; Verschuren 2007; Scholtes 2010).
6.4. Analysis.
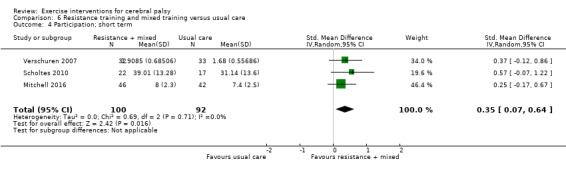
Comparison 6 Resistance training and mixed training versus usual care, Outcome 4 Participation; short term.
6.5. Analysis.
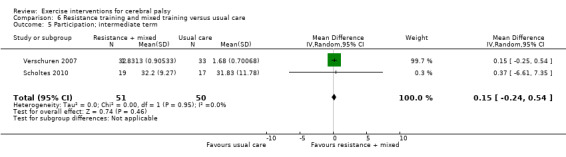
Comparison 6 Resistance training and mixed training versus usual care, Outcome 5 Participation; intermediate term.
Resistance training improved muscle strength more than usual care in the short term (SMD 0.38, 95% CI 0.01 to 0.76, P = 0.04, I2 = 66%, 397 children and adolescents, 11 studies, Analysis 6.6; Dodd 2003; Liao 2007; Seniorou 2007; Verschuren 2007; Lee 2008; Fowler 2010; Reid 2010; Scholtes 2010; Pandey 2011; Chen 2012; Mitchell 2016) but not in the intermediate term (SMD 0.28, 95% CI −0.16 to 0.71, P = 0.21, I2 = 37%, 149 children and adolescents, 4 studies, Analysis 6.7; Dodd 2003; Verschuren 2007; Lee 2008; Scholtes 2010).
6.6. Analysis.
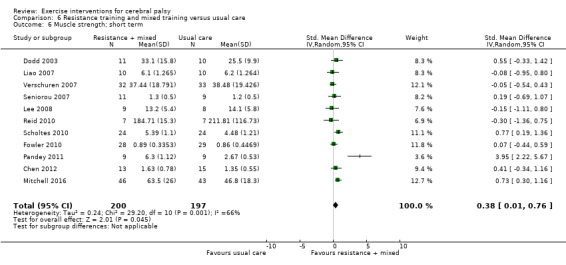
Comparison 6 Resistance training and mixed training versus usual care, Outcome 6 Muscle strength; short term.
6.7. Analysis.
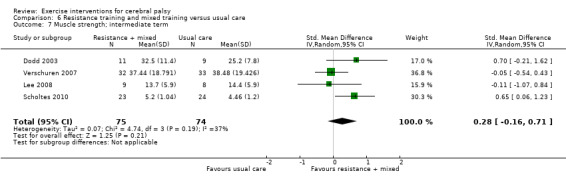
Comparison 6 Resistance training and mixed training versus usual care, Outcome 7 Muscle strength; intermediate term.
Sensitivity analysis
Using a fixed‐effect model rather than a random‐effects model impacted the effect sizes for strength in the short term (SMD 0.36, 95% CI 0.16 to 0.56, P = 0.001, I2 = 66%, 397 participants) and intermediate term (SMD 0.25, 95% CI −0.08 to 0.58, P = 0.13, I2 = 37%, 149 participants). Analyses not shown.
Summary of post hoc analyses
In agreement with the results of the analysis of aerobic exercise only compared to usual care, the pooled analysis showed some evidence that in children and adolescents with CP, aerobic exercise improves gross motor function in the short – but not intermediate – term, and no evidence that it improves short‐term gait speed, walking endurance or aerobic fitness.
The pooled analysis of resistance training and mixed training compared to usual care showed similar results to the analysis of resistance training only in terms of some evidence of an improvement in strength in the short term and no improvement in gross motor function or gait speed in the short term or strength in the intermediate term. The pooled analysis, however, found that participation improved following resistance training in the short term, which differed from findings in the primary analysis.
Discussion
Summary of main results
Overall, we are unable to provide firm conclusions regarding the effectiveness of exercise interventions for people with CP due to the poor quality of the available evidence, which was exacerbated by small sample sizes and, in turn, probably led to an underestimation of the extent of heterogeneity. As a result, there is substantial uncertainty related to the effect estimates presented in this review.
The results of the included trials suggest that aerobic exercise may result in a small improvement in gross motor function in the short and intermediate term and may improve physical activity. There is low‐ to very low‐quality evidence that aerobic exercise does not improve gait speed, walking endurance, or aerobic fitness, although these findings are mostly based on single studies. Generally, the results suggest that resistance training does not improve any aspect of activity or participation in people with CP but may improve muscle strength in children, adolescents and young adults in the short term and in children and adolescents in the intermediate term. In addition, a trial of 101 participants reported that resistance training improves walking endurance in children with CP in the short term (Mitchell 2016). Mixed training may improve participation, but at present, there is low‐quality evidence that it does not improve activity, muscle strength or aerobic fitness in children with CP. These conclusions, however, are largely based on the results of a single study (Verschuren 2007). Although only two trials compared resistance training and aerobic exercise in children and adolescents with CP (Johnston 2011; Olama 2011), the results indicate that there is no difference in terms of gait speed, gross motor function, quality of life or muscle strength between types of exercise in the short or intermediate term. Based on the data provided, exercise appears to be safe for people with CP, with no serious adverse events reported. The evidence for safety, however, is also incomplete because only 16 trials reported that they collected data on adverse events; it is unclear whether or not this reflects an absence of adverse events or a failure to report them. A small number of participants reported experiencing events potentially related to the intervention, including muscle soreness, mild knee, foot and ankle pain, shoulder pain from wearing weighted vests, fatigue, minor calf strain and falls (one of which resulted in a fracture).
Overall completeness and applicability of evidence
The evidence base for the use of exercise interventions for people with CP is incomplete. There is a lack of trials examining the effectiveness of both aerobic and resistance exercise in adults with CP. There is also a lack of trials investigating the intermediate‐ and long‐term effectiveness of exercise for people with CP; the longest follow‐up time point was four months postintervention (Verschuren 2007). Given the decline in mobility among young adults with CP (Bottos 2001; Day 2007; Opheim 2009), the effectiveness of exercise for slowing or preventing decline in activity and participation in the long term is a real concern (Shortland 2009). Few trials investigated the effectiveness of exercise for non‐ambulatory people with CP. Only 5 out of 23 trials that included an intervention that targeted the lower limbs included children and adolescents in GMFCS levels IV or V (Van den Berg‐Emons 1998; Johnston 2011; Smania 2011; Bryant 2013; Tedla 2014), and only one trial included children and adolescents in GMFCS levels IV and V only (Bryant 2013).
The evidence for the effectiveness of interventions at the level of participation on the International Classification of Functioning, Disability and Health (ICF) is incomplete (WHO 2001), with only 4 out of 29 included trials assessing participation (Verschuren 2007; Scholtes 2010; Johnston 2011; Mitchell 2016). Although three of these trials assessed participation using the CAPE, one trial reported the individual scores for each subscale of the CAPE, rather than the total score, making it difficult to pool results. Similarly, the evidence for the effect of exercise interventions on quality of life is incomplete. Of the four trials that assessed quality of life in children and adolescents, three assessed both participant‐ and parent‐reported quality of life, often with contradictory results (Engsberg 2006; Fowler 2010; Johnston 2011). Further, two trials reported the individual results for several subscales of the outcome measure and not an overall score, making it difficult to draw any firm conclusions regarding the impact of exercise on quality of life (Verschuren 2007; Fowler 2010).
Most trials assessed activity capacity, activity performance or both, with most assessing capacity rather than performance. The most commonly used measures of activity capacity were the GMFM and self‐selected gait speed. There was variation, however, in the component of the GMFM that trials assessed and reported (e.g. dimension E only, dimension D and E combined, total score) and in the methods they used to assess self‐selected gait speed (e.g. walking on a treadmill or walking over ground). Further, as a number of trials used the GMFM to assess motor capacity in adolescents outside of the age range for which the tool was developed (i.e. five months to 16 years) (Dodd 2003; Unnithan 2007; Verschuren 2007; Chrysagis 2009; Chrysagis 2012; Bryant 2013; Taylor 2013), the score may not accurately reflect participants' activity capacity. Perhaps unexpectedly, a number of trials did not assess physical fitness, particularly aerobic fitness; 3 of 14 trials assessed aerobic fitness following an aerobic exercise intervention, and 15 of 21 trials assessed muscle strength following a resistance training intervention.
There was consistently incomplete reporting of the comparator across trials. Although most trials reported that the control group received usual physiotherapy, reporting of the content and dose of usual care was poor. Reported physiotherapy varied considerably across individuals, and it was not clear from many trials if participants received the dose and content of physiotherapy prescribed.
We were unable to locate or extract data from three trials (Chrysagis 2009; Bryant 2013; Tedla 2014), which may have had an impact on the results of our meta‐analysis. In particular, the inclusion of data on muscle strength and gross motor function from Tedla 2014 would have increased the power of the analysis through the addition of a further 62 participants. Data from Bryant 2013 were only missing for the intermediate time point, but data on gross motor function from 31 participants in this trial may have impacted the outcome of the meta‐analysis.
The most effective dose of exercise for people with CP is currently unknown, making it difficult to prescribe exercise in this population. This is reflected in the large variation in the frequency, duration and intensity of exercise prescribed. There is a body of evidence supporting the effectiveness of exercise in general to improve aerobic fitness and muscle strength, which has contributed to the development of exercise prescription guidelines for the general population (American College of Sports Medicine 2009; Faigenbaum 2009; Garber 2011), and more recently, for people with CP (Verschuren 2016). While many included trials failed to report the volume and intensity of exercise prescribed, those that did report the dose of exercise prescribed did not meet current guidelines for exercise prescription for people with CP (Verschuren 2016). For example, of the 11 trials included in the pooled analysis of resistance training and mixed training in comparison to usual care, six were prescribed for a shorter duration than that suggested in guidelines (Dodd 2003; Liao 2007; Seniorou 2007; Lee 2008; Reid 2010; Pandey 2011), four trials either did not report the number of sets and repetitions performed or the number of sets and repetitions were lower than guideline recommendations (Verschuren 2007; Fowler 2010; Pandey 2011; Mitchell 2016), and five trials either did not report the intensity of the exercises or the intensity was lower than current guideline recommendations (Liao 2007; Verschuren 2007; Lee 2008; Scholtes 2010; Pandey 2011). Similarly, of the 14 trials that included aerobic exercise in the intervention, 12 did not state the intensity of exercise prescribed (Van den Berg‐Emons 1998; Verschuren 2007; Chrysagis 2009; Gharib 2011; Johnston 2011; Olama 2011; Smania 2011; Chen 2012; Chrysagis 2012; Bryant 2013; Mattern‐Baxter 2013; Emara 2015). It is possible that inadequate prescription of exercise volume and intensity across trials contributed to the limited or null improvement in physical fitness. At present, however, there is insufficient evidence to suggest that higher doses of exercise, in line with the current guidelines for people with and without CP (American College of Sports Medicine 2009; Faigenbaum 2009; Garber 2011, Verschuren 2016), result in improvements in physical fitness, activity or participation in people with CP. Further, the feasibility of delivering these higher doses of exercise to people with CP is not clear. There was variation in fidelity to the intervention among trials that reported this measure, perhaps because it was not feasible to deliver the dose prescribed in this population. This would suggest that failure to implement exercise interventions as planned contributed to the findings of this review. The findings of this review highlight the need for clearer reporting of the frequency, intensity and duration of exercise prescribed in future trials and further investigation into the effect and feasibility of following generic exercise guidelines for people with CP.
Quality of the evidence
Using the GRADE criteria (Guyatt 2008), we rated the quality of evidence for all comparisons as low or very low, mainly as a result of an overall high risk of bias for all trials and issues of imprecision.
We judged all trials as being at high risk of bias for blinding of participants. Unfortunately, while this is unavoidable when examining the effect of exercise in a trial, it does not negate the risk of introducing performance bias by not blinding participants and personnel. We also judged a large number of trials as being at high or unclear risk of bias for blinding of outcome assessment. It was recently reported that less than a quarter of physical therapy trials were adequately blinded and that blinding was poorly reported in trials (Armijo‐Olivo 2017). Our decision to classify studies that used a self‐report outcome as being at high risk of bias inflated the number of trials at high risk for blinding of outcome assessment. We believe that, although conservative, classifying these trials as being at high risk of bias was the correct decision given that lack of, or unclear, double blinding is associated with an average 22% exaggeration of intervention effects when assessing self‐report outcomes (Savović 2012). However, lack of blinding does not appear to exaggerate the intervention effect when assessing objective outcomes (Wood 2008; Savović 2012). Readers should therefore interpret the results for objective outcomes in these trials (judged at high risk of bias due to the inclusion of subjective outcomes) as being at low risk of detection bias. We judged most trials as being at unclear risk of bias for random sequence generation and allocation concealment; a recent study reported that only 39.7% and 11.5% of physical therapy trials achieved adequate random sequence generation and allocation concealment, respectively (Armijo‐Olivo 2015). Intervention effect estimates may be exaggerated by 11% in trials with inadequate or unclear sequence allocation and by 15% for trials with unclear or inadequate allocation concealment (Savović 2012). Effect sizes are exaggerated even further for self‐report outcomes whereas there is little evidence that inadequate or unclear sequence generation or allocation concealment exaggerates the intervention effect in trials with objective outcomes (Wood 2008; Savović 2012). A number of trials reported using sealed envelopes to generate a random sequence, which in itself appears to adequately prevent bias when compared to central randomisation (Herbison 2011). However, several of the trials in this review did not report enhanced security, such as use of opaque envelopes; intervention effects are exaggerated by, on average, 13% for trials that state that they use envelopes but do not give details of enhanced security (Herbison 2011).
Only four trials published a trial protocol prior to the publication of results. In addition, some of these trials were not the most recently published trials, suggesting that this issue is not improving with time. Chan 2004 found that statistically significant outcomes were 2.4 times more likely to be fully reported compared to non‐statistically significant outcomes. Although we judged most included trials as being at low risk of bias for incomplete outcome data, because of low levels of missing data, very few trials stated that they performed an intention‐to‐treat analysis. Excluding participants from the analysis can result in biased estimates of treatment effects (Nüesch 2009; Abraha 2015), although the direction of bias is unpredictable (Nüesch 2009).
Finally, 24 of the 29 included trials had small sample sizes (fewer than 50 participants), and as a result, data from a relatively small number of participants (65 to 397 participants) were included in pooled analyses. The small number of participants included in analyses may have impacted our findings in one of two ways, either inflating effect sizes or resulting in statistically non‐significant findings (Pereira 2012; Button 2013; Dechartres 2013). Effect sizes calculated on samples of fewer than 50 participants are, on average, 23% larger than estimates from trials with sample sizes of more than 50 participants (Dechartres 2013). However, the relatively small number of participants included in the analysis may have resulted in analyses that lacked statistical power. In addition, the small number of participants led to large within‐study variations and subsequently low between‐study variations; in many analyses heterogeneity was estimated as zero. Therefore, although the small sample sizes in trials may have resulted in analyses that lacked statistical power, our analyses likely underestimated the degree of heterogeneity, so we may have observed erroneously significant results.
Potential biases in the review process
We conducted an extensive search of all available literature, including grey literature. We included trials regardless of publication date or language and are therefore confident that this review includes all published evidence on the topic to date.
A broad and varied number of interventions may be described as exercise. The inclusion of certain interventions such as those described as 'gait training' or 'treadmill training' may be contentious. In order to ensure consistency when deciding if an intervention was classified as aerobic or resistance training, we referred to the definitions of exercise published by Caspersen 1985 and the US Department of Health and Human Services (USDHHS 2008). Repetitive movement of the lower limbs during walking on a treadmill or over ground meets the description of aerobic exercise, regardless of the support required to perform this repetitive movement. Similarly, we included any mode of exercise that involved repetitive movement of large muscle groups over a sustained period of time. For example, we included a swimming intervention where it was clear that participants swam continuously for a sustained period. We only included studies of resistance training if it was clear that the body's muscles were working or holding against an applied force, such as body weight, free weights, machine weights, or elastic bands (USDHHS 2008). We did not include studies that provided an intervention in addition to exercise, such as botulinum toxin, motivational interviewing or ankle foot orthoses, as it was not possible to distinguish the independent effect of exercise on the outcomes assessed.
We included postintervention means and SDs in our meta‐analysis if they were presented in the published report. We also calculated effect sizes for single studies where the effect size was not provided using postintervention means and SDs. This may have resulted in conservative estimates of the effect size as there was significant heterogeneity of the baseline scores in many studies. It may also explain the difference between the P values and CIs for effect estimates reported in some trials and those that we calculated.
Three authors are chartered physiotherapists and lecturers in physiotherapy. As professionals who might be involved in the delivery of exercise interventions, it is plausible that they might be perceived as having a bias favouring the effectiveness of exercise.
Agreements and disagreements with other studies or reviews
The results of this review are generally inconsistent with the results of previous reviews primarily because of differences in the number and type of included trials and because most previous reviews used narrative syntheses when drawing conclusions. Most authors accepted the statistical significance of comparisons reported in individual trials as indication of a positive effect. Rogers 2008 and Butler 2010 reviewed the literature on the effects of aerobic exercise for children with CP. Rogers 2008 presented the evidence from three trials, one RCT that was included in this review (Van den Berg‐Emons 1998), one cohort study and one RCT that we did not include in this review because the only difference between the intervention and control group was that the intervention group received active encouragement. Using a narrative synthesis of the literature, the authors concluded that aerobic exercise improved aerobic fitness and that no study assessed activity (Rogers 2008). Similarly, using a narrative synthesis of three trials included in this review (Van den Berg‐Emons 1998; Unnithan 2007; Verschuren 2007), Butler 2010 concluded that aerobic exercise may increase aerobic fitness but there was not enough evidence to indicate that it improved activity.
Two meta‐analyses assessed the effectiveness of resistance training for children and children and adults with CP (Scianni 2009; Park 2014b). Scianni 2009 reported that there was no short‐ or intermediate‐term improvement in strength, gross motor function or gait following resistance training in children with CP. Two of the six included studies are not included in the current review because they used electrical stimulation (Van der Linden 2003; Kerr 2006). Park 2014b reported that there was strong evidence for a large effect of strengthening interventions in people with CP, based on the results of 12 trials described in 13 reports. We included 11 of these trials in our review (Dodd 2003; Engsberg 2006; Unger 2006; Liao 2007; Unnithan 2007; Lee 2008; Maeland 2009; Fowler 2010; Scholtes 2010 (two reports); Chen 2012). However, similar to Scianni 2009, Park 2014b also included two trials that assessed the effect of electrical stimulation (Van der Linden 2003; Kerr 2006). Although Park 2014b did not report a clear plan for the meta‐analysis, it appears that the conclusion was based on a standardised effect size calculated by pooling all outcomes from all trials; this may explain the discrepancy between their results and ours. Further, although subgroup analyses were conducted according to outcome, it is not clear which trials contributed to each subgroup analysis, and it appears that more than one outcome measure from each trial was included in a single meta‐analysis. Park 2014b also combined the results of trials involving children and adults with CP, respectively.
A third systematic review investigated the effect of upper limb resistance training on all levels of the ICF Framework in children with CP (Rameckers 2014). Using a narrative synthesis, the authors concluded that strength training had a small to large effect on strength and that no study assessed the effect on activity or participation. These conclusions were informed by two trials included in the current review (McCubbin 1985; Reid 2010). However, we did not believe McCubbin 1985 assessed muscle strength and therefore did not include it in the meta‐analysis.
In contrast to our findings, a review of all physiotherapy interventions for adults with CP concluded that there was moderate‐quality evidence that strength training improves gait in adolescents and adults with CP (Jeglinsky 2010). However, this conclusion was based on the results of one RCT, Unger 2006, and therefore does not represent a thorough review of the literature as presented here.
Only one review to date has summarised the evidence for aerobic, anaerobic and resistance training for children with CP (Verschuren 2008). In contrast with our findings, the authors concluded that exercise may improve strength and aerobic capacity but that more trials are needed to determine the effects on activity, participation and quality of life. Authors drew these conclusions using a narrative synthesis of the results from both randomised and non‐randomised trials that took place prior to September 2006 and therefore do not represent the most recent or best quality evidence.
Authors' conclusions
Implications for practice.
This review shows that there is low‐ to very low‐quality evidence that aerobic exercise results in a small improvement in gross motor function but not aerobic fitness, and that resistance training results in a small improvement in muscle strength but not activity or participation in people with CP. Exercise appears to be safe for people with CP from the limited evidence available.
Historically, rehabilitation efforts for children with disabilities have focused on improving impairments in body structures and function in order to improve activity (Rosenbaum 2012). The results of this review do not support the hypothesis that improving impairments will improve activity, as we found no correlation between improvements in physical fitness and improvements in activity following an exercise intervention. The ICF framework highlights the complex interplay between body structures and functions, activity, participation, and environmental and personal factors (WHO 2001), and personal and environmental factors are likely to be key explanatory factors in this divergence between the effect on impairments and activity limitations.
Aerobic exercise may be important to improve activity capacity in terms of gross motor function, but for now it is not clear whether it improves activity performance. A gap exists between activity capacity and performance (Holsbeeke 2009), which raises an interesting question regarding the focus of exercise (i.e. to improve what a person can do in a controlled environment or to improve what a person actually does in their environment). Promoting participation in physical activity and aerobic exercise in general may be more important than prescribing an exercise programme for a fixed duration, not only for improving activity capacity, as demonstrated by the results of this review, but also for improving activity performance. Also, achieving a sustained impact on activity capacity through aerobic exercise is likely to require frequent participation and hence increased activity performance.
The studies included in this review show that exercise can be delivered through a range of modes, in a range of settings and by a range of providers. It is important not to think of exercise solely as therapy. While we were unable to examine the impact of different modes of delivery of exercise, such as group sessions, on outcomes in the current review, people, and particularly children, may be more likely to engage in exercise over their lifespan if they enjoy it, if they believe it has health benefits, and if they perceive it to be an opportunity for social interaction (Verschuren 2012). The studies included in this review used a wide variety of modes of aerobic exercise, such as cycling on a static bike at home, cycling on a static adapted bike in school, supported and unsupported treadmill walking, swimming and wheelchair driving, indicating that the type of exercise prescribed can be adapted according to the person's ability and preference. For resistance training, free weights or weight machines may increase the intensity of exercise, especially in adolescents and adults. This will likely require identifying opportunities to access this type of equipment in the community.
Implications for research.
The current evidence for exercise in people with CP is comprised of mostly small studies at high or unclear risk of bias. There is an urgent need for larger, more rigorous and more completely reported RCTs. Further, a number of key issues need to be addressed by future trials before any firm conclusions can be made regarding the effectiveness of exercise interventions for people with CP.
With regard to trial reporting, exercise interventions need to be prescribed, assessed, and adapted in a systematic fashion to enable development of an evidence‐based guideline specific to people with CP. Reporting fidelity to the intervention, in terms of the content as well as the dose of exercise, should be a prerequisite when publishing trials on the effectiveness of exercise interventions for people with CP in order to differentiate between intervention failure and implementation failure. We recommend that all authors of trials investigating exercise interventions in people with CP use the recently developed Template for Intervention Description and Replication Checklist (TIDieR) to ensure that they describe interventions in sufficient detail to allow replication (Hoffmann 2014). Adherence to this standard will also aid the implementation process of interventions that show clinical benefits. The research community should also reach a consensus on the content of the comparator when evaluating the effectiveness of exercise interventions for people with CP. If comparing the intervention to usual care, investigators should track and report the content and dose for both the intervention and comparison group. We acknowledge that usual care, particularly physiotherapy, can vary widely between countries, medical centres, and even health professionals in the same centre, and therefore it may not be possible to control its content and dose. However, clear guidance on how to record and report usual care will greatly improve the transparency of future trials.
With regard to trial design, future trials need to assess the effect of exercise interventions on quality of life and adverse events in people with CP on all levels of the ICF (WHO 2001). Guidance is urgently required regarding choice of outcome measures and reporting of data to allow for comparison across trials. Amongst the few trials that assessed participation and quality of life, there was inconsistency regarding the outcome measures used and the data reported, preventing pooled analysis of individual trials. Participation is an important outcome of any intervention for people with CP, and currently the impact of exercise on participation is not well understood because studies have not used outcome measures that specifically measure participation. Further, we propose that all trials assess activity capacity and activity performance. These are potentially different constructs (Holsbeeke 2009), but both are important to people with CP, their families and health professionals. While there were relatively few (and minor) adverse events that were potentially related to the intervention, the literature does not confirm that no serious adverse events occurred as a result of exercise, as approximately half of the included studies did not report monitoring this outcome. Implementation of a standardised method of recording and reporting adverse events would ensure more consistent and deliberative reporting. Further, trials need to include measures of body structures and functions in order to determine the likely process of change in activity, participation and quality of life. At present, exercise is believed to produce similar neuromuscular and physiological adaptations in people with and without CP. This assumption merits further examination in order to develop evidence‐based exercise prescription guidelines for people with CP.The results of this review are only representative of a subgroup of people with CP, as most included studies in children and adolescents fall into GMFCS levels I to III. We identified a stark dearth of studies involving adults with CP and people with moderate‐to‐severe CP. There are evident difficulties entailed in recruiting adults with CP into clinical trials, and people with moderate‐to‐severe CP may be not even be able to complete an exercise intervention as prescribed, so a feasibility study may be necessary to determine the appropriateness of trials on exercise interventions in these populations. It is essential, however, that the research community makes efforts towards identifying and overcoming these associated difficulties to prevent discrimination against this group of people.
With regard to future priorities, studies should investigate the long‐term effects of exercise on function and health in people with CP. A higher prevalence of a number of conditions such as pain, fatigue, depression, cardiovascular disease, type 2 diabetes mellitus and hypertension has been reported in adults with CP in comparison to the general population (Jahnsen 2004; Opheim 2009; Van Der Slot 2012; Peterson 2015). They also report experiencing a decline in muscle strength, aerobic fitness and mobility in young adulthood (Opheim 2009). The benefits of exercise for people with CP may be in the maintenance of physical fitness, activity and participation, and in the prevention of chronic disease, rather than the improvement of function alone. Future trials need to include long‐term follow‐up in order to thoroughly examine the effects of exercise throughout the lifespan of a person with CP.
Acknowledgements
We thank the Cochrane Developmental, Psychosocial, and Learning Problems editorial team, in particular Geraldine Macdonald and Joanne Wilson, for their guidance and support. We also thank Margaret Anderson, Information Specialist with Cochrane Developmental, Psychosocial, and Learning Problems, for her assistance with designing the search strategy and conducting the search. We are grateful to Georgia Spiliotopoulou and João De Aguiar Greca for their assistance with checking the eligibility of studies that were not published in English. We are also grateful to the authors who provided us with data to include in the review.
Appendices
Appendix 1. Gross Motor Function Classification System
Level I: walks without restrictions; limitations in more advanced gross motor skills
Before 2nd birthday: infants move in and out of sitting and floor sit with both hands free to manipulate objects. Infants crawl on hands and knees, pull to stand, and take steps holding onto furniture. Infants walk between 18 months and 2 years of age without the need for any assistive mobility device.
From age 2 to 4th birthday: children floor sit with both hands free to manipulate objects. Children perform movements in and out of floor sitting and standing without adult assistance. Children walk as the preferred method of mobility without the need for any assistive mobility device.
From age 4 to 6th birthday: children get into and out of and sit in a chair without the need for hand support. Children move from floor and chair sitting to standing without the need for objects for support. Children walk indoors and outdoors and climb stairs. Emerging ability to run and jump.
From age 6 to 12th birthday: children walk at home, school, outdoors and in the community. Children are able to walk up and down curbs without physical assistance and stairs without the use of a railing. Children perform gross motor skills, such as running and jumping, but speed, balance, and coordination are limited. Children may participate in physical activities and sports depending on personal choices and environmental factors.
From age 12: youth walks at home, school, outdoors and in the community. Youth is able to walk up and down curbs without physical assistance and stairs without the use of a railing. Youth performs gross motor skills, such as running and jumping, but speed, balance, and coordination are limited. Youth may participate in physical activities and sports depending on personal choices and environmental factors.
Level II: walks without assistive devices; limitations walking outdoors and in the community
Before 2nd birthday: infants maintain floor sitting but may need to use their hands for support to maintain balance. Infants creep on their stomach or crawl on hands and knees. Infants may pull to stand and take steps holding onto furniture.
From age 2 to 4th birthday: children floor sit but may have difficulty with balance when both hands are free to manipulate objects. Children perform movements in and out of sitting without adult assistance. Children pull to stand on a stable surface. Children crawl on hands and knees with a reciprocal pattern, cruise holding onto furniture and walk using an assistive mobility device as preferred methods of mobility.
From age 4 to 6th birthday: children sit in a chair with both hands free to manipulate objects. Children move from the floor to standing and from chair sitting to standing but often require a stable surface to push or pull up on with their arms. Children walk without needing any assistive mobility device indoors and for short distances on level surfaces outdoors. Children climb stairs holding onto a railing but are unable to run or jump.
From age 6 to 12th birthday: Children walk in most settings. Children may experience difficulty walking long distances and balancing on uneven terrain, on inclines, in crowded areas, in confined spaces, or when carrying objects. Children walk up and down stairs holding onto a railing or with physical assistance if there is no railing. Outdoors and in the community, children may walk with physical assistance or a hand‐held mobility device or use wheeled mobility when travelling long distances. Children have at best only minimal ability to perform gross motor skills such as running and jumping. Limitations in performance of gross motor skills may necessitate adaptations to enable participation in physical activities and sports.
From age 12: youth walk in most settings. Environmental factors (such as uneven terrain, inclines, long distances, time demands, weather, and peer acceptability) and personal preference influence mobility choices. At school or work, youth may walk using a hand‐held mobility device for safety. Outdoors and in the community, youth may use wheeled mobility when travelling long distances. Youth walk up and down stairs holding a railing or with physical assistance if there is no railing. Limitations in performance of gross motor skills may necessitate adaptations to enable participation in physical activities and sports.
Distinctions between levels I and II
Compared with children at level I, children at level II have limitations in the ease of performing movement transitions; walking outdoors and in the community; the need for assistive mobility devices when beginning to walk; quality of movement; and the ability to perform gross motor skills such as running and jumping.
Level III: walks with assistive mobility devices; limitations walking outdoors and in the community
Before 2nd birthday: infants maintain floor sitting when the low back is supported. Infants roll and creep forward on their stomachs.
From age 2 to 4th birthday: children maintain floor sitting often by 'W‐sitting' (sitting between flexed and internally rotated hips and knees) and may require adult assistance to assume sitting. Children creep on their stomach or crawl on hands and knees (often without reciprocal leg movements) as their primary methods of self‐mobility. Children may pull to stand on a stable surface and cruise short distances. Children may walk short distances indoors using an assistive mobility device and adult assistance for steering and turning.
From age 4 to 6th birthday: children sit on a regular chair but may require pelvic or trunk support to maximise hand function. Children move in and out of chair sitting using a stable surface to push on or pull up with their arms. Children walk with an assistive mobility device on level surfaces and climb stairs with adult assistance. Children are frequently transported when travelling for long distances or outdoors on uneven terrain.
From age 6 to 12th birthday: children walk using a hand‐held mobility device in most indoor settings. When seated, children may require a seat belt for pelvic alignment and balance. Sit‐to‐stand and floor‐to‐stand transfers require physical assistance of a person or support surface. When travelling long distances, children use some form of wheeled mobility. Children may walk up and down stairs holding onto a railing with supervision or physical assistance. Limitations in walking may necessitate adaptations to enable participation in physical activities and sports, including a self‐propelling manual wheelchair or powered mobility.
From age 12: youth is capable of walking using a hand‐held mobility device. In comparison with individuals at other levels, young people at level III demonstrate more variability in methods of mobility depending on physical ability and environmental and personal factors. When seated, youth may require a seat belt for pelvic alignment and balance. Sit‐to‐stand and floor‐to‐stand transfers require physical assistance from a person or support surface. At school, youth may self‐propel a manual wheelchair or use powered mobility. Outdoors and in the community, young people are transported in a wheelchair or use powered mobility. Youth may walk up and down stairs holding onto a railing with supervision or physical assistance. Limitations in walking may necessitate adaptations to enable participation in physical activities and sports, including self‐propelling a manual wheelchair or powered mobility.
Distinctions between levels II and III
Differences are seen in the degree of achievement of functional mobility. Children at level III need assistive mobility devices and frequently orthoses to walk, while children at level II do not require assistive mobility devices after age 4.
Level IV: self‐mobility with limitations; children are transported or use power mobility outdoors and in the community
Before 2nd birthday: infants have head control but require trunk support for floor sitting. Infants can roll to supine and may roll to prone.
From age 2 to 4th birthday: children floor sit when placed but are unable to maintain alignment and balance without using their hands for support. Children frequently require adaptive equipment for sitting and standing. Children achieve self‐mobility for short distances (within a room) through rolling, creeping on stomach, or crawling on hands and knees without reciprocal leg movement.
From age 4 to 6th birthday: children sit on a chair but need adaptive seating for trunk control and to maximise hand function. Children move in and out of chair sitting with assistance from an adult or a stable surface to push or pull up on with their arms. Children may at best walk short distances with a walker and adult supervision but have difficulty turning and maintaining balance on uneven surfaces. Children are transported in the community. Children may achieve self‐mobility using a power wheelchair.
From age 6 to 12th birthday: children use methods of mobility that require physical assistance or powered mobility in most settings. Children require adaptive seating for trunk and pelvic control and physical assistance for most transfers. At home, children use floor mobility (roll, creep, or crawl), walk short distances with physical assistance, or use powered mobility. When positioned, children may use a body support walker at home or school. At school, outdoors, and in the community, children are transported in a manual wheelchair or use powered mobility. Limitations in mobility necessitate adaptations to enable participation in physical activities and sports, including physical assistance, powered mobility or both.
From age 12: youth uses wheeled mobility in most settings. Youth requires adaptive seating for pelvic and trunk control. Youth requires physical assistance from one or two people for transfers. Youth may support weight with their legs to assist with standing transfers. Indoors, youth may walk short distances with physical assistance, use wheeled mobility, or, when positioned, use a body support walker. Youth are physically capable of operating a powered wheelchair. When a powered wheelchair is not feasible or available, youth are transported in a manual wheelchair. Limitations in mobility necessitate adaptations to enable participation in physical activities and sports, including physical assistance or powered mobility, or both.
Distinctions between levels III and IV
Differences in sitting ability and mobility exist, even allowing for extensive use of assistive technology. Children at level III sit independently, have independent floor mobility and walk with assistive mobility devices. Children at level IV function in sitting (usually supported), but independent mobility is very limited. Children at level IV are more likely to be transported or to use power mobility.
Level V: self‐mobility is severely limited even with the use of assistive technology
Before 2nd birthday: physical impairments limit voluntary control of movement. Infants are unable to maintain antigravity head and trunk postures in prone and sitting. Infants require adult assistance to roll.
From age 2 to 12th birthday: Children are transported in a manual wheelchair in all settings. Children are limited in their ability to maintain antigravity head and trunk postures and control arm and leg movements. Assistive technology is used to improve head alignment, seating, standing, and/or mobility, but limitations are not fully compensated for by equipment. Transfers require complete physical assistance of an adult. At home, children may move short distances on the floor or may be carried by an adult. Children may achieve self‐mobility using powered mobility with extensive adaptations for seating and control access. Limitations in mobility necessitate adaptations to enable participation in physical activities and sports, including physical assistance and using powered mobility.
From age 12: youth are transported in a manual wheelchair in all settings. Youth are limited in their ability to maintain antigravity head and trunk postures and control arm and leg movements. Assistive technology is used to improve head alignment, seating, standing, and mobility, but limitations are not fully compensated for by equipment. Transfers require physical assistance from one or two people or a mechanical lift. Youth may achieve self‐mobility using powered mobility with extensive adaptations for seating and control access. Limitations in mobility necessitate adaptations to enable participation in physical activities and sports, including physical assistance and using powered mobility.
Distinctions between levels IV and V
Children at level V lack independence even in basic antigravity postural control. Child achieves self‐mobility only if he or she can learn how to operate an electrically powered wheelchair.
Appendix 2. Manual Ability Classification System
Level I: handles objects easily and successfully. At most, limited in the ease of performing manual tasks requiring speed and accuracy. However, any limitations in manual abilities do not restrict independence in daily activities.
Level II: handles most objects but with somewhat reduced quality or speed of achievement, or both. May avoid or achieve with some difficulty certain activities; might use alternative ways of performance, but manual abilities do not usually restrict independence in daily activities.
Level III: handles objects with difficulty; needs help to prepare or modify activities, or both. The performance is slow and achieved with limited success regarding quality and quantity. Performs activities independently if they have been set up or adapted.
Level IV: handles a limited selection of easily managed objects in adapted situations. Performs parts of activities with effort and with limited success. Requires continuous support and assistance, adapted equipment or both, for even partial achievement of the activity.
Level V: does not handle objects and has severely limited ability to perform even simple actions. Requires total assistance.
Distinctions between levels I and II. Children at level I may have limitations in handling very small, heavy or fragile objects, which demand detailed fine motor control or efficient coordination between hands. Limitations may also involve performance in new and unfamiliar situations. Children at level II perform almost the same activities as children at level I, but the quality of performance is decreased or the performance is slower. Functional differences between hands can limit effectiveness of performance. Children at level II commonly try to simplify handling of objects, for example by using a surface for support instead of handling objects with both hands.
Distinctions between Levels II and III. Children at level II handle most objects, although slowly or with reduced quality of performance. Children at level III commonly need help to prepare the activity or require that adjustments be made to the environment, or both, since their ability to reach or handle objects is limited. They cannot perform certain activities and their degree of independence is related to the supportiveness of the environmental context.
Distinctions between Levels III and IV. Children at level III can perform select activities if the situation is prearranged and if they receive supervision and plenty of time. Children at level IV need continuous help during the activity and can at best participate meaningfully in only parts of an activity.
Distinctions between Levels IV and V. Children at level IV perform part of an activity with continuous help. Children at level V might at best participate with a simple movement in special situations, for example by pushing a button.
Appendix 3. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, which contains the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
#1[mh "cerebral palsy"] #2cerebral next pals* #3((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/5 spastic*) #4((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/3 ataxi*) #5"Little* disease" #6{or #1‐#5} #7[mh exercise] #8[mh "Exercise Movement Techniques"] #9[mh "Exercise Therapy"] #10[mh ^"Physical Education and Training"] #11[mh "Physical Endurance"] #12[mh ^"Physical Fitness"] #13[mh Sports] #14[mh Hydrotherapy] #15[mh "Equine‐Assisted Therapy"] #16aerobic* #17(cycle or cycling) #18[mh ergometry] #19ergometry #20(treadmill or tread next mill) #21(exercise* or strength* or fitness) #22(flexibility or stretching) #23((weight* near/1 lift*) or weight next training) #24(hippotherapy or equine* therapy or equine assist* or horse*) #25sport* #26(walking or running) #27(aquatic* or swim*) #28[mh "Physical Therapy Modalities"] #29(physiotherapy or physical next therap*) #30(resistance or resisted) #31physical next activit* #32{or #7‐#31} #33#6 and #32 in Trials
Ovid MEDLINE(R)
This strategy uses the Cochrane highly sensitive search strategy for identifying randomised trials in LInes 33 to 43 (Lefebvre 2011).
1 cerebral palsy/ 2 cerebral pals$.tw. 3 ((Hemiplegi$ or diplegi$ or quadriplegi$ or unilateral$) adj5 spastic$).tw. 4 ((Hemiplegi$ or diplegi$ or quadriplegi$ or unilateral$) adj3 ataxi$).tw. 5 Little$ disease.tw. 6 or/1‐5 7 exp Exercise/ 8 exp Exercise Movement Techniques/ 9 exp Exercise Therapy/ 10 exp "Physical Education and Training"/ 11 Physical Endurance/ 12 Physical Fitness/ 13 exp Sports/ 14 Hydrotherapy/ 15 Equine‐Assisted Therapy/ 16 aerobic$.tw. 17 (cycle or cycling).tw. 18 ergometry.tw. 19 (treadmill or tread‐mill).tw. 20 ergometry/ 21 (exercise$ or strength$ or fitness).tw. 22 (flexibility or stretching).tw. 23 ((weight$ adj1 lift$) or weight training).tw. 24 (hippotherapy or equine$ therapy or equine assist$ or horse$).tw. 25 sport$.tw. 26 (walking or running).tw. 27 (aquatic$ or swim$).tw. 28 Physical Therapy Modalities/ 29 (physiotherapy or physical therap$).tw. 30 (resistance or resisted).tw. 31 physical activit$.tw. 32 or/7‐31 33 randomized controlled trial.pt. 34 controlled clinical trial.pt. 35 randomi#ed.ab. 36 placebo$.ab. 37 drug therapy.fs. 38 randomly.ab. 39 trial.ab. 40 groups.ab. 41 or/33‐40 42 exp animals/ not humans.sh. 43 41 not 42 44 6 and 32 and 43
Embase Ovid
1 cerebral palsy/ 2 cerebral pals$.tw. 3 ((Hemiplegi$ or diplegi$ or quadriplegi$ or unilateral$) adj5 spastic$).tw. 4 ((Hemiplegi$ or diplegi$ or quadriplegi$ or unilateral$) adj3 ataxi$).tw. 5 Little$ disease.tw. 6 or/1‐5 7 exp exercise/ 8 exp kinesiotherapy/ 9 physical education/ 10 endurance/ 11 fitness/ 12 exp sport/ 13 hydrotherapy/ 14 hippotherapy/ 15 aerobic$.tw. 16 (cycle or cycling).tw. 17 (treadmill or tread‐mill).tw. 18 ergometry/ 19 ergometry.tw. 20 (exercise$ or strength$ or fitness).tw. 21 (flexibility or stretching).tw. 22 ((weight$ adj1 lift$) or weight training).tw. 23 (hippotherapy or equine$ therapy or equine assist$ or horse$).tw. 24 sport$.tw. 25 (walking or running).tw. 26 (aquatic$ or swim$).tw. 27 exp physiotherapy/ 28 (physiotherapy or physical therap$).tw. 29 physical activit$.tw. 30 (resistance or resisted).tw. 31 or/7‐30 32 6 and 31 33 Randomized controlled trial/ 34 controlled clinical trial/ 35 Single blind procedure/ 36 Double blind procedure/ 37 triple blind procedure/ 38 Crossover procedure/ 39 (crossover or cross‐over).tw. 40 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 41 Placebo/ 42 placebo.tw. 43 prospective.tw. 44 factorial$.tw. 45 random$.tw. 46 assign$.ab. 47 allocat$.tw. 48 volunteer$.ab. 49 or/33‐48 50 32 and 49
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S48 S32 AND S47 S47 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 S46 (MH "Treatment Outcomes") S45 (MH "Program Evaluation") S44 TI ("prospective study" or "prospective research") or AB("prospective study" or "prospective research") S43 TI ("follow‐up study" or "follow‐up research") or AB ("follow‐up study" or "follow‐up research") S42 AB((trebl* N1 mask*) or (trebl* N1 blind*)) S41 AB("cross over" or crossover) S40 (MH "Crossover Design") S39 AB((tripl* N1 mask*) or (tripl* N1 blind*)) S38 AB ((doubl* N1 mask*) or (doubl* N1 blind*)) S37 AB ((singl* N1 mask*) or(singl* N1 blind*)) S36 AB(trial) S35 AB(random*) S34 (MH "Random Assignment") S33 (MH "Clinical Trials+") S32 S6 AND S31 S31 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 S30 physical activit* S29 (resistance or resisted) S28 (physiotherapy or physical therap*) S27 (MH "Physical Therapy+") S26 (aquatic* or swim*) S25 (walking or running) S24 sport* S23 (hippotherapy or equine* therapy or equine assist* or horse*) S22 (weight* N1 lift*) or weight training S21 (flexibility or stretching) S20 (exercise* or strength* or fitness) S19 (treadmill or tread‐mill) S18 (cycle or cycling) S17 ergometry S16 (MH "Ergometry") S15 (MH "Sports+") S14 (MH "Physical Activity") S13 (MH "Physical Fitness+") S12 (MH "Physical Endurance+") S11 (MH "Physical Education and Training+") S10 (MH "Sports+") S9 (MH "Physical Fitness+") S8 (MH "Leisure Activities+") S7 (MH "Exercise+") S6 S1 OR S2 OR S3 OR S4 OR S5 S5 "Little* disease" S4 ((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) N3 ataxi*) S3 ((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) N5 spastic*) S2 cerebral pals* S1 (MH "Cerebral Palsy")
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library
#1[mh "cerebral palsy"] #2(cerebral next pals*):ti,ab #3((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/5 spastic*):ti,ab 251 #4((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/3 ataxi*):ti,ab #5("Little* disease"):ti,ab #6{or #1‐#5} #7[mh exercise] #8[mh "Exercise Movement Techniques"] #9[mh "Exercise Therapy"] #10[mh ^"Physical Education and Training"] #11[mh "Physical Endurance"] #12[mh ^"Physical Fitness"] #13[mh Sports] #14[mh Hydrotherapy] #15[mh "Equine‐Assisted Therapy"] #16aerobic*:ti,ab #17(cycle or cycling):ti,ab #18[mh ergometry] #19ergometry:ti,ab #20(treadmill or tread next mill):ti,ab #21(exercise* or strength* or fitness):ti,ab #22(flexibility or stretching):ti,ab #23((weight* near/1 lift*) or weight next training):ti,ab #24(hippotherapy or equine* next therapy or equine next assist* or horse*):ti,ab #25sport*:ti,ab #26(walking or running):ti,ab #27(aquatic* or swim*):ti,ab #28[mh "Physical Therapy Modalities"] #29(physiotherapy or physical next therap*):ti,ab #30(resistance or resisted):ti,ab #31(physical next activit*):ti,ab #32{or #7‐#31} #33#6 and #32 in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library
#1[mh "cerebral palsy"] #2(cerebral next pals*):ti,ab #3((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/5 spastic*):ti,ab #4((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) near/3 ataxi*):ti,ab #5("Little* disease"):ti,ab #6{or #1‐#5} #7[mh exercise] #8[mh "Exercise Movement Techniques"] #9[mh "Exercise Therapy"] #10[mh ^"Physical Education and Training"] #11[mh "Physical Endurance"] #12[mh ^"Physical Fitness"] #13[mh Sports] #14[mh Hydrotherapy] #15[mh "Equine‐Assisted Therapy"] #16aerobic*:ti,ab #17(cycle or cycling):ti,ab #18[mh ergometry] #19ergometry:ti,ab #20(treadmill or tread next mill):ti,ab #21(exercise* or strength* or fitness):ti,ab #22(flexibility or stretching):ti,ab #23((weight* near/1 lift*) or weight next training):ti,ab #24(hippotherapy or equine* next therapy or equine next assist* or horse*):ti,ab #25sport*:ti,ab #26(walking or running):ti,ab #27(aquatic* or swim*):ti,ab #28[mh "Physical Therapy Modalities"] #29(physiotherapy or physical next therap*):ti,ab #30(resistance or resisted):ti,ab #31(physical next activit*):ti,ab #32{or #7‐#31} #33#6 and #32 in Other Reviews
Science Citation Index Web of Science
# 14 #13 AND #12 # 13 TS=(random* or control NEAR/1 group* or assign* or allocat*) # 12 #11 AND #5 # 11 #10 OR #9 OR #8 OR #7 OR #6 # 10 TS= ("weight* lift*" or "weight training") # 9 TS= (physiotherapy or "physical therap*" or "physical education") # 8 TS=(aerobic* or aquatic* or cycle or cycling or ergometry or swim* or running or walking or treadmill or "tread mill" or sport* or horse* or equine* or hippotherapy) # 7 TS=(flexibility or stretching) # 6 Ts=(exercise* or strength* or fitness) # 5 #4 OR #3 OR #2 OR #1 # 4 TS= "littles disease" # 3 TS=((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) NEAR/3 ataxi*) # 2 TS=((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) NEAR/5 spastic*) # 1 TS=(cerebral pals*)
Conference Proceedings Citation Index ‐ Science Web of Science
# 14 #13 AND #12 # 13 TS=(random* or control NEAR/1 group* or assign* or allocat*) # 12 #11 AND #5 # 11 #10 OR #9 OR #8 OR #7 OR #6 # 10 TS= ("weight* lift*" or "weight training") # 9 TS= (physiotherapy or "physical therap*" or "physical education") # 8 TS=(aerobic* or aquatic* or cycle or cycling or ergometry or swim* or running or walking or treadmill or "tread mill" or sport* or horse* or equine* or hippotherapy) # 7 TS=(flexibility or stretching) # 6 Ts=(exercise* or strength* or fitness) # 5 #4 OR #3 OR #2 OR #1 # 4 TS= "littles disease" # 3 TS=((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) NEAR/3 ataxi*) # 2 TS=((Hemiplegi* or diplegi* or quadriplegi* or unilateral*) NEAR/5 spastic*) # 1 TS=(cerebral pals*)
LILACS (Latin American and Caribbean Health Science Information database)
(lilacs.bvsalud.org/en)
(tw:(cerebral pals*) OR mh:("Cerebral Palsy") OR tw:(littles disease)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Health Services Research Projects in Progress (HSRPRoj)
(wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm)
We searched for the following words and phrases individually and then manually de‐duplicated the records:
cerebral pals*; ataxi*; hemiplegi*; diplegi*; quadriplegi*; unilateral*; Little's disease; spastic*
OpenGrey
cerebral pals* OR hemiplegi* OR diplegi* OR quadriplegi*
National Rehabilitation Information Center
We searched for the following words and phrases individually and then manually de‐duplicated the records:
#1 Cerebral palsy #2 hemiplegia OR diplegia OR quadriplegia OR unilateral #3 spastic #4 ataxic
PEDro (Physiotherapy Evidence Database)
cerebral pals*
UKCRN Study Portfolio
(public.ukcrn.org.uk/search)
The following searches were conducted individually and the records were de‐duplicated manually: #1 cerebral palsy #2 cerebral pals* #3 cerebral pals$ #4 cerebral palsy OR hemiplegia #5 hemiplegia #6 diplegia #7 quadriplegia #8 ataxic #9 spastic #10 little disease
ClinicalTrials.gov
(clinicaltrials.gov)
cerebral palsy
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
We searched for the following words and phrases individually and then manually de‐duplicated the records:
#1 cerebral palsy (in the condition field) #2 cerebral palsy (in the title field) #3 hemiplegia OR diplegia OR quadriplegia OR unilateral OR ataxia OR spastic (in the condition) #4 hemiplegia OR diplegia OR quariplegia OR unilateral OR ataxia OR spastic (in the title) #5 Little disease (in the title field) #6 Little disease (in the condition)
Appendix 4. Record of searches
| Database | Search date | Database date range/issue/volume | Number of records | Limits |
| Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library | 7 May 2015 | 2015 Issue 4 | 76 | — |
| 14 June 2016 | 2016, Issue 5 | 0 | Publication year from 2015 to 2016 | |
| MEDLINE Ovid | 7 May 2015 | 1946 to May Week 1 2015 | 954 | — |
| 14 June 2016 | 1946 to June Week 1 2016 | 103 | ed=20150501‐20160602 | |
| Embase Ovid | 7 May 2015 | 1980 to 2015 Week 18 | 1095 | — |
| 14 June 2016 | 1980 to 2016 Week 24 | 172 | em=201519‐201624 | |
| CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature) | 7 May 2015 | 1937 to 7 May 2015 | 819 | — |
| 14 June 2016 | 1937 to 14 June 2016 | 65 | EM = 201504‐ | |
| Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library | 7 May 2015 | 2015 Issue 5 | 2 | — |
| 14 June 2016 | 2016 Issue 6 | 0 | — | |
| Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library | 7 May 2015 | 2015 Issue 2 | 11 | — |
| Not searched | No new content after 2015 Issue 2 | Not searched | Not searched | |
| Science Citation Index Web of Science | 7 May 2015 | 1970 to 6 May 2015 | 730 | — |
| 14 June 2016 | 1970 to 9 June 2016 | 160 | 2015‐2016 | |
| Conference Proceedings Citation Index ‐ Science Web of Science | 7 May 2015 | 1990 to 6 May 2015 | 36 | — |
| 14 June 2016 | 1990 to 9 June 2016 | 5 | 2015‐2016 | |
| LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en) | 7 May 2015 | All available years | 14 | — |
| 14 June 2016 | All available years | 4 | Deduplicated with previous records | |
| Health Services Research Projects in Progress (HSRPRoj; wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm) | 13 May 2015 | All available years | 103 | — |
| 23 June 2016 | All available years | 137 | — | |
| OpenGrey (www.opengrey.eu) | 6 May 2015 | All available years | 260 | — |
| 16 June 2016 | All available years | 0 | Deduplicated with previous records | |
| National Rehabilitation Information Center (www.naric.com) | 13 May 2015 | All available years | 1564 | — |
| 23 June 2016 | All available years | 417 | — | |
| PEDro (Physiotherapy Evidence Database; www.pedro.org.au) | 15 May 2015 | All available years | 320 | — |
| 17 June 2016 | All available years | 59 | — | |
| UKCRN Study Portfolio (public.ukcrn.org.uk/search) | 6 May 2015 | All available years | 41 | — |
| 16 May 2016 | All available years | 21 | — | |
| ClinicalTrials.gov (www.clinicaltrials.gov) | 6 May 2015 | All available years | 890 | — |
| 16 June 2016 | All available years | 112 | — | |
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en) | 10 June 2015 | All available years | 1998 | — |
| 20 June 2016 | All available years | 883 | — | |
| Total records | 11,100 | |||
Appendix 5. Risk of bias criteria: operational definitions
Adequate sequence generation?
Low risk of bias: based on a random component judged to be both appropriate and sufficiently well described.
High risk of bias: based on any non‐random component.
Unclear risk of bias: insufficient information regarding sequence generation process to permit judgement of low or high risk of bias.
Adequate allocation concealment?
Low risk of bias: method of concealment allocation employed prohibited foresight of participant assignment.
High risk of bias: method of concealment allocation employed permitted possible foresight of participant assignment.
Unclear risk of bias: method of concealment allocation not described or described in insufficient detail to permit judgement of low or high risk of bias.
Blinding of outcome assessment?
Low risk of bias: outcome assessor (including participants with respect to self‐reported outcomes) blinded to participants’ allocated intervention, and unlikely that blinding broken; OR no or incomplete blinding but judged that a given outcome unlikely to be influenced by lack of blinding.
High risk of bias: outcome assessor (including participants with respect to self‐report outcomes) unblinded to participants’ allocated intervention; OR outcome assessor blinded to allocated intervention but likely that blinding may have been broken (and a given outcome is likely to be influenced by lack of blinding).
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of participants?
Low risk of bias: participants blinded to allocated intervention and unlikely that blinding broken; OR no or incomplete blinding but judged that a given outcome unlikely to be influenced by lack of blinding.
High risk of bias: participants not blinded to allocated intervention; OR participants blinded to allocated intervention but likely that blinding may have been broken (and a given outcome is likely to be influenced by lack of blinding).
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of care provider?
Low risk of bias: care provider blinded to allocated intervention and unlikely that blinding broken; OR no or incomplete blinding but judged that a given outcome unlikely to be influenced by lack of blinding.
High risk of bias: care provider not blinded to allocated intervention; OR care provider blinded to allocated intervention but likely that blinding may have been broken (and a given outcome is likely to be influenced by lack of blinding).
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Incomplete outcome data addressed?
Low risk of bias: no missing outcome data. Reasons for missing data unlikely to be related to the true outcome. Missing outcome data balanced across intervention groups with similar reasons for omissions. Dichotomous outcomes: proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. Continuous outcomes: difference in means or standardised mean difference among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data imputed using appropriate methods. Intention‐to‐treat analysis undertaken. Less than or equal to 10% dropout rate.
High risk of bias: reason for missing outcome data likely to be related to the true outcome. Dichotomous outcomes: proportion of missing outcomes compared with observed event risk enough to induce a clinically relevant bias in intervention effect estimate. Continuous outcomes: difference in means or standardised mean difference among missing outcomes enough to induce a clinically relevant bias on observed effect size. As‐treated analysis undertaken with substantial departure of the intervention received from that assigned at randomisation. Equal to or greater than 30% dropout rate.
Unclear risk of bias: insufficient reporting of attrition or exclusions to permit judgement of low or high risk of bias. Greater than 10% and less than 30% dropout rate.
Selective outcome reporting?
Low risk of bias: all primary outcomes of interest adequately reported with point estimates and measures of variance for all time points.
High risk of bias: incomplete reporting of prespecified outcomes. One or more primary outcomes is reported using measurements, analysis methods, or subsets of data that were not prespecified. One or more reported primary outcomes were not prespecified. One or more outcomes of interest reported incompletely and cannot be entered into a meta‐analysis. Results for a key outcome expected to have been reported, excluded.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Free of other biases
Low risk of bias: appears free of other sources of bias.
High risk of bias: results may have been confounded by at least one important risk of bias (design‐specific, fraudulent, other).
Unclear risk of bias: other sources of bias may be present but there is either insufficient information to assess whether an important risk of bias exists; OR insufficient rationale or evidence regarding whether an identified problem will introduce bias.
Data and analyses
Comparison 1. Aerobic exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function, short term | 3 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.02, 1.04] |
| 2 Activity: gait speed, short term | 4 | 82 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.28] |
| 3 Activity: walking endurance; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Activity: gait speed, intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Activity: gross motor function, intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Activity: daily physical activity; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Aerobic fitness; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 2. Resistance training versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function, children and adolescents; short term | 7 | 164 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.19, 0.43] |
| 2 Activity: gross motor function, children and adolescents; intermediate term | 3 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.30, 0.55] |
| 3 Activity: gait speed, children and adolescents; short term | 8 | 185 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 4 Activity: gait speed, children and adolescents; intermediate term | 3 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.17, 0.11] |
| 5 Activity: gait speed, adults; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Activity: gross motor function, adults; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Activity: walking endurance, adults; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Participation, children and adolescents; short term | 2 | 127 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.01, 0.70] |
| 9 Participation, children and adolescents; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 Quality of life (parent‐reported), children and adolescents; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11 Quality of life (child‐reported), children and adolescents; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12 Muscle strength, children and adolescents; short term | 8 | 247 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.00, 1.06] |
| 13 Muscle strength, children and adolescents; intermediate term | 3 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.06, 0.94] |
| 14 Muscle strength, adults; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Mixed training versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function; short term | 4 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.29, 0.33] |
| 2 Activity: gait speed; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Activity: walking endurance; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Participation; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Participation; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Aerobic fitness; short term | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.39, 0.50] |
| 7 Muscle strength; short term | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.24, 0.40] |
| 8 Anaerobic fitness; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Aerobic fitness; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 Anaerobic fitness; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11 Muscle strength; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 4. Resistance training versus aerobic exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function; short term | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.50, 0.55] |
| 2 Activity: gait speed; short term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Activity: gait speed; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Activity: gross motor function; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Muscle strength; short term | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.64, 0.41] |
| 6 Muscle strength; intermediate term | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
4.1. Analysis.
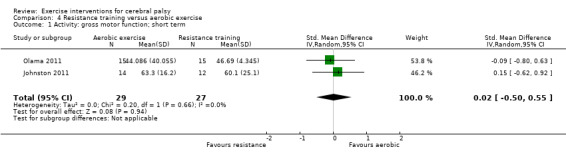
Comparison 4 Resistance training versus aerobic exercise, Outcome 1 Activity: gross motor function; short term.
Comparison 5. Aerobic exercise and mixed training versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function; short term | 7 | 228 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.09, 0.62] |
| 2 Activity: gross motor function, intermediate term | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.15, 1.64] |
| 3 Activity: gait speed; short term | 5 | 140 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.24] |
| 4 Activity: walking endurance; short term | 2 | 73 | Mean Difference (IV, Random, 95% CI) | 8.43 [‐12.38, 29.23] |
| 5 Aerobic fitness; short term | 3 | 98 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.34, 0.45] |
Comparison 6. Resistance training and mixed training versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity: gross motor function; short term | 11 | 327 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.01, 0.43] |
| 2 Activity: gross motor function; intermediate term | 4 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 3 Activity: gait speed; short term | 9 | 243 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| 4 Participation; short term | 3 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.07, 0.64] |
| 5 Participation; intermediate term | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.24, 0.54] |
| 6 Muscle strength; short term | 11 | 397 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.01, 0.76] |
| 7 Muscle strength; intermediate term | 4 | 149 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.16, 0.71] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
McCubbin 1985.
| Methods |
Design: randomised controlled trial Country of origin: USA Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 30 participants; 20 participants randomised to a resistance training intervention or repetitive movement exercise without resistance; 10 participants selected for matched control group not reported in this review Resistance exercise group n = 10; no resistance exercise group n = 10 Age: 10‐20 years. Mean age not reported. Sex: not reported Ethnicity: not reported GMFCS level: not reported MACS level: not reported Type of motor abnormality: spastic (n = 15), athetoid‐ataxic (n = 1), spastic‐athetoid (n = 1), mixed (n = 2), and athetoid (n = 1); resistance exercise group: spastic (n = 7), athetoid‐ataxic (n = 1), spastic‐athetoid (n = 1), mixed (n = 1); no resistance exercise group: spastic (n = 8), athetoid (n = 1), mixed (n = 1) Anatomical distribution of CP: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Aim of the intervention: not reported Type of exercise programme: resistance training Exercise mode: elbow extension exercise using Super Mini‐Gym Model 180, Mini Gym, Inc. Comparator: identical protocol of elbow extension exercise without resistance. All participants participated in regularly scheduled school activities, which included physical education, physical therapy, occupational therapy, speech therapy, and typical school classes. Subjects using medication prior to the experiment continued to use the medication recommended by their physician. Setting: not reported Intervention provider: primary researcher or registered physical therapist Duration of programme: 6 weeks Exercise dose: 3 sets of 10 repetitions, 3 days per week. Intensity not reported Tailoring of intervention to individual: not reported Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), interim (week 3), postintervention (week 6) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not reported Potential conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: the participants were "matched according to type and severity of cerebral palsy according to the classification system employed by the National Association of Sport for Cerebral Palsy . . . Following the classification the matched experimental subjects were randomly selected to either experimental group". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: the participants were "matched according to type and severity of cerebral palsy according to the classification system employed by the National Association of Sport for Cerebral Palsy . . . Following the classification the matched experimental subjects were randomly selected to either experimental group". Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: did not provide information to indicate if the assessor was blinded to group allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: no demographic data provided |
Van den Berg‐Emons 1998.
| Methods |
Design: randomised controlled trial Country of origin: Netherlands Intervention(s): aerobic exercise Unit of allocation: individual |
|
| Participants |
Number of participants: 20 randomised; exercise group: n = 10; control group: n = 10 Age: mean (SD) 9.2 (1.4) years; exercise group: mean (SD) = 9.5 (1.6) years; control group: mean (SD)= 8.8 (1.1) years Sex (male/female): 11/9 Ethnicity: authors reported 19 white participants, 1 participant born in Sri Lanka GMFCS level: ambulant (i.e. GMFCS levels I, II or III; n = 10) and 'wheelchair bound' (i.e. GMFCS levels IV or V; n = 10) Type of motor abnormality: spastic CP, 2 participants additionally had ataxia Anatomical distribution of CP: diplegia (n = 16) and tetraplegia (n = 4) Inclusion criteria: age 7‐13 years, diagnosis of spastic CP Exclusion criteria: not stated |
|
| Interventions |
Aim of the intervention: to increase daily physical activity, aerobic power, anaerobic power and muscle strength Type of exercise programme: aerobic Exercise mode: aerobic exercises such as cycling, wheelchair driving, running, swimming, training on a "flying saucer" and mat exercises Comparator: all participants participated in a normal school and therapy programme. School programme included 2, 45‐min gymnastic lessons per week. Therapy was based on personal needs and varied from no therapy to more than 2.5 h per week Setting: school Intervention provider: not stated Duration of programme: 18 months. After 9 months the participants in both groups were given the opportunity to participate in the next training programme of 2 times per week (45 min per session). 8 participants in exercise group and all participants in control group chose to participate. Data only extracted for first 9 months as after this participants were not randomised to a group Exercise dose: 4 sessions per week, 45 min per session. Heart rate (HR) was measured randomly to get an indication of training intensity. Mean (SD) HR during training sessions in first 2 months was mean (SD) 135 (10) bpm; mean (SD) percentage of time spent at ≥ 70% of HR reserve during training was 49 (17) % Fidelity to prescribed intervention: attendance was 84% (range 78 to 88%) in first 2 months Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (9 months) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[a]t the beginning of the first year (in September, after the summer holidays), the children were matched pairwise for physical ability, mental function, and if possible, for age, gender and body composition. After matching, the children of each pair were randomly assigned to an experimental group (EXP, n=10) or control group (CON, n=10)". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[a]t the beginning of the first year (in September, after the summer holidays), the children were matched pairwise for physical ability, mental function, and if possible, for age, gender and body composition. After matching, the children of each pair were randomly assigned to an experimental group (EXP, n=10) or control group (CON, n=10)". Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information is provided regarding who conducted assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[t]he project lasted 2 years and included two training periods of 9 months". Comment: only data from the first 9 month training period is reported as participants were not randomly allocated to a training group or control group for the second 9 month training period. No missing data for first 9 month training period |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Dodd 2003.
| Methods |
Design: randomised controlled trial Country of origin: Australia Intervention(s): resistance training programme of the ankle plantarflexors, knee extensors, and hip extensors Unit of allocation: individual |
|
| Participants |
Number of participants: 21 randomised; exercise group: n = 11; control group: n = 10 Group characteristics reported for all randomised participants. Age: 8‐18 years; exercise group: mean (SD) = 12.7 (2.8) years; control group: mean (SD) = 13.5 (3.4) years Sex (male/female): 10/11; exercise group: 4/7; control group: 6/4 Ethnicity: not stated GMFCS level: level I (n = 7), level II (n = 5), level III (n = 9); exercise group: level I (n = 2), level II (n = 2), level III (n = 7); control group: level I (n = 5), level II (n = 3), level III (n = 2) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: aged 8‐18 years, spastic diplegia, able to walk independently with or without a walking aid (GMFCS level I‐III), able to follow commands Exclusion criteria: fixed flexion deformity at the knee, hip greater than 25 degrees or fixed equinus of more than 10 degrees, current participation in other management strategies such as serial casting, botulinum toxin or orthopaedic surgery in the previous 12 months, participation in a strength‐training programme within the previous 3 months |
|
| Interventions |
Aim of the intervention: to increase muscle strength, physical activity and walking ability Type of exercise programme: resistance training. Participants also instructed to continue their normal activities including school and sport and attend usual physiotherapy provided the programme didn't include progressive resistance training (usual physiotherapy is a 45‐min consultation once or twice a month). Exercise mode: exercises to increase strength of ankle plantarflexors, knee extensors, and hip extensors i.e. bilateral heel raises, bilateral half squats, step‐ups Comparator: participants in control group: instructed to continue their normal activities including school and sport. Also attended usual physiotherapy provided the programme didn't include progressive resistance training Setting: participant's home. Unsupervised individual session Intervention provider: physiotherapist Duration of programme: 6 weeks Exercise dose: 3 sets of 8‐10 repetitions of each exercise (20‐30 min sessions). 3 times per week. Load was adjusted by adding free weights to a backpack worn by participant to ensure participants could complete 8‐12 repetitions to fatigue. At the end of 2nd and 4th week the physiotherapist visited participants and adjusted the load to ensure they can only complete between 8‐12 repetitions of each exercise. Tailoring of intervention to individual: at the first session the training load was adjusted to ensure that each participant obtained optimal strength training Fidelity to prescribed intervention: adherence monitored with a self‐report exercise diary. Mean (SD) sessions 16.8 (2.4) out of 18. Mean (SD) sets 147.7 (23.4) out of 162 Monitoring of adverse events: no adverse events reported that led to missing training sessions. 1 participant reported pressure on the shoulders from the loaded backpack. 2 participants reported mild foot and ankle discomfort during heel raises. Exercise was modified and enabled participants to continue without incident. Not stated how adverse events were monitored. |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6), 12 weeks postintervention (week 18) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]articipants were allocated randomly to either the strength training or control group using a concealed method. Twenty‐two identical pieces of paper were placed in an opaque container, 11 with the words 'experimental group', 11 with the words 'control group' written on them. In another opaque container, the name of each participant was written on 21 separate pieces of paper. Allocation was achieved by drawing a piece of paper from each container. This process continued until all participants were allocated to a group". |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[p]articipants were allocated randomly to either the strength training or control group using a concealed method. Twenty‐two identical pieces of paper were placed in an opaque container, 11 with the words 'experimental group', 11 with the words 'control group' written on them. In another opaque container, the name of each participant was written on 21 separate pieces of paper. Allocation was achieved by drawing a piece of paper from each container. This process continued until all participants were allocated to a group". Comment: unclear if an independent person did the allocation. Implementation of the sequence could be open to bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[a] physiotherapist who was blind to group allocation and experienced in assessing movement disorders took all outcome measures" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low rate of missing data. 1 participant (5%) withdrew from control group, reason given |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Engsberg 2006.
| Methods |
Design: randomised controlled trial Country of origin: USA Intervention(s): strength training Unit of allocation: individual |
|
| Participants |
Number of participants: 15 participants randomised. Data reported on 12 participants. Dorsiflexor exercise group: n = 3; plantarflexor group: n = 4; dorsi‐ and plantarflexor group: n = 2; control group: n = 3 Age: mean (SD) = 9.9 (3.5) years; dorsiflexor group: mean (SD) = 12.5 (5.4) years; plantarflexor group: mean (SD) = 8.6 (2.3) years; dorsi‐ and plantarflexor group: mean = 7.6 (unable to calculate SD); control group: mean (SD) 10.7 (2.2) years Sex (male/female): 5/10 (randomised) 3/9 (reported); dorsiflexor exercise group: 0/3; plantarflexor group: 2/2; dorsi‐ and plantarflexor group: 0/2; control group: 1/2 Ethnicity: not stated GMFCS level: level I (n = 5), level II (n = 5) and level III (n = 2); dorsiflexor exercise group: level I (n = 1), level II (n = 1), and level III (n = 1); plantarflexor group: level I (n = 2), level II (n = 1) and level III (n = 1); dorsi‐ and plantarflexor group: level I (n = 1) and level II (n = 1); control group: level I (n = 1) and level II (n = 2) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: diagnosis of spastic diplegia, GMFCS level I, II or III, cognitive skills to actively participate (follow simple commands), ability to perform 6‐8 repetitions of walking 9 m, passive dorsiflexion range of motion to at least ‐5 degrees with knee extended, ability to actively dorsi‐ and plantarflex the foot. Unclear whether hypertonicity of the plantar flexors as measured by the Ashworth scale was an inclusion or exclusion criterion Exclusion criteria: surgical intervention in last 12 months, casting procedures or botulinum toxin injection in last 6 months, selective dorsal rhizotomy, intrathecal baclofen, motor deficits secondary to neurological injury/illness beginning after the first month of life, or children with moderate‐to‐severe dystonia, athetosis, ataxia. |
|
| Interventions |
Aim of the intervention: to determine if an increase in ankle muscle strength results in an increase in function Type of exercise programme: resistance training Exercise mode: isokinetic dynamometer. Active assisted protocol in the passive mode was used. Participants were instructed to contribute as much force to the moving lever arm as possible. Comparator: comparison of dorsiflexor strength training, plantarflexor strength training, dorsi‐ and plantarflexor strength training, and control group undergoing no strength training (no more detail provided) Setting: physiotherapy clinic Intervention provider: physiotherapist Duration of programme: 12 weeks Exercise dose: all strength training groups (1, 2 and 3) performed 3 sessions each week. In each session they performed 3 sets of 5 repetitions at 30°/s concentrically and eccentrically and 3 sets of 5 repetitions at 90°/s concentrically and eccentrically. Total number of repetitions was 30 for a muscle group per session. Load was equal to or greater than 80% of maximum load determined at start of session Tailoring of intervention to individual: at the start of each session the participant had 3 attempts to meet or exceed previous maximum Fidelity to prescribed intervention: not stated Monitoring of adverse events: spasticity and end range dorsiflexion was monitored once a week. Spasticity did not increase and dorsiflexion range of motion did not reduce. No other adverse effects recorded |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 12) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: National Institute of Neurological Disorders and Stroke at the National Institute of Health (ROI NS 046434) Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote (from abstract): "Data were obtained from 12 children with spastic diplegia who were assigned randomly to a dorsiflexor group, a plantarflexor group, a dorsi‐ and plantarflexor group, or a control group." Quote: "A key factor for participation was the parents agreeing to permit random assignment to 1 of 4 groups: (1) dorsiflexion strength training (DF group), (2) plantarflexion strength training (PF group), (3) dorsi‐ and plantarflexion strength training (DF&PF group), and (4) control group undergoing no strength training program (control group)". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[i]t should also be noted that the investigators were not blinded to the training regimen of the subjects, since the study was too small to permit such a separation of tasks"
Quote: "[t]he same study therapist collected dorsiflexion end‐range outcome values for all subjects before and after the intervention" Comment: no indication who assessed outcomes other than "dorsiflexion end‐range outcome values". Both self‐reported and objective outcome measures were used. As participants and their parents were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "[h]owever, the final analysis included data from 12 of the 15 subjects" Quote: "[t]hree of the 15 subjects were eliminated from the investigation they did not demonstrate any gains in ankle strength (i.e. any increase above baseline values for a muscle being trained). The reasons for a lack of strength increases included: our inability to recognize the limited understanding of maximum effort for one subject, use of an older Biodex that did not permit an 80% target line, and a therapist who did not follow the protocol. As previously discussed the purpose of the investigation was to focus only on those subjects that increased in strength since we wanted to examine any relationship that might exist between strength gain and change in function". Comment: although the authors justify their exclusion of 3 participants, this represents a missing data rate of 20%. Further, reason for missing data is likely to be related to the true outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Unclear risk | Comment: demographic data provided only for those who completed the trial. Outcome data only provided for those who improved on measured outcomes |
Unger 2006.
| Methods |
Design: randomised controlled trial Country of origin: South Africa Intervention(s): strength training; specifically targeted at disadvantaged students Unit of allocation: individual |
|
| Participants |
Number of participants: 37 randomised; exercise group: n = 24; control group: n = 13. Data presented on 31 participants; exercise group: n = 21; control group: n = 10 Age: exercise group: mean (range) = 15.86 (13.5 to 18.92) years; control group: mean (range) = 16.28 (14.0 to 18.33) years Sex (male/female): 19/12; exercise group: 13/8; control group: 6/4 Ethnicity: not stated GMFCS level: no assistive devices (i.e. GMFCS level I or II; n = 29), and crutches or occasional use of wheelchair (i.e. GMFCS level III; n = 2); exercise group: no assistive devices (n = 19) and crutches or occasional use of wheelchair (n = 2); control group: no assistive devices (n = 10) Type of motor abnormality: spastic CP Anatomical distribution of CP: hemiplegia (n = 16), diplegia (n = 14), and triplegia (n = 1); exercise group: hemiplegia (n = 8), diplegia (n = 12), and triplegia (n = 1); control group:: hemiplegia (n = 8), diplegia (n = 2) Inclusion criteria: aged 13‐18 years, able to walk with or without a walking aid, be in good health, able to understand instructions Exclusion criteria: history of spasticity altering surgery such as a baclofen pump or selective dorsal rhizotomy, orthopaedic or neurosurgery in previous 12 months, botulinum toxin injection in last 6 months, participated in sport at provincial or international level |
|
| Interventions |
Aim of the intervention: to increase strength Type of exercise programme: resistance training Exercise mode: exercise circuit consisting of 28 stations targeting upper and lower limbs and trunk. 8 to 12 exercises selected. Body weight, free weights, dumbbells, ankle and wrist cuffs and bar with disc weights, elastic and rubber bands provided resistance. Balls were used for support or to provide an unstable surface Comparator: no information provided Setting: school Intervention provider: research assistant was given instructions on performance criteria by the researcher and assisted with the implementation and supervision of the exercise programmes Duration of programme: 8 weeks Exercise dose: 1‐3 sessions per week. 1 set of 6‐10 repetitions progressed to 3 sets of 12 repetitions Tailoring of intervention to individual: initial resistance was set to allow at least 1 set of 6 to 10 repetitions. Once participant could do 3 sets of 12 repetitions, resistance was increased and repetitions reduced. Fidelity to prescribed intervention: not reported The initial programme was recorded on a participation record, and subjects were responsible for updating and recording their own programme. Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 8) Primary outcome: no primary outcome measure stated. Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated. None perceived. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Following pre‐testing subjects were systematically randomised into either the experimental group or the control group with every third name drawn from the hat being allocated to the control group". Comment: description suggests sequence generation based on a non‐random component. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Following pre‐testing subjects were systematically randomised into either the experimental group or the control group with every third name drawn from the hat being allocated to the control group". Comment: insufficient information regarding allocation concealment was provided to make a judgement. Did not indicate who conducted sequence generation and how the allocation was concealed following sequence generation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The research assistants for both outcome measures were blinded to group allocation for both pretesting and at eight‐week testing". Quote: "A short, self‐administered questionnaires shown in the Appendix were used to assess perceptions of body image and functional competence. The themes relating to body image were identified from the physical appearance and attributes sub scale of the Piers Harris Children's Self‐Concept Scale. Themes for section B were decided on in consultation with the school therapists and included activities required by the child for successful functioning in his or her environment." Quote: "Subjects selected the most applicable phrase." Comment: both self‐reported and objective outcome measures were used. As participants were not blinded to group allocation at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: 6 participants (16%) were withdrawn in total. 3 participants (13%) withdrew from the intervention group; 2 were "withdrawn due to absenteeism" and 1 was withdrawn "sport participation". 3 participants (23%) were withdrawn from the control group due to "sport participation", "incorrect diagnosis", and "participated in PRE". Indicates a high rate of missing data that's not evenly distributed across groups. Reasons for withdrawal aren't clear. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Unclear risk | Comment: data analysis only included those who completed the trial not all those who enrolled. |
Liao 2007.
| Methods |
Design: randomised controlled trial Country of origin: Taiwan Intervention(s): resistance training (loaded sit‐to‐stand exercise) Unit of allocation: individual |
|
| Participants |
Number of participants: 24 randomised, 20 analysed. Exercise group: n = 10; control group: n = 10; Baseline characteristics provided for n = 20 analysed participants Age: exercise group: mean (SD) = 85.6 (20.8) months; control group: mean (SD) = 91.3 (17.5) months. Sex (male/female): 12/8; exercise group: 7/3; control group: 5/5 Ethnicity: not stated GMFCS level: level I (n = 10), level II (n = 10); exercise group: level I (n = 4) and level II (n = 6); control group: level I (n = 6) and level II (n = 4) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: aged 5‐12 years, spastic diplegia, GMFCS level I or II, able to stand up from a chair independently and maintain standing for more than 5 seconds, able to follow verbal instructions, without obvious limitation in passive range of motion of the lower limbs, able to attend physiotherapy at least once a week before and during the study, not received strength training in last 3 months, parental commitment to allow participation without altering current therapy or activity Exclusion criteria: orthopaedic intervention, selective dorsal rhizotomy or botulinum toxin injection to the lower extremities within 6 months, or orthopaedic problems or medical conditions that prevented participants from participating in the exercises |
|
| Interventions |
Aim of the intervention: to increase muscle strength Type of exercise programme: resistance training Exercise mode: loaded sit‐to‐stand exercise with weighted vest Comparator: all participants in both groups performed their regular physiotherapy programme which included passive range‐of‐motion exercises, positioning, balance training, functional training, and neurodevelopment training. At the start of the study both groups had 2 participants per group who had physiotherapy twice a week and 8 participants per group who received physiotherapy once a week. During a SARS epidemic participants in both groups decreased or stopped physiotherapy. In the control group, 1 participant received physiotherapy 2 days per week, 5 participants received physiotherapy 1 day per week, 1 participant received physiotherapy once every 2 weeks, and 3 participants didn't receive any physiotherapy. In the exercise group, 4 participants received physiotherapy 1 day per week, 2 participants received physiotherapy once every 2 weeks, and 4 participants discontinued physiotherapy Setting: home‐based programme Intervention provider: trainer taught participants and their caregivers how to perform the exercise and modify the exercise during a visit to the home or study site, every other week. Exercises at home supervised by caregiver Duration of programme: 6 weeks Exercise dose: 3 sets of sit‐to‐stand exercises; 2 sets of 10 repetitions at 20% of 1 repetition maximum (RM), 1 set performing as many repetitions as possible at 50% 1 RM until fatigue. 3 times per week Tailoring of intervention to individual: resistance applied was progressively increased to ensure the participant was performing exercises at 50% of 1 RM every 2 weeks Fidelity to prescribed intervention: trainer insured the compliance of exercises during the training period via telephone interview. An exercise diary was provided to the caregiver to document the participant's exercise date, weight and number of repetitions in each exercise session. Participants in the experimental group performed the loaded sit‐to‐stand exercise mean (SD) 18.0 (3.2) times (range 12 to 21 times) during the 6‐week period. All participants performed exercises at least twice a week and 3 participants performed exercises more than 3 times. Participants' average maximum repetitions of 50% of 1 RM sit‐to‐stand varied from 20 to 100 each session. Monitoring of adverse events: most participants in the exercise group reported pressure on the shoulders from the body vest during the loaded sit‐to‐stand. No pain or injury due to training was reported. |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 6) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: grant from the National Science Council, Taiwan Potential conflicts of interest: authors report no conflict of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[r]andomised block design" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[o]ne blinded tester (Y‐CL) who is a physical therapist with pediatric assessment experience (including GMFM‐88, gait speed) for 6 years, conducted the outcome measures and demographic data collection." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "[o]f 24 children, 4 children (2 in experimental group, 2 in control group) withdrew before the study's completion because parents were concerned about SARS and did not want their children to come to the laboratory, which was located inside a hospital, for a follow‐up test" Quote: "Some of the demographic data of these children who withdrew differed from the participant children. Compared with the participant children, the children who withdrew were statistically significantly older (109.8±6.4mo), heavier (26.1±4.3kg), and taller (127.0±10.9cm). However, their outcome measure data for the pre‐assessment were similar to the participant children in this study." Comment: attrition accounted for but high level of missing data (17%). Missing data evenly distributed across groups and no reported differences in baseline data between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: baseline outcome and demographic data only includes those who completed the trial |
Seniorou 2007.
| Methods |
Design: randomised controlled trial Country of origin: UK Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 21 participants randomised. 20 participants completed study and included in analysis. Resistance exercise group: n = 11; no resistance exercise group: n = 9 Age: mean (SD) = 12.5 (SD 2.5) years (range 7.9 to 16.0 years) Sex (male/female): 10/10 Ethnicity: not stated GMFCS level: level I (n = 3); level II (n = 13) and level III (n = 4) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: ambulant children and adolescents with spastic diplegic CP, surgery indicated Exclusion criteria: botulinum toxin or orthopaedic surgery in previous year |
|
| Interventions |
Aim of the intervention: to increase muscle strength Type of exercise programme: resistance training Exercise mode: exercises for the hip flexors, hip extensors, hip abductors, knee flexors and knee extensors bilaterally Comparator: identical programme performed with no weights (against gravity only). Setting: not reported Intervention provider: clinician. Duration of programme: 6 weeks. Exercise dose: 3 times per week. 3 sets of 10 repetitions for each muscle group using free weights. The weight was determined using a 10RM (repetition maximum) for each muscle group. Tailoring of intervention to individual: re‐assessment and incremental weight increase were dictated by the participant’s progress. Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6), 20 weeks postintervention (week 26) Primary outcomes: primary outcome not stated.
|
|
| Notes |
Source of funding: Oxfordshire Health Services Research Committee and the Wishbone Trust Potential conflicts of interest: authors report no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Six months post‐operatively, each child was allocated to one of two strengthening groups—AE or RS." Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "Six months post‐operatively, each child was allocated to one of two strengthening groups—AE or RS." Comment: insufficient information regarding allocation concealment was provided to make a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: did not provide information to indicate if the assessor was blinded to group allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "One child failed to complete the study for reasons unrelated to treatment. Therefore, 20 patients were included in the further analysis. 9 completed programme AE and 11 programme RS. One child failed to complete the 12‐month assessment". Comment: low rate of missing data (<10%). |
| Selective reporting (reporting bias) | Unclear risk | A protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Unnithan 2007.
| Methods |
Design: quasi‐randomised controlled trial Country of origin: Greece Intervention(s): resistance and aerobic interval training Unit of allocation: individual |
|
| Participants |
Number of participants: 13 allocated to groups; exercise group: n = 7; control group: n = 6. Data reported for all allocated participants. Age: exercise group: mean (SD) = 15.9 (1.5) years; control group: mean (SD) = 15.7 (1.2) years Sex (male/female): 4/9; exercise group: 2/5; control group: 2/4 Ethnicity: not stated GMFCS level: no walking aids (i.e. GMFCS level I or II) (n = 4) and anterior walker (i.e. GMFCS level III) (n = 9); exercise group: no walking aids (n = 2) and anterior walker (n = 5); control group: no walking aids (n = 2) and anterior walker (n = 4) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: diagnosis of spastic diplegia, able to walk with or without aids, aged 14‐18 years, not been subjected to any orthopaedic surgical operation and had not received botulinum toxin injections in the preceding year, attended a similar physical therapy programme that did not include any form of systematic exercise Exclusion criteria: not stated |
|
| Interventions |
Aim of the intervention: to increase strength and aerobic capacity Type of exercise programme: mixed training (resistance training and aerobic interval training) Exercise mode:
Comparator: all participants maintained normal physical therapy, which consisted of individualised physical therapy on 2 days per week for 45 min, carried out by a physical therapist, based on Bobath treatment Setting: not stated. Group, supervised sessions Intervention provider: not stated Duration of programme: 12 weeks Exercise dose: 3 sessions per week. Each session lasting approximately 70 min (10 min warm‐up, 20 min strength training protocol, 10 min game for general strengthening, 20‐22 min aerobic interval training, 7 min breathing and passive stretching)
Tailoring of intervention to individual: intensity was progressively increased every 3 weeks on an individualised basis by increasing the number of sets by 1 and repetitions by 5 while the weight remained stable. Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 12) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "[t]he subjects were recruited on a staggered basis during a 6‐month period and, therefore, were randomly allocated into either the training group (n=7) or control group (n=6) on the basis of timing of their recruitment into the study". Comment: description of method of sequence generation suggests it was based on a non‐random component |
| Allocation concealment (selection bias) | High risk |
Quote: "[t]he subjects were recruited on a staggered basis during a 6‐month period and, therefore, were randomly allocated into either the training group (n=7) or control group (n=6) on the basis of timing of their recruitment into the study". Comment: description of method of allocation suggests that it was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[t]his researcher was aware of which group each CP participant belonged to" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[a]ll subjects (training and control) participated in all the week 0 and week 12 tests, and none of the subject data were excluded from the subsequent data analyses". |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Verschuren 2007.
| Methods |
Design: randomised controlled trial Country of origin: Netherlands Intervention(s): mixed training; resistance training, aerobic and anaerobic training Unit of allocation: individual |
|
| Participants |
Number of participants: 68 randomised; exercise group: n = 34; control group: n = 34 Group characteristics reported for all randomised participants Age: exercise group: mean (SD) = 11.6 (2.5) year; control group: mean (SD) = 12.7 (2.7) year Sex (male/female): 44/24; exercise group: 20/14; control group: 24/10 Ethnicity: not stated GMFCS level: level I (n = 47) and level II (n = 21); exercise group: level I (n = 24) and level II (n = 10); control group: level I (n = 23) and II (n = 11) Type of motor abnormality: spastic CP Anatomical distribution of CP: unilateral (n = 45) and bilateral (n = 23); exercise group: unilateral (n = 23) and bilateral (n = 11); control group: unilateral (n = 22) and bilateral (n = 12) Inclusion criteria: aged 7‐20 years, diagnosed with spastic CP, GMFCS level I or II, able to follow simple verbal commands, receiving rehabilitation services at the time of the study. Exclusion criteria: orthopaedic surgery or neurosurgery and/or botulinum toxin injection in previous 6 months, cardiac or respiratory conditions that could be negatively affected by exercise |
|
| Interventions |
Aim of the intervention: to increase aerobic fitness (for first 4 months) and anaerobic fitness (for second 4 months) Type of exercise programme: mixed; aerobic, anaerobic, and muscle strengthening Exercise mode: 8 standardised aerobic exercises lasting 3‐6 min. 8 standardised anaerobic exercises lasting 20‐30 seconds. The task‐specific exercises,such as running and changing direction of the body abruptly, step‐ups, and negotiating stairs, were repeated throughout the programme and aimed to improve daily functioning. Participants also continued with usual care. Comparator: all participants received usual care, which ranged from no treatment to various therapeutic approaches. There was no difference in usual care between groups as tracked from medical records Setting: school; supervised groups of 4‐6 participants Intervention provider: led by 2 local paediatric physiotherapists who received standardised fitness programme training prior to the start of the programme Duration of programme: 8 months; aimed to increase aerobic fitness in the first 4 months and increase anaerobic fitness in second 4 months, Exercise dose: 2 days per week. Each session lasted 45 min (5 min warm up, 25 to 35 min functional aerobic, anaerobic, and muscle strengthening exercises performed in a circuit, 5 min cool down). Tailoring of intervention to individual: not stated Fidelity to prescribed intervention: median attendance was 56 out of a possible 60 sessions (93%). All participants attended at least 85% of the training sessions. Unclear how fidelity was monitored. Monitoring of adverse events: during a training session 1 participant fell and fractured her radius. She missed 4 training sessions because she was wearing a cast. |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (month 8), 4 months postintervention (month 12) Primary outcomes: primary outcomes were aerobic and anaerobic capacity
Secondary outcomes: agility, muscle strength, body mass index, self‐perception, gross motor function, participation, and health‐related quality of life
|
|
| Notes |
Source of funding: Dr WM Phelps foundation Potential conflicts of interest: authors declare no conflicts of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[p]articipants were randomly assigned to 2 groups using a 4‐block randomization protocol. Each block represented all participants from 1 school. The groups within each block consisted of children at level I or II on the GMFCS. From each block and group every participant was randomly allocated to the training group or the control group". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Low risk |
Quote: "[a]n independent off‐site researcher not involved in the assessments used a concealed method for allocation." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[t]o reduce bias 8 assessors who were not the treating therapist and who were blinded for the treatment modality undertook the testing without review of previous scores" Comment: participation and quality of life were assessed using self‐report outcome measures. As participants and their parents were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: all data analyses were carried out according to a pre‐established analysis plan and were performed according to the intention‐to‐treat principle". 4% attrition during baseline measures, evenly distributed, and accounted for. |
| Selective reporting (reporting bias) | Low risk | Comment: a summary of the study protocol is available and pre‐specified outcomes reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Lee 2008.
| Methods |
Design: randomised controlled trial Country of origin: Korea Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 17 randomised; exercise group: n = 9; control group: n = 8 Age: exercise group: mean (SD) = 6.3 (2.1) years; control group: mean (SD) = 6.3 (2.9) years Sex (male/female): 10/7; exercise group: 4/5; control group: 6/2 Ethnicity: not stated GMFCS level: II or III, distribution per group not stated Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia (n = 9) and hemiplegia (n = 8); exercise group: diplegia (n = 4) and hemiplegia (n = 5); control group: diplegia (n = 5) and hemiplegia (n = 3) Inclusion criteria: aged 4‐12 years, spastic diplegia or hemiplegia CP, GMFCS level II or III Exclusion criteria: unable to follow commands, fixed contracture at hip or knee joint of more than 25°, medical or orthopedic diseases that prevented them from exercising, received orthopedic surgery of the lower limb or injection of antispastic drug (e.g. botulinum toxin injection) |
|
| Interventions |
Aim of the intervention: to increase muscle strength in the lower limbs Type of exercise programme: resistance training Exercise mode: squat‐to‐stand, lateral step‐up, stair walk up and down, isotonic exercise of lower limb muscles, isokinetic exercises using a bike. Comparator: conventional physiotherapy including neurodevelopmental therapy, range of movement exercises and gait training. Dose not reported Setting: unclear Intervention provider: physiotherapist Duration of programme: 5 weeks Exercise dose: a 60 min session was delivered 3 times per week. For isotonic exercise 1 of 3 weights (0.25 kg, 0.45 kg or 0.9 kg) was used to provide resistance to voluntary muscle contraction during the exercise. Participants completed 2 sets of 10 repetitions in each muscle group. Tailoring of intervention to individual: selected weight depended on the ability of the participant to complete 2 sets of 10 repetitions. Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 5), 6 weeks postintervention (week 11) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not reported; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[p]articipants were allocated randomly to either the experimental group or control group using concealed methods" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[p]articipants were allocated randomly to either the experimental group or control group using concealed methods" Comment: insufficient information regarding the method of allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: did not provide information to indicate if the assessor was blinded to group allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: does not state if anyone withdrew or number of participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: unclear whether all demographic or outcome data for all participants are reported |
Chrysagis 2009.
| Methods |
Design: randomised controlled trial Country of origin: Greece Intervention(s): aquatic programme Unit of allocation: individual |
|
| Participants |
Number of participants: 12 randomised; exercise group: n = 6; control group: n = 6 Group characteristics were reported for all randomised participants Age: 13‐20 years; exercise group: mean (SD) = 16 (2.89) years; control group: mean (SD) = 16.66 (2.65) years. Sex (male/female): 7/5; exercise group: 4/2; control group: 3/3 Ethnicity: not stated GMFCS level: according to inclusion criteria levels I, II and III (i.e. able to walk with or without aids). Level of lower‐limb gross motor function of included participants is not stated Type of motor abnormality: spastic CP Anatomical distribution of motor abnormality: according to inclusion criteria tetraplegia and diplegia; anatomical distribution of included participants is not stated Inclusion criteria: diagnosis of spastic tetraplegia or diplegia, able to walk with or without aids, able to follow simple commands Exclusion criteria: undergone surgery in last 12 months or receive medication for spasticity |
|
| Interventions |
Aim of the intervention: to improve gross motor function and range of movement and to reduce spasticity Type of exercise programme: aerobic Exercise mode: swimming. Participants worked on the basic backstroke and crawl swimming styles Comparator: participants in the control group continued their normal activities and physiotherapy sessions provided from the school staff. Dose not reported. Setting: 25 m swimming pool. Supervised sessions. Unclear if individual or group session. Intervention provider: 2 physical educators trained in swimming skills for children with CP from the school staff were responsible for the swimming programme Duration of programme: 10 weeks Exercise dose: participants in the exercise group received 45 min of exercise (10 min warm up of stretching and 35 min of training), 2 days per week Tailoring of intervention to individual: training was individualised according to each participant's ability; floating devices were used if necessary Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0) and 4 weeks postintervention (week 14) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: none stated Potential conflicts of interest: none stated; no conflicts of interest perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[p]articipants were randomly allocated (sealed envelopes) to experimental and the control group" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[p]articipants were randomly allocated (sealed envelopes) to experimental and the control group". Comment: insufficient information regarding allocation concealment was provided to make a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "[t]wo members of the Laboratory of Adapted Physical Activity/Developmental and Physical Disabilities carried out the above measurements in the school gym at the beginning and at the end of the intervention program" Comment: insufficient information regarding assessment procedures was provided to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[t]here was no drop out during the study" Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Maeland 2009.
| Methods |
Design: randomised controlled trial Country of origin: Norway Intervention(s): resistance training using a seated leg press Unit of allocation: individual |
|
| Participants |
Number of participants: 12 randomised; exercise group: n = 6; control group: n = 6 Baseline characteristics reported on all randomised participants Age: exercise group: mean 41 years (range 32 to 69 years); control group: mean 45 years (range 27 to 65 years) Sex (male/female): 4/8; exercise group: 2/4; control group: 2/4 Ethnicity: not stated GMFCS level: level II (n = 7) and level III (n = 5); exercise group: level II (n = 4) and level III (n =2); control group: level II (n = 3) and level III (n = 3) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: age 18 years and older, spastic diplegia, GMFCS level II or III, experiencing difficulties walking but able to walk for 6 min with or without minimal support from another person, motivated to participate in progressive resistance training, able to understand and perform progressive resistance exercise training under supervision Exclusion criteria: participation in strength training for the lower limbs in the past 12 months, severe cognitive disorders |
|
| Interventions |
Aim of the intervention: to improve muscle strength Type of exercise programme: resistance training Exercise mode: seated leg press Comparator: participants in the control group continued their pre‐study individual ongoing symptom relief treatment or training regime. This consisted of mainly passive physiotherapy treatment such as stretching, mobilisation of joints and massage Setting: physiotherapy clinic; supervised individual session Intervention provider: physiotherapist Duration of programme: 8 weeks Exercise dose: 4 sets of 12‐15 repetitions at 60%‐75% of 1 repetition maximum (1 RM), 3 days per week for first 2 weeks. 4 sets of 4‐6 repetitions to fatigue (i.e. 4 RM to 6 RM, 85% of 1 RM), 3 days per week for 6 weeks Tailoring of intervention to individual: when the participants managed to complete 15 repetitions or 6 repetitions (depending on the week) in all 4 sets resistance was increased by 5 kg to 10 kg Fidelity to prescribed intervention: 90% of all sessions were completed Monitoring of adverse events: no adverse events or effects were reported or registered by the participants or physiotherapists |
|
| Outcomes |
Assessment time points: baseline (week 0), 2 weeks postintervention (week 10) Primary outcome:
Seconday outcomes:
|
|
| Notes |
Source of funding: Helse_Ost RHF (Eastern Norway Regional Health Authority) Potential conflicts of interest: authors report no conflict of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he randomisation was done by computerised generating of 12 Bernoulli trials (0s and 1s) using SPSS" |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[s]ealed numbered envelopes with information about the treatment were prepared by the statistician" Comment: did not state if envelopes were opaque or if the statistician was independent to the research team |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[a]ll functional outcome measures were carried out by two experienced senior physiotherapists who were blinded as to which group the participants belonged" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Fowler 2010.
| Methods |
Design: randomised controlled trial Country of origin: USA Intervention(s): stationary cycling programme Unit of allocation: individual |
|
| Participants |
Number of participants: 64 randomised; exercise group: n = 33; control group: n = 31; Baseline measures n = 62; exercise group: n = 31; control group: n = 31 Group characteristics reported for n = 62 Age: exercise group: mean (95% CI) = 11.1 (9.9 to 12.3) years; control group: mean (95% CI) = 11.6 (10.6 to 12.6) years Sex (male/female): 29/33; exercise group: 18/13; control group: 11/20. Ethnicity: African American (n = 8), white (n = 33), Asian (n = 6), other (not specified; n = 15); exercise group: African American (n = 5), white (n = 18), Asian (n = 1), other (n = 7); control group: African American (n = 3), white (n = 15), Asian (n = 5), other (n = 8) GMFCS level: levels I (n = 19), level II (n = 14), level III (29); exercise group: level I (n = 11), level II (n = 8), level III (n = 12). control group: level I (n = 8), level II (n = 6), level III (n = 17) Type of motor abnormality: spastic CP Anatomical distribution of motor abnormality: diplegia Inclusion criteria: diagnosis of spastic diplegia, aged 7‐18 years, ability to comply with simple verbal directions, GMFCS levels I‐III, selective motor control rating of good or fair Exclusion criteria: neurological surgery, orthopaedic surgery or implantation of a baclofen pump within the 12 months preceding enrolment; botulinum toxin injections within the preceding 3 months; serial casting or new orthotic devices within the preceding 3 months; initiation of oral medications that affect the neuromuscular system (e.g. baclofen) within the preceding 3 months; initiation of physical therapy, exercises, sports activity, or change in assistive devices for walking within the preceding 3 months; inability or unwillingness to maintain age‐appropriate behaviour; serious medical conditions such as diabetes, cardiovascular disease, or uncontrolled seizures; current participation in a fitness programme that included a minimum of once‐weekly cardiorespiratory endurance exercise; significant hip, knee, or ankle joint contractures preventing passive movement of the lower limbs through pedaling cycle; poor bilateral voluntary selective motor control |
|
| Interventions |
Aim of the intervention: to improve muscle strength and walking and running endurance Type of exercise programme: mixed (resistance and aerobic) Exercise mode: stationary cycling. In order to perform lower limb strengthening the bike seat was unlocked and allowed to slide backward. Up to 10 tension cords each providing 4.5 kg (10 lb) of force acted to pull the seat forward. Participant extended lower limbs to prevent the seat being pulled forward Comparator: no cycling. All participants completed physical activity diaries to assess levels of physical activity throughout duration of the study Setting: physiotherapy clinic Intervention provider: physical therapist Duration of programme: 12 weeks Exercise dose: 30, 60‐min sessions, 3 times a week. During cardiorespiratory phase participants exercised at 70%‐80% heart rate max (HRmax) (calculated using the Karvonen formula); aimed for 15‐30 min at this intensity. Mean (SD) typical exercise heart rate across all sessions was 147.2 (14.4) bpm (range 117‐176 bpm) representing a mean (SD) percentage of HRmax of 52.2 (12.2) (range = 8%‐77%). For lower limb strengthening the starting resistance was the attachment of 1 tensioning cord. Resistance was progressed to next cord when 10 revolutions were performed while keeping seat in desired zone. If a participant could not cycle with the seat unlocked or if the maximum resistance was reached a 'constant power' resistance mode was used. Number of revolutions prescribed not stated. The mean (SD) maximum load was 12.2 (12.1) kg (26.9 (26.6) lb) over the first 3 days of the intervention and 29.7 (15.5) kg (65.5 (34.2) lb) by the end of the intervention, representing a mean (SD) gain of 17.5 (11.7) kg (38.6 (25.7) lb) (range = 0‐40.1 kg (0‐90 lb)). Tailoring of intervention to individual: if participant could not cycle independently manual assistance was provided until independence was achieved. Resistance and pedaling rate were adjusted based on HR and Children's Effort Rating Table (scale 1‐10) Fidelity to prescribed intervention: attendance at cycling sessions was 89.6%. Monitoring of adverse events: did not state how adverse events were assessed. 28 mild events (for 18 participants) potentially related to the study: 6 observed falls, 17 complaints of soreness, muscle cramping or mild pain, 4 reports of fatigue, and 1 skin rash related to HR monitor. 30 events unrelated to study procedures: illness, tooth loss, headache, stomach ache, tonsillectomy, and skin irritation from orthotic use. |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 12) Primary outcomes
Secondary outcomes
Assessments were conducted by an evaluator who was blinded to participant group assignment and had to pass a rigorous standardization procedure for each outcome measurement protocol by demonstrating at least 90% competency. |
|
| Notes |
Source of funding: Foundation for Physical Therapy Potential conflicts of interest: not stated. Authors acknowledged other corporate funders, donors and discounts; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "[t]he participants were randomly assigned to a control (no intervention) or an intervention (cycling) group. Randomization was blocked by age (7‐11y, 12‐18y) and lower extremity selective voluntary motor control ability (good, fair) to minimize the effects of physical impairment and maturation. Quote: "[g]roup assignment was determined using a computerized random number generator." |
| Allocation concealment (selection bias) | Unclear risk |
Quote (from the protocol): "[a]n enrolment form containing the subject’s age and selective motor control ability will be submitted to the PTClinResNet Data Management Centre for subject randomisation. Families will be notified of their child’s assignment to the control or intervention group following baseline evaluation". Comment: although it appears randomisation was conducted by a third party, independent of the study, insufficient information is provided to make a clear judgement regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[e]valuators were blinded to group assignment" Quote: "[b]oth questionnaires were administered following a standardized protocol outlined in the study's manual of procedures". Quote: "[t]he youngest children in the study (7y) used the PedsQL young child version; older children (8‐12y) completed the child version and adolescents (13‐18y) used the teen version. The PODCI parent proxy versions for parents or guardians of children (2‐10y) or adolescents (11‐18y) were used." Quote: "[e]ach question and all possible answers were read to the participant and the evaluator recorded the selected answer". Comment: both self‐reported and objective outcome measures were used. As participants and their parents were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition and withdrawal accounted for. Low rate of missing data (< 10%) and evenly distributed across groups |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported |
| Other bias | Unclear risk | Comment: unaccounted for missing data for 1 participant (PODCI) |
Reid 2010.
| Methods |
Design: cross‐over randomised controlled trial Country of origin: Australia Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 14 randomised; exercise group: n = 7; control group: n = 7 Age: mean (SD) 11 (2) years (range 9 to 15 years) Sex (male/female): 6/8 Ethnicity: not stated GMFCS level: not stated MACS level: level I (n = 4), level II (n = 8) and level III (n = 2) Type of motor abnormality: spastic CP Anatomical distribution of CP: hemiplegia (n = 13) and triplegia (n = 1) Inclusion criteria: elbow flexor spasticity, the ability to follow 2‐step instructions, no previous upper‐limb surgery, and no upper‐limb strength training or pharmacological treatment for spasticity (botulinum toxin A) in the past 12 months Exclusion criteria: as stated in inclusion criteria |
|
| Interventions |
Aim of the intervention: predicted that eccentric strength training would improve peak torque and work, torque‐angle relationship, and electromyographic activation Type of exercise programme: resistance training Exercise mode: upper limb eccentric exercises using training rig that provided loaded assistance to draw the elbow into extension in a gravity‐eliminated position. The arm was returned to 110° elbow flexion at the end of each repetition by a partner so the participant did not perform a concentric muscle action Comparator: normal activity Setting: home‐based programme Intervention provider: training partner assisted (not stated who was training partner) Duration of programme: 6 weeks Exercise dose: 3 sessions per week, 3 sets of 10 repetitions, starting at 50% maximum eccentric torque and progressing to 70% maximum eccentric torque at by the last week in increments of 5% Tailoring of intervention to individual: each participant was provided with an individually adapted eccentric training rig that permitted eccentric extension of the elbow flexors only Fidelity to prescribed intervention: on average participants completed 16 out of a possible 18 eccentric training sessions Monitoring of adverse events: not reported: states no child reported muscle soreness as a consequence of eccentric training |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 6) Primary outcome: not stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[p]articipants with CP were randomised into two groups and completed the same eccentric training programme: group I (n=7) trained in the first 6 weeks of the study, while group II (n=7) acted as a control group, maintaining their normal activity for 6 weeks, and then completed the 6‐week eccentric training programme". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[p]articipants with CP were randomised into two groups and completed the same eccentric training programme: group I (n=7) trained in the first 6 weeks of the study, while group II (n=7) acted as a control group, maintaining their normal activity for 6 weeks, and then completed the 6‐week eccentric training programme". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: did not provide information to indicate if the assessor was blinded to group allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Scholtes 2010.
| Methods |
Design: randomised controlled trial Country of origin: Netherlands Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 51 participants randomised. Exercise group: n = 26; control group: n = 25 (49 participants included in analysis, some demographic data presented for 49 participants only) Age: mean (SD) = 10 years 5 months (1 year 10 months) (range 6 years 0 months to 13 years 10 months); exercise group: mean (SD) = 10 years 4 months (1 year 10 months); control group: mean (SD) = 10 years 3 months (2 years 3 months) Sex (male/female): 29/22; exercise group: 16/8; control group: 13/12 Ethnicity: not stated GMFCS level: level I (n = 25); level II (n = 17) and level III (n = 7); exercise group: level I (n = 13), level II (n = 8) and level III (n = 3); control group: level I (n = 12), level II (n = 9) and level III (n = 4) Type of motor abnormality: spastic CP Anatomical distribution of CP: unilateral (n = 17) and bilateral (n = 32); exercise group: unilateral (n = 7) and bilateral (n = 17); control group: unilateral (n = 10) and bilateral (n = 15) Inclusion criteria: aged 6‐13 years, able to accept and follow verbal instructions, able to walk independently indoors with or without walking aids (GMFCS level I, II or III), able to participate in a group training programme Exclusion criteria: unstable seizures, any treatment of spasticity or surgical procedures in last 3 months (for botox) or 6 months (for surgery), any change in medication expected during the study period and suffering from any other diseases that interfered with physical activity |
|
| Interventions |
Aim of the intervention: to increase muscle strength Type of exercise programme: resistance training Exercise mode: 2 loaded exercises: leg press and sit‐to‐stand. Unloaded or low loaded exercises: lateral step‐up, forward step‐up, half knee‐rise. Each participant completed 4 different exercises on a circuit of 5 stations. The intervention replaced usual care. Comparator: conventional physiotherapy programme, 1‐3 sessions per week, dose and content not recorded Setting: special school Intervention provider: physiotherapist Duration of programme: 12 weeks (33 sessions in total as 3 sessions cancelled) Exercise dose: each session was 45‐60 min. 3 sessions per week. Each session consisted of 4 different exercises performed as a station in a circuit. The exercises were a leg press exercise on a child‐adapted leg‐press, sit‐to‐stand, lateral step‐up and half knee‐rise. 3 sets of 8 repetitions of each exercise was performed. The stations were a combination of a high load exercise, a low load exercise and an unloaded exercise. The high load exercise (bilateral leg‐press) was performed at 100% 8 RM, the loaded exercise (bilateral sit‐to‐stand) was performed at 75% 8 RM, the loaded game (unilateral half‐knee rise, lateral step‐up or forward step‐up) was performed at 25% 8 RM, and the unloaded game (unilateral half‐knee rise, lateral step‐up or forward step‐up) was performed with no resistance. The first 6 weeks were intended to slowly build up training to these loads. The final 6 weeks were performed at these loads Tailoring of intervention to individual: training loads were adjusted to new individual levels of strength, if necessary, as determined by the 8 RM test Fidelity to prescribed intervention: mean compliance was 92.3% (71% to 100%). A mean of 32 out of 36 sessions (range 30 to 33) were attended. Reasons for absence were illness (41.4%), medical appointment (8.6%), vacation (6.9%), other/unknown (43.1%). Monitoring of adverse events: each week 1‐6 participants reported mild‐to‐moderate muscle soreness, recorded on a Likert scale (no, mild, moderate, severe, extremely severe) |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 12), 6 weeks postintervention (week 18) Primary outcomes: gross motor function, functional muscle strength, and walking ability
Secondary outcomes: muscle strength, anaerobic power, mobility, and participation
|
|
| Notes |
Source of funding: the Johanna Kinder‐Fonds (2005/0123‐357), the Adriaanstichting, and the Phelps Stichting (2006016) Potential conflicts of interest: not stated. In the published protocol the authors stated no competing interests; no conflicts of interest perceived. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[r]andomization was performed for each school separately by one independent physiatrist. The children were pre‐stratified according to 3 stratification variables: sex, GMFCS level (I, II‐III), age (youngest: 6‐9y; oldest 10‐13y), and subsequently randomised to one of two groups using sealed envelopes". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[r]andomization was performed for each school separately by one independent physiatrist. The children were pre‐stratified according to 3 stratification variables: sex, GMFCS level (I, II‐III), age (youngest: 6‐9y; oldest 10‐13y), and subsequently randomised to one of two groups using sealed envelopes" Comment: did not state if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[t]wo independent research assistants performed all assessments and data entry" Comment: both self‐reported and objective outcome measures were used. As participants and their parents were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[o]f the 51 included children, one dropped out before T0 (GMFCS III, girl, 13 years 1 month, intervention group) due to a hip‐injury which made testing and training impossible, and one was lost to follow‐up at T1 (GMFCS II, girl, 12 years 1 month, intervention group) due to an unexpected long term stay abroad. Consequently, data on 49 children were included in the analyses (intervention group: n = 24, control group: n = 25)". Quote: "[n]ot all children could be motivated to complete all tests on all occasions" Comment: missing data rate of approximately 4%. There is some (minimal) variation in the n reported for each outcome at each time point. Reasons for dropout accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Gharib 2011.
| Methods |
Design: randomised controlled trial Country of origin: Egypt Intervention(s): Gait Trainer assisted walking exercises Unit of allocation: individual |
|
| Participants |
Number of participants: 30 randomised; exercise group: n = 15; control group: n = 15 Group characteristics reported for all randomised participants Age: mean (SD) = 11.55 (1.11) years; exercise group: mean (SD) = 11.87 (1.06) years; control group: mean (SD) = 11.23 (1.11) years Sex (male/female): 16/14; exercise group: 10/5; control group: 6/9 Ethnicity: not stated GMFCS level: level II (n = 30) Type of motor abnormality: spastic CP Anatomical distribution of CP: hemiplegia Inclusion criteria: unclear if the following is stated as inclusion criteria or a description of participants included: age 10‐13 years, hemiparetic CP with a mild degree of spasticity in affected lower limbs (Modified Ashworth Scale score < 2), classified as Gross Motor Function Classification Scale level II Exclusion criteria: fracture, sprain or strain injury of the lower extremities in the past 6 months, neurological or orthopaedic surgery in the last 12 months, botulinum toxin application for at least 6 months before the study, exercise‐induced asthma, a congenital heart defect with cardiac compromise, aggressive or self‐harming behaviours, cognitive impairment (not being able to follow simple verbal commands and instructions during tests and training), uncontrolled seizure disorder |
|
| Interventions |
Aim of the intervention: to improve walking parameters Type of exercise programme: aerobic Exercise mode: Biodex Gait Trainer 2 (no body weight support) Comparator: all participants in both groups received a physical therapy exercise session of 30 min, 3 times per week. This included stretching, strengthening, practicing of activities of daily living, balance and gait exercises Setting: physiotherapy clinic Intervention provider: physiotherapist Duration of programme: 3 months Exercise dose: participants encouraged to walk continuously for 15 min, 3 times per week Tailoring of intervention to individual: a rest break of 1‐3 min was given to a participant if they requested it. The gait trainer belt speed was gradually increased for each participant. Fidelity to prescribed intervention: not reported; if a participant missed > 3 sessions because of medical reasons or an inability to participate the participant was considered as dropped from the study. No participant was excluded Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (3 months) Primary outcome: no primary outcome measure stated Outcomes: The following procedure was used to measure all gait parameters. The speed of the belt was slowly increased to a comfortable pace for the participant at which point the participant walked continuously for 3 min while data was collected. This was repeated 3 times and the average was taken for each gait parameter.
|
|
| Notes |
Source of funding: stated no funding received Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[c]hildren were randomly assigned to one of two groups" Quote: "[r]andom assignment of children was conducted into two stages. Stage one involved instructing two physical therapists who were working in the paediatric physical therapy outpatient clinic to report all children who fulfilled the inclusion criteria of the study (registration diagnosis, age, level of Gross Motor Function Classification System scale), and had no exclusion criteria. The second stage involved randomly assigning the children to either the experimental group or the control group by using sealed envelopes" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]he second stage involved randomly assigning the children to either the experimental group or the control group by using sealed envelopes. The randomisation process was carried out by a registration clerk who was not involved in any part of the study" Comment: insufficient information provided to determine if a person involved in the study could predict allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[a]ll children (in both groups) were evaluated prior to the commencement of baseline training and at the end of the three‐month training period (post‐treatment) by the same examiner who was blinded to which group each child was assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Johnston 2011.
| Methods |
Design: randomised controlled trial Country of origin: USA Intervention(s): a comparison of supported treadmill training and strength training Unit of allocation: individual |
|
| Participants |
Number of participants: 34 randomised. Outcome and demographic data only presented for participants who completed the study (n = 26); treadmil exercise group: n =14; strengthening exercise group: n = 12 Age: mean (SD) = 9 years 6 months (2 years 2 months); treadmill exercise group: mean (SD) = 9 years 7 months (2 years 2 months); strengthening exercise group: mean (SD) = 9 years 6 months (2 years 4 months) Sex (male/female): 14/12; treadmill exercise group: 7/7; strengthening exercise group: 7/5 Ethnicity: not stated GMFCS level: level II (n = 2), level III (n = 15), level IV (n = 9); treadmill exercise group: level II (n = 1), level III (n = 9), level IV (n = 4); strengthening exercise group: level II (n = 1), level III (n = 6), level IV (n = 5) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia (n = 12), triplegia (n = 2) and quadriplegia (n = 12); treadmill exercise group: diplegia (n = 8) and quadriplegia (n = 6); strengthening exercise group: diplegia (n = 4), triplegia (n = 2), and quadriplegia (n = 6) Inclusion criteria: spastic CP, marginal ambulatory function (defined as decreased gait velocity < 80% of age‐expected value regardless of GMFCS level, or GMFCS level III or IV), ability to take 8 steps independently with or without assistive devices, able to complete 3D gait analysis, body weight < 68 kg (150 lb), age 6‐13 years, ability to follow multiple‐step commands Exclusion criteria: medical condition that would be negatively affected by exercise; lower extremity orthopaedic surgery in the past year, botulinum toxin A in the past 6 months, dorsal rhizomotomy in the past 2 years; flexion contractures > 30° at the hip or > 20° at the knee or plantarflexion contractures > 15°, intrathecal baclofen, dystonia, athetoid or mixed types of CP |
|
| Interventions |
Aim of the intervention: to increase strength, motor control, gait, gross motor skills, and physical function, and improve quality of life, self‐concept, satisfaction and participation (not sure if needed) Type of exercise programme: aerobic exercise versus resistance training Exercise mode: treadmill exercise group: completed a programme of partial body‐weight‐supported treadmill training Comparator: strengthening exercise group completed a programme of step‐ups, squats, upper and lower limb progressive resistance exercise, and core strengthening Setting: home‐based programme. First 2 weeks were delivered at study site or in home. Final 10 weeks were delivered at home Intervention provider: physiotherapist delivered programme for 2 weeks and parents delivered it for 10 weeks Duration of programme: 12 weeks Exercise dose: all participants were prescribed 2 sessions of 30 min, 5 days/week for first 2 weeks and 1 session of 30 min, 5 days/week for final 10 weeks Tailoring of intervention to individual: Treadmill exercise group: each participant wore a harness and the parent or physiotherapist guided their leg if necessary, and ankle‐foot orthoses were used where necessary. Initial training speed based on participant's baseline gait speed and adjusted as needed based on participant's response. Aimed to decrease body‐weight support to < 30% and increase speed to normal values during the 2‐week induction period, based on the participant's ability to maintain normal gait pattern Strengthening exercise group: assistance was provided as needed, and assistive devices were allowed for weight bearing. The exercises were advanced on an individual basis by increasing the number of repetitions and then adding resistance by cuff weights. Fidelity to prescribed intervention: parents of participants kept weekly logs. All participants who completed the study attained an adherence rate of at least 80% Monitoring of adverse events: did not state how adverse events were assessed. 2 participants in the treadmill group complained of leg/knee discomfort which resolved without intervention. 1 participant developed a blister from the AFO during the induction period, unclear group allocation |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 12), 4 weeks postintervention (week 16) Primary outcome:
Outcomes:
|
|
| Notes |
Source of funding: Shriners Hospitals for Children (grant no. 9147) Potential conflicts of interest: authors report no financial conflict of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[c]hildren were randomly assigned to the SSTTEP or exercise group using a block randomization schedule at each site using blocks of eight group assignments (four of each intervention per block)." Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[t]wo sites were able to blind the evaluators to group assignment (representing 16 children) but the third site was unable to because of personnel issues. All evaluators were blinded to results and trained on each measure for which written study specific protocols were available at each site" Comment: 26 participants completed the study. Therefore 10 participants were assessed by evaluators not blinded to group allocation. Both self‐report and objective outcome measures were used. As participants and their parents were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "[d]id not receive allocated intervention (n=2)" Quote: "Lost to follow‐up (n=2). Discontinued participation part way through intervention period". Quote: "[s]ix participants were lost to follow‐up at the 12‐week point because of personal and family reasons not related to the intervention. Two participants (in the SSTTEP group) did not participate in data collection after the washout period." Quote: "[c]hildren were required to complete at least 40 out of 50 full sessions (80% adherence) to participate in data collection. Comment: 34 participants were randomised and only 26 participants were included in the analysis (24% missing data). Missing data were evenly distributed across groups (22% and 25%, respectively). All reasons for withdrawals not specified. As‐treated analysis undertaken with substantial departure of the intervention received from that assigned at randomisation |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: baseline and demographic data is only reported for those who completed the trial; not all completers' data is reported for all outcomes, no reason given |
Olama 2011.
| Methods |
Design: randomised controlled trial Country of origin: Egypt Intervention(s): a comparison of a treadmill training programme to a resistance training programme Unit of allocation: individual |
|
| Participants |
Number of participants: unclear. States 30 participants but also states that there were 12 males and 8 females, and 12 participants with left hemiplegia and 8 participants with right hemiplegia; treadmill exercise group: n = 15; Resistance exercise group: n =15 Age: mean (SD) = 13.73 (0.85) years Sex (male/female): 12/8 Ethnicity: not stated GMFCS level: not stated Type of motor abnormality: spastic CP Anatomical distribution of CP: hemiplegia Inclusion criteria: does not state inclusion criteria but describes sample as: age 12‐15 years, able to understand any command, with an IQ level within normal range, free from any associated disorders other than spasticity, spasticity in the range of 1+ and 2 on the Modified Ashworth Scale, free from structural changes of the joints of the lower limbs, able to walk independently Exclusion criteria: not stated |
|
| Interventions |
Aim of the intervention: to increase muscle strength Type of exercise programme: treadmill exercise group: aerobic; resistance exercise group: resistance training Exercise mode: treadmill exercise group: treadmill; resistance exercise group: resistance training of quadriceps and hamstrings Comparator: both groups received an exercise programme consisting of neurodevelopmental therapy, proprioceptive training, facilitation of righting reactions, stretching, and gait training, daily for 6 months Setting: not stated Intervention provider: not stated Duration of programme: unclear. States participants received 6 months of treatment but also states that the exercise procedure for the resistance training group was repeated 3 times per week for 3 months. Exercise dose: treadmill exercise group: daily (no indication of duration of treadmill training); resistance exercise group: 3 times per week Tailoring of intervention to individual: Treadmill exercise group: participants can hold on with 2 hands until they feel confident to walk without support. Resistance exercise group: the resistance added to the exercise was gradually increased starting at 10 repetitions at 50% of 10 RM, 10 repetitions at 75% of 10 RM and 10 repetitions at 100% of 10 RM Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (6 months) Primary outcome: no primary outcome measure stated. Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[t]he study sample was divided into two groups of equal size" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]he study sample was divided into two groups of equal size" Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "[d]ouble‐blind evaluation was conducted for each child individually before and after six months of treatment". Comment: insufficient information regarding who was blinded to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no indication of how many participants completed the study; no indication of how many participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: no demographic data provided |
Pandey 2011.
| Methods |
Design: randomised controlled trial Country of origin: India Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 18 randomised; exercise group: n = 9; control group: n = 9 Age: not stated Sex (male/female): 11/7 Ethnicity: not stated GMFCS level: not stated Type of motor abnormality: spastic Anatomical distribution of CP: diplegia Inclusion criteria: diagnosis of spastic CP; aged 5‐10 years, able to walk with or without aid, able to extend knee from 90° to 45° or more in sitting position with full passive range of motion in supine, no known cognitive impairment, able to flex knee to 90° in prone position, spasticity grade 2 or less than 2 on Modified Ashworth Scale (for hip adductors and abductors, knee flexors, and ankle plantarflexors) Exclusion criteria: known cognitive impairment, orthopaedic or medical condition that prevents exercising, cerebellar symptoms, known visual, speech, hearing disorders, systemic medical problem which prevents exercising, lower limb surgery within 12 months, on anticonvulsant, antispastic medication, non‐ambulatory |
|
| Interventions |
Aim of the intervention: to increase lower limb strength, improve segmental control of lower limbs, and improve balance Type of exercise programme: resistance training Exercise mode: bilateral heel raises, sit‐to‐stand exercises, standing balance exercises, step‐ups, vestibular ball supported half squat Comparator: not clearly stated. States "none were allowed to attend physiotherapy other than intervention protocol" Setting: not stated Intervention provider: physiotherapist Duration of programme: 4 weeks Exercise dose: 1 h session, twice per week. Number of sets, repetitions and resistance used not stated Tailoring of intervention to individual: not stated Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 4), follow‐up (not stated how long after intervention) Primary outcome: no primary outcome measure stated. Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[a]ll eligible subjects were randomly assigned to intervention group and control group, through use of random number generator with sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[a]ll eligible subjects were randomly assigned to intervention group and control group, through use of random number generator with sealed envelopes" Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "[t]he study employed a randomized single blind controlled trial design consisting of two groups and three measurements, training was conducted in one‐hour sessions twice a week for four weeks" Comment: unclear who was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no indication of how many participants completed the study; no indication of how many participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: there is no clear statement of numbers in each group or how many were analysed. Unclear whether baseline data is just for those who completed the trial |
Smania 2011.
| Methods |
Design: randomised controlled trial Country of origin: Italy Intervention(s): repetitive locomotor training Unit of allocation: individual |
|
| Participants |
Number of participants: 18 randomised; exercise group: n = 9; control group: n = 9 Group characteristics reported for all randomised participants Age: mean (SD) = 13.30 (2.91) years; exercise group: mean (SD) = 13.87 (2.79) years; control group: mean (SD) = 12.72 (3.08) years Sex (male/female): 10/8; exercise group: 4/5; control group: 6/3 Ethnicity: not stated GMFCS level: level I (n = 6), level II (n = 2), level III (n = 3), and level IV (n = 7); exercise group: level I (n = 3), level II (n = 2) and level IV (n = 4); control group: level I (n = 3), level III (n = 3) and level IV (n = 3) Type of motor abnormality: spastic CP Anatomical distribution of CP: tetraplegia (n = 7) and diplegia (n = 11); exercise group: tetraplegia (n = 4) and diplegia (n = 5); control group: tetraplegia (n = 3) and diplegia (n = 6) Inclusion criteria: bilateral lower limb (diplegia or tetraplegia) CP, aged 10‐18 years, GMFCS levels II, III or IV, ability to walk independently with or without an aid for at least 10 m, maintain a sitting position without assistance, follow instructions, participate in the programme Exclusion criteria: lower limb spasticity ≥ 2 on the Modified Ashworth Scale, severe lower limb contractures, cardiovascular diseases, orthopaedic surgery or neurosurgery in the past 12 months or botulinum toxin injections in past 6 months |
|
| Interventions |
Aim of the intervention: to increase walking speed and endurance Type of exercise programme: aerobic Exercise mode: gait trainer (Gait Trainer GT I). 30 min of repetitive locomotor therapy on the gait trainer and 10 min of passive joint mobilisation and stretching Comparator: usual physiotherapy: passive joint mobilisation and stretching of lower limbs, strengthening exercises including leg‐press and sit‐to‐stand exercises with resistance adapted for participant's ability, balance and gait exercises. Each type of exercise lasted 10, 15 and 15 min, respectively Setting: rehabilitation gym in medical centre Intervention provider: a physiotherapist delivered the exercise programme to both the exercise group and the control group Duration of programme: 2 weeks Exercise dose: both groups received 10 sessions of 40 min, 5 days per week. Tailoring of intervention to individual: gait speed and step length were individually set according to the gait parameters recorded at baseline. Walking speed was gradually increased over the course of the 2 weeks if the participant completed the last training session without discomfort or fatigue. Partial body weight support was decreased from 30% to 0% over the duration of the sessions. The criterion for reduction was the participants' ability to avoid their knees collapsing into flexion during the stance phase because of increased load of body weight. Fidelity to prescribed intervention: not reported Monitoring of adverse events: no adverse events that led to a missed session, no joint pain or muscle spasms were reported during or after the intervention. Unclear how adverse events were monitored. |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 2), 4 weeks postintervention (week 6) Primary outcome: gait speed and walking endurance
Secondary outcomes: gait parameters
|
|
| Notes |
Source of funding: the CariVerona Fondation (PACIS) Potential conflicts of interest: declares no conflicts of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[b]efore the start of the study participants were allocated to the experimental group or the control group via computerised randomisation. The randomisation sequence was generated by a research assistant not involved with the study and the group allocation was concealed using sealed numbered envelopes" |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]he randomisation sequence was generated by a research assistant not involved with the study and the group allocation was concealed using sealed numbered envelopes. The randomisation list was locked in a desk drawer accessible only to the principal investigator". Comment: PI has access to the randomised list, unclear whether envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[t]he participants were evaluated by the same examiner who was unaware of treatment allocation". Comment: both self‐reported and objective outcome measures were used. Although it does not state who completes the self‐report outcome measure, given its nature, we assume participants and/or their parents were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[n]o children withdrew from the study" Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Chen 2012.
| Methods |
Design: randomised controlled trial Country of origin: Taiwan Intervention(s): home‐based virtual cycling training programme Unit of allocation: individual |
|
| Participants |
Number of participants: 30 randomised; cycling group: n = 14; control group: n = 16. Age, sex, GMFCS level and anatomical distribution reported for 27 participants (cycling group, n = 13, and control group, n = 14) who completed the study. Age: 6‐12 years; cycling group: mean (SD) = 8.7 (2.1) years; control group: mean (SD) = 8.5 (2.2) years Sex (male/female): 18/9; cycling group: 9/4; control group: 9/5 Ethnicity: not reported GMFCS level: levels I and II; cycling group: level I (n = 10) and level II (n = 3); control group: level I (n = 11) and level II (n = 3) Type of motor abnormality: spastic CP Anatomical distribution of motor abnormality: diplegia (n = 19) and hemiplegia (n = 8); cycling group: diplegia (n = 10) and hemiplegia (n = 3); control group: diplegia (n = 9) and hemiplegia (n = 5) Inclusion criteria: aged 6‐12 years, diagnosis of spastic CP with GMFCS levels I‐II, in prepubertal stage, ability to walk independently, ability to undergo motor function and isokinetic muscle test, ability to comprehend commands and cooperate during an examination Exclusion criteria: children and adolescents with recognised chromosomal abnormalities, a progressive neurological disorder or severe concurrent illness or disease that is not typically associated with CP, active medical conditions such as pneumonia, any major surgery or nerve block in the preceding 3 months, hormonal disturbance, poor tolerance for performing the isokinetic test or a poor ability to cooperate during assessment |
|
| Interventions |
Aim of the intervention: to improve muscle strength, motor function and bone density Type of exercise programme: mixed (aerobic and resistance training) Exercise mode: cycling group: cycled on an ergometer and sit‐to‐stand exercises (n = 14). Virtual cycling system; CD‐ROM allowed participant to cycle in virtual world and guided participant through exercises Comparator: encouraged to perform general physical activity at home which involved walking, running, jogging or sports or recreational exercises at school or at home for 30‐40 min/day, 3 days/week. Supervised by parent Setting: home‐based programme; individual session Intervention provider: virtual cycling system Duration of programme: 12 week programme Exercise dose: cycling group: received 40 min of exercise (20 min of cycling and 2 sets of 10 repetitions of loaded sit‐to‐stand exercises), 3 days per week Tailoring of intervention to individual: the resistance of the bike was progressively increased depending on the ability of the participant. The loaded sit‐to‐stand exercises were adjusted by adding weight bags to a back‐pack worn by the participant, and the weight of the bags ranged from 0.5 to 3 kg. Participants trained with loads of 75% of 1 RM. Fidelity to prescribed intervention: compliance recorded for both groups by a research assistant. Parents and participants were interviewed about the implementation of the programmes by a research assistant via telephone every 1‐2 weeks. The participants and caregivers were followed up at the rehabilitation unit every month. Compliance data not reported. Monitoring of adverse events: no adverse effects observed during intervention for either group; not stated how adverse events were monitored |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 12) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: National Science Council of the Republic of China, Taiwan Potential conflicts of interest: authors state they have no conflicts of interest; no conflicts of interest perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[p]articipants were randomly assigned to the hVCT group or to the control group" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided to indicate if allocation was concealed or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "[a] physical therapist, who was not blinded to group allocation was trained to use an isokinetic dynamometer and the GMFM as a precondition of study participation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "[o]ne child in the control group and one child in the experimental groups dropped out due to lack of time to complete the study" Comment: in addition to the participants who dropped out of the study due to lack of time, 1 participant in the control group's data was excluded from the analysis; reasons not provided The reasons for missing data are not described in enough detail to make a judgement regarding the effect of attrition bias on results |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported |
| Other bias | Unclear risk | Comment: demographic and baseline data provided for those who completed the study only |
Chrysagis 2012.
| Methods |
Design: randomised controlled trial Country of origin: Greece Intervention(s): treadmill training programme Unit of allocation: individual |
|
| Participants |
Number of participants: 22 randomised; exercise group: n = 11; control group: n = 11 Group characteristics were reported for all randomised participants Age: 13‐19 years; exercise group: mean (SD) = 15.9 (1.97) years; control group: mean (SD) = 16.09 (1.51) years. Sex (male/female): 13/9; exercise group: 6/5; control group: 7/4 Ethnicity: not stated GMFCS level: level I (n = 5), level II (n = 9), level III (n = 8); exercise group: level I (n = 3), level II (n = 4), level III (n = 4); control group: level I (n = 2), level II (n = 5), level III (n = 4) Type of motor abnormality: spastic CP Anatomical distribution of motor abnormality: diplegia (n = 19) and tetraplegia (n = 3); exercise group: diplegia (n = 9), tetraplegia (n = 2); control group: diplegia (n = 10), tetraplegia (n = 1) Inclusion criteria: a diagnosis of spastic CP (tetraplegia or diplegia), GMFCS levels I‐III, ability to follow simple commands Exclusion criteria: undergone surgery in previous 12 months, received botulinum toxin injections in the previous 6 months, had cardiovascular disease or uncontrolled epilepsy |
|
| Interventions |
Aim of the intervention: to improve gross motor function and self‐selected walking speed Type of exercise programme: aerobic training. Parents and guardians were instructed to continue the daily activities of their participants, and not to initiate any additional interventions or increase the usual daily physical activities of their participants throughout the duration of the programme Exercise mode: treadmill training (no bodyweight support). Comparator: conventional physiotherapy delivered by a physical therapist. 3 sessions of 45 min each consisting of 3 sets of exercises with mat activities, balance, and gait training and functional gross motor activities; adherence not reported Setting: special school; individual supervised session Intervention provider: 2 physical therapists Duration of programme: 12 weeks Exercise dose: participants in the exercise group underwent treadmill training on 3 days per week for a total of 34 sessions (2 sessions cancelled). Each session consisted of a 10 min warm‐up, walking for a maximum of 30 min at a comfortable speed, and a 5 min cool down. Participants completed between 12.00 and 20.00 min (mean (SD) 16.43 (2.59) min) per session at the start, and between 23.90 and 29.80 min (mean (SD) 27.54 (2.12) min) per session, at the end of the training. Mean (SD) treadmill speed increased from 1.76 (0.41) km/h at the beginning to 3.00 (0.60) km/h at the end of the programme. Tailoring of intervention to individual: training was customised to each participant's ability and the physiotherapist's supervision of the participants' safety and facilitation of the walking pattern, without elicitation of abnormal movements. Adolescents held handrails when needed. Fidelity to prescribed intervention: therapists kept logbooks during each session for the exercise group. Adherence ranged from 26 to 34 sessions (mean (SD) 29.45 (2.84) sessions). Monitoring of adverse events: no change in spasticity as measured by Modified Ashworth Scale. No participant experienced a fall or complained of fatigue, soreness or pain as recorded by the therapist in a logbook. |
|
| Outcomes |
Assessment time points: baseline (week 0) and postintervention (week 12) Primary outcomes: gross motor function and self‐selected walking speed
Secondary outcomes:
|
|
| Notes |
Source of funding: none stated Potential conflicts of interest: authors report no conflict of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[t]he participants were stratified according to GMFCS level and sex and then randomly allocated to the experimental and control groups. The process was led by a PhD student working in the laboratory who was not involved with the participants or the experimental treatment. The student used sealed envelopes to assign the participants to the experimental and control conditions". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]he process was led by a PhD student working in the laboratory who was not involved with the participants or the experimental treatment. The student used sealed envelopes to assign the participants to the experimental and control conditions" Comment: did not state if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "[t]wo trained assessors who were blinded to the treatment allocations . . . carried out the measurements at the school" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[t]here were no dropouts" Comment: no missing data reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported. No convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Bryant 2013.
| Methods |
Design: randomised controlled trial Country of origin: UK Intervention(s): aerobic exercise intervention using a static bike or treadmill Unit of allocation: individual |
|
| Participants |
Number of participants: 35 randomised; cycling group: n = 11; treadmill group: n = 12; control group: n = 12 Group characteristics were reported for all randomised participants Age: 8‐17 years; mean (SD) = 13 years 9 months (2 years 3 months); cycling group: mean (SD) = 14.3 (1.9) years; treadmill group: mean (SD) = 13.5 (2.6) years; control group: mean (SD) = 13.8 (2.3) years Sex (male/female): 14/21; cycling group: 6/5; treadmill group: 3/9; control group: 5/7 Ethnicity: not reported GMFCS level: level IV (n = 23) and V (n = 12); cycling group: level IV (n = 8) and V (n = 3); treadmill group: level IV (n = 8) and V (n = 4); control group: level IV (n = 7) and V (n = 5). Type of motor abnormality: dyskinetic (n = 14) and spastic (n = 21); cycling group: dyskinetic (n = 4) and spastic (n = 7); treadmill group: dyskinetic (n = 3) and spastic (n = 9); control group: dyskinetic (n = 7) and spastic (n = 5) Anatomical distribution of motor abnormality: bilateral Inclusion criteria: aged 8‐17 years, CP at GMFCS level IV and V, able to pedal on adapted static bike and walk with partial bodyweight support on a treadmill Exclusion criteria: undergone orthopaedic surgery to the spine or lower limbs within the last year, cognitive or behavioural impairment preventing understanding or compliance with instructions |
|
| Interventions |
Aim of the intervention: to improve gross motor function Type of exercise programme: aerobic training Exercise mode: cycling group cycled on an adapted static bike (n = 11); treadmill group walked supported with a hoist on a treadmill, minimal operating speed 0.5km/h (n = 12) Comparator: control group received usual physiotherapy such as stretching and exercise on a mat, use of a standing frame and swimming, dose not reported (n = 12) Setting: 4 special schools; unclear if supervised individual or group session Intervention provider: not stated Duration of programme: all groups received a 6‐week programme Exercise dose: cycling group and treadmill group received 30 min of exercise (including transfers), 3 days per week. An exercise test was used to determine starting level of exercise and to monitor progress in ability. However, the exercise intensity is unclear. Tailoring of intervention to individual: bikes were adapted to provide extra postural support. Participants in treadmill group were supported during walking on treadmill with a hoist. Fidelity to prescribed intervention: the number of training sessions attended were recorded but it's not stated how attendance was recorded. Mean (SD) number of sessions attended was 14.6 (3.1) for cycling group and 13.8 (4.2) for treadmill group Monitoring of adverse events: reports no adverse events. In the treadmill group 1 participant withdrew due to hospitalisation because of gastric problems and 1 participant withdrew due to a reoccurrence of long‐standing hip pain; not stated how adverse events were monitored |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6), 6 weeks postintervention (week 12), 12 weeks postintervention (week 18) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: National Institute for Health Research (NIHR) Potential conflicts of interest: did not declare conflicts of interest; no perceived conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[t]he study administrator provided nine sealed envelopes (three for each group) to each site which were randomly placed by a third party in the participant files and were opened after the baseline assessment" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]he study administrator provided nine sealed envelopes (three for each group) to each site which were randomly placed by a third party in the participant files and were opened after the baseline assessment" Comment: unclear if the study administrator was independent to the study or if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "[o]ne researcher acted as assessor for all but three of the study assessments and was blinded as to which arm of the study each participant was allocated" Comment: assessor may not have been blinded as to which group all of the participants were allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low rate of missing data (<10%). Missing data evenly distributed across groups. Missing data accounted for |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Mattern‐Baxter 2013.
| Methods |
Design: quasi‐randomised controlled trial Country of origin: USA Intervention(s): locomotor treadmill training Unit of allocation: individual |
|
| Participants |
Number of participants: 15 randomised. Reported data on 12; exercise group: n = 6; control group: n = 6 Age: exercise group: mean (SD) = 21.76 (6.50) months; control group: mean (SD) = 21.25 (6.07) months Sex (male/female): 8/4; exercise group: 3/3; control group: 5/1 Ethnicity: African‐American (n = 2), Asian (n = 2), Hispanic (n = 2), white (n = 6); exercise group: Asian (n =1), Hispanic (n = 1), white (n = 4); control group: African‐American (n = 2), Asian (n = 1), Hispanic (n = 1), white (n = 2) GMFCS level: level I (n = 4) and level II (n = 8); exercise group: level I (n = 2) and level II (n = 4); control group: level I (n = 2) and level II (n = 4) Type of motor abnormality: spastic (n = 7) and hypotonic (n = 5); exercise group: spastic (n = 4) and hypotonic (n = 2); control group: spastic (n = 3) and hypotonic (n = 3) Anatomical distribution of CP: participants with spastic CP had hemiplegia and diplegia; not stated how many in each group. Inclusion criteria: diagnosis of CP in GMFCS level I or II, aged 9‐36 months, signs of walking readiness indicated by both the ability to sit for at least 30 seconds unsupported in ring sitting or W sitting, the ability to take 10 consecutive steps when held on hands or torso Exclusion criteria: diagnosis of a genetic syndrome, independent ambulation without an assistive device, previous or current use of treadmill intervention during physiotherapy, use of medication to control spasticity in the past 6 months |
|
| Interventions |
Aim of the intervention: to improve gross motor skills Type of exercise programme: aerobic Exercise mode: treadmill Comparator: all participants received their weekly scheduled physiotherapy sessions in their homes or in the clinic excluding treadmill training. Content not described, dose not recorded Setting: home‐based programme Intervention provider: parent assisted the participant. The parent was supervised once a week by a physiotherapist Duration of programme: 6 weeks Exercise dose: 2 sessions per day on 6 days per week. Each session lasted 10‐20 min Tailoring of intervention to individual: participants used their orthotics and bilateral hand rails to hold on while walking. Parents assisted the child in leg advancement if needed and provided as little manual support as needed at the pelvis. The starting treadmill speed was determined during the initial training session and was increased as quickly as possible throughout the sessions. The speed was determined as the fastest possible speed during which a participant could move his feet independently without dragging them for more than 5 seconds. A range of treadmill speeds was determined at each weekly visit and maintained throughout that week. Participants were encouraged to self‐correct before parent intervened Fidelity to prescribed intervention: mean completion rate of 87.5% for the 12 weekly training sessions. Participants walked an average 28.2 min/d (range 9.6 to 39.3 min/d) Monitoring of adverse events: parents were asked about adverse events weekly; no adverse events reported. |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6), 4 weeks postintervention (week 10), 12 weeks postintervention (week 18) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: supported by a research grant from the paediatric section of the American Physical Therapy Association Potential conflicts of interest: authors report no conflicts of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "[q]uasi‐randomization was conducted to achieve matched groups by age and GMFCS levels" Quote: "[t]he children were quasi‐randomized by the principal investigators and matched by GMFCS levels and age" Comment: description of method of sequence generation suggests it was based on a non‐random component |
| Allocation concealment (selection bias) | High risk |
Quote: "[t]he children were quasi‐randomized by the principal investigators and matched by GMFCS levels and age" Comment: description suggests the principal investigators were aware of group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[b]linding for the GMFM‐66 and PDMS‐2 was achieved by videotaping the children's gross motor skills at their homes. The videotapes were subsequently reviewed by a physical therapist who was blinded to group allocation" Quote: "[t]he timed 10‐m walk test (10MWT), the Functional Moblity Scale (FMS), and the number of alternating steps in 10 seconds were used as measures of walking function. They were scored by clinical observation by a non blinded assessor." Quote: "In order to gain the parents' perspective on their child's motor abilities, the mobility sub scale of the PEDI was administered via parent interview." Comment: although 1 outcome measure was assessed by an assessor blinded to group allocation, the other measure was not assessed by a blinded assessor. A self‐report measure was also used. As parents and assessors were not blinded to group allocation, at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "[a]ttrition (n=3). Illness (n=1). Family reasons (n=1). Change of diagnosis to genetic syndrome (n=1). Comment: missing data rate of 20%. All missing data in intervention group |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: demographic and baseline outcome data only provided for those who completed the trial |
Taylor 2013.
| Methods |
Design: randomised controlled trial Country of origin: Australia Intervention(s): resistance training Unit of allocation: individual |
|
| Participants |
Number of participants: 49 randomised; exercise group: n = 24; control group: n = 25 (demographic data reported on n = 48 as 1 person withdrew from the exercise group after allocation but before the start of training) Age: mean (SD) 18 years 1 month (1 year 11 months);exercise group: mean (SD) = 18 years 2 months (1 year 11 months); control group: mean (SD) = 18 years 7 months (2 years 11 months) Sex (male/female): 26/22; exercise group: 13/10; control group: 13/12 Ethnicity: not stated GMFCS level: level II (n = 29) and level III (n = 19); exercise group: level II (n = 13) and level III (n = 10); control group: level II (n = 16) and level III (n = 9) Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: diagnosis of spastic diplegia, aged 14‐22 years, GMFCS level II or III, able to follow simple instructions Exclusion criteria: participated in strength training in previous 6 months, single event multi‐level surgery in last 2 years, contractures > 20 degrees at hips and knees |
|
| Interventions |
Aim of the intervention: to improve mobility Type of exercise programme: resistance training Exercise mode: weights machine; 4‐6 individualised exercises to target deficits identified during gait analysis. Targeted muscles were the knee extensors (7 people), the plantarflexors (4 people), the hip extensors (3 people), the hip abductors (2 people) and generalised extensors represented by the leg press (7 people) Comparator: usual recreation and physiotherapy provided it did not include progressive resistance training Setting: community gym; individually or in pairs Intervention provider: physiotherapist Duration of programme: 12 weeks (24 sessions) Exercise dose: 2 sessions per week, 3 sets of 10‐12 repetitions to fatigue (i.e. 60%‐80% 1 RM), at least 5 on Borg Rating of Percieved Exertion scale Tailoring of intervention to individual: when the participant was able to complete 3 sets of 12 repetitions of an exercise the weight to be lifted was increased Fidelity to prescribed intervention: participants kept logbooks detailing exercise, weight lifted, number of repetitions, sets and details of injuries. Mean (SD) sessions performed 21.9 (2.4). The mean (SD) rating of perceived exertion at the end of each session was 6.9 (1.1). Participants increased their training load of exercises for targeted muscles from session 3 to session 24 by a mean (SD) of 183% (23%). Monitoring of adverse events: short‐term muscle soreness reported by most participants but resolved in a few days. 1 participant reported minor calf strain, and 1 participant reported minor discomfort to plantar fascia; the programme was adjusted but participants did not miss sessions. |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 12), 12 weeks postintervention (week 24) Primary outcome: mobility
Secondary outcomes: mobility‐related function, gross motor function, muscle performance
|
|
| Notes |
Source of funding: National Health and Medical Research Council of Australia Potential conflicts of interest: the authors report no conflict of interest; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[a] separate randomisation procedure was prepared for each stratum (GMFCS levels II and III) using permuted blocks. An independent researcher generated a block allocation sequence for each stratum by drawing pieces of paper from a sealed container and then sealing assignments in sequentially numbered opaque envelopes. The research coordinator allocated participants after enrolment and baseline testing". |
| Allocation concealment (selection bias) | Low risk | Quote: "[a] separate randomisation procedure was prepared for each stratum (GMFCS levels II and III) using permuted blocks. An independent researcher generated a block allocation sequence for each stratum by drawing pieces of paper from a sealed container and then sealing assignments in sequentially numbered opaque envelopes. The research coordinator allocated participants after enrolment and baseline testing". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[a]ssessments were completed in a hospital gait laboratory by an assessor blinded to group allocation". Quote: "[i]n addition, two participant‐rated mobility outcomes were assessed: the Functional Mobility Scale, which describes the level of assistance that children with CP required to cover different distances and environments; and the Functional Assessment Questionnaire, a 10‐level report of the level that best describes typical walking ability". Comment: both self‐reported and objective outcome measures were used. As participants were not blinded to group allocation at least 1 outcome is at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[t]he intention‐to‐treat principle was applied with available data of all participants who were allocated and commenced their programme included in analyses". Quote: "[o]ne participant withdrew from the intervention group after allocation but before the start of training because surgery was scheduled unexpectedly". Comment: low rate of missing data (2%) and intention‐to‐treat analysis undertaken |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Tedla 2014.
| Methods |
Design: randomised controlled trial Country of origin: Saudi Arabia Intervention(s): strength training of trunk and lower extremity muscles Unit of allocation: individual |
|
| Participants |
Number of participants: 62 randomised; exercise group: n = 31, control group: n = 31. Data presented on 60 participants; exercise group: n = 30; control group: n = 30 Age: mean (SD) = 8.94 (2.46) year; exercise group: mean (SD) = 9.14 (2.46) years; control group: mean (SD) = 8.74 (2.49) years Sex (male/female): 40/20 Ethnicity: not stated GMFCS level: presented as bar chart; unable to determine number of participants in each level Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: diagnosis of spastic diplegia, 5‐14 years, able to sit for 10 seconds with back unsupported and feet supported, minimum required score on the mini‐mental state examination, GMFCS levels I, II, III or IV, able to move the affected lower limbs at least in gravity eliminated position Exclusion criteria: orthopaedic surgery in past year, botulinum toxin injection in past 6 months, history of selective dorsal rhizotomy, receiving medication that alters muscle tone or strength |
|
| Interventions |
Aim of the intervention: to increase muscle strength Type of exercise programme: resistance training Exercise mode: muscles with > 50% weakness from the normal were targeted. Exercise group completed a circuit consisting of the following exercises: quadruped position upper extremity and lower extremity lifts for trunk extensors, curl‐ups and leg lifts for trunk flexors, trunk and lower extremity combined in wall squats and sit‐to‐stand with weighted vest, hip flexors, knee extensors and dorsiflexors with sand bags in high sitting position, hip extensors and knee flexors with sand bags in prone positions, hip abductors and hip adductors with sand bags in side lying position, heel rises on a step for plantar flexors with weighted vest Comparator: 3‐5 sessions a week of conventional physiotherapy consisting of range of motion exercises and stretching, positioning or adaptive equipment prescription, movement transitions and mobility training, functional activities and gait training. Average duration per session was 60‐90 min Setting: not stated; supervised programme Intervention provider: the investigator Duration of programme: 6 weeks Exercise dose: 3 sessions per week for a total of 18 sessions. All exercises performed at 80% of 1 repetition maximum (RM). The number of repetitions varied between exercises and individuals but ranging between 6 and 10 for 3 sets in the initial periods. Conventional physiotherapy continued as usual 1‐2 days/week Tailoring of intervention to individual: when the participant reached 3 sets of 12 repetitions without any difficulty or according to the new 80% of 1 RM at each week, the participant progressed to higher weights. With changed weight, the exercise repetitions were started again with 6‐10 in 3 sets Fidelity to prescribed intervention: parent/caregiver help was taken to maintain a log book. This recorded the type of exercise, the number of repetitions, the amount of weight lifted and any additional information; adherence not reported Monitoring of adverse events: muscle soreness was reported in a few subjects but subsided with rest and no additional treatment was required |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[i]n this randomized controlled trial subjects were randomly allocated either to 6 wk strengthening experimental group or to a conventional intervention control group". Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information regarding allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "[b]efore and after the training the assessments were conducted by the same investigator". Quote: "[i]n addition, the investigator did not review or have access to the pre‐training values at the post‐training assessments." Quote: "[i]deally the person conducting the assessment should have been blinded to the training schedule. Because limited manpower and long duration there was difficulty in blinding the tester." Comment: although not stated, text suggests assessor was not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[d]rop out due to absence (No‐1)" Quote: "[d]rop out due to surgery (No‐1)" Comment: figure 2 indicates that 1 person dropped out from each group. This represents a low rate of missing data (3%) that is evenly distributed across groups. |
| Selective reporting (reporting bias) | High risk | Comment: data on strength and gross motor function measure are reported incompletely and cannot be entered into a meta‐analysis. |
| Other bias | Low risk | Comment: no other sources of bias identified |
Emara 2015.
| Methods |
Design: randomised controlled trial Country of origin: Saudi Arabia Intervention(s): gait training programme using antigravity treadmill Unit of allocation: individual |
|
| Participants |
Number of participants: 36 randomised; exercise group: n = 17; control group: n = 17 30 completed the trial (exercise group: n = 15; control group: n = 15). Unclear if baseline data provided on 34 participants or 30 participants Age: 6‐8 years; exercise group: mean (SD) = 6.799 (0.77) years; control group: mean (SD) = 6.402 (0.68) years. Sex (male/female): 18/12; exercise group: 9/6; control group: 9/6 Ethnicity: not stated GMFCS level: not stated Type of motor abnormality: spastic CP Anatomical distribution of CP: diplegia Inclusion criteria: spasticity grades ranged from 1 to 1+ according to the modified Ashworth scale, no hearing defects, no fixed deformity of both lower limbs, absence of cognitive or visual impairment that could compromise the performance of the tasks Exclusion criteria: not stated |
|
| Interventions |
Aim of the intervention: to improve balance Type of exercise programme: aerobic exercise Exercise mode: gait training using the Alter G anti‐gravity treadmill in addition to the same physical therapy programme provided to the control group Comparator: physical therapy programme of 1 hour session, 3 times per week, based on neurodevelopmental approach, including facilitation, strengthening exercises, gait training in parallel bars, and negotiating obstacles Setting: participant's home. Unsupervised individual session. Antigravity treadmill training was provided in an outpatient department Intervention provider: not stated Duration of programme: 12 weeks Exercise dose: 20 min of walking on anti‐gravity treadmill, 3 times per week, at a comfortable walking speed Tailoring of intervention to individual: a comfortable treadmill speed was selected for all participants as 75% of their comfortable speed during over‐ground walking Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 12) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: authors reported no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[t]hey were divided randomly into two groups" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]hey were divided randomly into two groups". Comment: insufficient information regarding the method of allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: did not provide information to indicate if the assessor was blinded to group allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition and withdrawal accounted for. Low rate of missing data (< 10%) and evenly distributed across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Unclear risk | Comment: unclear whether all demographic or outcome data for all participants are reported |
Lee 2015.
| Methods |
Design: randomised controlled trial Country of origin: Korea Intervention(s): resistance training programme Unit of allocation: individual |
|
| Participants |
Number of participants: 26 randomised; exercise group: n = 13; control group: n = 13 Group characteristics reported for all randomised participants Age: 5‐10 years; exercise group: mean (SD) = 6.1 (2.7) years; control group: mean (SD) = 6.9 (2.5) years Sex (male/female): 13/13; exercise group: 8/5; control group: 5/8 Ethnicity: not stated GMFCS level: levels I to III Type of motor abnormality: spastic CP Anatomical distribution of CP: not stated Inclusion criteria: aged 5‐10 years, the ability to follow verbal instructions, able to walk independently indoors with or without a walking aid (GMFCS level I‐III) Exclusion criteria: unstable seizures, any treatment for spasticity or surgical procedures in the last 6 months, any change in medication expected during the study period, any other disease that would have interfered with physical activity |
|
| Interventions |
Aim of the intervention: to change lower limb muscle architecture and motor function Type of exercise programme: resistance training Exercise mode: 3 functional training items: loaded sit‐to‐stand for 5 min, loaded lateral step‐up and half knee‐rise for 10 min, unloaded lateral step‐up and half knee‐rise for 10 min Comparator: all participants received 30 min of general neurodevelopmental treatment (NDT), 3 sessions a week for 6 weeks Setting: not stated Intervention provider: not stated Duration of programme: 6 weeks Exercise dose: 3 sessions per week Tailoring of intervention to individual: the training load was initially set to 5% of participants' body weight and progressively increased base on repeated estimation of the 8 RM Fidelity to prescribed intervention: not reported Monitoring of adverse events: not reported |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 6) Primary outcome: no primary outcome measure stated Outcomes:
|
|
| Notes |
Source of funding: not stated Potential conflicts of interest: not stated; none perceived |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[t]his study . . . had a single‐blind randomized controlled design" Comment: insufficient information regarding the method of sequence allocation was provided to make a judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "[t]his study . . . had a single‐blind randomized controlled design" Comment: insufficient information regarding the method of allocation concealment was provided to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "[t]his study . . . had a single‐blind randomized controlled design" Comment: did not provide information to indicate if the assessor was blinded to group allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is not available for this study and therefore unable to determine if all expected outcomes are reported; no convincing text provided to indicate that published report includes all expected outcomes |
| Other bias | Low risk | Comment: no other sources of bias identified |
Mitchell 2016.
| Methods |
Design: randomised controlled trial Country of origin: Australia Intervention(s): web‐based multimodal therapy programme that included upper limb, cognitive, visual perceptual, and physical activity training Unit of allocation: individual |
|
| Participants |
Number of participants: 102 participants randomised. Baseline assessments conducted on 101 participants. Exercise group: n = 51; control group: n = 50 Group characteristics reported for 101 participants Age: mean (SD) 11 years 9 months (2 years 4 months), range 8 to 17 years; exercise group: mean (SD) = 11 years 3 months (2 years 4 months); control group: mean (SD) = 11 years 4 months (2 years 6 months) Sex (male/female): 52/49; exercise group: 26/25; control group: 26/24 Ethnicity: not stated GMFCS level: level I (n = 45), level II (n = 56); exercise group: level I (n = 20), level II (n = 31); control group: level I (n = 25), level II (n = 25) MACS level: level I (n = 24), level II (n = 76), level III (n = 1); exercise group: level I (n = 11), level II (n = 39), level III (n = 1); control group: level I (n = 13), level II (n = 37), level III (n = 0) Type of motor abnormality: spastic CP Anatomical distribution of CP: unilateral Inclusion criteria: aged 8‐18 years, unilateral CP, classified in GMFCS levels I and II, classified in MACS levels I to III, sufficient cooperation and cognitive understanding to perform required tasks. Computer and internet access were provided at home if participants did not have access on entry to the study Exclusion criteria: unstable epilepsy or medical conditions that precluded participation in training. Entry was delayed to trial if the participant had undergone upper‐limb botulinum neurotoxin A injections or surgery in the previous 2 months or 6 months, respectively |
|
| Interventions |
Aim of the intervention: to improve activity capacity and performance, reduce mobility limitations, improve recreational participation, improve occupational performance, upper limb function, and visual perception Type of exercise programme: resistance training Exercise mode: physical activity games were interspersed with upper‐limb and visual‐perceptual games. Gross‐motor exercises comprised of 40% of the overall programme and included sequences of repetitive multi‐joint bodyweight functional exercises (e.g. sit‐to‐stand, alternate lunging, step‐ups, side step‐ups onto a block, squatting, balancing on balance foam) Comparator: participants in the wait list control group continued care as usual for the duration of the programme 12 (23%) participants in the exercise group received physiotherapy totaling on average 0.47 hours over the 20 weeks. 15 (30%) participants in the control group received physiotherapy totaling on average 3.9 hours over the 20 weeks Setting: participant's home. Unsupervised individual session. Therapists were available to participants and their families via email, telephone or videoconferencing to provide encouragement and technical support. Intervention provider: web‐based programme Duration of programme: 20 weeks Exercise dose: 30‐min programme, 6 days per week (total potential dose of 60 hours) Tailoring of intervention to individual: the intensity of the lower‐limb strength exercises for week 1 were determined by setting tasks for approximately 75% of repetition maximum. These were incremented weekly by the physiotherapists remotely, by increasing the repetitions, speed, step height, and balance challenge in response to individual performance and feedback from the participants and their parents or caregivers.On average, week 1 started with 7 activities of 5‐10 repetitions, lasting approximately 60 seconds per activity, and progressed to 11 games of up to 20 repetitions lasting approximately 90 seconds with the addition of step blocks and balance foam. Fidelity to prescribed intervention: on average participants completed mean (SD) 32.4 (17.2) hours of training over the 20‐week period, logging in for 24.2 (5.5) min on 77.7 (35.7) days. The total dose of therapy ranged from 3.7 to 74.7 hours per participant. Monitoring of adverse events: 2 instances of minor musculoskeletal pain were recorded during the intervention; participants were instructed to modify the movement and continued with the programme. 1 participant in the intervention group had seizures but these were deemed unrelated to training |
|
| Outcomes |
Assessment time points: baseline (week 0), postintervention (week 22) Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Source of funding: financial support was obtained from Queensland Government Co‐Investment Program Grant "EBrain", a Financial Markets Foundation for Children research grant, an Australian Postgraduate Award, a National Health and Medical Research Council Career Development Fellowship, and a Smart State Fellowship. Potential conflicts of interest: the authors stated that they had no interests that might be perceived as posing a conflict or bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from trial protocol): "[c]hildren will be matched in pairs according to age, gender and level of functional ability based on MACS level . . . The randomisation process will involve randomly allocating a number '1' or '2' to each member of the pair. As each pair is entered, they will be allocated to the next consecutive envelope, which will be opened by the non‐study personnel who will read and record the treatment allocation from the paper inside the envelope. Treatment allocation will be recorded on a piece of folded paper inside each envelope, in random order". |
| Allocation concealment (selection bias) | Low risk | Quote (from trial protocol): "[c]hildren will be matched in pairs according to age, gender and level of functional ability based on MACS level . . . The randomisation process will involve randomly allocating a number '1' or '2' to each member of the pair. As each pair is entered, they will be allocated to the next consecutive envelope, which will be opened by the non‐study personnel who will read and record the treatment allocation from the paper inside the envelope. Treatment allocation will be recorded on a piece of folded paper inside each envelope, in random order". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: given the nature of the intervention participants were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "it should be noted that while the study's primary outcome (the Assessment of Motor and Process Skills) was blinded, study personnel were not able to be blinded to group allocation for other outcomes measures". Comment: the outcomes included in the results of this review were assessed by an unblinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[i]n the intervention group, 47 started training and were assessed at 20 weeks (92% retention); in the waitlist control group, 44 were assessed at 20 weeks (86% retention)" Quote: "[d]ata were analysed according to the intention‐to‐treat principle" |
| Selective reporting (reporting bias) | Unclear risk | Comment: a protocol is available for the trial. The majority of outcomes are reported in the 2 included reports. However, some outcomes are not reported to date e.g. quality of life, participation assessed with the Participation and Environment Measure for Children and Youth. |
| Other bias | Low risk | Comment: no other sources of bias identified |
bpm: beats per minute; CP: cerebral palsy; HR: heart rate; GMFCS: Gross Motor Function Classification System; GMFM: Gross Motor Function Measure; IQ: intelligence quotient; RER: respiratory exchange ratio; rpm: rotations per minute; RM: repetition maximum; SD: standard deviation; US: ultrasound; VO2 : volume of oxygen.
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
|---|---|
| Bar‐or 1976 | Not a randomised controlled trial |
| Hutzler 1998 | Not a randomised controlled trial |
| Jones 2001 | Not a randomised controlled trial |
| Ketelaar 2001 | Intervention did not meet pre‐stated definition of exercise |
| Benda 2003 | Intervention did not meet the pre‐stated definition of exercise |
| Blundell 2003 | Not a randomised controlled trial |
| Cherng 2004 | Intervention did not meet pre‐stated definition of exercise |
| Ahlborg 2006 | Comparison was whole body vibration therapy |
| Kandrali 2006 | Intervention did not meet the pre‐stated definition of exercise |
| Patikas 2006 | Intervention was provided following surgery; unable to determine independent effects of exercise |
| Reid 2006 | Intervention did not meet the pre‐stated definition of exercise |
| Dodd 2007 | Not a randomised controlled trial |
| Gordon 2007 | Intervention did not meet the pre‐stated definition of exercise |
| Ozer 2007 | Intervention did not meet the pre‐stated definition of exercise |
| Stackhouse 2007 | Compared strengthening with neuromuscular electrical stimulation to strengthening without neuromuscular electrical stimulation |
| Weindling 2007 | Intervention did not meet the pre‐stated definition of exercise |
| Bar‐Haim 2008 | Intervention did not meet the pre‐stated definition of exercise |
| Fernandes 2008 | Intervention did not meet the pre‐stated definition of exercise |
| Hornyak 2008 | This is a commentary |
| Jannink 2008 | Intervention did not meet the pre‐stated definition of exercise |
| Davis 2009 | Intervention did not meet pre‐stated definition of exercise |
| Katz‐Leurer 2009 | Included children with cerebral palsy and traumatic brain injury and presented data on combined group |
| McGibbon 2009 | Not a randomised controlled trial |
| Taylor 2009 | This is a correspondence not a study |
| Salem 2009 | Intervention did not meet the pre‐stated definition of exercise |
| Batista 2010 | Not a randomised controlled trial |
| Boyd 2010 | Protocol of constraint‐induced movement therapy compared to bimanual therapy (do not meet pre‐stated definition of exercise) |
| Brown 2010 | Not a randomised controlled trial |
| Harbourne 2010 | Compared different interventions (not exercise interventions) and different means of delivery. |
| Herrero 2010 | Intervention did not meet the pre‐stated definition of exercise |
| Kumar 2010 | Not a randomised controlled trial |
| Maher 2010 | Intervention did not meet the pre‐stated definition of exercise |
| Mehta 2010 | Compared Bobath therapy to conventional therapy. Neither intervention met the pre‐stated definition of exercise. |
| Sorsdahl 2010 | Not a randomised controlled trial |
| Speyer 2010 | Participants did not have a diagnosis of cerebral palsy |
| Willoughby 2010 | Compared two modes of aerobic exercise |
| Yonetsu 2010 | Intervention did not meet the pre‐stated definition of exercise |
| Batra 2011 | Intervention did not meet the pre‐stated definition of exercise |
| Choi 2011 | Intervention did not meet pre‐stated definition of exercise |
| Hung 2011 | Intervention did not meet the pre‐stated definition of exercise |
| Roberti 2011 | This is a literature review not a study |
| Sakzewski 2011a | Intervention did not meet the pre‐stated definition of exercise |
| Sakzewski 2011b | Intervention did not meet the pre‐stated definition of exercise. It compared constraint‐induced movement therapy to bimanual training. |
| Silva e Borges 2011 | Intervention did not meet the pre‐stated definition of exercise |
| Yabunaka 2011 | Intervention did not meet the pre‐stated definition of exercise |
| Bandholm 2012 | Compared resistance training and usual care following botulinum toxin treatment; unable to differentiate effects of exercise and botulinum toxin treatment |
| Dimitrijević 2012 | Intervention did not meet pre‐stated definition of exercise |
| Herrero 2012 | Intervention did not meet the pre‐stated definition of exercise |
| Kang 2012 | Intervention did not meet the pre‐stated definition of exercise |
| Kim 2012 | Compared 2 modes of resistance training |
| Nsenga Leunkeu 2012 | Participants not randomly allocated into groups |
| Olama 2012 | Compared resistance training to resistance training and myofeedback |
| Sakzewski 2012 | Intervention did not meet the pre‐stated definition of exercise |
| Salem 2012 | Participants did not have a diagnosis of cerebral palsy |
| Angulo‐Barroso 2013 | Participants did not have a diagnosis of cerebral palsy |
| Chang 2013 | Not a randomised controlled trial |
| Fedrizzi 2013 | Intervention included constraint‐induced movement therapy |
| Grecco 2013a | Compared two modes of aerobic exercise |
| Grecco 2013b | Compared two modes of aerobic exercise. |
| Green 2013 | Not a randomised controlled trial |
| Hutzler 2013 | Not a randomised controlled trial |
| Jeng 2013 | Not a randomised controlled trial |
| Kumar 2013 | Unable to determine the content of the intervention from the published report |
| Moreau 2013 | Compared 2 types of strength training (fast and slow training) |
| Nsenga Leunkeu 2013 | Participants not randomly allocated into groups |
| Rimmer 2013 | Intervention did not meet the pre‐stated definition of exercise |
| Su 2013 | Compared two modes of aerobic exercise |
| Williams 2013 | The participants in the intervention group received botulinum toxin type A treatment before or after strength training (this was not consistent for participants). Unable to determine independent effects of exercise |
| Abd El‐Kafy 2014 | Intervention did not meet the pre‐stated definition of exercise |
| Ayhan 2014 | Participants did not have a diagnosis of cerebral palsy |
| Chiu 2014 | Intervention did not meet pre‐stated definition of exercise |
| Franki 2014 | Intervention did not meet the pre‐stated definition of exercise |
| Hammond 2014 | Participants did not have a diagnosis of cerebral palsy |
| Hussein 2014 | Compared 2 modes of aerobic exercise |
| Lee 2014a | Intervention did not meet the pre‐stated definition of exercise |
| Lee 2014b | Not a randomised controlled trial |
| Park 2014a | Intervention did not meet pre‐stated definition of exercise |
| Schroeder 2014 | Not a randomised controlled trial |
| Slaman 2014 | Intervention group received 3 interventions; only 1 was exercise. Unable to determine independent effects of exercise |
| Van Wely 2014 | Intervention group did not receive exercise alone; unable to determine independent effects of exercise |
| Verschuren 2014 | This is a literature review not a study |
| Walsh 2014 | Not a randomised controlled trial but a case study |
| Williams 2014 | Participants did not have a diagnosis of cerebral palsy |
| AlSaif 2015 | Intervention did not meet the pre‐stated definition of exercise |
| Bohm 2015 | Intervention did not meet the pre‐stated definition of exercise |
| Capio 2015 | Not a randomised controlled trial |
| Chen 2015 | Not a randomised controlled trial |
| El‐Basatiny 2015 | Intervention did not meet pre‐stated definition of exercise |
| Gillaux 2015 | Intervention did not meet the pre‐stated definition of exercise |
| Hamah 2015 | Intervention did not meet the pre‐stated definition of exercise |
| Kim 2015 | Not a randomised controlled trial |
| Lai 2015 | Not a randomised controlled trial |
| Lowe 2015 | Participants do not have a diagnosis of cerebral palsy |
| Preston 2015 | Participants in intervention group received botulinum toxin and exercise; unable to determine independent effects of exercise |
| Sherief AEAA 2015 | Participants in intervention group received treadmill training and dynamic ankle‐foot orthoses; unable to determine independent effects of exercise |
| Swe 2015 | Compared 2 modes of aerobic exercise |
| Temcharoensuk 2015 | Intervention did not meet the pre‐stated definition of exercise |
| Declerck 2016 | Intervention did not meet the pre‐stated definition of exercise |
| Hsieh 2016 | Participants did not have a diagnosis of cerebral palsy |
Characteristics of studies awaiting assessment [author‐defined order]
Carlon 2014.
| Methods |
Design: randomised controlled trial Country of origin: Australia Intervention(s): aerobic training programme Unit of allocation: individual |
| Participants |
Number of participants: 19 Age: mean 13 years 10 months Sex: not stated Ethnicity: not stated GMFCS level: level I (n = 4), level II (n = 9) and level III (n = 6) Type of motor abnormality: not stated Anatomical distribution of CP: not stated Inclusion criteria: aged 8‐18 years, diagnosis of CP, classified in GMFCS level I, II or III, attending 1 of 3 specialist schools, reliable yes/no response Exclusion criteria: surgery or botulinum toxin A to the lower‐limbs, or aerobic training, in the previous 6 months |
| Interventions |
Aim of the intervention: not stated Type of exercise programme: aerobic training Exercise mode: individualised programme that considered student activity preference Comparator: arts programme of same duration Setting: school; supervised in ratio of 1:1 or 1:2 Intervention provider: not stated Duration of programme: 9 weeks Exercise dose: 3 weekly sessions of 30 min each Tailoring of intervention to individual: individualised programme that considered student activity preference Fidelity to prescribed intervention: participants attended, on average, 79% of sessions. Across the intervention there was 80% adherence to target heart rate (target heart rate not stated) Monitoring of adverse events: 3 non‐serious adverse events in 2 participants which were expected and related to the intervention. 1 participant hit her leg on a bike and 1 participant tripped on 2 separate instances with no adverse consequences. |
| Outcomes |
Assessment time points: unclear; baseline (week 0) and week 9 Primary outcomes: feasibility and safety
Secondary outcomes: cardiovascular function and physical activity
|
| Notes |
Source of funding: Dr WM Phelps Foundation Potential conflicts of interest: authors declare no conflicts of interest. |
CP: cerebral palsy; GMFCS: Gross Motor Function Classification System.
Characteristics of ongoing studies [author‐defined order]
Gillett 2015.
| Trial name or title | FAST CP |
| Methods |
Design: randomised controlled trial Country of origin: Australia Intervention(s): mixed training (lower limb resistance exercises and functional anaerobic exercises) Unit of allocation: individual |
| Participants |
Number of participants: 40 Inclusion criteria: aged 15 to 30 years, confirmed diagnosis of spastic hemiplegia or diplegia‐type CP, able to walk independently, GMFCS level I or II, maximum passive ankle dorsiflexion range of motion of < 5° (knee fully extended) Exclusion criteria: lower limb surgery in the past 2 years and/or botulinum toxin‐A injections to the lower extremities within the past 6 months, unable to provide sufficient cooperation and cognitive understanding to participate in the intervention, participated in lower limb resistance training within the past 6 months |
| Interventions |
Aim of the intervention: to alter skeletal muscle properties and improve muscle function Type of exercise programme: mixed training (lower limb resistance exercises and functional anaerobic exercises). Exercise mode: 5 resistance exercises: seated knee calf raise, leg press, seated straight knee calf press, tibialis anterior raise, standing calf raise. Functional anaerobic exercises including step‐ups, beanbag run, lateral step‐ups, 5 m sprint, obstacle course, shuttle sprint, up and down stairs, agility run, 4 cone run Comparator: no training; allowed to continue with usual activities Setting: tertiary institution gymnasium Intervention provider: 2 experienced trainers with tertiary qualifications in the field of exercise science Duration of programme: 12 weeks Exercise dose: 3 weekly sessions of 75 min each (total dosage 45 hours) Tailoring of intervention to individual: initial 12 RM load will be determined in the first week of training for each participant. Load will be added if the participant can complete more than the required number of repetitions in all sets of that exercise (i.e. not exercising to fatigue) Fidelity to prescribed intervention and monitoring of adverse events: each participant will complete a training diary to document their progress report injuries and fatigue levels. Any injuries or adverse outcomes attributable to the intervention or testing protocol will be documented and reported. Participants will report any leg pain, lower leg stiffness and level of leg fatigue prior to each training session using a visual analogue scale (0‐100 mm). |
| Outcomes |
Assessment time points: baseline (week 0), immediately postintervention (week 12), 12 weeks postintervention (week 24) Primary outcomes:
Secondary outcomes:
|
| Starting date | 23 January 2015 |
| Contact information | Jarred Gillett; email: j.gillett1@uq.edu.au |
| Notes |
Source of funding: the National Health and Medical Research Council of Australia Postgraduate Scholarship and Australian Rotary Health/Rotary Club of St Ives Funding Partner Scholarship Potential conflicts of interest: authors declare no conflicts of interest. |
ISRCTN90378161.
| Trial name or title | Feasibility and efficacy of resistance training in cerebral palsy (CP) |
| Methods |
Design: randomised controlled trial Country of origin: UK Intervention(s): resistance training compared to usual care Unit of allocation: individual |
| Participants |
Number of participants: 60 Inclusion criteria: adolescents with CP aged 10‐19 years (amended from 12‐19 years on 12/07/2016), the ability to walk independently with or without a mobility aid, the ability to activate the ankle plantarflexors Exclusion criteria: lower limb orthopaedic surgery in the past year, botulinum toxin type A (Botox) injections or serial casting in the past 6 months, receiving intrathecal baclofen, unable to comply with the protocol |
| Interventions |
Aim of the intervention: not stated Type of exercise programme: progressive resistance training Exercise mode: single‐joint plantarflexor exercises Comparator: all participants will be instructed to continue their usual physiotherapy programme and usual activities Setting: local physiotherapy department or gym and participants' homes Intervention provider: physiotherapist Duration of programme: 10 weeks Exercise dose: 30 sessions; 10 sessions in a class and 20 sessions at home. 4 sets of 8‐12 repetitions to fatigue per session. Tailoring of intervention to individual: the intensity of the exercise will be based on individual strength capacity to ensure that the participant can perform 8 to 12 repetitions to fatigue Fidelity to prescribed intervention and monitoring of adverse events: not reported |
| Outcomes |
Assessment time points: baseline (week 0), immediately postintervention (week 10), and 12 weeks postintervention (week 22) Primary outcomes:
Secondary outcomes:
|
| Starting date | August 2015 |
| Contact information | Jennifer.Ryan@brunel.ac.uk |
| Notes |
Source of funding: Action Medical Research and Chartered Society of Physiotherapy Charitable Trust Potential conflicts of interest: not reported |
RBR‐5rh6cg.
| Trial name or title | Aquatic physical therapy in the trunk control in children with cerebral palsy: randomized clinical trial |
| Methods |
Design: randomised controlled trial Country of origin: Brazil Intervention(s): resistance training versus aerobic Unit of allocation: individual |
| Participants |
Number of participants: 24 Inclusion criteria: clinical diagnosis of spastic diplegic CP; level IV Gross Motor Function Classification System (GMFCS); aged 4 years to 10 years and 11 months Exclusion criteria: uncooperative patients; unable to understand the proposed activities; undergoing orthopedic surgery or peripheral blocks less than 6 months previously |
| Interventions |
Aim of the intervention: not stated Type of exercise programme: aquatic exercises emphasising trunk control versus conventional aquatic therapy Exercise mode: trunk control exercises Comparator: conventional aquatic therapy Setting: not reported Intervention provider: not reported Duration of programme: 8 weeks Exercise dose: 16 sessions; 35 min twice a week Tailoring of intervention to individual: not reported Fidelity to prescribed intervention and monitoring of adverse events: not reported |
| Outcomes |
Assessment time points: pre‐ and postintervention measures Primary outcomes:
Secondary outcomes:
|
| Starting date | November 2015 |
| Contact information | Mirna Sayuri Kanashiro email: mitie_kakihata@hotmail.com |
| Notes |
Source of funding: institution: Associação de Assistência à Criança Deficiente Potential conflicts of interest: not reported |
NCT02766491.
| Trial name or title | Improving Stretching Interventions for Children with CP |
| Methods |
Design: randomised controlled trial Country of origin: UK Intervention(s): resistance training (stretching and strengthening exercises for the calf muscles) Unit of allocation: individual |
| Participants |
Number of participants: 30 Inclusion criteria: diagnosis of spastic CP, GMFCS level I‐III, able to perform at least 1 bilateral heel raise, aged 7‐14 years Exclusion criteria: orthopaedic or neural surgery to the lower limb 2 years prior to or planned during the intervention, botulinum toxin A injections 6 months prior to or planned during the intervention, a learning or behaviour impairment that prevents full participation in the intervention |
| Interventions |
Aim of the intervention: to stiffen the tendon and increase the amount of stretch in the muscle Type of exercise programme: resistance training (calf muscle resistance exercises) and stretching exercises Exercise mode: heel raises and stretching exercises Comparator: conventional stretching to the calf muscle, resisted upper limb bicep curls Setting: not reported Intervention provider: not reported Duration of programme: 10 weeks Exercise dose: strengthening exercises 4 times a week for 10 weeks, stretching exercises for the final 6 weeks of the intervention Tailoring of intervention to individual: exercise load can be reduced by changing to bilateral heel raises, giving external support, reducing the range of motion or performing the heel raises while seated. Exercise load will be progressively increased by adding weight in the form of water bottles to a rucksack worn on the participant's back. Extra load will be added to biceps curls by using water bottles held in the hand Fidelity to prescribed intervention and monitoring of adverse events: not reported |
| Outcomes |
Assessment time points: baseline (week 0), immediately postintervention (week 10) Primary outcomes:
Secondary outcomes:
|
| Starting date | June 2016 |
| Contact information | Email: B.M.Kalkman@2014.ljmu.ac.uk |
| Notes |
Source of funding: not reported Potential conflicts of interest: not reported |
NCT02754128.
| Trial name or title | BeFast or BeStrong: linking neuroplasticity with the outcomes of walking‐based interventions: a feasibility trial comparing a motor learning versus a strength‐based program in children with CP |
| Methods |
Design: randomised controlled trial Country of origin: Canada Intervention(s): motor learning‐based gait‐related training intervention versus a functional lower limb strength training intervention Unit of allocation: individual |
| Participants |
Number of participants: 22 Inclusion criteria: aged 7‐17 years, diagnosis of hemiplegic or diplegic CP, GMFCS level I or II, able to follow testing and motor imagery instructions, able to actively participate in a minimum of 45 min of physical activity, show evidence of independent dorsiflexion of both ankles, able to commit to attendance of sessions 2‐3 times weekly for 6 weeks Exclusion criteria: orthopaedic surgery within the last 9 months (muscle) or 12 months (bone), botulinum toxin‐A (BTX‐A) injections to lower limb in the last 4 months, inability to put BTX‐A on hold during trial, severe spasticity (may be a contraindication for neuroimaging procedures), seizure disorder (if not fully controlled by medication for 12 months), not prepared or unable to discontinue any formal lower limb therapy intervention or physical activity programme during the trial, involved in another intervention study, standard MRI contraindications (e.g. magnetic implants, inability to lay still, claustrophobia etc.) |
| Interventions |
Aim of the intervention: to compare a motor learning‐based gait‐related training intervention to a functional lower limb strength training intervention, and to evaluate functional, neural and participation outcomes for children and young people with CP Type of exercise programme: resistance training versus aerobic training Exercise mode: motor learning (ML)‐based gait‐related training programme, and a 3‐5 min mental motor imagery script to practice on days when there are no active training sessions. Exercises are designed to improve advanced gross motor skills and athleticism Comparator: functional strength training programme designed to improve gait‐related skills and a 3‐5 min home programme of strength exercises to practice on days when there are no active training sessions Setting: not reported Intervention provider: not reported Duration of programme: 6 weeks (a maximum of 7 weeks will be permitted) Exercise dose: 45‐min training sessions 2‐3 times a week, over 6 weeks for a total of 16 active sessions (training can extend to a 7th week if necessary), plus home based training for non‐active training days. Total training will be 5 times per week. Tailoring of intervention to individual: not reported Fidelity to prescribed intervention and monitoring of adverse events: Feasibility process indicators: retention rate, perceived intervention benefit (using a combination of participant and parent ratings) retention rate (number enrolled compared to number completed). Feasibility resource indicators: adherence rate (number of completed sessions), data collection time (projected versus actual), data collection completion (% missing data) Feasiblity management indicators: intervention fidelity (within session effort scores); intervention fidelity/contamination (video sessions every 2 weeks to assess session content: STRONG group: 1 RM lower limb strength progression, FAST group: session content via Motor Learning Strategy Rating Instrument (MLSRI); intervention fidelity (completion of diaries for motor imagery/strength home practice); intervention fidelity/contamination (log books for PA‐participation tracking/management in active intervention and 4 month follow‐up); treatment administration (PTA/RKin session summary form data); acceptability of intervention (aggregate score of: parent/staff satisfaction scale, child physical activity enjoyment scale (PACES), child intervention satisfaction score) Adverse events recorded as part of the feasibility science indicators through to study completion |
| Outcomes |
Assessment time points: 7 days pre/7 days post/4‐months post‐training intervention unless otherwise stated Primary outcomes:
Secondary outcomes:
Other outcome measures
|
| Starting date | June 2016 |
| Contact information | Alicia J Hilderley email: ahilderley@hollandbloorview.ca |
| Notes |
Source of funding: not reported Potential conflicts of interest: not reported |
CP: cerebral palsy; EMG: electromyography; GMFCS: Gross Motor Function Classification System; GMFM: Gross Motor Function Measure; PA: physical activity; PTA/RKin: Physiotherapist Assistant/Registered Kinesiologist.
Differences between protocol and review
The title has been changed from 'Exercise interventions for adults and children with cerebral palsy' to 'Exercise interventions for cerebral palsy', in accordance with guidance from the Cochrane Handbook (Higgins 2011b).
We planned to include studies that used any validated scale that measured the predefined primary and secondary outcomes. However, as trials used a range of outcome measures for these outcomes, we included any measure that purported to measure them, regardless of whether or not it was validated specifically in people with CP.
Although we proposed to classify general gross motor function as unaided walking, walking with aids, or unable to walk (Beckung 2008), most studies reported the GMFCS level of participants. Therefore, we reported the GMFCS level when available and the use of mobility aids when the GMFCS level was not available.
-
We were not able to use the following methods in the review, which we archived for use in future updates (see Table 5).
We planned to present the relative risk (or risk ratio (RR)) with 95% CI and calculate the number needed to treat for an additional beneficial outcome as an absolute measure of treatment effect, where studies used dichotomous outcomes (see Measures of treatment effect and Ryan 2015). We did not, however, identify any studies that reported dichotomous outcomes. Further, we will report the odds ratio (OR) with 95% CI in updates of this review as most studies report the OR rather than the RR if the outcome is dichotomous.
We planned to combine the results of cross‐over studies with those of parallel studies by imputing the post‐treatment between‐condition correlation coefficient from an included study that presented individual participant data, and using this to calculate the standard error of the SMD, using the generic inverse‐variance method (see Unit of analysis issues and Ryan 2015).
We planned to further explore possible clinical heterogeneity through preplanned subgroup analysis based on important clinical features. We predicted that some trials would include ambulatory participants only (i.e. people who could walk with or without a mobility aid; GMFCS level I, II, and III), and some studies would include participants who could walk without a mobility aid only (i.e. GMFCS level I and II). Where adequate data allowed, we planned to undertake two subgroup analyses for studies that include ambulatory people only (i.e. GMFCS level I, II, and III) and for studies that include ambulatory people who walk without a mobility aid only (i.e. GMFCS level I and II). However, due to the small number of trials amenable to meta‐analyses, we did not conduct subgroup analysis.
We planned to explore the impact of studies at high risk of bias by reanalysis with studies rated at overall high risk of bias excluded. However, we could not do this as all studies were rated at high risk of bias.
As stated in the 'Dealing with missing data' section, we planned to include all studies in the main analysis and exclude studies that were at high risk of bias for incomplete outcome data as a sensitivity analysis (Ryan 2015). However, this was not possible because of the small number of trials in each meta‐analysis.
We planned to explore the influence of using imputed correlation coefficients in our approach to including cross‐over and cluster trials (see Unit of analysis issues) by reanalysing these data with adjusted (higher and lower) coefficient values (Ryan 2015). However, we did not identify any cluster trials or include data from cross‐over trials in any pooled analyses.
We stated in the protocol that Dr Brian Timmons would validate the final list of studies (Ryan 2015). Dr Timmons did not validate the final list of studies, so we deleted this sentence from the 'Selection of studies' section in the review.
Following identification of included studies, we noted a large overlap between the content of aerobic exercise interventions and mixed training interventions and between the content of resistance training interventions and mixed training interventions, respectively. We therefore decided to conduct a post hoc pooled analysis of aerobic exercise and mixed training versus usual care and resistance training and mixed training versus usual care.
Contributions of authors
Jennifer M Ryan: conceived and designed the review protocol; implemented the search strategy, applied eligibility criteria, assessed studies, extracted and analysed data; assessed the quality of the evidence using the GRADE approach; led the write‐up of the review; and has overall responsibility for the review.
Elizabeth E Cassidy: informed the protocol design; applied eligibility criteria, assessed studies, extracted data; assessed the quality of the evidence using the GRADE approach; and assisted with the write‐up of the review.
Stephen G Noorduyn: informed the protocol design; acted as the third reviewer; and assisted with the write‐up of the review.
Neil E O'Connell: informed the protocol design; oversaw the data synthesis; acted as the third reviewer; and assisted with the write‐up of the review
Sources of support
Internal sources
-
Brunel University London, UK.
Provided JMR and NEO'C with the time required to undertake this review
External sources
None, Other.
Declarations of interest
Jennifer M Ryan, Elizabeth E Cassidy, and Neil E O'Connell are chartered physiotherapists and lecturers in physiotherapy. As professionals who might be involved in the delivery of exercise interventions, it is plausible that they might be perceived as having a bias favouring the effectiveness of exercise.
Jennifer M Ryan is receiving funding from Action Medical Research and the Chartered Society of Physiotherapy Charitable Trust, to evaluate the feasibility, acceptability and efficacy of resistance training for adolescents with CP.
Elizabeth E Cassidy: none known.
Stephen G Noorduyn: Stephen was lead author on Noorduyn 2011, which was screened by JR and EC.
Neil E O'Connell: none known.
New
References
References to studies included in this review
Bryant 2013 {published and unpublished data}
- Bryant E, Pountney T, Williams H, Edelman N. Can a six‐week intervention improve gross motor function for ambulant children with cerebral palsy? A pilot randomized controlled trial. Clinical Rehabilitation 2013;27(2):150‐9. [DOI: 10.1177/0269215512453061; PUBMED: 22850757] [DOI] [PubMed] [Google Scholar]
Chen 2012 {published and unpublished data}
- Chen CL, Chen CY, Liaw MY, Chung CY, Wang CJ, Hong WH. Efficacy of home‐based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy. Osteoporosis International 2013;24(4):1399‐406. [DOI: 10.1007/s00198-012-2137-0; PUBMED: 23052930] [DOI] [PubMed] [Google Scholar]
- Chen CL, Hong WH, Cheng HY, Liaw MY, Chung CY, Chen CY. Muscle strength enhancements following home‐based virtual cycling training in ambulatory children with cerebral palsy. Research in Developmental Disabilities 2012;33(4):1087‐94. [DOI: 10.1016/j.ridd.2012.01.017; PUBMED: 22502833] [DOI] [PubMed] [Google Scholar]
Chrysagis 2009 {published and unpublished data}
- Chrysagis N, Douka A, Nikopoulos M, Apostolopoulou F, Koutsouki D. Effects of an aquatic program on gross motor function of children with spastic cerebral palsy. Biology of Exercise 2009;5(2):13‐25. [DOI: 10.4127/jbe.2009.0027] [DOI] [Google Scholar]
Chrysagis 2012 {published and unpublished data}
- Chrysagis N, Skordilis EK, Stavrou N, Grammatopoulou E, Koutsouki D. The effect of treadmill training on gross motor function and walking speed in ambulatory adolescents with cerebral palsy: a randomized controlled trial. American Journal of Physical Medicine & Rehabilitation 2012;91(9):747‐60. [DOI: 10.1097/PHM.0b013e3182643eba; PUBMED: 22902937] [DOI] [PubMed] [Google Scholar]
Dodd 2003 {published data only}
- Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Developmental Medicine & Child Neurology 2003;45(10):652‐7. [PUBMED: 14515935] [DOI] [PubMed] [Google Scholar]
- Dodd KJ, Taylor NF, Graham HK. Strength training can have unexpected effects on the self‐concept of children with cerebral palsy. Paediatric Physical Therapy 2004;16(2):99‐105. [DOI: 10.1097/01.PEP.0000127566.90996.50; PUBMED: 17057534] [DOI] [PubMed] [Google Scholar]
Emara 2015 {published data only}
- Emara H. Effect of a new physical therapy concept on dynamic balance in children with spastic diplegic cerebral palsy. Egyptian Journal of Medical Human Genetics 2015;16(1):77‐83. [DOI: 10.1016/j.ejmhg.2014.09.001] [DOI] [Google Scholar]
Engsberg 2006 {published and unpublished data}
- Engsberg JR, Ross SA, Collins DR. Increasing ankle strength to improve gait and function in children with cerebral palsy: a pilot study. Paediatric Physical Therapy 2006;18(4):266‐75. [DOI: 10.1097/01.pep.0000233023.33383.2b; PUBMED: 17108800] [DOI] [PubMed] [Google Scholar]
Fowler 2010 {published and unpublished data}
- DeMuth SK, Knutson LM, Fowler EG. The PEDALS stationary cycling intervention and health‐related quality of life in children with cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2012;54(7):654‐61. [DOI: 10.1111/j.1469-8749.2012.04321.x; PUBMED: 22582760] [DOI] [PubMed] [Google Scholar]
- Fowler EG, Knutson LM, DeMuth SK, Siebert KL, Simms VD, Sugi MH, et al. Pediatric endurance and limb strengthening (PEDALS) for children with cerebral palsy using stationary cycling: a randomized controlled trial. Physical Therapy 2010;90(3):367‐81. [DOI: 10.2522/ptj.20080364; PUBMED: 20093327] [DOI] [PubMed] [Google Scholar]
- Fowler EG, Knutson LM, DeMuth SK, Sugi M, Siebert K, Simms V, et al. Pediatric endurance and limb strengthening for children with cerebral palsy (PEDALS) ‐ a randomized controlled trial protocol for a stationary cycling intervention. BMC Pediatrics 2007;7:14. [DOI: 10.1186/1471-2431-7-14; PMC1838902; PUBMED: 17374171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gharib 2011 {published and unpublished data}
- Gharib NM, El‐Maksoud GM, Rezk‐Allah SS. Efficacy of gait trainer as an adjunct to traditional physical therapy on walking performance in hemiparetic cerebral palsied children: a randomized controlled trial. Clinical Rehabilitation 2011;25(10):924‐34. [DOI: 10.1177/0269215511400768; PUBMED: 21427153] [DOI] [PubMed] [Google Scholar]
Johnston 2011 {published and unpublished data}
- Gates PE, Banks D, Johnston TE, Campbell SR, Gaughan JP, Ross SA, et al. Randomized controlled trial assessing participation and quality of life in a supported speed treadmill training exercise program vs. a strengthening program for children with cerebral palsy. Journal of Pediatric Rehabilitation Medicine 2012;5(2):75‐88. [DOI: 10.3233/PRM-2012-0199; PUBMED: 22699098] [DOI] [PubMed] [Google Scholar]
- Johnston TE, Watson KE, Ross SA, Gates PE, Gaughan JP, Lauer RT, et al. Effects of a supported speed treadmill training exercise program on impairment and function for children with cerebral palsy. Developmental Medicine & Child Neurology 2011;53(8):742‐50. [DOI: 10.1111/j.1469-8749.2011.03990.x; PUBMED: 21679357] [DOI] [PubMed] [Google Scholar]
Lee 2008 {published and unpublished data}
- Lee JH, Sung IY, Yoo JY. Therapeutic effects of strengthening exercise on gait function of cerebral palsy. Disability and Rehabilitation 2008;30(19):1439‐44. [PUBMED: 19230216] [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee M, Ko Y, Shin MMS, Lee W. The effects of progressive functional training on lower limb muscle architecture and motor function in children with spastic cerebral palsy. Journal of Physical Therapy Science 2015;27(5):1581‐4. [DOI: 10.1589/jpts.27.1581; PMC4483445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2007 {published and unpublished data}
- Liao HF, Liu YC, Liu WY, Lin YT. Effectiveness of loaded sit‐to‐stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial. Archives of Physical Medicine and Rehabilitation 2007;88(1):25‐31. [DOI: 10.1016/j.apmr.2006.10.006; PUBMED: 17207671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeland 2009 {published and unpublished data}
- Maeland S, Jahnsen R, Opheim A, Froslie KF, Moe‐Nilssen R, Stanghelle JK. No effect on gait function of progressive resistance exercise in adults with cerebral palsy ‐ a single‐blind randomized controlled trial. Advances in Physiotherapy 2009;11(4):227‐33. [DOI: 10.3109/14038190902912423] [DOI] [Google Scholar]
Mattern‐Baxter 2013 {published and unpublished data}
- Mattern‐Baxter K, McNeil S, Mansoor JK. Effects of home‐based locomotor treadmill training on gross motor function in young children with cerebral palsy: a quasi‐randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(11):2061‐7. [DOI: 10.1016/j.apmr.2013.05.012; PUBMED: 23747646] [DOI] [PubMed] [Google Scholar]
McCubbin 1985 {published data only}
- McCubbin JA, Shasby GB. Effects of isokinetic exercise on adolescents with cerebral palsy. Adapted Physical Activity Quarterly 1985;2(1):56‐64. [DOI: 10.1123/apaq.2.1.56] [DOI] [Google Scholar]
Mitchell 2016 {published data only}
- James S, Ziviani J, Ware RS, Boyd RN. Randomized controlled trial of web‐based multimodal therapy for unilateral cerebral palsy to improve occupational performance. Developmental Medicine & Child Neurology 2015;57(6):530‐8. [DOI: 10.1111/dmcn.12705; PUBMED: 25955443] [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Ziviani J, Boyd RN. A randomized controlled trial of web‐based training to increase activity in children with cerebral palsy. Developmental Medicine & Child Neurology 2016;58(7):767‐73. [DOI: 10.1111/dmcn.13065; PUBMED: 26877078] [DOI] [PubMed] [Google Scholar]
Olama 2011 {published and unpublished data}
- Olama KA. Endurance exercises versus treadmill training in improving muscle strength and functional activities in hemiparetic cerebral palsy. The Egyptian Journal of Medical Human Genetics 2011;12(2):193‐9. [DOI: 10.1016/j.ejmhg.2011.07.002] [DOI] [Google Scholar]
Pandey 2011 {published and unpublished data}
- Pandey DP, Tyagi V. Effect of functional strength training on functional motor performance in young children with cerebral palsy. Indian Journal of Physiotherapy and Occupational Therapy 2011;5(1):52‐5. [Google Scholar]
Reid 2010 {published and unpublished data}
- Reid S, Hamer P, Alderson J, Lloyd D. Neuromuscular adaptations to eccentric strength training in children and adolescents with cerebral palsy. Developmental Medicine & Child Neurology 2010;52(4):358‐63. [DOI: 10.1111/j.1469-8749.2009.03409.x; PUBMED: 19737297] [DOI] [PubMed] [Google Scholar]
Scholtes 2010 {published and unpublished data}
- Scholtes VA, Becher JG, Comuth A, Dekkers H, Dijk L, Dallmeijer AJ. Effectiveness of functional progressive resistance exercise strength training on muscle strength and mobility in children with cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2010;52(6):e107‐13. [DOI: 10.1111/j.1469-8749.2009.03604.x; PUBMED: 20132136] [DOI] [PubMed] [Google Scholar]
- Scholtes VA, Becher JG, Janssen‐Potten YJ, Dekkers H, Smallenbroek L, Dallmeijer AJ. Effectiveness of functional progressive resistance exercise training on walking ability in children with cerebral palsy: a randomized controlled trial. Research in Developmental Disabilities 2012;33(1):181‐8. [DOI: 10.1016/j.ridd.2011.08.026; PUBMED: 22093663] [DOI] [PubMed] [Google Scholar]
Seniorou 2007 {published data only}
- Seniorou M, Thompson N, Harrington M, Theologis T. Recovery of muscle strength following multi‐level orthopaedic surgery in diplegic cerebral palsy. Gait & Posture 2007;26(4):475‐81. [DOI: 10.1016/j.gaitpost.2007.07.008; PUBMED: 17855096] [DOI] [PubMed] [Google Scholar]
Smania 2011 {published and unpublished data}
- Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, et al. Improved gait after repetitive locomotor training in children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2011;90(2):137‐45. [DOI: 10.1097/PHM.0b013e318201741e; PUBMED: 21217461] [DOI] [PubMed] [Google Scholar]
Taylor 2013 {published and unpublished data}
- Bania TA, Dodd KJ, Baker RJ, Graham HK, Taylor NF. The effects of progressive resistance training on daily physical activity in young people with cerebral palsy: a randomised controlled trial. Disability and Rehabilitation 2016;38(7):620‐6. [DOI: 10.3109/09638288.2015.1055376; PUBMED: 26056856] [DOI] [PubMed] [Google Scholar]
- Taylor NF, Dodd KJ, Baker RJ, Willoughby K, Thomason P, Graham HK. Progressive resistance training and mobility‐related function in young people with cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2013;55(9):806‐12. [DOI: 10.1111/dmcn.12190; PUBMED: 23789741] [DOI] [PubMed] [Google Scholar]
Tedla 2014 {published and unpublished data}
- Tedla JS. Strength training effects on balance in spastic diplegia subjects: a randomized controlled trial. Journal of Pediatric Neurology 2014;12(1):15‐28. [DOI: 10.3233/JPN-140634] [DOI] [Google Scholar]
Unger 2006 {published and unpublished data}
- Unger M, Faure M, Frieg A. Strength training in adolescent learners with cerebral palsy: a randomized controlled trial. Clinical Rehabilitation 2006;20(6):469‐77. [DOI: 10.1191/0269215506cr961oa; PUBMED: 16892929] [DOI] [PubMed] [Google Scholar]
Unnithan 2007 {published and unpublished data}
- Unnithan VB, Katsimanis G, Evangelinou C, Kosmas C, Kandrali I, Kellis E. Effect of strength and aerobic training in children with cerebral palsy. Medicine & Science in Sport & Exercise 2007;39(11):1902‐9. [DOI: 10.1249/mss.0b013e3181453694; PUBMED: 17986896] [DOI] [PubMed] [Google Scholar]
Van den Berg‐Emons 1998 {published and unpublished data}
- Berg‐Emons RJ, Baak MA, Speth L, Saris WH. Physical training of school children with spastic cerebral palsy: effects on daily activity, fat mass and fitness. International Journal of Rehabilitation 1998;21(2):179‐94. [PUBMED: 9924680] [DOI] [PubMed] [Google Scholar]
Verschuren 2007 {published and unpublished data}
- Verschuren O, Ketelaar M, Gorter JW, Helders PJ, Uiterwaal CS, Takken T. Exercise training program in children and adolescents with cerebral palsy: a randomized controlled trial. Archives of Pediatric and Adolescent Medicine 2007;161(11):1075‐81. [DOI: 10.1001/archpedi.161.11.1075; PUBMED: 17984410] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd El‐Kafy 2014 {published data only}
- Abd El‐Kafy EM, El‐Basatiny HM. Effect of postural balance training on gait parameters in children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2014;93(11):938‐47. [DOI: 10.1097/PHM.0000000000000109; PUBMED: 24901761] [DOI] [PubMed] [Google Scholar]
Ahlborg 2006 {published and unpublished data}
- Ahlborg L, Andersson C, Julin P. Whole‐body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. Journal of Rehabilitation Medicine 2006;38(5):302‐8. [DOI: 10.1080/16501970600680262; PUBMED: 16931460] [DOI] [PubMed] [Google Scholar]
AlSaif 2015 {published data only}
- AlSaif AA, Alsenany S. Effects of interactive games on motor performance in children with spastic cerebral palsy. Journal of Physical Therapy Science 2015;27(6):2001‐3. [DOI: 10.1589/jpts.27.2001; PMC4500030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angulo‐Barroso 2013 {published and unpublished data}
- Angulo‐Barroso RM, Tiernan C, Chen LC, Valentin‐Gudiol M, Ulrich D. Treadmill training in moderate risk preterm infants promotes stepping quality ‐ results of a small randomised controlled trial. Research in Developmental Disabilities 2013;34(11):3629‐38. [DOI: 10.1016/j.ridd.2013.07.037; PUBMED: 24012586] [DOI] [PubMed] [Google Scholar]
Ayhan 2014 {published and unpublished data}
- Ayhan C, Unal E, Yakut Y. Core stabilisation reduces compensatory movement patterns in patients with injury to the arm: a randomized controlled trial. Clinical Rehabilitation 2014;28(1):36‐47. [DOI: 10.1177/0269215513492443; PUBMED: 23823711] [DOI] [PubMed] [Google Scholar]
Bandholm 2012 {published and unpublished data}
- Bandholm T, Jensen BR, Nielsen LM, Rasmussen H, Bencke J, Curtis D, et al. Neurorehabilitation with versus without resistance training after botulinum toxin treatment in children with cerebral palsy: a randomized pilot study. NeuroRehabilitation 2012;30(4):277‐86. [DOI: 10.3233/NRE-2012-0756; PUBMED: 22672941] [DOI] [PubMed] [Google Scholar]
Bar‐Haim 2008 {published data only}
- Bar‐Haim S, Harries N, Belokopytov M, Lahat E, Kaplanski J. Random perturbation: a potential aid in treatment of children with cerebral palsy. Disability and Rehabilitation 2008;30(19):1420‐8. [PUBMED: 19230215] [DOI] [PubMed] [Google Scholar]
Bar‐or 1976 {published and unpublished data}
- Bar‐or O, Inbar O, Spira R. Physiological effects of a sports rehabilitation program on cerebral palsied and post‐poliomyelitic adolescents. Medicine & Science in Sports & Exercise 1976;8(3):157‐61. [PUBMED: 979561] [DOI] [PubMed] [Google Scholar]
Batista 2010 {published and unpublished data}
- Batista KG, Lopes PO, Serradilha SM, Souza GAF, Bella GP, Souza RCT. Benefits of cardiorespiratory training in children or adolescents with cerebral palsy [Benefícios do condicionamento cardiorrespiratório em crianças ou adolescentes com paralisia cerebral]. Fisioterapia em Movimento 2010;23(2):201‐9. [DOI: 10.1590/S0103-51502010000200004] [DOI] [Google Scholar]
Batra 2011 {published data only}
- Batra M, Sharma VP, Malik GK, Batra V, Agarwal GG. Intervention based on dynamics of postural control in children with cerebral palsy ‐‐ an integral approach. Indian Journal of Physiotherapy and Occupational Therapy 2011;5(3):68‐73. [Google Scholar]
Benda 2003 {published data only}
- Benda W, McGibbon NH, Grant KL. Improvements in muscle symmetry in children with cerebral palsy after equine‐assisted therapy. The Journal of Alternative and Complemenary Medicine 2003;9(6):817‐25. [DOI: 10.1089/107555303771952163; PUBMED: 14736353] [DOI] [PubMed] [Google Scholar]
Blundell 2003 {published and unpublished data}
- Blundell SW, Shepherd RB, Dean CM, Adams RD, Cahill BM. Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4‐8 years. Clinical Rehabilitation 2003;17(1):48‐57. [DOI: 10.1191/0269215503cr584oa; PUBMED: 12617379] [DOI] [PubMed] [Google Scholar]
Bohm 2015 {published and unpublished data}
- Bohm H, Rammelmayr MK, Doderlein L. Effects of climbing therapy on gait function in children and adolescents with cerebral palsy ‐ a randomized, controlled crossover trial. European Journal of Physiotherapy 2015;17(1):1‐8. [DOI: 10.3109/21679169.2014.955525] [DOI] [Google Scholar]
Boyd 2010 {published data only}
- Boyd R, Sakzewski L, Ziviani J, Abbott DF, Badawy R, Gilmore R, et al. INCITE: a randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC Neurology 2010;10(4):15. [DOI: 10.1186/1471-2377-10-4; PMC2832893; PUBMED: 20064275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown SH, Lewis CA, McCarthy JM, Doyle ST, Hurvitz EA. The effects of Internet‐based home training on upper limb function in adults with cerebral palsy. Neurorehabilitation and neural repair 2010;24(6):575‐83. [DOI: 10.1177/1545968310361956; PUBMED: 20581338] [DOI] [PubMed] [Google Scholar]
Capio 2015 {published data only}
- Capio CM, Sit CH, Eguia KF, Abernethy B, Masters RS. Fundamental movement skills training to promote physical activity in children with and without disability: a pilot study. Journal of Sport and Health Science 2015;4(3):235‐43. [DOI: 10.1016/j.jshs.2014.08.001] [DOI] [Google Scholar]
Chang 2013 {published and unpublished data}
- Chang YJ, Han WY, Tsai YC. A Kinect‐based upper limb rehabilitation system to assist people with cerebral palsy. Research in Developmental Disabilities 2013;34(11):3654‐9. [DOI: 10.1016/j.ridd.2013.08.021] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen Y, Garcia‐Vergara S, Howard AM. Effect of a home‐based virtual reality intervention for children with cerebral palsy using Super Pop VR evaluation metrics: a feasibility study. Rehabilitation Research and Practice 2015;2015:9. [DOI: 10.1155/2015/812348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherng 2004 {published data only}
- Cherng R, Liao H, Leung HW, Hwang A. The effectiveness of therapeutic horseback riding in children with spastic cerebral palsy. Adapted Physical Activity Quarterly 2004;21(2):103‐21. [DOI: 10.1123/apaq.21.2.103] [DOI] [Google Scholar]
Chiu 2014 {published and unpublished data}
- Chiu HC, Ada L, Lee HM. Upper limb training using Wii Sports Resort for children with hemiplegic cerebral palsy: a randomized, single‐blind trial. Clinical Rehabilitation 2014;28(10):1015‐24. [DOI: 10.1177/0269215514533709; PUBMED: 24849793] [DOI] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi M, Lee D, Ro H. Effect of task‐oriented training and neurodevelopmental treatment on the sitting posture in children with cerebral palsy. Journal of Physical Therapy Science 2011;23(2):323‐5. [DOI: 10.1589/jpts.23.323] [DOI] [Google Scholar]
Davis 2009 {published data only}
- Davis E, Davies B, Wolfe R, Raadsveld R, Heine B, Thomason P, et al. A randomized controlled trial of the impact of therapeutic horse riding on the quality of life, health, and function of children with cerebral palsy. Developmental Medicine & Child Neurology 2009;51(2):111‐9. [DOI: 10.1111/j.1469-8749.2008.03245.x; PUBMED: 19191844] [DOI] [PubMed] [Google Scholar]
Declerck 2016 {published data only}
- Declerck M, Verheul M, Daly D, Sanders R. Benefits and enjoyment of a swimming intervention for youth with cerebral palsy: an RCT study. Pediatric Physical Therapy 2016;28(2):162‐9. [DOI: 10.1097/PEP.0000000000000235; PUBMED: 26871379] [DOI] [PubMed] [Google Scholar]
Dimitrijević 2012 {published data only}
- Dimitrijević L, Aleksandrović M, Madić D, Okičić T, Radovanović D, Daly D. The effect of aquatic intervention on the gross motor function and aquatic skills in children with cerebral palsy. Journal of Human Kinetics 2012;32:167‐74. [DOI: 10.2478/v10078-012-0033-5; PMC3590865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2007 {published and unpublished data}
- Dodd KJ, Foley S. Partial body‐weight‐supported treadmill training can improve walking in children with cerebral palsy: a clinical controlled trial. Developmental Medicine & Child Neurology 2007;49(2):101‐5. [DOI: 10.1111/j.1469-8749.2007.00101.x; PUBMED: 17253995] [DOI] [PubMed] [Google Scholar]
El‐Basatiny 2015 {published data only}
- El‐Basatiny HMY, Abdel‐Aziem AA. Effect of backward walking training on postural balance in children with hemiparetic cerebral palsy: a randomized controlled study [with consumer summary]. Clinical Rehabilitation 2015;29(5):457‐67. [DOI] [PubMed] [Google Scholar]
Fedrizzi 2013 {published and unpublished data}
- Fedrizzi E, Rosa‐Rizzotto M, Turconi AC, Pagliano E, Fazzi E, Pozza LV, et al. Unimanual and bimanual intensive training in children with hemiplegic cerebral palsy and persistence in time of hand function improvement: 6‐month follow‐up results of a multisite clinical trial. Journal of Child Neurology 2013;28(2):161‐75. [DOI: 10.1177/0883073812443004; PUBMED: 22580904] [DOI] [PubMed] [Google Scholar]
Fernandes 2008 {published data only}
- Fernandes LC, Chitra J, Metgud D, Khatri SM. Effectiveness of artificial horse riding on postural control in spastic diplegics ‐ RCT. Indian Journal of Physiotherapy and Occupational Therapy 2008;2(4):36‐40. [Google Scholar]
Franki 2014 {published data only}
- Franki I, Broeck C, Cat J, Tijhuis W, Molenaers G, Vanderstraeten G, et al. A randomized, single‐blind cross‐over design evaluating the effectiveness of an individually defined, targeted physical therapy approach in treatment of children with cerebral palsy. Clinical Rehabilitation 2014;28(10):1039‐52. [DOI: 10.1177/0269215514544984; PUBMED: 25147350] [DOI] [PubMed] [Google Scholar]
Gillaux 2015 {published data only}
- Gillaux M, Renders A, Dispa D, Holvoet D, Sapin J, Dehez B, et al. Upper limb robot‐assisted therapy in cerebral palsy: a single‐blind randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(2):183‐92. [DOI: 10.1177/1545968314541172; PUBMED: 25015650] [DOI] [PubMed] [Google Scholar]
Gordon 2007 {published and unpublished data}
- Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand‐arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Developmental Medicine & Child Neurology 2007;49(11):830‐8. [10.1111/j.1469‐8749.2007.00830.x; PUBMED: 17979861] [DOI] [PubMed] [Google Scholar]
Grecco 2013a {published and unpublished data}
- Grecco LA, Zanon N, Sampaio LM, Oliveira CS. A comparison of treadmill training and overground walking in ambulant children with cerebral palsy: randomized controlled clinical trial. Clinical Rehabilitation 2013;27(8):686‐96. [DOI: 10.1177/0269215513476721; PUBMED: 23503736] [DOI] [PubMed] [Google Scholar]
Grecco 2013b {published and unpublished data}
- Grecco LA, Tomita SM, Christovão TL, Pasini H, Sampaio LM, Oliveira CS. Effect of treadmill gait training on static and functional balance in children with cerebral palsy: a randomized controlled trial. Brazilian Journal of Physical Therapy/Revista Brasileira de Fisioterapia 2013;17(1):17‐23. [PUBMED: 23538455] [DOI] [PubMed] [Google Scholar]
Green 2013 {published data only}
- Green D, Schertz M, Gordon AM, Moore A, Schejter Margalit T, Farquharson Y, et al. A multi‐site study of functional outcomes following a themed approach to hand‐arm bimanual intensive therapy for children with hemiplegia. Developmental Medicine & Child Neurology 2013;55(6):527‐33. [DOI: 10.1111/dmcn.12113; PUBMED: 23458353] [DOI] [PubMed] [Google Scholar]
Hamah 2015 {published data only}
- Hamah E. Effect of a new physical therapy concept on dynamic balance in children with spastic diplegic cerebral palsy. The Egyptian Journal of Medical Human Genetics 2015;16(1):77‐83. [DOI: 10.1016/j.ejmhg.2014.09.001] [DOI] [Google Scholar]
Hammond 2014 {published and unpublished data}
- Hammond J, Jones V, Hill EL, Green D, Male I. An investigation of the impact of regular use of the Wii Fit to improve motor and psychosocial outcomes in children with movement difficulties: a pilot study. Child Care Health and Development 2014;40(2):165‐75. [DOI: 10.1111/cch.12029; PUBMED: 23363371] [DOI] [PubMed] [Google Scholar]
Harbourne 2010 {published data only}
- Harbourne RT, Willett S, Kyvelidou A, Deffeyes J, Stergiou N. A comparison of interventions for children with cerebral palsy to improve sitting postural control: a clinical trial. Physical Therapy 2010;90(12):1881‐98. [DOI: 10.2522/ptj.2010132; PUBMED: 20966212] [DOI] [PubMed] [Google Scholar]
Herrero 2010 {published data only}
- Herrero P, Asensio Á, García E, Marco Á, Oliván B, Ibarz A, et al. Study of the therapeutic effects of an advanced hippotherapy simulator in children with cerebral palsy: a randomised controlled trial. BMC Musculoskeletal Disorders 2010;11:71. [DOI: 10.1186/1471-2474-11-71; PMC2864204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrero 2012 {published data only}
- Herrero P, Gómez‐Trullén EM, Asensio A, García E, Casas R, Monserrat E, et al. Study of the therapeutic effects of a hippotherapy simulator in children with cerebral palsy: a stratified single‐blind randomized controlled trial. Clinical Rehabilitation 2012;26(12):1105‐13. [DOI: 10.1177/0269215512444633; PUBMED: 22610128] [DOI] [PubMed] [Google Scholar]
Hornyak 2008 {published data only}
- Hornyak JE, Hurvitz EA. Exercise training increases physical fitness for children with cerebral palsy. The Journal of Pediatrics 2008;152(5):739. [DOI: 10.1016/j.jpeds.2008.02.022; PUBMED: 18410788] [DOI] [PubMed] [Google Scholar]
Hsieh 2016 {published data only}
- Hsieh RL, Lee WC, Lin JH. The impact of short‐term video games on performance among children with developmental delays: a randomized controlled trial. PLOS ONE 2016;11(3):e0149714. [DOI: 10.1371/journal.pone.0149714; PMC4794225; PUBMED: 26983099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hung 2011 {published data only}
- Hung YC, Casertano L, Hillman A, Gordon AM. The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia. Research in Developmental Disabilities 2011;32(6):2724‐31. [DOI: 10.1016/j.ridd.2011.05.038; PUBMED: 21715141] [DOI] [PubMed] [Google Scholar]
Hussein 2014 {published data only}
- Hussein ZA, Abd‐Elwahab MS, El‐Shennawy SW. Effect of arm cycling on gait of children with hemiplegic cerebral palsy. The Egyptian Journal of Medical Human Genetics 2014;15(3):273‐9. [DOI: 10.1016/j.ejmhg.2014.02.008] [DOI] [Google Scholar]
Hutzler 1998 {published data only}
- Hutzler Y, Chacham A, Bergman U, Szeinberg A. Effects of a movement and swimming program on vital capacity and water orientation skills of children with cerebral palsy. Developmental Medicine & Child Neurology 1998;40(3):176‐81. [PUBMED: 9566654] [DOI] [PubMed] [Google Scholar]
Hutzler 2013 {published and unpublished data}
- Hutzler Y, Lamela Rodríguez B, Mendoza LN, Díez I, Barak S. The effects of an exercise training program on hand and wrist strength, and function, and activities of daily living, in adults with severe cerebral palsy. Research in Developmental Disabilities 2013;34(12):4343‐54. [DOI: 10.1016/j.ridd.2013.09.015; PUBMED: 24145046] [DOI] [PubMed] [Google Scholar]
Jannink 2008 {published data only}
- Jannink MJ, Wilden GJ, Navis DW, Visser G, Gussinklo J, Ijzerman M. A low‐cost video game applied for training of upper extremity function in children with cerebral palsy: a pilot study. CyberPsychology and Behavior 2008;11(1):27‐32. [DOI: 10.1089/cpb.2007.0014; PUBMED: 18275309] [DOI] [PubMed] [Google Scholar]
Jeng 2013 {published and unpublished data}
- Jeng SC, Yeh KK, Liu WY, Huang WP, Chuang YF, Wong AM, et al. A physical fitness follow‐up in children with cerebral palsy receiving 12‐week individualized exercise training. Research in Developmental Disabilities 2013;34(11):4017‐24. [DOI: 10.1016/j.ridd.2013.08.032; PUBMED: 24036390] [DOI] [PubMed] [Google Scholar]
Jones 2001 {published data only}
- Jones JA, Heinemann AW. Exercise & recreation for individuals with a disability: assessment and intervention. tinyurl.com/kt7dhy8 (accessed 10 April 2017).
Kandrali 2006 {published data only}
- Kandrali I, Katsimanis G, Christoulas K, Evaggelinou C, Aggelopoulou N. The influence of an adapted exercise program on the development of the gross motor function and performance in adolescents with spastic hemiplegia. Inquiries in Sport & Physical Education 2006;4(1):45‐56. [Google Scholar]
Kang 2012 {published data only}
- Kang H, Jung J, Yu J. Effects of hippotherapy on the sitting balance of children with cerebral palsy: a randomized control trial. Journal of Physical Therapy Science 2012;24(9):833‐6. [DOI: 10.1589/jpts.24.833] [DOI] [Google Scholar]
Katz‐Leurer 2009 {published and unpublished data}
- Katz‐Leurer M, Rotem H, Keren O, Meyer S. The effects of a 'home‐based' task‐oriented exercise programme on motor and balance performance in children with spastic cerebral palsy and severe traumatic brain injury. Clinical Rehabilitation 2009;23(8):714‐24. [DOI: 10.1177/0269215509335293; PUBMED: 19506005] [DOI] [PubMed] [Google Scholar]
Ketelaar 2001 {published data only}
- Ketelaar M, Vermeer A, Hart H, Petegem‐van Beek E, Helders PJM. Effects of a functional therapy program on motor abilities of children with cerebral palsy. Physical Therapy 2001;81:1534‐45. [PUBMED: 11688590] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim D‐A, Lee J‐A, Hwang P‐W, Lee M‐J, Kim H‐K, Park J‐J, et al. The effect of comprehensive hand repetitive intensive strength training (CHRIST) using motion analysis in children with cerebral palsy. Annals of Rehabilitation Medicine 2012;36(1):39‐46. [10.5535/arm.2012.36.1.39; PMC3309323] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim JH, Seo HJ. Effects of trunk‐hip strengthening on standing in children with spastic diplegia: a comparative pilot study. Journal of Physical Therapy Science 2015;27(5):1337‐40. [DOI: 10.1589/jpts.27.1337; PMC4483392; PUBMED: 26157214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar A, Kabeer S, Aikat R, Juneja M. Effect of strength training of muscles of lower limb of young children with cerebral palsy on gross motor function. Indian Journal of Physiotherapy and Occupational Therapy 2010;4(1):4‐7. [Google Scholar]
Kumar 2013 {published data only}
- Kumar C, Kataria S. Effectiveness of task oriented circuit training on functional mobility and balance in cerebral palsy. Indian Journal of Physiotherapy or Occupational Therapy 2013;7(4):23‐28. [DOI: 10.5958/j.0973-5674.7.4.116] [DOI] [Google Scholar]
Lai 2015 {published data only}
- Lai CJ, Liu WY, Yang TF, Chen CL, Wu CY, Chan RC. Pediatric aquatic therapy on motor function and enjoyment in children diagnosed with cerebral palsy of various motor severities. Journal of Child Neurology 2015;30(2):200‐208. [DOI: 10.1177/0883073814535491; PUBMED: 24907137] [DOI] [PubMed] [Google Scholar]
Lee 2014a {published data only}
- Lee CW, Kim SG, Na SS. The effects of hippotherapy and a horse riding simulator on the balance of children with cerebral palsy. Journal of Physical Therapy Science 2014;26(3):423‐5. [DOI: 10.1589/jpts.26.423; PMC3976017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014b {published data only}
- Lee DR, Kim YH, Kim DA, Lee JA, Hwang PW, Lee MJ, et al. Innovative strength training‐induced neuroplasticity and increased muscle size and strength in children with spastic cerebral palsy: an experimenter‐blind case study‐‐three‐month follow‐up. NeuroRehabilitation 2014;35(1):131‐6. [DOI: 10.3233/NRE-131036; PUBMED: 24419014] [DOI] [PubMed] [Google Scholar]
Lowe 2015 {published data only}
- Lowe L, McMillan AG, Yates C. Body weight support treadmill training for children with developmental delay who are ambulatory. Journal of Physical Therapy Science 2015;27(4):386‐94. [DOI: 10.1097/PEP.0000000000000172; PMC4580974; PUBMED: 26397083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maher 2010 {published and unpublished data}
- Maher CA, Williams MT, Olds T, Lane AE. An internet‐based physical activity intervention for adolescents with cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2010;52(5):448‐55. [DOI: 10.1111/j.1469-8749.2009.03609.x; PUBMED: 20132138] [DOI] [PubMed] [Google Scholar]
McGibbon 2009 {published data only}
- McGibbon NH, Benda W, Duncan BR, Silkwood‐Sherer D. Immediate and long‐term effects of hippotherapy on symmetry of adductor muscle activity and functional ability in children with spastic cerebral palsy. Archives Physical Medicine and Rehabilitation 2009;90(6):966‐74. [DOI: 10.1016/j.apmr.2009.01.011; PUBMED: 19480872] [DOI] [PubMed] [Google Scholar]
Mehta 2010 {published data only}
- Mehta P, Singh J, Vij J. Efficacy of intensive neurodevelopment therapy versus conventional physiotherapy in children with spastic cerebral palsy. Indian Journal of Physiotherapy and Occupational Therapy 2010;4(4):96‐101. [Google Scholar]
Moreau 2013 {published data only}
- Moreau NG, Holthaus K, Marlow N. Differential adaptations of muscle architecture to high velocity versus traditional strength training in cerebral palsy. Neurorehabilitation and Neural Repair 2013;27(4):325‐34. [DOI: 10.1177/1545968312469834; PUBMED: 23292847] [DOI] [PubMed] [Google Scholar]
Nsenga Leunkeu 2012 {published data only}
- Nsenga Leunkeu A, Shephard RJ, Ahmaidi S. Six‐minute walk test in children with cerebral palsy gross motor function classification system levels I and II: reproducibility, validity, and training effects. Archives of Physical Medicine and Rehabilitation 2012;93(12):2333‐9. [DOI: 10.1016/j.apmr.2012.06.005; PUBMED: 22721868] [DOI] [PubMed] [Google Scholar]
Nsenga Leunkeu 2013 {published data only}
- Nsenga Leunkeu A, Shephard RJ, Ahmaidi S. Aerobic training in children with cerebral palsy. International Journal of Sports Medicine 2013;34(6):533‐7. [DOI: 10.1055/s-0032-1321803; PUBMED: 23184482] [DOI] [PubMed] [Google Scholar]
Olama 2012 {published and unpublished data}
- Olama KA, Hegazy FA, Thabt NS. Combined effects of myofeedback and isokinetic training on hand function in spastic hemiplegic children. Egyptian Journal of Medical Human Genetics 2012;13(2):183‐8. [DOI: 10.1016/j.ejmhg.2012.03.005] [DOI] [Google Scholar]
Ozer 2007 {published and unpublished data}
- Ozer D, Nalbant S, Aktop A, Duman O, Keles I, Toraman NF. Swimming training program for children with cerebral palsy: body perceptions, problem behaviour, and competence. Perceptual & Motor Skills 2007;105(3):777‐87. [DOI: 10.2466/pms.105.3.777-787; PUBMED: 18229533] [DOI] [PubMed] [Google Scholar]
Park 2014a {published data only}
- Park ES, Rha DW, Shin JS, Kim S, Jung S. Effects of hippotherapy on gross motor function and functional performance of children with cerebral palsy. Yonsei Medical Journal 2014;55(6):1736‐42. [DOI: 10.3349/ymj.2014.55.6.1736; PMC4205717; PUBMED: 25323914] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patikas 2006 {published and unpublished data}
- Patikas D, Wolf SI, Armbrust P, Mund K, Schuster W, Dreher T, et al. Effects of a postoperative resistive exercise program on the knee extension and flexion torque in children with cerebral palsy: a randomized clinical trial. Archives of Physical Medicine and Rehabilitation 2006;87(9):1161‐9. [DOI: 10.1016/j.apmr.2006.05.014; PUBMED: 16935049] [DOI] [PubMed] [Google Scholar]
- Patikas D, Wolf SI, Mund K, Armbrust P, Schuster W, Döderlein L. Effects of a postoperative strength‐training program on the walking ability of children with cerebral palsy: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87(5):619‐26. [DOI: 10.1016/j.apmr.2006.01.023; PUBMED: 16635623] [DOI] [PubMed] [Google Scholar]
Preston 2015 {published data only}
- Preston N, Weightman A, Gallagher J, Levesley M, Mon‐Williams M, Clarke M, et al. A pilot single‐blind multicentre randomized controlled trial to evaluate the potential benefits of computer‐assisted arm rehabilitation gaming technology on the arm function of children with spastic cerebral palsy. Clinical Rehabilitation 2015;30(10):1004‐15. [DOI: 10.1177/0269215515604699; PUBMED: 26370148] [DOI] [PubMed] [Google Scholar]
Reid 2006 {published data only}
- Reid D, Campbell K. The use of virtual reality with children with cerebral palsy: a pilot randomized trial. Therapeutic Recreation Journal 2006;40(4):255‐68. [Google Scholar]
Rimmer 2013 {published and unpublished data}
- Rimmer JH, Wang E, Pellegrini CA, Lullo C, Gerber BS. Telehealth weight management intervention for adults with physical disabilities. American Journal of Physical Medicine & Rehabilitation 2013;92(12):1084‐94. [DOI: 10.1097/PHM.0b013e31829e780e; PUBMED: 24257266] [DOI] [PubMed] [Google Scholar]
Roberti 2011 {published data only}
- Roberti L, Biagini E. Effectiveness of static weight‐bearing exercises in children with cerebral palsy [Efficacia degli esercizi di carico statico nei bambini affetti da paralisi cerebrale infantile]. Scienza Riabilitativa Associazione Italiana Fisioterapisti 2011; Vol. 13, issue 1:1‐14.
Sakzewski 2011a {published data only}
- Sakzewski L, Carlon S, Shields N, Ziviani J, Ware RS, Boyd RN. Impact of intensive upper limb rehabilitation on quality of life: a randomized trial in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology 2012;54(5):415‐23. [DOI: 10.1111/j.1469-8749.2012.04272.x; PUBMED: 22429002] [DOI] [PubMed] [Google Scholar]
Sakzewski 2011b {published data only}
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Equivalent retention of gains at 1 year after training with constraint‐induced or bimanual therapy in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair 2011;25(7):664‐71. [DOI: 10.1177/1545968311400093; PUBMED: 21427273] [DOI] [PubMed] [Google Scholar]
Sakzewski 2012 {published and unpublished data}
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Participation outcomes in a randomized trial of 2 models of upper‐limb rehabilitation for children with congenital hemiplegia. Archives of Physical Medicine and Rehabilitation 2011;92(4):531‐9. [DOI: 10.1016/j.apmr.2010.11.022; PUBMED: 21440700] [DOI] [PubMed] [Google Scholar]
Salem 2009 {published data only}
- Salem Y, Godwin EM. Effects of task‐oriented training on mobility function in children with cerebral palsy. Neurorehabilitation 2009;24(4):307‐13. [DOI: 10.3233/NRE-2009-0483; PUBMED: 19597267] [DOI] [PubMed] [Google Scholar]
Salem 2012 {published data only}
- Salem Y, Gropack SJ, Coffin D, Godwin EM. Effectiveness of a low‐cost virtual reality system for children with developmental delay: a preliminary randomised single‐blind controlled trial. Physiotherapy 2012;98(3):189‐95. [DOI: 10.1016/j.physio.2012.06.003; PUBMED: 22898574] [DOI] [PubMed] [Google Scholar]
Schroeder 2014 {published data only}
- Schroeder AS, Homburg M, Warken B, Auffermann H, Koerte I, Berweck S, et al. Prospective controlled cohort study to evaluate changes of function, activity and participation in patients with bilateral spastic cerebral palsy after Robot‐enhanced repetitive treadmill therapy. European Journal of Paediatric Neurology 2014;18(4):502‐10. [DOI: 10.1016/j.ejpn.2014.04.012; PUBMED: 24821475] [DOI] [PubMed] [Google Scholar]
Sherief AEAA 2015 {published data only}
- Sherief AEAA, Abo Gazya AA, Abd el Gafaar MA. Integrated effect of treadmill training combined with dynamic ankle foot orthosis on balance in children with hemiplegic cerebral palsy. Egyptian Journal of Medical Human Genetics 2015;16(2):173‐9. [DOI: 10.1016/j.ejmhg.2014.11.002] [DOI] [Google Scholar]
Silva e Borges 2011 {published data only}
- Silva e Borges MB, Werneck MJ, Silva Mde L, Gandolfi L, Pratesi R. Therapeutic effects of a horse riding simulator in children with cerebral palsy [Efeitos terapêuticos de um simulador de equitação em crianças portadoras de paralisia cerebral]. Arquivos de Neuro‐Psiquiatria 2011;69(5):799‐804. [PUBMED: 22042184] [DOI] [PubMed] [Google Scholar]
Slaman 2014 {published data only}
- Slaman J, Roebroeck M, Dallmijer A, Twisk J, Stam H, Berg‐Emons R, Learn 2 Move Research Group. Can a lifestyle intervention programme improve physical behaviour among adolescents and young adults with spastic cerebral palsy? A randomized controlled trial. Developmental Medicine & Child Neurology 2015;57(2):159‐66. [DOI: 10.1111/dmcn.12602; PUBMED: 25303096] [DOI] [PubMed] [Google Scholar]
- Slaman J, Roebroeck M, Slot W, Twisk J, Wensink A, Stam H, et al. Learn 2 Move Research Group. Can a lifestyle intervention improve physical fitness in adolescents and young adults with spastic cerebral palsy? A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2014;95(9):1646‐55. [DOI: 10.1016/j.apmr.2014.05.011; PUBMED: 25067790] [DOI] [PubMed] [Google Scholar]
- Slaman J, Berg‐Emons HJ, Meeteren J, Twisk J, Markus F, Stam HJ, et al. A lifestyle intervention improves fatigue, mental health and social support among adolescents and young adults with cerebral palsy: focus on mediating effects. Clinical Rehabilitation 2015;29(7):717‐27. [DOI: 10.1177/0269215514555136; PUBMED: 25352613] [DOI] [PubMed] [Google Scholar]
Sorsdahl 2010 {published data only}
- Sorsdahl AB, Moe‐Nilssen R, Kaale HK, Rieber J, Strand LI. Change in basic motor abilities, quality of movement and everyday activities following intensive, goal‐directed, activity‐focused physiotherapy in a group setting for children with cerebral palsy. BMC Pediatrics 2010;10:26‐36. [DOI: 10.1186/1471-2431-10-26; PMC2878295; PUBMED: 20423507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Speyer 2010 {published data only}
- Speyer E, Vuillemin A, Herbinet A, Chastagner P, Briançon S. Effect of adapted physical activity on health‐related quality of life among hospitalized children and adolescents (the ACTIV'HOP randomized controlled trial): Design and methods. Contemporary Clinical Trials 2010;31(2):165‐71. [DOI: 10.1016/j.cct.2009.12.003; PUBMED: 20004740] [DOI] [PubMed] [Google Scholar]
Stackhouse 2007 {published data only}
- Stackhouse SK, Binder‐Macleod SA, Stackhouse CA, McCarthy JJ, Prosser LA, Lee SC. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabilitation & Neural Repair 2007;21(6):475‐85. [DOI: 10.1177/1545968306298932; PMC3069852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2013 {published data only}
- Su IY, Chung KK, Chow DH. Treadmill training with partial body weight support compared with conventional gait training for low‐functioning children and adolescents with nonspastic cerebral palsy: a two‐period crossover study. Prosthetics and Orthotics International 2013;37(6):445‐53. [DOI: 10.1177/0309364613476532; PUBMED: 23436693] [DOI] [PubMed] [Google Scholar]
Swe 2015 {published data only}
- Swe NN, Sendhilnnathan S, Berg M, Barr C. Over ground walking and body weight supported walking improve mobility equally in cerebral palsy: a randomised controlled trial. Clinical Rehabilitation 2015;29(11):1108‐16. [DOI: 10.1177/0269215514566249; PUBMED: 25636992] [DOI] [PubMed] [Google Scholar]
Taylor 2009 {published data only}
- Taylor NF. Is progressive resistance exercise ineffective in increasing muscle strength in young people with cerebral palsy?. Australian Journal of Physiotherapy 2009;55(3):222. [PUBMED: 19681747] [DOI] [PubMed] [Google Scholar]
Temcharoensuk 2015 {published data only}
- Temcharoensuk P, Lekskulchai R, Akamanon C, Ritruechai P, Sutcharitpongsa S. Effect of horseback riding versus a dynamic and static horse riding simulator on sitting ability of children with cerebral palsy: a randomized controlled trial. Journal of Physical Therapy Science 2015;27(1):273‐7. [DOI: 10.1589/jpts.27.273; PMC4305581; PUBMED: 25642090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Wely 2014 {published data only}
- Wely L, Balemans AC, Becher JG, Dallmeijer AJ. Physical activity stimulation program for children with cerebral palsy did not improve physical activity: a randomised trial. Journal of Physiotherapy 2014;60(1):40‐9. [DOI: 10.1016/j.jphys.2013.12.007; PUBMED: 24856939] [DOI] [PubMed] [Google Scholar]
- Wely L, Balemans AC, Becher JG, Dallmeijer AJ. The effectiveness of a physical activity stimulation programme for children with cerebral palsy on social participation, self‐perception and quality of life: a randomized controlled trial. Clinical Rehabilitation 2014;28(10):972‐82. [DOI: 10.1177/0269215513500971; PUBMED: 24047644] [DOI] [PubMed] [Google Scholar]
Verschuren 2014 {published data only}
- Verschuren O, Darrah J, Novak I, Ketelaar M, Wiart L. Health‐enhancing physical activity in children with cerebral palsy: more of the same is not enough. Physical Therapy 2014;94(2):279‐305. [DOI: 10.2522/ptj.20130214; PUBMED: 24092902] [DOI] [PubMed] [Google Scholar]
Walsh 2014 {published data only}
- Walsh SF, Scharf MG. Effects of a recreational ice skating program on the functional mobility of a child with cerebral palsy. Physiotherapy Theory and Practice 2014;30(3):185‐95. [DOI: 10.3109/09593985.2013.863414; PUBMED: 24328904] [DOI] [PubMed] [Google Scholar]
Weindling 2007 {published data only}
- Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ. Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial. Health Technology Assessment 2007;11(16):1‐71. [PUBMED: 17462166] [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only}
- Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disability and Rehabilitation 2013;35(7):596‐605. [DOI: 10.3109/09638288.2012.711898; PUBMED: 22928803] [DOI] [PubMed] [Google Scholar]
Williams 2014 {published data only}
- Williams G, Clark RA, Hansson J, Paterson K. Feasibility of ballistic strengthening exercises in neurologic rehabilitation. American Journal of Physical Medicine & Rehabilitation 2014;93(9):828‐33. [DOI: 10.1097/PHM.0000000000000139; PUBMED: 25137195] [DOI] [PubMed] [Google Scholar]
Willoughby 2010 {published data only}
- Willoughby KL, Dodd KJ, Shields N, Foley S. Efficacy of partial body weight‐supported treadmill training compared with overground walking practice for children with cerebral palsy: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2010;91(3):333‐9. [DOI: 10.1016/j.apmr.2009.10.029; PUBMED: 20298820] [DOI] [PubMed] [Google Scholar]
Yabunaka 2011 {published data only}
- Yabunaka Y, Kondo I, Sonoda S, Saitoh E, Tsuruta Y, Konaka M, et al. Evaluating the effect of intensive intervention in children with cerebral palsy using a hypothetical matched control group. American Journal of Physical Medicine & Rehabilitation 2011;90(2):128‐36. [DOI: 10.1097/PHM.0b013e3181fc7ddf; PUBMED: 20975523] [DOI] [PubMed] [Google Scholar]
Yonetsu 2010 {published data only}
- Yonetsu R, Shimizu J, Surya J. The effect of physiotherapy on sit‐to‐stand movements in a child with spastic diplegia. Disability and Rehabilitation 2010;32(7):598‐605. [DOI: 10.3109/09638280903171501; PUBMED: 19852706] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Carlon 2014 {published data only}
- Carlon SL, Taylor NF, Shields N, Dodd KJ. Aerobic training for young people with cerebral palsy in specialist schools: a pilot randomised controlled trial. Developmental Medicine & Child Neurology 2014;56(s2):12. [Google Scholar]
References to ongoing studies
Gillett 2015 {published data only}
- Gillett JG, Lichtwark GA, Boyd RN, Barber LA. FAST CP: protocol of a randomised controlled trial of the efficacy of a 12‐week combined Functional Anaerobic and Strength Training programme on muscle properties and mechanical gait deficiencies in adolescents and young adults with spastic‐type cerebral palsy. BMJ Open 2015;5(6):e008059. [DOI: 10.1136/bmjopen-2015-008059; PMC4486965; PUBMED: 26116614] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN90378161 {published data only}
- ISRCTN90378161. Feasibility and efficacy of resistance training in adolescents with cerebral palsy [An evaluation of the feasibility, acceptability and efficacy of resistance training in adolescents with cerebral palsy: a randomised controlled trial.]. www.isrctn.com/ISRCTN90378161 (first received 26 August 2015). [DOI: 10.1186/ISRCTN90378161] [DOI]
NCT02754128 {published data only}
- NCT02754128. BeFAST or BeSTRONG: brain change after fun athletic sports‐skill training or brain change after strength training focusing on gait [Linking neuroplasticity with the outcomes of walking‐based interventions: a feasibility trial comparing a motor learning versus a strength‐based program in children with cerebral palsy]. clinicaltrials.gov/ct2/show/NCT02754128 (first received 6 April 2016).
NCT02766491 {published data only}
- NCT02766491. Improving stretching interventions for children with cerebral palsy [Improving stretching interventions for children with cerebral palsy]. clinicaltrials.gov/ct2/show/NCT02766491 (first received 19 April 2016).
RBR‐5rh6cg {published data only}
- RBR‐5rh6cg. Benefits aquatic physical therapy in the trunk control in children with cerebral palsy [Aquatic physical therapy in the trunk control in children with cerebral palsy: randomized clinical trial]. www.ensaiosclinicos.gov.br/rg/RBR‐5rh6cg (first received 2 September 2015).
Additional references
Abraha 2015
- Abraha I, Cherubini A, Cozzolino F, Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta‐epidemiological study. BMJ (Clinical research ed.) 2015;350:h2445. [PUBMED: 26016488] [DOI] [PMC free article] [PubMed] [Google Scholar]
American College of Sports Medicine 2009
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine & Science in Sports & Exercise 2009;41(3):687‐708. [PUBMED: 11828249] 11828249 [Google Scholar]
Andersen 2011
- Andersen GL, Romundstad P, Cruz J, Himmelmann K, Sellier E, Cans C, et al. Cerebral palsy among children born moderately preterm or at moderately low birthweight between 1980 and 1998: a European register‐based study. Developmental Medicine & Child Neurology 2011;53(10):913‐9. [DOI: 10.1111/j.1469-8749.2011.04079.x; PUBMED: 21838820] [DOI] [PubMed] [Google Scholar]
Anttila 2008
- Anttila H, Autti‐Rämö I, Suoranta J, Mäkelä M, Malmivaara A. Effectiveness of physical therapy interventions for children with cerebral palsy: a systematic review. BMC Pediatrics 2008;8:14. [DOI: 10.1186/1471-2431-8-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armijo‐Olivo 2015
- Armijo‐Olivo S, Saltaji H, Costa BR, Fuentes J, Ha C, Cummings GG. What is the influence of randomisation sequence generation and allocation concealment on treatment effects of physical therapy trials? A meta‐epidemiological study. BMJ Open 2015;5(9):e008562. [PUBMED: 26338841] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armijo‐Olivo 2017
- Armijo‐Olivo S, Fuentes J, Costa BR, Saltaji H, Ha C, Cummings GG. Blinding in physical therapy trials and its association with treatment effects: a meta‐epidemiological study. American Journal of Physical Medicine & Rehabilitation 2017;96(1):34‐44. [DOI: 10.1097/PHM.0000000000000521; PUBMED: 27149591] [DOI] [PubMed] [Google Scholar]
Arnould 2004
- Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND‐Kids: a measure of manual ability in children with cerebral palsy. Neurology 2004;63(6):1045‐52. [PUBMED: 15452296] [DOI] [PubMed] [Google Scholar]
Ashwal 2004
- Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004;62(6):851‐63. [PUBMED: 15037681] [DOI] [PubMed] [Google Scholar]
Beckung 2008
- Beckung E, Hagberg G, Uldall P, Cans C, Surveillance of Cerebral Palsy in Europe. Probability of walking in children with cerebral palsy in Europe. Pediatrics 2008;121(1):e187‐92. [DOI: 10.1542/peds.2007-0068; PUBMED: 18070932] [DOI] [PubMed] [Google Scholar]
Bjornson 2008
- Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin J, Thomspon EA. The relationship of physical activity to health status and quality of life in cerebral palsy. Pediatric Physical Therapy 2008;20(3):247‐53. [DOI: 10.1097/PEP.0b013e318181a959; PMC3644992; PUBMED: 18703962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornson 2014
- Bjornson KF, Zhou C, Stevenson RD, Christakis D. Relation of stride activity and participation in mobility‐based life habits among children with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2014;95(2):360‐8. [DOI: 10.1016/j.apmr.2013.10.022; PMC3946862; PUBMED: 24231402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blair 2001
- Blair E, Watson L, Badawi N, Stanley FJ. Life expectancy among people with cerebral palsy in Western Australia. Developmental Medicine & Child Neurology 2001;43(8):508‐15. [DOI: 10.1111/j.1469-8749.2001.tb00753.x] [DOI] [PubMed] [Google Scholar]
Bottos 2001
- Bottos M, Feliciangeli A, Sciuto L, Gericke C, Vianello A. Functional status of adults with cerebral palsy and implications for treatment of children. Developmental Medicine & Child Neurology 2001;43(8):516‐28. [DOI: 10.1111/j.1469-8749.2001.tb00755.x] [DOI] [PubMed] [Google Scholar]
Brooks 2014
- Brooks JC, Strauss DJ, Shavelle RM, Tran LM, Rosenbloom L, Wu YW. Recent trends in cerebral palsy survival. Part II: individual survival prognosis. Developmental Medicine & Child Neurology 2014;56(11):1065‐71. [PUBMED: 25041081] [DOI] [PubMed] [Google Scholar]
Butler 2010
- Butler JM, Scianni A, Ada L. Effect of cardiorespiratory training on aerobic fitness and carryover to activity in children with cerebral palsy: a systematic review. International Journal of Rehabilitation Research 2010;33(2):97‐103. [DOI: 10.1097/MRR.0b013e328331c555] [DOI] [PubMed] [Google Scholar]
Button 2013
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience 2013;14(5):365‐76. [PUBMED: 23571845] [DOI] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Reports 1985;100(2):126‐31. [PUBMED: 3920711] [PMC free article] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan KS, Mangione‐Smith R, Burwinkle TM, Rosen M, Varni JW. The PedsQL: reliability and validity of the short‐form generic core scales and Asthma Module. Medical Care 2005;43(3):256‐65. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Craig 2003
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12‐country reliability and validity. Medicine & Science in Sports & Exercise 2003;35(8):1381‐95. [DOI: 10.1249/01.MSS.0000078924.61453.FB; PUBMED: 12900694] [DOI] [PubMed] [Google Scholar]
Daltroy 1998
- Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The PO‐SNA pediatric musculoskeletal functional questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. Journal of Pediatric Orthopaedics 1998;18:561‐71. [DOI] [PubMed] [Google Scholar]
Day 2007
- Day SM, Wu YW, Strauss DJ, Shavelle RM, Reynolds RJ. Change in ambulatory ability of adolescents and young adults with cerebral palsy. Developmental Medicine & Child Neurology 2007;49(9):647‐53. [DOI: 10.1111/j.1469-8749.2007.00647.x; PUBMED: 17718819] [DOI] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304; PUBMED: 23616031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dodd 2002
- Dodd KJ, Taylor NF, Damiano DL. A systematic review of the effectiveness of strength‐training programs for people with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2002;83(8):1157‐64. [PUBMED: 12161840] [DOI] [PubMed] [Google Scholar]
Eek 2008
- Eek MN, Beckung E. Walking ability is related to muscle strength in children with cerebral palsy. Gait & Posture 2008;28(3):366‐71. [DOI: 10.1016/j.gaitpost.2008.05.004; PUBMED: 18595712] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith DG. Meta‐analysis: potentials and promise. BMJ 1997;315(7119):1371‐4. [PMC2127866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eliasson 2006
- Eliasson AC, Krumlinde‐Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Developmental Medicine & Child Neurology 2006;48(7):549‐54. [DOI: 10.1017/S0012162206001162; PUBMED: 16780622] [DOI] [PubMed] [Google Scholar]
Engel 2002
- Engel JM, Kartin D, Jensen MP. Pain treatment in persons with cerebral palsy: frequency and helpfulness. American Journal of Physical Medicine & Rehabilitation 2002;81(4):291‐6. [PUBMED: 11953547] [DOI] [PubMed] [Google Scholar]
Faigenbaum 2009
- Faigenbaum AD, Kraemer WJ, Blimkie CJ, Jeffreys I, Micheli LJ, Nitka M, et al. Youth resistance training: updated position statement paper from the national strength and conditioning association. Journal of Strength and Conditioning Research 2009;23(5 Suppl):S60‐79. [PUBMED: 19620931] [DOI] [PubMed] [Google Scholar]
Fougeyrollas 1998
- Fougeyrollas P, Noreau L, Bergeron H, Cloutier R, Dion SA, St‐Michel G. Social consequences of long term impairments and disabilities: a conceptual approach and assessment of handicap. International Journal of Rehabilitation Research 1998;21(2):127‐41. [PUBMED: 9924676] [DOI] [PubMed] [Google Scholar]
Garber 2011
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise 2011;43(7):1334‐59. [DOI: 10.1249/MSS.0b013e318213fefb; PUBMED: 21694556] [DOI] [PubMed] [Google Scholar]
Giné‐Garriga 2014
- Giné‐Garriga M, Roqué‐Fíguls M, Coll‐Planas L, Sitjà‐Rabert M, Salvà A. Physical exercise interventions for improving performance‐based measures of physical function in community‐dwelling, frail older adults: a systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation 2014;95(4):753‐69.e3. [DOI: 10.1016/j.apmr.2013.11.007; PUBMED: 24291597] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 10 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD; PMC2335261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6: rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI: 10.1016/j.jclinepi.2011.01.012; PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Haley 1992
- Haley SM, Coster WJ, Ludlow LH, Haltiwanger JT, Andrellos PJ. Pediatric Evaluation of Disability Inventory (PEDI). Development, Standardization and Administration Manual. Boston (MA): New England Medical Centre, 1992. [Google Scholar]
Hanna 2009
- Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Developmental Medicine & Child Neurology 2009;51(4):295‐302. [PUBMED: 19391185] [DOI] [PubMed] [Google Scholar]
Hassani 2014
- Hassani S, Krzak JJ, Johnson B, Flanagan A, Gorton G 3rd, Bagley A, et al. One‐Minute Walk and modified Timed Up and Go tests in children with cerebral palsy: performance and minimum clinically important differences. Developmental Medicine & Child Neurology 2014;56(5):482‐9. [PUBMED: 24843890] [DOI] [PubMed] [Google Scholar]
Herbison 2011
- Herbison P, Hay‐Smith J, Gillespie WJ. Different methods of allocation to groups in randomized trials are associated with different levels of bias. A meta‐epidemiological study. Journal of Clinical Epidemiology 2011;64(10):1070‐5. [DOI: 10.1016/j.jclinepi.2010.12.018; PUBMED: 21474279] [DOI] [PubMed] [Google Scholar]
Herskind 2015
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S. Chapter 4: Guide to the contents of a Cochrane protocol and review. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsh 2011
- Hirsh AT, Kratz AL, Engel JM, Jensen MP. Survey results of pain treatments in adults with cerebral palsy. American Journal of Physical Medicine & Rehabilitation 2011;90(3):207‐16. [DOI: 10.1097/PHM.0b013e3182063bc9; PMC3036542; PUBMED: 21273894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: 10.1136/bmj.g1687; PUBMED: 24609605] [DOI] [PubMed] [Google Scholar]
Holsbeeke 2009
- Holsbeeke L, Ketelaar M, Schoemaker MM, Gorter JW. Capacity, capability, and performance: different constructs or three of a kind?. Archives of Physical Medicine and Rehabilitation 2009;90(5):849‐55. [DOI: 10.1016/j.apmr.2008.11.015; PUBMED: 19406307] [DOI] [PubMed] [Google Scholar]
Jahnsen 2004
- Jahnsen R, Villien L, Egeland T, Stanghelle JK, Holm I. Locomotion skills in adults with cerebral palsy. Clinical Rehabilitation 2004;18(3):309‐16. [DOI: 10.1191/0269215504cr735oa; PUBMED: 15137562] [DOI] [PubMed] [Google Scholar]
Jeglinsky 2010
- Jeglinsky I, Surakka J, Carlberg EB, Autti‐Rämö I. Evidence on physiotherapeutic interventions for adults with cerebral palsy is sparse. A systematic review. Clinical Rehabilitation 2010;24(9):771‐88. [DOI: 10.1177/0269215510367969; PUBMED: 20605857] [DOI] [PubMed] [Google Scholar]
Kerr 2006
- Kerr C, McDowell B, Cosgrove A, Walsh D, Bradbury I, McDonough S. Electrical stimulation in cerebral palsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2006;48(11):870‐6. [DOI: 10.1017/S0012162206001915; PUBMED: 17044952] [DOI] [PubMed] [Google Scholar]
Kerr 2011
- Kerr C, McDowell BC, Parkes J, Stevenson M, Cosgrove AP. Age‐related changes in energy efficiency of gait, activity, and participation in children with cerebral palsy. Developmental Medicine & Child Neurology 2011;53(1):61‐7. [DOI: 10.1111/j.1469-8749.2010.03795.x; PUBMED: 20875041] [DOI] [PubMed] [Google Scholar]
King 2004
- King G, Law M, King S, Hurley P, Hanna S, Kertoy M, et al. Children's Assessment of Participation and Enjoyment (CAPE) and Preferences for Activities of Children (PAC). San Antonio (TX): Harcourt Assessment Inc, 2004. [Google Scholar]
Kirby 2011
- Kirby RS, Wingate MS, Naarden Braun K, Doernberg NS, Arneson CL, Benedict RE, et al. Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network. Research in Developmental Disabilities 2011;32(2):462‐9. [DOI: 10.1016/j.ridd.2010.12.042; PUBMED: 21273041] [DOI] [PubMed] [Google Scholar]
Klingels 2012
- Klingels K, Demeyere I, Jaspers E, Cock P, Molenaers G, Boyd R, et al. Upper limb impairments and their impact on activity measures in children with unilateral cerebral palsy. European Journal of Paediatric Neurology 2012;16(5):475‐84. [DOI: 10.1016/j.ejpn.2011.12.008; PUBMED: 22244966] [DOI] [PubMed] [Google Scholar]
Krumlinde‐Sundholm 2003
- Krumlinde‐Sundholm L, Eliasson A. Development of the Assisting Hand Assessment: a Rasch‐built measure intended for children with unilateral upper limb impairments. Scandinavian Journal of Occupational Therapy 2003;10(1):16‐26. [DOI: 10.1080/11038120310004529] [DOI] [Google Scholar]
Krupp 1989
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. [PUBMED: 2803071] [DOI] [PubMed] [Google Scholar]
Landgraf 1998
- Landgraf JM, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian‐French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Quality of Life Research 1998;7(5):433‐45. [PUBMED: 9691723] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2009
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002759.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maenner 2013
- Maenner MJ, Smith LE, Hong J, Makuch R, Greenberg JS, Mailick MR. Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and Health Journal 2013;6(1):8‐17. [DOI: 10.1016/j.dhjo.2012.08.005; PMC3531884; PUBMED: 23260606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maher 2016
- Maher CA, Toohey M, Ferguson M. Physical activity predicts quality of life and happiness in children and adolescents with cerebral palsy. Disability and Rehabilitation 2016;38(9):865‐9. [DOI: 10.3109/09638288.2015.1066450; PUBMED: 26218617] [DOI] [PubMed] [Google Scholar]
McCormack 1988
- McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychological Medicine 1988;18(4):1007‐19. [PUBMED: 3078045] [DOI] [PubMed] [Google Scholar]
Mockford 2008
- Mockford M, Caulton JM. Systematic review of progressive strength training in children and adolescents with cerebral palsy who are ambulatory. Pediatric Physical Therapy 2008;20(4):318‐33. [PUBMED: 19011522] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieuwenhuijsen 2011
- Nieuwenhuijsen C, Slot WM, Dallmeijer AJ, Janssens PJ, Stam HJ, Roebroeck ME, et al. Physical fitness, everyday physical activity, and fatigue in ambulatory adults with bilateral spastic cerebral palsy. Scandinavian Journal of Medicine & Science in Sports 2011;21(4):535‐42. [PUBMED: 20459469] [DOI] [PubMed] [Google Scholar]
Nooijen 2014
- Nooijen C, Slaman J, Slot W, Stam H, Roebroeck M, Berg‐Emons R. Health‐related physical fitness of ambulatory adolescents and young adults with spastic cerebral palsy. Journal of Rehabilitation Medicine 2014;46(7):642‐7. [PUBMED: 24714702] [DOI] [PubMed] [Google Scholar]
Noorduyn 2011
- Noorduyn SG, Gorter JW, Verschuren O, Timmons BW. Exercise intervention programs for children and adolescents with cerebral palsy: a descriptive review of the current research. Critical Reviews on Physical and Rehabilitation Medicine 2011;23(1‐4):31‐47. [DOI: 10.1615/CritRevPhysRehabilMed.v23.i1-4.30] [DOI] [Google Scholar]
Novak 2013
- Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Developmental Medicine & Child Neurology 2013;55(10):885‐910. [PUBMED: 23962350] [DOI] [PubMed] [Google Scholar]
Nusselder 2005
- Nusselder WJ, Looman CW, Mackenbach JP. Nondisease factors affected trajectories of disability in a prospective study. Journal of Clinical Epidemiology 2005;58(5):484‐94. [PUBMED: 15845335] [DOI] [PubMed] [Google Scholar]
Nüesch 2009
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Bürgi E, Scherer M, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta‐epidemiological study. BMJ 2009;339:b3244. [DOI: 10.1136/bmj.b3244; PUBMED: 19736281] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Callaghan 2011
- O'Callaghan ME, MacLennan AH, Gibson CS, McMichael GL, Haan EA, Broadbent JL, et al. Australian Collaborative Cerebral Palsy Research Group. Epidemiologic associations with cerebral palsy. Obstetrics & Gynecology 2011;118(3):576‐82. [DOI: 10.1097/AOG.0b013e31822ad2dc; PUBMED: 21860286] [DOI] [PubMed] [Google Scholar]
Oeffinger 2008
- Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Developmental Medicine & Child Neurology 2008;50(12):918‐25. [DOI: 10.1111/j.1469-8749.2008.03150.x; PMC2990955; PUBMED: 19046185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Opheim 2009
- Opheim A, Jahnsen R, Olsson E, Stanghelle JK. Walking function, pain, and fatigue in adults with cerebral palsy: a 7‐year follow‐up study. Developmental Medicine & Child Neurology 2009;51(5):381‐8. [DOI: 10.1111/j.1469-8749.2008.03250.x; PUBMED: 19207296] [DOI] [PubMed] [Google Scholar]
Palisano 1997
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine & Child Neurology 1997;39(4):214‐23. [PUBMED: 9183258] [DOI] [PubMed] [Google Scholar]
Palisano 2008
- Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental Medicine & Child Neurology 2008;50(10):744‐50. [DOI: 10.1111/j.1469-8749.2008.03089.x; PUBMED: 18834387] [DOI] [PubMed] [Google Scholar]
Park 2013
- Park EY, Kim WH. Structural equation modelling of motor impairment, gross motor function, and the functional outcome in children with cerebral palsy. Research in Developmental Disabilities 2013;34(5):1731‐9. [DOI: 10.1016/j.ridd.2013.02.003; PUBMED: 23500167] [DOI] [PubMed] [Google Scholar]
Park 2014b
- Park EY, Kim WH. Meta‐analysis of the effect of strengthening interventions in individuals with cerebral palsy. Research in Developmental Disabilities 2014;35(2):239‐49. [DOI: 10.1016/j.ridd.2013.10.021; PUBMED: 24291625] [DOI] [PubMed] [Google Scholar]
Pereira 2012
- Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA 2012;308(16):1676‐84. [DOI: 10.1001/jama.2012.13444; PUBMED: 23093165] [DOI] [PubMed] [Google Scholar]
Peterson 2015
- Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic conditions in adults with cerebral palsy. JAMA 2015;314(21):2303‐5. [DOI: 10.1001/jama.2015.11025; PUBMED: 26624831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Platt 2007
- Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet 2007;369(9555):43‐50. [DOI: 10.1016/S0140-6736(07)60030-0; PUBMED: 17208641] [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff L. The CES‐D scale: a new self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385‐401. [Google Scholar]
Rameckers 2014
Randall 1999
- Randall M, Johnson L, Reddihough D. The Melbourne Assessment of Unilateral Upper Limb Function Test Administration Manual. Melbourne: Royal Children's Hospital, 1999. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riad 2012
- Riad J, Modlesky CM, Gutierrez‐Farewik EM, Broström E. Are muscle volume differences related to concentric muscle work during walking in spastic hemiplegic cerebral palsy?. Clinical Orthopaedics and Related Research 2012;470(5):1278‐85. [DOI: 10.1007/s11999-011-2093-6; PMC3314764; PUBMED: 21918799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2008
- Rogers A, Furler BL, Brinks S, Darrah J. A systematic review of the effectiveness of aerobic exercise interventions for children with cerebral palsy: an AACPDM evidence report. Developmental Medicine & Child Neurology 2008;50(11):808‐14. [DOI: 10.1111/j.1469-8749.2008.03134.x] [DOI] [PubMed] [Google Scholar]
Rosenbaum 2007
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology 2007;49(Suppl s109):8‐14. [DOI: 10.1111/j.1469-8749.2007.tb12610.x] [DOI] [PubMed] [Google Scholar]
Rosenbaum 2012
- Rosenbaum P, Gorter JW. The 'F‐words' in childhood disability: I swear this is how we should think!. Child: care, health and development 2012;38(4):457‐63. [PUBMED: 22040377] [DOI] [PubMed] [Google Scholar]
Ross 2007
- Ross SA, Engsberg JR. Relationships between spasticity, strength, gait, and the GMFM‐66 in persons with spastic diplegia cerebral palsy. Archives of Physical Medicine and Rehabilitation 2007;88(9):1114‐20. [PUBMED: 17826455] [DOI] [PubMed] [Google Scholar]
Russell 1989
- Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Developmental Medicine & Child Neurology 1989;31(3):341‐52. [PUBMED: 2753238] [DOI] [PubMed] [Google Scholar]
Sandström 2004
- Sandström K, Alinder J, Oberg B. Descriptions of functioning and health and relations to a gross motor classification in adults with cerebral palsy. Disability and Rehabilitation 2004;26(17):1023‐31. [DOI: 10.1080/09638280410001703503] [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI: 10.3310/hta16350; PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Schipper 1996
- Schipper H, Clinch JJ, Olweny CL. Quality of life studies: definitions and conceptual issues. In: Spilker B editor(s). Quality of Life and Pharmoeconomics in Clinical Trials. Vol. 2, Philadelphia: Lippincott‐Raven, 1996:11‐23. [Google Scholar]
Scianni 2009
- Scianni A, Butler JM, Ada L, Teixeira‐Salmela LF. Muscle strengthening is not effective in children and adolescents with cerebral palsy: a systematic review. Australian Journal of Physiotherapy 2009;55(2):81‐7. [PUBMED: 19463078] [DOI] [PubMed] [Google Scholar]
SCOPE 2002
- SCOPE. Prevalence and characteristics of children with cerebral palsy in Europe. Developmental Medicine & Child Neurology 2002;44(9):633‐40. [PUBMED: 12227618] [PubMed] [Google Scholar]
Sellier 2010
- Sellier E, Surman G, Himmelmann K, Andersen G, Colver A, Krageloh‐Mann I, et al. Trends in prevalence of cerebral palsy in children born with a birthweight of 2,500 g or over in Europe from 1980 to 1998. European Journal of Epidemiology 2010;25(9):635‐42. [DOI: 10.1007/s10654-010-9474-0; PUBMED: 20532622] [DOI] [PubMed] [Google Scholar]
Shortland 2009
- Shortland A. Muscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders?. Developmental Medicine & Child Neurology 2009;51(Suppl 4):59‐63. [DOI: 10.1111/j.1469-8749.2009.03434.x; PUBMED: 19740211] [DOI] [PubMed] [Google Scholar]
Solans 2008
- Solans M, Pane S, Estrada MD, Serra‐Sutton V, Berra S, Herdman M, et al. Health‐related quality of life measurement in children and adolescents: a systematic review of generic and disease‐specific instruments. Value in Health 2008;11(4):742‐64. [DOI: 10.1111/j.1524-4733.2007.00293.x; PUBMED: 18179668] [DOI] [PubMed] [Google Scholar]
Soyupek 2010
- Soyupek F, Aktepe E, Savas S, Askin A. Do the self‐concept and quality of life decrease in CP patients? Focussing on the predictors of self‐concept and quality of life. Disability and Rehabilitation 2010;32(13):1109‐15. [DOI: 10.3109/09638280903391120; PUBMED: 20131943] [DOI] [PubMed] [Google Scholar]
Strauss 1998a
- Strauss DJ, Shavelle RM, Anderson TW. Life expectancy of children with cerebral palsy. Pediatric Neurology 1998;18(2):143‐9. [PUBMED: 9535300] [DOI] [PubMed] [Google Scholar]
Strauss 1998b
- Strauss D, Shavelle R. Life expectancy of adults with cerebral palsy. Developmental Medicine & Child Neurology 1998;40(6):369‐75. [DOI: 10.1111/j.1469-8749.1998.tb08211.x] [DOI] [PubMed] [Google Scholar]
Taylor 2005
- Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Physical Therapy 2005;85(11):1208‐23. [PUBMED: 16253049] [PubMed] [Google Scholar]
Tollånes 2014
- Tollånes MC, Wilcox AJ, Lie RT, Moster D. Familial risk of cerebral palsy: population based cohort study. BMJ 2014;349:g4294. [DOI: 10.1136/bmj.g4294; PUBMED: 25028249] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trønnes 2014
- Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Developmental Medicine & Child Neurology 2014;56(8):779‐85. [DOI: 10.1111/dmcn.12430; PMC4107088; PUBMED: 24621110] [DOI] [PMC free article] [PubMed] [Google Scholar]
USDHHS 2008
- U.S. Department of Health and Human Services. Physical activity guidelines for Americans. www.health.gov/paguidelines (accessed 31 January 2014).
Van der Linden 2003
- Linden ML, Hazlewood ME, Aitchison AM, Hillman SJ, Robb JE. Electrical stimulation of gluteus maximus in children with cerebral palsy: effects on gait characteristics and muscle strength. Developmental Medicine & Child Neurology 2003;45(6):385‐90. [PUBMED: 12785439] [DOI] [PubMed] [Google Scholar]
Van Der Slot 2012
- Slot WM, Nieuwenhuijsen C, Berg‐Emons RJ, Bergen MP, Hilberink SR, Stam HJ, et al. Chronic pain, fatigue, and depressive symptoms in adults with spastic bilateral cerebral palsy. Developmental Medicine & Child Neurology 2012;54(9):836‐42. [PUBMED: 22809436] [DOI] [PubMed] [Google Scholar]
Vargus‐Adams 2011
- Vargus‐Adams JN, Martin LK. Domains of importance for parents, medical professionals and youth with cerebral palsy considering treatment outcomes. Child: Care, Health and Development 2011;37(2):276‐81. [DOI: 10.1111/j.1365-2214.2010.01121.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhoef 2014
- Verhoef JA, Bramsen I, Miedema HS, Stam HJ, Roebroeck ME. Development of work participation in young adults with cerebral palsy: a longitudinal study. Journal of Rehabilitation Medicine 2014;46(7):648‐55. [PUBMED: 24858956] [DOI] [PubMed] [Google Scholar]
Verschuren 2006
- Verschuren O, Takken T, Ketelaar M, Gorter JW, Helders PJ. Reliability and validity of data for 2 newly developed shuttle run tests in children with cerebral palsy. Physical Therapy 2006;86(8):1107‐17. [PUBMED: 16879044] [PubMed] [Google Scholar]
Verschuren 2008
- Verschuren O, Ketelaar M, Takken T, Helders PJ, Gorter JW. Exercise programs for children with cerebral palsy: a systematic review of the literature. American Journal of Physical Medicine & Rehabilitation 2008;87(5):404‐17. [PUBMED: 17993987] [DOI] [PubMed] [Google Scholar]
Verschuren 2009
- Verschuren O, Ketelaar M, Gorter JW, Helders PJ, Takken T. Relation between physical fitness and gross motor capacity in children and adolescents with cerebral palsy. Developmental Medicine & Child Neurology 2009;51(11):866‐71. [DOI: 10.1111/j.1469-8749.2009.03301.x] [DOI] [PubMed] [Google Scholar]
Verschuren 2010
- Verschuren O, Takken T. Aerobic capacity in children and adolescents with cerebral palsy. Research in Developmental Disabilities 2010;31(6):1352‐7. [PUBMED: 20674266] [DOI] [PubMed] [Google Scholar]
Verschuren 2012
- Verschuren O, Wiart L, Hermans D, Ketelaar M. Identification of facilitators and barriers to physical activity in children and adolescents with cerebral palsy. The Journal of Pediatrics 2012;161(3):488‐94. [DOI: 10.1016/j.jpeds.2012.02.042; PUBMED: 22494875] [DOI] [PubMed] [Google Scholar]
Verschuren 2016
- Verschuren O, Peterson MD, Balemans AC, Hurvitz EA. Exercise and physical activity recommendations for people with cerebral palsy. Developmental Medicine & Child Neurology 2016;58(8):798‐808. [PUBMED: 26853808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogels 2000
- Vogels T, Verrips GHW, Koopman HM, Theunissen NCM, Fekkes M, Kamphuis RP. TACQOL Manual. Parent Form and Child Form. Leiden: Leiden Center for Child Health and Pediatrics, 2000. [Google Scholar]
Voorman 2007
- Voorman JM, Dallmeijer AJ, Knol DL, Lankhorst GJ, Becher JG. Prospective longitudinal study of gross motor function in children with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2007;88(7):871‐6. [PUBMED: 17601467] [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kilinski M, Gandeck B. SF‐36 Health Survey Manual and Interpretation Guide. Boston (MA): The Health Institute, New England Medical Center, 1993. [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
WHO 2013
- WHO. Definition of key terms. www.who.int/hiv/pub/guidelines/arv2013/intro/keyterms/en/ (accessed 30 May 2017). [http://www.who.int/hiv/pub/guidelines/arv2013/intro/keyterms/en/]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2004
- Wu YW, Day SM, Strauss DJ, Shavelle RM. Prognosis for ambulation in cerebral palsy: a population‐based study. Pediatrics 2004;114(5):1264‐71. [PUBMED: 15520106] [DOI] [PubMed] [Google Scholar]
Young 2000
- Young NL, Williams JI, Yoshida KK, Wright JG. Measurement properties of the activities scale for kids. Journal of Clinical Epidemiology 2000;53(2):125‐37. [PUBMED: 10729684] [DOI] [PubMed] [Google Scholar]
Østensjø 2004
- Østensjø S, Carlberg EB, Vøllestad NK. Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Developmental Medicine & Child Neurology 2004;46(9):580‐9. [DOI: 10.1111/j.1469-8749.2004.tb01021.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ryan 2015
- Ryan JM, Cassidy EE, Noorduyn SG, O'Connell NE. Exercise interventions for adults and children with cerebral palsy. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011660] [DOI] [PMC free article] [PubMed] [Google Scholar]


