Abstract
Background
Anaemia is a common complication in people with chronic kidney disease (CKD) and mainly develops as a consequence of relative erythropoietin (EPO) deficiency. Anaemia develops early in the course of disease and peaks among people with end‐stage kidney disease (ESKD). Many types of EPO ‐ also called erythropoiesis‐stimulating agents (ESAs) ‐ are used to treat anaemia in people with ESKD.
ESAs have changed treatment of severe anaemia among people with CKD by relieving symptoms and avoiding complications associated with blood transfusion. However, no benefits have been found in relation to mortality rates and non‐cardiac fatal events, except quality of life. Moreover, a relationship between ESA use and increased cardiovascular morbidity and mortality in patients with CKD has been reported in studies with fully correcting anaemia comparing with partial anaemia correction. Until 2012, guidelines recommended commencing ESA treatment when haemoglobin was less than 11 g/dL; the current recommendation is EPO commencement when haemoglobin is between 9 and 10 g/dL. However, advantages in commencing therapy when haemoglobin levels are greater than 10 g/dL but less than 11 g/dL remain unknown, especially among older people whose life expectancy is limited, but in whom EPO therapy may improve quality of life.
Objectives
To assess the clinical benefits and harms of early versus delayed EPO for anaemia in patients with ESKD undergoing haemodialysis or peritoneal dialysis
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 22 May 2017 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
We planned to include randomised controlled trials (RCTs) and quasi‐RCTs evaluating at the clinical benefits and harms of early versus delayed EPO for anaemia in patients with ESKD undergoing haemodialysis or peritoneal dialysis. Studies comparing EPO with another EPO, placebo or no treatment were eligible for inclusion.
Data collection and analysis
It was planned that two authors would independently extract data from included studies and assess risk of bias using the Cochrane risk of bias tool. For dichotomous outcomes (all‐cause mortality, cardiovascular mortality, overall myocardial infarction, overall stroke, vascular access thrombosis, adverse effects of treatment, transfusion), we planned to use the risk ratio (RR) with 95% confidence intervals (CI). We planned to calculate the mean difference (MD) and CI 95% for continuous data (haemoglobin level) and the standardised mean difference (SMD) with CI 95% for quality of life if different scales had been used.
Main results
Literature searches yielded 1910 records, of these 1534 were screened after duplicates removed, of which 1376 were excluded following title and abstract assessment. We assessed 158 full text records and identified 18 studies (66 records) that were potentially eligible for inclusion. However, none matched our inclusion criteria and were excluded.
Authors' conclusions
We found no evidence to assess the benefits and harms of early versus delayed EPO for the anaemia of ESKD.
Plain language summary
Early versus delayed erythropoietin for the anaemia of end‐stage kidney disease
Anaemia (low haemoglobin) is a common complication among people with end‐stage kidney disease (ESKD) receiving dialysis treatment. Dialysis treatment removes toxic waste products from the blood when kidneys no longer function. Anaemia treatment is based on the use of manufactured erythropoietin (EPO, a hormone that increases haemoglobin), to improve fatigue and breathlessness which are common symptoms of severe anaemia. It is widely accepted that EPO treatment should be initiated when haemoglobin levels are less than or equal to 10 g/dL (delayed onset). However, it remains unknown if there are clinical benefits or harms when EPO treatment is commenced when haemoglobin levels are greater than 10 g/dL but less than 11 g/dL (early onset).
We conducted this review to try to determine if there are clinical benefits and harms associated with early versus delayed EPO.
We searched the literature to 8 July 2015 but found no studies that investigated early versus delayed EPO for ESKD‐related anaemia. Benefits and harms of early versus delayed EPO remain unknown.
Background
Description of the condition
Anaemia has been defined by the World Health Organization (WHO) as a haemoglobin concentration less than 13.0 g/dL and 12.0 g/dL respectively for males and non‐pregnant females aged 15 years or over (WHO 2008). This definition is widely accepted for people with anaemia caused by CKD (KDIGO 2012).
Anaemia is a common complication in people with CKD and develops early in the course of the disease. Anaemia increases in frequency with a corresponding decline in kidney function, and peaks in incidence among people with ESKD (Astor 2002). Kidney‐related anaemia develops mainly as a consequence of relative EPO deficiency in relation to haemoglobin levels; EPO levels are 10 to 100 times higher among anaemic patients with normal kidney function (Artunc 2007; Ly 2004; McGonigle 1984).
Description of the intervention
EPO is an essential growth factor for the recruitment, differentiation, maintenance and survival of erythroid progenitor cells. EPO is produced by hepatocytes in the foetal stage, and after birth is synthesised mainly by the kidneys in response to hypoxia (Glaspy 2009; Jelkmann 2011). After cloning the EPO gene in 1983, recombinant human technology enabled development and production of the first erythropoietin ‐ epoetin‐α ‐ which was approved for clinical use in 1989 (Eschbach 1988; Eschbach 1989). Since then, many EPO types ‐ also called erythropoiesis‐stimulating agents (ESAs) ‐ have been produced. According to their action time, they are classified as short‐acting and long‐acting (Horl 2013).
Short‐acting ESAs have a half‐life from six to eight hours intravenously and from 19 to 24 hours subcutaneously; most are administered two to three times weekly (Halstenson 1991). Short‐acting ESAs are more effective when administered subcutaneously. Alfa and beta are the most widely used short‐acting ESAs. Epoetin‐α biosimilars are used in Europe (Schellekens 2008).
Long‐acting ESAs have improved pharmacokinetic and pharmacodynamic characteristics. Dose requirements do not differ according to the route of administration. The combination of a significantly increased half‐life and lower binding affinity for the EPO receptor explains why long‐acting ESAs stimulate erythropoiesis for longer periods. Long‐acting ESAs used for the treatment of kidney‐related anaemia are darbepoetin‐α and the continuous EPO receptor activator (CERA). One darbepoetin dose is given every one or two weeks (Macdougall 1999; Padhi 2006) and CERA is administered biweekly or monthly (Macdougall 2006).
EPO therapy improves cognitive function and quality of life (Astor 2002; Drueke 2006; Pfeffer 2009; Ross 2002) and helps regression of left ventricular hypertrophy (Levin 2002; Parfrey 2009). However, EPO is not free of complications which are mainly hypertensive reactions, thrombosis of arteriovenous fistula in patients on haemodialysis, increased risk of stroke and faster tumour growth; appearance of severe anaemia as part of pure red cell aplasia, and seizures (Del Vecchio 2010; Rizzo 2010; Zhu 2006).
How the intervention might work
EPO acts as an essential growth factor. It regulates erythropoiesis, maintaining the survival of erythroid progenitors, stimulating their proliferation and differentiation in the bone marrow (Jelkmann 2011). EPO production is markedly up‐regulated by hypoxia via a negative feedback loop; hypoxia induces an increase in EPO hormone production in the kidneys, increasing the mass of circulating red blood cells, thereby increasing the oxygen‐carrying capacity of blood and suppressing further expression of EPO (Bunn 2013). Specifically, EPO binds to the EPO receptor through the high affinity isoform EPO, which is responsible for the erythropoietic effects by activation of several pathways (hypoxia‐inducible factor 1, 2 and 3, Janus kinase‐2, phosphatidylinositol 3‐kinase, protein kinase C, anti‐apoptotic protein) stimulating differentiation of erythroid precursor cells and inhibiting apoptosis of erythroid progenitors (Elliott 2008; Sinclair 2013).
Recombinant human EPO and human EPO have similar biological activity. Increase in red blood cell mass is dependent on the exposure time of the level of EPO; therefore, subcutaneous administration of short‐acting ESAs is more effective (Kaufman 1998). The response to administration of EPO may vary from patient to patient. The dose should be adjusted to reach a haemoglobin monthly increase between 1 and 2 g/dL. If the increase is less than expected, the dose is increased by 25%; if higher, it is decreased by 25%. After reaching the target haemoglobin level, the maintenance dose of EPO is adjusted according to monthly haemoglobin readings (Del Vecchio 2010; KDOQI 2006; KDIGO 2012).
Why it is important to do this review
The importance of this systematic review is based on the following premises.
Many of the clinical manifestations of CKD may be epiphenomena of anaemia, which is associated with the worsening of cognitive functions, exercise capacity, mental acuity, quality of life; depression and fatigue (Finkelstein 2009; Gerson 2004; Weisbord 2008). Increased risk of cardiovascular morbidity and mortality may also be present (Astor 2006; Glassock 2009; Locatelli 2004).
ESAs have changed treatment of kidney‐related anaemia by improving the signs and symptoms of severe anaemia, avoiding the complications of iron overload, transmission of viral diseases and sensitisation for future kidney transplants (Del Vecchio 2010). However, no benefits have been found in RCTs and meta‐analyses when correcting anaemia with regard to patients’ mortality and non‐cardiac fatal events, except for quality of life (Drueke 2006; Johansen 2010; Johansen 2012; Pfeffer 2009). Notwithstanding, a relationship between the use of ESAs and an increased cardiovascular morbidity and mortality in patients with CKD has been reported in studies with fully correcting anaemia comparing with partial anaemia correction (Palmer 2010; Phrommintikul 2007; Singh 2006). Patients with cancer also experience increased cardiovascular morbidity and mortality associated with ESA use (Rizzo 2010; Tonia 2012).
From 2000 to 2009 clinical guidelines from the United States and Europe recommended commencing ESA treatment when haemoglobin was less than 11 g/dL (EBPG 2004; ERBP 2009; ERBP 2010; KDOQI 2000; KDOQI 2006; KDOQI 2007; KDIGO 2008). The 2012 Anaemia Work Group of Kidney Disease Improving Global Outcomes (KDIGO) guidelines suggested starting treatment in dialysis patients when haemoglobin is between 9 to 10 g/dL (Grade 2B), and in some cases, starting treatment when haemoglobin is greater than 10 g/dL (not graded), primarily in older people among whom life expectancy is limited, being more relevant to improve quality of life (KDIGO 2012). The European Renal Best Practice (ERBP) Advisory Board and Canadian Society of Nephrology guidelines agree with KDIGO about whether and when to start ESA therapy in dialysis patients (ERBP 2013; Moist 2013).
Several systematic reviews have investigated ESA treatment for anaemia in patients with CKD and ESKD evaluating haemoglobin level, energy and physical function, fatigue, left ventricular mass index and mortality (Johansen 2010; Johansen 2012; Palmer 2010; Parfrey 2009; Vinhas 2012), but to date, a systematic review on the benefits and harms of early (haemoglobin < 11 g/dL but > 10 g/dL) versus delayed (haemoglobin ≤ 10 g/dL) ESA treatment for anaemia in dialysis patients with ESKD has not been published.
Objectives
To assess the clinical benefits and harms of early versus delayed EPO for anaemia in patients with ESKD undergoing haemodialysis or peritoneal dialysis.
Methods
Criteria for considering studies for this review
Types of studies
This review included all RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, the use of alternate medical records, date of birth or other predictable methods) looking at EPO in people with ESKD on dialysis with anaemia. Studies were considered without language restriction. Studies of at least 12 weeks follow‐up were to be included.
Types of participants
Inclusion criteria
Dialysis patients with anaemia due to ESKD irrespective of gender or age were eligible for inclusion. We planned to accept any definition of anaemia provided in individual studies.
Exclusion criteria
We excluded studies involving patients with functional or absolute iron deficiency.
Types of interventions
This review included studies of early (haemoglobin between 10 and 11 g/dL) versus delayed (haemoglobin ≤ 10 g/dL) treatment with any EPO or EPO against placebo/no treatment, by any route (subcutaneous or intravenous) or dose. The following comparisons were considered for inclusion.
EPO versus placebo/no treatment
EPO versus EPO
Types of outcome measures
We planned to evaluate all‐cause mortality and cardiovascular mortality according to end‐of‐study reports. We also planned to assess outcomes on mortality at the short (< 6 months), medium (from 6 to 12 months) and long term (> 12 months). We planned to evaluate numbers of adverse and cardiovascular events according to their occurrence during study follow‐up.
Primary outcomes
All‐cause mortality
Cardiovascular mortality
Quality of life (end of treatment scores obtained using validated tools such as the Kidney Disease Quality of Life tool or others as mentioned in the studies).
Secondary outcomes
Adverse events: hypertension (one or more hypertensive events requiring additional antihypertensive medication or as defined by the investigators); seizure ≥ 1 event)
Myocardial infarction (fatal or non‐fatal)
Stroke (ischaemic or haemorrhagic, either fatal or non‐fatal)
Thrombotic events (deep venous thrombosis, peripheral arterial thrombotic events, and dialysis vascular access thrombosis)
Blood transfusions requirements (number of individuals requiring one or more packed red blood cell transfusion)
Haemoglobin level reached at end of study.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 22 May 2017 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
Titles and abstracts were screened independently by two authors who discarded studies that were clearly not eligible and assessed the full text of potentially eligible studies to determine which satisfied inclusion criteria. Disagreements were to be resolved through discussion, with a third author if necessary.
Data extraction and management
Data extraction was to be carried out independently by two authors using standard forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were to be grouped together and the publication with the most complete data used in the analyses. Where relevant outcomes were only published in earlier versions these data were to be used. Any discrepancies between published versions were to be highlighted.
Assessment of risk of bias in included studies
We planned to assess risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011) (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all‐cause mortality, cardiovascular mortality, overall myocardial infarction, overall stroke, vascular access thrombosis, adverse effects of treatment, transfusion), we planned to use the risk ratio (RR) with 95% confidence intervals (CI). We planned to calculate the mean difference (MD) and CI 95% for continuous data (haemoglobin level) and the standardised mean difference (SMD) with CI 95% for quality of life if different scales had been used.
Dealing with missing data
We planned to request any further information required from the original author in writing and to include any relevant information obtained in the review.
Assessment of heterogeneity
We planned to analyse heterogeneity using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and the I² statistic (Higgins 2003).
Assessment of reporting biases
We planned to construct funnel plots to provide visual assessment of whether treatment estimates were associated with study size.
Data synthesis
We planned to pool data using the random‐effects model and to use the fixed‐effect model to ensure the robustness of the model chosen and the susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age and dialysis modality. Heterogeneity in treatments could be related to dose, type (short versus long acting) and duration of ESA treatment. Quality of life parameters were to be assessed based on the Kidney Disease Quality of Life tool, or as reported in studies (Hays 1997). We planned to perform the following subgroup analyses:
Patients on haemodialysis versus peritoneal dialysis
Paediatric versus adult participants
Use of EPO higher doses versus lower dose
EPO short‐acting versus long‐acting
Use the Kidney Disease Quality of Life tool versus others as mentioned in the studies.
Sensitivity analysis
We planned to perform sensitivity analysis to explore the influence of the following factors on the effect on size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking into account risk of bias
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding quasi‐RCTs.
'Summary of findings' tables
We were not able to assess quality evidence associated with primary outcomes (all‐cause mortality, cardiovascular mortality, quality of life, cardiovascular events, adverse events, haemoglobin level reached at the end of the study, and blood transfusions requirements) with the GRADE system (Guyatt 2008).
Results
Description of studies
Results of the search
We searched the literature to 8 July 2015 and identified 1910 potentially relevant records (Figure 1). We excluded duplicate records, and assessed titles and abstracts of 1534 records. Of these, 1376 were excluded (not RCT or quasi‐RCT; wrong population; wrong intervention). We obtained the full text of 158 papers for assessment, and excluded 92. We identified 18 potential studies (66 records) none of which met our inclusion criteria.
1.
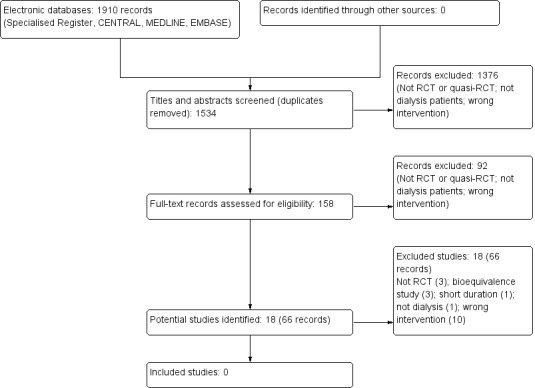
Study flow diagram
Excluded studies
We excluded 18 studies (66 records)
Three studies were not randomised (Besarab 1998; Linde 2001; Tareeva 1992)
Three were bioequivalence studies (Krivoshiev 2008; Krivoshiev 2010; Wizemann 2008).
Suzuki 1989 was short duration (8 weeks)
Teehan 1990 studied pre‐dialysis patients
Ten studies did not compare early versus delayed EPO for the anaemia of ESKD (Bahlmann 1991; Bennett 1991; Canadian EPO Study 1990; Foley 2000; Morris 1993; Muirhead 1992; Nissenson 1995; Parfrey 2005; Park 2014; Trembecki 1995a).
See Characteristics of excluded studies.
Risk of bias in included studies
Risk of bias assessment could not be conducted.
Effects of interventions
No studies met our inclusion criteria.
Discussion
Summary of main results
This Cochrane Review identified no studies assessing benefits or harms of early or delayed EPO to treat anaemia in patients on dialysis. Early versus delayed EPO for the treatment of anaemia among people with ESKD has unknown either benefits or harms.
Potential biases in the review process
We have been unable to identify evidence from RCTs supporting the use of early or delayed erythropoietin for treating anaemia of ESKD. The main limitation of this Cochrane Review is the paucity of evidence of the use of early or delayed erythropoietin for treating anaemia of ESKD.
Agreements and disagreements with other studies or reviews
We found no studies assessing early versus delayed EPO for anaemia associated with ESKD.
Authors' conclusions
Implications for practice.
We found no studies assessing early versus delayed EPO for ESKD‐related anaemia. Benefits and harms remain unknown.
Implications for research.
This Cochrane Review has highlighted a need for well‐designed, high‐quality RCTs to assess the benefits and harms of early versus delayed erythropoietin for the anaemia of end‐stage kidney disease. The potential study should include main clinical outcomes (patients‐oriented outcomes) such as all‐cause mortality, cardiovascular mortality, quality of life, adverse events and cardiovascular events according to their occurrence during study follow‐up.
The study should be reported according to the Consolidated standards of reporting trials (CONSORT) statement for improving the quality of reporting of efficacy and to get better reports of harms in clinical research (Ioannidis 2004; Moher 2010; Turner 2012). Future studies should be planned according to the recommendations of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Chan 2013a; Chan 2013b) and the Foundation of Patient‐Centered Outcomes Research (Gabriel 2012; PCORI 2012).
Future studies should be conducted by independent researchers and reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Ioannidis 2004; Moher 2010) and using the Foundation of Patient‐Centered Outcomes Research recommendations (Gabriel 2012; PCORI 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 1 June 2017 | Review declared as stable | New search undertaken in May 2017 ‐ no studies identified |
Notes
New search undertaken in May 2017 ‐ no studies identified.
Acknowledgements
We acknowledge the assistance of the Cochrane Kidney and Transplant and the Iberoamerican Cochrane Centre during the development of this review. We would also like to thank the referees for their kind feedback and advice during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Glossary
Epoetin Biosimilars: a biological medicinal product referring to an existing one and submitted to regulatory authorities for marketing authorization by an independent applicant after the existing agents' protection expires. Biosimilars can resemble the agents on which they are modelled but cannot fully copy their properties (Horl 2013; Schellekens 2008).
Erythropoiesis‐stimulating agents: an agent similar to the cytokine (erythropoietin) that stimulates red blood cell production, commonly abbreviated ESAs (Horl 2013).
Erythropoietin high dose: subcutaneous doses > 120 UI/kg/wk, or intravenous doses > 240 UI/kg/wk of epoetin alfa or beta or biosimilars; darbepoetin alfa corrected weekly ESAs doses can be calculated multiplying by 200; and the continuous erythropoietin receptor activator can be calculated as per the pharmacological equivalence (Kainz 2010; Tolman 2005).
European Best Practice Guidelines (EBPG): The first EBPG for the Management of Anaemia in Patients with Chronic Renal Failure were created to fulfil the need for recommendations that reflected current European clinical practice and experience. They were presented at the ERA‐EDTA Congress in Madrid in 1999. They were prompted from the need to analyse the huge volume of published data on anaemia management (Zoccali 2008).
European Renal Best Practice (ERBP): The ERA‐EDTA in 2008 opted to change the name EBPG to European Renal Best Practice (ERBP) as a means of acknowledging that, especially in nephrology, it is difficult to generate real ‘guidelines’ because of the lack of sufficient evidence (Zoccali 2008).
KDIGO: Kidney Disease Improving Global Outcomes (KDIGO) was established in 2003 as an independently incorporated non‐profit foundation governed by an international Board. The Mission is to improve the care and outcomes of kidney disease patients worldwide through the development and implementation of global clinical practice guidelines. KDIGO is led by an international Board comprised of approximately 50 members. The majority of the Board members are practicing nephrologists, but it also includes patient representatives, professionals from other medical specialties and disciplines – nephrology nurses, dieticians and social workers (KDIGO 2003).
KDOQI: In 1995, the National Kidney Foundation (NKF) began the development of what would become the first broadly accepted clinical practice guidelines in nephrology, now known as KDOQI—Kidney Disease Outcomes Quality Initiative. The first guidelines were published in 1997, and today there are 13 guidelines, which have made a major difference in the quality of care for kidney patients in the United States and worldwide (KDOQI 2014).
Long‐acting erythropoiesis‐stimulating agent: an agent with a half‐life from 24 to 134 hours when given intravenously and from 54 to 134 hours when given subcutaneously. It is administered once every two to four weeks to maintain haemoglobin levels (Horl 2013).
Short‐acting erythropoiesis‐stimulating agent: an agent with a half‐life from six to eight hours when given intravenously and from 19 to 24 hours when given subcutaneously. It is administered twice or three times weekly to maintain haemoglobin levels (Horl 2013).
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bahlmann 1991 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Bennett 1991 | Quasi‐RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Besarab 1998 | Open‐label study investigating EPO alfa to achieve normal or low haematocrit |
| Canadian EPO Study 1990 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Foley 2000 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Krivoshiev 2008 | RCT. Bioequivalence study comparing IV EPO zeta and EPO alfa |
| Krivoshiev 2010 | RCT. Bioequivalence study comparing SC EPO zeta and EPO alfa |
| Linde 2001 | Open‐label study investigated EPO alfa to reach normal haemoglobin or subnormal haemoglobin |
| Morris 1993 | Did not compare early versus delayed EPO for the anaemia of ESKD |
| Muirhead 1992 | Quasi‐RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Nissenson 1995 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Parfrey 2005 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Park 2014 | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Suzuki 1989 | RCT; was short duration (8 weeks). |
| Trembecki 1995a | RCT; did not compare early versus delayed EPO for the anaemia of ESKD |
| Wizemann 2008 | Bioequivalence study of IV erythropoietin zeta and erythropoietin alfa |
Differences between protocol and review
There were no differences between the protocol and the review.
Contributions of authors
Draft the review: JC, AMC, JR, NY, GU, AA, CL, CP
Study selection: JC, AA
Extract data from studies: JC, AA
Enter data into RevMan: JC, AMC, GU, CL
Carry out the analysis: JC, AA, JR, CL, NY, CP
Interpret the analysis: JC, AA, JR, CL, NY, CP
Draft the final review: JC, AMC, GU, CL
Disagreement resolution: JR
Update the review: JC, AMC
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cartagena, University, Colombia.
Full time professor salary
Declarations of interest
Jorge Coronado Daza: none known
Arturo Martí‐Carvajal: none known
Amaury Ariza García: none known
Joaquín Rodelo Ceballos: none known
Nancy Yomayusa González: none known
Carol Páez‐Canro: none known
César Loza Munárriz: none known
Gerard Urrútia: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Bahlmann 1991 {published data only}
- Bahlmann J, Schoter KH, Scigalla P, Gurland HJ, Hilfenhaus M, Koch KM, et al. Morbidity and mortality in hemodialysis patients with and without erythropoietin treatment: a controlled study. Contributions to Nephrology 1991;88:90‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett 1991 {published data only}
- Bennett WM. A multicenter clinical trial of epoetin beta for anemia of end‐stage renal disease. Journal of the American Society of Nephrology 1991;1(7):990‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Besarab 1998 {published data only}
- Berns JS, Rudnick MR, Cohen RM, Bower JD, Wood BC. Effects of normal hematocrit on ambulatory blood pressure in epoetin‐treated hemodialysis patients with cardiac disease. Kidney International 1999;56(1):253‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berns JS, Rudnick MR, Cohen RM, Maloney A. Effect of normal v. anemic hematocrit on ambulatory blood pressure (ABP) in erythropoietin‐treated hemodialysis (HD) patients [abstract]. Journal of the American Society of Nephrology 1995;6(3):520. [CENTRAL: CN‐00483215] [Google Scholar]
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New England Journal of Medicine 1998;339(9):584‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Besarab A, Goodkin DA, Nissenson AR, Normal Hematocrit Cardiac Trial Authors. The normal hematocrit study‐‐follow‐up. New England Journal of Medicine 2008;358(4):433‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Conlon P, Kovalik E, Minda SN, Schumm D, Gutman R, Schwab SJ. Normalizing hematocrit in hemodialysis patients does not increase blood pressure [abstract]. Journal of the American Society of Nephrology 1995;6(3):526. [CENTRAL: CN‐00483579] [Google Scholar]
- Conlon PJ, Kovalik E, Schumm D, Minda S, Schwab SJ. Normalization of hematocrit in hemodialysis patients does not affect silent ischemia. Renal Failure 2000;22(2):205‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Conlon PJ, Kovalik E, Schumm D, Minda S, Schwab SJ. Normalization of hematocrit in hemodialysis patients with cardiac disease does not increase blood pressure. Renal Failure 2000;22(4):435‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coyne DW. The health‐related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney International 2012;82(2):235‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin DA. The Normal Hematocrit Cardiac Trial revisited. Seminars in Dialysis 2009;22(5):495‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kilpatrick R, Critchlow C, Besarab A, Fishbane S, Stehman‐Breen C, Krishnan M, et al. Epoetin alfa (EPO) responsiveness predicts survival in the normal hematocrit study (NHS) [abstract no: TH‐PO382]. Journal of the American Society of Nephrology 2006;17(Abstracts):188A. [CENTRAL: CN‐00615892] [Google Scholar]
- Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman‐Breen C, Krishnan M, et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):1077‐83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canadian EPO Study 1990 {published data only}
- Anonymous. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. BMJ 1990;300(6724):573‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Effect of recombinant human erythropoietin therapy on blood pressure in hemodialysis patients. Canadian Erythropoietin Study Group. American Journal of Nephrology 1991;11(1):23‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Canadian Erythropoietin Study Group. A prospective randomized double‐blind study of re‐combinant human erythropoietin (r‐HuEPO) in chronic hemodialysis. [abstract]. Kidney International 1988;33(1):218. [Google Scholar]
- Canadian Erythropoietin Study Group. The clinical effects and side‐effects of recombinant human erythropoietin (EPO) in anaemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN‐00583134] [Google Scholar]
- Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life and exercise capacity of anemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN‐00583135] [Google Scholar]
- Keown PA. Quality of life in end‐stage renal disease patients during recombinant human erythropoietin therapy. The Canadian Erythropoietin Study Group. Contributions to Nephrology 1991;88:81‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Keown PA, Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life (QL) and functional capacity (FC) of anemic patients on chronic hemodialysis [abstract]. Kidney International 1989;35(1):195. [CENTRAL: CN‐00583136] [Google Scholar]
- Keown PA, Churchill DN, Poulin‐Costello M, Lei L, Gantotti S, Agodoa I, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2010;14(2):168‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Laupacis A. A randomized double‐blind study of recombinant human erythropoietin in anaemic hemodialysis patients. Canadian Erythropoietin Study Group. Transplantation Proceedings 1991;23(2):1825‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Laupacis A. Changes in quality of life and functional capacity in hemodialysis patients treated with recombinant human erythropoietin. The Canadian Erythropoietin Study Group. Seminars in Nephrology 1990;10(2 Suppl 1):11‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Laupacis A, Wong C, Churchill D. The use of generic and specific quality‐of‐life measures in hemodialysis patients treated with erythropoietin. The Canadian Erythropoietin Study Group. Controlled Clinical Trials 1991;12(4 Suppl):168S‐79S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muirhead N, Keown P, Churchill DN, Lei L, Gitlin M, Mayne TJ. An intent‐to‐treat (ITT) analysis of anemia symptoms in the Canadian Erythropoietin Study Group (CESG) [abstract no: PUB537]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):932A. [CENTRAL: CN‐00790629] [Google Scholar]
- Muirhead N, Keown P, Gitlin M, Mayne TJ, Churchill DN. A reanalysis of the Canadian Erythropoietin Study Group (CESG) patient‐reported outcomes (PRO) trial [abstract no: 177]. American Journal of Kidney Diseases 2008;51(4):A72. [CENTRAL: CN‐00790972] [Google Scholar]
- Muirhead N, Keown P, Lei L, Gitlin M, Mayne TJ, Churchill D. The relationship between achieved hemoglobin (HB) & exercise tolerance [abstract no: 161]. American Journal of Kidney Diseases 2008;51(4):A68. [CENTRAL: CN‐00796608] [Google Scholar]
- Muirhead N, Keown PA, Churchill DN, Poulin‐Costello M, Gantotti S, Lei L, et al. Dialysis patients treated with Epoetin alpha show improved exercise tolerance and physical function: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International 2011;15(1):87‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muirhead N, Laupacis A, Wong C. Erythropoietin for anaemia in haemodialysis patients: results of a maintenance study (the Canadian Erythropoietin Study Group). Nephrology Dialysis Transplantation 1992;7(8):811‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Foley 2000 {published data only}
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. A randomized controlled trial of complete vs partial correction of anemia in hemodialysis patients with asymptomatic concentric lV hypertrophy or lV dilation [abstract no: A1064]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):208A. [CENTRAL: CN‐00445361] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. Diastolic dysfunction in hemodialysis patients: the Canadian Normalization of Hemoglobin Study Group [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):261A. [CENTRAL: CN‐00550674] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney International 2000;58(3):1325‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Hemoglobin levels and hospitalization in hemodialysis patients without symptomatic cardiac disease [abstract no: SA‐PO818]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):432A. [CENTRAL: CN‐00445362] [Google Scholar]
- Wells GA, Coyne D, Lee KM, Foley RN, Parfrey PS, et al. Quality of life effects of normalization of hemoglobin in asymptomatic hemodialysis patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):230A. [CENTRAL: CN‐00448336] [Google Scholar]
Krivoshiev 2008 {published data only}
- Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R, et al. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Current Medical Research & Opinion 2008;24(5):1407‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiecek A, Koytchev R, Ahmed I. Long‐term follow‐up safety study of epoetin zeta: post hoc subanalysis by age of patients with chronic kidney disease [abstract no: M596]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Krivoshiev 2010 {published data only}
- Krivoshiev S, Wizemann V, Czekalski S, Schiller A, Pljesa S, Wolf‐Pflugmann M, et al. Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Advances in Therapy 2010;27(2):105‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Linde 2001 {published data only}
- Danielson BG, Furuland H, Ahlmen J, Christensson A, Linde T, Strombom U. Scandinavian study of normalizing hemoglobin with rHu‐EPO in end stage renal failure [abstract no: A0822]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):160A. [CENTRAL: CN‐00550642] [Google Scholar]
- Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre‐dialysis and dialysis patients. Nephrology Dialysis Transplantation 2003;18(2):353‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Cardiac function in patients with end‐stage renal disease after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN‐00445402] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Dialysis adequacy after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):296A. [CENTRAL: CN‐00445403] [Google Scholar]
- Furuland H, Linde T, Danielson BG. Physical exercise capacity in patients with end‐stage renal disease after normalizaton of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN‐00445404] [Google Scholar]
- Furuland H, Linde T, Sandhagen B, Andren B, Wikstrom B, Danielson BG. Hemorheological and hemodynamic changes in predialysis patients after normalization of hemoglobin with epoetin‐alpha. Scandinavian Journal of Urology & Nephrology 2005;39(5):399‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furuland H, Linde T, Wikstrom B, Danielson BG. Reduced hemodialysis adequacy after hemoglobin normalization with epoetin. Journal of Nephrology 2005;18(1):80‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Linde T, Ekberg H, Forslund T, Furuland H, Holdaas H, Nyberg G, et al. The use of pretransplant erythropoietin to normalize hemoglobin levels has no deleterious effects on renal transplantation outcome. Transplantation 2001;71(1):79‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Linde T, Wahlberg J, Furuland H, Danielson BG. Results of renal transplantation in patients randomized to EPO treatment aimed to reach a subnormal or normal Hb [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):684A. [CENTRAL: CN‐00446408] [Google Scholar]
- Strombom U, Ahlmen J, Danielsson B. The Scandinavian erythropoietin study: effects on quality of life of normalizing hemoglobin levels in uremic patients [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):269A. [Google Scholar]
Morris 1993 {published data only}
- Morris KP, Sharp J, Watson S, Coulthard MG. Non‐cardiac benefits of human recombinant erythropoietin in end stage renal failure and anaemia. Archives of Disease in Childhood 1993;69(5):580‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KP, Skinner JR, Hunter S, Coulthard MG. Short term correction of anaemia with recombinant human erythropoietin and reduction of cardiac output in end stage renal failure. Archives of Disease in Childhood 1993;68(5):644‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muirhead 1992 {published data only}
- Muirhead N, Churchill DN, Goldstein M, Nadler SP, Posen G, Wong C, et al. Comparison of subcutaneous and intravenous recombinant human erythropoietin for anemia in hemodialysis patients with significant comorbid disease. American Journal of Nephrology 1992;12(5):303‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nissenson 1995 {published data only}
- Nissenson AR, Korbet S, Faber M, Burkart J, Gentile D, Hamburger R, et al. Multicenter trial of erythropoietin in patients on peritoneal dialysis. Journal of the American Society of Nephrology 1995;5(7):1517‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parfrey 2005 {published data only}
- Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(4):726‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets and blood transfusions in hemodialysis patients without symptomatic cardiac disease receiving erythropoietin therapy. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(6):1669‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets, blood transfusions and quality of life in hemodialysis patients without symptomatic cardiac disease [abstract no: SA‐PO2745]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):730A. [CENTRAL: CN‐00756852] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(5):805‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Parfrey PS, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on left ventricular cavity volume in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:217.
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double‐blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. Journal of the American Society of Nephrology 2005;16(7):2180‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. Double‐blind comparison of full and partial anemia correction with erythropoietin in incident hemodialysis patients without symptomatic heart disease [abstract no: PUB002]. Journal of the American Society of Nephrology 2004;15(Oct):762A. [CENTRAL: CN‐00583759] [DOI] [PubMed] [Google Scholar]
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on quality of life in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:229.
Park 2014 {published data only}
- Park GS, Pollock A. Reduction of transfusions in dialysis patients taking epoetin alfa in a placebo‐controlled randomized clinical trial [abstract]. American Journal of Kidney Diseases 2014;63(5):A88. [EMBASE: 71448547] [Google Scholar]
Suzuki 1989 {published data only}
- Suzuki M, Hirasawa Y, Hirashima K. Comparative dose study of recombinant human erythropoietin therapy in renal anaemia [abstract]. Nephrology Dialysis Transplantation 1988;3(4):503. [CENTRAL: CN‐00260383] [Google Scholar]
- Suzuki M, Hirasawa Y, Hirashima K, Arakawa M, Odaka M, Ogura Y, et al. Dose‐finding, double‐blind, clinical trial of recombinant human erythropoietin (Chugai) in Japanese patients with end‐stage renal disease. Research Group for Clinical Assessment of rhEPO. Contributions to Nephrology 1989;76:179‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trembecki 1995a {published data only}
- Trembecki J, Kokot F, Wiecek A, Marcinkowski W, Rudka R. Improvement of sexual function in hemodialyzed male patients with chronic renal failure treated with erythropoietin (rHuEPO) [Poprawa czynnosci seksualnych u hemodializowanych mezczyzn chorych na przewlekla niewydolnosc nerek leczonych erytropoetyna (rHuEPO)]. Przeglad Lekarski 1995;52(9):462‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Wizemann 2008 {published data only}
- Baldamus C, Krivoshiev S, Wolf‐Pflugmann M, Siebert‐Weigel M, Koytchev R, Bronn A. Long‐term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Advances in Therapy 2008;25(11):1215‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R, Epoetin Zeta Study Group. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment.[Erratum appears in Curr Med Res Opin. 2008 Oct;24(10):3007], [Erratum appears in Curr Med Res Opin. 2008 Apr;24(4):1155]. Current Medical Research & Opinion 2008;24(3):625‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Artunc 2007
- Artunc F, Risler T. Serum erythropoietin concentrations and responses to anaemia in patients with or without chronic kidney disease. Nephrology Dialysis Transplantation 2007;22(10):2900‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Astor 2002
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988‐1994). Archives of Internal Medicine 2002;162(12):1401‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Astor 2006
- Astor BC, Coresh J, Heiss G, Pettitt D, Sarnak MJ. Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal 2006;151(2):492‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bunn 2013
- Bunn HF. Erythropoietin. Cold Spring Harbor Perspective in Medicine 2013;3(3):a011619. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013a
- Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013b
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Vecchio 2010
- Vecchio L, Locatelli F. Erythropoietin and iron therapy in patients with renal failure. Transfusion Alternatives in Transfusion Medicine 2010;11(1):20–9. [EMBASE: 2010285321] [Google Scholar]
Drueke 2006
- Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New England Journal of Medicine 2006;335(20):2071‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EBPG 2004
- Locatelli F, Aljama PA, Barany P, Canaud B, Carrera F, Eckardt KU, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrology Dialysis Transplantation 2004;19 Suppl 2:ii1‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elliott 2008
- Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Experimental Hematology 2008;36(12):1573‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ERBP 2009
- Locatelli F, Covic A, Eckardt K, Wiecek A, Vanholder R, ERA‐EDTA ERBP Advisory Board. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrology Dialysis Transplantation 2009;24(2):348‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ERBP 2010
- Locatelli F, Aljama P, Canaud B, Covic A, Francisco A, Macdougall IC, et al. Target haemoglobin to aim for with erythropoiesis‐stimulating agents: a position statement by ERBP following publication of the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Study. Nephrology Dialysis Transplantation 2010;25(9):2846‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ERBP 2013
- Locatelli F, Bárány P, Covic A, Francisco A, Vecchio L, Goldsmith D, et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrology Dialysis Transplantation 2013;28(6):1346‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eschbach 1988
- Eschbach JW, Adamson JW. Recombinant human erythropoietin: implications for nephrology. American Journal of Kidney Diseases 1988;11(3):203‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eschbach 1989
- Eschbach JW. The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney International 1989;35(1):134‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finkelstein 2009
- Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health‐related quality of life and hemoglobin levels in chronic kidney disease patients. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(1):33‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel SE, Normand SL. Getting the methods right ‐ the Foundation of Patient‐Centered Outcomes Research. New England Journal of Medicine 2012;367(9):787‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerson 2004
- Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, et al. Anemia and health‐related quality of life in adolescents with chronic kidney disease. American Journal of Kidney Diseases 2004;44(6):1017‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glaspy 2009
- Glaspy JA. Erythropoietin in cancer patients. Annual Review of Medicine 2009;60:181‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glassock 2009
- Glassock RJ, Pecoits‐Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clinical Journal of The American Society of Nephrology: CJASN 2009;4 Suppl 1:S79‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. What is “quality of evidence” and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halstenson 1991
- Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clinical Pharmacology & Therapeutics 1991;50(6):702‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hays 1997
- Hays RD, Kallich JD, Mapes D, Coons SL, Amin N, Carter WB, et al. Kidney Disease Quality of Life Short Form (KDQOL‐SFTM), Version 1.3: A manual for use and scoring. 1997. www.rand.org/content/dam/rand/pubs/papers/2006/P7994.pdf (accessed 9 December 2015).
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horl 2013
- Hörl WH. Differentiating factors between erythropoiesis‐stimulating agents: an update to selection for anaemia of chronic kidney disease. Drugs 2013;73(2):117‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jelkmann 2011
- Jelkmann W. Regulation of erythropoietin production. Journal of Physiology 2011;589(Pt 6):1251‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 2010
- Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ. Systematic review and meta‐analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis‐stimulating agents. American Journal of Kidney Diseases 2010;55(3):535‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johansen 2012
- Johansen KL, Finkelstein FO, Revicki DA, Evans C, Wan S, Gitlin M, et al. Systematic review of the impact of erythropoiesis‐stimulating agents on fatigue in dialysis patients. Nephrology Dialysis Transplantation 2012;27(6):2418‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kainz 2010
- Kainz A, Mayer B, Kramar R, Oberbauer R. Association of ESA hypo‐responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrology Dialysis Transplantation 2010;25(11):3701‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 1998
- Kaufman JS, Reda DJ, Fye CL, Goldfarb DS, Henderson WG, Kleinman JG, et al. Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients. New England Journal of Medicine 1998;339(9):578‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2003
- KDIGO. Kidney Disease: Improving Global Outcomes. kdigo.org/home/about‐us/ (accessed 9 December 2015).
KDIGO 2008
- Locatelli F, Nissenson AR, Barrett BJ, Walker RG, Wheeler DC, Eckardt KU, et al. Clinical practice guidelines for anemia in chronic kidney disease: problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 2008;74(10):1237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2012
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney International ‐ Supplement 2012;2(4):279‐335. [DOI: 10.1038/kisup.2012.37] [DOI] [Google Scholar]
KDOQI 2000
- Anonymous. IV. NKF‐K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2000.[Erratum appears in Am J Kidney Dis 2001 Aug;38(2):442]. American Journal of Kidney Diseases 2001;37(1 Suppl 1):S182‐238. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2006
- KDOQI, National Kidney Foundation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. American Journal of Kidney Diseases 2006;47(5 Suppl 3):S16‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2007
- KDOQI. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. American Journal of Kidney Diseases 2007;50(3):471‐530. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2014
- National Kidney Foundation. KDOQI history. www.kidney.org/professionals/kdoqi/abouthistory.cfm (accessed 9 December 2015).
Levin 2002
- Levin A. Anemia and left ventricular hypertrophy in chronic kidney disease populations: a review of the current state of knowledge. Kidney International ‐ Supplement 2002;61(Suppl 80):S35‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 2004
- Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS).[Erratum appears in Nephrol Dial Transplant. 2004 Jun;19(6):1666]. Nephrology Dialysis Transplantation 2004;19(1):121‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ly 2004
- Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. American Journal of Kidney Diseases 2004;44(4):715‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Macdougall 1999
- Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. Journal of the American Society of Nephrology 1999;10(11):2392‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macdougall 2006
- Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN 2006;1(6):1211‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McGonigle 1984
- McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney International 1984;25(2):437‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moist 2013
- Moist LM, Troyanov S, White CT, Wazny LD, Wilson JA, McFarlane P, et al. Canadian Society of Nephrology Commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. American Journal of Kidney Diseases 2013;62(5):860‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Padhi 2006
- Padhi D, Ni L, Cooke B, Marino R, Jang G. An extended terminal half‐life for darbepoetin alfa: results from a single‐dose pharmacokinetic study in patients with chronic kidney disease not receiving dialysis. Clinical Pharmacokinetics 2006;45(5):503‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2010
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Meta‐analysis: erythropoiesis stimulating agents in patients with chronic kidney disease. Annals of Internal Medicine 2010;153(1):23‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parfrey 2009
- Parfrey PS, Lauve M, Latremouille‐Viau D, Lefebvre P. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta‐analysis. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(4):755‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PCORI 2012
- Methodology Committee of the Patient‐Centered Outcomes Research Institute (PCORI). Methodological standards and patient‐centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pfeffer 2009
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 2009;361(21):2019‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phrommintikul 2007
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet 2007;369(9559):381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rizzo 2010
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Journal of Clinical Oncology 2010;28(33):4996‐5010. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ross 2002
- Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health‐related quality‐of‐life domains: a systematic review. Clinical Therapeutics 2002;25(6):1786‐805. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schellekens 2008
- Schellekens H. The first biosimilar epoetin: but how similar is it?. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(1):174‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sinclair 2013
- Sinclair AM. Erythropoiesis stimulating agents: approaches to modulate activity. Biologics 2013;7:161‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2006
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 2006;355(20):2085‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tolman 2005
- Tolman C, Richardson D, Bartlett C, Will E. Structured conversion from thrice weekly to weekly erythropoietic regimens using a computerized decision‐support system: a randomized clinical study. Journal of the American Society of Nephrology 2005;16(5):1463‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tonia 2012
- Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003407.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2012
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.MR000030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinhas 2012
- Vinhas J, Barreto C, Assunção J, Parreira L, Vaz A. Treatment of anaemia with erythropoiesis‐stimulating agents in patients with chronic kidney disease does not lower mortality and may increase cardiovascular risk: a meta‐analysis. Nephron Clinical Practice 2012;121(3‐4):c95‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weisbord 2008
- Weisbord SD, Kimmel PL. Health‐related quality of life in the era of erythropoietin. Hemodialysis International 2008;12(1):6‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHO 2008
- Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993‐2005: WHO Global Database on Anaemia. Geneva: World Health Organization, 2008. [ISBN: 978 92 4 159665 7] [Google Scholar]
Zhu 2006
- Zhu X, Perazella MA. Nonhematologic complications of erythropoietin therapy. Seminars in Dialysis 2006;19(4):279‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zoccali 2008
- Zoccali C, Abramowicz D, Cannata‐Andia JB, Cochat P, Covic A, Eckardt KU, et al. European best practice quo vadis? From European Best Practice Guidelines (EBPG) to European Renal Best Practice (ERBP). Nephrology Dialysis Transplantation 2008;23(7):2162‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Coronado Daza 2014
- Coronado Daza J, Ariza García A, Rodelo Ceballos J, Yomayusa González N, Urrútia G, Loza Munárriz C, et al. Early versus delayed erythropoietin for the anaemia of end‐stage kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011122] [DOI] [PMC free article] [PubMed] [Google Scholar]


