A “beautiful chimera” from the mid-Cretaceous sheds light on the evolution of novel forms and forces a rethink of what a crab is.
Abstract
Evolutionary origins of novel forms are often obscure because early and transitional fossils tend to be rare, poorly preserved, or lack proper phylogenetic contexts. We describe a new, exceptionally preserved enigmatic crab from the mid-Cretaceous of Colombia and the United States, whose completeness illuminates the early disparity of the group and the origins of novel forms. Its large and unprotected compound eyes, small fusiform body, and leg-like mouthparts suggest larval trait retention into adulthood via heterochronic development (pedomorphosis), while its large oar-like legs represent the earliest known adaptations in crabs for active swimming. Our phylogenetic analyses, including representatives of all major lineages of fossil and extant crabs, challenge conventional views of their evolution by revealing multiple convergent losses of a typical “crab-like” body plan since the Early Cretaceous. These parallel morphological transformations may be associated with repeated invasions of novel environments, including the pelagic/necto-benthic zone in this pedomorphic chimera crab.
INTRODUCTION
A full understanding of the evolution of novel body plans requires inference about their origins via (i) the study of genetic and anatomical variation in extant taxa and (ii) clues from the fossil record. However, the origins of morphological diversity in many highly successful groups are obscured by the scarcity of transitional fossils or reliable early occurrences placed in proper phylogenetic contexts. A particular example is true crabs, or Brachyura, one of the most speciose, disparate, and economically important groups of crustaceans, with nearly 7000 extant species described (1–3) and over 3000 known from fossils (4, 5). Yet, their evolutionary history and internal phylogenetic relationships remain unresolved [e.g., (2, 6–12)]. In addition, although the tropics hold much of the world’s modern biodiversity and have been considered cradles of diversity through time (13–17), little is known about the early tropical history of fossil crustaceans. This limited knowledge arises from enhanced tropical rock weathering, thick vegetation, and ground cover and fewer scientists working in tropical paleontology compared to modern high latitudes (5). Thus, only a few Lagerstätten are known from modern low latitudes (18), which results in considerable biases when attempting to address major paleogeographic, phylogenetic, and evolutionary questions. What role have megadiverse areas such as the Neotropics played in the evolution of novel forms through time? Which better predicts the distribution of convergent traits and across groups: phylogeny, development, or ecology? What are the relations among extinct and extant branches in the crab tree of life, and how can fossils inform about the time of origin of deep nodes?
Here, we describe a novel and exceptionally preserved body plan of marine arthropods from the mid-Cretaceous [Cenomanian-Turonian, ~95 to 90 million years (Ma) ago] of Colombia and the United States and the assemblage from which the type material was collected (see the Supplementary Materials). The Colombian Konservat-Lagerstätte, from the upper Cenomanian to lower Turonian Churuvita Group, includes hundreds of individuals of the earliest crown group Cumacea (comma shrimp), Caridea (true shrimp), and dozens of juvenile and adult specimens of a novel chimeric crab body plan that represents one of the most anatomically complete early crabs found to date (Figs. 1 to 4). Despite their small size (carapace width, ~4 to 10 mm), the new chimera crabs preserve many features rarely seen in the crustacean fossil record, including sexually dimorphic pleopods, first and second antennae, pediform mouthparts, and large compound eyes bearing facets and optical lobes.
Fig. 1. Ventral and appendicular features in Callichimaera perplexa n. gen. n. sp., from the mid-Cretaceous of Colombia.
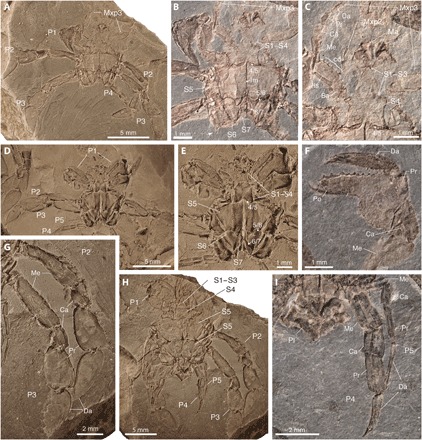
(A to C) Holotype IGM p881215. (A) Ventral view. (B) Close-up of sternal plates, sutures, and linea media (lm); the white arrow shows a spine in the third leg coxae. (C) Close-up of sternal crown, mandibles, and maxillipeds 2 and 3, the latter bearing a row of spines or crista dentata (cd) in the ischium. (D and E) Paratype IGM p881196. (D) Ventral view. (E) Close-up of sternum and sutures. (F) Paratype IGM p881185, showing details of the spanner-like claw. (G to I) Paratype IGM p881214. (G) Close-up of the large oar-like legs 2 and 3. (H) Ventral view showing the sternites, claws, legs, and pleon. (I) Close-up of the reduced and slender legs 4 and 5. Ba, basis; Ca, carpus; Da, dactylus; Pr, propodus; Ma, mandibula; Me, merus; Mxp2 and Mxp3, second and third maxillipeds, respectively; P1, claw or cheliped; P2 to P5, pereopods or legs 2 to 5; Po, pollex or fixed finger tip; Pl, pleon or “abdomen”; S1 to S7, sternites 1 to 7; 4/5–6/7, sternal sutures. All specimens were photographed dry; specimens in (A), (D), (E), (G), and (H) were coated with ammonium chloride; specimens in (B), (C), (F), and (I) were uncoated. Photos by J.L.
Fig. 4. Callichimaera perplexa n. genus. n. sp., reconstruction.
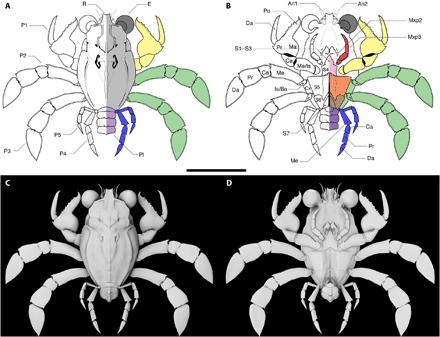
(A and B) Line drawing of dorsal (A) and ventral (B) views. Colors denote convergent traits with other decapod groups: light gray, dorsal carapace, similar to some lobsters and †Palaeocorystidae (Brachyura: Raninoida); dark gray, large eyes, similar to †Ekalakia (Dromiacea: †Glaessneropsidae) and several homoloids; red, pediform mxp3 bearing a crista dentata, similar to lobsters, most anomurans, and early-branching brachyurans (Homolodromioidea, most Homoloidea); yellow, spanner-like P1, similar to Hippoidea (Anomura), Raninoidea (Brachyura), and †Retrorsichela; green, flattened paddle-like legs P2 and P3, similar to Matutidae (Brachyura: Eubrachyura); blue, reduced legs P4 and P5, as in hermit crabs (Anomura: Paguroidea), podotreme brachyurans (e.g., Homolodromioidea and Cyclodorippoidea) and early-branching eubrachyurans (e.g., Dorippoidea); orange, sternites S5 and S6 similar to †Retrorsichela, and Heikeopsis (Eubrachyura: Dorippoidea); and purple, symmetrical pleon lacks articulated rings and uropods/uropodal plates, like most brachyurans. (C and D) Digital reconstruction. (C) Dorsal view. (D) Ventral view. A, pleon; Cx, coxa; Is, ischium; Ma, manus. Scale bar, 10 mm. See movies S1 and S2 and data file S2 for three-dimensional (3D) viewable and printable models. Line drawings by J.L. and 3D reconstructions by A.D.
Fig. 2. Dorsal, frontal, and ocular features in Callichimaera perplexa n. gen. n. sp., from the mid-Cretaceous of Colombia.
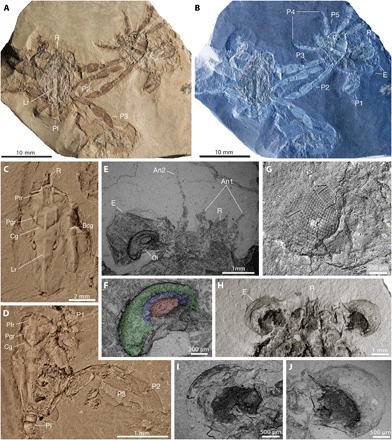
(A and B) Paratype MUN-STRI 27044-02, showing three specimens in dorsal view (left), ventral view (right), and an isolated dorsal carapace (bottom center). (A) Color image. (B) Inverted color image highlighting details of the carapace outlines, claws and legs, and large eyes. (C) Paratype IMG p881203, dorsal view, showing details of the carapace grooves and ridges and the lack of true orbits. (D) Paratype IMG p881218, dorsal view, showing details of dorsal carapace, claws, and large oar-like legs 2 and 3. (E and F) Paratype IGM p881209a. (E) Scanning electron microscope (SEM) image showing details of large compound eye, optical lobe, rostrum (R), and two pairs of short antennae between the eyes. (F) SEM showing a close-up of the optical lobe. (G) Paratype IGM p881220, SEM of large eye preserving mostly hexagonal facets in hexagonal arrangement (left box), although the proximal cornea bears squarish facets in orthogonal packing (right box). (H to J) Paratype IGM p881208, ventral view, (H) showing the large eyes and bifid rostrum, (I) SEM of ventral right eye, and (J) SEM of ventral left eye. An1, antenna 1 or antennula; An2, antenna 2; Bcg, branchio-cardiac groove; Cg, cervical groove; E, compound eye; Lr, longitudinal ridge; Ol, optical lobe; Pfr, postfrontal longitudinal ridge; Pgr, protogastric longitudinal ridge. All specimens were photographed dry; specimens in (C) and (D) were coated with ammonium chloride; specimens in (A), (B), and (E) to (J) were uncoated. Photos by J.L.
Phylogenetic analyses including all major living and fossil crab groups or sections revealed Callichimaera perplexa to be a unique lineage of ancient true crabs. It evolved during a period of extensive morphological experimentation in the mid-Cretaceous (Fig. 5) and represents the first marine arthropods to evolve highly modified, flattened oar-like thoracic legs for active swimming since the disappearance of paddle-legged eurypterids by the late Permian (~250 Ma ago) (19). Our findings suggest that (i) early crabs exhibited a considerable versatility of form during the Cretaceous, (ii) the novel chimeric body plan evolved via heterochronic retention of larval traits into adult stages, (iii) specialized swimming paddles in crabs can arise from repurposed, flattened limbs used for digging, and (iv) the loss of a typical “crab-like” body plan—or “decarcinization”—has occurred independently several times during the last 130 Ma among both false and true crabs (Fig. 6).
Fig. 5. Phylogenetic relationships among the main families, superfamilies, and sections of “true” crabs or Brachyura.
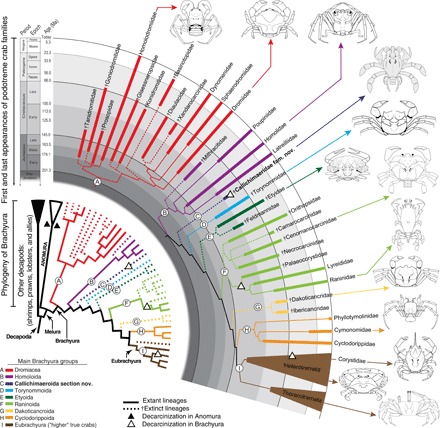
Tree topology after figs. S5 and S6. Each color and letter (encircled) represents one of the nine major brachyuran evolutionary branches. Dromiacea (red) (A) and Homoloida (purple) (B) are first known from the Jurassic, while †Callichimaeroida section nov. (dark blue) (C), †Torynommoida (light blue) (D), †Etyoida (dark green) (E), Raninoida (light green) (F), †Dakoticancroida (yellow) (G), Cyclodorippoida (orange) (H), and Eubrachyura or “higher” brachyurans (brown) (I) are all first known from the Cretaceous. Thick solid lines represent the ages of the first and last occurrences of each family within the main lineages. Dotted lines and daggers indicate extinct taxa; solid lines indicate living taxa. Black triangles indicate that in Anomura, decarcinization has occurred twice (anomuran clades not illustrated). White triangles indicate the three Brachyura lineages where decarcinization has occurred. Figure by J.L.
Fig. 6. Convergent decarcinized body forms in various families of false and true crabs and convergent appendages in swimming and/or fossorial arthropods.

(A to I) Decarcinized crabs. (A to D) Anomura. (A to C) Mole crabs, Hippoidea: (A) Hippidae, Hippa marmorata, Taiwan. (B) Albuneidae: Albunea occulta, Taiwan. (C) Blepharipodidae: Blepharipoda occidentalis. (D) Porcelain crabs, Galatheoidea: Porcellanidae: Euceramus panatelus, Panama. (E to I) Brachyura. (E and F) Frog crabs, Raninoidea: Raninidae: (E) Raninoides benedicti, Panamá. (F) Symethis sp. Panamá. (G and H) Masked burrowing crabs, Eubrachyura: Corystoidea: Corystidae: (G) Corystes cassivelaunus, Belgium. (H) Jonas distinctus, Taiwan. (I) Chimera crab, †Callichimaeroidea: Callichimaeridae: Callichimaera perplexa n. gen. n. sp., Cenomanian-Turonian (95 to 90 Ma ago) of Colombia. (J to Q) Other aquatic arthropods with modified appendages for swimming and/or digging. (J) Sea scorpions, Chelicerata: †Eurypterida: Eurypterus remipes, YPM 211521, upper Silurian, New York. (K to M) Insecta. (K) Coleoptera: Dytiscidae: Cybister fimbriolatus, YPM ENT.959234; (L) Hemiptera: Corixidae: Hesperocorixa kennicottii, YPM ENT.452851; (M) Hemiptera: Notonectidae: Notonecta undulate, last instar nymph, Alberta, Canada. (N) Isopoda: Munnopsidae: Munnopsis longiremis, USNM 113282, Baja California, Mexico. (O to Q) Brachyura. (O) Orithyioidea: Orithyiidae: Orithyia sinica, USNM 134243, China. (P) Calappoidea: Matutidae: Matuta victor. (Q) Portunoidea: Portunidae: Arenaeus cribrarius. Photos by T. Y. Chan (A, B, and H), C. Boyko (C), A. Anker (D, E, and N), J.L. (F and O), H. Hillewaert (G), J. Utrup (J), E. Lazo-Wasem (K and L), J. Acorn (M), and O. Radosta (P and Q).
RESULTS
Systematic paleontology
Arthropoda von Siebold, 1848
Decapoda Latreille, 1802
Brachyura Latreille, 1802
Callichimaeroida section nov
Included superfamily
Callichimaeroidea fam. nov.
Diagnosis
As for superfamily.
Callichimaeroidea superfam. nov.
Included family
Callichimaeridae fam. nov. Tentatively Retrorsichelidae Feldmann et al. (20).
Diagnosis
Crabs with carapaces longer than wide, small and fusiform (Callichimaeridae), or large and ovate (Retrorsichelidae). Sternites 1 to 4 are fused and visible ventrally, forming an elongated sternal crown; sternite 4 is not mesially depressed; sternites 5 to 7 are unfused and axially sulcate by linea media; sternite 5 is very wide, almost as wide as the carapace; suture 5/6 is complete, but lacks a true sterno-pleonal cavity. The pleon is symmetrical, sexually dimorphic, narrower in males than females, and in both sexes narrower than sternite 6 (Callichimaeridae). Pleonal somites are not fused, lacking articulating rings and bearing dorsal median tubercle or crest; pleonites 1 to 3 are exposed subdorsally; uropods or uropodal plates are absent. True orbits, orbital fissures, or any protective structures are absent. Eyes are large in Callichimaeridae but are likely small and reduced in Retrorsichelidae. Chelipeds (claws) are isochelous, and the manus is stout, with pollex or fixed finger slightly (Retrorsichelidae) or strongly (Callichimaeridae) deflected downward; chelipeds are folded ventrally and posteriorly beneath carapace. Pereopods (legs) P2 and P3 are much larger than P4 and P5; P4 and P5 are situated subdorsally and directed posteriorly, lacking spines, neither subchelate nor modified for carrying or grasping. In Callichimaeridae, P2 and P3 are large and wide and positioned laterally, with distal podomeres flattened and paddle-like; P4 and P5 are short and narrow, with the dorsal longitudinal keel neither flattened nor paddle-like; P5 is the smallest, well developed but reduced, and carried subdorsally. In Retrorsichelidae, P2 and P3 are apparently large and positioned ventrally; at least one pereopod bears flattened distal articles.
Callichimaeridae fam. nov.
LSID
urn:lsid:zoobank.org:act:A5D6688D-756B-4FB7-8098-5EB066C38383
Included genus
Callichimaera gen. nov.
Diagnosis
As for type genus and species.
C. perplexa gen. et sp. nov.
LSID
urn:lsid:zoobank.org:act:CD4585D1-B198-45E6-8485-45F94167BDEE
LSID
urn:lsid:zoobank.org:act:650E5046-C4FC-4485-A3B5-254DE785F80B
Etymology
The section, superfamily, family, and generic names are derived from the Greek prefix calli- “kalos” (beautiful), alluding to its exceptional preservation, and Chimera, the fabulous mythological beast commonly represented as composed of parts of different animals such as the lion, goat, and snake, alluding to its startling combination of traits present in separate higher decapod taxa, e.g., eubrachyurans, podotreme brachyurans, anomurans, and some macrurans. The specific epithet derives from the Latin “perplexus,” referring to its puzzling anatomy and phylogenetic affinities. The gender is feminine.
Diagnosis
Small crabs (carapace width, <10 mm; carapace length, <16 mm) that have a carapace longer than wide, fusiform, with distinct cervical and branchiocardiac grooves, and bearing axial longitudinal ridge and postfrontal ridges. Sternites 1 to 4 are visible ventrally; sternites 4 to 7 are unfused, with sutures distinct, and axially sulcate by linea media; all sternites are unique in shape and size; sternite 5 is very wide; suture 5/6 is complete, irregular, and sinuous, lacking true sterno-pleonal cavity; thoracic gonopores are not recognized in males or females. The pleon is symmetrical, sexually dimorphic, narrower in males than females, and in both sexes narrower than sternite 6. Pleonal somites are not fused, lacking articulating rings and bearing dorsal median tubercle; pleonites 1 to 3 are exposed subdorsally, and lacking pleonal, sternal, or appendicular locking mechanisms; uropods or uropodal plates are absent. The rostrum is bifid; first and second antennae are short, between the eyes; eyes are very large—the cornea is strongly dilated, subglobular, bearing mostly hexagonal facets, and a short ocular peduncle, lacking orbits, orbital fissures, or any protective structure; third maxillipeds are pediform, elongate, with “crista dentata”; lengths of the ischium and merus are slightly longer than the length of the palp, and the merus is positioned far back from anterior of carapace or basal antennal segments. Chelipeds (claws) are isochelous, the manus is stout, with fixed finger deflected ~90°; pereopods (legs P2 and P3) are large and wide, with propodus and dactylus flattened and paddle-like; P4 and P5 are short and narrow, with the dorsal longitudinal keel, lacking spines, not subchelate or modified to carry objects, and neither flattened nor paddle-like; P5 is the smallest, well developed but reduced, and carried subdorsally [modified from (21)].
Description
See the Supplementary Materials for a detailed description of C. perplexa gen. et sp. nov.
Holotype
IGM p881215, specimen preserved in ventral view (Fig. 1, A to C), deposited in the paleontological collections of the Colombian Geological Survey, Diagonal 53 #34-53, Bogotá DC, Colombia. Carapace length is 8.5 mm, and carapace width is 5.2 mm.
Additional material
Colombian paratypes IGM p881184 to IGM p881214 and IGM p881216 to IGM p881221 are deposited in the paleontological collections of the Colombian Geological Survey; paratypes MUN-STRI 27044-01 to MUN-STRI 27044-010 and MUN-STRI 27045-01 to MUN-STRI 27045-020 are deposited in the Mapuka Museum of Universidad del Norte, Barranquilla, Colombia. Additional nontype materials from the United States, specimens USNM 605049 to USNM 605056, are deposited in the Paleobiology Collections of the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Measurements
The range of measurements is as follows: Holotype has a carapace length of 8.5 mm and a carapace width of 5.2 mm, the smallest paratype IGM p881220 has a carapace length of 6.6 mm and a carapace width of 3.8 mm, and the largest paratype MUN-STRI 27045-015 has a carapace length of 15.1 mm and a carapace width of 9.6 mm.
Type locality, age, and horizon
Churuvita Group, upper Cenomanian to lower Turonian (~95 to 90 Ma ago), Pesca, Boyacá, Colombia (data files S1 and S2), from carapace- and appendage-rich surfaces. Other specimens are from the Frontier Formation, lower-middle Turonian (~90 Ma ago), WY, USA. See the Supplementary Materials for a detailed description of the geological, geographical, and paleontological context.
Systematic remarks
Although molecular and morphological phylogenetics bring powerful tools to the study of relatedness at the genotypic and phenotypic levels, the fossil record provides a unique view into the origins of such relatedness by revealing a past morphological diversity otherwise inaccessible. Furthermore, fossils are pivotal for understanding the evolution of key traits and provide geographic and chronologic data critical to the calibration of nodes of interest.
We consider Callichimaeroida section nov. a true crab or Brachyura instead of Anomura based on the following: (i) its short first and second antennae between the eyes, (ii) a symmetric, sexually dimorphic pleon, (iii) the absence of articulating rings between pleonites, (iv) a reduced telson, (v) complete absence of uropods or uropodal plates, (vi) the presence of modified male pleopods 1 and 2 as highly sclerotized gonopods but lacking pleopods 3 to 5, while the female bears pleopods 2 to 5, (vii) third maxilliped with well-defined ischium and merus, (viii) the presence of only one pair of chelae or claws (pereopod 1), thus pereopods 2 to 5 are achelate, and (ix) P5 is well developed, visible in dorsal view, and neither subchelate nor modified for carrying or grasping (Figs. 1 to 3). However, a precise phylogenetic placement of Callichimaera within Brachyura is problematic because of its “chimeric” nature, the unknown molting linea, and possession of multiple distinctive characters typical of several fossil and extant Brachyura and Anomura clades but not collectively seen in any one taxon. These characters include a lobster/raninid-like elongate carapace, pediform maxillipeds with a crista dentata, spanner-like chelipeds, large paddle-like legs P2 and P3, the dissimilar shape and size of its sternites, a symmetrical pleon lacking uropods or uropodal plates, a dorsally keeled carapace, and large eyes lacking true orbits and orbital fissures (Figs. 1 to 3).
Fig. 3. Pleon and sexual dimorphism in Callichimaera perplexa n. gen. n. sp., lower Upper Cretaceous, Colombia.
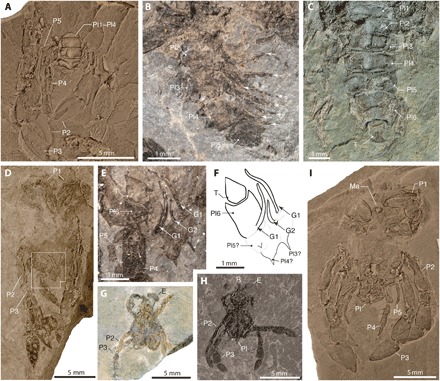
(A) Paratype IMG p881217, female, dorsal view showing the legs 2 to 5 and the pleonites 1 to 4, two of them bearing an axial tubercle. (B) Paratype IGM p881209b, female, showing the unfolded pleon in lateral view and pleonites 2 to 5 and multiple pairs of pleopods (white arrows). (C) Paratype MUN-STRI 27045-06, female, dorsal view, showing pleonites 1 to 6, the roundish telson, and the lack of uropods or uropodal plates. (D and E) Paratype IGM p881202, male. (D) Ventral view showing the chelipeds, pereopods, and pleon with sclerotized gonopods 1 and 2 [white box; see (E)]. (E) Close-up of sclerotized male gonopods 1 and 2 (white arrows), a narrow pleonite 6, an elongate telson, and the lack of uropods or uropodal plates. (F) Line drawing of (E). (G) Paratype MUN-STRI 27045-09, a very small individual, possibly a megalopa or early postlarval stage, preserving small and slender legs 2 and 3 compared to adult forms [see (I)]. (H) Paratype IGM p881220, juvenile, with legs 2 and 3, the pleon bearing an axial tubercle, and with a bifid rostrum and a pair of large compound eyes bearing hexagonal facets (Fig. 2G). (I) Paratype IGM p881206, adult female, ventral view preserving spanner-like chelipeds, long oar-like legs or P2 and P3, reduced and slender legs or P4 and P5, and a broad pleon. G1 and G2, male gonopods 1 and 2; Pl1 to Pl6, pleonites 1 to 6; T, telson. All specimens were photographed dry; specimens in (A), (C), (D), and (G) to (I) were coated with ammonium chloride; specimens in (B) and (E) were uncoated. Photos by J.L.
Phylogenetic remarks
Both molecular and morphological phylogenetic studies have recovered Brachyura, or true crabs, as a monophyletic and sister group to Anomura (false crabs and allies) (2, 10, 11, 22). Yet, phylogenetic relationships within Brachyura remain unsettled largely because of the lack of early, intermediate body forms. Results of our Maximum Parsimony (MP) and Maximum Likelihood (ML) analyses largely agree on the arrangement of ingroup taxa, including placement of Callichimaera as an independent lineage branching off before the extinct higher taxa †Torynommoida and †Etyoida (Fig. 5 and figs. S5, S6A, and S7). Although Callichimaera is also recovered in the same position under Implied Weight MP (IWMP) with moderate K values (K = 6) (fig. S6C), under low K values (K = 3), Callichimaera is pulled into a clade with Palaeocorystidae and Raninidae + Lyreididae as a result of a long-branch attraction due to the homoplastic characters shared among decarcinized crabs and, therefore, artificially inflating the fit value of the tree (fig. S6B). Similarly, under IWMP high K values (K = 12), Callichimaera is pulled into a clade with Orithopsidae and Necrocarcinidae + Cenomanocarcinidae as a result of a long-branch attraction due to the convergent carapaces ornamented with several dorsal longitudinal keels or carina (fig. S6D).
The Bayesian Inference (BI) consensus tree presents the least resolved topology with several major brachyuran lineages collapsed into a polytomy, yet shows a clear paraphyletic podotreme grade (fig. S8). Discrepancies between topologies recovered by probabilistic methods (ML and BI) under the same model of evolution (Mk) are not unusual considering differences in the criteria for selecting the best ML and BI topologies. Since BI uses marginal likelihoods to select the optimal topology (as opposed to the joined likelihood in the ML estimation), it is more sensitive to inconsistencies in a dataset.
Although BI has been shown to produce accurate topologies when dealing with morphological data (23, 24), the method remains sensitive to the consistency of the phylogenetic signal present in the dataset, selection of priors, and heterogeneity of evolutionary rates across different lineages on a tree. The lack of resolution in our BI consensus topology is best explained by high topological disparity in the posterior sample of trees, caused by multiple cases of convergent traits and disparity in evolutionary rates (pedomorphosis, high phenotypic plasticity, etc.) across lineages. In light of the ongoing debate over relative performance of parsimony and model-based approaches when analyzing morphological data (23–29), we present results of phylogenetic analyses under multiple optimality criteria, which agree in the placement of Callichimaeridae as a new, independent lineage.
DISCUSSION
Heterochronous development of the chimeric body plan
The diverse forms of the “crustacean” body are strongly regulated by homeobox-containing developmental genes [e.g., (30–32)] and modeled by the interplay of development, environment, and ecology (33, 34). Heterochrony, or changes in developmental timing and/or rates, has played an important role in the evolution of novel forms and functions in many taxa (33, 35), and pedomorphosis (i.e., the retention of juvenile or even larval traits into adulthood) has contributed to the evolution of disparate anatomies in eucrustaceans (36–38). The anatomical character richness seen in Callichimaera, the large sample size (n = 64), the wide size range (body width, 3.8 to 9.6 mm; body length, 6.6 to 15.1 mm) (Fig. 3, D and G to I), presence in localities of Colombia and United States (fig. S1), and the exquisite preservation (Figs. 1 to 4) provide us with a unique opportunity to study aspects of the growth, development, and functional morphology of the species and examine the role of development on the evolution of novel crab forms during the Cretaceous.
Callichimaera superficially resembles a larval stage known as a megalopa: the transitional (final) larval stage between the swimming planktonic zoea larva and the first benthic juvenile crab stage (34). Since megalopae are mostly a single larval stage, they tend to vary minimally in size and shape among conspecifics (39). The only fossil crab larvae currently known are one megalopa from the Late Jurassic Solnhofen lithographic limestones in Germany (~150 Ma) (carapace span, ~5 mm) (40) and a couple of minute Early Cretaceous zoea from the fossiliferous Santana Group in Brazil (~110 Ma) preserved in fish stomach contents (carapace span, >2 mm) [(41) and references therein]. Callichimaera is clearly not a zoea stage. However, it does share characteristics of some crab megalopae, such as general carapace shape or habitus, apparent lack of a clear molting linea, subdorsal extension of the pleon, leg-like maxillipeds armed with spines, and large unprotected and unconcealed eyes lacking orbits.
The new chimeric crab differs from a megalopa larva in several important ways. First, it exhibits a range of body sizes (carapace length, 6.6 to 15.1 mm; carapace width, 3.8 to 9.6 mm) consistent with several growth instars (Fig. 3, D and G to I). Second, brachyuran megalopae have uropods or relicts of them, lack the main sexual traits of adults, and are not sexually mature, thus lacking extreme sexual dimorphism (39). Callichimaera, on the contrary, lacks any trace of uropods and displays clear sexual dimorphism in both the pleon and pleopods in larger specimens; males bear a pair of well-developed sclerotized gonopods 1 to 2 but lack pleopods 3 to 5, and females bear unmodified pleopods 1 to 5 (Fig. 3). Last, Callichimaera has distinctive chelae that are more typical of juvenile and adult crabs like some frog crabs (Raninoidea) and the possible callichimaeroid †Retrorsichela than megalopa larvae do (39). Thus, we conclude that the megalopa-like anatomy of adult Callichimaera most likely originated via heterochronous development during early ontogenetic stages (42) and the early fixation of some juvenile traits in adulthood via pedomorphosis (43).
Convergence of paddle limbs in aquatic euarthropods
The peculiar oar-like P2 and P3 of Callichimaera are convergent with swimming/digging limbs of other euarthropods, such as the sixth prosomal appendages of some eurypterids (sea scorpions), the second and third thoracic legs of gyrinid beetles (whirligig beetles), corixid and notonectid hemipterans (backswimming true bugs), the fifth to seventh pereopods of deep-sea swimming munnopsid isopods, the fourth pereopod of extinct cenomanocarcinid crabs, and the fifth pereopod of orithyiid, matutid, and portunid crabs (tiger crabs, moon crabs, and swimming blue crabs, respectively) (Fig. 6, J to P). Although most of these structures are not homologous (they arise from different body metameres and may involve different podomeres), they are analogous as specialized multielemental modules suited for efficient swimming and/or digging. A few fossil swimming bugs from the Jurassic and Cretaceous already had long but slender legs seen across species of the family Notonectidae but not modified as paddles/oars for swimming as in eurypterids and swimming crabs. Curiously, after the disappearance of paddle-legged eurypterids by the late Permian around 250 Ma ago (19), no fossil arthropod, to our knowledge, had evolved such highly modified, enlarged, broad, and flattened thoracic limbs until the evolution of Callichimaera more than 95 Ma ago (Figs. 1 to 4). The absence of other aquatic arthropods with extremely enlarged and truly flattened uniramous swimming legs from deposits spanning this 150-Ma gap remains puzzling.
Swimming in most adult decapod crustaceans, such as shrimps and lobsters, is achieved via paddling with biramous pleopods and/or rapid flexion of their muscular pleon and caudal fan. The loss of a muscular pleon in the ancestors of crabs, as well as the reduction of the pleon, pleopods, and caudal fan in most groups, precludes them from active swimming in the same way. Instead, highly specialized groups such as swimming crabs (Eubrachyura: Portunoidea) and moon crabs (Eubrachyura: Matutidae) have evolved one or more pairs of legs with modified podomeres for digging, swimming, or both (Fig. 6) (44–46). The long, flattened oar-like legs P2 and P3 of Callichimaera resemble the spatulate legs of some moon crabs, but lack the oval-shaped, leaf-like, or scythe-like distal podomeres, and the nearly 90° angles formed between the meri and carpi podomeres seen in other swimming and digging crabs (Fig. 6, A to C, F, and G). Highly modified paddle- and shovel-like legs have evolved independently at least seven times in crabs, shaped by similar lifestyles, resulting in notable convergences of form and function (Fig. 6).
Paleontological and neontological information suggest that swimming via paddle-like legs in brachyurans has evolved several times via modification of specialized flattened shovel-like legs used for digging and repurposed into paddles for active swimming (47). Callichimaera appears to be structurally suited for active demersal/pelagic swimming, although it could also have been a facultative back burrower, as seen in extant pelagic swimming crabs such as Euphylax dovii or Charybdis smithii (48, 49).
Decarcinization or the departure from a crab-like body plan
Callichimaera lacks the typical crab-like body plan characterized by a shortened carapace, well-defined lateral margins, and a ventrally concealed pleon (50). A crab-like (carcinized) body plan has evolved independently at least four times among anomurans [e.g., in Aeglidae, Porcellanidae or porcelain crabs, Lithodidae or king crabs, and some Paguridae or hermit crabs (22, 51–57)] and multiple times among brachyuran crabs (e.g., in Dromioidea and Eubrachyura). It also likely evolved independently in most podotreme groups (Fig. 5). However, some lineages have “decarcinized” or lost the crab-like body form (50), typically associated with the evolution of fossoriality in groups such as mole crabs (Anomura: Hippoidea), frog crabs (Brachyura: Raninoidea), and masked crabs (Eubrachyura: Corystoidea) (Fig. 6, A to H) (44).
Although the fossil record of mole crabs is sparse and fragmentary, the exceptional fossil record of stem and crown raninoidans—ranging from Early Cretaceous to present—allows the direction of change of key morphological traits in the transition from carcinized to decarcinized to be investigated. For example, during the Early Cretaceous, as the carapace of some stem-group raninoidans lengthened and their thoracic sternum narrowed (i.e., †Palaeocorystidae), sternites 5 to 8 narrowed axially and with them the arthrodial cavities for their preriopods arthrodial cavities for their pereopods (41), while sternites 7 and 8 and the associated coxae of P4 and P5 migrated toward a more posterodorsal plane, thus forcing the pleon to unfold backward (41, 58–60). By the end of the Early Cretaceous, both †Palaeocorystidae and crown-group Raninoidea (Fig. 5) had already evolved flattened pereopods for back burrowing and legs with a ~90° angle of articulation between the merus and carpus (Fig. 6). Only crabs within the superfamily Raninoidea had narrow branchiostegites and exposed pleurites bridging their narrow posterior dorsal and ventral carapaces (59, 61, 62). The “naked” pleurites, or “gymnopleura,” are a synapomorphy exclusive of the crown-group Raninoidea (Lyreididae + Raninidae) because of their strong decarcinization and are absent in their closest relatives, the stem-group †Palaeocorystoidea and its relatives. Therefore, “Gymnopleura”, previously proposed as a name for the clade uniting Raninoidea and †Palaeocorystoidea (6), must be considered as a junior synonym of Raninoidea. This “naked pleura,” which is unique to Raninoidea, must have evolved in a most recent common ancestor not shared with †Palaeocorystoidea during the late Early Cretaceous at the latest [(58, 62, 63) and references herein].
The superficial resemblance of Callichimaera to other decarcinized crabs, particularly raninoids and palaeocorystids, might initially suggest a fossorial lifestyle. As personally observed by Luque in the raninid Raninoides benedicti, some of the advantages of a fossorial habit include avoiding visual detection by predators and prey and facilitation of ambush predation from a concealed position (unpublished observation). Yet, most traits of Callichimaera are unlike any other decarcinized crabs and suggest that they were not adaptations for burrowing or burying but are related with efficient swimming.
First, sternites 5 and 6 are very broad—nearly as wide as the carapace—and must have housed large thoracic muscles to control the large oar-like legs P2 and P3 (Figs. 1 to 4), unlike the rather narrow and often reduced sternites in truly fossorial crabs (41). Second, these oar-like legs also lack the ~90° angle of articulation between the carpus and merus seen in typical decarcinized crabs, which would prevent the distal segments from moving near the carapace to aid in back burrowing (Fig. 6). Third, legs P2 and P3 have articles with margins lined by setal pits where setae insert. Setae along these paddle-like legs would have increased the surface of the paddles, such as in blue crabs and munnopsid isopods, where they aid in the sculling stroke. Fourth, legs P4 and P5 differ markedly from legs P2 and P3; they are reduced, narrow, axially keeled, and directed dorsoposteriorly (Figs. 1 to 3) and so would be of little use for digging. In hippoids and raninoids, leg P4 is usually similar in shape to the preceding legs (P2 and P3), but leg P5 can either be concealed within the branchial chamber (hippoids) or exposed and modified for digging (raninoids) (Fig. 6). Fifth, Callichimaera does not exhibit obvious respiratory adaptations seen in many extant fossorial crabs, such as accessory exostegal channels or a sieving mechanism for water intake formed when chelipeds are tightly pressed ventrally against the subhepatic region, the pterygostome, and the buccal frame (44, 59). In hippoids and corystoids (Fig. 6, C, H, and I), the setae along the large second antennae interlock to form a tube or “snorkel” that filters and directs the water flow posteriorly; in mole crabs, the second antennae also aid in filter feeding.
Last, decarcinized burrowing crabs usually have a spinose fronto-orbital and/or anterolateral margins and have small eyes and slender eyestalks that retreat into orbits for protection or even eyes so reduced that they are barely exposed, as in Symethis (Fig. 6G). Callichimaera lacks these digging adaptations. Its eyes are unusually large, lack orbits, and are not protected by spines or any other structures, so they must have been permanently exposed even under times of stress.
Phylogenetic and evolutionary implications
Callichimaera perplexa blurs the boundaries of how a “crab” is defined. Both anomurans and brachyuran crabs are generally thought to have evolved crab-like body forms from weakly or uncarcinized ancestors. However, we show that a decarcinized body (loss of the crab-like form) (50) has occurred independently at least five times among both false and true crabs since at least the Early Cretaceous (Figs. 3 and 4). Callichimaera appears to be a unique example of a decarcinized crab well suited for active demersal/pelagic swimming instead of benthic fossorial habits. Although no other callichimaeroid taxa have been found beyond the putatively callichimaeroid-like †Retrorsichela, an actively swimming Callichimaera may well have evolved from a distant fossorial ancestor, as appears to have happened in several extant swimming crab groups such as portunids and matutids. The presence of coeval C. perplexa fossils in localities of Colombia and the United States, more than 4000 km apart today (fig. S1), suggests that many of its mosaic characters—so disparate from other adult decapod crustaceans—and the repurposing of flattened limbs for swimming are evolutionary novelties that must have stabilized by the late Cenomanian–early Turonian more than 90 Ma ago.
On the basis of our MP, ML, and BI results (Fig. 5 and figs. S5 to S8), as well as those from several recent works on larval, foregut, fossil and extant adult morphology, and molecular data (2, 10–12, 22, 64, 65), we conclude that podotreme brachyurans (i.e., where males and females have sexual openings at the base of the legs) do not form a natural group but rather a grade. Podotreme clades such as Dromiacea (Homolodromioidea, Dromioidea, and extinct relatives) and Homoloida branch closer to the root of Brachyura, while podotreme clades such as Raninoida, Cyclodorippoida, and extinct relatives, are recovered as sequential sister groups of Eubrachyura (Fig. 5 and figs. S5 to S8). The podotreme condition is plesiomorphic for decapod crustaceans, as it occurs in shrimps, lobsters, anomurans, and all brachyuran clades except for thoracotreme and female heterotreme Eubrachyura (12). Extinct clades such as Dakoticancroida and Componocancroidea also appear to be closer to some eubrachyurans (e.g., Dorippoidea) than to less inclusive podotreme brachyurans (i.e., Dromiacea and Homoloida). Alternatively, the presence of spermatheca in podotreme crabs may have valuable phylogenetic implications and support a monophyletic Podotremata (6, 66), but whether this character alone or other sexual characters were gained or lost several times within total-group Brachyura remains unknown (65). In addition, although extant heterotreme and thoracotreme eubrachyurans have been considered to form monophyletic assemblages, it is possible that the heterotreme and maybe the thoracotreme conditions had evolved in parallel more than once, but this is yet to be tested.
Regardless of tree topology, the enigmatic Callichimaera seems to occupy an intermediate position between the earliest podotreme brachyurans and more derived podotremes plus Eubrachyura (Fig. 5 and figs. S5 to S8), filling a major gap in the evolutionary history of true crabs. Callichimaera may well be neither brachyuran nor anomuran but rather its own infraorder Callichimaeridea lying between Anomura and Brachyura. More likely, our results suggest that Callichimaera represents a novel lineage of brachyurans that evolved when crabs were undergoing a major adaptive radiation that included extraordinary morphological experimentation, before settling into the more familiar body forms seen today. Crab diversity exploded during the “Cretaceous Crab Revolution” (~145 to 66 Ma ago), with nearly 80% of the higher clades first known from this period (Fig. 5) (67).
The tropics today hold much of the world’s biodiversity and have acted as cradle of diversity by producing and accumulating species through time (13, 14, 17). Thus, is not unusual that the fossil record from tropical settings should preserve snapshots of its past diversity. Recent discoveries from the Cretaceous of tropical and subtropical Americas include either the oldest or one of the oldest fossil records for several higher taxa (5) previously thought to have originated in higher latitudes (see the Supplementary Materials). Although our understanding of the origins of several true crab lineages is far from settled, these findings provide alternative hypotheses about the early evolution of several groups and suggest that the tropics overall might have played a key role in the origins and diversification for some groups since the Early Cretaceous or earlier (5).
MATERIALS AND METHODS
Origin of specimens
The type series was collected from carapace- and appendage-rich surfaces from the upper Cenomanian to lower Turonian (~95 to 90 Ma ago) Churuvita Group, Boyacá, Colombia, between the years 2005 and 2014. Additional nontype material was collected from the Turonian (~90 Ma) Frontier Formation, WY, USA. Specimens from the type series are generally compacted dorsoventrally. However, the thoracic sternites, pleonites, dorsal carapaces, mandibles, and even internal optical structures are represented in three dimensions in some specimens. The specimens were exposed using fine tungsten carbide needles and pin vises, dissecting scalpel blades, and fine pneumatic pencils. Broken or fragile samples were consolidated with the cyanoacrylate adhesive Paleo Bond PB40 and/or stabilized with Paraloid B72 and 95% EtOH as the solvent.
Imaging and illustration
Because of the very small size (in micrometers) of some external and internal features, specimens preserving fine-detailed eyes were studied under Zeiss Scanning Electron Microscope EVO 40 VP under variable pressure and back-scattered electron detector with acceleration voltages of 15 and 20 kV. For optical photography, some specimens were coated with sublimated NH4Cl before photographing to enhance relief and fine ornament. Sets of photographs at different focal points were taken with a Nikon Eclipse 80i and a Nikon Digital Camera Dxm 1200f, Olympus SZX16 Research Stereomicroscope with a digital camera Qimaging Retiga 2000R Fast 1394, Leica Macroscope with Spotflex digital camera, and/or a Nikon D3100 with MicroNikkor 60-mm lens. The resulting multilayered stacks of photos were merged in a single high-definition image using the stacking software Helicon Focus. The photo editing was completed in Adobe Photoshop CS5 and composite figure editing in Adobe Illustrator CS5. For the morphological reconstructions of Callichimaera, we digitized camera lucida line drawings using a Wacom Intuos4 Pen Tablet. Digital reconstructions and animations were performed in Autodesk Maya 2009 using standard polygon modeling tools and ultraviolet (UV) layout techniques. The structure, rendering, and topology of the base mesh were edited in Pixologic’s Zbrush 4.0 for digital sculpting and high-frequency detailing of the carapace. The final renders were performed with the Maya plug-in Arnold rendering system using a dome light with a studio lighting setup and an aiStandard material assigned to the mesh. An aiFacingRatio utility was connected to the color of the material to control the result of the Fresnel of the surface and accentuate the surface details.
Phylogenetic analyses
The dataset, containing 47 taxa and 85 adult morphological characters, was built in Mesquite 2.75 (68), modified from (10) (see the Supplementary Materials for details). Undetermined or not preserved characters were scored as “?” and inapplicable characters as “–.” Multiple character states present in a given terminal were scored as polymorphisms. The final dataset was analyzed under MP, ML, and BI search algorithms.
Maximum parsimony
The phylogenetic analyses were conducted in TNT v.1.5 (69), after 10,000 iterations under traditional search with random addition sequence, and replicated under equally weighted and different implied weights (K = 3, 6, and 12) as additional tests of placement of the new taxon. Bootstrap and jackknife values were calculated after 10,000 replications each and default settings. Bremer support values for the traditional search were calculated under tree bisection reconnection and retained trees suboptimal by 30 steps. All characters were unordered.
Maximum likelihood
The ML analysis was performed in IQ-TREE v. 1.5.6 (70, 71) using the Mk model of morphological character evolution (72) conditioned on sampling variable characters only (ascertainment bias correction; +ASC). The among-site rate variation was modeled using gamma distribution with eight discrete rate categories (+G8); the number of categories was selected from an empirically derived range of optimal values (73–75). Node support was estimated using ultrafast bootstrap and SH-aLRT options with 1000 replicates each (76).
Bayesian inference
We analyzed the dataset using BI as implemented in MrBayes v. 3.2.6 (77). The dataset was analyzed under the traditional Mk model (72) with an ascertainment bias correction to account for scoring only variable morphological characters. Each analysis was performed with two independent runs of 5 × 107 generations each. We used the default settings of four chains (one cold and three heated) per independent run. The relative burn-in fraction was set to 50%, and the chains were sampled every 1000 generations. We set the temperature parameter to 0.01 as determined by preliminary runs to achieve chain mixing values in the optimal range (0.4 to 0.8). Convergence of independent runs was assessed through the average SD of split frequencies (ASDSF << 0.01) and potential scale reduction factors [PSRF ≈ 1 for all parameters (78)]. We used Tracer v. 1.6 (79) to determine whether the runs reached stationary phase and to ensure that the effective sample size for each parameter was greater than 200. Results of the Bayesian runs were summarized as a majority-rule consensus tree of the post-burnin sample with a node support threshold of 75% (nodes with posterior probability support of <75% were collapsed).
Supplementary Material
Acknowledgments
We thank H. Bracken-Grissom, J. Wolfe, D. E. G. Briggs, J. Christy, J. W. Martin, J. M. Jaramillo, F. Etayo-Serna, D. Guinot, B. van Bakel, R. Fraaije, R. Lemaitre, M. Tavares, T. Simoes, N. Webster, H. Karasawa, H. Proctor, and T. Hegna for early discussions, comments, or proofreading the manuscript at different stages; J. C. Villegas, D. Schonwalder, J. Castellanos, C. Romero, C. Silva, N. Pérez, and G. A. Rodríguez-Abaunza for field assistance; L. Herrera and R. de Herrera for their hospitality; J. Ceballos for SEM assistance; J. Arenas, M. Pardo, and the Colombian Geological Survey for supply of export permits; A. Aguirre, M. Vrazo, J. Haug, and C. Haug for facilitating literature items; C. Boyko, A. Anker, T. Y. Chan, and H. Hillewaert for images of extant crabs; J. Utrup, E. Lazo-Wasem, and J. Acorn for images of eurypterids and extant insects; the Willi Hennig Society for the freely available software TNT; and R. Blakey (Colorado Plateau Geosystems) for the paleomaps herein used, and D. Erwin and two anonymous reviewers for their comments and suggestions. Funding: Partial funding for this study was provided to J.L. by the Smithsonian Tropical Research Institute Short-Term Fellowship Program (STRI-STF) (Panama), the American Museum of Natural History (AMNH) Lerner Gray grant, the Sedimentary Geology, Time, Environment, Paleontology, Paleoclimatology, and Energy (STEPPE) Student Travel Award Grant (USA), the Fondo Corrigan-ACGGP-ARES (Colombia), the Natural Science and Engineering Research Council of Canada Graduate Scholarship (NSERC CGS-D), the Izaak Walton Killam Memorial Scholarship, the Andrew Stewart Memorial Graduate Prize, the University of Alberta President’s Doctoral Prize of Distinction, the Devendra Jindal Graduate Scholarship, the Alberta Society of Professional Biologists Graduate Scholarship, and the Kay Ball Memorial Graduate Student Research Travel Award (Canada) and additional support to J.L. via an NSERC Discovery Grant RGPIN 04863 to A.R.P (Canada). J.L. also thanks the Natural Science and Engineering Research Council of Canada Postdoctoral Fellowship (NSERC PDF). Author contributions: J.L. conceived and designed the study. J.L. and M.S. found the Colombian and U.S. material, respectively. J.L. prepared and photographed specimens. J.L., R.M.F., C.E.S., and F.J.V. studied and described the material. J.L., R.M.F, and C.E.S. constructed the dataset. J.L. and O.V. analyzed the data. A.D. designed digital reconstructions and animations. R.M.F., C.E.S., K.A.K., C.B.C., A.R.P., and C.J. contributed logistics/materials/analysis tools. J.L. wrote the paper and made the line drawings, figures, and tables. J.L., K.A.K., R.M.F., C.E.S., O.V., F.J.V., C.B.C., A.R.P., and C.J. edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The data for this study are also available in Morphobank (http://morphobank.org/permalink/?P3404). This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature (http://zoobank.org). The Zoobank Life Science Identifiers (LSIDs) for this publication are urn:lsid:zoobank.org:pub:74A09A7E-A192-4481-A37E-A1023EBB300A.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav3875/DC1
Supplementary Text
Fig. S1. Paleogeographic map during early Late Cretaceous times (~95 to 90 Ma ago).
Fig. S2. Stratigraphic column of the Cenomanian-Turonian Churuvita Group in the studied area.
Fig. S3. Crustacean-dominated faunule at the studied section.
Fig. S4. Additional dorsal, ventral, and appendicular features in Callichimaera perplexa n. gen. n. sp., from the mid-Cretaceous of Colombia and the United States.
Fig. S5. MP-strict consensus of a single most parsimonious tree for the nine major brachyuran sections and podotreme brachyuran families, including Callichimaeridae n. fam.
Fig. S6. Results of equally weighted and IWMP analyses.
Fig. S7. ML topology with the nine major brachyuran sections and podotremous brachyuran families, including Callichimaeridae n. fam.
Fig. S8. Bayesian majority-rule consensus topology of the post-burnin sample of trees for fossil and extant podotremous brachyuran families, including Callichimaeridae n. fam.
Table S1. List of characters for phylogenetic analysis.
Table S2. Superfamilies and families of anomuran and brachyuran crabs included in the phylogenetic analysis.
Data file S1. Data matrix for phylogenetic analysis.
Data file S2. Printable 3D model file of Callichimaera perplexa n. gen. n. sp.
Movie S1. Dorsoventral view 3D reconstruction of Callichimaera perplexa n. gen. n. sp.
Movie S2. Side view 3D reconstruction of Callichimaera perplexa n. gen. n. sp.
REFERENCES AND NOTES
- 1.Ng P. K. L., Guinot D., Davie P. J. F., Systema brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. Raffles Bull. Zool. 17, 1–286 (2008). [Google Scholar]
- 2.Tsang L. M., Schubart C. D., Ahyong S. T., Lai J. C. Y., Au E. Y. C., Chan T.-Y., Ng P. K. L., Chu K. H., Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol. Biol. Evol. 31, 1173–1187 (2014). [DOI] [PubMed] [Google Scholar]
- 3.S. T. Ahyong, Z. Q. Zhang, in Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness, Z. Q. Zhang, Ed. (Zootaxa, 2011), pp. 165–191. [DOI] [PubMed] [Google Scholar]
- 4.C. E. Schweitzer, R. M. Feldmann, A. Garassino, H. Karasawa, G. Schweigert, Systematic List of Fossil Decapod Crustacean Species (Crustaceana Monographs, Brill, 2010), vol. 10, pp. 222. [Google Scholar]
- 5.Luque J., Schwitzer C. E., Santana W., Portell R. W., Vega F. J., Klompmaker A. A., Checklist of fossil decapod crustaceans from tropical America, part I: Anomura and Brachyura. Nauplius 25, e2017025 (2017). [Google Scholar]
- 6.Guinot D., Tavares M., Castro P., Significance of the sexual openings and supplementary structures on the phylogeny of brachyuran crabs (Crustacea, Decapoda, Brachyura), with new nomina for higher-ranked podotreme taxa. Zootaxa 3665, 1–414 (2013). [DOI] [PubMed] [Google Scholar]
- 7.P. J. F. Davie, D. Guinot, P. K. L. Ng, Phylogeny of Brachyura, in Treatise on Zoology-Anatomy, Taxonomy, Biology. The Crustacea, Volume 9 Part C (2 vols) (Brill, 2015), pp. 921–979. [Google Scholar]
- 8.J. W. M. Jagt, B. W. M. Van Bakel, D. Guinot, R. H. Fraaije, P. Artal, Fossil Brachyura, in Decapoda: Brachyura (Part 2). Treatise on Zoology—Anatomy, Taxonomy, Biology. The Crustacea, Vol. 9C, P. Castro, P. J. F. Davie, D. Guinot, F. R. Schram, J. C. v. Vaupel Klein, Eds. (Brill, Leiden & Boston, 2015), vol. 9C-II, pp. 847–920. [Google Scholar]
- 9.De Grave S., Pentcheff D. N., Ahyong S. T., Chan T.-Y., Crandall K. A., Dworschak P. C., Felder D. L., Feldmann R. M., Fransen C. H. J. M., Goulding L. Y. D., Lemaitre R., Low M. E. Y., Martin J. W., Ng P. K. L., Schweitzer C. E., Tan S. H., Tshudy D., Wetzer R., A classification of recent and fossil genera of decapod crustaceans. Raffles Bull. Zool. 21, 1–109 (2009). [Google Scholar]
- 10.Karasawa H., Schweitzer C. E., Feldmann R. M., Phylogenetic analysis and revised classification of podotrematous Brachyura (Decapoda) including extinct and extant families. J. Crustac. Biol. 31, 523–565 (2011). [Google Scholar]
- 11.Ahyong S. T., Lai J. C. Y., Sharkey D., Colgan D. J., Ng P. K. L., Phylogenetics of the brachyuran crabs (Crustacea: Decapoda): The status of Podotremata based on small subunit nuclear ribosomal RNA. Mol Phylogenet Evol. 45, 576–586 (2007). [DOI] [PubMed] [Google Scholar]
- 12.G. Scholtz, C. L. McLay, Is the Brachyura Podotremata a monophyletic group, in Decapod Crustacean Phylogenetics. Crustacean Issues, J. W. Martin, K. A. Crandall, D. L. Felder, Eds. (CRC Press, 2009), vol. 18, pp. 417–435. [Google Scholar]
- 13.Bowen B. W., Rocha L. A., Toonen R. J., Karl S. A.; ToBo Laboratory , The origins of tropical marine biodiversity. Trends Ecol. Evol. 28, 359–366 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Jablonski D., Roy K., Valentine J. W., Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Jablonski D., The tropics as a source of evolutionary novelty through geological time. Nature 364, 142–144 (1993). [Google Scholar]
- 16.Martin P. R., Bonier F., Tewksbury J. J., Revisiting Jablonski (1993): Cladogenesis and range expansion explain latitudinal variation in taxonomic richness. J. Evol. Biol. 20, 930–936 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Marshall C. R., Fossil record reveals tropics as cradle and museum. Science 314, 66–67 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Muscente A. D., Schiffbauer J. D., Broce J., Laflamme M., O’Donnell K., Boag T. H., Meyer M., Hawkins A. D., Huntley J. W., McNamara M., MacKenzie L. A., Stanley G. D. Jr., Hinman N. W., Hofmann M. H., Xiao S., Exceptionally preserved fossil assemblages through geologic time and space. Gondw. Res. 48, 164–188 (2017). [Google Scholar]
- 19.Tetlie O. E., Poschmann M., Phylogeny and palaeoecology of the Adelophthalmoidea (Arthropoda; Chelicerata; Eurypterida). J. Syst. Palaeontol. 6, 237–249 (2008). [Google Scholar]
- 20.Feldmann R. M., Tshudy D. M., Thomson M. R. A., Late Cretaceous and Paleocene decapod crustaceans from James Ross Basin, Antarctic Peninsula. Memoir 28, 1–41 (1993). [Google Scholar]
- 21.J. Luque, On the Origin and Evolution of True Crabs: Insights from Tropical America thesis, University of Alberta (2018), pp. 253. [Google Scholar]
- 22.J. M. Wolfe, J. W. Breinholt, K. A Crandall, A. R. Lemmon, E. Moriarty Lemmon, L. E. Timm, M. E. Siddall, H. D. Bracken-Grissom, A phylogenomic framework, evolutionary timeline, and genomic resources for comparative studies of decapod crustaceans. bioRxiv 466540 [Preprint]. 9 November 2018. 10.1101/466540. [DOI] [PMC free article] [PubMed]
- 23.Wright A. M., Hillis D. M., Bayesian analysis using a simple likelihood model outperforms parsimony for estimation of phylogeny from discrete morphological data. PLOS ONE 9, e109210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly J. E., Puttick M. N., Parry L., Tanner A. R., Tarver J. E., Fleming J., Pisani D., Donoghue P. C. J., Bayesian methods outperform parsimony but at the expense of precision in the estimation of phylogeny from discrete morphological data. Biol. Lett. 12, 20160081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goloboff P. A., Torres A., Arias J. S., Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics 34, 407–437 (2018). [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly J. E., Puttick M. N., Pisani D., Donoghue P. C. J., Probabilistic methods surpass parsimony when assessing clade support in phylogenetic analyses of discrete morphological data. Palaeontology 61, 105–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puttick M. N., O’Reilly J. E., Oakley D., Tanner A. R., Fleming J. F., Clark J., Holloway L., Lozano-Fernandez J., Parry L. A., Tarver J. E., Pisani D., Donoghue P. C. J., Parsimony and maximum-likelihood phylogenetic analyses of morphology do not generally integrate uncertainty in inferring evolutionary history: A response to Brown et al. Proc. R. Soc. Lond. B 284, 20171636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puttick M. N., O’Reilly J. E., Tanner A. R., Fleming J. F., Clark J., Holloway L., Lozano-Fernandez J., Parry L. A., Tarver J. E., Pisani D., Donoghue P. C. J., Uncertain-tree: Discriminating among competing approaches to the phylogenetic analysis of phenotype data. Proc. R. Soc. Lond. B 284, 20162290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puttick M. N., O’Reilly J. E., Pisani D., Donoghue P. C. J., Probabilistic methods outperform parsimony in the phylogenetic analysis of data simulated without a probabilistic model. Palaeontology 61, 105–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin A., Serano J. M., Jarvis E., Bruce H. S., Wang J., Ray S., Barker C. A., O’Connell L. C., Patel N. H., CRISPR/Cas9 mutagenesis reveals versatile roles of Hox genes in crustacean limb specification and evolution. Curr. Biol. 26, 14–26 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Averof M., Patel N. H., Crustacean appendage evolution associated with changes in Hox gene expression. Nature 388, 682–686 (1997). [DOI] [PubMed] [Google Scholar]
- 32.F. R. Schram, S. Koenemann, Developmental genetics and arthropod evolution: On body regions of Crustacea, in Evolutionary Developmental Biology of Crustacea, F. R. Schram, Ed. (Balkema, 2004), pp. 75–92. [Google Scholar]
- 33.Jablonski D., Evolutionary innovations in the fossil record: The intersection of ecology, development, and macroevolution. J. Exp. Zool. B Mol. Dev. Evol. 304, 504–519 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Wolfe J. M., Metamorphosis is ancestral for crown euarthropods, and evolved in the Cambrian or earlier. Integr. Comp. Biol. 57, 499–509 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Haug C., Haug J. T., Developmental paleontology and paleo-evo-devo. Enc. Evol. Biol. 1, 420–429 (2016). [Google Scholar]
- 36.F. R. Schram, Crustacea (Oxford Univ. Press, 1986), pp. 1–606. [Google Scholar]
- 37.Newman W. A., Origin of the Maxillopoda; urmalacostracan ontogeny and progenesis. Crust. Issues 1, 105–119 (1983). [Google Scholar]
- 38.Haug J. T., Maas A., Waloszek D., †Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution. Earth Env. Sci. T. R. So. 100, 311–350 (2010). [Google Scholar]
- 39.J. W. Martin, Brachyura, in Atlas of Crustacean Larvae, J. W. Martin, J. Olesen, J. T. Høeg, Eds. (Johns Hopkings Univ. Press, 2014), pp. 295–310. [Google Scholar]
- 40.Haug J. T., Martin J. W., Haug C., A 150-million-year-old crab larva and its implications for the early rise of brachyuran crabs. Nat. Commun. 6, 6417 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Luque J., A puzzling frog crab (Crustacea: Decapoda: Brachyura) from the Early Cretaceous Santana Group of Brazil: Frog first or crab first? J. Syst. Palaeontol. 13, 153–166 (2014). [Google Scholar]
- 42.Vermeij G. J., Forbidden phenotypes and the limits of evolution. Interface Focus 5, 20150028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.J. W. Martin, J. Olesen, J. T. Høeg, Atlas of Crustacean Larvae (JHU Press, 2014). [Google Scholar]
- 44.Bellwood O., The occurrence, mechanics and significance of burying behaviour in crabs (Crustacea: Brachyura). J. Nat. Hist. 36, 1223–1238 (2002). [Google Scholar]
- 45.Hartnoll R. G., The occurrence, methods and significance of swimming in the Brachyura. Anim. Behav. 19, 39–50 (1971). [Google Scholar]
- 46.Števčić Z., Revision of the Calappidae. Mem. Australian Mus. 18, 165–171 (1983). [Google Scholar]
- 47.S. F. Morris, The fossil arthropods of Jamaica, in Biostratigraphy of Jamaica. Geological Society of America Memoirs, R. M. Wright, E. Robinson, Eds. (Geological Society of America, 1993), vol. 182, pp. 115–124. [Google Scholar]
- 48.Norse E. A., Fox-Norse V., Studies on portunid crabs from the Eastern Pacific. II. Significance of the unusual distribution of Euphylax dovii. Mar. Biol. 40, 374–377 (1977). [Google Scholar]
- 49.Romanov E., Potier M., Zamorov V., Ménard F., The swimming crab Charybdis smithii: Distribution, biology and trophic role in the pelagic ecosystem of the western Indian Ocean. Mar. Biol. 156, 1089–1107 (2009). [Google Scholar]
- 50.Scholtz G., Evolution of crabs–history and deconstruction of a prime example of convergence. Contrib. Zool. 83, 87–105 (2014). [Google Scholar]
- 51.L. A. Borradaile, Crustacea. Part II. Porcellanopagurus: An instance of carcinization, in British Antarctic (“Terra Nova”) Expedition, 1910. Natural History Report. Zoology 3, 111–126 (1916).
- 52.McLaughlin P. A., Lemaitre R., Carcinization in the Anomura—Fact or fiction? I. Evidence from adult morphology. Contrib. Zool. 67, 79–123 (1997). [Google Scholar]
- 53.Anker A., Paulay G., A remarkable new crab-like hermit crab (Decapoda: Paguridae) from French Polynesia, with comments on carcinization in the Anomura. Zootaxa 3722, 283–300 (2013). [PubMed] [Google Scholar]
- 54.Hiller A., Viviani C. A., Werding B., Hypercarcinisation: An evolutionary novelty in the commensal porcellanid Allopetrolisthes spinifrons (Crustacea: Decapoda: Porcellanidae). Nauplius 18, 95–102 (2010). [Google Scholar]
- 55.Cunningham C. W., Blackstone N. W., Buss L. W., Evolution of king crabs from hermit crab ancestors. Nature 355, 539–542 (1992). [DOI] [PubMed] [Google Scholar]
- 56.Bracken-Grissom H. D., Cannon M. E., Cabezas P., Feldmann R. M., Schweitzer C. E., Ahyong S. T., Felder D. L., Lemaitre R., Crandall K. A., A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda). BMC Evol. Biol. 13, 128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsang L. M., Chan T.-Y., Ahyong S. T., Chu K. H., Hermit to king, or hermit to all: Multiple transitions to crab-like forms from hermit crab ancestors. Syst. Biol. 60, 616–629 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Luque J., Feldmann R. M., Schweitzer C. E., Jaramillo C., Cameron C. B., The oldest frog crabs (Decapoda: Brachyura: Raninoida) from the Aptian of northern South America. J. Crust. Biol. 32, 405–420 (2012). [Google Scholar]
- 59.van Bakel B. W. M., Guinot D., Artal P., Fraaije R. H. B., Jagt J. W. M., A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Decapoda, Brachyura, Podotremata). Zootaxa 3215, 1–216 (2012). [Google Scholar]
- 60.Karasawa H., Schweitzer C. E., Feldmann R. M., Luque J., Phylogeny and classification of Raninoida (Decapoda: Brachyura). J. Crust. Biol. 34, 216–272 (2014). [Google Scholar]
- 61.Bourne G. C., The raninidæ: A study in carcinology. J. Linn. Soc. Lon. Zool. 35, 25–79 (1922). [Google Scholar]
- 62.Van Bakel B. W., Preservation of internal pleurites in a new palaeocorystid crab (Crustacea, Brachyura, Raninoidia) from the Cenomanian (Upper Cretaceous) of Poitou-Charentes, France. Zootaxa 3701, 322–328 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Fraaije R. H. B., Van Bakel B. W. M., Jagt J. W. M., Viegas P. A., The rise of a novel, plankton-based marine ecosystem during the Mesozoic: A bottom-up model to explain new higher-tier invertebrate morphotypes. Bol. Soc. Geol. Mex. 70, 187–200 (2018). [Google Scholar]
- 64.Brösing A., Richter S., Scholtz G., Phylogenetic analysis of the Brachyura (Crustacea, Decapoda) based on characters of the foregut with establishment of a new taxon. J. Zoolog. Syst. Evol. Res. 45, 20–32 (2007). [Google Scholar]
- 65.Vehof J., Scholtz G., Becker C., Paradorippe granulata—A crab with external fertilization and a novel type of sperm storage organ challenges prevalent ideas on the evolution of reproduction in Eubrachyura (Crustacea: Brachyura: Dorippidae). Arthropod Struct. Dev. 47, 82–90 (2018). [DOI] [PubMed] [Google Scholar]
- 66.P. J. F. Davie, D. Guinot, P. K. L. Ng, Systematics and classification of Brachyura, in Treatise on Zoology-Anatomy, Taxonomy, Biology. The Crustacea, Volume 9 Part C (2 vols) (Brill, 2015), pp. 1049–1130. [Google Scholar]
- 67.Schweitzer C. E., Feldmann R. M., Faunal turnover and niche stability in marine Decapoda in the Phanerozoic. J. Crustac. Biol. 35, 633–649 (2015). [Google Scholar]
- 68.W. P. Maddison, D. R. Maddison, Mesquite: a modular system for evolutionary analysis. Version 3.51 (2018); http://www.mesquiteproject.org.
- 69.Goloboff P. A., Farris J. S., Nixon K. C., TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008). [Google Scholar]
- 70.Nguyen L.-T., Schmidt H. A., von Haeseler A., Minh B. Q., IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trifinopoulos J., Nguyen L.-T., von Haeseler A., Minh B. Q., W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44, W232–W235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis P. O., A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Yang Z., Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. J. Mol. Evol. 39, 306–314 (1994). [DOI] [PubMed] [Google Scholar]
- 74.Harrison L. B., Larsson H. C. E., Among-character rate variation distributions in phylogenetic analysis of discrete morphological characters. Syst. Biol. 64, 307–324 (2015). [DOI] [PubMed] [Google Scholar]
- 75.F. Ronquist, P. van der Mark, J. P. Huelsenbeck, Bayesian phylogenetic analysis using MrBayes, in The Phylogenetic Handbook, P. Lemey, M. Salemi, A.-M. Vandamme, Eds. (Cambridge Univ. Press, Cambridge, 2009), pp. 210–266. [Google Scholar]
- 76.Minh B. Q., Nguyen M. A. T., von Haeseler A., Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30, 1188–1195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P., MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gelman A., Rubin D. B., Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992). [Google Scholar]
- 79.Rambaut A., Drummond A. J., Xie D., Baele G., Suchard M. A., Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 67, 901–904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldmann R. M., Villamil T., Kauffman E. G., Decapod and stomatopod crustaceans from mass mortality lagerstatten: Turonian (Cretaceous) of Colombia. J. Paleontol. 73, 91–101 (1999). [Google Scholar]
- 81.Vega F. J., Nyborg T., Rojas-Briceño A., Patarroyo P., Luque J., Múzquiz H. P., Stinnesbeck W., Upper Cretaceous crustacea from Mexico and Colombia: Similar faunas and environments during Turonian times. Rev. Mex. Cien. Geol. 24, 403–422 (2007). [Google Scholar]
- 82.T. Villamil, C. Arango, Integrated stratigraphy of latest Cenomanian-Early Turonian facies of Colombia, in Eustasy and Tectonostratigraphic Evolution of Northern South Americ, J. Pindell, C. Drake, Eds. (SEPM Special Publication, 1998), vol. 58, pp. 129–159. [Google Scholar]
- 83.Vernygora O., Murray A. M., Luque J., Ruge M. L. P., Fonseca M. E. P., A new Cretaceous dercetid fish (neoteleostei: Aulopiformes) from the Turonian of Colombia. J. Syst. Palaeontol. 16, 1057–1071 (2017). [Google Scholar]
- 84.Guinot D., Vega F. J., Van Bakel B., Cenomanocarcinidae n. fam., a new Cretaceous podotreme family (Crustacea, Decapoda, Brachyura, Raninoidia), with comments on related families. Geodiversitas 30, 681–719 (2008). [Google Scholar]
- 85.Vega F. J., Nyborg T., Kovalchuk G., Luque J., Rojas Briceño A., Patarroyo P., Porras Múzquiz H., Armstrong A., Bermúde H., Garibay L., On some panamerican Cretaceous crabs (Decapoda: Raninoida). Bol. Soc. Geol. Mex. 62, 263–279 (2010). [Google Scholar]
- 86.F. Etayo-Serna, Zonation of the Cretaceous of Central Colombia by Ammonites (Ministerio de Minas y Energia, Instituto Nacional de Investigaciones Geológico-Mineras, Bogotá, Colombia, 1979), pp. 186. [Google Scholar]
- 87.Sánchez-Quiñonez C., Tchegliakova N., Foraminíferos planctónicos de la Formación San Rafael, Cretácico Superior, en los alrededores de Villa de Leiva, Boyacá, Colombia. Geología Colombiana 30, 99–126 (2005). [Google Scholar]
- 88.Etayo-Serna F., Sinopsis estratigráfica de la región de Villa de Leiva y zonas próximas. Bol. Geol. 21, 19–32 (1968). [Google Scholar]
- 89.Mann U., Stöhr D., Patarroyo P. C., Erste ergebnisse biostratigraphischer und lithstratigraphischer untersuchungen and kretazischen schwarzschiefern in villa de leiva, boyacá, kolumbien. Giessener Geol. Schr. 51, 149–164 (1994). [Google Scholar]
- 90.T. Villamil, Chronology, Relative Sea-Level History and a new Sequence Stratigraphic Model for Basinal Cretaceous Facies of Colombia, in Eustasy and Tectonostratigraphic Evolution of Northern South America, J. Pindell, C. Drake, Eds. (SEPM Special Publication, 1998), vol. 58, pp. 161–216. [Google Scholar]
- 91.C. Cáceres, F. Cediel, F. Etayo, Maps of sedimentary facies distribution and tectonic setting of Colombia through the Proterozoic and Phanerozoic. Publicación de Ingeominas (Bogotá, 2005), pp. 43. [Google Scholar]
- 92.Etayo-Serna F., El Sistema Cretáceo en la región de Villa de Leiva y zonas próximas. Geol. Colomb. 5, 5–74 (1968). [Google Scholar]
- 93.E. A. Merewether, P. D. Blackmon, J. C. Webb, “The mid-Cretaceous Frontier Formation near the Moxa arch, southwestern Wyoming,” U.S. Geol. Surv. Prof. Pap. (no. 1290) (1984).
- 94.M. A. Lanphere, D. L. Jones, Cretaceous time scale from North America, in The Geologic Time Scale, (AAPG Studies Geology, 1978), pp. 259–268. [Google Scholar]
- 95.P. A. Latreille, Histoire Naturelle, générale et particulière des Crustacés et des Insectes: Ouvrage faisant suite aux Oeuvres de Leclerc de Buffon, et Partie du Cours complet d'Histoire Naturelle rédigé par C. S. Sonnini, Membre de plusieurs Sociétés Savantes. (F. Dufart, 1802), vol. 3, pp. xii + 467.
- 96.Feldmann R. M., Schweitzer C. E., Green R. M., Unusual Albian (Early Cretaceous) Brachyura (Homoloidea: Componocancroidea new superfamily) from Montana and Wyoming, U.S.A. J. Crustac. Biol. 28, 502–509 (2008). [Google Scholar]
- 97.Artal P., Van Bakel B. W. M., Fraaije R. H. B., Jagt J. W. M., Klompmaker A. A., New Albian-Cenomanian crabs (Crustacea, Decapoda, Podotremata) from Monte Orobe, Navarra, northern Spain. Rev. Mex. Cien. Geol. 29, 398–410 (2012). [Google Scholar]
- 98.R. H. B. Fraaije, B. W. M. van Bakel, J. W. M. Jagt, P. Artal, New decapod crustaceans (Anomura, Brachyura) from mid-Cretaceous reefal deposits at Monte Orobe (Navarra, Northern Spain), and comments on related type-Maastrichtian material in Annie V. Dhondt Memorial Volume: Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, E. Steurbaut, J. W. M. Jagt, E. A. Jagt-Yazykova, Eds. (Sciences de la Terre, 2008), vol. 78, pp. 193–208. [Google Scholar]
- 99.Schweitzer C. E., Karasawa H., Luque J., Feldmann R. M., Phylogeny and classification of Necrocarcinoidea Förster, 1968 (Brachyura: Raninoida) with the description of two new genera. J. Crustac. Biol. 36, 338–372 (2016). [Google Scholar]
- 100.Alcock A., Materials for a carcinological fauna of india, no. 5. The Brachyura Primigenia or Dromiacea. J. Asiat. Soc. Bengal 68, 123–169 (1900). [Google Scholar]
- 101.Patrulius D., Contributions à la systématique des Décapodes néojurassiques. Rev. Geogr. Geol. 3, 249–257 (1959). [Google Scholar]
- 102.Števčić Z., The reclassification of brachyuran crabs (Crustacea: Decapoda: Brachyura). Nat. Croat. 14, 1–159 (2005). [Google Scholar]
- 103.Schweitzer C. E., Feldmann R. M., Revision of the Prosopinae sensu Glaessner, 1969 (Crustacea: Decapoda: Brachyura) including four new families, four new genera, and five new species. Ann. Naturhist. Mus. Wien 110, 55–121 (2009). [Google Scholar]
- 104.W. De Haan, in Fauna Japonica sive Descriptio Animalium, Quae in Itinere per Japoniam, Jussu et Auspiciis Superiorum, qui Summum in India Batava Imperium Tenent, Suscepto, Annis 1823–1830 Collegit, Notis, Observationibus et Adumbrationibus Illustravit, P. F. v. Siebold, Ed. (Lugduni-Batavorum, 1833–1850). [Google Scholar]
- 105.A. E. Ortmann, Die Dekapoden-Krebse des Strassburger Museums, in Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Thiere (1892), vol. 6, pp. 241–326. [Google Scholar]
- 106.Wright C. W., Collins J. S. H., British cretaceous crabs. Palaeontogr. Soc. Monogr. 126, 1–113 (1972). [Google Scholar]
- 107.C. L. McLay, Crustacea Decapoda: the sponge crabs (Dromiidae) of New Caledonia and the Philippines with a review of the genera, in Résultats des Campagnes MUSORSTOM, A. Crosnier, Ed. (Mémoires du Muséum National d’Histoire Naturelle, 1993), vol. 10, pp. 111–251. [Google Scholar]
- 108.C. L. McLay, Crustacea Decapoda: revision of the family Dynomenidae, in Résultats des Campagnes MUSORSTOM, A. Crosnier, Ed. (Mémoires du Muséum National d’Histoire Naturelle, 1999), vol. 20, pp. 427–569. [Google Scholar]
- 109.Guinot D., Tavares M., A new subfamilial arrangement for the dromiidae de haan, 1833, with diagnoses and descriptions of new genera and species (Crustacea, Decapoda, Brachyura). Zoosystema 25, 43–129 (2003). [Google Scholar]
- 110.Guinot D., A re-evaluation of the Dynomenidae Ortmann, 1892 (Crustacea, Decapoda, Brachyura, Podotremata), with the recognition of four subfamilies. Zootaxa 1850, 1–26 (2008). [Google Scholar]
- 111.Schweitzer C. E., Feldmann R. M., Sphaerodromiidae (Brachyura: Dromiacea: Dromioidea) in the fossil record. J. Crustac. Biol. 30, 417–429 (2010). [Google Scholar]
- 112.Stimpson W., Prodromus descriptionis animalium evertebratorum, quae in expeditione ad oceanum pacificum septentrionalem, a republica federata missa, Cadwaladaro Ringgold et johanne rodgers ducibus, observavit et descripsit. pars vii. crustacea anomura. Proc. Acad. Nat. Sci. Phila. 10, 93–110 (1858). [Google Scholar]
- 113.Guinot D., Établissement de la famille des Poupiniidae pour Poupina hirsuta gen. nov., sp. nov. de polynésie (crustacea decapoda brachyura homoloidea). Bull. Mus. Natl. Hist. Nat. Paris 12, 577–605 (1991). [Google Scholar]
- 114.Harlioglu M. M., Differences in the crista dentata structure of the ischium of third maxilliped in Astacus leptodactylus (eschscholtz, 1823). Folia Biol. (Kraków) 51, 111–116 (2003). [PubMed] [Google Scholar]
- 115.Harlioglu M. M., A scanning electron microscopic study on the appendage morphology of Astacus leptodactylus (eschscholtz, 1823) and Pacifastus leniusculus (dana, 1852) (Crustacea: Decapoda: Astacoidea). Int. J. Morphol. 26, 1035–1051 (2008). [Google Scholar]
- 116.Suthers I. M., Anderson D. T., Functional morphology of mouthparts and gastric mill of ibacus peronii (leach) (Palinura: Scyllaridae). Austr. J. Mar. Freshwat. Res. 32, 931–944 (1981). [Google Scholar]
- 117.Guerao G., Díaz D., Abello P., Morphology of puerulus and early juvenile stages of the spiny lobster Palinurus mauritanicus (Decapoda: Palinuridae). J. Crustac. Biol. 26, 480–494 (2006). [Google Scholar]
- 118.Martin J., Felgenhauer B. E., Grooming behaviour and the morphology of grooming appendages in the endemic south american crab genus Aegla (Decapoda, Anomura, Aeglidae). J. Zool. 209, 213–224 (1986). [Google Scholar]
- 119.Ahyong S. T., Baba K., Chirostylidae from north-western australia (Crustacea: Decapoda: Anomura). Mem. Mus. Vic. 61, 57–64 (2004). [Google Scholar]
- 120.Hoyoux C., Zbinden M., Samadi S., Gaill F., Compère P., Wood-based diet and microflora of a galatheid crab associated with pacific deep-sea wood falls. Mar. Biol. 156, 2421–2439 (2009). [Google Scholar]
- 121.McLaughlin P. A., Lemaitre R., A new classification for the Pylochelidae (Decapoda: Anomura: Paguroidea) and descriptions of new taxa. Raffles Bull. Zool. 20, 159–231 (2009). [Google Scholar]
- 122.McLay C. L., A new genus and two new species of unusual dromiid crabs (Brachyura: Dromiidae) from northern australia. Rec. Aust. Mus. 53, 1–8 (2001). [Google Scholar]
- 123.McLay C. L., Ng P. K. L., Revision of the indo-west pacific sponge crabs of the genus Petalomera stimpson, 1858 (Decapoda: Brachyura: Dromiidae). Raffles Bull. Zool. 55, 107–120 (2007). [Google Scholar]
- 124.Skilleter G. A., Anderson D. T., Functional morphology of the chelipeds, mouthparts and gastric mill of Ozius truncatus (Milne Edwards) (Xanthidae) and Leptograpsus variegatus (Fabricius) (Grapsidae) (Brachyura). Austr. J. Mar. Freshwat. Res. 37, 67–79 (1986). [Google Scholar]
- 125.Williams M. J., Opening of bivalve shells by the mud crab Scylla serrata Forskål. Austr. J. Mar. Freshwat. Res. 29, 699–702 (1978). [Google Scholar]
- 126.Marquez F. P. L., Pohle G. W., Vrbora L., On the larval stages of Macrocoeloma diplacanthum (Decapoda: Brachyura: Majidae), and a review of mithracine phylogenetic aspects. J. Crustac. Biol. 23, 187–200 (2003). [Google Scholar]
- 127.Wolff T., Description of a remarkable deep-sea hermit crab, with notes on the evolution of the Paguridae. Galathea Rep. 4, 11–32 (1961). [Google Scholar]
- 128.Hines A. H., Larval patterns in the life histories of brachyuran crabs (Crustacea, Decapoda, Brachyura). Bull. Mar. Sci. 39, 444–466 (1986). [Google Scholar]
- 129.DeBrosse G., Sulkin S., Jamieson G., Intraspecific morphological variability in megalopae of three sympatric species of the genus Cancer (Brachyura: Cancridae). J. Crustac. Biol. 10, 315–329 (1990). [Google Scholar]
- 130.Tanase H., Preliminary notes on zoea and megalopa of the giant spider crab, Macrocheira kaempferi de haan. Publ. Seto Mar. Biol. Lab. 15, 303–309 (1967). [Google Scholar]
- 131.Reese E. S., Kinzie R. A. III, The larval development of the coconut or robber crab Birgus latro (L.) in the laboratory (Anomura, Paguridea). Crustacaena 117–144 (1968). [Google Scholar]
- 132.A. Harvey, C. B. Boyko, P. A. McLaughlin, J. W. Martin, Anomura, in Atlas of Crustacean Larvae, J. W. Martin, J. Olesen, J. T. Høeg, Eds. (Johns Hopkings Univ. Press, 2014), pp. 283–294. [Google Scholar]
- 133.Flores A., Negreiros-Fransozo M. L., Allometry of the secondary sexual characters of the shore crab Pachygrapsus transversus (Gibbes, 1850) (Brachyura, Grapsidae). Crustaceana 72, 1051–1066 (1999). [Google Scholar]
- 134.Arruda D. C. B., Abrunhosa F., Redescription of megalopa and juvenile development of Pachygrapsus gracilis (Decapoda: Grapsidae) from the Amazon region, reared in the laboratory. Zoologia 28, 465–478 (2011). [Google Scholar]
- 135.Negreiros-Fransozo M. L., Wenner E. L., Knott D. M., Fransozo A., The megalopa and early juvenile stages of Calappa tortugae Rathbun, 1933 (Crustacea, Brachyura) reared in the laboratory from South Carolina neuston samples. Proc. Biol. Soc. Wash. 120, 469–485 (2007). [Google Scholar]
- 136.Guerao G., Rotllant G., Post-larval development and sexual dimorphism of the spider crab Maja brachydactyla (Brachyura: Majidae). Sci. Mar. 73, 797–808 (2009). [Google Scholar]
- 137.Negreiros-Fransozo M. L., Fernandes C. S., Januário Da Silva S. M., Fransozo A., Early juvenile development of Armases rubripes (Rathbun 1897) (Crustacea, Brachyura, Sesarmidae) and comments on the morphology of the megalopa and first crab. Invertebr. Reprod. Dev. 55, 53–64 (2011). [Google Scholar]
- 138.Guerao G., Anger K., Simoni R., Cannicci S., The early life history of Chiromantes ortmanni (crosnier, 1965) (Decapoda: Brachyura: Sesarmidae): Morphology of larval and juvenile stages. Zootaxa 3347, 1–35 (2012). [Google Scholar]
- 139.J. Luque, R. M. Feldmann, C. E. Schweitzer, H. Karasawa, C. Jaramillo, On the Origin and Evolution of True Crabs: Insights from Northern South America, paper presented at the Geological Society of America Annual Meeting: 125th Anniversary of GSA, Denver, Colorado. Abstract with Programs, 45, 459 (2013). [Google Scholar]
- 140.J. Luque, A new genus and species of raninoidian crab (Decapoda, Brachyura) from the Lower Cretaceous of Colombia, South America, in Proceedings of the 5th Symposium on Mesozoic and Cenozoic Decapod Crustaceans, Krakow, Poland 2013: A tribute to Pál Mihály Müller. Scripta Geologica,, R. H. B. Fraaije, M. Hyžný, J. W. M. Jagt, M. Krobicki, B. W. M. Van Bakel, Eds. (2014).
- 141.Prado L. A. C., Luque J., Barreto A. M. F., Palmer A. R., New brachyuran crabs from the Aptian–Albian Romualdo Formation, Santana Group of Brazil: Evidence for a Tethyan connection to the Araripe Basin. Acta Palaeontol. Pol. 63, 737–750 (2018). [Google Scholar]
- 142.Gomez A. J., Bermudez H., Vega F. J., A new species of Diaulax bell, 1863 (Brachyura: Dialucidae) in the Early Cretaceous of the Rosablanca Formation, Colombia. Bol. Soc. Geol. Mex. 67, 103–112 (2015). [Google Scholar]
- 143.Luque J., The oldest higher true crabs (Crustacea: Decapoda: Brachyura): Insights from the Early Cretaceous of the Americas. Palaeontology 58, 251–263 (2015). [Google Scholar]
- 144.B. W. M. van Bakel, D. Guinot, J. W. M. Jagt, R. H. B. Fraaije, Mithracites takedai, a new homoloid crab (Decapoda, Brachyura) from the Lower Cretaceous (Aptian) of Colombia, in Studies on Eumalacostraca: A Homage to Masatsune Takeda, H. Komatsu, J. Okuno, K. Fukuoka, Eds. (Crustaceana Monographs, BRILL, 2012), vol. 17, pp. 81–90. [Google Scholar]
- 145.Schweitzer C. E., Paleobiogeography of Cretaceous and Tertiary decapod Crustaceans of the North Pacific Ocean. J. Paleo. 75, 808–826 (2001). [Google Scholar]
- 146.Feldmann R. M., Schweitzer C. E., Paleobiogeography of southern hemisphere decapod Crustacea. J. Paleontol. 80, 83–103 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav3875/DC1
Supplementary Text
Fig. S1. Paleogeographic map during early Late Cretaceous times (~95 to 90 Ma ago).
Fig. S2. Stratigraphic column of the Cenomanian-Turonian Churuvita Group in the studied area.
Fig. S3. Crustacean-dominated faunule at the studied section.
Fig. S4. Additional dorsal, ventral, and appendicular features in Callichimaera perplexa n. gen. n. sp., from the mid-Cretaceous of Colombia and the United States.
Fig. S5. MP-strict consensus of a single most parsimonious tree for the nine major brachyuran sections and podotreme brachyuran families, including Callichimaeridae n. fam.
Fig. S6. Results of equally weighted and IWMP analyses.
Fig. S7. ML topology with the nine major brachyuran sections and podotremous brachyuran families, including Callichimaeridae n. fam.
Fig. S8. Bayesian majority-rule consensus topology of the post-burnin sample of trees for fossil and extant podotremous brachyuran families, including Callichimaeridae n. fam.
Table S1. List of characters for phylogenetic analysis.
Table S2. Superfamilies and families of anomuran and brachyuran crabs included in the phylogenetic analysis.
Data file S1. Data matrix for phylogenetic analysis.
Data file S2. Printable 3D model file of Callichimaera perplexa n. gen. n. sp.
Movie S1. Dorsoventral view 3D reconstruction of Callichimaera perplexa n. gen. n. sp.
Movie S2. Side view 3D reconstruction of Callichimaera perplexa n. gen. n. sp.


