Rehearsal shifts mnemonic processing from the hippocampus to the posterior parietal cortex, sleep stabilizes the transition.
Abstract
After encoding, memories undergo a transitional process termed systems memory consolidation. It allows fast acquisition of new information by the hippocampus, as well as stable storage in neocortical long-term networks, where memory is protected from interference. Whereas this process is generally thought to occur slowly over time and sleep, we recently found a rapid memory systems transition from hippocampus to posterior parietal cortex (PPC) that occurs over repeated rehearsal within one study session. Here, we use fMRI to demonstrate that this transition is stabilized over sleep, whereas wakefulness leads to a reset to naïve responses, such as observed during early encoding. The role of sleep therefore seems to go beyond providing additional rehearsal through memory trace reactivation, as previously thought. We conclude that repeated study induces systems consolidation, while sleep ensures that these transformations become stable and long lasting. Thus, sleep and repeated rehearsal jointly contribute to long-term memory consolidation.
INTRODUCTION
One of the brain’s most central capacities is the ability to form and maintain memories. The processes leading to memory formation have to be flexible enough to provide fast and faithful encoding of incoming information while also ensuring stable long-term storage. In addition, care must be taken that old memories are not overwritten by new information. The dual-storage model of memory assumes that at least two complementary memory systems have to exist for these requirements to be fulfilled (1). It proposes that the hippocampus constitutes a fast-learning system that can rapidly encode transient memory traces. Simultaneously, a second system gradually forms an additional long-term memory representation in the neocortex. Subsequently, the hippocampal system reinforces the trace in the neocortical system, leading to the stabilization and eventual independence of the neocortical memory trace. This process has been termed systems memory consolidation (1). Frankland and Bontempi (2) propose a model, in which a new memory initially resides in the connections from the hippocampus to the existing neocortical modules. These temporary connections are replaced over a consolidation period ranging from days to years by direct connections between these neocortical modules and additional connections to the prefrontal cortex, which might serve as an integrative node in the memory network in the absence of the hippocampus.
In a previous study, we found rapid changes in memory systems contributions over repeated rehearsal in a spatial navigation task, which were indicative of such a systems consolidation (3). Over repeated rehearsal, we observed fast increases in activity in the posterior parietal cortex (PPC), a brain region that has been implicated recently in episodic, spatial, and working memory (4–8). Changes in neocortical activity were accompanied by a rapid disengagement of the hippocampus, whose relevance for declarative memory is well established (9). These results suggest that transitions between the fast-learning hippocampal system and the slow-learning neocortex might occur on a much shorter time scale than previously assumed (10). It remains unclear whether this interaction occurs only in spatial memory, whether it is specific to hippocampal-PPC networks or can likewise arise between other regions, and whether resulting network alterations are only temporary or persist over time.
It has long been supposed that systems consolidation of memory is affected by sleep (2). Sleep has a beneficial effect on memory retention (11, 12). It offers an optimal environment for memory consolidation owing to an absence of external interference and changes in neurotransmitter levels that enable enhanced hippocampal-neocortical dialog, (12, 13). A possible mechanism by which memories could be gradually strengthened is their reactivation during sleep. Replay of hippocampal memory traces during sleep is thought to propagate into neocortical networks where it provides additional training and thus enhances the memory trace established during the initial encoding (14).
In the present study, we focused on the role of sleep in systems memory consolidation. We measured brain activity while participants repeatedly studied a word list. Each learning repetition was followed by a free recall of the material. Memory retrieval has been suggested to integrate memories with stored associative knowledge networks and thus to facilitate systems consolidation (10). Our experiment was explicitly designed to follow the stages of memory formation over rehearsal and consolidation. Participants learned lists of German words through seven learning-recall repetitions. After a 12-hour interval, during which they either stayed awake or were allowed to sleep for 8 hours, we compared brain responses during rehearsal of new words and during additional rehearsal of previously learned words (Fig. 1). On the basis of previous findings, we predicted that rehearsal induces a dynamics of brain activity corresponding to systems consolidation models, with the hippocampus losing in relevance and the PPC gaining in equal measure. Because rehearsal has assumed the systems consolidation function in this case, we hypothesized that the role of sleep will be mainly to stabilize the changes induced during learning.
Fig. 1. General design.
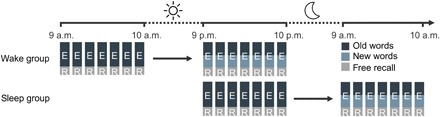
Participants visited the laboratory twice and spent the 12-hour interval in between either awake during the day (wake group, n = 16) or went to bed normally (sleep group, n = 15). Each session consisted of seven encoding repetitions (E) of a word list of 28 concrete German nouns. Every repetition was followed by a self-paced free recall (R) of all remembered words. We refer to the encoding-recall repetition as rehearsal. During the second session, the word list consisted of 14 words known from the first session (dark blue) and 14 new words (light blue). Words were presented in each repetition one at a time in randomized order.
RESULTS
Fast memory systems transition over repeated rehearsal
In the first analysis, we tested for the existence of a rapid transition in memory systems contributions over repeated rehearsal (see fig. S1 for memory performance). To track the neural response over time and learning repetitions, we analyzed the blood oxygen level–dependent (BOLD) signal acquired during learning of word lists. Consonant with our hypothesis, the left hippocampus and the medial prefrontal cortex (mPFC) showed a decrease in activity from the first to the last learning repetition (Fig. 2A and table S1). These results were confirmed by regression analyses of mean β estimates, which showed a linear decrease over repeated rehearsal (hippocampus: β = −0.864, t31 = −3.850, P = 0.012, R2 = 0.748; mPFC: β = −0.839, t31 = −3.452, P = 0.018, R2 = 0.704; Fig. 2B). Moreover, a linear decrease in β estimates was also observed in single-subject analyses (hippocampus: t31 = −4.291, P < 0.001, mean β = −0.3949; mPFC: t31 = −6.161, P < 0.001, mean β = −0.476; see Fig. 2C for individual regression slopes).
Fig. 2. Fast transition of memory systems contributions over repeated rehearsal.
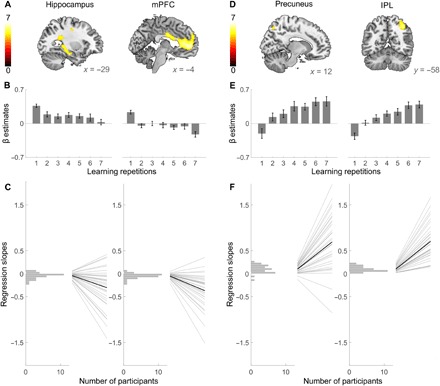
(A) The hippocampus (left) and the mPFC (right) showed decreasing activity between the first and the last learning repetition of the first session. (B) The β values within these clusters show a significant linear decline over all learning repetitions. (C) Single-subject regression slopes based on all learning repetitions confirm the decrease in activity within hippocampal and mPFC voxels. Each panel shows a histogram of regression slopes over all participants (left) and individual regression slopes plotted as regression lines over the seven learning repetitions (right, gray) and the average regression slope (right, black). (D) Activity in the precuneus (left) and in the IPL (right) showed significant increases between the first and the last learning repetition. (E) The β estimates based on the precuneus and the IPL clusters, respectively, show significant linear increases over all learning repetitions. (F) Single-subject regression slopes based on all learning repetitions confirm the increase in activity within the precuneus and the IPL clusters. Results are displayed as in (C). All T maps in (A) and (D) are displayed at a whole-brain family-wise error (FWE)–corrected threshold of PFWE ≤ 0.05 for clusters exceeding 20 voxels and are not masked. Error bars in (B) and (E) indicate SEM.
The opposite activity pattern—an activity increase between the first and the last learning repetition—emerged in the precuneus and in the inferior parietal lobule (IPL) (Fig. 2D and table S2). Regression analyses of the mean β estimates in these clusters showed a linear increase in activity over all repetitions (precuneus: β = 0.913, t31 = 5.014, P = 0.004, R2 = 0.834; IPL: β = 0.952, t31 = 6.984, P = 0.010, R2 = 0.907; Fig. 2E). Again, regression slopes in single-subject analyses confirmed this finding (precuneus: t31 = 6.673, P < 0.001, mean β = 0.561; IPL: t31 = 12.167, P < 0.001, mean β = 0.726; see Fig. 2F for individual regression slopes). When looking at recall instead of learning-related activity, we find a similar increase in activity in the precuneus (Fig. 3 and table S3). A decrease over recall was found in the mPFC but not in the hippocampus, which showed no significant retrieval-related activity to begin with [first repetition (first session), P > 0.5; last repetition (first session), P > 0.096 for all voxels within the hippocampus]. Neither increases nor decreases over repeated rehearsal showed a circadian modulation, i.e., there were no differences in any voxel between morning and evening sessions (all P > 0.001, uncorrected).
Fig. 3. Increasing precuneus activity over recall repetitions.
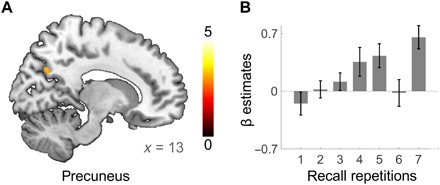
(A) The right precuneus showed increasing activity between the first and the last recall repetition of the first session. Further regions displaying significant increases at whole-brain FWE-corrected threshold of PFWE ≤ 0.05 are given in table S3. T maps are displayed at a whole-brain FWE-corrected threshold of PFWE ≤ 0.05 (B) The β estimates from the precuneus cluster. Single-subject regression statistics indicated a linear increase in the precuneus β estimates over recall repetitions (t30 = 4.385, P < 0.001, mean r = 0.469).
These fast changes of the BOLD signal over learning repetitions should predict memory performance if they are indeed indicative of underlying neural representations. Therefore, we examined the correlation between brain activity over repetitions and memory performance. The β estimates in the hippocampus, mPFC, IPL, and precuneus all predicted behavior on a single-subject level. Yet, whereas higher β estimates in the IPL and in the precuneus were associated with better memory, lower β estimates in the mPFC and in the hippocampus indicated superior performance (IPL: mean r = 0.674, P < 0.001; precuneus: mean r = 0.499, P < 0.001; hippocampus: mean r = −0.366, P = 0.001; mPFC: mean r = −0.469, P < 0.001). A similar pattern was found for recall activity (IPL: mean r = 0.2733, t30 = 3.080, P = 0.004; precuneus: mean r = 0.2642, t30= 3.109, P = 0.004; mPFC: mean r = −0.274, t30 = −2.968, P = 0.006). The hippocampus, which showed no retrieval-related activity, also did not correlate with memory performance (mean r = −0.1139, t30 = −1.222, P = 0.231).
To further investigate the interrelationship between the parietal regions and the hippocampus during encoding, we performed a bootstrapped Bayesian multilevel mediation analysis. We found that the hippocampus predicted memory performance but that this relation was significantly mediated by the IPL and the precuneus [indirect effect via precuneus: effect = −1.64, 95% confidence interval (CI) = −2.96 to −0.56; indirect effect via IPL: effect = −3.10, 95% CI = −5.34 to −1.17]. In the case of the precuneus, a small direct effect of the hippocampus remained (effect = −2.59, 95% CI = −5.18 to −0.02); in the case of the IPL, no independent contribution of the hippocampus survived (effect = −1.44, 95% CI = −3.90 to 0.98). Correlations of the precuneus and the IPL with memory performance were not mediated by the hippocampus. This analysis confirms the independent contribution of the parietal cortex to memory recall.
To replicate these findings of rapid systems consolidation over rehearsal, we additionally analyzed learning of new words in the second session. In this independent data from the same participants, we again observed fast shifts in systems memory contributions, with a rapid strengthening of the neocortical engagement in the IPL and in the precuneus over repetitions and a concurrent disengagement of the hippocampus and the mPFC (Fig. 4 and tables S4 and S5).
Fig. 4. Fast transition of memory systems contributions over repeated rehearsal of new words in the second session.
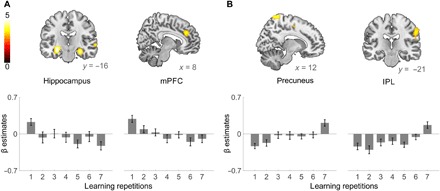
(A) The hippocampus (left) and the mPFC (right) showed decreasing activity between the first and the last learning repetition of the first session. The bottom panels show the mean β estimates within the left hippocampus and the mPFC for the seven repetitions. (B) Activity in the precuneus (left) and in the IPL (right) showed significant increases between the first and the last learning repetition. The bottom panels show the mean β estimates within the precuneus and the IPL for the seven learning repetitions. Because the number of stimuli was half that of session 1, only the hippocampus showed a whole-brain FWE-corrected effect. All T maps are displayed at P ≤ 0.001 for clusters exceeding 20 voxels and are not masked.
Stability of the parietal long-term memory representation
Next, we investigated whether these rapid changes in brain responsiveness were stable over long intervals. Twelve hours after the initial learning, the precuneus and the IPL responded more strongly to old words from the first session compared to new words (Fig. 5 and table S6). In contrast, stronger responses to new words were found in temporal regions that reached into the left hippocampus (table S7). The change in parietal responsiveness is specific to previously learned words and is long-term stable. It must therefore be memory related and depend on brain plasticity.
Fig. 5. Parietal long-term memory representations.
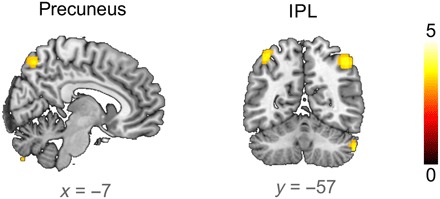
Both the precuneus and the IPL showed an increased BOLD response to the first presentation of old words compared to new words 12 hours after the initial learning (small-volume FWE corrected at PSVC ≤ 0.05, ROI based on all significant whole-brain–corrected voxels that decrease over learning repetitions in the first session; Fig. 2). T maps are displayed at P ≤ 0.001 for clusters exceeding 20 voxels.
This long-term memory-related increase in precuneus activity was observed across all participants, and no significant difference between sleep and wake groups was found (first repetition, old/new × sleep/wake; all precuneus voxels, P > 0.012, uncorrected). Furthermore, changes in precuneus activity over rehearsal repetitions in the second session were not dependent on whether words were old or new and on whether participants had slept or not in the consolidation interval [region of interest (ROI) analysis on structural right precuneus: (new words) sleep/wake × last/first repetition: F1,29 = 0.057, P = 0.813; (old words) sleep/wake × last/first repetition: F1,29 = 0.074, P = 0.787; old/new × last/first repetition: F1,30 = 0.065, P = 0.801; sleep/wake × old/new × last/first repetition: F1,29 = 0.001, P = 0.978]. However, the correlation of the precuneus β estimates with behavioral performance was influenced by sleep. The higher β estimates for old words after the 12-hour delay period predicted better memory in the sleep group (r15 = 0.588, P = 0.021) but not in the wake group (r16 = 0.102, P = 0.707).
Stabilization of hippocampal disengagement over sleep
To further investigate the impact of sleep on the stability of memory representations, we compared the change in brain responses after sleep and wakefulness to new and old words over the second session 12 hours after the initial encoding. This three-way interaction yielded a significant effect in a structural ROI of the left hippocampus (sleep/wake × old/new × first/last repetition: F1,29 = 6.680, P = 0.015; Fig. 6), which stems from distinct responses to old and new words in the sleep group (repetition × old/new: F1,14 = 15.470, P = 0.002), whereas both were indistinguishable in the wake group (repetition × old/new: F1,15 = 0.333, P = 0.572). In the wake group, old words showed a decrease in hippocampal activity over learning repetitions, and new words followed the same trend similar to what had been observed in the first session (old words: t15 = 2.863, P = 0.012; new words: t15 = 1.912, P = 0.075). Single-subject regression analyses over all learning repetitions confirmed significant declines of the β estimates in the wake group for both new and old words (old words: t15 = −2.509, P = 0.024, mean r = −0.296; new words: t15 = −2.813, P = 0.013, mean r = −0.298).
Fig. 6. Stabilization of hippocampal signaling over sleep.
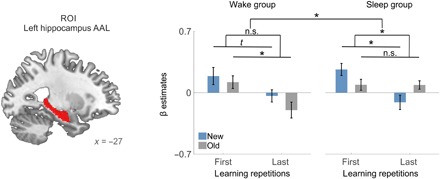
Sleep following repeated rehearsal alters hippocampal involvement during the memory task on the next day (sleep/wake × old/new × learning repetitions). This contrast was calculated for the mean β estimates in the left hippocampus (structural mask; left). The β estimates within the hippocampus showed a stabilization of the hippocampal response to old words after sleep: Initial activity in the sleep group is low and does not decrease significantly over repetitions. For new words in the sleep group, hippocampal activity mirrors that of the first session: Initial activity is high and decreases over repeated rehearsal. Similar activity decreases were observed for old words in the wake group but only showed a trend for new words (P = 0.075). Error bars indicate SEM, *P ≤ 0.05, t indicates P ≤ 0.1, n.s., not significant.
In the sleep group, this characteristic decline was significantly stronger in new words than in old words (new words: t14 = 3.632, P = 0.003; old words: t14 = 0.033, P = 0.974; see interaction reported above). Single-subject regression analyses on the left hippocampus β estimates from all learning repetitions confirmed stable hippocampal responses to old words and a decrease in the β estimates for new words in the sleep group (old words: t14 = 0.201, P = 0.843, mean β = 0.024; new words: t14 = −2.935, P = 0.011, mean β = −0.350), whereas both categories displayed a significant decrease in the β estimates in the wake group (old words: t15 = −2.509, P = 0.024, mean β = −0.296; new words: t15 = −2.813, P = 0.013, mean β = −0.298).
This stabilization of the hippocampal disengagement over sleep does not reflect a general circadian process but instead occurs exclusively for previously acquired memories. New words studied in the second session either in the morning after sleep or in the evening after wakefulness did not show such a modulation by time of day for any voxel in the brain (all P > 0.001, uncorrected). Together, our data show that the hippocampus resumes its initial encoding activity for previously studied old words after 12 hours of wakefulness but remains disengaged after a full night of sleep.
DISCUSSION
Using functional magnetic resonance imaging (fMRI) to track memory systems contributions over repeated learning sessions, we found that two complementary systems contribute to long-term memory, as predicted by the dual-storage model (1). Whereas the hippocampus and the mPFC showed fast decreases in activity over repeated rehearsal, the opposite pattern was found in the precuneus and the IPL. After a delay period of 12 hours, we show that old memories still activated posterior parietal areas, while new ones activated the hippocampus, reflecting long-term memory-related brain plasticity. Sleep changed the pattern of brain responses to old memories. Whereas old words suppressed hippocampal activity after sleep, the hippocampus had “forgotten” the old words after 12 hours of wakefulness and responded as strongly as for new words. Thus, while repeated rehearsal initiated systems memory consolidation, sleep was required to stabilize these changes.
A steady increase in PPC activity has previously been shown to reflect the gradual formation of a neocortical representation of spatial memory (5). Memory-related activity in the PPC is specific to the learned content, reflects memory performance, and remains stable for more than 12 hours (3). Here, we show that the same is true for verbal learning: Memory-specific activity gradually increases in the parietal cortex over the course of rehearsals. Simultaneously, we observe a fast decline in the contribution of the hippocampus. Our findings conform with the dual-storage model of memory, which predicts such an interaction between memory systems (1), although the time frame of this transition is faster than previously assumed (1, 10, 15). In this model, the hippocampus is required during the first stage of memory storage. Our data show that this initial stage might be of very short duration. A number of questions, however, remain open. For one, it is unclear whether both systems retain the same type of information or whether they store different aspects of memory. Previous models suggest that the hippocampus might store specific learning episodes, e.g., individual-learning repetitions, whereas the neocortex learns abstract relations, i.e., semantic knowledge. Our findings would also conform with a model of parallel encoding into the hippocampus and the neocortex. While the hippocampal trace would be faster but transient, the neocortical trace would develop independently over rehearsal repetitions. However, as most of the current literature, especially from patients with hippocampal lesions, suggests that neocortical encoding of declarative material is impossible, we would not suggest this conclusion without further evidence.
The PPC is involved in a wealth of memory tasks (4, 6, 8, 16). Its contribution has been shown to be independent of confidence (17), imagery (18), motor demands, and the nature of stimuli (19). Instead, the pattern of BOLD responses in the PPC suggests that it actually harbors a behaviorally relevant memory representation (3, 8, 20). This is in line with studies reporting deficits in recognition, recollection, episodic memory, and spatial learning after lesions to the PPC (21, 22). We found two regions in the PPC that change over rehearsal: the precuneus and the IPL. Precuneus activity appears consistently in our own and other studies on memory recall using different kinds of material (3, 23), and we could recently show a physical memory engram in that area (5). Thus, we believe it to be a central, material unspecific hub of semantic memory, which has a strong connectivity to the hippocampus (24). The IPL has substantial anatomical connections to the precuneus and the hippocampus. It is often linked to semantic comprehension of words (25, 26). The increase in IPL activity over repetitions indicates that it is not only related to stimulus processing, which should remain constant over rehearsal, but suggests that it is memory related. One possible process mediated by the IPL could be the integration of new information into the semantic network, as repeated rehearsal has been proposed to benefit this process (10). Generally speaking, our findings are compatible with the view that semantic memories are stored in the same location that processes this information and not—or not only—in dedicated memory areas.
The strong hippocampal response during initial rehearsal suggests a role of the hippocampus in memory encoding (3). This role of the hippocampus for fast encoding is consistent with its ability for single-trial learning (27). Together with its capacity to code space and time (28), it is particularly suited to encode episodic memories, which by their nature represent unique temporospatial events (29). Decreases in hippocampal activity over learning are sometimes attributed to repetition suppression (30, 31). Repetition suppression, which was initially proposed as a possible underlying mechanism of priming (32), is usually observed as an immediately decreased synaptic response to repeated stimulation. Although it can thus not directly explain long-lasting changes in brain activity (33), the concept has later been used to account for decreases in hippocampal BOLD responses over repeated learning (30). This interpretation is based on mechanisms such as sparse coding, which exist in the neocortex but have not been observed in the hippocampus so far (32). Notwithstanding, even if repeated encoding leads to sharpening and reduction of the neural response, residual hippocampal activity should still be detectable. However, we did not find any hippocampal response for well-learned words compared to a control condition that is known to maximally suppress hippocampal activity. We therefore argue that the most parsimonious interpretation is that decreased hippocampal response indicates an absence of new encoding. This view is compatible with the large body of evidence from patient studies with hippocampal lesions, showing that an intact hippocampus is required for fast acquisition of new declarative memories (9, 34), as those studies do not provide insight into the dynamics of hippocampal involvement over multiple learning repetitions.
In the present study, we mainly analyzed brain activity during repeated memory encoding. With the increasing number of repetitions and the concurrent buildup of memory representations, it is likely that later encoding trials contain a larger proportion of retrieval activity than earlier trials. We have therefore additionally analyzed the activity during successful free recall, which does not include new encoding. We find the same increase in activity in the precuneus that we see during encoding. Thus, the increase cannot be explained as a function of retrieval per se but only as a function of which brain network is used for retrieval. The pattern of activity also cannot be interpreted as an increasingly vivid “reinstatement” of encoding activity during retrieval because initial encoding should induce as much vividness as late retrieval. As precuneus activity emerges only slowly with the development of long-term memory in both encoding and retrieval, we believe that our data should be interpreted in terms of a systems interaction between decreasing hippocampus and increasing parietal cortex or in terms of an independent formation of a parietal memory trace.
Sleep only affected hippocampal responses in the present experiment. This is in line with other studies showing a change in hippocampal activity over sleep (35, 36). In previous studies, which also observed a decline in hippocampal activity with sleep over extended consolidation intervals, this was mainly interpreted as the result of sleep-dependent memory reactivation leading to a strengthening of the neocortical memory trace and a concurrent decrease in hippocampal involvement (35, 36). Those studies presented the learning material only once or twice, whereas participants saw each word seven times per session in the present study. Thus, we suggest that intensive rehearsal of the material leads to rapid neocortical plasticity, similar to what has been found with electroencephalography for auditory word learning (37). This induces a strong neocortical memory trace and removes the need for additional reactivation of neocortical circuits during sleep, which would promote systems consolidation for memories that did not receive rehearsal. We believe it is possible that especially the repeated alternation of encoding and retrieval causes this strong neocortical encoding (10). The lack of behavioral advantage of sleep might similarly be attributed to this high number of encoding repetitions, rendering additional reactivation during sleep obsolete. In a similar fashion, learning via fast mapping, which is supposed to induce direct neocortical memory, is not sleep dependent (38). In contrast, we see that the hippocampus returns to its naïve response when old words are restudied after 12 hours of wakefulness but remains stably disengaged if learning is followed by sleep. We believe that these results can be best explained by assuming that sleep most strongly affects the hippocampal memory response. However, the neural changes that underlie these effects of rehearsal and sleep are probably located in neocortical regions outside the hippocampus or in their connections to the hippocampus because the fMRI BOLD signal mainly reflects a region’s input and intrinsic processing rather than its output firing (39). Therefore, it is more likely that either the hippocampus receives less stimulation after rehearsal and sleep or that it is actively inhibited by a neocortical region than that it remains silent after input processing and detecting a lack of novelty. Together, our findings lead us to speculate that sleep has a role that goes beyond providing additional rehearsal through reactivation (40).
The mPFC plays a central role in some models of systems consolidation (41). It is supposed to take over the function of the hippocampus over time (2). This view would suggest a complementary role of the hippocampus and the mPFC. However, in the present data, these two regions are concurrently active during memory encoding, and their activity diminishes over repeated rehearsal. Thus, both areas seem to contribute to the same stage of memory. We suggest that the mPFC assumes mainly strategic executive functions during encoding and, if necessary, during retrieval. With stronger preexisting memory, this function is no longer required. However, with longer retrieval delays and weaker memory traces, strategic memory search might be required again, thus activating the mPFC (36).
We show that systems memory consolidation between the hippocampus and the PPC is initiated rapidly within repeated rehearsal. Our results support a crucial role of the hippocampus during fast memory encoding. Further maintenance of the memory might then depend on posterior parietal regions, which support the long-term storage of semantic memory. Moreover, our results suggest that sleep and rehearsal interact to ensure stable long-term memory storage: While repeated study is sufficient to trigger systems consolidation, these transitions can only be successfully stabilized if sleep follows learning.
MATERIALS AND METHODS
Experimental design and participants
The experiment followed a between-group design to compare the effects of sleep and wakefulness on the contribution of different memory systems to verbal memory. Thirty-two healthy young participants [sex, 21 females and 11 males; age, 23.81 ± 0.69 years (mean ± SD); 16 in the sleep group and 16 in the wake group] took part in the experiment. All participants were native German speakers. They reported to take no medication apart from oral contraceptives, to have no diagnosed psychiatric disorders, and to be nonsmokers. All participants followed a regular sleep schedule with habitual sleep durations between 6 and 9 hours, as reported in sleep diaries and the Munich ChronoType Questionnaire (42). Participants were randomly assigned to either the wake or the sleep group. Sample size was determined on the basis of previous imaging studies on sleep (36) and an a priori power calculation using GPower [repeated measures analysis of variance (ANOVA): effect size f = 0.5, α = 0.05, power 1-β = 0.9, number of groups = 2, number of measurements = 4] resulting in a total sample size of 30. To account for dropouts, 16 participants were scanned in each experimental group (total n = 32). Experimental procedures were approved by the ethics committee of the Department of Psychology, Ludwig-Maximilians-Universität München. All participants gave informed written consent before participating in the experiment. The second session of one participant in the sleep group was excluded from analyses because of technical problems with the MRI scanner.
General procedure and memory task
All participants visited the laboratory for two learning sessions, which were spaced 12 hours apart (see Fig. 1 for the general design). Participants in the wake group were initially scanned between 7:00 a.m. and 11:00 a.m. and instructed to stay awake until the second session, which took place between 7:00 p.m. and 11:00 p.m. For the sleep group, the first session took place between 7:00 p.m. and 11:00 p.m., and the second session took place between 7:00 a.m. and 11:00 a.m. Participants in the sleep group were instructed to sleep normally during the night. Experimental times were adapted to the habitual individual sleep schedules of each participant. Both wakefulness and sleep were monitored by actigraphy (ActiGraph), which confirmed that participants stayed awake (wake group) or slept (sleep group, mean sleep duration, 6.99 ± 0.05 hours).
While lying in the MRI scanner, participants completed a wordlist learning and recall task, which was repeated seven times in each session. During each learning repetition, 28 concrete German nouns were presented one at a time for 2.3 s in the middle of the screen on a colored background. Words were followed by a black screen for 200 ms. Participants were instructed to remember the words and the colors they were presented on. After each repetition, participants were asked to do a free recall of all words they could remember. They were asked to think of one of the words they remembered. When they succeeded, they pressed a button and then spoke the word. After another button press, they were asked to name the color that the word was presented on. Participants could indicate when they could not remember any more words and thereby stop the free recall procedure. The number of recalled words was registered to examine learning success. Word order was randomized within each learning repetition. In the second session, 14 words of the initial list and 14 new words were presented in an identical fashion. Because of technical difficulties, one participant completed only five learning repetitions in both sessions, and two additional participants completed only five learning repetitions during the first session.
An odd-even number judgment task interrupted rehearsal at jittered intervals between 30 and 75 s. Random digits between zero and nine were presented on the screen, and participants had to indicate whether the shown digit was odd or even by button press. Feedback on decisions was given. If response times exceeded 1.5 s, “too slow” appeared on the screen. This task was implemented to supply a baseline for analysis because it strongly suppresses hippocampal activity (9).
Data acquisition
Imaging data were acquired using a 3T scanner (Siemens TIM Trio) and a 32-channel head coil. Functional images were obtained with a T2*-weighted single-echo planar imaging sequence [voxel size = 3 mm isotropic, 36 slices, interslice gap = 3.3 mm; repetition time (TR) = 2250 ms, echo time (TE) = 30 ms, flip angle = 80°, acquisition matrix = 64 by 64, field of view (FOV) = 192 mm by 192 mm]. Scan numbers differed between participants as the free recall was self-paced (session 1: m = 747.77, SEM = 27.56; session 2: m = 751.13, SEM = 22.65). Structural images for registration purposes were acquired by a magnetization-prepared rapid gradient-echo sequence (voxel size = 1 mm by 1 mm, slice thickness = 1.2 mm, TR = 2300 ms, TE = 2.91 ms, flip angle = 9°, inversion time = 900 ms, FOV = 240 mm by 256 mm) in the beginning of the first scanning session.
Head motion was minimized by positioning participants firmly inside the scanner using foam cushions. Analysis of movement parameters showed no speech-related lateral movements >3 mm in most participants (4 mm in two participants) and no head rotation >1°.
Preprocessing
Preprocessing and statistical analyses of fMRI data were performed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK) on MATLAB R2012a (MathWorks, Sherborn, MA). The first five scans were discarded to allow for saturation effects. Preprocessing included realignment of the time series of images to the first volume of each session for each participant using a least-squares approach and a six-parameter rigid-body spatial transformation. Functional scans of both sessions were then registered to the participant’s individual structural scans before being normalized to standard stereotaxic Montreal Neurological Institute space and then smoothed with an 8-mm full width at half maximum (FWHM) Gaussian kernel to make activations between participants comparable.
Subject-level modeling
General linear models (GLMs) were fitted individually for each participant and session using a mixed-effects model. Low-frequency drifts were removed by implementing low-pass filtering at a cutoff of 128 s in the matrix design. Serial correlations in the fMRI signal were estimated by using a first-order autoregressive plus white noise model and a restricted maximum likelihood (ReML) algorithm. Changes in BOLD responses were estimated by convolving the modeled epochs of data with a canonical hemodynamic response function and estimating parameters using an ReML approach at each voxel for every participant.
Multiple first-level GLMs were calculated for our analyses. In all GLMs, six head motion parameters, their six derivatives, and the intervals during which participants were speaking were added as regressors of no interest. For the learning contrasts of the first session (Fig. 2), we used the following additional regressors: Each learning repetition was modeled as one regressor using the onsets and duration (2.3 s) of word presentation; all retrieval repetitions were modeled as one regressor that included onsets and durations until button press. We then tested increasing and decreasing linear contrasts over learning repetitions against an implicit baseline consisting of the odd-even number judgment task, which was not explicitly modeled. These summary statistic images were smoothed with a 6-mm FWHM Gaussian kernel before entering group-level statistics.
To test for effects of recall activity (Fig. 3), we modeled a GLM as above, but instead of learning, retrieval repetitions were modeled separately, i.e., all onsets and durations of retrieved words within each recall repetition were used as one regressor. We then tested increasing and decreasing linear contrasts over recall repetitions against the implicit baseline. Summary statistic images were smoothed with a 6-mm FWHM Gaussian kernel before entering group-level statistics.
The GLM for the second session (Figs. 4 to 6) was identical to the encoding model of the first session except that encoding repetitions were separately modeled for new and old words. Each encoding repetition for new words consisted of the onsets and durations of all new words within that encoding repetition, and each encoding repetition for old words consisted of the onsets and durations of all old words within that encoding repetition. We then tested increasing and decreasing linear contrasts of encoding repetitions for old and new words against the implicit baseline. These summary statistic images were smoothed with a 6-mm FWHM Gaussian Kernel before entering group-level statistics.
Group-level statistics
Data of all participants were combined in full factorial models to calculate main effects and interactions, which were tested with one-sided t contrasts. Changes in BOLD activity over repeated rehearsal in the first session were analyzed by comparing the first and the last learning repetitions, as we have done in a previous study (3). Results of these analyses were corrected for multiple testing by family-wise error (FWE) correction for the whole brain (PFWE ≤ 0.05) on clusters with an extent of at least 20 contiguous voxels. FWE-corrected clusters in the left hippocampus, mPFC, right precuneus, and left IPL were extracted as three-dimensional images and served as ROIs for subsequent analysis of independent data from the second session. The precuneus, hippocampus, and mPFC were regions of a priori interest because of their central roles in dual-storage models of memory (2, 3, 5). The IPL was selected for further analysis on the basis of its known involvement in visual word processing.
To show the detailed development of the BOLD signal over learning repetitions, we also extracted the mean β estimates for all seven repetitions from all voxels in these clusters. We then performed linear regression analyses on these β estimates to show linear decreases and increases in activity over repetitions within these clusters. Linear regression statistics were additionally performed on the β estimates in these clusters on the single-subject level. To test for significance, single-subject regression weights were then Fisher z-transformed and tested against zero by a two-sided t test. Mean regression weights were calculated by a back transformation, the mean of standardized single-subject regression weights. Furthermore, we analyzed differences between old and new words during the first repetition of the second session to investigate whether the changes induced in the first session reflected long-term memory.
In the last analysis, we examined the three-way interaction of the within-subject factors first/last repetition and old/new words and the between-subjects factor sleep/wake. Because this analysis has considerably lower power than the analyses of the first session (group size and number of stimuli were both half of those of the initial session), we decided to test this interaction not on the full volume but within anatomical ROIs that were indicated by the analyses of the first session (left hippocampus and right precuneus). Masks for the left hippocampus and the right precuneus were extracted from the automated anatomical labeling atlas (43). Mean β values were extracted from these ROIs. We used repeated measures ANOVAs and one-sided t tests on the β estimates to further delineate the effect observed in the three-way interaction. In addition, the β estimates from all learning repetitions were analyzed by single-subject linear regressions (as described above).
Correlation statistics of the β estimates and memory performance were done on the single-subject level. Correlation values were Fisher z-transformed to test correlations for significance across participants by a two-sided t test. Reported mean correlation values were calculated by a back transformation of the mean of standardized single-subject r values. To study the correlations between the β estimates and memory performance in more detail, we performed a bootstrapped Bayesian multilevel mediation analysis (R package “bmlm: Bayesian Multilevel Mediation”, M. Vuorre; https://cran.r-project.org/package=bmlm). The β estimates of the hippocampus, precuneus, and IPL were used as predictors and mediators; performance was used as outcome variable. The β estimates came from the seven repetitions of the first session. This analysis provides the direct influence of the predictor on the outcome when removing the influence of the mediator.
Supplementary Material
Acknowledgments
We thank V. Flanagin and K. Bothe for assistance with equipment and data acquisition. Funding: This study was supported by the Deutsche Forschungsgemeinschaft (grant GA730/3-1). Author contributions: L.H., M. Schönauer, and S.G. designed the experiment, collected and analyzed the data, and wrote the manuscript. D.P.J.H. and M. Schabus designed the experiment, collected the data, and contributed to writing the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav1695/DC1
Fig. S1. Memory performance.
Table S1. List of regions with decreasing activity over repeated learning (session 1).
Table S2. List of regions with increasing activity over repeated learning (session 1).
Table S3. List of regions with activity changes over repeated recall (session 1).
Table S4. List of regions with decreasing activity over repeated learning of new words (session 2).
Table S5. List of regions with increasing activity over repeated learning of new words (session 2).
Table S6. List of regions with a stronger response to old compared to new words (session 2).
Table S7. List of regions with a stronger response to new compared to old words (session 2).
REFERENCES AND NOTES
- 1.McClelland J. L., McNaughton B. L., O’Reilly R. C., Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Frankland P. W., Bontempi B., The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Brodt S., Pöhlchen D., Flanagin V. L., Glasauer S., Gais S., Schönauer M., Rapid and independent memory formation in the parietal cortex. Proc. Natl. Acad. Sci. U.S.A. 113, 13251–13256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilmore A. W., Nelson S. M., McDermott K. B., A parietal memory network revealed by multiple MRI methods. Trends Cogn. Sci. 19, 534–543 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Brodt S., Gais S., Beck J., Erb M., Scheffler K., Schonauer M., Fast track to the neocortex: A memory engram in the posterior parietal cortex. Science 362, 1045–1048 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rugg M. D., King D. R., Ventral lateral parietal cortex and episodic memory retrieval. Cortex 107, 238–250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ester E. F., Sprague T. C., Serences J. T., Parietal and frontal cortex encode stimulus-specific mnemonic representations during visual working memory. Neuron 87, 893–905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhl B. A., Chun M. M., Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J. Neurosci. 34, 8051–8060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark C. E. L., Squire L. R., Simple and associative recognition memory in the hippocampal region. Learn. Mem. 8, 190–197 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antony J. W., Ferreira C. S., Norman K. A., Wimber M., Retrieval as a fast route to memory consolidation. Trends Cogn. Sci. 21, 573–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schönauer M., Pawlizki A., Köck C., Gais S., Exploring the effect of sleep and reduced interference on different forms of declarative memory. Sleep 37, 1995–2007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahdat S., Fogel S., Benali H., Doyon J., Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. eLife 6, e24987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gais S., Born J., Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 101, 2140–2144 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji D., Wilson M. A., Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Takashima A., Petersson K. M., Rutters F., Tendolkar I., Jensen O., Zwarts M. J., McNaughton B. L., Fernández G., Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc. Natl. Acad. Sci. U.S.A. 103, 756–761 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnici H. M., Richter F. R., Yazar Y., Simons J. S., Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J. Neurosci. 36, 5462–5471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urgolites Z. J., Smith C. N., Squire L. R., True and false memories, parietal cortex, and confidence judgments. Learn. Mem. 22, 557–562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt D., Krause B. J., Mottaghy F. M., Halsband U., Herzog H., Tellmann L., Muller-Gärtner H. W., Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia 40, 457–470 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Schoo L. A., van Zandvoort M. J., Biessels G. J., Kappelle L. J., Postma A., de Haan E. H., The posterior parietal paradox: Why do functional magnetic resonance imaging and lesion studies on episodic memory produce conflicting results? J. Neuropsychol. 5 (Pt. 1), 15–38 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Jeong S. K., Xu Y., Behaviorally relevant abstract object identity representation in the human parietal cortex. J. Neurosci. 36, 1607–1619 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berryhill M. E., Phuong L., Picasso L., Cabeza R., Olson I. R., Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 27, 14415–14423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Zvi S., Soroker N., Levy D. A., Parietal lesion effects on cued recall following pair associate learning. Neuropsychologia 73, 176–194 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Addis D. R., McIntosh A. R., Moscovitch M., Crawley A. P., McAndrews M. P., Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage 23, 1460–1471 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Binder J. R., Conant L. L., Humphries C. J., Fernandino L., Simons S. B., Aguilar M., Desai R. H., Toward a brain-based componential semantic representation. Cogn. Neuropsychol. 33, 130–174 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Devlin J. T., Matthews P. M., Rushworth M. F. S., Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 15, 71–84 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Seghier M. L., The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19, 43–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutishauser U., Mamelak A. N., Schuman E. M., Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron 49, 805–813 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Eichenbaum H., Time (and space) in the hippocampus. Curr. Opin. Behav. Sci. 17, 65–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesner R. P., Rolls E. T., A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci. Biobehav. Rev. 48, 92–147 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Kovács G., Schweinberger S. R., Repetition suppression—An integrative view. Cortex 80, 1–4 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Desimone R., Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. U.S.A. 93, 13494–13499 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotts S. J., Incremental learning of perceptual and conceptual representations and the puzzle of neural repetition suppression. Psychon. Bull. Rev. 23, 1055–1071 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Gonsalves B. D., Kahn I., Curran T., Norman K. A., Wagner A. D., Memory strength and repetition suppression: Multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47, 751–761 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Insausti R., Annese J., Amaral D. G., Squire L. R., Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc. Natl. Acad. Sci. U.S.A. 110, E1953–E1962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takashima A., Nieuwenhuis I. L., Jensen O., Talamini L. M., Rijpkema M., Fernández G., Shift from hippocampal to neocortical centered retrieval network with consolidation. J. Neurosci. 29, 10087–10093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gais S., Albouy G., Boly M., Dang-Vu T. T., Darsaud A., Desseilles M., Rauchs G., Schabus M., Sterpenich V., Vandewalle G., Maquet P., Peigneux P., Sleep transforms the cerebral trace of declarative memories. Proc. Natl. Acad. Sci. U.S.A. 104, 18778–18783 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shtyrov Y., Nikulin V. V., Pulvermüller F., Rapid cortical plasticity underlying novel word learning. J. Neurosci. 30, 16864–16867 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himmer L., Müller E., Gais S., Schönauer M., Sleep-mediated memory consolidation depends on the level of integration at encoding. Neurobiol. Learn. Mem. 137, 101–106 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Logothetis N. K., Pauls J., Augath M., Trinath T., Oeltermann A., Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Schönauer M., Grätsch M., Gais S., Evidence for two distinct sleep-related long-term memory consolidation processes. Cortex 63, 68–78 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Euston D. R., Gruber A. J., McNaughton B. L., The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roenneberg T., Kuehnle T., Juda M., Kantermann T., Allebrandt K., Gordijn M., Merrow M., Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M., Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav1695/DC1
Fig. S1. Memory performance.
Table S1. List of regions with decreasing activity over repeated learning (session 1).
Table S2. List of regions with increasing activity over repeated learning (session 1).
Table S3. List of regions with activity changes over repeated recall (session 1).
Table S4. List of regions with decreasing activity over repeated learning of new words (session 2).
Table S5. List of regions with increasing activity over repeated learning of new words (session 2).
Table S6. List of regions with a stronger response to old compared to new words (session 2).
Table S7. List of regions with a stronger response to new compared to old words (session 2).


