Abstract
Much effort has been directed at studying the orexigenic actions of administered ghrelin and the potential effects of the endogenous ghrelin system on food intake, food reward, body weight, adiposity, and energy expenditure. Although endogenous ghrelin’s actions on some of these processes remain ambiguous, its glucoregulatory actions have emerged as well-recognized features during extreme metabolic conditions. The blood glucose–raising actions of ghrelin are beneficial during starvation-like conditions, defending against life-threatening falls in blood glucose, but they are seemingly detrimental in obese states and in certain monogenic forms of diabetes, contributing to hyperglycemia. Also of interest, blood glucose negatively regulates ghrelin secretion. This article reviews the literature suggesting the existence of a blood glucose–ghrelin axis and highlights the factors that mediate the glucoregulatory actions of ghrelin, especially during metabolic extremes such as starvation and diabetes.
The search for the receptor that mediates the effects of small molecule GH secretagogues led to cloning of the GH secretagogue receptor (GHSR) from swine and human pituitary cDNA libraries in 1996, and 3 years later, the discovery of the endogenous GHSR agonist ghrelin from rat stomachs (1, 2). Circulating ghrelin was subsequently proved to be derived primarily from the stomach (3, 4), although in the fetal period, ghrelin expression predominates in pancreatic islets (5–11). Ghrelin-producing islet ε-cells are difficult to find in adult mice, and in humans, they comprise only ∼1% of the endocrine islet cell types (8, 10, 12). The acylated (mostly octanoylated) form of ghrelin (referred to mainly as “ghrelin” throughout this review, or as acyl-ghrelin) works by binding to and activating GHSRs, which are expressed in selected neuronal populations, such as orexigenic arcuate hypothalamic agouti-related peptide (AgRP)/neuropeptide Y neurons, and in several peripheral tissues, such as the gastrointestinal tract and pancreatic islets (1, 13–24). GHSRs also have a high degree of constitutive—or rather, ghrelin-independent—activity, accounting for ∼50% of its maximal capacity to stimulate intracellular second messenger signaling cascades, when tested in vitro (25, 26). GHSRs also heterodimerize with and, in turn, modulate signaling by other G protein–coupled receptors [as reviewed in Edwards and Abizaid (27) and Howick et al. (28)]. Other important elements of the ghrelin system besides ghrelin and GHSR include ghrelin O-acyltransferase [GOAT; also known as membrane-bound O-acyltransferase 4 (MBOAT4)], which catalyzes the acylation of ghrelin (29, 30), and liver-enriched antimicrobial peptide 2 (LEAP2), which was described recently as an endogenous antagonist of GHSR (31). Also of note, a major portion of the circulating ghrelin pool is secreted in the unacylated form, which also can be derived by rapid deacylation of the circulating acyl-ghrelin by actions of enzymes including butyrylcholinesterase and acyl protein thioesterase 1 (32, 33). Unacyl-ghrelin has poor affinity for GHSR, and it is uncertain how its actions, which are in some instances opposite to those of acyl-ghrelin, are mediated (13).
Although much of the initial and ongoing efforts of ghrelin research relate to orexigenic effects of administered ghrelin and potential effects of the endogenous ghrelin system on food intake, food reward, body weight, adiposity, and energy expenditure [as reviewed in Müller et al. (13), Andrews (20), Mani and Zigman (34), Yanagi et al. (35), and Al Massadi et al. (36)], the focus of the current review is blood glucose. In particular, this review outlines and provides examples of the relationship between ghrelin and blood glucose, the factors mediating the glucoregulatory actions of ghrelin, the impact of ghrelin’s contributions to glucoregulation during metabolic extremes (such as starvation and diabetes), and the potential for ghrelin system modulators to treat conditions associated with uncontrolled blood glucose. We place particular emphasis on the recruitment by ghrelin of different sets of downstream effectors depending on the setting at hand. For other perspectives on this topic, we direct the readers to the excellent reviews of others (13, 37–40).
Ghrelin Regulates Blood Glucose
Early evidence of ghrelin’s effects on blood glucose includes studies in humans in which ghrelin administration acutely increased blood glucose (41, 42). Subsequent studies in rodents similarly have demonstrated acute blood glucose–raising actions of administered ghrelin (43). Ghrelin administration also worsens glucose tolerance in humans and rodents (44–46), as does high circulating ghrelin resulting from transgenic manipulations (47, 48). Thus, increases in circulating ghrelin have consistently been noted to raise blood glucose and worsen glucose tolerance. Conversely, administration of GHSR or GOAT antagonists lowers fasting blood glucose and improves glucose tolerance in mice (46, 49–51). Although in ad libitum–fed states genetic mouse models deficient in ghrelin function—including ghrelin-knockout (KO), GOAT-KO, GHSR-KO, and GHSR-null mice—exhibit relatively similar blood glucose levels as compared with wild-type mice, when challenged with administered glucose, they exhibit improved glucose tolerance when on regular chow diet or when on obesogenic high-fat diets (51–55). Furthermore, the improved glucose tolerance in the latter sets of mice fed a high-fat diet is not accompanied by major differences in body weight (54–59). Thus, even though plasma ghrelin is generally found to be lower in mouse models of diet-induced obesity (60–62), this lower amount of plasma ghrelin is sufficient to worsen glucose tolerance in a body weight–independent manner. Interestingly, 2-hour ghrelin infusion increases blood glucose in both lean humans and humans with obesity, with higher increments in blood glucose occurring in the subjects with obesity as compared with the lean subjects (63). Therefore, in contrast to the development of resistance to ghrelin actions to stimulate food intake in individuals with obesity (64), ghrelin’s blood glucose–raising actions persist—or even paradoxically become enhanced—in obese settings (53, 63, 65–67).
The glucoregulatory actions of ghrelin also have been investigated in the settings of caloric restriction and weight loss surgery. For example, mouse models lacking ghrelin function exhibit lower blood glucose after overnight fasting (53, 59, 68, 69). This escalates to life-threatening hypoglycemia under more chronic and severe calorie restriction conditions (55, 70, 71). In human subjects with obesity 2 weeks after having undergone Roux-en-Y gastric bypass (RYGB), ghrelin infusion was ineffective at increasing blood glucose, even though ghrelin infusion did increase blood glucose in the same individuals before RYGB (63). Furthermore, although plasma ghrelin levels dropped and glucose tolerance improved in wild-type mice following vertical sleeve gastrectomy, glucose tolerance also improved similarly in ghrelin-KO mice following vertical sleeve gastrectomy (72). These latter data thus suggest that in the setting of vertical sleeve gastrectomy, reduced ghrelin does not contribute to the improved glucose tolerance (72). In summary, the blood glucose–raising actions of ghrelin are highly dependent on the nutritional and metabolic state of the individual.
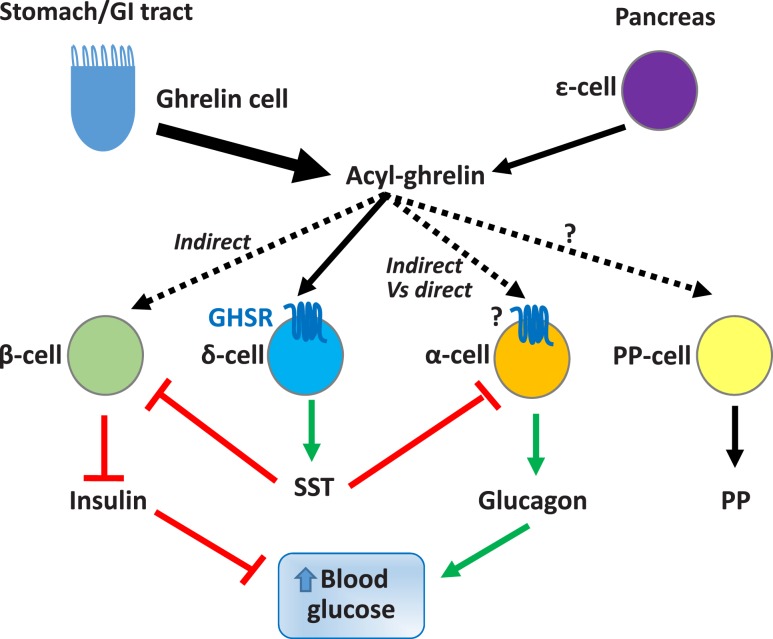
Ghrelin-induced effects on blood glucose are mediated via changes to multiple processes involving several downstream effectors (Fig. 1). These include reduction of insulin sensitivity. Also, these include either direct or indirect effects on several of the major pancreatic islet endocrine cell types (Fig. 2), for instance, suppression of insulin secretion from pancreatic β-cells. Other factors include modulation of glucagon secretion from pancreatic α-cells and stimulation of somatostatin (SST) secretion from pancreatic δ-cells, glucagon-like peptide-1 (GLP-1) secretion from intestinal L-cells, and GH secretion from pituitary somatotrophs. Stimulation of food intake is also likely important. The role of glucocorticoids, the levels of which are increased by ghrelin, in ghrelin-associated blood glucose regulation has not yet been thoroughly investigated (34). Although further research is needed to determine the requirement and sufficiency of each of these factors in mediating ghrelin’s glucoregulatory actions, what does seem apparent is that their recruitment by ghrelin is dependent on the nutritional and metabolic state of the individual. Below, we describe the major factors known to mediate ghrelin’s glucoregulatory actions.
Figure 1.
Factors mediating ghrelin actions on glucose homeostasis. Depending on the nutritional and metabolic setting, ghrelin actions trigger distinct sets of downstream effectors to modulate blood glucose. In individuals with obesity, ghrelin reduces insulin sensitivity, contributing to hyperglycemia and worsened glucose intolerance. In postprandial settings, ghrelin’s glucoregulatory actions involve inhibition of insulin secretion from pancreatic β-cells, reduction in insulin sensitivity, and “priming” of enteroendocrine L-cells to stimulate GLP-1 release. During fasting conditions, ghrelin increases blood glucose by stimulating glucagon secretion through its actions on pancreatic islets and the brain, and it enhances hepatic glucose production via actions on the brain. Stimulation of food intake is another plausible way by which central nervous system actions of ghrelin could support blood glucose. In fat-depleted, starvation-like states, stimulation of GH secretion resulting in sustained generation of gluconeogenic substrates for use by the liver appears to be key to preventing life-threatening hypoglycemia.
Figure 2.
Pancreas and ghrelin. The adult pancreatic islet has four major endocrine cell types: insulin-secreting β-cells, SST-secreting δ-cells, glucagon-secreting α-cells, and pancreatic polypeptide(PP)–secreting cells (also called F cells). A fifth endocrine islet cell type, the ghrelin-secreting ε-cell, is prominent in the fetal period in humans and mice, although its numbers gradually decrease postnatally, and the overall significance of islet-derived ghrelin as compared with ghrelin derived from the gastrointestinal (GI) tract has yet to be fully determined. The capacity for ghrelin to inhibit glucose-stimulated insulin secretion is almost uniformly reported and includes effects on islet endocrine cells. Although earlier studies suggest that ghrelin inhibits insulin secretion by direct engagement of GHSRs expressed on β-cells, more recent transcriptomic and functional studies strongly suggest that β-cells do not express GHSR and, furthermore, that ghrelin’s insulinostatic actions are instead mediated indirectly via stimulation of SST secretion from GHSR-expressing δ-cells. Ghrelin’s effects on glucagon secretion are mixed in the literature. Although GHSRs have been found in pools of isolated α-cells and in α-cell lines, this finding is not universal. Other studies suggest that ghrelin’s actions on glucagon release may also or instead be indirect via activation of GHSRs expressed in arcuate AgRP neurons (not depicted in the figure) or by δ-cells. The actions of ghrelin on PP secretion and GHSR expression by PP cells, and the overall role of PP as a potential downstream effector of ghrelin’s glucoregulatory actions remain to be clarified.
Insulin secretion and insulin sensitivity
Reduction of insulin secretion and/or insulin sensitivity forms a major conduit through which ghrelin raises blood glucose and induces glucose intolerance in postprandial settings and obese conditions. Most ghrelin administration studies demonstrate that ghrelin reduces insulin secretion, although there are some studies that alternatively suggest that ghrelin has no effect on insulin secretion or that ghrelin increases insulin secretion (38, 46, 51, 52, 73–75). In mice, for example, IP or IV injections of ghrelin dose-dependently blunt glucose-stimulated insulin secretion (67, 76). In contrast, pharmacologic inhibition of GOAT activity enhances glucose-stimulated insulin secretion and improves glucose tolerance in chow-fed mice (77). The improved glucose tolerance observed in GHSR-null and ghrelin-KO mice also is accompanied by enhanced glucose-stimulated insulin secretion (51, 52) and is not thought to be due to changes to the density, sizes, insulin protein content, or insulin mRNA level of their islets (52). Attenuation of glucose intolerance in high-fat diet–fed ghrelin-KO and GOAT-KO mice is associated with enhanced insulin responses (52, 55). Oppositely, transgenic mice with hyperghrelinemia due to overexpression of bioactive ghrelin exhibit reduced glucose-stimulated insulin secretion (47). Exogenous infusion of ghrelin also suppresses postprandial insulin secretion as well as glucose-stimulated insulin secretion in human subjects (41, 44, 78–80). The significance of endogenous ghrelin actions on insulin secretion, however, is understudied in humans.
The mechanisms by which ghrelin suppresses insulin most likely include direct effects on pancreatic islets. Supporting this notion, ghrelin suppresses insulin secretion from isolated rodent pancreata and freshly isolated or cultured pancreatic islets, whereas pharmacological blockade of GHSR actions enhances insulin release (46, 51, 52, 74, 76, 81, 82). Ghrelin suppression of glucose-stimulated insulin secretion is absent in pancreatic islets isolated from GHSR-null mice, suggesting that this action of ghrelin is dependent on GHSR expression (51). A handful of early studies suggested that these effects are mediated at least in part via direct ghrelin engagement of GHSRs expressed on pancreatic β-cells. For example, ghrelin, in a GHSR-dependent manner, has been shown to attenuate glucose-induced increases in intracellular calcium concentration ([Ca2+]i) and action potential firing in single pancreatic β-cells isolated from mouse islets and identified by insulin immunohistochemistry (46, 51). These changes to [Ca2+]i and action potential firing in β-cells were linked to changes in pertussis toxin–sensitive G protein Gαi2 signaling, nonselective cation currents through the transient receptor potential melastatin 2 (TRPM2), and voltage-dependent KV potassium currents (51, 73, 74). Also, selective GHSR expression only in pancreatic β-cells—made possible by crossing transgenic mice with rat insulin promoter–driven Cre recombinase (Ins-Cre) to mice with Cre-dependent GHSR expression—restores the insulinostatic actions of endogenous ghrelin and worsens glucose tolerance induced by exogenous ghrelin (51). GHSR expression also is detectable in a few insulinoma cell lines [INS-1SJ, INS-1 (832/13), INS-1E, βTC6, and MIN6] (6, 43, 83, 84). Furthermore, ghrelin can reduce glucose-stimulated insulin release from immortalized pancreatic β-cells (6, 84).
However, more recent studies make a strong case for indirect effects of ghrelin on β-cells, as opposed to the direct effects suggested by the earlier studies (85, 86). For instance, using transcriptomic profiling, these studies demonstrate that β-cells do not express GHSR (85, 86). Instead, using RNA sequencing, in situ hybridization histochemistry, and quantitative PCR, these studies found high expression of GHSRs by pancreatic δ-cells (85, 86). Additionally, ghrelin-mediated increases in [Ca2+]i within δ-cells in intact mouse pancreatic islets (86), ghrelin stimulation of SST secretion in perfused pancreas models (81, 85) and from isolated pancreatic islets (86), and the failure of ghrelin to inhibit insulin secretion in the presence of an SST receptor antagonist (85, 86) have been reported. Thus, these findings support a model by which ghrelin-inhibited insulin secretion occurs indirectly via stimulation of SST secretion from GHSR-expressing pancreatic δ-cells and, in turn, SST engagement of SST receptors localized to the β-cells (85, 86) (Fig. 2). We direct the reader to the papers cited above for a more thorough analysis of the data supporting indirect vs direct effects of ghrelin on insulin release.
Glucose clamp studies in mouse models deficient in ghrelin function reveal that ghrelin also affects insulin sensitivity. For instance, chow-fed ghrelin-KO mice (67) and diet-induced obese GHSR-KO mice (53, 65, 66) require higher glucose infusion rates during hyperinsulinemic-euglycemic clamps, suggesting that deletion of ghrelin or GHSR improves insulin sensitivity. Obese ghrelin-KO and GHSR-KO mice also exhibit lower hepatic glucose production and higher insulin-stimulated glucose disposal (66, 67). As an example, glucose-clamped obese ghrelin-KO mice demonstrate higher glucose uptake in several peripheral organs, including skeletal muscle, brown adipose tissue, and white adipose tissue (66). Blood glucose is lower following a pyruvate tolerance test in obese ghrelin-KO mice as compared with wild-type littermates, suggesting a lower rate of hepatic gluconeogenesis in ghrelin-KO mice (66). Similarly, chronic dosing of GHSR antagonists to obese wild-type mice markedly improves insulin sensitivity, as demonstrated using both glucose tolerance and hyperglycemic clamp tests, and as characterized by improved glucose disposal with decreased insulin secretion (53). Hyperinsulinemic-euglycemic clamp studies in healthy or gastrectomized humans show that ghrelin infusion lowers glucose disposal rate, suggesting that ghrelin increases insulin resistance (78, 87, 88). Another human study using hyperinsulinemic-euglycemic clamps demonstrates that ghrelin infusion increases insulin resistance in both lean humans and humans with obesity, and that those actions persist in the individuals with obesity 2 weeks after RYGB (63). Overall, results from rodent models and humans suggest that ghrelin worsens glucose tolerance, in part by lowering insulin sensitivity, and as associated with a lower rate of glucose disposal and a higher rate of hepatic glucose production.
Glucagon
Elevation of plasma ghrelin in mice either by chronic ghrelin administration or transgenic overexpression by ghrelinomas leads to higher plasma glucagon and increased blood glucose, whereas, in contrast, GHSR-null mice exhibit lower fasted plasma glucagon and reduced blood glucose (43). Combining these results with the observations of ghrelin-induced glucagon secretion from isolated mouse islets and from the pancreatic α-cell lines αTC1 and InR1G9 and the amplification of GHSR mRNA from those α-cell lines suggests that ghrelin can directly stimulate glucagon secretion from pancreatic α-cells to increase glycemia (43, 82). However, only one of the two transcriptomic studies on pancreatic islets mentioned above (85, 86) demonstrated GHSR mRNA in isolated α-cells, and its levels were fairly low, especially as compared with the expression levels in pooled populations of pancreatic δ-cells. Also, using a perfused mouse pancreas model, the same study demonstrated that ghrelin decreases (rather than increases) glucagon release in an SST-dependent manner, again suggesting the importance of GHSR-expressing pancreatic δ-cells (Fig. 2). That said, acyl-ghrelin administration increases plasma glucagon when administered in vivo to mice (43), suggesting that any indirect effects of ghrelin to inhibit glucagon secretion via SST are unlikely to be a predominant factor in ghrelin’s overall effects on circulating glucagon levels, at least in the setting of acyl-ghrelin administration. Indirect effects of ghrelin on glucagon release also appear to occur via the arcuate hypothalamic nucleus. In particular, fasted mice with arcuate AgRP/neuropeptide Y neuron-limited GHSR expression exhibit plasma glucagon and blood glucose levels similar to those of fasted wild-type mice, whereas fasted GHSR-null mice have lower plasma glucagon and blood glucose (43, 89). Plasma glucagon, however, is unaltered in ad libitum–fed GOAT-KO mice (55). Therefore, it appears that ghrelin’s potential to employ glucagon as a pathway to raise blood glucose is restricted to the setting of an overnight fast and does not occur during nonfasted conditions. Further studies are needed to determine the requirement of glucagon action in mediating ghrelin’s glucoregulatory actions during those conditions. In human subjects, by the way, an acute injection or a 2-hour infusion of ghrelin does not alter plasma glucagon (44, 90, 91), although these pharmacological studies do not rule out potential paracrine actions of islet-derived acyl-ghrelin on glucagon secretion.
GH
GH serves as an essential mediator of ghrelin’s glucoregulatory actions in the setting of chronic severe caloric restriction. In particular, when individually housed adult mice that lack key components of the ghrelin system (ghrelin, GOAT, GHSR) are provided access to only 40% of their usual daily calories in a week-long, starvation-like model, they develop marked hypoglycemia, leading to increased mortality (55, 70, 71, 89, 92). Deficiency of ghrelin secretion due to ghrelin cell–specific deletion of β1-adrenergic receptors also results in life-threatening hypoglycemia in mice (60), as does overexpression of the endogenous GHSR antagonist LEAP2 (31) and ablation of ghrelin cells from adult mice (93). In contrast, wild-type mice submitted to the same conditions are protected against the marked hypoglycemia and death due to stimulated ghrelin release, and, in turn, a several-fold progressive elevation of plasma GH. Blunted GH elevation has been observed in both GOAT-KO mice and ghrelin-KO mice over the course of the week-long protocol, whereas GH infusion prevents the marked hypoglycemia and increased mortality associated with the missing acyl-ghrelin response (55, 70, 71). These data not only highlight the critical glucoregulatory role of ghrelin during starvation-like states, but they also point to GH as a mediator of that function. Importantly, also note that during the week-long, starvation-like model, insulin and glucagon are regulated similarly in wild-type and GOAT-KO mice, suggesting that in contrast to GH, changes to those downstream effectors of ghrelin action are not used by ghrelin in the starvation-like environment to prevent hypoglycemia (55).
In chronic calorie-restricted GOAT-KO mice, the lower blood glucose is associated with lower plasma levels of the gluconeogenic substrates lactate and pyruvate (70). Exogenous administration of lactate or pyruvate to the calorie-restricted GOAT-KO mice reverses hypoglycemia, indicating that the blood glucose–protective actions of the ghrelin–GH axis during chronic caloric restriction involves sustained generation of these gluconeogenic substrates (70). Although the pathway or pathways through which ghrelin-engaged GH help sustain blood glucose and generate gluconeogenic substrates during this starvation-like state are not yet completely known, they do involve stimulation of hepatic autophagy (70, 71). In particular, chronic GH administration to GOAT-KO mice during the severe calorie restriction protocol is accompanied by a maintenance of hepatic autophagy, which otherwise is lacking in saline-administered controls (71). However, whereas a single injection of GH also stimulates hepatic autophagy in GOAT-KO mice during the severe calorie restriction protocol, it fails to reverse hypoglycemia, suggesting that the blood glucose–raising actions of the ghrelin–GH axis during chronic severe caloric restriction involve more than just stimulation of hepatic autophagy (55, 71).
The blood glucose–preserving actions of ghrelin during the severe caloric restriction protocol appear to depend on the starting fat mass content and age of the mice as well as the severity of caloric restriction. For instance, when 8-week-old GOAT-KO or GHSR-null mice with starting fat mass content of 8% to 10% are subjected to the above-described “severe” caloric restriction protocol (access to 40% of usual daily calories for a week), which leads to a drop in fat mass content to <2%, the mice become severely hypoglycemic (55, 89). When 9-month-old ghrelin-KO, GOAT-KO, GHSR-KO, or ghrelin-GHSR double KO mice, which have a higher starting fat mass, are subjected to a similar severe chronic caloric restriction protocol, the mice do not exhibit severe hypoglycemia (94). Also, when 10-week-old ghrelin-KO or GHSR-KO mice are subjected to a relatively less severe (50%) caloric restriction protocol for 40 days, blood glucose drops more than it does in similarly treated wild-type mice but nonetheless remains within the normoglycemic range (59). Similarly, overnight or 24-hour fasts in 8- to 22-week-old GHSR-null mice result in lower blood glucoses than in similarly treated wild-type mice, but those blood glucose levels remain in the lower end of the normal range (43, 57, 69, 89, 95).
The glucose-preserving function of ghrelin also appears to be critical in young mice. For instance, when 3-week-old wild-type mice are treated with the beta-blocker atenolol, which lowers plasma ghrelin, they become susceptible to ghrelin deficiency–associated hypoglycemia even after a short overnight fast (60). The blood glucose–raising actions of endogenous ghrelin also have been noted in neonatal Snord116del mice modeling Prader-Willi syndrome, which have a predisposition to hypoglycemia at a young age. Placing Snord116del mice on a ghrelin-KO background further exaggerates the lower blood glucose exhibited by male Snord116del neonates (96). Overall, these results strongly suggest glucose-preserving actions of ghrelin in starvation-like states and in other individuals who are susceptible to hypoglycemia.
Central nervous system actions
Apart from its actions to raise blood glucose via stimulation of food intake, site-specific ghrelin actions within the brain can regulate blood glucose homeostasis. For example, hypoglycemia observed in chronic calorically restricted GHSR-null mice is prevented by selective reexpression of GHSRs in arcuate AgRP neurons (89). This restoration of blood glucose is accompanied by elevated glucagon (described above) and induction of the hepatic gluconeogenic genes glucose-6-phosphatase, phosphoenolpyruvate carboxykinase, and hepatocyte nuclear factor 4α, suggesting that actions of endogenous ghrelin on AgRP neurons can induce hepatic gluconeogenesis (89). Similarly, selective reexpression of GHSR in Phox2B-expressing hindbrain neurons prevents the lower blood glucose otherwise observed in fasted GHSR-null mice (95). However, selective reexpression of GHSR in tyrosine hydroxylase–expressing neurons does not impact fasting blood glucose (68). Conversely, deletion of GHSRs either from all neurons or selectively from arcuate AgRP neurons reduces fasting blood glucose (97, 98). Also, chronic intracerebroventricular ghrelin infusion to mice (pair-fed to match the food intake of mice not receiving ghrelin) induces hepatic gluconeogenesis, suggesting that in conditions simulating negative energy balance, ghrelin action on the brain induces hepatic glucose production (99). These results also suggest that ghrelin’s actions on the brain can control liver function to increase blood glucose independently of its actions to stimulate food intake. Overall, these results suggest that ghrelin actions on the brain are necessary and sufficient to restore blood glucose in various extremes of calorie restriction.
GLP-1
Several data support a model in which preprandial increases in plasma ghrelin prime intestinal L-cells to stimulate nutrient-induced enhancement of GLP-1 secretion (100). Ghrelin administration enhances meal-induced increases in plasma GLP-1 in humans and glucose-induced increases in plasma GLP-1 in mice (79, 100, 101). Ghrelin directly stimulates GLP-1 secretion in murine GLUTag and human NCI-H716 L-cell lines, both of which express GHSR mRNA (100, 101). Ghrelin-induced increases in GLP-1 secretion improve glucose tolerance in mice (100), contrasting with the worsened glucose tolerance usually seen with ghrelin administration in humans (44, 79). In a human trial, combined administration of both ghrelin and the GLP-1 receptor antagonist exendin-(9,39) during a meal tolerance test caused greater postprandial glycemia than did either alone (45). These data suggest that the meal-induced increase in GLP-1 might mitigate ghrelin’s postprandial actions to suppress insulin secretion from pancreatic β-cells. More broadly, these data suggest that the overall effects of the ghrelin system on postprandial glucose turnover are determined by a complex interplay of several potential downstream effectors that include ghrelin and GLP-1, which have opposing actions on insulin secretion, food intake, and gastric emptying.
Unacyl-ghrelin
Unacyl-ghrelin also influences blood glucose homeostasis, although the reported findings have been less consistent than those for acyl-ghrelin. Although several human studies have shown that infusion of unacyl-ghrelin does not alter fasting or postprandial blood glucose or insulin, others have shown either increased postprandial insulin, decreased postprandial blood glucose, and/or improved insulin sensitivity (37, 42, 102–107). Data from some human studies suggest that although unacyl-ghrelin may not have independent glucoregulatory actions, it may oppose those of acyl-ghrelin. For example, coadministration of unacyl-ghrelin and acyl-ghrelin at equal concentrations abolishes the increase in blood glucose and decrease in plasma insulin induced by acyl-ghrelin alone, suggesting that unacyl-ghrelin may counterbalance the glucoregulatory actions of acyl-ghrelin (42). However, another clinical study showed that unacyl-ghrelin does not oppose the glucoregulatory actions of coadministered acyl-ghrelin, even when administered at a fourfold higher concentration than acyl-ghrelin (108). In rodent models, administration of unacyl-ghrelin or its analogs increase glucose-induced insulin secretion and improve insulin sensitivity (109–111). Overexpression of unacyl-ghrelin using a fatty acid–binding protein-4 (FABP-4) promoter in mice, without changes to plasma acyl-ghrelin, improves glucose tolerance and insulin sensitivity (112). Also, the stable unacyl-ghrelin analog AZP-531 has beneficial effects on glucose homeostasis in human subjects with diabetes (103). The receptor(s), sites, and downstream effectors mediating unacyl-ghrelin’s actions on blood glucose homeostasis remain to be identified.
Blood Glucose Regulates Plasma Ghrelin
Plasma ghrelin changes inversely with nutritional status of the individual: caloric restriction increases plasma ghrelin, whereas food ingestion causes a rapid fall in plasma ghrelin in humans and in rodent models (13, 113, 114). Stimulation of ghrelin secretion by the sympathetic nervous system is a principal driver of the former (60, 115–118), whereas increased availability of dietary macronutrients has been proposed to drive the latter. In fact, oral administration of individual dietary macronutrients, including glucose (which we focus on here), amino acids, and fatty acids, each can suppress plasma ghrelin (13, 119). For insights into regulation of ghrelin secretion by amino acids and fatty acids, we direct you to other relevant reviews (13, 119, 120).
Glucose causes a rapid and profound suppression of plasma ghrelin in humans and rodents when administered by either parental or oral routes (69, 121–125). Infusion of glucose or amino acids directly into the stomach, duodenum, or jejunum also suppresses plasma ghrelin in rats (126, 127). That said, it is likely that glucose within the stomach lumen does not itself directly suppress ghrelin release. Instead, several findings suggest that glucose impacts ghrelin release only after it has made its way into circulation. For instance, when gastric emptying is prevented in rats with a pyloric cuff, intragastric administration of glucose fails to suppress plasma ghrelin (127). Also, electron microscopy demonstrates that most gastric ghrelin cells are of the “closed-cell” variety that lack direct access to luminal stomach contents and that are positioned in close proximity to capillaries (128). Furthermore, glucose suppression of plasma ghrelin does not require oral or gastrointestinal administration, but it also can occur when glucose is administered by parenteral routes in humans and rodents (13, 122, 123, 125, 129, 130).
Reduction of plasma ghrelin by blood glucose is likely a result of several factors that include direct inhibition of ghrelin secretion by glucose taken up by and metabolized within ghrelin cells, actions of glucoregulatory hormones including insulin and glucagon that change with administration of glucose, and potential attenuation of the sympathetic drive to ghrelin cells. These are discussed below.
It is likely that a direct effect of glucose on ghrelin cells figures substantially in the overall inhibitory effect of glucose on ghrelin secretion. Exposure of mouse gastric mucosal cell primary cultures to culture medium containing high glucose (10 mM; simulating hyperglycemia) inhibits ghrelin secretion, whereas medium containing 0 mM or low glucose (1 mM; simulating hypoglycemia) stimulates ghrelin secretion as compared with medium containing 5 mM glucose (simulating normoglycemia) (69, 131). Similar to many enteroendocrine cells as well as glucose-sensing islet cells, ghrelin cells express machinery associated with glucose sensing and metabolism. This includes glucokinase, glucose transporters, the sulfonylurea receptor, and the ATP-sensitive potassium (KATP) channel (131). The glucoprivic agent 2-deoxy-d-glucose prevents the inhibitory effect of high glucose on ghrelin secretion in cultured gastric mucosal preparations, suggesting that glucose must enter and then be metabolized by the ghrelin cell to suppress ghrelin secretion (131). The exact metabolic pathways and molecular mechanisms within the ghrelin cell responsible for modulating ghrelin secretion are unclear, however. For example, despite the high expression of mRNAs encoding KATP channel components, pharmacological modulators of the KATP channel do not alter ghrelin secretion in mouse primary gastric mucosal cell cultures (131).
The suppression of ghrelin secretion with glucose administration is also likely mediated by changes to circulating levels of glucose-sensitive hormones, including insulin and glucagon. Both clinical and experimental evidence suggests that insulin can act independently of glucose to affect plasma ghrelin and/or ghrelin secretion. In a human study that used a stepped hyperinsulinemic glucose clamp procedure, insulin infusion caused an initial fall in plasma ghrelin during the euglycemic phase (132). Plasma ghrelin remained suppressed during subsequent periods of hypoglycemia and hyperglycemia, demonstrating that insulin can suppress plasma ghrelin independently of glucose (132). Also, a bolus injection of insulin to induce hyperinsulinemia suppressed plasma ghrelin in humans and rodents, regardless of whether individuals were maintained in hypoglycemic or normoglycemic ranges with concurrent glucose infusion (124, 133). Insulin can inhibit ghrelin secretion from mouse and neonatal rat gastric mucosal cell primary cultures when the glucose concentrations in the culture medium are in the hypoglycemic or normoglycemic range (117, 131). Also, insulin receptor mRNA is enriched in fluorescence-activated cell sorting–purified mouse gastric mucosal cells and ghrelinoma cell lines (131), insulin receptor subunits colocalize with ghrelin immunoreactivity in rat stomach (117), and insulin can phosphorylate and activate AKT, a serine-threonine kinase downstream of insulin receptor stimulation, in rat gastric ghrelin cells (117). It remains to be seen whether the insulin engagement and activation of insulin receptors expressed on ghrelin cells are necessary and sufficient for the ghrelin-suppressing actions of insulin. It also remains to be seen whether increased levels of insulin associated with obesity contribute to the obesity-associated reduction in plasma ghrelin.
Actions of glucagon to regulate plasma ghrelin are less consistent in the literature. In humans, studies have reported that administered glucagon either increases, decreases, or lacks an effect on plasma ghrelin (134–137). Glucagon stimulates ghrelin secretion from neonatal rat primary gastric mucosal cells and from isolated rat stomach when infused into the left gastric artery (138, 139). However, glucagon does not increase ghrelin secretion from mouse gastric mucosal cell primary cultures, primary cultures of fluorescence-activated cell sorting–purified mouse gastric ghrelin cells from ghrelin-GFP mice, or ghrelinoma cell lines (69, 115, 140, 141). Studies with glucagon receptor (GcgR)-KO mice or with mice administered anti-GcgR antibody suggest instead that glucagon action may have an overall effect to decrease ghrelin secretion. In particular, GcgR-KO mice, which have lower blood glucose but normal plasma insulin, have higher plasma ghrelin (69). Administration of anti-GcgR antibody increases plasma acyl-ghrelin in db/db mice and lowers blood glucose (69). Thus, perhaps disinhibition of glucagon’s normal effects on ghrelin secretion leads to higher plasma ghrelin. Importantly, we have proposed that ghrelin secretion increases in the setting of GcgR deletion or blockade to prevent the development of hypoglycemia (69).
Ghrelin in Diabetes
Several studies suggest that ghrelin might impact blood glucose levels in diabetes mellitus (DM). Plasma ghrelin is lower in individuals with obesity and individuals with type 2 DM (T2DM), many of whom may also be obese (63, 104, 122, 142–146). Diet-induced obese rodent models also have lower plasma ghrelin (60–62, 147). Yet, despite the lower plasma ghrelin, chronic pharmacological blockade of GHSR or genetic ablation of ghrelin system components improves glucose tolerance in diet-induced obese mice (described above) (52–55, 148, 149). Whereas leptin-deficient (ob/ob) mice are hyperphagic, obese, and diabetic, when ob/ob mice are crossed onto a ghrelin-KO background, lower blood glucose, lower plasma insulin, and improved glucose tolerance ensue (67). These occur without effects on food intake or body weight, suggesting that ghrelin contributes to the diabetic but not the obese phenotype of the ob/ob mice. A 6-day administration of a GHSR antagonist to ob/ob mice also reduces blood glucose [however, in that experiment, the reduced blood glucose was also accompanied by body weight loss (150)]. Similarly, chronic administration of an inverse agonist of GHSR also improves glucose tolerance in Zucker diabetic fatty (ZDF) rats (148). In contrast, ablation of GOAT in ob/ob mice does not alter glucose tolerance (151). Perhaps even more surprisingly, ablation of GHSR in ob/ob mice causes a paradoxical exaggeration of hyperglycemia and worsened glucose tolerance (152). The varied effects on blood glucose in ob/ob mice associated with each of the different ways of targeting the ghrelin system again suggests a complex interplay between ghrelin system components and blood glucose. Particularly, they lead us to speculate that GHSR actions attributed to its high constitutive activity and/or its heterodimerization with other G protein–coupled receptors or the actions of unacyl-ghrelin might help account for the differences observed in these models. In summary, ghrelin generally appears to have diabetogenic actions in individuals with obesity, independent of its actions on body weight. Therefore, there is potential for blockade of ghrelin system actions as an approach to improve glucose tolerance in patients with obesity/T2DM (see below).
Although consistent changes to plasma ghrelin in patients with type 1 DM (T1DM) have not been reported (153–159), T1DM modeled in rodents by chemical ablation of pancreatic β-cells with streptozotocin administration causes elevation of plasma acyl-ghrelin and total ghrelin (69, 160–162). Of interest, genetic ablation of ghrelin or pharmacological inhibition of GHSR causes significant reductions of streptozotocin-associated hyperphagia (69, 160–163). Genetic ablation of GHSR also lowers fasting blood glucose in streptozotocin-treated mice (69). These results suggest that elevated circulating ghrelin could contribute to hyperglycemia in individuals with T1DM. More studies are needed to assess ghrelin’s effects on blood glucose regulation in individuals with T1DM.
Elevated plasma ghrelin also is observed in humans with hepatocyte nuclear factor-1α–maturity-onset diabetes of the young (MODY; or MODY3) and glucokinase–MODY (or MODY2) forms of DM as compared with individuals with T1DM and T2DM (155). Plasma acyl-ghrelin and total ghrelin also are elevated in the mouse model of MODY3, which occurs as a result of deficient HNF1α (164). Notably, treatment with a GHSR antagonist reduces hyperglycemia, reverses the hypoinsulinemia, and improves glucose tolerance observed in these mice (164). Thus, increased ghrelin may contribute to the impaired insulin secretion and resultant aberrant blood glucose levels observed in MODY3 diabetes. The reason for the higher plasma ghrelin in these monogenic forms of diabetes is unknown.
Targeting the ghrelin system for glucose control
The diabetogenic actions of ghrelin in diet-induced obese models have sparked interest in the development of ghrelin antagonists and related compounds to minimize ghrelin-induced insulin resistance and glucose intolerance. A theoretical benefit of employing GHSR or GOAT antagonists instead of the available antidiabetic drugs would be their potential ability to improve glucose homeostasis without the propensity to cause significant hypoglycemia, which, based on mouse studies, presumably would occur only in certain settings, such as starvation-like states or perhaps when combined with therapies neutralizing the GcgR (34, 92). Although these agents have been efficacious in preclinical studies, and at least one such agent has progressed to phase 1 clinical trials, none of them has successfully made it to the market yet (49, 165–167). The recent discovery of LEAP2 as an endogenous GHSR antagonist (31) introduces new potential strategies and structure–activity relationships to target GHSR for glucose control. Overexpressing LEAP2 in mice lowers blood glucose during conditions of negative energy balance (31). It remains to be tested whether approaches that increase LEAP2 activity would improve blood glucose homeostasis in individuals with diabetes.
Administration of a stable unacyl-ghrelin peptide analog (AZP-531) has shown mixed success in terms of blood glucose control in individuals with obesity and T2DM (103). The compound also has been shown to improve postprandial glucose levels in adult subjects with Prader-Willi syndrome, independent of changes in body weight in a random placebo-control clinical trial (168). Therefore, administration of stable unacyl-ghrelin analogs presents another potential avenue for achieving blood glucose control, although much needs to be learned of how unacyl-ghrelin mediates its effects on glucose homeostasis.
Concluding Thoughts
Although not a major player in glucose homeostasis during ideal metabolic settings, ghrelin morphs into a vanguard for blood glucose control during extreme settings of metabolism and nutrition. We think that ghrelin’s glucoregulatory actions may have evolved as a system to support blood glucose during extreme negative energy balance such as starvation, during which its plasma levels naturally increase. Alternatively, ghrelin’s actions can be diabetogenic during diet-induced obesity and some monogenic forms of diabetes. We think that targeting the ghrelin system remains a feasible mechanism to achieve blood glucose control in individuals with diabetes.
Acknowledgments
We thank Sherri Osborne-Lawrence for reading through the manuscript and providing valuable comments.
Financial Support: This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01DK103884); the Diana and Richard C. Strauss Professorship in Biomedical Research; the Mr. and Mrs. Bruce G. Brookshire Professorship in Medicine; the Kent and Jodi Foster Distinguished Chair in Endocrinology, in Honor of Daniel Foster, MD; and institutional funds from the University of Texas Southwestern Medical Center to J.M.Z.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AgRP
agouti-related peptide
- [Ca2+]i
intracellular calcium concentration
- DM
diabetes mellitus
- GcgR
glucagon receptor
- GHSR
GH secretagogue receptor
- GLP-1
glucagon-like peptide-1
- GOAT
ghrelin O-acyltransferase
- KATP
ATP-sensitive potassium
- KO
knockout
- LEAP2
liver-enriched antimicrobial peptide 2
- MBOAT4
membrane-bound O-acyltransferase 4
- MODY
maturity-onset diabetes of the young
- RYGB
Roux-en-Y gastric bypass
- SST
somatostatin
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References
- 1. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. [DOI] [PubMed] [Google Scholar]
- 2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 3. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–4758. [DOI] [PubMed] [Google Scholar]
- 4. Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155(1–3):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol. 2013;52(1):R35–R49. [DOI] [PubMed] [Google Scholar]
- 6. Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52(3):301–310. [DOI] [PubMed] [Google Scholar]
- 7. Dominguez Gutierrez G, Kim J, Lee AH, Tong J, Niu J, Gray SM, Wei Y, Ding Y, Ni M, Adler C, Murphy AJ, Gromada J, Xin Y. Gene signature of the human pancreatic ε cell. Endocrinology. 2018;159(12):4023–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52(3):486–493. [DOI] [PubMed] [Google Scholar]
- 9. Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107(1–3):63–69. [DOI] [PubMed] [Google Scholar]
- 10. Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing β cells in two mouse models of pancreas development. Proc Natl Acad Sci USA. 2004;101(9):2924–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Genetic determinants of pancreatic ε-cell development. Dev Biol. 2005;286(1):217–224. [DOI] [PubMed] [Google Scholar]
- 12. Napolitano T, Silvano S, Vieira A, Balaji S, Garrido-Utrilla A, Friano ME, Atlija J, Collombat P. Role of ghrelin in pancreatic development and function. Diabetes Obes Metab. 2018;20(Suppl 2):3–10. [DOI] [PubMed] [Google Scholar]
- 13. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4(6):437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perelló M, Andrews ZB, Zigman JM. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522(16):3644–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48(1):23–29. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429(3):469–489. [DOI] [PubMed] [Google Scholar]
- 18. Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. [DOI] [PubMed] [Google Scholar]
- 19. Tannenbaum GS, Lapointe M, Beaudet A, Howard AD. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology. 1998;139(10):4420–4423. [DOI] [PubMed] [Google Scholar]
- 20. Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32(11):2248–2255. [DOI] [PubMed] [Google Scholar]
- 21. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50(11):2438–2443. [DOI] [PubMed] [Google Scholar]
- 22. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276(3):905–908. [DOI] [PubMed] [Google Scholar]
- 23. Mani BK, Osborne-Lawrence S, Mequinion M, Lawrence S, Gautron L, Andrews ZB, Zigman JM. The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Mol Metab. 2017;6(8):882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. [DOI] [PubMed] [Google Scholar]
- 25. Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor—identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–2210. [DOI] [PubMed] [Google Scholar]
- 26. Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25(3):113–117. [DOI] [PubMed] [Google Scholar]
- 27. Edwards A, Abizaid A. Clarifying the ghrelin system’s ability to regulate feeding behaviours despite enigmatic spatial separation of the GHSR and its endogenous ligand. Int J Mol Sci. 2017;18(4):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howick K, Griffin BT, Cryan JF, Schellekens H. From belly to brain: targeting the ghrelin receptor in appetite and food intake regulation. Int J Mol Sci. 2017;18(2):E273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. [DOI] [PubMed] [Google Scholar]
- 31. Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, Lichtman JS, Wang Y, Dalmas O, Yin Y, Tian H, Jermutus L, Grimsby J, Rondinone CM, Konkar A, Kaplan DD. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27(2):461–469.e6. [DOI] [PubMed] [Google Scholar]
- 32. Chen VP, Gao Y, Geng L, Brimijoin S. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes. 2017;41(9):1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Satou M, Nishi Y, Yoh J, Hattori Y, Sugimoto H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151(10):4765–4775. [DOI] [PubMed] [Google Scholar]
- 34. Mani BK, Zigman JM. Ghrelin as a survival hormone. Trends Endocrinol Metab. 2017;28(12):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metab. 2018;27(4):786–804. [DOI] [PubMed] [Google Scholar]
- 36. Al Massadi O, López M, Tschöp M, Diéguez C, Nogueiras R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 2017;40(3):167–180. [DOI] [PubMed] [Google Scholar]
- 37. Heppner KM, Tong J. Mechanisms in endocrinology: regulation of glucose metabolism by the ghrelin system: multiple players and multiple actions. Eur J Endocrinol. 2014;171(1):R21–R32. [DOI] [PubMed] [Google Scholar]
- 38. Verhulst PJ, Depoortere I. Ghrelin’s second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18(25):3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poher AL, Tschöp MH, Müller TD. Ghrelin regulation of glucose metabolism. Peptides. 2018;100:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32(11):2309–2318. [DOI] [PubMed] [Google Scholar]
- 41. Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–5086. [DOI] [PubMed] [Google Scholar]
- 42. Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89(6):3062–3065. [DOI] [PubMed] [Google Scholar]
- 43. Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol Endocrinol. 2011;25(9):1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Page LC, Gastaldelli A, Gray SM, D’Alessio DA, Tong J. Interaction of GLP-1 and Ghrelin on Glucose Tolerance in Healthy Humans. Diabetes. 2018;67(10):1976–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. [DOI] [PubMed] [Google Scholar]
- 47. Bewick GA, Kent A, Campbell D, Patterson M, Ghatei MA, Bloom SR, Gardiner JV. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58(4):840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reed JA, Benoit SC, Pfluger PT, Tschöp MH, D’Alessio DA, Seeley RJ. Mice with chronically increased circulating ghrelin develop age-related glucose intolerance. Am J Physiol Endocrinol Metab. 2008;294(4):E752–E760. [DOI] [PubMed] [Google Scholar]
- 49. Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, Bullock W, Daly M, Decarr L, Li Y, Milardo L, Molstad D, Zhu J, Gardell SJ, Livingston JN, Sweet LJ. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. [DOI] [PubMed] [Google Scholar]
- 50. Rudolph J, Esler WP, O’connor S, Coish PD, Wickens PL, Brands M, Bierer DE, Bloomquist BT, Bondar G, Chen L, Chuang CY, Claus TH, Fathi Z, Fu W, Khire UR, Kristie JA, Liu XG, Lowe DB, McClure AC, Michels M, Ortiz AA, Ramsden PD, Schoenleber RW, Shelekhin TE, Vakalopoulos A, Tang W, Wang L, Yi L, Gardell SJ, Livingston JN, Sweet LJ, Bullock WH. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem. 2007;50(21):5202–5216. [DOI] [PubMed] [Google Scholar]
- 51. Kurashina T, Dezaki K, Yoshida M, Sukma Rita R, Ito K, Taguchi M, Miura R, Tominaga M, Ishibashi S, Kakei M, Yada T. The β-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Sci Rep. 2015;5(1):14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55(12):3486–3493. [DOI] [PubMed] [Google Scholar]
- 53. Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Qi Y, DiStefano PS, Geddes BJ. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul Pept. 2008;150(1–3):55–61. [DOI] [PubMed] [Google Scholar]
- 54. Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115(12):3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101(21):8227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23(22):7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mani BK, Osborne-Lawrence S, Vijayaraghavan P, Hepler C, Zigman JM. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J Clin Invest. 2016;126(9):3467–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uchida A, Zechner JF, Mani BK, Park WM, Aguirre V, Zigman JM. Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass. Mol Metab. 2014;3(7):717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745–4755. [DOI] [PubMed] [Google Scholar]
- 63. Tamboli RA, Antoun J, Sidani RM, Clements A, Harmata EE, Marks-Shulman P, Gaylinn BD, Williams B, Clements RH, Albaugh VL, Abumrad NN. Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans. Diabetes Obes Metab. 2017;19(9):1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zigman JM, Bouret SG, Andrews ZB. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol Metab. 2016;27(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, Buras ED, Tong Q, Chan L, McGuinness OP, Sun Y. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10(6):996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qi Y, Longo KA, Giuliana DJ, Gagne S, McDonagh T, Govek E, Nolan A, Zou C, Morgan K, Hixon J, Saunders JO, Distefano PS, Geddes BJ. Characterization of the insulin sensitivity of ghrelin receptor KO mice using glycemic clamps. BMC Physiol. 2011;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3(5):379–386. [DOI] [PubMed] [Google Scholar]
- 68. Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121(7):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mani BK, Uchida A, Lee Y, Osborne-Lawrence S, Charron MJ, Unger RH, Berglund ED, Zigman JM. Hypoglycemic effect of combined ghrelin and glucagon receptor blockade. Diabetes. 2017;66(7):1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287(22):17942–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci USA. 2015;112(4):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013; 144:50–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dezaki K, Damdindorj B, Sone H, Dyachok O, Tengholm A, Gylfe E, Kurashina T, Yoshida M, Kakei M, Yada T. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet β-cells. Diabetes. 2011;60(9):2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dezaki K, Kakei M, Yada T. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. [DOI] [PubMed] [Google Scholar]
- 75. Sangiao-Alvarellos S, Cordido F. Effect of ghrelin on glucose-insulin homeostasis: therapeutic implications. Int J Pept. 2010;2010:234709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. [DOI] [PubMed] [Google Scholar]
- 77. Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschöp MH, Boeke JD, Cole PA. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330(6011):1689–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Damjanovic SS, Lalic NM, Pesko PM, Petakov MS, Jotic A, Miljic D, Lalic KS, Lukic L, Djurovic M, Djukic VB. Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab. 2006;91(7):2574–2581. [DOI] [PubMed] [Google Scholar]
- 79. Tong J, Davis HW, Gastaldelli A, D’Alessio D. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab. 2016;101(6):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Broglio F, Prodam F, Riganti F, Gottero C, Destefanis S, Granata R, Muccioli G, Abribat T, van der Lely AJ, Ghigo E. The continuous infusion of acylated ghrelin enhances growth hormone secretion and worsens glucose metabolism in humans [published correction appears in Trends Endocrinol Metab. 2016;27(5):348]. J Endocrinol Invest. 2008;31(9):788–794. [DOI] [PubMed] [Google Scholar]
- 81. Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol. 2002;146(2):241–244. [DOI] [PubMed] [Google Scholar]
- 82. Salehi A, Dornonville de la Cour C, Håkanson R, Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul Pept. 2004;118(3):143–150. [DOI] [PubMed] [Google Scholar]
- 83. Park S, Jiang H, Zhang H, Smith RG. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc Natl Acad Sci USA. 2012;109(46):19003–19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Colombo M, Gregersen S, Xiao J, Hermansen K. Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas. 2003;27(2):161–166. [DOI] [PubMed] [Google Scholar]
- 85. Adriaenssens AE, Svendsen B, Lam BY, Yeo GS, Holst JJ, Reimann F, Gribble FM. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. DiGruccio MR, Mawla AM, Donaldson CJ, Noguchi GM, Vaughan J, Cowing-Zitron C, van der Meulen T, Huising MO. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5(7):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57(12):3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JO, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab. 2008;93(2):438–444. [DOI] [PubMed] [Google Scholar]
- 89. Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2013;3(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M, Arvat E, Ghigo E. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88(4):1537–1542. [DOI] [PubMed] [Google Scholar]
- 91. Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55(3):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Goldstein JL, Zhao TJ, Li RL, Sherbet DP, Liang G, Brown MS. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76(0):121–127. [DOI] [PubMed] [Google Scholar]
- 93. McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yi CX, Heppner KM, Kirchner H, Tong J, Bielohuby M, Gaylinn BD, Müller TD, Bartley E, Davis HW, Zhao Y, Joseph A, Kruthaupt T, Ottaway N, Kabra D, Habegger KM, Benoit SC, Bidlingmaier M, Thorner MO, Perez-Tilve D, Tschöp MH, Pfluger PT. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7(2):e32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scott MM, Perello M, Chuang JC, Sakata I, Gautron L, Lee CE, Lauzon D, Elmquist JK, Zigman JM. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS One. 2012;7(8):e44089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rodriguez JA, Bruggeman EC, Mani BK, Osborne-Lawrence S, Lord CC, Roseman HF, Viroslav HL, Vijayaraghavan P, Metzger NP, Gupta D, Shankar K, Pietra C, Liu C, Zigman JM. Ghrelin receptor agonist rescues excess neonatal mortality in a Prader-Willi syndrome mouse model. Endocrinology. 2018;159(12):4006–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee JH, Lin L, Xu P, Saito K, Wei Q, Meadows AG, Bongmba OY, Pradhan G, Zheng H, Xu Y, Sun Y. Neuronal deletion of ghrelin receptor almost completely prevents diet-induced obesity. Diabetes. 2016;65(8):2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wu CS, Bongmba OY, Yue J, Lee JH, Lin L, Saito K, Pradhan G, Li DP, Pan HL, Xu A, Guo S, Xu Y, Sun Y. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int J Mol Sci. 2017;18(4):E832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stark R, Reichenbach A, Lockie SH, Pracht C, Wu Q, Tups A, Andrews ZB. Acyl ghrelin acts in the brain to control liver function and peripheral glucose homeostasis in male mice. Endocrinology. 2015;156(3):858–868. [DOI] [PubMed] [Google Scholar]
- 100. Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin is a novel regulator of GLP-1 secretion. Diabetes. 2015;64(5):1513–1521. [DOI] [PubMed] [Google Scholar]
- 101. Lindqvist A, Shcherbina L, Fischer AT, Wierup N. Ghrelin is a regulator of glucagon-like peptide 1 secretion and transcription in mice. Front Endocrinol (Lausanne). 2017;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benso A, St-Pierre DH, Prodam F, Gramaglia E, Granata R, van der Lely AJ, Ghigo E, Broglio F. Metabolic effects of overnight continuous infusion of unacylated ghrelin in humans. Eur J Endocrinol. 2012;166(5):911–916. [DOI] [PubMed] [Google Scholar]
- 103. Allas S, Delale T, Ngo N, Julien M, Sahakian P, Ritter J, Abribat T, van der Lely AJ. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZP-531, a first-in-class analogue of unacylated ghrelin, in healthy and overweight/obese subjects and subjects with type 2 diabetes. Diabetes Obes Metab. 2016;18(9):868–874. [DOI] [PubMed] [Google Scholar]
- 104. Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L, Guarnieri G. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(10):3935–3940. [DOI] [PubMed] [Google Scholar]
- 105. Özcan B, Neggers SJ, Miller AR, Yang HC, Lucaites V, Abribat T, Allas S, Huisman M, Visser JA, Themmen AP, Sijbrands EJ, Delhanty PJ, van der Lely AJ. Does des-acyl ghrelin improve glycemic control in obese diabetic subjects by decreasing acylated ghrelin levels? Eur J Endocrinol. 2014;170(6):799–807. [DOI] [PubMed] [Google Scholar]
- 106. Broglio F, Benso A, Gottero C, Prodam F, Gauna C, Filtri L, Arvat E, van der Lely AJ, Deghenghi R, Ghigo E. Non-acylated ghrelin does not possess the pituitaric and pancreatic endocrine activity of acylated ghrelin in humans. J Endocrinol Invest. 2003;26(3):192–196. [DOI] [PubMed] [Google Scholar]
- 107. Vestergaard ET, Jessen N, Møller N, Jørgensen JOL. Unacylated ghrelin does not acutely affect substrate metabolism or insulin sensitivity in men with type 2 diabetes [published online ahead of print 4 February 2019]. J Clin Endocrinol Metab. doi: 10.1210/jc.2018-02601. [DOI] [PubMed] [Google Scholar]
- 108. Tong J, Davis HW, Summer S, Benoit SC, Haque A, Bidlingmaier M, Tschöp MH, D’Alessio D. Acute administration of unacylated ghrelin has no effect on basal or stimulated insulin secretion in healthy humans. Diabetes. 2014;63(7):2309–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gauna C, Kiewiet RM, Janssen JA, van de Zande B, Delhanty PJ, Ghigo E, Hofland LJ, Themmen AP, van der Lely AJ. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab. 2007;293(3):E697–E704. [DOI] [PubMed] [Google Scholar]
- 110. Heppner KM, Piechowski CL, Müller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H, Tschöp MH, Sandoval DA, Perez-Tilve D. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014;63(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Delhanty PJ, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, Leenen PJ, Themmen AP, van der Lely AJ. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J. 2013;27(4):1690–1700. [DOI] [PubMed] [Google Scholar]
- 112. Zhang W, Chai B, Li JY, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149(9):4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. [DOI] [PubMed] [Google Scholar]
- 114. Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24(6):RC19–RC21. [DOI] [PubMed] [Google Scholar]
- 115. Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA. 2010;107(36):15868–15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mani BK, Chuang JC, Kjalarsdottir L, Sakata I, Walker AK, Kuperman A, Osborne-Lawrence S, Repa JJ, Zigman JM. Role of calcium and EPAC in norepinephrine-induced ghrelin secretion. Endocrinology. 2014;155(1):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gagnon J, Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology. 2012;153(8):3646–3656. [DOI] [PubMed] [Google Scholar]
- 118. Mundinger TO, Cummings DE, Taborsky GJ Jr. Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147(6):2893–2901. [DOI] [PubMed] [Google Scholar]
- 119. Iwakura H, Kangawa K, Nakao K. The regulation of circulating ghrelin—with recent updates from cell-based assays. Endocr J. 2015;62(2):107–122. [DOI] [PubMed] [Google Scholar]
- 120. Al Massadi O, Lear PV, Muller TD, Lopez M, Dieguez C, Tschop MH, Nogueiras R. Review of novel aspects of the regulation of ghrelin secretion. Curr Drug Metab. 2014;15(4):398–413. [DOI] [PubMed] [Google Scholar]
- 121. Djurhuus CB, Hansen TK, Gravholt C, Ørskov L, Hosoda H, Kangawa K, Jørgensen JO, Holst JJ, Schmitz O. Circulating levels of ghrelin and GLP-1 are inversely related during glucose ingestion. Horm Metab Res. 2002;34(7):411–413. [DOI] [PubMed] [Google Scholar]
- 122. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. [DOI] [PubMed] [Google Scholar]
- 123. Nakagawa E, Nagaya N, Okumura H, Enomoto M, Oya H, Ono F, Hosoda H, Kojima M, Kangawa K. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth-hormone-releasing peptide: responses to the intravenous and oral administration of glucose. Clin Sci (Lond). 2002;103(3):325–328. [DOI] [PubMed] [Google Scholar]
- 124. McCowen KC, Maykel JA, Bistrian BR, Ling PR. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. J Endocrinol. 2002;175(2):R7–R11. [DOI] [PubMed] [Google Scholar]
- 125. Briatore L, Andraghetti G, Cordera R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur J Endocrinol. 2003;149(5):403–406. [DOI] [PubMed] [Google Scholar]
- 126. Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146(2):845–850. [DOI] [PubMed] [Google Scholar]
- 127. Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144(7):2765–2767. [DOI] [PubMed] [Google Scholar]
- 128. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. [DOI] [PubMed] [Google Scholar]
- 129. Gomez G, Englander EW, Greeley GH Jr. Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept. 2004;117(1):33–36. [DOI] [PubMed] [Google Scholar]
- 130. Pérez-Fontán M, Cordido F, Rodríguez-Carmona A, García-Naveiro R, Isidro ML, Villaverde P, García-Buela J. Acute plasma ghrelin and leptin responses to oral feeding or intraperitoneal hypertonic glucose-based dialysate in patients with chronic renal failure. Kidney Int. 2005;68(6):2877–2885. [DOI] [PubMed] [Google Scholar]
- 131. Sakata I, Park WM, Walker AK, Piper PK, Chuang JC, Osborne-Lawrence S, Zigman JM. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab. 2012;302(10):E1300–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003;284(2):E313–E316. [DOI] [PubMed] [Google Scholar]
- 133. Lauritzen ES, Voss T, Kampmann U, Mengel A, Vendelbo MH, Jørgensen JO, Møller N, Vestergaard ET. Circulating acylghrelin levels are suppressed by insulin and increase in response to hypoglycemia in healthy adult volunteers. Eur J Endocrinol. 2015;172(4):357–362. [DOI] [PubMed] [Google Scholar]
- 134. Arafat MA, Otto B, Rochlitz H, Tschöp M, Bähr V, Möhlig M, Diederich S, Spranger J, Pfeiffer AF. Glucagon inhibits ghrelin secretion in humans. Eur J Endocrinol. 2005;153(3):397–402. [DOI] [PubMed] [Google Scholar]
- 135. Soule S, Pemberton C, Hunt P, Cole D, Raudsepp S, Inder W. Prandial regulation of ghrelin secretion in humans: does glucagon contribute to the preprandial increase in circulating ghrelin? Clin Endocrinol (Oxf). 2005;63(4):412–417. [DOI] [PubMed] [Google Scholar]
- 136. Hirsh D, Heinrichs C, Leenders B, Wong AC, Cummings DE, Chanoine JP. Ghrelin is suppressed by glucagon and does not mediate glucagon-related growth hormone release. Horm Res. 2005;63(3):111–118. [DOI] [PubMed] [Google Scholar]
- 137. Broglio F, Gottero C, Prodam F, Destefanis S, Gauna C, Me E, Riganti F, Vivenza D, Rapa A, Martina V, Arvat E, Bona G, van der Lely AJ, Ghigo E. Ghrelin secretion is inhibited by glucose load and insulin-induced hypoglycaemia but unaffected by glucagon and arginine in humans. Clin Endocrinol (Oxf). 2004;61(4):503–509. [DOI] [PubMed] [Google Scholar]
- 138. Gagnon J, Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154(2):666–674. [DOI] [PubMed] [Google Scholar]
- 139. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119(1-2):77–81. [DOI] [PubMed] [Google Scholar]
- 140. de la Cour CD, Norlén P, Håkanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept. 2007;143(1-3):118–126. [DOI] [PubMed] [Google Scholar]
- 141. Lu X, Zhao X, Feng J, Liou AP, Anthony S, Pechhold S, Sun Y, Lu H, Wank S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G367–G376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. [DOI] [PubMed] [Google Scholar]
- 143. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. [DOI] [PubMed] [Google Scholar]
- 144. Pöykkö SM, Kellokoski E, Hörkkö S, Kauma H, Kesäniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52(10):2546–2553. [DOI] [PubMed] [Google Scholar]
- 145. Katsuki A, Urakawa H, Gabazza EC, Murashima S, Nakatani K, Togashi K, Yano Y, Adachi Y, Sumida Y. Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2004;151(5):573–577. [DOI] [PubMed] [Google Scholar]
- 146. Ukkola O, Pöykkö SM, Antero Kesäniemi Y. Low plasma ghrelin concentration is an indicator of the metabolic syndrome. Ann Med. 2006;38(4):274–279. [DOI] [PubMed] [Google Scholar]
- 147. Chen VP, Gao Y, Geng L, Brimijoin S. Butyrylcholinesterase gene transfer in obese mice prevents postdieting body weight rebound by suppressing ghrelin signaling. Proc Natl Acad Sci USA. 2017;114(41):10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Abegg K, Bernasconi L, Hutter M, Whiting L, Pietra C, Giuliano C, Lutz TA, Riediger T. Ghrelin receptor inverse agonists as a novel therapeutic approach against obesity-related metabolic disease. Diabetes Obes Metab. 2017;19(12):1740–1750. [DOI] [PubMed] [Google Scholar]
- 149. Pfluger PT, Kirchner H, Günnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, Sleeman MW, Tschöp MH. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G610–G618. [DOI] [PubMed] [Google Scholar]
- 150. Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52(7):947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kirchner H, Heppner KM, Holland J, Kabra D, Tschöp MH, Pfluger PT. Ablation of ghrelin O-acyltransferase does not improve glucose intolerance or body adiposity in mice on a leptin-deficient ob/ob background. PLoS One. 2013;8(4):e61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ma X, Lin Y, Lin L, Qin G, Pereira FA, Haymond MW, Butte NF, Sun Y. Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am J Physiol Endocrinol Metab. 2012;303(3):E422–E431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bideci A, Camurdan MO, Cinaz P, Demirel F. Ghrelin, IGF-I and IGFBP-3 levels in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18(12):1433–1439. [DOI] [PubMed] [Google Scholar]
- 154. Soriano-Guillén L, Barrios V, Lechuga-Sancho A, Chowen JA, Argente J. Response of circulating ghrelin levels to insulin therapy in children with newly diagnosed type 1 diabetes mellitus. Pediatr Res. 2004;55(5):830–835. [DOI] [PubMed] [Google Scholar]
- 155. Nowak N, Hohendorff J, Solecka I, Szopa M, Skupien J, Kiec-Wilk B, Mlynarski W, Malecki MT. Circulating ghrelin level is higher in HNF1A–MODY and GCK–MODY than in polygenic forms of diabetes mellitus. Endocrine. 2015;50(3):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Holdstock C, Ludvigsson J, Karlsson FA. Abnormal ghrelin secretion in new onset childhood type 1 diabetes. Diabetologia. 2004;47(1):150–151. [DOI] [PubMed] [Google Scholar]
- 157. Huml M, Kobr J, Siala K, Varvařovská J, Pomahačová R, Karlíková M, Sýkora J. Gut peptide hormones and pediatric type 1 diabetes mellitus. Physiol Res. 2011;60(4):647–658. [DOI] [PubMed] [Google Scholar]
- 158. Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003;52(12):2923–2927. [DOI] [PubMed] [Google Scholar]
- 159. Prodam F, Cadario F, Bellone S, Trovato L, Moia S, Pozzi E, Savastio S, Bona G. Obestatin levels are associated with C-peptide and antiinsulin antibodies at the onset, whereas unacylated and acylated ghrelin levels are not predictive of long-term metabolic control in children with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(4):E599–E607. [DOI] [PubMed] [Google Scholar]
- 160. Gelling RW, Overduin J, Morrison CD, Morton GJ, Frayo RS, Cummings DE, Schwartz MW. Effect of uncontrolled diabetes on plasma ghrelin concentrations and ghrelin-induced feeding. Endocrinology. 2004;145(10):4575–4582. [DOI] [PubMed] [Google Scholar]
- 161. Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Role of ghrelin in streptozotocin-induced diabetic hyperphagia. Endocrinology. 2002;143(12):4934–4937. [DOI] [PubMed] [Google Scholar]
- 162. Masaoka T, Suzuki H, Hosoda H, Ota T, Minegishi Y, Nagata H, Kangawa K, Ishii H. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Lett. 2003;541(1–3):64–68. [DOI] [PubMed] [Google Scholar]
- 163. Dong J, Peeters TL, De Smet B, Moechars D, Delporte C, Vanden Berghe P, Coulie B, Tang M, Depoortere I. Role of endogenous ghrelin in the hyperphagia of mice with streptozotocin-induced diabetes. Endocrinology. 2006;147(6):2634–2642. [DOI] [PubMed] [Google Scholar]
- 164. Brial F, Lussier CR, Belleville K, Sarret P, Boudreau F. Ghrelin inhibition restores glucose homeostasis in hepatocyte nuclear factor-1α (MODY3)-deficient mice. Diabetes. 2015;64(9):3314–3320. [DOI] [PubMed] [Google Scholar]
- 165. Denney WS, Sonnenberg GE, Carvajal-Gonzalez S, Tuthill T, Jackson VM. Pharmacokinetics and pharmacodynamics of PF-05190457: the first oral ghrelin receptor inverse agonist to be profiled in healthy subjects. Br J Clin Pharmacol. 2017;83(2):326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kong J, Chuddy J, Stock IA, Loria PM, Straub SV, Vage C, Cameron KO, Bhattacharya SK, Lapham K, McClure KF, Zhang Y, Jackson VM. Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br J Pharmacol. 2016;173(9):1452–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bhattacharya SK, Andrews K, Beveridge R, Cameron KO, Chen C, Dunn M, Fernando D, Gao H, Hepworth D, Jackson VM, Khot V, Kong J, Kosa RE, Lapham K, Loria PM, Londregan AT, McClure KF, Orr ST, Patel J, Rose C, Saenz J, Stock IA, Storer G, VanVolkenburg M, Vrieze D, Wang G, Xiao J, Zhang Y. Discovery of PF-5190457, a potent, selective, and orally bioavailable ghrelin receptor inverse agonist clinical candidate. ACS Med Chem Lett. 2014;5(5):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Allas S, Caixàs A, Poitou C, Coupaye M, Thuilleaux D, Lorenzini F, Diene G, Crinò A, Illouz F, Grugni G, Potvin D, Bocchini S, Delale T, Abribat T, Tauber M. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: a randomized placebo-controlled trial. PLoS One. 2018;13(1):e0190849. [DOI] [PMC free article] [PubMed] [Google Scholar]