Abstract
Low aerobic capacity increases the risk for insulin resistance but the mechanisms are unknown. In this study, we tested susceptibility to acute (3-day) high-fat, high-sucrose diet (HFD)–induced insulin resistance in male rats selectively bred for divergent intrinsic aerobic capacity, that is, high-capacity running (HCR) and low-capacity running (LCR) rats. We employed hyperinsulinemic-euglycemic clamps, tracers, and transcriptome sequencing of skeletal muscle to test whether divergence in aerobic capacity impacted insulin resistance through systemic and tissue-specific metabolic adaptations. An HFD evoked decreased insulin sensitivity and insulin signaling in muscle and liver in LCR rats, whereas HCR rats were protected. An HFD led to increased glucose transport in skeletal muscle (twofold) of HCR rats while increasing glucose transport into adipose depots of the LCR rats (twofold). Skeletal muscle transcriptome revealed robust differences in the gene profile of HCR vs LCR on low-fat diet and HFD conditions, including robust differences in specific genes involved in lipid metabolism, adipogenesis, and differentiation. HCR transcriptional adaptations to an acute HFD were more robust than for LCR and included genes driving mitochondrial energy metabolism. In conclusion, intrinsic aerobic capacity robustly impacts systemic and skeletal muscle adaptations to HFD-induced alterations in insulin resistance, an effect that is likely driven by baseline differences in oxidative capacity, gene expression profile, and transcriptional adaptations to an HFD.
Low aerobic capacity is a powerful predictor of early mortality (1, 2) as well as increased susceptibility to chronic metabolic diseases including type 2 diabetes (3) and hepatic steatosis (4). Low aerobic capacity has also been shown to predict insulin resistance (5, 6). Individual aerobic capacity is driven by genetics (intrinsic), lifestyle, and age. Examining mechanisms by which intrinsic aerobic capacity impacts disease onset and progression, independent of exercise, is difficult in humans. Moreover, because polygenetic and multi-tissue effects drive intrinsic aerobic capacity, commonly used genetic mouse models of single gene mutations are not appropriate or generalizable.
We have used rats selectively bred for high-capacity running (HCR) and low-capacity running (LCR) to specifically examine the role of intrinsic aerobic capacity on metabolic disease susceptibility (7, 8). Selective breeding resulted in robust divergence in intrinsic aerobic capacity despite no exposure to exercise training (7). Mitochondrial content and oxidative capacity are significantly higher in HCR rats compared with LCR rats in skeletal muscle, adipose tissue, and liver (9–14). Moreover, HCR rats possess a thermogenic phenotype of higher resting energy expenditure (15), internal body temperature (16), skeletal muscle heat generation during exercise (17), and greater brown adipose mass than do LCR rats (12). All of these unique cellular and physiological traits track with protection against diet-induced obesity (13), insulin resistance, and hepatic steatosis in the HCR rat (18). In contrast, LCR rats display a metabolic syndrome phenotype at an early age and are highly susceptible to diet-induced obesity, insulin resistance, and hepatic steatosis (18). These robust differences between HCR and LCR phenotypes generated the “energy transfer hypothesis” contending that high intrinsic aerobic capacity leads to a higher rate of energy turnover. As a result, the hypothesis contends that any insult requiring a quick and adaptive response (e.g., dietary, chemical, environmental) will be much more robust in HCR rats compared with LCR rats (19). In accordance with this hypothesis, it has been shown that HCR rats are not only protected from metabolic dysfunction, but they also have a longer lifespan and are less susceptible to experimental induction of neurodegeneration, cancer, fatty liver, and other conditions compared with LCR rats (19).
It has been widely debated whether mitochondrial content and oxidative capacity in muscle and liver play a critical role in ectopic lipid storage and insulin resistance. However, obesity has been linked to reduced systemic, skeletal muscle (20), and hepatic fat oxidation (21). In contrast, other results strongly suggest that obesity or high-fat diets evoke increases in mitochondrial oxidative capacity in muscle (22) and liver (23) due to compensatory mechanisms. These studies have used cross-sectional comparisons of humans who are lean or obese or take measurements after obesity and insulin resistance is established by high-fat diets. However, the role of baseline differences in oxidative capacity and early metabolic adaptations that dictate protection or susceptibility to diet-induced metabolic dysfunction has been given less consideration.
The purpose of this study was to examine whether differences in baseline aerobic capacity impacts acute metabolic adaptations and susceptibility for systemic and tissue-specific insulin resistance measured by using hyperinsulinemic-euglycemic clamps (HECs) following a 3-day high-fat, high-sucrose diet (HFD). We hypothesized that HCR rats would maintain systemic insulin sensitivity following a transition to HFD conditions whereas the LCR rats would display worsened insulin resistance. We also hypothesized that systemic differences in insulin sensitivity would be driven by alterations in tissue glucose disposal, insulin signaling, and transcriptional adaptations.
Materials and Methods
Animals
The HCR/LCR rat model was developed and characterized as previously described (7–9, 11, 24). Generation 32 rats were bred at the University of Michigan and sent to the University of Missouri at 16 weeks of age. At 25 to 30 weeks of age, male animals (n = 5 to 7 per group) were singly housed and acclimatized to a control, low-fat diet (LFD; D12110704, 10% kcal fat, 3.0% kcal sucrose, Research Diets, New Brunswick, NJ) for at least 7 days prior to any surgical procedure and allowed to recover until attaining presurgical body weight (∼5 to 7 days) before the initiation of the 3-day HFD (D12451, 45% kcal fat, Research Diets) when appropriate. This 3-day HFD experimental paradigm allows for the investigation of adaptive physiological mechanisms involved in the susceptibility or protection from the development of metabolic disease states in response to increased energy intake (EI) and/or dietary fat, which may not be present or detectable after adaptation to chronic ad libitum HFD exposure. Food intake was monitored daily for at least 3 days prior to, and during, the 3-day HFD. For acute insulin studies (n = 4 to 7), animals received 3.8 U/kg IP insulin and were euthanized under pentobarbital anesthesia 15 minutes later. The Institutional Animal Care and Use Committee at the University of Missouri and the Subcommittee approved the animal protocols for Animal Safety at the Harry S. Truman Memorial VA Hospital.
HECs
HECs were performed in conscious rats following a 5-hour fast as described (25, 26). For surgical catheter placement, rats were anesthetized with isoflurane (1% to 4%/400 to 800 mL/min O2 carrier), the left common carotid artery and the right jugular vein were catheterized, and free ends of catheters were tunneled under the skin to the back of the neck where they were exteriorized and sealed with stainless steel plugs. Experiments were performed when rats had returned to presurgery weight (∼5 to 7 days). Baseline blood samples were taken (∼3-hour fasted), followed by a priming bolus (20 μCi) and then a constant infusion (0.2 μCi/min) of [3H-3]glucose for a 2-hour period and a second blood sample (∼5-hour fasted, Table 1) was taken to assess basal hepatic glucose output. A priming bolus of insulin (28 mU/kg) was given and a constant infusion of insulin (4 mU/kg/min) and glucose (50 g/100 mL) infusion rate was adjusted to maintain euglycemia from 80 to 120 minutes after the insulin bolus. Additionally, a constant infusion of [3H-3]glucose (0.4 μCi/min) was maintained to measure insulin suppression of hepatic glucose output. Plasma samples were taken for determination of insulin concentration and [3H-3]glucose concentration at t = 0, 80, 90, 100, 110, and 120 minutes. At the end of clamp, the animals were anesthetized with pentobarbital. Liver, skeletal muscle (gastrocnemius, soleus, and extensor digitorum longus), and fat pads (subcutaneous, retroperitoneal, epididymal, and omental) were taken and frozen for future analysis. Rates of whole-body glucose appearance and uptake were determined as the ratio of the [3H]glucose infusion rate to the specific activity of plasma glucose during the final 40 minutes of clamps. Hepatic glucose production during the clamps was determined by subtracting the glucose infusion rate from the whole-body glucose appearance.
Table 1.
Animal Characteristics
| HCR | LCR | |||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Anthropometrics | ||||
| Body weight, g | 396.8 ± 8.1 | 402.9 ± 7.3 | 505.4 ± 17.1a | 498.2 ± 17.0a |
| 3-d Weight gain, g | 7.2 ± 1.7 | 7.7 ± 1.8 | 5.3 ± 1.1 | 14.4 ± 1.2b,c |
| 3-d Food intake, g | 15.6 ± 0.9 | 14.5 ± 0.9 | 15.4 ± 1.0 | 15.2 ± 0.8 |
| 3-d EI, kcal | 59.9 ± 3.4 | 68.4 ± 4.2d | 57.3 ± 3.4 | 71.2 ± 4.0d |
| 3-d EI per body weight, kcal/g | 150.0 ± 6.8 | 168.4 ± 9.3d | 117.3 ± 6.0a | 144.6 ± 6.8a,d |
| Adipose tissue per body weight, mg/g | ||||
| Epididymal | 13.97 ± 0.75 | 14.04 ± 1.29 | 21.91 ± 1.00a | 24.33 ± 1.07a |
| Retroperitoneal | 14.04 ± 1.10 | 16.10 ± 1.20 | 26.82 ± 2.08a | 29.57 ± 1.84a |
| Omental | 2.77 ± 0.34 | 3.15 ± 0.30 | 4.46 ± 0.33a | 4.07 ± 0.29a |
| Fasting plasma | ||||
| Glucose, mg/dL | 128.8 ± 6.5 | 132.6 ± 4.0 | 130.4 ± 4.2 | 144.1 ± 4.3c |
| Insulin, ng/mL | 2.95 ± 0.60 | 2.98 ± 0.39 | 3.46 ± 0.48 | 4.52 ± 0.80 |
Final body weight and weight gain after 3 d of an HFD are expressed as means ± SEM (n = 10 to 15). Three-day food intake, EI, and EI per body weight are expressed as means ± SEM (n = 10 to 15). Fat pad mass per body weight is expressed as means ± SEM (n = 10 to 14). Fasting plasma glucose and insulin following 3 d of an HFD are expressed as means ± SEM (n = 5 to 7).
P < 0.05 for main effect HCR vs LCR rats.
P < 0.05 for HCR vs LCR rats within diet.
P < 0.05 for LFD vs HFD within strain.
P < 0.05 for main effect of LFD vs HFD.
Tissue [1-14C]2-deoxyglucose uptake
At the end of the HEC (120 minutes), rats received a bolus of [1-14C]2-deoxyglucose (390 μCi) for determination of tissue glucose uptake. Additional plasma samples were collected at 2, 5, 10, 15, and 25 minutes after [1-14C]2-deoxyglucose bolus for the determination of plasma disappearance of the 2-deoxyglucose tracer. Skeletal muscle (gastrocnemius, soleus, and extensor digitorum longus), adipose (subcutaneous and retroperitoneal), and liver tissues were assessed for the accumulation of free 2-deoxyglucose and phosphorylated 2-deoxyglucose as previously described (27). Briefly, ∼50 mg of skeletal muscle and liver and ∼300 mg of adipose tissues were homogenized in 1 mL of 0.5% perchloric acid, centrifuged (20 minutes, 2000g, 4°C), and the resultant supernatant was neutralized with potassium hydroxide. A 125-μL aliquot was taken for liquid scintillation counting (LSC) determination and represented the total 2-deoxyglucose radioactivity (free plus phosphorylated). To an additional 250-μL aliquot, 125 μL of both BaOH and ZnSO4 was added. The sample was vortexed and centrifuged (5 minutes, 16,000g, 4°C). Two hundred fifty microliters of the supernatant was mixed with 750 μL and H2O and analyzed by LSC, and this represents the free 2-deoxyglucose in the homogenate. The phosphorylated 2-deoxyglucose represents the total 2-deoxyglucose (free plus phosphorylated) minus the free 2-deoxyglucose. As highlighted in the previous work (27), this analysis prevents underestimation of the rate of glucose uptake (Rg) by allowing for the separation of the 2-deoxyglucose in solution from the amount of 2-deoxyglucose that is incorporated into glycogen.
Tracer incorporation into glycogen
Net incorporation of [3-3H]glucose in skeletal muscle (gastrocnemius) and liver tissue was determined as previously described (28). Briefly, between ∼15 and 30 mg of tissue was solubilized in 0.5 mL of 1 N NaOH (1 hour at 37°C, vortexed every 15 minutes). One microgram of carrier glycogen (33 μL of 60 mg/mL) was added to each sample. Then, 1.5 mL of 75% EtOH was added and the glycogen was precipitated overnight (4°C). Samples were centrifuged (10 minutes, 10,000g, 4°C), the supernatant was discarded, and the glycogen pellets were dried. The glycogen pellet was resuspended in 600 μL of H2O and vortexed until fully dissolved. The entire volume was analyzed by LSC. Glycogen synthesis was calculated as disintegrations per minute per milligram of tissue per specific activity of the tracer.
Tissue glycogen
Skeletal muscle glycogen content following the HEC was determined as previously described (29). Briefly, ∼15 to 30 mg of skeletal muscle was solubilized in 0.5 mL of 1 N HCl in boiling water for 1.5 to 2.0 hours. Samples were neutralized by adding 1.5 mL of 0.67 M NaOH. Free glycosyl units were determined spectrophotometrically using a glucose oxidase kit.
Gene expression analysis
Gastrocnemius muscle gene expression profiles were assessed via RNA sequencing (RNA-seq) as previously described (30). Total RNA was isolated from white gastrocnemius muscle using a combination of TRI Reagent and RNeasy mini columns (Qiagen, Valencia, CA), including on-column DNase digestion. Three biologically separate pools containing equal amounts of RNA from two to three individual rats per group were pooled. Isolation of polyadenylated RNA and construction of barcoded RNA-seq libraries were performed using TruSeq reagents according to the manufacturer’s protocols (Illumina, San Diego, CA). Quantification of the RNA-seq libraries was done using Qubit dsDNA high-sensitivity reagents, diluted, denatured, and sequenced using Illumina methodology (HiSeq 2500, 50-bp single reads). Following sequencing and demultiplexing, reads were trimmed for adapters, filtered based on Phred quality score, and aligned to the rat genome using the STAR aligner. Resulting .bam files were imported in SeqMonk for gene level quantification. Differential expression and other analyses, including principal component analysis, were performed using packages in base R and the limma-voom pipeline. RNA-seq quality metrics including proportion of reads aligning to genic regions were calculated. Pairwise comparisons between HCR and LCR groups within each diet type were performed and differentially expressed genes were identified (P < 0.05, and minimum plus twofold change). Multiple testing corrections were done using the false discovery rate (FDR) method. Additional analyses were performed using packages in the R statistical software, the ShinyGO application, and the gene set enrichment analysis java application (Broad Institute, Cambridge, MA). Supplemental data for RNA sequencing results are available in an online repository (31).
mRNA expression
RNA and cDNA were prepared as previously described (32). Real-time quantitative PCR analysis was performed utilizing QuantStudio 3 (Applied Biosystems, Foster City, CA) and SYBR Green rat primers [angiopoietin-like (ANGPTL)4, forward, 5′-TGTGAGATGACTTCAGAT-3′, reverse, 5′-CTTCCCAAGACTGATTGA-3′; IGF2, forward, 5′-AAACTATGGGTAGGAAGT-3′, reverse, 5′-ACATAAAATTTGGGGTCC-3′; DLK1, forward, 5′-CTGTGTTAATGGACTCTG-3′, reverse, 5′-CCCGAATATCTATTTCGC-3′]. All gene-specific values were normalized to relative cyclophilin B mRNA expression values.
Western Blot
Relative protein expression of liver and skeletal muscle following acute insulin delivery was determined by western blot analysis as previously described (18, 33). Rabbit monoclonal and polyclonal primary antibodies Akt (no. 9272, 1:1000) (34), phosphorylated (phospho-)Akt (Ser473; no. 9271, 1:1000) (35), phospho-Akt (Thr308; no. 9275; 1:1000) (36), AS160 (TBC1D4; no. 2670; 1:1000) (37), phospho-AS160 (Ser588; no. 8730; 1:1000) (38), GSK-3β (no. 9315; 1:1000) (39), phospho-GSK-3β (Ser9; no. 9322; 1:1000) (40), glycogen synthase (no. 3893; 1:1000) (41), phospho–glycogen synthase (Ser641; no. 3891; 1:1000) (42), and horseradish peroxidase–linked, anti-rabbit secondary antibody (no. 7074; 1:20,000) (43) were purchased from Cell Signaling Technologies (Danvers, MA). Individual protein bands were quantified using a densitometer (Bio-Rad Laboratories, Hercules, CA), and protein loading was corrected by 0.1% amido black (Sigma-Aldrich, St. Louis, MO) staining to determine total protein as previously described (33).
Statistical analysis
Animals within strain were randomized by weight into the two dietary groups. The main effects of phenotype and diet were tested by using two-way ANOVA (SPSS, Armonk, NY). Where significant main effects were observed, post hoc analysis was performed using the least significant difference to test for any specific pairwise differences. Statistical significance was set at P < 0.05.
Results
Animal characteristics
Endurance running distance was significantly different between strains [HCR, 1862 ± 47 m vs LCR, 162 ± 9 m (P < 0.05)]. As previously observed, LCR rats weighed ∼25% (P < 0.05) more than HCR rats (Table 1) (15, 44). Furthermore, LCR rats gained more weight during the 3-day HFD than the LFD (P < 0.05), whereas HCR rats were protected from weight gain as previously reported (15). Differences in HFD-induced weight gain between strains was not due to food or EI differences. Both strains significantly increased EI during the 3-day HFD (P < 0.05) due to greater energy density of the diet (Table 1). HCR rats displayed greater EI per body weight compared with LCR rats on the HFD (P < 0.05; Table 1). The LCR rats had greater epididymal, retroperitoneal, and omental fat pads per body weight compared with HCR rats regardless of diet (P < 0.05). No significant changes in fat pad mass due to diet were observed in either strain. Fasting glucose and insulin were not different between strains on the LFD, whereas the 3-day HFD increased fasting glucose in only the LCR rats (P < 0.05). LCR rats tended to have higher fasting insulin (P = 0.1), but fasting insulin was not impacted by an HFD.
Glucose infusion rate
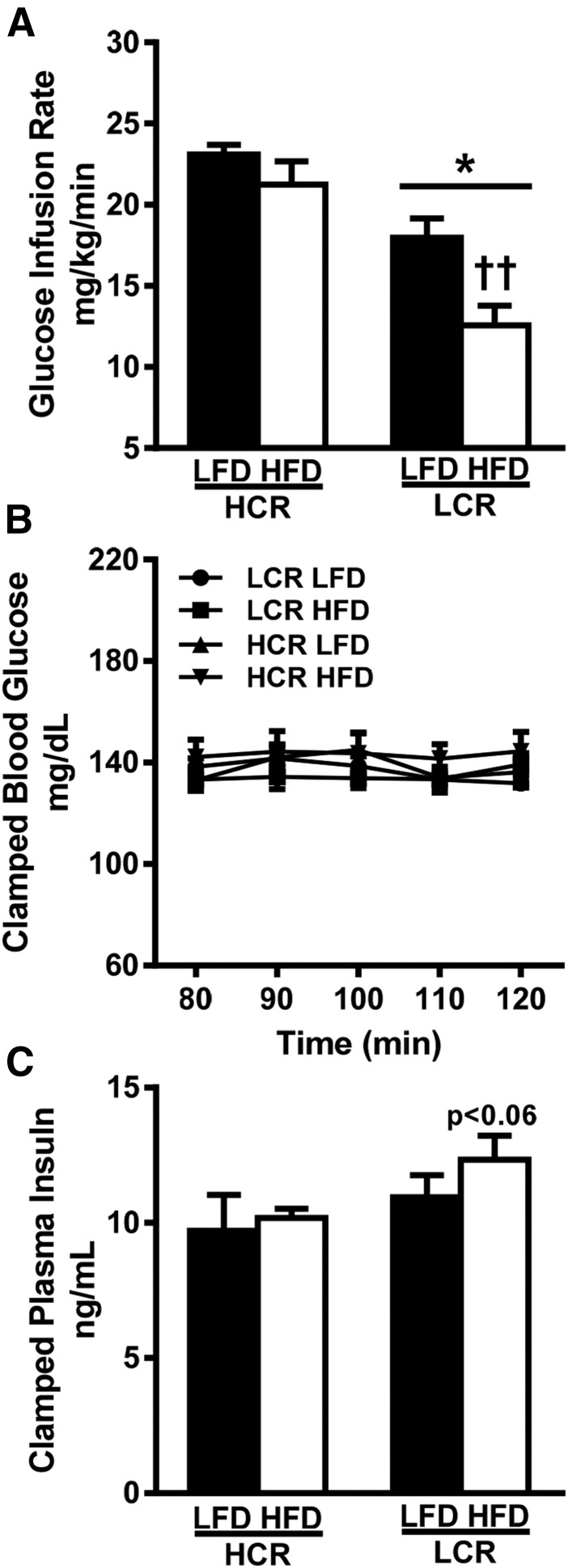
To assess whether divergent intrinsic aerobic capacity impacted insulin sensitivity following an acute HFD we performed HECs. Plasma glucose was maintained at ∼140 mg/dL during the last 40 minutes of the clamp, with no significant differences observed between groups (Fig. 1B). Clamped insulin was not significantly different between groups, but it trended to be higher in the HFD-fed LCR rats vs HCR rats (P = 0.06, Fig. 1C). As previously described in females (25), male HCR rats displayed a higher glucose infusion rate (GIR) on an LFD compared with LCR rats (∼28%, P < 0.05, Fig. 1A). Following the 3-day HFD the HCR rats sustained a higher GIR, whereas the LCR rats decreased the GIR by ∼30% (P < 0.05). As a result, the GIR in HFD-fed HCR rats was 40% higher than that in HFD-fed LCR rats (P < 0.05). Thus, LCR rats display decreased whole-body insulin sensitivity and greater susceptibility to HFD-induced insulin resistance. In contrast, HCR rats are protected against acute HFD-induced insulin resistance.
Figure 1.
High intrinsic aerobic capacity protects against acute (3-d) HFD-induced decreases in insulin sensitivity. (A) GIR was determined during HEC. (B) Plasma glucose and (C) plasma insulin were determined during the last 40 min of the HEC. Values are expressed as means ± SEM (n = 5 to 7). *P < 0.05 for main effect in HCR rats vs LCR rats; ††P < 0.05 for LFD vs HFD within strain.
Glucose clearance and endogenous glucose production
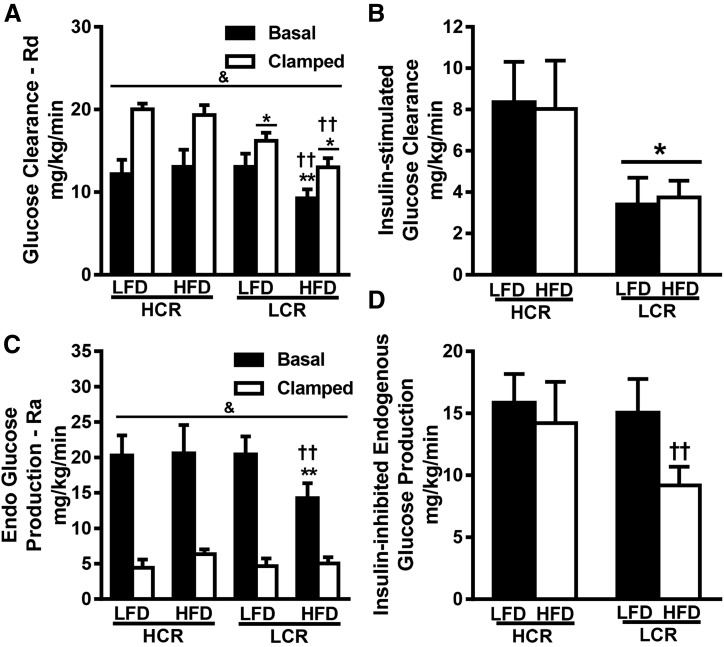
Systemic glucose clearance (GC) and endogenous glucose production (EGP) were determined under basal conditions (4-hour fast) and during hyperinsulinemia (last 40 minutes of HEC) (Fig. 2). During basal conditions, HFD-fed LCR rats had ∼30% of the GC compared with LFD-fed LCR rats and HFD-fed HCR rats (Fig. 2A, P < 0.05). No other significant differences were observed in GC during basal conditions. Hyperinsulinemia via the HEC increased GC in all groups (P < 0.05). LCR rats had significantly lower GC compared with HCR rats in both diet groups (P < 0.05, 19% and 30%, respectively), and the 3-day HFD resulted in a 19% reduction in GC in only the LCR rats (P < 0.05). Insulin-stimulated GC (GC during HEC − basal GC) demonstrated that the LCR rats have ∼55% less insulin-stimulated GC compared with HCR regardless of diet (Fig. 2B, P < 0.05). HFD-fed LCR rats also had ∼30% lower systemic EGP under basal conditions compared with LFD- and HFD-fed HCR rats (Fig. 2C, P < 0.05). The insulin inhibited EGP in both strains regardless of diet (Fig. 2C, P < 0.05). However, inhibition of EGP was ∼40% lower in the HFD-fed LCR rats compared with the LFD-fed LCR rats (Fig. 2D, P < 0.05), due entirely to the reduced basal EGP observed in HFD-fed LCR rats.
Figure 2.
Low intrinsic aerobic capacity is associated with decreased whole-body insulin-stimulated GC and inhibition of EGP. (A) Whole-body GC [rate of disappearance (Rd)] was determined during the basal state and during the last 40 min of the HEC, with (B) insulin-stimulated GC representing the difference of the clamp and basal values. (C) EGP [rate of appearance (Ra)] was determined during the basal state and during the last 40 min of the HEC. (D) Inhibition of EGP by insulin was determined as the difference between basal and clamped values. Values are expressed as means ± SEM (n = 5 to 7). &P < 0.05 for main effect of basal vs clamped; *P < 0.05 for main effect of HCR animals vs LCR animals; ††P < 0.05 for LFD vs HFD within strain; **P < 0.05 for HCR animals vs LCR animals within diet.
Tissue glucose uptake
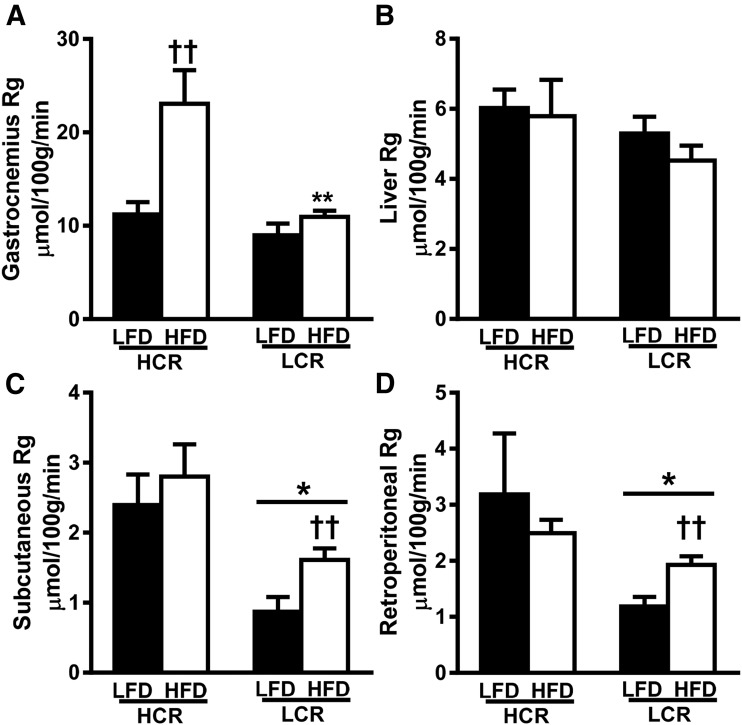
We examined tissue-specific differences in insulin action by measuring [14C]2-deoxyglucose transport in gastrocnemius muscle, liver, subcutaneous fat, and retroperitoneal fat at the end of the HEC. Insulin-stimulated glucose transport into gastrocnemius muscle was not different between strains on the LFD (Fig. 3A). However, the 3-day HFD induced a striking increase (>twofold, P < 0.05) in gastrocnemius glucose transport in the HCR rats. There was no effect of strain or diet on glucose transport in the liver (Fig. 3B). In both fat pads, glucose transport was ∼60% less in LCR rats than in HCR rats on the LFD (Fig. 3C, P < 0.05). Only the LCR rats demonstrated a ∼70% increase in insulin-stimulated glucose transport on an HFD compared with an LFD (P < 0.05). In summary, an acute HFD increases insulin-stimulated glucose transport into skeletal muscle of the HCR while increasing glucose transport into the adipose tissue of LCR rats.
Figure 3.
Divergent intrinsic aerobic capacity results in tissue-specific differences in glucose disposal during HEC. (A–D) Insulin-stimulated glucose uptake was determined during the last 40 min of the HEC by assessing tissue [14C]2-deoxyglucose uptake in (A) gastrocnemius, (B) liver, (C) subcutaneous fat, and (D) retroperitoneal fat. Values are expressed as means ± SEM (n = 5 to 7). *P < 0.05 for main effect of HCR rats vs LCR rats; ††P < 0.05 for LFD vs HFD within strain; **P < 0.05 for HCR rats vs LCR rats within diet.
Glucose incorporation into glycogen
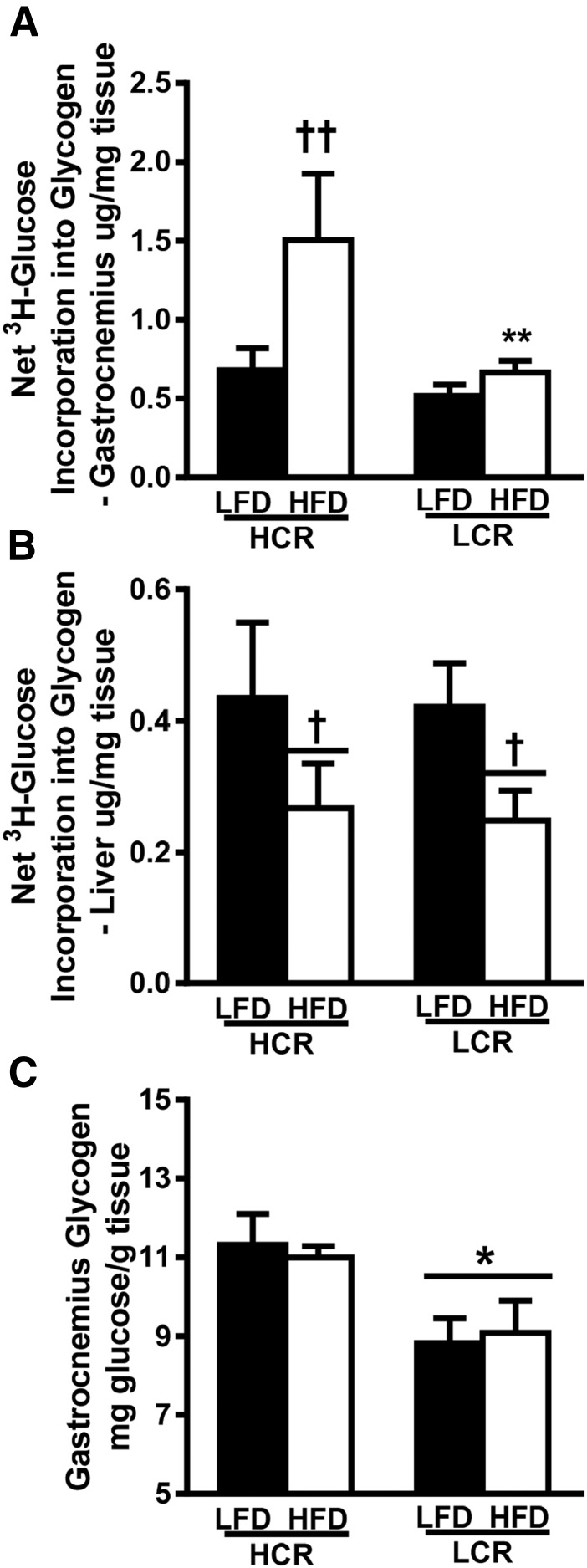
We also assessed insulin-stimulated incorporation of [3H]glucose into glycogen of gastrocnemius muscle and liver. Again, only the HCR rats displayed a >twofold increase in insulin-stimulated [3H]glucose incorporation into muscle glycogen compared with the LFD (Fig. 4A, P < 0.05). Both strains demonstrated a significant reduction (∼40%, P < 0.05) in insulin-stimulated net [3H]glucose incorporation into the liver following the acute HFD (Fig. 4B). Next, we assessed the amount of skeletal muscle glycogen in the groups following the clamp (Fig. 4C). LCR rats had lower skeletal muscle glycogen compared with HCR rats regardless of diet (∼20%, P < 0.05).
Figure 4.
Insulin-stimulated incorporation of glucose into glycogen in increased in HCR rats following acute HFD. (A and B) Insulin-stimulated incorporation of glucose into glycogen during the HEC was assessed by determining the amount of [3H]glucose in (A) gastrocnemius and (B) liver glycogen. (C) Total gastrocnemius glycogen content was measured spectrophotometrically. Values represent means ± SEM (n = 5 to 7). *P < 0.05 for main effect of HCR rats vs LCR rats; †P < 0.05 for main effect LFD vs HFD; ††P < 0.05 for LFD vs HFD within strain; **P < 0.05 for HCR rats vs LCR rats within diet.
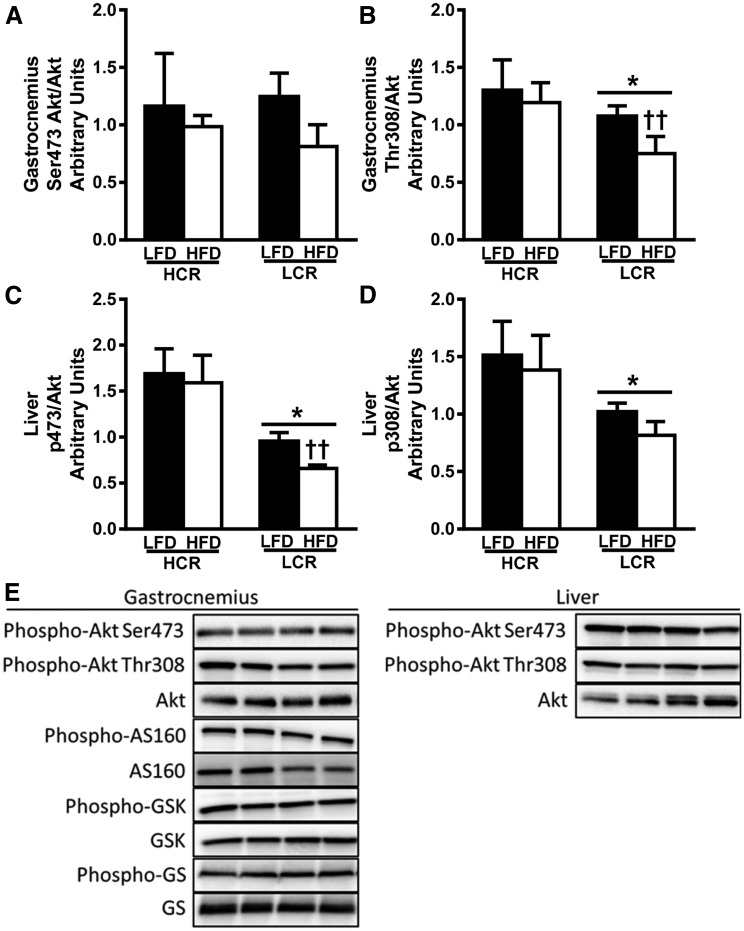
Skeletal muscle and liver insulin signaling
We assessed insulin signaling in gastrocnemius and liver in an additional cohort of 3-day HFD-fed HCR/LCR rats following an acute IP insulin bolus. Phosphorylation of Akt at Ser473 was not different between strains or diets in gastrocnemius (Fig. 5A), whereas Thr308 phosphorylation was reduced in the LCR rats on an LFD and further reduced by 3-days of an HFD (Fig. 5B, P < 0.05). Interestingly, both Ser473 and Thr308 phosphorylation was reduced in liver from LFD-fed LCR rats compared with HCR rats (Fig. 5C and 5D, P < 0.05). Only the LCR rats displayed reduced Ser473 phosphorylation following the 3-day HFD (P < 0.05). Further analysis of the insulin signaling pathway in gastrocnemius revealed no differences in phosphorylation status of AS160 (TBC1D4), GSK-3β, or glycogen synthase due to strain or diet (data not shown).
Figure 5.
Gastrocnemius and liver insulin signaling are reduced following acute insulin stimulation in LCR rats. (A–D) Insulin-stimulated activation of Akt was determined by western blot as the phosphorylation state of Ser473 or Thr308 relative to total Akt expression for gastrocnemius [(A) and (B), respectively] and liver [(C) and (D), respectively] following acute insulin bolus. (E) Representative blots. Values are expressed as means ± SEM (n = 4 to 7). *P < 0.05 for main effect HCR rats vs LCR rats; ††P < 0.05 for LFD vs HFD within strain.
Muscle transcriptome analysis using RNA-seq
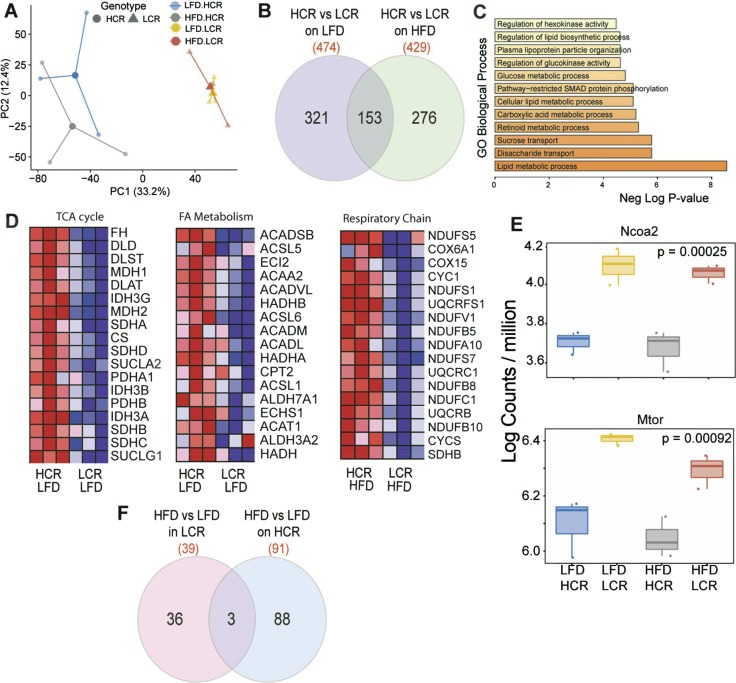
We assessed gastrocnemius muscle gene expression profiles in an unbiased manner via RNA-seq. RNA-seq analysis included an average of 90 million reads per replicate (three biologically distinct replicates per group) with 95% of reads mapping to exons. The range and distribution of reads (counts per million) were similar for all samples, indicating comparable transcriptomic coverage. Principal component analysis (Fig. 6A) of log count per million values revealed significant differences in groups based on aerobic capacity genotype (HCR vs LCR, P < 0.05) along principal component (PC)1 (explaining 33% of variance). Only HCR animals showed significant differences (along PC2), whereas LCR animals showed lack of global differences in gene expression. The PC2 results suggest a robust adaptation to the HFD in only HCR rats. Comparisons of HCR vs LCR groups identified 474 and 429 genes that were differentially expressed in LFD and HFD contexts, respectively (±1.5-fold, unadjusted P < 0.05). Of these, 221 genes in the LFD context and 275 genes in the HFD context were differentially expressed between HCR and LCR groups after correction for multiple testing using FDR [Fig. 6B; see the online repository (31)]. Venn analysis showed that 153 genes were regulated differently between HCR and LCR groups on both diets. Among the common differentially expressed genes (n = 153), gene ontology (GO) biological processes for lipid metabolic process, disaccharide and sucrose transport, lipid biosynthetic process, glucose metabolic process, and regulation of hexokinase activity were among the top statistically enriched processes (Fig. 6C, P < 0.0001). All of these genes were uniformly regulated the same direction in both diets, suggesting a fundamental alteration in HCR and LCR groups, and they were consistent with previous findings of greater lipid oxidation and glucose metabolism in HCR animals. To more broadly identify pathways affected between HCR and LCR groups, gene set enrichment analysis (GSEA) was used to identify biological processes, cellular components, and KEGG pathways enriched by genotype. GSEA does not rely on an arbitrary cutoff (such as fold change between groups) and is a computational method that determines whether an a priori–defined set of genes shows statistically significant, concordant differences between two biological states (32) and is hence valuable in detecting biological processes of modest but coordinated changes. These analyses also clearly identified the tricarboxylic acid (TCA) cycle, fatty acid metabolism, glucose metabolic process (not shown), and genes involved in respiratory chain and oxidative phosphorylation (not shown) to be different between HCR and LCR groups on an LFD (Fig. 6D). Most notably, coordinated upregulation of TCA cycle enzymes, fumarate hydratase, dihydrolipoamide dehydrogenase, malate dehydrogenase 1 and 2, and citrate synthase was noted in HCR muscle (Fig. 6D) on LFD-fed animals. HCR muscle also showed upregulation of fatty acid metabolic genes, including acyl–coenzyme A (CoA) synthetase long chain family member 5, acetyl-CoA acyltransferase 2, acyl-CoA dehydrogenase very long chain, acyl-CoA oxidase 2 (Acox2), and the mitochondrial trifunctional protein, hydroxyacyl-CoA dehydrogenase (Fig. 6D). Additionally, the HFD induced increases in the expression of respiratory complex genes including NDUFS5, COX1A1, and other reduced NAD:ubiquinone oxidoreductase subunits that occurred only in the HCR group, suggesting a transcriptional upregulation of mitochondrial oxidative phosphorylation following an HFD challenge.
Figure 6.
Muscle transcriptome analysis utilizing RNA-seq of white gastrocnemius muscle of HCR and LCR rats. (A) Principal component analysis of log count per million values of all genes. (B) Venn diagram of differentially expressed genes (±twofold, P < 0.05) between HCR and LCR strains on an LFD and HFD, respectively. (C) GO of enriched biological processes among differentially expressed genes common in both HCR and LCR rats. (D) GSEA of GO biological processes between HCR and LCR groups on LFD and HFD. (E) mRNA expression of Ncoa2 and Mtor in HCR and LCR groups. (F) Venn diagram showing differentially expressed genes following 3-d HFD in HCR and LCR rats, respectively. AACS2, acetyl-CoA acyltransferase 2; ACADVL, acyl-CoA dehydrogenase very long chain; Acox2, acyl-CoA oxidase 2; ACSL5, acyl-CoA synthetase long chain family member 5; CS, citrate synthase; DLD, dihydrolipoamide dehydrogenase; FA, fatty acid; FH, fumarate hydratase; HADHA, hydroxyacyl-CoA dehydrogenase; MDH1, malate dehydrogenase 1; MDH2, malate dehydrogenase 2.
Because HCR rats on an acute HFD showed greater glucose uptake in muscle, we examined changes in genes involved in the glucose metabolic process (GO: 006006), which includes 191 genes. Of these, we found significant differences in expression of 28 genes (FDR P < 0.05, ANOVA). These included transcriptional and signaling regulators critical in glucose metabolism, NCOA2, mTOR, and p38 MAPK (MAPK14) between HCR and LC rats (31) (Fig. 6E) (only NCOA2 and mTOR are shown). We also observed changes expression of IGF2 and INSR and differences in the expression of bifunctional 6-phosphofructo-2-kinase:fructose-2,6-biphosphatase gene (PFKFB1) and pyruvate dehydrogenase kinase 4 (PDK4) between HCR and LCR muscle. The overall effect of the HFD on gene expression was lower compared with the robust genotype (HCR vs LCR) differences. Nonetheless, HCR rats showed more pronounced gene expression adaptations to the 3-day HFD compared with LCR rats (Fig. 6F) (31). Although no genes survived multiple testing correction, the HFD influenced the expression of 91 genes in HCR animals and only 39 genes in LCR animals (±1.5-fold, unadjusted P < 0.05) (Fig. 6F).
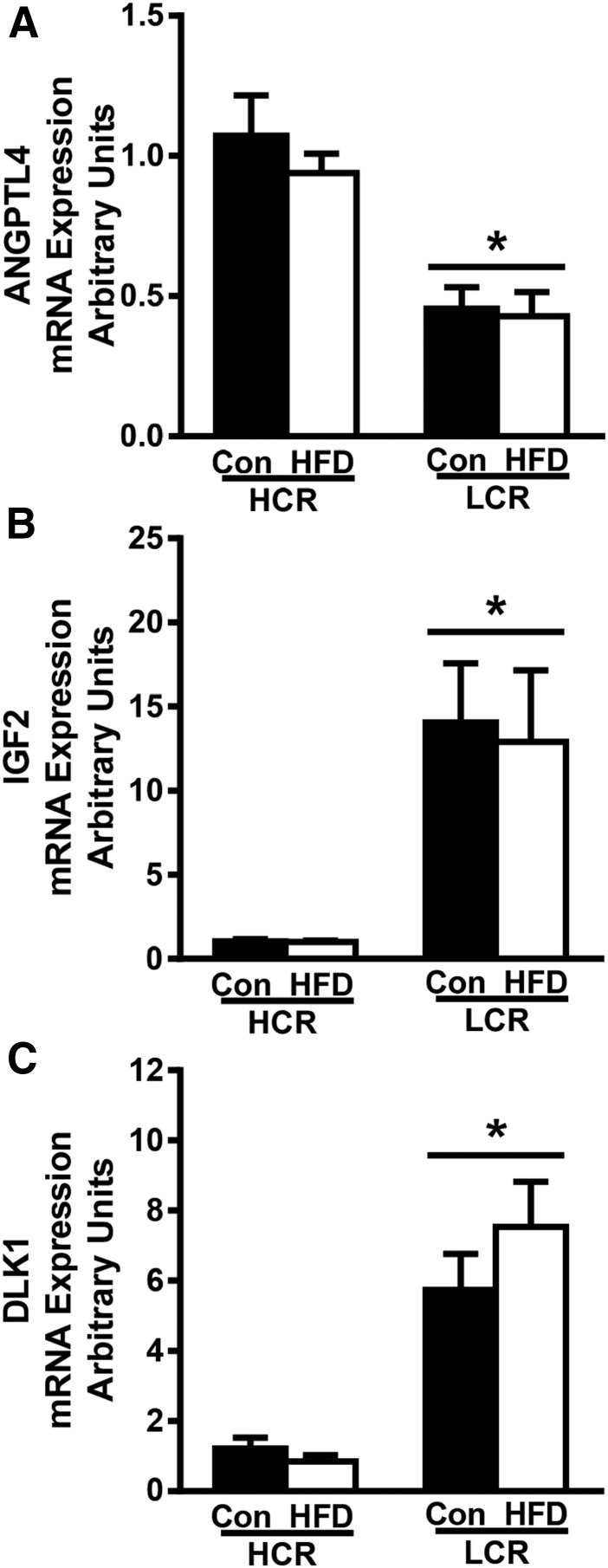
Finally, in addition to genes known to be involved in glucose metabolic pathways, we also identified several novel genes previously unrecognized to be altered in HCR/LCR skeletal muscle (ANGPTL4, IGF2, DLK1, SREBP-1, THRSP, NR5A1, DKK3, GRM4). We verified that ANGPTL4, IGF2, and DLK1 (also called PREF-1) expression were different via RT-PCR (Fig. 7), as these genes have been implicated in the modulation of lipid and glucose metabolism in skeletal muscle (45–47).
Figure 7.
Differential gene expression in white gastrocnemius of HCR and LCR rats. RT-PCR was used to determine gene expression in white gastrocnemius tissue. (A–C) Relative gene expression of genes for (A) ANGPTL4, (B) IGF2, and (C) DLK1 were normalized to cyclophilin B (PPIB) and are presented as means ± SEM (n = 7 to 8). *P < 0.05 for main effect of HCR vs LCR rats.
Discussion
Our results show that male HCR rats with high aerobic capacity are protected against diet-induced insulin resistance. In contrast, the LCR rats displayed reduced insulin sensitivity following an HFD marked by reduced systemic rates of whole-body GC and inhibition of EGP. Robust differences in tissue disposal were also noted between strains. The acute HFD led to an ∼twofold increase in glucose transport into adipose tissue in the LCR rats whereas there was a twofold increase in glucose transport into the skeletal muscle of HCR rats. Additionally, only the LCR rats displayed HFD-induced reductions in Akt signaling (phospho-Ser473 or Thr308) in liver and skeletal muscle, whereas no changes occurred in HCR rats. Finally, RNA-seq of skeletal muscle revealed robust transcriptional differences between strains (on both an LFD and HFD) and strain differences in response to the acute HFD of smaller magnitude. Interestingly, ANGPTL4, which has previously been linked to alterations in glucose metabolism (45), was significantly higher in HCR muscle, whereas IGF2 and DLK1 [e.g., preadipocyte factor-1 (Pref-1)], factors previously linked to elevated intramuscular lipids (48) and impaired adipogenesis (46), respectively, were dramatically upregulated in muscle of LCR rats.
The HCR/LCR rat model displays salient features analogous to the effects of aerobic capacity on human health (19). As previously stated, we posit that systems operating with higher rate and capacity for the metabolic transfer of energy (e.g., oxygen consumption, ATP turnover, substrate flux) have the potential for more robust and favorable adaptations to changes in diet (19). In the context of this study, the HCR animals displayed a higher rate of whole-body insulin sensitivity, GC, and insulin signaling in skeletal muscle and liver compared with LCR animals on the control LFD, matching results of previous studies (25, 49). These baseline differences in insulin-stimulated glucose utilization are likely driven by the elevated resting energy expenditure known to occur in HCR rats (15). Likewise, coordinated expression of genes involved in TCA cycle flux and glucose utilization was also greater in HCR rats on both the LFD and HFD diets. Results from human subjects also suggest that higher rates of mitochondrial content and function in skeletal muscle track with higher levels of insulin sensitivity in skeletal muscle, particularly when comparing chronic exercisers vs sedentary individuals (50). Unfortunately, we are unaware of studies in which insulin sensitivity and substrate metabolism have been examined in those with intrinsically low and high aerobic capacity who are matched for exercise or activity to determine whether these mechanisms occur in humans. However, human studies have shown aerobic capacity to be predictive of insulin sensitivity (5, 6).
We previously reported that the HCR rats display greater whole-body and tissue-specific adaptations in fatty acid oxidation and utilization patterns following a 3-day HFD that do not occur as robustly in the LCR rats (15). We also reported that the HCR animals had a higher trafficking of dietary fatty acids to skeletal muscle whereas the LCR animals had a higher trafficking of dietary lipids to adipose depots. In the present study, we report differences in tissue-specific glucose trafficking during hyperinsulinemic clamp conditions after an HFD. A primary difference was a twofold higher rate of glucose uptake into subcutaneous and retroperitoneal adipose of HCR rats vs LCR rats that were largely unmodified by an HFD in the HCR rats, but increased by 30% to 50% within the LCR rats. In contrast, the HFD-induced increase in glucose transport into adipose beds of LCR rats suggests upregulated storage. With regard to hepatic insulin sensitivity, our results do not match previous findings using a 3-day HFD model. Kraegen et al. (51) reported that hepatic insulin resistance (reduced insulin inhibition of hepatic glucose output) occurred after a 3-day HFD but that skeletal muscle insulin resistance was not observed until after 2 weeks of an HFD. When only examining insulin-inhibited EGP, our results would suggest a similar HFD-induced effect in LCR rats; however, it was only basal EGP that was altered by the HFD in the LCR rats whereas EGP rates during the clamp were not altered. In contrast, a chronic HFD increases EGP in both basal and insulin-inhibited conditions (52, 53). It is possible that chronically reduced hepatic mitochondrial fatty acid oxidation, respiratory capacity, and PGC-1α in LCR rats is the cause for reduced EGP following an HFD (11, 15, 44), as hepatic mitochondrial fat oxidation is necessary to fuel gluconeogenesis (54), and PGC-1α regulates both mitochondrial function (55) and gluconeogenesis (56).
Glucose transport and glycogen synthesis rate dramatically increased only in the HCR rat skeletal muscle following transition to the HFD, contrasting with numerous previous studies in which HFD challenges reduce insulin action in muscle (57). It was surprising that the HFD-induced increases in muscle glucose transport in HCR rats did not track with changes in whole-body insulin sensitivity, GC, or insulin signaling (Akt Ser473 and Thr308). Additionally, the HFD did not alter total muscle glycogen levels in a manner that would explain our findings. To better understand possible mechanisms controlling skeletal muscle glucose transport in the HCR and LCR rats, we performed global transcriptional profiling using RNA-seq in white gastrocnemius muscle. The white portion of gastrocnemius muscle was chosen (primarily type II muscle fibers) because we hypothesized that this would reveal the most robust transcriptional differences between high and low aerobic capacity. Indeed, RNA-Seq revealed large transcriptional differences between the HCR and LCR rats on the LFD. Additionally, the acute HFD also induced differences in transcriptional responses between the HCR and LCR rat skeletal muscle. Although principal component analysis of global expression profiles indicated a greater effect of aerobic capacity genotype (along PC1, explaining 33% of variance), a significant effect of the 3-day HFD challenge was observed only in HCR animals (along PC2, explaining 12% of variance). Because genotype differences in HCR and LCR rats dominated muscle gene expression, we evaluated the transcripts that differed between these groups in both diet contexts. These differentially expressed genes were enriched for biological processes, including lipid and glucose metabolic process, glucose transport, hexokinase activity, TCA cycle, and OXPHOS, based on GO term enrichment different between strains. Although the influence of aerobic capacity genotype was distinct based on the diet, we also identified a core set of genes that were altered by aerobic capacity genotype in both the LFD and HFD groups. These genes are likely important in the underlying metabolic differences and differential responses of HCR rats to metabolic challenges, including HFD feeding. To further confirm results obtained from exploratory RNA-seq analysis, we performed directed quantitative PCR confirmation of a selected set of targets with known roles in glucose and lipid metabolism and skeletal muscle development.
We found robust strain differences in the expression of three genes, ANGPTL4, IGF2, and DLK1, that have previously been implicated in the regulation of glucose metabolism and insulin action. ANGPTL4, also known as fasting-induced adipose factor (FIAF), is a member of the ANGPTL family of proteins that increases in circulation following fasting and increases plasma triacylglycerol by inhibiting lipoprotein lipase. Blocking ANGPTL proteins has been shown to lower plasma triacylglycerols in animal models (45, 58). ANGPTL4 is highly expressed in the liver and was recently shown to be released from the liver during exercise (59). The role of ANGPTL4 in skeletal muscle has received less attention, but a recent study showed that one-legged exercise evoked increased skeletal muscle ANGPTL4 mRNA expression in the contralateral resting leg (60). We found that ANGPTL4 expression was twofold higher in HCR rat skeletal muscle compared with LCR rat skeletal muscle but was not modified by the acute HFD. ANGPTL4/FIAF knockout mice were shown to have reduced PGC-1α expression along with reduced expression of fat oxidation genes in skeletal muscle and greater susceptibility to diet-induced obesity, matching the skeletal muscle and whole-body phenotype of LCR rats that we have reported previously (9, 10). The underlying mechanisms and functional role of differences in ANGPTL4 between the HCR and LCR rats deserve further study.
Skeletal muscle DLK1 and IGF-2 receptor expression was higher in LCR animals compared with HCR animals. DLK1, also known as Pref-1, is a protein that putatively blocks adipocyte differentiation through paracrine/endocrine mechanisms. DLK1 overexpression in mice induces a lipodystrophy phenotype accompanied with insulin resistance in peripheral tissues (46), but its effects in skeletal muscle are primarily linked to myogenic properties during development and regeneration (61). Deletion of DLK1 blunts muscle growth and size but does not alter morphology while improving adult muscle regeneration (62). The potential metabolic influence of increased DLK1 on LCR rat skeletal muscle glucose metabolism or insulin sensitivity is difficult to derive from existing work. Finally, IGF-2 is known to play a role in proliferation, differentiation, growth, and migration of cells and has been associated with increased lipid storage in skeletal muscle of domestic animals (47). The higher levels of IGF-2 in LCR skeletal muscle may occur as a result of reduced functioning IGF binding receptors or could be due to higher levels of intramuscular lipids, which have been reported to express IGF-2 when isolated from muscle cells (63).
Transcriptional responses to a 3-day HFD were quantitatively greater in HCR animals, consistent with phenotypic adaptations. Distinct changes with a short-term HFD in HCR rats included changes in protein and amide biosynthesis, mitochondrial ETC, and ATP metabolic process and muscle contraction–related genes (31). Specifically, expression of myosin heavy chain 3 (Myh3), cholinergic receptor nicotinic delta (Chrnd), and voltage-gated potassium channel subunit Kv1.5 (Kcna5) involved in skeletal muscle neurotransmitter release, contraction, and ATP synthesis were upregulated (>twofold) only in HCR rats after an HFD. These findings suggest that adaptive processes in the muscle following an HFD are differentially regulated in HCR rats bred for high intrinsic aerobic capacity.
Although aerobic capacity is positively associated with human insulin sensitivity (5, 6) and negatively associated with the risk of developing chronic metabolic diseases, including type 2 diabetes (3), the direct assessment of aerobic capacity as a mediator of basal and diet-induced changes in insulin sensitivity in human subjects is complicated by exercise and physical activity effects. In the present study, we demonstrate in a male exercise naive model that divergent intrinsic aerobic capacity not only impacts insulin sensitivity as previously described (5, 6, 25), but it directly impacts susceptibility to HFD-induced alterations in insulin sensitivity and glucose homeostasis. Importantly, the observed protection from 3-day HFD-induced insulin resistance in the high aerobic capacity HCR male rats was associated with a large increase in skeletal muscle glucose uptake and nonoxidative storage as glycogen compared with LFD-fed HCR rats and HFD-fed LCR rats. The HFD-induced decrease in insulin sensitivity observed in the low aerobic capacity bred LCR rat further highlights the previously described lack of metabolic adaptability in this strain (13, 15, 18, 44). A limitation of the current study was that it was only performed in male rates, but previous work from our group has confirmed profound differences in the insulin sensitivity of female HCR and LCR rats as well. Overall, these data provide novel insight into how aerobic capacity may modulate systemic and tissue-specific insulin sensitivity independent of any effects of exercise or physical activity. Furthermore, these data highlight the potential therapeutic value of aerobic capacity assessment as a tool for individualized medicine in the prevention and treatment of metabolic disease.
Acknowledgments
This work was supported with resources and the use of facilities at the Harry S. Truman Memorial VA Hospital in Columbia, MO. We thank the University of Kansas Medical Center Genomics Core for generating the sequence data sets. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. The LCR/HCR rat models were maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan (Ann Arbor, MI) (contact S.L.B. at brittons@umich.edu for information on these models).
Financial Support: This work was partially supported by National Institutes of Health Grants DK088940 (to J.P.T.) and 5T32AR48523-8 (to E.M.M.), American Heart Association Grant 14POST20110034 (to E.M.M.), and by US Department of Veterans Affairs Grant VHA-CDA2 IK2BX001299 (to R.S.R.). K.S. and U.D.W. are supported in part by the US Department of Agriculture–Agriculture Research Service Project 6251-51000-010-05S. The Kansas Intellectual and Developmental Disability Research Center (National Institutes of Health Grant U54 HD090216) and the Molecular Regulation of Cell Development and Differentiation (National Institutes of Health NIH COBRE Grant 5P20GM104936-10) supported RNA-seq. The LCR/HCR rat model system was funded by the Office of Research Infrastructure Programs through National Institutes of Health Grant P40OD021331 (to L.G.K. and S.L.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ANGPTL
angiopoietin-like
- CoA
coenzyme A
- EGP
endogenous glucose production
- EI
energy intake
- FDR
false discovery rate
- GC
glucose clearance
- GIR
glucose infusion rate
- GO
gene ontology
- GSEA
gene set enrichment analysis
- HCR
high-capacity running
- HEC
hyperinsulinemic-euglycemic clamp
- HFD
high-fat, high-sucrose diet
- LCR
low-capacity running
- LFD
low-fat diet
- LSC
liquid scintillation counting
- PC
principal component
- phospho-
phosphorylated
- RNA-seq
RNA sequencing
- TCA
tricarboxylic acid
References and Notes
- 1. Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. [DOI] [PubMed] [Google Scholar]
- 2. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. [DOI] [PubMed] [Google Scholar]
- 3. Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haufe S, Engeli S, Budziarek P, Utz W, Schulz-Menger J, Hermsdorf M, Wiesner S, Otto C, Haas V, de Greiff A, Luft FC, Boschmann M, Jordan J. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes. 2010;59(7):1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, Port JD, Glavin MT, Esponda RR, Nair KS, Lanza IR. Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J Clin Endocrinol Metab. 2016;101(2):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. [DOI] [PubMed] [Google Scholar]
- 7. Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. [DOI] [PubMed] [Google Scholar]
- 8. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52. [DOI] [PubMed] [Google Scholar]
- 9. Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293(1):E31–E41. [DOI] [PubMed] [Google Scholar]
- 10. Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, Koch LG, Jenkins NT, Crissey JM, Zidon T, Morris EM, Meers GM, Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308(6):R530–R542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthew Morris E, Meers GM, Koch LG, Britton SL, MacLean PS, Thyfault JP. Increased aerobic capacity reduces susceptibility to acute high-fat diet-induced weight gain. Obesity (Silver Spring). 2016;24(9):1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoin CS, White KS, Wilson DR, Meers GM, Koch LG, Britton SL, Thyfault JP, Geiger PC. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes. 2016;65(11):3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, Fletcher JA, Meers GM, Koch LG, Britton SL, Rector RS, Ibdah JA, MacLean PS, Thyfault JP. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2014;307(4):E355–E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karvinen SM, Silvennoinen M, Ma H, Törmäkangas T, Rantalainen T, Rinnankoski-Tuikka R, Lensu S, Koch LG, Britton SL, Kainulainen H. Voluntary running aids to maintain high body temperature in rats bred for high aerobic capacity. Front Physiol. 2016;7:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris EM, McCoin CS, Allen JA, Gastecki ML, Koch LG, Britton SL, Fletcher JA, Fu X, Ding WX, Burgess SC, Rector RS, Thyfault JP. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J Physiol. 2017;595(14):4909–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch LG, Britton SL. Theoretical and biological evaluation of the link between low exercise capacity and disease risk. Cold Spring Harb Perspect Med. 2018;8(1):a029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1111–R1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52(5):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 2008;105(22):7815–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. [DOI] [PubMed] [Google Scholar]
- 24. Koch LG, Britton SL. Theoretical and biological evaluation of the link between low exercise capacity and disease risk. Cold Spring Harb Perspect Med. 2018;8(1):a029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park YM, Rector RS, Thyfault JP, Zidon TM, Padilla J, Welly RJ, Meers GM, Morris ME, Britton SL, Koch LG, Booth FW, Kanaley JA, Vieira-Potter VJ. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am J Physiol Endocrinol Metab. 2016;310(3):E190–E199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rector RS, Morris EM, Ridenhour S, Meers GM, Hsu FF, Turk J, Ibdah JA. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology. 2013;57(6):2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shearer J, Fueger PT, Vorndick B, Bracy DP, Rottman JN, Clanton JA, Wasserman DH. AMP kinase-induced skeletal muscle glucose but not long-chain fatty acid uptake is dependent on nitric oxide. Diabetes. 2004;53(6):1429–1435. [DOI] [PubMed] [Google Scholar]
- 28. Virkamäki A, Rissanen E, Hämäläinen S, Utriainen T, Yki-Järvinen H. Incorporation of [3-3H]glucose and 2-[1-14C]deoxyglucose into glycogen in heart and skeletal muscle in vivo: implications for the quantitation of tissue glucose uptake. Diabetes. 1997;46(7):1106–1110. [DOI] [PubMed] [Google Scholar]
- 29. Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes. 2002;51(3):567–573. [DOI] [PubMed] [Google Scholar]
- 30. Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, Shankar K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One. 2017;12(4):e0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris EM, Meers GME, Ruegsegger GN, Wankhade UD, Robinson T, Koch LG, Britton SL, Rector RS, Shankar K, Thyfault JP. Data from: Intrinsic high aerobic capacity in male rats protects against diet-induced insulin resistance. figshare 2019. Deposited 14 February 2019 https://figshare.com/s/b8986409d2f715ecb870. [DOI] [PMC free article] [PubMed]
- 32. Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL, MacLean PS, Thyfault JP. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1α overexpression. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G868–G880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G979–G992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RRID:AB_329827, https://scicrunch.org/resolver/AB_329827.
- 35.RRID:AB_329825, https://scicrunch.org/resolver/AB_329825.
- 36.RRID:AB_329828, https://scicrunch.org/resolver/AB_329828.
- 37.RRID:AB_2199375, https://scicrunch.org/resolver/AB_2199375.
- 38.RRID:AB_10860251, https://scicrunch.org/resolver/AB_10860251.
- 39.RRID:AB_490890, https://scicrunch.org/resolver/AB_490890.
- 40.RRID:AB_2115196, https://scicrunch.org/resolver/AB_2115196.
- 41.RRID:AB_2279563, https://scicrunch.org/resolver/AB_2279563.
- 42.RRID:AB_2116390, https://scicrunch.org/resolver/AB_2116390.
- 43.RRID:AB_2099233, https://scicrunch.org/resolver/AB_2099233.
- 44. Morris EM, Meers GM, Koch LG, Britton SL, Fletcher JA, Fu X, Shankar K, Burgess SC, Ibdah JA, Rector RS, Thyfault JP. Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2016;311(4):E749–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davies BS. Can targeting ANGPTL proteins improve glucose tolerance? Diabetologia. 2018;61(6):1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Villena JA, Choi CS, Wang Y, Kim S, Hwang YJ, Kim YB, Cline G, Shulman GI, Sul HS. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57(12):3258–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aslan O, Hamill RM, Davey G, McBryan J, Mullen AM, Gispert M, Sweeney T. Variation in the IGF2 gene promoter region is associated with intramuscular fat content in porcine skeletal muscle. Mol Biol Rep. 2012;39(4):4101–4110. [DOI] [PubMed] [Google Scholar]
- 48. Cesar AS, Regitano LC, Koltes JE, Fritz-Waters ER, Lanna DP, Gasparin G, Mourão GB, Oliveira PS, Reecy JM, Coutinho LL. Putative regulatory factors associated with intramuscular fat content. PLoS One. 2015;10(6):e0128350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB III, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300(4):R835–R843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. [DOI] [PubMed] [Google Scholar]
- 51. Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40(11):1397–1403. [DOI] [PubMed] [Google Scholar]
- 52. Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Méndez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53(6):1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14(6):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Williamson JR, Scholz R, Browning ET. Control mechanisms of gluconeogenesis and ketogenesis. II. Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J Biol Chem. 1969;244(17):4617–4627. [PubMed] [Google Scholar]
- 55. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α)-deficient mice. J Biol Chem. 2006;281(28):19000–19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackson KC, Wohlers LM, Lovering RM, Schuh RA, Maher AC, Bonen A, Koves TR, Ilkayeva O, Thomson DM, Muoio DM, Spangenburg EE. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am J Physiol Regul Integr Comp Physiol. 2013;304(3):R206–R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA. 2007;104(28):11766–11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ingerslev B, Hansen JS, Hoffmann C, Clemmesen JO, Secher NH, Scheler M, Hrabĕ de Angelis M, Häring HU, Pedersen BK, Weigert C, Plomgaard P. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Mol Metab. 2017;6(10):1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cavalot F, Pagliarino A, Valle M, Di Martino L, Bonomo K, Massucco P, Anfossi G, Trovati M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34(10):2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Andersen DC, Petersson SJ, Jørgensen LH, Bollen P, Jensen PB, Teisner B, Schroeder HD, Jensen CH. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009;27(4):898–908. [DOI] [PubMed] [Google Scholar]
- 62. Andersen DC, Laborda J, Baladron V, Kassem M, Sheikh SP, Jensen CH. Dual role of delta-like 1 homolog (DLK1) in skeletal muscle development and adult muscle regeneration. Development. 2013;140(18):3743–3753. [DOI] [PubMed] [Google Scholar]
- 63. Gardan D, Gondret F, Louveau I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am J Physiol Endocrinol Metab. 2006;291(2):E372–E380. [DOI] [PubMed] [Google Scholar]