Abstract
Objective:
Explore interactive relations of lifetime discrimination burden and racial discrimination – chronic stressors among African Americans (AA) – and age with magnetic resonance imaging (MRI)-assessed white matter lesion volume (WMLV), a prognostic indicator of poor clinical brain health outcomes.
Methods:
AA (N= 71; 60.6% female, mean age = 50) participating in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) SCAN study underwent quantitative MRI coded for WMLV. Participants self-reported lifetime discrimination burden and racial discrimination approximately five years earlier. Multivariable regression models assessed interactions of linear and quadratic effects of discrimination and age with WMLV adjusted for sex and socioeconomic status.
Results:
Findings revealed significant interactive relations of age and (1) quadratic, lifetime discrimination burden, B = .05, p = .014, η2partial = .092, and (2) quadratic, racial discrimination, B = .03, p = .001, η2partial = .155 with WMLV. Among older AA, increases in lifetime discrimination burden and racial discrimination were associated with increases in WMLV (p’s < .03); in younger AA, decreasing levels of racial discrimination were related to increases in WMLV (p = .006).
Conclusions:
Among older AA, as lifetime discrimination burden and racial discrimination increased, so did WMLV. However, in younger AA, decreases in racial discrimination were associated with increased WMLV. Elucidation of complex mechanistic underpinnings, including potentially differential impacts of the acknowledgement versus suppression or underreporting of discriminatory experiences, among AA of different age cohorts, is critical to understanding the present pattern of findings.
Keywords: discrimination/racial discrimination, age, racial/ethnic minorities, subclinical cerebrovascular disease, white matter lesion volume (WMLV), MRI-brain health
Introduction
Compared with other racial and ethnic groups in the U.S., African Americans experience a disproportionate burden of poor brain clinical health outcomes, particularly earlier in adulthood (Harwood & Ownby, 2000; Mozaffarian et al., 2016). For instance, African Americans have almost twice the risk of an initial stroke compared with Whites and by 2032 are expected to experience a 134% increase in stroke compared with a 91% increase in Whites (Elkins & Johnston, 2003; Gillum, Kwagyan, & Obisesan, 2011; Morgenstern, Spears, Goff, Grotta, & Nicharman, 1997). This disparity in stroke risk is most striking in midlife; compared with Whites, African Americans are 2–5 times more likely to experience a stroke before the age of 55 and 3–4 times more likely to experience a stroke at 45 years of age (Centers for Disease Control [CDC], 2017; Morgenstern et al., 1997). African Americans are also at greater risk for dementia compared to Whites, American Indians/Alaskan Natives, Pacific Islanders, and Asian and Latino(a) Americans (Harwood & Ownby, 2000; Tatemichi et al., 1992). In a 14-year U.S. study including these six racial and ethnic groups, dementia incidence was highest for African Americans who had a 65% greater risk compared with Asian Americans, for whom incidence was lowest (Mayeda, Glymour, Quesenberry, & Whitmer, 2016). African Americans are also at greater risk for cognitive decline earlier in adulthood, evidencing 4 times greater risk for impairment at younger ages (i.e., 55–64) compared with Whites (Alzheimer’s Association, 2010).
African Americans also have worse subclinical brain health on markers identified as robust, prognostic indicators of future stroke, dementia, and cognitive decline. One such marker, white matter lesion volume (WMLV), is commonly derived from brain magnetic resonance imaging (MRI; Brickman et al., 2008). WMLV represents diffuse areas of non-specific injury indicating cerebral small vessel disease (Schmahmann, Smith, Eichler, & Filley, 2008). WMLV is a particularly pertinent indicator of future brain pathology: higher WMLV increases risk for and precedes vascular events, deteriorates neurocognitive functioning, and contributes to risk for dementia across adulthood (de Groot et al., 2002; Debette & Markus, 2010; Smith et al., 2008; Vermeer et al., 2003). Of note, risk for WMLV increases with age and is particularly prevalent at older ages (Habes et. al., 2016; Raz & Rodrigue, 2006). However, compared with other racial and ethnic groups African Americans are more vulnerable to earlier and greater severity of white matter disease (Liao et al., 1997).
African Americans bear a disproportionate burden of vascular risk factors, including smoking, obesity, diabetes, systolic and diastolic blood pressure, and pulse pressure (Hozawa, Folsom, Sharrett, & Chambless, 2007; Liao et al., 1997; Pathak & Sloan, 2009), which are predictors of poor subclinical (e.g., WMLV) and clinical brain health outcomes such as stroke (Simons, McCallum, Friedlander, & Simons, 1998; Tiehuis et al., 2008). However, the enhanced burden and earlier onset of poor brain health outcomes observed in African Americans has not been fully accounted for by other sociodemographic (e.g., age, education) or traditional (e.g., smoking, obesity, or blood pressure) vascular risk factors (Tang et al., 2001). Prospective studies have demonstrated that self-reported chronic stress – arising from specific types of stressors such as empty nest status or the mid-life transition – is associated with poor clinical and subclinical brain endpoints including atrophy and WMLV (e.g., Duan et al., 2017; Johansson et al., 2012). Similarly, chronic stress due to racial and ethnic bias, which is known to be more prevalent among and deleterious for African Americans, may partially underlie the observed racial and ethnic disparities in brain health endpoints (Alzheimer’s Association, 2016). Indeed, an extensive literature has theorized racial discrimination as a chronic stressor for African Americans (e.g., Clark, Anderson, Clark, & Williams, 1999) citing numerous negative health implications of exposure to these events in one-on-one interactions.
With respect to brain health outcomes, a single, prospective study of 6,508 middle-aged to older White, Black, Hispanic, and Chinese adults recently reported that lifetime discrimination and to a lesser extent, everyday discrimination predicted incident cardiovascular events, including stroke (Everson-Rose et al., 2015). To our knowledge, whether self-reported lifetime discrimination burden or racial discrimination are associated with MRI-assessed subclinical brain health outcomes has not been investigated. While both lifetime discrimination burden and racial discrimination can reflect chronic experiences of unfair treatment encountered in interpersonal interactions, lifetime discrimination burden encompasses experiences arising for any reason, whereas racial discrimination encompasses those experiences arising explicitly because of race or ethnicity (Essed, 1991; Williams, Yu, Jackson, & Anderson, 1997). Thus, lifetime discrimination burden is assessed in a context of general unfairness, and racial discrimination is assessed in a more specific context of unfairness underscored by power differentials driven by the sociohistorical implications of race and ethnicity. The negative health implications of both experiences of unfairness have been substantiated by a wealth of empirical data (see reviews by e.g., Paradies et al., 2015; Pascoe & Smart Richman, 2009).
Here, we examine both racial discrimination and lifetime discrimination burden for two reasons. First, although racial discrimination in interpersonal interactions is typically conveyed through discriminatory practices and behaviors, it is considered a specific and unique form of discrimination. Indeed, acts related to one’s race or ethnicity versus those related to some other reason – unrelated to the sociohistorical demarcation of stigmatized minority racial and ethnic status in this country – have a qualitatively and quantitatively different meaning for the target (Krieger, 2014; Shariff-Marco et al., 2011). Experiences of racial discrimination across settings (e.g., work, school, obtaining housing) that individuals traverse can lead to exclusion, rejection, and blocked opportunities for advancement. Understanding the frequency of such experiences and their linkage to health holds promise for understanding disparities and providing entry points for policy formation (Krieger, 2014). Second, we assess the lifetime discrimination burden as a way of gauging the collective weight of experiences that an individual may not readily make a specific attribution for but understands as mistreatment. Such an assessment may provide a better understanding of the implications of both minute and more exceptional experiences together, from the target’s perspective, considering their conceptualization of the impact or burden of these events over the entirety of their life. The promise of this focus – on both lifetime discrimination burden and racial discrimination – is underscored by recent reviews (Krieger, 2014; Lewis, Cogburn, & Williams, 2015) that have called for studies to expand beyond a focus on single dimensions of discrimination to concurrently assess multiple dimensions of this construct, particularly in the context of racial and ethnic disparities health research. Few studies (e.g., Everson-Rose et al., 2015; Sims et al., 2012) have done this in cardiovascular health research, and none have done this in the context of brain health.
We further explored whether there were nonlinear associations of self-reported lifetime discrimination burden and racial discrimination with WMLV. Some prior literature (Everage, Gjelsvik, McGarvey, Linkletter, & Louck, 2012; Krieger & Sidney, 1996; Ryan, Gee, & LaFlamme, 2006) has demonstrated that similar to increases in levels of self-reported racial discrimination and lifetime discrimination, decreasing levels may confer negative health outcomes (e.g., coronary artery calcification, hypertension) among African Americans. These findings have led researchers to posit that there are also negative health effects of suppression or lack of acknowledgement of experiences with racial discrimination in interpersonal interactions (Everage et al., 2012; Harrell, 2000; Krieger & Sidney, 1996). Therefore, it is possible that the association between discrimination and WMLV is nonlinear.
We also considered whether the linkages of lifetime discrimination burden and racial discrimination to brain health endpoints are patterned by age. Age has previously been conceptualized as an indicator of cumulative stress exposure (Beatty Moody et al., 2016; Gee, Walsemann, & Brondolo, 2012; Williams & Collins, 1995) that elucidates the accelerated onset of negative health outcomes for particular groups. Thus, it is plausible that these associations are more pronounced with increasing age due to greater lifetime discrimination burden and racial discrimination. In this regard, albeit equivocal, there is some evidence that older African Americans do report greater discrimination, including lifetime burden of discrimination (e.g., Sims et al., 2012) than their younger counterparts (e.g., for review see Paradies et al., 2006). At the same time, it is important to consider the potential for age cohort effects, given that African Americans from different generational cohorts would have been exposed to various dimensions of discrimination at differing levels of intensity and frequency. Therefore, age-dependent differences in the linkage between lifetime discrimination burden and racial discrimination with WMLV may reflect, at least in part, generational cohort effects (Gee et al., 2012). In addition, as noted earlier, there is a well-known increase in risk of WMLV with greater age (Xiong & Mok, 2011), perhaps reflecting increased vulnerability of the brain to exposure-related processes associated with adversity arising from social, environmental, and psychological conditions.
The purpose of the current study was to examine in a sample of socioeconomically diverse, urban dwelling African Americans: (1) linear and non-linear associations of self-reported lifetime discrimination burden and racial discrimination to WMLV and (2) whether age moderates these associations.
Method
Sample and Participants
Participants were drawn from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) SCAN study, an investigation of race- and SES-related disparities in subclinical brain health (Waldstein et al., 2017). HANDLS SCAN is an ancillary study of the larger HANDLS investigation, a prospective study of race- and SES-related health disparities among persons living in 13 select neighborhoods in Baltimore, Maryland. In addition to HANDLS parent study exclusions (see Evans et al., 2010), HANDLS SCAN exclusions were a history of dementia, stroke, transient ischemic attack, other neurological disease (e.g., multiple sclerosis), carotid endarterectomy, MRI contraindications (e.g., indwelling ferromagnetic material), terminal illness (e.g., metastatic cancer), and HIV positive status. In total, of the 147 participants who met inclusion criteria for HANDLS SCAN, 85 were African American. The present study’s analysis sample consisted of the 71 HANDLS SCAN participants who self-identified as African American or Black and had complete data for all relevant sociodemographic (age, poverty status, education), discrimination (i.e., lifetime discrimination burden & racial discrimination), and MRI measures. This analysis sample had an age range of 32.9–69.9 years.
The Institutional Review Boards of the University of Maryland School of Medicine and University of Maryland, Baltimore County approved the HANDLS SCAN study. Participants were provided $50 for their participation in addition to reimbursement of travel costs.
Measures
Sociodemographic Characteristics.
Age (in years), sex (0 = female; 1 = male), and socioeconomic status (SES) were assessed at study entry (data collection 2004–2009). SES was assessed using a composite variable (0 = higher SES; 1 = lower SES) that was derived from two measures: (1) dichotomous poverty status, which was defined as an annual household income above or below 125% of the 2004 Federal poverty level relative to family size (0 = non-poverty; 1 = poverty); and (2) dichotomous years of education (0 = greater than or equal to 12 years; 1 = fewer than 12 years; see Waldstein et al., 2017). Participants who were living above the poverty level and who had greater than or equal to 12 years of education were classified as higher SES. Conversely, participants who were living below the poverty line, had fewer than 12 years of education, or both were classified as lower SES.
Although optimal assessment of SES is more nuanced than a dichotomy, HANDLS investigators based their initial area probability recruitment on a division of household income based on 125% of the 2004 federal poverty level. The goal, which was achieved, was to recruit sufficient numbers of participants below and above 125% of the 2004 federal poverty level so that both low and moderate incomes were well represented. During the initial recruitment process, it was found that many HANDLS participants could not estimate their annual incomes, had no way to estimate their overall wealth, and were employed only sporadically (though not necessarily in low-status positions). Consequently, our best estimate of overall SES depends on the investigators’ initial ascertainment of poverty status and self-reported level of education. Here, we dichotomized as < high school and ≥ high school to best represent “low” and “high” levels of education in this sample. For parsimony, it was also more straightforward to combine a dichotomized version of education with poverty status than the categorical education ratings provided by participants. Importantly, there is no universally accepted measure of SES in health research. Rather, SES measurement should be expected to vary across studies according to the social group and outcomes of interest and what is feasible to measure, among other considerations (Braveman et al., 2005).
Self-Reported Lifetime Discrimination Burden and Racial discrimination.
Lifetime discrimination burden and racial discrimination were assessed at study entry. A single item was used to assess the lifetime discrimination burden: “Overall, how much harder has your life been because of discrimination?” Response options for this item were 1 (not at all), 2 (a little), 3 (some), or 4 (a lot). This item was sampled from the MacArthur Major Experiences of Discrimination Questionnaire (The John D. and Catherine T. MacArthur Foundation Research Network, 2008), which assesses discrimination across the lifetime. Furthermore, this item was previously included in a 3-item measure within the Jackson Heart Study Discrimination Instrument, which has been found to have strong psychometric properties (α = .78; Sims, Wyatt, Gutierrez, Taylor, & Williams, 2009). This item has previously been used in health disparities research with racially/ethnically diverse samples, such as in the Survey of Midlife Development in the United Status (MIDUS; e.g., Friedman, Williams, Singer, & Ryff, 2009).
Racial discrimination was assessed with six items that inquired about whether individuals had ever experienced racial discrimination at school, when getting a job, at work, when getting housing, when getting medical care, or from police or in courts (Krieger, 1990). Respondents could reply Yes (1) or No (0) to each of the 6 items. The possible scale range is 0–6, with a greater sum indicating greater racial discrimination. According to the scale’s author, items were designed to measure situations in which racial discrimination is well-documented (Krieger, 1990). These six items were initially introduced by Krieger (1990). They have been extensively used in the epidemiological Coronary Artery Risk Development in Young Adults study (CARDIA; e.g., Krieger & Sidney, 1996), and have since been drawn upon in health disparities research with racially and ethnically diverse samples, including the subsequently developed Experiences of Discrimination scale, which has strong internal consistency (α = 0.74) and test-retest reliability (0.70; see Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005). In our sample, this scale had strong internal consistency (Cronbach’s alpha = 0.84).
Clinical Variables.
Clinical variables were assessed at study entry during Wave 1. Hypertension was defined by self-reported history, use of antihypertensives, and/or resting systolic or diastolic blood pressures ≥ 140 mm Hg or ≥ 90 mm Hg, respectively. Levels of total serum cholesterol and fasting glucose were assessed by standard laboratory methods at Quest Diagnostics (Chantilly, VA; http://www.questdiagnostics.com). Blood samples were obtained from an antecubital vein after an overnight fast. Diabetes was defined as a fasting blood glucose level of ≥ 126 mg/dl, self-reported history, and/or use of relevant medications. Smoking status and alcohol use were dichotomized as never used (0) and ever used (1), which included former and current users. Body mass index was computed as weight divided by height squared (kg/m2) using height and weight obtained via calibrated equipment. Waist circumference was measured to the nearest 0.1 cm with a flexible tape measure placed at the midpoint between the lower rib margin and the iliac crest at the end of exhalation during normal breathing. Depressive symptoms were assessed using the Center for Epidemiologic Studies-Depression 20-item scale (Radloff, 1977). Clinical diagnostic data were documented by a HANDLS physician or nurse practitioner after a comprehensive physical examination and medical history.
Data imputation was previously performed for specific HANDLS variables with < 10% missing data within each race, poverty status, and sex subgroup. Multiple linear regression (i.e., using age, sex, race, and poverty status as predictors) was used for imputation for the purpose of replicability. Of the clinical variables used in the present study, data imputation was previously performed for depressive symptoms, waist circumference, and total cholesterol. These imputed data were used in the present statistical analyses.
Magnetic Resonance Imaging-Assessed (MRI) Lesion Volume.
Cranial magnetic resonance images were obtained using a Siemens Tim-Trio 3.0 Tesla unit approximately five years (mean = 1,942.43 days; SD = 297.83) after their participation in HANDLS Wave 3 (data collection 2009–2013). Volumetric T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) images covered the entire brain in the sagittal plane at 1.2 mm thickness for a total of 160 slices (TR/TE/TI = 2300/2.9/900 ms; FOV 25.6 cm) and were reformatted into axial sections. Axial fluid attenuated inversion recovery (FLAIR) images were obtained at a slice thickness of 3 mm with no gap in two concatenated groups of 24 slices each for a total of 48 slices (TR/TE/TI = 8000/7½500 ms; FOV = 23cm). A dual echo proton density (PD)/T2-weighted were acquired at 3 mm thickness with no gap using turbo spin-echo acquisition (TR/TE1/TE2 = 6600/9.4/93 ms; FOV = 23 cm; turbo factor 7).
Structural MRI scans were preprocessed by removal of extra-cranial material on T1-weighted image using a multi-atlas registration based method (Doshi, Erus, Ou, Gaonkar, & Davatzikos, 2013), followed by bias correction (Tustison et al., 2010). A supervised learning based multi-modal lesion segmentation technique was applied to segment ischemic lesions (Zacharaki et al. 2008). The method involved co-registration of T1, T2 and FL scans, histogram normalization to a template image, feature extraction, voxel wise label assignment using a model that was trained on an external training set with manually labeled ground-truth lesion masks, and false-positive elimination. The total white matter lesion volume (WMLV) is calculated for each subject from the segmented lesion mask.
Analytic Plan
Descriptive analyses were conducted to assess means, standard deviations, distributions, and linearity of variables. Multiple linear regression was used to examine the independent and interactive relations of age and both linear and quadratic lifetime discrimination burden and racial discrimination to WMLV using the Statistical Package for the Social Sciences (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Specifically, to assess linear associations of lifetime discrimination burden and WMLV, analyses were conducted by regressing WMLV on discrimination, age, sex, SES, and the interaction of age × discrimination. Additionally, to assess non-linear associations of lifetime discrimination burden and WMLV, a quadratic term for discrimination and an interaction of quadratic discrimination with age were entered into subsequent regression models. This approach was also employed to assess racial discrimination.
Significant interactions were probed using the PROCESS macro for SPSS version 2.16 (for the manual, see Hayes, 2013) to examine simple effect. Furthermore, Johnson-Neyman technique (Hayes, 2013; Johnson & Fay, 1950) was used to detect regions of significance of the moderator for the conditional effect of the predictor on the dependent variable, allowing a more precise inspection of the effect of the moderator. The Johnson-Neyman technique is commonly used to probe significant interactions when the moderator is a continuous variable, and may be preferable to indiscriminate identification of values of the moderator (see Hayes, 2013).
Initial examination of the data revealed that the distribution of WMLV was positively skewed (i.e., non-normally distributed), violating the normality assumption of parametric statistical tests. Natural-logarithmic data transformation resolved the skewness of the distribution. The transformed variable was used in all primary study analyses; however, in descriptive analyses and the related table (see Table 1) the non-transformed term for WMLV is utilized.
Table 1.
Participant Sociodemographic Characteristics and Self-Reported Lifetime Discrimination Burden and Racial Discrimination Descriptives (N = 71)
| Variable | Mean (SD)/N% | Range |
|---|---|---|
| Age | 50.58 (9.92) | 32.9 – 69.4 |
| % Female | 60.6% | — |
| % Lower SES | 54.9% | — |
| % <125% federal poverty level | 43.7% | — |
| Educational attainment (years) | 12.73 (2.64) | 3 – 20 |
| Racial discrimination summary score* | 1.31 (1.59) | 0 – 6 |
| Ever experience discrimination... | ||
| At school? | 11.3% | — |
| When getting a job? | 31.0% | — |
| At work? | 39.4% | — |
| When getting housing? | 14.1% | — |
| When getting medical care? | 7.0% | — |
| From the police or in courts | 28.2% | — |
| Lifetime discrimination burden | 2.00 (1.01) | 1 – 4 |
Lower SES = lower socioeconomic status. Lower SES reflects participants who were living below the poverty line (i.e., < 125% of the 2004 Federal poverty level relative to family size), had fewer than 12 years of education, or both.
% Reflects participants affirming these experiences.
Examination of the discrimination measures revealed positively skewed distributions for lifetime discrimination burden and racial discrimination. However, linear regression assumes that residuals are normally distributed, but makes no assumptions about the distribution of independent variables (Fox & Weisberg, 2011). As such, it is usually unnecessary to transform an independent variable on the basis of its distribution (Babyak, 2004), and there is no reason to suspect undue influence by outliers from the discrimination measures in the present analyses (Fox & Weisberg, 2011). Therefore, the distributions of the lifetime discrimination burden and racial discrimination were not transformed prior to analysis.
Sensitivity Analyses.
Subsequent analyses were conducted to assess respective contributions of hypertension (0 = absent, 1 = present), diabetes (0 = absent, 1 = present), cigarette use status (0 = never used, 1 = ever used), alcohol use status (0 = never used, 1 = ever used), total cholesterol, body mass index, waist circumference, and depressive symptoms as covariates in the aforementioned models. Due to concerns about reduced statistical power, each sensitivity variable was compared one at a time in separate regression analyses. This approach also allowed for determining the likelihood of potential mediation effects, which could be masked if all sensitivity variables were compared together in one analysis. Additionally, analyses were repeated excluding participants with a history of cardiovascular disease (i.e., coronary artery disease, myocardial infarction, or heart failure; n = 2) or kidney disease (n = 2). Finally, in lieu of the SES composite, all models were also run using poverty status. Poverty status was assessed using family income as a function of household size. This variable was then dichotomized using the 2004 Federal poverty threshold line (e.g., $18,850 per year for a family of four), where above poverty (0) was defined as having a family income above 125% of the poverty threshold, while below poverty (1) was defined as having a family income below or just above (between 100% and 124%) the poverty threshold.
Results
As shown in Table 1, 71 African American participants comprised the present study’s sample. The participants included 43 women and 28 men whose ages ranged from 33 to 69 years (M = 50.58, SD = 9.92), with a mean level of 12.73 (SD = 2.64) years of education, and 43.7% living below the poverty level. The sample was considered overweight with an average body mass index of 29.86 (SD = 6.76). Over one-third of participants was diagnosed as hypertensive, and over one-tenth were diabetic. Two participants reported a diagnosis of cardiovascular disease. Most participants affirmed a burden of lifetime discrimination (62.0%) and reported experiences with racial discrimination (53.5%). Lifetime discrimination burden was strongly correlated with the overall racial discrimination measure, r = .68, p < .001, and with most of the specific items comprising this measure (see Supplementary Table 1). Neither measure of discrimination was significantly related to WMLV, whereas lifetime discrimination burden was positively associated with depressive symptoms, r = .24, p = .047. Finally, greater WMLV was positively associated with greater age, r = .25, p = .034, and greater total cholesterol, r = .24, p = .042.
Supplementary Table 2 contains means and standard deviations of WMLV, across age tertile groups. A one-way analysis-of-variance was conducted to compare the effect of age tertile groups on WMLV, which revealed a significant effect, F(2, 68) = 4.60, p = .013. Subsequent analysis with a Tukey post-hoc test revealed that the mean WMLV of the oldest age tertile (M = 6.85, SD = 2.10) was significantly greater than the middle (M = 5.64, SD = 1.28) and youngest age tertiles (M = 5.67, SD = 1.14) at the p < .05 level. There was not a significant difference in WMLV between the middle and youngest age tertiles.
Findings from the models that contained only linear discrimination terms revealed no significant associations of WMLV with lifetime discrimination burden, B = .04, p = .844, age, B = .05, p = .235, or the interaction of lifetime discrimination burden and age, B = .002, p = .925. Likewise, findings from models that contained only linear racial discrimination terms revealed no significant association of WMLV with racial discrimination, B = .08, p = .510, or the interaction of racial discrimination and age, B = −.002, p = .896. As expected, this model revealed a significant main effect of age, B = .06, p = .032, such that as age increased WMLV increased.
Quadratic lifetime discrimination burden and racial discrimination terms and their interactions with age were added to subsequent models. Findings revealed two significant two-way interactions of age × quadratic lifetime discrimination burden, B = .05, p = .014, η2partial = .092; and age × quadratic racial discrimination, B = .03, p = .001, η2partial = .155 with WMLV (see Table 2). As demonstrated in Panel Figure 1, simple effects analyses indicated that, among older African Americans (i.e., 60 years of age), as lifetime discrimination burden increased, WMLV increased, B = .65, p = .016. Subsequent findings, using the Johnson-Neyman technique, revealed this association in particular between ages 55.7 to 69.4 years. The beta-values increased and p-values decreased incrementally with each increase in age above 55.7 years (these data are available in Supplementary Table 3).
Table 2.
Curvilinear Multiple Regression Models Estimating 2-way Interactions for Lifetime Discrimination Burden2 × Age and Racial Discrimination2 × Age with WMLV
| (a) Lifetime discrimination burden2 × Age | ||||
| Model predictors | Unstandardized B | SE | p-value | η2partial |
| Male sex | −0.19 | .40 | .629 | .004 |
| Lower SES | 0.59 | .41 | .157 | .031 |
| Age** | 0.28 | .10 | .005 | .116 |
| Lifetime discrimination burden | −0.71 | .93 | .453 | .009 |
| Lifetime discrimination burden × Age* | −0.24 | .10 | .016 | .089 |
| Lifetime discrimination burden2 | 0.15 | .19 | .433 | .010 |
| Lifetime discrimination burden2 × Age* | 0.05 | .02 | .014 | .092 |
| (b) Racial discrimination2 × Age | ||||
| Model predictors | Unstandardized B | SE | p-value | η2partial |
| Male sex | −0.36 | .38 | .349 | .014 |
| Lower SES | 0.41 | .41 | .314 | .016 |
| Age** | 0.09 | .03 | .002 | .148 |
| Racial discrimination | 0.30 | .34 | .373 | .013 |
| Racial discrimination × Age** | −0.12 | .04 | .002 | .141 |
| Racial discrimination2 | −0.08 | .08 | .301 | .017 |
| Racial discrimination2 × Age** | 0.03 | .01 | .001 | .155 |
Note.
p < .05,
p < .01
Figure 1.
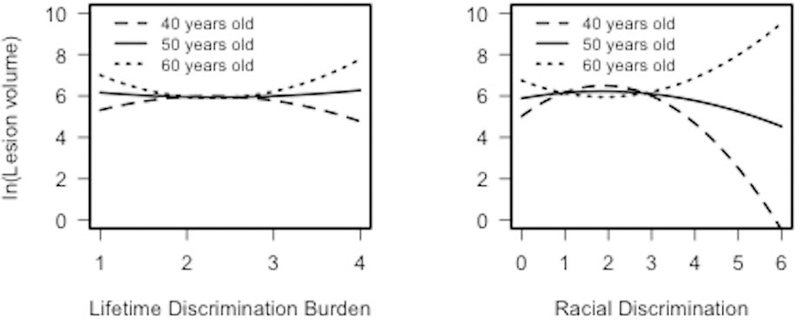
Panel Figures Demonstrating Significant Quadratic Interactions of Lifetime Discrimination Burden2 × Age and Racial Discrimination2 × Age Associated with log WMLV
As demonstrated in Panel Figure 1, simple effects analyses indicated that, among older African Americans (i.e., 60 years old), as racial discrimination increased, WMLV increased, B = .22, p = .024, whereas among younger African Americans (i.e., 40 years old), as racial discrimination increased, WMLV decreased, B = −.41, p = .006. Subsequent findings, using the Johnson-Neyman technique, further revealed that as racial discrimination increased, WMLV decreased between ages 58.6 years to 69.4 years. The Johnson-Neyman technique also revealed that as racial discrimination increased, WMLV decreased between ages 32.9 to 47.2 years (all p’s ≤ .05). Again, the beta-values increased and p-values decreased incrementally with each increase in age above 58.6 years and decrease in age below 47.2 years (these data are available in Supplementary Table 4). Notably, among middle-aged participants (i.e., 50 years old), there were no significant associations of linear or quadratic lifetime discrimination burden or racial discrimination with WMLV.
Finally, sensitivity analyses revealed that the aforementioned findings all remained significant after further statistical adjustment for the respective contributions of hypertension, diabetes, smoking, alcohol, total cholesterol, body mass index, waist circumference, and depressive symptoms (all p’s < .05). In addition, the results were not significantly changed when using the poverty status variable as the SES indicator instead of the SES composite variable (all p’s < .05) or when excluding participants with diagnoses of a cardiovascular disease (all p’s ≤ .05).
Discussion
This study is the first, to our knowledge, to report a linkage of discrimination to a subclinical brain endpoint. Here, we demonstrate that both self-reported lifetime discrimination burden and racial discrimination are associated with WMLV, a robust, prognostic indicator of future stroke, dementia, and cognitive decline for which African Americans carry a disproportionate burden. We observed a nonlinear association of these forms of discrimination to WMLV as a function of age. Specifically, the primary findings are that (1) among older African Americans, as lifetime discrimination burden and racial discrimination increased, WMLV increased, whereas (2) among younger African Americans, as racial discrimination increased, WMLV decreased. Notably, there were no observed significant associations between lifetime discrimination burden or racial discrimination with WMLV among middle-age African Americans. Altogether, these findings suggest that lifetime discrimination burden and racial discrimination – two established sources of chronic stress with particular salience among racial and ethnic minorities – are implicated in the disproportionate burden of poor brain health observed in African Americans.
The primary current findings extend prior evidence in at least three ways. First, prior reports have prospectively linked various forms of chronic stress to brain volume endpoints (e.g., Aggarwal et al., 2014; Gianaros et al., 2007; Johansson et al., 2012) in samples that have been predominately White and older. Specific to chronic stress and WMLV, findings have been mixed with some studies reporting a positive association (Duan et al., 2017; Johansson et al., 2012) and others reporting null results (e.g., Aggarwal et al., 2014; Gianaros et al., 2007). Inconsistencies in prior studies may be due to high rates of cardiovascular disease in very old samples (e.g., mean = 80 years of age; Aggarwal et al., 2014) or limited inclusion of participants from minority racial and ethnic groups (Gianaros et al., 2007), which may have obscured these potential relations.
Second, these findings extend on prior work by establishing associations of lifetime discrimination burden and racial discrimination – specific forms of chronic stress with potent impact in the lives of African Americans – to WMLV. Here, we see between the ages of 55 and 69 that associations between self-reported lifetime discrimination burden and racial discrimination with WMLV are significant and strengthen with increasing age for both measures of interpersonal-level discrimination. For instance, at age 58.6 years the unstandardized beta coefficient for racial discrimination on WMLV is .17, but triples to .51 by age 69.4 years as the significance doubles. These findings further underscore the position that the normative aging process may be compromised by exposure to adversity that gets “under the skin,” perhaps accelerating aging and/or shifting the aging process from normative to abnormal with disease onset. Further, age may also be useful as a marker of cumulative stress exposure, providing a lens into the way in which stress burden may translate into poor brain health.
The present findings indicate that, in the present sample, age moderation occurs at ages 56 and 59 for lifetime discrimination burden and racial discrimination, respectively. This pattern broadly overlaps with the time frame in which the epidemiological literature has shown the disproportionate emergence of clinical brain disease, primarily stroke, among African Americans (Boan et al., 2014; Howard et al., 2016). That is, prior reports indicate that middle-age is the period in which the incidence of poor brain health outcomes appear to be increasing among African Americans compared to other racial and ethnic groups (e.g., Boan et al., 2014). Although some studies have shown that onset of brain health disparities in African Americans occurs as early as 45 years of age (see Boan et al., 2014), this pattern also reaches into latter middle-age (e.g., 55–64 years of age; Howard et al., 2011) as well. Thus, the present findings may suggest that discrimination exposure may contribute to racial brain health disparities during later middle adulthood.
Similar patterns have also been noted for other indicators of declining brain health, such as mild cognitive impairment and dementia; that is, the onset of these indicators in African Americans is not limited to or primarily observed in older adulthood, but rather during middle-age (e.g., see Alzheimer’s Association, 2010). In this regard, the current work extends the single prior investigation linking discrimination to clinical cardiovascular disease endpoints including stroke in a racially and ethnically diverse sample of middle-aged U.S. adults (Everson-Rose et al., 2015) by explicating the contributions of discrimination as well as racial discrimination, to a subclinical brain health endpoint known to presage stroke, and doing so in a sample of African Americans.
The third way the present results extend prior evidence stems from our finding that among African Americans, as both age and lifetime discrimination burden (or racial discrimination) increased so did WMLV, which aligns with the concept of age-patterned exposures and suggests a possible age cohort effect (Gee et al., 2012). It is plausible that for African Americans in the U.S., increasing age is tantamount to having had greater opportunities for experiences with lifetime discrimination burden and racial discrimination across the life course. Thus, age may be useful as a marker of the cumulative exposure to lifetime discrimination- and racial discrimination-related stress among African Americans, serving as a lens into their overall stress burden and, in turn, the translation of these exposures into poorer brain health.
It is also plausible that the forms and frequencies of particular types of lifetime discrimination burden and racial discrimination may differ across generations and also change across an individual’s life course (Gee et al., 2012). Our older participants were born during the de jure segregation period in the United States (pre-1954) and/or came of age during the Civil Rights Movement (1954–1968), which included a myriad of racially-charged legal, political, and social transitions that stretched over a decade. Historical and anecdotal accounts demonstrate this generation was exposed to widespread discrimination, including racial discrimination, which was particularly hostile, aggressive, blatant, and frequent in presentation (Collins & Margo, 2004; Southern Poverty Law Center, 2011). However, our younger participants would have come of age towards the end of this particularly tumultuous period in which the residual effects of these earlier times continued but to a lesser extent. This may have contributed to an age cohort effect. Additionally, through the lens of what has been referred to as age-patterned exposures (Gee et al., 2012) to lifetime discrimination burden and racial discrimination, it is possible that earlier exposures reverberate across the life course and that the embodiment of those reverberations with a longer inculcation period have a more pronounced impact.
Our second primary finding that increases in WMLV were associated with decreases in self-reported racial discrimination in younger African Americans is reminiscent of several related findings on self-reported racial and other forms of discrimination with health demonstrated over the last two decades (Everage et al., 2012; Krieger & Sidney, 1996; Ryan, Gee, & LaFlamme, 2006). Specifically, prior reports (using largely linear modeling) have demonstrated that African Americans – who were also younger- to middle-aged adults – reporting that as reported levels of racial discrimination decreased, risk for hypertension (Krieger & Sidney, 1996) and coronary artery calcification (Everage et al., 2012) increased in the CARDIA study. These findings are unlikely to indicate that experiencing interpersonal-level racial discrimination is health-promoting or otherwise desirable. Rather, researchers have interpreted these findings to indicate that African Americans who affirm and acknowledge their experiences with racial discrimination may experience some benefit vis à vis labeling and identifying these stressful events (Krieger, 1990; Krieger & Sydney, 1996). In contrast, suppression or, lack of acknowledgement of experiences with racial discrimination in interpersonal interactions, internalized racial discrimination, or belief in one’s immunity from experiencing or being impacted by racial discrimination can elevate one’s risk for poorer health (Harrell et al., 2011; Krieger & Sidney, 1996).
To our knowledge, the present study was the first to demonstrate that an inverse association between racial discrimination and health endpoints may be age-dependent, as this finding emerged only in younger African American participants. This may point to a potential style of coping that warrants further evaluation, particularly in light of the early linkage observed with WMLV. For example, Harrell (2000) notes that the success of any given racial discrimination-related coping style involves creativity and flexibility across different circumstances. Therefore, the present findings in younger African American participants may reflect, at least in part, differences in flexible coping styles between those self-reporting lower versus higher racial discrimination. Other factors to consider are whether younger African Americans equate acknowledgement of these experiences with a destabilization of their locus of control, or with affirming and ascribing power to those who seek to disempower them through these acts, or whether they see these experiences as normative and a fact of their lives for which no recourse or solutions are available.
In addition, the age-varying association between racial discrimination and WMLV among older and younger African Americans may reflect age cohort effects. As described above, the older participants in the present study were born prior to and came of age during the Civil Rights Movement, and likely experienced particularly aggressive, hostile, and overt racial discrimination (Dovidio & Gaertner, 2004). In contrast, the younger participants, who were born following the Civil Rights Movement, may have been exposed to less overtly threatening, though not less distressing, experiences of racial discrimination. As a result, the younger African American participants may have required different strategies to effectively cope with increasingly nuanced experiences of interpersonal-level racial discrimination (Dovidio & Gaertner, 2004), among them acknowledging and labeling these experiences as such. Although speculative, these sociohistorical considerations indicate that a generational cohort effect may be implicated, at least in part, in the age-dependent effects observed in this study. It is also conceivable that, among younger respondents, lower levels of self-reported discrimination could be associated with greater burden of other risk factors (e.g., behavioral avoidance) not measured herein, that are not present in the older respondents. Alternatively, younger African Americans who report experiencing discrimination may have other protective resources (also not measured herein) that may be less evident among older African Americans reporting similar experiences.
The underlying environmental, neurobiological, psychosocial, and behavioral mechanisms that mediate the linkages of self-reported lifetime discrimination burden and racial discrimination as chronic stressors to brain endpoints, particularly WMLV, remain to be elucidated. Exposure to chronic stress has been associated with volumetric reductions in brain regions known to be involved in biological stress responses (Blix, Perski, Berglund, & Savic, 2013). Further, increases in acute stress-induced cardiovascular responses have been associated with increased greater levels of subclinical cerebrovascular disease including WMLV (Waldstein et al., 2004). Another promising focus is the possible epigenetic intergenerational transmission of stress effects and subsequent greater disease risk susceptibility, particularly in groups subjected to significant stressors such as historical atrocities (e.g., Bowers & Yehuda, 2016). Such work has mainly been conducted in Holocaust survivors and their offspring (e.g., Yehuda et al., 2016). However, extension to racial and ethnic disparities health research, particularly among African Americans is relevant and warranted given the sociohistorical backdrop against which their disproportionate and protracted burden of disease is unfolding in the U.S. It is plausible that observed health disparities in this group may not be an issue of ontogeny, but a recapitulation driven by generations of substantial stress exposure. Thus, the relative contributions of psychosocial, behavioral factors (e.g., smoking), and contemporary social determinants (e.g., SES) to health may be better understood in a context which elucidates biologically embedded intergenerational responses to unmitigated and unremitting significant stress exposures.
Psychosocial factors may also yield indirect implications for brain health via behavior. For instance, prior personal, vicarious, and historical (e.g., “Mississippi appendectomies,” Henrietta Lacks’ cells, or the Tuskegee Syphilis Experiment; Boston Women’s Health Collective, 1998; Skloot & Turpin, 2010; Washington, 2006) lifetime discrimination burden and racial discrimination may serve as barriers to seeking and maintaining relationships with the health care establishment. Medical mistrust should be considered as an ongoing threat to public health, particularly for health endpoints (e.g., cognitive stability and decline) concerning aspects of the body (e.g., brain) which have previously been demarcated as indicators of inferiority for certain racial or ethnic groups (e.g., “scientific racism”; Dennis, 1995; Shockley, 1992). Comorbid health factors could also serve as mechanistic pathways including depression, smoking, and various cardiovascular and metabolic risk factors. Although these were not explanatory factors in the present cross-sectional analyses, consideration of longer term trajectories of these variables vis à vis self-reported lifetime discrimination burden and racial discrimination and brain health may prove useful.
It is also plausible that discrimination acts upon brain health at other levels, beyond interpersonal-level discrimination. Prior research has identified structural-level discrimination and racism as deleterious sources of poor health among African Americans. For example, de facto residential segregation, which remains pervasive in urban communities throughout the United States (Bailey et al., 2017), inherently reflects structural discrimination and racism. Importantly, historically disadvantaged groups such as African Americans are more likely to live in neighborhoods with higher levels of residential segregation, which are typically characterized by greater levels of environmental pollutants, limited or poorer quality of food outlets, and increased availability of illicit or risky substances (Williams & Collins, 2001). Although not examined herein, it is conceivable that self-reported experiences of interpersonal-level discrimination are really nested within a broader system of discrimination and racism that not only shape these interpersonal-level experiences but are also simultaneously shaping boarder structural processes which, altogether impact brain health. Further, longer exposure to such environments, may yield more pronounced brain health deficits, which perhaps could be captured, in part, by greater chronological age.
This present study has some limitations. The sample size (N = 71) was relatively smaller, thus limiting statistical power to detect associations of interest. Next, participants may have under- or over-reported regarding their lifetime discrimination burden and experiences with racial discrimination. The present study could not evaluate whether self-reported lifetime discrimination burden and racial discrimination contributed to changes in WMLV over time. Self-reported lifetime discrimination burden and racial discrimination data were collected on average five years earlier than the MRI data. Nevertheless, the findings demonstrate that self-reports of lifetime discrimination burden and racial discrimination at an earlier time point are related to future MRI markers of subclinical disease in this sample. It would have been optimal to have measures of MRI and the indices of lifetime discrimination burden and racial discrimination at both time points. However, it is important to note that previous research has demonstrated that ratings of discrimination experiences across various indices including the racial discrimination measure utilized here, remain relatively stable across multiple measurement points (Borrell, Kiefe, Diez-Roux, Williams, Gordon-Larsen, 2013; Krieger et al., 2005; Lewis et al., 2013). Future research should seek to capture longitudinal covariation in discrimination and brain structure. Additionally, the use of a single item to measure lifetime discrimination burden is a limitation of this study, and future studies should seek to use multi-item measures of lifetime discrimination. Finally, given that cognitive assessments were only available for a subset of the present analysis sample, we were unable to examine the relation of WMLV to cognitive function in the present study. Future studies should examine the present findings in relation to cognitive function and decline. Also, participant recall of SES-related information precluded more comprehensive assessment of some SES indices as continuous variables.
This study had several strengths. The findings establish a temporal association and this is similar to how prior work linking chronic stress to MRI-assessed brain endpoints has been conducted (Aggarwal et al., 2014). Mounting evidence demonstrates that MRI detects early, subclinical brain pathology that correlates with concurrent functional status and is prognostic of future brain health outcomes. The use of MRI may shed critical light on the well-established racial and ethnic disparities in both subclinical and clinical cerebrovascular disease and other brain health outcomes. This study examined a well-documented, chronic stressor for racial and ethnic minorities in the U.S. Although more general measures of chronic stress allow us to assess such experiences across racial and ethnic groups, it remains critical to assess events that may be more particular to minority groups. Further, the assessment of age as a moderator of the relations of lifetime discrimination burden and racial discrimination to MRI outcomes allows us to better understand how two sources of stress may exacerbate each other to impact brain health. Finally, the present study’s significant findings did not attenuate after the addition of sensitivity variables that are known to correlate with stroke risk.
In this study, we have shown the first evidence of a linkage between lifetime discrimination burden and racial discrimination, age, and WMLV in the brains of African Americans living in an urban environment. We have not only reported that increases in lifetime discrimination burden and racial discrimination to increments in WMLV is pronounced among older African Americans, but among younger African Americans reporting more limited experiences with racial discrimination. The interactive relation of greater self-reported lifetime discrimination burden and racial discrimination with age in relation to greater WMLV may have particular prognostic significance for poor clinical brain health outcomes including stroke, dementia, and mild cognitive impairment (Smith et al., 2008; Vermeer et al., 2003). These findings warrant further investigation to better understand the implications of greater WMLV for this group in terms of future clinical events. In that regard, the current findings may shed some light on the disproportionate burden of clinical cerebrovascular disease (e.g., stroke) observed in African Americans. In other words, these findings may have clinical implications given our knowledge of this group’s epidemiological brain health profile and greater exposure to adversity arising from bias and a multitude of other sources. Screening for exposure to various dimensions of discrimination may permit identification of African Americans at risk for related adverse brain health outcomes. Another important next step in understanding the complexity of these factors as they shape African American health is to engage the broader system of structural racism and fully consider intergenerational processes, which may create greater adversity exposure and greater disease susceptibility. Such work may be particularly important and yield great value for elucidating upstream processes and facilitating policy implementation.
Supplementary Material
Acknowledgments
We would like to acknowledge our funding sources: K01AG043581 (Beatty Moody), R01AG034161 (Waldstein), the National Institute on Aging’s Intramural Research Program ZIAG000513 (Evans), and the Claude D. Pepper Older Americans Independence Center (P60-AG12583)
References
- Aggarwal NT, Clark CJ, Beck TL, de Leon CFM, DeCarli C, Evans DA, & Rose SAE (2014). Perceived stress is associated with subclinical cerebrovascular disease in older adults. The American Journal of Geriatric Psychiatry, 22, 53–62. doi: 10.1016/j.jagp.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2010. Alzheimer’s Disease facts and figures. Alzheimer’s & Dementia, 6, 158–194. doi: 10.1016/j.jalz.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2016. Alzheimer’s Disease facts and figures. Alzheimer’s & Dementia, 12, 459–509. doi: 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, & Bassett MT (2017). Structural racism and health inequities in the USA: evidence and interventions. The Lancet, 389(10077), 1453–1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- Babyak MA (2004). What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine, 66(3), 411–421. doi: 10.1097/01.psy.0000127692.23278.a9 [DOI] [PubMed] [Google Scholar]
- Beatty Moody DL, Waldstein SR, Tobin JN, Cassells A, Schwartz JC, & Brondolo E (2016). Lifetime racial/ethnic discrimination and ambulatory blood pressure: The moderating effect of age. Health Psychology, 35, 333–342. doi: 10.1037/hea0000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix E, Perski A, Berglund H, & Savic I (2013). Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLoS One, 8, e64065. doi: 10.1371/journal.pone.0064065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boan AD, Feng WW, Ovbiagele B, Bachman DL, Ellis C, Adams RJ, & Lackland DT (2014). Persistent racial disparity in stroke hospitalization and economic impact in young adults in the buckle of stroke belt. Stroke, 45, 1932–1938. doi: 10.1161/STROKEAHA.114.004853 [DOI] [PubMed] [Google Scholar]
- Borrell LN, Kiefe CI, Diez-Roux AV, Williams DR, & Gordon-Larsen P (2013). Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethnicity & health, 18(3), 227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston Women’s Health Collective. (1998). Our Bodies, Ourselves for the New Century. New York, NY: Simon & Schuster. [Google Scholar]
- Bowers ME, & Yehuda R (2016). Intergenerational transmission of stress in humans. Neuropsychopharmacology, 41, 232–244. doi: 10.1038/npp.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S (2005). Socioeconomic status in health research: One size does not fit all. JAMA, 14, 2879–88. doi: 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, & Stern Y (2008). Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Archives of Neurology, 65, 1202–1208. doi: 10.1001/archneur.65.9.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017, May 9). Stroke facts. Retrieved from https://www.cdc.gov/stroke/facts.htm
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist, 54, 805–816. [DOI] [PubMed] [Google Scholar]
- Collins WJ & Margo RA (2004). The Labor Market Effects of the 1960s Riots. Brookings-Wharton Papers on Urban Affairs, 2004, 1–46. [Google Scholar]
- Debette S, & Markus HS (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ, 341, c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis RM (1995). Social Darwinism, scientific racism, and the metaphysics of race. Journal of Negro Education, 64, 243–252. doi: 10.2307/2967206 [DOI] [Google Scholar]
- Doshi J, Erus G, Ou Y, Gaonkar B, & Davatzikos C (2013). Multi-Atlas Skull-Stripping. Academic Radiology, 20(12), 1566–76. doi: 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovidio JF, & Gaertner SL (2004). Race, class, and gender in the United States: An integrated study (pp. 132–142). New York, NY: Worth Publishers. [Google Scholar]
- Duan D, Dong Y, Zhang H, Zhao Y, Diao Y, Cui Y, & Liu Z (2017). Empty-nest-related psychological distress is associated with progression of brain white matter lesions and cognitive impairment in the elderly. Scientific Reports, 7, 43816. doi: 10.1038/srep43816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins JS, & Johnston SC (2003). Thirty-year projections for deaths from ischemic stroke in the United States. Stroke, 34, 2109–2112. doi: 10.1161/01.STR.0000085829.60324.DE [DOI] [PubMed] [Google Scholar]
- Essed P (1991). Understanding everyday racism: An interdisciplinary theory. Washington, DC: SAGE. [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, & Zonderman AB (2010). Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease, 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Everage NJ, Gjelsvik A, McGarvey ST, Linkletter CD, & Loucks EB (2012). Inverse associations between perceived racism and coronary artery calcification. Annals of Epidemiology, 22, 183–190. doi: 10.1016/j.annepidem.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, & Diez Roux AV (2015). Perceived discrimination and incident cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. American Journal of Epidemiology, 182, 225–234. doi: 10.1093/aje/kwv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, & Ryff CD (2009). Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain, Behavior, and Immunity, 23, 684–692. doi: 10.1016/j.bbi.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, & Fox J (2011). An R companion to applied regression. Thousand Oaks, California: SAGE Publications. [Google Scholar]
- Gee GC, Walsemann KM, & Brondolo E (2012). A life course perspective on how racism may be related to health inequities. American Journal of Public Health, 102, 967–974. doi: 10.2105/AJPH.2012.300666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, & Matthews KA (2007). Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage, 35, 795–803. doi: 10.1016/j.neuroimage.2006.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum RF, Kwagyan J, & Obisesan TO (2011). Ethnic and geographic variation in stroke mortality trends. Stroke, 42, 3294–3296. doi: 10.1161/STROKEAHA.111.625343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habes M, Habes M, Voelzke H, Erus G, Zhang T, Bryan N, & Homuth G (2016). White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain, 139, 1164–1179. doi: 10.1093/brain/aww008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CJP, Burford TI, Cage BN, Nelson TM, Shearon S, Thompson A, & Green S (2011). Multiple pathways linking racism to health outcomes. Du Bois Review: Social Science Research on Race, 8, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell SP (2000). A multidimensional conceptualization of racism‐related stress: Implications for the well‐being of people of color. American Journal of Orthopsychiatry, 70, 42–57. doi: 10.1037/h0087722 [DOI] [PubMed] [Google Scholar]
- Harwood DG, & Ownby RL (2000). Ethnicity & dementia. Current Psychiatry Reports, 2, 40–45. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Howard G, Moy CS, Howard VJ, McClure LA, Kleindorfer DO, Kissela BM, & Cushman M (2016). Where to Focus Efforts to Reduce the Black–White Disparity in Stroke Mortality. Stroke, 47, 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, & Howard G (2011). Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of Neurology, 69(4), 619–627. doi: 10.1002/ana.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozawa A, Folsom AR, Sharrett AR, & Chambless LE (2007). Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: Comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Archives of Internal Medicine, 167, 573–579. doi: 10.1001/archinte.167.6.573 [DOI] [PubMed] [Google Scholar]
- Johansson L, Skoog I, Gustafson DR, Olesen PJ, Waern M, Bengtsson C, & Guo X (2012). Midlife psychological distress associated with late-life brain atrophy and white matter lesions: A 32-year population study of women. Psychosomatic Medicine, 74, 120–125. doi: 10.1097/PSY.0b013e318246eb10 [DOI] [PubMed] [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman technique, its theory and application. Psychometrika, 15, 349–367. [DOI] [PubMed] [Google Scholar]
- Krieger N (1990). Racial and gender discrimination: Risk factors for high blood pressure? Social Science & Medicine, 30, 1273–1281. doi: 10.1016/0277-9536(90)90307-E [DOI] [PubMed] [Google Scholar]
- Krieger N (2014). Discrimination and health inequities. International Journal of Health Services, 44, 643–710. doi: 10.2190/HS.44.4.b [DOI] [PubMed] [Google Scholar]
- Krieger N, & Sidney S (1996). Racial discrimination and blood pressure: The CARDIA Study of young black and white adults. American Journal of Public Health, 86, 1370–1378. doi: 10.2105/AJPH.86.10.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science And Medicine, 61(7), 1576–1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11, 407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Troxel WM, Kravitz HM, Bromberger JT, Matthews KA, & Hall MH (2013). Chronic exposure to everyday discrimination and sleep in a multiethnic sample of middle-aged women. Health Psychology, 32(7), 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, & Heiss G (1997). The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: The ARIC Study. Neuroepidemiology, 16, 149–162. doi: 10.1159/000368814 [DOI] [PubMed] [Google Scholar]
- The John D and Catherine T MacArthur Foundation Research Network. (2008). MacArthur Midlife Survey: Major Experiences of Discrimination. Retrieved from b http://www.macses.ucsf.edu/research/psychosocial/midmac.php [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12, 216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern LB, Spears WD, Goff DC, Grotta JC, & Nichaman MZ (1997). African Americans and women have the highest stroke mortality in Texas. Stroke, 28, 15–18. doi: 10.1161/01.STR.28.1.15 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, & Howard VJ (2016). Executive summary: Heart disease and stroke statistics−−2016 update: A report from the American Heart Association. Circulation, 133, 447–454. doi: 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- Pathak EB, & Sloan MA (2009). Recent racial/ethnic disparities in stroke hospitalizations and outcomes for young adults in Florida, 2001–2006. Neuroepidemiology, 32(4), 302–311. doi: 10.1159/000208795 [DOI] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, & Gee G (2015). Racism as a determinant of health: A systematic review and meta-analysis. PloS One, 10, e0138511. doi: 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y (2006). A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology, 35(4), 888–901. doi: 10.1093/ije/dyl056 [DOI] [PubMed] [Google Scholar]
- Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raz N, & Rodrigue KM (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30, 730–748. doi: 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AM, Gee GC, & LaFlamme DF (2006). The association between self-reported discrimination, physical health and blood pressure: Findings from African Americans, Black immigrants, and Latino immigrants in New Hampshire. Journal of Health Care for the Poor and Underserved, 17, 116–132. doi: 10.1353/hpu.2006.0079 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, & Filley CM (2008). Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Annals of the New York Academy of Sciences, 1142, 266–309. doi: 10.1196/annals.1444.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff-Marco S, Breen N, Landrine H, Reeve BB, Krieger N, Gee GC, Williams DR, et al. (2011). Measuring Everyday Racial/Ethnic Discrimination in Health Surveys. Du Bois Review , 8 (1), 159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley WB (1992). Shockley on eugenics and race: The application of science to the solution of human problems. Washington, DC: Scott-Townsend. [Google Scholar]
- Simons LA, McCallum J, Friedlander Y, & Simons J (1998). Risk factors for ischemic stroke: Dubbo Study of the Elderly. Stroke, 29, 1341–1346. doi: 10.1161/01.STR.29.7.1341 [DOI] [PubMed] [Google Scholar]
- Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, & Taylor HA (2012). Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. American Journal of Public Health, 102, S258–S265. doi: 10.2105/AJPH.2011.300523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, Wyatt SB, Gutierrez ML, Taylor HA, & Williams DR (2009). Development and psychometric testing of a multidimensional instrument of perceived discrimination among African Americans in the Jackson Heart Study. Ethnicity & Disease, 19(1), 56–64. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2724869/ [PMC free article] [PubMed] [Google Scholar]
- Skloot R, & Turpin B (2010). The immortal life of Henrietta Lacks. New York, NY: Crown Publishers. [Google Scholar]
- Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, & Guttmann CR (2008). Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Archives of Neurology, 65, 94–100. doi: 10.1001/archneurol.2007.23 [DOI] [PubMed] [Google Scholar]
- Southern Poverty Law Center (2011). Ku Klux Klan: A History of Racism and Violence. 6th Edition Edited by Richard Baudouin. [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, & Mayeux R (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56, 49–56. doi: 10.1212/WNL.56.1.49 [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, & Figueroa M (1992). Dementia after stroke: Baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology, 42, 1185–1185. doi: 10.1212/WNL.42.6.1185 [DOI] [PubMed] [Google Scholar]
- Tiehuis AM, Van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, & Mali WP (2008). Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke, 39, 1600–1603. doi: 10.1161/STROKEAHA.107.506089 [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, & Gee JC (2010). N4ITK: Improved N3 Bias Correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. doi: 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, & Breteler MM (2003). Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine, 348, 1215–1222. doi: 10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Siegel EL, Lefkowitz D, Maier KJ, Pelletier Brown JR, Obuchowski AM & Katzel LI (2004). Stress-induced blood pressure reactivity and silent CVD. Stroke, 35, 1294–1298. doi: 10.1161/01.STR.0000127774.43890.5b [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Dore GA, Davatzikos C, Katzel LI, Gullapalli RP Seliger SL, & Zonderman AB. (2017). Differential associations of socioeconomic status with global brain volumes and white matter lesions in African American and White adults: The HANDLS SCAN study. Psychosomatic Medicine, 79, 327–335. doi: 10.1097/PSY.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington HA (2006). Medical apartheid: The dark history of medical experimentation on Black Americans from colonial times to the present. New York, NY: Doubeday Books. [Google Scholar]
- Williams DR, & Collins C (1995). U.S. socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology, 21, 349–386. doi: 10.1146/annurev.so.21.080195.002025 [DOI] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, & Anderson NB (1997). Racial differences in physical and mental health socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Xiong YY, & Mok V (2011). Age-related white matter changes. Journal of Aging Research, 2011. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, & Binder EB (2016). Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biological Psychiatry, 80, 372–380. doi: 10.1016/j.biopsych.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Zacharaki E, Kanterakis S, Bryan R, & Davatzikos C (2008). Measuring brain lesion progression with a supervised tissue classification system. Medical Image Computing and Computer Assisted Intervention. 11, 620–7. doi: 10.1007/978-3-540-85988-8_74 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


