Abstract
Background
Acute respiratory distress syndrome (ARDS) is a critical condition that is associated with high mortality and morbidity. Aerosolized prostacyclin has been used to improve oxygenation despite the limited evidence available so far.
This review was originally published in 2010 and updated in 2017.
Objectives
To assess the benefits and harms of aerosolized prostacyclin in adults and children with ARDS.
Search methods
In this update, we searched CENTRAL (2017, Issue 4); MEDLINE (OvidSP), Embase (OvidSP), ISI BIOSIS Previews, ISI Web of Science, LILACS, CINAHL (EBSCOhost), and three trials registers. We handsearched the reference lists of the latest reviews, randomized and non‐randomized trials, and editorials, and cross‐checked them with our search of MEDLINE. We contacted the main authors of included studies to request any missed, unreported or ongoing studies. The search was run from inception to 5 May 2017.
Selection criteria
We included all randomized controlled trials (RCTs), irrespective of publication status, date of publication, blinding status, outcomes published or language. We contacted trial investigators and study authors to retrieve relevant and missing data.
Data collection and analysis
Three authors independently abstracted data and resolved any disagreements by discussion. Our primary outcome measure was all‐cause mortality. We planned to perform subgroup and sensitivity analyses to assess the effect of aerosolized prostacyclin in adults and children, and on various clinical and physiological outcomes. We assessed the risk of bias through assessment of methodological trial components and the risk of random error through trial sequential analysis.
Main results
We included two RCTs with 81 participants.
One RCT involved 14 critically ill children with ARDS (very low quality of evidence), and one RCT involved 67 critically ill adults (very low quality evidence).
Only one RCT (paediatric trial) provided data on mortality and found no difference between intervention and control. However, this trial was eligible for meta‐analysis due to a cross‐over design.
We assessed the benefits and harms of aerosolized prostacyclin. One RCT found no difference in improvement of partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio (mean difference (MD) ‐25.35, 95% confidence interval (CI) ‐60.48 to 9.78; P = 0.16; 67 participants, very low quality evidence).
There were no adverse events such as bleeding or organ dysfunction in any of the included trials. Due to the limited number of RCTs, we were unable to perform the prespecified subgroup and sensitivity analyses or trial sequential analysis.
Authors' conclusions
We are unable to tell from our results whether the intervention has an important effect on mortality because the results were too imprecise to rule out a small or no effect. Therefore, no current evidence supports or refutes the routine use of aerosolized prostacyclin for people with ARDS. There is an urgent need for more RCTs.
Plain language summary
Aerosols of prostacyclin for management of acute respiratory distress syndrome (ARDS)
Background
Acute respiratory distress syndrome (ARDS) results in low oxygen levels in the blood and can develop when fluid builds up in the lungs because of inflammation. A direct or indirect injury to the lungs can cause ARDS in children and adults. Such injuries include sepsis (a serious condition where the body responds to infection by injuring to its own tissues and organs), viral infections, burns, massive blood transfusions, multiple trauma, entry of stomach contents into respiratory system, inflammation of the pancreas, inhalation injury, drug overdose and near drowning. ARDS is a major cause of death in critically ill people.
Prostacyclin can be administered as an aerosol to critically ill adults and children with ARDS to increase the blood oxygen levels and improve survival. It is a naturally occurring prostaglandin that relaxes blood vessels, stops blood platelets from clotting (antiplatelet aggregation) and has anti‐inflammatory properties in the lungs.
Study characteristics
This review was updated in 2017. We included two randomized controlled trials (RCT; clinical studies where people are randomly put into one of two or more treatment groups), one involving 14 critically ill children with ARDS and one involving 67 critically ill adults with ARDS. The trials did not measure severity of illness, resolution of organ dysfunction, length of stay in intensive care unit or hospital, and quality of life. The study authors did not report side effects such as bleeding, organ dysfunction, airway reactivity or side effects unrelated to the intervention.
Study funding source
None of the included trials reported receiving money from drug companies.
Key results
Only the RCT involving children provided data on deaths, with no clear difference with and without prostacyclin.
The RCT in adults reported a trend towards improved blood oxygen levels, for participants who were treated with alprostadil (prostaglandin E1).
Therefore, we could not identify a clear advantage of the use of aerosolized prostacyclin in critically ill children or adults with low blood oxygen levels.
Quality of the evidence
The quality of the evidence was very low because of the limited number of participants and poor trial design. The RCT involving children used a cross‐over design where one group received aerosolized prostacyclin first and the other group received saline (salt solution). They then changed over to the alternate treatment. There was insufficient information on the effect on death in both trials.
We conclude that there is a need for a large‐scale clinical trial with low risk of misleading information to investigate the advantages and harms of prostacyclin for critically ill people.
Summary of findings
Summary of findings for the main comparison. Aerosolized prostacyclin compared to placebo for acute respiratory distress syndrome (ARDS).
| Aerosolized prostacyclin compared to placebo for acute respiratory distress syndrome (ARDS) | ||||||
|
Patient or population: people with ARDS Setting: intensive care unit in the Netherlands and Pakistan Intervention: aerosolized prostacyclin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with aerosolized prostacyclin | |||||
| Mortality | Study population | RR 1.50 (0.17 to 12.94) | 14 (1 study) | ⊕⊝⊝⊝ Very low1,2,3,4 | Only 1 small paediatric trial with cross‐over design provided mortality data (Dahlem 2004). Thus, no meta‐analysis carried out. | |
| 167 per 1000 | 250 per 1000 (28 to 1000) | |||||
| PaO2/FiO2 ratio5 | ‐ | MD 25.35 lower (60.48 lower to 9.78 higher) | ‐ | 67 (1 study) | ⊕⊝⊝⊝ Very low6 | Only 1 trial provided data (Siddiqui 2013). Thus, no meta‐analysis was carried out. |
| Improvement in mean pulmonary arterial pressure | ‐ | ‐ | ‐ | ‐ | ‐ | No data is available for meta‐analysis (Characteristics of included studies, Siddiqui 2013) |
| Adverse events7 | ‐ | ‐ | ‐ | 81 (2 studies) |
⊕⊝⊝⊝ Very low8 | Only descriptive assessment of safety with no available data to carry out meaningful analyses (Dahlem 2004; Siddiqui 2013). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FiO2: fraction of inspired oxygen; MD: mean difference; PaO2: partial pressure of oxygen in arterial blood; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Mortality at 28 to 30 days.
2Required information size for paediatric population depending on the level of heterogeneity adjustment was between 2897 (I2 = 0) and 3862 (I2 = 25%). 3Required information size for the adult population depending on the same level of heterogeneity was between 1132 (I2 = 0) and 1508 (I2 = 25%).
4This outcome was downgraded from high to low quality of evidence due to limitations in design (small sample size, few events, cross‐over design) suggesting high likelihood of bias, indirectness of evidence and high probability of publication bias. (Dahlem 2004).
5Despite the fact that biochemical markers of clinical outcomes are often not included in SoF tables, we have chosen to include this outcomes since it is widely used in clinical practice to guide treatment.
6The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size, few events and wide 95% CI suggesting high likelihood of bias and indirectness of evidence. (Siddiqui 2013).
7Adverse events such as bleeding or organ dysfunction
8The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size and few events and since only descriptive assessment of safety and adverse events were provided in the included trials with no data being available for meta‐analyses.
Background
Description of the condition
Acute respiratory distress syndrome (ARDS) affects both children and adults. It is the result of an inflammatory process where the end‐organ affected by the inflammatory cascade is the lung and its alveolar‐capillary units (Anderson 2003). ARDS is a complication of a direct (primary) or indirect (secondary) lung injury caused by burns, massive transfusions, multiple trauma, aspiration of gastric contents, pancreatitis, inhalation injury, nosocomial pneumonia, sepsis, drug overdose and near drowning (Jain 2006; Ware 2000).
Since this review was first published (Afshari 2010), the definition of acute respiratory failure has changed. ARDS and acute lung injury (ALI) in adults or children older than one month of age were initially defined by the American‐European Consensus Conference (AECC) in 1994 (Bernard 1994). The ARDS Definition Task Force produced the latest definition and developed the Berlin definition (Ranieri 2012).
According to the Berlin definition, ARDS is defined by:
timing: within one week of a known clinical insult or new or worsening respiratory symptoms;
chest imaging: bilateral opacities ‐ not fully explained by effusions, lobar/lung collapse or nodules;
origin of oedema: respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (e.g. echocardiography) to exclude hydrostatic oedema if no risk factor present;
-
oxygenation:
Mild: PaO2/FIO2 greater than 200 mmHg to 300 mmHg with positive end expiratory pressure (PEEP) or continuous positive airway pressure 5 cm H2O or greater;
Moderate: PaO2/FIO2 greater than 100 mmHg to 200 mmHg or less with PEEP 5 cm H2O or greater;
Severe: PaO2/FIO2 100 mmHg or less with PEEP 5 cm H2O or greater.
ARDS is among the leading causes of death in critically ill people (Fröhlich 2013). Vohwinkel 2015 estimates an incidence of approximately 200,000 people annually in the US alone. Worldwide, Luhr 1999 and Rubenfeld 2005 reported the incidence to be between 14 and 86 people per 10,0000 per year in the general population. A report from Finland indicated a smaller incidence of ARDS of 10.6 per 100,000 per year for ALI (reported prior to 2012 definition of ARDS) and 5.0 per 100,000 per year for ARDS (Linko 2009). Mortality has gradually dropped to current figures of 25% to 58% throughout the last 30 years due to improved treatment regimens (Anderson 2003; MacCallum 2005). One systematic review indicated a mortality rate of 44.0% (95% confidence interval (CI) 40.1% to 47.5%) from observational studies, and 36.2% (95% CI 32.1% to 40.5%) from randomized controlled trials (RCT) (Phua 2009).
In paediatric settings, evidence indicates that the incidence of ARDS is around 12 cases per 100,000 population per year (Zimmerman 2009). Inhospital mortality is around 18% to 23%, with pneumonia, aspiration and sepsis as the primary causes of the condition (Dahlem 2003; Dahlem 2007; Flori 2005; Zimmerman 2009).
Inflammatory injuries disrupt the capillary endothelium and alveolar epithelia leading to neutrophil invasion and alveolar oedema and collapse (exudative phase). The next stage (proliferative stage) occurs at days seven to 21 with initiation of lung repair and increased surfactant production. People experience dyspnoea and hypoxaemia at this stage, with or without ventilatory support. Some people may then proceed to the fibrotic stage with a long‐term need for ventilatory assist or oxygen therapy (Ware 2000).
The worst prognosis is seen among people with sepsis or multiple organ failure, who are immunocompromised, and in whom oxygenation fails to improve after six days (TenHoor 2001; Ware 2000). Survivors tend to be young and their pulmonary function recovers gradually over one year (Piantadosi 2004). Many adult survivors have long‐term abnormalities in pulmonary function and impaired quality of life (Anderson 2003; Angus 2001).
Description of the intervention
Prostaglandins are lipid mediators that are synthesized from essential fatty acids by cellular enzymes and have strong physiological properties. They have important effects on endothelium, platelet, uterine and mast cells and are found in virtually all tissues and organs.
Prostacyclin (PGI2), generic name epoprostenol (brand name Flolan) is a member of the family of lipid molecules known as eicosanoids. Prostacyclin is a naturally occurring prostaglandin that has vascular smooth muscle relaxant and anti‐inflammatory properties (it inhibits platelet aggregation and neutrophil adhesion). It is synthesized by vascular endothelial and smooth muscle cells within the lung and has an in vivo half‐life of three to six minutes (Jain 2006). Prostacyclin is a potent vasodilator of the systemic and pulmonary vasculature resulting in reduction of right and left heart afterload (Siobal 2004) and can be administered by different routes such as: intravenous for pulmonary hypertension, and inhalational preparations for ARDS. Inhaled prostacyclin appears to improve oxygenation; lower pulmonary vascular resistance (PVR) and mean pulmonary arterial pressure (MPAP); and reduce pulmonary shunt fraction (Siobal 2004). It might have potential benefits in resolving hypoxaemia from ARDS and in the treatment of pulmonary hypertension and right heart failure, similar to the indications for inhaled nitric oxide (INO) (Siobal 2004).
Iloprost is a stable, synthetic analogue of prostacyclin. It has a plasma half‐life of 20 to 30 minutes, similar pulmonary and haemodynamic properties as prostacyclin and can be administered as an intravenous or inhalable solution (Hill 2015).
During mechanical ventilation lasting from hours to several days, inhalable prostacyclins require continuous aerosolization with specific nebulizers or periodic nebulization, for example, several times a day using long‐acting prostacyclin (e.g. iloprost) (Hill 2015; Siobal 2003).
Prostaglandin E1 (PGE1), generic name alprostadil (brand name Prostin) is a naturally occurring prostaglandin with anti‐inflammatory capabilities. PGE1 is an arterial vasodilator, a platelet aggregation inhibitor, and stimulates intestinal and uterine smooth muscle (Siobal 2004). It is mainly used to treat sexual dysfunction or as an intravenous treatment for neonates with congenital heart defects, to maintain patency of ductus arteriosus until surgery. Its half‐life is five to 10 minutes and it is primarily removed by the pulmonary vascular bed. PGE1 leads to a decrease in MPAP, mean arterial pressure, PVR and systemic vascular resistance. It can also be used as an inhalable solution with the potential to improve oxygenation in people with severe ARDS (Schuster 2008).
How the intervention might work
Aerosolized prostacyclins are potent vasodilators which reduce pulmonary arterial hypertension, improve right‐heart function, redistribute pulmonary blood flow to ventilated segments of the lung with matching improvements in ventilation and perfusion to result in better oxygenation (Hill 2015).
PGE1 and prostacyclin seem to reduce obstruction of pulmonary microcirculation in ARDS and modulate the underlying inflammation due to their ability to reduce leukocyte adhesion, and antithrombotic and platelet disaggregation properties (Wetzel 1995). Inhaled prostacyclins cause minimal systemic vasodilation and, due to minor transfer to the vascular system, they may even have systemic anti‐inflammatory and antithrombotic features and improve splanchnic oxygenation (Eichelbrönner 1996; Siobal 2004). However, the principle action of aerosolized prostacyclins is their property of selective vasodilation to reduce hypoxaemia.
Inhaled prostacyclins may result in an increased ventilation/perfusion mismatch, decreased oxygenation, systemic hypotension, bleeding, flushing, headache, nausea, vomiting and chest pain (Siobal 2004). However, there appear to be very few reported adverse effects from inhaled prostacyclins (Siobal 2004). Prostacyclin solution may act as a potential irritant due to its very alkaline pH. Prostacyclins have no known toxic metabolites.
Why it is important to do this review
ARDS is characterized by severe hypoxaemia from intrapulmonary shunting, pulmonary hypertension due to elevated PVR and areas with a low ventilation/perfusion ratio (Schuster 2008). Pulmonary hypertension is believed to be caused by mechanical obstruction of the pulmonary microcirculation by microthromboemboli (composed of platelets and leukocytes) and hypoxic pulmonary vasoconstriction due to alveolar and interstitial oedema triggered by inflammatory mediators (Moloney 2003).
Although people with ARDS are a heterogeneous population, they are all characterized by having local and systemic inflammation that causes lung damage and fluid leakage across the alveolar‐capillary barrier (Piantadosi 2004). This inflammation can result in multiple organ failure and death. Aerosolized prostacyclins are used because of the potential benefit of modifying the process of inflammation, preserving or restoring oxygen delivery and decreasing mortality in people with ARDS (Siobal 2004). Aerosolized prostacyclins are used as an alternative to INO due to various advantages (cost, setup and administration), but this strategy is controversial as the evidence for using prostacyclins is unclear. There are indications of harmful effects of INO in ARDS (Adhikari 2007; Barrington 2007; Gebistorf 2016).
There are no previous systematic reviews on this topic and, being a very costly treatment, the benefit and efficacy of aerosolized prostacyclin in people with ARDS is still controversial and debated. The aim of this updated review was to assess whether aerosolized prostacyclin therapy is beneficial for people with ARDS.
All abbreviations are explained in Appendix 1.
Objectives
To assess the benefits and harms of aerosolized prostacyclin in adults and children with ARDS
Methods
Criteria for considering studies for this review
Types of studies
We included parallel group, RCTs irrespective of publication status, date of publication, blinding status, outcomes published or language. We contacted trial investigators and study authors to ask for relevant data. We included unpublished trials only if trial data and methodological descriptions were provided in written form or could be retrieved from the trial authors.
We excluded trials using quasi‐randomization and observational studies.
Intravenous prostacyclin and PGE1 are potent systemic and pulmonary vasodilators that act as potent platelet aggregation inhibitors. They increase the risk of bleeding and adversely affect ventilation to perfusion matching and oxygenation (Siobal 2004). Since aerosolized prostacyclins are believed to have more selective properties with little systemic spillover, we chose not to include intravenous trials.
Types of participants
We included adults and children defined as having ALI or ARDS according to the various definitions presented in the literature. Despite the 2012 revision of terminology (Ranieri 2012), the previous version of this review aimed to include trials with ALI and ARDS (Afshari 2010). Thus, in case of detection of trials adhering to previous definitions of ARDS and ALI, we did not exclude these trials. We chose to accept the terms standard treatment of ARDS and critically ill people as reported by many authors, despite ongoing controversy. We excluded neonates with 'bronchopulmonary dysplasia' or 'chronic lung disease' due to the different pathophysiology, treatment, prognosis and progression of the disease.
Types of interventions
We included trials comparing aerosolized prostacyclins with placebo or no intervention. We chose to include any type or dose of aerosolized prostacyclin for any duration of administration. Any cointervention was allowed if it was administered in both groups. We excluded trials if the only aim was to compare the efficacy of different doses or types of prostacyclins and which did not have a control group without prostacyclin administration.
We did not intend to compare aerosolized prostacyclin with INO since, in our view, this comparison justifies the need for a separate systematic review in light of the controversy surrounding the use of INO for ARDS and ALI (Adhikari 2007; Gebistorf 2016). Indeed, we did not identify RCTs comparing aerosolized prostacyclin and INO.
Types of outcome measures
Primary outcomes
Overall mortality. We used the longest follow‐up data from each trial regardless of the period of follow‐up.
Overall 28‐day mortality (data presented at 30 days are to be incorporated in this analysis)
Secondary outcomes
Resolution of multiple organ failure (according to different organ dysfunction scores).
Bleeding events. We defined bleeding events as pulmonary or systemic bleeding requiring transfusion. We planned to count repeated transfusions in the same participant as a single event.
Complications during inpatient stay (e.g. hypotensive episodes, direct irritation on administration, thrombosis, congestive cardiac failure, myocardial infarction, renal failure, cerebrovascular accident).
Quality of life assessment, as defined by the authors in the included studies.
Duration of mechanical ventilation.
Improvement of respiratory failure (ventilator‐free days).
Mean pulmonary arterial pressure (MPAP) (mmHg).
Partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio.
Oxygenation index defined as [100 × mean airway pressure/(PaO2/FiO2)] or [(mean airway pressure × FiO2 × 100)/systemic arterial oxygen tension].
Number of days in hospital.
Mean length of stay in an intensive care unit (ICU).
Cost‐benefit analyses.
Search methods for identification of studies
Electronic searches
In this updated review, we extended the original review's search from December 2010 (Afshari 2010). Thus, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4). We updated our search of MEDLINE (OvidSP, to 5 May 2017), Embase (OvidSP, to 5 May 2017), CINAHL (EBSCOhost; to 5 May 2017), ISI Web of Science (to 5 May 2017), ISI BIOSIS Previews (to 5 May 2017) and Latin American Caribbean Health Sciences Literature (LILACS; via BIREME) (to 5 May 2017). The search is now from inception to 5 May 2017. For specific information regarding our search strategies and results, see Appendix 2.
We imposed no language restrictions.
Searching other resources
We searched for ongoing clinical trials and unpublished trials on the following Internet sites:
ISRCTN registry (www.controlled‐trials.com);
ClinicalTrials.gov (clinicaltrials.gov);
CenterWatch (www.centerwatch.com).
We handsearched the reference lists of reviews, randomized and non‐randomized trials, and editorials for additional trials. We contacted the main authors of trials in this field to ask for any missed, unreported or ongoing studies.
We applied no language restrictions to eligible reports. We conducted the latest search on 5 May 2017.
Data collection and analysis
Two review authors (MA, ABB) independently screened and classified all citations as potential primary studies, review articles or other. The two review authors also independently examined all potential primary studies and decided on their inclusion in the review. We independently abstracted and evaluated methodology and outcomes from each trial, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by consensus among the review authors.
Selection of studies
We assessed the reports identified from the described searches and excluded obviously irrelevant reports. We screened all articles by title and abstract, and then as full‐text articles for inclusion. We listed all excluded studies with reasons for their exclusion in the Characteristics of excluded studies table.
Two review authors (MA, ABB) independently examined the retrieved reports for eligibility. We performed this process without blinding to study authors, institution, journal of publication or results. We resolved disagreements by consensus among the review authors. We provide a detailed description of the search and assessment (Appendix 2).
Data extraction and management
We independently extracted and collected data from each trial without blinding to study authors, source institutions or publication source of trials. We resolved disagreements by discussion and approached all first authors of included trials for additional information on risks of bias. For more detailed information, see Contributions of authors.
Assessment of risk of bias in included studies
We evaluated the validity and design characteristics of each trial.
We evaluated trials for major potential sources of bias (random sequence generation, allocation concealment, blinding of participants, blinding of personnel, blinding of primary outcome assessor, blinding of secondary outcome assessor, incomplete outcome data, selective reporting and other bias; see Appendix 3). We assessed each trial quality factor separately and defined trials as having low risk of bias only if they adequately fulfilled all the criteria described in Appendix 3 and in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcomes). These included the following:
Primary outcome
Overall mortality.
Secondary outcomes
Resolution of multiple organ failure;
Bleeding events;
Complications during inpatient stay.
Continuous data
We used the mean difference (MD) if data were continuous and were measured in the same way between trials as follows:
Secondary outcomes
Quality of life assessment
Duration of mechanical ventilation
Improvement of respiratory failure (ventilator‐free days).
Mean pulmonary arterial pressure (MPAP) (mmHg).
PaO2/FiO2 ratio.
Oxygenation index defined as [100 × mean airway pressure/(PaO2/FiO2)] or [(mean airway pressure × FiO2 × 100)/systemic arterial oxygen tension].
Number of days in hospital.
Mean length of stay in an intensive care unit (ICU).
Cost benefit analyses
Unit of analysis issues
Cross‐over trials
We planned to exclude cross‐over trials from meta‐analyses because of the potential risk for 'carry‐over' of intervention effect. However, if considered important, these trials were to be included only in a descriptive manner in this review due to the lack of evidence.
Studies with multiple intervention groups
In studies designed with multiple intervention groups, we planned to combine groups to create a single pair‐wise comparison in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In trials with two or more groups receiving different doses, we aimed to combine data for primary and secondary outcomes.
Dealing with missing data
We contacted the authors of trials with missing data to retrieve the relevant information. For all included studies, we noted levels of attrition and any exclusions. In case of missing data, we chose 'complete‐case analysis' for our primary outcomes, which excludes from the analysis all participants with the outcome missing. Selective outcome reporting occurs when non‐significant results are selectively withheld from publication (Chan 2004), and is defined as the selection, on the basis of the results, of a subset of the original variables recorded for inclusion in publication of trials (Hutton 2000). The most important types of selective outcome reporting are: selective omission of outcomes from reports; selective choice of data for an outcome; selective reporting of different analyses using the same data; selective reporting of subsets of the data and selective under‐reporting of data (Higgins 2011).
Assessment of reporting biases
Publication bias occurs when the publication of research results depends on their nature and direction (Dickersin 1990). We planned to provide a funnel plot to detect either publication bias or a difference between smaller and larger studies ('small‐study effects') expressed by asymmetry (Egger 1997). To quantify this asymmetry in meta‐analyses with binary outcomes, we also intended to apply the arcsine test as proposed by Rücker 2008. This test has the advantage of including trials with no events.
According to the Cochrane Handbook for Systematic Reviews of Interventions a minimum of 10 trials has to be included before a statistical test is applied, to detect possible reporting bias, and results from tests for funnel plot asymmetry should be interpreted cautiously (Higgins 2011).
Data synthesis
Data analysis
We used Review Manager 5 software (RevMan 2014) and calculated MDs with 95% CIs for continuous outcomes and RRs with 95% CIs for dichotomous variables. We used the Chi2 test to obtain an indication of heterogeneity between studies, with P value ≤ 0.1 considered significant. We quantified the degree of heterogeneity observed in the results by using the I2 statistic, which can be interpreted as the proportion of total variation observed between studies that is attributable to differences between studies rather than to sampling error (Higgins 2011). An I2 statistic greater than 75% is considered as very heterogeneous. Suggested threshold values for the I2 statistic are: low (25% to 49%), moderate (50% to 74%) and high (75% or greater) (Higgins 2003). If I2 = 0, we planned to report only the results from the fixed‐effect model; in the case of an I2 greater than 0, we planned to report only the results from the random‐effects model unless one or two trials comprised more than 60% (weight %) of the total evidence provided, in which case the random‐effects model may be biased. The latter is to make the review more readable. We believe that there is little value in using a fixed‐effect model in cases of substantial heterogeneity possibly due to the various reasons leading to ARDS (clinical heterogeneity). Additionally, in the case of an I2 greater than 0 (mortality outcome), we planned to determine the cause of heterogeneity by performing relevant subgroup analyses. We intended to pool trial results only in the case of low clinical heterogeneity.
Trial sequential analysis
Risk of type 1 errors in meta‐analyses due to sparse data and repeated significance testing following updates with new trials remains a serious concern (Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009). As a result, spurious P values due to systematic errors from trials with high risk of bias, outcome reporting bias, publication bias, early stopping for benefit and small‐study bias may result in false conclusions. In a single trial, interim analysis increases the risk of type 1 errors. To avoid type 1 errors, group sequential monitoring boundaries (Lan 1983) are used to decide whether a trial could be terminated early because of a sufficiently small P value, thus the cumulative Z curve crosses the monitoring boundary.
Equally, sequential monitoring boundaries can be applied to meta‐analyses and are labelled 'trial sequential monitoring boundaries.' In 'trial sequential analysis' (TSA) (TSA 2010), the addition of each new trial to a cumulative meta‐analysis is viewed as an interim meta‐analysis, which provides useful information on the need for additional trials (Wetterslev 2008).
It is appropriate and wise to adjust new meta‐analyses for multiple testing on accumulating data to control overall type 1 error risk in cumulative meta‐analysis (Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008).
When TSA is performed, the cumulative Z curve crossing the boundary indicates that a sufficient level of evidence has been reached; as a consequence, one may conclude that no additional trials may be needed. However, evidence is insufficient to allow a conclusion if the Z curve does not cross the boundary or does not surpass the required information size.
To construct trial sequential monitoring boundaries, one needs a required information size, which is calculated as the least number of participants required in a well‐powered single trial with low risk of bias (Brok 2009; Pogue 1998; Wetterslev 2008).
In this updated review, we planned to adjust the required information size for heterogeneity by using the diversity adjustment factor (Wetterslev 2009). We aimed to apply TSA, as it prevents an increase in the risk of type 1 errors (20%). If the actual accrued information size was considered too small, we planned to provide the required information size in the light of actual diversity (Wetterslev 2009).
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Assessment of the benefits and harms of prostacyclins in participants with ARDS based on the cause (primary lung injury versus secondary lung injury).
Assessment of the benefits and harms of prostacyclins in children (paediatric, age less than 18 years) versus adults.
Assessment of the benefits and harms of prostacyclins based on the duration of drug administration.
If analyses of various subgroups were significant, we planned to perform a test of interaction (Altman 2003). We considered P < 0.05 as indicating significant interaction between the prostacyclin effect on mortality and subgroup category (Higgins 2011).
Sensitivity analysis
We planned the following sensitivity analyses for the primary outcomes.
Comparing estimates of the pooled intervention effect in trials with low risk of bias to estimates from trials with high risk of bias (i.e. trials having at least one inadequate risk of bias component).
Comparing estimates of the pooled intervention effect in trials based on different components of risk of bias (random sequence generation, allocation concealment, blinding, follow‐up, intention to treat (ITT)).
Comparing estimates of the pooled intervention effect in trials based on The ARDS Definition Task Force (Ranieri 2012).
Assessment of the benefits and harms of prostacyclins in participants with ARDS versus participants given placebo or usual care when excluding data from studies only published as abstracts.
Assessment of the benefits and harms of aerosolized prostacyclin in participants with ARDS versus participants given placebo or usual care when excluding trials with zero events.
Examining the role of funding bias when excluding trials that were exclusively sponsored by pharmaceutical companies.
We planned to calculate RR with 95% CI and apply complete case analysis, if possible, for our sensitivity and subgroup analyses based on our primary outcome measure (mortality).
'Summary of findings' table and GRADE
We used the principles of the GRADE approach to provide an overall assessment of evidence related to all our outcomes. We constructed Table 1 using GRADEpro software (GRADEpro). However, one may argue the true value of this table based on the limited number of RCTs and the quality of published data and their design limitations. As outcomes of public interest, we presented overall mortality (regardless of the follow‐up period), PaO2/FiO2 ratio and adverse events (see Table 1).
Results
Description of studies
Results of the search
Through electronic searches and from references of potentially relevant articles, we identified 3563 publications. We excluded 3517 publications as they were either duplicates or were clearly irrelevant (Figure 1). A total of 46 potentially relevant publications were retrieved for further assessment. From these, we excluded 43 trials. The previous review classified two trials as ongoing (Afshari 2010). One of them was terminated prior to enrolment (NCT00981591), and therefore excluded. The other was completed and included (Siddiqui 2013). We have now included two trials with 81 participants (Dahlem 2004; Siddiqui 2013). The three review authors (MA, ABB and AA) completely agreed on the selection of the included studies. We obtained additional information from the lead authors of both trials.
1.

Study flow diagram.
Included studies
Only two trials met the entry criteria for this systematic review (Dahlem 2004; Siddiqui 2013). Both trials were carried out at a single centre, one in the Netherlands and one in Pakistan. The details of the included studies are provided in the Characteristics of included studies table. One trial included only children (Dahlem 2004), and one trial included only adults aged 18 years or over (Siddiqui 2013). The two included trials involved 81 participants.
Dahlem 2004 had a cross‐over design; two groups of participants: one group initially treated with aerosolized prostacyclin followed by normal saline and the other group initially treated with normal saline followed by aerosolized prostacyclin . Due to the cross‐over design, mortality results therefore merely referred to the effect of the sequence of drug administration rather than potential benefits or harms of the drug per se.
Siddiqui 2013 used PGE1 (alprostadil) 20 μg as intervention and normal saline as control; both dispensed in 5 mL of saline nebulized continuously over 30 minutes.
The duration of intervention was less than 24 hours in both trials (Dahlem 2004; Siddiqui 2013). Length of follow‐up was 28 days in Dahlem 2004 and not stated in Siddiqui 2013. No other cointervention was applied besides standard critical care treatment in both trials (Dahlem 2004; Siddiqui 2013). There was no predefined protocol for mechanical ventilation in either trial. As described previously, we chose to include Dahlem 2004 in a descriptive manner since this trial also was included in the previous version of this review but was not considered eligible for meta‐analyses due to the cross‐over design.
Excluded studies
We excluded 27 publications (Abraham 1996; Abraham 1999; Archer 1996; Bein 1994; Boeck 2012; Bone 1989; Domenighetti 2001; Dunkley 2013; Eichelbrönner 1996; Holcroft 1986; Liu 2015; Meyer 1998; NCT00981591; Pappert 1995; Putensen 1998; Rossignon 1990; Sawheny 2013; Shoemaker 1986; Sood 2014; Torbic 2013; Van Heerden 1996; Van Heerden 2000; Vassar 1991; Vincent 2001; Walmrath 1995; Walmrath 1996; Zwissler 1996), for the reasons detailed in the Characteristics of excluded studies table.
One trial was an RCT enrolling only neonatal participants and was therefore excluded (Sood 2014).
In Sood 2014, all seven participants INO prior to enrolment. Four participants received pulmonary vasodilators (milrinone and sildenafil), one participant, randomized to high‐dose inhaled PGE1 received low‐dose inhaled PGE1 for the first 24 hours, thereafter high‐dose inhaled PGE1. Five (71.43%) participants received surfactant. Five (71.43%) received neuromuscular blockade, and four (57.14%) received steroids before randomization. Six (85.71%) received extracorporeal membrane oxygenation.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
The overall quality of the two included studies was evaluated based on the major sources of bias (domains) as described in Appendix 3 (Dahlem 2004; Siddiqui 2013).
We contacted the lead author of Dahlem 2004 but he was only able to provide a limited amount of relevant information. We also contacted the authors of Siddiqui 2013. After further contact, we became aware of incorrect reporting on an important outcome which has been revised in this paper (see Characteristics of included studies table).
Both Dahlem 2004 and Siddiqui 2013 could be considered as low risk of bias trials, despite its limitations due to size and design. For a more detailed description of individual trial methodology, see the Characteristics of included studies table. The various bias domains are presented in Figure 2 and Figure 3.
2.
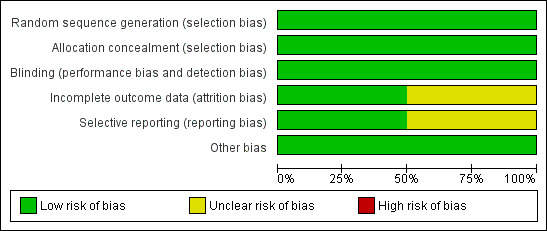
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
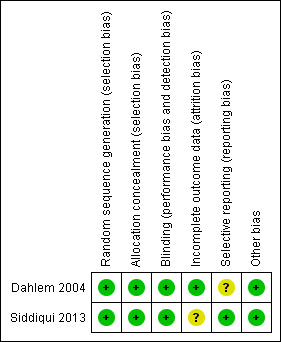
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Both trials reported generation of allocation sequence adequately (Dahlem 2004; Siddiqui 2013) (Figure 3).
Both trials reported allocation concealment adequately (Dahlem 2004; Siddiqui 2013) (Figure 3).
Blinding
Dahlem 2004 provided sufficient data to be categorized as double‐blinded (low risk of bias). In Siddiqui 2013, all investigators, staff and participants were masked to outcome measurements and allocation (low risk of bias) (Figure 3).
Incomplete outcome data
Both Dahlem 2004 and Siddiqui 2013 had adequate follow‐up (low risk) (Figure 3). There appeared to be complete follow‐up for the primary outcome in Dahlem 2004, but only for the length of follow‐up, which was 28 days. Siddiqui 2013 excluded five participants after randomization because they died before receiving the intervention. The remaining randomized participants seem accounted for in the tables. The authors performed analysis according to the ITT method in both trials (Dahlem 2004; Siddiqui 2013). However, in Siddiqui 2013, we became aware of inadequate reporting of data on the pulmonary artery pressure values (see Characteristics of included studies table).
Selective reporting
We were unable to retrieve the original protocol of Dahlem 2004 and thus were unable to examine selective outcome (unclear risk of bias). The trial registration of Siddiqui 2013 was available on ClinicalTrials.gov (low risk of bias) (see Characteristics of included studies table).
Other potential sources of bias
Neither trial was industry funded (Dahlem 2004; Siddiqui 2013). Sample size calculation was not reported and the trials were not powered to show a statistically significant benefit in primary outcome measures.
We were unable to conduct analyses such as the funnel plot, the arcsine‐Thompson test as proposed by Rücker (Rücker 2008), or the Egger's regression intercept test as only two RCTs were included, one of which was a cross‐over trial (Dahlem 2004), and the second did not present data on mortality (Siddiqui 2013).
Effects of interventions
See: Table 1
Primary outcomes
Overall mortality
As described above, data from Dahlem 2004 were not eligible for meta‐analysis due to the cross‐over design.
After completion of both treatment sequences, 2/8 participants in the intervention group died compared to 1/6 participants in the control group (RR 1.50, 95% CI 0.17 to 12.94; Characteristics of included studies table). Due to the cross‐over design of this trial, the results merely reflect the effect of the drug administration sequence rather than the intervention by itself. The results are thus not considered as a valid effect estimate for the primary endpoint of this review.
Furthermore, since no data were available from Siddiqui 2013, there was no option for meta‐analysis. However, no statistically significant difference was found in Dahlem 2004 when comparing the two groups (Table 1; Characteristics of included studies table). This outcome was downgraded from high to low quality of evidence due to limitations in design (small sample size, few events, cross‐over design) suggesting high likelihood of bias, indirectness of evidence and high probability of publication bias. (Dahlem 2004).
Sensitivity and subgroup analyses
We were unable to conduct our prespecified sensitivity and subgroup analyses due to lack of included RCTs. Additionally, the authors of the included trials conducted very few analyses relevant to our systematic review (Dahlem 2004; Siddiqui 2013).
Bias assessment: we were unable to conduct any relevant estimate of the intervention effect based on random sequence generation, allocation concealment, blinding, follow‐up, sample size calculation, early stopping, funding bias, other bias and the overall risk of bias since we only managed to find two relevant RCTs.
Secondary outcomes
Respiratory outcomes:Dahlem 2004 reported a 26% improvement in oxygenation index at 30 ng/kg/minute compared with placebo but there was no information on the oxygenation index based on different days. However, it is important to remember the limitations of this trial due to its design characteristics. Siddiqui 2013 reported a non‐significant improvement in PaO2/ FiO2 ratio (MD ‐25.35, 95% CI ‐60.48 to 9.78; 1 RCT, 67 participants, very low quality of evidence) (Table 1). The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size, few events and wide 95% CI suggesting high likelihood of bias and indirectness of evidence. (Siddiqui 2013).
Vascular outcomes: after further correspondence with the lead author of Siddiqui 2013, it became apparent that we were unable to carry out any meaningful analyses on our predefined secondary outcomes such as pulmonary artery pressure due to incorrect reporting (see Characteristics of included studies table).
Outcomes such as severity of illness, resolution of organ dysfunction, length of stay in ICU or hospital, quality of life assessment and cost‐benefit analyses were not conducted by the authors.
Adverse events and complications: the authors did not encounter any adverse events such as bleeding, organ dysfunction, airway reactivity or adverse events unrelated to the intervention (Dahlem 2004; Siddiqui 2013). The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size and few events and since only descriptive assessment of safety and adverse events were provided in the included trials with no data being available for meta‐analyses.
Quality of life and cost‐benefit analysis: the authors of both trials did not conduct any quality of life assessment or cost‐benefit analysis (Dahlem 2004; Siddiqui 2013).
Trial sequential analysis: trial sequential analysis was not considered appropriate since the one trial was cross‐over (Dahlem 2004) and its inclusion in this review may be considered debatable and since the other RCT was of a size that the proportion of the required information size was probably less than 1%. However, we tried to estimate the required information size for a conclusive meta‐analysis considering a type 1 error risk of 5%, a type 2 error risk of 20%, an anticipated relative risk reduction of 20%, and a mortality rate in the control group of a paediatric population of about 20% and 40% in the adult population.
The required information size for a paediatric population, depending on the level of heterogeneity adjustment, was between 2897 (I2 = 0) and 3862 (I2 = 25%). The required information size for the adult population with the same level of heterogeneity was between 1132 (I2 = 0) and 1508 (I2 = 25%).
Discussion
In this updated systematic review, we were only able to include two trials with 81 critically ill participants with ARDS that assessed the effect of aerosolized prostacyclin (Dahlem 2004; Siddiqui 2013).
Dahlem 2004 was a cross‐over trial, not considered eligible for meta‐analyses. This is insufficient to demonstrate any benefits or harms of inhaled prostacyclin therapy. We found no ongoing trials.
Based on the very limited data available, we were unable to show any benefits of aerosolized prostacyclin on survival or other clinical outcomes. The sparse data on mortality were not promising but were not evidence of the absence of a beneficial effect; neither did the data suggest the degree of a potentially beneficial or detrimental effect of inhaled prostacyclin.
Dahlem 2004 found the oxygenation index significantly improved in the prostacyclin group. Siddiqui 2013 found a non‐significant improvement in the PaO2/FiO2 ratio (P = 0.16) in the prostacyclin group. These two parameters are only surrogate outcomes and it is uncertain whether they predict any true clinical benefits, as previously illustrated by trials examining the role of INO (Adhikari 2007; Gebistorf 2016).
These results must therefore be interpreted with caution due to design issues, risk of bias, sample size limitations and the choice of surrogate outcomes.
The evidence for inhaled prostacyclin in adults with ARDS is currently based on observational studies and case reports examining the role of inhaled prostacyclin as a single intervention. Compared to INO or in conjunction with INO, all indicated improved oxygenation but with few data on survival (Domenighetti 2001; Meyer 1998; Putensen 1998; Van Heerden 1996; Van Heerden 2000; Walmrath 1996; Zwissler 1996).
One systematic review identified seven RCTs which applied intravenous PGE1 for the treatment of ARDS (Adhikari 2004). The authors found no evidence to support the routine administration of PGE1. However, there are important pharmacological differences that might not justify a direct comparison of intravenous and aerosolized treatment of PGE1. Intravenous PGE1 is a vasodilator that decreases both pulmonary and systemic blood pressure and at the same time increases the venous admixture. Inhaled prostacyclin is believed to selectively dilate the pulmonary vasculature in ventilated lung areas, thus improving the ventilation/perfusion ratio and oxygenation (Meyer 1998).
Summary of main results
This updated systematic review was unable to show any beneficial effect of aerosolized prostacyclin despite indications of improved oxygenation index, due to the limited number of RCTs at this stage. We were unable to conduct our prespecified multiple subgroup and sensitivity analyses. There is currently a lack of evidence to support the routine use of aerosolized prostacyclin for ARDS.
Quality of the evidence
We planned to apply several statistical methods to explore and reduce the risk of bias and risk of random error, such as complete case analysis, trial sequential analysis, overall methodological bias assessment and analyses of various relevant clinical and physiological outcomes. However, since only two trials were included in this updated review (Dahlem 2004; Siddiqui 2013), we were unable to carry out the analyses. Thus, we tried to estimate the required information for a conclusive meta‐analysis on this intervention in both paediatric and adult populations, with and without a 25% heterogeneity adjustment.
Our systematic review had several potential limitations. The findings and interpretations were limited by the quality and quantity of the available evidence. The risk of bias of the included trials was mainly assessed by using the published data, which ultimately may not reflect the truth. Also, our estimation of a required information size makes it possible to conclude that the risk of random error in the meta‐analysis is both imminent and manifest, as less than 1% of the required information size was actually randomized.
Potential biases in the review process
Since there is no previous systematic review on this topic, we were unaware of the number of existing trials. Most of the review authors were familiar with this intervention from their experience in various ICU settings. However, this did not influence our assessment of the existing data and to our knowledge there was no other additional bias in the review process.
Agreements and disagreements with other studies or reviews
One systematic review and meta‐analysis by Fuller and colleagues included 25 publications in the analysis, including: prospective, non‐randomized interventional studies, observational cohort studies, case series and case studies (Fuller 2015). The authors found that inhaled prostaglandins improved oxygenation and decreased pulmonary artery pressures and may be associated with adverse events. There was reported mortality in 17 of the 25 included studies and overall reported mortality was 295/522 (56.5%) people with ARDS receiving inhaled prostaglandins.
Fuller and colleagues found the same two RCTs as our search (Dahlem 2004; Siddiqui 2013), and described them with very brief exposure to study drug and no participant‐centred outcomes. They made no conclusions on the basis of the two trials separately.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support the routine use of aerosolized prostacyclin in people with acute respiratory distress syndrome (ARDS) and, equally important, insufficient information on the effect on mortality is available. Despite signs of improved oxygenation, there is no statistically significant effect on mortality or other clinical outcomes since only two RCTs have been carried out (Dahlem 2004; Siddiqui 2013). For the same reason, the overall quality of evidence is very low for mortality, partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio and adverse events, based on the GRADE method of assessment.
Implications for research.
There is a need for large randomized trials with low risk of bias and an information size of up to several thousand participants (children as well as adults), to evaluate aerosolized prostacyclin before this intervention can be definitely rejected or accepted for use in critically ill patients with ARDS.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 8, 2010
| Date | Event | Description |
|---|---|---|
| 5 May 2017 | New search has been performed | Title of the review changed. We searched the databases up to 5 May 2017. We have updated the Methods section, included a full risk of bias tables and added a summary of findings table. We searched the databases until 5 May 2017. We included one new trial of prostacyclin in this review update (Siddiqui 2013). This review now includes two studies in total (81 participants) (Dahlem 2004; Siddiqui 2013) |
| 5 May 2017 | New citation required but conclusions have not changed | Our conclusion remains the same. Two new review authors ‐ Anders Bastholm Bille and Mikkel Allingstrup ‐ have joined the team |
| 12 October 2010 | Amended | Contact details updated. |
| 11 August 2009 | Amended | Keus 2009: no longer in press; page numbers added |
Acknowledgements
We would like to thank Dr Peter Dahlem and Dr Shahla Siddiqui for providing further information about their trials.
From the Cochrane Anaesthesia, Critical and Emergency Care Group we would like to thank:
Prof Harald Herkner (content editor), Jing Xie (statistical editor), Neill Adhikari (peer reviewer), Janet Wale (consumer editor), Janne Vendt (trial search co‐ordinator) for their help and editorial advice during the preparation of this updated systematic review.
We would also like to thank Karen Hovhannisyan (former trials search co‐ordinator) for his assistance in providing our different search strategies and Jane Cracknell (Managing Editor) for her valuable assistance during the entire process.
Finally, special thanks to Prof Harald Herkner and Prof Nathan L Pace for their great editorial criticism and assistance, enabling us to improve the overall quality of the first edition of this paper (Afshari 2010).
Appendices
Appendix 1. Abbreviations
| ALI = acute lung injury; ARDS = acute respiratory distress syndrome; CI = confidence interval; CINAHL = Cumulative Index to Nursing & Allied Health Literature; COPD = chronic obstructive lung disease; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; INO = inhaled nitric oxide; ITT = intention to treat analysis; LILACS = Latin American Caribbean Health Sciences Literature; MD = mean difference; MPAP = mean arterial pulmonary pressure; PaO2 = partial pressure of oxygen in arterial blood; PEEP = positive end expiratory pressure; PGE1 = prostaglandin E1; PGI2 = prostacyclin or epoprostenol or Flolan; PVR = pulmonary vascular resistance; RCT = randomized controlled trial; RR = risk ratio; TSA = trial sequential analysis. |
Appendix 2. Search strategies
| Database | Search strategy |
| Handsearch | Citation search of included studies and relevant reviews |
| CENTRAL,the Cochrane Library, 2017, Issue 4 | #1 MeSH descriptor Epoprostenol explode all trees #2 MeSH descriptor Prostaglandins explode all trees #3 prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin* #4 (#1 OR #2 OR #3) #5 MeSH descriptor Respiratory Distress Syndrome, Adult explode all trees #6 ARDS #7 respirator* or distress #8 distress and syndrome #9 (#5 OR #6 OR #7 OR #8) #10 (#9 AND #4) |
| Embase (OvidSP) | 1. exp prostacyclin/ or exp prostaglandin/ 2. (prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin*).mp. 3. 1 or 2 4. exp adult‐respiratory‐distress‐syndrome/ 5. ARDS.mp. or (respirator* or distress).ti,ab. or (distress adj6 syndrome).mp. 6. 4 or 5 7. 6 and 3 8. (RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/ or (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab.) not (animals not (humans and animals)).sh. 9. 8 and 7 |
| ISI Web of Science | #1 TS = prostacyclin* or TS = prostaglandin* or TS = Iloprost or TS = Prostin or TS = Flolan or TS = Epoprostenol or TS = Beraprost or TS = Treprostinil Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years #2 TS = ARDS or TS = (respirator* NEAR distress) or TS = (distress NEAR syndrome) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years #3 (#1 AND #2) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years |
| ISI BIOSIS Previews | #1 TS = prostacyclin* or TS = prostaglandin* or TS = Iloprost or TS = Prostin or TS = Flolan or TS = Epoprostenol or TS = Beraprost or TS = Treprostinil Indexes=BIOSIS Previews Timespan=All years #2 TS = ARDS or TS = (respirator* NEAR distress) or TS = (distress NEAR syndrome) Indexes=BIOSIS Previews Timespan=All years #3 (#1 AND #2) Indexes=BIOSIS Previews Timespan=All years |
| LILACS (via BIREME) | ("EPOPROSTENOL" or "EPOPROSTENOL/" or "PROSTAGLANDINS" or "prostaglandin$" or "Iloprost" or "Prostin" or "Flolan" or "Epoprostenol" or "Beraprost" or "Treprostinil" or "prostacyclin$") and ("RESPIRATORY DISTRESS SYNDROME, ACUTE/" or "RESPIRATORY DISTRESS SYNDROME, ADULT/" or "respirator$" or "distress") |
| MEDLINE (Ovid SP) | 1. exp Epoprostenol/ or exp Prostaglandins/ 2. (prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin*).mp. 3. 1 or 2 4. exp Respiratory Distress Syndrome, Adult/ 5. (ARDS or (respirator* or distress) or (distress adj6 syndrome)).mp. 6. 5 or 4 7. 6 and 3 8. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 9. 8 and 7 |
| CINAHL (EBSCOhost) | ((MM "Respiratory Distress Syndrome, Acute") or (MH "Respiratory Distress Syndrome+") or ARDS or respirator* or distress ) and ( prostaglandin*or Iloprost or Prostin or Flolan or Epoprostenol or Beraprost or Treprostinil or prostacyclin*) |
Appendix 3. Assessment of risk of bias in included studies
1. Random sequence generation
Assessment of randomization: sufficiency of the method in producing two comparable groups before intervention.
Grade: 'low risk': a truly random process (e.g. random computer number generator, coin tossing, throwing dice); 'high risk': any non‐random process (e.g. date of birth, date of admission by hospital or clinic record number or by availability of the intervention) or 'unclear risk': insufficient information.
2. Allocation concealment
Allocation method prevented investigators or participants from foreseeing assignment.
Grade: 'low risk': central allocation or sealed opaque envelopes; 'high risk': use of open allocation schedule or other unconcealed procedure or 'unclear risk': insufficient information.
3. Blinding
Assessment of appropriate blinding of the team of investigators and participants: person responsible for participant care, participants and outcome assessors.
Grade: 'low risk': blinding considered adequate if participants and personnel were kept unaware of intervention allocations after inclusion of participants into the study, and if the method of blinding involved a placebo indistinguishable from the intervention, as mortality is an objective outcome; 'high risk': not double‐blind, categorized as an open‐label study or without use of a placebo indistinguishable from the intervention or 'unclear risk': blinding not described.
4. Incomplete outcome data
Completeness of outcome data, including attrition and exclusions.
Grade: 'low risk': numbers and reasons for dropouts and withdrawals in the intervention groups described, or no dropouts or withdrawals specified; 'high risk': no description of dropouts and withdrawals provided; 'unclear risk': report gave the impression of no dropouts or withdrawals, but this was not specifically stated.
5. Selective reporting
Possibility of selective outcome reporting.
Grade: 'low risk': reported outcomes were prespecified in an available study protocol, or, if this was not available, published report included all expected outcomes; 'high risk': not all prespecified outcomes reported, reported using non‐prespecified subscales, reported incompletely or report failed to include a key outcome that would have been expected for such a study or 'unclear risk': insufficient information.
6. Funding bias
Assessment of any possible funding bias.
Grade: 'low risk': reported no funding, funding from universities or public institutions; 'high risk': funding from private investors, pharmaceutical companies or trial investigator employed by the pharmaceutical company or 'unclear risk': insufficient information.
7. Other bias
Assessment of any possible sources of bias not addressed in domains 1 to 6.
Grade: 'low risk': report appeared free of such biases; 'high risk': at least one important bias was present that was related to study design, early stopping because of some data‐dependent process, extreme baseline imbalance, academic bias, claimed fraudulence or other problems; or 'unclear risk': insufficient information, or evidence that an identified problem will introduce bias.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dahlem 2004.
| Methods | 2‐group cross‐over RCT, 1 centre. ITT: yes. Overall study quality: low risk of bias. Sample size calculation: not reported. Country: the Netherlands. |
|
| Participants | 14 children included first after 24 hours of admission with ALI defined by the criteria of the American‐European Consensus Conference in 1994 (Bernard 1994). Inclusion criteria: acute onset of respiratory failure; PaO2/FIO2 ratio ≤ 300 torr; no clinical signs of atrial hypertension (suspected clinically); bilateral infiltrates on chest radiographs, children intubated with endotracheal tubes with an internal diameter > 3.5 mm. ALI classified as either primary (intrapulmonary) or secondary (extrapulmonary) lung injury. Exclusion criteria: congenital heart disease, decreased cardiac shortening fraction < 30%, mitral regurgitation, enlarged left atrium suspected to have raised left atrial pressure and cardiogenic pulmonary oedema, thrombocytopenia (< 50,000/L), bleeding diathesis, activated partial thromboplastin time > 43 seconds, intracranial haemorrhage, acute renal failure, chronic lung disease or poor prognosis with the probability of death, or withdrawal of therapy within the following 24 hours. |
|
| Interventions | Intervention group: 8 children, first treated with aerosolized prostacyclin (epoprostenol sodium), stepwise increase of doses (10, 20, 30, 40 and 50 ng/kg/minute) followed by normal saline (designated as placebo). Each dose administered over 20‐minute period, followed by 5‐minute period between each dose increment. To achieve washout, there was 30‐minute period between prostacyclin and placebo nebulization. Control group: 6 children, initially treated with 5 doses of normal saline followed by aerosolized prostacyclin. Ventilation strategy and weaning standardized. No cross‐over of treatment failures. Standard critical care therapy to both groups. |
|
| Outcomes | Primary outcomes: improved oxygenation. Secondary outcomes: mortality, adverse effects, oxygenation index, FiO2, improved ventilation and respiratory variables, primary versus secondary lung injury, changes in haemodynamics, bleeding. |
|
| Notes | Aerosolized prostacyclin over < 24 hours did not reduce overall mortality at 28 days (RR 1.50, 95% CI 0.17 to 12.94, 14 participants) compared with aerosolized saline (total of 3 deaths). Letter sent to authors in December 2009. Authors replied in December 2009. The authors were unable to provide additional information except data for the analysis of mortality based on origin of the lesion (primary versus secondary lung injury) without finding statistical significance. Length of longest follow‐up: 28 days. Authors conclusion: "Aerosolized prostacyclin improves oxygenation in children with acute lung injury. Future trials should investigate whether this treatment will positively affect outcome." Funding: not for profit. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by numbered envelopes, following a cross‐over randomization procedure. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators and carers blinded to assignment of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals specified. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess based on available information. |
| Other bias | Low risk | Appeared free of such biases. |
Siddiqui 2013.
| Methods | Single‐centre, RCT. Country: Pakistan. |
|
| Participants | 67 adults aged ≥ 18 years with ARDS. | |
| Interventions | Intervention group: PGE1 (alprostadil) 20 μg in 5 mL normal saline in a nebulizer continuously over 30 minutes. Control group: 5 mL normal saline in a nebulizer continuously over saline over 30 minutes. Used concealed syringes. |
|
| Outcomes | Primary endpoint: proportion of participants achieving 25% improvement in diastolic dysfunction, left ventricular end diastolic pressure, pulmonary artery systolic pressures and PaO2/FiO2 ratio from baseline as measured by repeat transthoracic echo and arterial blood gas analysis 30 minutes after treatment. No secondary outcomes. |
|
| Notes | Participant enrolment from May 2006 to February 2008. Contacted study author twice, 20 June 2016 and 24 March 2017 and received relevant response. Study took place in an adult, multidisciplinary, "open‐policy," 11 bed ICU in a tertiary care hospital of Karachi, Pakistan. The authors stated that measurement of pulmonary artery pressure was carried out with the application of echocardiography instead of pulmonary artery catheterization with the inherent risk of inaccuracies and bias in regards to measurements and inter‐observer variability. However, we were advised to approach the authors due to some questions in regards to the accuracy of the reported values of the pulmonary artery pressures in the publication (> 80 mmHg in both the intervention and control group). It became apparent that the authors had mistakenly reported on the systemic vascular mean systolic pressure and not the mean pulmonary artery systolic pressure. Furthermore, the authors provided additional information on lack of mortality data during the trial follow‐up. The trial was funded by the Pakistan Medical Research Council. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Parallel‐group study with balanced randomizations from a computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Independent pharmacists dispensed either the intervention or the control from pharmacy in a syringe form concealed with aluminium foil. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All investigators, staff and participants were masked to outcome measurements and allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomized participants seemed to be accounted for in the tables. However, the authors were unable to report data on mean artery pulmonary pressure and had provided data on systemic artery pressure instead in the manuscript. |
| Selective reporting (reporting bias) | Low risk | Trial registration available on ClinicalTrials.gov: NCT00314548. |
| Other bias | Low risk | Appeared free of other bias. |
For explanation of acronyms and abbreviations used in this table, see Appendix 1.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1996 | Randomized, multicentre, double‐blind, placebo‐controlled, phase II clinical trial of intravenous liposomal PGE1 versus placebo for people with ARDS. No inhalational therapy of prostacyclin. |
| Abraham 1999 | Multicentre, double‐blind, placebo‐controlled, phase III clinical trial; 350 people with ARDS randomized to receive either liposomal PGE1 or placebo. No inhalational therapy of prostacyclin. |
| Ammar 2015 | Retrospective, non‐interventional cohort study. 94 participants included, with 47 participants receiving prostacyclin and 47 receiving placebo. Reason for exclusion: retrospective study. |
| Archer 1996 | Randomized, placebo‐controlled trial of intravenous prostacyclin in acute respiratory failure in people with COPD. No inhalational therapy of prostacyclin. |
| Bein 1994 | Case report. No randomization. |
| Boeck 2012 | Randomized, double‐blind, cross‐over study; 16 people with COPD randomized to either a single dose of iloprost 10 mg (low dose), iloprost 20 mg (high dose) or placebo. All participants excluded because of chronic lung disease. |
| Bone 1989 | Randomized double‐blind, multicentre study of intravenous PGE1 in people with the ARDS versus placebo. No inhalational therapy of prostacyclin. There are multiple publications in different journals based on this trial. |
| Domenighetti 2001 | Prospective, non‐randomized interventional study examining the effect of inhaled prostacyclin in 15 consecutive, mechanically ventilated people with ARDS and severe hypoxaemia. |
| Dunkley 2013 | 16 participants in an observational study. Reason for exclusion: retrospective study. |
| Eichelbrönner 1996 | Randomized, interventional clinical study comparing INO and aerosolized prostacyclin on haemodynamics and gas exchange in people with septic shock and pulmonary hypertension. Excluded since majority of participants did not have ARDS or ALI. |
| Holcroft 1986 | Randomized, placebo‐controlled, double‐blind trial of intravenous PGE1 in surgical participants with ARDS. No inhalational therapy of prostacyclin. |
| Liu 2015 | 28 adults with end‐stage cirrhosis (18 men and 10 women) underwent modified piggyback liver transplantations. Reason for exclusion: observational study on elective patients. |
| Meyer 1998 | 15 people with ALI treated with PGE1 inhalation in addition to standard intensive care. No randomization. |
| NCT00981591 | Trial terminated prior to enrolment. Accessed 26 April 2016. |
| Pappert 1995 | Case report. No randomization. |
| Putensen 1998 | 10 people with ARDS received in random order: nitric oxide inhalation, aerosolized PGE1, infusion of PGE1 or no intervention. No control group and thus not an RCT. |
| Rossignon 1990 | Randomized double‐blind placebo‐controlled study on the activity of intravenous PGE1 in people with ARDS. No inhalational therapy with prostacyclin. |
| Sawheny 2013 | Prospective, non‐randomized interventional study examining the effect of nebulized iloprost in 20 people admitted to medical and surgical ICUs. No control group. |
| Shoemaker 1986 | Case report. PGE1 infusion. No randomization. |
| Sood 2014 | RCT enrolling only neonates and therefore excluded. All 7 participants INO prior to enrolment. 4 participants received pulmonary vasodilators (milrinone, sildenafil), 1 participant, randomized to high‐dose inhaled PGE1 received low‐dose inhaled PGE1 for the first 24 hours, thereafter high‐dose inhaled PGE1. 5/7 participants received surfactant. 5/7 received neuromuscular blockade and 4/7 received steroids before randomization. 6 participants received extracorporeal membrane oxygenation. |
| Torbic 2013 | Retrospective, single‐centre analysis of mechanically ventilated adults receiving INO or PGI2 for improvement in oxygenation; 105 mechanically ventilated people evaluated. Retrospective analysis and thus not an RCT. |
| Torbic 2016 | Same cohort as Torbic 2013. |
| Van Heerden 1996 | Case report. Comparison of INO and inhaled prostacyclin. No randomization. |
| Van Heerden 2000 | Unblinded, non‐randomized interventional, prospective clinical study of inhaled aerosolized prostacyclin in people with ARDS. |
| Vassar 1991 | Double‐blind, placebo‐controlled trial evaluating the efficacy of early infusion of PGE1 for reducing the incidence of severe respiratory failure and mortality. No inhalational prostacyclin therapy. |
| Vincent 2001 | Multicentre, randomized, double‐blind, placebo‐controlled clinical study evaluating the safety of intravenous liposomal PGE1 (TLC C‐53) in people with ARDS. No inhalational therapy of prostacyclin. |
| Walmrath 1995 | Trial examining the effects of aerosolized PGI2 on gas exchange and haemodynamics in mechanically ventilated people with severe community‐acquired pneumonia. Both groups received active treatment of inhalational prostacyclin. No control group. |
| Walmrath 1996 | 16 people with ARDS selected to receive initially either INO and then inhaled PGI2, or vice versa for very short period of time. No control group. |
| Zwissler 1996 | Case report of 8 participants receiving both inhaled prostacyclin and INO at various concentration. Not an RCT. |
For explanation of acronyms and abbreviations used in this table, see Appendix 1.
Differences between protocol and review
May 2017
The title of this review has been changed. Acute lung injury is no longer mentioned in the title of the review. As described in Description of the condition section, the term 'acute lung injury' no longer exists and has instead been replaced by a new ARDS definition based on the severity of hypoxaemia; the title of this updated review reflects this change.
Furthermore, in this updated review, we decided to expand our 'Risk of bias' table. Therefore, we added the following domains: blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. We also added two 'Risk of bias' figures (Figure 2; Figure 3).
We also applied the principles of the GRADE approach to provide an overall assessment of the evidence relating to our outcomes. We constructed a Table 1 table using GRADEpro software.
Contributions of authors
Updated review (2017)
AA, ABB and MA: involved in literature search, quality assessment and data abstraction of trials.
AA and MA: involved in writing the review.
Original published review
All four authors (Arash Afshari, Jesper Brok, Jørn Wetterslev and Ann Merete Møller) were involved in protocol development, literature searching, quality assessment and data abstraction of trials, and writing the review.
Sources of support
Internal sources
-
Cochrane Anaesthesia Critical and Emergency Care Group (CARG), Denmark.
Support from former trial search co‐ordinator (Karen Hovhannisyan) in designing search strategy
External sources
No sources of support supplied
Declarations of interest
AA: none known.
ABB: none known.
MA: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Dahlem 2004 {published and unpublished data}
- Dahlem P, Aalderen WM, Neef M, Dijkgraaf MG, Bos AP. Randomized controlled trial of aerosolized prostacyclin therapy in children with acute lung injury. Critical Care Medicine 2004;32(4):1055‐60. [PUBMED: 15071401] [DOI] [PubMed] [Google Scholar]
Siddiqui 2013 {published and unpublished data}
- Siddiqui S, Salahuddin N, Zubair S, Yousuf M, Azam I, Gilani AH. Use of inhaled PGE1 to improve diastolic dysfunction, LVEDP, pulmonary hypertension and hypoxia in ARDS ‐ a randomised clinical trial. Open Journal of Anesthesiology 2013;3(2):109‐15. [DOI: 10.4236/ojanes.2013.32027] [DOI] [Google Scholar]
References to studies excluded from this review
Abraham 1996 {published data only}
- Abraham E, Park YC, Covington P, Conrad SA, Schwartz M. Liposomal prostaglandin E1 in acute respiratory distress syndrome: a placebo‐controlled, randomized, double‐blind, multicenter clinical trial. Critical Care Medicine 1996;24(1):10‐5. [PUBMED: 8565513] [DOI] [PubMed] [Google Scholar]
Abraham 1999 {published data only}
- Abraham E, Baughman R, Fletcher E, Heard S, Lamberti J, Levy HN, et al. Liposomal prostaglandin E1 (TLC C‐53) in acute respiratory distress syndrome: a controlled, randomized, double‐blind, multicenter clinical trial. TLC C‐53 ARDS Study Group. Critical Care Medicine 1999;27(8):1478‐85. [PUBMED: 10470753 ] [DOI] [PubMed] [Google Scholar]
Ammar 2015 {published data only}
- Ammar MA, Bauer SR, Bass SN, Sasidhar M, Mullin R, Lam SW. Noninferiority of inhaled epoprostenol to inhaled nitric oxide for the treatment of ARDS. Annals of Pharmacotherapy 2015;49(10):1105‐12. [PUBMED: 26187741] [DOI] [PubMed] [Google Scholar]
Archer 1996 {published data only}
- Archer SL, Mike D, Crow J, Long W, Weir EK. A placebo‐controlled trial of prostacyclin in acute respiratory failure in COPD. Chest 1996;109(3):750‐5. [PUBMED: 8617086] [DOI] [PubMed] [Google Scholar]
Bein 1994 {published data only}
- Bein T, Pfeifer M, Riegger GA, Taeger K. Continuous intraarterial measurement of oxygenation during aerosolized prostacyclin administration in severe respiratory failure. New England Journal of Medicine 1994;331(5):335‐6. [PUBMED: 8022461] [DOI] [PubMed] [Google Scholar]
Boeck 2012 {published data only}
- Boeck L, Tamm M, Grendelmeier P, Stolz D. Acute effects of aerosolized iloprost in COPD related pulmonary hypertension ‐ a randomized controlled crossover trial. PloS One 2012;7(12):e52248. [PUBMED: 23300624 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bone 1989 {published data only}
- Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein MD, et al. Randomized double‐blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Prostaglandin E1 Study Group. Chest 1989;96(1):114‐9. [PUBMED: 2661155] [DOI] [PubMed] [Google Scholar]
- Silverman HJ, Slotman G, Bone RC, Maunder R, Hyers TM, Kerstein MD, et al. Effects of prostaglandin E1 on oxygen delivery and consumption in patients with the adult respiratory distress syndrome. Results from the prostaglandin E1 multicenter trial. The Prostaglandin E1 Study Group. Chest 1990;98(2):405‐10. [PUBMED: 2198140] [DOI] [PubMed] [Google Scholar]
- Slotman GJ, Kerstein MD, Bone RC, Silverman H, Maunder R, Hyers TM, et al. The effects of prostaglandin E1 on non‐pulmonary organ function during clinical acute respiratory failure. The Prostaglandin E1 Study Group. Journal of Trauma 1992;32(4):480‐8. [PUBMED: 1569622] [PubMed] [Google Scholar]
Domenighetti 2001 {published data only}
- Domenighetti G, Stricker H, Waldispuehl B. Nebulized prostacyclin (PGI2) in acute respiratory distress syndrome: impact of primary (pulmonary injury) and secondary (extrapulmonary injury) disease on gas exchange response. Critical Care Medicine 2001;29(1):57‐62. [PUBMED: 11176161] [DOI] [PubMed] [Google Scholar]
Dunkley 2013 {published data only}
- Dunkley KA, Louzon PR, Lee J, Vu S. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: a pilot study. Annals of Pharmacotherapy 2013;47(6):790‐6. [PUBMED: 23656748 ] [DOI] [PubMed] [Google Scholar]
Eichelbrönner 1996 {published data only}
- Eichelbrönner O, Reinelt H, Wiedeck H, Mezödy M, Rossaint R, Georgieff M, et al. Aerosolized prostacyclin and inhaled nitric oxide in septic shock ‐ different effects on splanchnic oxygenation?. Intensive Care Medicine 1996;22(9):880‐7. [PUBMED: 8905421] [DOI] [PubMed] [Google Scholar]
Holcroft 1986 {published data only}
- Holcroft JW, Vassar MJ, Weber CJ. Prostaglandin E(1) and survival in patients with the adult respiratory distress syndrome: a prospective trial. Seminars in Respiratory Medicine 1986;8 Suppl:40‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcroft JW, Vassar MJ, Weber CJ. Prostaglandin E1 and survival in patients with the adult respiratory distress syndrome. A prospective trial. Annals of Surgery 1986;203(4):371‐8. [PUBMED: 3516085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu D, Luo G, Luo C, Wang T, Sun G, Hei Z. Changes in the concentrations of mediators of inflammation and oxidative stress in exhaled breath condensate during liver transplantation and their relations with postoperative ARDS. Respiratory Care 2015;60(5):679‐88. [PUBMED: 25628453 ] [DOI] [PubMed] [Google Scholar]
Meyer 1998 {published data only}
- Meyer J, Theilmeier G, Aken H, Bone HG, Busse H, Waurick R, et al. Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesthesia and Analgesia 1998;86(4):753‐8. [PUBMED: 9539597] [DOI] [PubMed] [Google Scholar]
NCT00981591 {published and unpublished data}
- NCT00981591. Inhaled iloprost as an adjunct to inhaled nitric oxide in pediatric critical care patients. clinicaltrials.gov/ct2/show/NCT00981591 Date first received: 18 September 2009.
Pappert 1995 {published data only}
- Pappert D, Busch T, Gerlach H, Lewandowski K, Radermacher P, Rossaint R. Aerosolized prostacyclin versus inhaled nitric oxide in children with severe acute respiratory distress syndrome. Anesthesiology 1995;82(6):1507‐11. [PUBMED: 7793662] [DOI] [PubMed] [Google Scholar]
Putensen 1998 {published data only}
- Putensen C, Hörmann C, Kleinsasser A, Putensen‐Himmer G. Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1998;157(6 Pt 1):1743‐7. [PUBMED: 9620900] [DOI] [PubMed] [Google Scholar]
Rossignon 1990 {published data only}
- Rossignon MD, Khayat D, Royer C, Rouby JJ, Jacquillat C, Viars P. Functional and metabolic activity of polymorphonuclear leukocytes from patients with adult respiratory distress syndrome: results of a randomized double‐blind placebo‐controlled study on the activity of prostaglandin E1. Anesthesiology 1990;72(2):276‐81. [PUBMED: 2405741] [DOI] [PubMed] [Google Scholar]
Sawheny 2013 {published data only}
- Sawheny E, Ellis AL, Kinasewitz GT. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013;144(1):55‐62. [PUBMED: 23370599] [DOI] [PubMed] [Google Scholar]
Shoemaker 1986 {published data only}
- Shoemaker WC, Appel PL. Effects of prostaglandin E1 in adult respiratory distress syndrome. Surgery 1986;99(3):275‐83. [PUBMED: 3513357] [PubMed] [Google Scholar]
Sood 2014 {published and unpublished data}
- Sood B, Keszler M, Garg M, Klein JM, Ohls R, Ambalavanan N, et al. Inhaled PGE1 in neonates with hypoxemic respiratory failure: two pilot feasibility randomized clinical trials. Trials 2014;15:486. [PUBMED: 25496504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torbic 2013 {published data only}
- Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. Journal of Critical Care 2013;28(5):844‐8. [PUBMED: 23683572] [DOI] [PubMed] [Google Scholar]
Torbic 2016 {published data only}
- Torbic H, Szumita PM, Anger KE, Nuccio P, Lagambina S, Weinhouse G. Clinical and economic impact of formulary conversion from inhaled Flolan to inhaled veletri for refractory hypoxemia in critically ill patients. Annals of Pharmacotherapy 2016;50(2):106‐12. [PUBMED: 26668204] [DOI] [PubMed] [Google Scholar]
Van Heerden 1996 {published data only}
- Heerden PV, Blythe D, Webb SA. Inhaled aerosolized prostacyclin and nitric oxide as selective pulmonary vasodilators in ARDS ‐ a pilot study. Anaesthesia and Intensive Care 1996;24(5):564‐8. [PUBMED: 8909667] [DOI] [PubMed] [Google Scholar]
Van Heerden 2000 {published data only}
- Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose‐response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest 2000;117(3):819‐27. [PUBMED: 10713012] [DOI] [PubMed] [Google Scholar]
Vassar 1991 {published data only}
- Vassar MJ, Fletcher MP, Perry CA, Holcroft JW. Evaluation of prostaglandin E1 for prevention of respiratory failure in high risk trauma patients: a prospective clinical trial and correlation with plasma suppressive factors for neutrophil activation. Prostaglandins, Leukotrienes and Essential Fatty Acids 1991;44(4):223‐31. [PUBMED: 1667693] [DOI] [PubMed] [Google Scholar]
Vincent 2001 {published data only}
- Vincent JL, Brase R, Santman F, Suter PM, McLuckie A, Dhainaut JF, et al. A multi‐centre, double‐blind, placebo‐controlled study of liposomal prostaglandin E1 (TLC C‐53) in patients with acute respiratory distress syndrome. Intensive Care Medicine 2001;27(10):1578‐83. [PUBMED: 11685297] [DOI] [PubMed] [Google Scholar]
Walmrath 1995 {published data only}
- Walmrath D, Schneider T, Pilch J, Schermuly R, Grimminger F, Seeger W. Effects of aerosolized prostacyclin in severe pneumonia. Impact of fibrosis. American Journal of Respiratory and Critical Care Medicine 1995;151(3 Pt 1):724‐30. [PUBMED: 7881662] [DOI] [PubMed] [Google Scholar]
Walmrath 1996 {published data only}
- Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1996;153(3):991‐6. [PUBMED: 8630585] [DOI] [PubMed] [Google Scholar]
Zwissler 1996 {published data only}
- Zwissler B, Kemming G, Habler O, Kleen M, Merkel M, Haller M, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1996;154(6 Pt 1):1671‐7. [PUBMED: 8970353] [DOI] [PubMed] [Google Scholar]
Additional references
Adhikari 2004
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004477.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adhikari 2007
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ 2007;334(7597):779. [PUBMED: 17383982] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2003
- Anderson MR. Update on pediatric acute respiratory distress syndrome. Respiratory Care 2003;48(3):261‐76. [PUBMED: 12667276] [PubMed] [Google Scholar]
Angus 2001
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde‐Zwirble WT, Dremsizov TT, et al. Quality‐adjusted survival in the first year after the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2001;163(6):1389‐94. [PUBMED: 11371406] [DOI] [PubMed] [Google Scholar]
Barrington 2007
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000509.pub3] [DOI] [PubMed] [Google Scholar]
Bernard 1994
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American‐European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine 1994;149(3 Pt 1):818‐24. [PUBMED: 7509706] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. Journal of Clinical Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Dahlem 2003
- Dahlem P, Aalderen WM, Hamaker ME, Dijkgraaf MG, Bos AP. Incidence and short‐term outcome of acute lung injury in mechanically ventilated children. European Respiratory Journal 2003;22(6):980‐5. [PUBMED: 14680089] [DOI] [PubMed] [Google Scholar]
Dahlem 2007
- Dahlem P, Aalderen WM, Bos AP. Pediatric acute lung injury. Paediatric Respiratory Reviews 2007;8(4):348‐62. [PUBMED: 18005903] [DOI] [PubMed] [Google Scholar]
Dickersin 1990
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263(10):1385‐9. [PUBMED: 2406472] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flori 2005
- Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. American Journal of Respiratory and Critical Care Medicine 2005;171(9):995‐1001. [PUBMED: 15618461] [DOI] [PubMed] [Google Scholar]
Fröhlich 2013
- Fröhlich S, Murphy N, Ryan D, Boylan J. Acute respiratory distress syndrome: current concepts and future directions. Anaesthesia and Intensive Care 2013;41(4):463‐72. [PUBMED: 23808504] [DOI] [PubMed] [Google Scholar]
Fuller 2015
- Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta‐analysis. Chest 2015;147(6):1510‐22. [PUBMED: 25742022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gebistorf 2016
- Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD002787.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2015
- Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respiratory Care 2015;60(6):794‐802. [PUBMED: 26070575 ] [DOI] [PubMed] [Google Scholar]
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society. Series C, Applied Statistics 2000;49(3):359‐70. [DOI: 10.1111/1467-9876.00197] [DOI] [Google Scholar]
Jain 2006
- Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clinic Proceedings 2006;81(2):205‐12. [PUBMED: 16471076] [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses including zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [PUBMED: 19262513 ] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [uame‐roche.com/public/web/images/bibliografia/discrete_sequential_boundaries.pdf] [Google Scholar]
Linko 2009
- Linko R, Okkonen M, Pettilä V, Perttilä J, Parviainen I, Ruokonen E, et al. Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Medicine 2009;35(8):1352‐61. [PUBMED: 19526218] [DOI] [PubMed] [Google Scholar]
Luhr 1999
- Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. American Journal of Respiratory and Critical Care Medicine 1999;159(6):1849‐61. [PUBMED: 10351930] [DOI] [PubMed] [Google Scholar]
MacCallum 2005
- MacCallum NS, Evans TW. Epidemiology of acute lung injury. Current Opinion in Critical Care 2005;11(1):43‐9. [PUBMED: 15659944] [DOI] [PubMed] [Google Scholar]
Moloney 2003
- Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. European Respiratory Journal 2003;21(4):720‐7. [PUBMED: 12762363] [DOI] [PubMed] [Google Scholar]
Phua 2009
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. American Journal of Respiratory and Critical Care Medicine 2009;179(3):220‐7. [PUBMED: 19011152] [DOI] [PubMed] [Google Scholar]
Piantadosi 2004
- Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Annals of Internal Medicine 2004;141(6):460‐70. [PUBMED: 15381520] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomized controlled trials. Lancet 1998;351(9095):45‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Ranieri 2012
- ARDS Definition Task Force, Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526‐33. [PUBMED: 22797452] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubenfeld 2005
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. New England Journal of Medicine 2005;353(16):1685‐93. [PUBMED: 16236739] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Schuster 2008
- Schuster KM, Alouidor R, Barquist ES. Nonventilatory interventions in the acute respiratory distress syndrome. Journal of Intensive Care Medicine 2008;23(1):19‐32. [PUBMED: 18230633] [DOI] [PubMed] [Google Scholar]
Siobal 2003
- Siobal MS, Kallet RH, Pittet JF, Warnecke EL, Kraemer RW, Venkayya RV, et al. Description and evaluation of a delivery system for aerosolized prostacyclin. Respiratory Care 2003;48(8):742‐53. [PUBMED: 12890294] [PubMed] [Google Scholar]
Siobal 2004
- Siobal M. Aerosolized prostacyclins. Respiratory Care 2004;49(6):640‐52. [PUBMED: 15165299] [PubMed] [Google Scholar]
TenHoor 2001
- TenHoor T, Mannino DM, Moss M. Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest 2001;119(4):1179‐84. [PUBMED: 11296187] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
TSA 2010 [Computer program]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis, version 0.8. Copenhagen: Copenhagen Trial Unit, 2010.
Vohwinkel 2015
- Vohwinkel C, Hoegl S, Eltzschig H. Hypoxia signaling during acute lung injury. Journal of Applied Physiology (Bethesda, Md. : 1985) 2015;119(10):1157‐63. [PUBMED: 25977449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 2000
- Ware LB, Matthay MA. The acute respiratory distress syndrome. New England Journal of Medicine 2000;342(18):1334‐49. [PUBMED: 10793167] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetzel 1995
- Wetzel RC. Aerosolized prostacyclin. In search of the ideal pulmonary vasodilator. Anesthesiology 1995;82(6):1315‐7. [PUBMED: 7793644] [DOI] [PubMed] [Google Scholar]
Zimmerman 2009
- Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009;124(1):87‐95. [PUBMED: 19564287] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Afshari 2009
- Afshari A, Brok J, Møller AM, Wetterslev J. Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007733] [DOI] [PubMed] [Google Scholar]
Afshari 2010
- Afshari A, Brok J, Møller AM, Wetterslev J. Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD007733; PUBMED: 20687093] [DOI] [PubMed] [Google Scholar]


