Abstract
Background
Registry data shows that the incidence of acute rejection has been steadily falling. Approximately 10% to 35% of kidney recipients will undergo treatment for at least one episode of acute rejection within the first post‐transplant year. Treatment options include pulsed steroid therapy, the use of an antibody preparation, the alteration of background immunosuppression, or combinations of these options. Over recent years, new treatment strategies have evolved, and in many parts of the world there has been an increase in use of tacrolimus and mycophenolate and a reduction in the use of cyclosporin and azathioprine use as baseline immunosuppression to prevent acute rejection. There are also global variations in use of polyclonal and monoclonal antibodies to treat acute rejection. This is an update of a review published in 2006.
Objectives
The aim of this systematic review was to: (1) to evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for first episode of rejection in kidney transplant recipients; (2) evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for steroid‐resistant rejection in kidney transplant recipients; (3) determine how the benefits and adverse events vary for each type of antibody preparation; and (4) determine how the benefits and harms vary for different formulations of antibody within each type.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 18 April 2017 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) in all languages comparing all mono‐ and polyclonal antibody preparations, given in combination with any other immunosuppressive agents, for the treatment of cellular or humoral graft rejection, when compared to any other treatment for acute rejection were eligible for inclusion.
Data collection and analysis
Two authors independently assessed the risk of bias of the included studies and extracted data. Statistical analyses were performed using a random‐effects model and results expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
We included 11 new studies (18 reports, 346 participants) in this update, bring the total number of included studies to 31 (76 reports, 1680 participants). Studies were generally small, incompletely reported, especially for potential harms, and did not define outcome measures adequately. The risk of bias was inadequate or unclear risk for random sequence generation (81%), allocation concealment (87%) and other bias (87%). There were, however, a predominance of low risk of bias for blinding (75%) and incomplete outcome data (80%) across all the studies. Selective reporting had a mixture of low (58%), high (29%), and unclear (13%) risk of bias.
Seventeen studies (1005 participants) compared therapies for first acute cellular rejection episodes. Antibody therapy was probably better than steroid in reversing acute cellular rejection (RR 0.50, 95% CI 0.30 to 0.82; moderate certainty) and preventing subsequent rejection (RR 0.70, 95% CI 0.50 to 0.99; moderate certainty), may be better for preventing graft loss (death censored: (RR 0.80, 95% CI 0.57 to 1.12; low certainty) but there was little or no difference in death at one year. Adverse effects of treatment (including fever, chills and malaise following drug administration) were probably reduced with steroid therapy (RR 23.88, 95% CI 5.10 to 111.86; I2 = 16%; moderate certainty).
Twelve studies (576 patients) investigated antibody treatment for steroid‐resistant rejection. There was little or no benefit of muromonab‐CD3 over ATG or ALG in reversing rejection, preventing subsequent rejection, or preventing graft loss or death. Two studies compared the use of rituximab for treatment of acute humoral rejection (58 patients). Muromonab‐CD3 treated patients suffered three times more than those receiving either ATG or T10B9, from a syndrome of fever, chills and malaise following drug administration (RR 3.12, 95% CI 1.87 to 5.21; I2 = 31%), and experienced more neurological side effects (RR 13.10 95% CI 1.43 to 120.05; I2 = 36%) (low certainty evidence).
There was no evidence of additional benefit from rituximab in terms of either reversal of rejection (RR 0.94, 95% CI 0.54 to 1.64), or graft loss or death 12 months (RR 1.0, 95% CI 0.23 to 4.35). Rituximab plus steroids probably increases the risk of urinary tract infection/pyelonephritis (RR 5.73, 95% CI 1.80 to 18.21).
Authors' conclusions
In reversing first acute cellular rejection and preventing graft loss, any antibody is probably better than steroid, but there is little or no difference in subsequent rejection and patient survival. In reversing steroid‐resistant rejection there was little or no difference between different antibodies over a period of 12 months, with limited data beyond that time frame. In treating acute humoral rejection, there was no evidence that the use of antibody therapy conferred additional benefit in terms of reversal of rejection, or death or graft loss.
Although this is an updated review, the majority of newer included studies provide additional evidence from the cyclosporin/azathioprine era of kidney transplantation and therefore conclusions cannot necessarily be extrapolated to patients treated with more contemporary immunosuppressive regimens which include tacrolimus/mycophenolate or sirolimus. However, many kidney transplant centres around the world continue to use older immunosuppressive regimes and the findings of this review remain strongly relevant to their clinical practice.
Larger studies with standardised reproducible outcome criteria are needed to investigate the outcomes and risks of antibody treatments for acute rejection in kidney transplant recipients receiving contemporary immunosuppressive regimes.
Keywords: Humans; Kidney Transplantation; Acute Disease; Antibodies; Antibodies/therapeutic use; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Antilymphocyte Serum; Antilymphocyte Serum/therapeutic use; Drug Resistance; Graft Rejection; Graft Rejection/drug therapy; Immunologic Factors; Immunologic Factors/therapeutic use; Immunosuppressive Agents; Immunosuppressive Agents/therapeutic use; Muromonab‐CD3; Muromonab‐CD3/therapeutic use; Randomized Controlled Trials as Topic; Rituximab; Rituximab/therapeutic use
Plain language summary
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients
What is the issue? Kidney transplantation is the treatment of choice for most patients with end‐stage kidney disease. Strategies to increase donor organ availability and to prolong the transplanted kidney's survival have become priorities in kidney transplantation. About 10% to 35% of all kidney transplant recipients will experience one episode of acute rejection in the first year. Options for treating these episodes include pulsed steroid therapy, the use of an antibody preparation, the alteration of background immunosuppression, or combinations of these options.
What did we do? This review investigated the role of mono‐ or polyclonal antibodies in the treatment of acute cellular or acute humoral rejection in kidney transplant recipients. Thirty one studies (1680 patients) were included.
What did we find? We identified 31 studies enrolling 1680 people. Any antibody was better than steroid treatment for reversing the first acute cellular rejection episode and preventing graft loss, but showed little or difference in reversing steroid‐resistant rejection episodes. Polyclonal antibody‐treated patients were more likely to experience an immediate reaction of fever, chills and malaise than those receiving steroid treatment.
Conclusions Antibody treatment was better than steroid treatment for reversing first acute cellular rejection and preventing graft loss but this treatment was associated with a high incidence of adverse effects. The main limitation of this review is that many of the included studies were performed during the cyclosporin/azathioprine era of kidney transplantation and therefore conclusions cannot necessarily be extrapolated to patients treated with more contemporary immunosuppressive regimens which include tacrolimus/mycophenolate or sirolimus.
Summary of findings
Summary of findings for the main comparison. Antibody (T cell) versus steroid (stratified by antibody type) for the treatment of first rejection episodes in kidney transplant recipients.
| Antibody (T cell) versus steroid (stratified by antibody type) for the treatment of first rejection episodes in kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients: first rejection episode Setting: single and multicentre Intervention: antibody (T cell) Comparison: steroid (stratified by antibody type) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with steroid (stratified by antibody type) | Risk with antibody (T cell) | ||||
| Failure of reversal of acute rejection | Study population | RR 0.50 (0.30 to 0.82) | 405 (6) | ⊕⊕⊕⊝ MODERATE 1 | |
| 342 per 1,000 | 171 per 1,000 (102 to 280) | ||||
| Recurrent rejection Follow up: 12 months | Study population | RR 0.75 (0.56 to 1.00) | 508 (9) | ⊕⊕⊕⊝ MODERATE 1 | |
| 566 per 1,000 | 425 per 1,000 (317 to 566) | ||||
| Graft loss or death with a functioning graft Follow up: 12 months | Study population | RR 0.84 (0.58 to 1.22) | 490 (8) | ⊕⊕⊝⊝ LOW 1 2 | |
| 459 per 1,000 | 385 per 1,000 (266 to 560) | ||||
| Graft loss censored for death Follow up: 18 months | Study population | RR 0.80 (0.57 to 1.12) | 475 (8) | ⊕⊕⊝⊝ LOW 1 2 | |
| 409 per 1,000 | 327 per 1,000 (233 to 458) | ||||
| Death Follow up: 12 months | Study population | RR 0.98 (0.51 to 1.88) | 413 (7) | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| 83 per 1,000 | 81 per 1,000 (42 to 155) | ||||
| Treatment adverse events: fever, chill, or malaise after drug administration | Study population | RR 23.88 (5.10 to 111.86) | 280 (4) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Unclear/high risk in multiple studies for allocation concealment and selective reporting
2 CI includes null effect and potential for some harm and benefit
3 CI includes null effect and appreciable harm and benefit
4 High I2 (81%) and great variation in size of effect across all different treatment adverse effects
Summary of findings 2. Antibody (T cell) + steroid versus steroid alone for the treatment of first rejection episodes in kidney transplant recipients.
| Antibody (T cell) + steroid versus steroid alone for the treatment of first rejection episodes in kidney transplant recipients | |||||
| Patient or population: treatment of first rejection episodes in kidney transplant recipients: first rejection episode Setting: single centre Intervention: antibody (T cell) + steroid Comparison: steroid alone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with steroid alone | Risk with antibody (T cell) + steroid | ||||
| Failure of reversal of acute rejection episode | Study population | RR 0.42 (0.17 to 1.01) | 30 (1) | ⊕⊕⊝⊝ LOW 1 2 3 | |
| 688 per 1,000 | 289 per 1,000 (117 to 694) | ||||
| Recurrent rejection Follow up: 3 months | Study population | RR 0.07 (0.00 to 1.06) | 30 (1) | ⊕⊕⊝⊝ LOW 1 2 3 | |
| 500 per 1,000 | 35 per 1,000 (0 to 530) | ||||
| Graft loss or death with a functioning graft Follow up: 12 months | Study population | RR 0.35 (0.02 to 5.14) | 52 (2) | ⊕⊝⊝⊝ VERY LOW 1 3 4 5 | |
| 346 per 1,000 | 121 per 1,000 (7 to 1,000) | ||||
| Graft loss censored for death Follow up: 12 months | Study population | RR 0.33 (0.03 to 4.16) | 50 (2) | ⊕⊝⊝⊝ VERY LOW 1 3 4 5 | |
| 385 per 1,000 | 127 per 1,000 (12 to 1,000) | ||||
| Death Follow up: 12 months | Study population | RR 0.86 (0.53 to 1.39) | 50 (2) | ⊕⊕⊝⊝ LOW 1 3 6 | |
| 462 per 1,000 | 397 per 1,000 (245 to 642) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Small sample size and few number of events
2 Width of CI is very wide. CI includes null effect and is strongly one‐sided.
3 Unclear risk for random sequence generation and allocation concealment, and high risk for selective reporting
4 Big variation in size of effect with small overlap of CI and high I2 value
5 Width of CI is very wide. CI includes both null effect and appreciable benefit and harm
6 CI includes both null effect and appreciable benefit and harm
Summary of findings 3. Muromonab‐CD3 (T cell) versus other antibody (stratified by comparator) for the treatment of first rejection episodes in kidney transplant recipients.
| Muromonab‐CD3 (T cell) versus other antibody (stratified by comparator) for the treatment of first rejection episodes in kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients: first rejection episode Setting: single centre Intervention: muromonab‐CD3 (T cell) Comparison: other antibody (stratified by comparator) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with other antibody (stratified by comparator) | Risk with muromonab‐CD3 (T cell) | ||||
| Failure of acute rejection reversal | Study population | RR 1.84 (0.92 to 3.67) | 132 (2) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 134 per 1,000 | 247 per 1,000 (124 to 493) | ||||
| Recurrent rejection Follow up: 12 months | Study population | RR 1.06 (0.59 to 1.88) | 129 (2) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 254 per 1,000 | 269 per 1,000 (150 to 477) | ||||
| Treatment adverse events: fever, chills, malaise after drug administration | Study population | RR 3.12 (1.87 to 5.21) | 132 (2) | ⊕⊕⊝⊝ LOW 1 3 | |
| 269 per 1,000 | 838 per 1,000 (502 to 1,000) | ||||
| Treatment adverse events: neurological side effects | Study population | RR 13.10 (1.43 to 120.05) |
132 (2) | ⊕⊕⊝⊝ LOW 1 3 | |
| 15 per 1,000 | 196 per 1,000 (21 to 1,000) |
||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Small sample size and few number of events
2 CI includes null effect and potential for some harm and benefit
3 High I2 value and wide variation in size of effect
Summary of findings 4. Rituximab (B cell) + steroid versus steroid alone for the treatment of first rejection episodes in kidney transplant recipients.
| Rituximab (B cell) + steroid versus steroid alone for the treatment of first rejection episodes in kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients: first rejection episode Setting: single and multicentre Intervention: rituximab (B cell) + steroid Comparison: steroid alone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with steroid alone | Risk with rituximab (B cell) + steroid | ||||
| Failure of reversal of acute rejection | Study population | RR 0.94 (0.54 to 1.64) | 53 (2) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 500 per 1,000 | 470 per 1,000 (270 to 820) | ||||
| Graft loss or death with a functioning graft Follow up: 12 months | Study population | RR 1.00 (0.23 to 4.35) | 58 (2) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 103 per 1,000 | 103 per 1,000 (24 to 450) | ||||
| Death Follow up: 12 months | Study population | not estimable | 58 (2) | ⊕⊕⊕⊕ HIGH | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | ||||
| Treatment adverse events: UTI/pyelonephritis | Study population | RR 5.73 (1.80 to 18.21) | 38 (1) | ⊕⊕⊕⊝ MODERATE 1 | |
| 111 per 1,000 | 637 per 1,000 (200 to 1,000) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Small sample size and few number of events
2 CI includes both null effect and appreciable benefit and harm
Summary of findings 5. Antibody versus other antibody (stratified by antibody type) for the treatment of steroid‐resistant rejection episodes in kidney transplant recipients.
| Antibody versus other antibody (stratified by antibody type) for the treatment of steroid‐resistant rejection episodes in kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients: steroid‐resistant rejection episodes Setting: single centre Intervention: antibody Comparison: other antibody (stratified by antibody type) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with other antibody (stratified by antibody type) | Risk with antibody | ||||
| Failure of acute rejection reversal | Study population | RR 1.07 (0.63 to 1.81) | 244 (5) | ⊕⊕⊝⊝ LOW 1 2 | |
| 206 per 1,000 | 221 per 1,000 (130 to 373) | ||||
| Recurrent rejection | Study population | RR 0.78 (0.47 to 1.28) | 284 (5) | ⊕⊕⊝⊝ LOW 1 2 | |
| 356 per 1,000 | 278 per 1,000 (167 to 456) | ||||
| Graft loss censored for death Follow up: 12 months | Study population | RR 0.86 (0.34 to 2.17) | 244 (5) | ⊕⊕⊝⊝ LOW 1 2 | |
| 183 per 1,000 | 157 per 1,000 (62 to 396) | ||||
| Graft loss or death with a functioning graft Follow up: 12 months | Study population | RR 0.81 (0.43 to 1.51) | 211 (5) | ⊕⊕⊝⊝ LOW 1 2 | |
| 229 per 1,000 | 186 per 1,000 (99 to 346) | ||||
| Death Follow up: 12 months | Study population | RR 0.39 (0.09 to 1.65) | 175 (3) | ⊕⊕⊝⊝ LOW 1 2 3 | |
| 68 per 1,000 | 27 per 1,000 (6 to 112) | ||||
| Treatment adverse events: fever, chills, malaise after drug administration | Study population | ⊕⊕⊝⊝ LOW 1 4 | |||
| 342 per 1,000 | 870 per 1,000 (62 to 1,000) |
RR 2.54 (0.18 to 34.92) |
140 (3) | ||
| Treatment adverse events: bacterial infection | Study population | RR 8.64 (1.64 to 45.56) | 109 (2) | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| 17 per 1,000 | 149 per 1,000 (28 to 786) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 CI includes both null effect and appreciable benefit and harm
2 Unclear risk for random sequence generation and allocation across all studies
3 Small sample size and few number of events
4 High I2 value and wide variation in size of effect
Background
Description of the condition
Improvements in induction and maintenance immunosuppressive algorithms now mean that most recipients of kidney transplants can expect a greater than 90% chance of a functioning graft at one year. The impact of acute rejection on both graft survival in the short and longer term and on patient morbidity in the short and longer term is widely recognised (Jalalzadeh 2015; Joseph 2001; Koo 2015; Opelz 1997). The timing, severity, number of episodes of rejection, effectiveness of treatment and degree of recovery of kidney function are all important factors in determining outcome (Madden 2000; Opelz 2008).
The incidence of acute rejection in the first year post‐transplant has been steadily falling over the last 20 years. In 1990’s acute rejection episodes were reported in almost 50% of kidney transplant recipients (USRDS 2014) More recently, Registry data (Matas 2014; USRDS 2014) and randomised controlled trials (RCTs) of immunosuppressive interventions (Masson 2014) report the incidence of acute rejection during the first post‐transplant year to have stabilized at around 10%.
Clinically, acute rejection is defined as an acute deterioration in graft function associated with specific pathologic changes seen on transplant biopsy. The Banff classification (Solez 1993;Solez 2008) identifies acute cellular (T‐cell mediated) rejection which is classified according to presence and severity of interstitial inflammation, tubulitis and arteritis. Acute cellular rejection is caused by the cell mediated immune response ‐ which occurs when recipient T cells recognize donor antigens, T cells become activated and undergo clonal expansion, lymphocytes and other inflammatory cells then infiltrate the transplant and cause tissue damage (Ingulli 2010; Issa 2010; Nankivell 2010).
Acute humoral rejection is caused by humoral or antibody‐mediated responses and is an increasingly recognised cause of acute transplant dysfunction resistant to treatment with steroids and T cell specific immunosuppressive agents. Acute humoral rejection is caused by donor specific antibodies to Class I and Class II HLA antigens although other non‐HLA antigens have also been recognized (Dheda 2013).
The Banff criteria for classifying acute humoral rejection require the presence of (Colvin 2005; Haas 2016; Solez 1993;Solez 2008) (1) histological evidence of acute tissue injury e.g. acute tubular necrosis, capillaritis, tubulitis, arteritis, (2) presence of circulating donor‐specific antibodies (DSA), and (3) immunologic evidence of an antibody‐mediated process with positive peritubular capillary C4d staining reflecting complement activation via the classical pathway.
The increase in diagnosis of acute humoral rejection maybe due to improved detection techniques e.g. development of C4d staining, improved recognition of DSA and increase in highly sensitized recipients accepted for transplantation (Colvin 2005).
Acute cellular and humoral rejection may co‐exist. It may be difficult to distinguish between acute humoral rejection and severe acute cellular rejection, and it is not uncommon for treatment for the two conditions to be given concurrently or sequentially.
Histological evidence of acute rejection in the absence of deterioration in kidney function is defined in the Banff classification as subclinical rejection. It is unclear whether treatment of subclinical rejection improves long term transplant outcomes (Nankivell 2010).
The histological findings on transplant biopsy have important implications for prognosis and influence the treatment given to treat acute rejection.
Description of the intervention
There have been significant changes in the type of immunosuppressive agents and strategies used over the last 20 years. In 1996, almost 80% patients received cyclosporin (CsA) as first‐line calcineurin inhibitor (CNI), whereas in 2012, 92% patients received tacrolimus (TAC) as baseline immunosuppression. Similarly, mycophenolate preparations have replaced azathioprine (AZA) as the anti‐metabolite of choice. Registry data shows differing global trends in the use of induction antibodies. In the USA, Interleukin 2 antibody ((IL2a) use has fallen from a peak of 40% in 2002 to 20% in 2011, whilst the use of T‐cell depleting antibodies continues to increase (USRDS 2014). Whereas, in Australia, use of IL2 antibodies for induction has remained fairly stable at 81% to 93% and ATG 3% to 4% (ANZDATA 2012).
The treatment of acute cellular rejection requires a short course of more intensive immunosuppression, added to baseline immunosuppression therapy. Options include pulsed steroid therapy, the use of an antibody preparation, alteration of background immunosuppression, or combinations of these options, (Chon 2014; Denton 1999). Treatment of acute humoral rejection generally includes plasmapheresis, administration of intravenous immunoglobulin (IVIg), use of an antibody preparation, modification of background immunosuppression or a combination of these options (Bartel 2011).
There are several different preparations of horse and rabbit‐derived polyclonal antibodies against the human lymphocyte or thymocyte ‐ anti‐lymphocyte globulin (ALG), anti‐thymocyte globulin (ATG; horse or rabbit), and T10B9. A mouse monoclonal antibody against the CD3 receptor on activated T‐cells (muromonab‐CD3) also became commercially available in the late 1980s, but has since been withdrawn from the market in most countries. The integration of these antibodies into acute cellular rejection treatment protocols has developed as newer immunosuppressive agents have become available and immunosuppressive strategies evolved.
Recent studies have illustrated that a significant amount of B‐cell infiltrate is identified in T‐cell mediated tubulointerstitial rejection (Mengel 2007) and use of monoclonal antibody preparations which target different aspects of the immune system have been reported as described below.
How the intervention might work
ATG and mouse monoclonal antibodies against the CD3 receptor on activated T‐cells are preparations remove the functional T‐cell population from circulation, producing powerful saturation immunosuppression. These agents are useful for induction immunosuppression and for the management of acute rejection.
Recent reports describe the use of newer monoclonal antibodies targeting different aspects of the immune system (Chon 2014; Halloran 2004; Hardinger 2013). For example, alemtuzumab a monoclonal antibody directed against CD 52 (Campath – 1H) effects T and B lymphocytes and natural killer cells. The use of this agent to treat acute cellular rejection has been reported in a few small studies (Basu 2005; Csapo 2005)
Rituximab, a monoclonal antibody directed against CD20, causes depletion of mature B‐cells and has been used in treatment of acute cellular rejection with B‐cell infiltrates.
In the treatment of acute humoral rejection plasmapheresis and immunoadsorption are thought to act by physically removing circulating antibodies. IVIg has immunomodulatory properties, and suppresses the production of anti‐HLA antibodies and modifies complement activation (Dheda 2013; Jordan 2011). Other treatment strategies for treatment of acute humoral rejection are targeted towards reducing donor specific antibody titres by modifying B cell (rituximab) and plasma cell function (bortezomib), or reducing tissue damage induced by complement activation (eculizumab) (Dheda 2013; Roberts 2012).
Why it is important to do this review
However, these agents especially if used in combination or sequentially can cause profound and prolonged immunosuppression and be complicated by higher rates of infection and malignancy. It is important to consider the implications of cumulative immunosuppressive treatment in the context of a patient with lifelong end‐stage kidney disease who may have multiple transplants.
Objectives
The aim of this systematic review was to identify and summarise the evidence for the efficacy and adverse effects of using monoclonal or polyclonal antibodies to treat acute cellular or humoral rejection in kidney transplant recipients.
To evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for first episode of rejection in kidney transplant recipients
To evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for steroid‐resistant rejection in kidney transplant recipients
To determine how the benefits and adverse events vary for each type of antibody preparation
To determine how the benefits and harms vary for different formulations of antibody within each type.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs were included where an antibody was compared to any other treatment with the aim of reversing acute rejection. Eligibility for inclusion was not restricted on the basis of report language, age of recipients, or combinations of baseline immunosuppressive co‐interventions in either the control or intervention arm of the studies.
Types of participants
Adults and children who have had a kidney transplant Only studies involving kidney transplant as single organ were included; recipients of multi‐organ transplants were excluded from this review.
Types of interventions
All mono‐ and polyclonal antibody preparations, given in combination with any other immunosuppressive agents, for the treatment of acute graft rejection, compared to any other treatment for acute rejection. Treatments for acute cellular and humoral rejection were summarized separately. Comparisons examined were:
ATG versus ALG
ATG versus a different ATG (e.g. rabbit versus horse)
Monomurab‐CD3 versus ATG or ALG
Any antibody versus non‐antibody intervention
Any antibody in dosage comparisons
The class effect of anti‐lymphocyte preparations was initially assumed but differences in formulation were also examined (e.g. rabbit‐ versus horse‐based ATG formulations). All dosage regimens were included.
Types of outcome measures
Definitions used by each study for each outcome were recorded. Data on the following outcomes were collected wherever possible.
Primary outcomes
Reversal of acute rejection
Time to reversal
Recurrent rejection after the intervention rejection episode had been treated
Time to re‐rejection.
Secondary outcomes
Graft loss (censored and not censored for death)
Mortality
Graft function (measured by serum creatinine or calculated glomerular filtration rate (GFR))
Treatment failure necessitating a change in treatment either of the antibody or of the baseline immunosuppression
Immediate adverse effects of treatment
Occurrence of infection including cytomegalovirus disease (CMV)
Incidence of malignancy (including post‐transplant lymphoproliferative disorder (PTLD)).
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Failure of reversal of acute rejection
Recurrent rejection follow up: 12 months
Graft loss or death with a functioning graft follow up: 12 months
Graft loss censored for death follow up: 18 months
Death follow up: 12 months
Treatment adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 18 April 2017 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Hand searching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of hand searched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Clinical practice guidelines, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
This update (2017) was undertaken by five authors.
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by at least two authors, who discarded studies that were not applicable, however studies and reviews that may have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, where necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports be grouped together and the publication with the most complete data was used in the analyses. When relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions have been highlighted.
Assessment of risk of bias in included studies
For this update, the following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias (other bias)?
Measures of treatment effect
For dichotomous outcomes (e.g. rejection or no rejection) results were expressed as a risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. GFR), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used.
Unit of analysis issues
When analysing the risk ratio for the dichotomous outcomes, we took used each individual person/participant as the unit of analysis rather than each rejection event that occurred.
Dealing with missing data
Where necessary we contacted study authors for additional information about their studies. Four study authors (Drs Midtvedt, Almartine, Howard and Birkeland) provided additional information. We analysed available data and have referred to areas of missing data in the text (Alamartine 1994; Birkeland 1975; Howard 1977; Midtvedt 1996; Midtvedt 2003).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a CI for I2) (Higgins 2011).
Assessment of reporting biases
We planned to construct funnel plots to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
In this update, data have been grouped by their treatment for first cellular (T cell) rejection, first humoral (B cell) rejection, or steroid‐resistant rejection, and then by the possible outcomes measured, including: failure reversal of acute rejection, recurrent rejection, graft loss (censored and not censored for death), mortality, treatment failure requiring additional treatment, adverse events, occurrence of infections or malignancy, and graft function.
Within these categories, the outcome analysis was further stratified by antibody type. Forest plots were compiled using the Mantel‐Haenszel method for dichotomous outcomes, and the generic inverse variance method for continuous outcomes. Additional tables were made to analyse the effect of the intervention for both first cellular and steroid‐resistant rejection by the differences in formulation (rabbit‐derived ATG versus horse‐derived ATG), regimen (3 days versus 10 days ALG), dosage (half dose versus standard dose muromonab‐CD3), and non‐antibody intervention (IVIg, 15‐deoxyspergualin).
Subgroup analysis and investigation of heterogeneity
Heterogeneity was minimised as much as possible by grouping studies as described earlier. Data was pooled using the random effects model in all subgroup analyses.
Possible sources of heterogeneity identified a priori were study quality, specific formulation of antibody, and combination of baseline immunosuppression. Subgroup‐analysis was planned to formally identify important clinical differences among the studies that might potentially be expected to alter the magnitude of treatment effect, but this was not possible because of the sparseness of the data and many were old studies.
Sensitivity analysis
Sensitivity analysis was undertaken to assess the contribution of individual studies to heterogeneity and to assess any changes in results following exclusion of that study.
Results
Description of studies
Results of the search
We searched the Specialised Register to 18 April 2017 and identified 31 new reports. After full‐text assessment, 12 new studies (19 reports) were identified. Ten new studies (17 reports) were included (Alamartine 1994; Blumke 1989; Broyer 1987a; Campistol 1990; Okubo 1993; Olausson 1995; RITUX‐ERAH 2016; Simonian 1983; Toledo‐Pereyra 1985; Zarkhin 2008), one study was excluded (Kulkarni 2016), and one ongoing study was identified and will be assessed in a future update of this review (RIACT Study 2012).
We also identified 12 new reports of 10 existing included studies, nine of which offered no new data (Gaber 1998; Goldstein 1985; Hesse 1990; Hourmant 1985; Howard 1977; Johnson 1989; Spieker 1992; Waid 1991). One report was the completed protocol of Hoitsma 1982 and included more participants and outcomes.
See Figure 1 for flow chart of study selection.
1.

Study flow diagram.
Four studies (Alamartine 1994 (59 participants), Campistol 1990 (50 participants), Hilbrands 1996 (26 participants) and Simonian 1983 (20 participants)) were only reported as conference abstracts and the remaining 27 were reported in 10 different journals, published between 1975 and 2016. Twenty eight of the studies were reported in English, one was in German (Spieker 1992) and one in French (Hourmant 1985).
Included studies
Patient characteristics, baseline immunosuppression, randomised interventions and outcomes definitions varied across studies. There were two main groups of studies, those which evaluated interventions for first cellular or humoral rejection episodes and those which evaluated interventions in steroid‐resistant rejection episodes. There were no studies identified where IL2 receptor antagonists were investigated. Two studies investigated use of rituximab (RITUX‐ERAH 2016; Zarkhin 2008). One ongoing study (RIACT Study 2012), which will be included in a future update of this review, is also investigating rituximab.
Population demographics
Information on study population demographics was limited. Thirteen studies were conducted entirely in adult recipients (Alamartine 1994; Casadei 1998; Gaber 1998; Hesse 1990; Mariat 1998; Midtvedt 1996; Midtvedt 2003; Okubo 1993; Olausson 1995; RITUX‐ERAH 2016; Spieker 1992Streem 1983; Waid 1991) and four studies included a proportion (size not reported) of children (Broyer 1987a; Filo 1980; Howard 1977; Zarkhin 2008). Eight studies included a proportion (size not always stated) of patients with prior immunological sensitisation, as measured by panel reactive antibodies > 20 % (Alamartine 1994; Baldi 2000; Filo 1980; Gaber 1998; Goldstein 1985; Hoitsma 1982; Mariat 1998; Olausson 1995) and the remaining studies did not clearly define their recipient population. The proportion of grafts from deceased and living donor sources, and of recipients with prior failed transplants is given in the table of included studies.
Interventions
First cellular rejection
Seventeen studies (1005 participants) investigated the treatment of first cellular rejection episodes. For these studies, nine (530 participants) compared antibody to steroid (Broyer 1987a; Filo 1980; Glass 1983; Goldstein 1985; Hilbrands 1996; Hoitsma 1982; Shield 1979; Streem 1983; Theodorakis 1998); two (50 participants) compared antibody with steroid‐to‐steroid alone (Birkeland 1975; Simonian 1983); four (310 participants) compared antibody versus a different antibody (Baldi 2000; Johnson 1989; Toledo‐Pereyra 1985; Waid 1991). One (57 participants) compared ALG with IVIg (Howard 1977) and one (58 participants) compared ALG with steroid and a switch to CsA (Hourmant 1985).
ATG was rabbit‐derived for three studies, two manufactured by Fresenius (Baldi 2000; Theodorakis 1998) and the formulation unstated in Hilbrands 1996. Horse‐derived ATG was used for five studies, all Upjohn ATGAM (Filo 1980; Hoitsma 1982; Shield 1979; Simonian 1983; Toledo‐Pereyra 1985). ALG was entirely derived from horses, one manufactured by Merieux (Hourmant 1985), three by the University of Minnesota (Glass 1983; Streem 1983; Toledo‐Pereyra 1985), and formulations where unknown in three studies (Birkeland 1975; Broyer 1987a; Howard 1977).
Triple agent baseline immunosuppression with CsA, AZA and steroids was used in only one study (Baldi 2000), two studies used dual therapy with CsA and steroid (Hilbrands 1996; Theodorakis 1998) and the remainder used AZA and steroids, either with prior ALG induction therapy at the time of transplantation (Hourmant 1985; Streem 1983; Toledo‐Pereyra 1985) or without.
Humoral rejection
Two studies looking at humoral rejection using rituximab; Zarkhin 2008 (20 participants) compared rituximab with steroid‐to‐steroid alone, and RITUX‐ERAH 2016 (38 participants) compared rituximab with placebo. Zarkhin 2008 used anti‐CD 20 rituximab manufactured by BIOGEN‐IDEC Pharmaceuticals and Genentech Inc.
Steroid‐resistant rejection
Twelve studies (617 participants) investigated the treatment of steroid‐resistant rejection episodes; seven studies (339 participants) compared muromonab‐CD3 to treatment with another antibody (Alamartine 1994; Campistol 1990; Blumke 1989; Hesse 1990; Mariat 1998; Midtvedt 2003; Spieker 1992), one compared dosage schedules of muromonab‐CD3 (30 participants) (Midtvedt 1996), one compared dosage schedules of ATG (30 participants) (Olausson 1995), one compared muromonab‐CD3 to IVIg (30 participants) (Casadei 1998), and one compared muromonab‐CD3 to IV 15‐Deoxyspergualin (15‐DSP) (25 participants) (Okubo 1993).
ATG was rabbit‐derived for four studies, three manufactured by Genzyme (Gaber 1998; Mariat 1998; Midtvedt 2003), and one by Merieux (Alamartine 1994). Horse‐derived ATG was used by five studies, two used Upjohn ATGAM (Johnson 1989; Gaber 1998), two used Fresenius (Blumke 1989; Olausson 1995), and the formulation unstated in Spieker 1992. ALG was all horse‐derived, one used ALG manufactured by Merieux (Hesse 1990), and ALG was not defined in Campistol 1990.
Triple agent baseline immunosuppression with CsA, AZA and steroids was used for eight studies (Blumke 1989; Casadei 1998; Gaber 1998; Mariat 1998; Midtvedt 1996; Midtvedt 2003; Olausson 1995; Spieker 1992;) two studies used dual therapy with CsA and steroid (Campistol 1990; Hesse 1990) and one study used monotherapy with steroids (Alamartine 1994). No studies used tacrolimus or mycophenolate, or other antibody induction agents in either intervention rationale.
One study compared rabbit and horse preparations of ATG (163 participants) in recipients with mixed acute rejection scenarios; 33% had a previous rejection episodes, of which 40% had incomplete reversal at the time of randomisation to further treatment, and 11% had a first rejection episode that was steroid‐resistant (Gaber 1998).
Outcomes
The reporting of outcomes was variable (Figure 1) with graft‐focused outcomes reported more frequently (e.g. reversal of acute rejection, 23 studies) than patient‐focused complications of treatment (e.g. CMV infection, 16 studies) or specific adverse reactions. For many outcomes there was wide variation in the definitions used, the time post‐treatment at which the data was collected, and the detail provided for each definition. The variation in definitions used is illustrated in (Table 6, Table 7, Table 8). Data on time to reversal of acute rejection were often reported incompletely; although five studies reported mean time to rejection reversal and three studies the mean time to re‐rejection; only Filo 1980 reported the SD of the mean time, and so data could not be combined.
1. Inclusion criteria and outcome definitions used in studies of antibody for the treatment of first rejection episodes (cellular response).
| Study ID | Days since transplant | Timing of randomisation | Criteria for rejection* | Criteria for rejection reversal* |
| Antibody versus steroid | ||||
| Shield 1979 | < 35 | Rejection | Scoring algorithm of biochemical, and physical signs, with confirmatory “biopsy where possible” | Day 2 of “persistent creatinine fall” |
| Filo 1980 | < 90 | Rejection | “Clinical signs, imaging and renal function tests” | Increase in creatinine within 24 to 48 hours of bolus MP |
| Hoitsma 1982 | < 90 | Rejection | Increased creatinine, oliguria, sodium retention, weight gain, proteinuria, graft tenderness | Day 2 of 3 consecutive days of creatinine falling |
| Glass 1983 | ns | Transplantation | Clinical criteria including creatinine rise for 3 sequential days | Improvement in creatinine and clinical signs at 7th day of treatment |
| Streem 1983 | ns | Transplantation | Rise in creatinine and diminished function on I‐131 scan, with “supportive clinical findings” with confirmatory “biopsy where possible” | Day 2 of “persistent creatinine fall” |
| Goldstein 1985 | 6‐90 | Rejection | Scoring algorithm of biochemical, and physical signs, with confirmatory “biopsy where possible” | 3 day progressive fall in creatinine, or investigator judged clinical reversal. |
| Broyer 1987a | > 8 | Rejection | “Rise in plasma creatinine” and “changes in kidney echogenicity” on ultrasound. If unsure, “rejection was confirmed by kidney biopsy” | ns |
| Hilbrands 1996 | < 90 | Rejection | ns | ns |
| Theodorakis 1998 | ns | Rejection | Clinical ± biopsy confirmation | Not assessed. Severity of rejection episode judged by AUC of serial 10 day creatinine measurements. |
| Antibody and steroid versus steroid alone | ||||
| Birkeland 1975 | ns | Rejection | “Common clinical criteria”, with biopsy where possible | Day 2 of progressive rise in creatinine clearance |
| Simonian 1983 | ns | Rejection | ns | ns |
| Antibody versus other antibody | ||||
| Toledo‐Pereyra 1985 | ns | Transplantation | Primarily by laboratory signs of increase in SCr ≥ 0.3 mg/dL on any given day, or “clinical signs associated with rejection” and “an increase in kidney size on ultrasound” | ns |
| Waid 1991 | ns | Rejection | 4 of 7 clinical and biochemical signs, subsequently confirmed by biopsy | Absence of cross‐over, re‐treatment or graft loss |
| Baldi 2000 | ns | Rejection | 20% increase in creatinine with clinical suggestive signs, and biopsy if > 10 days from transplantation | ns |
| Formulation comparisons | ||||
| Johnson 1989 | ns | Rejection | Standard clinical indicators with supplementary “biopsy where possible” | 1st of 3 consecutive days of creatinine falling |
| Antibody versus other treatment | ||||
| Howard 1977 | ns | Rejection | Rise in creatinine of 0.3 mg/dL and deterioration of renogram, “mostly confirmed by biopsy” | ns |
| Hourmant 1985 | > 90 | 90 days post‐transplant | ns | ns |
* direct quotation from the text of study reports appears in quotation marks
AUC ‐ area under the curve; ns ‐ not stated and could not be clarified or deduced; MP ‐ methylprednisolone; SCr ‐ serum creatinine
2. Inclusion criteria and outcome definitions used in studies of antibody for the treatment of first rejection episodes (humoral response).
| Study ID | Days since transplant* | Timing of randomisation* | Criteria for rejection* | Criteria for rejection reversal* |
| Antibody versus placebo | ||||
| Zarkhin 2008 | ns | Rejection | “Biopsy proven” and Banff graded | “Recovery of graft function to within 20% of the baseline pre‐rejection value 1, 3, 6, and 12 months after the episode”, and “Resolution of the Banff biopsy grade” |
| RITUX‐ERAH 2016 | ns | Rejection | “Biopsy proven” | “Improvement of renal function at day 12” |
* direct quotation from the text of study reports appears in quotation marks
MP ‐ methylprednisolone; ns ‐ not stated and could not be clarified or deduced
3. Inclusion criteria and outcomes definitions used in studies of antibody for the treatment of resistant rejection episodes.
| Study ID | Days since transplant* | Timing of randomisation* | Criteria for rejection* | Initial treatment of rejection* | Criteria for resistant rejection* |
| Antibody versus other antibody | |||||
| Blumke 1989 | ns | “Steroid resistant rejection crisis” | ns | 3 bolus injections of cortisone | “Not sufficiently treated” with steroids |
| Campistol 1990 | ns | ns | Confirmed by renal biopsy | MP 1g for 3 days | ns |
| Hesse 1990 | < 42 | ns | Rise in creatinine of > 0.3 mg/dL and biopsy | MP 500 mg for 2 days | “Non response” |
| Spieker 1992 | “early” | ns | “Typical clinical symptoms”, renogram, and biopsy | MP 500‐1000 mg for 3 days | Lack of improvement in clinical and sonographic appearances |
| Alamartine 1994 | ns | At biopsy | Biopsy with “histological diagnosis” | MP 15 mg/kg, 2 bolus doses | “Absence of a clear response to the steroids” |
| Mariat 1998 | ns | At biopsy | Delayed graft function or rise in creatinine in presence of urine output < 1 L/d, low sodium excretion, weight gain > 1 kg/d or graft tenderness | MP 15 mg/kg, 2 doses alternate days | No decline in creatinine after 2 steroid boluses, followed by biopsy |
| Midtvedt 2003 | ns | Day 5 of treatment | Rise in creatinine > 20% in the absence of obvious cause and biopsy (Banff criteria) | MP 500 mg then 250 mg for 3 days | No decline in creatinine |
| Different formulations of antibody | |||||
| Gaber 1998 | ns | At biopsy | Biopsy, Banff graded | MP 500 mg, for 3 days | Creatinine increase of 10% after 3 days of MP |
| Different doses of same antibody | |||||
| Midtvedt 1996 | < 90 | Day 5 of treatment | Rise in creatinine > 20% in absence of obvious cause | MP boluses, cumulative dose 1‐1.5 g | No decline in creatinine after 5 days of treatment |
| Different duration of same antibody | |||||
| Olausson 1995 | ns | At biopsy | “Diagnosed clinically and verified with a core needle biopsy” | MP 250‐500 mg, for 4 days | Not responding with improved kidney function on 5th day of steroid treatment |
| Antibody versus other treatment | |||||
| Okubo 1993 | < 365 | Day 4 of treatment | Accelerated rejection: “progressive rise in SCr level was observed within 7 days of transplant”. Acute rejection: “rise in SCr of 0.5 mg/dl or higher” was seen anytime during post‐transplant course. Acute on chronic rejection: “a similar rise in SCr occurred in a patient with sustained creatinine level of ≥2.5mg/dl due to a documented previous acute rejection episode” | MP 500‐1000 mg, for 3 days | “Serum creatinine did not revert to the basal level within a week from the onset” |
| Casadei 1998 | ns | At biopsy | Clinical suspicion and biopsy | MP 500 mg for 3 days | “Failure to show improved renal function” within 7 days of starting MP |
* direct quotation from the text of study reports appears in quotation marks
MP ‐ methylprednisolone; ns ‐ not stated and could not be clarified or deduced
Excluded studies
Kulkarni 2016 was excluded as it assessed chronic not acute rejection.
Risk of bias in included studies
Allocation
Six studies (19%) reported adequate sequence generation (Filo 1980; Gaber 1998; Goldstein 1985; Hoitsma 1982; RITUX‐ERAH 2016; Waid 1991) two studies (6%) used inadequate sequence generation methods (Okubo 1993; Glass 1983) and the remaining 23 studies (75%) didn't provide sufficient information to assess the method of sequence generation.
Four studies (13%) reported adequate allocation concealment (Filo 1980; Gaber 1998; RITUX‐ERAH 2016; Waid 1991) two studies (6%) used inadequate allocation concealment (Blumke 1989; Okubo 1993) and the remaining 26 studies (81%) were randomised but gave no indication of the allocation method used.
Blinding
Twenty‐three studies (75%) adequately blinded (Alamartine 1994; Birkeland 1975; Blumke 1989; Broyer 1987a; Campistol 1990; Casadei 1998; Filo 1980; Gaber 1998; Glass 1983; Hesse 1990; Hilbrands 1996; Howard 1977; Midtvedt 1996; Midtvedt 2003; Olausson 1995; RITUX‐ERAH 2016; Shield 1979; Simonian 1983; Spieker 1992; Streem 1983; Theodorakis 1998; Waid 1991; Zarkhin 2008); six studies (19%) had no blinding (Baldi 2000; Goldstein 1985; Hoitsma 1982; Johnson 1989; Okubo 1993; Toledo‐Pereyra 1985) as evident by the differences in drug dosage, delivery and duration between the two groups; and two studies (6%) had insufficient information to suggest presence of absence of blinding (Hourmant 1985; Mariat 1998).
Incomplete outcome data
Three studies (10%) had incomplete data assessment having excluded randomised participants or had significant unexplained loss to follow‐up (Goldstein 1985; Howard 1977; Midtvedt 1996). Three studies (10%) had insufficient information to suggest the presence of incomplete outcome data (Johnson 1989, Theodorakis 1998, Waid 1991) and the remaining 25 studies (80%) had no loss to follow‐up or insignificant loss to follow‐up with intention‐to‐treat analysis performed.
Selective reporting
Nine studies (29%) had evidence of selective reporting (Birkeland 1975; Blumke 1989; Filo 1980; Goldstein 1985; Howard 1977; Johnson 1989; Midtvedt 1996; Olausson 1995; Toledo‐Pereyra 1985), and 18 studies (58%) had no risk of bias from selective reporting (Alamartine 1994; Baldi 2000; Broyer 1987a; Casadei 1998; Gaber 1998; Glass 1983; Hesse 1990; Hilbrands 1996; Hoitsma 1982; Mariat 1998; Midtvedt 2003; Okubo 1993; RITUX‐ERAH 2016; Shield 1979; Spieker 1992; Streem 1983; Waid 1991; Zarkhin 2008). Four studies (13%) had insufficient information to permit judgment about selective reporting (Campistol 1990; Hourmant 1985; Simonian 1983; Theodorakis 1998).
Other potential sources of bias
Seven studies (23%) had risk of bias due to possible conflict of interest from funding sources (Broyer 1987a; Filo 1980; Gaber 1998; Goldstein 1985; Olausson 1995; Shield 1979; Zarkhin 2008). Four studies (13%) were deemed as free of other biases (Hoitsma 1982; Howard 1977; RITUX‐ERAH 2016; Waid 1991) and the remaining 20 studies (64%) judged as having an unclear risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Antibody therapy for the first cellular rejection episode
Antibody versus steroids
Reversal of an initial episode of cellular rejection was probably better with antibody when compared to steroid alone (Analysis 1.1 (6 studies, 405 participants): RR 0.50, 95% CI 0.30 to 0.82; I2 = 36%; moderate certainty evidence). Recurrent rejection within the first year (Analysis 1.3 (9 studies, 508 participants): RR 0.75, 95% CI 0.56 to 1.00; I2 =54%; moderate certainty evidence) was probably slightly reduced with the use of antibody compared to steroid alone.
1.1. Analysis.
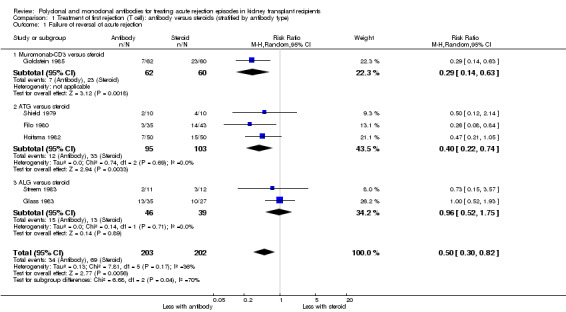
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 1 Failure of reversal of acute rejection.
1.3. Analysis.
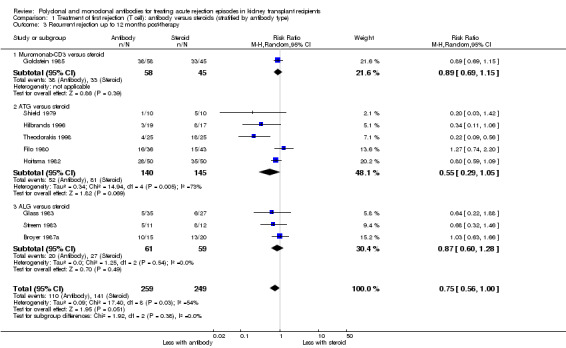
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 3 Recurrent rejection up to 12 months post‐therapy.
For the studies of antibody versus steroid, there was little or no difference in treatment failure necessitating additional treatment (Analysis 1.2), preventing graft loss, whether censored for deaths (Analysis 1.5 (8 studies, 475 participants): RR 0.80, 95% CI 0.57 to 1.12; I2 = 37%) or including death with a functioning graft (Analysis 1.4), deaths within a year (Analysis 1.6), and serum creatinine three months post‐treatment (Analysis 1.8). No studies reported malignancy data. Adverse effects of treatment (including fever, chills and malaise following drug administration) were probably reduced with steroid therapy (Analysis 1.7.1 (4 studies, 280 participants): RR 23.88, 95% CI 5.10 to 111.86; I2 = 16%; moderate certainty). There was probably little or no difference in infection (total), CMV infection, and avascular necrosis of the femoral head between antibody and steroid treatment (Analysis 1.7.2; Analysis 1.7.3; Analysis 1.7.4) (moderate certainty).
1.2. Analysis.

Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 2 Additional treatment needed.
1.5. Analysis.
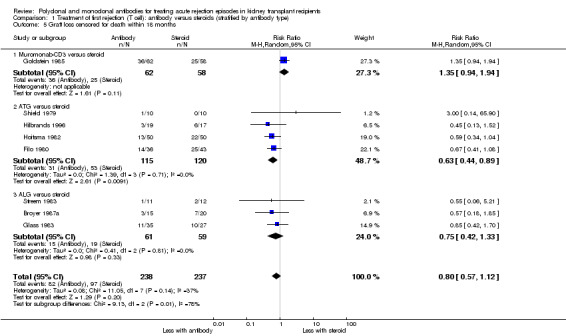
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 5 Graft loss censored for death within 18 months.
1.4. Analysis.
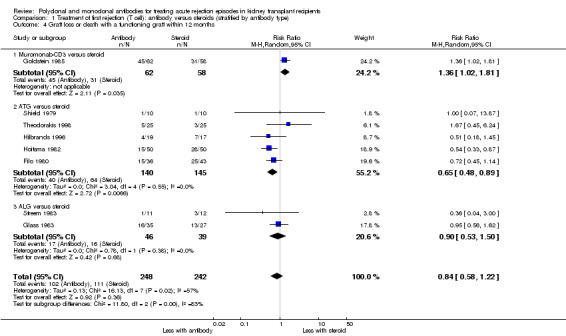
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 4 Graft loss or death with a functioning graft within 12 months.
1.6. Analysis.
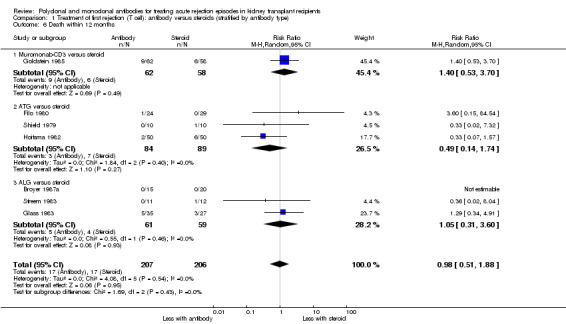
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 6 Death within 12 months.
1.8. Analysis.
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 8 Serum creatinine post treatment (3 months).
1.7. Analysis.
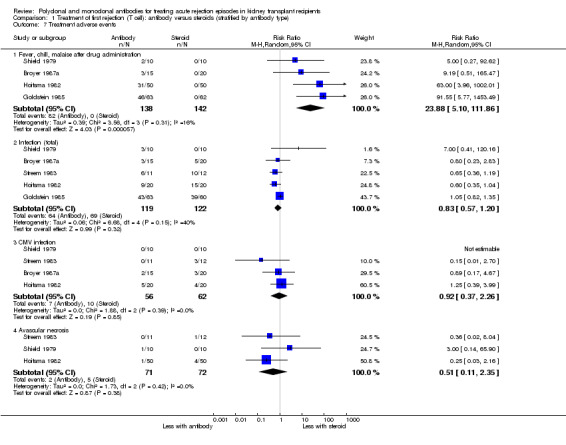
Comparison 1 Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type), Outcome 7 Treatment adverse events.
See Table 1.
Antibody plus steroids versus steroids alone
Two studies looked at antibody plus steroids versus steroids alone.
Antibody plus steroids may favour reversal of an initial episode of cellular rejection compared to steroids alone (Analysis 2.1 (1 study, 30 participants): RR 0.42, 95% CI 0.17 to 1.01; low certainty evidence). This was also the case for recurrent rejection within the first three months (Analysis 2.2 (1 study, 30 participants): RR 0.07, 95% CI 0.00 to 1.06).
2.1. Analysis.

Comparison 2 Treatment of first rejection (T cell): antibody + steroids versus steroids alone, Outcome 1 Failure of reversal of acute rejection (AR) episode.
2.2. Analysis.

Comparison 2 Treatment of first rejection (T cell): antibody + steroids versus steroids alone, Outcome 2 Recurrent rejection within 3 months post‐therapy.
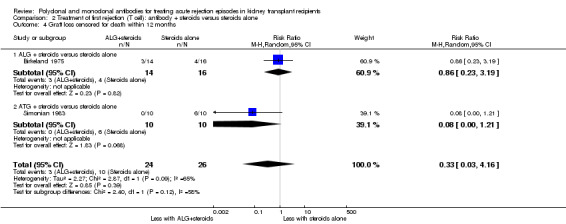
It was uncertain whether antibody plus steroids reduced graft loss (both censored for deaths and including death with a functioning graft) because the certainty of the evidence was very low (Analysis 2.3; Analysis 2.4). Antibody plus steroids may make little or no difference to death within a year (Analysis 2.5) (low certainty evidence).
2.3. Analysis.
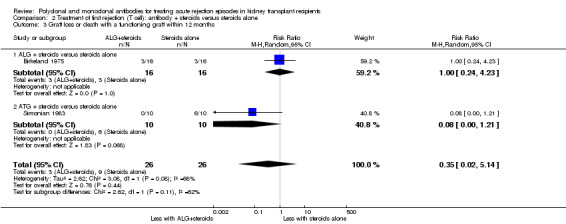
Comparison 2 Treatment of first rejection (T cell): antibody + steroids versus steroids alone, Outcome 3 Graft loss or death with a functioning graft within 12 months.
2.4. Analysis.
Comparison 2 Treatment of first rejection (T cell): antibody + steroids versus steroids alone, Outcome 4 Graft loss censored for death within 12 months.
2.5. Analysis.
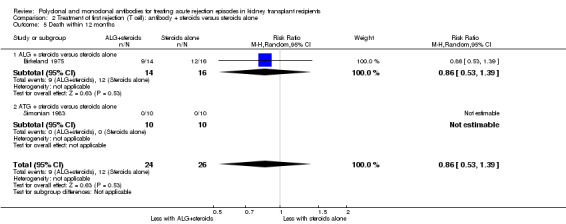
Comparison 2 Treatment of first rejection (T cell): antibody + steroids versus steroids alone, Outcome 5 Death within 12 months.
See Table 2.
Muromonab‐CD3 versus other antibody
For the two studies comparing muromonab‐CD3 with another antibody, there was probably little of no evidence of an advantage for muromonab‐CD3 in reversing rejection (Analysis 3.1 (2 studies, 132 participants): RR 1.84, 95% CI 0.92 to 3.67; I2 = 0%), the requirement for additional treatment to achieve reversal (Analysis 3.2 (2 studies, 132 participants): RR 1.67, 95% CI 0.77 to 3.63; I2 = 0%), subsequent recurrent rejection (Analysis 3.3 (2 studies, 129 participants): RR 1.06, 95% CI 0.59 to 1.88; I2 = 0%), infection (Analysis 3.4.4 (2 studies, 86 participants): RR 1.53, 95% CI 0.69 to 3.40; 12 = 37%), CMV infection (Analysis 3.4.5 (2 studies, 132 participants): RR 2.25, 95% CI 0.31 to 16.08; I2 = 48%), and malignancy (Analysis 3.4.6 (2 studies, 132 participants): RR 0.26, 95% CI 0.03 to 2.30) (moderate certainty evidence).
3.1. Analysis.
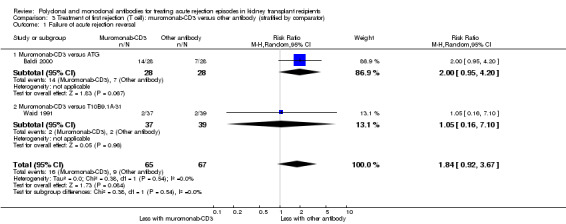
Comparison 3 Treatment of first rejection (T cell): muromonab‐CD3 versus other antibody (stratified by comparator), Outcome 1 Failure of acute rejection reversal.
3.2. Analysis.
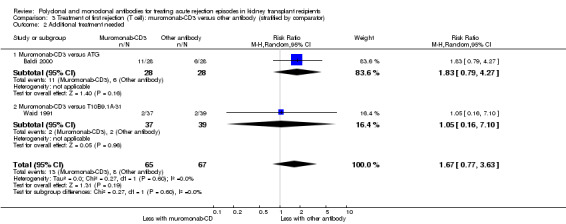
Comparison 3 Treatment of first rejection (T cell): muromonab‐CD3 versus other antibody (stratified by comparator), Outcome 2 Additional treatment needed.
3.3. Analysis.
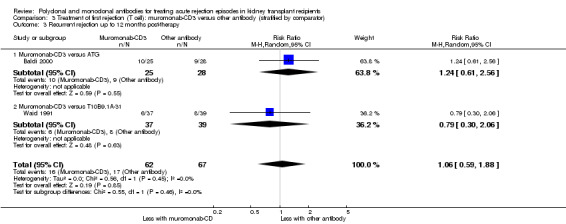
Comparison 3 Treatment of first rejection (T cell): muromonab‐CD3 versus other antibody (stratified by comparator), Outcome 3 Recurrent rejection up to 12 months post‐therapy.
3.4. Analysis.
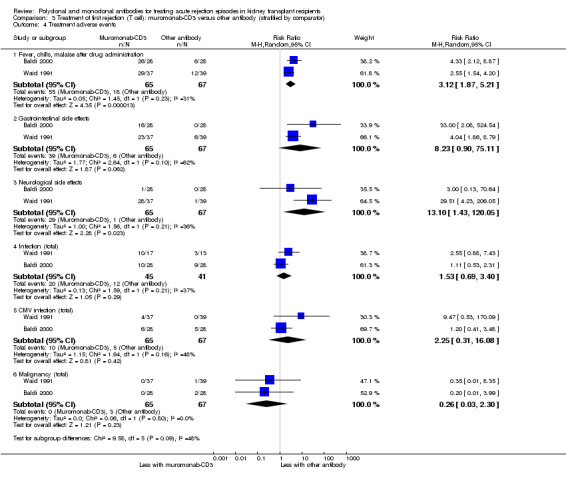
Comparison 3 Treatment of first rejection (T cell): muromonab‐CD3 versus other antibody (stratified by comparator), Outcome 4 Treatment adverse events.
Muromonab‐CD3 treated patients suffered three times more than those receiving either ATG or T10B9, from a syndrome of fever, chills and malaise following drug administration (Analysis 3.4.1 (2 studies, 132 participants): RR 3.12, 95% CI 1.87 to 5.21; I2 = 31%), and experienced more neurological side effects (Analysis 3.4.3 (2 studies, 132 participants): RR 13.10 95% CI 1.43 to 120.05; I2 = 36%) (low certainty evidence).
See Table 3.
Other comparisons
Three other RCTs compared three other different intervention algorithms using antibody in the treatment of first cellular rejection episodes. Where rabbit‐derived ATG was compared to horse‐derived ATG or where ALG was compared to IVIg, there were little or no differences in any outcomes assessed, with the exception for immediate treatment side effects of fevers, chills, and malaise with less with rabbit‐derived ATG than horse‐derived ATG (Table 9: RR 0.38, 95% CI 0.27 to 0.54). When ATG was compared to ALG, no difference was found in outcome assessed any of the outcomes assessed (Table 9).
4. Additional data and analysis (first rejection).
| Outcomes |
Comparisons Relative effect (95% CI) |
||
|
rabbit‐ATG versus horse‐ATG (1 study, 159 participants) |
ATG versus ALG (1 study, 50 participants) |
ALG versus IVIg (1 study, 45 participants) |
|
| Failure of reversal of acute rejection | RR 0.88 (0.41 to 1.87) | RR 0.95 (0.28 to 3.27) | RR 2.40 (0.27 to 21.35) |
| Recurrent rejection post‐therapy | RR 1.24 (0.77 to 1.99) | RR: 0.95 (0.48 to 1.87) | RR 0.62 (0.28 to 1.38) |
| Graft loss or death with a functioning graft (≤ 12 months) | RR 0.73 (0.37 to 1.44) | RR 1.09 (0.60 to 1.99) | RR 1.00 (0.49 to 2.05) |
| Graft loss censored for death (≤ 12 months) | Not reported | RR 0.89 (0.41 to 1.93) | RR 0.93 (0.37 to 2.34) |
| Death (≤ 12 months) | Not reported | RR 2.00 (0.40 to 9.95) | RR 1.20 (0.22 to 6.50) |
| Malignancy (total) | Not reported | Not reported | RR 2.42 (0.10 to 56.46) |
| Treatment side effects: fevers, chills, malaise following administration | RR 0.38 (0.27 to 0.54) | RR 0.75 (0.19 to 3.01) | Not reported |
| Treatment side effects: thrombocytopenia | Not reported | RR 1.00 (0.07 to 15.12) | Not reported |
ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; CI ‐ confidence interval; IVIg ‐ intravenous immunoglobulin; RR ‐ risk ratio
Antibody therapy for the first humoral rejection episode
Rituximab plus steroids versus steroids alone
Two studies compared rituximab plus steroids against steroids alone, however was no evidence of difference when adding rituximab in terms of reversal of initial episode of humoral rejection (Analysis 4.1 (2 studies, 53 participants): RR 0.94, 95% CI 0.54 to 1.64; I2 = 0%), additional treatment to achieve reversal (Analysis 4.2 (1 study, 20 participants): RR 1.33, 95% CI 0.40 to 4.49), graft loss including death with a functioning graft (Analysis 4.3 (2 studies, 58 participants): RR 1.00, 95% CI 0.23 to 4.35; I2 = 0%), and death within a year (Analysis 4.4). Rituximab plus steroids probably increases the risk of urinary tract infection/pyelonephritis (Analysis 4.5.3 (1 study, 38 participants): RR 5.73, 95% CI 1.80 to 18.21).
4.1. Analysis.
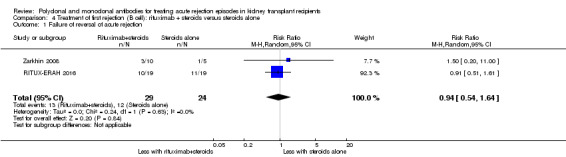
Comparison 4 Treatment of first rejection (B cell): rituximab + steroids versus steroids alone, Outcome 1 Failure of reversal of acute rejection.
4.2. Analysis.
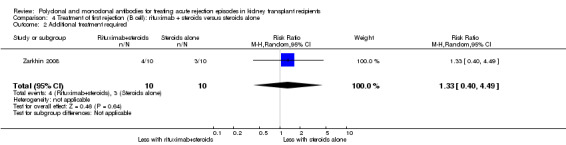
Comparison 4 Treatment of first rejection (B cell): rituximab + steroids versus steroids alone, Outcome 2 Additional treatment required.
4.3. Analysis.
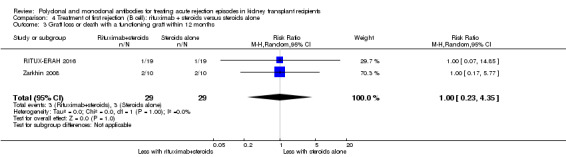
Comparison 4 Treatment of first rejection (B cell): rituximab + steroids versus steroids alone, Outcome 3 Graft loss or death with a functioning graft within 12 months.
4.4. Analysis.
Comparison 4 Treatment of first rejection (B cell): rituximab + steroids versus steroids alone, Outcome 4 Death within 12 months.
4.5. Analysis.
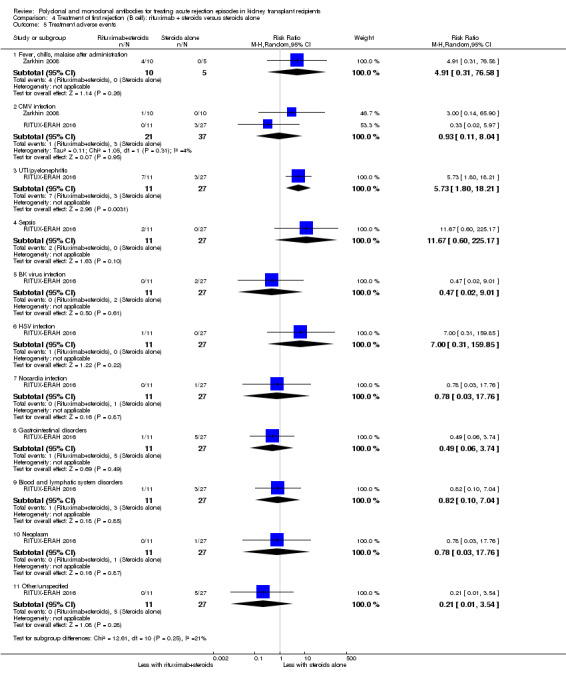
Comparison 4 Treatment of first rejection (B cell): rituximab + steroids versus steroids alone, Outcome 5 Treatment adverse events.
See Table 4.
Antibody therapy for steroid‐resistant rejection
Muromonab‐CD3 (OKT3) versus ATG or ALG
Muromonab‐CD3 may make little or no difference to reversing resistant rejection (Analysis 5.1 (5 studies, 244 participants): RR 1.07, 95% CI 0.63 to 1.81; I2 = 8%), requiring additional treatment achieve reversal (Analysis 5.2 (1 study, 11 participants): RR 1.16, 95% CI 0.40, 3.35), preventing subsequent rejection (Analysis 5.3 (5 studies, 284 participants): RR 0.78, 95% CI 0.47 to 1.28; I2 = 45%), or preventing graft loss (Analysis 5.4 censored for death (5 studies, 244 participants): RR 0.86, 95% CI 0.34 to 2.17; I2 = 42%) (Analysis 5.5 including death with a functioning graft (5 studies, 211 participants): RR 0.81, 95% CI 0.43 to 1.51; I2 = 15%) when compared to ALG or ATG (low certainty evidence). Similarly, there was probably little or no difference in death (Analysis 5.6 (3 studies, 175 participants): RR 0.39, 95% CI; I2 = 0%) or mean serum creatinine (Analysis 5.9 (4 studies, 179 participants): 5.93 µmol/L, 95% CI ‐18.46 to 30.32; I2 = 0%) at 12 months.
5.1. Analysis.
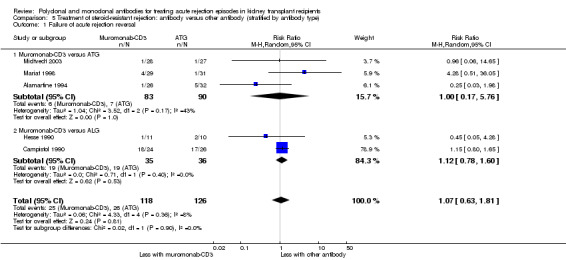
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 1 Failure of acute rejection reversal.
5.2. Analysis.
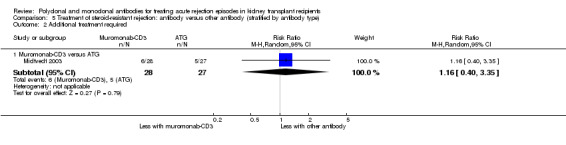
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 2 Additional treatment required.
5.3. Analysis.
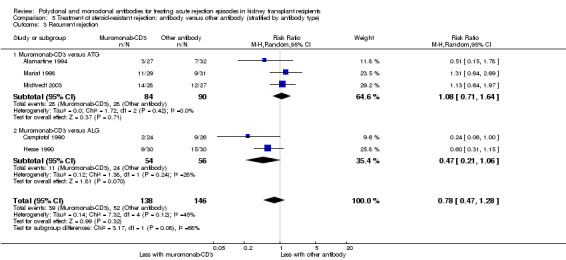
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 3 Recurrent rejection.
5.4. Analysis.
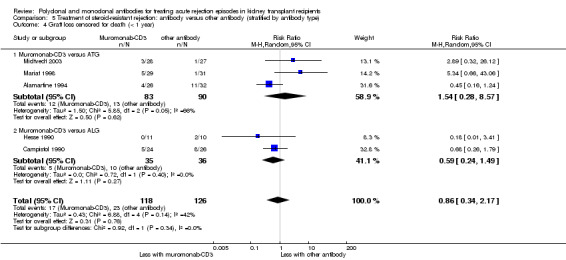
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 4 Graft loss censored for death (< 1 year).
5.5. Analysis.
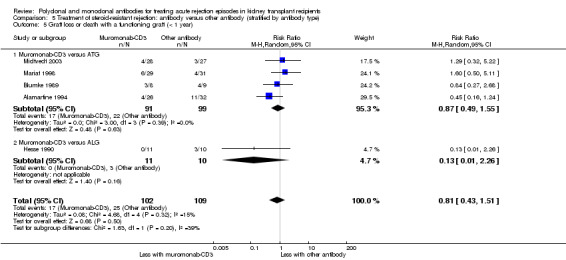
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 5 Graft loss or death with a functioning graft (< 1 year).
5.6. Analysis.
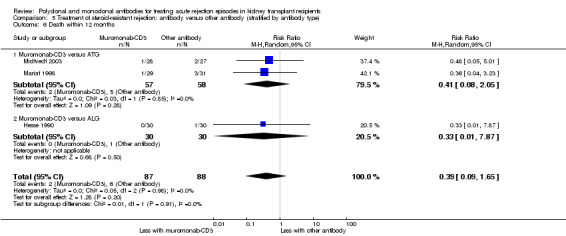
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 6 Death within 12 months.
5.9. Analysis.

Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 9 Serum creatinine at 12 months.
Patients taking muromonab‐CD3 were just as likely to experience a syndrome of fever, chills and malaise following drug administration (Analysis 5.7.1 (3 studies, 140 participants): RR 2.54, 95% CI 0.18 to 34.92; I2 = 93%), fungal infection (Analysis 5.7.4 (1 study, 50 participants): RR 7.56, 95% CI 0.41 to 139.17; I2 = 0%), CMV infection (Analysis 5.7.5 (5 studies, 284 participants): RR 0.93, 95% CI 0.60 to 1.43; I2 = 40%), and malignancy (Analysis 5.7.6 (2 studies, 115 participants): RR 2.09, 95% CI 0.28 to 15.66; I2 = 0%) as those treated with either ATG or ALG (low certainty evidence).
5.7. Analysis.
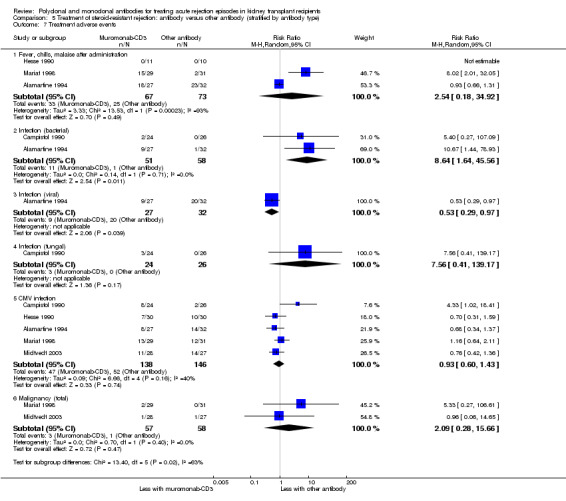
Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 7 Treatment adverse events.
It is uncertain if muromonab‐CD3 leads to more bacterial infection than either ATG or ALG because the certainty of this evidence is very low (Analysis 5.7.2 (2 studies, 109 participants): RR 8.64, 95% CI 1.64 to 45.56; I2 = 0%). Muromonab‐CD3 may slightly reduce viral infections (Analysis 5.7.3: (1 study, 59 participants): RR 0.53, 95% CI 0.29 to 0.97).
See Table 5.
Other comparisons
There were five additional studies each comparing unique paired interventions for treatment of steroid‐resistant rejection (Table 10). When rabbit‐derived ATG (thymoglobulin) was compared to horse‐derived ATG (ATGAM), rabbit‐derived ATG probably prevented recurrent rejection (RR 0.32 95% CI 0.15 to 0.66) compared to horse‐derived ATG. There was probably little or no difference for failure to reverse rejection, deaths, infections, or malignancy. Rabbit‐derived ATG probably slightly reduces graft loss compared to horse‐derived ATG (censored for death: RR 0.46, 95% CI 0.21 to 1.00).
5. Additional data and analysis (steroid‐resistant rejection).
| Outcome |
Comparisons Relative effect (95% CI) |
||||
|
rabbit‐ATG versus horse‐ATG (1 study, 163 participants) |
ATG 3 days versus ALG 10 days (1 study, 30 participants) |
Muromonab‐CD3half dose versus standard dose (1 study, 45 participants) |
Muromonab‐CD3versus IVIg (1 study, 30 participants) |
Muromonab‐CD3versus DSP (1 study, 25 participants) |
|
| Failure of reversal of acute rejection | RR 0.52 (0.26 to 1.05) | RR 0.88 (0.43 to 1.80) | RR 1.50 (0.29 to 7.73) | RR 0.50 (0.11 to 2.33) | RR 0.92 (0.35, 2.41) |
| Further treatment required | Not reported | RR 9.60 (0.56 to 163.58) | Not reported | Not reported | Not reported |
| Recurrent rejection post‐therapy | RR 0.32 (0.15 to 0.66) | Not reported | RR 0.50 (0.05 to 4.94) | RR 1.65 (0.80 to 3.41) | RR 1.48 (0.67 to 3.27) |
| Graft loss or death with a functioning graft (≤ 12 months) | RR 0.68 (0.37 to 1.26) | RR 0.86 (0.38 to 1.95) | RR 2.00 (0.43 to 9.32) | RR 1.00 (0.24 to 4.18) | Not reported |
| Graft loss censored for death (≤ 12 months) | RR 0.46 (0.21 to 1.00) | Not reported | RR 1.00 (0.16 to 6.20) | RR 2.00 (0.20 to 19.78) | Not reported |
| Death (≤ 12‐24 months) | RR 1.98 (0.51 to 7.63) | Not reported | RR 5.00 (0.26 to 96.13) | RR 0.50 (0.05 to 4.94) | Not reported |
| Treatment side effects: fevers, chills, malaise following administration | Not reported | Not reported | Not reported | RR 31.00 (2.02 to 475.12) | RR 5.54 (1.55 to 19.82) |
| Treatment side effects: leukopenia | RR 1.93 (1.32 to 2.84) | Not reported | Not reported | Not reported | RR 0.10 (0.02 to 0.69) |
| Treatment side effects: anorexia | Not reported | Not reported | Not reported | Not reported | RR 0.92 (0.15 to 5.56) |
| Treatment failure | RR 0.51 (0.25 to 1.04) | Not reported | Not reported | Not reported | Not reported |
| Infection (total) | RR 0.99 (0.73 to 1.34) | Not reported | Not reported | Not reported | Not reported |
| Infection (bacterial) | RR 0.79 (0.51 to 1.23) | Not reported | RR 3.00 (0.13 to 68.26) | Not reported | Not reported |
| Infection (viral) | RR 1.87 (0.88 to 3.94) | Not reported | Not reported | Not reported | Not reported |
| Infection (fungal) | RR 0.99 (0.36 to 2.69) | Not reported | Not reported | Not reported | Not reported |
| CMV infection (total) | RR 1.01 (0.86 to 1.18) | Not reported | RR 1.00 (0.51 to 1.95) | Not reported | Not reported |
| Malignancy (total) | RR 0.99 (0.21 to 4.75) | Not reported | Not reported | Not reported | Not reported |
| PTLD/Lymphoma | RR 1.48 (0.25 to 8.64) | Not reported | Not reported | Not reported | Not reported |
| SCr | Not reported | Not reported | MD ‐10.00 (‐60.15 to 40.15) (18 months after treatment) |
MD 0.47 (‐0.07 to 1.01) (3 months after treatment) |
MD 62.00 (‐107.08 to 231.08) (1 month after treatment) |
ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; CI ‐ confidence interval; CMV ‐ cytomegalovirus; DSP ‐ 15‐deoxyspergualin; IVIg ‐ intravenous immunoglobulin; MD ‐ mean difference; PTLD ‐ post‐transplant lymphoproliferative disease; RR ‐ risk ratio; SCr ‐ serum creatinine
When a 3‐day course of ATG was compared to a 10‐day course, there was little or difference in reversal of rejection, graft loss, or any further treatment required for reversal of rejection (Table 10).
When muromonab‐CD3 was compared at standard and half dose there was little or no differences in any of the outcomes measured (Table 10). When compared to IVIg or DSP, muromonab‐CD3 probably leads to more side effects post administration with respect to fever, chills, and malaise in both cases (versus IVIg: RR 31.00, 95% CI 2.02 to 475.12; versus DSP: RR 5.54, 95% CI 1.55 to 19.82). There were probably less leukopenia with muronomab‐CD3 than DSP (RR 0.10, 95% CI 0.02 to 0.69).
Discussion
Summary of main results
In kidney transplant recipients on dual baseline immunosuppressive therapy with either AZA and steroids or CsA and steroids, antibody therapy is 50% more effective at reversing a first acute cellular rejection episode, and 20% more effective at preventing graft loss than further steroid treatment, but significant benefit in patient survival has not been demonstrated.
In kidney transplant recipients on triple baseline immunosuppression with CsA, AZA and steroids, experiencing acute rejection resistant to further steroid treatment, there is no evidence that the effects of muromonab‐CD3 and ATG or ALG are different in reversal or recurrence of acute rejection, or patient or graft survival.
Patients treated with an antibody for acute cellular rejection were 23 times more likely to experience an immediate reaction of fever, chills and malaise than those receiving steroid, and muromonab‐CD3 treated patients were three times more likely to experience this reaction than those treated with other antibodies for the treatment of first cellular rejection episode. Other adverse effects of antibody therapy for treatment of acute cellular rejection were inconsistently reported and could not be easily summarised because of sparsely reported data.
In treating acute humoral rejection, there was no evidence that the use of rituximab improved graft or patient survival at one year and there were more adverse events, although these were not statistically different adverse events.
Overall completeness and applicability of evidence
This systematic review was undertaken with widely inclusive criteria, in order to highlight and summarise the totality of RCT evidence available. This approach led to identification of 31 studies involving 1680 participants, including unpublished and non‐English language data sources. This enhances the external and internal validity of our review, as confining a systematic review and meta‐analysis to published or English language data alone has been demonstrated to over‐estimate positive treatment effects (Egger 2001).
We did not identify any studies investigating antibody therapy for the treatment of acute cellular rejection where contemporary immunosuppressive agents such as tacrolimus, mycophenolate or sirolimus were employed which may impact upon the applicability of this evidence to modern day practice. One ongoing study investigating the use of Rituximab for treatment of acute cellular tubule‐interstitial rejection with B‐cell infiltrates will include data from patients receiving modern baseline immunosuppressive regimen (RIACT Study 2012).
In studies investigating the use of antibody therapy for treatment of acute humoral rejection, patients received contemporary immunosuppressive agents such as tacrolimus and mycophenolate which makes this evidence applicable to current practice (RITUX‐ERAH 2016; Zarkhin 2008). However, data regarding treatment of acute humoral rejection remain sparse and further collaborative randomised studies are required.
Quality of the evidence
The reporting of key components for evaluating of the validity of RCTs was not comprehensive and not in line with current standards of reporting. In many cases this reflected design features which are sub‐optimal such as inadequate or unclear sequence generation (81%), inadequate or unclear allocation concealment (87%), inadequate blinding of either personnel (participants or study personnel) or outcome assessors (25%), incomplete outcome data, selective reporting and bias from other sources. These features are associated with substantial bias in favour of the investigational intervention (Peduzzi 1993; Sackett 1979). Many clinically relevant outcomes were not reported at all or only within a very limited time frame; in particular, it is uncertain whether these agents improve graft survival beyond one year. Additionally, the definitions and criteria used to define rejection, steroid‐resistant rejection, and other outcomes were not always reported, were not provided in sufficient detail to be reproducible, and when reported were not uniform across studies. Unfortunately these inconsistencies are not limited to studies on this topic, or to the field of transplantation, but are widely recognised by other investigators across diverse medical fields (Chan 2005; Hollis 1999; Loke 2001).
This review is limited by the quantity and quality of existing published studies, so residual uncertainty about the true effects of these compounds remains.
Potential biases in the review process
The relatively low number of small studies published in this area means that there is considerable imprecision around all estimates of effect. For example, our data suggest that antibody therapy for acute rejection may prevent further recurrent rejection episodes by around 25% compared to steroids, a clinically important difference, but the width of the 95% CI are consistent with a 46% reduction or a 0% reduction. We have insufficient data to conclude with reasonable certainty that antibody treatment for acute cellular rejection prevents further rejection, but this possibility is suggested by our data. Imprecision is a particular problem with estimating the harms of the interventions.
Reporting of potential harms of treatment was very limited and inconsistently expressed, so the potential of meta‐analysis to increase both power and precision through combining study results to expose significant differences in harmful effects occurring at low frequency in individual studies was not realised. More than half the studies did not report treatment side effects, or other adverse events such as infection or malignancy. It should be recognised that absence of evidence does not equate to evidence of absence of effect, and we recognise that at present, with such scant study data, these outcomes may be better informed by available registry data. The value of increasing available evidence of potential harms associated with interventions (compared with potential benefits alone) has been widely recognised and is also not a problem peculiar to this review, but is common to many RCTs (Cuervo 2003; Tunis 2003)
Agreements and disagreements with other studies or reviews
There have been no other systematic reviews of RCTs of antibody therapy in treating acute cellular rejection in kidney recipients, although systematic reviews of antibodies used as induction immunosuppressive therapy, at the time of transplantation, with the aim of rejection prophylaxis have been undertaken (Szczech 1997; Szczech 1998; Webster 2004).
A previous systematic review of the treatment of acute humoral rejection included data from five RCTs and seven non‐RCTs. This review reported benefit from rituximab or bortezomib in the treatment of acute humoral rejection in four small non‐RCTs (10 to 26 patients). (Roberts 2012). However, in this review of RCTs there was no evidence of benefit from treatment with rituximab in treating acute humoral rejection. There been no other systematic reviews to date of RCTs of antibody therapy in treating acute humoral rejection
Authors' conclusions
Implications for practice.
In treatment of acute cellular rejection, especially where steroids have already failed, clinicians are faced with the option of using antibody therapy. There is no evidence from the pooled world literature of RCTs that muromonab‐CD3, ATG or ALG differ in beneficial or harmful effects over a period of 12 months, with limited data beyond that time frame. The most widely available agents in the current era are ATG formulations.
In treatment of acute humoral rejection, there is no evidence that the use of rituximab improved graft or patient survival at one year.
The majority of studies of first acute cellular rejection following kidney transplantation were published 10 to 30 years ago and used dual baseline immunosuppression that is now used very infrequently. All of the seven studies investigating the treatment of resistant rejection used triple baseline immunosuppression with CsA, AZA and steroids and this combination is no longer standard therapy in many countries. In 2012 in the USA, 92% of new transplant patients received tacrolimus, less than 10% CsA and fewer than 2% AZA. The CsA/AZA combination was used in only 3% in Australia and is not recommended in the UK (ANZDATA 2004; NICE 2004; USRDS 2014).
Whether the effects of antibody therapy are different when used with baseline immunosuppression that differs from that of the studies we identified cannot be answered with current evidence, so the results of our analysis may or may not be generalisable to the contemporary clinical practice of many countries.
Patients receiving antibody therapy may be at more at risk of infection and malignancy.
A recent review of adverse effects following use of monoclonal antibody treatment in kidney transplantation highlighted the increased risk of infection during induction therapy and in patients treated for some cancers (Zaza 2014). However, there was sparse evidence regarding the risk of infection and malignancy in the studies included in our review.
Throughout our analysis there are studies that reported non‐significant differences following the various interventions. However, it was unclear as to whether the studies included were adequately powered for such an endpoint. Therefore, we cannot be confident that the negative results are true negatives
This is a particularly important consideration in the modern era, where baseline immunosuppression is more potent and patients may receive one or more kidney transplants in their lifetime. Therefore, the relative risk of adding antibody therapy to treat acute cellular and humoral rejection needs to be carefully weighed against the benefits for a kidney transplant and for the patient. This may be particularly the case with newer antibody agents where profound and prolonged immunosuppression occurs and antibody therapies may be used concurrently or sequentially.
Implications for research.
Our goal was to summarise the evidence for the use of antibody therapy in the treatment of acute cellular and humoral rejection in kidney transplant recipients. Our meta‐analysis cannot answer the question of how best to treat rejection, but our systematic review does clearly establish and detail the entirety of study evidence that is available and has demonstrated that there is little evidence on which to base clinical decision making, and in treating acute cellular rejection, no evidence for antibody use in patients treated with tacrolimus, mycophenolate or sirolimus. To our knowledge, no peer‐reviewed journal has published data from any RCT of any intervention for the treatment of acute cellular rejection in kidney recipients for a number of years.
There is also sparse evidence to base clinical decision making in treating acute humoral rejection, although studies did include patients receiving tacrolimus and mycophenolate as baseline immunosuppression.
As the preparations for the treatment of acute cellular rejection are not new, there is no economic drive from the pharmaceutical industry to encourage and back new studies. A definitive answer will not arise until studies ask the question. To increase both the amount and the quality of evidence available from RCTs in this area, the drive must come from researchers.
There have, however, been numerous studies of newer immunosuppressive agents in acute humoral rejection and in primary, induction and maintenance therapy regimens designed with diverse primary outcomes.
Future studies investigating different antibody therapies for treatment of acute cellular and humoral rejection, or antibody therapy versus switch in baseline immunosuppression would inform clinical care, but must clearly define outcomes and adequately report harms of treatment to improve on current knowledge and allow more informative cross‐trial comparisons. In particular, the potential of antibody therapy to prevent graft loss compared with steroids alone to treat acute cellular rejection needs to be confirmed, as does the role of newer antibodies in the treatment of humoral rejection.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2017 | New citation required and conclusions have changed | New studies and interventions included |
| 15 June 2017 | New search has been performed | New search and authors |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 25 November 2014 | Amended | Search strategies updated |
| 14 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to acknowledge the help and support of all members of Cochrane Kidney and Transplant. They would also wish to thank Dr JC Craig, Dr T Pankhurst, Ms F Rinaldi, who were authors on the first publication of this review, but have not contributed to this update. We also wish to thank Dr N Webb, Dr J Mahan, and Ms L Orton, who contributed to advice and comment at the initial protocol development stage of the review. The authors also wishes to thank all report authors who responded to our enquiries about their work and those who provided further information about their studies, particularly Drs Midtvedt, Almartine, Howard and Birkeland.
This review was published in Transplantation (Webster 2006b).
Appendices
Appendix 1. Electronic search strategies
| Databases | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Treatment of first rejection (T cell): antibody versus steroids (stratified by antibody type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of reversal of acute rejection | 6 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.82] |
| 1.1 Muromonab‐CD3 versus steroid | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.63] |
| 1.2 ATG versus steroid | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.74] |
| 1.3 ALG versus steroid | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.75] |
| 2 Additional treatment needed | 4 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.15] |
| 2.1 ATG versus steroid | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.49, 1.30] |
| 2.2 ALG versus steroid | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.49] |
| 3 Recurrent rejection up to 12 months post‐therapy | 9 | 508 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.00] |
| 3.1 Muromonab‐CD3 versus steroid | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.15] |
| 3.2 ATG versus steroid | 5 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.05] |
| 3.3 ALG versus steroid | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.28] |
| 4 Graft loss or death with a functioning graft within 12 months | 8 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.58, 1.22] |
| 4.1 Muromonab‐CD3 versus steroid | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.02, 1.81] |
| 4.2 ATG versus steroid | 5 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.48, 0.89] |
| 4.3 ALG versus steroid | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.53, 1.50] |
| 5 Graft loss censored for death within 18 months | 8 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.57, 1.12] |
| 5.1 Muromonab‐CD3 versus steroid | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.94, 1.94] |
| 5.2 ATG versus steroid | 4 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.89] |
| 5.3 ALG versus steroid | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.33] |
| 6 Death within 12 months | 7 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.51, 1.88] |
| 6.1 Muromonab‐CD3 versus steroid | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.53, 3.70] |
| 6.2 ATG versus steroid | 3 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.74] |
| 6.3 ALG versus steroid | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.31, 3.60] |
| 7 Treatment adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Fever, chill, malaise after drug administration | 4 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 23.88 [5.10, 111.86] |
| 7.2 Infection (total) | 5 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.20] |
| 7.3 CMV infection | 4 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.26] |
| 7.4 Avascular necrosis | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.11, 2.35] |
| 8 Serum creatinine post treatment (3 months) | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐14.0 [‐37.53, 9.53] |
Comparison 2. Treatment of first rejection (T cell): antibody + steroids versus steroids alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of reversal of acute rejection (AR) episode | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 ALG + steroids versus steroids alone | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recurrent rejection within 3 months post‐therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 ALG + steroids versus steroids alone | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Graft loss or death with a functioning graft within 12 months | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 5.14] |
| 3.1 ALG + steroids versus steroids alone | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.24, 4.23] |
| 3.2 ATG + steroids versus steroids alone | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.21] |
| 4 Graft loss censored for death within 12 months | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 4.16] |
| 4.1 ALG + steroids versus steroids alone | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.23, 3.19] |
| 4.2 ATG + steroids versus steroids alone | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.21] |
| 5 Death within 12 months | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 5.1 ALG + steroids versus steroids alone | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 5.2 ATG + steroids versus steroids alone | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Treatment of first rejection (T cell): muromonab‐CD3 versus other antibody (stratified by comparator).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of acute rejection reversal | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.92, 3.67] |
| 1.1 Muromonab‐CD3 versus ATG | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.95, 4.20] |
| 1.2 Muromonab‐CD3 versus T10B9.1A‐31 | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 7.10] |
| 2 Additional treatment needed | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.77, 3.63] |
| 2.1 Muromonab‐CD3 versus ATG | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.79, 4.27] |
| 2.2 Muromonab‐CD3 versus T10B9.1A‐31 | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 7.10] |
| 3 Recurrent rejection up to 12 months post‐therapy | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.59, 1.88] |
| 3.1 Muromonab‐CD3 versus ATG | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.61, 2.56] |
| 3.2 Muromonab‐CD3 versus T10B9.1A‐31 | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.06] |
| 4 Treatment adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fever, chills, malaise after drug administration | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.87, 5.21] |
| 4.2 Gastrointestinal side effects | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 8.23 [0.90, 75.11] |
| 4.3 Neurological side effects | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 13.10 [1.43, 120.05] |
| 4.4 Infection (total) | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.69, 3.40] |
| 4.5 CMV infection (total) | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.31, 16.08] |
| 4.6 Malignancy (total) | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.30] |
Comparison 4. Treatment of first rejection (B cell): rituximab + steroids versus steroids alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of reversal of acute rejection | 2 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.54, 1.64] |
| 2 Additional treatment required | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.40, 4.49] |
| 3 Graft loss or death with a functioning graft within 12 months | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.23, 4.35] |
| 4 Death within 12 months | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Treatment adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fever, chills, malaise after administration | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 4.91 [0.31, 76.58] |
| 5.2 CMV infection | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.11, 8.04] |
| 5.3 UTI/pyelonephritis | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 5.73 [1.80, 18.21] |
| 5.4 Sepsis | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 11.67 [0.60, 225.17] |
| 5.5 BK virus infection | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.02, 9.01] |
| 5.6 HSV infection | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.31, 159.85] |
| 5.7 Nocardia infection | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.03, 17.76] |
| 5.8 Gastrointestinal disorders | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.06, 3.74] |
| 5.9 Blood and lymphatic system disorders | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.10, 7.04] |
| 5.10 Neoplasm | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.03, 17.76] |
| 5.11 Other/unspecified | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 3.54] |
Comparison 5. Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of acute rejection reversal | 5 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.81] |
| 1.1 Muromonab‐CD3 versus ATG | 3 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.17, 5.76] |
| 1.2 Muromonab‐CD3 versus ALG | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.78, 1.60] |
| 2 Additional treatment required | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Muromonab‐CD3 versus ATG | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.40, 3.35] |
| 3 Recurrent rejection | 5 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.28] |
| 3.1 Muromonab‐CD3 versus ATG | 3 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.71, 1.64] |
| 3.2 Muromonab‐CD3 versus ALG | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.06] |
| 4 Graft loss censored for death (< 1 year) | 5 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.17] |
| 4.1 Muromonab‐CD3 versus ATG | 3 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.28, 8.57] |
| 4.2 Muromonab‐CD3 versus ALG | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.24, 1.49] |
| 5 Graft loss or death with a functioning graft (< 1 year) | 5 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.51] |
| 5.1 Muromonab‐CD3 versus ATG | 4 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.55] |
| 5.2 Muromonab‐CD3 versus ALG | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.26] |
| 6 Death within 12 months | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.09, 1.65] |
| 6.1 Muromonab‐CD3 versus ATG | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.05] |
| 6.2 Muromonab‐CD3 versus ALG | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 7 Treatment adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Fever, chills, malaise after administration | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.18, 34.92] |
| 7.2 Infection (bacterial) | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 8.64 [1.64, 45.56] |
| 7.3 Infection (viral) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 7.4 Infection (fungal) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 7.56 [0.41, 139.17] |
| 7.5 CMV infection | 5 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.43] |
| 7.6 Malignancy (total) | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.28, 15.66] |
| 8 Serum creatinine post treatment (3 days) | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐0.25, 3.25] |
| 9 Serum creatinine at 12 months | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Muromonab‐CD3 versus ATG | 4 | 179 | Mean Difference (IV, Random, 95% CI) | 5.93 [‐18.46, 30.32] |
5.8. Analysis.

Comparison 5 Treatment of steroid‐resistant rejection: antibody versus other antibody (stratified by antibody type), Outcome 8 Serum creatinine post treatment (3 days).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alamartine 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Baldi 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "all kidney recipients...were randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Muromonab‐CD3 group received antihistamine 1 h prior to muromonab‐CD3 administration, outcome measurement (treatment side effects) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics except for higher proportion of immunized patients in the ATG group |
Birkeland 1975.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated randomly"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 randomised but excluded without ITT; however, only 10% people missing and balanced between groups, thus unlikely to affect outcome |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) and treatment adverse effects |
| Other bias | Unclear risk | The study was supported by grants from King Christian X Fund, Ingemann O. Buck's Fund, P. Carl Petersen's Fund, Engineer Soren Alfred Andersen's Fund, C. C. Klestrup and Wife's Legacy and State Medical Research Fund; similar baseline characteristics |
Blumke 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | High risk | Alternate allocation to treatment group |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | High risk | SD not reported for SCr and could not be meta‐analysed |
| Other bias | Unclear risk | Funding source not reported |
Broyer 1987a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | High risk | Funded by Merieux Institute; similar baseline characteristics |
Campistol 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized trial"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Casadei 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data at follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Filo 1980.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Card system of randomisation with allocation concealment |
| Allocation concealment (selection bias) | Low risk | Card system of randomisation with allocation concealment Quote: "primary care physicians had no control over the treatment group assigned to any given patient." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) or treatment adverse effects |
| Other bias | High risk | Funded by Upjohn ATGAM Company; similar baseline characteristics |
Gaber 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised using a centralised procedure...unique randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "each centre having a unique randomization code " Enrolments were stratified and randomised centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; only pharmacist at each centre was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | High risk | Funded by The Hardardt Group, Parssipanny, NJ, SangStat Medical Corp; similar baseline characteristics |
Glass 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Assigning ...patients consecutively in order of their admission to the hospital" Quote: "recipients over the age of 50, all of them were assigned to group B" Consecutive allocation to various groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but the outcome measurements are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Goldstein 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence at each centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Muromonab‐CD3 group received skin prick test before administration; drug regimen different between groups; outcome measurement (treatment side effects) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 patients lost to follow up and 1 patient excluded without reason |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) |
| Other bias | High risk | Funded by Ortho Pharmaceutical corporation as part of pilot trial; similar baseline characteristics |
Hesse 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized", method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported |
Hilbrands 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized trial"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Hoitsma 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "minimization method of Taves" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different drug administration regimes rabbit‐ATG + steroid vs steroid alone ‐ no blinding used Outcome measurement (treatment side effects) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication to suggest otherwise |
| Other bias | Low risk | Funded by Main Group for Health Research TNO Grant; similar baseline characteristics |
Hourmant 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was reported as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding source not reported |
Howard 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" but no mention of blinding methodology. However, outcome measurement is unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 patients excluded from analysis without ITT |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) and treatment adverse effect |
| Other bias | Low risk | Funded by US Public Health Services Grant |
Johnson 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different drug regimens and cross over was made possible Possible variation in maintenance immunosuppressant protocols Outcome measurement (treatment side effects) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent loss of data to follow up, however, final outcomes reported in percentages instead of actual numbers |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Mariat 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Different dose regimens. Antibody vials available in different quantities: OKT3 in 5ml vials and ALG in 25 ml. Outcome measurements (treatment side effects) did not have sufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics except cold ischaemia time which was significantly different |
Midtvedt 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Similar drug regimens; no mention made of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 patients lost to follow‐up due to graft loss excluded from analysis without ITT |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) and treatment adverse effect |
| Other bias | Unclear risk | Funding source not reported |
Midtvedt 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, prospective single centre"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug regimens are similar for priming and duration; no blinding mentioned, but outcome measurements are unlikely to be influenced lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all patients were followed from the day of inclusion until the end of 2000" |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Okubo 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternative allocation to treatment groups |
| Allocation concealment (selection bias) | High risk | Alternative allocation to treatment groups |
| Blinding (performance bias and detection bias) All outcomes | High risk | Drug regimens were of different durations, 10 consecutive days (muromonab‐CD3) vs 5 consecutive days (15‐deoxyspergualin); outcome measurements (treatment side effect) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics except for age, which was significantly different |
Olausson 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomised”; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding used, but outcome is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of study participants explained for, but no ITT analysis conducted. However, only 10% people missing and balanced between groups, thus unlikely to affect outcome |
| Selective reporting (reporting bias) | High risk | Unable to meta‐analyse data |
| Other bias | High risk | Possible cross‐over between groups, difficulty interpreting accuracy of results Funding sources from the Professor L‐E Gelin Memorial Foundation, Fresenius AG, Federal Republic of Germany, Riksforbundet for Njursjuka |
RITUX‐ERAH 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization and allocation concealment were achieved by use of a centralized, computer generated, interactive, Web‐response system managed by the Roche laboratory, which had no role in recruitment. Randomization was stratified by centre, with permutation blocks of variable sizes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization and allocation concealment were achieved by use of a centralized, computer generated, interactive, Web‐response system managed by the Roche laboratory, which had no role in recruitment. Randomization was stratified by centre, with permutation blocks of variable sizes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention made of blinding methodology, also unblinding of a third infusion of rituximab was planned and this occurred in 7/19 patients in placebo group. However, outcome measurements are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention made of blinding methodology, also unblinding of a third infusion of rituximab was planned and this occurred in 7/19 patients in placebo group. However, outcome measurements are unlikely to be influenced |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, no indications to suggest otherwise |
| Other bias | Low risk | Funding sources from the French Ministry of Health and grants from the Roche Laboratory; no conflict of interests were declared from authors |
Shield 1979.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, no indications to suggest otherwise |
| Other bias | High risk | Funded by Upjohn Company and US public Health Services Grant |
Simonian 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Spieker 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug regimens were similar. No mention of blinding, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, no indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Streem 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding mentioned, but outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported, no indications to suggest otherwise |
| Other bias | Unclear risk | Funding source not reported |
Theodorakis 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Open label...trial" But outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not sure of complete follow‐up as results are reported in % not numbers |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics except for minor criteria |
Toledo‐Pereyra 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | Drug regimen similar, but no mention of blinding; outcome measurement (treatment side effect) could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of data on follow‐up |
| Selective reporting (reporting bias) | High risk | No measure of graft function (SCr or GFR) |
| Other bias | Unclear risk | Funding source not reported; similar baseline characteristics |
Waid 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "(medication) was dispensed by the pharmacy according to a randomisation table" |
| Allocation concealment (selection bias) | Low risk | Quote: "(medication) was dispensed by the pharmacy according to a randomisation table"; pharmacy controlled allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind" Quote: " investigators, patients, nurses, and other personnel were all unaware of which mAb was being administered" Quote: "placebo injections...to maintain the blinded status" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons for missing data provided in cytokine expression analysis |
| Selective reporting (reporting bias) | Low risk | No indications suggest otherwise |
| Other bias | Low risk | Funded by National Institutes of Health; cross‐over between groups documented in protocol, reported in outcomes |
Zarkhin 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "open‐label" however, outcome measurements are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | No indications to suggest otherwise |
| Other bias | High risk | Funded by Genentech Inc. and BIOGEN‐IDEC Pharmaceuticals; similar baseline characteristics |
ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; AZA ‐ azathioprine; BP ‐ blood pressure; CsA ‐ cyclosporin; CMV ‐ cytomegalovirus; GFR ‐ glomerular filtration rate; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; ITT ‐ intention‐to‐treat; IV ‐ intravenous; IVIg ‐ intravenous immunoglobulin; M/F ‐ male/female; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; PRED ‐ prednisone/prednisolone; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; TAC ‐ tacrolimus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kulkarni 2016 | Wrong population: patients with chronic rather than acute rejection |
Characteristics of ongoing studies [ordered by study ID]
RIACT Study 2012.
| Trial name or title | Rationale and design of the RIACT‐study: a multi‐center placebo controlled double blind study to test the efficacy of Rituximab in Acute cellular tubulointerstitial rejection with B‐cell infiltrates in renal Transplant patients: study protocol for a randomized controlled trial |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Baseline immunosuppression (both groups)
Co‐interventions
|
| Outcomes |
|
| Starting date | May 2012 |
| Contact information | nephrologie@mh‐hannover.de |
| Notes | Funding source: German government grant (BMBF, Clinical studies Programme) Contact with study authors for additional information: yes |
Differences between protocol and review
Risk of bias assessment has replaced quality assessment checklist.
Contributions of authors
Writing of protocol and review: AW, TP, JRC, JCC, SC, SW
Screening of titles and abstracts: AW, TP, KT, SW, MP, SC
Assessment for inclusion: AW, TP, KT, SW, MP, SC
Quality assessment: AW, TP, KT, MP, SW, SC
Data extraction: AW, TP, KT, MP, SW
Data entry into RevMan: AW, TP, SW, KT
Data analysis: AW, TP, SW, KT
Disagreement resolution: AW, JRC, JCC, SC
Declarations of interest
AW, SW, KT, MP SC: no conflicts to declare.
JRC: has advisory board and clinical trial involvement with Novartis, Roche, Janssen‐Cilag, Fujisawa and Wyeth, and has also been an invited speaker at national and international meetings sponsored by these companies. These activities were unrelated to the production of this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alamartine 1994 {published and unpublished data}
- Alamartine E, Bellakoul R, Berthoux F. Randomized prospective study comparing OKT3 and antithymocyte globulins for treatment of the first acute cellular rejection of kidney allografts. Transplantation Proceedings 1994;26(1):273‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Baldi 2000 {published data only}
- Baldi A, Malaise J, Mourad M, Squifflet JP. A prospective randomized study comparing poly‐ATG to mono‐OKT3 clonal antibodies for the first rejection therapy after kidney transplantation: long‐term results. Transplantation Proceedings 2000;32(2):429‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Birkeland 1975 {published and unpublished data}
- Birkeland SA. A controlled clinical trial of treatment with ALG in established rejection of renal allografts. Acta Medica Scandinavica 1975;198(6):489‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Birkeland SA. The use of antilymphocyte globulin in renal allograft rejection. A controlled study. Postgraduate Medical Journal 1976;52(5 Suppl):82‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Blumke 1989 {published data only}
- Blumke M, Kirste G, Wanner U, Wilms H. Single center randomized trial using ATG v OKT3 treatment in steroid resistant rejection crises after kidney transplantation. Transplantation Proceedings 1989;21(1 Pt 2):1747. [MEDLINE: ] [PubMed] [Google Scholar]
Broyer 1987a {published data only}
- Broyer M, Niaudet P, Bijaoui M, Gagnadoux MF. Treatment of acute rejection crisis by antilymphocyte globulins: a randomized prospective study in pediatric kidney transplantation. Transplantation Proceedings 1987;19(1 Pt 3):1886‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Campistol 1990 {published data only}
- Campistol JM, Oppenheimer F, Vilardell J, Ricart MJ, Andreu J. Randomized trial between OK‐T3 monoclonal antibody and antilymphocyte globulin (ALG) for acute vascular graft rejection (AVGR) [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:514A. [CENTRAL: CN‐00716031]
- Poch E, Oppenheimer F, Darnell A, Campistol JM, Andreu J, Lopez‐Pedret J. A randomized prospective comparison of ALG with OKT3 for treatment of renal vascular rejection [abstract]. Nephrology Dialysis Transplantation 1992;7(7):785. [Google Scholar]
Casadei 1998 {published data only}
- Casadei D, Rial M, Argento J, Goldberg J, Raimondi E. Preliminary results from a randomized and prospective study about immunoglobulin (IVIg) high doses vs. MoAb in the rescue of steroid resistant rejections [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):677. [CENTRAL: CN‐00444696] [Google Scholar]
- Casadei D, Rial M, Argento J, Goldberg J, Raimondi E. Preliminary results from a randomized and prospective study of high‐dose immunoglobulin versus monoclonal antibody in the rescue of steroid‐resistant rejections. Transplantation Proceedings 1998;30(5):2164. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Casadei DH, C Rial M, Opelz G, Golberg JC, Argento JA, Greco G, et al. A randomized and prospective study comparing treatment with high‐dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid‐resistant rejection. Transplantation 2001;71(1):53‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Filo 1980 {published data only}
- Filo RS, Smith EJ, Leapman SB. Reversal of acute renal allograft rejection with adjunctive AG therapy. Transplantation Proceedings 1981;13(1 Pt 1):482‐90. [MEDLINE: ] [PubMed] [Google Scholar]
- Filo RS, Smith EJ, Leapman SB. Therapy of acute cadaveric renal allograft rejection with adjunctive antithymocyte globulin. Transplantation 1980;30(6):445‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaber 1998 {published data only}
- First MR, US Multicenter Thymoglobulin Study Group. Thymoglobulin successfully prevents recurrent rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:259. [CENTRAL: CN‐00509191]
- Gaber AO, First MR, Tesi RJ, Gaston RS, Mendez R, Mulloy LL, et al. Results of the double‐blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus ATGAM in the treatment of acute graft rejection episodes after renal transplantation. Transplantation 1998;66(1):29‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaber AO, US Multicenter Thymoglobulin Study Group. The 1996 double blinded randomized multicenter phase iii clinical trial of thymoglobulin versus ATGAM in the treatment of acute graft rejection following renal transplantation [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:261. [CENTRAL: CN‐00509206]
- Gaber LW, Moore LW, Gaber AO, Tesi RJ, Meyer J, Schroeder TJ. Correlation of histology to clinical rejection reversal: a thymoglobulin multicenter trial report. Kidney International 1999;55(6):2415‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaber LW, US Multicenter Thymoglobulin Study Group. Correlation of post treatment renal allograft biopsies to rejection reversal [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:238. [CENTRAL: CN‐00509207]
- Gaston RS, US Multicenter Thymoglobulin Study Group. A multicenter trial of thymoglobulin vs ATGAM as therapy for acute renal allograft rejection (AR) [abstract no: P1002]. Nephrology 1997;3(Suppl 1):S324. [CENTRAL: CN‐00460800] [Google Scholar]
- Irish WD, Canafax DM, Gaston RS, Thymoglobulin Multicenter Study Group. A multivarate logistic regression analysis of the U.S. multicenter, randomized trial of thymoglobulin (THYMO) versus ATGAM for treatment of acute renal allograft rejection [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):679A. [CENTRAL: CN‐00445859] [Google Scholar]
- Regan JF, Campbell K, Smith L, Le H, Schroeder T, Womble D, et al. Anti‐thymoglobulin IgI and anti‐ATGAM IgG in renal transplant patients undergoing treatment for acute rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:259. [CENTRAL: CN‐00509432]
- Regan JF, Campbell K, Smith L, Schroeder TJ, Womble D, Kano J, et al. Sensitization following Thymoglobulin and ATGAM rejection therapy as determined with a rapid enzyme‐linked immunosorbent assay. US Thymoglobulin Multi‐Center Study Group. Transplant Immunology 1999;7(2):115‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnitzler MA, Woodward RS, Lowell JA, Amir L, Schroeder TJ, Singer GG, et al. Economics of the antithymocyte globulins Thymoglobulin and ATGAM in the treatment of acute renal transplant rejection. Pharmacoeconomics 2000;17(3):287‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnitzler MA, Woodward RS, Lowell JA, Singer GG, Amir L, Horn HR, et al. Costs savings associated with thymoglobulin for treatment of acute renal transplant rejection in patient subsets. Transplantation Proceedings 1999;31(3B Suppl):7S‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnitzler MA, Woodward RS, Lowell JA, Singer GG, Brennan DC. High risk kidney transplant rejection treatment: cost savings from thymoglobulin. Transplantation Proceedings 1999;31(1‐2):269‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schnitzler MA, Woodward RS, Lowell JA, Singer GG, Shenoy S, Howard TK, et al. Economics of thymoglobulin versus ATGAM for induction immunosuppression in renal transplantation [abstract no: 578]. Transplantation 1999;67(7):S151. [CENTRAL: CN‐00402557] [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Moore LW, Gaber LW, Gaber AO, First MR. The US multicenter double‐blind, randomized, phase III trial of thymoglobulin versus ATGAM in the treatment of acute graft rejection episodes following renal transplantation: rationale for study design. Transplantation Proceedings 1999;31(3B Suppl):1S‐6S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tesi RJ, Kano JM, Horn HR, Schroeder T. Thymoglobulin reverses acute renal allograft rejection better than ATGAM‐‐a double‐blinded randomized clinical trial. Transplantation Proceedings 1997;29(7A):21S‐3S. [MEDLINE: ] [PubMed] [Google Scholar]
- Woodle ES, Canafax DM, Irish WD, Thymoglobulin Multicenter Study Group. Thymoglobulin (THYMO) may be more efficacious than ATGAM for reducing recurrent acute renal allograft rejection: results of the US multicenter, double‐blinded, comparative trial [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):703. [CENTRAL: CN‐00448417] [Google Scholar]
- Woodle S, Moore LW, Thymoglobulin Multicenter Study Group. 12 month intent to treat analysis of the double blind, randomized multicenter thymoglobulin vs ATGAM trial for the treatment of acute rejection following renal transplantation [abstract]. Transplantation 1998;65(12):S191. [CENTRAL: CN‐00448418] [Google Scholar]
Glass 1983 {published data only}
- Glass NR, Miller DT, Sollinger HW, Belzer FO. A comparative study of steroids and heterologous antiserum in the treatment of renal allograft rejection. Transplantation Proceedings 1983;15(1):617‐21. [EMBASE: 13086383] [Google Scholar]
Goldstein 1985 {published data only}
- A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. Ortho Multicenter Transplant Study Group. New England Journal of Medicine 1985;313(6):337‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cosimi AB. OKT3: First‐dose safety and success. Nephron 1987;46 Suppl 1:12‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldstein G, Norman DJ, Shield CF 3rd, Kreis H, Burdick J, Flye MW, et al. OKT3 monoclonal antibody reversal of acute renal allograft rejection unresponsive to conventional immunosuppressive treatments. Progress in Clinical & Biological Research 1986;224:239‐49. [MEDLINE: ] [PubMed] [Google Scholar]
Hesse 1990 {published data only}
- Hesse UJ, Wienand P, Baldamus C, Arns W. Preliminary results of a prospectively randomized trial of ALG vs OKT3 for steroid‐resistant rejection after renal transplantation in the early postoperative period. Transplantation Proceedings 1990;22(5):2273‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Hesse UJ, Wienand P, Baldamus C, Pollok M, Pichlmaier H. The risk of infection following OKT3 and antilymphocyte globulin treatment for renal transplant rejection: results of a single center prospectively randomized trial. Transplant International 1992;5 Suppl 1:S440‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stippel DL, Arns W, Pollok M, Beckurts KT, Hesse UJ, Holscher AH. ALG versus OKT3 for treatment of steroid‐resistant rejection in renal transplantation: ten‐year follow‐up results of a randomized trial. Transplantation Proceedings 2002;34(6):2201‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hilbrands 1996 {published data only}
- Hilbrands LB, Hoitsma AJ, Koene RA. Methylprednisolone versus ATG as initial treatment for acute rejections after renal transplantation [abstract]. Nephrology Dialysis Transplantation 1996;11(8):1675. [CENTRAL: CN‐00445721] [Google Scholar]
Hoitsma 1982 {published data only}
- Hoitsma AJ, Reekers P, Kreeftenberg JG, Lier HJ, Capel PJ, Koene RA. Treatment of acute rejection of cadaveric renal allografts with rabbit antithymocyte globulin. Transplantation 1982;33(1):12‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoitsma AJ, Lier HJ, Reekers P, Koene RA. Improved patient and graft survival after treatment of acute rejections of cadaveric renal allografts with rabbit antithymocyte globulin. Transplantation 1985;39(3):274‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hourmant 1985 {published data only}
- Hourmant M, Soulillou JP, Remi JP, Sagniez G, Guenel J. Use of cyclosporin A after antilymphocyte serum in renal transplantation [Utilisation de la cyclosporine A en relais du serum antilymphocytaire en transplantation renale]. Presse Medicale 1985;14(41):2093‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Schneider T, Fagnani F, Lanoe JL, Hourmant M, Soulillou JP. Economic analysis of an immunosuppressive strategy in renal transplantation. Health Policy 1988;9(1):75‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howard 1977 {published and unpublished data}
- Howard RJ, Condie RM, Sutherland DE, Simmons RL, Najarian JS. The use of antilymphoblast globulin in the treatment of renal allograft rejection. Transplantation Proceedings 1981;13(1 Pt 1):473‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Howard RJ, Condie RM, Sutherland DE, Simmons RL, Najarian JS. The use of antilymphoblast globulin in the treatment of renal allograft rejection: a double‐blind, randomized study. Transplantation 1977;24(6):419‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1989 {published data only}
- Johnson K, Niblack G, Richie R, MacDonell R, Nylander W, Walker P, et al. Multicenter comparison of rejection reversal: rabbit anti‐human lymphocyte serum (ATS) versus horse anti‐human lymphocyte globulin (ATGAM). Transplantation Proceedings 1989;21(1 Pt 2):1734‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Johnson K, Niblack G, Vaughn W. The effectiveness of rabbit anti‐thymocyte serum (ATS) in the reversal of acute allograft rejection ‐ a multicenter study [abstract]. Kidney International 1987;31(1):460. [CENTRAL: CN‐00583631] [Google Scholar]
Mariat 1998 {published data only}
- Mariat C, Alamartine E, Diab N, Filippis JP, Laurent B, Berthoux F. A randomized prospective study comparing low‐dose OKT3 to low‐dose ATG for the treatment of acute steroid‐resistant rejection episodes in kidney transplant recipients. Transplant International 1998;11(3):231‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mariat C, Alamartine E, Laurent‐Pilonchery B, Diab N, Filippis JP, Berthoux F. Randomized prospective study comparing low‐dose OKT3 to low‐dose antithymocyte globulins for treatment of the first acute rejection of kidney allografts [abstract]. Nephrology Dialysis Transplantation 1996;11(6):276. [CENTRAL: CN‐00261331] [Google Scholar]
Midtvedt 1996 {published and unpublished data}
- Midtvedt K, Tafjord AB, Hartmann A, Eide TC, Holdaas H, Nordal KP, et al. Half dose of OKT3 is efficient in treatment of steroid‐resistant renal allograft rejection. Transplantation 1996;62(1):38‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Midtvedt K, Tafjord AB, Nordal KP, Draganov B, Eide T, Hartmann A, et al. OKT3, doses of 2.5mg versus 5mg in steroid resistant renal allograft rejections [abstract]. Journal of the American Society of Nephrology 1995;6(3):1106. [CENTRAL: CN‐00485099] [Google Scholar]
Midtvedt 2003 {published and unpublished data}
- Fauchald P, Midtvedt K, Lien B, Hartmann A, Albrechtsen D, Bjerkely BL, et al. Randomized trial of T‐cell monitored administration of ATG vs OKT3 in steroid resistant kidney graft rejection [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415630]
- Midtvedt K, Fauchald P, Lien B, Hartmann A, Albrechtsen D, Bjerkely BL, et al. Individualized T cell monitored administration of ATG versus OKT3 in steroid‐resistant kidney graft rejection. Clinical Transplantation 2003;17(1):69‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Okubo 1993 {published data only}
- Okubo M, Tamura K, Kamata K, Tsukamoto Y, Nakayama Y, Osakabe T, et al. 15‐Deoxyspergualin "rescue therapy" for methylprednisolone‐resistant rejection of renal transplants as compared with anti‐T cell monoclonal antibody (OKT3). Transplantation 1993;55(3):505‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olausson 1995 {published data only}
- Olausson M, Mjornstedt L, Blohme I. Three‐day or ten‐day ATG treatment for steroid‐resistant rejection in kidney transplanted patients. Transplantation Proceedings 1995;27(6):3434‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Olausson M, Mjornstedt L, Brynger H, Blohme I. Steroid‐resistant rejection in kidney‐transplanted patients: is ATG treatment for three or ten days preferable?. Transplant International 1996;9 Suppl 1:S38‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RITUX‐ERAH 2016 {published data only}
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One year results of the effects of rituximab on acute antibody‐mediated rejection in renal transplantation: RITUX ERAH, a multicenter double‐blind randomized placebo‐controlled trial. Transplantation 2016;100(2):391‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One year results of the effects of rituximab on acute humoral rejection in renal transplantation: RITUX ERAH, a multicenter randomized placebo controlled trial [abstract no: 266]. American Journal of Transplantation 2013;13(Suppl S5):112. [EMBASE: 71056842] [DOI] [PubMed] [Google Scholar]
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. RITUX‐ERAH: Multicenter randomized trial of rituximab on acute humoral rejection in transplantation [abstract no:O218]. Transplant International 2013;26(Suppl 2):110. [EMBASE: 71359364] [Google Scholar]
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. RITUX‐ERAH: multicenter randomized trial of rituximab on acute antibody mediated rejection in transplantation [abstract no: O90]. Transplant International 2013;26(Suppl 3):22. [EMBASE: 71356165] [Google Scholar]
Shield 1979 {published data only}
- Shield CF 3rd, Cosimi AB, Tolkoff‐Rubin N, Rubin RH, Herrin J, Russell PS. Use of antithymocyte globulin for reversal of acute allograft rejection. Transplantation 1979;28(6):461‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Simonian 1983 {published data only}
- Simonian S, Lyons P, Chvala R, Swartz C, Onesti G, Bulova S. Reversal of acute cadaveric renal allograft rejection with adjunctive ATG treatment [abstract]. Kidney International 1983;23(1):295. [CENTRAL: CN‐00716036] [Google Scholar]
Spieker 1992 {published data only}
- Barenbrock M, Spieker C, Buchholz B, Heidenreich S, Zidek W, Rahn KH. Cardiovascular effects of the rejection therapy with antibodies against lymphocytes [Kardiovaskulare nebenwirkungen der abstossungsbehandlung mit lymphozytenantikorpern]. Nieren‐und Hochdruckkrankheiten 1994;23(2):84‐7. [EMBASE: 24091066] [Google Scholar]
- Spieker C, Barenbrock M, Buchholz B, Heidenreich S, Zidek W. Cardiovascular effects of ATG and OKT3 in renal allograft recipients. Transplantation Proceedings 1992;24(6):2594‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Streem 1983 {published data only}
- Streem SB, Novick AC, Braun WE, Steinmuller D, Greenstreet R. Low‐dose maintenance prednisone and antilymphoblast globulin for the treatment of acute rejection. A steroid‐sparing approach to immunosuppressive therapy. Transplantation 1983;35(5):420‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Theodorakis 1998 {published data only}
- Theodorakis J, Schneeberger H, Illner WD, Stangl M, Zanker B, Land W. Aggressive treatment of the first acute rejection episode using first‐line anti‐lymphocytic preparation reduces further acute rejection episodes after human kidney transplantation. Transplant International 1998;11 Suppl 1:S86‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toledo‐Pereyra 1985 {published data only}
- Toledo‐Pereyra LH, Bergren C, Mittal VK, Whitten JI, Baskin S, McNichol L. A prospective randomized comparison of antilymphoblast globulin versus antithymocyte globulin for cadaver kidney transplantation. Transplantation 1985;40(4):448‐50. [MEDLINE: ] [PubMed] [Google Scholar]
- Toledo‐Pereyra LH, Bergren C, Whitten J. Comparison of ALG and ATG for renal transplantation [abstract]. Kidney International 1985;28(2):388. [CENTRAL: CN‐00583280] [Google Scholar]
Waid 1991 {published data only}
- Lucas BA, Waid TH, Thompson JS, Brown SA, Munch LC, McKeown JW, et al. Comparison of T10Bg.1A‐31 and OKT3 in treating acute renal allograft rejection. Transplantation Proceedings 1993;25(1 Pt 1):543‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, Brown S, Munch LC, Kryscio R, et al. Treatment of acute rejection in renal allografts with t10b9.1a‐31 or OKT3 monoclonal antibody [abstract]. Journal of the American Society of Nephrology 1992;3(3):886. [CENTRAL: CN‐00461957] [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, Brown SA, Munch L, Prebeck RJ, et al. Treatment of acute cellular rejection with T10B9.1A‐31 or OKT3 in renal allograft recipients. Transplantation 1992;53(1):80‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown S, Kryscio R, et al. Treatment of renal allograft rejection with T10B9.1A31 or OKT3: final analysis of a phase II clinical trial. Transplantation 1997;64(2):274‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown SA. Improved graft and patient survival with t10b9 1‐a or OKT3 monoclonal antibody for treatment for primary rejection [abstract]. Journal of the American Society of Nephrology 1994;5(3):1042. [CENTRAL: CN‐00679081] [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown SA. T10B9.1A‐31 effectively reverses renal allograft rejection crises with fewer side effects, less cytokine release and no severe infections [abstract]. Journal of the American Society of Nephrology 1994;5(3):1042. [Google Scholar]
- Waid TH, Lucas BA, Thompson JS, Munch LC, Brown S, Kryscio R, et al. Treatment of acute rejection with anti‐T‐cell antigen receptor complex alpha beta (T10B9.1A‐31) or anti‐CD3 (OKT3) monoclonal antibody: results of a prospective randomized double‐blind trial. Transplantation Proceedings 1991;23(1 Pt 2):1062‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Zarkhin 2008 {published data only}
- Sarwal M, Zarkhin V, Mohile S, Kambham N, Li L, Martin J, et al. Randomized trial of Rituximab vs standard of care for B cell dense acute renal transplant rejection [abstract no: 538]. American Journal of Transplantation 2007;7(Suppl 2):287. [CENTRAL: CN‐00644179] [Google Scholar]
- Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. American Journal of Transplantation 2008;8(12):2607‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kulkarni 2016 {published data only}
- Kulkarni S, Kirkiles‐Smith NC, Deng YH, Formica RN, Moeckel G, Broecker V, et al. Eculizumab therapy for chronic antibody‐mediated injury in kidney transplant recipients: a pilot, randomized‐controlled trial. American Journal of Transplantation 2016;17(3):682‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
RIACT Study 2012 {published data only}
- Schiffer L, Schiffer M, Merkel S, Schwarz A, Mengel M, Jurgens C, et al. Rationale and design of the RIACT‐study: a multi‐center placebo controlled double blind study to test the efficacy of rItuximab in acute cellular tubulointerstitial rejection with B‐cell infiltrates in renal transplant patients: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2012;13:199. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ANZDATA 2004
- Excell L, Chadban S, McDonald S. ANZDATA 27th Annual Report. Chapter 8: Transplantation. www.anzdata.org.au/anzdata/AnzdataReport/27thReport/files/Ch08Transplantation.pdf (accessed 9 May 2017).
ANZDATA 2012
- Clayton P, Campbell S, Chadban S, McDonald S, Hurst K. ANZDATA 35th Annual Report. Chapter 8: transplantation. www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c08_transplants_v1.5.pdf (accessed 9 May 2017).
Bartel 2011
- Bartel G, Schwaiger E, Bohmig GA. Prevention and treatment of alloantibody‐mediated kidney transplant rejection. Transplant International 2011;24(2):1142‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basu 2005
- Basu A, Ramkumar M, Tan HP, Khan A, McCauley J, Marcos A, et al. Reversal of acute cellular rejection after renal transplantation with Campath‐1H. Transplantation Proceedings 2005;37(2):923‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ 2005;330(7494):753‐56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chon 2014
- Chon WJ, Brennan DC. Acute renal allograft rejection: treatment. www.uptodate.com/contents/acute‐renal‐allograft‐rejection‐treatment (accessed 9 May 2017).
Colvin 2005
- Colvin RB, Smith RN. Antibody‐mediated organ‐allograft rejection. Nature Reviews. Immunology 2005;5(10):807‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Csapo 2005
- Csapo Z, Benavides‐Viveros C, Podder H, Pollard V, Kahan BD. Campath‐1H as rescue therapy for the treatment of acute rejection in kidney transplant patients. Transplantation Proceedings 2005;37(5):2032‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cuervo 2003
- Cuervo LG, Clarke M. Balancing benefits and harms in health care. BMJ 2003;327(7406):65‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Denton 1999
- Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999;353(9158):1083‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dheda 2013
- Dheda S, Chong S, Williams RL, Wong G, Lim WH. Chapter 5: Detection of antibody‐mediated rejection in kidney transplantation and the management of highly sensitised kidney transplant recipients. In: Rath T editor(s). Current Issues and Future Direction in Kidney Transplantation. InTech, 2013. [DOI: 10.5772/54735] [DOI] [Google Scholar]
Egger 2001
- Egger M, Davey Smith G, Altman DG. Problems and limitations in conducting systematic reviews. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. 2nd Edition. London: BMJ Books, 2001:43‐68. [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2016
- Haas M. The revised (2013) Banff classification for antibody‐mediated rejection of renal allografts: update, difficulties, and future considerations. American Journal of Transplantation 2016;16(5):1352‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halloran 2004
- Halloran PF. Immunosuppressive drugs for kidney transplantation. [Review] [124 refs][Erratum appears in N Engl J Med. 2005 Mar 10;352(10):1056]. New England Journal of Medicine 2004;351(26):2715‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hardinger 2013
- Hardinger KL, Brennan DC. Novel immunosuppressive agents in kidney transplantation. World Journal of Transplantation 2013;3(4):68‐77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingulli 2010
- Ingulli E. Mechanism of cellular rejection in transplantation. Pediatric Nephrology 2010;25(1):61‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Issa 2010
- Issa F, Schiopu A, Wood KJ. Role of T cells in graft rejection and transplantation tolerance. Expert Review of Clinical Immunology 2010;6(1):155‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jalalzadeh 2015
- Jalalzadeh M, Mousavinasab N, Peyrovi S, Ghadiani MH. The impact of acute rejection in kidney transplantation on long‐term allograft and patient outcome. Nephrourology Monthly 2015;7(1):e24439. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2011
- Jordan SC, Toyoda M, Vo AA. Regulation of immunity and inflammation by intravenous immunoglobulin: relevance to solid organ transplantation. Expert Review of Clinical Immunology 2011;7(3):341‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Joseph 2001
- Joseph JT, Kingsmore DB, Junor BJ, Briggs JD, Mun Woo Y, Jaques BC, et al. The impact of late acute rejection after cadaveric kidney transplantation. Clinical Transplantation 2001;15(4):221‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koo 2015
- Koo EH, Jang HR, Lee JE, Park JB, Kim SJ, Kim DJ, et al. The impact of early and late acute rejection on graft survival in renal transplantation. Kidney Research & Clinical Practice 2015;34(3):160‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loke 2001
- Loke YK, Derry S. Reporting of adverse drug reactions in randomised controlled trials ‐ a systematic survey. BMC Clinical Pharmacology 2001;1(1):3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madden 2000
- Madden RL, Mulhern JG, Benedetto BJ, O'Shea MH, Germain MJ, Braden GL, et al. Completely reversed acute rejection is not a significant risk factor for the development of chronic rejection in renal allograft recipients. Transplant International 2000;13(5):344‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Masson 2014
- Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010699.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matas 2014
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. American Journal of Transplantation 2014;14 Suppl 1:11‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mengel 2007
- Mengel M, Gwinner W, Schwarz A, Bajeski R, Franz I, Brocker V, et al. Infiltrates in protocol biopsies from renal allografts. American Journal of Transplantation 2007;7(2):356‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nankivell 2010
- Nankivell BJ, Alexander SI. Rejection of the kidney allograft. New England Journal of Medicine 2010;363(15):1451‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE 2004
- Immunosuppressive therapy for renal transplantation in adults. London (UK): National Institute for Clinical Excellence (NICE); September 2004. Technology appraisal [TA85]. www.nice.org.uk/guidance/ta85 (accessed 9 May 2017).
Opelz 1997
- Opelz G. Critical evaluation of the association of acute with chronic graft rejection in kidney and heart transplant recipients. The Collaborative Transplant Study. Transplantation Proceedings 1997;29(1‐2):73‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Opelz 2008
- Opelz G, Dohler B, Collaborative Transplant Study Report. Influence of time of rejection on long‐term graft survival in renal transplantation. Transplantation 2008;85(5):661‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peduzzi 1993
- Peduzzi P, Wittes J, Detre K, Holford T. Analysis as‐randomized and the problem of non‐adherence: an example from the Veterans Affairs Randomized Trial of Coronary Artery Bypass Surgery. Statistics in Medicine 1993;12(13):1185‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roberts 2012
- Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody‐mediated rejection in kidney transplant recipients‐a systematic review. Transplantation 2012;94(8):775‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sackett 1979
- Sackett DL, Gent M. Controversy in counting and attributing events in clinical trials. New England Journal of Medicine 1979;301(26):1410‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Solez 1993
- Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney International 1993;44(2):411‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solez 2008
- Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American Journal of Transplantation 2008;8(4):753‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szczech 1997
- Szczech LA, Berlin JA, Aradhye S, Grossman RA, Feldman HI. Effect of anti‐lymphocyte induction therapy on renal allograft survival: a meta‐analysis. Journal of the American Society of Nephrology 1997;8(11):1771‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szczech 1998
- Szczech LA, Berlin JA, Feldman HI. The effect of antilymphocyte induction therapy on renal allograft survival. A meta‐analysis of individual patient‐level data. Anti‐Lymphocyte Antibody Induction Therapy Study Group. Annals of Internal Medicine 1998;128(10):817‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tunis 2003
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials; increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290(12):1624‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2014
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2014 USRDS Annual data report: Epidemiology of kidney disease in the United States Volume 2: End‐stage renal disease (ESRD) in the United States. Chapter 6: Transplantation. www.usrds.org/2014/view/v2_06.aspx (accessed 9 May 2017).
Zaza 2014
- Zaza G, Tomei P, Granata S, Boschiero L, Lupo A. Monoclonal antibody therapy and renal transplantation: focus on adverse effects. Toxins 2014;6(3):869‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Webster 2004
- Webster A, Chapman JR, Craig JC, Mahan J, Orton L, Pankhurst T, et al. Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004756.pub2] [DOI] [PubMed] [Google Scholar]
Webster 2006a
- Webster AC, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004756.pub3] [DOI] [PubMed] [Google Scholar]
Webster 2006b
- Webster AC, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Monoclonal and polyclonal antibody therapy for treating acute rejection in kidney transplant recipients: a systematic review of randomized trial data. Transplantation 2006;81(7):953‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


