Abstract
Background
This is an update of the original Cochrane Review, last published in 2012 (Loy 2012). Children and youths with disruptive behaviour disorders may present to health services, where they may be treated with atypical antipsychotics. There is increasing usage of atypical antipsychotics in the treatment of disruptive behaviour disorders.
Objectives
To evaluate the effect and safety of atypical antipsychotics, compared to placebo, for treating disruptive behaviour disorders in children and youths. The aim was to evaluate each drug separately rather than the class effect, on the grounds that each atypical antipsychotic has different pharmacologic binding profile (Stahl 2013) and that this is clinically more useful.
Search methods
In January 2017, we searched CENTRAL, MEDLINE, Embase, five other databases and two trials registers.
Selection criteria
Randomised controlled trials of atypical antipsychotics versus placebo in children and youths aged up to and including 18 years, with a diagnosis of disruptive behaviour disorders, including comorbid ADHD. The primary outcomes were aggression, conduct problems and adverse events (i.e. weight gain/changes and metabolic parameters). The secondary outcomes were general functioning, noncompliance, other adverse events, social functioning, family functioning, parent satisfaction and school functioning.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors (JL and KS) independently collected, evaluated and extracted data. We used the GRADE approach to assess the quality of the evidence. We performed meta‐analyses for each of our primary outcomes, except for metabolic parameters, due to inadequate outcome data.
Main results
We included 10 trials (spanning 2000 to 2014), involving a total of 896 children and youths aged five to 18 years. Bar two trials, all came from an outpatient setting. Eight trials assessed risperidone, one assessed quetiapine and one assessed ziprasidone. Nine trials assessed acute efficacy (over four to 10 weeks); one of which combined treatment with stimulant medication and parent training. One trial was a six‐month maintenance trial assessing symptom recurrence.
The quality of the evidence ranged from low to moderate. Nine studies had some degree of pharmaceutical support/funding.
Primary outcomes
Using the mean difference (MD), we combined data from three studies (238 participants) in a meta‐analysis of aggression, as assessed using the Aberrant Behaviour Checklist (ABC) ‒ Irritability subscale. We found that youths treated with risperidone show reduced aggression compared to youths treated with placebo (MD −6.49, 95% confidence interval (CI) −8.79 to −4.19; low‐quality evidence). Using the standardised mean difference (SMD), we pooled data from two risperidone trials (190 participants), which used different scales: the Overt Aggression Scale ‒ Modified (OAS‐M) Scale and the Antisocial Behaviour Scale (ABS); as the ABS had two subscales that could not be combined (reactive and proactive aggression), we performed two separate analyses. When we combined the ABS Reactive subscale and the OAS‐M, the SMD was −1.30 in favour of risperidone (95% CI −2.21 to −0.40, moderate‐quality evidence). When we combined the ABS Proactive subscale and OAS‐M, the SMD was −1.12 (95% CI −2.30 to 0.06, moderate‐quality evidence), suggesting uncertainty about the estimate of effect, as the confidence intervals overlapped the null value. In summary, there was some evidence that aggression could be reduced by risperidone. Data were lacking on other atypical antipsychotics, like quetiapine and ziprasidone, with regard to their effects on aggression.
We pooled data from two risperidone trials (225 participants) in a meta‐analysis of conduct problems, as assessed using the Nisonger Child Behaviour Rating Form ‒ Conduct Problem subscale (NCBRF‐CP). This yielded a final mean score that was 8.61 points lower in the risperidone group compared to the placebo group (95% CI −11.49 to −5.74; moderate‐quality evidence).
We investigated the effect on weight by performing two meta‐analyses. We wanted to distinguish between the effects of antipsychotic medication only and the combined effect with stimulants, since the latter can have a counteracting effect on weight gain due to appetite suppression. Pooling two trials with risperidone only (138 participants), we found that participants on risperidone gained 2.37 kilograms (kg) more (95% CI 0.26 to 4.49; moderate‐quality evidence) than those on placebo. When we added a trial where all participants received a combination of risperidone and stimulants, we found that those on the combined treatment gained 2.14 kg more (95% CI 1.04 to 3.23; 3 studies; 305 participants; low‐quality evidence) than those on placebo.
Secondary outcomes
Out of the 10 included trials, three examined general functioning, social functioning and parent satisfaction. No trials examined family or school functioning. Data on non‐compliance/attrition rate and other adverse events were available from all 10 trials.
Authors' conclusions
There is some evidence that in the short term risperidone may reduce aggression and conduct problems in children and youths with disruptive behaviour disorders There is also evidence that this intervention is associated with significant weight gain.
For aggression, the difference in scores of 6.49 points on the ABC ‒ Irritability subscale (range 0 to 45) may be clinically significant. It is challenging to interpret the clinical significance of the differential findings on two different ABS subscales as it may be difficult to distinguish between reactive and proactive aggression in clinical practice. For conduct problems, the difference in scores of 8.61 points on the NCBRF‐CP (range 0 to 48) is likely to be clinically significant. Weight gain remains a concern.
Caution is required in interpreting the results due to the limitations of current evidence and the small number of high‐quality trials. There is a lack of evidence to support the use of quetiapine, ziprasidone or any other atypical antipsychotic for disruptive behaviour disorders in children and youths and no evidence for children under five years of age. It is uncertain to what degree the efficacy found in clinical trials will translate into real‐life clinical practice. Given the effectiveness of parent‐training interventions in the management of these disorders, and the somewhat equivocal evidence on the efficacy of medication, it is important not to use medication alone. This is consistent with current clinical guidelines.
Plain language summary
Atypical antipsychotic drugs for disruptive behaviour disorders in children and youths
Review question
To review the effect and safety of atypical antipsychotics (which are newer‐generation major tranquillisers), compared to placebo (dummy pill), for treating disruptive behaviour disorders (e.g. defiance, disobedience, hostility) in children and youths.
Background
Children and youths with disruptive behaviour disorders often present with aggression and severe behaviour problems. These can result in families seeking health services, where atypical antipsychotics may be used to reduce these symptoms. There is increasing usage of atypical antipsychotics in the treatment of disruptive behaviour disorders.
Study characteristics
We reviewed the evidence for atypical antipsychotics, compared to placebo, for treating disruptive behaviour disorders in children and youths. The evidence is current to 19 January 2017. We found 10 studies. Of these studies, eight investigated the effect of risperidone, one investigated quetiapine and one investigated ziprasidone. Five studies were pilot studies (a small, preliminary study to assess the feasibility, including costs, of conducting a larger study). Five studies had 38 or fewer participants; one study had 50 participants, two studies had over 100 participants each, one had 168 participants and one had over 300 participants. Nine studies had a duration of four, six or 10 weeks. The tenth study was a six‐month maintenance trial. Nine out of 10 studies had some degree of pharmaceutical support/funding.
Key results and quality of evidence
Our analysis suggested that risperidone led to a reduction of aggression (low‐quality evidence) and conduct problems (moderate‐quality evidence), to some extent, after six weeks of treatment, and that risperidone appeared relatively safe in the short‐term. However, it was associated with significant weight gain (low‐ to moderate‐quality evidence). There are other side effects that have not been well studied and long‐term effects are not entirely clear. Clinicians prescribing such medication and families need to carefully consider the benefits and risks of medications. There were no studies with children under five years of age. There is a lack of studies of medications other than risperidone.
We recommend that more research be conducted to find out the long‐term effects and safety of these medications. More research is also needed for other medications besides risperidone. Ideally, medication should be used with or preceded by effective psychosocial treatments, like parent training, consistent with current clinical guidelines. It is important that medications are used at adequate doses and for an adequate duration. Careful thought needs to be given to usage of medications sequentially or in combination in order to optimise the therapeutic effect while minimising polypharmacy.
The findings need to be considered with caution because of the limitations of the evidence. The studies used different outcome measures, which limited our ability to combine the findings. Six out of 10 studies had small numbers of participants, which affected the power of the studies (the ability of the study to distinguish an effect of a certain size from chance). The quality of the evidence for the main outcomes of this review — aggression, conduct problems and weight gain — ranged from low to moderate quality using the GRADE considerations.
Summary of findings
Summary of findings for the main comparison. Risperidone compared to placebo for disruptive behaviours in children and youths.
| Risperidone compared to placebo for disruptive behaviours in children and youths | ||||||
| Patient or population: Disruptive behaviours in children and youths Setting: Mostly outpatient clinics Intervention: Risperidone Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with risperidone | |||||
| Aggression Assessed with: Aberrant Behaviour Checklist ‒ Irritability (ABC‐I) subscale Scale from: 0 to 45 Follow‐up: range 4 weeks to 6 weeks | The mean aggression ABC‐I score ranged across control groups from −4.40 to 0.10 | The mean aggression ABC‐I score in the intervention groups was, on average, 6.49 lower (8.79 lower to 4.19 lower) | ‐ | 238 (3 RCTs) | ⊕⊕⊝⊝ Low1 | Included studies: Aman 2002; Snyder 2002; Van Bellinghen 2001 |
| Aggression Assessed with: OAS‐M and ABS Proactive subscales Follow‐up: mean 6 weeks | The mean aggression OAS‐M and ABS Proactive score ranged across control groups from 8.10 to 15.10 | The mean aggression OAS‐M and ABS Proactive score in the intervention groups was, on average, 1.12 lower (2.30 lower to 0.06 higher) | ‐ | 190 (2 RCTs) | ⊕⊕⊕⊝ Moderate2 | Included studies: Buitelaar 2001; TOSCA study |
| Conduct Assessed with: Nisonger Child Behaviour Rating ‒ Conduct Problems subscale Scale from: 0 to 48 Follow‐up: mean 6 weeks | The mean conduct score ranged across control groups from −6.20 to 25.80 | The mean conduct score in the intervention groups was, on average, 8.61 lower (11.49 lower to 5.74 lower) | ‐ | 225 (2 RCTs) | ⊕⊕⊕⊝ Moderate3 | Included studies: Aman 2002; Snyder 2002 |
| Weight gain (treatment with antipsychotic only) Assessed with: mean change scores measured in kilograms | The mean weight gain (treatment with antipsychotic only) score in the control groups ranged from 0.74 to 0.90 | The mean weight gain score in the intervention groups was, on average, 2.37 higher (0.26 higher to 4.49 higher) | ‐ | 138 (2 RCTs) | ⊕⊕⊕⊝ Moderate4 | Included studies: Aman 2002; Findling 2000 |
| Weight gain (treatment with antipsychotic and stimulant) Assessed with: mean change scores measured in kilograms | The mean weight gain (treatment with antipsychotic and stimulant) score in the control groups ranged from −1.20 to 0.90 | The mean weight gain score in the intervention groups was, on average, 2.14 higher (1.04 higher to 3.23 higher) | ‐ | 305 (3 RCTs) | ⊕⊕⊝⊝ Low 5 | Included studies: Aman 2002; Findling 2000; TOSCA study |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; OR: Odds ratio ABS: Antisocial Behavior Scale;CI: Confidence interval; MD: Mean difference;OAS: Overt Aggression Scale;OAS‐M: Overt Aggression Scale ‒ Modified; RCT: Randomised controlled trial; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 2 levels because of unclear risk of bias due to lack of information on selection bias and detection bias in 2 studies, and unclear risk of bias due to lack of information and poor reporting standards in 1 study. 2 trials assessed outpatients, 1 trial assessed patients in residential care.
2 Unclear allocation concealment and unclear blinding of outcome assessment for 1 study and potential reporting bias in both studies.
3 Downgraded 1 level because of unclear allocation concealment and unclear blinding of outcome assessment for both studies and unclear attrition and potential reporting bias.
4 Downgraded 1 level because of unclear blinding of outcome assessment and potential reporting bias. Heterogeneity: Tau² = 2.22; Chi² = 20.77, df = 1 (P < 0.00001); I² = 95%.
5 Downgraded 2 levels because of unclear blinding of outcome assessment in 2 studies, potential reporting bias in 3 studies, and potential attrition bias in 2 studies. Heterogeneity: Tau² = 0.85; Chi² = 23.32, df = 2 (P < 0.00001); I² = 91%.
Background
Description of the condition
Disruptive behaviour disorders form a group of psychological problems that include conduct disorder, oppositional defiant disorder and disruptive behaviour disorder not otherwise specified (Findling 2008). Subclinical presentations of oppositional defiant disorder and conduct disorder were previously diagnosed as disruptive behaviour disorder not otherwise specified. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), disruptive behaviour disorder not otherwise specified became designated as "other specified disruptive disorder" (American Psychiatric Association 2013), when the number of symptoms does not meet the diagnostic threshold. Disruptive behaviour disorders are frequently comorbid with attention deficit hyperactivity disorder (ADHD) (Findling 2008).
According to the DSM‐5, conduct disorder is defined as a repetitive and persistent pattern of behaviour that violates the basic rights of others or violates major age‐appropriate societal rules or norms (American Psychiatric Association 2013). In the preceding 12 months, at least three out of 15 criteria must be present from any of the following four categories, with at least one criterion present in the last six months: aggression towards people or animals; destruction of property; deceitfulness or theft; or serious violation of rules. The behavioural disturbances must also cause clinically significant impairment in social, academic or occupational functioning. Conduct disorder can be classed as mild, moderate or severe (American Psychiatric Association 2013). It is also categorised into childhood onset, adolescent onset and unspecified onset subgroups (American Psychiatric Association 2013). The early onset group is believed to have a poorer prognosis with a more persistent course and more pervasive disturbances (Steiner 1997). A specifier was added in DSM‐5 for people with limited "prosocial emotion", showing callous and unemotional traits as research showed they tend to have a relatively more severe form of the disorder and a different treatment response (American Psychiatric Association 2013a).
Oppositional defiant disorder is diagnosed when a child has a minimum of four out of eight symptoms from the following three categories, for at least six months: angry/irritable mood; argumentative/defiant behaviour; and vindictiveness (American Psychiatric Association 2013). Oppositional defiant disorder is conceptualised as a potential precursor of conduct disorder if no interventions occur. This change highlights that the disorder reflects both emotional and behavioural symptomatology. The conduct disorder exclusion is deleted. The criteria were also changed with a note on frequency requirements and a specifier on current severity (American Psychiatric Association 2013a).
Prevalence of conduct disorder in the general population is estimated to be between 1.5% and 4% of children and adolescents using clinical interviewing as a method of detection (Steiner 1997). The ratio of boys to girls is between 5:1 and 3.2:1 depending on the age range (Steiner 1997). Reported community prevalence rates of oppositional defiant disorder range from 2% (Loeber 1998) to 16% (Cohen 1993), depending on the criteria and assessment methods used, the time period considered and the number of informants.
Disruptive mood dysregulation disorder (DMDD) was added as a new diagnosis to depressive disorders in DSM 5 (American Psychiatric Association 2013). This was to address concerns about the misdiagnosis and overtreatment of bipolar disorder in children and youths (Baweja 2016). This diagnosis remains somewhat controversial due to concerns about its construct validity and unclear treatment parameters (Baweja 2016; Freeman 2016). Youths with DMDD have significant overlap with symptoms of ODD (Freeman 2016). The development of ICD 11 aims to improve the diagnostic classification of irritability in youths (Evans 2017). The authors propose a different solution for ICD11: a subtype ODD with chronic irritability/anger (Evans 2017).
Comorbidity
Oppositional defiant disorder or conduct disorder may be comorbid in more than 50% of ADHD cases (Barkley 2006; Connor 2010). From psychology literature, there is evidence that children with comorbid ADHD, oppositional defiant disorder and conduct disorder experience multiple childhood and psychosocial risk factors that begin during infancy (Shaw 2001). Children with a history of trauma have greater oppositional defiant behaviours than children without exposure to trauma (Henry 2007). According to Steiner 2007, 14% of child patients have comorbid anxiety disorders and 9% have comorbid depressive disorder. Greene 2002 reported comorbidity of disruptive behaviour disorders with paediatric bipolar affective disorder of up to 40% to 50% (Greene 2002). However, there is a lack of clarity in the diagnosis of paediatric bipolar affective disorder and controversy in the literature, especially with emotionally dysregulated children and youths (Parens 2010).
Impact
A significant proportion of children (about 30%) with early onset of oppositional defiant disorder go on to develop conduct disorder (Waschbusch 2002). Oppositional defiant disorder significantly predicts compromised psychiatric, family and social functioning independently of the presence of conduct disorder (Biederman 1996; Greene 2002). In Biederman's study of oppositional defiant disorder in boys, oppositional defiant disorder was found to be associated with major depression, in the interval between the four‐year and 10‐year follow‐up (Biederman 2008).
Conduct disorder leads to multiple negative outcomes in adulthood (Moffitt 2002). From the Christchurch longitudinal study, Fergusson and Horwood demonstrated that children scoring in the top 5% for conduct problems at age eight years were at 4.8 times higher risk of leaving school without qualifications than children in the least disturbed 50%, and their rates of unemployment at 18 years of age were 2.9 times higher (Fergusson 1998). This study also indicated that conduct problems at seven to nine years of age were statistically significantly associated with a wide range of adverse psychosocial outcomes in adulthood, including crime, substance dependence, mental health problems and relationship difficulties, even after controlling for confounding factors (Fergusson 2004). Clinically, a significant proportion of children and youths with severe disruptive behaviour disorders may not be seen in psychiatric clinics, but are seen and dealt with by general practitioners, paediatricians, schools, welfare agencies, police, or courts, singly or in combination.
Psychosocial treatments
A range of psychosocial interventions are outlined in the NICE guidelines, including training programmes for parents and foster carers, child‐focused programmes and multimodal interventions for children and youths with, or at high risk of, developing oppositional defiant disorder and conduct disorders (NICE Clinical Guideline (CG158) 2013). For young children up to early adolescence, there are a variety of parent training programmes (Kazdin 1997; Weisz 2004; Kaminski 2008; Chorpita 2009). The programmes that do best are those that increase positive parent‐child interactions and emotional communication skills, teach parents to use time out and the importance of consistency, and those that require parents to practise new skills with their children (Kaminski 2008).
Screening for trauma is essential in clinical practice and thinking about the function of a child's behaviour is important. As succinctly put by Howard 2013: is a child distressed or deliberately defiant? Perry 2006 used the term "survival behaviours that include defiant behaviours" that are present in traumatized children. For those disruptive‐behaviour‐disorder children with comorbid trauma history, the additional treatment goals include ensuring safety, affect regulation and management, skills building, trauma resolution (Kuban 2011), and potentially more trauma‐specific therapies.
For youths, the main focus of interventions for conduct disorder is at the family or systemic level. They include functional family therapy and multi‐systemic therapy (Scott 2008). Functional family therapy is a treatment combining a family approach with cognitive and behavioural modification to improve family communication patterns and support functions, which has shown some effect (Scott 2008). A proposed Cochrane Review of functional family therapy remains at the protocol stage (Littell 2007). Multisystemic therapy (MST) is a family‐based treatment involving multiple systems (family, school, community). There were previous reports of effectiveness in some studies (Karnik 2007). An earlier Cochrane Review has reported that there is inconclusive evidence of the effectiveness of MST compared with other interventions in youths (Littell 2005). However, a more recent publication has summarised the effectiveness of MST outlining 55 published outcome, implementation and benchmarking studies, of which 25 are randomised trials (MST Services 2016). Out of the randomised trials, four are trials using MST with adolescents with serious conduct problems, and 11 are trials using MST with serious juvenile offenders. The authors suggest MST reduces long‐term re‐arrest rates in studies with serious juvenile offenders by a median of 42%. Out‐of‐home placements, across all MST studies, are reduced by a median of 54% (MST Services 2016).
Pharmacological treatments
The difficulties associated with disruptive behaviour disorders include problematic aggression and severe behavioural problems. These often result in presentation to psychiatric services, where a number of medications are used for disruptive behaviours, including off‐label use of some medications designed for other disorders, for example stimulant medications, mood stabilisers and antipsychotics (Tcheremissine 2006). None of these were originally developed for the treatment of disruptive behaviours.
Stimulant medications for the treatment of ADHD have been widely studied. There is evidence to support the use of extended‐release methylphenidate and amphetamine formulations, atomoxetine, and extended‐release guanfacine (α2‐adrenergic agonist) to improve symptoms of ADHD in adolescents in a recent systematic review (Chan 2016). There is also evidence for clonidine, another α2‐adrenergic agonist, and the US Food and Drug Administration (FDA) has approved an extended‐release clonidine to be used alone or with stimulants for the treatment of ADHD in paediatric patients aged 6 to 17 years since 2010 (Waknine 2010). There is convincing evidence that when ADHD co‐occurs with disruptive behaviour disorders and is treated with stimulant medications, improvements can be observed in disruptive behaviour disorder and aggression (Pappadopulos 2006; Ipser 2007).
The mood stabiliser lithium has been studied in inpatient settings for young people with conduct disorders. The evidence about its efficacy showed significant variability (Pappadopulos 2006; Ipser 2007). Two studies did not meet the inclusion criteria used in the systematic review by Pappadopulos 2006. One was a study of 20 youths with explosive temper and mood lability, in which sodium valproate was superior to placebo in reducing aggressive symptoms (Donovon 2000). Another was a seven‐week, cross‐over, randomised controlled trial of 71 youths with conduct disorder, in which participants receiving higher doses (500 mg/day to 1500 mg/day) of sodium valproate experienced greater global improvement scores and self‐reported impulse control than those randomised to low doses (250 mg/day) (Steiner 2003). Only one randomised controlled trial of 22 inpatient youths with conduct disorder indicated that carbamazepine was no different than placebo in reducing aggression and explosiveness (Cueva 1996), although, given the small sample size, is likely to be have been underpowered to show effect. Preliminary studies of alpha‐2 agonists (clonidine, guanfacine) suggest some effect on aggressive behaviour in patients with diagnoses of autism and ADHD with comorbid tics (Pappadopulos 2006).
Antipsychotic agents are used to control disruptive behaviour in clinical practice, particularly when aggression is a core feature. In the 1980s typical antipsychotics were studied (Findling 2008). However, interest has since shifted to atypical antipsychotics (Findling 2008). Of the atypical antipsychotics, risperidone is the most widely studied in the disruptive behaviour disorder population (Pappadopulos 2006). Currently, aripiprazole, olanzapine, quetiapine and risperidone have FDA‐ (Food and Drug Administration) approved paediatric indications for bipolar mania (10 to 17 years of age except for olanzapine, 13 to 17 years of age) and for schizophrenia (13 to 17 years of age) (FDA 2009; Correll 2010). In addition, aripiprazole and risperidone are also indicated for irritability and aggression associated with autistic disorder (six to 17 years of age) (Correll 2010; Ching 2012). Any usage for disruptive behaviour disorder is considered off‐label, except in Europe (European Medicines Agency 2011), and the individual clinician is medico‐legally responsible for the usage. There is a trend currently towards combination treatment with atypical antipsychotics and stimulant medication (Aman 2015; Kamble 2015).
Description of the intervention
This review focuses on atypical antipsychotics because of the clinical interest and usage in disruptive behaviour disorders (Doey 2007; Harrison‐Woolrych 2007). The atypical antipsychotics include risperidone, olanzapine, quetiapine, aripiprazole, amisulpiride, sertindole, ziprasidone, zotepine, clozapine, paliperidone, asenapine, iloperidone, and lurasidone. In treatment guidelines (Pappadopulos 2003), pharmacological management is used for emergency treatment of acute aggression in the short term (lasting days to weeks), or for chronic aggression where the duration of treatment is for at least six months.
How the intervention might work
A potential focus of the use of atypical antipsychotics is to target aggression in disruptive behaviour disorders (Findling 2008). Aggression is one of the diagnostic criteria for conduct disorders (American Psychiatric Association 2013); and a common presenting complaint in ODD (Turgay 2004). Reviewing the neurotransmitters of aggression, Swann 2003 postulates that the increased risk of impulsive behaviour may be associated with elevated dopaminergic or noradrenergic function. Results of animal studies suggest that trait impulsivity may result from an imbalance between dopamine and serotonin, where animals with serotonin depletions are impulsive due to release of a dopaminergic activation system from serotonin depletion (Harrison 1997). Siever 2008, in reviewing the neurobiology of aggression and violence, propose that aggression is mediated through insufficient serotonergic facilitation of "top‐down" control (executive regulation/control provided by the orbital frontal cortex and anterior cingulate cortex), excessive dopamine and noradrenaline stimulation and subcortical imbalances of glutamatergic/gabaminergic systems and dysfunction in the neuropeptide systems.
Atypical antipsychotics block dopamine and serotonin receptor systems and some investigators have proposed that their anti‐aggressive action comes from this effect (Schur 2003). Atypical antipsychotics that have the ability to antagonise the D2 receptor are said to reduce aggression (Nelson 2007). Siever 2008 proposes that atypical antipsychotics' anti‐aggressive effect is due to a reduction in dopaminergic stimulation and an increased effect on frontal inhibition. Pharmacologically, according to Stahl 2013, atypical antipsychotics, as a class, are defined as serotonin‐dopamine antagonists, in particular 5HT2a and D2 receptor antagonism. However, they also have partial agonist actions at 5HT1a receptors and D2 receptors. Interestingly, while atypical antipsychotics act as serotonin antagonist in the short term, chronic treatment may produce changes in the serotonin binding sites that are qualitatively and quantitatively similar to serotonin agonists (Krakowski 2006).
It is unclear if the mechanism of action for its antipsychotic effect is independent of its anti‐aggressive effect. Antipsychotic effect is achieved when 60% to 75% of D2 receptors have been blocked, while extrapyramidal side effects emerge when 80% or more D2 receptors have been blocked by an atypical antipsychotic (Ferrin 2015). This is the D2 occupancy theory (Remington 2014). The fast dissociation theory posits that antipsychotics come off the D2 receptor at very different rates with faster dissociation rates characterising the atypical antipsychotics (Remington 2014). It calls into question the need for continuous D2 binding to maintain antipsychotic response. The authors suggest exploring different types of antipsychotic dosing in order to achieve therapeutic effect and to reduce side effects (Remington 2014).
Thus, the pharmacological mechanism of action through which atypical antipsychotics may inhibit aggression is complex and further research is needed (Schur 2003; Siever 2008).
Why it is important to do this review
There are multiple studies showing increasing, widespread use of atypical antipsychotics amongst children and youths in different countries. They include Australia (Dean 2006), Canada (Doey 2007), New Zealand (Harrison‐Woolrych 2007), the United Kingdom (Rani 2008) and the USA (Olfson 2010).
This trend continues in more recent literature. An Australian study by Karanges and colleagues examined longitudinal trends in psychotropic medication dispensing from 2009 to 2012 by scrutinising the dispensing database maintained by the Department of Human Services (Karanges 2014). The overall trend (all ages) was a 22.7% increase in subsidised, antipsychotic prescriptions dispensed from 2009 to 2012 (from 2,573,833 prescriptions to 3,158,020 prescriptions). For atypical antipsychotics, the greatest increase was in the 10‐ to 14‐year age group (53.3% increase), followed by three to nine years (45%) and 15 to 19 years (40.9%). In 2012, risperidone was the most popular antipsychotic in those aged three to nine years and 10 to 14 years (90.1% and 72.6% respectively), with quetiapine most popular in those aged 15 to 19 years (34.1%). Seventy per cent of prescriptions for atypical antipsychotics were written by general practitioners and 20.1% by psychiatrists. There were no data on diagnoses. Bachmann and colleagues in Germany looked at antipsychotic prescription in children and adolescents, analysing data from a German statutory health insurance company from 2005 to 2012 (Bachmann 2014). They found that atypical antipsychotics were increasingly used off label to treat aggressive impulsive disorders. Most of the prescriptions were not written by child and adolescent psychiatrists. Risperidone was most commonly prescribed, given in 61.5% of cases to patients with ADHD and 35.5% of cases to patients with conduct disorders. Burcu and colleagues assessed antipsychotic prescribing patterns in the outpatient treatment of behavioural disorders in US youth (Burcu 2015). They used 2003 to 2010 national ambulatory medical care survey data and national hospital ambulatory medical care survey data (n = 4603). They found a different pattern — psychiatrists prescribed antipsychotics more than non‐psychiatrists (24.2% versus 4.6% respectively). In more than one third of the visits, antipsychotics were prescribed concomitantly with two or more psychotropic medication classes.
Other papers look at this issue through an ADHD treatment lens, in terms of combination treatment or concurrent use of atypical antipsychotics with stimulant medications (Amor 2014; Kamble 2015). Amor and colleagues assessed the one‐year period prevalence of stimulant combination therapy and 'switching' in children and adolescents with ADHD in Quebec, Canada (Amor 2014). They looked at a Quebec database from March 2007 to February 2012. They defined combination therapy as 30 consecutive days of concomitant use of multiple drugs. They found that among 9431 children and adolescents aged six to 17 years with ADHD, the one‐year period prevalence of combination therapy was 19.8% and that of switching was 18.7%. The most frequent combination categories were atypical antipsychotics (10.8%), followed by atomoxetine (5.5%) and clonidine (5.3%). The most frequently switched‐to categories were other stimulants (7.9%), atypical antipsychotics (5.5%) and atomoxetine (4.7%). There were no details available on the atypical antipsychotics used. In a US study, Kamble and colleagues examined the prevalence of concurrent use of long‐acting stimulants and atypical antipsychotics among children and adolescents aged six to 17 years with ADHD, retrospectively analysing 2003 to 2007 Medicaid data from four US states (Kamble 2015). They defined combination therapy as simultaneous receipt of both stimulant and atypical antipsychotic for at least 14 days. Among the 61,793 children and youths, 11,866 (19.2%) had combination treatment. The average length of concurrent use was 130 (± 98) days. Risperidone was used in about 61% of those children and youths.
Combination therapy has become more common in clinical practice (Aman 2015); and, from the papers above, potentially more so in the USA and Canada. Prescribing multiple combinations seems more the norm in a national survey of child and adolescent psychiatrists in the USA (Kearns 2014). Kreider 2014 also found that these combination treatments are common, of long duration and on the rise in the USA. Combinations of drugs with different mechanism of action and with some potentially reciprocally neutralising adverse events makes conceptual sense (Farmer 2011). However, it is not known how frequently patients receiving combination treatment are reviewed to assess the benefits and risks of their combination treatment (Olfson 2014). There is an emerging theme in the literature of a stepped care approach, as well as augmentation treatment, as part of the argument for combination therapy.
While there is a significant increase in the use of atypical antipsychotics in vulnerable child and adolescent populations, there is a lack of a corresponding increase in the clinical research evaluating efficacy or safety in this population (Greenhill 2003). The review seeks to address this important gap. In addition aggression itself, which is a common presenting symptom of disruptive behavioural disorders, remains an important clinical and social problem in the child and adolescent mental health field and is worthy of research (Aman 2015).
Objectives
To evaluate the effect and safety of atypical antipsychotics, compared to placebo, for treating disruptive behaviour disorders in children and youths. The aim was to evaluate each drug separately rather than the class effect, on the grounds that each atypical antipsychotic has different pharmacologic binding profile (Stahl 2013) and that this is clinically more useful.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled, double‐blinded trials.
Types of participants
Children and adolescents up to and including 18 years of age, in any setting, with a diagnosis of a disruptive behaviour disorder, including oppositional defiant disorder, conduct disorder and disruptive behaviour disorder not otherwise specified, as established using criteria from either the Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association 2000; American Psychiatric Association 2013) or International Statistical Classification of Diseases and Related Health Problems (ICD; WHO 2016). We included studies in which participants had comorbid diagnoses of ADHD, major depression, anxiety disorders or intellectual disability.
We excluded studies in which participants had a comorbid diagnosis of pervasive developmental disorder or autistic spectrum disorder (ASD), a comorbid psychotic disorder or bipolar affective disorder. This was to exclude those conditions in which antipsychotic agents may be treating symptoms other than disruptive behaviour, as in those cases clinical improvement may be related to improvement in the underlying psychopathology. In addition, there are other reviews dealing with atypical antipsychotics in ASD such as the recently updated review by Hirsch 2016.
Types of interventions
Any atypical antipsychotic, whether the mode of delivery was oral or intramuscular, compared with placebo. Trials including a combination of atypical antipsychotics combined with other medications or psychosocial interventions, or both, were also eligible. The rationale for including combination treatment in the review was that it mirrored clinical practice. No duration of treatment was specified a priori.
Types of outcome measures
Primary outcomes
Aggression: reduction in aggressive behaviour, measured through reduction in scores of validated rating scales.
Conduct problems: reduction in conduct problems or disruptive behaviour problems, measured through reduction in scores on relevant validated rating scales or subscales.
Adverse events: weight gain (absolute weight gain or changes in body mass index (BMI)) and metabolic parameters (specifically glucose and lipid profiles).
The hierarchy of preferred time points was: i) six‐week time point for initial efficacy and ii) six‐month time point after six months' maintenance treatment for long‐term efficacy (Jensen 2007a).
The rationale for the selection of the primary outcomes was that they were important and clinically relevant problems caregivers/families and clinicians grapple with. The justification for the time points arose from the recommendations from Jensen 2007a — a consensus report from the USA compiled from the contributions of multiple stakeholders including academics, researchers, the Food and Drug Administration (FDA), the National Institute of Mental Health (NIMH), industry sponsors and patient and family advocates. The report looked at impulsive aggression as a symptom across diagnostic categories in child psychiatry, with implications for medication studies.
Validated rating scales are those that accurately assess what they were designed to assess, are reliable and have normative data (Myers 2002). Collett 2003, Jensen 2007a and Steiner 2007 have listed scales that were suitable and have outlined the psychometric properties for the majority of them. The relevant scales are listed in Appendix 1.
For clinical and statistical reasons, it is usually necessary to obtain information that covers behaviour in different settings, including home and school, from different informants (Verhulst 2002). Both observer and self‐rated rating scales are used. Parents observe variations in behaviour across multiple situations while teachers note deviation from peers in the school setting (Myers 2002). Generally, for externalising problems, there is greater inter‐rater consistency between parent and teacher informants. (Clay 2008). For self‐reports, while children and adolescents can be reliable and valid self‐reporters, and may be useful for some difficult‐to‐observe behaviour such as stealing, there are potential limitations. These include children's and youths' linguistic skills, presence of learning difficulties, self‐reflection skills, ability to monitor one's behaviour and risks of under‐reporting undesirable behaviour or to respond in a socially desirable manner (Myers 2002; Collett 2003). For these reasons, observer‐rated data were preferable to self‐rated data for this review. If several measures of the same outcome were available, we selected the measure used as the primary outcome in a given trial.
For the measurement of weight gain, we selected measurement by kilogram.
Secondary outcomes
General functioning, as measured by the Children's Global Assessment Scale (CGAS) (Shaffer 1983).
Non‐compliance, measured as the proportion of participants discontinuing treatment.
Other adverse events, measured as the incidence of overall adverse events and breakdown by types of adverse events, taking into consideration frequency, severity and clinical importance, and including extrapyramidal side effects measured by standardised side‐effect scales, such as Simpson Angus Extrapyramidal Scale (SAES) (Simpson 1970), and common adverse events like sedation and hyperprolactinaemia.
Social functioning, as measured by, for example, the social adaptation subscale from the MacArthur Health and Behavior Questionnaire (HBQ) (Armstrong 2003).
Family functioning, as measured by, for example, Parenting Stress Index – Short Form (PSI‐SF) (Abidin 1995).
Parent satisfaction, as measured by, for example, Cleminshaw‒Guidubaldi Parent Satisfaction Scale (Guidubaldi 1985).
Functioning at school, as measured by, for example, the School Function Assessment (SFA) (Coster 2008).
Search methods for identification of studies
We ran database and trial register searches for the original review in June 2010 and August 2011 (see Appendix 2). For this version, we revised the search strategy by adding search terms for new drugs: iloperidone, asenapine, lurasidone and paliperidone (see Appendix 3). We did not limit our searches by date, language or publication type.
Electronic searches
We searched the databases and trial registers listed below in January 2015, February 2016 and January 2017. Details of the searches, including exact search dates, are reported in Appendix 4 .
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) in the Cochrane LIbrary, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialized Register (searched 19 January 2017).
MEDLINE Ovid (1946 to December Week 1 2016).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (18 January 2017).
Embase Ovid (1980 to 2017 Week 03).
PsycINFO Ovid (1806 to January Week 2 2017).
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to current).
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 1) in the Cochrane Library.
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) in the Cochrane Library. No new content added after this issue.
ClinicalTrials.gov (clinicaltrials.gov; searched 20 January 2017).
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 20 January 2017).
We searched the following sources in 2011, but not for this update:
Australian New Zealand Clinical Trials Registry (ANZCTR; anzctr.org.au/trialSearch.aspx). The content of this register is included in WHO ICTRP.
metaRegister of Controlled Trials (isrctn.com/page/mrct). Reported as "under review" in 2015 and 2016.
National Research Register Archive. This service is no longer available.
UK Clinical Research Network (UKCRN). This service is no longer available.
Searching other resources
We examined reference lists of included studies and other review articles to identify relevant studies. We contacted authors of the identified RCTs to request further information. We also contacted pharmaceutical companies to request information about any published or unpublished trials using atypical antipsychotics for disruptive behaviour disorders in children and youths.
Data collection and analysis
Selection of studies
Two review authors (JL and KS) independently examined the titles and abstracts of all records obtained through the search strategy. The same two authors obtained and independently assessed the full texts of relevant reports appearing to meet the inclusion criteria. The two authors discussed any conflicts of opinion and, if necessary, called upon another review author (SM) to arbitrate until consensus was reached. They recorded their decisions in a PRISMA diagram (Moher 2009).
Data extraction and management
Two authors (JH and KS) carried out data extraction independently. They discussed any disagreements with another review author (SM) until consensus was reached.
They extracted the data listed below.
Study methods
Randomisation method (i.e. sequence generation).
Method of allocation concealment.
Blinding method (for those giving the treatment, participants, outcome assessors).
Stratification factors (if relevant).
Participants
Inclusion and exclusion criteria.
Number (total or per group).
Age distribution.
Gender.
Ethnicity.
Comorbidity.
Setting.
Intervention
Type of medication.
Dosage.
Length of prescription.
Mode of delivery.
Outcome data
Reduction of aggression; scale used.
Reduction of conduct problems; scale used.
Social functioning; scale used.
General functioning; scale used.
Family functioning; measurement method.
Parent satisfaction; measurement method.
School functioning; measurement method.
Duration of follow‐up.
Loss to follow‐up and any reasons given by investigators for same.
Non‐compliance: proportion of participants discontinuing treatment.
Analysis data
Methods of analysis (intention‐to‐treat or per‐protocol analysis).
Comparability of groups at baseline (yes or no).
Any other statistical techniques used by the investigators.
Safety data
Adverse events (overall incidence).
Weight gain; lipid and glucose profile, if available.
Breakdown by type of adverse events, taking into consideration frequency, severity and clinical importance.
JL and KS individually entered data into Cochrane's software for developing reviews: Review Manager 5 (RevMan 5) (Review Manager 2014). We compared extracted data to ensure accuracy. We resolved any discrepancies by consensus.
Assessment of risk of bias in included studies
For each included study, two review authors (JH and KS) independently assessed risk of bias, using the seven domains set out below from Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (hereafter referred to as the Cochrane Handbook; Higgins 2011b), with ratings of low, high and unclear risk of bias. We assigned these ratings based on the guidelines included in Table 8.5.d: "Criteria for judging risk of bias" in the Cochrane Handbook (Higgins 2011b). Please see the Characteristics of included studies tables for full details.
Sequence generation – the method used to generate the allocation sequence to determine if it produced comparable groups.
Allocation concealment – the method used to conceal allocation sequence to ensure that participants and investigators enrolling participants could not foresee group assignment.
Blinding of participants and personnel – the methods used to ensure that participants and personnel were blind to treatment allocation.
Blinding of outcome assessors – the methods used to ensure that those assessing outcomes were blind to treatment allocation.
Incomplete outcome data – the methods for dealing with incomplete data and the extent of details on attrition and withdrawals.
Selective outcome reporting – the completeness of data reported in the published trial as compared to prespecified outcomes measures, protocol or trial registry.
Other risk of bias – whether the trial had other problems such as methodological shortcomings or reporting discrepancies.
We did not exclude studies from meta‐analysis on the basis of the 'Risk of bias' assessment.
Measures of treatment effect
For continuous outcomes, where studies used the same outcome measure for comparisons, we pooled data by calculating the mean difference (MD) with 95% confidence intervals (CIs). Where different measures were used to assess the same outcome, we considered whether to pool data by calculating the standardised mean difference (SMD), with 95% CI.
There were no dichotomous data to include in this version of the review. Please refer to our protocol (Loy 2010) and Table 2 for methods archived for future updates of this review.
1. Methods specified in protocol and not used in this review.
| Analysis | Method |
| Measures of treatment effect | For dichotomous data, we planned to analyse data on the intention‐to‐treat principle with dropouts included in the analysis. Out of the 10 studies, 1 used dichotomous outcomes (Armenteros 2007), therefore we were not able to perform further analyses. |
| Unit of analysis issues | For cross‐over trials, we planned to do paired analysis if data were presented. Otherwise, we planned to take all measurements from intervention periods and all measurements from control periods and analyse these as if the trial was a parallel‐group trial, acknowledging that there might be unit of analysis errors that could underestimate the precision of the estimate of the treatment effect (Deeks 2011). However, no cross‐over trials were identified. Also, there were no cluster‐randomised controlled trials, so we did not have to take this into account in our analyses. |
| Dealing with missing data ‒ missing participants | We intended to calculate the best‐ and worst‐case scenarios for the clinical response outcome, if possible. For example, the best‐case scenario assumed that dropouts in the intervention group had positive outcomes and those in the control group had negative outcomes. In the worst‐case scenario, dropouts in the intervention group had negative outcomes and those in the control group had positive outcomes. |
| Assessment of heterogeneity | Chapter 9 in the Cochrane Handbook recommends using a range for I² and a guide to interpretation (Deeks 2011). Had we found either moderate heterogeneity (I² in the range of 30% to 60%) or substantial heterogeneity (I² in the range of 50% to 90%), as specified in our protocol (Loy 2010), we planned to examine it using specified subgroup and sensitivity analyses (see Subgroup analysis and investigation of heterogeneity and Sensitivity analysis). |
| Assessment of reporting bias | We intended to draw funnel plots (effect size versus standard error) to assess publication bias if sufficient studies were found. Asymmetry of the plots may indicate publication bias, although they may also represent a true relationship between trial size and effect size. If such a relationship were identified, we planned to examine the clinical diversity of the studies as a possible explanation (Egger 1997). There were insufficient studies in our meta‐analysis to perform a funnel plot. |
| Subgroup analysis and investigation of heterogeneity | It was our intention to conduct separate analyses on the following subgroups, where possible.
There were too few studies in any of the analyses for us to carry out any subgroup analyses. |
| Sensitivity analysis | We intended to perform sensitivity analyses to explore whether the results of the review were robust in relation to certain study characteristics. We intended to exclude trials with 'no' or 'unclear' ratings for allocation concealment and use the fixed‐effect model for our primary outcome. We identified a limited number of trials and we did not exclude any of them based on the ratings of allocation concealment. We were not able to carry out a sensitivity analysis due to the small number of trials. |
ADHD: attention deficit hyperactivity disorder
Unit of analysis issues
We did not encounter any unit of analysis issues in this review. For methods to manage unit of analysis issues in future updates of this review, please refer to our protocol (Loy 2010) and Table 2.
Dealing with missing data
Missing statistics
In the first instance, we attempted to contact the original researchers for any missing data. If only standard error (SE) or P values were reported, we calculated standard deviations (SD) and have documented this in the review.
Missing participants
For continuous data, if available, we used intention‐to‐treat data and noted the methods used by authors for imputing missing data, such as last observation carried forward.
For additional methods archived for future updates of this review, please see our protocol (Loy 2010) and Table 2.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing differences in the distribution of important participant factors between trials (for example, age, gender, specific diagnosis, duration and severity of disorder, associated comorbidities). We assessed methodological heterogeneity by comparing trial factors (randomisation, concealment, blinding of outcome assessment, losses to follow‐up). We assessed statistical heterogeneity by performing the Chi² test of heterogeneity, where a significance level of less than 0.10 was interpreted as evidence of heterogeneity, and by using the I² statistic, which calculated the percentage of variability due to heterogeneity rather than sampling error. We also presented Tau² – an estimate of between‐study variance (see Differences between protocol and review).
Please refer to our protocol (Loy 2010) and to Table 2, for additional methods to assess heterogeneity, which have been archived for future updates of this review.
Assessment of reporting biases
In order to assess outcome reporting bias, we compared what the authors said they would report with what they actually reported for the main clinical outcomes. We assessed whether authors provided actual data for each outcome or just reported statistical significance without actual data, as missing data could indicate reporting bias. We corresponded with authors whenever possible to seek clarification regarding unclear detail in the publications.
For additional methods to assess reporting bias, which have been archived for future updates of this review, please refer to our protocol (Loy 2010) and to Table 2.
Data synthesis
We performed a meta‐analysis only where studies were considered to have sufficiently similar participants, interventions, comparators and outcome measures. We used a random‐effects model to pool data since there was expected clinical diversity.
According to Chapter 12 in the Cochrane Handbook (Schünemann 2011), where different outcome measures are used, the SMD should be used to pool results; and where the outcome measure is the same, the MD should be used. The statistical advice we had was that while we can pool outcomes based on different measures using the SMD, this can only be done using final scores and not with mean change scores. This is because using the SMD makes the assumption that there is an equal correlation between the baseline and final scores in each trial or for each measure, and information is seldom provided to confirm this. If some studies have a small correlation between baseline and final scores and others have a large correlation then pooling these makes the result meaningless. This is also the reason that change and final scores cannot be combined using SMD. Therefore, we undertook separate meta‐analyses of those studies reporting outcomes as change scores and those reporting them as final scores.
Summary of findings
We used the GRADEpro Guideline Development Tool (GRADEpro 2014) to construct Table 1 for the main comparison: medication versus placebo. The table contains information on the anticipated absolute magnitude of effect for three outcomes (aggression; conduct problems; and weight gain) and the number of participants and studies. It also includes a rating (high, moderate, low or very low) for the overall quality of the evidence, which we assessed using the GRADE approach (Schünemann 2011). Evidence from randomised controlled trials began as high quality but we downgraded according to the presence of the following criteria: limitations in the design and implementation; indirectness of evidence; inconsistency of results; imprecision of results; and high probability of publication bias.
Subgroup analysis and investigation of heterogeneity
There was inadequate information to perform subgroup analyses due to few relevant studies, small sample sizes and, therefore, low power. If adequately powered, we would consider the following subgroup analyses: differences by types of medications; duration of treatment; presence/absence of ADHD; presence/absence of psychostimulants; and presence/absence of intellectual disability.
Please see our protocol (Loy 2010) and Table 2 for subgroup analyses archived for future updates of this review.
Sensitivity analysis
Sensitivity analyses to assess the impact of study risk of bias on the results of meta‐analysis were inappropriate, as there were a limited number of studies. Please see our protocol (Loy 2010) and Table 2 for sensitivity analyses archived for future updates of this review.
Results
Description of studies
Results of the search
In the previous version of the review (Loy 2012), we ran searches in June 2010 and August 2011 and screened a total of 2992 citations by title and abstract. We obtained the full‐text reports of 106 records and assessed these for eligibility. Of these, we included eight studies (from 11 reports), identified two ongoing studies and excluded 11 studies.
For this update, we revised our search strategies to include four new drugs (iloperidone, asenapine, lurasidone and paliperidone), and added the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) to our list of sources. We conducted our revised search for the period from August 2011 to January 2015, and re‐ran this search in February 2016 and January 2017. Overall, we found 2390 records: 2283 from electronic searches and 107 from searching other sources (trial registers). Having removed 461 duplicates, we screened 1929 records against our inclusion criteria (Criteria for considering studies for this review), and eliminated 1837 on the basis of title and abstract. We next obtained and assessed 92 full‐text reports for eligibility. We excluded 76 reports, 10 of which are discussed in the Excluded studies section. We included 12 new reports of the TOSCA study; plus one additional report brought forward from the 2012 review (Loy 2012), when it had still been an ongoing trial; and one new report of another previously ongoing study in a conference poster (Fleischhaker 2011). See Included studies. In addition, we identified one new ongoing study (NCT00794625), and two studies of potential interest, which we have listed as 'awaiting classification' (NCT02063945; IRCT201211051743N10).
Please see Figure 1.
1.
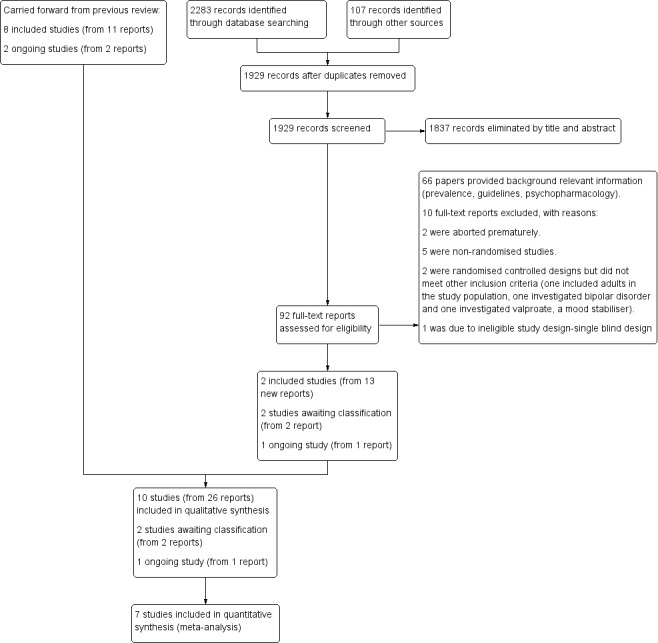
study flow diagram
Included studies
This review includes 10 trials (from 26 reports). Eight trials (11 reports) were included in the previous version of the review (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; Connor 2008). This update includes two new trials (Fleischhaker 2011; TOSCA study), from 15 reports. 13 of these reports were identified from the updated searches and two were carried forward from the original review having previously been ongoing studies
In the secondary paper to the TOSCA study, Gadow 2014 expanded on prior research by examining treatment effects on ADHD, disruptive behaviour symptoms and informant discrepancy. The main outcome was ADHD symptom severity rating assessed by the ADHD Symptom Checklist‐4 (ADHD‐SC4; Gadow 2008), Peer Conflict Scale (Gadow 1986), and the Child and Adolescent Symptom Inventory‐4R (CASI‐4R; Gadow 2005). Gadow 2014 reported that at least one half of the teacher data for the ADHD‐SC4 scale were missing for 50/117 children (43%) for various reasons, including difficulties in synchronizing the clinical trial with the child's school year, parent‐school conflicts and different levels of teacher involvement across sites (p 950). Intention‐to‐treat or 'last observation carried forward' analysis was used in Gadow 2014. We decided not to analyse this paper in depth for the following reasons: the significant amount of missing teacher data; the rating scales used in the study were not the ones commonly available or used; and the domains we are interested in, for example aggression, were based on a further subgroup analysis.
Location of studies
Four trials were multicentre (Aman 2002; Snyder 2002; Reyes 2006a; TOSCA study), and included data from several countries (Belgium, Canada, Germany, Great Britain, Israel, Netherlands, Poland, South Africa, Spain and the USA). The other trials were conducted in the USA (Findling 2000; Armenteros 2007; Connor 2008), the Netherlands (Buitelaar 2001), Belgium (Van Bellinghen 2001) and Germany (Fleischhaker 2011).
Study designs
All 10 included studies were randomised controlled trials. They spanned the period 2000 to 2014. Eight assessed risperidone (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; TOSCA study); one assessed quetiapine (Connor 2008); and one assessed ziprasidone (Fleischhaker 2011).
Sample sizes ranged from 13 to 335. In five trials, the total number of participants was 25 or fewer (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Armenteros 2007; Connor 2008). Five were pilot trials (Findling 2000; Van Bellinghen 2001; Armenteros 2007; Connor 2008, Fleischhaker 2011). Three trials had 115, 110 and 168 participants (Aman 2002; Snyder 2002; TOSCA study respectively), and one had 335 participants (Reyes 2006a).
All trials used inactive placebo as control.
The latest trial, 'treatment of severe childhood aggression' (referred to as the TOSCA trial), involved a complex trial design with three treatment components and may be viewed as an augmentation study or a combination treatment study (TOSCA study). It was a two‐stage, nine‐week parallel group, double‐blind, randomised controlled trial of risperidone ('augmented' = active) and placebo ('basic' = placebo) added to parent training and stimulant. The parents/guardians of all participants received parent training in strategies of behaviour management from baseline and throughout the nine weeks. Stage one consisted of three weeks' open‐label stimulant and stage two consisted of six weeks of a double‐blinded, placebo‐controlled comparison of added risperidone versus placebo. Only those participants who evidenced less‐than‐optimal response to stimulant were given the second medication (placebo or risperidone).
The timing of randomisation was one of the design challenges discussed in Farmer 2011. This was partly determined by the National Institute of Mental Health (NIMH) review process. The original proposal was to randomise less‐than‐optimal stimulant responders to either risperidone or placebo for six weeks (at the end of week three) but the NIMH review committee was concerned about attrition before the randomisation. Therefore, the final decision was to randomise enrolled participants at baseline (week 0) to the two treatment strategies — stimulant plus placebo versus stimulant plus risperidone — with the parents/guardians of all participants receiving parent training in strategies of behaviour management. Participants who responded optimally to the stimulant alone in stage one (i.e. in the first three weeks) were not given the second medication (risperidone or placebo).
The trial of ziprasidone was a double‐blinded, parallel‐group, randomised controlled trial, including a three‐week baseline period for finding the best individual dose, a six‐week treatment period and a two‐week washout period (Fleischhaker 2011).
One trial was a three‐stage trial that included a six‐week open‐label phase, followed by a six‐week single‐blind phase of risperidone and then a six‐month maintenance, double‐blind, randomised controlled trial (Reyes 2006a). It is important to note that randomisation occurred not at the acute phase but only after participants responded to active treatment. The objective of the study was to evaluate long‐term maintenance treatment. Participants were excluded from the trial once they had symptom recurrence.
The remainder of the studies were between four weeks and 10 weeks in duration, with follow‐up from four weeks in two trials (Van Bellinghen 2001; Armenteros 2007) to six weeks in five trials (Buitelaar 2001; Aman 2002; Snyder 2002; Connor 2008; TOSCA study), nine weeks in one trial (Fleischhaker 2011; three weeks of titration followed by six weeks of fixed‐dose medication), and 10 weeks in another trial (Findling 2000).
Three trials used a one‐week placebo run‐in (Aman 2002; Snyder 2002; Connor 2008) or ‘single‐blind placebo phase’ (Connor 2008), after which placebo responders were excluded from further participation in the trial.
Participants
Participants were between five and 18 years of age. In eight trials, there were significantly more males than females (Findling 2000; Buitelaar 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; Connor 2008, TOSCA study). Eight trials included outpatients while one trial included children from residential care (Van Bellinghen 2001) and one trial included inpatients (Buitelaar 2001).
Six studies included a significant number of participants with sub‐average to borderline intelligence quotient (IQ; 36 to 84) (not Armenteros 2007; Connor 2008 or TOSCA study). One study stipulated that patients must have an IQ over 55 but did not report demographic features of the participants, including average IQ (Fleischhaker 2011).
Studies varied in their inclusion criteria for participants. Aman 2002, Snyder 2002 and Reyes 2006a included participants with DSM‐IV criteria for conduct disorder, oppositional defiant disorder and disruptive behaviour disorder not otherwise specified (American Psychiatric Association 2000), and included participants with comorbid ADHD. Buitelaar 2001 included participants with DSM‐IV criteria for conduct disorder, oppositional defiant disorder and ADHD (American Psychiatric Association 2000). Findling 2000 and Connor 2008 included participants with conduct disorder only. Armenteros 2007 specifically looked at participants with DSM‐IV criteria for ADHD (American Psychiatric Association 2000) and a specific aggression criterion, as it was an ADHD augmentation trial, while Van Bellinghen 2001 included participants with symptoms of "persistent behavioural disturbances including hostility, aggression, irritability, agitation and hyperactivity" rather than DSM‐IV diagnoses. The TOSCA study included participants with DSM‐IV (American Psychiatric Association 2000) diagnoses of ADHD with comorbid conduct disorder or oppositional defiant disorder. Fleischhaker 2011 included participants with DSM‐IV diagnoses of conduct disorder, oppositional defiant disorder and disruptive behaviour disorder not otherwise specified (American Psychiatric Association 2000); there was no detail provided about comorbidity in this trial.
Six trials included ADHD comorbidity. In the TOSCA study, all participants had ADHD with comorbid conduct disorder or oppositional defiant disorder, and all participants had stimulant treatment titrated in the three‐week lead‐in period.
In Armenteros 2007, ADHD was the main diagnosis. In Van Bellinghen 2001, ADHD comorbidity was not stated other than that one participant in the placebo group was on Ritalin, which was discontinued during the trial. In Findling 2000, there was no information on ADHD comorbidity. With the exception of the TOSCA study, doses of concomitant stimulant medications were not mentioned in the trials.
Interventions
In one study for quetiapine, the mean dose at endpoint was 294 (± 78) mg/day, with a range of 200 to 600 mg/day (Connor 2008).
In one study for ziprasidone, the maximum intended daily dose was 20 mg for patients with a body weight of 50 kg or less, and 40 mg for patients with a body weight more than 50 kg. No endpoint mean dose was reported (Fleischhaker 2011).
In the earlier studies (2000 to 2007) the mean doses of risperidone at endpoint ranged from 0.98 mg/day to 1.5 mg/day. In the most recent study (TOSCA study), the mean endpoint dose of risperidone was 1.7 (± 0.75) mg/day in the active arm and 1.9 (± 0.72) mg/day in the placebo arm.
All trials used the oral method of antipsychotic administration, four of them using risperidone solution (Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a), and the rest using oral preparation.
The duration of intervention was four weeks in two trials (Van Bellinghen 2001; Armenteros 2007); six weeks in six trials (Buitelaar 2001; Aman 2002; Snyder 2002; Connor 2008; Fleischhaker 2011; TOSCA study); and 10 weeks in one trial (Findling 2000).
One trial investigated risperidone maintenance (Reyes 2006a). In this trial, after two 6‐week phases (open‐label followed by single‐blind risperidone treatment), all responders were randomised to six months' maintenance of risperidone or placebo. The primary efficacy measure was time‐to‐symptom recurrence.
In the TOSCA study, the mean endpoint dose of methylphenidate (stimulant) for the placebo group was 44.8 (± 14.6) mg/day compared with 46.1 (± 16.8) mg/day in the active group. The parents/guardians of all participants (both active and placebo arms) received parent training in strategies of behaviour management throughout the trial.
With the exception of the TOSCA study (as detailed above), the rest of the trials dealt with psychosocial interventions in the following way: one trial specified that participants had to have failed psychosocial treatment (contingency management and social skills training) before starting medication (Buitelaar 2001). Aman 2002 permitted behavioural therapy that had started 30 days before the trial. Armenteros 2007 and Connor 2008 allowed "pre‐existing or current psychosocial interventions". There was no information in the rest of the trials as to how many participants actually had concomitant psychosocial treatments or further details of those treatments.
With the exception of the TOSCA study, doses of concomitant stimulant medications were not mentioned in the trials.
Outcomes
Primary outcomes
Details of the standardised scales used to assess aggression and conduct problems are presented in additional Table 3 and Table 4.
2. Rating scales used in included trials to assess aggression.
| Name of rating scale | Description | Construction | Study | Source of Information used in the study |
| Aberrant Behaviour Checklist (ABC) (Aman 1985a; Aman 1985b) | Symptom checklist for assessing problem behaviours of children and adults with mental retardation. It is also used for classifying problem behaviours of children and adolescents with mental retardation. | 58 items, 5 scales.
|
Van Bellinghen 2001 Aman 2002 Snyder 2002 |
Parent/caregiver |
| Child Behaviour Checklist (CBCL) (Achenbach 1991) |
Checklist for evaluating maladaptive behavioural and emotional problems. | 113 items, 8 subscales.
|
Findling 2000 | Parent |
| Overt Aggression Scale (OAS) (Yudofsky 1986) | Assesses the severity and frequency of overt aggression. | 25 items, 4 subscales.
Within each category, aggressive behaviour is rated according to its severity. |
Connor 2008 | Parent |
| Overt Aggression Scale ‒ Modified (OAS‐M) (Kay 1988) | Assesses the severity and frequency of overt aggression. | 20 items, 4 subscales.
5‐point interval scale that represents increasing level of aggression. The total aggression score is obtained by multiplying the 4 individual scales by weights of 1, 2, 3 or 4 and then summing the 4 weighted scores. |
Buitelaar 2001 | Nurse or teacher |
| Rating of aggression against people and/or property scale (RAAP) (Kemph 1993) | ‐ | Global rating scale, 1 item. Scored from 1 (no aggression reported) to 5 (intolerable behaviour). |
Findling 2000 | Clinician |
| Children's Aggression Scale ‒ Parent (CAS‐P; Halperin 2002) and Teacher (CAS‐T; Halperin 2003) | Retrospectively measures the frequency and severity of 4 categories of aggression: verbal aggression; aggression against objects and animals; provoked physical aggression; and initiated physical aggression | Respondents (parents/guardians and teachers) complete a Likert scale to evaluate the frequency of an act. The frequency of aggressive events is multiplied by its designated severity weight factor and then summed to yield a total score. | Armenteros 2007 | Parent and teacher |
| Antisocial Behavior Scale (ABS) Proactive and Reactive Subscales (Brown 1996) | Instrument used to differentiate reactive/affective from proactive subtypes of aggression | 28 items. Proactive Aggression subscale: 5 proactive items and 5 covert antisocial items. Reactive Aggression subscale: 6 items. |
TOSCA study | Parent |
3. Rating scales used in the reviewed trials to assess conduct problems.
| Name of rating scale | Description | Construction | Study | Source of information used in the study |
| Conners' Parent Rating Scale (CPRS) (Conners 1989) | Checklist for assessing behavioural and emotional difficulties. | 48 items, 6 subscales.
|
Findling 2000 Connor 2008 |
Parent |
| Nisonger Child Behaviour Rating Form (NCBRF) (Aman 1996; Tassé 1996) | Assesses behaviour of children and adolescents with intellectual disability or autism spectrum disorders, or both. | 76 items, 8 subscales.
|
Findling 2000 Aman 2002 Snyder 2002 Reyes 2006a |
Parent |
| Nisonger Child Behavior Rating Form ‒ Typical IQ D‐Total (includes conduct problems and oppositional subscales) | Typical IQ version: assesses behaviour of children and adolescents with normal IQ. | 10 items, 1 prosocial subscale.
54 items, 6 problem behaviour subscales.
|
TOSCA study | Parent |
IQ: intelligence quotient.
Aggression
In one study, Reyes 2006a, aggression was not a specific outcome.
Eight studies — Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Armenteros 2007; Connor 2008; TOSCA study — assessed aggression using the rating scales below (endpoints fall between four and 10 weeks).
Aberrant Behaviour Checklist (ABC) ‒ Irritability subscale (Aman 1985a; Aman 1985b), used in three studies (Van Bellinghen 2001; Aman 2002; Snyder 2002).
Child Behaviour Checklist (CBCL) ‒ Aggression subscale (Achenbach 1991), used in one study (Findling 2000).
Overt Aggression Scale (OAS) (Yudofsky 1986), used in one study (Connor 2008).
Overt Aggression Scale ‒ Modified (OAS‐M) (Kay 1988), used in one study (Buitelaar 2001).
Rating of aggression against people and/or property scale (RAAP) (Kemph 1993), used in one study (Findling 2000).
Children's Aggression Scale ‒ Parent (CAS‐P) and Teacher (CAS‐T) (Halperin 2002; Halperin 2003), used in one study (Armenteros 2007).
Antisocial Behavior Scale (ABS) ‒ Proactive and Reactive Behavior subscales (Brown 1996), used in one study (TOSCA study).
Conduct problems
Three studies did not measure conduct problems (Buitelaar 2001; Van Bellinghen 2001; Armenteros 2007).
Seven studies — Findling 2000; Aman 2002; Snyder 2002; Reyes 2006a; Connor 2008; Fleischhaker 2011; TOSCA study — assessed conduct problems using the rating scales listed below (endpoints fall between six weeks to six months).
Nisonger Child Behaviour Rating Form ‒ Conduct Problem subscale (NCBRF‐CP) (Aman 1996; Tassé 1996), used in four studies (Findling 2000; Aman 2002; Snyder 2002; Reyes 2006a).
Conners' Parent Rating Scale ‒ Conduct Problem subscale (CPRS‐CP) (Conners 1989), used in one study (Connor 2008).
Nisonger Child Behaviour Rating Form (NCBRF) Typical IQ, D‐Total (consisting of conduct disorder and oppositional defiant disorder subscales) (Aman 2008), used in two studies (Fleischhaker 2011; TOSCA study).
Adverse events
With the exception of one study, Fleischhaker 2011, which did not report on weight gain, all trials assessed weight gain in kilograms. Three studies presented mean weight gain and SDs (Findling 2000; Aman 2002; Reyes 2006a). One study, the TOSCA study, reported baseline and endpoint mean weight and SDs for both arms. Metabolic parameters were only available in two trials (Reyes 2006a; TOSCA study).
Secondary outcomes
General functioning
Only one trial, Reyes 2006a, assessed general functioning, using the Children's Global Assessment Scale (CGAS) (Shaffer 1983).
Non‐compliance
Data on non‐compliance and attrition rate were available from all 10 trials (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; Connor 2008; Fleischhaker 2011; TOSCA study), and are reported in the 'Risk of bias' tables, beneath the Characteristics of included studies tables.
Other adverse events
Data on other adverse events were available from all 10 trials (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; Connor 2008; Fleischhaker 2011; TOSCA study), and are presented in Table 5.
4. Other adverse events.
| Study ID | General | Neurological | Gastrointestinal | Respiratory | Cardiovascular/Metabolic |
Serious adverse event (unspecified) |
Other |
|
Armenteros 2007 (risperidone = 12, placebo = 13) |
|
|
|
‐ | Not reported | ‐ | ‐ |
|
Buitelaar 2001 (risperidone = 19, placebo = 19) |
|
|
|
|
Not reported | ‐ | ‐ |
|
Connor 2008 (quetiapine = 9, placebo = 10) |
|
|
‐ | ‐ | No differences across groups found on ECG QRS or QTc intervals. | ‐ | ‐ |
|
Findling 2000 (risperidone = 10, placebo = 10) |
|
‐ |
|
‐ | No clinically significant changes in ECG. | ‐ |
|
|
Van Bellinghen 2001 (risperidone = 6, placebo = 7) |
No side effects reported in any category. | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Aman 2002 (risperidone = 55, placebo = 63) |
|
|
|
|
|
‐ | ‐ |
|
Reyes 2006a (risperidone = 172, placebo = 163) |
|
|
|
|
|
|
‐ |
|
Snyder 2002 (risperidone = 53, placebo = 57) |
|
|
|
|
|
|
|
|
TOSCA study (risperidone = 73, placebo = 80) |
|
|
|
|
|
‐ |
|
|
Fleischhaker 2011 (ziprasidone = 25, placebo = 25) |
|
|
|
|
|
|
|
Bpm: beats per minute; ECG: electrocardiogram; URTI: upper respiratory tract infection; EPSE: Extrapyramidal side effects; QRS: the name for the 3 waves (Q wave, R wave and S wave) on an electrocardiogram; QTc: correct QT (start of Q wave to end of T wave) interval
Social functioning
One trial, Van Bellinghen 2001, assessed social functioning, using the Personal Assessment Checklist (PAC), part of which rated social relationships. It was unclear if PAC was a validated measure.
Family functioning
No trial set out to examine this outcome.
Parent satisfaction
A secondary paper to the TOSCA study examined participants' parents' satisfaction with the TOSCA study overall, with special attention to parents' satisfaction with the parent training component (Rundberg‐Rivera 2015). No other trial set out to examine this as an outcome.
Functioning at school
No trial set out to examine this outcome.
Excluded studies
For full details, please see Characteristics of excluded studies.
In the previous version of this review (Loy 2012), we excluded 11 studies because they did not meet all our inclusion criteria (Buitelaar 2000; Soderstrom 2002; Turgay 2002; Findling 2004; Croonenberghs 2005; Findling 2006; Handen 2006; Masi 2006; Reyes 2006b; Haas 2008; Tyrer 2008). Tyrer 2008 was a RCT of risperidone, haloperidol and placebo in the treatment of aggressive, challenging behaviour in adults with intellectual disability. Three studies were on olanzapine; one was a clinical case series of six aggressive youths (Soderstrom 2002), one was a retrospective chart review of olanzapine treatment in adolescents with conduct disorder (Masi 2006), and one was an open‐label prospective trial of olanzapine in youths with disruptive behaviour disorder and below‐average intelligence (Handen 2006). One study was an open‐label trial of quetiapine in aggressive children with conduct disorder (Findling 2006). There was one open‐label study of risperidone in inpatient children and youths with psychiatric disorders associated with aggressive behaviour (Buitelaar 2000). There were five studies that were long‐term, open‐label studies of risperidone in children with disruptive behaviour disorders, four of which were up to a year's duration (Turgay 2002; Findling 2004; Croonenberghs 2005; Haas 2008), and one of which was up to three years' duration (Reyes 2006b).
In this updated review, we excluded eight trials for not meeting our inclusion criteria. NCT00550147 was an open‐label study of quetiapine added to methylphenidate in the treatment of ADHD and aggressive behaviour. Teixeira 2013a was an open, naturalistic study of clozapine in seven boys with severe conduct disorder over 26 weeks. Blader 2013 examined open titration and optimisation of stimulant monotherapy in 160 children. Holzer 2013 was an open‐label trial of atomoxetine and olanzapine in ADHD with comorbid disruptive behaviour disorder in children and adolescents. Kuperman 2010 was an open‐label trial of aripiprazole in the treatment of conduct disorder in adolescents. In Tramontina 2009, the investigators assessed response to treatment with aripiprazole in children and adolescents with bipolar disorder and comorbid ADHD in a pilot randomised controlled trial. Blader 2009 examined adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Divalproex is considered to be a mood stabiliser and not an antipsychotic and does not fall under the scope of the review. Safavi 2016 was excluded due to ineligible methodology; it was a single‐blind design, rather than double blind, and compared the effects of methylphenidate, and methylphenidate and risperidone combined, in preschool children with attention deficit hyperactivity disorder.
Two additional trials would have met our criteria but were aborted prematurely. NCT00279409 was terminated due to slow rate of recruitment. It was originally called "Treatment of Children With ADHD Who do Not Fully Respond to Stimulants (TREAT)". The active comparator (also called the "combination arm") was parent training plus continued treatment on a stimulant plus augmentation with aripiprazole. Placebo comparator (also called the "simple treatment" arm) was parent training plus continued treatment on a stimulant plus a placebo matching aripiprazole. The ISRCTN95609637 study — a trial which was part of the Pediatric European Risperidone Project (PERS) — was abandoned according to the information on the research registry with no further information given. According to the PERS project website (Pediatric European Risperidone Studies (PERS) 2016), it was put on hold with no patients enrolled since 2013, as the British regulatory agency (Medicines & Healthcare products Regulatory Agency (MHRA)) put a recall on their medicine, followed by withdrawal of the trial sponsor in 2014. The reason, at the time, was that risperidone was one of 16 prescription medicines made at an Indian factory which failed a routine inspection. In the inspection, they found some risk of cross‐contamination due to poor cleaning practices, defects in building fabric and the ventilation systems at the site. There was also evidence of forged documents relating to staff training records, which had been rewritten (British Broadcasting Corporation (BBC) 2013).
Studies awaiting assessment
We identified two studies awaiting assessment (NCT02063945; IRCT201211051743N10). One study has not yet been published and it is unclear if it will be published in Persian or English, as it is an Iranian study (IRCT201211051743N10). We are awaiting publication of NCT02063945 to assess for possible exclusion, as it is likely to be an open‐label trial.
Ongoing studies
We identified one ongoing study called "Effectiveness of Combined Medication Treatment for Aggression in Children With Attention Deficit With Hyperactivity Disorder (The SPICY Study)" (NCT00794625). It is a double‐blinded, randomised controlled trial involving children (male and female), aged six to 12 years, with attention deficit disorder with hyperactivity (ADHD). The purpose of this trial is to determine the advantages and disadvantages of adding one of two different types of drugs to stimulant treatment for reducing aggressive behaviour in children with ADHD. During phase one, participants will receive a stimulant medication. If they do not respond to the stimulant, valproate and behavioural family counselling will be added to their treatment during phase two. If they do not respond to valproate, they will be switched to risperidone. The primary outcome is aggressive behaviour. The secondary outcomes are ADHD symptoms. The time frame is weekly follow‐up for 11 to 16 weeks. The recruitment status of this study is unknown. We have written to the author but have not received a reply at time of updating this review (Loy 2016c [pers comm]).
Risk of bias in included studies
We provide a brief description of our 'Risk of bias' assessment below. For more detailed information, please see the 'Risk of bias' tables, beneath the Characteristics of included studies tables, Figure 2 and Figure 3.
2.
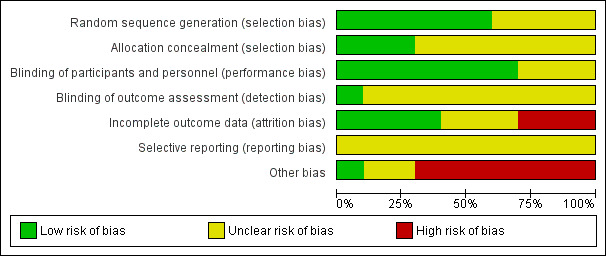
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
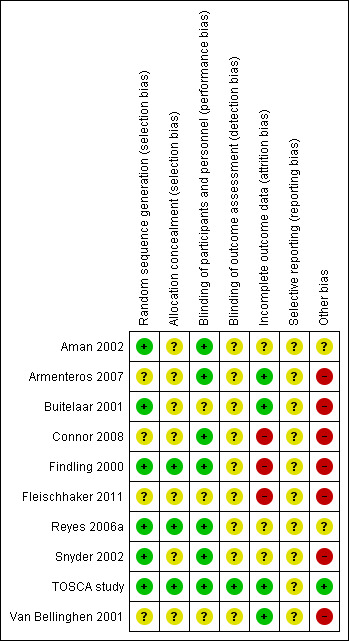
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged sequence generation to be at low risk of bias for six trials (Findling 2000; Buitelaar 2001; Snyder 2002; Reyes 2006a; Armenteros 2007; TOSCA study). In the remaining four trials, we judged the risk of bias to be unclear: sequence generation was not described in two trials (Van Bellinghen 2001; Connor 2008); and there was insufficient information available for two other trials (Aman 2002; Fleischhaker 2011).
Allocation concealment
We judged allocation concealment to be at low risk of bias for three trials (Findling 2000; Reyes 2006a; TOSCA study). Findling 2000 used a random number list. The list was kept in the Center for Drug Research and was not accessible to either the primary investigator or other study raters. Reyes 2006a used treatment numbers allocated at each investigative centre in chronological order. Randomisation assignment in the TOSCA study was completed through a secured website. The unblinded medication dispenser entered the appropriate information into the website and an email with the participant’s treatment assignment was sent to the dispenser and to the statistician at the time. Randomisation assignment for each participant was printed and sealed in an envelope for study emergency only (Loy 2016a [pers comm]). In the other seven trials, the method of concealment was not described and we judged the risk of bias to be unclear (Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Armenteros 2007; Connor 2008; Fleischhaker 2011).
Blinding
Blinding of participants and personnel
We judged seven trials to be a low risk of bias for blinding of participants and personnel (Findling 2000; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007; Connor 2008; TOSCA study). For two trials, details of blinding were not described (Van Bellinghen 2001; Fleischhaker 2011), and for another trial we judged details of blinding to be inadequate (Buitelaar 2001), and therefore rated all three studies at unclear risk of bias.
Blinding of outcome assessors
Only one study provided significant detail on the blinding of outcome assessors (albeit some of this information was obtained by email correspondence (Loy 2016b [pers comm]) and we rated it at low risk of bias (TOSCA study). The other studies did not include sufficient details on the blinding of outcome assessors and therefore we deemed the risk of bias to be unclear for all.
Incomplete outcome data
Overall, we judged four trials — Buitelaar 2001, Van Bellinghen 2001, Armenteros 2007, and the TOSCA study — to be at low risk of attrition bias for the following reasons. Van Bellinghen 2001 did not have any dropouts in either the treatment (6/6) or placebo (7/7) arms. Buitelaar 2001 and Armenteros 2007 clearly stated the reasons for all the dropouts in their articles. TOSCA study displayed detailed reasons for participant attrition in table S1 and S2 (p 60e1). Complete patient flow charts were available for Armenteros 2007 and the TOSCA study.
We judged three trials to be at high risk of attrition bias (Findling 2000; Connor 2008; Fleischhaker 2011). Both Findling 2000and Connor 2008 had 70% attrition rate in their placebo arm. The reasons for the attrition were clearly articulated. A patient flow chart was available for Connor 2008. The reasons for attrition in Fleischhaker 2011 were inadequately described and the narrative description and the flow of participants' diagram did not match. Furthermore, Fleischhaker 2011 did not report participant allocation and attrition using CONSORT standards.
We judged three trials to be at unclear risk of attrition bias (Aman 2002; Snyder 2002; Reyes 2006a). For Aman 2002, no efficacy data were recorded for three patients in the treatment arm and hence they were not included in any efficacy analyses (but the authors claimed to have used an intention‐to‐treat analysis). For Snyder 2002, there was discrepancy in dropouts between the table data, the narrative and graph in the published article. In Reyes 2006a, the reasons were only partially described (24 in the treatment arm discontinued, four due to side effects, 20 others not described; 25 in the placebo arm discontinued, four due to side effects, 21 others not described). A patient flow chart was available for Reyes 2006a.
Other than Van Bellinghen 2001 mentioned above who had no dropouts, for the other nine trials the attrition rate ranged from 0% to 40% in the treatment arm and 8% to 70% in the placebo arm (Aman 2002 ‒ 31 withdrawn (12 active treatment; 19 placebo) out of sample size of 118 (55 active treatment, 63 placebo); TOSCA study ‒ 14 withdrawn (11 active treatment, three placebo) out of sample size of 168 (84 active treatment, 84 placebo); Armenteros 2007 ‒ two withdrawn (one active treatment, one placebo) out of sample size of 25 (12 active treatment, 13 placebo); Buitelaar 2001 ‒ two withdrawn (from placebo) out of sample size of 38 (19 active treatment, 19 placebo); Connor 2008 ‒ eight withdrawn (one active treatment, seven placebo) out of sample size of 19 (nine active treatment, 10 placebo); Findling 2000 ‒ 11 withdrawn (four active treatment, seven placebo) out of sample size of 20 (10 active treatment, 10 placebo); Snyder 2002 ‒ 25 withdrawn (six active treatment, 19 placebo) out of sample size of 110 (53 active treatment, 57 placebo).
There was imbalance in the proportion missing between the treatment and placebo arms in three studies in particular (Findling 2000; Snyder 2002; Connor 2008). In Findling 2000, three out of 10 youths assigned to risperidone were withdrawn by their guardian because of lack of effect, and one youth who received risperidone was withdrawn from the study during week four due to the development of a rash (side effect). Four out of 10 patients assigned to placebo were withdrawn by their guardians because of lack of benefit, two more were withdrawn from the study by the principal investigator because of non‐compliance with study procedures, and one youth randomly assigned to placebo was lost to follow‐up. For Connor 2008, one out of nine participants withdrew from the medication (quetiapine) group due to side effects. Out of 10 participants in the placebo group, five withdrew due to lack of efficacy and two withdrew due to protocol violation. In Snyder 2002, six out of 53 participants dropped out of the risperidone group and 19 out of 57 participants dropped out from the placebo group. Reasons for discontinuation included: (i) insufficient response (two from risperidone group and 19 from placebo group); (ii) loss to follow‐up (one from risperidone group); and (iii) loss of parental consent (three from risperidone group). Thus in two of the studies (Snyder 2002; Connor 2008), the imbalance between the treatment and placebo arms was attributable to the lack of effect from the placebo.
In the TOSCA study, where the non‐responders only were given risperidone or placebo, the authors stated that they included data from all randomised participants based on intention‐to‐treat principles, and last observation carried forward was used to check the sensitivity of the primary analysis to the assumption that data were missing at random (Farmer 2011). The TOSCA study reported a completers' analysis only and this was confirmed in correspondence with the authors (Loy 2016b [pers comm]. In the TOSCA study paper it was stated that, "various sensitivity analyses were conducted to examine the robustness of results" (TOSCA study p 51); however, the sensitivity analysis was not published in the paper for comparison. From the correspondence (Loy 2016b [pers comm], the statistician of the TOSCA study confirmed that it was carried out and was found to be non‐significant (did not impact on the other results). The published attrition figures are as follows: 30 out of 168 participants were lost to follow‐up (baseline basic or placebo = 84; augmented or risperidone added = 84; week nine basic or placebo = 71; week nine augmented or risperidone added = 66).
In the reporting of missing data, no study in the review did a comparison between key baseline characteristics between individuals with missing and observed outcomes.
In the analysis of missing data, five studies used the last observation carried forward as an imputation method to address incomplete data (Buitelaar 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007). Criticisms against this method are based on the underlying assumption that an individual’s missing value has not changed from the previously measured value, that it fails to account for the uncertainty about missing values (Wood 2004; Sterne 2009), and that there is a risk that the resulting standard errors will be too small (Sterne 2009), or that CIs are too narrow (Higgins 2011a). One study used mixed‐effect longitudinal analysis (Connor 2008); and one study was not explicit in the method used (Findling 2000).
The assumptions made in the main analysis were not made explicit in the studies except for Connor 2008. No formal sensitivity analyses were performed by any study to explore the effect of departures from the assumptions made in the main analysis.
Selective reporting
No complete protocol was available for any of the 10 trials and we therefore judged all studies to be of unclear risk of bias. We considered the TOSCA study to be unclear as the primary outcomes were consistent but secondary outcomes were not listed fully. In addition, there may be a possible reporting bias in Armenteros 2007, as dichotomous results were presented while no differences in mean scores were detected.
Other potential sources of bias
There are further design issues that may introduce bias by overestimating the true intervention effect.
As the completer analysis was the only other potential source of bias, we rated one trial at low risk of bias on this domain (TOSCA study).
We judged two trials to be at unclear risk of other potential sources of bias (Aman 2002; Reyes 2006a). For Aman 2002, there was the use of a one‐week, placebo run‐in design. In general, the use of enriched designs, such as the use of placebo run‐in periods to exclude responders, may artificially inflate the numbers responding to the active drug. For Reyes 2006a, only patients who responded to the initial treatment were randomised, potentially introducing a selection bias. This was addressed, in part, by including a single‐blind phase.
We judged seven trials to be at high risk of other potential sources of bias (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Snyder 2002; Armenteros 2007; Connor 2008; Fleischhaker 2011). Findling 2000, Buitelaar 2001, Van Bellinghen 2001, Armenteros 2007, Connor 2008 and Fleischhaker 2011 had small numbers of participants, and therefore had limited power to detect differences. For Buitelaar 2001, we were unsure why 145 potential participants were approached and only 49 were found to be eligible. Both Snyder 2002 and Connor 2008 also used the one‐week, placebo run‐in design. In addition, for Snyder 2002 it was unclear if the two international sites in South Africa followed the same protocol. The quality of reporting for Fleischhaker 2011 is consistent with a poster presentation but not to the level of a peer‐review journal article.
The authors of nine trials, except for Fleischhaker 2011, stated that intention‐to‐treat analyses (ITT) had been undertaken; however, as pointed out above, the TOSCA study publication includes a completers' analysis only. A full application of the intention‐to‐treat principle is only possible when complete outcome data are available for all randomised participants (Hollis 1999). As noted above, no efficacy data were recorded for three participants in the treatment arm of Aman 2002, and subsequently the Aman 2002 study presented the data for 52 out of the 55 participants randomised to the active arm. (Sample size: 118; 55 active treatment arm (results reported for 52 participants); 63 placebo arm).
None of the studies reported any conflict of interest with regard to the funding of the studies.
Effects of interventions
See: Table 1
Our initial aim was to have two meta‐analyses overall, one for aggression and one for conduct problems. However, the studies identified for inclusion reported results in two ways: some reported pre‐intervention and postintervention means, while others presented mean change scores. Despite our best efforts, we were unable to obtain data from the authors to allow us to get the data in a consistent format. In addition, some studies used the same measures while others used different measures.
According to Chapter 12 in the Cochrane Handbook (Schünemann 2011), where different outcomes measures are used, the SMD should be used to pool results; and where the outcome measures are the same, the MD should be used. However, it is more complex when the results are presented differently, as outlined above.
We were advised that while we can pool outcomes based on different measures using the SMD, this can only be done using final scores or mean change scores but not a combination of both. This is because using the SMD makes the assumption that there is an equal correlation between the baseline and final scores in each trial or for each measure but information is seldom provided to confirm this. If some studies have a small correlation between baseline and final scores and others have a large correlation then pooling these studies makes the result meaningless. This is also the reason that change and final scores cannot be combined using SMD.
Therefore, we undertook separate analyses. For aggression, the first meta‐analysis uses the MD method to pool data, as we had several studies that used the same outcome measure. The second uses the SMD to combine outcome data from two different measures (both reported final scores). We used a similar approach for the outcome 'conduct problems'.
Comparison: medication versus placebo
Primary outcomes
1. Aggression
The first meta‐analysis of aggression included three trials (Van Bellinghen 2001; Aman 2002; Snyder 2002), which employed the Aberrant Behaviour Checklist (ABC) ‒ Irritability subscale (15 items yielding a maximum of 45 points; Aman 1985a). Results for the ABC‐Irritability subscale yielded a final mean score on treatment that was 6.49 points lower on this subscale than with placebo, in favour of risperidone (95% CI −8.79 to −4.19, Tau² = 0, I² = 0%, P < 0.00001, 3 trials, 238 participants, low‐quality evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 1 Aggression: ABC irritability (mean change scores).
In the next meta‐analysis we combined two trials (TOSCA study; Buitelaar 2001). We used the SMD as the trials used different rating scales. The rating scales were OAS‐M (Coccaro 1991); and Antisocial Behaviour Scales (ABS) (Elliot 1985). The ABS consisted of Reactive and Proactive subscales (parent‐rated), which could not be combined to give a total score. Therefore, we conducted separate meta‐analyses with ABS Reactive and ABS Proactive subscales. The Reactive items measured "hot" aggression while the Proactive subscale was said to measure "cold" aggression (TOSCA study). Combining data from the ABS Reactive subscale and the OAS‐M, yielded an SMD of −1.30, suggesting a significant effect in favour of risperidone (95% CI −2.21 to −0.40, Tau² = 0, I² = 0, P value = 0.005, 190 participants, moderate‐quality evidence; Analysis 1.2). In contrast, combining data from the ABS Proactive subscale and the OAS‐M, yielded an SMD of −1.12 (95% CI −2.30 to 0.06, Tau² = 0, I² = 0, P value = 0.06, 190 participants, moderate‐quality evidence; Analysis 1.3), suggesting uncertainty about the estimate of effect, as the CIs overlapped the null value.
1.2. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 2 Aggression: OAS‐M, ABS Reactive subscale (final scores).
1.3. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 3 Aggression: OAS‐M, ABS Proactive subscale (final scores).
The data for meta‐analyses for aggression came from short‐term studies (Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; TOSCA study).
2. Conduct problems
We combined data from two trials, Aman 2002 and Snyder 2002, both of which employed the Nisonger Child Behaviour Rating Form ‒ Conduct Problem subscale (NCBRF‐CP; 16 items yielding a maximum of 48; Aman 1996) in a meta‐analysis. The results yielded a mean score at the end of the intervention period that was 8.61 points lower than that on placebo, in favour of risperidone (95% CI −11.49 to −5.74, Tau² = 0, I² = 0, P < 0.00001, 225 participants, moderate‐quality evidence; Analysis 1.4).
1.4. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 4 Conduct: NCBR‐CP (mean change scores).
We were unable to extract any appropriate data from the TOSCA study to include in the meta‐analysis of conduct problems. The TOSCA study used a new form of Nisonger Child Behavior Rating Scale Form ‒ Typical IQ (NCBRF; Aman 2008) while the earlier trials (Aman 2002 and Snyder 2002) used the low IQ NCBRF scale (Aman 1996). The TOSCA study utilised D‐Total as a primary outcome, which is a combination of conduct disorder and oppositional defiant disorder symptoms, while Aman 2002 and Snyder 2002 used the conduct disorder single subscales of the NCBRF ‒ low IQ version. Upon comparing the individual items between the low‐IQ and normal‐IQ subscales, we discovered there was insufficient overlap between the low‐IQ and normal‐IQ subscales to allow us to assume they measure the same construct. In effect, they needed to be treated as different rating scales for the purpose of the meta‐analysis. Because our existing conduct problems meta‐analysis was based on the MD method, we were unable to combine it with the TOSCA study data.
We excluded Reyes 2006a from the meta‐analysis, as the study's objectives and methodology were significantly different from the other trials (investigation of symptom recurrence up to six months, rather than six weeks' acute efficacy).
The data for meta‐analyses of conduct problems were from short‐term studies (Aman 2002; Snyder 2002).
3. Adverse events
i) Weight gain
We performed two meta‐analyses of weight gain.
In the first meta‐analysis, we pooled data from two trials that used antipsychotic medication only (Findling 2000; Aman 2002). Using the MD method, the results revealed that participants on risperidone gained, on average, 2.37 kilograms (kg) more than those in the placebo group over the treatment period of six to 10 weeks (MD 2.37, 95% CI 0.26 to 4.49, Tau² = 2.22, I² = 95%, P value = 0.03, 138 participants, moderate‐quality evidence; Analysis 1.5).
1.5. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 5 Weight gain (antipsychotic only): Kg (mean change scores).
In the second meta‐analysis, we pooled data from three trials (Findling 2000; Aman 2002; TOSCA study), noting that in the TOSCA study trial, all participants received stimulants as well as a risperidone (or placebo) during the course of the treatment. Stimulant medication has a counteracting effect on weight gain due to its appetite suppression. Using the MD method, the results revealed that participants in the intervention group gained, on average, 2.14 kg more than those in the placebo group over the treatment period of six to 10 weeks (MD 2.14; 95% CI 1.04 to 3.23, Tau² = 0.85, I² = 91%, P < 0.0001, 305 participants, low‐quality evidence; Analysis 1.6). There is substantial heterogeneity with I² = 91%, most likely due to clinical heterogeneity. Specifically, while in three trials the medication was risperidone, the characteristics of participants and methodologies varied considerably. Findling 2000 included participants with IQ above 70, excluded moderate‐to‐severe ADHD, and it was unclear whether psychosocial interventions were offered. Aman 2002 included participants with IQ range of 36 to 84, and more than half of the sample had comorbid ADHD and were allowed concurrent stimulant medication (no dosage specified) and behavioural therapy. Finally, the TOSCA study included participants with a mean IQ of 97.1 (SD 14.1), and all participants had comorbid ADHD, which was actively treated with specified stimulant dosage, while all families received parent training.
1.6. Analysis.
Comparison 1 Risperidone versus placebo, Outcome 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores).
We excluded Reyes 2006a from the analysis of weight gain for the same reason as noted above. The authors reported that over the six‐month maintenance phase, the mean weight increase was 2.1 kg (SD = 2.7) for risperidone‐treated patients; while participants receiving placebo had a decrease in mean weight of 0.2 kg (SD = 2.2) (Reyes 2006a).
ii) Metabolic parameters
Two trials reported data for this outcome (Reyes 2006a; TOSCA study). Reyes 2006a reported "no clinically significant changes in mean fasting glucose levels during treatment" but no specific data were provided in the published study. There was no information reported on glucose or lipid profiles from other trials. The TOSCA study reported there were four clinically‐significant abnormal values: two with the risperidone (augmented) treatment group (triglyceride 389 mg/dL and prolactin 112 microg/L) and two with the placebo (basic) group (fasting glucose 144 mg/dL and fasting insulin 24 microIU/mL).
The TOSCA study also analysed prolactin concentrations at screening and endpoint. The values were very similar at screening (5.7 (± 3.9) µg/L and 5.9 (± 3.0) µg/L, for placebo/basic and risperidone/augmented treatment respectively), but significantly different at endpoint (placebo/basic treatment 7.1 (± 9.3) µg/L; risperidone/augmented treatment 36.0 (± 27.5) µg/L; Wilcoxon ranked sum test, P < 0.001). Using upper limits higher than 18.0 ng/mL for boys and higher than 30 ng/mL for girls, 68% assigned to risperidone (augmented) treatment had elevated prolactin levels compared with 5% assigned to placebo (basic) treatment. None were considered to be causing sexual or other adverse events according to the authors.
In Fleischhaker 2011, the reported metabolic data were only limited to incidence of hyperprolactinaemia (3/25 in the ziprasidone group and 1/25 in the placebo group). No blood results were reported.
In the first version of this review (Loy 2012), we wrote to all eight authors and had replies from four of them. Professor Connor and Dr Coppola, from Johnson and Johnson, responded on behalf of three authors: Aman, Synder and Reyes. The response for Reyes for glucose was: "No statistical testing was performed on laboratory analyses for this study. The statement was based on clinical assessment of mean changes. Note that the protocol specified that blood samples for clinical laboratory evaluations were to be obtained after an overnight fast. Although the majority (n = 368) of subjects were considered fasting, 138 subjects were identified as not fasting in regard to glucose changes from screening. The results described here (in the raw data) were for all data regardless of the fasting conditions." (Loy 2011a [pers comm]). We were unable to interpret the raw data, which had a mixture of fasting and non‐fasting glucose values.
Secondary outcomes
1. General functioning
Only one trial reported on general functioning (Reyes 2006a), using the Children's Global Assessment Scale (CGAS; Shaffer 1983). Participants treated with risperidone improved significantly more on CGAS than those on placebo.
The TOSCA study reported that there were no significant differences between scores at the end of treatment for groups on the Clinical Global Impression ‒ Improvement (CGI‐I; Guy 1976) and Clinical Global Impression ‒ Severity (CGI‐S; Rapoport 1985).
2. Non‐compliance
The number of participants (n) who withdrew due to adverse events was small (n = 15: one in Findling 2000; two in Aman 2002; eight in Reyes 2006a; one in Connor 2008; three in the TOSCA study). Overall, very few participants (n = 13) withdrew due to non‐compliance with treatment protocol (two in Findling 2000; three in Aman 2002; two in Armenteros 2007; two in Connor 2008; four (categorised as "terminated for non‐adherence") in the TOSCA study). The TOSCA study provided detailed tables explaining participant attrition; in the earlier studies, however, the details of non‐compliance were not defined.
3. Other adverse events
Table 5 summarises other adverse events besides weight gain and metabolic parameters, which are reported above. We grouped them into 'general', 'neurological', 'gastrointestinal', 'respiratory', 'cardiovascular' and 'other' side effects. Both the TOSCA study and Fleischhaker 2011 provided detailed information on adverse events in their respective publications. Two trials reported unspecified, serious adverse events but provided no details on what they were (Snyder 2002; Reyes 2006a). Van Bellinghen 2001 reported no data on adverse events.
4. Social functioning
One trial reported that scores on the Personal Assessment Checklist significantly favoured risperidone over placebo in terms of social relationships (mean change at endpoint = 1.3 for risperidone‐treated group compared with mean change at endpoint = 0.1 for placebo‐treated group) (Van Bellinghen 2001), but no SDs were provided by the authors, and the number of participants in this study was low (n = 13).
5. Parent satisfaction
Rundberg‐Rivera 2015 (a secondary publication to the TOSCA study) reported findings regarding parent satisfaction of those who had participated in the TOSCA study. Parents completed the Parent Satisfaction Questionnaire (PSQ), consisting of 18 items (Stallard 1996), to evaluate the: (1) overall levels of satisfaction in the study, (2) differences in satisfaction between responders and non‐responders, (3) differences in satisfaction based on treatment assignment (placebo versus active treatment), and (4) relation of study condition with parental confidence in managing problem behaviours. The authors reported no statistically significant differences between group allocation and parent satisfaction using three items on the PSQ (items six, seven and 18; P value greater than or equal to 0.72).
6. Family functioning, parent satisfaction, functioning at school
There was no information available on family functioning and functioning at school.
Subgroup analysis
The systematic review became focused on risperidone, as there was only one pilot study on quetiapine (Connor 2008), and one on ziprasidone (Fleischhaker 2011), compared with the eight trials on risperidone. In the first version of this review (Loy 2012), which spanned studies from 2000 to 2008, there was no clinically significant diversity in doses of risperidone between studies. The mean doses at endpoint ranged from 0.98 mg/day to 1.5 mg/day. The TOSCA study used a higher dose of risperidone: for children weighing less than 25 kg risperidone was dosed at 0.5 to 2.5 mg/day and for those weighing more than 25kg dosing ranged from 0.5 to 3.5 mg/day.
There was inadequate information from the original papers to do subgroup analyses by presence/absence of ADHD, presence/absence of psychostimulants, or presence/absence of intellectual disability. We note that Aman 2002 and Snyder 2002 conducted a post‐hoc analysis in a separate paper (Aman 2004), assessing risperidone effects in the presence/absence of psychostimulant medicine in participants with ADHD and disruptive behaviour disorders, and showed significant reductions in both disruptive behaviour and hyperactivity compared to placebo, regardless of concomitant stimulant use.
With regard to the duration of treatment, two pilot risperidone studies were 10 weeks and four weeks (Findling 2000 and Van Bellinghen 2001 respectively). Subsequent studies were mainly six weeks in duration (Buitelaar 2001; Aman 2002; Snyder 2002; TOSCA study); Armenteros 2007 lasted four weeks. The pilot study on quetiapine was six weeks (Connor 2008); and the pilot study on ziprasidone had a three‐week baseline period for finding the best individual dose, a six‐week treatment period and two‐week washout period (Fleischhaker 2011). There was only one study looking at six‐month risperidone maintenance treatment and 'time‐to‐symptom' recurrence (Reyes 2006a). Trial authors reported that 'time‐to‐symptom' recurrence was significantly longer in patients who continued risperidone than in those switched to placebo.
Discussion
Summary of main results
Overall, there was some evidence of limited efficacy of risperidone in reducing aggression and conduct problems in the short term in children and youths (aged five to 18 years) with disruptive behaviour disorders (four to 10 weeks), from a small number of studies in which there was some risk of bias of overestimating the true intervention effect. There were significant methodological limitations (Quality of the evidence), which impact on our confidence in the estimation of effect. There is no current evidence for the efficacy of quetiapine and ziprasidone on aggression and conduct problems in children and youths. Earlier studies did not include psychosocial interventions and stimulant medication treatments for comorbid ADHD in disruptive behaviour disorders. Recently, however, there has been some effort to address this in response to clinical guidelines and best clinical practice. So far there is only one trial which investigates the risperidone augmentation/combination with simultaneous stimulant medication and parent training in disruptive behavioural disorders. Risperidone treatment is associated with significant adverse events of weight gain and metabolic dysfunction.
For aggression, the difference in scores of 6.49 points on the ABC ‒ Irritability subscale (range 0 to 45) may be clinically significant. Owen 2009 used a difference of seven points on the ABC ‒ Irritability subscale as a measure of a clinically significant difference between treatment and placebo for children and adolescents with autistic disorder. Hassiotis 2009 used a difference of eight points on the ABC ‒ Irritability subscale between the treatment and control group to detect a clinically significant difference for challenging behaviour in adults with intellectual disabilities. The meaning of the differential findings on the two different ABS subscales is unclear. The scale splits aggression into two conceptually different constructs: reactive ("hot") and proactive ("cold"). From a clinical perspective, it can be difficult to distinguish between the two types of aggression. When we look at the risperidone‐only studies (Buitelaar 2001; TOSCA study), the differential findings on the two different ABS subscales remain, though the effect becomes larger and more precise as the heterogeneity of the meta‐analysis is reduced when we take out the quetiapine study (Connor 2008). In the future, we will consider pooling only risperidone studies.
For conduct problems, the difference in mean scores of 8.61 points on the NCBRF‐CP (range 0 to 48) is likely to be clinically significant. A difference of seven or more points on NCBRF‐CP by Reyes 2006a, and a difference of at least eight points on NCBRF‐CP by Tassé 1996, were considered to be clinically relevant differences between treatment and placebo in children and adolescents with disruptive behaviour disorders.
For weight gain, our meta‐analyses suggest a range in the average differences in weight gain from 2.14 kg to 2.37 kg over the treatment period, depending on whether there was concomitant administration of stimulant medication, which acts as an appetite suppressant. While weight is well documented, other metabolic side effects — including hyperprolactinaemia — were not well studied in the trials. However, these still need to be factored in clinically, as part of the risk benefit consideration.
Please refer to Table 1.
Overall completeness and applicability of evidence
Ten RCTs assessed the efficacy of atypical antipsychotics in disruptive behaviour disorders in children and youths. Of these, eight trials assessed risperidone, one assessed quetiapine and one assessed ziprasidone. There were no RCTs in disruptive behavioural disorders for olanzapine, aripiprazole and the other newer atypical antipsychotics. Of the eight risperidone studies, three were pilot trials. One was a pilot trial of risperidone augmentation for treatment‐resistant aggression in ADHD (Armenteros 2007); one was a study of risperidone in the treatment of conduct disorder (Findling 2000); and one was a pilot trial of risperidone in the treatment of behavioural disturbances in children and youths in residential care with borderline intellectual functioning (Van Bellinghen 2001). The quetiapine trial was a pilot study of quetiapine in the treatment of conduct disorder (Connor 2008). The ziprasidone trial was also a pilot study of ziprasidone in the treatment of severe conduct disorder/disruptive behaviour disorders in children and youths (Fleischhaker 2011). Thus out of the 10 included trials, five are pilot trials, which has implications in terms of number of participants per trial.
There were small numbers of participants in five trials (38 participants or fewer) (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Armenteros 2007; Connor 2008). This had a significant impact on the power of the studies and the precision of the estimate of the effect. One trial had 50 participants (Fleischhaker 2011); two trials had over 100 participants each (Aman 2002; Snyder 2002); one had 168 participants (TOSCA study); and one had over 300 participants (Reyes 2006a). Participants in the trials were between five and 18 years of age. There were no data for children under five years. The study on quetiapine (Connor 2008), which had a small sample size and was inadequately powered, produced a non‐significant result for aggression. The study on ziprasidone was also underpowered and found no significant effect of the active agent (ziprasidone) compared with the placebo group at the end of treatment (Fleischhaker 2011).
Aman 2002 and Snyder 2002 were larger, outpatient, multicentre, randomised, double‐blind controlled trials of risperidone and placebo in children and youths with disruptive behaviour disorders and sub‐average IQ. Buitelaar 2001 was a randomised, double‐blind controlled trial of risperidone and placebo in inpatient youths with disruptive behaviour disorders and an IQ between 60 and 90. The TOSCA study was a large, multicentre trial. It had a complex study design with three treatment components and may be viewed as an augmentation study or a combination treatment study. What is important about the trial design in terms of the findings is that it sought to replicate ideal clinical treatment in the trial, with all participants already receiving stimulants for ADHD and parent training before the addition of risperidone.
Nine trials focused on acute efficacy (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Armenteros 2007; Connor 2008, Fleischhaker 2011; TOSCA study), with the duration of intervention at four, six and 10 weeks. The tenth study was a six‐month maintenance trial looking at 'time‐to‐symptom' recurrence (Reyes 2006a). The duration of a RCT is an important consideration given the episodic nature of aggression, the natural waxing and waning of conduct problems, and the stability and the chronicity of the diagnosis of disruptive behaviour disorder, and in particular of conduct disorder (Steiner 1997; Steiner 2007; Connor 2008). Thus far, the follow‐up period in the trials is not long enough to demonstrate whether or not there is a sustained effect. We await the publication of the follow‐up results from the TOSCA study.
Due to the short duration of treatment in most trials there was a focus on short‐term adverse events. Earlier trials focused on weight gain, extrapyramidal side effects and prolactin levels. Metabolic side effects were poorly addressed in earlier trials; they were better documented in later trials (TOSCA study). Some trials continued as open‐label studies looking at safety, tolerability and continuation of effects of risperidone, but they were outside the parameters of this review. The long‐term, open‐label studies of risperidone in children with disruptive behaviour disorders were Turgay 2002, Croonenberghs 2005, Findling 2006 and Haas 2008, with up to one year's follow‐up; and Reyes 2006b, with up to three years' follow‐up.
Seven trials — Findling 2000, Buitelaar 2001, Aman 2002, Snyder 2002, Reyes 2006a, Connor 2008, and the TOSCA study — focused on a DSM‐IV diagnosis of a disruptive behaviour disorder with comorbid ADHD (American Psychiatric Association 2000); one trial focused on treatment‐resistant aggression in ADHD (Armenteros 2007); one trial measured behavioural symptoms instead of diagnoses (Van Bellinghen 2001); and one trial focused on primary DSM‐IV diagnoses of conduct disorder, oppositional defiant disorder and disruptive behaviour disorder not otherwise specified (Fleischhaker 2011). Trials that used DSM‐IV diagnoses would be more applicable clinically. The majority of the trials included participants with moderate‐to‐severe symptom severity and of clinical concern. This reflected clinical practice and circumstances where medication was warranted.
Seven trials included a significant number of participants with sub‐average to borderline IQ (range 36 to 84) (Findling 2000; Buitelaar 2001; Van Bellinghen 2001; Aman 2002; Snyder 2002; Reyes 2006a; Fleischhaker 2011), and only two trials, Reyes 2006a and Fleischhaker 2011, provided information on numbers, with approximately two‐thirds of participants having an IQ greater than 84 and IQ greater than 55, respectively. Armenteros 2007, Connor 2008 and the TOSCA study specifically excluded participants with sub‐average IQ. Thus the studies represented a mixed sample. In clinical practice, disruptive behavioural disorders are found in children and youths with both sub‐average and normal IQ and so the sample from the reviewed trials appears to be generalisable to the ones encountered in a clinical context.
With regards to the setting: eight studies were conducted with an outpatient population; one study looked at children in residential care (Van Bellinghen 2001); and one was carried out with inpatients (Buitelaar 2001). Outpatient management would be the most common in clinical practice and some children and youths with intellectual disability/mental retardation may be in residential care.
An important limitation in the evidence base was that earlier trials did not address the issue of pre‐existing or concurrent use of psychosocial treatments for disruptive behaviour disorders with medications, which is applicable in clinical practice. In one trial (Buitelaar 2001), it was specified that the participants had previously failed psychosocial treatment (contingency management and social skills training) before starting intervention with antipsychotic medication. Aman 2002 permitted behavioural therapy if it had started 30 days before the trial. Armenteros 2007 and Connor 2008 allowed "pre‐existing or current psychosocial interventions". The remaining trials did not comment on psychosocial interventions. There was no information in any of the trials about how many participants actually had concomitant psychosocial treatments and no further details of those treatments were described. This limitation was addressed directly by the TOSCA study, where all parents received evidence‐based parent training with monitoring of fidelity. This reflects ideal, optimal clinical treatment and the implication is that future clinical trials should incorporate psychosocial treatment into their designs.
Another limitation of the evidence was the comorbid treatment of ADHD with stimulant medication. There was no information available in earlier papers on the doses of stimulant medications used by participants: this was addressed by the TOSCA study to some extent. From baseline, there was a three‐week, open‐label, stimulant titration followed by six weeks of a double‐blinded, placebo‐controlled comparison of added risperidone versus placebo. Blader 2014 critiqued the three‐week duration of the pre‐treatment as not being long enough to establish an optimal stimulant regime. In the Blader 2013 study of adjunctive sodium valproate for ADHD children with aggression, the stimulant dosing took five to seven weeks or longer (alongside behaviour therapy) and, consequently, at least half the patients (82 out of 160 patients) showed remission of their aggressive behaviour. This compares with eight out 138 patients in the TOSCA study trial who responded to stimulants in the lead‐in phase. Blader 2014 also noted that adherence to stimulant treatment was not specifically addressed in the TOSCA study trial. ADHD symptom outcome was reported in a secondary paper (Gadow 2014).
Quality of the evidence
Important methodological limitations were present in the trials included in this review. Some of the trials were carried out pre‐CONSORT statement of 2001 (Moher 2001), thus the level of reporting currently expected was not available in the papers describing these studies, particularly with regard to the allocation concealment, randomisation and blinding processes. Fleischhaker 2011 did not follow the CONSORT standards. Reyes 2006a reported that they were unable to exclude the possibility of selection bias as only patients who responded to the initial acute treatment were subsequently randomised. No protocol was available for any of the trials. Three of the eight trials implemented a one‐week placebo run‐in to exclude placebo responders (Aman 2002; Snyder 2002; Connor 2008). One critique of this design was that placebo washout causes artificial inflation of the numbers apparently responding to the active drug and reduction of the numbers apparently responding to placebo (Jackson 2005, cited in Timimi 2008).
The overall attrition rate was relatively high, varying between 0% and 40% in the intervention group and 0% and 70% in the control group. This group of participants is difficult to study as, by definition, children and youths with disruptive behaviour disorders may not adhere to the rules of the treatment and research protocol; they may be oppositional and may not come for follow‐up appointments, and they may be itinerant. Therefore high dropout rates may be expected.
There were shortcomings in dealing with incomplete outcome data, in particular the use of the last observation carried forward as an imputation method to address incomplete data (Buitelaar 2001; Aman 2002; Snyder 2002; Reyes 2006a; Armenteros 2007). Criticisms against this method are that it is based on the underlying assumption that an individual’s missing value has not changed from the previously measured value and that it fails to account for the uncertainty about missing values (Wood 2004; Sterne 2009) and risk, resulting in standard errors that are too small (Sterne 2009) or CIs that are too narrow (Higgins 2011a). On the other hand, a limitation of the TOSCA study is that the published data are based on analysis of those who completed the study only, and the sensitivity analyses are not available for comparison. The overall implication is a potential risk of attrition bias, which may lead to an overestimation of the effect size.
Another methodological shortcoming is that there are significant overlaps in theoretical construct and measurement of aggression and conduct problems (Jensen 2007a; Calles 2011). There are no current gold standard measures of aggression or conduct problems. One implication is that by using different measures and reporting standards, it is challenging to pool the results for the purpose of a meta‐analysis or to understand the results from a clinical perspective.
All earlier trials had some degree of pharmaceutical support or sponsorship. Fleischhaker 2011 was an investigator‐initiated trial, financially supported by a pharmaceutical company. One trial specifically stated that the authors analysed all the data and completed all the writing (Connor 2008). From email correspondence (Loy 2016a [pers comm], we learned that the TOSCA study originally requested a pharmaceutical company to sponsor the study medication. Due to lack of agreement over certain issues, this fell through, except for one trial site. Subsequently, the authors obtained funding from the National Institute of Mental Health (NIMH) to purchase the study medications (both stimulant and risperidone and placebo) from an independent pharmacy. Only one study site, from which approximately 50 participants were recruited, received medication sponsored by the pharmaceutical company. It is possible that the centre used pharmaceutical‐supplied medication for one or two participants only. There were 168 participants in the trial. The lead author's view from this trial was that the pharmaceutical involvement was virtually nonexistent. There was no information available regarding this for the remainder of the trials.
For each trial included in the review, there were some areas of unclear bias and these are listed in the 'Risk of bias' tables, beneath the Characteristics of included studies tables. The overall quality of four trials was better than the others (Aman 2002; Snyder 2002; Reyes 2006a; TOSCA study), due to these trials being adequately powered and more methodologically rigorous, and therefore more trustworthy. Overall, we graded the quality of evidence as low, with the exception of the TOSCA study (Figure 3). Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate, given all the study limitations as well as methodological shortcomings. These are also listed in Table 1.
Potential biases in the review process
We conducted a comprehensive review of published literature from databases and trial registers and extracted and synthesized the available information. However, we cannot be confident about the grey literature. We acknowledged the authors who replied to our queries for further clarification in the original review (Loy 2012); and acknowledge those who replied in this update (see Acknowledgements). There were authors who did not reply to our requests for further information and, therefore, we had to classify some aspects of the trials as 'unclear' especially with regards to methodological issues. This has an impact on our 'Risk of bias' assessment and GRADE assessment in terms of downgrading the evidence.
Agreements and disagreements with other studies or reviews
Differences to the previous version of this review, Loy 2012, include the addition of two new trials (one of which was published in a peer review journal (TOSCA study), and the other published as a conference poster (Fleischhaker 2011)); the synthesis of additional and combined data into one analysis; the discovery of two trials that were terminated at the recruitment stage and were never completed (one due to slow recruitment (NCT00279409), and the other due to drug formulation availability issues (ISRCTN95609637)); and recent updates in the literature.
Since the publication of our review in 2012 (Loy 2012), a number of new, clinical guidelines have been developed. The original guideline was the "TRAAY‐ Treatment recommendations for the use of antipsychotics for aggressive youth" (Pappadopulos 2003). Since 2012, the Treatment of Maladaptive Aggression in Youth (T‐MAY) Center for Education and Research on Mental Health Therapeutics (CERT) Guidlines I and II have been developed (Knapp 2012; Scotto Rosato 2012). Scotto Rosato 2012 recommended using evidence‐based, psychosocial interventions as the first line of treatment; targeting the underlying disorder first; considering individual psychosocial and medical factors in the selection of drugs, if evidence‐based medication treatment is initiated; avoiding the use of multiple, psychotropic medications simultaneously; and careful monitoring of treatment response by using structured rating scales, as well as careful monitoring for side effects.
Canadian guidelines on pharmacotherapy for disruptive and aggressive behaviour in children and adolescents with ADHD, oppositional defiant disorder and conduct disorder suggest that atypical antipsychotics are introduced only if psychosocial interventions have not shown adequate improvement (Gorman 2015). Risperidone received a conditional recommendation in this guideline due to risk of adverse effects. The results of this review are consistent with the role of risperidone given in these clinical guidelines. Only one trial in this review — the TOSCA study — closely resembles best, real world, clinical practice as outlined in the treatment recommendations.
A review by Pappadopulos 2006 reported a mean effect size of 0.9 for aggression for risperidone in treating disruptive behaviour disorders in children and adolescents. The studies in Pappadopulos 2006, which required effect‐size calculations for multiple raters, were averaged to determine the overall effect size. There were two duplications in this review as both Aman 2004 and LeBlanc 2005 were not stand‐alone studies but described post‐hoc analyses of the data from Aman 2002 and Snyder 2002 combined. The review also included some studies of children and youths with autistic spectrum disorder and disruptive behaviour disorders in the same analysis while autism spectrum disorder was not in our inclusion criteria. The effect size reported in Pappadopulos 2006 is large compared to the small effect size from our meta‐analysis for aggression for risperidone. The difference in our view is probably due to the inclusion of studies of children and youths with autistic spectrum disorder and comorbid disruptive behaviour disorders. Pappadopulos 2006 summarised and averaged the published effect sizes instead of conducting a meta‐analysis and this may have led to an overestimation of the effect size. The analysis by Jensen 2007b was similar to our review. There were six studies in the Jensen 2007b review with the exception of Armenteros 2007 and Connor 2008. Another issue was that the two reviews — Pappadopulos 2006 and Jensen 2007b — did not present CIs alongside the effect sizes. Since 2009, the sixth edition of the American Psychological Association's Publication Manual states that “estimates of appropriate effect sizes and confidence intervals are the minimum expectations” (American Psychological Association 2010, p 33).
Seida 2012 published a report called, "Antipsychotics for children and young adults: a comparative effectiveness review". This is a broad (non‐Cochrane) systematic review of the effectiveness and safety of first‐ (FGA) and second‐generation antipsychotics (SGA) for patients aged 24 years and younger with psychiatric and behavioural conditions. Because the authors focused on breadth, there is less depth in their analysis. A useful feature of this review is the authors' highlight of the number of studies looking at effectiveness (FGA versus SGA) and efficacy (drug versus placebo). They also identified the same eight studies for disruptive behaviour disorder. Their conclusion is that youths treated with risperidone had greater improvement on behavioural symptom measures and on CGI measures compared to placebo, with moderate strength of evidence. This is in contrast with our more conservative estimate of the effect.
Pringsheim 2012 reported a non‐Cochrane systematic review in December 2012, subsequent to our Cochrane Review, with the same eight trials and similar findings to ours. Duhig 2013 is a non‐Cochrane, non‐systematic review, specifically examining the efficacy of risperidone studies in children and adolescents with disruptive behavioural disorders. The authors cited the same seven trials of risperidone included in this review with similar findings. Pringsheim 2015 is a non‐Cochrane, systematic review of RCTs of antipsychotics, lithium and anticonvulsants. They included 11 RCTs of antipsychotics and seven RCTs of lithium and anticonvulsants. The authors concluded that there is "moderate quality evidence that risperidone has a moderate‐to‐large effect in conduct problems and aggression in youth with sub‐average IQ and ODD [oppositional defiant disorder], CD [conduct disorder] or DBD‐NOS [disruptive behaviour disorder ‐ not otherwise specified], with and without ADHD, and high‐quality evidence that risperidone has a moderate effect on disruptive and aggressive behaviour in youth with average IQ and ODD or CD, with or without ADHD". The authors also concluded that the evidence to support the use of antipsychotics and mood stabilizers is of low quality except for risperidone. Again, our findings are more conservative.
Our review does not include general expert opinion, commentary or partial reviews (Nevels 2010; Teixeira 2013b); or inpatient treatment of aggression in youths (Deshmukh 2010).
The TOSCA study group have published the results of their 12‐month naturalistic follow‐up (Gadow 2016), with an aim to report on the treatment regime, behavioural outcomes and adverse events. Following the nine‐week trial, blinded treatment of clinical responders continued until week 21 (Findling 2017). (Results were not available at the cut‐off date for this review, 19 January 2017, and therefore have not been included.) After week 21, study blind was broken and study staff assisted families to find ongoing care. According to the authors, after week 21, treatment was no longer free of charge to the families (Gadow 2016). At 12 months, there were 108 participants available for the follow‐up (basic = 55; augmented = 53), comprising 64% of the original sample. Of the participants, 43% in the augmented group and 36% in the basic group adhered to their assigned medication regimen; 23% in the augmented group and 11% in the basic group were not taking medications. About half of the participants were receiving modified drug treatment consisting of multiple drugs, including alpha agonists, selective serotonin reuptake inhibitors (SSRIs) and anti‐epileptics, in addition to stimulants and atypical antipsychotics in various permutations. While both treatment strategies were associated with clinical improvement at follow‐up, and primary behavioural outcomes did not differ significantly, parents still rated 45% of participants as impaired 'often or very often' from ADHD and as non‐compliant or aggressive. The authors raised issues of the impact of terminating parent training after week 21, and the impact of the unaffordability of the recommended treatment on long‐term outcomes (Gadow 2016).
It is important to note the theoretical concerns and themes in the literature regarding short‐ and long‐term side effects, and ultimately safety concerns for children and youth, to have a sense of the extent and depth of the problem (Devlin 2015). This will affect the risk‒benefit consideration and impact on decision on starting, adhering to, and persisting in treatment (Murphy 2015).
The weight gain reported in our review is of concern. It is unclear if the effect will attenuate over time. Correll 2009 suggests that interpretation of data may be hampered by variables prior to antipsychotic medication exposure, which can obscure cardiometabolic effects. In his prospective cohort study of weight and metabolic changes in paediatric patients naive to antipsychotic medication, 22.1% (60 participants) of the sample (272 participants) had disruptive or aggressive behaviour disorders. The rest had mood or schizophrenia spectrum disorders. After a median of 10.8 weeks of treatment, weight increased by 5.3 kg (95% CI 4.8 to 5.9 kg) in those treated with risperidone (135 participants). However, other authors of open‐label studies of risperidone for disruptive behaviour disorders in children and youth suggest that weight gain was greatest early on and levelled off between six and 12 months (Croonenberghs 2005, 504 participants; Turgay 2002, 77 participants) and two years (Reyes 2006b, 35 participants). About half of the mean weight gain of participants on risperidone at year one was attributable to developmentally expected growth (Turgay 2002; Findling 2004; Croonenberghs 2005).
In the 12‐month follow‐up of the TOSCA trial (Gadow 2016), the group exposed to risperidone treatment (augmented group) experienced an increase in weight from the end of acute treatment (week nine) till week 52, and weight gain was maintained at week 52, while the non‐exposed group (basic group) experienced decreased weight over time. Both groups had prior, concurrent stimulant treatment. The augmented group also had elevated prolactin levels (59%) compared to the basic group (5%) at 52 weeks (Gadow 2016). In a systematic review of cardiometabolic and endocrine side effects of atypical antipsychotics in children and adolescents, De Hert 2011 reported that risperidone was associated with intermediate weight gain (1.76 kg, 95% CI 1.27 to 2.25; 1200 participants).
Apart from weight gain, other cardiometabolic side effects like abdominal adiposity, hypertension, dyslipidaemia, insulin resistance and metabolic syndrome have been reported in children and youths treated with atypical antipsychotics (Devlin 2015). Recent estimates from longitudinal studies suggest that SGA‐treated children have a threefold greater risk of developing type 2 diabetes compared with untreated children. If there are problems of childhood obesity, it is associated with vascular damage in childhood, and with greater risk of cardiovascular disease in adulthood and at an earlier onset (Devlin 2015). Research into some genetic variants that may be useful in predicting cardiometabolic side effects in SGA‐treated children is still in its infancy (Devlin 2015).
It is known that risperidone can increase prolactin levels (Perkins 2004). One issue is that children may not have more common side effects of hyperprolactinaemia like galactorrhoea, amenorrhoea and sexual dysfunction. One concern is that hyperprolactinaemia at levels leading to hypogonadism may be associated with osteoporosis (Almandil 2011). Other (unconfirmed) potential risks from childhood hyperprolactinaemia are risk of breast cancer and pituitary tumours (Perkins 2004; Correll 2008). Little is known about the long‐term effects of risperidone treatment received early in the developmental course. In one animal study, adult rats treated with risperidone during development are hyperactive (Bardgett 2013). The authors queried whether chronic antipsychotic drug use in a paediatric population may modify brain development and alter neural set points for future specific behaviours.
Vitiello 2009 proposed that short‐term use of atypical antipsychotics includes a treatment course of less than six months, an intermediate treatment course of 18 months and long‐term use as beyond that. The authors' view is that distal benefit/risk ratio remains to be determined for long‐term treatment for atypical antipsychotics in general for children and adolescents. Caccia 2011 did a review on antipsychotic toxicology in children. The authors raised multiple, pertinent issues relating to further research on mechanisms underlying adverse effects, genetic risk of specific toxicities induced by antipsychotics and, because long‐term safety data are limited, advocated for permanent, systematic and collaborative epidemiological assessments and quantification for long‐term monitoring in this age group.
This challenge of a pharmacovigilance system has been taken up by the SENTIA (Safety of Neuroleptics in Infancy and Adolescence) project, which is an online monitoring registry in Spain (Palanca‐Maresca 2014). Another initiative, called the PERS (Pediatric European Risperidone Studies) project, plans to look at long‐term tolerability in a two‐year pharmacovigilance in children and adolescents with conduct disorder and normal intelligence (Glennon 2014).
Authors' conclusions
Implications for practice.
There is some limited evidence that risperidone reduces aggression (low‐quality evidence) and conduct problems (moderate‐quality evidence) in the short term in children and youths (aged five to 18 years) with disruptive behaviour disorders. A recent study has added some weight to this evidence but has not changed the overall findings (TOSCA study). The overall effect from our meta‐analysis is small and may be clinically meaningful. The children and adolescents in the trials were recruited from both inpatient and outpatient populations so findings are potentially generalisable. There is currently no evidence to support the use of quetiapine, ziprasidone or any other atypical antipsychotics for disruptive behaviour disorders in children and adolescents. There are no research data for children under five years of age.
These findings, however, are based on a small number of studies conducted in clinical sites in which there was some risk of bias resulting in a overestimation of the true intervention effect due to methodological shortcomings. These include: lack of detail in the reporting of the implementation of the trials; usage of enriched designs; the risk of selection bias; and the risk of attrition bias. Trials that were inadequately powered had non‐significant results. There is large heterogeneity of the population, including sub‐average and borderline IQ. Only one controlled trial investigated long‐term efficacy (Reyes 2006a), suggesting that the therapeutic effects of risperidone may be maintained to some extent (with a small effect size) for up to six months. The trials do not address the issue of pre‐existing or comorbid psychosocial interventions except for the TOSCA study. Comorbid stimulant medication and its dosage is poorly addressed, although the recent trial by the TOSCA study provides some data on this. Overall, the limitations of the evidence and the very limited number of high‐quality trials conducted (only four out of 10 trials) requires caution in the interpretation of findings.
The participants taking risperidone gained, on average, between 2.14 kg and 2.37 kg (low‐quality evidence) over the treatment period of six to 10 weeks. It is unclear if this effect will attenuate over time. There are a paucity of data regarding metabolic side effects in these trials.
The use of risperidone ultimately needs to be contextualised and be regarded as one of the potential, carefully considered, targeted and time‐limited interventions in the whole continuum of care available for children and youths with disruptive behaviour disorders. The TOSCA study utilized risperidone augmentation/combination treatment strategy with stimulant and parent training and is the closest to ideal, real world clinical practice and current clinical guidelines.
A key question posed by Jensen 2014 is: "How much 'pretreatment' should be provided and for how long, before augmentation with the addition of atypical antipsychotic?" (p 950). Aggression treatment guidelines (TRAAY (Pappadopulos 2003) and T‐MAY (Scotto Rosato 2012)) generally suggest more intensive or longer periods of psychosocial treatment, longer periods of stimulant medication, more flexible dosing of stimulant optimisation in the monotherapy lead‐in phase (Blader 2013; Blader 2014), and testing of alternative stimulant medications (Jensen 2014) before augmentation with risperidone is considered.
Implications for research.
In our original review (Loy 2012), we flagged that, from an ethical perspective, add‐on medication studies may be the preferred clinical design for future trials. This is to ensure that participants are being optimally treated for comorbid ADHD or have previously received/are receiving concurrent psychosocial intervention, or both (Jensen 2007a). It is important to have details of the concomitant interventions made explicit as well. In this review, the TOSCA study is the only trial to have done this so far. A key implication for research is to have more trials of this design in the future. The follow‐up results of the TOSCA study, reported in Gadow 2016, also raised questions about the long‐term effectiveness and the possible effect of long‐term parent training on outcomes.
Another implication for research concerns the issue of measurement. There remains some uncertainty and lack of consensus regarding nosology in aggression literature, due to heterogeneity in aggressive behaviour and multiple subtypes of aggression proposed (Collett 2003). The rating scales for aggression may measure traits of impulsive aggression or aggressive acts. Jensen 2007a has recommended that both should be assessed in clinical trials. This has not occurred in trials to date. Further work to delineate consensus in measurement or establish a gold standard method to measure aggression and conduct problems would be useful in this field.
Further research on disruptive mood dysregulation disorder (DMDD) is required to further clarify its construct validity and treatment parameters. In the future, it is unclear if DMDD will remain under the depressive disorder classification or becomes a subtype of ODD (Baweja 2016, Freeman 2016, Evans 2017).
A future area of research may include screening for trauma history and its treatment and investigation into whether trauma is a contributor to treatment resistance in this group of patients. The role of anxiety as a mediator in the treatment of disruptive behaviours in children and youth is worth exploring further (Arnold 2015). While outside the parameters of this review, an intriguing finding from the TOSCA study is that an improvement in anxiety mediates an improvement in disruptive behaviour disorders (Arnold 2015), with the authors suggesting that anxiety results in aggression due to the fight or flight response. This has potential implications for focusing treatment on existing anxiety in children and youths presenting with disruptive behaviour disorders and aggression.
It would be useful for future research to include measures of school and family functioning to assess social functioning of this patient group in more detail. Furthermore, it would be informative to further investigate the effectiveness of interventions in particular subgroups such as girls with disruptive behaviour disorder and those with disruptive behaviour disorder and intellectual disability. Qualitative studies with youths with disruptive behaviour disorders and their families are needed to understand the acceptability of atypical antipsychotics from their perspectives.
Recently, there is a concern of under‐representation of harm associated with possible miscoding of data in the area of antidepressant treatment for children and adolescents (Cipriani 2016; Sharma 2016). The authors have called for greater transparency and access to individual patient‐level data and case report forms, rather than a reliance on published data, to minimise this risk. The European Medicines Agency has drafted a policy document for public consultation recommending that there should be greater access to analysable data from future clinicals trials (European Medicines Agency 2014).
Overall, this review highlights the limited evidence base regarding the use of atypical antipsychotics for disruptive behaviour disorders in children and youths. There is a need for more adequately powered and high‐quality trials of longer duration and trials on other atypical antipsychotics besides risperidone. Pharmacovigilance studies are currently underway, which will hopefully shed more light on the long‐term effects and safety of atypical antipsychotics in children and youths with disruptive behaviour disorders.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2017 | New citation required but conclusions have not changed | This updated review has 2 new trials (making a total of 10 trials) and an updated literature review. We can only pool new data from one recent trial into our existing meta‐analysis. There were 2 other trials that would have met our criteria but they were terminated prematurely and we have listed them under excluded trials. The overall findings have not changed substantively in 2016 compared to 2012. |
| 19 January 2017 | New search has been performed | We updated this review following a new search in February 2016 and Janaury 2017. |
Acknowledgements
The authors wish to thank Anne Wilson, from the University of Auckland, for assistance with search strategies. We wish to thank the authors who replied to our correspondences, including Professor Aman, Nicole Brown, Dr Hennighausen, Professor Schulz, Professor Connor, Dr Danielle Coppola and Dr Magali Haas from Johnson and Johnson for queries on Van Bellinghen 2001, Aman 2002, Snyder 2002, Reyes 2006a, Connor 2008, Fleischhaker 2011 and the TOSCA study. We also thank our colleague Chohye Park for her contribution to the protocol (Loy 2012). The first author also want to acknowledge Associate Professor David Menkies for his encouragement in the early days. We would also like to thank the authors of the studies included in this review who have provided additional data and comments in response to our requests for information. We want to thank Dr Joanne Wilson, Managing Editor, Cochrane, for her helpful suggestions.
Appendices
Appendix 1. Rating scales
Scales for aggression (observer rated)
Children’s Aggression Scale – Parent’s version (CAS‐P) (Halperin 2002).
Children’s Aggression Scale – Teacher’s version (CAS‐T) (Halperin 2003).
Overt Aggression Scale (OAS) (Yudofsky 1986).
Modified Overt Aggression scale (MOAS) (Sorgi 1991).
Overt Aggression Scale Modified (OAS‐M) (Coccaro 1991).
Proactive and Reactive Aggression Scale (PRA) (Dodge 1987).
Revised Teacher Rating Scale for Reactive and Proactive Aggression (R‐TRPA) (Brown 1996).
Vitiello Aggression Questionnaire (VAQ) (Vitiello 1997).
Children's Social Behaviour Scale (CSBS) (Crick 1995).
General Behavior Inventory (Parent Version) (P‐GBI) (Youngstrom 2001).
Life History of Aggression (LHA) (Coccaro 1997).
Scale for aggression (self‐report)
Buss‐Durkee Hostility Inventory (BDHI) (Buss 1957).
Aberrant Behaviour Checklist (ABC) – Irritability subscale (Aman 1985a).
Child Behaviour Checklist (CBCL) – Aggression subscale (Achenbach 1991).
Barratt Aggressive Acts Questionnaire (AAQ) (Barratt 1999).
Spielberger Anger and Expression of Anger Inventory (STAXI) (Fuqua 1991).
Anger, Irritability, and Aggression Questionnaire (AAIQ) (Coccaro 1991).
Buss‐Perry Aggression Questionnaire (B‐PAQ) (Buss 1992).
Aggression Questionnaire (AQ) (Vitiello 1990; Malone 1998).
Scales for disruptive behaviour disorders or conduct disorder
Nisonger Child Behaviour Rating Form (NCBRF) – Conduct Problem subscale (Aman 1996).
Connors' Parent Rating Scale – Revised (CPRS‐R) (Conners 1997a).
Conners' Teacher Rating Scale – Revised (CTRS‐R) (Conners 1997a).
Swanson, Nolan and Pelham scale, Fourth Edition (SNAP‐IV) (Swanson 1992).
Vanderbilt ADHD Diagnostic Parent Rating Scale (VADPRS) (Wolraich 2003).
Vanderbilt ADHD Diagnostic Teacher Rating Scale (VADTRS) (Wolraich 1998).
Achenbach Child Behavior Checklist (CBCL) (Achenbach 1991).
Eyberg Child Behaviour Inventory (ECBI) (Eyberg 1999).
New York Teacher Rating Scale for Disruptive and Antisocial Behaviour (NYTRS) (Miller 1995).
Home and School Questionnaire (HSQ/SSQ) (Barkley 1997).
Antisocial Process Screening Device (APSD) (Frick 2001).
Sutter‐Eyberg Student Behavior Inventory Revised (SESBI‐R) (Eyberg 1999).
Youth Self Report (YSR) (Achenbach 1991).
Conners/Wells' Adolescent Self Report of Symptoms (CASS) – conduct problem and anger control problem subscales (Conners 1997b).
Parent Daily report (PDA) (Kazdin 1986).
Interview for Antisocial Behaviour (IAB) (Chamberlain 1991).
Appendix 2. Search strategies up to 2011
Cochrane Central Register of Controlled Studies (CENTRAL) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
#1 MeSH descriptor Child Behavior Disorders, this term only #2 MeSH descriptor Attention Deficit and Disruptive Behavior Disorders, this term only #3 MeSH descriptor Attention Deficit Disorder with Hyperactivity, this term only #4 MeSH descriptor Conduct Disorder, this term only #5 conduct NEAR/5 (disorder* or disturb*) #6 oppositional* #7 MeSH descriptor Antisocial Personality Disorder, this term only #8 (antisocial or anti NEXT social) NEAR/5 (behav* or histor* or conduct*) #9 attention NEAR/5 deficit* #10 ad/hd #11 adhd or "ad/hd" or adhkd or addh or adhs #12 hyperactiv* #13 MeSH descriptor Hyperkinesis, this term only #14 hyperkine* #15 MeSH descriptor Aggression, this term only #16 aggress* #17 MeSH descriptor Agonistic Behavior, this term only #18 agonistic* #19 MeSH descriptor Anger, this term only #20 MeSH descriptor Rage, this term only #21 anger or angry #22 malic* #23hostil* #24 deceit* #25 cruel* #26 MeSH descriptor Juvenile Delinquency, this term only #27 threaten* #28 (dangerous* or disrupt* ) NEAR/5 (behav* or histor* or conduct*) #29 MeSH descriptor Impulsive Behavior, this term only #30 impulse* or impulsiv* #31 MeSH descriptor Crime explode all trees #32 criminal* or violen* or unlawful* or delinquen* #33 MeSH descriptor Violence, this term only #34 offend* or offence* or offense* #35 correctional* or penal* #36 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35) #37 MeSH descriptor Clozapine, this term only #38 MeSH descriptor Risperidone, this term only #39 risperidon* #40 clozapin* #41 amisulprid* #42 amisulpirid* #43 aripiprazol* #44 olanzapin* #45 quetiapin* #46 sertindol* #47 ziprasidon* #48 zotepin* #49 atypical NEAR/3 (anti next psychotic* or antipsychotic*) #50(#37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49) #51 MeSH descriptor Infant, this term only #52 MeSH descriptor Child explode all trees #53 MeSH descriptor Adolescent, this term only #54 MeSH descriptor Minors, this term only #55 (child* or boy* or girl* or baby or babies or toddler* or infant* or teen* or adolescen* or juvenile* or preschool* or pre next school* or schoolchild*) #56 youth* or young* people #57 young* people #58 (#51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57) #59 (#36 AND #50 AND #58)
OVID MEDLINE(R)
1 Child Behavior Disorders/ 2 Attention Deficit Disorder with Hyperactivity/ 3 "Attention Deficit and Disruptive Behavior Disorders"/ 4 Conduct Disorder/ 5 (conduct adj5 (disorder$ or disturb$)).mp. 6 oppositional$.mp. 7 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 8 Antisocial Personality Disorder/ 9 (attention adj5 deficit$).mp. 10 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 11 hyperactiv$.mp. 12 Hyperkinesis/ 13 hyperkine$.mp. 14 Aggression/ 15 Agonistic behavior/ 16 aggress$.mp. 17 agonistic$.mp. 18 Anger/ 19 Rage/ 20 (anger or angry).mp. 21 malic$.mp. 22 hostil$.mp. 23 ((dangerous$ or disrupt$ or defiant$) adj3 (behav$ or histor$ or conduct$)).mp. 24 deceit$.mp. 25 cruel$.mp. 26 juvenile delinquency/ 27 delinquen$.mp. 28 threaten$.mp. 29 Impulsive Behavior/ 30 impulsiv$.mp. 31 impulse$.mp. 32 unlawful$.mp. 33 Violence/ 34 violen$.mp. 35 exp Crime/ 36 criminal$.mp. 37 penal$.mp. 38 (offend$ or offenc$ or offens$).mp. 39 correctional.mp. 40 or/1‐39 41 amisulprid$.mp. 42 amisulpirid$.mp. 43 aripiprazol$.mp. 44 Clozapine/ 45 clozapin$.mp. 46 olanzapin$.mp. 47 quetiapin$.mp. 48 Risperidone/ 49 risperidon$.mp. 50 sertindol$.mp. 51 ziprasidon$.mp. 52 zotepin$.mp. 53 (atypical$ adj3 (antipsychotic$ or anti‐psychotic)).mp. 54 or/41‐53 55 (child$ or boy$ or girl$ or baby or babies or toddler$ or infant$ or teen$ or adolescen$ or juvenile$ or preschool$ or pre‐school$ or schoolchild$).mp. 56 (youth$ or young$ people).mp. 57 Child/ or Child, preschool/ 58 Infant/ 59 Adolescent/ 60 Minors/ 61 or/55‐60 62 40 and 54 and 61
The search terms "iloperidone" "asenapine", "lurasidone" and "paliperidone" were included in the searches from 2015 onwards to reflect the availablity of new drugs.
Embase Ovid
1 behavior disorder/ or attention deficit disorder/ or disruptive behavior/ or impulse control disorder/ or oppositional defiant disorder/ 2 (conduct adj5 (disorder$ or disturb$)).mp. 3 oppositional$.mp. 4 antisocial behavior/ 5 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 6 ((antisocial$ or anti‐social$) adj personality adj disorder$).mp. 7 (attention adj5 deficit$).mp. 8 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 9 hyperactiv$.mp. 10 hyperkinesia/ 11 hyperkine$.mp. 12 exp aggression/ 13 aggress$.mp. 14 agonistic$.mp. 15 (anger or angry).mp. 16 malic$.mp. 17 hostil$.mp. 18 ((dangerous$ or disrupt$) adj3 (behav$ or histor$ or conduct$)).mp. 19 deceit$.mp. 20 cruel$.mp. 21 delinquen$.mp. 22 threaten$.mp. 23 impulsiveness/ 24 impulse control disorder/ 25 (impulsiv$ or impulse$).mp. 26 unlawful$.mp. 27 exp violence/ 28 violen$.mp. 29 exp crime/ 30 criminal$.mp. 31 penal$.mp. 32 (offend$ or offenc$ or offens$).mp. 33 correctional.mp. 34 or/1‐33 35 exp atypical antipsychotic agent/ 36 amisulprid$.mp. 37 amisulpirid$.mp. 38 aripiprazol$.mp. 39 clozapin$.mp. 40 olanzapin$.mp. 41 quetiapin$.mp. 42 risperidon$.mp. 43 sertindol$.mp. 44 ziprasidon$.mp. 45 zotepin$.mp. 46 (atypical$ adj3 (antipsychotic$ or anti‐psychotic$)).mp. 47 or/35‐46 48 (baby or babies or infant$ or toddler$ or child$ or boy$ or girl$ or preschool$ or pre school$ or teen$ or adolescen$ or juvenile$ or minor$ or youth$ or young people).tw. 49 infant/ 50 child/ 51 school child/ 52 preschool child/ 53 adolescent/ 54 or/49‐53 55 34 and 47 and 54
PsycINFO strategies
During the preparation of the 2012 review the supplier for PsycINFO changed from EBSCOhost to Ovid . The search stategy was therefore adapted in 2011 for the Ovid platform.
PsycINFO EBSCOhost: 2010 search
S52 S47 and S48 and S51 S51 S49 or S50 S50 baby or babies or infant* or toddler* or child* or boy* or girl* or preschool* or pre school* or schoolchild* or juvenile* or minor* or teen* or adolescen* or youth* or young* people S49 (ZG "adolescence (13‐17 yrs)") or (ZG "childhood (birth‐12 yrs)") or (ZG "infancy (2‐23 mo)") or (ZG "preschool age (2‐5 yrs)") or (ZG "schoolage (6‐12 yrs)") S48 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 S47 S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 S46 atypical* N3 anti psychotic* S45 atypical* N3 antipsychotic* S44 zotepin* S43 ziprasidon* S42 sertindol* S41 risperidon* S40 quetiapin* S39 olanzapin* S38 clozapin* S37 aripiprazol* S36 amisulpirid* S35 amisulprid* S34 DE "Aripiprazole" or DE "Clozapine" or DE "Olanzapine" or DE "Quetiapine" or DE "Risperidone" S33 impulsiv* or impulse* S32 DE "Impulsiveness" S31 (DE "Violent Crime") or (DE "Violence") S30 DE "Crime" S29 DE "Criminal Behavior" OR DE "Juvenile Delinquency" S28 offend* or offenc* or offens* S27 violen* or criminal* or penal* or correctional* S26 unlawful* S25 threaten* S24 delinquen* S23 (disrupt* N3 behav* ) or (disrupt* N3 histor*) or (disrupt* N3 conduct*) S22 (dangerous* N3 behav* ) or (dangerous* N3 histor*) or (dangerous* N3 conduct*) S21 cruel* S20 deceit* S19 hostil* S18 malic* S17 anger or angry or rage* S16 aggress* or agonistic* S15 hyperkine* S14 hyperactiv* S13 adhd or "ad/hd" or adhkd or addh or adhs S12 attention N5 deficit* S11 (anti social* N5 behav*) or (anti social* N5 histor*) or (anti social* N5 conduct*) S10 (antisocial* N5 behav*) or (antisocial* N5 histor*) or (antisocial* N5 conduct*) S9 oppositional* S8 (conduct N5 disturb*) S7 (conduct N5 disorder*) S6 DE "Anger" OR DE "Hostility" S5 (DE "Attention Deficit Disorder with Hyperactivity" or DE "Attention Deficit Disorder" or DE "Hyperkinesis" or DE "Impulsiveness" or DE "Oppositional Defiant Disorder") S4 DE "Aggressive Behavior" or DE "Aggressiveness" S3 DE "Conduct Disorder" S2 DE "Behavior Disorders" S1 (DE "Antisocial Personality Disorder")
PsycINFO Ovid: 2011 search
1 antisocial behavior/ 2 Antisocial Personality Disorder/ 3 Behavior Disorders/ 4 Conduct Disorder/ 5 Aggressive Behavior/ 6 Aggressiveness/ 7 Impulsiveness/ 8 Oppositional Defiant Disorder/ 9 exp attention deficit disorder/ 10 Hyperkinesis/ 11 Anger/ 12 Hostility/ 13 (conduct adj5 (disorder$ or disturb$)).mp. 14 oppositional$.mp. 15 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 16 (attention adj5 deficit$).mp. 17 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 18 hyperactiv$.mp. 19 hyperkine$.mp. 20 aggress$.mp. 21 agonistic$.mp. 22 (anger or angry).mp. 23 malic$.mp. 24 hostil$.mp. 25 ((dangerous$ or disrupt$ or defiant$) adj3 (behav$ or histor$ or conduct$)).mp. 26 deceit$.mp. 27 cruel$.mp. 28 Juvenile Delinquency/ 29 delinquen$.mp. 30 threaten$.mp. 31 impulsiv$.mp. 32 impulse$.mp. 33 unlawful$.mp. 34 Violence/ 35 violen$.mp. 36 exp Crime/ 37 criminal$.mp. 38 penal$.mp. 39 (offend$ or offenc$ or offens$).mp. 40 correctional.mp. 41 or/1‐40 42 Clozapine/ 43 clozapin$.mp. 44 exp Olanzapine/ 45 olanzapin$.mp. 46 Quetiapine/ 47 quetiapin$.mp. 48 Risperidone/ 49 risperidon$.mp. 50 sertindol$.mp. 51 ziprasidon$.mp. 52 zotepin$.mp. 53 amisulprid$.mp. 54 amisulpirid$.mp. 55 aripiprazole/ 56 aripiprazol$.mp. 57 (atypical$ adj3 (antipsychotic$ or anti‐psychotic$)).mp. 58 or/42‐57 59 (child$ or boy$ or girl$ or baby or babies or toddler$ or infant$ or teen$ or adolescen$ or juvenile$ or preschool$ or pre‐school$ or schoolchild$).mp. 60 (youth$ or young$ people).mp. 61 ("100" or "120" or "140" or "160" or "180" or "200" or "320").ag. 62 59 or 60 or 61 63 41 and 58 and 62
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S53 S33 and S47 and S52 S52 (S48 or S49 or S50 or S51) S51 AG preschool S50 AG adolescent S49 AG child S48 baby or babies or infant* or toddler* or child* or boy* or girl* or preschool* or pre school* or schoolchild* or juvenile* or minor* or teen* or adolescen* or youth* or young* people S47 S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 S46 atypical* N3 anti psychotic* S45 atypical* N3 antipsychotic* S44 zotepin* S43 ziprasidon* S42 sertindol* S41 risperidon* S40 quetiapin* S39 olanzapin* S38 clozapin* S37 aripiprazol* S36 amisulpirid* S35 amisulprid* S34 (MH "Aripiprazole") or (MH "Clozapine") or (MH "Olanzapine") or (MH "Quetiapine") or (MH "Risperidone") S33 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 impulse* or impulsiv* S31 (MH "Impulse Control Disorders") S30 (MH "Violence") or (MH "School Violence") or (MH "Sibling Violence") S29 (MH "Juvenile Delinquency") or (MH "Juvenile Offenders+") S28 (MH "Crime") S27 (MH "Public Offenders") S26 offend* or offenc* or offens* S25 violen* or criminal* or penal* or correctional* or unlawful* or threaten* S24 delinquen* S23 (disrupt* N3 behav* ) or (disrupt* N3 histor*) or (disrupt* N3 conduct*) S22 (dangerous* N3 behav* ) or (dangerous* N3 histor*) or (dangerous* N3 conduct*) S21 cruel* S20 deceit* S19 hostil* S18 malic* S17 anger or angry or rage* S16 aggress* or agonistic* S15 hyperkine* S14 hyperactiv* S13 adhd or "ad/hd" or adhkd or addh or adhs S12 attention N5 deficit* S11 (anti social* N5 behav*) or (anti social* N5 histor*) or (anti social* N5 conduct*) S10 (antisocial* N5 behav*) or (antisocial* N5 histor*) or (antisocial* N5 conduct*) S9 oppositional* S8 (conduct N5 disorder*) OR (conduct N5 disturb*) S7 (MH "Anger") S6 (MH "Aggression") S5 (MH "Hyperkinesis") S4 (MH "Disruptive Behavior") S3 (MH "Antisocial Personality Disorder") S2 (MH "Attention Deficit Hyperactivity Disorder") S1 (MH "Child Behavior Disorders")
ClinicalTrials.gov
(clinicaltrials.gov)
adhd OR dispruptive OR oppositional OR conduct OR impulsive OR anger OR rage OR antisocial | aripiprazole OR olanzapine OR quetiapine OR sertindole OR ziprasidone OR zotepine OR clozapine OR risperidone OR amisulpride OR amisulpirid | Child, Adult |
CenterWatch
Advanced search: (amisulpride or amisulpiride or aripiprazole or clozapine or olanzapine or quetiapine or risperidone or sertindole or ziprasidone or zotepine ) AND (adhd or disruptive or oppositional or "conduct disorder" or impulsive or anger or rage or antisocial
WHO ICTRP
(apps.who.int/trialsearch)
Intervention| aripiprazole OR olanzapine OR quetiapine OR sertindole OR ziprasidone OR zotepine OR clozapine OR risperidone OR amisulpride OR amisulpiride
metaRegister of Controlled Trials
antipsychotic* AND child* AND behav*
UK Clinical Research Network (UKCRN)
atypical antipsychotics
Appendix 3. Search strategies from 2011 onwards
CENTRAL, in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register
#1MeSH descriptor: [Child Behavior Disorders] this term only #2MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] this term only #3MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] this term only #4MeSH descriptor: [Conduct Disorder] this term only #5(conduct near/5 (disorder* or disturb*)) #6oppositional* #7MeSH descriptor: [Antisocial Personality Disorder] this term only #8((antisocial or anti next social) near/5 (behav* or histor* or conduct*)) #9(attention near/5 deficit*) #10(adhd or "ad/hd" or adhkd or addh or adhs) #11hyperactiv* or hyper next activ* #12hyperkine* or hyper next kin* #13aggress* #14agonistic* #15MeSH descriptor: [Anger] this term only #16MeSH descriptor: [Rage] this term only #17(anger or angry) #18anger or angry #19malic* #20hostil* #21deceit* #22cruel* #23(dangerous* or disrupt*) near/5 (behav* or histor* or conduct*) #24MeSH descriptor: [Impulsive Behavior] this term only #25impulse* or impulsiv* #26MeSH descriptor: [Crime] explode all trees #27criminal* or violen* or unlawful* or delinquen* #28MeSH descriptor: [Violence] this term only #29offend* or offence* or offense* #30correctional* or penal* #31(offend* or offenc* or offens*) #32{or #1‐#31} #33MeSH descriptor: [Risperidone] this term only #34MeSH descriptor: [Clozapine] this term only #35amisulpirid* #36amisulprid* #37aripiprazol* #38asenapin* #39clozapin* #40iloperidon* #41lurasidon* #42olanzapin* #43paliperid* #44quetiapin* #45risperidon* #46sertindol* #47ziprasidon* #48zotepin* #49atypical near/3 (anti next psychotic* or antipsychotic*) #50{or #33‐#49} #51MeSH descriptor: [Infant] this term only #52MeSH descriptor: [Child] explode all trees #53MeSH descriptor: [Adolescent] this term only #54MeSH descriptor: [Minors] this term only #55(child* or boy* or girl* or baby or babies or toddler* or infant* or teen* or adolescen* or juvenile* or preschool* or pre next school* or schoolchild*) #56youth* or young* people #57{or #51‐#56} #58#32 and #50 and #57
Ovid MEDLINE(R)
1 Child Behavior Disorders/ 2 Attention Deficit Disorder with Hyperactivity/ 3 "Attention Deficit and Disruptive Behavior Disorders"/ 4 Conduct Disorder/ 5 (conduct adj5 (disorder$ or disturb$)).mp. 6 oppositional$.mp. 7 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 8 Antisocial Personality Disorder/ 9 (attention adj5 deficit$).mp. 10 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 11 hyperactiv$.mp. 12 Hyperkinesis/ 13 hyperkine$.mp. 14 Aggression/ 15 Agonistic behavior/ 16 aggress$.mp. 17 agonistic$.mp. 18 Anger/ 19 Rage/ 20 (anger or angry).mp. 21 malic$.mp. 22 hostil$.mp. 23 ((dangerous$ or disrupt$ or defiant$) adj3 (behav$ or histor$ or conduct$)).mp. 24 deceit$.mp. 25 cruel$.mp. 26 delinquen$.mp. 27 threaten$.mp. 28 Impulsive Behavior/ 29 impulsiv$.mp. 30 impulse$.mp. 31 unlawful$.mp. 32 Violence/ 33 violen$.mp. 34 Crime/ 35 criminal$.mp. 36 penal$.mp. 37 (offend$ or offenc$ or offens$).mp. 38 correctional.mp. 39 or/1‐38 40 amisulprid$.mp. 41 amisulpirid$.mp. 42 aripiprazol$.mp. 43 asenapin$.mp. 44 Clozapine/ 45 clozapin$.mp. 46 iloperidon$.mp. 47 lurasidon$.mp. 48 olanzapin$.mp. 49 paliperidon$.mp. 50 quetiapin$.mp. 51 Risperidone/ 52 risperidon$.mp. 53 sertindol$.mp. 54 ziprasidon$.mp. 55 zotepin$.mp. 56 (atypical$ adj3 (antipsychotic$ or anti‐psychotic)).mp. 57 or/40‐56 58 (child$ or boy$ or girl$ or baby or babies or toddler$ or infant$ or teen$ or adolescen$ or juvenile$ or preschool$ or pre‐school$ or schoolchild$).mp. 59 (youth$ or young$ people).mp. 60 Child/ or Child, preschool/ 61 Infant/ 62 Adolescent/ 63 Minors/ 64 or/58‐63 65 39 and 57 and 64
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations
1 (conduct adj5 (disorder$ or disturb$)).mp. 2 oppositional$.mp. 3 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 4 (attention adj5 deficit$).mp. 5 (adhd or "ad/hd" or adhkd or addh or adhs).mp. 6 hyperactiv$.mp. 7 hyperkine$.mp. 8 aggress$.mp. 9 agonistic$.mp. 10 (anger or angry).mp. 11 malic$.mp. 12 hostil$.mp. 13 ((dangerous$ or disrupt$ or defiant$) adj3 (behav$ or histor$ or conduct$)).mp. 14 deceit$.mp. 15 cruel$.mp. 16 delinquen$.mp. 17 threaten$.mp. 18 impulsiv$.mp. 19 impulse$.mp. 20 unlawful$.mp. 21 violen$.mp. 22 criminal$.mp. 23 penal$.mp. 24 (offend$ or offenc$ or offens$).mp. 25 correctional.mp. 26 amisulprid$.mp. 27 amisulpirid$.mp. 28 aripiprazol$.mp. 29 asenapin$.mp. 30 clozapin$.mp. 31 iloperidon$.mp. 32 lurasidone.mp. 33 olanzapin$.mp. 34 paliperid$.mp. 35 quetiapin$.mp. 36 risperidon$.mp. 37 sertindol$.mp. 38 ziprasidon$.mp. 39 zotepin$.mp. 40 (atypical$ adj3 (antipsychotic$ or anti‐psychotic)).mp. 41 (child$ or boy$ or girl$ or baby or babies or toddler$ or infant$ or teen$ or adolescen$ or juvenile$ or preschool$ or pre‐school$ or schoolchild$).mp. 42 (youth$ or young$ people).mp. 43 or/1‐25 44 or/26‐40 45 or/41‐42 46 43 and 44 and 45
Embase Ovid
1 behavior disorder/ or attention deficit disorder/ or disruptive behavior/ or impulse control disorder/ or oppositional defiant disorder/ 2 (conduct adj5 (disorder$ or disturb$)).mp. 3 oppositional$.mp. 4 antisocial behavior/ 5 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 6 ((antisocial$ or anti‐social$) adj personality adj disorder$).mp. 7 (attention adj5 deficit$).mp. 8 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 9 hyperactiv$.mp. 10 hyperkinesia/ 11 hyperkine$.mp. 12 exp aggression/ 13 aggress$.mp. 14 agonistic$.mp. 15 (anger or angry).mp. 16 malic$.mp. 17 hostil$.mp. 18 ((dangerous$ or disrupt$) adj3 (behav$ or histor$ or conduct$)).mp. 19 deceit$.mp. 20 cruel$.mp. 21 delinquen$.mp. 22 threaten$.mp. 23 impulsiveness/ 24 impulse control disorder/ 25 (impulsiv$ or impulse$).mp. 26 unlawful$.mp. 27 exp violence/ 28 violen$.mp. 29 exp crime/ 30 criminal$.mp. 31 penal$.mp. 32 (offend$ or offenc$ or offens$).mp. 33 correctional.mp. 34 or/1‐33 35 exp atypical antipsychotic agent/ 36 amisulprid$.mp. 37 amisulpirid$.mp. 38 aripiprazol$.mp. 39 asenapin$.mp. 40 clozapin$.mp. 41 iloperidon$.mp. 42 lurasidone.mp. 43 olanzapin$.mp. 44 paliperid$.mp. 45 quetiapin$.mp. 46 risperidon$.mp. 47 sertindol$.mp. 48 ziprasidon$.mp. 49 zotepin$.mp. 50 (atypical$ adj3 (antipsychotic$ or anti‐psychotic$)).mp. 51 or/35‐50 52 (baby or babies or infant$ or toddler$ or child$ or boy$ or girl$ or preschool$ or pre school$ or teen$ or adolescen$ or juvenile$ or minor$ or youth$ or young people).tw. 53 infant/ 54 child/ 55 school child/ 56 preschool child/ 57 adolescent/ 58 or/53‐57 59 34 and 51 and 58
PsycINFO Ovid
1 antisocial behavior/ 2 Antisocial Personality Disorder/ 3 Behavior Disorders/ 4 Conduct Disorder/ 5 Aggressive Behavior/ 6 Aggressiveness/ 7 Impulsiveness/ 8 Oppositional Defiant Disorder/ 9 exp attention deficit disorder/ 10 Hyperkinesis/ 11 Anger/ 12 Hostility/ 13 (conduct adj5 (disorder$ or disturb$)).mp. 14 oppositional$.mp. 15 ((antisocial$ or anti‐social$) adj5 (behav$ or histor$ or conduct)).mp. 16 (attention adj5 deficit$).mp. 17 (adhd or "ad/hd" or adhkd or addh or adhs).tw. 18 hyperactiv$.mp. 19 hyperkine$.mp. 20 aggress$.mp. 21 agonistic$.mp. 22 (anger or angry).mp. 23 malic$.mp. 24 hostil$.mp. 25 ((dangerous$ or disrupt$ or defiant$) adj3 (behav$ or histor$ or conduct$)).mp. 26 deceit$.mp. 27 cruel$.mp. 28 Juvenile Delinquency/ 29 delinquen$.mp. 30 threaten$.mp. 31 impulsiv$.mp. 32 impulse$.mp. 33 unlawful$.mp. 34 Violence/ 35 violen$.mp. 36 exp Crime/ 37 criminal$.mp. 38 penal$.mp. 39 (offend$ or offenc$ or offens$).mp. 40 correctional.mp. 41 or/1‐40 42 Clozapine/ 43 clozapin$.mp. 44 exp Olanzapine/ 45 olanzapin$.mp. 46 Quetiapine/ 47 quetiapin$.mp. 48 Risperidone/ 49 risperidon$.mp. 50 sertindol$.mp. 51 ziprasidon$.mp. 52 zotepin$.mp. 53 amisulprid$.mp. 54 amisulpirid$.mp. 55 aripiprazole/ 56 aripiprazol$.mp. 57 asenapin$.mp. 58 iloperidon$.mp. 59 lurasidone.mp. 60 paliperid$.mp. 61 (atypical$ adj3 (antipsychotic$ or anti‐psychotic)).mp. 62 or/42‐61 63 (child$ or boy$ or girl$ or baby or babies or toddler$ or infant$ or teen$ or adolescen$ or juvenile$ or preschool$ or pre‐school$ or schoolchild$).mp. (829362) 64 (youth$ or young$ people).mp. (94048) 65 ("100" or "120" or "140" or "160" or "180" or "200" or "320").ag. 66 63 or 64 or 65 ) 67 41 and 62 and 66
CINAHL Plus EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S1 (MH "Child Behavior Disorders") S2 (MH "Attention Deficit Hyperactivity Disorder") S3 (MH "Antisocial Personality Disorder") S4 (MH "Disruptive Behavior") S5 (MH "Hyperkinesis") S6 (MH "Aggression") S7 (MH "Anger") S8 (conduct N5 disorder*) OR (conduct N5 disturb*) S9 oppositional* S10 (antisocial* N5 behav*) or (antisocial* N5 histor*) or (antisocial* N5 conduct*) S11 (anti social* N5 behav*) or (anti social* N5 histor*) or (anti social* N5 conduct*) S12 attention N5 deficit* S13 adhd or "ad/hd" or adhkd or addh or adhs S14 hyperactiv* S15 hyperkine* S16 aggress* or agonistic* S17 anger or angry or rage* S18 malic* or hostil* S19 deceit* S20 cruel* S21 (dangerous* N3 behav* ) or (dangerous* N3 histor*) or (dangerous* N3 conduct*) S22 (disrupt* N3 behav* ) or (disrupt* N3 histor*) or (disrupt* conduct*) S23 delinquen* S24 violen* or criminal* or penal* or correctional* or unlawful* or threaten* S25 offend* or offenc* or offens* S26 (MH "Public Offenders") S27 (MH "Crime") S28 (MH "Juvenile Delinquency") or (MH "Juvenile Offenders+") S29 (MH "Violence") or (MH "School Violence") or (MH "Sibling Violence") S30 (MH "Impulse Control Disorders") S31 impulse* or impulsiv* S32 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33 (MH "Aripiprazole") or (MH "Clozapine") or (MH "Olanzapine") or (MH "Quetiapine") or (MH "Risperidone") S34 (MH "Asenapine") S35 (MH "Iloperidone") S36 (MH "Paliperidone") S37 amisulpirid* S38 amisulprid* S39 aripiprazol* S40 asenapin* S41 clozapin* S42 iloperidon* S43 lurasidon* S44 olanzapin* S45 paliperid* S46 quetiapin* S47 risperidon* S48 sertindol* S49 ziprasidon* S50 zotepin* S51 (atypical n3 (antipsychotic* or anti‐psychotic*)) S52S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 S53 S32 AND S52 S54 baby or babies or infant* or toddler* or child* or boy* or girl* or preschool* or pre school* or schoolchild* or juvenile* or minor* or teen* or adolescen* or youth* or young* people S55 AG child S56 AG adolescent S57 AG preschool S58 S54 OR S55 OR S56 OR S57 S59 S53 AND S58
Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library
#1 MeSH descriptor: [Child Behavior Disorders] this term only #2 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] this term only #3 MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] this term only #4 MeSH descriptor: [Conduct Disorder] this term only #5 (conduct near/5 (disorder* or disturb*)):ti,ab,kw #6 (oppositional*):ti,ab,kw #7 MeSH descriptor: [Antisocial Personality Disorder] this term only #8 ((antisocial or anti next social) near/5 (behav* or histor* or conduct*)):ti,ab,kw #9 (attention near/5 deficit*):ti,ab,kw #10 (adhd or "ad/hd" or adhkd or addh or adhs):ti,ab,kw #11 hyperactiv*:ti,ab,kw #12 hyperkine*:ti,ab,kw #13 aggress*:ti,ab,kw #14 agonistic*:ti,ab,kw #15 MeSH descriptor: [Anger] this term only #16 MeSH descriptor: [Rage] this term only #17(anger or angry):ti,ab,kw #18 malic*:ti,ab,kw #19 hostil*:ti,ab,kw #20 deceit*:ti,ab,kw #21 cruel*:ti,ab,kw #22 ((dangerous* or disrupt*) near/5 (behav* or histor* or conduct*)):ti,ab,kw #23 MeSH descriptor: [Impulsive Behavior] this term only #24 (impulse* or impulsiv*):ti,ab,kw #25 MeSH descriptor: [Crime] explode all trees #26 (criminal* or violen* or unlawful* or delinquen*):ti,ab,kw #27 MeSH descriptor: [Violence] this term only #28 (offend* or offence* or offens*):ti,ab,kw #29 (correctional* or penal*):ti,ab,kw #30 {or #1‐#29} #31 MeSH descriptor: [Risperidone] this term only #32 MeSH descriptor: [Clozapine] this term only #33 amisulpirid*:ti,ab,kw #34 amisulprid*:ti,ab,kw #35 aripiprazol*:ti,ab,kw #36 asenapin*:ti,ab,kw #37 clozapin*:ti,ab,kw #38 iloperidon*:ti,ab,kw #39 lurasidon*:ti,ab,kw #40 olanzapin*:ti,ab,kw #41 paliperid*:ti,ab,kw #42 quetiapin*:ti,ab,kw #43 risperidon*:ti,ab,kw #44 sertindol*:ti,ab,kw #45 ziprasidon*:ti,ab,kw #46 zotepin*:ti,ab,kw #47 (atypical near/3 (anti next psychotic* or antipsychotic*)):ti,ab,kw #48 {or #31‐#47} #49 MeSH descriptor: [Infant] this term only #50 MeSH descriptor: [Child] explode all trees #51 MeSH descriptor: [Adolescent] this term only #52 MeSH descriptor: [Minors] this term only #53 (child* or boy* or girl* or baby or babies or toddler* or infant* or teen* or adolescen* or juvenile* or preschool* or pre next school* or schoolchild*):ti,ab,kw #54 (youth* or young* next people):ti,ab,kw #55 {or #49‐#54} #56#48 and #55 and #30
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library
#1 MeSH descriptor: [Child Behavior Disorders] this term only #2 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] this term only #3 MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] this term only #4 MeSH descriptor: [Conduct Disorder] this term only #5 (conduct near/5 (disorder* or disturb*)):ti,ab,kw #6 (oppositional*):ti,ab,kw #7 MeSH descriptor: [Antisocial Personality Disorder] this term only #8 ((antisocial or anti next social) near/5 (behav* or histor* or conduct*)):ti,ab,kw #9 (attention near/5 deficit*):ti,ab,kw #10 (adhd or "ad/hd" or adhkd or addh or adhs):ti,ab,kw #11 hyperactiv*:ti,ab,kw #12 hyperkine*:ti,ab,kw #13 aggress*:ti,ab,kw #14 agonistic*:ti,ab,kw #15 MeSH descriptor: [Anger] this term only #16 MeSH descriptor: [Rage] this term only #17(anger or angry):ti,ab,kw #18 malic*:ti,ab,kw #19 hostil*:ti,ab,kw #20 deceit*:ti,ab,kw #21 cruel*:ti,ab,kw #22 ((dangerous* or disrupt*) near/5 (behav* or histor* or conduct*)):ti,ab,kw #23 MeSH descriptor: [Impulsive Behavior] this term only #24 (impulse* or impulsiv*):ti,ab,kw #25 MeSH descriptor: [Crime] explode all trees #26 (criminal* or violen* or unlawful* or delinquen*):ti,ab,kw #27 MeSH descriptor: [Violence] this term only #28 (offend* or offence* or offens*):ti,ab,kw #29 (correctional* or penal*):ti,ab,kw #30 {or #1‐#29} #31 MeSH descriptor: [Risperidone] this term only #32 MeSH descriptor: [Clozapine] this term only #33 amisulpirid*:ti,ab,kw #34 amisulprid*:ti,ab,kw #35 aripiprazol*:ti,ab,kw #36 asenapin*:ti,ab,kw #37 clozapin*:ti,ab,kw #38 iloperidon*:ti,ab,kw #39 lurasidon*:ti,ab,kw #40 olanzapin*:ti,ab,kw #41 paliperid*:ti,ab,kw #42 quetiapin*:ti,ab,kw #43 risperidon*:ti,ab,kw #44 sertindol*:ti,ab,kw #45 ziprasidon*:ti,ab,kw #46 zotepin*:ti,ab,kw #47 (atypical near/3 (anti next psychotic* or antipsychotic*)):ti,ab,kw #48 {or #31‐#47} #49 MeSH descriptor: [Infant] this term only #50 MeSH descriptor: [Child] explode all trees #51 MeSH descriptor: [Adolescent] this term only #52 MeSH descriptor: [Minors] this term only #53 (child* or boy* or girl* or baby or babies or toddler* or infant* or teen* or adolescen* or juvenile* or preschool* or pre next school* or schoolchild*):ti,ab,kw #54 (youth* or young* next people):ti,ab,kw #55 {or #49‐#54} #56#48 and #55 and #30
ClinicalTrials.gov
(clinicaltrials.gov)
Interventional Studies | antisocial OR disruptive OR conduct OR oppositional OR attention deficit OR adhd | aripiprazole OR olanzapine OR quetiapine OR sertindole OR ziprasidone OR zotepine OR clozapine OR risperidone OR amisulpride OR amisulpirid OR asenapine OR iloperidone OR lurasidone OR paliperidone OR atypical | Child |
WHO ICTRP
(apps.who.int/trialsearch)
Condition: antisocial OR disruptive OR conduct OR oppositional OR attention OR adhd AND Intervention: aripiprazole OR olanzapine OR quetiapine OR sertindole OR ziprasidone OR zotepine OR clozapine OR risperidone OR amisulpride OR amisulpirid OR asenapine OR iloperidone OR lurasidone OR paliperidone OR atypical AND Clinical Trials in children= selected AND Recruitment status =all
Appendix 4. Search summary 2011 onwards
| Database | Search date | Database date range or issue | Number of records | Limits applied |
| Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register | 26 January 2015 | 2014, Issue 12 (December) | 42 | Limited to 2011‐2015 and new drugs for all years |
| 17 February 2016 | 2016, Issue 1 | 12 | Limited to 2015‐2016 | |
| 19 January 2017 | 2016, Issue 11 | 19 | Limited to 2015‐2017 and de‐duped with previous records | |
| Ovid MEDLINE | 23 January 2015 | 1946 to January Week 3 2015 | 158 | ED=20110801‐20150123 and new drugs for all years |
| 17 February 2016 | 1946 to February Week 1 2016 | 47 | ED=20150101‐20160204 | |
| 19 January 2017 | 1946 to December Week 1 2016 | 52 | ED=20150101‐20161114 and de‐duped with previous records | |
| Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations | 23 January 2015 | 22 January 2015 | 56 | No limits |
| 17 February 2016 | 16 February 2016 | 70 | No limits | |
| 19 January 2017 | 18 January 2017 | 53 | De‐dupedlicated with previous records | |
| Embase Ovid | 28 January 2015 | 1980 to 2015 Week 03 | 721 | 2011 to current and new drugs for all years |
| 17 February 2016 | 1980 to 2016 Week 07 | 311 | 2015 to current | |
| 19 January 2017 | 1980 to 2017 Week 03 | 240 | 2015 to current. De‐duped with previous records | |
| PsycINFO Ovid | 23 January 2015 | 1806 to January Week 3 2015 | 245 | UP=20110801‐20150115 and new drugs for all years |
| 17 February 2016 | 1806 to February Week 1 2016 | 67 | UP=20150115‐20160201 | |
| 19 January 2017 | 1806 to January Week 2 2017 | 60 | UP=20150115‐20170109 de‐duped with previous records | |
| CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature) | 27 January 2015 | 1937 to current | 73 | EM = 2011801 onwards plus new drugs for all years |
| 17 February 2016 | 1937 to current | 29 | EM = 20150101 onwards | |
| 19 January 2017 | 1937 to current | 17 | EM 20150101onwards . De‐duped with previous records | |
| Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library | 26 January 2015 | 1 of 12 2015 | 5 | No date limits. Not searched in 2011 |
| 17 February 2016 | 2016 Issue 2 (February) | 0 | 2015‐2016 | |
| 19 January 2017 | 2017, Issue 1 (January) | 1 | 2016‐2017 | |
| Database of Abstracts of Reviews of Effect (DARE), part of the Cochrane Library | 26 January 2015 | 2014 Issue 4 (October) | 5 | No date limits; not searched previously |
| 17 February 2016 | 2015 Issue 2 (April) | 0 | 2014‐2015. Not searched in later years because there is no new content after 2015 Issue 2 | |
| ClinicalTrials.gov (clinicaltrials.gov) | 26 January 2015 | All available years | 6 | Limited to trials registered since 1 August 2011 |
| 18 February 2016 | All available years | 42 | No limits | |
| 20 January 2017 | All available years | 12 | Limited to trials received from 18 February 2016 to 20 January 2017 | |
| World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP;apps.who.int/trialsearch) | 27 January 2015 | All available years | 8 | Limited to trials registered since 1 August 2011 |
| 18 February 2016 | All available years | 32 | No limits | |
| 20 January 2017 | All available years | 7 | Limited to trials registered since 18 February 2017 | |
| Total records | 2390 | |||
Data and analyses
Comparison 1. Risperidone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aggression: ABC irritability (mean change scores) | 3 | 238 | Mean Difference (IV, Random, 95% CI) | ‐6.49 [‐8.79, ‐4.19] |
| 2 Aggression: OAS‐M, ABS Reactive subscale (final scores) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.21, ‐0.40] |
| 3 Aggression: OAS‐M, ABS Proactive subscale (final scores) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.30, 0.06] |
| 4 Conduct: NCBR‐CP (mean change scores) | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐8.61 [‐11.49, ‐5.74] |
| 5 Weight gain (antipsychotic only): Kg (mean change scores) | 2 | 138 | Mean Difference (IV, Random, 95% CI) | 2.37 [0.26, 4.49] |
| 6 Weight gain (antipsychotic and stimulant): Kg (mean change scores) | 3 | 305 | Mean Difference (IV, Random, 95% CI) | 2.14 [1.04, 3.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aman 2002.
| Methods | Double‐blind, randomised controlled trial of risperidone solution and placebo | |
| Participants |
Setting: outpatients, multicentre Sample size: 118; 55 active treatment (reported for 52 participants); 63 placebo Sex: 97 males, 21 females Age range: 5 to 12 years Mean age: active treatment 8.7 (SD = 2.1) years; placebo 8.1 (SD = 2.3) years IQ range: 36 to 84 Inclusion criteria: ≥ 24 (70th percentile) on the Conduct Problem subscale of the Nisonger Child Behaviour Rating Form Diagnosis: DSM‐IV diagnosis of conduct disorder, oppositional defiant disorder, or disruptive behaviour disorder not otherwise specified Comorbidity: 70 ADHD (33 active treatment; 37 placebo) Withdrawn/dropouts: 31 withdrawn (12 active treatment; 19 placebo) Other interventions
|
|
| Interventions |
Intended dose: risperidone solution 0.01 mg/kg increasing to 0.02 mg/kg on day 3 Mean dose at endpoint: 1.16 mg/day (mean 0.037 mg/kg per day) |
|
| Outcomes |
Primary outcomes: Conduct Problem subscale of the Nisonger Child Behaviour Rating Form Secondary outcomes: Aberrant Behaviour Checklist, Behaviour Problem Inventory, Clinical Global Impression ‒ Severity Rating Scale, Clinical Global Impression ‒ Change Scores, Visual Analogue Scale of the target symptoms Follow‐up interval: 6 weeks |
|
| Notes |
Imputation method for incomplete data: last observation carried forward (LOCF) (p 1339) Funding/support
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned" (p 1338) ‒ insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Appearance and taste of solutions were identical. All trial medications were labelled with the protocol number, medication number, lot number and strata. A tear‐off label was provided on each box of study medication which contained the medication code. The label was placed in the Case Report Form on the appropriate page. The code should only be broken in case of an emergency (Loy 2011b [pers comm]). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 participants (22%) in the risperidone and 19 (30%) in the placebo group withdrew. 4 in the risperidone and 15 in the placebo group due to insufficient response, 2 in risperidone because of adverse events, 3 in risperidone due to non‐compliance, 1 in risperidone and 3 in placebo lost to follow‐up, 1 in risperidone and 1 in placebo withdrew consent and 1 in risperidone lost medication. No efficacy data were recorded for 3 patients in the risperidone group and hence they were not included in any efficacy analyses (but the authors stated to have used an intention‐to‐treat analysis). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | Unclear risk | 1‐week placebo run‐in "to rule out placebo responders" (p 1338). |
Armenteros 2007.
| Methods | Double‐blind, randomised controlled trial of risperidone and placebo, added to pre‐existing stimulants | |
| Participants |
Setting: outpatients, 1 centre Sample size: 25; 12 active treatment, 13 placebo Sex: 22 males and 3 females Age range: 7 to 12 years Mean age: active treatment 7.3 (SD = 3.7) years; placebo 8.8 (SD = 3.1) years IQ range: IQ ≥ 75 Inclusion criteria: DSM‐IV criteria for ADHD; aggression criteria documented by the presence of 3 acts of aggression in the past week, 2 of which had to be acts of physical aggression against other people, objects or self. Patients had an Aggression Questionnaire Predatory ‒ Affective index score of 0 or below indicating primarily an affective or impulsive type of aggression, Clinical Global Impressions ‒ Severity score ≥ 4 (moderately ill) Comorbidity: All ADHD Withdrawn/dropouts: 2 withdrawn (1 active treatment, 1 placebo) Other interventions
|
|
| Interventions |
Intended dose: 0.5 mg/day at bedtime individually regulated until optimum efficacy. Max 2 mg a day Mean end dose: 1.08 mg/day |
|
| Outcomes |
Primary outcomes: Children's Aggression Scale ‒ Parents, Children's Aggression Scale ‒ Teachers Secondary outcomes: Conners' Parent Rating Scale, Conners' Teacher Rating Scale, Clinical Global Impression ‒ Improvement, Clinical Global Impression ‒ Severity Follow‐up interval: 28 days |
|
| Notes |
Imputation method for incomplete data: "data from last known prior session were used for subsequent missing time points" (LOCF) Funding/support: "This study was supported by Janssen Pharmaceuticals" (p 558). Declaration: Dr Armenteros has received research support and is on the speakers' panel of Janssen Pharmaceuticals. The other authors have no financial relationships to disclose (p 564). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assignment of subjects to treatment groups was carried out by following a table of random permutations, which balanced the number of subjects in each group. The research staff was not informed of the length of the permutations." (p 560). |
| Allocation concealment (selection bias) | Unclear risk | No stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All of the subjects and clinical and research staff were blind to treatment condition" (p 560). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant (8%) in the risperidone group dropped out of the study after 1 week of treatment and 1 participant (8%) in the placebo group also dropped out of the study after 2 weeks of treatment. In both cases, participants failed to comply with treatment regulations. The rest of the sample completed the 28 days of treatment as per protocol (p 561). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. Possible reporting bias as dichotomous results were presented while no differences in mean scores were detected. |
| Other bias | High risk | Small number of participants therefore a limited power to detect differences. |
Buitelaar 2001.
| Methods | Double‐blind, randomised controlled trial of risperidone and placebo | |
| Participants |
Setting: inpatients (2 centres) Sample size: 38; 19 active treatment, 19 placebo Sex: 33 males, 5 females Age range: 12 to 18 years Mean age: active treatment 14 (SD = 1.5) years; placebo 13.7 (SD = 2.0) years IQ range: 60 to 90 Inclusion criteria: persistent overt aggressive behaviour as evidenced by ≥ 1 on Overt Agression Scale ‒ Modified; failure of behavioural treatment. Participants were included if "their aggressive behavior failed to respond to behavioral treatment approaches (typically, these behavioral treatments involve contingency management and social skills training delivered on an individual basis for at least 2 months)." (p 240) Diagnosis: DSM‐IV criteria conduct disorder, oppositional defiant disorder Comorbidity: ADHD (14 active treatment, 12 placebo) Withdrawn/dropouts: 2 withdrawals (2 placebo) |
|
| Interventions |
Intended dose: from 0.5 mg twice daily increased by 1 mg up to 5 mg. As fixed as possible, could be adjusted down if adverse event present. Mean end dose: 63% on 3 mg a day, mean 2.9 mg (1.5 to 4 mg) |
|
| Outcomes |
Primary outcomes: Clinical Global Impression ‒ Severity scale Secondary outcomes: Overt Aggression Scale ‒ Modified, Aberrant Behaviour Checklist (all scales nurse and teacher‐rated) Follow‐up interval: 6 weeks |
|
| Notes |
Imputation method for incomplete data: last observation carry forward (LOCF) (p 242) Funding/support: "Supported by Janssen‐Cilag, BV, Tilburg, the Netherlands" (p 239) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code had been generated by computer in block of four numbers" (p 241). |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Dosage was adjusted by the responsible psychiatrist who was blind to the treatment" (p 241). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two participants (11%) in the placebo group stopped treatment during the double‐blind period because of lack of therapeutic effects and uncontrollable aggressive behavior" (p 242). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | No reason given why 145 approached and 49 found to be eligible. "Greater severity of psychosocial stressors in the risperidone group" (p 242). |
Connor 2008.
| Methods | Double‐blind randomised controlled trial of oral quetiapine and placebo | |
| Participants |
Setting: single site, outpatients Sample size: 19; 9 active treatment, 10 placebo Sex: 74% male Age range: 12 to 17 years Mean age: 14.1 (1.6) years IQ range: excluded significantly sub‐average IQ, assessed by the clinician, based on school history Inclusion criteria: primary psychiatric diagnosis of conduct disorder, moderate to severe aggressive behaviour as evidenced by Overt Aggression Scale score ≥ 25, Clinical Global Impressions ‒ Severity score ≥ 4 Comorbidity: ADHD in active 8 and control 7 Withdrawn/dropouts: 8 withdrawals (1 active treatment, 7 placebo) Other interventions: current psychosocial therapies were allowed in the protocol as long as therapy was not changed during the study |
|
| Interventions |
Intended dose: 25 mg twice daily, by day 14 at least 200 mg, after day 14 up to 800 mg at the discretion of a clinician Mean dose at endpoint: 294 (± 78) mg/day, range 200 to 600 mg/day, average weight adjusted 4.5 (± 2.5) mg/kg per day |
|
| Outcomes |
Primary outcomes: Clinical Global Impression ‒ Severity, Clinical Global Impression ‐ Improvement scales Secondary outcomes: Overt Aggression Scale ‒ parent‐rated, Conners' Parent Rating Scale ‒ conduct problem subscale, Quality of Life Enjoyment and Satisfaction Questionnaire Follow‐up interval: 6 weeks |
|
| Notes |
Imputation method for incomplete data: used mixed‐effect longitudinal analysis (p 146) Funding/support: this study was supported by an Investigator Initiated Grant from Astra Zeneca Pharmaceuticals (p 153). Dr Connor and McLaughlin analysed all the data and completed all the writing of this submission (p 153). First author is a consultant for Shire Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study medication was blinded and encapsulated by placing whole tablets into identical‐looking tablets by institutional research pharmacist" (p 144). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant (11%) withdrew from the medication (quetiapine) group due to side effects. 5 participants withdrew from the placebo group due to lack of efficacy and 2 withdrew due to protocol violation (70%). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | 1‐week single‐blind placebo to start with. Small sample size and therefore limited power to detect differences. |
Findling 2000.
| Methods | Randomised double‐blind control trial of risperidone and placebo | |
| Participants |
Setting: outpatients, 1 centre, inner city academic outpatient medical centre Sample size: 20; 10 active treatment, 10 placebo Sex: 19 male, 1 female Age range: 5 to 15 years Mean age: 9.2 (2.9) years (range 6 to 14 years) IQ range: IQ more than 70 Diagnosis: DSM‐IV of conduct disorder Inclusion criteria: Clinical Global Impression ‒ Severity score moderate severity; Child Behaviour Checklist aggression subscale T‐score 2 SD or more above mean for age and gender‐matched peers Comorbidity: moderate to severe ADHD excluded Withdrawn/dropouts: 11 withdrew (4 active treatment, 7 placebo) Other Interventions: psychosocial interventions not mentioned |
|
| Interventions |
Intended dose: for under 50 kg, 0.25 mg increasing to 1.5 mg; and for over 50 kg, 0.5 mg increasing to 3 mg Mean dose at endpoint: 0.028 (± 0.004) mg/kg per day (range 0.75 to 1.50 mg/day) |
|
| Outcomes |
Primary outcomes: Rating of Aggression Against People and Property Scale Secondary outcomes: Conners' Parent Rating Scale ‒ conduct problem subscale, Child Behaviour Checklist, Clinical Global Impression ‐ Severity, Clinical Global Impression ‐ Improvement Follow‐up interval: 10 weeks |
|
| Notes |
Imputation method for incomplete data: unclear from the published study Funding/support
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number list. The list was kept in the Center for Drug Research and not was not accessible to either PI or other study raters. |
| Allocation concealment (selection bias) | Low risk | A random number list. The list was kept in the Center for Drug Research and not was not accessible to either PI or other study raters. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Risperidone and placebo matched in appearance. The blind was not broken during the course of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 10 youths assigned to risperidone, 6 completed the entire study (40% attrition): "3 youths who were assigned to receive risperidone were withdrawn by their guardian because of lack of effect, and 1 youth who received risperidone was withdrawn from the study during week 4 because of the development of a rash" (p 511). Only 3 youths who received placebo finished the trial (70% attrition): "4 patients assigned to placebo were withdrawn from the protocol by their guardians because of lack of benefit, 2 more were withdrawn from the study by the PI because of non‐compliance with study procedures, and 1 youth randomly assigned to placebo was lost to follow‐up" (p 511). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | Small sample size and therefore limited power to detect differences. |
Fleischhaker 2011.
| Methods | Randomised double‐blind control trial of ziprasidone and placebo | |
| Participants |
Setting: outpatients; single site Sample size: 50; 25 active treatment and 25 placebo Sex: not reported Age range: 7 to 17 years Mean age: active treatment 11.5 (± 2.4) years, placebo 12.2 (±2.5) years IQ range: no average IQ reported; IQ greater than 55 Inclusion criteria: primary diagnosis of conduct disorder or oppositional defiant disorder or disruptive disorder not otherwise specified; score of > 21 on Nisonger Child Behavior Rating Form Typical IQ Version scales for conduct and oppositional behaviour disorders at screening Comorbidity: no specific report on the assessment of comorbid ADHD. Withdrawn/dropouts: 22 (44%) participants completed the study; 12 in the active arm and 10 in the placebo arm Other interventions: psychosocial interventions not mentioned |
|
| Interventions |
Intended dose: oral suspension of ziprasidone (Zeldox) vs placebo. Patients with a body weight ≤ 50 kg received an initial oral course starting with 5 mg/d Ziprasidone or placebo titrated over a course of 3 weeks to a maximum daily dose of 20 mg. Patients with a body weight > 50 kg received 10 mg/d Ziprasidone or placebo, which was titrated over 3 weeks to a maximum of 40 mg/day. The total dose was split into 2 half doses for administration twice a day (morning and evening) Mean dose at endpoint: none reported |
|
| Outcomes |
Primary outcome: Nisonger Child Behaviour Rating Form ‒ Typical IQ Version D‐Total (conduct disorder and oppositional defiant disorder subscales) rated by the participant’s primary caregiver. Secondary outcomes: included Clinical Global Impressions ‐ Severity of Illness and the Clinical Global Impressions‐Improvement scales. Follow up interval: the study consisted of a 3‐week baseline period for finding the best individual dose followed by a 6‐week treatment period and 2‐week washout period. |
|
| Notes | The authors of this paper queried whether the chosen dosage level was too low. Funding/support: the study medication and the trial were financially supported by a pharmaceutical company (it was an investigator‐initiated trial). Christian Fleischhaker received grants from government (Federal Ministry of Education and Research [BMBF]) and by Bristol‐Myers‐Squibb, Novartis, Shire, Otsuka and Pfizer. Eberhard Schulz received grants from government (Federal Ministry of Education and Research [BMBF]) and by Janssen‐Cilag, Eli Lilly, Novartis, Shire und Pfizer (as described in the Conflict of interest section) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment assignments were made in accordance with the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors reported that 22/50 participants (44%) completed the study but the reasons for withdrawal or dropout were not stated for 16 of those who had not completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. A number of factual errors noted in the AACAP abstract and conference poster provided to the reviewers via correspondence (Stasiak 2015 [pers comm]). |
| Other bias | High risk | Unpublished data and thus not peer reviewed. Small sample size and no power calculation described, thus the study may have been underpowered. Quality of reporting consistent with a poster presentation but not to the level of a peer‐review journal article. |
Reyes 2006a.
| Methods | Randomised double‐blind controlled trial of risperidone and placebo | |
| Participants |
Setting: international, multicentre, 3‐stage; outpatients Sample size: 527 (acute, 6 weeks, open label); 436 (continuation, 6 weeks, single blind, risperidone), 335 (maintenance, 6 months, double‐blind, RCT). N = 335 (for the 6‐month, double blind, maintenance treatment); 172 active treatment, 163 placebo Sex: 290 male, 45 female Age range: 5 to 17 years Mean age: risperdone 10.9 (SD 2.93) years; placebo 10.8 (SD 2.94) years IQ range: 216 with IQ > 84; 119 with IQ < 84 Inclusion criteria: Nisonger Child Behaviour Rating Form score > 24 Diagnosis: DSM‐IV for conduct disorder, oppositional defiant disorder or disruptive behaviour disorder not otherwise specified Comorbidity: 227 with comorbid ADHD (overall, 24% treated with concomitant stimulant, p 409) Withdrawn/dropouts: 162 completed treatment; 124 experienced symptom recurrence; 49 discontinued (out of these, 8 experienced an adverse event) Other interventions: pre‐existing or comorbid psychosocial interventions not mentioned |
|
| Interventions | Oral risperidone solution Intended dose: 1 mg/ml oral solution once or twice daily, same dose as in continuation phase, max < 50 kg = 0.75 mg or > 50 kg = 1.5 mg Mean dose at endpoint: < 50 kg = 0.81 mg (0.34 mg), > 50 kg = 1.22 mg (0.36 mg) |
|
| Outcomes |
Primary outcomes: time to symptom recurrence, deterioration of ≥ 2 points on Clinical Global Impression ‒ Improvement score or 7 points on conduct problem subscale Secondary outcomes: rate of discontinuation, Nisonger Children's Behaviour Rating Form, Clinical Global Impression ‒ Severity, visual analogue scale of the most troublesome symptoms, Children's Global Assessment Scale Follow‐up interval: 6 months |
|
| Notes |
Imputation method for incomplete data: LOCF (page 405) Funding/support: this study was supported by Johnson & Johnson R&D (p 410). The first author's correspondence address is at J & J Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was generated by the study sponsor" (p 403‐4). |
| Allocation concealment (selection bias) | Low risk | "...treatment numbers allocated at each investigative centre in chronological order" (p 404). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo and risperidone oral solutions were identical in appearance and flavour" (p 404). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the risperidone group, 24 discontinued treatment and that included 4 participants who experienced an adverse event. In the placebo group, 25 discontinued and that included 4 participants who had experienced an adverse event. The reasons for discontinuations (besides those stopping due to adverse events) were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | Unclear risk | "...only patients who responded to initial treatment were randomized, potentially introducing a selection bias. This was, in part, addressed by including a single‐blind period prior to double‐blind randomization..." (p 409). |
Snyder 2002.
| Methods | Randomised double‐blind controlled trial of risperidone and placebo | |
| Participants |
Setting: outpatients; multicentre (10 sites in Canada, 4 in USA and 2 in South Africa) Sample size: 110; 53 active treatment, 57 placebo Sex: 83 male, 27 female Age range: 5 to 12 years IQ range: 36 to 84 (n = 53 borderline, n = 42 mild, n = 15 moderate ID) Inclusion criteria: Nisonger Childrens Behaviour Rating Form ‒ parent version > 24 Diagnosis: DSM‐IV for conduct disorder, oppositional defiant disorder, or disruptive behaviour disorder not otherwise specified Comorbidity: ADHD 80% (n = 84), 45 were treated Withdrawn/dropouts: 25 withdrawals (6 risperidone, 19 placebo) Other interventions: psychosocial intervention was not mentioned but its design was stated to be identical to Aman 2002; behavioural therapy was permitted if it was initiated at least 30 days before the start of the study; no changes to behavioural therapy were allowed during the trial. |
|
| Interventions |
Intended dose: max 0.06 mg/kg in the morning Mean dose at endpoint: 0.98 mg/day (SE = 0.06), which equalled 0.033 mg/kg (SE = 0.001) range 0.40 to 3.80 mg/day |
|
| Outcomes |
Primary outcome: Nisonger Childrens Behaviour Rating Form ‒ Conduct Disorder subscale Secondary outcomes: Aberrant Behaviour Checklist, Behaviour Problem Inventory, Clinician Global Impression ‒ Improvement, visual analogue scale symptom (parent‐rated) Follow‐up interval: 6 weeks |
|
| Notes |
Imputation method for incomplete data: "last post randomisation assessments were used in endpoint analysis" (LOCF) Funding/support: Janssen Research Foundation provided randomisation and training, company central co‐ordination |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Janssen Research Foundation prepared the randomization list". (p 1029) |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Appearance and taste of solutions were identical. All trial medications were labelled with the protocol number, medication number, lot number and strata. A tear‐off label was provided on each box of study medication, which contained the medication code. The label was placed in the Case Report Form on the appropriate page. The code should only be broken in case of an emergency (Loy 2011b [pers comm]). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 (11.3%) participants dropped out of the risperidone group and 19 (33%) dropped out from the placebo group. Reasons for discontinuance included: (1) insufficient response (2 from risperidone group and 19 from placebo group); (2) loss to follow‐up (1 from risperidone group); and (3) loss of parental consent (3 from risperidone group). Discrepancy in dropouts (n = 6) between the table data and the narrative and graph. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | 1‐week placebo run‐in. 2 sites in South Africa ‒ unclear if followed the same protocol. |
TOSCA study.
| Methods | 2‐stage 9 week parallel group, double‐blind, randomised controlled trial of risperidone ('augmented' = active treatment) and placebo ('basic' = placebo) added to parent training and stimulant. Stage 1: 3 weeks, open‐label stimulant and parent training; Stage 2: 6 weeks of a double‐blinded, placebo‐controlled comparison of added risperidone versus placebo. | |
| Participants |
Setting: outpatients, multicentre (University clinics) Sample size: 168; 84 active treatment, 84 placebo Sex: 129 boys (77%) and 39 girls; 65 boys and 19 girls in the active arm and 64 boys and 20 girls in the placebo arm Age range: 6 to 12 years Mean age: active treatment 9.03 (SD = 2.05) years; placebo 8.75 (SD = 1.98) years IQ range: not reported. Mean IQ 97.1 (SD = 14.1) Inclusion criteria: DSM‐IV disruptive behaviour disorder diagnosis (conduct disorder or oppositional defiant disorder); DSM‐IV diagnosis of ADHD (any subtype); evidence of serious physical aggression as rated on the Overt Aggression Scale–M (score greater or equal to 3 on assaults against other people, objects, or self); and evidence of seriously disruptive behaviour as determined by a parent or guardian rating of at least 27 (90th percentile) on the Nisonger Child Behaviour Rating Form ‒ Typical IQ Version D‐Total (Conduct Disorder and Oppositional Defiant Disorder subscales combined). In addition, a Clinical Global Impressions ‒ Severity scale score of at least 4 (“moderately ill” or higher) for aggression was required by blinded clinicians. Participants needed to be free of psychotropic medicines for 2 weeks for most drugs (such as most antidepressants, a‐agonists, b‐blockers, anxiolytics, mood stabilizers, oral antipsychotics, and antihistamines) and 4 weeks for depot antipsychotics or fluoxetine. This rule was occasionally relaxed (to as few as 3 to 7 days) for extreme cases who could not tolerate being unmedicated the full time, as approved by the cross‐site steering committee. Diagnosis: 124 (74%) oppositional defiant disorder; 44 (265) conduct disorder Comorbidity: All have ADHD Sample characteristics: 53% White European ancestry; living with working parents (52% mothers, 53% fathers); some college education (66% mothers; 35% fathers); family incomes of USD 40,000 or less a year (57%) Withdrawn/dropouts: 22 participants dropped out before active treatment was introduced or because they were deemed not to need it (i.e. they were clinical responders to methylphenidate alone). In the active arm, 11 participants dropped out in the first 3 weeks (5 were clinical responders to methylphenidate alone) and in the placebo arm 3 dropped out in the first 3 weeks (3 were clinical responders to methylphenidate alone). Other Interventions:
|
|
| Interventions |
Intended dose: if residual symptoms remained, then randomised placebo or risperidone was added to treatment at weeks 4 through 6. For children weighing less than 25 kg, risperidone was dosed at 0.5 to 2.5 mg/day; for children heavier than 25 kg, dosing ranged from 0.5 to 3.5 mg/day. The risperidone titration schemes allowed for dose increases every 3 to 7 days, following a schedule that specified maximum dose increases over 29 days of titration; doses could always be held constant or decreased if a satisfactory clinical response occurred or if indicated by adverse event. Mean dose at endpoint: at week 9 (endpoint), in the active group the mean risperidone dose was 1.7 (± 0.75) mg/day and in the placebo group it was 1.9 (± 0.72) mg/day. |
|
| Outcomes |
Primary outcomes: Nisonger Child Behavior Rating Form ‐ Typical IQ version D‐Total Secondary outcomes: Positive Social, Overly sensitive ADHD, Withdrawn‐dysphoric subscales of the Nisonger Child Behavior Rating Form, Antisocial Behavior Scale consisting of Proactive and Reactive subscales; Clinical Global Impression ‒ Improvement and Clinical Global Impression ‒ Severity scales Follow‐up interval: 9 weeks (risperidone and placebo were introduced in week 4) |
|
| Notes |
Funding/support: the trial was funded by National Institute of Mental Health (NIMH). From correspondence (Loy 2016a [pers comm]), the authors originally requested a pharmaceutical company to sponsor the study medication. Due to lack of agreement over certain issues, this fell through except for 1 trial site. Subsequently, the authors obtained funding from the NIMH to purchase the study medications (both stimulant and risperidone and placebo) from an independent pharmacy. Only 1 study site, from which approximately 50 participants were recruited, received medication sponsored by the pharmaceutical company. It is possible that the centre used pharmaceutical‐supplied medication for 1 or 2 participants only. There were 168 participants in the trial. The lead author's view was that the pharmaceutical involvement was virtually nonexistent. Disclosures: the lead author has "received research contracts, consulted with, or served on advisory boards" of various pharmaceutical companies as listed on p 59. Full disclosure for other co‐authors is available on p 59. Long‐term outcomes to be published. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation was used: blocks of size 2 were allocated to each stratum formed by the cross‐classification of the levels of the stratifying factors: site and DSM‐IV diagnosis (conduct disorder versus no conduct disorder) (Loy 2016b [pers comm]). |
| Allocation concealment (selection bias) | Low risk | Randomisation assignment was completed through a secured website. The unblinded medication dispenser entered the appropriate information into the website and an email with the participant’s treatment assignment was sent to the dispenser and to the statistician at the time. Randomisation assignment for each participant was printed and sealed in an envelope for study emergency only (Loy 2016b [pers comm]). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each child was followed by a primary clinician and a blinded clinician. Risperidone and placebo were absolutely identical. Different colour capsules were used to signify different doses (Loy 2016b [pers comm]). No details published about the details of the blinding procedure of the participants (children and parents) (Loy 2016b [pers comm]). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each child was followed by a primary clinician and a blinded clinician. The primary clinician assessed the participants for adverse events and titrated dosage, whereas the blinded clinician assessed the children for clinical improvement (i.e. was responsible for monitoring therapeutic effects on Nisonger Child Behaviour Rating Form ‒ Typical IQ Version, Clinical Global Impression‐Improvement (CGI‐Improvement), Clinical Global Impression‐Severity (CGI‐Severity). Parents and participating children were told not to discuss side effects or (when the blind was broken) medication assignment with the blinded clinicians. Blinded clinicians were barred from asking about side effects, appetite, sleep patterns, or seeing any of the completed Adverse Effect forms. There was a Medication Knowledge Form to determine both the parents' and blinded clinicians' knowledge regarding the identity of risperidone recipients. Blinded clinicians were never unblinded until the entire study was completed and all study data were locked (Loy 2016b [pers comm]). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed reasons for participant attrition are displayed in table S1 and S2 (p 60 e1). |
| Selective reporting (reporting bias) | Unclear risk | Published results are based on completers‐only sample (i.e. no imputation of missing data was carried out in the published manuscript). Sensitivity analysis was said to be conducted (Loy 2016b [pers comm]) but not published or available. The reported outcome measures are consistent with those listed on the Clinical Trials registry (NCT00796302). |
| Other bias | Low risk | Completers analysis published only. |
Van Bellinghen 2001.
| Methods | Double‐blind randomised controlled trial of risperidone and placebo | |
| Participants |
Setting: residential care Sample size: 13; 6 active treatment, 7 placebo Sex: 5 males and 8 females Age range: 6 to 18 years Mean age: active treatment 10.5 (range 6 to 14) years; placebo 11 (range 7 to 14) years IQ range: 45 to 85 Inclusion criteria: "Persistent behavioural disturbance" (hostility, aggressive behaviour, irritability, agitation, hyperactivity) symptoms. Primary psychiatric diagnoses were not specified. Comorbidity: ADHD comorbidity not reported other that 1 participant in placebo group was on ritalin but this was discontinued during the trial; and 1 (in the active group) received concurrent antiepileptic (valproate) Withdrawn/dropouts: no withdrawals Other interventions: no description of pre‐existing or comorbid psychosocial interventions |
|
| Interventions |
Intended dose: once daily, evenings, week 1 0.01 to 0.04 mg/kg/day, week 2 to 4 flexible dosing Mean dose at endpoint: 0.05 mg/kg (range 0.03 to 0.06 mg/kg or 1.2 mg/day) |
|
| Outcomes |
Primary outcome: no pre‐specified primary endpoint (from email correspondence) Secondary outcomes: Aberrant Behaviour Checklist, Personal Assessment Checklist, Clinical Global Impression, visual analogue scale for the most disturbing symptom Follow‐up interval: 4 weeks |
|
| Notes |
Imputation method for incomplete data: LOCF (page 7) Funding/support: "Support for this work was received from Janssen Pharmaceutica, Berchem, Belgium" (p 5) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients completed the study" (p 7). |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable. |
| Other bias | High risk | Pilot study, limited by a small sample size and thus limited power to detect differences. No SDs or SEs reported. |
AACAP: American Academy of Child and Adolescent Psychiatry; ADHD: attention deficit hyperactivity disorder; DSM IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; IQ: intelligence quotient; LOCF: last observation carried forward; PI: principal investigator; RCT: randomised controlled trial; SD: standard deviation; SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blader 2009 | Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Divalproex is considered to be a mood stabiliser. Ineligible intervention. |
| Blader 2013 | Open titration and optimisation of stimulant monotherapy in 160 children. Ineligible study design (not randomised) and ineligible intervention. |
| Buitelaar 2000 | Open‐label study of risperidone treatment in 26 hospitalised children and youths with borderline or sub‐average IQs with mixed diagnoses and aggressive behaviour. Ineligible study design (not randomised). |
| Croonenberghs 2005 | 1‐year, multi‐site, open‐label study looking at safety and effectiveness of risperidone in 504 children and youths aged 5 to 14 with disruptive behaviour disorders and below average IQs. Ineligible study design (not randomised). |
| Findling 2004 | 48‐week open‐label study of risperidone in 107 children aged 5 to 12 with severe disruptive behaviour disorders and below average IQs. Ineligible study design (not randomised). |
| Findling 2006 | 8‐week pilot, open‐label, outpatient trial of quetiapine in 17 aggressive children with conduct disorder aged 6 to 12 years old. Ineligible study design (not randomised). |
| Haas 2008 | 1‐year, open‐label, safety extension study in 232 children with disruptive behaviour disorders treated with risperidone. Ineligible study design (not randomised). |
| Handen 2006 | Open‐label trial of olanzapine in 16 youths with sub‐average IQ and disruptive behaviour disorders. Ineligible study design (not randomised). |
| Holzer 2013 | Open‐label atomoxetine and olanzapine in ADHD with comorbid disruptive behaviour disorder in children and adolescents. Ineligible study design (not randomised). |
| ISRCTN95609637 | Study on hold since 2013. Relapse prevention in children and adolescents with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) Conduct Disorder treated with risperidone: a randomised double‐blind, placebo‐controlled, discontinuation study (www.isrctn.com/ISRCTN95609637). Discontinued study/no data available. |
| Kuperman 2010 | Open‐label trial of aripiprazole in the treatment of conduct disorders in adolescents. Ineligible study design (not randomised). |
| Masi 2006 | A retrospective chart review of olanzapine treatment in adolescents with conduct disorder. Ineligible study design (not randomised). |
| NCT00279409 | Treatment of Children With ADHD Who do Not Fully Respond to Stimulants (TREAT). Active comparator (also called the "combination arm") consists of parent training plus continued treatment on a stimulant, plus augmentation with aripiprazole. Placebo comparator (also called the "simple treatment" arm) will consist of parent training plus continued treatment on a stimulant plus a placebo matching aripiprazole. Study was terminated due to slow rate of recruitment. Discontinued study/no data available. ClinicalTrials.gov Identifier: NCT00279409. |
| NCT00550147 | An open‐label study of quetiapine added to OROS methylphenidate in the treatment of ADHD and aggressive behaviour. Ineligible study design (not randomised). |
| Reyes 2006b | Open‐label study over a cumulative period of 3 years, looking at safety and tolerability of risperidone in 35 children with disruptive behaviour disorders and borderline and sub‐average IQs. Ineligible study design (not randomised). |
| Safavi 2016 | Ineligible study design (single‐blind design). |
| Soderstrom 2002 | A clinical case series of 6 extremely aggressive youths treated with olanzapine. Ineligible study design (not randomised). |
| Teixeira 2013a | Open, naturalistic study of clozapine in 7 boys with severe conduct disorder over 26 weeks. Ineligible study design (not randomised). |
| Tramontina 2009 | Response to treatment with aripiprazole in children and adolescents with bipolar disorder and comorbid ADHD. Ineligible study design (not randomised). |
| Turgay 2002 | 48‐week open‐label study of risperidone for the treatment of disruptive behaviour disorders in 77 children with sub‐average IQs. Ineligible study design (not randomised). |
| Tyrer 2008 | RCT in adults of risperidone, haloperidol and placebo in the treatment of aggressive challenging behaviour in adult patients with intellectual disability. Ineligible population (adults). |
ADHD: attention deficit hyperactivity disorder. IQ: intelligence quotient. RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
IRCT201211051743N10.
| Methods | Double blinded, randomised controlled trial, interventional study |
| Participants | Children aged 6 to 12 years with ADHD, male and female |
| Interventions |
Active treatment: methylphenidate with maximum dose of 30 mg per day and risperidone with maximum dose of 1 mg per day for 8 weeks Comparator: methylphenidate with maximum dose of 30 mg per day and placebo for 8 weeks |
| Outcomes | Improvement in ADHD symptoms as assessed using the Clinical Global Impression (CGI) scale and Conners' Parents Rating Scale (CDRS) for ADHD. |
| Notes | IRCT identifier: IRCT201211051743N10: A double‐blind, placebo‐controlled study on the efficacy of risperidone adjunctive to methylphenidate in patients with ADHD. Limitation is that this is an Iranian study and whether it will be published in English or Persian. It may not measure the outcomes relevant to the review, which are aggression and conduct problems. |
NCT02063945.
| Methods | Randomised controlled trial, open label |
| Participants | Children and adolescents with attention deficit/hyperactivity disorder, oppositional defiant disorder or conduct disorder |
| Interventions |
Drug 1: methylphenidate Drug 2: risperidone |
| Outcomes |
Primary outcome measure: change from baseline of aggressive behaviours. The Retrospective Modified Overt Aggression Scale (R‐MOAS) will be used for the assessment of aggressive behaviours and their response to treatment. Time frame: baseline, 2 weeks, 4 weeks, 8 weeks |
| Notes | ClinicalTrials.gov identifier: NCT02063945: Methylphenidate vs. Risperidone for the Treatment of Children and Adolescents With ADHD and Disruptive Disorders. Open label rather than double blinded according to the ClinicalTrials.gov web site. Likely to end up as an excluded trial. (clinicaltrials.gov/ct2/show/NCT02063945) |
ADHD: attention deficit hyperactivity disorder. IQ: intelligence quotient. IRCT: Iranian Registry of Clinical Trials. vs: versus.
Characteristics of ongoing studies [ordered by study ID]
NCT00794625.
| Trial name or title | Effectiveness of Combined Medication Treatment for Aggression in Children With Attention Deficit With Hyperactivity Disorder (The SPICY Study) |
| Methods | Intervention; double‐blinded randomised controlled trial |
| Participants | Children aged 6 to 12 years with attention deficit disorder with hyperactivity, male and female |
| Interventions |
Drug 1: valproate Drug 2: risperidone Drug 3: placebo Drug 4: stimulant medication Behavioral: behavioural family counselling |
| Outcomes |
Primary outcome: aggressive behaviour Secondary outcome: ADHD symptoms Time frame: measured weekly for 11 to 16 weeks |
| Starting date | November 2008 Estimated completion date: April 2013 |
| Contact information |
Name: Joseph Blader Email: joseph.blader@stonybrook.edu Address: University of Texas, Northwell Health |
| Notes | Purpose: this study will determine the advantages and disadvantages of adding 1 of 2 different types of drugs to stimulant treatment for reducing aggressive behaviour in children with ADHD. During phase 1, participants will receive a stimulant medication. If they do not respond to the stimulant, valproate and behavioural family counselling will be added to their treatment during phase 2. If they do not respond to valproate, they will be switched to risperidone. The recruitment status of this study is unknown because the information has not been verified recently |
ADHD: attention deficit hyperactivity disorder.
Differences between protocol and review
-
Methods. Types of participants. For clarity, we added that:
a diagnosis of a disruptive behaviour disorder must have been established "using criteria from either the Diagnostic and and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association 2000; American Psychiatric Association 2013) or International Statistical Classification of Diseases and Related Health Problems (ICD; WHO 2016)"; and
we excluded studies where participants had a comorbid diagnosis of autism spectrum disorder (ASD), because "there are other reviews dealing with atypical antipsychotics in ASD such as the recently updated review by Hirsch 2016".
Methods. Types of interventions. For clarity, we added that: "Trials including a combination of atypical antipsychotics combined with other medications or psychosocial interventions, or both, were also eligible. No duration of treatment was specified a priori."
-
Methods. Types of outcome measures. Primary outcomes.
We deleted the following text, which was in our original protocol: "Steiner 2007 ranked some of the above scales in three categories: Excellent psychometric properties: cohesion, convergent, discriminant and predictive validity had all been tested in representative samples. Good psychometric properties: as above, but studies had one or two criteria missing. Adequate psychometric properties: more than two of the criteria listed above were not met but the scale was conceptually interesting or particularly suitable for clinical practice."
We added the following paragraph: "For clinical and statistical reasons, it is usually necessary to obtain information that covers behaviour in different settings, including home and school, from different informants (Verhulst 2002). Both observer and self‐rated rating scales are used. Parents observe variations in behaviour across multiple situations while teachers note deviation from peers in the school setting (Myers 2002). Generally, for externalising problems, there is greater inter‐rater consistency between parent and teacher informants. (Clay 2008). For self reports, while children and adolescents can be reliable and valid self reporters, and may useful for some difficult to observe behaviour such as stealing, there are potential limitations. These include children and youth's linguistic skills, presence of learning difficulties, self reflection skills, ability to monitor one's behaviour and risks of under‐reporting undesirable behaviour or to respond in a socially desirable manner (Myers 2002; Collett 2003). For these reasons, observer‐rated data were preferable to self‐rated data for this review. If several measures of the same outcome were available, we selected the measure used as the primary outcome in a given trial."
Methods. Types of outcome measures. Secondary outcomes. General functioning. We used the Clinical Global Impression ‒ Severity scale (CGI‐S; Rapoport 1985) as a very indirect approximation of functioning, as no data were available for CGAS.
Methods. Selection of studies. We reported that we recorded our decisions in a PRISMA diagram (Moher 2009).
Methods. Assessment of heterogeneity. We specified that: "We also reported Tau² ‒ an estimate of between‐study variance" in the updated review.
Methods. 'Summary of findings'. We included a 'Summary of findings' section to reflect the updated Cochrane guidelines. This information is summarised in Table 1.
Methods. Sensitivity analysis. We did not exclude trials with 'no' or 'unclear' ratings for allocation concealment because we identified a limited number of trials. This method has been archived for use in future updates of this review. Please refer to our protocol (Loy 2010) and Table 2.
Results. Effects of interventions. In the previous version of this review, we pooled data on quetiapine and risperidone in meta‐analyses on aggression and conduct disorders (see analysis 1.2 and 1.4, respectively, in Loy 2012). To be consistent with our objective, which states that we will evaluate each drug separately (as opposed to assessing the class effect), we removed this study, Connor 2008, from these analyses in this version of the review. Thus, this version of the review reports the effects of risperidone only versus placebo.
Contributions of authors
Jik Loy, Sally Merry and Sarah Hetrick prepared the protocol. Jik Loy, Sally Merry, Chohye Park and Sarah Hetrick revised the protocol. Jik Loy and Karolina Stasiak did the data collection and extraction in the 2012 and 2016 reviews. Jik Loy, Sally Merry, Sarah Hetrick and Karolina Stasiak contributed to the data analysis in the 2012 and 2016 reviews. Jik Loy, Sally Merry, Sarah Hetrick and Karolina Stasiak contributed to the writing in the 2012 and 2016 reviews.
Jik Loy is the guarantor for the review.
Sources of support
Internal sources
-
Waikato District Health Board, New Zealand.
Provision of time and release from other clinical responsibilities for the first author.
External sources
None requested, Other.
Declarations of interest
Jik H Loy is a co‐investigator on a Health Research Grant (grant number 13/331) to evaluate the effectiveness of a modular, psychosocial intervention — Modular Approach to Treatment of Children ‒ Anxiety, Depression,Trauma and Conduct (MATCH‐ADTC) — in secondary mental health services for children and adolescents. It is unrelated to this review.
Sally N Merry (SM) is a board member for the International Society for Research in Internet Interventions, which is an unpaid position. SM is a consultant on a psychosocial intervention being carried out as an addition to a trial on antidepressant medication being carried out at Cambridge University. SM declares that both positions are unrelated to this review. SM is a co‐developer of SPARX — an online cognitive behavioural therapy for adolescents with depression. The intellectual property belongs to Uniservices Ltd Auckland (the commercial arm of the University of Auckland) and if SPARX were commercialised, SM stands to gain financially. SM is a Principal Investigator on the Health Research Grant (grant number 13/331) to evaluate the effectiveness of a modular psychosocial intervention (MATCH‐ADTC) in secondary mental health services for children and adolescents. It is unrelated to this review. SM is a co‐investigator with Karolina Stasiak on a project funded by the Ministry of Business, Innovation and Employment (MBIE) to develop and trial an e‐health platform to deliver interventions for common mental health problems in adolescence. SM declares this project is not related to this review.
Sarah E Hetrick's (SEH) institution receives funds from the Federal Government of Australia and the Colonial Foundation for this review update. SH received a Clinical Training Fellowship from the Australian National Health and Medical Research Council, which supports research focused on implementation of evidence for youth depression. She is a named investigator on several randomised controlled trials (RCTs) funded by the Australian National Health and Medical Research Council, but none are related to this review.
Karolina Stasiak (KS) is Senior Research Fellow at the Department of Psychological Medicine at the University of Auckland, where is she involved in applied research in the area of child, youth and family mental health. KS is a co‐developer of SPARX ‐ an online cognitive behavioural therapy for adolescents with depression. The intellectual property belongs to Uniservices Ltd Auckland (the commercial arm of the University of Auckland) and if SPARX were commercialised, KS stands to gain financially. In addition, KS is a co‐investigator with Sally Merry on a project funded by the MBIE to develop and trial an e‐health platform to deliver interventions for common mental health problems in adolescence. KS is also a co‐investigator in the Health Research Grant (grant number 13/331), to evaluate the effectiveness of a modular psychosocial intervention (MATCH‐ADTC) in secondary mental health services for children and adolescents, and a co‐investigator on a project funded by the Oakley Mental Health Foundation & Starship Foundation, to develop and evaluate an e‐health intervention to support psychological needs of children living with long‐term physical illness. KS is a co‐principal investigator on an open trial, funded by the Oakley Mental Health Foundation, to test the feasibility of an internet‐based cognitive behavioural therapy self‐help resource for children and adolescents in the aftermath of Christchurch earthquakes. KS has also worked on two projects funded by the Counties Manukau District Health Board to evaluate an intensive parenting programme for parents experiencing significant parenting problems with infants and young children. KS declares that none of these projects are related to this review. In the interests of transparency, KS declares travel grants from the Auckland Medical Research Foundation, to present a paper at the fifth European Conference on Positive Psychology; and the Maurice Phyllis Paykel Trust, to present a paper at the fifth European Conference on Positive Psychology.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aman 2002 {published data only}
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children in ADHD, other disruptive disorders, and subaverage IQ. Journal of Child and Adolescent Psychopharmacology 2004;14(2):243‐54. [DOI: 10.1089/1044546041649020; PUBMED: 15319021] [DOI] [PubMed] [Google Scholar]
- Aman MG, Smedt G, Derivan A, Lyons B, Findling RL, Risperidone Disruptive Behavior Study Group. Double‐blind, placebo‐controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. American Journal of Psychiatry 2002;159(8):1337‐46. [DOI: 10.1176/appi.ajp.159.8.1337; PUBMED: 12153826] [DOI] [PubMed] [Google Scholar]
Armenteros 2007 {published data only}
- Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment‐resistant aggression in attention‐deficit/hyperactivity disorder: a placebo‐controlled pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(5):558‐65. [DOI: 10.1097/chi.0b013e3180323354; PUBMED: 17450046] [DOI] [PubMed] [Google Scholar]
Buitelaar 2001 {published data only}
- Buitelaar JK, Gaag RJ, Cohen‐Kettenis P, Melman CT. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. Journal of Clinical Psychiatry 2001;62(4):239‐48. [PUBMED: 11379837] [DOI] [PubMed] [Google Scholar]
Connor 2008 {published data only}
- Connor DF, McLaughlin TJ, Jeffers‐Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(2):140‐56. [DOI: 10.1089/cap.2006.0007; PUBMED: 18439112] [DOI] [PubMed] [Google Scholar]
Findling 2000 {published data only}
- Findling RL, McNamara NK, Branicky LA, Schluchter MD, Lemon E, Blumer JL. A double‐blind pilot study of risperidone in the treatment of conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(4):509‐16. [DOI: 10.1097/00004583-200004000-00021; PUBMED: 10761354] [DOI] [PubMed] [Google Scholar]
Fleischhaker 2011 {unpublished data only}
- Fleischhaker C, Hennighausen K, Schneider‐Momm K, Schulz E. Ziprasidone for severe conduct and other disruptive behavior disorders in children and adolescents — a placebo‐controlled, randomized, double‐blind clinical trial. American Academy of Child and Adolecent Psychiatry (AACAP) and Canadian Academy of Child and Adolescent Psychiatry (CACAP) Joint Meeting; 2011 Oct 18‐23; Toronto (CA). 2011 (first received 9 May 2008).
- NCT00676429. Ziprasidone for severe conduct and other disruptive behavior disorders. clinicaltrials.gov/ct2/show/NCT00676429 (first received 9 May 2008).
Reyes 2006a {published data only}
- Reyes M, Buitelaar J, Toren P, Augustyns I, Eerdekens M. A randomized, double‐blind, placebo‐controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. American Journal of Psychiatry 2006;163(3):402‐10. [DOI: 10.1176/appi.ajp.163.3.402; PUBMED: 16513860] [DOI] [PubMed] [Google Scholar]
Snyder 2002 {published data only}
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children in ADHD, other disruptive disorders and subaverage IQ. Journal of Child and Adolescent Psychopharmacology 2004;14(2):243‐54. [DOI] [PubMed] [Google Scholar]
- LeBlanc JC, Binder CE, Armenteros JL, Aman MG, Wang JS, Hew H, et al. Risperidone reduces aggression in boys with disruptive behaviour disorder and below average intelligence quotient: analysis of two placebo‐controlled randomized trials. International Clinical Psychopharmacology 2005;20:275‐83. [DOI] [PubMed] [Google Scholar]
- Snyder R, Turgay A, Aman M, Binder C, Fisman S, Carroll A, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. Journal of American Academy of Child and Adolescent Psychiatry 2002;41(9):1026‐36. [DOI] [PubMed] [Google Scholar]
TOSCA study {published data only (unpublished sought but not used)}
- Aman MG. Treatment of Severe Childhood Aggression (TOSCA) Studies. Journal of Child and Adolescent Psychopharmacology 2015;25(3):201‐202. [DOI: 10.1089/cap.2015.2532] [DOI] [PubMed] [Google Scholar]
- Aman MG, Bukstein OG, Gadow KD, Arnold LE, Molina BSG, McNamara NK, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention‐deficit/hyperactivity disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):47‐60. [DOI: 10.1016/j.jaac.2013.09.022; PMC3984501; PUBMED: 24342385] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LE, Gadow KD, Farmer CA, Findling RL, Bukstein O, Molina BS, et al. Comorbid anxiety and social avoidance in treatment of severe childhood aggression: response to adding risperidone to stimulant and parent training; mediation of disruptive symptom response. Journal of Child and Adolescent Psychopharmacology 2015;25(3):203‐12. [DOI: 10.1089/cap.2014.0104; PMC4403224; PUBMED: 25885010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader JC. Not just another antipsychotic‐for‐conduct‐problems trial. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):17‐20. [DOI: 10.1016/j.jaac.2013.10.007; PUBMED: 24342382] [DOI] [PubMed] [Google Scholar]
- Farmer CA, Arnold LE, Bukstein OG, Findling RL, Gadow KD, Li XB, et al. The treatment of severe child aggression (TOSCA) study: design challenges. Child and Adolescent Psychiatry and Mental Health 2011;5(1):36. [10.1186/1753‐2000‐5‐36] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer CA, Brown NV, Gadow KG, Arnold LE, Kolko DG, Findling RL, et al. Comorbid symptomatology moderates response to risperidone, stimulant, and parent training in children with severe aggression, disruptive behavior disorder, and attention‐deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2015;25(3):213‐24. [DOI: 10.1089/cap.2014.0109; PMC4403232; PUBMED: 25885011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Townsend L, Brown NV, Arnold LE, Gadow KD, Kolko DJ, et al. The treatment of severe childhood aggression study: 12 weeks of extended, blinded treatment in clinical responders. Journal of Child and Adolescent Psychopharmacology 2017;27(1):52‐65. [DOI: 10.1089/cap.2016.0081; PMC5327034; PUBMED: 28212067] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Arnold LE, Molina BS, Findling RL, Bukstein OG, Brown NV, et al. Risperidone added to parent training and stimulant medication: effects on attention‐deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):948‐59. [DOI: 10.1016/j.jaac.2014.05.008; PMC4145805; PUBMED: 25151418] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Brown NV, Arnold LE, Buchan‐Page KA, Bukstein OG, Butter E, Farmer CA, Findling RL, Kolko DJ, Molina BS, Rice RR Jr, Schneider J, Aman MG. Severely Aggressive Children Receiving Stimulant Medication Versus Stimulant and Risperidone: 12‐Month Follow‐Up of the TOSCA Trial. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(6):469‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Brown NV, Arnold LE, Buchan‐Page KA, Bukstein OG, Butter E, et al. Severely aggressive children receiving stimulant medication versus stimulant and risperidone: 12‐month follow‐up of the TOSCA trial. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(6):469‐78. [DOI: 10.1016/j.jaac.2016.03.014; PMC4886346; PUBMED: 27238065] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS. TOSCA: no longer just an opera. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):938‐41. [DOI: 10.1016/j.jaac.2014.06.008; PUBMED: 25151415] [DOI] [PubMed] [Google Scholar]
- NCT00796302. Treatment of severe childhood aggression (the TOSCA study) [Stimulant and risperidone in children with severe physical aggression]. clinicaltrials.gov/show/NCT00796302 (first received 21 November 2008).
- Rundberg‐Rivera EV, Townsend LD, Schneider J, Farmer CA, Molina BB, Findling RL, et al. Participant satisfaction in a study of stimulant, parent training, and risperidone in children with severe physical aggression. Journal of Child and Adolescent Psychopharmacology 2015;25(3):225‐33. [DOI: 10.1089/cap.2014.0097; PMC4403019; PUBMED: 25885012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Bellinghen 2001 {published data only}
- Bellinghen M, Troch C. Risperidone in the treatment of behavioral disturbances in children and adolescents with borderline intellectual functioning: a double‐blind, placebo‐controlled pilot trial. Journal of Child and Adolescent Psychopharmacology 2001;11(1):5‐13. [DOI: 10.1089/104454601750143348; PUBMED: 11322745] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blader 2009 {published data only}
- Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. American Journal of Psychiatry 2009;166(12):1392‐401. [DOI: 10.1176/appi.ajp.2009.09020233; PMC2940237; PUBMED: 19884222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blader 2013 {published data only}
- Blader JC, Pliszka SR, Kafantaris V, Foley CA, Crowell JA, Carlson GA, et al. Callous‐unemotional traits, proactive aggression, and treatment outcomes of aggressive children with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(12):1281‐93. [DOI: 10.1016/j.jaac.2013.08.024; PMC4530123; PUBMED: 24290461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitelaar 2000 {published data only}
- Buitelaar JK. Open‐label treatment with risperidone of 26 psychiatrically hospitalized children and adolescents with mixed diagnoses and aggressive behavior. Journal of Child and Adolescent Psychopharmacology 2000;10(1):19‐26. [DOI: 10.1089/cap.2000.10.19; PUBMED: 10755578] [DOI] [PubMed] [Google Scholar]
Croonenberghs 2005 {published data only}
- Croonenberghs J, Fegert JM, Findling RL, Smedt G, Dongen S, Risperidone Disruptive Behavior Study Group. Risperidone in children with disruptive behavior disorders and subaverage intelligence: a 1‐year, open‐label of 504 patients. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(1):64‐72. [DOI: 10.1097/01.chi.0000145805.24274.09; PUBMED: 15608545] [DOI] [PubMed] [Google Scholar]
Findling 2004 {published data only}
- Findling RL, Aman MG, Eerdekens M, Derivan A, Lyons B, Risperidone Disruptive Behavior Study Group. Long‐term, open‐label study of risperidone in children with severe disruptive behavior disorders and below‐average IQ. The American Journal of Psychiatry 2004;161(4):677‐84. [DOI: 10.1176/appi.ajp.161.4.677; PUBMED: 15056514] [DOI] [PubMed] [Google Scholar]
Findling 2006 {published data only}
- Findling RL, Reed MD, O'Riordan MA, Demeter CA, Stansbrey RJ, McNamara NK. Effectiveness, safety and pharmacokinetics of quetiapine in aggressive children with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(7):792‐800. [DOI: 10.1097/01.chi.0000219832.23849.31; PUBMED: 16832315] [DOI] [PubMed] [Google Scholar]
Haas 2008 {published data only}
- Haas M, Karcher K, Pandina GJ. Treating disruptive behavior disorders with risperidone: a 1‐year, open‐label safety study in children and adolescents. Journal of Child Adolescent Psychopharmacology 2008;18(4):337‐45. [DOI: 10.1089/cap.2007.0098; PUBMED: 18759643] [DOI] [PubMed] [Google Scholar]
Handen 2006 {published data only}
- Handen BL, Hardan AY. Open‐label, prospective trial of olanzapine in adolescents with subaverage intelligence and disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(8):928‐35. [DOI: 10.1097/01.chi.0000223312.48406.6e; PUBMED: 16865035] [DOI] [PubMed] [Google Scholar]
Holzer 2013 {published data only}
- Holzer B, Lopes V, Lehman R. Combination use of atomoxetine hydrochloride and olanzapine in the treatment of attention‐deficit/hyperactivity disorder with comorbid disruptive behavior disorder in children and adolescents 10‐18 years of age. Journal of Child and Adolescent Psychopharmacology 2013;23(6):415‐8. [DOI: 10.1089/cap.2013.0029; PUBMED: 23952189] [DOI] [PubMed] [Google Scholar]
ISRCTN95609637 {published data only}
- ISRCTN95609637. Relapse prevention in children and adolescents with aggressive behaviour problems treated with risperidone [Relapse prevention in children and adolescents with Siagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) Conduct Disorder treated with risperidone: a randomized double blind, placebo‐controlled, discontinuation Study]. www.isrctn.com/ISRCTN95609637 (first received 27 January 2012). [DOI: 10.1186/ISRCTN95609637] [DOI]
Kuperman 2010 {published data only}
- Kuperman S, Calarge C, Kolar A, Holman T, Barnett M, Perry P. An open‐label trial of aripiprazole in the treatment of aggression in male adolescents diagnosed with conduct disorder. Annals of Clinical Psychiatry 2011;23(4):270‐6. [22073384] [PubMed] [Google Scholar]
Masi 2006 {published data only}
- Masi G, Milone A, Canepa G, Millepiedi S, Mucci M, Muratori F. Olanzapine treatment in adolescents with severe conduct disorder. European Psychiatry 2006;21(1):51‐7. [DOI: 10.1016/j.eurpsy.2004.11.010; PUBMED: 16487906] [DOI] [PubMed] [Google Scholar]
NCT00279409 {published data only}
- NCT00279409. Treatment of children with ADHD who do not fully respond to stimulants (TREAT) [Developing more efficacious treatments for children with ADHD who are "partial" or "non‐responders" to stimulants]. clinicaltrials.gov/ct2/show/NCT00279409 (first received 18 January 2006).
NCT00550147 {published data only}
- NCT00550147. Quetiapine and concerta in the treatment for ADHD and aggressive behavior [An open‐label study of quetiapine added to oros methylphenidate in the treatment of ADHD and aggressive behavior]. clinicaltrials.gov/ct2/show/NCT00550147 (first received 25 October 2007). [www.druglib.com/trial/47/NCT00550147.html]
Reyes 2006b {published data only}
- Reyes M, Olah R, Csaba K, Augustyns I, Eerdekens M. Long‐term safety and efficacy of risperidone in children with disruptive behaviour disorder: results of a 2‐year extension study. European Child & Adolescent Psychiatry 2006;15(2):97‐104. [DOI] [PubMed] [Google Scholar]
Safavi 2016 {published data only}
- Safavi P, Dehkordi AH, Ghasemi, N. Comparison of the effects of methylphenidate and the combination of methylphenidate and risperidone in preschool children with attention‐deficit hyperactivity disorder. Journal of Advanced Pharmaceutical Technology & Research 2016;7(4):144‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soderstrom 2002 {published data only}
- Soderstrom H, Rastam M, Gillberg C. A clinical case series of six extremely aggressive youths treated with olanzapine. European Child and Adolescent Psychiatry 2002;11(3):138‐41. [PUBMED: 12369774] [DOI] [PubMed] [Google Scholar]
Teixeira 2013a {published data only}
- Teixeira EH, Celeri EV, Jacintho AC, Dalgalarrondo P. Clozapine in severe conduct disorder. Journal of Child and Adolescent Psychopharmacology 2013;23(1):44‐8. [DOI: 10.1089/cap.2011.0148; PUBMED: 23347126] [DOI] [PubMed] [Google Scholar]
Tramontina 2009 {published data only}
- Tramontina S, Zeni CP, Ketzer CR, Pheula GF, Narvaez J, Rohde LA. Aripiprazole in children and adolescents with bipolar disorder comorbid with attention‐deficit/hyperactivity disorder: a pilot randomized clinical trial. Journal of Clinical Psychiatry 2009;70(5):756‐64. [DOI: 10.4088/JCP.08m04726; NCT00116259; PUBMED: 19389329] [DOI] [PubMed] [Google Scholar]
Turgay 2002 {published data only}
- Turgay A, Binder C, Snyder R, Fisman S. Long‐term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics 2002;110(3):e34. [PUBMED: 12205284] [DOI] [PubMed] [Google Scholar]
Tyrer 2008 {published data only}
- Tyrer P, Oliver‐Africano PC, Ahmed Z, Bouras N, Cooray S, Deb S, et al. Risperidone, haloperidol and placebo in the treatment of aggressive challenging behaviour in patients with intellectual disability: a randomised controlled trial. Lancet 2008;371(9606):57‐63. [DOI: 10.1016/S0140-6736(08)60072-0; PUBMED: 18177776] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT201211051743N10 {published data only}
- Holsboer‐Trachsler E, Jahangard L, Akbarian S, Haghighi M, Ahmadpanah M, Keshavarzi A, et al. Children with ADHD and symptoms of opposite defiant disorder improved in behavior when treated with methylphenidate and adjuvant risperidone, though weight gain was also observed. Neuropsychopharmacology 2016;41:S134. [DOI: 10.1038/npp.2016.240; IRCT201211051743N10] [DOI] [PubMed] [Google Scholar]
- IRCT201211051743N10. A double‐blind, placebo‐controlled study on the efficacy of risperidone adjunctive to methylphenidate in patients with ADHD. www.irct.ir/searchresult.php?id=1743&number=10 (first received 26 January 2013).
NCT02063945 {published data only}
- NCT02063945. Methylphenidate vs. risperidone for the treatment of children and adolescents with ADHD and disruptive disorders [The assessment of efficacy and tolerability of methylphenidate vs. risperidone in the treatment of children and adolescents with ADHD and disruptive disorders]. clinicaltrials.gov/ct2/show/NCT02063945 (first received 11 February 2014).
References to ongoing studies
NCT00794625 {published data only}
- NCT00794625. Effectiveness of combined medication treatment for aggression in children with attention deficit with hyperactivity disorder (the SPICY study) [Adjunctive treatment with divalproex or risperidone for aggression refractory to stimulant monotherapy among children with ADHD]. www.clinicaltrials.gov/ct2/show/NCT00794625 (first received 19 November 2008).
Additional references
Abidin 1995
- Abidin, RR. Parenting Stress Index, Third Edition: Professional Manual.. Psychological Assessment Resources. Odessa (Florida): Psychological Assessment Resources, 1995. [Google Scholar]
Achenbach 1991
- Achenbach TM. Manual for the Child Behavior Checklist/4‐18 and 1991 Profile. Burlington (VT): University of Vermont, Department of Psychiatry, 1991. [Google Scholar]
Almandil 2011
- Almandil NB, Wong ICK. Review on the current use of antipsychotic drugs in children and adolescents. Archives of Disease in Childhood. Education and Practice Edition 2011;96(5):192‐6. [DOI: 10.1136/archdischild-2011-300054; PUBMED: 21771730] [DOI] [PubMed] [Google Scholar]
Aman 1985a
- Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency 1985;89(5):485‐91. [PUBMED: 3993694] [PubMed] [Google Scholar]
Aman 1985b
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. American Journal of Mental Deficiency 1985;89(5):492‐502. [PUBMED: 3158201] [PubMed] [Google Scholar]
Aman 1996
- Aman MG, Tassé MJ, Rojahn J, Hammer D. The Nisonger CBRF: a child behavior rating form for children with developmental disabilities. Research in Developmental Disabilities 1996;17(1):41‐57. [PUBMED: 8750075] [DOI] [PubMed] [Google Scholar]
Aman 2004
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive disorders, and subaverage IQ. Journal of Child and Adolescent Psychopharmacology 2004;14(2):243‐54. [DOI: 10.1089/1044546041649020; PUBMED: 15319021] [DOI] [PubMed] [Google Scholar]
Aman 2008
- Aman MG, Leone S, Lecavalier L, Park L, Buican B, Coury D. The Nisonger Child Behavior Rating Form: typical IQ version. International Clinical Psychopharmacology 2008;23(4):232‐42. [DOI: 10.1097/YIC.0b013e3282f94ad0] [DOI] [PubMed] [Google Scholar]
Aman 2015
- Aman MG. Treatment of Severe Childhood Aggression (TOSCA) Studies. Journal of Child and Adolescent Psychopharmacology 2015;25(3):201‐2. [DOI: 10.1089/cap.2015.2532] [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM‐IV‐TR. 4th Edition. Washington (DC): APA, 2000. [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM‐5). 5th Edition. Arlington (VA): American Psychiatric Publishing, 2013. [Google Scholar]
American Psychiatric Association 2013a
- American Psychiatric Association. Highlights of changes from DSM‐IV‐TR to DSM‐5. www.dsm5.org/documents/changes%20from%20dsm‐iv‐tr%20to%20dsm‐5.pdf (accessed 1 May 2013).
American Psychological Association 2010
- American Psychological Association. Publication Manual of the American Psychological Association. 6th Edition. Washington (DC): American Psychological Association, 2010. [Google Scholar]
Amor 2014
- Amor LB, Sikirica V, Cloutier M, Lachaine J, Guerin A, Carter V, et al. Combination and switching of stimulants in children and adolescents with Attention Deficit/Hyperactivity Disorder in Quebec. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2014;23(3):157‐66. [PMC4197516; PUBMED: 25320609] [PMC free article] [PubMed] [Google Scholar]
Armstrong 2003
- Armstrong JM, Goldstein LH, The MacArthur Working Group on Outcome Assessment. The MacArthur Health and Behavior Questionnaire (HBQ 1.0). Pittsburgh: University of Pittsburgh, 2003. [Google Scholar]
Arnold 2015
- Arnold LE, Gadow KD, Farmer CA, Findling RL, Bukstein O, Molina BS, et al. Comorbid anxiety and social avoidance in treatment of severe childhood aggression: response to adding risperidone to stimulant and parent training; mediation of disruptive symptom response. Journal of Child and Adolescent Psychopharmacology 2015;25(3):203‐12. [DOI: 10.1089/cap.2014.0104; PMC4403224; PUBMED: 25885010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachmann 2014
- Bachmann CJ, Lempp T, Glaeske G, Hoffmann F. Antipsychotic prescription in children and adolescents. An analysis of data from a German statutory health insurance company from 2005 to 2012. Deutsches Ärzteblatt International 2014;111(3):25‐34. [DOI: 10.3238/arztebl.2014.0025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bardgett 2013
- Bardgett ME, Franks‐Henry JM, Colemire KR, Juneau KR, Stevens RM, Marczinski CA, et al. Adult rats treated with risperidone during development are hyperactive. Experimental and Clinical Psychopharmacology. 2013;21(3):259‐67. [DOI: 10.1037/a0031972; PMC4041194; PUBMED: 23750695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkley 1997
- Barkley RA. Defiant Children: A Clinician's Manual for Assessment and Parent Training. 2nd Edition. New York: Guilford Press, 1997. [Google Scholar]
Barkley 2006
- Barkley RA. Attention‐Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 3rd Edition. New York: Guilford Press, 2006. [Google Scholar]
Barratt 1999
- Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self‐reported acts. Psychiatry Research 1999;31(2):163‐73. [PUBMED: 10397418] [DOI] [PubMed] [Google Scholar]
Baweja 2016
- Baweja R, Mayes SD, Hameed U, Waxmonsky JG. Disruptive mood dysregulation disorder: current insights. Neuropsychiatric Disease and Treatment 2016;24(12):2115‐24. [DOI: 10.2147/NDT.S100312; PMC5003560; PUBMED: 27601906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biederman 1996
- Biederman J, Faraone SV, Milberger S, Jetton JG, Chen L, Mick E, et al. Is childhood oppositional defiant disorder a precursor to adolescent conduct disorder? Findings from a four year follow up study of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(9):1193‐204. [DOI: 10.1097/00004583-199609000-00017; PUBMED: 8824063] [DOI] [PubMed] [Google Scholar]
Biederman 2008
- Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, et al. The long‐term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10‐year prospective longitudinal follow‐up study. Psychological Medicine 2008;38(7):1027‐36. [DOI: 10.1017/S0033291707002668; PUBMED: 18205967] [DOI] [PubMed] [Google Scholar]
Blader 2014
- Blader JC. Not just another antipsychotic‐for‐conduct‐problems trial. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):17‐20. [DOI: 10.1016/j.jaac.2013.10.007; PUBMED: 24342382] [DOI] [PubMed] [Google Scholar]
British Broadcasting Corporation (BBC) 2013
- BBC. Medicines watchdog recalls drugs made in India. www.bbc.com/news/health‐23273101 (accessed 11 July 2013).
Brown 1996
- Brown K, Atkins MS, Osborne ML, Milnamow M. A revised teacher rating scale for reactive and proactive aggression. Journal of Abnormal Child Psychology 1996;24(4):473‐80. [PUBMED: 8886943] [DOI] [PubMed] [Google Scholar]
Burcu 2015
- Burcu M, Safer DJ, Zito JM. Antipsychotic prescribing for behavioral disorders in US youth: physician specialty, insurance coverage, and complex regimens. Pharmacoepidemiology and Drug Safety 2016;25(1):26‐34. [DOI: 10.1002/pds.3897; PUBMED: 26507224] [DOI] [PubMed] [Google Scholar]
Buss 1957
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting and Clinical Psychology 1957;21(4):343–9. [PUBMED: 13463189] [DOI] [PubMed] [Google Scholar]
Buss 1992
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology 1992;63(4):452–9. [PUBMED: 1403624] [DOI] [PubMed] [Google Scholar]
Caccia 2011
- Caccia S, Clavenna A, Bonati M. Antipsychotic drug toxicology in children. Expert Opinion on Drug Metabolism and Toxicology 2011;7(5):591‐608. [DOI: 10.1517/17425255.2011.562198] [DOI] [PubMed] [Google Scholar]
Calles 2011
- Calles JL Jr. Psychopharmacologic control of aggression and violence in children and adolescents. Pediatric Clinics of North America 2011;58(1):73‐84. [DOI: 10.1016/j.pcl.2010.11.002; PUBMED: 21281849] [DOI] [PubMed] [Google Scholar]
Chamberlain 1991
- Chamberlain P, Reid JB. Using a specialized foster care community treatment model for children and adolescents leaving the state mental hospital. Journal of Community Psychology 1991;19(3):266‐76. [DOI: 10.1002/1520-6629(199107)19:3%3C266::AID-JCOP2290190310%3E3.0.CO;2-5] [DOI] [Google Scholar]
Chan 2016
- Chan E, Fogler JM, Hammerness PG. Treatment of attention‐deficit/hyperactivity disorder in adolescents: a systematic review. Journal of the American Medical Association 2016;315(18):1997‐2008. [DOI: 10.1001/jama.2016.5453; PUBMED: 27163988] [DOI] [PubMed] [Google Scholar]
Ching 2012
- Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009043.pub2] [DOI] [PubMed] [Google Scholar]
Chorpita 2009
- Chorpita BF, Weisz JR. (MATCH‐ADTC): Modular Approach to Therapy for Children with Anxiety, Depression, Trauma, or Conduct problems. Satellite Beach: PracticeWise LLC, 2009. [Google Scholar]
Cipriani 2016
- Cipriani A, Zhou X, Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta‐analysis. Lancet 2016;388(10047):881‐90. [DOI: 10.1016/S0140-6736(16)30385-3; PUBMED: 27289172] [DOI] [PubMed] [Google Scholar]
Clay 2008
- Clay H, Surgenor LJ, Frampton C. Assessing emotional and behavioural problems in children: factors associated with multiple informant consistency in New Zealand. New Zealand Journal of Psychology 2008;37(1):10‐16. [Google Scholar]
Coccaro 1991
- Coccaro EF, Harvey PD, Kupshaw‐Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry and Clinical Neurosciences 1991;3(2):S44‐51. [PUBMED: 1821222] [PubMed] [Google Scholar]
Coccaro 1997
- Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Research 1997;73(3):147‐57. [PUBMED: 9481806] [DOI] [PubMed] [Google Scholar]
Cohen 1993
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, et al. An epidemiological study of disorders in late childhood and adolescence: age‐ and gender‐specific prevalence. Journal of Child Psychology and Psychiatry and Allied Disciplines 1993;34(6):851‐67. [PUBMED: 8408371] [DOI] [PubMed] [Google Scholar]
Collett 2003
- Collett BR, Ohan JL, Myers KM. Ten‐year review of rating scales. VI: scales assessing externalising behaviors. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(10):1143‐70. [DOI: 10.1097/00004583-200310000-00006; PUBMED: 14560165] [DOI] [PubMed] [Google Scholar]
Conners 1989
- Conners CK. Manual for Conners Rating Scales: Instruments for Use with Children and Adolescents. North Tonawanda (NY): Multi‐Health Systems, 1989. [Google Scholar]
Conners 1997a
- Conners CK. Conners Rating Scales‐Revised: Technical Manual. North Tonawanda (NY): Multi‐Health Systems, 1997. [Google Scholar]
Conners 1997b
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self‐report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. Journal of Abnormal Child Psychology 1997;25(6):487–97. [PUBMED: 9468109] [DOI] [PubMed] [Google Scholar]
Connor 2010
- Connor DF, Steeber J, McBurnett K. A review of attention‐deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. Journal of Developmental and Behavioral Pediatrics 2010;31(5):427‐40. [DOI: 10.1097/DBP.0b013e3181e121bd] [DOI] [PubMed] [Google Scholar]
Correll 2008
- Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. Journal of Clinical Psychiatry 2008;69(Suppl 4):26‐36. [PUBMED: 18533766] [PubMed] [Google Scholar]
Correll 2009
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second generation antipsychotic medications during first time use in children and adolescents. Journal of the American Medical Association 2009;302(16):1765‐73. [DOI: 10.1001/jama.2009.1549; PMC3055794; PUBMED: 19861668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Correll 2010
- Correll CU. Antipsychotic use in youth. www.medscape.com/viewarticle/718519?src=mp&spon=12&uac=123108CX (accessed 26 March 2010).
Coster 2008
- Coster W, Deeney T, Haltiwanger J, Haley S. School Function Assessment User’s Manual. San Antonio (TX): Pearson Education, 2008. [Google Scholar]
Crick 1995
- Crick NR, Grotpeter JK. Relational aggression, gender and social‐psychological adjustment. Child Development 1995;66(3):710‐22. [PUBMED: 7789197 ] [DOI] [PubMed] [Google Scholar]
Cueva 1996
- Cueva JE, Overall JE, Small AM, Armenteros JL, Perry R, Campbell M. Carbamazepine in aggressive children with conduct disorder: a double‐blind and placebo‐controlled study. Journal of American Academy of Child and Adolescent Psychiatry 1996;35(4):480‐90. [DOI: 10.1097/00004583-199604000-00014; PUBMED: 8919710] [DOI] [PubMed] [Google Scholar]
De Hert 2011
- Hert M, Dobbelaere M, Sheridan EM, Cohen D, Correll CU. Metabolic and endocrine adverse effects of second‐generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. European Psychiatry 2011;26(3):144‐58. [DOI: 10.1016/j.eurpsy.2010.09.011; PUBMED: 21295450] [DOI] [PubMed] [Google Scholar]
Dean 2006
- Dean AJ, McDermott BM, Marshall RT. Psychotropic medication utilization in a child and adolescent mental health service. Journal of the American Academy of Child and Adolescent Psychiatry 2006;16(3):273‐85. [DOI: 10.1089/cap.2006.16.273; PUBMED: 16768635] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Deshmukh 2010
- Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry 2010;7(2):32‐40. [PMC2848469; PUBMED: 20376274] [PMC free article] [PubMed] [Google Scholar]
Devlin 2015
- Devlin AM, Panagiotopoulos C. Metabolic side effects and pharmacogenetics of second‐generation antipsychotics in children. Pharmcogenomics 2015;16(9):981‐96. [DOI: 10.2217/pgs.15.55; PUBMED: 26107755] [DOI] [PubMed] [Google Scholar]
Dodge 1987
- Dodge KA, Coie JD. Social‐information‐processing factors in reactive and proactive aggression in children's peer groups. Journal of Personality and Social Psychology 1987;53(6):1146‐58. [PUBMED: 3694454] [DOI] [PubMed] [Google Scholar]
Doey 2007
- Doey T, Handelman K, Seabrook JA, Steele M. Survey of atypical antipsychotic prescribing by Canadian child psychiatrists and developmental pediatricians for patients aged under 18 years. Canadian Journal of Psychiatry 2007;52(6):363‐8. [PUBMED: 17696022] [DOI] [PubMed] [Google Scholar]
Donovon 2000
- Donovan SJ, Stewart JW, Nunes EV, Quitkin FM, Parides M, Daniel W, et al. Divalproex treatment for youth with explosive temper and mood lability: a double‐blind, placebo‐controlled crossover design. American Journal of Psychiatry 2000;157(5):18‐20. [DOI: 10.1176/appi.ajp.157.5.818; PUBMED: 10784478] [DOI] [PubMed] [Google Scholar]
Duhig 2013
- Duhig MJ, Saha S, Scott JG. Efficacy of risperidone in children with disruptive behavioural disorders. Journal of Paediatrics and Child Health 2013;49(1):19‐26. [DOI: 10.1111/j.1440-1754.2011.02200.x; PUBMED: 22050179] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphic test. British Medical Journal 1997;315(7):629‐34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliot 1985
- Elliott DS, Huizinga DE, Ageton SS. Explaining delinquency and drug use. Beverly Hills (CA): Sage, 1985. [Google Scholar]
European Medicines Agency 2011
- European Medicines Agency. European Medicines Agency decision P/167/2011. www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500110116.pdf (accessed prior to 23 November 2016).
European Medicines Agency 2014
- European Medicines Agency. Publication and access to clinical‐trial data. www.ema.europa.eu/docs/en_GB/document_library/Other/2013/06/WC500144730.pdf (accessed prior to 23 November 2016).
Evans 2017
- Evans SC, Burke JD, Roberts MC, Fite PJ, Lochman JE, Peña FR, Reed GM. Irritability in child and adolescent psychopathology: an integrative review for ICD‐11. Clinical Psychology Review 2017;53:29‐45. [DOI: 10.1016/j.cpr.2017.01.004; PUBMED: 28192774] [DOI] [PubMed] [Google Scholar]
Eyberg 1999
- Eyberg SM, Pincus D. Eyberg Child behaviour Inventory and Sutter‐Eyberg Student Behaviour Inventory‐Revised. Odessa (FL): Psychological Assessment Resources, 1999. [Google Scholar]
Farmer 2011
- Farmer CA, Arnold LE, Bukstein OG, Findling RL, Gadow KD, Li X, et al. The treatment of severe child aggression (TOSCA) study: design challenges. Child and Adolescent Psychiatry and Mental Health 2011;5(36):1‐11. [DOI: 10.1186/1753-2000-5-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2009
- FDA US Food and Drug Administration. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM193200.pdf (accessed 8 June 2010).
Fergusson 1998
- Fergusson DM, Horwood LJ. Early conduct problems and later life opportunities. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1998;39(8):1097‐108. [PUBMED: 9844980] [PubMed] [Google Scholar]
Fergusson 2004
- Fergusson DM, Horwood LJ, Ridder E. Show me the child at seven: the consequences of conduct problems in childhood for psychosocial functioning in adulthood. Journal of Child Psychology and Psychiatry and Allied Disciplines 2004;46(8):837‐49. [DOI: 10.1111/j.1469-7610.2004.00387.x; PUBMED: 16033632] [DOI] [PubMed] [Google Scholar]
Ferrin 2015
- Ferrin M, Gosney H, Marconi A, Rey JM. Using antipsychotic medication for the treatment of schizophrenia in children and adolescents. In: Rey JM editor(s). IACAPAP e‐Textbook of Child and Adolescent Mental Health. Geneva: International Association for Child and Adolescent Psychiatry and Allied Professions, 2015. [Google Scholar]
Findling 2008
- Findling RL. Atypical antipsychotic treatment of disruptive behavior disorders in children and adolescents. Journal of Clinical Psychiatry 2008;69(Suppl 4):9‐14. [PUBMED: 18533763] [PubMed] [Google Scholar]
Findling 2017
- Findling RL, Townsend L, Brown NV, Arnold LE, Gadow KD, Kolko DJ, et al. The treatment of severe childhood aggression study: 12 weeks of extended, blinded treatment in clinical responders. Journal of Child and Adolescent Psychopharmacology 2017;27(1):52‐65. [DOI: 10.1089/cap.2016.0081; PUBMED: 28212067] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2016
- Freeman AJ, Youngstrom EA, Youngstrom JK, Findling RL. Disruptive mood dysregulation disorder in a community mental health clinic: prevalence, comorbidity and correlates. Journal of Child and Adolescent Psychopharmacology 2016;26(2):123‐30. [DOI: 10.1089/cap.2015.0061; PMC4800380; PUBMED: 26745325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frick 2001
- Frick PJ, Hare RD. Antisocial Process Screening Device (APSD) Technical Manual. North Tonawanda (NY): Multi‐Health Systems, 2001. [Google Scholar]
Fuqua 1991
- Fuqua DR, Leonard E, Masters MA, Smith RJ, Campbell JL, Fischer PC. A structural analysis of the State‐Trait Anger Expression Inventory. Educational and Psychological Measurement 1991;51(2):439–46. [DOI: 10.1177/0013164491512018] [DOI] [Google Scholar]
Gadow 1986
- Gadow KD. Peer Conflict Scale. Stony Brook (NY): State University of New York, Department of Psychiatry, 1986. [Google Scholar]
Gadow 2005
- Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory‐4R. Stony Brook (NY): Checkmate Plus, 2005. [Google Scholar]
Gadow 2008
- Gadow KD, Sprafkin J. ADHD Symptom Checklist‐4 2008 Manual. Stony Brook (NY): Checkmate Plus, 2008. [Google Scholar]
Gadow 2014
- Gadow KD, Arnold LE, Molina BS, Findling RL, Bukstein OG, Brown NV, et al. Risperidone added to parent training and stimulant medication: effects on attention‐deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):948‐59. [DOI: 10.1016/j.jaac.2014.05.008; PMC4145805; PUBMED: 25151418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadow 2016
- Gadow KD, Brown NV, Arnold LE, Buchan‐Page KA, Bukstein OG, Butter E, et al. Severely aggressive children receiving stimulant medication versus stimulant and risperidone: 12‐Month follow‐Up of the TOSCA Trial. Journal of American Academy of Child and Adolescent Psychiatry. 2016;55(6):469‐78. [DOI: 10.1016/j.jaac.2016.03.014; PMC4886346; PUBMED: 27238065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glennon 2014
- Glennon J, Purper‐Ouakil D, Bakker M, Zuddas A, Hoekstra P, Schulze U, et al. Paediatric European Risperidone Studies (PERS): context, rationale, objectives, strategy, and challenges. European Child & Adolescent Psychiatry 2014;23(12):1149‐60. [DOI: 10.1007/s00787-013-0498-3; PMC4246122; PUBMED: 24337449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorman 2015
- Gorman DA, Gardner DM, Murphy AL, Feldman M, Bélanger SA, Steele MM, et al. Canadian guidelines on pharmacotherapy for disruptive and aggressive behaviour in children and adolescents with attention‐deficit hyperactivity disorder, oppositional defiant disorder, or conduct disorder. Canadian Journal of Psychiatry 2015;60(2):62‐76. [PMC4344948; PUBMED: 25886657] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- Grade Working Group, McMaster University. GRADEpro GDT. Version accessed 12 January 2017. Hamilton (ON): Grade Working Group, McMaster University, 2014.
Greene 2002
- Greene RW, Biederman J, Zerwas S, Monuteaux MC, Goring JC, Faraone SV. Psychiatric comorbidity, family dysfunction, and social impairment on referred youth with oppositional defiant disorder. American Journal of Psychiatry 2002;159(7):1214‐24. [DOI: 10.1176/appi.ajp.159.7.1214; PUBMED: 12091202] [DOI] [PubMed] [Google Scholar]
Greenhill 2003
- Greenhill LL, Vitiello B, Riddle MA, Fisher P, Shockey E, March JS, et al. Review of safety assessment methods used in pediatric psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(6):627‐33. [DOI: 10.1097/01.CHI.0000046841.56865.37; PUBMED: 12921469] [DOI] [PubMed] [Google Scholar]
Guidubaldi 1985
- Guidubaldi J, Cleminshaw HK. The development of the Cleminshaw‐Guidubaldi Parent Satisfaction Scale. Journal of Clinical Child Psychology 1985;14(4):293‐98. [DOI: 10.1207/s15374424jccp1404_4] [DOI] [Google Scholar]
Guy 1976
- Guy W, National Institute of Mental Health (US) Psychopharmacology Research Branch, Early Clinical Drug Evaluation Program. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Heath, Education, and Welfare Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
Halperin 2002
- Halperin J, McKay KE, Newcorn JH. Development, reliability, and validity of the Children's Aggression Scale ‐ Parent Version. Journal of American Academy of Child and Adolescent Psychiatry 2002;41(3):245‐52. [DOI: 10.1097/00004583-200203000-00003; PUBMED: 11886018] [DOI] [PubMed] [Google Scholar]
Halperin 2003
- Halperin J, McKay K, Grayson R, Newcorn J. Reliability, validity, and preliminary normative data for the Children's Aggression Scale ‐ Teacher Version. Journal of American Academy of Child and Adolescent Psychiatry 2003;42(8):965‐71. [DOI] [PubMed] [Google Scholar]
Harrison 1997
- Harrison AA, Everitt BJ, Robbins TW. Central 5‐HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 1997;133(4):329‐42. [PUBMED: 9372531] [DOI] [PubMed] [Google Scholar]
Harrison‐Woolrych 2007
- Harrison‐Woolrych M, Garcia‐Quiroga J, Ashton J, Herbison P. Safety and usage of atypical antipsychotic medicines in children: a nationwide prospective cohort study. Drug Safety 2007;30(7):569‐79. [PUBMED: 17604408] [DOI] [PubMed] [Google Scholar]
Hassiotis 2009
- Hassiotis A, Robotham D, Canagasabey A, Romeo R, Langridge D, Blizard R, et al. Randomized, single‐blind, controlled trial of a specialist behavior therapy team for challenging behavior in adults with intellectual disabilities. American Journal of Psychiatry 2009;166(11):1278‐85. [DOI: 10.1176/appi.ajp.2009.08111747; PUBMED: 19687128] [DOI] [PubMed] [Google Scholar]
Henry 2007
- Henry J, Sloane, M, Black‐Pond C. Neurobiology and neurodevelopmental impact of childhood traumatic stress and prenatal alcohol exposure. Language, Speech, and Hearing Services in Schools 2007;38(2):99‐108. [DOI: 10.1044/0161-1461(2007/010); PUBMED: 17428956] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirsch 2016
- Hirsch LE, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD009043.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. British Medical Journal 1999;319:670‐4. [DOI: 10.1136/bmj.319.7211.670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2013
- Howard JA. Distressed or Deliberately Defiant? Managing Challenging Student Behaviour Due to Trauma and Disorganised Attachment. Bowen Hills (QLD): Australian Academic Press, 2013. [DOI: 10.1017/jse.2014.2] [DOI] [Google Scholar]
Ipser 2007
- Ipser J, Stein DJ. Systematic review of pharmacotherapy of disruptive behaviour disorders in children and adolescents. Psychopharmacology 2007;191(1):127‐40. [DOI: 10.1007/s00213-006-0537-6; PUBMED: 16983542] [DOI] [PubMed] [Google Scholar]
Jensen 2007a
- Jensen PS, Youngstrom EA, Steiner H, Findling RL, Meyer RE, Malone RP, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(3):309‐22. [DOI: 10.1097/chi.0b013e31802f1454; PUBMED: 17314717] [DOI] [PubMed] [Google Scholar]
Jensen 2007b
- Jensen PS, Buitelaar J, Pandina GJ, Binder C, Haas M. Management of psychiatric disorders in children and adolescents with atypical antipsychotics: a systematic review of published clinical trials. European Child and Adolescent Psychiatry 2007;16(2):104‐20. [DOI: 10.1007/s00787-006-0580-1; PUBMED: 17075688] [DOI] [PubMed] [Google Scholar]
Jensen 2014
- Jensen PS. TOSCA: no longer just an opera. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):938‐941. [DOI: 10.1016/j.jaac.2014.06.008; PUBMED: 25151415] [DOI] [PubMed] [Google Scholar]
Kamble 2015
- Kamble P, Chen H, Johnson ML, Bhatara V, Aparasu RR. Concurrent use of stimulants and second‐generation antipsychotics among children with ADHD enrolled in Medicaid. Psychiatric Services (Washington, D.C.) 2015;66(4):404‐10. [DOI: 10.1176/appi.ps.201300391; PUBMED: 25828983] [DOI] [PubMed] [Google Scholar]
Kaminski 2008
- Kaminski JW, Valle LA, Filene JH, Boyle CL. A meta‐analytic review of components associated with parent training program effectiveness. Journal of Abnormal Child Psychology 2008;36(4):567‐89. [DOI: 10.1007/s10802-007-9201-9; PUBMED: 18205039] [DOI] [PubMed] [Google Scholar]
Karanges 2014
- Karanges EA, Stephenson CP, McGregor IS. Longitudinal trends in the dispensing of psychotropic medications in Australia from 2009‐2012: focus on children, adolescents and prescriber specialty. Australian and New Zealand Journal of Psychiatry 2014;48(10):917‐31. [DOI: 10.1177/0004867414538675; PUBMED: 24927734] [DOI] [PubMed] [Google Scholar]
Karnik 2007
- Karnik NS, Steiner H. Evidence for interventions for young offenders. Child and Adolescent Mental Health 2007;12(4):154‐9. [DOI: 10.1111/j.1475-3588.2007.00460.x] [DOI] [PubMed] [Google Scholar]
Kay 1988
- Kay SR, Wolkenfeld F, Murrill LM. Profiles of aggression among psychiatric patients. I. Nature and prevalence. Journal of Nervous and Mental Disease 1988;176(9):539‐46. [PUBMED: 3418327] [DOI] [PubMed] [Google Scholar]
Kazdin 1986
- Kazdin AE, Esveldt‐Dawson K. The interview for antisocial behavior: psychometric characteristics and concurrent validity with child psychiatric inpatients. Journal of Psychopathology and Behavioral Assessment 1986;8:289–303. [DOI: 10.1007/BF00960727] [DOI] [Google Scholar]
Kazdin 1997
- Kazdin AE. Parent management training: evidence, outcomes, and issues. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(10):1349‐56. [DOI: 10.1097/00004583-199710000-00016; PUBMED: 9334547] [DOI] [PubMed] [Google Scholar]
Kearns 2014
- Kearns MA, Hawley KM. Predictors of polypharmacy and off‐label prescribing of psychotropic medications: a national survey of child and adolescent psychiatrists. Journal of Psychiatry Practice 2014;20(6):438‐47. [DOI: 10.1097/01.pra.0000456592.20622.45; PUBMED: 25406048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemph 1993
- Kemph JP, DeVane CL, Levin GM, Jarecke R, Miller RL. Treatment of aggressive children with clonidine: results of an open pilot study. Journal of American Academy of Child and Adolescent Psychiatry 1993;32(3):577‐81. [DOI: 10.1097/00004583-199305000-00013; PUBMED: 8496122] [DOI] [PubMed] [Google Scholar]
Knapp 2012
- Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS, T‐MAY Steering Group. Treatment of maladaptive aggression in youth: CERT guidelines I. Engagement, assessment, and management. Pediatrics 2012;129(6):e1562‐76. [DOI: 10.1542/peds.2010-1360; PUBMED: 22641762] [DOI] [PubMed] [Google Scholar]
Krakowski 2006
- Krakowsk MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry 2006;63(6):622‐9. [DOI: 10.1001/archpsyc.63.6.622; PUBMED: 16754835] [DOI] [PubMed] [Google Scholar]
Kreider 2014
- Kreider AR, Matone M, Bellonci C, dosReis S, Feudtner C, Huang YS, et al. Growth in the concurrent use of antipsychotics with other psychotropic medications in Medicaid‐enrolled children. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):960‐70. [DOI: 10.1016/j.jaac.2014.05.010; PUBMED: 25151419] [DOI] [PubMed] [Google Scholar]
Kuban 2011
- Kuban, C. Oppositional Defiant Disorder and Trauma. The National Institute for Trauma and Loss in Children. www.lianalowenstein.com/articlesODDKuban.pdf (accessed prior to 23 November 2016).
LeBlanc 2005
- LeBlanc JC, Binder CE, Armenteros JL, Aman MG, Wang JS, Hew H, et al. Risperidone reduces aggression in boys with a disruptive behaviour disorder and below average intelligence quotient: analysis of two placebo‐controlled randomized trials. International Clinical Psychopharmacology 2005;20(5):275‐83. [PUBMED: 16096518] [DOI] [PubMed] [Google Scholar]
Littell 2005
- Littell JH, Popa M, Forsythe B. Multisystemic therapy for social, emotional, and behavioral problems in youth aged 10‐17. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004797.pub4] [DOI] [PubMed] [Google Scholar]
Littell 2007
- Littell JH, Winsvold A, Bjørndal A, Hammerstrøm KT. Functional Family Therapy for families of youth (age 11‐18) with behaviour problems. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006561] [DOI] [Google Scholar]
Loeber 1998
- Loeber R, Farrington DP, Stouthamer‐Loeber M, Kammen WB (editors). Antisocial Behavior and Mental Health Problems: Explanatory Factors in Childhood and Adolescence. Mahwah, NJ: Erlbaum, 1998. [Google Scholar]
Loy 2011a [pers comm]
- Loy J. Query for Cochrane Review [personal communication]. Email to: M Reyes 6 July 2011.
Loy 2011b [pers comm]
- Loy J. Query for Cochrane Review [personal communication]. Email to: C Binder 8 June 2011.
Loy 2016a [pers comm]
- Loy J. Enquiry regarding TOSCA trial [personal communication]. Email to: M Aman 07 January 2016.
Loy 2016b [pers comm]
- Loy J. TOSCA statistic related questions [personal communication]. Email to: N Brown 19 January 2016.
Loy 2016c [pers comm]
- Loy J. Enquiry re SPICY study [personal communication]. Email to: J Blader 4 August 2016.
Malone 1998
- Malone RP, Bennett DS, Luebbert JF, Rowan AB, Biesecker KA, Blaney BL, et al. Aggression classification and treatment response. Psychopharmacology Bulletin 1998;34(1):41‐5. [PUBMED: 9564197] [PubMed] [Google Scholar]
Miller 1995
- Miller LS, Klein RG, Piacentini J, Abikoff H, Shah MR, Samoilov A, et al. The New York Teacher Rating Scale for disruptive and antisocial behavior. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(3):359‐70. [DOI: 10.1097/00004583-199503000-00022; PUBMED: 7896678] [DOI] [PubMed] [Google Scholar]
Moffitt 2002
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life‐course‐persistent and adolescence‐limited antisocial pathway: follow up at age 26 years. Development and Psychopathology 2002;14(1):179‐207. [PUBMED: 11893092] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Lancet 2001;357(9263):1191‐4. [PUBMED: 11323066] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DF, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
MST Services 2016
- Multisystemic Therapy ® (MST ®) Research at a Glance January 2016. mstservices.com/files/outcomestudies_condensed.pdf 2016.
Murphy 2015
- Murphy AL, Gardner DM, Kisely S, Cooke C, Kutcher SP, Hughes J. A qualitative study of antipsychotic medication experiences of youth. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2015;24(1):61‐9. [PMC4357337; PUBMED: 26336383] [PMC free article] [PubMed] [Google Scholar]
Myers 2002
- Myers K, Winters NC. Ten year review of rating scales. I: overview of scale functioning, psychometric properties, and selection. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(2):114‐22. [DOI: 10.1097/00004583-200202000-00004; PUBMED: 11837400] [DOI] [PubMed] [Google Scholar]
Nelson 2007
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Reviews. Neuroscience 2007;8(7):536‐46. [DOI: 10.1038/nrn2174; PUBMED: 17585306] [DOI] [PubMed] [Google Scholar]
Nevels 2010
- Nevels RM, Dehon EE, Alexander K, Gontkovsky ST. Psychopharmacology of aggression in children and adolescents with primary neuropsychiatric disorders: a review of current and potentially promising treatment options. Experimental and Clinical Psychopharmacology 2010;18(2):184‐201. [DOI: 10.1037/a0018059; PUBMED: 20384430] [DOI] [PubMed] [Google Scholar]
NICE Clinical Guideline (CG158) 2013
- NICE National Institute for Health and Care Excellence Clinical guideline CG158. Antisocial behaviour and conduct disorders in children and young people: recognition and management. www.nice.org.uk/guidance/cg158/chapter/1‐Recommendations#psychosocial‐interventions‐treatment‐and‐indicated‐prevention 2013, issue March.
Olfson 2010
- Olfson M, Crystal S, Huang C, Gerhard T. Trends in antipsychotic drug use by very young, privately insured children. Journal of the American Academy of Child and Adolescent Psychiatry 2010;49(1):13‐23. [PUBMED: 20215922] [DOI] [PubMed] [Google Scholar]
Olfson 2014
- Olfson M. Combination psychotropic regimens in community practice. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(9):942‐4. [DOI: 10.1016/j.jaac.2014.05.018] [DOI] [PubMed] [Google Scholar]
Owen 2009
- Owen R, Sikich L, Marcus RN, Corey‐Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009;124(6):1533‐40. [DOI: 10.1542/peds.2008-3782; PUBMED: 19948625] [DOI] [PubMed] [Google Scholar]
Palanca‐Maresca 2014
- Palanca‐Maresca I, Ruiz‐Antorán B, Centeno‐Soto G, Jiménez‐Fernandez S, García‐Murillo L, Siles A, et al. SENTIA: a systematic online monitoring registry for children and adolescents treated with antipsychotics. SpringerPlus 2014;3:187. [DOI: 10.1186/2193-1801-3-187; PMC4000597; PUBMED: 24790830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pappadopulos 2003
- Pappadopulos E, Macintyre Ii JC, Crismon ML, Findling RL, Malone RP, Derivan A, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. Journal of American Academy of Child and Adolescent Psychiatry 2003;42(2):145‐61. [PUBMED: 12544174] [DOI] [PubMed] [Google Scholar]
Pappadopulos 2006
- Pappadopulos E, Woolston S, Chait B, Perkins M, Connor DF, Jensen PS. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. Journal of the American Academy of Child and Adolescent Psychiatry 2006;15(1):27‐39. [PMC2277275; PUBMED: 18392193] [PMC free article] [PubMed] [Google Scholar]
Parens 2010
- Parens E, Johnston J. Controversies concerning the diagnosis and treatment of bipolar disorder in children. Child and Adolescent Psychiatry and Mental Health 2010;10(4):9. [DOI: 10.1186/1753-2000-4-9; PMC2846895; PUBMED: 20219111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pediatric European Risperidone Studies (PERS) 2016
- PERS. Pediatric European Risperidone Studies. www.pers‐project.com/ (accessed 9 May 2016).
Perkins 2004
- Perkins DO. Antipsychotic‐induced hyperprolactinemia: pathophysiology and clinical consequences. Advanced Studies in Medicine 2004;4(10F):S982–6. [Google Scholar]
Perry 2006
- Perry, BD. Applying principles of neurodevelopment to clinical work with maltreated and traumatized children: the neurosequential model of therapeutics. In: Webb NB editor(s). Working with Traumatized Children in Child Welfare. New York: Guildford Press, 2006:27‐52. [Google Scholar]
Pringsheim 2012
- PringsheimT, Gorman D. Second‐generation antipsychotics for the treatment of disruptive behaviour disorders in children: a systematic review. Canadian Journal of Psychiatry 2012;57(12):722‐7. [PUBMED: 23228230] [DOI] [PubMed] [Google Scholar]
Pringsheim 2015
- Pringsheim T, Hirsch L, Gardner D, Gorman DA. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention‐deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta‐analysis. Part 2: antipsychotics and traditional mood stabilizers. Canadian Journal of Psychiatry 2015;60(2):52‐61. [PMC4344947; PUBMED: 25886656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rani 2008
- Rani F, Murray ML, Byrne PJ, Wong ICK. Epidemiologic feature of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics 2008;121(5):1002‐9. [DOI: 10.1542/peds.2007-2008; PUBMED: 18450906] [DOI] [PubMed] [Google Scholar]
Rapoport 1985
- Rapoport J, Conners CK, Reatig N. Rating scales and assessment for use in paediatric psychopharmacology research. Psychopharmacology Bulletin 1985;21:1077‐80. [PubMed] [Google Scholar]
Remington 2014
- Remington G, Fervaha G, Foussias G, Agid O, Turrone P. Antipsychotic dosing: found in translation. Journal of Psychiatry and Neuroscience 2014;39(4):223‐31. [PMC4074233; PUBMED: 24467943] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rundberg‐Rivera 2015
- Rundberg‐Rivera EV, Townsend LD, Schneider J, Farmer CA, Molina BB, Findling RL, et al. Participant satisfaction in a study of stimulant, parent training, and risperidone in children with severe physical aggression. Journal of Child and Adolescent Psychopharmacology 2015;25(3):225‐33. [DOI: 10.1089/cap.2014.0097; PMC4403019; PUBMED: 25885012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schur 2003
- Schur SB, Sikich L, Findling RL, Malone RP, Crismon ML, Derivan A, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part 1: a review. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(2):132‐44. [PUBMED: 12544173] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scott 2008
- Scott S. An update on interventions for conduct disorder. Advances in Psychiatric Treatment 2008;14(1):61‐70. [DOI: 10.1192/apt.bp.106.002626] [DOI] [Google Scholar]
Scotto Rosato 2012
Seida 2012
- Seida JC, Schouten JR, Boylan K, Newton AS, Mousavi SS, Beaith A, et al. Antipsychotics for children and young adults: a comparative effectiveness review. Pediatrics 2012;129(3):e771‐84. [DOI: 10.1542/peds.2011-2158] [DOI] [PubMed] [Google Scholar]
Shaffer 1983
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children's global assessment scale (CGAS). Archives of General Psychiatry 1983;40(11):1228‐31. [DOI: 10.1001/archpsyc.1983.01790100074010] [DOI] [PubMed] [Google Scholar]
Sharma 2016
- Sharma T, Guski LS, Freund F, Gøtzsche PC. Suicidality and aggression during antidepressant treatment: systematic review and meta‐analyses based on clinical study reports. British Medical Journal 2016;352:i65. [DOI: 10.1136/bmj.i65] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2001
- Shaw DS, Owens EB, Giovannelli J, Winslow EB. Infant and toddler pathways leading to early externalizing disorders. Journal of American Academy of Child and Adolescent Psychiatry 2001;40(1):36‐43. [DOI: 10.1097/00004583-200101000-00014; PUBMED: 11195559] [DOI] [PubMed] [Google Scholar]
Siever 2008
- Siever LJ. Neurobiology of aggression and violence. American Journal of Psychiatry 2008;165(4):429‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica Supplement 1970;212:11‐9. [PUBMED: 4917967] [DOI] [PubMed] [Google Scholar]
Sorgi 1991
- Sorgi P, Ratey J, Knoedler DW, Markert RJ, Reichman M. Rating aggression in the clinical setting. A retrospective adaptation of the Overt Aggression Scale: preliminary results. Journal of Neuropsychiatry and Clinical Neurosciences 1991;3(2):S52‐6. [PUBMED: 1687961] [PubMed] [Google Scholar]
Stahl 2013
- Stahl SM. Chapter 5 Antipsychotic agents. Stahl's Essential Psychopharmacology Neuroscientific Basis and Practical Applications. Fourth. Cambridge: Cambridge University Press, 2013:129‐236. [Google Scholar]
Stallard 1996
- Stallard P. Validity and reliability of the Parent Satisfaction Questionnaire. British Journal of Clinical Psychology 1996;35(Pt 2):311‐8. [PUBMED: 8773805] [DOI] [PubMed] [Google Scholar]
Stasiak 2015 [pers comm]
- Stasiak K. Seeking information on your trial "Ziprasidone for Severe Conduct and Other Disruptive Behavior Disorders" (NCT00676429)(personal communication). Email to: K Hennighausen and E Schulz 12 June 2015.
Steiner 1997
- Steiner H, The Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(Suppl 10):122S‐39S. [PUBMED: 9334568] [DOI] [PubMed] [Google Scholar]
Steiner 2003
- Steiner H, Saxena K, Chang K. Psychopharmacologic strategies for the treatment of aggression in juveniles. CNS Spectrums 2003;8(4):298‐308. [PUBMED: 12679744] [DOI] [PubMed] [Google Scholar]
Steiner 2007
- Steiner H, Remsing L, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with oppositional defiant disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(1):126‐41. [DOI: 10.1097/01.chi.0000246060.62706.af; PUBMED: 17195736] [DOI] [PubMed] [Google Scholar]
Sterne 2009
- Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. British Medical Journal 2009;338(b2393):157‐160. [DOI: 10.1136/bmj.b2393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swann 2003
- Swann AC. Neuroreceptor mechanisms of aggression and its treatment. Journal of Clinical Psychiatry 2003;64(Suppl 4):26‐35. [PUBMED: 12672262] [PubMed] [Google Scholar]
Swanson 1992
- Swanson J. School based assessments and interventions for ADD students. Irvine (CA): KC Publishing, 1992. [Google Scholar]
Tassé 1996
- Tassé MJ, Aman MG, Hammer D, Rojahn J. The Nisonger Child Behavior Rating Form: age and gender effect and norms. Research in Developmental Disabilities 1996;17(1):59‐75. [PUBMED: 8750076] [DOI] [PubMed] [Google Scholar]
Tcheremissine 2006
- Tcheremissine OV, Lieving LM. Pharmacological aspects of the treatment of conduct disorder in children and adolescents. CNS Drugs 2006;20(7):549‐65. [PUBMED: 16800715] [DOI] [PubMed] [Google Scholar]
Teixeira 2013b
- Teixeira EH, Jacintho A, Celeri HV, Dalgalarrondo P. Atypical antipsychotics in the treatment of pathological aggression in children and adolescents: literature review and clinical recommendations. Trends in Psychiatry and Psychotherapy 2013;35(3):151‐9. [PUBMED: 25923387] [DOI] [PubMed] [Google Scholar]
Timimi 2008
- Timimi S. Child psychiatry and its relationship with the pharmaceutical industry: theoretical and practical issues. Advances in Psychiatric Treatment 2008;14(1):3‐9. [DOI: 10.1192/apt.bp.105.000901] [DOI] [Google Scholar]
Turgay 2004
- Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Review of Neurotherapeutics 2004;4(4):623‐32. [DOI: 10.1586/14737175.4.4.623; PUBMED: 15853581] [DOI] [PubMed] [Google Scholar]
Verhulst 2002
- Verhulst FC, Ende J. Rating Scales. In: Rutter M, Taylor E editor(s). Child and Adolescent Psychiatry. Malden (MA): Blackwell Publishing, 2002:70‐86. [Google Scholar]
Vitiello 1990
- Vitiello B, Behar D, Hunt J, Stoff D, Ricciuti, A. Subtyping aggression in children and adolescents. Journal of Neuropsychiatry and Clinical Neurosciences 1990;2(2):189‐92. [DOI: 10.1176/jnp.2.2.189; PUBMED: 2136074] [DOI] [PubMed] [Google Scholar]
Vitiello 1997
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(3):307‐15. [DOI: 10.1097/00004583-199703000-00008] [DOI] [PubMed] [Google Scholar]
Vitiello 2009
- Vitiello B, Correll C, Zwieten‐Boot B, Zuddas A, Parellada M, Arango C. Antipsychotics in children and adolescents: increasing use, evidence for efficacy and safety concerns. European Neuropsychopharmacology 2009;19(9):629‐35. [DOI: 10.1016/j.euroneuro.2009.04.008; PUBMED: 19467582] [DOI] [PubMed] [Google Scholar]
Waknine 2010
- Waknine Y. FDA approves extended‐release clonidine for pediatric ADHD. www.medscape.com/viewarticle/730140 (accessed prior to 25 July 2017).
Waschbusch 2002
- Waschbusch DA, Pelham WE Jr, Jennings JR, Greiner AR, Tarter RE, Moss HB. Reactive aggression in boys with disruptive behaviour disorders: behaviour, physiology, and affect. Journal of Abnormal Child Psychology 2002;30(6):641‐56. [PUBMED: 12481977] [DOI] [PubMed] [Google Scholar]
Weisz 2004
- Weisz JR. Psychotherapy for Children and Adolescents, Evidence‐Based Treatments and Case Examples. New York: Cambridge University Press, 2004. [Google Scholar]
WHO 2016
- WHO 2016. International Classification of Diseases and Related Health Problems, 10th Revision. apps.who.int/classifications/icd10/browse/2016/en (accessed 12 January 2017).
Wolraich 1998
- Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM‐IV. Journal of Abnormal Child Psychology 1998;26(2):141‐52. [PUBMED: 9634136] [DOI] [PubMed] [Google Scholar]
Wolraich 2003
- Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. Journal of Pediatric Psychology 2003;28(8):559‐67. [PUBMED: 14602846] [DOI] [PubMed] [Google Scholar]
Wood 2004
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clinical Trials 2004;1(4):368‐76. [PUBMED: 16279275] [DOI] [PubMed] [Google Scholar]
Youngstrom 2001
- Youngstrom EA, Findling RL, Danielson CK, Calabrese JR. Discriminative validity of parent report of hypomanic and depressive symptoms on the General Behavior Inventory. Psychological Assessment 2001;13(2):267‐76. [PUBMED: 11433802] [PubMed] [Google Scholar]
Yudofsky 1986
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The overt aggression scale for the objective rating of verbal and physical aggression. American Journal of Psychiatry 1986;143(1):35‐9. [DOI: 10.1176/ajp.143.1.35; PUBMED: 3942284] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Loy 2010
- Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behavioural disorders ininpatient or outpatient children and youths. Cochrane Database of Systematic Reviews 2010, Issue 6 . Art. No.: CD008559. DOI: 10.1002/14651858.CD008559. [DOI: 10.1002/14651858.CD008559] [DOI] [Google Scholar]
Loy 2012
- Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database of Systematic Reviews 2012;9:Art. No.: CD008559. DOI: 10.1002/14651858.CD008559.pub2. [DOI] [PubMed] [Google Scholar]


