Abstract
Background
Patients with longstanding ulcerative colitis and colonic Crohn's disease have an increased risk of colorectal cancer (CRC) compared with the general population. This review assessed the evidence that endoscopic surveillance may prolong life by allowing earlier detection of CRC or its pre‐cursor lesion, dysplasia, in patients with inflammatory bowel disease (IBD).
Objectives
To assess the effectiveness of cancer surveillance programs for diagnosis of IBD‐associated colorectal cancer and in reducing the mortality rate from colorectal cancer in patients with IBD.
Search methods
We searched MEDLINE, EMBASE, CENTRAL and clinical clinicaltrials.gov from inception to 19 September 2016. We also searched conference abstracts and reference lists to identify additional studies.
Selection criteria
Potentially relevant articles were reviewed independently and unblinded by two authors to determine eligibility. Randomised controlled trials (RCTs) or observational studies (cohort or case control) assessing any form of endoscopic surveillance aimed at early detection of CRC were considered for inclusion. Studies had to have a no surveillance comparison group to be eligible for inclusion.
Data collection and analysis
Eligible studies were reviewed in duplicate and the results of the primary research trials were independently extracted by two authors. The primary outcome was detection of CRC. Secondary outcomes included death from CRC, time to cancer detection, time to death and adverse events. Deaths from CRC were derived from life tables, survival curves or where possible, by calculating life tables from the data provided. The presence of significant heterogeneity among studies was tested by the chi‐square test. Because this is a relatively insensitive test, a P value of less than 0.1 was considered statistically significant. Provided statistical heterogeneity was not present, the fixed effects model was used for the pooling of data. The 2x2 tables were combined into a summary test statistic using the pooled odds ratio (OR) and 95% confidence intervals as described by Cochrane and Mantel and Haenszel. The methodological quality of the included studies was assessed using the Newcastle‐Ottawa scale for non‐randomised studies The overall quality of the evidence supporting the primary and selected secondary outcomes was assessed using the GRADE criteria.
Main results
No RCTs were identified. Five observational studies (N = 7199) met the inclusion criteria. The studies scored well on the Newcastle‐Ottawa scale, but due to the nature of observational studies, a high risk of bias was assigned to all the studies. Three studies were pooled to assess the rate of cancer detected in the surveillance group compared to the non‐surveillance group. The studies found a significantly higher rate of cancer detection in the non surveillance group compared to the surveillance group. CRC was detected in 1.83% (53/2895) of patients in the surveillance group compared to 3.17% (135/4256) of patients in the non‐surveillance group (OR 0.58, 95% CI 0.42 to 0.80; P = 0.0009). Four studies were pooled to assess the death rate associated with CRC in patients who underwent surveillance compared to patients who did not undergo surveillance. There was a significantly lower death rate associated with CRC in the surveillance group compared to the non‐surveillance group. Eight per cent (15/176) of patients in the surveillance group died from CRC compared to 22% (79/354) of patients in the non‐surveillance group (OR 0.36, 95% CI 0.19 to 0.69, P=0.002). Data were pooled from two studies to examine the rate of early stage versus late stage colorectal cancer (Duke stages A & B compared to Duke stages C & D) in patients who underwent surveillance compared to patients who do not undergo surveillance. A significantly higher rate of early stage CRC (Duke A & B) was detected in the surveillance group compared to the non‐surveillance group. Sixteen per cent (17/110) of patients in the surveillance group had early stage CRC compared to 8% (9/117) of patients in the non‐surveillance group (OR 5.40, 95% CI 1.51 to 19.30; P = 0.009). A higher rate of late stage CRC (Duke C & D) was observed in the non‐surveillance group compared to the surveillance group. Nine per cent (10/110) of patients in the surveillance group had late stage CRC compared to 16% (19/117) of patients in the non‐surveillance group (OR 0.46, 95% CI 0.08 to 2.51; P = 0.37). A GRADE analysis indicated that the quality of the data was very low for all of these outcomes. The included studies did not report on the other pre‐specified outcomes including time to cancer detection, time to death and adverse events.
Authors' conclusions
The current data suggest that colonoscopic surveillance in IBD may reduce the development of both CRC and the rate of CRC‐associated death through early detection, although the quality of the evidence is very low. The detection of earlier stage CRC in the surveillance group may explain some of the survival benefit observed. RCTs assessing the efficacy of endoscopic surveillance in people with IBD are unlikely to be undertaken due to ethical considerations.
Plain language summary
Strategies for detecting colon cancer in patients with inflammatory bowel disease
What is inflammatory bowel disease?
Inflammatory bowel disease (IBD) is composed of two main disorders Crohn’s disease (CD) and ulcerative colitis (UC). These diseases are chronic inflammatory disorder of the gastrointestinal tract. Common symptoms may include abdominal pain, cramping, diarrhoea, and blood in stools. People with CD may also experience intestinal strictures (a narrowing of a section of the intestine that causes problems by slowing or blocking the movement of food), abscesses (a collection of pus that has built up within the tissue) and fistulae (an abnormal channel or passageway connecting one internal organ to another, or to the outside surface of the body).
What is colon cancer?
Long term inflammation associated with IBD leads to an increased risk of colon cancer compared to the risk in people without IBD. Colon cancer is a malignant tumour arising from the inner wall of the large intestine (the colon).
What is endoscopic surveillance?
An endoscopy is a non‐surgical procedure used to view the digestive tract using a camera. The doctor who performs the endoscopy can take tissue samples of suspicious lesions or growths during the procedure. Endoscopic surveillance is used to identify pre‐cancerous growths (called dysplasia) or colon cancer in patients with IBD. Endoscopy may help to identify colon cancer at an earlier stage and help prolong survival and lower the death rate due to colon cancer
What did the researchers investigate?
The researchers reviewed published studies comparing people with IBD who had endoscopic surveillance to people who did not have endoscopic surveillance to see whether surveillance provided any benefit in terms of diagnosing colon cancer at an earlier stage or reducing the death rate due to colon cancer. The medical literature was searched and analysed up to 19 September 2016.
What did the researchers find?
Five observational studies with 7199 patients were used to compare endoscopic surveillance to non‐surveillance. The key findings of the review were that a higher rate of cancer occurred in the non‐surveillance group compared to the surveillance group, and that a lower rate of colon cancer‐associated death was demonstrated in the surveillance group compared to the non‐surveillance group. In patients undergoing surveillance, the odds of colon cancer development were reduced by 42% and the odds of death associated with colon cancer was reduced by 64%. Surveillance resulted in detection of a higher rate of early stage colorectal cancer in the surveillance group compared to the non surveillance group which may explain the improved survival seen with surveillance. The overall quality of the evidence is very low due to the nature of observation studies and the low number of events. Nonetheless, these results suggest that endoscopic surveillance in people with IBD may reduce the development of colon cancer through early detection and may also reduce the chances of dying from colon cancer.
Summary of findings
Summary of findings for the main comparison. Surveillance compared to non surveillance for detecting colon cancer in patients with inflammatory bowel disease.
| Surveillance compared to non surveillance for detecting colon cancer in patients with inflammatory bowel disease | ||||||
| Patient or population: detecting colon cancer in patients with inflammatory bowel disease Setting: Intervention: Surveillance Comparison: non surveillance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non surveillance | Risk with Surveillance | |||||
| Cancer detection | 32 per 1,0001 | 19 per 1,000 (14 to 26) | OR 0.58 (0.42 to 0.80) | 7151 (3 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 | |
| Death from colorectal cancer | 223 per 1,0001 | 94 per 1,000 (52 to 165) | OR 0.36 (0.19 to 0.69) | 530 (4 observational studies) | ⊕⊝⊝⊝ VERY LOW 3 | |
| Cancer stage‐ Duke A or B | 77 per 1,0001 | 310 per 1,000 (112 to 617) | OR 5.40 (1.51 to 19.30) | 227 (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 4 | |
| Cancer stage‐ Duke C or D | 162 per 1,0001 | 82 per 1,000 (15 to 327) | OR 0.46 (0.08 to 2.51) | 227 (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials
2 Downgraded one level due to sparse data (188 events)
3 Downgraded one level due to sparse data (94 events)
4 Downgraded due to very sparse data (26 events)
5 Downgraded due to very sparse data (29 events) and heterogeneity (I2 = 71%)
Background
Patients with longstanding ulcerative colitis (UC) and colonic Crohn's disease (CD) have an increased risk of colorectal cancer (CRC) compared to the general population. This review assessed the evidence that endoscopic surveillance may prolong life by allowing earlier detection of CRC or its pre‐cursor lesion, dysplasia, in patients with inflammatory bowel disease (IBD).
To assess such issues, it is important to understand;
1. The size of the cancer risk in IBD (UC and CD);
2. Risk factors for developing CRC in IBD to enable selection of a high‐yield population to be targeted for screening; and
3. The definition, grades and natural history of dysplasia.
Description of the condition
IBD includes UC and CD. IBD is a chronic, relapsing and remitting condition that targets the gastrointestinal system and causes significant long term co‐morbidity. The incidence of IBD is increasing worldwide (Kaplan 2015). The cause of IBD is unknown, but is speculated to be due to an interaction between genetic, environmental and immunoregulatory factors ( Hanauer 2006). Patients with IBD experience symptoms including diarrhoea, fever, fatigue, abdominal pain, cramping, reduced appetite and weight loss.
Patients with longstanding UC and CD have an increased risk of CRC compared to the general population (Eaden 2001a; Lashner 1991). First described in 1925 (Crohn 1925), CRC is a recognised complication of chronic colonic inflammation as a result of IBD. Colitis‐associated CRC has a unique clinical profile compared to sporadic CRC in the general community, owing to its distinct manner of carcinogenesis. Age of onset is generally younger in colitis‐associated CRC (average age 50 to 60 years) compared with sporadic CRC in the general population (average age 65 to 75 years) (Baars 2012; Rutter 2006). Disease location is more likely to be proximal if the CRC is related to Crohn’s colitis or primary sclerosing cholangitis (PSC) (Bansal 1996). Colitis associated cancer is more often synchronous and has an increased frequency of mucinous or signet ring cell histology (Itzkowitz 2004). A recent population‐based meta‐analysis has downgraded the risk of CRC in IBD from previous estimates (standardized incidence ratio 2.4, 95% CI 2.1 to 2.7), although the risk still remains above that of the general population (Jess 2012).
Description of the intervention
Surveillance is performed via colonoscopy. The purpose of the colonoscopy is to detect dysplasia or CRC at an earlier stage, which may lead to an improved prognosis. Strategies are emerging to optimise surveillance. The timing of surveillance, effective bowel preparation, use of high resolution endoscopic equipment and use of chromoendoscopy may all optimise surveillance.
How the intervention might work
Endoscopic surveillance programs aim to reduce the mortality of CRC by detecting CRC at an earlier stage or through the detection of CRC precursor dysplastic lesions which allow definitive management and thus reduction in CRC incidence.
Why it is important to do this review
Although endoscopic surveillance for CRC in IBD patients has been used for over four decades, direct evidence of a benefit in terms of a reduction in mortality is lacking. However, advances in the availability of effective biological therapies for IBD as well as endoscopic techniques for detection of dysplastic lesions are unlikely to have been reflected in previous analyses. With the increasing incidence of IBD, the burden of ongoing surveillance healthcare costs will continue to increase and surveillance programs may result in recurrent patient intervention which is not without risk. An understanding of the efficacy and potential ways to optimise surveillance is crucial for patients and health systems alike. This systematic review is an update of a previously published Cochrane review (Collins 2006).
Objectives
The primary objectives were to assess the effectiveness of cancer surveillance programs for diagnosis of IBD‐associated CRC and in reducing the mortality rate from colorectal cancer in patients with IBD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were considered for inclusion. Cohort and case‐control studies were also eligible for inclusion.
Types of participants
Patients of any age, with a diagnosis of ulcerative colitis or colonic Crohn's disease defined by conventional clinical, endoscopic, and histologic criteria who have been selected for surveillance, based solely on the duration and extent of disease were eligible for inclusion.
Types of interventions
Any form of endoscopic surveillance aimed at early detection of CRC was considered for inclusion. Studies had to have a no surveillance comparison group to be eligible for inclusion.
Types of outcome measures
Primary outcomes
Comparative rates of diagnosis of CRC between the surveillance and non‐surveillance group.
Secondary outcomes
Secondary outcomes included:
a) The proportion of patients who died from CRC with or without colonoscopy surveillance;
b)The time to cancer detection;
c)The time to death;
d) The proportion of patients with adverse events;
e) The proportion of patients with serious adverse events; and
d) The proportion of patients who withdrew due to adverse events
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant studies:
1. MEDLINE (Ovid, 1946 to 19 September 2016);
2. EMBASE (Ovid, 1984 to 19 September 2016);
3. CENTRAL; and
4. The Cochrane IBD Group Specialized Register.
The search strategies are listed in Appendix 1.
Searching other resources
We searched the reference lists of potentially relevant trials and papers to identify additional studies. Conference proceedings from Digestive Disease Week, United European Gastroenterology Week and the European Crohn's and Colitis Organisation Congress were hand searched to identify studies reported in abstract form only.
Data collection and analysis
Selection of studies
Potentially relevant articles were reviewed independently and unblinded by three authors (WB; TN; CP) to determine eligibility. Each article was rated as being eligible, ineligible, or without sufficient information to determine eligibility. Any disagreement between reviewers was resolved by consensus. Any trials published in abstract form were only considered if it was possible to obtain full details of the protocol and results from the authors.
Data extraction and management
Two authors (WB and TN) independently extracted the results of the primary research trials. The proportion of patients dying from CRC in the surveillance and control groups of each study was derived from life tables, survival curves, or where possible, by calculating life tables from the data provided.
Assessment of risk of bias in included studies
The methodological quality of each included study was independently evaluated by two authors (WB and TN) using the Newcastle‐Ottawa Quality Assessment Scale (NOS; Wells 2017). Factors assessed for cohort studies included:
1) Selection
a) Representativeness of the exposed cohort:
b) Selection of the non‐exposed cohort:
c) Ascertainment of exposure; and
d) Demonstration that outcome of interest was not present at start of study.
2) Comparability
a) Comparability of cohorts on the basis of the design or analysis.
3)Outcome
a) Assessment of outcome
b) Appropriate length of follow‐up for outcomes to occur
c) Adequacy of follow up of cohorts
Factors assessed for case control studies included:
1) Selection
a) Adequate case definition;
b) Representativeness of the cases;
c) Selection of controls; and
d) Definition of controls.
2) Comparability
a) Comparability of cases and controls on the basis of design or analysis.
3) Exposure
a) Ascertainment of exposure;
b) Same method of ascertainment for cases and controls; and
c) Non‐response rate.
The GRADE approach was used to evaluate the overall quality of evidence supporting the primary and secondary outcomes (Guyatt 2008; Schünemann 2011). Evidence from RCTs is considered high quality evidence and evidence from observational studies is considered low quality. The quality of the evidence can be downgraded due to:
1) risk of bias;
2) indirect evidence;
3) inconsistency (unexplained heterogeneity);
4) imprecision; and
5) publication bias.
The overall quality of evidence for each outcome was classified as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low quality (i.e. we are very uncertain about the estimate).
Measures of treatment effect
Data were analysed using Review Manager (RevMan 5.3.5). For dichotomous outcomes we calculated the odds ratio (OR) and corresponding 95% confidence interval (CI). For continuous outcomes we calculated the mean difference (MD) and corresponding 95% CI.
Unit of analysis issues
When studies reported multiple observations for the same outcome, the outcomes were combined for fixed intervals of follow‐up.
Dealing with missing data
Where possible, study authors were contacted to request missing data. An available case analysis was conducted when missing data could not be obtained.
Assessment of heterogeneity
We assessed heterogeneity among studies using the Chi2 test (a P value of 0.10 was considered statistically significant) and the I2 statistic. An I2 value of 25% indicates low heterogeneity, 50% indicates moderate heterogeneity and 75% indicates high heterogeneity (Higgins 2011). We used sensitivity analyses to explore potential explanations for heterogeneity.
Assessment of reporting biases
We assessed potential reporting bias by comparing outcomes listed in protocols to published manuscripts. If protocols were not available we compared outcomes listed in the methods section of published manuscripts to those reported in the results section. If a sufficient number of studies were included (i.e. > 10) in the pooled analyses, we planned to investigate potential publication bias using funnel plots (Egger 1997).
Data synthesis
Data from individual trials were combined for meta‐analysis when the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). The pooled OR and 95% CI was calculated for dichotomous outcomes. For continuous outcomes the pooled MD and corresponding 95% CI was calculated. We calculated the standardized mean difference (SMD) and 95% CI when different scales were used to measure the same underlying construct. A fixed‐effect model was used to pool data unless heterogeneity existed between the studies. A random‐effects model was be employed if heterogeneity exits (I2 50 to 75%). We did not pool data for meta‐analysis if a high degree of heterogeneity (I2 ≥ 75%) was detected.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included:
1) study characteristics (location, setting); and
2) patient characteristics (sex, age, disease onset, disease duration, disease severity, disease stage, concomitant medication, previous exposure to anti‐TNF drugs).
Sensitivity analysis
Planned sensitivity analyses included:
1) random‐effects versus fixed‐effect modelling;
2) low risk of bias versus unclear or high risk of bias;
3) relevant loss to follow up (>10%): base‐case versus worst‐case scenario; and
4) full‐text manuscripts versus abstract or unpublished studies.
Results
Description of studies
Results of the search
The literature search was conducted on September 19 2016 and identified 12,896 records. After duplicates were removed, 9499 records were screened for inclusion. Of the studies that were screened, 41 studies were selected for full text review. Thirty‐four studies were excluded resulting in 7 reports of 5 trials that met the inclusion criteria (Figure 1). No additional studies were identified.
1.
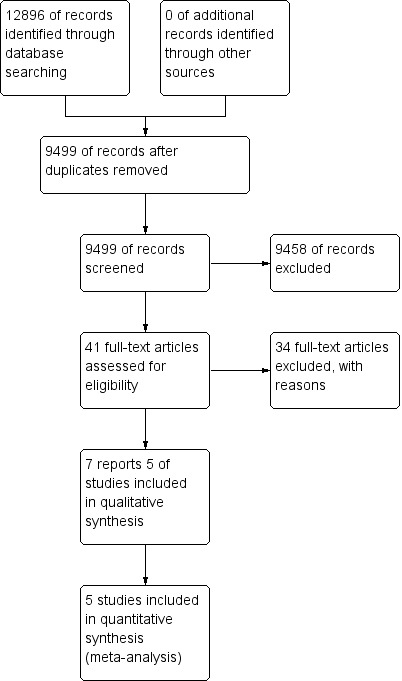
Study flow diagram.
Included studies
The search did not find any RCTs. The study yielded 41 reports of 39 studies attempting to address the impact of surveillance on survival (Akbar 2015; Ananthakrishnan 2014; Arthurs 2012; Basseri 2012; Biasco 2002; Bopanna 2016; Brostrom 1986; Carballal 2014; Choi 2015;Choi 1993; Eaden 2000; Friedman 2001; Gonzalez 2016; Gunther 2011; Hata 2003; Hernandez 2013; Higashi 2011; Hiroyuki 2014; Jonsson 1994; Karlen 1998a; Lashner 1990; Lindberg 2005; Lofberg 1990; Lutgens 2009; Lynch 1993; Manninen 2013; Matsuoka 2013; Monzur 2013; Mooiweer 2015; Nugent 1991; Panara 2016; Riegler 2003; Rodino 2011; Rosenstock 1985; Rutegard 2016; Rutter 2006; Saoula 2013; Stolwijk 2013; Velayos 2006). Eaden 2000 performed a case‐control study of 102 patients with colitis‐associated cancer and 102 controls matched for sex, age, extent and duration of disease. They showed that performance of colonoscopy after diagnosis was not a significant protective factor when adjusted for mesalazine usage and contact with a hospital doctor, but this study was not designed to assess the impact of formal colonoscopic surveillance with multiple biopsies. Nineteen of these studies were retrospective descriptive analyses without control groups (Akbar 2015; Arthurs 2012; Friedman 2001Gonzalez 2016;Hata 2003; Hernandez 2013; Hiroyuki 2014; Higashi 2011Jonsson 1994; Lofberg 1990; Manninen 2013; Matsuoka 2013;Monzur 2013; Nugent 1991; Panara 2016; Riegler 2003; Rosenstock 1985; Rodino 2011;Velayos 2006). Fourteen of these studies were prospective analyses without a control group (Basseri 2012; Biasco 2002; Bopanna 2016; Brostrom 1986; Carballal 2014; Choi 2015; Gunther 2011; Lindberg 2005; Lynch 1993; Mooiweer 2015; Rutegard 2016;Rutter 2006; Saoula 2013; Stolwijk 2013). Of the remaining studies, four were cohort studies (Ananthakrishnan 2014; Choi 1993; Lashner 1990; Lutgens 2009) and one was a case control study (Karlen 1998a). These studies are discussed in more detail below.
Karlen 1998a carried out a population‐based, nested case control study in 4664 patients with UC. The presence or absence of colonoscopy surveillance in all 40 patients with UC who died from colorectal cancer after 1975 was compared to 102 controls matched for age, sex, extent and duration of disease. The relationship between colonoscopic surveillance and CRC mortality was assessed by the relative risk and expressed as the odds ratio. Matched analyses were performed using conditional logistic regression. The estimated standard deviations of the regression coefficient estimates were used to calculate 95% confidence limits. Only colonoscopies performed with the intention of cancer surveillance were included in the study. Index colonoscopies and those performed because of clinical symptoms or signs were excluded.
Choi 1993 reviewed results of a prospective surveillance programme in which patients with a disease duration of eight years or greater and extension of disease proximal to the sigmoid colon were enrolled from a heterogeneous group of 2050 patients with UC. In total 41 patients developed carcinoma of whom 19 had been undergoing colonoscopic surveillance and 22 had not. The two groups were compared for differences in survival and cancer detection (Duke's stage). Survival distributions were estimated using the product‐limit method of Kaplan and Meier. The statistical significance of differences between distributions was assessed using the Tarone‐Ware method. Differences in age distribution at the time of onset of UC and the diagnosis of CRC, and the duration of UC before development of CRC were analysed using the Mann‐Whitney test. Surveillance was defined as colonoscopic biopsy study performed with an intent to screen for neoplasia based on long duration of disease without any concomitant symptoms or signs to suggest neoplasia before the procedure.
Lashner 1990 reported a retrospective cohort analysis in which they identified 91 screened and 95 control UC patients who had extensive disease for at least eight years. The two groups were compared for differences in survival, CRC detection and colectomy. Crude analyses were performed using Kaplan‐Meier product‐limit survival curves and curves were compared with the log rank test. Differences in entry variables between the two groups were adjusted to remove confounding effects (age at symptom onset, sex, and duration of disease) using a Cox proportional hazards model. The CRC surveillance program recommended yearly colonoscopy with biopsy. Patients were excluded if they were referred with CRC, if CRC was found at first evaluation or if no follow‐up information was obtained. Controls were excluded if CRC was found at first referral.
Ananthakrishnan 2014 reported a retrospective cohort analysis where data from 6823 patients were analysed. The proportion of patients who underwent recent colonoscopy within 36 months was compared to the proportion of patients that have not undergone recent colonoscopy. The primary aim of the study was to determine whether recent colonoscopy impacts on the risk of CRC in patients with IBD, and whether outcomes after CRC diagnosis are different in patients who had recent colonoscopies. The statistical significance between groups was calculated using the Chi2 test for categorical outcomes and the t‐test for continuous outcomes. The Mann‐Whitney test was used for non‐parametric comparisons.
Lutgens 2009 reviewed results from a nationwide pathology database to identify IBD patients who were treated at all eight universities in the Netherlands over a period of 15 years. Patients who had undergone surveillance colonoscopies before their CRC diagnosis were assigned to the treatment group and patients who had not undergone surveillance colonoscopies were assigned to the control group. One hundred and forty‐nine patients with IBD‐associated CRC were identified, of which 23 had surveillance colonoscopies before their diagnosis of CRC. The primary objective of this study was to compare the tumour stage and survival of IBD patients with CRC who were in the surveillance program compared to those who were not in the program. The Chi2 test, Fisher's exact test and Student's t‐test were used to compare characteristics between the treatment and control groups. In addition, the Kaplan Meier and cox regression tests were used for survival calculations.
Excluded studies
The majority of excluded studies were excluded for not having a control group (See Characteristics of excluded studies).
Risk of bias in included studies
The NOS was used to assess the quality of non randomised trials (Wells 2017). The NOS uses a star system to assess the quality of cohort and case control studies based on three different domains. To assess the quality of cohort studies, the first domain is the selection of study groups (four items), the second domain is the comparability between the study group (two items) and the control group, and the last domain is the ascertainment of the exposure or outcome of interest (three items), for a maximum score of nine stars. To assess the quality of the case control studies, the first domain is the selection of study groups (four items), the second domain is the comparability between the cases and controls (two items), and the last domain is the ascertainment of exposure, for a maximum of nine stars.
All of these studies scored well based on the selection of the study groups, the comparability between the treatment group and control group and the outcome assessment. For the cohort studies included in this review, Ananthakrishnan 2014 scored nine; Lashner 1990 scored nine; Choi 1993 scored nine and lastly, Lutgens 2009 scored nine (see Table 2). Karlen 1998a was the only case control study included in this review and this study scored nine (See Table 3).
1. Risk of bias assessment using the Newcastle‐Ottawa Scale (Cohort).
| Study ID | Selection | Comparability | Outcome | Total | |||||
| Representativeness of the exposed cohort ( /1) |
Selection of the non‐exposed cohort (/1) |
Ascertainment of exposure (/1) |
Demonstration that outcome of interest not present at start (/1) |
Comparability of cohorts on design or analysis (/2) |
Assessment of outcome (/1) |
Appropriate length of follow‐up (/1) |
Adequacy of follow‐up (/1) |
||
| Ananthakrishnan 2014 | * | * | * | * | ** | * | * | * | 9 |
| Lashner 1990 | * | * | * | * | ** | * | * | * | 9 |
| Choi 1993 | * | * | * | * | ** | * | * | * | 9 |
| Lutgens 2009 | * | * | * | * | ** | * | * | * | 9 |
2. Risk of bias assessment using the Newcastle‐Ottawa Scale (Case control).
| Study ID | Selection | Comparability | Exposure | Total | |||||
| Adequate case definition |
Representativeness of cases |
Selection of controls |
Definition of controls |
Comparability of cases and controls on design or analysis |
Ascertainment of exposure |
Same method of ascertainment for cases and controls |
Non‐response rate |
||
| Karlen 1998a | * | * | * | * | ** | * | * | * | 9 |
Effects of interventions
See: Table 1
Does surveillance work ‐ direct evidence Karlen 1998a came the closest to providing direct evidence for a survival benefit. Only 2/40 of the patients dying of colorectal cancer had undergone surveillance colonoscopy on at least one occasion compared with 18/102 of the controls. This difference however, did not reach statistical significance (OR 0.25, 95% CI 0.05 to 1.11).
In the Choi 1993 study CRC was detected at a significantly earlier stage in the surveillance group; 15/19 had Duke's A or B carcinoma in the surveillance group compared to 9/22 in the non‐surveillance group (P = 0.039). The 5‐year survival rate was 77.2% for cancers occurring in the surveillance group and 36.3% for the non‐surveillance group (P = 0.026). Four of 19 patients in the surveillance group died from CRC compared to 11 of 22 patients in the non‐surveillance group (OR 0.27, 95% CI 0.07 to 1.06).
In the Lashner 1990 study, there were 14 deaths in the non‐surveillance group compared with six deaths in the surveillance group, but overall there were two more CRC‐related deaths in the surveillance group. Four of 91 patients in the surveillance group died from colorectal cancer compared to 2 of 95 patients in the non‐surveillance group (OR 2.14, 95% CI 0.38 to 11.97). Colectomy was less common in the surveillance group, 33 compared to 51 (P < 0.05) and was performed four years later (after 10 years of disease) in the surveillance group. Although surveillance was associated with improved survival, this improvement was not related to reduced mortality from colorectal cancer.
In the Ananthakrishnan 2014 study 2764 patients underwent a recent colonoscopy and 4059 patients didn't undergo a recent colonoscopy. Out of the 6823 patients, 154 patients developed CRC. A total of 43 (1.6%) patients in the colonoscopy group developed CRC compared to 111 (2.7%) patients in the no‐colonoscopy group (OR 0.56; 95% CI, 0.39 to 0.80). In addition, although there was no information on the cause of death, the study showed that IBD patients who developed CRC but had a recent colonoscopy had a significantly reduced overall mortality. Among the patients who were diagnosed with CRC, 6 of the 43 (14%) patients with CRC who underwent a recent colonoscopy died, compared to 37 of 111 (34%) of patients in the CRC without recent colonoscopy (OR 0.32, 95% CI 0.13 to 0.84).
In the Lutgens 2009 study 1 of 23 patients (4.34%) in the surveillance group died as a result of CRC compared to 29 of 126 (23.01%) patients that died in the control group (OR 0.15, 95% CI 0.02 to 1.18). The overall 5 year survival rate in the surveillance group was 100%, compared to the 74% survival rate in the non‐surveillance group (OR 0.15; 95% CI, 0.02 to 1.18).
Pooled data analysis
Cancer detection:
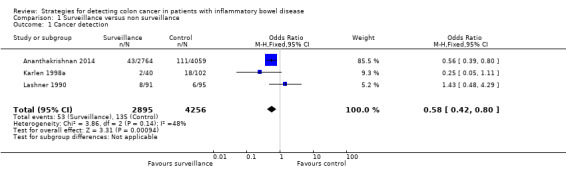
Three studies had available data for rates of cancer detection (Ananthakrishnan 2014; Karlen 1998a; Lashner 1990). A fixed‐effect model was used to estimate the OR. A total of 7151 patients were enrolled in the studies with 2895 patients in the surveillance group and 4256 patients in the non‐surveillance group. Cancer was detected in 53/2895 (1.83%) of patients in the surveillance group compared to 135/4256 (3.17%) of the patients in the non‐surveillance group (OR 0.58, 95% CI 0.42 to 0.80, P = 0.0009).
Death rate:
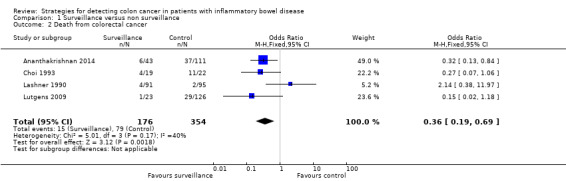
Four studies had available data for the death rate due to CRC (Ananthakrishnan 2014; Choi 1993; Lashner 1990; Lutgens 2009) . A fixed‐effect model was used to estimate the OR. A total of 530 patients were enrolled in the studies with 354 patients in the surveillance group and 176 patients in the non‐surveillance group. Death occurred in 15 of 176 (8.52%) of patients in the surveillance group compared to 79 of 354 (22.31%) of patients in the non‐ surveillance group (OR 0.36, 95% CI 0.19 to 0.69, P = 0.002).
A GRADE analysis indicated that the quality of evidence supporting the outcomes of cancer detection and death rate was very low (See Table 1).
Tumour stage:
Data was pooled from two studies to examine the detection rate of early stage versus late stage CRC (Duke Stages A & B compared to Duke Stages C & D) in patients who underwent surveillance compared to patients who did not undergo surveillance (Choi 1993; Lashner 1990). A significantly higher rate of early stage CRC (Duke A & B) was detected in the surveillance group compared to the non‐surveillance group. Sixteen per cent (17/110) of patients in the surveillance group showed early stage colorectal cancer, compared to 8% (9/117) patients in the non‐surveillance group (OR 5.40, 95% 1.51 to 19.30; P = 0.009). A lower rate of late stage colorectal cancer (Duke C & D) was observed in the surveillance group, compared to the non‐surveillance group. Nine per cent (10/110) of patients in the surveillance group had late stage colorectal cancer compared to 16% (19/117) patients in the non‐surveillance group (OR 0.46, 95% 0.08 to 2.51, P = 0.37). A random‐effects model was used for this analysis due to significant heterogeneity (I2 = 71%). A GRADE analysis indicates that the quality of evidence supporting these outcomes was very low (See Table 1).
Time to cancer detection:
The included studies did not include data on time to cancer detection.
Time to death:
The included studies did not include data on time to death.
Adverse events:
The included studies did not include data on adverse events.
Serious adverse events:
The included studies did not include data on serious adverse events.
Withdrawal due to adverse events:
The included studies did not include data on withdrawals due to adverse events.
Subgroup analysis
Subgroup analyses based on study characteristics and patient characteristics (other than disease stage) were not conducted due to lack of data.
Sensitivity analysis
We used fixed‐effect models to pool most study results due to low heterogeneity. However, using a more conservative random‐effects model the results were similar but not statistically significant for death from colorectal cancer (OR 0.40, 95% CI 0.16 to 1.01; P=0.05) or cancer detection (OR 0.62, 95% CI 0.30 to 1.30; P = 0.21). The random‐effects model for early stage CRC (Duke A & B) still demonstrated statistical significance (OR 5.40, 95% CI 1.52 to 19.17; P = 0.009) .
All studies were full text articles, and were assessed to be at low risk of bias as assessed by the Newcastle‐Ottawa scale. We were unable to ascertain loss to follow‐up.
Discussion
The data regarding the effectiveness of cancer surveillance programs for the early diagnosis of IBD‐associated CRC and for reducing the death rate from CRC are limited by an absence of any randomised controlled trials designed to assess the true impact of therapeutic intervention as a result of colonoscopic surveillance. The theoretical survival benefit of CRC surveillance in IBD is due to an intervention in the dysplastic colon before adenocarcinoma develops, be this through endoscopic therapy or surgical intervention, or to detect cancer at a curative stage. A long‐term, randomised control trial of surveillance to assess survival outcomes in which surveillance is conducted using high quality endoscopy with uniform management of dysplasia including advanced endoscopic resection or surgery compared to a control arm with no surveillance has not been performed. Furthermnore, a randomised trial is unlikely due to ethical considerations. Without data from such a trial, conclusions of survival benefit are limited to evidence from observational studies in which various inferences from data are necessary to comment on effectiveness of surveillance programs.
Despite the accumulation of a large body of additional evidence concerning CRC surveillance in IBD since the previous version of this review (Collins 2006), the use of data from observational studies limits the conclusions that can be drawn rather than providing any evidence that surveillance does not work. Further, the rapid evolution of confounding factors on CRC survival such as the impact of improved medical therapy, advancing endoscopic technology and increasingly sensitive diagnostic methods of detecting dysplasia and CRC make the interpretation of survival benefit derived solely from long‐term data from observational studies difficult.
The included studies in this review provide suggest a survival benefit by demonstrating that CRC tends to be detected at an earlier stage in patients with IBD who are undergoing surveillance, and that these patients have a correspondingly better prognosis (Ananthakrishnan 2014; Choi 1993; Karlen 1998a; Lashner 1990; Lutgens 2009). Further, the Lashner 1990 study reported colectomy rates as indicated for either CRC, dysplasia or active disease in the surveillance group compared to the non‐surveillance group. The odds of colectomy were significantly lower in the surveillance group (OR 0.49, 95% CI 0.27 to 0.88; P = 0.02). This result must be qualified however, in that endoscopic techniques for identifying lesions and techniques to endoscopically excise dysplasia have evolved significantly since this study.
In view of the very low quality evidence for a survival benefit, questions remain as to the best method to survey populations in order to optimise survival and to ensure feasibility of surveillance programs in the context of health care systems with limited resources.
To assess such issues, it is important to understand;
1. The size of the cancer risk in IBD (UC and Crohn’s disease);
2. Risk factors for developing CRC in IBD to enable selection of a high‐yield population to be targeted for screening; and
3. The definition, grades and natural history of dysplasia.
Incidence of IBD‐associated CRC
In the 1920's Crohn and Rosenberg documented a case of rectal carcinoma complicating ulcerative colitis and postulated that the lesion developed as a late sequela of the disease (Crohn 1920). Only 3 years later 17 such cases were reported and carcinoma of the colon and rectum was hypothesised to be a complication of UC (Bargen 1928).
The first meta‐analysis on the incidence of IBD‐associated CRC in 2001 reported a cumulative risk of 2% at 10 years, 8% at 20 years, and 18% at 30 years of disease duration in patients with UC (Eaden 2001a). A more recent meta‐analysis, utilising more homogenous cohorts and stringent study design has downgraded this risk, and reported a standardised incidence ratio (SIR) of 1.7 (95% CI, 1.2 to 2.2) in the overall IBD population based on studies which included 9 population studies with 259,266 person‐years at risk (Lutgens 2013). Individual SIRs for UC and CD were 1.7 (95% CI 1.03 to 2.4) and 1.7 (95% CI 1.01 to 2.5) respectively.
Of interest is a large Swedish cohort study of 7607 patients (198,227 person‐years) with IBD who were diagnosed between 1954 and 1989, which demonstrated a decreased incidence of IBD‐associated CRC over time (Soderlund 2009). The relative risk of incident CRC compared to the general population declined from a five‐fold increase in CRC risk for patients with IBD during the 1960s to a doubled risk of CRC for the follow‐up period between 2000 and 2004 (P = 0.006). Similar or lower risks of CRC have been reported from North American health maintenance organization administrative data set studies and a large Danish population based study (Herrinton 2012; Jess 2012).
Although the data may be skewed by an ageing population in which high‐risk patients were diagnosed earlier in the study periods, it is hypothesised that the decrease in incidence of CRC is due to a combination of enhanced medical therapies which have improved control of active inflammation (particularly the use of biologic agents), the impact of improving efficacy of colonoscopic surveillance and possible chemoprophylaxis protection from 5‐aminosalicylates. Important in the context of considering CRC surveillance target populations is the observation that the CRC risk varies in certain subgroups which may allow risk stratification and populations to target with surveillance.
Risk factors for developing cancer in IBD
To greater understand the risk factors that drive IBD‐associated colitis it is important to recognise the evolutionary process driving tumorigenesis and appreciate the differences in those factors that drive sporadic CRC. The relapsing and remitting nature of IBD results in repeated cycles of epithelial wounding and repair. Such repetitive inflammation result in mutant clones that select for cells, such as those resistant to apoptosis and with accelerated growth, which are better suited to the hostile microenvironment (Choi 2017). This pathogenesis is unique from that responsible for sporadic CRC and thus accounts for differences in IBD‐ associated CRC phenotype such as presentation at an earlier age, a higher prevalence in the proximal colon, more commonly synchronous and an increased frequency of mucinous signet ring cell histology.
Risk factors for sporadic CRC in IBD patients remain the same as those in the general population and include increasing age beyond 50 years, a history of a first‐degree family member with CRC as well as male sex. A familial history of sporadic CRC doubles the risk of CRC when compared to patients with IBD without a familial history (Askling 2001). However, analysis of large cohorts, either nationally, through registries or referral centres has identified certain clinical features of IBD disease as risk factors for IBD‐associated CRC which are consistent with the hypotheses of clonal evolution driven by inflammation. IBD‐associated CRC risk is increased by greater disease extent, duration and severity of colonic inflammation (Beaugeri 2015). Importantly, patients without colonic involvement of disease or those with UC limited to the rectum are not at increased risk of CRC (Ekbom 1990a).
The possibility of a linear correlation of inflammatory burden and CRC risk has been explored (Ullman 2011). One study demonstrated a significant correlation between colonoscopic and histological inflammation scores and the risk of neoplasia, although multivariate analysis revealed that only histologic inflammation score remained a significant risk factor (Rutter 2004b). A further retrospective cohort study of 418 patients undergoing colonoscopic surveillance for UC demonstrated that with every increase in histological inflammation score (based on a 4‐point scale) there was a 3.8‐fold increase in the risk for high‐grade dysplasia or CRC over time (Gupta 2007). Further, a history of pseudopolyps, as an indirect marker of severe inflammation, increased the risk of CRC in UC by 2.5‐fold (95% CI 1.4 to 4.6) (Velayos 2006).
Previous data have suggested patients with UC and PSC have a four‐fold increased risk of CRC compared to those without (Soetikno 2002). However, a more recent population‐based study from the Netherlands involving 590 PSC patients demonstrated a 10‐fold increased risk of CRC in PSC‐IBD patients compared to ulcerative colitis controls. CRC developed at an earlier age (39 years; range 26 to 64) compared to IBD controls (59 years; range 34 to 73; P = 0.019) (Boonstra 2013). For PSC‐IBD patients, cumulative risk of CRC after 10, 20 and 30 years since IBD diagnosis was 1% (95% CI:0 to 15), 6% (95% CI 1 to 22), and 13% (95% CI:2 to 37), respectively. The risk of CRC is increased from the time of diagnosis. This may be explained by observations that patients with UC tend to have disease onset earlier in life, are more likely to have pancolitis and often have quiescent IBD manifestations (Boberg 2011).
Identifying risk factors enables risk stratification which is important for the design of efficacious and economically viable surveillance programs.
Dysplasia
Definition and Grade
Dysplasia, as first defined by Riddell 1983, is an unequivocal neoplastic alteration of the epithelium that remains confined within the basement membrane within which it originated (Riddell 1983). Dysplasia is the best and most reliable marker of an increased risk of malignancy in patients with IBD (Goldman 1996).
The grade of dysplasia is relevant for surveillance programs as it influences the sensitivity and specificity of the presence of future development of CRC. Although dysplasia is generally classified into three distinct morphologic categories; 'indefinite', 'low grade' or 'high grade', dysplasia should be considered to have a scale of evolution that may progress or regress. Such a spectrum of change means that the interpretation of grade of dysplasia is subject to a significant degree of variability, even amongst, specialist and experienced gastrointestinal pathologists (Eaden 2001b; Odze 2002). Interpretation of indefinite for dysplasia has the highest level of inter‐observer variability followed by low grade dysplasia (LGD) (Odze 2002, Eaden 2001b, Riddell 1983). This is likely explained by the difficulty in distinguishing inflammation‐associated regenerative changes from LGD.
Reproducibility and reduced inter‐observer variability is seen in the extremes: negative for dysplasia and high grade dysplasia (HGD). This is somewhat fortuitous in that the recommendations for invasive intervention are strongest for the findings of HGD. Nonetheless, most international guidelines will suggest the use of either a gastro‐intestinal pathologist or a confirmed second pathologist opinion before any invasive intervention (Feakins 2013; Laine 2015; Magro 2013).
With improving endoscopic technology used to detect dysplasia, the macroscopic patterns of dysplasia are becoming increasingly important in stratifying risk of progression to CRC and thus need to be considered in forming management strategies. The nomenclature of lesions has evolved and persistence of some terms has led to some confusion. In part, the increasing ability to visualise lesions endoscopically has made previous labels redundant, in fact most dysplastic lesions should now be visible (Blonski 2008; Rutter 2004b). The SCENIC guidelines have suggested the use of the Paris classification (polypoid – pedunculated, sessile; non‐polypoid – slightly elevated, flat, depressed) to classify the macroscopic appearance of dysplasia (Laine 2015; Shergill 2015). Dysplasia Associated Lesion or Mass (DALM) should no longer be used. When detected, dysplasia should be characterised as 'endoscopically resectable' or 'non‐endoscopically resectable'.
The definition of endoscopically resectable indicates that:
1. Distinct margins of the lesion could be identified;
2. The lesion appears to be completely removed on visual inspection after endoscopic resection;
3. Histologic examination of the resected specimen is consistent with complete removal; and
4. Biopsy specimens taken from mucosa immediately adjacent to the resection site are free of dysplasia on histologic examination (Laine 2015).
Natural History
IBD‐associated CRC is believed to arise from dysplasia. Colonoscopic surveillance in IBD is therefore aimed at detecting dysplasia, allowing removal before the development of CRC. All surveillance programs in medicine are dependent on a sound understanding of the natural history of a disease and identifying a precursor therapeutic window in which surveillance can intervene and halt disease progression. The natural history of dysplasia in IBD, particularly low grade dysplasia is not well understood. Not all dysplasia has an equivalent risk for progression to CRC and not all patients possess the risk factors which drive progression. This undermines the feasibility of a 'one‐size fits all' surveillance program.
The largest meta‐analysis on the natural history of LGD included 20 studies with 508 LGD lesions and found an overall prevalence of LDG of 9.4% (95% CI 1.1 to 51), with a nine fold higher risk of developing CRC once LGD is diagnosed compared with patients with no dysplasia; and a 12‐fold higher risk of developing an advanced lesion (HGD or CRC) (OR 11.9, 95% CI 5.2 to 27) (Thomas 2007). This was updated recently for an annual risk of developing CRC after diagnosis of LGD in colitis of 0.8% (95% CI 0.4 to 1.3%) (Fumery 2017).
In one cohort from St Marks Hospital, London, 172 patients with histologically confirmed extensive UC, who were diagnosed with LGD between 1993 and 2012 were followed up for a median of 48 months from the date of initial LGD diagnosis (Choi 2015). Overall cumulative incidence of HGD or CRC development at 1 and 5 years after initial LGD diagnosis was 10.9% and 19.5% respectively. When adjusted for the number of risk factors, including lesion shape (nonpolypoid the greatest risk followed by invisible and then polypoid), size (> 1 cm greatest risk), preceding dysplasia in the first ten years from the date of initial LGD diagnosis, there was a significant positive correlation between the number of risk factors present and the cumulative risk of developing HGD or CRC. The cumulative incidence of HGD or CRC at 1 and 5 years after initial LGD was 0 to 1.8% for no risk factor (Hazard ratio (HR), 0.3%; standard error (SE) 0.2%), 9.6 and 17.7% for one risk factor (HR 4.9%; SE 1.8%) and 29.0 and 53.4% for two risk factors (HR 13.6; SE 3.3%). For those with three risk factors, cumulative risk of HGD or CRC development was 61.6% and 80.7% at 1 and 2 years respectively.
Although the natural history of dysplasia remains poorly elucidated it is important to differentiate the type of lesion in which the LGD was detected as its malignant potential may vary significantly.
Summary of main results
Five observational studies (N = 7199) met inclusion criteria and were used for analysis. Data from three studies were pooled to assess the rate of CRC detection in the surveillance group compared to the non‐surveillance group. The studies found a significantly higher rate of cancer in the non‐surveillance group compared to the surveillance group. The pooled analysis showed 53/2895 (1.83%) patients in the surveillance group detected positively for cancer, compared to 135/4256 (3.17%) patients in the non‐surveillance group (OR 0.58, 95% CI 0.42 to 0.80; P = 0.0009).
Data from four studies were pooled to assess the rate of death associated with CRC. A significantly lower rate of death associated with CRC was demonstrated in the surveillance group compared to the non‐surveillance group. The pooled analysis showed 15/176 (8.5%) patients in the surveillance group died from CRC compared to 79/354 (22.3%) patients in the non‐surveillance group (OR 0.36, 95% CI 0.19 to 0.69, P = 0.002).
Lastly, data was pooled from two studies to examine the rate of detection of early stage versus late stage colorectal cancer (Duke Stages A & B compared to Duke Stages C & D) in patients who underwent surveillance compared to patients who didn't undergo surveillance. The data shows a significantly higher rate of early stage colorectal cancer (Duke A & B) detected in the surveillance group compared to the non‐surveillance group). Sixteen per cent (17/110) of patients in the surveillance group were detected with early stage CRC compared to 8% (9/117) patients in the non‐surveillance group (OR 5.40, 95% CI 1.52 to 19.17; P = 0.009). The data showed a higher rate of late stage CRC (Duke C & D) in the non‐surveillance group compared to the surveillance group. Nine per cent (10/110) of patients in the surveillance group had late stage CRC compared to 16% (19/117) patients in the non‐surveillance group (OR 0.46, 95% CI 0.08 to 2.51; P = 0.37).
Overall completeness and applicability of evidence
The results of this review are applicable for patients with a diagnosis of ulcerative colitis or colonic Crohn's disease. Very low quality evidence suggests that surveillance may be effective for detection of early stage CRC as well as reducing death rates from CRC.
Quality of the evidence
GRADE analysis indicated that the overall quality of the evidence supporting the outcomes of CRC detection and death from CRC are of very low quality due to the nature of observational studies and imprecision. The GRADE analysis also indicated that the overall quality of the evidence supporting the subgroup analyses on CRC stage (i.e. Duke A or B and Duke C or D) was very low.
Potential biases in the review process
To reduce the amount of potential biases during the review process we had two authors independently screen the results and extract the data. We also performed an exhaustive literature search in an attempt to identify all applicable studies. We did not identify any RCTs, thus the main limitation of this review was the inclusion of observational studies (cohort and case control). Due to the design of observational studies, this resulted in an increased risk of bias and the very low quality of evidence presented in the GRADE analysis. It should be noted, however, that ethical considerations would not allow for RCTs of this intervention.
Agreements and disagreements with other studies or reviews
The results of our review agree with other published reviews on effectiveness of cancer surveillance programs for the early diagnosis of IBD‐associated CRC and in reducing the death rate from CRC in patients with ulcerative colitis and colonic Crohn's disease (Choi 1993; Eaden 2000; Karlen 1998a; Lutgens 2009; Nugent 1991). Our review adds further low quality evidence that surveillance is likely to be effective at reducing the risk of death from IBD‐associated colorectal cancer.
Authors' conclusions
Implications for practice.
These data suggest that the ongoing use of colonoscopy based surveillance in IBD may reduce both CRC development and CRC‐associated death through early detection, although the quality of the evidence is very low. The detection of earlier stage CRC in the surveillance group may explain some of the survival benefit observed.
Implications for research.
It is very unlikely that anyone will now undertake (or give ethical permission for) a prospective randomised study comparing colonoscopic surveillance with no surveillance in patients with longstanding colitis. There will probably therefore never be clear evidence for a survival advantage from a prospective RCT. Even evidence from retrospective case control studies is becoming very difficult to obtain, since it would require sampling a very large population of patients who had not undergone surveillance, although this may be possible from large administrative databases. Nevertheless, comparative studies that use white light surveillance versus advanced endoscopic imaging (e.g. chromo endoscopy), or that prescribe differing surveillance schedules (e.g. risk stratified versus fixed strategies) should be performed. Very large studies will be needed if cancer or survival outcomes are to be investigated.
Future research could focus on less invasive tests such as faecal DNA analysis or rectal mucosal FISH analysis of chromosomal instability to identify patients at high risk for development of CRC. Other possibilities for less invasive tests include aneuploidy, mutations in p53 and KRAS, methylation status, microbiome and glycosylation abnormalities (Kisiel 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 23 September 2017 | Amended | Correction of minor error in Figure 1 |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 19 September 2016 | New search has been performed | New literature search performed on 19 September 2017. Two new studies were added |
| 19 September 2016 | New citation required and conclusions have changed | Updated review with changes to conclusions and new authors |
Acknowledgements
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
Dr. James East was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. Search strategies
EMBASE
1. Exp Inflammatory bowel disease/
2. Crohn*.mp.
3. Ulcerative colitis*.mp
4. IBD.mp.
5. Inflammatory bowel disease*.mp.
6. Or/1‐5
7. Colon.mp.
8. Colorectal.mp.
9. Rectal.mp.
10. Or/7‐9
11. Cancer*.mp.
12. Neoplas*.mp.
13. Dysplasia.mp.
14. Or/11‐13
15. Detect*.mp.
16. Screen*.mp.
17. Diagnos*.mp.
18. Assess*.mp.
19. Surveillance.mp.
20. Or/15‐19
21. 6 and 10 and 14 and 20
MEDLINE
1. Exp Inflammatory bowel disease/
2. Crohn*.mp.
3. Ulcerative colitis*.mp
4. IBD.mp.
5. Inflammatory bowel disease*.mp.
6. Or/1‐5
7. Colon.mp.
8. Colorectal.mp.
9. Rectal.mp.
10. Or/7‐9
11. Cancer*.mp.
12. Neoplas*.mp.
13. Dysplasia.mp.
14. Or/11‐13
15. Detect*.mp.
16. Screen*.mp.
17. Diagnos*.mp.
18. Assess*.mp.
19. Surveillance.mp.
20. Or/15‐19
21. 6 and 10 and 14 and 20
Cochrane CENTRAL
#1 MeSH: [Inflammatory bowel disease] explode all trees
#2 Crohn
#3 Ulcerative colitis
#4 IBD
#5 #1 or #2 or #3 or #4
#6 colon cancer
#7 colorectal cancer
#8 rectal cancer
#9 dysplasia
#10 #6 or #7 or #8 or #9
#11 Detection
#12 Screen
#13 Diagnose
#14 assess
#15 surveillance
#16 #11 or #12 or #13 or #14 or #15
#17 #5 and #10 and #16
Data and analyses
Comparison 1. Surveillance versus non surveillance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cancer detection | 3 | 7151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.80] |
| 2 Death from colorectal cancer | 4 | 530 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.69] |
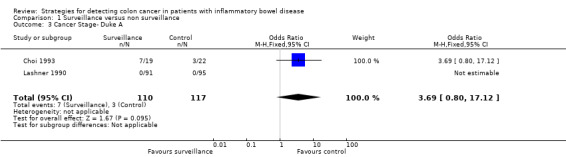
| 3 Cancer Stage‐ Duke A | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.80, 17.12] |
| 4 Cancer Stage‐ Duke B | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.73, 7.73] |
| 5 Cancer stage‐ Duke C | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 0.98] |
| 6 Cancer stage‐ Duke D | 1 | 186 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.25, 4.31] |
| 7 Cancer stage‐ Duke A or B | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.40 [1.51, 19.30] |
| 8 Cancer stage‐ Duke C or D | 2 | 227 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.51] |
| 9 Colectomy | 1 | 186 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.88] |
1.1. Analysis.
Comparison 1 Surveillance versus non surveillance, Outcome 1 Cancer detection.
1.2. Analysis.
Comparison 1 Surveillance versus non surveillance, Outcome 2 Death from colorectal cancer.
1.3. Analysis.
Comparison 1 Surveillance versus non surveillance, Outcome 3 Cancer Stage‐ Duke A.
1.4. Analysis.
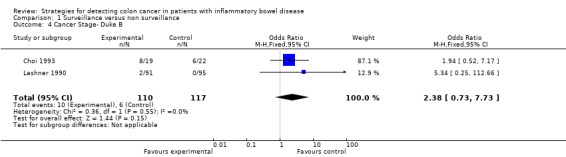
Comparison 1 Surveillance versus non surveillance, Outcome 4 Cancer Stage‐ Duke B.
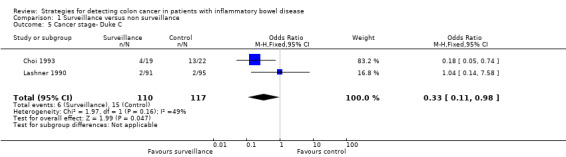
1.5. Analysis.
Comparison 1 Surveillance versus non surveillance, Outcome 5 Cancer stage‐ Duke C.
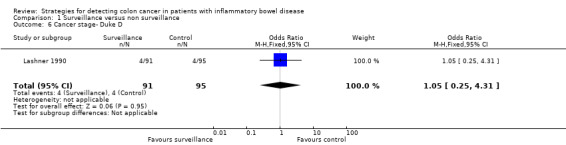
1.6. Analysis.
Comparison 1 Surveillance versus non surveillance, Outcome 6 Cancer stage‐ Duke D.
1.7. Analysis.
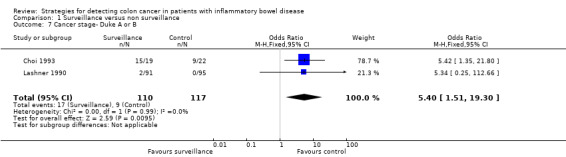
Comparison 1 Surveillance versus non surveillance, Outcome 7 Cancer stage‐ Duke A or B.
1.8. Analysis.
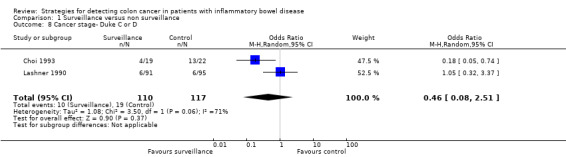
Comparison 1 Surveillance versus non surveillance, Outcome 8 Cancer stage‐ Duke C or D.
1.9. Analysis.
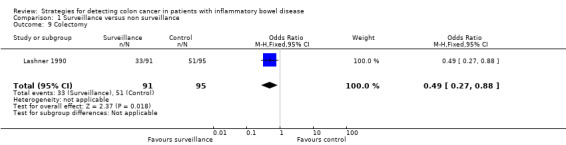
Comparison 1 Surveillance versus non surveillance, Outcome 9 Colectomy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ananthakrishnan 2014.
| Methods | Retrospective population based cohort study | |
| Participants | 6823 patients were followed 2754 patients had undergone colonoscopy within the last 36 months and 4059 patients had not undergone colonoscopy within the last 36 months |
|
| Interventions | Colonoscopic surveillance within 36 months versus no colonoscopic surveillance within 36 months | |
| Outcomes | Primary outcome: Diagnosis of CRC determined by diagnosis codes for colon or rectal cancer | |
| Notes | Attempts to control for bias: 1. Satistical analysis: (a) The Mann‐Whitney test was used for non‐parametric comparisons (b) Examined the association between colonoscopy and risk of CRC by stratifying the cohort according to sex, type of IBD, and a diagnosis of PSC 2. The characteristics between the recent surveillance and non‐recent surveillance groups were compared. Patients with a recent colonoscopy were more likely to be younger, had a slightly longer duration of follow‐up evaluation, and were less likely to be women or have a diagnosis of UC. There was no difference in racial distribution between the two groups |
|
Choi 1993.
| Methods | Prospective cohort study | |
| Participants | 41 ulcerative colitis patients who developed colorectal cancer 19 patients had been undergoing colonoscopic surveillance and 22 had not Patients with a duration of disease of 8 years or more with extension of the disease proximal to the sigmoid colon were enrolled in the prospective surveillance program All patients with high grade dysplasia, a dysplasia‐associated lesion or mass, or carcinoma were advised to undergo colectomy |
|
| Interventions | Colonoscopic surveillance versus no surveillance | |
| Outcomes | 1. Duke's stage 2. 5‐year survival rate 3. death | |
| Notes | Attempts to control for bias: 1. statistical analysis: (a) survival distributions were calculated by the product‐limit method of Kaplan and Meier (b) statistical significance of differences between distributions was analysed by the Tarone‐Ware method. (c) differences in age distribution at the time of onset of ulcerative colitis and the diagnosis of carcinoma, and duration of ulcerative colitis before development of carcinoma were analysed using the Mann‐Whitney test 2. Surveillance was defined as colonoscopic biopsy study performed with an intent to screen for neoplasia based on long duration of disease without any concomitant symptoms or signs to suggest neoplasia before the procedure | |
Karlen 1998a.
| Methods | Case control study | |
| Participants | 142 patients with ulcerative colitis of at least 5 years duration 40 patients who died from colorectal cancer were compared to 102 control patients matched for age, sex, extent and duration of ulcerative colitis | |
| Interventions | None | |
| Outcomes | Exposure to at least one surveillance colonoscopy | |
| Notes | Attempts to control for bias:
1. Control group was matched for age, sex, extent and duration of disease. Controls had to be alive at the time of death of the patient and to have some part of their colon intact five years prior to the diagnosis of the cancer of the patient
2. Statistical analyses: the relationship between colonoscopic surveillance and colorectal cancer mortality was analysed by the relative risk obtained by the odds ratio Matched analyses were performed using conditional logistic regression analyses The estimated standard deviations of the regression coefficient estimates were used to calculate 95% confidence limits. 3. Only colonoscopies performed with the intention of cancer surveillance were included, index colonoscopies and those being made due to clinical symptoms or signs were excluded Confounders not controlled for: 1. drug treatment (e.g. SASP treatment has been shown to reduce the risk of colorectal cancer) |
|
Lashner 1990.
| Methods | Retrospective cohort study | |
| Participants | 186 patients with extensive ulcerative colitis of at least 8 years duration 91 patients have been undergoing surveillance and 95 had not Total colectomy was advised when cancer, high grade dysplasia or low grade dysplasia associated with a mass was found |
|
| Interventions | Colonoscopic surveillance versus no surveillance | |
| Outcomes | 1. Survival 2. Death 3. Cancer detection 4. Colectomy rates | |
| Notes | Attempts to control for bias:
1. Statistical analysis: crude analyses were performed using Kaplan‐Meier product‐limit survival curves and curves were compared with the log rank test Differences in entry variables between the two groups were adjusted to remove confounding effects (age at symptom onset, sex, and duration of disease) using a Cox proportional hazards model 2. The cancer surveillance program recommended yearly colonoscopy with biopsy Patients were excluded if they were referred with cancer, if cancer was found at first evaluation or if no follow‐up information was obtained Controls were excluded if cancer was found on initial referral |
|
Lutgens 2009.
| Methods | Cohort study | |
| Participants | 149 patients with IBD‐associated CRC were identified Patients were assigned to the surveillance group when they had undergone one or more surveillance colonoscopies before a diagnosis of CRC Patients who had not undergone surveillance served as controls |
|
| Interventions | Colonoscopic surveillance versus no surveillance | |
| Outcomes | 1. CRC related 2. Tumour stage 3. Death |
|
| Notes | Attempts to control for bias: 1. Statistical analysis: (a) Patient characteristics using the X2 test, Fischer's exact test and Student's t‐test compared the patient characteristics between the two groups (b) Kaplan‐Meier and cox regression analyses were used for survival calculations |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbar 2015 | No control group |
| Arthurs 2012 | No control group |
| Basseri 2012 | No control group |
| Biasco 2002 | No control group |
| Bopanna 2016 | No control group |
| Brostrom 1986 | No control group |
| Carballal 2014 | No control group |
| Choi 2015 | No control group |
| Eaden 2000 | Not designed to assess colonoscopic surveillance |
| Friedman 2001 | No control group |
| Gonzalez 2016 | No control group |
| Gunther 2011 | No control group |
| Hata 2003 | No control group |
| Hernandez 2013 | No control group |
| Higashi 2011 | No control group |
| Hiroyuki 2014 | No control group |
| Jonsson 1994 | No control group |
| Lindberg 2005 | No control group |
| Lofberg 1990 | No control group |
| Lynch 1993 | No control group |
| Manninen 2013 | No control group |
| Matsuoka 2013 | No control group |
| Monzur 2013 | No control group |
| Mooiweer 2015 | No control group |
| Nugent 1991 | No control group |
| Panara 2016 | No control group |
| Riegler 2003 | No control group |
| Rodino 2011 | No control group |
| Rosenstock 1985 | No control group |
| Rutegard 2016 | No control group |
| Rutter 2006 | No control group |
| Saoula 2013 | No control group |
| Stolwijk 2013 | No control group |
| Velayos 2006 | No control group |
Differences between protocol and review
The differences between the protocol and review include:
Title and primary outcome: The title of the review was changed from "Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease" to "Strategies for detecting colon cancer in patients with inflammatory bowel disease". The primary outcome In addition, dysplasia detection was removed as an outcome of interest.
Primary and secondary outcomes: The primary and secondary outcomes were not well defined in the protocol. The primary outcome in this review is the comparative rates of diagnosis of colorectal cancer between the surveillance and non‐surveillance group. The secondary outcomes in this review are:
a) The proportion of patients who died from colorectal cancer with or without colonoscopy surveillance
b)The time to cancer detection
c)The time to death
d) The proportion of patients with adverse events
e) The proportion of patients with serious adverse events
d) The proportion of patients who withdrew due to adverse events
The primary and secondary outcomes of this review are similar to those of the previously published version of this review.
Risk of bias: The Ottawa‐Castle Scale was used to assess the studies instead of the Cochrane risk of bias tool, due to the lack of RCTs.
Contributions of authors
Dr. Bye and Tran Nguyen wrote the initial draft that was then extensively edited by Dr. East and Dr. Jairath. All authors approved the final draft.
Declarations of interest
William Bye‐No known conflicts of interest
Tran Nguyen‐ No known conflicts of interest
James East‐ Clinical advisory boards: Lumendi, Boston Scientific.
Vipul Jairath‐ has received scientific advisory board fees from AbbVie, Sandoz, Takeda, Janssen; speaker’s fees from Takeda, Janssen, Shire, Ferring
Claire Parker‐ No known conflicts of interest
Edited (no change to conclusions)
References
References to studies included in this review
Ananthakrishnan 2014 {published data only}
- Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clinical Gastroenterology & Hepatology 2015;13(2):322‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, et al. Colonoscopy is associated with a reduced risk of colon cancer and morality in patients with inflammatory bowel diseases. American Journal of Gastroenterology 2014;109:S639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 1993 {published data only}
- Choi PM, Nugent FW, Schoetz DJ Jr, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology 1993;105:418‐24. [DOI] [PubMed] [Google Scholar]
Karlen 1998a {published data only}
- Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut 1998;42:711‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lashner 1990 {published data only}
- Lashner BA, Kane SV, Hanauer SB. Colon cancer surveillance in chronic ulcerative colitis: Historical cohort study. American Journal of Gastroenterology 1990;85(9):1083‐7. [PubMed] [Google Scholar]
Lutgens 2009 {published data only}
- Lutgens MW, Oldenburg B, Siersema PD, Bodegraven AA, Dijkstra G, Hommes DW, et al. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. British Journal of Cancer 2009;101(10):1671‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens MW, Vleggaar FP, Schipper ME, Stokkers PC, Woude CJ, Hommes DW, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut 2008;57(9):1246‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akbar 2015 {published data only}
- Akbar T, Rahmany S, Harris R, Cotton S, McCabe LA, Lloyd DA, et al. Colorectal cancer in inflammatory bowel disease‐is surveillance working?. United European Gastroenterology Journal 2015;1:A626. [Google Scholar]
Arthurs 2012 {published data only}
- Arthurs E, Burley K, Gholkar B, Williams L, Lockett M. Colorectal cancer surveillance in inflammatory bowel disease: A retrospective audit of primary and secondary care. Journal of Crohn's and Colitis 2012;6:S139. [Google Scholar]
Basseri 2012 {published data only}
- Basseri RJ, Basseri B, Vassilaki ME, Melmed GY, Ippoliti A, Vasiliauskas EA, et al. Colorectal cancer screening and surveillance in Crohn's colitis. Journal of Crohn's and Colitis 2012;6(8):824‐9. [DOI] [PubMed] [Google Scholar]
Biasco 2002 {published data only}
- Biasco G, Rossini FP, Hakim R, Brandi G, Battista M, Febo G, et al. Cancer surveillance in ulcerative colitis: critical analysis of long‐term prospective programme. Digestive and Liver Disease 2002;34(5):339‐42. [DOI] [PubMed] [Google Scholar]
Bopanna 2016 {published data only}
- Bopanna S, Das P, Dattagupta S, Pratap Mouli V, Kedia S, Dhingra R, et al. High incidence of ulcerative colitis related colorectal cancer in a low incidence area of sporadic colon cancer. Gastroenterology 2016;1:S765. [Google Scholar]
Brostrom 1986 {published data only}
- Brostrom O, Lofberg R, Ost A, Reichard H. Cancer surveillance of patients with longstanding ulcerative colitis: A clinical, endoscopical, and histological study. Gut 1986;27(12):1408‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carballal 2014 {published data only}
- Carballal S, Lopez‐Ceron M, Ricart E, Miguel CR, Cuatrecasas M, Romero I, et al. Results of a chromoendoscopy‐based surveillance program for long‐standing colonic inflammatory bowel disease. Gastrointestinal Endoscopy 2014;1:AB464. [Google Scholar]
Choi 2015 {published data only}
- Choi CH, Rutter M, Askari A, Lee GH, Warusavitarne J, Moorghen M, et al. Forty‐year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: An updated overview. American Journal of Gastroenterology 2015;110:1022‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eaden 2000 {published data only}
- Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case‐control study. Alimentary Pharmacology & Therapeutics 2000;14:145‐53. [DOI] [PubMed] [Google Scholar]
Friedman 2001 {published data only}
- Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology 2001;120:820‐6. [DOI] [PubMed] [Google Scholar]
Gonzalez 2016 {published data only}
- Gonzalez R, Pereyra L, Gomez EJ, Mella JM, Omodeno M, Panigadi N, et al. Neoplasia Detection During Colonoscopic Surveillance of Patients With Inflammatory Bowel Disease: Does the Endoscopist's and Endoscopie's Characteristics Matter?. Gastrointestinal Endoscopy 2016;1:AB291. [Google Scholar]
Gunther 2011 {published data only}
- Gunther U, Kusch D, Heller F, Burgel N, Leonhardt S, Daum S, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: Comparison of random biopsy vs. targeted biopsy protocols. International Journal of Colorectal Disease 2011;26(5):667‐72. [DOI] [PubMed] [Google Scholar]
Hata 2003 {published data only}
- Hata K, Watanabe T, Kazama S, Suzuki K, Shinozaki M, Yokoyama T, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23‐year surveillance programme in the Japanese population. British Journal of Cancer 2003;89(7):1232‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2013 {published data only}
- Hernandez N, Penate M, Saiz P, Monescillo A, Jimenez E, Sanchez MA, et al. Dysplasia and colorectal cancer in surveillance colonoscopies of ulcerative colitis in a single center: Retrospective study. Journal of Crohn's and Colitis 2013;7:S112. [Google Scholar]
Higashi 2011 {published data only}
- Higashi D, Futami K, Ishibashi Y, Egawa Y, Maekawa T, Matsui T, et al. Clinical course of colorectal cancer in patients with ulcerative colitis. Anticancer Research 2011;31(7):2499‐504. [PubMed] [Google Scholar]
Hiroyuki 2014 {published data only}
- Hiroyuki A, Shinsuke K, Takashi N, Toshiaki T, Junichiro T, Tomomichi K, et al. Effectiveness of 34‐year surveillance colonoscopy program for long‐standing ulcerative colitis in a single institution. Inflammatory Bowel Diseases 2014;20:S42. [Google Scholar]
Jonsson 1994 {published data only}
- Jonsson B, Ahsgren L, Andersson LO, Stenling R, Rutegard J. Colorectal cancer surveillance in patients with ulcerative colitis. British Journal of Surgery 1994;81:689‐91. [DOI] [PubMed] [Google Scholar]
Lindberg 2005 {published data only}
- Lindberg B, Persson B, Veress B, Ingelman‐Sundberg H, Granqvist S. Efficiency of colorectal cancer surveillance in patients with ulcerative colitis: 26 Years' experience in a patient cohort from a defined population area. Scandinavian Journal of Gastroenterology 2005;40(9):1076‐80. [DOI] [PubMed] [Google Scholar]
Lofberg 1990 {published data only}
- Lofberg R, Brostrom O, Karlen P, Tribukait B, Ost A. Colonoscopic surveillance in long‐standing total ulcerative colitis ‐ a 15‐year follow‐up study. Gastroenterology 1990;99:1021‐31. [DOI] [PubMed] [Google Scholar]
Lynch 1993 {published data only}
- Lynch D, Lobo AJ, Sobala GM, Dixon MF, Axon AT. Failure of colonoscopic surveillance in ulcerative colitis. Gut 1993;34(8):1075‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manninen 2013 {published data only}
- Manninen P, Karvonen AL, Huhtala H, Aitola P, Hyoty M, Nieminen I, et al. The risk of colorectal cancer in patients with inflammatory bowel disease in Finland: A follow‐up of 20 years. Journal of Crohn's and Colitis 2013;7:551‐7. [DOI] [PubMed] [Google Scholar]
Matsuoka 2013 {published data only}
- Matsuoka H, Ikeuchi H, Uchino M, Bando T, Takesue Y, Nishigami T, et al. Clinicopathological features of ulcerative colitis‐associated colorectal cancer pointing to efficiency of surveillance colonoscopy in a large retrospective Japanese cohort. International Journal of Colorectal Disease 2013;28:829‐34. [DOI] [PubMed] [Google Scholar]
Monzur 2013 {published data only}
- Monzur F, Jennings J, Mattar M, Charabaty A. IBD‐associated CRC: Are we screening often enough?. American Journal of Gastroenterology 2013;108:S501‐2. [Google Scholar]
Mooiweer 2015 {published data only}
- Mooiweer E, Meulen AE, Woude CJ. Incidence of interval colorectal cancer among inflammatory bowel disease patients enrolled in a colonoscopic surveillance program. Gastroenterology 2014;1:S632. [DOI] [PubMed] [Google Scholar]
Nugent 1991 {published data only}
- Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology 1991;100:1241‐8. [PubMed] [Google Scholar]
Panara 2016 {published data only}
- Panara AJ, Zhang HC, Waljee AK, Gaidos J, Feagins LA, Govani SM, et al. Presence of colonic dysplasia prior to colorectal cancer diagnosis is associated with early stage cancer among patients with inflammatory bowel disease: A national cohort study. Gastroenterology 2016;1:S561. [Google Scholar]
Riegler 2003 {published data only}
- Riegler G, Bossa F, Caserta L, Pera A, Tonelli F, Sturniolo GC, et al. Colorectal cancer and high grade dysplasia complicating ulcerative colitis in Italy: A retrospective co‐operative IG‐IBD study. Digestive and Liver Disease 2003;35:628‐34. [DOI] [PubMed] [Google Scholar]
Rodino 2011 {published data only}
- Rodino S, D'Amico T, Sebkova L, Sacca N. Fifteen years colonoscopy surveillance for colorectal cancer in patients with inflammatory bowel disease: Efficacy of endoscopic polypectomy. Gastrointestinal Endoscopy 2011;1:AB439. [Google Scholar]
Rosenstock 1985 {published data only}
- Rosenstock E, Farmer RG, Petras R, Sivak MV Jr, Rankin GB, Sullivan BH. Surveillance for colonic carcinoma in ulcerative colitis. Gastroenterology 1985;89:1342‐6. [DOI] [PubMed] [Google Scholar]
Rutegard 2016 {published data only}
- Rutegard M, Palmqvist R, Stenling R, Lindberg J, Rutegard J. Efficiency of Colorectal Cancer Surveillance in Patients With Ulcerative Colitis: 38 Years' Experience in a Patient Cohort From a Defined Population Area. Scandinavian Journal of Surgery 2016;18:18. [DOI] [PubMed] [Google Scholar]
Rutter 2006 {published data only}
- Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, et al. Thirty‐Year Analysis of a Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis. Gastroenterology 2006;130(4):1030‐8. [DOI] [PubMed] [Google Scholar]
Saoula 2013 {published data only}
- Saoula H, Boutaleb AF, Aissaoui M, Mahiou H, Salah A, Zmiri Y, et al. Screening for dysplasia and colorectal cancer in ulcerative colitis. United European Gastroenterology Journal 2013:A211. [Google Scholar]
Stolwijk 2013 {published data only}
- Stolwijk JAM, Langers AMJ, Hardwick JC, Veenendaal RA, Verspaget HW, Hogezand RA, et al. A thirty‐year follow‐up surveillance study for neoplasia of a Dutch ulcerative colitis cohort. Scientific World Journal 2013:Article Number: 274715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velayos 2006 {published data only}
- Velayos F, Loftus E, Jess T, Harmsen S, Bida J, Zinsmeister A, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case‐control study. Gastroenterology 2006;130:1941‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Askling 2001
- Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 2001;120:1356‐62. [DOI] [PubMed] [Google Scholar]
Baars 2012
- Baars JE, Kuipers EJ, Haastert M, Nicolai JJ, Poen AC, Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long‐term survey. Journal of Gastroenterology 2012;47:1308‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansal 1996
- Bansal P, Sonnenburg A. Risk factors of colorectal cancer in inflammatory bowel disease. American Journal of Gastroenterology 1996;91:44‐8. [PubMed] [Google Scholar]
Bargen 1928
- Bargen JA. Chronic ulcerative colitis associated with malignant disease. Archives of Surgery 1928;17(4):561‐76. [DOI] [PubMed] [Google Scholar]
Beaugeri 2015
- Beaugeri L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. New England Journal of Medicine 2015;372(15):1441‐52. [DOI] [PubMed] [Google Scholar]
Blonski 2008
- Blonksi W, Kundu R, Lewis J, Aberra F, Osterman M, Lichtenstein GR. Is dysplasia visible during surveillance colonoscopy in patients with ulcerative colitis?. Scandinavian Journal of Gastroenterology 2008;43:698‐703. [DOI] [PubMed] [Google Scholar]
Boberg 2011
- Boberg KM, Lind GE. Primary sclerosing cholangitis and malignancy. Best Practice & Research. Clinical Gastroenterology 2011;25(6):753‐64. [DOI] [PubMed] [Google Scholar]
Boonstra 2013
- Boonstra K, Weersma RK, Erpecum KJ, Rauws EA, Spanier BW, Poen AC, et al. Population‐Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis. Hepatology 2013;58(6):2045‐55. [DOI] [PubMed] [Google Scholar]
Choi 2017
- Choi CR, Al BakirI, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nature Reviews Gastroenterology and Hepatology 2017;14(4):218‐29. [DOI] [PubMed] [Google Scholar]
Crohn 1920
- Crohn BB, Rosenburg H. The sigmoidoscopic picture of chronic ulcerative colitis (non‐specific). Amercian Journal of Medical Science 1920;170:220‐8. [Google Scholar]
Crohn 1925
- Crohn BB, Rosenburg H. The sigmoidoscopic picture of chronic ulcerative colitis (non‐specific). American Journal of the Medical Sciences 1925;170:220. [Google Scholar]
Eaden 2001a
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta‐analysis. Gut 2001;48:526‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eaden 2001b
- Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter‐observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. Journal of Pathology 2001;194:152‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekbom 1990a
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population‐based study. New England Journal of Medicine 1990;323:1228‐33. [DOI] [PubMed] [Google Scholar]
Feakins 2013
- Feakins RM. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. Journal of Clinical Pathology 2013;66(12):1005‐26. [DOI] [PubMed] [Google Scholar]
Fumery 2017
- Fumery M, Dulai PS, Gupta S, Prokop LJ, Ramamoorthy S, Sandborn WJ. Incidence, Risk Factors, and Outcomes of Colorectal Cancer in Patients with Ulcerative Colitis with Low‐Grade Dysplasia: A systematic Review and Meta‐analysis. Clinical Gastroenterology and Hepatology 2017;15(5):665‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 1996
- Goldman H. Significance and detection of dysplasia in chronic colitis. Cancer 1996;789:2261‐3. [DOI] [PubMed] [Google Scholar]
Gupta 2007
- Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007;133(4):1099‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2006
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases 2006;12(5):S3‐9. [DOI] [PubMed] [Google Scholar]
Herrinton 2012
- Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012;143(2):382‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.. [Google Scholar]
Itzkowitz 2004
- Itzkowitz SH, Steven H, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology. Gastrointestinal and Liver Physiology 2004;287(1):7‐17. [DOI] [PubMed] [Google Scholar]
Jess 2012
- Jess T, Rungoe C, Peyrin‐Biroulet L. Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta‐analysis of Population‐Based Cohort Studies. Clinical Gastroenterology and Hepatology 2012;10:639‐45. [DOI] [PubMed] [Google Scholar]
Kaplan 2015
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology 2015;12:720‐27. [DOI] [PubMed] [Google Scholar]
Kisiel 2013
- Kisiel JB, Ahlquist DA. Stool DNA testing for cancer surveillance in inflammatory bowel disease: an early view. Therapeutic Advances in Gastroenterology 2013;6(5):371‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laine 2015
- Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148(3):639‐51. [DOI] [PubMed] [Google Scholar]
Lashner 1991
- Lashner BA. Colon cancer surveillance in ulcerative colitis. Seminars in Gastrointestinal Disease 1991;2:126‐31. [Google Scholar]
Lutgens 2013
- Lutgens MW, Oijen MG, Heijden GJ, Vleggar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta‐analysis of population‐based cohort studies. Inflammatory Bowel Diseases 2013;19(4):789‐99. [DOI] [PubMed] [Google Scholar]
Magro 2013
- Magro R, Langer C, Driessen A, Ensari K, Geboes K, Mantzaris GJ, et al. European consensus on the histopathology of inflammatory bowel disease. Journal of Crohns and Colitis 2013;7(10):827‐51. [DOI] [PubMed] [Google Scholar]
Odze 2002
- Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Inter‐observer variability in the diagnosis of ulcerative colitis‐associated dysplasia by telepathology. Modern Pathology 2002;15:379‐86. [DOI] [PubMed] [Google Scholar]
Riddell 1983
- Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical application. Human Pathology 1983;14:931‐68. [DOI] [PubMed] [Google Scholar]
Rutter 2004b
- Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointestinal Endoscopy 2004;60(3):334‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from¬www.cochrane‐handbook.org, 2011. [Google Scholar]
Shergill 2015
- Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, et al. The role of endoscopy in inflammatory bowel disease. Gastrointestinal Endoscopy 2015;81(5):1101‐21. [DOI] [PubMed] [Google Scholar]
Soderlund 2009
- Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, et al. Decreasing time‐trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 2009;136:1561‐7. [DOI] [PubMed] [Google Scholar]
Soetikno 2002
- Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta‐analysis. Gastrointestinal Endoscopy 2002;56(1):48‐54. [DOI] [PubMed] [Google Scholar]
Thomas 2007
- Thomas T, Abrams KA, Robinson RJ, Mayberry JF. Meta‐analysis: cancer risk of low‐grade dysplasia in chronic ulcerative colitis. Alimentary Pharmacology & Therapeutics 2007;25(6):657‐68. [DOI] [PubMed] [Google Scholar]
Ullman 2011
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807‐16. [DOI] [PubMed] [Google Scholar]
Wells 2017
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 31 Aug 2017).
References to other published versions of this review
Collins 2006
- Collins P, Mpofu C, Watson A, Rhodes J. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000279.pub3] [DOI] [PubMed] [Google Scholar]


