Abstract
Background
Post‐thrombotic syndrome (PTS) is a long‐term complication of deep vein thrombosis (DVT) that is characterised by chronic pain, swelling, and skin changes in the affected limb. One of every three people with DVT will develop post‐thrombotic complications within five years. Several non‐pharmaceutical measures are used for prevention of post‐thrombotic syndrome during the acute phase of DVT. These include elevation of the legs and compression therapy. Clinicians and guidelines differ in their assessment of the utility of compression therapy for treatment of DVT. This is an update of a review first published in 2003.
Objectives
To determine relative effectiveness and rate of complications when compression therapy is used in people with deep vein thrombosis (DVT) for prevention of post‐thrombotic syndrome (PTS).
Search methods
For this update, the Cochrane Vascular Information Specialist (CIS) searched the Cochrane Vascular Specialised Register (20 March 2017) and CENTRAL (2017, Issue 2). The CIS also searched trial registries for details of ongoing or unpublished studies.
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) of compression therapy, such as bandaging and elastic stockings, in people with clinically confirmed DVT. The primary outcome was the occurrence of PTS.
Data collection and analysis
Two review authors (DK and EvL) identified and assessed titles and abstracts for relevance, and a third review author (DA) verified this assessment independently. Review authors imposed no restrictions on date or language of publications. Three review authors (DA, DK, EvL) used data extraction sheets to independently extract study data. We resolved disagreements by discussion.
Main results
We identified 10 RCTs with a total of 2361 participants that evaluated compression therapy. The overall methodological quality of these trials was low. We used only five studies in meta‐analysis owing to differences in intervention types and lack of data. Three studies compared elastic compression stockings (pressure of 30 to 40 mmHg at the ankle) versus no intervention. Two studies compared elastic compression stockings (pressure 20 to 40 mmHg) versus placebo stockings. Overall, use of elastic compression stockings led to a clinically significant reduction in the incidence of PTS (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.38 to 1.01; P = 0.05; 1393 participants; 5 studies; low‐quality evidence); no reduction in the incidence of severe PTS (RR 0.78, 95% CI 0.53 to 1.15; P = 0.21; 1224 participants; 4 studies; low‐quality evidence); and no clear difference in DVT recurrence (RR 0.94, 95% CI 0.69 to 1.28; 1212 participants; 4 studies; P = 0.69; low‐quality evidence). We did not pool data on the incidence of pulmonary embolism because this information was poorly reported, but we observed no differences between groups included in individual studies (low‐quality evidence).
Two studies evaluated effects of compression in the acute phase versus no compression treatment and found no differences in the incidence of PTS (RR 0.76, 95% CI 0.49 to 1.16; P = 0.2; 101 participants). One study reported that thigh‐length stockings did not provide better protection against development of PTS than knee‐length stockings (RR 0.92, 95% CI 0.66 to 1.28; P = 0.6; 267 participants). Another trial reported that wearing compression stockings for two years seemed to be superior to wearing them for one year in terms of PTS incidence.
Two of the 10 included studies described patient satisfaction and quality of life (moderate‐quality evidence), using different measurement systems. The first study showed significant improvement in well‐being and DVT‐related quality of life with compression treatment (P < 0.05) compared with bed rest, and the second study showed no differences in quality of life scores between compression and placebo groups. Four studies poorly reported side effects (low‐quality evidence) that included itching, erythema, and other forms of allergic reaction and described no serious adverse events. Compliance with wearing of compression stockings was generally high but varied across studies.
Authors' conclusions
Low‐quality evidence suggests that elastic compression stockings may reduce the occurrence of PTS after DVT. We downgraded the quality of evidence owing to considerable heterogeneity between studies and lack of or unclear risk of blinding due to clinical assessment scores. No serious adverse effects occurred in these studies. Large randomised controlled trials are needed to confirm these findings because of current lack of high‐quality evidence and considerable heterogeneity.
Plain language summary
Compression therapy for prevention of post‐thrombotic syndrome
Background
Deep vein thrombosis (DVT) occurs when a blood clot blocks blood flow through a vein. One in every three people with DVT will develop chronic pain, swelling, and skin changes in the legs, called post‐thrombotic syndrome (PTS). Compression therapy with, for example, elastic compression stockings is used to try to reduce swelling and improve blood flow in the veins of the leg. People with DVT can reduce the chance of developing PTS by wearing elastic compression stockings. Our objective was to determine effectiveness and rate of complications when compression therapy is used in people with DVT for prevention of PTS.
Study characteristics and key results
We identified 10 randomised controlled trials with a total of 2361 participants that evaluated compression therapy (current until March 2017). We combined five trials to assess our main outcome ‐ PTS. We found that people with DVT who wear elastic compression stockings are less likely to develop PTS, and that compression did not lead to reduced incidence of severe PTS. We found no clear differences in occurrence of pulmonary embolism (blockage of the artery in the lung) nor in reports of recurrent DVT. Compression in the acute phase of DVT compared with "no compression" treatment did not significantly lower PTS incidence. Thigh‐length stockings did not provide better protection against development of PTS than knee‐length stockings. One trial reported that wearing compression stockings for two years seemed to be superior to wearing them for one year in terms of PTS incidence. Compression treatment did not seem to improve quality of life, except during the first nine days after DVT, but we could draw no real conclusions regarding this outcome. Side effects included itching, erythema, and other forms of allergic reaction. The study investigators reported no serious adverse events and indicated that compliance with use of compression stockings was generally high but varied across studies.
Quality of the evidence
Although studies show a reduction in the number of people developing PTS, the quality of evidence is low because of considerable differences between studies and lack of or unclear risk of blinding due to clinical assessment scores. Overall, the included studies were of poor methodological quality.
Summary of findings
Summary of findings for the main comparison. Does compression treatment prevent post‐thrombotic syndrome?
|
Compression therapy for prevention of post‐thrombotic syndrome Participants or patients: adults with objectively diagnosed DVT Setting: outpatient or clinical Intervention: compression (elastic stockings, Unna boots, bandages) Comparison: no compression or placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no compression or with placebo compression | Risk with compression | |||||
|
Cumulative incidence of any PTS (follow‐up 2 to 6.3 years) |
Study population | RR 0.62 (0.38 to 1.01) | 1393 (5 RCTs)a | ⊕⊕⊝⊝ lowb,c | ||
| 400 per 1000 | 248 per 1000 (152 to 404) | |||||
|
Cumulative incidence of severe PTS (follow‐up 2 to 6.3 years) |
Study population |
RR 0.78 (0.53 to 1.15) |
1224 (4 RCTs)d | ⊕⊕⊝⊝ lowb,c | ||
| 86 per 1000 | 67 per 1000 (46 to 99) | |||||
|
VTE: recurrence of DVT occurring in the first 2 weeks after initiation of treatment (follow‐up 2 to 6.3 years) |
Study population | RR 0.94 (0.69 to 1.28) | 1212 (4 RCTs)e | ⊕⊕⊝⊝ lowb,c | ||
| 118 per 1000 | 111 per 1000 (81 to 151) | |||||
|
VTE: incidence of PE occurring in the first 2 weeks after initiation of treatment (follow‐up 2 to 5 years) |
PE occurrence was low and exactly when the PE occurred was unclear. Partsch reported no difference in PE occurrence between groups (Unna boot group: 2, stocking group: 1, bed rest group: 1) (Partsch 2004). Kahn reported 9 occurrences in the active stockings group and 12 in the placebo group (Kahn 2014). Prandoni reported recurrent VTE in 12 patients in the stockings group and in 13 controls; 7/25 were PE, but it was unclear when these occurred, and if they were from the control or stockings group (Prandoni 2004). In a later study, Prandoni reported no PE (Prandoni 2012) | 1296 (4 RCTs)f | ⊕⊕⊝⊝ lowb,c,g | |||
|
Adverse effects (follow‐up 2 to 5 years) |
No serious adverse events occurred. Side effects (i.e. itching, erythema, or other forms of allergic reaction) were poorly reported in 4 studies. The largest study found that 2% in either group reported itching (Kahn 2014). Another study reported itching in about 6% of the elastic stockings group (Prandoni 2004). Two studies found more frequent occurrence of side effects (Partsch 2004; Prandoni 2012). Of participants allocated to the thigh‐length group, 40.7% developed side effects, as did 27.3% of those randomised to the below‐knee group (P = 0.017) (Prandoni 2012). Combining the bandaging and elastic stockings group revealed that 25% had minor skin changes or itching, which did not lead to discontinuation (Partsch 2004). | 1296 (4 RCTs)h |
⊕⊕⊝⊝ lowb,c,g,i | We present data from all studies and all comparisons in the review reporting on adverse effects. | ||
|
Patient satisfaction or QOL (follow‐up 2 years) |
Kahn 2014 (active stockings vs placebo): no significant difference in SF‐36 physical score (P = 0.12) and mental score (P = 0.79). No significant difference in VEINES‐QOL score (P = 0.81) Mol 2016 (1 year vs 2 years of compression stockings): no significant difference in median quality of life at end of follow‐up (P = 0.99) or mean intraindividual change in VEINES‐QOL (P = 0.21) and VEINES‐Sym (P = 0.12) Partsch 2004: significantly faster and more pronounced improvement in well‐being and DVT‐related quality of life with compression than with bed rest (P < 0.05) in the first 9 days after DVT. Psychological and somatic quality of life did not differ between groups. |
1377 (3 RCTs)j |
⊕⊕⊕⊝ moderateb,k | We present data from all studies and all comparisons in the review reporting on patient satisfaction or QOL. | ||
|
Compliance (percentage compliance with treatment during 2‐year study periodl) |
Aschwanden 2008: 92% stockings Brandjes 1997: 76% stockings Jayaraj 2015: 60% stockings Kahn 2014: 56% in active stockings group and 55% in placebo group (56% for both) Mol 2016: 85% stockings Partsch 2004: 73% in bed rest group and 50% in active group, with both receiving the same compression stockings for 2 years Prandoni 2004: 80% stockings Prandoni 2012: 67% in thigh‐length stockings group and 83% in knee‐length stockings group |
1639 (8 RCTs)m | ⊕⊕⊝⊝ lowb,c,n | We present data from all studies and all comparisons in the review reporting on compliance. | ||
| CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; PTS: post‐thrombotic syndrome; QOL: quality of life; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAschwanden 2008; Brandjes 1997; Ginsberg 2001; Kahn 2014; Prandoni 2004. bDowngraded owing to high heterogeneity between studies. cDowngraded owing to lack of or unclear risk of blinding due to assessment scores and reasons for loss to follow‐up not clearly described. dBrandjes 1997; Ginsberg 2001; Kahn 2014; Prandoni 2004. eAschwanden 2008; Brandjes 1997; Kahn 2014; Prandoni 2004. fKahn 2014; Partsch 2004; Prandoni 2004; Prandoni 2012. gScarcely and not always clearly reported. Therefore not pooled. hKahn 2014; Partsch 2004; Prandoni 2004; Prandoni 2012. iDowngraded owing to risk of bias concerns over selective reporting. jKahn 2014; Mol 2016; Partsch 2004. kDifferent measurements were used by studies reporting on this outcome. lPercentage compliant with allocated treatment (compression stockings, placebo stockings, bandages). mAschwanden 2008; Brandjes 1997; Jayaraj 2015; Kahn 2014; Mol 2016; Partsch 2004; Prandoni 2004; Prandoni 2012. nProportions were described narratively because of heterogeneity between studies and differences in control groups, with only one study placebo controlled and most having no treatment as control. Therefore, results were not pooled.
Background
Description of the condition
One out of every three people with deep vein thrombosis (DVT) will develop post‐thrombotic sequelae (complications resulting from a disease) within five years (Brandjes 1997; Prandoni 2009). Although no consensus has been reached on the definition provided in the literature, several scoring systems are used to define post‐thrombotic syndrome (PTS) (Kahn 2009; Porter 1995). The main item included in all definitions is (documented) DVT preceding chronic complaints of the leg(s). Complaints may include subjective symptoms of the legs (e.g. pain, cramps, heaviness, pruritus (itching), paraesthesia (sensation that makes it impossible to keep the leg still)); signs of stasis (pretibial oedema, redness, induration (hardening) of the skin, hyperpigmentation, new venous ectasia (dilatation), and pain during calf compression); and venous leg ulceration. All these complaints have an impact on quality of life (Kahn 2002).
Post‐thrombotic syndrome seems to develop as the result of several factors such as deep vein obstruction, venous reflux (back flow of blood in the veins), calf muscle pump dysfunction, and abnormal microvasculature or lymphatic function (Haenen 2002; Prandoni 2009). In the acute phase of DVT, a fresh thrombus in the deep vein produces an obstruction. During the first few months following onset of DVT, recanalisation ‐ a complex process involving fibrinolysis (dissolving of a blood clot), thrombus organisation, and neovascularisation (proliferation of blood vessels) ‐ may occur (Meissner 2002). This process can result in valve destruction. Damaged valves, insufficient closure, and/or occlusion of the veins increase pressure within the veins (venous hypertension). It is postulated that this venous hypertension disturbs normal blood flow in small capillaries, resulting in increased capillary filtration leading to ankle flare, oedema (swelling of tissue caused by excessive fluid in the subcutaneous tissue) in the lower leg, and various skin changes including dermatitis, lipodermatosclerosis, and white skin atrophy. (Lipodermatosclerosis is the term given to hardening of the skin, which may gain a red/brown pigmentation, that is accompanied by wasting of the subcutaneous fat. White skin atrophy consists of small depressions of ivory‐coloured hard skin with stippled red spots.) Finally, this disturbed microcirculation may lead to a decrease in cutaneous oxygen concentration (TcPO2), contributing to delayed healing after injury to the skin, or leading to spontaneous breakdown of the skin (venous leg ulceration) (Guex 1994; Neumann 1984). These changes are called 'chronic venous insufficiency', and when DVT is the source of this insufficiency, the condition is called 'post‐thrombotic syndrome (PTS)'.
Description of the intervention
Several non‐pharmaceutical measures are used for prevention of PTS in the acute phase of DVT. These include elevation of the legs and use of compression therapy. Compression therapy may consist of circular pressure on the leg applied with removable bandages or permanent bandages such as an adherent bandage or an Unna paste boot, medical elastic compression stockings, antithrombosis stockings, or an external intermittent pneumatic device (Brakkee 1988; Neumann 1993; Partsch 1984; Partsch 2001). Medical elastic compression stockings are classified according to the amount of pressure that they exert (European Committee for Standardization (CEN)).
How the intervention might work
The mechanism of action of compression therapy of the legs is produced by one or more of the following: reduction of oedema, acceleration of venous blood flow, and improvement in venous pump function (Partsch 1991). Very tight non‐elastic bandages are used in an attempt to reverse venous hypertension (Partsch 1984b). Although compression therapy is generally harmless, it can lead to complications. Pressure sores may develop if pressure is given at an extremely high level because such pressure will reduce blood supply to the skin (Kay 1986). Similarly, application of moderate pressure in people with impaired arterial blood supply to the legs may result in exacerbation of arterial insufficiency (Callam 1987).
Why it is important to do this review
Opinions of clinicians and guidelines of their professional organisations differ in their assessment of the utility of compression therapy for treatment of DVT; therefore a review of the literature is needed (Blattler 1995; Guyatt 2001; Kahn 2016; Kearon 2008; Kearon 2012).
Objectives
To determine relative effectiveness and rate of complications when compression therapy is used in people with deep vein thrombosis (DVT) for prevention of post‐thrombotic syndrome (PTS).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) evaluating compression therapy for treatment of DVT. We classified as CCTs trials that used allocation processes that were transparent before assignment, such as open lists of random numbers, case records, days of the week, surnames, etc. Anticoagulant therapy and/or surgical intervention for DVT had to be provided equally among participant groups in these trials.
Types of participants
Adult men and women of any age with DVT that was objectively diagnosed. Methods considered acceptable for diagnosis of DVT included ultrasonography and venography.
Types of interventions
The primary intervention was compression therapy. Medical elastic stockings, compression bandages, bed rest, and no intervention were compared. Also included were comparisons between different kinds of elastic stockings and different durations of use. We included only trials that assessed development of PTS as an outcome.
Types of outcome measures
Primary outcomes
Incidence of PTS up to two years (Definitions used for PTS in each trial paper were accepted, provided they included a systematic clinical history and scoring of physical examinations.)
Secondary outcomes
Venous thromboembolism (VTE), including recurrent DVT and pulmonary embolism (PE)
Adverse effects, including discomfort, pain, and pressure sores
Patient satisfaction and quality of life: generic or specific measures obtained from quality of life questionnaires
Compliance rate
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (20 March 2017).
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings that have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies, using the terms 'postthrombotic OR post‐thrombotic'.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
International Standard Registered Clinical/Social Study Number (ISRCTN) Register (www.isrctn.com/).
Data collection and analysis
Selection of studies
Two review authors (DK and EvL) independently assessed titles and abstracts of studies identified in terms of their relevance and design, according to review selection criteria. We obtained full versions of articles if review authors determined on initial assessment that they might satisfy review inclusion criteria. We checked full papers to identify those fitting the criteria. Another review author (DA) repeated this procedure independently, for verification purposes. Review authors resolved disagreements by discussion.
Data extraction and management
Two review authors (DA and EvL) independently extracted data, and a third review author (DK) checked data for accuracy. Review authors extracted study details and summarised them using data extraction sheets. If data were missing from reports, we attempted to contact study authors to request the missing information. We included only once all studies published in duplicate.
We appraised each study using a standard checklist to assess the validity of methods used. We collected data on the following.
Trial setting (country, and whether primary or secondary care).
Method of randomisation.
Concealment of allocation.
Objective outcome, method of assessment, and whether the study was blinded.
Length of follow‐up.
Number of participants (or limbs) randomised and analysed.
Inclusion criteria.
Exclusion criteria.
Descriptions of interventions and co‐interventions.
Baseline characteristics of groups for important variables.
Definition of post‐thrombotic syndrome.
Duration of follow‐up.
We also collected data on the following.
Intention‐to‐treat analysis.
Numbers of and reasons for withdrawal.
Source of funding.
Use of an a priori sample size/power calculation.
We intended to compare elastic compression stockings versus control (no compression or placebo compression), along with the following.
Mechanical compression therapy versus no intervention.
Mechanical compression therapy versus compression stockings.
Different brands or types of compression stockings versus one another.
Assessment of risk of bias in included studies
Three review authors (DA, DK, and EvL) independently assessed bias in all studies using Cochrane's 'Risk of bias' tool (Higgins 2011), resolving disagreements by discussion. We examined five key domains for bias: selection bias; performance bias; attrition bias; detection bias; and reporting bias. We assessed these and classified them as having low risk of bias or high risk of bias. When a study provided insufficient detail for assessment of risk, we reported this as 'unclear' risk. In addition, we reported any other form of bias noted in the study.
Measures of treatment effect
We expressed dichotomous outcomes as risk ratios (RRs) with accompanying 95% confidence intervals (CIs) if studies reported these measures. In our analyses, we used RRs with accompanying 95% CIs for pooled outcomes.
Unit of analysis issues
We used participants as the unit of analysis.
Dealing with missing data
We contacted authors of included studies to enquire about missing or incomplete data, such as information on participants who dropped out of the study, and missing statistics. If we had concerns about missing data, we planned to exclude them from the meta‐analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity through visual inspection of forest plots and performance of Chi2 and I2 tests. We considered I2 values less than 50% as indicative of low heterogeneity, I2 values between 50% and 75% as indicative of moderate heterogeneity, and I2 values greater than 75% as indicative of significant heterogeneity (Higgins 2011). When we identified substantial clinical, methodological, or statistical heterogeneity, we considered whether pooling of results in a meta‐analysis or using a narrative approach to data synthesis was appropriate. If we identified substantial heterogeneity, we explored possible reasons for this by grouping trials that included similar populations.
Assessment of reporting biases
We intended to construct a funnel plot to test for reporting bias in meta‐analyses that included 10 or more studies (Higgins 2011). It was not possible to do this, as we did not identify 10 studies for inclusion in our meta‐analysis.
Data synthesis
In the absence of clinical or statistical heterogeneity, we used a fixed‐effect model to calculate pooled treatment effect data and 95% CIs for dichotomous outcome variables, as detailed under Measures of treatment effect. We used a random‐effects model if we found significant heterogeneity (defined as I2 > 75%). We created a forest plot for each treatment effect, as per Cochrane Vascular guidelines.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was based on populations of similar size. We assessed statistical heterogeneity through visual inspection of forest plots and performance of Chi2 and I2 tests.
Sensitivity analysis
To determine whether compression in the acute phase of DVT had an effect on our pooled results, we conducted a sensitivity analysis that included Partsch 2004 and Roumen‐Klappe 2009 (Analysis 5.1; Analysis 5.2; Analysis 5.3). This validated the robustness of the pooled estimate because it did not change the results that we first obtained through meta‐analysis.
5.1. Analysis.
Comparison 5 Sensitivity analysis: effect of compression treatment on PTS incidence and recurrence of DVT, Outcome 1 Cumulative incidence of any post‐thrombotic syndrome.
5.2. Analysis.
Comparison 5 Sensitivity analysis: effect of compression treatment on PTS incidence and recurrence of DVT, Outcome 2 Cumulative incidence of severe post‐thrombotic syndrome.
5.3. Analysis.
Comparison 5 Sensitivity analysis: effect of compression treatment on PTS incidence and recurrence of DVT, Outcome 3 Recurrence of deep venous thrombosis.
'Summary of findings'
We prepared a 'Summary of findings' table to present evidence for the use of compression in preventing PTS among participants with a diagnosis of DVT. We used no external information in generating the 'Assumed risk' column. We used the GRADE approach to evaluate the evidence and to assign one of four levels of quality: high, moderate, low, or very low (Guyatt 2008; Higgins 2011). We required no departures from standard methods in generating these tables. We included the primary and secondary endpoints described under Types of outcome measures: incidence of PTS; recurrence of DVT and PE; adverse effects; patient satisfaction and quality of life; and compliance rates. We chose these endpoints because we deemed them to be most clinically relevant. Outcomes such as adverse effects, patient satisfaction, and compliance reflect details of all comparisons and studies included in the review. See Table 1.
Results
Description of studies
Results of the search
See Figure 1.
1.
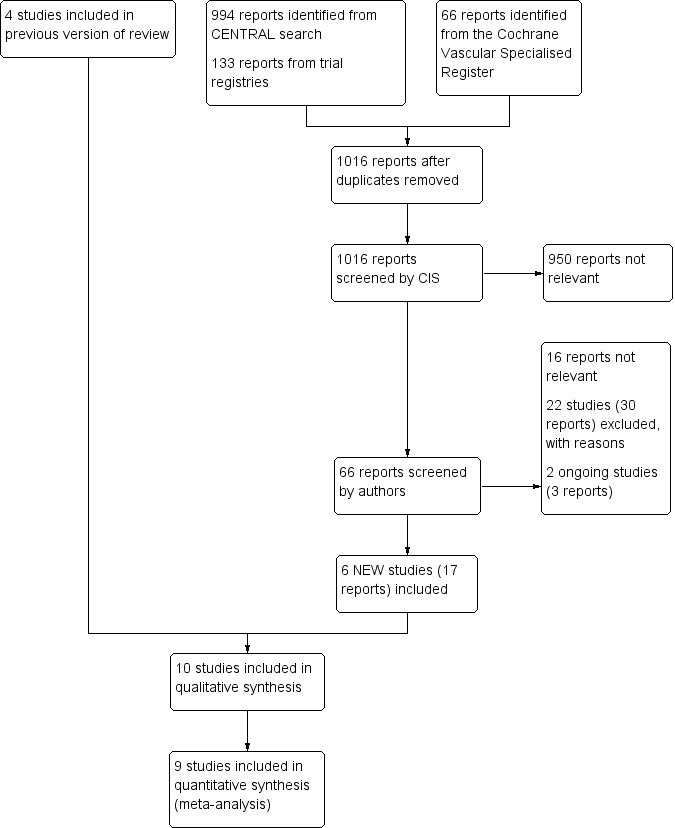
Study flow diagram.
Included studies
We identified six studies for inclusion in this review update (Aschwanden 2008; Jayaraj 2015; Kahn 2014; Mol 2016; Prandoni 2012; Roumen‐Klappe 2009).
We identified 10 RCTs that met all inclusion criteria with mean follow‐up ranging from 2 to 6.3 years, and with a total of 2361 participants (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Jayaraj 2015; Kahn 2014; Mol 2016; Partsch 2004; Prandoni 2004; Prandoni 2012; Roumen‐Klappe 2009). We identified no CCTs.
Two trials measured the incidence of PTS, comparing compression stockings versus placebo stockings (Ginsberg 2001; Kahn 2014).
Ginsberg included 47 participants one year after a diagnosed proximal DVT and compared the effectiveness of below‐knee elastic compression stockings (20 to 30 mmHg pressure at the ankle) versus placebo stockings (one to two sizes too large) (Ginsberg 2001). At the time of inclusion, participants did not have pain or swelling of the leg, but all had venous valvular insufficiency measured by plethysmography and/or Doppler ultrasonography. The primary outcome was treatment failure defined as pain and swelling of the leg appearing during subsequent follow‐up. The study included people with symptomatic (n = 33) and asymptomatic (n = 12) DVT; for two participants, the clinical presentation was unknown. Kahn included 806 patients presenting with a first symptomatic, proximal DVT, objectively confirmed on ultrasonography (Kahn 2014). Investigators treated DVT using standard anticoagulant therapy, equally for both groups. They compared active stockings (30 to 40 mmHg) versus placebo stockings. They applied stockings within two weeks of DVT diagnosis and asked participants to wear them daily for two years. Trial results show assessment of PTS from 6 to 24 months' follow‐up, using Ginsberg’s criteria (leg pain and swelling of ≥ 1 month's duration) and Villalta's score (score ≥ 5 indicates PTS). Other secondary outcomes were presence of leg ulcers, objectively confirmed recurrent venous thromboembolism, death, adverse events, venous valvular reflux, and quality of life.
Four trials compared compression therapy versus no intervention (Aschwanden 2008; Brandjes 1997; Jayaraj 2015; Prandoni 2004).
Aschwanden included 169 adults with first or recurrent proximal DVT confirmed by duplex ultrasonography (Aschwanden 2008). Comparing compression stockings versus no stockings, they assessed post‐thrombotic skin changes according to C4 or higher (clinical, etiology, anatomy, pathophysiology (CEAP) classification). As secondary outcomes, they assessed occurrence of symptoms related to PTS such as pain, heaviness, heat sensation, tension, and tiredness. Investigators randomised participants to either group after they had received a standard treatment regimen for six months after DVT, which also included compression stockings. Follow‐up examinations took place every three months during the first year after inclusion, and from then on, every six months. Trialists censored recurrent DVT during follow‐up from further analysis because of the need for compression stockings. The Brandjes and Prandoni trials consisted of 194 and 180 participants, respectively (Brandjes 1997; Prandoni 2004). Both studies compared graduated elastic compression stockings with ankle pressure of 30 to 40 mmHg versus no intervention. Researchers randomised participants to either group one to three weeks after first proximal DVT. Assessment took place at least six‐monthly for up to five years. The primary outcome was the cumulative incidence of PTS. Both studies used a self‐developed scale for assessment of PTS (Brandjes Scale and Villalta Scale) that was based on symptoms and appearance of the affected leg. As secondary outcomes, they assessed both venous thromboembolism recurrence and treatment compliance.
Jayaraj included 69 participants within 48 hours of diagnosis of a first acute proximal DVT by duplex ultrasonography (Jayaraj 2015). Participants underwent standard anticoagulation therapy for DVT and were randomised to treatment with graduated compression stockings (30 to 40 mmHg) or no stockings. Investigators used the Venous Clinical Severity Score (VCSS) and the Villalta score to assess PTS at 3, 6, 12, 18, and 24 months. They maintained compliance by providing telephone follow‐up between study co‐ordinator and participants, and by instructing participants to use a compliance calendar. They assessed no other secondary outcomes.
Two studies compared compression therapy versus no intervention in the acute stage of DVT (Partsch 2004; Roumen‐Klappe 2009).
In their three‐part study, Partsch et al randomised 53 participants with confirmed first proximal DVT to three groups (Partsch 2004). They compared inelastic compression bandages (Unna boots on the lower leg, adhesive bandages on the thigh), thigh‐length compression stockings (class II, 30 mmHg), and bed rest without compression for nine days. They instructed groups A and B to stay mobile. At first, primary outcomes were pain reduction and leg circumference between baseline and day nine; on both days, investigators objectively assessed the presence of PE and the extent of DVT by performing ventilation‐perfusion lung scintography and duplex ultrasonography, respectively. They assessed patient well‐being and quality of life in the first nine days after DVT. After nine days, all participants received class II elastic compression stockings (thigh‐length or knee‐length, depending on thigh oedema). Independent observers re‐evaluated 37 study participants after two years: 11 in the bed rest group, 13 in the bandage group, and 13 in the stocking group. They assessed PTS incidence using the Villalta Scale, for which they combined bandaging and stocking groups of the acute phase (= active group) for comparison with groups treated with bed rest without compression. They carried out clinical and venous duplex investigations.
Roumen‐Klappe evaluated compression treatment in the acute phase of first proximal and distal DVT (Roumen‐Klappe 2009). Researchers included 69 patients with acute symptomatic DVT. All participants received standard anticoagulant DVT therapy for three months and were randomised to immediate multi‐layer bandaging or no bandaging in the acute phase. Investigators encouraged all participants to walk as much as possible. After reduction of oedema, they applied sized‐to‐fit elastic stockings for all participants after 7 to 14 days and instructed participants to wear these for at least two years. When participants were assigned to no immediate bandaging but oedema was persistent after 10 days, study authors applied bandaging after all. They planned follow‐up after 7, 30, and 90 days and at 1 year. After 1 year, they evaluated participants for clinical PTS using both the clinical CEAP classification (with PTS defined as C3 or higher) and the Villalta Scale. They did not assess recurrence of DVT or PE, nor did they evaluate compliance with compression therapy.
Another study compared thigh‐length versus knee‐length compression stockings, both worn for two years (30 to 40 mmHg), starting the first week after DVT (Prandoni 2012). This study included 267 participants with a first episode of symptomatic proximal DVT, as confirmed by ultrasonography. The primary outcome was 3‐year cumulative incidence of PTS, which investigators assessed at 3, 6, 12, 24, and 36 months after the acute episode, using the Villalta Scale (score ≥ 5 indicates PTS). They recorded recurrent venous thromboembolism as well. In addition, researchers assessed compliance with assigned compression stockings and their tolerability (including adverse effects) by instructing participants to record this information every day in a booklet. Compliance was satisfactory if stockings were used at least 70% of the time during the day.
The non‐inferiority trial of Mol compared 1‐year versus 2‐year use of compression stockings; the main outcome measure was incidence of PTS 24 months after diagnosis of first proximal DVT, assessed by the Villalta Scale (Mol 2016). Secondary outcomes were adherence to use of stockings during study follow‐up, recurrent DVT, death, and quality of life assessed using the VEINES‐QOL/Sym questionnaire. Investigators randomly assigned participants to continue or stop wearing compression stockings 1 year after the index DVT. They scheduled follow‐up visits at 3, 6, and 12 months from the baseline visit.
None of the included studies assessed treatment costs.
Excluded studies
We excluded 22 additional studies from this review update (Adam 1994; Cairols 2011; Chylarecki 1996; Dennis 2013; Enden 2009; Gonzalez‐Fajardo 2008; Hull 2009; ISRCTN81127756; Junger 2006; Kahn 2003; Kahn 2005; Kahn 2011; Lewis 1976; Markevicius 2004; Moser 1976; NCT02148029; Rahman 2009; Ratiu 2009; Romera 2005; Schulman 2006; Schwarz 1998; Vedantham 2013). See Characteristics of excluded studies for details.
Ongoing studies
We identified three studies as ongoing (NCT01429714; NCT01578122; NCT03039517). Details can be found in the Ongoing studies section.
Risk of bias in included studies
2.
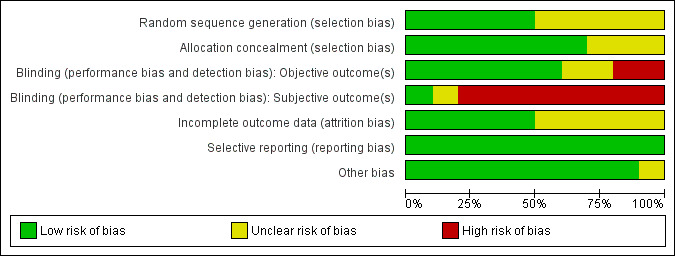
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
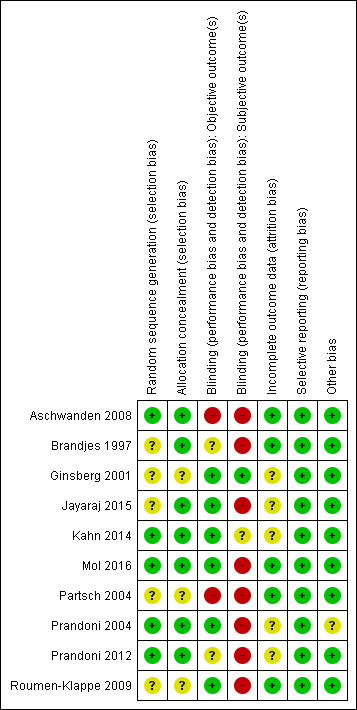
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The included studies had low to moderate overall risk of bias; highest risk involved performance bias for subjective outcomes because participants were not blinded (Aschwanden 2008; Brandjes 1997; Jayaraj 2015; Mol 2016; Partsch 2004; Prandoni 2004; Prandoni 2012; Roumen‐Klappe 2009). Selection bias was generally low because most studies concealed allocation to treatments, but risk was unclear for three studies (Ginsberg 2001; Partsch 2004; Roumen‐Klappe 2009). Risk of attrition bias was unclear among half of the included studies because researchers did not always describe loss to follow‐up clearly (Ginsberg 2001; Jayaraj 2015; Kahn 2014; Prandoni 2004; Prandoni 2012), but most studies used survival curves to deal with loss to follow‐up, and we therefore believe that this approach did not introduce high risk of bias. We found no risk of selective reporting bias in the included studies. Use of co‐interventions for some participants might have influenced the main outcome in one study (Prandoni 2004).
Allocation
In one study, investigators concealed treatment allocation, and a study nurse not involved in the trial allocated treatment using a computer‐generated randomisation list (Aschwanden 2008). Two studies performed randomisation by using sealed envelopes (Brandjes 1997; Jayaraj 2015). Another two studies randomly assigned participants to study groups using a Web‐based randomisation system, which ensured concealed allocation (Kahn 2014; Mol 2016). Another investigator used a computer‐generated list for randomisation that was accessible only to a trial nurse who informed study physicians on treatment allocation after participants provided informed consent (Prandoni 2004; Prandoni 2012). Three studies provided unclear information on allocation concealment (Ginsberg 2001; Partsch 2004; Roumen‐Klappe 2009).
Blinding
Eight studies had high risk of performance bias for subjective outcomes (participants' symptoms) owing to lack of participant blinding (Aschwanden 2008; Brandjes 1997; Jayaraj 2015; Mol 2016; Partsch 2004; Prandoni 2004; Prandoni 2012; Roumen‐Klappe 2009). Two studies did not blind outcome assessors (Aschwanden 2008; Partsch 2004). Investigators assessed the outcome PTS by using scoring systems that contained both subjective and objective criteria. Subjective criteria, most of which consist of patient‐reported items, introduce high risk of bias when participants are not blinded. Use of placebo stockings, as done by two studies could potentially avoid this bias (Ginsberg 2001; Kahn 2014). However, participant awareness of the allocated treatment could still influence subjective outcomes in a way. Kahn correctly blinded participants, but 41% were allocated to the active stockings group and correctly guessed the group to which they belonged, compared with 17% of the placebo group (Kahn 2014). For this reason, we assessed this bias as unclear. For objective criteria, lack of blinding of outcome assessors may influence the incidence of PTS.
Incomplete outcome data
Five studies had unclear risk of bias, and the other five had low risk of bias. When necessary, appropriate survival analysis with censoring of loss to follow‐up was performed. Reasons for loss to follow‐up were usually mentioned in flow diagrams. Numbers and reasons for loss to follow‐up were generally balanced across groups. When sufficiently reported and balanced across groups, loss to follow‐up did not represent a risk. We excluded one study from meta‐analysis owing to lack of data because investigators did not report exact numbers and presented only hazard ratios (Jayaraj 2015).
One study did not fully describe loss to follow‐up, but indicated that three people in the active stockings group died (Ginsberg 2001). Another study had a high rate of attrition in both intervention and control groups, with the latter showing greatest loss to follow‐up (Jayaraj 2015). However, investigators conducted Kaplan‐Meier analyses to deal with censored cases; therefore, we did not classify this study as being at high risk. In another study, 186 participants were lost owing to withdrawal, death, and loss to follow‐up (Kahn 2014). Although researchers conducted a Kaplan‐Meier survival analysis to deal with censored cases, the description of reasons for loss to follow‐up was very brief, so it is unclear whether reasons for withdrawal were related to outcomes. Prandoni presented a flow diagram that did not provide clear reasons for exclusion (Prandoni 2004). Six participants withdrew, which still leaves unexplained losses to follow‐up. Trial authors stated that three participants died of PE but did not describe their treatment groups, omitting relevant information. In a later study, Prandoni did not further specify reasons for loss to follow‐up, which was higher in the thigh‐length group than in the below‐knee group (eight vs three) (Prandoni 2012). A total of 31 participants died (equal among treatment groups), none as the result of PE. Trial authors used Kaplan‐Meier analysis to deal with censored cases.
Selective reporting
All included studies reported on the prespecified outcomes and predictor variables. In the study of Partsch 2004, all primary outcomes are reported. PTS was not the primary outcome in this study. Investigators described PTS scores as bed rest versus mobile. They provided no information on comparison of PTS scores among the three treatment groups, but review authors judged it unlikely that this introduced bias.
Other potential sources of bias
One of the included studies provided co‐interventions (Prandoni 2004). Twelve out of 90 participants in the no stockings group (control) wore various types of elastic stockings (most providing 12 to 18 mmHg of pressure at the ankle) for varying periods ranging from six weeks to six months, by self‐prescription or by prescription of their attending physician. Use of anticoagulants such as aspirin or non‐steroidal anti‐inflammatory drugs (NSAIDs) was greater in the control group (15 vs 6 in the treatment group). This could account for better outcomes in the control group. All other studies used anticoagulants equally among groups, and we identified no other potential sources of bias.
Effects of interventions
See: Table 1
Primary outcome
Incidence of post‐thrombotic syndrome
All studies reported on post‐thrombotic syndrome, but we were unable to combine all 10 in our meta‐analysis.
We did not include two studies in the overall meta‐analysis, as investigators studied effects of different kinds of compression therapy during the acute phase of DVT, after which all participants received compression stockings (Partsch 2004; Roumen‐Klappe 2009). This treatment regimen differed from that used in the other studies. Therefore we pooled these data separately. We did not include three other studies in our meta‐analysis, the first because investigators did not include a control group that would enable them to determine any beneficial effect of compression therapy (placebo stockings or no treatment) but instead compared thigh‐length versus knee‐length compression stockings (Prandoni 2012). We omitted another study from our meta‐analysis because researchers compared two different durations of use of the same compression stockings (Mol 2016). We excluded a third study because researchers did not present specific numbers and we could not retrieve risk ratios (Jayaraj 2015). We have reported the results of these studies narratively below.
Our meta‐analysis consisted of five of the included studies (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Kahn 2014; Prandoni 2004).
Overall use of elastic compression stockings was associated with a reduction in the incidence of any PTS (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.38 to 1.01; P = 0.05; 1393 participants; 5 studies; low‐quality evidence; random‐effects model). Meta‐analysis showed considerable heterogeneity (I2 = 80%; P = 0.0005). See Analysis 1.1.
1.1. Analysis.
Comparison 1 Compression stockings vs placebo or no stockings, Outcome 1 Cumulative incidence of any post‐thrombotic syndrome.
After performing subgroup analysis for which we grouped studies with similar populations, we found that one study was the main cause of heterogeneity in our meta‐analysis (subgroup difference P < 0.0001), possibly because it included a much larger study population than was included in the other studies (Kahn 2014). The first subgroup showed a significant effect of compression treatment on the incidence of PTS (RR 0.50, 95% CI 0.38 to 0.66; P < 0.00001) (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Prandoni 2004). The second subgroup contained data from only one study and showed no significant differences between compression and control groups (RR 1.01, 95% CI 0.86 to 1.18; P = 0.91) (Kahn 2014).
Sensitivity analysis that included two studies did not change the results of our meta‐analysis, which validated the robustness of the pooled estimate (Analysis 5.1; Analysis 5.2; Analysis 5.3) (Partsch 2004; Roumen‐Klappe 2009).
Four studies reported on severe PTS, noting that the incidence of severe PTS was not reduced in the compression stocking group (RR 0.78, 95% CI 0.53 to 1.15; P = 0.21; 1224 participants; 4 studies; low‐quality evidence) (Brandjes 1997; Ginsberg 2001; Kahn 2014; Prandoni 2004). See Analysis 1.2.
1.2. Analysis.
Comparison 1 Compression stockings vs placebo or no stockings, Outcome 2 Cumulative incidence of severe post‐thrombotic syndrome.
Two studies investigated use of compression therapy in the acute phase and reported that this was not associated with a significant reduction in the incidence of PTS (RR 0.76, 95% CI 0.49 to 1.16; P = 0.2; 101 participants; 2 studies). See Analysis 2.1.
2.1. Analysis.
Comparison 2 Compression in the acute phase of deep venous thrombosis to prevent post‐thrombotic syndrome, Outcome 1 Cumulative incidence of any post‐thrombotic syndrome.
Thigh‐length stockings did not provide better protection than knee‐length stockings (RR 0.92, 95% CI 0.66 to 1.28; P = 0.6; 267 participants; 1 study). See Analysis 3.1.
3.1. Analysis.
Comparison 3 Thigh‐length vs knee‐length compression stockings, Outcome 1 Cumulative incidence of any post‐thrombotic syndrome.
Results showed a difference favouring wearing of compression stockings for two years rather than one year after DVT, in terms of PTS incidence (RR 1.54, 95% CI 1.03 to 2.29; P = 0.03; 518 participants; 1 study). See Analysis 4.1.
4.1. Analysis.
Comparison 4 One year versus two years of compression stockings, Outcome 1 Cumulative incidence of any post‐thrombotic syndrome.
Results of individual studies are detailed below.
In the Aschwanden study, 11 participants (13.1%) in the stockings group and 17 (20.0%) in the control group developed PTS (P < 0.19) (Aschwanden 2008). Trialists did not provide exact differentiation between mild, moderate, and severe PTS. Seventeen participants in the control group developed dermatoliposclerosis (C4b). Study authors reported no venous ulcerations.
In the study by Brandjes, PTS occurred in 19 (20%) of 96 participants treated with stockings and was considered severe in 11 participants; corresponding numbers in the control group were 46 (47%) of 98 participants, 23 of whom had severe PTS (Brandjes 1997). Prandoni obtained similar results in his study (hazard ratio (HR) 0.49; 95% CI 0.29 to 0.84) (Prandoni 2004). PTS occurred in 23 (26%) of 90 participants treated with stockings and was considered severe in three participants. In the control group, 44 (49%) of 90 participants developed PTS and 10 participants had severe PTS. Risk reduction for any, and for severe, PTS was highly statistically significant at P < 0.001. Although 12 participants In the control group wore stockings (12 to 18 mmHg), a significant difference in outcomes favoured the stockings group (Prandoni 2004).
In the Ginsberg trial, none of the 24 participants in the active stocking group were considered treatment failures (PTS was considered treatment failure) compared with one (4.3%) of 23 participants treated with placebo stockings (Ginsberg 2001).
In another study, the cumulative incidence of PTS at two years (750 days) was 14.2% in the active stockings group and 12.7% in the placebo group (HR 1.13, 95% CI 0.73 to 1.76; P = 0.58) (Kahn 2014). Using the Villalta Scale, investigators found a two‐year cumulative incidence of PTS of 52.6% in the active stockings group and 52.3% in the placebo group (HR 1.00, 95% CI 0.81 to 1.24; P = 0·96). PTS was severe in 27 and 20 participants in the stockings group and the placebo group, respectively. PTS severity did not differ between groups. In each group, 17 ulcers developed.
Jayaraj reported that the cumulative incidence of PTS depended on which cutoff point was used during follow‐up for diagnosis of PTS (Jayaraj 2015). Study authors did not report specific data but found no statistically significant difference in PTS incidence between the two groups. At two years' follow‐up, general cumulative incidence based on the Villalta score was high (65% to 80%) compared with that based on the Venous Clinical Severity Score (VCSS) (20% to 40%).
Partsch found significantly lower median Villalta scores in the mobile group than in the bed rest group (P < 0.01) (Partsch 2004). PTS developed in 54% (14 of 26) in the mobile group (bandages or stockings) and in 82% (9 of 11) in the bed rest group. This difference was not significant. Investigators reported no cases of severe PTS.
Another trial used two measures to assess PTS up to a year (Roumen‐Klappe 2009). Using CEAP classification, investigators found a PTS incidence of 39% in participants with bandages and 42% in those without bandages (RR 0.92, 95% CI 0.55 to 1.56). The Villalta score showed that the incidence of PTS was 29% and 33%, respectively (RR 0.87, 95% CI 0.41 to 1.8). Severity of PTS did not differ between groups. Study authors reported no cases of severe PTS.
Prandoni found that the three‐year cumulative incidence of PTS was 33.9% in the thigh‐length group and 36.7% in the below‐knee group (HR 0.93, 95% CI 0.62 to 1.41) (Prandoni 2012). PTS developed in 32.6% (44 of 135) of participants in the thigh‐length compression stockings group and in 35.6% (47 of 132) of those in the below‐knee group. Severe PTS developed in three participants in each group.
Mol reported that wearing compression stockings for two years seemed to be superior to wearing them for one year in terms of PTS incidence after DVT (HR 1.6, 95% CI 1.02 to 2.5; RR 1.54, 95% CI 1.03 to 2.29; P = 0.03) (Mol 2016).
Secondary outcomes
VTE (DVT recurrence and PE occurrence in the first two weeks after initiation of treatment)
Four studies reported pulmonary embolism (PE) (Kahn 2014; Partsch 2004; Prandoni 2004; Prandoni 2012). We report the data narratively. Occurrence of pulmonary emboli was low; only one study was clear as to when exactly the emboli occurred (Partsch 2004). These investigators reported no differences in PE occurrence between groups during the first nine days after DVT (two in the Unna boot group, one in the stocking group, and one in the bed rest group). Another trial found no difference in PE occurrence between groups (9 in the active stockings group and 12 in the placebo group) (Kahn 2014). The first study of Prandoni reported that recurrent VTE developed in 12 participants in the stockings group and in 13 controls, and that seven of these 25 were pulmonary emboli (three caused death) (Prandoni 2004). It was unclear when these occurred and if those affected were controls, or if they belonged to the stockings group. In a later study by Prandoni, no pulmonary emboli occurred (Prandoni 2012). Six studies did not report PE (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Jayaraj 2015; Mol 2016; Roumen‐Klappe 2009).
We were able to pool the data for recurrent DVT from four studies (Aschwanden 2008; Brandjes 1997; Kahn 2014; Prandoni 2004). Use of compression stockings did not lower the incidence of recurrent DVT (RR 0.94, 95% CI 0.69 to 1.28; P = 0.69; 1212 participants; 4 studies; I2 = 0%; low‐quality evidence). See Analysis 1.3. Aschwanden reported 15 DVT recurrences (eight in the stockings group and seven in the control group) (Aschwanden 2008). Brandjes found DVT recurrence in 14.6% (14 of 96) of the stocking group and in 13.3% (13 of 98) of the control group (not significant) (Brandjes 1997). Kahn described recurrent VTE in 33 participants in the active stockings group (45 events ‐ 36 DVT and 9 PE) and in 38 participants in the placebo group (44 events ‐ 32 DVT and 12 PE) (Kahn 2014).
1.3. Analysis.
Comparison 1 Compression stockings vs placebo or no stockings, Outcome 3 Recurrence of deep venous thrombosis.
Results from remaining individual studies are detailed below.
Mol reported that 14 participants developed recurrent DVT: 8 (3.1%) in the one‐year stockings group and 6 (2.3%) in the two‐year stockings group (RR 1.36, 95% CI 0.48 to 3.88; P = 0.56; 518 participants; 1 study) (Mol 2016). See Analysis 4.2.
4.2. Analysis.
Comparison 4 One year versus two years of compression stockings, Outcome 2 Recurrence of deep venous thrombosis.
Prandoni found no difference in recurrent DVT between thigh‐length and knee‐length stocking groups (Prandoni 2012). Twelve in each group (8.9% in the thigh‐length group and 9.0% in the below‐knee group) developed recurrent DVT (RR 0.98, 95% CI 0.46 to 2.10; P = 0.95; 267 participants; 1 study). See Analysis 3.2. Of two studies assessing effects of compression in the acute phase, only one described DVT recurrence (Partsch 2004). These investigators found that, after two years of follow‐up, three participants in the stockings group and none in the other groups developed recurrent DVT.
3.2. Analysis.
Comparison 3 Thigh‐length vs knee‐length compression stockings, Outcome 2 Recurrence of deep venous thrombosis.
Three studies provided no information on recurrence of DVT (Ginsberg 2001; Jayaraj 2015; Roumen‐Klappe 2009).
Adverse effects and intervention‐related complaints
Four studies poorly reported side effects (i.e. itching, erythema, or other forms of allergic reaction) (Kahn 2014; Partsch 2004; Prandoni 2004; Prandoni 2012). Therefore, we did not pool data for this outcome but instead reported this narratively. No serious adverse events occurred. The largest study found 2% itching in both groups (Kahn 2014). Another study reported itching in about 6% of the elastic stockings group (Prandoni 2004). Two studies reported more frequent occurrence of side effects (Partsch 2004; Prandoni 2012). Among participants allocated to the thigh‐length group, 40.7% developed side effects compared with 27.3% of those randomised to the below‐knee group (P = 0.017; Prandoni 2012). When investigators combined bandaging and elastic stockings groups, they found that 25% of participants had minor skin changes or itching, which did not lead to discontinuation (Partsch 2004).
Patient satisfaction and quality of life
Three studies reported this outcome (Kahn 2014; Mol 2016; Partsch 2004). One trial reported significantly faster and more pronounced improvement of well‐being and DVT‐related quality of life with compression than with bed rest in the first nine days after DVT (P < 0.05) (Partsch 2004). Psychological and somatic quality of life did not differ between groups (Partsch 2004). In another trial, Short Form (SF)‐36 scores did not differ significantly between the active stockings group and the placebo group (P = 0.12 for improvement in physical scores; P = 0.79 for mental scores), nor did results show a significant difference in VEINES‐QOL score (P = 0.81) (Kahn 2014). Mol found similar results between the one‐year group and the two‐year group in median quality of life at end of follow‐up (P = 0.99) or in mean intraindividual change in VEINES‐QOL (P = 0.21) and VEINES‐Sym (P = 0.12) scores (Mol 2016).
Compliance rates
We describe the proportions of compliance narratively. Overall compliance was high but varied among the included studies. One study reported that compliance was 92% (Aschwanden 2008). Investigators reported that men had significantly worse compliance with stockings treatment than women (P = 0.05). Another trial found a high compliance rate of 85% at two years (Mol 2016). Adherence to stockings was 76% (73 of 96) up to two years in another trial (Brandjes 1997). Jayaraj reported compliance with stockings of 80% at six months, 70% at one year, and 60% at two years (Jayaraj 2015). Kahn reported that compliance with either stockings was 70% at one‐year follow‐up and 56% at two years (Kahn 2014). Partsch found better compliance in the bed rest group than in the active group (73% vs 50%) (Partsch 2004). In their first study, Prandoni and colleagues stated that 78 (out of 90 participants) wore their stockings for at least 80% of daytime hours during the study period (Prandoni 2004). In their other study, Prandoni provided data showing that rates of compliance with thigh‐length and knee‐length stockings were 67% (90 of 135) and 83% (109 of 132), respectively (P = 0.003) (Prandoni 2012). The remaining studies did not report compliance (Ginsberg 2001; Roumen‐Klappe 2009).
Discussion
Summary of main results
Results of this systematic review show that use of elastic compression stockings reduces the incidence of post‐thrombotic syndrome (PTS) after deep vein thrombosis (DVT) (risk ratio (RR) 0.62, 95% confidence interval (CI) 0.38 to 1.01; P = 0.05; low‐quality evidence) but not the severity of PTS (RR 0.78, 95% CI 0.53 to 1.15; P = 0.21; low‐quality evidence).
Compression in the acute phase of DVT did not seem to reduce PTS incidence, but these results were based on two studies with small sample sizes (Partsch 2004; Roumen‐Klappe 2009). In both studies, all participants received compression stockings after initial compression treatment during the acute phase of DVT. This could have led to a lower overall incidence of PTS in both groups and could have diminished between‐group differences. Compliance with stockings in was higher in the bed rest group than in the active group in Partsch 2004. This may have masked a potential beneficial effect of early compression therapy on later PTS incidence in the active group. Another group used DVT symptoms as one of its outcomes (Roumen‐Klappe 2009). However, investigators did not fully specify which symptoms were used and how they scored these. This made it difficult to interpret presented results on this outcome. Aschwanden also included patients with recurrent DVT, which in theory could lead to a higher incidence of PTS (Aschwanden 2008). However, this group defined post‐thrombotic skin changes of C4 or higher (clinical, etiology, anatomy, pathophysiology (CEAP) classification) as its endpoint and did not include changes of C3 or lower. This group also added compression stockings to standard DVT therapy before randomising participants to treatment groups. These factors may have led to a lower incidence of PTS in this study. Results showed that only women seemed to benefit from wearing stockings for prevention of PTS; this could be explained by the much lower rate of compliance among men in this study (Aschwanden 2008). Another group described a substantial difference between PTS incidence rates based on the Ginsberg or Villalta Scale score, with the latter accounting for a five times higher incidence (Kahn 2014). This can be explained by the fact that the Ginsberg score is more specific for measuring severe PTS (Kahn 2006; Soosainathan 2013).
Included studies showed considerable heterogeneity, with only one study finding no statistically significant effects of compression stockings (Kahn 2014). This study included one of the largest sample sizes but described low compliance. Although study authors found no significant difference in PTS incidence when analysing subgroups of compliant and frequent stocking users (hazard ratio (HR) 0.96, 95% CI 0.53 to 1.74), their conclusion that compression stockings do not lower PTS incidence has to be interpreted with caution. We performed subgroup analyses based on study populations of similar size; one subgroup contained four studies and showed a reduction of PTS in the compression group (RR 0.50, 95% CI 0.38 to 0.66; P < 0.00001); the second subgroup contained only one study and showed no differences in PTS (RR 1.01, 95% CI 0.86, 1.18; P = 0.91) (Kahn 2014). See Analysis 1.1. Other possible contributors to this heterogeneity are inclusion of unblinded studies (see section on blinding in studies under Assessment of risk of bias in included studies), variety in follow‐up, and variation in length of time following diagnosis of DVT before randomisation for compression. Researchers started compression therapy during the first few weeks after DVT, but one study provided compression starting one year after diagnosis of DVT (Ginsberg 2001). This could in theory lead to a higher overall incidence of PTS.
We found no differences in DVT recurrence (P = 0.69; low‐quality evidence). We did not pool data on the incidence of pulmonary embolism (PE) because this was poorly reported, but we observed no differences between groups in individual studies (low‐quality evidence). One study indicated that high‐length stockings did not provide better protection against PTS than knee‐length stockings (RR 0.92, 95% CI 0.66 to 1.28; P = 0.6) (Prandoni 2012).
Two of the 10 included studies reported patient satisfaction and quality of life (moderate‐quality evidence), using different measurement systems; the first study noted significant improvement in well‐being and DVT‐related quality of life when comparing compression treatment versus bed rest (P < 0.05), and the second study reported no significant differences in quality of life scores between compression and placebo groups. Compliance with compression stockings was generally high but varied across studies. Researchers reported no serious adverse effects after compression therapy was initiated.
In general, adverse effects of stockings do not often occur. Most frequently reported adverse effects (in four studies) were itching, erythema, rash, and other forms of allergic reaction (low‐quality evidence).
We included only one trial investigating the duration of compression treatment; these researchers concluded that wearing compression stockings for one year was not non‐inferior to wearing them for two years (Mol 2016). One large trial that is also studying the duration of compression treatment is ongoing; we will include this trial in future updates (NCT01429714).
Overall completeness and applicability of evidence
Our literature search, which included all languages, yielded 10 randomised controlled trials dedicated to the prevention of such a prevalent disorder as PTS. At first glance, it seemed that these studies were conflicting. Ginsberg concluded that most participants had no PTS one year after proximal DVT and did not require use of elastic compression stockings (Ginsberg 2001). However, this study included people with asymptomatic DVT who carry low risk for PTS. In fact, in a subpopulation of participants with symptomatic DVT, these investigators assessed the prevalence of PTS (using a different, non‐validated, and possibly less sensitive scale) as 27%, and as not distinctly different from both other studies. Another important difference is the use of stockings one to two sizes too large instead of no intervention. This might have diminished the contrast between treatment groups, in that even a stocking that is too large will certainly provide some compression (Compression Bulletin). Indeed, it has been shown that stockings that are one or two sizes too large exert considerable pressure when compared with stockings without elastic threads. Moreover, the therapeutic stocking used exerted pressure of 20 to 30 mmHg at the ankle region. This is less than the pressure applied in other studies, which compared a stocking with an ankle pressure of 30 to 40 mmHg. In addition, in this study, stockings were provided only at one year after the index thrombosis (i.e. at a time point when most people might already have developed PTS), and a much less preventive effect could be achieved. In the previous version of this review, we analysed data on thigh‐high and knee‐length stockings together because study authors reported no superiority of thigh‐high stockings upon measuring venous pressure and foot volume (Partsch 1984). A more recent study also found that thigh‐length stockings did not provide better protection than below‐knee stockings, and that lower tolerability of thigh‐length stockings can lead to discontinuation (Prandoni 2012). All included trials and results are relevant to our review questions. High‐quality evidence needed to fully support the use of compression therapy in prevention of PTS is lacking, and any conclusions drawn from current evidence should be interpreted with care.
Quality of the evidence
Our review is limited by considerable clinical and statistical heterogeneity among the included studies and by use of different scoring systems for assessment of PTS. Therefore we judged evidence for the incidence of PTS and severe PTS, recurrence of DVT, incidence of PE, adverse effects, and compliance to be of low quality. We judged the outcomes of patient satisfaction and quality of life to be of moderate quality. We downgraded these because of risk of bias and significant heterogeneity among studies. For this reason, pooled results of our meta‐analyses should be interpreted with caution. See Table 1.
Potential biases in the review process
Methods used in this review such as study selection, data extraction, and quality assessment were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we judged them not likely to cause any bias. Searching of the literature was extensive, but it remains possible that relevant data were not included in this review because they were not published or were not found during the search.
Agreements and disagreements with other studies or reviews
We found six other reviews that used the same main outcome measure (Berntsen 2016; Giannoukas 2006; Jin 2016; Kakkos 2006; Musani 2010; Tie 2015).
Two reviews ‐ Kakkos 2006 and Musani 2010 ‐ included studies that we also included in our review (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Partsch 2004; Prandoni 2004). These review authors concluded that compression treatment reduced the incidence of PTS, particularly of severe PTS. We did not reach the same conclusion for severe PTS, presumably because we included one, more recent study for this outcome (Kahn 2014). Two other reviews ‐ Giannoukas 2006 and Tie 2015 ‐ drew very similar conclusions to those presented in our review and addressed the same problems that we encountered (mostly heterogeneity), as they included the same studies (Aschwanden 2008; Brandjes 1997; Ginsberg 2001; Jayaraj 2015; Kahn 2014; Partsch 2004; Prandoni 2004; Roumen‐Klappe 2009). Both of these reviews concluded that compression therapy reduced the overall incidence of PTS, but not of severe PTS specifically. Two recent reviews were also reluctant to draw firm conclusions based on current evidence (Berntsen 2016; Jin 2016). These review authors found no reduction in the incidence of PTS with compression treatment. One of these reviews ‐ Jin 2016 ‐ analysed only studies using the Villalta or Ginsberg scoring system, for which they made two subgroups (Ginsberg 2001; Kahn 2014; Prandoni 2004). These findings confirm the need to use a single scoring system in future trials; review authors recommend using the Villalta Scale.
Authors' conclusions
Implications for practice.
Low‐quality evidence suggests that elastic compression stockings reduce the occurrence of post‐thrombotic syndrome (PTS) after deep vein thrombosis (DVT). Included studies have reported no serious adverse effects and generally high compliance. Thigh‐length stockings do not provide better protection than knee‐length stockings against development of PTS and recurrence of DVT, according to one study. Quality of life was not significantly improved with compression stockings, except in the first nine days after DVT. In terms of PTS incidence, wearing these stockings for two years is likely the better choice over wearing them for one year, according to one non‐inferiority trial.
Implications for research.
Larger randomised double‐blind placebo‐controlled trials are needed to confirm the findings of this review because currently high‐quality evidence is lacking and heterogeneity is considerable. It is uncertain whether stockings should be prescribed immediately or after compression bandaging during the acute phase (one to two weeks) of DVT. In addition, it is important to study how long stockings must be worn. We found only one trial on this topic that we could include (Mol 2016) and one ongoing trial that is studying duration of compression (NCT01429714), highlighting the need for additional trials conducted to explore this matter. We also identified a need for researchers to compare elastic compression stockings versus different compression classes for prevention of PTS. None of the included studies assessed costs. Most compared the use of compression stockings versus other forms of compression therapy or versus no compression therapy. Future trials conducted to compare stockings and to evaluate their use versus other forms of non‐pharmaceutical treatment (e.g. intermittent pneumatic compression) are warranted. Assessment of cost perspectives could prove useful for future researchers. Differences among measurement scales used give rise to variability of PTS incidence. This heterogeneity makes it difficult to correctly combine data. Future studies should use one single measurement scale, for which we advise using the Villalta Scale (Kahn 2009).
Feedback
Results: Secondary outcomes
Summary
I found this review interesting and very useful.
You mentioned in the Results section, secondary outcomes: No information was available in those three studies about recurrence of deep venous thrombosis, pulmonary embolism, compliance with compression therapy, or costs.
I wonder whether this involves Brandjes study 1997, as in this study the authors found no difference in the recurrence rate of DVT between both groups of the study (14 of the 96 patients in the stocking group and 13 of the 98 patients in the control group developed documented recurrence of venous thromboembolism)?
Regarding assessment of compliance the authors of the same study reported that 73 of the 96 patients always wore their stockings a further 16 patients usually wore the stockings.
References: 1. Kolbach DN, Sandbrink MWC, Hamulyak K, Neumann HAM, Prins MH. Non‐pharmaceutical measures for prevention of post‐thrombotic syndrome. The Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No.: CD004174. DOI: 10.1002/14651858.CD004174.pub2.
2.Brandjes DP, Buller HR, Heijboer H, Huisman MV, de Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal‐vein thrombosis. Lancet 1997;349(9054):759‐62.
Reply
For reply, see next question.
Contributors
Feedback: S Sawan, Research Fellow, saldin.sawan@ghnt.nhs.uk
Response: Diebrecht Appelen, Eva van Loo, Martin H Prins, Martino HAM Neumann, and Dinanda N Kolbach
Results: Secondary outcomes
Summary
Further to my previous correspondence (earlier today 25/08/05), I have had chance to read Pandoni's 2004 paper. Again about the same point of secondary outcomes, the authors of this study reported 25 cases of confirmed recurrent venous thromboembolic complications (12 patients in the elastic group and 13 in the control group).
The authors reported as well that 78 patients out of 90 used their stockings for at least 80% of daytime hours; I think this is a good compliance rate.
Thanks in advance for clarifying those points.
References: 1. Kolbach DN, Sandbrink MWC, Hamulyak K, Neumann HAM, Prins MH. Non‐pharmaceutical measures for prevention of post‐thrombotic syndrome. The Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No.: CD004174. DOI: 10.1002/14651858.CD004174.pub2.
2. Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Annals of Internal Medicine 2004;141(4):249‐56.
Reply
Dear Dr Sawan, We thank you for your appraisal and correct input for this review. In reply to your questions, as part of updating this review we assessed the points you mentioned and adjusted any errors or missing information in the review text. If you have any further remarks or suggestions, we are eager to hear them.
Kind regards,
Contributors
Feedback: S Sawan, Research Fellow, saldin.sawan@ghnt.nhs.uk Response: Diebrecht Appelen, Eva van Loo, Martin H Prins, Martino HAM Neumann, and Dinanda N Kolbach
What's new
| Date | Event | Description |
|---|---|---|
| 9 August 2017 | New search has been performed | Search updated. Six new studies included and 22 new studies excluded. Three new ongoing studies identified |
| 9 August 2017 | New citation required but conclusions have not changed | Search updated. Six new studies included and 22 new studies excluded. Three new ongoing studies identified. Text amended to reflect current Cochrane standards, and 'Summary of findings' table added. Feedback addressed. No change to conclusions |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 25 August 2016 | Feedback has been incorporated | Feedback addressed |
| 22 April 2008 | Amended | Converted to new review format |
| 15 November 2005 | Amended | Five possible trials awaiting assessment, 1 of which provides follow‐up data for Partsch 2000. For personal reasons, update has been delayed for 6 months. Now expected May 2006 |
| 15 November 2005 | Feedback has been incorporated | Two comments added to the "Comments and criticism" section. A response will be added when the review is updated. |
| 3 November 2004 | Amended | Full article of Prandoni trial (Prandoni 2004) added to "Included studies" references |
| 17 November 2003 | New citation required and conclusions have changed | First review version |
Acknowledgements
The Cochrane Vascular Information Specialist assisted with searching of the literature for relevant trials.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Postthrombotic Syndrome EXPLODE ALL TREES | 24 |
| #2 | postthrombotic:TI,AB,KY | 64 |
| #3 | (post near3 thrombot*):TI,AB,KY | 123 |
| #4 | PTS*:TI,AB,KY | 7161 |
| #5 | MESH DESCRIPTOR Postphlebitic Syndrome EXPLODE ALL TREES | 23 |
| #6 | postphlebit*:TI,AB,KY | 35 |
| #7 | (post near3 phlebit*):TI,AB,KY | 15 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 7304 |
| #9 | MESH DESCRIPTOR Bandages | 1362 |
| #10 | MESH DESCRIPTOR Compression Bandages EXPLODE ALL TREES | 222 |
| #11 | (stocking* or hosiery or tights or sock*):TI,AB,KY | 1321 |
| #12 | MESH DESCRIPTOR Exercise EXPLODE ALL TREES | 15607 |
| #13 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES | 7979 |
| #14 | MESH DESCRIPTOR Physical Exertion EXPLODE ALL TREES | 3485 |
| #15 | MESH DESCRIPTOR Exercise Movement Techniques EXPLODE ALL TREES | 1262 |
| #16 | MESH DESCRIPTOR Locomotion EXPLODE ALL TREES | 4960 |
| #17 | exercis*:TI,AB,KY | 48183 |
| #18 | (physical near3 (exertion or endurance or therap* or conditioning or activit* or fitness)):TI,AB,KY | 22945 |
| #19 | mobil*:TI,AB,KY | 10385 |
| #20 | walk*:TI,AB,KY | 12173 |
| #21 | compress*:TI,AB,KY | 5288 |
| #22 | MESH DESCRIPTOR Bed Rest | 374 |
| #23 | (bed rest):TI,AB,KY | 834 |
| #24 | prevent*:TI,AB,KY | 129650 |
| #25 | #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 | 195354 |
| #26 | #8 AND #25 | 994 |
Appendix 2. Trial registries searches
ClinicalTrials.gov
85 studies found for postthrombotic OR post‐thrombotic
World Health Organization International Clinical Trials Registry Platform
39 records for 35 trials found for postthrombotic OR post‐thrombotic
ISRCTN Register
9 results postthrombotic OR post‐thrombotic
Data and analyses
Comparison 1. Compression stockings vs placebo or no stockings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative incidence of any post‐thrombotic syndrome | 5 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.01] |
| 1.1 Smaller population | 4 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.66] |
| 1.2 Larger population | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.86, 1.18] |
| 2 Cumulative incidence of severe post‐thrombotic syndrome | 4 | 1224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 2.1 Smaller population | 3 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.80] |
| 2.2 Larger population | 1 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.74, 2.28] |
| 3 Recurrence of deep venous thrombosis | 4 | 1212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.28] |
Comparison 2. Compression in the acute phase of deep venous thrombosis to prevent post‐thrombotic syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative incidence of any post‐thrombotic syndrome | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.49, 1.16] |
Comparison 3. Thigh‐length vs knee‐length compression stockings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative incidence of any post‐thrombotic syndrome | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 2 Recurrence of deep venous thrombosis | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.46, 2.10] |
Comparison 4. One year versus two years of compression stockings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative incidence of any post‐thrombotic syndrome | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.03, 2.29] |
| 2 Recurrence of deep venous thrombosis | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.48, 3.88] |
Comparison 5. Sensitivity analysis: effect of compression treatment on PTS incidence and recurrence of DVT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cumulative incidence of any post‐thrombotic syndrome | 7 | 1494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.92] |
| 1.1 Smaller population | 6 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.69] |
| 1.2 Larger population | 1 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.18] |
| 2 Cumulative incidence of severe post‐thrombotic syndrome | 5 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.15] |
| 2.1 Smaller population | 4 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.80] |
| 2.2 Larger population | 1 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.74, 2.28] |
| 3 Recurrence of deep venous thrombosis | 5 | 1249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aschwanden 2008.
| Methods | Study design: randomised controlled trial Method of randomisation: computer‐generated randomisation list Concealment of allocation: yes Blinding: no Follow‐up: 3.2 to 2.9 years (median 3.2) Losses to follow‐up: 55 participants due to recurrent thrombosis, severe illness, migration, withdrawal of informed consent, and death |
|
| Participants | Country: Switzerland No. of participants: 169 Age: intervention group: median age 64.1 years (range 52.9 to 72.5); control group: median age 63.8 years (range 45.3 to 70.8) Sex: intervention group: 54 men (64.3%) and 45 women (35.7%); control group: 45 men (52.9%) and 40 women (47.1%) Inclusion criteria: age > 18 years and first or recurrent proximal (popliteal vein or more proximal) DVT confirmed by duplex ultrasound (DUS) imaging Exclusion criteria: CVI C4 to C6 by CEAP classification, advanced malignancy, death anticipated to occur < 2 years, long‐lasting immobilisation, geographic inaccessibility, dementia, peripheral artery disease contraindicating compression therapy, anticipated lack of compliance, and refused informed consent |
|
| Interventions | Intervention group (n = 84): compression stockings after 6 months of standard care (including compression stockings) Comparison group (n = 85): no compression stockings after 6 months of standard care (including compression stockings) |
|
| Outcomes | Primary: post‐thrombotic skin changes according to C4 or higher Secondary: PTS‐related symptoms |
|
| Notes | Standard care consisted of heparin in initial phase, followed by oral anticoagulation and compressing stockings with ankle pressure 26.3 to 36.1 mmHg for 6 months. Patients were screened for study inclusion after finishing 6 months of therapy. Any skin‐related endpoint detected for the first time had to be confirmed by a consensus of 2 outcome assessors at a second visit 3 months later. This second date was used as the event date for analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Citation: “Treatment allocation to compression stocking (intervention) or no stocking (control) was concealed and performed by a study nurse not involved in the trial by means of a computer‐generated randomization list.” |
| Allocation concealment (selection bias) | Low risk | Citation: “Treatment allocation to compression stocking (intervention) or no stocking (control) was concealed and performed by a study nurse not involved in the trial by means of a computer‐generated randomization list.” |
| Blinding (performance bias and detection bias) Objective outcome(s) | High risk | Outcome PTS: no blinding of outcome assessors (specialists in vascular medicine) |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | The subjective outcome 'PTS‐associated symptoms' could be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Survival analysis with censoring of loss to follow‐up. Reasons for follow‐up in flow‐diagram. Withdrawal of informed consent is not further specified; unsure whether this could be related to outcome. However, numbers and reasons are balanced across groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes and predictor variables are reported. |
| Other bias | Low risk | Baseline imbalances in sex. Women may have better results with compression stockings, some other studies suggest. However, in this study, the control group wore no stockings, so no influence of sex should be evident. |
Brandjes 1997.
| Methods | Study design: randomised controlled trial Method of randomisation: sealed envelopes, blocks of 8 Concealment of allocation: not stated Blinding: participants not blinded. Observer and outcome assessor: blinding unclear Follow‐up: 5 to 8 years (median 6.3) Losses to follow‐up: treatment group, 4 were lost to follow‐up and 19 died; non‐intervention group, 2 were lost to follow‐up and 18 died |
|
| Participants | Country: The Netherlands No. of participants: 194 Age: mean age 60 years Sex: males 109; females 86 Inclusion criteria: consecutive outpatients with a first episode of venogram‐proven proximal DVT Exclusion criteria: life expectancy < 6 months, paralysis of the leg, bilateral thrombosis, leg ulcers or extensive varicosity, and current use of compression stockings |
|
| Interventions | Intervention group (n = 96): below‐knee elastic compression stockings, made to measure (Neodurelna Varitex) with an ankle pressure of 40 mmHg Control group (n = 98): no intervention Start of compression stockings: 2 to 3 weeks after DVT diagnosis |
|
| Outcomes | Primary: cumulative incidence of mild to moderate PTS Secondary: VTE recurrence, compliance |
|
| Notes | Follow‐up assessments were scheduled at 3 and 6 months, then every 6 months. At each follow‐up visit, presence and severity of PTS were scored on a standardised scale. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation. Quote:"Randomisation was done by a sealed envelope technique in blocks of eight." |
| Allocation concealment (selection bias) | Low risk | Quote:"Randomisation was done by a sealed envelope technique in blocks of eight." |
| Blinding (performance bias and detection bias) Objective outcome(s) | Unclear risk | Quote: "Patients were asked not to wear their stockings on the day of assessment. Each assessment was done by an independent investigator who was unaware of the measurements recorded at the previous assessment and who was not involved in the final scoring." |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | The outcome PTS was based on a scoring system containing subjective and objective criteria. Subjective criteria, most of which were patient‐reported items, are at high risk of bias because participants were not blinded. For objective criteria, risk of bias is unclear. Blinding of investigators is not described clearly. Furthermore, stockings could leave typical imprints on the skin, which could lead to breaking of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for loss of follow‐up are reported and balanced across groups. A Kaplan‐Meier analysis was done to deal with censored cases. |
| Selective reporting (reporting bias) | Low risk | No study protocol is available. All outcomes of the "Methods" section of the article are reported. |
| Other bias | Low risk | Study appears to be free of other potential sources of bias. |
Ginsberg 2001.
| Methods | Study design: randomised double‐blind clinical trial Method of randomisation: prerandomisation stratification applied, randomisation not stated Concealment of allocation: not stated Blinding: observer and outcome assessor blinded Follow‐up: mean 4.6 to 4.9 years Losses to follow‐up: not reported, but 3 participants in the treatment group died (none in the control group) |
|
| Participants | Country: Ontario, Canada No. of participants: 47 Age: mean age 61 years Sex: males 26; females 21 Inclusion criteria: asymptomatic postphlebitic syndrome and venous valvular incompetence (as assessed by photoplethysmography or venous Doppler) Exclusion criteria: previous graduated compression stocking therapy, geographic inaccessibility, and failure to provide informed consent |
|
| Interventions | Intervention group (n = 24): elastic compression stockings; below‐knee elastic compression stockings with ankle pressure of 20 to 30 mmHg at the ankle region Control (n = 23): placebo stocking Start of compression stockings: 1 year after DVT diagnosis |
|
| Outcomes | Cumulative incidence of PTS | |
| Notes | Three‐part study; only data from study 2 are taken into account Study was terminated after an interim analysis owing to slow recruitment of patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Participants and outcome assessors were blinded. For participants, placebo stockings were provided. Participants were informed not to wear stockings to the assessments. |
| Blinding (performance bias and detection bias) Subjective outcome(s) | Low risk | Participants and outcome assessors were blinded. For participants, placebo stockings were provided. Participants were informed not to wear stockings to the assessments. The definition of PTS contained both objective and subjective items. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data are not described, but 3 participants (in the active stocking group) died. |
| Selective reporting (reporting bias) | Low risk | Only PTS was a prespecified outcome; this is reported. |
| Other bias | Low risk | Study seems to be free of other risk of bias. |
Jayaraj 2015.
| Methods | Study design: randomised controlled trial Method of randomisation: sealed envelopes, with separate blocks for each of the 2 participating institutions Concealment of allocation: yes Blinding: single‐blind. The investigator scoring the presence and severity of PTS was blinded to treatment allocation and results of previous measurements. Follow‐up: 2 years Losses to follow‐up: not accurately described. Seventeen in the treatment group were lost to follow‐up or died; 20 in the non‐intervention group were lost to follow‐up or died. |
|
| Participants | Country: United States Number of participants: 69 Age: mean age 48 years Sex: 34 (49%) female Inclusion criteria: acute proximal DVT of the lower extremity documented by duplex ultrasonography. In patients with bilateral acute proximal vein thrombosis, the limb with more extensive disease was designated as the study limb. Exclusion criteria: inability to provide consent, return for follow‐up, or be followed in an anticoagulation clinic; previous DVT, DVT isolated to the calf veins, contraindications to standard anticoagulation, arterial insufficiency (ABI < 0.6), pre‐existing CVI (CEAP class C4 to C6), and current use of compression stockings |
|
| Interventions | Intervention group (n = 36): graduated compression stockings (below knee, 30 to 40 mmHg) Control group (n = 33): no stockings Time from diagnosis to randomisation (start of compression stockings): within 48 hours |
|
| Outcomes | Primary outcome: assessment of the impact of graduated compression stockings on prevention of PTS Secondary outcomes: comparison of different instruments used to assess PTS including VCSS and VPS instruments |
|
| Notes | Complete published version of Jayaraj 2011, including results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation Quote:"Consenting patients were randomised at the time of enrolment by means of sealed envelopes, with separate blocks for each of the two participating institutions affiliated with the University of Washington." |
| Allocation concealment (selection bias) | Low risk | Quote:"Consenting patients were randomised at the time of enrolment by means of sealed envelopes, with separate blocks for each of the two participating institutions affiliated with the University of Washington." |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Quote: "Patients were instructed not to wear their stockings at their initial or follow‐up clinic visits and not to reveal their treatment allocation at such visits. Each assessment was performed by an investigator (different from the investigator who initially saw patient at the time of randomisation) who was aware of the side of the index DVT but was blinded to treatment allocation or results of previous measurements." |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | The outcome PTS was based on different scoring systems containing both subjective and objective criteria. Subjective criteria, most of which were patient‐reported items, are at high risk of bias because participants were not blinded. For objective criteria, risk of bias is rather low because of the above mentioned investigator blinding. However, the stockings could leave typical imprints on the skin, which could lead to breaking of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High amount of attrition in both groups, with the control group having greatest loss to follow‐up. Kaplan‐Meier analysis was conducted to deal with censored cases. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Study seems to be free of other risk of bias. |
Kahn 2014.
| Methods | Study design: multi‐centre randomised placebo‐controlled trial Method of randomisation: Web‐based randomisation system, stratified by centre and using varying block sizes of 4 and 8 Concealment of allocation: yes Blinding: Treatment allocation was masked from participants, healthcare providers, study personnel, and study statisticians. Follow‐up: 2 years Losses to follow‐up: 186 due to withdrawal, death, and loss |
|
| Participants | Country: Canada and USA Number of participants: 803 Age: mean age 55.1 years (SD 15.5 years) Sex: 483 of 803 participants (60.1%) were men. Inclusion criteria: consecutive patients with a first symptomatic objectively confirmed proximal DVT diagnosed within the previous 14 days (with or without concurrent distal DVT or PE), age > 18 years, no contraindications to standard treatment with heparin and/or warfarin, and provided informed consent to participate Exclusion criteria: contraindication to use of compression stockings (allergy, claudication), limited lifespan (estimated < 6 months), geographic inaccessibility preventing return for follow‐up visits, inability to apply stockings daily and unavailability of a caregiver to apply stockings daily, and treatment of acute DVT with thrombolytic agents |
|
| Interventions | Intervention group (n = 409): knee‐length, 30 to 40 mmHg (Class II), graduated ECS worn on the DVT‐affected leg daily for 2 years Comparison group (n = 394): knee‐length, inactive (i.e. no compression/< 5 mmHg) stocking, identical in appearance to active ECS, worn on the DVT‐affected leg daily for 2 years Start of compression stockings: within 2 weeks of DVT diagnosis |
|
| Outcomes | Primary: cumulative incidence of PTS from 6 to 24 months' follow‐up Secondary: presence of leg ulcers, VTE recurrence, death, adverse events, venous valvular reflux, quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Web‐based randomisation system, stratified by centre and used varying block sizes of four and eight.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were randomly assigned to study groups with a web‐based randomisation system (TrialStat, Jubilant Clinsys, Ottawa, ON, Canada), which ensured concealed allocation.” |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Outcome: (severity) of PTS, adverse events, enough measures taken to ensure blinding of participants and personnel |
| Blinding (performance bias and detection bias) Subjective outcome(s) | Unclear risk | Outcome: quality of life. This outcome could be influenced if participants guessed their allocated treatment correctly, but this was the case in only 41% of the active ECS group and 17% of the placebo group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Kaplan‐Meier survival analysis was done to deal with censored cases. However, descriptions of reasons for loss to follow‐up are very brief/unclear, so it is unclear whether reasons for withdrawal are related to outcomes. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available, and all prespecified outcomes have been reported. |
| Other bias | Low risk | Some baseline imbalances between treatment groups were noted: Active stocking group contains slightly more men (62.4% vs 57.9%). This likely does not affect study results. |
Mol 2016.
| Methods | Study design: multi‐centre single‐blind non‐inferiority randomised controlled trial Method of randomisation: online randomisation module; stratified by sex, study centre, and timing of randomisation per season of the year Concealment of allocation: yes Blinding: Treatment allocation was masked from study personnel during follow‐up. Participants who were allocated to standard care were asked to not wear the stocking on the day of follow‐up, and all study participants were instructed not to reveal their allocation at study visits. Follow‐up: 1 year Losses to follow‐up: 33 due to withdrawal, death, and loss |
|
| Participants | Country: The Netherlands Number of participants: 518 Age: mean age 56.5 years (SD 14 years) Sex: males 307; females 211 Inclusion criteria: compliant with compression therapy for 12 months after symptomatic and ultrasound‐proven proximal DVT of the leg Exclusion criteria: recurrent ipsilateral DVT, calf vein thrombosis, PTS diagnosed in the first year after DVT before study inclusion (Villalta score ≥ 5 points), use of ECS before the index DVT diagnosis for other reasons, contraindication to wearing compression stockings, life expectancy < 6 months, comorbid conditions or geographical inaccessibility precluding return for follow‐up visits, and no informed consent |
|
| Interventions | Intervention group (n = 256): cessation of ECS after 1 year of use (34 to 46 mmHg (Class III)) Comparison group (n = 262): continuation of ECS for 1 more year (34 to 46 mm Hg (Class III)) Start of compression stockings: uncertain, but most likely in the acute phase |
|
| Outcomes | Primary: incidence of PTS 24 months after diagnosis of DVT, as assessed by the standardised Villalta Scale Secondary: compliance (≥ 6 days a week), recurrent DVT in the same leg as the index event, death, within‐patient changes in quality of life over the study period as assessed by the VEINES‐QOL/Sym questionnaire |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomisation module; stratified for sex, study centre, and timing of randomisation per season of the year |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation used an online randomisation module provided by the Dutch Julius Centre for Health Sciences and Primary Care in Utrecht, the Netherlands, which ensured concealed allocation." |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Study personnel were blinded to treatment. Quote: "Patients who were allocated to standard care were asked not to wear the stocking on the day of follow‐ up, and all study participants were instructed not to reveal their allocation at study visits." |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | Participants were not blinded owing to the nature of this study. Subjective criteria, most of which were patient‐reported items, are at high risk of bias because participants were not blinded. For objective criteria, risk of bias is rather low because of the above mentioned investigator blinding. However, the stockings could leave typical imprints on the skin, which could lead to breaking of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Assuming a loss to follow‐up of 10%, including deaths and recurrent ipsilateral deep venous thrombosis events, the total study population was set at 516 people." All losses to follow‐up were accurately described and equal among groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported. |
| Other bias | Low risk | Study seems to be free of other risk of bias. |
Partsch 2004.
| Methods | Study design: randomised clinical trial (3 treatment arms) Method of randomisation: sealed envelopes Concealment of allocation: yes, sealed envelopes were supplied by a statistician Blinding: no Follow‐up: 2 years Losses to follow‐up: 3 died, 1 had an ulcer, 7 were reported to be well without specific problems, and 5 could not be contacted |
|
| Participants | Country: Vienna, Austria No. of participants: 53 in primary study. For assessment of PTS incidence, 37 participants were reinvestigated 2 years later. Age: mean age 58 years (baseline data available from 45 participants in first study, Partsch 2000) Sex: males 29; females 16 (baseline data available from 45 participants in first study, Partsch 2000) Inclusion criteria: proximal DVT, confirmed by compression ultrasound scan or phlebography in mobile patients older than 18 years. Duration of symptoms had to be less than 14 days. Exclusion criteria: compression therapy or heparin therapy already started; massive symptomatic PE or need for thrombolysis or thrombectomy; ABI < 0.8; pregnancy or breastfeeding; and contraindication for compression, anticoagulants, or both Participants included 7 patients with previous DVT in the same leg and 11 with previous DVT in the contralateral leg. |
|
| Interventions | Group A (n = 18): inelastic compression bandages (Unna boots on the lower leg, adhesive bandages on the thigh) Group B (n = 18): thigh‐length compression stockings (class II, 30 mmHg) Group C (n = 17): bed rest for 9 days without compression After 9 days, all participants were encouraged to walk with compression class II stockings. |
|
| Outcomes | Reduced pain and leg circumference on days 0 and 9; on both days, PE and extent of DVT
Clinical score calculated for the following symptoms: pain during walking; painful foot sole; painful calf palpation; subfascial oedema; increase in skin temperature; redness/cyanosis. Severity score comprised 4 stages: absent, mild, marked, and severe. Cumulative incidence of PTS |
|
| Notes | Information on cumulative incidence of PTS is available only for the mobile group (treatment arms A + B combined) vs the group with bed rest (arm C). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available on allocation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was performed with sealed envelopes. Methods of allocation and sequence generation are not described, and concealment of allocation cannot be judged. |
| Blinding (performance bias and detection bias) Objective outcome(s) | High risk | Participants and investigators were not blinded. |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | Participants and investigators were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for incomplete outcome data are described. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes are reported. PTS was not a primary outcome. PTS scores are described as bed rest vs mobile. No information is available on the comparison of PTS scores for the 3 treatment groups, but it is judged unlikely that this introduces bias. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Prandoni 2004.
| Methods | Study design: randomised controlled trial Method of randomisation: computer‐generated list, blocks of 20 Concealment of allocation: yes, list was accessible only by a trial nurse Blinding: participants not blinded, observer and outcome assessor blinded Follow‐up: 5 years Losses to follow‐up: treatment group ‐ 2 participants, plus 6 participants died; control group ‐ 13 participants. Follow‐up assessments were scheduled at 3 months and 6 months, then every 6 months. At each follow‐up visit, presence and severity of PTS were scored on a standardised scale. |
|
| Participants | Country: Italy No. of participants: 180 (consecutive) Age: mean age 62 years Sex: males 77; females 103 Inclusion criteria: first episode of symptomatic proximal DVT, confirmed by compression ultrasonography Exclusion criteria: recurrent ipsilateral thrombosis, short life expectancy, bilateral thrombosis, leg ulcers or signs of CVI, and contraindication for use of ECS |
|
| Interventions | Intervention group: below‐knee ready‐made flat knitted ECS with ankle pressure of 30 to 40 mmHg (Flebysan, Rovigo) at hospital discharge (± 1 week after admission) Control group: no stockings |
|
| Outcomes | Primary: cumulative incidence of PTS Secondary: VTE recurrence, compliance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomly assigned in blocks of 20 to the elastic stockings group .. or to the control group. Study group assignment was based on a computer‐generated list that was accessible only to a trial nurse, who informed study physicians about treatment allocation after patients had provided informed consent." |
| Allocation concealment (selection bias) | Low risk | See quote above. Low risk, assuming that the trial nurse who had access to the list was not involved in recruitment of patients and in judging eligibility |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Participants were instructed not to wear their stockings on the day of assessment and not to reveal their treatment location to the assessor. Participants were also instructed to use a notebook to record the length of time they wore the assigned stockings, and to bring the notebook to study physicians. It seems possible that assessors could read the notebook and blinding would be broken, but study authors describe that this information on protocol adherence was collected independently by another physician. |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | PTS was scored on the Villalta‐Prandoni Scale, which uses symptoms (subjective criteria) as well as clinical signs (objective). Subjective symptoms could be influenced because participants were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons for incomplete outcome data are described. Three participants died of PE; their treatment group is not described. The flow diagram is not clear on reasons for exclusion after 2 years. Six participants withdrew; this leaves 16 participants excluded from further study participation unexplained. |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section are reported. |
| Other bias | Unclear risk | In the control group, 12 participants wore stockings (12 to 18 mmHg); use of anticoagulant agents such as aspirin or NSAIDs during the first 21 days was greater in the control group (15 vs 6 in treatment group); the control group included a greater percentage of women (61.1% vs 53.3% in treatment group) (in other studies, stockings have been found to be more beneficial for women). Significant effect favouring ECS group was reported. |
Prandoni 2012.
| Methods | Study design: randomised controlled trial Method of randomisation: computer‐generated list. Randomisation was stratified by centre, and permuted blocks of various sizes were used. Concealment of allocation: Randomisation list was accessible only by a trial nurse who informed study physicians on treatment allocation after participants provided informed consent. Blinding: Participants were not blinded. The outcome assessor was blinded. Follow‐up: 3 years Losses to follow‐up: 66 participants |
|
| Participants | Country: Italy No. of participants: 267 Age: mean age 68 years Sex: 142 male; 125 female Inclusion criteria: first episode of symptomatic proximal DVT, as confirmed by compression ultrasonography Exclusion criteria: recurrent ipsilateral DVT, pre‐existing leg ulcers or signs of CVI, bilateral thrombosis, and short life expectancy or contraindication for the use of ECS (e.g. advanced‐stage peripheral arterial insufficiency, allergy to stockings) |
|
| Interventions | Thigh‐length group (n = 135): thigh‐length graded ECS Below‐knee length group (n = 132): below‐knee graded ECS Both started during the first week after DVT diagnosis. |
|
| Outcomes | Primary: 3‐year cumulative incidence of PTS Secondary: VTE recurrence, adverse effects, compliance and tolerability |
|
| Notes | Study included no control group (i.e. treatment group with placebo or with no intervention); different lengths of compression stockings are compared with each other. All participants wore elastic bandages the first few (4 to 5) days during hospital admission. Compression stockings were provided at hospital discharge. PTS was assessed on the Villalta Scale. Participants were followed for up to 3 years. Follow‐up visits occurred 3 and 6 months from the index event and every 6 months thereafter. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by centre, and permuted blocks of various sizes were used." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was according to a computer‐generated list that was accessible only to a trial nurse who informed study physicians on treatment allocation after patients had provided informed consent." |
| Blinding (performance bias and detection bias) Objective outcome(s) | Unclear risk | Study personnel: blinded, plus it is stated that the outcome assessor was not involved in care of the participant. However, participants held a diary, which they brought to study physicians; this diary may contain hints of the allocated treatment. Participants were instructed to not wear ECS to the assessment and to not disclose information on treatment allocation, but it is not clear whether they complied. |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | Participants: not blinded; can be a problem as subjective symptoms were also (but not exclusively) recorded to assess primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for loss to follow‐up are not further specified, with a difference of 5 participants between groups. Kaplan‐Meier analysis was conducted to deal with censored cases. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are addressed. Subgroup analyses of the primary outcome: unclear whether these were prespecified Not prespecified outcome reported: analysis of compliant participants only |
| Other bias | Low risk | Thigh‐length group included slightly more participants with recent trauma or surgery (22 (16.3%) vs 16 (12.1%)). These participants may be more immobile, leading to less effect of stockings and underestimating effects of thigh‐length stockings. Also, location of DVT was different in the 2 treatment groups, but adjustments for this factor were made with the Cox‐regression model. |
Roumen‐Klappe 2009.
| Methods | Study design: randomised controlled trial Method of randomisation: unclear Concealment of allocation: unclear Blinding: no Follow‐up: 1 year Losses to follow‐up: 4 (2 participants died, 2 were lost to follow‐up) |
|
| Participants | Country: The Netherlands No. of participants: 69 Age: bandaging group mean age 64 (range 25 to 80), control group mean age 53 (range 20 to 86) Sex: 32 male, 37 female Inclusion criteria: consecutive patients with symptomatic proximal and distal DVT, confirmed by compression ultrasonography Exclusion criteria: previous DVT, PE, short life expectancy (< 2 years), paralysis of the leg, pre‐existing leg ulcers or signs of venous insufficiency, bilateral thrombosis, arterial insufficiency (ABI < 0.9), malignant disease, pregnancy or puerperium, use of anti‐inflammatory medication, and other severe medical illnesses |
|
| Interventions | Intervention group: multi‐layer bandaging on the first day Comparison group: no multi‐layer bandaging |
|
| Outcomes | Development of clinical PTS after 1 year (CEAP and Villalta scores) | |
| Notes | After reduction of oedema in the acute phase, all participants received sized‐to‐fit graded ECSs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on generation of allocation sequence is provided in the published report. |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment of allocation is provided in the published report. |
| Blinding (performance bias and detection bias) Objective outcome(s) | Low risk | Quote: "This study has some limitations. Firstly randomisation was not blind. However, the vascular technicians and the dermatologist who classified PTS after 1 year were not informed." Blinding of outcome assessors is unclear; it is not clear whether vascular technicians and dermatologists were uninformed (treatment allocation or previous outcomes of venous examination). In our opinion, this does not have a large effect on assessment of the clinical score of CEAP, as this involves assessing the presence of objectively noticeable factors. |
| Blinding (performance bias and detection bias) Subjective outcome(s) | High risk | No blinding to allocated interventions of participants. Blinding of participants is not possible owing to the nature of the intervention. Both CEAP and Villalta scoring were used, including subjective items. Therefore, lack of blinding of participants could influence outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions and reasons for missing participant data are similar between treatment groups, so bias is unlikely. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in the Methods section are reported. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
ABI: ankle brachial index. CEAP: clinical, etiology, anatomy, pathophysiology. CVI: chronic venous insufficiency. DUS: duplex ultrasonography. DVT: deep vein thrombosis. ECS: elastic compression stockings. NSAIDs: non‐steroidal anti‐inflammatory drugs. PE: pulmonary embolism. PTS: post‐thrombotic syndrome. SD: standard deviation. VCSS: Venous Clinical Severity Score. VPS: Villalta‐Prandoni Scale. VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1994 | Participants were patients who had a hip replacement and were at risk of DVT. |
| Cairols 2011 | No compression used, pharmacological interventions compared |
| Chylarecki 1996 | Participants were trauma patients. |
| Dennis 2013 | Participants were stroke patients. |
| Enden 2009 | Compares CDT with standard anticoagulation |
| Gonzalez‐Fajardo 2008 | Compares different anticoagulants |
| Hull 2009 | Compares different anticoagulants |
| ISRCTN81127756 | Study stopped owing to poor recruitment. No further information |
| Junger 2006 | All participants wore compression stockings. Mobilisation was compared with immobilisation. |
| Kahn 2003 | Assessed compression stockings for acute leg symptoms and signs during exercise in patients with DVT. Almost 50% of the study population already had PTS when the intervention was applied. In the group without PTS, development of PTS was not assessed. |
| Kahn 2005 | Compares different doses of warfarin |
| Kahn 2011 | Compares exercise vs no exercise |
| Lewis 1976 | Participants were not DVT patients. |
| Markevicius 2004 | Comparing CDT vs standard anticoagulation |
| Moser 1976 | Participants were not DVT patients. |
| NCT02148029 | Compares exercise vs no exercise |
| Rahman 2009 | All participants received stockings, and all groups received different kinds and dosages of anticoagulants. Occurrence of PTS was not assessed. |
| Ratiu 2009 | Compares exercise vs no exercise |
| Romera 2005 | Compares early immobilisation and stockings in patients with bed rest for incidence of PE. Occurrence of PTS was not assessed. |
| Schulman 2006 | Comparing anticoagulant duration for treatment of VTE |
| Schwarz 1998 | Compared mobilisation vs immobilisation |
| Vedantham 2013 | Comparing catheter‐directed thrombolysis vs standard anticoagulation |
CDT: catheter‐directed thrombolysis. DVT: deep vein thrombosis. PE: pulmonary embolism. PTS: post‐thrombotic embolism. VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
NCT01429714.
| Trial name or title | The IDEAL DVT Study |
| Methods | Study design: randomised controlled trial Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: single‐blind (caregiver) Primary purpose: prevention Losses to follow‐up: not available yet |
| Participants | Countries: The Netherlands and Italy n = 864 Inclusion criteria:
Exclusion criteria:
|
| Interventions | All participants are advised to wear the ECS during the first 6 months after DVT Intervention group (n = 432): Duration of ECS therapy is individually tailored on the basis of Villalta scores.
Comparison group (n = 432): ECS daily for a total duration of 2 years |
| Outcomes | Primary outcome: development of PTS at 24 months after DVT Secondary outcomes: ‐ Health‐related quality of life (HRQOL) ‐ Costs ‐ Recurrent thrombosis ‐ VTE‐related death during follow‐up ‐ Patient preferences |
| Starting date | March 2011 |
| Contact information | Principal Investigator: Arina J Ten Cate‐Hoek, MD, PhD, MpH Maastricht University Medical Centre |
| Notes |
NCT01578122.
| Trial name or title | Elastic Compression Stockings for Prevention of Post‐thrombotic Syndrome (CELEST) |
| Methods | Study design: randomised controlled trial Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (participant, investigator) Primary purpose: treatment Losses to follow‐up: not available yet |
| Participants | Country: France n = 350 ≥ 18 years (adult, senior) Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | June 2012 |
| Contact information | Contact: Jean‐Luc BOSSON, MD, PhD + 33 476765040 JLBosson@chu‐grenoble.fr Contact: Carole ROLLAND + 33 476765040 CarRolland@chu‐grenoble.fr |
| Notes |
NCT03039517.
| Trial name or title | Post‐Thrombotic Syndrome Prevention Study |
| Methods | Study design: randomised controlled trial Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: no masking Primary purpose: treatment Losses to follow‐up: not available yet |
| Participants | Country: United States n = 84 ≥ 18 years of age (adult, senior) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Control group will receive care using elastic compression stocking, and intervention group will use the ACTitouch device (pneumatic compression).
|
| Outcomes | Primary outcome: development of PTS Villalta score [time frame: 2 years] The Villalta score is a disease score specific for PTS that can be used to both diagnose and categorise the severity of the condition. It was developed in a cross‐sectional study of 100 patients who were assessed 6 to 36 months after DVT.
|
| Starting date | January 2017 |
| Contact information | Principal investigator: Thomas Maldonado, MD, New York University School of Medicine. Contact: Juanita Erbjuanita.erb@nyumc.org Contact: Gina Bernardezgina.bernardez@nyumc.org |
| Notes |
ABI: ankle brachial index. BMI: body mass index. CEAP: clinical, etiology, anatomy, pathophysiology. CTV: computed tomographic venography. CVI: chronic venous insufficiency. DVT: deep vein thrombosis. ECS: elastic compression stockings. HRQOL: health‐related quality of life. IVC: inferior vena cava. MR: magnetic resonance. MRV: magnetic resonance venography. PAD: peripheral arterial disease. PE: pulmonary embolism. PTS: post‐thrombotic syndrome. SF‐36: Short Form‐36 quality of life questionnaire. VTE: venous thromboembolism.
Differences between protocol and review
We amended the title and the objective of the review to reflect its intention to assess effectiveness and complications involved in using compression therapy for people with deep vein thrombosis (DVT) for prevention of post‐thrombotic syndrome (PTS). We removed impedance plethysmography as an acceptable tool for diagnosis of DVT, as this is no longer an acceptable standard, and we have further clarified the planned types of interventions.
We have added compliance as a secondary outcome. Although we did not prespecify in our protocol any subgroups for analysis, we did perform subgroup analyses to explore causes of heterogeneity.
Contributions of authors
DA: assessed studies, extracted data, performed analyses, and wrote the update. DK: identified and assessed studies, and extracted data. EvL: assessed studies and extracted data. MHAMN: checked rejected studies and helped to clarify key points in the discussion. MHP: checked rejected studies.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Goverment, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
DA: none known. DK: none known. EvL: none known. MHAMN: none known. MHP: has declared that his institution received payments from companies (Daichi Sankyo, Pfizer, Bayer, Sanofi, Boeringer Ingelheim) involved in development of direct anticoagulants for treatment of thrombosis.
No review authors have present or past affiliations nor other involvement in any organisation or entity with an interest in the outcome of this review that might lead to a real or perceived conflict of interest. This includes acting as an investigator for a study that might be included in this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aschwanden 2008 {published data only}
- Aschwanden M, Jeanneret C, Koller MT, Thalhammer C, Bucher HC, Jaeger KA. Effect of prolonged treatment with compression stockings to prevent post‐thrombotic sequelae: a randomized controlled trial. Journal of Vascular Surgery 2008;47(5):1015‐21. [DOI] [PubMed] [Google Scholar]
Brandjes 1997 {published data only}
- Brandjes DP, Buller HR, Heijboer H, Huisman MV, Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal‐vein thrombosis. Lancet 1997;349(9054):759‐62. [DOI] [PubMed] [Google Scholar]
- Brandjes DPM, Rutten GCFM, Heijboer H, Huisman MV, Borm JJJ, Cate JW, et al. Elastic compression stockings in the prevention of the post thrombotic syndrome in patients with a proximal deep vein thrombosis; an interim analysis. Thrombosis and Haemostasis 1989;62:130. [Google Scholar]
- Rutten GCFM, Brandjes DPM, Huisman M, Heyboer H, Jagt H, Buller HR, et al. The effect of a size to fit graded compression stocking on the development of the post thrombotic syndrome (PTS) in patients with a proximal deep vein thrombosis, measured with a clinical score: an interim analysis. British Journal of Haematology 1990;76(Suppl 1):19. [Google Scholar]
Ginsberg 2001 {published data only}
- Ginsberg JS, Hirsh J, Julian J, Vander Laan de Vries M, Magier D, MacKinnon B, et al. Prevention and treatment of postphlebitic syndrome: results of a 3‐part study. Archives of Internal Medicine 2001;161(17):2105‐9. [DOI] [PubMed] [Google Scholar]
Jayaraj 2015 {published data only}
- Jajaray A, Natiello C, Nicholls S, Meissner M. Impact of graduated compressive stockings on the prevention of post‐thrombotic syndrome: results of a randomized trial. Journal of Vascular Surgery 2011;53(103s):SS33. [Google Scholar]
- Jayaraj A, Meissner M. Impact of graduated compression stockings on the prevention of post‐thrombotic syndrome ‐ results of a randomized controlled trial. Phlebology / Venous Forum of the Royal Society of Medicine 2015;30(8):541‐8. [DOI] [PubMed] [Google Scholar]
- Jayaraj A, Natiello C, Nicholls S, Meissner M. Impact of graduated compressive stockings on the prevention of P‐thrombotic syndrome: results of a randomized trial. Journal of Vascular Surgery 2011;53(6 Suppl 1):103S‐4S. [Google Scholar]
Kahn 2014 {published data only}
- Houweling AHS. Blinding strategies and their effect in a randomized placebo‐controlled trial (RCT) of elastic compression stockings (ECS) for prevention of post‐thrombotic syndrome (PTS). Clinical Trials 2013;10(S50):S51. [Google Scholar]
- Kahn SR, Shapiro S, Ducruet T, Wells PS, Rodger MA, Kovacs MJ, et al. Graduated compression stockings to treat acute leg pain associated with proximal DVT. A randomised controlled trial. Thrombosis and Haemostasis 2014;112(6):1137‐1141. [DOI] [PubMed] [Google Scholar]
- Kahn SR, Shapiro S, Ducruet T, Wells PS, Rodger MA, Kovacs MJ, et al. Graduated compression stockings to treat acute leg pain associated with proximal DVT: a randomised controlled trial. Thrombosis and Haemostasis 2014;112(6):1137‐41. [DOI] [PubMed] [Google Scholar]
- Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet 2014;383(9920):880‐8. [DOI] [PubMed] [Google Scholar]
- Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson RD, et al. A multicenter randomized placebo controlled trial of compression stockings to prevent the post‐thrombotic syndrome after proximal deep venous thrombosis: the S.O.X. trial. Blood 2012;120:21. [Google Scholar]
- Kahn SR, Shbaklo H, Shapiro S, Wells PS, Kovacs MJ, Rodger MA, et al. Effectiveness of compression stockings to prevent the post‐thrombotic syndrome (the SOX Trial and Bio‐SOX biomarker substudy): a randomized controlled trial. BMC Cardiovascular Disorders 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00143598. The SOX trial: compression stockings to prevent post thrombotic syndrome. https://clinicaltrials.gov/ct2/show/NCT00143598 (first received 1 September 2005).
- Rabinovich A, Cohen JM, Cushman M, Houweling AH, Sharpiro S, Wells PS, et al. Inflammation markers and the risk of post‐thrombotic syndrome: results from the Bio‐Sox Study. Blood 2013;122:36. [Google Scholar]
Mol 2016 {published data only}
- Mol GC, Ree MA, Klok FA, Tegelberg MJ, Sanders FBM, Koppen S, et al. One versus two years of elastic compression stockings for prevention of post‐thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ 2016;353:i2691. [DOI: 10.1136/bmj.i2691.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol GC, Ree MN, Klok FA, Tegelberg MJAM, Sanders FBM, Koppen S, et al. Optimal duration of compression therapy as prevention of chronic venous insufficiency after deep venous thrombosis. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1442 (date registered: 4 September 2008).
Partsch 2004 {published data only}
- Blättler W, Partsch H. Leg compression and ambulation is better than bed rest for the treatment of acute deep venous thrombosis. International Angiology 2003;22(4):393‐400. [PubMed] [Google Scholar]
- Partsch H. The role of compression in preventing postthrombotic syndrome. 2003 UIP World Congress Chapter Meeting; San Diego, California. 2003 August 27‐31.
- Partsch H. Thrombophlebitis: bed rest or walking exercise?. Wiener Medizinische Wochenschrift 1999;149(2‐4):50‐3. [PubMed] [Google Scholar]
- Partsch H, Blättler W. Compression and walking versus bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin. Journal of Vascular Surgery 2000;32(5):861‐9. [CRSREF: 2819992] [DOI] [PubMed] [Google Scholar]
- Partsch H, Kaulich M, Mayer W. Immediate mobilisation in acute vein thrombosis reduces post‐thrombotic syndrome. International Angiology 2004;23(3):206‐12. [PubMed] [Google Scholar]
Prandoni 2004 {published data only}
- Prandoni P. Below‐knee compression stockings for prevention of the post‐thrombotic syndrome. A randomized study. Proceedings of the XIX Congress of the International Society on Thrombosis and Haemostasis incorporating the 49th Annual SSC Meeting; 2003 July 12‐18; Birmingham, AL. Blackwell Publishing, 2003.
- Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Annals of Internal Medicine 2004;141(4):249‐56. [DOI] [PubMed] [Google Scholar]
Prandoni 2012 {published data only}
- NCT00426075. Full leg versus below knee elastic stockings for prevention of the post thrombotic syndrome. https://clinicaltrials.gov/ct2/show/NCT00426075 (first received January 23 2007).
- Prandoni P, Noventa F, Quintavalla R, Bova C, Cosmi B, Siragusa S, et al. Thigh‐length versus below‐knee compression elastic stockings for prevention of the post‐thrombotic syndrome in patients with proximal‐venous thrombosis: a randomized trial. Blood 2012;119(6):1561‐5. [DOI] [PubMed] [Google Scholar]
Roumen‐Klappe 2009 {published data only}
- Roumen‐Klappe E, Heijer M, Holewijn S, Wollersheim H. Compression bandaging in the acute phase of deep‐vein thrombosis reduces symptoms but does not decrease venous outflow resistance and thrombosis score as a proxy of the post‐thrombotic syndrome. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P1425. [Google Scholar]
- Roumen‐Klappe EM, Heijer M, Rossum J, Wollersheim H, Vleuten C, Thien T, et al. Multilayer compression bandaging in the acute phase of deep‐vein thrombosis has no effect on the development of the post‐thrombotic syndrome. Journal of Thrombosis and Thrombolysis 2009;27(4):400‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1994 {published data only}
- Adam O, Kormann B, Fisch R, Wiseman M. Prevention of deep vein thrombosis with intermittent sequential pneumatic leg compression (ISC) in patients undergoing total hip replacement. Annals of Hematology 1994; Vol. 68, issue Suppl 1:A91.
Cairols 2011 {published data only}
- Cairols MA, Romera A, Garcia Pelegri S, Martinez‐Rico C, Rizza N. Is long‐term LMWH better than oral anticoagulation in reducing the postthrombotic syndrome in patient with symptomatic DVT. 12th Meeting of the European Venous Forum Ljubljana, Slovenia. 2011 30 June ‐ 3 July.
Chylarecki 1996 {published data only}
- Chylarecki C, Hierholzer G, Rudofsky G. Can ankle motion device replace heparin in the prevention of thromboembolism in traumatology?. Orthopaedic Transactions 1996; Vol. 20:131.
- Chylarecki C, Hierholzer G, Rudofsky G. Can heparin be replaced by a movable ankle splint in thrombo‐embolism prophylaxis? Results of a clinical study. Hefte zur der Unfallchirurg 1996;257:119‐27. [Google Scholar]
Dennis 2013 {published data only}
- The CLOTS Trials Collaboration, Dennis M, Sandercock P, Reid J, Graham C, Murray G, et al. The effect of graduated compression stockings on long‐term outcomes after stroke. The CLOTS Trials 1 and 2. Stroke 2013;44(4):1075‐9. [DOI] [PubMed] [Google Scholar]
Enden 2009 {published data only}
- Enden T, Klow NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, et al. Catheter‐directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short‐term patency. Journal of Thrombosis and Haemostasis 2009;7(8):1268‐75. [DOI] [PubMed] [Google Scholar]
- Enden T, Sandvik L, Klow N‐E, Hafsahl G, Holme PA, Holmen LO, et al. Catheter‐directed Venous Thrombolysis in acute iliofemoral vein thrombosis ‐ the CaVenT Study: rationale and design of a multicenter, randomized, controlled, clinical trial (NCT00251771). American Heart Journal 2007;154(5):808‐14. [DOI] [PubMed] [Google Scholar]
- Enden T, Wik HS, Kvam AK, Haig Y, Kløw NE, Sandset PM. Health‐related quality of life after catheter‐directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non‐blinded, parallel‐group CaVenT study. BMJ Open 2013;3(8):e002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enden TH, Holme G. Long‐term outcome after additional catheter‐directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379(9810):31‐8. [DOI] [PubMed] [Google Scholar]
- Enden TR, Slagsvold CE, Klow NE, Sandset PM. Additional catheter‐directed venous thrombolysis in iliofemoral deep vein thrombosis; short‐term results from the CaVenT study, a multicenter, randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(Suppl 2):Abstract no: AS‐MO‐007. [Google Scholar]
- Enden TRH. Improved functional outcome after additional catheter‐directed thrombolysis for acute iliofemoral deep vein thrombosis: results of a randomized controlled clinical trial (The CaVenT Study). Blood 2011;118(21):LBA‐1. [Google Scholar]
Gonzalez‐Fajardo 2008 {published data only}
- Gonzalez‐Fajardo JA, Martin‐Pedrosa M, Castrodeza J, Tamames S, Vaquero‐Puerta C. Effect of the anticoagulant therapy in the incidence of post‐thrombotic syndrome and recurrent thromboembolism: comparative study of enoxaparin versus coumarin. Journal of Vascular Surgery 2008;48(4):953‐9. [DOI] [PubMed] [Google Scholar]
Hull 2009 {published data only}
- Hull RD, Pineo GF, Brant R, Liang J, Cook R, Solymoss S, et al. Home therapy of venous thrombosis with long‐term LMWH versus usual care: patient satisfaction and post‐thrombotic syndrome. American Journal of Medicine 2009;122(8):762‐9. [DOI] [PubMed] [Google Scholar]
ISRCTN81127756 {published data only}
- ISRCTN81127756. Does early compression therapy following deep vein thrombosis prevent development of post‐thrombotic limb syndrome?. ISRCTN Registry (date first received 10 August 2005).
Junger 2006 {published data only}
- Junger M, Diehm C, Storiko H, Hach‐Wunderle V, Heidrich H, Karasch T, et al. Mobilization versus immobilization in the treatment of acute proximal deep venous thrombosis: a prospective, randomized, open, multicentre trial. Current Medical Research and Opinion 2006;22(3):593‐602. [DOI] [PubMed] [Google Scholar]
- Junger M, Sannwald GA, Steins A, Storiko H. Prospective randomized study comparing mobilization and immobilization of patients with acute venous thrombosis of the leg. Annals of Hematology 2003;82:S42. [Google Scholar]
Kahn 2003 {published data only}
- Kahn SRA. Effect of graduated elastic compression stockings on leg symptoms and signs during exercise in patients with deep venous thrombosis: a randomized cross‐over trial. Journal of Thrombosis and Haemostasis 2003;1(3):494‐9. [DOI] [PubMed] [Google Scholar]
Kahn 2005 {published data only}
- Kahn SR, Kearon C, Julian JA, MacKinnon B, Kovacs MJ, Wells P, et al. Predictors of the post‐thrombotic syndrome during long‐term treatment of proximal deep vein thrombosis. Journal of Thrombosis and Haemostasis 2005;3(4):718‐23. [DOI] [PubMed] [Google Scholar]
- Kahn SR, Kearon C, Julian JA, MacKinnon B, Kovacs MJ, Wells P, et al. Prevalance and predictors of the post‐thrombotic syndrome during long‐term treatment of proximal deep vein thrombosis: results from a randomized trial comparing two intensities of anticoagulation. Blood 2003;102:57a. [DOI] [PubMed] [Google Scholar]
Kahn 2011 {published data only}
- Kahn SR, Shrier I, Shapiro S, Houweling AH, Hirsch AM, Reid RD, et al. Six‐month exercise training program to treat post‐thrombotic syndrome: a randomized controlled two‐centre trial. CMAJ Canadian Medical Association Journal 2011;183(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1976 {published data only}
- Lewis CE Jr, Antoine J, Mueller C, Talbot WA, Swaroop R, Edwards WS. Elastic compression in the prevention of venous stasis. A critical reevaluation. American Journal of Surgery 1976;132(6):739‐43. [DOI] [PubMed] [Google Scholar]
Markevicius 2004 {published data only}
- Markevicius N, Apanavicius G, Scerbinskas S. Comparison between long‐term results of catheter‐directed thrombolysis and anticoagulation in the treatment of acute iliofemoral deep vein thrombosis. Phlebology 2004; Vol. 19, issue 3:148‐9.
Moser 1976 {published data only}
- Moser G, Froidevaux A, Moser G, Froidevaux A. Prophylaxis of post‐operative deep venous thrombosis using small sub‐cutaneous heparin doses, associated or not with compressive stockings: comparative study and results. Schweizerische Rundschau fur Medizin Praxis 1976;65(33):1015‐20. [PubMed] [Google Scholar]
NCT02148029 {published data only}
- NCT02148029. Role of a novel exercise program to prevent post‐thrombotic syndrome (EFFORT2). clinicaltrials.gov/ct2/show/NCT02148029 (date first received 21 May 2014).
Rahman 2009 {published data only}
- Rahman A, Colak MC, Ustunel L, Koc M, Kocakoc E, Colak C. A comparison of different treatment managements in patients with acute deep vein thrombosis by the effects on enhancing venous outflow in the lower limb. Medical Science Monitor 2009;15(11):CR588‐93. [PubMed] [Google Scholar]
Ratiu 2009 {published data only}
- Ratiu A, Motoc A, Pascut D, Crisan DC, Anca T, Pascut M. Compression and walking compared with bed rest in the treatment of proximal deep venous thrombosis during pregnancy. Revista Medico‐Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2009;113(3):795‐8. [PubMed] [Google Scholar]
Romera 2005 {published data only}
- Romera V, Vila R, Perez‐Piqueras A, Marti X, Cairois MA. Early mobilization in patients with acute deep vein thrombosis: does it increase the incidence of symptomatic pulmonary embolism?. Phlebology 2005; Vol. 20, issue 3:141.
- Romera‐Villegas A, Cairols‐Castellote MA, Vila‐Coll R, Gómez AP, Martí‐Mestre X, Bonell‐Pascual A, et al. Early mobilisation in patients with acute deep vein thrombosis does not increase the risk of a symptomatic pulmonary embolism. International Journal of Angiology 2008;27(6):494‐9. [PubMed] [Google Scholar]
Schulman 2006 {published data only}
- Schulman S, Lindmarker P, Holmstrom M, Lafars G, Carlsson A, Nicol P, et al. Post‐thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. Journal of Thrombosis and Haemostasis 2006;4(4):734‐42. [DOI] [PubMed] [Google Scholar]
Schwarz 1998 {published data only}
- Schwarz T, Schellong SM, Kropp J, Prescher Y, Daniel WG. Time of mobilization does not influence the incidence of pulmonary embolism (PE) in patients with deep venous thrombosis (DVT). Preliminary results of a prospective randomized trial. Annals of Hematology 1998;76(Suppl I):A57. [Google Scholar]
Vedantham 2013 {published data only}
- Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. Rationale and design of the ATTRACT study: a multicenter randomized trial to evaluate pharmacomechanical catheter‐directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. American Heart Journal 2013;165(4):523‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01429714 {published data only}
- Bouman A, Cate‐Hoek A, Dirksen C, Joore M. Patients' preferences regarding elastic compression stocking therapy for the prevention of post thrombotic syndrome. Journal of Thrombosis and Haemostasis 2015;13(Suppl S2):432. [Google Scholar]
- NCT01429714. The Ideal deep venous thrombosis (DVT) study (IDEAL). clinicaltrials.gov/ct2/show/NCT01429714 (date first received 24 August 2011).
- Cate‐Hoek AJ, Bouman AC, Joore MA, Prins M, Cate H. The IDEAL DVT study, individualised duration elastic compression therapy against long‐term duration of therapy for the prevention of post‐thrombotic syndrome: protocol of a randomised controlled trial. BMJ Open 2014;4(9):e005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01578122 {published data only}
- NCT01578122. Elastic compression stockings for prevention of post‐thrombotic syndrome (CELEST). clinicaltrials.gov/ct2/show/NCT01578122 (date first received 13 April 2012).
NCT03039517 {published data only}
- NCT03039517. Post thrombotic syndrome prevention study. clinicaltrials.gov/ct2/show/NCT03039517 (date first received 30 January 2017).
Additional references
Berntsen 2016
- Berntsen CF, Kristiansen A, Akl EA, Sandset PM, Jacobsen EM, Guyatt G, et al. Compression stockings for preventing the postthrombotic syndrome in patients with deep vein thrombosis. American Journal of Medicine 2016;129(4):447.e1‐447.e20. [DOI: 10.1016/j.amjmed.2015.11.031] [DOI] [PubMed] [Google Scholar]
Blattler 1995
- Blattler W. Compression therapy of deep vein thrombosis. Proceedings of the Union Internationale de Phlebologie X11 World Congress. 1995 September 3‐8; London, UK. Phlebology '95. Springer, 1995:41‐6.
Brakkee 1988
- Brakkee AJM, Kuiper JP. The influence of compressive stockings on the haemodynamics in the lower extremities. Phlebology 1988;3:147‐53. [Google Scholar]
Callam 1987
- Callam MJ, Ruckley CV, Dale JJ, Harper DR. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. BMJ (Clinical Research Edition) 1987;295(6610):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Compression Bulletin
- Partsch H, Rabe E. Comment on 'Prevention and treatment of postphlebitic syndrome. Results of a 3‐part‐study'. Compression Bulletin. Robert Stemmer Library on Compression Therapy. Available at www.sigvaris.com. 2002 (date accessed 15 September 2017).
European Committee for Standardization (CEN)
- European Committee for Standardization (CEN). Non‐active medical devices. Working Group 2 ENV 12718: European Prestandard ‘Medical elastic compression hosiery’ CEN/TC205, Brussels, Belgium. 2001.
Giannoukas 2006
- Giannoukas AD, Labropoulos N, Michaels JA. Compression with or without early ambulation in the prevention of post‐thrombotic syndrome: a systematic review. European Journal of Vascular and Endovascular Surgery 2006;32:217–21. [DOI] [PubMed] [Google Scholar]
Guex 1994
- Guex JJ. Physiopathology of post‐thrombotic syndrome. Update 1994 [Physiopathologie du syndrome post‐thrombotic. Actualisation 1994]. Journal des Maladies Vasculaires 1994;19(1):12‐6. [PubMed] [Google Scholar]
Guyatt 2001
- Guyatt G, Schunemann H, Cook D, Jaeschke R, Pauker S, Bucher H. American College of Chest Physicians. Grades of recommendation for antithrombotic agents. Chest 2001;119(1 Suppl):3S‐7S. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haenen 2002
- Haenen JH, Janssen MC, Wollersheim H, Van't Hof MA, Rooij MJ, Langen H, et al. The development of post‐thrombotic syndrome in relationship to venous reflux and calf muscle pump dysfunction at 2 years after the onset of deep venous thrombosis. Journal of Vascular Surgery 2002;35(6):1184‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2016
- Jin YW, Ye H, Li FY, Xiong FZ, Cheng NS. Compression stockings for prevention of post‐thrombotic syndrome: a systematic review and meta‐analysis. Vascular and Endovascular Surgery 2016;50(5):328‐34. [DOI: 10.1177/1538574416652242] [DOI] [PubMed] [Google Scholar]
Kahn 2002
- Kahn SR, Hirsch A, Shrier I. Effect of post‐thrombotic syndrome on health‐related quality of life after deep venous thrombosis. Archives of Internal Medicine 2002;162(10):1144‐8. [DOI] [PubMed] [Google Scholar]
Kahn 2006
- Kahn SR, Desmarais S, Ducruet T, Arsenault L, Ginsberg JS. Comparison of the Villalta and Ginsberg clinical scales to diagnose the post‐thrombotic syndrome: correlation with patient‐reported disease burden and venous valvular reflux. Journal of Thrombosis and Haemostasis 2006;4:907‐8. [DOI] [PubMed] [Google Scholar]
Kahn 2009
- Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post‐thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. Journal of Thrombosis and Haemostasis 2009;7(5):879‐83. [DOI] [PubMed] [Google Scholar]
Kahn 2016
- Kahn SR, Galanaud JP, Vedantham S, Ginsberg JS. Guidance for the prevention and treatment of the post‐thrombotic syndrome. Journal of Thrombosis and Thrombolysis 2016;41:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakkos 2006
- Kakkos SK, Daskalopoulou SS, Daskalopoulos ME, Nicolaides AN, Geroulakos G. Review on the value of graduated elastic compression stockings after deep vein thrombosis. Thrombosis and Haemostasis 2006;96(4):441‐5. [PubMed] [Google Scholar]
Kay 1986
- Kay TW, Martin FI. Heel ulcers in patients with long‐standing diabetes who wear antiembolism stockings. Medical Journal of Australia 1986;145(6):290‐1. [DOI] [PubMed] [Google Scholar]
Kearon 2008
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):454S‐545S. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed. American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S‐94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meissner 2002
- Meissner MH, Zierler BK, Bergelin RO, Chandler WL, Strandness DE Jr. Coagulation, fibrinolysis, and recanalization after acute deep venous thrombosis. Journal of Vascular Surgery 2002;35(2):278‐85. [DOI] [PubMed] [Google Scholar]
Musani 2010
- Musani MH, Matta F, Yaekoub AY, Liang J, Hull RD, Stein PD, et al. Venous compression for prevention of postthrombotic syndrome: a meta‐analysis. American Journal of Medicine 2010;123(8):735‐40. [DOI] [PubMed] [Google Scholar]
Neumann 1984
- Neumann HA, Leeuwen M, Broek MJ, Berretty PJ. Transcutaneous oxygen tension in chronic venous insufficiency syndrome. VASA 1984;13(3):213‐9. [PubMed] [Google Scholar]
Neumann 1993
- Neumann HAM, Tazelaar DJ. Compression therapy. In: Bergan JJ, Golman MP editor(s). Varicose Veins and Telangiectasias. Diagnosis and Treatment. 1st Edition. St. Louis: Quality Medical Publishing Inc., 1993:103‐22. [Google Scholar]
Partsch 1984
- Partsch H. Do we need firm compression stockings exerting high pressure?. VASA 1984;13(1):52‐7. [PubMed] [Google Scholar]
Partsch 1984b
- Partsch H. Improving the venous pumping function in chronic venous insufficiency by compression as dependent on pressure and material [Besserung der venosen Pumpleistung bei chronischer Veneninsuffizienz durch Kompression in Abhangigkeit von Andruck und Material]. VASA 1984;13(1):58‐64. [PubMed] [Google Scholar]
Partsch 1991
- Partsch H. Compression therapy of the legs. A review. Journal of Dermatologic Surgery & Oncology 1991;17(10):799‐805. [DOI] [PubMed] [Google Scholar]
Partsch 2001
- Partsch H, Rabe E, Stemmer R. Compression Therapy of the Extremities. 1st Edition. Vol. 1, Paris: Editions Phleboligiques Francaises, 2001:167‐173; 213‐218; 243‐259; 297‐300. [Google Scholar]
Porter 1995
- Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. Journal of Vascular Surgery 1995;21(4):635‐45. [DOI] [PubMed] [Google Scholar]
Prandoni 2009
- Prandoni P, Kahn SR. Post‐thrombotic syndrome: prevalence, prognostication and need for progress. British Journal of Haematology 2009;145(3):286‐95. [DOI: 10.1111/j.1365-2141.2009.07601.x] [DOI] [PubMed] [Google Scholar]
Soosainathan 2013
- Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post‐thrombotic syndrome. Journal of Vascular Surgery 2013;57:254‐61. [DOI] [PubMed] [Google Scholar]
Tie 2015
- Tie HT, Luo MZ, Luo MJ, Li K, Li Q, Wu QC. Compression therapy in the prevention of post‐thrombotic syndrome: a systematic review and meta‐analysis. Medicine (Baltimore) 2015;94 (31):e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kolbach 2002
- Kolbach DN, Sandbrink MWC, Hamulyak K, Neumann HAM, Prins MH. Non‐pharmaceutical measures for prevention of post‐thrombotic syndrome. Cochrane Database of Systematic Reviews 2002, Issue 9. [DOI: 10.1002/14651858.CD004174] [DOI] [PubMed] [Google Scholar]
Kolbach 2003
- Kolbach DN, Sandbrink MWC, Hamulyak K, Prins MH, Heumann MHAM. Non‐pharmaceutical measures for prevention of post‐thrombotic syndrome. Cochrane Database of Systematic Reviews 2003, Issue 9. [DOI: 10.1002/14651858.CD004174.pub2] [DOI] [PubMed] [Google Scholar]


