Abstract
Background
Strong evidence supports the use of metered‐dose inhalers combined with a spacer for delivering rapid‐acting inhaled beta‐2 agonists in the treatment of acute exacerbations of asthma in children. The high cost and lack of availability of commercially produced spacers however, have limited their use in developing countries.
Objectives
The aim of this review was to compare the response to inhaled beta‐2 agonists delivered through metered‐dose inhaler using home‐made spacers, to the use of commercially produced spacers, in children with acute exacerbations of wheezing or asthma.
Search methods
We searched the Cochrane Airwyas Group Register (up to August 2010), Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library, MEDLINE , EMBASE, CINHAL, LILACS and reference lists of included studies. We contacted authors and known experts in the field, and approached pharmaceutical companies that manufacture inhalation spacers to identify additional published or unpublished data. No language restrictions were applied.
Selection criteria
Trials comparing treatment with rapid acting beta 2‐agonists delivered through a MDI attached to home‐made spacers, versus the same bronchodilator therapy delivered with a MDI and commercially produced spacers, in children under 18 years with acute exacerbations of wheezing or asthma.
Data collection and analysis
Two review authors independently extracted the data and assessed trial quality. Missing data were obtained from the authors or estimated from information available in published reports.
Main results
Six trials with 658 participants met the inclusion criteria. At the time of this review, five trials were published in full text, and one study was available in abstract form only. No significant differences were demonstrated between the two delivery methods in terms of the need for hospital admission (RR 1.00, 95% CI 0.63 to 1.59), change in oxygen saturation (SMD ‐0.03, 95% CI ‐0.39 to 0.33), PEFR (SMD 0.04, 95% CI ‐0.72 to 0.80), clinical score (WMD 0.00, 95% CI ‐0.37 to 0.37), in terms of need for additional treatment (RR 1.18, 95% CI 0.84 to 1.65), or regarding change in heart rate per minute (SMD 0.09, 95% CI ‐0.24 to 0.42).
Authors' conclusions
Overall, this review did not identify a statistically significant difference between these two methods for delivering bronchodilator therapy to children with acute asthma or lower airways obstruction attacks. Care should be taken in the interpretation and applicability of our results because of the small number of RCTs along with few events available meeting the criteria for inclusion in the review, absence of the primary outcome of interest and other clinically important outcomes in the majority of included studies. The possible need for a face‐mask in younger children using home‐made spacers should also be considered in practice.
Plain language summary
Commercial versus home‐made spacers in delivering bronchodilator therapy for acute therapy in children
The aim of this review was to compare the response to inhaled beta‐2 agonists delivered through a metered‐dose inhaler (MDI) attached to home‐made spacers, compared with beta‐2 agonists delivered through a MDI attached to commercially produced spacers in children with acute exacerbations of wheezing or asthma. Six randomized clinical trials (RCTs) with 658 participants met the inclusion criteria of the review. Overall, this review fails to identify a difference between these two delivery methods for delivering bronchodilator therapy to children with acute asthma or lower airways obstruction attacks. However, given the small total sample and wide confidence intervals, equivalence between the treatments cannot be claimed.
Background
Asthma is a major cause of childhood morbidity (Braman 2006) and disability (Newacheck 2000), with acute exacerbations of the disease being a common reason for emergency department (ED) visits and hospital admissions (Akinbami 2002). The main drugs used in the management of acute asthma are rapid‐acting beta‐agonists and systemic corticosteroids. Ipratropium bromide (Plotnick 2000), inhaled glucocorticosteroids (Edmonds 2000; Edmonds 2003), and intravenous magnesium (Rowe 2000) may also confer benefit in acute asthma. Antibiotics (Graham 2001), inhaled mucolytics (Sethi 1998), aminophylline (Mitra 2005), sedation (Sethi 1998) and antihistamines (Van Ganse 1997), have no established role in the treatment of exacerbations. Although rapid‐acting inhaled beta 2‐agonists are generally administered by nebulization, equivalent (Cates 2005) or even greater bronchodilatation (Rodríguez 2004), with a more rapid onset, fewer side effects, and less time spent in the ED can be achieved using a metered‐dose inhaler (MDI) with spacer.
Effective use of MDI requires synchronization of inhalation with actuation of the device. Since synchronization is difficult in children, spacer devices are used to overcome this poor co‐ordination problem. Spacers have further advantages in that they improve efficacy (increase lung deposition and decrease oropharyngeal deposition) and reduce side effects from inhaled drugs (Amirav 1997; Singhal 2001). For this reason, inhaled therapy using an MDI with attached spacer has been increasingly recognized as the optimal method for delivering rapid acting beta 2‐agonists for acute exacerbations of wheezing or asthma. A wide variety of commercially produced spacers are available; these differ in shape, size, material out of which they are constructed, and the presence of valves (Zar 2002a). The high cost and lack of availability of commercial produced spacers have limited their use in developing countries (Zar 2002a).
As an alternative, rapid acting beta 2‐agonists via MDI have been delivered attached to home‐made spacers for treating children with acute exacerbations of wheezing or asthma (Zar 1999; Singhal 2001; Zar 2002a). A wide variety of home‐made spacers have been developed, including plastic cold‐drink bottles, plastic mineral water bottles, polystyrene cups, plastic zip‐up bags, and paper spacers. In spite of the wide use of these home‐made spacers in developing countries, there are only a few studies comparing their use for delivery of rapid acting beta 2‐agonists via MDI versus the same bronchodilator therapy with commercially produced spacers for treating acute exacerbations of wheezing or asthma in children under 18 years of age.
Although several studies have concluded that rapid acting beta 2‐agonists via MDI given attached to home‐made spacers produce similar bronchodilation to the same bronchodilator therapy delivered with commercially produced spacers for treating acute exacerbations of wheezing or asthma in children (Panicker 2001; Singhal 2001; Quetulio 2002; Obgaidze 2005), some of these studies were not powered sufficiently to detect differences between the two devices. We therefore aimed to review the literature to determine if the existing evidence allows concluding that the bronchodilatory response to beta 2‐agonists, delivered through metered‐dose inhaler (MDI) attached to home‐made spacers, is equivalent to the bronchodilatory response to beta 2‐agonists delivered through MDI attached to commercially produced spacers, in children with acute exacerbations of wheezing or asthma.
Objectives
The aim of this review was to compare the response to inhaled beta‐2 agonists delivered through metered‐dose inhaler (MDI) attached to home‐made spacers, to beta‐2 agonists delivered through MDI attached to commercially produced spacers, in children with acute exacerbations of wheezing or asthma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCT) including open and blinded study designs.
Types of participants
Children under 18 years with acute exacerbations of wheezing or asthma presenting to an ED or equivalent care setting.
Types of interventions
Intervention: Rapid acting beta 2‐agonists via MDI given attached to home‐made spacers. Combination treatment with anti‐cholinergic agents was permitted. Controls: The same bronchodilator therapy delivered with commercially produced spacers. Combination treatment with anti‐cholinergic agents was permitted.
Types of outcome measures
Primary outcomes
The primary outcome measure was the need for hospital admission.
Secondary outcomes
Secondary outcomes measures were changes from baseline in peak expiratory flow rate (PEFR), forced expiratory volume in one second (FEV1), oxygen saturation (SaO2), respiratory rate (RR), clinical scores, and physical signs, such as dyspnea, accessory muscle use, and wheezing. Other secondary outcomes measures were intensive care unit (ICU) admission rates, emergency department length of stay, need for additional treatment upon completion of the intervention protocol, and adverse effects such as heart rate (HR), dysrhythmia, tremor, and nausea.
Search methods for identification of studies
For the original version of this review potentially relevant trials relevant trials were identified through searches of the electronic databases MEDLINE (From 1966 to August 2009), EMBASE (From 1974 to August 2009), CINHAL (From 1982 to August 2009), Cochrane Central Register of Controlled Trials (CENTRAL) ‐ Issue 3/2009, and LILACS (From 1982 to August 2009). The full strategies are in Appendix 1. Controlled trials were identified by using the Cochrane highly sensitive search filter (Robinson 2002). A search of the Cochrane Airways Group Register was also conducted with the following search strategy:
(spacer* or MDI or bronchodilat* or nebuli* or vapori* or aerosol* or inhal* or "holding chamber" or holding‐chamber) and (bottle* or home‐made* or "home made" or homemade or alternative* or improvi* or cup or plastic or paper or polystyrene)
For the latest update of this review (2010), the Airways Register has been searched up to August 2010.The Airways Group Register is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts.
We included citations in any language. We also reviewed the bibliographies of the randomized trials identified, contacted the authors of included trials and known experts in the field, and approached pharmaceutical companies that manufacture inhalation spacers to identify additional published or unpublished data.
Data collection and analysis
I. Trial selection
Two review authors (CRM, MPS) scanned the abstracts and titles of articles retrieved by the electronic and handsearches for eligibility, according to the inclusion criteria above. One of the review authors retrieved full copies of all those deemed potentially eligible for closer examination. Two review authors determined whether or not they met eligibility criteria; if necessary we sought advice from other authors. Disagreement was resolved by consensus.
II. Data extraction and management
We developed our data abstraction forms a priori to capture specific items of data needed for this review. We pilot‐tested the data extraction form with a sample of three included studies to ensure clarity, completeness and ease of use. We extracted data on study design, details on participants (including age, gender, number in each group), description of the interventions (including process), and description of outcomes, including timing of assessment, and adverse effects. Two review authors (CRM, MPS) independently extracted data from trials to the specially designed form, one review author (CRM) then entered into RevMan, and this was checked by a second using the double data entry facility. If data were not reported in abstractable form, we contacted one of the authors of the included study for additional information. For binary outcome measures we calculated a pooled estimate of the relative risk (RR) of the treatment effect for each outcome. For continuous outcomes, we recorded either mean change from baseline for each group or mean values after intervention, and their respective standard deviations or standard errors, and calculated mean differences. If standard deviations or standard errors were missing, we tried to extract them from other relevant information reported in the paper (P‐values, confidence intervals, etc). If the authors could not be contacted or if the information was no longer available, this was reported.
III. Trial quality assessment
Two review authors (CRM, MPS) independently assessed the methodological quality using two methods. Firstly, all included trials were scored using the Cochrane approach to assessment of allocation concealment, using the following principles (Schulz 1995): Grade A: Adequate concealment Grade B: Uncertain Grade C: Clearly inadequate concealment
Secondly, the methodological quality of the eligible RCTs was also assessed with a modified version of a 5‐point scoring instrument, proposed by Jadad (Jadad 1996), and summarized as follows: 1. Was the trial described as randomized (1 = yes; 0 = no)?; 2. Was the trial described as double‐blind (1 = yes; 0 = no)?; 3. Was there a description of withdrawals and dropouts (1 = yes; 0 = no)?; 4. Was the method of randomisation well described and appropriate (1 = yes;0 = no); 5. Was the method of blinding well described and appropriate (1 = yes; 0 = no)?; 6. One point was deducted if methods for randomization or blinding were inappropriate.
The modification of the original Jadad scoring instrument consisted of giving one additional point for each of the two items of (1) description and appropriateness of the method of generating the sequence of randomization and (2) description and appropriateness of the method of double blinding, as opposed to the original Jadad scale, which would only award one point even if both items were fulfilled.
Inter‐rater reliability was measured by using simple agreement and kappa weighted statistics.
IV. Data analysis
Cochrane Review Manager software (RevMan 2008) was used to compile and analyze the data. For binary outcomes, we calculated relative risks (RR) and their 95% confidence intervals (CI) for each study. For continuous outcomes, we calculated the weighted mean difference (WMD) (for variables measured using the same scale), or the standardised mean differences (SMD) (for variables measured using different scales) and 95% CI. We measured heterogeneity among trials using I2 statistic (Higgins 2002), heterogeneity test Q and by comparing the fixed‐effect model (where only within‐study variation is considered to influence the uncertainty of the weighted mean results) and random‐effects model results (in which both within‐study and between‐study variations are included in the assessment of the uncertainty of the overall mean results). If statistical heterogeneity was not found, a fixed‐effect model was used with 95% confidence interval (CI). If significant heterogeneity was detected, we devoted further research to identify possible causes of heterogeneity for the following characteristics: methodological quality of included studies, severity of asthma attack at presentation, age, presence of valves in commercially produced spacers, material of home‐made spacer, and dose of bronchodilator. We explored the impact of these characteristics on heterogeneity and the effectiveness of the intervention by means of subgroup analyses.
In the sensitivity analysis we evaluated the impact of the study quality by separating studies according to low risk of bias (characteristic = "adequate") or medium/high risk of bias (characteristic = "unclear/inadequate") for allocation concealment. We also performed sensitivity analysis with respect to the modified version of the Jadad scale.
We intended to assess publication bias with funnel plots (Egger 1997).
Results
Description of studies
Results of the search
The computerised search has yielded a total of 1789 citations (see Appendix 2 for the search history). A total of 31 studies were examined in full text for possible inclusion. One paper was translated from Russian into English. This left six trials and data for 658 children and adolescents available for meta‐analysis. There was total agreement between the two independent reviewers on inclusion of studies. At the time of this report, five trials were published in full text (Zar 1999; Panicker 2001; Singhal 2001; Chong Neto 2005; Zar 2007), and one study was available in abstract form only (Quetulio 2002). Confirmation of methods and data extraction was obtained from the authors of two trials, including voluntary disclosure of data for one unpublished study.
Included studies
Most studies were relatively small randomized controlled trials, and were relatively homogeneous in the age and sex of the participants. Four trials enrolled children older than four years of age (Zar 1999; Singhal 2001; Quetulio 2002; Chong Neto 2005), and two enrolled children younger than four (Panicker 2001; Zar 2007). The oldest included study was published in 1999 (Zar 1999) and the most recent in 2007 (Zar 2007). Studies that were included were conducted mainly in developing countries: two were from India (Panicker 2001; Singhal 2001), two from South Africa (Zar 1999; Zar 2007), one from Brazil (Chong Neto 2005), and one from the Philippines (Quetulio 2002). The exact method of randomization was described in only four of the seven studies (Zar 1999; Panicker 2001; Chong Neto 2005; Zar 2007), and the investigators performing the clinical assessment were blind as to which delivery system patients were randomized to in five studies (Zar 1999; Singhal 2001; Quetulio 2002; Chong Neto 2005; Zar 2007). In one study it was not possible to determine whether those assessing the outcomes of interest were blind to the delivery system allocation (Panicker 2001). Two studies included patients with mild‐to‐moderate acute exacerbations of wheezing or asthma (Singhal 2001; Chong Neto 2005), one study with mild‐to‐severe exacerbations (Zar 1999), one with moderate‐to‐severe exacerbations (Panicker 2001), and two studies failed to report wheezing or asthma exacerbations severity (Quetulio 2002; Zar 2007).
Five studies tested 500‐750 ml plastic drink bottles (Zar 1999; Singhal 2001; Panicker 2001; Chong Neto 2005; Zar 2007), and one study tested a cardboard cone as home‐made spacers (Quetulio 2002). One study tested three types of home‐made spacers (sealed 500 ml plastic drink bottle, unsealed 500 ml plastic drink bottle, and 200 ml polystyrene cup). (Zar 1999). Commercial spacers were Cipla (750 ml) in two studies (Singhal 2001; Panicker 2001), Aerochamber (145‐165 ml) in three studies (Zar 1999; Chong Neto 2005; Zar 2007), and NebuChamber (250 ml) in one study (Quetulio 2002). All commercial spacers were valved devices. Salbutamol was the rapid acting beta 2‐agonist used in four studies (Singhal 2001; Panicker 2001; Chong Neto 2005; Zar 2007), terbutaline in one (Quetulio 2002), and fenoterol in one (Zar 1999). There was a wide range of doses of beta‐2 agonist used in the included studies. For salbutamol: single doses of 100 mcg to cumulative doses up to 2400 µg. Fenoterol: single doses of 100 µg to cumulative doses up to 600 µg. One study was available in abstract form only, so the cumulative doses of terbutaline could not be established (Quetulio 2002).
There was complete short‐term follow up of all randomized children in the included studies. No long‐term follow‐up data were presented in any of the studies. The most frequently reported outcomes were change in PEFR (four studies) (Zar 1999; Singhal 2001; Panicker 2001; Quetulio 2002), and need for additional treatment (three studies) (Zar 1999; Panicker 2001; Zar 2007). Hospitalization rate was used in only one study (Zar 2007), and the reporting of adverse effects was variable. One study included patients with acute lower airway obstruction, without a diagnosis of asthma (Zar 2007). One of the studies included in the review was reported as an abstract and we wrote to one of the authors in an attempt to obtain complete data from the study, but the authors did not respond to requests for further information (Quetulio 2002).
Excluded studies
A total of 25 studies were excluded due to the following reasons: not acute asthma (N = 6) (Becker 1985; Vichyanond 1992; Kerac 1998; Schleufe 1998; Lipworth 2002; Rajkumar 2002), no commercial spacer involved in study comparison (N = 4) (Henry 1983; Carson 1985; Teo 1988; Duarte 2002), absence of outcomes of interest (N = 5) (Zar 1998a; Zar 1998b; Fowler 2001; Kissoon 2001; Lipworth 2002), review article (N = 4) (Mazur 2000a; Motala 2000; Zar 2002a; Zar 2002b), study included adult patients (N = 5) (El‐Kassimi 1987; Samaranayake 1998; Schleufe 1998; Fowler 2001; Willemse 2003), duplicate study (N = 2) (Mazur 2000b; Mazur 2000c), non‐randomized trial (Obgaidze 2005) and no outcomes presented in a usable form, and no response from authors (N = 1) (Quetulio 2004).
Risk of bias in included studies
The methodological quality of the included studies was variable (see table 'Characteristics of included studies'). In general the sample size of the majority of studies was small (range 30 to 400 participants).
One study was given a modified Jadad score of four (Chong Neto 2005), two studies were given a modified Jadad score of three (Zar 1999; Zar 2007), and three studies were given a score of two (Singhal 2001; Panicker 2001; Quetulio 2002). One study was reported in abstract and was therefore devoid of substantial details for critical appraisal (Quetulio 2002). Only one study was described as double‐blind (Chong Neto 2005). Only one study of the included studies reported that intention to treat analysis was employed (Zar 2007).
Agreement between the two independent assessments of study quality was as follows:
Randomisation: Kappa = 1
Double‐blind: Kappa = 1
Withdrawals/Dropouts: Kappa = 0.8
Method of Randomisation: Kappa = 0.8
Method Blinded: Kappa = 0.8
There was total agreement between two independent assessments of study quality using the Cochrane approach. Five studies were graded A according to the Cochrane approach to concealment of allocation (Zar 1999; Singhal 2001; Quetulio 2002; Chong Neto 2005; Zar 2007). One study were graded B since the method of concealment of allocation was not described, and details were not able to be obtained from the authors (Panicker 2001).
Effects of interventions
Primary outcome: admission to hospital
The primary outcome of hospital admission was available only from one study (Zar 2007). In this study the proportion of admitted patients was identical in the home‐made and commercial spacer groups (15% in each group), so no significant differences were demonstrated between the two spacers in terms of this outcome (RR 1.0, CI 95% 0.63 to 1.59, Analysis 1.1).
1.1. Analysis.

Comparison 1 Home‐made spacers versus commercial spacers, Outcome 1 Hospital admission.
Oxygen saturation
Two studies (Panicker 2001; Singhal 2001) (120 patients) had complete data on measurements of change in SaO2. When comparing home‐made spacers and commercial spacers, no significant differences were demonstrated between the two delivery methods in terms of change in SaO2 (SMD ‐0.03, 95% CI ‐0.39 to 0.33, Analysis 1.2). Two additional trials could not be pooled owing to skewness of the data and lack of report of mean and standard deviation for this outcome (Zar 1999; Zar 2007). These studies showed that the median change in SaO2 was not significantly different between the two spacers (P = 0.52 and P = 0.53, respectively).
1.2. Analysis.
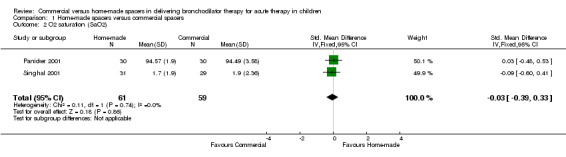
Comparison 1 Home‐made spacers versus commercial spacers, Outcome 2 O2 saturation (SaO2).
Pulmonary function tests
Two studies involving 90 patients reported change in PEFR (Panicker 2001; Quetulio 2002). No significant differences were demonstrated between the two spacers in terms of this outcome based on a random effects model (SMD 0.04, 95% CI ‐0.72 to 0.80, Analysis 1.3). These results should be interpreted with caution because significantly heterogeneity (X2 = 2.99, df =1, I2 = 66.6%, P = 0.08) was identified among trials. Two additional studies could not be pooled owing to skewness of the data and lack of report of mean and standard deviation for this outcome (Zar 1999; Singhal 2001). These studies did not show significant differences in the median change in PEFR between home‐made and commercial spacer groups (P = 0.95 and P = 0.4, respectively).
1.3. Analysis.
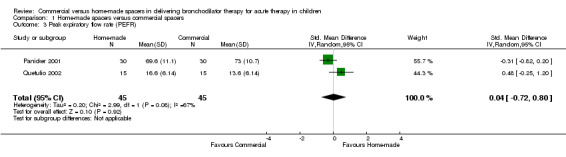
Comparison 1 Home‐made spacers versus commercial spacers, Outcome 3 Peak expiratory flow rate (PEFR).
Clinical score
No significant differences were demonstrated between the two delivery methods in terms of change in clinical score (WMD 0.0, 95% CI ‐0.37 to 0.37, Analysis 1.4). This finding is based on one study containing 20 participants (Chong Neto 2005). Two additional studies could not be pooled owing to skewness of the data and lack of report of mean and standard deviation for this outcome (Zar 1999; Zar 2007). These studies did not show significant differences in the median number of change in clinical score between home‐made and commercial spacer groups (P = 0.60 and P = 0.53, respectively).
1.4. Analysis.
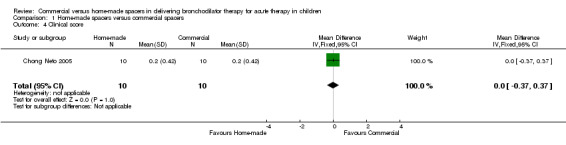
Comparison 1 Home‐made spacers versus commercial spacers, Outcome 4 Clinical score.
Vital signs
Three studies totaling 140 patients reported change in HR (Singhal 2001; Panicker 2001; Chong Neto 2005). No significant differences were demonstrated between the two spacers in terms of this outcome (SMD 0.09, 95% CI ‐0.24 to 0.42, Analysis 1.5).
1.5. Analysis.
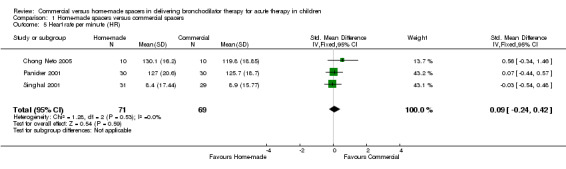
Comparison 1 Home‐made spacers versus commercial spacers, Outcome 5 Heart rate per minute (HR).
Clinical outcomes
Three studies involving 552 patients had complete data on the proportion of children who needed additional treatment (Zar 1999; Panicker 2001; Zar 2007). When comparing home‐made spacers and commercial spacers, no significant differences were demonstrated between groups in terms of this outcome (RR 1.18, 95% CI 0.84 to 1.65, Analysis 1.6).
1.6. Analysis.
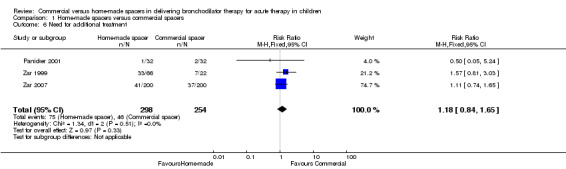
Comparison 1 Home‐made spacers versus commercial spacers, Outcome 6 Need for additional treatment.
These results did not change significantly when studies of lower methodological quality or studies that included patients without asthma diagnosis were excluded. Heterogeneity was found among trials pooled for change in PEFR (X2 = 2.99, df = 1, I2 = 66.6%, P = 0.08) but not for other outcomes.
There was insufficient information to pool outcomes such as change in RR, in accessory muscle use, in grading of dyspnea, and in breath sounds due to the insufficient number of trials reporting these outcomes. Analysis of the only trial which tested these outcomes did not show significant differences between patients treated with home‐made spacers when comparing with patients treated with commercial devices (P > 0.05) (Panicker 2001). One additional trial could not be pooled owing to skewness of the data and lack of report of mean and standard deviation for change in RR. (Singhal 2001). This study showed a significantly greater decline in RR with the commercial spacer compared with the home‐made device (P = 0.003).
No data were available for the following outcome measures in any study: emergency department length of stay, dysrhythmia and ICU admission.
We wrote to five authors of the six included studies for further information and received three replies. Any replies or data received from authors after publication of this review will be incorporated into future updates of the review.
Discussion
This systematic review constitutes an effort to incorporate the best evidence available up to August 2010 on the role of home‐made spacers compared to commercial spacers in delivering bronchodilator therapy to children with acute asthma or lower airway obstruction attacks. Overall, this review fails to identify a difference between these two delivery methods for delivering bronchodilator therapy to children with acute asthma or lower airways obstruction attacks. When comparing home‐made spacers and commercial spacers, no significant differences were demonstrated between the two delivery methods in terms of hospital admission, change in SaO2, clinical score, change in PEFR, and need for additional treatment. Moreover, sensitivity analysis did not show any significant influences of quality of methods in the efficacy of the commercially produced spacers compared to home‐made devices in delivering bronchodilator therapy to children with acute exacerbations of wheezing or asthma.
In our meta‐analysis we also looked at adverse effects but these were difficult to analyse because there was insufficient information to be pooled. The only one of these measurements that could be pooled was change in HR. When comparing home‐made spacers and commercial spacers, no significant differences were demonstrated between groups in terms of this outcome.
These are very relevant findings since acute exacerbations of asthma account for the largest part of direct health costs for asthma in most countries, and the fact that inhaled therapy using a MDI with attached spacer has been increasingly recognized as the optimal method for delivering rapid acting beta 2‐agonists for acute exacerbations of wheezing or asthma, and that high cost and lack of availability of commercially produced spacers have limited their use in developing countries.
The following outcome measures did not show any heterogeneity when data from different trials were combined: change in SaO2, change in HR, and need for additional treatment. Statistical heterogeneity was found among trials pooled for change in PEFR (X2 = 2.99, df = 1, I2 = 66.6%, P = 0.08). The statistical heterogeneity for this outcome is difficult to explain due to the limited number of trials reporting this outcome in a way that could be pooled (only two trials). No significant differences were demonstrated between the two spacers in terms of this outcome based on a random effects model (SMD 0.04, 95% CI ‐0.72 to 0.80). However, these results should be interpreted with caution because of this statistical heterogeneity among trials, and because there are very few studies, and very few events for this outcome.
Interpretation and applicability of our results needs to be cautious for several reasons: (1) The small number of RCTs along with few events available meeting the criteria for inclusion in the review, (2) absence of the primary outcome of interest (hospital admission, available from only one study) and other clinically important outcomes in the majority of included studies, (3) only limited analysis of outcomes such as change in SaO2, change in clinical score, and change in PEFR was possible due to data being presented as medians, and (4) the careful preparation and use of delivery devices in some studies (Zar 2007 used a facemask with the home‐made spacer in younger children and took measures to reduce static in both spacers and bottles). Valved commercial spacers contain a one‐way, low resistance valve that allows the aerosol to remain within the device until the patient's inhalation effort opens the valve. The theoretical advantages of these valved holding spacers compared to non‐valved devices consist in that the former improve coordination with inspiratory flow, and eliminate the cold‐Freon effect (Rubin 2005). Moreover, if the child exhales through non‐valved spacers, whether home‐made or not, any remaining drug which would have been inhaled on the second inspiration may be lost. However, it's important to take into account that it also has been described advantages of the non‐valved spacers over valved holding chambers: the former devices may increase pulmonary aerosol deposition, especially in young children or those with airway obstruction (since overcoming the resistance of the valve on inspiration may be difficult for such patients), and may also minimize the amount of dead space in the spacer (Zar 2002b).
A possible weakness of this review is the inaccessibility of data on outcomes known to have been measured (but unreported), and data not presented in a form that can be combined in the meta‐analysis. This may be a confounding factor in the results and thus the conclusions. However, outcomes that could not be entirely pooled owing to skewness of the data and lack of report of mean and standard deviation in some studies (change in SaO2, change in clinical score, and change in PEFR), were not significantly different between the two spacer devices in these studies.
In summary, this review has demonstrated that all of the analyzed outcomes were not significantly different when home‐made spacers were compared with commercial devices in delivering bronchodilator therapy to children with acute exacerbations of wheezing or asthma. However, further studies are needed in order to support or to refute the use of home‐made spacers in delivering bronchodilator therapy to these children. These additional studies should include assessment of clinically important outcomes such as rate of hospitalization or rate of ICU admission, and should be designed and adequately powered to test equivalence (null hypothesis that one device is superior to the other), and also should be designed to compare the two spacers, using lower doses of bronchodilators. Additionally, cost‐effectiveness analysis and patient preferences are important considerations that require still further assessment, and must be considered in these additional studies.
Authors' conclusions
Implications for practice.
Overall, this review did not identify a difference between these two delivery methods for delivering bronchodilator therapy to children with acute asthma or lower airways obstruction attacks. However, because of the small number of RCTs along with few events available meeting the criteria for inclusion in the review, absence of the primary outcome of interest and other clinically important outcomes in the majority of included studies, we consider that the results of this review should be interpreted with caution and need confirmation through further, larger trials. In the meanwhile, selection of the spacer device for an individual patient should begin with a commercial spacer with home‐made spacers being used if commercial device is not available. The possible need for a face‐mask in younger children using home‐made spacers should be considered.
Implications for research.
Further studies are needed before we can confidently draw conclusions about the efficacy of home‐made spacers in delivering bronchodilator therapy to children with acute exacerbations of wheezing or asthma. These additional studies should include assessment of clinically important outcomes such as rate of hospitalization or rate of ICU admission, and should be designed and adequately powered to test equivalence (null hypothesis that one device is superior to the other), in order to evaluate whether the theoretical advantages of commercial spacers over non‐valved devices (including home‐made devices) are so important as to influence the bronchodilator response in evaluating clinically important outcomes. The use of a mask with home‐made spacers in small children also needs further clarification. Additionally, cost‐effectiveness and patient preferences are important considerations that require further assessment, and should be considered in these additional studies.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2017 | Amended | New literature search run to assess the need to update this review. Seven potentially eligible studies added to Studies awaiting classification. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 18 August 2010 | New search has been performed | Literature search run, no new studies found. |
| 13 August 2008 | New search has been performed | Literature search re‐run; no new studies were identified. |
| 17 April 2008 | Amended | Converted to new review format. |
| 3 January 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The reviewers would like to acknowledge the assistance provided by the Review Group Coordinator (Toby Lasserson) for his continued encouragement, by the Trials Search Coordinator (Elizabeth Arnold) for her help in identifying the trials and obtaining copies of the papers, Dr Tatiana Kit and Dr Jesus Agreda for their assistance in translation of a paper from Russian, and Georgia Salanti (MRC Biostatistics Unit, Cambridge, UK) for her helpful guidance and comments. We would like to acknowledge Dr Heather Zar for providing the data from of her last study for inclusion into the review prior to publication. We would also like to thank Dr SK Kabra, Dr Herberto Chong Neto, Dr Heather Zar, Dr Shamim Qazi, Glaxo Wellcome (UK), Boehringer‐Ingelheim (Colombia), and Trudell Medical International (Colombia) who responded enthusiastically to our enquiries and requests for data.
Appendices
Appendix 1. Search strategies
| CENTRAL | MEDLINE/CINAHL | EMBASE |
| #1. ASTHMA explode tree 1 (MeSH) #2. BRONCHIAL SPASM single term (MeSH) #3. asthma* #4. wheez* #5. (bronch* near spas*) #6. bronchospas* #7. bronchoconstrict* #8. (#1 or #2 or #3 or #4 or #5 or #6 or #7) #9. NEBULIZERS AND VAPORIZERS explode all trees (MeSH) #10. spacer* #11. mdi #12. nebuli* #13. vapori* #14. aerosol* #15. inhal* #16. bronchodilat* #17. (#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #18. (#8 and #17) #19. home‐made* #20. (home next made*) #21. alternative* #22. improvi* #23. bottle* #24. ((plastic or paper) and cup*) #25. polystyrene #26. (#19 or #20 or #21 or #22 or #23 or #24 or #25) #27. (#26 and #18) | 1. exp asthma/ 2. bronchial spasm/ 3. asthma$.mp. 4. wheez$.mp. 5. (bronch$ adj3 spas$).mp. 6. bronchospas$.mp. 7. bronchoconstrict$.mp. 8. or/1‐7 9. exp nebulizers/ and vaporizers/ 10. spacer$.mp. 11. MDI.mp. 12. nebuli$.mp. 13. vapori$.mp. 14. aerosol$.mp. 15. inhal$.mp. 16. bronchodilat$.mp. 17. or/9‐16 18. "home made".mp. 19. alternative$.mp. 20. improvi$.mp. 21. bottle$.mp. 22. ((plastic$ or paper$) adj3 cup).mp. 23. polystyrene.mp. 24. or/18‐23 25. 8 and 17 and 24 | 1. exp asthma/ 2. Bronchospasm/ 3. asthma$.mp. 4. wheez$.mp. 5. (bronch$ adj3 spas$).mp. 6. bronchospas$.mp. 7. bronchoconstrict$.mp. 8. or/1‐7 9. exp nebulizer/ 10. spacer$.mp. 11. MDI.mp. 12. nebuli$.mp. 13. vapori$.mp. 14. aerosol$.mp. 15. inhal$.mp. 16. bronchodilat$.mp. 17. or/9‐16 18. "home made".mp. 19. alternative$.mp. 20. (improvis$ or improviz$).mp. 21. bottle$.mp. 22. ((plastic$ or paper$) adj3 cup).mp. 23. polystyrene.mp. 24. or/18‐23 25. 8 and 17 and 24 |
Appendix 2. Search history
1) Original search 2006/2007: 1531 references
2) 2008 search update: 81 references
3) 2009‐2010 search update: 81 references
Data and analyses
Comparison 1. Home‐made spacers versus commercial spacers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital admission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 O2 saturation (SaO2) | 2 | 120 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.39, 0.33] |
| 3 Peak expiratory flow rate (PEFR) | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.72, 0.80] |
| 4 Clinical score | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.37, 0.37] |
| 5 Heart rate per minute (HR) | 3 | 140 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.24, 0.42] |
| 6 Need for additional treatment | 3 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chong Neto 2005.
| Methods | Design: Randomized, placebo‐controlled study (four arms). Randomisation: Children were asked to draw a slip of paper that determined which group they were in. Blinding: double‐blind. Excluded: described. Withdrawals: none. Baseline characteristics: comparable. Power calculation: calculation of sample size necessary to establish significant differences (P < 0.05) between groups for a 15% increase in FEV1 with a power of 80%. Modified Jadad score: 4 | |
| Participants | Setting: Emergency health unit affiliated with the City Hall of Curitiba. 40 children (10 in each arm) aged 6 to 18 years (average age 11.01 years). Inclusion criteria: patients with acute asthma attacks who sought medical care. Exclusion criteria: history of cardiac and pulmonary diseases other than asthma, clinical score < 3, FEV1 < 20% and greater than 80% of the predicted value, smokers, children treated with short‐acting and long‐acting beta‐2 agonists in the last 24 hours, corticosteroids in the last 7 days, and those receiving xanthines. | |
| Interventions | Beta‐agonist: salbutamol (albuterol). Home‐made spacer: sealed 500 ml mineral water bottle. Dosage: 4 puffs (400 µg) given every 20 minutes during one hour (total dosage: 1200). Co‐interventions: not stated Beta‐agonist: salbutamol (albuterol). Commercial spacer: Aerochamber 145 ml. Dosage: 4 puffs (400 µg) given every 20 minutes during one hour (total dosage: 1200). Co‐interventions: not stated. | |
| Outcomes | Primary outcomes: Change in clinical score, FEV1, and heart rate per minute. Secondary outcomes (adverse effects): tremor, nausea and/or vomiting, and hypokalaemia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details on how randomised sequence was generated were not available. |
| Allocation concealment (selection bias) | High risk | Children drew slips of paper to determine which group they were allocated to |
Panicker 2001.
| Methods | Design: Parallel group study. Randomisation: computer generated random numbers. Blinding: no details. Excluded: described. Withdrawals: none. Baseline characteristics: comparable. Power calculation: not given. Modified Jadad score: 2 | |
| Participants | Setting: India. Emergency room department. 60 children (30 in each group) aged 1 to 12 years (average age 4.8 years). Inclusion criteria: over two previous attacks of asthma exacerbation, and children seeking treatment for an acute exacerbation of bronchial asthma. Exclusion criteria: pulmonary tuberculosis, emphysema, other cardiac, hepatic, pulmonary, or skeletal disease involving the spine, any neuromuscular disorder involving intercostal muscle or diaphragm, or children who had already received steroids before going to hospital. | |
| Interventions | Beta‐agonist: salbutamol (albuterol). Home‐made spacer: 750 ml (final volume) plastic water bottle. Dosage: 2 puffs (200 µg) given every 5 ‐ 10 minutes during one hour (total dosage: 1200 ‐ 2400 µg). Co‐interventions: humidified oxygen was given to all patients. Cases with incomplete or poor response at 60 minutes were given further treatment. Beta‐agonist: salbutamol (albuterol). Commercial spacer: Cipla 750 ml. Dosage: 2 puffs (200 µg) given every 5 ‐ 10 minutes during one hour (total dosage: 1200 ‐ 2400 µg). Co‐interventions: humidified oxygen was given to all patients. Cases with incomplete or poor response at 60 minutes were given further treatment. | |
| Outcomes | Primary outcomes: Change in grading of dyspnoea, ability to speak, heart rate per minute, respiratory rate, cyanosis, accessory muscle use, breath sounds, rhonchi, PEFR, pulsus paradoxus, arterial blood gas, and oxygen saturation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Quetulio 2002.
| Methods | Design: Parallel group study. Randomisation: method not given. Blinding: single blind. Excluded: not described. Withdrawals: not described. Baseline characteristics: not described. Power calculation: not given. Modified Jadad score: 2 | |
| Participants | Setting: Philippines. Pediatric out‐patient department and children´s asthma unit. 30 children aged 5 to 18 years. Inclusion criteria: acute exacerbation of bronchial asthma. Exclusion criteria: not given. | |
| Interventions | Beta‐agonist: terbutaline. Home‐made spacer: cardboard cone. Dosage: not stated. Co‐interventions: not stated. Beta‐agonist: terbutaline. Comercial Spacer: NebuChamber 250 ml. Dosage: not stated. Co‐interventions: not stated. | |
| Outcomes | Primary outcomes: Change in respiratory rate, subcostal and intercostal retractions, air exchange, wheezes and PEFR. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Singhal 2001.
| Methods | Design: Parallel group study. Randomisation: method not given. Blinding: single blind. Excluded: described. Withdrawals: none. Baseline characteristics: comparable. Power calculation: 27 for each spacer was calculated to ensure detection of a 15% change in PEFR between the two spacers at a significance level 0.05 with a power of 95%. Modified Jadad score: 2 | |
| Participants | Setting: India. Pediatric Chest Clinics of all India Institute of Medical Sciences. 60 children (31 in home‐made spacer, and 29 in commercial spacer group) aged 5 to 15 years (average age 9.6 years). Inclusion criteria: asthmatic who presented with an acute exacerbation. Exclusion criteria: severe or life threatening attack characterized by either inability to speak, severe recessions, very poor air entry, PEFR < 20% of predicted, and oxygen saturation < 92% | |
| Interventions | Beta‐agonist: salbutamol (albuterol). Home‐made spacer: 700ml (final volume) plastic mineral water. Dosage: 10 puffs (1000 µg) given at a rate of 1 puff every 30‐60 seconds. Co‐interventions: not stated. Beta‐agonist: salbutamol (albuterol). Spacer: Aerochamber 150 ml. Dosage: 10 puffs (1000 µg) given at a rate of 1 puff every 30‐60 seconds. Co‐interventions: not stated. | |
| Outcomes | Primary outcome: change in PEFR. Secondary outcomes: change in oxygen saturation, heart rate per minute and respiratory rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Zar 1999.
| Methods | Design: Parallel group study (four arms). Randomisation: block randomisation. Blinding: single blind. Excluded: described. Withdrawals: none. Baseline characteristics: comparable. Power calculation: 22 for each spacer to ensure detection of a 15% change in PEFR at a significance level of 0.05 with a power of 90% for each type of spacer. Modified Jadad score: 3 | |
| Participants | Setting: South Africa. Red Cross Children´s Hospital. 88 children (22 in each arm) aged 5 to 13 years. Inclusion criteria: children with a known history of asthma who presented to the Hospital with an acute asthma attack. Exclusion criteria: inability to use an MDI and spacer or to reliably undergo pulmonary function tests, PEFR < 20% of the predicted normal, arterial oxygen saturation < 92% in air, underlying cardiac or other chronic chronic pulmonary disease, treatment with oral corticosteroids for more than 5 days before presentation, and use of beta‐agonists within 4hours of presentation. | |
| Interventions | Beta‐agonist: fenoterol hydrobromide. Home‐made spacers: sealed 500 ml plastic cold‐drink bottle, unsealed 500 ml plastic cold‐drink bottle, and 200 ml polystyrene cup. Dosage: 4 puffs (400 µg) for children who weighed 25 kg or less, and 6 puffs (600 µg) for children who weighed more than 25 kg, given at a rate of 1 puff every 10 seconds. Co‐interventions: fenoterol 1000 µg in 2 ml normal saline via a jet nebuliser, and oxygen at a flow rate of 5 L per min in children who after bronchodilator treatment had PEFR < 70% of the predicted value. Beta‐agonist: fenoterol hydrobromide. Commercial spacer: Aerochamber 145 ml. Dosage: 4 puffs (400 µg) for children who weighed 25 kg or less, and 6 puffs (600 µg) for children who weighed more than 25 kg, given at a rate of 1 puff every 10 seconds. Co‐interventions: fenoterol 1000 µg in 2 ml normal saline via a jet nebuliser, and oxygen at a flow rate of 5 L per min in children who after bronchodilator treatment had PEFR < 70% of the predicted value. | |
| Outcomes | Primary outcomes: changes in clinical score and pulmonary function, and need for and response to nebulisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method to generate randomised sequence not reported; investigators employed block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Zar 2007.
| Methods | Design: Parallel group study. Randomisation: computer‐generated. Blinding: single blind. Excluded: described. Withdrawals: none. Baseline characteristics: comparable. Power calculation: 198 children were required in each group to demonstrate that hospitalisation with bottle spacer was not more than 10% higher, 80% power and a one‐tailed significance level of 0.05. Modified Jadad score: 3 | |
| Participants | Setting: South Africa. Red Cross Children´s Hospital. 400 children (200 in each group) aged 2 months to 5 years. Inclusion criteria: children with history of cough or difficulty breathing within the prior 5 days and clinical signs of acute lower airways obstruction (expiratory wheeze on auscultation or hyperinflation of the chest). Exclusion criteria: use of bronchodilator within the preceding 4 hours, known underlying cardiac or chronic pulmonary disease (other than asthma), presence of stridor or daily treatment with oral corticosteroids for more than 2 days prior. | |
| Interventions | Beta‐agonist: salbutamol (albuterol). Home‐made spacer: modified 500 ml plastic bottle. Dosage: 5 puffs (500 µg) given at a rate of 1 puff every 10 seconds, and given until three times at 15 minute intervals. Co‐interventions: oral corticosteroids were prescribed for children who required hospitalisation or who required 2 or more bronchodilator treatments. If additional treatments were required after third inhalation, patients were nebulised (5 mg salbutamol in 2.5 mls normal saline) using a jet nebuliser. Anti‐cholinergic agents were not used. Beta‐agonist: salbutamol (albuterol). Commercial spacer: Aerochamber 150 ml. Dosage: 5 puffs (500 µg) given at a rate of 1 puff every 10 seconds, and given until three times at 15 minute intervals. Co‐interventions: oral corticosteroids were prescribed for children with recurrent wheeze who required hospitalisation or who required 2 or more bronchodilator treatments. If additional treatments were required after third inhalation, patients were nebulised (5 mg salbutamol in 2.5 ml normal saline) using a jet nebuliser. Anti‐cholinergic agents were not used. | |
| Outcomes | Primary outcome: hospitalization. Secondary outcomes: change in clinical score, oximetry, number of bronchodilator treatments required prior to discharge (if not hospitalised), and need for systemic corticosteroids. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
FEV1: forced expiratory volume in one second PEFR: peak expiratory flow rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker 1985 | Not acute asthma. |
| Carson 1985 | Study comparison was a home‐made spacer vs. oxygen‐driven nebulizer, no commercial spacer involved. |
| Duarte 2002 | Study comparison was a home‐made spacer vs. oxygen‐driven nebulizer, no commercial spacer involved. |
| El‐Kassimi 1987 | Study included adult patients |
| Fowler 2001 | Study included adult patients, and did not include outcomes of interest. |
| Henry 1983 | Study comparison was salbutamol versus placebo via a home‐made spacer, no commercial spacer involved. |
| Kerac 1998 | Not acute asthma |
| Kissoon 2001 | No outcomes of interest in this study. |
| Lipworth 2002 | Not a randomised controlled trial, not acute asthma, and absence of outcomes of interest. |
| Mazur 2000a | Not a randomised controlled trial, but a review and commentary of a previous published paper. |
| Mazur 2000b | Same study as Zar 1999. |
| Mazur 2000c | Same study as Zar 1999. |
| Motala 2000 | Review article. |
| Obgaidze 2005 | Non‐randomized trial |
| Quetulio 2004 | Probably some patients included in Quetulio 2002, no outcomes presented in this abstract in a usable form, and no response from authors to request for clarification. |
| Rajkumar 2002 | Not acute asthma. |
| Samaranayake 1998 | Mixed population of patients (pediatric and adult participants), not possible to separate data from pediatric patients and no response from authors. |
| Schleufe 1998 | Not a randomised controlled trial, design more suitable to before and after study. Not acute asthma. Probably adult patients, no response from authors to request for clarification. |
| Teo 1988 | Study comparison was salbutamol versus placebo via a home‐made spacer, no commercial spacer involved. |
| Vichyanond 1992 | Not acute asthma. |
| Willemse 2003 | Study included adult patients. Not acute asthma. |
| Zar 1998a | Absence of outcomes of interest. |
| Zar 1998b | Absence of outcomes of interest. |
| Zar 2002a | Review article. |
| Zar 2002b | Review article. |
vs: versus
Contributions of authors
CM: initiation and development of the review; study assessment, data extraction, data entry, write‐up MS: Study assessment, data extraction, write‐up JL: Write‐up
Declarations of interest
The authors have no financial interest in any of the devices used to deliver beta‐2‐agonists in acute asthma and no involvement with the primary studies.
Edited (no change to conclusions)
References
References to studies included in this review
Chong Neto 2005 {published data only}
- Chong Neto HJ, Chong‐Silva DC, Marani DM, Kuroda F, Olandosky M, Noronha L. Different inhaler devices in acute asthma attacks: a randomized, double‐blind, placebo‐controlled study. Jornal de Pediatria 2005;81:298‐304. [DOI] [PubMed] [Google Scholar]
Panicker 2001 {published data only}
- Panicker J, Sethi GR, Sehgal V. Comparative efficiency of commercial and improvised spacer device in acute bronchial asthma. Indian Pediatrics 2001;38:340‐8. [PubMed] [Google Scholar]
Quetulio 2002 {unpublished data only}
- Quetulio JJ, Rivera CR, Go OC. A practical alternative spacer for delivery of inhaled bronchodilators for children with acute exacerbations of bronchial asthma. Chest. 2002:P115.
Singhal 2001 {published data only}
- Singhal T, Garg H, Arora HS, Lodha R, Pandey RM, Kabra SK. Efficacy of a home‐made spacer with acute exacerbation of bronchial asthma: a randomized controlled trial. Indian Journal of Pediatrics 2001;68:37‐40. [DOI] [PubMed] [Google Scholar]
Zar 1999 {published data only}
- Zar HJ, Brown G, Donson H, Brathwaite N, Mann MD, Weinberg EG. Home‐made spacers for bronchodilator therapy in children with acute asthma: a randomized trial. Lancet 1999;354:979‐82. [DOI] [PubMed] [Google Scholar]
Zar 2007 {published data only}
- Zar HJ, Streun S, Levin M, Weinberg EG, Swingler GH. Randomised controlled trial of the efficacy of a metered‐dose inhaler with bottle spacer for bronchodilator treatment in acute lower airway obstruction. Archives of Disease in Childhood 2007;92:142‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Becker 1985 {published data only}
- Becker AB, Simons ER, Benoit TC, Gillespie CA. Terbutaline by metered‐dose inhaler: conventional inhaler versus tube spacer for children with asthma. Annals of Allergy 1985;55:724‐8. [PubMed] [Google Scholar]
Carson 1985 {published data only}
- Carson JWK, Hiller EJ. "Cup‐mask" salbutamol in acute asthma in children. Irish Medical Journal 1985;78:5‐6. [PubMed] [Google Scholar]
Duarte 2002 {published data only}
- Duarte M, Camargos P. Efficacy and safety of a home‐made non‐valved spacer for bronchodilator therapy in acute asthma. Acta Paediatrica 2002;91:909‐13. [DOI] [PubMed] [Google Scholar]
El‐Kassimi 1987 {published data only}
- El‐Kassimi FA. "Aerosol‐in‐bag" administration of inhaled bronchodilators. European Journal of Respiratory Disease 1987;70:234‐8. [PubMed] [Google Scholar]
Fowler 2001 {published data only}
- Fowler SJ, Wilson AM, Griffiths EA, Lipworth BJ. Comparative in vivo lung delivery of hydrofluoroalkane‐salbutamol formulation via metered‐dose inhaler alone, with plastic spacer, or with cardboard tube. Chest 2001;119:1018‐20. [DOI] [PubMed] [Google Scholar]
Henry 1983 {published data only}
- Henry RL, Milner AD. Simple drug delivery system for use by young asthmatics. BMJ 1983;286:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerac 1998 {published data only}
- Kerac M, Montgomery H, Johnson N. A low cost spacer device used for asthma treatment in a Calcuta street clinic to improve efficacy of metered dose inhalers. Tropical Doctor 1998;28:228‐9. [DOI] [PubMed] [Google Scholar]
Kissoon 2001 {published data only}
- Kisoon N, Teelucksingh S, Blake KV, Kesser B, Murphy SP, Geller D. Pastic bottles as spacers for a pressurized metered‐dose inhaler: In vitro characteristics. West Indian Medical Journal 2001;50:189‐93. [PubMed] [Google Scholar]
Lipworth 2002 {published data only}
- Lipworth BJ, Lee DKC, Anhoj J, Bisgaard H. Effect of plastic spacer handling on salbutamol lung deposition in asthmatic children. British Journal of Clinical Pharmacology 2002;54:544‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazur 2000a {published data only}
- Mazur LJ. Spacers made from sealed cold‐drink bottles were as effective as conventional spacers in children with acute asthma. Evidence Based Medicine 2000;5:79. [Google Scholar]
Mazur 2000b {published data only}
- Mazur LJ, Zar HJ, Brown G, Donson H. Are spacers made from sealed cold‐drink bottles as effective as conventional spacers?. Western Journal of Medicine 2000;173:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazur 2000c {published data only}
- Mazur LJ. Home‐made spacers for bronchodilator therapy in children with asthma: A randomized trial. Journal of Pediatrics 2000;136:415‐6. [Google Scholar]
Motala 2000 {published data only}
- Motala C, Kling S, Gie R, Potter PC, Manjra A, Vermeulen J, et al. Guideline for the management of chronic asthma in children ‐ 2000 update. SAMJ 2000;90:524‐39. [PubMed] [Google Scholar]
Obgaidze 2005 {published data only}
- Obgaidze T, Nemsadze K, Chkaidze L, Peradze D. Effectiveness of treatment of broncho‐obstruction in children with acute respiratory infections using home‐made spacers. Georgian Medical News 2005;1:46‐9. [PubMed] [Google Scholar]
Quetulio 2004 {published data only}
- Quetulio JJ. Inexpensive spacer for bronchodilator therapy in young asthmatics [Abstract]. Chest 2004;126(4 Suppl):910Sb, 911. [Google Scholar]
Rajkumar 2002 {published data only}
- Rajkumar, Vatsa HK, Gaur SN. Comparative evaluation of market spacer and home made spacer in the management of bronchial asthma. Journal of the Association of Physicians of India 2002;50:397‐9. [PubMed] [Google Scholar]
Samaranayake 1998 {published data only}
- Samaranayake SW, Perera BJC. Paper spacers coupled to metered dose inhalers in family practice. Ceylon Medical Journal 1998;43:147‐50. [PubMed] [Google Scholar]
Schleufe 1998 {published data only}
- Schleufe P, Reiffen HP, Piepenbrock S. Effective application of bronchodilator aerosols from mereted‐dose inhalers (MDI) via resuscitator‐bag and adapter. Resuscitation 1998;39:175‐8. [DOI] [PubMed] [Google Scholar]
Teo 1988 {published data only}
- Teo J, Kwang LW, Yip WC. An inexpensive spacer for use with metered‐dose bronchodilators in young asthmatic children. Pediatric Pulmonology 1988;5:244‐6. [DOI] [PubMed] [Google Scholar]
Vichyanond 1992 {published data only}
- Vichyanond P, Chokephaibulkit K, Kerdsomnuig S, Visitsuntorn N, Tuchinda M. Clinical evaluation of the "Siriraj spacer" in asthmatic Thai children. Annals of Allergy 1992;69:433‐8. [PubMed] [Google Scholar]
Willemse 2003 {published data only}
- Willemse BW, Toelle BG, Li JSM, Shah S, Peat JK. Use of a paper disposable cup as a spacer is effective for the first‐aid management of asthma. Respiratory Medicine 2003;97:86‐9. [DOI] [PubMed] [Google Scholar]
Zar 1998a {published data only}
- Zar HJ, Liebenberg M, Weinberg EG, Binns HJ, Mann MD. The efficacy of alternative spacer devices for delivery of aerosol therapy to children with asthma. Annals of Tropical Paediatrics 1998;18:75‐9. [DOI] [PubMed] [Google Scholar]
Zar 1998b {published data only}
- Zar HJ, Liebenberg M, Weinberg E, Binns HJ, Mann MD. Alternative spacer devices for delivery of aerosol therapy to children with asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3 Suppl):A709. [DOI] [PubMed] [Google Scholar]
Zar 2002a {published data only}
- Zar HJ, Weinberg EG. Spacer devices for the developing world. ACI International 2002;14:13‐6. [Google Scholar]
Zar 2002b {published data only}
- Zar HJ, Asmus MJ, Weinberg EG. A 500‐ml plastic bottle: An effective spacer for children with asthma. Pediatric Allergy & Immunology 2002;13:217‐22. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chong 2005 {published data only}
- Chong Neto HJ, Chong‐Silva DC, Marani DM, Kuroda F, Olandosky M, Noronha Ld. Different inhaled devices in acute asthma attacks: a randomized, double‐blinded, placebo controlled study. Jornal de pediatria 2005;81(4):298‐304. [DOI] [PubMed] [Google Scholar]
Leelathipkul 2016 {published data only}
- Leelathipkul L, Tanticharoenwiwat P, Ithiawatchakul J, Prommin D, Sirisalee P, Junhunee P, et al. MDI with DIY spacer versus nebulizer for bronchodilator therapy in children admitted with asthmatic attack. Chotmaihet thangphaet [Journal of the Medical Association of Thailand]. Medical Association of Thailand (E‐mail: math@loxinfo.co.th), 2016; Vol. 99:S265‐74. [PubMed]
Mazur 2000 {published data only}
- Mazur L J. Home‐made spacers for bronchodilator therapy in children with acute asthma: a randomized trial. Journal of pediatrics 2000;136(3):415‐6. [Google Scholar]
Poachanukoon 2016 {published data only}
- Poachanukoon O, Leelathipkul L, Tanticharoenwiwat P, Ithiawachakul J. PMDI with DIY spacer vs nebulizer for bronchodilator therapy in children admitted with asthmatic attack. Allergy. Blackwell Publishing Ltd, 2016; Vol. 71:604. [PubMed]
Santati 2000 {published data only}
- Santati S, Prethipan A. Clinical comparison of homemade fruit juice spacer and two standard spacers in children. American journal of respiratory and critical care medicine 2000; Vol. 161, issue 3 Suppl:A343.
Schor 2017 {published data only}
- Schor D, Rizzo JA, Medeiros D, Dela Bianca AC, Silva AR, Nunes C, et al. Home‐made spacer as an auxiliary device in administration of beclomethasone via pressurized metered dose inhaler for asthma control. A randomized controlled pragmatic trial. Respiratory medicine. England: W.B. Saunders Ltd, 2017; Vol. 126:52‐8. [DOI] [PubMed]
Yasmin 2012 {published data only}
- Yasmin S, Mollah AH, Basak R, Islam KT, Chowdhury YS. Efficacy of salbutamol by nebulizer versus metered dose inhaler with home‐made non‐valved spacer in acute exacerbation of childhood asthma. Mymensingh medical journal : MMJ. Bangladesh, 2012; Vol. 21, issue 1:66‐71. [PubMed]
Additional references
Akinbami 2002
- Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics 2002;110:315‐22. [DOI] [PubMed] [Google Scholar]
Amirav 1997
- Amirav I, Newhouse MT. Metered dose inhaler accessory devices in acute asthma: efficacy and comparison with nebulizers: a literature review. Archives of Pediatric and Adolescent Medicine 1997;151:876‐82. [DOI] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S‐12S. [DOI] [PubMed] [Google Scholar]
Cates 2005
- Cates CJ, Bara A, Crilly JA, Rowe BH. Holding chambers versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database of Systematic Reviews 2006, Issue 2. [Art. No.: CD000052. DOI: 10.1002/14651858.CD000052.pub2.] [DOI] [PubMed] [Google Scholar]
Edmonds 2000
- Edmonds ML, Camargo CA Jr, Brenner BE, Rowe BH. Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database of Systematic Reviews 2000, Issue 3. [Art. No.: CD002316. DOI: 10.1002/14651858.CD002316] [DOI] [PubMed] [Google Scholar]
Edmonds 2003
- Edmonds ML, Camargo CA Jr, Pollack CV Jr, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002308. DOI: 10.1002/14651858.CD002308] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2001
- Graham V, Lasserson TJ, Rowe BH. Antibiotics for acute asthma. Cochrane Database of Systematic Reviews 2001, Issue 2. [Art. No.: CD002741. DOI: 10.1002/14651858.CD002741.] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Mitra 2005
- Mitra A, Bassler D, Watts K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newacheck 2000
- Newacheck PW, Halfon N. Prevalence, impact, and trends in childhood disability due to asthma. Archives of Pediatric and Adolescent Medicine 2000;154:287‐93. [DOI] [PubMed] [Google Scholar]
Plotnick 2000
- Plotnick LH, Ducharme FM. Combined inhaled anticholinergics and beta2‐agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2000, Issue 3. [Art. No.: CD000060. DOI: 10.1002/14651858.CD000060.] [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2006.
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal Epidemiology 2002;31:150‐3. [DOI] [PubMed] [Google Scholar]
Rodríguez 2004
- Castro‐Rodríguez JA, Rodrigo GJ. Beta‐agonists through metered‐dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta‐analysis. Journal of Pediatrics 2004;145:172‐7. [DOI] [PubMed] [Google Scholar]
Rowe 2000
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2000, Issue 1. [Art. No.: CD001490. DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubin 2005
- Rubin BK, Fink JB. Optimizing Aerosol Delivery by Pressurized Metered‐Dose Inhalers. Respiratory Care 2005;50:1191‐7. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Sethi 1998
- Sethi GR, Bajaj M, Sehgal V. Management of acute asthma. Indian Pediatrics 1998;35:745‐62. [PubMed] [Google Scholar]
Van Ganse 1997
- Ganse E, Kaufman L, Derde MP, Yernault JC, Delaunois L, Vincken W. Effects of antihistamines in adult asthma: a meta‐analysis of clinical trials. European Respiratory Journal 1997;10:2216‐24. [DOI] [PubMed] [Google Scholar]


