Abstract
Background
Neuropathic or neurogenic bladder describes a process of dysfunctional voiding as the result of injury in the brain, spinal cord or nerves innervating the bladder. People with neuropathic bladder, such as from spinal cord injury (SCI), are at significant risk of morbidity from urinary tract infections (UTI). Effective methods to prevent UTI in people with SCI have been sought for many years. Probiotics (micro‐organisms that exert beneficial health effects in the host) have been recommended for bacterial interference of the urological tract to reduce colonisation by uropathogen and to manage the dual problems of infection and antibiotic resistance.
Objectives
This review looked at the benefits and harms of probiotics in preventing symptomatic UTI in people with neuropathic bladder compared with placebo, no therapy, or non‐antibiotic prophylaxis (cranberry juice, methenamine hippurate, topical oestrogen).
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register up to 10 March 2017 through contact with the Information Specialist using search terms relevant to this review. Studies in the Specialised Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs), quasi‐RCTs and cross‐over RCTs looking at the use of probiotics for the prophylaxis of UTI in people with neuropathic bladders was considered for inclusion. Men, women and children of all ages with neuropathic bladders from neurological injury such as suprapontine, supra sacral and sacral aetiologies was included. All bladder management types, including reflex voiding, time voiding, indwelling and intermittent catheterization were eligible for this review.
Studies comparing probiotics to placebo, no treatment or other non‐antibiotic prophylaxis was included. Studies comparing probiotics with antibiotics or in combination with antibiotics were excluded.
Data collection and analysis
Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) and 95% CI were planned for continuous outcomes.
Main results
This review includes a total of three studies (one cross‐over and two parallel RCTs) which involved 110 participants. All three studies looked at intravesical instillation of a low virulent Escherichia coli (E. coli) strain in reducing the risk of symptomatic UTI in participants with neuropathic bladder, predominantly from SCI. Two studies used the E. coli 83972 strain and one study used the E. coli HU2117 strain.
We did not find any RCTs involving other probiotics or other routes of administration for preventing UTI in people with neuropathic bladder.
There was consistency in definition of symptomatic UTI in all three studies. Symptoms that all studies considered were relevant to diagnose UTI were adequately defined. All three studies defined microbiological diagnosis of symptomatic UTI.
Asymptomatic bacteriuria was not considered an outcome measure in any of the included studies; however it was defined in two studies to establish successful inoculation.
It is uncertain if the risk of symptomatic UTI is reduced with bladder inoculation using E. coli because the certainty of the evidence is very low (3 studies, 110 participants: RR 0.32, 95% CI 0.08 to 1.19; I2 = 82%).
Two studies reported adverse events. One study reported one episode of autonomic dysreflexia. One study reported three symptomatic UTI occurring in two patients, and two studies mentioned the absence of septicaemia and pyelonephritis. Intravesical instillation was reported as "generally safe". One study reported high attrition rates in participants due to the need to adhere to strict instillation protocols.
The overall quality of the studies was poor. All three studies had high risk of attrition bias due to failure of an intention‐to‐treat analysis which undermines the randomisation process and weakened the results of the studies. All three studies also had high risk of reporting bias.
Authors' conclusions
In this review, there were no studies identified addressing oral probiotics in preventing UTI in people with neuropathic bladder. It is uncertain if the risk of symptomatic UTI is reduced in people with neuropathic bladders via intravesical instillation of non‐pathogenic E. coli as data were derived from small studies with high risk of bias.
Although very minimal levels of harm was reported with this procedure, due to variable success rates, the need for strict adherence to instillation protocols together with high attrition rates in these studies, it is doubtful bladder instillation will be a widely accepted intervention in its current form.
It is recommended that further appropriately powered RCTs with more robust methodological reporting be carried out.
Plain language summary
Probiotics for preventing urinary tract infections in people with bladder dysfunction after a nervous system injury
What is the issue?
Bladder function can be altered after an injury to the nervous system and most often happens in conditions like multiple sclerosis, spinal cord injury or stroke. This type of bladder dysfunction is termed ‘neuropathic bladder’. The bladder dysfunction in people with a nervous system injury increases their risk of frequent bladder infections. Currently, there is no effective way to prevent bladder infections in these people. Long‐term use of antibiotics is not encouraged as it results in reduced effectiveness of that antibiotic. Probiotics are bacteria that can have a beneficial effect on the body. Some evidence already exists to suggest that probiotics can prevent bladder infections in post‐menopausal women.
What did we do?
This review investigates the evidence for the effectiveness of probiotics on the prevention of bladder infections in people with bladder dysfunction after a nervous system injury.
What did we find?
We conducted a literature review up to March 2017 and three studies were included according to our selection criteria. The three studies reported data on 110 participants. All three studies investigated whether introducing probiotics directly into the bladder to create a non‐harmful colony will prevent urinary tract infections in people with bladder dysfunction, predominantly people with spinal cord injury. Two studies reported that this method was generally safe. This review found that generally, the studies were poor quality with high risk of bias. We found the effectiveness of colonisation with probiotics in preventing bladder infection in people with bladder dysfunction is uncertain. Furthermore, the success of colonisation was variable, and the colonisation process is invasive and demands a high level of commitment on the part of the participant.
We did not identify any studies investigating whether other probiotics and other administration routes is effective in preventing urinary tract infections in people with bladder dysfunction.
Conclusions
It is uncertain if probiotics prevent urine infections in people with bladder dysfunction after a nervous system injury. Further robustly designed studies are necessary.
Summary of findings
Summary of findings for the main comparison. Intravesical bacterial interference versus placebo for preventing urinary tract infection in people with neuropathic bladder.
| Intravesical bacterial interference versus placebo for preventing urinary tract infection in people with neuropathic bladder | |||||
| Patient or population: people with neuropathic bladder Setting: outpatient Intervention: intravesical bacterial interference Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with placebo | Risk with intravesical bacterial interference | ||||
| Symptomatic UTI Follow‐up: mean 12 months | 613 per 1,000 | 196 per 1,000 (49 to 729) | RR 0.32 (0.08 to 1.19) | 110 (3) | ⊕⊝⊝⊝1 VERY LOW |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1Risk of bias was assessed at high in most domains, with heterogeneity and small studies, suggesting that results overestimate intravesical bacterial interference versus placebo
Background
Description of the condition
Neuropathic or neurogenic bladder describes a process of dysfunctional voiding as the result of neurological injury (Kaynan 2008). The International Continence Society states that neuropathic bladder can only be diagnosed in the presence of neurological pathology (Stohrer 1999). Neurologic control of bladder function is at multiple levels throughout the central nervous system and subject to multiple pathophysiologic processes (Kaynan 2008). Neurological diseases that affect bladder function can be classified according to the location of the lesion: suprapontine, supra sacral or sacral. Examples of neurological disorders leading to voiding dysfunction include cerebral palsy, stroke, Parkinson's disease, dementia, spinal cord injury (SCI), multiple sclerosis, transverse myelitis, poliomyelitis, and radical pelvic surgery (Kaynan 2008). Tumours affecting the central nervous system can also cause voiding dysfunction.
Traumatic and non‐traumatic causes of SCI can lead to neuropathic bladders. Aetiologies of non‐traumatic SCI are congenital, vascular, demyelinating, toxic, infectious, inflammatory, or malignancy (Kim 2010). People with neuropathic bladder from SCI are at significant risk of morbidity from urinary tract infection (UTI); the average is about two episodes annually (Waites 1993). The problem of frequent UTI is amplified by the high prevalence of multiresistant organism(s) (MRO) in SCI populations which combine to exacerbate the clinical, social and economic consequences of disease for this patient population. This makes UTI more expensive and difficult to treat and MRO prevalence more difficult to control (Haran 2007; Kitagawa 2002; Lee 2008; Murphy 2003).
An effective way to prevent UTI in people with neuropathic bladder, especially people with SCI has been sought for many years. In 1998, 73% of patients in an Australian SCI unit were attempting non‐antibiotic based UTI prevention (Lee 2007). A recent randomised controlled trial (RCT) of 305 subjects with SCI conclusively demonstrated that the current common methods of non‐antibiotic UTI prevention were ineffective (Lee 2007). UTI prevention, particularly the more difficult to treat MRO‐UTI, is a clinical imperative for people with SCI and neuropathic bladder.
Thom 1999 showed that up to 73% of patients with SCI in the US become MRO‐colonised during inpatient treatment; and Mylotte 2000 found 44% were colonised with multiresistant Staphylococcus aureus in surveillance cultures. The problem is not confined to hospitals only; Waites 2000 found that 33% of an outpatient SCI population were MRO colonised. High colonisation rates have been attributed to neuropathic bladder and bladder management type (particularly indwelling catheterization), high rates of antibiotic use, mechanical ventilation and pressure ulcers (Girard 2006; Mylotte 2000; Thom 1999; Waites 2000). Girard 2006 found that UTI accounted for the high prevalence of nosocomial infections among people with SCI in rehabilitation units. In most settings continence is most commonly managed using bladder management techniques such as intermittent, indwelling, suprapubic and urethral catheterization; external collection device; or reflex voiding.
Description of the intervention
Currently there is no proven prophylaxis for UTI in people with neuropathic bladder, such as people with SCI. A recent research directions document recommended probiotic studies in SCI for “bacterial interference” of the urological tract to “reduce colonisation by uropathogen and manage the dual problems of infection and pathogen resistance to anti‐microbials” (Hayes 2007). Probiotics are defined as “a preparation of or a product containing viable, defined micro‐organisms in sufficient numbers, which alter the microflora (by implantation or colonisation) in a compartment of the host and by that exert beneficial health effects in this host” (Schrezenmeir 2001). There are many species and strains of probiotics available, prepared in different formulations, and administered through a variety of routes. Falagas 2006 suggested that Lactobacillus rhamnose GR‐1 and Lactobacillus reuteri RC‐14 could be the most effective probiotics for UTI prevention. A dose‐finding RCT determined that oral doses above 8 x 108 colony forming units (CFU)/d were necessary to colonise the vagina and prevent urogenital infection in women (Reid 2001).
How the intervention might work
The most common route of urinary infection is the ascension of host pathogens from the rectum and vagina to the urethra and bladder. Similarly, naturally occurring probiotic organisms in healthy people can spread from the rectum and perineum and form a barrier to uropathogen (Reid 2006). It may be possible to artificially boost probiotic colonisation through probiotic instillation via oral, vaginal or intravesical routes.
The Lactobacillus GR‐1 probiotic strain produces bacteriocins that influence the growth and biofilm development of uropathogen through down‐regulation of the inflammatory processes (Reid 2006). It is reported that a single intravaginal insertion of Lactobacillus GR‐1 can also up‐regulate host defence factors known to be important in fighting infection (Reid 2006). Similarly, studies with Lactobacillus RC‐14 strain have shown that it can up‐regulate mucin production which may act as a barrier to infection (Reid 2006), and down‐regulate virulence factor expression in pathogens such as staphylococci (Laughton 2006). The organism also affects cell membrane components in Escherichia coli and produces biosurfactants that inhibit their adhesion to surfaces (Valraeds 1998). Lactobacillus acidophilus has also been shown to coat biomaterial surfaces and thus decrease the adhesion of uropathogen (Hawthorn 1990) and inhibit Enterococcus faecalis, E. coli and Staphylococcus epidermidis from a urine suspension to silicone rubber (surlactin) (Valraeds 1998).
Why it is important to do this review
For people with a neuropathic bladder, the treatment of a simple UTI is becoming more complicated as the prevalence of MRO‐UTI rises. Such infections can be life threatening, or at the very least, expensive to treat with intravenous antibiotics with the possibility of side‐effects and increased demands on resources (Murphy 2003; Romero‐Vivas 1995). These resource demands include use of isolation rooms and specific isolation wards, more stringent use of personal protective equipment and auditing, plus an increased risk of transmission to other patients. Colonising MRO have the potential to adversely affect the individual should their immune system become compromised or if there is cross‐contamination of body systems (most commonly bowel flora contaminating the urological tract). The most common clinical infection for people with a neuropathic bladder is UTI, for which there are no urinary prophylactic substances with proven efficacy (Falagas 2006; Girard 2006; Haran 2005; Haran 2007; Lee 2008).
Many people with SCI have neuropathic bladder. The high prevalence of MRO colonisation puts people with SCI at high risk of both entry to the hospital system, but also attendant care visits necessary for SCI care among people in the community. MRO clusters that frequently occur in SCI units put other services such as emergency, investigative, ward based, theatre, radiology and outpatient at risk of cross infection and require additional resources and training to manage. Current practice for SCI‐UTI management is that asymptomatic bacteriuria is not treated because it is not associated with adverse urological outcomes in the spinal injured population (Cardenas 1995; NIDRRCS 1992) and contributes to antibiotic resistance.
UTI are likely to pose more problems in the future as organisms continue to become more resistant to available antibiotics. UTI are increasingly difficult and expensive to treat, and therefore, exploring the use of probiotics to reduce MRO colonisation and resultant UTI is crucial to reducing significant health system demands from current and future prevalence. Non‐antibiotic prophylaxis is needed to prevent UTI in people with neuropathic bladder.
Objectives
This review looked at the benefits and harms of probiotics in preventing symptomatic UTI in people with neuropathic bladder compared with placebo, no therapy, or non‐antibiotic prophylaxis (cranberry juice, methenamine hippurate, topical oestrogen).
Methods
Criteria for considering studies for this review
Types of studies
All RCTs, quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) and randomised cross‐over studies looking at the use of probiotics for the prophylaxis of UTI in people with neuropathic bladders were considered for inclusion.
Types of participants
Inclusion criteria
Men, women and children of all ages with neuropathic bladders from neurological injury such as suprapontine, supra sacral and sacral aetiologies were included. All bladder management types, including reflex voiding, time voiding, indwelling and intermittent catheterization were eligible for this review.
Exclusion criteria
Men, women and children with normal bladders were excluded. Normal bladder is defined as absence of a neurological condition causing neuropathic bladder.
Types of interventions
Inclusion criteria
Studies evaluating probiotic products administered with the objective of preventing UTI were sought. There were no restrictions based on the dose, frequency, formulation, duration or mode of administration of the product. Studies comparing probiotics to placebo, no treatment or other non‐antibiotic prophylaxis were included.
Exclusion criteria
Studies involving treatment of symptomatic UTI with probiotics were excluded.
Studies comparing probiotics with antibiotics or in combination with antibiotics as prophylaxis were beyond the scope of this review and were excluded.
Types of outcome measures
Numbers of occurrences of each of the following outcome measures were examined. A comparison with control were made during and after the treatment period.
Primary outcomes
-
Symptomatic urinary tract infection
The primary outcome measure was the number of symptomatic UTI according to defined clinical criteria, along with a positive quantitative urine culture. This is the most clinically significant outcome measure for populations at long‐term risk of recurrent UTI, such as people with neuropathic bladder.
Symptoms of UTI in the general population include dysuria, frequency, urgency, voiding of small volumes, abrupt onset, suprapubic pain, and presence of pyuria (Stamm 1988). In SCI patients, relevant symptoms should be explained by other intercurrent pathology and include: temperature, autonomic dysreflexia, increased frequency of muscle spasms or spasticity, failure of usual control of urinary incontinence, and new abdominal discomfort (NIDRRCS 1992).
-
Quantitative urine culture
Numbers of UTI confirmed by appropriate microbiological criteria. Bacteriuria on quantitative urine analysis of more than 100,000 organisms of a single species/mL was the accepted standard; however, the colony count may vary from 100 to 100,000 depending on the clinical setting (Stamm 1988). Therefore in some situations, such as a clean suprapubic tap, a colony count of less than 100,000 was acceptable.
Secondary outcomes
Numbers with at least one asymptomatic bacterial UTI
Asymptomatic bacteriuria refers to isolation of a specified quantitative count of bacteria in an appropriately collected urine specimen from a person without symptoms of signs referable to urinary infection (Nicolle 2005). According to the Infectious Disease Society of America (Nicolle 2005):
For asymptomatic women, bacteriuria is defined as two consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts ≥ 100,000 CFU/mL
A single, clean‐catch voided urine specimen with one bacterial species isolated in a quantitative count ≥ 100,000 CFU/mL identifies bacteriuria in men
A single catheterised urine specimen with one bacterial species isolated in a quantitative count ≥ 100 CFU/mL identifies bacteriuria in women or men
In children any bacteria isolated, regardless of the quantitative count on suprapubic aspiration or catheter urine identifies bacteriuria (Kilham 2009).
-
Adverse reactions
Adverse reactions were recorded as the proportion of people who reported side effects, and a description of these side effects such as bloating, flatulence, diarrhoea or any alteration to bowel movements (Fioramonti 2003). We also reported numbers of people who withdrew from studies due to side effects; a description of these side effects were included.
Numbers of patients with at least one confirmed case of bacteraemia or fungaemia on blood cultures were reported. Rare cases of bacteraemia, fungaemia, and infections in other body organs have been reported (Marteau 2003).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 10 March 2017 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contain studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategies described were used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction were carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study exists, reports were grouped together and the publication with the most complete data was used in the analyses. When relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions were highlighted.
Assessment of risk of bias in included studies
The following items were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (selection bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. symptomatic UTI, bacteriuria, positive urine culture) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement are used to assess the effects of treatment (e.g. time to UTI), the mean difference (MD) were to be used, or the standardised mean difference (SMD) if different scales had been used.
Unit of analysis issues
Care was taken in situations where total numbers of events are reported. Authors did not have to be contacted for clarification to distinguish the number of people who had events from the total number of events. Clarification did not need to be sought from authors to differentiate numbers of people who had events and numbers of events/person. Authors were contacted in situations where data were incomplete or censored. In cases where clarification could not be obtained from primary study data to resolve episodes of participant discrepancy, a narrative summary of data was presented, but these data were not included in the meta‐analysis for that outcome.
In studies that have two or more experimental arms, we investigated the most appropriate approach to pool results for meta‐analysis. If a consensus approach could not be reached, statistical support was sought to resolve the method to be applied. For randomised cross‐over studies, data to the point of first cross‐over were used for pooling into a meta‐analysis.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
If possible, funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data was pooled using the random‐effects model but the fixed‐effect model were also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis were used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age, duration since SCI and presence of abnormal renal tract pathology. Subgroup analyses may be performed for those with and without SCI, and those with neuropathic bladders of supra sacral or sacral origin. Heterogeneity in treatments could be related to route of administration of the probiotics (oral versus direct bladder inoculation), duration of therapy (less or more than one month), number of probiotics and concurrent use of probiotics and other non‐antibiotic urinary antiseptics. Adverse effects were tabulated and assessed with descriptive techniques, as they are likely to be different for the various agents used. Where possible, the risk difference with 95% CI were calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
Sensitivity analysis to assess for whether conclusions are robust were undertaken separately for the following categories.
Studies with proper randomisation and concealment of allocation compared to those without these characteristics.
Studies performed with and without intention‐to‐treat analyses.
Data are missing from the treatment arm and these patients were assumed to have the worst possible outcome.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcome in the 'Summary of findings' tables.
Symptomatic UTI
Other outcomes like quantitative urine culture, asymptomatic UTI and adverse events were unable to be included in the Summary of Findings table due to lack of data.
Results
Description of studies
Results of the search
We searched the Specialised Register and identified 63 records. After checking the references lists an addition eight records were identified. Titles and abstracts were screened and 49 records were excluded (not RCT, wrong population or intervention). The remaining 22 records represented 12 studies. After full‐text review, three studies (five records) were included and eight studies (15 records) were excluded.
One ongoing study was identified (ProSCIUTTU Study 2016) and will be assessed in a future update of this review (Figure 1).
1.
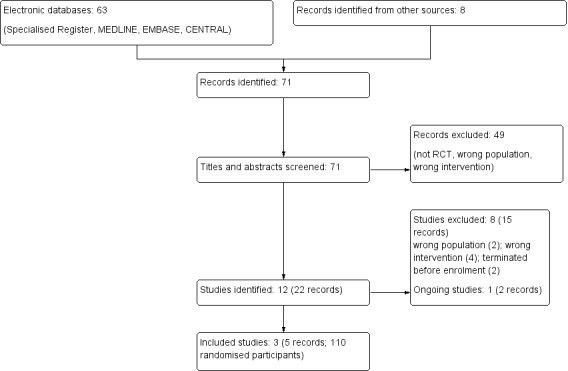
Study flow diagram.
Included studies
Three studies (Darouiche 2005; Darouiche 2011; Sunden 2010) were included enrolling 110 participants (see Characteristics of included studies). All three studies were randomised, placebo‐controlled, double‐blind RCTs involving intravesical instillation of non‐pathogenic E. coli for the prevention of UTI in people with neuropathic bladder. We did not identify any study which evaluated other routes of administration or other types of probiotics.
Two E. coli strains were used ‐ E. coli HU2117 (Darouiche 2011) and E. coli 83972 (Darouiche 2005; Sunden 2010). All three studies involved inserting 10 to 30 mL of fluid containing either normal saline or E. coli suspension directly into the bladder once or twice/day for three consecutive days. Two studies enrolled persons with known neuropathic bladder (Darouiche 2005; Darouiche 2011). More than 40% of the participants in Sunden 2010 had participants with known neuropathic bladder. There was heterogeneity in bladder management type between the three studies with intermittent catheterization being predominant in Sunden 2010 and indwelling or suprapubic catheterization being predominant in Darouiche 2005. All three studies had an adequate follow‐up period of one year or more.
Symptomatic UTI was measured in all three studies. Two studies (Darouiche 2005; Darouiche 2011) used similar criteria for diagnosing symptomatic UTI which was a combination of symptoms as well as microbiological urine culture. Bacteriuria of ≥ 105 CFU/mL with symptoms were classified as UTI. Sunden 2010 defined UTI on self‐reported symptoms by participants only, although they did perform urine cultures for confirmation.
Two studies reported adverse events (Darouiche 2005; Sunden 2010). Sunden 2010 reported that there was an absence of serious side effects such as pyelonephritis. Darouiche 2005 reported one episode of autonomic dysreflexia occurring in one patient during bladder instillation which was resolved after the catheter used for instillation was unclamped. Sunden 2010 reported symptomatic UTI in participants who had long‐term colonisation with E. coli 83972 but these symptoms were easily resolved with appropriate antibiotic therapy.
Two studies had grants from non‐profit organisation (Darouiche 2005; Darouiche 2011). Sunden 2010 had their majority of funding from non‐profit organisation although they did disclose some funding from a commercial company.
Excluded studies
Eight studies were excluded (see Characteristics of excluded studies). Two studies did not enrol participants with neuropathic bladder (Czaja 2007; Kontiokari 2001); four compared probiotics to antibiotics, which was outside the scope of this review (Lee 2007a; Mohseni 2013; NAPRUTI Study II 2006; Reid 1992); and two studies were terminated before the participants were enrolled (NCT00767988; NCT00789464).
Risk of bias in included studies
2.
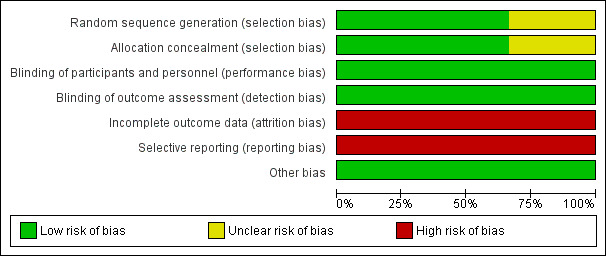
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
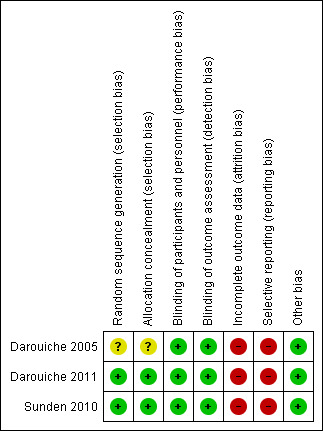
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was computer generated in two studies (low risk) (Darouiche 2011; Sunden 2010) and not reported in the third study (unclear risk) (Darouiche 2005).
Allocation concealment was not reported in one study (unclear risk) (Darouiche 2005) and was adequate in two studies (low risk) (Darouiche 2011; Sunden 2010).
Blinding
Blinding of participants was reported in all three studies (Darouiche 2005; Darouiche 2011; Sunden 2010). It was stated that assessors were also blinded in all three studies (Darouiche 2005; Darouiche 2011; Sunden 2010).
Incomplete outcome data
All three studies (Darouiche 2005; Darouiche 2011; Sunden 2010) had high risk of bias due to attrition as they either excluded analysing patients who failed bladder colonisation with E. coli in the results they analysed or included them in the placebo arm to analyse post randomisation which violates the intention to treat principle. Darouiche 2011 had more than 50% dropouts post randomisation and majority were in the treatment arm.
Selective reporting
Two studies did not have secondary outcomes of interest to our review (Darouiche 2005; Sunden 2010) and Darouiche 2011 outlined the practical limitations of this procedure in preventing UTI. Our prespecified secondary outcome of asymptomatic UTI were not addressed by any of the studies.
Other potential sources of bias
All studies appeared to be free of other biases.
Effects of interventions
See: Table 1
Symptomatic urinary tract infection
There was consistency in definition of symptomatic UTI in all three studies. Symptomatic UTI were defined as a constellation of symptoms as well as presence of bacteriuria or pyuria (Darouiche 2005; Darouiche 2011; Sunden 2010). Symptoms that all studies considered were relevant to diagnose UTI were adequately defined e.g. fever, suprapubic pain, frequency, urgency and for spinal cord patients, autonomic dysreflexia and increased spasticity.
Successful bladder colonisation with E. coli varied with different strains. The E. coli 83972 strain had success rates of 60% to 70% (Darouiche 2005; Sunden 2010) but the E. coli HU2117 only had a 37% success rate of inoculation (Darouiche 2011). In those patients who had successful colonisation with E. coli:
The time to first symptomatic UTI post successful colonisation was longer: Sunden 2010 reported median 11.3 months in treatment group versus 5.7 months in placebo, P = 0.013 with E. coli 83972
The number of symptomatic UTI in the follow up one year period was lower: Darouiche 2005 reported 46% in treatment group versus 93% in placebo group reported at least 1 episode of UTI (P = 0.01) with E. coli 83972; Darouiche 2011 reported 29% in treatment group versus 70% in placebo group reported at least one episode of UTI, (P = 0.49) with E. coli HU2117.
It is uncertain if the risk of symptomatic UTI is reduced with bladder inoculation using E. coli because the certainty of the evidence is very low (Analysis 1.1 (3 studies, 110 participants): RR 0.32, 95% CI 0.08 to 1.19; I2 = 82%). As Sunden 2010 was a cross‐over study, for the meta‐analysis, only the results of the first part of the cross‐over were included.
1.1. Analysis.
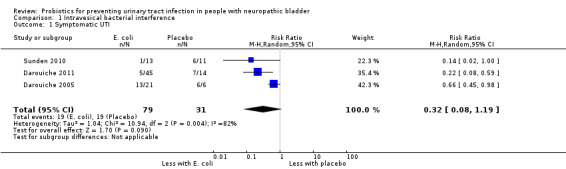
Comparison 1 Intravesical bacterial interference, Outcome 1 Symptomatic UTI.
Quantitative urine culture
Two studies (Darouiche 2005; Darouiche 2011) defined microbiological diagnosis of symptomatic UTI i.e. bacteriuria on quantitative urine analysis of more than 100,000 organisms or CFU/mL. Two studies (Darouiche 2005; Darouiche 2011) also accepted symptoms and the presence of pyuria (> 10 white blood cells/high power field) as diagnostic of UTI. Sunden 2010 did not define what they considered "uropathogenic growth" or how they established successful colonisation although they mentioned that urine cultures were taken.
Asymptomatic urinary tract infections
Asymptomatic bacteriuria was not considered an outcome measure in any of the included studies. It was defined in two studies (Darouiche 2005; Darouiche 2011) to establish successful inoculation. As both studies were from the same group of investigators, they defined it as growth of ≥ 103 CFU/mL.
None of the studies reported on the rate of asymptomatic bacteriuria or asymptomatic UTI post intervention.
Adverse events
Reporting of adverse events is limited as only two studies reported adverse events (Darouiche 2005; Sunden 2010). Darouiche 2005 reported one episode of autonomic dysreflexia as well as absence of septicaemia and UTI attributable for E. coli 83972. Sunden 2010 commented that there were no significant side effects including pyelonephritis. In a later second publication, Sunden 2010 reported symptomatic UTI in participants who had long‐term colonisation with E. coli 83972 but these symptoms were easily resolved with appropriate antibiotic therapy.
While being reported as "generally safe", bladder instillation is an invasive form of intervention which demands a high level of commitment on the part of patients. The secondary outcome in Darouiche 2011 investigated the practical limitations of inducing and maintaining bladder colonisation. They reported declining participant participation as instillation cycles increased as it required three full day clinic visits per instillation cycle and cycles may have to be repeated.
Discussion
Summary of main results
In this review, there were no studies identified that evaluated oral probiotics in preventing UTI in people with neuropathic bladder. Most of the evidence for preventing or prolonging UTI in people with neuropathic bladders are derived from three studies involving intravesical instillation of non‐pathogenic E. coli involving mainly the population with neuropathic bladder from SCI.
It is uncertain if the risk of symptomatic UTI is reduced with bladder inoculation using E. coli because the certainty of the evidence is very low (RR 0.32, 95% CI 0.08 to 1.19). Heterogeneity was high, numbers of participants per study small, and there was a high risk of attrition and reporting bias.
We conclude that the quality of evidence is very low to determine whether intravesical instillation with E. coli compared to placebo will lead to UTI prevention in people with neuropathic bladder.
Moreover, the success of intravesical installation with non‐pathogenic bacteria is variable. There are also practical limitations to carrying out the procedure as it involves high patient compliance and dedication. The procedure also needs to be repeated as the bacteria can be spontaneously cleared, especially if there is concurrent use of antibiotics.
Adverse events were also poorly reported in all studies although the procedure was reported as "generally safe". Hence, there are outstanding uncertainties as to whether the benefits of intravesical instillation outweigh the harms.
Overall completeness and applicability of evidence
This review suggests that there is a lack of evidence (few studies) that intravesical instillation with non‐pathogenic bacteria in adults with neuropathic bladder is effective in preventing symptomatic UTI.
Due to multiple exclusion criteria in these small studies, especially in relation to immunosuppression, presence of other infections, and other urogenital tract intervention/abnormalities, the limited evidence is only applicable to a selective group of the adult population with neuropathic bladder. In addition, there is heterogeneity between bladder management types as well as male predominance between the studies.
From the evidence presented and due to variable success rates, the need for strict adherence to instillation protocols together with high attrition rates in these studies, intravesical instillation with non‐pathogenic bacteria is unlikely to be a widely accepted intervention. However, with innovative therapy and with additional support for participants undergoing this procedure, it may be worthy of further study.
Quality of the evidence
Overall, the studies were of poor quality. All three studies (Darouiche 2005; Darouiche 2011; Sunden 2010) investigated patients with neuropathic bladder had adequate performance and detection bias, but had high attrition and reporting bias. The main weakness in all three studies was exclusion from analysis of patients who failed inoculation or analysing them in the placebo arm post randomisation (i.e. not analysing via intention‐to‐treat). Failure of an intention‐to‐treat analysis undermines the randomisation process and weakened the results of the studies. There was also a high gender bias in two studies. (Darouiche 2005; Darouiche 2011).
Potential biases in the review process
Publication bias could not be fully excluded. Bias from the literature search was attempted to be controlled by: Cochrane independently performing literature searches as well as two other authors using hand searching and searching multiple databases as well as references from included studies. No language restrictions were applied. Letters, abstracts and unpublished studies were accepted to reduce publication bias. If a duplicate publication was suspected, it was screened by two authors, and if confirmed, the publication with the most and/or the longest follow‐up data was used for the review.
[Escherichia coli (explode)] was not used as a search term in this review as it did not yield any additional studies.
Agreements and disagreements with other studies or reviews
To date, there is no known systematic review conducted in regards to probiotics in patients with neuropathic bladder. There are also no known reported studies of bladder inoculation of non‐pathogenic bacteria in children or normal adult population with recurrent UTI.
From literature review, there are other pilot non‐RCTs which have been conducted (Darouiche 2001; Hull 2000; Prasad 2009) mainly by the same research group which reported lower rates of UTI per year in participants with successful colonisers compared to the year prior to intervention. There is also one small pilot non‐RCT (Uehera 2006) which reported a reduction in UTI recurrence in patients with neuropathic bladder with daily insertion of Lactobacillus vaginal suppositories. To date, no other studies involving probiotic vaginal suppositories in women with neurogenic bladders have been conducted.
The authors are aware that there is a Cochrane review regarding probiotics for preventing UTI in adults and children (Schwenger 2015). Our review also concurs with theirs that there is currently low evidence of probiotics in preventing UTI, with overestimation of treatment effect and high risk of bias although their conclusion apply to a broader patient population. In regards to the efficacy of probiotics in other patient population, in the systemic review conducted by Barrons 2008, the authors conclude that studies of lactobacilli for prophylaxis in UTI in women remain inconclusive. NAPRUTI Study II 2006 reported that oral capsules of Lactobacillus rhamnose GR‐1 and Lactobacillus reuteri RC‐14 did reduced the mean number of symptomatic UTI in post‐menopausal women with recurrent UTI. However, the combination probiotics did not meet the non‐inferiority criteria in preventing UTI compared with trimethoprim‐sulfamethoxazole. In children, a review by Nickavar 2011 concluded that there is insufficient data about the preventative effects of probiotics in recurrent UTI for that population.
Authors' conclusions
Implications for practice.
There is lack of evidence that intravesical instillation with non‐pathogenic strains of E. coli is beneficial in reducing episodes of UTI in patients with neuropathic bladder. In addition, the evidence available is of such low quality that it cannot determine whether a benefit exists. There is no studies identified of less invasive methods of treatment such as oral or enteral route of probiotics to reduce infections in people with neuropathic bladders.
The adverse effects of intravesical instillation are poorly reported. It is clear from the three studies that it is an invasive form of intervention which demands a high level of commitment on the part of patients as the procedure needs to be repeated once or twice daily within consecutive days.
Implications for research.
It is recommended that further appropriately powered, methodologically robust RCTs be carried out. This includes preparations that can be delivered and self‐administered via oral and alternative routes.
In addition, future RCTs that involve bladder instillation with non‐pathogenic bacteria should adhere to the intention‐to‐treat analysis to prevent bias and improve the quality of studies.
Acknowledgements
We would like to acknowledge Cochrane Kidney and Transplant for their assistance with this review. We are also grateful for comments and feedback from the peer referees during preparation of the review. Also Professor Wullt for providing further data and clarification.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Intravesical bacterial interference.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic UTI | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Darouiche 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that patients were blinded. It was stated that nurse administering the bladder instillation was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated clearly that evaluator was blinded to randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patients accounted for up to 1 year follow‐up. For patients randomised into the treatment group, if they failed inoculation, they were then combined with the patients in the placebo group for statistical comparisons. High risk of bias as it not fully intention to treat |
| Selective reporting (reporting bias) | High risk | Only adverse event reported was one participant had autonomic dysreflexia. Generally stated that there was lack of evidence of septicaemia and UTI attributable to E. coli 83972 |
| Other bias | Low risk | Study appears free of other biases |
Darouiche 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clearly stated randomisation was computer generated for each centre |
| Allocation concealment (selection bias) | Low risk | Stated that placebo inoculum visually identical to bacterial inoculum |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clearly stated patients and investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clearly stated outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clinical characteristics of the 27 patients who were followed up till 1 year was similar. However, no characteristics of the 32 patients who had initial bladder inoculation but dropped out was mentioned. There were more drop outs in the treatment than placebo group 28 versus 4. Authors did mention that patient compliance with inoculation protocol was low to account for the reason that losses to follow‐up was higher in the treatment group Although 59 patients received bladder inoculation, only 27 patients were evaluated and analysed up to the 1 year follow‐up period. The survival analysis were only based on patients who completed the one year follow‐up period and did not account for the loss to follow‐ups; high risk of bias as not fully intention to treat |
| Selective reporting (reporting bias) | High risk | No reporting of adverse events except that female gender was associated with bladder colonisation failure |
| Other bias | Low risk | Study appears free of other biases |
Sunden 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Wullt corresponded in 2015 that both active and placebo inoculum have the same appearance, a clear fluid that could not be differentiated by a non‐informed observer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patient were blinded as to the nature of inoculum; investigators were aware of whether patients were in intervention or control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Wullt corresponded in 2015 that study team was unaware of urine culture results but were aware of patient's symptoms in view of outcome being self‐reported UTI symptoms. Wullt corresponded in 2015 that the investigator who surveyed the urine culture was aware of type of inoculum |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients with unsuccessful colonisation post inoculation were excluded from analysis ‐ not fully intention to treat ‐ only analysed 20/26 patients. Wullt corresponded in 2015 that an intention‐to‐treat analysis was not conducted due to the small number of patients enrolled |
| Selective reporting (reporting bias) | High risk | Only reported that it is safe due to absence of pyelonephritis. In a later article published in 2014, the authors reported symptomatic UTI in 2 patients who were successfully inoculated with E. coli 83972 |
| Other bias | Low risk | Study appears free of other biases |
CFU ‐ colony‐forming units; DM ‐ diabetes mellitus; HPF ‐ high powered field; RCT ‐ randomised controlled trial; UTI ‐ urinary tract infection; VUR ‐ vesicoureteric reflux; WBC ‐ white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Czaja 2007 | Wrong population: young premenopausal women with no urologic abnormalities; authors also state study is not powered to evaluate the effect of probiotics on the rate of UTI recurrence |
| Kontiokari 2001 | Wrong population: only 5/150 participants had urinary tract abnormality |
| Lee 2007a | Wrong intervention: probiotics versus antibiotics, beyond scope of protocol |
| Mohseni 2013 | Wrong intervention: probiotics + antibiotics combination versus antibiotics, beyond scope of the review |
| NAPRUTI Study II 2006 | Wrong intervention: probiotics versus antibiotics; study did enrol patients with neuropathic bladder |
| NCT00767988 | Study withdrawn prior to participant enrolment; no reason provided |
| NCT00789464 | Study withdrawn prior to participant enrolment; no reason provided |
| Reid 1992 | Wrong intervention: probiotics versus antibiotics and no mention of patients with neuropathic bladders although excluded patients with urinary tract complications |
UTI ‐ urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
ProSCIUTTU Study 2016.
| Trial name or title | Probiotics prophylaxis of Spinal Cord Injury Urinary Tract Infection Therapeutic Trial |
| Methods | Randomised, placebo‐controlled, double‐blind study with factorial design |
| Participants | Participants with known neuropathic bladder with stable spinal cord injuries or stable known demyelinating lesion |
| Interventions | All patients randomised into 1 of 4 groups
|
| Outcomes | Time from randomisation to first occurrence of symptomatic UTI or those with no UTI, until 6 months post randomisation |
| Starting date | April 2011 |
| Contact information | b.lee@neura.edu.au |
| Notes |
LGG ‐ Lactobacillus rhamnose GG; RC14 ‐ Lactobacillus reuteri RC‐14; UTI ‐ urinary tract infection
Differences between protocol and review
Summary of findings table has been incorporated.
Contributions of authors
Draft the protocol: CBR, ST
Study selection: CBR, ST
Extract data from studies: CBR, ST
Enter data into RevMan: CBR, ST
Carry out the analysis: CBR, ST
Interpret the analysis: CBR, ST, JMS, KC
Draft the final review: CBR, ST, KC
Disagreement resolution: BL
Update the review: CBR, ST
Sources of support
Internal sources
No sources of support supplied
External sources
-
NHMRC, Australia.
Project Grant Application: 630448
Declarations of interest
Dr Lee is the lead investigator of NHMRC Project Grant Application: 630448 Probiotic Prophylaxis of Spinal Cord Injury Urinary Tract‐Infection TherapeUtic‐Trial (ProSCIUTTU), which is an Australian government‐sponsored study looking at the effectiveness of Probiotic therapy in preventing urinary tract infection and multidrug resistance. Dr Toh is completing a PhD looking at whether probiotics can prevent UTI in patients with spinal cord injury and neurogenic bladders. Dr Toh and Dr Boswell‐Ruys are also assisting in the ProSCIUTTU trial. No other authors had any declarations of interest.
New
References
References to studies included in this review
Darouiche 2005 {published data only}
- Darouiche RO, Thornby JI, Cerra‐Stewart C, Donovan WH, Hull RA. Bacterial interference for prevention of urinary tract infection: a prospective, randomized, placebo‐controlled, double‐blind pilot trial. Clinical Infectious Diseases 2005;41(10):1531‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Darouiche 2011 {published data only}
- Darouiche RO, Green BG, Donovan WH, Chen D, Schwartz M, Merritt J, et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 2011;78(2):341‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sunden 2010 {published data only}
- Köves B, Salvador E, Grönberg‐Hernández J, Zdziarski J, Wullt B, Svanborg C, et al. Rare emergence of symptoms during long‐term asymptomatic Escherichia coli 83972 carriage without an altered virulence factor repertoire. Journal of Urology 2014;191(2):519‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sunden F, Hakansson L, Ljunggren E, Wullt B. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. Journal of Urology 2010;184(1):179‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sundén F, Håkansson L, Ljunggren E, Wullt B. Bacterial interference‐‐is deliberate colonization with Escherichia coli 83972 an alternative treatment for patients with recurrent urinary tract infection?. International Journal of Antimicrobial Agents 2006;28 Suppl 1:S26‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Czaja 2007 {published data only}
- Czaja CA, Stapleton AE, Yarova‐Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infectious Diseases in Obstetrics & Gynecology 2007:35387. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontiokari 2001 {published data only}
- Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry‐lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322(7302):1571. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007a {published data only}
- Lee SJ, Kim HJ, Shim YH, Lee JW. The effect of probiotic prophylaxis for preventing recurrent urinary tract infection in children with persistant primary vesicoureteral reflux [abstract no: COD. PP 81]. Pediatric Nephrology 2006;21(10):1542. [CENTRAL: CN‐00724966] [Google Scholar]
- Lee SJ, Shim YH, Cho SJ, Lee JW. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatric Nephrology 2007;22(9):1315‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohseni 2013 {published data only}
- Mohseni MJ, Aryan Z, Emamzadeh‐Fard S, Paydary K, Mofid V, Joudaki H, et al. Combination of probiotics and antibiotics in the prevention of recurrent urinary tract infection in children. Iranian Journal of Pediatrics 2013;23(4):430‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
NAPRUTI Study II 2006 {published data only}
- Beerepoot MA. Recurrent urinary tract infections. Antibiotic resistance and non‐antibiotic prophylaxis [abstract no:SP34‐2]. International Journal of Antimicrobial Agents 2013;42(Suppl 2):S38. [EMBASE: 71102681] [Google Scholar]
- Beerepoot MA, Stobberingh EE, Geerlings SE. A study of non‐antibiotic versus antibiotic prophylaxis for recurrent urinary‐tract infections in women (the NAPRUTI study) [Onderzoek naar niet‐antibiotische versus antibiotische profylaxe bij vrouwen met recidiverende urineweginfecties (de NAPRUTI‐studie)]. Nederlands Tijdschrift voor Geneeskunde 2006;150(10):574‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Beerepoot MA, Heijer CD, Penders J, Prins JM, Stobberingh EE, Geerlings SE. Predictive value of Escherichia coli susceptibility in strains causing asymptomatic bacteriuria for women with recurrent symptomatic urinary tract infections receiving prophylaxis. Clinical Microbiology & Infection 2012;18(4):E84‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beerepoot MA, ter Riet G, Nys S, Wal WM, Borgie CA, Reijke TM. Lactobacilli versus antibiotics to prevent urinary tract infections: a randomized, double‐blind, noninferiority trial in postmenopausal women [Lactobacillen versus antibiotica ter preventie van urineweginfecties: 'Non‐in feriority'‐studie bij post men opauzale vrouwen]. Nederlands Tijdschrift voor Geneeskunde 2013;157(10):A5674. [EMBASE: 2013248979] [Google Scholar]
- Beerepoot MA, ter Riet G, Nys S, Wal WM, Borgie CA, Reijke TM, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double‐blind, noninferiority trial in postmenopausal women. Archives of Internal Medicine 2012;172(9):704‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beerepoot MA, Wal WM, Nys S. Lactobacillus rhamnosus gr‐1 and l. Reuteri rc‐14 versus trimethoprim‐sulfamethoxazole (TMP/SMX) in the prevention of recurrent urinary tract infections (RUTIs) in postmenopausal women: a randomized double‐blind non‐inferiority trial [abstract no: L1‐1656a]. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2009 Sep 12‐15; San Francisco (CA). 2009.
- Schaeffer EM. Re: Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double‐blind, noninferiority trial in postmenopausal women. Journal of Urology 2013;189(4):1332‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00767988 {published data only}
- Jones EA. Probiotics improvement of gastrointestinal and genitourinary health in girls with spina bifida (H‐23245). clinicaltrials.gov/ct2/show/NCT00767988 (first received 8 October 2008).
NCT00789464 {published data only}
- Roth DR. H‐23187: Probiotic prophylaxis against recurrent pediatric urinary tract infection. clinicaltrials.gov/ct2/show/NCT00789464 (first received 10 November 2008).
Reid 1992 {published data only}
- Reid G, Bruce AW, Taylor M. Influence of three‐day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clinical Therapeutics 1992;14(1):11‐6. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
ProSCIUTTU Study 2016 {published data only}
- Lee BB, Toh SL, Ryan S, Simpson JM, Clezy K, Bossa L, et al. Probiotics [LGG‐BB12 or RC14‐GR1] versus placebo as prophylaxis for urinary tract infection in persons with spinal cord injury [ProSCIUTTU]: a study protocol for a randomised controlled trial. BMC Urology 2016;16:18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh SL, Lee BS, Ryan S, Simpson J. Prophylaxis of spinal cord injury urinary tract infection therapeutic trial (ProSCIUTTU): protocol [abstract]. 53rd ISCoS Annual Scientific Meeting; 2014 Sep 2‐4; Maastricht, Netherlands. 2014.
Additional references
Barrons 2008
- Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clinical Therapeutics 2008;30(3):453‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cardenas 1995
- Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Archives of Physical Medicine & Rehabilitation 1995;76(3):272‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Darouiche 2001
- Darouiche RO, Donovan WH, Terzo M, Thornby JI, Rudy DC, Hull RA. Pilot trial of bacterial interference for preventing urinary tract infection. Urology 2001;58(3):339‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Falagas 2006
- Falagas ME, Betsi GI, Tokas T, Athanasiou S. Probiotics for prevention of recurrent urinary tract infections in women: a review of the evidence from microbiological and clinical studies. Drugs 2006;66(9):1253‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fioramonti 2003
- Fioramonti J, Theodorou V, Bueno L. Probiotics: what are they? What are their effects on gut physiology?. Best Practice & Research Clinical Gastroenterology 2003;17(5):711‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girard 2006
- Girard R, Mazoyer MA, Plauchu MM, Rode G. High prevalence of nosocomial infections in rehabilitation units accounted for by urinary tract infections in patients with spinal cord injury. Journal of Hospital Infection 2006;62(4):473‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haran 2005
- Haran MJ, Lee BB, King MT, Marial O, Stockler MR. Health status rated with the medical outcomes study 36‐item short‐form health survey after spinal cord injury. Archives of Physical Medicine & Rehabilitation 2005;86(12):2290‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haran 2007
- Haran MJ, King MT, Stockler MR, Marial O, Lee BB. Validity of the SF‐36 Health Survey as an outcome measure for trials in people with spinal injury. www.uts.edu.au/sites/default/files/wp2007_4.pdf (accessed 3 July 2017).
Hawthorn 1990
- Hawthorn LA, Reid G. Exclusion of uropathogen adhesion to polymer surfaces by Lactobacillus acidophilus. Journal of Biomedical Materials Research 1990;24(1):39‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hayes 2007
- Hayes KC, Bassett‐Spiers K, Das R, Ethans KD, Kagan C, Kramer JL, et al. Research priorities for urological care following spinal cord injury: recommendations of an expert panel. Canadian Journal of Urology 2007;14(1):3416‐23. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hull 2000
- Hull R, Rudy D, Donovan W, Svanborg C, Wieser I, Stewart C, et al. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. Journal of Urology 2000;163(3):872‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Kaynan 2008
- Kaynan AM, Perkash I. Neurogenic bladder. In: Frontera WR, Silver JK, Rizzo TD editor(s). Essentials of Physical Medicine and Rehabilitation: Musculoskeletal Disorders, Pain and Rehabilitation. 2nd Edition. Philadelphia: Saunders Elsevier, 2008. [ISBN: 9781437721751] [Google Scholar]
Kilham 2009
- Kilham H, Alexander S, Woods N, Isaacs D. Paediatrics Manual: The Children's Hospital at Westmead Handbook. 2nd Edition. Sydney: McGraw‐Hill, 2009. [Google Scholar]
Kim 2010
- Kim RC. Spinal cord pathology. In: Lin VW editor(s). Spinal Cord Medicine: Principles and Practice. 2nd Edition. New York: Demos Medical Publishing, 2010:22‐34. [Google Scholar]
Kitagawa 2002
- Kitagawa T, Kimura T. The influence of complications on rehabilitation of spinal cord injuries: economical minus effects and physical disadvantages caused by urinary tract infection and decubitus ulcer. Journal of Nippon Medical School [Nihon Ika Daigahu Zasshi] 2002;69(3):268‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laughton 2006
- Laughton J, Devillard E, Heinrichs DE, Reid G, McCormick JK. Inhibition of expression of a staphylococcal superantigen‐like protein by a soluble factor from Lactobacillus reuteri. Microbiology 2006;152(Pt 4):1155‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee BB, Haran MJ, Hunt LM, Simpson JM, Marial O, Rutkowski SB, et al. Spinal‐injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord 2007;45(8):542‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee BB, King MT, Simpson JM, Haran MJ, Stockler MR, Marial O, et al. Validity, responsiveness, and minimal important difference for the SF‐6D health utility scale in a spinal cord injured population. Value in Health 2008;11(4):680‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marteau 2003
- Marteau P, Shanahan F. Basic aspects and pharmacology of probiotics: an overview of pharmacokinetics, mechanisms of action and side‐effects. Best Practice & Research Clinical Gastroenterology 2003;17(5):725‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 2003
- Murphy DP, Lampert V. Current implications of drug resistance in spinal cord injury. American Journal of Physical Medicine & Rehabilitation 2003;82(1):72‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mylotte 2000
- Mylotte JM, Kahler L, Graham R, Young L, Goodnough S. Prospective surveillance for antibiotic‐resistant organisms in patients with spinal cord injury admitted to an acute rehabilitation unit. American Journal of Infection Control 2000;28(4):291‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nickavar 2011
- Nickavar A, Sotoudeh K. Treatment and prophylaxis in pediatric urinary tract infection. International Journal of Preventive Medicine 2011;2(1):4‐9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Nicolle 2005
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults.[Erratum appears in Clin Infect Dis. 2005 May 15;40(10):1556]. Clinical Infectious Diseases 2005;40(5):643‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NIDRRCS 1992
- Anonymous. The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. Journal of the American Paraplegia Society 1992;15(3):194‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 2009
- Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord 2009;47(7):565‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2001
- Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunology & Medical Microbiology 2001;32(1):37‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reid 2006
- Reid G, Bruce AW. Probiotics to prevent urinary tract infections: the rationale and evidence. World Journal of Urology 2006;24(1):28‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romero‐Vivas 1995
- Romero‐Vivas J, Rubio M, Fernandez C, Picazo JJ. Mortality associated with nosocomial bacteremia due to methicillin‐resistant Staphylococcus aureus. Clinical Infectious Diseases 1995;21(6):1417‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrezenmeir 2001
- Schrezenmeir J, Vrese M. Probiotics, prebiotics, and synbiotics‐‐approaching a definition. American Journal of Clinical Nutrition 2001;73(2 Suppl):361S‐4S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schwenger 2015
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD008772.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stamm 1988
- Stamm WE. Protocol for diagnosis of urinary tract infection: reconsidering the criterion for significant bacteriuria. Urology 1988;32(2 Suppl):6‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Stohrer 1999
- Stohrer M, Goepel M, Kondo A, Kramer G, Madersbacher H, Millard R, et al. The standardization of terminology in neurogenic lower urinary tract dysfunction: with suggestions for diagnostic procedures. International Continence Society Standardization Committee. Neurourology & Urodynamics 1999;18(2):139‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thom 1999
- Thom JD, Wolfe V, Perkash I, Lin VW. Methicillin‐resistant staphylococcus aureus in patients with spinal cord injury. Journal of Spinal Cord Medicine 1999;22(2):125‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uehera 2006
- Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. International Journal of Antimicrobial Agents 2006;28 Suppl 1:S30‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valraeds 1998
- Velraeds MM, Belt‐Grittter B, Mei HC, Reid G, Busscher HJ. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. Journal of Medical Microbiology 1998;47(12):1081‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waites 1993
- Waites KB, Canupp KC, DeVivo MJ. Epidemiology and risk factors for urinary tract infection following spinal cord injury. Archives of Physical Medicine & Rehabilitation 1993;74(7):691‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waites 2000
- Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram‐negative bacteria isolated from the urinary tract in community‐residing persons with spinal cord injury. Archives of Physical Medicine & Rehabilitation 2000;81(6):764‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Boswell‐Ruys 2013
- Boswell‐Ruys CL, Toh SL, Lee BS, Simpson JM, Clezy KR. Probiotics for preventing urinary tract infection in people with neuropathic bladder. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010723] [DOI] [PMC free article] [PubMed] [Google Scholar]


