Abstract
Background
For management of pneumothorax that occurs without underlying lung disease, also referred to as primary spontaneous pneumothorax, simple aspiration is technically easier to perform than intercostal tube drainage. In this systematic review, we seek to compare the clinical efficacy and safety of simple aspiration versus intercostal tube drainage for management of primary spontaneous pneumothorax. This review was first published in 2007 and was updated in 2017.
Objectives
To compare the clinical efficacy and safety of simple aspiration versus intercostal tube drainage for management of primary spontaneous pneumothorax.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library; MEDLINE (1966 to January 2017); and Embase (1980 to January 2017). We searched the World Health Organization (WHO) International Clinical Trials Registry for ongoing trials (January 2017). We checked the reference lists of included trials and contacted trial authors. We imposed no language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) of adults 18 years of age and older with primary spontaneous pneumothorax that compared simple aspiration versus intercostal tube drainage.
Data collection and analysis
Two review authors independently selected studies for inclusion, assessed trial quality, and extracted data. We combined studies using the random‐effects model.
Main results
Of 2332 publications obtained through the search strategy, seven studies met the inclusion criteria; one study was ongoing and six studies of 435 participants were eligible for inclusion in the updated review. Data show a significant difference in immediate success rates of procedures favouring tube drainage over simple aspiration for management of primary spontaneous pneumothorax (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.69 to 0.89; 435 participants, 6 studies; moderate‐quality evidence). Duration of hospitalization however was significantly less for patients treated by simple aspiration (mean difference (MD) ‐1.66, 95% CI ‐2.28 to ‐1.04; 387 participants, 5 studies; moderate‐quality evidence). A narrative synthesis of evidence revealed that simple aspiration led to fewer adverse events (245 participants, 3 studies; low‐quality evidence), but data suggest no differences between groups in terms of one‐year success rate (RR 1.07, 95% CI 0.96 to 1.18; 318 participants, 4 studies; moderate‐quality evidence), hospitalization rate (RR 0.60, 95% CI 0.25 to 1.47; 245 participants, 3 studies; very low‐quality evidence), and patient satisfaction (median between‐group difference of 0.5 on a scale from 1 to 10; 48 participants, 1 study; low‐quality evidence). No studies provided data on cost‐effectiveness.
Authors' conclusions
Available trials showed low to moderate‐quality evidence that intercostal tube drainage produced higher rates of immediate success, while simple aspiration resulted in a shorter duration of hospitalization. Although adverse events were reported more commonly for patients treated with tube drainage, the low quality of the evidence warrants caution in interpreting these findings. Similarly, although this review observed no differences between groups when early failure rate, one‐year success rate, or hospital admission rate was evaluated, this too needs to be put into the perspective of the quality of evidence, specifically, for evidence of very low and low quality for hospitalization rate and patient satisfaction, respectively. Future adequately powered research is needed to strengthen the evidence presented in this review.
Plain language summary
Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults
Why is this comparison important?
Air that collects between the lung and the chest wall (the pleural space) is described as a pneumothorax. A pneumothorax may be caused by trauma or lung disease, but sometimes it happens spontaneously and has no obvious cause. When this happens, the lungs cannot expand properly, making it difficult to breathe effectively. The person can become breathless and may have chest pain. It is important to treat the pneumothorax by removing collected air and allowing healing of the pleura ‐ a thin membrane that covers the lungs and acts as a lining for them within the chest. For initial management when medical intervention is needed, air can be removed by drawing it out through a thin tube (simple aspiration), or by inserting a much larger chest tube into the space between the ribs (intercostal tube drainage).
How did we gather evidence for this comparison?
We searched the medical literature (January 2017) and identified seven studies that met the inclusion criteria; one study was ongoing and six studies were eligible for inclusion in the updated review.
What did we find?
The six included studies comprised a total of 435 participants with primary spontaneous pneumothorax; 208 of these underwent simple aspiration and 227 underwent intercostal tube drainage. Study results show that tube drainage produced a better rate of immediate treatment success when compared with simple aspiration for primary spontaneous pneumothorax. However, simple aspiration was associated with shorter duration of hospitalization and may have led to fewer adverse events. Researchers noted no significant differences between the two treatments with regard to hospitalization rate, early failure rate, one‐year success rate, or patient satisfaction. However, the quality of evidence presented in this review ranged between very low and moderate, making it difficult for review authors to come to definitive conclusions.
Conclusions
Results of this review indicate that tube drainage has a better immediate success rate than simple aspiration for treating people with primary spontaneous pneumothorax. However, simple aspiration results in a shorter hospital stay and, although the evidence presented for this outcome is of low quality, may lead to fewer adverse events than are reported with tube drainage. The overall quality of evidence ranges from very low to moderate, and future research is needed to strengthen the evidence presented in this review.
Summary of findings
Summary of findings for the main comparison. Simple aspiration compared with intercostal tube drainage for primary spontaneous pneumothorax.
| Simple aspiration compared with intercostal tube drainage for primary spontaneous pneumothorax | ||||||
|
Patient or population: adults with primary spontaneous pneumothorax Settings: university teaching hospitals, tertiary care hospitals, and general hospitals Intervention: simple aspiration Comparison: intercostal tube drainage | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Intercostal tube drainage | Simple aspiration | |||||
| Immediate success rate Follow‐up: 3 days to 24 months | 714 per 1000 | 557 per 1000 (493 to 635) | RR 0.78 (0.69 to 0.89) | 435 (6 studies) | ⊕⊕⊕⊝ moderatea | |
| One‐year success rate Follow‐up: 12 to 24 months | 766 per 1000 | 820 per 1000 (735 to 904) | RR 1.07 (0.96 to 1.18) | 318 (4 studies) | ⊕⊕⊕⊝ moderatea | |
| Hospitalization rate Follow‐up: 3 days to 24 months | 862 per 1000 | 517 per 1000 (215 to 1000) | RR 0.60 (0.25 to 1.47) | 245 (3 studies) | ⊕⊝⊝⊝ very lowa,b,c | |
| Duration of hospital stay Follow‐up: 12 to 24 months | Mean duration of hospital stay ranged across control groups from 4.04 to 7 days. | Mean duration of hospital stay in the intervention groups was 1.66 lower (‐2.28 to ‐1.04). | — | 387 (5 studies) | ⊕⊕⊕⊝ moderatea | |
| Adverse events Follow‐up: 3 days to 24 months | Overall, fewer adverse events occurred when patients were treated by simple aspiration than by tube drainage, including lesser perceived pain and lower pain scores, reduced need for thoracoscopic pleurodesis, and fewer technical adverse events (e.g. tube blockage when treated by tube drainage). | Not estimable | 245 (3 studies) | ⊕⊕⊝⊝ lowa,d | ||
| Cost‐effectiveness Not reported | See comment. | See comment. | Not estimable | ‐ | See comment. | No studies reported cost‐effectiveness data. |
| Patient satisfaction Follow‐up: mean 3 days | Median patient satisfaction among those treated with intercostal tube drainage was 8 on a visual analogue scale of 1 to 10 (Interquartile range 6.25 to 9.00), and median patient satisfaction among those treated by simple aspiration was 0.5 points lower (Interquartile range 5.00 to 9.00). | 48 (1 study) | ⊕⊕⊝⊝ lowa,e | |||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aResults for all outcomes downgraded one level as a result of study and/or reporting limitations, specifically, the large number of unclear or high risks of bias in the presented studies.
bDowngraded one level as a result of significant heterogeneity (I² = 91%). cDowngraded one level as a result of imprecision: low numbers of events and large confidence intervals. dDowngraded one level as a result of inconsistency in and lack of reporting of adverse events. eDowngraded one level as a result of imprecision: reported by only one small study of 48 participants.
Background
Description of the condition
Pneumothorax is defined as trapping of air or gas in the space between the lung and the chest wall (the pleural space) (Light 2007; Noppen 2010). Spontaneous pneumothoraces may have no identifiable cause (e.g. trauma), making this a topic of ongoing debate among healthcare professionals (Sahn 2000; Tschopp 2015). Two main types of spontaneous pneumothoraces may occur: primary and secondary. Primary spontaneous pneumothorax (PSP) is a pneumothorax that occurs in a patient with clinically unapparent lung disease. Secondary spontaneous pneumothoraces occur in patients with underlying lung disease, most often chronic obstructive pulmonary disease (Currie 2007; Light 1994). Although their exact cause remains unknown, PSPs are believed to be associated with diffuse, often bilateral, abnormalities (including but not limited to subpleural blebs or bullae) within the pleura (Grundy 2012; Noppen 2008).
Primary spontaneous pneumothorax is a significant global problem, with a reported incidence of 18 to 28 per 100,000 men per year and 1.2 to 6 per 100,000 women per year (Grundy 2012; Melton 1979). Primary spontaneous pneumothorax is more prevalent among individuals who smoke (Bense 1987; Grundy 2012). Combined global hospital admission rates for primary and secondary pneumothoraces have been reported at 16.7 per 100,000 men per year and 5.8 per 100,000 women per year (Gupta 2000). The United Kingdom (UK) has reported mortality rates of 1.26 per million men per year and 0.62 per million women per year (Gupta 2000). Meanwhile, in the USA, PSP affects more than 20,000 individuals per year (Melton 1979) and accounts for nearly USD130,000,000 in annual healthcare expenditures (Baumann 2001).
Description of the intervention
People with small PSPs may require only observation with supplemental oxygen. However, recommendations often call for active intervention for a large pneumothorax, with an apex‐to‐cupola distance ≥ 3 cm or measuring ≥ 15%/20% on the Light Index (Noppen 2001), or for one that causes substantial dyspnoea, chest pain, or both (Noppen 2003; Pasquier 2013). Percutaneous needle aspiration, hereafter referred to as simple aspiration, is performed by placing an intravenous catheter into the pleural space at the intersection of the midclavicular line and the second or third intercostal space, then using a large syringe to withdraw air or gas from the pleural space. This procedure can be performed in the emergency department (ED) without the need for hospital admission (BMJ 2016).
Alternatively, intercostal tube drainage (hereafter referred to as tube drainage) involves insertion of a small‐bore catheter that is attached to a one‐way flutter valve into the pleural space. Most suction devices employ a water‐filled chamber, through which air removed from the pleural space bubbles, allowing easy identification of a persistent air leak (BMJ 2016). After insertion, patients can be discharged from the ED with the catheter in situ, as long as the flutter valve is functioning properly (BMJ 2016). In some instances, patients require negative‐pressure suction to resolve the pneumothorax and hospitalization may be necessary.
How the intervention might work
For initial management of PSP requiring medical intervention, an air evacuation technique is required, specifically, simple aspiration (thoracocentesis) or insertion of a chest tube (tube thoracostomy), as described above (Noppen 2003). Goals of pneumothorax treatment include the following: to eliminate the intrapleural air collection, to facilitate pleural healing, and to attempt to prevent recurrence (Baumann 1997; Grundy 2012).
Why it is important to do this review
Two clinical trials conducted in the 1960s led to opposing recommendations for initial management of PSP (Ruckley 1966; Stradling 1966); since that time, debate has continued, and current guidelines provide contradictory recommendations (Baumann 2001; Bintcliffe 2015; MacDuff 2010). The guideline consensus process of the American College of Chest Physicians (ACCP) revealed that simple aspiration is rarely appropriate in any clinical circumstance (Baumann 2001). In contrast, current British Thoracic Society (BTS) guidelines for management of PSP recommend simple aspiration as first‐line treatment for all primary pneumothoraces requiring intervention (MacDuff 2010). More recently, the European Respiratory Society (ERS) task force recommended aspiration for the first episode of PSP among symptomatic patients (Tschopp 2015). Despite publication of these specific but contradictory guidelines, management of PSP continues to be characterized by empiricism rather than by evidence‐based practices (Janssen 1994; Mendis 2002; Selby 1994; Soulsby 1998; Yeoh 2000). This variability is due in part to the paucity of high‐quality evidence and to variable management recommendations within different sets of guidelines (Bintcliffe 2015). Thus, compliance with guideline recommendations remains poor (Grundy 2012). This review aims to consolidate available evidence for management of PSP by simple aspiration versus intercostal tube drainage, and to determine the best treatment approach as suggested by current evidence.
Objectives
To compare the clinical efficacy and safety of simple aspiration versus intercostal tube drainage for management of PSP.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), defined as studies in which investigators allocated participants to treatment groups on the basis of a random or quasi‐random method (e.g. use of random numbers tables, a hospital number, date of birth).
Types of participants
We included adults 18 years of age and older with PSP.
Types of interventions
Targeted interventions included simple aspiration or intercostal tube drainage for a first episode of PSP. We included only studies in which the technique incorporated immediate catheter removal after aspiration. We excluded studies in which investigators used kits containing aspiration catheters and a one‐way valve (e.g. Heimlich valve) and did not remove the catheter immediately after aspiration.
Types of outcome measures
Primary outcomes
Immediate success rate of the procedure, as defined by individual study authors (definitions for each study provided in Characteristics of included studies tables)
Secondary outcomes
Early failure rate of simple aspiration/chest tube drainage as defined by study authors
Cost‐effectiveness
One‐year success rate
Adverse events (e.g. pain during procedure, daily pain score, number undergoing pleurectomy or other procedures for lung pleurodesis within one year, mortality)
Hospital utilization (length of stay, re‐admission rate)
Patient satisfaction during the procedure
Dyspnoea score
We planned to broadly divide adverse events into four categories: procedural, non‐procedural, technical, and mortality. We defined procedural adverse events as penetration of major organs (such as lung, stomach, spleen, liver, heart, and great vessels), haemorrhage from intercostal vessels, and pain experienced during the procedure. We defined non‐procedural adverse events as pleural infection (empyema) and surgical emphysema. We defined technical adverse events as malpositioned, kinked, blocked, or clamped tubes.
Search methods for identification of studies
Electronic searches
We identified RCTs by searching the literature using systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply restrictions to language or publication status. We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1).
MEDLINE (Ovid SP, 1966 to January week 1 2017).
Embase (Ovid SP, 1988 to January week 1 2017).
We developed a subject‐specific search strategy for MEDLINE and used this as the basis for search strategies applied for the other listed databases. When appropriate, we expanded the search strategy using search terms for identifying RCTs. All search strategies can be found in Appendix 1.
Searching other resources
We made additional efforts to locate potentially relevant RCTs from the following data sources.
World Health Organization International Clinical Trial Registry Portal (WHO ICTRP) for potential ongoing studies (http://apps.who.int/trialsearch/default.aspx; 1 January 2017), using the search terms 'pneumothorax' AND ((aspiration OR thoracocentesis) AND (thoracotomy OR "chest drain" OR "chest drains" OR "chest tube" OR "chest tubes" OR "intercostal drainage")).
Clinicaltrials.gov for potential ongoing AND unpublished studies (https://clinicaltrials.gov; 1 January 2017), using the search terms 'pneumothorax' AND ((aspiration OR thoracocentesis) AND (thoracotomy OR "chest drain" OR "chest drains" OR "chest tube" OR "chest tubes" OR "intercostal drainage")).
Reference lists of included studies and primary publications.
Other unpublished sources known to lead authors of BTS and ACCP guidelines on management of pneumothorax (sought by personal communication).
Raw data from published trials (sought by personal communication).
Data collection and analysis
Selection of studies
Two review authors (from KC, JA, GM, MB, and AW) independently reviewed the literature by searching titles, abstracts, or descriptors; excluded all studies that clearly did not meet the inclusion criteria of the review; and reviewed full texts of retrieved articles to assess eligibility for inclusion. Review authors reached complete agreement (after discussion) regarding inclusion and exclusion criteria for all full‐text studies obtained for closer examination.
Data extraction and management
Two review authors (KC, JA) independently extracted data for each study onto standardized data collection forms. We requested unpublished data from primary authors when necessary. We then entered the data into Review Manager 5.3 software for analysis (RevMan 5.3). We extracted data using a standardized pilot‐tested data collection form.
Assessment of risk of bias in included studies
Two review authors (KV, JA) independently assessed each study for risk of bias in relation to allocation sequence generation, allocation concealment, blinding of participants and outcome assessors, handling of missing data, selective outcome reporting, and other threats to validity, in line with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For continuous outcomes, we calculated mean differences (MDs) with 95% confidence intervals (CIs) and pooled MDs or standardized mean differences (SMDs). For dichotomous outcomes, we calculated risk ratios (RRs) with 95% CIs.
Unit of analysis issues
We did not encounter unit of analysis issues, as we included no cross‐over studies, no cluster‐randomized studies, and no multiple observational studies. Had we identified a cluster‐randomized study for inclusion, we would have performed analysis at the level of the individual, while accounting for clustering within data, as per recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4) (Higgins 2011).
Dealing with missing data
We evaluated missing information regarding participants on an available case analysis basis, as described in Chapter 16.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When statistics essential for analysis were missing (e.g. group means and standard deviations for both groups were not reported) and could not be calculated from other data, we attempted to contact study authors to obtain data. We assumed that loss of participants that occurred before baseline measurements were obtained would have no effect on eventual outcome data of the study. We assessed and discussed on an intention‐to‐treat basis any losses reported after baseline measurements were taken. We performed a narrative synthesis for each of the included studies.
Assessment of heterogeneity
In meta‐analyses of outcomes based on pooling of two or more studies, we tested heterogeneity estimates using the Der Simonian and Laird method, with P < 0.05 and I² ≥ 50% considered statistically significant, and we visually inspected the data (Higgins 2011). We reported all results using a random‐effects model; however, in the presence of considerable heterogeneity (I² ≥ 75%), we believed that pooling of data may not have been suitable and that pooled data should be interpreted with caution.
Assessment of reporting biases
Provided we identified a minimum of 10 studies for inclusion, we planned to explore potential reporting biases using a funnel plot. Asymmetry in the plot could have been attributed to publication bias but also could have been due to true heterogeneity, poor methodological design, poor quality, or artefact. If we identified fewer than 10 studies, as occurred in this review, we planned to extrapolate potential reporting biases within the 'Other bias' section in the 'Risk of bias' tables.
Data synthesis
We analysed outcomes as continuous or dichotomous data using standard statistical techniques with a random‐effects model for all studies deemed similar enough to be pooled. We analysed data from all trials using Review Manager 5.3. For continuous variables, we calculated mean differences (MDs) and 95% confidence intervals (CIs) for each study outcome. For dichotomous variables, we calculated risk ratios (RRs) with 95% CIs. We also performed a narrative synthesis for each included study.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for size of pneumothorax and study quality (allocation concealment, blinding of participants, and blinding of outcome assessors) in the presence of significant heterogeneity (defined by the Der Simonian and Laird method, with P < 0.05 and I² ≥ 50% considered statistically significant), together with visual inspection of the data.
Sensitivity analysis
We conducted sensitivity analyses on studies at high risk of selection bias for sequence generation or allocation concealment, or both, and on studies with significant differences following visual inspection of the data producing anomalies. We performed a sensitivity analysis to re‐analyse potential bias in studies comparing recurrent pneumothorax versus a first episode of pneumothorax.
'Summary of findings' table and GRADE
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and to generate a 'Summary of findings' table (Guyatt 2008). We downgraded evidence by one level in cases of serious, and by two levels in cases of very serious, risk of bias, indirectness of evidence, imprecision (e.g. small sample size), publication bias, or serious inconsistency between results (high heterogeneity). The 'Summary of findings' table includes the following outcomes.
Immediate success rate.
Hospitalization rate.
Duration of hospital stay.
Adverse events.
Cost‐effectiveness.
Patient satisfaction.
One‐year success rate.
Results
Description of studies
Results of the search
Using the search strategies listed above, we identified 2332 unique records from CENTRAL, Embase, and MEDLINE. In addition, we identified four records by searching clinicaltrials.gov and 95 (90 trials) by searching WHO ICTRP. Independent assessment of the search results mentioned above led to identification of 11 potential studies (14 records), one of which was ongoing (Desmettre 2011) and ten that were completed. Of these 10 studies, we deemed four ineligible for inclusion (Burgaud 1985; Faruqi 2004; Hernandez Ortiz 1999), leading to identification of six studies (Andrivet 1995; Ayed 2006; Harvey 1994; Ho 2011; Noppen 2002; Parlak 2012) that met the inclusion criteria for this review (Figure 1).
1.
Study flow diagram.
Included studies
In this updated review, we included six studies (Andrivet 1995; Ayed 2006; Harvey 1994; Ho 2011; Noppen 2002; Parlak 2012) with 435 participants (see Characteristics of included studies table). We added five of those six studies (Andrivet 1995; Ayed 2006; Harvey 1994; Ho 2011; Parlak 2012) when performing the 2017 update of this review.
Design
All included studies were randomized controlled trials (RCTs) with pretest and post‐test control groups. Investigators randomized participants to simple aspiration or intercostal tube drainage. All studies employed an intention‐to‐treat analysis method.
Population
Studies were conducted in France (Andrivet 1995), Kuwait (Ayed 2006), the UK (Harvey 1994), Singapore (Ho 2011), Belgium (Noppen 2002), and the Netherlands (Parlak 2012).
Ayed 2006, Harvey 1994, and Ho 2011 were single‐site studies performed at a tertiary care hospital and a general hospital, respectively. Andrivet 1995 was conducted across four medical intensive care units, three of which were located in university teaching hospitals. Noppen 2002 took place at a tertiary hospital and at four regional general hospitals, and Parlak 2012 was conducted in a hospital group.
The six studies included 435 participants according to similar diagnostic criteria. Ayed 2006 included participants who were symptomatic (regardless of size of the pneumothorax) or for whom pneumothoraces made up > 20% of the hemithorax. Neither Andrivet 1995 nor Harvey 1994 reported specific criteria, except to say that PSP occurred in participants without underlying lung disease (Andrivet 1995), or that the study included participants presenting with PSP thought by the admitting team to require a drainage procedure (Harvey 1994). Noppen 2002 and Parlak 2012 had similar inclusion criteria: 20% as determined by the Light Index (Light 2007) and symptomatic pneumothorax (e.g. dyspnoea, chest pain). Ho 2011 included participants with pneumothorax ≥ 3 cm from the apex (< 3 cm from the apex was an exclusion criterion).
Three included studies (Ayed 2006; Ho 2011; Noppen 2002) tested only participants with first episode PSP, two examined participants with first episode PSP and those with recurrent PSP (Andrivet 1995; Harvey 1994), and the fifth (Parlak 2012) tested participants with first episode PSP or traumatic primary pneumothorax. It was not possible to separate participants with PSP from those with traumatic pneumothorax, and this should be taken into account when review results are interpreted.
Participant numbers per trial ranged from 48 to 137, with five studies (Andrivet 1995; Harvey 1994; Ho 2011; Noppen 2002; Parlak 2012) reporting fairly similar smaller participant numbers, and the sixth (Ayed 2006) including a greater number of participants (n=137).
All studies tested a majority of male participants. Andrivet 1995 included 50 males and 11 females, Ayed 2006 enrolled 128 males and 9 females, and Harvey 1994 included 57 males and 16 females. Ho 2011 reported that a majority of participants were male (92% in the aspiration group and 91.3% in the tube drainage group). Noppen 2002 performed simple aspiration on 20 males (vs 7 females) and tube drainage on 28 males (vs 5 females). Parlak 2012 performed simple aspiration on 17 males and 8 females compared with 23 males and 8 females for the tube drainage condition.
Participants in the aspiration group in Ayed 2006 had an average age of 24.4, and average age of those in the tube drainage group was 23.5. Ho 2011 and Noppen 2002 included participants of similar age: 26 and 28.2 years for the simple aspiration group, and 24.3 and 28.9 years for the chest tube drainage group, respectively. Parlak 2012 described a slightly older participant group, with a mean age of 47 for the aspiration group and 40 for the chest tube drainage group.
Interventions
Andrivet 1995 reported simple aspiration (maximum 30 minutes) performed after local anaesthesia with a plastic catheter (calibre 16 or 18F) inserted into the second anterior intercostal space at the midclavicular line, with a chest tube (calibre 20F) connected to a vacuum source via a two‐bottle regulated system.
Investigators in Ayed 2006 performed simple aspiration for 30 minutes, or until cessation of air to the one‐bottle water seal vacuum system occurred. They performed chest tube drainage under local anaesthesia at the fourth or fifth interspace at the midaxillary line, and directed towards the apex.
Harvey 1994 used a 16‐ to 18‐gauge catheter for aspiration with a three‐way tap, and managed the chest tube according to the usual practice of the participating physician; researchers provided no other information.
Ho 2011 performed simple aspiration via a 16G cannula in the second intercostal space at the midclavicular line. Investigators performed chest tube drainage via a tube connected to a Heimlich valve.
In Noppen 2002, researchers performed simple aspiration through a three‐way tap with a small‐calibre 16‐gauge polyethane intravenous catheter under local anaesthesia in the second or third intercostal space, at the midclavicular line. They performed chest tube drainage by inserting 16‐ or 20‐gauge plastic tubes under local anaesthesia at the anterior midclavicular second interspace, or at the fourth or fifth interspace, at the midaxillary line, and directed to the apex.
Parlak 2012 performed simple aspiration in the second or third intercostal space with a 1.3‐mm catheter, or with a pneumocatheter in cases of extreme obesity. Researchers performed chest tube drainage in a similar fashion but via a tube thoracostomy with two reservoirs.
If simple aspiration was unsuccessful in Andrivet 1995, Ayed 2006, or Noppen 2002 (as determined by chest radiography), investigators made a second attempt. If the second attempt was unsuccessful, the participant received a tube thoracostomy. If simple aspiration was unsuccessful in Harvey 1994, Ho 2011, or Parlak 2012, participants underwent chest tube drainage after the first attempt (no second attempt would be made).
Ho 2011 used a different approach to tube drainage compared with the other studies. In Andrivet 1995, Ayed 2006, Harvey 1994, Noppen 2002, and Parlak 2012, all participants treated with tube drainage were automatically hospitalized, but this was not the case for participants in Ho 2011.
Length of follow‐up
Follow‐up ranged widely from 3 days (Ho 2011) to 3 months (Andrivet 1995) to 12 months (Harvey 1994; Noppen 2002; Parlak 2012) to 24 months (Ayed 2006).
Outcome measures
Immediate success rate
Andrivet 1995, Ayed 2006, Harvey 1994, and Noppen 2002 reported immediate success rate, defined as (near) complete lung expansion following simple aspiration. Investigators stated no time limit for an immediate success rate of simple aspiration. Parlak 2012 defined immediate success rate for simple aspiration as "complete success after the first attempt with discharge after 24 hours." All studies provided similar definitions of immediate success rate for tube drainage, including absence of air leakage, complete expansion of the lung, and chest tube removal within 72 hours after insertion of the chest tube. Ho 2011 did not report directly on immediate success rates (although immediate failure rates reported allow for calculation of immediate success rates).
Early failure rate of simple aspiration/chest tube
Ho 2011 reported on rate of early failure and defined this as "recurrence of pneumothorax, need for a second procedure, or need for surgical intervention." Study authors further specified this as worsening of pneumothorax for the tube drainage group, and as pneumothorax greater than 10% after the observation period of six hours for the simple aspiration group. A similar observation period applies to the tube drainage group.
Cost‐effectiveness
No studies provided information on cost‐effectiveness.
One‐year success rate
Ayed 2006, Harvey 1994, Noppen 2002, and Parlak 2012 provided information on one‐year success rate, defined as lack of recurrence of pneumothorax. Investigators in all trials treated participants with tube drainage if the first attempt (Andrivet 1995; Ho 2011; Parlak 2012) or the first two attempts (Ayed 2006; Harvey 1994; Noppen 2002) at simple aspiration failed; this can influence the success rate beyond immediate success (owing to intention‐to‐treat).
Adverse events (pain during procedure, daily pain score, number undergoing pleurectomy or other procedures for lung pleurodesis within one year, mortality)
Andrivet 1995 did not report any adverse events. Ayed 2006 did not explicitly report on procedural adverse events but did report on the need for analgesia in the form of pethidine to reflect the level of pain experienced during the procedure. Ho 2011 reported on pain scores and bleeding (haemothorax), and Noppen 2002 and Parlak 2012 did not refer to specific procedural adverse events. Ayed 2006 and Ho 2011 mentioned non‐procedural adverse events including the need for video‐assisted thoracoscopic surgery to deal with persistent air leaks and occurrence of subcutaneous emphysema. Ayed 2006 furthermore reported on exit site infection. Harvey 1994 and Noppen 2002 reported only on the need for thoracoscopy for pleurodesis, and Parlak 2012 made no mention of non‐procedural events. Ayed 2006 mentioned tube blockage as the only technical adverse event. Ho 2011 mentioned problems related to placement of the chest tube, and Noppen 2002 and Parlak 2012 did not report on technical adverse events. Ayed 2006 and Noppen 2002 did not report on mortality, and Ho 2011 and Parlak 2012 mentioned mortality in the text.
Hospital utilization (length of stay, re‐admission rate)
Ayed 2006, Ho 2011, and Noppen 2002 provided information on hospitalization rate and duration, and Andrivet 1995, Harvey 1994, and Parlak 2012 provided information only on duration of hospitalization.
Participant satisfaction during the procedure
Ho 2011 was the only study that reported satisfaction rates (via visual analogue scales).
Dyspnoea score
No studies provided information on dyspnoea.
Other endpoints mentioned
Studies reported several endpoints that were not defined in this review. Ayed 2006 reported one‐week success, recurrence rates at follow‐up of three months and two years, and inability to work. Noppen 2002 reported one‐week success and participant safety. Ho 2011 reported on rates of full recovery at outpatient follow‐up, as well as rates of recurrence after six hours.
Excluded studies
We excluded four studies, three because they were not RCTs ((Burgaud 1985; Faruqi 2004; Hernandez Ortiz 1999), and one because both primary and secondary pneumothoraces were included (Korczynski 2015). For a detailed description of the reason for each exclusion, see the Characteristics of excluded studies table.
Ongoing studies
One study (Desmettre 2011) is currently ongoing (but is not recruiting) and most likely can be considered in subsequent versions of this review. (See Characteristics of ongoing studies.)
Studies awaiting classification
No studies are awaiting classification.
Risk of bias in included studies
We have provided methodological details for the six included studies in 'Risk of bias' tables at the end of the Characteristics of included studies tables. We have summarized key methodological features in Figure 2 and Figure 3.
2.
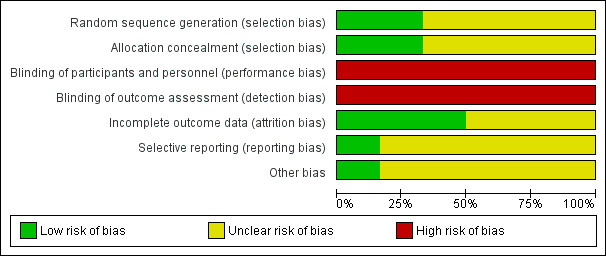
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
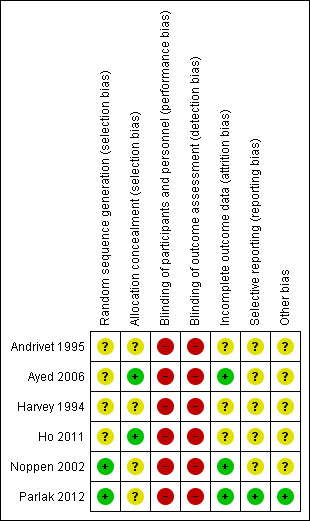
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Noppen 2002 and Parlak 2012 reported adequate methods of sequence generation; the remaining four studies did not provide enough information to reveal whether sequence generation was adequate and therefore remained at unclear risk. Adequate methods include randomization via a computer minimization programme and via a random number list provided by a computer‐generated table.
Allocation concealment
Two studies were at low risk of bias owing to allocation concealment, as concealment was done via sealed envelopes (Ayed 2006; Ho 2011). However, the remaining four studies did not report methods used to conceal the allocation of participants and therefore were rated as having unclear risk.
Blinding
Blinding of participants or personnel did not occur in any of the included studies. Both procedures require local anaesthesia, meaning that the participant is aware of the procedure that is being conducted. Therefore, all studies were at high risk of performance and detection bias.
Incomplete outcome data
Three studies (Ayed 2006; Noppen 2002; Parlak 2012) conducted analysis on an intention‐to‐treat basis, and all three articles discussed attrition. Andrivet 1995, Harvey 1994, and Ho 2011 also used an intention‐to‐treat analysis but did not report sufficient information to show risk of attrition bias.
Selective reporting
Parlak 2012 is included as an entry on clinicaltrials.gov, leading review authors to assess risk of reporting bias as low. Remaining studies did not provide sufficient information to permit a judgement.
Other potential sources of bias
Parlak 2012 is included as an entry on clinicaltrials.gov, leading review authors to assess risk of other bias as low. The remaining five studies (Andrivet 1995; Ayed 2006; Harvey 1994; Ho 2011; Noppen 2002) did not provide sufficient information to permit a judgement.
Effects of interventions
See: Table 1
Immediate success rate for the procedure (primary outcome)
The primary endpoint of this review was immediate success rate for both the simple aspiration group and the intercostal tube drainage group. Five studies reported on this outcome directly (Andrivet 1995; Ayed 2006; Harvey 1994; Noppen 2002; Parlak 2012), and Ho 2011 allowed the number to be inferred from failure rate data (the study did not perform a second simple aspiration after a failed attempt, meaning that the number mentioned for failure rate in this study can be recalculated to determine the immediate success rate). Overall, results show a statistically significant difference in immediate success rates favouring chest tube drainage (RR 0.78, 95% CI 0.69 to 0.89; P = 0.0001; 435 participants, 6 studies) (Figure 4). We assessed the quality of this evidence as moderate.
4.
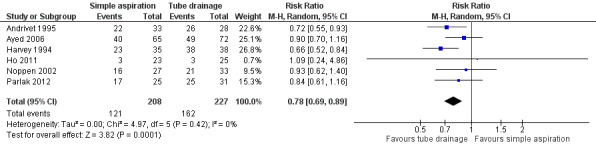
Forest plot of comparison: 1 Simple aspiration versus intercostal tube drainage, outcome: 1.1 Immediate success rate of procedure as defined by study authors.
Early failure rate for simple aspiration or chest tube drainage
The first secondary endpoint of this review was failure rate for simple aspiration versus intercostal tube drainage, which was measured by all studies (Andrivet 1995; Ayed 2006, Harvey 1994; Ho 2011; Noppen 2002; Parlak 2012). As Ayed 2006, Noppen 2002, and Harvey 1994 made a second attempt after a failed first attempt, failure rates are not equal to the results described above. Data show no differences in failure rate between simple aspiration and chest tube drainage (RR 1.24, 95% CI 0.91 to 1.68; 427 participants, 6 studies; Analysis 1.2).
1.2. Analysis.
Comparison 1 Simple aspiration versus intercostal tube drainage, Outcome 2 Early failure rate of procedure (incomplete lung expansion after the procedure).
Cost‐effectiveness
None of the included studies reported on cost‐effectiveness.
One‐year success rate
Four studies (Ayed 2006; Harvey 1994; Noppen 2002; Parlak 2012) mentioned one‐year success rate, as determined by recurrence of pneumothorax, and this information could subsequently be added to a meta‐analysis showing no differences between simple aspiration and chest tube drainage in one‐year success rates (RR 1.07, 95% CI 0.96 to 1.18; 318 participants, 4 studies; Analysis 1.3). We assessed the quality of this evidence as moderate.
1.3. Analysis.
Comparison 1 Simple aspiration versus intercostal tube drainage, Outcome 3 One‐year success rate (number of participants with no recurrence of pneumothorax at 1 year).
Adverse events
We could not combine adverse events in a meta‐analysis, as studies reported this information quite differently from one another. However, we prepared a narrative synthesis separating adverse events into the four categories below. We judged the quality of the evidence provided as low.
Procedural adverse events
Ayed 2006 indirectly reported on perceived pain during treatment by reporting the need for analgesia in 62 participants ‐ 22 simple aspiration participants and 40 tube drainage participants. Although this difference was significant at P = 0.01, results show no differences between amounts of analgesia required. Harvey 1994 found that pain during the procedure was not significant (P = 0.33), but that average daily pain score was significantly higher in the tube drainage arm than in the aspiration arm (P < 0.001). Ho 2011 reported that no cases of haemothorax occurred during either treatment and showed no differences on pain scales between the two intervention groups (P = 0.308). Andrivet 1995, Noppen 2002, and Parlak 2012 did not report procedural adverse events.
Non‐procedural adverse events
Ayed 2006 (seven vs nine), Harvey 1994 (zero vs seven), Ho 2011 (six vs three), and Noppen 2002 (two vs five) reported on the need for (thoracoscopic) pleurodesis in simple aspiration and tube drainage, respectively, after previous unsuccessful treatment attempts. Ayed 2006 reported two participants with subcutaneous emphysema and one with exit site infection in the tube drainage group versus one participant with subcutaneous emphysema in the simple aspiration group. Ho 2011 reported that two participants developed subcutaneous emphysema after simple aspiration versus none in the tube drainage condition. Andrivet 1995 and Parlak 2012 did not report on non‐procedural adverse events.
Technical adverse events
Ayed 2006 reported two cases of tube blockage in the tube drainage condition. Ho 2011 found that one participant developed signs of tension pneumothorax owing to misplacement of a Heimlich valve in the tube drainage condition. The remaining four studies did not report technical adverse events.
Mortality
Ho 2011 reported no mortality; Parlak 2012 mentioned one death (due to heart failure) in the text but did not indicate to which group the participant belonged. The remaining four studies did not report on mortality.
Hospital utilization
Three studies (Ayed 2006; Ho 2011; Noppen 2002) mentioned the need for hospitalization after the procedure. Pooling of results showed no differences between the two interventions (RR 0.60, 95% CI 0.25 to 1.47; 245 participants, 3 studies). However, these results must be placed in the context of extremely high heterogeneity (I² = 91%) and a low total event rate (245 events), and therefore should be interpreted with caution; no decisive conclusions can be drawn from the current evidence. We judged the quality of the evidence to be very low.
Five studies reported hospitalization duration, which was significantly less for participants undergoing simple aspiration than for those treated with chest tube drainage (MD ‐1.66, 95% CI ‐2.28 to ‐1.04; P < 0.00001; 387 participants, 5 studies; Analysis 1.5). We rated the quality of this evidence as moderate (after downgrading due to a low total event rate of 387 events). Although Ho 2011 reported duration of hospitalization, it was not possible to include these data in the meta‐analysis, as numbers were given in medians and interquartile distances, and the reported information was not sufficient to allow recalculation. Study authors found no differences between simple aspiration and tube drainage in duration of hospitalization when participants were hospitalized after immediate treatment failure (P = 0.344), or after re‐assessment at 72 hours in an outpatient clinic (P = 0.814).
1.5. Analysis.
Comparison 1 Simple aspiration versus intercostal tube drainage, Outcome 5 Duration of hospital stay in days.
Patient satisfaction during the procedure
Ho 2011 reported no differences in patient satisfaction (P = 0.583), as determined by visual analogue scales, with patients treated by simple aspiration indicating a median score of 7.5 (interquartile ratio (IQR) 5 to 9) and patients treated by tube drainage indicating a median score of 8 (IQR 6.25 to 9.00). We judged the quality of this evidence to be low.
Dyspnoea score
None of the included studies reported on levels of dyspnoea after the procedure.
Subgroup analysis
Hospitalization was the only outcome that produced considerable heterogeneity warranting subgroup analysis. However, analysis based on pneumothorax size was not possible because a limited number of studies reported heterogeneity in pneumothorax size (Ho 2011 reported 73.9% in the simple aspiration group and 80.0% in the tube drainage group, and Noppen 2002 reported a Light Index of 62.1% in the simple aspiration group and 63.6% in the tube drainage group).
Sensitivity analyses
Risk of bias
Hospital utilization
Sensitivity analyses for risk of bias for blinding were not possible because we considered all studies to be at high risk of bias. However, for allocation concealment, the sensitivity analysis did not identify the cause of heterogeneity (Analysis 2.1).
2.1. Analysis.
Comparison 2 Sensitivity analysis for risk of bias, Outcome 1 Hospitalization (number of participants hospitalized).
Visual inspection of anomalies in the data
Hospital utilization
Although visual inspection of the plots seems to indicate that Ho 2011 may be the study contributing to high heterogeneity of the hospitalization rate, removing this study left an I² of 85% for the two remaining studies (Analysis 3.1).
3.1. Analysis.
Comparison 3 Sensitivity analysis following visual inspection of data, Outcome 1 Hospitalization (number of participants hospitalized).
Recurrent versus first episode pneumothorax
Immediate success rate of the procedure
Recurrent episodes of PSP were significantly more likely to produce immediate success with tube drainage compared with simple aspiration (P = 0.04; I² = 76.4%; Analysis 4.1).
4.1. Analysis.
Comparison 4 Sensitivity analysis for studies with recurrent pneumothorax, Outcome 1 Immediate success rate of procedure as defined by study authors.
Early failure rate of simple aspiration or chest tube
Data show no differences between recurrent and first episode pneumothorax for early failure rate of the procedure (P = 0.72; Analysis 4.2).
4.2. Analysis.
Comparison 4 Sensitivity analysis for studies with recurrent pneumothorax, Outcome 2 Early failure rate of procedure (incomplete lung expansion after the procedure).
One‐year success rate
Data show no differences between recurrent and first episode pneumothorax for one‐year success rate (P = 0.47; Analysis 4.3).
4.3. Analysis.
Comparison 4 Sensitivity analysis for studies with recurrent pneumothorax, Outcome 3 One‐year success rate (number of participants with no recurrence of pneumothorax at 1 year).
Hospital utilization
Data show no differences between recurrent and first episode pneumothorax for duration of hospital stay (P = 0.73; Analysis 4.4).
4.4. Analysis.
Comparison 4 Sensitivity analysis for studies with recurrent pneumothorax, Outcome 4 Duration of hospital stay in days.
Discussion
Summary of main results
For this update, we found five new studies, complementing the data obtained from the single study identified in the first review (Wakai 2007) comparing simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax (PSP) in adults. We therefore included a total of six studies in this updated review. Furthermore, we identified one ongoing trial (Desmettre 2011) that can be taken into account in future updates of this review.
The six included trials demonstrated significant improvement in the immediate success rate for tube drainage compared with simple aspiration. Simple aspiration on the contrary led to fewer adverse events and a shorter duration of hospitalization. Furthermore, results show no differences in early failure rate, one‐year success rate, patient satisfaction, or hospitalization between simple aspiration and tube drainage. No studies reported directly on cost‐effectiveness, but the shorter duration of hospitalization and the fact that simple aspiration is technically easier to perform might indirectly indicate potential benefit in cost‐effectiveness for the use of simple aspiration to treat individuals with PSP. These results must be placed in the context of the quality of evidence provided, which ranged from very low to moderate.
Overall completeness and applicability of evidence
For this review update, we identified five additional studies examining whether simple aspiration or chest tube drainage should be preferred as initial treatment for PSP. This review provides information on several relevant outcomes, specifically, immediate treatment success, long‐term treatment success, and hospital utilization, for which simple aspiration was performed in an equal or superior way to tube drainage, with the exception of immediate success rate, which was greater in patients treated by tube drainage. Although review authors identified a total of six randomized controlled trials (RCTs) of adequate quality, the total number of participants involved in the combined studies was low. Furthermore, absence of clear‐cut data for some of the secondary outcome measures (cost‐effectiveness, patient satisfaction, dyspnoea, and even adequate description of adverse events in some trials) specified in the protocol limits the robustness of conclusions presented in this review.
Although none of the included studies measured cost‐effectiveness directly, the shorter duration of hospitalization associated with simple aspiration compared with tube drainage hints towards favourable cost‐effectiveness of simple aspiration resulting from the considerable costs associated with hospitalization. This finding is supported by some early studies on the costs of pneumothorax treatment, which show a large proportion of costs going towards hospitalization when video‐assisted thoracoscopic surgery (VATS) was compared with tube drainage (Schramel 1996; Tschopp 2002), and when inpatient care was compared with outpatient care (Bense 1991; Gurley 1998).
Only one study (Ho 2011) reported patient satisfaction, making adequate judgement regarding this outcome difficult. Satisfaction is expected to be higher among patients who do not require hospitalization and is expected to be lower when treatment attempts fail (and therefore have to be repeated). Therefore, it will be useful for researchers to take this assessment into account in future studies. Dyspnoea is not always present and is generally mild in PSP, but it plays a more prominent role in secondary spontaneous pneumothorax (Noppen 2010). Therefore, it is not completely surprising that none of the included RCTs reported on dyspnoea as an endpoint.
Although most studies reported on several adverse events, one study (Parlak 2012) did not mention any associated complications. Investigators have reported potentially fatal penetration of major organs or blood vessels with both intercostal tube drainage (Daly 1985; Holden 1982; Iberti 1992; Miller 1987; Symbas 1989) and simple aspiration (Rawlins 2003). Other reported complications of intercostal tube drainage include pleural cavity infection (empyema, with a reported incidence of 1%) (Chan 1997), surgical emphysema (Maunder 1984), and problems related to displacement or blockage of the tube (Davies 2008). Camuset 2006 reported a transient vagal reaction due to needle aspiration during treatment of PSP as a complication associated with the procedure.
Quality of the evidence
The quality of studies included in this review and their risk of bias are adequate but can be considered an issue, as all studies had at least one source of high risk of bias and (multiple) sources of unclear risk. The biggest problem affecting the quality of the evidence is related to inability to perform blinding during treatment. Furthermore, the final participant number of 435 across six studies is not very high. These results led the review author team to downgrade the quality of evidence provided by these studies by one level for all outcomes.
We deemed that evidence on the two significant outcomes included in the meta‐analyses ‐ immediate success rate favouring tube drainage and hospitalization duration favouring simple aspiration ‐ was of moderate quality. Downgrading of this evidence resulted from the overall one‐level downgrade required by risk of bias of the included studies.
The quality of evidence for the other (non‐significant) outcomes, namely, one‐year success rate, hospitalization rate, adverse events, and patient satisfaction, ranged from very low to moderate. We deemed evidence on hospitalization rate to be of very low quality as a result of very high heterogeneity (I² = 91%) and imprecision of results due to small numbers of events and large confidence intervals. We regarded the quality of evidence for adverse events and patient satisfaction as low. The quality of evidence on adverse events suffered from inconsistency between reported results and an overall lack of appropriate reporting of adverse events, and only one small study including 48 participants reported on patient satisfaction. We downgraded the quality of evidence on one‐year success rate only as a result of the overall downgrade due to risk of bias, as reported above. As no trials reported on cost‐effectiveness, we could assign no judgement on the quality of this evidence.
Potential biases in the review process
Similar to all systematic reviews, this review is limited by the quality of data provided by reported trials (Khan 1996; Khan 2011). However, our search has been executed by a trial specialist, and we have searched for extra studies by going through the reference lists of included studies and other reviews on the topic. We are confident that this review has included all RCTs so far conducted on treatment of primary spontaneous pneumothorax by simple aspiration or tube drainage. All review authors for this review are experienced in producing Cochrane Reviews and come from a variety of backgrounds, which further adds to the robustness of the execution of this review.
Agreements and disagreements with other studies or reviews
Aguinagalde 2010, Devanand 2004, and Zehtabchi 2008 conducted three different systematic reviews on this topic. Aguinagalde 2010 included Andrivet 1995, Ayed 2006, Harvey 1994, and Noppen 2002 but not the more recent Ho 2011, which was not available at the time of publication. The authors of Aguinagalde 2010 reported that including Andrivet 1995 in particular caused the risk ratio for the immediate success rate to favour tube drainage in their meta‐analysis, but that this advantage disappeared after a week. The difference between these results and those reported in this review, as we review authors have noted ourselves, might be due to a more strict definition of treatment failure in simple aspiration than in tube drainage. We further noted that allowing multiple simple aspiration attempts (to match the longer 10‐day interval of continuous aspiration in the tube drainage group) might lead to a better success rate for simple aspiration, which, for instance, was shown in Ayed 2006 (13 out of 25 second attempt simple aspirations were successful) but not in Noppen 2002 (0 out of 6 were successful). The second difference noted in Aguinagalde 2010 lies in the definition of 'treatment success', which specifically affects an outcome that was not specified in the current review: one‐week success. Study authors in Ayed 2006 and authors of the current review consider treatment in the simple aspiration group to have occurred even if the participant received tube drainage as a result of failed previous attempts at simple aspiration. Aguinagalde 2010 did not use this definition and accepted treatment success by simple aspiration only. As our review is based on intention‐to‐treat, we view success as belonging to the treatment to which participants were initially randomized, which can be considered a limitation owing to the specific protocols chosen by trial authors.
The Zehtabchi 2008 review included Ayed 2006, Harvey 1994, and Noppen 2002, and the Devanand 2004 review included Andrivet 1995, Harvey 1994, and Noppen 2002. Findings from both reviews as well as their evidence ratings are mostly in line with those of the current review. The discussion point of Zehtabchi 2008 on limitations of RCTs versus expertise‐based RCTs for non‐pharmaceutical trials such as pneumothorax treatment (see Cook 2015 for a review) is interesting and should be considered for investigation in future trials.
Furthermore, the current review found no advantage for hospitalization rate, although all three of the other reviews reported such an advantage. Inclusion of Ho 2011 led to significant heterogeneity. Sensitivity analysis revealed that heterogeneity is likely due to admission of all patients for tube drainage in Ayed 2006 and Noppen 2002, although Ho 2011 did not automatically admit all patients who underwent a tube drainage procedure.
Authors' conclusions
Implications for practice.
For management of primary spontaneous pneumothorax (PSP) in adults, trials show significant benefit for immediate success rate of the procedure with tube drainage and no differences between groups for early failure rate, one‐year success rate, or hospital admission rate. However, researchers found that simple aspiration was associated with reduced duration of hospitalization and fewer adverse events when compared with intercostal tube drainage. The quality of evidence for this review ranged from very low to moderate, with downgrading due to risk of bias, low event numbers, inconsistent reporting, and substantial heterogeneity.
Taken together with findings from prospective studies and other excluded trials, and given that simple aspiration is relatively easier to perform, simple aspiration seems to be an attractive first‐line treatment option that can reduce the burden on the healthcare system by preventing hospital admissions when they are not needed. The main results of this review validate existing guidelines and consensus statements, which point to simple aspiration as first‐line treatment for all individuals with PSP requiring intervention (MacDuff 2010; Tschopp 2015).
Implications for research.
Well‐powered randomized controlled trials (RCTs) of good methodological design are needed to further validate the primary findings of this review and to address the following.
Future trials should look into (or account for) the effect of tube drainage after failed simple aspiration attempts on future outcomes (e.g. one‐year success rate).
Unaddressed topics of this review should be addressed, namely, cost‐effectiveness and patient satisfaction. A major limitation of currently available RCTs is that they do not consistently report complications associated with simple aspiration and intercostal tube drainage in the management of PSP; future clinical trials should address complications as an important outcome measure. Although the ongoing trial of Desmettre 2011 might bolster participant numbers and thus the power of this review, outcomes specified on clinicaltrials.gov seem to focus only on success rate and size at different intervals, leaving some topics unexamined.
Future trials must consider adding a control group that receives only observation as treatment, as observation has been shown to be effective in treating patients with PSP (Kelly 2008).
Future studies need to look into the role of expertise of the treating clinician (i.e. by deploying an expertise‐based design). Especially tube drainage is susceptible to complications due to technical error, and this can further add to morbidity and unnecessary hospital utilization.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 9 September 2017 | Amended | Typos corrected in plain language summary, excluded studies section, PRISMA figure and search. |
| 20 January 2017 | New citation required and conclusions have changed | We updated the literature search, updated methods, added 'Risk of bias' and 'Summary of findings' tables, updated Background and Discussion sections with new references, changed Conclusions to reflect new studies, changed the Abstract, and updated the 'Characteristics of included studies' table. We added three authors to the review author line. |
| 20 January 2017 | New search has been performed | Updated search: We identified 529 new journal records and 99 new online clinical trial registry records after removal of duplicates. We included five new included studies, and found one study that is ongoing. |
| 7 April 2008 | Amended | We converted this review to new review format |
Acknowledgements
We wish to acknowledge and thank Frédérique Noble for translating the Desmettre 2010 publication into English. For their tireless efforts, we wish to thank all staff and editors of the Cochrane Anaesthesia, Crticial and Emergency Care Group, in particular Jane Cracknell, who aided review completion and provided valuable feedback and suggestions.
We would like to thank Harald Herkner (Content Editor); Jing Xie (Sophia) (Statistical Editor); Nick Maskell and Teresa Williams (Peer Reviewers); Janne Vendt (Information Specialist); and Brian Stafford (Consumer Referee) for help and editorial advice provided during preparation of this updated systematic review.
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor Chest Tubes, this term only #2 MeSH descriptor Drainage, this term only #3 MeSH descriptor Thorax, this term only #4 MeSH descriptor Suction explode all trees #5 MeSH descriptor Needles explode all trees #6 MeSH descriptor Catheterization explode all trees #7 MeSH descriptor Thoracotomy, this term only #8 MeSH descriptor Thoracostomy, this term only #9 chest tube* or drainage or intercostal or thorax or thoracic or chest drain* or suction or Simple or manual or needle* or catheter* or aspiration or Thoracotomy or thoracostomy #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #8 OR #9) #11 MeSH descriptor Pneumothorax, this term only #12 MeSH descriptor Pneumothorax, Artificial explode all trees #13 pneumothorax #14 (#11 OR #12 OR #13) #15 (#10 AND #14)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp chest tubes/ or exp drainage/ or intercostals.mp. or exp thorax/ or chest drain*.mp. or exp suction/ or exp needles/ or exp catheterization/ or exp thoracotomy/ or exp thoracostomy/ or aspiration.mp. or (simple or manual).ti. 2. exp pneumothorax, artificial/ or exp pneumothorax/ or pneumothorax*.mp. 3. 1 and 2 4. ((randomised controlled trial or controlled clinical trial).pt. or randomised.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Embase (Ovid SP) search strategy
1. exp chest tubes/ or exp thorax/ or exp catheterization/ or land drainage/ or drainage.mp. or thora*.ti,ab. or suction.mp. or suction/ or (simple or manual).ti. or (catheter* or needle*).ti,ab. or thoracostomy.mp. or exp thorax drainage/ or thoracotomy.mp. or exp thoracotomy/ or aspiration/ or aspiration.mp. 2. exp pneumothorax/ or pneumothorax.mp. or exp artificial pneumothorax/ or exp spontaneous pneumothorax/ 3. 1 and 2 4. (randomised‐controlled‐trial/ or randomisation/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Data and analyses
Comparison 1. Simple aspiration versus intercostal tube drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immediate success rate of procedure as defined by study authors | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.89] |
| 2 Early failure rate of procedure (incomplete lung expansion after the procedure) | 6 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.68] |
| 3 One‐year success rate (number of participants with no recurrence of pneumothorax at 1 year) | 4 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.18] |
| 4 Hospitalization rate (number of participants hospitalized) | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.47] |
| 5 Duration of hospital stay in days | 5 | 387 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.28, ‐1.04] |
1.1. Analysis.
Comparison 1 Simple aspiration versus intercostal tube drainage, Outcome 1 Immediate success rate of procedure as defined by study authors.
1.4. Analysis.
Comparison 1 Simple aspiration versus intercostal tube drainage, Outcome 4 Hospitalization rate (number of participants hospitalized).
Comparison 2. Sensitivity analysis for risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalization (number of participants hospitalized) | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.47] |
| 1.1 Unclear risk of bias | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.37, 0.75] |
| 1.2 Low risk of bias | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.61] |
Comparison 3. Sensitivity analysis following visual inspection of data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalization (number of participants hospitalized) | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.47] |
| 1.1 All participants admitted for tube drainage | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.76] |
| 1.2 Not all participants admitted for tube drainage | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.89, 3.91] |
Comparison 4. Sensitivity analysis for studies with recurrent pneumothorax.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immediate success rate of procedure as defined by study authors | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.89] |
| 1.1 Recurrent primary spontaneous pneumothorax (PSP) | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.58, 0.82] |
| 1.2 First episode PSP | 4 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.75, 1.06] |
| 2 Early failure rate of procedure (incomplete lung expansion after the procedure) | 6 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.68] |
| 2.1 Recurrent PSP | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.11, 42.99] |
| 2.2 First episode PSP | 4 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.93, 1.77] |
| 3 One‐year success rate (number of participants with no recurrence of pneumothorax at 1 year) | 4 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.18] |
| 3.1 Recurrent PSP | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.90, 1.52] |
| 3.2 First episode PSP | 3 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.17] |
| 4 Duration of hospital stay in days | 5 | 387 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.28, ‐1.04] |
| 4.1 Recurrent PSP | 2 | 134 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐3.31, 0.67] |
| 4.2 First episode PSP | 3 | 253 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.40, ‐0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrivet 1995.
| Methods |
Country: France Design: first protocol: RCT; second protocol: observational study Objective: to evaluate the efficacy and complications of needle aspiration vs tube drainage and to delineate their elective indications Study site for recruitment: 4 medical intensive care units, 3 of them in university teaching hospitals Method of analysis: Student’s t test for unpaired data; comparisons between qualitative parameters were made with the Chi² test; intention‐to‐treat analysis used |
|
| Participants |
Eligible for study: not reported Randomized: aspiration n = 33; tube drainage n = 28 Completed: aspiration n = 29; tube drainage n = 24 Age: aspiration mean 32 ± 16; tube drainage mean 33 ± 13 Gender: aspiration male n = 26, female n = 7; tube drainage male n = 24, female n = 4 Diagnosis criteria: primary pneumothorax defined as occurring in a patient without underlying lung disease Recruitment: In the first part of the trial, participants with spontaneous pneumothorax were randomized to 1 of 2 therapeutic methods (i.e. delayed needle aspiration or immediate tube drainage); in the second part, an additional group of participants were treated by immediate needle aspiration, performed as soon as possible after hospital admission; participants were admitted to 1 of 4 medical intensive care units. Diseases included: none reported Reasons for patient exclusion: post‐traumatic, iatrogenic, or bilateral pneumothorax; third ipsilateral episode or more; moderate to major associated pleural effusion or haemothorax; contralateral emphysematous bullae; suspected or proven lung cancer; lung abscess or consolidated pneumonia; diffuse interstitial pneumonitis; body temperature > 38.5°C; moderate to severe haemostasis defects; need for mechanical ventilation; prior ipsilateral thoracotomy; proven or suspected HIV infection Reasons for patient inclusion: older than 18 years; suffering from a first episode or first recurrence of a complete spontaneous pneumothorax Baseline values: not clearly described; aspiration primary 88% (n = 29); tube drainage primary 86% (n = 24) |
|
| Interventions |
Setting: ICU of 4 hospitals Aspiration description: performed after local anaesthesia with a plastic catheter (calibre 16F or 18F) inserted into the second anterior intercostal space at the midclavicular line, with participants lying in a semirecumbent position; the catheter was connected to a 1‐bottle water seal vacuum system, regulated to generate depressurization of 10 to 15 cm water; aspiration was performed until cessation of bubbling in the water seal bottle or for a maximum of 30 minutes Chest tube description: performed after local anaesthesia, using blunt dissection of the fourth or fifth intercostal space, on the midaxillary line; chest tube (calibre 20F) was directed toward the lung apex and was connected to the vacuum source via a 2‐bottle regulated system adjusted to create a depression of 20 to 25 cm H2O. After 2 hours of bubbling in the water seal bottle, the tube was clamped for an additional 24‐hour period and then was withdrawn if the chest radiograph revealed no recurrent pneumothorax. |
|
| Outcomes |
Prespecified outcomes: primary endpoints: (1) success rates of needle aspiration and chest tube, (2) recurrence rate of complete pneumothorax within first 24 hours after the last procedure
Secondary endpoints: (1) daily pain, (2) dyspnoea scores from visual analogue scales Follow‐up period: 3 months |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned but method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding did not occur. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis performed; attrition numbers reported but reasons not described; methods of handling missing outcome data from questionnaires (if any) not described; no PRISMA provided |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement; pain and dyspnoea scores not reported in a way that would allow meta‐analysis; no prespecified protocol available for comparison; all specified outcomes mentioned in methods are reported |
| Other bias | Unclear risk | Information insufficient to permit a judgement |
Ayed 2006.
| Methods |
Country: Kuwait Design: randomized controlled trial Objective: to evaluate the efficacy and complications of aspiration vs tube thoracostomy in patients with first episode of primary spontaneous pneumothorax Study site for recruitment: tertiary care hospital Method of analysis: intention‐to‐treat |
|
| Participants |
Eligible for study: aspiration n = 208; tube drainage n = 208 Randomized: aspiration n = 65; tube drainage n = 72 Completed: aspiration n = 65; tube drainage n = 72 Age: aspiration mean 24.38 ± 4.4; tube drainage mean 23.58 ± 4.8 Gender: aspiration male n = 59, female n = 6; tube drainage male n = 69, female n = 3 Diagnosis criteria: If pneumothorax is > 20% or patient is symptomatic, evacuation of air from air space via aspiration or tube thoracostomy is indicated. Recruitment: recruited through chest clinic at tertiary centres Diseases included: first episode of primary spontaneous pneumothorax Reasons for patient exclusion: previous pneumothorax; secondary pneumothorax; tension pneumothorax; bilateral pneumothorax; iatrogenic pneumothorax; < 20% or asymptomatic pneumothorax; haemopneumothorax Reasons for patient inclusion: primary spontaneous pneumothorax for evaluation of efficacy of aspiration or tube thoracostomy Baseline values: not clearly described; aspiration left n = 20, right n = 45; tube drainage left n = 30, right n = 42 |
|
| Interventions |
Setting: patients with first episode of primary spontaneous pneumothorax at a tertiary care hospital Duration: patient dependent Aspiration description: Simple aspiration was performed whilst participants were lying in a semi‐supine position. The catheter was connected to a 1‐bottle water seal vacuum system, regulated to generate a negative pressure of 10 to 15 cm H2O. Aspiration was performed until cessation of air occurred in the water seal bottle or for a maximum of 30 minutes. Thereafter, the catheter was withdrawn and a chest radiograph was performed. If lung expansion was complete, or if only a small rim of apical pneumothorax was present, the participant was discharged. If no lung expansion or only partial expansion was attained, a second aspiration attempt through a newly inserted catheter, at the same skin site, was performed immediately. If a second attempt was successful, the participant was discharged. If a second attempt was unsuccessful, or if a continuous air leak was observed at the 1‐bottle water seal vacuum system, a tube thoracostomy was performed. After discharge, participants were seen at 1 week; at 3, 6, 12, and 24 months; or when indicated. Chest tube description: A tube thoracostomy was performed with 20F plastic tubes (Argyle; Sherwood Medical, Argyle, New York). The chest tube was inserted under local anaesthesia at the fourth or fifth interspace at the midaxillary line, and was directed towards the apex. The drain was connected to an underwater seal suction with negative pressure of 20 cm H2O. When the air had stopped bubbling and a chest radiograph had confirmed lung expansion, the drain was left connected to the water seal for 24 additional hours. After a chest radiograph, the drain was removed and the participant was allowed home. Chest tube drainage was prolonged if the air leak persisted and incomplete lung expansion remained after 7 days. Thereafter, subsequent treatment (VATS) was performed. After discharge, participants were seen at 1 week; at 3, 6, 12, and 24 months; or earlier if indicated. Any recurrence was proved by a chest radiograph during a follow‐up visit. Pethidine, an intramuscular analgesic, was administered every 4 to 6 hours, according to the participant's request, and oral analgesia (acetaminophen) was administered as needed. |
|
| Outcomes |
Prespecified outcomes: primary endpoint: immediate success
Secondary endpoints: (1) 1‐week success; (2) recurrence rate at 3 months, 1 year, and 2 years; (3) hospitalization (% of participants); (4) duration of hospital stay; (5) analgesia requirements and quantity of analgesia in 24 hours; (6) complications; (7) inability to work Follow‐up period: 24 months |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned but method not described |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by self‐selecting a sealed envelope that indicated respective treatments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding did not occur. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed; attrition discussed |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Unclear risk | Information insufficient to permit a judgement |
Harvey 1994.
| Methods |
Country: United Kingdom Design: randomized controlled trial Objective: to assess acceptability and outcomes of a randomized comparison of simple aspiration vs intercostal drainage at 1 year Study site for recruitment: patients presenting to hospital Method of analysis: not reported, although logistical regression was used to measure association with failed aspiration; intention‐to‐treat data reported |
|
| Participants |
Eligible for study: not reported Randomized: aspiration n = 35; tube drainage n = 38 Completed: aspiration n = 35; tube drainage n = 38 Age: aspiration mean 34.6 ± 15.0; tube drainage mean 34.6 ± 13.1 Gender: aspiration male n = 28, female n = 7; tube drainage male n = 29, female n = 9 Diagnosis criteria: patients presenting with spontaneous pneumothorax for whom the admitting team thought a drainage procedure was required Recruitment: not specifically reported, although participants presenting with spontaneous pneumothorax were randomized to either group Diseases included: not reported Reasons for patient exclusion: patients with signs of tension pneumothorax or with lung disease other than previous pneumothorax Reasons for patient inclusion: patients presenting with spontaneous pneumothorax for whom the admitting team thought a drainage procedure was required Baseline values: aspiration: previous pneumothorax n = 6; radiographic appearance: left side n = 13/30; size: small rim n = 3, partial collapse n = 16, complete collapse n = 10 Tube drainage: previous pneumothorax n = 8; radiographic appearance: left side n = 12/32; size: small rim n = 1, partial collapse n = 12, complete collapse n = 18 |
|
| Interventions |
Setting: not specifically reported, hospital setting assumed Aspiration description: undertaken by inserting a 16‐ to 18‐gauge catheter under local anaesthetic and aspirating air through a 3‐way tap with the exit tube under water; procedure continued until no more air could be aspirated, participant became uncomfortable, or maximum of 3 litres had been removed Chest tube description: managed according to the participating physician’s usual practice; no sclerosing drugs allowed with either technique |
|
| Outcomes |
Prespecified outcomes: (1) pain experienced during procedure; (2) length of hospital stay; (3) procedure success; (4) recurrence; (5) procedure failure Follow‐up period: 12 months |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization reported but method not described |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis performed; no attrition mentioned; however, information insufficient to permit a judgement |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Unclear risk | Information insufficient to permit a judgement |
Ho 2011.
| Methods |
Country: Singapore Design: randomized controlled trial Objective: to compare outcomes and complications associated with needle aspiration and mini‐chest tube insertion vs Heimlich valve attachment for treatment of primary spontaneous pneumothorax at an emergency department Study site for recruitment: Singapore General Hosptial Method of analysis: intention‐to‐treat |
|
| Participants |
Eligible for study: not described Randomized: aspiration n = 23; tube drainage n = 25 Completed: aspiration n = 23; tube drainage n = 25 Age: aspiration mean 26.0 ± 8.6; tube drainage mean 24.3 ± 6.1 Gender: aspiration male 92.0%; tube drainage male 91.3% Diagnosis criteria: spontaneous pneumothorax in a patient with no previous lung disease Recruitment: emergency department Diseases included: first episode of primary spontaneous pneumothorax Reasons for patient exclusion: tension pneumothorax; pneumothorax secondary to trauma; minor pneumothorax; unstable patients; presence of chest effusion; bleeding disorder; underlying lung disease; smoker older than 50 years; indication for chest tube Reasons for patient inclusion: none specified Baseline values: aspiration: primary first episode 73.9%, primary recurrence 26.1%, < 3 cm from apex = 4.3, 3 to 5 cm from apex = 30.4, > 5 cm from apex = 65.2, right %= 60.9; tube drainage: primary first episode 80.0%, primary recurrence 20.0%, < 3 cm from apex = 4, 3 to 5 cm from apex = 60, > 5 cm from apex = 36, right % = 68 |
|
| Interventions |
Setting: tertiary care hospital; patients in the aspiration arm underwent aspiration done by a resident under the supervision of an ED physician Duration: patient dependent; patients were observed for 6 hours after intervention and were discharged if chest x‐ray showed < 10% pneumothorax Aspiration description: A 16G cannula was inserted into the second intercostal state at the midclavicular line. Chest x‐ray was done to confirm re‐expansion. Chest tube description: Participants in the tube drainage arm had mini‐chest tube inserted in the ED, tube was connected to Heimlich valve, and chest x‐ray was done to confirm re‐expansion and tube placement. |
|
| Outcomes |
Prespecified outcomes: primary endpoints: failure rate and admission rate (procedure failure was defined as recurrence of pneumothorax, need for second procedure, or need for surgical intervention)
Secondary endpoint: recurrence, defined as worsening pneumothorax or > 10% pneumothorax (aspiration group) after 6‐hour period of observation Follow‐up period: 3 days |
|
| Notes | "We acknowledge the support of the SingHealth Research Secretariat (CC032/2002) in providing the research grant" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned but method not described |
| Allocation concealment (selection bias) | Low risk | Blocked randomization using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding did not occur. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit a judgement |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Unclear risk | Information insufficient to permit a judgement |
Noppen 2002.
| Methods |
Country: Belgium Design: randomized controlled trial Objective: to evaluate the success rate of simple aspiration vs chest tube drainage in first episode of primary spontaneous pneumothorax Study site for recruitment: 1 tertiary care academic hospital and 4 regional general hospitals Method of analysis: intention‐to‐treat |
|
| Participants |
Eligible for study: not reported Randomized: aspiration n = 27; tube drainage n = 33 Completed: aspiration n = 27; tube drainage n = 33 Age: aspiration mean 28.2 ± 11.6; tube drainage mean 28.9 ± 8.9 Gender: aspiration male n = 20, female n = 7; tube drainage male n = 28, female n = 5 Diagnosis criteria: Primary spontaneous pneumothorax is defined as a spontaneously occurring pneumothorax in a person without clinically apparent underlying lung disease. Recruitment: 60 consecutive patients at 1 tertiary care hospital and 4 regional general hospitals were recruited for the trial. Diseases included: first episode of primary spontaneous pneumothorax Reasons for patient exclusion: none specified Reasons for patient inclusion: documented first episode of spontaneous pneumothorax; symptomatic regardless of size of the pneumothorax; pneumothorax > 20% under Light Index criteria Baseline values: aspiration: Light Index % = 62.1 ± 26.9, right/left % = 61/39; chest tube: Light Index % = 63.6 ± 24.7, right/left % = 64/36 |
|
| Interventions |
Setting: patients with first episode of primary spontaneous pneumothorax Duration: patient dependent Aspiration description: Simple aspiration was performed as follows: Participants were seated in semi‐supine position. After skin disinfection and field preparation, a small‐calibre polyethane intravenous needle catheter (Intracath, ga; Becton Dickinson, Sandy, Utah) was introduced after local anaesthesia with 2% lidocaine in the second or third intercostal space, at the midclavicular line. After the needle had entered the pleural space (which was witnessed by bubbling of air in the lidocaine‐filled syringe), the needle was directed apical and the catheter was advanced into the pleural space 5 to 10 cm. While the catheter was held in place, the needle was withdrawn. The catheter was fixed to the skin with sterile adhesive tape and was connected via a 3‐way valve to a 50‐mL syringe. Air was manually aspirated until resistance was felt and air was no longer aspirated. Thereafter, a chest x‐ray was performed with the catheter in place, or the catheter was immediately withdrawn and a chest x‐ray was performed afterwards, depending on local logistical capacity. When lung expansion was complete, or when only a very small rim of apical air was present, the participant was discharged. If no lung expansion was attained, a second aspiration attempt through the original aspiration catheter when left in place or through a newly inserted catheter at the same skin site (in case of removal of the original catheter for participant transportation) was performed, or subsequent treatment was proposed at the discretion of the local pulmonologist. If a second attempt was successful, the participant was discharged. After discharge, a control chest x‐ray was ordered within 48 hours and at 1 week. Thereafter, chest x‐rays were ordered at 2, 6, and 12 months, or when indicated. Chest tube description: Chest tube drainage was performed with 16F or 20F plastic (Argyle; Sherwood Medical, Tullamore, Ireland) tubes. The chest tube was inserted under local anaesthesia, at the anterior midclavicular second interspace or at the fourth or fifth interspace, at the midaxillary line, and directed to the apex. The drain was connected to a 4‐chamber system at water seal (0 cm H2O) or slight aspiration (5 cm H2O). When air bubbling had stopped and a chest x‐ray had confirmed complete lung expansion, the drain was left at water seal for 24 hours. After a control chest x‐ray, the drain was removed at expiration, and the participant was allowed to leave the hospital. Chest drainage was prolonged as long as air leakage persisted and/or no complete lung expansion was attained for a maximum of 7 days. Thereafter, subsequent treatment (e.g. thoracoscopy, thoracotomy) was left to the discretion of the attending pulmonologist. After discharge, participants were seen at 1 week, 2 months, 6 months, and 12 months, or earlier when indicated. |
|
| Outcomes |
Prespecified outcomes: primary endpoints: (1) immediate success rate; (2) 1‐week success rate; (3) 1‐year success rate
Secondary endpoints: (1) safety; (2) hospitalization (% of participants); (3) duration of hospitalization Follow‐up period: 12 months |
|
| Notes | This is a pilot study. The "forum Vlaamse Longartsen" provided logistical support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized at each centre with a separate random number list to 1 of 2 treatment groups with a computer‐generated table numerically corresponding to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding did not occur, |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed; attrition discussed |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit a judgement |
| Other bias | Unclear risk | Information insufficient to permit a judgement |
Parlak 2012.
| Methods |
Country: The Netherlands Design: randomized controlled trial Objective: to evaluate the efficacy of simple aspiration in comparison with tube thoracostomy in spontaneous pneumothorax therapy, whether simple aspiration will shorten hospital admission, and whether the lung will expand as assessed by clinical and radiological findings Study site for recruitment: hospital group Method of analysis: intention‐to‐treat |
|
| Participants |
Eligible for study: not reported Randomized: aspiration n = 25; tube drainage n = 31 Completed: aspiration n = 25; tube drainage n = 31 Age: aspiration mean 47 ± 19; tube drainage n = 40 ± 20 Gender: aspiration n = 17 male, n = 8 female; tube drainage n = 23 male, n = 8 female Diagnosis criteria: asymptomatic pneumothorax with a size of 20% as estimated by the Light Index formula (1 ‒ length ÷ height × 100) recruited Recruitment: admitted through ED Diseases included: first episode of primary spontaneous pneumothorax Reasons for patient exclusion: pregnancy; severe comorbidity; prior randomization; recurrent or tension pneumothorax; limited decision making; chronic lung disease; HIV or Marfan syndrome Reasons for patient inclusion: none specified Baseline values: aspiration mean size 60.5 ± 25.4 side, n = 8 right, n = 17 left; tube drainage mean size 63.8 ± 22.8 side, n = 15 right, n = 16 left |
|
| Interventions |
Setting: hospital group in the East of the Netherlands Duration: patient dependent Aspiration description: An angio intravenous catheter with a diameter of 1.3 mm was introduced after local anaesthesia (lidocaine 1%). After fixation to the skin, the intravenous catheter was connected with a 3‐way valve to a 50‐mL syringe and air was manually aspirated until resistance was felt and no air was acquired any longer. In cases of success with an expanded lung at chest x‐ray, the drainage system was disconnected and the participant was observed over 24 hours. If simple aspiration had failed, no second attempt was made and drainage was chosen. After the observation period, a new chest x‐ray was taken for final evaluation. When the lung was still expanded at the chest x‐ray, discharge followed. When no lung expansion was reached, or in cases of absorption of > 2000 mL air (prolonged air leak), tube drainage was performed. Chest tube description: Chest tube drainage was initially performed in a manner similar to simple aspiration. The difference consisted of connection of a drain to a drainage system (tube thoracostomy with 2 reservoirs; Abbott Laboratories; Lake Bluff, Illinois) with negative pressure of 10 mmHg H2O. When airway leakage had ceased, expansion of the lung was radiologically evaluated. The drain was clipped for 4 hours when the lung had expanded. When expansion of the lung continued after 4 hours, the drain was removed and the participant was discharged. After discharge, participants were seen at day 7, and after 1 year at the outpatient clinic, with a chest x‐ray performed to evaluate possible recurrence of the pneumothorax. Otherwise, participants with complaints were seen earlier. |
|
| Outcomes |
Prespecified outcomes: primary endpoint: immediate success, duration of hospital stay
Secondary endpoints: none specified Follow‐up period: 12 months |
|
| Notes | No sponsors were involved. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done via a computer minimization programme between simple aspiration and tube drainage initiated by the doctor at the ED. Minimization programme balanced participants for the following factors: cause of PTX (spontaneous or traumatic), smoking status (past, actual), and gender (male/female) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Local anaesthesia; no mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding did not occur. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed; attrition discussed |
| Selective reporting (reporting bias) | Low risk | Trial has been placed on clinicaltrials.gov. |
| Other bias | Low risk | Trial has been placed on clinicaltrials.gov; no other biases were identified. |
20F: friction factor of bore. ED: emergency department. H: hydrogen. Hg: mercury. HIV: human immunodeficiency virus. H2O: water. ICU: intensive care unit. N: nitrogen. PTX: pneumothorax. RCT: randomized controlled trial. VATS: video‐assisted thoracoscopic surgery.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burgaud 1985 | Retrospective observational study |
| Faruqi 2004 | Not a prospective randomized study. All included participants were treated with simple aspiration as the initial treatment modality |
| Hernandez Ortiz 1999 | Not a prospective randomized controlled study; intercostal tube drainage group was part of an historical series |
| Korczynski 2015 | Both primary and secondary pneumothoraces included |
Characteristics of ongoing studies [ordered by study ID]
Desmettre 2011.
| Trial name or title | Comparison of Exsufflation Versus Drainage in Primary Spontaneous Pneumothorax (EXPRED) |
| Methods |
Country: France Design: randomized controlled trial Objective: to compare the therapeutic efficacy of simple aspiration vs chest tube drainage for a first large spontaneous pneumothorax Study site for recruitment: university tertiary teaching hospitals Method of analysis: intention‐to‐treat |
| Participants |
Eligible for study: not described Randomized: not described Completed: aspiration n = 200; tube drainage n = 200 Age: range 18 to 50 years Gender: not described Diagnosis criteria: criteria defining a large primary pneumothorax requiring a procedure for evacuation of intrapleural air: must be homogeneous, simple, indisputable, validated, and assessable on chest x‐ray for all patients; detachment to full height (complete) on chest x‐ray when standing up and facing, or from profile Recruitment: not described Diseases included: first episode of primary spontaneous pneumothorax Reasons for patient exclusion: under trusteeship, under curatorship, or under judicial protection; incapable adults; pregnant or breastfeeding women; younger than 18 years of age and over 50 years of age; non‐large primary pneumothorax; suffocating pneumothorax; recurrent pneumothorax; traumatic pneumothorax; primitive pneumothorax associated with effusion; bilateral pneumothorax; secondary pneumothorax related to lung disease; pneumothorax that occurred more than 48 hours ago Reasons for patient inclusion: over 18 years of age and younger than 50 years of age with a first primitive large primary PSP defined by a detached lung on the entire height of the pulmonary field Baseline values: not described |
| Interventions |
Setting: university tertiary teaching hospitals Duration: patient dependent Aspiration description: The aspiration system was a single‐use sterile device specially conceived for this indication (tri‐compartmental chambers of Pleurevac type in case of drainage or monocompartmental suction bottle from BPDS‐700 type, Medical Teleflex, in case of aspiration). Chest tube description: In the drainage group, chest tube insertion was carried out with a 16F or 20F drain of Joly or Monod type according to the habits of the operator. |
| Outcomes |
Prespecified outcomes: primary endpoint: immediate success of the procedure
Secondary endpoints: (1) efficacy and safety; (2) hospitalization; (3) sick leave; (4) economic impact Follow‐up period: 12 months |
| Starting date | June 2009 |
| Contact information | Principal Investigator: Thibaut TJ Desmettre |
| Notes |
16F, 20F: friction factor of bore. PSP: primary spontaneous pneumothorax.
Differences between protocol and review
We have changed the review author line since the protocol (Wakai 2003) and the original review (Wakai 2007) were prepared, by adding Kristin Carson‐Chahhoud, Joseph van Agteren, Malcolm Brinn, and Brian Smith.
We used the GRADE approach to assess the quality of the evidence in this update.
We reduced the number of secondary outcomes to adhere to Cochrane recommendations to include as small a total number of outcomes as possible, while keeping them clinically relevant (went from 13 to 7 secondary outcomes).
We incorporated a 'Summary of findings' table into the review.
We changed the analysis method from a fixed‐effect to a random‐effects model for all outcomes.
We included in the review studies investigating participants with a recurrent pneumothorax and conducted a sensitivity analysis to examine potential biases.
Contributions of authors
Kristin Carson‐Chahhoud ‐ updated the protocol, reviewed the literature, identified studies for inclusion, extracted data, entered and analysed data, completed editorial amendments, and updated the text of the manuscript.
Joseph van Agteren ‐ updated the protocol, reviewed the literature, identified studies for inclusion, extracted data, entered and analysed data, and updated the text of the manuscript.
Abel Wakai ‐ authored the original review, updated the text of the manuscript, and supervised the update.
Brian Smith ‐ updated the text of the manuscript and supervised the latest update.
Grainne McCabe ‐ authored the original review and identified studies for inclusion in the update.
Malcolm Brinn ‐ updated search and identified studies for inclusion.
Ronan O'Sullivan ‐ authored the original review.
Sources of support
Internal sources
Department of Emergency Medicine, St. Vincent's University Hospital, Dublin, Ireland.
External sources
Higher Specialist Training Scheme in Emergency Medicine, Ireland.
Declarations of interest
Kristin Carson‐Chahhoud: no conflicts of interest to declare.
Abel Wakai: no conflicts of interest to declare.
Joseph van Agteren: no conflicts of interest to declare.
Brian Smith: no conflicts of interest to declare.
Grainne McCabe: no conflicts of interest to declare.
Malcolm Brinn: no conflicts of interest to declare.
Ronan O'Sullivan: no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Andrivet 1995 {published data only}
- Andrivet P, Djedaini K, Teboul JL, Brochard L, Dreyfuss D. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest 1995;108:335‐9. [PUBMED: 7634863] [DOI] [PubMed] [Google Scholar]
Ayed 2006 {published data only}
- Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. European Respiratory Journal 2006;27:477‐82. [DOI: 10.1183/09031936.06.00091505; PUBMED: 16507846] [DOI] [PubMed] [Google Scholar]
Harvey 1994 {published data only}
- Harvey J, Prescott RJ. Simple aspiration versus intercostal tube drainage for spontaneous pneumothorax in patients with normal lungs. British Thoracic Society Research Committee. BMJ 1994;309:1338‐9. [PUBMED: 7755720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2011 {published data only}
- Ho KK, Ong MEH, Koh MS, Wong E, Raghuram J. A randomized controlled trial comparing minichest tube and needle aspiration in outpatient management of primary spontaneous pneumothorax. American Journal of Emergency Medicine 2011;29:1152‐7. [DOI: 10.1016/j.ajem.2010.05.017; PUBMED: 20716475 ] [DOI] [PubMed] [Google Scholar]
Noppen 2002 {published data only}
- Noppen M, Alexander P, Driesen P, Slabbynck H, Verstraeten A. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. American Journal of Respiratory and Critical Care Medicine 2002;165:1240‐4. [DOI: 10.1164/rccm.200111-078OC; PUBMED: 11991872 ] [DOI] [PubMed] [Google Scholar]
Parlak 2012 {published data only}
- Parlak M, Uil SM, Berg JWK. A prospective, randomised trial of pneumothorax therapy: manual aspiration versus conventional chest tube drainage. Respiratory Medicine 2012;106:1600‐5. [DOI: 10.1016/j.rmed.2012.08.005; PUBMED: 22925840 ] [DOI] [PubMed] [Google Scholar]
- Berg JWK, Klinieken I. Pneumothorax therapy: manual aspiration versus conventional chest tube drainage (pneumothorax). Clinicaltrials.gov (accessed 9 November 2007). [NCT00556335]
References to studies excluded from this review
Burgaud 1985 {published data only}
- Burgaud JC, Offenstadt G, Bencharif G, Hericord P, Amstutz P. Spontaneous pneumothorax: drainage by catheter or chest tube?. Revue de Pneumologie Clinique 1985;41:317‐9. [PUBMED: 4095427] [PubMed] [Google Scholar]
Faruqi 2004 {published data only}
- Faruqi S, Gupta D, Aggarwal AN, Jindal SK. Role of simple needle aspiration in the management of pneumothorax. The Indian Journal of Chest Diseases and Allied Sciences 2004;46:183‐90. [PUBMED: 15553207] [PubMed] [Google Scholar]
Hernandez Ortiz 1999 {published data only}
- Hernandez OC, Zugasti GK, Emparanza KJ, Boyero UA, Ventura HI, Isaba SL, et al. Idiopathic spontaneous pneumothorax: treatment by small‐caliber catheter aspiration compared to drainage through a chest tube. Archivos de Bronchoneumologia 1999;35:179‐81. [PUBMED: 10330539] [PubMed] [Google Scholar]
Korczynski 2015 {published data only}
- Korczynski P, Gorska K, Nasilowski J, Chazan R, Krenke R. Comparison of small bore catheter aspiration and chest tube drainage in the management of spontaneous pneumothorax. Advances in Experimental Medicine and Biology. 2015;866:15‐23. [PUBMED: 26022901] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Desmettre 2011 {published data only}
- Desmettre T. Comparison of exsufflation versus drainage in primary spontaneous pneumothorax (EXPRED). Clinicaltrials.gov (accessed 9 September 2009). [NCT01008228]
- Desmettre T, Meurice J‐C, Woronoff M‐C, Tiffet O, Ferretti G, Dalphin J‐C. EXPRED study: aspiration versus drainage of a first pneumothorax; comparison of simple aspiration versus standard drainage in the treatment of large primary spontaneous pneumothorax. Revue des Maladies Respiratoires 2011;28(3):336‐43. [DOI: 10.1016/j.rmr.2010.10.030] [DOI] [PubMed] [Google Scholar]
- Desmettre T, Meurice JC, Tapponnier R, Pretalli JB, Dalphin JC. The EXPRED study: where are we? [Étude EXPRED (EXsufflation d’un PREmier pneumothorax versus Drainage): où en sommes‐nous?]. Revue des Maladies Respiratoires 2015;30(1):18‐21. [DOI: 10.1016/j.rmr.2012.09.019] [DOI] [PubMed] [Google Scholar]
Additional references
Aguinagalde 2010
- Aguinagalde B, Zabaleta J, Fuentes M, Bazterargui N, Hernandez C, Izquierdo JM, et al. Percutaneous aspiration versus tube drainage for spontaneous pneumothorax: systematic review and meta‐analysis. European Journal of Cardio‐thoracic Surgery 2010;37(5):1129‐35. [DOI: 10.1016/j.ejcts.2009.12.008; PUBMED: 20060735 ] [DOI] [PubMed] [Google Scholar]
Baumann 1997
- Baumann MH, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach?. Chest 1997;112:789‐804. [PUBMED: 9315817] [DOI] [PubMed] [Google Scholar]
Baumann 2001
- Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590‐602. [PUBMED: 11171742] [DOI] [PubMed] [Google Scholar]
Bense 1987
- Bense L, Wiman LG, Hedenstierna G. Onset of symptoms in spontaneous pneumothorax: correlations to physical activity. European Journal of Respiratory Diseases 1987;71:181‐6. [PUBMED: 3678419] [PubMed] [Google Scholar]
Bense 1991
- Bense L, Wiman LG, Jendteg S, Lindgren B. Economic costs of spontaneous pneumothorax. Chest 1991;99:260‐1. [PUBMED: 1984974] [DOI] [PubMed] [Google Scholar]
Bintcliffe 2015
- Bintcliffe OJ, Hallifax RJ, Edey A, Feller‐Kopman D, Lee YCG, Marquette CH, et al. Spontaneous pneumothorax: time to rethink management?. The Lancet Respiratory Medicine 2015;3(7):578‐88. [PUBMED: 26170077] [DOI] [PubMed] [Google Scholar]
BMJ 2016
- BMJ Best Practice. Pneumothorax. http://bestpractice.bmj.com/best‐practice/monograph/504/treatment/step‐by‐step.html 2016 (accessed February 2016).
Camuset 2006
- Camuset J, Laganier J, Brugière O, Dauriat G, Jebrak G, Thabut G, et al. Needle aspiration as first‐line management of primary spontaneous pneumothorax. La Presse Médicale 2006;35(5):765‐8. [PUBMED: 16710143] [DOI] [PubMed] [Google Scholar]
Chan 1997
- Chan L, Reilly KM, Henderson C, Khan F, Salluzzo RF. Complication rates of tube thoracostomy. American Journal of Emergency Medicine 1997;15:368‐70. [PUBMED: 9217527] [DOI] [PubMed] [Google Scholar]
Cook 2015
- Cook JA, Elders A, Boachie C, Bassinga T, Fraser C, Altman DG, et al. A systematic review of the use of an expertise‐based randomised controlled trial design. Trials 2015;16(1):241. [PUBMED: 26025450 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Currie 2007
- Currie GP, Alluri R, Christie GL, Legge JS. Pneumothorax: an update. Postgraduate Medical Journal 2007;83(981):461‐5. [PUBMED: 17621614 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 1985
- Daly RC, Mucha P, Pairolero PC, Farnell MB. The risk of percutaneous chest tube thoracostomy for blunt thoracic trauma. Annals of Emergency Medicine 1985;14:865‐70. [PUBMED: 4025984] [DOI] [PubMed] [Google Scholar]
Davies 2008
- Davies HE, Merchant S, McGown A. A study of the complications of small bore 'Seldinger' intercostal chest drains. Respirology 2008;13(4):603‐7. [PUBMED: 18422864] [DOI] [PubMed] [Google Scholar]
Devanand 2004
- Devanand A, Koh MS, Ong TH, Low SY, Phua GC, Tan KL, et al. Simple aspiration versus chest‐tube insertion in the management of primary spontaneous pneumothorax: a systematic review. Respiratory Medicine 2004;98(7):579‐90. [PUBMED: 15250222] [DOI] [PubMed] [Google Scholar]
Grundy 2012
- Grundy S, Bentley A, Tschopp J. Primary spontaneous pneumothorax: a diffuse disease of the pleura. Respiration 2012;83(3):185‐9. [PUBMED: 22343477 ] [DOI] [PubMed] [Google Scholar]
Gupta 2000
- Gupta D, Hansell A, Nichols T, Duong T, Ayers JG, Strachan D. Epidemiology of pneumothorax in England. Thorax 2000;55:666‐71. [PUBMED: 10899243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurley 1998
- Gurley MB, Richli WR, Waugh KA. Outpatient management of pneumothorax after fine‐needle aspiration: economic advantages for the hospital and patient. Radiology 1998;209(3):717‐22. [PUBMED: 9844664] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research edition) 2008;336:924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org. [Google Scholar]
Holden 1982
- Holden MP. Management of Intercostal Drainage Tubes. Bristol: John Wright, 1982. [Google Scholar]
Iberti 1992
- Iberti TJ, Stern PM. Chest tube thoracostomy. Critical Care Clinics 1992;14:879‐95. [PUBMED: 1393756] [PubMed] [Google Scholar]
Janssen 1994
- Janssen JP, Cuesta MA, Postmus PE. Treatment of spontaneous pneumothorax: survey among Dutch pneumonologists. Nederlands Tijdschrift voor Geneeskunde 1994;138:661‐4. [PUBMED: 8152496] [PubMed] [Google Scholar]
Kelly 2008
- Kelly A‐M, Kerr D, Clooney M. Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest 2008;134(5):1033‐6. [PUBMED: 18583511 ] [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jahad AR. The importance of quality primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. [PUBMED: 8629879] [PubMed] [Google Scholar]
Khan 2011
- Khan K, Kunz R, Kleijnen J, Antes G. Systematic Reviews to Support Evidence‐Based Medicine. Boca Raton, FL: CRC Press, 2011. [Google Scholar]
Light 1994
- Light RW. Pneumothorax. In: Murray JF, Nadel JA editor(s). Textbook of Respiratory Medicine. Philadelphia: WB Saunders, 1994:2193‐210. [Google Scholar]
Light 2007
- Light RW. Pleural Diseases. Baltimore, MD: Lippincott Williams & Wilkins, 2007. [Google Scholar]
MacDuff 2010
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65(Suppl 2):ii18‐31. [PUBMED: 20696690 ] [DOI] [PubMed] [Google Scholar]
Maunder 1984
- Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis and management. Archives of Internal Medicine 1984;144:1447‐53. [PUBMED: 6375617] [PubMed] [Google Scholar]
Melton 1979
- Melton LJ, Hepper NCG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950‐1974. American Review of Respiratory Disease 1979;29:1379‐82. [PUBMED: 517861] [DOI] [PubMed] [Google Scholar]
Mendis 2002
- Mendis D, El‐Shanawany T, Mathur A, Redington AE. Management of spontaneous pneumothorax: are British Thoracic Society guidelines being followed?. Postgraduate Medical Journal 2002;78:80‐4. [PUBMED: 11807188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1987
- Miller KS, Sahn FA. Chest tubes: indications, technique, management and complications. Chest 1987;91:258‐64. [PUBMED: 3542404] [DOI] [PubMed] [Google Scholar]
Noppen 2001
- Noppen M, Alexander P, Driesen P, Slabbynck H, Verstraeten A. Quantification of the size of primary spontaneous pneumothorax: accuracy of the Light Index. Respiration 2001;68:396‐9. [PUBMED: 11464087] [DOI] [PubMed] [Google Scholar]
Noppen 2003
- Noppen M, Baumann MH. Pathogenesis and treatment of primary spontaneous pneumothorax: an overview. Respiration 2003;70(4):431‐8. [PUBMED: 14512683] [DOI] [PubMed] [Google Scholar]
Noppen 2008
- Noppen M, Keukeleire T. Pneumothorax. Respiration 2008;76(2):121‐7. [PUBMED: 18708734 ] [DOI] [PubMed] [Google Scholar]
Noppen 2010
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. European Respiratory Review 2010;19(117):217‐9. [PUBMED: 20956196] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasquier 2013
- Pasquier M, Hugil O, Carron P. Videos in clinical medicine. Needle aspiration of primary spontaneous pneumothorax. New England Journal of Medicine 2013;368(19):e24. [PUBMED: 23656667 ] [DOI] [PubMed] [Google Scholar]
Rawlins 2003
- Rawlins R, Brown KM, Carr CS, Cameron CR. Life threatening haemorrhage after anterior needle aspiration of pneumothoraces. A role for lateral needle aspiration in emergency decompression of spontaneous pneumothorax. Emergency Medicine Journal 2003;20:383‐4. [PUBMED: 12835367] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5) Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruckley 1966
- Ruckley CV, McCormack RJM. The management of spontaneous pneumothorax. Thorax 1966;21:139‐44. [PUBMED: 5935841] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahn 2000
- Sahn SA, Heffner JE. Spontaneous pneumothorax. New England Journal of Medicine 2000;342(12):868‐74. [PUBMED: 10727592 ] [DOI] [PubMed] [Google Scholar]
Schramel 1996
- Schramel FM, Sutedja TG, Braber JC, Mourik JC, Postmus PE. Cost‐effectiveness of video‐assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. European Respiratory Journal 1996;9(9):1821‐5. [PUBMED: 8880097] [DOI] [PubMed] [Google Scholar]
Selby 1994
- Selby CD, Sudlow MF. Deficiencies of management of spontaneous pneumothoraces. Scottish Medical Journal 1994;39:75‐7. [PUBMED: 8720768] [DOI] [PubMed] [Google Scholar]
Soulsby 1998
- Soulsby T. British Thoracic Society guidelines for the management of spontaneous pneumothorax: do we comply with them and do they work?. Journal of Accident & Emergency Medicine 1998;15:317‐21. [PUBMED: 9785159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stradling 1966
- Stradling P, Poole G. Conservative management of spontaneous pneumothorax. Thorax 1966;21:145‐9. [PUBMED: 5935842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Symbas 1989
- Symbas PN. Chest drainage tubes. Surgical Clinics of North America 1989;69:41‐6. [PUBMED: 2643182] [DOI] [PubMed] [Google Scholar]
Tschopp 2002
- Tschopp JM, Boutin C, Astoul P, Janssen JP, Grandin S, Bolliger CT, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost‐effective than drainage: a randomised study. European Respiratory Journal 2002;20(4):1003‐9. [PUBMED: 12412696] [DOI] [PubMed] [Google Scholar]
Tschopp 2015
- Tschopp J, Bintcliffe O, Astoul P, Canalis E, Driesen P, Janssen J, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. European Respiratory Journal 2015;46:321‐35. [DOI: 10.1183/09031936.00219214; PUBMED: 26113675 ] [DOI] [PubMed] [Google Scholar]
Yeoh 2000
- Yeoh JH, Ansari S, Campbell IA. Management of spontaneous pneumothorax ‐ a Welsh survey. Postgraduate Medical Journal 2000;76:496‐9. [PUBMED: 10908379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zehtabchi 2008
- Zehtabchi S, Rios CL. Management of emergency department patients with primary spontaneous pneumothorax: needle aspiration or tube thoracostomy?. Annals of Emergency Medicine 2008;51(1):91‐100. [PUBMED: 18166436] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wakai 2003
- Wakai A, O'Sullivan RG, Deasy C, McCabe G. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakai 2007
- Wakai A, O'Sullivan R, McCabe G. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004479.pub2] [DOI] [PubMed] [Google Scholar]


