Abstract
Background
There is evidence from observational studies that whole grains can have a beneficial effect on risk for cardiovascular disease (CVD). Earlier versions of this review found mainly short‐term intervention studies. There are now longer‐term randomised controlled trials (RCTs) available. This is an update and expansion of the original review conducted in 2007.
Objectives
The aim of this systematic review was to assess the effect of whole grain foods or diets on total mortality, cardiovascular events, and cardiovascular risk factors (blood lipids, blood pressure) in healthy people or people who have established cardiovascular disease or related risk factors, using all eligible RCTs.
Search methods
We searched CENTRAL (Issue 8, 2016) in the Cochrane Library, MEDLINE (1946 to 31 August 2016), Embase (1980 to week 35 2016), and CINAHL Plus (1937 to 31 August 2016) on 31 August 2016. We also searched ClinicalTrials.gov on 5 July 2017 and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) on 6 July 2017. We checked reference lists of relevant articles and applied no language restrictions.
Selection criteria
We selected RCTs assessing the effects of whole grain foods or diets containing whole grains compared to foods or diets with a similar composition, over a minimum of 12 weeks, on cardiovascular disease and related risk factors. Eligible for inclusion were healthy adults, those at increased risk of CVD, or those previously diagnosed with CVD.
Data collection and analysis
Two review authors independently selected studies. Data were extracted and quality‐checked by one review author and checked by a second review author. A second review author checked the analyses. We assessed treatment effect using mean difference in a fixed‐effect model and heterogeneity using the I2 statistic and the Chi2 test of heterogeneity. We assessed the overall quality of evidence using GRADE with GRADEpro software.
Main results
We included nine RCTs randomising a total of 1414 participants (age range 24 to 70; mean age 45 to 59, where reported) to whole grain versus lower whole grain or refined grain control groups. We found no studies that reported the effect of whole grain diets on total cardiovascular mortality or cardiovascular events (total myocardial infarction, unstable angina, coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty, total stroke). All included studies reported the effect of whole grain diets on risk factors for cardiovascular disease including blood lipids and blood pressure. All studies were in primary prevention populations and had an unclear or high risk of bias, and no studies had an intervention duration greater than 16 weeks.
Overall, we found no difference between whole grain and control groups for total cholesterol (mean difference 0.07, 95% confidence interval ‐0.07 to 0.21; 6 studies (7 comparisons); 722 participants; low‐quality evidence).
Using GRADE, we assessed the overall quality of the available evidence on cholesterol as low. Four studies were funded by independent national and government funding bodies, while the remaining studies reported funding or partial funding by organisations with commercial interests in cereals.
Authors' conclusions
There is insufficient evidence from RCTs of an effect of whole grain diets on cardiovascular outcomes or on major CVD risk factors such as blood lipids and blood pressure. Trials were at unclear or high risk of bias with small sample sizes and relatively short‐term interventions, and the overall quality of the evidence was low. There is a need for well‐designed, adequately powered RCTs with longer durations assessing cardiovascular events as well as cardiovascular risk factors.
Plain language summary
Whole grain cereals for cardiovascular disease
Background
Whole grain foods encompass a range of products and include whole grain wheat, rice, maize, and oats. The term 'whole grain' also includes milled whole grains such as oatmeal and wholemeal wheat.
Study characteristics
We evaluated nine randomised studies assessing the effects of whole grain diets compared to diets with refined grains or a usual diet on levels of cholesterol in the blood or blood pressure (major risk factors for cardiovascular disease including heart attacks or stroke). The evidence is current to August 2016.
Key results
The diets were followed for at least 12 weeks, but most studies had some methodological limitations, numbers of participants were small, and the overall quality of the evidence was low. We found no studies reporting on the effect of whole grains on deaths from cardiovascular disease or cardiovascular events. All nine included studies reported the effects of whole grain diets on levels of cholesterol in the blood or blood pressure. We found no effects on blood cholesterol or blood pressure in favour of whole grain diets. Four studies were funded by independent national and government funding bodies, while the remaining studies reported funding or partial funding by organisations with commercial interests in cereals.
Conclusion
There is insufficient evidence from randomised controlled trials to date to recommend consumption of whole grain diets to reduce the risk of cardiovascular disease, or lower blood cholesterol, or blood pressure.
Summary of findings
Summary of findings 1. Whole grain cereals for the primary or secondary prevention of cardiovascular disease.
| Whole grain cereals for the primary prevention of cardiovascular disease (no studies were available to examine secondary prevention) | ||||||
|
Patient or population: Free‐living adults who were healthy, had established cardiovascular disease or risk factors for cardiovascular disease
Settings: Europe and USA
Intervention: Higher levels of whole grain dietary intake1 Control: Refined grains or lower levels of wholegrain | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Refined grains or lower levels of whole grain dietary intake | Refined grains or higher levels of whole grain dietary intake | |||||
| Total cardiovascular mortality | See comment | See comment | See comment | See comment | See comment | No trials reported total CVD mortality for the primary prevention of CVD. |
| Fatal and non‐fatal myocardial infarction | See comment | See comment | See comment | See comment | See comment | No trials reported total myocardial infarction for the primary prevention of CVD. |
| Unstable angina | See comment | See comment | See comment | See comment | See comment | No trials reported unstable angina for the primary prevention of CVD. |
| Coronary artery bypass graft surgery | See comment | See comment | See comment | See comment | See comment | No trials reported coronary artery bypass graft surgery for the primary prevention of CVD. |
| Percutaneous transluminal coronary angioplasty | See comment | See comment | See comment | See comment | See comment | No trials reported percutaneous transluminal coronary angioplasty for the primary prevention of CVD. |
| Stroke | See comment | See comment | See comment | See comment | See comment | No trials reported total stroke for the primary prevention of CVD. |
|
Total cholesterol change (mmol/L)
Objectively measured Follow‐up: 12 to 16 weeks |
The mean total cholesterol change ranged across lower levels of whole grain dietary intake groups from ‐0.4 to 0.3. | The mean total cholesterol change (mmol/L) in the intervention groups was 0.07 higher (0.07 lower to 0.21 higher). | ‐ | 722 (6 studies) | ⊕⊕⊝⊝ low2,3 | See Appendix 1 for total cholesterol change checklist. |
| Abbreviations: CI: confidence interval; CVD: cardiovascular disease | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The term 'whole grain' includes foods based on milled whole grains, such as wholemeal or oatmeal. 2Downgraded for inconsistency (see Appendix 1 for checklist to aid consistency and reproducibility of GRADE assessments). 3Downgraded for imprecision (see Appendix 1 for checklist to aid consistency and reproducibility of GRADE assessments).
Background
This was an update and expansion of the original review published in 2007 (Kelly 2007).
Description of the condition
Cardiovascular diseases (CVD) are a group of conditions that affect the heart and blood vessels and include coronary heart disease, cerebrovascular disease, and peripheral arterial disease (WHO 2013). One of the main mechanisms thought to cause CVD is atherosclerosis, where the arteries become clogged by atheromas or plaques (NHS 2012). Cardiovascular disease occurs when the arteries are completely blocked or when blood flow is restricted by a narrowed artery, limiting the amount of blood and oxygen delivered to organs or tissue (BHF 2014). Arteries may naturally become harder and narrower with age, although this process may be accelerated by such factors as a sedentary lifestyle, obesity, ethnicity, smoking, high cholesterol, and high blood pressure (NHS 2012). Another cause of CVD is unstable plaque rupturing. It is thought that unstable plaques activate an inflammatory response in the body that causes the structure of atherosclerotic plaque to weaken and rupture, leading to the formation of blood clots (Spagnoli 2007).
Cardiovascular disease is the number one cause of death and disability globally (WHO 2013). Around 30% of total global deaths can be attributed to CVD (WHO 2013), and it is estimated to cause 17 million deaths per year (Bovet 2012). The World Health Organization reports that by 2030, CVDs will account for almost 23.3 million deaths per year (WHO 2013). This burden is set to increase as a consequence of ageing populations and increasing levels of sedentary lifestyles and obesity.
One key public health priority in the prevention of CVD is targeting modifiable risk factors. One such risk factor is diet, which plays a major role in the aetiology of many chronic conditions, including CVD. Indeed, a number of dietary factors have been found to be associated with a decrease in CVD risk, such as a low sodium intake (Aburto 2013), a low‐carbohydrate diet (Hu 2014), intake of whole grains (Ye 2012), and a high consumption of fruits and vegetables (Oude 2010). Such factors are important, not only because they have been linked to CVD development, but also because they can be modified. This makes them one of the main targets for interventions aimed at primary prevention and management of CVD.
Description of the intervention
A whole grain contains the entire edible parts of a natural grain kernel. The structure of all whole grains is similar and includes the endosperm, germ, and bran. Whole grains are rich in dietary fibre, antioxidants, resistant starch, phyto‐oestrogens, and other important micronutrients such as vitamins and folic acid (Slavin 2003). In the grain‐refining process, most of the bran and some of the germ is removed, resulting in the loss of dietary fibre, vitamins, minerals, lignans, phyto‐oestrogens, phenolic compounds, and phytic acid. The remaining starchy endosperm is ground to produce refined white flours.
Important grains in the Western diet include wheat, rice, maize, oats, barley, and rye. Wholemeal foods are made from whole grains that have been milled to a finer texture rather than leaving them whole in the final product. Both whole grain and wholemeal cereal foods are grain foods that include the outer layers of the grain, including the bran and germ. The EU HEALTHGRAIN consortium definition of whole grain is "whole grains shall consist of the intact, ground, cracked or flaked kernel after the removal of inedible parts such as the hull and husk. The principal anatomical components ‐ the starchy endosperm, germ and bran ‐ are present in the same relative proportions as they exist in the intact kernel" (van der Kamp 2014). This definition also allows for small losses of components during processing. The HEALTHGRAIN definition also lists specific grains included as whole grain. Research has shown that such processing of whole grains does not remove biologically important compounds (Slavin 2001). Nutritionally, whole grain and wholemeal foods are similar.
For foods made from whole grain such as breads, breakfast cereals, pasta, biscuits, and grain‐based snack foods, a standard definition for what constitutes a whole grain food has been recommended as a minimum of 8 g whole grains/30 g serving (27 g/100 g) (Ferruzzi 2014). This was in response to a lack of consistency in previous definitions of whole grain foods across "countries, governments, regulatory agencies, private and commercial organisations" (Ferruzzi 2014).
A recent comprehensive systematic review and meta‐analysis of prospective studies of the relationship between whole grain intake and cardiovascular disease found significant reductions in risk for cardiovascular disease, stroke, and coronary heart disease per 90 g/day (3 servings) increase of whole grain intake (Aune 2016). Evidence from two different meta‐analyses of observational cohort studies suggests that those consuming 48 to 80 g/day (3 to 5 servings/day) compared to lower consumers of whole grains (Ye 2012), or 2.5 servings/day compared to 0.2 servings/day have a 21% lower risk of CVD (Mellen 2008). The 10‐year Nurses' Health Study, a large prospective study of 75,521 women aged 38 to 63, found that increased whole grain intake was associated with decreased risk of coronary heart disease (Liu 1999). The lower risk associated with higher whole grain intake was not fully explained by the contribution of the diet to intakes of dietary fibre, folate, vitamin B6, and vitamin E. The Atherosclerosis Risk in Communities (ARIC) study found a beneficial relationship between whole grain consumption and the risk of total mortality and incidence of coronary artery disease but not the risk of ischaemic stroke (Steffen 2003). The study followed 15,792 people aged 45 to 64 for 11 years. A review of the relationship between whole grains and CVD risk concluded that there is an increasing body of evidence (Seal 2006), including from observational studies, suggesting a strong inverse relationship between increased consumption of whole grain foods and CVD risk. Associations between whole grain consumption and risk factors for coronary heart disease have also been reported. In the Framingham Offspring study, diets rich in whole grains were inversely associated with total cholesterol, low‐density lipoprotein (LDL) cholesterol and body mass index (McKeown 2002).
While cereal fibre has been associated with reduced risk of CVD (Rimm 1996; Wolk 1999), the relative effects of fibre or other components of whole grains such as phytochemicals and micronutrients, Fardet 2010; Okarter 2010, on CVD and risk factors is unclear (Ferruzzi 2014). A recent systematic review of RCTs found no effect of whole grains on body weight outcomes, although there was some evidence of small changes in body fat (Pol 2013). A systematic review of the effect of whole grains on type 2 diabetes and risk factors, Priebe 2008, found only one relevant randomised trial relating to a small improvement in insulin sensitivity (Pereira 2002).
Why it is important to do this review
Recent meta‐analyses examining whole grains and CVD events included only prospective cohort studies. Evidence seems to suggest a benefit of foods and diets containing whole grain on CVD risk factors. However, evidence has come largely from observational studies, which may be prone to confounding and other biases. We undertook this systematic review to examine the evidence of the effects of whole grains on CVD events and major risk factors from RCTs.
Objectives
The aim of this systematic review was to assess the effect of whole grain foods or diets on total mortality, cardiovascular events, and cardiovascular risk factors (blood lipids, blood pressure) in healthy people or people who have established cardiovascular disease or related risk factors, using all eligible RCTs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Cross‐over and parallel‐group study designs were eligible for inclusion. Minimum study duration for inclusion was 12 weeks.
Types of participants
Free‐living adults (age 18 years or older) were eligible for inclusion if they were healthy, had established cardiovascular disease, or one or more of the following risk factors: abnormal blood lipid levels (high‐density lipoprotein (HDL) and LDL cholesterol, triglycerides, and total cholesterol), raised blood pressure/hypertension, overweight (body mass index (BMI) > 25 kg/m2) or obesity (BMI > 30 kg/m2), metabolic syndrome, or diabetes.
Types of interventions
We included studies if they compared the effect of individual whole grain foods or diets high in whole grain foods with other diets or foods with lower levels or no whole grains. Comparisons were between diets with similar overall macronutrient (energy, carbohydrate, fat, and protein) levels. For the purposes of this review, the term 'whole grain' includes foods based on milled whole grains, such as wholemeal or oatmeal. Studies had to have a minimum 12‐week intervention period (or follow‐up period following dietary advice).
We did not include studies if they were multiple‐component interventions, or interventions that incorporated factors other than whole grain foods or diets, unless the effect of whole grain foods or diets could be separated from the other factors. We did not include studies on foods that were based only on individual components (e.g. bran, germ, or other components) of the grain. We did not include studies that examined the effect of high fibre, dietary fibre, or cereal fibre where the specific effect of whole grain foods or diets could not be distinguished.
Types of outcome measures
Primary outcomes
Total cardiovascular mortality.
Cardiovascular events (e.g. fatal and non‐fatal myocardial infarction, unstable angina, coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty, stroke).
Secondary outcomes
Blood lipid levels (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides).
Blood pressure.
Quality of life.
Adverse events (e.g. bloating, nausea, weight gain, difficulty in eating out).
Body weight data and BMI were recorded as potential parameters that might influence the above outcomes, but studies that reported only weight or BMI outcomes (without cardiovascular, lipid, or blood pressure outcomes) were not included.
Since the last update of this review, a separate Cochrane Review has been published focusing on the effects of whole grain foods for the prevention of type 2 diabetes mellitus (Priebe 2008). Hence, we excluded studies with diabetes as an outcome or changes in related risk factors including impaired glucose tolerance, insulin resistance or sensitivity, glucose or insulin outcomes or weight, BMI, and other anthropometric outcomes if they did not also measure lipids or blood pressure.
Search methods for identification of studies
Electronic searches
We updated and ran the searches on 31 August 2016 in the following databases; the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 8, 2016) in the Cochrane Library, MEDLINE (Ovid, 1946 to 31 August 2016), Embase (Ovid, 1980 to week 35, 2016), and CINAHL Plus (EBSCO, 1937 to 31 August 2016).
We also searched ClinicalTrials.gov (www.clinicaltrials.gov, 5 July 2017) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/, 6 July 2017).
We amended the searches from the previous review (Appendix 2) to account for the broader inclusion criteria of this update. We ran the updated search strategies (Appendix 3) without date limits and applied no language restrictions. The Cochrane sensitivity‐maximising RCT filter was applied to MEDLINE and terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions were applied to Embase (Lefebvre 2011).
Searching other resources
We checked the reference lists of all relevant studies. We also sought relevant published reviews as a source of RCTs.
Data collection and analysis
Selection of studies
Two review authors (SK and LH/HL/CC/LA/HJ) independently scanned the titles and abstracts of retrieved records and rejected only those that the review author definitively determined did not meet the inclusion criteria. We obtained full texts for any studies that could not be rejected with certainty. Two review authors (SK and RG/HL/HJ/LA) then independently assessed each paper. We used an in/out form to assess the inclusion (or otherwise) of full papers into the review. If a trial was excluded after the full paper was obtained, the study and its reason for exclusion were recorded. Differences in selection were resolved by discussion or by consulting a third review author (KR).
Data extraction and management
One review author (SK, JC, or LA) extracted original reports of trial results, which a second review author (KR or EL) checked. Differences between review authors' extraction results were resolved by discussion and, when necessary, by consulting a third review author.
We extracted data as follows, which are reported in the Characteristics of included studies table.
General information: published/unpublished, title, authors, source, country, year of publication, trial dates, additional publications.
Trial characteristics: design, setting, duration, randomisation (and method), allocation concealment (and method), blinding (outcome assessors), check of blinding, funding/conflict of interest.
Participants: inclusion criteria, exclusion criteria, total number and number in comparison groups, sex/age, ethnicity, BMI, lipid levels, blood pressure, similarity of groups at baseline, withdrawals/losses to follow‐up, assessment of adherence, medications used, smoking status when provided.
Intervention: dietary information/diet provided, length of intervention, comparison interventions, macronutrient composition of diets.
Outcomes: outcomes as specified above, the main outcome assessed in the study, other events, length of follow‐up.
Results: for outcomes and times of assessment.
Assessment of risk of bias in included studies
We assessed risk of bias of included studies by examining the random sequence generation and allocation concealment, description of dropouts and withdrawals, blinding (outcome assessment), and selective outcome reporting. We based assessment on the Cochrane 'Risk of bias' tool (Higgins 2011), however we did not assess blinding of participants, as it is difficult to blind participants to their intervention arm in this type of dietary trial. We additionally assessed whether intention‐to‐treat analysis was conducted. We categorised risk of bias as 'low', 'unclear', or 'high'. One review author (SK or LA) assessed the risk of bias, which a second review author (EL) checked. Any disagreements were resolved by a third review author (KR).
We did not exclude studies on the basis of risk of bias. In particular, we examined the following factors.
Method of randomisation
Allocation concealment
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Intention‐to‐treat analysis
Selective reporting (reporting bias)
Groups comparable at baseline
Other (e.g. power analysis, analysis issues)
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For continuous outcomes, we compared net changes (i.e. intervention group minus control group differences) and calculated a mean difference (MD) and 95% CI for each study.
Cholesterol and triglyceride levels were expressed as mmol/L (and converted from mg/dL by multiplying by conversion factors of 0.0259 for total, HDL, and LDL cholesterol, and by 0.0113 for triglycerides where necessary) (JAMA 2004).
We converted standard errors to standard deviations by the equation given in the Cochrane Handbook where necessary.
We included studies reporting multiple comparison groups in this review. Where this was the case, we used the data for the control group for each intervention group comparison and reduced the weight assigned to the control group by dividing the control group N by the number of intervention groups. We included two studies that had two interventions. One did not provide mean values and could not be included in the meta‐analysis (Brownlee 2010), and the control group N of the second study was halved (Tighe 2010‐W).
We aimed to include cluster‐randomised trials in this review by using the unit of randomisation (cluster) as the number of observations. Where necessary, we would have utilised individual‐level means and standard deviations adjusted for clustering together with the number of clusters in the denominator, in order to weight the trials appropriately. We found no trials where participants were randomised by cluster/groups.
We entered data presented as a scale with a consistent direction of effect, with the exception of HDL cholesterol, where an increase in this outcome is a positive finding.
Assessment of heterogeneity
For each outcome, we conducted tests of heterogeneity using the Chi2 test of heterogeneity and the I2 statistic. Where heterogeneity was low, we performed a fixed‐effect meta‐analysis. If we detected substantial heterogeneity (I2 of 50% or greater), we looked for possible explanations (e.g. participants and intervention). If we were unable to explain the source of heterogeneity, we considered the following options: provide a narrative overview and not aggregate the studies at all, or use a random‐effects model with appropriate cautious interpretation.
Data synthesis
We carried out statistical analysis using Cochrane's statistical software, Review Manager 5 (RevMan 2014). We entered continuous data as the change in means and standard deviations from baseline to follow‐up measurements.
We aimed to categorise studies into two subgroups: primary‐prevention populations (healthy individuals or those at high risk of CVD) or secondary‐prevention populations (those with a pre‐existing diagnosis of CVD). However, we identified no studies in secondary‐prevention populations.
Studies reported results either as absolute values at the endpoint or as change from baseline. For the pooled analysis, we reported change from baseline values. Where papers did not report results as change from baseline, we calculated this and for the standard deviation differences followed the methods presented in Section 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions for imputing these (Higgins 2011), and assumed a correlation of 0.5 between baseline and follow‐up measures as suggested by Follman (Follmann 1992).
We planned to use sensitivity analysis to take into account the influence of various factors, for example (a) risk of bias, and (b) exclusion of particularly small and underpowered trials. However, all studies were of similar risk of bias and size, and therefore no sensitivity analyses were undertaken. We also planned to undertake assessment of funnel plots and tests of asymmetry to assess possible publication bias, but there were insufficient studies included in the review (Egger 1997).
Quality of the evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. Two review authors (LA, SK) rated the quality for each outcome. We presented a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We presented results on the outcomes as described in Types of outcome measures.
In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' to help with standardisation of the 'Summary of findings' table (Appendix 1) (Meader 2014).
Results
Description of studies
Results of the search
The updated searches identified 15,283 potentially relevant records. Records from the databases were imported into EndNote reference management software and combined. The combined articles were then de‐duplicated using the EndNote software, leaving 9635 combined hits. A further 42 potentially relevant records were identified from handsearching relevant reviews, and 1427 from searching clinical trials registers. A total of 414 full‐text articles were assessed for eligibility, and 9 studies (reported in 13 references) were included in the review, of which 8 were included in meta‐analysis.
For full details of the searches and selection of trials for the review, see Figure 1 (Moher 2009).
1.
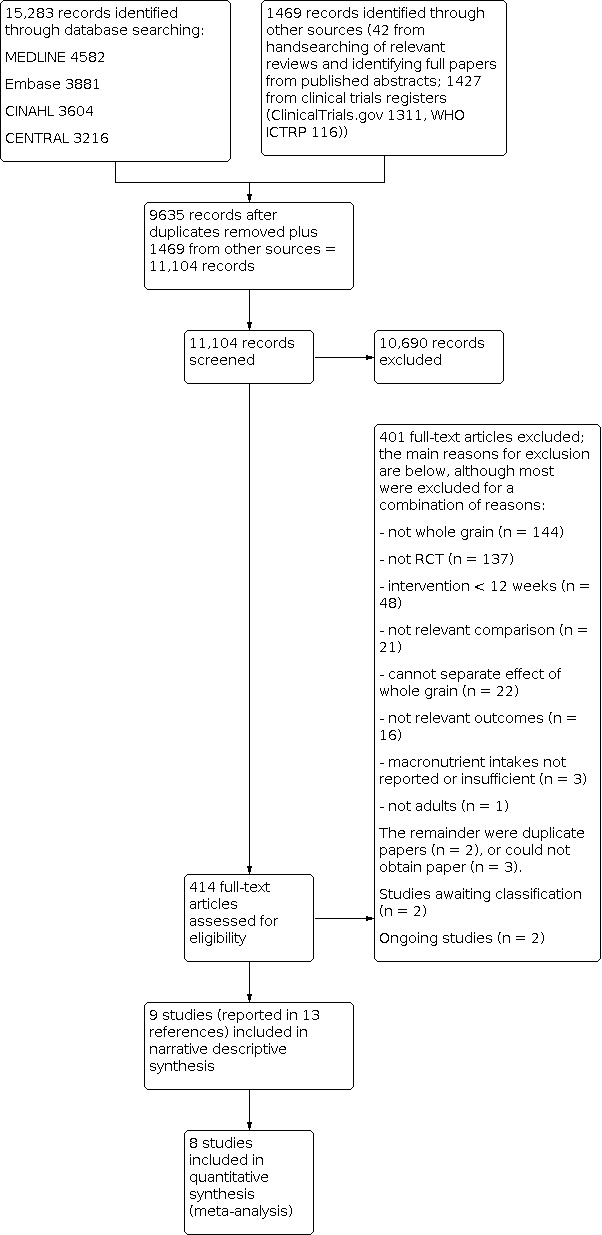
Study flow diagram for updated searches 2016.
Included studies
Details of the studies included in the review are shown in the Characteristics of included studies table.
We identified nine different studies that met the inclusion criteria for this review (the study by Tighe reported two different relevant intervention arms in the same paper) (Brownlee 2010; Giacco 2013; Harris 2014; Katcher 2008; Kristensen 2012; Lankinen 2014; Maki 2010; Tighe 2010‐W; Tighe 2010‐WO; Zhang 2011). We found no studies that reported the effect of whole grain foods or diets on CVD mortality or CVD events and morbidity (total myocardial infarction, unstable angina, coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty, total stroke). All nine included studies reported the effect of whole grain foods or diets on major risk factors for CVD (lipids and blood pressure) according to the inclusion criteria for this review outlined in Types of outcome measures. All nine studies were parallel RCTs. The nine included studies involved a total of 1414 participants. Four of the nine studies had total sample sizes below 100 participants (Giacco 2013; Harris 2014; Katcher 2008; Kristensen 2012).
In four studies, both the whole grain and control diets were energy‐restricted weight loss diets (Harris 2014; Katcher 2008; Kristensen 2012; Maki 2010).
In one study, the whole grain was oats (Maki 2010). In five studies, the diets included a range of whole grain foods mainly based on wheat (Brownlee 2010; Harris 2014; Katcher 2008; Kristensen 2012; Lankinen 2014); one study used a mixture of rye and wheat products (Giacco 2013); one study used whole grain brown rice (Zhang 2011); and one study had two intervention arms, one based on whole grain wheat products and the other a mixture of whole grain wheat and oats (Tighe 2010‐W).
Seven studies described their control group diet as refined foods; one study described the control diet as usual diet (Brownlee 2010); and in the remaining study the control was white rice (Zhang 2011).
The length of the intervention was 12 weeks in seven studies and 16 weeks in two studies (Brownlee 2010; Zhang 2011).
Population and setting
Three studies included participants who were overweight or obese (Brownlee 2010; Kristensen 2012; Maki 2010); two studies included participants with metabolic syndrome (Giacco 2013; Lankinen 2014); one study included participants who were overweight or obese and had metabolic syndrome (Katcher 2008); and one study included participants who had risk factors for metabolic syndrome (Harris 2014), including obesity. One study recruited participants with BMI from 18.5 to 35 kg/m2 or with signs of metabolic syndrome or moderate hypercholesterolaemia (Tighe 2010‐W). One study included participants with metabolic syndrome or diabetes (Zhang 2011).
Three studies were conducted in the USA (Harris 2014; Katcher 2008; Maki 2010), two in the UK (one in Scotland, Tighe 2010‐W, and one in England, Brownlee 2010), one in Finland (Lankinen 2014), one in Denmark (Kristensen 2012), one in both Italy and Finland (Giacco 2013), and one in China (Zhang 2011).
Intervention
The interventions in eight of the nine studies included the provision of whole grain foods to the study participants (Brownlee 2010; Giacco 2013; Harris 2014; Kristensen 2012; Lankinen 2014; Maki 2010; Tighe 2010‐W; Zhang 2011). In the remaining study the participants were given a list and description of foods and a daily serving of whole grain foods to aim for depending on individualised energy requirements set out by a study dietitian (Katcher 2008). In two studies (Brownlee 2010; Kristensen 2012), a range of foods were available, and the studies aimed to allow participants to choose their whole grain foods. The Giacco 2013 study prespecified a proportion of whole grain that was required to be in the form of bread (this differed slightly between the two different study centres), and participants were also provided with oat biscuits for snacks. In the study by Harris 2014, a range of grain types were provided including wheat, oats and rice, with 77% from wheat products. In this study prepared meals and snacks were provided to the study participants. The study by Maki 2010 also provided ready‐to‐eat cereals. In the study by Tighe 2010‐W, participants had three food servings replaced by study foods (either whole grain wheat or whole grain wheat and oats). In all studies except Brownlee 2010, control participants also received like‐for‐like food or advice but with refined products.
Three studies reported that participants also received advice from a dietitian (Katcher 2008; Kristensen 2012; Maki 2010), and two studies encouraged participants to undertake a prescribed amount of physical activity each week (Katcher 2008; Maki 2010).
All nine trials reported daily dietary components of the intervention diets. One study reported daily intake of energy as a change from baseline (Brownlee 2010), where the energy intake increased in the two intervention groups but decreased in the control group. In the four studies that aimed to reduce energy intake as part of their intervention (Harris 2014; Katcher 2008; Kristensen 2012; Maki 2010), only two reported baseline and end‐of‐study daily energy, where as expected results showed a reduction in energy consumption (Katcher 2008; Maki 2010). Of the remaining studies, there was an increase in energy consumption in both groups by the end of the intervention in the study by Giacco 2013 and no difference in energy intake across or between groups in the studies by Tighe 2010‐W, Lankinen 2014, and Zhang 2011. As can be seen in Table 2, most studies reported similar dietary components between intervention and control groups (or did not report the statistical significance, but data appear to be similar). There was a difference in carbohydrates and fibre (g/day) between the whole grain groups and the control groups in two studies (Brownlee 2010; Kristensen 2012), but no other dietary components differed. In the Giacco 2013 study, a difference in protein intake was seen between those in the whole grain group and those in the control group. Zhang 2011 reported lower intake of carbohydrates (P = 0.03) and dairy products in the brown rice group (P = 0.02), and Lankinen 2014 reported that total fat decreased in the whole grain group.
1. Comparability of diets: whole grain versus control.
| Study ID | Dietary component | Whole grain | Control | P value |
| Brownlee 2010 | Energy (kJ/day) | Baseline: Not reported Data are change from baseline. Intervention 1 (lower WG) Wk 8: 379 (SD/SEM NR) Wk 16: 387 (SD/SEM NR) Intervention 2 (higher WG) Wk 8: ‐399 (SD/SEM NR) Wk 16: 587 (SD/SEM NR) |
Baseline: Not reported Data are change from baseline. Wk 8: ‐430 (SD/SEM NR) Wk 16: ‐679 (SD/SEM NR) |
Intervention 1 (lower WG) Wk 8: 0.015 Wk 16: NS Intervention 2 (higher WG) Wk 8: 0.32 Wk 16: 0.005 |
| Carbohydrate (g/day) | Baseline: Not reported Data are change from baseline. Intervention 1 (lower WG) Wk 8: 22.6 (SD/SEM NR) Wk 16: 37.1 (SD/SEM NR) Intervention 2 (higher WG) Wk 8: 14.8 (SD/SEM NR) Wk 16: 53.8 (SD/SEM NR) |
Baseline: Not reported Data are change from baseline. Wk 8: ‐1.97 (SD/SEM NR) Wk 16: ‐14.8 (SD/SEM NR) |
Intervention 1 (lower WG) 0.004 0.007 Intervention 2 (higher WG) 0.026 < 0.001 |
|
| Fat (g/day) | Baseline: Not reported Data are change from baseline. Intervention 1 (lower WG) Wk 8: 0.245 (SD/SEM NR) Wk 16: ‐2.96 (SD/SEM NR) Intervention 2 (higher WG) Wk 8: ‐8.12 (SD/SEM NR) Wk 16: ‐1.63 (SD/SEM NR) |
Baseline: Not reported Data are change from baseline. Wk 8: ‐2.87 (SD/SEM NR) Wk 16: ‐4.05 (SD/SEM NR) |
Intervention 1 (lower WG) NS NS Intervention 2 (higher WG) NS NS |
|
| Protein (g/day) | Baseline: Not reported Data are change from baseline. Intervention 1 (lower WG) Wk 8: 6.15 (SD/SEM NR) Wk 16: 5.05 (SD/SEM NR) Intervention 2 (higher WG) Wk 8: 1.75 (SD/SEM NR) Wk 16: 6.99 (SD/SEM NR) |
Baseline: Not reported Data are change from baseline. Wk 8: ‐3.17 (SD/SEM NR) Wk 16: ‐4.25 (SD/SEM NR) |
Intervention 1 (lower WG) NS NS Intervention 2 (higher WG) NS NS |
|
| NSP/fibre (g/day) | Baseline: Not reported Data are change from baseline. Intervention 1 (lower WG) Wk 8: 4.69 (SD/SEM NR) Wk 16: 5.70 (SD/SEM NR) Intervention 2 (higher WG) Wk 8: 6.23 (SD/SEM NR) Wk 16: 11.0 (SD/SEM NR) |
Baseline: Not reported Data are change from baseline. Wk 8: ‐0.144 (SD/SEM NR) Wk 16: ‐0.438 (SD/SEM NR) |
Intervention 1 (lower WG) < 0.001 < 0.001 Intervention 2 (higher WG) < 0.001 < 0.001 |
|
| Whole grain (g/day) |
Working definition of whole grain product: commercially available whole grain products readily available in the UK, ranging from 34 to 80.8 g/100 g dry weight or 11.2 g cooked weight of whole grain Data approximated from graph; SD/SEM not available. Intervention 1 (lower WG) (mean intake g/day) Wk 8: 75 Wk 16: 70 Intervention 2 (higher WG) (mean intake g/day) Wk 8: 80 Wk 16: 115 |
Data taken from graph; SD/SEM not available. < 20 g/day (mean intake g/day) |
NR NR |
|
| Giacco 2013 | Energy (kilocalories/day) | Baseline: 1702 (SEM 62) Wk 12: 1900 (SEM 57) |
Baseline: 1719 (SEM 63) Wk 12: 1965 (SEM 57) |
NR NS |
| Carbohydrate (% E) | Baseline: 46 (SEM 0.6) Wk 12: 48 (SEM 0.6) |
Baseline: 48 (SEM 0.7) Wk 12: 49 (SEM 0.6) |
NR NS |
|
| Fat (% E) | Baseline: 33.5 (SEM 0.6) Wk 12: 31 (SEM 0.7) |
Baseline: 31.8 (SEM 0.6) Wk 12: 30.8 (SEM 0.7) |
NR NS |
|
| Protein (% E) | Baseline: 18 (SEM 0.4) Wk 12: 18.7 (SEM 0.3) |
Baseline: 18 (SEM 0.4) Wk 12: 17.8 (SEM 0.3) |
NR < 0.05 |
|
| Fibre (g/day) |
Total fibre Baseline: 22.7 (SEM 0.8) Wk 12: 32.6 (SEM 0.7) Cereal fibre Baseline: 11.9 (SEM 0.8) Wk 12: 24.3 (SEM 0.9) |
Total fibre Baseline: 21.6 (SEM 0.8) Wk 12: 19.8 (SEM 0.7) Cereal fibre Baseline: 11.4 (SEM 0.6) Wk 12: 10.4 (SEM 0.3) |
NR < 0.05 NR < 0.05 |
|
| Whole grain (g/day) Whole grain level (plasma total alkylresorcinol concentration nmol/L) |
Study states that Working definition of whole grain: 51% whole grain per day, dry weight was used according to HealthGrain forum definition and analysis of whole grain but no data reported for wholegrain levels. However plasma total alkylresorcinol as a proxy for wholegrain content was reported as below: | NR ‐19.7 (n = 26) |
NR 88.3 (n = 28) |
|
| Harris 2014 | Energy (kilocalories/day) | Calculated from menus. All food provided. 86% compliance reported. Mean/day 2079 |
Mean/day 2023 | NR |
| Carbohydrate (g/day) | 299 | 280 | NR | |
| Fat (g/day) | 62 | 64 | NR | |
| Protein (g/day) | 97 | 90 | NR | |
| Fibre (g/day) | 38 | 22 | NR | |
| Whole grain (g/day) |
Working definition of whole grain: whole grain products made from milled flour were required to have > 51% of dry weight from whole grain flour. When possible, whole grain products with the 100% whole grain stamp were selected, which indicated that each grain serving contained at least 16 g whole grain and used 100% whole grain flour. Whole grain content of diets ranged from 163 to 301 g/day, as energy content of diets were adjusted for individual requirements. Energy of diets was 1600 to 3600 kilocalories/day. Based on energy intake of 2100 kilocalories/day, typical whole grains supplied were 187 g/day (7 servings/day). |
0 servings/day 0 g/day |
NR NR |
|
| Katcher 2008 | Energy (kilocalories/day) | Baseline: 1967 (SD 545) Wk 4: 1812 (SD 505) Wk 8: 1744 (SD 533) Wk 12: 1611 (SD 377) |
Baseline: 2265 (SD 744) Wk 4: 1616 (SD 468) Wk 8: 1562 (SD 398) Wk 12: 1575 (SD 500) |
NS |
| Carbohydrate (% E) | Baseline: 47.8 (SD 8.3) Wk 4: 54.0 (SD 7.1) Wk 8: 53.9 (SD 9.1) Wk 12: 54.6 (SD 6.8) |
Baseline: 47.5 (SD 8.7) Wk 4: 49.6 (SD 10.7) Wk 8: 47.5 (SD 10.2) Wk 12: 49.9 (SD 9.7) |
NS | |
| Fat (% E) | Baseline: 35.4 (SD 5.9) Wk 4: 28.7 (SD 6.6) Wk 8: 29.6 (SD 7.2) Wk 12: 27.8 (SD 6.9) |
Baseline: 36.2 (SD 6.8) Wk 4: 32.3 (SD 8.4) Wk 8: 33.8 (SD 8.5) Wk 12: 30.5 (SD 8.0) |
NS | |
| Protein (% E) | Baseline: 16.9 (SD 3.2) Wk 4: 18.2 (SD 2.7) Wk 8: 18.4 (SD 3.4) Wk 12: 19.1 (SD 4.3) |
Baseline: 16.5 (SD 3.2) Wk 4: 18.7 (SD 4.5) Wk 8: 19.2 (SD 4.8) Wk 12: 20.0 (SD 4.8) |
NS | |
| Fibre (g/1000 kilocalories) | Baseline: 8.6 (SD 3.7) Wk 4: 12.6 (SD 3.2) Wk 8: 13.3 (SD 3.4) Wk 12: 12.9 (SD 2.2) |
Baseline: 9.1 (SD 3.7) Wk 4: 10.0 (SD 3.0) Wk 8: 9.5 (SD 2.0) Wk 12: 9.7 (SD 3.5) |
Significant difference between WG and RG at wks 8 and 12 (P < 0.05) | |
| Whole grain (g/day) | Definition of whole grain product: "grain product was identified as a wholegrain if a wholegrain was listed as the first ingredient on the food label". Data taken from graph so approximate: 1 serving whole grain equivalent to 1 slice wholemeal bread, or 28 g (1 oz ready‐to‐eat cereal), or 1/2 cup cooked cereal, rice, or pasta (2005 dietary guidelines for Americans). Baseline: 1.5 servings/day Wk 12: 5 servings/day |
Baseline: ~1.5 servings/day Week 12: 0.2 servings/day |
NR | |
| Kristensen 2012 | Energy (kJ/day) | Wks 1 to 6: 5830 (SEM 190) Wks 7 to 12: 6060 (SEM 150) |
Wks 1 to 6: 5900 (SEM 280) Wks 7 to 12: 6330 (SEM 180) |
NR NR |
| Carbohydrate (g/day) | 86.8 | 95.8 | NR | |
| Fat (g/day) | 6.8 | 6.6 | NR | |
| Protein (g/day) | 16.6 | 16.0 | NR | |
| Fibre (g/day) | 11.0 | 4.5 | NR | |
| Whole grain (g/day) Alkylresorcinol (mg/day) |
Working definition of whole grain: mean whole grain intake: 105 g/day 25.5 |
Mean whole grain intake: 0 g/day AR: 3.1 |
NR | |
| Lankinen 2014 | Energy (kJ/day) | Baseline: 6995 ± 2373 Wk 12: 7654 ± 2395 |
Baseline: 7282 ± 2011 Wk 12: 8533 ± 1693 |
0.119 |
| Carbohydrate (% E/day) | Baseline: 45.6 ± 6.3 Wk 12: 47.2 ± 7.5 |
Baseline: 47.8 ± 5.6 Wk 12: 47.3 ± 5.1 |
0.268 | |
| Fat (% E/day) | Baseline: 33.6 ± 5.2 Wk 12: 3 ± 6.3 |
Baseline: 31.3 ± 5.3 Wk 12: 31.9 ± 5.9 |
0.012 | |
| Protein (% E/day) | Baseline: 19.1 ± 3.2 Wk 12: 18.8 ± 2.5 |
Baseline: 18.8 ± 3.7 Wk 12: 18.3 ± 2.5 |
0.950 | |
| Fibre (g/day) | Baseline: 24.6 ± 7.0 Wk 12: 26.5 ± 5.4 |
Baseline: 22.5 ± 7.0 Wk 12: 18.0 ± 4.2 |
2.7x107 | |
| Whole grain (g/day) | Working definition of whole grain: whole grain breads and a bread with low glycaemic index products covered 20% to 25% of total energy intake and were delivered to the participants. The fibre contents of the breads were 6.9% (endosperm rye bread), 6.4% (whole grain wheat bread), and 10% to 14% (commercial whole grain rye breads). Wholemeal pasta. | Refined wheat breads and other cereal products with low fibre. Participants were allowed to eat maximum of 1 to 2 portions of rye products per day. | ‐ | |
| Maki 2010 | Energy (kilocalories/day) | Baseline: 1939 (SEM 97) Wk 4: 1563 (SEM 50) Wk 12: 1529 (SEM 44) |
Baseline: 1853 (SEM 70) Wk 4: 1395 (SEM 44) Wk 12: 1443 (SEM 45) |
0.690 0.009 0.256 |
| Carbohydrate (% E) | Baseline: 44.8 (SEM 0.9) Wk 4: 50.6 (SEM 0.9) Wk 12: 52.2 (SEM 0.9) |
Baseline: 45.6 (SEM 1.2) Wk 4: 49.8 (SEM 1.0) Wk 12: 49.8 (SEM 1.0) |
0.660 0.625 0.017 |
|
| Total fat (% E) | Baseline: 36.9 (SEM 0.8) Wk 4: 30.4 (SEM 0.9) Wk 12: 29.6 (SEM 0.8) |
Baseline: 35.6 (SEM 0.8) Wk 4: 30.0 (SEM 0.8) Wk 12: 29.8 (SEM 0.7) |
0.297 0.697 0.718 |
|
| Protein (% E) | Baseline: 18.2 (SEM 0.5) Wk 4: 20.1 (SEM 0.5) Wk 12: 19.7 (SEM 0.5) |
Baseline: 17.9 (SEM 0.6) Wk 4: 20.0 (SEM 0.6) Wk 12: 20.1 (SEM 0.6) |
0.330 0.971 0.623 |
|
| Fibre (g/day) | Baseline: 15.8 (SEM 1.0) Wk 4: 21.0 (SEM 0.5) Wk 12: 21.7 (SEM 0.5) |
Baseline: 14.8 (SEM 0.8) Wk 4: 11.8 (SEM 0.6) Wk 12: 12.7 (SEM 0.6) |
0.612 < 0.001 < 0.001 |
|
| Whole grain (g/day) | Working definition of whole grain: the whole grain used was a whole grain oat ready‐to‐eat cereal (Cheerios, General Mills, Minneapolis, MN), 2 portions per day (approximately 80 g/day), containing equivalent of 3 g oat β‐glucan. | ‐ | ‐ | |
| Tighe 2010‐W | Energy (kilocalories/day) |
Whole grain wheat group Baseline: 2115 (SEM 64) Wk 12: 2121 (SEM 75) Whole grain wheat + oats group Baseline: 2115 (SEM 58) Wk 12: 2142 (SEM 69) |
Baseline: 2036 (SEM 79) Wk 12: 2080 (SEM 83) |
Baseline: 0.650 Wk 12: 0.843 |
| Carbohydrate (g/day) |
Whole grain wheat group Baseline: 256 (SEM 9) Wk 12: 253 (SEM 9) Whole grain wheat + oats group Baseline: 252 (SEM 7) Wk 12: 243 (SEM 8) |
Baseline: 238 (SEM 10) Wk 12: 245 (SEM 10) |
Baseline: 0.324 Wk 12: 0.633 |
|
| Fat (g/day) |
Whole grain wheat group Baseline: 80.8 (SEM 2.8) Wk 12: 79.7 (SEM 3.3) Whole grain wheat + oats group Baseline: 78.6 (SEM 2.9) Wk 12: 82.1 (SEM 3.5) |
Baseline: 78.7 (SEM 3.6) Wk 12: 79.9 (SEM 4.3) |
Baseline: 0.847 Wk 12: 0.871 |
|
| Protein (g/day) |
Whole grain wheat group Baseline: 85.2 (SEM 3.0) Wk 12: 89.1 (SEM 3.5) Whole grain wheat + oats group Baseline: 83.1 (SEM 2.4) Wk 12: 87.0 (SEM 2.8) |
Baseline: 81.3 (SEM 2.9) Wk 12: 84.0 (SEM 2.4) |
Baseline: 0.627 Wk 12: 0.496 |
|
| Fibre (NSP) (g/day) |
Whole grain wheat group Baseline: 12.3 (SEM 0.4) Wk 12: 18.5 (SEM 0.5) Whole grain wheat + oats group Baseline: 12.4 (SEM 0.4) Wk 12: 16.8 (SEM 0.5) |
Baseline: 10.9 (SEM 0.5) Wk 12: 11.3 (SEM 0.4) |
Baseline: 0.049 Wk 12: < 0.001 |
|
| Whole grain (g/day) |
Working definition of whole grain: not reported Whole grain wheat group 3 servings whole grain foods: 70 to 80 g wholemeal bread and 30 to 40 g whole grain cereals/day Whole grain wheat + oats group 3 servings whole grain foods: 1 serving whole grain wheat products and 2 servings whole grain oat foods/day |
‐ | ‐ | |
| Zhang 2011 | Energy (MJ/day) |
Brown rice Baseline: 8.72 (SD 2.30) Wk 4: 8.31 (SD 1.75) Wk 8: 8.05 (SD 1.89) Wk 12: 8.00 (SD 1.86) Wk 16: 8.22 (SD 1.80) |
White rice Baseline: 8.65 (SD 2.38) Wk 4: 8.16 (SD 2.23) Wk 8: 8.25 (SD 2.01) Wk 12: 8.46 (SD 2.04) Wk 16: 8.60 (SD 2.01) |
‐ |
| Carbohydrate (% E) |
Brown rice Baseline: 53.9 (SD 7.3) Wk 4: 53.3 (SD 6.5) Wk 8: 52.9 (SD 5.5) Wk 12: 51.8 (SD 7.0) Wk 16: 51.6 (SD 7.3) |
White rice Baseline: 54.9 (SD 7.2) Wk 4: 51.8 (SD 6.4) Wk 8: 53.8 (SD 6.3) Wk 12: 52.8 (SD 5.8) Wk 16: 53.3 (SD 6.8) |
‐ | |
| Fat (% E) |
Brown rice Baseline: 32.5 (SD 6.7) Wk 4: 32.3 (SD 5.9) Wk 8: 33.0 (SD 4.5) Wk 12: 34.2 (SD 6.4) Wk 16: 35.0 (SD 6.4) |
White rice Baseline: 31.7 (SD 6.4) Wk 4: 33.1 (SD 6.2) Wk 8: 31.5 (SD 5.8) Wk 12: 32.7 (SD 5.7) Wk 16: 32.7 (SD 6.6) |
‐ | |
| Protein (% E) |
Brown rice Baseline: 15.8 (SD 2.8) Wk 4: 17.2 (SD 2.8) Wk 8: 17.0 (SD 3.1) Wk 12: 16.8 (SD 2.7) Wk 16: 16.1 (SD 2.8) |
White rice Baseline: 15.7 (SD 2.7) Wk 4: 17.4 (SD 2.8) Wk 8: 17.0 (SD 2.7) Wk 12: 16.7 (SD 2.4) Wk 16: 16.0 (SD 2.2) |
‐ | |
| Fibre (g/1000 kJ) |
Brown rice Baseline: 1.34 (SD 0.49) Wk 4: 1.65 (SD 0.40) Wk 8: 1.69 (SD 0.42) Wk 12: 1.64 (SD 0.42) Wk 16: 1.57 (SD 0.51) |
White rice Baseline: 1.32 (SD 0.38) Wk 4: 1.34 (SD 0.42) Wk 8: 1.37 (SD 0.49) Wk 12: 1.28 (SD 0.39) Wk 16: 1.20 (SD 0.39) |
‐ | |
| Whole grain (g/day) | Authors stated that the brown rice was a whole grain in the paper, and Table 1 of the paper reports nutrient comparison of the brown rice versus the white rice used in the study. | ‐ | ‐ |
% E: percentage energy NR: Not reported NS: Not statistically significant NSP: non‐starch polysaccharide RG: refined grains SD: standard deviation SEM: standard error of the mean WG: whole grains
We relied on the study authors' definition of whole grain for this review. Definitions and level of whole grain in the diets where reported are shown in Table 2. This varied between studies. In two studies a whole grain product was defined as at least 51% whole grain by dry weight (Giacco 2013; Harris 2014); in one study it was 50% by dry weight (Kristensen 2012); in one study the whole grain content of commercially available whole grain foods used ranged from 34 to 80.8 g per 100 g dry weight (and one product was included with 11.2 g per 100 g cooked weight) (Brownlee 2010); and in one study products were identified as whole grain if a whole grain was listed as the first ingredient on the food label (Katcher 2008). Two studies did not report the definition of whole grain used (Lankinen 2014; Tighe 2010‐W), but one of these did report the number of servings (Tighe 2010‐W). One study used exclusively whole grain oats (Maki 2010), and another study defined the brown rice used as whole grain (Zhang 2011).
Funding
Four studies were funded wholly by independent sources, two by the UK Food Standards Agency (Brownlee 2010; Tighe 2010‐W), one by a European Commission grant (Giacco 2013), and one through Chinese national funding organisations (Zhang 2011), although in three of these studies some of the test foods were supplied by commercial companies (Brownlee 2010; Giacco 2013; Tighe 2010‐W). All of the other five studies reported funding or some partial funding by organisations with interests in cereals.
Excluded studies
We excluded two studies because the macronutrient content of the diets was not reported adequately (Chang 2013; Wang 2013). We excluded 13 studies because the length of the intervention was less than 12 weeks (Charlton 2012; Davidson 1991; Johnston 1998; Karmally 2005; Keenan 2002; Kim 2008; Leinonen 2000; McGeoch 2013; Reynolds 2000; Van Horn 1988; Van Horn 1991; Vitaglione 2015; Zhang 2012). This update of the review only included studies with an intervention length of 12 weeks or longer. We also excluded studies that only reported outcomes related to diabetes and measures of glucose and insulin or body weight alone that did not also report lipid or blood pressure outcomes. Consequently, we have excluded some short‐term studies that we included in the original version of this review (Kelly 2007): Davidson 1991, Johnston 1998, Karmally 2005, Keenan 2002, Leinonen 2000, Reynolds 2000, Van Horn 1988, and Van Horn 1991. Additionally, one study in the original review no longer met the inclusion criteria as it did not report relevant outcomes (Pereira 2002). We excluded a further study because reporting of macronutrient intake was insufficient to determine if the diet composition was similar in the intervention and control groups (Pins 2002); this was marked as unclear in the original review, but as we were unable to obtain any further data from the reported supplementary table or from the authors, this study has been excluded for this update. These changes mean that none of the studies in the original review, Kelly 2007, are included in this update.
Risk of bias in included studies
Overview
The reporting of methods was poor, therefore we rated the risk of bias as unclear in the majority of studies (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies reported an adequate method of randomisation (Brownlee 2010; Giacco 2013; Harris 2014; Katcher 2008; Kristensen 2012; Lankinen 2014; Tighe 2010‐W), but only two of these also clearly reported concealment of allocation to treatment groups (Giacco 2013; Tighe 2010‐W). The remaining studies did not provide information on allocation process.
Blinding
Only three studies reported adequate blinding of outcome assessment (Katcher 2008; Tighe 2010‐W; Zhang 2011). We rated all of the remaining studies as unclear for this domain as no information was provided.
Incomplete outcome data
A number of studies had high levels of dropouts/losses to follow‐up, and reporting of the details of these dropouts was poor. Three studies fully reported numbers and reasons for incomplete data (Katcher 2008; Lankinen 2014; Zhang 2011), whereas five studies had differential dropouts between treatment groups and were therefore rated as at high risk of attrition bias (Brownlee 2010; Giacco 2013; Harris 2014; Maki 2010; Tighe 2010‐W). One study reported an adequate intention‐to‐treat analysis for outcomes of interest to this review (Zhang 2011). One further study provided some data as a modified intention‐to‐treat population, but did not provide this in a format that was useable for the review (baseline values for this population were not reported) (Maki 2010).
Selective reporting
While all studies appeared to report all outcomes as intended, not enough information was available to judge the potential risk of selective reporting bias. Of the nine included studies, eight reported that the study protocol was registered, and one did not report if a protocol was registered (Maki 2010).
Other potential sources of bias
Power
We rated three studies as being at low risk of other sources of bias (Brownlee 2010; Maki 2010; Tighe 2010‐W), as the power calculations were based on relevant outcomes (LDL cholesterol or total cholesterol, or both), and the predicated sample size was met. We rated all of the other studies as unclear on this factor. The remaining six studies based their power calculations on outcomes that were not relevant to this review (Giacco 2013; Harris 2014; Katcher 2008; Kristensen 2012; Lankinen 2014; Zhang 2011), and it is therefore uncertain if they were fully powered to detect a difference in the key outcomes of relevance here.
Compliance to diet
None of the studies specifically reported any problems with compliance to the diets. In one study (Kristensen 2012), the authors reported high compliance with the diet, but this was only reported in 57 of 72 female participants who completed food diaries. The Zhang 2011 study reported mean adherence 90.0 +/‐ 17.1% in the white rice group and 88.7 +/‐ 23.3% in the brown rice group. However, a number of studies reported high levels of dropouts and losses to follow‐up, and full details of reasons for dropout were not fully reported.
Comparability at baseline
Five studies had comparable groups at baseline (Brownlee 2010; Giacco 2013; Harris 2014; Kristensen 2012; Lankinen 2014). The remaining four studies reported some differences between intervention and control for some baseline variables (Katcher 2008; Maki 2010; Tighe 2010‐W; Zhang 2011); it was unclear whether these were of any significance, as they may have occurred by chance. Details are reported in the 'Risk of bias' table in Characteristics of included studies for each study.
Potential confounders
Body weight
Differential changes in body weight between intervention and control groups could lead to differences in lipid levels between groups. However, we found no evidence of differences in body weight between intervention and control groups. Six included studies reported data on body weight, and five of these could be summarised in a meta‐analysis (see Analysis 1.1). The four studies with energy‐restricted diets all showed weight loss in both arms of their studies. In three studies this was greater in the intervention arm (Harris 2014; Kristensen 2012; Maki 2010), and in the fourth study this was greater in the control arm (Katcher 2008). The remaining studies did not show any clear pattern of weight loss between intervention and control groups. There was no difference between groups in the pooled study estimate of weight loss (mean difference (MD) ‐0.41, 95% confidence interval (CI) ‐1.04 to 0.23). In the study that could not be pooled (Brownlee 2010), the median weight change in the whole grain intervention group was a gain of 0.2 (standard deviation (SD) 13.1) kg compared to a zero weight change (SD 13.9) in the control arm. Five studies reported data on the BMI of participants, and there was no difference between groups in the pooled study estimate (MD ‐0.12, 95% CI ‐0.24 to 0.01) (Analysis 1.2).
1.1. Analysis.

Comparison 1: Whole grain versus control, Outcome 1: Body weight change (kg)
1.2. Analysis.

Comparison 1: Whole grain versus control, Outcome 2: BMI change
Dietary fibre
Any reported differences in dietary fibre content between the treatment and control groups have been reported in Table 2. The focus of this review was to assess whether whole grain diets have a beneficial effect on CVD and major risk factors for CVD in order to inform recommendations as to whether more whole grain foods should be included in the diets of the general public. All whole grains contain dietary fibre. Dietary fibre content may potentially be a contributing factor to any differences between whole grain diets and the control diets, but it was not the primary focus of this review.
Effects of interventions
See: Table 1
Primary outcomes
We found no studies that reported the effect of whole grain foods or diets on:
total cardiovascular mortality; or
cardiovascular events (e.g. fatal and non‐fatal myocardial infarction, unstable angina, coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty, stroke).
Secondary outcomes
Blood lipids
Total cholesterol
Eight studies reported total cholesterol, and data from six studies (seven comparisons) could be summarised in a meta‐analysis (Analysis 1.3). The pooled analysis showed no effect on total cholesterol (MD 0.07, 95% CI ‐0.07 to 0.21; 722 participants; 6 studies (7 comparisons); low‐quality evidence). There was no substantial heterogeneity.
1.3. Analysis.

Comparison 1: Whole grain versus control, Outcome 3: Total cholesterol change (mmol/L)
Two studies could not be summarised in the meta‐analysis. In one study this was because data were presented as medians only. In this study there was no difference in total cholesterol between participants in the two whole grain groups and participants in the control group (Brownlee 2010). The remaining study reported percentage change in total cholesterol levels rather than absolute levels, and data were aggregated over all follow‐up points (from 4 to 12 weeks). The data in this study were reported for a per‐protocol population with approximately 24% of participants in the whole grain arm and 35% in the control arm excluded from the analysis (fewer than reported to have completed the study). The authors of this study found that the total cholesterol level was reduced more with whole grain ready‐to‐eat oat cereal than with control (5.4% (standard error (SE) 0.8%) versus 2.9% (SE 0.9%), P = 0.038) during the treatment period (Maki 2010).
LDL cholesterol
Nine studies reported LDL cholesterol, and data from seven studies (eight comparators) could be summarised in a meta‐analysis (Analysis 1.4). The pooled analysis showed that there was no effect on LDL cholesterol (MD 0.06, 95% CI ‐0.05 to 0.16; 770 participants). There was no substantial heterogeneity.
1.4. Analysis.

Comparison 1: Whole grain versus control, Outcome 4: LDL cholesterol change (mmol/L)
Two studies could not be summarised in the meta‐analysis. In one study this was because data were presented as medians only. In this study there was no difference in LDL cholesterol between participants in the two whole grain groups and participants in the control group (Brownlee 2010). The remaining study reported percentage change in LDL cholesterol levels rather than absolute levels, and data were aggregated over all follow‐up points (from 4 to 12 weeks). The data in this study were reported for a per‐protocol population with approximately 24% of participants in the whole grain arm and 35% in the control arm excluded from the analysis (fewer than reported to have completed the study). The authors of this study found that LDL cholesterol level was reduced more with whole grain ready‐to‐eat oat cereal than with control (8.7% (SE 1.0%) versus 4.3% (SE 1.1%), P = 0.005) during the treatment period (Maki 2010).
HDL cholesterol
Eight studies reported HDL cholesterol, and data from seven studies (eight comparators) could be summarised in a meta‐analysis (Analysis 1.5). The pooled analysis showed no effect on HDL cholesterol (MD ‐0.02, 95% CI ‐0.05 to 0.01; 772 participants). There was no substantial heterogeneity. In the one study that could not be summarised in the meta‐analysis, there were no differences in HDL cholesterol between the whole grain groups and the control group (Brownlee 2010).
1.5. Analysis.

Comparison 1: Whole grain versus control, Outcome 5: HDL cholesterol change (mmol/L)
Triglycerides
Eight studies reported triglyceride levels, and data from seven studies (eight comparators) could be summarised in a meta‐analysis (Analysis 1.6). The pooled analysis showed no effect on triglycerides (MD 0.03, 95% CI ‐0.08 to 0.13; 771 participants). There was no heterogeneity. In the one study that could not be summarised in the meta‐analysis, there were no differences in triglyceride levels between the whole grain groups and the control group (Brownlee 2010)..
1.6. Analysis.

Comparison 1: Whole grain versus control, Outcome 6: Triglycerides change (mmol/L)
Blood pressure
Systolic blood pressure
Eight studies reported systolic blood pressure, and data from seven studies (eight comparators) could be summarised in a meta‐analysis (Analysis 1.7).The pooled analysis showed no effect on systolic blood pressure (MD 0.04, 95% CI ‐1.67 to 1.75; 768 participants). There was no substantial heterogeneity. In the study that could not be included in the meta‐analysis (Brownlee 2010), there were no effects of whole grain foods compared with the control group.
1.7. Analysis.

Comparison 1: Whole grain versus control, Outcome 7: Systolic blood pressure change (mmHg)
Diastolic blood pressure
Eight studies reported diastolic blood pressure, and data from seven studies (eight comparators) could be summarised in a meta‐analysis (Analysis 1.8). The pooled analysis showed no effect on diastolic blood pressure (MD 0.16, 95% CI ‐0.89 to 1.21; 768 participants). There was no heterogeneity. In the study that could not be included in the meta‐analysis (Brownlee 2010), there were no effects of whole grain foods compared with the control group.
1.8. Analysis.

Comparison 1: Whole grain versus control, Outcome 8: Diastolic blood pressure (mmHg)
Quality of life
No studies reported quality of life as an outcome measure, although one study did report measures of satisfaction with the diets (Katcher 2008). There were no differences between intervention and control groups for "sense of a healthy lifestyle", "convenience", "family dynamics", "preoccupation with food", and "negative feelings", but those participants on the whole grain diets did report more (P = 0.04) difficulty with meal planning and preparation.
Adverse events
Two studies reported adverse events. In one study (Maki 2010), the frequencies of adverse events of any type (whether related to the study products or not) were reported to be similar between groups (59.8% for the whole grain group and 52.4% for the control group, P = 0.321). The most common adverse events in both groups were respiratory tract infection, sinusitis, and pharyngitis. Adverse events the authors considered to be related to the study products were: nausea (2 participants in the whole grain oat group), flatulence (2 participants in the whole grain oat group), gastroenteritis (1 participant in the control group), gastroesophageal reflux (1 participant in the control group), and vomiting (1 participant in the control group). Adverse events that led to drop out from the study were an infectious cyst (1 control) and spinal stenosis (1 control), but the study authors did not consider these to be related to the study product. In the Brownlee 2010 study, 3 participants in both whole grain groups (different levels of whole grain) and no participants in the control group reported intolerance to study foods.
Discussion
Summary of main results
This systematic review summarised nine RCTs examining the effect of whole grain foods or diets compared with refined grain foods or a usual diet control on risk factors for cardiovascular disease over 12 weeks or longer. All studies were conducted in primary‐prevention populations. None of the studies reported on our primary outcomes of mortality (total or cardiovascular) or cardiovascular events. The nine included studies reported the effect of whole grain diets on the secondary outcomes of this review, that is lipids or blood pressure, or both. We assessed all included studies as being at high or unclear risk of bias. The duration of the interventions in the included studies ranged from 12 to 16 weeks.
Overall, our meta‐analysis found no differences between whole grain and control groups for any lipid or blood pressure outcomes, with similar macronutrient contents between whole grain and control groups. Weight or BMI (as potential confounders) also did not differ between groups. Heterogeneity was low or non‐substantial.
Overall completeness and applicability of evidence
While there is growing evidence from observational studies that whole grains have benefits for cardiovascular disease, we found insufficient evidence from RCTs of a duration of 12 weeks or longer to draw any conclusions.
We found no studies on the effect of whole grains on CVD mortality or events. The included studies reported lipid and blood pressure outcomes. Only a few trials reported quality of life or adverse events.
We included studies in this review if there was a comparison between a whole grain food or diet and a diet or food containing no whole grains or fewer whole grains. We did not aim to assess the effects of dietary fibre, although any beneficial effects of whole grains may well be associated with the dietary fibre content (Brown 1999). We have reported the dietary fibre content of the foods or diets in Table 2, and, as would be expected, it is consistently higher in the whole grain diets than in the control diets.
Most of the studies reported the effect of predominantly whole grain wheat, with one study reporting oats (Maki 2010), one brown rice (Zhang 2011), and one study reporting the effect of combined whole grain wheat and oats (Tighe 2010‐WO). There is a lack of evidence to date from longer‐term studies (> 12 weeks) of the effect of wholegrain oats. The one trial that studied oats exclusively found reductions in total and LDL cholesterol with whole grain oat cereal compared to the control (Maki 2010). Oats (and barley) also typically contain soluble non‐starch polysaccharide such as β‐glucan, which has been associated with a cholesterol‐lowering effect of oats (Whitehead 2014). Due to the presence of β‐glucan, it is possible that whole grain oats may have differential effects than other whole grains. There is a need for more studies of longer duration on the effect of whole grain oats on CVD and cardiovascular risk factors (the review by Whitehead 2014 included β‐glucan in a range of different forms, not just as whole grain oats). Regarding studies that examined whole grain wheat, there were no differences between whole grain and control groups for individual studies or when studies were pooled in meta‐analysis (Analysis 1.3; Analysis 1.4).
It is possible that body weight change may account for any observed changes in cardiovascular risk factors. While we have reported weight data in this review, most of the studies had insufficient power to measure small changes in weight. We also included studies in the review if the only difference in dietary composition was substitution of refined grains by whole grains, and therefore the macronutrient contents and the overall energy intake of the diets were similar.
Quality of the evidence
Most of the included studies had a number of methodological uncertainties, and all of the included studies were at high or unclear risk of bias. However, heterogeneity was low or non‐substantial. The duration of interventions included in the review was short, sample sizes were small, and many trials had a large number of dropouts. Adverse events were generally not reported. These factors make overall interpretation of the findings of the review difficult.
We aimed to assess the overall quality of the evidence for each primary outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. The included studies were RCTs, but they did not report the primary outcomes (n = 6) of this review, therefore we could not assess the overall quality (Table 1). For secondary outcomes, we assessed total cholesterol, which we downgraded by two levels: for inconsistency due to variability in the direction of effect (Appendix 1), and for imprecision because "clinical action would differ if the upper versus the lower boundary of the confidence interval represented the truth" (Guyatt 2011)
Potential biases in the review process
We carried out a comprehensive search across major databases for interventions involving whole grains for this review. In addition, we screened the reference lists of systematic reviews and contacted authors for information when needed. Two review authors carried out all screening, inclusion and exclusion, and data abstraction, and one review author conducted analyses, which a second review author checked.
We excluded a number of trials of short duration (< 12 weeks), as we were interested in the sustained and longer‐term effects of increased whole grain intake, which are more relevant to public health interventions. However, this did limit the number of trials eligible for inclusion.
Agreements and disagreements with other studies or reviews
Evidence from two different meta‐analyses of observational cohort studies suggests that those consuming 48 to 80 g/day (3 to 5 servings/day) compared to rare consumers of whole grains (Ye 2012), or 2.5 servings/day compared to 0.2 servings/day, have a 21% lower risk of CVD (Mellen 2008). However, observational studies can be confounded by variables that cannot be controlled or that remain unaccounted for, which can impact on the results. However, to our knowledge there are no previous systematic reviews of RCTs examining the effects of whole grains on CVD risk factors and clinical events. The intervention length in the studies included in this review ranged from 12 to 16 weeks. In contrast, many of the observational studies that report beneficial effects of whole grains have follow‐up periods of many years (Ye 2012).
The current review is limited in its findings. All trials were short term and hence none reported clinical events, and there was insufficient information on CVD risk factors to be able to draw any conclusions about the effect of whole grain diets on cardiovascular health.
Short‐term trials (< 6 weeks) have reported beneficial effects of whole grain oats on lipids (Johnston 1998; Karmally 2005), but these are very short‐term studies, and it is unclear if such effects are sustained over the long term.
Authors' conclusions
Implications for practice.
We found no evidence from randomised controlled trials (RCTs) of the effect of whole grain diets or foods on cardiovascular mortality or events. Only evidence relating to the effect of whole grains on lipids or blood pressure is currently available, and most studies were at unclear or high risk of bias, with the longest study followed up to only 16 weeks. It is unclear to what extent whole grain foods contribute to cardiovascular risk. The results from this review do not support changing dietary habits of patients for short periods of time to obtain better control of cardiovascular risk factors. There may be a benefit if diet is changed on a long‐term basis but the RCT evidence from longer term studies is not currently available.
Implications for research.
There is currently no evidence from RCTs of the effect of whole grain diets on cardiovascular mortality or events. This is a clear gap in the evidence, and well‐designed, adequately powered studies are needed of sufficient duration to assess the impact of whole grain diets on cardiovascular mortality or events. The Global Burden of Disease Study attributed 1.7 million deaths worldwide in 2015 to ischaemic heart disease, and 3.1 million deaths from all causes to low whole grain diets (Global Burden of Disease Study 2015). These figures were derived from longitudinal cohort studies, and there is a need for RCTs in this area. While this review found 9 studies that reported the effects of whole grain diets on lipids or blood pressure, most studies were at unclear or high risk of bias, and no study followed up for longer than 16 weeks. There is also a need for well‐designed, adequately powered, long‐term (follow‐up at one year or more) RCTs to ascertain the effects of whole grains in the primary and secondary prevention of cardiovascular disease. While there are a number of short‐term studies on oats (< 12 weeks) (Kelly 2007), there are few longer‐term studies on oats (12 weeks or longer). The higher soluble non‐starch polysaccharide content, such as ß‐glucan, of oats (and barley) has previously been linked with beneficial effects on risk factors for cardiovascular disease, but there are insufficient trials of 12 weeks or longer with oats.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2021 | Amended | Due to recent systematic reviews on the same topic, albeit with slightly different inclusion criteria, but with similar findings, the authors concluded that an update of this Cochrane review is not currently a priority (https://doi.org/10.1186/s12872-020-01337-z; https://doi.org/10.1016/j.jand.2020.06.021; https://doi.org/10.1016/S0140-6736(18)31809-9). |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 31 August 2016 | New search has been performed | Search for update run on 31 August 2016. |
| 31 August 2016 | New citation required and conclusions have changed | The review was updated and the inclusion criteria were expanded to include all cardiovascular disease and not just coronary heart disease and studies examining both primary and secondary prevention of cardiovascular disease.. Since the last update of this review, a separate Cochrane Review has been published focusing on the effects of whole grain foods for the prevention of type 2 diabetes mellitus (Priebe 2008). Hence, we excluded studies with diabetes as an outcome or changes in related risk factors including impaired glucose tolerance, insulin resistance or sensitivity, glucose or insulin outcomes. We excluded studies reporting only weight, body mass index, and other anthropometric outcomes if they did not also measure lipids or blood pressure. We included studies in healthy participants to capture both primary and secondary prevention of cardiovascular disease. As more longer‐term trials are currently available, we have excluded very short‐term studies and included only those of at least 12 weeks' duration. We specified an eligible participant age of inclusion of 18 years or older (previously ≥ 16 years). |
| 15 February 2008 | Amended | Converted to new review format. |
| 15 January 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Current update
We are grateful to Nicole Martin for conducting the searches for this review. With thanks also to Dr Frank Thies for providing additional data for the trial on which he was an author (Tighe 2010‐W; Tighe 2010‐WO).
Previous versions of this review
Margaret Burke, Cochrane Heart Group for advice on the search strategy and for assistance with translation of a paper. Lone Gale for assistance with translation of a paper.
We acknowledge the contribution of Carolyn Summerbell, Audrey Brynes, and Victoria Whittaker to the previous versions of this review.
Appendices
Appendix 1. Checklist to aid consistency and reproducibility of GRADE assessments
| Total cholesterol change | ||
| Study limitations (risk of bias) | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Unclear (half of the studies low risk and the other half unclear) | |
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | N/A (not applicable in lifestyle interventions) | |
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | No (does not affect objective outcomes) | |
| 5. Was an objective outcome used? | Yes | |
| 6. Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?a | Yes | |
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | |
| 8. No other biases reported (i.e. no potential of other bias)? | Possible risk of attrition bias | |
| 9. Did the trials end up as scheduled (i.e. not stopped early)? | Yes | |
| Inconsistency | 1. Point estimates did not vary widely? | No (some variability) (↓) |
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier, where the confidence intervals of some of the studies do not overlap with those of most included studies)? | Some | |
| 3. Was the direction of effect consistent? | No (↓) | |
| 4. What was the magnitude of statistical heterogeneity (as measured by I2) ‐ low (I2 < 40%), moderate (I2 40% to 60%), high (I2 > 60%)? | Low | |
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | No | |
| Indirectness | 1. Were the populations in included studies applicable to the decision context? | Yes |
| 2. Were the interventions in the included studies applicable to the decision context? | Yes | |
| 3. Was the included outcome not a surrogate outcome? | No (however relevant risk factors) | |
| 4. Was the outcome timeframe sufficient? | Yes | |
| 5. Were the conclusions based on direct comparisons? | Yes | |
| Imprecision | 1. Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) |
| 2. What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: <100 participants)?a | Intermediate (103 participants) | |
| 3. What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?a | Moderate | |
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | |
| Publication bias | 1. Was a comprehensive search conducted? | Yes |
| 2. Was grey literature searched? | No | |
| 3. Were no restrictions applied to study selection on the basis of language? | No | |
| 4. There was no industry influence on studies included in the review? | Some industry support but all declared | |
| 5. There was no evidence of funnel plot asymmetry? | N/A | |
| 6. There was no discrepancy in findings between published and unpublished trials? | N/A | |
|
aDepends on the context of the systematic review area. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of findings' table(s); N/A: not applicable | ||
Appendix 2. Search strategies for previous versions of this review
MEDLINE OVID (previous version of this review)
1. wholegrain$.ab,ti. 2. wholemeal$.ab,ti. 3. wholewheat$.ab,ti. 4. (whole adj3 grain$).ab,ti. 5. (whole adj3 meal).ab,ti. 6. (whole adj3 wheat).ab,ti. 7. (whole adj3 food$).ab,ti. 8. (wheat adj3 meal).ab,ti. 9. cereal$.ab,ti. 10. bread$.ab,ti. 11. wheat$.ab,ti. 12. oat$.ab,ti. 13. rye$.ab,ti. 14. barley$.ab,ti. 15. maize$.ab,ti. 16. corn.ab,ti. 17. cornmeal.ab,ti. 18. popcorn.ab,ti. 19. sorghum$.ab,ti. 20. (bulgar or bulghar).ab,ti. 21. couscous$.ab,ti. 22. grain$.ab,ti. 23. porridge.ab,ti. 24. rice$.ab,ti. 25. millet$.ab,ti. 26. exp CEREALS/ 27. exp BREAD/ 28. exp Dietary Fiber/ 29. exp Coronary Disease/ 30. exp Cardiovascular Diseases/ 31. heart disease$.tw. 32. coronary disease$.tw. 33. chd.tw. 34. cardiovascular.tw. 35. angina.tw. 36. cvd.tw. 37. exp CHOLESTEROL/ 38. exp Blood Pressure/ 39. exp Obesity/ 40. exp Insulin Resistance/ 41. exp Diabetes Mellitus/ 42. exp LIPIDS/ 43. insulin resistance.ab,ti. 44. insulin sensitivity.ab,ti. 45. (glyc?emic adj3 control).ab,ti. 46. or/1‐28 47. or/29‐45 48. 46 and 47
Embase OVID (previous version of this review)
1. wholegrain$.ab,ti. 2. wholemeal$.ab,ti. 3. wholewheat$.ab,ti. 4. (whole adj3 grain$).ab,ti. 5. (whole adj3 meal$).ab,ti. 6. (whole adj3 wheat).ab,ti. 7. (whole adj3 food$).ab,ti. 8. (wheat adj3 meal).ab,ti. 9. cereal$.ab,ti. 10. bread.ab,ti. 11. breads.ab,ti. 12. wheat$.ab,ti. 13. oat$.ab,ti. 14. rye$.ab,ti. 15. barley$.ab,ti. 16. maize.ab,ti. 17. corn.ab,ti. 18. cornmeal.ab,ti. 19. popcorn.ab,ti. 20. sorghum$.ab,ti. 21. (bulgar or bulghar).ab,ti. 22. couscous.ab,ti. 23. grain.ab,ti. 24. grains.ab,ti. 25. porridge.ab,ti. 26. exp Cereal/ 27. exp BREAD/ 28. exp Dietary Fiber/ 29. exp Coronary Artery Disease/ 30. exp Cardiovascular Disease/ 31. heart disease$.tw. 32. coronary disease$.tw. 33. chd.tw. 34. cardiovascular.tw. 35. angina.tw. 36. cvd.tw. 37. exp CHOLESTEROL/ 38. exp Blood Pressure/ 39. exp OBESITY/ 40. exp Insulin Resistance/ 41. (glyc?emic adj3 control).ab,ti. 42. exp Diabetes Mellitus/ 43. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 44. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 45. 43 and 44 46. Controlled Study/ 47. Clinical Trial/ 48. random$.tw. 49. compar$.ab,ti. 50. control$.ab,ti. 51. study.ab,ti. 52. follow$ up.ab,ti. 53. clinic$.ab,ti. 54. blind$.ab,ti. 55. Double Blind Procedure/ 56. double$.ab,ti. 57. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 58. 45 and 57 59. limit 58 to human
CINAHL (previous version of this review)
1. wholegrain$.ab,ti. 2. wholemeal$.ab,ti. 3. wholewheat$.ab,ti. 4. (whole adj3 grain$).ab,ti. 5. (whole adj3 meal).ab,ti. 6. (whole adj3 wheat).ab,ti. 7. (whole adj3 food$).ab,ti. 8. (wheat adj3 meal).ab,ti. 9. cereal$.ab,ti. 10. bread$.ab,ti. 11. wheat$.ab,ti. 12. oat$.ab,ti. 13. rye$.ab,ti. 14. barley$.ab,ti. 15. maize$.ab,ti. 16. corn.ab,ti. 17. cornmeal.ab,ti. 18. popcorn.ab,ti. 19. sorghum$.ab,ti. 20. (bulgar or bulghar).ab,ti. 21. couscous$.ab,ti. 22. grain$.ab,ti. 23. porridge.ab,ti. 24. rice$.ab,ti. 25. millet$.ab,ti. 26. exp CEREALS/ 27. exp BREAD/ 28. exp Dietary Fiber/ 29. exp Coronary Disease/ 30. exp Cardiovascular Diseases/ 31. heart disease$.tw. 32. coronary disease$.tw. 33. chd.tw. 34. cardiovascular.tw. 35. angina.tw. 36. cvd.tw. 37. exp CHOLESTEROL/ 38. exp Blood Pressure/ 39. exp Obesity/ 40. exp Insulin Resistance/ 41. exp Diabetes Mellitus/ 42. exp LIPIDS/ 43. insulin resistance.ab,ti. 44. insulin sensitivity.ab,ti. 45. (glyc?emic adj3 control).ab,ti. 46. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 26 or 27 or 28 47. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 43 or 44 or 45 48. 46 and 47 49. clinical trial.pt. 50. exp Clinical Trials/ 51. (clin$ adj25 trial$).tw. 52. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. 53. exp PLACEBOS/ 54. placebo$.tw. 55. random$.tw. 56. exp Evaluation Research/ 57. exp Prospective Studies/ 58. exp Random Assignment/ 59. exp Random Sample/ 60. exp Crossover Design/ 61. exp Comparative Studies/ 62. 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 63. 48 and 62
CENTRAL (previous version of this review)
1. wholegrain$.af. 2. wholemeal$.af. 3. wholewheat$.af. 4. (whole adj3 grain$).af. 5. (whole adj3 meal$).af. 6. (whole adj3 wheat$).af. 7. (whole adj3 food$).af. 8. (wheat adj3 meal$).af. 9. cereals.af. 10. bread$.af. 11. wheat$.af. 12. oat$.af. 13. rye$.af. 14. barley$.af. 15. maize$.af. 16. corn.af. 17. cornmeal.af. 18. popcorn.af. 19. sorghum$.af. 20. (bulgar or bulghar).af. 21. couscous$.af. 22. grain$.af. 23. porridge$.af. 24. (rice$:ti or rice$:ab).af. 25. millet$.af. 26. cereals.sh. 27. bread.sh. 28. dietary fiber.sh. 29. (diet$ adj fiber$).af. 30. (diet$ adj fiber$).af. 31. or/1‐30 32. cardiovascular diseases.sh. 33. (heart adj disease$).af. 34. chd.af. 35. (coronary adj3 disease$).af. 36. cardiovascular.af. 37. angina.af. 38. cvd.af. 39. cholesterol.sh. 40. cholesterol.tw. 41. (blood adj pressure).af. 42. blood pressure.sh. 43. hypertension.sh. 44. hypertension.tw. 45. obesity.sh. 46. obesity.tw. 47. obese.af. 48. insulin resistance.af. 49. (insulin adj resistance).af. 50. (metabolic adj syndrome).af. 51. diabetes mellitus.sh. 52. diabetes.af. 53. (insulin adj sensitivity).af. 54. (glycemic adj3 control).af. 55. (glycaemic adj3 control).af. 56. hyperlipidemia.af. 57. hyperlipidaemia.af. 58. hyperlipidemia.sh. 59. or/32‐58 60. 31 and 59 1. wholegrain$.af. 2. wholemeal$.af. 3. wholewheat$.af. 4. (whole adj3 grain$).af. 5. (whole adj3 meal$).af. 6. (whole adj3 wheat$).af. 7. (whole adj3 food$).af. 8. (wheat adj3 meal$).af. 9. cereals.af. 10. bread$.af. 11. wheat$.af. 12. oat$.af. 13. rye$.af. 14. barley$.af. 15. maize$.af. 16. corn.af. 17. cornmeal.af. 18. popcorn.af. 19. sorghum$.af. 20. (bulgar or bulghar).af. 21. couscous$.af. 22. grain$.af. 23. porridge$.af. 24. (rice$:ti or rice$:ab).af. 25. millet$.af. 26. cereals.sh. 27. bread.sh. 28. dietary fiber.sh. 29. (diet$ adj fiber$).af. 30. (diet$ adj fiber$).af. 31. or/1‐30 32. cardiovascular diseases.sh. 33. (heart adj disease$).af. 34. chd.af. 35. (coronary adj3 disease$).af. 36. cardiovascular.af. 37. angina.af. 38. cvd.af. 39. cholesterol.sh. 40. cholesterol.tw. 41. (blood adj pressure).af. 42. blood pressure.sh. 43. hypertension.sh. 44. hypertension.tw. 45. obesity.sh. 46. obesity.tw. 47. obese.af. 48. insulin resistance.af. 49. (insulin adj resistance).af. 50. (metabolic adj syndrome).af. 51. diabetes mellitus.sh. 52. diabetes.af. 53. (insulin adj sensitivity).af. 54. (glycemic adj3 control).af. 55. (glycaemic adj3 control).af. 56. hyperlipidemia.af. 57. hyperlipidaemia.af. 58. hyperlipidemia.sh. 59. or/32‐58 60. 31 and 59
ProQuest Digital Dissertations (previous version of this review)
AB (wholegrain*) or AB (wholemeal) or AB (grain*) or AB (wheat*) or AB (cereal*) or AB ( bread* ) or AB (oat*) or AB (rye*) or AB (barley*) or AB (maize*) or AB (corn*) or AB ( sorghum*) or AB (bulgar*) or AB (bulghar*) or AB (couscous*) or AB (grain*) or AB (porridge*) or AB ( rice*) or AB (millet*)
Appendix 3. Search strategies for this updated review
CENTRAL
#1wholegrain* #2wholemeal* #3wholewheat* #4(whole near/3 meal*) #5(whole near/3 food*) #6cereal* #7bread* #8wheat* #9oat* #10rye* #11barley* #12maize* #13corn #14cornmeal #15popcorn #16sorghum* #17bulgar or bulghar #18couscous* #19grain* #20porridge* #21rice* #22millet* #23MeSH descriptor: [Cereals] explode all trees #24MeSH descriptor: [Bread] this term only #25MeSH descriptor: [Dietary Fiber] explode all trees #26(diet* next fiber*) #27bulgur #28roughage* #29triticale #30farro #31emmer #32einkorn #33spelt #34quinoa #35amaranth #36teff #37#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #38#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 #39#21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 #40#31 or #32 or #33 or #34 or #35 or #36 #41#37 or #38 or #39 or #40 #42#37 or #38 or #39 or #40 #43MeSH descriptor: [Cardiovascular Diseases] explode all trees #44heart near/2 disease* #45coronary near/3 disease* #46chd #47cardiovascular #48angina #49cvd #50MeSH descriptor: [Cholesterol] explode all trees #51cholesterol #52blood near/2 pressure #53MeSH descriptor: [Blood Pressure] explode all trees #54MeSH descriptor: [Hypertension] explode all trees #55hypertensi* #56MeSH descriptor: [Obesity] explode all trees #57obes* #58insulin next resistan* #59metabolic next syndrome* #60MeSH descriptor: [Diabetes Mellitus] explode all trees #61diabetes #62insulin next sensitiv* #63glycemic near/3 control* #64glycaemic near/3 control* #65MeSH descriptor: [Hyperlipidemias] explode all trees #66MeSH descriptor: [Overweight] explode all trees #67MeSH descriptor: [Glucose Metabolism Disorders] explode all trees #68MeSH descriptor: [Hyperinsulinism] explode all trees #69cardio* near/6 risk* #70overweight #71over‐weight #72hdl or ldl #73hyperlip* #74lipid* #75hyperglycem* #76hyperglycaem* #77#43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 #78#53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 #79#63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 #80#77 or #78 or #79 #81#42 and #80
MEDLINE Ovid
1. wholegrain*.tw. 2. wholemeal*.tw. 3. wholewheat*.tw. 4. (whole adj3 meal*).tw. 5. (whole adj3 food*).tw. 6. cereal*.tw. 7. bread*.tw. 8. wheat*.tw. 9. oat*.tw. 10. rye*.tw. 11. barley*.tw. 12. maize*.tw. 13. corn.tw. 14. cornmeal.tw. 15. popcorn.tw. 16. sorghum*.tw. 17. (bulgar or bulghar).tw. 18. couscous*.tw. 19. grain*.tw. 20. porridge.tw. 21. rice*.tw. 22. millet*.tw. 23. exp Dietary Fiber/ 24. Bread/ 25. exp Cereals/ 26. bulgur.tw. 27. (dietary adj2 fiber*).tw. 28. roughage*.tw. 29. triticale.tw. 30. farro.tw. 31. emmer.tw. 32. einkorn.tw. 33. spelt.tw. 34. quinoa.tw. 35. amaranth.tw. 36. teff.tw. 37. or/1‐36 38. exp Cardiovascular Diseases/ 39. (heart adj2 disease*).tw. 40. (coronary adj2 disease*).tw. 41. chd.tw. 42. cardiovascular.tw. 43. angina*.tw. 44. cvd.tw. 45. exp Cholesterol/ 46. exp blood pressure/ 47. exp Obesity/ 48. exp Hyperinsulinism/ 49. exp Hyperlipidemias/ 50. exp Glucose Metabolism Disorders/ 51. insulin resistan*.tw. 52. insulin sensitiv*.tw. 53. (glyc?emic adj3 control).tw. 54. exp Hypertension/ 55. exp Overweight/ 56. (cardio* adj6 risk*).tw. 57. (blood adj2 pressure).tw. 58. overweight.tw. 59. obes*.tw. 60. over‐weight.tw. 61. cholesterol.tw. 62. (hdl or ldl).tw. 63. hyperlip*.tw. 64. lipid*.tw. 65. hyperglyc?em*.tw. 66. hypertens*.tw. 67. diabet*.tw. 68. or/38‐67 69. 37 and 68 70. randomized controlled trial.pt. 71. controlled clinical trial.pt. 72. randomized.ab. 73. placebo.ab. 74. drug therapy.fs. 75. randomly.ab. 76. trial.ab. 77. groups.ab. 78. 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 79. exp animals/ not humans.sh. 80. 78 not 79 81. 69 and 80
Embase Ovid
1. wholegrain*.tw. 2. wholemeal*.tw. 3. wholewheat*.tw. 4. (whole adj3 meal*).tw. 5. (whole adj3 food*).tw. 6. cereal*.tw. 7. bread*.tw. 8. wheat*.tw. 9. oat*.tw. 10. rye*.tw. 11. barley*.tw. 12. maize*.tw. 13. corn.tw. 14. cornmeal.tw. 15. popcorn.tw. 16. sorghum*.tw. 17. (bulgar or bulghar).tw. 18. couscous*.tw. 19. grain*.tw. 20. porridge.tw. 21. exp cereal/ 22. dietary fiber/ 23. rice*.tw. 24. millet*.tw. 25. bulgur.tw. 26. (dietary adj2 fiber*).tw. 27. roughage*.tw. 28. triticale.tw. 29. farro.tw. 30. emmer.tw. 31. einkorn.tw. 32. spelt.tw. 33. quinoa.tw. 34. amaranth.tw. 35. teff.tw. 36. or/1‐35 37. exp coronary artery disease/ 38. exp cardiovascular disease/ 39. (heart adj2 disease*).tw. 40. (coronary adj2 disease*).tw. 41. chd.tw. 42. cardiovascular.tw. 43. angina*.tw. 44. cvd.tw. 45. exp cholesterol/ 46. exp blood pressure/ 47. exp Obesity/ 48. exp "disorders of carbohydrate metabolism"/ 49. (glyc?emic adj3 control).tw. 50. insulin resistan*.tw. 51. insulin sensitiv*.tw. 52. exp Hypertension/ 53. exp Overweight/ 54. (cardio* adj6 risk*).tw. 55. (blood adj2 pressure).tw. 56. overweight.tw. 57. obes*.tw. 58. over‐weight.tw. 59. cholesterol.tw. 60. (hdl or ldl).tw. 61. hyperlip*.tw. 62. lipid*.tw. 63. hyperglyc?em*.tw. 64. hypertens*.tw. 65. exp hyperinsulinism/ 66. exp hyperlipidemia/ 67. diabet*.tw. 68. or/37‐67 69. 36 and 68 70. random$.tw. 71. factorial$.tw. 72. crossover$.tw. 73. cross over$.tw. 74. cross‐over$.tw. 75. placebo$.tw. 76. (doubl$ adj blind$).tw. 77. (singl$ adj blind$).tw. 78. assign$.tw. 79. allocat$.tw. 80. volunteer$.tw. 81. crossover procedure/ 82. double blind procedure/ 83. randomized controlled trial/ 84. single blind procedure/ 85. 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 86. (animal/ or nonhuman/) not human/ 87. 85 not 86 88. 69 and 87 89. limit 88 to embase
CINAHL Plus
S68 S37 AND S67
S67 S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66
S66TI diabet* or AB diabet*
S65 TI hypertens* or AB hypertens*
S64 TI hyperglycaem* or AB hyperglycaem*
S63 TI hyperglycem* or AB hyperglycem*
S62 TI lipid* or AB lipid*
S61 TI hyperlip* or AB hyperlip*
S60 (TI hdl or ldl) or (AB hdl or ldl)
S59 TI cholesterol or AB cholesterol
S58 TI obes* or AB obes*
S57 TI over‐weight or AB over‐weight
S56 TI overweight or AB overweight
S55 TI blood N2 pressure or AB blood N2 pressure
S54 TI cardio* N6 risk* or AB cardio* N6 risk*
S53 (MH "Hypertension")
S52 TI glycaemic N3 control* or AB glycaemic N3 control*
S51 TI glycemic N3 control* or AB glycemic N3 control*
S50 TI insulin N2 sensitiv* or AB insulin N2 sensitiv*
S49 TI insulin N2 resist* or AB insulin N2 resist*
S48 (MH "Metabolic Diseases+")
S47 (MH "Obesity")
S46 (MH "Blood Pressure+")
S45 (MH "Cholesterol")
S44 TI cvd or AB cvd
S43 TI angina* or AB angina*
S42 TI cardiovascular or AB cardiovascular
S41 TI chd or AB chd
S40 (TI coronary N2 disease*) or (AB coronary N2 disease*)
S39 (TI heart N2 disease*) or (AB heart N2 disease*)
S38 (MH "Cardiovascular Diseases+")
S37 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36
S36 TI teff or AB teff
S35 TI amaranth or AB amaranth
S34 TI quinoa or AB quinoa
S33 TI spelt or AB spelt
S32 TI einkorn or AB einkorn
S31 TI emmer or AB emmer
S30 TI farro or AB farro
S29 TI triticale or AB triticale
S28 TI roughage* or AB roughage*
S27 TI dietary N2 fiber* or AB dietary N2 fiber*
S26 TI bulgur or AB bulgur
S25 (MH "Dietary Fiber")
S24 (MH "Bread")
S23 (MH "Cereals+")
S22 TI millet* or AB millet*
S21 TI rice* or AB rice*
S20 TI porridge* or AB porridge*
S19 TI grain* or AB grain*
S18 TI couscous* or AB couscous*
S17 (TI bulgar or bulghar) or (AB bulgar or bulghar)
S16 TI sorghum* or AB sorghum*
S15 TI popcorn or AB popcorn
S14 TI cornmeal or AB cornmeal
S13 TI corn or AB corn
S12 TI maize* or AB maize*
S11 TI barley* or AB barley*
S10 TI rye* or AB rye*
S9 TI oat* or AB oat*
S8 TI wheat* or AB wheat*
S7 TI bread* or AB bread*
S6 TI cereal* or AB cereal*
S5 (TI whole N3 food*) or (AB whole N3 food*)
S4 (TI whole N3 meal*) or (AB whole N3 meal*)
S3 TI wholewheat* or AB wholewheat*
S2 TI wholemeal* or AB wholemeal*
S1 TI wholegrain* or AB wholegrain*
ClinicalTrials.gov
Search terms: cardiovascular diseases, diet (under interventions), limited to interventions, adults and seniors, all study results chosen.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
Search terms: cardiovascular disease (condition), diet (intervention)
Data and analyses
Comparison 1. Whole grain versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Body weight change (kg) | 5 | 439 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.04, 0.23] |
| 1.2 BMI change | 5 | 516 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.24, 0.01] |
| 1.3 Total cholesterol change (mmol/L) | 7 | 722 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 1.4 LDL cholesterol change (mmol/L) | 8 | 770 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.05, 0.16] |
| 1.5 HDL cholesterol change (mmol/L) | 8 | 772 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 1.6 Triglycerides change (mmol/L) | 8 | 771 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.08, 0.13] |
| 1.7 Systolic blood pressure change (mmHg) | 8 | 768 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐1.67, 1.75] |
| 1.8 Diastolic blood pressure (mmHg) | 8 | 768 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.89, 1.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brownlee 2010.
| Study characteristics | ||
| Methods |
Setting: UK (Cambridge and Newcastle) Design: randomisation by minimisation with even distribution of participants in each group by age sex and BMI, parallel group. Dates: trial dates not reported Intervention duration: 16 weeks Follow‐up: no postintervention follow‐up Focus: to compare the effects of different levels of whole grain diets (2 intervention groups) to replace refined grains in the diets of overweight and obese people who were not whole grain consumers on markers of cardiovascular risk. |
|
| Participants |
N: 316 randomised (to 3 groups) (85/105 completers in the lower‐dose intervention group, 81/105 in the higher‐dose intervention group, and 100/106 in the control group). Inclusion criteria: overweight or obese adults, aged 18 to 65 years, BMI > 25 kg/m2, consuming < 30 g whole grains/day. Exclusion criteria: BMI < 25 kg/m2; habitual consumption of > 30 g/day whole grain; CVD, diabetes, or hyperlipidaemia; smoking > 20 cigarettes/day; history of substance abuse; recent weight change; desire to diet; or absence during a period of the intervention. Age (years): intervention (lower dose): 45.9 SD 10.1; intervention (higher dose): 45.7 SD 9.9; control: 45.6 SD 10.0 Sex (% men): intervention (lower dose): 50.0%; intervention (higher dose): 51.2%%; control: 49.0% Ethnicity: British (ethnic composition not reported) Baseline cardiovascular risk status: all reported as medians. BMI (kg/m2): intervention (lower dose): 30.0 SD 3.7; intervention (higher dose): 30.3 SD 4.5; control: 30.0 SD 4.0 Total cholesterol (mmol/L): intervention (lower dose): 5.1 SD 0.8; intervention (higher dose): 5.3 SD 1.0; control: 5.2 SD 1.0 HDL cholesterol (mmol/L): intervention (lower dose): 1.3 SD 0.3; intervention (higher dose): 1.3 SD 0.2; control: 1.3 SD 0.3 LDL cholesterol (mmol/L): intervention (lower dose): 3.2 SD 0.7; intervention (higher dose): 3.3 SD 0.8; control: 3.2 SD 0.9 Systolic blood pressure (mmHg): intervention (lower dose): 125.5 SD 16.1; intervention (higher dose): 129.5 SD 15.5; control: 127.3 SD 14.8 Diastolic blood pressure (mmHg): intervention (lower dose): 79.0 SD 9.8; intervention (higher dose): 79.0 SD 9.3; control: 79.8 SD 10.2 Medications used: not reported |
|
| Interventions |
Whole grain group 1: (lower whole grain; 60 g WG/d as 3 servings for 16 weeks) (n = 105 randomised, 85 completed) Whole grain group 2: (higher whole grain; 60 g WG/d as 3 servings for 8 weeks, then 120 g WG/d as 5 servings for 8 weeks) (n = 105 randomised, 81 completed) Control: (usual diet) (n = 106 randomised, 100 completed) Description of dietary intervention: the 2 intervention groups were provided with a range of whole grain foods (whole wheat bread, Shredded Wheat Fruitful, Cheerios, porridge oats, brown basmati rice, whole wheat pasta, Weetabix, porridge, oat bars, and whole grain crisps) and asked to substitute ‘like for like’ for refined grain foods to a prescribed amount. The approach was chosen to reflect choices of whole grains in a free‐living population. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: FFQ (7‐day recall) at weeks 8 and 16 Was the diet energy reduced? no Comparability of diet composition: There was an (P < 0.05) increase in carbohydrate intake in both whole grain groups at week 8 and week 16, and between control and whole grain group 1 and whole grain group 2. There was also an increase in total energy intake in whole grain group 1 at week 8 and wholegrain group 2 at week 16. There was an increase in protein in whole grain group 1 at week 8 and whole grain group 2 at week 16. Percentage energy from fat decreased in whole grain group 2 at weeks 8 and 16. Dietary fibre increased in both whole grain groups at both week 8 and week 16. (See Table 2.) Change in diet over time: Whole grain intake (estimated from FFQ data): at baseline WG intake was < 20 g/day for each group; for the control group, WG intake averaged 19 (SD 19.9) g/d; for whole grain group 1, WG intake was 74 (SD 28.5) g/d averaged for weeks 8 and 16 (no significant difference between week 8 and 16); for whole grain group 2, WG intake was 83 (SD 3) g/d at week 8 and 115 (SD 39.6) g/d at week 16. (See Table 2.) There were differences between baseline and intervention groups in frequency of consumption of breads and breakfast cereals at week 16 (P < 0.05):
A difference between whole grain group 2 and control was seen for fruit intake after 16 weeks (p<0.005). There were no differences in other food groups (meat, fish, potatoes, rice and pasta, dairy and egg products, milk, spreads, sweets and snacks, vegetables. |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triacylglycerides (TAG; mmol/L), weight (kg), body fat (%), systolic BP (mm Hg), diastolic BP (mmHg), NEFA (mmol/L), markers of insulin sensitivity, endothelial function, inflammatory and coagulatory status. States no conflicts of interest for any authors. | |
| Funding/conflicts of interest | Fully funded by UK Food Standards Agency (project N02036). Study foods were provided by commercial suppliers (Cereal Partners Worldwide, Weetabix, Allied Bakeries, PepsiCo). | |
| Notes | The authors noted that "the participants appeared to include the whole grain foods as a dietary addition as opposed to the dietary substitution that was explicitly requested". This conclusion is supported by the diet composition data. Adverse effects: 3 participants in both whole grain groups reported intolerance to study foods, none in control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated (using an MS‐DOS based computer program at each study centre)"; minimisation was used to ensure even distribution within each group by age, sex, and BMI |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researcher collecting anthropometric data was not blind to allocation (secondary outcome). Lipid analysis not specifically reported to have been blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition greater in both whole grain intervention groups than in control group (85/105 completers in the lower‐dose intervention group, 81/105 in the higher‐dose intervention group, and 100/106 in the control group). Characteristics of those lost to follow‐up not completed. Some reasons provided but not for all dropouts. |
| Intention to treat analysis | Unclear risk | Not reported. Only outcomes for completers reported, so probably not done. |
| Selective reporting (reporting bias) | Unclear risk | Reports all relevant outcomes, but not enough information to judge. |
| Groups comparable at baseline | Low risk | Comparable for lipids (total, HDL, LDL, TAG), blood pressure, and anthropometry |
| Other bias | Low risk | Sample size of 254 participants was calculated to detect a 10% decrease in LDL cholesterol with a 0.05 significance level and 80% power. Allowing for an estimated dropout of 15%, a target of 100 participants per treatment group was proposed. This was achieved. |
Giacco 2013.
| Study characteristics | ||
| Methods |
Setting: Finland (Kuopio) and Italy (Naples) Design: parallel groups (with stratification for sex, age, and BMI). Dates: March 2008 to May 2014 Intervention duration: 12 weeks Follow‐up: no postintervention follow‐up Focus: to compare the effects of diets containing whole grain rye or wheat cereals compared to refined grains on markers of metabolic syndrome in people with metabolic syndrome. |
|
| Participants |
N: 146 randomised (62/71 completers in the whole grain intervention group and 61/75 in the control group). Inclusion criteria: male and female, aged 40 to 65 years, with metabolic syndrome (diagnosed on National Cholesterol Education Program criteria). Exclusion criteria: diabetes and/or renal failure (serum creatinine > 1.5 mg/dL), liver abnormalities (ALT/AST ratio 2 times above normal values), anaemia (haemoglobin < 12 g/dL), any other chronic disease, or if they used any drug able to influence glucose and lipid metabolism and inflammation (corticosteroid hormones other than inhaled corticosteroids, hypolipidaemic and/or anti‐inflammatory drugs); however, in the Kuopio study centre the use of cholesterol lowering medications (statins) was allowed. Age (years): 40 to 65 years, otherwise not reported. Sex (% men): whole grain intervention: 46.9%; control: 47.5% Ethnicity: Italian and Finnish (ethnic composition not reported) Baseline cardiovascular risk status: BMI (kg/m2): whole grain intervention: 31.6 (4.6); control: 31.3 (4.4) Total cholesterol (mmol/L): whole grain intervention: 5.15 (1.09); control: 5.28 (0.93) HDL cholesterol (mmol/L): whole grain intervention: 6 (0.36); control: 4 (0.31) LDL cholesterol (mmol/L): whole grain intervention: 3.26 (0.98); control: 3.41 (0.80) Systolic blood pressure (mmHg): whole grain intervention: 133 (15); control: 135 (14) Diastolic blood pressure (mmHg): whole grain intervention: 84 (9); control: 86 (8) Medications used: in the Kuopio study centre, some individuals were using cholesterol‐lowering medication (10 in the whole grain intervention group and 10 in the control group), however sensitivity analyses were conducted after excluding these people. |
|
| Interventions |
Whole grain diet group: (wheat and rye whole grain) Control: (refined cereal foods), differed between centres Description of dietary intervention: whole grain wheat and rye products (and smaller amounts of oat and barley), most with a low postprandial glucose and/or insulin response. The only difference between the whole grain and the control diet was the inclusion of a fixed amount of whole grain or refined cereal products as the main carbohydrate source. Aimed to include 90% sourdough bread and 10% endosperm rye bread. Naples ‐ whole grain products including whole wheat bread (plus some endosperm rye bread), whole wheat pasta, barley kernels, whole grain oat biscuits and breakfast cereals (all bran sticks and flakes). Kuopio ‐ whole grain and control diets aimed to include 20% to 25% of the total daily energy intake as study breads. The type of bread consumed by the Kuopio participants was 50% commercial whole grain rye bread, 40% endosperm rye bread, and 10% sourdough whole wheat bread, and participants were advised to replace their habitual potato consumption with 210 g dry weight of whole wheat pasta per week, and were given whole oat biscuits for snacks. Test products in both diets provided free of charge on a weekly basis. Also given written instructions and recipes. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: 4‐day and 7‐day food records and plasma alkylresorcinols Was the diet energy reduced? no Comparability of diet composition: energy intake increased in intervention and control groups P < 0.02 from baseline, but no significant difference between groups at 12 wks. Significant increased protein, PUFA, total fibre, and cereal fibre between WG group and control at 12 wks (P < 0.05). (See Table 2.) Change in diet over time: not reported |
|
| Outcomes | Peripheral insulin sensitivity assessed by FSIGT, lipids and inflammatory markers, body weight, blood pressure, waist circumference, short‐chain fatty acids | |
| Funding/conflicts of interest | European Commission (6th Framework Programme, project HEALTHGRAIN FOOD‐CT‐2005‐514008), Raisio plc Research Foundation (Raisio is a commercial organisation that makes cereal products), the Nordic Centre of Excellence (projects HELGA and SYSDIET). Barilla G&R F.lli.SpA, Parma, Italy and Raisio Nutrition Ltd, Finland provided some of the cereal products for the study participants. | |
| Notes | Stable body weight, body fat composition, and waist circumference maintained during the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by means of a computerised random allocation list. |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out by personnel not involved in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Anthropometric data collection does not appear to have been blind (secondary outcome). Lipid analysis appears to have been blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential rates of attrition between groups, and characteristics of those lost to follow‐up not reported (62/71 completers in the whole grain intervention group and 61/75 in the control group). |
| Intention to treat analysis | Unclear risk | Not reported. Only outcomes for completers reported, so probably not done. |
| Selective reporting (reporting bias) | Unclear risk | Reports all relevant outcomes, but not enough information to judge. |
| Groups comparable at baseline | Low risk | Comparable for lipids (total, HDL, LDL, TAG), blood pressure, anthropometry, and age |
| Other bias | Unclear risk | Power calculations used insulin sensitivity as outcome variable (not a primary outcome of this review). Power relevant to lipids, blood pressure, or BMI not reported. The 2 centres had different nutritional constituent parts and respective controls. 10 participants in each group had lipid‐lowering medications. |
Harris 2014.
| Study characteristics | ||
| Methods |
Setting: USA Design: randomised parallel study Dates: March 2009 to May 2011 Intervention duration: 12 weeks (6 weeks weight maintenance diet, followed by 6 weeks weight loss diet) Follow‐up: no postintervention follow‐up Focus: to compare the effects of whole grain diets compared to refined grains in people with or at risk of metabolic syndrome (overweight and obese individuals with increased waist circumference and 1 or more other MetS criteria). |
|
| Participants |
N: 60 randomised (25/28 completers in the whole grain intervention group and 25/32 in the control group). Inclusion criteria: male and female, aged 35 to 55 years (overweight and obese individuals with BMI 25 to 42 kg/m2; with increased waist circumference >/= 102 cm in men and >/= 88 cm in women; and 1 or more other MetS criteria: fasting plasma glucose >/= 100 mg/dL, fasting serum triglycerides >/= 150 mg/dL, BP >/= 130/85 mmHg and/or fasting serum HDL cholesterol < 50 mg/dL in women or < 40 mg/dL in men). Exclusion criteria: use of medications affecting glucose or lipid metabolism, frequent (> 4 times/wk) use of anti‐inflammatory medications, pregnancy or lactation, smoking, high alcohol intake (> 14 drinks/wk), and diagnosed CVD, diabetes, or inflammatory disease. Age (years): 35 to 55 years: whole grain: 46.4 (SD 5.9); control: 45.8 (SD 6.0) Sex (% men): whole grain: 48%; control: 52% Ethnicity: US (ethnic composition not reported) Baseline cardiovascular risk status: BMI (kg/m2): whole grain intervention: 32.9 (3.5); control: 33.5 (4.0) Total cholesterol (mmol/L): not reported HDL cholesterol (mmol/L): whole grain intervention: 6 (0.28); control: 1.06 (0.39) LDL cholesterol (mmol/L): not reported Systolic blood pressure (mmHg): whole grain intervention: 123 (12); control: 125 (12) Diastolic blood pressure (mmHg): whole grain intervention: 83 (10); control: 85(6) Medications used: whole grain group: 48%; control: 40%. Blood pressure medications were allowed. |
|
| Interventions |
Whole grain diet group (whole grain products, variety of grain types) Control (refined grain products) Description of dietary intervention: the whole grain diet group consumed all WG products from a variety of grain types; those receiving the control diet consumed the refined grain counterpart. The top 3 grains consumed were wheat, oats, and rice with the whole grain diet and wheat, rice, and corn with the refined grain diet. Wheat products constituted 77% of the whole grain diets and 63% of the control diets. Diets were tailored to individual energy requirements; the whole grain diets contained between 163 and 301 g/whole grain per day, and the refined grain diets no whole grains. Both diets comprised an isocaloric weight maintenance diet for 6 weeks followed by an energy‐reduced diet for the next 6 weeks (reduced by ~500 kilocalories/d). All meals and snacks were prepared at metabolic kitchens on the university campus. Participants had to go to the kitchen to pick up or eat their meals. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: compliance forms (all food provided) Was the diet energy reduced? yes Comparability of diet composition: both diets were designed to have the same macronutrient composition and to meet National Cholesterol Education Program guidelines for saturated fat (< 7% E), mono‐ and polyunsaturated fats (~10% and ~7% E), total cholesterol (< 200 mg/d), and total fibre (> 20 g/d). Participants consumed all study foods and did not consume any additional non‐study foods on 86% of reported days. Physical activity levels were stable. (See Table 2.) Change in diet over time: not reported |
|
| Outcomes | Primary: weight, BMI, waist circumference, triglycerides, HDL cholesterol, glucose, systolic BP, diastolic BP. Secondary: total adiponectin, HMW adiponectin, leptin, TC:HDL ratio, LDL cholesterol, CRP, IL‐6, TNF‐alpha, insulin, HOMA‐IR, RMR, % adipose tissue. | |
| Funding/conflicts of interest | Bell Institute of Health and Nutrition (General Mills, Inc.) and National Institutes of Health grant M01RR10732. The first author was supported by a Nestle fellowship, alkylresorcinol analysis was funded by Cereal Partners Worldwide (a joint venture between General Mills, Inc. and Nestle SA). | |
| Notes | To enhance compliance, participants were given the option of taking a 1‐ to 2‐week break after the first 6‐week diet period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned ... using a computer‐generated random number assignment" |
| Allocation concealment (selection bias) | Unclear risk | "An unblinded study coordinator stratified participants by age, sex and BMI" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome assessors (i.e. nurses and technicians) were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for attrition reported: 7 lost to follow‐up in control group (none due to diet issues), 3 lost to follow‐up in whole grain group (2 due to diet issues), similar rates of loss to follow‐up, but differential rates in those withdrawing because of the diet. |
| Intention to treat analysis | Unclear risk | "Per protocol analysis included only the data from participants who completed both phases of the study" |
| Selective reporting (reporting bias) | Unclear risk | Reports all relevant outcomes but not enough information to judge. |
| Groups comparable at baseline | Low risk | Yes, except for triglycerides, which were higher in the control group. Unclear risk for triglycerides |
| Other bias | Unclear risk | Power calculations used body composition (% of abdominal AT) as outcome variable (not a primary outcome of this review), which required a sample size of 50. This was met, but power relevant to measuring changes in lipids or blood pressure not reported. |
Katcher 2008.
| Study characteristics | ||
| Methods |
Setting: USA Design: parallel RCT with stratified randomisation by sex (male or female) and BMI status (BMI < 40 or >/= 40 kg/m2). Dates: trial opened to accrual September 2005, enrolment completed August 2006. Intervention duration: 12 weeks Follow‐up: no postintervention follow‐up Focus: to determine whether including whole grain foods in a hypocaloric (reduced by 500 kilocalories/d) diet enhances weight loss and improves CVD risk factors in obese adults with metabolic syndrome. |
|
| Participants |
N: 50 randomised (24/25 completers in the whole grain intervention group and 23/25 in the control group). Inclusion criteria: men and women were eligible if they had a BMI (in kg/m2) >/= 30 and met 3 of 5 National Cholesterol Education Program Adult Treatment Panel III criteria for metabolic syndrome:
Exclusion criteria: diagnosed with type 1 or 2 diabetes, CVD, cancer, or any other serious medical condition or if they were using any medications known to affect glucose, insulin, cholesterol, or reproductive hormones; people who smoked, drank > 2 alcoholic beverages/d, or consumed a diet high in whole grains (> 3 servings/d); or who were pregnant or lactating. Age (years): 24 to 63 years. Whole grain group mean age: 45.4 (SD 8); refined grain group mean age 46.6 (SD 9.7). Sex (% men): whole grain group: 48%; refined grain group: 52% Ethnicity: US (48/50 white; 1/50 African‐American; 1/50 Hispanic) Baseline cardiovascular risk status: BMI (kg/m2): whole grain intervention: 35.54 (4.1); control: 36.1 (4.9) Total cholesterol mmol/L: whole grain intervention: 4.91 (1.22); control: 4.86 (0.64) LDL cholesterol, mmol/L: whole grain intervention: 3.08 (1.02); control: 2.96 (0.51) HDL cholesterol, mmol/L: whole grain intervention: 1.07 (0.23); control: 1.06 (0.2) Systolic blood pressure (mmHg): whole grain intervention: 123 (9.4); control: 130.3 (13.3), P = 0.03 Diastolic blood pressure (mmHg): whole grain intervention: 83.2 (8.3); control: 82.0 (7.5) Medications used: not reported, other than that participants on medications known to affect glucose, insulin, cholesterol, or reproductive hormones were excluded. |
|
| Interventions |
Whole grain diet group: based on a range of whole grains Control: refined cereal foods Description of dietary intervention: The whole grain group were given a target number of daily whole grain servings (4, 5, 6, or 7 servings/d) based on their energy needs. The whole grain group were given a list and description of whole grain foods to help them identify foods to include in their diet, and they were encouraged to select foods for which a whole grain was listed as the first ingredient. They were advised to consume 3 daily servings of whole grain foods for the first 2 wks of the study, and then to increase to their target number of daily whole grain servings for the remaining 10 wks. Participants in the refined grain group were also given a list of whole grain foods and were asked not to consume any of these foods during the study period. A registered dietitian met individually with each participant at baseline to discuss the dietary intervention and provided educational materials. Additionally, both groups were asked to eat 5 servings of fruits and vegetables, 3 servings of low‐fat dairy products, and 2 servings of lean meat, fish, or poultry, per day, based on 2005 dietary guidelines for Americans. The target macronutrient composition was 55% of energy as carbohydrate, 30% of energy as fat (with an emphasis on unsaturated fats), and 15% of energy as protein. Incentives: not reported Co‐interventions in both groups: participants in both groups were encouraged to engage in moderate physical activity for 30 min per session 3 times/wk. Assessment of dietary adherence: 3‐day diet record (and a diet satisfaction questionnaire) Was the diet energy reduced? yes (target energy deficit ~500 kilocalories/d) Comparability of diet composition: yes. Energy intake decreased (P < 0.001) from baseline in both diet groups (as both were weight loss diets). The percentage of energy from carbohydrate and protein increased (P < 0.01), and from fat decreased (P < 0.001) in both diet groups compared with baseline. Participants in the whole grain group increased their intake of total, insoluble, and soluble fibre by 50%, 52%, and 47%, respectively, and those in the refined grain group increased their intakes by 7%, 5%, and 14%, respectively. (See Table 2.) Change in diet over time: as above |
|
| Outcomes | Systolic and diastolic BP; lipids (LDL cholesterol, HDL cholesterol, triacylglycerol); anthropometric measures, including weight and BMI; apolipoprotein A1/B; glycaemic measures; satisfaction with diet. | |
| Funding/conflicts of interest | General Mills Bell Institute of Health and Human Nutrition, grant no. K24 HD01476 and M01 RR10732 from the National Institutes of Health, and construction grant no. C06 RR016499 (to the General Clinical Research Center of The Pennsylvania State University). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned to either a whole grain or refined‐grain hypocaloric diet with the use of a stratified randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons for loss to follow‐up fully reported. |
| Intention to treat analysis | Unclear risk | Analysis only reported for completers. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes fully reported, but not enough information to judge. |
| Groups comparable at baseline | Unclear risk | Yes, except for systolic BP and the percentage of the LDL‐III subclass, which were higher (P=0.03) at baseline in the refined grain group than in the whole grain group; however, there were no other differences at baseline between the groups. |
| Other bias | Unclear risk | Power calculations used weight as outcome variable (not a primary outcome of this review), which required a sample size of 50. This was met, but power relevant to measuring changes in lipids or blood pressure not reported. |
Kristensen 2012.
| Study characteristics | ||
| Methods |
Setting: Denmark (Copenhagen) Design: parallel‐group RCT Dates: not reported Intervention duration: 12 weeks (after a 2‐week run‐in period) Follow‐up: no postintervention follow‐up Focus: to compare energy‐restricted diets based on whole grain wheat compared to refined grain wheat in overweight or obese postmenopausal women. |
|
| Participants |
N: 79 were randomised after the run‐in period, and a total of 72 women completed the study (38/42 in the whole grain wheat group and 34/37 in the refined grain wheat group). Inclusion criteria: BMI 27 to 37 kg/m2, age 45 to 70 years, and 1‐year postmenopausal (self reported). Exclusion criteria: smoking, chronic illnesses (diabetes or CVD), untreated hypertension (> 160/100 mmHg), elevated fasting total cholesterol (> 6.5 mmol/L) or glucose (> 7.0 mmol/L), use of dietary supplements, food dislikes or intolerances relevant to the study, and use of medications (except antihypertensives). Age (years): 45 to 70 years, otherwise not reported. Sex (% men): 0% (all female) Ethnicity: Danish (ethnic composition not reported) Baseline cardiovascular risk status: The study reports there was no difference in any of the baseline characteristics. BMI (kg/m2): whole grain intervention: 30.0 (SEM 0.4); control: 30.4 (SEM 0.6) Total cholesterol (mmol/L): whole grain intervention: 5.57 (SEM 0.16); control: 5.61 (SEM 0.14) HDL cholesterol (mmol/L): whole grain intervention: 1.24 (0.04 SEM); control: 1.28 (0.04 SEM) LDL cholesterol (mmol/L): whole grain intervention: 3.75 (0.16 SEM); control: 3.75 (0.13 SEM) Systolic blood pressure (mmHg): whole grain intervention: 133 (2 SEM); control: 138 (4 SEM) Diastolic blood pressure (mmHg): whole grain intervention: 85.5 (1.4 SEM); control: 87.3 (1.6 SEM) Medications used: 10 women in each group used antihypertensive medications. |
|
| Interventions |
Whole grain diet group: whole grain wheat Control: refined wheat foods Description of dietary intervention: whole grain wheat foods as part of an energy‐restricted diet to provide 105 g of whole grains daily. The whole grain or refined grain foods were intended to replace ~2 MJ of the participants' habitual diet, and both groups were asked to consume 62 g of bread, 60 g pasta (uncooked), and 28 g of biscuits daily. There was no restriction on consumption of other cereal products. Both groups were asked to consume an energy‐restricted diet with a deficit of at least 1250 kJ/d but not less than 5000 kJ/d, with minimum protein intake of 60 g/d. The participants met with a dietitian at least 5 times during the study. Food provided biweekly. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: food diary (self reported) (note only 57 of 72 women who completed the study kept food diaries eligible for data analysis). Was the diet energy reduced? yes (~1250 kJ/d deficit) Comparability of diet composition: total self reported energy intake similar at weeks 1 to 6: whole grain 5830 kJ/d (SEM 190) and refined grain 5900 kJ/d (SEM 280), but higher in refined grain group for weeks 7 to 12: whole grain 6060 kJ/d (SEM 150) and refined grain 6330 (SEM 180) kJ/d. Carbohydrate also appears to be higher in the refined grain group, but SEM and P values not reported. (See Table 2.) Change in diet over time: reported at 4, 8, 12 weeks. (See Table 2.) |
|
| Outcomes | Systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, insulin, HOMA‐IR, glycated haemoglobin, hsCRP, IL‐6, body weight, BMI, waist circumference, FFM. | |
| Funding/conflicts of interest | European Commission in the Communities 6th Framework Programme, Project HEALTHGRAIN (FOOD‐CT‐2005‐514008), the University of Copenhagen, Faculty of Life Sciences and LMC FOOD Research School. Authors M Petronio and G Riboldi are employed by Barilla, and AB Ross is employed by Nestle. | |
| Notes | There was a concomitant increase in energy intake and carbohydrate intake in the refined grain group. Authors report that non‐compliance in the refined grain group did not reflect a lack of intake of refined grain foods, but instead was due to high intake of whole grain products other than the foods provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomly allocated", but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up and reasons for dropout similar in both groups. Whole grain 4/42 lost, refined grain 3/37 lost. However, only 57 of 72 food diaries eligible for analysis, reasons not reported. |
| Intention to treat analysis | Unclear risk | Intention‐to‐treat analysis performed for anthropometric outcomes, no differences in ITT analysis compared to completers. Intention‐to‐treat analysis not done for lipids and blood pressure outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Only 57 of 72 food diaries eligible for analysis, reasons not reported. |
| Groups comparable at baseline | Low risk | States no difference. |
| Other bias | Unclear risk | Power calculations used weight as outcome variable (not a primary outcome of this review), which required a sample size of 72 (36 in each group). This was met, but power relevant to measuring changes in lipids or blood pressure not reported. |
Lankinen 2014.
| Study characteristics | ||
| Methods |
Setting: Finland Design: parallel‐group RCT Dates: recruitment and screening October 2007 to November 2008, intervention periods were carried out during January 2008 to June 2009. Intervention duration: 12 weeks Follow‐up: no postintervention follow‐up Focus: to investigate the effects of whole grain, fish, and bilberries on serum metabolic profile and lipid transfer protein activities in people with metabolic syndrome. |
|
| Participants |
N: 131 were randomised (34/42 completers in the whole grain intervention and 35/45 completers in the control group). Inclusion criteria: 40 to 70 years of age, impaired glucose metabolism (FPG 5.6 to 6.9 mmol/L) or in OGTT 2 hour (plasma glucose 7.8 to 11.0 mmol/L) and 2 of the following: BMI 26 to 39 kg/m2, waist circumference > 102 cm in men and > 88 cm in women, serum TG > 1.7 mmol/L, HDL < 1.0 mmol/L in men and < 1.3 mmol/L in women, or blood pressure >= 130 or >= 85 mmHg. Exclusion criteria: BMI > 40 kg/m2; fasting serum triglyceride concentration > 3.5 mmol/L; fasting serum cholesterol > 8 mmol/L; type 1 or 2 diabetes; abnormal liver, kidney, or thyroid function; large alcohol intake (women > 16, men > 24 doses (4 cL liquor or equivalent) during week); inflammatory bowel disease; disease that prevents participation; neuroleptic cortisone medication. Age (years): 40 to 70 years; mean age in whole grain enriched diet group 58 (SD 8) and in control group 59 (SD 7). Sex (% men): whole grain intervention: 50%, control: 51% Ethnicity: Caucasian (understood to be white) Baseline cardiovascular risk status: The study reports there was no difference in any of the baseline characteristics. BMI (kg/m2): whole grain intervention: 31.4 (SD 3.4); control: 31.0 (SD 3.6) Total cholesterol (mmol/L): whole grain intervention: 5.1 (SD 1.0); control: 5.4 (SD 1.0) HDL cholesterol (mmol/L): whole grain intervention: 1.2 (SD 0.4); control: 1.3 (SD 0.3) LDL cholesterol (mmol/L): whole grain intervention: 3.2 (SD 0.8); control: 3.4 (SD 0.8) Systolic blood pressure (mmHg): whole grain intervention: 135 (SD 16); control: 139 (SD 12) Diastolic blood pressure (mmHg): whole grain intervention: 86 (SD 8); control: 88 (SD 7) Medications used: Statins: 10/34 (29%) intervention; 9/35 (26%) control Hormonal replacement therapy: 4/34 (11%) intervention; 3/34 (9%) control Beta‐blocker or diuretics: 12/34 (35%) intervention; 9/35 (25.7%) control |
|
| Interventions |
Whole grain diet group: whole grain wheat and rye bread Control: refined wheat foods Description of dietary intervention: the whole grain group replaced their habitual grain products with whole grain breads and a bread with low postprandial insulin response. Products covered 20% to 25% of total energy intake and were delivered to the participants. The fibre contents of the breads were 6.9% (endosperm rye bread), 6.4% (whole grain wheat bread), and 10% to 14% (commercial whole grain rye breads). 1 portion of habitually used grain product, e.g. a slice of low‐fibre wheat bread, was allowed daily to increase compliance. Pasta with a fiber content of 6% was also delivered and was instructed to be consumed at the dose equal to 3.5 dL of uncooked pasta per week. Participants were given whole grain oat biscuits of which they were allowed to consume 1 portion per day on a voluntary basis. Biscuits contained 8 to 8.5 g/100 g of dietary fibre and 16 to 18 g/100 g of fat, of which 4.3 to 7.7 g was saturated. The control group replaced their habitually used breads with refined wheat breads (dietary fiber 3 to 4.3 g/100 g) and other cereal products, e.g. pasta, with low‐fibre products (< 6 g/100 g dietary fibre). Participants were allowed to eat a maximum of 1 to 2 portions of rye products per day. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: 4‐day food record at baseline, three 4‐day food records at weeks 3, 7, and 11. Was the diet energy reduced? no Comparability of diet composition: yes. In the whole grain group, the intake of total fat decreased, but there was no change in the quality of dietary fat. Fibre intake increased in the whole grain group, whereas it decreased in the control group. (See Table 2.) Change in diet over time: reported at baseline, 12 weeks. (See Table 2.) |
|
| Outcomes | Gene expression, glucose metabolism, plasma lipidomic profiles | |
| Funding/conflicts of interest | Funding was provided by Academy of Finland (The Research Program on Nutrition, Foods and Health (ELVIRA), Decision number 117844 for MU and 117996 for MO), European Commission in the Communities 6th Framework Programme, Project HEALTHGRAIN (FOOD‐CT‐2005‐514008, for HM and KP), Sigrid Juselius Foundation, The Finnish Diabetes Research Foundation, Nordic Centre of Excellence on Systems Biology in Controlled Dietary Interventions and Cohort Studies (SYSDIET, 070014), TEKES 70103/06, The EVO‐fund of Kuopio University Hospital (5254), Fazer bakeries Oy, Vaasan & Vaasan Oy, KE Leipa¨ Oy, Leipomo Ruista¨hka¨, Leipomo Koskelonseutu, Raisio Oyj, Pakkasmarja Oy, Joswola Oy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. | |
| Notes | This was a 3‐arm RCT (HealthyDiet, not relevant to this review: whole grain and low postprandial insulin response grain products, fatty fish 3 times a week, and bilberries 3 portions per day; whole grain: whole grain and low postprandial insulin response grain products; control: refined wheat breads and cereal products). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was conducted based on a randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | "Participants were randomly assigned by the study nurse to one of the following groups: HealthyDiet, wholegrain or control" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. In the CONSORT checklist they mention N/A. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition were reported and numbers provided: 24.4% in the control and 19% in the intervention. |
| Intention to treat analysis | Unclear risk | Analysis only reported for completers. |
| Selective reporting (reporting bias) | Low risk | All outcomes intended to measure in the protocol were reported. |
| Groups comparable at baseline | Low risk | Yes. "There were no significant differences in the characteristics between the groups at baseline" |
| Other bias | Unclear risk | Power calculations used glucose metabolism as outcome variable (not a primary outcome of this review), which required a sample size of 111 (37 in each group). This was nearly met, but power relevant to measuring changes in lipids or blood pressure not reported. |
Maki 2010.
| Study characteristics | ||
| Methods |
Setting: USA (Bloomington, IN and St Petersburg, FL) Design: randomised parallel‐arm controlled trial Dates: trial dates not reported Intervention duration: 12 weeks Follow‐up: no postintervention follow‐up Focus: to compare the effect on LDL cholesterol and other cardiovascular disease markers of whole grain oat ready‐to‐eat cereal compared to energy‐matched low‐fibre foods as part of a dietary program for weight loss in overweight and obese adults. |
|
| Participants |
N: 204 randomised, 80/101 completed in whole grain oat ready‐to‐eat cereal group; 73/103 completed in the control group. Inclusion criteria: free‐living overweight and obese adults, BMI 25 to 40 kg/m2 with baseline LDL cholesterol 3.4 to 5.2 mmol/L, aged 20 to 65 years. Exclusion criteria: participants who reported a weight change of 4.5 kg during the previous 2 months; use of weight loss medications within 2 months before screening or supplements, programs, or meal replacement products within 2 weeks before screening; use of drugs (within 4 weeks before screening), supplements, or foods (within 2 weeks before screening) known to alter lipid levels; use of fibre‐containing supplements within 2 weeks before screening; daily consumption of oat or barley products (e.g. ready‐to‐eat oat‐based cereals, oatmeal, or oat bran) or frequent consumption of foods rich in viscous fibre within 2 weeks of screening; clinically significant abnormal laboratory test results (e.g. triglycerides >/= 4.5 mmol/L, glucose >/= 7.0 mmol/L, creatinine >/= 114.4 μmol/L, and alanine aminotransferase and aspartate aminotransferase levels 1.5 times the upper limit of normal), uncontrolled hypertension (systolic/diastolic blood pressures >/= 160/100 mmHg); a history of cardiac, renal, hepatic, endocrine, pulmonary, biliary, pancreatic, gastrointestinal, or neurologic disorders, or cancer in the past 2 years; known sensitivity to any of the ingredients in the study foods; a history of weight‐reducing surgery; a history of eating disorders or alcohol abuse; or use of thyroid hormones (except stable dose replacement therapy) or systemic corticosteroids. Age (years): whole grain oat group: 50.1 (SEM); control: 47.5 (SEM 1.3) Sex (% men): whole grain oat group: 24.7%; control: 17.9% Ethnicity: US:
Baseline cardiovascular risk status: BMI (kg/m2): whole grain intervention: 32.2 (SEM 0.6); control: 32.0 (SEM 0.5) Total cholesterol (mmol/L): whole grain intervention: 6.00 (SEM 0.08); control: 5.92 (SEM 0.08) HDL cholesterol (mmol/L): whole grain intervention: 1.24 (0.03); control: 1.24 (0.04) LDL cholesterol (mmol/L): whole grain intervention: 4.02 (0.05); control: 4.00 (0.06) Triglyceride (mmol/L): whole grain intervention: 1.65 (SEM 0.0.9); control: 1.48 (SEM 0.0.8) Systolic blood pressure (mmHg): whole grain intervention: 127.2 (SEM 1.2); control: 122.6 (SEM 1.3) (P = 0.01) Diastolic blood pressure (mmHg): whole grain intervention: 79.4 (0.9); control: 78.3 (1.00) Medications used: not reported |
|
| Interventions |
Whole grain diet group: whole grain oat ready‐to‐eat cereal Control: low‐fibre cereal foods Description of dietary intervention: 2 portions per day of whole grain ready‐to‐eat oat cereal or energy‐matched low‐fibre foods (control) such as corn cereals, white toast, plain bagels, English muffins, pretzels, soda crackers, or rice cakes, as part of a reduced‐energy (~500 kilocalories/day deficit) dietary program that encouraged limiting consumption of foods high in energy and fat, portion control, and regular physical activity. All participants were asked to avoid foods rich in viscous soluble fibre such as barley, oatmeal and oat bran products except for the study products provided to the whole grain oat cereal group. The whole grain oat ready‐to‐eat cereal (Cheerios, General Mills, Minneapolis, MN) was packaged in ~40 g portions by the manufacturer. A registered dietitian met biweekly with participants to monitor and reinforce dietary changes. Incentives: not reported Co‐interventions in both groups: regular physical activity (30 to 60 min/day) was encouraged in both groups as part of the intervention. Physical activity was assessed using a 7‐day recall questionnaire. At baseline, the mean activity scores above resting were 118.4 (SEM 7.6) metabolic equivalent hours in the whole grain oat group and 118.3 (SEM 10.0) in the control group. Physical activity scores increased by 9.1% (SEM 4%) in the whole grain group and 15% (SEM 5.2%) (P = 0.710) in the control group. Assessment of dietary adherence: 3‐day self reported diaries recording compliance with provided foods, participant interview, and review of unused foods. The whole grain oat group consumed 96.8% (SEM 0.6) of the expected servings of study foods, and the control group consumed 95.7% (SEM 0.7) (P = 0.202). Was the diet energy reduced? yes (~500 kilocalories/day deficit) Comparability of diet composition: (See Table 2.) As designed, both groups reduced energy intakes; there was no difference between groups at week 12, although the control group showed a larger reduction in energy intake at week 4. The percentage of total daily energy intake from carbohydrate was greater at week 12 in the whole grain oat group than in the control group (P = 0.017). Total and soluble fibre increased as expected. Change in diet over time: as above for energy. (See Table 2.) |
|
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, non‐HDL cholesterol, triglycerides, BMI, waist circumference, midarm circumference, triceps skinfold thickness | |
| Funding/conflicts of interest | General Mills Bell Institute of Health and Nutrition, Minneapolis, MN. 3 of the authors are employees of General Mills, 3 authors are employees of Provident Clinical Research and received research grant support from General Mills to conduct the study. 1 author is an employee of Meridien Research and received research grant support from General Mills. | |
| Notes | Adverse effects: the frequencies of adverse events (of any type, whether related to the study products or not) were reported to be similar between groups (59.8% for the whole grain group and 52.4% for the control group, P = 0.321). The most common adverse events in both groups were respiratory tract infection, sinusitis, and pharyngitis. Most adverse events were mild and not related to the study product. Adverse events the authors considered related to the study products were nausea (2 people in the whole grain oat group), flatulence (2 people in the whole grain oat group), gastroenteritis (1 person in the control group), gastroesophageal reflux (1 person in the control group), and vomiting (1 person in the control group). Adverse events that led to drop out from the study were an infectious cyst (1 control) and spinal stenosis (1 control), but the study authors did not consider these to be related to the study product. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomly assigned"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported for anthropometry or lab measurements |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More participants completed study in whole grain oat group than in control group. 20 withdrew consent in control group, 11 in whole grain oat group; both groups lost n = 7 to follow‐up; 2 in control group reported adverse events; 1 in WG group reported product too filling. There was differential dropout between groups. |
| Intention to treat analysis | Unclear risk | Modified intention‐to‐treat analyses done. Of 101 participants randomised to whole grain oat group, 86 were included in MITT analysis (15 were excluded as they did not have at least 1 postrandomisation lipid value or the blood draw was invalid). Of 103 randomised to control group, 87 were included in MITT analysis (16 were excluded as they did not have at least 1 postrandomisation lipid value or the blood draw was invalid). Sensitivity analyses conducted using MITT and completers data. Results also presented as per‐protocol subset of MITT. |
| Selective reporting (reporting bias) | Unclear risk | Blood pressure data not fully reported; no outcome data given. |
| Groups comparable at baseline | Unclear risk | Yes, for all reported measures, except for systolic blood pressure, which was higher in the whole grain oat group compared to control (127.2 SEM 1.2 vs 122.6 SEM 1.3). However, outcome data not reported for systolic blood pressure. Reported only for the per‐protocol subset. |
| Other bias | Low risk | Power calculations: a sample size of 128 (64 per arm) was expected to provide 80% power to detect a difference of 5% between groups in the per cent change from baseline in LDL cholesterol, assuming a 10% pooled standard deviation for the LDL cholesterol response. This sample size was achieved in both the analysis on completers and the MITT analysis. |
Tighe 2010‐W.
| Study characteristics | ||
| Methods |
Setting: UK (Aberdeen, Scotland) Design: parallel‐group RCT, stratified by sex, age, and BMI Dates: September 2005 to December 2008 Intervention duration: 12 weeks (following 4‐week run‐in) Follow‐up: no postintervention follow‐up Focus: to assess the effects of consumption of 3 daily portions of whole grains (2 intervention groups: wheat only or a mixture of wheat and oats) compared to refined grains on markers of cardiovascular disease risk in people at relatively high risk. |
|
| Participants |
N: 226 randomised (73/77 completers in the whole grain wheat intervention group; 70/73 in the whole grain wheat and oats group; and 63/76 in the control group). Inclusion criteria: male and female, aged 40 to 65 years with a BMI between 18.5 and 35. Sedentary or moderately active (< 2 aerobic sessions/wk) people or those with signs of metabolic syndrome or moderate hypercholesterolaemia. Exclusion criteria: people with CVD, diabetes, fasting blood glucose > 7.0 mmol/L, asthma, systolic BP > 160 mmHg and diastolic BP > 99 mmHg, thyroid conditions, eating disorders, high intake of whole grain foods, or taking regular medication or supplements known to affect any of the outcomes. Age (years): 40 to 65 years recruited: whole grain wheat group mean age 51.6 (SEM 0.8); whole grain wheat + oats: 52.1 (SEM 0.9); refined grain (control group): 51.8 (SEM 0.8). Sex (% men): whole grain wheat group: 52%; whole grain wheat + oats: 51%; refined grain (control group): 48%. Ethnicity: Scottish (ethnic composition not reported) Baseline cardiovascular risk status: BMI (kg/m2): whole grain wheat group: 28.0 (SEM 0.5); whole grain wheat + oats: 27.0 (SEM 0.4); control: 28.0 (SEM 0.5); P = 0.221 Total cholesterol (mmol/L): whole grain wheat group: 5.46 (SEM 0.14); whole grain wheat + oats: 5.57 (SEM 0.12); control: 5.94 (SEM 0.14); P = 0.087 HDL cholesterol (mmol/L): whole grain wheat group: 1.55 (SEM 0.04); whole grain wheat + oats: 1.62 (SEM 0.05); control: 1.62 (SEM 0.06); P = 0.506 LDL cholesterol (mmol/L): whole grain wheat group: 3.45 (SEM 0.11); whole grain wheat + oats: 3.45 (SEM 0.11); control: 3.66 (SEM 0.12); P = 0.365 Triglycerides (TAG): whole grain wheat group: 1.27 (SEM 0.08); whole grain wheat + oats: 2 (SEM 0.06); control: 1.49 (SEM 0.11); P = 0.012 Systolic blood pressure (mmHg): whole grain wheat group: 125.9 (SEM 1.4); whole grain wheat + oats: 131.7 (SEM 1.4); control: 131.2 (SEM 1.4); P = 0.019 Diastolic blood pressure (mmHg): whole grain wheat group: 75.7 (SEM 0.8); whole grain wheat + oats: 78.4 (SEM 0.8); control: 79.1 (SEM 0.8); P = 0.26 Medications used: monitored but not reported |
|
| Interventions |
Whole grain diet group: 2 intervention groups: whole grain wheat and whole grain wheat and oats Control: refined grain foods Description of dietary intervention: after a 4‐week run‐in period on the refined grain diet, the intervention groups had 3 servings/d of refined cereal foods replaced by either 3 servings of whole wheat foods (70 to 80 g wholemeal bread and 30 to 40 g whole grain cereals) or with 1 serving of whole wheat foods and 2 servings of oats. The study foods, both refined and whole grain, were products widely available from UK food retailers. Apart from the whole grain foods supplied, participants selected their own foods to eat and advice on which foods to replace with whole grain servings was standardised to each participant's regular diet. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: 7‐day food diaries, 3 times during the intervention. Was the diet energy reduced? no Comparability of diet composition: no differences in macronutrients, except for fibre (as expected). (See Table 2.) Change in diet over time: reported at baseline and week 12. (See Table 2.) |
|
| Outcomes | Total cholesterol, triglycerides (TAG), HDL cholesterol, LDL cholesterol, Apo A‐I, Apo B, BMI waist circumference, systolic BP, diastolic BP, pulse pressure, heart rate, stiffness index, insulin, glucose, HOMA‐IR, revised QUICKI, h CRP, IL‐6 | |
| Funding/conflicts of interest | UK Food Standards Agency (grant NO2035), Scottish government (Rural and Environment Research and Analysis Directorate). Oat cakes were supplied by Paterson Arran Ltd. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation system, random permuted blocks stratified by age, gender, and BMI |
| Allocation concealment (selection bias) | Low risk | Off‐site allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up higher in control group: 63/76 completers, 11 voluntarily withdrew, 2 excluded; whole grain wheat group 73/77 completers, 4 voluntarily withdrew; whole grain wheat + oats group 70/73 completers, 2 voluntarily withdrew, 1 excluded. Reasons for higher withdrawal in control group not clear. |
| Intention to treat analysis | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Blood pressure outcomes provided by author as not reported in publications except graphically. Unclear if all variables reported at baseline are outcomes. |
| Groups comparable at baseline | Unclear risk | Yes, except for both systolic and diastolic blood pressure, which were lower in the whole grain wheat group than in the whole grain wheat + oats group and the control group |
| Other bias | Low risk | Sample size was based on total cholesterol and LDL cholesterol. Power calculations indicated 60 participants per group would give sufficient power to detect effects of 5% to 7%. This was met. |
Tighe 2010‐WO.
| Study characteristics | ||
| Methods | See above | |
| Participants | See above | |
| Interventions | See above | |
| Outcomes | See above | |
| Funding/conflicts of interest | See above | |
| Notes | See above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See above |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | See above |
| Intention to treat analysis | Unclear risk | See above |
| Selective reporting (reporting bias) | Unclear risk | See above |
| Groups comparable at baseline | Unclear risk | See above |
| Other bias | Low risk | See above |
Zhang 2011.
| Study characteristics | ||
| Methods |
Setting: China Design: parallel‐group RCT (stratified by sex and 5‐year age category) Dates: 2009 onwards (initial screening conducted in 2009) Intervention duration: 16 weeks Follow‐up: no postintervention follow‐up Focus: to compare the effects of replacing white rice with brown rice in the diets of middle‐aged Chinese men and women with diabetes or a high risk for diabetes. |
|
| Participants | Faculty and staff of a large university in Shanghai who had metabolic syndrome. N: 202 randomised (to 2 groups); 193 completed overall. 98/101 completers in brown rice group; 95/101 completers in white rice group. Inclusion criteria: people with MetS defined as presenting with at least 3 of the following components:
Exclusion criteria: history of severe kidney disease, cardiovascular disease, stroke, cancer, or psychological disorders as well as pregnant or lactating women were excluded. Age (years): white rice group: 49.8 SD 7.1; brown rice group: 49.6 SD 6.7 Sex (% men): white rice group: 53.5%; brown rice group: 53.5% Ethnicity: Chinese Baseline cardiovascular risk status: mean (SD) or (95% confidence interval (CI)) BMI (kg/m2): white rice: 25.4 SD 2.5; brown rice: 25.9 SD 3.4; P = 0.22 Total cholesterol (mmol/L): white rice: 5.55 SD 1.33; brown rice: 5.44 SD 1.27; P = 0.55 HDL cholesterol (mmol/L): white rice: 1.31 SD 0.38; brown rice: 1.22 SD 0.34; P = 0.08 LDL cholesterol (mmol/L): white rice: 3.93 SD 8; brown rice: 3.81 SD 1; P = 0.46 Triglycerides (mmol/L): white rice: 1.78 (95% CI 1.21 to 2.39); brown rice: 1.81 (95% CI 1.30 to 2.53); P = 0.48 Systolic blood pressure (mmHg): white rice: 129 SD 15; brown rice: 129 SD 16; P = 0.82 Diastolic blood pressure (mmHg): white rice: 85 SD 10; brown rice: 86 SD 10; P = 0.42 Medications used: antihypertensive agents (% using): white rice: 44.6; brown rice: 29.7; P = 0.03 hypoglycaemic agents (% using): white rice: 5.0; brown rice: 4.0; P = 0.73 lipid‐lowering agents (% using): white rice: 3.0; brown rice: 3.0; P = 1.00 |
|
| Interventions |
White rice: the 2 types of rice in the study were from the same batch; the white rice was produced by further milling the brown rice. Both types of rice were cooked in the same steam box under the same conditions. Brown rice: from the same batch as the white rice as described above. Description of dietary intervention: the cooked rice was packaged into 225 g servings (equivalent to 100 g cooked rice) and provided to participants at designated university campus cafeterias during the lunch hour from Monday to Fridays. Participants took cooked rice home for dinner and meals on Saturdays. They were encouraged to eat ad libitum and were permitted to consume other staple foods only on Sundays. They were instructed to maintain their usual dietary pattern regarding other food selections. Incentives: not reported Co‐interventions in both groups: none Assessment of dietary adherence: compliance was monitored by researchers weighing leftovers in the cafeteria and was calculated as the frequency of consumption of the prescribed type of rice divided by the frequency of consumption of total staple carbohydrates throughout the intervention. Participants recorded the amount of rice they consumed at home using electronic scales provided by the researchers. Dietary intake measured using a 3‐day diet record was obtained at baseline and every 4 weeks during follow‐up. Was the diet energy reduced? no Comparability of diet composition: adherence to diets was high: mean adherence 90.0 +/‐ 17.1% in the white rice group and 88.7 +/‐ 23.3% in the brown rice group; P for difference = 0.20. There was no difference in energy intake between groups over the intervention period, but lower intake of carbohydrates (P = 0.03) and dairy products in the brown rice group (P = 0.02). There was no difference in protein, fat, dietary cholesterol, vegetables, fruits, red meat, poultry, or seafood. Dietary fibre was higher in the brown rice group, as would be expected (P < 0.0001). (See Table 2.) Change in diet over time: reported at weeks 4, 8, 12, and 16. (See Table 2.) |
|
| Outcomes | BMI, waist circumference, systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, total:HDL cholesterol ratio, triglycerides, glucose, insulin, HOMA‐IR | |
| Funding/conflicts of interest | Supported by Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, National Basic Research Program of China, National Natural Science Foundation of China. | |
| Notes | No between‐group differences were found for any markers except serum LDL concentration, which decreased more in the white rice group that in the brown rice group (P = 0.02). However, this effect was only observed among participants with diabetes (n = 47). Among participants with diabetes, a greater reduction in diastolic blood pressure was observed in the brown rice group than in the white rice group (P = 0.02). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned (stratified by sex and 5y age category)", but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All researchers not directly in contact with study participants (dietitians, laboratory technicians, and statisticians) were unaware of group allocations. Not possible for participants to be unaware of their group assignment due to differences in appearance and texture of the brown and white rice. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98/101 completers in brown rice group; 95/101 completers in white rice group. The 6 withdrawals in the white rice group were due to busy schedule (n = 4); loss of interest (n = 1); or stroke unrelated to intervention. The 3 withdrawals in the brown rice group were due to a busy schedule or heart disease unrelated to the intervention. |
| Intention to treat analysis | Low risk | Intention‐to‐treat analysis done for all relevant outcomes. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes of relevance to this review that were reported at baseline were reported at follow‐up. |
| Groups comparable at baseline | Unclear risk | Higher proportion of participants with diabetes (P = 0.03) and participants on antihypertensive medication (P = 0.03) in the white rice group at baseline |
| Other bias | Unclear risk | Sample size calculation based on fasting glucose outcome, which is not a relevant outcome for this review. |
% E: percentage energy ALT/AST: alanine transaminase/aspartate transaminase BMI: body mass index BP: blood pressure CVD: cardiovascular disease FFQ: Food Frequency Questionnaire FPG: fasting plasma glucose FSIGT: frequently sampled intravenous glucose tolerance test HDL: high‐density lipoprotein ITT: intention‐to‐treat LDL: low‐density lipoprotein MITT: modified intention‐to‐treat NEFA: non‐esterified fatty acids OGTT: oral glucose tolerance test PUFA: polyunsaturated fatty acid RCT: randomised controlled trial SD: standard deviation SEM: standard error of the mean TAG: triacylglyceride TC/HDL: total cholesterol/HDL cholesterol TG: triglycerides WG: whole grain
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrahamsson 1994 | Not a primary study |
| Ampatzoglou 2015 | Short‐term intervention |
| Anderson 1978 | Not specifically whole grain Not RCT or CCT |
| Anderson 1979 | Not specifically whole grain Participants not free‐living Not RCT or CCT Intervention < 4 weeks |
| Anderson 2009 | Not RCT |
| Andersson 2007 | Intervention < 12 weeks |
| Asp 1981 | Not specifically whole grain Cannot isolate the effect of whole grain Intervention < 4 weeks |
| Azadbakht 2005 | Cannot separate effect of whole grain |
| Beer 2000 | Not whole grain |
| Behall 2004a | Cannot isolate the effect of whole grain. (Intervention groups are barley, barley and whole grain, or whole grain. Not clear if all the barley‐based foods are whole grain, but any comparison would be whole grain versus whole grain.) |
| Behall 2004b | Cannot isolate the effect of whole grain. (Intervention groups are barley, barley and whole grain, or whole grain. Not clear if all the barley‐based foods are whole grain, but any comparison would be whole grain versus whole grain.) |
| Behall 2006 | All 3 diets are whole grain, so comparison is one type of whole grain against another. |
| Berg 2003 | Inpatients |
| Birkeland 1991 | From translation of paper, both the intervention and control groups were given products containing oat bran, which did not meet the definition of whole grain for this review. |
| Birketvedt 2000 | Not whole grain |
| Bodinham 2011 | Only 3‐week intervention |
| Bourdon 1999 | Not whole grain Intervention < 4 weeks |
| Braaten 1994 | Not whole grain |
| Brock 2006 | Postprandial study only |
| Brownlee 2013 | Not lipid or blood pressure outcomes. Main outcomes are reported in Brownlee 2010, which is an included study. This study reports longer‐term effects of the intervention on whole grain consumption. |
| Bruce 2000 | Not whole grain |
| Brussaard 1981 | Not whole grain |
| Bruttomesso 1989 | Not specifically whole grain |
| Burley 1987 | Not RCT |
| Burr 1989 | Not specifically whole grain |
| Buyken 2000 | Not RCT |
| Cairella 1995 | Not specifically whole grain |
| Cara 1992 | Not whole grain |
| Carvalho‐Wells 2010 | Intervention < 4 weeks |
| Chandalia 2000 | Not specifically whole grain |
| Chang 2013 | Macronutrients not reported. |
| Charlton 2012 | Intervention < 12 weeks |
| Chen 2006 | Not whole grain |
| Chi 2012 | Not whole grain, mixture of different carbohydrates including corn and soya |
| Cohen 1980 | Not whole grain (guar gum, bran) |
| Collier 1982 | Intervention < 4 weeks |
| Comi 1995 | Not specifically whole grain |
| Connell 1975 | Not whole grain |
| Connolly 2016 | Relevant study, RCT, relevant outcomes, but only 6‐week intervention |
| Costabile 2008 | < 4 weeks, outcomes not relevant |
| Crapo 1981 | Not whole grain Intervention < 4 weeks |
| Cugnet‐Anceau 2010 | Not whole grain |
| Data 1980 | Intervention < 4 weeks |
| Dattilo 1992 | Not RCT |
| Davidson 1991 | Intervention < 12 weeks |
| Davy 2002a | Comparison is whole grain versus whole grain; cannot isolate effect of whole grain. |
| Davy 2002b | Cannot isolate the effect of whole grain |
| Demark‐Wahnefried 1990 | Not whole grain |
| de Mello 2011 | Does not report lipid or BP outcomes |
| Di Capua 2010 | Only 3‐week intervention |
| Dixit 2011 | Not RCT |
| Ebell 2000 | Not specifically whole grain |
| Eliasson 1992 | Not whole grain |
| Ellis 2005 | Not RCT |
| Fappa 2013 | Outcomes not relevant, < 12 weeks |
| Fehily 1986 | Intervention is whole grain and bran. 4‐week intervention |
| Fordyce‐Baum 1989 | Product is described as a whole wheat protein isolate. We contacted the authors but were unable to obtain any further information on the nature of the product. |
| Fung 2002 | Not RCT |
| Giacco 2010 | Intervention < 4 weeks |
| Golay 1992 | Not specifically whole grain Intervention < 4 weeks |
| Guzic 1994 | Not whole grain |
| Hagander 1985 | Intervention < 4 weeks |
| Hagander 1988 | Not specifically whole grain |
| He 1995 | Not RCT |
| Heaton 1976 | Participants not diagnosed with CHD or risk factors. Not concurrent control? |
| Hoffman 1982 | Not specifically whole grain Participants not free‐living Not RCT Intervention < 4 weeks |
| Hollenbeck 1986 | Not specifically whole grain Cannot isolate effect of whole grain |
| Hunninghake 1994 | Not whole grain |
| Jacobs 2002 | Outcome is serum enterolactone, which was not a specified outcome for this review. |
| Jang 2001 | Not specifically whole grain Cannot isolate the effect of whole grain |
| Jenkins 1985 | Not specifically whole grain Not RCT |
| Jenkins 1993 | Not specifically whole grain |
| Jenkins 2008 | Comparison is high cereal fibre vs low glycaemic index, but not all differences due to whole grain. |
| Johnston 1998 | Intervention < 12 weeks |
| Judd 1981 | Cannot isolate effect of whole grains Not RCT Intervention < 4 weeks |
| Juntunen 2002 | Intervention < 4 weeks |
| Juntunen 2003 | Intervention group consumed wholemeal rye bread enriched with rye bran; cannot isolate the effect of whole grain. Confirmation of composition of bread received from authors (K Juntunen). |
| Kabir 2002 | Cannot isolate the effect of whole grain |
| Karl 2016 | Relevant study, RCT, relevant outcomes, but only 6‐week intervention |
| Karlstrom 1984 | Not specifically whole grain Participants not free‐living Intervention < 4 weeks |
| Karmally 2005 | Intervention < 12 weeks (6 weeks) |
| Katz 2001a | Cannot isolate the effect of whole grain |
| Katz 2001b | Intervention < 4 weeks |
| Kay 1977 | Not whole grain Not RCT Intervention < 4 weeks |
| Kay 1981 | Not specifically whole grain Participants not free‐living Intervention < 4 weeks |
| Keenan 2002 | Intervention < 12 weeks |
| Kesaniemi 1990 | Not specifically whole grain |
| Kim 2008 | Intervention < 12 weeks |
| Kirwan 2016 | Relevant study, RCT, relevant outcomes, but only 8‐week intervention |
| Kleemola 1999 | Not whole grain |
| Kris‐Etherton 2002 | Not specifically whole grain |
| Lakshmi 1996 | Intervention < 4 weeks |
| Lankinen 2010 | Compares 2 diets containing whole grain, but whole grain not the only component, so cannot identify effect of whole grain |
| Leinonen 1999 | Intervention < 4 weeks |
| Leinonen 2000 | Intervention < 12 weeks |
| Liese 2003 | Not RCT |
| Lousley 1984 | Not specifically whole grain Different macronutrient (carbohydrate) compositions |
| MacKay 2012 | Comparison is WG sourdough versus refined white (not equivalent comparison). Intervention < 12 weeks |
| MacMahon 1998 | Not whole grain |
| Maki 2003 | Cannot isolate effect of whole grain |
| Maki 2007 | Oat β‐glucan but not whole grain |
| Manhire 1981 | Intervention is the effect of whole grain plus the withdrawal of refined sugars; cannot isolate effect of whole grain. |
| Mathur 1968 | Not specifically whole grain Not RCT |
| McGeoch 2013 | Intervention < 12 weeks |
| McIntosh 1991 | Cannot specifically isolate the effect of whole grain |
| McIntosh 2003 | No included outcomes. Intervention < 12 weeks |
| Melanson 2006 | No included outcomes |
| Meydani 2016 | Relevant study, RCT, lipid outcomes, but 6‐week intervention |
| Moazzami 2012 | Comparison is whole grain vs whole grain. |
| Montonen 2003 | Not RCT |
| Nielsen 1988 | Not whole grain Intervention < 4 weeks |
| O'Kell 1988 | Dietary intakes not reported, and compliance unclear |
| Odes 1993 | Not whole grain cereal |
| Pacy 1986 | Not RCT |
| Pereira 2002 | No lipid or blood pressure outcomes. The primary outcomes of this study relate to insulin sensitivity. As there is now a separate published review on whole grains and diabetes outcomes in which this study was included (Priebe 2008), it has now been excluded from the present review, for which the main outcomes relate to CVD and lipid and blood pressure outcomes. |
| Pins 2002 | Excluded due to a lack of clarity about the relative macronutrient content of the diets. There is a reference to the macronutrient data in the paper (Table reference T2) to the journal website. However, the data were not available there, so we attempted to contact the authors, but were unable to find up‐to‐date contact details. In the original version of this review this study was included as it aimed for the same macronutrient content in both arms. It was marked as awaiting further information. We have been unable to verify the relative macronutrient of the diets (as above), so the study is now excluded. |
| Poulter 1993 | Cannot isolate the effect of whole grain |
| Rave 2007 | Not relevant comparison ‐ whole grain vs nutrient replacement product |
| Reynolds 1989 | Abstract only ‐ no full paper found We attempted to contact authors, but were unable to obtain further information. Results quoted appear to be average of 2‐ and 4‐week results, not end results after 4 weeks. |
| Reynolds 2000 | Intervention < 12 weeks |
| Rigaud 1990 | Not whole grain |
| Ross 2012 | Outcomes not relevant ‐ reports plasma alkylresorcinols |
| Roth 1985 | Not RCT |
| Russ 1985 | High fibre versus low fibre; not specifically whole grain |
| Rytter 1996 | Not specifically whole grain Not RCT |
| Saltzman 2001a | Not all participants free‐living |
| Saltzman 2001b | 6‐week intervention |
| Schlamowitz 1987 | Not whole grain |
| Talati 2009 | Not whole grain barley: "administered in various forms, including pearled barley, barley bran flour, oil extracts in capsules, barley concentrates, barley‐containing beverages, and gelling agents" |
| Tighe 2013 | Same study as Tighe 2010, relevant results reported in Tighe 2010 (included study) |
| Turnbull 1987 | Comparison is rolled oats versus wheat, but it is not clear from the paper whether the wheat products used in the comparison were whole grain or refined grain. We contacted the authors but obtained no further details. |
| Turnbull 1989 | Comparison is rolled oats versus wheat, but it is not clear from the paper whether the wheat products used in the comparison were whole grain or refined grain. We contacted the authors but obtained no further details. |
| Turpeinen 2000 | 4‐week intervention; comparison is wholemeal rye versus low‐fibre wheat, which is not an equivalent comparison |
| Van Horn 1986 | 6‐week intervention. Intervention is oatmeal and oat bran. |
| Van Horn 1988 | Intervention < 12 weeks |
| Van Horn 1991 | Intervention < 12 weeks |
| Van Horn 2001 | Cannot isolate the effects of whole grain (intervention is oats and oat bran) |
| Vitaglione 2015 | 8‐week intervention only |
| Wang 2013 | Does not report energy intake or macronutrients |
| Willms 1987 | Participants not free‐living Intervention < 4 weeks |
| Wolever 2003 | Not specifically whole grain |
| Wolever 2016 | Oat β‐glucan rather than WG, 4‐week intervention only |
| Wolffenbuttel 1992 | Intervention not specifically whole grain |
| Wursch 1991 | Not specifically whole grain Intervention < 4 weeks |
| Zhang 2012 | Intervention < 12 weeks (6 weeks) |
BP: blood pressure CCT: controlled clinical trial CHD: coronary heart disease CVD: cardiovascular disease RCT: randomised controlled trial WG: whole grain
Characteristics of studies awaiting classification [ordered by study ID]
Bi 2013.
| Methods | Randomised controlled trial |
| Participants | Unclear if free‐living ‐ 100 people with impaired fasting glucose |
| Interventions | Oatmeal vs barley flake, 3 months |
| Outcomes | Reduction in total cholesterol and LDL cholesterol (P < 0.001 and P = 0.002, respectively) |
| Notes | Unsure if barley flake is from pearl barley or whole grain barley, and unclear if free‐living participants were involved. Contacted authors on 19 August 2016, but have received no response. |
Li 2016.
| Methods | Randomised controlled trial |
| Participants | 298 overweight participants with type 2 diabetes mellitus |
| Interventions | 30‐day centralised intervention, then participants returned home and and were asked to continue with their diet. Participants were randomly allocated to 1 of the following 4 groups.
|
| Outcomes | Anthropometric measurements, glucose profile, lipid profile |
| Notes | To be included in the update of this review |
LDL: low‐density lipoprotein
Characteristics of ongoing studies [ordered by study ID]
NCT02615444.
| Study name | An investigation of the effects of consuming oatcakes containing 4 g of oat beta‐glucan on physiological parameters in individuals at risk of developing metabolic syndrome |
| Methods | Intervention study, double‐blind, parallel assignment |
| Participants | ‐ |
| Interventions | The effects of beta‐glucan enriched oatcake consumption on metabolic disease risk factors |
| Outcomes |
Secondary outcome measures: Blood pressure [ Time Frame: 6 weeks ] Fasting serum triglycerides [ Time Frame: 6 weeks ] Oral glucose tolerance test [ Time Frame: 6 weeks ] Fasting serum HDL cholesterol [ Time Frame: 6 weeks ] Fasting serum total cholesterol [ Time Frame: 6 weeks ] Interleukin‐6 [ Time Frame: 6 weeks ] Energy intakes (kilocalories) and macronutrient intakes (grams) from food diaries [ Time Frame: 6 weeks ] |
| Starting date | ‐ |
| Contact information | Suzanne Zaremba, Queen Margaret University |
| Notes | 6‐week intervention only proposed ‐ monitor |
Wedick 2015.
| Study name | ‐ |
| Methods | RCT non‐blinded, cross‐over design |
| Participants | South Indian adults Inclusion: age 25 to 65 years, BMI >= 23 kg/m2, waist >= 90 cm in men or >= 80 cm in women, daily rice intake >= 100 g/day Exclusion: fasting glucose >= 126 mg/dL, postprandial glucose >= 200 mg/dL, having any chronic disease |
| Interventions | Brown rice vs white rice |
| Outcomes | Anthropometrics, glucose profile, lipid profile, blood pressure, dietary intake, and physical activity |
| Starting date | ‐ |
| Contact information | drmohans@diabetes.ind.in |
| Notes |
BMI: body mass index HDL: high‐density lipoprotein RCT: randomised controlled trial
Differences between protocol and review
We expanded the inclusion criteria for this review to include the primary and secondary prevention of coronary heart disease. Since the last update of this review in 2007, a separate Cochrane Review has been published focusing on the effects of whole grain foods for the prevention of type 2 diabetes mellitus (Priebe 2008). Hence, we excluded studies with diabetes as an outcome or changes in related risk factors including impaired glucose tolerance, insulin resistance or sensitivity, glucose or insulin outcomes. We excluded studies reporting weight, body mass index, and other anthropometric outcomes if they did not also measure lipids or blood pressure. As more trials are currently available, we have excluded short‐term studies and included only those of at least 12 weeks' duration. We specified an eligible participant age of inclusion of 18 years or older (previously ≥ 16 years).
Contributions of authors
SK, LH, CC, RG, HL, and HJ screened titles and abstracts and assessed studies for formal inclusion and exclusion.
SK, LA, EL, and JC abstracted data and assessed methodological rigour.
Analyses were conducted by EL and LA and checked by JC and KR.
SK wrote the first draft of the review, which was updated by EL, KR, LA, and SK.
LA retrieved trial records and conducted GRADE assessment and 'Summary of findings' table, which was checked by SK.
GF critically read the final draft.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK
-
University of Teesside, Middlesbrough, UK
Supported the original version of the review
-
Hammersmith Hospital, UK
Supported the original version of the review
External sources
NIHR Cochrane Programme Grant, UK
Lena Al‐Khudairy and Helen M Jones are supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK
Declarations of interest
SK: None known.
LH: None known.
EL: None known.
JC: None known.
HJ: None known.
LA: None known.
CC: None known.
RG: None known.
HL: None known.
GF: None of the relationships described are felt to be a conflict of interest regarding this publication. Consultancy for appetite regulation with Unilever; grant application to Nestle on modified cereal fibre and glycaemic control (awaiting outcome); patent on compounds and their effects on appetite control and insulin sensitivity (WO2014020344 A1).
KR: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Brownlee 2010 {published data only}
- Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, et al. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. British Journal of Nutrition 2010;104(1):125-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giacco 2013 {published data only}
- Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases 2014;24(8):837-44. [DOI] [PubMed] [Google Scholar]
- Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, et al. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clinical Nutrition 2013;32:941-9. [DOI] [PubMed] [Google Scholar]
- Vetrani C, Costabile G, Luongo D, Naviglio D, Rivellese AA, Riccardi G, et al. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition 2016;32(2):217-21. [DOI] [PubMed] [Google Scholar]
Harris 2014 {published data only}
- Harris JK, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, et al. Effects of whole and refined grains in a weight loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled feeding trial. American Journal of Clinical Nutrition 2014;100:577-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katcher 2008 {published data only}
- Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, et al. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. American Journal of Clinical Nutrition 2008;87(1):79-90. [DOI] [PubMed] [Google Scholar]
Kristensen 2012 {published data only}
- Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. Journal of Nutrition 2012;142(4):710-6. [DOI] [PubMed] [Google Scholar]
Lankinen 2014 {published data only}
- Lankinen M, Kolehmainen M, Jääskeläinen T, Paananen J, Joukamo L, Kangas AJ, et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS ONE 2014;9(2):e90352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen M, Schwab U, Kolehmainen M, Paananen J, Poutanen K, Mykkänen H, et al. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLoS ONE 2011;6(8):e22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00573781. Dietary modulation of gene expression and metabolic pathways in glucose metabolism (Sysdimet). clinicaltrials.gov/ct2/show/NCT00573781 (first received 13 December 2007).
Maki 2010 {published data only}
- Maki KC, Beiseigel JM, Jonnalagadda SS, Gugger CK, Reeves MS, Farmer MV, et al. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. Journal of the American Dietetic Association 2010;110(2):205-14. [DOI] [PubMed] [Google Scholar]
Tighe 2010‐W {published data only}
- Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S et al. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. American Journal of Clinical Nutrition 2010;92(40):733-40. [DOI] [PubMed] [Google Scholar]
Tighe 2010‐WO {published data only}
- Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S et al. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. American Journal of Clinical Nutrition 2010;40:733-40. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang G, Pan A, Zong G, Yu Z, Wu H, Chen X, et al. Substituting white rice with brown rice for 16 weeks does not substantially affect metabolic risk factors in middle-aged Chinese men and women with diabetes or a high risk for diabetes. Journal of Nutrition 2011;141:1685-90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrahamsson 1994 {published data only}
- Abrahamsson L, Goranzon H, Karlstrom B, Vessby B, Aaman P. Metabolic effects of oat bran and wheat bran in healthy women. Scandinavian Journal of Nutrition/Naringsforskning 1994;38:5-10. [Google Scholar]
Ampatzoglou 2015 {published data only}
- Ampatzoglou A, Atwal KK, Maidens CM, Williams CL, Ross AB, Thielecke F, et al. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. Journal of Nutrition 2015;145(2):215-21. [DOI] [PubMed] [Google Scholar]
Anderson 1978 {published data only}
- Anderson JW, Ward K. Long-term effects of high-carbohydrate, high-fiber diets on glucose and lipid metabolism: a preliminary report on patients with diabetes. Diabetes Care 1978;1(2):77-82. [DOI] [PubMed] [Google Scholar]
Anderson 1979 {published data only}
- Anderson JW, Ward KW. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. American Journal of Clinical Nutrition 1979;32:2312-21. [DOI] [PubMed] [Google Scholar]
Anderson 2009 {published data only}
- Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A et al. Health benefits of dietary fiber. Nutrition Reviews 2009;67(4):188-205. [DOI] [PubMed] [Google Scholar]
Andersson 2007 {published data only}
- Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S et al. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. Journal of Nutrition 2007;137(6):1401-7. [DOI] [PubMed] [Google Scholar]
Asp 1981 {published data only}
- Asp N-G, Agardh C-D, Ahren B, Dencker I, Johansson C-G, Lundquist I, et al. Dietary fibre in Type II diabetes. Acta Medica Scandinavica 1981;Suppl 656:47-50. [DOI] [PubMed] [Google Scholar]
Azadbakht 2005 {published data only}
- Azadbakht L, Haghighatdoost F, Feizi A, Esmaillzadeh A. Breakfast eating pattern and its association with dietary quality indices and anthropometric measurements in young women in Isfahan. Nutrition 2013;29:420-5. [DOI] [PubMed] [Google Scholar]
Beer 2000 {published data only}
- Beer MU, Clayton D, Pelletier X. Cholesterol reducing effects of a food containing oat bran and soy germ in mildly hypercholesterolemic subjects. Atherosclerosis 2000;151:117. [Google Scholar]
Behall 2004a {published data only}
- Behall KM, Scholfield DJ, Hallfrisch J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. American Journal of Clinical Nutrition 2004;80(5):1185-93. [DOI] [PubMed] [Google Scholar]
Behall 2004b {published data only}
- Behall KM, Scholfield DJ, Hallfrisch J. Lipids significantly reduced by diets containing barley in moderately hypercholesterolemic men. Journal of the American College of Nutrition 2004;23(1):55-62. [DOI] [PubMed] [Google Scholar]
Behall 2006 {published data only}
- Behall KM, Scholfield DJ, Hallfrisch J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. Journal of the American Dietetic Association 2006;106(9):1445-9. [DOI] [PubMed] [Google Scholar]
Berg 2003 {published data only}
- Berg A, König D, Deibert P, Grathwohl D, Berg A, Baumstark MW et al. Effect of an oat bran enriched diet on the atherogenic lipid profile in patients with an increased coronary heart disease risk. A controlled randomized lifestyle intervention study. Annals of Nutrition and Metabolism 2003;47:306-11. [DOI] [PubMed] [Google Scholar]
Birkeland 1991 {published data only}
- Birkeland KI, Gullestad L, Torsvik H. Cholesterol-lowering effect of oats. Tidsskrift for Den Norske Laegeforening 1991;111(17):2081-5. [PubMed] [Google Scholar]
Birketvedt 2000 {published data only}
- Birketvedt GS, Auseth J, Florholmen JR, Ryttig K. Long term effect of fibre supplement and reduced energy intake on body weight and blood lipids in overweight subjects. Acta Medica 2000;43(3):129-32. [PubMed] [Google Scholar]
Bodinham 2011 {published data only}
- Bodinham CL, Hitchen KL, Youngman PJ, Frost GS, Robertson MD. Short-term effects of whole-grain wheat on appetite and food intake in healthy adults: a pilot study. British Journal of Nutrition 2011;106(3):327-30. [DOI] [PubMed] [Google Scholar]
Bourdon 1999 {published data only}
- Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, et al. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. American Journal of Clinical Nutrition 1999;69:55-63. [DOI] [PubMed] [Google Scholar]
Braaten 1994 {published data only}
- Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P et al. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. European Journal of Clinical Nutrition 1994;48(7):465-74. [PubMed] [Google Scholar]
Brock 2006 {published data only}
- Brock DW, Davis CK, Irving BA, Rodriguez J, Barrett EJ, Weltman A et al. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care 2006;29(10):2313-5. [DOI] [PubMed] [Google Scholar]
Brownlee 2013 {published data only}
- Brownlee IA, Kuznesof SA, Moore C, Jebb SA, Seal CJ. The impact of a 16-week dietary intervention with prescribed amounts of whole-grain foods on subsequent, elective whole grain consumption. British Journal of Nutrition 2013;110(5):943-8. [DOI] [PubMed] [Google Scholar]
Bruce 2000 {published data only}
- Bruce B, Spiller GA, Klevay LM, Gallagher SK. A diet high in whole and unrefined foods favorably alters lipids, antioxidant defenses, and colon function. Journal of the American College of Nutrition 2000;19(1):61-7. [DOI] [PubMed] [Google Scholar]
Brussaard 1981 {published data only}
- Brussaard JH, Raaij JM, Stasse-Wolthuis M, Katan MB, Hautvast JG. Blood pressure and diet in normotensive volunteers: absence of an effect of dietary fiber, protein, or fat. American Journal of Clinical Nutrition 1981;34(10):2023-9. [DOI] [PubMed] [Google Scholar]
Bruttomesso 1989 {published data only}
- Bruttomesso D, Briani G, Bilardo G, Vitale E, Lavagnini T, Marescotti C, et al. The medium-term effect of natural or extractive dietary fibres on plasma amino acids and lipids in type 1 diabetics. Diabetes Research and Clinical Practice 1989;6:149-55. [DOI] [PubMed] [Google Scholar]
Burley 1987 {published data only}
- Burley VJ, Leeds AR, Blundell JE. The effect of high and low-fibre breakfasts on hunger, satiety and food intake in a subsequent meal. International Journal of Obesity & Related Metabolic Disorders 1987;11:87-93. [PubMed] [Google Scholar]
Burr 1989 {published data only}
- Burr ML, Gilbert JF, Holliday RM, Elwood PC, Fehily AM, Rogers S et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989;2:757-61. [DOI] [PubMed] [Google Scholar]
Buyken 2000 {published data only}
- Buyken AE, Toeller M, Heitkamp G, Irsigler K, Holler C, Santeusanio F et al. Carbohydrate sources and glycaemic control in Type 1 diabetes mellitus. EURODIAB IDDM Complications Study Group. Diabetic Medicine 2000;17(5):351-9. [DOI] [PubMed] [Google Scholar]
Cairella 1995 {published data only}
- Cairella G, Cairella M, Marchini G. Effect of dietary fibre on weight correction after modified fasting. European Journal of Clinical Nutrition 1995;49(Suppl 3):S325-7. [PubMed] [Google Scholar]
Cara 1992 {published data only}
- Cara L, Armand M, Borel P, Senft M, Portugal H, Pauli AM et al. Long-term wheat germ intake beneficially affects plasma lipids and lipoproteins in hypercholesterolemic human subjects. Journal of Nutrition 1992;122:317-26. [DOI] [PubMed] [Google Scholar]
Carvalho‐Wells 2010 {published data only}
- Carvalho-Wells AL, Helmolz K, Nodet C, Molzer C, Leonard C, McKevith B et al. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: a human feeding study. British Journal of Nutrition 2010;104(9):1353-6. [DOI] [PubMed] [Google Scholar]
Chandalia 2000 {published data only}
- Chandalia M, Garg A, Lutjohann D, Von Bergman K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. New England Journal of Medicine 2000;342:1392-8. [DOI] [PubMed] [Google Scholar]
Chang 2013 {published data only}
- Chang HC, Huang CN, Yeh DM, Wang SJ, Peng CH, Wang CJ. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 2013;68(1):18-23. [DOI] [PubMed] [Google Scholar]
Charlton 2012 {published data only}
- Charlton KE, Tapsell LC, Batterham MJ, O'Shea J, Thorne R, Beck E et al. Effect of 6 weeks' consumption of -glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. British Journal of Nutrition 2012;107(7):1037-47. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen J, He J, Wildman RP, Reynolds K, Streiffer RH, Whelton PK. A randomized controlled trial of dietary fiber intake on serum lipids. European Journal of Clinical Nutrition 2006;60(1):62-8. [DOI] [PubMed] [Google Scholar]
Chi 2012 {published data only}
- Chi J, Zhang Q, Zhai CK, Zhang H, Han SF, Liu YQ et al. Influences of compound whole grain on oxidative stress to hyperlipidemia population. Chung-Hua Yu Fang i Hsueh Tsa Chih [Chinese Journal of Preventive Medicine] 2012;46:143-7. [PubMed] [Google Scholar]
Cohen 1980 {published data only}
- Cohen M, Leong VW, Salmon E, Martin FI. Role of guar and dietary fibre in the management of diabetes mellitus. Medical Journal of Australia 1980;1:59-61. [DOI] [PubMed] [Google Scholar]
Collier 1982 {published data only}
- Collier G, O'Dea K. Effect of physical form of carbohydrate on the postprandial glucose, insulin, and gastric inhibitory responses in type 2 diabetes. American Journal of Clinical Nutrition 1982;36:10-4. [DOI] [PubMed] [Google Scholar]
Comi 1995 {published data only}
- Comi D, Brugnani M, Gianino A. Metabolic effects of hypocaloric high-carbohydrate/high-fibre diet in non-insulin dependent diabetic patients. European Journal of Clinical Nutrition 1995;49(Suppl 3):S242-4. [PubMed] [Google Scholar]
Connell 1975 {published data only}
- Connell AM, Smith CL, Somsel M. Absence of effect of bran on blood-lipids. Lancet 1975;1:496-7. [DOI] [PubMed] [Google Scholar]
Connolly 2016 {published data only}
- Connolly ML, Tsounis X, Tuohy KM, Lovegrove JA. Hypocholesterolemic and prebiotic effects of a whole-grain oat based granola breakfast cereal in a cardiometabolic 'at-risk' population. Frontiers in Microbiology 2016;7:Article 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costabile 2008 {published data only}
- Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. British Journal of Nutrition 2008;99(1):110-20. [DOI] [PubMed] [Google Scholar]
Crapo 1981 {published data only}
- Crapo PA, Insel J, Sperling M, Kolterman OG. Comparison of serum glucose, insulin, and glucagon responses to different types of complex carbohydrate in noninsulin-dependent diabetic patients. American Journal of Clinical Nutrition 1981;34:184-90. [DOI] [PubMed] [Google Scholar]
Cugnet‐Anceau 2010 {published data only}
- Cugnet-Anceau C, Nazare JA, Biorklund M, Le Coquil E, Sassolas A, Sothier M et al. A controlled study of consumption of beta-glucan-enriched soups for 2 months by type 2 diabetic free-living subjects. British Journal of Nutrition 2010;103(3):422-8. [DOI] [PubMed] [Google Scholar]
Data 1980 {published data only}
- Data PG, Cacchio M, Sergiacomo P, Di Tano G. Investigation of the importance of dietetic fibers in the regulation of cholesterolemia. Bollettino della Societa Italiana di Biologia Sperimentale 1980;16:1545-50. [PubMed] [Google Scholar]
Dattilo 1992 {published data only}
- Dattilo AM, Dugan L, Burns J, Davidson MH, Synecki C. Exploring the lipid-lowering effects of oats. Journal of Cardiopulmonary Rehabilitation 1992;12:164-6. [Google Scholar]
Davidson 1991 {published data only}
- Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The hypocholesterolemic effects of beta-glucan in oatmeal and oat bran. JAMA 1991;265(14):1833-9. [PubMed] [Google Scholar]
Davy 2002a {published data only}
- Davy BM, Melby CL, Beske SD, Ho RC, Davrath LR, Davy KP. Oat consumption does not affect resting casual and ambulatory 24-h arterial blood pressure in men with high-normal blood pressure to stage I hypertension. Journal of Nutrition 2002;132(3):394-8. [DOI] [PubMed] [Google Scholar]
Davy 2002b {published data only}
- Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. American Journal of Clinical Nutrition 2002;76:351-8. [DOI] [PubMed] [Google Scholar]
Demark‐Wahnefried 1990 {published data only}
- Demark-Wahnefried W, Bowering J, Cohen PS. Reduced serum cholesterol with dietary change using fat-modified and oat bran supplemented diets. Journal of the American Dietetic Association 1990;90:223-9. [PubMed] [Google Scholar]
de Mello 2011 {published data only}
- Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K et al. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia 2011;54(11):2755-67. [DOI] [PubMed] [Google Scholar]
Di Capua 2010 {published data only}
- Di Capua L, Bozzetto L, De Natale C, Giacco R, Patti L, Maione S, et al. Effects on systemic inflammation of dietary approaches useful for cardiovascular risk reduction [Effetti sull'infiammazione sistemica di approcci nutrizionali utili per la riduzione del rischio cardiovascolare]. Giornale Italiano di Diabetologia e Metabolismo 2010;30(1):13-8. [Google Scholar]
Dixit 2011 {published data only}
- Dixit AA, Azar KM, Gardner CD, Palaniappan LP. Incorporation of whole, ancient grains into a modern Asian Indian diet to reduce the burden of chronic disease. Nutrition Reviews 2011;69(8):479-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebell 2000 {published data only}
- Ebell M. Does a diet high in fiber improve disease-oriented end points in patients with type 2 diabetes mellitus? Evidence-Based Practice 2000;3(10, insert):12p. [Google Scholar]
Eliasson 1992 {published data only}
- Eliasson K, Ryttig KR, Hylander B, Rossner S. A dietary fibre supplement in the treatment of mild hypertension. A randomized, double-blind, placebo-controlled trial. Journal of Hypertension 1992;10:195-9. [DOI] [PubMed] [Google Scholar]
Ellis 2005 {published data only}
- Ellis J, Johnson MA, Fischer JG, Hargrove JL. Nutrition and health education intervention for whole grain foods in the Georgia Older Americans Nutrition Program. Journal of Nutrition for the Elderly 2005;24:67-83. [DOI] [PubMed] [Google Scholar]
Fappa 2013 {published data only}
- Fappa E, Georgiadi T, Mestana S, Vasiliki B. Increasing whole grain food consumption seems to be a promising strategy in promoting weight loss in overweight or obese adults. Annals of Nutrition and Metabolism 2013;63:1371. [Google Scholar]
Fehily 1986 {published data only}
- Fehily AM, Burr M, Butland BK, Eastham RD. A randomised controlled trial to investigate the effect of a high fibre diet on blood pressure and plasma fibrinogen. Journal of Epidemiology & Community Health 1986;40:334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fordyce‐Baum 1989 {published data only}
- Fordyce-Baum MK, Langer L, Mantero-Atienza E, Crass R, Beach RS. Use of an expanded whole-wheat product in the reduction of body weight and serum lipids in obese females. American Journal of Clinical Nutrition 1989;50(1):30-6. [DOI] [PubMed] [Google Scholar]
Fung 2002 {published data only}
- Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. American Journal of Clinical Nutrition 2002;76:535-40. [DOI] [PubMed] [Google Scholar]
Giacco 2010 {published data only}
- Giacco R, Clemente G, Cipriano D, Luongo D, Viscovo D, Patti L, et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutrition, Metabolism and Cardiovascular Diseases 2010;20(3):186-94. [DOI] [PubMed] [Google Scholar]
Golay 1992 {published data only}
- Golay A, Koellreutter B, Bloise D, Assal J-P, Wursch P. The effect of muesli or cornflakes at breakfast on carbohydrate metabolism in type 2 diabetic patients. Diabetes Research & Clinical Practice 1992;15:135-42. [DOI] [PubMed] [Google Scholar]
Guzic 1994 {published data only}
- Guzic B, Sundell IB, Keber I, Keber D. The effect of oat husk supplementation in diet on plasminogen activator inhibitor type 1 in diabetic survivors of myocardial infarction. Fibrinolysis 1994;8(Suppl 2):44-6. [Google Scholar]
Hagander 1985 {published data only}
- Hagander B, Bjorck I, Asp N-G, Lunquist I, Nilsson-Ehle P, Schrezenmeir J, et al. Hormonal and metabolic responses to breakfast meals in NIDDM: comparison of white and whole-grain wheat bread and corresponding extruded products. Human Nutrition. Applied Nutrition 1985;39A:114-23. [PubMed] [Google Scholar]
Hagander 1988 {published data only}
- Hagander B, Asp N-G, Efendic S, Nilsson-Ehle P, Schersten B. Dietary fiber decreases fasting blood glucose levels and plasma LDL concentration in non-insulin-dependent diabetes mellitus patients. American Journal of Clinical Nutrition 1988;47:852-8. [DOI] [PubMed] [Google Scholar]
He 1995 {published data only}
- He J, Klag MJ, Whelton PK, Mo J-P, Qian M-C, Mo P-S, et al. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. American Journal of Clinical Nutrition 1995;61:366-72. [DOI] [PubMed] [Google Scholar]
Heaton 1976 {published data only}
- Heaton KW, Manning AP, Hartog M. Lack of effect on blood lipid and calcium concentrations of young men on changing from white to wholemeal bread. British Journal of Nutrition 1976;35:55-60. [DOI] [PubMed] [Google Scholar]
Hoffman 1982 {published data only}
- Hoffman CR, Fineberg SE, Howey DC, Clark CM, Pronsky Z. Short-term effects of a high-fiber, high-carbohydrate diet in very obese diabetic individuals. Diabetes Care 1982;5(6):605-11. [DOI] [PubMed] [Google Scholar]
Hollenbeck 1986 {published data only}
- Hollenbeck CB, Coulston AM, Reaven GM. To what extent does increased dietary fiber improve glucose and lipid metabolism in patients with noninsulin-dependent diabetes mellitus (NIDDM)? American Journal of Clinical Nutrition 1986;43:16-24. [DOI] [PubMed] [Google Scholar]
Hunninghake 1994 {published data only}
- Hunninghake DB, Miller VT, LaRosa JC, Kinosian B, Brown V, Howard WJ, et al. Hypocholesterolemic effects of a dietary fiber supplement. American Journal of Clinical Nutrition 1994;59:1050-4. [DOI] [PubMed] [Google Scholar]
Jacobs 2002 {published data only}
- Jacobs DR, Pereira MA, Stumpf K, Pins JJ, Adlercreutz H. Whole grain food intake elevates serum enterolactone. British Journal of Nutrition 2002;88:111-6. [DOI] [PubMed] [Google Scholar]
Jang 2001 {published data only}
- Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arteriosclerosis Thrombosis & Vascular Biology 2001;21:2065-71. [DOI] [PubMed] [Google Scholar]
Jenkins 1985 {published data only}
- Jenkins DJA, Wolever TMS, Kalmusky J, Giudici S, Giordano C, Wong GS, et al. Low glycemic index carbohydrate foods in the management of hyperlipidemia. American Journal of Clinical Nutrition 1985;42:604-17. [DOI] [PubMed] [Google Scholar]
Jenkins 1993 {published data only}
- Jenkins D, Wolever T, Rao V, Hegele RA, Mitchell SJ, Ransom T, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. New England Journal of Medicine 1993;329:21-6. [DOI] [PubMed] [Google Scholar]
Jenkins 2008 {published data only}
- Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA 2008;300(23):2742-53. [DOI] [PubMed] [Google Scholar]
Johnston 1998 {published data only}
- Johnston L, Reynolds HB, Hunninghake DB, Schultz K, Westereng B. Cholesterol-lowering benefits of a whole grain oat ready to eat cereal. Nutrition Clinical Care 1998;1(1):6-12. [Google Scholar]
Judd 1981 {published data only}
- Judd PA, Truswell AS. The effect of rolled oats on blood lipids and fecal steroid excretion in man. American Journal of Clinical Nutrition 1981;34:2061-7. [DOI] [PubMed] [Google Scholar]
Juntunen 2002 {published data only}
- Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkanen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. American Journal of Clinical Nutrition 2002;75:254-62. [DOI] [PubMed] [Google Scholar]
Juntunen 2003 {published data only}
- Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkanen HM. High-fiber rye bread and insulin secretion and sensitivity in healthy menopausal women. American Journal of Clinical Nutrition 2003;77:385-91. [DOI] [PubMed] [Google Scholar]
Kabir 2002 {published data only}
- Kabir M, Oppert J-M, Vidal H, Bruzzo F, Fiquet C, Wursch P, et al. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism: Clinical & Experimental 2002;51(7):819-26. [DOI] [PubMed] [Google Scholar]
Karl 2016 {published data only}
- Connolly Ml, Tzounis X, Tuohy KM, Lovegrove JA. Hypocholesterolemic and prebiotic effects of a whole-grain oat-based granola breakfast cereal in a cardio-metabolic "at risk" population. Frontiers in Microbiology 2016;7:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlstrom 1984 {published data only}
- Karlstrom B, Vessby B, Asp N-G, Boberg M, Gustafsson I-B, Lithell H, et al. Effects of increased content of cereal fibre in the diet of Type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1984;26:272-7. [DOI] [PubMed] [Google Scholar]
Karmally 2005 {published data only}
- Karmally W, Montez WG. Cholesterol-lowering benefits of oat-containing cereal in Hispanic Americans. Journal of the American Dietetic Association 2005;105(6):967-70. [DOI] [PubMed] [Google Scholar]
Katz 2001a {published data only}
- Katz DL, Nawaz H, Boukhalil J, Chan W, Ahmadi R, Giannamore V, et al. Effects of oat and wheat cereals on endothelial responses. Preventive Medicine 2001;33:476-84. [DOI] [PubMed] [Google Scholar]
Katz 2001b {published data only}
- Katz DL, Nawaz H, Boukhalil J, Giannamore V, Chan W, Ahmadi R, et al. Acute effects of oats and vitamin E on endothelial responses to ingested fat. American Journal of Preventive Medicine 2001;20(2):124-9. [DOI] [PubMed] [Google Scholar]
Kay 1977 {published data only}
- Kay RM, Truswell AS. The effect of wheat fibre on plasma lipids and faecal steroid excretion in man. British Journal of Nutrition 1977;37:227-35. [DOI] [PubMed] [Google Scholar]
Kay 1981 {published data only}
- Kay RM, Grobin W, Track NS. Diets rich in natural fibre improve carbohydrate tolerance in maturity-onset, non-insulin dependent diabetics. Diabetologia 1981;20:18-21. [DOI] [PubMed] [Google Scholar]
Keenan 2002 {published data only}
- Keenan JM, Pins JJ, Frazel C, Moran A, Turnquist L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: a pilot trial. Journal of Family Practice 2002;51(4):369. [PubMed] [Google Scholar]
Kesaniemi 1990 {published data only}
- Kesaniemi YA, Tarpila S, Miettinen TA. Low vs high dietary fiber and serum, biliary, and fecal lipids in middle-aged men. American Journal of Clinical Nutrition 1990;51:1007-12. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim JY, Kim JH, Lee DH, Kim SH, Lee SS. Meal replacement with mixed rice is more effective than white rice in weight control, while improving antioxidant enzyme activity in obese women. Nutrition Research 2008;28(2):66-71. [DOI] [PubMed] [Google Scholar]
Kirwan 2016 {published data only}
- Kirwan JP, Malin SK, Scelsi AR, Kullman El, Navaneethan SD, Pagadala MR, et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. Journal of Nutrition 2016;146(11):2244-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleemola 1999 {published data only}
- Kleemola P, Puska P, Vartiainnen, Roos E, Luoto R, Ehnholm C. The effect of breakfast cereal on diet and serum cholesterol: a randomized trial in North Karelia, Finland. European Journal of Clinical Nutrition 1999;53:716-21. [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 2002 {published data only}
- Kris-Etherton PM, Shaffer-Taylor D, Smicklas-Wright H, Mitchell DC, Bekhuis TC, Olson BH et al. High-soluble-fiber foods in conjunction with a telephone-based, personalized behavior change support service result in favorable changes in lipids and lifestyles after 7 weeks. Journal of the American Dietetic Association 2001;102:503-10. [DOI] [PubMed] [Google Scholar]
Lakshmi 1996 {published data only}
- Lakshmi KB, Vimala V. Hypoglycemic effect of selected sorghum recipes. Nutrition Research 1996;16(10):1651-8. [Google Scholar]
Lankinen 2010 {published data only}
- Lankinen M, Schwab U, Gopalacharyulu PV, Seppänen-Laakso T, Yetukuri L, Sysi-Aho M et al. Dietary carbohydrate modification alters serum metabolic profiles in individuals with the metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases 2010;20(4):249-57. [DOI] [PubMed] [Google Scholar]
Leinonen 1999 {published data only}
- Leinonen K, Liukkonen K, Poutanene K, Uusitupa M, Mykkanen H. Rye bread decreases postprandial insulin response but does not alter glucose response in healthy Finnish subjects. European Journal of Clinical Nutrition 1999;53(4):262-7. [DOI] [PubMed] [Google Scholar]
Leinonen 2000 {published data only}
- Leinonen KS, Poutanen KS, Mykkanen HM. Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. Journal of Nutrition 2000;130:164-70. [DOI] [PubMed] [Google Scholar]
Liese 2003 {published data only}
- Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB, Mayer-Davis E. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. American Journal of Clinical Nutrition 2003;78(5):965-71. [DOI] [PubMed] [Google Scholar]
Lousley 1984 {published data only}
- Lousley SE, Jones DB, Slaughter P, Carter RD, Jelfs R, Mann JI. High carbohydrate-high fibre diets in poorly controlled diabetes. Diabetic Medicine 1984;1(1):21-5. [DOI] [PubMed] [Google Scholar]
MacKay 2012 {published data only}
- MacKay KA, Tucker AJ, Duncan AM, Graham TE, Robinson LE. Whole grain wheat sourdough bread does not affect plasminogen activator inhibitor-1 in adults with normal or impaired carbohydrate metabolism. Nutrition, Metabolism and Cardiovascular Diseases 2012;22:704-11. [DOI] [PubMed] [Google Scholar]
MacMahon 1998 {published data only}
- MacMahon M, Carless J. Ispaghula husk in the treatment of hypercholesterolaemia: a double blind controlled study. Journal of Cardiovascular Risk 1998;5(3):167-72. [PubMed] [Google Scholar]
Maki 2003 {published data only}
- Maki KC, Davidson MH, Ingram KA, Veith PE. Lipid responses to consumption of a beta-glucan containing ready-to-eat cereal in children and adolescents with mild-to-moderate primary hypercholesterolemia. Nutrition Research 2003;23(11):1527-35. [Google Scholar]
Maki 2007 {published data only}
- Maki KC, Galant R, Samuel P, Tesser J, Witchger MS, Ribaya-Mercado JD et al. Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. European Journal of Clinical Nutrition 2007;61(6):786-95. [DOI] [PubMed] [Google Scholar]
Manhire 1981 {published data only}
- Manhire A, Henry CL, Hartog M, Heaton KW. Unrefined carbohydrate and dietary fibre in treatment of diabetes mellitus. Journal of Human Nutrition 1981;35:99-101. [DOI] [PubMed] [Google Scholar]
Mathur 1968 {published data only}
- Mathur KS, Khan MA, Sharma RD. Hypocholesterolaemic effect of Bengal gram: a long-term study in man. British Medical Journal 1968;1:30-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGeoch 2013 {published data only}
- McGeoch SC, Johnstone AM, Lobley GE, Adamson J, Hickson K, Holtrop G et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in Type 2 diabetes. Diabetic Medicine 2013;30(11):1314-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 1991 {published data only}
- McIntosh GH, Whyte J, McArthur R, Nestel PJ. Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. American Journal of Clinical Nutrition 1991;53:1205-9. [DOI] [PubMed] [Google Scholar]
McIntosh 2003 {published data only}
- McIntosh GH, Noakes M, Royle PJ, Foster PR. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. American Journal of Clinical Nutrition 2003;77(4):967-74. [DOI] [PubMed] [Google Scholar]
Melanson 2006 {published data only}
- Melanson KJ, Angelopoulos TJ, Nguyen VT, Martini M, Zukley L, Lowndes J. Consumption of whole-grain cereals during weight loss: effects on dietary quality, dietary fiber, magnesium, vitamin B-6, and obesity. Journal of the American Dietetic Association 2006;106(9):1380-8. [DOI] [PubMed] [Google Scholar]
Meydani 2016 {published data only}
- Meydani M, Thomas M, Barnett JB, Vanegas S, Chen O, Dolnikowski G, et al. Short term consumption of whole grain foods independent of weight loss does not affect surrogate markers of CVD. FASEB Journal 2016;30(1 Suppl):678.10. [Google Scholar]
Moazzami 2012 {published data only}
- Moazzami AA, Bondia-Pons I, Hanhineva K, Juntunen K, Antl N, Poutanen K, et al. Metabolomics reveals the metabolic shifts following an intervention with rye bread in postmenopausal women - a randomized control trial. Nutrition Journal 2012;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montonen 2003 {published data only}
- Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. American Journal of Clinical Nutrition 2003;77:622-9. [DOI] [PubMed] [Google Scholar]
Nielsen 1988 {published data only}
- Nielsen GL, Thuesen H. Blood glucose responses to sweetcorn and potato meals. Diabetic Medicine 1988;5(6):598-9. [DOI] [PubMed] [Google Scholar]
O'Kell 1988 {published data only}
- O'Kell RT, Duston AA. Lack of effect of dietary oats on serum cholesterol. Missouri Medicine 1988;85(11):726-8. [PubMed] [Google Scholar]
Odes 1993 {published data only}
- Odes HS, Lazovski H, Stern I, Madar Z. Double-blind trial of a high dietary fiber, mixed grain cereal in patients with chronic constipation and hyperlipidemia. Nutrition Research 1993;13:979-85. [Google Scholar]
Pacy 1986 {published data only}
- Pacy PJ, Dodson PM, Taylor MP. The effect of a high fibre, low fat, low sodium diet on diabetics with intermittent claudication. British Journal of Clinical Practice 1986;40(8):313-7. [PubMed] [Google Scholar]
Pereira 2002 {published data only}
- Pereira MA, Jacobs JR, Pins JJ, Raatz SK, Gross MD, Slavin JL, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. American Journal of Clinical Nutrition 2002;75:848-55. [DOI] [PubMed] [Google Scholar]
Pins 2002 {published data only}
- Keenan JM, Pins JJ, Geleva D, Frazel C, O'Connor PJ, Cherney LM. Whole-grain oat cereal consumption reduces antihypertensive medication need: a cost analysis. Preventive Medicine in Managed Care 2002;3(1):9-17. [Google Scholar]
- Pins JJ, Geleva DRD, Keenan JM, Frazel C, O'Connor PJ, Cherney LM. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? Journal of Family Practice 2002;51(4):353-9. [PubMed] [Google Scholar]
Poulter 1993 {published data only}
- Poulter N, Chang CL, Cuff A, Poulter C, Sever P, Thom S. Lipid profiles after the daily consumption of an oat-based cereal: a controlled crossover trial. American Journal of Clinical Nutrition 1993;58:66-9. [DOI] [PubMed] [Google Scholar]
Rave 2007 {published data only}
- Rave K, Roggen K, Dellweg S, Heise T, Dieck H. Improvement of insulin resistance after diet with a whole-grain based dietary product: results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. British Journal of Nutrition 2007;98(5):929-36. [DOI] [PubMed] [Google Scholar]
Reynolds 1989 {published data only}
- Reynolds HR, Lindeke E, Hunninghake DB. Effect of oat bran on serum lipids. Journal of the American Dietetic Association 1989;89(Suppl):A112. [Google Scholar]
Reynolds 2000 {published data only}
- Reynolds HR, Quiter E, Hunninghake DB. Whole grain oat cereal lowers serum lipids. Topics in Clinical Nutrition 2000;15(4):74-83. [Google Scholar]
Rigaud 1990 {published data only}
- Rigaud D, Ryttig KR, Angel LA, Apfelbaum M. Overweight treated with energy restriction and a dietary fibre supplement: a 6-month randomized, double-blind, placebo-controlled trial. International Journal of Obesity 1990;14:763-9. [PubMed] [Google Scholar]
Ross 2012 {published data only}
- Ross AB, Bourgeois A, Macharia HN, Kochhar S, Jebb SA, Brownlee IA et al. Plasma alkylresorcinols as a biomarker of whole-grain food consumption in a large population: results from the WHOLEheart Intervention Study. American Journal of Clinical Nutrition 2012;95(1):204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 1985 {published data only}
- Roth G, Leitzmann C. Long-term influence of breakfast cereals rich in dietary fibres on human blood lipid values [Langzeieinfluss ballast-stoffreicher Fruhstuck-cerealien auf die Blutlipide beim Menschen]. Aktuelle Ernahringsmedizin Klinik und Praxis 1985;10(3):106-9. [Google Scholar]
Russ 1985 {published data only}
- Russ CS, Atkinson RL. Use of high fiber diets for the outpatient treatment of obesity. Nutrition Reports International 1985;32(1):193-8. [Google Scholar]
Rytter 1996 {published data only}
- Rytter E, Erlanson-Albertsson C, Lindahl L, Lundquist I, Viberg U, Akesson B, et al. Changes in plasma insulin, enterostatin, and lipoprotein levels during an energy-restricted dietary regimen including a new oat-based liquid food. Annals of Nutrition and Metabolism 1996;40:212-20. [DOI] [PubMed] [Google Scholar]
Saltzman 2001a {published data only}
- Saltzman E, Moriguti JC, Das SK, Corrales A, Fuss P, Greenberg AS, et al. Effects of a cereal rich in soluble fiber on body composition and dietary compliance during consumption of a hypocaloric diet. Journal of the American College of Nutrition 2001;20(1):50-7. [DOI] [PubMed] [Google Scholar]
Saltzman 2001b {published data only}
- Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ, et al. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. Journal of Nutrition 2001;131:1465-70. [DOI] [PubMed] [Google Scholar]
Schlamowitz 1987 {published data only}
- Schlamowitz P, Halberg T, Warnoe O, Wilstrup F, Ryttig K. Treatment of mild to moderate hypertension with dietary fibre. Lancet 1987;2:622-3. [DOI] [PubMed] [Google Scholar]
Talati 2009 {published data only}
- Talati R, Baker WL, Pabilonia MS, White CM, Coleman CI. The effects of barley-derived soluble fiber on serum lipids. Annals of Family Medicine 2009;7(2):157-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tighe 2013 {published data only}
- Tighe P, Duthie G, Brittenden J, Vaughan N, Mutch W, Simpson WG, et al. Effects of wheat and oat-based whole grain foods on serum lipoprotein size and distribution in overweight middle aged people: a randomised controlled trial. PLoS ONE 2913;8(6):e70436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 1987 {published data only}
- Turnbull WH, Leeds AR. Reduction of total and LDL-C cholesterol in plasma by oats. Journal of Clinical Nutrition Gastroenterology 1987;2:177-81. [Google Scholar]
Turnbull 1989 {published data only}
- Turnbull WH, Leeds AR. The effect of rolled oats and a reduced/modified fat diet on apolipoproteins A1 and B. Journal of Clinical Nutrition - Gastroenterology 1989;1:15-9. [Google Scholar]
Turpeinen 2000 {published data only}
- Turpeinen AM, Juntunen K, Mutanen M, Mykkanen H. Similar responses in hemostatic factors after consumption of wholemeal rye bread and low-fiber wheat bread. European Journal of Clinical Nutrition 2000;54:418-23. [DOI] [PubMed] [Google Scholar]
Van Horn 1986 {published data only}
- Van Horn LV, Liu K, Parker D, Emidy L, Liao Y, Pan WH, et al. Serum lipid response to oat product intake with a fat-modified diet. Journal of the American Dietetic Association 1986;86(6):759-64. [PubMed] [Google Scholar]
Van Horn 1988 {published data only}
- Van Horn L, Emidy LA, Liu K, Liao Y, Ballew C, King J, et al. Serum lipid response to a fat-modified, oatmeal-enhanced diet. Preventive Medicine 1988;17:377-86. [DOI] [PubMed] [Google Scholar]
Van Horn 1991 {published data only}
- Van Horn L, Moag-Stahlberg A, Liu K, Ballew C, Ruth K, Hughes R, et al. Effects on serum lipids of adding instant oats to usual American diets. American Journal of Public Health 1991;81(2):183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Horn 2001 {published data only}
- Van Horn L, Liu K, Gerber J, Garside D, Schiffer L, Gernhofer N, et al. Oats and soy in lipid-lowering diets for women with hypercholesterolemia: is there synergy? Journal of the American Dietetic Association 2001;101:1319-25. [DOI] [PubMed] [Google Scholar]
Vitaglione 2015 {published data only}
- Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. American Journal of Clinical Nutrition 2015;101(2):251-61. [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang B, Medapalli R, Xu J, Cai W, Chen X, He JC et al. Effects of a whole rice diet on metabolic parameters and inflammatory markers in prediabetes. e-SPEN Journal 2013;8:e15-20. [Google Scholar]
Willms 1987 {published data only}
- Willms B, Arends J. Comparison of isolated (guar) and natural (Musli) dietary fiber in the treatment of type II diabetes [Verglech von isolierten (Guar) und naturlichen (Musli) Ballastoffen in der therapie des Typ-II-Diabetes]. Medizinische Klinik 1987;12/13:429-31. [PubMed] [Google Scholar]
Wolever 2003 {published data only}
- Wolever TMS, Tshilias EB, McBurney MI, Le N-A. Long-term effect of reduced carbohydrate or increased fiber intake on LDL particle size and HDL composition in subjects with type 2 diabetes. Nutrition Research 2003;23:15-26. [Google Scholar]
Wolever 2016 {published data only}
- Wolever TMS, Raederstorff D, Duss R. Oat beta-glucan reduces serum LDL cholesterol in humans with serum LDL cholesterol < 160 mg/dL. Immunology, Endocrine & Metabolic Agents in Medicinal Chemistry 2016;16(2):122-8. [Google Scholar]
Wolffenbuttel 1992 {published data only}
- Wolffenbuttel BHR, Sels J-PJE, Heesen BJ, Menheere PPCA, Nieuwenhuijzen-Kruseman AC. The effects of dietary fibre and insulin treatment on the serum levels of lipids and lipoprotein (a) in patients with diabetes mellitus type II. Nederlands Tijdschrift voor Geneeskunde 1992;136(15):739-42. [PubMed] [Google Scholar]
Wursch 1991 {published data only}
- Wursch P, Koellreutter B, Haesler E, Felber JP, Golay A. Metabolic effects of slow release starch in non-insulin dependent diabetic patients. Diabetes, Nutrition & Metabolism 1991;4(3):195-9. [Google Scholar]
Zhang 2012 {published data only}
- Zhang J, Li L, Song P, Wang C, Man Q, Meng L et al. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutrition Journal 2012;11(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bi 2013 {published data only}
- Bi M, Niu Y, Li X, Li Y, Sun C. Effects of barley flake on metabolism of glucose and lipids in patients with impaired fasting glucose. Wei sheng yan jiu [Journal of Hygiene Research] 2013;42(5):719-23. [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li X, Cai X, Ma X, Jing L, Gu J, Bao L, et al. Short-and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients 2016;8(9):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02615444 {published data only}
- NCT02615444. The effects of beta-glucan enriched oatcake consumption on metabolic disease risk factors. clinicaltrials.gov/ct2/show/NCT02615444 (first received 5 November 2015).
Wedick 2015 {published data only}
- Wedick NM, Sudha V, Spiegelman D, Bai MR, Malik VS, Venkatachalam SS, et al. Study design and methods for a randomized crossover trial substituting brown rice for white rice on diabetes risk factors in India. International Journal of Food Sciences and Nutrition 2015;66(7):797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aburto 2013
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aune 2016
- Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
BHF 2014
- British Heart Foundation. Cardiovascular disease 2014. www.bhf.org.uk/heart-health/conditions/cardiovascular-disease.aspx (accessed prior to 15 August 2017).
Bovet 2012
- Bovet P, Paccaud F. Cardiovascular disease and the changing face of global public health: a focus on low and middle income countries. Public Health Reviews 2012;33(2):397-415. [Google Scholar]
Brown 1999
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. American Journal of Clinical Nutrition 1999;69:30-42. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fardet 2010
- Fardet A. New hypothesis for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutrition Research Reviews 2010;23:65-134. [DOI] [PubMed] [Google Scholar]
Ferruzzi 2014
- Ferruzzi MG, Jonnalagadda SS, Liu S, Marquart L, McKeown N, Reicks M et al. Developing a standard definition of whole-grain foods for dietary recommendations: summary report of a multidisciplinary expert roundtable discussion. Advances in Nutrition 2014;5:164-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769-73. [DOI] [PubMed] [Google Scholar]
Global Burden of Disease Study 2015
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2015;388(10053):1659-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hu 2014
- Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutrition, Metabolism and Cardiovascular Diseases 2014;24(4):337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
JAMA 2004
- JAMA. Author Instructions: Système International (SI) Conversion Factors for Selected Laboratory Components. jama.ama-assn.org/misc/auinst_si.dtl 2004.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 1999
- Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson J, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses Health Study. American Journal of Clinical Nutrition 1999;70:412-9. [DOI] [PubMed] [Google Scholar]
McKeown 2002
- McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. American Journal of Clinical Nutrition 2002;76(2):390-8. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mellen 2008
- Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutrition, Metabolism and Cardiovascular Diseases 2008;18(4):283-90. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS 2012
- National Health Service. Atherosclerosis 2012. www.nhs.uk/conditions/Atherosclerosis/Pages/Introduction.aspx#commentCountLink (accessed prior to 15 August 2017).
Okarter 2010
- Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Critical Reviews in Food Science and Nutrition 2010;50(3):193-208. [DOI] [PubMed] [Google Scholar]
Oude 2010
- Oude Griep LM, Gelejinse JM, Kronhout D, Ocke MC, Verschuern WM. Raw and processed fruit and vegetable consumption and 10-year coronary heart disease incidence in a population-based cohort study in the Netherlands. PLoS ONE 2010;5:e13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pol 2013
- Pol K, Christensen R, Bartels E, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. American Journal of Clinical Nutrition 2013;98(4):872-84. [DOI: 10.3945/ajcn.113.064659] [DOI] [PubMed]
Priebe 2008
- Priebe M, Binsbergen J, Vos R, Vonk RJ. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD006061. [DOI: 10.1002/14651858.CD006061.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimm 1996
- Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer M, Willet W. Vegetable, fruit and cereal fibre intake and risk of coronary heart disease among men. JAMA 1996;59:1386-94. [DOI] [PubMed] [Google Scholar]
Seal 2006
- Seal CJ. Wholegrains and CVD risk. Proceedings of the Nutrition Society 2006;65:1-12. [DOI] [PubMed] [Google Scholar]
Slavin 2001
- Slavin JL, Jacobs D, Marquart L, Wiemer K. The role of whole grains in disease prevention. Journal of the American Dietetic Association 2001;101:780-5. [DOI] [PubMed] [Google Scholar]
Slavin 2003
- Slavin J. Why wholegrains are protective: biological mechanisms. Proceedings of the Nutrition Society 2003;62:129-34. [DOI] [PubMed] [Google Scholar]
Spagnoli 2007
- Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. Journal of Nuclear Medicine 2007;11:1800-15. [DOI] [PubMed] [Google Scholar]
Steffen 2003
- Steffen LM, Jacobs DR, Stevens J, Shahar E, Carithers T, Folsom A. Associations of whole-grain, refined grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. American Journal of Clinical Nutrition 2003;78:383-90. [DOI] [PubMed] [Google Scholar]
van der Kamp 2014
- Kamp JW, Poutanen K, Seal CJ, Richardson DP. The HEALTHGRAIN definition of 'whole grain'. Food and Nutrition Research 2014;58:22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehead 2014
- Whitehead A, Beck EJ, Tosh S, Wolever TM. Cholesterol-lowering effects of oat beta-glucan: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2014;100(6):1413-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Cardiovascular diseases (CVDs). Fact Sheet Number 317. March 2013. www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed prior to 15 August 2017).
Wolk 1999
- Wolk A, Manson JE, Stampfer MJ, Colditz GA. Long-term intake of dietary fiber and increased risk of coronary heart disease among women. JAMA 1999;281:1998-2004. [DOI] [PubMed] [Google Scholar]
Ye 2012
- Ye EQ, Chacko SA, Clou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease and weight gain. Journal of Nutrition 2012;142(7):1304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2007
- Kelly SAM, Summerbell CD, Brynes A, Whittaker V, Frost G. Wholegrain cereals for coronary heart disease. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005051. [DOI: 10.1002/14651858.CD005051.pub2] [DOI] [PubMed] [Google Scholar]


