Key Points
Question
What is the safety of drug-coated devices vs non–drug-coated devices for femoropopliteal artery revascularization?
Findings
In this cohort study of 16 560 Centers for Medicare and Medicaid Services beneficiaries who underwent femoropopliteal artery revascularization, the cumulative incidence of all-cause mortality was lower among those who were treated with drug-coated devices vs non–drug-coated devices through 600 days (32.5% vs 34.3%, respectively; log-rank P = .007). After multivariable adjustment, there was no association between drug-coated devices and mortality.
Meaning
Among Centers for Medicare and Medicaid Services patients, we found no signal of increased all-cause mortality following femoropopliteal artery revascularization with drug-coated devices.
Abstract
Importance
In a recent meta-analysis of randomized clinical trials, femoropopliteal artery revascularization with paclitaxel drug-coated devices was associated with increased long-term all-cause mortality compared with non–drug-coated devices. However, to our knowledge, these findings have not been replicated in other data sources and may be subject to confounding from missing data associated with patient withdrawal and loss to follow-up.
Objective
To evaluate differences in all-cause mortality between patients who were treated with drug-coated devices vs non–drug-coated devices for femoropopliteal artery revascularization.
Design, Setting, and Participants
This nationwide, multicenter retrospective cohort study included 16 560 Centers for Medicare and Medicaid Services beneficiaries who were admitted for femoropopliteal artery revascularization from January 1, 2016, to December 31, 2016. All-cause mortality was analyzed through September 30, 2017.
Exposures
Drug-coated devices (drug-eluting stent [DES] or drug-coated balloon [DCB]) compared with non–drug-coated devices (bare metal stent or uncoated percutaneous transluminal angioplasty balloon).
Main Outcomes and Measures
The primary outcome was all-cause mortality analyzed through the end of follow-up.
Results
Among 16 560 patients treated at 1883 hospitals, the mean (SD) age was 72.9 (11) years, 7734 (46.7%) were men, 12 232 (73.9%) were white, 8222 (49.7%) currently or had previously used tobacco, 9817 (59.3%) had diabetes, and 8450 (51.0%) had critical limb ischemia (CLI). Drug-coated devices were used in 5989 participants (36.2%). The median follow-up was 389 days (interquartile range, 277-508 days). Among all patients, treatment with drug-coated devices was associated with a lower cumulative incidence of all-cause mortality compared with treatment with non–drug-coated devices through 600 days postprocedure (32.5% vs 34.3%, respectively; log-rank P = .007). Similar survival trends were observed when treatment was stratified by using a DCB alone or DES with or without DCB. After multivariable adjustment, drug-coated devices were not associated with a difference in all-cause mortality compared with non–drug-coated devices (hazard ratio [HR], 0.97; 95% CI, 0.91-1.04; P = .43). These findings were consistent among those with CLI (HR, 0.93; 95% CI, 0.85-1.01; P = .09) or without CLI (HR, 0.94; 95% CI, 0.85-1.03; P = .20), and for those treated with DCB alone (HR, 0.94; 95% CI, 0.86-1.03; P = .17) or DES with or without DCB (HR, 0.97; 95% CI, 0.89-1.06; P = .48).
Conclusions and Relevance
In this large nationwide analysis of Centers for Medicare and Medicaid Services beneficiaries, there was no evidence of increased all-cause mortality following femoropopliteal artery revascularization with drug-coated devices compared with non–drug-coated devices.
This cohort study examines survival following treatment with drug-coated devices compared with non–drug-coated devices for Centers for Medicare and Medicaid Services beneficiaries who were admitted for femoropopliteal artery revascularization.
Introduction
In a recent meta-analysis of randomized clinical trials,1 paclitaxel drug-coated devices (drug-eluting stent [DES] and drug-coated balloon [DCB]) that are used for lower extremity peripheral artery revascularization were associated with decreased long-term survival compared with non–drug-coated devices (bare metal stent [BMS] and uncoated percutaneous transluminal angioplasty balloon [PTA]). These findings have culminated in the pausing of ongoing randomized trials,2 an expedited safety review by US and UK regulatory authorities, and a cautionary message to treating physicians from the US Food and Drug Administration.3 However, to our knowledge, the meta-analysis of summary-level results has not been replicated in other data sources. Furthermore, this study did not use survival analysis methods to account for patient withdrawal and loss to follow-up,4,5 which may be relevant as many of the included trials were not designed for long-term monitoring.6,7,8,9
Methods
Data Source, Study Population, and Study Outcome
Using data of all hospitalizations among Centers for Medicare and Medicaid Services (CMS) fee-for-service beneficiaries from the Medicare Provider Analysis and Review (MedPAR) files from January 1, 2016, through December 31, 2016, we identified patients with admissions that were associated with an International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) code for femoropopliteal artery revascularization with a DES, BMS, DES with DCB, BMS with DCB, DCB alone, or PTA alone (eTable 1 in the Supplement). For patients who underwent multiple revascularization procedures during the same index hospitalization, patients were included in the non–drug-coated device treatment group only if they did not receive treatment with any drug-coated devices. For patients with repeated admissions for femoropopliteal artery revascularization within the study period, only the first admission was selected. The primary outcome was all-cause mortality, which was analyzed through September 30, 2017. The study was approved by the institutional review board of Beth Israel Deaconess Medical Center, with a waiver of informed consent for retrospective data analysis.
Patient, Procedure, and Hospital Characteristics
Baseline covariates were ascertained using index diagnosis codes, as defined in eTable 2 in the Supplement, that were coded as “present on admission” as well as diagnosis codes from all hospitalizations within the 3 months preceding the date of admission for the index hospitalization for each patient (from October 1, 2015, to October 1, 2016). Comorbidities were determined using CMS hierarchical conditions as well as additional diagnosis codes for tobacco use and critical limb ischemia (eTable 2 in the Supplement). Procedure characteristics were identified using ICD-10-PCS codes (eTable 1 in the Supplement). Race/ethnicity was reported as collected and classified by the CMS MedPAR files. Hospital characteristics included hospital teaching status, region, bed capacity, and femoropopliteal artery revascularization procedure volume in 2016.
Statistical Analysis
Categorical variables were reported as counts and percentages, and continuous variables as means with standard deviations or medians with interquartile ranges (IQRs). Between-group differences were assessed using χ2 tests for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables. Kaplan-Meier methods were used to estimate long-term survival between devices and log-rank tests were used to evaluate for differences between groups. A multivariable Cox proportional hazards regression model was used to calculate hazard ratios (HRs) and the model adjusted for age, sex, comorbidities (Table 1), atherectomy use, and hospital characteristics (Table 2). In addition, to account for hospital-level variation in drug-coated device use, the model was adjusted for each hospital’s ratio of drug-coated to non–drug-coated device use for 2016. Analyses were stratified by treatment with a balloon only (DCB vs PTA) or stent (DES with or without DCB vs BMS). In addition, stratified results were reported for patients with or without critical limb ischemia. P < .05 was considered significant. Statistical analyses were performed using Stata, version 15.0 (Stata Corp).
Table 1. Baseline Characteristics of Patients Admitted for Femoropopliteal Artery Revascularization, Stratified by Treatment With Drug-Coated Devices vs Non–Drug-Coated Devices.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (N = 16 560) | Non–Drug-Coated Devices (n = 10 571) | Drug-Coated Devices (n = 5989) | ||
| Age, mean (SD), y | 72.9 (11.0) | 72.9 (11.1) | 73.0 (10.9) | .56 |
| Male | 7734 (46.7) | 4826 (45.7) | 2908 (48.6) | <.001 |
| Race/ethnicity | .12 | |||
| White | 12 232 (73.9) | 7806 (73.8) | 4426 (73.9) | |
| African American | 3050 (18.4) | 1978 (18.7) | 1072 (17.9) | |
| Other | 1278 (7.7) | 787 (7.4) | 491 (8.2) | |
| Current or prior tobacco use | 8222 (49.7) | 5241 (49.6) | 2981 (49.8) | .81 |
| Critical limb ischemia | 8450 (51.0) | 5400 (51.1) | 3050 (50.9) | .85 |
| Congestive heart failure | 4946 (29.9) | 3089 (29.2) | 1857 (31.0) | .02 |
| Cardiac arrhythmias | 5035 (30.4) | 3237 (30.6) | 1798 (30.0) | .42 |
| Prior myocardial infarction | 2467 (14.9) | 1562 (14.8) | 905 (15.1) | .56 |
| Valvular heart disease | 1681 (10.2) | 1053 (10.0) | 628 (10.5) | .28 |
| Hypertension | 14 475 (87.4) | 9205 (87.1) | 5270 (88.0) | .09 |
| Hemiplegia or paraplegia | 124 (0.7) | 78 (0.7) | 46 (0.8) | .83 |
| Other neurological disorders | 1222 (7.4) | 814 (7.7) | 408 (6.8) | .04 |
| Chronic obstructive pulmonary disease | 4305 (26.0) | 2762 (26.1) | 1543 (25.8) | .61 |
| Diabetes | 9817 (59.3) | 6164 (58.3) | 3653 (61.0) | <.001 |
| Liver disease | 327 (2.0) | 227 (2.1) | 120 (2.0) | .54 |
| Hypothyroidism | 2455 (14.8) | 1522 (14.4) | 933 (15.6) | .04 |
| Renal failure | 6810 (41.1) | 4344 (41.1) | 2466 (41.2) | .92 |
| Rheumatoid arthritis/collagen vascular disease | 602 (3.6) | 387 (3.7) | 215 (3.6) | .81 |
| Coagulopathy | 917 (5.5) | 624 (5.9) | 293 (4.9) | .006 |
| Obesity | 1929 (11.6) | 1225 (11.6) | 704 (11.8) | .75 |
| Weight loss | 1156 (7.0) | 779 (7.4) | 377 (6.3) | .009 |
| Fluid and electrolyte disorders | 4136 (25.0) | 2677 (25.3) | 1459 (24.4) | .17 |
| Deficiency anemia | 675 (4.1) | 401 (3.8) | 274 (4.6) | .02 |
| Alcohol use | 407 (2.5) | 277 (2.6) | 130 (2.2) | .07 |
| Drug use | 238 (1.4) | 158 (1.5) | 80 (1.3) | .41 |
| Psychoses | 103 (0.6) | 61 (0.6) | 42 (0.7) | .33 |
| Depression | 1718 (10.4) | 1077 (10.2) | 641 (10.7) | .30 |
| Dementia | 1464 (8.8) | 986 (9.3) | 478 (8.0) | .003 |
| Cancer | 555 (3.4) | 365 (3.5) | 190 (3.2) | .34 |
| Adjunctive atherectomy | 3503 (21.2) | 1880 (17.8) | 1623 (27.1) | <.001 |
Table 2. Hospital Characteristics of Patients Admitted for Femoropopliteal Artery Revascularization, Stratified by Treatment With Drug-Coated Devices vs Non–Drug-Coated Devices.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (N = 16 560) | Non–Drug-Coated Devices (n = 10 571) | Drug-Coated Devices (n = 5989) | ||
| Bed size, mean (SD) | 458 (342) | 446 (326) | 480 (367) | <.001 |
| Femoropopliteal artery revascularization procedure volume in 2016, mean (SD) | 21.6 (29.9) | 19.3 (21.7) | 25.6 (40.4) | <.001 |
| Teaching status | ||||
| Metropolitan teaching | 10 229 (61.8) | 6428 (60.8) | 3801 (63.5) | .003 |
| Metropolitan nonteaching | 5876 (35.5) | 3843 (36.4) | 2033 (33.9) | |
| Rural | 455 (2.8) | 300 (2.8) | 155 (2.6) | |
| Region | ||||
| West | 2336 (14.1) | 1524 (14.4) | 812 (13.6) | <.001 |
| Midwest | 3719 (22.5) | 2330 (22.0) | 1389 (23.2) | |
| South | 6841 (41.3) | 4539 (42.9) | 2302 (38.4) | |
| Northeast | 3664 (22.1) | 2178 (20.6) | 1486 (24.8) | |
| Ratio of use of drug-coated/non–drug-coated devices, mean (SD) | 0.36 (0.22) | 0.29 (0.19) | 0.49 (0.21) | <.001 |
Results
Baseline Characteristics
Among 16 560 patients who underwent femoropopliteal artery revascularization at 1883 hospitals during the study period, DCB alone was used in 2709 (16.4%), PTA alone in 5796 (35.0%), DES with DCB in 851 (5.1%), BMS with DCB in 727 (4.4%), DES in 1702 (10.3%), and BMS in 4775 (28.8%). Adjunctive atherectomy was used in 3503 procedures (21.2%) (Table 1). For the total population, the mean (SD) age was 72.9 (11) years, 7734 (46.7%) were men, 12 232 (73.9%) were white, 8222 (49.7%) currently or had previously used tobacco, 9817 (59.3%) had diabetes, and 8450 (51.0%) had critical limb ischemia. Patients who were treated with drug-coated devices were more often men and had a higher prevalence of congestive heart failure, diabetes, hypothyroidism, and anemia (Table 1). They were also more frequently treated with adjunctive atherectomy (27.1% vs 17.8% with non–drug-coated devices; P < .01). Otherwise, there was no significant difference in baseline rates of cardiovascular disorders and risk factors, including critical limb ischemia and tobacco use, between groups.
Hospital characteristics varied between patients who were treated with drug-coated and non–drug-coated devices. Patients treated with drug-coated devices were more often cared for at hospitals located in the Northeast and Midwest and at institutions with greater bed capacities, teaching affiliations, and larger femoropopliteal artery revascularization procedure volumes (Table 2).
Survival by Device Type After Femoropopliteal Artery Revascularization
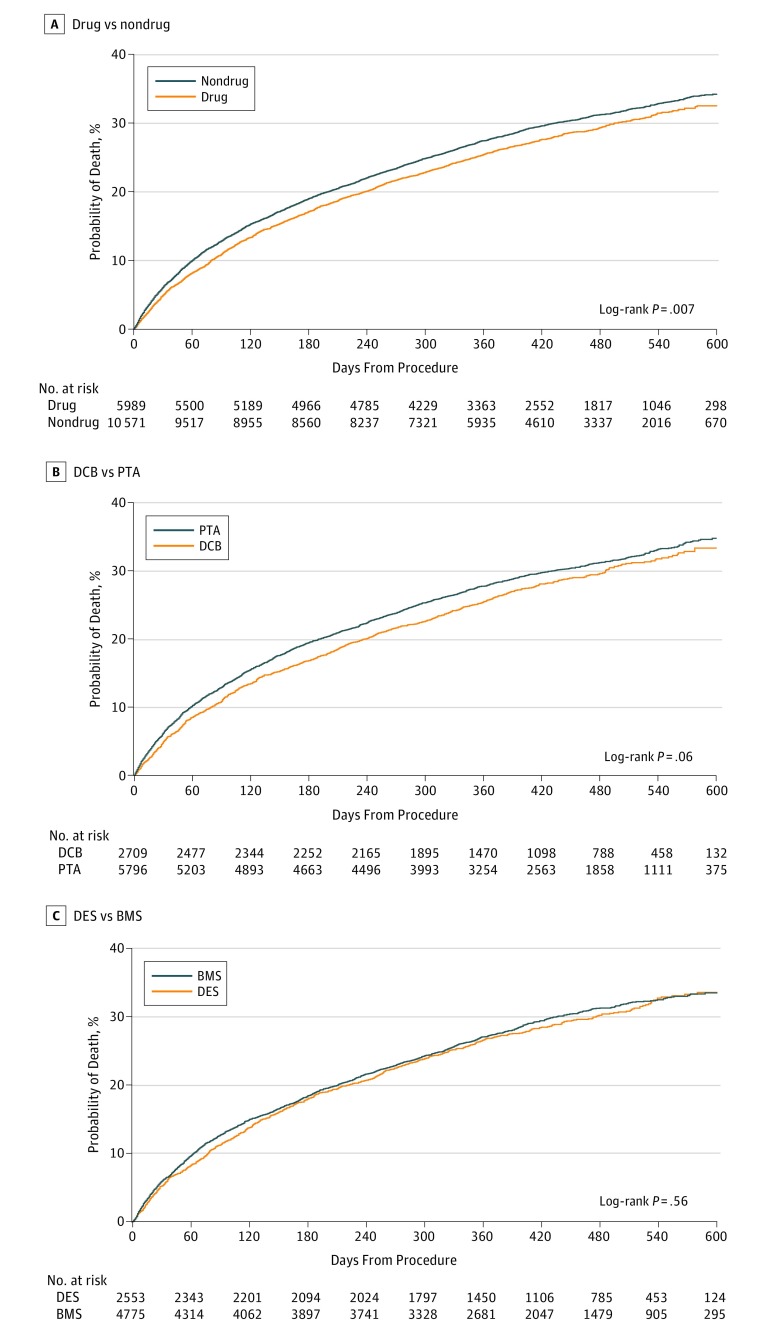
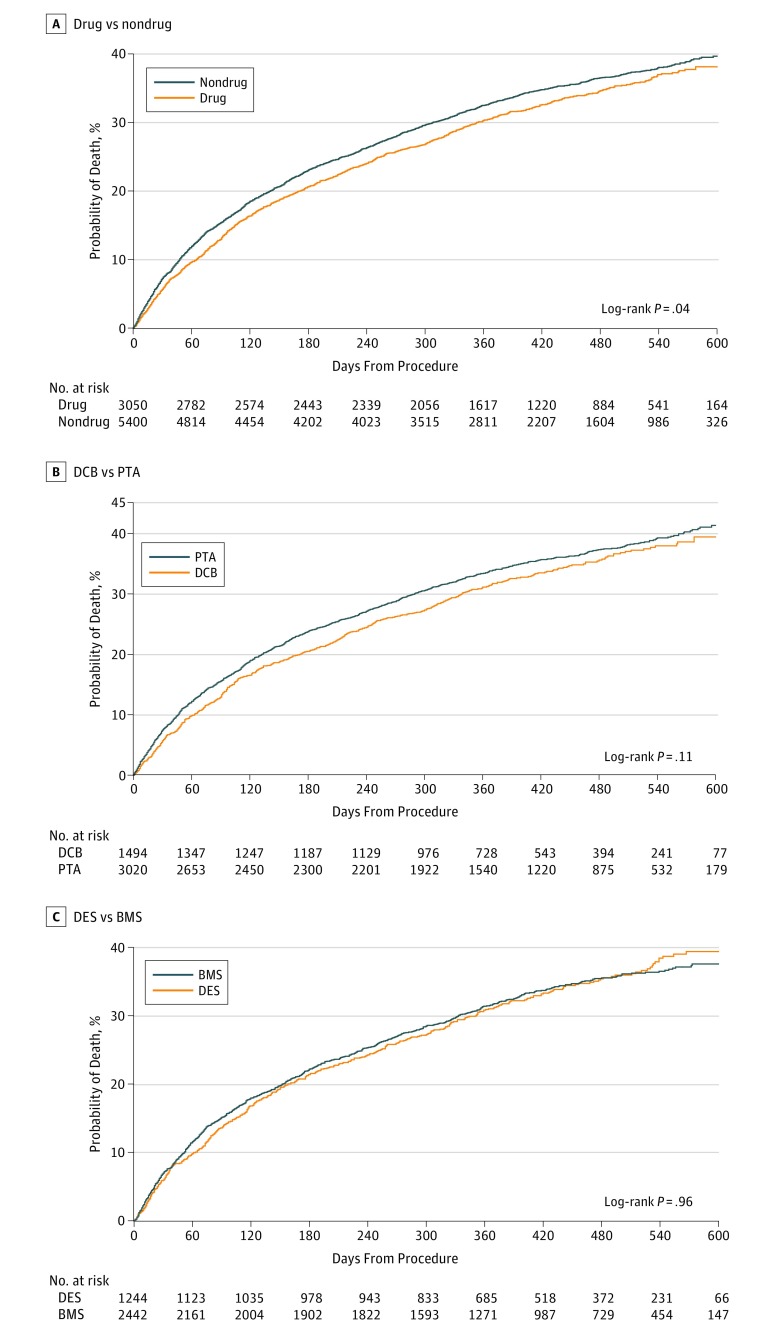
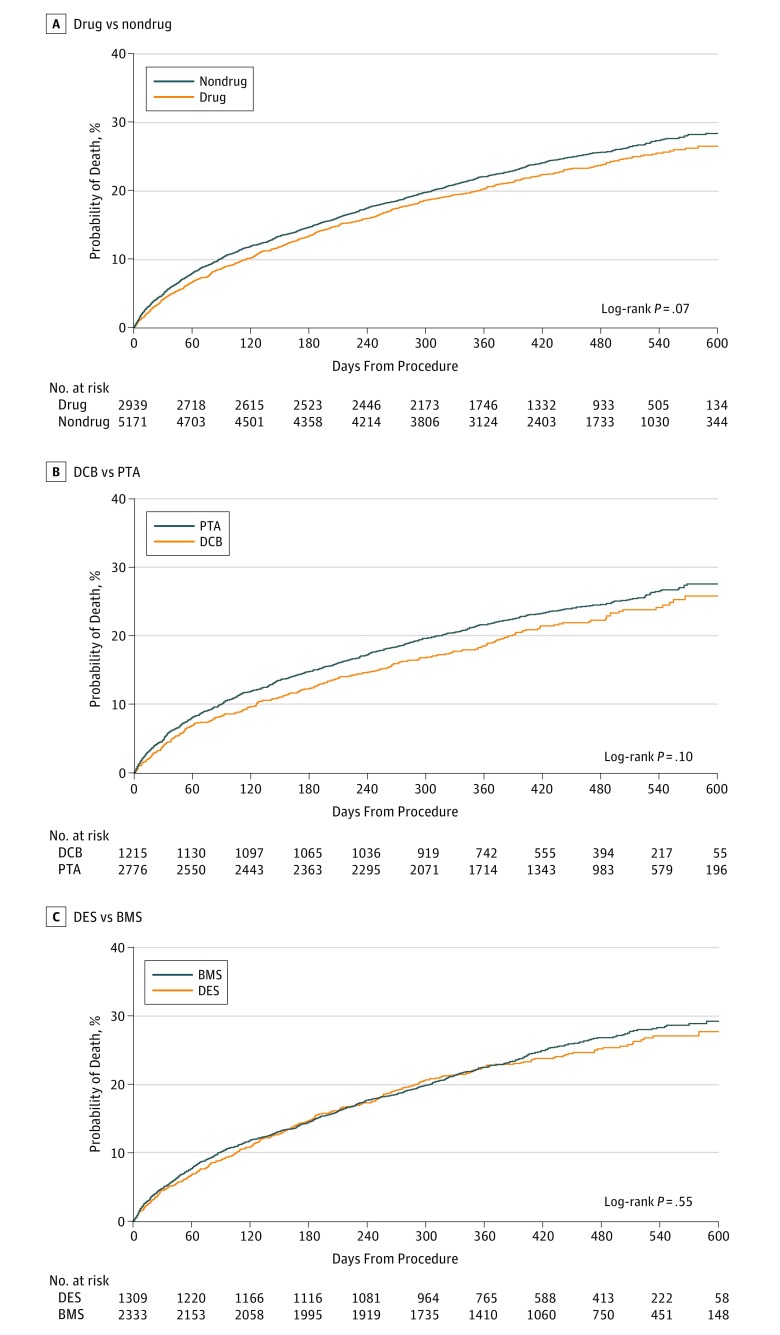
After femoropopliteal artery revascularization, the median follow-up was 389 days (IQR, 277-508 days). Among all patients, treatment with drug-coated devices was associated with a lower cumulative incidence of all-cause mortality compared with treatment with non–drug-coated devices through 600 days postprocedure (32.5% vs 34.3%, respectively; log-rank P = .007; Figure 1A). When stratified by the type of device used for revascularization, similar survival trends were observed for patients who were treated with drug-coated vs non–drug-coated balloon angioplasty (log-rank P = .06; Figure 1B), and DES vs BMS (log-rank P = .56; Figure 1C). Among patients with critical limb ischemia, revascularization with drug-coated devices was also associated with a lower cumulative incidence of mortality through 600 days postprocedure (38.1% vs 40.1% for non–drug-coated devices; log-rank P = .04; Figure 2A), with no difference in survival trends observed between device types (Figure 2B and C). For patients without critical limb ischemia, there was no statistical difference in the cumulative incidence of survival between drug-coated and non–drug-coated device treatment (26.5% vs 29.0%, respectively; log-rank P = .07; Figure 3).
Figure 1. Long-term Survival Following Femoropopliteal Artery Revascularization With Drug-Coated Devices Compared With Non–Drug-Coated Devices.
Displayed are the cumulative incidence curves for all-cause mortality after femoropopliteal artery revascularization, stratified by treatment with drug-coated devices (drug) vs non–drug-coated devices (nondrug) (A), drug-coated balloons (DCB) vs uncoated balloons (PTA) (B), and drug-eluting stents (DES) vs bare metal stents (BMS) (C).
Figure 2. Long-term Survival Following Femoropopliteal Artery Revascularization With Drug-Coated Devices Compared With Non–Drug-Coated Devices Among Patients With Critical Limb Ischemia.
Displayed are the cumulative incidence curves for all-cause mortality after femoropopliteal artery revascularization among patients with critical limb ischemia, stratified by treatment with drug-coated devices (drug) vs non–drug-coated devices (nondrug) (A), drug-coated balloons (DCB) vs uncoated balloons (PTA) (B), and drug-eluting stents (DES) vs bare metal stents (BMS) (C).
Figure 3. Long-term Survival Following Femoropopliteal Artery Revascularization With Drug-Coated Devices Compared With Non–Drug-Coated Devices Among Patients Without Critical Limb Ischemia.
Displayed are the cumulative incidence curves for all-cause mortality after femoropopliteal artery revascularization among patients without critical limb ischemia, stratified by treatment with drug-coated devices (drug) vs non–drug-coated devices (nondrug) (A), drug-coated balloons (DCB) vs uncoated balloons (PTA) (B), and drug-eluting stents (DES) vs bare metal stents (BMS) (C).
After multivariable adjustment, there was no association between drug-coated devices and all-cause mortality (adjusted HR, 0.97; 95% CI, 0.91-1.04; P = .43). In addition, there was no difference in the adjusted mortality risk following treatment with DCBs alone (adjusted HR, 0.94; 95% CI, 0.86-1.03; P = .17) or DES with or without DCB (adjusted HR, 0.97; 95% CI, 0.89-1.06; P = .48). Similar findings were observed among those with critical limb ischemia (adjusted HR, 0.93; 95% CI, 0.85-1.01; P = .09) and those without critical limb ischemia (adjusted HR, 0.94; 95% CI, 0.85-1.03; P = .20).
Discussion
In this analysis of more than 16 000 CMS beneficiaries who were admitted for femoropopliteal artery revascularization, we found no evidence of reduced survival following treatment with drug-coated devices compared with non–drug-coated devices through a median of 389 days of follow-up (IQR, 277-508). This finding was consistent among patients who were treated with either balloon angioplasty alone or with stenting and the presence or absence of critical limb ischemia. Furthermore, our findings persisted after adjustment for patient, procedure, and hospital characteristics.
Drug-coated devices have become a mainstay for treating lower extremity peripheral artery disease, with more than 40% of lesions treated with either a DCB or DES since 2016.10,11 In particular, DCBs have demonstrated improved long-term patency compared with PTA6,7,12,13 and have been recommended as an initial strategy for above-the-knee revascularization.14
Recently, a meta-analysis of randomized clinical trials has raised concerns regarding the safety of paclitaxel drug-coated devices in the periphery.1 This meta-analysis found that all-cause mortality rates at 2 and 5 years were greater among patients treated with these devices.1 Although cause-specific mortality was not reported, potential mechanisms of death included increased risks of infection, pulmonary disease, and malignancy.7 These findings, if confirmed, have substantial implications for clinical practice. In response to this study, 2 ongoing clinical trials have been paused,2 and regulatory bodies in the United States and United Kingdom have initiated an urgent internal safety review with all companies that produce drug delivery technologies for femoropopliteal artery disease. In addition, the US Food and Drug Administration recently issued a message of caution to physicians with advice to consider the risks and benefits before treatment with drug-coated devices and instructions to report any adverse events.3
However, the referenced meta-analysis has limitations that mitigate its implication. Mortality rates were estimated using the trial enrollment population, which fails to account for patient withdrawal and loss to follow-up.4,5 In addition, outcomes were based on summary-level trial data, which cannot account for missing data at the individual level and may lack up-to-date follow-up information.15 As such, the significance of this harm signal requires replication in other populations to substantiate or alleviate the perceived safety risk.
Strengths and Limitations
In this study, we leveraged CMS beneficiary data to provide a real-world examination of survival following peripheral artery revascularization with drug-coated and non–drug-coated devices. The strengths of using these data include ICD-10-PCS claims codes specific for femoropopliteal artery revascularization and the availability of time-to-event mortality data, which allowed for the use of survival analysis methods. In addition, this analysis was sufficiently powered to examine important subgroups, in particular those with critical limb ischemia, who represented a minority of the patients who were included in the Katsanos et al meta-analysis.1
The results of this analysis must also be considered in the context of the study design. Our analysis lacked the extended follow-up observed in the Katsanos et al meta-analysis1 (through 5 years), and it is possible that the risks of drug-coated devices could become apparent over greater follow-up. Furthermore, this study was observational in design, and thus the treatment effect was nonrandomized. As such, we cannot account for treatment selection bias by operators or residual unmeasured confounding. A patient-level meta-analysis of randomized clinical trials with survival methods would be useful in overcoming these limitations and confirming our findings. In addition, our analysis was limited by the use of claims data. For instance, we were unable to report granular patient and procedure characteristics, such as the presence of multilevel peripheral artery disease or intervention and device brands, and claims codes could have been subject to misclassification. We also lacked data on revascularization procedures that were performed in the outpatient setting, which may partially account for the high proportion of patients with critical limb ischemia. Lastly, our analysis only included Medicare fee-for-service beneficiaries, and this population was older and had higher rates of comorbidities, including critical limb ischemia, compared with patients in the Katsanos et al meta-analysis.1 The high rates of mortality observed in our study likely reflect these differences in patient characteristics. As our analysis could not differentiate cause-specific mortality, we were unable to account for the competing risk of death between cardiovascular and noncardiovascular causes or evaluate for associations between drug exposure and specific causes of death.
Conclusions
In this large nationwide analysis of CMS beneficiaries, we found no signal of increased all-cause mortality following femoropopliteal artery revascularization with drug-coated devices compared with non–drug-coated devices. Further review of patient-level trial data and real-world registries will be valuable in providing greater evidence of the safety of drug-coated devices used for peripheral artery revascularization.
eTable 1. ICD-10-PCS Codes of Femoropopliteal Artery Revascularization
eTable 2. ICD-10-CM Codes of Baseline Characteristics
References
- 1.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24):e011245. doi: 10.1161/JAHA.118.011245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeown LA. Two trials halted in wake of study linking paclitaxel-coated devices to deaths in PAD. https://www.tctmd.com/news/two-trials-halted-wake-study-linking-paclitaxel-coated-devices-deaths-pad. Accessed December 19, 2018.
- 3.US Food & Drug Administration. Treatment of peripheral arterial disease with paclitaxel-coated balloons and paclitaxel-eluting stents potentially associated with increased mortality—letter to health care providers. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm629589.htm. Accessed January 21, 2019.
- 4.Singh R, Mukhopadhyay K. Survival analysis in clinical trials: basics and must know areas. Perspect Clin Res. 2011;2(4):145-148. doi: 10.4103/2229-3485.86872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SR, Schoenfeld DA. Survival methods. Circulation. 2007;115(1):109-113. doi: 10.1161/CIRCULATIONAHA.106.614859 [DOI] [PubMed] [Google Scholar]
- 6.Tepe G, Laird J, Schneider P, et al. ; IN.PACT SFA Trial Investigators . Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495-502. doi: 10.1161/CIRCULATIONAHA.114.011004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider PA, Laird JR, Tepe G, et al. ; IN.PACT SFA Trial Investigators . Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11(1):e005891. doi: 10.1161/CIRCINTERVENTIONS.117.005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118(13):1358-1365. doi: 10.1161/CIRCULATIONAHA.107.735985 [DOI] [PubMed] [Google Scholar]
- 9.Iida O, Soga Y, Urasawa K, et al. ; MDT-2113 SFA Japan Investigators . Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25(1):109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohapatra A, Bertges DJ, Madigan MC, Al-Khoury GE, Makaroun MS, Eslami MH. Nationwide trends in drug-coated balloon and drug-eluting stent utilization in the femoropopliteal arteries. J Vasc Surg. 2018;68(2):e15-e16. doi: 10.1016/j.jvs.2018.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones S. Use and outcomes with drug-coated balloons in patients with lower extremity peripheral artery disease. Paper presented at: Transcatheter Scientific Sessions of the Cardiovascular Research Foundation; September 25-29, 2018; San Diego, CA. https://www.tctmd.com/slide/use-and-outcomes-drug-coated-balloons-patients-lower-extremity-peripheral-artery-disease. Accessed January 4, 2019. [Google Scholar]
- 12.Giacoppo D, Cassese S, Harada Y, et al. Drug-coated balloon versus plain balloon angioplasty for the treatment of femoropopliteal artery disease: an updated systematic review and meta-analysis of randomized clinical trials. JACC Cardiovasc Interv. 2016;9(16):1731-1742. doi: 10.1016/j.jcin.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Rosenfield K, Jaff MR, White CJ, et al. ; LEVANT 2 Investigators . Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373(2):145-153. doi: 10.1056/NEJMoa1406235 [DOI] [PubMed] [Google Scholar]
- 14.Feldman DN, Armstrong EJ, Aronow HD, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions [published online April 24, 2018]. Catheter Cardiovasc Interv. doi: 10.1002/ccd.27635 [DOI] [PubMed] [Google Scholar]
- 15.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10-PCS Codes of Femoropopliteal Artery Revascularization
eTable 2. ICD-10-CM Codes of Baseline Characteristics