Abstract
Background:
Restless leg syndrome (RLS) is a common symptom of some diseases specially observed during hemodialysis. Cooling the dialysate is a safe and nonpharmacological method. The aim of this study was to assess the effect of cool dialysate on RLS in hemodialysis patients.
Materials and Methods:
A total of 79 patients were selected for screening based on the four main criteria set by the RLS International Association. Finally, in line with the inclusion and exclusion criteria, 63 hemodialysis patients were recruited and participated in this clinical trial. The patients were randomly assigned to the intervention group (n = 32) and the control group (n = 31). The intervention group received 35.5°C dialysate and the control group received 37°C dialysate three times a week for a period of 1 month. The severity of RLS was measured in both groups using a standardized RLS questionnaire. Using R software version 3.3.1, the data were analyzed using the Student's t-test, and Wilcoxon test, at 95% confidence interval.
Results:
In terms of RLS severity, there was no significant difference between intervention and control groups before the intervention (t = -2.11, p > 0.05). After the intervention, the mean (SD) of RLS severity in the control group was 28.77 (5.45) and in the intervention group was 11.66 (4.69), in which t test showed a significant difference between two groups (t = 14.03, p= 0.001).
Conclusions:
Using cool dialysate as a nonpharmacological treatment may reduce the severity of RLS in patients on hemodialysis. Therefore, using this method to improve RLS in hemodialysis patients is recommended.
Keywords: Chronic, dialysis solutions, kidney failure, renal dialysis, restless leg syndrome
Introduction
Hemodialysis (HD) patients usually have multiple complications. Neuromuscular complications, including restless leg syndrome (RLS) is one of them.[1] RLS is a neurological disorder characterized by an intense tendency to move the legs during rest and unpleasant sensation in the legs such as throbbing, burning, or creeping.[2,3]
RLS is one of the most commonly undiagnosed diseases with the prevalence of 28–62% in patients on hemodialysis.[1] During a 4-hour hemodialysis session, patients are trained to minimize their movements in order to reduce the possible complications associated with hemodialysis catheters (arterial and venous).[4] According to the most accepted assumptions for RLS, factors associated with the development or worsening of restless leg syndrome include peripheral neuropathy, dysfunction in the dopaminergic system, and the iron deficiency in certain regions of the brain.[5,6] Results of a recent study showed that the subclinical peripheral neuropathies were present in most patients with uremic RLS compared to idiopathic RLS, which indicates the potential role of damaged peripheral nerves in the pathophysiology of uremic RLS.[4,7] RLS increases the incidence of mental disorders, decreases the quality of life, and increases the risk of heart disease, depression, anxiety, and fatigue.[8]
The basis of RLS treatment is the use of primary care, medications, and kidney transplantation.[9] The efficacy of opioids in the treatment of RLS has been recognized, but the potential misuse of the drug and its side effects, including respiratory depression and constipation, limits its use to RLS.[10] Dopamine agonists can cause drowsiness and impulse control disorders, while the common side effects of α 2-adrenergic agonists include weight gain, dizziness, and gait imbalance.[11,12] Medication tolerance and augmentation are two main factors causing the failure of a prolonged RLS treatment.[13,14] Regular exercises such as walking, stretching, massage, hot and cold water baths, acupuncture, and Transcutaneous Electrical Nerve Stimulation (TENS) may alleviate the RLS symptoms.[15] Because RLS can affect the sleep quality of the patients, it seems that reducing body temperature in patients on hemodialysis can be effective in relieving these complications.[16] Patients on hemodialysis who due to the removal of urea from the body, are usually hypothermic, experience increasing central body temperature during the treatment session (0.5°C).[17] A rise in central body temperature usually results in reflex dilatation of the central vessels, increased venous capacitance and decreased cardiac output.[18] Increased body temperature seems to occur due to increased activity of the sympathetic system and reduced ability of the body to lose heat, which along with the removal of the fluid, causes severe vascular contraction. Therefore, a slight decrease of 1–2° C in the dialysate temperature returns the above-mentioned changes with increasing central venous and arterial vascular tone, venous return, and cardiac output. Thus, reducing the heat load and improving hemodynamic status during and after hemodialysis using a cool dialysate improve the blood flow of the skin at night and positively affect nighttime sleep.[16] Therefore, considering that RLS worsens at rest, especially in the evening and night and during hemodialysis,[19] this mechanism of action can be used to reduce the severity of RLS symptoms.
The benefits of using a cool dialysate include: reducing the incidence of hypotension during dialysis, making a constant hemodynamic, reducing ischemic brain damage, and maintaining white matter, improving cardiac function and morphologic indices, controlling arterial blood pressure during and after hemodialysis, Reducing fatigue after hemodialysis, increasing energy levels, does not change the effectiveness of dialysis, improving overall understanding of the body health and reducing the number of nursing interventions during hemodialysis, can be done at no extra cost and can be implemented globally.[11,20] Nurses can take several interventions to reduce the restless leg syndrome, such as using stretching exercises, TENS, acupuncture, as well as reflexology. But the cold dialysis method may have more benefits and cover other complications related to RLS as mentioned above. So, hemodialysis with the cool dialysate as a simple, safe, and cost-effective approach, needs to be investigated in terms of effectiveness in improving symptoms of RLS. Therefore, this study was conducted to evaluate the effect of cool dialysate on RLS in patients on hemodialysis.
Materials and Methods
The present study is a randomized, triple-blind, clinical trial (IRCT20170722035232N1) with a parallel group design, which was conducted on 63 patients on hemodialysis with RLS for a month on March to May 2018 at two dialysis centers in Sabzevar (hemodialysis Ward of Vaseei Hospital and Kashefi Center). The sample size was determined using the G Power software according to the data obtained from ten preliminary sample in each group. The confidence level, test power, and the dropping rate was considered to be 95%, 80%, and 20%, respectively. 79 patients out of 140 patients selected from two dialysis centers (Vaseei = 105 and Kashefi = 35), screened based on the four main criteria of the International Association of RLeg Syndrome, suffered from RLS. Finally, based on the inclusion and exclusion criteria and using the random allocation with permuted block method, according to sample size (63 patients), 32 and 31 patients were assigned to the intervention group and the control group, respectively.
Inclusion criteria were giving informed consent for participation in the study, aged 18–75 years, having complete consciousness and acceptable hearing and speech ability to answer questions, no history of paralysis and physical disability, no ulcer and redness of the limbs, patients with chronic renal failure (patients who have been undergoing hemodialysis for 3 months), access to the arteries through arteriovenous fistula, permanent catheter and arteriovenous graft, patients who were undergoing hemodialysis three time per week and each for 4 hours, history of RLS during hemodialysis during the last 2 months, no history of itchy skin diseases, not taking medication or treatment that may affect the RLS during dialysis, KT/V greater than or equal to 1 (KT/V is a number used to quantify hemodialysis and peritoneal dialysis treatment adequacy), hemoglobin level higher than 10 mg/dL, no catabolic diseases such as malignancies, no endocrine disorders (such as hypothyroidism, hyperparathyroidism).
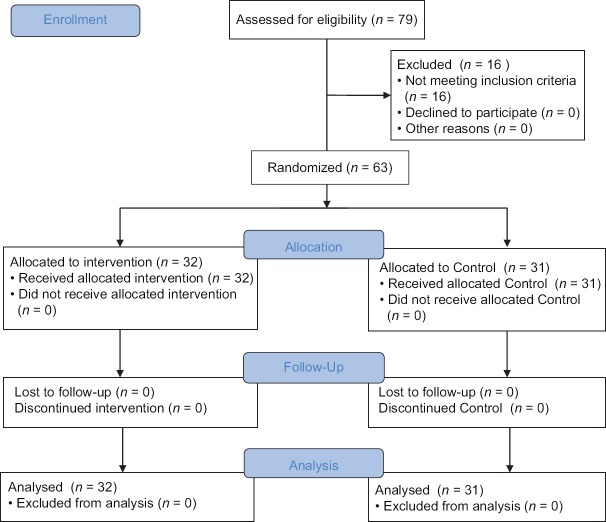
Exclusion criteria were discontinuation of dialysis of patients due to acute complications, renal transplantation, changes in hemodialysis frequency and hours, cool dialysis intolerance, withdrawal from the study, fever, diabetic neuropathy, chronic infections, heart failure, severe anemia, coronary artery disease, and substance abuse [Figure 1].
Figure 1.
Flowchart of the design, group, and participants in the study
The data for this study were gathered using a demographic questionnaire, patient's medical records, and the standardized questionnaire for RLS. This questionnaire was designed by the International Restless Leg Syndrome Study Group in 2003 and consists of 10 four-point questions. The severity of RLS is classified into five categories, based on the points obtained, including: without difficulty (0), mild (1–10), moderate (11–20), severe (21–30), and very severe (31 to 40).[3] The content validity of the RLS questionnaire was quantitavely determined by calculating a content validity index (CVI) based on a panel of 10 experts. The mean score of CVI was 0.81. So, content validity of the questionnaire was approved. The reliability of this questionnaire using the Cronbach's alpha formula was calculated to be 0.94.
Using a single filter type, all patients underwent HD. Before the intervention, hemodialysis devices were calibrated in terms of temperature settings. The data were collected from dialysis centers in three shifts: morning shift from 7:15 to 13:00, afternoon shift from 13:00 to 18:00, and evening shift from 18:00 to 23:00. First, for screening, the four criteria introduced by the International Association for RLS Diagnosis were checked in patients and if a patient met all of the four criteria, s/he was considered as a patient with RLS. In the next step, in order to rule out the differential diagnosis, the patients were examined by a neurologist to ensure that there was no neuropathy and diabetic neuropathy. Then, the recruitment form was completed by the researcher assistant through interviews with patients, while they were relaxed during the course of dialysis and eligible patients were selected. In order to accurate blinding, a research assistance helped in data gathering. She did not know the type of intervention and random allocation of the patients to intervention and control groups. The participants were also not aware of their assignment to the intervention or control group. In this study, to prevent the participants' bias, the information about the dialysate temperature was covered with 2 × 1 cm paper; therefore, the patients in both groups couldn't see this information during the study. At the beginning of the study, i.e., preintervention stage, both the intervention and control groups underwent hemodialysis with the usual method (dialysis with a dialysate temperature of 37°C) for three times per week and for 4 hours each time. At the end the third session of hemodialysis, RLS status was recorded using the RLS questionnaire (as before the intervention data gathering (baseline)). Then, during the intervention stage, patients in the intervention group underwent hemodialysis with cool dialysate (35.5°C) for 1 month (three days per week, each for 4 hours). During the same period, patients in the control group were treated with normal dialysis (dialysis with a dialysate temperature of 37°C) and at the end of the 12th HD session, the RLS status was recorded through the RLS questionnaire.
In this study, the statistician was also blinded to the study. Because the patients were coded as A and B, he was not aware of the random allocation of the patients into intervention and the control groups. After completion of the data collection, the forms were coded and entered into the computer and after ensuring the correct data entry, all data were analyzed using the R software version 3.3.1. First, Kolmogorov–Smirnov test was used to investigate the normal distribution of the data. Mann–Whitney U test, t test, Chi-square test, and Fisher's exact test used to analyze demographic characteristics and disease-related information difference between two groups. In order to determine the differences between the intragroup and the intergroup in the distribution of the measured variables, Independent t-test, and Wilcoxon test were used. As a significance level for all the statistical analyses, p value was considered to be <0.05.
Ethical considerations
This study was approved by the Ethics Committee of Sabzevar University of Medical Sciences (Code IR. Medsab. Rec. 1395.92). The patients' information remained confidential throughout the study. Informed consent was obtained from the participants. The aims of the study were explained in detail to the participants. Participation in this study was completely voluntary and free from any obligation to the physician, nursing staff, or researchers.
Results
Demographic characteristics and information of the medical chart of the two groups are presented in Table 1. Mann–Whitney U test, t test, Chi-square test, and Fisher's exact test showed no significant difference between the groups with respect to the demographic characteristics and disease-related information. Before intervention, the mean and standard deviation (SD) of the RLS severity in the control group was 28.48 (6.93) and in the intervention group was 31.51 (3.92), Independent t test indicated no significant difference between the two groups (t = -2.11, p > 0.05). After intervention (1 month later), the mean (SD) in the control group was 28.77 (5.45) and in the intervention group was 11.66 (4.69), which Independent t test showed a significant difference between the two groups (t = 14.03, p = 0.001). The mean of the RLS severity in the intervention group decreased from 31.51 (3.92) before intervention to 11.66 (4.69) after intervention, Wilcoxon test showed significant differences between them (p < 0.001), whereas there was no significant difference in the control group before and after the intervention with Wilcoxon test (p > 0.05) [Table 2].
Table 1.
Characteristics of the patients in the intervention and control groups
| Group Variable |
Intervention | Control | Statistical results | df | p | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||
| Age (year) | 56.25 (12.28) | 55.61 (15.53) | t=1.18 | 58 | 0.863* | |
| Duration of hemodialysis treatment (Month) | 36.72 (22.65) | 35.10 (26.10) | t=2.13 | 58 | 0.793** | |
| (%) Number | (%) Number | |||||
| Gender | Male | 20 (62.50) | 15 (48.40) | χ2=1.27 | 1 | 0.315*** |
| Female | 12 (37.50) | 16 (51.60) | ||||
| Duration of the onset of RLS during dialysis | Less than 2 weeks | 0 (0.00) | 0 (0.00) | χ2=1.46 | 2 | 0.482*** |
| 2 weeks- 6 months | 9 (28.10) | 8 (25.80) | ||||
| 6-12 months | 8 (25.00) | 12 (38.70) | ||||
| More than a year | 15 (46.90) | 11 (35.50) | ||||
| History of taking medication to relieve RLS symptoms | Oral medication | 19 (59.40) | 21 (67.70) | Exact test=2.14 | 2 | 0.661**** |
| Supplementary medication | 3 (9.40) | 1 (3.10) | ||||
| No medication | 10 (31.20) | 9 (29.00) | ||||
| Factor exacerbating RLS during dialysis | Rest | 9 (28.10) | 11 (35.50) | Exact test=2.82 | 3 | 0.897**** |
| Stress & rest | 7 (21.90) | 5 (16.10) | ||||
| Heat & rest | 15 (46.90) | 14 (45.20) | ||||
| Stress, Heat & rest | 1 (3.10) | 1 (3.20) | ||||
*Mann-Whitney U test, **t-test, ***Chi-square test, **** Fisher’s exact test
Table 2.
Comparison of Mean (SD) of RLS severity before intervention, and after the end of the intervention in two groups
| Group | Mean (SD) |
t | df | p* | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Before intervention (pretest) | 31.51 (3.92) | 28.45 (6.93) | -2.11 | 58 | >0.05 |
| After intervention (posttest) | 11.66 (4.69) | 28.77 (5.45) | 14.03 | 58 | 0.001 |
| (Z, p)** | (-2.52, 0.001) | (1.11, 0.541) | - | ||
*t-test, **Wilcoxon test
Discussion
The results of this study showed that using cool dialysate can significantly decrease the severity of RLS in patients undergoing hemodialysis. There has been little study on the effect of cool dialysate on RLS, and most studies have discussed its effects on hemodynamic changes in the body and vital signs such as preventing or improving blood pressure during hemodialysis.[21] It is very interesting to note that increased release of beta-endorphin is associated with reduced RLS-related-movements in patients with idiopathic RLS and improve RLS symptoms by performing chronic or acute exercise.[13] However, increased levels of beta-endorphin after 4 hours of hemodialysis with cool temperatures (34.5°C) in patients without RLS was reported.[21] Similarly, it can be said that beta-endorphin release with cold dialysis can reduce Restless Leg Syndrome in Patients on hemodialysis.
Using cool dialysate in this study not only did not have the side effects of Levodopa and Gabapentin, but also during dialysis, the improvement of RLS symptoms could be related with an increase in cerebrovascular perfusion and decrease in hypoxia levels (there is recent report about strong association between the environmental hypoxia and the severity of RLS).[22] Happe et al. showed reduction of the local and whole body temperature with cryotherapy has been successful in reducing RLS symptoms. They observed that local cryotherapy was less effective than whole body cooling[23] which is in line with the results of the recent study. Improvement symptoms of RLS by reducing body temperature may be due to decreased function of sensory receptors, leading to a reduction in neuronal activity with extensive anesthesia.[24] In addition, the cold can lead to a smaller amplitude and lower frequency of incoming impulses to the nerve endings, resulting in a reduction in perception of pain that lead to an analgesic response.[25] Another possible explanation for decreasing RLS severity, especially in cooling the whole body by cool dialysate, might be modulation in the spinal cord excitability. Hence, spinal cord excitability can affect the onset of RLS.[26]
Using cool dialysate was well tolerated by patients. With the same amount of ultrafiltration that may occur in dialysis with a 37°C temperature, cool dialysate increases systolic blood pressure and reduces heart rate, and during dialysis, it acts like catecholamines and increases peripheral vascular and cardiac contractility that results in the stability of hemodynamic and mean arterial pressure (MAP) and ultimately prevents hypotension during dialysis.[21] Using cool dialysate in this study not only does not require the preparation of patients and direct nursing supervision, but also minimizes the number of nursing interventions during hemodialysis.
This study has a few limitations such as small sample size that reduces the generalizability of the results. The RLS threshold varies from one patient to another. Therefore, to better control this issue in this research, the pre and post method was used to determine the changes in RLS status in each person.
Conclusion
The results of this study shows that using cool dialysate can reduce the RLS severity during dialysis. Therefore, cool dialysate could be considered as a safe none pharmacological method in the treatment of RLS in patients undergoing hemodialysis. The results of this study can be used in planning to maintain and improve the health status of hemodialysis patients. Using the results of this study can prevent and reduce the complications during hemodialysis, in particular RLS, and thus, prevent from medication's side effects and decrease nursing interventions. It is considered that doctors use this intervention in order to decrease nursing workload.
Financial support and sponsorship
Sabzevar University of Medical Sciences.
Conflicts of interest
Nothing to declare.
Acknowledgements
This study would not have been possible without the support of the vice chancellor for Research Dept. of Sabzevar University of Medical Sciences. I am also grateful to all the patients who participated in this study.
References
- 1.Rad M, Shomoossi N, Mirhosseini Z, Kashani E. Can cool dialysate alleviate restless leg syndrome in hemodialysis patients? J Res Med Sci. 2017;22:124. doi: 10.4103/jrms.JRMS_587_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology. 2016;86:1336–43. doi: 10.1212/WNL.0000000000002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Borreguero D, Patrick J, DuBrava S, Becker PM, Lankford A, Chen C, et al. Pregabalin versus pramipexole: Effects on sleep disturbance in restless legs syndrome. Sleep. 2014;37:635–43. doi: 10.5665/sleep.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Pantzaris MC, Stefanidis I, Sakkas GK. Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: An evidence-based review. Kidney Int. 2014;85:1275–82. doi: 10.1038/ki.2013.394. [DOI] [PubMed] [Google Scholar]
- 5.Scherer JS, Combs SA, Brennan F. Sleep disorders, restless legs syndrome, and uremic pruritus: Diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis. 2017;69:117–28. doi: 10.1053/j.ajkd.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yilmaz O, Şengül Y, Şengül HS, Parlakkaya FB, Öztürk A. Investigation of alexithymia and levels of anxiety and depression among patients with restless legs syndrome. Neuropsychiatr Dis Treat. 2018;14:2207. doi: 10.2147/NDT.S174552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baiardi S, Mondini S, Antognini AB, Santoro A, Cirignotta F. Survival of dialysis patients with restless legs syndrome: A 15-year follow-up study. Am J Nephrol. 2017;46:224–30. doi: 10.1159/000479938. [DOI] [PubMed] [Google Scholar]
- 8.Haibo YU, Wei F, Jiang A, Wang Z, Dong H, Meng J, et al. Efficacy and safety of neurotropin in treatment of restless legs syndrome in patients undergoing maintenance hemodialysis. Chinese J Nephrol. 2017;33:745–9. [Google Scholar]
- 9.Altunayoglu Cakmak V, Gazioglu S, Can Usta N, Ozkorumak E, Ayar A, Topbas M, et al. Evaluation Of temperament and character features as risk factors for depressive symptoms in patients with restless legs syndrome. J Clin Neurol. 2014;10:320–7. doi: 10.3988/jcn.2014.10.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oertel WH, Hallström Y, Saletu-Zyhlarz GM, Hopp M, Bosse B, Trenkwalder C, et al. RELOXYN Study Group-Sleep and quality of life under prolonged release oxycodone/naloxone for severe restless legs syndrome: An analysis of secondary efficacy variables of a double-blind, randomized, placebo-controlled study with an open-label extension. CNS Drugs. 2016;30:749–60. doi: 10.1007/s40263-016-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anguelova GV, Vlak MH, Kurvers AG, Rijsman RM. Pharmacologic and nonpharmacologic treatment of restless legs syndrome. Sleep Med Clin. 2018;13:219–30. doi: 10.1016/j.jsmc.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Hornyak M, Scholz H, Kohnen R, Bengel J, Kassubek J, Trenkwalder C. What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med Rev. 2014;18:153–64. doi: 10.1016/j.smrv.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Wu L, Wang S, Ding L, Xu L, Wang Y, et al. Incidence of augmentation in primary restless legs syndrome patients may not be that high: Evidence from a systematic review and meta-analysis. Medicine. 2016;95:e2504. doi: 10.1097/MD.0000000000002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bo Y, Jiaruo X, Qiang X, Tingting W, Jing X, Chaoyang Y, et al. Non-pharmacological intervention for improving sleep quality in patients on dialysis: Systematic review and meta-analysis. Sleep Med Rev. 2015;23:68–82. doi: 10.1016/j.smrv.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Quach K, Gandhi M, Brimble K, Walsh M. The Effectiveness Of Pharmacological And Non-Pharmacological Interventions In Improving Restless Legs Syndrome Severity Among Patients On Dialysis: A Systematic Review. BMJ Publishing Group Ltd. 2015 [Google Scholar]
- 16.Salminen AV, Rimpilä V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom Disease) Neurology. 2014;82:1856–61. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 17.Rad M, Jaghouri E, Sharifipour F, Rakhshani MH. The effects of cool dialysate on pruritus status during hemodialysis of patients with chronic renal failure: A controlled randomized clinical trial. Iran Red Crescent Med J. 2017;19:e34759. [Google Scholar]
- 18.Eldehni M, Odudu A, McIntyre C. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–65. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker K, Bailey J, Rye D, Bliwise D, Van Someren E. Lowering dialysate temperature improves sleep and alters nocturnal skin temperature in patients on chronic hemodialysis. J Sleep Res. 2007;16:42–50. doi: 10.1111/j.1365-2869.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Wu J, Jia Q. Efficacy of intravenous iron sucrose in hemodialysis patients with Restless Legs Syndrome (RLS): A randomized, placebo- controlled study. Med Sci Monit. 2017;23:1254–60. doi: 10.12659/MSM.900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustafa RA, Bdair F, Akl EA, Garg AX, Thiessen-Philbrook H, Salameh H, et al. Effect of lowering the dialysate temperature in chronic hemodialysis: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016;11:442–57. doi: 10.2215/CJN.04580415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paiva T, Gaspar T, Matos MG. Sleep deprivation in adolescents: Correlations with health complaints and health-related quality of life. Sleep Med. 2015;16:521–7. doi: 10.1016/j.sleep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Happe S, Evers S, Thiedemann C, Bunten S, Siegert R. Whole body and local cryotherapy in restless legs syndrome: A randomized, single-blind, controlled parallel group pilot study. J Neurol Sci. 2016;370:7–12. doi: 10.1016/j.jns.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Downie G, Krimsky W. Response to spray cryotherapy in a patient with adenocarcinoma in the parietal pleura. Respiration. 2010;80:73–7. doi: 10.1159/000271866. [DOI] [PubMed] [Google Scholar]
- 25.Lange U, Uhlemann C, Müller-Ladner U. Serial whole-body cryotherapy in the criostream for inflammatory rheumatic diseases. A pilot study. Medizinische Klinik. 2008;103:383–8. doi: 10.1007/s00063-008-1056-5. [DOI] [PubMed] [Google Scholar]
- 26.Palmieri-Smith RM, Leonard-Frye JL, Garrison CJ, Weltman A, Ingersoll CD. Peripheral joint cooling increases spinal reflex excitability and serum norepinephrine. Int J Neurosci. 2007;117:229–42. doi: 10.1080/00207450600582702. [DOI] [PubMed] [Google Scholar]