Abstract
Photoacoustic imaging is a rapidly maturing imaging modality in biological research and medicine. This modality uses the photoacoustic effect (“light in, sound out”) to combine the contrast and specificity of optical imaging with the high temporal resolution of ultrasound. The primary goal of image-guided therapy, and theranostics in general, is to transition from conventional medicine to precision strategies that combine diagnosis with therapy. Photoacoustic imaging is well-suited for noninvasive guidance of many therapies and applications currently being pursued in three broad areas. These include the image-guided resection of diseased tissue, monitoring of disease states, and drug delivery. In this review, we examine the progress and strategies for development of photoacoustics in these three key areas with an emphasis on the value photoacoustics has for image-guided therapy.
Keywords: photoacoustic imaging, ultrasound, image-guided, therapy, surgery, drug delivery
1. Introduction
1.1. History of Photoacoustic Imaging
In the past few decades, photoacoustic imaging (PAI) has emerged as a novel imaging modality with a variety of promising applications in medicine. In contrast to traditional X-ray imaging, positron-emission tomography (PET), and computed tomography (CT), PAI is capable of revealing structural, functional, and molecular information of biological systems without ionizing radiation by integrating optical excitation with ultrasound detection. This hybrid technique relies on the photoacoustic effect—the generation of acoustic waves as light is converted to heat following optical absorption. In the body, PAI can be used to image intrinsic components of tissue such as hemoglobin and melanin, as well as extrinsic contrast agents targeted to biomarkers or specific regions of the body.
Today, PAI is being developed for a wide range of image-guided applications including the monitoring and therapy of disease states, drug delivery, and surgical guidance. A number of review articles on PAI exist in the literature. These range from emphases on contrast agents or specific applications to more general overviews 1-6. For contrast agents, both broad surveys 7-10 and spotlights on particular types such as ratiometric probes 11, activatable probes 12, inorganic nanomaterials 13-16, photothermal probes 17, NIR-II probes 18, 19, and organic probes 19-23 are recommended to the reader. In addition, applications reviewed include imaging the brain 24, 25, microvasculature 26, cancer 27, and drug delivery 28. The purpose of this review is to highlight image-guided applications of PAI, assess developments in the technology, and examine progress towards clinical translation.
Alexander Graham Bell first reported the photoacoustic (PA) effect in 1880 following his experiments on the photophone, the world's first wireless communications device 29. He described the generation of sound waves by various materials in response to pulses of sunlight 30. These observations were overlooked for many years, but have since been studied extensively, and the physics of the PA effect were expounded by Rosencwaig and Gersho, of Bell Labs, for photoacoustic spectroscopy 31. They predicted its utility for biomedical imaging because of its ability to obtain optical absorption spectra for almost all solids and semisolids, its insensitivity to light scattering, and its noninvasive nature. Not long after, a method was developed for acquiring 1D PA signal in synthetic scattering media using a single transducer element following a pulse of ionizing radiation 32. In the 1990s, non-ionizing light was harnessed for signal propagation, and the technology was extended to 3D imaging in biological tissue, along with theoretical underpinnings of PA ultrasound reconstruction tomography 33-35 and microwave-induced acoustic tomography 36. Since then, major developments in PAI have included photoacoustic tomography (PAT), photoacoustic computed tomography (PACT), photoacoustic microscopy (PAM), handheld imaging devices, and customized contrast agents 37. In this review, we provide an overview of the emerging roles these PA technologies are playing in image-guided therapy, surgery, and drug delivery (Figure 1).
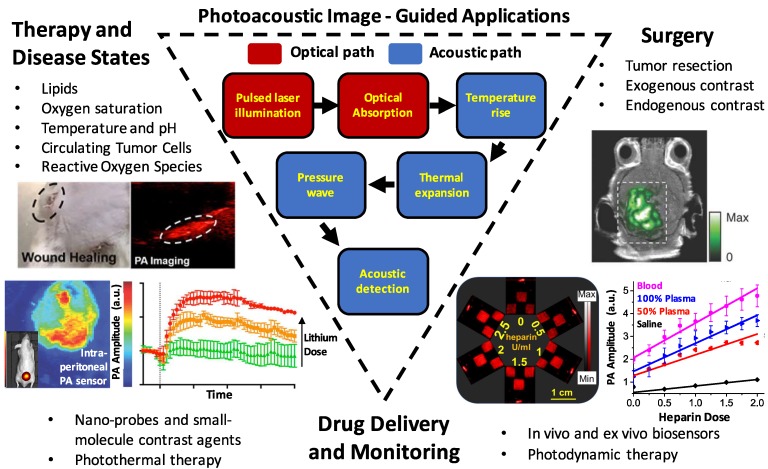
Figure 1.
Summary schematic of the topics examined in this article: image-guided surgery (including tumor margination and resection, endogenous contrast mechanisms, and endogenous contrast agents), drug delivery and monitoring (including photothermal/photodynamic therapies, nanoparticle and molecular contrast agents, and biosensors), and monitoring therapy and disease states (including targets such as reactive oxygen species, hemodynamics/blood oxygen saturation, temperature/pH, circulating tumor cells, and lipids).
1.2. Principles of PAI
PAI is a hybrid imaging modality because it combines the high contrast and specificity of optical imaging with the strong temporal resolution and increased penetration depth of ultrasound. PAI (sometimes called optoacoustic imaging) takes advantage of the photoacoustic effect and typically uses near-infrared (NIR) or visible light to leverage the optical window— a range of non-ionizing wavelengths that allow for deeper penetration in human tissue 38, 39. The photoacoustic effect consists of three distinct steps in soft matter 40:
The target absorbs a photon.
The target increases in temperature, inducing a local pressure increase via thermoelastic expansion.
The pressure perturbation is propagated throughout the surrounding medium by elastic interactions.
The generation of acoustic waves requires thermal expansion to be time variant. To achieve this in PAI, a pulsed laser is used (1-100 ns), or a continuous-wave (CW) laser is intensity modulated to excite a light-absorbing target 41, 42. Pulsed lasers are the most common because they have a better signal to noise ratio than CW lasers at constant fluence and power 43. In addition, laser pulses are shorter than the thermal and stress confinement times of absorbers, meaning thermal diffusion and volume expansion can be neglected during illumination 4.
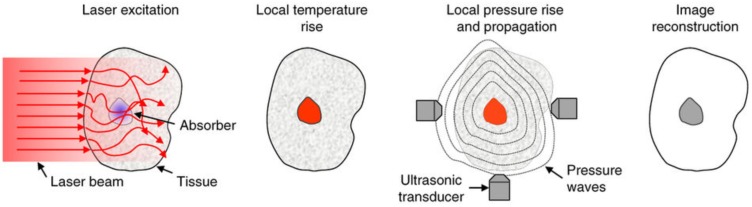
In tissue, the interaction of photons with cellular structures results in elastic scattering. The molecules struck by these scattered photons undergo thermoelastic expansion generating wideband acoustic waves (ultrasonic) in the surrounding medium 44. These waves are detectable by an array of piezoelectric transducers and images can be generated by measuring the waves' amplitudes and arrival times to reconstruct the initial photoacoustic pressure distribution. This process is represented schematically in Figure 2.
Figure 2.
Schematic of the principles of PAI. Light is absorbed by a target, raising the local temperature. Thermoelastic expansion propagates pressure waves in the ultrasonic range through surrounding tissue. Ultrasound transducers detect the waves and images are constructed based on their strength and times of arrival. Adapted with permission from 5, copyright 2016 Nature Publishing Group.
The initial amplitudes of PA waves can be determined according to Equation 1.
| P0 =ΓηthμaF | (1) |
Here, P0 is the initial local pressure rise (PA amplitude) that initiates ultrasonic wave propagation. The PA efficiency is dictated by the Grüneisen parameter Γ (dimensionless), a thermodynamic property that scales with temperature and differs between absorbing targets, and ηth, the heat conversion efficiency (equivalent to the amount of nonradiative energy decay following the laser pulse, or, 1 minus the fluorescence quantum yield) 4, 28. Because Γ is temperature dependent, PAI can be used to monitor temperature 45. The optical absorption coefficient, μa (cm-1), is proportional to the concentration of the chromophore target, and F is the local optical fluence (J/m2). Together, μa and F can be understood as the total energy deposition (J/m3), or absorbed optical energy 6, 28, 40. Equation 1 shows that the absorption coefficient of an object can be determined from pressure measurements if the optical fluence is known.
1.3. PAI Implementation: Tomography, Microscopy, and Endoscopy
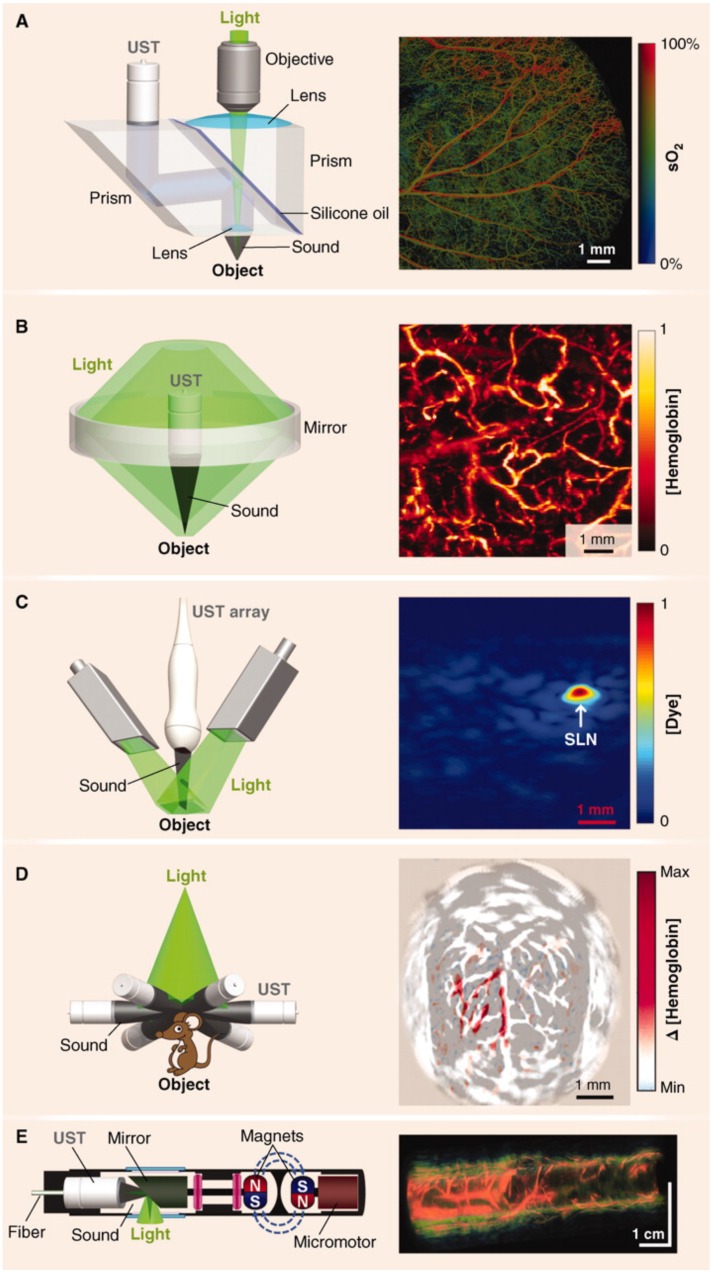
The principles of PAI have been primarily adapted in major forms to photoacoustic microscopy (PAM), photoacoustic tomography (PAT), photoacoustic computed tomography (PACT), and photoacoustic endoscopy (PAE) 46. These configurations are represented schematically in Figure 3 and summarized in Table 1. PAI systems may operate in reflection (backward) mode or transmission (forward) mode 47. In reflection mode, the irradiation source and transducer are on the same side of the sample. The irradiation source and transducer are typically coupled, making operation similar to B-mode ultrasound imaging. In forward mode, the irradiation source and transducer are on opposite sides of the sample. This mode is usually limited to thin tissue sections or ex vivo samples because of the strong attenuation of light through tissue.
Figure 3.
Major hardware configurations for PAI. (A) OR-PAM of oxygen saturation in a mouse ear. The light source is focused by an objective lens and sound is focused by a concave lens at the bottom of the prism where it is reflected to the ultrasound transducer (UST). (B) AR-PAM of hemoglobin concentration in a human palm. Dark-field illumination creates a cone of light rather than a point. (C) Linear Array PACT of methylene blue localized to a rat sentinel lymph node. Diffuse homogenous light illuminates the sample and is collected by a scanning line of transducer elements. (D) Circular Array PACT of cerebral [hemoglobin] dynamics. The array of ultrasound transducers can detect acoustic waves generated by the sample in all directions of a given plane. (E) PAE of a rabbit esophagus where components have been miniaturized for internal imaging. Adapted with permission from 3, copyright 2012 AAAS.
Table 1.
Major hardware configurations of PAI with representative performance metrics.
| Technique | Full Name | Brief Description | Resolution | Imaging Depth |
|---|---|---|---|---|
| OR-PAM | Optical Resolution - Photoacoustic Microscopy 49, 50 | A laser is focused to a diffraction-limited spot with a microscope objective and the generated acoustic signal is focused with a concave acoustic lens to be detected by a broadband ultrasound transducer. | ~0.5 - 3.0 μm | ~1.2 mm |
| AR-PAM | Acoustic Resolution - Photoacoustic Microscopy 3 | A dark-field ring of light is focused onto tissue and a concave acoustic lens focuses the acoustic signal to a broadband ultrasound transducer. | ~45 μm | ~5 mm |
| PAT/PACT | Photoacoustic Tomography / Computed Tomography 5 | A pulsed illumination beam excites a region of interest and a linear or circular transducer arrayreceives acoustic signal for image generationusing inverse/back-projection algorithms. | 0.1 - 1.0 mm | Multiple centimeters possible |
| PAE | Photoacoustic Endoscopy 60 | Optical excitation, acoustic transduction, and mechanical scanning components are confined to the distal tip of an endoscope. | 31 - 170 μm | 1 - 7 mm |
PAM is a reflection or transmission mode technique in which a single element transducer raster scans the sample and it is capable of millimeter imaging depths and micron resolutions48. In this mode, the optical excitation and acoustic detection create dual foci that are both focused to increase sensitivity 3. A single laser pulse is capable of returning a 1D ultrasonic signal with resolution axial to the laser. When scanning laterally, the laser transduces these signals across the area of the sample, generating 3D images. Axial resolution depends on the acoustic time of flight and lateral resolution relies on the distance between foci 3. PAM is further classified by the modality that governs resolution: OR-PAM (optical resolution) or AR-PAM (acoustic resolution). OR-PAM is limited in depth by the optical diffusion limit (~1.2 mm), but can image cellular and subcellular structures from hundreds of nanometers to a few micrometers 49, 50. The capabilities of OR-PAM have recently been extended by Lan et al., who reported frame rates of 900 Hz with a wide field of view (12 mm) using a hexagon-mirror scanner 51, 52. AR- PAM uses dark-field optical excitation, and is capable of penetrating millimeters beyond the diffusion limit (though this depth sacrifices lateral resolution) 53. A 45-μm lateral resolution and 3-mm imaging depth are achievable values for AR-PAM 3. PAM systems are typically benchtop configurations that are most conveniently used for ex vivo applications; however, they can certainly be used for in vivo work. For example, subcutaneous melanomas in mice, oxygen saturation in rats, and total hemoglobin concentration in the human palm have been imaged with PAM 48. Furthermore, a handheld PAM probe using a waterproof MEMS scanner (total weight: 162 g, diameter: 17 mm), was recently developed and shown to be effective for clinically relevant, in vivo applications such as delineating the 3D boundaries of a human mole 54. These miniaturizations will improve the clinical feasibility of PAM systems.
PAT is a technique that follows the principles described in Section 1.2— it is widely used in research studies and multiple commercial systems are on the market. In focused scanning PAT, a focused ultrasonic transducer mechanically scans the area of the sample. PACT was developed to decrease the long imaging times (tens of minutes for a full mouse body) associated with such raster scanning modes 28. It implements a wide beam of homogenous light to illuminate the entire region of interest and an array (linear or circular) of ultrasound detectors for signal acquisition. Back-projection algorithms are then used to pinpoint the ultrasonic sources and reconstruct 2D or 3D images 55. PACT is faster than scanning PAM and can image more deeply, but typically has lower resolution 28. In general, tomographic systems have resolutions in the 0.1 - 1.0 mm range but can achieve multiple centimeter imaging depths in soft tissue. Another advantage of tomography is that transducers (e.g. linear arrays) can be handheld, as in conventional ultrasound imaging. A recently published review covers advancements in handheld PAI in detail 56.
PAE has also been developed for imaging in difficult locations such as the gastrointestinal tract, lungs, trachea, and mediastina 57. These internal organs can be imaged using miniaturized components confined to the tip of an endoscopic probe a few millimeters in diameter 58, 59. In such cases, typically an apparatus combining the light generator (optical fiber) and ultrasound detector is driven by motors to circumscribe the target resulting in cross-sectional and volumetric images. The size and capabilities of these PAE probes will improve as designs using optically transparent transducer components become more common and are further miniaturized. Intraoperative PAE applications are discussed more in Section 2.1.
1.4. Strengths and Limitations of PAI
PAI demonstrates numerous unique features and advantages over traditional imaging techniques. For example, by relying on NIR and visible light, PAI eliminates the risks associated with high-frequency ionizing radiation. It is capable of imaging at multiple scales, resolving single cells, organelles, and capillaries in vivo 61, 62. In PAM, imaging depths and resolutions can be scaled from the micro to macro regime by tuning the numerical aperture of the objective lens or the central frequency of the transducer 3, 4. Unlike most optical imaging techniques, PAI is capable of breaking the optical diffusion limit, which is defined by the transport mean free path (~1 mm in tissue) 63. Photons scatter ballistically above this depth and diffusely below it. Most optical techniques cannot achieve adequate resolution below this depth, but PAI overcomes this limit by detecting the ultrasonic waves induced by diffuse photons 46. This fact demonstrates that PA signal is fundamentally based on absorption (and subsequent thermoelastic expansion). In effect, background can be reduced in PAI because non-absorbing objects do not generate PA signal and spectral imaging/signal thresholding can be used to minimize signal from endogenous absorbers.
Both non-fluorescent and fluorescent targets can be imaged, and a number of endogenous chromophores have strong signal such as oxygenated/ deoxygenated hemoglobin 64, melanin 65, lipids 66, and water because of their differential absorbance spectra in the NIR. A wide variety of exogenous contrast agents have also been developed and are designed to boost contrast while expanding the range of biological processes that can be imaged. Some examples include small-molecule dyes, metallic and polymeric nanoparticles, up-conversion nanoparticles, carbon nanotubes, microbubbles, quantum dots and numerous others 7, 67, 68. Because a diverse array of materials and strategies have been investingated as contrast agents, it can be useful to distinguish them according to certain traits: for instance, activatable vs. non-activatable (passive), nanoparticle vs. small-molecule, inorganic vs. organic, or NIR-I absorbing vs. NIR-II absorbing. Activatable probes benefit from higher specificity and signal-to- background ratios than passive ones because they only activate PA signal following a biological stimulus. Nanoparticles are ideal for carrying cargoes and have unique optical properties, but small- molecules tend to have better biocompatibility and lower toxicity/faster clearance. Similarly, organic nanomaterials offer better biodegradability than inorganics, but may have lower absorption coefficients or more complex syntheses.
One important consideration for nanoparticle design is the size: typically, particles < 6 nm will be rapidly cleared by the kidneys, whereas particles > 8 nm cannot undergo glomerular filtration, and sizes between 30-200 nm can achieve long circulation times for enhanced accumulation and retention in tumors 69, 70. It is also known that for photothermally active gold nanomaterials (e.g. spheres, rods, shells), the magnitude of extinction increases with size (e.g. from 20 to 80 nm for spheres) 71. Specifically, the contribution of scattering to the total extinction increases relative to absorption. Feis et al. investigated this effect with gold nanoparticles and saw that larger particles at larger wavelengths had lower photoacoustic amplitudes than predicted by the extinction spectrum 72. This discrepancy was attributed to the insensitivity of PAI to scattering effects. Therefore, the individual contributions of absorption and scattering, which is often size dependent, should also be considered during PA nanoparticle design. Overall, desirable properties for effective contrast agents include high molar absorption coefficients, absorption at long NIR wavelengths (ideally non-overlapping with endogenous absorbers), stability, affordability, targetability, and safety 10.
Using exogenous contrast, imaging depths in PACT have been reported as high as 11.6 cm in chicken breast tissue and 5 cm in the human arm 73. However, deep imaging (>1 cm) with high resolution still remains a challenge for most PA applications. Specific deep-lying organs that present challenges for PAI because of their location include the heart (location under the rib cage), lungs (beneath rib cage and gas-tissue interface), stomach (a hollow structure and under rib cage), and pancreas (depth) 28. Penetration depth issues can be countered, however, with exogenous agents (especially in the second optical window) and unique hardware designs. For example, novel transducers and intraoperative probes have been designed to image trans-vaginally 74, trans-rectally 75, 76, and trans-esophageal 77. Of course, all hardware designs can be combined with molecular imaging strategies using exogenous contrast agents to maximize performance (see Table 2 for representative molecular imaging agents). Furthermore, one of the emerging trends in recent years has been the increased use of the second optical window (NIR II: 1000-1700 nm) for fluorescence imaging and subsequently PAI 78, 79. This range is highly attractive for biophotonic applications because of the diminished light-tissue interactions— specifically, scattering and autofluorescence— at longer wavelengths 80. Compared to the first optical window (650-950 nm), these factors allow imaging at higher penetration depths. Additionally, because these photons have lower energies, the maximum permissible exposure (MPE) is higher for clinical applications which can boost PA performance and reduce signal-to-background ratios. The first NIR-II PA contrast agents explored have been inorganic nanomaterials, such as plasmonic precious metal nanoparticles and copper sulfide nanoparticles 81- 83. However, recent progress has been made in organic NIR-II probes, including semiconducting polymer nanoparticles 22, 79 and small molecules 19. Still, the second optical window presents new challenges that must be overcome. For example, water has a significant absorption peak at 1450 nm, existing NIR-II agents have low quantum yields, and few organic materials have been reported that can absorb above 1064 nm in the NIR-II 22.
Table 2.
Representative exogenous contrast agents used for applications in PA-image guided surgery.
| Photoacoustic Agent | Description | Wavelength (nm) | Target | Image-Guided Outcome | Reference |
|---|---|---|---|---|---|
| B7-H3-ICG | Anti-B7-H3 conjugated with Indocyanine Green | 800-825 | Breast Tumor | In vivo murine tumor delineation | Wilson et al. 2017 104 |
| Gold Nanorods | Gold nanorods coatedwith PEG, 41 nm x 12 nm, 3.5 aspect ratio | 756 | Ovarian Tumor | In vivo murine tumor delineationand resection guidance | Jokerst et al. 2012 105 |
| Iron Oxide NPs (Endorem®) | Superparamagnetic IronOxide NPs (80-150 nm diameter) | 720 | Lymphoma | Nodal stagingof resected human lymph nodes | Grootendorst et al. 2013 106 |
| Coomassie Blue Polyacrylamide NPs | Polyacrylamide hydrogel nanoparticles crosslinked with Coomassie-Blue dye (80 nm diameter) | 590 | Brain Tumor | Ex vivo murine tumor delineation | Ray et al. 2011 107 |
| ICG-Coated Gold Nanorods | Gold nanorods coated with liposomal indocyanine green, 31 nm x 10 nm, 3.1 aspect ratio | 795 | Liver Tumor | Pre-operative PA detection and intraoperative fluorescence-guide resection | Guan et al. 2017 108 |
| Black Hole Quencher 3-Fluorescein-labeled Trastuzumab | Chemotheraeutic trastuzumab dual-labeled with BHQ3 (photoacoustic) and fluorescein (fluorescence) | 700 | Breast Tumor | Pre-operative PA detection and post-operative fluorescence-guided resection validation | Maeda et al. 2015 109 |
| Silica-coated gold nanoparticles with Raman/MRI-active layers | Gold nanoparticles (60 nm) coated with trans-1,2-bis(4-pyridyl)-ethylene (Raman), silica, and DOTA-Gadolinium (MRI) | 532 | Brain Tumor | Intra-operative PA and Raman-guided resection | Kircher et al. 2012 110 |
2. Metastastis Detection & Surgical Resection
2.1. Endogenous Targets for Guided Surgery
A substantial portion of PAI research is devoted to cancer applications. The vast majority of cancer deaths are caused by metastatic progression of the disease. To mitigate these cases, it is important to determine both the extent of metastasis and the margins of tumors to guide surgical intervention 84. Intraoperative PAI shows potential to improve upon existing methods (immunohistochemistry, histopathological assessment) by determining these variables in real time. Many groups are investigating the potential for PAI to detect metastasis and enhance tumor imaging. Melanoma is an appropriate target for such studies because melanoma cells contain highly absorbent melanin that acts as an endogenous PA absorber.
Using PAT, Grootendorst et al. compared intraoperative PA images of melanoma metastases from resected, human sentinel lymph nodes (SLN: the first lymph node to which a tumor drains) to hematoxylin-eosin histologic images (the routine method for prognosis that takes days). This showed that intraoperative detection of melanoma was possible although distinctions between malign and benign nodes were not obvious 85. In order to discriminate between signals from blood versus melanin, the signal from ex vivo nodes were characterized spectrally and separated with an unmixing algorithm 86. However, the accuracy of this method depends entirely on the accuracy of the PA reference spectra measured for blood and melanin. The potential benefit of intraoperative PAI of ex vivo SLNs is that detection of metastasis would allow an immediate node dissection and prevent the need for a second surgery. Luke & Emelianov showed that metastasis in lymph nodes from other (non-melanoma) primary tumor types could also be imaged 87. In a mouse model for oral cancer, they took advantage of the decreased blood oxygen saturation (SO2) associated with metastatic foci in SLNs and imaged deoxygenated/oxygenated hemoglobin to evaluate the metastasis. The differential absorption between oxygenated and deoxygenated blood provides one of the most straightforward mechanisms of endogenous PA contrast 64, 88, 89.
To accelerate clinical translation, intraoperative handheld PA probes have been designed that streamline operation of the technology. Xi et al. demonstrated a handheld microelectromechanical systems (MEMS) probe for real time inspection of 3D tumor margins in mice during resection 90. Here, they used the imaging system at 532 nm to evaluate the completeness of tumor removal following surgery and histologically validated the margins as measured with photoacoustics with < 6.5% error. One issue with using endogenous contrast for resection validation, especially at 532 nm, is the strong absorption of blood, which will produce background during surgeries. Longer wavelengths can mitigate this effect but it is difficult to match the signal to background ratios achievable with exogenous agents.
Neuschmelting et al. evaluated both 2D and 3D handheld MSOT (multispectral optoacoustic tomography) devices developed by iThera Medical by imaging murine brain melanomas at 4 MHz and 700 - 900 nm 91. The authors implanted B16F10 melanoma cells into the right frontal lobe, where tumors 3.5 - 7.0 mm in diameter grew within 13 days; the size of these tumors were independently measured with MRI. The 2D system was deemed more accurate (bias = 0.24 mm) than the 3D system (bias = 2.35 mm) but was limited to axial tumor depths of 4 mm. The 3D probe was limited by tumor size (< 3.5 mm) in all dimensions, but the acquisition time was shorter. The performance disparity between the 2D and 3D systems was attributed to a non-ideal distribution of diffused light in the transducer array of the 3D system. These handheld systems also showed superior signal to noise ratios and limits of detection and for metastatic melanoma cells (5 cells/uL at 8 mm tissue depths in mice) to PET/CT using fluorine 18 fluorodeoxyglucose 92. However, this sensitivity would be difficult to achieve if applied to human lymph nodes located centimeters below the skin. Additionally, to our knowledge, these systems have not yet been used to monitor resection during or after an operation.
Miniaturized and endoscopic probes can offer advantages for image-guided surgeries due to their adaptability and form factor. One example is radical prostatectomy, a common treatment for prostate cancer in which the prostate and seminal vesicles are fully removed 93. Oncological success (full tumor elimination) must be balanced with minimizing functional damage (e.g. incontinence and impotence). A recent study was conducted to determine the best scanning geometry for intraoperative PA prostate imaging 94. It was demonstrated through simulation and experiment (using phantoms) that a convex, “pick-up” transducer (28 mm width, 10 MHz) controlled by a da Vinci robot was a promising approach for prostate imaging. In this setup, the transducer is located anterior to the prostate and can form a cylindrical detection surface while conformally scanning the prostate to generate a 3D image. The integration of PAI with a da Vinci robot for guided surgery was also investigated by Gandhi et al 95. They used phantom studies to show that by connecting an optical fiber (powered by a pulsed laser diode) to a surgical arm of the robot, and a transducer to another arm, that the system could distinguish separation distances blood vessels and nerves in real-time while also visualizing the tool tip itself96. These technologies are still in their early stages, but show promise for improving patient outcomes during future in vivo and human studies.
PAE applications have been studied for over a decade, but miniaturizing the hardware while maintaining high image quality is a challenge. Nevertheless, systems have been proposed and constructed for detection of atherosclerotic plaques in cardiovascular disease 97-100, esophageal imaging 101, and gastrointestinal diseases 58. However, many of these designs take a side-viewing approach, whereby a single element transducer is attached to the distal end of an optical fiber and generates a field of view perpendicular to the fiber axis. This approach is not as desirable as a forward-viewing design, which is more useful for image-guided intraoperative procedures 102. For PAE, this presents a design challenge because forward-viewing necessitates either lateral scanning of the transducer head (which is more challenging then rotational scanning of a side-view design), or the use of a 2-D array of transducer elements. Unfortunately, traditional ultrasound transducer elements are opaque to light; therefore, they need to be offset from the light source which increases the overall probe size.
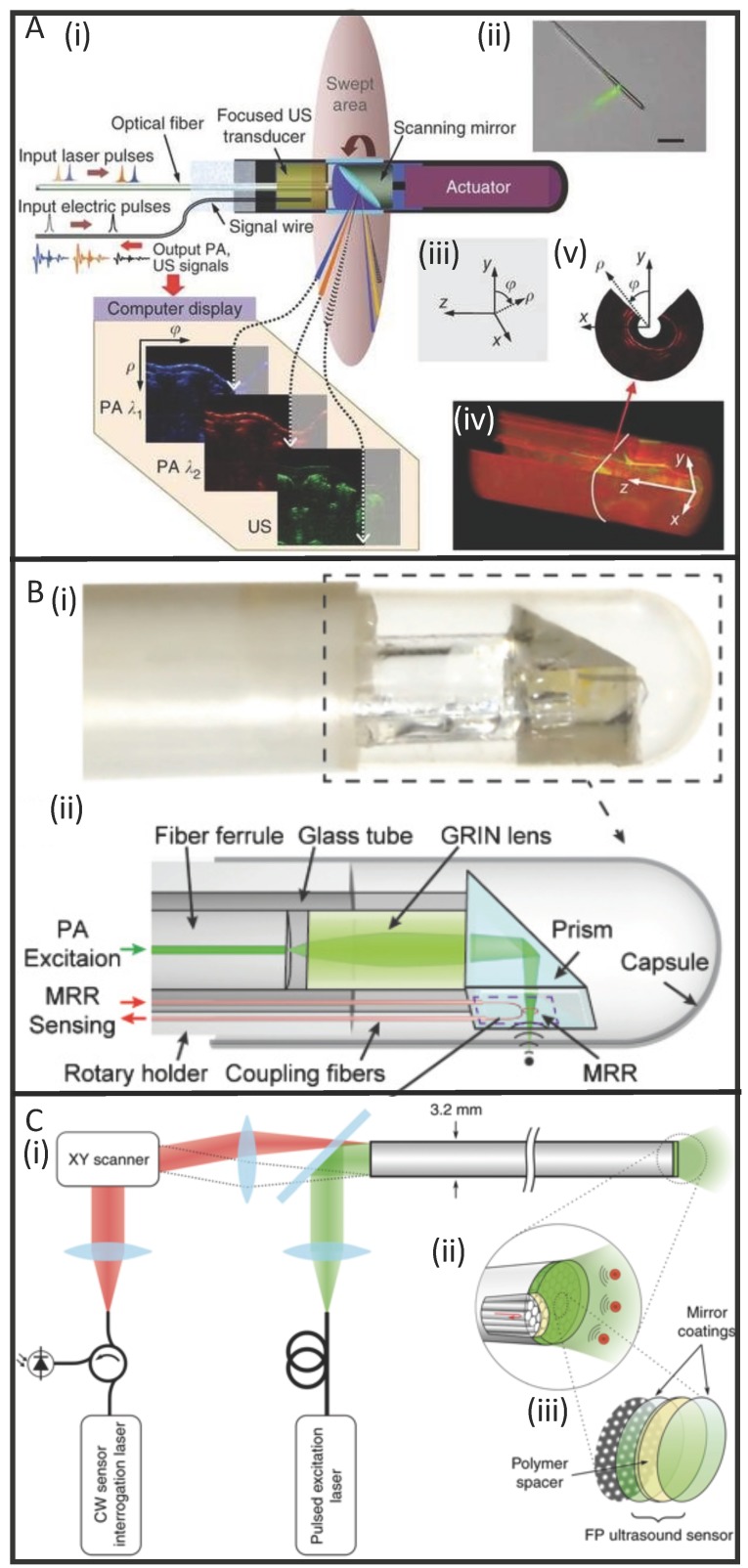
To solve this issue, optically transparent ultrasound elements can be used. Dong et al. used a polymeric microring resonator ultrasound sensor and demonstrated phantom imaging with sub-10- μm axial resolution and 15.7 μm tangential resolution but retained a side-view design 103. Furthermore, Ansari et al. recently demonstrated a novel, forward- view PAE system with a 3.2 mm diameter footprint that uses a Fabry-Pérot polymer film sensor, capable of resolving microvascular anatomy in impressive detail 60. The sensor acts as a high-density transducer array with 50,000 individual elements and allows transmission of light with 45-170 μm on-axis lateral resolution, 31 μm vertical resolution, and 1-7 mm imaging depths. While still in its early stages, this new class of instrumentation shows promise for accelerating the use of PAE in image-guided laparoscopies and surgeries (Figure 4).
Figure 4.
Developments in PAE instrumentation. (A) A side-viewing photoacoustic endoscope with 3.8 mm diameter (i, ii) that uses a conventional single-crystal transducer (LiNbO3, 36 MHz) and achieves circumferential scanning with a rotating mirror that reflects ultrasound waves and laser pulses. Representative images from rabbit esophagi with Cartesian and cylindrical coordinate systems (iii) are shown in (iv) and (v) 58. (B) A photograph (i) and schematic (ii) of a side-viewing endoscope (4.5 mm diameter) that uses an optically transparent, micro-ring resonator transducer. 103. (C) A schematic of a forward-viewing endoscope with 3.2 mm diameter that uses an optically transparent Fabry-Pérot ultrasound sensor attached to the distal end of the device. A magnified view of the distal end (i) shows individual fiber-optic cores beneath the Fabry-Pérot sensor which is revealed in (iii): it is made from two dielectric mirror coatings that sandwich a Parylene C polymer spacer (15 µm) 60.
2.2. Exogenous Contrast Agents for Guided Surgery
Many exogenous contrast agents have been designed to accumulate at target sites and enhance the photoacoustic signal of diseased tissues. These are typically small molecules or nano-scale agents injected into the bloodstream, but they can be administered via other routes. This section highlights some of the vehicles used specifically for tumor identification and resection (Table 2).
Variations of gold nanoparticles are popular because of their strong optical absorption (orders of magnitude greater than organic dyes), and geometry-dependent resonant frequencies that exploit the NIR window 13, 14. Gold nanorods were employed in 2012 as dual PA/Raman contrast agents for passive targeting of ovarian xenografts in mice 105. Here, maximum PA signal was observed after 3 h (persisting for 48 h). The detection limit was 0.40 nM, and the margins between tumor and normal tissue were clearly visualized. Silica-coated gold nanoparticles coated with Raman and MRI-active layers have also been developed as triple modality (PA/MRI/Raman) contrast agents for glioblastoma tagging and resection, shown in Figure 5 110. They exhibited optical absorbance coefficients 200-fold higher than previously studied carbon nanotubes, allowing for 3D imaging of deep-lying tumor tissue (via PA) in conjunction with the Raman modality's high sensitivity to microscopic tumor deposits. Guan et al. combined gold nanorods with indocyanine green dye (ICG, a NIR absorbing fluorescent dye) to integrate fluorescent imaging and photoacoustics with utility for liver cancer diagnosis 108.
Figure 5.
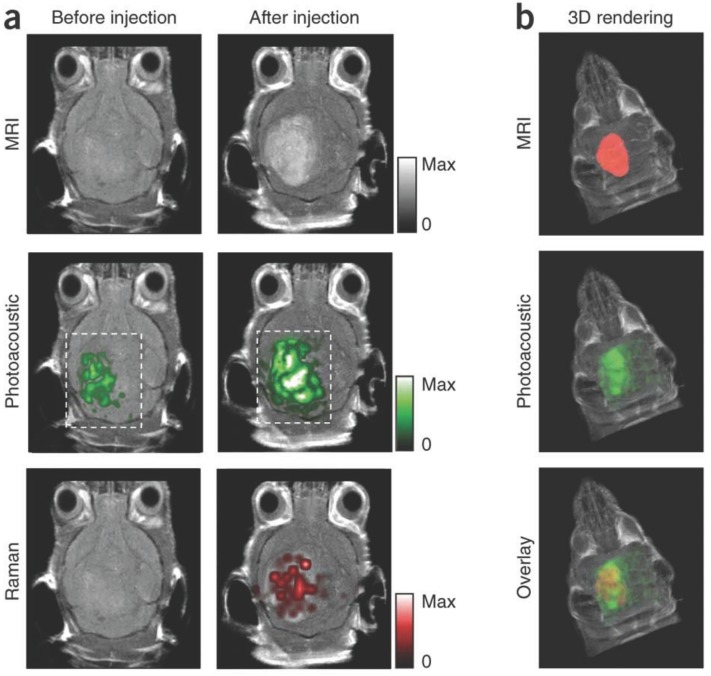
Murine brain tumor imaged in vivo with modified gold-silica nanoparticles for PAI, MRI, and Raman spectroscopy for guided surgery. (A) Tumor-bearing mice were given IV injections of the particles and imaged axially in 2D both pre- and post-injection at 2 h (PA), 3 h (Raman), and 4 h (MRI). PA and Raman images were overlaid with MRI demonstrating clear tumor visualization and co-localization between techniques. (B) 3D construction of MR with segmented tumor (red: top), 3D PA overlay with MRI (green: middle), and overlay of segmented tumor, PA, and MRI (bottom). Adapted with permission from 110, copyright 2012 Nature Publishing Group.
A variety of other nanoparticles for PA-based tumor tagging have been studied as well 8. Magnetic iron oxide nanoparticles (IONPs) are biodegradable with low toxicity and have been used previously in humans in conjunction with MRI 111. They enhance PA signal in lymph nodes following uptake by nodal macrophages although metastatic versus benign tissue could not be distinguished 106. However, Xi et al. reported the successful imaging of breast cancers with IONPs conjugated with NIR-dye labeled peptide fragments in a murine model 112. Other groups have reported tumor targeting antibodies labeled with PA contrast agents (such as the Black Hole Quencher 3 dye) 109 and self-assembling nanovesicles containing lipid-based dyes (J-aggregates) 113.
One challenge in PAI is differentiating the signal from the contrast agents from that of endogenous photoabsorbers. With spectroscopic photoacoustic imaging (sPA), it is possible to reduce noise from background photoabsorbers using knowledge of their wavelength-specific absorption spectra and relative tissue concentrations to isolate the signal of the exogenous agent 114, 115. Wilson et al. used sPA and an ICG-labeled antibody to target and image a novel breast cancer surface receptor, B7-H3 104. They could distinguish agent that had located and penetrated the target cells because of the spectral shift in absorption following its endocytosis and degradation by malignant cells.
3. Monitoring Drug Delivery
3.1. Nanocarriers and Co-Loaded Contrast Agents
A major challenge in drug delivery is the noninvasive and real-time monitoring of biodistribution and pharmacokinetics. In humans, radiotracers in conjunction with PET/CT scanning can be used 116. In animals, pharmacokinetics are usually determined via radiolabeling followed by dissection or imaging for biodistribution analysis 117. PAI shows potential in small animals for both localized drug monitoring and whole-body imaging in a non-invasive format without ionizing radiation 118. Unfortunately, very few clinically prescribed drugs, if any, exhibit intrinsic absorption in the NIR—an important trait for PAI 28. This shortcoming can be overcome, however, with the use of co-loaded contrast agents and nanocarriers and that act as vehicles for drugs. Furthermore, some of these materials can act therapeutically themselves, in the case of photothermal and photodynamic therapies. Examples of contrast agents discussed in this section are highlighted in Table 3.
Table 3.
Representative exogenous PA contrast agents with applications in drug delivery and monitoring.
| Photoacoustic Agent | Description | Wavelength (nm) | Target | Image-Guided Outcome | Reference |
|---|---|---|---|---|---|
| Semiconducting Polymer Nanoparticles | Photothermal SPNs doped with fullerene | 750 | Breast Tumor | Photothermal therapy | Lyu et al. 2016 119 |
| Nanonaps | Frozen micelles of hydrophobic, napthalocyanine dyes (~20 nm diameter) | 600, 707, 793, 863 (Tunable) | Intestine | Diagnosis/treatment of intestinal motility disorders | Zhang et al. 2014 120 |
| MoS2 Nanosheets | PEGylated, 2-D nanosheets loaded with photodynamic Ce6 | 800 | Breast Tumor | Photothermal therapy, photodynamic therapy | Liu et al. 2014 121 |
| RbxWO3 Nanorods | Rubidium tungsten bronze nanorods with doxorubicin payload, 20-40 nm x 5 nm | 808 | Breast Tumor | Combined chemo/photo-thermal therapy | Tian et al. 2014 122 |
| Gold Nanocages | Gold nanocages loaded with1-tetradecanol (phase-change)and organic dyes | 750 | Non-specific | HIFU/Temperature-triggered drug release and monitoring | Moon et al. 2011 123 |
| Fucoidan CappedGold Nanoparticles | Gold nanoparticles coatedwith fucoidan and doxorubicin | 566 | Breast Tumor | Chemotherapy | Manivasagan et al. 2016 124 |
| Perfluorocarbon Nanodroplets | Perfluorocarbon nanodropletsloaded with gold nanorods and paclitaxel | 770 | Cervical Tumor | Combined chemo/photo-acoustic therapy | Zhong et al. 2016 125 |
| Porphyrinoid-LoadedPLGA Nanoparticles | Poly(lactic-co-glycolic) acid nanoparticles loaded withporphyrinoid macrocycles | 910 | Uranium | Quantification of in vivo uranium exposure | Ho et al. 2015 126 |
| Optode Nanosensors | Intradermal injectable nanosensorcontaining a crown ether ionophoreand a chromoionophore | 515, 660 | Lithium | Drug monitoring of systemiclithium dose | Cash et al. 2015 127 |
| Methylene Blue or Nile Blue | Cellulose-based, PEG-infused substrates containing Nile Blue | 680 | Heparin | Drug monitoring of systemic heparin dose | Jeevarathinam et al. 2018 128 |
In 2011, hollow gold nanocages were loaded with a phase change material (1-tetradecanol) and either methylene blue (44 g/L solubility in H2O) or Rhodamine 6G (20 g/L solubility in H2O) to demonstrate a controlled release drug delivery system that can be monitored with PAI 123. 1-Tetradecanol has a melting temperature of 38°C and the authors showed that high-intensity focused ultrasound (HIFU) or heating could melt the material, allowing both the hydrophilic methylene blue and 2-fold less hydrophilic Rhodamine 6G to leak from pores in the nanocages at a controllable rate. Another group has shown that the hydrophobic cancer drug, doxorubicin, can be loaded with fucoidan capped gold nanoparticles for PA imaging of anti-tumor therapy in breast cancer cell lines 124. However, it was not demonstrated in vivo. One interesting approach is to use the pulsed NIR excitation itself as a stimulus for drug release—e.g. using a low laser fluence for imaging and a higher fluence for triggered release. Wilson et al. first developed this platform by loading phase-change particles (perfluorocarbon droplets) with gold nanorods (for optical absorption), showing that both US and PA signal could be drastically boosted following the vaporization of the particles; the technique was then extended by Zhong et al. who incorporated paclitaxel for an activatable release mechanism at a laser fluence of 14 mJ/cm2 125, 129. Organic vesicle-forming carriers also show potential for drug delivery imaging via their capacity for passive loading and biodegradability. Supra- molecular assemblies of the highly absorbent porphyrin (porphysomes and porphyrin shell microbubbles) can accumulate in tumors of xenograft- bearing mice while exhibiting high contrast 130, 131. Ho et al. showed that polymeric nanoparticles containing porphyrinoids could boost PA signal upon complexing with uranyl ions, demonstrating a path towards sensitive and specific imaging of radioactive isotopes in vivo 126.
Another promising method for direct PA drug monitoring was demonstrated by Cash et al. in 2015 127. They developed a nanosensor capable of detecting systemic lithium. Upon recognition by a crown ether ionophore, a lithium cation deprotonates a chromoionophore of the nanosensor, changing its absorption, PA, and fluorescence properties. This injectable nanosensor allows for the continuous PA and fluorescence monitoring of lithium, which has both a narrow therapeutic window and low toxic dose (Figure 6). An injectable nanosensor has also been developed for cell permeable calcium ion imaging 132. This group synthesized a metallochromic compound based on fura-2, a fluorescent indicator for calcium, but replaced the chromophore with a semi- cyanine chromophore to shift the absorption to longer wavelengths while maintaining a photoinduced charge transfer mechanism. Furthermore, the first application of photoacoustics for non-invasive imaging of the ubiquitous anticoagulant, heparin, was recently reported 128, 133, 134. Currently, heparin concentrations in patients undergoing surgery are monitored with the activated partial thromboplastin time (aPTT) per nomogram, an in vitro surrogate test for the deactivation of clotting factors 135. Here, the concentration of heparin was monitored in blood using the FDA approved dye methylene blue, a method that benefits from a fast turnaround time, strong sensitivity to heparin, and correlation to the aPTT. Methylene blue is an attractive PA agent because of its clinical approval 136, 137— it has previously been used for PAI of sentinel lymph nodes 138 and has also demonstrated PA lifetime and intensity shifts via self-quenching and interactions with micelles 139, 140.
Figure 6.
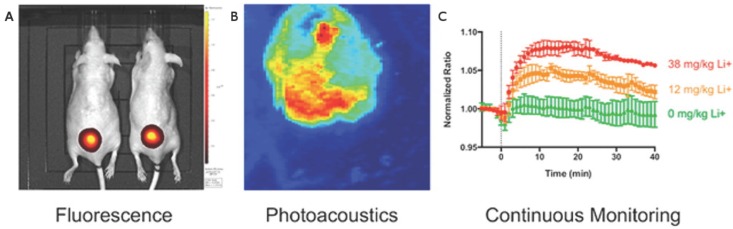
Photoacoustic and fluorescent monitoring of systemic lithium concentration in vivo using injected bimodal nanosensors. (A) NIR fluorescence was used to image lithium concentration at the site of subcutaneous nanosensors administration. (B) Photoacoustic tomography shows the boundary of the subcutaneous nanosensor injection. (C) Photoacoustics and fluorescence were both used to measure nanosensor activation. Dose-dependent curves show continuous monitoring demonstrating that higher lithium concentrations yielded stronger signal. This also revealed that peak lithium was achieved at 18 min. Adapted with permission from 127, copyright 2015 American Chemical Society.
Non-IR-absorbing carriers can be used for drug monitoring if the payload includes fluorescent/PA- active dyes. Multispectral Optoacoustic Tomography (MSOT) was developed to image fluorescent proteins in vivo. It can track, at high speeds, the biodistribution of fluorescent agents (e.g. ICG, carboxylate dyes) through circulation and uptake in the liver, gallbladder, and kidneys 141, 142. These agents are commonly loaded within delivery vehicles to boost contrast. Perfluorocarbon (PFC) nano-droplets have been loaded with ICG to enable photoacoustic and ultrasound imaging; signal is enhanced by the optically triggered phase transition of the droplets to generate PFC microbubbles 143. This controlled event also allows for drug release. Another unique example is the use of PAT with micelle-forming naphthalocyanines, “nanonaps”, to address the challenges of non-invasive GI tract imaging 120. These hydrophobic chromophores self-assemble in biocompatible surfactant (PEO-PPO-PEO) to form 20 nm spheres with high PA signal. Organic dyes are promising in PAI for their biocompatibility, multimodal nature, small size, and general stability. Current efforts devoted to synthesizing new fluorophores for optical imaging in the deep NIR (second optical window) will also have significant utility for PAI 144 For the time being, payload strategies that incorporate already FDA approved dyes such as methylene blue and ICG may have the quickest path to clinical approval. However, much work remains towards demonstrating the full potential of PAI for drug delivery and monitoring. In humans, because penetration depths are heavily surface-weighted and tissue-transducer coupling is required, it is likely that drug monitoring applications will continue to take the form of localized imaging and monitoring approaches rather than full-body biodistributions.
3.2. Photothermal Contrast Agents
Certain nanocarriers can exert their own therapeutic effects by exciting them via NIR laser radiation to release heat. Photothermal therapy (PTT) refers to the use of these photothermal agents for targeted tissue ablation. In recent decades, thermal ablation has been used to destroy diseased tissue using radiofrequencies 145 and lasers without contrast agents 146. Recently, dual contrast/PTT agents have become attractive for their ability to combine diagnostic and therapeutic capabilities into a single agent 147. Desirable traits for these agents include the ability to locate and identify tumor margins, monitor the treatment in real time, and determine post-treatment effectiveness afterwards. Photoacoustic imaging is particularly suited for monitoring these tasks.
In 2006, gold nanorods functionalized with antibody were shown to selectively target malignant cells in vitro 148. Photothermal destruction of the cells could be triggered using an 800 nm NIR laser, and malignant cells (with higher nanorod uptake) required half the laser power of non-malignant cells to cause necrosis. It was subsequently shown that PTT could be integrated with PAI using agents such as quantum dots 149, graphene 150, gold nanoshelled microcapsules 151, gold nanovesicles 152, iron oxide-polydopamine nanocomposites 153 porphysomes 130, 154, and semiconducting polymers 119. Quantum dots extended their application in fluorescent imaging to PAI/PTT, but their absorption spectra marginally overlapped with that of blood. In contrast, nanographene sheets (coated with polyethylene glycol, “PEGylated”) showed excellent NIR absorption and passive targeting of tumors, while requiring a relatively low laser power density for effective ablation.
Transitional metal dichalcogenides (TMDCs) are another noteworthy class of 2D nanomaterials; they have gained popularity in electronics and energy storage because of their unique optical and electronic properties. These materials are now being studied for their utility in medicine. In one such example, PEGylated WS2 nanosheets passively targeted tumors while maintaining strong photothermal activity. This enabled tumor ablation via NIR light-activation 155. Both groups hypothesized that the 2D geometries of nanosheets are well suited to take advantage of the experimentally observed enhanced permeability and retention (EPR) effect of growing tumors.
Organic carriers can also be useful in PTT for their biocompatibility and capacity for drug loading. Porphysomes have passive tumor targeting and PA signal while also being photothermally active 130, 154. Some polymer carriers have been combined with inorganic components: gold-nanoshelled microcapsules, for example, consist of poly-lactic acid cores coated with gold nanoparticles to impart both photoacoustic and photothermal functionality with drug-loading capabilities. Recently, semiconducting polymer nanoparticles, engineered at the molecular orbital level to favor photoinduced electron transfer, were shown to localize in tumors while increasing photoacoustic signal and PTT efficiency 119.
In 2017, the temperature responsive polymer poly(N-isospropylacrylamide) (PNIPAM), was loaded with photothermally active gold nanorods to achieve sensitive PA monitoring of photothermal therapy 156. PNIPAM shrinks when heated, allowing the gold nanorods to approach one another and shift their NIR absorbance (and PA signal) via plasmonic coupling (Figure 7). These dynamic constructs were used to selectively monitor photothermal activation in murine prostate tumors while negating background from blood. Overall, these advances in PTT demonstrate practical ways that PAI can be leveraged with synergistic contrast agents to both deliver and monitor drugs in real time.
Figure 7.
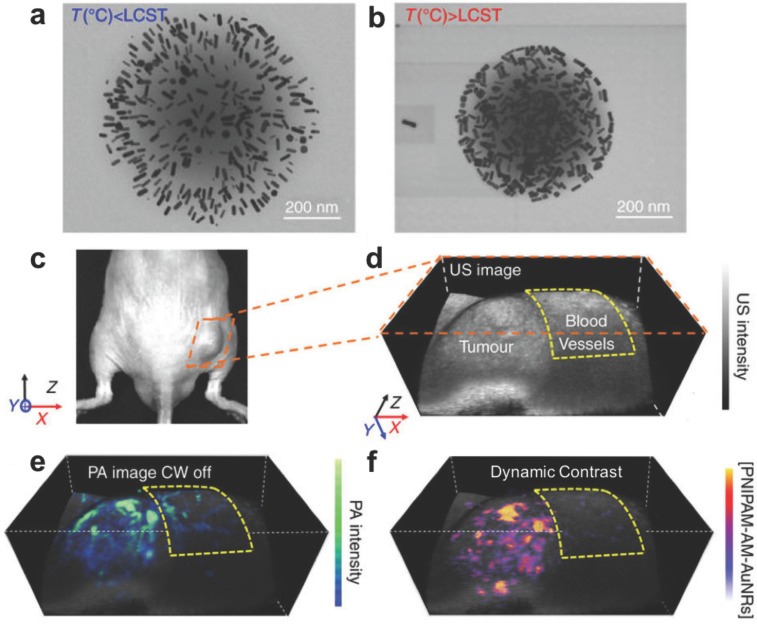
Gold nanorod (AuNR) loaded PNIPAM nanoparticles for PA monitoring of photothermal therapy. (A) Expanded form of PNIPAM-AuNRs below the lower critical solution temperature (LCST). (B) Compressed form of PNIPAM-AuNRs above the LCST, allowing plasmonic coupling between the gold nanorods. (C) Image of a prostate tumor in a mouse. (D) Ultrasound image of tumor with surrounding region of normal blood vessels highlighted. (E) PA image of tumor following injection of PNIPAM-AuNRs prior to photothermal heating (continuous wave (CW) laser off). (F) PA image with dynamic contrast following photothermal heating (CW laser on) where PNIPAM-AuNRs have compressed to shift their PA signal via plasmonic coupling and the pre-heating signal has been subtracted to remove the background from adjacent blood vessels. Adapted with permission from 156, copyright 2017 Nature Publishing Group.
3.3. Photosensitizers and Photodynamic Therapy
Photodynamic therapy (PDT) is another therapeutic approach that can work in tandem with PAI. PDT was the first drug-device combination to be approved by the FDA over two decades ago 157. It has been used clinically to address a wide range of conditions, such as acne 158, microbial infections 159, and macular degeneration 160. Currently, researchers are showing the potential for PDT to address a number of unmet needs in cancer therapy 161. It requires light, oxygen, and a photosensitizer, an agent that can absorb light and initiate a photochemical reaction to generate the highly reactive singlet oxygen, a type of reactive oxygen species (ROS). Singlet oxygen is characterized by the opposite spin of a pair of its electrons, reducing its stability and lending it cytotoxic effects. During the procedure, a photosensitizer is systemically or locally administered, localizing in a neoplastic region, followed by irradiation from a light source. Because NIR light penetrates tissue deeper than other optical wavelengths, ideal photosensitizers would be strong absorbers in the NIR range.
PAI also uses NIR absorbing agents, and some photosensitizers exhibit PA contrast in vivo. This allows the monitoring of therapy in real time 162. Ho et al. demonstrated the photoacoustic activity of five photosensitizers— the most promising molecule, zinc phthalocyanine, localized in tumors within 10 min, peaked at 1 h, and cleared after 24 h. Unfortunately, the activation absorption spectrum of many photosensitizers is in the visible range. In order to extend the usable depth of these agents in human tissue, it is possible to convert deep penetrating NIR light to visible wavelengths while enabling activation of the photosensitizers. Idris et al. accomplished this using mesoporous-silica-coated upconverting fluorescent nanoparticles that functioned as “nanotransducers” to convert NIR light to visible in vivo 163. Creative strategies such as this one will be required to enable PDT in deep-tissue applications. Alternatively, because 1) PDT requires oxygen to generate ROS and 2) PAI can be used to image oxygen saturation (via the ratiometric absorption of oxygenated and dioxygenated hemoglobin), the efficacy of PDT can be monitored label-free. Shao et al. used AR-PAM to image SO2 in the microvasculature of a rat ear following tail vein injection of the photosensitizer Verteporfin with 80 mW/cm2 light irradiance for 60 min 164. They observed an initial decrease in oxygenation followed by a gradual recovery during the treatment course; however, the data was limited to a sample size of n = 3. Nevertheless, this study showed the potential for PDT monitoring with endogenous contrast.
As with other PA applications, the therapeutic agent does not strictly require inherent contrast. The active agent (the photosensitizer, in PDT) can be loaded into NIR-absorbing vehicles to enable PA signal. Srivatsan et al. showed that the photosensitizer HPPH could be loaded onto the surface of gold nanocages via non-covalent entrapment with a PEG monolayer 165. These conjugates were stable in saline and began to degrade in protein solutions. This allowed for slow release of HPPH under biological conditions. PAI was harnessed to track the progress of delivery and suppressed tumor growth in mice—likely because of the passive targeting of the nanocages and generation of singlet oxygen at tumor sites by HPPH. Similarly, gold nanovesicles loaded with the photosensitizer Ce6 have been shown to infiltrate tumors in xenograft-bearing mice while drastically reducing the growth of tumors after 14 d 166. This study highlights the synergistic effects of agents that allow for both thermal ablation (PTT) and ROS generation (PDT). In fact, TMDC nanosheets, previously discussed for their photothermal applications, have also been employed for PDT. Liu et al. took advantage of the high surface area of MoS2 nanosheets to load Ce6, which then passively targeted and suppressed tumors via ROS generation and photothermal ablation 121. One more strategy for synergistic therapy is to use a multifunctional material such as Cu2(OH)PO4 quantum dots to achieve a “one-for-all” technique for PAI-guided PTT/PDT 167. Notably, these particles absorb well into the second optical window (up to 1700 nm). In the clinic, these could be implemented for image- guided therapy by delivering the agent to the site of a tumor, imaging the distribution of the agent for treatment guidance, and then pulsing NIR light for PTT/ PDT therapy followed by PAI for short and long-term monitoring. However, the toxicological effects of quantum dots vary widely and more work is needed to establish their potential utility in humans 168.
4. Monitoring Disease States and Therapeutic Effects
Diseases are multi-scale processes that exert effects from the molecular to the visible. Diseases often begin with a molecular event and gradually cascade as larger macroscopic effects emerge. As a molecular imaging modality, PAI is capable of revealing the early stages of pathogenesis. Early detection of these pre-disease states is critical when the therapeutic intervention can have the greatest impact. Importantly, by imaging changes in specific aberrations characteristic of disease—such as hemodynamics or ROS generation—the utility of PAI can extend beyond initial diagnosis to monitoring the results of therapeutic intervention. This section covers applications that use both endogenous and exogenous contrast, and examples are highlighted in Table 4.
Table 4.
Representative endogenous and exogenous targets used for PA monitoring of therapies and disease states.
| Endogenous/Exogenous PA Target | Description | Wavelength (nm) | Image-Guided Outcome | Reference |
|---|---|---|---|---|
| Hemoglobin | Label-free 3D monitoring of colorectal xenografts in response to vascular disruptor Oxi4503 | 600, 640, 758 | Evaluation of anti- angiogenic therapy | Laufer et al. 2012 169 |
| Hemoglobin | Label-free 3D monitoring of orthotopic ovarian tumors following trebananib treatment | 757, 797, 837 | Evaluation of anti- angiogenic therapy | Bohndiek et al. 2015 170 |
| Circulating Tumor Cells | Label-free, real-time monitoring of flow rate and distribution of melanoma CTCs in the mouse brain | 680 | Tracking of metastatic cancer cell fate for malignancy indication | Li et al. 2017 118 |
| Organic SemiconductingNanoprobes (OSNs) | Boronate-caged boron-dipyrromethane dyes were doped with bulky boranes for imaging peroxynitrite | 675 | ROS monitoring | Zhang et al. 2017 171 |
| Silver-Coated Gold Nanorods | Au/AgNRs were etched by ROS at sites of bacterial infection for Ag ion release and PA imaging | 800 | ROS monitoring and anti-bacterial treatment | Kim et al. 2018 172 |
| Lipid Rich Plaques | Atheromatous lesions of human aortas were spectrally distinguished from healthy areas | 970, 1210 | Vulnerable plaque identification for atherosclerosis | Allen et al. 2012 66 |
| J-Aggregating Nanoparticles | Light-harvesting porphyrins were intercalated with phospholipids to form PA-active nanovesicles sensitive to temperature | 680, 824 | Monitoring thermal therapy of tumors | Ng et al. 2014 173 |
| Oligomerizable Fluorescent Peptide Probes | Furin substrates were linked with an Atto740 fluorophore and cleaved to form aggregated, PA active nanostructures | 740 | Monitoring proteolytic furin and furin-like activity in tumors | Dragulescu-Andras et al. 2013 174 |
4.1. Blood and Vasculature
PAI is well suited for vascular imaging because it is optically sensitive to oxygen saturation of hemoglobin at visible and NIR wavelengths, and it can operate at length scales from a few microns to millimeters. PAI can improve upon current methods for vascular imaging, which have various drawbacks including the use of ionizing radiation (CT), requisite contrast agents (pulse-echo US, MRI, CT), minimum flow velocities (speckle variance tracking), and low penetration depths (optical methods).
Angiogenesis refers to the growth of new blood vessel networks and is often correlated to the progression of a tumor. Laufer et al. used a PA scanner and a time-reversal image reconstruction program to create accurate 3D images of angiogenesis in vivo at depths of nearly 10 mm and with sub-100 μm spatial resolution 169. Tumor margins were clearly visualized while showing dynamic changes such as the recruitment of feeding vessels from surrounding tissue and vessel tortuosity. In addition, by administering the targeted vascular disrupting agent, OXi4503, PA images showed that features of necrosis associated with this agent could be tracked longitudinally. Trebanabib is another drug that disrupts angiogenesis. Bohndiek and colleagues showed that PAT could be used to monitor the antiangiogenic efficacy of trebanabib by monitoring the tumor hemoglobin concentration and oxygenation in an orthotopic mouse model of ovarian cancer 170.
In 2014, a handheld system was developed that could provide volumetric imaging of spectrally resolved vascular parameters in real time 175. In a healthy human wrist, the spectral signals from melanin, oxygenated hemoglobin, and deoxygenated hemoglobin were separated and imaged within a few seconds. In mice, favorably low concentrations (10 nM) of the dye ICG were employed to boost contrast and monitor perfusion in the brain, allowing for an overall improvement of sensitivity in deep tissue while using less agent. The application of PAI to functional brain imaging was further developed by Yao et al., who were able to image blood oxygenation, flow, vascular morphology, and O2 metabolism using PAM 62. They achieved capillary level resolution while imaging through the intact mouse skull.
Very recently, Li et al. reported the development of a novel PA system, Single-Impulse Panoramic Photoacoustic Computed Tomography (SIP-PACT), which has already been used to image the full mouse body, brain functional connectivity, and track circulating tumor cells in the living vasculature (Figure 8). This scheme improves upon many of the issues encountered with PACT thus far, especially low spatiotemporal resolution beyond 10 mm depths caused by multiplexed signal acquisition, partial spatial sampling, and gaps in acoustic detection space. In its current form, SIP-PACT is designed and optimized for full body imaging of the mouse, combining a ring shaped, 512-element transducer array with multi-source, confocal laser propagation, providing frame rates of 50 Hz and the ability to image vasculature, organs, and sub-organ structures at high spatiotemporal resolution. The team ultimately aims to use it to resolve individual action potentials throughout the brain, which will require even greater advances in spatiotemporal resolution as well as contrast agents sensitive to voltage. It should be understood, however, that full body imaging requires a water bath for coupling, which limits the design at this stage to small animals.
Figure 8.
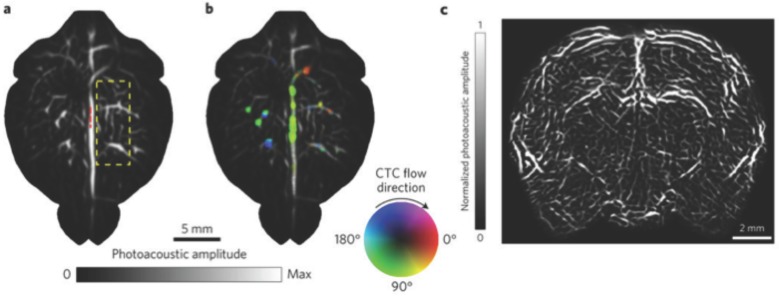
Whole brain vasculature imaging and tracking circulating tumor cells (CTCs) in the mouse brain with SIP-PACT using endogenous contrast (680 nm). (A) Cortical vasculature prior to melanoma cell injection. (B) Vasculature following CTC injection, where flow speed is denoted by hue saturation, encoded radially (where a greater radius designates faster flow speed). (C) Coronal section of the entire rat brain vasculature. Adapted with permission from 118, copyright 2017 Nature Publishing Group.
4.2. Reactive Oxygen Species
ROS are highly reactive molecules formed by the reaction of molecular oxygen with electrons and include O2-, H2O2, -OH, and 1O2 (singlet oxygen) 176. They have been implicated in the etiology of dozens of disease states as well as normal biological functions 177-179. Because their presence can be linked to both the onset and progression of many diseases, they are powerful targets for monitoring pathogenesis in real-time and measuring therapeutic effects.
In 2014, initial steps were taken to develop real-time probes of pathogenesis in the form of semiconducting π-conjugated polymer nanoparticles (SPNs) 180. These agents are NIR-active nanoparticles capable of generating bright PA signal. Because of their stability towards ROS, they were conjugated to a cyanine dye (IR775S), sensitive to oxidation by ROS, allowing for spectral differentiation between various ROS. It was shown in murine macrophage cells that activated SPNs could return to their normal state when their environment was no longer saturated with ROS illustrating the real-time monitoring potential for these agents. The SPNs were subsequently proven to highlight regions of increased ROS production in murine models of acute edema. Importantly, these agents could undergo signal evolution following a molecular event, making them activatable. An example of a ROS-sensitive, activatable nanoparticle was demonstrated in 2018— here, a silver-coated gold nanorod was used for simultaneous imaging and treatment of bacterial infection 172. In this strategy, the PA signal from gold was initially quenched while coated with silver, but was gradually turned on as the silver was etched and released by ROS into sites of infection, exerting bactericidal effects. Activatable probes differentiate themselves from many of the agents used for guided surgeries, which are typically based on accumulation at a target site (relying on the EPR effect) with signal-to-noise ratios proportional to their concentration differences between target and background tissue.
Another activatable probe was recently developed for peroxynitrite. Peroxynitrite (ONOO-) is a particularly reactive species, capable of nitrating numerous amino acids, leading to mutation of protein function. It has also shown to play an important role in the immunosuppression of the tumor microenvironment, making it a highly desirable target for understanding the mechanisms that protect tumors from the host immune system and developing effective therapies 181. Recently, Zhang et al. employed organic semiconducting nanoprobes doped with bulky boranes to enable ratiometric detection of ONOO- in murine tumors with PAI 171. In short, this strategy allowed the ROS-sensitive component, a NIR-active dye, to shift its absorption upon reaction with ONOO- (enabling imaging), while the bulky borane prevented reaction with unwanted ROS and maintained a favorable pH in the local environment.
4.3. Lipids
Lipids are essential for many biological functions (energy storage, membrane structure, cell signaling, regulation) but can also perform destructive roles. Unfortunately, existing methods are lacking in their ability to image and quantitatively track lipid molecules in living cells 182. Cardiovascular health in particular would benefit from such a method. There are currently no reliable techniques to detect vulnerable or unstable arterial plaques prone to rupture (leading to stroke or heart attack) 183. One of the early studies in biomedical PAI showed that atheromatous (lipid rich) arterial tissue could be distinguished from healthy tissue using wavelengths in the visible range 184. The characteristic spectra associated with lipids are generated by the overtones of the stretching vibration mode of C-H bonds abundant in their structures 66. These spectral profiles of lipids are easily distinguished from other vascular constituents, such as collagen, elastin and water 185. However, visible wavelengths are weakly penetrating and interfere with the absorbance of hemoglobin. Photoacoustic catheter designs can be used to mitigate this issue 186. Another approach is to use the upper wavelength range of the second NIR window 144 to penetrate tissue more deeply 187, where lipids have peaks around 1200, 1400, and 1700 nm 188.
In 2012, Allen et al. used NIR wavelengths around 1210 nm to image the entire thickness (3 mm) of an atheromatous region of aorta differentiating it from healthy tissue 66. In addition, they demonstrated the feasibility of imaging through ~3 mm of blood, increasing the ease of use for intravascular implementation. Detection of atheroma in its early stages would allow for therapeutic and lifestyle interventions to prevent the onset of fully staged cardiovascular disease. Similarly, Wang et al. developed a rotational, catheter-based intravascular PA probe for the detection of lipid deposits within plaques of the aorta of rabbits 97. Their data suggested that imaging of lipids at 1720 nm provided superior contrast to 1210 nm while also requiring lower laser output energy. The imaging speed was limited, however, to about 50 s per frame. To address this limitation, another group constructed a 2-kHz master oscillator power amplifier -pumped, barium nitrite Raman laser 189. Because of its high repetition rate, pulse-to-pulse stability, and beam quality, this laser was able to image two orders of magnitude faster than prior reports (1.0 s per frame). These developments in intravascular lipid imaging, especially in concert with improvements in PAE probes, will likely continue to facilitate clinical translation.
4.4. Temperature and pH
Standard thermometers and pH meters can both be used for intravital readings but they cannot obtain precise spatiotemporal data. PAI is fundamentally sensitive to temperature (Equation 1) and can serve as a non-invasive approach for in vivo temperature mapping. Additionally, contrast agents for enhanced sensitivity can be used to obtain highly localized measurements of temperature/pH gradients as well as long term temporal monitoring 190. In comparison to their optical counterparts, PA probes are well-suited for this application because of their deeper penetration depths in tissue and ability to overlap with ultrasound anatomy.
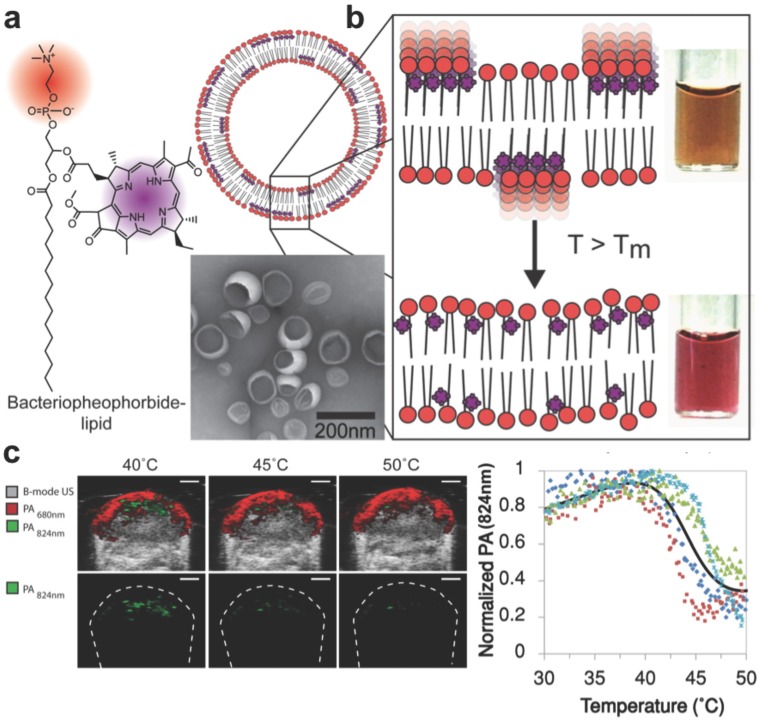
In order to detect temperature changes in tissue following thermal therapy, Ng et al. developed a probe capable of spectral shifting via phase transition 173. Photosynthetic organisms show a remarkable ability to optimize light absorption using ordered arrangements of pigment molecules within lipid and protein scaffolds. By mimicking this phenomenon with lipid-conjugated, light- absorbing porphyrins, the group assembled aggregates (nanovesicles) with tunable temperature thresholds, allowing for the generation of spatial maps of temperature thresholds to monitor heat therapy with PAI (Figure 9). The exploited mechanism of reversible dye coupling (J-aggregation) can also apply to biological stimuli beyond temperature, such as enzyme and pH detection.
Figure 9.
Light-harvesting porphyrin dyes were conjugated within lipid bilayers for measuring temperature via reversible J-aggregation. (A) The structure of a lipid-dye subunit (bacteriopheophorbide-lipid) and representative TEM image are shown. (B) The nanovesicles were composed of 15% Bchl-lipid, 80% host phospholipid and 5% mPEG2000-DPPE. (C) PA-Ultrasound images of a murine tumor xenograft show the PA intensity shift in response to local temperature change by a surrounding water bath, quantified in the last panel where each color is data from one animal (n = 4). Adapted with permission from 173, copyright 2014 American Chemical Society.
Advancements have also been made in label-free temperature mapping and thermal detection of flow speeds. Typically, it is assumed that a linear correlation exists between temperature and the Grüneisen parameter, and that the temperature is independent from optical fluence 191, 192. This allows relative temperature mapping based either on calibration measurements or ratiometric changes in PA signals, and avoids quantifying optical tissue parameters 193. Recently, however, in order to achieve absolute temperature mapping, a multi-illumination PAT method was proposed and simulated that obtains optical parameters, such as the fluence, using multiple illumination sources and an optical diffusion model to quantify temperature directly 194. This technique achieved relative errors between 10.6-15.7%, but has not yet been applied beyond simulation. Another interesting temperature- based PA technique was demonstrated by Liu et al., who developed a flowmetry method based on OR-PAM that uses a pulsed light source for both “thermal tagging” and photoacoustic excitation 195. It leverages the correlation between the Grüneisen parameter and local temperature to measure thermal dynamics and flow speed of homogenous or nonhomogeneous media. The authors were able to measure flow speeds (e.g. 2.55, 0.96, 0.99 mm/s) in the microvasculature of a mouse ear based on thermal fluctuations.
Aberrations in physiological parameters such as pH are associated with a number of conditions and diseases, including ischemia, inflammation, cancer, chronic obstructive pulmonary disease, and kidney failure 196. Existing methods for in vivo pH detection rely primarily on dye fluorescence, which is hindered by light scattering, cytosolic leakage, and varying photostabilities 197. In 2016, Miao et al. developed an activatable probe for the detection of pH, combining a semiconducting oligomer matrix (for PA signal) with a boron-dipyrromethene dye (for pH indication and PA enhancement) 198. The dye backbone contained hydroxyl groups allowing for protonation upon exposure to acids, endowing the probe with sensitivity to pH. The researchers showed that in mice, a dose of 25 μg was sufficient for real time, ratiometric mapping of pH in tumors.
In 2013, PAI was used to monitor and image enzyme activity. Dragulescu-Andrasi et al. targeted the protease, furin, which plays a role in tumor regulation and normal cell function 174. They devised an enzyme-sensitive oligomerizable reactive PA probe consisting of a peptide substrate, two pro-reactive moieties, and the NIR active PA molecule Atto740, sensitive even in deep tissue. The ability to image furin activity was shown in mice, and the probe could be theoretically adapted to interrogate other enzymes of interest. In another enzyme-responsive system, Chen et. al developed a micellar probe combining indocyanine green with an alkaline phosphatase-specific peptide for combination PAI and PTT. Here, the probes had prolonged circulation/ tumor accumulation and could convert to a nanofiber morphology once the peptides were phosphorylated by alkaline phosphatase in the tumor microenvironment 199. In this form, the probes had 25-fold higher tumor uptake than free indocyanine green at 4 h after injection, while facilitating PTT (0.8 W/cm2, 5 min) with high therapeutic efficacy. Enzyme-sensitive contrast agents hold significant biological interest but PA applications are still in their early stages. It is likely that strategies from other imaging modalities can be borrowed; for example, FRET-based mechanisms, responsive hydrogels, and assembly/ disassembly of micelles 200. However, some challenges include honing the specificity of engineered probes for their target enzymes (especially against members of the same enzyme family, e.g. matrix metalloproteinases), and choosing feasible targets that do not require deep tissue penetration.
5. Outlook and Future Developments
The applications and capabilities of photoacoustic technology are growing at a rapid pace. The development of novel PA systems such as SIP-PACT (Section 4.1), forward-viewing PAE (Section 2.1), and handheld PAM (Section 1.3) are promising examples of hardware innovations 54, 60, 118. These advances are pushing the state of the art toward finer resolutions and new areas of application. It is feasible that the spatial resolutions of PAI will continue to improve as transducer elements become more affordable and image reconstruction algorithms are refined to address problems such as the heterogeneity of the speed of sound through various tissues 201. Additionally, lower cost systems with excitation sources such as laser diodes 202 and light emitting diodes (LEDs) 203, 204, which are more stable, may increase the accessibility of PAI for researchers and clinicians alike. Furthermore, contrast agents with absorbance maxima at longer wavelengths in the second optical window will be increasingly developed to improve the depths at which PAI is effective. This will likely mirror the recent progress in fluorescence imaging, where new NIR-II fluorophores have been pushing the field forward 18, 205, 206. Analogous PA materials should become more common as the chemistry develops. Fortunately, PA systems do not need to implement expensive, high quantum efficiency detectors (InGaAs or HgCdTe) that are required for fluorescence imaging in the second optical window 19. Instead, however, they must implement longer wavelength lasers—e.g., the commercial Vevo LAZR-X includes a 1200-2000 nm laser in addition to a 680-970 nm source (though this combination excludes the 1000-1200 nm range) 18, 79. In the short-term, we believe that biodegradable contrast agents, small molecule dyes (especially those with existing FDA approval), and techniques that do not require intravenous injection will have the shortest paths to the clinic.
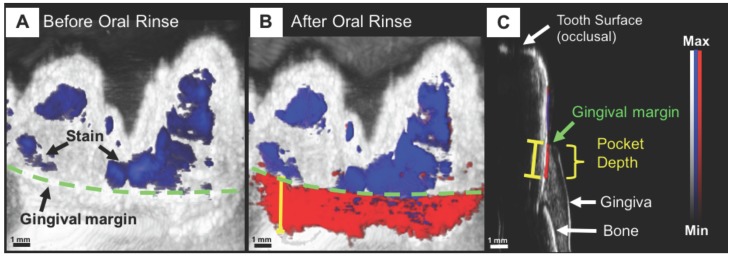
As the technology itself continues to develop, so does the application space. Work is underway by numerous groups on alternative transducer designs for specialized applications, including through-needle imaging for guided surgery 207, PA-guided surgical drilling 96, and human finger joint imaging 208. “Wearable” PA devices are another example. The concept was recently demonstrated for dental imaging, specifically, non-invasive periodontal pocket depth measurements for early gum disease diagnosis (Figure 10). Here, a contrast agent derived from food-grade cuttlefish ink (rich in melanin nanoparticles) was used with PAT to image the pockets between gum and teeth, showing potential for a PA mouthpiece device 209, 210.
Figure 10.
Non-invasive and precise measurement of porcine periodontal pocket depths using PAI. (A) PA/Ultrasound image of the gum line (green) and tooth with stains (blue) at 680 nm, prior to oral rinse of contrast agent. (B) Image following oral rinse with contrast agent, revealing the pocket (red) under the gum line. (C) Sagittal view of a 3D PA scan of the tooth, distinguishing hard and soft tissue, and revealing the PA signal from the contrast agent beginning at the gingival margin and extending through the pocket depth 209.
It is also worth recognizing progress in Magneto- Acoustic Imaging (MAI), a technique similar to PAI, but in which magnetic contrast agents are used to label tissue, whereafter a pulsed magnetic field is used to induce motion of the agents within the tissue, detectable by ultrasound 211. Such a technique would circumvent the need for optical penetration. Proof-of-concept has been demonstrated in cells and tissue phantoms and MAI remains in its early stages, but may see progress in the near future that parallels the recent growth of photoacoustics 212.
PAI will likely never become as widespread as MRI or CT, but it is highly complementary to ultrasound, already one of the most widely available imaging tools in medicine. While ultrasound generates anatomical images, PAI adds to this the molecular and functional contrast that is normally associated with optical imaging techniques. The clinical appeal of this combination will be a driving force for the continued growth of photoacoustic imaging.
Acknowledgments
We acknowledge support from the National Institutes of Health through DP2 HL 137187 and T32 EB 009380.
Abbreviations
- APTT
activated partial thromboplastin time
- AR-PAM
acoustic resolution-photoacoustic microscopy
- AuNR
gold nanorod
- CT
computed tomography
- CTC
circulating tumor cell
- CW
continuous wave
- EPR
enhanced permeability and retention
- FDA
food and drug administration
- GI
gastrointestinal
- HIFU
high intensity focused ultrasound
- ICG
indocyanine green
- IONPs
iron oxide nanoparticles
- IV
intravascular
- LCST
lower critical saturation temperature
- LEDs
light emitting diodes
- MAI
magnetic acoustic imaging
- MEMS
micro-electro- mechanical systems
- MRI
magnetic resonance imaging
- MSOT
multispectral optoacoustic tomography
- NIR
near infrared
- OR-PAM
optical resolution- photoacoustic microscopy
- PA
photoacoustic
- PACT
photoacoustic computed tomography
- PAE
photoacoustic endoscopy
- PAI
photoacoustic imaging
- PAM
photoacoustic microscopy
- PAT
photoacoustic tomography
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PET
positron emission tomography
- PFC
perfluorocarbon
- PNIPAM
poly(N-isospropylacrylamide)
- PTT
photothermal therapy
- ROS
reactive oxygen species
- SIP-PACT
single-impulse panoramic photoacoustic computed tomography
- SLN
sentinel lymph node
- SO2
oxygen saturation
- sPA
spectroscopic photoacoustic imaging
- SPNs
semiconducting polymer nanoparticles
- TMDCs
transition metal dichalcogenides.
References
- 1.Ntziachristos V, Razansky D. Molecular Imaging by Means of Multispectral Optoacoustic Tomography (MSOT) Chemical Reviews. 2010;110:2783–94. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 2.Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chemical reviews. 2010;110:2756–82. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LV, Hu S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science (New York, Ny) 2012;335:1458–62. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia J, Yao J, Wang LV. Photoacoustic tomography: principles and advances. Electromagnetic waves (Cambridge, Mass) 2014;147:1–22. doi: 10.2528/pier14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Meth. 2016;13:627–38. doi: 10.1038/nmeth.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–31. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke GP, Yeager D, Emelianov SY. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Annals of biomedical engineering. 2012;40:422–37. doi: 10.1007/s10439-011-0449-4. [DOI] [PubMed] [Google Scholar]
- 8.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Meth. 2016;13:639–50. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 9.Nie L, Chen X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chemical Society Reviews. 2014;43:7132–70. doi: 10.1039/c4cs00086b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Q, Zhu R, Song J, Yang H, Chen X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Advanced Materials. 2018;0:1805875. doi: 10.1002/adma.201805875. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Song J, Yung BC, Huang X, Xiong Y, Chen X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chemical Society Reviews. 2018;47:2873–920. doi: 10.1039/C7CS00612H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao Q, Pu K. Emerging Designs of Activatable Photoacoustic Probes for Molecular Imaging. Bioconjugate Chemistry. 2016;27:2808–23. doi: 10.1021/acs.bioconjchem.6b00641. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine. 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manohar S, Ungureanu C, Van Leeuwen TG. Gold nanorods as molecular contrast agents in photoacoustic imaging: the promises and the caveats. Contrast Media & Molecular Imaging. 2011;6:389–400. doi: 10.1002/cmmi.454. [DOI] [PubMed] [Google Scholar]
- 15.Cho EC, Glaus C, Chen J, Welch MJ, Xia Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends in molecular medicine. 2010;16:561–73. doi: 10.1016/j.molmed.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L-S, Song J, Yang H-H, Chen X. Yolk-Shell Nanostructures: Design, Synthesis, and Biomedical Applications. Advanced Materials. 2018;30:1704639. doi: 10.1002/adma.201704639. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Reviews; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang K, Zhang Y, Lin J, Huang P. Nanomaterials for photoacoustic imaging in the second near-infrared window. Biomaterials science; 2018. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Pu K. Molecular Fluorescence and Photoacoustic Imaging in the Second Near-Infrared Optical Window Using Organic Contrast Agents. Advanced Biosystems. 2018;2:1700262. doi: 10.1002/adbi.201700262. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Pu K. Advanced photoacoustic imaging applications of near-infrared absorbing organic nanoparticles. Small. 2017;13:1700710. doi: 10.1002/smll.201700710. [DOI] [PubMed] [Google Scholar]
- 21.Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chemical Society Reviews. 2014;43:6570–97. doi: 10.1039/c4cs00014e. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chemical Society Reviews. 2019;48:38–71. doi: 10.1039/c8cs00001h. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Pu K. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Accounts of Chemical Research. 2018;51:1840–9. doi: 10.1021/acs.accounts.8b00242. [DOI] [PubMed] [Google Scholar]
- 24.Hu S, Wang LV. Neurovascular photoacoustic tomography. Frontiers in neuroenergetics. 2010;2:10. doi: 10.3389/fnene.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Wu Y, Xia J. Review on photoacoustic imaging of the brain using nanoprobes. Neurophotonics. 2016;3:010901. doi: 10.1117/1.NPh.3.1.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu S, Wang LV. Photoacoustic imaging and characterization of the microvasculature. Journal of biomedical optics. 2010;15:011101. doi: 10.1117/1.3281673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends in biotechnology. 2011;29:213–21. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J, Kim C, Lovell JF. Opportunities for Photoacoustic-Guided Drug Delivery. Curr Drug Targets. 2015;16:571–81. doi: 10.2174/1389450116666150707100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell AG. The photophone. Journal of the Franklin Institute. 1880;110:237–48. [Google Scholar]
- 30.Bell AG. The Production of Sound by Radiant Energy. Science. 1881;2:242–53. doi: 10.1126/science.os-2.49.242. [DOI] [PubMed] [Google Scholar]
- 31.Rosencwaig A, Gersho A. Theory of the photoacoustic effect with solids. Journal of Applied Physics. 1976;47:64–9. [Google Scholar]
- 32.Bowen T. Radiation-induced thermoacoustic imaging. Google Patents; 1983. [Google Scholar]
- 33.Kruger RA. Photoacoustic ultrasound. Medical Physics. 1994;21:127–31. doi: 10.1118/1.597367. [DOI] [PubMed] [Google Scholar]
- 34.Kruger RA, Liu P, Fang YR, Appledorn CR. Photoacoustic ultrasound (PAUS)—Reconstruction tomography. Medical Physics. 1995;22:1605–9. doi: 10.1118/1.597429. [DOI] [PubMed] [Google Scholar]
- 35.Hoelen C, De Mul F, Pongers R, Dekker A. Three-dimensional photoacoustic imaging of blood vessels in tissue. Optics letters. 1998;23:648–50. doi: 10.1364/ol.23.000648. [DOI] [PubMed] [Google Scholar]
- 36.Wang LV, Zhao X, Sun H, Ku G. Microwave-induced acoustic imaging of biological tissues. Review of scientific instruments. 1999;70:3744–8. [Google Scholar]
- 37.Zackrisson S, van de Ven S, Gambhir S. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer research. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordillo LA, Pu Y, Pratavieira S, Budansky Y, Alfano RR. Deep optical imaging of tissue using the second and third near-infrared spectral windows. Journal of Biomedical Optics. 2014;19:056004. doi: 10.1117/1.JBO.19.5.056004. - [DOI] [PubMed] [Google Scholar]
- 39.Frangioni JV. In vivo near-infrared fluorescence imaging. Current opinion in chemical biology. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Cox B, Laufer JG, Arridge SR, Beard PC. Quantitative spectroscopic photoacoustic imaging: a review. Journal of Biomedical Optics. 2012;17:0612021–06120222. doi: 10.1117/1.JBO.17.6.061202. [DOI] [PubMed] [Google Scholar]
- 41.Maslov K, Wang LV. Photoacoustic imaging of biological tissue with intensity-modulated continuous-wave laser. Journal of biomedical optics. 2008;13:024006–5. doi: 10.1117/1.2904965. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature biotechnology. 2003;21:803–6. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 43.Lashkari B, Mandelis A. Comparison between pulsed laser and frequency-domain photoacoustic modalities: signal-to-noise ratio, contrast, resolution, and maximum depth detectivity. Review of Scientific Instruments. 2011;82:094903. doi: 10.1063/1.3632117. [DOI] [PubMed] [Google Scholar]
- 44.Xu M, Wang LV. Photoacoustic imaging in biomedicine. Review of scientific instruments. 2006;77:041101. [Google Scholar]
- 45.Pramanik M, Wang LV. Thermoacoustic and photoacoustic sensing of temperature. Journal of biomedical optics. 2009;14:054024–7. doi: 10.1117/1.3247155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nature photonics. 2009;3:503–9. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Wang Y. Comparison of Various Imaging Modes for Photoacoustic Tomography. Springer Berlin Heidelberg; 2009. pp. 121–4. [Google Scholar]
- 48.Zhang HF, Maslov K, Stoica G, Wang LV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnology. 2006;24:848. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 49.Hu S, Maslov K, Wang LV. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Optics Letters. 2011;36:1134. doi: 10.1364/OL.36.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Maslov KI, Hu S, Wang LV, Chen R, Zhou Q, Reflection-mode submicron-resolution in vivo photoacoustic microscopy. SPIE; 2012. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan B, Liu W, Wang Y-c, Shi J, Li Y, Xu S. et al. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomedical Optics Express. 2018;9:4689–701. doi: 10.1364/BOE.9.004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan B, Liu W, Yao J. High-speed wide-field photoacoustic microscopy. Clinical and Translational Biophotonics: Optical Society of America; 2018. JTu3A. 68. [Google Scholar]
- 53.Favazza CP, Jassim O, Cornelius LA, Wang LV. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus. Journal of Biomedical Optics. 2011;16:016015. doi: 10.1117/1.3528661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park K, Kim JY, Lee C, Jeon S, Lim G, Kim C. Handheld Photoacoustic Microscopy Probe. Scientific Reports. 2017;7:13359. doi: 10.1038/s41598-017-13224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu M, Wang LV. Universal back-projection algorithm for photoacoustic computed tomography. Physical Review E. 2005;71:016706. doi: 10.1103/PhysRevE.71.016706. [DOI] [PubMed] [Google Scholar]
- 56.Schellenberg MW, Hunt HK. Hand-held optoacoustic imaging: A review. Photoacoustics. 2018;11:14–27. doi: 10.1016/j.pacs.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J-M, Maslov K, Yang H-C, Zhou Q, Shung KK, Wang LV. Photoacoustic endoscopy. Optics letters. 2009;34:1591–3. doi: 10.1364/ol.34.001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J-M, Favazza C, Chen R, Yao J, Cai X, Maslov K. et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nature medicine. 2012;18:1297–302. doi: 10.1038/nm.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nikoozadeh A, Choe JW, Kothapalli S-R, Moini A, Sanjani SS, Kamaya A, Photoacoustic imaging using a 9F microLinear CMUT ICE catheter. Ultrasonics Symposium (IUS), 2012 IEEE International: IEEE; 2012. pp. 24–7. [Google Scholar]
- 60.Ansari R, Zhang EZ, Desjardins AE, Beard PC. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy. Light: Science & Applications. 2018;7:75. doi: 10.1038/s41377-018-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang HF, Maslov K, Wang LV. In vivo imaging of subcutaneous structures using functional photoacoustic microscopy. Nature protocols. 2007;2:797–804. doi: 10.1038/nprot.2007.108. [DOI] [PubMed] [Google Scholar]
- 62.Yao J, Wang L, Yang J-M, Maslov KI, Wong TTW, Li L. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat Meth. 2015;12:407–10. doi: 10.1038/nmeth.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang LV, Wu H-i. Biomedical optics: principles and imaging: John Wiley & Sons; 2012.
- 64.Zhang HF, Maslov K, Sivaramakrishnan M, Stoica G, Wang LV. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Applied physics letters. 2007;90:053901. [Google Scholar]
- 65.Oh J-T, Li M-L, Zhang HF, Maslov K, Stoica G, Wang LV. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. Journal of biomedical optics. 2006;11:034032–4. doi: 10.1117/1.2210907. [DOI] [PubMed] [Google Scholar]
- 66.Allen TJ, Hall A, Dhillon AP, Owen JS, Beard PC. Spectroscopic photoacoustic imaging of lipid-rich plaques in the human aorta in the 740 to 1400 nm wavelength range. Journal of biomedical optics. 2012;17:0612091–06120910. doi: 10.1117/1.JBO.17.6.061209. [DOI] [PubMed] [Google Scholar]
- 67.Lemaster JE, Jokerst JV. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology; 2017. p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically approved nanoparticle imaging agents. Journal of Nuclear Medicine. 2016;57:1833–7. doi: 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annual Review of Biomedical Engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 70.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angewandte Chemie International Edition. 2014;53:12320–64. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 71.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. The Journal of Physical Chemistry B. 2006;110:7238–48. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 72.Feis A, Gellini C, Salvi PR, Becucci M. Photoacoustic excitation profiles of gold nanoparticles. Photoacoustics. 2014;2:47–53. doi: 10.1016/j.pacs.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y, Wang D, Zhang Y, Chitgupi U, Geng J, Wang Y. et al. A Phosphorus Phthalocyanine Formulation with Intense Absorbance at 1000 nm for Deep Optical Imaging. Theranostics. 2016;6:688–97. doi: 10.7150/thno.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salehi HS, Kumavor PD, Li H, Alqasemi U, Wang T, Xu C. et al. Design of optimal light delivery system for co-registered transvaginal ultrasound and photoacoustic imaging of ovarian tissue. Photoacoustics. 2015;3:114–22. doi: 10.1016/j.pacs.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Gohary SH, Metwally MK, Eom S, Jeon SH, Byun KM, Kim T-S. Design study on photoacoustic probe to detect prostate cancer using 3D Monte Carlo simulation and finite element method. Biomedical Engineering Letters. 2014;4:250–7. [Google Scholar]
- 76.Bell MAL, Kuo NP, Song DY, Kang JU, Boctor EM. In vivo visualization of prostate brachytherapy seeds with photoacoustic imaging. Journal of biomedical optics. 2014;19:126011. doi: 10.1117/1.JBO.19.12.126011. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang J-M, Favazza CP, Yao J, Chen R, Zhou Q, Shung KK, Three-dimensional photoacoustic and ultrasonic endoscopic imaging of two rabbit esophagi. SPIE BiOS: International Society for Optics and Photonics; 2015. pp. 932334–8. [Google Scholar]
- 78.Smith AM, Mancini MC, Nie S. Bioimaging: second window for in vivo imaging. Nature nanotechnology. 2009;4:710–1. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang Y, Upputuri PK, Xie C, Lyu Y, Zhang L, Xiong Q. et al. Broadband Absorbing Semiconducting Polymer Nanoparticles for Photoacoustic Imaging in Second Near-Infrared Window. Nano Letters. 2017;17:4964–9. doi: 10.1021/acs.nanolett.7b02106. [DOI] [PubMed] [Google Scholar]
- 80.Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature nanotechnology. 2009;4:773–80. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JE, Kim M, Hwang JH, Nam JM. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications based on the Second Near-Infrared Window. Small Methods. 2017;1:1600032. [Google Scholar]
- 82.Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. Acs Nano. 2012;6:7489–96. doi: 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Homan KA, Souza M, Truby R, Luke GP, Green C, Vreeland E. et al. Silver nanoplate contrast agents for in vivo molecular photoacoustic imaging. ACS nano. 2012;6:641–50. doi: 10.1021/nn204100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature clinical practice Oncology. 2008;5:206–19. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grootendorst D, Jose J, Wouters M, van Boven H, Van der Hage J, Van Leeuwen T. et al. First experiences of photoacoustic imaging for detection of melanoma metastases in resected human lymph nodes. Lasers in surgery and medicine. 2012;44:541–9. doi: 10.1002/lsm.22058. [DOI] [PubMed] [Google Scholar]
- 86.Langhout GC, Grootendorst DJ, Nieweg OE, Wouters MWJM, Hage JAvd, Jose J. et al. Detection of melanoma metastases in resected human lymph nodes by noninvasive multispectral photoacoustic imaging. Journal of Biomedical Imaging. 2014;2014:5. doi: 10.1155/2014/163652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luke GP, Emelianov SY. Label-free Detection of Lymph Node Metastases with US-guided Functional Photoacoustic Imaging. Radiology. 2015;277:435–42. doi: 10.1148/radiol.2015141909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Hu S, Maslov K, Zhang Y, Xia Y, Wang LV. In vivo integrated photoacoustic and confocal microscopy of hemoglobin oxygen saturation and oxygen partial pressure. Optics letters. 2011;36:1029–31. doi: 10.1364/OL.36.001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ning B, Kennedy MJ, Dixon AJ, Sun N, Cao R, Soetikno BT. et al. Simultaneous photoacoustic microscopy of microvascular anatomy, oxygen saturation, and blood flow. Optics letters. 2015;40:910–3. doi: 10.1364/OL.40.000910. [DOI] [PubMed] [Google Scholar]
- 90.Xi L, Grobmyer SR, Wu L, Chen R, Zhou G, Gutwein LG. et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Optics express. 2012;20:8726–31. doi: 10.1364/OE.20.008726. [DOI] [PubMed] [Google Scholar]
- 91.Neuschmelting V, Burton NC, Lockau H, Urich A, Harmsen S, Ntziachristos V. et al. Performance of a Multispectral Optoacoustic Tomography (MSOT) System equipped with 2D vs. 3D Handheld Probes for Potential Clinical Translation. Photoacoustics. 2016;4:1–10. doi: 10.1016/j.pacs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neuschmelting V, Lockau H, Ntziachristos V, Grimm J, Kircher MF. Lymph Node Micrometastases and In-Transit Metastases from Melanoma: In Vivo Detection with Multispectral Optoacoustic Imaging in a Mouse Model. Radiology. 2016;280:137–50. doi: 10.1148/radiol.2016160191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horiguchi A, Tsujita K, Irisawa K, Kasamatsu T, Hirota K, Kawaguchi M. et al. A pilot study of photoacoustic imaging system for improved real-time visualization of neurovascular bundle during radical prostatectomy. The Prostate. 2016;76:307–15. doi: 10.1002/pros.23122. [DOI] [PubMed] [Google Scholar]
- 94.Moradi H, Tang S, Salcudean SE. Toward Intra-Operative Prostate Photoacoustic Imaging: Configuration Evaluation and Implementation Using the da Vinci Research Kit. IEEE Transactions on Medical Imaging. 2019;38:57–68. doi: 10.1109/TMI.2018.2855166. [DOI] [PubMed] [Google Scholar]
- 95.Gandhi N, Allard M, Kim S, Kazanzides P, Lediju Bell MA. Photoacoustic-based approach to surgical guidance performed with and without a da Vinci robot. Journal of Biomedical Optics. 2017;22:121606. [Google Scholar]
- 96.Eddins B, Bell MAL. Design of a multifiber light delivery system for photoacoustic-guided surgery. Journal of biomedical optics. 2017;22:041011. doi: 10.1117/1.JBO.22.4.041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B, Karpiouk A, Yeager D, Amirian J, Litovsky S, Smalling R. et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Optics letters. 2012;37:1244–6. doi: 10.1364/OL.37.001244. [DOI] [PubMed] [Google Scholar]
- 98.Wang B, Su JL, Karpiouk AB, Sokolov KV, Smalling RW, Emelianov SY. Intravascular photoacoustic imaging. IEEE Journal of selected topics in Quantum Electronics. 2010;16:588–99. doi: 10.1109/JSTQE.2009.2037023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jansen K, Van Der Steen AF, van Beusekom HM, Oosterhuis JW, van Soest G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Optics letters. 2011;36:597–9. doi: 10.1364/OL.36.000597. [DOI] [PubMed] [Google Scholar]
- 100.Wu M, Springeling G, Lovrak M, Mastik F, Iskander-Rizk S, Wang T. et al. Real-time volumetric lipid imaging in vivo by intravascular photoacoustics at 20 frames per second. Biomedical optics express. 2017;8:943–53. doi: 10.1364/BOE.8.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang J-M, Li C, Chen R, Zhou Q, Shung KK, Wang LV. Catheter-based photoacoustic endoscope. Journal of biomedical optics. 2014;19:066001. doi: 10.1117/1.JBO.19.6.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iwashita T, Nakai Y, Lee JG, Park DH, Muthusamy VR, Chang KJ. Newly-developed, forward-viewing echoendoscope: A comparative pilot study to the standard echoendoscope in the imaging of abdominal organs and feasibility of endoscopic ultrasound-guided interventions. Journal of Gastroenterology and Hepatology. 2012;27:362–7. doi: 10.1111/j.1440-1746.2011.06923.x. [DOI] [PubMed] [Google Scholar]
- 103.Dong B, Chen S, Zhang Z, Sun C, Zhang HF. Photoacoustic probe using a microring resonator ultrasonic sensor for endoscopic applications. Optics letters. 2014;39:4372–5. doi: 10.1364/OL.39.004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson KE, Bachawal SV, Abou-Elkacem L, Jensen K, Machtaler S, Tian L. et al. Spectroscopic Photoacoustic Molecular Imaging of Breast Cancer using a B7-H3-targeted ICG Contrast Agent. Theranostics. 2017;7:1463–76. doi: 10.7150/thno.18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS nano. 2012;6:10366–77. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grootendorst DJ, Jose J, Fratila RM, Visscher M, Velders AH, Ten Haken B. et al. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast media & molecular imaging. 2013;8:83–91. doi: 10.1002/cmmi.1498. [DOI] [PubMed] [Google Scholar]
- 107.Ray A, Wang X, Lee Y-EK, Hah HJ, Kim G, Chen T. et al. Targeted blue nanoparticles as photoacoustic contrast agent for brain tumor delineation. Nano research. 2011;4:1163–73. doi: 10.1007/s12274-011-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guan T, Shang W, Li H, Yang X, Fang C, Tian J. et al. From Detection to Resection: Photoacoustic Tomography and Surgery Guidance with Indocyanine Green Loaded Gold Nanorod@liposome Core-Shell Nanoparticles in Liver Cancer. Bioconjugate Chemistry. 2017;28:1221–8. doi: 10.1021/acs.bioconjchem.7b00065. [DOI] [PubMed] [Google Scholar]
- 109.Maeda A, Bu J, Chen J, Zheng G, DaCosta RS. Dual in vivo Photoacoustic and Fluorescence Imaging of HER2 Expression in Breast Tumors for Diagnosis, Margin Assessment, and Surgical Guidance. Molecular Imaging. 2015;14:7290.2014.00043. doi: 10.2310/7290.2014.00043. [DOI] [PubMed] [Google Scholar]
- 110.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E. et al. A Brain Tumor Molecular Imaging Strategy Using A New Triple-Modality MRI-Photoacoustic-Raman Nanoparticle. Nature medicine. 2012;18:829–34. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH. et al. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. New England Journal of Medicine. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 112.Xi L, Zhou G, Gao N, Yang L, Gonzalo DA, Hughes SJ. et al. Photoacoustic and Fluorescence Image-Guided Surgery Using a Multifunctional Targeted Nanoprobe. Annals of surgical oncology. 2014;21:1602–9. doi: 10.1245/s10434-014-3541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shakiba M, Ng KK, Huynh E, Chan H, Charron DM, Chen J. et al. Stable J-aggregation enabled dual photoacoustic and fluorescence nanoparticles for intraoperative cancer imaging. Nanoscale. 2016;8:12618–25. doi: 10.1039/c5nr08165c. [DOI] [PubMed] [Google Scholar]
- 114.Mallidi S, Larson T, Tam J, Joshi PP, Karpiouk A, Sokolov K. et al. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano letters. 2009;9:2825–31. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sethuraman S, Amirian JH, Litovsky SH, Smalling RW, Emelianov SY. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Optics express. 2008;16:3362–7. doi: 10.1364/oe.16.003362. [DOI] [PubMed] [Google Scholar]
- 116.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart H, Hadaschik B. et al. PET imaging with a [68 Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. European journal of nuclear medicine and molecular imaging. 2013;40:486–95. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 117.Yang K, Wan J, Zhang S, Zhang Y, Lee S-T, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS nano. 2010;5:516–22. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 118.Li L, Zhu L, Ma C, Lin L, Yao J, Wang L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nature Biomedical Engineering. 2017;1:0071. doi: 10.1038/s41551-017-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lyu Y, Fang Y, Miao Q, Zhen X, Ding D, Pu K. Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy. ACS Nano. 2016;10:4472–81. doi: 10.1021/acsnano.6b00168. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y. et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nature nanotechnology. 2014;9:631–8. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu T, Wang C, Cui W, Gong H, Liang C, Shi X. et al. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale. 2014;6:11219–25. doi: 10.1039/c4nr03753g. [DOI] [PubMed] [Google Scholar]
- 122.Tian G, Zhang X, Zheng X, Yin W, Ruan L, Liu X. et al. Multifunctional RbxWO3 Nanorods for Simultaneous Combined Chemo-photothermal Therapy and Photoacoustic/CT Imaging. Small. 2014;10:4160–70. doi: 10.1002/smll.201401237. [DOI] [PubMed] [Google Scholar]
- 123.Moon GD, Choi S-W, Cai X, Li W, Cho EC, Jeong U. et al. A New Theranostic System Based on Gold Nanocages and Phase-Change Materials with Unique Features for Photoacoustic Imaging and Controlled Release. Journal of the American Chemical Society. 2011;133:4762–5. doi: 10.1021/ja200894u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manivasagan P, Bharathiraja S, Bui NQ, Jang B, Oh Y-O, Lim IG. et al. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. International journal of biological macromolecules. 2016;91:578–88. doi: 10.1016/j.ijbiomac.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 125.Zhong J, Yang S, Wen L, Xing D. Imaging-guided photoacoustic drug release and synergistic chemo-photoacoustic therapy with paclitaxel-containing nanoparticles. Journal of Controlled Release. 2016;226:77–87. doi: 10.1016/j.jconrel.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 126.Ho I-T, Sessler JL, Gambhir SS, Jokerst JV. Parts per billion detection of uranium with a porphyrinoid-containing nanoparticle and in vivo photoacoustic imaging. Analyst. 2015;140:3731–7. doi: 10.1039/c5an00207a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cash KJ, Li C, Xia J, Wang LV, Clark HA. Optical Drug Monitoring: Photoacoustic Imaging of Nanosensors to Monitor Therapeutic Lithium in Vivo. ACS Nano. 2015;9:1692–8. doi: 10.1021/nn5064858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeevarathinam AS, Pai N, Huang K, Hariri A, Wang J, Bai Y. et al. A cellulose-based photoacoustic sensor to measure heparin concentration and activity in human blood samples. Biosensors and Bioelectronics. 2019;126:831–7. doi: 10.1016/j.bios.2018.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson K, Homan K, Emelianov S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nature Communications. 2012;3:618. doi: 10.1038/ncomms1627. [DOI] [PubMed] [Google Scholar]
- 130.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10:324–32. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 131.Huynh E, Jin CS, Wilson BC, Zheng G. Aggregate Enhanced Trimodal Porphyrin Shell Microbubbles for Ultrasound, Photoacoustic, and Fluorescence Imaging. Bioconjugate Chemistry. 2014;25:796–801. doi: 10.1021/bc5000725. [DOI] [PubMed] [Google Scholar]
- 132.Roberts S, Seeger M, Jiang Y, Mishra A, Sigmund F, Stelzl A, Calcium Sensor for Photoacoustic Imaging. Journal of the American Chemical Society; 2017. [DOI] [PubMed] [Google Scholar]
- 133.Wang J, Chen F, Arconada-Alvarez SJ, Hartanto J, Yap L-P, Park R. et al. A Nanoscale Tool for Photoacoustic-based Measurements of Clotting Time and Therapeutic Drug Monitoring of Heparin. Nano Letters. 2016;16:6265–71. doi: 10.1021/acs.nanolett.6b02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J, Jeevarathinam AS, Humphries K, Jhunjhunwala A, Chen F, Hariri A, A Mechanistic Investigation of Methylene Blue and Heparin Interactions and Their Photoacoustic Enhancement. Bioconjugate chemistry; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a standard care nomogram: a randomized controlled trial. Annals of Internal Medicine. 1993;119:874–81. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 136.Jeon M, Song W, Huynh E, Kim J, Kim J, Helfield BL. et al. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. Journal of biomedical optics. 2014;19:016005. doi: 10.1117/1.JBO.19.1.016005. [DOI] [PubMed] [Google Scholar]
- 137.Ashkenazi S. Photoacoustic lifetime imaging of dissolved oxygen using methylene blue. Journal of Biomedical Optics. 2010;15:040501. doi: 10.1117/1.3465548. [DOI] [PubMed] [Google Scholar]
- 138.Song KH, Stein EW, Margenthaler JA, Wang LV. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. Journal of biomedical optics. 2008;13:054033. doi: 10.1117/1.2976427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgounova E, Shao Q, Hackel BJ, Thomas DD, Ashkenazi S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. Journal of biomedical optics. 2013;18:056004. doi: 10.1117/1.JBO.18.5.056004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J, Lin C-y, Moore C, Jhunjhunwala A, Jokerst JV. Switchable Photoacoustic Intensity of Methylene Blue via Sodium Dodecyl Sulfate Micellization. Langmuir; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taruttis A, Morscher S, Burton NC, Razansky D, Ntziachristos V. Fast Multispectral Optoacoustic Tomography (MSOT) for Dynamic Imaging of Pharmacokinetics and Biodistribution in Multiple Organs. PLOS ONE. 2012;7:e30491. doi: 10.1371/journal.pone.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Razansky D, Distel M, Vinegoni C, Ma R, Perrimon N, Köster RW. et al. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. nature photonics. 2009;3:412. [Google Scholar]
- 143.Hannah A, Luke G, Wilson K, Homan K, Emelianov S. Indocyanine Green-Loaded Photoacoustic Nanodroplets: Dual Contrast Nanoconstructs for Enhanced Photoacoustic and Ultrasound Imaging. ACS Nano. 2014;8:250–9. doi: 10.1021/nn403527r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nature Biomedical Engineering. 2017;1:0010. [Google Scholar]
- 145.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G. et al. Circumferential radiofrequency ablation of pulmonary vein ostia. Circulation. 2000;102:2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 146.Pacella CM, Bizzarri G, Spiezia S, Bianchini A, Guglielmi R, Crescenzi A. et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology. 2004;232:272–80. doi: 10.1148/radiol.2321021368. [DOI] [PubMed] [Google Scholar]
- 147.Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Annals of biomedical engineering. 2012;40:438–59. doi: 10.1007/s10439-011-0472-5. [DOI] [PubMed] [Google Scholar]
- 148.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of the American Chemical Society. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 149.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano letters. 2008;8:3953–8. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano letters. 2010;10:3318–23. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 151.Ke H, Wang J, Dai Z, Jin Y, Qu E, Xing Z. et al. Gold-Nanoshelled Microcapsules: A Theranostic Agent for Ultrasound Contrast Imaging and Photothermal Therapy. Angewandte Chemie. 2011;123:3073–7. doi: 10.1002/anie.201008286. [DOI] [PubMed] [Google Scholar]
- 152.Huang P, Lin J, Li W, Rong P, Wang Z, Wang S. et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angewandte Chemie. 2013;125:14208–14. doi: 10.1002/anie.201308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin L-S, Cong Z-X, Cao J-B, Ke K-M, Peng Q-L, Gao J. et al. Multifunctional Fe3O4@Polydopamine Core-Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano. 2014;8:3876–83. doi: 10.1021/nn500722y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lovell JF, Jin CS, Huynh E, MacDonald TD, Cao W, Zheng G. Enzymatic Regioselection for the Synthesis and Biodegradation of Porphysome Nanovesicles. Angewandte Chemie International Edition. 2012;51:2429–33. doi: 10.1002/anie.201108280. [DOI] [PubMed] [Google Scholar]
- 155.Cheng L, Liu J, Gu X, Gong H, Shi X, Liu T. et al. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Advanced Materials. 2014;26:1886–93. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]
- 156.Chen Y-S, Yoon SJ, Frey W, Dockery M, Emelianov S. Dynamic contrast-enhanced photoacoustic imaging using photothermal stimuli-responsive composite nanomodulators. Nature communications. 2017;8:15782. doi: 10.1038/ncomms15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lightdale CJ, Heier SK, Marcon NE, McCaughan JS, Gerdes H, Overholt BF. et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd: YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointestinal endoscopy. 1995;42:507–12. doi: 10.1016/s0016-5107(95)70002-1. [DOI] [PubMed] [Google Scholar]
- 158.Hongcharu W, Taylor CR, Aghassi D, Suthamjariya K, Anderson RR, Chang Y. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. Journal of Investigative Dermatology. 2000;115:183–92. doi: 10.1046/j.1523-1747.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 159.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers in surgery and medicine. 2011;43:755–67. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bressler N. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Archives of ophthalmology (Chicago, Ill: 1960) 2001;119:198–207. [PubMed] [Google Scholar]
- 161.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO. et al. Photodynamic therapy of cancer: an update. CA: a cancer journal for clinicians. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ho CJH, Balasundaram G, Driessen W, McLaren R, Wong CL, Dinish U, Multifunctional photosensitizer-based contrast agents for photoacoustic imaging. Scientific reports; 2014. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature medicine. 2012;18:1580–5. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 164.Shao P, Chapman DW, Moore RB, Zemp RJ. Monitoring photodynamic therapy with photoacoustic microscopy. SPIE; 2015. p. 6. [DOI] [PubMed] [Google Scholar]
- 165.Srivatsan A, Jenkins SV, Jeon M, Wu Z, Kim C, Chen J. et al. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics. 2014;4:163. doi: 10.7150/thno.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin J, Wang S, Huang P, Wang Z, Chen S, Niu G. et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS nano. 2013;7:5320–9. doi: 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo W, Qiu Z, Guo C, Ding D, Li T, Wang F. et al. Multifunctional Theranostic Agent of Cu2(OH)PO4 Quantum Dots for Photoacoustic Image-Guided Photothermal/Photodynamic Combination Cancer Therapy. ACS Applied Materials & Interfaces. 2017;9:9348–58. doi: 10.1021/acsami.6b15703. [DOI] [PubMed] [Google Scholar]
- 168.Clift MJD, Stone V. Quantum dots: an insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics. 2012;2:668–80. doi: 10.7150/thno.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Laufer J, Johnson P, Zhang E, Treeby B, Cox B, Pedley B. et al. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. Journal of biomedical optics. 2012;17:0560161–8. doi: 10.1117/1.JBO.17.5.056016. [DOI] [PubMed] [Google Scholar]
- 170.Bohndiek SE, Sasportas LS, Machtaler S, Jokerst JV, Hori S, Gambhir SS. Photoacoustic Tomography Detects Early Vessel Regression and Normalization During Ovarian Tumor Response to the Antiangiogenic Therapy Trebananib. J Nucl Med. 2015;56:1942–7. doi: 10.2967/jnumed.115.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang J, Zhen X, Upputuri PK, Pramanik M, Chen P, Pu K. Activatable Photoacoustic Nanoprobes for In Vivo Ratiometric Imaging of Peroxynitrite. Advanced Materials. 2017;29:1604764. doi: 10.1002/adma.201604764. [DOI] [PubMed] [Google Scholar]
- 172.Kim T, Zhang Q, Li J, Zhang L, Jokerst JV. A Gold/Silver Hybrid Nanoparticle for Treatment and Photoacoustic Imaging of Bacterial Infection. ACS Nano. 2018;12:5615–25. doi: 10.1021/acsnano.8b01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ng KK, Shakiba M, Huynh E, Weersink RA, Roxin Á, Wilson BC. et al. Stimuli-Responsive Photoacoustic Nanoswitch for in Vivo Sensing Applications. ACS Nano. 2014;8:8363–73. doi: 10.1021/nn502858b. [DOI] [PubMed] [Google Scholar]
- 174.Dragulescu-Andrasi A, Kothapalli S-R, Tikhomirov GA, Rao J, Gambhir SS. Activatable Oligomerizable Imaging Agents for Photoacoustic Imaging of Furin-Like Activity in Living Subjects. Journal of the American Chemical Society. 2013;135:11015–22. doi: 10.1021/ja4010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luis Dean-Ben X, Razansky D. Adding fifth dimension to optoacoustic imaging: volumetric time-resolved spectrally enriched tomography. Light Sci Appl. 2014;3:e137. [Google Scholar]
- 176.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. The American journal of medicine. 1991;91:S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 177.Alfadda AA, Sallam RM. Reactive Oxygen Species in Health and Disease. Journal of Biomedicine and Biotechnology. 2012;2012:14. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brieger K, Schiavone S, Miller Jr FJ, Krause K-H. Reactive oxygen species: from health to disease. Swiss medical weekly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 179.Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP. et al. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biology. 2015;6:372–85. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z. et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat Nano. 2014;9:233–9. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoon Y, Lee PJ, Kurilova S, Cho W. In situ quantitative imaging of cellular lipids using molecular sensors. Nat Chem. 2011;3:868–74. doi: 10.1038/nchem.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kullo IJ, Trejo-Gutierrez JF, Lopez-Jimenez F, Thomas RJ, Allison TG, Mulvagh SL, A Perspective on the New American College of Cardiology/American Heart Association Guidelines for Cardiovascular Risk Assessment. Mayo Clinic Proceedings. 89: 1244-56. [DOI] [PubMed]
- 184.Beard PC, Mills T. Characterization of post mortem arterial tissue using time-resolved photoacoustic spectroscopy at 436, 461 and 532 nm. Physics in medicine and biology. 1997;42:177. doi: 10.1088/0031-9155/42/1/012. [DOI] [PubMed] [Google Scholar]
- 185.Allen TJ, Beard PC. Photoacoustic characterisation of vascular tissue at NIR wavelengths. 2009: 71770A-A-9.
- 186.Abran M, Cloutier G, Cardinal M-HR, Chayer B, Tardif J-C, Lesage F. Development of a photoacoustic, ultrasound and fluorescence imaging catheter for the study of atherosclerotic plaque. IEEE transactions on biomedical circuits and systems. 2014;8:696–703. doi: 10.1109/TBCAS.2014.2360560. [DOI] [PubMed] [Google Scholar]
- 187.Sangha GS, Phillips EH, Goergen CJ. In vivo photoacoustic lipid imaging in mice using the second near-infrared window. Biomedical optics express. 2017;8:736–42. doi: 10.1364/BOE.8.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wilson RH, Nadeau KP, Jaworski FB, Tromberg BJ, Durkin AJ. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. Journal of Biomedical Optics. 2015;20:030901. doi: 10.1117/1.JBO.20.3.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang P, Ma T, Slipchenko MN, Liang S, Hui J, Shung KK. et al. High-speed intravascular photoacoustic imaging of lipid-laden atherosclerotic plaque enabled by a 2-kHz barium nitrite raman laser. Scientific reports. 2014;4:6889. doi: 10.1038/srep06889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen YS, Frey W, Walker C, Aglyamov S, Emelianov S. Sensitivity enhanced nanothermal sensors for photoacoustic temperature mapping. Journal of biophotonics. 2013;6:534–42. doi: 10.1002/jbio.201200219. [DOI] [PubMed] [Google Scholar]
- 191.Larina IV, Larin KV, Esenaliev RO. Real-time optoacoustic monitoring of temperature in tissues. Journal of Physics D: Applied Physics. 2005;38:2633. [Google Scholar]
- 192.Ke H, Tai S, Wang LV. Photoacoustic thermography of tissue. Journal of biomedical optics. 2014;19:026003. doi: 10.1117/1.JBO.19.2.026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang S-H, Wei C-W, Jee S-H, Li P-C. Photoacoustic temperature measurements for monitoring of thermal therapy. Photons Plus Ultrasound: Imaging and Sensing 2009: International Society for Optics and Photonics; 2009. p. 71771. S. [Google Scholar]
- 194.Zhou Y, Tang E, Luo J, Yao J. Deep-tissue temperature mapping by multi-illumination photoacoustic tomography aided by a diffusion optical model: a numerical study. SPIE; 2018. p. 10. [DOI] [PubMed] [Google Scholar]
- 195.Liu W, Lan B, Hu L, Chen R, Zhou Q, Yao J. Photoacoustic thermal flowmetry with a single light source. SPIE; 2017. p. 6. [DOI] [PubMed] [Google Scholar]
- 196.Gillies R, Raghunand N, Garcia-Martin M, Gatenby R. pH imaging: A review of pH measurement methods and applications in cancers; 2004. [DOI] [PubMed]
- 197.Han J, Burgess K. Fluorescent Indicators for Intracellular pH. Chemical Reviews. 2010;110:2709–28. doi: 10.1021/cr900249z. [DOI] [PubMed] [Google Scholar]
- 198.Miao Q, Lyu Y, Ding D, Pu K. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Advanced Materials. 2016;28:3662–8. doi: 10.1002/adma.201505681. [DOI] [PubMed] [Google Scholar]
- 199.Huang P, Gao Y, Lin J, Hu H, Liao H-S, Yan X. et al. Tumor-Specific Formation of Enzyme-Instructed Supramolecular Self-Assemblies as Cancer Theranostics. ACS Nano. 2015;9:9517–27. doi: 10.1021/acsnano.5b03874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mu J, Lin J, Huang P, Chen X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chemical Society Reviews; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu Y, Wang LV. Effects of acoustic heterogeneity in breast thermoacoustic tomography. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2003;50:1134–46. doi: 10.1109/tuffc.2003.1235325. [DOI] [PubMed] [Google Scholar]
- 202.Hariri A, Fatima A, Mohammadian N, Mahmoodkalayeh S, Ansari MA, Bely N. et al. Development of low-cost photoacoustic imaging systems using very low-energy pulsed laser diodes. Journal of biomedical optics. 2017;22:075001. doi: 10.1117/1.JBO.22.7.075001. [DOI] [PubMed] [Google Scholar]
- 203.Hariri A, Lemaster J, Wang J, Jeevarathinam AS, Chao DL, Jokerst JV. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics. 2018;9:10–20. doi: 10.1016/j.pacs.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu Y, Xu G, Yuan J, Jo J, Gandikota G, Demirci H. et al. Light emitting diodes based photoacoustic imaging and potential clinical applications. Scientific reports. 2018;8:9885. doi: 10.1038/s41598-018-28131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Antaris AL, Chen H, Diao S, Ma Z, Zhang Z, Zhu S. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nature communications. 2017;8:15269. doi: 10.1038/ncomms15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu S, Yang Q, Antaris AL, Yue J, Ma Z, Wang H. et al. Molecular imaging of biological systems with a clickable dye in the broad 800-to 1,700-nm near-infrared window. Proceedings of the National Academy of Sciences. 2017;114:962–7. doi: 10.1073/pnas.1617990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Finlay MC, Mosse CA, Colchester RJ, Noimark S, Zhang EZ, Ourselin S. et al. Through-needle all-optical ultrasound imaging in vivo: a preclinical swine study. Light: Science &Amp; Applications. 2017;6:e17103. doi: 10.1038/lsa.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xi L, Jiang H. Integrated photoacoustic and diffuse optical tomography system for imaging of human finger joints in vivo. Journal of Biophotonics. 2016;9:213–7. doi: 10.1002/jbio.201500197. [DOI] [PubMed] [Google Scholar]
- 209.Lin CY, Chen F, Hariri A, Chen CJ, Wilder-Smith P, Takesh T, Photoacoustic Imaging for Noninvasive Periodontal Probing Depth Measurements. Journal of Dental Research; 2017. p. 0022034517729820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moore C, Bai Y, Hariri A, Sanchez JB, Lin C-Y, Koka S. et al. Photoacoustic imaging for monitoring periodontal health: A first human study. Photoacoustics. 2018;12:67–74. doi: 10.1016/j.pacs.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Aliroteh M, Scott G, Arbabian A. Frequency-modulated magneto-acoustic detection and imaging. Electronics letters. 2014;50:790–2. [Google Scholar]
- 212.Mehrmohammadi M, Oh J, Aglyamov SR, Karpiouk AB, Emelianov SY. Pulsed magneto-acoustic imaging. Engineering in Medicine and Biology Society, 2009 EMBC 2009 Annual International Conference of the IEEE: IEEE; 2009. pp. 4771–4. [DOI] [PubMed] [Google Scholar]