Abstract
The glycome acts as an essential interface between cells and the surrounding microenvironment. However, changes in glycosylation occur in nearly all breast cancers, which can alter this interaction. Here we report that profiles of glycosylation vary between ER-positive and ER-negative breast cancers. We found that genes involved in the synthesis of sialyl-Lewis x (sLex) (FUT3, FUT4 and ST3GAL6) are significantly increased in estrogen receptor alpha negative (ER-negative) tumours compared to ER-positive ones. SLex expression had no influence on the survival of patients whether they had ER-negative or ER-positive tumours. However, high expression of sLex in ER-positive tumors was correlated with metastasis to the bone where sLex receptor E-selectin is constitutively expressed. The ER-positive ZR-75-1 and the ER-negative BT20 cell lines both express sLex but only ZR-75-1 cells could adhere to activated endothelial cells under dynamic flow conditions in a sLex and E-selectin dependent manner. Moreover, L/P-selectins bound strongly to ER-negative MDA-MB-231 and BT-20 cell lines in a heparan sulfate-dependent manner that was independent of sLex expression. Expression of glycosylation genes involved in heparan biosynthesis (EXT1 and HS3ST1) was increased in ER-negative tumors. Taken together, our results suggest that the context of sLex expression is important in determining its functional significance and that selectins may promote metastasis in breast cancer through protein-associated sLex and heparan sulfate glycosaminoglycans.
Keywords: selectin, sialyl-Lewis x, estrogen receptor, breast cancer metastasis, heparan sulfate
Introduction
The 5 year survival of patients with distant metastatic disease is low and once detected, metastatic breast cancer is incurable. Distant metastases arise from circulating cancer cells that emigrate from the blood stream to colonise a distant organ (1). Thus, identifying the molecular mechanism involved in this process is one of the crucial challenges for breast cancer treatment. Selectins are calcium-dependent lectins involved in immune cells trafficking (2). E(ndothelial)-selectin expression is induced by inflammation in most organs (3–5) but is constitutively expressed in the microvasculature of bone marrow and skin (6). L(eukocyte)-selectin is constitutively expressed by myeloid and naïve T cells (3,7) while P(latelet)-selectin expression is induced in activated platelets and endothelial cells (3). Selectins share a common ligand, the carbohydrate sialyl-Lewis x (sLex), though their affinity is modulated by the nature of the carbohydrate scaffold and the backbone that carries sLex. E-selectin weakly binds to sLex-containing glycolipids (2) but has a strong affinity for O-glycan associated sLex expressed by neutrophils (8). L-selectin binds preferentially to a sulfated form of sLex (6-sulfo-sLex) while P-selectin recognizes with high affinity sLex presented on Core2 O-glycans carried on threonine 57 of P-selectin Glycoprotein Ligand-1 (2). L/P-selectins also bind glycosaminoglycans such as heparan and chondroitin sulfate (9). SLex and glycosaminoglycans may engage with selectins via different binding domains (10).
There is evidence that both classes of selectin ligands could promote metastasis. SLex is a marker of poor prognosis in colorectal and prostatic carcinomas (11). SLex expression in breast cancer has been associated with higher risk of metastasis (12) but its prognostic value remains debatable (13). Furthermore, sLex expression on colon (14) and prostate (15) cancer cells mediates adhesion to activated endothelial cells under flow conditions. However, heparin-derived reagents can inhibit L/P-selectins binding to both human (16, 17) and mouse (18) mammary cancer cell lines, resulting in decreased lung metastasis in animal models.
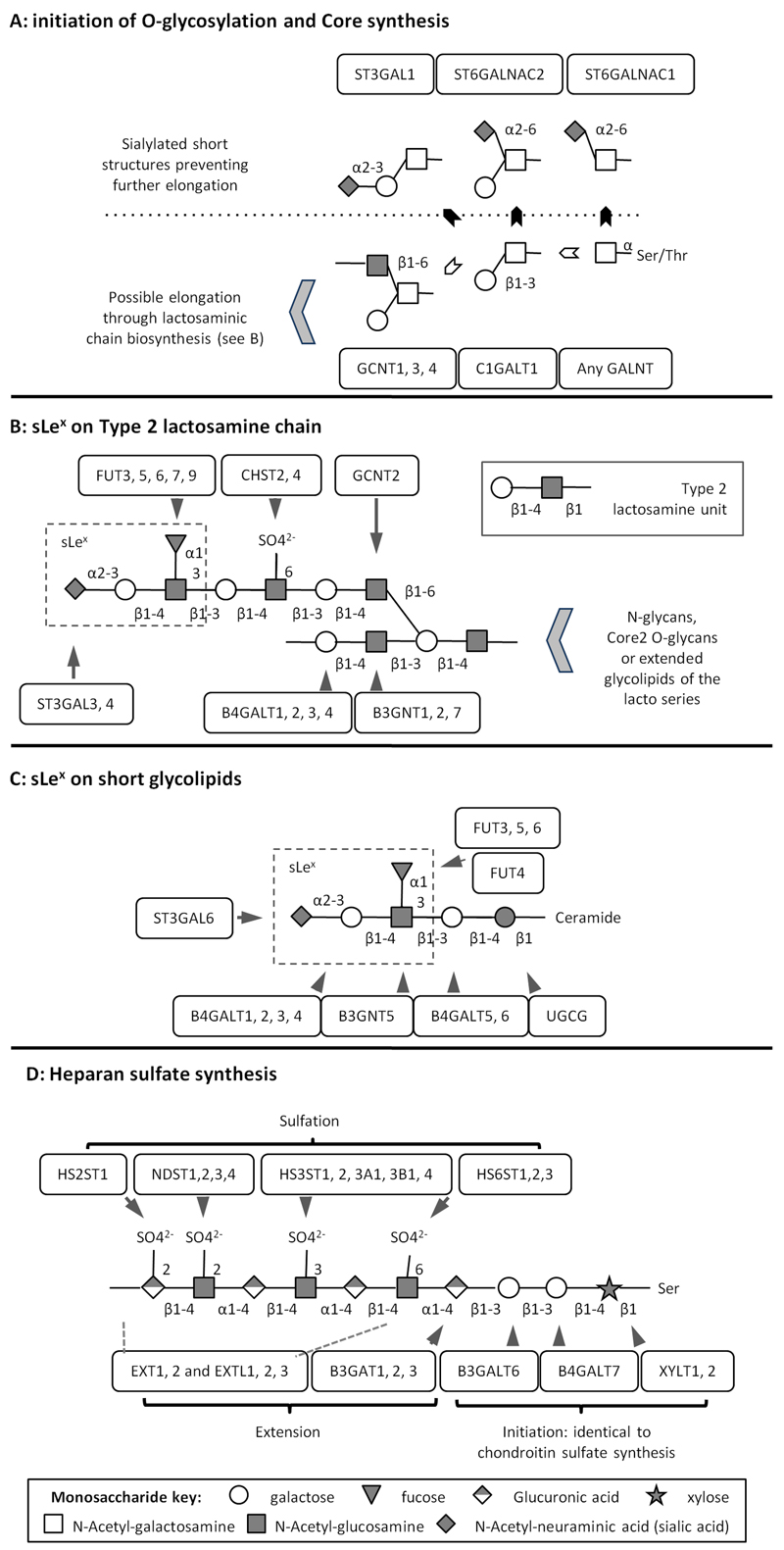
Glycan biosynthesis involves multiple glycosyltransferases in a complex multistep process that builds up carbohydrate structures on proteins and lipids. Each glycosyltransferase is specific for the acceptor structure, the transferred monosaccharide and the position and anomery of the linkage it creates (see figure 1) (19). Due to redundant specificities, some structures can be generated by several glycosyltransferases. For example, there are five fucosyl-transferases (FUT3, 4, 5, 6, and 7) potentially involved in sLex synthesis (figure 1B and 1C). Based on the profile of expression of glycosyltransferases in cells or tissues, the hypothetical structures of their glycans may be predicted. Expression array screening of glycosyltransferases has recently shown that many enzymes are aberrantly expressed in malignant breast tissue, which may account for the expression of cancer-associated carbohydrate antigens (20).
Figure 1: Representation of glycosylation pathways relevant to the study adapted from KEGG pathway website (48).
A: competition between sialyl-transferases creating short sialylated structures (black arrows) and elongating glycosyltransferases (white arrows). B and C: synthesis of sLex on lactosamine chain or glycolipid backbone. D: synthesis of linear chains of HS. Ser: serine, Thr: Threonine, SO4-: sulfate group.
Here, we investigated the expression profile of glycosyltransferases involved in sLex and heparan sulfate (HS) biosynthesis in human primary breast cancers. We found a significant difference in the expression of glycosyltransferases involved in the synthesis of sLex and expression of the glycan between ER-positive and ER-negative breast cancers, with significantly more ER-negative tumours expressing sLex. Although presence or absence of sLex was not associated with prognosis, high sLex expression in ER-positive tumours was significantly associated with bone metastasis. In vitro studies demonstrated functional differences between sLex expressed on ER-positive or ER-negative cell lines. In addition, HS dependent binding of P- and L-selectin was observed in the absence of sLex expression. Our data suggest that the functional significance of sLex is dependent on the context of its expression and that two mechanisms involving E-selectin/sLex and L/P-selectins/glycosaminoglycans could be independently involved in the extravasation step required for metastatic spread.
Material and methods
Microarray analysis
Total RNA was extracted from 10 ER-negative and 8 ER-positive breast cancers and submitted to microarray analysis on the GlycoV4 oligonucleotide array, a custom Affymetrix GeneChip (Affymetrix, Santa Clara, CA) designed for the Consortium for Functional Glycomics and having probes for 1260 glyco-genes. Data normalization was performed using RMA Express 1.0 with quantile normalization, median polish and background adjustment (21). The data have been deposited in GEO, accession number GSE32394. The generated data and two published datasets from Chin et al. (22) and van de Vijver et al. (23) were analysed using Prism 5 (Graph-Pad Software). Probes considered for the analysis from each dataset are listed in supplementary Table 1. Levels of glyco-gene expression were compared between ER-positive or negative breast tumours using unpaired Student t test. Multiple testing correction was applied using the Westfall and Young permutation in a step-down procedure. All p-values were two-tailed and p<0.05 was considered as statistically significant.
RNA extraction, reverse transcription and quantitative real-time-PCR (qRT-PCR)
Cell pellets were snap frozen in 50µl of RNA Later (Qiagen, Crawley, UK). Total RNA was extracted using the Nucleospin RNA II kit (Macherey Nagel, Düren, Germany), according to manufacturer’s instructions. The integrity of the RNA was controlled using a Bioanalyser (Agilent Technologies, Wokingham, UK). DNased RNA (0.6µg) was reverse transcribed using random hexamer primers and superscript III reverse transcriptase (Invitrogen, Paisley, UK). QRT-PCR was performed using SYBR Green I technology (Sigma-Aldrich, Poole, UK, see supplementary table 2 for primers) as described previously (24).
Tissue MicroArray (TMA) construction and analysis
Sections (3µm) from tissue microarrays consisting of 400 consecutive cases of invasive breast cancer from the Guy’s & St Thomas’ Breast Tissue & Data Bank were subjected to standard immunohistochemistry using the anti-sLex monoclonal antibody HECA-452 (BD biosciences, Oxford, UK). Expression of sLex was independently scored by two observers (SJ and SEP) and calculated as the average of I x P, where I is intensity (0, 1, 2 or 3) and P percentage (0-100%) of tumour cells staining (see figure S1). Scores were validated for 352 tumours. Tumours were classified as ‘negative’ when scored below 2.5 and as expressing high levels of sLex if they scored above 60. Statistical analyses were carried on using Prism5 (Graph-Pad Software).
Selectin binding and flow cytometry
Cell monolayers were detached using 5mM EDTA in PBS. When necessary, cells were treated with 500mU of type V neuraminidase from Clostridium perfringens (Sigma-Aldrich) or 1.6U of heparinase I (Sigma-Aldrich) in serum-free medium for 1hr at 37°C. SLex antigen was detected using HECA-452 followed by FITC conjugated anti-rat Ig (Dako, Ely, UK). Selectin ligands were detected using soluble recombinant human E/L/P-selectins (R&D Systems, Abingdon, UK) diluted at 40μg.mL-1, followed with FITC anti-human Ig (Dako). 5×105 cells were incubated with lectins or antibodies for 1hour on ice in PBS containing 0.5% bovine serum albumin and 1mM CaCl2. Where necessary, 5 mM EDTA was added during the incubation. For the sodium chlorate treatment, cells were incubated for 48hours in the presence or absence of 50mM sodium chlorate, detached from the dish and stained with L-selectin as described above. Samples were analysed on an EPICS-XL Flow Cytometer (Beckman-Coulter, High Wycombe, UK).
Parallel plate flow chamber assay
Confluent Human Umbilical Vein Endothelial Cells (HUVEC, Lonza) were stimulated with 10ng.mL-1 TNF-α (R&D systems) for 4-6 hours. Cancer cells were labelled with 1μM green or orange Cell Tracker® (Invitrogen) fluorescent dyes to distinguished them from endothelial cells and perfused at a density of 0.5×106 cells per mL at a shear rate of 1.25dynes/cm2 for 10 minutes and monitored using fluorescence timelapse microscopy (Olympus IX81). The entire flow chamber (Glycotech, Maryland) was imaged after 10min of perfusion and adherent cells were counted and expressed as cell number per field of view. Co-perfusion studies were performed using a total of 1×106 cells per mL of orange and green labelled cells. Swapping dyes did not affect cell behaviour (data not shown). Sialidase treatment was performed as described above prior to the dying step. To block E-selectin, HUVEC were incubated with 5μg.mL-1 anti-E-selectin mAb (Invitrogen, clone C126C10B7) for 2 hours. Control experiments were performed under the same conditions, by co-perfusion, but in the absence of sialidase or blocking mAb.
Western blotting
Cell pellets were lysed in 50mM Tris-HCl, 150mM NaCl buffer containing 1% Triton X-100, loaded onto 4 to 8% gradient acrylamide gel, submitted to SDS-PAGE under reducing conditions and electro-transferred to nitrocellulose membranes (Biotrace NT; Gelman Science, Ann Arbor, MI). SLex was detected with HECA-452, followed by alkaline phosphatase-conjugated secondary antibodies (Stratech, Newmarket, UK) and NBT/X-phosphate revelation reagent (Roche, Lewes, UK).
Selectin-precipitation
Total lysates (500µg per incubation in a volume of 100µl) were incubated (4hours, 4°C) with HECA-452 antibody (50µg/ml) or of recombinant E-selectin (200µg/ml) followed by 200μL of A/G-protein agarose beads (Thermo Scientific, Cramlington, UK) overnight at 4°C. Immuno-precipitated proteins were collected by centrifugation, washed and subjected to SDS-PAGE as described above. Transferred proteins were stained with HECA-452 or anti-BST-2 (H-135 from Santa Cruz Biotechnology, Heidelberg, Germany) antibody followed by horseradish peroxidase-conjugated secondary antibodies (DAKO).
LC/MS/MS Analysis of SDS PAGE 1D Gel bands
Lysates of ZR-75-1 cells were precipitated with E-selectin in the presence or absence of calcium, run on SDS-PAGE as described above and stained with silver nitrate. Eight bands of interest defined by comparing silver nitrate staining to Western blots with anti-sLex of E-selectin IPs, were excised and analysed by LC/MS/MS (detailed method can be found in the supplementary material).
Human samples
All tissue samples used were from the Guy’s and St Thomas’ Breast Tissue & Data Bank and had been approved for use in this study in accordance with their NHS REC approval (REC No: 07/H0874/131).
Cell culture
Breast cancer cell lines MDA-MB-231, BT-20 and ZR-75-1 were cultured in DMEM medium (Lonza, Cambridge, UK) containing 10% foetal calf serum and kept in culture for a maximum of 2-3months. ZR-75-1 cells were a gift from Dr Engels and BT20 from Dr E. Lasfargues. Both lines were authenticated by LGF Standards (Middlesex, UK) in February 2011 using the short tandem repeat profile of 16 loci. These 16 loci contain the 9 used by ATCC and both lines exactly matched these profiles. MDA-MB-231 were obtained from ATCC (HTB-26). For all cellular and biochemical studies cell monolayers were detached using 5mM EDTA in PBS for 5 minutes and then washed in PBS.
Results
The expression of certain glyco-genes correlates with ER status
To investigate the expression of glyco-genes in breast cancer we initially analysed two published microarray datasets (Chin et al. (22) and van de Vijver et al. (23), focusing on a set of 70 genes potentially involved in biosynthetic pathways of selectin ligands (supplementary table 1). A number of glyco-genes (29/70) were significantly over-expressed or under-expressed in ER-negative tumours compare to ER-positive tumours (table 1 and supplementary table 3). C1GALT1 (core1 synthase) and GCNT1 (core2 synthase) were both over-expressed in ER-negative tumours, while ST6GALNAC2 expression was lower in these tumours. As GCNT1 and ST6GALNAC2 compete for the same acceptor substrate (figure 1A), the results suggest that ER-negative tumours may be more likely to display a pattern of Core2 O-glycans than ER-positive ones.
Table 1: Correlation of glyco-genes expression with ER status or distant metastasisa.
| ER statusb | Distant metastasisc | |||||||
|---|---|---|---|---|---|---|---|---|
| Datasetsd | A | B | C | A | B | |||
| ERpos n= | 75 | 226 | 8 | DMpos n= | 87 | 194 | ||
| ERneg n= | 43 | 69 | 10 | DMneg n= | 31 | 101 | ||
| Pathways | Gene Symbol | p valuese | p values | |||||
|
A: O-glycan Core synthesis |
||||||||
| C1GALT1 | 0.0009 | 0.0005 | ||||||
| GCNT1 | <0.0001 | <0.05 | ||||||
| ST6GALNAC2 | 0.0542 | |||||||
|
B: sLex type 2 lactosamines chain |
||||||||
| FUT3 | <0.0001 | <0.05 | ||||||
| FUT6 | 0.059 | |||||||
| C: sLex on glycolipids | ||||||||
| B4GALT5 | 0.0216 | 0.0027 | 0.0158 | <0.01 | ||||
| B3GNT5 | NA | <0.0001 | 0.0415 | |||||
| FUT4 | 0.0019 | <0.0001 | 0.064 | |||||
| ST3GAL6 | 0.1275 | 0.0186 | ||||||
|
D: Synthesis of heparan sulfate |
||||||||
| B3GAT1 | 0.0793 | <0.05 | ||||||
| EXT1 | <0.0001 | 0.0343 | 0.0221 | <0.05 | ||||
| HS3ST1 | 0.0002 | 0.070 | ||||||
| HS3ST3B1 | 0.0674 | |||||||
See supplementary table 3 for a comprehensive version of the table.
ER: estrogen receptor alpha. Glyco-genes significantly more expressed in ER-negative tumours than ER-positive are shaded, those significantly less expressed are on white background. Blank: no statistical significance found.
Glyco-genes significantly more expressed in metastatic tumours (DMpos) than in non metastatic ones (DMneg) are shaded, those significantly less expressed are on white background. Blank: no statistical significance found.
A: Chin et al (22), B: van de Vijver et al. (23); C: present study. || Pathways described in Figure 1
Statistical significance was tested with the unpaired Student’s t-test. All p values were two tailed. For ER status, multiple testing correction was applied using the Westfall and Young permutation in a step-down procedure. For distant metastasis, only the original p values obtained by simple Student test are reported, and can be considered as a trend. p<0.05 was considered as statistically significant. Borderline p values are indicated in italic.
The expression of FUT3, FUT4 and ST3GAL6 were found to be higher in ER-negative tumours in the van Vijver dataset, suggesting these tumours could synthesize more sLex. The biosynthesis of sLex on glycolipids might also be increased in ER-negative tumours as most of the enzymes involved were over-expressed in these tumours from both datasets. Expression array analysis was then performed on an additional cohort of breast carcinoma samples, using a custom-made glyco-gene array. This analysis also showed the over-expression of FUT3, although significance was lost when multiple testing correction was applied. However, it should be noted that the number of samples in this cohort was small. Increased expression of two of the genes involved sLex expression on glycolipids in ER-negative tumours was also observed (table 1). These were among twelve glyco-genes that were consistently differentially expressed between ER-negative and ER-positive breast cancer in at least two datasets (supplementary table 3). Moreover, we found that ER-negative tumours had increased expression of enzymes involved in the extension of HS chains (B3GAT1, EXT1), sulfated on carbon 3 of GlcNAc (HS3ST1) (supplementary table 3).
Interestingly, glyco-genes involved in sLex expression (FUT3, 4 and 6) or in HS extension/sulfation (EXT1, B3GAT1 and HS3ST1) were also over-expressed in tumours of patients who subsequently developed distant metastasis.
To verify these results, qRT-PCR was performed on an independent cohort of 73 breast cancers for some of the glycosyltransferases. In concordance with the expression arrays, FUT3, FUT4, and ST3GAL6 were all found significantly more expressed in ER-negative tumours (figure S2).To summarise, based on the glyco-gene expression profiling, ER-negative tumours are predicted to express more sLex antigen on lactosamine chains (Core2 O-glycans or N-glycans) as well as on glycolipids than ER-positive tumours. They are also more likely to express longer and more sulfated heparan chains.
SLex expression is more frequently seen in ER-negative breast cancers
Using tissue microarrays 352 primary breast cancers were stained for sLex using the HECA-452 mAb. SLex expression was found to be significantly associated with ER-negative status, confirming the prediction made from the glyco-gene expression data. SLex expression was also associated with lymph node involvement and high histological grade of the tumours (table 2) as previously reported (12). Although ER-negative tumours metastasised more often than ER-positive ones (41.3% and 22.3% respectively, p<0.01 in Fisher’s exact test), sLex expression did not correlate with distant metastases (table 2).
Table 2: Correlation of sLex expression with biological features of the tumours.
| Morphological and clinical features | sLex negativea n (%) | sLex positive n (%) | p value |
|---|---|---|---|
| Estrogen receptor | |||
| positive | 160 (59.5) | 109 (40.5) | <0.001b |
| negative | 27 (35.5) | 49 (65.5) | |
| Lymph node involvement | |||
| negative | 98 (61.6) | 61 (38.4) | <0.01b |
| positive | 84 (47.2) | 94 (52.8) | |
| Grade | |||
| G1 | 40 (62.5) | 24 (37.5) | <0.001c |
| G2 | 78 (63.4) | 45 (36.4) | |
| G3 | 51 (38.9) | 80 (61.1) | |
| Tumour size | |||
| <2cm | 58 (60.4) | 38 (39.6) | 0.231b |
| >2cm | 137 (53.1) | 121 (46.9) | |
| Age at diagnosis | |||
| <50years | 44 (47.8) | 48 (52.1) | 0.219c |
| 50-70 years | 81 (58.7) | 57 (41.3) | |
| >70years | 36 (50.0) | 36 (50.0) | |
| Distant metastasis | |||
| negative | 145 (75.5) | 47 (24.5) | 0.334b |
| positive | 113 (70.6) | 47 (29.4) | |
Based on cut-off score of 2.5 as detailed in material and methods section.
Correlation calculated using Fisher’s exact test on Prism5 software.
Correlation calculated using Chi-square test on Prism5 software.
High SLex expression in ER-positive tumours is associated with bone metastasis
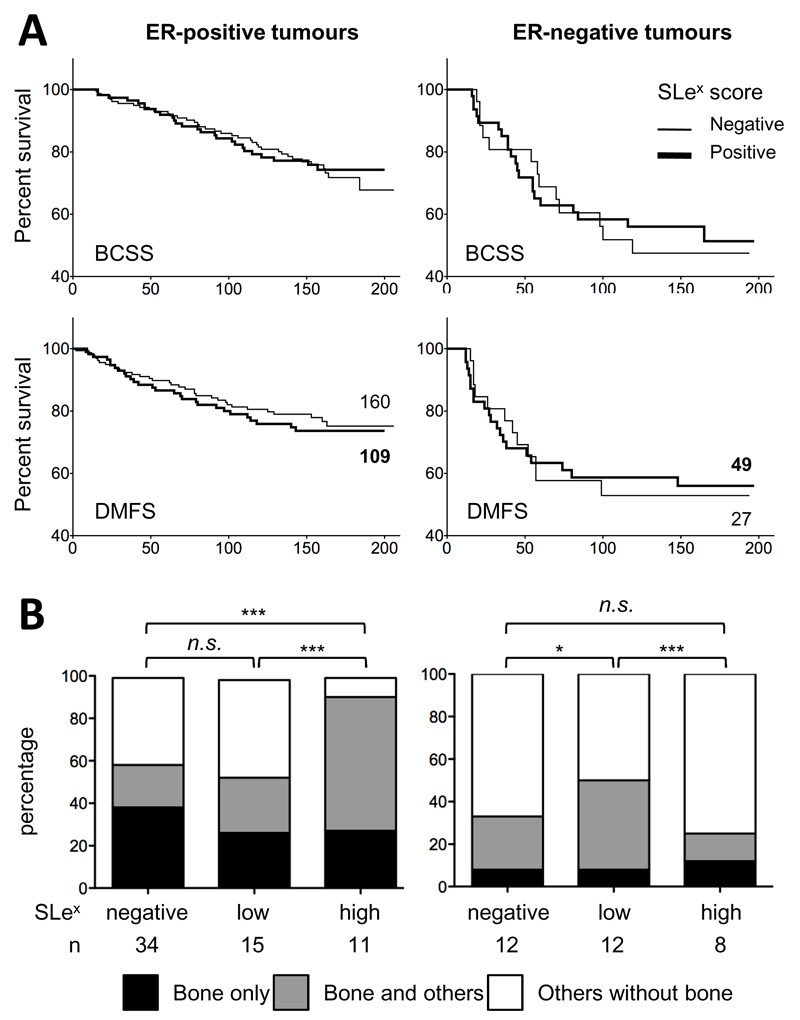
Correlation of sLex expression with breast cancer specific survival and metastasis free survival showed no significant difference between patients with sLex negative and sLex positive tumours whether these were ER-negative or ER-positive (figure 2A). However, when the site of metastatic spread was analysed (figure 2B), of the patients who developed distant metastasis, those with ER-positive tumours that expressed high levels of sLex had a significantly higher frequency of bone metastasis than those with low or no expression (p<0.0001). All but one of the ER-positive tumours with high levels of sLex showed bone metastasis. In contrast in the ER-negative cases 75% of the tumours that expressed high levels of sLex did not metastasise to the bone.
Figure 2: Correlation of sLex expression with survival and metastasis.
A: Kaplan-Meier analysis of breast cancer specific survival (BCSS) and distant metastasis free survival (DMFS) according to ER status and sLex expression. Number of patients included is indicated next to each DMFS curve. P value was calculated using Grehan-Breslow-Wilcoxon test (Prism5). B: Organotropism of distant metastasis according to ER status and sLex score (see Material and Methods). Other metastasis includes lung, liver, brain, skin, contralateral lymphatics, mediastine and pleura‥Statistical significance was tested using Chi-square test on 3x3 contingency table (Prism5), n.s. not significant, *: p<0.05, *** p<0.0001.
Selectins can bind to cell lines independently of sLex
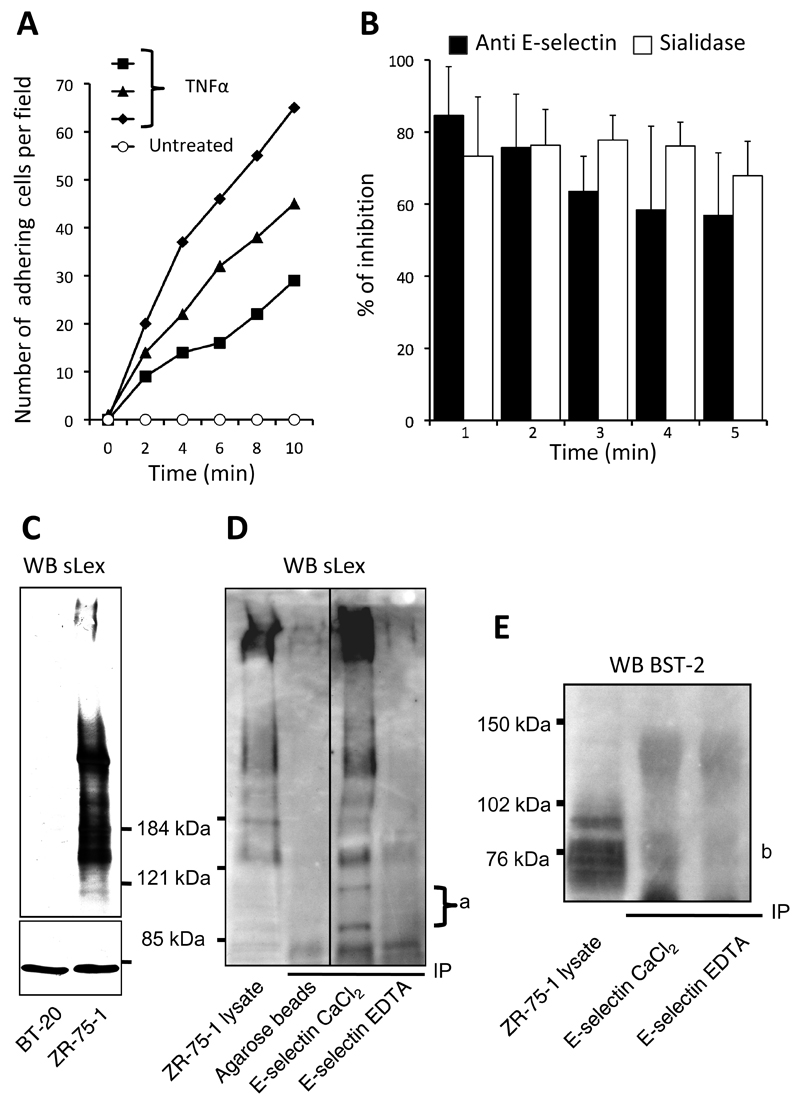
We investigated the functional relevance of sLex expression in ER-positive and ER-negative breast cancer cell lines. The ER-positive cell line ZR-75-1 and the ER-negative cell line BT20 both express sLex while the ER-negative cell line MDA-MB-231 does not (figure 3A). However, E-selectin only bound to ZR-75-1 (figure 3A) where both sialidase and EDTA treatments abrogated the binding, demonstrating the respective requirement for sialic acid and calcium. This indicates the ligand for E-selectin on ZR-75-1 is indeed a sLex-containing moiety. The lack of E-selectin binding to BT-20 suggests that the determinant carrying sLex in these cells shows low or no affinity for E-selectin.
Figure 3: Binding of E- and L-selectins to breast cancer cell lines.
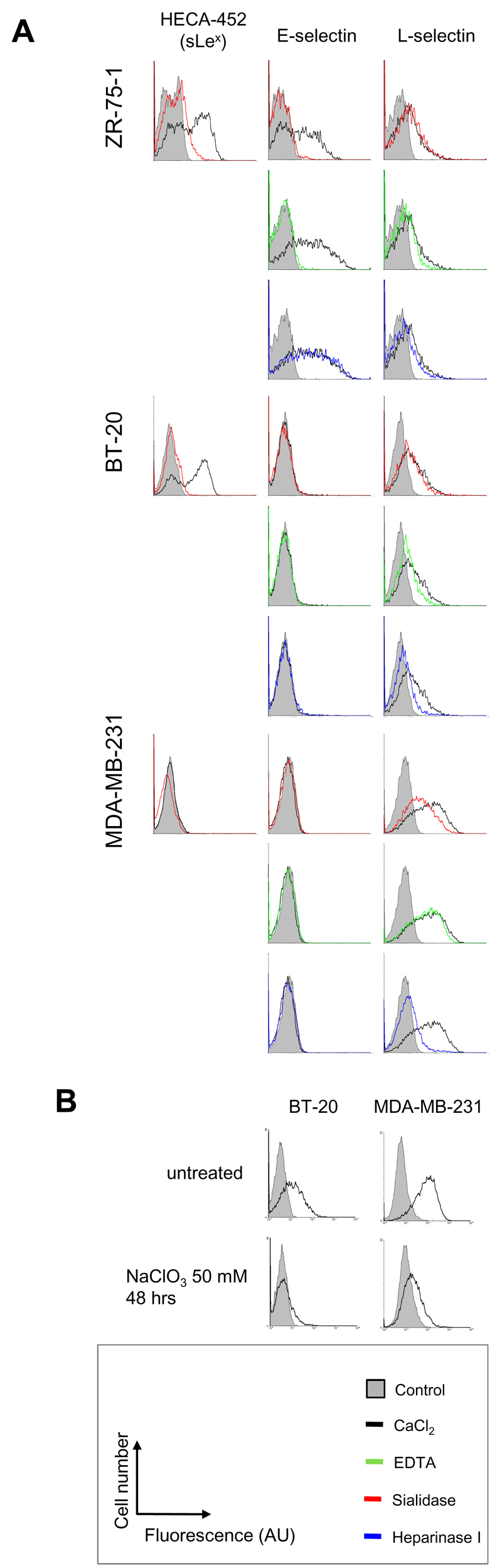
A: Fluorescent signal obtained in presence of CaCl2 (black) is overlaid with signals measured after treatment with sialidase (red), EDTA (green) or heparinase I (blue). SLex expression is reported on the left side of the figure. B: Effect of sodium chlorate treatment on L-selectin binding to MDA-MB-231 and BT20. Control signal (grey box) was obtained by omitting primary probes during the staining procedure. Each histogram is representative of at least three independent experiments. AU: arbitrary unit.
L/P-selectins bound to MDA-MB-231 and marginally to BT-20 (figure 3 and figure S3 for P-selectin binding). This binding was independent of sialic acid but was sensitive to heparinase I treatment (figures 3 and S3). The binding of L/P-selectins was not abrogated by 5mM EDTA treatment, suggesting calcium was not required (figures 3A and S3). However, EDTA at 20mM did decrease the binding of L-selectin indicating a small concentration of calcium may modulate its binding (data not shown). L-selectin binding was also abrogated by the inhibition of sulfation following sodium chlorate treatment (figure 3B). To confirm the binding of L-selectin to MDA-MB-231, a murine cell line transfected with human L-selectin was used (25). The sLex-negative MDA-MB-231 interacted with these cells in L-selectin specific manner (figure S4). However, this cell-cell adhesion could not be observed when tested in shear stress condition using a parallel-plateflow chamber assay (data not shown). Taken together, these results indicate that the ligand for L-selectin on MDA-MB-231 was not sLex but rather HS chains.
Differential expression of glyco-genes in breast cancer cell lines
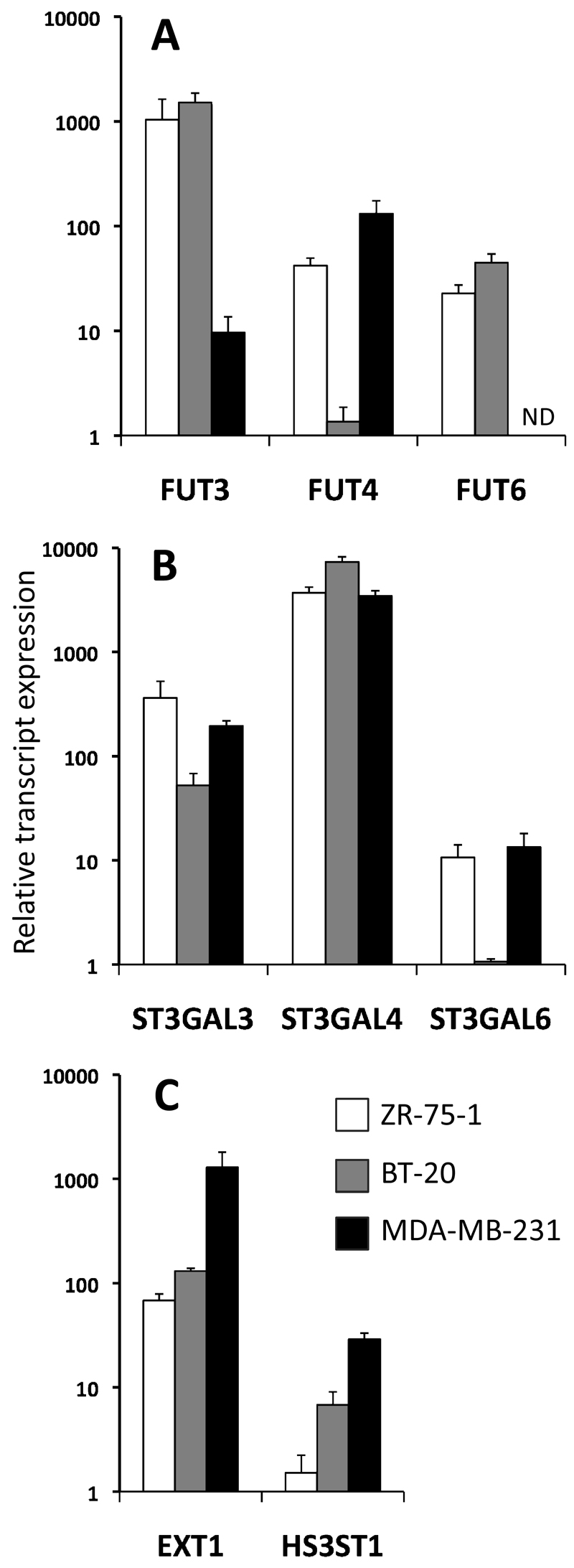
Levels of expression of glyco-genes potentially involved in the biosynthesis of either sLex or HS chains were investigated by qRT-PCR in ZR-75-1, BT-20 and MDA-MB-231. SLex-positive cell lines ZR-75-1 and BT-20 both expressed FUT3 and FUT6 at higher level than sLex-negative MDA-MB-231 (figure 4A), implying sLex expression was dependent on the activity of at least one of these glycosyltransferases. Sialyltransferases were found to be similarly expressed in all cell lines, apart from ST3GAL6 which was less expressed in BT-20 (figure 4B). Glycosyltransferases involved in the extension (EXT1) and in the sulfation (HS3ST1) of HS chains were also analysed (figure 4C). The expression of EXT1 was 10 fold higher in MDA-MB-231 than in BT-20 or ZR-75-1 and HS3ST1 was 10 and 100 fold more expressed in MDA-MB-231 than in BT-20 and ZR-75-1 respectively. This indicates than MDA-MB-231 has an accrued potential for synthesising long and 3-sulfated chains of heparan. These profiles fit well with the degree of binding of L/P-selectins (figures 3 and S3) suggesting FUT3, FUT6, EXT1 and HS3ST1 may be involved in the synthesis of selectin ligands.
Figure 4: qRT-PCR analysis of the expression of glycosyltransferases potentially involved in the synthesis of selectin ligands in breast cancer cell lines.
A: fucosyl-transferases. B: sialyl-transferases. C: glycosyltransferases involved in HS synthesis. All amplification have been standardised against PUM1 and then related to the lowest detected transcript for each histogram, FUT4 in BT-20 (panel A), ST3GAL6 in BT-20 (panel B) and HS3ST1 in ZR-75-1 (panel C). Bars represent the standard deviation for the value of three technical replicates. ND: not detected.
Function of selectin ligands in adhesion of breast cancer cells on HUVEC
We assessed the adhesion of ZR-75-1, BT-20 and MDA-MB-231 to TNFα activated HUVEC under conditions of physiological shear stress. When perfused individually or co-perfused over the HUVEC monolayer, we found that ZR-75-1 but not BT-20 or MDA-MB-231 firmly adhered to the endothelial cells (supplementary data movie 1 and 2). However, BT-20 cells were able to transiently interact with HUVEC (movie 1) while MDA-MB-231 did not seem to connect at all (movie 2). Adhesion of ZR-75-1 was consistently observed when HUVEC were expressing E-selectin upon activation with TNFα (figure 5A). Adhesion was drastically decreased by sialidase treatment of ZR-75-1 cells or by E-Selectin blocking (mAb treatment of HUVEC monolayers), both resulting in about 75% inhibition (figure 5B). Taken together, these results confirm that sLex/E-selectin interactions induce adhesion of ER-positive ZR-75-1 to HUVEC.
Figure 5: Adhesion of the ER-positive breast cancer cell line ZR-75-1 to HUVEC.
A: adhesion of fluorescently labelled ZR-75-1 on HUVEC was measured by cell counting performed at specific times of the video record. Time 0 was defined as the picture taken just before the first adhesion event. B: inhibitory effect of E-selectin blocking (anti E-selectin) or sLex enzymatic degradation (sialidase). For each experiment, cells were counted in a control perfusion condition paired with an inhibition test. Percentage of inhibition of adhesion was calculated for each control/test pair and averaged in the histogram. Bars represent the standard deviation of three independent experiments. C: Western-blot analysis of sLex-containing glycoproteins in breast cancer cell lysates (cropped). Lanes were loaded with 100μg of total proteins and separated proteins were blotted with HECA-452 mAb to detect sLex. HSC70 staining was used as loading control (cropped). D: Total ZR-75-1 cell lysate (500μg of proteins) were precipitated using recombinant soluble E-selectin. Precipitated proteins were submitted to SDS-PAGE and blotted with HECA-452 mAb (cropped). a: glycoproteins highly enriched in E-selectin precipitate expressing sLex. E: E-selectin precipitated proteins blotted anti-BST2. “b” indicates the presence of bands precipitated with E-selectin in a calcium dependent manner that bind to anti-BST2. Blots are representative of two (E) or three (C and D) independent experiments.
Characterisation of the sLex-containing ligand for E-selectin
ZR-75-1 cell-lysate contained several glycoproteins positively stained with the anti-sLex mAb HECA-452 (figure 5C). In contrast, BT-20 cell-lysate did not show any reactivity, even though live cells were positively stained with HECA-452 in flow cytometry experiments (figures 5C and 3). This suggests that sLex antigen expressed by BT-20 is not carried by glycoproteins but presumably by glycolipids on the surface of the cells. Immunoprecipitations of ZR-75-1 demonstrated that most of the glycoproteins identified by Western-blot with HECA-452 were also precipitated in a calcium specific manner by E-selectin (figure 5D). Furthermore, two minor glycoproteins of about 90 and 110kDa, invisible in the crude cell lysate, were highly enriched by E-selectin precipitation (annotated ‘a’ on figure 5D) suggesting a stronger affinity of E-selectin for these proteins. To identify these glycoproteins we submitted E-selectin-precipitated proteins separated on PAGE to mass-spectrometry analysis. A large number of non-specific proteins were identified as determined by the lack of dependency on calcium (see supplementary data for comprehensive protocol and results). However, we did identify the protein BST2 (Bone marrow stromal antigen 2) as one of the membrane bound proteins binding to E-selectin in a calcium specific manner. This finding was further confirmed by western blot using an anti-BST2 to probe E-selectin precipitate (figure 5E).
Discussion
Changes in glycosylation leading to the expression of aberrant glycans in cancer cells are well documented (12,26,27). Abnormal expression of sLex has attracted particular interest because of its function in cancer cell extravasation, mimicking a molecular mechanism involved in leukocyte extravasation (4,28). This has been extensively investigated in colorectal and prostate cancers (4,14,17,26,29) with relatively fewer studies addressing sLex in breast cancer metastasis. Here we report that sLex expression is associated with ER-negative status, lymph node involvement and high grade of breast tumours, but not with the survival of patients. However, in the ER-positive tumours high expression of sLex appears to have functional significance, correlating with bone metastasis.
Higher incidence of sLex in ER-negative breast tumours coincided with higher expression of glycosyltransferases FUT3, FUT4 and ST3GAL6. Interestingly, FUT3 was included in a 16 genes signature predicting distant metastasis in lymph node negative, ER-negative breast cancer (30). Moreover, a bone-colonizing variant of ER-negative MDA-MB-231 was reported to over-express FUT3 (31). However, our TMA analysis showed that expression of sLex by primary tumours had no influence metastasis or breast cancer free survival of patients, irrespective of their ER status. On the other hand, ER-positive breast cancers metastasised more often to the bone, where E-selectin is constitutively expressed, when sLex expression was high (p<0.0001), or when compared to ER-negative tumours expressing high sLex. Taken together, these data suggest that in breast cancer it is not the expression of sLex per se that contributes to organotropism but rather the context in which the glycan is expressed. Interestingly, our results suggest that BST2, which is associated with bone metastasis in breast cancer (32), can bind E-selectin. Further studies are warranted to confirm its role as an E-selectin ligand involved in adhesion to endothelial cells.
Using two sLex-positive breast cancer cell lines, we showed that the ER-positive line ZR-75-1 could adhere to activated HUVEC (which express E- but not P-selectin (33)) while ER-negative BT-20 only transiently interacted with HUVEC. This difference may be due to the fact that ZR-75-1 expresses sLex on a variety of glycoproteins, while BT-20 displays sLex antigen on glycolipids (34). This difference in the scaffold underlying sLex may modulate its affinity to E-selectin as seen in other systems (35). Protein-associated and lipid-associated sLex could not be distinguished in our TMA analysis. However, microarray data suggest that ER-negative tumours have an increased potential to synthesize sLex on a lipid scaffold. Thus, sLex in ER-negative tumours, if mostly carried by glycolipids, might have a lower affinity for E-selectin and therefore less involvement in the metastatic cascade.
We found that both sLex-negative and sLex-low tumours metastasise. Furthermore, the ER-negative MDA-MB-231 cell line, widely used as a metastatic model (see (31) as an example), was sLex-negative and unable to adhere to TNFα activated HUVEC. These observations suggest that sLex/E-selectin interaction is not mandatory for breast cancer metastasis and other interaction(s) must exist to support cancer cell extravasation.
Here we show that L- and P-selectins bind to HS expressed by MDA-MB-231 and BT-20 in a calcium independent manner and L-selectin could mediate cell-cell interaction under static but not flow conditions. This interaction could involve a positively charged domain of the CRD of selectins that is distinct from the sLex-specific calcium dependent domain (36). Proteoglycans carrying HS, such as Syndecan-I and Glypican-I are over-expressed in breast cancer and associated with poor prognosis (37,38). Moreover, inhibition of L/P-selectins binding by heparin significantly decreased metastasis in animal models (16). The pro-metastatic role of L/P-selectins may operate through the formation of ‘metastatic emboli’ of cancer cells aggregated with platelets and leukocytes (28,39,40). In this model, the suggested ligand for selectins is almost invariably sLex. Our data support the idea that L/P-selectin mediated metastasis may be dependent on cancer-associated 3-sulfated HS chains rather than sLex. Since both leukocytes and circulating cancer cells are carried by the blood flow, shear forces between these cells may be sufficiently low to allow interaction between selectins and HS as observed in our static adhesion assay. Supporting this model, platelet adhesion to metastatic mammary cancer cells can be mediated by P-selectin/glycosaminoglycan interactions in animal models (41). Moreover, we observed that EXT1 and HS3ST1 involved in HS biosynthesis, were more highly expressed in ER-negative tumours that had a higher incidence of metastasis. Strikingly, ER-negative tumours tend to metastasise predominantly in organs were leukocyte extravasation is independent of sLex/E-selectin interaction (e.g. lung or liver) (42–47).
In conclusion, our data suggest that sLex/E-selectin may be involved in metastasis of ER-positive tumours to the bone. Additionally, we propose that HS/L/P-selectin interaction involved in the formation of metastatic emboli may be another selectin-based mechanism promoting metastatic events in breast cancer. Further structural characterisation of these ligands could prove useful in the design of therapeutic approaches aiming to impede breast cancer extravasation.
Supplementary Material
Acknowledgements
The authors thank Dr Margreet Luchtenborg for the retrieval of the human tissues and clinical data from the Guy’s and St Thomas’s Research Breast Tissue Bank, as well as Mr John Brown and Mrs Leticia Bosshard-Carter for the preparation and staining of tissue microarrays. We would also like to thank the Proteomics Facility at KCL, especially Dr Steven Lynham.
Footnotes
Grant Support
This work was supported by Cancer Research UK, La Ligue Contre le Cancer, Breakthrough Breast Cancer and the British Heart Foundation. The authors also acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King’s College Hospital NHS Foundation Trust.
References
- 1.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 2.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230(1):97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4(5):325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 4.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11(11):1473–91. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148(1):165–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Investig Dermatol Symp Proc. 2004;9(3):215–23. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–56. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 8.Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc Natl Acad Sci U S A. 2010;107(20):9204–9. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994;91(16):7390–7. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103(3):467–79. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 11.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95(5):377–84. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010;12(3):204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sozzani P, Arisio R, Porpiglia M, Benedetto C. Is Sialyl Lewis x antigen expression a prognostic factor in patients with breast cancer? Int J Surg Pathol. 2008;16(4):365–74. doi: 10.1177/1066896908324668. [DOI] [PubMed] [Google Scholar]
- 14.Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol. 2003;284(4):C977–87. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 15.Barthel SR, Gavino JD, Wiese GK, Jaynes JM, Siddiqui J, Dimitroff CJ. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18(10):806–17. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsig L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb Res. 2010;125(Suppl 2):S66–71. doi: 10.1016/S0049-3848(10)70017-7. [DOI] [PubMed] [Google Scholar]
- 17.Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, Vlodavsky I, et al. P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. Faseb J. 2007;21(13):3562–72. doi: 10.1096/fj.07-8450com. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel J, Zeisig R, Fichtner I. Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell-platelet interactions. Clin Exp Metastasis. 2010;27(1):25–34. doi: 10.1007/s10585-009-9299-y. [DOI] [PubMed] [Google Scholar]
- 19.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7(6):599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potapenko IO, Haakensen VD, Luders T, Aslaug H, Bukholm I, Sorlie T, et al. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol Oncol. 2009;4(2):98–118. doi: 10.1016/j.molonc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10(6):529–41. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 23.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 24.Julien S, Grimshaw MJ, Sutton-Smith M, Coleman J, Morris HR, Dell A, et al. Sialyl-Lewisx on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: A mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. Journal of Immunology. 2007;179(9):5701–10. doi: 10.4049/jimmunol.179.9.5701. [DOI] [PubMed] [Google Scholar]
- 25.Ivetic A, Florey O, Deka J, Haskard DO, Ager A, Ridley AJ. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J Biol Chem. 2004;279(32):33263–72. doi: 10.1074/jbc.M312212200. [DOI] [PubMed] [Google Scholar]
- 26.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 27.Burchell JM, Mungul A, Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001;6(3):355–64. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- 28.Laubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–77. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 31.Carcel-Trullols J, Stanley JS, Saha R, Shaaf S, Bendre MS, Monzavi-Karbassi B, et al. Characterization of the glycosylation profile of the human breast cancer cell line, MDA-231, and a bone colonizing variant. Int J Oncol. 2006;28(5):1173–83. [PubMed] [Google Scholar]
- 32.Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W, et al. Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer. 2009;9:102. doi: 10.1186/1471-2407-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood. 1999;94(11):3820–8. [PubMed] [Google Scholar]
- 34.Shirure VS, Henson KA, Schnaar RL, Nimrichter L, Burdick MM. Gangliosides expressed on breast cancer cells are E-selectin ligands. Biochem Biophys Res Commun. 2011;406(3):423–9. doi: 10.1016/j.bbrc.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 35.Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem. 2006;281(20):13899–905. doi: 10.1074/jbc.M513617200. [DOI] [PubMed] [Google Scholar]
- 36.Green PJ, Yuen CT, Childs RA, Chai W, Miyasaka M, Lemoine R, et al. Further studies of the binding specificity of the leukocyte adhesion molecule, L-selectin, towards sulphated oligosaccharides--suggestion of a link between the selectin- and the integrin-mediated lymphocyte adhesion systems. Glycobiology. 1995;5(1):29–38. doi: 10.1093/glycob/5.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda K, Maruyama H, Guo F, Kleeff J, Itakura J, Matsumoto Y, et al. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001;61(14):5562–9. [PubMed] [Google Scholar]
- 38.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98(3):474–83. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 39.Maraveyas A, Johnson MJ, Xiao YP, Noble S. Malignant melanoma as a target malignancy for the study of the anti-metastatic properties of the heparins. Cancer Metastasis Rev. 2010;29(4):777–84. doi: 10.1007/s10555-010-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ, et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161(3):749–53. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monzavi-Karbassi B, Stanley JS, Hennings L, Jousheghany F, Artaud C, Shaaf S, et al. Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int J Cancer. 2007;120(6):1179–91. doi: 10.1002/ijc.22424. [DOI] [PubMed] [Google Scholar]
- 42.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–8. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 43.Maki DD, Grossman RI. Patterns of disease spread in metastatic breast carcinoma: influence of estrogen and progesterone receptor status. AJNR Am J Neuroradiol. 2000;21(6):1064–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 45.Strell C, Entschladen F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal. 2008;6:10. doi: 10.1186/1478-811X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37(1):24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 47.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 48.Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories at Kyoto University and the University of Tokyo Japan; c1995-2011. Available from http://www.genome.jp/kegg/pathway.html [database on the internet] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.