Abstract
Background
T NADPH oxidase, by generating reactive oxygen species, is involved in the pathophysiology of many cardiovascular diseases and represents a therapeutic target for the development of novel drugs. A single-nucleotide polymorphism (SNP) C242T of the p22phox subunit of NADPH oxidase has been reported to be negatively associated with coronary heart disease (CHD) and may predict disease prevalence. However, the underlying mechanisms remain unknown.
Methods and Results
Using computer molecular modelling we discovered that C242T SNP causes significant structural changes in the extracellular loop of p22phox and reduces its interaction stability with Nox2 subunit. Gene transfection of human pulmonary microvascular endothelial cells showed that C242T p22phox reduced significantly Nox2 expression but had no significant effect on basal endothelial O2.- production or the expression of Nox1 and Nox4. When cells were stimulated with TNFα (or high glucose), C242T p22phox inhibited significantly TNFα-induced Nox2 maturation, O2.- production, MAPK and NFκB activation and inflammation (all p<0.05). These C242T effects were further confirmed using p22phox shRNA engineered HeLa cells and Nox2-/- coronary microvascular endothelial cells. Clinical significance was investigated using saphenous vein segments from non CHD subjects after phlebectomies. TT (C242T) allele was common (prevalence of ~22%) and compared to CC, veins bearing TT allele had significantly lower levels of Nox2 expression and O2.- generation in response to high glucose challenge.
Conclusions
C242T SNP causes p22phox structural changes that inhibit endothelial Nox2 activation and oxidative response to TNFα or high glucose stimulation. C242T SNP may represent a natural protective mechanism against inflammatory cardiovascular diseases.
Keywords: genetics, enzymes, structure, inflammation, endothelium, vessels
Introduction
Single-nucleotide polymorphisms (SNP) are one base variations in DNA sequences that occur in at least 1% of the population1. Although most SNPs have little or no effect on human health, some can predispose individuals to disease or have a major impact on the physiological response to environmental challenges or to drugs1, 2. Vascular endothelial cells express constitutively a Nox2 (also called gp91phox) containing NADPH oxidase, which by generating superoxide (O2.-) plays an important role in oxidative stress-related endothelial dysfunction and cardiovascular diseases (CVD)3–7. The Nox2 catalytic subunit requires p22phox in a 1:1 ratio to form the cytochrome b558 complex to produce O2.-, and structural changes of p22phox may affect its interaction with Nox2. In the context of Nox2-derived oxidative stress and CVD, SNPs of the p22phox have attracted significant attention recently.
The p22phox is encoded by the CYBA gene located on the long arm of chromosome 16 at position 24. It spans 8.5 kb and is composed of six exons and five introns encoding an open reading frame of approximately 600 bp8. The p22phox has three structural domains: 1) a hydrophobic N-terminal domain consisting of three transmembrane helices; 2) a hydrophilic extracellular region; and 3) a cytosolic tail featuring a proline rich region (PRR)9, 10. So far, seven SNPs of the p22phox gene have been reported i.e. C242T, A640G; C549T; A930G; A675T; C852G and C536T11, and only two of them (C242T and C549T) are translated into the protein. The C549T SNP changes an Ala174 to Val174 without any reported significant functional effect11. In contrast, the C242T SNP changes His72 to Tyr72 that is located in the extracellular loop of the putative Nox2 binding region12 and has been reported in several studies to be negatively associated with CVD, such as hypertension, atherosclerosis, and myocardial infarction and to reduce oxidative burst in neutrophils11,13–15. However, others have found that the C242T variant had no effect on cardiovascular disease progression16 or even increased ROS production in CVD arteries17,18. Given the contradictory evidence, it is important to elucidate the molecular mechanism responsible for the effects of p22phox C242T SNP on inhibiting endothelial Nox2 activity and vascular oxidative stress in order to ascertain its effect on CVD.
In this study, we used computer structural modeling plus molecular and biochemical approaches to elucidate the mechanism of p22phox C242T SNP inhibition of endothelial Nox2 activation and oxidative stress in response to TNFα or high glucose stimulation. Results were further confirmed using engineered p22phox depleted (p22phox-depl) HeLa cells, primary coronary microvascular endothelial cells (CMEC) isolated from Nox2-/- mice and saphenous vein segments of patients without history of coronary heart diseases (CHD) after phlebectomies. We report for the first time that C242T SNP is linked to the p22phox extracellular domain morphological change that interferes with Nox2 binding stability and inhibits endothelial oxidative stress and inflammatory response to TNFα or high glucose challenges.
Materials and methods
The in silico p22phox model was generated as reported previously by incorporating data from online prediction servers with the molecular operating environment (MOE; Chemical Computing Group Inc., Canada) and subjected to energy minimization using the AMBER99 forcefield9,10. The docking of p22phox with Nox2 peptides was performed in the MOE10. HapMap analysis of the CYBA gene was performed using the International HapMap Project (HapMap phase II+III; dbSNP b126) and six common CYBA SNPs were chosen from the National Centre for Biotechnology Information (NCBI) SNP database (dbSNP). Haplotype association was calculated using the Haplo-view analysis program (version 4.2) in the northern and western European population (CEU). Human pulmonary microvascular endothelial cells (HPMEC-ST1.6R) were a kind gift from Dr R. Unger (Johannes Gutenberg University, Germany)19. Mouse CMEC were isolated from the hearts of ~12 week old Nox2-/- and WT mice and cultured as described previously20,21. The shRNA p22phox (p22phox-depl) and shRNA scrambled control HeLa cells were generated as described previously22 in the laboratory of Professor Krönke (University of Cologne, Germany). Segments of human saphenous vein were collected from 36 patients (without history of CHD) undergoing phlebectomies at a specialist vein unit. Informed consents were obtained from the patients and the project was approved by the local NHS and the university ethical committees according to UK regulation. The IRB approval was obtained according to the guidelines noted in the Circulation Instruction to Authors. The C to T substitution at position 242 in the CYBA coding sequence was examined as described previously23. ROS production was measured using four independent methods as described previously24: 1) Lucigenin-chemiluminescence; 2) DHE fluorescence HPLC assay with or without superoxide dismutase–polyethylene glycol (PEG-SOD)4,25; 3) DHE flow cytometry; and 4) DCF fluorescence.
See the online-only Data Supplement for a full description of materials and methods.
Statistics
Data were presented as means ± SD in figures. For cell culture experiments, results were taken from at least 3 independent cell cultures and gene transfections for each condition. In the case of CMEC isolation, 6 mice/per group were used for each isolation and the data presented were the means ± SD from at least 3 separate CMEC isolations. Comparisons were made by ANOVA with Bonferroni post hoc correction or as indicated in the figure legend. Values of P<0.05 were considered statistically significant.
Results
Structural changes in p22phox associated with C242T SNP
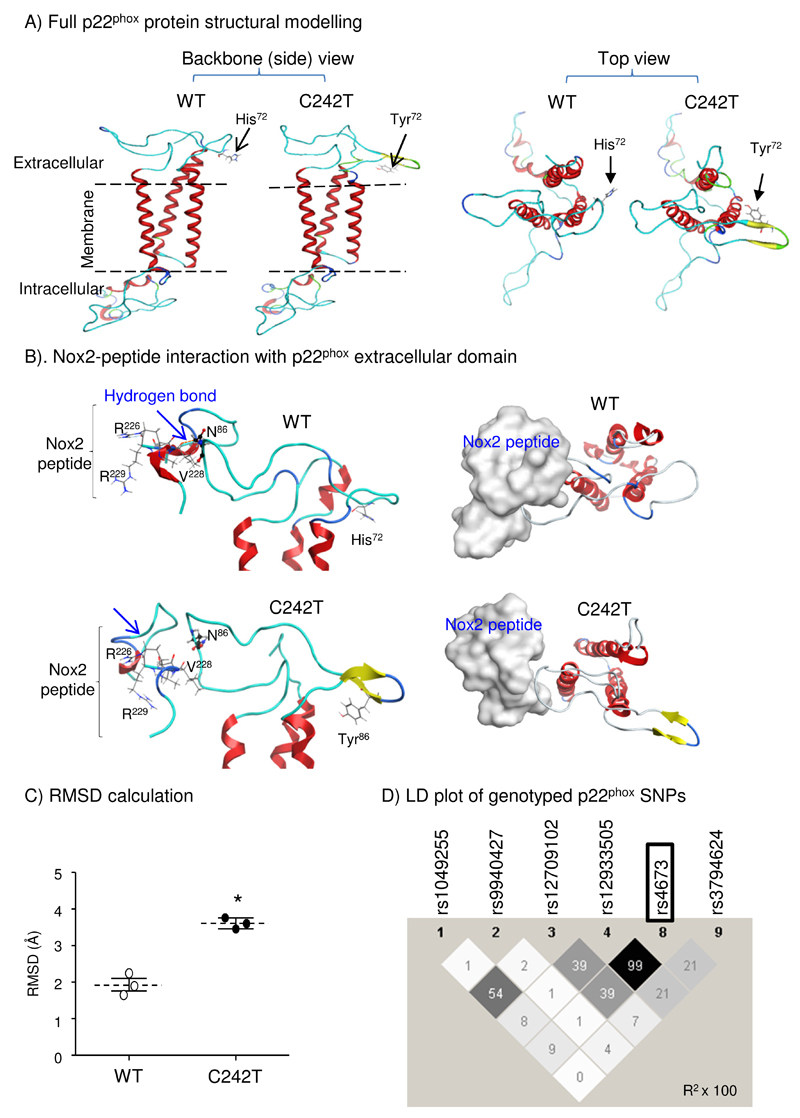
Computer modeling was used to investigate potential protein structural changes linked to p22phox C242T SNP9. Our consensus p22phox model showed that His72 (WT p22phox) is located within the extra-cellular region of the p22phox (Figure 1A), which has been identified to be essential for interaction with Nox212,26. The histidine residue contains a polar hydrophilic imidazole in comparison to the aromatic and hydrophobic tyrosine residue. The substitution of His72 to Tyr72 alters the conformation of the extracellular region as shown by the backbone side-view (Figure 1A, left panels) and top-view (Figure 1A, right panels). Root mean squared deviation (RMSD) calculations of extracellular structures of WT and C242T p22phox following energy minimization revealed significant difference between two structures (Figure 1C).
Figure 1. Computer modelling of p22phox structural changes associated with C242T SNP.
(A) Morphological differences between WT and C242T p22phox structures (ribbon presentation). (B) Docking of a Nox2-peptide (222-HGAERIVR-229) (skeleton in left panels and silver-space-fill in right panels) with p22phox extracellular domain (ribbon). Nox2 peptide interacts and forms an H-bond with the N86 (ball and stick presentation) of WT p22phox but not with the N86 of C242T p22phox. (C) RMSD calculations of the extracellular domain structural differences between WT and C242T p22phox following energy minimization. n=3 independent investigators. *p<0.05 for C242T values versus WT values (Mann-Whitney U-test). (D) Linkage disequilibrium (LD) plot from Haplo-view of common genotyped SNPs within the CYBA (p22phox) gene. The darker a diamond appears, the greater the correlation (r2 × 100) between the respective genotyped CYBA polymorphisms. The C242T SNP (rs4673) is outlined.
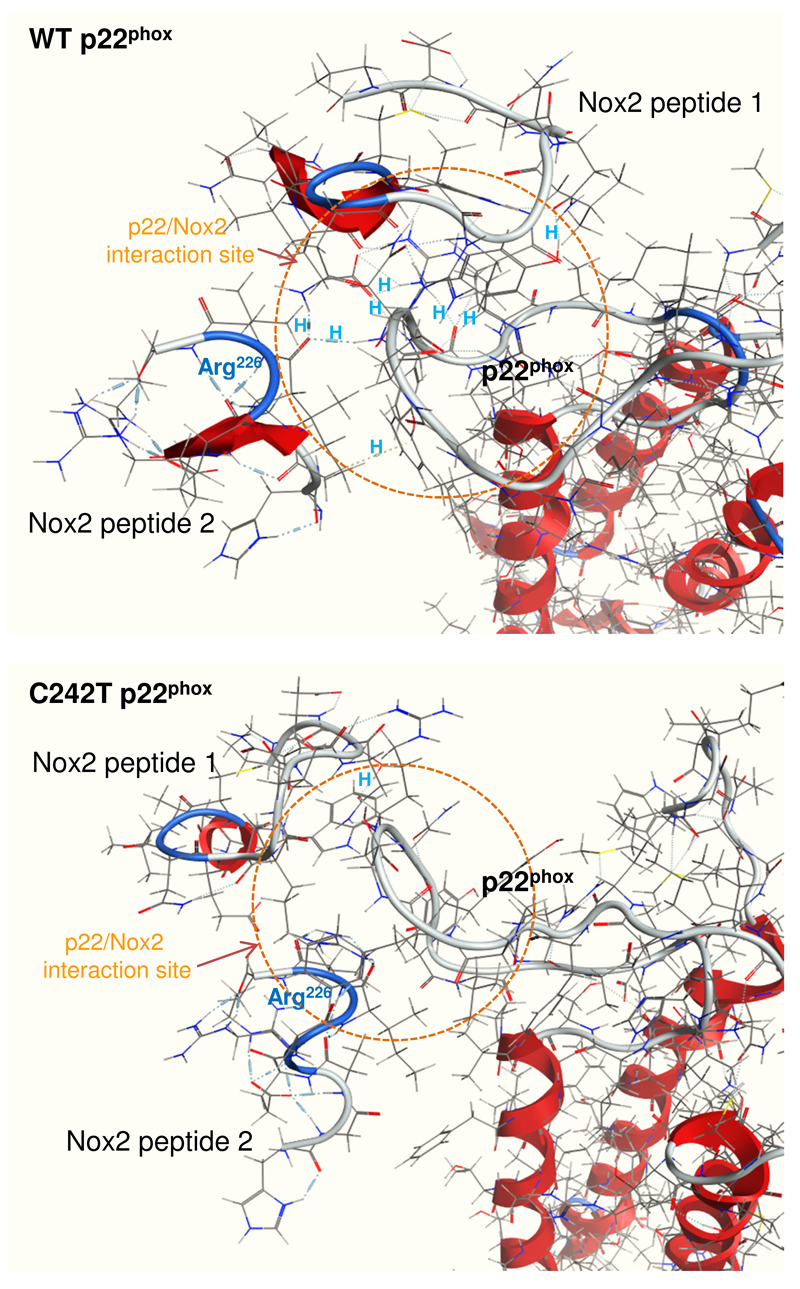
We next performed protein/protein docking of p22phox with a known Nox2 peptide (222-HGAERIVR-229) that corresponds to the Nox2 extracellular region involved in binding to p22phox in a previous report27. We found that the Nox2 peptide interacted successfully with the WT p22phox extracellular domain and formed a hydrogen-bond between Arg226 of the Nox2 and Asn86 of the p22phox (Figure 1B, upper panels). However, this interaction was lost in the C242T model (Figure 1B, lower panels). Further docking experiments were performed using two Nox2 peptides (peptide 1: 154-NFARKRIKNPEGGLY-168; peptide 2: 222-HGAERIVRG-230), which are located in the Nox2 extracellular domain and have been reported previously to be recognized by a Nox2 antibody (7D5)27 (Figure 2). We found several important hydrogen bonds (labeled by H) formed between the Nox2 peptides and the WT p22phox configuration, which further illustrated the importance of Nox2 Arg226 in stabilizing the interaction with WT p22phox. However, these links were disrupted by the C242T p22phox configuration. We also performed HapMap analysis of the CEU (European) population, and found that the C242T SNP is only in strong correlation (r2 = 0.99) with one SNP located within a non-coding region of intron 4 (rs12933505; Figure 1D). Moreover, the genotyped allele frequency of the CC (WT) and the CT and TT variants is 53% and 47%, respectively. This confirms the functional effect of C242T SNP is independent of other known p22phox SNPs at the protein level.
Figure 2. Docking of two Nox2 peptides with p22phox extracellular domain.
Images are shown as a tiled 45 degrees side-view/top-view. Two Nox2 peptides (peptide 1: 154-NFARKRIKNPEGGLY-168; peptide 2: 222-HGAERIVRG-230) used for the docking have been reported previously to be the extracellular epitopes of Nox2 recognised by an antibody (7D5) to Nox2. WT p22phox configuration forms several hydrogen bonds (short grey dashed lines labelled by H) with Nox2 peptides. The location of Nox2 Arg226 is indicated. However, these interactions were lost in the C242T p22phox configuration. Red ribbons represent alpha helix.
Effect of C242T SNP on endothelial Nox2 activity
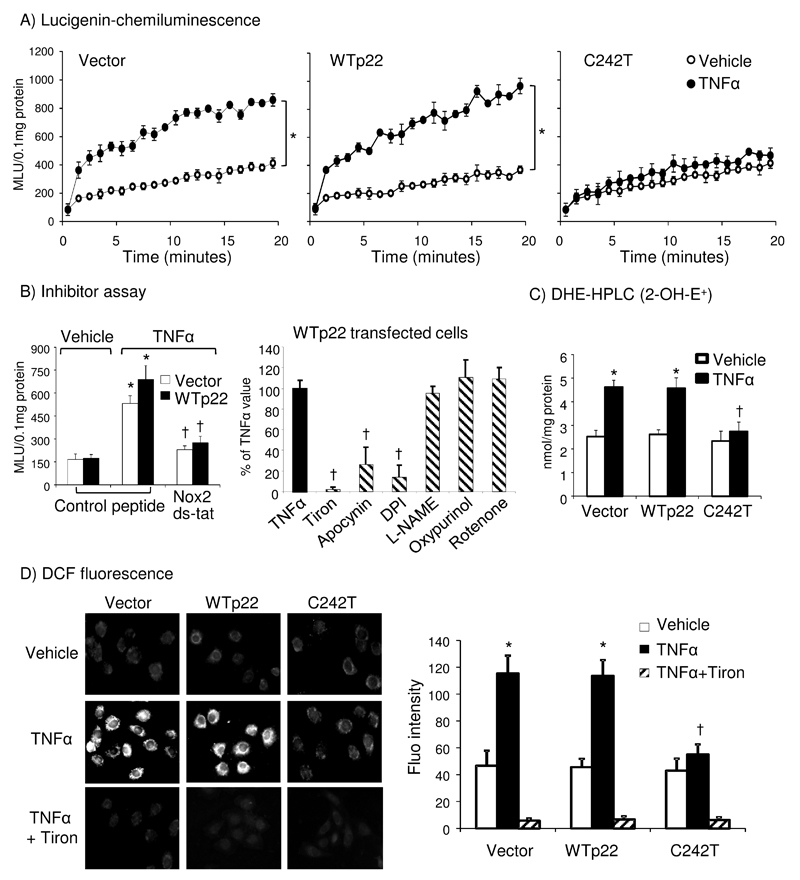
The functional effect of p22phox C242T SNP on human endothelial Nox2 activity was firstly investigated by site-directed mutagenesis followed by gene transfection of human pulmonary microvascular endothelial cells (HPMECs) with or without acute TNFα (100 unit/ml; 30 min) stimulation. HPMECs displayed low levels of O2.- production at basal conditions (without TNFα stimulation) whereas TNFα increased significantly the levels of O2.- production in cells either transfected with an empty vector or with WT p22phox, without significant differences between them (Figure 3A). However, compared to WT p22phox, C242T p22phox had no significant effect on endothelial basal O2.- production, but did significantly inhibit TNFα-induced O2.- production (Figure 3A; right panel). The enzymatic sources of TNFα-induced O2.- production in vector or WT p22phox transfected cells was confirmed using human Nox2ds-tat peptide (a specific inhibitor of Nox2)28 and different enzyme inhibitors (Figure 3B). TNFα-induced O2.- production was inhibited significantly by Nox2ds-tat but not by NoxA1ds peptide (a specific inhibitor of Nox1, see Supplemental Figure 1) and was completely abolished by an O2.- scavenger (Tiron; 10mmol/L) which confirmed the detection of O2.-. It was significantly inhibited by apocynin (100µmol/L, a Nox2 inhibitor) and diphenyliodium (DPI 20µmol/L, a flavoprotein inhibitor), but not by L-Nitroarginine-methyl-ester (L-NAME 100µmol/L, an eNOS inhibitor), oxypurinol (100µmol/L, a xanthine oxidase inhibitor) or rotenone (50µmol/L, a mitochondrial electron transport chain inhibitor) (Figure 3B). Compared to WT p22phox transfected cells, C242T p22phox had no significant effect on Nox4 activity (H2O2 production) as shown using HEK293 cells (no endogenous Nox4 expression) co-transfected with Nox4 plus p22phox (Supplemental Figure 2). These data further confirmed a crucial role of Nox2-containing NADPH oxidase in TNFα-induced endothelial O2.- production.
Figure 3. The effect of p22phox C242T SNP on HPMEC ROS production.
(A) Kinetic measurement of O2.- production. Area under curve (AUC) was calculated for each sample. *p<0.05 for TNFα AUC values verses vehicle AUC values analyzed by unpaired t-test with Welch's correction. (B) Inhibition of O2.- production measured by lucigenin-chemiluminescence. Left panel: Nox2ds-tat peptide; Right panel: Effects of different enzyme inhibitors presented as percentages to TNFα values without inhibitor (filled bar). *p<0.05 for indicated values versus vehicle values under the same gene transfection. †p<0.05 for indicated values verses TNFα values (filled bar) under the same gene transfection. (C) O2.- production (2-OH-E) measured by DHE-HPLC. The amount of 2-OH-E was quantified against a standard curve and expressed as nmol/mg protein. (D) Intracellular ROS production examined by DCF fluorescence microscopy. Tiron was used to confirm the detection of O2.-. (C-D) *p<0.05 for TNFα values verses vehicle values under the same gene transfection. †p<0.05 for indicated values versus WTp22 TNFα values. Sample size (A-C): n=4 independent experiments; (D): n=3 independent experiments.
The inhibitory effect of p22phox C242T on TNFα-induced endothelial O2.- production was further confirmed by two independent methods: DHE high-performance liquid chromatography (HPLC) detection of 2-OH-E+ (Figure 3C) and the tiron-inhibitable DCF-fluorescence microscopy using intact adherent endothelial cells (Figure 3D).
Effects of C242T p22phox on endothelial Nox subunit expression and binding to p22phox
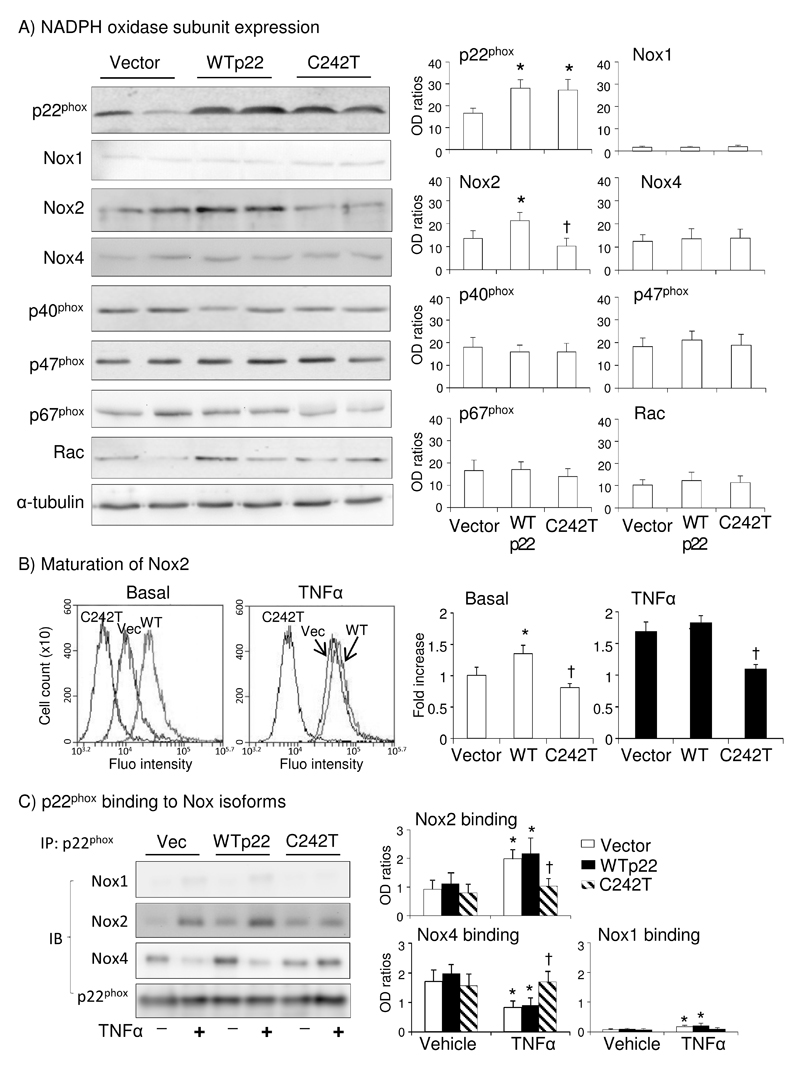
The effects of p22phox C242T SNP on the basal levels of NADPH oxidase subunit expression was examined by Western blot. Compared to vector-transfected cells, the levels of p22phox expression were significantly increased in cells transfected with WT or C242T p22phox, which confirmed the success of gene transfection (Figure 4A). Nox1 expression was nearly undetectable. We found that C242T p22phox reduced significantly Nox2 expression without significant effect on Nox4 or Nox2 regulatory subunits i.e. p47phox, p67phox, p40phox and Rac1/2 and Nox4 as compared to cells transfected with vector or WT p22phox (Figure 4A).
Figure 4. NADPH oxidase subunit expression, Nox2 maturation and binding to p22phox in HPMEC.
A) Western blots. Optical densities (OD) of protein bands of p22phox, Nox1, Nox2, Nox4, p47phox, p67phox, p40phox and Rac1/2 were quantified and normalized to the levels of α-tubulin (loading control) detected in the same samples. (B) Flow cytometry for the binding of Nox2 antibody (7D5). *p<0.05 for indicated values versus to vector values. †p<0.05 for indicated values versus WT values. (C) Left panel: p22phox was immunoprecipited (IP) followed by immunoblotting (IB) of Nox1, Nox2 and Nox4. Right panels: Optical densities (OD) of protein bands were quantified and normalized to the levels of total p22phox detected in the same samples. *p<0.05 for indicated values versus vehicle values under the same gene transfection. †P<0.05 for indicated values versus WT TNFα values. n=4 independent experiments.
Nox2 antibody 7D5 recognizes specifically an extracellular domain of human Nox2 and the levels of antibody 7D5 binding have been successfully used previously to indicate the levels of Nox2 maturation (cell surface membrane expression and stable cytochrome b formation) in cells27,29,30. Using flow cytometry, we found that TNFα increased significantly antibody 7D5 binding to endothelial cells, and C242T p22phox reduced significantly the antibody 7D5 binding at both basal and in response to TNFα stimulation as compared to cells transfected with vector or WT p22phox (Figure 4B). We then examined the effects of C242T p22phox on p22phox binding to Nox1, Nox2 and Nox4 by immuno-pull-down assay using p22phox antibody coated beads (Figure 4C). Supplemental Figure 3 showed the molecular weights on full gel presentation, and the protein levels detected in the whole cell homogenates were shown in Supplemental Figure 4. The levels of Nox1 pulled-down by p22phox were barely detectable, and we could not see a significant effect of C242T p22phox on Nox1. Under basal condition (vehicle stimulated), there was no significant difference in the levels of Nox2 or Nox4 pulled-down by p22phox beads between cells transfected with vector or WT or C242T p22phox (Figure 4C). TNFα stimulation (24 h) increased significantly the levels of Nox2 and reduced the levels of Nox4 pulled-down by p22phox beads in vector or WT p22phox transfected cells. However, in cells transfected with C242T p22phox, TNFα-induced Nox2 binding to p22phox was significantly reduced, while the levels of Nox4 binding to p22phox remained at vehicle levels as compared to vector or WT p22phox transfected cells. Taken together our results strongly suggested that p22phox C242T morphology inhibits specifically the interaction stability between Nox2 and p22phox and reduced TNFα-induced Nox2 activation in endothelial cells.
Effects of C242T p22phox on TNFα-induced p22phox/p47phox binding and redox signaling through MAPKs and NF-κB in endothelial cells
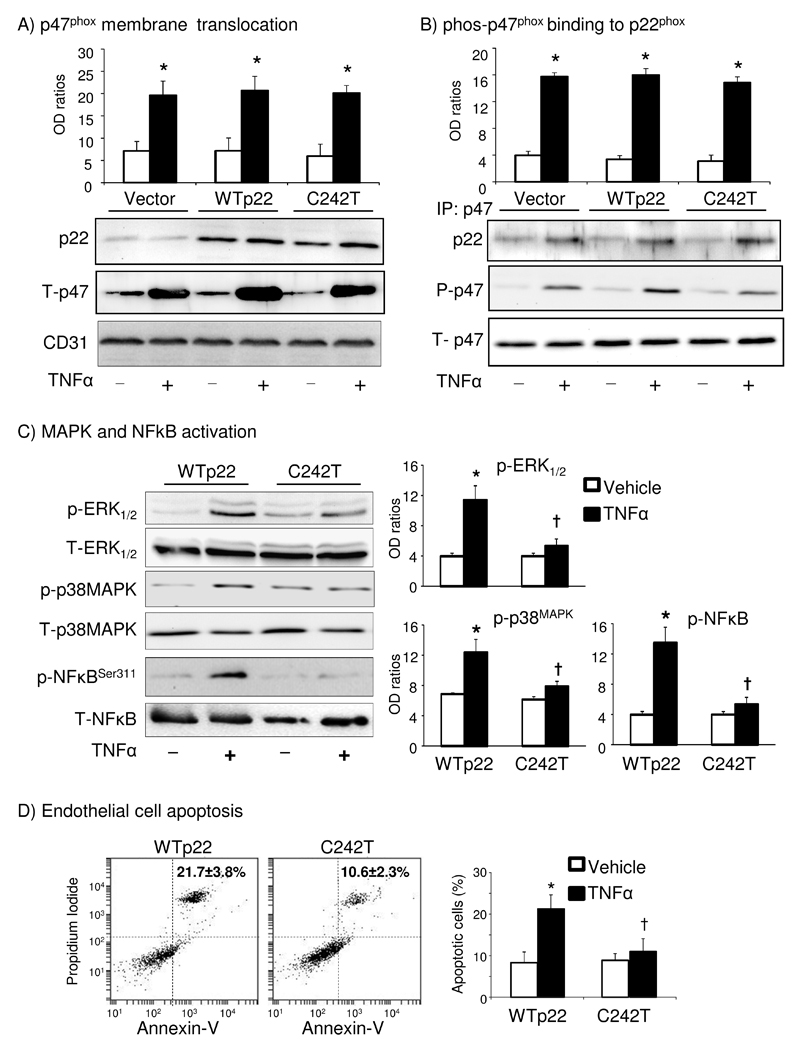
p22phox, through its intracellular C-terminal domain, anchors p47phox to Nox2 in the membrane to activate NADPH oxidase. In our computer model, C242T SNP was predicted not to affect p47phox membrane translocation and binding to p22phox. To confirm this, we prepared endothelial cell membrane fraction and examined for the membrane expression of p47phox by immunoblot (Figure 5A). Compared to vehicle control cells, TNFα stimulation significantly increased the levels of p47phox membrane expression without significant difference between vector and WT or C242T p22phox transfected cells. We then immuno-precipitated p47phox and examined the levels of p22phox pulled down by p47phox beads and the levels of p47phox phosphorylation (Figure 5B). Compared to vehicle control cells, TNFα stimulation increased the levels of p22phox/p47phox complex formation and p47phox phosphorylation without significant difference between cells transfected with vector and WT or C242T p22phox. Membrane ROS production was shown in the Supplemental Figure 5. Once again, our data confirmed that the C242T p22phox inhibition of TNFα-induced endothelial O2.- production is specific to the Nox2 catalytic subunit.
Figure 5. TNFα-induced p47phox membrane translocation and binding to p22phox, redox signaling and cell apoptosis in HMEC.
(A) Western blot. The optical densities (OD) of protein bands were quantified and normalized to the levels of CD31 (an endothelial membrane marker) detected in the same samples. (B) p47phox was immunoprecipitated (IP) and detected by Western blot for the presence of p22phox and p47phox phosphorylation (P-p47). The optical densities (OD) of protein bands were quantified and normalized to the levels of total p47phox (T-p47) detected in the same samples. (C) Western blots. The phospho-bands were quantified and normalized to the total levels of the same protein detected in the same samples. (D) Right panels: Annexin-V/propidium iodide detection of cell apoptosis (upper right square) by flow cytometry. Apoptotic cells were presented as a percentage of total cells (20,000). *p<0.05 for indicated value versus vehicle values under the same gene transfection. †p<0.05 for indicated values versus WTp22 TNFα values. n=4 independent experiments..
Next, we examined the effect of C242T p22phox on TNFα-induced redox activation of extracellular regulated kinase-1/2 (ERK1/2), p38MAPK and NFκB in endothelial cells (Figure 5C). Compared to vehicle treated cells, TNFα stimulation increased significantly the levels of ERK1/2, p38MAPK and NFκBSer311 phosphorylation in WT p22phox transfected cells (Figure 5C), and these were accompanied by significant increases in endothelial apoptosis detected by annexin-V flow cytometry (Figure 5D). Interestingly, these TNFα effects were significantly inhibited in cells transfected with C242T p22phox. The levels of JNK phosphorylation was very low with no significant changes being detected after TNFα stimulation (data not shown).
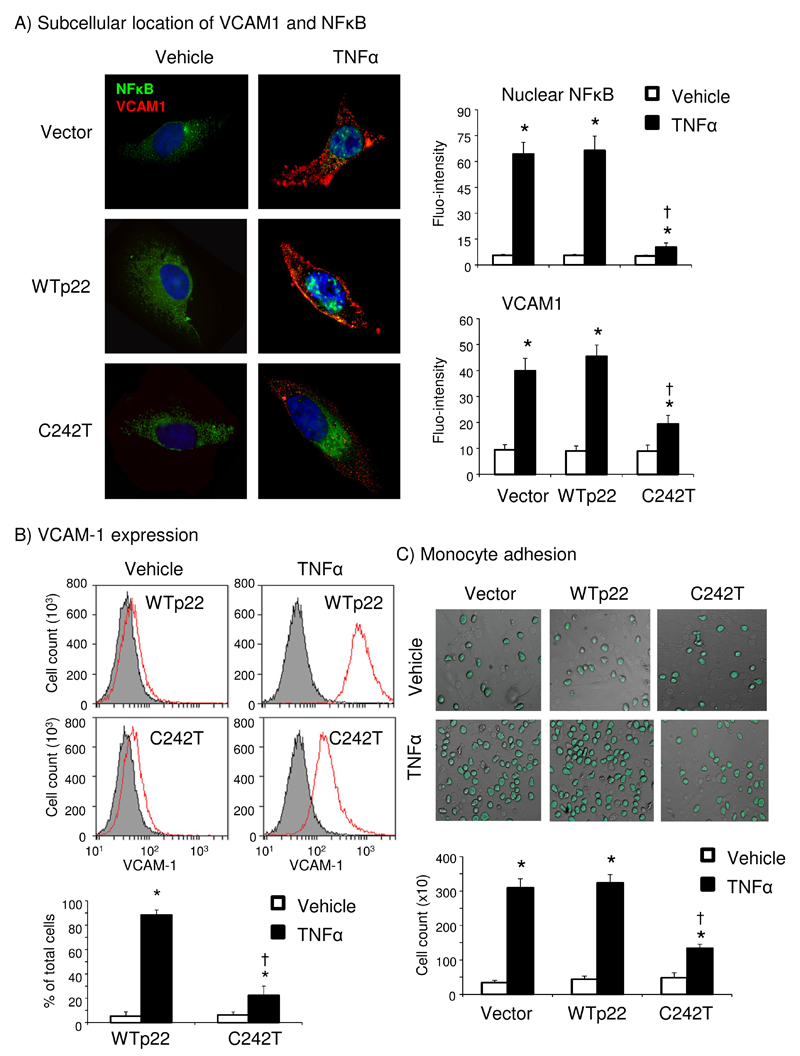
p22phox C242T inhibition of TNFα-induced endothelial vascular cell adhesion molecule-1 (VCAM-1) expression and inflammation
TNFα plays a key role in the pathogenesis of atherosclerosis and many other cardiovascular disorders. In order to explore the clinical significance of C242T p22phox in inhibiting inflammatory endothelial dysfunction, we examined TNFα (24 h)-induced endothelial expression of VCAM-1 and NFĸB nuclear translocation by immunofluorescence (Figure 6A). Compared to vehicle treated cells, TNFα increased significantly the levels of NFκB (green) in the nuclei and VCAM-1 expression (red) mainly around the plasma membrane in cells transfected with vector or WT p22phox, but not with C242T p22phox (Figure 6A). The inhibitory effect of p22phox C242T on TNFα-induced endothelial VCAM-1 expression was further confirmed by flow cytometry (Figure 6B).
Figure 6. TNFα-induced NFκB nuclear translocation, VCAM1 expression and monocyte adherence in HPMECs.
(A) Immunofluorescence microscopy detection of NFĸB (green) nuclear translocation and VCAM1 (red) expression. Nuclei were labelled with DAPI (blue) to visualize cells. The fluorescence intensity was quantified from 20 images/per group. n=3 independent experiments. (B) Flow cytometry detection of VCAM-1 expression. (C) U937 monocytes were labeled with FITC (green) and the number of monocytes adhering to the HPMEC monolayer was counted for quantification. *p<0.05 for indicated value versus vehicle values under the same gene transfection. †p<0.05 for indicated values versus WTp22 TNFα values. n=4 independent experiments (B-C) .
We examined also TNFα-induced monocyte adhesion to endothelial cells using FITC-labelled U937 cells, and found that 24 h of TNFα stimulation increased significantly the number of monocytes attached to the endothelial monolayer in vector or WT p22phox transfected HPMEC but not in C242T p22phox transfected cells (Figure 6C). The inhibitory effect of C242T p22phox on endothelial Nox2 activation was further confirmed using cells stimulated with high level of glucose (25 mmol/L) (Supplemental Figure 6)
Experiments using engineered p22phox-depl HeLa cells
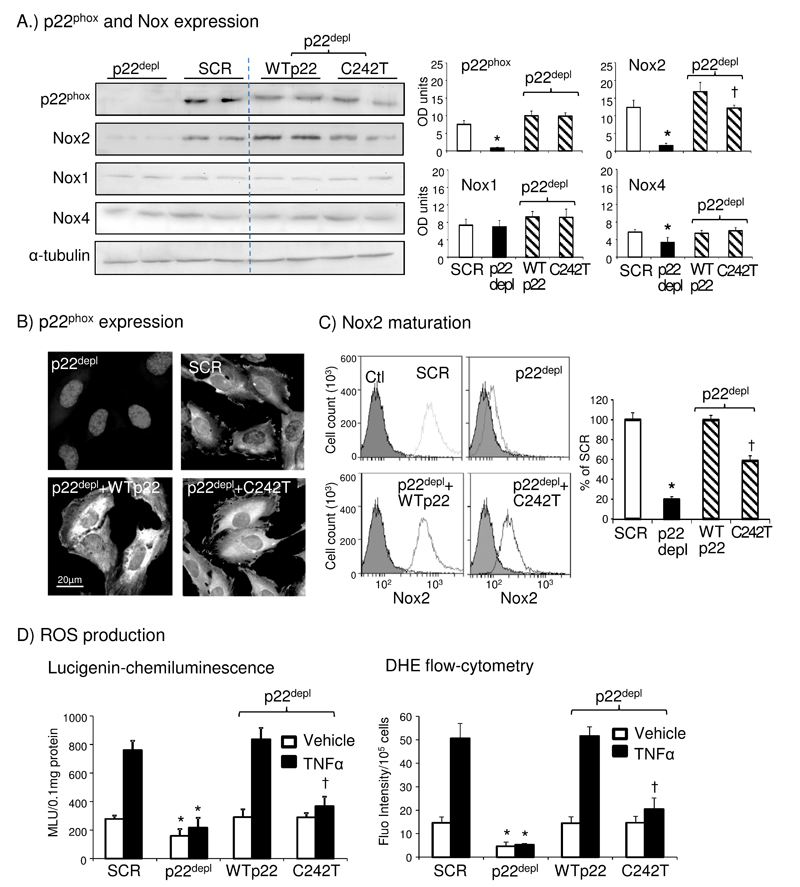
In order to confirm that our results of C242T p22phox would not be affected by the intrinsic p22phox protein expressed in HPMECs, we used engineered HeLa cells after p22phox depletion using a specific short-hairpin RNA (shRNA). A scrambled shRNA was used as controls in all experiments. The absence of p22phox protein in p22phox-depl cells was confirmed by immunoblotting (Figure 7A) and immunofluorescence (Figure 7B and Supplemental Figure 7). The p22phox protein was detected in scrambled shRNA treated cells and in p22phox-depl cells after gene transfection of WT or C242T p22phox (Figure 7A and B). Compared to scrambled shRNA treated control cells, the levels of both Nox2 and Nox4 were low in p22phox-depl cells, and increased to control levels after p22phox gene transfection. However, compared to WT p22phox transfected cells, the levels of Nox2 (but not Nox4) remained significantly lower in C242T p22phox transfected cells (Figure 7A). The levels of Nox1 expression was unaffected by the absence of p22phox. We then examined Nox2 maturation recognized by Nox2 antibody 7D5 and analyzed by flow cytometry (Figure 7C). Compared to scrambled shRNA control cells, p22phox-depl cells had significantly lower (19.5±2.8%) levels of Nox2 maturation, and this was restored to the control levels after WT p22phox transfection. However, the levels of Nox2 maturation remained significantly lower (58.7±2.3%) in C242T p22phox transfected cells.
Figure 7. Experiments using p22phoxdepl HeLa cells.
(A) Western blots. Optical densities (OD) of protein bands were quantified and normalized to the levels of α-tubulin detected in the same sample. (B) Immunofluorescence detection of p22phox expression. The color images and quantification of p22phox fluorescence intensities are presented in the Supplemental Figure 7. (C) Flow cytometry detection of anti-Nox2 antibody (7D5) binding to HeLa cells. Shaded area represents control cells without Nox2 antibody labelling. (D) O2.- production. SCR: scrambled shRNA. *p<0.05 for indicated values versus SCR values under the same treatment. †p<0.05 for indicated values versus WTp22 values under the same treatment. n=4 independent experiment.
The levels of O2.- production by these cells were examined by lucigenin chemiluminescence in cell homogenates (Figure 7D, left panel) and the tiron-inhibitable DHE fluorescence flow cytometry (Figure 7D, right panel). Compared to scrambled shRNA treated control cells, p22phox-depl cells had significantly lower levels of O2.- production under basal condition and lost completely the O2.- response to TNFα stimulation. TNFα-induced O2.- production was restored to the scrambled shRNA control levels after gene transfection of WT p22phox. However, gene transfection of C242T p22phox only restored the basal but not the TNFα-induced O2.- production (Figure 7D). Reduced interaction between C242T p22phox and Nox2 (but not Nox4) after TNFα stimulation was further confirmed in p22phox-depl cells (Supplemental Figure 8).
Experiments using Nox2-/- coronary microvascular endothelial cells (CMEC)
In order to further confirm if C242T p22phox affected only Nox2 (but not Nox4 and/or Nox1), we isolated primary CMEC from wild-type and Nox2 knockout mice. Cells were then subjected to gene transfection and examined for O2.- production with or without TNFα stimulation (Supplemental Figure 9). Under basal conditions, Nox2-/- cells produced less O2.- compared to WT cells without significant differences between cells transfected with vector or WT p22phox or C242T p22phox. TNFα stimulation greatly increased the levels of O2.- production in WT CMEC transfected with vector or WT p22phox. Compared to WT p22phox transfected cells, p22phox C242T inhibited significantly the levels of TNFα-induced O2.- production (40±14% reduction) (Supplemental Figure 9). In contrast, TNFα increased only slightly (but still statistically significant) the levels of O2.- production by Nox2-/- CMEC and there was no significant difference between cells transfected with vector, WT or C242T p22phox. It was clear that C242T p22phox had no significant effect on TNFα-induced endothelial O2.- production in the absence of Nox2.
C242T SNP frequency and inhibition of Nox2 expression and high-glucose induced ROS production in human saphenous veins
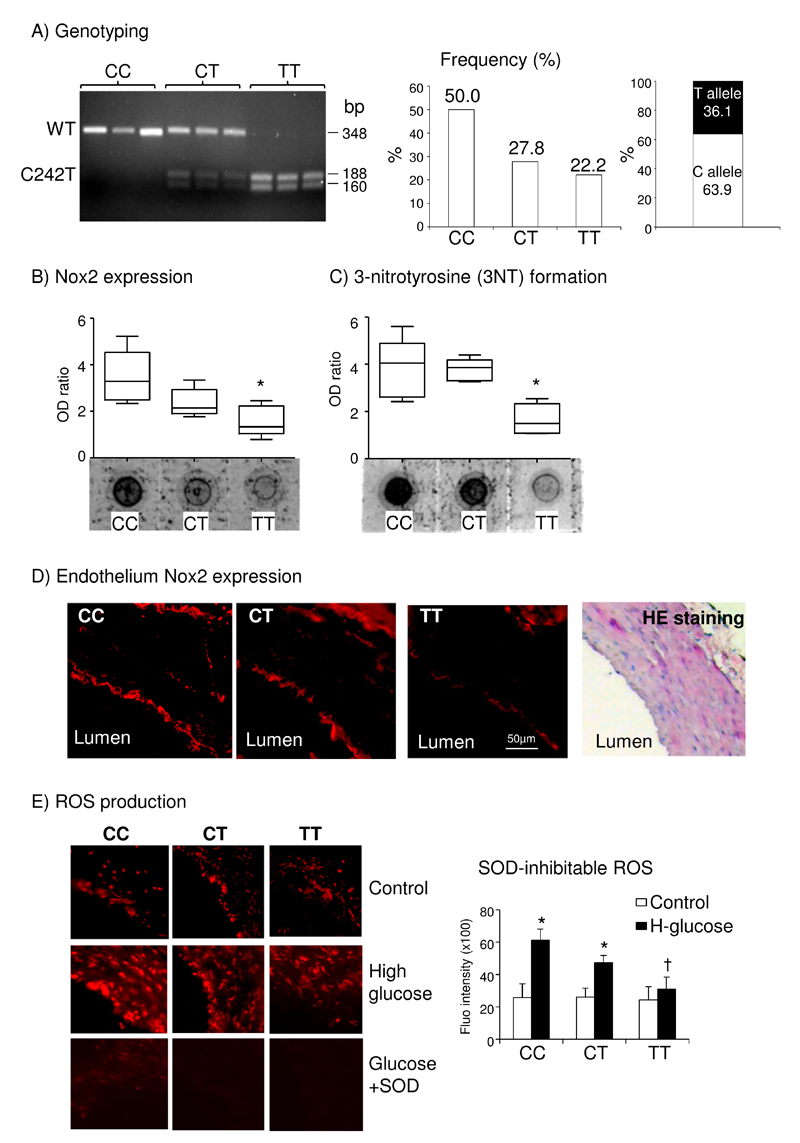
In order to investigate the clinical significance, we genotyped 36 saphenous vein samples collected from patients after phlebectomies who had no history of coronary heart disease. We found that 50% samples were wild-type (CC), ~27.8% were heterozygotes (CT) and ~22.2% had p22phox C242T SNP (TT) with ~36.1% frequency of T allele appearance (Figure 8A). Dot-blot showed a significant reduction of Nox2 expression in TT vessels in comparison to CC vessels (Figure 8B). Immunofluorescence images revealed that the reduction of Nox2 was mainly in the endothelium (Figure 8D). It is well known that superoxide (O2.-) reacts with nitric oxide (NO) to form peroxynitrite which modifies tyrosine residue to form 3-nitrotyrosine (3NT), a biomarker of tissue oxidative damage. We found significantly lower levels of 3-nitrotyrosine (3NT) detected in TT samples as compared to CC samples (Figure 8C). When the vessels were challenged ex vivo with high level of glucose (25 mmol/L) for 24 h, TT samples had significantly less ROS production in comparison to CC sample (Figure 8E). Superoxidase dismutase was used to confirm the detection of O2.-.
Figure 8. The effects of C242T p22phox SNP on Nox2 expression and high-glucose induced ROS production in human saphenous veins.
A) Genotyping of C242T p22phox SNP (left panel) and calculated frequency in a total of 36 samples. WT p22phox appeared as a single band of 348 bp and C242T SNP had two bands at 188 and 160 bp. B) Nox2 expression detected by dot-blotting. C) 3NT formation detected by dot-blotting. *p<0.05 for indicated values versus CC values. D) Nox2 (red color) expression detected by Immunofluorescence. Lumen was labeled for the location of the endothelium. Vessel structure was shown by H&E staining (right panel). E) Left panels: Representative images of vessel ROS production with or without high-glucose (H-glucose) challenge (24 h) detected by DHE fluorescence on vessel sections. Superoxide dismutase (SOD) was used to confirm the detection of O2.-. *p<0.05 for indicated values versus control values in the same genetic group. †p<0.05 for indicated values versus CT values under H-glucose.
Discussion
The NADPH oxidase, by generating O2.-, plays a crucial role in the inflammatory response involved in the pathogenesis of many cardiovascular diseases. The p22phox is an integral membrane-associated subunit of NADPH oxidase responsible for assembling and stabilising Nox subunits into an active enzyme complex for ROS production8. A number of clinical studies have reported that individuals bearing a naturally-occurring p22phox C242T SNP have diminished O2.- production and are less susceptible to inflammatory cardiovascular diseases11,23,31,32. However, little progress has been made to clarify the underlying molecular mechanisms. For the first time, our study provides scientific evidence that p22phox C242T SNP causes p22phox structural changes in the extracellular domain that are unfavorable for Nox2 maturation and activation, and inhibits endothelial O2.- production in response to TNFα or high glucose stimulation.
A number of findings have been made by the current study in understanding the mechanism of p22phox C242T SNP inhibition of Nox2 activation: 1) There is a significant structural difference between WT and the C242T p22phox protein extracellular region that is important for Nox2 maturation12,33; 2) C242T p22phox structure compromises its binding stability with Nox2. The complex formed between Nox2 and C242T p22phox is less stable due to the lacking of crucial hydrogen bonds to bind them together. Unstable binding may led to Nox2 protein degradation and this may explain the low levels of Nox2 detected in C242T p22phox transfected cells and in vein samples of TT individuals.
The C242T SNP does not affect Nox1 because the Arg226 (crucial for the hydrogen bond formation to stabilize the interaction between Nox2 and WT p22phox) is replaced by Gly in Nox1. Furthermore, the putative epitope 1 (160-IKNP-163) that is required to interact with p22phox27 is absent in Nox1 (Supplemental Table 1). Nevertheless, Nox1 expression is extremely low in endothelial cells and is not a major source of endothelial ROS production in inflammation. Nox4 is highly expressed in endothelial cells. Nox4 generates H2O2 and has been found to be involved mainly in cellular growth and normal function, and is protective to cardiovascular function34,35. Using immunoblotting, real-time PCR (Supplemental Figure 10) and co-immunoprecipitation we have shown that C242T p22phox does not inhibit Nox4 expression or its complex formation with p22phox. Furthermore, using HEK293 cells that do not express endogenous Nox4, we have demonstrated that (after Nox4 gene transfection) C242T p22phox has no effect on Nox4 activity (Supplemental Figure 2). Under the basal condition (without TNFα), C242T p22phox has no effect on endothelial Nox2 mRNA expression (Supplemental Figure 10) but reduces the protein levels of Nox2 expression and Nox2 maturation. These results strongly suggest that the effect of C242T p22phox on Nox2 expression is post-translational. When the cells were challenged by TNFα, the inhibitory effect of C242T p22phox on TNFα-induced Nox2 mRNA expression (Supplemental Figure 10) is more likely due to reduced levels of oxidative stress and redox signaling (such as NFκB) that impair Nox2 transcription in C242T p22phox transfected cells.
Nox2 has low basal activity in endothelial cells and generates a small quantity of O2.- mainly used for redox-signaling under physiological conditions. Another important finding by the current study is that C242T p22phox configuration can still allow Nox2 to bind and support a low basal level of O2.- production. However, when the cells face challenge by TNFα C242T p22phox could not support a full Nox2 activation and thereby inhibits endothelial oxidative response to TNFα and protects endothelial cells from inflammation and cell apoptosis. The inhibitory effect of C242T p22phox on TNFα-induced endothelial ROS production by Nox2 was examined using four independent complementary methods: lucigenin-chemiluminecscence; DHE-HPLC; DHE-flow cytometry and DCF fluorescence microscopy and further confirmed by experiments using Nox2ds-tat, several enzyme inhibitors and Nox2-/- CMEC.
Previously, C242T SNP was reported to form a haplotype correlated with two other p22phox SNPs: namely the C521T (rs1049254) and A24G (rs1049255) that reduced Nox2-activity in Epstein-Barr virus-transformed B-lymphocytes isolated from 50 healthy individuals of an Utah pedigree33. However, our HapMap analysis could not identify a close correlation of C242T with any other SNPs of p22phox currently identified within the HapMap database. Our results strongly suggest that the functional effects of C242T SNP on NADPH oxidase are independent of any other known protein-translated p22phox SNPs.
Using engineered p22phox-depl HeLa cells we further demonstrated that in the absence of p22phox, both Nox2 and Nox4 expressions were reduced, and could be restored after gene transfection of WT p22phox. However, gene transfection of C242T p22phox into p22phox-depl cells only fully restored the expression of Nox4 (but not Nox2). When cells were subjected to TNFα challenge, C242T p22phox transfected cells failed to increase O2.- production. C242T p22phox had no significant effect on its interaction with p47phox or on TNFα-induced p47phox phosphorylation. However, due to the inhibitory effect of C242T p22phox on Nox2 activation, TNFα-induced ROS production and redox-signaling through MAPK and NFκB were compromised in endothelial cells, which protected endothelial cells from oxidative damage and inflammation.
Increased VCAM-1 expression and monocyte adherence to endothelial cells are crucial steps in the pathogenesis of inflammatory cardiovascular diseases. The clinical significance of C242T p22phox was demonstrated by showing that TNFα-induced endothelial VCAM-1 expression and monocyte adherence to endothelial cells were significantly inhibited in C242T p22phox transfected cells. Saphenous veins are commonly used as conduits for bypass surgery to treat coronary artery diseases. Information on the p22phox genetic variation can predict graft oxidative response to environment challenge and help clinical management of vein grafts. We found a genetic frequency of 22.2% for TT genotype (36.1% for T allele) in our samples, which is in accordance to those reported previously for control samples or for the general population36–38. Using both dot-blotting and immunofluorescence, we confirmed that p22phox C242T SNP is indeed associated with reduced Nox2 expression mainly in the endothelium, and TT vessels had significantly less ROS generation in response to high glucose challenge as compared to CC vessels.
Limitations need to be considered in the interpretation of our p22phox molecular models. Although our computer models are supported by extensive cell and molecular experimental data, the structural models still need to be confirmed by X-ray crystallography or nuclear magnetic resonance spectroscopy.
In summary, this is the first report to describe the mechanism of p22phox C242T SNP in inhibiting Nox2 activity and protecting endothelial cells from TNFα-induced oxidative damage and inflammation. p22phox C242T SNP represents a natural protective mechanism against inflammatory cardiovascular diseases. The molecular mechanism reported here can be further explored for novel drug development.
Supplementary Material
Clinical Perspective.
NADPH oxidase, by generating reactive oxygen species, is involved in the pathophysiology of many cardiovascular diseases and represents a therapeutic target for the development of novel drugs. A number of clinic studies have reported that individuals bearing a naturally-occurring p22phox C242T single-nucleotide polymorphism (SNP) have diminished ROS production in the cardiovascular system and are less susceptible to inflammatory cardiovascular diseases. However, little progress has been made to clarify the underlying molecular mechanisms. The current study using computer molecular modelling plus cell and molecular techniques provides scientific evidence that p22phox C242T SNP causes p22phox structural changes in the extracellular domain that compromises its binding stability with Nox2 (the catalytic subunit of the NADPH oxidase) and reduces the levels of Nox2 protein expression and maturation. In response to TNFα challenge, C242T p22phox inhibits endothelial NADPH oxidase O2.- production, inflammatory VCAM-1 expression and monocyte adherence to endothelial cells. Clinical significance was investigated using saphenous vein segments from non-coronary heart disease subjects after phlebectomies. TT (C242T) allele was common (prevalence of ~22%) and compared to CC, veins bearing TT allele had significantly lower levels of Nox2 expression and O2.- generation in response to high glucose challenge. C242T SNP may represent a natural protective mechanism against inflammatory cardiovascular diseases. The molecular mechanism reported in this study can be further explored for novel drug development.
Funding sources
This work was funded by the British Heart Foundation (PG/14/85/31161) and the Wellcome Trust (Project Grant 07863/Z/05/Z).
Footnotes
Journal Subject Terms: Genetic, Association Studies; Cell Biology/Structural Biology; Oxidant Stress.
The authors declare no completing financial interests.
Disclosures: None.
References
- (1).Shastry BS. SNP alleles in human disease and evolution. J Hum Genet. 2002;47:561–6. doi: 10.1007/s100380200086. [DOI] [PubMed] [Google Scholar]
- (2).Thanassoulis G, Vasan RS. Genetic cardiovascular risk prediction. Will we get there? Circulation. 2010;122:2323–34. doi: 10.1161/CIRCULATIONAHA.109.909309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lassègue B, Martin AS, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidase in the cardiovascular system. Circ Res. 2012;110:1364–90. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fan LM, Douglas G, Bendall JK, McNeill E, Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, Choudhury RP, et al. Endothelial Cell-Specific ROS Production Increases Susceptibility to Aortic Dissection. Circulation. 2014;129:2661–72. doi: 10.1161/CIRCULATIONAHA.113.005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Du J, Fan LM, Mai A, Li J-M. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptor and endothelium in dietary obesity of middle-aged mice. Br J Pharmacol. 2013;170:1064–77. doi: 10.1111/bph.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Li J-M, Fan LM, George VT, Brooks G. Nox2 regulates endothelial cell cycle arrest and apoptosis via p21cip1 and p53. Free Radic Biol Med. 2007;43:976–86. doi: 10.1016/j.freeradbiomed.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Li J-M, Shah AM. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- (8).Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosome location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86:1729–37. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Meijles D, Howlin BJ, Li J-M. Consensus in silico computational modelling of the p22phox subunit of the NADPH oxidase. Comput Biol Chem. 2012;39:6–13. doi: 10.1016/j.compbiolchem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- (10).Meijles DN, Fan LM, Howlin BJ, Li JM. Molecular insights of p47phox phosphorylation dynamics in the regulation of NADPH oxidase activation and superoxide production. J Biol Chem. 2014;289:22759–70. doi: 10.1074/jbc.M114.561159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).San José G, Fortuño A, Beloqui O, Diez J, Zalba G. NADPH oxidase CYBA polymorphisms, oxidative stress and cardiovascular diseases. Clin Sci. 2008;114:173–82. doi: 10.1042/CS20070130. [DOI] [PubMed] [Google Scholar]
- (12).Zhu Y, Marchal CC, Casbon AJ, Stull N, von LK, Knaus UG, Jesaitis AJ, McCormick S, Nauseef WM, Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem. 2006;281:30336–46. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
- (13).Soccio M, Toniato E, Evangelista V, Carluccio M, De Caterina R. Oxidatrive stress and cardiovascular risk: the role of vascular NAD(P)H oxidase and its genetic variants. Eur J Clin Invest. 2005;35:305–14. doi: 10.1111/j.1365-2362.2005.01500.x. [DOI] [PubMed] [Google Scholar]
- (14).Jones LC, Hingorani AD. Genetic regulation of endothelial function. Heart. 2005;91:1275–7. doi: 10.1136/hrt.2005.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wythe KE, Wang S, Griendling KK, Dikalov SI, Austin H, Rao S, Fink B, Harrison DG, Zafari AM. C242T CYBA polymorphism of the NADPH oxidase is associated with reduced respiratory burst in human neutrophils. Hypertension. 2004;43:1246–51. doi: 10.1161/01.HYP.0000126579.50711.62. [DOI] [PubMed] [Google Scholar]
- (16).Zafari AM, Davidoff MN, Austin H, Valppu L, Cotsonis G, Lassegue B, Griendling KK. The A640G and C242T p22(phox) polymorphisms in patients with coronary artery disease. Antioxid Redox Signal. 2002;4:675–80. doi: 10.1089/15230860260220184. [DOI] [PubMed] [Google Scholar]
- (17).Cahilly C, Ballantyne CM, Lim D-S, Gotto A, Marian AJ. A variant of p22, involved in generation of reactive oxygen species in the vessel wall, is associated with progression of coronary atherosclerosis. Circ Res. 2000;86:391–5. doi: 10.1161/01.res.86.4.391. [DOI] [PubMed] [Google Scholar]
- (18).Nasti S, Spallarossa P, Altieri P, Garibaldi S, Fabbi P, Polito L, Bacino L, Brunelli C, Barsotti A, Ghigliotti G. C242T polymorphism in CYBA gene (p22phox) and risk of coronary artery disease in a population of Caucasian Italians. Dis Markers. 2006;22:167–73. doi: 10.1155/2006/458587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: Comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvascular Res. 2002;64:384–97. doi: 10.1006/mvre.2002.2434. [DOI] [PubMed] [Google Scholar]
- (20).Li J-M, Mullen AM, Shah AM. Phenotypic properties and characteristics of superoxide production by mouse coronary microvascular endothelial cells. J Mol Cell Cardiol. 2001;33:1119–31. doi: 10.1006/jmcc.2001.1372. [DOI] [PubMed] [Google Scholar]
- (21).Fan LM, Teng L, Li J-M. Knockout of p47phox uncovers a critical role of p40phox in reactive oxygen species production in microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:1651–6. doi: 10.1161/ATVBAHA.109.191502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermohlen O, Bruning JC, et al. Roboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–63. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- (23).Guzik TJ, West NEJ, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation. 2000;102:1744–7. doi: 10.1161/01.cir.102.15.1744. [DOI] [PubMed] [Google Scholar]
- (24).Fan LM, Li JM. Evaluation of methods of detecting cell reactive oxygen species production for drug screening and cell cycle studies. J Pharmacol Toxicol Methods. 2014;70:40–7. doi: 10.1016/j.vascn.2014.03.173. [DOI] [PubMed] [Google Scholar]
- (25).Teng L, Fan LM, Meijles D, Li J-M. Divergent effects of p47phox phosphorylation at S303-4 or S379 on tumor necrosis factor-α signaling via TRAF4 and MAPK in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1488–96. doi: 10.1161/ATVBAHA.112.247775. [DOI] [PubMed] [Google Scholar]
- (26).Campion Y, Jesaitis AJ, Nguyen MVC, Grichine A, Herenger Y, Baillet A, Berthier S, Morel F, Paclet M-H. New p22-phox monoclonal antobodies: Identification of a conformational probe for cytochromeb558. J Innate Immun. 2009;1:556–69. doi: 10.1159/000231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Burritt JB, Deleo FR, McDonald CL, Prigge JR, Dinauer MC, Nakamura M, Nauseef WM, Jesaitis AJ. Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J Biol Chem. 2001;276:2053–61. doi: 10.1074/jbc.M006236200. [DOI] [PubMed] [Google Scholar]
- (28).Csanyi G, Cifuentes-Pagano E, Al GI, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–25. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yamauchi A, Yu L, Potgens AJ, Kuribayashi F, Nunoi H, Kanegasaki S, Roos D, Malech HL, Dinauer MC, Nakamura M. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol Immunol. 2001;45:249–57. doi: 10.1111/j.1348-0421.2001.tb02614.x. [DOI] [PubMed] [Google Scholar]
- (30).Taylor RM, Dratz EA, Jesaitis AJ. Invariant local conformation in p22phox p.Y72H polymorphisms suggested by mass spectral analysis of crosslinked human neutrophil flavocytochrome b. Biochimie. 2011;93:1502–9. doi: 10.1016/j.biochi.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Inoue N, Kawashima S, Yamada K, Akita H, Yokoyama M. Polymorphism of the NADH/NADPH oxidase p22 phox gene in patients with coronary artery disease. Circulation. 1998;97:135–7. doi: 10.1161/01.cir.97.2.135. [DOI] [PubMed] [Google Scholar]
- (32).Schächinger V, Britten MB, Dimmeler S, Zeiher AM. NADH/NADPH oxidase p22 phox gene polymorphism is associated with improved coronary endothelial vascular function. Eur Heart J. 2001;22:96–101. doi: 10.1053/euhj.2000.2123. [DOI] [PubMed] [Google Scholar]
- (33).Bedard K, Attar H, Bonnefont J, Jaquet V, Borel C, Plastre O, Stasia M-J, Antonarakis SE, Krause K-H. Three common polymorphisms in the CYBA gene from a haplotype associated with decreased ROS generation. Hum Mutat. 2009;30:1123–33. doi: 10.1002/humu.21029. [DOI] [PubMed] [Google Scholar]
- (34).Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, et al. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. International J Obesity. 2012;36:1503–13. doi: 10.1038/ijo.2011.279. [DOI] [PubMed] [Google Scholar]
- (35).Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is reguired for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).de Oliveira Alvim R, Santos PC, Dias RG, Rodrigues MV, de Sa Cunha R, Mill JG, Junior WN, Krieger JE, Pereira AC. Association between the C242T polymorphism in the p22phox gene with arterial stiffness in the Brazilian population. Physiol Genomics. 2012;44:587–92. doi: 10.1152/physiolgenomics.00122.2011. [DOI] [PubMed] [Google Scholar]
- (37).Najafi M, Alipoor B, Shabani M, Amirfarhangi A, Ghasemi H. Association between rs4673 (C/T) and rs13306294 (A/G) haplotypes of NAD(P)H oxidase p22phox gene and severity of stenosis in coronary arteries. Gene. 2012;499:213–7. doi: 10.1016/j.gene.2012.02.032. [DOI] [PubMed] [Google Scholar]
- (38).Xu Q, Yuan F, Shen X, Wen H, Li W, Cheng B, Wu J. Polymorphisms of C242T and A640G in CYBA gene and the risk of coronary artery disease: a meta-analysis. PLoS One. 2014;9:e84251. doi: 10.1371/journal.pone.0084251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.