Abstract
Increasing CO2 atmospheric levels lead to increasing ocean acidification, thereby enhancing calcium carbonate dissolution of calcifying species. We gathered peer-reviewed experimental data on the effects of acidified seawater on calcifying species growth, reproduction, and survival. The data were used to derive species-specific median effective concentrations, i.e., pH50, and pH10, via logistic regression. Subsequently, we developed species sensitivity distributions (SSDs) to assess the potentially affected fraction (PAF) of species exposed to pH declines. Effects on species growth were observed at higher pH than those on species reproduction (mean pH10 was 7.73 vs 7.63 and mean pH50 was 7.28 vs 7.11 for the two life processes, respectively) and the variability in the sensitivity of species increased with increasing number of species available for the PAF (pH10 standard deviation was 0.20, 0.21, and 0.33 for survival, reproduction, and growth, respectively). The SSDs were then applied to two climate change scenarios to estimate the increase in PAF (ΔPAF) by future ocean acidification. In a high CO2 emission scenario, ΔPAF was 3 to 10% (for pH50) and 21 to 32% (for pH10). In a low emission scenario, ΔPAF was 1 to 4% (for pH50) and 7 to 12% (for pH10). Our SSDs developed for the effect of decreasing ocean pH on calcifying marine species assemblages can also be used for comparison with other environmental stressors.
Introduction
Increase in atmospheric carbon levels has led to increasing uptake of CO2 by oceans.1 This process, characterized by enhancement of dissolved CO2 levels and the decrease in ocean pH, leads also to amplification of carbonate dissolution in oceans.2 The building of shells and skeletons of calcifying species is favored by oversaturated carbonate minerals in oceans.3,4 Thereby, changes in carbonate chemistry following ocean acidification may pose a particular threat to these species.5 Since the process of calcification is observed in a wide variety of taxa (including phytoplankton, corals, and crustaceans), detrimental effects of ocean acidification may result in a decline of species richness and the various ecosystem services those species provide, including nursery for fish and protection against erosion and storms by corals, fisheries of urchins and invertebrates, and food provisioning for predators.3
Many laboratory experiments assessed the effects of ocean acidification on individual species, which allowed for various metaanalyses of the effects of acidification on marine species.6–9 Those studies have shown that acidification effects on marine species, particularly on the calcifying ones, are mainly detrimental,8 although the responses of individual species are far from uniform.7
In environmental risk assessments, species-specific responses to environmental stressors can be used in the derivation of the so-called species sensitivity distributions (SSDs). They are probabilistic models of the interspecies variation to a specific stressor, where a statistical function illustrates the increasing fraction of species affected by increasing levels of the stressor.10,11 They are commonly applied in risk assessment of toxicants, such as metals or pesticides, but recently they have also been developed for marine species with respect to increasing CO2 exposure.12,13 They are particularly useful for (1) the estimation of the overall response (and the variability in responses) of an assemblage of species in the environment and (2) the derivation of acceptable or “safe” levels of a stressor for the protection of the environment.10 Despite recent developments in the risk assessment of ocean acidification,12,13 probabilistic models related to pH declines (a commonly monitored indicator of ocean acidity) have not yet been reported.
Here, we developed species sensitivity distributions (SSDs) based on three life processes (i.e., growth, reproduction, and survival) and apply the SSDs to two global climate change scenarios of the Intergovernmental Panel on Climate Change, IPCC.14 Growth, reproduction, and survival are often indicators of the maintenance of species populations in the environment as they reflect the performance of species at important life processes.15
Material and Methods
Data Inventory
We collected experimental data of ocean acidification effects on calcifying species from literature studies. Those studies were listed in previous meta-analyses7–9 and were complemented by a keyword search of studies published afterward and until September 2012 (see keyword selection in appendix S1 of the Supporting Information, SI). We only included experiments where the taxonomic level of the tested organism was species and where the total scale pH (hereafter described as pH) was manipulated by changing CO2 partial pressure (pCO2). In experiments where pCO2 but not pH was reported, we converted pCO2 to pH (see conversion in SI Appendix S2). The reported responses in each study were categorized into one of three life processes: growth, reproduction, and survival. Responses allocated to growth included calcification and growth rates (e.g., as mass of calcium carbonate or body size increase per time), body size, or body weight; to reproduction, they included fertilization success, percentage of normal larvae, and hatching rates; and to survival, they included mortality and survival rates. We excluded responses reporting metabolic changes (for example, O2 productivity, and photosynthetic rate or amount of RuBisCO) since they could not be directly related to any of the three life processes. If multiple responses, different conditions (for example, duration or temperature of the experiment), different tested organisms (for example, different generations or life stages) were reported by a single study, then the experiments of the study were kept separate. This resulted in more experiments than studies. Finally, we recorded the species response and the pH associated with that response. In each experiment, we excluded pH levels which were above 8.35. Also, we excluded experiments where the highest tested pH level was lower than 7.95 since those conditions do not represent contemporary but past or future ocean pH levels.2,16
Derivation of pH50 and pH10
First, we adjusted the responses reported in each experiment to empirical relative responses17 as follows:
| (1a) |
where eRRi, Ri, and Rr are the relative response at pH i and the reported responses at pH i and at the reference situation (i.e., the highest reported pH level), respectively. For example, the relative response of growth rate at pH i is 0.5 if Ri and Rr are given as calcification rates of 500 and 1000 mg CaCO3·day−1, respectively. The equation was modified if the reported response did not promote but reduced growth, reproduction, or survival (for example, rate of mortality or weight loss). In such cases, eRRi was calculated as follows:
| (1b) |
Second, we employed eRRi and the corresponding pHi to fit a logistic regression where the calculated relative response in each experiment was as follows:
| (2) |
where pH50 is the pH causing an effect of 50% to a given life process of a species (i.e., growth, reproduction, or survival) and β is slope of the logistic function. For each experiment, the effect pH declines on the species was then recorded as detrimental (if β < 0 at a 95% confidence level), beneficial (if β > 0), or uncertain (where β was nondifferent from zero or if the p value of the regression was higher than 0.05) using SAS 9.2. Only experiments with 3 or more RRi and pH conditions were included since the logistic function describe in eq 2 has 2 parameters (i.e., pH50 and β) and requires 1 degree of freedom.
For detrimental effects, we proceeded by calculating the pH causing an effect of 10% to a life process of a species as follows:
| (3) |
If multiple pH50 and pH10 results were available for a given life process of a species, then the highest value of pH50 and pH10 was employed for the derivation of the SSD. This is a conservative approach since the effect on a species is given at the highest reported pH value. We tested if the pH50 and pH10 results employed in the SSD were correlated with the duration and the temperature at which the experiment was conducted using Pearson correlation (SAS 9.2).
Species Sensitivity Distributions
For each life process (i.e., growth, reproduction, and survival) and for each effect concentration (i.e., pH50 and pH10, hereafter defined as severe and subtle effects, respectively), the SSD was constructed using a cumulative normal distribution function described as the potentially affected fraction of species (PAF). The PAF at pH i was given as follows:
| (4) |
where μ and σ are the average and standard deviation of species-specific pH50 or pH10 value. We determined the statistical uncertainty around the PAF along the pH gradient using a Monte Carlo exercise (10 000 simulations) using Oracle Crystal Ball. This was attained by first determining the uncertainty around μ and σ, which was executed following the procedure described by Roelofs et al.18 In this procedure, the uncertainty around the μ and σ coefficients is augmented for SSDs encompassing fewer species.
Climate Change Scenarios
Two climate change scenarios proposed by the Special Report on Emissions Scenarios, SRES14 were employed in order to estimate the fraction of species potentially affected by future ocean acidification. The scenarios B1 and A2 describe, respectively, low and high greenhouse gas emission scenarios and differing with respect to social-economic and technological developments expected in the future.14 In addition to the decline of 0.1 pH unit, which has already occurred since the industrial revolution,19 current global average pH at the ocean’s surface is roughly 8.1.20 Under the B1 and A2 climate change scenarios, pH is projected to be roughly 7.95 and 7.8 by 2100.20
The change in PAF was calculated for each life process and effect concentration for future scenarios S as follows:
| (5) |
where PAF0 is the current PAF, and PAFS is the PAF at pH projected under global climate change scenario S. The uncertainty in ΔPAF results was calculated as the range in 5th and 95th in a Monte Carlo simulation.
Results
Species Sensitivity Distributions
Our data inventory included 72 studies and 346 experiments, of which 73 logistic regressions showed detrimental effects of ocean acidification on growth (40 in total), on reproduction (25 in total), and on survival (8 in total) while the remaining experiments did not show a significant logistic distribution (at a 95% confidence), SI Table S3.1. SI Appendix S3 provides further details of the logistic regression results per experiment.
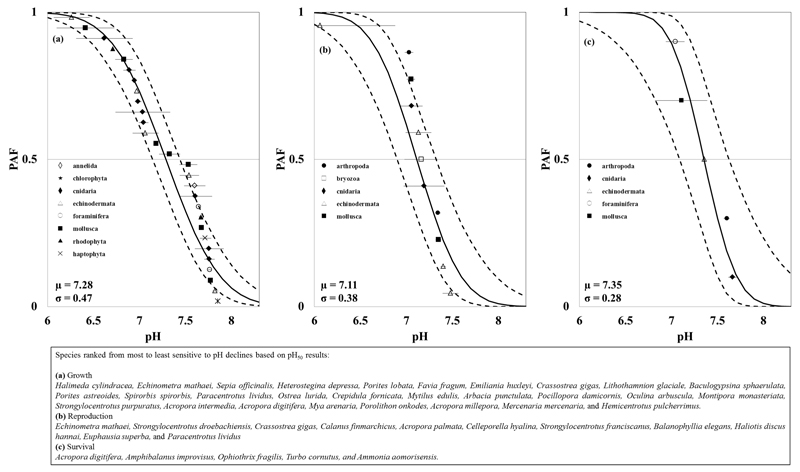
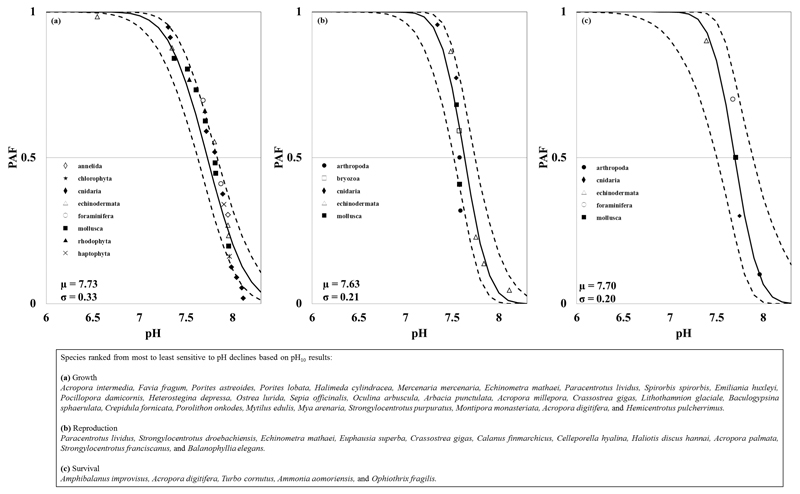
A total of 82 species were included in our data set, in which 40 species had detrimental effects of ocean pH declines confirmed in our logistic regressions. The PAF functions for growth, reproduction, and survival comprised a total of 28, 11, and 5 calcifying species, respectively, belonging to 10 different phyla. Figure 1 shows that the pH at which 50% of the calcifying species have a severe effect (described by the pH50) varied between 7.11 and 7.35, depending on the life process. Likewise, the pH at which 50% of the species show a subtle effect (described by the pH10) varied from 7.63 to 7.73, Figure 2. The variability in both pH50 and pH10 results was higher for effects on growth, followed by effects on reproduction and survival (σgrowth > σreproduction > σsurvival).
Figure 1.
Potentially affected fraction (PAF, continuous line) using pH50 values for (a) growth, (b) reproduction, and (c) survival. The error bars illustrate the 95% confidence intervals around the pH50 determined with the logistic regression and the μ and σ are the mean and standard deviation of pH50 used in the derivation of the PAF. The bottom right box shows the rankings of species sensitivity to pH declines.
Figure 2.
Potentially affected fraction (PAF, continuous line) using pH10 values for (a) growth, (b) reproduction, and (c) survival. The μ and σ are the mean and standard deviation of pH10 used in the derivation of the PAF. The bottom right box shows the rankings of species sensitivity to pH declines.
The corals Acropora intermedia, Favia fragum, and Porites astreoides shows the highest pH10 related to growth effects (indicating high sensitivity to pH declines) while the sea urchin Hemicentrotus pulcherrimus and the coral Acropora digitifera were the least sensitive to pH declines. The chlorophyte Halimeda cylindracea and the sea urchin Echinometra mathaei showed the highest pH50 related to growth effects whereas the bivalve Mercenaria mercenaria and the sea urchin Hemicentrotus pulcherrimus were the least sensitive to pH declines.
The duration of experiments ranged from less than 1 day (especially in reproduction experiments testing egg hatching success, for example) to 320 days while ocean water temperatures ranged from –1.9 to 34 °C (SI Table S3.2). However, we found no significant correlation between duration or temperature and pH50 or pH10 results (SI Appendix S4).
Climate Change Scenarios
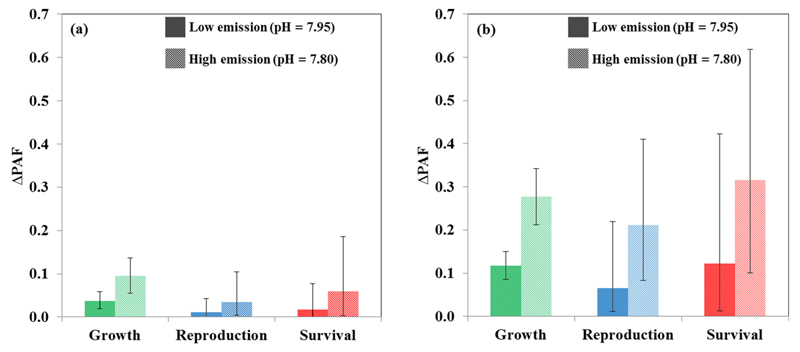
In the low emission scenario (B1), represented by a decline in pH from 8.1 to 7.95, we found an increase in the PAF by 12%, 7%, and 12% in subtle effects (i.e., pH10) and an increase in the PAF by 4%, 1%, and 2% in severe effects (i.e., pH50) on growth, reproduction, and survival of species, respectively (Figure 3). In the high emission scenario (A2), represented by a decline in pH from 8.10 to 7.80, we found an increase in the PAF by 28%, 21%, and 32% in subtle effects (i.e., pH10) and an increase in the PAF by 10%, 3%, and 6% in severe effects (i.e., pH50) on growth, reproduction, and survival of species, respectively. The effects on species survival were found to be the most uncertain since the ratios between the 95th and 5th percentiles of PAF results obtained by the Monte Carlo simulation ranged from 1.26 to 1.58 for growth, 1.94 to 3.97, for reproduction, and 1.96 to 4.57 for survival (depending on the effect severity and emission scenario).
Figure 3.
Change in the potentially affected fraction (ΔPAF, eq 5) from current conditions (pH = 8.1) to a low emission (B1) and high emission (A2) scenario of climate change, where pH is estimated to decline by 0.15 and 0.30 units, respectively. The error bars are the 5th and the 95th percentiles around ΔPAF, and the results are given for (a) severe (i.e., pH50) and (b) subtle (i.e., pH10) effects.
Discussion
We gathered data on ocean acidification experiments reported by peer-reviewed studies. In each experiment, the effect of pH decreases on individual species was allocated one of three life processes: growth, reproduction, and survival. Species-specific pH50 and pH10 were employed in the construction of SSDs and we estimated the change in the PAF of calcifying species under two global climate change scenarios. Below, we discuss the main uncertainties of the analysis and interpret our results.
Uncertainties
We constructed SSDs based on pH50 and pH10 derived from logistic regressions where detrimental effects of pH declines on the three life processes of calcifying species were confirmed. However, for most regressions, we found no significant relationship between pH declines and effects to life processes of species (SI Table S3.1), which considerably decreased the number of species available in the derivation of the SSDs. Still, for some species, multiple pH50 results were obtained for individual species (SI Table S3.1). For example, we found 16 experiments testing ocean acidification effects on the growth of the coral Strongylocentrotus purpuratus, 4 of which had detrimental effects confirmed with logistic regressions. In those cases, we used a conservative approach in which acidification effects are considered at the highest reported pH10 and pH50 levels. Had we chosen the lower reported pH10 and pH50, the mean μ describing the PAF curves would be lower than those we reported.
Unconfirmed or dissimilar results for a given species across regressions may have been triggered by various factors, including the lack of standardization between the experiments with respect to their duration, temperature, salinity, and so forth.12 For example, De Vries et al.12 found that effect levels were correlated with the duration of experiments for marine species (both calcifying and noncalcifying), although, in our study, we found no evidence of this correlation. The discrepancy in the influence of experiment duration between our study and that of De Vries et al.12 may have been caused by the higher range in the duration of experiments employed by De Vries et al.12 (0.07 to 365 days) compared to those of our study (0.01 to 320 days). The lack of experiment standardization is also reflected in the discrepancy between the numbers of pH treatments to which the species was exposed. (The statistical power for the logistic regression declines considerably when few samples, i.e., pH levels, are available for the logistic regression, which may explain the high number of regressions with uncertain species effects of acidification.) We also found no correlation of pH50 and temperature of experiments, both antagonistic and synergistic effects of temperature and ocean acidification have been shown in other studies.21,22 Species-specific factors which could contribute to the discrepancy between logistic regressions for a given species include differences in species populations across experiments,23 metabolic plasticity of species exposed to ocean acidification,24,25 altering of physiological processes to counterweight declines in calcium carbonate skeleton weights, e.g., reduction of motility.25
In the derivation of logistic regressions in each experiment, we only included the effects at pH levels below 8.35. The choice of discarding more alkaline conditions is based on the fact that marine waters seldom reach 8.35 or higher.16,26 For the calculations of expected changes in the PAF from current to estimated pH scenarios, we assumed current pH levels of 8.10, which is the level of monthly averages of ocean pH reported by Takahashi and Sutherland26 at different world’s locations.
The SSDs we developed do not take into account spatial variability or seasonal differences across species assemblages. However, such differences are known to occur. For example, while some of the species considered have a global distribution (e.g., Mytilus edulis), others have narrower distribution (e.g., Oculina arbuscula). Even though there is an increasing body of literature on ocean acidification experiments, including studies developing SSDs for pCO2,12,13 deriving ecosystem-specific SSDs would considerably decrease the confidence around PAF estimates.
Interpretation of Results
Subtle growth effects on species (for which responses include organism size, weight, and calcification rate) follow changes in water chemistry by reducing the accretion of calcium carbonate in the tissue of organisms.8 This effect may be accompanied by an energy-saving strategy whereby metabolic rates are suppressed in order to maintain other life processes under short-term acidified seawater27. This process has been documented for individual species, such as Acropora sp. and Mytilus galloprovincialis.27,28 Nevertheless, detrimental effects on growth may as well be artifact of oxygen displacement following the bubbling of CO2.12,29
Detrimental effects on the growth of crustaceans were not found in our study. Their lack of sensitivity to ocean acidification may be due to the more extensive biogenic covering, thereby offsetting the dissolution of CaCO3 from their exoskeleton.13,30 By contrast, species encompassing structures which are heavily calcified or which have a high content of highly soluble aragonite may be particularly sensitive to pH declines.13,31
Under a high emission scenario of climate change (A2), the uncertainty in the change in the PAF is higher than under a low emission scenario (B1) because the uncertainty in the PAF function at 7.80 is higher than at 7.95. This discrepancy is enhanced for the SSDs of life processes for which lesser species are available (i.e., reproduction and survival). Still, the increase in the fraction of species for which negative effects of ocean acidification were reported is comparable to those of Wittman and Pörtner.13 In their study, Wittman and Pörtner13 found that an increase in 5 to 10% in the fraction of species affected by an increase in pCO2 levels from 344 to 511 μatm (which are roughly the CO2 levels currently and under the B1 scenario, respectively).
Implications of Environmental Risk Assessment
Our study shows how species-specific responses of calcifying species to pH declines can be implemented in environmental risk assessments. Our results show that responses to ocean acidification vary across different calcifying taxa (molluscs, echinoderms, and cnidarians, among others) as well as across life processes (in our case, growth, reproduction, and survival). Subtle effects on 50% of species occur within a range of 7.63 to 7.73 whereas severe effects are observed to 50% of species in pH levels ranging from 7.11 to 7.35. The employment of SSDs of ocean pH may be especially useful to assessments of ocean acidification risks to calcifying species since they convey the interspecies variability responses to gradual pH declines and they allow for the estimation of the potential fraction of the species assemblage affected in different global climate change scenarios.
Supporting Information
Appendix S1 shows the keyword used to complement the data collection with more recent literature. Appendix S2 shows the conversion from CO2 partial pressure (pCO2) to ocean pH. Appendix S3 describes the experimental design and pH50 and pH10 results of each logistic regression (Table S3.1), the literature list included by our study (Table S3.2), and the location of each experiment (Figure S3.1). Appendix S4 shows the result of the influence of temperature and experiment duration on pH50 results. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank Thomas M. W. J. van Goethem for his support in calculating the uncertainty ranges of the results and three anonymous reviewers for their helpful comments on the manuscript. This research was funded by the European Research Council under the Synergy Grant 2013: IMBALANCE-P, grant agreement number 14-139.
Footnotes
Notes
The authors declare no competing financial interest.
References
- (1).Houghton JT, Jenkins GJ, Ephraums JJ, editors. IPCC. Report prepared for Intergovernmental Panel on Climate Change by Working Group. Cambridge University Press; Cambridge/New York/Melbourne: 1990. p. 410. [Google Scholar]
- (2).Feely RA, Doney SC, Cooley SR. Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography. 2009;22(4):36–47. [Google Scholar]
- (3).Secretariat of the Convention on Biological Diversity. Scientic synthesis of the impacts of ocean acidification on marine biodiversity. Montreal: 2009. p. 61. [Google Scholar]
- (4).Fabry VJ. Marine calcifiers in a high-CO2 ocean. Science. 2008;320(5879):1020–1022. doi: 10.1126/science.1157130. [DOI] [PubMed] [Google Scholar]
- (5).Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. In Annual Review of Marine Science. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- (6).Chan NCS, Connolly SR. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Global Change Biol. 2013;19(1):282–290. doi: 10.1111/gcb.12011. [DOI] [PubMed] [Google Scholar]
- (7).Hendriks IE, Duarte CM, Alvarez M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuarine, Coastal Shelf Sci. 2010;86(2):157–164. [Google Scholar]
- (8).Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Lett. 2010;13(11):1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- (9).Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Global Change Biol. 2013;19(6):1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Posthuma L, Traas T, Suter GW. General introduction to species sensitivity distributions. In: Posthuma L, Suter GW, Traas TP, editors. Species Sensitivity Distributions in Ecotoxicology. CRC Press; Boca Raton, FL: 2002. pp. 3–10. [Google Scholar]
- (11).Posthuma L, Suter GW, II, Traas TP. Species Sensitivity Distributions in Ecotoxicology. Lewis Publishers; 2002. p. 616. [Google Scholar]
- (12).de Vries P, Tamis JE, Foekema EM, Klok C, Murk AJ. Towards quantitative ecological risk assessment of elevated carbon dioxide levels in the marine environment. Mar Pollut Bull. 2013;73(2):516–523. doi: 10.1016/j.marpolbul.2013.06.039. [DOI] [PubMed] [Google Scholar]
- (13).Wittmann AC, Pörtner HO. Sensitivities of extant animal taxa to ocean acidification. Nature Climate Change. 2013;3(11):995–1001. [Google Scholar]
- (14).IPCC. Emission scenarios: Special report of working group III, Summary for policy makers. Geneva: IPCC; 2000. [Google Scholar]
- (15).Schiel DR. Growth, Survival and Reproduction of Two Species of Marine Algae at Different Densities in Natural Stands. J Ecol. 1985;73(1):199–217. [Google Scholar]
- (16).Rhein M, Rintoul SR, Aoki S, Campos E, Chambers D, Feely RA, Gulev S, Johnson GC, Josey SA, Kostianoy A, Mauritzen C, et al. Observations: Ocean. IPCC; Cambridge/New York: 2013. [Google Scholar]
- (17).Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80(4):1150–1156. [Google Scholar]
- (18).Roelofs W, Huijbregts MAJ, Jager T, Ragas AMJ. Prediction of ecological no-effect concentrations for initial risk assessment: Combining substance-specific data and database information. Environ Toxicol Chem. 2003;22(6):1387–1393. doi: 10.1897/1551-5028(2003)022<1387:poenec>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- (19).Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437(7059):681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- (20).Joos F, Frölicher TL, Steinarcher M, Plattner G-K. Impact of climate change mitigation on ocean acidification projections. In: Gattuso J-P, Hansson L, editors. In Ocean Acidification. Oxford University Press; Oxford: 2011. pp. 272–290. [Google Scholar]
- (21).Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, Davis AR. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc R Soc B: Biol Sci. 2009;276(1663):1883–1889. doi: 10.1098/rspb.2008.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Benner I, Diner RE, Lefebvre SC, Li D, Komada T, Carpenter EJ, Stillman JH. Emiliania huxleyi increases calcification but not expression of calcification-related genes in long-term exposure to elevated temperature and pCO2. Philos Trans R Soc B: Biol Sci. 2013;368:1627. doi: 10.1098/rstb.2013.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Beaufort L, Probert I, de Garidel-Thoron T, Bendif EM, Ruiz-Pino D, Metzl N, Goyet C, Buchet N, Coupel P, Grelaud M, Rost B, et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature. 2011;476(7358):80–83. doi: 10.1038/nature10295. [DOI] [PubMed] [Google Scholar]
- (24).Godbold JA, Calosi P. Ocean acidification and climate change: advances in ecology and evolution. Philos Trans R Soc B: Biol Sci. 2013;368(1627) doi: 10.1098/rstb.2012.0448. 20120448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wood HL, Spicer JI, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B-Biol Sci. 2008;275(1644):1767–1773. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Takahashi T, Sutherland SC. Climatological mean distribution of pH and carbonate ion concentration in global ocean surface waters in the unified pH scale and mean rate of their changes in selected areas. Lamont-Doherty Earth Observatory of Columbia University; 2013. Nov 15th, http://www.ldeo.columbia.edu/res/pi/CO2/ [Google Scholar]
- (27).Nakamura M, Ohki S, Suzuki A, Sakai K. Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS One. 2011;6(1):e14521. doi: 10.1371/journal.pone.0014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Michaelidis B, Ouzounis C, Paleras A, Pörtner HO. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol: Prog Ser. 2005;293:109–118. [Google Scholar]
- (29).Truchot JP, Duhamel-Jouve A. Oxygen and carbon dioxide in the marine intertidal environment: Diurnal and tidal changes in rockpools. Respir Physiol. 1980;39(3):241–254. doi: 10.1016/0034-5687(80)90056-0. [DOI] [PubMed] [Google Scholar]
- (30).Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37(12):1131–1134. [Google Scholar]
- (31).Beesley A, Lowe DM, Pascoe CK, Widdicombe S. Effects of CO2-induced seawater acidification on the health of Mytilus edulis. Clim Res. 2008;37(2–3):215–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.