Abstract
Background
Medication‐related adverse events in primary care represent an important cause of hospital admissions and mortality. Adverse events could result from people experiencing adverse drug reactions (not usually preventable) or could be due to medication errors (usually preventable).
Objectives
To determine the effectiveness of professional, organisational and structural interventions compared to standard care to reduce preventable medication errors by primary healthcare professionals that lead to hospital admissions, emergency department visits, and mortality in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, three other databases, and two trial registries on 4 October 2016, together with reference checking, citation searching and contact with study authors to identify additional studies. We also searched several sources of grey literature.
Selection criteria
We included randomised trials in which healthcare professionals provided community‐based medical services. We also included interventions in outpatient clinics attached to a hospital where people are seen by healthcare professionals but are not admitted to hospital. We only included interventions that aimed to reduce medication errors leading to hospital admissions, emergency department visits, or mortality. We included all participants, irrespective of age, who were prescribed medication by a primary healthcare professional.
Data collection and analysis
Three review authors independently extracted data. Each of the outcomes (hospital admissions, emergency department visits, and mortality), are reported in natural units (i.e. number of participants with an event per total number of participants at follow‐up). We presented all outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). We used the GRADE tool to assess the certainty of evidence.
Main results
We included 30 studies (169,969 participants) in the review addressing various interventions to prevent medication errors; four studies addressed professional interventions (8266 participants) and 26 studies described organisational interventions (161,703 participants). We did not find any studies addressing structural interventions. Professional interventions included the use of health information technology to identify people at risk of medication problems, computer‐generated care suggested and actioned by a physician, electronic notification systems about dose changes, drug interventions and follow‐up, and educational interventions on drug use aimed at physicians to improve drug prescriptions. Organisational interventions included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians.
There is a great deal of diversity in types of professionals involved and where the studies occurred. However, most (61%) of the interventions were conducted by pharmacists or a combination of pharmacists and medical doctors. The studies took place in many different countries; 65% took place in either the USA or the UK. They all ranged from three months to 4.7 years of follow‐up, they all took place in primary care settings such as general practice, outpatients' clinics, patients' homes and aged‐care facilities. The participants in the studies were adults taking medications and the interventions were undertaken by healthcare professionals including pharmacists, nurses or physicians. There was also evidence of potential bias in some studies, with only 18 studies reporting adequate concealment of allocation and only 12 studies reporting appropriate protection from contamination, both of which may have influenced the overall effect estimate and the overall pooled estimate.
Professional interventions
Professional interventions probably make little or no difference to the number of hospital admissions (risk ratio (RR) 1.24, 95% confidence interval (CI) 0.79 to 1.96; 2 studies, 3889 participants; moderate‐certainty evidence). Professional interventions make little or no difference to the number of participants admitted to hospital (adjusted RR 0.99, 95% CI 0.92 to 1.06; 1 study, 3661 participants; high‐certainty evidence). Professional interventions may make little or no difference to the number of emergency department visits (adjusted RR 0.71, 95% CI 0.50 to 1.02; 2 studies, 1067 participants; low‐certainty evidence). Professional interventions probably make little or no difference to mortality in the study population (adjusted RR 0.98, 95% CI 0.82 to 1.17; 1 study, 3538 participants; moderate‐certainty evidence).
Organisational interventions
Overall, it is uncertain whether organisational interventions reduce the number of hospital admissions (adjusted RR 0.85, 95% CI 0.71 to 1.03; 11 studies, 6203 participants; very low‐certainty evidence). Overall, organisational interventions may make little difference to the total number of people admitted to hospital in favour of the intervention group compared with the control group (adjusted RR 0.92, 95% CI 0.86 to 0.99; 13 studies, 152,237 participants; low‐certainty evidence. Overall, it is uncertain whether organisational interventions reduce the number of emergency department visits in favour of the intervention group compared with the control group (adjusted RR 0.75, 95% CI 0.49 to 1.15; 5 studies, 1819 participants; very low‐certainty evidence. Overall, it is uncertain whether organisational interventions reduce mortality in favour of the intervention group (adjusted RR 0.94, 95% CI 0.85 to 1.03; 12 studies, 154,962 participants; very low‐certainty evidence.
Authors' conclusions
Based on moderate‐ and low‐certainty evidence, interventions in primary care for reducing preventable medication errors probably make little or no difference to the number of people admitted to hospital or the number of hospitalisations, emergency department visits, or mortality. The variation in heterogeneity in the pooled estimates means that our results should be treated cautiously as the interventions may not have worked consistently across all studies due to differences in how the interventions were provided, background practice, and culture or delivery of the interventions. Larger studies addressing both professional and organisational interventions are needed before evidence‐based recommendations can be made. We did not identify any structural interventions and only four studies used professional interventions, and so more work needs to be done with these types of interventions. There is a need for high‐quality studies describing the interventions in more detail and testing patient‐related outcomes.
Plain language summary
Actions to reduce medication errors in adults in primary care
What is the aim of this review?
The aim of this Cochrane Review was to find out the best way to reduce medication errors by primary healthcare professionals in adult patients that lead to hospital admissions, emergency department visits, and death. We wanted to know whether targeting individual health professionals (e.g. with educational materials and reminders about drug dosage etc.), changing the organisation of primary care (e.g. revising professional roles, such as nurse‐ or pharmacist‐led prescribing etc.), or structural actions, such as organising quality monitoring services can reduce medication errors by primary healthcare professionals. We collected and analysed relevant studies to answer this question and found 30 studies.
Key messages
The 30 studies (169,969 participants) in this Cochrane Review showed that actions aimed at reducing medication errors, such as medication reviews by pharmacists or physicians probably make little or no difference to the number of people admitted to hospital, number of hospital admissions, number of emergency department visits, or death. In general, all the actions described in the review were found to have unclear benefits. We did not find any studies that fitted the criteria of structural actions. The main limitation of this review is the small number of studies addressing each method and the low‐certainty of the evidence.
What was studied in the review?
Prescribing medications is one of the most powerful tools available to general practitioners (GPs) in the prevention and treatment of disease. Medication‐related adverse events could be the result of people either experiencing adverse drug reactions (not usually preventable) or as a result of medication errors (usually preventable). We studied the effectiveness of professional and organisational methods compared to standard care in primary care settings (examples of primary care settings include general practices, community pharmacies, patient homes, community settings, outpatient clinics, and aged‐care facilities) to reduce preventable medication errors that lead to hospital admissions, emergency department visits, and death in adults who are prescribed medication in primary care.
What are the main results of the review?
We included 30 studies in our analysis. We classified 26 studies as organisational and the remaining four as professional actions. We found no structural actions in our search. The studies included in this Cochrane Review showed that based on moderate‐ and low‐certainty evidence, actions in primary care for reducing preventable medication errors probably make little or no difference to the number of people admitted to hospital or the number of hospitalisations, emergency department visits, or death. Most of the studies took place in the UK and the USA; studies undertaken in high‐income countries with disadvantaged populations, and in low‐ and middle‐income countries, were underrepresented. This might affect the generalisation of the results.
Certainty of the evidence
We found the overall certainty of evidence for the professional actions to vary considerably across the reported outcomes: moderate‐certainty for number of hospital admissions, high‐certainty for number of people admitted to hospital, low‐certainty for number of emergency department visits, and moderate‐certainty for deaths. The certainty of evidence for organisational actions was less varied: very low‐certainty for number of hospital admissions, low‐certainty for number of people admitted to hospital, and very low‐certainty for number of emergency department visits and deaths.
More work needs to be done in improving the quality of the studies regarding selection of participants and adequate blinding of participants and study assessors. Participants dropping out of the studies was another concern in the certainty of evidence. Funding of the included studies came from various sources and it is difficult to decide whether the funding affected the results of the studies.
How up‐to‐date is this review?
We searched for studies that had been published up to 4 October 2016.
Summary of findings
Summary of findings 1. Professional interventions compared to standard/usual care for prevention of medication errors.
| Professional interventions compared to standard/usual care for prevention of medication errors | ||||||
| Patient or population: adults receiving medication in primary care Setting: primary and community care Intervention: professional interventions (using health information technology to identify people at risk or using it to generate a patient care plan) Comparison: standard/usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard/usual care | Risk with professional interventions | |||||
| Number of hospital admissions | Study population | RR 1.24 (0.79 to 1.96) | 3889 (2 RTs) | ⊕⊕⊕⊝ Moderate1 | The two studies had wide confidence intervals. | |
| 17 per 1000 | 21 per 1000 (13 to 33) | |||||
| Number of people admitted to hospital | Study population | RR 0.99 (0.92 to 1.06) | 3661 (1 RT) | ⊕⊕⊕⊕ High2 | ||
| 448 per 1000 | 443 per 1000 (412 to 475) | |||||
| Number of emergency department visits | Study population | RR 0.71 (0.50 to 1.02) | 1067 (2 RTs) | ⊕⊕⊝⊝ Low1,3 | The two studies had wide confidence intervals and selection bias. | |
| 118 per 1000 | 85 per 1000 (59 to 121) | |||||
| Mortality | Study population | RR 0.98 (0.82 to 1.17) | 3538 (1 RT) | ⊕⊕⊕⊝ Moderate3 | ||
| 122 per 1000 | 119 per 1000 (100 to 142) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RT: randomised trial | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level due to imprecision. 2We did not downgrade the outcomes because all included studies had low risk of bias and narrow confidence intervals. 3We downgraded one level due to risk of bias (selection bias).
Summary of findings 2. Organisational interventions compared to standard/usual care for prevention of medication errors.
| Organisational interventions compared to standard/usual care for prevention of medication errors | ||||||
| Patient or population: adults receiving medication in primary care Setting: primary care Intervention: organisational interventions (provision of pharmaceutical care, medication reviews, follow‐up visits by a healthcare professional including a pharmacist, nurse or physician) Comparison: standard/usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard/usual care | Risk with organisational interventions | |||||
| Number of hospital admissions | Study population | RR 0.85 (0.71 to 1.03) | 6203 (11 RTs) | ⊕⊝⊝⊝ Very low1,2,3 | Some studies had unclear risk of bias (selection and attrition), high heterogeneity and wide confidence intervals. | |
| 274 per 1000 | 233 per 1000 (194 to 282) | |||||
| Number of people admitted to hospital | Study population | RR 0.92 (0.86 to 0.99) | 152,237 (13 RTs) | ⊕⊕⊝⊝ Low1,3 | Some studies had unclear risk of bias (selection, attrition and performance bias) and wide confidence intervals. | |
| 13 per 1000 | 13 per 1000 (11 to 14) | |||||
| Number of emergency department visits | Study population | RR 0.75 (0.49 to 1.15) | 1819 (5 RTs) | ⊕⊝⊝⊝ Very low1,2,3 | Studies had unclear risk of bias (selection, performance and attrition bias), high heterogeneity and wide confidence intervals. | |
| 234 per 1000 | 176 per 1000 (115 to 269) | |||||
| Mortality | Study population | RR 0.94 (0.85 to 1.03) | 154,962 (12 RTs) | ⊕⊝⊝⊝ Very low3,4 | Studies had high risk of selection, attrition and performance bias and wide confidence intervals. | |
| 50 per 1000 | 47 per 1000 (43 to 52) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; RT: randomised trial. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded one level for unclear risk of bias (selection and attrition bias). 2We downgraded one level for inconsistency (high heterogeneity across studies). 3We downgraded one level for imprecision. 4We downgraded two levels for high risk of bias (selection, performance and attrition bias).
Background
Description of the condition
Medication‐related (drug‐related) adverse events in primary care represent an important cause of hospital admissions and mortality (Howard 2003). Medication‐related adverse events could be the result of people either experiencing adverse drug reactions (not usually preventable) or as a result of medication errors (usually preventable) (Bates 1995; Ioannidis 2001).
According to Edwards 2000, adverse drug reactions can be defined as "an appreciably harmful or unpleasant reaction resulting from an intervention related to the use of a medicinal product." Medication errors on the other hand, are mostly preventable. A medication error is defined by Ferner 2006 as "a failure in the treatment process that leads to, or has the potential to lead to, harm to the patient." They are mainly due to prescribing or medication management errors. A reduction of these types of prescribing/medication errors has been a high priority for healthcare policy in order to improve the safety profile of the healthcare delivery system (Howard 2003; Soe 2013).
A prospective cohort study has shown that within four weeks of receiving a primary care prescription, 25% of participants experienced an adverse drug event, 11% of which were judged preventable (Gandhi 2003). A systematic review and meta‐analysis by Winterstein 2002 reported that a median 7.1% (inter‐quartile range 5.7% to 16.2%) of hospital admissions resulted from drug‐related problems, of which 59% were considered preventable (i.e. attributable to error), while Howard 2007 reported that a median of 3.7% of hospital admissions were preventable and drug‐related. Improving patient safety is, as a consequence, now a government priority in many high‐income and middle‐ and low‐income countries, including the UK, USA and five African countries; Egypt, South Africa, Morocco, Tanzania and Zimbabwe (Brown 2008; WHO 2004).
Description of the intervention
In this review we examined interventions in primary care to reduce preventable medication errors that resulted in hospital admissions, emergency department visits, and mortality. The three main types of interventions that we examined included professional, organisational, and structural interventions as described by Cochrane Effective Practice and Organisation of Care (EPOC) (Appendix 1). Professional interventions included quality assurance tools that provided educational interventions for practitioners or participants, such as teaching the use of structured assessments with general practitioners (GPs). Organisational interventions included revision of professional roles (e.g. nurse‐ or pharmacist‐led chronic disease clinics and nurse prescribing) and revision of clinical multidisciplinary teams (e.g. pharmacist‐managed medication reviews). Structural interventions included the organisation of quality monitoring services. We used these interventions for any type of primary care‐based population, irrespective of their characteristics. The comparator was no intervention or standard or usual care. The selected outcomes included in the review were the number of hospital admissions, emergency department visits, and mortality. These outcomes were selected as they are tangible and mostly reported in primary studies. We did not consider patient‐oriented or patient‐mediated outcomes in this review due to the complexity of the included interventions. We will consider these outcomes in the updated review.
How the intervention might work
The three main interventions, mentioned above, used different approaches to achieve a reduction in medication errors that led to hospital admissions, emergency department visits, and mortality.
Professional interventions included continuing education and quality assurance that provided educational interventions for practitioners or participants, such as teaching the use of structured assessments with GPs. Other examples of professional interventions included drug education programmes for physicians that were run by physicians, electronic health record systems that provided information about drugs and gave recommendations about changing doses, health technology that identified care home residents at risk of falls, and computer‐based drug‐ordering systems that gave suggestions to physicians and pharmacists.
Organisational interventions included revision of professional roles (e.g. nurse‐ or pharmacist‐led chronic disease clinics and nurse prescribing) and revision of clinical multidisciplinary teams (e.g. pharmacist‐managed medication reviews). Organisational interventions may have included telephone consultations along with home‐based medication reviews by pharmacists or nurses. Such interventions aimed at engaging workers in the management of risk to increase patient safety.
Structural interventions included the organisation of quality monitoring services. Examples of these interventions included structural approaches such as social, economic, and political interventions that could improve public health outcomes by increasing the willingness and ability of individuals to practice prevention. An example of the latter would be the introduction of financial incentives to healthcare workers to reduce medication errors. By looking at all of these interventions in the current review, we can begin to address the multiple perspectives of various stakeholders who provide health care to individuals in primary care (Benning 2011).
Why it is important to do this review
Prescribing medications is one of the most powerful tools available to GPs in the prevention and treatment of disease, and alleviation of symptoms (Spencer 2014). However, medication‐related adverse events arising as a result of primary care prescribing are an important source of participant morbidity, much of which could be prevented by higher‐quality prescribing and medicines management (Howard 2007). To date, there is little information on the interventions mentioned above, aimed at reducing preventable medication‐related adverse events in primary care due to errors. A review undertaken by Ioannidis 2001, addressed interventions of all types of medical errors in both primary and secondary care. It highlighted the complexity in studying those types of interventions aimed at minimising errors in healthcare delivery. Other reviews by Durieux 2012 and O'Brien 2008 focused on interventions to improve professional practice and healthcare outcomes, including prescribing. A review by Royal 2006 found that there was weak evidence to support pharmacist‐led medication interventions being effective in reducing hospital admissions. However, none of these reviews have focused on other types of interventions at the professional, organisational or structural level that could possibly reduce medication errors in the primary care setting.
Given that preventable medication errors in primary care are associated with hospital admissions, emergency department visits, and mortality, it is important to know whether there are any interventions that have been found to be effective in reducing the occurrence of these outcomes. While members of our team published a related systematic review on this topic (Royal 2006), there has been no Cochrane Review of interventions aimed at reducing the incidence of preventable medication errors that lead to hospital admissions, emergency department visits, and mortality.
Objectives
To determine the effectiveness of professional, organisational and structural interventions compared to standard care to reduce preventable medication errors by primary healthcare professionals that lead to hospital admissions, emergency department visits, and mortality in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials in this review. We excluded controlled before‐after studies and other non‐randomised designs as they provided much weaker evidence due to the non‐randomisation of participants to experimental and control groups. We did not impose any restriction on the language or country in which studies were carried out.
Types of participants
We included studies directed at healthcare professionals and organisations involved in the provision of primary care in the community setting who were authorised to prescribe, sell or administer medications, including primary care physicians (general practitioners (GPs), family doctors, family physicians, family practitioners), dental practitioners, community nurses, nurse practitioners, community pharmacists, dispensers in community pharmacies and any other relevant healthcare providers. We included all adult participants who were receiving a medication through the intervention of the aforementioned primary healthcare professionals.
Examples of community settings included general practice, community pharmacies, and nursing and residential homes. We excluded studies of interventions for outpatients in a clinic attached to a hospital or a day hospital unless these were specifically described as primary care clinics.
Types of interventions
Using the taxonomy of interventions developed by EPOC, we categorised interventions that improved patient safety by reducing hospital admissions, emergency department visits, and mortality (Appendix 1). We compared the interventions with inactive control interventions such as no treatment, or standard or conventional care. We divided interventions into the following categories.
Professional interventions
Professional interventions included the use of health information technology to identify people at risk of medication problems, computer‐generated care suggested and actioned by a physician, electronic notification systems about dose changes, drug interventions and follow‐up, and educational interventions on drug use aimed at physicians to improve drug prescriptions.
Organisational interventions
Examples of organisational interventions included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians.
Structural interventions
Structural interventions included the organisation of quality monitoring services. Structural approaches included social, economic, and political interventions that could improve public health outcomes by increasing the willingness and ability of individuals to practice prevention. An example of the latter would be the introduction of financial incentives to healthcare workers to reduce medication errors.
Types of outcome measures
We included studies that addressed preventable medication errors with the following outcomes. All the outcomes below are included in Table 1 and Table 2.
Primary outcomes
Number of hospital admissions (this outcome takes into account that one patient can have multiple admissions)
Number of people admitted to hospital (this outcome reports on the number of people admitted to hospital irrespective of the number of times they were admitted during the study period)
Secondary outcomes
Number of emergency department visits
Mortality
Search methods for identification of studies
EPOC's Information Specialist, Paul Miller, developed the search strategies in consultation with the review authors. The Information Specialist searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews, and the databases listed below for primary studies.
Electronic searches
We searched the following databases on 4 October 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9), in the Cochrane Library.
MEDLINE Ovid (including epub ahead of print, in‐process and other non‐indexed citations) (1946 to 4 October 2016).
Embase, Ovid (1974 to 3 October 2016).
Health Technology Assessment Database (NHSEED; 2015, Issue 2), in the Cochrane Library.
NHS Economic Evaluation Database (NHSEED; 2015, Issue 2), in the Cochrane Library.
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1981 to 4 October 2016).
Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language or time limits. We searched all databases from database start date to date of search. All search strategies used are provided in Appendix 2.
Searching other resources
Grey literature
On 4 October 2016 we conducted a grey literature search to identify studies not indexed in the databases listed above. Sources included the sites listed below. We documented additional sources, if any, in the review.
Open Grey (opengrey.eu).
Grey Literature Report (New York Academy of Medicine) (greylit.org).
Agency for Healthcare Research and Quality (AHRQ) (ahrq.gov).
Joanna Briggs Institute (joannabriggs.edu.au).
National Institute for Health and Care Excellence (NICE) (nice.org.uk).
Trial registries
We searched the following trial registries on 4 October 2016.
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) (who.int/ictrp).
ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov).
We undertook the following.
Screened individual journals and conference proceedings (e.g. handsearch).
Reviewed reference lists of all included studies, relevant systematic reviews/primary studies/other publications.
Contacted authors of relevant studies or reviews to clarify reported published information/seek unpublished results/data.
Contacted researchers with expertise relevant to the review topic/EPOC interventions.
Conducted cited reference searches for all included studies in citations indexes.
Data collection and analysis
Selection of studies
Three review authors (HK, HC and BB) independently screened the titles and abstracts to assess studies against the inclusion criteria. We obtained full‐text copies of all papers considered to be of potential relevance and we contacted first authors of studies for clarification, where necessary. We resolved disagreements about relevance by discussion between the review authors. We entered all included studies in Review Manager 5 software (Review Manager 2014).
Data extraction and management
Three review authors (HK, HC and BB) independently completed data extraction using a customised version of the EPOC data collection checklist (EPOC 2017a). All three review authors met frequently to discuss progress, with discrepancies resolved by discussion between the review authors. We grouped studies together on the basis of similar interventions and common outcomes and used Review Manager 5 software to manage and pool data (Review Manager 2014), as mentioned in chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We documented the selection process in sufficient detail to complete a PRISMA flow chart (Liberati 2009), and a Characteristics of excluded studies table.
Assessment of risk of bias in included studies
Three review authors (HK, HC and BB) independently assessed the risk of bias of all included studies using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved differences through discussion.
We assessed seven parameters including random sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective reporting, and other bias including protection against contamination and publication bias. We discussed the inclusion of the selected studies in the meta‐analysis based on their risk of bias. We assessed studies on the basis of having low, unclear or high risk of bias. We included all trials in the final meta‐analysis.
Measures of treatment effect
For each of the primary outcomes listed above, we reported outcomes for each study in natural units (i.e. number of participants with an event per total number of participants at follow‐up). We examined funnel plots for evidence of publication bias and analysed data using Review Manager 5 (Review Manager 2014). We presented results with 95% confidence intervals (CIs) and estimates for dichotomous data (number of people admitted to hospital) as risk ratios (RRs).
Unit of analysis issues
We examined the methods of analysis of all study types critically. All randomised trials were appropriately analysed. We analysed cluster‐randomised trials at the same level as the allocation, thereby avoiding unit‐of‐analyses errors (Alvarez 2001; Coleman 1999; Gernant 2016; Kaczorowski 2011; Lapane 2011; Lowrie 2012; Malet‐Larrea 2016; Roberts 2001). Therefore, we did not need to reanalyse the results and it was appropriate to combine them with other randomised trials.
Dealing with missing data
We did not exclude any studies from the meta‐analysis due to a differential loss to follow‐up or missing data. Most studies had adequate reporting of the participants in their samples. We were able to extract all the data needed for analysis from the included studies. We did not need to contact any study authors for more information.
Assessment of heterogeneity
Because trials may not have been carried out according to a common protocol, there were usually variations in participant groups, clinical settings, concomitant care, etc. Therefore, we assessed heterogeneity between trial results. We considered trial data to be heterogeneous where the I2 statistic was greater than 40% (Higgins 2003). For analyses, we used the random‐effects method. We attempted to explain the differences between studies on the basis of the characteristics of interventions in the included studies.
Assessment of reporting biases
We carefully assessed all studies for reporting bias. Reporting bias was especially likely with outcomes that used participant self‐reports or self‐administered surveys.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We used a random‐effects meta‐analysis for combining data due to the clinical and methodological heterogeneity between studies. We grouped studies based on the two main interventions (i.e. professional and organisational). Where appropriate, we carried out meta‐analyses to establish the effects of interventions on medication‐related hospital admissions, emergency department visits, and mortality. We found no studies addressing structural interventions and hence no analysis was undertaken.
'Summary of findings table' and GRADE
We included two 'Summary of findings' tables for the main intervention comparisons: 'professional interventions compared to usual care' (Table 1); and 'organisational interventions compared to usual care' (Table 2). The 'Summary of findings' tables include the justification for our decisions to downgrade or upgrade the evidence for an outcome, along with comments to help the reader understand the process. We included the following outcomes in the 'Summary of findings' tables: number of hospital admissions, number of people admitted to hospital, number of emergency department visits, and mortality.
Three review authors (HK, HC and BB) used the GRADE tool to independently judge the certainty of the evidence (high, moderate, low, and very low) with respect to five criteria (risk of bias, inconsistency, indirectness, imprecision, and publication bias), with disagreements resolved through discussion (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 11 of the Cochrane Handbook for Systematic Reviews of interventions and GRADEpro GDT software (GRADE pro GDT 2015; Higgins 2011b; Schünemann 2011). In addition, we used the EPOC worksheets to write plain language statements to report these findings in the review (EPOC 2017b).
Subgroup analysis and investigation of heterogeneity
We conducted the analyses based on the types of interventions (professional, organisational, structural) as described by Deeks 2011. We undertook analyses for the following interventions.
Professional interventions, such as provision of educational interventions for practitioners or participants.
Organisational interventions, including revision of professional roles (e.g. nurse‐ or pharmacist‐led chronic disease clinics, nurse prescribing) and clinical multidisciplinary teams (e.g. pharmacist‐managed medication reviews).
We found no studies addressing structural interventions and therefore, we did not include this type of intervention in our review. There was no other subgroup analysis undertaken in the review.
Sensitivity analysis
We used a sensitivity analysis to explore the influence of the following on effect size: repeating the analysis; and excluding any high risk of bias studies to see how they influenced the results. We did this in order to help understand whether the results of the review are robust.
Results
Description of studies
Results of the search
Searches of the main electronic databases led to identification of 14,604 titles. A search of the grey literature and of trial registries yielded a total of five articles that did not make it in the final included studies. Handsearching of the references listed did not yield new studies.
We identified a total of 11,019 references after removal of duplicates. From reading titles and abstracts, we eliminated 10,960 as being not relevant to the review. Reasons for exclusions included irrelevant interventions, study designs and populations (i.e. not primary care settings). We obtained full papers for 89 references. From these 89 papers, we excluded 59 papers for reasons such as study design, study reported elsewhere and study not conducted in a primary care setting, irrelevant outcomes and protocols (see Characteristics of excluded studies). We included a total of 30 papers reporting on 30 trials (see Characteristics of included studies). We have provided an overview of the selection process in a PRISMA flow diagram, Figure 1 (Liberati 2009).
1.
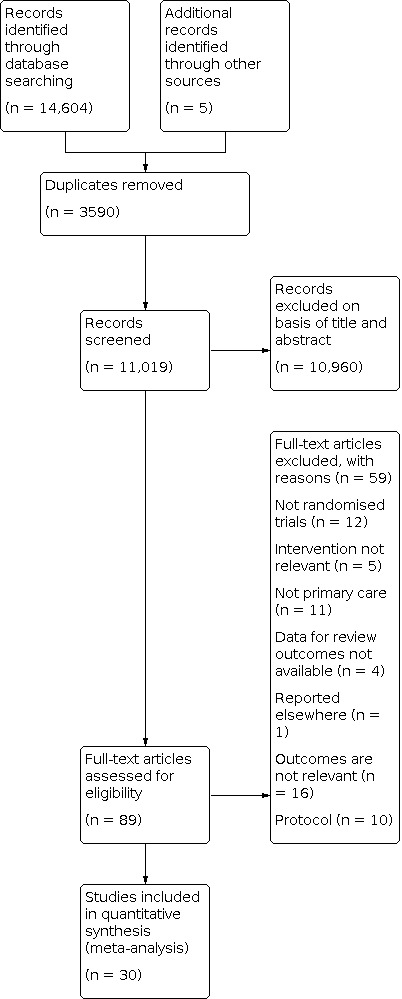
Study flow diagram
Included studies
Method (design)
We included a total of 30 studies (169,969 participants) in this review. Four studies addressed professional interventions (8266 participants) and 26 studies described organisational interventions (161,703 participants). Overall, there were eight cluster‐randomised trials (Alvarez 2001; Coleman 1999; Gernant 2016; Kaczorowski 2011; Lapane 2011; Lowrie 2012; Malet‐Larrea 2016; Roberts 2001), and 22 randomised trials (Bernsten 2001; Campins 2016; Frankenthal 2014; Garcia‐Gollarte 2014; Gurwitz 2014; Hawes 2014; Holland 2005; Ibrahim 2013; Korajkic 2011; Krska 2001; Lenaghan 2007; Malone 2000; Moertl 2009; Murray 2004; Nabagiez 2013; Okamoto 2001; Olesen 2014; Pai 2009; Rytter 2010; Triller 2007; Zermansky 2001; Zermansky 2006. They all ranged from three months to 4.7 years of follow‐up. A full description of the interventions of each study is included in the 'Characteristics of included studies', Table 3 and Table 4. All cluster‐randomised trials were appropriately analysed. Alvarez 2001 reported randomisation at the pharmacy level. They used adjusted Pearson's Chi2 to compare means. Coleman 1999 used statistical techniques that accounted for potential within‐practice correlation that results from randomisation of practices. For continuous variables, they used a mixed model analysis of covariance and regression analysis and for binary values, they used generalised estimating equations. They derived P values from a t‐distribution rather than a normal distribution. Gernant 2016 used a multivariable logistic regression model using generalised estimating equations to examine the effect of the intervention on the probability of 60‐day all‐cause emergency department utilisation. The analysis was approved by the Purdue University Institutional Review Board. Kaczorowski 2011 fitted linear regression models by using the Poisson distribution. Lapane 2011 analysed their results using a Poisson regression model and accounted for the cluster trial design to provide estimates adjusted for potential confounders. In contrast, Lowrie 2012 compared the main outcomes between the intervention and control groups using a Cox proportional hazards frailty model, which accounted for the cluster‐randomisation design. Malet‐Larrea 2016 included a random intercept for pharmacies nested within a group, to account for clustering of participants within pharmacies, and was adjusted by covariate that could affect hospital admissions (age, gender and number of health problems). Differences between groups in hospital costs were analysed by hospital admission and by participant, and the latter ones adjusted by analysis of covariance (ANCOVA) for the number of health problems. Roberts 2001 used robust variance estimation techniques (SUDAAN 2012), in which the effect of clustering within nursing homes on the variance was accounted for.
1. Tentative description of interventions (part 1).
| Study | Name | Theory | Materials | Procedures | Who provided intervention | Modes of delivery |
| Alvarez 2001 | Pharmaceutical care | Pharmaceutical care is the provision of drug therapy for the purpose of achieving outcomes that improve a person’s quality of life. | Pharmacies in the intervention group provided pharmaceutical care, which consisted of offering the pharmaceutical care service to participants and to their corresponding GPs. | An Initial interview and assessment of the therapeutic plan was undertaken, registration of data during the subsequent visits to allow the identification of medication‐related problems, and an intervention to solve the problem. The intervention involved proposing changes in the medication participants received, which had to be communicated to the patient’s GP. | Pharmacists provided the intervention. | Individual and face‐to‐face |
| Bernsten 2001 | Pharmaceutical care | Pharmaceutical care is the provision of drug therapy for the purpose of achieving outcomes that improve a person's quality of life, although little research has been conducted in community‐based pharmaceutical care with elderly people. | Training of pharmacists was done with a study manual. The manual contained an overview of the concept of pharmaceutical care and its provision to elderly people. No reference was provided for the study manual. | The intervention group of pharmacists identified actual and potential drug‐related problems using a structured approach. These pharmacists utilised a number of data sources in this assessment including the participant, the participant's GP, and pharmacy records. Following this assessment, pharmacists were instructed to formulate an intervention and monitoring plan. | Community pharmacists were trained to provide the structured pharmaceutical care intervention. A study manual helped facilitate this process. It contained an overview of the concept of pharmaceutical care, its provision to elderly people, information on the therapeutic management of a number of disease states common in the elderly, together with other issues pertinent to drug therapy in the elderly. | Individual face‐to‐face |
| Campins 2016 | Drug evaluation and recommendation | Several instruments, criteria, and algorithms have been developed to enable more rational and appropriate use of medication, but limited evidence exists with regard to the outcomes that were investigated. | The Good Palliative‐Geriatric Practice algorithm Garfinkel 2007) and the STOPP/START criteria were used (O'Mahony 2015). Both of these tools assess the appropriate use of medication in older people. | The intervention was composed of 3 phases. In the first phase, an experienced pharmacist evaluated all prescriptions using the GP‐GP algorithm and based their decision about appropriateness on the STOPP/START criteria. In the second phase, the pharmacist discussed recommendations for each drug with the participant's physician in order to come up with a final list of recommendations. Finally, the recommendations were discussed with the participant and a final decision was agreed by physicians and participants. | The intervention was delivered by a trained and experienced pharmacist. No details are provided concerning what is a "trained and experienced" pharmacist. | Individual and face‐to‐face |
| Coleman 1999 | Chronic care clinics | Chronic care clinics redesign the structure and content of primary care services through the delivery of scheduled visits devoted to chronic disease management. This mode of service delivery has the potential to improve outcomes for elderly people. | The chronic care clinics included an extended visit with the physician and nurse dedicated to planning chronic disease management, a pharmacist visit that emphasised reduction of polypharmacy and high‐risk medications, and a patient self‐management group. | Frail older people were invited to participate in visits with the primary care team. During these visits, a shared treatment plan was developed, a session was conducted with the pharmacist that addressed polypharmacy and medications associated with functional decline, patient self‐management group sessions were conducted, and the provision of health status assessment information was provided to the practice team. | The team that provided the intervention consisted of the participant’s physician, a team nurse, and a pharmacist. Physicians and team nurses received training in population‐based medicine and management strategies of geriatric syndromes. Team nurses received on‐the‐job coaching from study staff. | The intervention was delivered individually and in groups in a face‐to‐face format. |
| Frankenthal 2014 | Medication review and drug recommendations | Potentially inappropriate prescriptions are prevalent in older people and are associated with adverse drug events. The STOPP/START criteria are designed to detect potentially inappropriate prescriptions in elderly people. However, little is known about the effects of an intervention involving the application of the STOPP/START criteria on clinical outcomes. | The STOPP/START criteria were used to deliver the intervention (Gallagher 2008). The STOPP criteria focus on avoiding the use of drugs that are potentially inappropriate for older people and the START criteria identify undertreatment or prescribing omissions in older people. | Medication reviews were conducted by the study pharmacist for all residents. Recommendations made by the pharmacist were discussed with the chief physician. The physician then decided whether to accept these recommendations and implement prescribing changes. | The intervention was conducted by the study pharmacist who applied the STOPP/START criteria during the medication review. The pharmacist also discussed the recommendations from the intervention with the chief physician, who decided whether to accept these recommendations and implement prescribing changes. | Intervention was delivered individually and face‐to‐face. |
| Garcia‐Gollarte 2014 | Structured educational intervention | Inappropriate drug prescription is a common problem in people living in nursing homes and is linked to adverse health outcomes. This study assessed the effect of an educational intervention directed to nursing home physicians in reducing inappropriate prescription and improving health outcomes and resource utilisation. |
Educational material and references were given to physicians and two 1‐h workshops were used to review cases and promote practice changes. The STOPP/START criteria were reviewed with a random sample of 10 residents cared for by each physician (Gallagher 2008). The content of the educational intervention is provided in an appendix (Garcia‐Gollarte 2014). | The educational intervention included general aspects of prescription and drug use in geriatric patients, how to reduce the number of drugs, to perform a regular review of medications, to avoid inappropriate drug use, to discontinue drugs that do not show benefits, and to avoid under treatment with drugs that have shown benefits. It also discussed some drugs frequently related to adverse drug reactions in older people. | A nursing home physician delivered the structured educational intervention. | Face‐to‐face intervention delivered in a group and individual format. |
| Gernant 2016 | Medicine reconciliation and action plan | Emergency department overcrowding has been linked to increased mortality, costs, and length of stay. This study evaluated the effectiveness of a telephone‐based, medicines‐management service on reducing emergency department utilisation. | Medication therapy management was provided to participants (APA 2008). A pharmacy technician completed telephonic medication reconciliation, after which a trained pharmacist consulted with the participant or caregiver via telephone to complete a scheduled, comprehensive medication therapy review to identify and resolve any medication‐related problems. The pharmacist constructed a personal medication record and a medication‐related action plan for the participant. The action plan was a participant‐centred document that assisted participants, caregivers, and the pharmacist in the resolution of identified medication‐related problems. | The intervention commenced with a pharmacy technician completing medication reconciliation with the participant over the telephone. Then, a pharmacist consulted with the participant by telephone for an average of 30 min to complete a comprehensive medication review to identify and resolve medication‐related problems. The pharmacist constructed a person medication‐related action plan and followed‐up with the participant's prescriber. | A pharmacy technician delivered the initial medicine reconciliation with the participant. A trained pharmacist conducted the medication therapy review, constructed a personal medication record, and a medication‐related action plan. The pharmacist also followed up with the participant's prescriber for resolution of problems that could not be resolved with the participant. | The intervention was conducted individually on the telephone. |
| Gurwitz 2014 | Automated system to facilitate flow of information and provide warnings, alerts, and recommendations | Transitions between the impatient and outpatient setting is a period of high risk for older adults. Most approaches to improving transitions require a substantial commitment of resources but automating these processes may improve the quality and safety of care. | An automated system was used to facilitate the flow of information to the medical group's primary care providers about individuals who were discharged to home from the hospital (Field 2012). | An automated system was developed to facilitate the flow of information to the medical group's primary care providers. A computer interface linked the primary care provider's electronic health records to the hospital records, which provided information about admissions and discharges. The system also provided information about new drugs at discharge, warnings about drug‐drug interactions, recommendations about dose changes and laboratory monitoring of high‐risk medications, and alerts to the provider's support staff to schedule a post‐hospitalisation office visit within 1 week of discharge if not already scheduled. | The automated system delivered the intervention. | The intervention was delivered electronically. |
| Hawes 2014 | Care transitions clinic visit | Medication errors related to hospital discharge result in rehospitalisations and emergency department visits, which may be reduced by pharmacist involvement during postdischarge transitions of care. This study evaluated the impact of a transitional care clinic visit conducted by a pharmacist. |
The Best Possible Medication Discharge List was used to identify medication discrepancies (Wong 2008). It served as the gold standard for the list of medications that the participant should take after discharge. | Participants in the intervention group were scheduled for a care transitions clinic visit approximately 72 h after hospital discharge. The visit involved performing a complete medication history, identifying and resolving medication discrepancies, creating a current medication list, and counselling on appropriate medication use. | Clinical pharmacists provided the intervention. They collaborated with the inpatient medical team to create the Best Possible Medication Discharge List. | The intervention was delivered individually and face‐to‐face. |
| Holland 2005 | Pharmacist home visits | Older people often have trouble adhering to their medications. This study evaluated the effectiveness of a home‐based medication review on hospital admissions among elderly people. | A standardised visit form was used to record the home visit but no reference was provided. | Pharmacists arranged home visits with the participant during which they assessed the participant's ability to self‐medicate and drug adherence. They educated the participant, removed out‐of‐date drugs, reported drug reactions or interactions to the physician, and reported the need for a compliance aid. | Pharmacists conducted the home visits. Pharmacists held a postgraduate qualification in pharmacy practice or had recent continuing professional development in therapeutics. The pharmacists participated in a 2‐day training course, which included lectures on adverse drug reactions, prescribing in elderly people, improving concordance, and communication skills. | The intervention was delivered individually and face‐to‐face. |
| Ibrahim 2013 | Telephone consultation with home visits | Adherence to warfarin treatment and monitoring guidelines may be suboptimal among patients and staff. This study assessed the improvement in adherence to warfarin therapy with telephone and home visits. | A predesigned set of questions was used in the telephone consultation, but no reference or any additional details were provided. | The intervention group was counselled with once‐a‐week telephone consultations and 2 home visits per month by either a nurse or a pharmacist that dealt with warfarin use. | A pharmacist or a nurse provided the home visits. The telephone consultation was conducted by a pharmacist. | The intervention was delivered individually using a face‐to‐face format and telephone calls. |
| Kaczorowski 2011 | Cardiovascular risk assessment and education sessions | Strategies for managing blood pressure are essential as high blood pressure is the leading risk factor for death. The study authors evaluated the effectiveness of a community‐based cardiovascular health promotion and disease prevention programme in reducing morbidity. | The Cardiovascular Health Awareness Program was a standardised intervention that consisted of 10 weeks of cardiovascular risk assessment, blood pressure measurements, and education sessions (CHAP 2017). | The intervention consisted of 10 weeks of cardiovascular risk factor assessment and educational sessions. Volunteers were recruited to help participants measure their blood pressure and supported self‐management by providing participants with their risk profile, risk‐specific educational materials and information about access to local services. At the end of the 10‐week programme and 6 months after the programme ended, the results were forwarded to family physicians who rank‐ordered their participants by their most recent systolic blood pressure reading. | Volunteers were recruited and trained to carry out the intervention. The volunteers were trained according to a standardised curriculum developed by a public health nurse and delivered by nurses working in the intervention community. | The intervention was conducted individually in a face‐to‐face manner. |
| Korajkic 2011 | Educational intervention with pharmacist | Few studies have examined a pharmacist's contribution to improving diuretic compliance and reducing rehospitalisation and health care use. This study aimed to determine the impact of a pharmacist‐led intervention on patient‐guided diuretic dose adjustment. | The intervention group adjusted their diuretic dose using a flexible frusemide dose‐adjustment guide that was provided in the paper. | The intervention consisted of a 30‐min educational session and focused on improving participant self‐care, recognising symptoms of fluid retention, measuring weight daily, self‐adjusting the diuretic dose and improving knowledge of heart failure and heart failure medications. | A pharmacist provided the intervention. The frusemide dose‐adjustment guide was developed in collaboration with cardiologists. | Conducted individually in a face‐to‐face fashion. |
| Krska 2001 | Pharmaceutical care plan | Regular medication reviews can reduce the risk of medication‐related problems. This study aimed to evaluate the effect of a pharmacist‐led medication review on pharmaceutical care issues and hospitalisations. | Clinically‐trained pharmacists completed a detailed profile for each participant using medical notes and computer records. All participants were interviewed in their home about their use of and responses to medication and their use of health and social services. No references provided | A pharmaceutical care plan was drawn up listing all pharmaceutical care issues together with all the actions planned to achieve the outcomes of any pharmaceutical care issue. Copies of the plan were given to the GP who was asked to agree, after which the pharmacist implemented the plan. | The pharmacist performed the medication review. The participants' GP indicated their level of agreement with each pharmaceutical care issue and with the actions taken. | The mode of delivery was individual and face‐to‐face. |
| Lapane 2011 | Use of health information technology to identify people at risk for delirium and falls, implement monitoring plans, and provide reports to pharmacists | Falls and delirium pose the greatest threats to resident safety in nursing homes and contributes to further functional decline. Medication use is associated with greater risk of delirium and falls. Therefore, this study used health information technology to identify residents at risk for delirium and falls due to adverse drug events. | A Geriatric Risk Assessment MedGuide was a database designed to identify medications that potentially contributed to delirium and fall risk (Tobias 1999). It also facilitated early recognition of signs and symptoms indicative of potential medication‐related problems. Training was provided to nursing staff and pharmacists in how to use the reports generated by the Geriatric Risk Assessment MedGuide. | Health information technology was used to identify residents at risk for delirium and falls, implement monitoring plans, and provide reports to pharmacists in conducting medication reviews. The consultant pharmacist shared the reports with the nurse contact at the facility and used the reports in their monthly drug review. | The intervention was an automated system that provided reports to pharmacists and nurses, who were trained to use these reports. The training for nurses provided information regarding medications that cause, aggravate, or contribute to the risk of falls and delirium. The course also reviewed symptoms and signs of adverse medication effects and reinforced the importance of the early observation of symptoms and signs of adverse medication effects. Pharmacists were trained to provide a targeted drug review for all participants who experienced delirium and falls. | The intervention was delivered individually and face‐to‐face. |
| Lenaghan 2007 | Home‐based medication review | Home‐based medication reviews are convenient for the patient and provide an opportunity to understand their medication‐taking in their home environment. Therefore, this study looked at whether home‐based medication reviews with elderly people could reduce hospital admissions. | The intervention comprised 2 home visits by a community pharmacist who educated the participant/carer about their medicines, noted any pharmaceutical care issues and assessed the need for an adherence aid. | At the home visit, the pharmacist educated the participant, removed out‐of‐date drugs, and assessed the need for an adherence aid. The pharmacist held regular meetings with the GP where changes to the participant's medications were discussed and amendments were implemented by the GP. | A pharmacist with a post‐graduate qualification in pharmacy practice conducted the home‐based medication review. They had regular meetings with the lead GP. Possible changes to the participant's medication were discussed and agreed amendments were implemented by the GP. | The intervention was delivered individually and face‐to‐face. |
| Lowrie 2012 | Pharmacist medication review | Although angiotensin‐converting enzyme inhibitors and beta‐blockers reduce morbidity and mortality in people with heart failure, these treatments are underused. Pharmacists may improve treatment through medication review. This study investigated whether a pharmacist intervention would reduce hospital admission and death for people with heart problems. | Pharmacists received training covering the aetiology, symptoms, and evidence‐based management of heart failure. They also participated in monthly discussions of specific cases. The pharmacist used guidelines to optimise treatment for participants with left ventricular systolic dysfunction. All of these materials are available at onlinelibrary.wiley.com/journal | Participants were offered a 30‐min appointment with the pharmacist If there was agreement between the pharmacist and the participant, and subsequently with the doctor, medications were initiated, discontinued, or modified by the pharmacist during 3‐4 weekly or fortnightly consultations. | The pharmacists, who delivered the medication review, had between 3 and 16 years of post‐qualification experience, had experience delivering primary care‐based medication review clinics for people receiving multiple‐drug treatment and attended an in‐house training day covering the aetiology, symptoms, and evidence‐based management of heart failure. An additional session covered the methods of the trial. | The intervention was delivered individually and face‐to‐face. |
| Malet‐Larrea 2016 | Pharmacist medication review | Aging and the use of polypharmacy are risk factors for drug‐related problems and medication‐related hospital admissions. Therefore, this study assessed the impact of a community pharmacist‐led medication review on hospital admissions in older people. | Pharmacists in the intervention group received a training course that covered the clinical management of older people and the medication review method. No reference was provided. | The medication review consisted of the pharmacist collecting information about the participant's health problems, medication use, lifestyle habits, and concerns about diseases and medications. The pharmacist then identified negative clinical outcomes related to medicines and drug‐related problems. Subsequently, an action plan was agreed upon which focused on participant outcomes and the medication use process. | Pharmacists provided the medication review. They received a 3‐day training course covering clinical management of elderly people, the medication review with follow‐up method, communication with participants and doctors, study protocol and documentation forms. | The intervention was delivered individually and face‐to‐face. |
| Malone 2000 | Pharmacist visits | Pharmacists have adopted pharmaceutical care, which is the provision of drug therapy to improve a person's quality of life, to reduce morbidity and mortality. Unlike previous studies that did not focus on people who were most likely to benefit, this study examined veterans who were at high risk for a medication‐related problem. | Contacts between the pharmacist and participant were recorded on a data collection form, which contained the method of contact, time spent, medical problems addressed, drug‐related problems addressed, and drug‐related problems resolved. This form was not referenced. | The intervention participants received consultation and follow‐up care from a clinical pharmacist. | Pharmacists conducted the intervention. Most had a Doctor of Pharmacy degree and over 70% were either receiving or had completed postgraduate training. | The intervention was delivered individually and face‐to‐face. |
| Moertl 2009 | Home‐based nurse care |
Home‐based nurse care can reduce adverse events in people with chronic heart failure. High levels of natriuretic peptides in people with heart failure are predictors of death and hospitalisations. The study authors looked at whether high levels of these peptides can predict whether people with heart failure benefit from a home‐based nurse intervention. | The nurse checked for and, in co‐ordination with the treating physician, implemented guideline‐based medication (Remme 1997; Remme 2001). | At home visits, the nurse checked and recorded weight, recorded symptoms and signs of heart failure as well as heart rate and blood pressure, and organised and reviewed blood analyses on demand. The nurse also gave the patient education and self‐management skills. | Nurses who specialised in caring for people with heart failure provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Murray 2004 | Computerised care suggestions | Hypertension is associated with cardiovascular morbidity and mortality, but is difficult to control. Guidelines on hypertension are complicated and can become outdated quickly, so this study investigated the benefits of evidence‐based treatment for hypertension using a computerised system. | This study used the pharmacist intervention recording system, which was used to document all pharmaceutical care interventions (Overhage 1999). This system gave the pharmacist care suggestions, which they could pass on to the physician. The physician used an order writing workstation to write orders for drugs, tests, nursing activities, and consultations (McDonald 1999). The workstation gave the physician care suggestions for the treatment of hypertension. |
The pharmacist intervention recording system was used by intervention pharmacists to receive care suggestions. The pharmacist could fill the prescription as written, discuss the suggestions with the participant and encourage discussions between the participant and physician, or contact the ordering physician. The physician intervention used an order‐writing workstation to write orders for drugs, tests, nursing activities and consultations and display care suggestions. All hypertension care suggestions were displayed as suggested orders along with possible actions and a brief explanation of the rationale for the suggestion. |
Pharmacists and physicians provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Nabagiez 2013 | Home visits by physician assistants | Studies suggest that people who have undergone coronary artery bypass graft surgery benefit from a home intervention, but there are few studies of home visits by physicians or physician assistants. Therefore, this study examined the hospital readmissions of people who received home visits by physician assistants. | A physician assistant home care form/checklist was used to record all findings from the home visit. A copy of this form was provided in the paper. | Cardiothoracic physician assistants conducted home visits during which they performed a physical examination and reviewed the participant's medications. Adjustments were made to the participant's medications and new medications were prescribed as needed. The surgical wounds were examined and participant concerns were addressed. Prescriptions were written for antibiotics, blood work, or imaging studies. | Physician assistants provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Okamoto 2001 | Pharmacist‐managed hypertension clinic | Hypertension can be controlled, but this study investigated whether it can be managed at a reasonable cost with minimal adverse effects by pharmacists. | Sitting blood pressure was measured with a Datascope Accutorr automated sphygmomanometer (Datascope Corporation Montvale, NJ, USA). 2 readings were taken for each participant and the average of the 2 readings was recorded (Datascope Patient Monitoring 1996). | Participants were counselled by a pharmacist who told them that efforts would be made to decrease the number of antihypertensive drugs or alter their therapy by giving more appropriate or less expensive drugs to achieve similar or improved blood pressure control. The pharmacist determined the most appropriate antihypertensive regimen for each participant, ordered laboratory tests as needed, and provided education on nonpharmacological ways to control blood pressure. | Clinical pharmacists provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Olesen 2014 | Pharmacist medication review | Pharmacists work with participants in designing, implementing and monitoring therapeutic plans, but elderly people may have problems with adhering to their medication. This study looked at treatment adherence, as well as hospitalisations and mortality, in elderly people who received a home visit by a pharmacist along with telephone follow‐up. | Pharmacists adhered to a manual to deliver the intervention (Medication Review‐Managing Medicine Manual, Danmarks Apotekerforening, Pharmakon. Medicingennemgang 2004). This manual helps pharmacists identify and resolve drug‐related problems (Danmarks 2004). |
Participants were visited at home by a pharmacist who examined the medicines list with regard to side‐effects, interactions and administration. The pharmacist tried to make the regime less complex, informed participants, and motivated adherence. | Pharmacists who had some practical experience or courses in medication review provided the intervention. | The intervention was delivered individually. It was conducted by telephone and face‐to‐face. |
| Pai 2009 | Pharmacist medication review | People with end‐stage renal disease take multiple drugs and experience multiple co morbidities, which places them at greater risk of drug‐related problems. This paper looked at the effects of a pharmacist‐led intervention on drug‐related problems and hospitalisations in ambulatory patients undergoing haemodialysis. | Drug‐related problems were recorded, evaluated and assigned to 10 possible categories (Hepler 1990). The drug‐related problems were also categorised into therapeutic drug classes and the outcome related to the drug‐related problem intervention was captured. | Participants assigned to pharmaceutical care had drug therapy reviews conducted by a nephrology‐trained pharmacist. The pharmacist conducted a participant interview, generated a drug therapy profile, identified and addressed drug‐related problems, and provided healthcare‐provider and participant education. The pharmacist also provided consultative services that focused on optimising drug therapy. | The clinical pharmacists who conducted the intervention were either nephrology‐trained or completing postdoctoral training in nephrology pharmacotherapy. | The intervention was delivered individually and face‐to‐face. |
| Roberts 2001 | Medication review, nurse education, and development of professional relationships | Pharmacist‐conducted medication reviews and nurse education about medication use may have an impact on drug use in nursing homes. This study looked at the effect of medication review and nurse education on mortality and hospitalisations in nursing homes. | Problem‐based educational sessions were provided to nurses and addressed basic geriatric pharmacology and some common problems in long‐term care. No referenced documentation is provided for these sessions. | The intervention introduced a new professional role to stakeholders with relationship building, nurse education, and a medication review by pharmacists. Professional contact between nursing home staff and pharmacists on issues such as drug policy and resident problems was conducted along with problem‐based educational sessions for nurses. These sessions addressed geriatric pharmacology and problems in long‐term care. The medication reviews highlighted adverse drug effects, ceasing or adding drugs, better use of specific drug therapy, non‐drug interventions, and adverse effect and drug response monitoring. | Clinical pharmacists delivered the intervention. | The intervention was delivered individually and in groups over the phone and face‐to‐face. |
| Rytter 2010 | Structured home visits by GP and nurse | Many hospital admissions are due to inappropriate medical treatment, and the discharge of fragile elderly patients is associated with a high risk of readmission. This study examined whether home visits by GPs and district nurses reduced the risk of readmission of discharged elderly patients. | The joint home visits were guided by an agenda. During the structured home visit the agenda included checking the discharge letter for recommended follow‐up, checking the need for adjustment of medication, checking if social and personal support was arranged, and checking the family’s medicine cabinet. This agenda was provided in the article. | There was a joint home visit by the GP and district nurse approximately one week after discharge from the hospital. 2 more contacts were conducted by the GP in the GP's clinic or as a home visit. These visits included checking the discharge letter, checking the need for adjustment of medication, checking if social and personal support was arranged, and checking the family's medicine cabinet. | GPs and district nurses provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Triller 2007 | Pharmacist medication reviews | Adverse drug events are frequently caused by cardiovascular drugs. Pharmacists can identify and resolve drug‐related problems for people at home and reduce re‐hospitalisation rates. This study investigated whether a pharmacist‐led intervention could reduce re‐hospitalisations and death in people with heart failure. | Using a predefined checklist, the pharmacist tried to reduce the use of inappropriate mediations, encourage smoking cessation, suggest improvements in the participant’s diet, and promote medication adherence, self‐monitoring, and vaccination. The checklist is not provided in the paper. | The pharmacist in the intervention group conducted an in‐home medication assessment and 2 follow‐up visits. This involved assessing and reviewing physician notes and laboratory test values and interacting with prescribers on behalf of the participants. The pharmacist catalogued all medications and interviewed the participant regarding medication use. | A clinical pharmacist, who had over 20 years of combined experience as a hospital and community pharmacist and had received a doctor of pharmacy degree and completed a 1‐year clinical residency in home care, provided the intervention. | The intervention was delivered individually and face‐to‐face. |
| Zermansky 2001 | Pharmacist medication review | Repeat prescribing is poorly managed in the UK, which puts people at risk. Pharmacists could review these prescriptions and reduce the pressure on GPs. This study tested whether pharmacists can review repeat prescriptions to reduce hospital admissions and deaths. | The process for reviewing repeat prescriptions involved discussing each condition with the participant and asking about symptoms (Lowe 2000). If clinical or pathological monitoring was due, the pharmacist directed the participant to the practice nurse or doctor. Participants with new clinical problems were referred to the doctor. | The pharmacists conducted a medication review during which they evaluated the therapeutic efficacy of each drug and the progress of the conditions being treated. Compliance, actual and potential adverse effects, interactions, and the participant’s understanding of the condition and its treatment were considered. The outcome of the review was a decision about the continuation of the treatment. | A pharmacist provided the medication review. | The intervention was delivered individually and face‐to‐face. |
| Zermansky 2006 | Pharmacist medication review | Elderly people take multiple medicines, which increases the risk of adverse drug events. Pharmacists can improve medicine management for elderly people in the community. In this study, the authors looked at whether a pharmacist‐led review would reduce hospitalisations and deaths among elderly people in nursing homes. | The clinical medication review (Lowe 2000), which was conducted by the pharmacist, comprised a review of the GP clinical record, and a consultation with the participant and carer. The pharmacist made recommendations and passed them on a written proforma to the GP for acceptance and recommendation. | The pharmacist conducted a medication review in which the pharmacist identified the drugs that were taken, identified the original indication for each drug, assessed adherence to medication, and identified unaddressed medical problems. They also considered the continuing need for each drug, identified side effects, identified drug interactions or contraindications, and considered costs. Finally, the pharmacist implemented and documented any changes. | The study pharmacist provided the intervention. | The intervention was delivered individually and face‐to‐face. |
GP: general practitioner
2. Tentative description of interventions (part 2).
| Study | Location of intervention | When and how much of the intervention was delivered | Tailoring | Modifications | Adherence planning | Adherence assessment |
| Alvarez 2001 | 83 community pharmacies in the provinces of Asturias, Barcelona, Madrid and Biscay | The intervention was delivered once. | There was no tailoring made to the intervention. | Two additional seminars were given to the intervention group on real cases in order to approve the intervention. | Not undertaken | Not undertaken |
| Bernsten 2001 | Community pharmacies in 7 European countries; Denmark, Germany, The Netherlands, Northern Ireland (co‐ordinating centre), Portugal, Republic of Ireland and Sweden. A minimum of 12 sites per country were chosen according to specific criteria set within each participating country relating to the population of elderly people who visited the pharmacy, staffing levels within the pharmacy and working relationships with local GPs. |
The intervention was delivered at least once according to the study manual. However, Each site was free to provide as much information as possible to the intervention group as per the study manual. | A study manual describing the intervention was developed for all the participating countries. Each country translated the manual into their own language. | Each country adapted the manual, translating and modifying sections where appropriate, according to differing national practices. | Not undertaken | Not undertaken |
| Campins 2016 | 7 Primary Health care clinics in Mataró and Argentona | The intervention included 3 phases and the participants were followed up for 12 months. It is not clear if the intervention was repeated more than once. | There was no tailoring made to the intervention. | There were no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Coleman 1999 | 9 primary care physician practices in Washington State. Clinics were allowed to select their target condition of focus: frail older adults or people with diabetes. The physicians were board certified in Family Practice and did not have formal training or certification in geriatric medicine. |
The intervention was undertaken once. However there was variability in the frequency of one of its components. | There was no tailoring made to the intervention. | There were no modifications made to the intervention during the study. | A priori process of care measures for each of the geriatric syndromes were developed with decision rules for acceptable documentation by the study reviewers for the interventions. |
The chart abstraction of assessing the documentation for the interventions was performed by one member of the study team along with an additional reviewer blinded to knowledge of the study group and study hypothesis. The overall level of agreement between the 2 reviewers was acceptable based on published ranges (kappas for geriatric syndrome process measures 0.75 to 0.85) |
| Frankenthal 2014 | Chronic care geriatric facilities in Central Israel | The intervention was done once at 6 months and 12 months later. | There was no tailoring made to the intervention. | There were no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Garcia‐Gollarte 2014 | A private organisation of 37 nursing homes in Spain | It is unclear how many times the intervention was given as the educator offered further on‐demand advice on prescription for the next 6 months. |
There was no tailoring made to the intervention. | There was no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Gernant 2016 | Home health patients within a medicare insured home health population in Canada | The intervention was undertaken at least once however, some participants received more than one phone call as additional telephone follow‐up was provided as needed per the pharmacists' discretion during the first 30 days of the 60‐day home healthcare episode. |
Some participants received additional follow‐up depending on their conditions. | There were no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Gurwitz 2014 | Large multispecialty group practice employing 265 physicians, including 66 primary care providers caring for adults in the outpatient setting |
Daily records generated by the computer system were examined. | There was no tailoring made to the intervention. | There were no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Hawes 2014 | 804‐bed academic medical centre in North Carolina, USA | The intervention took place once. | There was no tailoring made to the intervention. | Only hospitalisations and ED visits at the study institution were included for those participants who were not able to be contacted after 3 phone call attempts. |
Not undertaken | Not undertaken |
| Holland 2005 | Home‐based medication review after discharge from acute or community hospitals in Norfolk and Suffolk, UK. | The intervention was performed once. | It is possible that a small number of participants in both groups may have had their medication reviewed during the follow‐up period by their GP or community pharmacist. | There were no modifications made to the intervention during the study. | Not undertaken. No data on adherence were collected. | Not undertaken |
| Ibrahim 2013 | Telephone consultation with home visits | The intervention was performed once. | Any additional contact as requested by the participant in the intervention group was undertaken. | There were no modifications made to the intervention during the study. | Not undertaken | Not undertaken |
| Kaczorowski 2011 | Community‐based pharmacies in Canada | The intervention was performed once as planned. | The local lead organisations used several strategies to recruit volunteer peer health educators. These strategies included using the local lead organisation's existing volunteer base, advertising in the local media, and giving presentations at local seniors’ clubs. |
When required, Cardiovascular Health Awareness Program support staff produced and mailed invitation letters on behalf of participating physicians (CHAP 2017). | Feedback of results was given to primary healthcare providers. | Evaluation data collected for the purpose of ongoing evaluation and quality improvement: 1. Success of different advertising/invitation strategies 2. Attendance, consent, completed assessments 3. Nurse assessments, pharmacist consults, fax/call to family physician the same day. Feedback to family physicians, pharmacists, and participants |
| Korajkic 2011 | Outpatients clinic in Melbourne, Australia | The intervention was performed once as planned. | There was no tailoring made to the intervention. | There were no modifications made to the intervention during the study. | There were written instructions on how to adjust the dose of frusemide per weight increase. | Data on dosage adjustment of frusemide were collected and compared against the initial criteria. |
| Krska 2001 | General medical practices in the Grampian region of Scotland | The intervention was performed once as planned. | In the control group, when pharmacists considered a review to be serious and beneficial to the participants, an independent medical assessor decided on the need to withdraw the participants on clinical grounds. | There were no modifications made to the intervention during the study. | Any outstanding care issues in both groups were communicated to the participant's GP. | Not undertaken |
| Lapane 2011 | 25 nursing homes serviced by 2 long‐term care pharmacies in Northern Ireland | It is unclear the number of times the reports were generated and used by the pharmacists for every resident. | The Geriatric Risk Assessment MedGuide database software for falls and delirium was integrated into the pharmacies’ commercial pharmacy software system (Rescot LTCP System) for the intervention homes (Tobias 1999). |
It is unclear if there were any modifications to the interventions. | The computer system did not capture if the recommendations done by the pharmacist were accepted. | Not undertaken |
| Lenaghan 2007 | A GP setting in Norfolk, UK | It is unclear how many times the pharmacist and the GP met to discuss participant's care plan. | A follow‐up visit with the participant occurred 6‐8 weeks later to reinforce the original advice, and assess whether there were any further pharmaceutical care issues to address with the GP. |
It is unclear if there were any modifications to the interventions. | Not undertaken | Not undertaken |
| Lowrie 2012 | The study was conducted within the NHS which provides free health care to the population of the UK. 27 primary care‐based pharmacists employed by the NHS to work with family doctors | It is unclear how many times the pharmacist met the participant and the GP. | If there was agreement between the pharmacist and the participant during the consultation and subsequently with the family doctor, medications were initiated, discontinued, or modified by the pharmacist during 3‐4 subsequent weekly or fortnightly consultations. | It is unclear if there were any modifications to the interventions. | Not undertaken | Not undertaken |
| Malet‐Larrea 2016 | The study was conducted in 178 community pharmacies in Spain | It is unclear how many times the intervention was undertaken. | A specifically trained pharmacist called a practice change facilitator helped pharmacists of the intervention group in the provision of the medication review with follow‐up service, identifying barriers specific to each pharmacy and providing solutions. | It is unclear if there were any modifications to the interventions. | The practice change facilitator ensured fidelity to the intervention and supported pharmacists of both study groups on queries about documentation forms. |
The experts were requested to answer individually for each case and the degree of agreement between them was later established. Inter‐rater reliability was measured using Fleiss's kappa. |
| Malone 2000 | 9 Veterans Affairs medical centres in the USA | It is unclear how many times participants were seen by the pharmacist in the intervention group as the protocol indicated that each participant should have at least 3 visits with the clinical pharmacist during the study, but participants could be seen as frequently as deemed necessary to ensure appropriate care. | To prevent contamination, some sites marked medical records of intervention and control participants to alert clinical pharmacists that participants were in the study. Other sites noted this distinction in electronic medical records. One site distributed a list of participants enrolled in the study to all pharmacists providing primary care. |
Clinical pharmacist intervention, however, occurred in one control participant; this participant was withdrawn from the study and his data were not included in the results. | Each contact with the participant was recorded on a standard data collection form that contained information about the method of contact, estimated time spent with the participant, medical problems addressed, drug‐related problems addressed, and drug‐related problems resolved. | Each month after enrolment the co‐ordinating centre received electronic data on each participant's prescription drugs dispensed in the preceding month. When participants either completed the study or died, data on resource use from enrolment to termination were retrieved. |
| Moertl 2009 | Ambulatory patients participating in the EuroHeart Failure Survey programme in Vienna | It is unclear how many times the nurse visited the intervention participants as more visits were made optional for participants. | More frequent contacts such as visits or telephone calls between the nurse and the participants were optional in case the participant or the nurse considered them necessary. |
The nurse was in charge of individualised participant and caregiver education and enhancement of self‐management. If the nurse noted any deterioration in the participant's status, she reported to the treating physician or advised the participant to visit the treating physician. |
Not undertaken | Not undertaken |
| Murray 2004 | Academic primary care internal medicine practice in the USA | It is unclear how many times the intervention was undertaken. | There was no tailoring made to the intervention. | There were no modifications made to the interventions. | Data necessary to generate care suggestions were derived from the computer programme. Treatment suggestions fell into 5 major categories. | Not undertaken |
| Nabagiez 2013 | Ambulatory patients discharged from a large 702‐bed hospital in Staten University Hospital, USA | It is unclear how many times the physician visited each participant in the home after their discharge. | There was no tailoring made to the intervention. | There were some modifications done to the intervention due to the participants not being available at the weekend. Participants were not seen directly after discharge as per the study protocol. | All findings were documented on the intervention visit form. | It is unclear if this was undertaken. |
| Okamoto 2001 | Managed care organisation in California, USA | It is unclear how many times participants were seen by the pharmacist in the intervention group as additional follow‐up was organised by the pharmacists for some participants. | Additional follow‐up was organised by the pharmacists for some participants. | The intervention was not modified. | Not undertaken | Not undertaken |
| Olesen 2014 | Patients living at home in the municipality of Aarhus, Denmark | The intervention was performed at the intended follow‐up. | Pharmacists could consult the project physician if they considered a participant's medication problems to be life‐threatening. |
The intervention was not modified. | Adherence to the medications were assessed by a pill‐count in all participants during 1 year. | Pill count was undertaken |
| Pai 2009 | The study took place in a non‐profit university‐affiliated dialysis clinic in Albany, USA. | It is unclear if all participants received the same number of follow‐up visits by the pharmacist or the physician in the intervention group. | It is unclear if there was any tailoring made to the intervention. | The intervention was not modified. | Not undertaken | Not undertaken |
| Roberts 2001 | 52 nursing homes located in south‐east Queensland and north‐east New South Wales, Australia | There was variability in the number of educational sessions provided to staff in each nursing home as well as the number of visits by the intervention pharmacists. | It is unclear if there was any tailoring made to the intervention. | It is unclear if the intervention was modified. | Validation of prescription claim data with participants' medications profiles. | To validate prescription claims data, a sample of 1328 cross‐sectional medication profiles were collected for 8 nursing home clusters for control and intervention homes at post‐intervention. An audit, comparing original post‐intervention medication data with the same data recollected up to 6 weeks later for a 6%, random sample, showed an overall reproducibility of 97% (range 92% to 100%) |
| Rytter 2010 | Patients discharged from Glostrup Hospital, Denmark. | The intervention was performed as prescribed. | There was no tailoring made to the intervention. | There was no modification made to the intervention. | Not undertaken | Not undertaken |
| Triller 2007 | Heart failure patients discharged from hospital in Albany, Scotland | The intervention was performed as prescribed. | The clinical pharmacist accessed and reviewed all pertinent physician notes and laboratory test values via the National Endowment for the Humanities data system and interacted with prescribers on behalf of the participants, as necessary. | There was no modification made to the intervention. | Not undertaken | Not undertaken |
| Zermansky 2001 | 4 GPs in Leeds, UK | It is unclear how many times the pharmacist visited the participant. | Immobile participants were visited at home. Non‐attenders were invited once more by telephone. | The study authors agreed with each practice the level of intervention that the pharmacist could make without seeking prior approval | It is unclear if this was undertaken. | It is unclear if this was undertaken. |
| Zermansky 2006 | 65 care homes for the elderly in Leeds, UK | It is unclear how many times the pharmacist reviewed each participant. | There was no tailoring made to the intervention. | There was no modification made to the intervention. | Pharmacists filled in a proforma sheet including their recommendations. | GP acceptance of the recommendations was signified by ticking a box on the proforma. |
ED: emergency department; GP: general practitioner; NHS: National Health Service
Participants and study setting
There is a great deal of diversity in types of professionals involved and where the studies occurred. However, most (61%) of the interventions were conducted by pharmacists or a combination of pharmacists and medical doctors. The studies took place in many different countries; 65% took place in either the USA or the UK. The study settings included general practices (Coleman 1999; Gurwitz 2014; Krska 2001; Lowrie 2012; Murray 2004;Zermansky 2001), community pharmacies (Alvarez 2001; Bernsten 2001; Malet‐Larrea 2016), patient homes or community settings (Campins 2016; Gernant 2016; Kaczorowski 2011; Holland 2005; Ibrahim 2013; Lenaghan 2007; Olesen 2014; Rytter 2010; Triller 2007), outpatient clinics (Hawes 2014; Korajkic 2011; Malone 2000; Moertl 2009; Okamoto 2001;Pai 2009), and aged care facilities (Frankenthal 2014; Garcia‐Gollarte 2014; Lapane 2011; Roberts 2001; Triller 2007;Zermansky 2006).
Interventions
We included a total of 30 studies (169,969 participants) in this review.
Four studies (8266 participants) reported on professional interventions (Garcia‐Gollarte 2014; Gurwitz 2014; Lapane 2011; Murray 2004). Two of these studies (3889 participants) reported on the number of hospital admissions (Lapane 2011; Murray 2004), one study (3661 participants) reported on the number of people admitted to hospital (Gurwitz 2014), one study (3538 participants) reported on mortality (Lapane 2011), and two studies (1067 participants) reported on the number of emergency department visits (Garcia‐Gollarte 2014; Murray 2004).
A total of 26 studies (161,703 participants) reported on organisational interventions (Alvarez 2001; Bernsten 2001; Campins 2016; Coleman 1999; Frankenthal 2014; Gernant 2016; Hawes 2014; Holland 2005; Ibrahim 2013; Kaczorowski 2011; Korajkic 2011; Krska 2001; Lenaghan 2007; Lowrie 2012; Malet‐Larrea 2016; Malone 2000; Moertl 2009; Nabagiez 2013; Okamoto 2001; Olesen 2014; Pai 2009; Roberts 2001; Rytter 2010; Triller 2007; Zermansky 2001; Zermansky 2006). Eleven trials (6203 participants) reported on number of hospital admissions (Coleman 1999; Holland 2005; Ibrahim 2013; Krska 2001; Lenaghan 2007; Lowrie 2012; Malone 2000; Moertl 2009; Nabagiez 2013; Okamoto 2001;Rytter 2010). A total of 13 studies (152,237 participants) reported on the number of people admitted to hospital (Alvarez 2001; Bernsten 2001; Campins 2016; Frankenthal 2014; Hawes 2014; Kaczorowski 2011; Korajkic 2011; Malet‐Larrea 2016; Nabagiez 2013; Olesen 2014; Triller 2007; Zermansky 2001; Zermansky 2006). Five studies (1819 participants) reported on emergency department visits (Alvarez 2001; Coleman 1999; Gernant 2016; Hawes 2014; Ibrahim 2013), and 12 studies (154,962 participants) reported on mortality (Campins 2016; Holland 2005; Kaczorowski 2011; Lenaghan 2007; Lowrie 2012; Moertl 2009; Olesen 2014; Pai 2009; Roberts 2001; Triller 2007; Zermansky 2001; Zermansky 2006).
We did not find any studies that fitted the criteria of structural interventions. This was in concordance with the EPOC taxonomy of interventions (Appendix 1).
The 'Characteristics of included studies' tables provide a summary of the interventions and comparisons. The interventions varied. Professional interventions included the use of health information technology to identify people at risk of medication problems: computer‐generated care suggested and actioned by physicians; electronic notification system about dose changes, drug interventions and follow‐up; and educational interventions on drug use aimed at physicians to improve drug prescriptions. Organisational interventions included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics and home visits by clinicians.
Outcomes
The primary outcomes were number of hospital admissions and number of people admitted to hospital. A total of 13 studies (10,092 participants) reported on number of hospital admissions (Coleman 1999; Holland 2005; Ibrahim 2013; Krska 2001; Lapane 2011; Lenaghan 2007; Lowrie 2012; Malone 2000; Moertl 2009; Murray 2004; Nabagiez 2013; Okamoto 2001; Rytter 2010); and 14 studies (155,898 participants) reported on number of people admitted to hospital (Alvarez 2001; Bernsten 2001; Campins 2016; Frankenthal 2014; Kaczorowski 2011; Gurwitz 2014; Hawes 2014; Korajkic 2011; Malet‐Larrea 2016; Nabagiez 2013; Olesen 2014; Triller 2007; Zermansky 2001;Zermansky 2006).
The secondary outcomes were the number of emergency department visits and mortality. Seven studies (2886 participants) reported on the number of emergency department visits (Alvarez 2001; Coleman 1999; Garcia‐Gollarte 2014; Gernant 2016; Hawes 2014; Ibrahim 2013; Murray 2004); and 13 studies (158,500 participants) reported on mortality (Campins 2016; Holland 2005; Kaczorowski 2011; Lenaghan 2007; Lapane 2011; Lowrie 2012; Moertl 2009; Olesen 2014; Pai 2009; Roberts 2001; Triller 2007; Zermansky 2001; Zermansky 2006).
Excluded studies
We have summarised the 59 excluded studies, with the reasons for their exclusion in the 'Characteristics of excluded studies' table. We excluded studies with an unsuitable design (Furniss 2000; Graffen 2004; Hugtenburg 2009; Lee 1996; Leendertse 2011; Leendertse 2013; Mills 2001; Montero‐Balosa 2016; Moreno 2016; Ni 2016; Safran 1993; Saltzberg 2011). Other reasons for exclusion were studies that did not occur in a primary care setting (Alassaad 2014; Barker 2012; Bell 2016; Bonnet‐Zamponi 2013; Briggs 2015; Gorgas 2012; Hanlon 1996; Keane 2014; Naunton 2003; Neven 2016; Xin 2014); results reported elsewhere (Sturgess 2003); data for outcomes not available (Cowper 1998; Knowlton 1994; Liu 2010; Yuan 2003); interventions not relevant to the review (Al‐Arifi 2014; Benard‐Laribiere 2015; Carrington 2013; Fredericks 2013; Pinnock 2013); outcomes not relevant to the review (Barker 2016; Barnes 2014; Basheti 2016; Billington 2015; Clyne 2015; Clyne 2016; Dhalla 2014; Geurts 2016; Guthrie 2016; Hallsworth 2016; Huiskes 2014; Malin 2016; Perula 2014; Setter 2009; Sinnott 2015; Wolf 2015); and 10 studies were published protocols (Alicic 2016; Bhatt 2014; Clyne 2013; Desveaux 2016; Elliott 2014; Forster 2015; Phung 2013; Przytula 2015; Stingl 2016; Wooster 2016), as described in the PRISMA diagram (see Figure 1).
Risk of bias in included studies
We have presented details of risk of bias in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
A total of 21 studies reported adequate sequence generation (Bernsten 2001; Campins 2016; Frankenthal 2014; Garcia‐Gollarte 2014; Gernant 2016; Gurwitz 2014; Hawes 2014; Holland 2005; Kaczorowski 2011; Krska 2001; Lowrie 2012; Malet‐Larrea 2016; Malone 2000; Okamoto 2001; Olesen 2014; Pai 2009; Roberts 2001; Rytter 2010; Triller 2007; Zermansky 2001; Zermansky 2006); and 18 reported adequate concealment of allocation (Bernsten 2001; Campins 2016; Frankenthal 2014; Gernant 2016; Gurwitz 2014; Hawes 2014; Holland 2005; Kaczorowski 2011; Lenaghan 2007; Lowrie 2012; Malone 2000; Malet‐Larrea 2016; Murray 2004; Pai 2009; Roberts 2001; Rytter 2010; Triller 2007;Zermansky 2001).
Blinding
Thirteen studies adequately blinded measurements of participants and personnel delivering the intervention (Bernsten 2001; Campins 2016; Coleman 1999; Garcia‐Gollarte 2014; Gernant 2016; Gurwitz 2014; Kaczorowski 2011; Krska 2001; Lapane 2011; Lowrie 2012; Murray 2004; Pai 2009; Triller 2007), whereas adequate blinding of outcome assessment was undertaken in 19 studies (Bernsten 2001; Campins 2016; Frankenthal 2014; Garcia‐Gollarte 2014; Gurwitz 2014; Hawes 2014; Holland 2005; Kaczorowski 2011; Korajkic 2011; Krska 2001; Malet‐Larrea 2016; Murray 2004; Nabagiez 2013; Olesen 2014; Pai 2009; Roberts 2001; Rytter 2010; Triller 2007;Zermansky 2006). Eleven studies reported an unclear risk of detection bias (Alvarez 2001; Coleman 1999; Gernant 2016; Ibrahim 2013; Lapane 2011; Lenaghan 2007; Lowrie 2012; Malone 2000; Moertl 2009; Okamoto 2001;Zermansky 2001).
Incomplete outcome data
A total of five studies had high risk of attrition bias (Alvarez 2001; Bernsten 2001; Hawes 2014; Nabagiez 2013; Pai 2009). Twenty‐two studies adequately addressed problems with incomplete outcomes (Campins 2016; Frankenthal 2014; Gernant 2016; Gurwitz 2014; Holland 2005; Ibrahim 2013; Kaczorowski 2011; Korajkic 2011; Krska 2001; Lapane 2011; Lenaghan 2007; Lowrie 2012; Malet‐Larrea 2016; Malone 2000; Moertl 2009; Okamoto 2001; Olesen 2014; Roberts 2001; Rytter 2010; Triller 2007; Zermansky 2001; Zermansky 2006), that is, these studies reported complete outcome data or they replaced any missing outcome data using a recognised statistical method, such as last observation carried forward with participants remaining in the group to which they had been allocated.
Selective reporting
There was no selective reporting in the included studies. All studies assessed their predefined primary and secondary outcomes.
Other potential sources of bias
Other potential sources of bias included protection against contamination, publication bias and other bias.
Protection against contamination bias
A total of 12 studies adequately protected against contamination bias (Bernsten 2001; Coleman 1999; Gernant 2016; Lapane 2011; Lowrie 2012; Murray 2004; Nabagiez 2013; Olesen 2014; Pai 2009; Roberts 2001; Rytter 2010; Triller 2007); whereas 16 studies, had unclear risk of protection against contamination (Alvarez 2001; Campins 2016; Frankenthal 2014; Gurwitz 2014; Hawes 2014; Holland 2005; Ibrahim 2013; Kaczorowski 2011; Korajkic 2011; Lenaghan 2007; Malet‐Larrea 2016; Malone 2000; Moertl 2009; Okamoto 2001; Zermansky 2001; Zermansky 2006), and two studies clearly did not adequately protect against contamination bias (Garcia‐Gollarte 2014; Krska 2001). Contamination bias occurs when members of the control group are inadvertently exposed to the intervention, thus potentially minimising the difference in outcomes between the two groups (Higgins 2011b).
Publication bias
Publication bias did not take place amongst the professional interventions due to the small number of studies included in the review. We have shown funnel plots of the main outcomes for the organisational interventions as follows: number of hospital admissions (Figure 3); number of people admitted to hospital (Figure 4); number of emergency department visits (Figure 5); and mortality (Figure 6). There was no evidence of publication bias.
3.

Funnel plot of comparison: 2 Organisational interventions, outcome: 2.1 Number of hospital admissions
4.

Funnel plot of comparison: 2 Organisational interventions, outcome: 2.2 Number of people admitted to hospital
5.

Funnel plot of comparison: 2 Organisational interventions, outcome: 2.3 Number of emergency department visits
6.

Funnel plot of comparison: 2 Organisational interventions, outcome: 2.4 Mortality
Other bias
A total of 22 studies had an unclear risk of 'other bias' (Bernsten 2001; Campins 2016; Coleman 1999; Gernant 2016; Gurwitz 2014; Hawes 2014; Ibrahim 2013; Korajkic 2011; Krska 2001; Lapane 2011; Lowrie 2012; Malet‐Larrea 2016; Malone 2000; Murray 2004; Nabagiez 2013; Okamoto 2001; Olesen 2014; Pai 2009; Roberts 2001; Rytter 2010; Zermansky 2001;Zermansky 2006). Seven studies had a high risk of 'other bias' (Alvarez 2001; Frankenthal 2014; Garcia‐Gollarte 2014; Kaczorowski 2011; Lenaghan 2007; Moertl 2009;Triller 2007), while only one study had a low risk of 'other bias' (Holland 2005). Other biases included inappropriate administration of the intervention, such as the method of training used to deliver the intervention or level of knowledge of the health professional delivering the intervention. Short length of the intervention was a bias in some studies (Kaczorowski 2011;Triller 2007), with the level of knowledge of the pharmacist or health professional delivering the intervention a bias in other studies. For example, in the study by Lenaghan 2007, research was carried out in one rural general practice with a single experienced review pharmacist, which may have had a bearing on the generalisability of the results.
In Bernsten 2001, one aspect of the study that was not rigorously controlled was the training of participating pharmacists. A study manual was provided to each participating pharmacist, followed by a one‐day training session. Further training was provided in individual countries; however, the extent of this was driven by available resources. Other biases include small sample sizes in the intervention arms; with 48 participants in Moertl 2009 and 77 participants in Triller 2007, and poor pharmacist‐prescriber communication, which may have reduced the efficacy of the intervention (Triller 2007). Alvarez 2001 did not report on any pre‐intervention data for most of the outcome measures. Garcia‐Gollarte 2014 had a short intervention of a six‐month period and a short follow‐up of three months. Frankenthal 2014 conducted the study in only one geriatric centre.
There was a total of eight cluster‐randomised studies included in the review (Alvarez 2001; Coleman 1999; Gernant 2016; Kaczorowski 2011; Lapane 2011; Lowrie 2012; Malet‐Larrea 2016; Roberts 2001). Cluster‐randomised studies include other potential biases such as recruitment bias and complete loss of a cluster in a trial. The extent of this type of bias was not fully reported and therefore we considered it under this section.
Effects of interventions
Professional interventions
We performed a meta‐analysis on the number of hospital admissions and the number of emergency department visits, as they were the only outcomes reported by more than one study. We have presented effect estimates and certainty of evidence for each outcome in Table 1 (see Appendix 3 for the full GRADE evidence profile). We obtained all reported data from the published papers.
Primary outcomes
We measured hospital admissions as either the number of hospital admissions or the number of people admitted to hospitals.
1. Number of hospital admissions
Two studies (3889 participants) reported on the number of hospital admissions (Lapane 2011; Murray 2004). Overall, professional interventions in health information technology to identify people at risk of medication problems in the case of Lapane 2011, or the computer‐generated care suggested and actioned by the physician described by Murray 2004, reported an increase in the number of hospital admissions, but the 95% confidence interval (CI) indicates that it probably makes little or no difference (risk ratio (RR) 1.24, 95% CI 0.79 to 1.96; moderate‐certainty evidence). There was no significant heterogeneity (I2 = 0%, P = 0.44) across the studies, as shown in Table 1.
2. Number of people admitted to hospital
One study (3661 participants) reported on number of people admitted to hospital (Gurwitz 2014). The study authors found that the intervention, which included an electronic notification system about dose changes, drug interactions, and follow‐up, made little or no difference to the number of people admitted to hospital (adjusted RR 0.99, 95% CI 0.92 to 1.06; high‐certainty evidence).
Secondary outcomes
1. Number of emergency department visits
Two studies (1067 participants) reported on this outcome (Garcia‐Gollarte 2014; Murray 2004). Garcia‐Gollarte 2014 described an educational intervention on drug use aimed at physicians to improve drug prescriptions. Murray 2004 described an intervention including computer‐generated care suggested and actioned by the physician. Both professional interventions described by the study authors may make little or no difference to the number of emergency department visits (adjusted RR 0.71, 95% CI 0.50 to 1.02; low‐certainty evidence). There was no significant heterogeneity among the two studies (I2 = 0%, P = 0.64).
2. Mortality
One study (3538 participants) reported on the number of deaths (Lapane 2011). The health information technology to identify people at risk of medication problems probably makes little or no difference to the number of deaths in the study population (adjusted RR 0.98, 95% CI 0.82 to 1.17; moderate‐certainty evidence).
Organisational interventions
We performed a meta‐analysis on the number of hospital admissions, number of people admitted to hospital, the number of emergency department visits, and mortality. We have presented effect estimates and certainty of evidence for each outcome in Table 2 (see Appendix 4 for the full GRADE evidence profile). We obtained all reported data from the published papers.
Primary outcome
1. Number of hospital admissions
Eleven trials (6203 participants) reported on the number of hospital admissions (Coleman 1999; Holland 2005; Ibrahim 2013; Krska 2001; Lenaghan 2007; Lowrie 2012; Malone 2000; Moertl 2009; Nabagiez 2013; Okamoto 2001; Rytter 2010). The organisational interventions included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians. Most interventions included optimisation of the medications that participants were taking or home visits by healthcare practitioners, or both. Overall, it is uncertain whether organisational interventions (which included pharmaceutical care or medication reviews by a doctor, a pharmacist, or a nurse, home visits, educational interventions with a pharmacist) reduce the number of hospital admissions (adjusted RR 0.85, 95% CI 0.71 to 1.03; very low‐certainty evidence. There was significant heterogeneity (I2 = 75%, P < 0.0001) across the studies (Analysis 2.1). The direction of the effect was consistent in 10 out of 11 trials. Holland 2005 reported an increase in the total number of hospital admissions (adjusted RR 1.31, 95% CI 1.13 to 1.50; based on very low‐certainty evidence. The study authors explained these findings by indicating that their study was not statistically powered to detect changes in hospital admissions and new admissions. The study authors explained the unusual increase in hospital admissions among participants by concluding that the participants were better informed about adverse events through the pharmacist intervention, and this promoted help‐seeking behaviour, which resulted in an admission.
2.1. Analysis.

Comparison 2: Organisational interventions versus standard care, Outcome 1: Number of hospital admissions
We undertook a sensitivity analysis by removing studies at high risk of bias (Holland 2005; Krska 2001; Moertl 2009; Nabagiez 2013), and again it is uncertain whether organisational interventions (which included pharmacist home visits, pharmaceutical care plan, home‐based nurse care and home visits by physician assistants) reduce the number of hospital admissions (adjusted RR 0.86, 95% CI 0.72 to 1.03). Ibrahim 2013, Moertl 2009 and Rytter 2010 showed a reduction in hospital admissions with a relatively narrow confidence interval: adjusted RR 0.40, 95% CI 0.22 to 0.74 (Ibrahim 2013); RR 0.38, 95% CI 0.19 to 0.72 (Moertl 2009); and RR 0.76, 95% CI 0.61 to 0.95 (Rytter 2010), in favour of the interventions. These three studies were characterised by frequent follow‐up by the clinical pharmacists. In the case of Ibrahim 2013, there was a three‐month follow‐up and a once‐a‐week telephone conversation; Moertl 2009 had frequent follow‐up by the clinical pharmacists at three, six, nine, and 12 months; and Rytter 2010 also had three follow‐up contacts by GPs and district nurses.
2. Number of people admitted to hospital
A total of 13 studies (152,237 participants) reported on the number of people admitted to hospital (Alvarez 2001; Bernsten 2001; Campins 2016; Frankenthal 2014; Hawes 2014; Kaczorowski 2011; Korajkic 2011; Malet‐Larrea 2016; Nabagiez 2013; Olesen 2014; Triller 2007; Zermansky 2001; Zermansky 2006). Most of the organisational interventions described included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians. Overall, organisational interventions may make little or no difference to the total number of people admitted to hospital in favour of the intervention group compared with the control group (adjusted RR 0.92, 95% CI 0.86 to 0.99; low‐certainty evidence) with significant heterogeneity (I2 = 47%, P = 0.03). Three studies showed that the organisational interventions reduced the total number of people admitted to hospital, as the RR was less than 1 (Bernsten 2001; Hawes 2014; Malet‐Larrea 2016).
We undertook a sensitivity analysis by removing studies at high risk of bias (Alvarez 2001; Bernsten 2001; Frankenthal 2014; Hawes 2014; Kaczorowski 2011; Malet‐Larrea 2016; Nabagiez 2013; Triller 2007), and again, the intervention made little or no difference to the number of people admitted to hospital (adjusted RR 0.98, 95% CI 0.85 to 1.13) with low, non‐significant heterogeneity between studies (I2 = 28%, P = 0.23).
Secondary outcomes
1. Number of emergency department visits
Five studies (1819 participants) reported on emergency department visits (Alvarez 2001; Coleman 1999; Gernant 2016; Hawes 2014; Ibrahim 2013). Overall, it is uncertain whether organisational interventions including medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians reduce emergency department visits in favour of the intervention group compared with the control group (adjusted RR 0.75, 95% CI 0.49 to 1.15; very low‐certainty evidence). Please refer to Table 2.
There was also significant heterogeneity between the studies (I2 = 73%, P = 0.005). We undertook a sensitivity analysis by removing studies at high risk of bias (Alvarez 2001; Hawes 2014). Pharmaceutical care and care transition clinic interventions may make little or no difference in emergency department visits (adjusted RR 0.68, 95% CI 0.37 to 1.27) with significant heterogeneity between studies (I2 = 79%, P = 0.009).
All studies showed wide confidence intervals, and although Alvarez 2001 showed an increase in the number of emergency department visits in favour of the intervention, this study had high risk of bias as there was a high proportion of incomplete data in the outcomes measured.
2. Mortality
A total of 12 studies (154,962 participants) reported on mortality (Campins 2016; Holland 2005; Kaczorowski 2011; Lenaghan 2007; Lowrie 2012; Moertl 2009; Olesen 2014; Pai 2009; Roberts 2001; Triller 2007; Zermansky 2001; Zermansky 2006). Overall, it is uncertain whether organisational interventions, which included medication reviews by pharmacists, nurses or physicians, clinician‐led clinics, and home visits by clinicians reduce mortality in favour of the intervention group (adjusted RR 0.94, 95% CI 0.85 to 1.03; very low‐certainty evidence) with non‐significant heterogeneity (I2 = 37%, P = 0.10). Please refer to Table 2.
We undertook sensitivity analysis by removing studies at high risk of bias (Holland 2005; Kaczorowski 2011; Lenaghan 2007; Moertl 2009; Olesen 2014; Roberts 2001), and we found that interventions addressing home visits by pharmacists, educational sessions to assess cardiovascular risk, medication reviews, home‐based nurse care, and nurse education probably made little or no difference to mortality (adjusted RR 1.02, 95% CI 90 to 1.17). There was no significant heterogeneity between studies after the removal of the six studies (I2 = 0%, P = 0.45).
Structural interventions
We did not find any studies that fitted the criteria of structural interventions.
Discussion
Summary of main results
The studies included in this Cochrane Review showed that, based on moderate‐ and low‐certainty evidence, interventions in primary care for reducing preventable medication errors probably make little or no difference to the number of people admitted to hospital or the number of hospitalisations, emergency department visits, or mortality. Most of the interventions took place in the UK and the USA; studies undertaken in high‐income countries with disadvantaged populations, and in low‐ and middle‐income countries, were underrepresented. This might affect the generalisability of the results. We undertook sensitivity analysis by removing studies at high risk of bias and detecting whether there was any difference on the overall effect size. Overall, there is no evidence of an effect in any of the outcomes.
Overall completeness and applicability of evidence
The types of interventions included in this review were based on the taxonomy of interventions developed by Cochrane EPOC (Appendix 1); four studies used professional interventions and 26 studies used organisational interventions. The professional interventions included the use of health information technology to identify people at risk of medication problems: computer‐generated care suggested and actioned by a physician; electronic notification systems about dose changes, drug interventions and follow‐up; and educational interventions on drug use aimed at physicians to improve drug prescriptions. Organisational interventions consisted of medication reviews by pharmacists, nurses or physicians, clinician‐led clinics and home visits by clinicians.
The interventions described in the review were complex and generally multifaceted, which resulted in significant heterogeneity. The variation in heterogeneity in the pooled estimates means that our results should be treated cautiously as the interventions may not have worked consistently across all studies due to differences in how the interventions were provided, background practice, setting, healthcare system, or delivery of the interventions. Another potential limitation is the quality of the studies. The methods sections of the studies provided varying levels of detail on how complex interventions were developed, the design of the trials or how staff were trained to deliver the interventions. There was also evidence of potential bias in some studies, with only 18 studies reporting adequate concealment of allocation and only 12 studies reporting appropriate protection from contamination, both of which may have influenced the overall effect estimate and the overall pooled estimate.
Certainty of the evidence
The certainty of the evidence obtained from the 'Summary of findings' tables using the GRADE system highlights the very low to high certainty of the evidence reported by the studies. The primary outcomes of number of hospital admissions and number of people admitted to hospital were reported to have very low‐ to high‐certainty evidence, respectively. Mortality was reported in 13 studies (1 study involving professional interventions, moderate‐certainty evidence; and 12 studies involving organisational interventions, very low‐certainty evidence), with the main type of biases being detection and performance bias. We considered studies reporting on emergency department visits to have low‐certainty evidence for professional interventions and very low‐certainty evidence for organisational interventions using the GRADE system, due to study design and heterogeneity. Further research and better study designs are likely to change the overall estimate reported using these outcomes.
The Methods sections provided few details about study methodology and how complex interventions were delivered. The overall quality of the evidence presented in this review is either at high risk or unclear risk of bias. The main limitations were the heterogeneity between studies, the imprecision in results due to the wide confidence intervals amongst studies, unclear selection bias, performance and detection bias, and attrition bias.
Potential biases in the review process
The number of studies that we were able to combine in the meta‐analysis was somewhat small due to subclassification of the interventions and because not all studies reported on all the outcomes of interest mentioned in the review. We did not place any language restrictions on the search strategy. The review included one study written in Spanish (Alvarez 2001). We were able to pool the data from this study with the help of a Spanish‐speaking colleague. Despite the limited number of studies that were included, funnel plots of studies reporting the outcomes of interest showed no apparent publication bias (Figure 3; Figure 4; Figure 5; Figure 6). Another limitation of the review included the definitions of 'pharmaceutical care' and 'pharmaceutical review' described in the studies, which may have led to different interventions. We also did not consider studies where participants were treated in the emergency department of hospitals, although we are aware that at times people could receive treatment in the emergency department without being admitted to hospital. We will consider these types of studies in our updated review. Finally, a sensitivity analysis with a separate comparison of cluster‐ and individual randomised trials may have yielded different results, and we will consider including this in our updated review.
Agreements and disagreements with other studies or reviews
Few studies have examined whether the types of interventions that were investigated in this review lead to reductions in hospital admissions, emergency department visits, or mortality. One of the few reviews that studied this problem (Royal 2006), found that pharmacist‐led medication reviews were effective in reducing hospital admissions, although restricting the analysis to randomised trials did not produce a significant benefit.
Previous observational studies addressing similar interventions also provide limited evidence of their effectiveness. A controlled study by Hugtenburg 2009, which included 37 community pharmacists and 715 participants, and examined the impact of medication reviews and participant counselling at discharge from the hospital by community pharmacists, found that the intervention was not effective at reducing mortality. Another open controlled study, conducted by Leendertse 2013, examined the effect of reviewing medications in primary care by pharmacists. They found that the intervention did not significantly reduce medication‐related hospital admissions. Moreover, a study by Safran 1993 examined the effect of an electronic medical record used by physicians to care for people with HIV on hospitalisation, emergency visits and mortality. The study authors found that the intervention was significant for emergency department visits, but not for mortality or hospitalisations. Our study mirrors these findings in that the interventions investigated in this review had little or no effect on the number of people admitted to hospital, number of hospital admissions, number of emergency department visits, or mortality.
Authors' conclusions
Implications for practice.
The evidence from this review does not fully support the benefits of interventions to reduce medication‐related preventable errors with respect to any of the outcomes of interest that were reported in this review. Both professional and organisational interventions had little or no significant effect on the outcomes of interest. Therefore, organisations implementing interventions to improve medication safety in primary care should be aware that the evidence endorsing these interventions is limited, both in number and methodological quality.
Implications for research.
Larger studies addressing both professional and organisational interventions and reporting on the number of people admitted to hospital and emergency department visits are needed before evidence‐based recommendations can be made, given that only one study with 3661 participants addressing professional interventions and 13 studies with 8960 participants reported on the number of people admitted to hospital in primary care following organisational interventions. Emergency department visits were only reported by two studies (1067 participants) describing professional interventions and five studies (1819 participants) describing organisational interventions.
Further, large studies exploring which interventions involving healthcare professionals (nurse, physician or pharmacist) are likely to have a beneficial effect in preventing errors in primary care should also be addressed. Furthermore, longer time frames for interventions and a focus on high risk participants/therapies would also help. The quality of the studies needs to be improved as the certainty of the evidence was very low to high. The methods sections of the studies provided varying levels of detail on how complex interventions were developed, the design of the trials, or how staff were trained to deliver the interventions. We did not identify any structural interventions and only four studies used professional interventions, so more work needs to be done with these types of interventions. Most of the studies did not provide details of what constituted 'usual care', so this can also be improved in future studies.
History
Protocol first published: Issue 4, 2002 Review first published: Issue 10, 2017
| Date | Event | Description |
|---|---|---|
| 14 January 2013 | New citation required and major changes | New authors list added and new contact author for this review is listed. Title changed from 'Interventions for reducing preventable drug‐related hospital admissions or preventable drug‐related morbidity in primary care'. The search strategy has been significantly revised from the original protocol. |
Acknowledgements
The authors would like to thank Brian Serumaga for his work on earlier versions of the review. In addition, the authors would like to thank Paul Miller (EPOC Information Specialist) for support with the search strategies, Emma Tavender and Clare Dooley (EPOC Managing Editors) for their support with the review, Ignacio Ricci Cabello for his translation of the Spanish article (Alvarez 2001), Eric Harvey (Peer Reviewer), Luke Vale (EPOC Economics Editor), Burnard Bernard (EPOC Editor), Christiana Kartsonaki (EPOC Statistician), Pierre Durieux (EPOC Contact Editor), and Denise Mitchell (Copy‐editor).
National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Effective Practice and Organisation of Care (EPOC). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
The Australasian Satellite of Cochrane EPOC is funded by the Australian Government National Health and Medical Research Council (NHMRC); it is a partnership between Cochrane EPOC and Monash University.
Appendices
Appendix 1. EPOC Taxonomy
Professional interventions
Distribution of educational materials (distribution of published or printed recommendations for clinical care, including clinical practice guidelines, audiovisual materials and electronic publications; the materials may have been delivered personally or through mass mailings).
Educational meetings (healthcare providers who have participated in conferences, lectures, workshops or traineeships)
Local consensus processes (inclusion of participating providers in discussion to ensure that they agreed that the chosen clinical problem was important and the approach to managing the problem was appropriate).
Educational outreach visits (use of a trained person who met with providers in their practice settings to give information with the intent of changing the provider's practice. The information given may have included feedback on the performance of the providers).
Local opinion leaders (use of providers nominated by their colleagues as 'educationally influential'. The investigators must have explicitly stated that their colleagues identified the opinion leaders).
Patient‐mediated interventions (new clinical information (not previously available) collected directly from patients and given to the provider e.g. depression scores from an instrument).
Audit and feedback (any summary of clinical performance of health care over a specified period of time. The summary may also have included recommendations for clinical action. The information may have been obtained from medical records, computerised databases, or observations from patients).
The following interventions are excluded
Provision of new clinical information not directly reflecting provider performance which was collected from patients, e.g. scores on a depression instrument, abnormal test results. These interventions should be described as patient‐mediated.
Feedback of individual patients' health record information in an alternate format (e.g. computerised). These interventions should be described as organisational.
Reminders (patient‐ or encounter‐specific information, provided verbally, on paper or on a computer screen, which is designed or intended to prompt a health professional to recall information. This would usually be encountered through their general education; in the medical records or through interactions with peers, and so remind them to perform or avoid some action to aid individual patient care. Computer‐aided decision support and drugs dosage are included).
Marketing (use of personal interviewing, group discussion ('focus groups'), or a survey of targeted providers to identify barriers to change and subsequent design of an intervention that addresses identified barriers)
Mass media (i. varied use of communication that reached great numbers of people including television, radio, newspapers, posters, leaflets, and booklets, alone or in conjunction with other interventions; ii. targeted at the population level)
Other (other categories to be agreed in consultation with the EPOC editorial team)
Financial interventions
Provider
Fee‐for‐service (provider has been paid for number and type of service delivered)
Prepaid (no other description)
Capitation (provider was paid a set amount per patient for providing specific care)
Provider salaried service (provider received basic salary for providing specific care)
Prospective payment (provider was paid a fixed amount for health care in advance)
Provider incentives (provider received direct or indirect financial reward or benefit for doing specific action)
Institution incentives (institution or group of providers received direct or indirect financial rewards or benefits for doing specific action)
Provider grant/allowance (provider received direct or indirect financial reward or benefit not tied to specific action)
Institution grant/allowance (institution or group of providers received direct or indirect financial reward or benefit not tied to specific action)
Provider penalty (provider received direct or indirect financial penalty for inappropriate behaviour)
Institution penalty (institution or group of providers received direct or indirect financial penalty for inappropriate behaviour)
Formulary (added or removed from reimbursable available products)
Other (other categories to be agreed in consultation with the EPOC editorial team)
Patient
Premium (patient payment for health insurance. It is important to determine if the patient paid the entire premium, or if the patient’s employer paid some of it. This includes different types of insurance plans).
Co‐payment (patient payment at the time of healthcare delivery in addition to health insurance, e.g. in many insurance plans that cover prescription medications, the patient may pay AUD 5 per prescription, with the rest covered by insurance).
User fee (patient payment at the time of healthcare delivery)
Patient incentives (patient received direct or indirect financial reward or benefit for doing or encouraging them to do specific action)
Patient grant/allowance (patient received direct or indirect financial reward or benefit not tied to specific action)
Patient penalty (patient received direct or indirect financial penalty for specified behaviour, e.g. reimbursement limits on prescriptions)
Other (other categories to be agreed in consultation with the EPOC editorial team)
Organisational interventions
Provider‐orientated
Revision of professional roles (also known as 'professional substitution', 'boundary encroachment' and includes the shifting of roles among health professionals. For example, nurse midwives providing obstetrical care; pharmacists providing drug counselling that was formerly provided by nurses and physicians; nutritionists providing nursing care; physical therapists providing nursing care. Also includes expansion of role to include new tasks).
Clinical multidisciplinary teams (creation of a new team of health professionals of different disciplines or additions of new members to the team who work together to care for patients)
Formal integration of services (bringing together of services across sectors or teams or the organisation of services to bring all services together at one time also sometimes called 'seamless care')
Skill mix changes (changes in numbers, types or qualifications of staff)
Continuity of care (including one or many episodes of care for inpatients or outpatients)
Arrangements for follow‐up
Case management (including co‐ordination of assessment, treatment and arrangement for referrals)
Satisfaction of providers with the conditions of work and the material and psychic rewards (e.g. interventions to 'boost morale')
Communication and case discussion between distant health professionals (e.g. telephone links; telemedicine; there is a television/video link between specialist and remote nurse practitioners)
Other (other categories to be agreed in consultation with the EPOC editorial team)
Patient‐orientated
Mail order pharmacies (e.g. compared to traditional pharmacies)
Presence and functioning of adequate mechanisms for dealing with patients' suggestions and complaints
Consumer participation in governance of healthcare organisation
Other (other categories to be agreed in consultation with the EPOC editorial team)
Structural
Changes to the setting/site of service delivery (e.g. moving a family planning service from a hospital to a school)
Changes in physical structure, facilities and equipment (e.g. change of location of nursing stations, inclusion of equipment where technology in question is used in a wide range of problems and is not disease specific, for example an MRI scanner)
Changes in medical records systems (e.g. changing from paper to computerised records, patient‐tracking systems)
Changes in scope and nature of benefits and services
Presence and organisation of quality monitoring mechanisms
Ownership, accreditation, and affiliation status of hospitals and other facilities
Staff organisation
Other (other categories to be agreed in consultation with the EPOC editorial team)
Regulatory interventions
Any intervention that aims to change health service delivery or costs by regulation or law. (These interventions may overlap with organisational and financial interventions).
Changes in medical liability
Management of patient complaints
Peer review
Licensure
Other (other categories to be agreed in consultation with the EPOC editorial team)
Appendix 2. Search Strategies
MEDLINE
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present
Searched 4 October 2016
| No. | Search terms | Results |
| 1 | *community pharmacy services/ | 2635 |
| 2 | (prevent* adj2 medication error?).ti,ab. | 436 |
| 3 | (((medication or drug) adj2 event) and ((primary adj2 care) or ((family or general) adj (practice or practitioner?)))).ti,ab. | 46 |
| 4 | ("safety of medications" or prescribing safety or safe prescribing or (safely adj prescribing)).ti,ab. | 325 |
| 5 | ((structured adj2 (assessment? or care)) and drug?).ti,ab. | 175 |
| 6 | (changes adj4 prescription?).ti,ab. | 568 |
| 7 | (medication adherence/ or patient compliance/) and pharmacists/ | 634 |
| 8 | (pharmacist? adj2 (driven or directed or managed)).ti. | 243 |
| 9 | or/1‐8 | 4923 |
| 10 | ((adverse drug or adverse medication?) adj2 (admission? or readmission? or event or reduce? or prevent*)).ti,ab. | 1126 |
| 11 | ((medication related or drug related) adj2 (event? or adverse or admission? or readmission? or readmitted or admitted or problem?)).ti,ab. | 4316 |
| 12 | ((prevent* or reduce? or reducing or reduction or improve or lower or fewer) adj3 adverse drug).ti,ab. | 890 |
| 13 | dt.fs. and ((readmission? or readmit*) adj4 (avoid* or fewer or improv* or less or lower or preventable or rate or rates or reduce? or reduction? or related or unnecessary or avoidable)).ti,ab. | 504 |
| 14 | patient readmission/ and ((drug or prescription? or medication?) adj2 (error? or inappropriat* or problem? or related)).ti,ab. | 85 |
| 15 | inappropriate prescribing/ | 1557 |
| 16 | medication reconciliation/ | 580 |
| 17 | community pharmacy services/ | 3350 |
| 18 | (community pharmacy or community pharmacist?).ti. | 1577 |
| 19 | (exp drug therapy/ or (pharmaceutical? or prescrib* or prescription? or medication?).ti,ab,hw.) and (near miss or near misses or never event?).ti,ab. | 274 |
| 20 | (changes adj3 (medication? or prescription? or prescribing)).ti,ab. | 2865 |
| 21 | patient readmission/ and (preventing or preventable or prevent? or unnecessary or avoidable or reduce? or reducing or reduction? or fewer).ti. | 740 |
| 22 | (self administration/ or medication adherence/) and medication errors/ | 239 |
| 23 | medication errors/ and (prevent*.ti. or (preventable or avoidable).ab.) | 931 |
| 24 | (medication adherence and self care).ti,ab. | 199 |
| 25 | medication adherence.ti,ab. and (self care.ti,ab. or patient education.ti,ab,hw.) | 848 |
| 26 | (medication review and (community or home)).ti. or (medication review adj2 (community or home)).ab. | 48 |
| 27 | (pharmacist? adj2 (driven or directed or managed)).ab. | 390 |
| 28 | decision support systems, clinical/ and ((drug? or medication?) adj3 (management or prevent* or adverse or event? or related)).ti,ab. | 284 |
| 29 | or/10‐28 | 17871 |
| 30 | medication errors/ | 11484 |
| 31 | (medication review or medication reconciliation).ti,ab. or clinical audit/ | 2574 |
| 32 | exp *drug therapy/ and ((adverse and event?).ti. or (adverse drug event? or adverse medication*).ab.) | 1070 |
| 33 | ((problem? or hospitali?ation? or mortality or morbidity or illness*) adj2 (drug related or drug induced)).ti,ab. | 1571 |
| 34 | ((error? or mistake? or wrong or adverse event? or near miss or near misses or never event? or incorrect* or inappropriat*) adj3 (drug? or dose or doses or dosage or dosing or pharmaceutical or medication? or prescription? or prescribing)).ti,ab. | 17195 |
| 35 | (dispensing adj2 (error? or mistake? or wrong)).ti,ab. | 262 |
| 36 | (drug therapy/ or prescription drugs/ or drug prescriptions/ or pharmaceutical preparations/ or drug dosage calculations/ or drug repositioning/ or drug substitution/ or "off‐label use"/ or "drug therapy, combination"/ or "drug therapy, computer‐assisted"/ or polypharmacy/) and (mortality/ or (((preventable or avoidable or prescrib* or medication) adj2 (error? or event?)) or mistake? or wrong or incorrect* or inappropriat*).ti,ab.) | 4290 |
| 37 | or/30‐36 | 29692 |
| 38 | primary health care/ or general practice/ or family practice/ or general practice, dental/ or primary care nursing/ | 130955 |
| 39 | ((primary adj4 (care or healthcare)) or ((general or family) adj2 practice)).ti,ab. | 148502 |
| 40 | (primary care or family medic* or general practice or family practi*).jn. | 8882 |
| 41 | community medicine/ or community health nursing/ or community health services/ or community health centers/ or home care services/ | 79553 |
| 42 | (community care or community healthcare).ti,ab. | 4095 |
| 43 | ambulatory care facilities/ or ambulatory care/ | 54315 |
| 44 | ((ambulatory or walk‐in or neighbo?rhood or community) adj2 (clinic? or care centre or care centres or care center? or health* centre or health* centres or health* center?)).ti,ab. | 10784 |
| 45 | maternal‐child health centers/ or outpatient clinics, hospital/ or pain clinics/ or community mental health centers/ | 21343 |
| 46 | nursing homes/ or intermediate care facilities/ or skilled nursing facilities/ | 34654 |
| 47 | (nursing home? or care facility).ti,ab. | 30670 |
| 48 | or/38‐47 | 398235 |
| 49 | general practitioners/ or physicians, family/ or physicians, primary care/ | 22546 |
| 50 | ((general or family) adj2 (practitioner? or physician? or doctor?)).ti,ab. | 65949 |
| 51 | nurse practitioners/ or physician assistants/ | 19913 |
| 52 | (physician? assistant? or doctor? assistant? or physician? extender? or feldsher?).ti,ab. and (ambulatory or community or outpatient? or out‐patient?).ti,ab,hw. | 672 |
| 53 | (structured assessment? or structured care or case management).ti,ab. | 10118 |
| 54 | nurses.ti. or (nurse? adj2 prescrib*).ti,ab. | 57795 |
| 55 | *pharmacists/ | 8741 |
| 56 | pharmacist?.ti. | 10013 |
| 57 | or/49‐56 | 174871 |
| 58 | pharmacists/ or pharmacists' aides/ or pharmaceutical services/ or drug information services/ or clinical pharmacy information systems/ | 23085 |
| 59 | medication review.ti,ab. | 667 |
| 60 | (pharmaceutical care or pharmacy or pharmacies or pharmacist? or prescriber? or prescribing or prescription? or drug therapy).ti. | 61398 |
| 61 | ((pharmacist? or prescription? or prescribing or medication?) adj3 (consult* or review* or service or services)).ab. | 6688 |
| 62 | ((medication? or prescrib* or pharmac*) adj2 (manage? or management or service? or system?)).ti,ab. | 18502 |
| 63 | (drug? assess* or drug? audit? or drug? reconcil*).ti,ab. | 387 |
| 64 | (("drug therapy" or dosage? or dose? or medication? or prescription? or prescrib* or pharmacist? or pharmaceutical care) adj2 (managing or management or monitor*)).ti,ab. | 9624 |
| 65 | ((improv* or optimi?ing or optimi?e? or optimal*) and (dosing or dosage)).ti. or ((improv* or optimi?ing or optimi?e? or optimal*) adj2 (pharmaceutical care or pharmacy or prescrib* or prescript*)).ab. | 3363 |
| 66 | ((drug therapy or drug regime? or medication? or medicines or pharmacy or pharmacist? or pharmaceutical or prescrib* or prescription?) adj2 (audit* or monitor* or reconcil* or review?)).ti,ab. | 7239 |
| 67 | drug utilization review/ | 3363 |
| 68 | medication adherence/ or self administration/ | 21980 |
| 69 | or/58‐68 | 118774 |
| 70 | ((unexpected or return) adj2 (emergency adj2 (department? or room? or visit?))).ti,ab. | 62 |
| 71 | (emergency adj3 (visit? or room? or clinic? or admission?) adj3 (reduc* or fewer or lower)).ti,ab. | 922 |
| 72 | reduc* hospital admissions.ti,ab. | 362 |
| 73 | (readmission? adj3 (reduc* or fewer or lower)).ti,ab. | 2124 |
| 74 | ((hospital admission? or (readmit* or readmission?)) adj3 (reduc* or fewer or lower)).ti,ab. or patient readmission/ | 13305 |
| 75 | ((preventable or avoidable) adj3 (admission? or readmission? or readmit*)).ti,ab. | 573 |
| 76 | patient compliance.ti,ab. | 7649 |
| 77 | (adverse drug or adverse event?).ti,ab. | 122534 |
| 78 | (improve or improvement or improv* patient? or patient outcomes).ti,ab. | 1028191 |
| 79 | (ambulatory or outpatient? or out‐patient?).ti,ab,hw. | 267824 |
| 80 | or/70‐79 | 1376183 |
| 81 | (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. | 1071692 |
| 82 | exp animals/ not humans.sh. | 4323394 |
| 83 | 81 not 82 | 988060 |
| 84 | 37 and (or/48,57) | 4572 |
| 85 | 69 and (or/48,57) and 80 | 7995 |
| 86 | or/9,29 | 19108 |
| 87 | 84 or 85 or 86 | 27506 |
| 88 | (clinical decision and drug?).ti. | 83 |
| 89 | (collaborative and (drug? or medication?) and management).ti. | 45 |
| 90 | ((safe or safety) and ((medication? or drug?) adj management)).ti. | 26 |
| 91 | ((reduce or reducing or reduced or reduction) and ((medication? or prescrib*) adj2 (error? or mistake or adverse or event?))).ti. | 269 |
| 92 | or/88‐91 | 422 |
| 93 | 87 and 83 | 4692 |
| 94 | 92 or 93 | 5101 |
| 95 | ((prevent* or reduce or reducing) adj2 (medication error? or adverse drug or adverse medication)).ti,ab. | 1483 |
| 96 | medication errors/ and (avoid* or intervention or prevent* or reducing or rate or improv* or quality).ti,ab,hw. | 5124 |
| 97 | ((medication? or drug or prescription?) adj (error? or mistake?) adj5 (avoid* or intervention? or prevent* or reducing or quality improv*)).ti,ab. | 1052 |
| 98 | inappropriate prescribing/ and (avoid* or intervention or prevent* or reducing or rate or improv* or quality).ti,ab,hw. | 759 |
| 99 | decision support systems, clinical/ and ((drug? or medication?) adj2 (management or adverse or event? or related)).ti,ab. | 241 |
| 100 | ((structured adj2 (assessment? or care)) and (drug? or medication?)).ti,ab. | 277 |
| 101 | (structured assessment? or structured care or case management).ti,ab. and (drug therapy or medication? or pharmaco* therapy or prescription? or prescribing).ti,ab,hw. | 780 |
| 102 | ("safety of medications" or prescribing safety or safe prescribing or (safely adj prescribing)).ti,ab. | 325 |
| 103 | ((safe or safety or quality improv*) and ((medication? or drug?) adj management)).ti. | 31 |
| 104 | (quality improv* adj10 ((medication? or drug?) adj management)).ab. | 8 |
| 105 | (clinical decision and drug?).ti. | 83 |
| 106 | ((clinical decision making or decision support) adj4 (prescribing or drug therap* or drug management or medication management or (managing adj2 (drug? or drug therapy or medication?)))).ti,ab. | 170 |
| 107 | (collaborative and (drug? or medication?) and management).ti. | 45 |
| 108 | medication reconciliation/ | 580 |
| 109 | (drug? assess* or drug? audit? or drug? reconcil* or ((medication or drug or prescribing or prescription?) adj2 (reconciliation or review* or audit))).ti,ab. | 5413 |
| 110 | ((medication? or prescrib* or pharmac*) adj2 (manage? or management or service? or system?)).ti,ab. | 18502 |
| 111 | (("drug therapy" or dosage? or dose? or medication? or prescription? or prescrib* or pharmacist? or pharmaceutical care) adj2 (managing or management or monitor*)).ti,ab. | 9624 |
| 112 | drug utilization review/ | 3363 |
| 113 | community pharmacy.ti. or (community adj (pharmacy or pharmacist? or pharmacies)).ab. | 4021 |
| 114 | pharmacist?.ti. or (pharmacist? adj2 (collaborat* or driven or directed or led or managed or team*)).ab. | 10533 |
| 115 | ((pharmacist? or prescription? or prescribing or medication?) adj3 (consult* or review* or service or services)).ab. | 6688 |
| 116 | patient readmission/ and (prescription? or drug therapy).ti,hw. | 179 |
| 117 | patient readmission/ and (((adverse drug or adverse medication?) adj2 (event or related)) or ((medication related or drug related) adj2 (event? or problem?))).ti,ab. | 22 |
| 118 | ((drug related or medication related or adverse drug or adverse medication) adj5 (emergency department? or emergency unit? or emergency centre? or emergency center? or emergency room? or afterhours or after hours or (emergency adj2 (admission? or admitting)))).ti,ab. | 163 |
| 119 | ((drug related or medication related or adverse drug or adverse medication) adj5 (readmission? or readmitted or emergency visit or unexpected visit?)).ti,ab. | 51 |
| 120 | (((hospital admission? or (readmit* or readmission?)) adj3 (reduc* or fewer or lower)) or ((avoidable or preventable or reduced or reducing) adj5 (admission? or readmission?))).ti,ab. and (drug or medication? or prescription?).ti,ab,hw. | 922 |
| 121 | or/95‐120 | 52001 |
| 122 | community pharmacy services/ | 3350 |
| 123 | primary health care/ or general practice/ or family practice/ or general practice, dental/ or primary care nursing/ | 130955 |
| 124 | ((primary adj2 care) or ((general or family) adj2 practice)).ti,ab. | 141092 |
| 125 | (primary care or family medic* or general practice or family practi*).jn. | 8882 |
| 126 | community medicine/ or community health nursing/ or community health services/ or community health centers/ or home care services/ | 79553 |
| 127 | (community or ambulatory).ti,ab,hw. | 556987 |
| 128 | ambulatory care facilities/ or ambulatory care/ | 54315 |
| 129 | ((walk‐in or neighbo?rhood) adj2 (clinic? or care centre or care centres or care center? or health* centre or health* centres or health* center?)).ti,ab. | 848 |
| 130 | maternal‐child health centers/ or outpatient clinics, hospital/ or pain clinics/ or community mental health centers/ | 21343 |
| 131 | nursing homes/ or intermediate care facilities/ or skilled nursing facilities/ | 34654 |
| 132 | ((patient? adj2 (home or homes)) or home visit?).ti,ab. | 14823 |
| 133 | or/123‐132 | 798316 |
| 134 | general practitioners/ or physicians, family/ or physicians, primary care/ | 22546 |
| 135 | ((general or family) adj2 (practitioner? or physician? or doctor?)).ti,ab. | 65949 |
| 136 | ((primary care or family or general practice or community or home care) adj2 (nurse or nurses)).ti,ab. | 6697 |
| 137 | or/134‐136 | 82852 |
| 138 | 121 and (or/133,137) | 13867 |
| 139 | 138 or 122 | 14799 |
| 140 | (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. | 1071692 |
| 141 | exp animals/ not humans.sh. | 4323394 |
| 142 | 140 not 141 | 988060 |
| 143 | 139 and 142 | 1916 |
| 144 | 94 or 143 | 5768 |
| 145 | (pharmacist? and (adverse drug event? or adverse medication event?)).ti. | 23 |
| 146 | (pharmacist? and adverse drug reaction?).ti. | 58 |
| 147 | (admission? and (adverse drug event? or adverse medication event? or adverse drug reaction?)).ti. | 101 |
| 148 | (prevent$ and medication error?).ti. | 224 |
| 149 | (prevent$ and (adverse drug event? or adverse medication event? or adverse drug reaction?)).ti. | 214 |
| 150 | or/145‐149 | 597 |
| 151 | 83 and 150 | 32 |
| 152 | 144 or 151 | 5774 |
Embase
Embase 1974 to 2016 October 03
Searched 4 October 2016
| No. | Search terms | Results |
| 1 | (((primary adj2 care) or general practi*) and (adverse drug? or adverse medication? or medication related or drug related or preventable drug? or preventable medication? or (avoidable and (drug? or medication? or pharmacother*)))).ti. | 101 |
| 2 | ((ambulatory or outpatient?) and (adverse drug? or adverse medication? or medication related or drug related or preventable drug? or preventable medication? or (avoidable and (drug? or medication? or pharmacother*)))).ti. | 158 |
| 3 | or/1‐2 | 257 |
| 4 | ((prevent* or reduce or reducing) adj2 (drug related or medication related or medication error? or adverse drug or adverse medication)).ti,ab. | 2780 |
| 5 | *"drug use"/ and (adverse or readmission? or readmit* or emergency or problem or safety or safely or harm*).ti,ab. | 4163 |
| 6 | ((drug? or medication?) adj4 (emergency or readmission* or readmit* or (urgent adj2 (care or visit?)))).ti,ab. | 3206 |
| 7 | *medication error/ and (avoid* or intervention or prevent* or reducing or rate or improv* or quality).ti,ab. | 3616 |
| 8 | ((medication? or drug or prescription?) adj (error? or mistake?) adj5 (avoid* or intervention? or prevent* or reducing or quality improv*)).ti,ab. | 1733 |
| 9 | *inappropriate prescribing/ and (avoid* or intervention or prevent* or reducing or rate or quality improv*).ti,ab,hw. | 404 |
| 10 | (*clinical decision making/ or *medical decision making/) and (drug? therapy or medication? management or prescribing or pharmaceutical care or adverse drug or adverse medication or medication related).ti,ab. | 286 |
| 11 | ((clinical decision making or decision support) adj4 (prescribing or drug therap* or drug management or medication management or (managing adj2 (drug? or drug therapy or medication?)))).ti,ab. | 198 |
| 12 | ((structured adj2 (assessment? or care)) and (drug? or medication?)).ti,ab. | 423 |
| 13 | (case management and (drug therapy or medication? or pharmaco* therapy or prescription? or prescribing)).ti,ab. | 883 |
| 14 | ("safety of medications" or prescribing safety or safe prescribing or (safely adj prescribing)).ti,ab. | 554 |
| 15 | ((safe or safety or quality improv*) and ((medication? or drug?) adj2 management)).ti. | 87 |
| 16 | (quality improv* adj10 ((medication? or drug?) adj management)).ab. | 15 |
| 17 | (collaborative and (drug? or medication?) and management).ti. | 73 |
| 18 | (computer assisted drug therapy/ or *drug therapy/ or *drug choice/ or *drug dose regimen/ or *pharmaceutical care/) and (((readmission? or readmit*) adj3 (patient? or rate or rates or reduce? or avoid* or prevent*)) or medication related or (emergency adj3 (vist? or admission? or admitt* or readmission? or readmit*)) or medication related or ((adverse or avoid* or drug or medication or prevent*) adj2 event?)).ti,ab. | 4260 |
| 19 | *medication therapy management/ | 2813 |
| 20 | (drug? assess* or drug? audit? or drug? reconcil* or ((medication or drug or prescribing or prescription?) adj2 (reconciliation or review* or audit))).ti,ab. | 9698 |
| 21 | ((medication? or prescrib* or pharmac*) adj2 (manage? or management or service? or system?)).ti,ab. | 30705 |
| 22 | (("drug therapy" or dosage? or dose? or medication? or prescription? or prescrib* or pharmacist? or pharmaceutical care) adj2 (managing or management or monitor*)).ti,ab. | 14843 |
| 23 | *"drug use"/ and (adverse or avoidable or emergency or preventable or readmission? or mortality).ti,ab. | 2412 |
| 24 | (community adj (pharmacy or pharmacist? or pharmacies) adj5 (quality improv* or readmission? or readmitt* or mortalitly or (adverse adj2 (reduc* or prevent* or avoid*)))).ab. | 20 |
| 25 | pharmacist?.ti. or (pharmacist? adj2 (collaborat* or driven or directed or led or managed or team*)).ab. | 21725 |
| 26 | ((pharmacist? or prescription? or prescribing or medication?) adj3 (consult* or review* or service or services)).ab. | 12144 |
| 27 | *hospital readmission/ and (adverse drug or adverse medication or medication related or adverse event? or ((avoidable or preventable) adj2 (adverse or event?))).ti,ab. | 240 |
| 28 | ((drug related or medication related or adverse drug or adverse medication) adj5 (emergency department? or emergency unit? or emergency centre? or emergency center? or emergency room? or afterhours or after hours or (emergency adj2 (admission? or admitting)))).ti,ab. | 247 |
| 29 | ((drug related or medication related or adverse drug or adverse medication) adj5 (readmission? or readmitted or emergency visit or unexpected visit?)).ti,ab. | 87 |
| 30 | ((((hospital admission? or (readmit* or readmission?)) adj3 (reduc* or fewer or lower)) or ((avoidable or preventable or reduced or reducing) adj5 (admission? or readmission?))) and (drug? or medication? or prescription?)).ti,ab. | 1724 |
| 31 | ((problem? or hospitali?ation? or mortality or morbidity or illness*) adj2 (drug related or drug induced)).ti,ab. | 2687 |
| 32 | *medication error/ and (reduc*.ti. or ((prevent* or avoid* or rate or rates or reduc* or fewer) adj3 (admission? or readmission? or emergency or prevent* or quality improv*)).ti,ab.) | 2027 |
| 33 | medication therapy management/ and (adverse or avoidable or emergency or preventable or readmission? or mortality).ti,ab. | 1596 |
| 34 | *drug therapy/ and adverse event?.ti,ab. | 2290 |
| 35 | ((*hospital readmission/ and dt.fs.) or (dt.fs. and (readmission? or readmit*)).ti,ab.) and (preventable or avoidable or adverse).ti,ab. | 392 |
| 36 | (drug therapy/ or (pharmaceutical? or prescrib* or prescription? or medication?).ti,ab.) and (near miss or near misses or never event?).ti,ab. | 399 |
| 37 | ((appropriat* or inappropriate or unsafe) adj3 (medicine? or prescrib* or drug therap* or pharmacotherap*)).ti,ab. | 5847 |
| 38 | or/4‐37 | 94819 |
| 39 | *primary health care/ or *primary medical care/ or *general practice/ | 97127 |
| 40 | ((primary adj2 care) or ((general or family) adj2 practice)).ti,ab. | 171036 |
| 41 | (primary care or family medic* or general practice or family practi*).jn. | 10436 |
| 42 | *community medicine/ or *community health nursing/ or *community care/ or *home care/ | 67132 |
| 43 | (community or ambulatory).ti,ab. | 514922 |
| 44 | *outpatient/ or *outpatient care/ or *outpatient department/ or *community mental health center/ | 40935 |
| 45 | ((walk‐in or neighbo?rhood) adj2 (clinic? or care centre or care centres or care center? or health* centre or health* centres or health* center?)).ti,ab. | 997 |
| 46 | *nursing home/ or *residential home/ | 28324 |
| 47 | ((patient? adj2 (home or homes)) or home visit?).ti,ab. | 20542 |
| 48 | *general practitioner/ | 21735 |
| 49 | ((general or family) adj2 (practitioner? or physician? or doctor?)).ti,ab. | 82552 |
| 50 | or/39‐49 | 857629 |
| 51 | (ecstasy or marijuana or methamphet* or illegal* or street drug? or cocaine? or cannabis or inject* drug or drug user?).ti,ab,hw. | 136236 |
| 52 | ((drug? or alcohol?) adj2 (abus* or addict* or dependence)).ti,ab,hw. | 165048 |
| 53 | (placebo or "head to head").ti,ab. | 251431 |
| 54 | or/51‐53 | 504531 |
| 55 | multicenter study/ | 152623 |
| 56 | controlled clinical trial/ or controlled study/ or randomized controlled trial/ | 5318787 |
| 57 | randomi?ed.ti. or ((random* or control) adj3 (group? or cohort? or patient? or hospital* or department?)).ab. or (controlled adj2 (study or trial)).ti. | 894626 |
| 58 | (random sampl* or random digit* or random effect* or random survey or random regression).ti,ab. not randomized controlled trial/ | 74604 |
| 59 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 18016161 |
| 60 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not 59 | 5847628 |
| 61 | (or/55‐57) not (or/58,60) | 3860921 |
| 62 | medication safety.ti,ab. | 1951 |
| 63 | ((optim* or evidence based or rational*) adj2 prescrib*).ti,ab. | 1711 |
| 64 | hospitali?ation?.ti,ab. | 181464 |
| 65 | (emergency or urgent care or visit? or adverse or medication related or drug related or safely or safety or fewer or drug therapy or pharmacotherap* or improve? patient? outcome or readmission? or ((lower* or reduc*) adj2 admission?)).ti,ab,hw. | 2550209 |
| 66 | *drug interaction/ and (prevent* or avoid* or reduc*).ti. | 1132 |
| 67 | (drug drug interaction? adj4 (reduc* or avoid* or prevent*)).ti,ab. | 382 |
| 68 | or/62‐67 | 2664679 |
| 69 | ((38 and 50 and 61 and 68) not 54) or 3 | 2877 |
| 70 | (2013* or 2014* or 2015* or 2016*).dp,dd,yr,em. | 29074507 |
| 71 | 69 and 70 | 2695 |
The Cochrane Library
Searched 4 October 2016
| No. | Search terms | Results |
| #1 | ((prevent* or reduce or reducing) near/2 (medication error? or adverse drug or adverse medication)):ti,ab | 61 |
| #2 | [mh "medication errors"] and (avoid* or intervention or prevent* or reducing or rate or improv* or quality):ti,ab,kw | 250 |
| #3 | ((medication? or drug or prescription?) near/1 (error? or mistake?) near/5 (avoid* or intervention? or prevent* or reducing or quality improv*)):ti,ab | 2 |
| #4 | [mh "inappropriate prescribing"] and (avoid* or intervention or prevent* or reducing or rate or improv* or quality):ti,ab,kw | 64 |
| #5 | [mh "decision support systems, clinical"] and ((drug? or medication?) near/2 (management or adverse or event? or related)):ti,ab | 0 |
| #6 | ((structured near/2 (assessment? or care)) and (drug? or medication?)):ti,ab | 18 |
| #7 | (structured assessment? or structured care or case management):ti,ab and (drug therapy or medication? or pharmaco* therapy or prescription? or prescribing):ti,ab,kw | 1074 |
| #8 | ("safety of medications" or prescribing safety or safe prescribing or (safely next prescribing)):ti,ab | 259 |
| #9 | ((safe or safety or quality improv*) and ((medication? or drug?) near/1 management)):ti | 0 |
| #10 | (quality improv* near/10 ((medication? or drug?) near/1 management)):ab | 1 |
| #11 | (clinical decision and drug?):ti | 0 |
| #12 | ((clinical decision making or decision support) near/4 (prescribing or drug therap* or drug management or medication management or (managing near/2 (drug? or drug therapy or medication?)))):ti,ab | 19 |
| #13 | (collaborative and (drug? or medication?) and management):ti | 0 |
| #14 | [mh "medication reconciliation"] | 40 |
| #15 | (drug? assess* or drug? audit? or drug? reconcil* or ((medication or drug or prescribing or prescription?) near/2 (reconciliation or review* or audit))):ti,ab | 14511 |
| #16 | ((medication? or prescrib* or pharmac*) near/2 (manage? or management or service? or system?)):ti,ab | 626 |
| #17 | (("drug therapy" or dosage? or dose? or medication? or prescription? or prescrib* or pharmacist? or pharmaceutical care) near/2 (managing or management or monitor*)):ti,ab | 222 |
| #18 | [mh "drug utilization review"] | 134 |
| #19 | community pharmacy:ti or (community next pharmac*):ab | 491 |
| #20 | pharmacist?:ti or (pharmacist? near/2 (collaborat* or driven or directed or led or managed or team*)):ab | 268 |
| #21 | ((pharmacist? or prescription? or prescribing or medication?) near/3 (consult* or review* or service or services)):ab | 200 |
| #22 | [mh "patient readmission"] and (prescription? or drug therapy):ti,kw | 59 |
| #23 | [mh "patient readmission"] and (((adverse drug or adverse medication?) near/2 (event or related)) or ((medication related or drug related) near/2 (event? or problem?))):ti,ab | 4 |
| #24 | ((drug related or medication related or adverse drug or adverse medication) near/5 (emergency department? or emergency unit? or emergency centre? or emergency center? or emergency room? or afterhours or after hours or (emergency near/2 (admission? or admitting)))):ti,ab | 3 |
| #25 | ((drug related or medication related or adverse drug or adverse medication) near/5 (readmission? or readmitted or emergency visit or unexpected visit?)):ti,ab | 8 |
| #26 | (((hospital admission? or (readmit* or readmission?)) near/3 (reduc* or fewer or lower)) or ((avoidable or preventable or reduced or reducing) near/5 (admission? or readmission?))):ti,ab and (drug or medication? or prescription?):ti,ab,kw | 124 |
| #27 | {or #1‐#26} | 17390 |
| #28 | [mh "community pharmacy services"] | 250 |
| #29 | [mh "primary health care"] or [mh "general practice"] or [mh "family practice"] or [mh "general practice, dental"] or [mh "primary care nursing"] | 8243 |
| #30 | ((primary near/2 care) or ((general or family) near/2 practice)):ti,ab | 14278 |
| #31 | [mh "community medicine"] or [mh "community health nursing"] or [mh "community health services"] or [mh "community health centers"] or [mh "home care services"] | 12860 |
| #32 | (community or ambulatory):ti,ab,kw | 36494 |
| #33 | [mh "ambulatory care facilities"] or [mh "ambulatory care"] | 5379 |
| #34 | ((walk‐in or neighbo?rhood) near/2 (clinic? or care centre or care centres or care center? or health* centre or health* centres or health* center?)):ti,ab | 4 |
| #35 | [mh "maternal‐child health centers"] or [mh "outpatient clinics, hospital"] or [mh "pain clinics"] or [mh "community mental health centers"] | 842 |
| #36 | [mh "nursing homes"] or [mh "intermediate care facilities"] or [mh "skilled nursing facilities"] | 1204 |
| #37 | ((patient? near/2 (home or homes)) or home visit?):ti,ab | 3045 |
| #38 | {or #29‐#37} | 61131 |
| #39 | [mh "general practitioners"] or [mh "physicians, family"] or [mh "physicians, primary care"] | 747 |
| #40 | ((general or family) near/2 (practitioner? or physician? or doctor?)):ti,ab | 3101 |
| #41 | ((primary care or family or general practice or community or home care) near/2 (nurse or nurses)):ti,ab | 457 |
| #42 | {or #39‐#41} | 3950 |
| #43 | #27 and (#38 or #42) | 2668 |
| #44 | #28 or #43 | 2719 |
CINAHL (EBSCO)
Searched 4 October 2016
| No. | Search terms | Results |
| S1 | MH Medication errors | 8,902 |
| S2 | TI ((medication review or medication reconciliation)) OR AB ((medication review or medication reconciliation)) | 1,484 |
| S3 | MH "Drug Therapy+" AND (TI adverse event# OR AB adverse event#) | 2,408 |
| S4 | TI (((problem# or hospitali#ation# or mortality or morbidity or illness*) N2 (drug related or drug induced))) OR AB (((problem# or hospitali#ation# or mortality or morbidity or illness*) N2 (drug related or drug induced))) | 512 |
| S5 | TI (((error# or mistake# or wrong or adverse event# or near miss or near misses or never event# or incorrect* or inappropriat*) N3 (drug# or dose or doses or dosage or dosing or pharmaceutical or medication# or prescription# or prescribing))) OR AB (((error# or mistake# or wrong or adverse event# or near miss or near misses or never event# or incorrect* or inappropriat*) N3 (drug# or dose or doses or dosage or dosing or pharmaceutical or medication# or prescription# or prescribing))) | 6,946 |
| S6 | TI ((dispensing N2 (error# or mistake# or wrong))) OR AB ((dispensing N2 (error# or mistake# or wrong))) | 62 |
| S7 | ((MH Mortality) AND (MH Drug therapy OR MH Prescriptions, Drug OR MH Drugs, Prescription OR MH Dosage Calculation OR MH Drugs, Off‐Label OR MH "Drug Therapy, Combination" OR MH "Drug Therapy, Computer‐Assisted" OR MH Polypharmacy)) OR (TI ((((preventable or avoidable or prescrib* or medication) N2 error#) or mistake# or wrong or incorrect* or inappropriat*)) OR AB ((((preventable or avoidable or prescrib* or medication) N2 error#) or mistake# or wrong or incorrect* or inappropriat*))) | 20,695 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 31,409 |
| S9 | MH Primary Health Care OR MH Family practice | 43,304 |
| S10 | TI (((primary N4 (care or healthcare)) or ((General or family) N2 practice))) OR AB (((primary N4 (care or healthcare)) or ((General or family) N2 practice))) | 47,581 |
| S11 | MH Community medicine OR MH community health nursing OR MH community health services OR MH community health centers OR MH home health care | 49,837 |
| S12 | TI ((community care or community healthcare)) OR AB ((community care or community healthcare)) | 14,011 |
| S13 | MH Ambulatory Care Facilities OR MH Ambulatory Care | 9,934 |
| S14 | TI (((ambulatory or walk‐in or neighbo#rhood or community) N2 (clinic# or care centre or care centres or care center# or health* centre or health* centres or health* center#))) OR AB (((ambulatory or walk‐in or neighbo#rhood or community) N2 (clinic# or care centre or care centres or care center# or health* centre or health* centres or health* center#))) | 4,226 |
| S15 | MH Pain Clinics OR MH Nursing Homes OR MH Skilled Nursing Facilities | 18,610 |
| S16 | TI ((nursing home# or care facility)) OR AB ((nursing home# or care facility)) | 22,849 |
| S17 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 162,691 |
| S18 | MH Physicians, Family | 9,090 |
| S19 | TI (((general or family) N2 (practitioner# or physician# or doctor#))) OR AB (((general or family) N2 (practitioner# or physician# or doctor#))) | 12,972 |
| S20 | (MH nurse practitioners OR MH physician Assistants) AND (TI community OR AB community OR MW community) | 834 |
| S21 | (TI ((physician# assistant# or doctor# assistant# or physician# extender# or feldsher#)) OR AB ((physician# assistant# or doctor# assistant# or physician# extender# or feldsher#))) AND ((TI community OR AB community OR MW community)) | 170 |
| S22 | S18 OR S19 OR S20 OR S21 | 20,115 |
| S23 | MH Pharmacists OR MH Pharmacy Technicians OR MH Drug Information Services OR MH Clinical Pharmacy Information Systems | 6,238 |
| S24 | TI medication review OR AB medication review | 1,119 |
| S25 | TI (pharmaceutical care or pharmacy or pharmacies or pharmacist# or prescriber# or prescribing or prescription# or drug therapy) | 19,149 |
| S26 | AB ((pharmacist# or prescription# or prescribing or medication#) N3 (consult* or review* or service or services)) | 2,074 |
| S27 | TI (((medication# or prescrib* or pharmac*) N2 (manage# or management or service# or system#))) OR AB (((medication# or prescrib* or pharmac*) N2 (manage# or management or service# or system#))) | 6,368 |
| S28 | TI ((drug# assess* or drug# audit# or drug# reconcil*)) OR AB ((drug# assess* or drug# audit# or drug# reconcil*)) | 2,283 |
| S29 | TI ((("drug therapy" or dosage# or dose# or medication# or prescription# or prescrib* or pharmacist# or pharmaceutical care) N2 (managing or management or monitor*))) OR AB ((("drug therapy" or dosage# or dose# or medication# or prescription# or prescrib* or pharmacist# or pharmaceutical care) N2 (managing or management or monitor*))) | 3,702 |
| S30 | TI (((improv* or optimi#ing or optimi#e# or optimal*) and (dosing or dosage))) OR AB (((improv* or optimi#ing or optimi#e# or optimal*) N2 (pharmaceutical care or pharmacy or prescrib* or prescript*))) | 846 |
| S31 | TI (((drug therapy or drug regime# or medication# or medicineS or pharmacy or pharmacist# or pharmaceutical or PRESCRIB* or prescription#) N2 (audit* or monitor* or reconcil* or review#))) OR AB (((drug therapy or drug regime# or medication# or medicineS or pharmacy or pharmacist# or pharmaceutical or PRESCRIB* or prescription#) N2 (audit* or monitor* or reconcil* or review#))) | 3,183 |
| S32 | MH Drug Utilization | 4,070 |
| S33 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 | 35,911 |
| S34 | TI ((emergency N3 (visit# or room# or clinic# or admission#) N3 (reduc* or fewer or lower))) OR AB ((emergency N3 (visit# or room# or clinic# or admission#) N3 (reduc* or fewer or lower))) | 412 |
| S35 | TI reduc* hospital admissions OR AB reduc* hospital admissions | 552 |
| S36 | TI ((readmission# N3 (reduc* or fewer or lower))) OR AB ((readmission# N3 (reduc* or fewer or lower))) | 989 |
| S37 | MH Readmission AND (TI (((hospital admission# or (readmit* or readmission#)) N3 (reduc* or fewer or lower))) OR AB (((hospital admission# or (readmit* or readmission#)) N3 (reduc* or fewer or lower)))) | 725 |
| S38 | S34 OR S35 OR S36 OR S37 | 1,902 |
| S39 | S8 and S17 | 2,345 |
| S40 | S8 and S22 | 464 |
| S41 | S33 and S17 | 4,462 |
| S42 | S33 and S22 | 945 |
| S43 | S33 and S38 | 102 |
| S44 | S39 OR S40 OR S41 OR S42 OR S43 | 6,625 |
| S45 | (MM "Clinical Trials+") OR (MH "Multicenter Studies") | 23,509 |
| S46 | TI (“clinical study” or “clinical studies”) or AB (“clinical study” or “clinical studies”) | 8,089 |
| S47 | TI random* or AB random* | 130,238 |
| S48 | TI (control group or control groups OR control* experiment* or control* design or controlled study) OR AB (control group OR control groups or control* cohort* or controlled experiment* controlled design or controlled study) | 58,722 |
| S49 | TI (cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment*) OR AB (cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment*) | 2,286 |
| S50 | TI multicentre or multicenter or multi‐centre or multi‐center | 25,954 |
| S51 | AB ((multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*)) or AB ((multi‐cent* n2 design*) or (multi‐cent* n2 study) or (multi‐cent* n2 studies) or (multi‐cent* n2 trial*)) | 8,384 |
| S52 | TI controlled AND TI (trial or trials or study or experiment* or intervention) | 23,532 |
| S53 | S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 | 192,373 |
| S54 | S44 and S53 | 835 |
| S55 | TI ((((medication or drug) N2 event) and ((primary N2 care) or ((family or general) N2 (practice or practitioner#))))) OR AB ((((medication or drug) N2 event) and ((primary N2 care) or ((family or general) N2 (practice or practitioner#))))) | 58 |
| S56 | S54 OR S55 | 871 |
| S57 | S56 Limiters ‐ Exclude MEDLINE records | 116 |
Appendix 3. GRADE evidence profile: professional interventions compared to standard care for preventation of medication errors
| Grade evidence profile: professional interventions compared to standard/usual care for prevention of medication errors | |||||||||
| Patient or population: adults receiving medication in primary care Setting: primary and community care Intervention: professional interventions (using health information technology to identify patients at risk or help to generate a care plan for patients) Comparison: standard/usual care | |||||||||
| Quality assessment | Effect | Quality | Importance | ||||||
| № of participants/studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative/absolute (95% CI) | ||
| Number of hospital admissions | |||||||||
| PI: 41/1949 (2.1%) SC: 33/1940 (1.7%) (2 studies) |
Randomised trials | Not serious | Not serious | Not serious | Seriousa | None |
Relative: RR 1.24 (0.79 to 1.96) Absolute: 4 more per 1000 (from 4 fewer to 16 more) |
⊕⊕⊕⊝ Moderate | Not Important |
| Number of people admitted to hospital | |||||||||
| PI: 827/1870 (44.2%) SC: 802/1791 (44.8%) (1 study) |
Randomised trials | Not serious | Not serious | Not serious | Not serious | None |
Relative: RR 0.99 (0.92 to 1.06) Absolute: 5 fewer per 1000 (from 27 more to 36 fewer) |
⊕⊕⊕⊕ High | Not Important |
| Number of emergency department visits | |||||||||
| PI: 47/552 (8.5%) SC: 61/515 (11.8%) (2 studies) |
Randomised trials | Seriousb | Not serious | Not serious | Seriousa | None |
Relative: RR 0.71 (0.50 to 1.02) Absolute: 33 fewer per 1000 (from 2 more to 59 fewer) |
⊕⊕⊝⊝ Low | Important |
| Mortality | |||||||||
| PI: 211/1769 (11.9%) SC:215/1769 (12.2%) (1 study) |
Randomised trials | Seriousb | Not serious | Not serious | Not serious | None |
Relative: RR 0.98 (0.82 to 1.17) Absolute: 3 fewer per 1000 (from 21 more to 22 fewer) |
⊕⊕⊕⊝ Moderate | Not Important |
CI: confidence interval; PI: professional intervention; RR: risk ratio; SC: standard care
aWe downgraded one level due to imprecision. bWe downgraded one level due to risk of bias (selection bias).
Appendix 4. GRADE evidence profile: organisational Interventions compared to standard care for prevention of medication errors
| Organisational interventions compared to standard/usual care for prevention of medication errors | |||||||||
| Patient or population: adults receiving medication in primary care Setting: primary care Intervention: organisational interventions (provision of pharmaceutical care, medication reviews, follow‐up visits by a healthcare professional (e.g. pharmacist, nurse or physician) Comparison: standard/usual care | |||||||||
| Quality assessment | Effect | Quality | Importance | ||||||
| № of participants/ studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative/absolute (95% CI) | ||
| Number of hospital admissions | |||||||||
| OI: 817/3100 (26.4%) SC: 850/3103 (27.4%) (11 studies) |
Randomised trials | Seriousa | Seriousb | Not serious | Seriousc | None |
Relative: RR 0.85 (0.71 to 1.03) Absolute: 41 fewer per 1000 (from 8 more to 79 fewer) |
⊕⊝⊝⊝ Very low | Critical |
| Number of people admitted to hospital | |||||||||
| OI: 2846/73,313 (3.9%) SC: 3221/78,924 (4.1%) (13 studies) |
Randomised trials | Seriousa | Not serious | Not serious | Seriousc | None |
Relative: RR 0.92 (0.86 to 0.99) Absolute: 0 fewer per 1000 (from 0 fewer to 6 fewer) |
⊕⊕⊝⊝ Low | Important |
| Number of emergency departments visits | |||||||||
| OI: 171/849 (20.1%) SC: 227/970 (23.4%) (5 studies) |
Randomised trials | Serious a | Seriousb | Not serious | Seriousc | None |
Relative: RR 0.75 (0.49 to 1.15) Absolute: 58 fewer per 1000 (from 35 more to 119 fewer) |
⊕⊝⊝⊝ Very low | Critical |
| Mortality | |||||||||
| OI: 3180/74,017 (4.3%) SC: 4085/80,945 (5.0%) (12 studies) |
Randomised trials | Very seriousd | Not serious | Not serious | Seriousc | None |
Relative: RR 0.94 (0.85 to 1.03) Absolute: 3 fewer per 1000 (from 2 more to 8 fewer) |
⊕⊝⊝⊝ Very low | Important |
CI: confidence interval; OI: organisational intervention; RR: risk ratio; SC: standard care
aWe downgraded one level for unclear risk of bias (selection and attrition bias). bWe downgraded one level for inconsistency (high heterogeneity across studies). cWe downgraded one level for imprecision. dWe downgraded one level for high risk of bias (selection, performance and attrition bias).
Data and analyses
Comparison 1. Professional interventions versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Number of hospital admissions | 2 | 3889 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.79, 1.96] |
| 1.2 Number of people admitted to hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Number of emergency department visits | 2 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.50, 1.02] |
| 1.4 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: Professional interventions versus standard care, Outcome 1: Number of hospital admissions
1.2. Analysis.

Comparison 1: Professional interventions versus standard care, Outcome 2: Number of people admitted to hospital
1.3. Analysis.

Comparison 1: Professional interventions versus standard care, Outcome 3: Number of emergency department visits
1.4. Analysis.

Comparison 1: Professional interventions versus standard care, Outcome 4: Mortality
Comparison 2. Organisational interventions versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Number of hospital admissions | 11 | 6203 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.03] |
| 2.2 Number of people admitted to hospital | 13 | 152237 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.99] |
| 2.3 Number of emergency department visits | 5 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.15] |
| 2.4 Mortality | 12 | 154962 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.03] |
2.2. Analysis.

Comparison 2: Organisational interventions versus standard care, Outcome 2: Number of people admitted to hospital
2.3. Analysis.

Comparison 2: Organisational interventions versus standard care, Outcome 3: Number of emergency department visits
2.4. Analysis.

Comparison 2: Organisational interventions versus standard care, Outcome 4: Mortality
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alvarez 2001.
| Study characteristics | ||
| Methods | Cluster‐RT (randomisation at the pharmacy level) Study duration: 1 year |
|
| Participants | 735 at beginning of the study, 600 at the end of the study (data are on the 600 reported below) Setting: community pharmacies Diagnostic criteria: CHD Age (years) (mean): intervention group: 64.8 years; control group: 65.8 years Sex female n (%): intervention group: 79 (29.5%); control group: 94 (29%). Country: Spain Comorbidity: not reported Sociodemographics: not reported Ethnicity: not reported Date of study: not reported |
|
| Interventions | 1 intervention group Intervention group: pharmacies allocated to that group provided pharmaceutical care, consisting of the prevention, identification and solution of medication‐related problems. Control group: care as usual Pharmaceutical care consisted of the following: offering the pharmaceutical care service to participants and to their corresponding GPs, initial interview and assessment of the therapeutic plan, registration of data during the subsequent visits in order to allow the identification of medication‐related problems, and intervention to solve the problem. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Pharmacists may have been blinded, as they all received training on methods to treat CHD (in order to ensure that differences after the intervention are due to the intervention per se and not due to differences in theoretical knowledge on methods to treat CHD). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High proportion of incomplete outcome data for most to the measures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination bias | Unclear risk | Unclear |
| Other bias | High risk | For most of the outcome measures no pre‐intervention data were collected. We considered other bias due to cluster randomisation. |
Bernsten 2001.
| Study characteristics | ||
| Methods | RT Study duration: 18 months Setting: community pharmacy |
|
| Participants | 2454 participants were recruited: 1290 intervention participants and 1164 control participants were assessed at baseline although there were subsequent dropouts. Diagnostic criteria: participants were eligible if they were ≥ 65 years, taking 4 or more prescribed medicines, and oriented with respect to time, place, and person. They were required to be community dwelling and regular visitors to a community pharmacy. Participants could not be housebound or in a nursing facility. Age (years) (mean ± SD): intervention: 735 (58%); control: 663 (57%); no significant difference Sex female n (%): intervention: 735 (58%); control: 663 (57%); no significant difference Country: 7 European countries; Denmark, Germany, The Netherlands, Northern Ireland (co‐ordinating centre), Portugal, Republic of Ireland and Sweden. Comorbidity: there were no significant differences between intervention and control participants at baseline. Sociodemographics: none of note, although participants from 2 countries (Republic of Ireland and Portugal) did not complete the study Ethnicity: not reported Date of study: unclear although published in 2001 |
|
| Interventions | 104 intervention pharmacies A pharmaceutical care programme was involved and a manual was distributed to all the intervention sites detailing the intervention. Pharmacists assessed participants to identify drug‐related problems using a structured approach. Pharmacists used several sources of information including informal questioning of the participant, the participant's GP, and pharmacy records. Pharmacists also formulated a monitoring and intervention plan for each participant, which included participant education about drugs and their medical condition, using improvement in medication compliance strategies, and simplifying drug regimens. Control group: participants were treated as per the usual care with no pharmaceutical care plan provided. |
|
| Outcomes | Data relating to health and economic outcomes were collected for each participant at baseline, 6, 12 and 18 months. These included hospital admissions. | |
| Notes | The study authors note that the training of pharmacists was not rigorously controlled. Although a study manual was provided along with a 1‐day training session, additional training was not consistently provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sites were randomly allocated as control or intervention sites |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate as sites rather than individual participants were randomly allocated. Also, all units were allocated at the start of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As pharmacies were the unit of randomisation, this appears to be low risk. Control pharmacists provided usual care and intervention pharmacists only provided the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Those participants who withdrew from the study were significantly older and in poorer health. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is less important for our purpose as we are looking at objective outcomes (hospitalisations, ED visits, and mortality). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Low risk | No evidence of contamination of the intervention group with the control |
| Other bias | Unclear risk | 1 aspect of the study that was not rigorously controlled was the training of participating pharmacists. A study manual was provided to each participating pharmacist, followed by a 1‐day training session. Further training was provided in individual countries; however, the extent of this was driven by the available resources. |
Campins 2016.
| Study characteristics | ||
| Methods | RT 15 months |
|
| Participants | 503 participants: 252 intervention, 251 control; final sample 242 intervention, 246 control Setting: primary care centres Diagnostic criteria: elderly people (> 70 years) on ≥ 8 drugs |
|
| Interventions | The intervention consisted of 3 consecutive phases. First, a trained and experienced clinical pharmacist evaluated all drugs prescribed to each participant using the GP‐GP algorithm and based their decision about appropriateness on the STOPP/START criteria. Second, the pharmacist discussed recommendations for each drug with the participant’s physician in order to come up with a final set of recommendations. Finally, these recommendations were discussed with the participant, and a final decision was agreed by physicians and their patients in a face‐to‐face visit. Control group participants followed the usual treatments and control procedures of their physicians. |
|
| Outcomes | Main outcome measures regarding intervention effectiveness were as follows
Adherence was measured using the Morisky‐Green test. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were blindly randomised to 1 or other of the 2 study arms. Assignment was based on a list of random numbers generated by a statistical programme. |
| Allocation concealment (selection bias) | Low risk | Each family physician received 10 sealed, opaque envelopes with identification numbers (assigned consecutively in strict chronological order of recruitment) on the back. Each envelope contained a card with the same identification number and the intervention group to which the subject was assigned. Envelopes were not prepared in primary care centres but in the research unit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Low risk because pharmacists only treated intervention participants and did not know that the participants they interacted with were in a study. Also, participants did not appear to know whether they were receiving an intervention or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few people dropped out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes of interest were objective |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Unclear risk | Prescribing physicians who received recommendations from the pharmacist regarding intervention group participants also had participants in the control group, so the control group could have benefited from the intervention. |
| Other bias | Unclear risk | Study has limited statistical power to detect effects for outcomes of interest |
Coleman 1999.
| Study characteristics | ||
| Methods | Cluster‐RT. The unit of randomisation was the physician practice Study duration: 2 years |
|
| Participants | Total participants: 169 participants, 9 physician groups Participants aged ≥ 65 in ambulatory setting, chronic‐care clinics Age: intervention 77.3%; control 77.4%; no SD provided; P = 0.70 Sex female (%): intervention 47.9%; control 49.6%; no SD provided; P = 0.81 Country: USA Diabetes: intervention 53.2%; control 48.6%; P = 0.62 Education > 12 years: intervention 77.1%; control 66.7%; P = 0.1 Married: intervention 55.2%; control 58.3%; P = 0.63 Income < USD 15,000: intervention 15.8%; control 14%; P = 0.75 Hospitalised in prior year: intervention 46.7%; control 39.7%; P = 0.15 Mean chronic disease score: intervention 7.3; control 7.7; P = 0.06 Mean risk score: intervention 0.55; control 0.53; P = 0.35 Ethnicity: non‐white: intervention 2.8%; control 4.1%; P = 0.54 |
|
| Interventions | Intervention practices (5 physicians, 96 participants) held half‐day, chronic‐care clinics every 3‐4 months. These clinics included an extended visit with the physician and nurse dedicated to planning chronic‐disease management, a pharmacist visit that emphasised reduction of polypharmacy and high‐risk medications, and a patient self‐management group. Control practices (4 physicians, 73 participants) received usual care. |
|
| Outcomes | Emergency visits (mean/year) and hospitalisations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was not mentioned nor how it was done. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was not mentioned nor how it was done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This would be impossible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some participants did not complete the study and were reported in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No explanation given |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination bias | Low risk | It is hard to determine if the intervention group interacted with the control group. |
| Other bias | Unclear risk | Unclear. We considered other bias due to cluster randomisation. |
Frankenthal 2014.
| Study characteristics | ||
| Methods | RT 18 months Chronic‐care geriatric facility |
|
| Participants | 359 participants: 183 intervention, 176 control; final sample: 160 intervention, 146 control | |
| Interventions | The intervention consisted of a medication review by the study pharmacist for all residents at study opening and 6 and 12 months later using the The STOPP/START criteria. Interventional recommendations that the study pharmacist made for residents in the intervention group but not in the control group were discussed with the chief physician at study opening and after 6 months. The chief physician decided whether to accept these recommendations and implement prescribing changes. Control: usual care |
|
| Outcomes | Outcome measures included:
Outcomes were measured at the beginning of the study and at the 12‐month follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fixed, stratified randomisation was used to allocate residents to groups according to the 3 types of residents: ADL‐dependent, ADL‐independent, and primarily cognitively impaired. Participants who were ADL‐dependent with impaired cognition were assigned to the ADL‐dependent group. Randomisation for each level was according to simple list randomisations. |
| Allocation concealment (selection bias) | Low risk | A physician who was not part of the study randomised participants. Group allocation was concealed from the study pharmacist, and participants were assigned to 1 of the 2 groups using sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacists were aware that they were interacting with the intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participants completed the study and there was no apparent difference between intervention and control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Number of hospitalisations is an objective measure. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Unclear risk | The intervention pharmacist only interacted with intervention participants, but may have interacted with pharmacists in the control group |
| Other bias | High risk | Only 1 geriatric centre was investigated |
Garcia‐Gollarte 2014.
| Study characteristics | ||
| Methods | RT | |
| Participants | 1018 residents: 516 intervention, 502 control Final sample: 59 physicians, 716 nursing home residents Intervention: 29 doctors, 372 nursing home residents Control: 30 doctors, 344 nursing home residents Diagnostic criteria: residents aged ≥ 65 years and clinically stable (no change in prescription in last 2 months) Setting: private organisation |
|
| Interventions | 6 months professional intervention A nursing home physician, expert in drug use in older people, delivered a structured educational intervention. The programme included: general aspects of prescription and drug use in geriatric patients, how to reduce the number of drugs, to perform a regular review of medications, to avoid inappropriate drug use, to discontinue drugs that do not show benefits, and to avoid under‐treatment with drugs that have shown benefits. It also discussed in detail some drugs frequently related to adverse drug reactions in older people. Educational material and references were given to participants. Finally, two, 1‐h workshops reviewed practical, real life cases and promoted practice changes in participants. The educator offered further on‐demand advice on prescriptions for the next 6 months. This intervention was reinforced through a single review by the researchers, using standard appropriateness criteria, STOPP‐START. Control: physicians in the control group did not receive any intervention or information about an educational intervention delivered in other centres. |
|
| Outcomes | Outcome measures were as follows:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | There was no mention made of sequence concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians in both groups were informed that there was a company programme aimed to improve drug prescription (to explain why data on prescriptions was collected in their centres) but were blinded to the fact that the educational intervention was being assessed. Also, participants did not know they were receiving an intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30% of participants were lost to the study, but it is unclear if there was differential attrition in intervention and control groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Number of emergency room visits and length of hospitalisations are objective outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | High risk | Although nursing homes in the intervention and control groups were separate, some cross‐contamination because of informal contacts between physicians may have occurred. |
| Other bias | High risk | Short intervention period (6 months) and short follow‐up (3 months) |
Gernant 2016.
| Study characteristics | ||
| Methods | Cluster‐RT | |
| Participants | 656 home care participants (intervention n = 297, usual care n = 359) were available for this study | |
| Interventions | The intervention began approximately 3 days after in‐home health admission when a pharmacy technician completed telephonic medication reconciliation with the participant and/or caregiver. Then a trained pharmacist would consult with the participant or caregiver via telephone for an average of 30 min to complete a scheduled comprehensive medication therapy review to identify and resolve any medication‐related problems. The pharmacist constructed a personal medication record and a medication‐related action plan for the participant. The action plan was a participant‐centred document that assisted participants, caregivers, and the pharmacist in the resolution of identified medication‐related problems. Control group: standard/usual care |
|
| Outcomes | The primary outcome of this study was participant‐level, 60‐day, all‐cause ED utilisation. This outcome was defined as a dichotomous variable (i.e. the participant visited the ED 1 or more times following the intervention or they did not). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Site and participant randomisation was a 2‐step process. Firstly a simple random sample of 40 co‐ordinating home healthcare centres, with a monthly census of ≥ 20 admitted participants, was selected among 419 care centres from a nationwide Home Health Agency (Amedisys, Inc, Baton Rouge, LA). Then, at each study site, using blocks of 7 participants, and constrained for equal allocation to study intervention or usual care groups. |
| Allocation concealment (selection bias) | Low risk | Home Health Agency nurses were blinded to their participants’ group assignment to prevent bias during the initial in‐home admissions assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants did not know to which group they were allocated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination bias | Low risk | Cluster‐randomisation with a small chance of contamination |
| Other bias | Unclear risk | Unclear |
Gurwitz 2014.
| Study characteristics | ||
| Methods | RT, total study duration not provided | |
| Participants | 5077 hospital discharges: 2563 intervention discharges, 2514 control discharges Final sample: 1870 intervention, 1791 control Setting: large multispecialty group practice Diagnostic criteria: ≥ 65 years discharged from hospital to home |
|
| Interventions | Professional intervention Intervention: an automated system was developed to facilitate the flow of information to the medical group’s primary care providers about individuals who were discharged to home from the hospital. In addition to notifying providers about an individual’s discharge, the system provided information about new drugs at the time of hospital discharge, warnings about selected drug‐drug interactions, recommendations for consideration of dose changes and laboratory monitoring of high‐risk medications, and alerts to the provider’s support staff to schedule a post hospitalisation office visit within 1 week of discharge. Control: care as usual |
|
| Outcomes | Whether discharged individuals had an office visit with a primary care physician in the 7‐, 14‐, and 30‐day periods after hospital discharge was determined, as was whether a participant was rehospitalisation within 30 days. Information related to office visits and hospitalisations was ascertained from the medical group’s electronic health record and from health plan data, which allowed for determination of whether a rehospitalisation had occurred at any hospital and not just the primary hospital that served individuals under the care of the medical group. Analysts blinded to intervention status determined these outcomes at least 6 months after completion of the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator was used to assign a discharge to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | Computer allocated discharges |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Automated system was used. Also participants were not aware of which group they were in |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comparable rates of attrition in intervention and control groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analysts blinded to intervention status determined the outcomes. (The trialist reviewing the data (JHG) was unaware of which type of unit the event had occurred on.) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Protection against contamination bias | Unclear risk | The study is a cluster design and the authors stated the following, "Efforts were made to limit crossover of prescribers between intervention and control units, however, some prescribers worked simultaneously on both intervention and control units." |
| Other bias | Unclear risk | Unclear risk |
Hawes 2014.
| Study characteristics | ||
| Methods | RT 18 months |
|
| Participants | 61 participants: 24 intervention, 37 control Unclear how many participants were analysed Setting: healthcare system's outpatient family medicine centre Diagnostic criteria: In the first year of the study, inclusion criteria had to be 1 of the following 3 criteria:
In the second year, the criteria were changed to the following: ≥ 8 scheduled medications anticipated at discharge |
|
| Interventions | Organisational. Participants in the intervention group were scheduled for a care transitions clinic visit with a clinical pharmacist approximately 72 h post discharge, and prior to the post‐hospitalisation, primary care‐provider visit. The visit involved performing a complete medication history, identifying and resolving medication discrepancies, creating a current medication list for both the medical record and the participant, and counselling on appropriate medication use. During these visits, the pharmacist identified discrepancies between the best possible medication discharge list and the discharge summary, and characterised medication discrepancies using predefined categories. Study participants in the usual care group were scheduled to see their primary care provider for a post‐hospitalisation visit with no interim pharmacist intervention. Medication discrepancies of study participants not attending care transitions visits were identified and characterised by study personnel in the same manner as those in the intervention group. Study personnel reviewed study participants’ medical records to quantify 30‐day ED visits and rehospitalisation at the study institution. All study participants received a phone call approximately 30 days after discharge to report hospitalisations or ED visits at outside institutions. Only hospitalisations and ED visits at the study institution were included for those participants who were not able to be contacted after 3 phone call attempts. Both the intervention and control group received clinical pharmacy services for the family medicine inpatient service and outpatient family medicine clinic. Inpatient clinical pharmacists conducted rounds with the medical team daily, reviewed and monitored medications for effectiveness and safety, and made recommendations to the physician staff to optimise medications. Participants in both groups received this usual care from the inpatient pharmacist. The role of the inpatient pharmacist in the study was to collaborate with the inpatient medical team to create a BPMDL for all study participants just prior to discharge. The BPMDL was used to identify medication discrepancies, and it served as the gold standard list of medications that the participant should take after discharge. The BPMDL accounted for home medications, medication changes made during the hospitalisation, and medications that should be initiated or discontinued on discharge. |
|
| Outcomes | The 3 prespecified primary outcomes of this study were a composite of the occurrence of a hospital admission or an ED visit within 30 days after hospital discharge and the resolution of medication discrepancies before the primary care provider visit. Secondary outcomes include the individual rates of rehospitalisation and ED visits within 30 days after discharge. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | For the first year of study, a random number generator was used to randomise participants. For the second year, block randomisation with a block size of 4 was used. |
| Allocation concealment (selection bias) | Low risk | A computer was used to randomise |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants did not know they were receiving the intervention, however, pharmacists may have been aware that they were delivering the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Half of the intervention participants did not participate in the clinic visit. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Hospital admissions and emergency room visits are objective outcomes. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Protection against contamination bias | Unclear risk | It cannot be determined if the control group was contaminated by the intervention. |
| Other bias | Unclear risk | Small sample size. Also, recall bias may have operated as participants provided a self‐report of hospitalisations and ED visits outside of the study institution. It is not clear what was the final analysis sample. |
Holland 2005.
| Study characteristics | ||
| Methods | RT Setting: home visit study (primary care) Study duration: 6 months |
|
| Participants | 872 participants in a home visit study. Researchers recruited patients from four general hospitals and six community hospitals if they were aged ≥ 80 years, admitted as an emergency, intended to be discharged to their own home or warden‐controlled accommodation, and prescribed ≥ 2 drugs on discharge. Exclusion criteria: participants received dialysis treatment and participation in an intensive discharge service on 1 site Age (years) (mean ± SD): intervention 85.4 (4); control 85.5 (4); not significant Sex female n (%): intervention 262 (61.1); control 272 (63.8); not tested for significance Country: UK Comorbidity: baseline diagnosis: cardiovascular (total): intervention 134 (31.2), control 144 (33.8); myocardial infarction/angina: intervention 57 (13.3), control 65 (15.3); heart failure: intervention 38 (8.9), control 34 (8.0); musculoskeletal (total): intervention 61 (14.2), control 65 (15.3); fracture: intervention 37 (8.6), control 40 (9.4); gastrointestinal (total): intervention 47 (11.0), control 54 (12.7); respiratory (total): intervention 48 (11.2), control 49 (11.5); COPD/asthma: intervention 15 (3.5), control 13 (3.1); lower respiratory tract infection: intervention 16 (3.7), control 22 (5.2); neurological: intervention 40 (9.3), control 25 (5.9); stroke/transient ischaemic attack: intervention 16 (3.7), control 14 (3.3); senility/dementia: intervention 16 (3.7), control 6 (1.4); genitourinary: intervention 17 (4.0), control 16 (3.8); cancer (total): intervention 15 (3.5), control 7 (1.6); other or unclassified: intervention 67 (15.6), Control 66 (15.5) Sociodemographic: not mentioned Ethnicity: not mentioned Participants recruited between October 2000 and December 2002 |
|
| Interventions | Initial referral to a review pharmacist included a copy of the participant's discharge letter. Pharmacists arranged home visits at times when they could meet participants and carers. Pharmacists assessed participants' ability to self‐medicate and drug adherence, and they completed a standardised visit form. Where appropriate, they educated the participant and carer, removed out of date drugs, reported possible drug reactions or interactions to the general practitioner, and reported the need for a compliance aid to the local pharmacist. Where a compliance aid was recommended, this was provided within the trial and a filling fee was paid to the local pharmacist. 1 follow‐up visit occurred at 6‐8 weeks after recruitment to reinforce the original advice. Control participants received usual care. |
|
| Outcomes | The primary outcome was total number of emergency admissions to hospital over 6 months. Secondary outcomes included deaths, admissions to residential homes and nursing homes, and self‐assessed QoL measured using the EQ‐5D. Participants also rated their health on a visual analogue scale from 100 (perfect health) to 0 (worst imaginable health). The EQ‐5D and visual analogue scales were collected at baseline, 3 months, and 6 months. Data were collected on emergency admissions from hospital episode statistics. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Third party telephone randomisation based on a computer‐generated sequence in blocks of varying length. Randomisation appeared adequate |
| Allocation concealment (selection bias) | Low risk | Sequence was concealed based on what is noted above about sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not done as stated in the manuscript, "Because of the nature of the intervention, no “placebo” could be provided. Participants were told after randomisation which group they were in." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up of the main outcome (hospital admissions) was good—only 3% of participants withdrew or were lost to follow‐up. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is less important for our purpose as we are looking at objective outcomes (hospitalisations, ED visits, and mortality). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Unclear risk | It can not be determined if the control group has been contaminated by intervention |
| Other bias | Low risk | There is no other bias. |
Ibrahim 2013.
| Study characteristics | ||
| Methods | RT Setting: community participants Follow up: 3 months |
|
| Participants | 240 participants discharged to the community for the first time on oral anticoagulant warfarin (regardless of strength, gender, or age) There was no significant difference between the groups in terms of age, all participants 60.2 years ± 17.84 Sex of participants: male 160 (58.3%). There was no statistically significant difference found between the groups based on indication for anticoagulation, with atrial fibrillation representing the most common indication. All participants lived close to the participating medical centre and could access it easily. Country: United Arab Emirates Comorbidity: atrial fibrillation: 82 (34.2%), valve replacement: 37(15.4%), CHF:32 (13.3%), peripheral artery disease: 8 (3.33%), left ventricular thrombus: 7 (2.91%), stroke: 9 (3.75%) |
|
| Interventions | Intervention (Group A) was the 'counselled' group, whereas, control (Group B) was the 'non‐counselled' group. After initial physician/pharmacist consultation in a standard care setting, 1 group was thoroughly counselled, defined by the following:
The other group received no follow‐up consultation other than what was ordered by their own physician in a standard care setting. This group was asked only to visit the anticoagulation clinic twice a month for 3 months to evaluate international normalised ratio levels. |
|
| Outcomes | Number of adverse events, emergency visits and inpatients admissions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | The first 240 participants discharged or prescribed for the first time warfarin (regardless of strength, gender, or age) were divided randomly and assigned a intervention or control group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not fully described: separation of intervention and control groups is not exclusive. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants from the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described how and who measured the outcomes |
| Selective reporting (reporting bias) | Low risk | The study reported the proposed outcome. |
| Protection against contamination bias | Unclear risk | It is not clear if the control group was contaminated by the intervention. |
| Other bias | Unclear risk | Not applicable |
Kaczorowski 2011.
| Study characteristics | ||
| Methods | 2‐arm, community, cluster‐randomised trial Study duration: 3 years 39 eligible communities were stratified, geographically defined according to municipal boundaries, by population size (3 strata) and geographical location (4 strata). An independent expert in cluster‐randomised trials then used a random number generator to randomly allocate communities in each stratum to receive either CHAP or no intervention. |
|
| Participants | 39 communities (148,589 participants) initially and 145,441 participants after follow‐up post intervention Setting: 39 eligible communities, geographically defined according to municipal boundaries, by population size (3 strata) and geographical location (4 strata); community‐based Sex male (%): intervention communities 42.65 ± 1.19, control communities 42.92 ± 2.16 Country: Ontario, Canada Comorbidity: No. of prescription drugs in previous year: control communities: 7.25 (0.49), intervention communities: 6.98 (0.54) No. of comorbidity groups in previous 2 years: control communities: 7.31 (0.30), intervention communities: 7.17 (0.50) Charlson comorbidity index in previous 2 years: control communities: 0.57 (0.09), intervention communities: 0.58 (0.11) Diabetes (%): control communities: 22.16 (2.34), intervention communities: 21.20 (2.79) History of congestive heart failure (%): control communities: 12.19 (1.91), intervention communities: 12.45 (2.34). Rurality index: control communities 28.96 (13.60), intervention communities: 31.63 (14.09) Low‐income status (%): control communities: 16.95 (8.55), intervention communities: 18.57 (11.33) Ethnicity: Not stated. |
|
| Interventions | Communities were randomised to receive CHAP (n = 20) or no intervention (n = 19) In CHAP communities, residents aged ≥ 65 were invited to attend volunteer‐run cardiovascular risk assessment and education sessions held in community‐based pharmacies over a 10‐week period; automated blood pressure readings and self‐reported risk factor data were collected and shared with participants and their family physicians and pharmacists. In both intervention and control arms, residents received the usual health promotion and healthcare services available to all Ontarians under its publicly financed universal health insurance programme. |
|
| Outcomes | Rates of hospital admission for acute myocardial infarction, CHF, and stroke in these 39 communities and the 2001 census population estimates for people aged ≥ 65 years and over for power calculations | |
| Notes | A potential limitation of CHAP is the short duration of the intervention. The 10‐week exposure to CHAP may be too short to affect hospital admission rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 39 eligible communities were stratified by size of the population ≥ 65 years (3 groups) and geographic location (4 groups), forming seven substrata. Communities within each stratum were randomly allocated to either the intervention (n = 20) or control arm of the study (n = 19) by an independent expert in cluster‐randomised trials not associated with the study. |
| Allocation concealment (selection bias) | Low risk | The independent expert in cluster‐randomised trials was not associated with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although intervention community members (older adults, family physicians, volunteers, pharmacists) were clearly aware of their group assignment, the names of control communities were not publicised and control community members were not notified that the study was taking place. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported. Cluster‐randomised trial of communities reporting rates of hospitalisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Though unlikely, community members' knowledge of the evaluation could influence outcomes, but hospitalisation rates were retrieved from a population‐based administrative health dataset. |
| Selective reporting (reporting bias) | Low risk | Data retrieved from routinely collected, population‐based administrative health data |
| Protection against contamination bias | Unclear risk | It is hard to determine if the control group is contaminated with the intervention |
| Other bias | High risk | Short duration of the intervention may have had an impact on detection of outcomes such as hospital admissions. We considered other bias due to cluster randomisation |
Korajkic 2011.
| Study characteristics | ||
| Methods | RT Setting: ambulatory setting 9 months |
|
| Participants | 70 participants Ambulatory setting Attendees at a heart failure outpatient clinic, > 18 years, had New York Heart Association class II, III or IV heart failure, stable signs and symptoms of heart failure, clinically euvolaemic, daily frusemide dose up to a maximum of 320 mg, treatment with other drugs such as beta‐blockers, digoxin, vasodilators and spironolactone was permitted. Participants were excluded if they were not on frusemide; were on a daily frusemide dose above 320 mg and/or thiazide diuretic; had baseline renal impairment (serum creatinine concentration > 200 µmol/L or on dialysis); had a severe psychiatric illness or moderate‐severe dementia; life expectancy of < 3 months; severe hearing impairment or legal blindness; or had difficulty understanding and speaking English and did not have an interpreter or family member to assist. Other exclusions included scheduled cardiac surgery; heart transplant candidacy; inability to give informed consent; and no access to a telephone. |
|
| Interventions | Pharmacist intervention focused on participants improving self‐care, recognising symptoms of fluid retention, measuring weight daily and self‐adjusting diuretic dose using frusemide. Intervention group: participants assigned to the intervention group received usual care plus pharmacist intervention. The intervention was provided to every participant in the intervention group and consisted of a 30‐min educational session during the clinic appointment. The pharmacist intervention focused on participants improving self‐care, recognising symptoms of fluid retention, measuring weight daily and self‐adjusting diuretic dose using a flexible frusemide dose‐adjustment regimen, and improving knowledge and understanding of heart failure and heart failure medications. Usual care (control group): usual care was provided to all of the eligible participants by a cardiologist, heart failure nurse co‐ordinators and a dietitian during the clinic appointment. Usual care consisted of assessment of clinical status and medications, education on daily weight measurement, diet, fluid and sodium management, and recognition of signs and symptoms of fluid retention and dehydration. In case of a sudden increase in weight of more than 1 kg/d for 2 d, participants were encouraged to contact the heart failure nurse co‐ordinators for advice in consultation with the cardiologist to self‐adjust their frusemide dose. The heart failure nurse co‐ordinators followed up participants 48 h after a dose adjustment to assess if their weight had decreased and condition improved. The key difference between the groups was that the control group called a heart failure nurse co‐ordinator to discuss frusemide dose modification, while the intervention group adjusted the diuretic dose themselves. |
|
| Outcomes | Hospital readmissions due to fluid overload: measured at 1st, 2nd and 3rd months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred after selection criteria had been observed. No description of the randomisation method/sequence generation was presented. |
| Allocation concealment (selection bias) | Unclear risk | A significant number of heart failure participants were not good candidates for the intervention. Only 1 in 3 participants who met inclusion criteria remained eligible after application of exclusion criteria. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments and readmissions were evaluated and confirmed by an independent doctor blinded to the randomisation using data from participants, hospital admissions records and medical records. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as mentioned at the start of the trial. |
| Protection against contamination bias | Unclear risk | It can not be determined if the intervention group interacted with the control group. |
| Other bias | Unclear risk | The intervention was delivered by the same pharmacist. It precludes study of other factors, such as pharmacist attitudes or behaviours that may have promoted delivery of the intervention and limit the generalisability of the intervention. This study was conducted at a single institution, and the results may reflect local population characteristics and patterns of care. |
Krska 2001.
| Study characteristics | ||
| Methods | RT Duration of study: 3 months |
|
| Participants | 332 participants completed study (168 intervention and 164 control) Setting: general practice Diagnostic criteria: the inclusion criteria for participants were ≥ 65 years, regular request for ≥ 4 medicines via the computerised repeat prescribing system and ≥ 2 chronic diseases. Exclusion criteria were dementia and being considered by the GP to be unable to cope with the study. Age (years) (mean ± SD): intervention 74.8 (6.2), control 75.2 (6.6) Sex female n (%): intervention 95 (56.5%), control 106 (64.6%) Country: UK Comorbidity: mean no. of chronic diseases: intervention 3.9 (1.4), control 3.8 (1.4), P = 0.968 Sociodemographics: nothing of note Ethnicity: not mentioned Date of study: not mentioned but paper received by journal on 23 December 1999 |
|
| Interventions | 1 intervention group Intervention group: a pharmaceutical care plan was drawn up for each intervention group participant, listing all potential and actual pharmaceutical care issues, together with the desired output(s), the action(s) planned to achieve the output(s) and the outcomes of any potential pharmaceutical care issues already resolved by the pharmacist. Copies of the plan were inserted in the participants' medical notes and given to their GP, who was asked to indicate their level of agreement with each pharmaceutical care issue identified and with the actions. The pharmacist then implemented all remaining agreed actions, assisted by other practice staff where appropriate. Control participants were similarly interviewed and pharmaceutical care issues identified, although no pharmaceutical care plan was implemented. Participants were advised to consult any usual carers or health‐care professionals in response to direct queries during interview. |
|
| Outcomes | Number of hospital admissions | |
| Notes | The pharmacists undertaking the medication review also administered the SF‐36 questionnaire and identified all care issues. There is also potential for GPs receiving recommendations for some participants to increase their tendency to note similar issues in control participants. In some cases the care plan was not fully implemented by 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random number tables, 1 practice from each of the 6 resultant categories was selected and invited to participate. 1 practice refused and a further practice was randomly selected. Participants were randomly allocated to the intervention or control group. |
| Allocation concealment (selection bias) | Unclear risk | Although a random number table was used to select practices, it was not clear whether participant assignment to intervention and control groups was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was adequate because pharmacists only treated intervention participants and did not know that the participants they interacted with were in a study. Also, participants did not appear to know whether they were receiving an intervention or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal numbers of participants in the control and intervention groups withdrew from the study. Around 14% to 15 % of the participants withdrew in each group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is less important for our purpose as we are looking at objective outcomes (hospitalisations, ER visits, and mortality) |
| Selective reporting (reporting bias) | Low risk | There is no evidence that there was selective reporting of results |
| Protection against contamination bias | High risk | It can not be determined if the control group was contaminated with the intervention group. |
| Other bias | Unclear risk | In some cases, the care plan had not been fully implemented by the 3‐month follow‐up. |
Lapane 2011.
| Study characteristics | ||
| Methods | Cluster‐RT Study duration: 2 years |
|
| Participants | 6523 participants Final sample: 1769 control, 1769 intervention Diagnostic criteria: not relevant as homes were the unit of analysis not individuals Age (years) (mean ± SD): average age of residents was not reported. At baseline 16% of the residents in both intervention and control homes were aged 65‐74 years, 36% in intervention homes and 35% in usual care homes were 75‐84 years, and 40% of the residents in the intervention homes and 36% in the usual care homes were ≥ 85 years. During the intervention period, 15% in both groups were 65‐74 years, 39% were 75‐84 years, and 39% were ≥ 85 years Sex, female n (%): at baseline, 72% of the residents in the intervention homes and 68% in the usual‐care homes were female. During the intervention period, 74% of the residents in the intervention and usual‐care homes were female. Country: USA Comorbidity: (intervention, control), dementia (35.4, 43.4); Alzheimer's disease (12.7, 14.6), cancer (8.3, 12.1), diabetes mellitus (27.5, 31.0), cerebrovascular accident (22.2, 22.4), heart failure (26.5, 28.5), coronary artery disease (18.6, 16.2), arrhythmia (15.8, 15.8), hypertension (64.9, 61.8), other cardiovascular disease (23.6, 28.0) Sociodemographics: nothing reported other than race Ethnicity: 18% in intervention group and 11% in usual care group were minority race at baseline. During the intervention period, 19% of both groups were minority race Date of study: 2003‐2004 |
|
| Interventions | Professional intervention The overarching idea was to use health information technology to engage consultant pharmacists and nursing staff to identify residents at risk for delirium and falls, implement proactive monitoring plans as appropriate, and provide reports to assist consultant pharmacists in conducting the medication regimen review. Intervention: A Geriatric Risk Assessment MedGuide database for falls and delirium was integrated into the pharmacies' commercial pharmacy software system (Rescot LTCP System) for the intervention homes. Control: usual care |
|
| Outcomes | Incidence of potential delirium, falls, hospitalisations potentially due to adverse drug events, and mortality | |
| Notes | Residents in the intervention homes experienced fewer falls, less potential delirium, and death, but more hospitalisations than in the comparison homes. In new admissions, there appeared to be a trend toward lower mortality (adjusted hazard ratio 0.88, 95% CI 0.66 to 1.16) and a lower overall hospitalisation rate (adjusted hazard ratio 0.89, 95% CI 0.72 to 1.09) and a clear reduction in the rate of potential delirium (adjusted hazard ratio 0.42, 95% CI 0.35 to 0.52) in the intervention homes than the comparison homes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Cluster‐randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination bias | Low risk | It can not be determined if the intervention group was contaminated with the control group. |
| Other bias | Unclear risk | There is no evidence of the presence of other bias. We considered other bias due to cluster randomisation. |
Lenaghan 2007.
| Study characteristics | ||
| Methods | RT 6 months |
|
| Participants | 136 participants registered with 1 general practice (1 participant from each group withdrew shortly after randomisation) Home‐based > 80 years, living at home, taking ≥ 4 oral medications, and had ≥ 1 additional medicine‐related risk factor Participants were excluded if they were resident in a care home or if there was documented use of an adherence aid. Age: intervention 84.5 years, control 84.1 years (no SD supplied) Gender female: intervention 46 (67.6%), control 42 (63.6%) Country: UK Sociodemographics: living alone: intervention 44 (64.7%), control 43 (65.1%); social class (I, II, III): intervention 33 (48.5%), control 29 (43.9%); 9% of practice were aged over 80 years (twice the national average) Ethnicity: 98.5% of the local town population were white, compared to 90.9% for England |
|
| Interventions | Comparing home‐based medication review with standard care The intervention: the pharmacist was asked to identify cases where adverse drug reactions or drug interactions may be occurring. This was noted using a tick box on the medication review form after detailed information had been gained from the participant regarding all over‐the‐counter and prescribed drugs The control group received standard care |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of random sequencing |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by a third party and was stratified by whether the participant lived alone. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported and reasons for attrition presented |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome data on hospital admissions were provided by hospital episode statistics (not self‐report) and are therefore unlikely to be biased. |
| Selective reporting (reporting bias) | Low risk | Outcome data on hospital admissions were provided by hospital episode statistics (not self‐report) and are therefore unlikely to be biased. |
| Protection against contamination bias | Unclear risk | Unclear |
| Other bias | High risk | Research was carried out in 1 rural general practice with a single experienced review pharmacist, which has a bearing on the generalisability of the results. |
Lowrie 2012.
| Study characteristics | ||
| Methods | Study design: a cluster‐randomised design, this provides protection against contamination across trial groups when trial participants are managed within the same setting. Participants in practices in the UK were managed by all GPs within the practice; the control intervention was mediated by GPs, this precluded individual, participant‐level randomisation. Study duration: median follow‐up was 4.7 years |
|
| Participants | 2164 participants (174 practices) Setting: general practice Diagnostic criteria: consenting participants were eligible if aged ≥ 18 years and had left ventricular systolic dysfunction confirmed by cardiac imaging conducted at a local hospital (transthoracic echocardiography in 90% of cases). Participants did not have to have symptoms or signs of heart failure. Family doctors received a semi quantitative report of left ventricular systolic function (normal, mild, moderately or severely reduced) instead of ejection fraction. Age (years) (mean ± SD): pharmacist intervention, 70.6 (10.3) and control 70.6 (10.1) Sex female n (%): pharmacist intervention 320 (29%), control 329 (31%) Country: UK Comorbidity: hypertension, myocardial infarction, pharmaceutical care issue, coronary artery bypass grafting, atrial fibrillation or flutter, diabetes mellitus, stroke, respiratory disease, asthma Sociodemographics: not mentioned Ethnicity: not mentioned Date of study: from 25 October 2004‐6 September 2007 |
|
| Interventions | 1 intervention and 1 control Participants from practices assigned to the intervention were offered a 30‐min appointment with a pharmacist. The main aim of this review was optimisation of medical treatment for left ventricular systolic dysfunction according to guidelines (supplementary material online). If there was agreement between the pharmacist and the participant during the consultation and subsequently with the family doctor, medications were initiated, discontinued, or modified by the pharmacist during 3‐4 subsequent weekly or fortnightly consultations. Family doctors provided usual care thereafter. No instructions were given to family doctors in the usual care practices. The study pharmacists did not collect information on symptoms or examine the participants as this was not part of their professional training. |
|
| Outcomes |
|
|
| Notes | There was no difference in mortality or hospital admissions between the intervention and the control group. (Mortality from heart failure should be reported in the final analysis as this intervention was targeting heart failure management) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by a computer |
| Allocation concealment (selection bias) | Low risk | Computer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Cluster randomisation was undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination bias | Low risk | It can not be determined if the intervention group mixed with the control group |
| Other bias | Unclear risk | Unclear. We considered other bias due to cluster randomisation. |
Malet‐Larrea 2016.
| Study characteristics | ||
| Methods | Cluster‐RT (pharmacies were the cluster unit of randomisation) | |
| Participants | 31; 17 intervention and 14 control, this was also the final sample that was analysed Setting: community pharmacists Diagnostic criteria: participants were ≥ 65, used ≥ 5 medications for ≥ 6 months, with the ability to complete the EuroQol 5D questionnaire |
|
| Interventions | Organisational IIntervention group: pharmacists allocated to the intervention group provided the medication with follow‐up service according to national guidelines. The medication review with follow‐up service started with a comprehensive interview undertaken in a private area of the pharmacy. The pharmacist collected relevant information about the participant’s health problems, medicines used, clinical and biological parameters (gathered through medical records provided by the participant or measured in the pharmacy), medication use, lifestyle habits and concerns about diseases and medications. Pharmacists also assessed the level of control of health problems by using information referred by participants’ and/or clinical and biological parameters, depending on the type of health problem (i.e. pain versus hyperlipidaemia) and classified every health problem as controlled, uncontrolled or unknown. After performing a comprehensive medication review, the pharmacist identified negative clinical outcomes related to medicines and drug‐related problems. Subsequently, an action plan was agreed upon by the participant and the physician if required. This medication review with follow‐up service was focused on both participants’ outcomes and medication use process and required a commitment to follow‐up. The usual care consisted of dispensing medicines prescribed by physicians and advice on minor ailments. |
|
| Outcomes | Medication‐related hospital admission was the primary outcome of this sub analysis. Hospital admissions were recorded in participants’ visits to the pharmacies and the medication related ones were identified through the expert panel after the fieldwork. Kappa values ranging from 0.61 to 1 were considered as an acceptable incidence rate ratio to measure the agreement among experts. The cost of hospital admissions estimated by diagnosis‐related group was a secondary outcome and the diagnosis‐related groups were recorded after the fieldwork. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacies were randomised to the intervention or control group by an independent researcher. |
| Allocation concealment (selection bias) | Low risk | An independent researcher performed randomisation using a computer‐generated list of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor pharmacists were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was little attrition and comparable rates for intervention and control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the sub analysis, the expert panel was blind as to which group the participants belonged so whether a hospital admission was medication‐related was not affected. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Unclear risk | Although pharmacies were randomised to control and intervention groups, informal contact between pharmacists may have led to contamination. |
| Other bias | Unclear risk | There is no evidence of other bias. |
Malone 2000.
| Study characteristics | ||
| Methods | A prospective, multisite RT Duration of study: 12 months |
|
| Participants | Of 1054 participants enrolled at the 9 Veterans Affairs clinics, 523 were randomised to the intervention and 531 to the control group. Of these, 950 participants completed 6‐month follow‐up questionnaires and 931 completed the study. Of participants completing the study, 447 were in the intervention group and 484 were in the control group. Setting: Veterans Affairs clinics Interventions: clinical input by pharmacists Diagnostic criteria: participants were considered at high risk for drug‐related problems if they met ≥ 3 of the following criteria: were taking ≥ 5, were taking ≥ 12 doses/d, had ≥ 3 chronic medical conditions, had ≥ 4 changes in their drug regimen over the past year, had a history of noncompliance with drug therapy, or were taking an agent that required therapeutic drug monitoring. Age: (years) mean ± SD: 67 ± 10.1 Sex n (%): intervention group 21 (0.04%), control, 20 (0.04%) Country: USA Comorbidity: hypertension, angina, hyperlipidaemia, arthritis, diabetes and COPD Sociodemographics: not mentioned Ethnicity: not mentioned |
|
| Interventions | 1 intervention and 1 control The intervention group was given a protocol to follow; the protocol indicated that each participant should have ≥ 3 visits with the clinical pharmacist during the study, but participants could be seen as frequently as deemed necessary to ensure appropriate care. Visits were to occur between or concurrent with appointments with the primary care provider or other physicians. The control group followed the usual care with no specific protocol given to clinicians. |
|
| Outcomes | Number of hospitalisations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a central computer. |
| Allocation concealment (selection bias) | Low risk | Computer‐based |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some participants did not complete the study and were reported in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear how it was done |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination bias | Unclear risk | It is not possible to determine if the control group was contaminated with the intervention group. |
| Other bias | Unclear risk | It is unclear if there are other biases. |
Moertl 2009.
| Study characteristics | ||
| Methods | Prospective, randomised study design Study duration: 2 years The major limitation of the study was selection bias because only participants who responded to a letter of invitation had the opportunity to take part in the study. |
|
| Participants | 96 participants took part in the study; 48 were randomised to the nurse group and 48 to the non‐nurse group. Setting: outpatient heart failure clinic Diagnostic criteria: participants who survived index hospitalisation were invited by letter to a visit for treatment optimisation at the outpatient heart failure unit. Among the participants who appeared at the ambulatory visit, those with a verified heart failure diagnosis and residing < 50 km from Vienna were eligible for the nurse intervention and therefore offered to participate in the present study. Baseline evaluation was performed by a cardiologist specialising in the management of heart failure. The ambulatory visit comprised a patient history, physical examination, electrocardiogram, a routine blood analysis, and, if necessary, an echocardiography. Furthermore, blood samples were taken for later analysis of natriuretic peptides. The participants were thoroughly informed about the disease of CHF and recommendations were made regarding medication, self‐assessment of weight, blood pressure and pulse, and diet and exercise management. The baseline demographic, clinical, and therapeutic characteristics were not statistically different between the nurse group and the non‐nurse group. Age (years) (mean ± SD): non‐nurse, control (66 ± 13); nurse, intervention 70 ± 12 Country: Vienna, Austria Comorbidity: hypertension, diabetes, respiratory diseases Sociodemographics: not reported Ethnicity: Austrian (unclear if they were all white) |
|
| Interventions | 1 intervention and 1 control. There were 48 participants in each group. Intervention: home‐based nurse care Participants in the nurse group were visited by a nurse specialised in caring for people with heart failure on the initial visit at the outpatient heart failure unit and then at their home 3, 6, 9, and 12 months after randomisation. At home visits, the nurse checked and recorded weight, symptoms and signs of heart failure, heart rate and blood pressure, and organised and reviewed blood analyses on demand, especially of electrolytes and renal parameters. Furthermore, the nurse had to check for and, in co‐ordination with the treating physician, implement guideline‐based medication. Moreover, the nurse was in charge of individualised participant and caregiver education and enhancement of self‐management. If the nurse noted any deterioration in the participant's status, she reported to the treating physician or advised the participant to visit the treating physician. Control group received the usual care provided |
|
| Outcomes | Admission for heart failure at 12 months and 24 months Mortality at 12 months and 24 months |
|
| Notes | The major limitation of the study is selection bias because only participants who responded to a letter of invitation had the opportunity to take part in the study. See notes to other relevant studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is unclear how the sequence generation was done. |
| Allocation concealment (selection bias) | High risk | The major limitation of the study was selection bias because only participants who responded to a letter of invitation had the opportunity to take part in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no incomplete outcome data. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent data collector |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as mentioned at the start of the trial. |
| Protection against contamination bias | Unclear risk | Unclear |
| Other bias | High risk | Small sample size |
Murray 2004.
| Study characteristics | ||
| Methods | RT with a 2 x 2 factorial design using physician and pharmacist interventions, which resulted in 4 groups of participants: physician intervention only, pharmacist intervention only, intervention by physician and pharmacist, and intervention by neither physician nor pharmacist (control) Study duration: 1 year |
|
| Participants | Total participants 712 with uncomplicated hypertension Control: (n = 171), pharmacist intervention: (n = 180), physician intervention: (n = 181), dual intervention: (n = 180). Setting: large, inner‐city, academic, internal medicine practice affiliated with the Indiana University School of Medicine. The primary venues for this study were the general medicine practice and the Wishard Memorial Hospital outpatient pharmacy, which at the time of the study were located 1 floor apart in the Regenstrief Health Centre. Eligibility for this study required that participants had evidence in their electronic medical records of hypertension as an active outpatient diagnosis or, in the absence of such a diagnosis, all of the following: ≥ 2 systolic blood pressure measurements of ≥ 140 mm Hg, ≥ 2 diastolic blood pressure measurements of ≥ 90 mm Hg, and a prescription for ≥ 1 antihypertensive agent. Qualifying antihypertensive agents were ace‐converting enzyme inhibitors, b‐blockers, calcium channel blockers, oral clonidine and topical patch, diuretics, and other less commonly prescribed drugs such as methyldopa and reserpine. Participants were excluded from taking part if they had evidence (diagnoses or test results) indicating the presence of a cardiovascular complication such as coronary artery disease, myocardial infarction, stroke, heart failure, or renal insufficiency. Age mean ± SD: control: 54 ± 11, pharmacist intervention: 54 ± 11, physician intervention: 56 ± 11, dual intervention: 54 ± 11 Gender female n (%): control: 75 (44%), pharmacist intervention: 79 (44%), physician intervention: 78 (43%), dual intervention: 81 (45%) Country: USA Comorbidity: none stated Sociodemographics: potential participants who were able to communicate (could hear and speak English and understand instructions), had access to a working telephone, and were willing to provide written informed consent were enrolled. Formal education, mean ± SD (years), control: 11 ± 3, pharmacist intervention: 10 ± 3, physician intervention: 11 ± 3, dual intervention: 11 ± 3 Married (%), control: 30, pharmacist intervention: 29, physician intervention: 28, dual intervention: 30 Number of people in household, mean ± SD, control: 2.3 ± 1.5, pharmacist intervention: 2.6 ± 1.7, physician intervention: 2.3 ± 1.4, dual intervention: 2.2 ± 1.2 Live alone (%), control: 32, pharmacist intervention: 28, physician intervention: 32, dual intervention: 30. Ethnicity: unknown; control: 57 (33%), pharmacist intervention: 61 (34%), physician intervention: 58 (32%), dual intervention: 58 (32%) |
|
| Interventions | Physician intervention The computer‐based ordering system generated care suggestions for both intervention and control groups; however, the suggestions were displayed by the computer to physicians and/or pharmacists for participants randomised to the appropriate intervention groups. This allowed the researchers to assess the numbers and types of interventions that the control group was eligible to receive as well as those in the 3 intervention groups. For participants in the physician intervention group, all care suggestions based solely on earlier Regenstrief medical records system data were generated at the time that the encounter form was printed and were displayed at the end of the drug list. All hypertension care suggestions for intervention participants were displayed as “suggested orders” on physicians’ workstations when they wrote orders after participant visits. This computer screen displayed the actual suggested order, possible actions for each order (order or omit), and a brief explanation of the rationale for the order. Physicians could list full guidelines and literature citations associated with the specific suggestions by using the workstation’s 'help' key. Pharmacist Intervention When any participant brought a new or refill prescription (written in any affiliated clinic, physician’s office, or Wishard Hospital emergency department) to the Wishard outpatient pharmacy, a pharmacy technician entered the data into the Regenstrief medical records system pharmacy module. This was required for all prescriptions because it was the only way to generate and complete a financial transaction for prescriptions in the outpatient pharmacy. After entering prescription data, a high‐speed printer created a label to affix to the participant’s drug container. The technician who filled the prescription notified the pharmacist for all intervention participants. The labelled drug product was checked by a pharmacist who dispensed the agent to the participant and provided counselling. For this study the researchers created the pharmacist intervention recording system. This software programme was used by all Wishard pharmacists to document all pharmaceutical care interventions provided to any outpatient. For participants enrolled in this study only (regardless of study group), care suggestions generated by the Regenstrief medical records system or the outpatient workstations (in response to data entered by the physician, e.g. new antihypertensive prescriptions) were stored in the pharmacist intervention recording system. For participants randomised to receive care from an intervention pharmacist who had such care suggestions, the high‐speed printer printed a note together with drug container labels directing the pharmacist to the pharmacist intervention recording system to display care suggestions that were identical to those viewed by intervention physicians. Physician and pharmacist (dual) Intervention Control group: the control group did not receive any interventions by either physician nor pharmacist |
|
| Outcomes | The primary end point was generic health‐related QoL. Secondary end points were symptom profile and side effects from antihypertensive drugs, number of emergency department visits and hospitalisations, blood pressure measurements, participant satisfaction with physicians and pharmacists, drug therapy compliance, and health care charges. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were taken on a next‐in‐line basis – no random sequence, but sequentially allocated to either intervention or control. Physicians were randomly assigned to practices. There were no details of how randomisation was generated. |
| Allocation concealment (selection bias) | Low risk | Participating physicians and pharmacists were unaware of the study hypothesis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participating physicians and pharmacists were unaware of the study hypothesis. All research assistants and interviewers were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data extracted from Regenstrief medical records system records for ED visits, hospitalisations and mortality |
| Selective reporting (reporting bias) | Low risk | All selected outcomes were reported. Random audit (10%) of all paper records from intervention and control groups |
| Protection against contamination bias | Low risk | It is hard to determine if the control group was contaminated with the intervention group. |
| Other bias | Unclear risk | Unclear. There is no evidence of other bias |
Nabagiez 2013.
| Study characteristics | ||
| Methods | RT Study duration: 13 months |
|
| Participants | 701 participants Setting: home Diagnostic criteria: all participants discharged to home following coronary artery bypass graft procedure and/or valve repair or replacement and/or aneurysm repair, or other cardiac procedure Age (years) (mean ± SD): intervention group: 62.8 (10.6), control group: 63.2 (10.9) Sex female n (%): intervention group: 73 (21.5%), control group: 88 (24.4%) Country: USA Comorbidity n (%): diabetes mellitus: intervention 123 (34.0), control 111 (32.6); hypertension: intervention 268 (74.2), control 283 (83.2); dyslipidaemia: intervention 263 (72.8), control 274 (80.5); dialysis: intervention 8 (2.2), control 7 (2.0); cerebrovascular accident: intervention 15 (4.1), control 9 (2.6); COPD: intervention 44 (12.1), control 30 (8.8); peripheral vascular disease: intervention 29 (8.0); control 25 (7.3); previous myocardial infarction: intervention 146 (40.4), control 144 (42.3); CHF: intervention 51 (14.1), control 48 (14.1); arrhythmia: intervention 37 (10.2), control 39 (11.4) Sociodemographics: not stated Ethnicity: intervention group: 289 (84.4%) white, 53 (15.5%) non‐white; control group: 318 (88%) white, 43 (11.9%) non‐white Date of study: August 2009‐September 2011 |
|
| Interventions | 1 intervention group 340 participants, control 361 participants Hospital‐employed, cardiothoracic physician assistants conducted home visits on post‐discharge days 2 and 5, with occasional variation due to participant availability and Sundays, on which no house calls were made. The same hospital‐based physician assistants responsible for perioperative and intraoperative care were assigned to make house calls. During a house call, the physician assistant performed a focused physical exam and reviewed the participant’s medications. Adjustments were made to the participant’s medications, and new medications were prescribed as necessary. The surgical wounds were examined carefully and all participant concerns were addressed. Prescriptions were written for antibiotics, blood work, or imaging studies when indicated. Arrangements were made if the participant needed to be evaluated as an inpatient. All findings were documented on the visit form. Both groups were seen in the office on post‐discharge weeks 2 and 4. The control group was seen at home by standard visiting nurses without any specialty training or expertise in caring for people with cardiac surgery |
|
| Outcomes | Hospital admissions/number of people admitted to hospital | |
| Notes | Not sure of randomisation and these were hospital‐based physician assistants working in homes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear from the document |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the document |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear from the document |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% of the participants in the intervention group refused to participate or failed to respond to requests to participate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is less important for our purpose as we are looking at objective outcomes (hospitalisations, ED visits, and mortality). |
| Selective reporting (reporting bias) | Low risk | No evidence of this |
| Protection against contamination bias | Low risk | No contamination |
| Other bias | Unclear risk | Unclear. There is no evidence of other bias |
Okamoto 2001.
| Study characteristics | ||
| Methods | Prospective, randomised, comparative study Duration: 6 months |
|
| Participants | 330 participants with mild‐to‐moderate essential hypertension Age, years (mean ± SD): intervention 61.95 ± 11.4, control 61.71 ± 11.3, P = 0.85 Sex female n (%): intervention 72 (44%), control 90 (54%) Country: USA, California No statistically significant differences were noted between the groups. Concurrent disease (number of participants) intervention 98, control 95, P = 0.74 Smoker (number of participants): intervention 15, control 9, P = 0.18 Alcohol consumer (number of participants): intervention 12, control 9, P = 0.47 Sociodemographics: not reported Ethnicity: not reported |
|
| Interventions | Hypertension care provided by either the pharmacist‐managed hypertension clinic or physician‐managed general medical clinics In the pharmacist‐managed hypertension clinic, a clinical pharmacist managed the treatment of participants, who made up the experimental group. Physicians were contacted and provided consent for any therapeutic changes but were asked not to adjust drug therapy unless a lack of intervention would be dangerous for the participant. In the physician‐managed clinic, physicians managed the treatment of participants independently with no pharmacy intervention; this was the control group. Participants randomly assigned to the pharmacist‐managed hypertension clinic group were counselled by the clinical pharmacist. The pharmacist informed the participants that an effort would be made to decrease the number of drugs they took for hypertension or to alter their therapy by administering more appropriate or less expensive drugs to achieve similar or improved blood pressure control. The pharmacist determined the most appropriate antihypertensive regimen for the participant and ordered laboratory tests as needed. The pharmacist also provided education on nonpharmacologic ways to control blood pressure. Control group: participants randomised to the physician‐managed clinic group were referred back to their primary care provider for hypertension treatment. These participants received no intervention, and physicians treated them in the customary manner |
|
| Outcomes | Number of hospitalisations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation, but no description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not clear. The document does not state whether the allocation was concealed or not |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is unclear if participants were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates in both groups are comparable for various reasons. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of data collection is not clear. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as mentioned at the start of the trial. |
| Protection against contamination bias | Unclear risk | It is hard to determine if the intervention group was contaminated with the control group. |
| Other bias | Unclear risk | It would have been more desirable to have only newly diagnosed participants, but the sample size was already small. Results cannot be extrapolated to other physician groups. |
Olesen 2014.
| Study characteristics | ||
| Methods | RT Duration: not provided |
|
| Participants | 630 participants, 315 intervention, 315 control. Final sample analysed 253 intervention, 264 control Setting: pharmaceutical care was provided at home Diagnostic criteria: participants aged ≥ 65 on 5 prescription medications taken without assistance |
|
| Interventions | Organisational Participants in the ‘pharmaceutical care’ group were visited at home by a pharmacist at the beginning of the project. The pharmacist examined the medicines list with regard to possible side‐effects, interactions, and administration, then tried to make the regime less complex, informed the participants meanwhile about the drugs, listened to questions concerning the drugs, handed over information leaflets, and motivated adherence. Nine different pharmacists were involved and adhered to the Danish manual for pharmaceutical care: ‘Medication Review ‐ Managing Medicine Manual’. The aim of the ‘Medication Review ‐ Managing Medicine’ is to prevent, identify, and resolve drug‐related problems and to contribute to rational pharmacotherapy for participants and society. Control participants were not provided any intervention. |
|
| Outcomes | The primary endpoint was treatment adherence assessed by a pill‐count in all participants during 1 year. Only oral prescription drugs taken throughout the study period were included in the adherence calculation. In addition, a project nurse visited all participants initially, then at 6 and 12 months to photograph pills to be counted later by a ‘counter pen’ (a combination of a marker and a digital counter). The adherence rate (%) per drug was calculated as mean adherence rate during 1 year. We also calculated adherence rates for the intervals of 0‐6 and 6‐12 months. Secondary outcome measures included drug‐related problems, hospitalisations and mortality measured during the intervention year and at 2‐year follow‐up. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to control and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | No mention is made of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pharmacist was aware of whether the participant was in the intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Little differential attrition between intervention and control groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Hospitalisations and mortality are objective outcomes. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Protection against contamination bias | Low risk | Control group participants were not provided any pharmaceutical intervention. |
| Other bias | Unclear risk | According to the study authors, control participants were not exposed to any intervention, but something was done and this was not specified. |
Pai 2009.
| Study characteristics | ||
| Methods | Prospective, randomised, controlled, longitudinal, 2‐year pilot study | |
| Participants | 104 participants, nonprofit university‐affiliated dialysis clinic Participants > 18 years with end‐stage renal disease who were undergoing a stable haemodialysis regimen for at least 3 months Age (yrs ± SD): intervention 56.3 ± 15, control 60.5 ± 14.7 Sex female n (%): intervention 22 (39), control 28 (60), P < 0.03 Country: USA, New Mexico Baseline clinical characteristics: length of time participant had been receiving haemodialysis (years): intervention 2.8 ± 1.8, control 2.4 ± 2.2 Number of drugs used: intervention 10 ± 4, control 10 ± 4 Cost of drugs (USD); intervention 430 ± 197, control 451 ± 267 Comorbidity: end‐stage renal disease aetiology Diabetes mellitus n (%): intervention 22 (39), control 23 (49) Hypertension n (%): intervention 18 (32), control 12 (26) White n (%): intervention 13 (23), control 16 (34) Hispanic n (%): intervention 17 (30), control 15 (32) Native American n (%): intervention 13 (23), control 5 (11) |
|
| Interventions | Intervention group: effects of pharmaceutical care, consisting of 1‐1, in‐depth drug therapy reviews conducted by a clinical pharmacist, versus Control group: standard care, consisting of brief drug therapy reviews conducted by a nurse on several participant outcomes in ambulatory participants undergoing haemodialysis. Participants assigned to pharmaceutical care had drug therapy reviews conducted by a nephrology‐trained clinical pharmacist or 1 of 2 pharmacists completing postdoctoral training in nephrology pharmacotherapy. Types of drug‐related problems were recorded and evaluated by using a previously described method. All drug‐related problems were assigned to 10 possible categories: untreated indications, improper drug selection, sub therapeutic dosage, overdose, adverse drug reactions, drug interactions, failure to receive drugs, medical record discrepancy, inadequate education of participant or health care professional, and drug use without indication. The drug‐related problems were further categorised into therapeutic drug classes, and the outcome related to the drug‐related problem intervention was captured. The standard care group served as the control group. The participants in the standard care group received periodic drug profile updates by dialysis nursing staff as mandated by the dialysis clinic policy and procedure. These are typically brief interactions in which participants are queried as to whether any drugs have changed since the last review. |
|
| Outcomes | Mortality | |
| Notes | The study experienced high attrition due to death, transplantation, or transfer to a different facility, with about 50% of participants remaining at the end of study. The study also did not conduct an assessment of the relationship between drug‐related problem resolution and hospitalisations, which could provide useful information as to whether targeted pharmaceutical care interventions would be helpful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, by dialysis shift but no description of sequence generation |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by the clinic nurse manager, who had no affiliation with the study, by drawing the shift name from an opaque envelope and assigning the first 3 drawn shifts to pharmaceutical care and the remainder to standard care. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding as far as participants and personnel were not communicating ‐ different shifts |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a high level of attrition due to death, transplantation or transfer to a different facility, with about 50% of participants remaining at the end of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data on hospital admissions were provided by hospital episode statistics (not self‐report) and are therefore unlikely to be biased. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as mentioned at the start of the trial. |
| Protection against contamination bias | Low risk | It can not be determined if the intervention group was contaminated with the control group. |
| Other bias | Unclear risk | Unclear. It is unclear if there is other bias. |
Roberts 2001.
| Study characteristics | ||
| Methods | Cluster‐RT Study duration: 12 months |
|
| Participants | 3230 participants Setting: nursing homes Diagnostic criteria: none provided Age: participant characteristics not provided in terms of mean age, just percent of sample in intervention and control groups that were in particular age ranges Sex: participant characteristics not provided Country: Australia Comorbidity: not provided Ethnicity: participant characteristics not provided Date of study: unknown although paper was initially received for publication in May 2000 |
|
| Interventions | 1 intervention group The 12‐month intervention involved 3 phases: introducing a new professional role to stakeholders with relationship building, nurse education, and medication review by pharmacists who had a postgraduate diploma in clinical pharmacy. The clinical pharmacy service model introduced to each nursing home was supported with activities such as focus groups facilitated by a research nurse, written and telephone communication, and face‐to‐face professional contact between nursing home staff and clinical pharmacists on issues such as drug policy and specific resident problems, together with education and medication review. This was a multifaceted intervention directly targeting nursing homes. Most of the contact with GPs was indirect using the existing relationships between nursing homes and visiting GPs. A number of focus groups and personal interviews about the project were conducted with GPs. Control nursing homes continued with usual care. |
|
| Outcomes | Mortality was collected at the end of the 12‐month study. | |
| Notes | No significant changes were observed in annual mortality rates or frequency of hospitalisations between intervention and control nursing home groups. It is unclear from Table 5, which shows the mortality and hospitalisation data, how the study authors arrived at their figures or their conclusions. Therefore, we were unable to use the data to calculate hospitalisations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Nursing homes were selected for the intervention treatment by random draws from a hat. |
| Allocation concealment (selection bias) | Low risk | Not clear if this was done although the homes were independently assigned to the control or intervention groups. However, according to the EPOC criteria, the risk of bias for this study is low because units, in this case nursing homes, were assigned rather than individuals. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear, although with the objective outcomes that we are interested in this is less of a concern (according to EPOC risk of bias criteria). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Control and intervention groups did not appear to differ in terms of attrition. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is less important for our purpose as we are looking at objective outcomes (hospitalisations, ED visits, and mortality). |
| Selective reporting (reporting bias) | Low risk | No evidence of this |
| Protection against contamination bias | Low risk | There is indication to suggest that the intervention was contaminated by the control group. |
| Other bias | Unclear risk | The limited duration of the study and size of the sample may have compromised the ability to detect an effect. We considered other bias due to cluster randomisation. |
Rytter 2010.
| Study characteristics | ||
| Methods | RT Study duration: 26 weeks |
|
| Participants | 148 intervention, 145 control Setting: primary care Age: median, intervention 84 years, control 83 years Sex female n (%): intervention 66%, control 66% Country: Denmark Diagnosis: cardiovascular disease: intervention 45 (30%), control 28 (19%); other intervention 103 (70%), control 117 (81%); P = 0.02 Sociodemographics: housing: living in private home intervention: 95%, control 97%; widow/widower: intervention 59%, control 57%; married: intervention 30%, control 29%; divorced/single: intervention 11%, control 14% Ethnicity: unclear, possibly white Danish Date of study: November 2003‐June 2005 |
|
| Interventions | 1 intervention and 1 control The intervention follow‐up consisted of 3 contacts. The main intervention was a joint home visit involving both the GP and the district nurse. It was conducted approximately 1 week after discharge and was guided by an agenda. Control group: standard care |
|
| Outcomes | The primary outcome measures were hospital readmissions of any kind and the concordance between the GP's knowledge of the medical treatment and what the participant was actually taking. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by a computer. |
| Allocation concealment (selection bias) | Low risk | Computer |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This would be impossible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent team |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination bias | Low risk | There is no indication to suggest that the intervention was contaminated by the control group. |
| Other bias | Unclear risk | It is unclear if there are other risks not accounted for. |
Triller 2007.
| Study characteristics | ||
| Methods | RT Study duration: 201 days (3‐week intervention and 180 days of follow‐up) |
|
| Participants | 154 participants Setting: home Diagnostic criteria: participants had to have a primary or secondary diagnosis of heart failure and were referred to receive skilled nursing services Age (years) (mean ± SD): control: 78.1 (11.2), intervention: 81.3 (9.3), participants had to be ≥ 21 years Female n (%): control: 55 (72), intervention: 56 (73) Country: USA Comorbidity: heart failure Sociodemographics: the catchment area provided participants from urban, suburban, and rural environs and from across all socioeconomic classes. According to census data, 89% of the population of these 3 counties combined is white, and 87% of adults have a high school diploma. Median household income for the counties is approximately USD 47,000 (2003 data). Non‐English‐speaking participants were included if adequate translation services were available from family members or friends. Ethnicity: unknown; it is difficult to ascertain the ethnicity from the information given. control 68 (88%), intervention 75 (97%) Date of study: 1 July 2002 to end 2004 |
|
| Interventions | 1 intervention: pharmaceutical care services Pharmaceutical care services consisted of an initial comprehensive in‐home medication assessment (concurrent with agency admission) and 2 follow‐up visits (7‐10 and 18‐21 days later). The follow‐up visits were contingent on the participant’s continued receipt of visiting nurse services (i.e. participants discharged from the visiting nurse before 21 days would not receive all of the pharmacist’s planned visits). Throughout the 3‐week intervention period, the clinical pharmacist accessed and reviewed all pertinent physician notes and laboratory test values via the National Endowment for the Humanities data system and interacted with prescribers on behalf of the participants as necessary. Control participants received the usual care provided by the visiting nurse association. Visiting nurse services (provided to both groups) included basic nursing care and a brief physical assessment and medical history. |
|
| Outcomes | Hospitalisations and mortality were assessed during a 180 day follow‐up period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was adequate: "Patients provided informed consent for study participation and were randomised to receive usual care or usual care plus pharmaceutical care by means of a computer‐generated random numbers table in blocks of four." |
| Allocation concealment (selection bias) | Low risk | This was adequate: "Once informed consent was received, the nurse obtained a baseline quality‐of life assessment (using the SF‐12) and then accessed a sealed envelope containing the group assignment from the intake office." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding or a lack of blinding was unlikely to affect the outcome because the usual care group received usual care from nurses whereas the intervention group received usual care plus the services of a pharmacist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition in both the intervention and control groups was comparable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is of less concern in our case as all of our outcomes are objective (according to the EPOC risk of bias criteria). |
| Selective reporting (reporting bias) | Low risk | No evidence of a problem |
| Protection against contamination bias | Low risk | There is no evidence of contamination between the intervention and the control groups |
| Other bias | High risk | Small sample may have produced low power to detect an effect. Poor pharmacist‐prescriber communication may have reduced efficacy of intervention. |
Zermansky 2001.
| Study characteristics | ||
| Methods | RT of clinical medication review by a pharmacist against normal general practice review Length of study: 12 months (study conducted: June 1999‐June 2000) |
|
| Participants | Participants from general practices 1188 participants aged ≥ 65 or over who were receiving at least 1 repeat prescription and living in the community. Age: mean (SD) intervention, 74 (6.6) control, 73 (6.4) Sex female n (%): intervention 339 (56%), control 325 (56%) Country: UK Comorbidity: not reported Sociodemographics: not reported Ethnicity: not reported |
|
| Interventions | 1 intervention and 1 control; 601 participants in the intervention and 580 participants in the control group. Intervention group: participants were invited to a consultation at which the pharmacist reviewed their medical conditions and current treatment according to a specific algorithm which includes history taking and data gathering, evaluation and implementation stages. Control group: participants in the control group continued to receive normal care from their GP and primary healthcare staff. Participants were recalled for review of treatment by the GP according to normal custom in the practice. |
|
| Outcomes | Hospital admission and mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by a computer. |
| Allocation concealment (selection bias) | Low risk | Practice based allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This would be impossible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some participants did not complete the study and were reported in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No explanation given. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination bias | Unclear risk | It is unclear if there was a contamination between the intervention and the control group. |
| Other bias | Unclear risk | It is unclear to determine if there are other biases. |
Zermansky 2006.
| Study characteristics | ||
| Methods | An open randomised controlled trial of clinical medication review by a pharmacist of elderly care home residents against usual care Study duration: 6 months |
|
| Participants | 661 participants (331 intervention group but only 315 received Intervention) and 330 participants in the control group Setting: aged care facilities in Leeds, UK (nursing, residential and mixed care homes for older people in Leeds, UK) Diagnostic criteria: residents aged ≥ 65, seeking to recruit all residents taking ≥ 1 repeat medicines Age (years) (mean ± SD): age mean (interquartile range), Intervention 85.3 (81 to 90) and control 84.9 (80 to 90) Sex male n (%): intervention 75 (22.7), control 79 (23.9) Country: UK Comorbidity: not stated Sociodemographics: not stated Ethnicity: not stated Date of study: not reported, but paper first published 12/8/2006 |
|
| Interventions | 1 intervention A clinical medication review was conducted by the study pharmacist within 28 days of randomisation. It comprised a review of the general practice clinical record and a consultation with the participant and carer. The pharmacist formulated recommendations with the participant and carer and passed them on a written proforma to the GP for acceptance and implementation. GP acceptance was signified by ticking a box on the proforma. Control participants received usual GP care |
|
| Outcomes | The primary outcome measure was the number of changes in medication per participant. Secondary outcome measures were the following:
|
|
| Notes | Randomisation was curtailed on 30 June 2003 when it became clear that the intended sample size was not achievable within the available timescale. Data were analysed on an intention‐to‐treat basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly sized blocks. |
| Allocation concealment (selection bias) | Unclear risk | This is not mentioned in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear if the participants were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some participants did not complete the study and were reported in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A nurse blind to the study assessed participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination bias | Unclear risk | It is difficult to determine if the intervention group was contaminated by the control group. |
| Other bias | Unclear risk | It is difficult to determine if there are other biases. |
ADL: activities of daily living; BPMDL: best possible medication discharge list; CI: confidence interval; CHAP: Cardiovascular Health Awareness Program; CHD: coronary heart disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; ED: emergency department; EuroQoL‐5D: EuroQol Group Association ("The EuroQol Group") comprises a network of international, multilingual, multidisciplinary researchers; EQ‐5D: a standardised instrument for use as a measure of health outcome; GP: general practitioner; ICU: intensive care unit; QoL: quality of life; RT: randomised trial; SD: standard deviation; SF‐12: Short Form‐12; SF‐36: Short Form‐36; STOPP: Screening Tool of Older Persons Prescriptions; START: Screening Tool to Alert Doctors to Right Treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Arifi 2014 | Irrelevant intervention |
| Alassaad 2014 | Not primary care |
| Alicic 2016 | Protocol of a study |
| Barker 2012 | This study does not appear to be a primary care intervention. |
| Barker 2016 | Outcomes not relevant |
| Barnes 2014 | Outcomes not relevant |
| Basheti 2016 | Outcomes not relevant |
| Bell 2016 | Not primary care |
| Benard‐Laribiere 2015 | Irrelevant intervention |
| Bhatt 2014 | Study protocol |
| Billington 2015 | Outcomes not relevant |
| Bonnet‐Zamponi 2013 | This was not a primary care intervention; it was done by geriatricians. |
| Briggs 2015 | Not primary care |
| Carrington 2013 | Irrelevant intervention |
| Clyne 2013 | Outcomes not relevant |
| Clyne 2015 | Outcomes not relevant |
| Clyne 2016 | Outcomes not relevant |
| Cowper 1998 | Cost‐effectiveness study that had data in a form not enabling data extraction. |
| Desveaux 2016 | Study protocol |
| Dhalla 2014 | Outcomes not relevant |
| Elliott 2014 | Study protocol |
| Forster 2015 | Study protocol |
| Fredericks 2013 | Irrelevant intervention |
| Furniss 2000 | Study was for a pre‐post design not included in the protocol |
| Geurts 2016 | Outcomes not relevant |
| Gorgas 2012 | This study did not occur in primary care. |
| Graffen 2004 | Study was for a pre‐post design not included in the protocol |
| Guthrie 2016 | Outcomes not relevant |
| Hallsworth 2016 | Outcomes not relevant |
| Hanlon 1996 | This study did not occur in primary care. |
| Hugtenburg 2009 | This is not a randomised trial (it is described as a controlled intervention study and there is no evidence of randomisation). |
| Huiskes 2014 | Outcomes not relevant |
| Keane 2014 | Not primary care |
| Knowlton 1994 | Not possible to extract appropriate data |
| Lee 1996 | This study was not a randomised trial. |
| Leendertse 2011 | This study was not a randomised trial. |
| Leendertse 2013 | This study was not a randomised trial. |
| Liu 2010 | This study was a conference abstract only and did not address adverse drug reactions. |
| Malin 2016 | Outcomes not relevant |
| Mills 2001 | This study is reported elsewhere (see Furniss 2000, also excluded) |
| Montero‐Balosa 2016 | Not a randomised trial |
| Moreno 2016 | Not a randomised trial |
| Naunton 2003 | This is a hospital intervention and not done in primary care. It appears that the study pharmacist is recruited from the hospital. As it says, the pharmacist complied with the Society of Hospital Pharmacist clinical pharmacy services. It is also published in a hospital journal. |
| Neven 2016 | Not primary care |
| Ni 2016 | Not a randomised trial |
| Perula 2014 | Outcomes not relevant |
| Phung 2013 | Study protocol |
| Pinnock 2013 | Irrelevant intervention |
| Przytula 2015 | Study protocol |
| Safran 1993 | This study was not a randomised trial |
| Saltzberg 2011 | This study was not a randomised trial |
| Setter 2009 | The outcomes reported are not appropriate for this study |
| Sinnott 2015 | Outcomes not relevant |
| Stingl 2016 | Study protocol |
| Sturgess 2003 | Reported elsewhere (see Bernsten 2001) |
| Wolf 2015 | Outcomes not relevant |
| Wooster 2016 | Study protocol |
| Xin 2014 | Not primary care |
| Yuan 2003 | Complex study, which made data extraction not possible. |
Differences between protocol and review
For the primary outcome, we included all the studies that reported on the number of hospital admissions and the number of participants admitted to hospitals. The two outcomes are different since some people can have more than one hospital admission.
We also included all the trials in the final meta‐analysis, irrespective of their qualities. Removing them from the analysis would give rise to selective reporting.
Contributions of authors
Anthony Avery, Hanan Khalil and Aziz Sheikh were involved in the conception of this review, the drafting of the initial protocol and providing critical feedback on drafts of the review.
Helen Chambers retrieved the studies and provided support with the searching.
Hanan Khalil, Helen Chambers and Brian Bell selected the studies for inclusion/exclusion and critically appraised the included studies.
Hanan Khalil undertook the analysis described in the review and wrote the review. Brian Bell helped with editing the review.
Sources of support
Internal sources
Monash University, Faculty of Medicine, Nursing and Health Sciences, School of Rural Health, Clayton, Vic 3168, Australia
External sources
No sources of support supplied
Declarations of interest
Hanan Khalil has no conflicts of interest to declare. Brian Bell has no conflicts of interest to declare. Helen Chambers has no conflicts of interest to declare. Aziz Sheikh received a WHO grant addressing patient safety in primary care. Tony Avery received BUPA Foundation funding in 2001 to 2002 on a much earlier version of this review (Smeaton 2002).
New
References
References to studies included in this review
Alvarez 2001 {published data only}
- Alvarez de Toledo F, Arcos Gonzalez P, Eyaralar Riera T, Abal Ferrer FF, Dago Martinez A, Cabiedes Miragaya L, et al. Pharmaceutical care in people who have had acute coronary episodes (TOMCOR study). Revista Espanola de Salud Publica 2001;75:375-87. [PubMed] [Google Scholar]
Bernsten 2001 {published data only}
- Bernsten C, Bjorkman I, Caramona M, Crealey G, Frokjaer B, Grundberger E, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs & Aging 2001;18:63-77. [DOI] [PubMed] [Google Scholar]
Campins 2016 {published data only}
- Campins L, Serra-Prat M, Gozalo I, Lopez D, Palomera E, Agusti C, et al, REMEI Group. Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Family Practice 2016;34(1):36-42. [DOI] [PubMed] [Google Scholar]
Coleman 1999 {published data only}
- Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. Journal of the American Geriatrics Society 1999;47:775-83. [DOI] [PubMed] [Google Scholar]
Frankenthal 2014 {published data only}
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658-65. [DOI] [PubMed] [Google Scholar]
Garcia‐Gollarte 2014 {published data only}
- Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cuenllas-Diaz A, Cruz-Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. Journal of the American Medical Directors Association 2014;15(12):885-91. [DOI] [PubMed] [Google Scholar]
Gernant 2016 {published data only}
- Gernant SA, Snyder ME, Jaynes H, Sutherland JM, Zillich AJ. The effectiveness of pharmacist-provided telephonic medication therapy management on emergency department utilization in home health patients. Journal of Pharmacy Technology 2016;32(5):179-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurwitz 2014 {published data only}
- Gurwitz JH, Field TS, Ogarek J, Tjia J, Cutrona SL, Harrold LR, et al. An electronic health record-based intervention to increase follow-up office visits and decrease rehospitalization in older adults. Journal of the American Geriatrics Society 2014;62(5):865-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawes 2014 {published data only}
- Hawes EM, Maxwell WD, White SF, Mangun J, Lin FC. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. Journal of Primary Care & Community Health 2014;5(1):14-8. [DOI] [PubMed] [Google Scholar]
Holland 2005 {published data only}
- Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ Open 2005;330:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim R, Saber-Ayad M. Continuous outpatient warfarin counseling and its effects on adherence. Asian Journal of Pharmaceutical and Clinical Research 2013;6:101-4. [Google Scholar]
Kaczorowski 2011 {published data only}
- Kaczorowski J, Chambers LW, Dolovich L, Paterson JM, Karwalajtys T, Gierman T, et al. Improving cardiovascular health at population level: 39 community cluster randomised trial of cardiovascular health awareness program (CHAP). BMJ 2011;342:d442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korajkic 2011 {published data only}
- Korajkic A, Poole SG, MacFarlane LM, Bergin PJ, Dooley MJ. Impact of a pharmacist intervention on ambulatory patients with heart failure: a randomised controlled study. Journal of Pharmacy Practice & Research 2011;41:126-31. [Google Scholar]
Krska 2001 {published data only}
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PRS, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age and Ageing 2001;30:205-11. [DOI] [PubMed] [Google Scholar]
Lapane 2011 {published data only}
- Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. Journal of the American Geriatrics Society 2011;59:1238-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenaghan 2007 {published data only}
- Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care--the POLYMED randomised controlled trial. Age & Ageing 2007;36:292-7. [DOI] [PubMed] [Google Scholar]
Lowrie 2012 {published data only}
- Lowrie R, Mair FS, Greenlaw N, Forsyth P, Jhund PS, McConnachie A, et al. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. European Heart Journal 2012;33:314-24. [DOI] [PubMed] [Google Scholar]
Malet‐Larrea 2016 {published data only}
- Malet-Larrea A, Goyenechea E, Garcia-Cardenas V, Calvo B, Arteche JM, Aranegui P, et al. The impact of a medication review with follow-up service on hospital admissions in aged polypharmacy patients. British Journal of Clinical Pharmacology 2016;82(3):831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malone 2000 {published data only}
- Malone DC, Carter BL, Billups SJ, Valuck RJ, Barnette DJ, Sintek CD, et al. An economic analysis of a randomized, controlled, multicenter study of clinical pharmacist Interventions for high-risk veterans: the IMPROVE study. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2000;20:1149-58. [DOI] [PubMed] [Google Scholar]
Moertl 2009 {published data only}
- Moertl D, Berger R, Hammer A, Huelsmann M, Hutuleac R, Pacher R. B-type natriuretic peptide predicts benefit from a home-based nurse care in chronic heart failure. Journal of Cardiac Failure 2009;15:233-40. [DOI] [PubMed] [Google Scholar]
Murray 2004 {published data only}
- Murray MD, Harris LE, Overhage JM, Zhou XH, Eckert GJ, Smith FE, et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: results of a randomized controlled trial. Pharmacotherapy 2004;24:324-37. [DOI] [PubMed] [Google Scholar]
Nabagiez 2013 {published data only}
- Nabagiez JP, Shariff MA, Khan MA, Molloy WJ, McGinn Jr JT. Physician assistant home visit program to reduce hospital readmissions. Journal of Thoracic and Cardiovascular Surgery 2013;145:225-33. [DOI] [PubMed] [Google Scholar]
Okamoto 2001 {published data only}
- Okamoto MP, Nakahiro K. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2001;21:1337-44. [DOI] [PubMed] [Google Scholar]
Olesen 2014 {published data only}
- Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. International Journal of Clinical Pharmacy 2014;36(1):163-71. [DOI] [PubMed] [Google Scholar]
Pai 2009 {published data only}
- Pai AB, Boyd A, Depczynski J, Chavez IM, Khan N, Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2009;29:1433-40. [DOI] [PubMed] [Google Scholar]
Roberts 2001 {published data only}
- Roberts MS, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. British Journal of Clinical Pharmacology 2001;51:257-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rytter 2010 {published data only}
- Rytter L, Jakobsen HN, Ronholt F, Hammer AV, Andreasen AH, Nissen A, et al. Comprehensive discharge follow-up in patients' homes by GPs and district nurses of elderly patients. A randomized controlled trial. Scandinavian Journal of Primary Health Care 2010;28:146-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Triller 2007 {published data only}
- Triller DM, Hamilton RA. Effect of pharmaceutical care services on outcomes for home care patients with heart failure. American Journal of Health-System Pharmacy 2007;64:2244-9. [DOI] [PubMed] [Google Scholar]
Zermansky 2001 {published data only}
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ 2001;323:1340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zermansky 2006 {published data only}
- Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes - randomised controlled trial. Age & Ageing 2006;35:586-91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Arifi 2014 {published data only}
- Al-Arifi M, Abu-Hashem H, Al-Meziny M, Said R, Aljadhey H. Emergency department visits and admissions due to drug related problems at Riyadh military hospital (RMH), Saudi Arabia. Saudi Pharmaceutical Journal 2014;22(1):17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alassaad 2014 {published data only}
- Alassaad A, Bertilsson M, Gillespie U, Sundstrom J, Hammarlund-Udenaes M, Melhus H. The effects of pharmacist intervention on emergency department visits in patients 80 years and older: subgroup analyses by number of prescribed drugs and appropriate prescribing. PLoS ONE 2014;9(11):e111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alicic 2016 {published data only}
- Alicic RZ, Short RA, Corbett CL, Neumiller JJ, Gates BJ, Daratha KB, et al. Medication intervention for chronic kidney disease patients transitioning from hospital to home: study design and baseline characteristics. American Journal of Nephrology 2016;44(2):122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2012 {published data only}
- Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. International Journal of Cardiology 2012;159:139-43. [DOI] [PubMed] [Google Scholar]
Barker 2016 {published data only}
- Barker EA, Pond ST, Zaiken K. Impact of medication onboarding: a clinical pharmacist-run "onboarding" telephone service for patients entering a primary care practice. Journal of Pharmacy Technology 2016;32(1):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2014 {published data only}
- Barnes KD, Tayal NH, Lehman AM, Beatty SJ. Pharmacist-driven renal medication dosing intervention in a primary care patient-centered medical home. Pharmacotherapy 2014;34(12):1330-5. [DOI] [PubMed] [Google Scholar]
Basheti 2016 {published data only}
- Basheti IA, Tadros OK, Aburuz S. Value of a community-based medication management review service in Jordan: a prospective randomized controlled study. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2016;36(10):1075-86. [DOI] [PubMed] [Google Scholar]
Bell 2016 {published data only}
- Bell SP, Schnipper JL, Goggins K, Bian A, Shintani A, Roumie CL, et al. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. Journal of General Internal Medicine 2016;31(5):470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benard‐Laribiere 2015 {published data only}
- Benard-Laribiere A, Miremont-Salame G, Perault-Pochat MC, Noize P, Haramburu F, centres EMIR Study Group on behalf of the French network of pharmacovigilance. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundamental & Clinical Pharmacology 2015;29(1):106-11. [DOI] [PubMed] [Google Scholar]
Bhatt 2014 {published data only}
- Bhatt V, Masilamani S, Hardin-Oliver C. Initiation of pharmaceutical services for the management of asthma and chronic obstructive pulmonary disease in a community pharmacy. Journal of the American Pharmacists Association 2014;54(2):e171. [Google Scholar]
Billington 2015 {published data only}
- Billington J, Coster S, Murrells T, Norman I. Evaluation of a nurse-led educational telephone intervention to support self-management of patients with chronic obstructive pulmonary disease: a randomized feasibility study. COPD: Journal of Chronic Obstructive Pulmonary Disease 2015;12(4):395-403. [DOI] [PubMed] [Google Scholar]
Bonnet‐Zamponi 2013 {published data only}
- Bonnet-Zamponi D, d'Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al. Drug-related readmissions to medical units of older adults discharged from acute geriatric units: results of the optimization of medication in aged multicenter randomized controlled trial. Journal of the American Geriatrics Society 2013;61:113-21. [DOI] [PubMed] [Google Scholar]
Briggs 2015 {published data only}
- Briggs S, Pearce R, Dilworth S, Higgins I, Hullick C, Attia J. Clinical pharmacist review: a randomised controlled trial. Emergency Medicine Australasia 2015;27(5):419-26. [DOI] [PubMed] [Google Scholar]
Carrington 2013 {published data only}
- Carrington MJ, Chan YK, Calderone A, Scuffham PA, Esterman A, Goldstein S, et al. A multicenter, randomized trial of a nurse-led, home-based intervention for optimal secondary cardiac prevention suggests some benefits for men but not for women: the Young at Heart study. Circulation. Cardiovascular quality and outcomes 2013;6(4):379-89. [DOI] [PubMed] [Google Scholar]
Clyne 2013 {published data only}
- Clyne B, Bradley MC, Smith SM, Hughes CM, Motterlini N, Clear D, et al. Effectiveness of medicines review with web-based pharmaceutical treatment algorithms in reducing potentially inappropriate prescribing in older people in primary care: a cluster randomized trial (OPTI-SCRIPT study protocol). Trials 2013;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clyne 2015 {published data only}
- Clyne B, Smith SM, Hughes CM, Boland F, Bradley MC, Cooper JA, et al. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT study). Annals of Family Medicine 2015;13(6):545-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clyne 2016 {published data only}
- Clyne B, Cooper JA, Hughes CM, Fahey T, Smith SM. A process evaluation of a cluster randomised trial to reduce potentially inappropriate prescribing in older people in primary care (OPTI-SCRIPT study). Trials 2016;17(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowper 1998 {published data only}
- Cowper PA, Weinberger M, Hanlon JT, Landsman PB, Samsa GP, Uttech KM, et al. The cost-effectiveness of a clinical pharmacist intervention among elderly outpatients. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 1998;18:327-32. [PubMed] [Google Scholar]
Desveaux 2016 {published data only}
- Desveaux L, Gomes T, Tadrous M, Jeffs L, Taljaard M, Rogers J, et al. Appropriate prescribing in nursing homes demonstration project (APDP) study protocol: pragmatic, cluster-randomized trial and mixed methods process evaluation of an Ontario policy-maker initiative to improve appropriate prescribing of antipsychotics. Implementation Science 2016;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhalla 2014 {published data only}
- Dhalla IA, O'Brien T, Morra D, Thorpe KE, Wong BM, Mehta R, et al. Effect of a postdischarge virtual ward on readmission or death for high-risk patients: a randomized clinical trial. JAMA 2014;312(13):1305-12. [DOI] [PubMed] [Google Scholar]
Elliott 2014 {published data only}
- Elliott RA, Putman KD, Franklin M, Annemans L, Verhaeghe N, Eden M, et al. Cost effectiveness of a pharmacist-led information technology intervention for reducing rates of clinically important errors in medicines management in general practices (PINCER) (Provisional abstract). PharmacoEconomics 2014;32(6):573-90. [DOI] [PubMed] [Google Scholar]
Forster 2015 {published data only}
- Forster AJ, Erlanger TE, Jennings A, Auger C, Buckeridge D, Walraven C, et al. Effectiveness of a computerized drug-monitoring program to detect and prevent adverse drug events and medication non-adherence in outpatient ambulatory care: study protocol of a randomized controlled trial. Trials 2015;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fredericks 2013 {published data only}
- Fredericks S, Yau T. Educational intervention reduces complications and rehospitalizations after heart surgery. Western Journal of Nursing Research 2013;35(10):1251-65. [DOI] [PubMed] [Google Scholar]
Furniss 2000 {published data only}
- Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. British Journal of Psychiatry 2000;176:563-7. [DOI] [PubMed] [Google Scholar]
Geurts 2016 {published data only}
- Geurts MM, Stewart RE, Brouwers JR, De Graeff PA, De Gier JJ. Implications of a clinical medication review and a pharmaceutical care plan of polypharmacy patients with a cardiovascular disorder. International Journal of Clinical Pharmacy 2016;38(4):808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorgas 2012 {published data only}
- Gorgas TM, Pez VF, Camos RJ, De Puig CE, Jolonch SP, Homs PE, et al. Integrated pharmaceutical care programme in patients with chronic diseases. Farmacia Hospitalaria 2012;36:229-39. [DOI] [PubMed] [Google Scholar]
Graffen 2004 {published data only}
- Graffen M, Kennedy D, Simpson M. Quality use of medicines in the rural ambulant elderly: a pilot study. Rural & Remote Health 2004;4:184. [PubMed] [Google Scholar]
Guthrie 2016 {published data only}
- Guthrie B, Kavanagh K, Robertson C, Barnett K, Treweek S, Petrie D, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ 2016;354:i4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallsworth 2016 {published data only}
- Hallsworth M, Chadborn T, Sallis A, Sanders M, Berry D, Greaves F, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016;387(10029):1743-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 1996 {published data only}
- Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. American Journal of Medicine 1996;100:428-37. [DOI] [PubMed] [Google Scholar]
Hugtenburg 2009 {published data only}
- Hugtenburg JG, Borgsteede SD, Beckeringh JJ. Medication review and patient counselling at discharge from the hospital by community pharmacists. Pharmacy World and Science 2009;31:630-7. [DOI] [PubMed] [Google Scholar]
Huiskes 2014 {published data only}
- Huiskes VJ, Kruijtbosch M, Ensing R, Meijs M, Meijs VM, Van den Bemt BJ. The effectiveness of a medication review on the number of drug related problems in outpatient cardiology patients: a randomized clinical trial. British Journal of Clinical Pharmacology 2014;78(4):766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keane 2014 {published data only}
- Keane K. Reducing medication errors by educating nurses on bar code technology. MEDSURG Nursing 2014;23(5):Suppl 1, 10-1. [PubMed] [Google Scholar]
Knowlton 1994 {published data only}
- Knowlton CH, Knapp DA. Community pharmacists help HMO cut drug costs. American Pharmacy 1994;NS34:36-42. [DOI] [PubMed] [Google Scholar]
Lee 1996 {published data only}
- Lee YP, Schommer JC. Effect of a pharmacist-managed anticoagulation clinic on warfarin-related hospital readmissions. American Journal of Health-System Pharmacy 1996;53:1580-3. [DOI] [PubMed] [Google Scholar]
Leendertse 2011 {published data only}
- Leendertse AJ, De Koning GH, Goudswaard AN, Belitser SV, Verhoef M, De Gier JJ, et al. The effect of a pharmaceutical care process on medication related hospital admissions in the elderly in an integrated primary care setting: results of the Pharm study. International Journal of Clinical Pharmacy 2011;33 (2):337. [Google Scholar]
Leendertse 2013 {published data only}
Liu 2010 {published data only}
- Liu MY, Li YJ, Zhu W, Wei M. Effects of intensive clinic follow-up on management and short-term outcome for patients with heart failure. Circulation 2010;122 (2):e33-4. [Google Scholar]
Malin 2016 {published data only}
- Malin A. Use of electronic medication administration records to reduce perceived stress and risk of medication errors in nursing homes. Journal of Forensic Nursing 2016;34(7):329. [DOI] [PubMed] [Google Scholar]
Mills 2001 {published data only}
- Mills A. Pharmacist medication review decreased death, and number and cost of drugs prescribed for residents in nursing homes. Evidence Based Mental Health 2001;4:63. [Google Scholar]
Montero‐Balosa 2016 {published data only}
- Montero-Balosa MC, Palma-Morgado D, Sanchez-Blanco J, Perez-Fuentes MF, Vela-Marquez MC, Lacalle-Remigio JR. Intervention to reduce potential events in elderly patients. International Journal of Clinical Pharmacy 2016;38 (6):492-3. [Google Scholar]
Moreno 2016 {published data only}
- Moreno G, Fu JY, Chon J, Whitmire N, Tseng CH, Grotts J, et al. Impact of pharmacists on the primary care team on emergency room visits and hospitalizations for poorly controlled patients with diabetes. Journal of General Internal Medicine 2016;31(2 SUPPL. 1):S263-4. [Google Scholar]
Naunton 2003 {published data only}
- Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. Journal of Pharmacy Practice & Research 2003;33:176-82. [Google Scholar]
Neven 2016 {published data only}
- Neven D, Paulozzi L, Howell D, McPherson S, Murphy SM, Grohs B, et al. A randomized controlled trial of a citywide emergency department care coordination program to reduce prescription opioid related emergency department visits. Journal of Emergency Medicine 2016;51(5):498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2016 {published data only}
- Ni W, Colayco D, Hashimoto J, Komoto K, Gowda C, Wearda B, et al. Impact of an ambulatory care pharmacy-based transition of care program on hospital readmissions and cost. Value in Health 2016;19(3):A28. [Google Scholar]
Perula 2014 {published data only}
- Perula TL, Pulido OL, Perula TC, Gonzalez LJ, Olaya CI, Ruiz MR, et al. Efficacy of motivational interviewing for reducing medication errors in chronic patients over 65 years with polypharmacy: results of a cluster randomized trial. Medicina Clinica 2014;143(8):341-8. [DOI] [PubMed] [Google Scholar]
Phung 2013 {published data only}
- Phung J. Reducing hospital readmissions: a pilot study of pharmacists' role on an American Indian reservation. Journal of the American Pharmacists Association 2013;53(2):e41. [Google Scholar]
Pinnock 2013 {published data only}
- Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Przytula 2015 {published data only}
- Przytula K, Bailey SC, Galanter WL, Lambert BL, Shrestha N, Dickens C, et al. A primary care, electronic health record-based strategy to promote safe drug use: study protocol for a randomized controlled trial. Trials 2015;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safran 1993 {published data only}
- Safran C, Rind DM, Davis RM, Currier J, Ives D, Sands DZ, et al. An electronic medical record that helps care for patients with HIV infection. In: The Annual Symposium on Computer Applications in Medical Care. 1993:224-8. [PMC free article] [PubMed]
Saltzberg 2011 {published data only}
- Saltzberg M, Arhinful E, Lynch S, Olurin O, Nordenson M. Control your heart for the future study (CHF): unique partnership to reduce resource utilization among high risk heart failure patients. Journal of the American College of Cardiology 2011;1:E1213. [Google Scholar]
Setter 2009 {published data only}
- Setter SM, Corbett CF, Neumiller JJ, Gates BJ, Sclar DA, Sonnett TE. Effectiveness of a pharmacist-nurse intervention on resolving medication discrepancies for patients transitioning from hospital to home health care. American Journal of Health-System Pharmacy 2009;66:2027-31. [DOI] [PubMed] [Google Scholar]
Sinnott 2015 {published data only}
- Sinnott C, Mercer SW, Payne RA, Duerden M, Bradley CP, Byrne M. Improving medication management in multimorbidity: development of the multimorbidity collaborative medication review and decision making (MY COMRADE) intervention using the behaviour change wheel. Implementation Science 2015;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stingl 2016 {published data only}
- Stingl JC, Kaumanns KL, Claus K, Lehmann ML, Kastenmuller K, Bleckwenn M, et al. Individualized versus standardized risk assessment in patients at high risk for adverse drug reactions (IDRUG) - study protocol for a pragmatic randomized controlled trial. BMC Family Practice 2016;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturgess 2003 {published data only}
- Sturgess IK, McElnay JC, Hughes CM, Crealey G. Community pharmacy based provision of pharmaceutical care to older patients. Pharmacy World & Science 2003;25:218-26. [DOI] [PubMed] [Google Scholar]
Wolf 2015 {published data only}
- Wolf C, Pauly A, Mayr A, Gromer T, Lenz B, Kornhuber J, et al. Pharmacist-led medication reviews to identify and collaboratively resolve drug-related problems in psychiatry - a controlled, clinical trial. PLoS ONE 2015;10(11):e0142011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wooster 2016 {published data only}
- Wooster J, Gurney M, Hecht K, Kucera A. Impact of community pharmacist-performed medication reconciliations in post-discharge patients on 30-day hospital readmission rates. Journal of the American Pharmacists Association 2016;56(3):e92. [Google Scholar]
Xin 2014 {published data only}
- Xin C, Ge X, Yang X, Lin M, Jiang C, Xia Z. The impact of pharmaceutical care on improving outcomes in patients with type 2 diabetes mellitus from China: a pre- and postintervention study. International Journal of Clinical Pharmacy 2014;36(5):963-8. [DOI] [PubMed] [Google Scholar]
Yuan 2003 {published data only}
- Yuan Y, Hay JW, McCombs JS. Effects of ambulatory-care pharmacist consultation on mortality and hospitalization. American Journal of Managed Care 2003;9:45-56. [PubMed] [Google Scholar]
Additional references
APA 2008
- American Pharmacists Association, National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). Journal of the American Pharmacists Association 2008;48(3):341-53. [DOI: 10.1331/JAPhA.2008.08514] [DOI] [PubMed] [Google Scholar]
Bates 1995
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Prevention study group. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA 1995;274(1):29-34. [PubMed] [Google Scholar]
Benning 2011
- Benning A, Ghaleb M, Suokas A, Dixon-Woods M, Dawson J, Barber N, et al. Large scale organisational intervention to improve patient safety in four UK hospitals: mixed method evaluation. BMJ 2011;342:d195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2008
- Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ 2008;337(a2764):159-63. [DOI] [PubMed] [Google Scholar]
CHAP 2017
- CHAP - Cardiovascular Health Awareness Program. www.chapprogram.ca (accessed prior to 26 June 2017).
Danmarks 2004
- Danmarks Apotekerforening, Pharmakon. Medication review. Managing Medication Manual 2004;1(1):1-21.
Datascope Patient Monitoring 1996 [Computer program]
- Passport Service Manual. Datascope Patient Monitoring. Montvale, NJ: Datascope Corp, 1996. pp. 3-1–3-80.
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Durieux 2012
- Durieux P, Trinquart L, Colombet I, Nies J, Walton RT, Rajeswaran A, et al. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD002894. [DOI: 10.1002/14651858.CD002894] [DOI] [PubMed] [Google Scholar]
Edwards 2000
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356(9237):1255-9. [DOI] [PubMed] [Google Scholar]
EPOC 2017a
- Cochrane Effective Practice and Organisation of Care (EPOC). Screening, data extraction and management. EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2017.
EPOC 2017b
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors. Available from epoc.cochrane.org/epoc-specific-resources-review-authors 2017.
Ferner 2006
- Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug Safety 2006;29(11):1011-22. [DOI] [PubMed] [Google Scholar]
Field 2012
- Field TS, Garber L, Gagne SJ, Tjia J, Preusse P, Donovan JL, et al. Technological resources and personnel costs required to implement an automated alert system for ambulatory physicians when patients are discharged from hospitals to home. Journal of Innovation in Health Informatics 2013;20(2):87-93. [DOI] [PubMed] [Google Scholar]
Gallagher 2008
Gandhi 2003
- Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse drug events in ambulatory care. New England Journal of Medicine 2003;348(16):1556-64. [DOI] [PubMed] [Google Scholar]
GRADE pro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 31 January 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hepler 1990
- Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. American Journal of Hospital Pharmacy 1990;47(3):533-43. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 2003
- Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Quality & Safety in Health Care 2003;12(4):280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2007
- Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. British Journal of Clinical Pharmacology 2007;63(2):136-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2001
- Ioannidis JP, Lau J. Evidence on interventions to reduce medical errors: an overview and recommendations for future research. Journal of General Internal Medicine 2001;16(5):325-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2000
- Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. British Journal of Clinical Pharmacology 2000;50(2):172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 1999
- McDonald CJ, Overhage JM, Tierney WM, Dexter PR, Martin DK, Suico JG, et al. The Regenstrief Medical Record System: a quarter century experience. International Journal of Medical Informatics 1999;54(3):225-53. [DOI] [PubMed] [Google Scholar]
O'Brien 2008
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD000409. [DOI: 10.1002/14651858.CD000409] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Mahony 2015
- O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age & Ageing 2015;44(2):213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overhage 1999
- Overhage JM, Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists' clinical activities. American Journal of Health-System Pharmacy 1999;56(23):2444. [DOI] [PubMed] [Google Scholar]
Remme 1997
- Members of the Task Force from the Working Group on Heart Failure of the European Society of Cardiology. The treatment of heart failure. European Heart Journal 1997;18(5):736. [DOI] [PubMed] [Google Scholar]
Remme 2001
- Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. European Heart Journal 2001;22(23):1527-60. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royal 2006
- Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Quality and Safety in Health Care 2006;15(1):23-31. [DOI: 10.1136/qshc.2004.012153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Soe 2013
- Soe A, Apampa B, Fernando B, Maaskant J, Neubert A, Thayyil S, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD006208. [DOI: 10.1002/14651858.CD006208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2014
- Spencer R, Bell B, Avery AJ, Gookey G, Campbell SM. Identification of an updated set of prescribing-safety indicators for GPs. British Journal of General Practice 2014;64:e181-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
SUDAAN 2012 [Computer program]
- Research Triangle Park SUDAAN: professional software for survey data analysis. Version 11. NC: Research Triangle Park, 2012.
Tobias 1999
- Tobias DE, Feinberg JL, Troutman WG. The MDS-Med Guide: a Tool for the new millennium. Consultant Pharmacist 1999;14(8):831-60. [Google Scholar]
WHO 2004
- World Health Organization (WHO). Pharmacovigilance: ensuring the safe use of medicines. WHO Policy Perspectives on Medicines 2004;No. 009.
Winterstein 2002
- Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug related hospital admissions. Annals of Pharmacotherapy 2002;36(7-8):1238-48. [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong JD, Bajcar JM, Wong GG, Alibhai SMH, Huh J-H, Cesta A, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Annals of Pharmacotherapy 2008;42(10):1373-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Khalil 2013
- Khalil H, Avery AJ, Chambers H, Bell B, Serumaga B, Sheikh A. Interventions in primary care for reducing preventable medication errors that lead to hospital admissions, mortality and emergency department visits. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD003942. [DOI: 10.1002/14651858.CD003942] [DOI] [Google Scholar]
Smeaton 2002
- Smeaton L, Sheikh A, Avery A, Royal S, Hurwitz B, Dewey M. Interventions for reducing preventable drug-related hospital admissions or preventable drug-related morbidity in primary care. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD003942. [DOI: 10.1002/14651858.CD003942] [DOI] [Google Scholar]


