Abstract
Pollinators are in global decline, and agricultural pesticides are a potential driver of this. Recent studies have suggested that pesticides may significantly impact bumblebee colonies, an important and declining group of pollinators. Here we show that colony founding queens, a critical yet vulnerable stage of the bumblebee lifecycle, are less likely to initiate a colony after exposure to thiamethoxam, a neonicotinoid insecticide. Bombus terrestris queens were exposed to field-relevant levels of thiamethoxam, and two natural stressors, the parasite Crithidia bombi, and varying hibernation durations. Exposure to thiamethoxam produced a 26% reduction in the proportion of queens that laid eggs, and advanced the timing of colony initiation, although we did not detect impacts of any experimental treatment on the ability of queens to produce adult offspring during the 14-week experimental period. As expected from previous studies, hibernation duration also had an impact on egg laying, but there was no significant interaction with insecticide treatment. Modelling the impacts of a 26% reduction in colony founding on population dynamics dramatically increased the likelihood of population extinction. This shows that neonicotinoids can affect this critical stage in the bumblebee lifecycle, and may have significant impacts on population dynamics.
Bees play a vital role as pollinators in both agricultural and natural systems1–4. However, there is increasing concern about the state of wild bee populations. Nearly 10% of European bee species are currently considered threatened5, and bumblebees are declining on a global scale5–9. The cause of these declines is thought to be a combination of factors, particularly habitat loss10, parasites and diseases11–13, invasive species14, and climate change15,16. Pesticide use is also considered a major threat to wild bees17–20, and both laboratory21–25, semi-field26–32 and field studies33 have found negative impacts of pesticides on bumblebee behaviour, reproduction, and colony success. However, we are still lacking information on the impacts of pesticides on key life history stages of wild bees. Bumblebees, like solitary bees, have an annual lifecycle whereby reproductive females (queens) initiate a colony in the spring34. Bumblebee queens are functionally solitary at this stage, and do not have a colony to buffer them from environmental stress. Success depends entirely upon the queen’s survival and ability to initiate a colony, and as such this represents a critical but vulnerable period in the lifecycle. Although bumblebee queens are likely to be exposed to a range of pesticides throughout their lifecycle, particularly when foraging in the early spring on flowering crops such as oilseed rape, to date there has been no research into the impacts of pesticides on founding queens and their ability to initiate a colony. Rundlöf et al.33 found that neonicotinoid treatment of oilseed rape crops resulted in a lack of brood-cell building in solitary bees, but the mechanism remained unexplored. Negative impacts of neonicotinoids on honeybee Apis mellifera queen reproduction have also been found35,36, but honey bee colonies are perennial and how this relates to the annual cycle in bumblebees remains unknown. However, given these results, it is vital that we understand the potential impacts of pesticides on bumblebee queens37, and the resultant implications for wild populations.
We examined the impact of thiamethoxam (a neonicotinoid insecticide) exposure on colony founding bumblebee (Bombus terrestris) queens. Neonicotinoids are the most widely used class of pesticide in the world38, and thiamethoxam is one of three neonicotinoids currently under an EU moratorium for use on flowering, bee attractive crops. Neonicotinoids have been implicated in the decline of wild bees20, butterflies39 and other taxa40. A range of regulations on the use of neonicotinoids have also recently come into force in North America. Therefore research into the risks to beneficial insects associated with exposure to these compounds has important global policy implications.
In addition to the potential threat from pesticide exposure, bumblebee queens are faced with a range of environmental stressors which can reduce their survival and fitness. Before initiating a colony in the spring, queens must first survive hibernation over winter, during which time they can lose up to 80% of their fat reserves41, which may make them vulnerable to additional stress. Little is known about overwintering survival of bumblebee queens in the wild, but studies in the laboratory have shown that a range of factors, such as pre-hibernation weight42,43, hibernation duration43, and genotype of the queen and of her mate44,45, can be important. Furthermore, exposure to parasites and pesticides can also impact hibernation survival37, and parasites have been shown to affect post-hibernation success of queens. For example, Crithidia bombi, a prevalent trypanosome parasite of bumblebees, has a context-dependent impact on its queen host46. Under laboratory conditions, parasitized queens lost up to 11% more mass during hibernation, and had up to a 40% reduction in fitness compared to uninfected queens46.
In natural environments, bumblebee queens face not only potential pesticide impacts, but also other simultaneous environmental stressors. To reflect this, we investigated the effects of thiamethoxam exposure on B. terrestris queens, and tested for interactions with two natural environmental stressors: infection with the parasite C. bombi, and variation in hibernation duration. To extrapolate our results to field populations, we used a Bayesian framework to assess their implications for population sustainability.
Results
Are bumblebee queens exposed to neonicotinoids in the field?
Bumblebee queens forage on oilseed rape (OSR: Brassica napus) treated with neonicotinoids. Transect walks in Oxfordshire, UK, identified seven species of queen in the vicinity of winter OSR crops, and six of these were actively foraging on OSR flowers (Table 1). B. terrestris and B. lapidarius were the species most commonly observed foraging directly on the crop. In order to establish whether neonicotinoids have an impact on queens during this colony founding period, we conducted a laboratory trial, exposing B. terrestris queens to a range of potential stressors, including dietary thiamethoxam.
Table 1.
Species of bumblebee queen observed foraging in and around oilseed rape (OSR) fields during two visits in April 2014. Numbers indicate the average number of each species observed per hour of searching (5.5 hours in total).
| Species | Number of queens observed per hour of searching | |
|---|---|---|
| Foraging on OSR | Foraging on other flowers* | |
| Bombus terrestris | 3.6 | 0.4 |
| Bombus lapidarius | 9.1 | 1.5 |
| Bombus lucorum agg. | 0.4 | 0.0 |
| Bombus hortorum | 1.5 | 0.5 |
| Bombus pratorum | 0.0 | 0.2 |
| Bombus hypnorum | 0.2 | 0.2 |
| Bombus pascuorum | 0.4 | 0.4 |
Other flowers were Lamium album, Glechoma hederacea, and Veronica chamaedrys.
Impacts of multiple stressors on B. terrestris queens
Mated B. terrestris queens were experimentally exposed to C. bombi or a control, hibernated for one of two durations, and treated with the neonicotinoid thiamethoxam or a control, in a fully crossed design. Survival and colony initiation (egg laying) were then monitored.
Colony Initiation
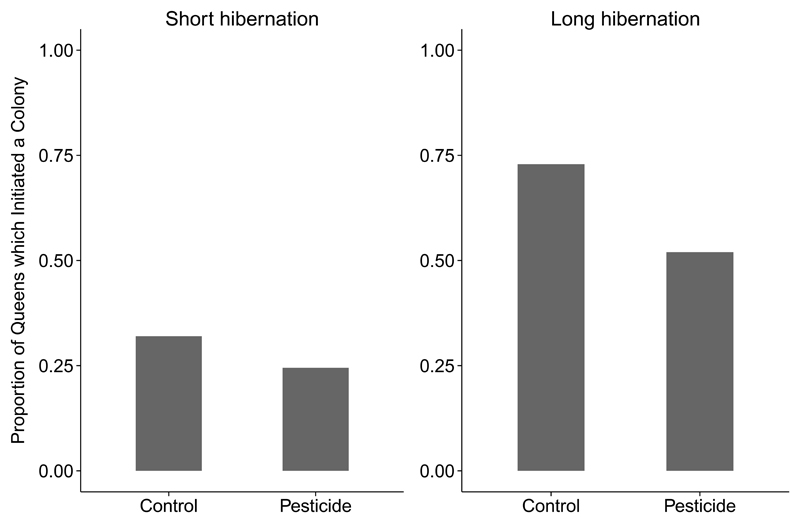
Colony initiation was reduced by 26% in queens exposed to thiamethoxam (2.4 ppb for two weeks) during the colony founding period (Figure 1). Hibernation duration was also an important predictor of colony initiation, with fewer queens laying eggs after a six-week hibernation (28% of all 231 queens in the experiment), compared to a twelve-week hibernation (62% of all 231 queens in the experiment). Both pesticide treatment (estimate = -0.628, 95% CI [-1.240, -0.017]) and hibernation treatment (estimate = -1.514, 95% CI [-2.131, -0.898]), were included in the final composite generalised linear model (GLM) for egg laying (Table 2). Neither parasite treatment, nor any of the interactions, were included in the final model, as they did not improve model fit.
Figure 1.
The proportion of B. terrestris queens that had undergone a short (6 week) or long (12 week) hibernation, and had either been exposed to the pesticide thiamethoxam or a control, which initiated a colony (by laying eggs) within ten weeks of emergence from hibernation. Total sample size for each treatment group: short hibernation, control group = 50; short hibernation, pesticide group = 49; long hibernation, control group = 48; long hibernation, pesticide group = 50 queens (includes queens which survived the whole experiment only).
Table 2.
Summary of models used in analysis of data on the impact of the three treatments on B. terrestris queens. Table includes details of models used in a model selection process (see Analysis section of the Methods for full details), the specific r packages used, as well as the parameters and estimates from the final or composite models. The importance and reliability of each parameter in the final models was assessed by checking for confidence intervals which did not cross zero (highlighted in bold in the model summaries below).
| Model types | Fixed Factors | Random Factors | R packages used | Final / Composite model | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | Lower | Upper | |||||
| Presence or absence of egg laying | GLM, GLMM | Hibernation, Pesticide, Parasite, Thorax | Qcolony, Mcolony | lme4 | Intercept | 5.969 | 5.200 | -4.171 | 16.110 |
| Hibernation | -1.514 | 0.316 | -2.131 | -0.898 | |||||
| Pesticide | -0.628 | 0.313 | -1.240 | -0.017 | |||||
| Thorax | -0.990 | 0.582 | -2.124 | 0.144 | |||||
| Timing of egg laying +* | Cox Regression | Hibernation, P1, P2, Parasite, Thorax | Qcolony, Mcolony | survival | Hibernation | -1.044 | 0.233 | -1.499 | -0.590 |
| P1 | 1.400 | 0.577 | 0.275 | 2.525 | |||||
| P2 | -0.573 | 0.236 | -1.034 | -0.112 | |||||
| Infection | 0.096 | 0.228 | -0.349 | 0.540 | |||||
| Thorax | -0.553 | 0.378 | -1.291 | 0.185 | |||||
| Presence or absence of adult offspring ◊ | GLM, GLMM | Hibernation, Pesticide, Parasite, Thorax | Qcolony, Mcolony | lme4 | Intercept | -0.750 | 0.360 | -1.453 | -0.048 |
| Pesticide | 1.214 | 0.458 | 0.320 | 2.107 | |||||
| Hibernation | 0.963 | 0.495 | -0.003 | 1.928 | |||||
| Weight lost during hibernation† | lm, lme | Hibernation, Parasite, Thorax | Qcolony, Mcolony | nlme | Intercept | 17.895 | 3.284 | 11.491 | 24.298 |
| Hibernation | -5.379 | 0.677 | -6.700 | -4.059 | |||||
| Parasite | 1.323 | 0.676 | 0.006 | 2.641 | |||||
| Thorax | -0.658 | 1.204 | -3.006 | 1.691 | |||||
| Hibernation survival | GLM, GLMM | Hibernation, Parasite, Thorax, PreWeight | Qcolony, Mcolony | lme4 | Intercept | 2.355 | 1.597 | -0.759 | 5.470 |
| Preweight | -7.195 | 2.546 | -12.159 | -2.231 | |||||
| Parasite | 0.447 | 0.497 | -0.522 | 1.415 | |||||
| Hibernation | 0.778 | 2.786 | -4.655 | 6.211 | |||||
| Preweight*Hib. | -6.945 | 5.606 | -17.877 | 3.987 | |||||
| Post-hibernation survival + | Cox Regression | Hibernation, Pesticide, Parasite, Thorax | Qcolony, Mcolony | lme4 | Null model | ||||
| Syrup consumption | lm, lme | Hibernation, Pesticide, Parasite | Qcolony, Mcolony | nlme | Intercept | 0.802 | 0.037 | 0.729 | 0.874 |
| Hibernation | -0.278 | 0.049 | -0.373 | -0.183 | |||||
| Pesticide | 0.024 | 0.488 | -0.927 | 0.974 | |||||
For Cox Regression models, random factors were included as frailty terms48, and model selection was undertaken as described for mixed models.
Timing of egg laying was analysed using a cox regression with proportional hazards. Examination of the residuals showed that the Pesticide factor did not meet the assumption of proportional hazards. To deal with this, the interaction between Pesticide and time was considered, and separate hazard functions were calculated for the period during pesticide exposure (P1: the first 17 days), and for the period after exposure (P2: 17 days - end)48. These two interaction terms were included instead of Pesticide in the model selection process.
For analyses during hibernation, the fixed factor Parasite indicates the exposure of queens to the parasite or a control (as infection status was unknown at this stage). All other analyses including Parasite used data on infection status (whether the queen was successfully infected or not).
Analysis included egg layers only.
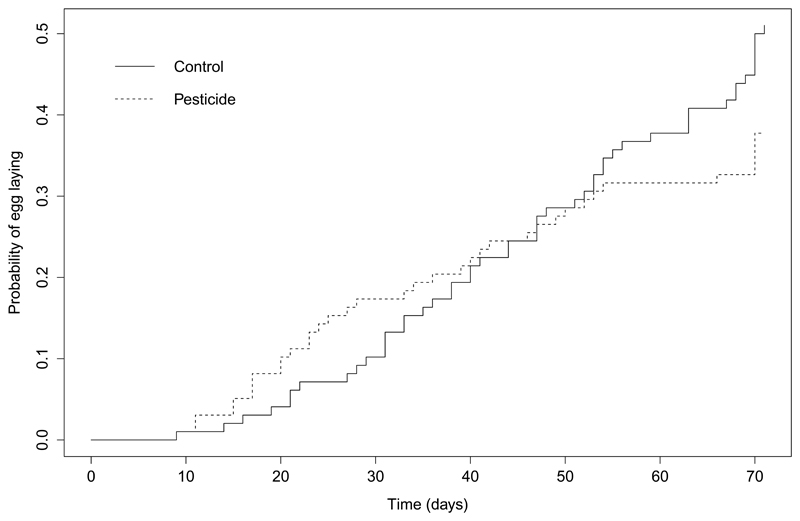
Pesticide treated queens laid eggs earlier in the experiment than untreated queens. Because pesticide treatment violated the assumption of proportional hazards in initial models, episode splitting was used to estimate separate hazard ratios; during pesticide treatment (P1), and after treatment (P2)43. During treatment (P1), more pesticide treated queens laid eggs (estimate = 1.400, 95% CI [0.275, 2.525]), whereas after treatment (P2), the reverse effect was seen (estimate = -0.573, 95% CI [-1.034, -0.112]) (Figure 2).
Figure 2.
The event history curve showing the cumulative probability of egg laying from the end of hibernation (time = 0 days) until the first egg was laid, by B. terrestris queens exposed to either the pesticide thiamethoxam, or a control. Total sample size for each treatment group: control group = 98; pesticide group = 99 (includes queens which survived the whole experiment only).
At a population level, no experimental factors predicted the presence or absence of adult workers. However, when only egg laying queens were considered, pesticide was an important factor (estimate = 1.214, 95% CI [0.320, 2.107]), and unsurprisingly, due to the timing of egg laying (see above) a higher proportion of egg laying queens in the pesticide treatment group had adult offspring by the end of the experiment.
The proportion of queens in the pesticide treatment groups, hibernation treatment groups, and both of these combined, which laid eggs and reared adult offspring are shown in Table S1.
Syrup Consumption
There is no evidence that pesticide exposure affected the amount of syrup consumed by queens (estimate = 0.024, 95% CI [-0.927, 0.974), suggesting that thiamethoxam does not inhibit or promote feeding behaviour. However, queens that had hibernated for longer, consumed more syrup post-hibernation (mean daily syrup consumption (ml) ± SE = 0.805 ± 0.031 (long), 0.527 ± 0.036 (short)) (estimate = -0.278, 95% CI [-0.373, -0.183]). Consequently, the average daily amount of active ingredient consumed by pesticide treated queens in the long hibernation group was 1.977 ng per day, compared to 1.405 ng in the short hibernation group. Again, there were no effects of parasite treatment, or any of the interactions, on syrup consumption, and thus these factors were not included in the final model. The average daily syrup consumption for queens in each treatment group is shown in Table S2.
Survival and weight loss
The only factor that predicted survival during hibernation was initial queen weight (estimate = -7.195, 95% CI [-12.159, -2.231]), with heavier queens being more likely to survive. Post-hibernation queen survival was not predicted by any experimental treatments.
Queens lost more of their body weight after a long hibernation (mean % weight loss ± SE = 17.2% ±0.50 (long), 11.8% ± 0.45 (short))) (estimate = -5.379, 95% CI [-6.700, -4.059]). Parasite exposure also caused an increase in weight loss, although this was a much smaller effect (mean % weight loss ± SE = 15.19% ± 0.55 (parasite), 13.86% ± 0.53 (control)) (estimate = 1.323, 95% CI [0.006, 2.641]).
Population modelling
To extrapolate our results to field populations, the experimental procedure described above would, ideally, be carried out on populations in the field. This is practically unfeasible to assess. To overcome this problem, and demonstrate how estimates can be obtained for the effect of pesticide in the field, we integrate our results with existing data using a Bayesian framework to assess their implications for population sustainability.
For a bumblebee population to survive in the natural environment, each year a colony should produce, on average, at least one daughter colony in the absence of density dependence. Making an analogy with metapopulation theory, we call the long term average number of new colonies produced by an existing colony the colony capacity47. This number depends on the chances of a new queen mating, surviving hibernation, finding a nest site, and initiating a new colony, combined with the number of queens produced by the colony. The data we have on all these factors is limited, and therefore we cannot know the value of the colony capacity with certainty. Using published data on hibernation survival, colony initiation, and new queen production (see Methods), we used a Bayesian framework to integrate this existing empirical data and its associated uncertainty, and map its dependence on the probability with which queens successfully mate and find nest-sites in the wild (pnm); a parameter for which no empirical data are currently available. This allows us to capture the existing information on bumblebee biology and integrate it with our results, whilst quantifying the certainty we have about the value of the colony capacity. We estimated the probability that colony capacity takes a certain value (Figure 3a), and how this value will change if we take into account the effect of thiamethoxam on colony initiation (Figure 3b).
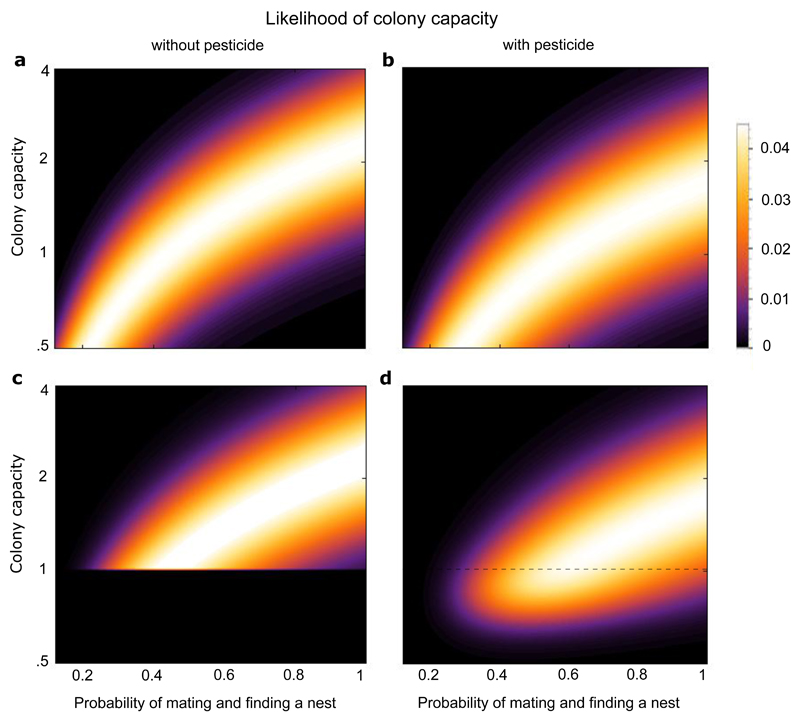
Figure 3.
Heat maps showing the likelihood profile for the colony capacity with (panels b and d) or without thiamethoxam exposure (panels a and c). The heat maps in Figure 3 represent the probability distribution (normalised likelihoods) of the colony capacity (where lighter colours indicate higher probability, see scale to right of graphs) as a function of pnm, the probability with which queens successfully mate and find a new nest site in the natural environment, and the colony capacity itself. The colony capacity is the product of pnm with the number of gynes produced76, the probability to survive hibernation42 and the probability to be able initiate a colony following survival.
Panel (a) depicts the probability that the colony capacity takes a certain value, for different values of pnm, (using data from75, 42). Panel (b) shows the likelihood profile of colony capacity after exposure to thiamethoxam: the distribution is lower than in panel (a) due to the reduction in colony initiation (as found in our empirical results, see Supplementary Material for methodology). Panel (c) shows the likelihood profile of the colony capacity without exposure to thiamethoxam, conditioned to take a value of at least one to take account that the natural bumblebee population of B. terrestris is extant. The colony capacity must therefore be at least one. The likelihoods for the colony capacities below one are therefore set to zero, and the profile renormalised. Panel (d) shows the likelihood profile for the colony capacity as in (c) after the effect of thiamethoxam exposure was taken into account. Because of the reduction in colony initiation caused by thiamethoxam exposure colony capacities below one a have positive probability. The total probability of a colony capacity below one is found by integrating all probabilities below the dashed line in Fig. 3d.
For bumblebee populations to persist, the colony capacity must be at least one in natural environments. The data we have used for the number of new queens produced by a colony come from one study, in one particular year76. Population persistence requires the colony capacity to be at least one when suitably averaged over both good and bad years. Even though we lack information about the extent of annual variability in colony capacity, the data we used came from a favourable location, Swiss meadows, and from a year that was favourable for bumblebee reproduction (see Methods). As the Swiss, and broader European B. terrestris population is extant, the colony capacity in the year from which these data were collected must, therefore, have been at least one. Using our Bayesian framework we can add this information to our estimate of the probability of the colony capacity.
Figure 3c shows the probability heat map for colony capacity, accounting for the fact that colony capacity must exceed one in self-sustaining natural populations. At a minimum, the colony capacity must exceed one in good years. As noted above, the data we have used represent a favourable year, and therefore we set the likelihood of colony capacities below one to zero in Figure 3c. If we now add the impact of thiamethoxam exposure on colony initiation, we see that values of colony capacity below one become likely (Figure 3d). By adding all the probabilities for colony capacities below one together we find that the reduction in the chance of initiating a colony caused by thiamethoxam exposure results in a colony capacity with value below one with a probability of 28%.
If the colony capacity is less than one, the population will eventually go extinct. We can therefore say that, based on these data, that widespread thiamethoxam use leads to eventual population extinction with a probability of at least 28%. This is likely to be a conservative estimate as it is based on the data taken in a favourable location in a year with good conditions and does not take the small probabilities of extinction for colony capacities exceeding one into account. Note that for intermediate ranges of pnm, there is a substantially higher probability of neonicotinoid exposure leading to population extinction.
Discussion
We show for the first time that exposure to field relevant levels of the neonicotinoid pesticide thiamethoxam significantly reduces successful colony founding in B. terrestris queens. Two weeks of insecticide exposure resulted in a shift in the timing of colony initiation, and ultimately a 26% reduction in the proportion of queens that laid eggs by the end of the experiment. These results add significantly to our understanding of neonicotinoid impacts on a key life-stage in an essential agricultural pollinator. Including this 26% reduction in models of population dynamics indicated a dramatically increased risk of population extinction after pesticide exposure.
Interestingly, despite its overall negative effect, thiamethoxam exposure caused an increase in the number of queens laying eggs early in the experiment (Figure 2). However, by day 40, colony initiation by pesticide and control treated queens had levelled off, and by the end of the experiment (day 70) a higher proportion of control queens had laid eggs. There is evidence that individuals from various taxa respond to natural enemies by shifting reproductive effort earlier, for example the snail, Biomphalaria glabrata, increases oviposition soon after exposure to a trematode worm49, while Daphnia species lay larger clutches earlier when exposed to a microsporidian parasite50, and mature at an earlier instar after exposure to predatory fish cues51. Such plasticity in life-history traits are thought to be adaptive responses to threats to survival or reproduction. Moret and Schmid-Hempel52 found that bumblebee colonies will shift reproduction earlier in response to immune challenge of workers, and also to harsh conditions (lower temperature). This resulted in an increase in the production of sexual offspring early on in these treatment groups, followed by a drop compared to controls later in the experiment. Although this effect was seen at the colony level rather than the individual level, it shows that social insects such as bumblebees exhibit life-history changes in response to physiological stress. Our study is the first to suggest that similar processes may occur in response to pesticide exposure. Pesticides cause metabolic changes in honeybees, including the regulation of genes associated with immune function and detoxification53,54. It therefore seems likely that the shift in timing of colony initiation observed in the current experiment is a response to physiological stress from pesticide exposure.
Pesticide impacts on reproduction have also been observed in bumblebee workers21,55, and solitary bees33,56. However, the mechanisms behind reduced colony initiation in our study, and impacts on workers and solitary bees in previous studies, remain unclear. If the metabolic cost of detoxification is high, this could lead to the reallocation of nutrients such as proteins, reducing nutrient availability for other biological processes (e.g. ovary development). Studies of other taxa, for example the southern armyworm Spodoptera eridania have found that nicotine detoxification imposes a significant metabolic cost, leading to a reduction in growth57. It is known that honeybees and bumblebees can clear ingested pesticide rapidly58, however, the metabolic costs of the detoxification process in bees remain unknown.
We did not detect impacts of any of the experimental treatments on the ability of queens to produce adult offspring. However, when only egg laying queens were considered, a higher proportion of thiamethoxam treated queens had adult offspring by the end of the experiment. This is likely to be due to a higher proportion of treated queens laying eggs early in the experiment, giving them more time to rear adult offspring. Given the temporal patterns in egg-laying identified in our study, this pattern would most likely have reversed if the experiment had been continued beyond 14 weeks. Our results suggest that a pulse of thiamethoxam exposure after hibernation creates two populations of queens, with presumably weaker queens being effectively castrated, whilst stronger queens respond by bringing reproduction forward. Early initiation could have benefits, such as earlier access to foraging resources, a longer growth period and thus higher reproductive output. However, it could also put colonies out of synchronisation with the broader population, and the flowering times of key forage plants.
Our observations in the field indicate that bumblebee queens of multiple species will forage on and around oilseed rape crops which have been treated with a neonicotinoid seed treatment. Previous studies have shown that neonicotinoids are present in both the pollen and nectar of crops and forage plants in the surrounding area59–61. Bumblebee queens foraging in agricultural environments are therefore being exposed to neonicotinoids whilst foraging in the early spring. The crops observed in this study had been treated with Modesto seed treatment which contains clothianidin. In the laboratory trial we tested the impacts of thiamethoxam (which is the most widely used neonicotinoid on oilseed rape crops62). Neither thiamethoxam nor clothianidin are repellent to bumblebees63, and we found no evidence for a reduction in feeding associated with thiamethoxam treatment in this study. The extent of pesticide exposure faced by bumblebee queens in the field, and how this impacts colony initiation under natural foraging conditions, requires further investigation.
A two-week exposure period was used in this experiment, to represent the period of foraging immediately after hibernation and before colony initiation is likely to begin, and therefore specifically targeting the queens rather than developing brood34. However, wild foraging queens could be exposed to thiamethoxam and other pesticides for longer periods, and potentially well into the development of the colony. It would be useful to explore this further, as the impacts on colony initiation observed in this study may be further exacerbated if queens were exposed for longer periods or to a range of pesticides.
Using a Bayesian model for bumblebee population dynamics we show that the impacts of pesticide exposure during colony-founding carry a considerable risk of population extinction. Due to the lack of field data on mating success and the availability of nest sites (although given bumblebee sex ratios at least the former is likely to have a probability of 1: e.g.12,21), we cannot give a precise prediction on whether or not populations will go extinct. However, our model shows that unless mating success and finding a nest site are assured, reductions in colony initiation resulting from thiamethoxam exposure are likely to lead to appreciable probabilities of population failure. Given the landscape of pesticide usage, this could lead to local extinctions and drive a source-sink network in bumblebee populations. Detailed population genetic studies would be required to test for such a large-scale impact.
Queens that underwent a 12-week hibernation period were more likely to lay eggs compared to queens in the 6-week hibernation group, something which has been observed in laboratory studies before43,46. Wild B. terrestris queens in the UK are likely to need to hibernate for up to 6-9 months through the winter41, and so this may reflect an adaptation allowing them to perform better under longer hibernation conditions. The relatively short hibernation periods used in the current study were chosen to maximise sample size for the pesticide phase of the experiment. Queens that undergo a longer, more stressful hibernation in the wild, may therefore be even more susceptible to the effects of pesticide exposure. This hypothesis is supported by observations that queens exposed to neonicotinoids were more likely to die after longer hibernation periods of up to 4 months, and lost weight more rapidly over this period37. None of the treatments or covariates measured in this study had an impact on the survival of queens after hibernation, although queens that were heavier before hibernation were more likely to survive to the end of hibernation, as has been found in a number of previous studies42,43. Neonicotinoids have been found to have an impact on queen survival in several previous studies24,37,64. However, in these cases, this effect was seen at much higher doses (20 ppb or higher in Scholer and Krischik64), during hibernation37, or much later in the colony life cycle24. Nevertheless, the current study was conducted under optimal laboratory conditions making our results conservative, and more stressful field conditions may increase pesticide impacts.
Exposure to C. bombi resulted in greater weight loss during hibernation, as was the case in Brown, Schmid-Hempel and Schmid-Hempel46. However, there were no parasite impacts on the other traits measured. Brown, Schmid-Hempel & Schmid-Hempel46 found an impact of C. bombi on colony founding and development, with fewer infected queens initiating a colony, and those which did producing fewer workers, males, and gynes. However, the shorter hibernation period used in the current study is likely to explain this difference.
These results provide the first evidence that chronic exposure to thiamethoxam reduces colony initiation by bumblebee queens, with knock-on effects for population sustainability and extinction. In addition, there is also considerable evidence that neonicotinoids can impair the development of bumblebee colonies and reduce the number of queens produced later in the lifecycle22,24,27,28,33 which could additionally deplete populations. Further research is needed to explore the long-term impacts of the observed reduction in egg laying on colony success, and population dynamics in the field. However, imminent policy decisions relating to the use of neonicotinoids should take into consideration the timing and mode of application of these compounds, and the organisms, and crucially, lifecycle stages, which are likely to be impacted by exposure.
Methods
Are bumblebee queens exposed to neonicotinoids in the field?
Two visits were made to two winter oilseed rape (Brassica napus) fields (variety PR46W21), at Shiplake Farm, Oxfordshire, UK (Latitude: 51.504696, Longitude: -0.90030080) during early April 2014 when the crop was in flower. Crop seeds had been treated with Modesto seed treatment (clothianidin and β-cyfluthrin, Bayer CropScience, Cambridge, UK), and planted the previous year. Transects around the edge of the fields (distance around each field = 2 km and 0.94 km) and through the centre of the crop (0.3 km and 0.4 km) were walked between 11am and 3pm on days when weather conditions were suitable (sunny and dry with minimal wind). Transects were walked once per visit, at a steady pace (total walking time per visit = 3 hours), and all bumblebee species within 2 metres of the transect were recorded, along with the caste and activity of each bee. Queens of the B. lucorum complex (B. lucorum, B. cryptarum and B. magnus) cannot be reliably separated using morphological features alone65, and so these were recorded as B. lucorum agg.
Impacts of multiple stressors on B. terrestris queens
Colonies
Fifteen Bombus terrestris audax colonies were obtained from Koppert Ltd (Haverhill, UK). Colonies were kept in the laboratory in darkness (red light was used for colony manipulation), at 22 °C. Colonies were fed ad libitum with 50% Ambrosia (E H Thorne Ltd), an inverted sugar syrup solution (from now on referred to as syrup), and frozen honeybee-collected pollen pellets (Koppert Ltd, Haverhill, UK). On arrival, 10% of the workers from each colony were dissected and screened microscopically for the parasites Crithidia bombi (Trypanosomatidae), Nosema bombi (Microsporidia), and Apicystis bombi (Neogregarinida), using a Nikon eclipse (50i) compound microscope at 400x magnification. No parasite infections were detected at this stage.
Mating
Males and gynes (reproductive females) were removed from colonies as callows (newly emerged bees), and kept communally in single sex wooden boxes (24 x 14 x 10.5 cm) with nest mates of the same age, and fed ad libitum with pollen and syrup.
Four days after eclosion, gynes were mated with unrelated males of at least four days of age. Mating took place in a 60 x 50 x 50 cm wooden framed arena, with plastic mesh sides, under natural light, at a temperature of 22 °C. Up to 25 males from a single colony were placed into the arena, and left to acclimatise for 10 minutes. Unrelated gynes from another single colony and age group were then added to the arena. Mating pairs were removed from the arena immediately, and the time, date, male and female colony, and age were recorded. Once mating was complete, the male was removed, and frozen at -20 °C. The mated queen was kept in an individual plastic box (13 x 11 x 6.8 cm) containing a small amount of tissue paper to remove excess moisture, and immediately provided with 100µl of inoculum (see below for inoculum preparation). When this full amount had been consumed, the queens were provided with ad libitum food (pollen and syrup), for between 2 and 4 days after mating (depending on how quickly the inoculum was consumed), at which point they were weighed, and placed into hibernation (see below). Queens that did not consume the full amount of inoculum within 4 days were excluded from the experiment.
Gynes that did not mate on the first attempt were kept in their communal boxes as described above, and further mating attempts (up to 5 attempts per gyne) were made (with different groups of males), until mating took place. Males were also kept until mating had occurred, and mating attempts continued until males were 2 weeks of age, at which point they were frozen at -20 °C.
Preparation and delivery of C. bombi inoculum
Crithidia bombi was obtained from naturally infected wild B. terrestris queens, collected from Windsor Great Park, Surrey, UK (Latitude: 51.417432, Longitude: -0.60481256) during the spring of 2013. Queens were also screened for N. bombi, S. bombi, and A. bombi; any queens co-infected by these parasites were removed. Crithidia infected queens were kept in the laboratory in Perspex queen rearing boxes (13.3 x 8 x 5.6 cm) with ad libitum syrup and pollen, and kept in a dark room at a constant temperature of 28°C and 50% humidity (conditions suitable for colony initiation). Eleven naturally infected queens (and their colonies in 6 cases) were available at the start of the experiment, and 10 µl of faeces was collected from each of these, combined, and used to infect 20 stock worker bees collected from each of the experimental colonies. This ensured that a wide range of naturally occurring strains of C. bombi was available for the infection of experimental queens. All faeces collected were combined, and diluted with 0.9% Ringer’s solution to make 1ml of solution. Crithidia bombi cells were filtered using a modified protocol for purification23 originally developed by Cole66. This process was repeated using wild caught queens from the same population that were not infected with C. bombi, A. bombi, N. bombi, or S. bombi in order to provide a control.
The stock bees were taken from the experimental colonies in order to account for any filtering of the parasite strains by workers prior to infection of the experimental queens67. Workers were removed from each colony and starved for a period of four hours. Each stock bee was then individually fed a 10 µl drop of inoculum (containing 10,000 C. bombi cells), and observed until all of the liquid had been consumed. These stock bees were then kept communally in wooden boxes with their nest-mates, and fed ad libitum pollen and syrup. The same process was repeated using faeces from the uninfected wild queens, to create a control stock.
To make the inoculum for the experimental queens, an equal amount of faeces (10 µl) was collected from each box of stock bees each day that inoculation took place. This was combined and purified as described above. The resulting solution was diluted with syrup, and 100 µl of this inoculum (containing at least 20,000 C. bombi cells) was provided in a feeding tube for each queen. The same process was repeated using the C. bombi free faeces from the control stock bees.
Hibernation
Mated queens (only those which had consumed the full amount of inoculum), were weighed and placed into 50 ml tubes (Falcon) with damp sterilised sand, and kept in a dark incubator at a constant temperature of 4 °C for either a six-week period, or a 12-week period. After this hibernation period, the queens were removed from the tubes and re-weighed. Surviving queens were then placed into Perspex queen rearing boxes (13.3 x 8 x 5.6 cm) with ad libitum syrup and pollen, and kept in a dark room at a constant temperature of 28 °C and 50% humidity.
Pesticide exposure
A total of 319 mated queens were placed into hibernation. Of these, 20 died during hibernation, and a further 68 were excluded from the final analysis. Exclusion was due to a lack of replication for their natal colony (as a result of nest-mates being lost (n = 60)), accidental infection with C. bombi (n = 6), and accidental death (n = 2). The remaining 231 queens (from eight colonies) were allocated to either the pesticide or control treatment. The distribution of queens across the eight treatment groups is shown in Table 3.
Table 3.
Summary of queen numbers allocated to the eight treatment groups in the experiment. Hibernation (long: 12-week; short: 6-week), Pesticide (exposure to thiamethoxam or no exposure) and Parasite (exposure to C. bombi or no exposure) are the three treatments, Infection status indicates the number and percentage for each Parasite group that was successfully infected by the end of the experiment.
| Hibernation | Pesticide | Parasite | n | Infection | n | % Infected |
|---|---|---|---|---|---|---|
| Long | Pesticide | Parasite | 31 | Infected | 20 | 64.5 |
| Control | 27 | |||||
| Control | Parasite | 29 | Infected | 18 | 62.1 | |
| Control | 27 | |||||
| Short | Pesticide | Parasite | 30 | Infected | 23 | 76.7 |
| Control | 30 | |||||
| Control | Parasite | 28 | Infected | 22 | 78.6 | |
| Control | 29 |
Three days after emergence from hibernation, queens in the pesticide treatment group were provided with syrup containing 2.4 ppb thiamethoxam, which is the equivalent to that found in stored nectar in bumblebee colonies foraging in agricultural environments in the UK68 and significantly below mean levels reported in stored pollen from bumblebee colonies foraging in agricultural environments in the UK69. As established in the field trial above, bumblebee queens will forage on neonicotinoid treated oilseed rape crops, and are therefore likely to be exposed to these pesticides as they establish a colony in the spring.
Analytical standard thiamethoxam (Pestanal, Sigma Aldrich) was mixed with Acetone (Fluka, Sigma Aldrich) to give a stock solution of 100 mg/ml. Aliquots of this stock were diluted with syrup, to give a final concentration of 2.4 ppb thiamethoxam. Acetone alone was diluted in the same way, to provide a solvent control. Solution was freshly made each day of the experiment. Samples of treated syrup from two dates in the experiment were collected and analysed for thiamethoxam residues using LC-MS (Food and Environment Research Agency, Sand Hutton, York). Average residues were found to be 2.5 µg/Kg (ppb) ± 0.085.
Queens were provided with the pesticide (or acetone in the case of the control group) treated syrup for 14 days, and the amount consumed by the queen during this time measured twice (once after 7 days, at which point the feeder was replenished with fresh treated syrup, and again after 14 days) using a 25 ml measuring cylinder to an accuracy of 0.25 ml. Average evaporation rate was measured by keeping feeders (n = 10) in empty rearing boxes for a week, and calculating volume lost during this time – syrup consumption data were then corrected for evaporation. Queens were provided with ad libitum untreated syrup for the remainder of the experiment.
Post-hibernation monitoring
After hibernation, all queens were provided with a pollen ball (ground pollen pellets mixed with syrup to form a soft dough, shaped into a cylinder of approximately 1cm in height and diameter), in which to lay their eggs and as a source of food. Unused pollen balls (which contained no eggs or brood) were changed twice a week, in order to provide a source of fresh pollen for the queens. Pollen balls containing brood were left in the box, and an additional pollen ball or dish of loose pollen provided twice a week.
Queens were monitored daily for mortality and egg laying. All bees which died during the experiment were frozen at -20 °C on the day of death. The first date of egg laying (colony initiation) was recorded, as was the date that the first adult worker eclosed. Queens which had not initiated a colony 10 weeks after emergence from hibernation were frozen at -20 °C. Queens which had brood were kept for an additional 4 weeks, in order to monitor development of the brood into adult workers.
Each queen was checked for the presence of C. bombi (by microscopic examination of a fresh faecal sample) three times during the experiment. The first check occurred 4 days after the end of hibernation, the second 11 days after hibernation, and the third check 30 days after hibernation.
Dissection
All dissections were performed using a Nikon microscope (SM2800) at a magnification of x10 to x30. At the end of the experiment, all queens were dissected, and checked microscopically for the presence of C. bombi (as described for the parasite screening above). Queens were also screened for N. bombi and A. bombi in order to verify the earlier colony screening results. Neither of these parasites were found at this stage.
Analysis
Models were constructed for each analysis using some or all of the following factors: Hibernation (short or long), Pesticide (pesticide or control), Parasite (exposed to the parasite or not exposed), Infection (Infected or uninfected – this was assessed through the four parasite checks – if C. bombi was detected during any of these, the individual was considered to be infected). The following covariates were also considered: Preweight (pre-hibernation weight), Postweight (post-hibernation weight), Weightloss (proportion of weight lost during hibernation), and Thorax (thorax width). The natal colony of the queen, and of her mate (QColony and MColony) were considered as random factors in mixed models, and compared to equivalent models without random factors. In analysis of egg laying and colony development, all queens that died during the experiment were excluded, as they had not been present during the entire 10 (or 14) week observation period. Details of each analysis are summarised in Table 2.
All analyses were performed in R (Version 3.1.170) using the packages lme471 and survival72.
Model selection
In order to select the optimal model for each analysis, AICc values (AIC values corrected for small sample sizes) were compared for a set of candidate models. Firstly, mixed models with one or both of the random factors Qcolony and Mcolony were compared to equivalent models with no random factors73. This was used to decide on the random structure used in further model selection (one random factor, both random factors, or no random factors). Candidate models were then constructed including biologically meaningful combinations of the fixed factors listed above. These were compared with the null model (no fixed factors), and full model (all fixed factors). Two random factors, queen colony and male colony, were included in initial comparisons, but did not improve fit of any of the models, and so were not included here. Two way interactions between treatments were considered, but due to lack of coverage, three way interactions were not. Interactions between covariates and treatments were included if data visualisation indicated this may be useful. The AICc values were used (these were chosen over AIC values due to small sample sizes), and the optimal model (with the lowest AICc) was selected. When AICc values for different models were within two units of the lowest, model averaging was undertaken74 (except in cases where the null model was amongst these, in which case the null was assumed to be optimal). Final models were verified graphically for fit and to ensure all assumptions had been met73,75. Interpretation of the importance of factors within the final models was based on the size of the estimate (the larger the estimate, the greater the effect size of that factor), and 95% confidence intervals (those which did not cross zero were considered reliable and important to the model). Model selection tables are available in the Supplementary Information Tables S2-S8.
Modelling methods
The colony capacity is the average number of colonies produced over one season. It is calculated as the product of the likelihood of the average number of gynes produced per colony, the likely probability to survive 6 months in hibernation and consequently initiate a colony, and the probability of successfully mating, and finding a new nest site – a variable which we call pnm. Although we do not know the value of any of the components of pnm with absolute certainty, several studies have quantified the success of various stages of the life cycle. Baer and Schmid-Hempel76 studied the number of gynes produced by colonies under field conditions. They found that 18 colonies produced 155 gynes, an average of 8.61 gynes per colony. We will assume that the number of gynes produced follows a geometric distribution with mean mg. The probability to survive hibernation (which we will call ph) and the probability to be able initiate a colony following survival (which we will call pc) were studied by Beekman et al.43. They found that out of 45 queens, 23 survived a 6-month hibernation period, from which 11 (of 23) initiated a colony. We have assumed that both the number of survivors and the number of colony initiating queens is binomially distributed. Here we use this information to calculate the likelihood of the colony capacity.
Following emergence from the natal colony, gynes need to mate, survive hibernation, and then find a nest site. The probability of mating and finding a nest site (pnm) has not been studied quantitatively, and we therefore have no information about the value of this parameter. Figure 3a shows the probability of the colony capacity to take a certain value.
Next we estimated the effect of pesticide exposure on the population. In this study, the effect of thiamethoxam on queen survival after 3 months of hibernation was tested experimentally using control queens and queens treated with pesticide. Assuming that the numbers surviving are binomially distributed, we established the likelihood of the multiplicative effect of pesticide exposure.
To do this we conservatively assumed that the effect of thiamethoxam on colony initiation after hibernation is the same for 6 months as it is for 3 months (in reality it is likely to be higher) and inferred the likelihood of this effect. Subsequently, we normalized the likelihood before combining the results to establish the probability of the colony capacity after neonicotinoid exposure (Figure 3b). For intermediate values of pnm, it can be seen that the colony capacity is likely to be reduced below 1 (Figure 3d), which means that populations will become extinct.
As B. terrestris populations have persisted in natural environments, we know that the colony capacity of an extant population has to be at least one. The expected number of colonies produced per colony can vary over the years, and the information we have is restricted to an observation in a single year. However, even though we have no comparable data on the number of gynes produced, there are additional studies which indicate that the productivity of B. terrestris in the same location was ~50% lower in a previous year76,77. This indicates that the data used here to estimate the colony capacity represent a relatively good year. We can thus reason that if the number of daughter colonies falls below one in a good year, a population cannot persist, and thus, that persistence of B. terrestris in the wild tells us that our estimate of the colony capacity based on a good year has to be at least one. We therefore conditioned the result on this and set the probability of colony capacities below one to zero and normalised (see Figure 3c). We then took the effect of pesticide into account and calculated the probability of the colony capacity under pesticide exposure (Figure 3d). The total probability of the colony capacity estimated to be to less than one came to 28%. As we made several restrictive assumptions to reach this result, this is very likely a conservative estimate.
Full details of these probability calculations are given in the Supplementary Information.
Supplementary Material
Acknowledgements
We thank Susan Baldwin, Barry McCrea, Oscar Ramos-Rodriguez, and David Wells for assistance in the laboratory, and Lisa Evans, Matthias Fürst, Catherine Jones, Elli Leadbeater, Fabio Manfredini, Karen Smith, and Dara Stanley for comments. We thank The Crown Estate for permission to collect wild bumblebees at Windsor Great Park, and Steven Doble for permission to survey fields at Shiplake Farm. This study was supported by UK Insect Pollinators Initiative grants BB/I000178/1 awarded to NER and BB/1000151/1 awarded to MJFB and VAAJ (funded jointly by the Living with Environmental Change programme, Biotechnology and Biological Sciences Research Council (BBSRC), Wellcome Trust, Scottish Government, Department for Environment, Food and Rural Affairs (Defra) and Natural Environment Research Council (NERC)), and a NERC studentship to GLB. NER is supported as the Rebanks Family Chair in Pollinator Conservation by The W. Garfield Weston Foundation.
Footnotes
Author Contributions
GLB, MJFB and NER conceived the project and designed the experiment; GLB carried out the experiment and statistical analyses; VAAJ carried out modelling, and all authors contributed to writing the paper.
Author Information
The authors declare no competing financial interests.
Data availability
The datasets generated and analysed during the current study are available as supplementary data 1, and the Mathematica notebook used to generate Figure 3 is available from the corresponding author on request.
References
- 1.Corbet SA, Williams IH, Osborne JL. Bees and the pollination of crops and wild flowers in the European community. Bee World. 1991;72:47–59. [Google Scholar]
- 2.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc Lond B Biol. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120:321–326. [Google Scholar]
- 4.Garibaldi LA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- 5.Nieto A, et al. European Red List of Bees. IUCN: Publication Office of the European Union; Luxembourg: 2014. [Google Scholar]
- 6.Williams PH. The distribution and decline of British bumble bees (Bombus Latr) J Apic Res. 1982;21:236–245. [Google Scholar]
- 7.Fitzpatrick Ú, et al. Rarity and decline in bumblebees - a test of causes and correlates in the Irish fauna. Biol Cons. 2007;136:185–194. [Google Scholar]
- 8.Cameron SA, et al. Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA. 2011;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid-Hempel R, et al. The invasion of southern South America by imported bumblebees and associated parasites. J Anim Ecol. 2014;83:823–837. doi: 10.1111/1365-2656.12185. [DOI] [PubMed] [Google Scholar]
- 10.Carvell C, Roy DB, Smart SM, Pywell RF, Preston CD, Goulson D. Declines in forage availability for bumblebees at a national scale. Biol Cons. 2006;132:481–489. [Google Scholar]
- 11.Meeus I, Brown MJF, De Graaf DC, Smagghe G. Effects of invasive parasites on bumble bee declines. Cons Biol. 2011;25:662–671. doi: 10.1111/j.1523-1739.2011.01707.x. [DOI] [PubMed] [Google Scholar]
- 12.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature. 2014;506:364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon DP, Fürst MA, Caspar J, Theodorou P, Brown MJF, Paxton RJ. A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. J Anim Ecol. 2015;84:615–624. doi: 10.1111/1365-2656.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stout JC, Morales CL. Ecological impacts of invasive alien species on bees. Apidologie. 2009;40:388–409. [Google Scholar]
- 15.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecol Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 16.Kerr JT, et al. Climate change impacts on bumblebees converge across continents. Science. 2015;349:177–180. doi: 10.1126/science.aaa7031. [DOI] [PubMed] [Google Scholar]
- 17.Vanbergen AJ, et al. Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ. 2013;11:251–259. [Google Scholar]
- 18.Godfray HCJ, et al. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc Lond B Biol. 2014;281:20140558. doi: 10.1098/rspb.2014.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfray HCJ, et al. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc Lond B Biol. 2015;282:20151821. doi: 10.1098/rspb.2015.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodcock BA, et al. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat Commun. 2016;7:12459. doi: 10.1038/ncomms12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laycock I, Lenthall KM, Barratt AT, Cresswell JE. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris) Ecotoxicology. 2012;21:1937–1945. doi: 10.1007/s10646-012-0927-y. [DOI] [PubMed] [Google Scholar]
- 22.Bryden J, Gill RJ, Mitton RAA, Raine NE, Jansen VAA. Chronic sublethal stress causes bee colony failure. Ecol Lett. 2013;16:1463–1469. doi: 10.1111/ele.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron GL, Raine NE, Brown MJF. Impact of chronic exposure to a pyrethroid pesticide on bumblebees and interactions with a trypanosome parasite. J Appl Ecol. 2014;51:460–469. [Google Scholar]
- 24.Fauser-Misslin A, Sadd BM, Neumann P, Sandrock C. Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J Appl Ecol. 2014;51:450–459. [Google Scholar]
- 25.Stanley DA, Smith KE, Raine NE. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci Rep. 2015;5:16508. doi: 10.1038/srep16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley DA, Raine NE. Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct Ecol. 2016;30:1132–1139. doi: 10.1111/1365-2435.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill RJ, Ramos-Rodriguez O, Raine NE. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature. 2012;491:105–108. doi: 10.1038/nature11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336:351–352. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- 29.Gill RJ, Raine NE. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct Ecol. 2014;28:1459–1471. [Google Scholar]
- 30.Feltham H, Park K, Goulson D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology. 2014;23:317–323. doi: 10.1007/s10646-014-1189-7. [DOI] [PubMed] [Google Scholar]
- 31.Stanley DA, et al. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J Appl Ecol. 2016;53:1440–1449. doi: 10.1111/1365-2664.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley DA, et al. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature. 2015;528:548–550. doi: 10.1038/nature16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rundlöf M, et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature. 2015;521:77–80. doi: 10.1038/nature14420. [DOI] [PubMed] [Google Scholar]
- 34.Sladen FWL. The Humble-bee: its life-history and how to domesticate it with descriptions of all the British species of Bombus and Psithyrus. Macmillan; London: 1912. [Google Scholar]
- 35.Williams GR, et al. Neonicotinoid pesticides severely affect honey bee queens. Sci Rep. 2015;5:14621. doi: 10.1038/srep14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu-Smart J, Spivak M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci Rep. 2016;6:32108. doi: 10.1038/srep32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauser A, Sandrock C, Neumann P, Sadd BM. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol Entomol. 2017;42:306–314. [Google Scholar]
- 38.Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol. 2013;50:977–987. [Google Scholar]
- 39.Forister ML, et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol Lett. 2016;12:2016047. doi: 10.1098/rsbl.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallmann CA, Foppen RP, van Turnhout CA, de Kroon H, Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511:341–343. doi: 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- 41.Alford DV. A study of the hibernation of bumblebees (Hymenoptera: Bombidae) in southern England. J Anim Ecol. 1969;38:149–170. [Google Scholar]
- 42.Holm SN. Weight and life length of hibernating bumble bee queens (Hymenoptera: Bombidae) under controlled conditions. Entomol Scand. 1972;3:313–320. [Google Scholar]
- 43.Beekman M, van Stratum P, Lingeman R. Diapause survival and post-diapause performance in bumblebee queens (Bombus terrestris) Entomol Exp Appl. 1998;89:207–214. [Google Scholar]
- 44.Korner P, Schmid-Hempel P. Effects of sperm on female longevity in the bumble-bee Bombus terrestris L. Proc R Soc Lond B Biol. 2003;270:S227–S229. doi: 10.1098/rsbl.2003.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yourth CP, Brown MJF, Schmid-Hempel P. Effects of natal and novel Crithidia bombi (Trypanosomatidae) infections on Bombus terrestris hosts. Insectes Soc. 2008;55:86–90. [Google Scholar]
- 46.Brown MJF, Schmid-Hempel R, Schmid-Hempel P. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. J Anim Ecol. 2003;72:994–1002. [Google Scholar]
- 47.Hanski I, Ovaskainen O. The metapopulation capacity of a fragmented landscape. Nature. 2000;404:755–758. doi: 10.1038/35008063. [DOI] [PubMed] [Google Scholar]
- 48.Mills M. Introducing Survival and Event History Analysis. SAGE Pulications Ltd; London: 2011. [Google Scholar]
- 49.Thornhill JA, Jones JT, Kusel JR. Increased oviposition and growth in an immature Biomphalaria glabrata after exposure to Schistoma mansoni. Parasitology. 1986;93:443–450. doi: 10.1017/s0031182000081166. [DOI] [PubMed] [Google Scholar]
- 50.Chadwick W, Little TJ. A parasite-mediated life-history shift in Daphnia magna. Proc R Soc Lond B Biol. 2005;272:505–509. doi: 10.1098/rspb.2004.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakwinska O. Response to fish kairomone in Daphnia galeata life history traits relies on shift to earlier instar at maturation. Oecologia. 2002;131:409–417. doi: 10.1007/s00442-002-0901-0. [DOI] [PubMed] [Google Scholar]
- 52.Moret Y, Schmid-Hempel P. Social life-history response to individual immune challenge of workers of Bombus terrestris L.: a possible new cooperative phenomenon. Ecol Lett. 2004;7:146–152. [Google Scholar]
- 53.Boncristiani H, et al. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J Insect Physiol. 2012;58:613–620. doi: 10.1016/j.jinsphys.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Aufauvre J, et al. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One. 2014;9:e91686. doi: 10.1371/journal.pone.0091686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elston C, Thompson HM, Walters KFA. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie. 2013;44:563–574. [Google Scholar]
- 56.Sandrock C, et al. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric For Entomol. 2014;16:119–128. [Google Scholar]
- 57.Cresswell JE, Merritt SZ, Martin MM. The effect of dietary nicotine on the allocation of assimilated food to energy metabolism and growth in fourth-instar larvae of the southern armyworm, Spodoptera eridania (Lepidoptera: Noctuidae) Oecologia. 1992;89:449–453. doi: 10.1007/BF00317425. [DOI] [PubMed] [Google Scholar]
- 58.Cresswell JE, Robert F-XL, Florance H, Smirnoff N. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris) Pest Manag Sci. 2014;70:332–337. doi: 10.1002/ps.3569. [DOI] [PubMed] [Google Scholar]
- 59.Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One. 2012;7:e29268. doi: 10.1371/journal.pone.0029268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart SD, et al. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-Southern United States. Environ Sci Technol. 2014;48:9762–9769. doi: 10.1021/es501657w. [DOI] [PubMed] [Google Scholar]
- 61.Botías C, et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ Sci Technol. 2015;49:12731–12740. doi: 10.1021/acs.est.5b03459. [DOI] [PubMed] [Google Scholar]
- 62.Garthwaite D, et al. Arable Crops in the United Kingdon 2014. FERA Pesticide Usage Survey Report. 2014;236 [Google Scholar]
- 63.Kessler SC, et al. Bees prefer foods containing neonicotinoid pesticides. Nature. 2015;521:74–76. doi: 10.1038/nature14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholer J, Krischik V. Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens . PLoS One. 2014;9:e91573. doi: 10.1371/journal.pone.0091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carolan JC, et al. Colour patterns do not diagnose species: quantitative evaluation of a DNA barcoded cryptic bumblebee complex. PLoS One. 2012;7:e29251. doi: 10.1371/journal.pone.0029251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cole RJ. Application of triangulation method to purification of Nosema spores from insect tissues. J Invertebr Pathol. 1970;15:193–195. [Google Scholar]
- 67.Ulrich Y, Sadd BM, Schmid-Hempel P. Strain filtering and transmission of a mixed infection in a social insect. J Evol Biol. 2011;24:354–362. doi: 10.1111/j.1420-9101.2010.02172.x. [DOI] [PubMed] [Google Scholar]
- 68.Thompson H, et al. Effects of neonicotinoid seed treatments on bumble bee colonies under field conditions. Food and Environment Research Agency; York: 2013. [Google Scholar]
- 69.David A, et al. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ Int. 2016;88:169–178. doi: 10.1016/j.envint.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 70.R Core Team. R: A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 71.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. 2014 [Google Scholar]
- 72.Therneau T. A Package for Survival Analysis in S. R package version 2.37-7. 2014 [Google Scholar]
- 73.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]
- 74.Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Zuur A, Hilbe JM, Ieno EN. A Beginner's Guide to GLM and GLMM with R. Highland Statistics Ltd; Newburgh: 2013. [Google Scholar]
- 76.Baer B, Schmid-Hempel P. Unexpected consequences of polyandry for parasitism and fitness in the bumblebee, Bombus terrestris. Evolution. 2001;55:1639–1643. doi: 10.1111/j.0014-3820.2001.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 77.Baer B, Schmid-Hempel P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature. 1999;397:151–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.