Abstract
Background
Neuropathic pain is thought to arise from damage to the somatosensory nervous system. Its prevalence is increasing in line with many chronic disorders such as diabetes. All treatments have limited effectiveness. Given the evidence regarding psychological treatment for distress and disability in people with various chronic pain conditions, we were interested to investigate whether psychological treatments have any effects for those with chronic neuropathic pain.
Objectives
To assess the effects of psychological treatments on pain experience, disability, mood, and health‐care use in adults with chronic neuropathic pain.
Search methods
We searched for randomised controlled trials (RCTs) published in any language in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and PsycINFO, from database inception to March 2015.
Selection criteria
Full publications of RCTs on psychological interventions for neuropathic pain. Trials had to have lasted at least three months, had at least 20 participants in each arm at the end of treatment, and compared a psychological intervention with any active or inactive intervention.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
Two small studies (enrolling a total of 105 participants) met the inclusion criteria. One was a standard cognitive behavioural treatment (CBT) programme for 61 people with pain from spinal cord injury, followed up for three months, and compared with a waiting list. The other was weekly group psychotherapy for 44 people with burning mouth syndrome, compared with a daily placebo tablet. The overall risk of bias was high in both trials.
The CBT study assessed participants for pain, disability, mood, and quality of life, with improvement in treatment and control groups. However, there was no more improvement in the treatment group than in the control for any outcome, either post‐treatment or at follow‐up. The group psychotherapy study only assessed pain, classifying participants by pain severity. There is a lack of evidence on the efficacy and safety of psychological interventions for people with neuropathic pain.
Authors' conclusions
There is insufficient evidence of the efficacy and safety of psychological interventions for chronic neuropathic pain. The two available studies show no benefit of treatment over either waiting list or placebo control groups.
Plain language summary
Psychological treatments for chronic pain involving damage or disease to nerves responsible for pain
Many people experience pain from an injury or disease that goes away within three months, but for some people the pain continues. When the pain involves changes to nerves we call the pain 'neuropathic'. Although the condition is increasingly common, the treatments we have help only a few people. Following unsuccessful surgical or pharmacological treatment, people with chronic pain may be offered psychologically‐based rehabilitation to improve their quality of life. While we know that this can help people with other types of chronic pain, this treatment for neuropathic pain alone has received less research attention.
In this review, we were interested in finding out whether psychological treatments improve pain, distress, and disability in people with chronic neuropathic pain. We searched the academic literature to March 2015 and identified two randomised controlled trials (the gold standard design for clinical trials) on psychological interventions for chronic neuropathic pain. The two studies included 105 participants: one trial of 61 people with pain from spinal cord injury and the other of 44 people with burning mouth syndrome.
Our confidence in the results of the individual trials was limited by several potential biases in how they were conducted. We were not able to analyse the results of the two trials together because the experiences of people with spinal cord injury or burning mouth are too different from each other. On their own, the trials were too small for us to undertake any statistical analysis. However, neither trial found any clear benefit of treatment. We conclude that there is currently no evidence that will help practitioners and patients to decide whether to use these treatments. We discuss what studies are needed.
Background
Neuropathic pain is an increasingly prevalent problem. Given the limited effectiveness of existing therapies, it is appropriate to offer psychologically based treatments that aim to rehabilitate patients despite continuing pain or to reduce pain and its effects on everyday life. This review draws on new criteria for what constitutes reliable evidence in chronic pain, established specifically for reviews of drugs for neuropathic pain (Moore 2010). We have also used material from a recent review of psychological treatments for all chronic pain in adults when developing this review (Williams 2012).
Description of the condition
The 2011 International Association for the Study of Pain defines neuropathic pain as "pain arising as a direct consequence of a lesion or disease affecting the somatosensory system" (Haanpää 2011; Jensen 2011). Neuropathic pain can be a severe and complex consequence of nerve damage, which is often followed by changes in the central nervous system (Apkarian 2011; Moisset 2007; Tracey 2011). Many people with neuropathic pain conditions are significantly disabled, with moderate or severe pain for many years (Doth 2010; Smith 2012; Williams 2011).
In primary care in the UK, the estimated incidence of postherpetic neuralgia is 28 per 100,000 person‐years (95% confidence interval (CI 27 to 30), while this figure stands at 27 (95% CI 26 to 29) for trigeminal neuralgia, 0.8 (95% CI 0.6 to 1.1) for phantom limb pain and 21 (95% CI 20 to 22) for painful diabetic neuropathy (Hall 2008). Because of the small numbers of cases, estimates vary between studies. In a systematic review of studies published since 2000 Moore 2014 reported the overall prevalence of neuropathic pain (all types) to be about 7%, while Haanpää 2011 reported prevalence ranging from 3.3% to 8.2%.
Neuropathic pain is difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention (Kalso 2014; Moore 2013). Thus, practitioners are increasingly taking a multidisciplinary approach that combines pharmacological treatments with physical therapy, cognitive interventions, or both. Different drugs and drug combinations are used, with efficacy varying by condition (Kalso 2014). A minority of people experience at least a 50% reduction in pain intensity, with numbers needed to treat for an additional beneficial outcome (NNT) usually falling between 4 and 10 (Moore 2013). The only Cochrane review of non‐pharmacological interventions (Boldt 2014) found a single cognitive behavioural intervention (Heutink 2012).
In earlier reviews of all chronic pain except headache (Eccleston 2009; Williams 2012), we searched for all published RCTs of interventions described as psychological in nature, finding mainly trials of behavioural therapy (BT) or cognitive behavioural therapy (CBT). We excluded RCTs of interventions for headache for consistency with the previous reviews and because CBT for headache aims primarily to reduce the frequency, duration, and intensity of headache pain, rather than to rehabilitate despite ongoing pain (Eccleston 2009; Williams 2012). Readers are referred to other reviews specific to migraine and headache (Nestoriuc 2007; Nestoriuc 2008); Cochrane systematic reviews for headache are in progress (McGuire 2014). We also excluded Internet trials because they are the subject of an existing review (Eccleston 2014).
The Williams 2012 review found 42 trials, of which 35 provided data (N = 4788) for meta‐analysis. CBT showed small positive effects on disability and catastrophising, but not on pain or mood, when compared with active controls. CBT showed small to moderate effects on pain, disability, mood, and catastrophising when compared with treatment as usual or waiting list. Only a small effect on mood persisted at follow‐up. The only positive effect of BT was on catastrophising immediately after treatment, but there were relatively few behavioural therapy studies. The quality of trials and trial reporting is improving (Morley 2006; Williams 2012), although the quality of treatment remains variable (Williams 2012). Of the eight trials of mixed chronic pain conditions identified in that review, one identified 31% of participants with neuropathic pain, but it did not analyse this subgroup separately (Wetherell 2011); another trial described 10% of patients as having 'neurological' pain (Puder 1988).
Although combining multiple chronic pain conditions in our review made sense in terms of measuring the effect of psychological interventions, the proliferation of trials and the trend to improve participant homogeneity by selecting a single condition, such as fibromyalgia, suggests that psychological interventions for chronic pain would be best addressed by diagnostic group. This choice has the added advantage of making the results accessible to overviews that include other types of intervention, mainly pharmacological and physical. Given the ongoing Cochrane headache review (McGuire 2014) and the recent publication of a Cochrane review of CBT for fibromyalgia (Bernady 2013), we decided to focus this review on another important area: neuropathic pain.
Description of the intervention
A broad family of treatments fall under the umbrella term 'psychological'. In essence, these treatments are specifically designed to alter the psychological processes that contribute to pain, distress and disability (Eccleston 2011; Eccleston 2013; Morley 1999; Morley 2013). Specific theories relating to the aetiology of human behaviour normally underpin the design of psychological treatments, though some treatments have a more pragmatic basis in the observation and study of response to intervention (Eccleston 2013). In practice, a wide variety of interventions are used, and not all have been evaluated for their efficacy (Morley 2006; Morley 2013). The evidence base for psychological therapies stems mainly from studies of programmatic and protocolised treatments rooted in the behavioural or cognitive behavioural tradition of clinical psychology (Eccleston 2013). Practitioners may choose psychological therapies after orthodox treatments have failed, when the treatment goal shifts from removing or alleviating pain to managing pain and its myriad adverse consequences on quality of life (Lumley 2011; Morley 2006).
How the intervention might work
Most, but not all, psychological treatments consist of cognitive or behavioural therapies. A typical treatment protocol for cognitive behavioural therapy (CBT) uses methods aimed directly at assessing the thoughts and psychological biases associated with pain as well as the extent of avoiding unpleasant thoughts (Morley 1999). Behavioural methods focus on identifying behaviour that is contingent on pain or on events that suggest pain relief or comfort; interventions aim to reduce avoidance or persistence behaviour that impedes progress towards valued goals (Van Damme 2015). Most therapies involve education, and many are incorporated within larger treatment programmes involving physical, occupational, or vocational therapy (Morley 2006). Non‐CBT interventions may also be relevant to the extent that they are based on defined psychological theories and treatment methods with a foundation in the broader field of clinical psychology (Eccleston 2013).
Why it is important to do this review
Five of the 11 top‐ranking conditions for years lived with disability in 2010 were chronic painful conditions (Vos 2012), which are responsible for considerable loss of quality of life, loss of employment, and increased health costs (Moore 2014). Neuropathic pain is under‐recognised (see Description of the condition and Smith 2012), and some forms, such as from diabetic neuropathy and postsurgical chronic pain (often neuropathic in origin), are increasing in prevalence (Hall 2008). Neuropathic pain has a particularly severe impact on quality of life (Smith 2007), and its origins and persistence are difficult for patients to understand. For psychologically based pain management, it is not usual to distinguish between diagnoses; however, the predominant model of musculoskeletal pain may serve those with neuropathic pain rather poorly. It is time to disaggregate the compound review of psychologically based pain management for all chronic pain except headache (Williams 2012) and to instead review specific types of chronic pain, consistent with those of medical interventions. There is an increased need to evaluate psychological treatments in this field. The only existing review (Wetering 2010) is outside of Cochrane and of very poor quality (Eccleston 2010). However, there is a review of all non‐pharmacological interventions, including psychological interventions, for one neuropathic pain condition: spinal cord injury (Boldt 2014).
Objectives
To assess the effects of psychological treatments on pain experience, disability, mood, and healthcare use in adults with chronic neuropathic pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing a credible psychological treatment, or a compound treatment with primary psychological content, versus placebo, another active treatment, treatment as usual, or waiting list control. We judged a psychological treatment to be credible if it was based on an extant psychological model or framework, and if a qualified healthcare professional in psychology delivered or supervised it.
Studies were included if they:
were available as a full publication or report of an RCT;
had a design that considered a psychological treatment as an active treatment;
were published (or electronically pre‐published) in a peer‐reviewed scientific journal;
had 20 or more participants in each treatment arm at the end of the treatment or follow‐up assessment.
Types of participants
We included studies involving adult participants (≥18 years) who had been experiencing neuropathic pain for at least three months. They may have had a wide range of chronic neuropathic pain conditions, including:
painful diabetic neuropathy (PDN);
postherpetic neuralgia;
trigeminal neuralgia;
phantom limb pain;
postoperative or traumatic neuropathic pain;
complex regional pain syndrome (CRPS), Types I and II;
cancer‐related neuropathy;
human immunodeficiency virus (HIV) neuropathy;
spinal cord injury.
In case studies that included participants with more than one type of neuropathic pain, we planned to analyse results according to the primary condition.
Types of interventions
We included studies if at least one trial arm consisted of a psychological intervention and one comparator arm used a placebo, another active treatment, treatment as usual, or waiting list control. We define psychological intervention as using psychotherapeutic methods specifically designed to alter psychological processes believed to contribute to pain, distress, or disability (Eccleston 2013; Morley 1999; Morley 2013). We included methods underpinned by specific theories of the aetiology of human behaviour for which there is some evidence of efficacy in the broader field of clinical psychology (Eccleston 2013).
Types of outcome measures
We aimed to use outcomes in the domains of pain experience, disability, negative mood, and healthcare use as well as adverse events. We anticipated that studies would use a variety of outcome measures, with the majority of studies using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity, pain affect, or both. We were particularly interested in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). This project defined moderate benefit in the domain of pain experience as at least 30% pain relief over baseline and substantial benefit as at least 50% pain relief over baseline; at least half a standard deviation, or 1 point on a 0 to 10 mean of the seven pain interference items as measured by the Brief Pain Inventory (Cleeland 1994); and at least half a standard deviation or a change of at least 5 points for depression as measured by the Beck Depression Inventory (Beck 1961; Morley 2002).
Primary outcomes
Pain experience, as measured by a self‐rating of pain intensity or pain distress according to VAS or NRS.
Mood, as measured by a standard anxiety scale such as Spielberger State/Trait Anxiety Inventory (STAI; Spielberger 1983), standard depression scale such as Beck Depression Inventory (BDI; Beck 1961), or a standard distress scale such as the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983).
Disability or quality of life, as measured by the Brief Pain Inventory (BPI; Cleeland 1994) or the 36‐ or 12‐item Short Form Health Survey (SF‐36 or SF‐12; Medical Outcomes Study).
Adverse events and dropouts.
Secondary outcomes
Healthcare use (e.g. medication use, healthcare visits, healthcare procedures, hospital stays).
Search methods for identification of studies
We searched electronic databases, journal pre‐publication websites, and reference lists.
Electronic searches
We searched the following databases, with no restriction on language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via the CRSO to March 2015.
MEDLINE via Ovid 1946 to March 2015.
EMBASE via Ovid 1974 to March 2015.
PsycINFO via Ovid 1806 to March 2015.
We describe our search strategies in Appendix 1.
At least two review authors reviewed all abstracts, consulting the third review author when necessary to decide on inclusion or exclusion.
Searching other resources
We reviewed the bibliographies of identified RCTs and review articles, and we searched the USA National Institutes of Health clinical trial database (clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
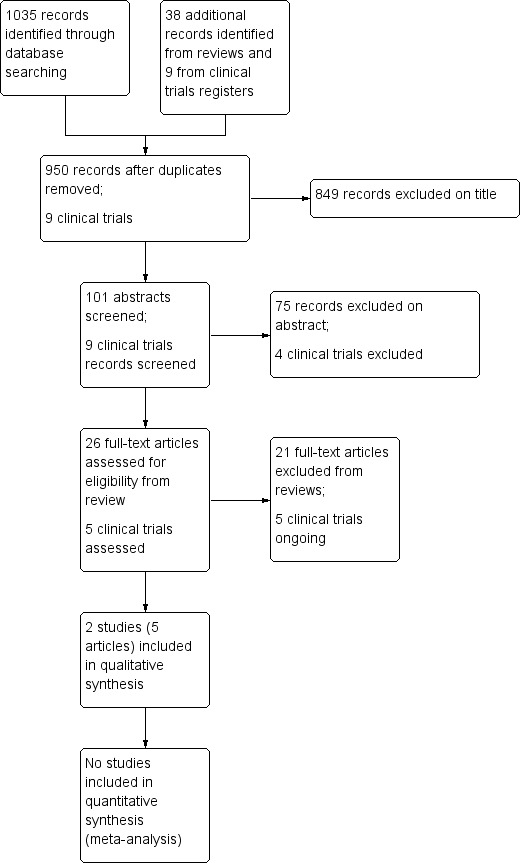
We determined eligibility by reading the abstract of each study identified in our search. We eliminated studies that clearly did not satisfy the inclusion criteria, and we obtained full copies of the remaining studies; two review authors (LH, AW) independently assessed the full text to determine eligibility, reaching agreement by discussion. We did not anonymise the studies before assessment. We created a PRISMA flow chart to describe the process (Figure 1).
1.
Study flow diagram.
Data extraction and management
Two review authors (LH, AW) independently extracted data using a standard form and checked for agreement before entering data into Review Manager (RevMan 2014). We included information about the pain condition, the number of participants treated, type of intervention, study design (type of control), study duration and follow‐up, outcome measures used, data for primary measure in each domain, attrition, and adverse events.
Assessment of risk of bias in included studies
We assessed risk of bias using the recommended Cochrane methods (Higgins 2011) and the five suggested 'Risk of bias' categories: random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Although we included the risk 'blinding participants and personnel', it is not possible to blind therapists or patients in trials of psychologically based treatment, so we planned to follow the procedure we used in Williams 2012 of applying a quality rating scale specifically designed for psychological interventions in pain (Yates 2005).
Measures of treatment effect
Where different trials used different instruments to measure an outcome domain, we planned to analyse the effect size for continuous outcomes by calculating the standardised mean differences (SMD) with 95% confidence intervals (CI). Where appropriate, we planned to apply categories of improvement as defined by the IMMPACT group (Dworkin 2008) and analyse dichotomously. For dichotomous outcomes, we planned to calculate the odds ratio (OR) with 95% CI.
Unit of analysis issues
For trials with more than one treatment arm, we planned to combine the arms provided that the treatments were sufficiently similar.
Dealing with missing data
For continuous variables we could not assign participants who were excluded from analysis; we used the data from the published reports. We distinguished between intention‐to‐treat (ITT) and per protocol (PP) analyses when judging quality.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by only combining studies that examined similar conditions. We expected to assess statistical heterogeneity using the I2 statistic, and where I2 was greater than 50%, we would discuss possible reasons.
Assessment of reporting biases
We searched relevant reviews and the reference sections of eligible trials and planned a funnel plot if there were sufficient studies.
Data synthesis
We planned to use a random‐effects model for meta‐analysis, assuming significant clinical heterogeneity. We also planned a summary of findings table. However, because data synthesis of included studies was not relevant, we instead present a narrative summary.
Subgroup analysis and investigation of heterogeneity
Ideally, we hoped to be able to perform subgroup analysis by type of neuropathic pain (specifically painful diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, phantom limb pain, postoperative or traumatic neuropathic pain, complex regional pain syndrome, cancer‐related neuropathy, human immunodeficiency virus (HIV) neuropathy, and spinal cord injury), but we anticipated too few data for this on the basis of previous reviews of chronic pain. We did not plan any other subgroup analyses. We planned to use the I2statistic to test for heterogeneity.
Sensitivity analysis
We did not plan a sensitivity analysis because we knew that the evidence base was too small to allow reliable analysis. We planned to examine details of dose escalation schedules in the unlikely event that this could provide a basis for a sensitivity analysis.
Results
Description of studies
Results of the search
Our database searches yielded 1035 records, which were reduced to 912 after removing duplicates. In addition, we handsearched the bibliographies of 60 reviews, three of which required translation from German and one from Japanese, and we identified 38 potentially eligible studies not previously identified. Of these 950 records, we excluded 849 based on title alone, leaving 101 abstracts to screen. Of these, we rejected 75 and obtained the full text of 26 (see Figure 1). After assessing these, we included two eligible studies (Heutink 2012; Miziara 2009) in five papers. In addition, we identified nine potentially relevant studies from the clinical trials registries, finding five relevant ongoing studies.
Included studies
Two studies met all of our inclusion criteria. Heutink 2012 consisted of a published protocol and separate short‐ and long‐term outcome and process papers. The intervention under study was 30 hours of CBT over 10 weeks for outpatients with neuropathic pain from spinal cord injury. A drug company funded the study, which took place in the Netherlands; two‐thirds of participants were male, with a mean age of 59. There were 61 participants at the start of the study, with 54 finishing treatment, but investigators were able to collect data from 59 at three‐month follow‐up, after which the comparator waiting list group entered treatment. A subsequent process study found some associations between changes in cognitive and practical strategies and improvements in pain and disability.
Johannsen 2016a reported a study of women who have >3 on a numeric rating scale, three months after breast cancer surgery. One hundred and twenty‐nine women were randomised to mindfulness based cognitive therapy or a waiting‐list control. Women in the treatment group received eight weekly sessions lasting two years in mindfulness, yoga, and cognitive exercises.
Miziara 2009 reported their results in a single paper for a trial of group psychotherapy sessions delivered once a week for three months for outpatients with burning mouth syndrome, conceptualised as a psychosomatic disorder. The study took place in Brazil; two‐thirds of participants were female, with a mean age of 55. The study involved 44 participants and had no dropouts; the comparison group took a single placebo tablet daily for a month. Investigators did not declare the source of funding.
Excluded studies
Of the 23 studies that we excluded after reviewing the full text, two are new to this update (Johannsen 2016; Pozeg 2017). There were two (non‐randomised) trials studying neuropathic pain: Edelson 1989 treated subjects with mixed neuropathic pain by hypnosis or CBT, and Perry 2010 treated subjects with neuropathic pain related to spinal cord injury with a multidisciplinary cognitive behavioural pain management programme.
Twelve studies did not assess psychological treatments. These included five of mirror therapy (Brodie 2007; Cacchio 2009a; Cacchio 2009b; Chan 2007; Michielsen 2011), three of graded motor imagery (Moseley 2004; Moseley 2005; Moseley 2006), one of Iyengar yoga (Garfinkel 1998), one of 'therapeutic touch' (Blankfield 2001), one virtual reality therapy (Pozeg 2017), and one mindfulness therapy (Johannsen 2016).
Although Wetering 2010 did include Jensen 2001 in their review, this study did not focus on neuropathic pain. Jensen 2009a mixed neuropathic and non‐neuropathic pain, and Kip 2014 did not ascertain neuropathic pain but estimated from pain descriptors that about 80% of their sample had it; the study objectives also included treatment for post‐traumatic stress disorder, for which they considered pain to be a secondary symptom. Two more excluded studies described related RCTs of psychological treatment in HIV‐positive people with neuropathic pain, but it was not clear that the neuropathic pain was chronic (lasting for more than three months), and we received no answers from the authors on this question (Davis 2004; Evans 2003). In any case, these two studies did not meet our criterion for a minimum of 20 participants per arm at the end of treatment, so we would have excluded them regardless. One further study of sciatic pain recruited acute pain patients (Hasenbring 1999).
We also excluded three other studies due to an insufficient number of participants: Jensen 2009b treated subjects with multiple sclerosis‐related pain using self‐hypnosis, Meize‐Grochowski 2012 treated subjects with postherpetic neuralgia by mindfulness meditation, and Rickard 2004 treated subjects with stump or phantom limb pain by hypnosis.
Ongoing studies
The trials register searches produced nine trials, of which five were potentially eligible for inclusion (NCT00829387; NCT00830011; NCT00731614; NCT02125006; NCT01884662). Three are completed, but the results are not yet available (NCT00829387; NCT00830011; NCT00731614).
Studies awaiting classification
There were no studies awaiting classification.
Risk of bias in included studies
We used the standard Cochrane methods for assessing risk of bias; because of the low number of eligible studies and their lack of amenability to meta‐analysis, we did not proceed with the Yates 2005 quality rating as planned (Figure 2, Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Neither study provided details on randomisation method nor information on allocation concealment, so we rated the bias for this domain as unclear.
Blinding
There was no information in either study about attempts to blind patients to allocation or to assess their expectations of benefit, and while Heutink 2012 used a waiting list control that would be unlikely to generate expectations of benefit to the active treatment, Miziara 2009 compared group psychotherapy, which might not have been very credible to patients as an intervention for pain, with a placebo tablet, which might have generated some expectation of benefit. However, neither study sampled participant expectations. Further, all assessment was by self‐report, whereas a blinded assessor should ideally have evaluated the outcomes under study.
Incomplete outcome data
Heutink 2012 used ITT analysis but did not provide details about how they generated missing values; Miziara 2009 reported no attrition.
Selective reporting
Both studies reported all the outcome measures listed in their Methods. No further investigation was possible because no record of the trial protocols was found.
Other potential sources of bias
Neither control condition (waiting list for Heutink 2012, a placebo tablet for Miziara 2009) shared any general characteristics with the active treatment arm (30 hours CBT for Heutink 2012, weekly group psychotherapy for 3 months for Miziara 2009) in terms of time, therapist contact, or group support.
Effects of interventions
It was not possible to combine any of the outcomes from the two included papers.
For descriptive purposes we used RevMan to calculate the fixed‐effect mean difference (MD). For the Heutink 2012 study, there were no immediate effects post‐treatment on pain intensity, disability, or anxiety. Pain intensity data had an MD of ‐2.00 (95% CI − 9.65 to 5.65; Analysis 1.1). Disability data had a MD of ‐ 6.20 (95% CI − 20.37 to 7.97; Analysis 1.2). Anxiety data had a MD of ‐0.10 (95% CI −1.99 to 1.79; Analysis 1.3). Investigators reported the same pattern of data showing no effects at follow‐up. Pain intensity data had a MD of 0.40 (95% CI − 7.69 to 8.49; Analysis 2.1). Disability data had a MD of ‐ 3.90 (95% CI − 17.79 to 9.99; Analysis 2.2). Anxiety data had a MD of 0.30 (95% CI − 1.63 to 2.23; Analysis 2.3).
1.1. Analysis.

Comparison 1: CBT versus control post‐treatment, Outcome 1: Pain intensity
1.2. Analysis.

Comparison 1: CBT versus control post‐treatment, Outcome 2: Pain disability
1.3. Analysis.

Comparison 1: CBT versus control post‐treatment, Outcome 3: Anxiety
2.1. Analysis.

Comparison 2: CBT versus control at follow up, Outcome 1: Pain intensity
2.2. Analysis.

Comparison 2: CBT versus control at follow up, Outcome 2: Pain disability
2.3. Analysis.

Comparison 2: CBT versus control at follow up, Outcome 3: Anxiety
For the Miziara 2009 study we were able to extract dichotomous data using the category of 'no worse than mild pain'. There was no effect of treatment compared to placebo in patients in this category at the end of treatment. The relative risk was 1.50 (95% CI 0.60 to 3.76; Analysis 3.1).
3.1. Analysis.

Comparison 3: Group psychotherapy versus placebo post‐treatment, Outcome 1: No worse than mild pain
There were no data on adverse events.
We can make no conclusion on the efficacy or safety of psychological treatments for neuropathic pain from these two small, different studies.
Discussion
Summary of main results
We found only two eligible studies, which we could not combine for meta‐analysis because they differed in treatment method, participants, and outcomes. Neither study provided evidence supporting psychological therapy for neuropathic pain. Group therapy for burning mouth syndrome was not effective in changing pain status, but the comparison group was not a credible comparison, being placebo designed for an oral pharmacological treatment not a psychotherapy treatment (Miziara 2009). The more conventional CBT for spinal cord injury pain did not mitigate pain either, nor (using our calculations) did it improve disability or anxiety (the other two primary outcomes) by the end of treatment or at three month follow‐up (Heutink 2012). Overall, we are unable to conclude anything from these two small, negative, and very different studies.
We excluded three further studies solely because there were fewer than 20 participants in each arm at the point of assessment: two used hypnosis −Jensen 2009b in people with multiple sclerosis‐related pain, and Rickard 2004 with stump or phantom limb pain) − while a third used mindfulness meditation in people with postherpetic neuralgia (Meize‐Grochowski 2012). These illustrate the diversity of methods and neuropathic pain populations in the wider field. Some of the excluded trials could be eligible for inclusion in other reviews of therapies aimed at reducing pain directly: mirror therapy, used predominantly for complex regional pain syndrome (CRPS); and hypnosis, often used for spinal cord injury or phantom pain.
Overall completeness and applicability of evidence
We found most but not all of the neuropathic pain groups we had anticipated among the included, excluded, and ongoing studies; we did not find studies in people experiencing postoperative pain or cancer‐related neuropathic pain. CRPS was treated most often with mirror therapy, motor imagery, or both, conceived and delivered mostly in non‐psychological frameworks (although Moseley came closer to psychological methods than others; Moseley 2004; Moseley 2005; Moseley 2006). We also included a study of burning mouth syndrome, a diagnosis we had not anticipated among neuropathic pain groups. Although chronic pain is a significant problem in many of the people falling under our inclusion criteria, some of those people may receive all or most of their care within specialist services defined by the disease, such as diabetes or HIV, rather than in specialist pain services, where psychologically based treatment is more often available. Treating physicians may refer patients to specialist pain services only when they have no specific diagnosis, which is not the case for people suffering from neuropathic pain, or when they attribute patients' distress and disability to psychological factors. We do not endorse this way of implicitly dividing pain‐related problems into inaccurate categories of 'organic' and 'psychological', because it is typically based on flawed science (Crombez 2009), but we recognise that it continues to guide referral in many health services.
While one of the studies reviewed used a mainstream chronic pain intervention, CBT, the other used an unconventional therapy drawn from mental health interventions and delivered within that framework, identifying burning mouth as a 'non‐organic' pain problem on the basis of the lack of physical findings. Among the three studies excluded on the basis of their number of participants, two used hypnosis/self‐hypnosis. Hypnosis is relatively rare in the treatment of low back or mixed chronic pain where the target of therapy is reactivation despite chronic pain; however, it is more common in neuropathic pain where the target of therapy is pain itself (Williams 2012).
The range of outcomes was adequate in Heutink 2012 but not in Miziara 2009; neither study reported adverse events, nor did they assess effects on healthcare use, our secondary outcome.
Quality of the evidence
There are no estimates of effect so a Grading of Recommendations Assessment, Development and Evaluation (GRADE) judgement of the quality of the evidence was not relevant, except by definition the quality of the evidence base was 'low' due to single small studies with extant biases.
Neither trial had sufficient information to determine selection bias, nor was it feasible (for a psychologically based trial) to blind therapists to treatment group. Both trials assessed by self‐report, so they did not introduce detection bias but neither did they use third party or objective measures, such as function. Both avoided attrition bias and reporting bias, although outcomes were narrow, particularly for Miziara 2009, and both failed to include adverse events.
Potential biases in the review process
There were no obvious sources of bias in our search, but we had to assess a number full text reports to ascertain their eligibility for this review, as many abstracts did not provide sufficient information to make a firm determination. This may have introduced a bias in the representation of studies in the excluded category. We did not have difficulty applying criteria: we decided that mirror therapy and motor imagery did not constitute psychological treatment but rather education followed by intensive repeated exercises supervised by a physiotherapist, with no involvement of a psychologist. There were no discrepancies in data extraction that required the involvement of the third author.
Agreements and disagreements with other studies or reviews
Our study disagrees with the only other review we found, Wetering 2010, which included 14 studies but only three RCTs (Jensen 2001; Brodie 2007; Evans 2003). We excluded all of these: Brodie 2007 because it studied mirror therapy, with no psychological content; Evans 2003 because it focused on acute or sub‐acute pain, and had fewer than 20 participants per arm; and Jensen 2001, by far the largest of the three, because the paper makes it clear that participants were experiencing musculoskeletal back and neck pain, not neuropathic pain (Wetering 2010 apparently included the study on the description of the participants as having 'spinal pain'). Wetering 2010 reported a conclusion of the positive and promising effects of psychological interventions for neuropathic pain. We believe this was based on flawed methods.
A Cochrane review of all non‐pharmacological interventions for chronic pain in people with spinal cord injury included a comparison of cognitive behavioural therapy, also analysing Heutink 2012. Our results were in agreement (Boldt 2014).
Our results, such as they are, compare poorly with those from psychologically based interventions for mixed chronic pain (Williams 2012), which reported clear benefits for distress and disability in participants. The Heutink 2012 trial was certainly comparable with many of the trials included in that systematic review, but its negative findings were not offset by positive results from other trials in the present review. Our results also fall short of the general conclusions of numerous reviews of predominantly pharmacological treatments for specific neuropathic pain conditions, which find unambiguous—if not impressive—benefits for at least some patients.
Authors' conclusions
Implications for practice.
The data recovered in this search and analysis do not provide any meaningful implications for individual practice or policy. Rather, our review demonstrates clearly the lack of direct evidence for or against the effectiveness of psychological interventions in altering the experience of pain, disability and impaired mood associated with chronic neuropathic pain. Thus, for now, there is still a large and growing population of people disabled by chronic neuropathic pain from diabetic neuropathy, cancer treatments, and other causes, who have limited options for treatment.
Implications for research.
There is a worrying lack of trial data in this area of need. We need RCTs of specific psychological treatments tailored for neuropathic pain populations. Good candidate conditions for effective trial design and delivery are
Peripheral Diabetic Neuropathy. An optimal treatment could be cognitive and behavioural methods aiming to increase activity and participation in society, with specific content to address mood disorder, sleep problems, and increased pain on activity. This treatment could be one component in the management of complications from diabetes.
HIV neuropathy, treated with cognitive and behavioural methods focused on social emotional functioning, compared with no treatment, aiming to address beliefs about pain, low mood, and participation in society, including work. This may be a difficult group to engage and the delivery of rehabilitative treatment might be more effective outside direct health service settings (Davis 2004).
Postherpetic neuralgia, treated with cognitive behavioural therapy incorporating some of the exposure methods used with CRPS, compared with nonspecific support and medication. Outcomes of interest might include better management of provoked pain, more engagement in valued activities, and less anxiety and depression concerning the pain (Daniel 2008).
All trials would need to be sufficiently powered (200 patients or more) and involve service users in their design, which would include acceptable comparison groups and options for supplementary content using electronic media. Placebo comparison groups should control for patient trust and belief in therapy outcome (i.e. be equally credible), time of exposure to therapy, and general educational content. Primary endpoints should follow other neuropathic pain treatments in terms of pain, but investigators should define potential improvements in distress, disability, quality of life, pain, sleep, and acceptability/adherence in advance, also routinely reporting adverse events. See Morley 2013 for further discussion.
More generally, and to facilitate future reviews, it would be helpful if abstracts contained essential information about what treatment is used, for which pain problem, in how many participants, and what outcomes are reported, as well as whether the study was randomised and what the comparison conditions were. Reviewers can assess the studies that include this information in the abstract more effectively than those that do not.
History
Protocol first published: Issue 8, 2014 Review first published: Issue 10, 2015
| Date | Event | Description |
|---|---|---|
| 22 June 2020 | Review declared as stable | See Published notes. |
| 30 September 2019 | Amended | Clarification added to Declarations of interest. |
| 13 November 2018 | Review declared as stable | See Published notes |
| 12 November 2018 | Amended | See Published notes |
| 26 September 2017 | Review declared as stable | See Published notes. |
Notes
2018
A restricted search in June 2018 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised for two years following discussion with the authors and editors. If appropriate, we will update the review earlier if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
2020
We performed another restricted search in May 2020 which did not identify any potentially relevant studies likely to change the conclusions. This is not an active area of research and so this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Thanks to Clare Daniel for discussing experience of psychological treatment of people with specific forms of neuropathic pain.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (CRSO)
MESH DESCRIPTOR Psychotherapy EXPLODE ALL TREES
MESH DESCRIPTOR Behavior Therapy
MESH DESCRIPTOR cognitive therapy EXPLODE ALL TREES
MESH DESCRIPTOR Biofeedback, Psychology EXPLODE ALL TREES
( behavio?r therap*):TI,AB,KY
( cognitive therap*):TI,AB,KY
((relax* near3 therap*)):TI,AB,KY
((relax* near3 technique*)):TI,AB,KY
meditat*:TI,AB,KY
psychotherap*:TI,AB,KY
((psychological near2 (treatment* or therap*))):TI,AB,KY
("group therapy"):TI,AB,KY
("self‐regulation training"):TI,AB,KY
("coping skill*"):TI,AB,KY
("pain‐related thought*"):TI,AB,KY
((behavio?r* near3 rehabilitat*)):TI,AB,KY
((psychoeducation near2 group*)):TI,AB,KY
((psycho‐education near2 group*)):TI,AB,KY
(("mind and body relaxation technique*" or "mind‐body relaxation technique*")):TI,AB,KY
MESH DESCRIPTOR mind‐body therapies EXPLODE ALL TREES
MESH DESCRIPTOR relaxation therapy
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES
MESH DESCRIPTOR Chronic Pain
((neuralgia* or neurodynia)):TI,AB,KY
(((neuropathic or nerve*) near3 pain*)):TI,AB,KY
#23 OR #24 OR #25 OR #26
#22 AND #27
MEDLINE (OVID)
1 exp Psychotherapy/ 2 behavior therapy/ or cognitive therapy/ 3 exp Biofeedback, Psychology/ 4 behavio#r therap*.tw. 5 cognitive therap*.tw. 6 (relax* adj3 technique*).tw. 7 (relax* adj3 (technique* or therap*)).tw. 8 meditat*.tw. 9 psychotherap*.tw. 10 (psychological adj2 (treatment* or therap*)).tw. 11 "group therapy".tw. 12 "self‐regulation training".tw. 13 "coping skill*".tw. 14 "pain‐related thought*".tw. 15 (behavio#r* adj3 rehabilitat*).tw. 16 (psychoeducation adj2 group*).tw. 17 (psycho‐education adj2 group*).tw. 18 ("mind and body relaxation technique*" or "mind‐body relaxation technique*").tw. 19 exp mind‐body therapies/ or relaxation therapy/ 20 or/1‐19 21 exp Neuralgia/ 22 Chronic Pain/ 23 (neuralgia* or neurodynia).tw. 24 ((neuropathic or nerve*) adj3 pain*).tw 25 or/21‐24 26 20 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomized.ab. 30 placebo.ab. 31 drug therapy.fs. 32 randomly.ab. 33 trial.ab. 34 or/27‐33 35 exp animals/ not humans.sh. 36 34 not 35 37 26 and 36
EMBASE (OVID)
1. exp Psychotherapy/
2. behavior therapy/ or cognitive therapy/
3. exp Biofeedback, Psychology/
4. behavio#r therap*.tw.
5. cognitive therap*.tw.
6. (relax* adj3 technique*).tw.
7. (relax* adj3 (technique* or therap*)).tw.
8. meditat*.tw.
9. psychotherap*.tw.
10. (psychological adj2 (treatment* or therap*)).tw.
11. "group therapy".tw.
12. "self‐regulation training".tw.
13. "coping skill*".tw.
14. "pain‐related thought*".tw.
15. (behavio#r* adj3 rehabilitat*).tw.
16. (psychoeducation adj2 group*).tw.
17. (psycho‐education adj2 group*).tw.
18. ("mind and body relaxation technique*" or "mind‐body relaxation technique*").tw.
19. exp mind‐body therapies/ or relaxation therapy/
20. or/1‐19
21. exp Neuralgia/
22. Chronic Pain/
23. (neuralgia* or neurodynia).tw.
24. ((neuropathic or nerve*) adj3 pain*).tw.
25. or/21‐24
26. 20 and 25
27. random$.tw.
28. factorial$.tw.
29. crossover$.tw.
30. cross over$.tw.
31. cross‐over$.tw.
32. placebo$.tw.
33. (doubl$ adj blind$).tw.
34. (singl$ adj blind$).tw.
35. assign$.tw.
36. allocat$.tw.
37. volunteer$.tw.
38. Crossover Procedure/
39. double‐blind procedure.tw.
40. Randomized Controlled Trial/
41. Single Blind Procedure/
42. or/27‐41
43. (animal/ or nonhuman/) not human/
44. 42 not 43
45. 26 and 44
PsycINFO (OVID)
1. exp Psychotherapy/
2. behavior therapy/ or cognitive therapy/
3. exp Biofeedback/
4. behavio#r therap*.tw.
5. cognitive therap*.tw.
6. (relax* adj3 technique*).tw.
7. (relax* adj3 (technique* or therap*)).tw.
8. meditat*.tw.
9. psychotherap*.tw.
10. (psychological adj2 (treatment* or therap*)).tw.
11. "group therapy".tw.
12. "self‐regulation training".tw.
13. "coping skill*".tw.
14. "pain‐related thought*".tw.
15. (behavio#r* adj3 rehabilitat*).tw.
16. (psychoeducation adj2 group*).tw.
17. (psycho‐education adj2 group*).tw.
18. ("mind and body relaxation technique*" or "mind‐body relaxation technique*").tw.
19. exp mind‐body therapies/ or relaxation therapy/
20. or/1‐19
21. exp Neuralgia/
22. Chronic Pain/
23. (neuralgia* or neurodynia).tw.
24. ((neuropathic or nerve*) adj3 pain*).tw.
25. or/21‐24
26. 20 and 25
27. clinical trials/
28. (randomis* or randomiz*).tw.
29. (random$ adj3 (allocat$ or assign$)).tw.
30. ((clinic$ or control$) adj trial$).tw.
31. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
32. (crossover$ or "cross over$").tw.
33. random sampling/
34. Experiment Controls/
35. Placebo/
36. placebo$.tw.
37. exp program evaluation/
38. treatment effectiveness evaluation/
39. ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw.
40. or/27‐39
41. 26 and 40
Data and analyses
Comparison 1. CBT versus control post‐treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain intensity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Pain disability | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Anxiety | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. CBT versus control at follow up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain intensity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Pain disability | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 Anxiety | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Group psychotherapy versus placebo post‐treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 No worse than mild pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Heutink 2012.
| Study characteristics | ||
| Methods | RCT, treatment versus waiting list, multicentre trial | |
| Participants |
Neuropathic Pain condition: NP after SCI, recruited from Dutch rehabilitation centres at least 1 year after first discharge from inpatient rehabilitation; minimum 40/100 pain score over last week; median time since SCI 5.4 years; NP for at least 6 months; median time in pain, 4.5 years Number of participants: 61 at start of treatment, 54 at end of treatment, but data for 59 at follow‐up Mean age (SD): 59 (± 11) Sex: 39 men; 22 women |
|
| Interventions | Cognitive behavioural therapy versus waiting list; 10 sessions of 3 hours over 10 weeks = 30 hours. Participants were randomly assigned to either: Experimental arm: "educational, cognitive and behavioral elements" with detailed protocol and one day's training Duration: 10 sessions of 3 h over 10 weeks = 30 h Treatment protocol: refer to biopsychosocial model and model linking events, beliefs, and consequences Therapists: psychologist and physiotherapist in 3 centres, nurse‐practitioner and physiotherapist in 1 centre Comparator arm: waiting list, after which invited to join treatment programme |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Timepoints for assessment: baseline, end of treatment (3 months), 3‐month follow‐up |
|
| Notes | Data available for all outcomes, all 3 time points Study period: unknown but probably in 2010 Country: Netherlands Funding source: unrestricted grant from Pfizer Declarations of interest among the primary researchers: no competing interests Dutch Trial Register NTR1580 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated to the intervention group or to the waiting list control group within each participating rehabilitation center." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment all by self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis: no information on how investigators generated data for missing participants ‐ EF: shouldn't this be high? |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No further information on therapist qualifications; one day training and detailed protocol, and one of authors attended 2 sessions at each of 4 centres to ensure adherence to protocol No information on therapist allegiance |
Miziara 2009.
| Study characteristics | ||
| Methods | RCT, treatment versus daily placebo medication | |
| Participants |
Neuropathic Pain condition: Burning mouth syndrome, no identifiable organic cause Number of participants: 44 (recruited from 64 consecutive outpatients); no dropouts Mean age (SD): 55 (± 7) Sex: 15 men; 29 women |
|
| Interventions | Group psychotherapy, 1 session per week for 3 months Participants were randomly assigned to either: Experimental arm: 24 participants, psychotherapy sessions in groups of 4 Duration: once weekly for 3 months Treatment protocol: no information Therapist: psychologist Comparator arm: 20 participants Duration: 30 days Treatment protocol: one placebo capsule per day Therapist: none No data on adherence |
|
| Outcomes |
Pain: short‐form McGill Pain Questionnaire PPI, classified as improved versus not improved, and in 5 groups at assessment (no pain, mild, discomforting, distressing, horrible) Timepoints for assessment: before randomisation and at end of treatment (3 months for treatment group, 1 month for control group) |
|
| Notes |
Study period: 2002‐2007 Country: Brazil Funding source: none declared Declarations of interest among the primary researchers: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The other 44 patients with primary BMS were randomized and distributed into two groups." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment by self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Single outcome reported |
| Other bias | Unclear risk | No information on therapist training, instructions with placebo, therapist allegiance, or treatment fidelity |
BMS: burning mouth syndrome; HADS: Hospital anxiety and depression scale; NP: neuropathic pain; PPI: present pain intensity;RCT: randomised controlled trial; SCI: spinal cord injury.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blankfield 2001 | Not a psychological intervention (therapeutic touch works by 'energy exchange') |
| Brodie 2007 | Not a psychological intervention (mirror therapy) |
| Cacchio 2009a | Not a psychological intervention (mirror therapy) |
| Cacchio 2009b | Not a psychological intervention (mirror therapy) |
| Chan 2007 | Not a psychological intervention (mirror therapy) |
| Davis 2004 | N < 20 at end of treatment Unclear if NP > 3 months |
| Edelson 1989 | Not RCT |
| Evans 2003 | N < 20 at end of treatment Unclear if NP > 3 months |
| Garfinkel 1998 | Not a psychological intervention (Iyengar yoga) |
| Hasenbring 1999 | N < 20 at end of treatment Unclear if NP > 3 months |
| Jensen 2001 | Not NP (spinal pain) |
| Jensen 2009a | Exclusions: N < 20 at start of treatment Not clear if all NP |
| Jensen 2009b | N < 20 |
| Johannsen 2016 | Insufficient psychotherapeutic content |
| Kip 2014 | 80% thought to be NP by pain descriptors Treatment aimed at PTSD not pain: pain was secondary outcome |
| Meize‐Grochowski 2012 | Exclusions: N < 20 Data not yet published |
| Michielsen 2011 | Not a psychological intervention (mirror therapy) |
| Moseley 2004 | Not a psychological intervention (graded motor imagery) |
| Moseley 2005 | Not a psychological intervention (graded motor imagery) |
| Moseley 2006 | Not a psychological intervention (graded motor imagery) |
| Perry 2010 | Not RCT |
| Pozeg 2017 | |
| Rickard 2004 | N < 20 |
NP: neuropathic pain; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT00731614.
| Study name | Cognitive behavior therapy (CBT) and mirror training for phantom limb pain |
| Methods | RCT |
| Participants | Phantom limb pain |
| Interventions | CBT + mirror therapy versus supportive care |
| Outcomes | Phantom Limb Pain Questionnaire SF‐12 |
| Starting date | November 2008 |
| Contact information | John McQuaid: John.McQuaid@va.gov |
| Notes | NCT00731614. Closed December 2012. Results not expected to be published until late 2015. |
NCT00829387.
| Study name | Cognitive behavioral therapy for diabetic neuropathic pain |
| Methods | RCT |
| Participants | Diabetic peripheral NP |
| Interventions | Brief CBT + pharmacotherapy versus education + pharmacotherapy |
| Outcomes | Pain (NRS); pain interference (MPI) |
| Starting date | Completed but no results |
| Contact information | Alicia Heapy: alicia.heapy@va.gov |
| Notes | NCT00829387 |
NCT00830011.
| Study name | Cognitive behavioral therapy for painful diabetic neuropathy |
| Methods | RCT |
| Participants | Diabetic NP of lower limb(s) |
| Interventions | CBT + standard medical care versus standard medical care |
| Outcomes | Pain intensity (NRS); pain‐related disability (MPI) |
| Starting date | 2004‐2011 |
| Contact information | Robert Kerns: robert.kerns@va.gov |
| Notes | NCT00830011 completed but not yet published |
NCT01884662.
| Study name | Virtual walking for neuropathic pain in spinal cord injury |
| Methods | RCT |
| Participants | People with traumatic spinal cord injury with neuropathic pain |
| Interventions | Virtual reality walking versus tape of wheeling (wheelchair) |
| Outcomes | Change in pain (NRS); change in pain interference (NRS); change in neuropathic pain (NPS); cortical somatosensory plasticity (fMRI) |
| Starting date | 2012‐2016 |
| Contact information | John S Richards, University of Alabama at Birmingham |
| Notes | NCT01884662 |
NCT02125006.
| Study name | The effect of an inter‐disciplinary program, including MBSR, in breast cancer survivors with chronic neuropathic pain (in depth) |
| Methods | RCT |
| Participants | Breast cancer survivors |
| Interventions | Medical treatment and MSBR versus waitlist |
| Outcomes | Pain interference (BPI); pain intensity (NPSI); pain severity (BPI); mood (POMS); depression (PHQ); catastrophising (PCS); mindfulness; stress; quality of life (SF‐12); cortisol; immune function; white matter integrity; brain volume; patient global impression of change |
| Starting date | 2013‐2016 |
| Contact information | Patricia Poulin, Ottawa Hospital Research Institute |
| Notes | NCT02125006 |
BPI: brief pain inventory; CBT: cognitive behavioural therapy;fMRI: functional magnetic resonance imaging; MBSR: mindfulness‐based stress reduction;MPI: multidimensional pain inventory; NP: neuropathic pain; NPS: neuropathic pain scale; NPSI: neuropathic pain symptom inventory; NRS: numerical rating scale; PCS: pain catastrophising scale; PHQ: patient health questionnaire; POMS: profile of mood states; RCT: randomised controlled trial; SF‐12: 12‐item Short Form Health Survey.
Differences between protocol and review
We used a fixed‐effects rather than a random‐effects model to extract an effect size from the single study in the analysis; likewise, we presented mean rather than standardised mean difference.
We reported a relative risk rather than an odds ratio for the single study in the analysis that offered dichotomous data.
We dropped the adverse events outcome in order to specify a single aim.
Because of the low number of eligible studies and their lack of amenability to meta‐analysis, we did not proceed with the Yates 2005 scale as planned.
There is no 'Summary of findings' table as there are insufficient data to create one.
Contributions of authors
CE directed the project and is responsible for updates. CE, LH, and AW all contributed to conceptualising and authoring the review.
Sources of support
Internal sources
None, Other
External sources
-
The National Institute for Health Research (NIHR), UK
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
Declarations of interest
Christopher Eccleston (CE) receives funding support from the UK National Institute for Health Research for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain. This title is part of this research programme. Since CE is an author as well as the PaPaS Co‐ordinating Editor at the time of writing, we acknowledge the input of Andrew Moore who acted as Sign Off Editor for this review. CE had no input into the editorial decisions or processes for this review.
Leslie Hearn: none known.
Amanda Williams: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Heutink 2012 {published data only}
- Heutink M, Post MW, Luthart P, Schuitemaker M, Slangen S, Sweers J, et al. Long-term outcomes of a multidisciplinary cognitive-behavioural programme for coping with chronic neuropathic spinal cord injury pain. Journal of Rehabilitation Medicine 2014;46(6):540-45. [DOI] [PubMed] [Google Scholar]
- Heutink M, Post MWM, Bongers-Janssen HMH, Dijkstra CA, Snoek GJ, Spijkerman DCM, et al. The CONNECSI trial: results of a randomized controlled trial of a multidisciplinary cognitive-behavioral program for coping with chronic neuropathic pain after spinal cord injury. Pain 2012;153(1):120-8. [DOI] [PubMed] [Google Scholar]
- Heutink M, Post MWM, Luthart P, Pfennings LEMA, Dijkstra CA, Lindeman E. A multidisciplinary cognitive behavioural programme for coping with chronic neuropathic pain following spinal cord injury: the protocol of the CONNECSI trial. BMC Neurology 2010;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutink M, Post MWM, Overdulve CW, Pfennings LEMA, Van de Vis W, Vrijens NLH, et al. Which pain coping strategies and cognitions are associated with outcomes of a cognitive behavioral intervention for neuropathic pain after spinal cord injury? Topics in Spinal Cord Injury & Rehabilitation 2013;19(4):330-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miziara 2009 {published data only}
- Miziara ID, Filho BCA, Oliveira R, Dos Santos RMR. Group psychotherapy: an additional approach to burning mouth syndrome. Journal of Psychosomatic Research 2009;67(5):443-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blankfield 2001 {published data only}
- Blankfield RP, Sulzmann C, Fradley LG, Tapolyai AA, Zyzanski SJ. Therapeutic touch in the treatment of carpal tunnel syndrome. Journal of the American Board of Family Practice 2001;14(5):335-42. [PubMed] [Google Scholar]
Brodie 2007 {published data only}
- Brodie EE, Whyte A, Niven CA. Analgesia through the looking glass? A randomized controlled trial investigating the effect of viewing a 'virtual' limb upon phantom limb pain, sensation and movement. European Journal of Pain 2007;11(4):428-36. [DOI] [PubMed] [Google Scholar]
Cacchio 2009a {published data only}
- Cacchio A, De Blasis E, De Blasis V, Santilli V, Spacca G. Mirror therapy in complex regional pain syndrome Type 1 of the upper limb in stroke patients. Neurorehabilitation and Neural Repair 2009;23(8):792-9. [DOI] [PubMed] [Google Scholar]
Cacchio 2009b {published data only}
- Cacchio A, De Blasis E, Necozione S, di Orio F, Santilli V. Mirror therapy for chronic complex regional pain syndrome type 1 and stroke. New England Journal of Medicine 2009;361(6):634-6. [DOI] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. New England Journal of Medicine 2007;357(21):2206-7. [DOI] [PubMed] [Google Scholar]
Davis 2004 {published data only}
- Davis L, Evans S, Fishman B, Haley A, Spielman LA. Predictors of attrition in HIV-positive subjects with peripheral neuropathic pain. AIDS Care: Psychological and Sociomedical Aspects of AIDS/HIV 2004;16(3):395-402. [DOI] [PubMed] [Google Scholar]
Edelson 1989 {published data only}
- Edelson J, Fitzpatrick JL. A comparison of cognitive-behavioral and hypnotic treatments of chronic pain. Journal of Clinical Psychology 1989;45(2):316-23. [DOI] [PubMed] [Google Scholar]
Evans 2003 {published data only}
- Evans S, Fishman B, Spielman L, Haley A. Randomized trial of cognitive behavior therapy versus supportive psychotherapy for HIV-related peripheral neuropathic pain. Psychosomatics 2003;44(1):44-50. [DOI] [PubMed] [Google Scholar]
Garfinkel 1998 {published data only}
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR. Yoga-based intervention for carpal tunnel syndrome. JAMA 1998;280(18):1601-3. [DOI] [PubMed] [Google Scholar]
Hasenbring 1999 {published data only}
- Hasenbring M, Ulrich HW, Hartmann M, Soyka D. The efficacy of a risk factor-based cognitive behavioral intervention and electromyographic biofeedback in patients with acute sciatic pain. Spine 1999;24(23):2525-35. [DOI] [PubMed] [Google Scholar]
Jensen 2001 {published data only}
- Jensen IB, Bergström G, Ljungquist T, Bodin L, Nygren ÅL. A randomized controlled component analysis of a behavioral medicine rehabilitation program for chronic spinal pain: are the effects dependent on gender? Pain 2001;91(1-2):65-78. [DOI] [PubMed] [Google Scholar]
Jensen 2009a {published data only}
- Jensen MP, Barber J, Romano JM, Hamley MA, Raichle KA, Molton IR, et al. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. International Journal of Clinical and Experimental Hypnosis 2009;57(3):239-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2009b {published data only}
- Jensen MP, Barber J, Romano JM, Molton IR, Raichle KA, Osborne TL, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. International Journal of Clinical and Experimental Hypnosis 2009;57(2):198-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johannsen 2016 {published data only}
- Johannsen M, O'Connor M, O'Toole MS, Jensen AB, Højris I, Zachariae R. Efficacy of mindfulness-based cognitive therapy on late post-treatment pain in women treated for primary breast cancer: a randomized controlled trial. Journal of Clinical Oncology 2016;34(28):3390-9. [DOI] [PubMed] [Google Scholar]
Kip 2014 {published data only}
- Kip KE, Rosenzweig L, Hernandez DF, Shuman A, Diamond DM, Girling SA, et al. Accelerated Resolution Therapy for treatment of pain secondary to symptoms of combat-related posttraumatic stress disorder. European Journal of Psychotraumatology 2014;5:doi: 10.3402/ejpt.v5.24066. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meize‐Grochowski 2012 {published data only}
- Meize-Grochowski R, Prasad A, Murray-Krezan C, Schrader R, DuVal M, Smith B, et al. Mindfulness meditation in community dwelling older adults with postherpetic neuralgia (Poster abstract). BMC Complementary and Alternative Medicine 2012;12(Suppl 1):139. [Google Scholar]
Michielsen 2011 {published data only}
- Michielsen ME, Selles RW, Van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(3):223-33. [DOI] [PubMed] [Google Scholar]
Moseley 2004 {published data only}
- Moseley GL. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 2004;108(1-2):192-8. [DOI] [PubMed] [Google Scholar]
Moseley 2005 {published data only}
- Moseley GL. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain 2005;114(1-2):54-61. [DOI] [PubMed] [Google Scholar]
Moseley 2006 {published data only}
- Moseley GL. Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology 2006;67(12):2129-34. [DOI] [PubMed] [Google Scholar]
Perry 2010 {published data only}
- Perry KN, Nicholas MK, Middleton JW. Comparison of a pain management program with usual care in a pain management center for people with spinal cord injury-related chronic pain. Clinical Journal of Pain 2010;26(3):206-16. [DOI] [PubMed] [Google Scholar]
Pozeg 2017 {published data only}
- Pozeg P, Palluel E, Ronchi R, Solcà M, Al-Khodairy AW, Jordan X, et al. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology 2017;89(18):1894-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rickard 2004 {published data only}
- Rickard JA. Effects of hypnosis in the treatment of residual stump pain and phantom limb pain [PhD thesis]. Pullman (WA): Washington State University, 2004. [Google Scholar]
References to ongoing studies
NCT00731614 {published data only}
- NCT00731614. Cognitive behavior therapy (CBT) and mirror training for phantom limb pain. http://clinicaltrials.gov/show/NCT00731614 (accessed 23 September 2015).
NCT00829387 {published data only}
- NCT00829387. Cognitive behavioral therapy for diabetic neuropathic pain. https://clinicaltrials.gov/ct2/show/NCT00829387 (accessed 23 September 2015).
NCT00830011 {published data only}
- NCT00830011. Cognitive behavioral therapy for painful diabetic neuropathy. https://clinicaltrials.gov/ct2/show/NCT00830011 (accessed 23 September 2015).
NCT01884662 {published data only}
- NCT01884662. Virtual walking for neuropathic pain in spinal cord injury. https://clinicaltrials.gov/ct2/show/NCT01884662 (accessed 23 September 2015).
NCT02125006 {published data only}
- NCT02125006. The effect of an inter-disciplinary program, including MBSR, in breast cancer survivors with chronic neuropathic pain (in depth). https://clinicaltrials.gov/ct2/show/NCT02125006 (accessed 23 September 2015).
Additional references
Apkarian 2011
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152(3 Suppl):S49-64. [DOI: 10.1016/j.pain.2010.11.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock N, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
Bernady 2013
- Bernardy K, Klose P, Busch AJ, Choy EHS, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD009796. [DOI: 10.1002/14651858.CD009796.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boldt 2014
- Boldt I, Eriks-Hoogland I, Brinkhof MWG, De Bie R, Joggi D, Von Elm E. Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD009177. [DOI: 10.1002/14651858.CD009177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleeland 1994
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine 1994;23(2):129-38. [PubMed] [Google Scholar]
Crombez 2009
- Crombez G, Beirens K, Van Damme S, Eccleston C, Fontaine J. The unbearable lightness of somatisation: a systematic review of the concept of somatisation in empirical studies of pain. Pain 2009;145(1-2):31-5. [DOI] [PubMed] [Google Scholar]
Daniel 2008
- Daniel HC, Narewska J, Serpell M, Hoggart B, Johnson R, Rice ASC. Comparison of psychological and physical function in neuropathic pain and nociceptive pain: implications for cognitive behavioral pain management programs. European Journal of Pain 2008;12(6):731-41. [DOI] [PubMed] [Google Scholar]
Doth 2010
- Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain 2010;149(2):338-44. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Eccleston 2009
- Eccleston C, Williams ACDC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007407. [DOI: 10.1002/14651858.CD007407] [DOI] [PubMed] [Google Scholar]
Eccleston 2010
- Eccleston C, Moore RA, Derry S, Bell RF, McQuay H. Improving the quality and reporting of systematic reviews. European Journal of Pain 2010;14(7):667-9. [DOI] [PubMed] [Google Scholar]
Eccleston 2011
- Eccleston C. A normal psychology of chronic pain. The Psychologist 2011;26(6):422-5. [Google Scholar]
Eccleston 2013
- Eccleston C, Morley S, Williams A. Psychological approaches to chronic pain management: evidence and challenges. British Journal of Anaesthesia 2013;111(1):59-63. [DOI] [PubMed] [Google Scholar]
Eccleston 2014
- Eccleston C, Fisher E, Craig L, Duggan GB, Rosser BA, Keogh E. Psychological therapies (Internet-delivered) for the management of chronic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD010152. [DOI: 10.1002/14651858.CD010152.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haanpää 2011
- Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152(1):14-27. [DOI] [PubMed] [Google Scholar]
Hall 2008
- Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002-2005. BMC Family Practice 2008;9:26. [DOI: 10.1186/1471-2296-9-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jensen 2011
Kalso 2014
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2014;348:34-7. [DOI] [PubMed] [Google Scholar]
Lumley 2011
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, et al. Pain and emotion: a biopsychosocial review of recent research. Journal of Clinical Psychology 2011;67:942-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuire 2014
- McGuire B, Williams AC de C, Lynch J, Nicholas M, Morley S, Newell J, et al. Psychological therapies for frequent episodic and chronic tension-type headache in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD011309. [DOI: 10.1002/14651858.CD011309] [DOI] [Google Scholar]
Medical Outcomes Study
- RAND Health. Medical Outcomes Study: Measures of Quality of Life Core Survey from RAND Health. http://www.rand.org/health/surveys_tools/mos.html (accessed 23 September 2015).
Moisset 2007
- Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimaging 2007;37(Suppl 1):S80-8. [DOI: 10.1016/j.neuroimage.2007.03.054] [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain - establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386-9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79-94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Morley 1999
- Morley SJ, Eccleston C, Williams A. Systematic review and meta-analysis of randomised controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80(1-2):1-13. [DOI] [PubMed] [Google Scholar]
Morley 2002
- Morley SJ, Williams AC, Black S. A confirmatory analysis of the Beck Depression Inventory in chronic pain. Pain 2002;99(1-2):289-98. [DOI] [PubMed] [Google Scholar]
Morley 2006
- Morley SJ, Williams AC. RCTs of psychological treatments for chronic pain: progress and challenges. Pain 2006;121(3):171-2. [DOI] [PubMed] [Google Scholar]
Morley 2013
- Morley S, Williams A, Eccleston C. Examining the evidence of psychological treatments for chronic pain: time for a paradigm shift? Pain 2013;154(10):1929-31. [DOI] [PubMed] [Google Scholar]
Nestoriuc 2007
- Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain 2007;118(1-2):111-27. [DOI] [PubMed] [Google Scholar]
Nestoriuc 2008
- Nestoriuc Y, Rief W, Martin A. Meta-analysis of biofeedback for tension type headache: efficacy, specificity, and treatment moderators. Journal of Consulting and Clinical Psychology 2008;76(3):379-96. [DOI] [PubMed] [Google Scholar]
Puder 1988
- Puder RS. Age analysis of cognitive-behavioral group therapy for chronic pain outpatients. Psychology & Aging 1988;3(2):204-7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2007
- Smith BH, Torrance N, Bennett MI, Lee AJ. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clinical Journal of Pain 2007;23(2):143-9. [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith BH, Torrance N, Johnson M. Assessment and management of neuropathic pain in primary care. Pain Management 2012;2(6):553-9. [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1983. [Google Scholar]
Tracey 2011
- Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nature Reviews Neurology 2011;7(3):173-81. [DOI: 10.1038/nrneurol.2011.4] [DOI] [PubMed] [Google Scholar]
Van Damme 2015
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetering 2010
- Van de Wetering EJ, Lemmens KMM, Nieboer AP, Huijsman R. Cognitive and behavioral interventions for the management of chronic neuropathic pain in adults - a systematic review. European Journal of Pain 2010;14(7):670-81. [DOI] [PubMed] [Google Scholar]
Wetherell 2011
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain 2011;152(9):2098-107. [DOI] [PubMed] [Google Scholar]
Williams 2011
- Williams ACdeC. Pain – the patient's viewpoint. The Psychologist 2011;24(6):426-7. [Google Scholar]
Williams 2012
- Williams ACDC, Eccleston C, Morley SP. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD007407. [DOI: 10.1002/14651858.CD007407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yates 2005
- Yates SL, Morley S, Eccleston C, Williams A. A scale for rating the quality of psychological trials for pain. Pain 2005;117(3):314-25. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]


