Abstract
Acquired evasive resistance is a major limitation of hepatocellular carcinoma (HCC) treatment with the tyrosine kinase inhibitor (TKI) sorafenib. Recent findings suggest that resistance to sorafenib may have a reversible phenotype. In addition, loss of responsiveness has been proposed to be due to a gradual decrease in sorafenib plasma levels in patients. Here, the possible mechanisms underlying reversible sorafenib resistance were investigated using a Hep3B-hCG orthotopic human xenograft model of locally advanced HCC. Tissue and plasma sorafenib and metabolite levels, downstream antitumor targets, and toxicity were assessed during standard and dose-escalated sorafenib treatment. Drug levels were found to decline significantly over time in mice treated with 30 mg/kg sorafenib, coinciding with the onset of resistance but a greater magnitude of change was observed in tissues compared with plasma. Skin rash also correlated with drug levels and tended to decrease in severity over time. Drug level changes appeared to be partially tumor dependent involving induction of tumoral CYP3A4 metabolism, with host pretreatment alone unable to generate resistance. Escalation from 30 to 60 mg/kg sorafenib improved antitumor efficacy but worsened survival due to excessive body weight loss. Microvessel density was inhibited by sorafenib treatment but remained suppressed over time and dose increase. In conclusion, tumor CYP3A4 induction by sorafenib is a novel mechanism to account for variability in systemic drug levels; however, declining systemic sorafenib levels may only be a minor resistance mechanism. Escalating the dose may be an effective treatment strategy, provided toxicity can be controlled.
Introduction
The oral antiangiogenic tyrosine kinase inhibitor (TKI) sorafenib remains the only approved systemic treatment for advanced hepatocellular carcinoma (HCC). Sorafenib is an inhibitor of Raf serine/threonine kinases and receptor tyrosine kinases associated with VEGFR2 and 3, platelet-derived growth factor receptor (PDGFR)-β, Flt-3, and c-Kit (1) and other signaling pathways, including STAT3 (2). In two randomized phase III trials in advanced HCC patients, sorafenib treatment improved the time to progression and extended overall survival by 2.8 (3) and 2.3 months (4) compared with placebo. These benefits are unfortunately modest, with upfront (innate/intrinsic) and acquired (evasive/secondary) drug resistance being major contributing factors. Toxicity is also an issue, leading to a high rate of dose reductions and treatment interruptions in patients (5, 6).
Proposed mechanisms for resistance, based primarily on preclinical studies, are diverse and numerous. Typically the host tumor microenvironment has been shown to be involved in an adaptive or evasive response to antiangiogenic treatment leading, for instance, to reinduction of tumor vascularization and adaptation or escape from tumor hypoxia (7). Cancer cells intrinsically may also drive resistance, particularly in HCC (8).
There has also been the suggestion that “pharmacokinetic resistance” could develop to TKIs (9, 10). Standard doses of oral sorafenib lead to high (∼50%) interindividual variability in drug exposure (11–13), suggesting that some patients may be underdosed. The absence of certain toxicities that are associated with improved clinical outcomes [e.g., hand–foot skin reaction (HSFR); refs. 14, 15] may also indicate underexposure and underdosing (16, 17). Inadequate target inhibition and tumor regrowth could result, appearing as intrinsic resistance. In addition, sorafenib plasma levels have been shown to decline over time. In a study of 15 patients with HCC, sorafenib exposure was found to decrease significantly from 1 month of treatment (AUC 60.3 mg·h/L) to the time of disease progression (33.2 mg·h/L; P = 0.007; ref. 10). This trend was later reported in other malignancies (12, 18, 19). Such drug level changes could lead to reduced toxicities and acquired resistance and managed simply by long-term dosage adjustments. The cause of declining sorafenib exposure is unknown.
In patients, resistance to TKIs occasionally presents as a reversible rather than permanent phenotype (20). Patients with renal cell carcinoma (RCC) who had progressed on/acquired resistance to sorafenib (21) or sunitinib (22) have been reported to respond to rechallenge with the same agents. Similarly, it has been argued that clinical trials evaluating the strategy of switching to another antiangiogenic TKI after “resistance” has developed should contain an arm evaluating continuation of Kuczynski et al. treatment with the same TKI since this strategy is often efficacious (23). Reversible sorafenib resistance has been modeled preclinically in HCC using mice bearing intrahepatic human xenografts of Hep3B-hCG cells (24). These mice demonstrate initial marked sensitivity to sorafenib that is followed by tumor rebound after 1 month, based on levels of the secreted urinary protein tumor biomarker βhCG (β human chorionic gonadotropic hormone) and tumor weight changes (24). Once ostensibly resistant tumor cells were adapted to culture and reimplanted into new hosts, the resultant tumors were completely resensitized to retreatment (24). Reversible resistance may be a common but underappreciated phenomenon but how it relates to other resistance mechanisms is unclear.
Using the Hep3B-hCG model of HCC, mechanisms underlying reversible resistance to sorafenib were investigated. Similar to patients, sorafenib plasma levels were found to gradually decline—an effect that was found to be partially tumor dependent. Dose escalation inhibited tumor growth, but was unable to prevent further tumor progression and was too toxic without incorporation of therapy breaks. This study supports the involvement of declining sorafenib plasma levels as a minor or contributing resistance mechanism.
Materials and Methods
Orthotopic mouse model
Athymic nude mice (nu/nu; Harlan) were used for experiments assessing sorafenib concentrations. CB17 SCID mice (Charles River) were used for sorafenib “preconditioning” and in-house bred yellow fluorescent protein expressing CB17 SCID mice were used for transfer of resistant phenotype experiments (breeding pairs a gift from Dr. Janusz Rak, McGill University, Montreal, Quebec) . Mice were male ages 6 to 8 weeks. Animal procedures were in accordance with institutional animal care and maintenance guidelines.
The Hep3B-hCG model of HCC was described previously (25). Briefly, HCCs were established by injecting 1 to 2 × 106 Hep3B-hCG cells/10 µL volume into the left liver lobe of anesthetized mice (25). Individual mouse urine βhCG levels (henceforth, “hCG”) normalized to creatinine served as a noninvasive surrogate biomarker for tumor burden (25). The Hep3B-hCG cell line was authenticated by STR DNA analysis (Genetica DNA Laboratories) and was found to be Mycoplasma free (Lonza).
Sorafenib dosing and toxicity monitoring
Sorafenib tosylate was obtained from Bayer with the assistance of Dr. Dennis Healy and was prepared according to the manufacturer's recommendations. Unless otherwise indicated, oral gavage treatment of 30 mg/kg sorafenib or vehicle control began at hCG > 0 (tumor diameter ∼1–2 mm). Tumor response was defined as hCG stabilization (decline from prior assessment and/or hCG < 200 mIU/mg) and a progression defined as hCG > 200 mIU/mg. In cases where mice progressed early, treatment was continued to confirm tumor response. Dose was switched to 60 mg/kg on treatment day 29 approximating the average time of tumor progression.
Therapy was temporarily stopped at 10% average body weight loss in SCID mice and reinitiated following recovery to ≤5% (3–5 days of therapy breaks) mimicking the way toxicity is frequently managed clinically (5). For sorafenib preconditioning, tumor-free SCID mice were treated with sorafenib or vehicle as per tumor-bearing mice for 45 or 65 days, were given a 2-day washout period then were orthotopically implanted with parental Hep3B-hCG cells and randomized. After 5 days of surgery recovery, treatment was resumed according to the schedule of the group experiencing the most toxicity. Continuous daily dosing was possible for athymic nu/nu mice, which tolerate TKIs better than SCID mice (24). Skin toxicity was monitored biweekly by briefly anesthetizing and photographing nu/nu mice. Rash was graded as “rash,” “no rash,” or “resolved rash.” Resolved rash appeared as clear skin or rashes that diminished significantly in area (>∼95%) and redness from prior assessment.
Plasma and tissue sampling
Flash-frozen tumor and heparin-plasma samples (by cardiac puncture) were initially obtained 24 hours after dosing (ctrough). In subsequent experiments, mice were fasted for 3 hours, dosed, and heparin-plasma was obtained from the retro-orbital sinus 3 hours later to achieve maximal drug/metabolite concentrations (tmax 1–3 hours in mice; refs. 26, 27). Plasma samples were taken from different tumor-free and HCC mice 2 weeks pretreatment, days 1, 4, 7, and 11. Serial biweekly samples were obtained from a different set of mice for weeks 2+. Weighed flash-frozen liver and tumor and formalin-fixed tumor samples were obtained at sacrifice (3 hours after dosing) on day 14 (sensitive) and at endpoint (control, resistance, and dose-escalation phases). Plasma or homogenized tissue samples (100 μL volume) were analyzed by HPLC tandem mass spectrometry (HPLC-MS/MS) to determine sorafenib and estimate N-oxide metabolite levels as previously described (28, 29).
Protein analysis
Protein expression in liver and tumor lysates was analyzed by Western blot analysis (40 µg protein/well) using the following antibodies: phospho-STAT3 (Tyr705; 4113), STAT3 (4904), mouse/human CYP3A4 (13384), phospho-ERK (Thr202/Tyr204; 4376), ERK (4696; all Cell Signaling Technology), and β-actin (A5441; Sigma-Aldrich).
IHC
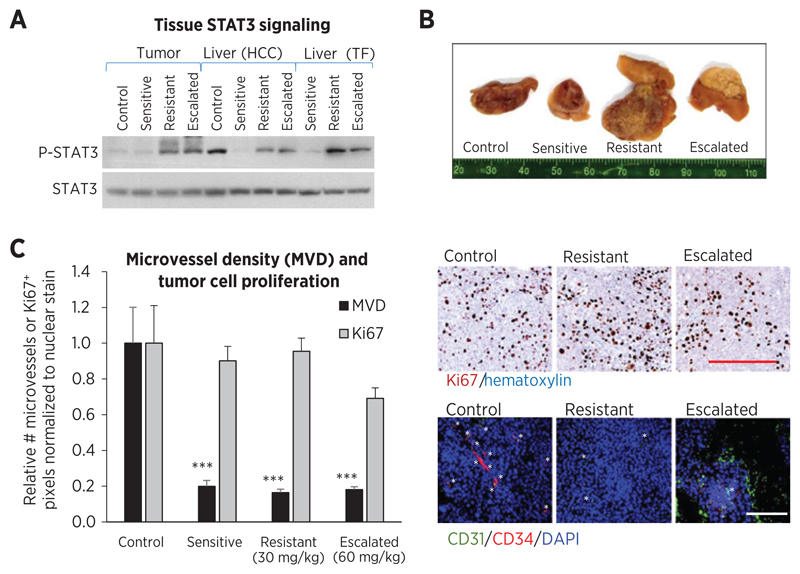
Formalin fixed, paraffin-embedded tumor sections were immunostained using the following reagents: For microvessel density (MVD; CD34+ and CD31+ vessels), 1:150 CD34 (LS-C47878; LifeSpan Biosciences), 1:100 CD31 (sc-1506; Santa Cruz Biotechnology), Cy3- and Alexa488-conjugated secondary antibodies (Jackson Immunoresearch) and DAPI (Invitrogen); for cell proliferation (human Ki-67), 1:1,000 Ki-67 antibody (VP-K451; Vector Laboratories), LSAB+ and DAB+ kits (Dako), and hematoxylin (Surgipath). Approximately 20 images/section were obtained (n = 4–9/group) at ×100 (Ki-67) or ×200 (MVD). CD34+ or CD31+ microvessel counts and Ki-67 stain were normalized to nuclear stain using ImageJ (v.1.46r) software.
Statistical analysis
Experimental group differences were evaluated by the Student t test or one-way ANOVA with Bonferroni correction for multiple comparisons. The effects of tumor presence and time on drug concentrations were assessed by two-way ANOVA. Correlations between hCG and drug concentrations were determined by linear regression. Survival was evaluated by log-rank test using Graph-Pad Prism (v.4.00). Data are presented as the mean and SEM. Significance level was set at P = 0.05.
Results
Plasma sorafenib declines over time
Variants from in vivo sorafenib (30 mg/kg)-resistant Hep3B-hCG tumors were first assessed in vitro for resistant properties; however, no indication of resistance was observed (Supplementary Fig. S1A–S1E). Because the resistant phenotype was also lost by transferring resistant tumor fragments or chronically sorafenib-exposed variants into tumor-naïve hosts, host treatment appeared important for resistance (Supplementary Fig. S1F and S1G and unpublished results). Host-wide pharmacokinetic changes are associated with disease progression in patients (10, 12, 18, 19), therefore this was investigated as a mediator of the reversible sorafenib-resistant phenotype in mice.
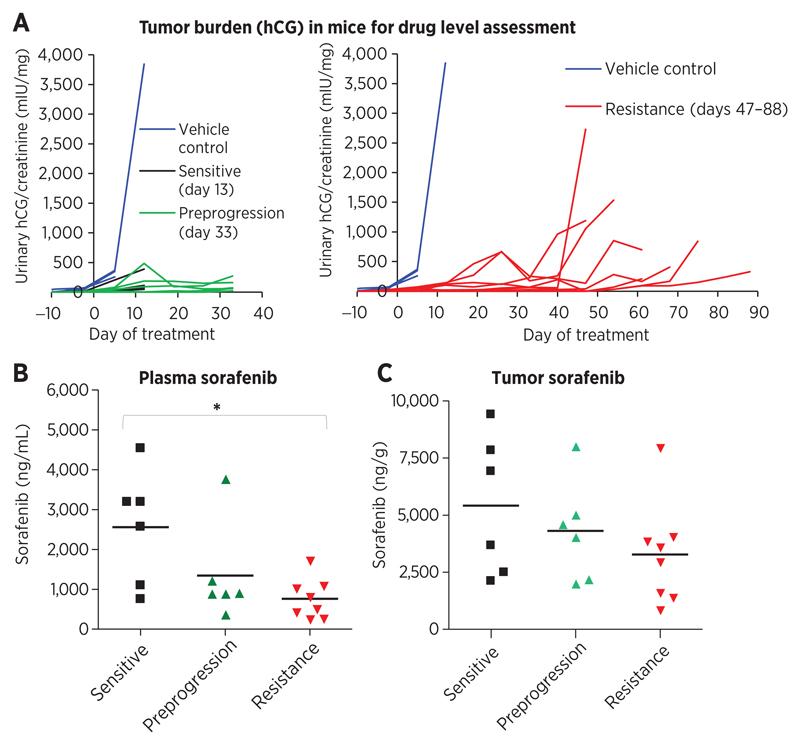
Drug levels in tumor and plasma samples were analyzed from athymic nu/nu mice bearing Hep3B-hCG xenografts treated daily with 30 mg/kg sorafenib. Samples were obtained from sensitive (treatment day 13, n = 6), preprogression (day 33, n = 6), and acquired resistant mice (days 47–88, n = 8; Fig. 1A). Trough (ctrough, 24 hours) measurements were taken to estimate steady state levels (shown to correlate with TKI treatment outcome; refs. 30, 31), and to overcome the obstacle of obtaining multiple timed plasma samples. As per patients with HCC (10), a significant reduction in plasma sorafenib concentration was observed in mice that had acquired resistance relative to responsive mice (70.6% decline, P < 0.05; ANOVA P = 0.0204; Fig. 1B). Tumor sorafenib levels tended to decline but this was not statistically significant (ANOVA P = 0.309; Fig. 1C). Similar trends were also observed in 4 mice that progressed without a prolonged initial response to sorafenib (plasma P = 0.0451; tumor P = 0.142; Supplementary Fig. S2A and S2B). Overall, declining systemic drug levels correlated with resistance in mice with HCC.
Figure 1.
Decline in plasma sorafenib levels is associated with resistance in HCC. A, athymic nu/nu mice bearing intrahepatic xenografts of Hep3B-hCG cells eventually acquire resistance to daily 30 mg/kg sorafenib treatment as demonstrated by urinary hCG levels normalized to creatinine. Plasma and tumors were obtained 24 hours after dosing for drug level assessment from drug-sensitive, preprogression, and acquired resistant mice (days 13, 33, and 47–88, respectively; n = 6–8). B, sorafenib plasma concentration was found to decline significantly from sensitive to resistant time points (ANOVA, P = 0.0204; *, P < 0.05), indicating a potential pharmacokinetic resistance mechanism. C, corresponding tumor concentrations of sorafenib also tended to decline but not significantly (ANOVA, P = 0.309).
The presence of tumor intensifies declining sorafenib levels
Both host and tumor factors could conceivably contribute to the observed decline in sorafenib. In the host, 5% of sorafenib is oxidated by hepatic p450 enzyme CYP3A4 (32) and 15% glucuronidated by UGT1A9 (33) contributing to fecal and urinary elimination, respectively. The oxidated N-oxide metabolite is pharmacologically active but more hydrophobic than the parent compound and represents the dominant circulating metabolite in humans (9%–16% of total sorafenib; ref. 33) and pharmacologic induction of CYP3A4 has been shown to decrease systemic sorafenib concentrations (32, 34). In contrast, drug efflux transporters P-glycoprotein and ABCG2 are thought to play minor roles in sorafenib pharmacokinetics (35, 36). In the tumor, drugs may accumulate in cancer cells (such as in acidic lysosomes for sunitinib; ref. 9) or cancer cells may themselves highly express drug metabolizing enzymes (37) thereby potentially influencing exposure levels. Thus, concentrations of sorafenib and its major metabolite were determined in the presence and absence of tumor to explore the mechanism of sorafenib decline.
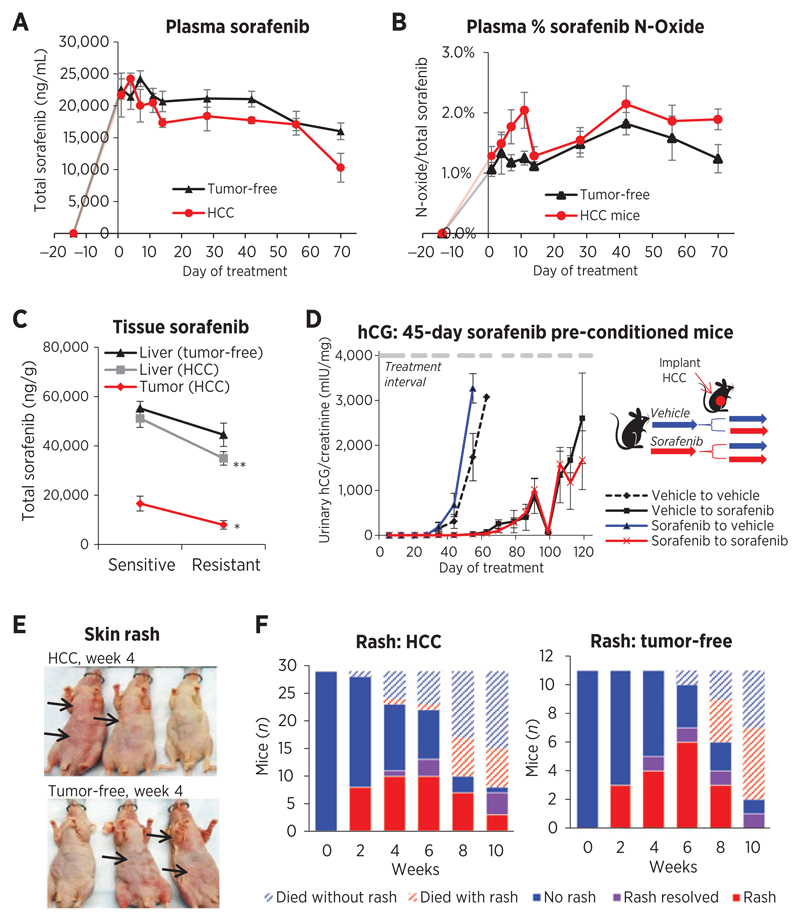
Tumor-free and Hep3B-hCG tumor-bearing (HCC) mice were treated daily with sorafenib (30 mg/kg) and after 29 days half of mice were switched to 60 mg/kg, and plasma was sampled 3 hours after dosing to maximize drug concentrations. Peak plasma sorafenib was achieved days 4 to 7 in mice treated with 30 mg/kg sorafenib (Fig. 2A), which then declined in both HCC (R2 = 0.723, P = 0.0075) and tumor-free mice (R2 = 0.813, P = 0.0055); however, total sorafenib levels tended to be higher in tumor-free mouse plasma (Fig. 2B). Both time (P < 0.0001) and tumor presence (P = 0.0196) were significantly associated with plasma levels by two-way ANOVA. Tumor presence also significantly affected %N-oxide levels (P = 0.0063 vs. time P = 0.1107) as %N-oxide peaked by day 11 in tumor-bearing mice only, but thereafter remained marginally higher than in tumor-free mice. The decline in sorafenib concentrations from sensitive (day 14) to resistance (endpoint) phases was more striking in HCC mouse tissues: a 52.1% (P = 0.0192) and 31.7% drop (P = 0.00184) was observed in tumors and in livers, respectively, whereas a nonsignificant 19.4% decline was observed in tumor-free livers (P = 0.232; Fig. 2C). Tissue levels of %sorafenib N-oxide did not significantly change (P > 0.05; Supplementary Fig. S3A).
Figure 2.
Tumor impact on drug concentrations and resistance, and variation in rash development. A, total sorafenib plasma concentrations sampled 3 hours after dosing tended to decline in tumor free (R2 = 0.813 days 7–70; P = 0.0055) and Hep3B-hCG–bearing (HCC) mouse plasma (R2 = 0.723 days 4–70; P = 0.0075) but drug levels were generally higher in tumor-free mice. The tumor (P = 0.0196) and time (P < 0.0001) significantly impacted drug levels (two-way ANOVA). B, the %N-oxide metabolite/total sorafenib was marginally higher in HCC mouse plasma (two-way ANOVA, P = 0.0063 tumor; P = 0.1107 time) and peaked in HCC mice day 11 (t test, P = 0.0372). C, sorafenib concentrations declined significantly from sensitive to resistant phases in the liver (P = 0.00184; n = 10–11) and tumor (P = 0.0192; n = 8–9) of HCC mice but not in tumor-free mouse livers (P = 0.232; n = 6). *, P < 0.05; **, P < 0.01. D, SCID mice preconditioned for 45 days with 30 mg/kg sorafenib and subsequently implanted orthotopically with parental tumors developed resistance at the same rate as vehicle preconditioned mice (n = 4–5). E, skin rash toxicity frequently developed in tumor-free and HCC athymic nu/nu mice (arrow) when mice were sorafenib responsive. F, rash eventually improved weeks 4+ in mice, potentially relating to drug level decline.
The host effect on resistance is minimal
To determine the extent to which host-induced changes contributed to resistance, tumor-free SCID mice were preconditioned with sorafenib for 45 or 65 days before tumor implantation, corresponding to early and late onset of resistance (Supplementary Fig. S3B). SCID mice were given brief toxicity-associated therapy breaks allowing extended treatment times (24). Sorafenib preconditioning worsened weight loss but did not accelerate the onset of resistance relative to vehicle preconditioning (Fig. 2D and Supplementary Fig. S3C). Thus, the presence of HCC tumor worsened the gradual decline in sorafenib but the host effect alone appeared minimal as host treatment was insufficient to generate resistance.
Incidence of mouse skin toxicity parallels decline of sorafenib levels
Skin toxicities, including rash and HSFR, are the most commonly reported adverse events in sorafenib-treated patients (5). Skin toxicity has been correlated with drug exposures (17, 38) and improved clinical outcomes (14, 39) but has paradoxically been reported to decrease in severity over time (12, 40, 41). Seventy-four percent of HCC (n = 43) and 75% of tumor-free mice (n = 20) developed a nonirritated red skin on the ventral skin surface, ranging from small spots to nearly the entire surface (Fig. 2E). Rash was a treatment effect since stopping therapy caused complete rash resolution (and weight gain) in 7 of 7 mice within 1 to 2 weeks (results not shown). While weight loss was slow and progressive in sorafenib (30 mg/kg) treated mice, rash initially developed weeks 2 to 6 but tended to resolve beginning week 4 (Fig. 2F). Forty-two percent of HCC mice treated >6 weeks (n = 12) and 44% tumor-free mice (n = 9) showed rash improvement. Although delayed in time, this result may reflect declining tissue drug levels.
Tumor-mediated sorafenib metabolism correlates with declining drug levels
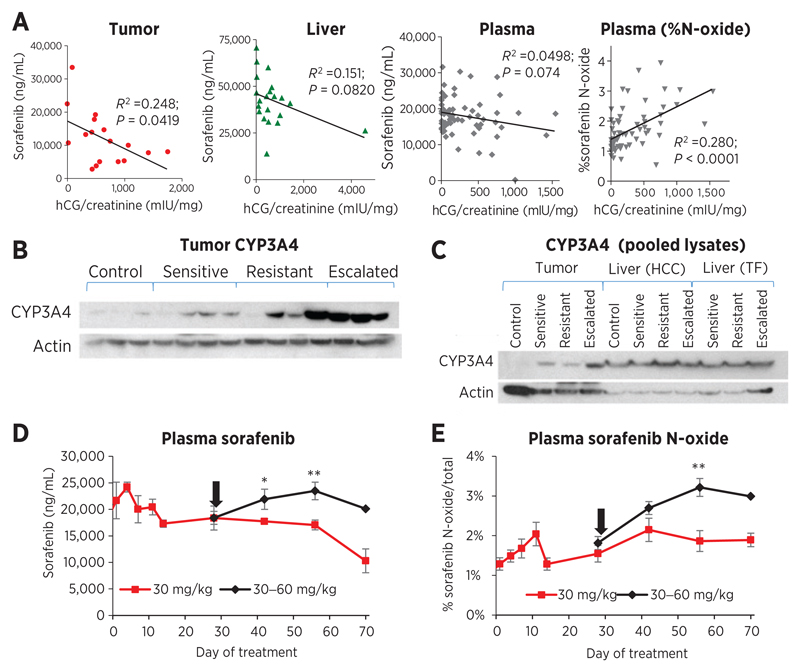
The effect of the tumor on drug levels was further explored. Negative correlations were observed between sorafenib concentrations and tumor burden/hCG. This relationship was strongest in the tumor (R2 = 0.248, P = 0.0419) and approached significance in the liver (R2 = 0.1507, P = 0.0820) and the plasma (R2 = 0.0498, P = 0.074; Fig. 3A). Contrastingly, plasma %N-oxide correlated positively with hCG (R2 = 0.280, P < 0.0001). Thus, local tumor drug levels and plasma %N-oxide associated well with the degree of tumor burden, but associations with plasma drug concentrations and tumor responses were weak.
Figure 3.
sorafenib levels are associated with tumoral drug metabolism. A, tumor burden (hCG) in 30 mg/kg sorafenib-treated mice (all time points for plasma; sensitive and resistance time points for tissues) correlated significantly with tumor total sorafenib concentrations (R2 = 0.248; P = 0.0419) and plasma %N-oxide (R2 = 0.280; P < 0.0001), suggesting a link with drug metabolism. Correlations for total sorafenib approached significance in the liver (R2 = 0.151; P = 0.0820) and plasma (R2 = 0.0498; P = 0.074). B, CYP3A4 protein in individual tumors was induced by 30 mg/kg sorafenib treatment and reinduced by escalation to 60 mg/kg. C, analysis of pooled lysates confirmed induction of CYP3A4 in tumor but not in livers (controls, n = 4; HCC, n = 9–10; tumor-free, n = 5–6). D and E, dose escalation increased sorafenib plasma concentrations (D) and the percentage N-oxide metabolite (E) in HCC mice, consistent with CYP3A4 expression (n = 10–12; *, P < 0.05; **, P < 0.01 vs. 30 mg/kg). Arrow, switch to 60 mg/kg.
Tumor and liver lysates were analyzed to determine expression levels of CYP3A4 enzyme. CYP3A4 protein levels were found to increase during sorafenib treatment in tumors (individual and pooled lysates) compared with vehicle-treated controls (Fig. 3B and C) and was further induced in dose-escalated tumors. In contrast, CYP3A4 remained stable in HCC and tumor-free livers (Fig. 3C). Although dose escalation increased plasma sorafenib concentrations (Fig. 3D), it also induced %N-oxide metabolite, which appeared to relate to CYP3A4 levels (Fig. 3E). Thus, autoinduction of sorafenib metabolism may explain, at least in part, the tumor's contribution to systemic sorafenib level decline.
Sorafenib dose escalation inhibits tumor growth at the expense of increased toxicity
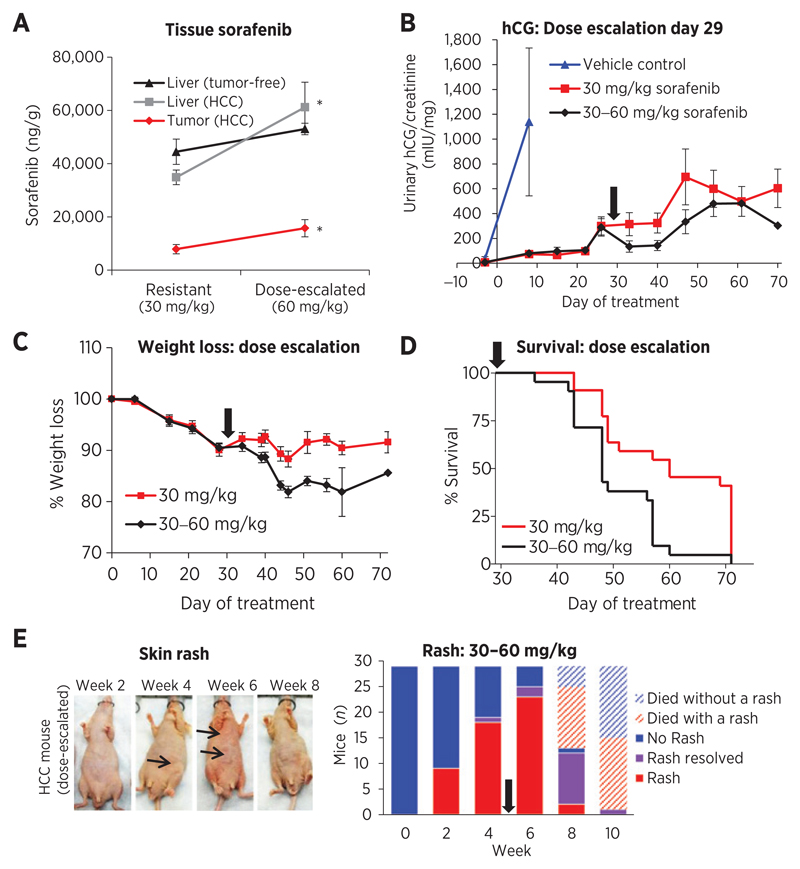
When the dose of sorafenib was doubled to 60 mg/kg on day 29 (beginning at the time of ctrough decline Fig. 1B and average hCG progression), plasma levels increased by only 23.6% from pre-escalation levels. This escalation strategy appeared effective in correcting drug level decline since plasma concentrations were restored to early timepoints (Fig. 3D) and concentrations significantly increased in the livers (P = 0.00895) and tumors (P = 0.0387) of HCC mice (Fig. 4A; though this did not correspond with significant %N-oxide increases except in tumor-free livers; Supplementary Fig. S4A).
Figure 4.
Impact of sorafenib dose escalation. A, dose-escalating sorafenib to 60 mg/kg significantly increased drug levels in the livers (P = 0.0089; n = 11, 9) and tumors (P = 0.0387; n = 8–9) of HCC mice, but not in the livers of tumor-free mice (P = 0.161; n = 5–6). *, P < 0.05. B, escalating the dose at disease progression slowed tumor growth (n = 5 control; 21–22 sorafenib). C, sorafenib-treated mice lost weight (~10% loss day 44), which was accelerated by dose escalation and led to animal termination. D, mice maintenance on 30 mg/kg sorafenib had superior survival (median 60 days) compared with switching to 60 mg/kg (48 days, log-rank P = 0.002; n = 21–22). E, skin rash (arrowhead) worsened by week 6 after dose escalation, then tended to improve. Arrow, switch to 60 mg/kg.
Dose escalation was evaluated for its ability to treat or prevent resistant disease. By day 29, progressive disease was achieved in 17 of 43 mice, a difference that appeared weakly (nonsignificantly) to lower tissue sorafenib levels (Supplementary Fig. S4B and S4C). In all mice, dose escalation inhibited hCG (Fig. 4B) in addition to tumor plus liver mass (P < 0.01; Supplementary Fig. S4D). This strategy was not, however, effective in reversing resistance since disease continued to progress at a rate similar to nonescalated mice. Dose escalation also resulted in excessive weight loss (Fig. 4C), which was the primary reason for termination (20% weight loss endpoint), leading to significantly decreased median survival (log rank P = 0.002; Fig. 4D). Tumor presence had no significant impact on weight loss or survival (P > 0.05; Supplementary Fig. S4E–S4G); therefore, drug toxicity was a critical factor in survival. Dose escalation worsened skin rash by week 6, then 58% of HCC mice (n = 12) and 50% of tumor-free mice (n = 8) experienced rash improvement (Fig. 4E).
Inhibition of angiogenesis is not associated with drug level changes
The possibility that circulating drug concentrations were suboptimal for target inhibition and antitumor effect was investigated. No inhibition in Raf (ERK), VEGFR2, or PDGFRβ signaling by sorafenib treatment was detected in tumor lysates (Supplementary Fig. S5A and S5B). Only P-STAT3 was inhibited by initial sorafenib treatment, which subsequently increased during resistance in livers from both HCC and tumor-free mice (Fig. 5A and Supplementary Fig. S5C), potentially related to changes in local sorafenib levels. Similar findings were observed in tumors although initial P-STAT3 inhibition by sorafenib treatment was not observed. Escalation to 60 mg/kg sorafenib had little effect on P-STAT3 despite increases in tissue drug concentrations (Fig. 5A).
Figure 5.
Effects of sorafenib on cell signaling and antitumor activity. A, sorafenib treatment inhibited phosphorylation of downstream target STAT3 (P-STAT3) in livers (pooled lysates). P-STAT3 increased in tumors and livers of resistance phase HCC and tumor-free mice, but remained high in dose-escalated tissues, indicating some correlation with local drug levels. B, fixed tumor cross-sections show hemorrhagic control tumors and increasingly pale treated tumors. Dose escalated tumors were small and appeared necrotic/white. C, tumor cell proliferation (human Ki-67 immunostaining) did not significantly change during treatment (ANOVA, P = 0.082) but tended to decrease during dose escalation (t test, P = 0.0135 vs. resistant). Tumor microvessel density [CD31 (green) and CD34 (red) vessel counts normalized to DAPI (blue)] was significantly inhibited throughout 30 and 60 mg/kg sorafenib treatment (ANOVA, P < 0.0001; ***, P < 0.001), showing little association with drug levels. Right, representative images. Bar, 500 µm.
Of those tested, the predominant antitumor mechanism for sorafenib treatment appeared to be antiangiogenesis. Sorafenib-treated tumors were less hemorrhagic than controls, but dose-escalated tumors were smaller and appeared more white/necrotic (Fig. 5B). Sorafenib treatment significantly inhibited microvessel density (P < 0.001 vs. controls, ANOVA P < 0.0001), but during resistance there was no evidence of resumption of angiogenesis. Likewise, hypoxia-responsive carbonic anhydrase IX (CAIX) protein remained elevated throughout treatment (Supplementary Fig. S5D). In contrast, tumor cell proliferation was not significantly affected by treatment (ANOVA P = 0.082; Fig. 5C), except for minor inhibition during dose escalation (t test, P = 0.0135 vs. resistance). Altogether, systemic drug levels appeared sufficient for inhibiting angiogenesis therefore factors other than an increase in microvessel density must be responsible for causing HCC tumor progression.
Discussion
In recent clinical studies, sorafenib exposure was reported to decline by up to 50% at the time of disease progression in small groups of patients with HCC (10), melanoma (19), and other solid tumors (12, 18). A similar observation has been made in GIST patients treated with the TKI imatinib (42), but as with sorafenib, the underlying causes of it are unknown. Here, clinical observations were confirmed in mice bearing HCC xenografts and a possible mechanism was identified. However, the involvement of this phenomenon in reversible resistance appears complex.
Mechanism of reduced drug exposure over time
In patients, pharmacologic induction of CYP3A4 by anti-epileptic drugs or rifampin significantly decreased sorafenib exposure (32, 34), suggesting a key role for this metabolic pathway. CYP3A4 inhibition by ketoconazole or midazolam had little effect on exposure except for reducing metabolite levels (43, 44), which is expected given that only 5% of an oral dose is metabolized by CYP3A4 (32). Autoinduction of drug metabolism in the tumor has largely been an underappreciated contributor to resistance (37). In vivo tumor induction of CYP3A4 by sorafenib was found to be a possible contributor to declining drug levels for the first time. This result is consistent with findings that TKIs gefitinib and sunitinib could induce expression of p450 enzyme CYP1A1 in cancer cell lines (45, 46), which has not yet been shown for sorafenib. Despite the key role for hepatic metabolism, no liver induction of CYP3A4 was observed during treatment, in agreement with prior observations (26, 27).
Tumoral CYP3A4 induction does not explain the weaker sorafenib decline in tumor-free mice. It is possible that additional factors, such as drug-binding plasma proteins levels, decreased intestinal absorption, and involvement of other metabolic pathways (e.g., UGT1A9) contribute to sorafenib level changes in the host and in patients. Because of the high tumor:body mass ratio of mice in xenograft studies, the importance of a tumor-dependent mechanism may have been exaggerated here, further highlighting the need for follow-up studies.
The sorafenib dose used here (30 mg/kg) is considered low following conversion to a human equivalent dose (47). Plasma concentrations (∼20,000 ng/mL) were in the range of mouse studies (26) but higher than in patients (<10,000 ng/mL with 400 mg twice daily dosing; ref. 11). In humans, the N-oxidation pathway is more pronounced than in mice (27), which is validated by the N-oxide levels observed here (1%–3% vs. 9%–16% in humans; ref. 33). Although higher %N-oxide might be expected from CYP3A4 induction, it cannot be ruled out that generated metabolite was cleared too rapidly for direct quantification. Alternatively, the observed metabolite levels may indicate a minor impact of the tumor on drug levels. Indeed P-STAT3 and rash patterns (potential pharmacodynamic markers) were similar regardless of tumor presence, but the relationship between pharmacokinetics and pharmacodynamics can be complex.
Dose escalation as a therapeutic strategy
In selected populations of RCC and HCC patients, slowly ramping up the dose of sorafenib (41) or increasing the dose at progression (48, 49) has demonstrated tolerability and antitumor activity. Dose escalating may also serve to reestablish adequate exposure levels (10, 12, 18, 19). Here, dose was doubled concurrently for all mice regardless of weight loss status. This did not prove to be effective: antitumor activity increased but tolerability was poor. Lower-than-predicted plasma drug levels also occurred as is common in dose-escalated patients, which may indicate saturated drug absorption (11, 13) or poor drug solubility at higher doses (27).
On the basis of the data presented here, therapeutic plasma drug monitoring could underrepresent drug levels changes within the tissues, which could mean missed opportunities to optimize the dosage. Dose escalating according to toxicity is an alternative. For the first time shown here, skin rash in mice recapitulated observations of rash and HSFR in TKI-treated patients. Rash developed at a high rate for up to 6 weeks correlating with early treatment response (14, 15) and tended to improve in approximately 50% of cases mimicking the reported decreasing severity of skin toxicity (12, 40, 41). Rash improvement may therefore be directly related to declining drug levels as suggested by correlations with AUC in patients (12, 19). The uncoupling between patterns of weight loss and rash appears consistent with clinical findings and may relate to higher unabsorbed drug concentrations in the gut (19). To manage excessive weight loss, dose interruptions should be incorporated, which may also help resensitize the tumor (20). Optimized strategies that combine dose increases with brief therapy breaks may hold at least some antitumor activity while prolonging survival. Extensions in PFS and OS have been achieved in a retrospective study using a similar strategy with sunitinib in RCC (50).
Declining sorafenib levels as a resistance mechanism
At first glance, systemic drug level changes appear to correlate with resistance and hence provide a possible mechanism for the reversible resistant phenotype that cannot be propagated to new hosts (24). Tissue sorafenib levels related to tumor progression and P-STAT signaling. Interaction between the host and tumor cells was also found to be critical for resistance. However, the lack of microvessel density change during treatment suggests that drug levels remained sufficient for long-term angiogenesis inhibition, assuming that microvessel density is directly correlated to the antitumor effect. Thus, declining drug levels could be a minor or a contributing factor to resistance. Likewise, dose escalation slowed tumor growth but did not directly prevent tumor progression or effectively treat resistant disease. This might explain why the benefits of sorafenib dose escalation are often transient (18, 41).
In conclusion, sorafenib levels declined over time in mice, but its role in resistance is unclear. Escalating the dose may be an effective strategy; however, more tolerable regimens are needed, particularly for patients with HCC with impaired liver function. A relationship was also observed between skin rash in “nude” mice and drug levels. Given the frequency of rash as a side effect of many biologic anticancer agents, these results could be extended to study the impact of rash as a potential biomarker of drug efficacy. Although drug resistance remains a complex issue, individualizing treatment regimens with toxicity-guided approaches has potential to enhance the activity of currently available TKIs for cancer treatment.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Acknowledgments
The authors thank Bayer through Dr. Dennis Healy for providing sorafenib, Dr. Terence Tang for developing the Hep3B-hCG model, and Dr. Georg Bjarnason for helpful discussion.
Grant Support
This work was supported by Canadian Liver Foundation graduate studentship (E.A. Kuczynski), Canadian Institutes of Health Research Grant #5814, National Institutes of Health (CA41233; R.S. Kerbel), and International Association for Cancer Research (Worldwide Cancer Research; R.S. Kerbel)
Footnotes
Disclosure of Potential Conflicts of Interest
R.S. Kerbel has received speakers bureau honoraria from Boehringer Ingelheim and Eli Lilly; is a consultant/advisory board member for Angiocrine Biosciences, Cerulean, MolMed, and Triphase Accelerator. No potential conflicts of interest were disclosed by the authors.
Authors' Contributions
Conception and design: E.A. Kuczynski, S. Man, R.S. Kerbel
Development of methodology: E.A. Kuczynski, S. Man, E. Chen
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): E.A. Kuczynski, C.R. Lee, E. Chen
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): E.A. Kuczynski, E. Chen, R.S. Kerbel
Writing, review, and/or revision of the manuscript: E.A. Kuczynski, C.R. Lee, S. Man, E. Chen, R.S. Kerbel
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): E.A. Kuczynski, C.R. Lee
Study supervision: R.S. Kerbel
References
- 1.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Rosmorduc O, Desbois-Mouthon C. Targeting STAT3 in hepatocellular carcinoma: sorafenib again…. J Hepatol. 2011;55:957–9. doi: 10.1016/j.jhep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A, Kang Y, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Riechelmann RP, Chin S, Wang L, Tannock IF, Berthold DR, Moore MJ, et al. Sorafenib for metastatic renal cancer: the Princess Margaret experience. Am J Clin Oncol. 2008;31:182–7. doi: 10.1097/COC.0b013e3181574084. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Eisen T, Fishman M, Quinn D. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2011;78:24–32. doi: 10.1016/j.critrevonc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berasain C. Hepatocellular carcinoma and sorafenib: too many resistance mechanisms? Gut. 2013;62:1674–5. doi: 10.1136/gutjnl-2013-304564. [DOI] [PubMed] [Google Scholar]
- 9.Gotink KJ, Broxterman HJ, Labots M, de Haas RR, Dekker H, Honeywell RJ, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res. 2011;17:7337–46. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:2046–9. doi: 10.1007/s10637-011-9764-8. [DOI] [PubMed] [Google Scholar]
- 11.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 12.Boudou-Rouquette P, Ropert S, Mir O, Coriat R, Billemont B, Tod M, et al. Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist. 2012;17:1204–12. doi: 10.1634/theoncologist.2011-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornecker M, Blanchet B, Billemont B, Sassi H, Ropert S, Taieb F, et al. Saturable absorption of sorafenib in patients with solid tumors: a population model. Invest New Drugs. 2012;30:1991–2000. doi: 10.1007/s10637-011-9760-z. [DOI] [PubMed] [Google Scholar]
- 14.Shin SY, Lee YJ. Correlation of skin toxicity and hypertension with clinical benefit in advanced hepatocellular carcinoma patients treated with sorafenib. Int J Clin Pharmacol Ther. 2013;51:837–46. doi: 10.5414/CP201907. [DOI] [PubMed] [Google Scholar]
- 15.Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–24. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–6. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellesoeur A, Carton E, Mir O, Groussin L, Blanchet B, Billemont B, et al. Critical role of sorafenib exposure over time for its antitumor activity in thyroid cancer. Invest New Drugs. 2014;32:569–72. doi: 10.1007/s10637-013-0052-7. [DOI] [PubMed] [Google Scholar]
- 19.Pécuchet N, Lebbe C, Mir O, Billemont B, Blanchet B, Franck N, et al. Sorafenib in advanced melanoma: a critical role for pharmacokinetics? Br J Cancer. 2012;107:455–61. doi: 10.1038/bjc.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczynski EA, Sargent DJ, Grothey A, Kerbel RS. Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat Rev Clin Oncol. 2013;10:571–87. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozawa M, Yamamoto Y, Minami T, Shimizu N, Hatanaka Y, Tsuji H, et al. Sorafenib rechallenge in patients with metastatic renal cell carcinoma. BJU Int. 2012;110(6 Pt B):E228–34. doi: 10.1111/j.1464-410X.2011.10905.x. [DOI] [PubMed] [Google Scholar]
- 22.Zama IN, Hutson TE, Elson P, Cleary JM, Choueiri TK, Heng DY, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer. 2010;116:5400–6. doi: 10.1002/cncr.25583. [DOI] [PubMed] [Google Scholar]
- 23.Burotto M, Wilkerson J, Stein W, Motzer R, Bates S, Fojo T. Continuing a cancer treatment despite tumor growth may be valuable: sunitinib in renal cell carcinoma as example. PLoS ONE. 2014;9:e96316. doi: 10.1371/journal.pone.0096316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang TC, Man S, Xu P, Francia G, Hashimoto K, Emmenegger U, et al. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12:928–40. doi: 10.1593/neo.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang TC, Man S, Lee CR, Xu P, Kerbel RS. Impact of metronomic UFT/cyclophosphamide chemotherapy and antiangiogenic drug assessed in a new preclinical model of locally advanced orthotopic hepatocellular carcinoma. Neoplasia. 2010;12:264–74. doi: 10.1593/neo.91872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawaskar D, Straubinger R, Fetterly G, Hylander BH, Repasky EA, Ma WW, et al. Physiologically based pharmacokinetic models for everolimus and sorafenib in mice. Cancer Chemother Pharmacol. 2013;71:1219–29. doi: 10.1007/s00280-013-2116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saber-Mahloogi H, Morse DE. Pharmacology review—sorafenib. Rockville, MD: Center for Drug Evaluation and Research; 2005. [Google Scholar]
- 28.Honeywell R, Yarzadah K, Giovannetti E, Losekoot N, Smit EF, Walraven M, et al. Simple and selective method for the determination of various tyrosine kinase inhibitors used in the clinical setting by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1059–68. doi: 10.1016/j.jchromb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Zhao M, Navid F, Pratz K, Smith BD, Rudek MA, et al. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3033–8. doi: 10.1016/j.jchromb.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klümpen H, Samer CF, Mathijssen RHJ, Schellens JHM, Gurney H. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat Rev. 2011;37:251–60. doi: 10.1016/j.ctrv.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Yeap S, Clements A, Balakrishnar B, Wong M, Gurney H. Evidence for therapeutic drug monitoring of targeted anticancer therapies. J Clin Oncol. 2012;30:4017–25. doi: 10.1200/JCO.2012.43.5362. [DOI] [PubMed] [Google Scholar]
- 32.European medicines agency. EMEA report on sorafenib. [accessed 13 april 2014]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000690/WC500027704.pdf.
- 33.Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother. 2010;11:1943–55. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- 34.Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang SC, de Vries N, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) gene dosage on plasma pharmacokinetics and brain accumulation of dasatinib, sorafenib, and sunitinib. J Pharmacol Exp Ther. 2013;346:486–94. doi: 10.1124/jpet.113.205583. [DOI] [PubMed] [Google Scholar]
- 36.Lagas JS, van Waterschoot RAB, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9:319–26. doi: 10.1158/1535-7163.MCT-09-0663. [DOI] [PubMed] [Google Scholar]
- 37.Rochat B. Role of cytochrome P450 activity in the fate of anticancer agents and in drug resistance: focus on tamoxifen, paclitaxel and imatinib metabolism. Clin Parmacokint. 2005;44:349–66. doi: 10.2165/00003088-200544040-00002. [DOI] [PubMed] [Google Scholar]
- 38.Boudou-Rouquette P, Narjoz C, Golmard JL, Thomas-Schoemann A, Mir O, Taieb F, et al. Early sorafenib-induced toxicity is associated with drug exposure and UGTIA9 genetic polymorphism in patients with solid tumors: a preliminary study. PLoS ONE. 2012;7:e42875. doi: 10.1371/journal.pone.0042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poprach A, Pavlik T, Melichar B, Puzanov I, Dusek L, Bortlicek Z, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23:3137–43. doi: 10.1093/annonc/mds145. [DOI] [PubMed] [Google Scholar]
- 40.Flaherty KT, Brose MS. Sorafenib-related hand-foot skin reaction improves, not worsens, with continued treatment. Clin Cancer Res. 2009;15:7749. doi: 10.1158/1078-0432.CCR-09-1190. [DOI] [PubMed] [Google Scholar]
- 41.Amato R, Zhai J, Willis J, Saxena S, DeFoe M. A phase II trial of intrapatient dose-escalated sorafenib in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2012;10:153–8. doi: 10.1016/j.clgc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Judson I, Ma P, Peng B, Verweij J, Racine A, di Paola ED, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC soft tissue and bone sarcoma group. Cancer Chemother Pharmacol. 2005;55:379–86. doi: 10.1007/s00280-004-0876-0. [DOI] [PubMed] [Google Scholar]
- 43.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–92. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 44.Flaherty K, Lathia C, Frye R, Schuchter L, Redlinger M, Rosen M, et al. Interaction of sorafenib and cytochrome P450 isoenzymes in patients with advanced melanoma: a phase I/II pharmacokinetic interaction study. Cancer Chemother Pharmacol. 2011;68:1111–8. doi: 10.1007/s00280-011-1585-0. [DOI] [PubMed] [Google Scholar]
- 45.Alfieri RR, Galetti M, Tramonti S, Andreoli R, Mozzoni P, Cavazzoni A, et al. Metabolism of the EGFR tyrosin kinase inhibitor gefitinib by cytochrome P450 1A1 enzyme in EGFR-wild type non small cell lung cancer cell lines. Mol Cancer. 2011;10:143. doi: 10.1186/1476-4598-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maayah ZH, El Gendy MA, El-Kadi AO, Korashy HM. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome P450 1A1 gene in human breast cancer MCF7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch Toxicol. 2013;87:847–56. doi: 10.1007/s00204-012-0996-y. [DOI] [PubMed] [Google Scholar]
- 47.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 48.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–9. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 49.Rimassa L, Pressiani T, Boni C, Carnaghi C, Rota Caremoli E, Fagiuoli S, et al. A phase II randomized dose escalation trial of sorafenib in patients with advanced hepatocellular carcinoma. Oncologist. 2013;18:379–80. doi: 10.1634/theoncologist.2012-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjarnason GA, Khalil B, Hudson JM, Williams R, Milot LM, Atri M, et al. Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol. 2014;32:480–7. doi: 10.1016/j.urolonc.2013.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.