Abstract
Background
Multiple sclerosis (MS) is a widespread neurological autoimmune disease that includes episodes of demyelination in the central nervous system (CNS). The accumulated evidence has suggested that aryl hydrocarbon receptor (Ahr), a ligand-activated transcription factor, is a promising treatment target for MS. Thus, the current study aimed to identify a novel Ahr ligand with anti-inflammatory potential in experimental autoimmune encephalomyelitis (EAE).
Methods
An in silico analysis was carried out to predict interactions between Ahr and potential natural ligands. The effects of a predicted interaction were examined in vitro using CD4+ T cells under T helper17 (Th17) cell-polarizing conditions and lipopolysaccharide (LPS)-stimulated macrophages. Silencing Ahr and microRNA (miR)-132 was achieved by electroporation. Myelin oligodendrocyte glycoprotein (MOG)35-55 and the adoptive transfer of encephalitogenic CD4+ T cells were used to induce EAE.
Results
Molecular docking analysis and in vitro data identified gallic acid (GA) as a novel Ahr ligand with potent activation potential. GA induced the expression of Ahr downstream genes, including cytochrome P450 family 1 subfamily A member 1 (Cyp1a1) and the miR-212/132 cluster, and promoted the formation of the Ahr/Ahr nuclear translocator (Arnt) complex. In vivo, GA-treated mice were resistant to EAE and exhibited reduced levels of proinflammatory cytokines and increased levels of transforming growth factor-β (TGF-β). Furthermore, GA reduced infiltration of CD4+CD45+ T cells and monocytes into the CNS. The anti-inflammatory effects of GA were concomitant with miR-132-potentiated cholinergic anti-inflammation and the regulation of the pathogenic potential of astrocytes and microglia. Inducing EAE by adoptive transfer revealed that CD4+ T cells were not entirely responsible for the ameliorative effects of GA.
Conclusion
Our findings identify GA as a novel Ahr ligand and provide molecular mechanisms elucidating the ameliorative effects of GA on EAE, suggesting that GA is a potential therapeutic agent to control inflammation in autoimmune diseases such as MS.
Introduction
Multiple sclerosis (MS) is the most common neurological autoimmune disease of the central nervous system (CNS). MS patients present variable patterns of relapsing remittance characterized by intermittent exacerbations. Such exacerbations and disease progression have often been reduced with disease-modifying therapies [1, 2]. However, some of these therapies exert negative side effects [3].
The immune response in autoimmunity is regulated by both genetic and environmental factors. Although significant progress has been achieved in identifying the genetic control of MS pathogenesis, limited information is available about the contribution of environmental factors [4]. In this context, aryl hydrocarbon receptor (Ahr) represents a valuable model to investigate therapeutic immunomodulation by natural ligands. Ahr, a member of the basic helix-loop-helix (bHLH) family, is implicated in several events of the immune response and autoimmunity [5–7]. It is activated by a variety of exogenous ligands from the diet and environment [8, 9]. Therefore, Ahr signaling integrates the effects of the environment and metabolism on the immune response [10]. In response to ligation, Ahr forms a heterodimer with Ahr nuclear translocator (Arnt) and translocates into the nucleus to induce several downstream genes such as cytochrome P450 family 1 subfamily A member 1 (Cyp1a1) [8, 9]. We have also identified the microRNA (miR)-212/132 cluster as a downstream gene of Ahr that mediates some of the immunomodulatory properties of Ahr in autoimmunity [11, 12].
Several studies have introduced endogenous and exogenous ligands that interact with Ahr to attenuate the inflammatory response in animal models of autoimmunity [13, 14]. Two of the potential mechanisms of the reported therapeutic potential of Ahr ligands is promoting the generation of regulatory T (Treg) cells and suppressing proinflammatory mediators. For example, 2-(1' H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), an endogenous Ahr ligand, ameliorates autoimmune inflammation by inducing Treg cells and reducing proinflammatory cytokine levels and the macrophage frequency in experimental models of colitis [13] and uveoretinitis [15]. 3,3'-Diindolylmethane (DIM), a dietary Ahr ligand, shifts the balance among T helper 2 (Th2)/Th17/Treg cells toward Treg cells to ameliorate colitis [16]. Moreover, norisoboldine, a natural Ahr ligand identified by means of in silico, in vitro and in vivo investigations, alleviates autoimmune inflammation by inducing the generation of Treg cells and suppressing proinflammatory cytokines in experimental models of arthritis [17] and colitis [18].
In an experimental model of MS, DIM- and indole-3-carbinol (I3C)-activated Ahr were shown to inhibit clinical symptoms and cellular infiltration within the CNS by promoting the generation of Treg cells while suppressing myelin oligodendrocyte glycoprotein (MOG)-specific Th17 cells [19]. Laquinimod, an oral drug being evaluated for the treatment of MS, attenuates experimental autoimmune encephalomyelitis (EAE) by inducing the generation of Treg cells and suppression of proinflammatory cytokines in an Ahr-dependent fashion [20]. Furthermore, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) activates Ahr to induce miR-132-mediated cholinergic anti-inflammatory processes in EAE [11]. It has been recently shown that type I interferons (IFN-Is) in combination with indole, indoxyl-3-sulfate (I3S), indole-3-propionic acid (IPA) and indole-3-aldehyde (IAld) activate Ahr signaling in astrocytes to suppress CNS inflammation in EAE [21].
In the current study, we used a combination of in silico, in vitro and in vivo approaches to identify a natural Ahr ligand with therapeutic potential in EAE. For the first time, we introduce gallic acid (GA) as a novel Ahr ligand of natural origin and provide a mechanistic explanation for the anti-inflammatory properties of GA.
Materials and methods
In silico analysis
The alignment of the Ahr PAS-A sequence (UniProt; P30561) with several orthologues was performed by ClustalX 2.0 [22]. The Ahr PAS-A three-dimensional (3D) structure was obtained from the RCSB Protein Data Bank (ID: 4M4X; http://www.rcsb.org), and the chemical structure of GA was obtained from the PubChem database (CID_370; www.ncbi.nlm.nih.gov/pccompound). The sequence of mouse PAS-B (NP_038492.1) was obtained from NCBI (https://www.ncbi.nlm.nih.gov/protein/). Modeling of the 3D structure was established for the stereochemical value by SAVES version v5.0 software (https://services.mbi.ucla.edu/SAVES/), and the 3D structure of PAS-B was coordinated by using PS2 software (http://www.ps2.life.nctu.edu.tw/) [23]. The confirmation of model overlap with the retrieved human C-terminal PAS domain of HIF2a was performed by using SALIGN software (https://modbase.compbio.ucsf.edu/salign/).
The molecular docking simulation of GA against Ahr domains was performed by using SYBYLX 2.1 (Tripos Associates Inc.). The docking conditions included applying the ChemPLP scoring function within the genetic algorithm docking program GOLD 5.2 (Cambridge Crystallographic Data Centre). Based on the obtained scores and the molecular orientation within the binding pocket, the molecule with the best score was selected and merged into the receptor. The model-ligand complexes were energetically adjusted using the Tripos Force Field (Gasteiger-Hückel charges, distance-dependent dielectric constant = 4.0) to optimize the interactions between the ligand and receptor within the binding cavity.
Mice and ethics statement
The female 6-8-week-old C57BL/6 mice for all experiments were purchased originally from Charles River Laboratories and maintained under specific pathogen-free conditions. All in vivo and in vitro experiments were performed in accordance with protocols approved by the Research Ethics Committee (KFU-REC/2018-3-1) of King Faisal University, Saudi Arabia. The humane endpoints included ≥ 25% body weight loss, paresis or forelimbs paralysis for 24 h.
Cell isolation and differentiation
Naïve T cells were isolated from the spleen by using a MACS CD4+CD62L+ isolation kit (Miltenyi Biotec). The differentiation of Th17, Treg and type 1 regulatory T (Tr1) cells was induced as previously described [6, 24]. The purified CD4+CD62L+ T cells were cultured in the presence of Dynabeads Mouse T-Activator CD3/CD28 (Invitrogen). The cell culture contained IL-6 (30 ng/mL), TGF-β1 (4 ng/mL), anti-IFN-γ and anti-IL-4 (10 μg/mL) to generate Th17 cells; TGF‐β1 (4 ng/mL) and IL‐2 (20 U/mL) to generate Treg cells; and TGF‐β1 (2 ng/mL) and IL‐27 (30 ng/mL) to generate Tr1 cells. All recombinant cytokines were purchased from R&D Systems, and anti-IFN-γ and anti-IL-4 antibodies were obtained from BioLegend. Peritoneal macrophages were stimulated with 0.5 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich). GA, DIM (30 μmol/L) and phytohemagglutinin (PHA; 5 μg/mL) were purchased from Sigma-Aldrich. To isolate microglia, astrocytes and monocytes from the CNS, tissue samples were prepared using the MACS Neuronal Dissociation Tissue Kit, and then cells were isolated using MACS isolation kits. The absolute number of Ly-6Chi monocytes was assessed by flow cytometry. To isolate CD4+ and CD4+CD45+ cells from the CNS, mononuclear cells were separated from the CNS using a gradient comprising 30% and 70% Percoll solutions (GE Healthcare) as previously described [25], and then the cells were isolated using MACS CD4+ and CD45+ cell isolation kits following the manufacturer's instructions.
Quantitative real-time PCR
cDNA was synthesized by using a TaqMan reverse transcription kit and amplified by using a ViiA7 system and TaqMan gene expression assays for Cyp1a1 (Mm00487218_m1), Rorc (Mm01261022_m1), Foxp3 (Mm00475162_m1), Il6 (Mm00446190_m1), Il10 (Mm01288386_m1), Ccl2 (Mm00441242_m1), Csf2 (Mm01290062_m1), Nos2 (Mm00440502_m1) and Gapdh (Mm99999915_g1) for coding genes or TaqMan microRNA assays for has-miR-132 (ID: 000457), has-miR-212 (ID: 002551) and RNU6B (ID: 001093) for noncoding genes. Kits, probes and reagents were obtained from Applied Biosystems. The relative expression of mRNAs was calculated by the ΔΔCt method.
Transfection and luciferase activity
CD4+CD62L+ cells and peritoneal macrophages were transfected with oligonucleotides using Primary Cell Nucleofector kits and a 4D-Nucleofector system (Lonza). An siRNA specific for Ahr (siAhr, 75 nmol/L), a nonspecific siRNA (siNS, 75 nmol/L), an antisense (as)-miR-132 (250 μmol/L) and a scrambled control (250 μmol/L) were obtained from Ambion. qPCR and immunoblotting were used to confirm transfection efficiencies. Reporter plasmid and luciferase activities in the presence of siAhr were assessed following a modified method described elsewhere [26]. A 4,553 bp fragment of the mouse miR-212/132 promoter [27] was amplified using the following oligonucleotide primers: forward, 5'-AGATCGCCGTGTAATTCTAGAGGGAAGGTTCTGTCTTCAAATGAGGAACTC-3' and reverse, 5'-TTCTCGCCACCTTAGGCAGCGATACCCGGCCGCCCCGACTCTAGA-3'. The purified PCR product was cloned into the XbaI restriction site of the pGL3 plasmid (Promega) using the In-Fusion HD Cloning Kit (Clontech). The empty or miR-212/132 promoter-encoding pGL3 vector (100 ng) was cotransfected with siNS or siAhr into CD4+CD62L+ cultured under Th17 cell-conditions and macrophages by electroporation. Luciferase activity was quantified using the Dual-Luciferase Reporter System (Promega) following the manufacturer's instructions.
Protein quantification
Cell lysates were prepared using the RIPA Lysis Buffer System and fractionated by SDS-PAGE. Target proteins in the lysates and in immunoprecipitation eluates were detected by rabbit polyclonal antibodies specific for Cyp1a1, Ahr, Arnt and AChE (dilution; 1:500), mouse monoclonal antibodies specific for β-actin (dilution; 1:1,000) and the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000). The Lysis Buffer System and antibodies were purchased from Santa Cruz Biotechnology. Band intensity was quantified by ImageJ software (version 1.48; https://imagej.nih.gov/ij/download.html). To quantify the serum and supernatant cytokine levels, ELISA kits for IL-17a, IL-6, TNF-α, TGF-β (Invitrogen), and IL-10 (GenWay) were used following the manufacturer’s instructions.
Flow cytometry
Isolated CD4+ T cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (Sigma-Aldrich) and 800 ng/mL ionomycin (Sigma-Aldrich) for 5 h, with Protein Transport Inhibitor (Invitrogen) added for the final 2 h. An Intracellular Staining kit (Life Technologies) and phycoerythrin (PE)-conjugated anti-IL-17 antibodies (eBioscience) were used following the manufacturer’s instructions. Foxp3 was stained by using a Foxp3 Staining kit (Invitrogen) including fluorescein isothiocyanate (FITC)-conjugated anti-Foxp3 according to the manufacturer’s instructions. For surface staining, PerCP-Cy5.5-conjugated anti-CD4 antibodies and FITC-conjugated anti-CD45 antibodies from eBioscience were used. The analysis was performed by using a FlowSight system (Amnis).
EAE models
Mice were immunized with MOG35-55 (150 μg; Peptide International) emulsified in complete Freund's adjuvant (CFA; Sigma-Aldrich) containing 10 mg/mL heat‐killed Mycobacterium tuberculosis H37Ra (Difco Laboratories). The mice were injected intraperitoneally with pertussis toxin (List Biological Laboratories; 500 ng) on days 0 and 2. The adoptive transfer of encephalitogenic CD4+ T cells isolated 9 days after immunization was also used to induce EAE. The encephalitogenic CD4+ T cells were restimulated with MOG35‐55 (30 μg/mL) and IL‐23 (20 ng/mL; R&D Systems) for 72 h before being injected in naïve mice at a dose of 1×107 cell/mouse. Scoring of clinical symptoms was as follows: 0, no clinical signs; 1, hind limb weakness or limp tail; 2, paralyzed hind limb; 3, paralyzed forelimb; 4, complete paralysis; and 5, death. The mice were injected intraperitoneally (i.p.) with GA (2 mg/day) or vehicle (corn oil) for 10 days starting one day before the MOG35-55 immunization.
Statistical analysis
Data were pooled and are shown as the mean ± SD from three independent experiments performed in triplicate using three mice per experiment unless otherwise indicated. The mean values were tested for statistical significance by one‐way ANOVA. The statistical significance of the differences between EAE clinical scores was analyzed by two‐way ANOVA, and the X2 test was used to test differences between EAE incidence rates (%). *p < 0.05.
Results
GA is a potential Ahr ligand
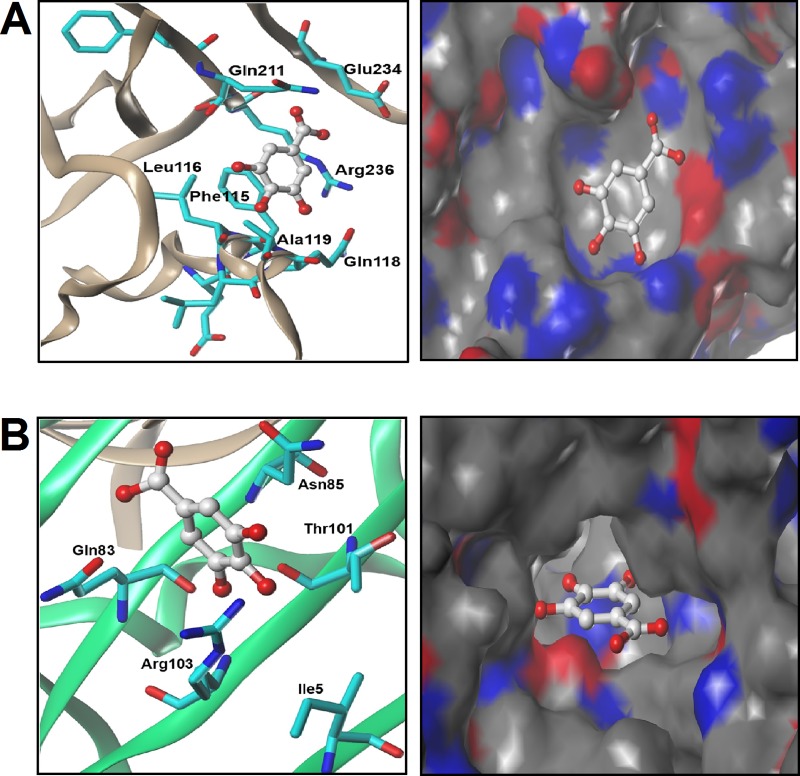
In an attempt to identify a novel Ahr ligand, we first ran an in silico screening experiment using molecular docking simulation. Ahr, like other members of the bHLH family, contains PAS-A and PAS-B domains that are involved in transforming Ahr into its transcriptionally active form by the formation of the Ahr/Arnt complex. Therefore, both domains were used to perform docking simulation with a customized screening library that included natural aromatic hydrocarbons. Among the tested molecules, GA formed a relatively strong interaction with Ahr PAS-A residues with a binding score of 39.4 ChemPLP. It formed five hydrogen bonds with the PAS-A residues Phe115, Leu116, Ala119, Gln211 and Glu234 (Fig 1A and 1B). Furthermore, GA formed an ionic interaction between its negatively charged carboxylate group and the positively charged guanidine moiety of Arg236 (Fig 1A).
Fig 1. GA has binding potential to Ahr domains.
The binding mode of docked GA (ball and stick model) in mouse Ahr (A) PAS-A and (B) PAS-B binding sites. The labelled residues in left panel represent the major interacting amino acids in the binding site that interact with GA. Right panel represents contour maps for the binding site of GA showing polar areas (blue and red) and hydrophobic areas (gray).
The PAS-B domain of Ahr plays a critical role in Ahr activation by serving as a ligand-binding domain. Docking analysis revealed that GA formed a relatively strong interaction with PAS-B residues with a binding score of 46.24 ChemPLP. GA formed one hydrogen bond with Gln83, one hydrogen bond with Asn85 and two hydrogen bonds with Arg103 (Fig 1B). In addition, a hydrophobic interaction was predicted to occur between Thr101 and Ile5. Taken together, these observations suggest that GA is a potential Ahr ligand. The 3D coordinates of PAS-B, a Ramachandran plot and the analysis of G-factor parameters are presented in S1 Fig.
GA induces the expression of Ahr downstream genes
Ahr classically forms a heterodimeric complex with Arnt and translocates to the nucleus to induce the transcription of downstream genes such as Cyp1a1. Therefore, we first examined the effect of GA on Cyp1a1 gene expression in naïve CD4+ T cells cultured under Th17 conditions cells and LPS-stimulated macrophages, the cells implicated in EAE pathogenesis. The efficiency of the effector CD4+ cells-inducing milieus and the stimulation of macrophages were confirmed (S2 Fig).
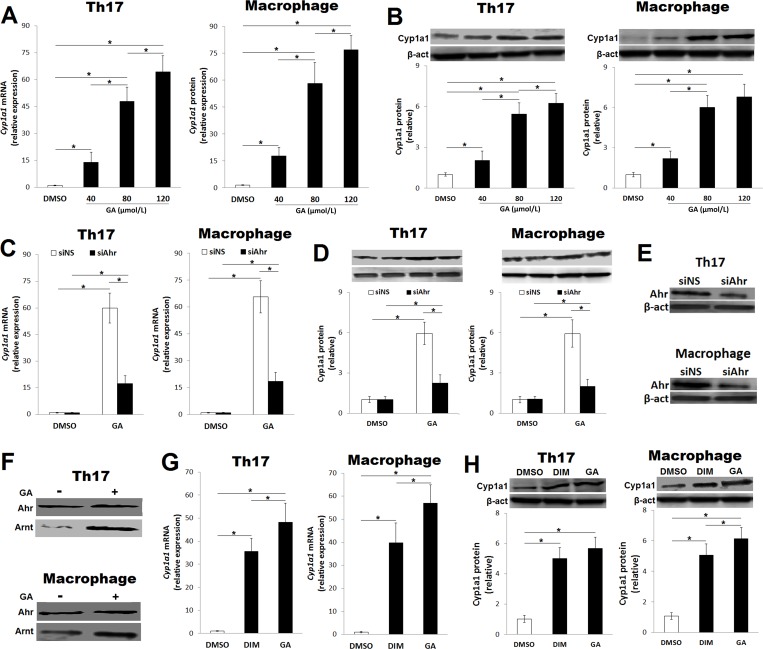
As depicted in Fig 2A and 2B, GA (40–120 μmol/L) upregulated Cyp1a1 mRNA and protein expression in differentiating Th17 cells and macrophages in a concentration-dependent fashion. Studying the effects of GA on cell viability showed a reduction in cell viability at a dose of 120 μmol/L GA (S3 Fig). Thus, 80 μmol/L GA was used hereafter. The depletion of Ahr by RNA interference abolished the enhancing effects of GA (80 μmol/L) on Cyp1a1 gene expression (Fig 2C and 2D), suggesting an Ahr-dependent mode of action. The efficiency of Ahr depletion by a specific siRNA (siAhr) was confirmed (Fig 2E).
Fig 2. GA induces gene expression of Cyp1a1 and formation of Ahr/Arnt complex.
The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. The expression of Cyp1a1 mRNA was assessed by quantitative real‐time PCR and normalized to Gapdh mRNA, and protein level was assessed by immunoblot using Actin as a loading control. (A) Relative expression of Cyp1a1 mRNA at 12 h in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages in presence of GA (40–120 μmol/L) compared to vehicle (DMSO). (B) Immunoblot and relative protein level of Cyp1a1 at 48 h in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages in presence of GA (40–120 μmol/L) compared to DMSO. (C) Relative expression of Cyp1a1 mRNA at 12 h in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages electroporated with Ahr siRNA (siAhr) or non-specific siRNA (siNS) in presence of GA (80 μmol/L) compared to DMSO. (D) Immunoblot and relative protein level of Cyp1a1 at 48 h in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages electroporated with siAhr or siNS in presence of GA (80 μmol/L) compared to DMSO. (E) Efficiency of siAhr in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages was confirmed by immunoblot. (F) Detection of Arnt protein by immunoblot in the eluates pulled down by Ahr antibodies using CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages. (G and H) Relative expression of (G) Cyp1a1 mRNA and (H) protein in presence of DIM (25 μmol/L) or GA (80 μmol/L) in CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages compared to DMSO. Data were pooled from three independent experiments with three mice per experiment and shown as mean ± SD. *p < 0.05, (one‐way ANOVA); horizontal bars denote statistical comparison.
Because the dimerization of Ahr with Arnt is required to activate the transcriptional activity of Ahr, we examined the formation of the heterodimer by immunoprecipitation using anti-Ahr antibodies. Detection of the Arnt protein in an eluate confirmed the formation of the Ahr/Arnt complex in GA-treated differentiating Th17 cells and macrophages (Fig 2F). Finally, we compared the effects of GA and DIM, a well-known natural ligand of Ahr, on the expression of the Cyp1a1 gene. Interestingly, the Cyp1a1 mRNA and protein levels were significantly higher with GA treatment than DIM treatment (Fig 2G and 2H).
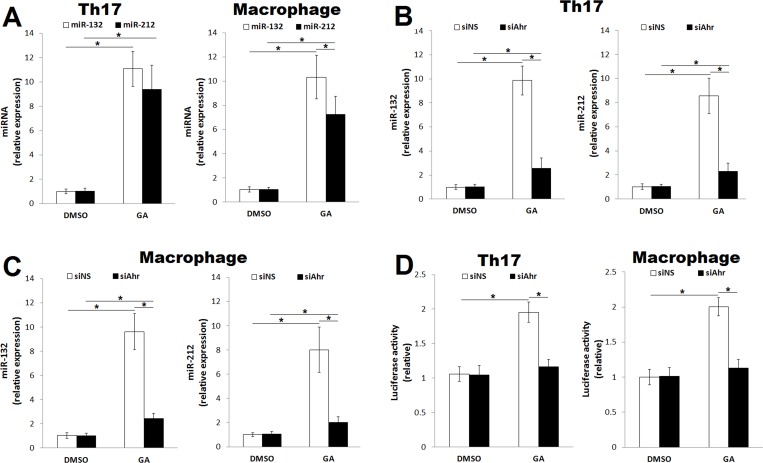
We previously identified the miR-212/132 cluster as a downstream gene of ligand-activated Ahr [11, 12]. Therefore, we tested whether GA-activated Ahr induces the expression of miR-132 and miR-212. As predicted, GA treatment (80 μmol/L) induced the expression of miR-132 and miR-212 in both polarized Th17 cells and macrophages (Fig 3A), and knocking down Ahr expression abrogated these effects (Fig 3B and 3C). To examine whether Ahr has a direct transcriptional activity on miR-212/132, differentiating Th17 cells and macrophages were cotransfected with a reporter plasmid encoding the miR-212/132 promoter and siAhr. As depicted in Fig 3D, GA-activated Ahr induced luciferase activity driven by the miR-212/132 promoter, whereas knocking down Ahr expression abolished this effect, indicating a direct transcriptional activation role for Ahr on miR-212/132. Taken together, these findings suggest that GA is a novel Ahr agonist.
Fig 3. GA-activated Ahr induces the expression of miR-212/132 cluster.
The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-polarizing conditions, and peritoneal macrophages were stimulated with LPS. The expression of miR-132 and miR-212 were assessed by quantitative real‐time PCR and normalized to RNU6B mRNA. (A) Relative expression of miR-132 and miR-212 in CD4+CD62L+ T cells cultured under Th17-polarizing conditions (72 h) and macrophages (36 h) in presence of GA (80 μmol/L) compared to vehicle (DMSO). (B) Relative expression of miR-132 and miR-212 in CD4+CD62L+ T cells cultured under Th17-polarizing conditions (72 h) and electroporated with Ahr siRNA (siAhr) or non-specific siRNA (siNS) compared to DMSO. (C) Relative expression of miR-132 and miR-212 in macrophages (36 h) electroporated with siAhr or siNS compared to DMSO. (D) Relative luciferase activity of miR-212/132 promoter reporter cotransfected with siNS or siAhr into CD4+CD62L+ T cells cultured under Th17-polarizing conditions and macrophages compared to DMSO. Data were pooled from three independent experiments with three mice per experiment and shown as mean ± SD. *p<0.05, (one‐way ANOVA); horizontal bars denote statistical comparison.
GA ameliorates EAE
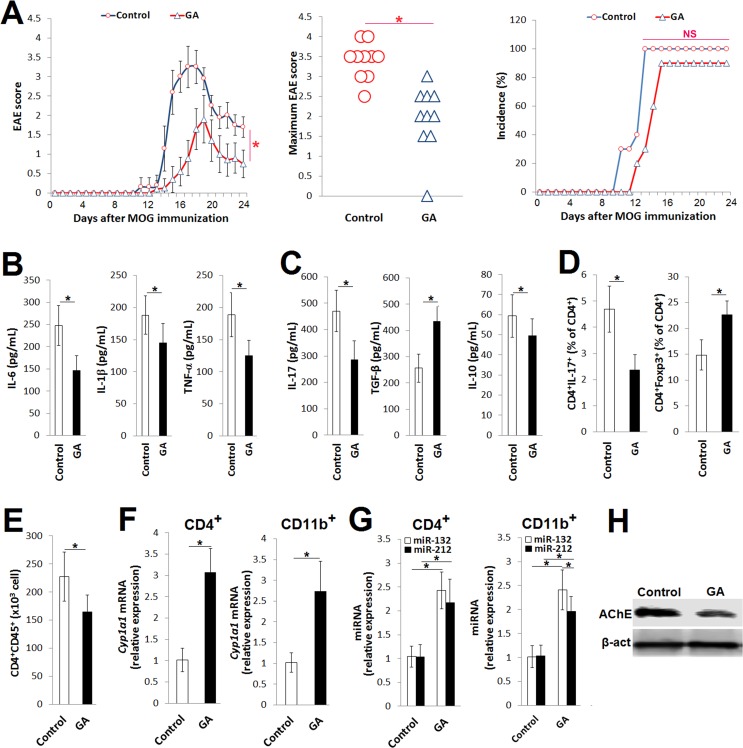
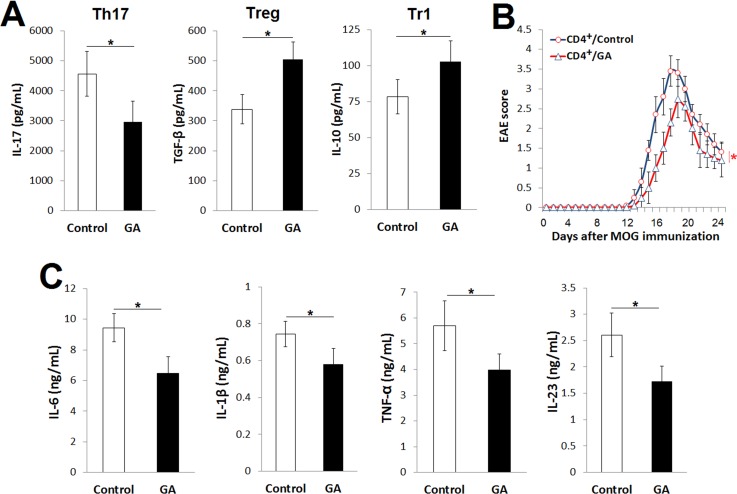
To ascertain the biological significance of Ahr activation by GA in vivo, we first compared disease severity between control EAE (Control) and GA-treated EAE (GA) mice. Studying the effects of different GA doses on the weight of the spleen and liver showed a significant increase in liver weight at a GA dosage of 4 mg/day (S3 Fig). GA treatment for 10 days, starting one day prior to MOG35-55 immunization, attenuated clinical and maximum EAE scores but not disease incidence (Fig 4A). The attenuated EAE symptoms were associated with lower cytokine levels of IL‐6, IL-1β and TNF‐α on day 24 after MOG35-55 immunization (Fig 4B). Moreover, the CD4+ T cells isolated from the inguinal lymph nodes of the GA mice 9 days after immunization produced less IL‐17 in response to restimulation with MOG35-55 than the CD4+ T cells from the Control mice (Fig 4C). Notably, GA enhanced the production of TGF-β but decreased that of IL-10 (Fig 4C). Consistent with the ameliorated EAE symptoms and altered levels of IL-17 and TGF-β, a flow cytometry analysis revealed that GA reduced the frequency of CD4+IL-17+ T cells and increased that of CD4+Foxp3+ T cells (Fig 4D). Because the infiltration of activated T cells is important for clinical EAE symptoms, we compared the number of CD4+CD45+ T cells in the CNS between the Control and GA EAE mice on day 12 after immunization. As predicted, the total number of CD4+CD45+ T cells was significantly lower in the CNS of the GA EAE mice (Fig 4E).
Fig 4. GA-activated Ahr alleviates EAE severity.
The mice were immunized with MOG35-55 emulsified in CFA. The mRNA expression of Cyp1a1 and miR-212/132 cluster were assessed in isolated CD4+ T cells and CD11b+ cells by quantitative real-time PCR and normalized to the mRNAs of Gapdh for Cyp1a1 and RNU6B for miRNAs. The levels of cytokines were quantified by ELISA. (A) EAE clinical score, maximum score and incidence (%) of Control EAE (Control) and GA EAE mice, n = 10 each. (B) Serum levels of IL-6, IL-1β and TNF-α in EAE mice 24 days after MOG35-55 immunization. (C) Levels of IL-17, TGF-β and IL-10 in culture supernatant of encephalitogenic CD4+ T cells restimulated with MOG35-55 and IL-23 for 72 h. (D) Frequency (%) of CD4+IL-17+ and CD4+Foxp3+ T cells in total CD4+ T cells isolated from inguinal lymph nodes 24 days after MOG35-55 immunization. (E) Absolute number of CD4+CD45+ T cells in the CNS 18 days after MOG35-55 immunization. (F) Relative expression of Cyp1a1 mRNA in CD4+ T cells and CD11b+ cells isolated from spleen 10 days after MOG35-55 immunization compared to Control. (G) Relative expression of miR-132 and miR-212 in CD4+ T cells and CD11b+ cells isolated from spleen 10 days after MOG35-55 immunization compared to Control. (H) Representative immunoblot of AChE in spleen WBCs 10 days after MOG35-55 immunization. Data were pooled from three independent experiments and shown as mean ± SD. *p<0.05; (A) EAE score, two‐way ANOVA; (A) incidence (%), X2; (A) maximum score and (B-G), one-way-ANOVA; horizontal bars denote statistical comparison.
We next examined whether GA induces the transcription of the Cyp1a1 gene in CD4+ T cells and CD11b+ macrophages collected from the spleen of EAE mice. In line with the in vitro results, the mRNA expression of Cyp1a1 was upregulated in the examined cells (Fig 4F). Furthermore, GA induced the expression of miR-132 and miR-212 in CD4+ T cells and CD11b+ macrophages from the spleen of EAE mice (Fig 4G). The activation of Ahr in vivo induces acetylcholinesterase (AChE)-targeting miR-132 to potentiate cholinergic anti-inflammatory processes [11, 28]. Interestedly, the AChE protein level was reduced in the spleens from the GA EAE mice (Fig 4H), suggesting that GA could potentiate cholinergic anti-inflammatory processes. This finding was supported by the in vitro data showing that GA suppressed the AChE activity in PHA-stimulated CD4+ T cells and LPS-stimulated macrophages (S4 Fig). Collectively, these results indicate that the ameliorative effects of GA are at least partially attributed to Ahr activation.
The ameliorative effects of GA on EAE are not entirely mediated by CD4+ T cells
Th17 cells, which correlate reciprocally with Treg cells, play a pivotal role in the pathogenesis of EAE. Therefore, we studied the effects of GA on the effector functions of polarized Th17, Treg and Tr1 cells. Consistent with the in vivo observations, GA (80 μmol/L) suppressed the production of IL-17 and enhanced that of TGF-β (Fig 5A). However, GA treatment showed a discrepancy in IL-10 production between the in vitro and in vivo experiments. Subsequently, we studied the intrinsic role of CD4+ T cells in EAE. Thus, we induced EAE by adoptively transferring encephalitogenic CD4+ T cells isolated from the Control or GA EAE mice. These CD4+ T cells were restimulated with MOG35‐55 and IL‐23 for 72 h before being transferred into naïve mice. As shown in Fig 5B, the encephalitogenic CD4+ T cells from the GA mice induced mildly ameliorated EAE symptoms, indicating that the ameliorative effects of GA on EAE symptoms were not solely attributable to CD4+ T cells.
Fig 5. GA alleviates EAE by CD4+-dependent and -independent mechanisms.
The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-, Treg- or Tr1-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. (A) Levels of IL-17, TGF-β and IL-10 at 72 h in culture supernatant of polarized Th17, Treg or Tr1cells, respectively. (B) Clinical scores of EAE induced by adoptive transfer of encephalitogenic CD4+ T cells from control EAE (Control) or GA EAE mice, n = 10 each. (C) Levels of IL-6, IL-1β, TNF-α and IL-23 at 16 h in culture supernatant of macrophages. Data were pooled from three independent experiments with three mice per experiment and shown as mean ± SD. *p<0.05; (A and C), one-way-ANOVA; (B), two-way ANOVA.
Macrophages contribute to EAE pathogenesis by producing proinflammatory mediators. Therefore, we studied the effects of GA on proinflammatory cytokines in the culture supernatant of LPS-stimulated macrophages. Consistent with the in vivo data, GA (580 μmol/L) suppressed the production of IL-6, IL-1β and TNF-α (Fig 5C). We also found that GA suppressed IL-23 production in response to LPS stimulation (Fig 5C). Therefore, the anti-inflammatory effects of GA on macrophages could be involved in the anti-inflammatory effects of GA on EAE.
GA modulates the pathogenic activities of astrocytes
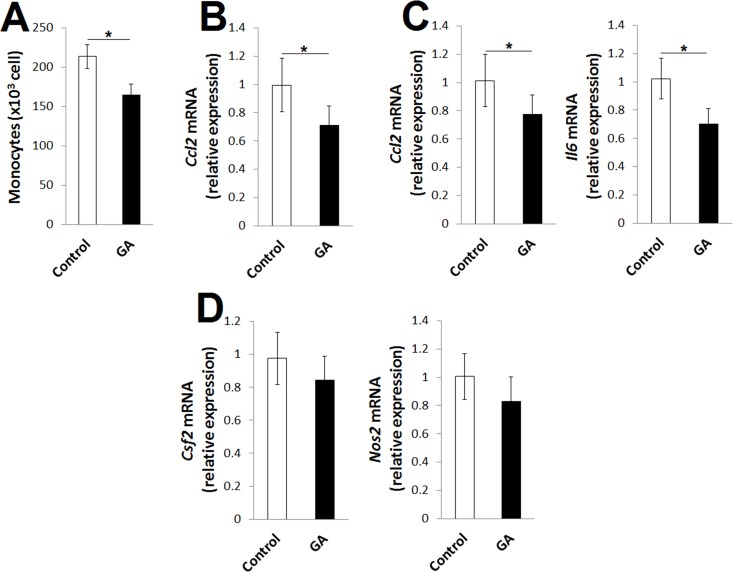
IFN-Is in combination with Ahr ligands induce Ahr signaling in astrocytes, and this signaling attenuates CNS inflammation in EAE by suppressing colony stimulating factor 2 (Csf2), nitric oxide synthase 2 (Nos2) and monocyte chemotactic and activating factor (Ccl2) [21]. CCl2 exacerbates inflammation in EAE by recruiting monocytes to the site of inflammation [29]. Based on these observations, we examined whether GA affects monocyte infiltration within the CNS. The GA EAE mice showed significantly reduced numbers of infiltrating monocytes within the CNS on day 18 after MOG35-55 immunization (Fig 6A). This observation prompted us to quantify the mRNA expression of Ccl2 in astrocytes from EAE mice. Interestingly, a significant reduction in the mRNA expression of Ccl2 was found with GA treatment (Fig 6B).
Fig 6. GA modulates pathogenic activities of astrocytes and microglia in EAE mice.
The mice were immunized with MOG35-55 emulsified in CFA, and samples were collected 18 days later. The mRNA expressions of Ccl2, Il6, Csf2 and Nos2 were assessed by quantitative real‐time PCR and normalized to Gapdh mRNA. (A) Absolute number of infiltrated monocytes within the CNS of control EAE (Control) and GA EAE mice. (B) Relative expression of Ccl2 mRNA in isolated astrocytes compared to Control. (C) Relative mRNA expression of Ccl2 and Il6 in isolated microglia compared to Control. (D) Relative expression of Csf2 and Nos2 in isolated astrocytes compared to Control. Data were pooled from two independent experiments with three mice per experiment and shown as mean ± SD. *p<0.05, (one‐way ANOVA); horizontal bars denote statistical comparison.
Ahr signaling in astrocytes modulates the polarization and activation of microglia and monocytes [21]. Therefore, we quantified the mRNA expression of Ccl2 and Il6 in microglia from the CNS of EAE mice 18 days after MOG35-55 immunization. Interestingly, GA also repressed the mRNA expression of Ccl2 and Il6 in these cells (Fig 6C), suggesting that the modulatory effects of GA on the pathogenic activities of astrocytes might contribute to the ameliorative effects of GA on EAE. Notably, further investigation showed that the inhibitory effects of GA on the mRNA expression of Csf2 and Nos2 in astrocytes did not achieve statistical significance (Fig 6D).
Discussion
Despite the remarkable progress in understanding the genetic control of MS [30], limited evidence is available about the roles of environmental factors [4]. In this context, Ahr exemplifies a unique link between the immune system and environment by interacting with a variety of exogenous aromatic hydrocarbons. The accumulated evidence clearly demonstrates that the activation of Ahr by exogenous ligands attenuates inflammation in a murine model of MS [19–21]. Therefore, identifying new Ahr ligands is a promising treatment strategy to control proinflammatory mediators in MS patients. Herein, we identified GA as a novel Ahr agonist of natural origin using in silico, in vitro and in vivo approaches.
Ahr includes two PAS domains, PAS-A and PAS-B, of which PAS-A is critically involved in Ahr/Arnt dimerization [31]. Molecular docking simulation analysis revealed the formation of five hydrogen bonds with five residues in the PAS-A domain. Importantly, three of these residues, Phe115, Leu116, and Ala119, are hydrophobic residues that play important roles in the formation of the Ahr/Arnt complex [32]. The PAS-B domain of Ahr is essential for sensing the environment and activating Ahr through interactions with ligands [31, 33, 34]. Furthermore, PAS-B plays roles in the initiation of Ahr/Arnt dimerization [33]. An analysis of the predicted interaction between PAS-B and GA revealed relatively strong interactions between PAS-B and GA. It has been demonstrated that the formation of the Ahr/Arnt heterodimer, which transforms Ahr into a transcriptionally active form, initiates the transcription of downstream genes such as Cyp1a1 [35, 36] and the miR-212/132 cluster [8, 37]. Consistently, our data showed that GA promotes the formation of the Ahr/Arnt complex and enhances the expression of Cyp1a1 and the miR-212/132 cluster.
A number of reports have studied the modulatory effects of GA on different aspects of the immune response [38, 39]. GA attenuates experimental colitis by suppressing IL-6, IL-1β, TNF-α, IL-17 and IFN-γ production and inhibiting p65-NF-κB and IL-6/p-STAT3 (Y705) activation [40]. GA suppresses the expression of several proinflammatory mediators, such as IL-6, IL-1β, CCL2 and CCL7, in fibroblast-like synoviocytes from rheumatoid arthritis patients [41]. However, to our knowledge, the effects of GA on the pathogenesis of autoimmune encephalomyelitis have never been investigated. In the present study, we found that the activation of Ahr by GA in vivo ameliorated EAE severity by suppressing the production of IL-6, IL-1β, TNF-α and IL-17 and enhancing that of TGF-β. In addition, GA reduced the frequency of CD4+IL-17+ T cells and increased that of Foxp3+CD4+ T cells. Consistently, previous studies have demonstrated that the activation of Ahr by exogenous ligands attenuates autoimmune inflammation in EAE by inhibiting IL-17-producing Th17 cells and proinflammatory cytokines and promoting the generation of TGF-β-producing Treg cells [19, 42, 43]. Several mechanisms have been proposed to elucidate the ameliorative effects of TGF-β on EAE. For instance, TGF-β suppresses the proliferation, differentiation and effector functions of encephalitogenic effector CD4+ T cells [44]. Furthermore, the activation of TGF-β-secreting Treg cells suppresses the infiltration of pathogenic T cells into the CNS [45]. Based on these observations, the GA-mediated increases in the TGF-β level and CD4+Foxp3+ cell frequency might have contributed to the reduced Th17 cell frequency, IL-17 production and number of infiltrating CD4+CD45+ cells in the CNS of the GA EAE mice. However, further investigation is required to identify the exact mechanism through which GA-induced TGF-β ameliorates EAE.
AChE catalyzes the breakdown of acetylcholine (ACh) and mitigates its anti-inflammatory potential. Therefore, targeting AChE augments cholinergic anti-inflammatory processes [46]. It has been demonstrated that the activation of Ahr upregulates the expression of AChE-targeting miR-132, followed by augmented cholinergic anti-inflammatory processes, in which IL-6, IL-1β, TNF-α and IL-17 levels are downregulated [11, 28]. Herein, we found that the GA EAE mice exhibited upregulation of miR-132 expression in CD4+ T cells and macrophages concomitant with downregulation of AChE, IL-6, IL-1β, TNF-α and IL-17 expression. Furthermore, GA inhibited AChE catalytic activity in CD4+ T cells and macrophages in vitro in a miR-132-dependent fashion, suggesting that GA could potentiate cholinergic anti-inflammatory processes. Consistent with the dual anti-inflammatory effects of GA on CD4+ T cells and macrophages in vitro, the adoptive transfer of encephalitogenic CD4+ T cells from the GA EAE mice induced mildly ameliorated disease symptoms. Taken together, our data indicate that GA potentiates cholinergic anti-inflammatory processes in CD4+ T cells and macrophages that contribute partially to the anti-inflammatory effects of GA on EAE.
Interestingly, we found that GA suppressed the expression of Ccl2 in astrocytes and microglia from EAE mice. Recent supporting observations have shown that Ahr controls the proinflammatory functions of astrocytes during EAE by limiting the recruitment of NF-κB to the promoters of responding factors such as Ccl2, Csf2 and Nos2 [21]. CCl2 is implicated in the recruitment of monocytes [47] and T cells [48] to the site of inflammation. Therefore, the reduced expression of Ccl2 in these CNS-resident cells might have contributed to the reduced number of infiltrating monocytes and CD4+CD45+ T cells. Moreover, the reduced number of infiltrating monocytes could be a part of the GA-potentiated cholinergic anti-inflammatory processes. This suggestion is supported by recent findings showing that the ligation of nicotinic acetylcholine receptor inhibits the infiltration of monocytes and neutrophils into the CNS and suppresses the mRNA expression of Ccl2 and chemokine C-X-C motif ligand 2 (CXCL2) in the brain [49]. It has been demonstrated that Ahr signaling in astrocytes regulates their neurotoxic activities during EAE, the activation of microglia and monocytes and the recruitment of monocytes to the CNS [21]. These observations may partially explain the reduced expression of Il6 in microglia. Notably, IL-6 and IL-23a are associated with differentiation and effector functions in Th17 cells [50]. Overall, the cholinergic anti-inflammatory processes and modulation of the pathogenic activity of astrocytes might have contributed to the anti-inflammatory effects of GA on EAE.
In summary, our findings identify GA as a novel Ahr ligand of natural origin and clearly indicate that GA has anti-inflammatory properties in EAE. The mechanisms underlying the ameliorative effects of GA on EAE involve promoting Treg cell generation, potentiating miR-132-mediated cholinergic anti-inflammatory processes and modulating the pathogenic activities of astrocytes. These findings suggest that GA is a promising candidate to control inflammation in autoimmune diseases such as MS.
Supporting information
A) Superimposition of predicted 3D structure of mouse Ahr PAS-B domain with the human C-terminal PAS domain of HIF2a (PDB ID: 1p97). (B) Ramachandran plot of predicted mouse Ahr PAS-B domain.
(PDF)
The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-, Treg or Tr1-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. The mRNA expression of Rorc, Il6, FoxP3 and Il10 were assessed by quantitative real‐time PCR and normalized to Gapdh mRNA. (A) Relative expression of Rorc in CD4+CD62L+ T cells cultured under Th17-conditions for 48 hr compared to Th0. (B) Relative expression of Il6 mRNA in macrophages stimulated with LPS for 4 hr. (C) Relative expression of FoxP3 mRNA in CD4+CD62L+ T cells cultured under Treg-polarizing conditions for 48 hr. (D) Relative expression of Il10 mRNA in CD4+CD62L+ T cells cultured under Tr1-polarizing conditions for 56 hr. Data were pooled from independent experiments and shown as mean ± SD.
(PDF)
(A and B) The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. Cell viability of (A) differentiating Th17 and (B) peritoneal macrophages 48 hr after stimulation in presence of GA (20–120 μmol/L). (C and D) The EAE was induced by immunizing mice with MOG35-55 emulsified in CFA. The mice were injected intraperitoneally with vehicle (corn oil) or GA (1–4 mg/day) for 14 days starting one day before MOG35-55 immunization. Weight of (C) spleen and (D) liver were measured 24 h after last dose, n = 6. Data were pooled from independent experiments and shown as mean ± SD. *p < 0.05.
(PDF)
AChE catalytic activity in culture supernatant of (A) CD4+ T cells isolated from naive mice and stimulated with PHA and (B) peritoneal macrophages were stimulated with LPS. The PHA-stimulated CD4+ T cells and LPS-stimulated macrophages were electroporated with antisense (as)-miR-132, and cells treated with PHA, PHA+GA, LPS and LPS+GA were electroporated with scramble hairpin inhibitor. Data were pooled from independent experiments and shown as mean ± SD. *p < 0.05, PHA+GA versus PHA, and LPS+GA versus LPS; ǂp < 0.05, PHA+GA+as-miR-132 versus PHA, and LPS+GA+as-miR-132 versus LPS; #p < 0.05, PHA+GA versus PHA+GA+as-miR-132, and LPS+GA versus LPS+GA+as-miR-132.
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by the Deanship of Scientific Research, King Faisal University through grants number 150222 and 180090 to HH.
References
- 1.Guarnera C, Bramanti P, Mazzon E. Comparison of efficacy and safety of oral agents for the treatment of relapsing-remitting multiple sclerosis. Drug Des Devel Ther. 2017; 11:2193–2207. 10.2147/DDDT.S137572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milo R. Effectiveness of multiple sclerosis treatment with current immunomodulatory drugs. Expert Opin Pharmacother. 2015; 16:659–673.OpenUrl 10.1517/14656566.2015.1002769 [DOI] [PubMed] [Google Scholar]
- 3.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012; 366:1870–1880. 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- 4.Gutiérrez-Vázquez C, Quintana FJ. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 2018; 48:19–33. 10.1016/j.immuni.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, Nohara K, et al. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci U S A. 2011; 108:14222–14227. 10.1073/pnas.1111786108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinen I, Nakahama T, Kimura A, Nguyen NT, Takemori H, Kumagai A, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015; 27:405–415. 10.1093/intimm/dxv015 [DOI] [PubMed] [Google Scholar]
- 7.Chuang HC, Tsai CY, Hsueh CH, Tan TH. GLK-IKKβ signaling induces dimerization and translocation of the AhR-RORγt complex in IL-17A induction and autoimmune disease. Sci Adv. 2018; 4:eaat5401 10.1126/sciadv.aat5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanieh H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends. Biomed Res Int. 2014; 2014:520763 10.1155/2014/520763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock KW. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem Pharmacol. 2018; 155:419–424. 10.1016/j.bcp.2018.07.032 [DOI] [PubMed] [Google Scholar]
- 10.Quintana FJ. Astrocytes play a crucial role in the formation and evolution of MS lesions-Commentary. Mult Scler. 2018; 23:1352458518796693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanieh H, Alzahrani A. MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: a new Ahr-based exploration. Eur J Immunol. 2013; 43:2771–2782. 10.1002/eji.201343486 [DOI] [PubMed] [Google Scholar]
- 12.Nakahama T, Hanieh H, Nguyen NT, Chinen I, Ripley B, Millrine D, et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc Natl Acad Sci U S A. 2013; 110:11964–11969. 10.1073/pnas.1311087110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abron JD, Singh NP, Mishra MK, Price RL, Nagarkatti M, Nagarkatti PS, et al. An endogenous aryl hydrocarbon receptor ligand, ITE, induces regulatory T cells and ameliorates experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2018; 315:G220–G230. 10.1152/ajpgi.00413.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammadi S, Memarian A, Sedighi S, Behnampour N, Yazdani Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity. 2018; 5:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Nugent LF, Shi G, Vistica BP, Ogbeifun O, Hinshaw SJ, Gery I. ITE, a novel endogenous nontoxic aryl hydrocarbon receptor ligand, efficiently suppresses EAU and T-cell-mediated immunity. Invest Ophthalmol Vis Sci. 2013; 54:7463–7469. 10.1167/iovs.12-11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, Jiang Y, Yang Y, Shao J, Sun X, Chen J, et al. 3,3'-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol. 2013; 53:335–344. 10.1016/j.molimm.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Tong B, Yuan X, Dou Y, Wu X, Chou G, Wang Z, et al. Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. Int J Biochem Cell Biol. 2016; 75:63–73. 10.1016/j.biocel.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 18.Lv Q, Qiao SM, Xia Y, Shi C, Xia YF, Chou GX, et al. Norisoboldine ameliorates DSS-induced ulcerative colitis in mice through induction of regulatory T cells in colons. Int. Immunopharmacol. 2015; 29:787–797. 10.1016/j.intimp.2015.08.040 [DOI] [PubMed] [Google Scholar]
- 19.Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol. 2013; 169:1305–1321. 10.1111/bph.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaye J, Piryatinsky V, Birnberg T, Hingaly T, Raymond E, Kashi R, et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2016; 113:E6145–E6152. 10.1073/pnas.1607843113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016; 22:586–597. 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007; 23:2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 23.Thirugnanasambantham K, Muralidaran S, Mandal AK. Molecular cloning, computational and expression analysis of anthocyanidin reductase in tea (Camellia sinensis). Appl Biochem Biotechnol. 2014; 174:130–145. 10.1007/s12010-014-1038-4 [DOI] [PubMed] [Google Scholar]
- 24.Hanieh H, Masuda K, Metwally H, Chalise JP, Mohamed M, Nyati KK, Standley DM, et al. Arid5a stabilizes OX40 mRNA in murine CD4+ T cells by recognizing a stem-loop structure in its 3 UTR. Eur J Immunol. 2018; 48:593–604. 10.1002/eji.201747109 [DOI] [PubMed] [Google Scholar]
- 25.Pino PA, Cardona AE. Isolation of brain and spinal cord mononuclear cells using percoll gradients. J Vis Exp. 2011; 48: 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanieh H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol Cancer. 2015; 14:172 10.1186/s12943-015-0443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remenyi J1, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS. Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J. 2010. May 13;428(2):281–91. 10.1042/BJ20100024 [DOI] [PubMed] [Google Scholar]
- 28.Alzahrani AM, Hanieh H, Ibrahim HM, Mohafez O, Shehata T, Bani Ismail M, et al. Enhancing miR-132 expression by aryl hydrocarbon receptor attenuates tumorigenesis associated with chronic colitis. Int Immunopharmacol. 2017; 52:342–351. 10.1016/j.intimp.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 29.Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin Immunol. 2002; 103:125–131 10.1006/clim.2001.5167 [DOI] [PubMed] [Google Scholar]
- 30.Patsopoulos NA. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb Perspect Med. 2018; 8:a028951 10.1101/cshperspect.a028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soshilov A, Denison MS. Role of the Per/Arnt/Sim domains in ligand-dependent transformation of the aryl hydrocarbon receptor. Biol Chem. 2008; 283:32995–33005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Potluri N, Kim Y, Rastinejad F. Structure and Dimerization Properties of the Aryl Hydrocarbon Receptor PAS-A Domain. Mol Cell Biol. 2013; 33:4346–4356. 10.1128/MCB.00698-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995; 270:29270–29278. [DOI] [PubMed] [Google Scholar]
- 34.Corrada D, Soshilov AA, Denison MS, Bonati L. Deciphering Dimerization Modes of PAS Domains: Computational and Experimental Analyses of the AhR:ARNT Complex Reveal New Insights Into the Mechanisms of AhR Transformation. PLoS Comput Biol. 2016; 12:e1004981 10.1371/journal.pcbi.1004981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukasawa K, Kagaya S, Maruyama S, Kuroiwa S, Masuda K, Kameyama Y, et al. A novel compound, NK150460, exhibits selective antitumor activity against breast cancer cell lines through activation of aryl hydrocarbon receptor. Mol Cancer Ther. 2015; 14:343–354. 10.1158/1535-7163.MCT-14-0158 [DOI] [PubMed] [Google Scholar]
- 36.Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y, et al. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD+/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018; 9:258 10.1038/s41419-018-0297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanieh H, Mohafez O, Hairul-Islam VI, Alzahrani A, Bani Ismail M, Thirugnanasambantham K. Novel Aryl Hydrocarbon Receptor Agonist Suppresses Migration and Invasion of Breast Cancer Cells. PLoS One. 2016; 11:e0167650 10.1371/journal.pone.0167650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haute GV, Caberlon E, Squizani E, de Mesquita FC, Pedrazza L, Martha BA, et al. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol In Vitro. 2015; 30:309–317. 10.1016/j.tiv.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 39.Pereira ADS, de Oliveira LS, Lopes TF, Baldissarelli J, Palma TV, Soares MSP, et al. Effect of gallic acid on purinergic signaling in lymphocytes, platelets, and serum of diabetic rats. Biomed Pharmacother. 2018; 101:30–36. 10.1016/j.biopha.2018.02.029 [DOI] [PubMed] [Google Scholar]
- 40.Pandurangan AK, Mohebali N, Esa NM, Looi CY, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int Immunopharmacol. 2015; 28:1034–1043. 10.1016/j.intimp.2015.08.019 [DOI] [PubMed] [Google Scholar]
- 41.Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013; 80:274–279. 10.1016/j.jbspin.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Xie O, Zhao B. The Role of AhR in Autoimmune Regulation and Its Potential as a Therapeutic Target against CD4 T Cell Mediated Inflammatory Disorder. Int J Mol Sci. 2014; 15: 10116–10135. 10.3390/ijms150610116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen TL, Makhlouf NT, Anthony BA, Teague RM, DiPaolo RJ. In vitro induced regulatory T cells are unique from endogenous regulatory T cells and effective at suppressing late stages of ongoing autoimmunity. PLoS One. 2014; 13;9:e104698 10.1371/journal.pone.0104698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee PW1, Severin ME1, Lovett-Racke AE1. TGF-β regulation of encephalitogenic and regulatory T cells in multiple sclerosis. Eur J Immunol. 2017. March;47(3):446–453. Epub 2017 Feb 10. 10.1002/eji.201646716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tischner D., Weishaupt A., van den Brandt J., Müller N., Beyersdorf N., Ip C.W., Toyka K.V., Hünig T., Gold R., Kerkau T., Reichardt H.M. Polyclonal expansion of regulatory T-cells interferes with effector cell migration in a model of multiple sclerosis. Brain 2006, 129, 2635–2647. 10.1093/brain/awl213 [DOI] [PubMed] [Google Scholar]
- 46.Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T. Anti-inflammatory properties of cholinergic up-regulation: A new role for acetylcholinesterase inhibitors. Neuropharmacology. 2006; 50:540–547. 10.1016/j.neuropharm.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 47.Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014; 274:53–61. 10.1016/j.jneuroim.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cédile O, Wlodarczyk A, Owens T. CCL2 recruits T cells into the brain in a CCR2-independent manner. APMIS. 2017; 125:945–956. 10.1111/apm.12740 [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, St-Pierre S, Roy P, Morley BJ, Hao J, Simard AR. Infiltration of CCR2+Ly6Chigh Proinflammatory Monocytes and Neutrophils into the Central Nervous System Is Modulated by Nicotinic Acetylcholine Receptors in a Model of Multiple Sclerosis. J Immunol. 2016; 196:2095–2108. 10.4049/jimmunol.1501613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med. 2011; 43:503–511. 10.3109/07853890.2011.577093 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Superimposition of predicted 3D structure of mouse Ahr PAS-B domain with the human C-terminal PAS domain of HIF2a (PDB ID: 1p97). (B) Ramachandran plot of predicted mouse Ahr PAS-B domain.
(PDF)
The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-, Treg or Tr1-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. The mRNA expression of Rorc, Il6, FoxP3 and Il10 were assessed by quantitative real‐time PCR and normalized to Gapdh mRNA. (A) Relative expression of Rorc in CD4+CD62L+ T cells cultured under Th17-conditions for 48 hr compared to Th0. (B) Relative expression of Il6 mRNA in macrophages stimulated with LPS for 4 hr. (C) Relative expression of FoxP3 mRNA in CD4+CD62L+ T cells cultured under Treg-polarizing conditions for 48 hr. (D) Relative expression of Il10 mRNA in CD4+CD62L+ T cells cultured under Tr1-polarizing conditions for 56 hr. Data were pooled from independent experiments and shown as mean ± SD.
(PDF)
(A and B) The CD4+CD62L+ T cells were isolated from the spleen and cultured under Th17-polarizing conditions, and peritoneal macrophages were cultured in presence of LPS. Cell viability of (A) differentiating Th17 and (B) peritoneal macrophages 48 hr after stimulation in presence of GA (20–120 μmol/L). (C and D) The EAE was induced by immunizing mice with MOG35-55 emulsified in CFA. The mice were injected intraperitoneally with vehicle (corn oil) or GA (1–4 mg/day) for 14 days starting one day before MOG35-55 immunization. Weight of (C) spleen and (D) liver were measured 24 h after last dose, n = 6. Data were pooled from independent experiments and shown as mean ± SD. *p < 0.05.
(PDF)
AChE catalytic activity in culture supernatant of (A) CD4+ T cells isolated from naive mice and stimulated with PHA and (B) peritoneal macrophages were stimulated with LPS. The PHA-stimulated CD4+ T cells and LPS-stimulated macrophages were electroporated with antisense (as)-miR-132, and cells treated with PHA, PHA+GA, LPS and LPS+GA were electroporated with scramble hairpin inhibitor. Data were pooled from independent experiments and shown as mean ± SD. *p < 0.05, PHA+GA versus PHA, and LPS+GA versus LPS; ǂp < 0.05, PHA+GA+as-miR-132 versus PHA, and LPS+GA+as-miR-132 versus LPS; #p < 0.05, PHA+GA versus PHA+GA+as-miR-132, and LPS+GA versus LPS+GA+as-miR-132.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.