Abstract
Lumbar foraminal spinal stenosis (LFSS) is defined as the narrowing of the nerve root exit associated with a herniated intervertebral disc, osteoarthritic changes in the facet joints, or a hypertrophied ligamentum flavum, which can provoke neurogenic claudication. To achieve effective and safe decompression of the lumbar spinal foramen, a specially designed instrument (Claudicare, SEAWON Meditech, Bucheon-si, Gyeonggi-do, Republic of Korea) for percutaneous lumbar foraminoplasty (PLF) was invented. The purpose of this study was to evaluate the clinical efficacy and safety of the newly devised instrument in patients with LFSS.
PLF was performed for LFSS by a single pain physician. For each patient, an 11-point numerical rating scale (NRS) pain score—the Oswestry Disability Index (ODI)—and the duration of walking without radicular pain were evaluated at the 3-month follow-up. The successful responder percentage was defined as ≥50% reduction from the baseline NRS score with improvement in ODI and duration of walking.
Among 24 patients who underwent PLF, 15 patients showed successful responses. The NRS pain score and duration of walking without radicular pain were improved significantly from baseline at the 3-month follow-up (P < .01). The ODI was also decreased, but the difference was not statistically significant (P = .09). The NRS pain score and walking duration without pain at 3 months were statistically significantly different between the groups (P < .001 and P = .01, respectively), whereas there was no statistically significant difference in improvement in ODI between the groups (P = .23). No serious adverse events occurred in the study.
In conclusion, PLF using the Claudicare device may be an optimal and safe option for managing intractable LFSS on an outpatient basis.
Keywords: claudicare, lumbar foraminal spinal stenosis, neurogenic claudication, percutaneous lumbar foraminoplasty
1. Introduction
Lumbar foraminal spinal stenosis (LFSS) is a common cause of lumbar radiculopathy with a 10% incidence rate among the global population.[1] LFSS is defined as the narrowing of the nerve root exit[2–4] associated with a herniated intervertebral disc, osteoarthritic changes in the facet joints, or hypertrophied ligamentum flavum, which can provoke neurogenic claudication.[5–7] The pain in LFSS is thought to arise from the dorsal root ganglion (DRG) that is impinged within the foramen, given the high concentration of substance P in the DRG[8] and its sensitivity to external pressure.[9,10]
The pain of LFSS can initially be conservative treatment, including oral medications, epidural steroid injections, and/or physical therapy. However, patients who do not respond to those therapies are usually recommended to undergo surgery as the next step. Conventional surgical methods for LFSS may be categorized into decompression techniques such as total or partial facetectomy, with or without fusion, and facet-preserving foraminoplasty.[11] Among these techniques, facetectomy offers sufficient decompression around the nerve root; however, it often leads to segmental instability.[6,12,13] Moreover, this surgical approach requires general anesthesia, and occasionally, a long hospital stay and slow recovery, and poses the risk of procedure-related complications.[14,15] On the contrary, minimally invasive techniques, such as percutaneous foraminoplasty via a paraspinal approach, facilitate direct access to a foraminal lesion with the least manipulation of the facet joint and less postoperative pain.[16] It can be performed under local anesthesia, allowing direct feedback from the patient to avoid possible nerve damage during and after the procedure.[17]
Use of foraminoplasty has been proposed to widen the foramen by undercutting the ventral part of the superior articular process (SAP), with ablation of the foraminal ligament, by using bone trephines or an endoscopic drill.[18] Although classified as a minimally invasive technique, percutaneous endoscopic foraminoplasty is a time-consuming procedure that requires a working channel for the rigid endoscope and expensive equipment, and involves a steep learning curve.[19] In the mid-2000, Schubert and Hoogland[20] introduced percutaneous lumbar foraminoplasty (PLF) that uses transforaminal percutaneous reamers with a guidewire to drill to the tip of the SAP. During PLF, a trephine and a bone reamer allows rapid resection of the hypertrophied SAP or osteophyte under fluoroscopic guidance.[21] This may be more convenient and time-saving than the endoscopic procedure; however, it does entail safety issues, such as the risk of injury to the exiting and traversing nerve root, due to mechanical or thermal irritation during the procedure.[22]
Given the above, a specially designed instrument, Claudicare (SEAWON Meditech, Bucheon-si, Gyeonggi-do, Republic of Korea), which can improve safety during PLF, has been invented. It has a tip with a blunt end and a shield to protect the nerve root during the procedure and was approved by the Korean Food and Drug Administration (approval number was 16-368) in 2016. As the maximum outer diameter of the device is only 3.5 mm, this procedure might be relatively nontraumatic and time-saving. In this study, we aimed to evaluate the clinical efficacy and safety outcomes of percutaneous lumbar foraminoplasty with Claudicare in 24 patients with LFSS.
2. Methods
2.1. Patients
This retrospective study was approved by the institutional review board (IRB) of the Seoul Metropolitan Government Seoul National University Boramae Medical Center (No. 20180509/10-2018-56/061). The study adhered to the applicable STROBE guidelines.[23] The need for obtaining informed consent from the patients was waived because of the retrospective nature of the study.
After obtaining IRB approval, the medical records of patients who underwent PLF using the Claudicare between January 1, 2017 and December 31, 2017 were reviewed. Inclusion criteria in this study were as follows: patients with LFSS causing radiculopathy and neurogenic claudication, which was confirmed by magnetic resonance imaging (MRI); patients between 45 and 85 years of age who underwent PLF for LFSS, by a single pain physician (S.E.S.) using the Claudicare; pain duration >3 months; predominant radicular or referred leg pain rather than low back pain; a score of ≥4 on an 11-point numerical rating scale (NRS) pain score[24] after receiving at least 3 months’ conservative treatment, including oral medication, physical therapy, and epidural steroid injections; and failed pain relief or short-term pain relief for <1 month from a previous transforaminal epidural steroid injection (TFESI).
Exclusion criteria were as follows: a large contained or sequestered disc herniation or severe central canal stenosis in lumbar MRI scans; segmental instability at the level of the symptomatic disc; patients with red flags for back pain,[25] such as cauda equina syndrome, cancer, fracture, and infection; absence of lumbar spinal MRI within the previous 3 months; psychological disorders that may affect treatment outcome; concurrent medical condition that could interfere with follow-up care or evaluation; and allergies to local anesthetics or contrast dyes, pregnancy, coagulation disorder, general infection, fever, or local infection at the puncture site.
For the 3-month follow-up period, no additional medication or therapies, including physical therapy, trigger point injections, and epidural injections, were allowed. However, administration of oral or transdermal analgesics, such as opioids, tramadol, and/or nonsteroidal anti-inflammatory drugs (NSAIDs), was allowed to continue during the follow-up period. In addition, the dosages of these medications as rescue or regular (around-the-clock) analgesics were modified in accordance with pain intensity. Use of adjuvants, such as anticonvulsants and antidepressants that were used to manage radicular pain, was also continued. After the 3-month follow-up, patients were allowed to undergo physical therapy or interventional procedures other than epidural steroid injections, such as TFESI, and to take additional medications, including NSAIDs, antidepressants, anticonvulsants, and/or opioids.
2.2. PLF using claudicare
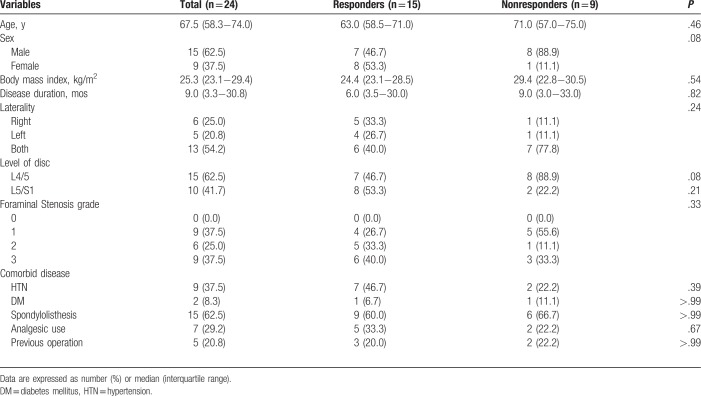
To perform PLF, we used the Claudicare device, which consists of a guidewire with a less than 1-mm diameter, a dilator with a 2-mm diameter, and a working cannula (inner/outer diameter: 3 mm/3.5 mm) (Fig. 1). The distal end of the working drill has a blunt-shaped tip and a protection shield, allowing it to proceed through the intervertebral foramen below the SAP while minimizing mechanical damage to the nerve root inside the target foramen. The tip of the working cannula was developed to affix to the posterior aspect of the superior endplate of the distal vertebra to avoid moving itself into the neural foramen. The drill, which is introduced over a guidewire with a protective working cannula, contains a half-covered shield that is designed to prevent damage to exiting and traversing nerve roots (Fig. 1).
Figure 1.
The specially designed instrument for the percutaneous lumbar foraminoplasty procedure. The Claudicare device consists of a guidewire with a less than 1-m diameter, a dilator with a 2-mm diameter, a working cannula (inner/outer diameter: 3 mm/3.5 mm), a drill with a blunt-shaped end and a protection shield, and a disposable battery.
After entering the procedure room, patients were placed in a prone position with a cushion under the abdomen to reduce the lumbar lordosis, and electrocardiography, heart rate, noninvasive blood pressure, and peripheral oxygen saturation were monitored. All procedures were performed in a strict, sterile manner using local anesthetics. After preparing the skin of the lower back with betadine soap and betadine solution, sterilized drapes were applied to the target area. Throughout the Claudicare procedure, C-arm fluoroscopy was used in the anteroposterior (AP), oblique, and lateral planes to confirm the target disc, align the endplates of the vertebral bodies, and direct instrument placement onto the disc surface. The patients were kept conscious during the procedure to monitor any changes in the symptoms and signs.
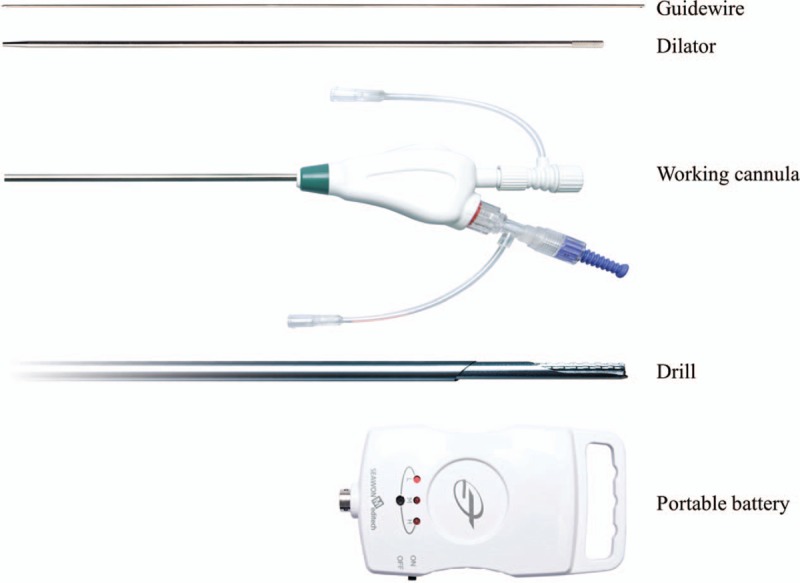
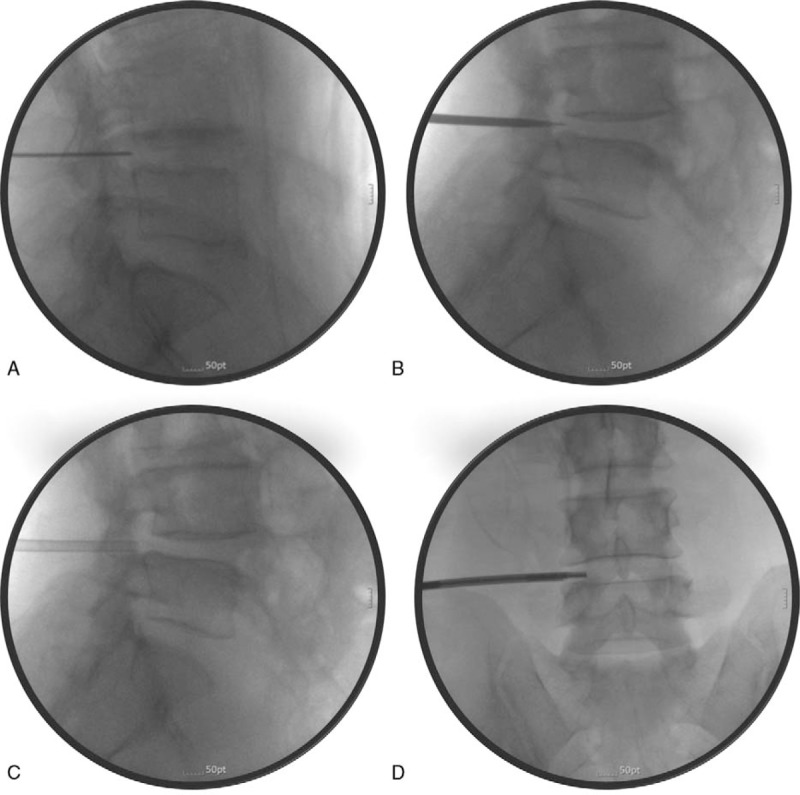
The skin entry point was approximately 8 to 10 cm off the midline. After skin infiltration with 1% lidocaine through the trajectory, a skin incision was made, and a guidewire was inserted into Kambin triangle, using a 45-degree ipsilateral oblique view. In the lateral fluoroscopic view, the tip of the guidewire was advanced until it was located at the anterior three-fourths of the neck of the SAP. After touching the SAP with the guidewire, an AP image was taken to confirm that the tip of the guidewire was located at the medial border of the SAP. Then, it was advanced approximately 0.5 cm further, anteriorly, into the target epidural foramen in the lateral fluoroscopic view (Fig. 2A). Next, a dilator with a 2-mm diameter was inserted via the guidewire to the target foramen until it touched the anterior border of the SAP (Fig. 2B). After removing the guidewire, a working cannula with a 3.5-mm outer diameter was inserted via the dilator. The final position of the working cannula was the anterior border of the hypertrophied SAP (Fig. 2C). Next, a Claudicare drill with a shield on the tip was inserted via the working cannula and connected to a specially designed disposable battery (Fig. 1). After ensuring that there was no sensory or motor discomfort of the leg, partial removal of the hypertrophied capsule of the SAP commenced from the lateral to the medial direction in the AP fluoroscope image, using the drill at a speed of 12,500 rpm (low-power mode) to 17,500 rpm (high-power mode) (Fig. 2D). Simultaneously, lateral fluoroscope images were obtained to confirm the location of the tip on the Claudicare drill. After drilling back and forth approximately 3 to 5 times, a slight reduction in resistance at the tip of the device was noticed, with thinning of the anterior capsule of the SAP. This process was repeated about 3 to 4 times until the tip of the Claudicare drill reached the medial border of the pedicle in the AP fluoroscopic view (Fig. 2D). During the procedure, the shield on the drill tip was always directed at the nerve root to protect against possible nerve damage (Fig. 3). While drilling and grinding the hypertrophied capsule of the SAP with part of the thickened transforaminal ligament in the target foraminal space, the ground fragments of the capsule were removed via the working channel. After finishing the grinding process, the working channel was removed and the incision was sutured. No additional drug or steroid was injected into the target foraminal space. All patients were observed in the recovery room for at least 2 hours to monitor for occurrence of any complications. Patients were then discharged on the day of the procedure and attended the outpatient clinic for the 3-month follow-up evaluation.
Figure 2.
Fluoroscopic images obtained during the percutaneous lumbar foraminoplasty procedure with the Claudicare device. (A) The guidewire was advanced approximately 0.5 cm anteriorly into the target epidural foramen, in the lateral fluoroscopic view. (B) A dilator was inserted via the guidewire into the target foramen until it touched the anterior border of the superior articular process (SAP). (C) After removing the guidewire, a working cannula was inserted via the dilator. The final position of the working cannula was the anterior border of the hypertrophied SAP. (D) A Claudicare drill was inserted via the working cannula and partial removal of the hypertrophied capsule of the SAP commenced from the lateral to medial direction in the AP fluoroscope image.
Figure 3.
Schematic drawing of the Claudicare drill working inside the target lesion.
2.3. Outcome measurement
Patient demographics (age, sex, body mass index [BMI], and coexisting disease, including hypertension and/or diabetes mellitus) and other clinical data, such as pain characteristics, including its duration and severity, and concurrent medications, were obtained from medical record reviews and patient-based outcome questionnaires or telephonic interviews. The severity of LFSS on MRI was shown with perineural fat obliteration or nerve root collapse based on the practical grading system for lumbar foraminal stenosis reported by Lee et al,[26] where grade 0 refers to the absence of foraminal stenosis; grade 1 refers to mild foraminal stenosis showing perineural fat obliteration in 2 opposing directions, vertical or transverse; grade 2 refers to moderate foraminal stenosis showing perineural fat obliteration in 4 directions without morphologic changes, both vertical and transverse directions; and grade 3 refers to severe foraminal stenosis showing nerve root collapse or morphologic changes. In addition, we collected procedure-related information, such as the level and side of operation.
The patients’ pain severity was assessed using an NRS pain score with 0 as the lowest score (no pain at all) and 10 as the highest score (unbearable pain). Functional status was assessed using the Korean version of the 9-item Oswestry Disability Index (ODI; range from 0 to 100, where 0 means no disability). Patient satisfaction was also assessed using a 5-point Likert scale (1 = extremely dissatisfied; 2 = somewhat dissatisfied; 3 = neutral; 4 = somewhat satisfied; and 5 = extremely satisfied). In addition, the patient's symptoms were evaluated. The time of walking without radicular pain was assessed by asking “How long can you walk without having to stop to rest due to your leg pain?” via a questionnaire. At the 3-month follow-up, the patients completed questionnaires that reflected their functional status and pain intensity.
Regarding safety, complications that occurred within 3 months after the procedure were reviewed either in person or by telephone. Any complications that occurred after the procedure (motor weakness of the lower legs, sensory change, infection, etc) were recorded.
2.4. Statistical analysis
Preprocedural and postprocedural NRS pain scores, ODI, and duration of walking without radicular pain, and changes in NRS pain score (%), ODI (%), and duration of walking without radicular pain between baseline and the 3-month follow-up visit were analyzed with the Wilcoxon signed-rank test. Outcomes are shown as mean (interquartile range [IQR]), or frequency (%), as appropriate. We assessed the proportion of successful responders, defined as at least 50% decrease in the NRS pain score, accompanied by improvement in the ODI (%) and duration of walking, by the 3-month follow-up visit. Then, differences in outcomes were compared between responders and nonresponders, using the Mann–Whitney test for nonparametric data and Fisher exact test for parametric data.
Statistical analysis was performed using SPSS Statistics version 23.0 for Windows (IBM Corp., Armonk, NY). A P value <.05 was considered statistically significant.
3. Results
We thoroughly reviewed the medical records of 29 consecutive patients who underwent PLF using the Claudicare; 5 of these patients were excluded due to an absence of MRI within a 3-month period (n = 2) or an unclear history of TFESI before PLF (n = 3). Finally, 24 patients were included in the study. Of these patients, more than a half (n = 15) were classified as successful responders according to the predefined criteria, with the remaining patients (n = 9) classified as nonresponders (Fig. 4).
Figure 4.
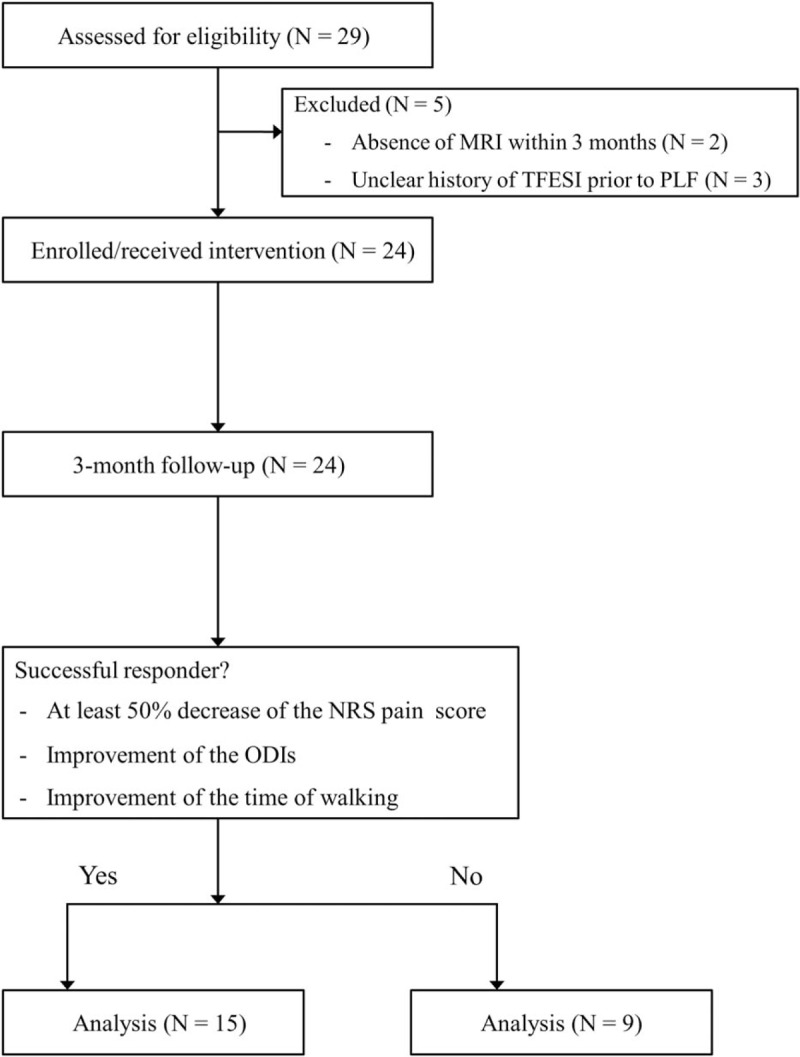
Flow chart of patients in this study.
Demographics and perioperative clinical parameters of all patients, including responders and nonresponders, are summarized in Table 1. The mean age of the patients overall was 67.5 years (IQR 58.3–74.0), and 15 were male and 9 were female. The mean BMI was 25.3 kg/m2 (IQR 23.1–29.4). Procedure levels were at L4-5 in 15 patients and at L5-S1 in 10 patients. Using MRI, grade 1 LFSS (mild foraminal stenosis) was observed in 9 patients, grade 2 (moderate foraminal stenosis) in 6 patients, and grade 3 (severe foraminal stenosis) in 9 patients, based on Lee et al's[26] MRI grading system for LFSS. In addition, 15 patients (62.5%) showed concomitant spondylolisthesis and LFSS at the same level, and 5 (20.8%) had previously undergone open surgery at other levels. The procedures were performed via a left-sided approach in 5 patients, a right-sided approach in 6, and a bilateral approach in 13, based on radiologic findings and clinical symptoms. Seven patients were prescribed a weak opioid, tramadol, and acetaminophen mixture at baseline; there was no statistically significant difference in opioid prescription use between the responder (n = 5) and nonresponder groups (n = 2; P = .67). None of the patients required strong opioid medication.
Table 1.
Baseline characteristics and demographics.
Table 2 demonstrates the changes in outcome variables from baseline to the 3-month follow-up. The preoperative and postoperative NRS pain scores at 3 months were 8.0 (IQR 7.0–8.8) and 3.5 (IQR 2.0–6.8), respectively, which represented an overall significant decrease of 41.4% (P < .001). The ODI also decreased from baseline (34.5; IQR 29.5–43.3) to the 3-month follow-up (27.0; IQR 24.0–34.5) by 13.5%; however, this decrease was not statistically significant (P = .09). The duration of walking without radicular pain significantly increased from 5.0 minutes (IQR 1.3–5.0) at baseline to 17.5 minutes (IQR 5.0–30.0) at the 3-month follow-up visit (P < .001).
Table 2.
Clinical outcomes of responder analysis at three-month follow-up.
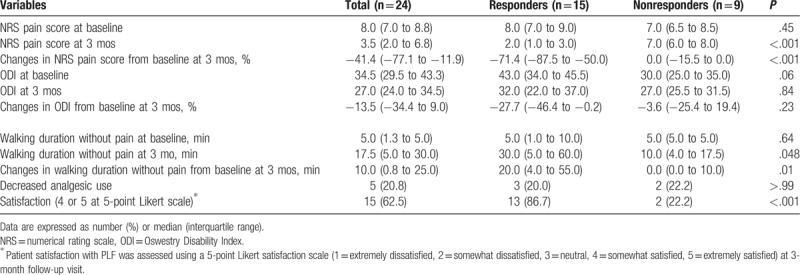
The NRS pain score and duration of walking without pain at 3 months were statistically significantly different between the responder and nonresponder groups (P < .001 and P = .01, respectively), whereas there was no significant difference in improvement of ODI (%) between the groups (P = .23). Among the patients overall, however, only 5 patients (20.8%) experienced the reduced weak opioid analgesics; 3 were in the responder group and 2 were in the nonresponder group (P > .99). According to the 5-point Likert scale, 3 months after the procedure, 15 patients (62.5%) reported satisfaction (score of 4 or 5 on the 5-point Likert scale); there was a statistically significant difference between the responders and nonresponders in terms of patient satisfaction (P < .001).
All adverse events that occurred during the study period were minor and temporary. One-fourth (25%, n = 6) of patients reported temporary pain during the procedure, but this was tolerable and did not require additional medication or discontinuation of the procedure. Four patients (16.7%) complained of procedure-related pain for 2 to 3 days postprocedurally, but it was spontaneously relieved without any neurological sequelae. There was no report of transient paresthesia in the lower extremity or other adverse events, such as hematoma formation, persistent motor or sensory impairment, severe pain, paresthesia, or infection.
4. Discussion
In this study, PLF was performed with a specially designed device, the Claudicare, on an outpatient basis in 24 patients who were diagnosed with LFSS. This relatively simple and easy procedure significantly improved pain intensity and duration of walking without radicular pain in 63% of the patients (n = 15) at the 3-month follow-up visit. All patients were discharged on the day of the procedure without any serious adverse events.
There may be several advantages in using this device for PLF. First, because the largest external diameter of the instrument is as small as 3.5 mm (in the working cannula), it minimizes injury to the nerve and the surrounding tissues during the procedure via a transforaminal approach. Second, the drill is designed with a special tip and a nerve-protecting shield, allowing a physician to grind away the hypertrophied capsule of the SAP and part of the transforaminal ligaments, safely. In addition, as it is driven by a small disposable, portable battery, rather than a large standing power source, the device can be handled conveniently.
Lumbar foraminal spinal stenosis is a major cause of neuropathic low back pain. Hypertrophy of the facet joint, particularly the SAP, and transforaminal ligaments has been suggested to be a common source of LFSS, resulting in reduction of spinal canal and foraminal dimensions and compression of neural elements. A systematic review concluded that there is insufficient evidence to recommend any specific type of treatment for LFSS.[27] Schneider et al[28] showed that a combination of conservative therapy provides greater short-term improvement in symptoms and physical function and walking capacity. However, several different surgical procedures should be considered to treat patients who do not improve with nonoperative therapies.[29] When focusing on the surgical options, decompression, fusion, and minimally invasive procedures are possible treatments. With the recent move toward adopting minimally invasive techniques, TFESI or percutaneous epidural adhesiolysis has been commonly used as the initial step for managing LFSS in daily clinical practice. However, TFESI may not effectively alleviate pain, particularly in cases accompanied by lateral recess stenosis, which is also frequently caused by hypertrophy of the SAP. Furthermore, although percutaneous epidural adhesiolysis has been suggested as an alternative to TFESI, it has not yielded a concretely positive outcome in LFSS patients. As a basic approach for managing LFSS, removal of the hypertrophied capsule and/or ligament by percutaneous endoscopic lumbar foraminotomy has been proposed to enlarge the narrowed lumbar foramen.[30,31] Although several studies have described the efficacy of the endoscopic technique, it involves a steep learning curve for physicians and requires hospitalization for patients, due to the relative invasiveness of the procedure, and poses a risk of procedure-related adverse events. Recently, Lee et al[32] introduced percutaneous lumbar extraforaminotomy to resect the foraminal ligaments around the target nerve by using a specially designed instrument, which may be analogous to the concept of PLF. Lee et al's procedure showed similar efficacy as that achieved in our study; however, in comparison, PLF using the Claudicare device is relatively simple, does not require hospitalization, and is safe, with less procedure-associated pain than extraforaminotomy.
In Lee et al's study, the proportion of successful outcomes (≥50% pain reduction at 3-month follow-up visit after lumbar extraforaminotomy) was 60%, which is similar to the success rate in our study (63%, n = 15). However, we predefined successful outcomes as a combination of improvement in pain intensity and functional ability: at least 50% reduction in pain intensity, with improvement of ODI (%) and duration of walking without radicular pain. Although ODI (%) was not considerably improved, walking duration was significantly increased in the patients overall after the procedure, which suggests that PLF using the Claudicare device may contribute to improving neurogenic claudication in patients with lumbar spinal stenosis. In addition, as compared with the Lee et al's study, the prevalence of adverse events, including temporary pain during the procedure and procedure-related pain in the postprocedural period, was relatively low in this study (65% vs 25% and 45% vs 16.7%, respectively). Furthermore, no transient paresthesia in the lower extremity during the procedure or follow-up period was reported in our patients. Taken together, we deduce that PLF using the Claudicare device may be the optimal option for managing intractable LFSS on a routine outpatient basis.
During the 3-month follow-up period, none of the patients in this study underwent any additional interventional procedures, such as lumbar epidural block, which may have confounded evaluation of the effectiveness of the procedure. However, a major limitation of this study is its nonrandomized, retrospective nature, which may have been affected by characteristic confounders that include bias and variability in the quality of the available information. Furthermore, the small sample size and the short follow-up period are drawbacks in this study. Similar to other minimally invasive procedures, the proficiency of the practitioner may have influenced the outcome in this study. The duration of drilling is not standardized and could be changed by the operator. These factors might have influenced the outcomes of the study. Furthermore, we could not verify an overall reduction of analgesics use in patients, despite the fact that pain intensity and duration of walking without radicular pain were significantly improved at 3 months after the procedure. Nonetheless, out of 7 patients who took weak opioid medications, the majority (n = 5) reported a decrease in their analgesic use at the 3-month follow-up visit. Taken together, appropriately blinded, prospective clinical trials, with an acceptable number of patients monitored over a longer period of time, are warranted to verify our results.
5. Conclusions
In conclusion, PLF using the Claudicare device could be helpful for improving symptoms from lumbar foraminal stenosis. More than half of the patients in this study (63%) experienced at least 50% or more pain reduction, accompanied by improvement in functions, including the duration of walking without radicular pain by 3 months after the procedure. The prevalence of procedure-related discomfort or adverse events was trivial. Although further clinical evidence should be accumulated, we propose that PLF using the Claudicare device is an advantageous option for managing intractable LFSS on an outpatient basis.
Author contributions
Conceptualization: Sung Eun Sim.
Data curation: Sojeong Yoon, Seok Min Kwon.
Formal analysis: Jee Youn Moon.
Investigation: Yongjae Yoo.
Methodology: Yongjae Yoo, Jee Youn Moon.
Supervision: Jee Youn Moon, Sung Eun Sim.
Writing – original draft: Yongjae Yoo, Jee Youn Moon.
Writing – review & editing: Yongjae Yoo, Jee Youn Moon.
Footnotes
Abbreviations: AP = anteroposterior, DRG = dorsal root ganglion, LFSS = lumbar foraminal spinal stenosis, MRI = magnetic resonance imaging, NRS = numeric rating scale, PLF = percutaneous lumbar foraminoplasty, SAP = superior articular process, TFESI = transforaminal epidural steroid injection.
YY and JYM are contributed equally to this manuscript.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Dr S.E. Sim participated in developing the Claudicare and has a shared patent of the device with the company. The other authors have no conflicts of interest to disclose.
References
- [1].Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage 2012;20:1103–8. [DOI] [PubMed] [Google Scholar]
- [2].Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine 1991;16:1312–20. [DOI] [PubMed] [Google Scholar]
- [3].Lee SY, Kim TH, Oh JK, et al. Lumbar stenosis: a recent update by review of literature. Asian Spine J 2015;9:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Porter RW, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine 1984;9:418–21. [DOI] [PubMed] [Google Scholar]
- [5].Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am 1995;77:32–8. [PubMed] [Google Scholar]
- [6].Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine 2000;25:389–94. [DOI] [PubMed] [Google Scholar]
- [7].Splendiani A, Ferrari F, Barile A, et al. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: demonstration with dedicated upright MRI system. Radiol Med 2014;119:164–74. [DOI] [PubMed] [Google Scholar]
- [8].Kobayashi S, Kokubo Y, Uchida K, et al. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine 2005;30:276–82. [DOI] [PubMed] [Google Scholar]
- [9].Vanderlinden RG. Subarticular entrapment of the dorsal root ganglion as a cause of sciatic pain. Spine 1984;9:19–22. [DOI] [PubMed] [Google Scholar]
- [10].Weinstein J. Report of the 1985 ISSLS Traveling Fellowship. Mechanisms of spinal pain. The dorsal root ganglion and its role as a mediator of low-back pain. Spine 1986;11:999–1001. [DOI] [PubMed] [Google Scholar]
- [11].Ahn Y, Oh HK, Kim H, et al. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery 2014;75:124–33. [discussion 132–123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine 1990;15:1142–7. [DOI] [PubMed] [Google Scholar]
- [13].Epstein NE. Foraminal and far lateral lumbar disc herniations: surgical alternatives and outcome measures. Spinal Cord 2002;40:491–500. [DOI] [PubMed] [Google Scholar]
- [14].Polikandriotis JA, Hudak EM, Perry MW. Minimally invasive surgery through endoscopic laminotomy and foraminotomy for the treatment of lumbar spinal stenosis. J Orthop 2013;10:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schoenfeld AJ, Ochoa LM, Bader JO, et al. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am 2011;93:1577–82. [DOI] [PubMed] [Google Scholar]
- [16].Chang SB, Lee SH, Ahn Y, et al. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine 2006;31:1163–7. [DOI] [PubMed] [Google Scholar]
- [17].Chun EH, Park HS. A modified approach of percutaneous endoscopic lumbar discectomy (PELD) for far lateral disc herniation at L5-S1 with foot drop. Korean J Pain 2016;29:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine 2008;33:E508–515. [DOI] [PubMed] [Google Scholar]
- [19].Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disc herniation. Oper Orthop Traumatol 2005;17:641–61. [DOI] [PubMed] [Google Scholar]
- [21].Li ZZ, Hou SX, Shang WL, et al. Modified percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar discectomy: instrument design, technique notes, and 5 years follow-up. Pain Phys 2017;20:E85–98. [PubMed] [Google Scholar]
- [22].Sencer A, Yorukoglu AG, Akcakaya MO, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: short-term clinical results of 163 surgically treated patients. World Neurosurg 2014;82:884–90. [DOI] [PubMed] [Google Scholar]
- [23].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- [24].Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008;101:17–24. [DOI] [PubMed] [Google Scholar]
- [25].Samanta J, Kendall J, Samanta A. 10-minute consultation: chronic low back pain. BMJ 2003;326:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol 2010;194:1095–8. [DOI] [PubMed] [Google Scholar]
- [27].Zaina F, Tomkins-Lane C, Carragee E, et al. Surgical versus non-surgical treatment for lumbar spinal stenosis. Spine 2016;41:E857–68. [DOI] [PubMed] [Google Scholar]
- [28].Schneider MJ, Ammendolia C, Murphy DR, et al. Comparative clinical effectiveness of nonsurgical treatment methods in patients with lumbar spinal stenosis: a randomized clinical trial. JAMA Netw Open 2019;2:e186828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yeung A, Gore S. Endoscopic foraminal decompression for failed back surgery syndrome under local anesthesia. Int J Spine Surg 2014;8doi: 10.14444/1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wen B, Zhang X, Zhang L, et al. Percutaneous endoscopic transforaminal lumbar spinal canal decompression for lumbar spinal stenosis. Medicine 2016;95:e5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee SC, Kim WJ, Lee CS, et al. Effectiveness of percutaneous lumbar extraforaminotomy in patients with lumbar foraminal spinal stenosis: a prospective, single-armed, observational pilot study. Pain Med 2017;18:1975–86. [DOI] [PubMed] [Google Scholar]