Abstract
Background:
Due to decreasing eradication rate and increasing side effects, probiotics have gradually become an important supplement to standard eradication regimens for Helicobacter pylori.
Objective:
To evaluate the effectiveness and safety of probiotics in facilitating the eradication of H pylori and to explore the best timing and duration of probiotic supplementation, use of eradication regimens, strains, locations, and common side effects.
Methods:
Eligible studies were retrieved from the PubMed, EMBASE, Cochrane Library, Web of Science, and CNKI databases, and we applied the Stata 12.0 software for the standard meta-analysis and network meta-analysis.
Results:
Forty eligible studies with 8924 patients were included in the analysis. We used a random-effects model (I2 = 52.1% and I2 = 81.4%) to analyze the eradication rate and the incidence of total side effects by intention to treat (ITT). Compared with the control group, a higher eradication rate (relative risk [RR] 1.140, 95% confidence interval (CI) 1.101–1.180, P < .001) and lower incidence of total side effects (RR 0.470, 95% CI 0.391–0.565, P < .001) were observed in the probiotic group. In the subgroup analysis, we evaluated the surface under the cumulative ranking curve scores for the before + same (75.2%), >2 weeks (92.6%), probiotic + quadruple regimen (99.9%), Lactobacillus (73.6%), multiple strains (72.1%), China (98.5%) groups. The rankings of common side effects are shown in Table 6. SUCRA scores for diarrhea (39.7%), abdominal pain (43.9%), nausea (78.8%), taste disturbance (99.6%), vomiting (7.1%), and constipation (30.9%) were reported. The consistency of all comparison groups was good.
Conclusions:
Probiotics improved the eradication rate and reduced side effects when added to the treatments designed to eradicate H pylori. The use of probiotics before the eradication treatment and throughout the eradication treatment, and also the use of probiotics for more than 2 weeks, exerted better eradication effects. Probiotics combined with the bismuth quadruple regimen was the best combination. Lactobacillus and multiple strains were better choices of probiotic strains. The eradication effect observed in China was better than the effect observed in other countries.
Keywords: eradication, Helicobacter pylori, network meta-analysis, probiotics, side effect
1. Introduction
Helicobacter pylori has been extensively studied by scientists worldwide since its isolation and cultivation by J. RobinWarren and Barry J. Marshall in 1983.[1] The infection rate of H pylori is nearly 50% across the global and 41.5% to 72.3% in China.[2] At present, H pylori is recognized as the main cause of many digestive diseases, including chronic active gastritis, gastroduodenal ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer.[3,4] It is urgent to find the satisfied eradication regimens. In the past few years, the recommended eradication regimens include clarithromycin triple regimen, bismuth quadruple regimen, concomitant regimen, and sequential regimen.[5] Because of highly resistant to antibiotics, the quadruple regimen containing bismuth was recommended to increase the sensibility.[6] However, the side effects during treatment, such as antibiotic-associated diarrhea, nausea, or vomiting, may reduce patients’ compliance.[5,7] Therefore, high resistance and poor compliance have hindered the satisfactory eradication effects of standard regimens.[8]
As an adjuvant of eradication treatment, probiotics have been recommended in some current guidelines, such as Lactobacilli, fecal bacteria, Bifidobacterium, Saccharomyces, and Bacillus licheniformis.[4,5,9] On one hand, probiotics help to competitive inhibit the colonization of H pylori and produce bacteriostatic substances.[7] On the contrary, probiotics have a positive effect on reducing the side effects of treatment, such as antibiotic-associated diarrhea.[10–14] However, other studies hold the opposite views on the efficiency and safety of probiotics in assisting with the eradication.[15–17] Although some previous studies was focused on the probitic addition, the timing and duration of probiotic addition remain unclear.[18–21] Therefore, we performed a meta-analysis of the most recent and most favorable evidence to evaluate the efficacy and safety, and explore the optimal timing and duration of probiotics in assisting with the eradication of H pylori.
2. Methods
2.1. Search strategy
The literature search was performed up to July, 2018. Reviewers systematically searched PubMed, EMBASE, Cochrane Library, Web of Science, and CNKI databases using the following terms: (probiotic OR probiotics OR yeast OR yeasts OR yogurt OR Lactobacillus OR Bifidobacterium OR Saccharomyces) AND (Helicobacter pylori OR H. pylori).
2.2. Inclusion criteria and exclusion criteria
Inclusion criteria were the following: randomized controlled trial; patients’ aged ≥18 years, receiving the first anti-H pylori treatment; confirmation of eradication results by histology or H pylori fecal antigen tests performed at least 4 weeks after the end of eradication; the use of at least 2 groups, including the control group (placebo or no other intervention) and the experimental group (H pylori standard eradication regimen plus probiotics) (H pylori standard eradication regimen refers to standard triple regimen, sequential regimen, standard quadruple regimen); the probiotic strains were Lactobacilli, Bifidobacterium, Saccharomyces, or a mixture of the 3; and the eradication rates were available.
Exclusion criteria were the following: an uncertain eradication rate; the use of an agent other than probiotics as an auxiliary treatment for H pylori infection in the intervention group; studies for which the complete text was not available; studies that were not published in Chinese or English; repeated studies; inappropriate randomization method; no description of withdrawals and dropout rates; intention to treat (ITT) was not used when withdrawals and dropouts occurred; and the original data were incorrect.
2.3. Study quality assessment
Two researchers independently screened the studies and evaluated the quality of the included studies. Disagreements were resolved by a third researcher. Quality was mainly assessed using the Jadad scale,[22,23] based on the following three criteria: randomization, double blinding, and description of withdrawals and dropout. The maximum number of points was 5: a low-quality study scored ≤2 and a high-quality study scored >2.[22,23]
2.4. Data extraction
The data were extracted using a self-made form, and the extracted contents are listed below. Patients meeting at least 1 of the following criteria were defined as H pylori-positive patients: 13C-urea breath test (UBT), rapid urea test (RUT), H pylori antibody, histopathology, or stool antigen test. The primary outcome of this meta-analysis was the successful eradication of H pylori, which was confirmed by 13C-UBT or other generally accepted methods 4 weeks after the end of treatment. Secondary outcomes were side effects during H pylori eradication. The data extracted and assessed in the meta-analysis were: author and publication year; number of patients in the study (experimental group/control group); type and duration of the first H pylori eradication treatment (triple or quadruple); number of probiotic strains and probiotic species; timing and duration of addition; and an ITT analysis of the H pylori eradication rate and incidence of side effects (nausea, vomiting, abdominal pain, constipation, diarrhea, taste disturbance, and total side effects).
2.5. Statistical analysis
2.5.1. Standard meta-analysis
For direct comparisons, we used the metan command in Stata 12.0 for the standard meta-analysis. The eradication rate and the incidence of total side effects were analyzed using two-category data. Relative risk (RR), an effector, and 95% confidence intervals (95% CIs) were calculated. The heterogeneity between the results of the study was examined using the Q test (test level is α = 0.1), and the magnitude of heterogeneity was judged by combining the findings with the I2 test.[24] If heterogeneity between studies was not observed (P > .10 or I2 ≤ 50%), the fixed-effect model was used for analysis; if heterogeneity existed (P ≤ .10 or I2 > 50%), the heterogeneity source was analyzed. If significant clinical heterogeneity was not observed, a random-effects model was used for the analysis.
2.6. Network meta-analysis
We used the mvmeta command in Stata 12.0 to perform the network meta-analysis of subgroups and common side effects.[25] First, we constructed a network of evidence for the comparison of treatments.[26] Inconsistency factors (IFs) and 95% CIs were used to evaluate the consistency of each closed loop. The 95% CI lower limit was equal to 0, which was considered consistent. Otherwise, the closed loop was considered obviously inconsistent.[27] In the present study, the outcome index was used as the count data. Therefore, the RR was used to combine the effect sizes, and the interval estimation was performed with 95% CIs, where the upper limit of the 95% CI was less than 1 or the lower limit was greater than 1, which indicated a statistically significant difference; otherwise the difference was not statistically significant. The PrBest and surface under the cumulative ranking curve (SUCRA) functions were used to rank the results of the network meta-analysis.[28] The SUCRA score was 100%. In the subgroup analysis, a greater SUCRA score indicated a better treatment effect. PrBest indicated the probability that the treatment would be the best treatment. In the side effect analysis, a greater SUCRA score indicated more common side effects. PrBest was the probability that this side effect became the most common side effect.
For data displaying significant clinical heterogeneity, a subgroup analysis or sensitivity analysis was conducted, and only a descriptive analysis was performed, if necessary. Publication bias was assessed using a funnel plot and an Egger linear regression analysis.
This study aimed to compare the incidence of the H pylori eradication rate and side effects between the probiotic group and the control group, and conducted the following subgroup analyses: probiotic addition timing: compared before (used before the eradication treatment), same (started and ended at the same time as the eradication treatment), after (used when the eradication treatment ended), before + same (used before the eradication treatment and ending with the eradication treatment), and same + after (starting at the same time as the eradication treatment and continuing when the eradication treatment was complete); probiotic duration: ≤2 weeks and >2 weeks; different eradication regimens: triple regimen, bismuth quadruple regimen, probiotic + triple regimen and probiotic + bismuth quadruple regimen; different probiotic species compared with multiple strains; eradication treatments used in China and other countries; and the occurrence of common side effects (including nausea, vomiting, abdominal pain, constipation, diarrhea, and taste disturbance).
2.7. Ethical statement
All analyses were based on previous published studies; thus no ethical approval and patient consent were required.
3. Results
3.1. Study identification and selection
We retrieved 905 studies, but excluded 530 nonclinical studies. Two hundred fifty-four replicated studies were excluded after a primary screen. Of the 121 studies obtained after screening, 81 studies did not meet the inclusion criteria (5 studies used inappropriate randomization methods, patients in 23 studies were younger than 18 years, 19 studies used rescue regimens, 8 studies used other drugs, 2 studies were not published in Chinese or English, 1 study listed incorrect data, and 17 studies did not describe withdrawals and dropouts. When withdrawals and dropouts occurred, 6 studies did not use the ITT analysis. Finally, 40 randomized controlled trials were eligible, including 16 Chinese studies and 24 English studies[29–68] (Fig. 1).
Figure 1.
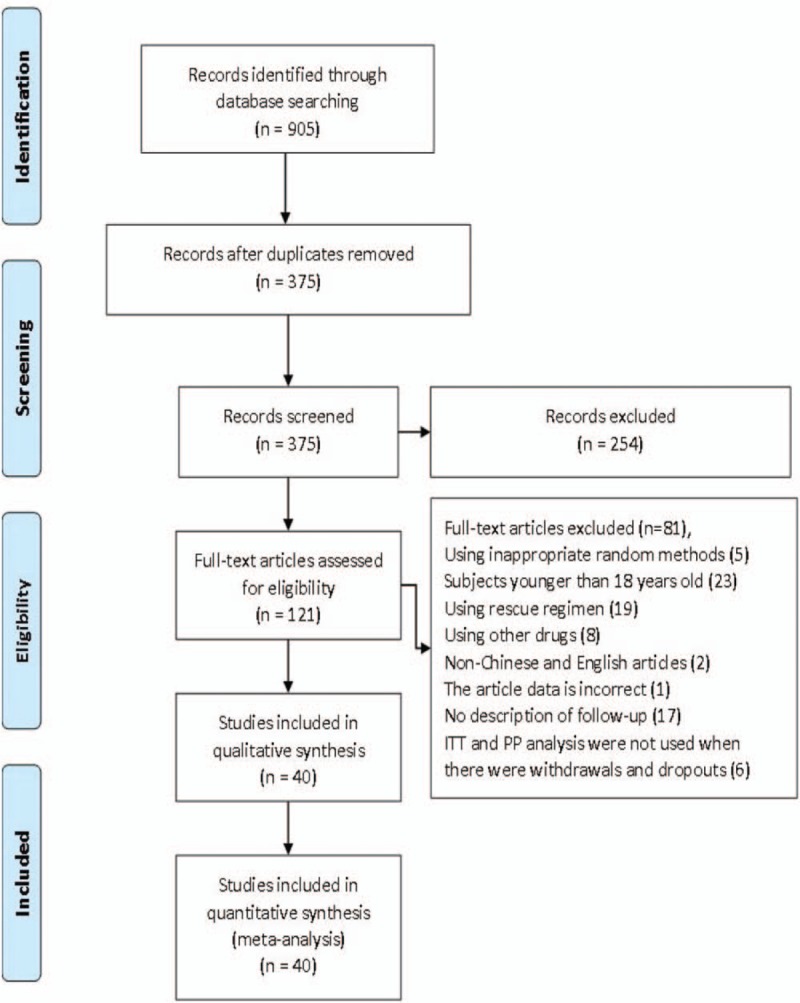
Flow diagram of the trials identified and selected.
3.2. Study characteristics and quality
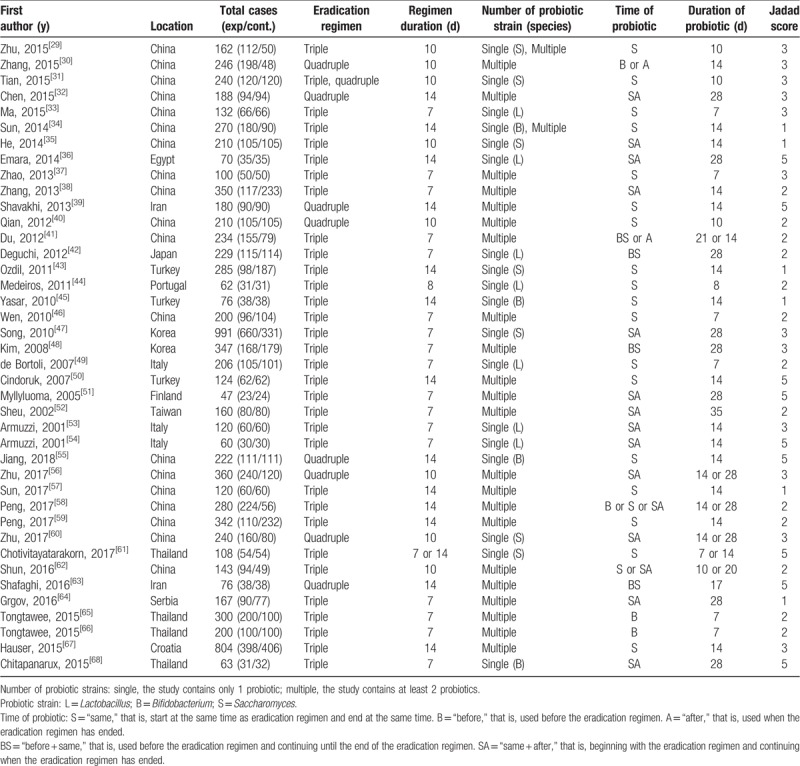
Forty randomized controlled trials with 8924 patients were analyzed in our study. Among these patients, 4903 were in the probiotic group and 4021 in the control group. We summarized the baseline characteristics of the included studies (Table 1).
Table 1.
Characteristics of the included studies.
3.3. Standard meta-analysis
3.3.1. H pylori eradication
The H pylori eradication rate was obtained from 40 randomized controlled trials. The eradication rates of the probiotic group and the control group obtained from the ITT analysis were 81.5% and 71.6%, respectively. Greater heterogeneity between studies (P < .001, I2 = 52.1%) necessitated the use of the random-effects model for meta-analysis, which showed that the difference between the probiotic group and the control group was statistically significant (RR 1.140, 95% CI 1.101–1.180, P < .001) (Fig. 2).
Figure 2.
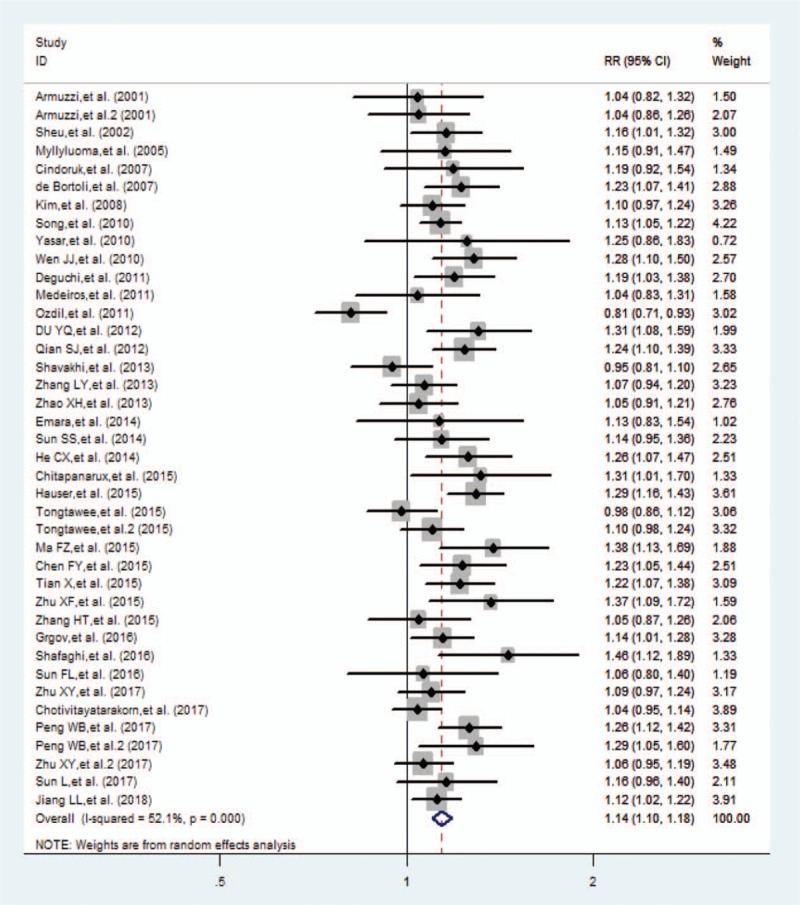
Forest plot comparing the eradication rate of probiotic addition by intention-to-treat analysis. CI = confidence interval, RR = relative risk.
3.4. Total side effects
Total side effects were described by 31 studies. The incidence of total side effects in the probiotic group obtained from the ITT analysis was 18.9%. The incidence of total side effects in the control group was 39.0%. The heterogeneity was greater (P < .001, I2 = 81.4%), and the result was obtained with the random-effects model was (RR 0.470, 95% CI 0.391–0.565, P < .001) (Fig. 3).
Figure 3.
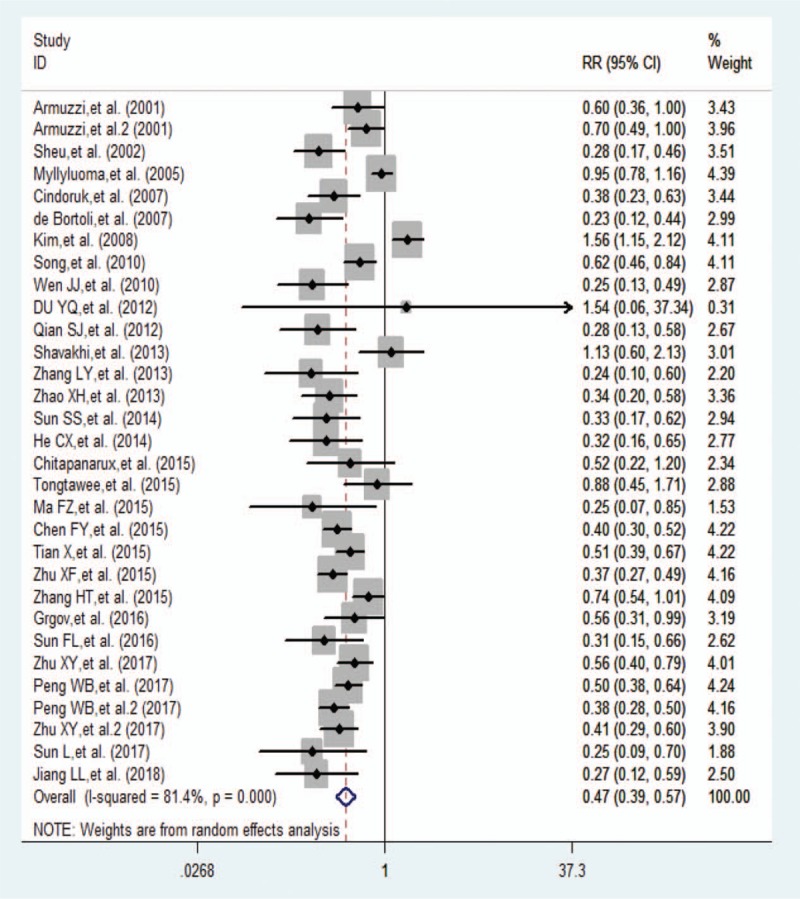
Forest plot comparing the total side effects of probiotic addition by intention-to-treat analysis. CI = confidence interval, RR = relative risk.
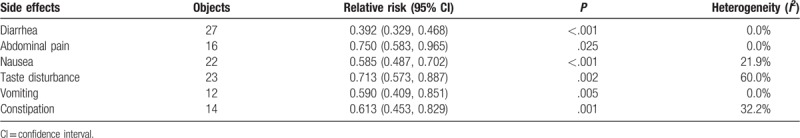
On the basis of the results from the standard meta-analysis, the incidence of diarrhea, abdominal pain, nausea, taste disturbance, vomiting, and constipation was significantly decreased in the probiotic group compared with the control group. Using a fixed-effect model, the following results were obtained: diarrhea (RR 0.392, 95% CI 0.329–0.468, P < .001), abdominal pain (RR 0.750, 95% CI 0.583–0.965, P = .025), nausea (RR 0.585, 95% CI 0.487–0.702, P < .001), vomiting (RR 0.590, 95% CI 0.409–0.851, P = .005), and constipation (RR 0.613, 95% CI 0.453–0.829, P < .001). The taste disturbance was analyzed using a random-effects model (RR 0.713, 95% CI 0.573–0.887, P = .002) (Table 2).
Table 2.
Standard meta-analysis of side effects.
3.5. Network meta-analysis
3.5.1. Network evidence
The options tested in the network were: probiotic addition time: before, same, after, before + same, and same + after; duration of probiotic addition: ≤2 weeks and >2 weeks; eradication regimens: triple regimen, quadruple regimen, probiotic + triple regimen, and probiotic + quadruple regimen; species of probiotics: Lactobacillus, Saccharomyces, Bifidobacterium, and multiple strains, location: China and abroad; and common side effects: diarrhea, abdominal pain, nausea, taste disturbance, vomiting, and constipation. Network plots for various treatment methods and common side effects were constructed (Fig. 4).
Figure 4.
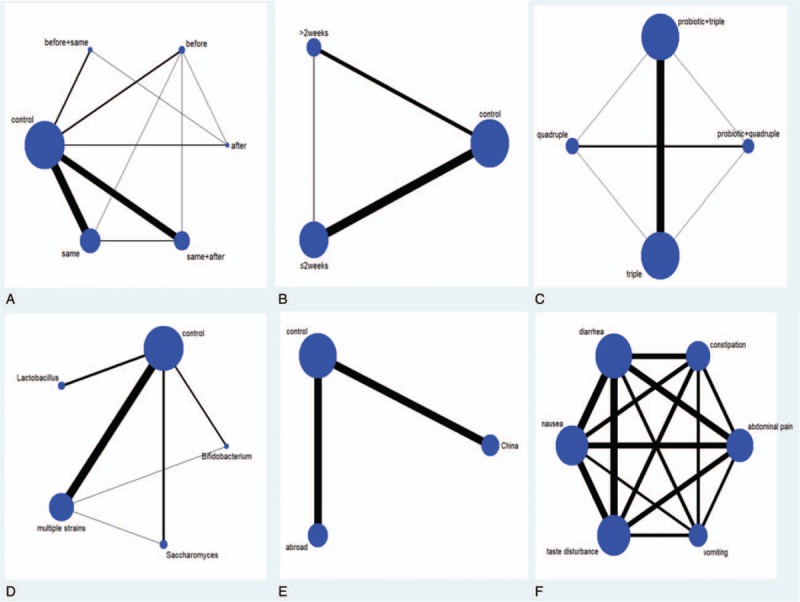
Network plot of subgroup and common side effects. (A) Probiotic addition time; (B) duration of probiotic addition; (C) eradication regimens; (D) species of probiotics; (E) location; (F) common side effects.
3.6. Statistical analysis
3.6.1. H pylori eradication
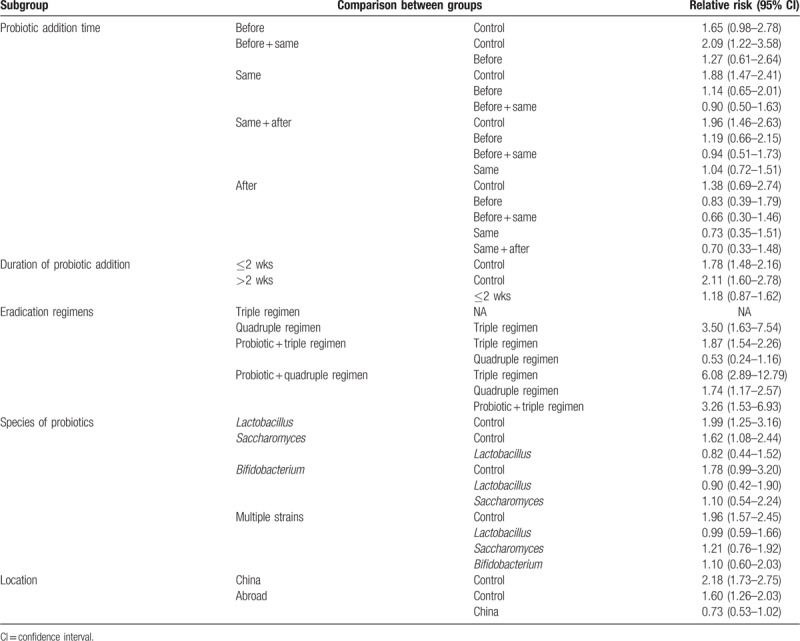
In the probiotic addition timing subgroup, the before + same (RR 2.09, 95% CI 1.22–3.58), same (RR 1.88, 95% CI 1.47–2.41), and same + after (RR 1.96, 95% CI 1.46–2.63) groups all yielded statistically significant differences from the control group. In the duration of probiotic addition subgroup.≤2 weeks (RR 1.78, 95% CI 1.48–2.16) and >2 weeks (RR 2.11, 95% CI 1.60–2.78) both produced statistically significant differences compared with the control group. When we compared the quadruple regimen in the eradication regimens subgroup with the probiotic + triple regimen (RR 0.53, 95% CI 0.24–1.16), a statistically significant difference was not observed between the 2 subgroups. Statistically significant differences between the other eradication regimens were observed. Compared with the control group, Lactobacillus (RR 1.99, 95% CI 1.25–3.16), Saccharomyces (RR 1.62, 95% CI 1.08–2.44), and multiple strains (RR 1.96, 95% CI 1.57–2.45) exhibited statistically significant differences in the analysis of the probiotic species. Regarding different locations, China (RR 2.18, 95% CI 1.73–2.75) and other countries (RR 1.60, 95% CI 1.26–2.03) were statistically significantly different from the control group. The results of the network meta-analysis are presented in Table 3.
Table 3.
Network meta-analysis results of subgroup eradication rate.
3.7. Common side effects
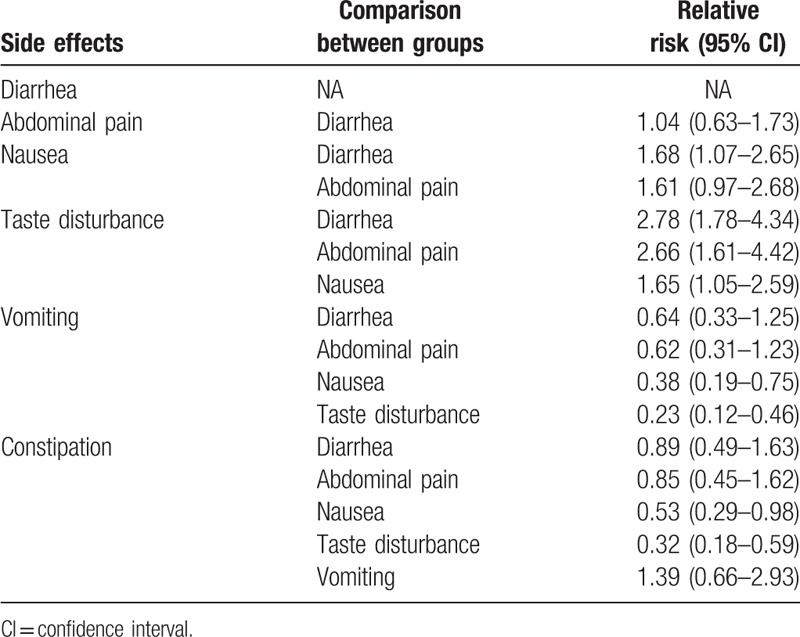
For the analysis of common side effects, nausea versus diarrhea (RR 1.68, 95% CI 1.07–2.65), taste disturbance versus diarrhea (RR 2.78, 95% CI 1.78–4.34), taste disturbance versus abdominal pain (RR 2.66, 95% CI 1.61–4.42), taste disturbance versus nausea (RR 1.65, 95% CI 1.05–2.59), vomiting versus nausea (RR 0.38, 95% CI 0.19–0.75), and constipation versus nausea (RR 0.53, 95% CI 0.29–0.98), vomiting versus taste disturbance (RR 0.23, 95% CI 0.12–0.46), and constipation versus taste disturbance (RR 0.32, 95% CI 0.18–0.59) produced statistically significant differences. The results of the network meta-analysis of side effects are shown in Table 4.
Table 4.
Network meta-analysis results of side effects.
3.8. Inconsistency analysis
In the location subgroup, no closed loop was formed, and no inconsistency analysis was performed. In the subgroups, closed loops were formed. The IFs for each subgroup were: probiotic addition timing (IF 0.04–0.18), duration of probiotic addition (IF 0.05), eradication regimens (IF 0.04–0.18), and species of probiotics (IF 0.06–0.17). The IFs for common side effects ranged between 0.00 and 0.90. The lower limit of the 95% CI for the subgroups and common side effects were 0, indicating that the closed loop consistency was better (Fig. 5).
Figure 5.

Inconsistency plot of subgroup and common side effects. (A) Probiotic addition time; (B) duration of probiotic addition; (C) eradication regimens; (D) species of probiotics; (E) common side effects. A = abdominal pain, C = constipation, D = diarrhea, N = nausea, T = taste disturbance, V = vomiting.
3.9. Ranking probability
The rankings of the various treatment modalities in the subgroups are shown in Table 5. The SUCRA scores for the probiotic subgroups were: before (49.6%), same (65.2%), after (33.6%), before + same (75.2%), and same + after (71.9%); duration of probiotic addition: ≤2 weeks (57.4%) and >2 weeks (92.6%); eradication regimens: triple regimen (0.0%), quadruple regimen (65.1%), probiotic + triple regimen (35.0%), and probiotic + quadruple regimen (99.9%); species of probiotics: Lactobacillus (73.6%), Saccharomyces (43.9%), Bifidobacterium (59.4%), and multiple strains (72.1%); location: China (98.5%) and abroad (51.5%). The rankings of common side effects are shown in Table 6. The SUCRA scores for diarrhea (39.7%), abdominal pain (43.9%), nausea (78.8%), taste disturbance (99.6%), vomiting (7.1%), and constipation (30.9%) were calculated. The SUCRA ranking plots were constructed according to the SUCRA curve (Fig. 6).
Table 5.
Ranking according to subgroup analysis of SUCRA curves.
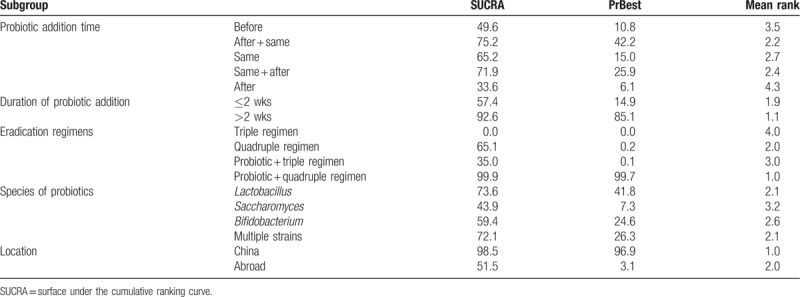
Table 6.
Ranking according to the common side effects of the SUCRA curve.
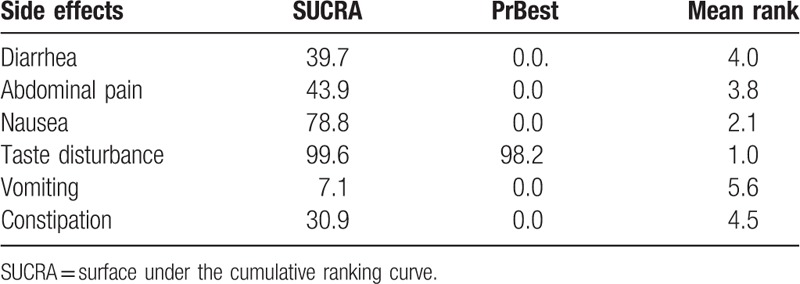
Figure 6.
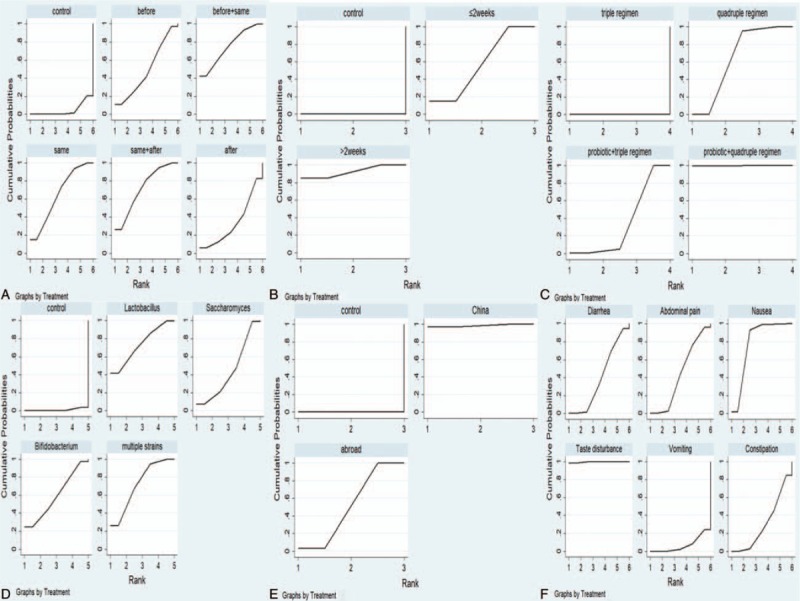
SUCRA plot of subgroup and common side effects. (A) Probiotic addition time; (B) duration of probiotic addition; (C) eradication regimens; (D) species of probiotics; (E) location; (F) common side effects. SUCRA = surface under the cumulative ranking curve.
3.10. Publication bias
The funnel plot obtained by an intentional analysis of the eradication rates of the 40 studies included was asymmetric. However, after the Egger test, no publication bias was detected (Fig. 7).
Figure 7.
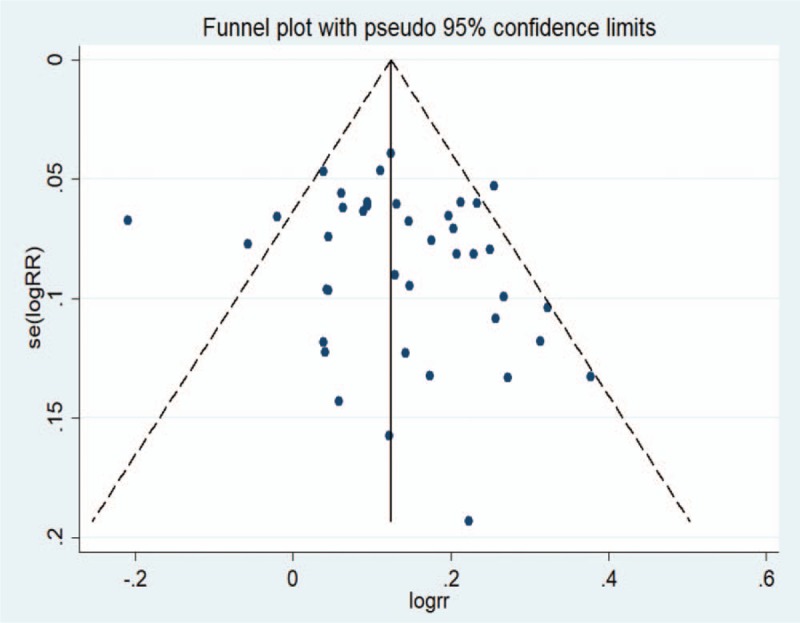
Funnel plot of the included studies for eradication rate.
3.11. Sensitivity analysis
A sensitivity analysis was applied because the included studies were heterogeneous. When performing a sensitivity analysis according to ITT, the 95% CIs of each study overlapped, and thus the difference was not significant. When the most different study was removed,[43] the CI and RR did not change significantly. Therefore, the results of the meta-analysis were reliable.
3.12. Heterogeneity
When we conducted a meta-analysis of the total side effects, the heterogeneity was greater. Therefore, we used a meta-regression analysis to assess heterogeneity. The probiotic addition duration, study language, and study quality were the main sources of heterogeneity.
4. Discussion
This study investigated the efficacy and safety of probiotics in the eradication of H pylori. Our study indicated that probiotics improved the eradication rate and reduced the incidence of side effects when administered with treatments of eradicating H pylori, especially combined with the bismuth quadruple regimen. Better eradication effects were exerted when using probiotics before and throughout the eradication treatment or using probiotics for more than 2 weeks. Also, the eradication effect of Chinese was better than other countries.
In the past years, the decreasing eradication rate and increasing side effects made it urgent to find the optimized eradicative regimens of H pylori.[6–8] Probiotics have been receiving more and more attention as an adjuvant of eradication treatment. Probiotics, initially proposed by Lilly and Sttillwel in 1965, were defined as factors derived from microorganisms that stimulate the proliferation of other beneficial bacteria.[69] It have been used on the treatment of variety diseases, including IBD, irritable bowel syndrome, and diarrhea.[70] Bhatia et al firstly shown that H pylori growth was inhibited by Lactobacillus acidophilus in vitro.[71–73] Also, the mechanism might be related to the reduction in urease activity mediated by short-chain fatty acids produced by probiotics, an enhancement of the acidic environment of the stomach, damages of the cell wall of H pylori strains, and inhibition of the colonization of H pylori in the gastric mucosa.[74–74,49,75] What is more, probiotics had a positive effect on inhibiting the inflammatory response which mediated by interleukin (IL)-8 after an H pylori infection.[76,77] Meanwhile, probiotics helped to alleviate antibiotic-related gastrointestinal reactions and improved drug compliance.[78]
Combined probiotics with the bismuth quadruple regimen exerted the best eradication effect in our study. Because the eradication rate of combined probiotics and standard triple regimen was inferior to that in the bismuth quadruple regimen, probiotics were not able to replace bismuth. The results were the same as those reported by Chinese scholars.[79,80] On the basis of the strong antibacterial effect of bismuth quadruple regimen, adding probiotics could increase the eradication rate. However, internists should have noticed that bismuth has an inhibitory effect on probiotics. To avoid this effect, bismuth and probiotics should be taken at different times.
Lactobacillus and multiple strains exerted better eradication effects. Previous analyses also supported this result.[18,81] This might be related to the species specificity of the probiotics.[82] The metabolites of Lactobacillus exert a strong antibacterial effect, potentially increasing humoral and cellular immunity. Moreover, the use of multiple strains included the characteristics of other probiotics. However, Saccharomyces needed to cooperate with other probiotics to more substantially improve the eradication effect. In China, the multiple probiotic strains and bismuth quadruple regimen were used widely. Therefore, the eradication effect of Chinese was better than other countries.
Our study indicated that using the probiotics before and throughout the eradication treatment achieved a higher eradication rate. Also, the optimal duration of probiotics was more than 2 weeks. Using probiotics alone could improve the eradication rate, but the effect was not satisfied, which also indicated the characteristics of probiotics as an adjuvant for eradication treatment.[83] Excluded the effects of using probiotics alone, a potential mechanism was that probiotics helped to reduce the load of H pylori before eradication and continued to remove residual H pylori after eradication.[7] Although previous studies supported this result,[19,84] our study had a more detailed subgroup on the timing and duration of probiotic addition.
We analyzed the incidence of 6 common side effects in the probiotic group. Taste disturbance was the most common side effect, whereas vomiting and diarrhea were relatively less frequent. This difference may explain why probiotics reduced antibiotic-related gastrointestinal side effects, but the mitigation of taste disturbance was not good.[10,11,85]
In terms of the efficacy and safety of probiotics in eradicating H pylori, the results from previous meta-analyses were similar to the present study.[18,19,83,86–89] However, some studies did not address the timing and duration of probiotic addition, and excluded the bismuth quadruple regime. In contrast, our study had the following strengths. First, we used the network meta-analysis method to rank the subgroup results that were not able to directly compared, and the timing of probiotic supplementation was more comprehensive. Second, we investigated the situation of eradicating H pylori in China and abroad. Last, we also analyzed the occurrence of common side effects such as diarrhea, abdominal pain, nausea, taste disturbance, vomiting, and constipation.
This study also had some limitations. First, a high heterogeneity was observed on the analysis of total side effects. Although we had used a meta-regression analysis to assess heterogeneity, the source of heterogeneity could not be completely explained. The data on side effects were collected during the follow-up period. Therefore, subjectivity and inconsistency may cause substantial heterogeneity. Second, the small sample size of the study would lead to an overestimation of treatment effects. Last, the subjects analyzed in the present study did not include children. Therefore, more studies with larger sample sizes and higher-quality trials were needed for further analysis.
5. Conclusions
Probiotics improved the eradication rate and reduced side effects when assisting with the eradication of H pylori. The use of probiotics before and throughout the eradication treatment, and the use of probiotics for more than 2 weeks exerted a better eradication effect. Probiotics combined with the bismuth quadruple regimen was the best combination. Lactobacillus and multiple strains were the better choices for probiotic strains. The eradication effect reported in China was better than the rates reported in other countries.
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant no. 81660093). We are grateful for the generous support of the hospital.
Author contributions
Conceptualization: Xiaoguang Shi, Xue Huang.
Data curation: Xiaoguang Shi, Jialing Shi.
Formal analysis: Xiaoguang Shi.
Funding acquisition: Junhong Zhang, Xue Huang.
Investigation: Xiaoguang Shi.
Methodology: Xiaoguang Shi, Lingshan Mo, Jialing Shi, Mengbin Qin.
Project administration: Junhong Zhang, Xue Huang.
Resources: Xiaoguang Shi, Xue Huang.
Software: Xiaoguang Shi, Lingshan Mo.
Supervision: Mengbin Qin, Xue Huang.
Validation: Xiaoguang Shi, Jialing Shi, Mengbin Qin, Xue Huang.
Writing – original draft: Xiaoguang Shi.
Writing – review & editing: Xiaoguang Shi, Jialing Shi, Xue Huang.
Footnotes
Abbreviations: CI, confidence interval IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; ITT, intention to treat; RR, relative risk; SUCRA = surface under the cumulative ranking curve.
The authors report no conflicts of interest in this work.
References
- [1].Abbasi J, Barry Marshall MD. H pylori 35 years later. JAMA 2017;317:1400–2. [DOI] [PubMed] [Google Scholar]
- [2].Xia Y, Meng G, Zhang Q, et al. Dietary patterns are associated with Helicobacter pylori infection in Chinese adults: a cross-sectional study. Sci Rep 2016;6:32334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goli YD, Moniri R. Efficacy of probiotics as an adjuvant agent in eradication of Helicobacter pylori infection and associated side effects. Benef Microbes 2016;7:519–27. [DOI] [PubMed] [Google Scholar]
- [4].Gisbert JP, Molina-Infante J, Amador J, et al. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol Hepatol 2016;39:697–721. [DOI] [PubMed] [Google Scholar]
- [5].Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- [6].Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut 2012;61:646–64. [DOI] [PubMed] [Google Scholar]
- [7].Song HY, Zhou L, Liu DY, et al. What roles do probiotics play in the eradication of Helicobacter pylori? Current knowledge and ongoing research. Gastroenterol Res Pract 2018;2018:9379480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol 2018;102:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother 2011;45:960–6. [DOI] [PubMed] [Google Scholar]
- [10].Goldenberg JZ, Mertz D, Johnston BC. Probiotics to prevent Clostridium difficile infection in patients receiving antibiotics. JAMA 2018;320:499–500. [DOI] [PubMed] [Google Scholar]
- [11].Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology 2017;152:1889–900. e1889. [DOI] [PubMed] [Google Scholar]
- [12].Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016;65:1906–15. [DOI] [PubMed] [Google Scholar]
- [13].Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World J Gastrointest Pathophysiol 2014;5:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther 2010;32:1069–79. [DOI] [PubMed] [Google Scholar]
- [15].Talebi Bezmin Abadi A, Kusters JG. Future of Helicobacter pylori and its feasibility. Expert Rev Anti Infect Ther 2018;16:733–5. [DOI] [PubMed] [Google Scholar]
- [16].Lu C, Sang J, He H, et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: a meta-analysis. Sci Rep 2016;6:23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Navarro-Rodriguez T, Silva FM, Barbuti RC, et al. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: a prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol 2013;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu M, Yu S, Deng J, et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: a meta-analysis of randomized controlled trials. PloS One 2016;11:e0163743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lv Z, Wang B, Zhou X, et al. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: a meta-analysis. Exp Ther Med 2015;9:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matsushima M, Takagi A. Is it effective?” to “How to use it?”: the era has changed in probiotics and functional food products against Helicobacter pylori infection. J Gastroenterol Hepatol 2012;27:851–3. [DOI] [PubMed] [Google Scholar]
- [21].Boyanova L, Mitov I. Coadministration of probiotics with antibiotics: why, when and for how long? Expert Rev Anti Infect Ther 2012;10:407–9. [DOI] [PubMed] [Google Scholar]
- [22].Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539-1558. [DOI] [PubMed] [Google Scholar]
- [25].White IR. Multivariate Random-effects meta-analysis. Stata J 2009;9:40–56. [Google Scholar]
- [26].Zhang C, Xu C, Zeng X. Drawing of network plots of network meta-analysis. Chin J Evid-Based Med 2013;13:1382–6. [Google Scholar]
- [27].Zhang C, Yan J, Sun F, et al. Differentiation and handling of homogeneity in network meta-analysis. Chin J Evid-Based Med 2014;14:884–8. [Google Scholar]
- [28].Chaimani A, Higgins JPT, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PloS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhu XF, Li C, Han SS, et al. Curative effect observation of triple therapy in combination with different probiotics in treatment of anti-H pylori. Chin J Biochem Pharm 2015;35:110–2. [Google Scholar]
- [30].Zhang HT, Pu YF, Zhang XH, et al. Clinical study on the eradication of Helicobacter pylori by compound Lactobacillus acidophilus tablets and Bifidobacterium triple live capsules combined with quadruple therapy. Chin J Integr Tradit West Med Digest 2015;23:581–3. +585. [Google Scholar]
- [31].Tian X, Li C, Zhu XF, et al. Therapeutic effect of Saccharomyces boulardii on eradication of Helicobacter pylori. J Hebei Med Univ 2015;36:939–41. [Google Scholar]
- [32].Chen FY, Zhang JL, Xu HF. The efficacy of Livzon Weisan+Esomeprazole combined with probiotics against Helicobacter pylori. Modern J Integr Tradit Chin West Med 2015;24:1211–3. [Google Scholar]
- [33].Ma F, Zhou C, Wang J, et al. Probiotics in the treatment of peptic ulcer infected by helicobacter pylory and its safety. Pak J Pharm Sci 2015;28(3 suppl):1087–90. [PubMed] [Google Scholar]
- [34].Sun SS, Du SS. Observation on the efficacy of bifidobacterium and Lactobacillus acidophilus combined with triple therapy for eradication of Helicobacter pylori. Shandong Med J 2014;54:39–40. [Google Scholar]
- [35].He CX, Liu GF, Zhu XY, et al. Therapeutic effect of Saccharomyces boulardii Sachets combined with triple therapy for Helicobacter pylori eradication. Chin J Microecol 2014;26:306–9. [Google Scholar]
- [36].Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap Adv Gastroenterol 2014;7:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao XH, Zhang J, Feng Q, et al. The clinical study of compound lactobacillus capsule combining with triple therapy in the radical treatment of Helicobacter pylori. China Modern Med 2013;20:28–9. +31. [Google Scholar]
- [38].Zhang LY, Wu ZJ. Sequential therapy and triple therapy combined with probiotics for eradication of Helicobacter pylori infection: a controlled clinical study. Chin J Gastroenterol 2013;18:286–91. [Google Scholar]
- [39].Shavakhi A, Tabesh E, Yaghoutkar A, et al. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter 2013;18:280–4. [DOI] [PubMed] [Google Scholar]
- [40].Qian SJ, Wei XJ, Wang L, et al. Clinical observation of modified sequential therapy combined with Si Liankang eradication of Helicobacter pylori. Hebei Med J 2012;34:3546–7. [Google Scholar]
- [41].Du YQ, Su T, Fan JG, et al. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol 2012;18:6302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Deguchi R, Nakaminami H, Rimbara E, et al. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol 2012;27:888–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ozdil K, Calhan T, Sahin A, et al. Levofloxacin based sequential and triple therapy compared with standard plus probiotic combination for Helicobacter pylori eradication. Hepatogastroenterology 2011;58:1148–52. [DOI] [PubMed] [Google Scholar]
- [44].Medeiros JA, Goncalves TM, Boyanova L, et al. Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis 2011;30:555–9. [DOI] [PubMed] [Google Scholar]
- [45].Yasar B, Abut E, Kayadibi H, et al. Efficacy of probiotics in Helicobacter pylori eradication therapy. Turk J Gastroenterol 2010;21:212–7. [DOI] [PubMed] [Google Scholar]
- [46].Wen JQR. Clinical study on the efficacy of bifid triple live bacterial preparation in eradication of Helicobacter pylori. J Gannan Med Univ 2010;30:902–3. [Google Scholar]
- [47].Song MJ, Park DI, Park JH, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter 2010;15:206–13. [DOI] [PubMed] [Google Scholar]
- [48].Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008;13:261–8. [DOI] [PubMed] [Google Scholar]
- [49].de Bortoli N, Leonardi G, Ciancia E, et al. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol 2007;102:951–6. [DOI] [PubMed] [Google Scholar]
- [50].Cindoruk M, Erkan G, Karakan T, et al. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter 2007;12:309–16. [DOI] [PubMed] [Google Scholar]
- [51].Myllyluoma E, Veijola L, Ahlroos T, et al. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy: a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther 2005;21:1263–72. [DOI] [PubMed] [Google Scholar]
- [52].Sheu BS, Wu JJ, Lo CY, et al. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther 2002;16:1669–75. [DOI] [PubMed] [Google Scholar]
- [53].Armuzzi A, Cremonini F, Ojetti V, et al. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 2001;63:1–7. [DOI] [PubMed] [Google Scholar]
- [54].Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2001;15:163–9. [DOI] [PubMed] [Google Scholar]
- [55].Jiang L, Zhu W. Probiotics improved the effectiveness and safety of the quadruple Helicobacter pylori eradication therapy. Biomed Res (India) 2018;29:2053–6. [Google Scholar]
- [56].Zhu XY, Du J, Zhao WJ, et al. Efficacy of compound Lactobacillus tablets combined with bismuth-containing quadruple therapy for Helicobacter pylori eradication. Chin J Microecol 2017;29:909–12. [Google Scholar]
- [57].Sun L. Comparative observation of conventional triple therapy combined with probiotics for the treatment of Helicobacter pylori infection. Peoples Military Surg 2017;60:272–4. +278. [Google Scholar]
- [58].Peng WB, Rong HY, Sha WH, et al. The clinical efficacy of different adding times, treatment courses and doses of probiotics for Helicobacter pylori eradication. J Pract Med 2017;33:395–8. [Google Scholar]
- [59].Peng WB, Rong HY, Sha WH, et al. Efficacy of quadruple therapy containing probiotics on eradication of Helicobacter pylori. Guangzhou Med J 2017;48:38–41. [Google Scholar]
- [60].Zhu XY, Du J, Wu J, et al. [Influence of Saccharomyces boulardii Sachets combined with bismuth quadruple therapy for initial Helicobacter pylori eradication]. Zhonghua Yi Xue Za Zhi 2017;97:2353–6. [DOI] [PubMed] [Google Scholar]
- [61].Chotivitayatarakorn P, Mahachai V, Vilaichone RK. Effectiveness of 7-day and 14-day moxifloxacin-dexlansoprazole based triple therapy and probiotic supplement for Helicobacter pylori eradication in thai patients with non-ulcer dyspepsia: a double-blind randomized placebo-controlled study. Asian Pac J Cancer Prev 2017;18:2839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shun FL, Zhang QS, Guo GA, et al. The eradication of Helicobacter pylori using compound Lactobacillus acidophilus tablets combined with triple therapy. Chin J Microecol 2016;28:1080–3. [Google Scholar]
- [63].Shafaghi A, Pourkazemi A, Khosravani M, et al. The effect of probiotic plus prebiotic supplementation on the tolerance and efficacy of Helicobacter pylori eradication quadruple therapy: a randomized prospective double blind controlled trial. Middle East J Digest Dis 2016;8:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Grgov S, Tasic T, Radovanovic-Dinic B, et al. Can probiotics improve efficiency and safety profile of triple Helicobacter pylori eradication therapy? A prospective randomized study. Vojnosanitetski pregled 2016;73:1044–9. [DOI] [PubMed] [Google Scholar]
- [65].Tongtawee T, Dechsukhum C, Leeanansaksiri W, et al. Improved Helicobacter pylori eradication rate of tailored triple therapy by adding Lactobacillus delbrueckii and Streptococcus thermophilus in northeast region of Thailand: a prospective randomized controlled clinical trial. Gastroenterol Res Pract 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tongtawee T, Dechsukhum C, Leeanansaksiri W, et al. Effect of pretreatment with Lactobacillus delbrueckii and Streptococcus thermophillus on tailored triple therapy for Helicobacter pylori eradication: a prospective randomized controlled clinical trial. Asian Pac J Cancer Prev 2015;16:4885–90. [DOI] [PubMed] [Google Scholar]
- [67].Hauser G, Salkic N, Vukelic K, et al. Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Medicine 2015;94:e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chitapanarux T, Thongsawat S, Pisespongsa P, et al. Effect of Bifidobacterium longum on PPI-based triple therapy for eradication of Helicobacter pylori: a randomized, double-blind placebo-controlled study. J Funct Foods 2015;13:289–94. [Google Scholar]
- [69].Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- [70].Alvarez-Calatayud G, Margolles A. Dual-coated lactic acid bacteria: an emerging innovative technology in the field of probiotics. Future Microbiol 2016;11:467–75. [DOI] [PubMed] [Google Scholar]
- [71].Aiba Y, Ishikawa H, Tokunaga M, et al. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol Lett 2017;364: [DOI] [PubMed] [Google Scholar]
- [72].Enany S, Abdalla S. In vitro antagonistic activity of Lactobacillus casei against Helicobacter pylori. Braz J Microbiol 2015;46:1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Buhling A, Radun D, Muller WA, et al. Influence of anti-Helicobacter triple-therapy with metronidazole, omeprazole and clarithromycin on intestinal microflora. Aliment Pharmacol Ther 2001;15:1445–52. [DOI] [PubMed] [Google Scholar]
- [74].Vijayaram S, Kannan S. Probiotics: The marvelous factor and health benefits. Biomed Biotechnol Res J (BBRJ) 2018;2:1–8. [Google Scholar]
- [75].Mukai T, Asasaka T, Sato E, et al. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol 2002;32:105–10. [DOI] [PubMed] [Google Scholar]
- [76].Panpetch W, Spinler JK, Versalovic J, et al. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori-stimulated gastric epithelial cells. BMC Microbiol 2016;16:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sultana R, McBain AJ, O’Neill CA. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol 2013;79:4887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhou BG, Xiao Z, Wang CQ, et al. Meta-analysis of probiotics for the treatment of Helicobacter pylori infection was re-evaluated. WCJD 2015;23:3326–36. [Google Scholar]
- [79].Tan XL, Xie CH, Zhao C, et al. Efficacy of compound Lactobacillus combined with triple or quadruple therapy in eradicating Helicobacter pylori. Clin Med Eng 2015;22:1054–5. [Google Scholar]
- [80].Li X, Cai YM, Jin HS. Analysis of eradication rate of Helicobacter pylori by Bifidobacterium quadruple bacteria combined with triple and quadruple therapy. China Foreign Med Treat 2014;33:100–1. [Google Scholar]
- [81].Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007;25:155–68. [DOI] [PubMed] [Google Scholar]
- [82].Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 2011;50:1–7. [DOI] [PubMed] [Google Scholar]
- [83].Losurdo G, Cubisino R, Barone M, et al. Probiotic monotherapy and Helicobacter pylori eradication: a systematic review with pooled-data analysis. World J Gastroenterol 2018;24:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Szajewska H, Kolodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther 2015;42:1149–57. [DOI] [PubMed] [Google Scholar]
- [85].Zhu R, Chen K, Zheng YY, et al. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J Gastroenterol 2014;20:18013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang F, Feng J, Chen P, et al. Probiotics in Helicobacter pylori eradication therapy: systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol 2017;41:466–75. [DOI] [PubMed] [Google Scholar]
- [87].Lau CS, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis. Infect Drug Resist 2016;9:275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang MM, Qian W, Qin YY, et al. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol 2015;21:4345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gong Y, Li Y, Sun Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:6530–43. [PMC free article] [PubMed] [Google Scholar]


