Abstract
Background
Previous systematic reviews and meta‐analyses consistently show the positive effect of exercise‐based rehabilitation for heart failure (HF) on exercise capacity; however, the direction and magnitude of effects on health‐related quality of life, mortality and hospital admissions in HF remain less certain. This is an update of a Cochrane systematic review previously published in 2010.
Objectives
To determine the effectiveness of exercise‐based rehabilitation on the mortality, hospitalisation admissions, morbidity and health‐related quality of life for people with HF. Review inclusion criteria were extended to consider not only HF due to reduced ejection fraction (HFREF or 'systolic HF') but also HF due to preserved ejection fraction (HFPEF or 'diastolic HF').
Search methods
We updated searches from the previous Cochrane review. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue1, 2013) from January 2008 to January 2013. We also searched MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCO) and PsycINFO (Ovid) (January 2008 to January 2013). We handsearched Web of Science, bibliographies of systematic reviews and trial registers (Controlled‐trials.com and Clinicaltrials.gov).
Selection criteria
Randomised controlled trials of exercise‐based interventions with six months' follow‐up or longer compared with a no exercise control that could include usual medical care. The study population comprised adults over 18 years and were broadened to include individuals with HFPEF in addition to HFREF.
Data collection and analysis
Two review authors independently screened all identified references and rejected those that were clearly ineligible. We obtained full‐text papers of potentially relevant trials. One review author independently extracted data from the included trials and assessed their risk of bias; a second review author checked data.
Main results
We included 33 trials with 4740 people with HF predominantly with HFREF and New York Heart Association classes II and III. This latest update identified a further 14 trials. The overall risk of bias of included trials was moderate. There was no difference in pooled mortality between exercise‐based rehabilitation versus no exercise control in trials with up to one‐year follow‐up (25 trials, 1871 participants: risk ratio (RR) 0.93; 95% confidence interval (CI) 0.69 to 1.27, fixed‐effect analysis). However, there was trend towards a reduction in mortality with exercise in trials with more than one year of follow‐up (6 trials, 2845 participants: RR 0.88; 95% CI 0.75 to 1.02, fixed‐effect analysis). Compared with control, exercise training reduced the rate of overall (15 trials, 1328 participants: RR 0.75; 95% CI 0.62 to 0.92, fixed‐effect analysis) and HF specific hospitalisation (12 trials, 1036 participants: RR 0.61; 95% CI 0.46 to 0.80, fixed‐effect analysis). Exercise also resulted in a clinically important improvement superior in the Minnesota Living with Heart Failure questionnaire (13 trials, 1270 participants: mean difference: ‐5.8 points; 95% CI ‐9.2 to ‐2.4, random‐effects analysis) ‐ a disease specific health‐related quality of life measure. However, levels of statistical heterogeneity across studies in this outcome were substantial. Univariate meta‐regression analysis showed that these benefits were independent of the participant's age, gender, degree of left ventricular dysfunction, type of cardiac rehabilitation (exercise only vs. comprehensive rehabilitation), mean dose of exercise intervention, length of follow‐up, overall risk of bias and trial publication date. Within these included studies, a small body of evidence supported exercise‐based rehabilitation for HFPEF (three trials, undefined participant number) and when exclusively delivered in a home‐based setting (5 trials, 521 participants). One study reported an additional mean healthcare cost in the training group compared with control of USD3227/person. Two studies indicated exercise‐based rehabilitation to be a potentially cost‐effective use of resources in terms of gain in quality‐adjusted life years (QALYs) and life‐years saved.
Authors' conclusions
This updated Cochrane review supports the conclusions of the previous version of this review that, compared with no exercise control, exercise‐based rehabilitation does not increase or decrease the risk of all‐cause mortality in the short term (up to 12‐months' follow‐up) but reduces the risk of hospital admissions and confers important improvements in health‐related quality of life. This update provides further evidence that exercise training may reduce mortality in the longer term and that the benefits of exercise training on appear to be consistent across participant characteristics including age, gender and HF severity. Further randomised controlled trials are needed to confirm the small body of evidence seen in this review for the benefit of exercise in HFPEF and when exercise rehabilitation is exclusively delivered in a home‐based setting.
Keywords: Adult, Aged, Humans, Middle Aged, Young Adult, Exercise Therapy, Exercise Therapy/mortality, Chronic Disease, Exercise Tolerance, Health Status, Heart Failure, Heart Failure/mortality, Heart Failure/rehabilitation, Hospitalization, Hospitalization/statistics & numerical data, Quality of Life, Randomized Controlled Trials as Topic
Exercise‐based rehabilitation for heart failure
Background
People with heart failure experience marked reductions in their exercise capacity, which has detrimental effects on their activities of daily living, health‐related quality of life and ultimately their hospital admission rate and mortality.
Study characteristics
We searched the scientific literature for randomised controlled trials (experiments in which two or more interventions, possibly including a control intervention or no intervention, are compared by being randomly allocated to participants) looking at the effectiveness of exercise‐based treatments compared with no exercise on heart failure in adults over 18 years of age. The inclusion criteria of this updated review were extended to consider not only HF due to reduced ejection fraction (HFREF or 'systolic HF') (ejection fraction is a measure of how well your heart is pumping), but also HF due to preserved ejection fraction (HFPEF or 'diastolic HF'). The search is current to January 2013.
Key results
We found 33 RCTs that included 4740 participants. The findings of this update are consistent with the previous (2010) version of this Cochrane review and show important benefits of exercise‐based rehabilitation that include a reduction in the risk of hospital admissions due to HF and improvements in health‐related quality of life compared with not undertaking exercise. There was a high level of variation across studies in health‐related quality of life outcome. While the majority of evidence was for exercise‐based rehabilitation in people with HFREF, this update did identify a broader evidence base that included higher risk (New York Heart Association class IV) and older people, people with HFPEF and more programmes conducted in a home‐based setting. We found no evidence to suggest that exercise training programmes cause harm in terms of an increase in the risk of death in either the short or longer term. A small body of economic evidence was identified indicating exercise‐based rehabilitation to be cost‐effective. Further evidence is needed to understand the effect of exercise training in people with HFPEF better and the costs and effects of exclusively home‐based exercise rehabilitation programmes.
Quality of evidence
The general lack of reporting of methods in the included trial reports made it difficult to assess their methodological quality and thereby judge their risk of possible bias.
Background
Description of the condition
People with heart failure (HF) present with a variety of symptoms most of which are non‐specific (Watson 2000). The most frequently presenting symptom is exertional breathlessness. Other important symptoms are fatigue and lethargy in addition to swelling of the feet and ankles. There is no single diagnostic test for HF and diagnosis relies on clinical judgement based on a combination of history, physical examination and appropriate investigations. The symptoms and functional exercise capacity are used to classify the severity of HF, using the New York Heart Association (NYHA) classification (NYHA 1994), and to judge responsiveness to treatment. While diagnosis is based upon symptoms, disease severity can be quantified using objective measures, for example echocardiographic assessment of ejection fraction.
People with HF experience marked reductions in their exercise capacity, which has detrimental effects on their activities of daily living, health‐related quality of life (HRQoL), and ultimately their hospital admission rate and mortality (WGCR 2001). While survival after HF diagnosis has improved (AHA 2014), HF has a poor prognosis as 30% to 40% of people diagnosed with HF die within one year although thereafter the mortality is less than 10% per year (AHA 2014). Hospital admission rates for HF in the US appear to have fallen between 1998 and 2008 (Chen 2011). However, in the UK, despite a progressive reduction in age‐adjusted hospital admission rates since 1992 to 1993, admissions due HF are projected to rise by 50% over the next 25 years, largely due to the ageing of the population (NICE 2010). It is estimated that the total annual cost of HF to the UK National Health Service (NHS) is around GBP1 billion, or around 2% of the total UK NHS budget; approximately 70% of this total is due to the costs of hospitalisation (Editorial 2011; NICE 2010).
The prevalence and incidence of HF is steadily increasing, with approximately 825,000 new cases annually in the US (AHA 2014). While improved management of hypertension has reduced this condition as an aetiological factor in the development of HF, the increased survival rate from myocardial infarction has led to a subsequent increase in the number of cases of HF (Kostis 1997), as has increasing longevity in developed countries. Estimates of the prevalence of HF in the US range from 0.7% to 1.5% in adults aged 40 to 59 years; over 80 years of age the prevalence of HF is in the region of 8.6% to 11.5% (AHA 2014).
It has been increasingly recognised that HF has two subcategories. People with HF can be categorised as having impaired left ventricular contraction, which results in a reduced ejection fraction (less than 35% to 50%), known as HF with reduced ejection fraction (HFREF) or 'left ventricular dysfunction' or 'systolic HF'. The other category is HF with preserved ejection fraction (HFPEF) with an ejection fraction of greater than 35% to 50% and also known as 'diastolic HF' (Lam 2011; Owen 2006). Prognosis in HFPEF is better than HFREF. One meta‐analysis reported a mortality of 32.1% in HFPEF versus 40.6% in HFREF (risk ratio (RR) 0.79) over a mean of 47 months' follow‐up (Somaratne 2009). Although individuals with HFPEF are thought to contribute 54% of all people with HF, most trials to date of drug and medical device therapies have recruited only people with HFREF. This limited number of studies examining the effect of different pharmacological agents with proven use in HFREF has largely been disappointing in the HFPEF group (Holland 2011).
National and international evidence‐based guidelines have been developed to help improve diagnosis and treatment for people with HF. These guidelines cover aetiology, prevention, diagnostic modalities and therapeutic interventions that increasingly include exercise rehabilitation (ACCF/AHA 2013; McMurray 2012; NICE 2010).
Description of the intervention
While there are many definitions of cardiac rehabilitation (CR), the following presents their combined key elements: "The coordinated sum of activities required to influence favourably the underlying cause of cardiovascular disease, as well as to provide the best possible physical, mental and social conditions, so that the patients may, by their own efforts, preserve or resume optimal functioning in their community and through improved health behaviour, slow or reverse progression of disease" (BACPR 2012). A central component of CR is exercise training (Piepoli 1998). However, in addition to exercise, programmes are encouraged also provide risk factor and lifestyle education on risk factor management plus counselling and psychological support, so‐called 'comprehensive CR' (Corra 2005).
Based on current evidence of clinical outcomes and costs, national and international guidelines on the management of HF including the American College of Cardiology/American Heart Association, European Society of Cardiology and National Institute for Health and Care Excellence (NICE) in the UK consistently recommend CR as an effective and safe intervention (ACCF/AHA 2013; McMurray 2012; NICE 2010). However, these guidelines are not fully implemented in practice and the current uptake of CR for HF appears to be suboptimal (Dalal 2012; Tierney 2011). A key driver of this poor uptake has shown to be that CR programmes are not offering rehabilitation to people with HF due to lack of resources and exclusion of HF from local commissioning agreements (Dalal 2012).
How the intervention works
The precise mechanism(s) through which exercise training benefits people with HF remains unclear. One explanation, applicable to people with Ischaemic causes of HF, is that exercise training improves myocardial perfusion by alleviating endothelial dysfunction, therefore dilating coronary vessels and by stimulating new vessel formation by way of intermittent ischaemia (ExTraMatch 2004). Indeed, Belardinelli and colleagues have demonstrated that aerobic training improves myocardial contractility and diastolic filling (Belardinelli 1998). One meta‐analysis by Haykowsky et al. demonstrated the benefits of exercise training on cardiac remodelling as measured by ejection fraction, end‐diastolic volume and end‐systolic volume (Haykowsky 2007). Regardless of cause, there are important neurohormonal and musculoskeletal abnormalities in HF. Exercise training may reduce adrenergic tone and increase vagal tone, as suggested by an assessment of variability in heart rate. Skeletal muscle dysfunction and wasting may also respond to exercise training (ExTraMatch 2004). Hambrecht et al. have demonstrated that regular physical activity in people with HF stimulates vasodilation in the skeletal muscle vasculature (Hambrecht 1998).
Why it is important to do this review
This is an update of a Cochrane review published in 2010. The first Cochrane systematic review of exercise‐based interventions for HF in 2004 concluded that exercise training clearly improved short‐term (up to one‐year follow‐up) exercise capacity compared with no exercise control (Rees 2004; Smart 2004). However, only one of the 29 included randomised controlled trials (RCTs) was powered to report hospitalisations and mortality. Few trials assessed HRQoL. Accepting the evidence for improvement in short‐term exercise capacity, the updated 2010 Cochrane review focused on trials of follow‐up of six‐months or longer that reported clinical events (mortality, hospitalisation) or HRQoL (Davies 2010). The 2010 review of 19 RCTs (3647 participants) showed no difference between exercise and control in either short or long‐term all‐cause mortality, a reduction in HF‐related hospitalisations (RR 0.72; 95% CI 0.52 to 0.99) and improvement in patient‐reported HRQoL (standardised mean difference (SMD) 20.63; 95% CI 20.37 to 20.80) with exercise therapy. The majority of trials included in the 2010 review were in men at low‐to‐medium risk (NYHA class II to III). None of the trials included people with HFPEF and programmes delivered in a centre‐based setting.
Using additional RCT evidence, since the 2010 Cochrane review, the aim of this update was to reassess the effectiveness of exercise‐based rehabilitation on mortality, hospital admissions, morbidity and HRQoL of people with HF compared with no exercise training. In particular, we sought to identify additional evidence: 1. for those individuals poorly represented in previous reviews (i.e. older individuals, females and people with HFPEF), 2. for programmes specifically delivered in a home‐ or community‐based setting and 3. on costs and cost‐effectiveness.
Objectives
To determine the effectiveness of exercise‐based rehabilitation on the mortality, hospitalisation admissions, morbidity and health‐related quality of life for people with HF. Review inclusion criteria were extended to consider not only HF due to reduced ejection fraction (HFREF or 'systolic HF') but also HF due to preserved ejection fraction (HFPEF or 'diastolic HF').
Methods
Criteria for considering studies for this review
Types of studies
RCTs of either a parallel group or cross‐over design where the follow‐up was at least six months post‐randomisation.
Types of participants
Adults aged 18 years or older with HF.
We widened the inclusion criteria to include studies with individuals with HFPEF in addition to those with HFREF who were included in the previous versions of this review. We excluded studies that included participants who had previously received exercise rehabilitation.
Types of interventions
Exercise‐based interventions either alone or as a component of comprehensive CR (defined as programmes including components such as health education and psychological interventions in addition to exercise interventions). The control group must not have received exercise training but may have received active intervention (i.e. education, psychological intervention) or usual medical care alone.
Types of outcome measures
To be included the study must include one or more of the following outcomes.
Primary outcomes
Mortality and safety: all‐cause mortality, deaths due to HF and sudden death.
Hospital admission or re‐hospitalisation, and whether this was due to HF.
Secondary outcomes
HRQoL assessed by a validated outcome measure (e.g. 36‐item Short Form (SF‐36), Minnesota Living with Heart Failure (MLWHF) questionnaire), costs and cost‐effectiveness.
Search methods for identification of studies
Electronic searches
For the previous reviews (Davies 2010; Rees 2004), the review authors searched the Cochrane Controlled Trials Register (Issue 1, 2001; Issue 1, 2007), MEDLINE, EMBASE and CINAHL (1984 to January 2008) (see Appendix 1; Appendix 2). The search strategy developed in 2008 included broader terms as this search was part of review strategy that sought to identify evidence for CR that included an update of this review and exercise‐based rehabilitation for coronary heart disease (Heran 2011), and home‐ versus centre‐based CR (Taylor 2010).
This search was updated from the last version (2008) and included the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2013), MEDLINE (Ovid, January 2013, week 4 2013), MEDLINE In‐Process (Ovid, 5 February 2013), EMBASE (Ovid, January 2013, week 5), CINAHL (EBSCOhost, 5 February 2013) and PsycINFO (Ovid, January 2013, week 5). A small addition to the search strategy was made to reflect the more recent use of the terms 'HFPEF' and 'HFREF'.
We searched conference proceedings on Web of Science (2008 to January 2013) and trial registers (Clinicaltrials.gov; Controlled‐trials.com).
We limited searches to RCTs and applied filters to limit to humans and year 2008 onwards. We imposed no language or other limitations. We considered variations in terms used and the spelling of terms in different countries, so that studies were not missed by the search strategy. We designed the search strategies with reference to those of the previous systematic review (Davies 2010), and in accordance with the Cochrane Handbook of Reviews of Interventions (Higgins 2011) (see Appendix 3).
Searching other resources
We searched reference lists of all eligible trials and identified systematic reviews for additional studies.
Data collection and analysis
Selection of studies
Two review authors (VAS, RST) screened the references identified by the search strategy by title and abstract and discarded clearly irrelevant studies. For selection, abstracts had to clearly identify the study design, an appropriate population and relevant components of the intervention as described above. We obtained the full‐text reports of all potentially relevant trials and two review authors (VAS and RST) independently assessed them for eligibility based on the defined inclusion criteria. We resolved any disagreements by discussion. EJD, KR and RST undertook data study selection in previous review versions.
Data extraction and management
We extracted relevant data regarding inclusion criteria (study design; participants; interventions including type of exercise, frequency, duration, intensity and modality; comparisons and outcomes), risk of bias (randomisation, blinding, attrition and control) and results. One review author (VAS) extracted data and a second review author (RST) checked entries. We contacted study authors to seek clarification on issues of reporting or to obtain further outcome details. Excluded studies and reasons for their exclusion are detailed in the Characteristics of excluded studies table. EJD, KR and RST undertook data extraction in previous review versions.
Assessment of risk of bias in included studies
Factors considered included the quality of the random sequence generation and allocation concealment, incomplete outcome data, analysis by intention‐to‐treat, blinding (participants, personnel and outcome assessors) and selective outcome reporting (Higgins 2011). One review author (VAS) assessed the risk of bias in eligible trials and a second review author (RST) verified the decision. EJD, KR and RST undertook risk of bias in previous review versions.
Measures of treatment effect
We expressed dichotomous outcomes as RR and 95% CI for each study. For continuous variables, we compared net changes (i.e. exercise group minus control group to give differences) and calculated mean difference (MD) or SMD and 95% CI for each study. For each trial, we sought the mean change (and standard deviation (SD)) in outcome between baseline and follow‐up for both exercise and control groups and when not available, we instead used the absolute mean (and SD) outcome at follow‐up for both groups. For trials with more than one relevant intervention arm, we divided the number randomised in the control group by the number of intervention arms to obtain the denominator for data analysis. Where trials reported more than one HRQoL outcome, we included the first outcome reported in the paper in the meta‐analysis. We tabulated all reported HRQoL outcomes at all follow‐up times for each included study. We reported outcome results at two time points: 1. up to and including 12 months' follow‐up and 2. longer than 12 months' follow‐up. The latest follow‐up was used in each of these time point analyses.
Assessment of heterogeneity
We explored heterogeneity among included studies qualitatively (by comparing the characteristics of included studies) and quantitatively (using the Chi2 test of heterogeneity and the I2 statistic). Where appropriate, we combined the results from included studies for each outcome to give an overall estimate of treatment effect.
Assessment of reporting biases
We used funnels plots and Egger tests to assess potential small‐study effects and publication bias for those outcomes with an adequate number of trials (i.e. all‐cause mortality, hospital admissions and HRQoL) (Egger 1997).
Data synthesis
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used a fixed‐effect meta‐analysis except where we identified statistical heterogeneity (I2 statistic greater than 50%), where we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We explored the potential heterogeneity in exercise‐based rehabilitation by two approaches: 1. within‐trial subgroup analyses (supported by subgroup x intervention/control interaction terms) and 2. between‐trial analyses using meta‐regression. Meta‐regression was used to examine the association between the effect of exercise on all‐cause mortality, all hospitalisation and HRQoL (MLWHF or other measures) up to 12 months as these three outcomes contained the most trials. Specific study covariates included in the meta‐regression included: mean per cent left ventricular ejection fraction (LVEF); dose of aerobic exercise (calculated as the overall number of weeks of training multiplied by the mean number of sessions per week multiplied by the mean duration of sessions in minutes); type of exercise (aerobic training alone or aerobic plus resistance training); mean age; sex (per cent male); setting (hospital only, home only, both hospital and home); type of rehabilitation (exercise only versus comprehensive); overall risk of bias ('low', i.e. absence of bias in five or more of eight of risk of bias items; 'high', i.e. absence of bias in fewer than five of eight of risk of bias items); single versus multicentre; and publication date. We added year of publication as an additional study level factor (pre versus post 2000) in order to assess the potential effect of a change in the standard of usual care over time, that is to reflect when beta‐blockers, angiotensin‐receptor blockers and angiotensin‐converting enzyme inhibitors became established therapies for HF (Shekelle 2003). Given the relatively small ratio of trials to covariates, meta‐regression was limited to univariate analysis (Higgins 2011). The permute option in STATA was used to allow for multiple testing in meta‐regression.
Results
Description of studies
The 2004 and 2010 versions of this Cochrane review contributed eight (Rees 2004) and 19 trials (Davies 2010) to this latest update. Several trials from the 2004 review were excluded in the 2010 review as their follow‐up was less than six months or they reported only exercise capacity outcomes. This 2014 update identified a further 14 trials. The study selection process is summarised in the QUORUM flow diagram shown in Figure 1.
Figure 1.
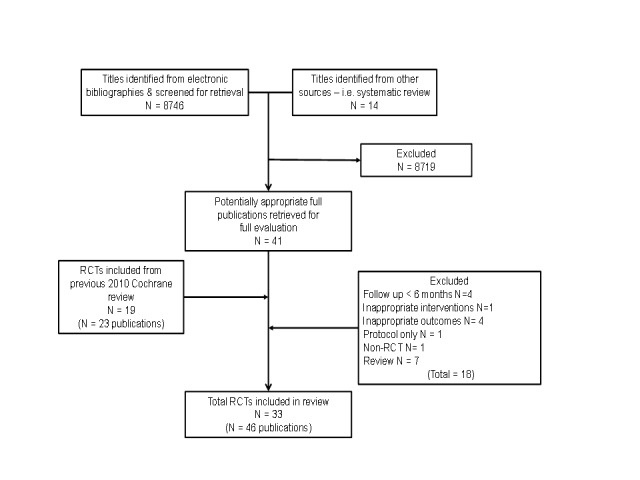
The included 33 trials randomised 4740 participants predominantly with HFREF and NYHA classes II and III. Four trials included a (undefined) proportion of people with HFPEF (Davidson 2010; Gary 2010 (comp); Gary 2010 (exalone); Nilsson 2008; Wall 2010). The majority of trials were small (26 trials had fewer than 100 participants) and single centre (30 trials), with one large trial contributing about 50% (2331 participants) of all included participants (HF ACTION 2009). The mean age of participants across the included studies ranged from 51 to 81 years. Studies recruited predominantly men (median 87%), although there was evidence that more females were recruited in recent trials. Only four trials reported on ethnicity and 62% to 100% of the study population was white. Eleven trials reported follow‐up in excess of 12 months (Austin 2005; Belardinelli 1999; Belardinelli 2012; Davidson 2010; Dracup 2007; HF ACTION 2009; McKelvie 2002; Mueller 2007; Myers 2000; Nilsson 2008; Wall 2010). Two trials had more than one exercise intervention arm. These two trials were treated as each contributing two separate comparative arms for the purpose of the meta‐analysis (Gary 2010 (comp); Gary 2010 (exalone); Klocek 2005 (Const); Klocek 2005 (Prog)).
All trials evaluated an aerobic intervention and 11 also included resistance training (Austin 2005; DANREHAB 2008; Dracup 2007; Jolly 2009; Jónsdóttir 2006a; Koukouvou 2004; McKelvie 2002; Norman 2012; Pozehl 2008; Witham 2005; Witham 2012). Exercise training was most commonly delivered in either an exclusively centre‐based setting or a centre‐based setting in combination with some home exercise sessions. Five studies were conducted in an exclusively home‐based setting (Dracup 2007; Gary 2010 (comp); Gary 2010 (exalone); Jolly 2009; Passino 2006; Wall 2010). The dose of exercise training ranged widely across studies with session duration of 15 to 120 minutes, one to seven sessions/week, intensity of 40% to 80% of maximal heart rate to 50% to 85% of maximal oxygen uptake (VO2 max) to Borg rating of 12 to 18, over a period of 15 to 120 weeks. In addition to exercise training,12 trials included other ('comprehensive rehabilitation') elements that included education and psychological interventions (Bocalini 2008; DANREHAB 2008; Davidson 2010; Gary 2010 (exalone); Jolly 2009; Jónsdóttir 2006a; Mueller 2007; Myers 2000; Nilsson 2008; Pozehl 2008; Witham 2012).
Details of the studies included in the review are shown in the Characteristics of included studies table. Reasons for exclusion are presented in the Characteristics of excluded studies table. The status of ongoing trials are detailed in the Characteristics of ongoing studies table.
Risk of bias in included studies
The overall risk of bias was moderate. A number of trials (particularly those published prior to 2000) failed to give sufficient detail to assess their potential risk of bias (Figure 2; Figure 3). Details of generation and concealment of random allocation sequence and blinding of outcomes were particularly poorly reported. Only the studies of Austin 2005; DANREHAB 2008; HF ACTION 2009; Jolly 2009; McKelvie 2002; and Witham 2012 provided an adequate description of the randomisation process. Nevertheless, none of the studies had objective evidence of imbalance in baseline characteristics. Most studies performed an intention‐to‐treat analysis, comparing exercise and control group outcomes according to the initial random allocation. Given the nature of an exercise intervention, is not possible to blind participants and carers. However, several studies reported blinding of outcome assessment (Davidson 2010; Gary 2010 (exalone); Gary 2010 (comp); HF ACTION 2009; McKelvie 2002, Koukouvou 2004; Nilsson 2008; Willenheimer 2001; Witham 2005; Yeh 2011). By not reporting co‐intervention details for both exercise and control groups, some studies may be prone to performance bias (Belardinelli 1999; Giannuzzi 2003; Gielen 2003; Hambrecht 1995; Hambrecht 2000; Keteyian 1996; Klecha 2007; Klocek 2005 (Prog); Klocek 2005 (Const); McKelvie 2002; Nilsson 2008; Pozehl 2008). There was evidence of improvement in reporting and lower risk of bias in more recent trials.
Figure 2.
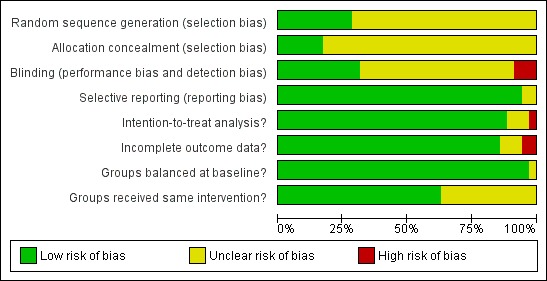
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 3.
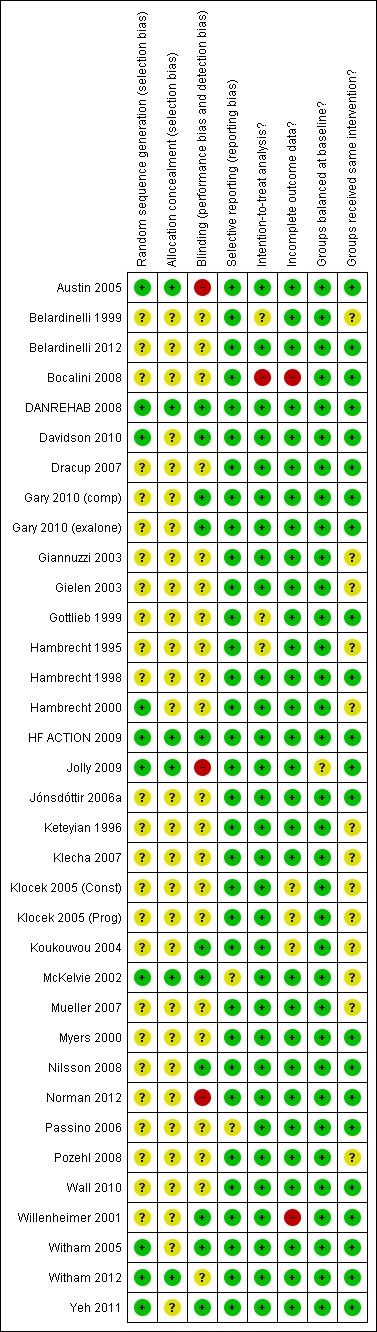
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Mortality
Twenty‐two studies reported all‐cause mortality at up to 12‐months' follow‐up. The trials of Gielen 2003 and Klecha 2007 reported no deaths in either the exercise or control arm. There was no significant difference in pooled mortality up to 12 months' follow‐up between groups (RR 0.93; 95% CI 0.69 to 1.27; P value = 0.59, I2 = 0%; Chi2 = 12.37, P value = 0.26, fixed‐effect analysis) (Analysis 1.1). The studies of Austin 2005; Belardinelli 1999;HF ACTION 2009;Jónsdóttir 2006a; and Mueller 2007 reported mortality at 60, 26, 30, 28, and 74 months, respectively. Although not reported in their original publication (Belardinelli 2012), we obtained mortality data at 10 years by contacting the study authors. There was a trend towards a reduction in all‐cause mortality when pooled across longest follow‐up point of the six trials with more 12 months' follow‐up (RR 0.88; 95% CI 0.75 to 1.02; P value = 0.07, I2 = 34%; Chi2 = 7.54, P value = 0.18, fixed‐effect analysis) (Analysis 1.2). Studies did not consistently report deaths due to HF or sudden death.
Analysis 1.1.
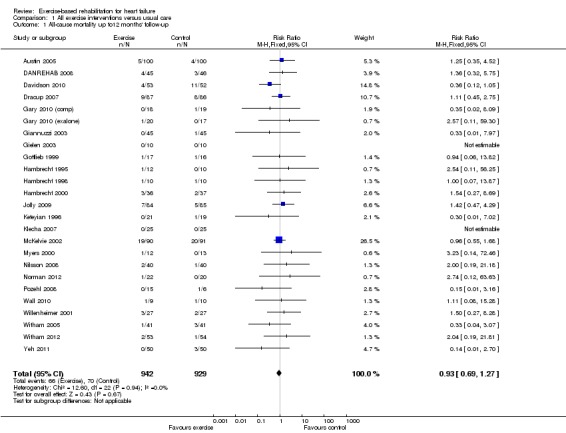
Comparison 1 All exercise interventions versus usual care, Outcome 1 All‐cause mortality up to12 months' follow‐up.
Analysis 1.2.
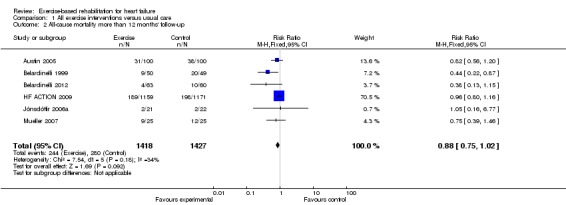
Comparison 1 All exercise interventions versus usual care, Outcome 2 All‐cause mortality more than 12 months' follow‐up.
Hospital admissions
There were reductions in the number of people experiencing hospital admissions with exercise compared with control up to 12 months' follow‐up, all hospital admissions up to 12 months' follow‐up (15 trials, RR 0.75; 95% CI 0.62 to 0.92; P value = 0.005, I2 = 0%; Chi2 = 11.71, P value = 0.55, fixed‐effect analysis) (Analysis 1.3) and HF‐specific admissions (12 trials, RR 0.61; 95% CI 0.46 to 0.80; P value = 0.002, I2 = 34%; Chi2 = 16.70, P value = 0.12) (Analysis 1.4). There was no difference in all hospital admissions in trials with more than 12 months' follow‐up (5 trials, RR 0.92; 95% CI 0.66 to 1.29; P value = 0.63, I2 = 63%; Chi2 = 10.90, P value = 0.03, random‐effects analysis) (Analysis 1.5)
Analysis 1.3.
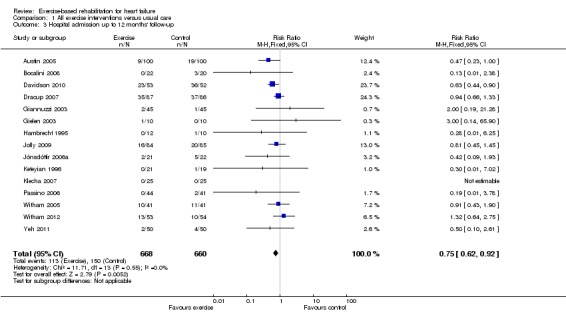
Comparison 1 All exercise interventions versus usual care, Outcome 3 Hospital admission up to 12 months' follow‐up.
Analysis 1.4.
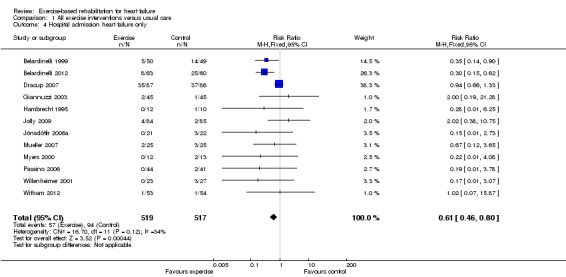
Comparison 1 All exercise interventions versus usual care, Outcome 4 Hospital admission heart failure only.
Analysis 1.5.
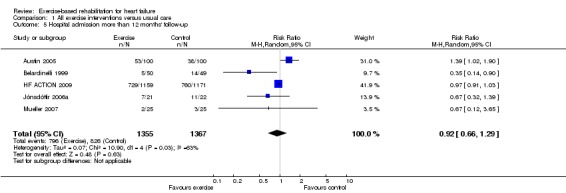
Comparison 1 All exercise interventions versus usual care, Outcome 5 Hospital admission more than 12 months' follow‐up.
Health‐related quality of life
Nineteen out of the 33 included trials (20 comparisons) reported a validated HRQoL measure (see Table 2). The majority of studies reported disease‐specific quality of life using the MLWHF, the HF ACTION 2009 trial using the Kansas City Cardiomyopathy Questionnaire (KCCQ). Generic HRQoL was also assessed using the EuroQoL (EQ‐5D), SF‐36, Psychological General Wellbeing index (PGWB), Patient's Global Assessment of Quality of Life (PGAQoL) and Spritzer's Quality of Life Index (QLI). The study by Gottlieb 1999 reported HRQoL values at follow‐up for the exercise group but not the controls. Eleven of the 19 trials (58%) reported superior HRQoL at follow‐up in people who exercised compared with controls and in no case was HRQoL score lower with exercise than control (see Table 2).
Table 1.
Health‐related quality of life results
| Trial first author (year) | Follow‐up | Measure |
Outcome values (or change from baseline) at follow‐up Mean (standard deviation) Control vs. exercise; between‐group P value |
Between‐group difference |
| Austin (2005/8) | 6 months 5 years |
MLWHF Physical Emotional Total EQ‐5D MLWHF Physical Emotional Total EQ‐5D |
20.4 (12.2) vs. 12.6 (9.7); P value < 0.0001* 8.0 (7.1) vs. 4.4 (10.4); P value < 0.01* 36.9 (24.0) vs. 22.9 (17.8); P value < 0.001* 0.58 (0.19) vs. 0.70 (0.16); P value < 0.0001* 19.3 (23.5) vs. 18.3 (11.2); P value = 0.66* 7.6 (7.1) vs. 7.4 (6.5); P value = 0.88* 37.1 (24.9) vs. 35.5 (21.7); P value = 0.72* 0.58 (0.22) vs. 0.64 (0.19); P value = 0.12* |
Exercise > Control Exercise > Control Exercise > Control Exercise > Control Exercise = Control Exercise = Control Exercise = Control Exercise = Control |
| Belardinelli (1999) | 15 months 29 months |
MLWHF total | 52 (20) vs. 39 (20); P value < 0.001 54 (22) vs. 44 (21); P value < 0.001 |
Exercise > Control Exercise > Control |
| DANREHAB (2008) | 12 months | SF‐36 PCS MCS |
37.4 (11.4) vs. 42.7 (9.1)*; P value = 0.14 50.5 (10.0) vs. 49.7 (8.8)*; P value = 0.81 |
Exercise = Control Exercise = Control |
| Davidson (2010) | 12 months | MLWHF total | 56.4 (18.3) vs. 52.9 (15.7); P value = 0.33 | Exercise = Control |
| Dracup (2007) | 6 months | MLWHF Physical Emotional Total |
19.4 (11.5) vs. 16.1 (10.0); P value = 0.04* 10.5 (7.4) vs. 7.8 (6.6); P value = 0.01* 43.2 (26.5) vs. 35.7 (23.7); P value = 0.05 |
Exercise > Control Exercise > Control Exercise > Control |
| Gary (2010) Comp | 6 months | MLWHF total | 34.3 (23.6) vs. 24.2 (16.3); P value = 0.18* | Exercise = Control |
| Gary (2010) Exer | 6 months | MLWHF total | 28.9 (29.9) vs. 25.6 (19.7); P value = 0.71* | Exercise = Control |
| Gottlieb (1999) | 6 months | MLWHF Total MOS PF RL GH |
NR (NR) vs. 22 (20) NR ‐ NR (NR) vs. 68 (28) NR NR (NR) vs. 50 (42) NR NR (NR) vs. 361 (224) NR |
NR ‐ NR NR NR |
| HF‐ACTION (2009) | 3 months | KCCQ+ | 5.21 (95% CI 4.42 to 6.00) vs. 3.28 (2.48 to 4.09); P value < 0.001 | Exercise > control |
| Jolly (2009) | 6 months 12 months |
MLWHF total EQ‐5D MLWHF total EQ‐5D |
34.5 (24.0) vs. 36.3 (24.1); P value = 0.30 0.62 (0.32) vs. 0.66 (0.24); P value = 0.004 34.9 (24.8) vs. 37.6 (21.0); P value = 0.80 0.69 (0.28) vs. 0.68 (0.21); P value = 0.07 |
Exercise = Control Exercise > Control Exercise = Control Exercise = Control |
| Jónsdóttir (2006) | 6 months | Icelandic Quality of Life Questionnaire | 4.10 (14.04) vs. 47.55 (8.7); P value = 0.34 | Exercise = Control |
| Klocek (2005) | 6.5 months | PGWB total | 99.0 vs. 109.0 (training group A) vs. 71.7 (training group B); P value < 0.01 | Exercise > Control |
| Koukouvou (2004) | 6 months | MLWHF total Spritzer QLI total |
34.1 (13.0) vs. 45.1 (9.9); P value = 0.05* 7.1 (1.1) vs. 9.1 (1.1); P value < 0.0001* |
Exercise > Control Exercise > Control |
| McKelvie (2002) | 12 months | MLWHF total+ | ‐3.3 (13.9) vs. ‐3.4 (18.1); P value = 0.98 | Exercise = Control |
| Nilsson (2008) | 12 months | MLWHF total | 28 (20) vs. 22 (12); P value = 0.003 | Exercise > Control |
| Norman (2012) | 6 months | KCCQ | 77.9 (11.6) vs. 81.0 (18.2); P value = 0.78 | Exercise = Control |
| Passino (2006) | 9.75 months | MLWHF total | 53 (32) vs. 32 (26.5); P value < 0.0001* | Exercise > Control |
| Willenheimer (2001) | 10 months | PGAQoL | 0 (1) vs. 0.7 (0.9); P value = 0.023 | Exercise > Control |
| Witham (2005) | 6 months | GCHFQ | 69 (13) vs. 65 (10); P value = 0.48 | Exercise = Control |
| Yeh (2011) | 12 months | MLWHF total | 18 (6) vs. 13 (4); P value < 0.0001 | Exercise > Control |
*P values calculated by authors of this paper; +: change in outcome from baseline.
GCHFQ: Guyatt chronic heart failure questionnaire; GH: General health; KCCQ: Kansas City Cardiomyopathy Questionnaire; MCS: mental component score; MLWHF: Minnesota Living with Heart Failure questionnaire; MOS: Medical Outcomes Study; NR: not reported; PCS: physical component score; PF: physical functioning; PGAQoL: Patient's Global Assessment of Quality of life; PGWB: Psychological General Wellbeing Index; QLI: Quality of Life Index; RL: role limitation; SF‐36: 36‐item Short Form.
Exercise = Control: no statistically significant difference (P value > 0.05) in HRQoL between exercise and control groups at follow‐up.
Exercise > Control: statistically significant (P value ≤ 0.05) higher HRQoL in exercise compared to control group at follow‐up.
Exercise < Control: statistically significant (P value ≤ 0.05) lower HRQoL in exercise versus control group at follow‐up.
There was evidence of high levels of statistical heterogeneity in the exercise‐control difference in MLWHF scores at follow‐up across studies. When pooled across the 13 studies that reported the total MLWHF score up to 12 months' follow‐up, there was a clinically important improvement with exercise (MD ‐5.8; 95% CI ‐9.2 to ‐2.4; P value = 0.0007, I2 = 70%; Chi2 = 40.24, P value < 0.0001, random‐effects analysis) (Analysis 1.6). Pooling across all studies, regardless of the HRQoL measure used, there was also evidence of a significant improvement with exercise (19 trials [21 comparisons], SMD ‐0.46; 95% CI ‐0.66 to ‐0.26; P value < 0.0001, I2 = 80%; Chi2 = 93.86, P value < 0.0001, random‐effects analysis) (Analysis 1.7). The three trials that reported MLWHF score at follow‐up greater than 12 months also showed greater improvement compared with control (MD ‐9.5; 95% CI ‐17.5 to ‐1.5; P value < 0.0001, I2 = 73%; Chi2 = 7.33, P value < 0.02, random‐effect analysis) (Analysis 1.8). Where studies reported more than one total HRQoL measure score, we selected the first cited score reported in the trial publication for meta‐analysis to prevent double counting of a study; the inference of the SMD meta‐analysis did not change when selecting the alternative HRQoL measure score.
Analysis 1.6.
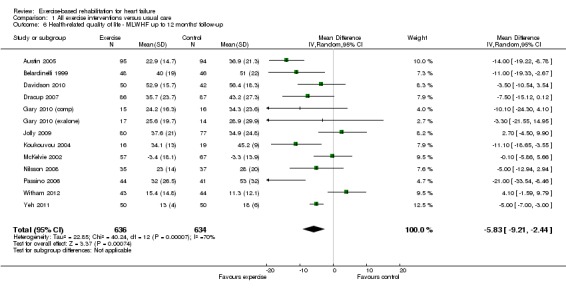
Comparison 1 All exercise interventions versus usual care, Outcome 6 Health‐related quality of life ‐ MLWHF up to 12 months' follow‐up.
Analysis 1.7.
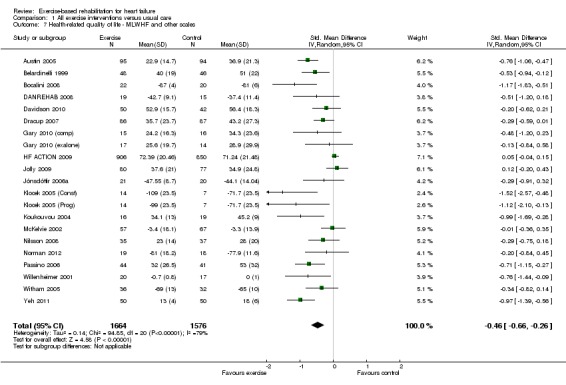
Comparison 1 All exercise interventions versus usual care, Outcome 7 Health‐related quality of life ‐ MLWHF and other scales.
Analysis 1.8.
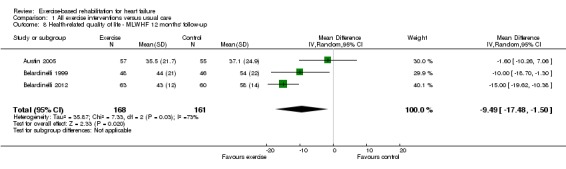
Comparison 1 All exercise interventions versus usual care, Outcome 8 Health‐related quality of life ‐ MLWHF 12 months' follow‐up.
Cost and cost‐effectiveness
Three studies reported economic data, two undertaking a cost‐effectiveness analysis (Flynn 2009; Georgiou 2001), and one reporting costs (Witham 2012) (see Table 3). Based on the Belardinelli trial (Belardinelli 1999), Georgiou and colleagues estimated an additional mean healthcare cost in the training group compared with controls of USD3227/person (Georgiou 2001). This cost was calculated by subtracting the averted hospitalisation cost, USD1336/person, from the cost of exercise training and wages lost due to exercise training, estimated at USD4563/person. Using exponential survival modelling to 15.5 years, the estimated increment in life expectancy with exercise was 1.82 years/person compared with people in the control group and an incremental cost‐effectiveness ratio of USD1773/life‐year saved. The HF ACTION group estimated a mean gain in QALY of 0.03 at an additional mean cost of USD1161/person at 2.5 years' follow‐up (Flynn 2009). Although an incremental cost‐effectiveness ratio was not reported, the authors stated that there was a 89.9% probability that exercise training was more cost‐effective than usual care at a maximum willingness to pay threshold of USD50,000. Witham and colleagues reported the mean cost in the exercise group were lower (‐GBP477.85/person) than the control group at six months' follow‐up (Witham 2012). This cost difference was primarily the result of a reduction in the days of hospital admission in the exercise group compared with the control group. None of the between‐group differences in costs or outcomes across these three studies achieved statistical significance at P value 0.05 or less level.
Table 2.
Costs and cost‐effectiveness
| Author (year) | Georgiou (2001) |
HF‐ACTION Reed (2010) |
Witham (2012) |
| Year of costs Country Currency |
1998 US USD |
2008 US USD |
2010 UK GBP |
| Intervention cost | |||
| Mean costs/participant | USD4563 | USD 6482 (SD 4884) | GBP474.75 |
| Costs considered | Staffing, space rental, equipment, participant's lost wages | Staffing, participant time, travel, parking | Staffing, equipment, staff and participant travel |
| Cost‐effectiveness | |||
| Follow‐up period | 15.5 years | Mean 2.5 years | 6 months |
| Total mean healthcare cost/participant (exercise) | USD5282* | USD57,338 (SD 81,343)+ | GBP1888.24 (SD 3111) |
| Total mean healthcare costs/participant (control) | USD2055* | USD56,177 (SD 92,749)+ | GBP1943.93 (SD 4551) |
| Incremental healthcare costs | 3227* | USD1161 (95% CI ‐6205 to 8404) | GBP‐447.85 (95% CI ‐1696.00 to 931.00) |
| Additional healthcare costs considered | Hospitalisations | Medication, procedures, outpatient visits, emergency visits, hospitalisations, tests | Inpatient and outpatient admissions, primary care contacts, medication |
| Mean healthcare benefit (exercise) | 10.24 life years | 2.02 QALYs (SD 1.00) | ‐ |
| Mean healthcare benefit (control) | 7.96 life years | 1.99 QALYS (SD 1.01) | ‐ |
| Incremental mean healthcare benefit | 1.82 life years* | 0.03 (95% CI ‐0.06 to 0.11) | ‐ |
| Incremental cost‐effectiveness ratio | USD1773 per life year saved | Not reported | ‐ |
CI: confidence interval; GBP: GB pounds; QALY: quality adjusted life year; SD: standard deviation; USD: US dollars.
Meta‐regression
Predictors of all‐cause mortality, hospitalisation and HRQoL intervention effects (12 months or less of follow‐up) were examined using univariate meta‐regression. No significant associations were seen on all‐cause mortality, all hospitalisation and HRQoL at the P less than 0.05 level with the exception of risk of bias and setting for HRQoL (see Table 4). The HRQoL mean effect size for studies with a higher risk of bias was larger than for studies with lower risk of bias (MLWHF MD: high risk: ‐14.4 vs. low risk ‐4.2, P value = 0.04): and higher for single‐centre studies (all HRQoL SMD: single centre: ‐0.90 vs. multicentre ‐0.35, P value = 0.04).
Table 3.
Univariate meta‐regression analysis
|
All‐cause mortality P value |
All hospitalisations P value |
MLWHF P value |
All HRQoL outcomes P value |
|
| Mean left ventricular ejection fraction (%) | 0.39 | 0.26 | 0.42 | 0.82 |
| Mean age (years) | 0.29 | 0.93 | 0.09 | 0.88 |
| Sex (% male) | 0.54 | 0.16 | ‐ | 0.69 |
| Type of rehabilitation (exercise only vs. comprehensive) | 0.76 | 0.77 | 0.23 | 0.28 |
| Type of exercise (aerobic training alone vs. aerobic plus resistance training) | 0.74 | 0.56 | 0.28 | 0.54 |
| Exercise dose (number of weeks x number of sessions/week x mean duration of session in hours) | 0.15 | 0.80 | 0.15 | 0.28 |
| Exercise setting I (hospital only, home only, both hospital and home) | 0.23 | 0.11 | 0.85 | 0.23 |
| Exercise setting II (single centre vs. multicentre) | 0.94 | 0.70 | 0.14 | 0.01 |
| Publication date | 0.54 | 0.54 | 0.46 | 0.60 |
| Risk of bias* | 0.40 | 0.57 | 0.04 | 0.08 |
*'low' risk of bias trial: absence of bias in > 5 out 8 of risk of bias items vs. 'high' risk of trial: absence of bias in < 5 out 8 items.
HRQoL: health‐related quality of life; MLWHF: Minnesota Living with Heart Failure questionnaire.
Within‐trial subgroup analyses
Several studies reported that they had undertaken subgroup analyses. However, most of these analyses were not based on a formal subgroup interaction test with the intervention effect but instead a cross‐sectional association between particular participant characteristics and outcome (e.g. association between participant age at baseline and mortality (regardless of exercise or control group allocation)) (Austin 2005; Belardinelli 1999; Belardinelli 2012; Davidson 2010; Klocek 2005 (Const); Klocek 2005 (Prog)). Two studies reported subgroup analyses where the methods were unclear (Pozehl 2008; Yeh 2011). Only the large HF ACTION trial undertook a pre‐defined formal interaction tests of differences in intervention effects between subgroups. The HF ACTION authors reported no evidence of difference in the intervention effects as assessed on either the primary outcome (all‐cause mortality or hospitalisation) or HRQoL (KCCQ overall score) across a number of participant‐defined subgroups (see Table 5). The HF ACTION group also undertook a large post hoc observational analysis in those people assigned to exercise training (Keteyian 2012). This analysis showed that the volume of exercise undertaken by participants was associated with the risk for clinical events and moderate levels (3 to 7 MET‐h per week) of exercise was needed to observe a clinical benefit.
Table 4.
Within trial subgroup analyses
| Author (year) | Outcome(s) | Subgroup(s) | Results (P value) | Data analysis methods |
|
HF ACTION (O'Connor, 2009) |
Composite primary end point of all‐cause mortality or hospitalisation, median follow‐up 30 months | Age (≤ 70 yr vs. > 70 yr), gender (males vs. females), race (white vs. non‐white), heart failure aetiology (ischaemic vs. non‐ischaemic), baseline LVEF (≤ 25% vs. > 25%), baseline NYHA (Class II vs. Class III/IV), previous revascularisation, history of MI, on ACE or beta‐blocker at baseline |
"there was no significant interaction of exercise training with any of the factors defining these subgroups" (P value > 0.05) |
Interaction test on hazard ratio |
|
HF ACTION (Flynn, 2009) |
KCCQ overall score up to 36 months | Age, LVEF (≤ 25% or > 25%), previous revascularisation (coronary artery bypass graft surgery or percutaneous coronary intervention, or no previous revascularisation), history of MI, and KCCQ overall summary score at baseline (0‐50, 50‐75 or 75‐100) | No significant subgroup interactions (P value > 0.05) | Interaction test |
|
HF ACTION Keteyian (2012) |
All‐cause mortality or hospitalisation and cardiovascular mortality or HF hospitalisation at median follow‐up 28.2 months |
Exercise volume defined as metabolic equivalent [MET]‐hr per week i.e. product of exercise intensity (where 1 MET is 3.5 mL VO2/ kg/min) and the hours of exercise/week |
Exercise volume was logarithmic predictor (P value = 0.03) for all‐cause mortality or hospitalisation. For cardiovascular mortality or heart failure hospitalisation, exercise volume was a significant (P value < 0.001) linear and logarithmic predictor Moderate exercise volumes of 3‐5 MET‐hr and 5‐7 MET‐hr/week were associated with reductions in subsequent risk that exceeded 30% |
Regression‐based methods (based only on exercise group data) |
ACE: angiotensin‐converting enzyme; hr: hour; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEF: left ventricular ejection fraction; MET: metabolic equivalent; MI: myocardial infarction; NYHA: New York Heart Association; VO2: oxygen consumption; yr: year.
Small‐study bias
There was no evidence of funnel plot asymmetry for all‐cause mortality (Egger test P value = 0.805) (Figure 4) or WLWHF (Egger test P value = 0.606) (Figure 5). The funnel plots for SMD HRQoL showed evidence of asymmetry (Egger test P value < 0.0001) (Figure 6).
Figure 4.
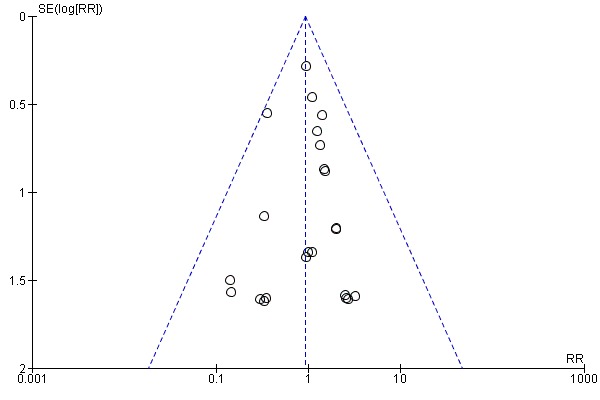
Funnel plot of comparison: 1 All exercise interventions versus usual care, outcome: 1.1 All‐cause mortality up to 12 months' follow‐up.
Figure 5.
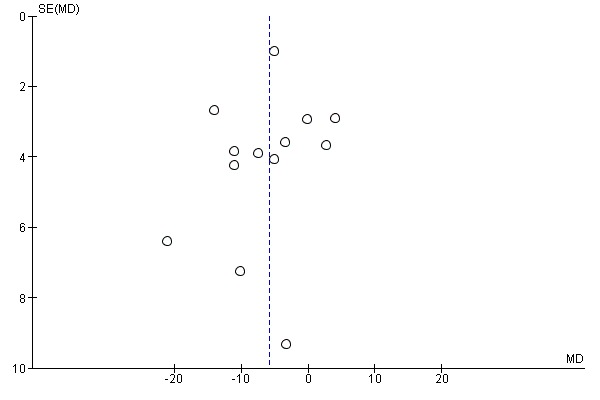
Funnel plot of comparison: 1 All exercise interventions versus usual care, outcome: 1.6 Health‐related quality of life ‐ Minnesota Living with Heart Failure (MLWHF) questionnaire up to 12 months' follow‐up.
Figure 6.
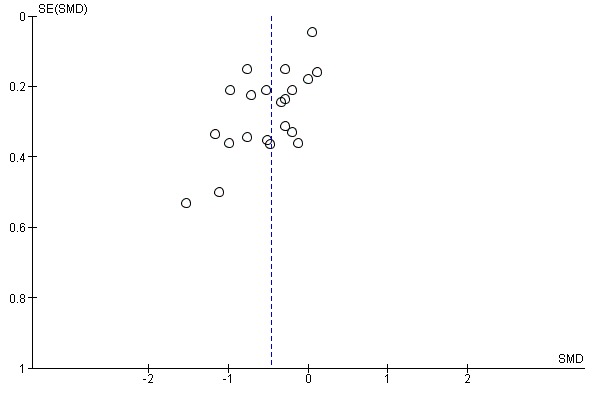
Funnel plot of comparison: 1 All exercise interventions versus usual care, outcome: 1.7 Health‐related quality of life ‐ Minnesota Living with Heart Failure (MLWHF) questionnaire and other scales.
Discussion
Summary of main results
This update review shows that, when compared with no exercise control, exercise‐based rehabilitation did not significantly impact on short‐term (up to 12‐months' follow‐up) all‐cause mortality. There was trend towards a reduction in all‐cause mortality in trials with follow‐up in excess of 12 months. We also found a reduction in hospitalisations related due to HF and higher levels of HRQoL following exercise training programmes compared with no exercise control. It is important to note that there was significant heterogeneity in our observations on HRQoL. Univariate meta‐regression analysis shows that the benefits of exercise‐based rehabilitation to be independent of participant age, gender, degree of left ventricular dysfunction, type of CR (exercise only versus comprehensive), mean dose of exercise intervention, length of follow‐up, overall risk of bias and trial publication date. Whilst the majority of included participants in this review were HFREF and NYHA class II to III, more recent trials have recruited those who with HFPEF and NHYA IV and a greater proportion of females and older patients. Evidence from two trials support the cost‐effectiveness of exercise‐based rehabilitation.
Overall completeness and applicability of evidence
The generalisability of the previous version of this review was limited as most included studies recruited only low‐ to moderate‐risk younger men. However, with the inclusion of more women, older age and people with HFPEF in recent trials, the findings of this updated review have potential greater external validity.
Quality of the evidence
The general lack of reporting of methods in the included RCT reports made it difficult to assess their methodological quality and thereby judge their risk of bias. There was evidence of large treatment effect for HRQoL outcomes in studies judged to be overall higher risk of bias compared with lower risk of bias studies, suggesting that risk of bias may be a major driver of the substantive statistical heterogeneity seen across trials in this outcome. There appeared to be improvement in the quality of reporting in more recent trials.
Potential biases in the review process
We believe this is the most comprehensive systematic review to date of RCT‐based evidence for the impact of exercise‐based rehabilitation for people with HF. However, our review has some limitations. Funnel plot asymmetry for HRQoL is indicative of small‐study bias and possible publication bias. Although a specific goal of this updated review was to clarify the impact of exercise training programmes on clinical events, many included trials were relatively small and of short‐term follow‐up so that the number of deaths and hospitalisations reported by most trials was small. Indeed, in many studies, we located event data in the trial descriptions of losses to follow‐up and exclusions rather that as reported outcomes per se.
Agreements and disagreements with other studies or reviews
Based on an individual participant data pooled analysis, the ExTraMATCH Collaborative Group concluded that exercise training for HF significantly reduced overall mortality (hazard ratio 0.65; 95% CI 0.46 to 0.92) at mean follow‐up of approximately two years (ExTraMatch 2004). The ExTraMATCH study was based on a limited bibliographic literature search (MEDLINE plus handsearching of selected leading cardiac journals), was limited to trials that reported survival data, and included unpublished data. Therefore, it has been difficult to verify the data and the comprehensiveness of this meta‐analysis; in addition, several of the RCTs included in the Cochrane review were not included in the ExTraMATCH review. Re‐analysis of the ExTraMATCH data using formal meta‐analytic methods (taking account of outcome clustering at the trials level) has shown that the effect of exercise training was not statistically significant when compared with control (RR 0.88; 95% CI 0.70 to 1.10) (Gotzsche 2005).
The impact of exercise training on mortality in people with HF may depend on the length of follow‐up and age of studies. While we found no improvement (or worsening) in overall survival with exercise compared with control in trials with short‐term follow‐up, there was a trend towards an improved survival with exercise in trials with follow‐up beyond 12 months. More recent trials included in this review have been conducted in the era of optimal medical therapy. For example, at entry to the HF‐ACTION trial, 94% of participants were receiving beta‐blockers and angiotensin‐receptor blockers or angiotensin‐converting enzyme inhibitors (Whellan 2007). Forty‐five per cent had an implantable cardioverter defibrillator or implanted biventricular pacemaker at the time of enrolment. Given the proven survival advantage of these medical treatments (Shekelle 2003), any incremental all‐cause mortality benefit with exercise is likely to be small.
This update review found the exercise group scored on average 5.8 points higher than the control group at up 12 months' follow‐up on the MLWHF questionnaire. A difference of four points or larger on the MLWHF questionnaire has been shown to represent a clinically important, meaningful difference for patients (McAlister 2004). The improvements in HRQoL seen with exercise training are in accordance with the previous systematic review of van Tol and colleagues (van Tol 2006), but not with that of Chien, which focused on home‐based exercise training and concluded that exercise training compared with usual care or activity did not improve the HRQoL of people with HF (Chien 2008). Five studies included in this update review were conducted in an exclusively home‐based setting (Dracup 2007; Gary 2010 (comp); Gary 2010 (exalone); Jolly 2009; Passino 2006; Wall 2010). Our meta‐regression analysis showed no difference in the reduction in hospitalisations and improvement in HRQoL with exercise training between those studies based in a hospital versus home based setting.
Authors' conclusions
This review shows that exercise rehabilitation provides important benefits by improving health‐related quality of life and reducing heart failure (HF)‐related hospitalisation in people predominantly with reduced left ventricular ejection fraction (HFREF or 'systolic HF') ranging from New York Heart Association (NYHA) class I to IV. We found no evidence to support that exercise training programmes increase (or decrease) the risk of death in the short term but there was trend towards reduced mortality in trials with follow‐up beyond 12 months. The benefits of exercise training programmes appears to be independent of participant characteristics (e.g. age, gender, degree of left ventricular dysfunction) and the characteristics and setting of the exercise programmes. Programmes are typically based on aerobic exercise training with or without a resistance exercise element. Despite clinical guidelines stating their support of exercise‐based rehabilitation in the management of HF, the provision and uptake of rehabilitation in HF remains poor. Future robust evidence of the economic value (costs and cost‐effectiveness) of cardiac rehabilitation is likely to be important to encourage hospital and primary care providers to extend the current provision of exercise‐based programmes for HF
The majority of trials in this review have investigated exercise training as a single intervention and against a no exercise control. However, in practice, exercise‐based rehabilitation is often an adjunct to other HF management interventions, such as specialist HF nurse support or disease management programmes. While trials have demonstrated the benefits of such HF management interventions alone, few trials have compared such interventions with and without adding a structured exercise training programme (Jolly 2009; Mudge 2011). This is an important clinical question for the future design of HF services, because the addition of an exercise programme adds considerably to staffing and equipment costs. Future clinical trials of exercise rehabilitation in HF also need to consider: the generalisability of trial populations (women, older people and people with HFPEF remain under‐represented in trial populations); interventions to enhance the long‐term maintenance of exercise training; and outcomes, costs and cost‐effectiveness of exercise‐based programmes delivered exclusively in a home‐based setting.
Acknowledgements
We thank for the following authors who provided additional outcome data included in this update review:
Prof Romualdo Belardinelli, Ancona, Italy (Belardinelli 2012) ‐ mortality data.
Dr Kathryn Flynn, Department of Medicine Center for Patient Care & Outcomes Research Medical College of Wisconsin, USA (HF ACTION 2009) ‐ health‐related quality of life data.
Dr Miles Witham, University of Dundee, UK (Witham 2012) ‐ health‐related quality of life data.
Dr Ann‐Dorte Zwisler, Project Manager CopenHeart, Copenhagen, Denmark (DANREHAB 2008) ‐ mortality and health‐related quality of life data for people with HF.
Appendices
Appendix 1. Search strategy 2001
Cochrane Controlled Trials Register (2001, Issue 2)
1. HEART‐FAILURE‐CONGESTIVE*:ME 2. (HEART and FAILURE) 3. (CARDIAC and FAILURE) 4. ((#1 or #2) or #3) 5. REHABILITATION*:ME 6. EXERCISE*:ME 7. EXERCISE‐THERAPY*:ME 8. SPORTS*:ME 9. PHYSICAL‐EDUCATION‐AND‐TRAINING*:ME 10. EXERTION*:ME 11. REHABILITAT* 12. (PHYSICAL* near FIT) 13. (PHYSICAL* near FITNESS) 14. (PHYSICAL near TRAIN*) 15. (PHYSICAL* near ACTIVIT*) 16. (TRAIN* near STRENGTH*) 17. (TRAIN* near AEROBIC*) 18. (AEROBIC* near EXERCISE*) 19. KINESIOTHERAP* 20. (EXERCISE* near TRAIN*) 21. (((((((((((((((#5 or #6) or #7) or #8) or #9) or #10) or #11) or #12) or #13) or #14) or #15) or #16) or #17) or #18) or #19) or #20) 22. (#4 and #21)
Appendix 2. Search strategies 2008
CENTRAL on The Cochrane Library 2007, Issue 4
#1MeSH descriptor Myocardial Ischemia explode all trees #2(myocard* NEAR isch*mi*) #3isch*mi* NEAR heart #4MeSH descriptor Coronary Artery Bypass explode all trees #5coronary #6MeSH descriptor Coronary Disease explode all trees #7MeSH descriptor Myocardial Revascularization explode all trees #8MeSH descriptor Myocardial Infarction explode all trees #9myocard* NEAR infarct* #10heart NEAR infarct* #11MeSH descriptor Angina Pectoris explode all trees #12angina #13MeSH descriptor Heart Failure, Congestive explode all trees #14heart and (failure or attack) #15MeSH descriptor Heart Diseases explode all trees #16heart and disease* #17myocard* #18cardiac* #19CABG #20PTCA #21stent* AND (heart or cardiac*) #22MeSH descriptor Heart Bypass, Left explode all trees #23MeSH descriptor Heart Bypass, Right explode all trees #24(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25MeSH descriptor Rehabilitation Centers, this term only #26MeSH descriptor Exercise Therapy explode all trees #27MeSH descriptor Sports, this term only #28MeSH descriptor Exertion explode all trees #29rehabilitat* #30(physical* NEAR (fit* or train* or therap* or activit*)) #31MeSH descriptor Exercise explode all trees #32(train*) near (strength* or aerobic or exercise*) #33((exercise* or fitness) NEAR/3 (treatment or intervent* or program*)) #34MeSH descriptor Rehabilitation explode all trees #35MeSH descriptor Patient Education explode all trees #36(patient* NEAR/3 educat*) #37((lifestyle or life‐style) NEAR/3 (intervent* or program* or treatment*)) #38MeSH descriptor Self Care explode all trees #39MeSH descriptor Ambulatory Care explode all trees #40MeSH descriptor Psychotherapy explode all trees #41psychotherap* #42psycholog* NEAR intervent* #43relax* #44MeSH descriptor Mind‐Body and Relaxation Techniques explode all trees #45MeSH descriptor Counseling explode all trees #46counsel*ing #47MeSH descriptor Cognitive Therapy explode all trees #48MeSH descriptor Behavior Therapy explode all trees #49(behavio*r*) NEAR/4 (modif* or therap* or rehab* or change) #50MeSH descriptor Stress, Psychological explode all trees #51stress NEAR manage* #52cognitive* NEAR therap* #53MeSH descriptor Meditation explode all trees #54meditat* #55MeSH descriptor Anxiety, this term only #56(manage*) NEAR (anxiety or depres*) #57CBT #58hypnotherap* #59goal NEAR/3 setting #60(psycho‐educat*) or (psychoeducat*) #61motivat* NEAR interv* #62MeSH descriptor Psychopathology explode all trees #63psychopathol* #64MeSH descriptor Autogenic Training explode all trees #65autogenic* #66self near (manage* or care or motivat*) #67distress* #68psychosocial* or psycho‐social #69MeSH descriptor Health Education explode all trees #70(nutrition or diet or health) NEAR education #71heart manual #72(#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37) #73(#38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71) #74(#72 OR #73) #75(#74 AND #24)
MEDLINE DIALOG to WEEK 1 2008
1. SEARCH: MYOCARDIAL‐ISCHEMIA#.DE. 2. SEARCH: MYOCARD$4 NEAR (ISCHAEMI$2 OR ISCHEMI$2) 3. SEARCH: (ISCHAEMI$2 OR ISCHEMI$2) NEAR HEART 4. SEARCH: CORONARY‐ARTERY‐BYPASS#.DE. 5. SEARCH: CORONARY.TI,AB. 6. SEARCH: CORONARY‐DISEASE#.DE. 7. SEARCH: MYOCARDIAL‐REVASCULARIZATION#.DE. 8. SEARCH: MYOCARDIAL‐INFARCTION#.DE. 9. SEARCH: MYOCARD$5 NEAR INFARCT$5 10. SEARCH: HEART NEAR INFARCT$5 11. SEARCH: ANGINA‐PECTORIS#.DE. 12. SEARCH: ANGINA.TI,AB. 13. SEARCH: HEART‐FAILURE‐CONGESTIVE#.DE. 14. SEARCH: HEART NEAR FAILURE 15. SEARCH: 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 16. SEARCH: HEART‐DISEASES#.DE. 17. SEARCH: (HEART NEAR DISEASE$2).TI,AB. 18. SEARCH: MYOCARD$5.TI,AB. 19. SEARCH: CARDIAC$2.TI,AB. 20. SEARCH: CABG 21. SEARCH: PTCA 22. SEARCH: STENT$4 AND (HEART OR CARDIAC$4) 23. SEARCH: HEART‐BYPASS‐LEFT#.DE. OR HEART‐BYPASS‐RIGHT#.DE. 24. SEARCH: 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 25. SEARCH: REHABILITATION‐CENTERS.DE. 26. SEARCH: EXERCISE‐THERAPY#.DE. 27. SEARCH: REHABILITATION.W..DE. 28. SEARCH: SPORTS#.W..DE. 29. SEARCH: EXERTION#.W..DE. 30. SEARCH: EXERCISE#.W..DE. 31. SEARCH: REHABILITAT$5.TI,AB. 32. SEARCH: PHYSICAL$4 NEAR (FIT OR FITNESS OR TRAIN$5 OR THERAP$5 OR ACTIVIT$5) 33. SEARCH: TRAIN$5 NEAR (STRENGTH$3 OR AEROBIC OR EXERCIS$4) 34. SEARCH: (EXERCISE$4 OR FITNESS) NEAR (TREATMENT OR INTERVENT$4 OR PROGRAM$2 OR THERAPY) 35. SEARCH: PATIENT‐EDUCATION#.DE. 36. SEARCH: PATIENT$2 NEAR EDUCAT$4 37. SEARCH: (LIFESTYLE OR LIFE‐STYLE) NEAR (INTERVENT$5 OR PROGRAM$2 OR TREATMENT$2) 38. SEARCH: SELF‐CARE.DE. 39. SEARCH: SELF NEAR (MANAGE$5 OR CARE OR MOTIVAT$5) 40. SEARCH: AMBULATORY‐CARE.DE. 41. SEARCH: PSYCHOTHERAPY#.W..DE. 42. SEARCH: PSYCHOTHERAP$2.TI,AB. 43. SEARCH: PSYCHOLOG$5 NEAR INTERVENT$5 44. SEARCH: RELAX$6.TI,AB. 45. SEARCH: RELAXATION‐TECHNIQUES#.DE. OR MIND‐BODY‐AND‐RELAXATION‐TECHNIQUES#.DE. 46. SEARCH: COUNSELING#.W..DE. 47. SEARCH: (COUNSELLING OR COUNSELING).TI,AB. 48. SEARCH: COGNITIVE‐THERAPY#.DE. 49. SEARCH: BEHAVIOR‐THERAPY#.DE. 50. SEARCH: (BEHAVIOR$4 OR BEHAVIOUR$4) NEAR (MODIFY OR MODIFICAT$4 OR THERAP$2 OR CHANGE) 51. SEARCH: STRESS‐PSYCHOLOGICAL#.DE. 52. SEARCH: STRESS NEAR MANAGEMENT 53. SEARCH: COGNITIVE NEAR THERAP$2 54. SEARCH: MEDITAT$4 55. SEARCH: MEDITATION#.W..DE. 56. SEARCH: ANXIETY#.W..DE. 57. SEARCH: MANAGE$5 NEAR (ANXIETY OR DEPRES$5) 58. SEARCH: CBT.TI,AB. 59. SEARCH: HYPNOTHERAP$5 60. SEARCH: GOAL NEAR SETTING 61. SEARCH: GOAL$2 NEAR SETTING 62. SEARCH: PSYCHO‐EDUCAT$5 OR PSYCHOEDUCAT$5 63. SEARCH: MOTIVAT$5 NEAR (INTERVENTION OR INTERV$3) 64. SEARCH: PSYCHOPATHOLOGY#.W..DE. 65. SEARCH: PSYCHOPATHOL$4.TI,AB. 66. SEARCH: PSYCHOSOCIAL$4.TI,AB. 67. SEARCH: DISTRESS$4.TI,AB. 68. SEARCH: HEALTH‐EDUCATION#.DE. 69. SEARCH: HEALTH NEAR EDUCATION 70. SEARCH: HEART ADJ MANUAL 71. SEARCH: AUTOGENIC‐TRAINING#.DE. 72. SEARCH: AUTOGENIC$5.TI.AB. 73. SEARCH: 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 74. SEARCH: 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72 75. SEARCH: 15 OR 24 76. SEARCH: 73 or 74 77. SEARCH: 75 AND 76 78. SEARCH: RANDOMIZED‐CONTROLLED‐TRIALS#.DE. 79. SEARCH: PT=RANDOMIZED‐CONTROLLED‐TRIAL 80. SEARCH: PT=CONTROLLED‐CLINICAL‐TRIAL 81. SEARCH: CONTROLLED‐CLINICAL‐TRIALS#.DE. 82. SEARCH: RANDOM‐ALLOCATION#.DE. 83. SEARCH: DOUBLE‐BLIND‐METHOD#.DE. 84. SEARCH: SINGLE‐BLIND‐METHOD#.DE. 85. SEARCH: (RANDOM$ OR PLACEBO$).TI,AB. 86. SEARCH: ((SINGL$3 OR DOUBL$3 OR TRIPL$3 OR TREBL$3) NEAR (BLIND$3 OR MASK$3)).TI,AB. 87. SEARCH: RESEARCH‐DESIGN#.DE. 88. SEARCH: PT=CLINICAL‐TRIAL# 89. SEARCH: CLINICAL‐TRIALS#.DE. 90. SEARCH: (CLINIC$3 ADJ TRIAL$2).TI,AB. 91. SEARCH: 77 AND 90 92. SEARCH: (ANIMALS NOT HUMANS).SH. 93. SEARCH: 91 NOT 92 94. SEARCH: LIMIT 93 TO 2001‐DATE
EMBASE DIALOG to WEEK 1 2008
1. HEART‐DISEASE#.DE. 2. (MYOCARD$4 NEAR (ISCHAEMI$2 OR ISCHEMI$2)).TI,AB. 3. ((ISCHAEMI$2 OR ISCHEMI$2) NEAR HEART).TI,AB. 4. CORONARY‐ARTERY‐DISEASE#.DE. 5. TRANSLUMINAL‐CORONARY‐ANGIOPLASTY#.DE. 6. (CORONARY NEAR (DISEASE$2 OR BYPASS$2 OR THROMBO$5 OR ANGIOPLAST$2)).TI,AB. 7. HEART‐INFARCTION#.DE. 8. (MYOCARD$4 NEAR INFARCT$5).TI,AB. 9. (HEART NEAR INFARC$5).TI,AB. 10. HEART‐MUSCLE‐REVASCULARIZATION#.DE. 11. ANGINA‐PECTORIS#.DE. 12. ANGINA.TI,AB. 13. CONGESTIVE‐HEART‐FAILURE#.DE. 14. (HEART NEAR FAILURE).TI,AB. 15. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 16. (HEART NEAR DISEASE$2).TI,AB. 17. CARDIAC$2.TI,AB. 18. CABG.TI,AB. 19. PTCA.TI,AB. 20. STENT$4.TI,AB. AND HEART.TI,AB. 21. EXTRACORPOREAL‐CIRCULATION#.DE. 22. 16 OR 17 OR 18 OR 19 OR 20 OR 21 23. 15 OR 22 24. PSYCHOTHERAPY#.W..DE. 25. PSYCHOTHERAP$2.TI,AB. 26. PSYCHOLOG$5 NEAR INTERVENT$5 27. RELAX$6.TI,AB. 28. RELAXATION‐TRAINING#.DE. 29. COUNSELING#.W..DE. 30. (COUNSELLING OR COUNSELING).TI,AB. 31. (BEHAVIOR$4 OR BEHAVIOUR$4) NEAR (MODIFY OR MODIFICAT$4 OR THERAPY$2 OR CHANGE) 32. STRESS‐MANAGEMENT#.DE. 33. STRESS NEAR MANAGEMENT 34. MEDITATION#.W..DE. 35. MEDITAT$5.TI,AB. 36. MANAGE$5 NEAR (ANXIETY OR DEPRES$5) 37. CBT.TI,AB. 38. HYPNOTHERAP$2.TI,AB. 39. GOAL$2 NEAR SETTING 40. PSYCHO‐EDUCAT$5 OR PSYCHOEDUCAT$5 41. MOTIVAT$5 NEAR INTERVENT$6 42. PSYCHOSOCIAL‐CARE#.DE. OR PSYCHOSOCIAL‐REHABILITATION#.DE. 43. PSYCHOSOCIAL.TI,AB. 44. HEALTH‐EDUCATION#.DE. 45. HEALTH NEAR EDUCATION 46. HEART ADJ MANUAL 47. AUTOGENIC‐TRAINING#.DE. 48. AUTOGENIC.TI,AB. 49. REHABILITATION#.W..DE. 50. REHABILITATION‐CENTER#.DE. 51. REHABIL$.TI,AB. 52. SPORT#.W..DE. 53. KINESIOTHERAPY#.W..DE. 54. EXERCISE#.W..DE. 55. PHYSIOTHERAPY#.W..DE. 56. PHYSICAL$4 NEAR (FIT OR FITNESS OR TRAIN$5 OR THERAP$5 OR ACTIVIT$5) 57. TRAIN$5 NEAR (STRENGTH$3 OR AEROBIC OR EXERCIS$4) 58. (EXERCISE$4 OR FITNESS) NEAR (TREATMENT OR INTERVENT$4 OR PROGRAM$2 OR THERAPY) 59. AEROBIC$4 NEAR EXERCISE$4 60. (KINESIOTHERAPY OR PHYSIOTHERAPY).TI,AB. 61. PATIENT‐EDUCATION#.DE. 62. PATIENT$2 NEAR EDUCAT$4 63. (LIFESTYLE OR LIFE ADJ STYLE OR LIFE‐STYLE) NEAR (INTERVENT$5 OR PROGRAM$2 OR TREATMENT$2) 64. SELF‐CARE#.DE. 65. SELF NEAR (MANAGE$5 OR CARE OR MOTIVAT$5) 66. AMBULATORY‐CARE#.DE. 67. PSYCHO‐EDUCAT$5 OR PSYCHOEDUCAT$5 68. MOTIVAT$5 NEAR INTERVENT$6 69. PSYCHOSOCIAL‐CARE#.DE. OR PSYCHOSOCIAL‐REHABILITATION#.DE. 70. PSYCHOSOCIAL.TI,AB. 71. HEALTH‐EDUCATION#.DE. 72. HEALTH NEAR EDUCATION 73. HEART ADJ MANUAL 74. AUTOGENIC‐TRAINING#.DE. 75. AUTOGENIC.TI,AB. 76. PSYCHO‐EDUCAT$5 OR PSYCHOEDUCAT$5 77. MOTIVAT$5 NEAR INTERVENT$6 78. PSYCHOSOCIAL‐CARE#.DE. OR PSYCHOSOCIAL‐REHABILITATION#.DE. 79. PSYCHOSOCIAL.TI,AB. 80. HEALTH‐EDUCATION#.DE. 81. HEALTH NEAR EDUCATION 82. HEART ADJ MANUAL 83. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 84 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72 OR 73 OR 74 OR 75 OR 76 OR 77 OR 78 OR 79 OR 80 OR 81 OR 82 85. 83 OR 84 86. (RANDOM$ OR PLACEBO$).TI,AB. 87. (SINGL$4 OR DOUBLE$4 OR TRIPLE$4 OR TREBLE$4).TI,AB. AND (BLIND$4 OR MASK$4).TI,AB. 88. (CONTROLLED ADJ CLINICAL ADJ TRIAL).TI,AB. 89. RANDOMIZED‐CONTROLLED‐TRIAL#.DE. 90. 1 OR 2 OR 3 OR 4 91. 23 AND 85 92. 91 AND 92 93. LIMIT 92 TO 2001‐2008
CINAHL DIALOG to WEEK 1 2008
1. ((MYOCARD$4 OR HEART) NEAR (ISCHAEMI$2 OR ISCHEMI$2)).TI,AB. 2. CORONARY.TI,AB. 3. ((MYOCARD$4 OR HEART) NEAR INFARC$5).TI,AB. 4. ANGINA.TI,AB. 5. (HEART NEAR FAILURE).TI,AB. 6. (HEART NEAR DISEAS$2).TI,AB. 7. CARDIAC$2.TI,AB. 8. CABG 9. PTCA 10. STENT$4.TI,AB. AND (HEART OR CARDIAC$4).TI,AB. 11. MYOCARDIAL‐ISCHEMIA#.DE. 12. MYOCARDIAL‐INFARCTION#.DE. 13. CORONARY‐ARTERY‐BYPASS#.DE. 14. CORONARY‐DISEASE#.DE. 15. CARDIAC‐PATIENTS#.DE. 16. MYOCARDIAL‐DISEASES#.DE. 17. MYOCARDIAL‐REVASCULARIZATION#.DE. 18. HEART‐DISEASES#.DE. 19. CARDIOVASCULAR‐DISEASES#.DE. 20. HEART‐FAILURE‐CONGESTIVE#.DE. 21. ANGINA‐PECTORIS#.DE. 22. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 23. REHABILITATION#.W..DE. 24. SPORTS#.W..DE. 25. EXERCISE#.W..DE. 26. PHYSICAL‐ACTIVITY#.DE. 27. MUSCLE‐STRENGTHENING#.DE. 28. AEROBIC‐EXERCISES#.DE. 29. PHYSICAL‐FITNESS#.DE. 30. PATIENT‐EDUCATION#.DE. 31. THERAPEUTIC‐EXERCISE#.DE. 32. REHABILITAT$5.TI,AB. 33. (PHYSICAL$4 NEAR (FIT OR FITNESS OR TRAIN$4 OR THERAP$5 OR ACTIVIT$4)).TI,AB. 34. (TRAIN$4 NEAR (STRENGTH$3 OR AEROBIC OR EXERCIS$4)).TI,AB. 35. ((EXERCISE$4 OR FITNESS) NEAR (TREATMENT OR INTERVENT$4 OR PROGRAM$2 OR THERAPY)).TI,AB. 36. (PATIENT$2 NEAR EDUCAT$4).TI,AB. 37. ((LIFESTYLE OR LIFE‐STYLE) NEAR (INTERVENT$5 OR PROGRAM$2 OR TREATMENT$2)).TI,AB. 38. SELF‐CARE#.DE. 39. (SELF NEAR (MANAGE$5 OR CARE OR MOTIVAT$5)).TI,AB. 40. AMBULATORY‐CARE#.DE. 41 AEROBIC.TI,AB. 42. RESISTANCE ADJ TRAIN$4 43. MUSCLE ADJ STRENGTH$5 44. AEROBIC.TI,AB. 45. RESISTANCE ADJ TRAIN$4 46. MUSCLE ADJ STRENGTH$5 47. PSYCHOTHERAPY#.W..DE. 48. PSYCHOTHERAP$2.TI,AB. 49. (PSYCHOLOG$5 NEAR INTERVENT$5).TI,AB. 50. RELAX.TI,AB. 51. RELAXATION‐TECHNIQUES#.DE. 52. (COUNSELLING OR COUNSELING).TI,AB. 53. COUNSELING#.W..DE. 54. ((BEHAVIOR$4 OR BEHAVIOUR$4) NEAR (MODIFY OR MODIFICAT$4 OR THERAP$2 OR CHANGE)).TI,AB. 55. STRESS‐MANAGEMENT#.DE. 56. (STRESS NEAR MANAG$5).TI,AB. 57. (COGNITIVE NEAR THERAP$2).TI,AB. 58. MEDITATION#.W..DE. 59. MEDITAT$5.TI,AB. 60. ANXIETY#.W..DE. 61. (MANAGE$5 NEAR (ANXIETY OR DEPRESS$5)).TI,AB. 62. CBT.TI,AB. 63. HYPNOTHERAP$5.TI,AB. 64. (GOAL$2 NEAR SETTING).TI,AB. 65. (PSYCHO‐EDUCAT$5 OR PSYCHOEDUCAT$5).TI,AB. 66. (MOTIVAT$5 NEAR (INTERV$3 OR INTERVENT$5)).TI,AB. 67. PSYCHOSOCIAL$4.TI,AB. 68. HEALTH‐EDUCATION#.DE. 69. (HEALTH NEAR EDUCAT$5).TI,AB. 70. HEART ADJ MANUAL 71. AUTOGENIC$3.TI,AB. 72. 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 73. 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 74. 72 OR 73 75. 22 AND 74 76. PT=CLINICAL‐TRIAL 77. CLINICAL‐TRIALS#.DE. 78. (RANDOM$5 OR PLACEBO$2).TI,AB. 79. (SINGL$ OR DOUBLE$ OR TRIPLE$ OR TREBLE$).TI,AB. AND (BLIND$ OR MASK$).TI,AB. 80. CONTROLLED ADJ CLINICAL ADJ TRIALS 81. 76 OR 77 OR 78 OR 79 OR 80 82. 75 AND 81 83. LIMIT 82 TO 2001‐2008
PsycINFO DIALOG TO JAN WEEK 1
1. SEARCH: HEART‐DISORDERS#.DE. 2. SEARCH: MYOCARDIAL‐INFARCTIONS.DE. 3. SEARCH: ISCHEMIA#.W..DE. 4. SEARCH: HEART‐SURGERY.DE. 5. SEARCH: ANGIOPLASTY 6. SEARCH: HEART ADJ BYPASS 7. SEARCH: CORONARY.TI,AB. 8. SEARCH: (ISCHEMI$3 OR ISCHAEMI$3).TI,AB. 9. SEARCH: (MYOCARD$5 NEAR INFARCT$5).TI,AB. 10. SEARCH: (HEART NEAR (INFARC$5 OR FAILURE OR ATTACK)).TI,AB. 11. SEARCH: ANGINA.TI,AB. 12. SEARCH: (HEART NEAR DISEASE$2).TI,AB. 13. SEARCH: MYOCARD$5.TI,AB. 14. SEARCH: CARDIAC$4.TI,AB. 15. SEARCH: CABG.TI,AB. 16. SEARCH: PTCA.TI,AB. 17. SEARCH: 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 18. SEARCH: PHYSICAL‐ACTIVITY#.DE. 19. SEARCH: SPORTS#.W..DE. 20. SEARCH: PHYSICAL‐EDUCATION.DE. 21. SEARCH: HEALTH‐BEHAVIOR#.DE. 22. SEARCH: PHYSICAL‐FITNESS.DE. 23. SEARCH: (PHYSICAL ADJ EDUCATION).TI,AB. 24 SEARCH: EXERTION.TI,AB. 25. SEARCH: REHABILITAT$6.TI,AB. 26. SEARCH: (PHYSICAL NEAR (FIT$5 OR TRAIN$5 OR THERAP$5 OR ACTIVIT$4)).TI,AB. 27. SEARCH: (TRAIN$4 NEAR (STRENGTH$4 OR AEROBIC OR EXERCISE$2)).TI,AB. 28. SEARCH: ((EXERCISE$3 OR FITNESS) NEAR (TREATMENT OR INTERVENT$4 OR PROGRAM$4 OR THERAP$2)).TI,AB. 29. SEARCH: (PATIENT WITH EDUCATION).TI,AB. 30. SEARCH: CLIENT‐EDUCATION#.DE. 31. SEARCH: HEALTH‐PROMOTION#.DE. 32. SEARCH: ((LIFESTYLE OR LIFE‐STYLE) NEAR (INTERVENT$5 OR PROGRAM$2 OR TREATMENT$2)).TI,AB. 33. SEARCH: OUTPATIENT‐TREATMENT#.DE. 34. SEARCH: 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 35. SEARCH: PSYCHOTHERAPY#.W..DE. 36 SEARCH: PSYCHOTHERAP$2.TI,AB. 37 SEARCH: TREATMENT#.W..DE. 38 SEARCH: (PSYCHOLOG$4 NEAR INTERVENT$5).TI,AB. 39 SEARCH: COUNSELING#.W..DE. 40 SEARCH: COPING‐BEHAVIOR#.DE. 41 SEARCH: MEDITATION.W..DE. 42 SEARCH: AUTOGENIC‐TRAINING.DE. 43 SEARCH: HEALTH‐EDUCATION#.DE. 44. SEARCH: RELAX$6.TI,AB. 45. SEARCH: (COUNSELLING OR COUNSELING).TI,AB. 46. SEARCH: ((BEHAVIOUR OR BEHAVIOR) NEAR (MODIF$5 OR THERAP$5 OR REHABILIT$5 OR CHANGE)).TI,AB. 47. SEARCH: (STRESS NEAR MANAGE$5).TI,AB. 48. SEARCH: MEDITAT$5.TI,AB. 49. SEARCH: (MANAGE$5 NEAR (ANXIETY OR DEPRES$5)).TI,AB. 50. SEARCH: (CBT OR COGNITIV$2 NEAR THERAP$3).TI,AB. 51. SEARCH: HYPNOTHERAP$3.TI,AB. 52. SEARCH: (PSYCHO‐EDUCAT$6 OR PSYCHOEDUCAT$6).TI,AB. 53. SEARCH: (MOTIVAT$5 NEAR INTERVENT$5).TI,AB. 54. SEARCH: (SELF NEAR MANAG$6).TI,AB. 55. SEARCH: AUTOGENIC$3.TI,AB. 56. SEARCH: (GOAL NEAR SETTING).TI,AB. 57. SEARCH: (HEALTH NEAR EDUCATION).TI,AB. 58. SEARCH: (HEART ADJ MANUAL).TI,AB. 59. SEARCH: 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 60. SEARCH: 17 AND (34 OR 59) 61. SEARCH: (RANDOM$5 OR PLACEBO$5).TI,AB. 62. SEARCH: (DOUBLE$4 OR SINGLE$4 OR TRIPLE$4).TI,AB. AND (BLIND$4 OR MASK OR SHAM$4 OR DUMMY).TI,AB. 63. SEARCH: RCT.TI,AB. 64. SEARCH: AT=TREATMENT$ 65. SEARCH: 61 OR 62 OR 63 OR 64 66. SEARCH: 60 AND 66 67. SEARCH: LIMIT 66 TO YRS=2001‐2008
ISI Proceedings, search date 1 April 2008
# 7 807 #5 and #6 Databases=STP Timespan=2001‐2008 # 6 29,517 TS=(rehab* or educat*) Databases=STP Timespan=2001‐2008 # 5 52,687 #4 OR #3 OR #2 OR #1 Databases=STP Timespan=2001‐2008 # 4 27,506 TS=(angina or cardiac* or PTCA or CABG) Databases=STP Timespan=2001‐2008 # 3 11,226 TS=((heart) SAME (infarct* or isch?emia or failure or attack)) Databases=STP Timespan=2001‐2008 # 2 12,618 TS=((coronary* or heart*) SAME (by?pass or disease*)) Databases=STP Timespan=2001‐2008 # 1 11,809 TS=((myocard*) SAME (isch?emia or infarct* or revasculari?*)) Databases=STP Timespan=2001‐2008
Appendix 3. Search strategies 2013
CENTRAL on The Cochrane Library 2013, Issue 1
1. MeSH descriptor: [Myocardial Ischemia] explode all trees
2. (myocard* near isch*mi*):ti or (myocard* near isch*mi*):ab
3. (isch*mi* near heart):ti or (isch*mi* near heart):ab
4. MeSH descriptor: [Coronary Artery Bypass] explode all trees
5. (coronary):ti or (coronary):ab
6. MeSH descriptor: [Coronary Disease] explode all trees
7. MeSH descriptor: [Myocardial Revascularization] explode all trees
8. MeSH descriptor: [Myocardial Infarction] explode all trees
9. (myocard* near infarct*):ti or (myocard* near infarct*):ab
10. (heart near infarct*):ti or (heart near infarct*):ab
11. MeSH descriptor: [Angina Pectoris] explode all trees
12. (angina):ti or (angina):ab
13. MeSH descriptor: [Heart Failure] explode all trees
14. (heart and (failure or attack)):ti or (heart and (failure or attack)):ab
15. (Heart diseases):ti or (Heart diseases):ab
16. MeSH descriptor: [Heart Diseases] explode all trees
17. (heart and (disease*)):ti or (heart and (disease*)):ab
18. (myocard*):ti or (myocard*):ab
19. (cardiac*):ti or (cardiac*):ab
20. (CABG):ti or (CABG):ab
21. (PTCA):ti or (PTCA):ab
22. (stent* and (heart or cardiac*)):ti or (stent* and (heart or cardiac*)):ab
23. MeSH descriptor: [Heart Bypass, Left] explode all trees
24. (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF"):ti or (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF"):ab
25. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
26. MeSH descriptor: [Rehabilitation Centers] this term only
27. MeSH descriptor: [Exercise Therapy] explode all trees
28. MeSH descriptor: [Sports] this term only
29. MeSH descriptor: [Physical Exertion] explode all trees
30. (rehabilitat*):ti or (rehabilitat*):ab
31. (physical* near (fit* or train* or therap* or activit*)):ti or (physical* near (fit* or train* or therap* or activit*)):ab
32. MeSH descriptor: [Exercise] explode all trees
33. (train*) near (strength* or aerobic or exercise*):ti or (train*) near (strength* or aerobic or exercise*):ab
34. ((exercise* or fitness) near/3 (treatment or intervent* or program*)):ti or ((exercise* or fitness) near/3 (treatment or intervent* or program*)):ab
35. MeSH descriptor: [Rehabilitation] explode all trees
36. MeSH descriptor: [Patient Education as Topic] this term only
37. (patient* near/3 educat*):ti or (patient* near/3 educat*):ab
38. ((lifestyle or life‐style) near/3 (intervent* or program* or treatment*)):ti or ((lifestyle or life‐style) near/3 (intervent* or program* or treatment*)):ab
39. MeSH descriptor: [Self Care] explode all trees
40. MeSH descriptor: [Ambulatory Care] explode all trees
41. MeSH descriptor: [Psychotherapy] explode all trees
42. (psychotherap*):ti or (psychotherap*):ab
43. (psycholog* near intervent*):ti or (psycholog* near intervent*):ab
44. (relax*):ti or (relax*):ab
45. MeSH descriptor: [Mind‐Body Therapies] explode all trees
46. ((Mind or Body) and (Relaxation Techniques)):ti or ((Mind or Body) and (Relaxation Techniques)):ab
47. MeSH descriptor: [Counseling] explode all trees
48. (counseling or counselling):ti or (counseling or counselling):ab
49. MeSH descriptor: [Cognitive Therapy] explode all trees
50. MeSH descriptor: [Behavior Therapy] explode all trees
51. ((behavio*r*) near/4 (modif* or therap* or rehab* or change)):ti or ((behavio*r*) near/4 (modif* or therap* or rehab* or change)):ab
52. MeSH descriptor: [Stress, Psychological] explode all trees
53. (stress near manage*):ti or (stress near manage*):ab
54. (cognitive* near therap*):ti or (cognitive* near therap*):ab
55. MeSH descriptor: [Meditation] explode all trees
56. (meditat*):ti or (meditat*):ab
57. MeSH descriptor: [Anxiety] this term only
58. ((manage*) near (anxiety or depres*)):ti or ((manage*) near (anxiety or depres*)):ab
59. (CBT):ti or (CBT):ab
60. (hypnotherap*):ti or (hypnotherap*):ab
61. (goal near/3 (setting)):ti or (goal near/3 (setting)):ab
62. ((psycho‐educat*) or (psychoeducat*)):ti ((psycho‐educat*) or (psychoeducat*)):ab
63. (motivat* near (interv*)):ti or (motivat* near (interv*)):ab
64. MeSH descriptor: [Psychopathology] explode all trees
65. (psychopathol*):ti or (psychopathol*):ab
66. MeSH descriptor: [Autogenic Training] explode all trees
67. (autogenic*):ti or (autogenic*):ab
68. (self near (manage* or care or motivat*)):ti or (self near (manage* or care or motivat*)):ab
69. (distress*):ti or (distress*):ab
70. (psychosocial* or psycho‐social):ti or (psychosocial* or psycho‐social):ab
71. MeSH descriptor: [Health Education] explode all trees
72. (nutrition or diet or health near (education)):ti or (nutrition or diet or health near (education)):ab
73. (heart manual):ti or (heart manual):ab
74. (#26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37)
75. (#38 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73)
76. (#74 or #75)
77. (#76 and #25)
78. #77 from 2008, in Trials
MEDLINE(R) Ovid 1946 to January week 4 2013
1. exp Myocardial Ischemia/
2. (myocard$4 adj5 (ischaemi$2 or ischemi$2)).ti,ab.
3. ((ischaemi$2 or ischemi$2) adj5 heart).ti,ab.
4. exp Coronary Artery Bypass/
5. coronary.ti,ab.
6. exp Coronary Disease/
7. exp Myocardial Revascularization/
8. Myocardial Infarction/
9. (myocard$5 adj5 infarct$5).ti,ab.
10. (heart adj5 infarct$5).ti,ab.
11. exp Angina Pectoris/
12. angina.ti,ab.
13. exp Heart Failure/
14. (heart adj5 failure).ti,ab.
15. (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF").ti,ab.
16. or/1‐15
17. exp Heart Diseases/
18. (heart adj5 disease$2).ti,ab.
19. myocard$5.ti,ab.
20. cardiac$2.ti,ab.
21. CABG.ti,ab.
22. PTCA.ti,ab.
23. (stent$4 and (heart or cardiac$4)).ti,ab.
24. Heart Bypass, Left/ or exp Heart Bypass, Right/
25. or/17‐24
26. *Rehabilitation Centers/
27. exp Exercise Therapy/
28. *Rehabilitation/
29. exp Sports/
30. Physical Exertion/ or exertion.ti,ab.
31. exp Exercise/
32. rehabilitat$5.ti,ab.
33. (physical$4 adj5 (fit or fitness or train$5 or therap$5 or activit$5)).ti,ab.
34. (train$5 adj5 (strength$3 or aerobic or exercise$4)).ti,ab.
35. ((exercise$4 or fitness) adj5 (treatment or intervent$4 or programs$2 or therapy)).ti,ab.
36. Patient Education as Topic/
37. (patient$2 adj5 educat$4).ti,ab.
38. ((lifestyle or life‐style) adj5 (intervent$5 or program$2 or treatment$2)).ti,ab.
39. *Self Care/
40. (self adj5 (manage$5 or care or motivate$5)).ti,ab.
41. *Ambulatory Care/
42. exp Psychotherapy/
43. psychotherap$2.ti,ab.
44. (psycholog$5 adj5 intervent$5).ti,ab.
45. relax$6.ti,ab.
46. exp Relaxation Therapy/ or exp Mind‐Body Therapies/
47. exp Counseling/
48. (counselling or counseling).ti,ab.
49. exp Cognitive Therapy/
50. exp Behavior Therapy/
51. ((behavior$4 or behaviour$4) adj5 (modify or modificat$4 or therap$2 or change)).ti,ab.
52. *Stress, Psychological/
53. (stress adj5 management).ti,ab.
54. (cognitive adj5 therap$2).ti,ab.
55. meditat$4.ti,ab.
56. *Meditation/
57. exp Anxiety/
58. (manage$5 adj5 (anxiety or depress$5)).ti,ab.
59. CBT.ti,ab.
60. hypnotherap$5.ti,ab.
61. (goal adj5 setting).ti,ab.
62. (goal$2 adj5 setting).ti,ab.
63. (psycho‐educat$5 or psychoeducat$5).ti,ab.
64. (motivat$5 adj5 (intervention or interv$3)).ti,ab.
65. Psychopathology/
66. psychopathol$4.ti,ab.
67. psychosocial$4.ti,ab.
68. distress$4.ti,ab.
69. exp Health Education/
70. (health adj5 education).ti,ab.
71. (heart adj5 manual).ti,ab.
72. Autogenic Training/
73. autogenic$5.ti,ab.
74. or/26‐39
75. or/40‐73
76. 16 or 25
77. 74 or 75
78. 76 and 77
79. randomized controlled trial/
80. randomized controlled trial.pt.
81. controlled clinical trial.pt.
82. controlled clinical trial/
83. Random Allocation/
84. Double‐Blind Method/
85. single‐blind method/
86. (random$ or placebo$).ti,ab.
87. ((singl$3 or doubl$3 or tripl$3 or trebl$3) adj5 (blind$3 or mask$3)).ti,ab.
88. exp Research Design/
89. Clinical Trial.pt.
90. exp clinical trial/
91. (clinic$3 adj trial$2).ti,ab.
92. or/79‐91
93. 78 and 92
94. (Animals not Humans).sh.
95. 93 not 94
96. limit 95 to yr="2008 ‐Current"
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid 5 February 2013
1. (myocard$4 adj5 (ischaemi$2 or ischemi$2)).ti,ab.
2. ((ischaemi$2 or ischemi$2) adj5 heart).ti,ab.
3. coronary.ti,ab.
4. (myocard$5 adj5 infarct$5).ti,ab.
5. (heart adj5 infarct$5).ti,ab.
6. angina.ti,ab.
7. (heart adj5 failure).ti,ab.
8. (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF").ti,ab.
9. or/1‐8
10. (heart adj5 disease$2).ti,ab.
11. myocard$5.ti,ab.
12. cardiac$2.ti,ab.
13. CABG.ti,ab.
14. PTCA.ti,ab.
15. (stent$4 and (heart or cardiac$4)).ti,ab.
16. or/10‐15
17. Physical Exertion/ or exertion.ti,ab.
18. rehabilitat$5.ti,ab.
19. (physical$4 adj5 (fit or fitness or train$5 or therap$5 or activit$5)).ti,ab.
20. (train$5 adj5 (strength$3 or aerobic or exercise$4)).ti,ab.
21. ((exercise$4 or fitness) adj5 (treatment or intervent$4 or programs$2 or therapy)).ti,ab.
22. (patient$2 adj5 educat$4).ti,ab.
23. ((lifestyle or life‐style) adj5 (intervent$5 or program$2 or treatment$2)).ti,ab.
24. (self adj5 (manage$5 or care or motivate$5)).ti,ab.
25. psychotherap$2.ti,ab.
26. (psycholog$5 adj5 intervent$5).ti,ab.
27. relax$6.ti,ab.
28. (counselling or counseling).ti,ab.
29. ((behavior$4 or behaviour$4) adj5 (modify or modificat$4 or therap$2 or change)).ti,ab.
30. (stress adj5 management).ti,ab.
31. (cognitive adj5 therap$2).ti,ab.
32. meditat$4.ti,ab.
33. (manage$5 adj5 (anxiety or depress$5)).ti,ab.
34. CBT.ti,ab.
35. hypnotherap$5.ti,ab.
36. (goal adj5 setting).ti,ab.
37. (goal$2 adj5 setting).ti,ab.
38. (psycho‐educat$5 or psychoeducat$5).ti,ab.
39. (motivat$5 adj5 (intervention or interv$3)).ti,ab.
40. psychopathol$4.ti,ab.
41. psychosocial$4.ti,ab.
42. distress$4.ti,ab.
43. (health adj5 education).ti,ab.
44. (heart adj5 manual).ti,ab.
45. autogenic$5.ti,ab.
46. or/17‐45
47. 9 or 16
48. 46 and 47
49. (random$ or placebo$).ti,ab.
50. ((singl$3 or doubl$3 or tripl$3 or trebl$3) adj5 (blind$3 or mask$3)).ti,ab.
51. (clinic$3 adj trial$2).ti,ab.
52. 49 or 50 or 51
53. 48 and 52
54. limit 53 to yr="2008 ‐Current"
EMBASEOvid 1980 to 2013 week 5
1. exp heart disease/
2. (myocard$4 adj5 (ischaemi$2 or ischemi$2)).ti,ab.
3. ((ischaemi$2 or ischemi$2) adj5 heart).ti,ab.
4. exp coronary artery disease/
5. transluminal coronary angioplasty/
6. (coronary adj5 (disease$2 or bypass$2 or thrombo$5 or angioplasty$2)).ti,ab.
7. exp heart infarction/
8. (myocard$5 adj5 infarct$5).ti,ab.
9. (heart adj5 infarct$5).ti,ab.
10. heart muscle revascularization/
11. exp Angina Pectoris/
12. angina.ti,ab.
13. exp congestive heart failure/
14. (heart adj5 failure).ti,ab.
15. (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF").ti,ab.
16. or/1‐15
17. (heart adj5 disease$2).ti,ab.
18. cardiac$2.ti,ab.
19. CABG.ti,ab.
20. PTCA.ti,ab.
21. (stent$4 and heart).ti,ab.
22. exp extracorporeal circulation/
23. or/17‐22
24. 16 or 23
25. *Psychotherapy/
26. psychotherapy$2.ti,ab.
27. (psycholog$5 adj5 intervent$5).ti,ab.
28. relax$6.ti,ab.
29. relaxation training/
30. *counselling/
31. (counselling or counseling).ti,ab.
32. ((behavior$4 or behaviour$4) adj5 (modify or modificat$4 or therap$2 or change)).ti,ab.
33. stress management/
34. (stress adj5 management).ti,ab.
35. *Mediation/
36. meditat$5.ti,ab.
37. (manage$5 adj5 (anxiety or depress$5)).ti,ab.
38. CBT.ti,ab.
39. hypnotherap$2.ti,ab.
40. (goal$2 adj5 setting).ti,ab.
41. (psycho‐educat$5 or psychoeducat$5).ti,ab.
42. (motivat$5 adj5 intervent$6).ti,ab.
43. exp psychosocial care/ or exp psychosocial rehabilitation/
44. psychosocial.ti,ab.
45. exp health education/
46. (health adj5 education).ti,ab.
47. (heart adj5 manual).ti,ab.
48. autogenic training/
49. autogenic.ti,ab.
50. *Rehabilitation/
51. rehabilitation center/
52. rehabil$.ti,ab.
53. exp Sport/
54. exp Kinesiotherapy/
55. exp Exercise/
56. exp Physiotherapy/
57. (physical$4 adj5 (fit or fitness or train$5 or therap$5 or activit$5)).ti,ab.
58. (train$5 adj5 (strength$3 or aerobic or exercise$4)).ti,ab.
59. ((exercise$4 or fitness) adj5 (treatment or intervent$4 or programs$2 or therapy)).ti,ab.
60. (aerobic$4 adj5 exercise$4).ti,ab.
61. (kinesiotherapy or physiotherapy).ti,ab.
62. patient education/
63. (patient$2 adj5 educat$4).ti,ab.
64. ((((lifestyle or life) adj1 style) or life‐style) adj5 (intervent$5 or program$2 or treatment$2)).ti,ab.
65. exp self care/
66. (self adj5 (manage$5 or care or motivate$5)).ti,ab.
67. exp ambulatory care/
68. (psycho‐educat$5 or psychoeducat$5).ti,ab.
69. (motivat$5 adj5 intervent$6).ti,ab.
70. psychosocial care/ or psychosocial rehabilitation/
71. psychosocial.ti,ab.
72. exp health education/
73. (health adj5 education).ti,ab.
74. (heart adj5 manual).ti,ab.
75. autogenic training/
76. autogenic$5.ti,ab.
77. (psycho‐educat$5 or psychoeducat$5).ti,ab.
78. (motivat$5 adj5 intervent$6).ti,ab.
79. psychosocial care/ or psychosocial rehabilitation/
80. psychosocial.ti,ab.
81. exp health education/
82. (health adj5 education).ti,ab.
83. (heart adj5 manual).ti,ab.
84. or/25‐50
85. or/51‐83
86. 84 or 85
87. (random$ or placebo$).ti,ab.
88. ((singl$4 or doubl$4 or tripl$4 or trebl$4) adj5 (blind$4 or mask$4)).ti,ab.
89. (controlled adj1 clinical adj1 trial).ti,ab.
90. randomized controlled trial/
91. or/87‐90
92. 24 and 86
93. 91 and 92
94. (animal$ not human$).sh,hw.
95. 93 not 94
96. limit 95 to yr="2008 ‐Current"
PsycINFO Ovid 1806 to January week 5 2013
1. exp heart disorders/
2. *Myocardial Infarctions/
3. exp Ischemia/
4. *Heart Surgery/
5. angioplasty.ti,ab.
6. (heart adj1 bypass).ti,ab.
7. coronary.ti,ab.
8. (ischemi$3 or ischaemi$3).ti,ab.
9. (myocard$5 adj5 infarct$5).ti,ab.
10. (heart adj5 (infarct$5 or failure or attack)).ti,ab.
11. angina.ti,ab.
12. (heart adj5 disease$2).ti,ab.
13. myocard$5.ti,ab.
14. cardiac$4.ti,ab.
15. CABG.ti,ab.
16. PTCA.ti,ab.
17. (HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF").ti,ab.
18. or/1‐17
19. exp Physical Activity/
20. exp Sports/
21. *Physical Education/
22. exp Health Behavior/
23. *Physical Fitness/
24. (physical adj1 education).ti,ab.
25. exertion$6.ti,ab.
26. rehabilitat$6.ti,ab.
27. (physical adj5 (fit$5 or train$5 or therap$5 or activit$4)).ti,ab.
28. (train$4 adj5 (strength$4 or aerobic or exercise$2)).ti,ab.
29. ((exercise$3 or fitness) adj5 (treatment or intervent$4 or program$4 or therap$2)).ti,ab.
30. patient with education.ti,ab.
31. exp Client Education/
32. exp Health Promotion/
33. ((lifestyle or life‐style) adj5 (intervent$5 or program$2 or treatment$2)).ti,ab.
34. exp Outpatient Treatment/
35. or/19‐34
36. exp Psychotherapy/
37. psychotherapy$2.ti,ab.
38. exp Treatment/
39. (psycholog$4 adj5 intervent$5).ti,ab.
40. exp Counseling/
41. exp Coping Behavior/
42. *Meditation/
43. *Autogenic Training/
44. exp Health Education/
45. relax$6.ti,ab.
46. (counselling or counseling).ti,ab.
47. ((behavior or behaviour) adj5 (modif$5 or therap$5 or rehabilit5 or change)).ti,ab.
48. (stress adj5 management).ti,ab.
49. meditat$5.ti,ab.
50. (manage$5 adj5 (anxiety or depress$5)).ti,ab.
51. ((cbt or cognitive$2) adj5 therap$3).ti,ab.
52. hypnotherap$3.ti,ab.
53. (psycho‐educat$6 or psychoeducat$6).ti,ab.
54. (motivat$5 adj5 intervent$5).ti,ab.
55. (self adj5 manag$6).ti,ab.
56. autogenic$3.ti,ab.
57. (goal adj5 setting).ti,ab.
58. (health adj5 education).ti,ab.
59. (heart adj1 manual).ti,ab.
60. or/36‐59
61. 18 and (35 or 60)
62. (random$5 or placebo$5).ti,ab.
63. ((single$4 or double$4 or triple$4) and (blind$4 or mask or sham$4 or dummy)).ti,ab.
64. RCT.ti,ab.
65. or/62‐64
66. 61 and 65
67. limit 66 to yr="2008 ‐Current"
CINAHL EBSCOhost, search date 5 February 2013
1. TI((myocard* N5 ischaemi*) or (myocard* N5 ischemi*) or (heart N5 ischaemi*) or (heart N5 ischemi*)) OR AB((myocard* N5 ischaemi*) or (myocard* N5 ischemi*) or (heart N5 ischaemi*) or (heart N5 ischemi*))
2. TI(coronary) or AB(coronary)
3. TI((myocard* N5 infarc*) or (heart N5 infarc*)) or AB((myocard* N5 infarc*) or (heart N5 infarc*))
4. TI(angina) OR AB(angina)
5. TI(heart N5 failure) or AB(heart N5 failure)
6. TI(heart N5 diseas*) or AB(heart N5 diseas*)
7. TI(cardiac) or AB(cardiac)
8. TI(CABG) or AB(CABG)
9. TI(PTCA) or AB(PTCA)
10. TI(Stent* and (heart or cardiac*)) or AB(Stent* and (heart or cardiac*))
11. (MH "Myocardial Ischemia+")
12. (MH "Myocardial Infarction+")
13. (MH "Coronary Artery Bypass+")
14. (MH "Coronary Disease+")
15. TI(cardiac N5 patient*) or AB(cardiac N5 patient*)
16. TI(Cardiomyopathies) or AB(Cardiomyopathies)
17. (MH "Myocardial Revascularization+")
18. (MH "Heart Diseases+")
19. (MH "Cardiovascular Diseases+")
20. (MH "Heart Failure+")
21. (MH "Angina Pectoris+")
22. TI(HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF") or AB(HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF")
23. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
24. (MM "Rehabilitation")
25. (MM "Sports")
26. (MM "Physical Activity")
27. (MH "Muscle Strengthening+")
28. (MH "Aerobic Exercises+")
29. (MH "Physical Fitness+")
30. (MH "Patient Education+")
31. (MH "Therapeutic Exercise+")
32. TI(rehabilitat*) or AB(rehabilitat*)
33. TI((physical* N5 fit) or (physical N5 fitness) or (physical N5 train*) or (physical N5 therap*) or (physical N5 activit*)) or AB((physical* N5 fit) or (physical N5 fitness) or (physical N5 train*) or (physical N5 therap*) or (physical N5 activit*))
34. TI((train N5 strength) or (train N5 aerobic) or (train N5 exercis*)) or AB((train N5 strength) or (train N5 aerobic) or (train N5 exercis*))
35. TI((exercise N5 treatment) or (fitness N5 treatment) or (exercise N5 intervent*) or (fitness N5 intervent*) or (exercise N5 program*) or (fitness N5 program) or (exercise N5 therapy) or (fitness N5 therapy)) or AB((exercise N5 treatment) or (fitness N5 treatment) or (exercise N5 intervent*) or (fitness N5 intervent*) or (exercise N5 program*) or (fitness N5 program) or (exercise N5 therapy) or (fitness N5 therapy))
36. TI(patient* N5 educat*) or AB(patient* N5 educat*)
37. TI ((lifestyle N5 intervent*) or (life‐style N5 intervent*) or (lifestyle N5 program*) or (life‐style N5 program*) or (lifestyle N5 treatment) or (life‐style N5 treatment)) OR AB ((lifestyle N5 intervent*) or (life‐style N5 intervent*) or (lifestyle N5 program*) or (life‐style N5 program*) or (lifestyle N5 treatment) or (life‐style N5 treatment))
38. (MH "Self Care+")
39. TI((self N5 manage*) or (self N5 care) or (self N5 motivat*)) or AB((self N5 manage*) or (self N5 care) or (self N5 motivat*))
40. (MM "Ambulatory Care")
41. TI(aerobic) or AB(aerobic)
42. TI(resistance W1 train*) or AB(resistance W1 train*)
43. TI(muscle W1 strength*) or AB(muscle W1 strength*)
44. TI(resistance W1 train*) or AB(resistance W1 train*)
45. TI(muscle W1 strength*) or AB(muscle W1 strength*)
46. (MH "Psychotherapy+")
47. TI(psychotherap*) or AB(psychotherap*)
48. TI(psycholog* N5 intervent*) or AB(psycholog* N5 intervent*)
49. TI(relax) or AB(relax)
50. (MH "Relaxation Techniques+")
51. TI(counselling or counseling) or AB(counselling or counseling)
52. (MH "Counseling+")
53. TI((behavio?r* N5 modify) or (behavio?r* N5 modificat*) or (behavio?r* N5 therap*) or (behavio?r* N5 change)) or AB((behavio?r* N5 modify) or (behavio?r* N5 modificat*) or (behavio?r* N5 therap*) or (behavio?r* N5 change))
54. (MM "Stress Management")
55. TI(stress N5 manag*) or AB(stress N5 manag*)
56. TI(cognitive N5 therap*) or AB(cognitive N5 therap*)
57. (MM "Meditation")
58. TI(meditat*) or AB(meditat*)
59. (MH "Anxiety+")
60. TI((manage* N5 anxiety) or (manage* N5 depress*)) or AB((manage* N5 anxiety) or (manage* N5 depress*))
61. TI(CBT) or AB(CBT)
62. TI(hypnotherap*) or AB(hypnotherap*)
63. TI(goal* N5 setting) or AB(goal* N5 setting)
64. TI(psycho‐educat* or psychoeducat*) or AB(psycho‐educat* or psychoeducat*)
65. TI((motivat* N5 interv*) or (motivate* N5 intervent*)) or AB((motivat* N5 interv*) or (motivate* N5 intervent*))
66. TI(psychosocial*) or AB(psychosocial*)
67. (MH "Health Education+")
68. TI(health N5 educat*) or AB(health N5 educat*)
69. TI(heart W1 manual) or AB(heart W1 manual)
70. TI(autogenic*) or AB(autogenic*)
71. S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45
72. S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70
73. S71 OR S72
74. S23 AND S73
75. PT CLINICAL TRIAL
76. (MH "Clinical Trials+")
77. TI (random* or placebo*) or AB (random* or placebo*)
78. TI(singl* or double* or triple* or treble* and (blind* or mask*)) or AB(singl* or double* or triple* or treble* and (blind* or mask*))
79. TI(controlled w1 clinical w1 trials) or AB(controlled w1 clinical w1 trials)
80. S75 OR S76 OR S77 OR S78 OR S79
81. S74 AND S80 date limit=2008‐current
Web of Science, search date 6 February 2013
1. TS=((myocard*) SAME (isch?emia or infarct* or revasculari?*))
2. TS=((coronary* or heart*) SAME (by?pass or disease*))
3. TS=((heart) SAME (infarct* or isch?emia or failure or attack))
4. TS=(angina or cardiac* or PTCA or CABG)
5. TS=(HFNEF or HFPEF or HFREF or "HF NEF" or "HF PEF" or "HF REF")
6. #1 OR #2 OR #3 OR #4 OR #5
7. TS=(rehab* or educat*)
8. #6 AND #7
9. TS=(random* or placebo*)
10. TS=((singl* or doubl* or tripl* or trebl*) SAME (blind* or mask*))
11. TS=("clinic* trial*")
12. #9 OR #10 OR #11
13. #8 AND #12
Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=2008‐2013
Data and analyses
Comparison 1.
All exercise interventions versus usual care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality up to12 months' follow‐up | 25 | 1871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.27] |
| 2 All‐cause mortality more than 12 months' follow‐up | 6 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.02] |
| 3 Hospital admission up to 12 months' follow‐up | 15 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.92] |
| 4 Hospital admission heart failure only | 12 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 5 Hospital admission more than 12 months' follow‐up | 5 | 2722 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.29] |
| 6 Health‐related quality of life ‐ MLWHF up to 12 months' follow‐up | 13 | 1270 | Mean Difference (IV, Random, 95% CI) | ‐5.83 [‐9.21, ‐2.44] |
| 7 Health‐related quality of life ‐ MLWHF and other scales | 21 | 3240 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.66, ‐0.26] |
| 8 Health‐related quality of life ‐ MLWHF 12 months' follow‐up | 3 | 329 | Mean Difference (IV, Random, 95% CI) | ‐9.49 [‐17.48, ‐1.50] |
What's new
Last assessed as up‐to‐date: 30 June 2013.
| Date | Event | Description |
|---|---|---|
| 19 October 2017 | Amended | Tables moved to correct section of the review. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 1 November 2013 | New citation required but conclusions have not changed | This update review identified a further 14 trials. Whilst conclusions of the review do not change, this update provides broader body of evidence of the benefit of exercise‐based interventions that includes HFPEF patients and delivery in a home‐based setting |
| 14 February 2013 | New search has been performed | Searches updated |
| 18 May 2004 | New citation required and conclusions have changed | Substantive amendment |
Differences between protocol and review
Compared with previous version of this review, the inclusion criteria extended to include HFPEF.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 200 (exercise 100; control 100) Diagnosis (% of participants): Aetiology: ischaemic 77%; hypertension 15.5%; DCM 5.5%; other 2% NYHA: Class II 51.5%; Class III 48.5% LVEF: 40‐35%: 16.5%; < 35‐30%: 45%; < 30%: 38.5% Case mix: 100% as above Age (yr): exercise 71.9 (SD 6.3); control 71.8 (SD 6.8) Male: 43% White: not reported Inclusion/exclusion criteria Inclusion: age > 60 yr, NYHA Class II or III, and LVSD < 40%, confirmed by echocardiography Exclusion: diastolic dysfunction, significant co‐morbidity preventing entry into study because of terminal disease or an inability to exercise (e.g. severe musculoskeletal disorder, unstable IHD, advanced valvular disease), resident outside the catchment area or in a long‐term care establishment |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: aerobic endurance training and low resistance training/high repetitive muscular strength work Frequency: 2 sessions/wk (for 8 wk), 1 session/wk (16 wk) plus 3 sessions/wk at home Duration: 2.5 hr class (8 wk) and 1 hr class (next 16 wk) Intensity: not reported Modality: not reported Setting: hospital and home Other: none |
|
| Outcomes | HRQoL (MLWHFQ and EuroQol/EQ‐5D); healthcare utilisation (length of stay of hospital, admissions arising from heart disease, prescribed HF medication); mortality | |
| Comparison | Standard care group (including monitoring of clinical status, explanation of HF and its treatment self monitoring; dietary advice and contact details of clinical nurse specialist) | |
| Country and setting | UK Single centre |
|
| Follow‐up | 6 months and 5 yr (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer was used to generate a list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "The numbers, placed in plain sealed envelopes by a university colleague prior to patient recruitment, were allocated to the participants by a hospital colleague unconnected with the study. The allocation schedule was not broken until the trial was completed" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No, for HRQoL. Data on deaths, admissions from the hospital records department |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported |
| Intention‐to‐treat analysis? | Low risk | Although term ITT not stated it appears from CONSORT diagram that ITT analysis undertaken |
| Incomplete outcome data? | Low risk | CONSORT diagram presented showing participant flow. No imputation or sensitivity analysis to assess impact of loss or follow‐up |
| Groups balanced at baseline? | Low risk | "There are no significant differences in the baseline parameters of the standard care and experimental groups" |
| Groups received same intervention? | Low risk | Yes, both groups received usual medical care and the only difference between groups was the exercise intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 99 (exercise 50; control 49) Diagnosis (% of participants): Aetiology: ischaemic cardiomyopathy 85%; idiopathic DCM 15% NYHA: Class II 49%; Class III 34%; Class IV 17% LVEF: exercise 28.4 (SD 6); control 27.9 (SD 5) Case mix: see above Age (yr): exercise 56 (SD 7); control 53 (SD 9) Male: 89% White: not reported Inclusion/exclusion criteria Inclusion: HF, LVEF < 40%, and sinus rhythm, diagnosis of CHF based on clinical symptoms and signs with or without radiological evidence of pulmonary congestion Exclusion: unstable angina, recent acute MI, decompensated congestive HF, haemodynamically significant valvular heart disease, significant chronic pulmonary illness, uncontrolled hypertension, renal insufficiency (serum creatinine > 2.5 mg/dL), and orthopaedic or neurological limitations) |
|
| Interventions |
Exercise:Total duration: 14 month; 8 wk supervised then 12 months maintenance Aerobic/resistance/mix: aerobic Frequency: 2‐3 sessions/wk Duration: 40 min/session Intensity: 60% max VO2 Modality: cycling Setting: hospital‐based programme Other: all sessions were supervised by a cardiologist |
|
| Outcomes | HRQoL (MLWHFQ); mortality; morbidity; cost‐effectiveness | |
| Comparison | Standard medical care | |
| Country and setting | Italy Single centre |
|
| Follow‐up | 14 and 26 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods, reported in results |
| Intention‐to‐treat analysis? | Unclear risk | Not reported |
| Incomplete outcome data? | Low risk | Losses to follow‐up reported |
| Groups balanced at baseline? | Low risk | "The baseline characteristics of the study population are shown in Table 1. The 2 groups were well balanced with respect to most characteristics, including peak VO2, New York Heart Association functional class, and left ventricular ejection fraction. There were no differences in type and doses of medications, blood chemistry, and previous cardiac events" |
| Groups received same intervention? | Unclear risk | Not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 123 (exercise 63; control 60) Diagnosis (% of participants): Aetiology: ischaemic 80%; non‐ischaemic 20% NYHA: Class II 59%; Class III 41% LVEF: 37 (SD 8) Case mix: see above Age (yr): 59 (SD 14) Male: 78% White: not reported Inclusion/exclusion criteria Inclusion: clinical stability for 3 months before enrolment, LVEF < 40% and ability to exercise Exclusion: haemodynamically significant valvular heart disease, uncontrolled DM and hypertension, orthopaedic or neurological problems, and renal insufficiency (creatinine > 2.5 mg/dL) |
|
| Interventions |
Exercise:Total duration: 10 yr; 8 wk supervised then 12 months maintenance Aerobic/resistance/mix: aerobic Frequency: 2‐3 sessions/wk Duration: 40 min/session Intensity: 60% max VO2 for first 2 months, and thereafter at 70% max VO2 Modality: cycling Setting: Hospital and home‐based Other: trained participants were encouraged to exercise without supervision at home at least a third time, performing aerobic activities at the same HR as the other 2 supervised sessions. Exercises sessions held at the hospital were supervised by cardiologists. Authors emphasise that the supervised element was maintained over the 10 yr of follow‐up. |
|
| Outcomes | HRQoL (MLWHQ), mortality, morbidity (including hospitalisation), cost‐effectiveness | |
| Comparison | Standard medical care. Participants were instructed to continue with their usual home daily physical activities, avoiding exercise training in a supervised environment. They were free to perform aerobic activities such as walking, cycling (home or outside), and swimming, avoiding a duration of longer than 30 min. Authors advised control group participants to walk and perform usual physical activities. | |
| Country and setting | Italy Single centre |
|
| Follow‐up | 10 yr (every 12 months) (after randomisation) | |
| Notes | Every 6 months, participants exercised at the hospital, and then they returned to a coronary club, where they exercised the rest of the year. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods, reported in results |
| Intention‐to‐treat analysis? | Low risk | "All analysis were performed with an intention‐to‐treat principle" |
| Incomplete outcome data? | Low risk | Losses to follow‐up reported. Drop‐out rate was 3% on average in the exercise group. 2/63 did not complete the protocol, 1 because of a car accident and the other for personal reasons. 3/60 in control group decided to withdraw from study for reasons unrelated to their clinical status. |
| Groups balanced at baseline? | Low risk | "The baseline characteristics of the study population are shown in Table 1. The 2 groups were well balanced with respect to most characteristics, including peak VO2, New York Heart Association functional class, left ventricular ejection fraction. There were no difference in type and doses of medication, blood chemistry, and previous cardiac events." |
| Groups received same intervention? | Low risk | Both groups appeared to receive same interventions apart from CR intervention. |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 42 (exercise 22; control 20) Diagnosis (% of participants): Aetiology: MI 45.2%; systemic hypertension 19%; dilated Chagas' cardiomyopathy 11.9%; DM 4.8%; other 19.1% NYHA: Class II or III LVEF: ≤ 45% Case mix: 100% as above Age (yr): exercise 61 (SD 12); control 60 (SD 11) Male: 88% White: not reported Inclusion/exclusion criteria Inclusion: EF < 45%, symptoms of NYHA functional Class II or III, optimised pharmacological therapy established at least 4 wk before inclusion in the study, and compensated HF state at least 2 months prior Exclusion: age < 50 yr, NYHA functional Class IV, clinical instability in the preceding 2 months, non‐optimised therapy, uncontrolled arrhythmias, MI within the last 2 months, surgery‐associated cardiomyopathy, pulmonary disease or other co‐morbid conditions that limit physical exercise, accentuated severe cardiac symptoms (hypotension, complex ventricular arrhythmia, progressive worsening of dyspnoea and significant ischaemia at low rates) during ergometric tests, regular participation in some exercise programme within the last 6 months and a frequency in training protocol of < 80% |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: 90 min Intensity: target HR (50% of work in the max HR) Modality: walking on a treadmill Setting: not reported Other: relaxation and stretching exercises before and after every session |
|
| Outcomes | HRQoL (shortened version of World Health Organization Quality of Life questionnaire), hospitalisation | |
| Comparison | Usual medical therapy ‐ individual dietary guidance and pharmacological therapy | |
| Country and setting | Brazil Single‐centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | Initially randomised 53 participants, excluded data from participants who withdrew, lost to follow‐up, etc. and hence 42 participants were analysed Although setting not reported, the exercise programme was described as "supervised" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | High risk | "During the follow‐up, medicine doses were not modified except for those that presented impairment of symptoms and, consequently, these patients were excluded from the analysis" |
| Incomplete outcome data? | High risk | "…3 patients from the untrained group experienced an impairment of symptoms and were hospitalized" |
| Groups balanced at baseline? | Low risk | Table 1 of the publication shows groups are well balanced |
| Groups received same intervention? | Low risk | "All patients continued with pharmacological therapy and individual dietary guidance" |
| Methods | Parallel group RCT | |
| Participants |
N randomised: 91 (exercise 45; control 46) Age (yr): exercise: median 66 (range 33‐91); control median (range 29‐94) Male: 90% White: not reported Inclusion/exclusion criteria Inclusion: present symptoms of CHF and objective findings or effect of medication Exclusion: mental disorders and social problems (such as dementia, alcoholism or drug addiction). Transferred to other department or hospital at discharge. Severe illness, including NYHA Class IV. Living at nursing home. Did not speak Danish. Refused consent |
|
| Interventions |
Exercise:Total duration: 12 wk Aerobic/resistance/mix: mix Frequency: 3 sessions/wk Duration: 90 min/session Intensity: 50% max HR Modality: not reported Setting: supervised centre‐based plus home‐based also encouraged to continue Other: the physical exercise was conducted as a mixture of endurance and strengthening training using various upper and lower body modalities easily implemented as activities that the participants could perform at home. CR included participant education, exercise training, dietary counselling, smoking cessation, psychosocial support, and risk factor management and clinical assessment. All components included theoretical and practical approaches followed by individual follow‐up and feedback. The lifestyle intervention strategy was based on the stages of change model and self efficacy theory. The lifestyle intervention was designed as group intervention, but individual counselling was included |
|
| Outcomes | Primary: composite outcome measure included overall mortality, MI or acute first‐time re‐admission due to heart disease other than MI Secondary: collected data using an adapted standardised interview questionnaire and a postal questionnaire (e.g. SF‐36, HADS), clinical examination and blood tests |
|
| Comparison | Usual care participants were offered follow‐up treatment prescribed by the discharging physician either as outpatient control or by the general practitioner. The pharmaceutical treatment followed routine clinical practice based on current national guidelines. The discharging nurse or physician determined whether participants were referred to smoking cessation and dietary counselling parallel to outpatient treatment | |
| Country and setting | Demark Single centre |
|
| Follow‐up | 12‐months | |
| Notes | HF subset of 770 participants randomised, other participants with coronary heart disease and were high risk but no disease. Randomisation stratified by indication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients who gave informed consent were randomized using a centralized randomization procedure administered by the Copenhagen Trial Unit. The randomization was stratified according to risk group (CHF, IHD, or HR) based on a random‐permuted multiblock within‐stratum method" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Because of the nature of CR, the interventions were open to the investigators and the patients. Investigator independent outcome data from registries were chosen to ensure blinded assessment and outcome analysis" |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | ITT analysis stated |
| Incomplete outcome data? | Low risk | 81% overall follow‐up at 12‐months |
| Groups balanced at baseline? | Low risk | "Patients were well matched at entry" |
| Groups received same intervention? | Low risk | Both groups received control care |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 105 (exercise 53; control 52) Diagnosis (% of participants): Aetiology: not reported NYHA: Class I: exercise 2%; control 0%; Class II: exercise 38%; control 33%; Class III: exercise 60%; control 67%; Class IV: exercise 0%; control 0% LVEF: not reported Case mix: as above Age (yr): exercise 71.6 (SD not reported); control 73.9 (SD not reported) Male: 67% White: not reported Inclusion/exclusion criteria Inclusion: participants were of any age with a diagnosis of HF of any aetiology, and NYHA Class I‐IV. All participants cleared by their physician to participate in the exercise group Exclusion: participants with unstable angina pectoris were ineligible to participate |
|
| Interventions |
Exercise:Total duration: 12 wk Aerobic/resistance/mix: aerobic Frequency: 1 session/wk Duration: 30‐50 min Intensity: not reported Modality: gymnasium: treadmills, stationary cycles, recumbent cycles Home‐based: hall walks, stairs and sporting activities such as lawn bowls Setting: supervised gymnasium, home‐based programme tailored to participant's need Other: also attended a nurse‐coordinated CR clinic with emphasis of self‐management. A group‐based educational session was conducted for study participants and their families. Exercise group attended the nurse‐co‐ordinated CR clinic, where comprehensive assessment was performed by the physiotherapist, CR co‐ordinator and occupational therapist |
|
| Outcomes | HRQoL (MLWHFQ), all‐cause and cardiovascular‐related hospital admission, mortality | |
| Comparison | Information session and then usual medical care | |
| Country and setting | Australia Single‐centre |
|
| Follow‐up | 12 months (after randomisation) | |
| Notes | The trial had to be stopped prematurely at 12 months following introduction of chronic and complex care for people with CHF by the New South Wales Health Department. "In view of trends in favour of the intervention group and emerging evidence from other studies, it was considered unethical and untenable to continue randomization in view of the policy mandate. When the trial was stopped there were 53 participants in the intervention group and 52 participants in the usual care group" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to either the intervention or control group by means of a computer‐generated program" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The randomization technique was blinded to the investigators until the close of the study" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported |
| Intention‐to‐treat analysis? | Low risk | Although not reported as ITT analysis, groups did appear to be analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | "No participants were lost to follow‐up" |
| Groups balanced at baseline? | Low risk | "…there were few differences between intervention and usual care groups, indicating success of randomization. The most important difference on clinical variable was that a significantly greater proportion of people in the intervention group were taking spironolactone at baseline" |
| Groups received same intervention? | Low risk | Both groups appeared to receive same interventions apart from CR intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 173 (exercise 86; control 87) Diagnosis (% of participants): Aetiology: ischaemic; idiopathic; valvular; DCM; other NYHA: Class II‐IV LVEF: 26.4 (SD 6.8) Case mix: 100% as above Age (yr): 54 (SD 12.5) Male: 71.7% White: 60.1 Inclusion/exclusion criteria Inclusion: English‐speaking, age 18‐80 yr, NYHA II‐IV and LVSD with LVEF < 40% as documented by echocardiogram or radionuclide ventriculography within < 6 months, and sinus rhythm Exclusion: MI or recurrent angina within < 3 months, orthopaedic impediments to exercise, severe obstructive pulmonary disease with a forced expiratory volume < 1 L in 1 second as measured by spirometry, stenotic valvular disease as measured by echocardiogram, history of uncontrolled ventricular tachyarrhythmias (documented by electrophysiology study or 24‐hr Holter monitor), or absence of an implantable cardioverter‐defibrillator despite a history of sudden cardiac death |
|
| Interventions |
Exercise:Total duration: unclear (6 months or 1 year) Aerobic/resistance/mix: mix Frequency: 4 sessions/wk Duration: 10‐45 min Intensity: 40‐60% max HR Modality: walking Setting: home‐based Other: "After six weeks resistive training component involved both upper and lower extremity strengthening. Resistance training was prescribed at 80% of one repetition maximum, which is the maximal weight lifted one time, for 2 sets of 10 repetitions using seated biceps curls to strengthen the arms & seated lateral raises to strengthen shoulders. A second set of 10 repetitions at 80% of one repetition maximum was also prescribed…" |
|
| Outcomes | HRQoL (MLWHFQ), mortality, hospitalisation | |
| Comparison | Maintained usual level of daily activities. No exercise component | |
| Country and setting | USA Single centre |
|
| Follow‐up | 6 and 12 months (after randomisation) | |
| Notes | Home‐based exercise programme Subgroup analysis reported: Evangelista 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding reported for physical activity (accelerometer) outcome but not reported for other outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported |
| Intention‐to‐treat analysis? | Low risk | Although not reported as ITT analysis, groups did appear to be analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | "Two patients (one from the experimental and one from the control group) were lost to follow‐up within the first three months of enrollment. One was incarcerated and the second left the geographic area with no forwarding information. The remaining 173 patients compose the final study" |
| Groups balanced at baseline? | Low risk |
Current version: "There were no differences between the control and exercise groups at baseline with respect to sociodemographic variables (Table I) and most clinical characteristics. However, patients in the exercise group had a significantly higher likelihood of having a history of coronary heart disease and taking antiplatelet medication than in the control group" Our version: "There were no significant differences in any of baseline characteristics between the 2 groups, except for angiotensin‐converting enzyme (ACE) inhibitor; adherers were more likely to use ACE inhibitors than nonadherers (84% vs 60%; P = 0.039)" |
| Groups received same intervention? | Low risk | "Research nurses made home visits weekly for the first two weeks and then monthly to assess protocol adherence, correct use of the pedometer, and tolerance to the exercise program. The home visits also served as a form of attention control in the care‐ as‐usual group. All clinical questions were referred to the patient's cardiologist" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 28 (CBT 10; CBT and exercise 18) Diagnosis (% of participants): Aetiology: not reported NYHA: Class II 43.3%; Class III 56.7% (as a whole) LVEF: ≥ 15% Case mix: 100% as above Age (yr): 65.8 (SD 13.5) Male: 41.9% White: not reported Inclusion/exclusion criteria Inclusion: 1. documented medical diagnosis of HF; 2. LVEF ≥ 15% documented within the last year by echocardiogram, cardiac catheterisation ventriculography or radionuclide ventriculography; 3. receiving therapy for HF according to guidelines published by the American College of Cardiology American Heart recommendations (angiotensin‐converting enzyme inhibitors, diuretics, beta‐blockers, angiotensin receptor blockers, hydralazine and nitrate combination, etc.); 4. Hamilton Rating Scale for Depression (HAM‐D) score ≥ 11; 5. positive results on the Mini International Neuropsychiatric Interview (Mini) for minor or major depression and 6. DSM‐IV diagnosis for depression for 14 days; or 7 days if history of major depressive disorder in the last 6 months. Participants also had to be 1. English speaking, 2. living independently (non‐institutionalised) within 100 miles of Atlanta, GA, 3. able to respond to questions appropriately, 4. able to hear adequately to respond to verbal questions, 5. not involved in any structured exercise programme or walking 3 times/wk for a minimum of 20 min, 6. not participating in any psychotherapy and 7. not hospitalised within the last 60 days Exclusion: 1. suicide ideation according to psychiatric assessment or Mini evaluation; 2. major psychiatric co‐morbidity such as schizophrenia, personality disorder or dementia; 3. planned surgery; 4. not diagnosed with HF in the past 3 months; 5. renal insufficiency (serum creatinine >2.5 mg/dL); 6. uncontrolled hypertension; 7. acute bereavement or loss of significant other within the last month or currently involved in family crisis such as divorce; 8. any disorder interfering with independent ambulation; and 9. terminal illness such as cancer |
|
| Interventions |
Exercise:Total duration: 12 wk Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: 30‐45 min/session, max 1 hr Intensity: Borg < 15 ('moderate') Modality: walking Setting: home‐based Other: exercise + CBT group also received 12 wk weekly 1‐hr sessions of CBT for 12 wk. No other co‐interventions mentioned |
|
| Outcomes | HRQoL (MLWHFQ) and mortality | |
| Comparison | Usual care "Participants assigned to the UC [usual care] group received no information or counselling from their health care provider other than that normally provided" |
|
| Country and setting | USA Single‐centre |
|
| Follow‐up | 24 wk (after randomisation) | |
| Notes | Exercise group participants had 12 weekly face‐to‐face home visits by research nurse to monitor walking progress and to tailor the exercise prescription. "At the first home visit for EX, the research nurse (1) educated the patient on the rationale for EX in HF; (2) instructed on self‐monitoring of symptoms [dyspnea, heart rate (HR), fatigue] during walking; (3) provided the patient with a Polar monitor and instruction on how to use it; (4) provided patient with EX logs and instructions; (5) instructed on use of the 6‐ to 20‐point Borg's rate of perceived exertion (RPE) scale; (6) provided patient with blood pressure cuff and weight scale, if not available; and (7) observed participant response to walking out side home" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Data collectors were blinded to group assignment" |
| Selective reporting (reporting bias) | Low risk | Outcome described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | Although not stated, CONSORT diagram suggests groups analysed according to initial randomised allocation |
| Incomplete outcome data? | Low risk | QUORUM diagram and details of losses to follow‐up reported. In exercise group, 1 patient died and 3 withdrew at 24 wk. In usual care group, 2 participants and 1 participant withdraw at 12 and 24 wk, respectively. In combined CBT/exercise group 2 withdrew at 12 wk. 1 lost to follow‐up and 1 withdrew at 24 wk. In CBT group, 1 withdrew at 12 wk and 24 wk. 1 died and 1 lost to follow‐up at 24 wk |
| Groups balanced at baseline? | Low risk | "There were no BL differences between groups on any demographic or outcome variables" |
| Groups received same intervention? | Low risk | Groups appeared to receive same care other that exercise and CBT interventions |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 37 (exercise alone 20; control 17) CBT only group not included to this review Diagnosis (% of participants): Aetiology: not reported NYHA: Class II 43.3%; Class III 56.7% LVEF: ≥ 15% Case mix: 100% as above Age (yr): 65.8 (SD 13.5) Male: 41.9% White: not reported Inclusion/exclusion criteria Inclusion: 1. documented medical diagnosis of HF; 2. LVEF of ≥ 15% documented within the last year by echocardiogram, cardiac catheterisation ventriculography or radionuclide ventriculography; 3. receiving therapy for HF according to guidelines published by the American College of Cardiology American Heart recommendations (angiotensin‐converting enzyme inhibitors, diuretics, beta‐blockers, angiotensin receptor blockers, hydralazine and nitrate combination, etc.); 4. Hamilton Rating Scale for Depression (HAM‐D) score ≥ 11; 5. positive results on the Mini International Neuropsychiatric Interview (Mini) for minor or major depression; and 6. DSM‐IV diagnosis for depression for 14 days; or 7 days if history of major depressive disorder in the last 6 months. Participants also had to be 1. English speaking, 2. living independently (non‐institutionalised) within 100 miles of Atlanta, GA, 3. able to respond to questions appropriately, 4. able to hear adequately to respond to verbal questions, 5. not involved in any structured exercise programme or walking 3 times/wk for a minimum of 20 min, 6. not participating in any psychotherapy, and 7. not hospitalised within the last 60 days Exclusion: 1. suicide ideation according to psychiatric assessment or Mini evaluation; 2. major psychiatric co‐morbidity such as schizophrenia, personality disorder or dementia; 3. planned surgery; 4. not diagnosed with HF in the past 3 months; 5. renal insufficiency (serum creatinine >2.5 mg/dL); 6. uncontrolled hypertension; 7. acute bereavement or loss of significant other within the last month or currently involved in family crisis such as divorce; 8. any disorder interfering with independent ambulation; and 9. terminal illness such as cancer |
|
| Interventions |
Exercise:Total duration: 12 wk Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: 30‐45 min/session, max 1 hr Intensity: Borg < 15 ('moderate') Modality: walking Setting: home‐based Other: none reported |
|
| Outcomes | HRQoL (MLWHFQ) and mortality | |
| Comparison | Usual care "Participants assigned to the UC [usual care] group received no information or counselling from their health care provider other than that normally provided." |
|
| Country and setting | USA Single‐centre |
|
| Follow‐up | 24 wk | |
| Notes | Exercise group participants had 12 weekly face‐to‐face home visits by research nurse to monitor walking progress and to tailor the exercise prescription. "At the first home visit for EX, the research nurse (1) educated the patient on the rationale for EX in HF; (2) instructed on self‐monitoring of symptoms [dyspnea, heart rate (HR), fatigue] during walking; (3) provided the patient with a Polar monitor and instruction on how to use it; (4) provided patient with EX logs and instructions; (5) instructed on use of the 6‐ to 20‐point Borg's rate of perceived exertion (RPE) scale; (6) provided patient with blood pressure cuff and weight scale, if not available; and (7) observed participant response to walking out side home" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Data collectors were blinded to group assignment" |
| Selective reporting (reporting bias) | Low risk | Outcome described in methods were reported in results |
| Intention‐to‐treat analysis? | Low risk | Although not stated, CONSORT diagram suggests groups analysed according to initial randomised allocation |
| Incomplete outcome data? | Low risk | QUORUM diagram and details of losses to follow‐up reported. In exercise group, 1 participant died and 3 withdrew at 24 wk. In usual care group, 2 participants and 1 participant withdrew at 12 and 24 wk, respectively. In combined CBT/exercise group, 2 withdrew at 12 wk. 1 lost to follow‐up and 1 withdrew at 24 wk. In CBT group, 1 withdrew at 12 wk and 24 wk. 1 died and 1 lost to follow‐up at 24 wk |
| Groups balanced at baseline? | Low risk | "There were no BL differences between groups on any demographic or outcome variables" |
| Groups received same intervention? | Low risk | Groups appeared to receive same care other that exercise and CBT interventions |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 90, 45 each group Diagnosis (% of participants): Aetiology: HF secondary to idiopathic DCM; ischaemic heart disease; valvular disease NYHA: Class II‐III LVEF: exercise 25% (SD 4); control 25% (SD 4) Case mix: 100% Age (yr): exercise 60 (SD 7); control 61 (SD 7) Male: not reported White: not reported Inclusion/exclusion criteria Inclusion: 1. HF secondary to idiopathic DCM, ischaemic heart disease or valvular disease; 2. echocardiographic ejection fraction < 35%; 3. clinical stability for at least 3 months under optimised therapy; 4. NYHA functional Class II to III; 5. peak oxygen uptake (VO2) < 20 mL/kg/min; and 6. echocardiographic images of adequate quality for quantitative analysis Exclusion: any systemic disease limiting exercise, hypertrophic cardiomyopathy, valvular disease requiring surgery, angina pectoris, sustained ventricular arrhythmias, severe hypertension, excess variability (> 10%) at baseline cardiopulmonary exercise test and inability to participate in a prospective study for any logistic reason |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: aerobic Frequency: 3‐5 sessions/wk Duration: 30 min Intensity: 60% peak VO2 Modality: exercise cycle, daily brisk walk, callisthenic. In addition, requested to take brisk daily walk of > 30 min Setting: supervised cycling sessions at rehabilitation centre and unsupervised at home Other: not reported |
|
| Outcomes | Mortality and morbidity | |
| Comparison | Educational support but no formal exercise protocol | |
| Country and setting | Italy Multicentre (15 CR units) |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods are reported |
| Intention‐to‐treat analysis? | Low risk | Although not stated, it is clear from CONSORT diagram that 2 groups were analysed according to ITT |
| Incomplete outcome data? | Low risk | 45/45 (100%) exercise training group and 44/45 (98%) available at 6 months' follow‐up |
| Groups balanced at baseline? | Low risk | "No significant differences were observed between the 2 groups with respect to demographic and clinical data, including age, weight, cause of heart failure, or New York Heart Association functional class. Furthermore, there was no difference between the 2 groups in the medications received during the 6‐month period of the study" |
| Groups received same intervention? | Unclear risk | Not clearly stated if co‐treatments (i.e. cardiovascular medication) in 2 groups were the same |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 20 (exercise 10; control 10) Diagnosis (% of participants): Aetiology: IHD; DCM NYHA: Class II 90%; Class III 10% LVEF: exercise mean 26.1% (SD 6); control mean 24.7% (SD 8) Case mix: 100% as above Age (yr): exercise 55 (SD 6); control 53 (SD 9) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: age < 70 yr with CHF (NYHA II to III) as result of DCM or IHD as assessed by cardiac catheterisation. All had clinical, radiological and echocardiographic signs of CHF and an LVEF 40% as assessed by ventriculography and clinically stable condition for > 3 months before enrolment Exclusion: significant valvular heart disease, uncontrolled hypertension, peripheral vascular disease, pulmonary disease or musculoskeletal abnormalities precluding exercise training |
|
| Interventions |
Exercise:Total duration: 2 wk inpatient followed by 6 months as outpatient Aerobic/resistance/mix: aerobic Frequency: 7 sessions/wk Duration: 20 min/session Intensity: 70% symptom limited VO2 max Modality: cycle ergometers Setting: supervised sessions at hospital and home‐based unsupervised sessions Other: expected to participate in 1 group training session (walking, callisthenics and non‐competitive ball games) of 60 min each wk. Participants were asked to exercise for 20 min/day at home |
|
| Outcomes | Mortality | |
| Comparison | Continued their sedentary lifestyle and remained on their individually tailored cardiac medication supervised by their private physicians | |
| Country and setting | Switzerland Single centre |
|
| Follow‐up | 26 wk (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | Although ITT analysis not reported, groups do appear to be analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | No loss to follow‐up |
| Groups balanced at baseline? | Low risk | "Patients in the training group and in the control group showed a significantly reduced left ventricular ejection fraction (training group: 26.1 ±3.1%, control group: 24.7± 2.4%; NS [not significant]) and exercise capacity as determined by peak oxygen uptake (training group: 20.3 ±1.0 ml/kg min, control group: 17.9 ±1.6 ml/kg min; P NS)" |
| Groups received same intervention? | Unclear risk | Details of co‐interventions not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 33 Diagnosis (% of participants): Aetiology: ischaemic or primary NYHA: Class II or III LVEF: exercise 22% (SD 8); control 25% (SD 10) Case mix: 100% as above Age (yr): exercise 67 (SD 7); control 64 (SD 10) Male: exercise 15/16 (94%); control 11/14 (79%); total 87% White: not reported Inclusion/exclusion criteria Inclusion: NYHA Class II‐III for at least 3 months and were on stable medications for the past 1 month. All participants were on maximal medical therapy with angiotensin‐converting enzyme inhibitors, diuretic and digoxin. All participants had EF < 40% by nuclear ventriculography. No participants had obstructive valvular disease, MI within 3 months, or limitation of exercise secondary to angina or new arrhythmias Exclusion: not reported |
|
| Interventions |
Exercise:Total duration: 3 months Aerobic/resistance/mix: aerobic Frequency: 3 session/wk Duration: 30 min Intensity: Borg 12‐13 Modality: bike and treadmill Setting: supervised sessions at medical centre by a nurse or exercise physiologist Other: Care provided by specialist HF physician |
|
| Outcomes | HRQoL (MLWHFQ and MOS SF‐36 questionnaire), mortality, morbidity | |
| Comparison | Usual medical care Other: care provided by specialist HF physicians |
|
| Country and setting | USA Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | MLWHF, MOS SF‐36 results not reported for the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported |
| Intention‐to‐treat analysis? | Unclear risk | Not reported |
| Incomplete outcome data? | Low risk | Yes, QUORUM flow diagram reported Unclear how loss to follow‐up, drop‐out and cross‐over dealt with |
| Groups balanced at baseline? | Low risk | "There were no differences at baseline between patients randomised to the control group and those randomised to the exercise program" |
| Groups received same intervention? | Low risk | "Medical follow‐up of both the control and intervention patients groups was provided by specialized heart failure physicians" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 22 (exercise 12; control 10) Diagnosis (% of participants): Aetiology: DCM 86%, ischaemic heart disease 14% NYHA: Class II (55%); Class III (45%) LVEF: exercise 26% (SD 9); control 27% (SD 10) Case mix: 100% as above Age (yr): exercise 50 (SD 12); control 52 (SD 8) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: EF < 40% as assessed by radionucleotide scintigraphy, and a reduced fractional shortening < 30% assessed by echocardiography; willingness to participate in the study for the next 6 months; and a permanent residence within 25 km of the training facility. Physical work capacity at baseline > 25 watts without signs of myocardial ischaemia (i.e. angina or ST segment depression). Clinically stable > 3 months Exclusion: exercise‐induced myocardial ischaemia or ventricular tachyarrhythmias (higher then Lown Class IVa), valvular heart disease, uncontrolled hypertension, peripheral vascular disease, COPD, and orthopaedic or other conditions precluding regular participation in exercise sessions |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 4‐6 sessions/wk Duration: 10‐60 min/session, 1 hr at home Intensity: 70% VO2max Modality: cycling, walking, ball games and callisthenics Setting: first 3 wk supervised hospital‐based training; thereafter home‐based Other: none |
|
| Outcomes | Morbidity and mortality | |
| Comparison | After discharge medical therapy continued and participants supervised by private physician | |
| Country and setting | Germany Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods, reported in results |
| Intention‐to‐treat analysis? | Unclear risk | Not reported |
| Incomplete outcome data? | Low risk | Drop‐outs and clinical events are fully reported for both groups. No imputation undertaken |
| Groups balanced at baseline? | Low risk | "There were no significant differences in baseline variables between the training and control groups" |
| Groups received same intervention? | Unclear risk | The exercise group had 3 wk of hospital stay, the control only 3 days. The control group follow‐up with private physician. No comment on follow‐up of intervention group |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 20 (exercise 10, control 10) Diagnosis (% of participants): Aetiology: IHD 35%; DCM 65% NYHA: Class II 65%; Class III 35% LVEF: exercise mean 24% (SD 13); control mean 23% (SD 10%) Case mix: as above Age (yr): exercise 54 (SD 9); control 56 (8) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: age < 70 yr, with CHF as a result of DCM or IHD, LVEF < 40% Exclusion: DM, hypertension, overt atherosclerotic PVD, hypercholesterolaemia, ventricular tachycardia, COPD and primary valvular disease |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 2‐6 sessions/day Duration: 10‐20 min/session Intensity: 70% VO2 max Modality: bike ergometer Setting: supervised hospital‐based sessions and unsupervised home‐based sessions Other: not reported |
|
| Outcomes | Mortality | |
| Comparison | Description: stayed on previous medication, continued sedentary lifestyle, and supervised by their private physicians | |
| Country and setting | Germany Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | It appears that groups are analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | Detailed description of losses to follow‐up and drop‐outs reported |
| Groups balanced at baseline? | Low risk | "At baseline, patients in the control group did not differ significantly from those in the training group with respect to age, aetiology of heart failure, NYHA functional class, duration of heart failure, LVEF [lett ventricular ejection fraction] or LVEDD [Left Ventricular End Diastolic Diameter]" |
| Groups received same intervention? | Low risk | "Patients were on angiotensin‐converting enzyme inhibitors (100% in both groups), diuretics (training group 82%, control 70%), and digoxin (training 73%, control 70%, P5NS). Drug treatment did not change between 4 weeks before enrolment and study termination" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 73 (exercise 36; control 37) Diagnosis (% of participants): Aetiology: IHD 16%; DCM 84% NYHA: Class I and II 74%; Class III 26% LVEF: 29% (SD 9) Case mix: 100% as above Age (yr): exercise 54 (SD 9); control 54 (SD 8) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: documented HF by signs, symptoms and angiographic evidence of reduced left ventricular function (LVEF < 40%) as a result of DCM or IHD; physical work capacity at baseline > 25 watts, clinical stability >=3 months before study start Exclusion: significant valvular heart disease, uncontrolled hypertension, DM, hypercholesterolaemia, PVD, pulmonary disease, musculoskeletal abnormalities precluding exercise training |
|
| Interventions |
Exercise:Total duration: 6‐months Aerobic/resistance/mix: aerobic Frequency: 6 or 7 sessions/wk Duration: 10‐20/session Intensity: 70% of peak VO2 Modality: cycle ergometer Setting: first 2 wk in hospital, remainder home based Other: plus group sessions 1 hr twice weekly, walking, ball games and callisthenics |
|
| Outcomes | Mortality | |
| Comparison | Continued individually tailored cardiac medications, supervised by their physicians | |
| Country and setting | Germany Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to either a training group or an inactive group sing a list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Not reported |
| Incomplete outcome data? | Low risk | QUORUM diagram and details of losses to follow‐up reported |
| Groups balanced at baseline? | Low risk | "No significant differences were observed between the two groups with regard to demographic or clinical data, including age, weight, LVEF, LVEDD [Left Ventricular End Diastolic Diameter], NYHA or maximum oxygen uptake" |
| Groups received same intervention? | Unclear risk | The co‐interventions in the control group not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 2331 (exercise 1159; control 1172) Diagnosis (% of participants): Aetiology: IHD 51% NYHA: Class II 63%; Class III 35%; Class IV 1% LVEF: 25% (SD not reported) Case mix: 100% as above Age (yr): exercise 59 (SD not reported); control 59 (SD not reported) Male: 72% White: 62% Inclusion/exclusion criteria Inclusion: LVEF < 35%, NYHA Class II‐IV HF for the previous 3 months despite a 6‐wk period of treatment, optimal HF therapy at stable doses for 6 wk before enrolment or documented rationale for variation, including intolerance, contraindication, participant preference and personal physicians judgement, sufficient stability, by investigator judgement, to begin an exercise programme Exclusion: (selected) age <18 yr, co‐morbid disease or behavioural or other limitations that interfere with performing exercise training or prevent the completion of 1 yr of exercise training, major cardiovascular event or cardiovascular procedure, including implantable cardioverter defibrillator use and cardiac resynchronisation, within the previous 6 wk |
|
| Interventions |
Exercise:Total duration: 30 months Aerobic/resistance/mix: aerobic Frequency: 3‐5 sessions/wk Duration: 15‐35 min/session Intensity: 60‐70% of HR reserve Modality: cycling or walking Setting: First 36 sessions were supervised then advised to follow 5 day/wk home‐based exercise programme Other: none reported |
|
| Outcomes | Mortality, hospitalisation, HRQoL (KCCQ), cost‐effectiveness | |
| Comparison | Usual care: all participants, regardless of group allocation, received self management educational materials consistent with guidelines of American College of Cardiology and American Heart Association | |
| Country and setting | USA Multicentre |
|
| Follow‐up | Median 30.1 months (after randomisation) | |
| Notes | Authors contacted for further details of outcome findings but no information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The trial uses a permuted block randomization scheme stratified by center and by the etiology of the patient's heart failure (ischemic vs nonischemic)" |
| Allocation concealment (selection bias) | Low risk | "Patients are randomized at the enrolling centers using an interactive voice response" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Event outcomes were blinded |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | |
| Incomplete outcome data? | Low risk | QUORUM diagram and details of losses to follow‐up reported |
| Groups balanced at baseline? | Low risk | Table 1 of the publication shows 2 groups are well balanced |
| Groups received same intervention? | Low risk | "All patients, regardless of group allocation, received self‐management educational materials...consistent with guidelines of American College of Cardiology and American Heart Association" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 169 (exercise 84; control 85) Diagnosis (% of participants): Aetiology: data not available NYHA: Class I 6%; Class II 74%; Class III 20% LVEF: ≤ 40% Age (yr): exercise 65.9 (SD 12.5); control 70 (SD 12.5) Male: 75% White: 85.1% Inclusion/exclusion criteria Inclusion: LVEF ≤ 40% on echocardiogram and had a severity of at least NYHA group II in the previous 24 months. They had to have been clinically stable for 4 wk and in receipt of optimal medical treatment and in care of a specialist HF nurse team from 2 acute hospital trusts and 1 primary care trusts and not considered high‐risk for a home‐based exercise programme Exclusion: NYHA Class IV, MI or revascularisation within the past 4 months, hypotension, unstable angina, ventricular or symptomatic arrhythmias, obstructive abortive valvular disease, COPD, hypertrophic obstructive cardiomyopathy, severe musculoskeletal problems preventing exercise, and case‐note reported dementia or current severe psychiatric disorder |
|
| Interventions | Exercise:Total duration: 6 months programme progressive with aim that participants achieved the following: Aerobic/resistance/mix: mix Frequency: 5 times/wk Duration: 20‐30 min Intensity: 70% of peak VO2 or Borg 12‐13 Modality: aerobic and resistance elements (upper and lower limb exercises) Setting: first 3 sessions supervised centre‐based followed by home‐based programme with home‐visits by nurse at 4, 10 and 20 wk and telephone support at 6, 15 and 24 wk. Intervention manual provided Other: specialist HF nurse care | |
| Outcomes | HRQoL (MLWHFQ), composite of death, hospital admissions, generic quality of life (EQ‐5D) | |
| Comparison | Specialist HF nurse care | |
| Country and setting | UK West‐midlands, community |
|
| Follow‐up | 6‐ and 12‐month follow‐up (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent clinical trials unit using a computerized programme undertook randomization after each patient had consented and undergone the baseline tests and questionnaire" |
| Allocation concealment (selection bias) | Low risk | "An independent clinical trials unit using a computerized programme undertook randomization after each patient had consented and undergone the baseline tests and questionnaire" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "…, the nurse undertaking the assessment was blinded to the treatment allocation of the patient, but owing to staffing issues, this occurred in only 62% of participants followed up at 6 months" |
| Selective reporting (reporting bias) | Low risk | All of primary and majority of secondary outcomes described in methods reported Stated in methods that blood pressure and incremental shuttle walking test were not collected at 12 months |
| Intention‐to‐treat analysis? | Low risk | "...between‐ and within‐group analyses for primary and secondary outcomes at 6 and 12 months were performed according to intention to treat" |
| Incomplete outcome data? | Low risk | Drop‐outs and clinical events are fully reported Outcome available for 161 (95%) participants at 6 months and 157 (92%) participants at 12 months. Non‐imputed data reported and sensitivity analysis undertaken to examine impact of missing data |
| Groups balanced at baseline? | Unclear risk | "Baseline characteristics were broadly comparable, the exception being that the exercise group was somewhat younger and had higher HADS depression scores and a lower systolic blood pressure" |
| Groups received same intervention? | Low risk | "Both groups received specialist heart failure nurse input in primary and secondary care through clinic and home visits that included the provision of information about heart failure, advice about self‐management and monitoring of their condition, and titration of beta‐blocker therapy" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 43 (exercise 21; control 22) Diagnosis (% of participants): Aetiology: ischaemic 79%; AF 12%; valvular 7%; hypertension 2% NYHA: Class II and III LVEF: exercise 41.5 (SD 13.6); control 40.6% (SD 13.7) Case mix: as above Age (yr): exercise 68 (SD 7); control 69 (SD 5) Male: 79% White: not reported Inclusion/exclusion criteria Inclusion: CHF diagnosis, on CHF medication, clinical symptoms of CHF, clinically stable > 3 months before study entrance, fulfil 1 of the following criteria: previous MI, hospitalised because of CHF, lung oedema and cardiac enlargement on X‐ray Exclusion: chronic obstructive lung disease, orthopaedic disabilities, psychiatric disabilities, cancer, senility and age > 80 yr |
|
| Interventions |
Exercise:Total duration: 5 months Aerobic/resistance/mix: mix Frequency: 2 sessions/wk Duration: 45 min Intensity: not reported Modality: cycling, free weights and elastic rubber‐bands (Thera‐bands) Setting: hospital outpatients, supervised by physiotherapists Other: training group had 3 educational lectures, about nutrition, physical activity and relaxation in addition to the exercise programme |
|
| Outcomes | Rehospitalisation and mortality | |
| Comparison | Usual medical care (continued their previous level of physical activity, which varied from performing little physical activity up to taking a daily walk outdoors) | |
| Country and setting | Iceland Single centre |
|
| Follow‐up | 12 and 28 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Although not reported as ITT analysis, groups did appear to be analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | No losses to follow‐up |
| Groups balanced at baseline? | Low risk | Table 2 of the publication suggests 2 groups are well balanced |
| Groups received same intervention? | Low risk | Yes, both groups appeared to receive same interventions apart from CR intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 40 (exercise 21; control 19) Diagnosis (% of participants): Aetiology: DCM 40%; IHD 60% NYHA: Class II 67.5%; Class III 32.5% LVEF: 21% (SD 7) Case mix: 100% as above Age (yr): 56 (SD 11) Male: 100% White: 62.5% (remainder black) Inclusion/exclusion criteria Inclusion: NYHA Class II or III, resting EF < 35% measured by echocardiography or gated equilibrium radionuclide angiography and no change in medical therapy >=30 days before randomisation. Exclusion: AF, acute MI ?3 months, angina pectoris at rest or induced by exercise, current enrolment in another clinical trial, and current participation in a regular exercise programme (at least twice weekly) |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk (rate of perceived exertion 12‐14) Duration: 33 min Intensity: 60‐80% peak HR Modality: treadmills, stationary cycles, rowing machines and arm ergometers Setting:outpatient clinic Other: none reported |
|
| Outcomes | Morality and hospital admissions | |
| Comparison | Usual medical care Participants were instructed to maintain their normal daily activity habits and not to begin an exercise regimen |
|
| Country and setting | North America Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | Authors contacted for further details of outcome findings but no information provided. Each participant's physician was asked not to change drug regimen during the study, if possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the exercise group or the control group" |
| Allocation concealment (selection bias) | Unclear risk | "Each patient's assignment was sealed in an envelope until completion of the second exercise test" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | "Of the 40 patients entered into the study, only those who also completed the exercise tests at weeks 12 and 24 were considered in the data analysis" |
| Incomplete outcome data? | Low risk | "Fifteen patients in the exercise group completed the study. Two patients dropped out because of noncardiac medical conditions (progressive, limiting arthritis in one patient and newly diagnosed cancer in the other) that developed within 1 month of the start of the exercise program. One patient developed atrial fibrillation between week 12 and week 24; 3 other patients stopped exercising for personal reasons before week 12 and refused follow‐up testing. Fourteen of the 19 patients in the control group completed the study. Two dropped out for personal reasons and refused follow‐up testing, one developed atrial fibrillation between week 12 and week 24, one was hospitalized at week 22 for an acute myocardial infarction, and one died suddenly" |
| Groups balanced at baseline? | Low risk | "Among patients who completed the study, no differences in demographic characteristics were seen between the two study groups after randomization" |
| Groups received same intervention? | Unclear risk | The co‐interventions in the control group not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 50 (exercise 25; control 25) Diagnosis (% of participants): Aetiology: IHD 100% NYHA: Class II: exercise 56%; control 60%; Class III: exercise 44%; control 40% LVEF: exercise mean 27.4% (SD 5.7); control: 28.5% (SD 5.2) Case mix: 100% as above Age (yr): exercise 59.6 (SD 10.2); control 61.2 (SD 9.5) Male: exercise 80%; control 72% White: not reported Inclusion/exclusion criteria Inclusion: ischaemic HF in NYHA Classes II and III of > 6 months, clinically stable > 6 wk and LVEF < 35% Exclusion: uncontrolled arterial hypertension; history of major ventricular arrhythmias, acute coronary syndrome, percutaneous coronary intervention or brain event 3 months prior to the study; AF or other arrhythmia making it impossible to perform MRI; previous coronary artery bypass grafting; implantable cardiodefibrillator; permanent pacemaker or the presence of metal parts in the body; signs of osteoarticular dysfunction excluding participation in physical training; DM; COPD and anaemia |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: 25 min/session Intensity: 80% predicted HR at VO2 max Modality: cycling Setting: centre‐based Other: none reported |
|
| Outcomes | Mortality | |
| Comparison | Standard medical care only | |
| Country and setting | Poland Single centre |
|
| Follow‐up | 26 wk (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Not implicit but numbers used suggest that groups analysed according to randomised allocation |
| Incomplete outcome data? | Low risk | No participants lost to follow‐up |
| Groups balanced at baseline? | Low risk | "At baseline the groups did not differ significantly in clinical characteristics. The only exception was smoking, the training group consisted of significantly more ex‐smokers" |
| Groups received same intervention? | Unclear risk | Not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 42 (exercise group A 14; control 14) Diagnosis (% of participants): Aetiology: ischaemic 100% NYHA: Class II/III exercise group A 55%; control 100% LVEF: exercise group A: mean 33.6% (SD 3.6); control 33.2% (SD 3.8) Case mix: 100% as above Age (yr): exercise group A 54 (SD 7); control 55 (SD 9) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: stable CHF, LVEF < 40% on echocardiography =<1 month before inclusion, age < 65 yr Exclusion: moderate or severe pulmonary disease, orthostatic blood pressure fall (> 20 mmHg), or with MI, unstable angina, heart surgery or coronary angioplasty within 3 months prior to inclusion as well as inability to perform bicycle training |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: group A ‐ 20 min/session (4‐min constant workload with 1 min rest repeated 5 times) Intensity: group A ‐ 60% max HR Modality: cycle ergometer Setting: CR, outpatient unit under supervision of the physician and rehabilitation specialist Other: none reported |
|
| Outcomes | HRQoL (Psychological General Wellbeing Index) | |
| Comparison | Description: controls were asked not to change their degree of physical activity during the study | |
| Country and setting | Poland Single centre |
|
| Follow‐up | 26 wk (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Results of baseline QoL examinations were not known to the patients and their physicians or to the persons performing the randomisation" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | It appears that groups were analysed according to initial random allocation |
| Incomplete outcome data? | Unclear risk | No information presented on loss on loss to follow‐up or drop‐outs |
| Groups balanced at baseline? | Low risk | "At baseline there were no significant differences in between groups in left ventricular ejection fraction and other basic parameters of left ventricular function." "At the start of the study, mean PGWB [Psychological General Wellbeing Index] total index was similar in groups A and B. Controls had lower total index than patients in group B" |
| Groups received same intervention? | Unclear risk | Details of co‐interventions not reported although degree of follow‐up was stated to be equivalent |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 42 (exercise group B 14; control 14) Diagnosis (% of participants): Aetiology: ischaemic 100% NYHA: Class II/III exercise group B 75%; control 100% LVEF: exercise group B: mean 34.2% (SD 4.2); control 33.2% (SD 3.8) Case mix: 100% as above Age (yr): exercise group B: 57 (SD 8); control 55 (SD 9) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: stable CHF, LVEF < 40% on echocardiography =< 1 month before inclusion, age < 65 yr Exclusion: moderate or severe pulmonary disease, orthostatic blood pressure fall (> 20 mmHg), or with MI, unstable angina, heart surgery or coronary angioplasty within 3 months prior to inclusion as well as inability to perform bicycle training |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: group B: 25 min/session (exercise workload gradually increased after each 5‐min training period to a total of 25 min) Intensity: group B: up to 75% max HR Modality: cycle ergometer Setting: CR, outpatient unit under supervision of the physician and rehabilitation specialist Other: none reported |
|
| Outcomes | HRQoL (Psychological General Wellbeing Index) | |
| Comparison | Controls were asked not to change their degree of physical activity during the study | |
| Country and setting | Poland Single centre |
|
| Follow‐up | 26 wk (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Results of baseline QoL examinations were not known to the patients and their physicians or to the persons performing the randomisation" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | It appears that groups were analysed according to initial random allocation |
| Incomplete outcome data? | Unclear risk | No information presented on loss on loss to follow‐up or drop‐outs |
| Groups balanced at baseline? | Low risk | "At baseline there were no significant differences in between groups in left ventricular ejection fraction and other basic parameters of left ventricular function." "At the start of the study, mean PGWB [Psychological General Wellbeing Index] total index was similar in groups A and B. Controls had lower total index than patients in group B" |
| Groups received same intervention? | Unclear risk | Details of co‐interventions not reported although degree of follow‐up was stated to equivalent |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 26 (exercise 16; control 10) Diagnosis (% of participants): Aetiology: DCM 7%; ischaemic 100% NYHA: Class II 58%; Class III 42% LVEF: < 40% Case mix: 100% as above Age (yr): exercise 52 (SD 9); control 53 (SD 11) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion:aetiology of CHF was either ischaemic heart disease or DCM. Diagnosis of CHF was mainly based on clinical signs (NYHA Class II and III), radiological findings, and echocardiographically determined EF < 40% and shortening fraction < 30% Exclusion: recent MI or unstable angina; aortic stenosis; DM; uncontrolled hypertension; musculoskeletal limitations or other contraindications for participating in an exercise training programme; documented exercise‐induced severe ischaemia or serious arrhythmias or both |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: mix Frequency: 3 or 4 sessions/wk Duration: 60 min/session Intensity: 50‐75% peak VO2 Modality: cycle ergometer, walking or jogging, stair climber and step‐aerobics Plus 'light' resistance exercise (not defined) Setting: supervised exercise training programme at institution Other: none reported |
|
| Outcomes | HRQoL (MLWHFQ and Spritzer Quality of Life Index) | |
| Comparison | Not reported | |
| Country and setting | Greece Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The psychological tests were assessed from all patients in the first week of admission, before randomization to study groups and the end of the study by the same physician, who was not familiar with the patients" |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in methods are reported |
| Intention‐to‐treat analysis? | Low risk | Not stated explicitly but appear to analysed according to initial group allocation |
| Incomplete outcome data? | Unclear risk | Losses to follow‐up, drop‐outs not reported |
| Groups balanced at baseline? | Low risk | "The two groups of patients participating in the study were similar as regards their clinical data" |
| Groups received same intervention? | Unclear risk | Not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 181 (exercise 90; control 91) Diagnosis (% of participants): Aetiology: ischaemic 76%; hypertensive 7%; valvular 5%; other 12% NYHA: Class I‐III LVEF: < 40% Case mix: 100% as above Age (yr): exercise 64.8±1.1 (SD 10.5); control 66.1 (SD 9.4) Male: control 80; exercise 82 White: not reported Inclusion/exclusion criteria Inclusion: documented clinical signs and symptoms of HF; LVEF < 40%; NYHA Functional Class I‐III; 6‐min walk test distance < 500 m Exclusion: inability to attend regular exercise training sessions; exercise testing limited by angina or leg claudication; abnormal blood pressure response to exercise testing (systolic blood pressure during exercise > 250 mm Hg or diastolic blood pressure response > 15 mm Hg, systolic blood pressure response decrease of > 20 mm Hg after a normal increase or decrease below the resting level); cerebrovascular or musculoskeletal disease preventing exercise testing or training; respiratory limitation (forced expired volume in 1 second, or vital capacity < 60% of predicted, or both); poorly controlled cardiac arrhythmias and any non‐cardiac condition affecting regular exercise training or decreasing survival |
|
| Interventions |
Exercise:Total duration: 9 months (3 supervised, 6 home based) Aerobic/resistance/mix: mix Frequency: 2 sessions/wk Duration: aerobic; 30 min/session Intensity: aerobic: 60‐70% max HR. Resistance: 40% of 1‐repetition maximum, with 10 repetitions for the arm exercises and 15 repetitions for the leg exercises, with an increase over 5 wk to an intensity of 60% of 1‐repetition maximum and a total of 3 sets of each exercise per session Modality: aerobic: cycle, treadmill and arm ergometry exercise. Resistance: arm curl, knee extension and leg press performed individually with each limb After 3 months of supervised training, participants in the exercise group were provided an exercise cycle and set of free weights with instructions to continue training at home 3 times/wk for the remainder of the study Setting:Supervised for 3 months at rehabilitation centre and unsupervised for 9 months at home Other: none reported |
|
| Outcomes | HRQoL (MLWHFQ), mortality, composite of mortality and hospital admission for HF | |
| Comparison | Usual medical care. Control participants were not provided with a formal exercise prescription but were encouraged to continue their usual level of physical activity and were not discouraged from regular physical activity | |
| Country and setting | Canada Multicentre |
|
| Follow‐up | 12 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The predetermined allocation sequence was based on a stream of computer‐generated pseudorandom numbers from a uniform distribution stratified by center and with a blocking factor of 4" |
| Allocation concealment (selection bias) | Low risk | "Eligible patients were registered in a log and treatment group determined by opening the next sequential study allocation envelope" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Outcome measures were performed in a blinded fashion. Individuals responsible for supervising and recording the results of the outcome measurements were unaware of the patients group assignment" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | Although ITT analysis not reported, groups do appear to analysed according to original randomised allocation |
| Incomplete outcome data? | Low risk | "In the control group, 83 patients completed 3 months of follow‐up (reasons for incompletion: death 3; other problems 4; worsening heart failure 1) and 75 patients completed 12months of follow‐up (reasons for incompletion: death 8; withdrawal 2; other problems 3; worsening heart failure 2; refused testing 1) For the exercise group, 80 patients completed 3 months of follow‐up (reasons for incompletion: death 1; withdrawal 5; other problems 1; worsening failure 2; refused testing 1) and 64 patients completed 12 months of follow‐up (reasons for incompletion: death 9; withdrawal 6; other problems 7; worsening heart failure 3; refused testing 1)" No imputation or sensitivity analysis undertaken to assess impact of loss to follow‐up |
| Groups balanced at baseline? | Low risk | "There were no differences between the control and exercise training groups with respect to age, resting ejection fraction, New York Heart Association class, cause of heart failure, or duration of heart failure" |
| Groups received same intervention? | Unclear risk | "All patients were reviewed monthly throughout the study" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 50 (exercise 25; control 25) Diagnosis (% of participants): Aetiology: ischaemic; DCM (% not reported) NYHA: not reported LVEF: < 40% (% not reported) Case mix: 100% as above Age (yr): 55 (SD 10) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: CHF documented by clinical, angiographic or echocardiographic criteria; and resting EF < 40% Exclusion: not reported |
|
| Interventions |
Exercise:Total duration: 1 month Aerobic/resistance/mix: aerobic Frequency: 5 sessions/wk Duration: 30 min/session cycling, 90 min walking each day Intensity: Borg 12‐14 (60‐80% max HR) Modality: cycling and walking Setting: indoor cycling sessions were supervised directly by a medical resident and outdoor walking sessions were supervised by exercise physiologists Other: resided at the rehabilitation centre for 1 month. Programme component also included education and low‐fat meals prepared daily by the centre's cook |
|
| Outcomes | Morbidity and mortality | |
| Comparison | Usual medical care | |
| Country and setting | Switzerland Single centre |
|
| Follow‐up | 6.2 yr (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods are reported in the results |
| Intention‐to‐treat analysis? | Low risk | ITT not stated explicitly. However, groups appear to analysed according to original allocation |
| Incomplete outcome data? | Low risk | "Data from one patient in the control group was not available at the two‐month evaluation due to refusal to complete testing." "Among subjects in the exercise group, 9 died, and one refused repeat testing. Among patients in the control group, 12 died and two refused repeat testing. Therefore, 14 and 13 patients performed six‐year evaluations in the exercise and control groups, respectively." QUORUM diagram reported and detailed text. No imputation undertaken |
| Groups balanced at baseline? | Low risk | "No differences were observed between the exercise and control groups initially in clinical or demographic data, including age, height, weight, pulmonary function or medication status" |
| Groups received same intervention? | Unclear risk | "Patients in the exercise group resided at the rehabilitation centre for one month. Control subjects received usual clinical care, including verbal encouragement to remain physically active" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 25 (exercise 12; control 13) Diagnosis (% of participants): Aetiology: ischaemic 100% NYHA: not reported LVEF: exercise 31.5% (SD 7); control 33.3% (SD 6) Case mix: 100% as above Age (yr): exercise 56 (SD 5); control 55 (SD 7) Male: 100% White: not reported Inclusion/exclusion criteria Inclusion: MI, diagnosis of HF and stable symptoms, LVEF < 40% Exclusion: pulmonary disease |
|
| Interventions |
Exercise:Total duration: 2 months Aerobic/resistance/mix: aerobic Frequency: walking: 2 sessions/daily; cycling: 4 sessions/wk Duration: walking: 1 hr; cycling: 45 min Intensity: walking: not reported; cycling: 60‐70% peak VO2 Modality: walking and cycling Setting: centre based with supervised by physicians Other: exercise groups received education sessions and low‐fat meals prepared 3 times daily |
|
| Outcomes | Hospitalisation and mortality | |
| Comparison | Usual clinical follow‐up | |
| Country and setting | Switzerland Single‐centre |
|
| Follow‐up | 2 and 12 months (after randomisation) | |
| Notes | "After the initial 2‐months exercise training or control period, both groups were encouraged to remain physically active over the subsequent 10 months, although no formal program was implemented" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Although not explicit, participants appeared to be analysed according to initial random allocation |
| Incomplete outcome data? | Low risk | Lost to follow‐up reported |
| Groups balanced at baseline? | Low risk | "No differences were observed between the 2 groups initially in clinical or demographic data, including age, height, weight, resting blood pressure, pulmonary function, ejection fraction, or maximal oxygen uptake" |
| Groups received same intervention? | Low risk | Yes, both groups appeared to receive same interventions apart from CR intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 80 (exercise 40; control 40) Diagnosis (% of participants): Aetiology: ischaemic cardiomyopathy 69%; idiopathic DCM 18%; hypertensive HF 13% NYHA: Class II 47%; Class III 35% LVEF: exercise 31% (SD 8); control 31% (SD 9) Case mix: 100% as above Age (yr): 70.1 (SD 7.9) Male: 79% White: not reported Inclusion/exclusion criteria Inclusion: stable CHF and a LVEF < 40% or ≥ 40% with clinical symptoms of diastolic HF Exclusion: acute MI within 4 wk; unstable angina pectoris; serious rhythm disturbance; symptomatic PVD; severe CPOD, with a forced expiratory vital capacity < 50% of expected measured by spirometry; 6‐min walking distance > 550 m; and work load on the cycle ergometer test > 110 watts, significant co‐morbidities that would prevent entry into the study due to terminal disease or an inability to exercise (e.g. severe musculoskeletal disorder, advanced valvular disease) or were in long‐term care establishments |
|
| Interventions |
Exercise:Total duration: 4 months Aerobic/resistance/mix: aerobic Frequency: 2 sessions/wk Duration: 50 min Intensity: 15‐18 on Borg scale Modality: fast walking, side stepping and leg lifts in combination with overhead arm reaches Setting: hospital outpatient department Other: 15‐30 min counselling for participants in exercise group with CHF nurse (4 hr in total) |
|
| Outcomes | HRQoL (MLWHFQ) and mortality | |
| Comparison | The control group was not provided with exercise prescriptions and encouraged to continue their usual levels of physical activity | |
| Country and setting | Norway Single centre |
|
| Follow‐up | 12 months (after randomisation) | |
| Notes | All training sessions were supervised by physiotherapist, a specialist in heart rehabilitation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "computer‐generated table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Three physicians and 3 nurses who were blinded to the clinical data and group assignments of the patients carried out all the follow‐up tests. Patients were told not to reveal to which groups they belonged" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | "Intention‐to‐treat analyses were performed" |
| Incomplete outcome data? | Low risk | 35/40 (88%) exercise training group and 37/40 (93%) control group available at 12 months |
| Groups balanced at baseline? | Low risk | Table 1 of the publication suggests no difference between the 2 groups |
| Groups received same intervention? | Low risk | Yes |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 42 (exercise 22; control 20) Diagnosis (% of participants): Aetiology: ischaemic 50%; non‐ischaemic 50% NYHA: Class II: exercise 64%; control 45%; Class III: exercise 36%; control 55% LVEF: exercise: mean 33% (SD 7); control: mean 32% (SD) Age (yr): exercise 57 (SD 12); control 63 (SD 15) Male: 57.5% White: not reported Inclusion/exclusion criteria Inclusion: age ≥ 21 yr, with HF; orientated to person, place and time; able to speak and read English; resting LVEF ≤ 40% and stable on optimal medical therapy for at least 30 days Exclusion: clinical evidence of decompensated HF, unstable angina pectoris, MI, coronary artery bypass surgery, biventricular pacemaker < 3 months ago, orthopaedic or neuromuscular limitations preventing participation in aerobic or resistance exercise training, and participation in an aerobic exercise programme during the past 12 months |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: mix Frequency: aerobic 3 days/wk, resistance 2 days/wk Duration: aerobic: 30 min/session (30 min warm‐up); resistance: 8‐10 exercises (upper and lower extremity) performed for 1 set of 10‐15 repetitions Intensity: aerobic: 40‐70% HR reserve, or Borg 11‐14; resistance: not reported Modality: aerobic: not reported; resistance: weight machines, free weights or elastic bands based on their exercise performance Setting: 3 wk: supervised; 21 wk: hospital's wellness centre or home Other: group meetings that addressed the same education topics as the control group but in addition included information on problem‐solving barriers to exercise, relapse management and symptoms experienced during exercise |
|
| Outcomes | HRQoL (KCCQ), SF‐36 and mortality | |
| Comparison | "Attention control" Instructions to continue with their normal level of activity. No instructions were given to withhold or stop activity |
|
| Country and setting | USA Single centre |
|
| Follow‐up | 24 wk (after randomisation) | |
| Notes | Study conducted in 2 sequential 12‐wk phases Phase 1: separate weekly group meetings of both groups during wk 1‐3, then separate biweekly meetings during wk 4‐12 Phase 2: following the groups for an additional 12 wk without group sessions Other trial report: Pozehl B, Duncan K, Hertzog M, Norman JF. Heart failure exercise and training camp: effects of a multicomponent exercise training intervention in patients with heart failure. Heart Lung 2010;39(6 Suppl):S1‐13 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Research assistants who were blinded to group assignment assisted in some of the data collection. However, because of budget constraints, the investigators who were not blinded to group assignment were also involved in data collection" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Not stated but groups analysed according to randomised allocation |
| Incomplete outcome data? | Low risk | Due to mortality and drop out KCCQ scores available in 37 patients (88%) at 24 wk |
| Groups balanced at baseline? | Low risk | "…no significant difference noted between groups" |
| Groups received same intervention? | Low risk | Both groups received group sessions (attention control) so only difference between groups was exercise based intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 85 (training 44; control 41) Diagnosis (% of participants): * Aetiology: ischaemic 59%; DCM 41% NYHA: Class I 16%; Class II 69%; Class III 34% LVEF: training: 35% (SD 9.3); control 32.3 (SD 14.1) Case mix: 100% as above Age (yr): exercise 60 (SD 13); control 61 (SD 13) Male: 87% White: not reported Inclusion/exclusion criteria Inclusion: impaired left ventricular systolic function (EF < 45%) and exercise capacity (peak VO2 < 25 mL/min/kg) Exclusion: NYHA Class IV, MI or unstable angina < 6 months before the examination, exercise‐limiting diseases, and severe pulmonary or renal disease * baseline data only available for 85 participants |
|
| Interventions |
Exercise:Total duration: 9 months Aerobic/resistance/mix: aerobic Frequency: > 3 sessions/wk Duration: 30 min/session Intensity: 65% max VO2 Modality: cycle Setting: home‐based Other: not reported |
|
| Outcomes | HRQoL (MLWHFQ) Morbidity |
|
| Comparison | Not reported | |
| Country and setting | Italy Not reported |
|
| Follow‐up | 9 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Exercise test assessor blinded |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis? | Low risk | Although ITT not stated, groups appeared to be analysed according to original randomisation |
| Incomplete outcome data? | Low risk | Outcomes described in methods reported in results |
| Groups balanced at baseline? | Low risk | "The two groups did not differ as to age, gender, NYHA functional class, EF, pharmacologic treatment, or HF etiology (Table 1)" |
| Groups received same intervention? | Low risk | "Patients in [control] group underwent follow‐up visits at the third and ninth month to exclude changes in their usual lifestyle and physical activity" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 21 (exercise 15; control 6) Diagnosis (% of participants): Aetiology: ischaemic 71%; non‐ischaemic 29% NYHA: Class II 39%; Class III 52%; Class IV 9% LVEF: exercise 27.9% (SD 7.0); control 29.7% (SD 8.7) Case mix: 100% as above Age (yr): exercise 66.3 (SD 9.6); control 66 (SD 12.6) Male: 90% White: 100% Inclusion/exclusion criteria Inclusion: able to speak and read English; stable NYHA Class II‐IV no change in medical therapy for 30 days; resting LVEF < 40% measured by echocardiography or gated equilibrium radionuclide angiography; medical diagnosis of HF either ischaemic or non‐ischaemic; and standard pharmacological therapy for HF (diuretics, angiotensin‐converting enzyme inhibitors and beta‐blockers) Exclusion: participation in a formal exercise programme < 30 days prior to this study; clinical evidence decompensated HF; and any of the following medical conditions: AF, acute MI < 3 months, unstable angina pectoris, end‐stage renal disease or orthopaedic impediments to exercise |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: mix Frequency: 3 sessions/wk Duration: 30 min aerobic, 20 min resistance Intensity: 60‐85% max VO2, 12‐14 Borg scale Modality: aerobic: treadmill, stationary bike, rower, arm ergometer; resistance: light upper‐body exercises (military press, biceps curl and lateral deltoid raises) and lower‐body exercises (knee extension, side hip raise and hip extension) with 1‐10 lb hand and ankle weights. Wall push‐ups, abdominal curl‐ups, pelvic tilts, or a combination Setting: first 12 wk at the hospital and remaining sessions were unsupervised at rehabilitation centre Other: strategies from social learning theory (goal‐setting, feedback and problem‐solving guidance) utilised to facilitate, improve adherence to the training programme |
|
| Outcomes | Mortality | |
| Comparison | Usual medical care | |
| Country and setting | USA Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | Although not stated, groups appear to analysed according to initial randomised allocation |
| Incomplete outcome data? | Low risk | "One subject in the control group died of myocardial infarction and one subject in the exercise training group was diagnosed with cancer and unable to continue the exercise training."No imputation undertaken |
| Groups balanced at baseline? | Low risk | "Subjects did not differ in fatigue or dyspnea by type of HF (ischemic vs. nonischemic) or years since diagnosis of HF (length of time since diagnosis)" |
| Groups received same intervention? | Unclear risk | Not reported |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 19 (exercise 9; control 10) Diagnosis (% of participants): Aetiology: not reported NYHA: mean: exercise 2 (SE 0); control 2.13 (SE 0.13) LVEF: ≤ 60% Case mix: as above Age (yr): exercise 69 (SD 4.44); control 70 (SD 4.05) Male: 58% White: 100% Inclusion/exclusion criteria Inclusion: 1. a diagnosis of NYHA Class I‐III congestive HF, 2. an EF ≤ 60%, 3. systolic dysfunction, 4. physician approval and 5. the ability to complete a minimum of 3 min of a modified Bruce‐protocol stress test Exclusion: failure to meet any of the inclusion criteria, inability to speak English or having noticeable cognitive impairment |
|
| Interventions |
Exercise:Total duration: 12 months Aerobic/resistance/mix: aerobic Frequency: 3 sessions/wk Duration: > 15 min Intensity: not reported Modality: treadmill Lifestyler® treadmill provided for 1 year of in‐home use, 3 supervised exercise sessions at hospital with CR specialist. Weekly in‐home exercise visits with CR specialist, Month 1. Monthly in‐home exercise visits with CR specialist, Months 2‐12. Also received comprehensive disease management programme Setting: 3 hospital based and the remainder at home Other: not reported |
|
| Outcomes | Disease‐specific HRQoL (Chronic Heart Failure Questionnaire), mortality | |
| Comparison | Comprehensive disease management ‐ by dedicated case manager (participant education on nutrition, medications, and disease management; an oximetry assessment; and constant monitoring of symptomatic changes and disease status | |
| Country and setting | USA Single‐centre |
|
| Follow‐up | 12 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported |
| Intention‐to‐treat analysis? | Low risk | Although not stated, it is clear from CONSORT diagram that 2 groups were analysed according to ITT |
| Incomplete outcome data? | Low risk | QUORUM flow diagram report suggests 19 were included in the analysis 15 participants (79%) completed final follow‐up measures at month 12 |
| Groups balanced at baseline? | Low risk | Table 3 of the publication suggests there is no difference between the 2 groups (except dyspnoea score) |
| Groups received same intervention? | Low risk | Both groups received comprehensive disease management |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 54 (exercise 27; control 27) Diagnosis (% of participants): Aetiology: ischaemic 80%; non‐ischaemic 20% NYHA: exercise 2.1 (SD 0.7); control 2.4 (0.7) LVEF: exercise 35% (SD 12); control 38% (SD 10) Case mix: 100% as above Age (yr): exercise 64 (SD 5); control 64 (SD 9) Male: exercise 73%; control 70% White: not reported Inclusion/exclusion criteria Inclusion: 1. 8 points on Boston heart failure criteria; 2. LVEF 0.45 at the most recent radionuclide or echocardiographic examination (not older than 1 year at inclusion) and 3. age 75 yr Exclusion: 1. change of clinical status or medication (or both) within 4 wk prior to inclusion; 2. MI, heart surgery or coronary angioplasty within 3 months prior to inclusion; 3. inability to perform a bicycle test; 4. exercise‐terminating angina pectoris, ST‐depressions (> 2 mm in > 1 lead), blood pressure fall (>.10 mm Hg), or arrhythmia (e.g. ventricular tachycardia/fibrillation, ventricular extrasystoles, supraventricular tachycardia > 170 bpm) at the most recent maximal exercise test (including the baseline test); 5. pulmonary disease judged to be the main exercise‐limiting factor or peak expiratory flow rate < 50% of the age‐ and sex‐adjusted reference value, or both; 6. NYHA Class IV and 7. clinically significant aortic stenosis |
|
| Interventions |
Exercise:Total duration: 4 months Aerobic/resistance/mix: aerobic/interval Frequency: 2‐3 sessions/wk Duration: 15 min/session increasing to 45 min/session Intensity: 80% peak VO2, or 15 on Borg score Modality: cycle ergometry Setting: group sessions supervised by physiotherapist Other: none |
|
| Outcomes | HRQoL (Patient's Global Assessment of Quality of Life), mortality | |
| Comparison | Control participants were asked not to change their degree of physical activity during the active study period. Neither training participants nor controls were instructed regarding physical activity during the 6‐month extended follow‐up | |
| Country and setting | Sweden Single centre |
|
| Follow‐up | 10 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors blinded. Participants, clinical carers not blinded |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Although ITT not implicit, it appears that groups are analysed according to original randomised allocation |
| Incomplete outcome data? | High risk | Outcome available in only 43/54 (80%) participants randomised at 10 months' follow‐up. No imputation or sensitivity analysis undertaken to assess effect of loss to follow‐up. Authors state that participants available at 10 months' follow‐up are representative |
| Groups balanced at baseline? | Low risk | "There was no difference between training (n =22) and control (n =27) patients as regards baseline variables" |
| Groups received same intervention? | Low risk | "No change in medication allowed during study" |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 82 (exercise 41; control 41) Diagnosis (% of participants): Aetiology: IHD 66% NYHA: Class II 56%; Class III 44% LVEF: not reported Case mix: as above Age (yr): exercise 80 (SD 6); control 81 (SD 4) Male: 55% White: not reported Inclusion/exclusion criteria Inclusion: age ≥ 70 yr with clinical diagnosis of CHF according to European Society of Cardiology guidelines, NYHA Class II or III symptoms and evidence of LVSD on echocardiography, contrast ventriculography or radionuclide ventriculography. Evidence of LVSD Exclusion: uncontrolled AF, significant aortic stenosis, sustained ventricular tachycardia, recent MI, inability to walk without human assistance, abbreviated mental score < 6 of 10, or people currently undergoing physiotherapy or rehabilitation |
|
| Interventions |
Exercise:Total duration: 6 months Aerobic/resistance/mix: mix Frequency: 2‐3 sessions/wk Duration: 20 min Intensity: Borg 11‐13 Modality: walking and wrist/ankle weights Setting: 3 months; hospital based by senior physiotherapist, 3 months; home‐based After 3 months of supervised training, participants in the exercise group were asked to continue performing exercises at home 2 or 3 times/wk with the aid of video or audio cassette with demonstrations, instructions and music. No face‐to‐face contact with the physiotherapist during this period Other: not reported |
|
| Outcomes | A disease specific health‐related quality‐of‐life (Guyatt chronic heart failure questionnaire), mortality, hospitalisation | |
| Comparison | Usual medical care | |
| Country and setting | UK Single centre |
|
| Follow‐up | 6 months (after randomisation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A researcher not otherwise connected with the operation of the study prepared cards contained in numbered, sealed envelopes from computer‐generated random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "An experienced research nurse who was blinded to treatment allocation performed all assessments" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods are reported in results |
| Intention‐to‐treat analysis? | Low risk | It appeared that groups were analysed according to initial random allocation from QUORUM diagram |
| Incomplete outcome data? | Low risk | 75/82 (91%) and 68/82 (83%) available at 3 and 6 months' follow‐up, respectively |
| Groups balanced at baseline? | Low risk | Table 1 of the publication shows groups are well balanced |
| Groups received same intervention? | Low risk | Yes, both group appeared to receive usual medical care and the only difference between groups was the exercise intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 107 (exercise 53; control 54) Diagnosis (% of participants): Aetiology: ischaemic 62.6% NYHA: Class II 79%; Class III 21% LVEF: not reported Case mix: as above Age (yr): exercise 80.4 (SD 5.8); control 79.5 (SD 4.9) Male: exercise 35%; control 37% White: 100% Inclusion/exclusion criteria Inclusion: age ≥ 70 yr with a confirmed diagnosis of HF due to LVSD (NYHA Class II and III) and a history of symptoms and signs of congestive HF Exclusion: wheelchair bound, unwilling or unable to give informed, had aortic stenosis with peak gradient > 30 mmHg, experienced sustained ventricular tachycardia or ventricular fibrillation outside the context of an acute MI, and currently (within the past month) had unstable angina or AF with a ventricular rate of > 100/min |
|
| Interventions |
Exercise:Total duration: 24 wk Aerobic/resistance/mix: mix Frequency: 2 sessions/wk Duration: ≤ 60 min Intensity: not reported Modality: home; walking Setting: hospital and home* Other: cognitive and behavioural techniques were incorporated into first 8‐wk hospital‐based rehabilitation. Resistance training with elasticised bands |
|
| Outcomes | Disease‐specific HRQoL (MLWHFQ), HRQoL (EuroQoL‐5D), mortality, hospital admission, cost | |
| Comparison | Usual medical care (given a booklet with general advice on diet, exercise and lifestyle). Not discouraged from exercising if they were already in the habit of doing so | |
| Country and setting | UK Single‐centre |
|
| Follow‐up | 24 wk (after randomisation) | |
| Notes | *8 wk in hospital delivered by experienced physiotherapist, 16‐wk home‐based (telephoned every 2 wk for 8 wk by the physiotherapists, then monthly for the final 8 wk) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using off‐site telephone randomization service, randomization was performed without stratification and with block sizes between 8 and 16, depending on the size of each planned exercise class" |
| Allocation concealment (selection bias) | Low risk | "…the project coordinator passed the participants'details to the research physiotherapist who obtained group allocation, ensuring that the project coordinator remained blind to group assignments" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | Analyses were by ITT |
| Incomplete outcome data? | Low risk | 89/104 (86%) and 87/104 (83%) available for follow‐up at 8 and 24 wk, respectively |
| Groups balanced at baseline? | Low risk | Table 1 of the publication suggests no difference between the 2 groups |
| Groups received same intervention? | Low risk | It appeared that both groups received same care expect exercise intervention |
| Methods | Parallel group RCT | |
| Participants |
N Randomised: 100 (Tai Chi (exercise) 50; education (control) 50) Diagnosis (% of participants): Aetiology: ischaemic 54%; non‐ischaemic 46% NYHA: Class I 20%; Class II 63%; Class III 17% LVEF: mean 29% (SD 8%) Case mix: 100% as above Age (yr): exercise 68.1 (SD 11.9); control 66.6 (SD 12.1) Male: 64% White: 86% Inclusion/exclusion criteria Inclusion: EF < 40% or lower in past 2 yr, stable medical regimen, NYHA Class I‐III HF Exclusion: unstable angina, MI or major surgery in past 3 months; history of cardiac arrest in the past 6 months, history of cardiac resynchronisation therapy in the past 3 months; unstable serious ventricular arrhythmias; unstable structural valve disease; current participation in conventional CR programme; diagnosis of peripartum cardiomyopathy within the preceding 6 months; inability to perform a bicycle stress test; lower extremity amputation or other inability to ambulance owing to condition other than HF; severe cognitive dysfunction (Mini‐Mental State Examination score ≤ 24); inability to speak English and regular practice of Tai Chi |
|
| Interventions |
Exercise:Total duration: 12 wk Aerobic/resistance/mix: aerobic Frequency: 2 sessions/wk (for 12 wk) and encouraged to practice at home at least 3 times/wk Duration: 1 hr class (30 min warm‐up) Intensity: not reported Modality: Tai Chi movements 1. Wk 2‐5: warm‐up + raising the power, withdraw and push 2. Wk 6‐9: 1 + grasp sparrow's tail, brush knee twist step 3. Wk 10‐12: 2 + wave hands like clouds Participants were given 45‐min instructional videotape that outlined the exercises presented in class as an aid to practice Participants also received same educational pamphlets used in education (control) group with a brief (< 5 min) explanation towards end of 1 Tai Chi session weekly Setting: centre‐based and home‐based Other: none reported |
|
| Outcomes | HRQoL (MLWHFQ), mortality, hospital admission | |
| Comparison | Education group ('attention control'): nurse practitioner lead education session (same duration and frequency as the Tai Chi group classes) Participants were asked not to start Tai Chi classes during the study |
|
| Country and setting | USA Multisite |
|
| Follow‐up | 12 wk and 6 months (after randomisation) | |
| Notes | Single blind | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The trial uses a permuted block randomization with variable block size to generate treatment assignment" |
| Allocation concealment (selection bias) | Unclear risk | "Patients who chose to were randomly assigned to receive a 12‐week tai chi exercise program or a heart health education program (attention control)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "We masked all the study staff performing all tests to each participant's group allocation" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods reported in results |
| Intention‐to‐treat analysis? | Low risk | All participants were included in the analysis regardless of their attendance |
| Incomplete outcome data? | Low risk | Figure 1 of the publication shows 91% to 96% complete data across HRQoL and exercise outcomes |
| Groups balanced at baseline? | Low risk | "The 2 groups were generally similar in demographics, clinical classification of heart disease severity, and rates of comorbidities" |
| Groups received same intervention? | Low risk | Yes, both groups received comprehensive disease management |
AF: atrial fibrillation; bpm: beats/minute; CBT: cognitive behavioural therapy; CHF: chronic heart failure; CONSORT: CONsolidated Standards of Reporting Trials; COPD: chronic obstructive pulmonary disease; CR: cardiac rehabilitation; DCM: dilated cardiomyopathy; DM: diabetes mellitus; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; EF: ejection fraction; HADS: Hospital Anxiety and Depression Scale; HF: heart failure; hr: hour; HR: heart rate; HRQoL: health‐related quality of life; ITT: intention to treat; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; max: maximum; MI: myocardial infarction; min: minute; MOS: Medical Outcomes Survey; MLWHFQ: Minnesota Living with Heart Failure Questionnaire; MRI: magnetic resonance imaging; NYHA: New York Heart Association; PVD: peripheral vascular disease; RCT: randomised controlled trial; SD: standard deviation; SE: standard error; SF‐36: 36‐item Short Form; VO2: oxygen consumption; wk: week; yr: year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamopoulos 2001 | Relevant outcomes not reported |
| Alves 2012 | Relevant outcomes not reported |
| Barrow 2008 | < 6 months' follow‐up |
| Belardinelli 2005 | < 6 months' follow‐up |
| Briffa 2005 | Not heart failure |
| Brotons 2009 | Not exercise‐based cardiac rehabilitation intervention |
| Chang 2005 | Relevant outcomes not reported |
| Coats 1992 | < 6 months' follow‐up |
| Collins 2004 | < 6 months' follow‐up |
| Corvera‐Tindel 2004 | < 6 months' follow‐up |
| Cowie 2011 | < 6 months' follow‐up |
| Deng 2006 | Relevant outcomes not reported |
| Dingli 2002 | Relevant outcomes not reported |
| Edelmann 2011 | < 6 months' follow‐up |
| Erbs 2003 | Relevant outcomes not reported |
| Erbs 2010 | Relevant outcomes not reported |
| ExTraMATCH 2004 | Meta‐analysis |
| Franco 2006 | < 6 months' follow‐up |
| Gary 2004 | Relevant outcomes not reported |
| Gary 2007 | < 6 months' follow‐up |
| Haykowsky 2007 | Meta‐analysis |
| Inglis 2006 | Exercise advice only |
| Jolly 2007 | Protocol only |
| Jónsdóttir 2006b | < 6 months' follow‐up |
| Kiilavuori 1999 | Relevant outcomes not reported |
| Kitzman 2010 | < 6 months' follow‐up |
| Kobayashi 2003 | Relevant outcomes not reported |
| Korzeniowska‐Kubacka 2010 | Not a randomised controlled trial |
| Lloyd‐Williams 2002 | Meta‐analysis |
| Meyer 2005 | Relevant outcomes not reported |
| Molloy 2006 | Relevant outcomes not reported |
| Mudge 2011 | Protocol |
| Myers 2001 | Relevant outcomes not reported |
| Myers 2002 | Relevant outcomes not reported |
| Myers 2007 | Relevant outcomes not reported |
| Niebauer 2005a | Relevant outcomes not reported |
| Niebauer 2005b | Relevant outcomes not reported |
| Oka 2000 | Relevant outcomes not reported |
| Owen 2000 | < 6 months' follow‐up |
| Parnell 2002 | < 6 months' follow‐up |
| Passino 2008 | Relevant outcomes not reported |
| Ponikowski 1997 | < 6 months' follow‐up |
| Pozehl 2003 | < 6 months' follow‐up |
| Pu 2001 | Relevant outcomes not reported |
| Sabelis 2004 | Relevant outcomes not reported |
| Sarullo 2006 | < 6 months' follow‐up |
| Selig 2004 | < 6 months' follow‐up |
| Senden 2005 | Relevant outcomes not reported |
| Smart 2004 | Meta‐analysis |
| Smart 2007 | < 6 months' follow‐up |
| Stewart 1998 | Exercise advice only |
| Taylor‐Piliae 2004 | Meta‐analysis |
| Tyni‐Lenne 2001 | < 6 months' follow‐up |
| van den Berg‐Emons 2004 | < 6 months' follow‐up |
| van Tol 2006 | Meta‐analysis |
| Vasiliauskas 2007 | Relevant outcomes not reported |
| Wielenga 1998 | < 6 months' follow‐up |
| Williams 2007 | Relevant outcomes not reported |
| Wisløff 2007 | < 6 months' follow‐up |
| Yeh 2004 | < 6 months' follow‐up |
| Zhang 2003 | < 6 months' follow‐up |
| Zhao 2005 | Relevant outcomes not reported |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Exercise Training in Diastolic Heart Failure: a Prospective, Randomized, Controlled Study to Determine the Effects of Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction (Ex‐DHF) |
| Methods | RCT |
| Participants | Stable symptomatic HF with preserved ejection fraction (diagnosis according to criteria of the European Society of Cardiology (Paulus 2007)) |
| Interventions | Experimental intervention: individually prescribed, supervised, combined endurance/strength training for 12 months (≥ 3 times/week) Control intervention: usual care |
| Outcomes | Primary
Secondary
|
| Starting date | 1 September 2011 |
| Contact information | Dr Frank Edelmann: fedelmann@med.uni‐goettingen.de |
| Notes | Trial still recruiting. Recruitment completion expected in 2014 (author email reply 21 July 2013) |
| Trial name or title | The Exercise Joins Education: Combined Therapy to Improve Outcomes in Newly‐discharged Heart Failure (EJECTION‐HF) |
| Methods | RCT |
| Participants | 350 recently hospitalised people with HF with impaired and preserved left ventricular systolic function |
| Interventions | Supervised exercise training programme and disease management programme vs. disease management programme alone |
| Outcomes | Primary outcome
Secondary outcomes at 6 and 12 months
|
| Starting date | Not reported (150 recruited at time of publication) "Enrolment will be completed in 2013" |
| Contact information | Corresponding author: telephone: +61 7 36360854, fax: +61 7 36360272, Email: Alison_Mudge@health.qld.gov.au Internal Medicine and Aged Care, Royal Brisbane and Women's Hospital, Butterfield St, Herston, Queensland 4029 Australia |
| Notes | Trial completed recruiting. Publication of primary outcomes expected in late 2014 (author email reply 29 July 2013) |
| Trial name or title | Home Walking Exercise (HWE) Training in Advanced Heart Failure |
| Methods | RCT |
| Participants | 79 participants with stable HF in the past 3 months |
| Interventions | 12‐week nurse‐managed progressive home walking exercise protocol versus usual activity |
| Outcomes | Pre‐ and post‐study assessment of: Functional status (peak VO2 and ventilatory threshold via complete physical examination, 6‐min walk test and a Heart Failure Functional Status Inventory), quality of life (Cardiac Quality of Life Index, SF‐36, and Dyspnea‐Fatigue Index with global rating of symptoms) and autonomic tone (norepinephrine (noradrenaline) and heart rate variability) |
| Starting date | December 2001 |
| Contact information | Teresita E Corvera‐Tindel, PhD RN MN, VA Greater Los Angeles Health Care System, USA |
| Notes | Contact email sent ‐ no reply |
| Trial name or title | Exercise Effect on Aerobic Capacity and QOL in Heart Failure |
| Methods | RCT |
| Participants | About 84 participants with left LVEF ≤ 40%. Stable HF |
| Interventions | Exercise group: 36 weeks of exercise training Control group: weekly visits with a nurse for 12 weeks |
| Outcomes | At 12 weeks, exercise capacity (peak VO2) and HRQoL (SF‐36) |
| Starting date | Not reported |
| Contact information | Eileen G Collins, PhD RN, Edward Hines Jr. VA Hospital, USA |
| Notes | Contact email sent ‐ no reply (as of 20 September 2013) |
| Trial name or title | Exercise for Patients with Heart Failure in Primary Care: the EFICAR |
| Methods | RCT |
| Participants | Inclusion criteria:
600 participant target |
| Interventions | Experimental: exercise supervised exercise + optimised treatment according to the European Society of Cardiology guidelines No intervention: control optimised treatment according to the European Society of Cardiology guidelines |
| Outcomes | Primary outcomes:
Secondary outcomes:
All at 12 months |
| Starting date | January 2011 |
| Contact information | Contact: Dr Gonzalo Grandes |
| Notes | Zuazagoitia A, Grandes G, Torcal J, Lekuona I, Echevarria P, Gómez MA, Domingo M, de la Torre MM, Ramírez JI, Montoya I, Oyanguren J, Pinilla RO; EFICAR Group (Ejercicio Físico en la Insuficiencia Cardiaca). Rationale and design of a randomised controlled trial evaluating the effectiveness of an exercise program to improve the quality of life of patients with HF in primary care: The EFICAR study protocol. BMC Public Health. 2010;10:33 Trial still recruiting. Recruitment completion expected June 2014 (author email reply 20 July 2013) |
HF: heart failure; HRQoL: health‐related quality of life; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; min: minute; RCT: randomised controlled trial; SF‐36: 36‐item Short Form; VCO2: carbon dioxide consumption; VE: ventilatory efficiency; VO2: oxygen consumption.
Contributions of authors
Rod Taylor and Viral Sagar led the design of the update review.
Simon Briscoe developed the updated the searches.
Viral Sagar and Rod Taylor undertook study selection, data extraction, assessment of risk of bias and data analysis.
Viral Sagar and Rod Taylor wrote the first draft of the update review, and all co‐authors commented on a draft of the report.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Rod Taylor and Hayes Dalal are co‐lead investigators on an ongoing National Institute for Health Research (NIHR) Programme Grants for Applied Research funded study ‐ Rehabilitation Enablement in Chronic Heart Failure (REACH‐HF) ‐ to develop and evaluate the costs and outcomes of a home‐based self help heart failure exercise rehabilitation manual (RP‐PG‐1210‐12004).
Notes
None.
Edited (no change to conclusions)
References
References to studies included in this review
- Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. European Journal of Heart Failure 2005;7(3):411‐7. [DOI] [PubMed] [Google Scholar]; Austin J, Williams WR, Hutchison S. Multidisciplinary management of elderly patients with chronic heart failure: five year outcome measures in death and survivor groups. European Journal of Cardiovascular Nursing: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 2009;8(1):34‐9. [PUBMED: 18534911] [DOI] [PubMed] [Google Scholar]; Austin J, Williams WR, Ross L, Hutchison S. Five‐year follow‐up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. European Journal of Cardiovascular Prevention and Rehabilitation 2008;15(2):162‐7. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long‐term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99(9):1173‐82. [DOI] [PubMed] [Google Scholar]; Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost‐effectiveness analysis of long‐term moderate exercise training in chronic heart failure. American Journal of Cardiology 2001;87:984‐8. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10‐year exercise training in chronic heart failure: a randomized controlled trial. Journal of the American College of Cardiology 2012;60:1521‐8. [DOI] [PubMed] [Google Scholar]
- Bocalini DS, dos Santos L, Serra AJ. Physical exercise improves the functional capacity and quality of life in patients with heart failure. Clinics (Sao Paulo, Brazil) 2008;63:437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwisler AD, Schou L, Soja AM, Brønnum‐Hansen H, Gluud C, Iversen L, et al. A randomized clinical trial of hospital‐based, comprehensive cardiac rehabilitation versus usual care for patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease (the DANREHAB trial) ‐ design, intervention, and population. American Heart Journal 2005;899:e16. [DOI] [PubMed] [Google Scholar]; Zwisler AD, Soja AM, Rasmussen S, Frederiksen M, Abedini S, Appel J, et al. Hospital‐based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12‐month results of a randomized clinical trial. American Heart Journal 2008;155:1106‐13. [DOI] [PubMed] [Google Scholar]
- Davidson PM, Cockburn J, Newton PJ, Webster JK, Betihavas V, Howes L, et al. Can a heart failure‐specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high‐risk patients?. European Journal of Cardiovascular Prevention & Rehabilitation 2010;17:393‐402. [DOI] [PubMed] [Google Scholar]
- Dracup K, Evangelista LS, Hamilton MA, Erickson V, Hage A, Moriguchi J, et al. Effects of a home‐based exercise program on clinical outcomes in heart failure. American Heart Journal 2007;154(5):877‐83. [DOI] [PubMed] [Google Scholar]; Evangelista LS, Doering LV, Lennie T, Moser DK, Hamilton MA, Fonarow GC, et al. Usefulness of a home‐based exercise program for overweight and obese patients with advanced heart failure. The American journal of cardiology 2006;97(6):886‐90. [PUBMED: 16516595] [DOI] [PubMed] [Google Scholar]; Evangelista LS, Hamilton MA, Fonarow GC, Dracup K. Is exercise adherence associated with clinical outcomes in patients with advanced heart failure?. Physician & Sports Medicine 2010;38:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. Journal of psychosomatic research 2010;69(2):119‐31. [PUBMED: 20624510] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. Journal of Psychosomatic Research 2010;69:119‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannuzzi P, Temporelli PL, Corra U, Tavazzi L. Antiremodeling effect of long‐term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD‐CHF) Trial. Circulation 2003;108(5):554‐9. [DOI] [PubMed] [Google Scholar]
- Gielen S, Adams V, Mobius‐Winkler S, Linke A, Erbs S, Yu J, et al. Anti‐inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. Journal of the American College of Cardiology 2003;42(5):861‐8. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, Fisher ML, Freudenberger R, Robinson S, Zietowski G, Alves L, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. Journal of Cardiac Failure 1999;5(3):188‐94. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. Journal of the American College of Cardiology 1995;25(6):1239‐49. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998;98(24):2709‐15. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 2000;283(23):3095‐101. [DOI] [PubMed] [Google Scholar]
- Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;153:1451‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O'Connor CM, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. Journal of the American College of Cardiology 2012;60:1899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]; O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;301:1439‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reed SD, Whellan DJ, Li Y, Friedman JY, Ellis SJ, Pina IL, et al. Economic evaluation of the HF‐ACTION (Heart failure: a controlled trial investigating outcomes of exercise training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circulation: Cardiovascular Quality and Outcomes 2010;3:374‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reed Shelby D, Li Yanhong, Dunlap Mark E, Kraus William E, Samsa Gregory P, Schulman Kevin A, et al. In‐hospital resource use and medical costs in the last year of life by mode of death (from the HF‐ACTION randomized controlled trial). American Journal of Cardiology 2012;110:1150‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF‐ACTION): design and rationale. American Heart Journal 2007;153:201‐11. [DOI] [PubMed] [Google Scholar]
- Jolly K, Taylor RS, Lip GY, Davies M, Davis R, Mant J, et al. A randomized trial of the addition of home‐based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM‐CHF) study. European Journal of Heart Failure 2009;11:205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsdóttir S, Andersen KK, Sigurðsson AF, Sigurðsson SB. The effect of physical training in chronic heart failure. European Journal of Heart Failure 2006;8(1):97‐101. [DOI] [PubMed] [Google Scholar]
- Keteyian SJ, Levine AB, Brawner CA, Kataoka T, Rogers FJ, Schairer JR, et al. Exercise training in patients with heart failure. A randomized, controlled trial. Annals of Internal Medicine 1996;124(12):1051‐7. [DOI] [PubMed] [Google Scholar]
- Klecha A, Kawecka‐Jaszcz K, Bacior B, Kubinyi A, Pasowicz M, Klimeczek P, et al. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14(1):85‐91. [DOI] [PubMed] [Google Scholar]
- Klocek M, Kubinyi A, Bacior B, Kawecka‐Jaszcz K. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. International Journal of Cardiology 2005;103(1):323‐9. [DOI] [PubMed] [Google Scholar]
- Klocek M, Kubinyi A, Bacior B, Kawecka‐Jaszcz K. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. International Journal of Cardiology 2005;103(1):323‐9. [DOI] [PubMed] [Google Scholar]
- Koukouvou G, Kouidi E, Iacovides A, Konstantinidou E, Kaprinis G, Deligiannis A. Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. Journal of Rehabilitation Medicine 2004;36:36‐41. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). American Heart Journal 2002;144(1):23‐30. [DOI] [PubMed] [Google Scholar]
- Mueller L, Myers J, Kottman W, Oswald U, Boesch C, Arbrol N, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clinical Rehabilitation 2007;21(10):923‐31. [DOI] [PubMed] [Google Scholar]
- Myers J, Goebbels U, Dzeikan G, Froelicher V, Bremerich J, Mueller P, et al. Exercise training and myocardial remodeling in patients with reduced ventricular function: one‐year follow‐up with magnetic resonance imaging. American Heart Journal 2000;139(2 Pt 1):252‐61. [PUBMED: 10650298] [DOI] [PubMed] [Google Scholar]
- Nilsson BB, Westheim A, Risberg MA. Long‐term effects of a group‐based high‐intensity aerobic interval‐training program in patients with chronic heart failure. American Journal of Cardiology 2008;102:1220‐4. [DOI] [PubMed] [Google Scholar]
- Norman JF, Pozehl BJ, Duncan KA, Hertzog MA, Krueger SK. Effects of exercise training versus attention on plasma B‐type natriuretic peptide, 6‐minute walk test and quality of life in individuals with heart failure. Cardiopulmonary Physical Therapy Journal 2012;23:19‐25. [PMC free article] [PubMed] [Google Scholar]; Pozehl B, Duncan K, Hertzog M, Norman JF. Heart failure exercise and training camp: effects of a multicomponent exercise training intervention in patients with heart failure. Heart & Lung 2010;39:S1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, et al. Aerobic training decreases B‐type natriuretic peptide expression and adrenergic activation in patients with heart failure. Journal of the American College of Cardiology 2006;47:1835‐9. [DOI] [PubMed] [Google Scholar]
- Pozehl B, Duncan K, Hertzog M. The effects of exercise training on fatigue and dyspnea in heart failure. European Journal of Cardiovascular Nursing 2008;7(2):127‐32. [DOI] [PubMed] [Google Scholar]
- Wall HK, Ballard J, Troped P, Njike VY, Katz DL. Impact of home‐based, supervised exercise on congestive heart failure. International Journal of Cardiology 2010;145:267‐70. [DOI] [PubMed] [Google Scholar]
- Willenheimer R, Rydberg E, Cline C, Broms K, Hillberger B, Oberg L, et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. International Journal of Cardiology 2001;77(1):25‐31. [DOI] [PubMed] [Google Scholar]
- Witham MD, Gray JM, Argo IS, Johnston DW, Struthers AD, McMurdo ME. Effect of a seated exercise program to improve physical function and health status in frail patients > or = 70 years of age with heart failure. American Journal of Cardiology 2005;95(9):1120‐4. [PUBMED: 15842989] [DOI] [PubMed] [Google Scholar]
- Witham MD, Fulton RL, Greig CA, Johnston DW, Lang CC, Pol M, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circulation: Heart Failure 2012;5:209‐16. [DOI] [PubMed] [Google Scholar]
- Yeh GY, McCarthy EP, Wayne PM, Stevenson LW, Wood MJ, Davis RB, et al. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Archives of Internal Medicine 2011;171:750‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adamopoulos S, Parissis J, Kroupis C, Georgiadis M, Karatzas D, Karavolias G, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. European Heart Journal 2001;22(9):791‐7. [DOI] [PubMed] [Google Scholar]
- Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, et al. Exercise training improves diastolic function in heart failure patients. Medicine & Science in Sports & Exercise 2012;44:776‐85. [DOI] [PubMed] [Google Scholar]
- Barrow DE, Bedford A, Ives G, O'Toole L, Channer KS. An evaluation of the effects of Tai Chi Chuan and Chi Kung training in patients with symptomatic heart failure: a randomised controlled pilot study. Postgraduate Medical Journal 2008;83(985):717‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli R, Lacalaprice F, Faccenda E, Purcaro A, Perna G. Effects of short‐term moderate exercise training on sexual function in male patients with chronic stable heart failure. International Journal of Cardiology 2005;101(1):83‐90. [DOI] [PubMed] [Google Scholar]
- Briffa TG, Eckermann SD, Griffiths AD, Harris PJ, Heath MR, Freedman SB, et al. Cost‐effectiveness of rehabilitation after an acute coronary event: a randomised controlled trial. Medical Journal of Australia 2005;183(9):450‐5. [DOI] [PubMed] [Google Scholar]
- Brotons C, Falces C, Alegre J, Ballarín E, Casanovas J, Catà T, et al. Randomized clinical trial of the effectiveness of a home‐based intervention in patients with heart failure: the IC‐DOM study. Revista Espanola de Cardiologia 2009;62:400‐8. [DOI] [PubMed] [Google Scholar]
- Chang B, Zhang X, Peng W. Movement training for exercise tolerance and cardiac function in patients with chronic heart failure. Chinese Journal of Clinical Rehabilitation 2005;9(23):241‐2. [Google Scholar]
- Coats A, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, haemodynamics, ventilation and autonomic function. Circulation 1992;85(6):2119‐31. [DOI] [PubMed] [Google Scholar]
- Collins E, Langbein WE, Dilan‐Koetje J, Bammert C, Hanson K, Reda D, et al. Effects of exercise training on aerobic capacity and quality of life in individuals with heart failure. Heart Lung 2004;33(3):154‐61. [DOI] [PubMed] [Google Scholar]
- Corvera‐Tindel T, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. American Heart Journal 2004;147(2):339‐46. [DOI] [PubMed] [Google Scholar]
- Cowie A, Thow MK, Granat MH, Mitchell SL. A comparison of home and hospital‐based exercise training in heart failure: immediate and long‐term effects upon physical activity level. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18:158‐66. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang H, Wang F, lui S, Bi Y. Effects of six‐minutes walking training on left ventricular ejection fractions and motor ability in chronic heart failure patients. Chinese Journal of Clinical Rehabilitation 2006;7(18):2542‐3. [Google Scholar]
- Xu D, Wang B, Hou Y, Hui H, Meng S, Liu Y. The effects of exercise training on plasma tumor necrosis factor‐alpha, blood leucocyte and its components in congestive heart failure patients. Chinese Journal of Internal Medicine 2002;41(4):237‐40. [PubMed] [Google Scholar]
- Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex‐DHF (exercise training in diastolic heart failure) pilot study. Journal of the American College of Cardiology 2011;58:1780‐91. [DOI] [PubMed] [Google Scholar]
- Erbs S, Linke A, Gielen S, Fiehn E, Walther C, Yu J, et al. Exercise training in patients with severe chronic heart failure: impact on left ventricular performance and cardiac size. A retrospective analysis of the Leipzig Heart Failure Training Trial. European Journal of Cardiovascular Prevention and Rehabilitation 2003;10(5):223‐44. [DOI] [PubMed] [Google Scholar]
- Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circulation. Heart failure 2010;3(4):486‐94. [PUBMED: 20430934] [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328(7433):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, et al. Effects of home‐based exercise training on neurovascular control in patients with heart failure. European Journal of Heart Failure 2006;8(8):851‐5. [DOI] [PubMed] [Google Scholar]
- Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, et al. Home‐based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung 2004;33(4):210‐8. [DOI] [PubMed] [Google Scholar]
- Gary R, Lee SY. Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Progress in Cardiovascular Nursing 2007;22(2):72‐80. [PUBMED: 17541316] [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta‐analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. Journal of the American College of Cardiology 2007;19:2329‐36. [DOI] [PubMed] [Google Scholar]
- Inglis SC, Pearson S, Treen S, Gallasch T, Horowitz JD, Stewart S. Extending the horizon in chronic heart failure: effects of multidisciplinary, home‐based intervention relative to usual care. Circulation 2006;114(23):2466‐73. [DOI] [PubMed] [Google Scholar]
- Jolly K, Taylor RS, Lip GYH, Greenfield SM, Davies MK, Davis RC, et al. home based exercise rehabilitation in addition to specialist heart failure nurse care: design, rationale and recruitment to the Birmingham Rehabilitation Uptake Maximisation study for patients with congestive heart failure (BRUM‐CHF): a randomised controlled trial. BMC Cardiovascular Disorders 2007;7:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsdóttir S, Andersen KK, Sigurosson AF, Sigurosson SB. The effect of physical training in chronic heart failure. European Journal of Heart Failure 2006;1(97):97‐101. [DOI] [PubMed] [Google Scholar]
- Kiilavuori K, Näveri H, Salmi T, Härkönen M. The effect of physical training on hormonal status and exertional hormonal response in patients with chronic congestive heart failure. European Heart Journal 1999;20:456‐64. [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. Circulation: Heart Failure 2010;3:659‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N, Hashimoto S, Yasu T, et al. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities . Circulation Journal 2003;67(6):505‐10. [DOI] [PubMed] [Google Scholar]
- Korzeniowska‐Kubacka I, Bilińska M, Michalak E, Kuśmierczyk‐Droszcz B, Dobraszkiewicz‐Wasilewska B, Piotrowicz R. Influence of exercise training on left ventricular diastolic function and its relationship to exercise capacity in patients after myocardial infarction. Cardiology Journal 2010;23:136‐42. [PubMed] [Google Scholar]
- Lloyd‐Williams F, Mair FS, Leitner M. Exercise training and heart failure: a systematic review of current evidence. British Journal of General Practice 2002;52(474):47‐55. [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Görge G, Schwaab B, Hildebrandt K, Walldorf J, Schäfer C, et al. An alternative approach for exercise prescription and efficacy testing in patients with chronic heart failure: a randomized controlled training study. American Heart Journal 2005;149(5):e1‐7. [DOI] [PubMed] [Google Scholar]
- Molloy GJ, Johnston DW, Gao C, Witham MD, Gray JM, Argo IS, et al. Effects of an exercise intervention for older heart failure patients on caregiver burden and emotional distress. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(3):381‐7. [DOI] [PubMed] [Google Scholar]
- Mudge AM, Denaro CP, Scott AC, Atherton JJ, Meyers DE, Marwick TH, et al. Exercise training in recently hospitalized heart failure patients enrolled in a disease management programme: design of the EJECTION‐HF randomized controlled trial. European Journal of Heart Failure 2011;13:1370‐5. [DOI] [PubMed] [Google Scholar]
- Myers J, Gianrossi R, Schwitter J, Wagner D, Dubach P. Reduced ventricular function oxygen uptake kinetics in patients with effect of exercise training on postexercise. Chest 2001;120(4):1206‐11. [DOI] [PubMed] [Google Scholar]
- Myers J, Wagner D, Schertler T, Beer M, Luchinger R, Klein M, et al. Effects of exercise training on left ventricular volumes and function in patients with nonischemic cardiomyopathy: application of magnetic resonance myocardial tagging. American Heart Journal 2002;144(4):719‐25. [DOI] [PubMed] [Google Scholar]
- Myers J, Hadley D, Oswald U, Bruner K, Kottman W, Hsu L, et al. Effects of exercise training on heart rate recovery in patients with chronic heart failure. American Heart Journal 2007;152(6):1056‐63. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Clark AL, Webb‐Peploe KM, Coats AJ. Exercise training in chronic heart failure: effects on pro‐inflammatory markers. European Journal of Heart Failure 2005;7(2):189‐93. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Clark AL, Webb‐Peploe KM, Böger R, Coats AJ. Home‐based exercise training modulates pro‐oxidant substrates in patients with chronic heart failure. European Journal of Heart Failure 2005;7(2):183‐8. [DOI] [PubMed] [Google Scholar]
- Oka RK, Marco T, Haskell WL, Botvinick E, Dae MW, Bolen K, et al. Impact of a home‐based walking and resistance training program on quality of life in patients with heart failure. American Journal of Cardiology 2000;85(3):365‐9. [DOI] [PubMed] [Google Scholar]
- Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. European Journal of Heart Failure 2000;2(1):65‐70. [DOI] [PubMed] [Google Scholar]
- Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clinical Science (London) 2002;102(1):1‐7. [PubMed] [Google Scholar]
- Passino C, Ry S, Severino S, Gabutti A, Prontera C, Clerico A, et al. C‐type natriuretic peptide expression in patients with chronic heart failure: effects of aerobic training. European Journal of Cardiovascular Prevention & Rehabilitation 2008;15:168‐72. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Szelemej R, Kowalska‐Superlak M, Kratochwil D, Sobkowicz B, Sebzda T, et al. Exercise rehabilitation in patients with moderate‐severe chronic heart failure. Kardiologia Polska 1997;47(10):291‐300. [Google Scholar]
- Pozehl B, Duncan K, Krueger S, VerMaas P. Adjunctive effects of exercise training in heart failure patients receiving maximum pharmacologic therapy. Progress in Cardiovascular Nursing 2003;18(4):177‐83. [DOI] [PubMed] [Google Scholar]
- Pu CT, Johnson MT, Forman DE, Hausdorff JM, Roubenoff R, Foldvari M, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. Journal of Applied Physiology 2001;90(6):2341‐50. [DOI] [PubMed] [Google Scholar]
- Sabelis LW, Senden PJ, Boekhorst BC, Hulzebos HJ, Wiel A, Haeften TW, et al. Does physical training increase insulin sensitivity in chronic heart failure patients?. Clinical Science (London) 2004;106(5):459‐66. [DOI] [PubMed] [Google Scholar]
- Sarullo MF, Gristina T, Brusca I, Milia S, Raimondi R, Sajeva M, et al. Effect of physical training on exercise capacity, gas exchange and N‐terminal pro‐brain natriuretic peptide levels in patients with chronic heart failure. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(5):812‐7. [DOI] [PubMed] [Google Scholar]
- Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, et al. Moderate‐intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. Journal of Cardiac Failure 2004;10(1):21‐30. [DOI] [PubMed] [Google Scholar]
- Senden PJ, Sabelis LW, Zonderland ML, Hulzebos EH, Bol E, Mosterd WL. The effect of physical training on workload, upper leg muscle function and muscle areas in patients with chronic heart failure. International Journal of Cardiology 2005;100(2):293‐300. [DOI] [PubMed] [Google Scholar]
- Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. American Journal of Medicine 2004;116:693‐706. [DOI] [PubMed] [Google Scholar]
- Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. American Heart Journal 2007;119:1097. [DOI] [PubMed] [Google Scholar]
- Stewart S, Pearson S, Horowitz JD. Effects of a home‐based intervention among patients with congestive heart failure discharged from acute hospital care. Archives of Internal Medicine 1998;158(10):1067‐72. [DOI] [PubMed] [Google Scholar]
- Taylor‐Piliae RE, Froelicher ES. The effectiveness of Tai Chi exercise in improving aerobic capacity: a meta‐analysis. Holistic Nursing Practice 2004;18(5):254‐63. [DOI] [PubMed] [Google Scholar]
- Tyni‐Lenné R, Dencker K, Gordon A, Jansson E, Sylvén C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. European Journal of Heart Failure 2001;3(1):47‐52. [DOI] [PubMed] [Google Scholar]
- Berg‐Emons R, Balk A, Bussmann H, Stam H. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure?. European Journal of Heart Failure 2004;6(1):95‐100. [DOI] [PubMed] [Google Scholar]
- Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta‐analysis. European Journal of Heart Failure 2006;8:841‐50. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas D, Benetis R, Jasiukeviciene L, Grizas V, Marcinkeviciene J, Navickas R, et al. Exercise training after coronary angioplasty improves cardiorespiratory function. Scandinavian Cardiovascular Journal 2007;41(3):142‐8. [PUBMED: 17487762] [DOI] [PubMed] [Google Scholar]
- Wielenga RP, Erdman RA, Huisveld IA, Bol E, Dunselman PH, Baselier MR, et al. Effect of exercise training on quality of life in patients with chronic heart failure. Journal of Psychosomatic Research 1998;45(5):459‐64. [DOI] [PubMed] [Google Scholar]
- Williams AD, Carey MF, Selig S, Hayes A, Krum H, Patterson J, et al. Circuit resistance training in chronic heart failure improves skeletal muscle mitochondrial ATP production rated a randomized controlled trial. Journal of Cardiac Failure 2007;13(2):79‐85. [DOI] [PubMed] [Google Scholar]
- Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation 2007;115(24):3042‐94. [DOI] [PubMed] [Google Scholar]
- Yeh GY, Wood MJ, Lorell BH, Stevenson LW, Eisenberg DM, Wayne PM, et al. Effects of tai chi mind‐body movement therapy on functional status and exercise capacity in patients with chronic heart failure: a randomized controlled trial. American Journal of Medicine 2004;117(8):541‐8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Lui J, Zhang S, Yuan M. Effect of walking movement on plasma TNF and receptor in chronic heart failure patients. Chinese Journal of Clinical Rehabilitation 2003;7(24):2248‐9. [Google Scholar]
- Zhao X. Effects of exercise training on the improvement of cardiac function and exercise endurance in patients with chronic heart failure. Chinese Journal of Clinical Rehabilitation 2005;9(16):170‐1. [Google Scholar]
References to ongoing studies
- ISRCTN86879094. Exercise training in diastolic heart failure: a prospective, randomized, controlled study to determine the effects of exercise training in patients with heart failure and preserved ejection fraction (Ex‐DHF). www.controlled‐trials.com/ISRCTN86879094/ (accessed 11 April 2014).
- Mudge AM, Denaro CP, Scott AC, Atherton JJ, Meyers DE, Marwick TH, et al. Exercise training in recently hospitalized heart failure patients enrolled in a disease management programme: design of the EJECTION‐HF randomized controlled trial. European Journal of Heart Failure 2011;13:1370‐5. [DOI] [PubMed] [Google Scholar]
- NCT00012883. Home walking exercise training in advanced heart failure. ClinicalTrials.gov/show/NCT00012883 (accessed 11 April 2014).
- NCT00013221. Exercise effect on aerobic capacity and QOL in heart failure. ClinicalTrials.gov/show/NCT00013221 (accessed 11 April 2014).
- NCT01033591. Exercise for patients with heart failure in primary care: the EFICAR. ClinicalTrials.gov/show/NCT01033591 (accessed 11 April 2014).
Additional references
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;15:e147‐239. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics ‐ 2014 update: a report from the American Heart Association. Circulation 2014;129:e28‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Association for Cardiovascular Prevention and Rehabilitation (BACPR). The BACPR standards and core components for cardiovascular disease prevention and rehabilitation, 2nd edition, 2012. www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf (accessed 11 April 2014).
- Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low‐dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 1998;97:553‐61. [DOI] [PubMed] [Google Scholar]
- Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998‐2008. JAMA 2011;306:1669‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home‐based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Australian Journal of Physiotherapy 2008;54:87‐93. [DOI] [PubMed] [Google Scholar]
- Corra U, Giannuzzia, P, Adamopoulos S, Bjornstad Bjarnason‐Wehernsd HB, Cohen‐Solale A, Dugmore D, et al. Executive summary of the position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology (ESC): core components of cardiac rehabilitation in chronic heart failure. European Journal of Cardiovascular Prevention and Rehabilitation 2005;12:32‐105. [DOI] [PubMed] [Google Scholar]
- Dalal HM, Wingham J, Palmer J, Taylor R, Petre C, Lewin R. Why do so few patients with heart failure participate in cardiac rehabilitation? A cross‐sectional survey from England, Wales and Northern Ireland. BMJ Open 2012;2:e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial. On the horizon in heart failure. Lancet 2011;378:637. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneiger M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista LS, Hamilton MA, Fonarow GC, Dracup K. Is exercise adherence associated with clinical outcomes in patients with advanced heart failure?. Physician and Sportsmedicine 2010;38:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training metaanalysis of trials in patients with chronic heart failure (ExTraMATCH). Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. [Erratum in: JAMA 2009;302(21):2322]. JAMA 2009;301:1451‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost‐effectiveness analysis of long‐term moderate exercise training in chronic heart failure. American Journal of Cardiology 2001;87:984‐8. [DOI] [PubMed] [Google Scholar]
- Gotzsche P. Does exercise training lower mortality in patients with chronic heart failure? 2005. www.bmj.com/rapid‐response/2011/10/30/does‐exercise‐training‐lower‐mortality‐patients‐chronic‐heart‐failure (accessed 11 April 2014).
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. A meta‐analysis. Journal of the American College of Cardiology 2011;19:1676‐86. [DOI] [PubMed] [Google Scholar]
- Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O'Connor CM, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. Journal of the American College of Cardiology 2012;60:1899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostis JB, Davis BR, Cutler J. Prevention of heart failure antihypertensive drug treatment in older persons with isolated systemic hypertension. SHEP Cooperative Research Group. JAMA 1997;278(3):212‐6. [PubMed] [Google Scholar]
- Lam CSP, Donal E, Kraigher‐Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. European Journal of Heart Failure 2011;13:18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E. Cardiac resynchronization therapy for congestive heart failure. Evidence Report/Technology Assessment (Summary) 2004;November:1‐8. [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2012;14:803‐69. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Chronic heart failure. Management of chronic heart failure in adults in primary and secondary care. guidance.nice.org.uk/CG108 (accessed 11 April 2014).
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th edition. Boston: Little, Brown & Co, 1994. [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine 2006;355:251‐9. [DOI] [PubMed] [Google Scholar]
- Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure.. J Card Fail. 2001;7:176‐82. [DOI] [PubMed] [Google Scholar]
- Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European Heart Journal 2007;28:2539‐50. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Maugeri FS, Campana M, Ferrari R, Giordano A, Scalvini S, et al. Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. European Heart Journal 1998;19:466‐75. [DOI] [PubMed] [Google Scholar]
- Shekelle P, Morton S, Atkinson S, Suttorp M, Tu W, Heidenreich P, et al. Pharmacologic management of heart failure and left ventricular systolic dysfunction: effect in female, black, and diabetic patients, and cost‐effectiveness. Evidence Report/Technology Assessment (Summary) 2003;82:1‐6. [PMC free article] [PubMed] [Google Scholar]
- Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature‐based meta‐analysis. European Journal of Heart Failure 2009;11:855‐62. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007130.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney S, Mamas M, Skelton D, Woods S, Rutter MK, Gibson M, et al. What can we learn from patients with heart failure about exercise adherence? A systematic review of qualitative papers. Health Psychology 2011;30:401‐10. [DOI] [PubMed] [Google Scholar]
- Watson RDS, Gibbs CR, Lip GYH. ABC of heart failure: clinical features and complications. BMJ 2000;320:236‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Group on Cardiac Rehabilitation and Exercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise training in chronic heart failure patients. European Heart Journal 2011;22:125‐35. [Google Scholar]
- Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF‐ACTION): design and rationale. American Heart Journal 2007;153:201‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI] [PubMed] [Google Scholar]
- Rees K, Taylor RS, Singh S, Coats AJS, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


