Abstract
Background
Chronic pain is common and can be challenging to manage. Despite increased utilisation of opioids, the safety and efficacy of long‐term use of these compounds for chronic non‐cancer pain (CNCP) remains controversial. This overview of Cochrane Reviews complements the overview entitled 'High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane Reviews'.
Objectives
To provide an overview of the occurrence and nature of adverse events associated with any opioid agent (any dose, frequency, or route of administration) used on a medium‐ or long‐term basis for the treatment of CNCP in adults.
Methods
We searched the Cochrane Database of Systematic Reviews (the Cochrane Library) Issue 3, 2017 on 8 March 2017 to identify all Cochrane Reviews of studies of medium‐ or long‐term opioid use (2 weeks or more) for CNCP in adults aged 18 and over. We assessed the quality of the reviews using the AMSTAR criteria (Assessing the Methodological Quality of Systematic Reviews) as adapted for Cochrane Overviews. We assessed the quality of the evidence for the outcomes using the GRADE framework.
Main results
We included a total of 16 reviews in our overview, of which 14 presented unique quantitative data. These 14 Cochrane Reviews investigated 14 different opioid agents that were administered for time periods of two weeks or longer. The longest study was 13 months in duration, with most in the 6‐ to 16‐week range. The quality of the included reviews was high using AMSTAR criteria, with 11 reviews meeting all 10 criteria, and 5 of the reviews meeting 9 out of 10, not scoring a point for either duplicate study selection and data extraction, or searching for articles irrespective of language and publication type. The quality of the evidence for the generic adverse event outcomes according to GRADE ranged from very low to moderate, with risk of bias and imprecision being identified for the following generic adverse event outcomes: any adverse event, any serious adverse event, and withdrawals due to adverse events. A GRADE assessment of the quality of the evidence for specific adverse events led to a downgrading to very low‐ to moderate‐quality evidence due to risk of bias, indirectness, and imprecision.
We calculated the equivalent milligrams of morphine per 24 hours for each opioid studied (buprenorphine, codeine, dextropropoxyphene, dihydrocodeine, fentanyl, hydromorphone, levorphanol, methadone, morphine, oxycodone, oxymorphone, tapentadol, tilidine, and tramadol). In the 14 Cochrane Reviews providing unique quantitative data, there were 61 studies with a total of 18,679 randomised participants; 12 of these studies had a cross‐over design with two to four arms and a total of 796 participants. Based on the 14 selected Cochrane Reviews, there was a significantly increased risk of experiencing any adverse event with opioids compared to placebo (risk ratio (RR) 1.42, 95% confidence interval (CI) 1.22 to 1.66) as well as with opioids compared to a non‐opioid active pharmacological comparator, with a similar risk ratio (RR 1.21, 95% CI 1.10 to 1.33). There was also a significantly increased risk of experiencing a serious adverse event with opioids compared to placebo (RR 2.75, 95% CI 2.06 to 3.67). Furthermore, we found significantly increased risk ratios with opioids compared to placebo for a number of specific adverse events: constipation, dizziness, drowsiness, fatigue, hot flushes, increased sweating, nausea, pruritus, and vomiting.
There was no data on any of the following prespecified adverse events of interest in any of the included reviews in this overview of Cochrane Reviews: addiction, cognitive dysfunction, depressive symptoms or mood disturbances, hypogonadism or other endocrine dysfunction, respiratory depression, sexual dysfunction, and sleep apnoea or sleep‐disordered breathing. We found no data for adverse events analysed by sex or ethnicity.
Authors' conclusions
A number of adverse events, including serious adverse events, are associated with the medium‐ and long‐term use of opioids for CNCP. The absolute event rate for any adverse event with opioids in trials using a placebo as comparison was 78%, with an absolute event rate of 7.5% for any serious adverse event. Based on the adverse events identified, clinically relevant benefit would need to be clearly demonstrated before long‐term use could be considered in people with CNCP in clinical practice. A number of adverse events that we would have expected to occur with opioid use were not reported in the included Cochrane Reviews. Going forward, we recommend more rigorous identification and reporting of all adverse events in randomised controlled trials and systematic reviews on opioid therapy. The absence of data for many adverse events represents a serious limitation of the evidence on opioids. We also recommend extending study follow‐up, as a latency of onset may exist for some adverse events.
Plain language summary
Side effects of opioid drugs when used to treat chronic non‐cancer pain in the medium‐ or long‐term
Bottom line
There is good‐quality evidence showing that side effects can occur in people with chronic non‐cancer pain who use opioid medicines for longer than two weeks.
Background
Opioids are a type of pain medicine related to opium. We conducted an overview of Cochrane Reviews, which are a type of scientific paper, to learn what these papers said about the side effects of opioid drugs. We were interested in the medium‐ and long‐term side effects with this treatment for pain in adults who use opioid medicines who have chronic pain that is not due to cancer. We studied opioid medications compared to pills that do not contain any medicine (placebos) and opioid medications compared to other treatments.
Key results
In March 2017, we found 16 Cochrane Reviews of 14 different opioid medicines, including codeine, morphine, and oxycodone. These papers included 61 studies with more than 18,000 participants. We found that people who take opioids have a higher risk of having any side effect, such as constipation, dizziness, and nausea, as well as having a serious side effect. We did not find any information in the Cochrane Reviews about many of the known and sometimes serious side effects of opioids, such as addiction, depression, and sleep problems.
Quality of the reviews and the evidence
We rated the quality of the included reviews out of 10 points. As all of the reviews scored 9 or 10 out of 10, we are confident that the quality of the included reviews is very good. We also rated the quality of the evidence from studies using four levels: very low, low, moderate, or high; these ratings showed how sure we could be about our results for the side effects of opioids. Very low‐quality evidence meant that we are very unsure about the results. High‐quality evidence meant that we are very sure of the results. All of our ratings were between very low and moderate.
Background
Description of the condition
Pain is described as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" (Merskey 1994). Chronic pain is typically described as pain on most days for at least three months. Chronic non‐cancer pain (CNCP) is any chronic pain that is not due to a malignancy. Chronic non‐cancer pain is frequently divided into neuropathic pain (i.e. pain originating in nerves) and non‐neuropathic or nociceptive pain, which is often musculoskeletal in origin and arises from structures such as muscles, bones, or ligaments.
Chronic non‐cancer pain is very common in adults. A recent review estimated the prevalence of CNCP (of moderate or severe intensity, lasting more than three months) at approximately 20%, with considerable variation between studies (Moore 2014). The impact of CNCP on life is substantial, affecting quality of life and activities of daily living, social life, and work, with approximately 20% of people with chronic pain unable to work due to pain (Moore 2014).
The personal and subjective nature of pain makes objective measurement impossible; the assessment of pain is subjective and based on individual report (Breivik 2008). Different instruments are used to measure pain and to determine its impact on the physical, social, emotional, and spiritual aspects of life.
Description of the interventions
The treatment of pain may encompass a variety of approaches, including pharmacological management. Effective pain therapy has been described in terms of a reduction in pain intensity of at least 50% over study baseline, and results in consistent improvements in fatigue, sleep, depression, quality of life, and work ability (Moore 2014). Opioid therapy is used for the treatment of both acute and chronic pain conditions. There is a large number of policies and guidelines to assist with the use of opioids for the management of chronic pain. The World Health Organization (WHO) published a field‐tested analgesic ladder to guide the use of sequentially stronger pain medications for the relief of cancer pain, including opioids and non‐opioids such as non‐steroidal anti‐inflammatory drugs (NSAIDs) (WHO 1996). This tool is now applied generally for people who require analgesic treatment and is widely used for both cancer and non‐cancer pain (Vargas‐Schaffer 2010).
Long‐term opioid use may be associated with problematic patterns of use, leading to clinically significant impairment or distress, including substance use disorders (i.e. abuse and dependence). Opioid use may also be associated with somatic and psychological sequelae, including depressive disorders, anxiety disorders, sleep disorders, sexual dysfunction, and delirium (APA 2013). Furthermore, chronic opioid use is associated with a risk of fatal and non‐fatal overdose, as well as cardiovascular events, endocrinological harms, and motor vehicle accidents (Dowell 2016). High‐quality evidence demonstrates that an increased risk of vehicle crashes exists with the use of opioids (Hegmann 2014a). Operating a vehicle is considered a surrogate for safety‐sensitive work tasks, and hence the use of opioids is usually deemed incompatible with working in safety‐sensitive positions (Hegmann 2014a), and may also be incompatible with decision‐critical tasks. Opioid use may therefore have direct implications on ability to work and economic productivity.
The American College of Occupational and Environmental Medicine's Evidence‐based Practice Guidelines conclude that quality evidence (moderate or high quality) does not support the concept of superiority of opioids over NSAIDs or other medications for the treatment of CNCP (Hegmann 2014b). Estimates of efficacy may also be inflated by inappropriate imputation methods (McQuay 2012). Furthermore, there is a relative dearth of literature available on how to discontinue opioids in high‐dose users (Windmill 2013).
In view of the absence of dependable, high‐quality evidence for long‐term benefits with the use of opioids for CNCP, the Centers for Disease Control and Prevention suggest utilising the lowest effective dose, with careful reassessment of benefits versus risks when increasing the dose to 50 morphine milligram equivalents or more per day (Dowell 2016).
How the intervention might work
This overview focused on the use of opioids for their key function of analgesia. Opium is a plant‐derived substance, with pharmacologically active ingredients including morphine and codeine. The term 'opioids' can refer to either naturally occurring compounds ('opiates') or synthetic compounds. Opioids act by binding to opioid receptors; mu, kappa, and delta opioid receptors are widely distributed throughout the nervous system (Rachinger‐Adam 2011). Opioids bring about complex changes at the cellular and molecular level, decreasing pain perception and increasing tolerance to painful stimuli (Borg 2014).
Other opioid actions include euphoria (Schulteis 1996), sedation, drowsiness, and endocrine dysregulation (Vuong 2010). Opioids alter sleep regulation, and are associated with poor sleep quality, insomnia, respiratory depression, sleep apnoea, and sleep‐disordered breathing (Zutler 2011). Physiological dependence on opioids may develop rapidly after the initiation of opioid use, leading to opioid abuse and dependence (opioid use disorder). Increasing doses of opioids over time are a common and significant concern with this group of medications (Kosten 2002).
A number of effects have been identified with the acute administration of opioids or in opioid‐naive people; it has been suggested that chronic opioid use results in fewer medical problems (Rass 2014). However, there are serious and potentially lethal adverse effects that may occur with long‐term use.
Why it is important to do this overview
Opioids are now commonly and increasingly used for the treatment of pain, including CNCP (Zutler 2011). In fact, there has been a large increase in the use of opioids for CNCP in recent years despite safety concerns and a lack of convincing evidence of effectiveness (Kidner 2009; Chapman 2010; Bohnert 2012). Evidence of utilisation of larger doses of opioids for the treatment of CNCP is emerging. For example, one analysis of Workers' Compensation Board (WCB) data (where the vast majority of claimants with pain would have non‐malignant pain) from Manitoba, Canada, demonstrated a dramatic increase in the average opioid dose prescribed over time: from less than 500 morphine milligram equivalents per person per year in 1998 to over 6000 morphine milligram equivalents per person per year in 2010. Moreover, compared to other Manitobans, the WCB claimant population was about twice as likely to be prescribed doses above 120 morphine milligram equivalents per day (Kraut 2015). Opioid use often continues post‐claim, and both duration and dose of post‐claim opioid use are correlated with the dose during the claim (Shafer 2015). Dramatic increases in the number of opioid prescriptions have been seen across the world since the 2000s, for example in the UK (Zin 2014), Australia (Leong 2009), and the USA (Manchikanti 2012a). The rate of dispensing of high‐dose opioids specifically (i.e. doses of 200 morphine milligram equivalents per day or greater) increased in Canada by 23% between 2006 and 2011 (Gomes 2014).
The previous perception of the adverse event profile associated with opioid use may have contributed to the current opioid use and overdose epidemic in North America, which has been decades in the making. A letter published in The New England Journal of Medicine in 1980 examined the incidence of narcotic addiction in 39,946 hospitalised medical patients and suggested that addiction was rare in patients treated with opioids (Porter 1980). Later in the 1980s, Portenoy and Foley described an addiction risk of lower than 1% (Portenoy 1986). By current standards, most of the patients in that study were not on high‐dose opioids: two‐thirds (n = 25) required a dose of less than 20 morphine milligram equivalents per day, while only four participants received more than 40 morphine milligram equivalents per day (Portenoy 1986). Another survey of 100 participants receiving opioids for CNCP (mean treatment duration 224 days) suggested partial or good relief for almost 80% of those participants, with the most common adverse events listed as nausea and constipation, but no reported cases of respiratory depression or addiction (Zenz 1992). Guidelines for managing CNCP published in 1995 in the Canadian Family Physician cited evidence in support of an extremely low risk of addiction and evidence of high rates of efficacy of opioids for CNCP, as well as a relative paucity of adverse events in people first receiving opioids for medical reasons (Hagen 1995).
However, the liberal use of opioids for CNCP has come under scrutiny due to questions about their effectiveness and the potential for adverse events, abuse, and addiction (NOUGG 2010; Franklin 2014; Häuser 2014; Nuckols 2014; Katz 2015). There has recently been considerable criticism of earlier publications and their role in contributing to the opioid epidemic. One seminal paper, Porter 1980, was described as having been "heavily and uncritically cited as evidence that addiction was rare with long‐term opioid therapy. We believe that this citation pattern contributed to the North American opioid crisis by helping to shape a narrative that allayed prescribers’ concerns about the risk of addiction associated with long‐term opioid therapy" (Leung 2017).
The updated Canadian opioid guidelines, Busse 2017, had also come under criticism for potential financial conflicts of interest (Howlett 2017), highlighting the need for independent and unbiased summaries of the evidence such as those provided by Cochrane.
In contrast to the early and more permissive approaches to opioid use for CNCP, more recent evidence suggests that opioid abuse and addiction are well documented among people with chronic pain (Vowles 2015). There is a potential for opioid addiction to develop even if these compounds are used for the management of severe pain (Kosten 2002; Huffman 2015; Vowles 2015). The risk for addiction increases with increasing opioid doses. Huffman and colleagues reported that a 50‐milligram increase in oral morphine milligram equivalent dose almost doubled the risk of addiction; a 100‐milligram dose increase was associated with a three‐fold increase in that risk (Huffman 2015).
There is furthermore the potential for serious adverse events. Serious adverse events, as defined by the US Food and Drug Administration, are those with patient outcomes of life‐threatening effects, hospitalisation, disability or permanent damage, intervention to prevent permanent impairment, drug dependence or abuse, death, or another event that jeopardises the patient or requires treatment to prevent one of the other outcomes (FDA 2016). Some outcomes, including sleep‐disordered breathing and respiratory depression, may result in opioid‐associated deaths and demonstrate a clear relationship to dose (Walker 2007; Jungquist 2012). Drug interactions are another concern, as is interaction with alcohol, which can result in several types of serious adverse events (McCance‐Katz 2010).
Hegmann and colleagues summarised the substantial increase in the use of opioids and the increase in deaths associated with opioids (Hegmann 2014b). Opioid‐related deaths are common and can occur even when the prescription is in accordance with guidelines. Most opioid‐related deaths in the USA (60%) occurred in people given prescriptions based on prescribing guidelines by medical boards (with 20% of deaths at doses of 100 morphine milligram equivalents per day or less, and 40% in people who received doses above that threshold). The remaining 40% of deaths occurred in people abusing the drugs (Manchikanti 2012a). Abuse of opioids may be related to multiple prescriptions/'double‐doctoring', requesting early refills, and drug diversion.
A consensus is emerging that long‐term opioid therapy for CNCP may be appropriate only for well‐selected populations (Manchikanti 2012b). Furthermore, agreement is building that high‐dose opioid treatment should be used with extreme caution for indications other than cancer pain.
Objectives
To provide an overview of the occurrence and nature of adverse events associated with any opioid agent (any dose, frequency, or route of administration) used on a medium‐ or long‐term basis for the treatment of CNCP in adults.
Methods
Criteria for considering reviews for inclusion
We included all Cochrane Reviews that assessed medium‐ or long‐term opioid use for CNCP due to any condition in adults. The reviews must have reported our specified adverse event outcomes. We planned to analyse data from trials of opioids versus placebo and opioids versus non‐opioid treatments. We planned to analyse data from randomised controlled trials (RCTs) and other study designs separately.
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (the Cochrane Library), Issue 3, 2017, on 8 March 2017, using the search strategy presented in Appendix 1.
Data collection and analysis
Selection of reviews
Medium‐ and long‐term opioid use have been variably defined. For our overview, we defined opioid use between two weeks and two months as medium‐term and two months or longer as long‐term use. We would expect trial durations of two weeks or more to be relevant for a chronic painful condition. Included reviews therefore assessed RCTs of opioid use versus placebo or active (non‐opioid) comparator for two weeks or longer, for CNCP due to any condition in adults.
The reviews must also have reported the inclusion and exclusion criteria used to select studies and the presence or absence of one or more of our specified adverse event outcomes. We only included trials from the reviews that met our criteria in the analyses.
Data extraction and management
Two overview authors (of CE, VL, and TJ) independently screened the results of the electronic search by title and abstract to assess reviews for inclusion. We obtained the full‐text versions of reviews deemed potentially relevant and subsequently applied the eligibility criteria to determine final inclusion. Reasons for exclusion are detailed in Table 1. Any disagreements were resolved by discussion or by consulting a third overview author (SSt).
1. Reasons for exclusion.
| Review | Reason for exclusion |
| Challapalli 2005 | Trials either included cancer pain, did not use opioids, or were not at least 2 weeks in duration. |
| Duehmke 2006 | Did not exclude cancer pain |
| Gaskell 2014 | Review update published as Gaskell 2016. |
| Moore 2015a | No opioids studied. |
| Mujakperuo 2010 | No opioids studied. |
| Ramiro 2011 | Trials with opioids were less than 2 weeks in duration. |
| Rubinstein 2012 | No opioids studied. |
| Seidel 2013 | Trials with opioids were for acute pain. |
We piloted a standardised data extraction form on three reviews and revised this for clarity and comprehensiveness. At least two overview authors (of CE, TJ, DK, VL, BS, and FK) then independently extracted data using this form and assessed methodological quality. After completion of the analyses, two overview authors (CE, TJ) independently assessed the quality of the evidence for the outcomes of interest. Where we were unable to achieve consensus, we consulted a third overview author (SSt).
We extracted data on the following:
citation details;
conditions studied;
number of included studies;
study and participant characteristics;
opioid medications used, formulation, doses, and frequencies of administration;
adverse event outcomes;
which studies were eligible, if there were studies from the review that did not meet all of the eligibility criteria.
The adverse event outcomes of interest were:
number of participants with any adverse event;
number of participants with any serious adverse event;
number of participants who withdrew from the studies due to adverse events;
number of deaths;
number of participants who experienced the following specific adverse events (of any severity):
addiction;
cognitive dysfunction;
constipation;
depressive symptoms or other mood disturbances;
hypogonadism or other endocrine dysfunction;
infection;
respiratory depression;
sexual dysfunction;
sleep apnoea or sleep‐disordered breathing;
xerostomia.
We added the following adverse events, which were not originally identified by us as outcomes of interest, but were reported in the included reviews and deemed relevant:
anorexia (loss of appetite);
diarrhoea;
dizziness;
drowsiness;
fatigue;
headache;
hot flushes;
increased sweating;
nausea;
pruritus;
sinusitis;
unspecified gastrointestinal events;
unspecified neurological events;
vomiting.
We consulted the original study reports where necessary to clarify discrepancies in data across reviews, or where only partial data was presented in the reviews. Some adverse events were variably named between the trials and reviews; we accepted different terminology as long as it pertained to similar concepts. For example, we combined "drowsiness" and "somnolence". We added other specific adverse events that were described in the reviews, as listed above. If the reporting of adverse events had been specified by sex or ethnicity in any of the reviews, we would have extracted these data as well. We aimed primarily to compare opioids to control groups receiving placebo; we also undertook comparisons of opioids versus non‐opioid treatments.
Where data from a trial were presented in more than one review, these data were only included once and were ascribed to the review that was published first. There was one exception where the earliest review, Chaparro 2013, did not include data on adverse events for one study (O'Donnell 2009), and in this case we used the study data as presented in a later review (Enthoven 2016). Without such de‐duplication, studies would have been counted multiple times, as shown in Table 2. We have included an outcome matrix to show which outcomes were extractable from which reviews (see Table 3 for outcomes reported for opioids versus placebo, and Table 4 for opioids versus active comparators).
2. Number of trials in reviews with quantitative data.
| Review | Total number of trials | Number of eligible trials | Number of trials also in other reviews | Number of de‐duplicated trials |
| Cepeda 2006 | 11 | 8 | 0 | 8 |
| Chaparro 2012 | 21 | 5 | 4 | 5 |
| Chaparro 2013 | 15 | 10 | 2 | 9 |
| da Costa 2014 | 22 | 19 | 2 | 18 |
| Derry 2015 | 6 | 1 | 1 | 0 |
| Derry 2016 | 1 | 1 | 0 | 1 |
| Enthoven 2016 | 13 | 1 | 0 | 1 |
| Gaskell 2016 | 5 | 5 | 4 | 1 |
| Haroutiunian 2012 | 3 | 2 | 2 | 2 |
| McNicol 2013 | 31 | 13 | 10 | 6 |
| Noble 2010 | 26 | 6 | 1 | 6 |
| Rubinstein 2011 | 3 | 1 | 0 | 1 |
| Santos 2015 | 4 | 4 | 2 | 2 |
| Stannard 2016 | 1 | 1 | 0 | 1 |
| Whittle 2011 | 11 | 2 | 2 | 2 |
| Totals | 173 | 79 | 29 | 63 |
3. Outcome matrix: opioids versus placebo for reviews contributing quantitative outcomes.
| Events reported | Cepeda 2006 | Chaparro 2012 | Chaparro 2013 | da Costa 2014 | Derry 2016 | Gaskell 2016 | Haroutiunian 2012 | McNicol 2013 | Noble 2010 | Santos 2015 | Stannard 2016 | Whittle 2011 | Totals |
| Any adverse event | X | X | X | X | X | X | 6 | ||||||
| Any serious adverse event | X | X | X | X | X | X | 6 | ||||||
| Withdrawals due to adverse events | X | X | X | X | X | X | X | X | X | X | 10 | ||
| Deaths | X | X | X | X | X | X | X | X | X | 9 | |||
| Anorexia | X | 1 | |||||||||||
| Constipation | X | X | X | X | 4 | ||||||||
| Diarrhoea | X | 1 | |||||||||||
| Dizziness | X | X | X | X | X | 5 | |||||||
| Drowsiness or somnolence | X | X | X | X | 4 | ||||||||
| Fatigue | X | 1 | |||||||||||
| Gastrointestinal (unspecified) | X | 1 | |||||||||||
| Headache | X | 1 | |||||||||||
| Hot flushes | X | 1 | |||||||||||
| Increased sweating | X | 1 | |||||||||||
| Infection | X | X | 2 | ||||||||||
| Nausea | X | X | X | X | 4 | ||||||||
| Nervous system (unspecified) | X | 1 | |||||||||||
| Pruritus | X | 1 | |||||||||||
| Sinusitis | X | 1 | |||||||||||
| Vomiting | X | X | X | 3 | |||||||||
| Xerostomia | X | 1 |
An "X" indicates that the outcome was reported (whether or not any participants experienced it).
In Cepeda 2006, "serious adverse events" were defined as adverse events that resulted in withdrawals. These data are therefore included in both categories for the review in question.
4. Outcome matrix: opioids versus active comparator.
| Events reported | Cepeda 2006 | Chaparro 2012 | Enthoven 2016 | Haroutiunian 2012 | McNicol 2013 | Rubinstein 2011 | Totals |
| Any adverse event | X | X | 2 | ||||
| Any serious adverse event | X | 1 | |||||
| Withdrawals due to adverse events | X | X | X | X | 4 | ||
| Constipation | X | X | 2 | ||||
| Dizziness | X | 1 | |||||
| Drowsiness or somnolence | X | 1 | |||||
| Nausea | X | 1 | |||||
| Vomiting | X | 1 |
An "X" indicates that the outcome was reported (whether or not any participants experienced it).
Assessment of methodological quality of included reviews
An overview of Cochrane Reviews on adverse events associated with treatments for acute pain has established appropriate criteria (adapted from the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) guidance), Shea 2007, for the quality assessment of the Cochrane Reviews to be included in an overview (Moore 2015b). Following this example, we assessed the reviews with the following questions.
Was an a priori design provided?
Was there duplicate study selection and data extraction?
Was a comprehensive literature search performed?
Were published and unpublished studies included irrespective of language of publication?
Was a list of studies (included and excluded) provided?
Were the characteristics of the included studies provided?
Was the scientific quality of the included studies assessed and documented?
Was the scientific quality of the included studies used appropriately in formulating conclusions?
Were the methods used to combine the findings of studies appropriate?
Was a conflict of interest stated?
Data synthesis
We performed qualitative and quantitative evidence syntheses as appropriate. For meta‐analysis, we used either a fixed‐effect or alternatively a random‐effects model as determined by between‐study heterogeneity. In addition to assessing statistical heterogeneity (I² statistic), we also considered clinical heterogeneity between the studies. We used a fixed‐effect model when there was no evidence of significant heterogeneity of either type. We calculated risk ratios (RRs) and numbers needed to treat for an additional harmful outcome (NNTH) from the pooled number of events using the method of Cook and Sackett (Cook 1995). We did not calculate an NNTH where the RR was not significant (the 95% confidence interval (CI) of the RR included 1). We also calculated the proportion of participants experiencing adverse events and associated 95% CIs; if the lower bound of such a 95% CI was calculated as negative, we reported it as 0, following the methodology of Moore and McQuay (Moore 2005). We conducted the methodology for our overview and for meta‐analyses according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed our analyses for all opioid agents together. We had planned to conduct supplementary analyses for the individual opioid drugs and by trial duration. In some reviews, outcomes were reported only for a treatment group and not for the placebo or comparator group. We have therefore presented additional summary data for adverse events experienced with opioids from studies with or without reported comparators.
We assessed the quality of the evidence on adverse events associated with medium‐ and long‐term use of opioids for CNCP using the GRADE approach as applied in Cochrane Reviews (Higgins 2011). See Appendix 2 for a further description of the GRADE system.
Results
Our searches of the Cochrane Database of Systematic Reviews identified 421 records. We excluded 397 records based on titles and abstracts, and obtained the full texts of the remaining 24 records. We excluded eight reviews for reasons such as the reviews not reporting non‐cancer pain separately, not studying opioids, or investigating acute rather than chronic pain (Table 1).
We included 16 Cochrane Reviews in total. For a further description of our screening process, see the study flow diagram (Figure 1).
1.
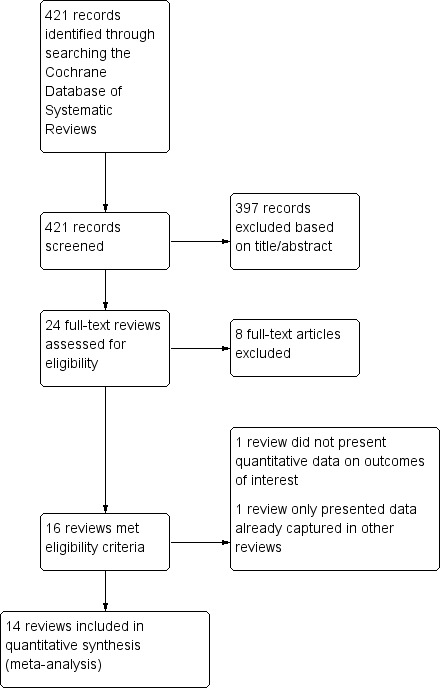
Study selection.
Description of included reviews
We included a total of 16 reviews in the overview, of which 15 presented quantitative data, and 14 of these presented quantitative data that was not already included in a previously published review. The 14 Cochrane Reviews containing novel quantitative data investigated 14 different opioid agents (buprenorphine, codeine, dextropropoxyphene, dihydrocodeine, fentanyl, hydromorphone, levorphanol, methadone, morphine, oxycodone, oxymorphone, tapentadol, tilidine, and tramadol) that were administered for a period of at least two weeks for CNCP and reported adverse events (Figure 1 and Table 5). The opioid agents and doses studied in the included reviews are detailed in Table 6. Conversions were performed to calculate the equivalent milligrams of morphine per 24 hours for each opioid studied, according to the sources in Table 7. Conversion factors for transdermal fentanyl were computed from the manufacturer's monograph (Fentanyl monograph 2017).
5. Characteristics of reviews.
| Review | Date assessed as up‐to‐date | Condition(s) studied | Participant characteristics | Inclusion criteria | Exclusion criteria | Duration of treatment in eligible studies |
| Alviar 2011 | Oct‐11 | Phantom limb pain | Participants of any age with established phantom limb pain | Pharmacologic agents given singly or in combination | Stump/residual limb pain alone, or postamputation pain that was not phantom pain, or phantom pain mixed with other neuropathic pains; pharmacologic interventions aimed at preventing phantom limb pain | 10 weeks (no quantitative data reported on outcomes of interest) |
| Cepeda 2006 | May‐06 | Osteoarthritis | Adults with primary or secondary osteoarthritis of the hip or knee | Tramadol or tramadol plus paracetamol used | Other types of arthritis; non‐osteoarthritic joint pain or back pain | 14 to 91 days |
| Chaparro 2012 | Apr‐12 | Neuropathic pain | Adults with neuropathic pain | Compared combinations of 2 or more drugs against placebo or another comparator | Studies with a neuraxial approach or that included injection therapies, transcutaneous electrical stimulation, or vitamins | 5 to 36 weeks (includes a cross‐over trial of 9 weeks with 4 conditions) |
| Chaparro 2013 | Apr‐13 | CLBP | Adults with persistent pain in the low back for at least 12 weeks | Any opioid prescribed in an outpatient setting for 1 month or longer | Participants with cancer, infections, inflammatory arthritic conditions, compression fractures, or studies where less than 50% of participants had CLBP | 4 to 15 weeks |
| da Costa 2014 | Aug‐12 | Osteoarthritis | Adults with osteoarthritis of the knee or hip | Any type of opioid except tramadol | Trials with inflammatory arthritis exclusively or with less than 75% of participants having osteoarthritis of the knee or hip | 2 to 30 weeks |
| Derry 2015 | Jan‐15 | Neuropathic pain | Adults with a chronic neuropathic pain condition | Nortriptyline at any dose, by any route, compared to placebo or any active comparator | Nortriptyline given in combination with other drugs, without separate reporting | 28 weeks (no unique data was reported) |
| Derry 2016 | Jun‐16 | Neuropathic pain | Adults with postherpetic neuralgia, complex regional pain syndrome, or chronic postoperative pain | Fentanyl at any dose, by any route | Treatment of < 2 weeks | 94 to 113 days |
| Enthoven 2016 | Jun‐15 | CLBP | Adults with non‐specific CLBP for at least 12 weeks | 1 or more types of NSAIDs used | Trials of NSAIDs no longer available on the market; participants with sciatica or with specific low back pain caused by pathological entities, e.g. infection, neoplasm, metastases, osteoporosis, rheumatoid arthritis, or fractures | 6 weeks |
| Gaskell 2016 | Dec‐15 | Chronic neuropathic pain | Adults with painful diabetic neuropathy or postherpetic neuralgia | Any dose or formulation of oxycodone | Fewer than 10 participants per treatment arm, or less than 2 weeks of treatment | 12 weeks |
| Haroutiunian 2012 | Apr‐12 | CNCP | Adults having any type of CNCP | Methadone by any route in randomised or quasi‐randomised studies | Studies with fewer than 10 participants | 40 to 119 days |
| McNicol 2013 | Aug‐13 | Neuropathic pain | Adults with central or peripheral neuropathic pain of any aetiology | Opioid agonists used in an RCT | Partial opioid agonists or agonist‐antagonists used | 6 to 16 weeks (includes a 6‐ and 8‐week cross‐over trial with 2 conditions) |
| Noble 2010 | May‐09 | CNCP | Adults with chronic pain for at least 3 months | Treament for at least 6 months | Fewer than 10 participants | 2 weeks to 13 months |
| Rubinstein 2011 | Dec‐09 | CLBP | Adults with CLBP, with or without radiating pain | Mean duration of CLBP > 12 weeks | Single‐treatment studies; studies examining specific pathologies (e.g. sciatica) | 6 weeks |
| Santos 2015 | Mar‐14 | CNCP | Adults with osteoarthritis of the knee or hip, CLBP | Tapentadol ER in doses of 100 to 500 mg/day | Pain for less than 3 months or that was not moderate to severe | 15 to 52 weeks |
| Stannard 2016 | Nov‐15 | Neuropathic pain | Adults with 1 or more chronic neuropathic pain conditions | Hydromorphone at any dose, by any route | Treatment of < 2 weeks | 14 to 16 weeks |
| Whittle 2011 | May‐10 | Rheumatoid arthritis pain | Adults with rheumatoid arthritis | Opioids of any formulation at any dose, by any route | Studies of opioid therapy for rheumatoid arthritis in the immediate postoperative setting | 6 to 10 weeks |
CLBP: chronic low back pain CNCP: chronic non‐cancer pain NSAIDs: non‐steroidal anti‐inflammatory drugs RCT: randomised controlled trial Tapentadol ER: tapentadol extended‐release
6. Opioids in included reviews reporting unique quantitative data.
| Drug | Formulations | Dosing Schedule | Dose (lowest) | Dose (highest) | MEq (lowest) | MEq (highest) | Cepeda 2006 | Chaparro 2012 | Chaparro 2013 | da Costa 2014 | Derry 2016 | Enthoven 2016 | Gaskell 2016 | Haroutiunian 2012 | McNicol 2013 | Noble 2010 | Rubinstein 2011 | Santos 2015 | Stannard 2016 | Whittle 2011 |
| Buprenorphine | Transdermal patch (µg/h) | ‐ | 5 µg/h | 40 µg/h | 12 | 96 | X | X | ||||||||||||
| Codeine | Contin | Twice a day, 3 times a day | 32 | 200 | 4.8 | 30 | X | X | ||||||||||||
| Dextropropoxyphene | ‐ | 3 times a day | 300 | ‐ | 30 | ‐ | X | |||||||||||||
| Dihydrocodeine | LA | Every 12 hours | 30 | 240 | 3 | 24 | X | X | ||||||||||||
| Fentanyl | Transdermal patch (µg/h) | ‐ | 12.5 µg/h | 250 µg/h | 45 | 944 | X | X | X | |||||||||||
| Hydromorphone | ER, OROS | Once a day | 4 | 64 | 16 | 256 | X | X | ||||||||||||
| Levorphanol | ‐ | 3 times a day | 0.45 | 15.75 | 4.95 | 173.5 | X | |||||||||||||
| Methadone | ‐ | Twice a day | 5 | 80 | 15 | 240 | X | X | ||||||||||||
| Morphine | Avinza, Contin, CR, ER, LA, SR | Twice a day, once a day, every 12 hours, as needed | 15 | 300 | 15 | 300 | X | X | X | X | X | X | X | |||||||
| Oxycodone | CR, ER, LA, MR, PR, immediate‐release, liquid | Twice a day, 3 times a day to 6 times a day | 10 | 160 | 15 | 240 | X | X | X | X | X | X | X | |||||||
| Oxycodone and naloxone | PR | ‐ | ‐ | ‐ | ‐ | ‐ | X | |||||||||||||
| Oxycodone and naltrexone | ‐ | 4 times a day | 10 | 40 | 15 | 60 | X | |||||||||||||
| Oxymorphone | ER | Twice a day, every 12 hours | 10 | 140 | 30 | 420 | X | X | ||||||||||||
| Tapentadol | ER, immediate‐release | Twice a day, 3 times a day to 6 times a day | 100 | 500 | 40 | 200 | X | X | X | |||||||||||
| Tilidine and naloxone | ‐ | ‐ | 4 | 12 | 10 | 30 | X | |||||||||||||
| Tramadol | ER, LP, Retard | Twice a day, as needed, 3 times a day, 4 times a day, once a day, every 12 hours | 37.5 | 400 | 3.75 | 40 | X | X | X | X | X |
Dose is given in milligrams, except for transdermal opioids, which are given in micrograms.
CR: controlled‐release ER: extended‐release LA: long‐acting LP: sustained‐release (libération prolongée) MEq: the equivalent number of milligrams of morphine per 24‐hour period MR: modified‐release OROS: extended‐release (registered trademark)
PR: Prolonged release Retard: prolonged‐release SR: sustained‐release
7. Opioid dose conversions.
| Opioid | Source | Equivalent dose of oral morphine, in mg, per 1 mg of the converted opioid |
| Buprenorphine (transdermal) | EMRPCC 2016 | 100 |
| Codeine | OARRS 2016 | 0.15 |
| Dextropropoxyphene | Van Griensven | 0.1 |
| Dihydrocodeine | NHS Wales | 0.1 |
| Fentanyl (transdermal) | Fentanyl monograph 2017 | 158* |
| Hydromorphone | OARRS 2016 | 4 |
| Levorphanol | University of Alberta 2017 | 7.5 |
| Methadone | OARRS 2016 | 3 |
| Oxycodone | OARRS 2016 | 1.5 |
| Oxymorphone | OARRS 2016 | 3 |
| Tapentadol | OARRS 2016 | 0.4 |
| Tilidine | Radbruch 2013 | 0.2 |
| Tramadol | OARRS 2016 | 0.1 |
Transdermally delivered opioid doses (buprenorphine and fentanyl) are usually expressed as an hourly rate of delivery, but were converted to the dose per 24 hours before being converted into morphine equivalents.
*Calculated as the mean conversion factor from data in Fentanyl monograph 2017.
On appraising the reviews, we added the following adverse events to our prespecified list of specific adverse events: anorexia, diarrhoea, dizziness, drowsiness, fatigue, headache, hot flushes, increased sweating, nausea, pruritus, sinusitis, unspecified gastrointestinal events, unspecified neurological events, and vomiting.
Seven of our prespecified adverse events were not reported in any of the included reviews: addiction, cognitive dysfunction, depressive symptoms or mood disturbance, hypogonadism or other endocrine dysfunction, respiratory depression, sexual dysfunction, and sleep apnoea or sleep‐disordered breathing. In our overview, we extracted data on serious adverse events if they were reported as such in the included Cochrane Reviews.
We found no data for adverse events analysed by sex or ethnicity. We excluded data from studies under two weeks' duration from the analysis.
In some reviews, outcomes were reported only for a treatment group and not for the placebo group. We have therefore presented summary data for opioids used with or without a comparator as supplementary analyses (Table 8, Table 9, and Table 10).
8. Any adverse event with opioids (from studies with or without comparators).
| Review | Events | Total | Event rate (%) | |
| Average | 95% CI | |||
| Cepeda 2006 | 481 | 1613 | 29.8 | 27.6 to 32.1 |
| da Costa 2014 | 2145 | 2725 | 78.7 | 77.2 to 80.3 |
| Enthoven 2016 | 454 | 785 | 57.8 | 54.4 to 61.3 |
| Gaskell 2016 | 40 | 48 | 83.3 | 72.8 to 93.9 |
| Rubinstein 2011 | 1 | 17 | 5.9 | ‐5.3 to 17.1 |
| Santos 2015 | 766 | 894 | 85.7 | 83.4 to 88 |
| Stannard 2016 | 21 | 43 | 48.8 | 33.9 to 63.8 |
| Total events | 3908 | 6622 | 59.0 | 57.8 to 60.2 |
CI: confidence interval
9. Any serious adverse event with opioids (from studies with or without comparators).
| Review | Events | Total | Event rate (%) | |
| Average | 95% CI | |||
| Cepeda 2006 | 196 | 899 | 21.8 | 19.1 to 24.5 |
| da Costa 2014 | 9 | 355 | 2.5 | 0.9 to 4.2 |
| Gaskell 2016 | 4 | 48 | 8.3 | 0.5 to 16.2 |
| Santos 2015 | 73 | 1767 | 4.1 | 3.2 to 5.1 |
| Stannard 2016 | 6 | 134 | 4.5 | 1 to 8 |
| Total events | 288 | 3203 | 9.0 | 8 to 10 |
CI: confidence interval
10. Withdrawals due to adverse events with opioids (from studies with or without comparators).
| Review | Events | Total | Event rate (%) | |
| Average | 95% CI | |||
| Cepeda 2006 | 196 | 899 | 21.8 | 19.1 to 24.5 |
| Chaparro 2012 | 63 | 526 | 12.0 | 9.2 to 14.8 |
| da Costa 2014 | 1169 | 4398 | 26.6 | 25.3 to 27.9 |
| Enthoven 2016 | 132 | 785 | 16.8 | 14.2 to 19.5 |
| Gaskell 2016 | 3 | 48 | 6.3 | 0 to 13.1 |
| Haroutiunian 2012 | 11 | 90 | 12.2 | 5.5 to 19 |
| McNicol 2013 | 19 | 177 | 10.7 | 6.2 to 15.3 |
| Noble 2010 | 620 | 1830 | 33.9 | 31.7 to 36.1 |
| Santos 2015 | 480 | 1770 | 27.1 | 24.9 to 29.3 |
| Stannard 2016 | 3 | 43 | 7.0 | 7 to 7 |
| Whittle 2011 | 3 | 11 | 27.3 | 27.3 to 27.3 |
| Total events | 2699 | 10,577 | 25.5 | 25.5 to 25.5 |
In some reviews, specific adverse event outcomes were reported only as qualitative data. For example, a review on phantom limb pain noted that constipation, nausea, and drowsiness were commonly reported in opioid trials (Alviar 2011). The occurrence of these adverse events as most common or frequent was echoed by two other reviews (Cepeda 2006; Whittle 2011), which presented quantitative data only for generic adverse event outcomes.
Methodological quality of included reviews
The AMSTAR quality assessment found that only two of the criteria were not met by all reviews (Table 11). Two reviews did not explicitly describe duplicate, independent study selection and data extraction (Chaparro 2012; Enthoven 2016). Three reviews did not state that they included non‐English, unpublished, and/or grey literature in their searches (Noble 2010; Chaparro 2013; McNicol 2013).
11. Results of AMSTAR quality assessment.
| AMSTAR criteria | Alviar 2011 | Cepeda 2006 | Chaparro 2012 | Chaparro 2013 | da Costa 2014 | Derry 2015 | Derry 2016 | Enthoven 2016 | Gaskell 2016 | Haroutiunian 2012 | McNicol 2013 | Noble 2010 | Rubinstein 2011 | Santos 2015 | Stannard 2016 | Whittle 2011 |
| 1. A priori design | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Duplicate selection and extraction | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Comprehensive literature search | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4. Published and unpublished, no language restrictions | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| 5. List of studies provided | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6. Characteristics of studies provided | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Scientific quality assessed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8. Scientific quality used in formulating conclusions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9. Methods used to combine appropriate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10. Conflict of interest stated | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score/10 | 10 | 10 | 9 | 9 | 10 | 10 | 10 | 9 | 10 | 10 | 9 | 9 | 10 | 10 | 10 | 10 |
AMSTAR: Assessing the Methodological Quality of Systematic Reviews
GRADE quality judgement
The GRADE quality judgements, which are detailed in Table 12, Table 13, Table 14, and Table 15, revealed the following:
12. GRADE quality judgement: opioids versus placebo.
|
Participants (reviews) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Overall quality of evidence | |
| Any adverse event | 1583 (1 review) | Serious | Not serious | Not serious | Not serious | None | +++◯ MODERATE |
| Any serious adverse event | 108 (1 review) | Serious | Not serious | Not serious | Not serious | None | +++◯ MODERATE |
| Withdrawals due to adverse events | 2375 (4 reviews) | Serious | Not serious | Not serious | Not serious | None | +++◯ MODERATE |
13. GRADE quality judgement: opioids versus placebo, specific adverse events.
|
Participants (reviews) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Overall quality of evidence | |
| Constipation | 4255 (4 reviews) |
Serious | Not serious | Serious | Not serious | Strong association | +++◯ MODERATE |
| Dizziness | 4130 (4 reviews) |
Serious | Not serious | Serious | Not serious | Strong association | +++◯ MODERATE |
| Drowsiness or somnolence | 3856 (3 reviews) |
Serious | Not serious | Serious | Not serious | Strong association | +++◯ MODERATE |
| Fatigue | 1589 (1 review) |
Serious | Not serious | Very serious | Not serious | None | +◯◯◯ VERY LOW |
| Hot flushes | 593 (1 review) |
Serious | Not serious | Very serious | Not serious | None | +◯◯◯ VERY LOW |
| Increased sweating | 1350 (1 review) |
Serious | Not serious | Very serious | Not serious | Very strong association | +++◯ MODERATE |
| Nausea | 4346 (3 reviews) |
Serious | Not serious | Serious | Not serious | Strong association | +++◯ MODERATE |
| Pruritus | 2865 (1 review) |
Serious | Not serious | Very serious | Not serious | None | +◯◯◯ VERY LOW |
| Vomiting | 3368 (2 reviews) |
Serious | Not serious | Very serious | Not serious | Strong association | ++◯◯ LOW |
14. GRADE quality judgement: opioids versus active pharmacological comparator.
|
Participants (reviews) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Overall quality of evidence | |
| Any adverse event | 1583 (1 review) | Serious | Not serious | Not serious | Not serious | None | +++◯ MODERATE |
| Any serious adverse event | 108 (1 review) | Serious | Not serious | Not serious | Very serious | None | +◯◯◯ VERY LOW |
| Withdrawals due to adverse events | 2375 (4 reviews) | Serious | Not serious | Not serious | Not serious | None | +++◯ MODERATE |
15. GRADE quality judgement: opioids versus non‐pharmacological intervention.
|
Participants (reviews) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Overall quality of evidence | |
| Any adverse event | 32 (1 review) |
Very serious | Not serious | Not serious | Not serious | None | +◯◯◯ VERY LOW |
Opioids compared to placebo
Any adverse event: moderate quality of evidence
Any serious adverse event: moderate quality of evidence
Withdrawal due to adverse events: moderate quality of evidence
Constipation: moderate quality of evidence
Dizziness: moderate quality of evidence
Drowsiness or somnolence: moderate quality of evidence
Fatigue: very low quality of evidence
Hot flushes: very low quality of evidence
Increased sweating: moderate quality of evidence
Nausea: moderate quality of evidence
Pruritus: very low quality of evidence
Vomiting: low quality of evidence
Opioids compared to active (non‐opioid) pharmacological comparators
Any adverse event: moderate quality of evidence
Any serious adverse event: very low quality of evidence
Withdrawal due to adverse events: moderate quality of evidence
Opioids compared to non‐pharmacological interventions
Any adverse event: very low quality of evidence
Effect of interventions
Opioids compared to placebo
Number of participants with any adverse event
There was a significantly increased risk of experiencing any adverse event with opioids compared to placebo (risk ratio (RR) 1.42, 95% confidence interval (CI) 1.22 to 1.66; Figure 2 and Table 16). The absolute event rate was 78% (Table 17).
2.
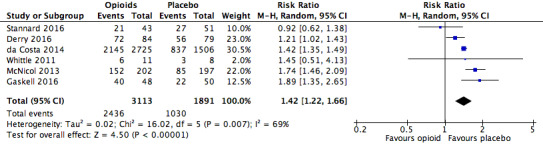
Analysis 1.1: Opioids versus placebo, any adverse event.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
16. Opioids versus placebo: risk ratio and number needed to treat for an additional harmful outcome (NNTH) for generic adverse events.
| Adverse event | Studies | Participants | Statistical method | Risk ratio | NNTH |
| Any adverse event | 6 | 5004 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 (1.22, 1.66) | 4.20 (3.78, 4.74) |
| Any serious adverse event | 6 | 4324 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 (2.06, 3.67) | 28.71 (20.50, 47.88) |
| Withdrawals due to adverse events | 10 | 11,510 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 (3.02, 3.82) | 5.55 (5.19, 5.97) |
CI: confidence interval M‐H: Mantel‐Haenszel method of meta‐analysis
17. Absolute event rates: opioids versus placebo.
| Opioid | Placebo | ||||||||
| Number of participants | Event rate (%) | Number of participants | Event rate (%) | ||||||
| Analysis | Adverse event | With AE | Total | Average | 95% CI | With AE | Total | Average | 95% CI |
| 1.1 | Any adverse event | 2436 | 3113 | 78.3 | 76.8 to 79.7 | 1030 | 1891 | 54.5 | 52.2 to 56.7 |
| 1.2 | Any serious adverse event | 216 | 2893 | 7.5 | 6.5 to 8.4 | 57 | 1431 | 4.0 | 3 to 5 |
| 1.3 | Withdrawals due to adverse events | 1836 | 7316 | 25.1 | 24.1 to 26.1 | 297 | 4194 | 7.1 | 6.3 to 7.9 |
| 2.1 | Constipation | 285 | 2513 | 11.3 | 10.1 to 12.6 | 94 | 1742 | 5.4 | 4.3 to 6.5 |
| 2.6 | Dizziness | 284 | 2448 | 11.6 | 10.3 to 12.9 | 71 | 1682 | 4.2 | 3.3 to 5.2 |
| 2.7 | Drowsiness or somnolence | 237 | 2313 | 10.3 | 9 to 11.5 | 57 | 1543 | 3.7 | 2.8 to 4.6 |
| 2.8 | Fatigue | 57 | 796 | 7.2 | 5.4 to 8.9 | 29 | 793 | 3.7 | 2.4 to 5 |
| 2.10 | Hot flushes | 14 | 295 | 4.8 | 2.3 to 7.2 | 5 | 298 | 1.7 | 0.2 to 3.1 |
| 2.11 | Increased sweating | 32 | 674 | 4.7 | 3.1 to 6.3 | 2 | 676 | 0.3 | 0.0 to 0.7 |
| 2.12 | Nausea | 535 | 2556 | 20.9 | 20.9 to 20.9 | 151 | 1790 | 8.4 | 8.4 to 8.4 |
| 2.13 | Pruritus | 155 | 1809 | 8.6 | 8.6 to 8.6 | 52 | 1056 | 4.9 | 4.9 to 4.9 |
| 2.15 | Vomiting | 184 | 2058 | 8.9 | 8.9 to 8.9 | 28 | 1310 | 2.1 | 2.1 to 2.1 |
AE: adverse event CI: confidence interval
Number of participants with any serious adverse event
There was an increased risk of experiencing any serious adverse event with opioids compared to placebo (RR 2.75, 95% CI 2.06 to 3.67; Figure 3). The absolute event rate was 7.5% (Table 17).
3.
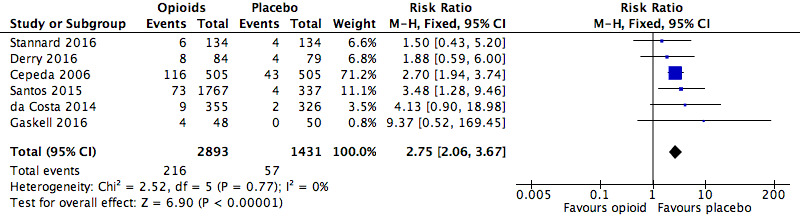
Analysis 1.2: Opioids versus placebo, any serious adverse event.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
Number of participants who withdrew from the studies due to adverse events
We found that the risk of participants withdrawing from the trials due to adverse events was significantly increased with opioid treatment compared to placebo (RR 3.40, 95% CI 3.02 to 3.82; Figure 4). The absolute event rate was 25% (Table 17).
4.
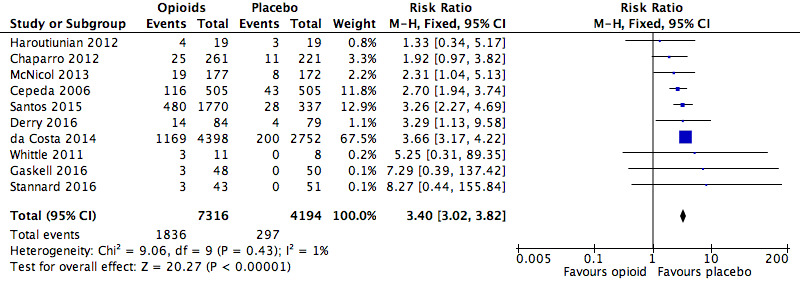
Analysis 1.3: Opioids versus placebo, withdrawals due to adverse events.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
Number of deaths
A total of two deaths were reported in the included reviews (da Costa 2014 and Gaskell 2016). In one RCT, Afilalo 2010, reviewed in da Costa 2014, the death occurred in the oxycodone group and was ascribed to myocardial infarction. The death in Gimbel 2003, reviewed in Gaskell 2016, was also in an oxycodone group and was ascribed to acute renal failure.
Number of participants who experienced specific adverse events (of any severity)
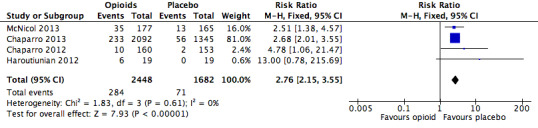
We also found significantly increased risk ratios with opioids compared to placebo for a number of specific adverse events: constipation (Figure 5), dizziness (Figure 6), drowsiness (Figure 7), fatigue (Figure 8), hot flushes (Figure 9), increased sweating (Figure 10), nausea (Figure 11), pruritus (Figure 12), and vomiting (Figure 13). Table 18 summarises the specific adverse events with opioids and placebo.
5.
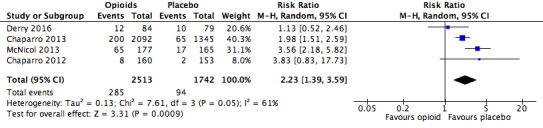
Analysis 2.1: Opioids versus placebo, constipation.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
6.
Analysis 2.6: Opioids versus placebo, dizziness.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
7.
Analysis 2.7: Opioids versus placebo, drowsiness.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
8.
Analysis 2.8: Opioids versus placebo, fatigue.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
9.
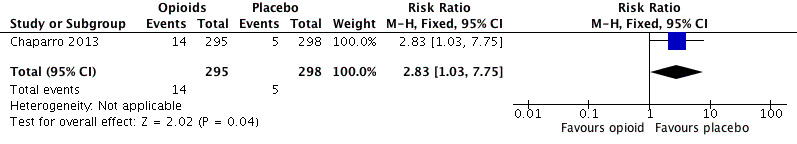
Analysis 2.10: Opioids versus placebo, hot flushes.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
10.

Analysis 2.11: Opioids versus placebo, increased sweating.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
11.
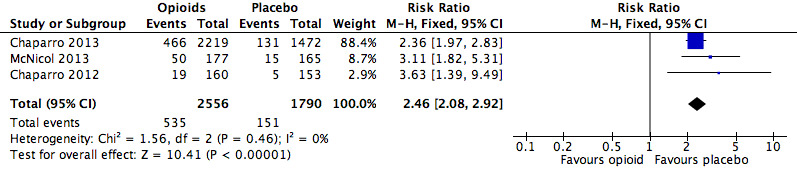
Analysis 2.12: Opioids versus placebo, nausea.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
12.
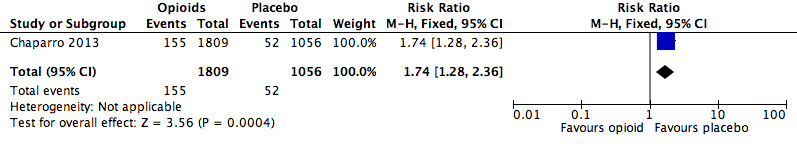
Analysis 2.13: opioids versus placebo, pruritus.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
13.
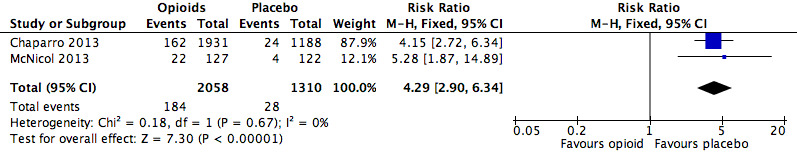
Analysis 2.15: Opioids versus placebo, vomiting.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
18. Opioids versus placebo: risk ratio and number needed to treat for an additional harmful outcome (NNTH) for specific adverse events.
| Adverse event | Studies | Participants | Statistical method | Risk ratio | NNTH |
| Anorexia | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.64 (0.77, 240.21) | ‐ |
| Constipation | 4 | 4255 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 (1.39, 3.59) | 16.82 (13.20, 23.19) |
| Diarrhoea | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 (0.69, 9.43) | ‐ |
| Dizziness | 4 | 4130 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 (2.15, 3.55) | 13.55 (11.15, 17.28) |
| Drowsiness, sleepiness, somnolence, or anergia | 3 | 3856 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 (2.19, 3.83) | 15.26 (12.34, 20.00) |
| Fatigue | 1 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 (1.27, 3.03) | 28.54 (17.48, 77.71) |
| Gastrointestinal (unspecified) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 (0.90, 3.47) | ‐ |
| Headache | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 (0.33, 1.84) | ‐ |
| Hot flushes | 1 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 (1.03, 7.75) | 32.60 (16.95, 421.76) |
| Increased sweating | 1 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.05 (3.86, 66.69) | 22.46 (16.37, 35.78) |
| Infection | 2 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 (0.47, 1.61) | ‐ |
| Nausea | 3 | 4346 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 (2.08, 2.92) | 8.00 (6.88, 9.56) |
| Nervous system disorders (unspecified) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 (0.95, 6.56) | ‐ |
| Pruritus | 1 | 2865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 (1.28, 2.36) | 27.44 (18.25, 55.27) |
| Sinusitis | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 (0.52, 4.67) | ‐ |
| Vomiting | 2 | 3368 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 (2.90, 6.34) | 14.70 (12.10, 18.72) |
| Xerostomia | 1 | 1668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 (0.47, 2.57) | ‐ |
CI: confidence interval M‐H: Mantel‐Haenszel method of meta‐analysis
Opioids versus active pharmacological comparators
Table 19 outlines the active comparators in the included reviews.
19. Active comparators in included reviews.
| Drug | Total dose per day | Dosing schedule | Cepeda 2006 | Chaparro 2012 | Enthoven 2016 | Haroutiunian 2012 |
| Celecoxib | 400 mg | ‐ | X | |||
| Desipramine | 10 to 160 mg | ‐ | X | |||
| Diclofenac | 25 to 150 mg | Up to 3 times a day | X | |||
| Gabapentin | 1200 to 3600 mg | 3 times a day | X | |||
| Lorazepam | 0.7 to 1.6 mg | Twice a day and 3 times a day | X | |||
| Naproxen | 250 to 1000 mg | ‐ | X | |||
| Nortriptyline | 10 to 160 mg | Twice a day | X | X |
An "X" indicates that the drug was used as an active comparator to opioids in the review.
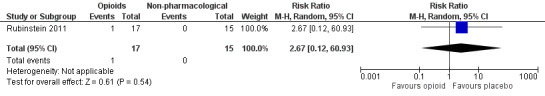
Rubinstein 2011 used a non‐pharmacological comparator (spinal manipulative therapy).
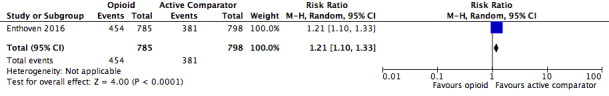
Number of participants with any adverse event
The absolute event rate for any adverse event with opioids compared with active pharmacological comparators was 58% (Figure 14 and Table 20).
14.
Analysis 3.1: Opioids versus active pharmacological comparator, any adverse event.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
20. Absolute event rates: opioids versus active pharmacological comparator.
| Opioid | Active comparator | ||||||||
| Number of participants | Event rate (%) | Number of participants | Event rate (%) | ||||||
| Analysis | Adverse event | With AE | Total | Average | 95% CI | With AE | Total | Average | 95% CI |
| 1.1 | Any adverse event | 454 | 785 | 57.8 | 54.4 to 61.3 | 381 | 798 | 47.7 | 44.3 to 51.2 |
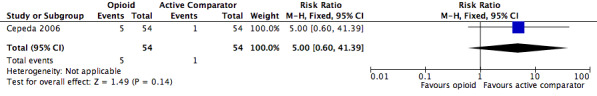
| 1.2 | Any serious adverse event | 5 | 54 | 9.3 | 1.5 to 17 | 1 | 54 | 1.9 | 0 to 5.4 |
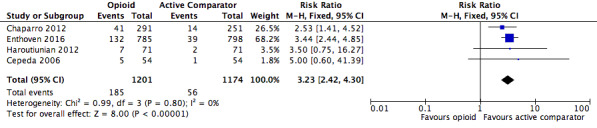
| 1.3 | Withdrawals due to adverse events | 185 | 1201 | 15.4 | 13.4 to 17.4 | 56 | 1174 | 4.8 | 3.6 to 6 |
AE: adverse event CI: confidence interval
Number of participants with any serious adverse event
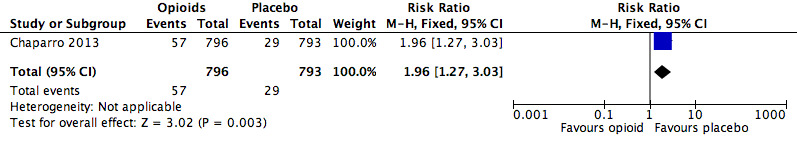
The absolute event rate for any serious adverse event with opioids compared with active pharmacological comparators was 9.3% (Figure 15 and Table 20).
15.
Analysis 3.2: Opioids versus active pharmacological comparator, any serious adverse event.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
Number of participants who withdrew from the studies due to adverse events
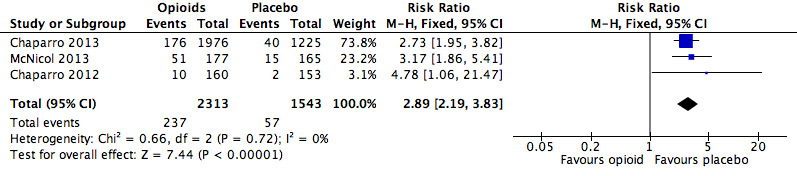
The risk of withdrawals from the trials due to adverse events was increased in participants treated with opioids compared to other active pharmacological interventions (RR 3.23, 95% CI 2.42 to 4.30; Figure 16 and Table 21). The absolute event rate for withdrawal from studies due to adverse events for those taking opioids was 15% (Table 20).
16.
Analysis 3.3: Opioids versus active pharmacological comparator, withdrawals due to adverse events.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
21. Opioids versus active pharmacological comparator: risk ratio and number needed to treat for an additional harmful outcome (NNTH) for generic adverse events.
| Adverse event | Studies | Participants | Statistical method | Risk ratio | NNTH |
| Any adverse event | 1 | 1583 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 (1.10, 1.33) | 9.91 (6.67, 19.24) |
| Any serious adverse event | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 (0.60, 41.39) | ‐ |
| Withdrawals due to adverse events | 4 | 2375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 (2.42, 4.30) | 9.40 (7.69, 12.11) |
CI: confidence interval M‐H: Mantel‐Haenszel method of meta‐analysis
Number of deaths
No deaths were reported.
Number of participants who experienced specific adverse events (of any severity)
No data was reported.
Opioids versus active non‐pharmacological comparators
Number of participants with any adverse event
The absolute event rate for any adverse event with opioids compared with active non‐pharmacological comparators was 5.8% (Figure 17 and Table 22).
17.
Analysis 4.1: Opioids versus active non‐pharmacological comparator, any adverse event.
CI: confidence interval df: degrees of freedom M‐H: Mantel‐Haenszel method of meta‐analysis P: probability Z: Z score (standard score)
22. Absolute event rates: opioids versus active non‐pharmacological comparator.
| Opioid | Active comparator | ||||||||
| Number of participants | Event rate (%) | Number of participants | Event rate (%) | ||||||
| Analysis | Adverse event | With AE | Total | Average | 95% CI | With AE | Total | Average | 95% CI |
| 1.1 | Any adverse event | 1 | 17 | 5.8 | 0 to 17.1 | 0 | 15 | 0 | 0 to 0 |
AE: adverse event CI: confidence interval
Discussion
Given the current evidence of limited efficacy and risk for serious adverse events, primary care providers often find the treatment of the common condition of CNCP to be challenging (Dowell 2016). There are substantial variations in clinical practice, influenced by politics, economics, and socioeconomic variables, which complicate generalisation of solutions (Moore 2010). Similarly, physicians' opioid prescribing practices are impacted by several factors, including societal and normative values as well as prescribers' perception of efficacy of the opioid and the risk of adverse events. Earlier studies, which have not always been conducted to current methodological standards, suggest a relative paucity of opioid‐related adverse events, including reports of a low risk of addiction with the use of opioids (Porter 1980; Portenoy 1986).
The perception of infrequent adverse events and even more infrequent serious adverse events, along with advocacy by special interest groups aimed at remedying an undertreatment of chronic pain, and with marketing efforts by opioid manufacturers, arguably resulted in a bolstering of prescribing by physicians. Reliance on earlier studies with less robust methodology may have contributed to the current opioid use epidemic and opioid overdoses and deaths. In the midst of a public health crisis related to opioid use and overdoses, there was a need to examine the existing evidence to determine the true nature of occurrence of adverse events, as well as the efficacy of commonly used approaches to the management of CNCP. This overview suggests that the occurrence of adverse events with opioids for CNCP is both common and clinically relevant.
Summary of main results
This overview included 16 Cochrane Reviews, of which 15 reported quantitative data, and 14 of these contained data not already presented in earlier reviews. The 14 Cochrane Reviews reporting unique quantitative data had 18,679 participants, and investigated 14 different opioids for a variety of chronic non‐cancer painful conditions where opioids were administered for longer than two weeks. There is a 42% higher risk of any adverse events and a 175% increased risk of serious adverse events associated with opioid use when compared to placebo.
The risks of specific adverse events were increased for constipation, dizziness, drowsiness, fatigue, hot flushes, increased sweating, nausea, pruritus, and vomiting.
Overall completeness and applicability of evidence
This overview of Cochrane Reviews suggests that the occurrence of adverse events related to the medium‐ and long‐term use of opioids is common, but unlike what is observed in clinical practice, the included reviews reported a limited range of specific adverse events. The overview authors consider addiction, cognitive dysfunction, hypogonadism or other endocrine dysfunction, respiratory depression, and sleep apnoea or sleep‐disordered breathing as significant harms, none of which were reported in the reviews, and the absence of reporting represents a serious limitation.
Only two deaths were reported in the reviews, both of which were in participants randomised to oxycodone, and were ascribed to myocardial infarction and renal failure, respectively.
The reviews included in this overview also did not report adverse events by sex or ethnicity. For some adverse events, such as endocrinological harms, sex‐specific reporting would have been especially informative.
Quality of the evidence
We utilised AMSTAR to evaluate the included reviews. The methodological quality of the 16 systematic reviews included in this overview was high overall. The quality of the evidence for the outcomes according to GRADE ranged from very low to moderate, with risk of bias and imprecision identified for the following generic adverse event outcomes: any adverse event, any serious adverse event, and withdrawals due to adverse events. A GRADE assessment of the quality of evidence for specific adverse events led to a downgrading to very low to moderate due to risk of bias, indirectness, and imprecision.
Potential biases in the overview process
To limit the potential for bias in this overview, two overview authors conducted the key steps, involving a third overview author to resolve discrepancies. We adhered to the methodology described in the protocol.
Agreements and disagreements with other studies or reviews
The Canadian guideline for opioid therapy and chronic non‐cancer pain reports the presence of substantial risks associated with the use of opioids (Busse 2017). This guideline is a departure from the 2010 Canadian guideline, where 200 mg morphine equivalent per day was considered a "watchful dose", and where doses in excess of this were supported under some circumstances (NOUGG 2010). Although some differences emerged, the present overview yielded evidence that was mostly consistent with the 2016 Centers for Disease Control and Prevention (CDC) guideline for prescribing opioids for chronic pain (Dowell 2016).
The prevalence of opioid‐use disorders in primary care settings in some recent studies ranged from 3% to 26% (Fleming 2007; Banta‐Green 2009; Boscarino 2010). The CDC guideline reports the association of long‐term opioid use with the development of opioid abuse and dependence, whereas the reviews included in this overview did not report opioid abuse or dependence. This should not be misinterpreted as the absence of risk for the development of a substance use disorder. Possible explanations are that these outcomes were not reported in the RCTs or the Cochrane Reviews, or that the inclusion criteria were sufficiently stringent to have screened out potential participants with risk factors for addiction or abuse. Furthermore, trial duration may not have been sufficient for these adverse events to manifest. In the CDC guideline, there is mention of an increased risk of fatal and non‐fatal overdose (Dunn 2010; Gomes 2011). Yet, the Cochrane Reviews included in this overview did not report overdoses, either fatal or non‐fatal. Similarly, there were no instances of endocrinological harms in the reviews included in this overview, despite the salient caution in this regard in the CDC guideline.
In their position paper, the American Academy of Neurology suggests that no substantial evidence exists for maintained pain relief or improved function with chronic opioid use for CNCP without incurring serious risk of developing adverse events (Franklin 2014).
The Washington State guideline on prescribing opioids for pain suggests that the most commonly reported adverse events in RCTs included constipation, nausea and vomiting, dizziness, and drowsiness, but that more serious long‐term adverse events have only been identified from observational studies (AMDG 2015).
These observations from major guideline groups are broadly consistent with the findings of the present overview of Cochrane Reviews.
Authors' conclusions
Implications for practice.
For people with chronic non‐cancer pain
A number of adverse events can occur, including serious adverse events, when opioids are used for CNCP in adults.
For clinicians
Clinicians should be aware that a significant risk increase exists for a number of adverse events when opioids are used for CNCP in adults. As there is limited evidence to support the efficacy of long‐term use of opioids in CNCP, an absence of evidence of improvement in function and pain scores when high doses of opioids are used, and robust evidence of harm associated with medium‐ to long‐term opioid use, prescribers should proceed with caution prior to initiating treatment with opioids and with even greater caution when transitioning from short‐term to medium‐ and long‐term use of opioids for people with CNCP. The evidence is severely limited at this time due to the absence of reporting on expected and clinically significant adverse events from the Cochrane Reviews. This limits our ability to evaluate the harms of opioid medications when used in the medium or long term.
For policymakers
There are a number of adverse events, including serious adverse events, when opioids are used for CNCP. This should be considered in policy decisions.
For funders of the intervention(s)
Funders may consider supporting the use of opioids for CNCP only in exceptional circumstances or after failure of other therapeutic modalities, when the benefit outweighs the risks. Funders of opioid research may consider requiring more detailed reporting of adverse events.
Implications for research.
A number of adverse events that we would have expected to occur with opioid use were not reported in the included Cochrane Reviews. Going forward, we recommend consistent reporting of all relevant adverse events in randomised controlled trials and systematic reviews on opioid therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2018 | Amended | Minor amendment to Declarations of interest section |
Acknowledgements
This overview complements another Cochrane overview entitled 'High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane Reviews' (Els 2017). For consistency and following discussion with the Cochrane Pain, Palliative and Supportive Care editorial office, we have utilised text from that overview for the present overview.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategy
#1 MeSH descriptor: (Pain) explode all trees
#2 pain*:ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: (Analgesics, Opioid) explode all trees
#5 opioid*:ti,ab,kw
#6 codeine or oxycodone or tramadol or hydromorphone or morphine or fentanyl:ti,ab,kw
#7 meperidine or pethidine or dextropropoxyphene or methadone or buprenorphine or pentazocine or hydrocodone or opium or butorphanol:ti,ab,kw
#8 tapentadol or papaveretum or meptazinol or dipipanone or dihydrocodeine or diamorphine:ti,ab,kw
#9 #4 or #5 or #6 or #7 or #8
#10 #3 and #9 in Cochrane Database of Systematic Reviews
Appendix 2. GRADE Assessment
The GRADE system uses the following criteria for assigning grade of evidence.
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Grade of evidence is decreased if the following are present.
Serious (‐1) or very serious (‐2) limitation to study quality.
Important inconsistency (‐1).
Some (‐1) or major (‐2) uncertainty about directness.
Imprecise or sparse data (‐1).
High probability of reporting bias (‐1).
Contributions of authors
SSt conceived of the idea. All authors except TJ were involved in writing the protocol. TJ joined the project after the protocol was published. TJ did the searching with the help of the Cochrane Pain, Palliative and Supportive Care Review Group. Two authors (of CE, VL, and TJ) screened the abstracts. At least two authors (of CE, TJ, DK, VL, BS, and FK) decided on eligibility of the identified Cochrane Reviews and extracted data. Two authors (CE, TJ) assessed the quality of the evidence, consulting a third author (SSt) where needed to make a majority decision. TJ and SSt performed the analysis; CE, TJ, and SSt drafted the full overview. All authors approved the final version of the overview.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Workers' Compensation Board of Alberta, Canada.
We acknowledge funding from the Workers' Compensation Board of Alberta for the project 'Adverse events associated with medium‐ and long‐term use of opioids for chronic non‐cancer pain: an overview of Cochrane Reviews'.
Declarations of interest
Charl Els: none known.
Tanya D Jackson: none known; Tanya D Jackson is a clinical psychologist whose practice includes patients with chronic pain.
Diane Kunyk: none known.
Vernon G Lappi: none known; Vernon G Lappi is a specialist occupational medicine physician. His past practice included assessment of patients with chronic pain.
Barend Sonnenberg: none known.
Reidar Hagtvedt: none known.
Sangita Sharma: none known.
Fariba Kolahdooz: none known.
Sebastian Straube's institution (University of Alberta) received fees for his contribution to an advisory board from Daiichi Sankyo, Inc. (2015). Sebastian Straube is a specialist occupational medicine physician and some of the patients he assesses have chronic pain.
Edited (no change to conclusions)
References
References to included reviews
Alviar 2011
- Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD006380.pub2] [DOI] [PubMed] [Google Scholar]
Cepeda 2006
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005522.pub2] [DOI] [PubMed] [Google Scholar]
Chaparro 2012
- Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD008943.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro LE, Furlan AD, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low‐back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004959.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Costa 2014
- Costa BR, Nuesch E, Kasteler R, Husni E, Welch V, Rutjes AWS, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2015
- Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2016
- Derry S, Stannard C, Cole P, Wiffen PJ, Knaggs R, Aldington D, et al. Fentanyl for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD011605.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enthoven 2016
- Enthoven WT, Roelofs PD, Deyo RA, Tulder MW, Koes BW. Non‐steroidal anti‐inflammatory drugs for chronic low back pain. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaskell 2016
- Gaskell H, Derry S, Stannard C, Moore RA. Oxycodone for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD010692.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haroutiunian 2012
- Haroutiunian S, McNicol ED, Lipman AG. Methadone for chronic non‐cancer pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008025.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2013
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006146.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noble 2010
- Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006605.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2015
- Santos J, Alarcão J, Fareleira F, Vaz‐Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD009923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stannard 2016
- Stannard C, Gaskell H, Derry S, Aldington D, Cole P, Cooper TE, et al. Hydromorphone for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD011604.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittle 2011
- Whittle SL, Richards BL, Husni E, Buchbinder R. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD003113.pub3] [DOI] [PubMed] [Google Scholar]
References to excluded reviews
Challapalli 2005
- Challapalli V, Tremont‐Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003345.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duehmke 2006
- Duehmke RM, Hollingshead J, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003726.pub3] [DOI] [PubMed] [Google Scholar]
Gaskell 2014
- Gaskell H, Moore RA, Derry S, Stannard C. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010692.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2015a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD008242.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mujakperuo 2010
- Mujakperuo HR, Watson M, Morrison R, Macfarlane TV. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD004715.pub2] [DOI] [PubMed] [Google Scholar]
Ramiro 2011
- Ramiro S, Radner H, Heijde D, Tubergen A, Buchbinder R, Aletaha D, et al. Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis). Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008886.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinstein 2012
- Rubinstein SM, Terwee CB, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for acute low‐back pain. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD008880.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidel 2013
- Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004844.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Afilalo 2010
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Hove I, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee. A randomized, double‐blind, placebo‐ and active‐controlled phase III study. Clinical Drug Investigation 2010;30(8):489‐505. [DOI: 10.2165/11533440-000000000-00000] [DOI] [PubMed] [Google Scholar]
AMDG 2015
- The Washington State Agency Medical Directors’ Group (AMDG). Interagency guideline on prescribing opioids for pain. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf (accessed 10 May 2017).
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington: American Psychiatric Association, 2013. [Google Scholar]
Banta‐Green 2009
- Banta‐Green CJ, Merrill JO, Doyle SR, Boudreau DM, Calsyn DA. Opioid use behaviors, mental health and pain: development of a typology of chronic pain patients. Drug and Alcohol Dependence 2009;104(1‐2):34‐42. [DOI: 10.1016/j.drugalcdep.2009.03.021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohnert 2012
- Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. American Journal of Psychiatry 2012;169(1):64‐70. [DOI: 10.1176/appi.ajp.2011.10101476] [DOI] [PubMed] [Google Scholar]
Borg 2014
- Borg L, Buonora M, Butelman ER, Ducat E, Ray BM, Kreek MJ. The pharmacology of opioids. In: Ries RK, Fiellin DA, Miller SC, Saitz R editor(s). The ASAM Principles of Addiction Medicine. 5th Edition. Philadelphia: Wolters Kluwer Health, 2014:135‐50. [Google Scholar]
Boscarino 2010
- Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, et al. Risk factors for drug dependence among out‐patients on opioid therapy in a large US health‐care system. Addiction 2010;105(10):1776‐82. [DOI: 10.1111/j.1360-0443.2010.03052.x] [DOI] [PubMed] [Google Scholar]
Breivik 2008
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad, Hals EB, et al. Assessment of pain. British Journal of Anaesthesia 2008;101(1):17‐24. [DOI: 10.1093/bja/aen103] [DOI] [PubMed] [Google Scholar]
Busse 2017
- Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189(18):E659‐66. [DOI: 10.1503/cmaj.170363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2010
- Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, et al. Opioid pharmacotherapy for chronic non‐cancer pain in the United States: a research guideline for developing an evidence‐base. Journal of Pain 2010;11(9):807‐29. [DOI: 10.1016/j.jpain.2010.02.019] [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI: 10.1136/bmj.310.6977.452] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowell 2016
- Dowell D, Haegerich TM, Chou R. CDC Guidelines for prescribing opioids for chronic pain ‐ United States, 2016. JAMA 2016;15:1624‐45. [DOI: 10.1001/jama.2016.1464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2010
- Dunn, KM, Saunders KW, Rutter CM, Banta‐Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Annals of Internal Medicine 2010;152(2):85‐92. [DOI: 10.1059/0003-4819-152-2-201001190-00006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Els 2017
- Els C, Hagtvedt R, Kunyk D, Sonnenberg B, Lappi VG, Straube S. High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012299] [DOI] [Google Scholar]
EMRPCC 2016
- Eastern Metropolitan Region Palliative Care Consortium (EMRPCC). Opioid conversion ratios: Guide to palliative care practice. www.emrpcc.org.au/wp‐content/uploads/2016/05/Opioid‐Conversions‐May‐3‐2016‐final.pdf (accessed 12 May 2017).
FDA 2016
- U.S. Food, Drug Administration. What is a serious adverse event?. www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (accessed 9 August 2017).
Fentanyl monograph 2017
- Lexi‐Comp, Inc. Fentanyl. In: Lexi‐Drugs http://online.lexi.com/lco/action/home/switch (accessed 12 May 2017).
Fleming 2007
- Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. Journal of Pain 2007;8(7):573‐82. [DOI: 10.1016/j.jpain.2007.02.432] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franklin 2014
- Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83(14):1277‐84. [DOI: 10.1212/WNL.0000000000000839] [DOI] [PubMed] [Google Scholar]
Gimbel 2003
- Gimbel JS, Richards P, Portenoy RK. Controlled‐release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 2003;60(6):927–34. [DOI: 10.1212/01.WNL.0000057720.36503.2C] [DOI] [PubMed] [Google Scholar]
Gomes 2011
- Gomes T, Mamdani MM, DhallaI A, Paterson JM, Juurlink DN. Opioid dose and drug‐related mortality in patients with nonmalignant pain. Archives of Internal Medicine 2011;171(7):686‐91. [DOI: 10.1001/archinternmed.2011.117] [DOI] [PubMed] [Google Scholar]
Gomes 2014
- Gomes T, Mamdani MM, Paterson JM, Dhalla IA, Juurlink DN. Trends in high‐dose opioid prescribing in Canada. Canadian Family Physician 2014;60(9):826‐32. [DOI: 10.1001/archinternmed.2011.117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagen 1995
- Hagen N, Flynne P, Hays H, MacDonald N. Guidelines for managing chronic non‐malignant pain. Canadian Family Physician 1995;41:49‐53. [PUBMED: PMC2145959] [PMC free article] [PubMed] [Google Scholar]
Hegmann 2014a
- Hegmann KT, Weiss MS, Bowden K, Branco F, DuBrueler K, Els C, et al. ACOEM practice guidelines: opioids and safety‐sensitive work. Journal of Occupational and Environmental Medicine 2014;56(7):e46‐53. [DOI: 10.1097/JOM.0000000000000237] [DOI] [PubMed] [Google Scholar]
Hegmann 2014b
- Hegmann KT, Weiss MS, Bowden K, Branco F, DuBrueler K, Els C, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. Journal of Occupational and Environmental Medicine 2014;56(12):e143‐59. [DOI: 10.1097/JOM.0000000000000352] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. The Cochrane Collaboration, Available from handbook.cochrane.org.
Howlett 2017
- Howlett K. Opioid panel chair admits conflict‐of‐interest lapse. www.theglobeandmail.com/news/national/opioid‐panel‐chair‐admits‐conflict‐of‐interest‐lapse/article35073017/ 19 May 2017.
Huffman 2015
- Huffman KL, Shella ER, Sweis G, Griffith SD, Scheman J, Covington EC. Nonopioid substance use disorders and opioid dose predict therapeutic opioid addiction. Journal of Pain 2015;16(2):126‐34. [DOI: 10.1016/j.jpain.2014.10.011] [DOI] [PubMed] [Google Scholar]
Häuser 2014
- Häuser W, Bock F, Engeser P, Tölle T, Willweber‐Strumpf A, Petzke F. Clinical practice guideline: long‐term opioid use in non‐cancer pain. Deutsches Ärzteblatt International 2014;111:732‐40. [DOI: 10.3238/arztebl.2014.0732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jungquist 2012
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Management Nursing 2012;13(2):70‐9. [DOI: 10.1016/j.pmn.2010.04.003] [DOI] [PubMed] [Google Scholar]
Katz 2015
- Katz JA, Swerdloff MA, Brass SD, Argoff CE, Markman J, Backonja M, et al. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2015;84(14):1503‐5. [DOI: 10.1212/WNL.0000000000001485] [DOI] [PubMed] [Google Scholar]
Kidner 2009
- Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. Journal of Bone and Joint Surgery 2009;91(4):919‐27. [DOI: 10.2106/JBJS.H.00286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosten 2002
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Science & Practice Perspectives 2002;1(1):13‐20. [DOI: 10.1151/spp021113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraut 2015
- Kraut A, Shafer LA, Raymond CB. Proportion of opioid use due to compensated workers' compensation claims in Manitoba, Canada. American Journal of Industrial Medicine 2015;58(1):33‐9. [DOI: 10.1002/ajim.22374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leong 2009
- Leong M, Murnion B, Haber PS. Examination of opioid prescribing in Australia from 1992 to 2007. Internal Medicine Journal 2009;39(10):676‐81. [DOI: 10.1111/j.1445-5994.2009.01982.x] [DOI] [PubMed] [Google Scholar]
Leung 2017
- Leung PT, Macdonald EM, Stanbrook MB, Dhalla IA, Juurlink DN. A 1980 letter on the risk of opioid addiction. New England Journal of Medicine 2017;376(22):2194‐5. [DOI: 10.1056/NEJMc1700150] [DOI] [PubMed] [Google Scholar]
Manchikanti 2012a
- Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Physician 2012;15(3 Suppl):ES9‐38. [PUBMED: 22786464] [PubMed] [Google Scholar]
Manchikanti 2012b
- Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non‐cancer pain: part 2 ‐ guidance. Pain Physician 2012;15(3 Suppl):S67‐116. [PUBMED: 22786449] [PubMed] [Google Scholar]
McCance‐Katz 2010
- McCance‐Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. American Journal on Addictions 2010;19(1):4‐16. [DOI: 10.1111/j.1521-0391.2009.00005.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2012
- McQuay HJ, Derry S, Eccleston C, Wiffen PJ, Moore RA. Evidence for analgesic effect in acute pain ‐ 50 years on. Pain 2012;153(7):1364‐7. [DOI: 10.1016/j.pain.2012.01.024] [DOI] [PubMed] [Google Scholar]
Merskey 1994
- Merskey H, Lindblom U, Mumford JM, Nathan PW, Sunderland S. Part III: pain terms, a current list with definitions and notes on usage. In: Merskey H, Bogduk N editor(s). Classification of Chronic Pain. 2nd Edition. Seattle: International Association for the Study of Pain Press, 1994:209‐14. [Google Scholar]
Moore 2005
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non‐malignant pain: systematic review of randomised trials of oral opioids. Arthritis Research & Therapy 2005;7(5):R1046. [DOI: 10.1186/ar1782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore AR, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2015b
- Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011407.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS Wales
- NHS Wales. Opiate conversion doses. www.wales.nhs.uk/sites3/Documents/814/OpiateConversionDoses%5BFinal%5DNov2010.pdf (accessed 12 May 2017).
NOUGG 2010
- National Opioid Use Guideline Group (NOUGG). Canadian guideline for safe and effective use of opioids for chronic non‐cancer pain. nationalpaincentre.mcmaster.ca/opioid/documents.html (accessed 12 May 2017).
Nuckols 2014
- Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Capua P, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Annals of Internal Medicine 2014;160(1):38‐47. [DOI: 10.7326/0003-4819-160-1-201401070-00732] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 2009
- O'Donnell JB, Ekman EF, Spalding WM, Bhadra P, McCabe D, Berger MF. The effectiveness of a weak opioid medication versus a cyclo‐oxygenase‐2 (COX‐2) selective non‐steroidal anti‐inflammatory drug in treating flare‐up of chronic low‐back pain: results from two randomized, double‐blind, 6‐week studies. Journal of International Medical Research 2009;37(6):1789‐802. [DOI: 10.1177/147323000903700615] [DOI] [PubMed] [Google Scholar]
OARRS 2016
- State of Ohio Board of Pharmacy. Ohio Automated Rx Reporting System. www.ohiopmp.gov/Portal/MED_Calculator.aspx (accessed 12 May 2017).
Portenoy 1986
- Portenoy R, Foley K. Chronic use of opioid analgesics in non‐malignant pain: report of 38 cases. Pain 1986;25(2):171‐86. [DOI: 10.1016/0304-3959(86)90091-6] [DOI] [PubMed] [Google Scholar]
Porter 1980
- Porter J, Jick H. Addiction rare in patients treated with narcotics. New England Journal of Medicine 1980;302(2):123. [DOI: 10.1056/nejm198001103020221] [DOI] [PubMed] [Google Scholar]
Rachinger‐Adam 2011
- Rachinger‐Adam B, Conzen P, Azad SC. Pharmacology of peripheral opioid receptors. Current Opinion in Anaesthesiology 2011;24(4):408‐13. [DOI: 10.1097/aco.0b013e32834873e5] [DOI] [PubMed] [Google Scholar]
Radbruch 2013
- Radbruch L, Glaeske G, Grond S, Munchberg F, Scherbaum N, Storz E, et al. Topical review on the abuse and misuse potential of tramadol and tilidine in Germany. Substance Abuse 2013;34(3):313‐20. [DOI: 10.1080/08897077.2012.735216] [DOI] [PubMed] [Google Scholar]
Rass 2014
- Rass O, Schacht RL, Marvel CL, Mintzer MZ. Opioids. In: Allen DN, Woods SP editor(s). Neuropsychological Aspects of Substance Use Disorders: Evidence‐Based Perspectives. New York: Oxford University Press, 2014:231‐53. [Google Scholar]
Schulteis 1996
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochemical Research 1996;21(11):1437‐54. [DOI: 10.1007/bf02532385] [DOI] [PubMed] [Google Scholar]
Shafer 2015
- Shafer LA, Raymond C, Ekuma O, Kraut A. The impact of opioid prescription dose and duration during a workers compensation claim, on post‐claim continued opioid use: a retrospective population‐based study. American Journal of Industrial Medicine 2015;58(6):650‐7. [DOI: 10.1002/ajim.22453] [DOI] [PubMed] [Google Scholar]
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:1‐10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
University of Alberta 2017
- University of Alberta multidisciplinary pain centre. Opioid conversion guide. https://www.ualberta.ca/medicine/institutes‐centres‐groups/multidisciplinary‐pain‐clinic/for‐healthcare‐professionals/opioid‐conversion‐guide (accessed 19 October 2017).
Van Griensven
- Griensven H, Strong J, Unruh A. Pain: a Textbook for Health Professionals. 2nd Edition. Edinburgh: Churchill Livingstone, 2014. [ISBN: 9780702034787] [Google Scholar]
Vargas‐Schaffer 2010
- Vargas‐Schaffer G. Is the WHO analgesic ladder still valid?. Canadian Family Physician 2010;56(6):514‐7. [PMC free article] [PubMed] [Google Scholar]
Vowles 2015
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015;156(4):569‐76. [DOI: 10.1097/01.j.pain.0000460357.01998.f1] [DOI] [PubMed] [Google Scholar]
Vuong 2010
- Vuong C, Uum SHM, O'Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocrine Reviews 2010;31(1):98‐132. [DOI: 10.1210/er.2009-0009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2007
- Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. Journal of Clinical Sleep Medicine 2007;3(5):455‐61. [PUBMED: PMC1978331] [PMC free article] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd Edition. Geneva: WHO, 1996. [ISBN: 9241544821] [Google Scholar]
Windmill 2013
- Windmill J, Fisher E, Eccleston C, Derry S, Stannard C, Knaggs R, et al. Interventions for the reduction of prescribed opioid use in chronic non‐cancer pain. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010323.pub2] [DOI] [PubMed] [Google Scholar]
Zenz 1992
- Zenz M, Strumpf M, Tryba M. Long‐term oral opioid therapy in patients with chronic non‐malignant pain. Journal of Pain and Symptom Management 1992;7(2):69‐77. [PUBMED: 1573287] [DOI] [PubMed] [Google Scholar]
Zin 2014
- Zin CS, Chen LC, Knaggs RD. Changes in trends and pattern of strong opioid prescribing in primary care. European Journal of Pain 2014;18(9):1343‐51. [DOI: 10.1002/j.1532-2149.2014.496.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zutler 2011
- Zutler M, Holty JE. Opioids, sleep, and sleep‐disordered breathing. Current Pharmaceutical Design 2011;17(15):1443‐9. [PUBMED: 21476955] [DOI] [PubMed] [Google Scholar]


