Abstract
The freshwater planarian Dugesia japonica has recently emerged as an animal model for developmental neurotoxicology and found to be sensitive to organophosphorus (OP) pesticides. While previous activity staining of D. japonica, which possess a discrete cholinergic nervous system, has shown acylthiocholine catalysis, it is unknown whether this is accomplished through an acetylcholinesterase (AChE), butyrylcholinesterase (BChE), or a hybrid esterase and how OP exposure affects esterase activity. Here, we show that the majority of D. japonica cholinesterase (DjChE) activity departs from conventional AChE and BChE classifications. Inhibition by classic protonable amine and quaternary reversible inhibitors (ethopropazine, donepezil, tacrine, edrophonium, BW284c51, propidium) shows that DjChE is far less sensitive to these inhibitors than human AChE, suggesting discrete differences in active center and peripheral site recognition and structures. Additionally, we find that different OPs (chlorpyrifos oxon, paraoxon, dichlorvos, diazinon oxon, malaoxon) and carbamylating agents (carbaryl, neostigmine, physostigmine, pyridostigmine) differentially inhibit DjChE activity in vitro. DjChE was most sensitive to diazinon oxon and neostigmine and least sensitive to malaoxon and carbaryl. Diazinon oxon inhibited DjChE could be reactivated by the quaternary oxime, pralidoxime (2-PAM), and the zwitterionic oxime, RS194B, with RS194B being significantly more potent. Sodium fluoride (NaF) reactivates OP-DjChE faster than 2-PAM. As one of the most ancient true cholinesterases, DjChE provides insight into the evolution of a hybrid enzyme before the separation into distinct AChE and BChE enzymes found in higher vertebrates. The sensitivity of DjChE to OPs and capacity for reactivation validate the use of planarians for OP toxicology studies.
Keywords: acetylcholinesterase kinetics and inhibition, planarians, fluoride and oxime reactivation, organophosphorus pesticides
Introduction
Acetylcholinesterase (AChE) is a serine hydrolase of the α-hydrolase-fold family catalyzing hydrolysis of the neurotransmitter acetylcholine (ACh) that controls various central nervous system (CNS) cognitive, peripheral autonomic, and somatic motor functions. AChE regulates cholinergic neurotransmission by catalyzing the hydrolysis of released synaptic ACh in a sub-millisecond to second time frame (Rosenberry 1975; Quinn 1987; Taylor P 2016). Because of this crucial role, AChE has long been an important pharmacological and toxicological target. For example, carbamylating AChE inhibitors, such as physostigmine and neostigmine, have been used pharmacologically to treat CNS disorders such as Alzheimer’s disease and peripheral autonomic disorders, affecting secretion and smooth muscle tone, and somatic motor disorders (myasthenia gravis) (Pope et al. 2005; King and Aaron 2015).
Moreover, AChE is the primary target of organophosphorus and carbamylating pesticides, the most commonly used classes of insecticides worldwide (Pope et al. 2005; Grube et al. 2011; Russom et al. 2014; King and Aaron 2015). OPs inhibit AChE through alkylphosphorylation of the active site serine, thus leading to ACh accumulation and cholinergic overstimulation, resulting in decreased heart and respiration rates, muscle tremors, and eventually paralysis and death (Taylor P 2016; Russomţ et al. 2014; King and Aaron 2015). A most insidious use of certain OPs has been in terrorism by rogue terrorist groups and despotic regimes (Okumura et al. 1996; Ohbu et al. 1997; King and Aaron 2015).
Apart from lethal acute toxicity at high doses, the wide use and availability of OPs in agricultural and domestic use raise questions about the safety of long-term exposure at the currently approved levels (Gatto et al. 2009; Muñoz-Quezada et al. 2013; Shelton et al. 2014; González-Alzaga et al. 2014). In particular, recent studies have suggested developmental toxic manifestations that may be linked not only to cholinesterases, but related serine hydrolases or other protein targets (Pope 1999; Pope et al. 2005; Pancetti et al. 2007; Muñoz-Quezada et al. 2013; González-Alzaga et al. 2014).
We have recently shown that the freshwater planarian Dugesia japonica is a valuable in vivo model for neurotoxicity studies (Hagstrom et al. 2015; Hagstrom et al. 2016). The planarian’s capacity to regenerate after asexual reproduction or amputation - due to its large population of stem cells - make it well suited to study perturbations in neurodevelopment (Hagstrom et al. 2016). Because full and regenerating worms are of similar size, the planarian system allows for a direct comparison of the effects of neurotoxicants on brain development and function using the same behavioral endpoints (Hagstrom et al. 2015; Hagstrom et al. 2016). Using a custom planarian screening platform (Hagstrom et al. 2015), we found that planarians are sensitive to OPs. Exposure to chlorpyrifos or dichlorvos at sub-lethal concentrations elicits behavioral phenotypes with reduced rates of locomotion. We observed that planarians exposed to chlorpyrifos exhibited an increased frequency of sharp turns and head motions (Hagstrom et al. 2015) suggestive of altered neuromuscular communication through OP-mediated cholinesterase inhibition. Regenerating worms displayed increased sensitivity compared to full/intact animals, suggesting additional neurodevelopmental effects of these OPs (Hagstrom et al. 2015).
Cholinergic neurons contribute to control of motor functions in D. japonica. When exposed to physostigmine, planarians contract (Nishimura et al. 2010). This suggests that planarians use cholinesterase to regulate ACh levels at neuromuscular junctions and perhaps also at sites within the CNS. Further support for this hypothesis comes from activity staining using an acetylthiocholine (ATCh) substrate, which revealed specific localization in the planarian nervous system (Zheng et al. 2011). To be a suitable model for mammalian and aquatic organism toxicity, the molecular, structural, and biochemical properties of the planarian cholinesterase(s) and related targets require thorough investigation.
Here, we characterize the cholinesterase activity of D. japonica tissue homogenates and find that the predominant D. japonica cholinesterase (DjChE) activity has recognition and catalytic properties characteristic of an AChE-BChE hybrid. To compare the properties of DjChE with mammalian AChE and thus gain insight into structural differences, we probe how DjChE activity is inhibited by classic reversible inhibitors, OPs, and carbamylating agents. Finally, we study oxime (the quaternary, 2-PAM, and the zwitterion, RS194B) and fluoride mediated reactivation after inhibition by diazinon oxon. We find a greater potency for RS194B and enhanced fluoride mediated reactivation after inhibition with the OP. As an ancient true cholinesterase, DjChE provides insight into the evolution of distinct AChE and BChE enzymes from a hybrid enzyme ancestor.
Materials and Methods
Planarian culture
Freshwater planarians of the species Dugesia japonica were used for all experiments. Planarians were stored in 1× Instant Ocean (Instant Ocean, Blacksburg, VA) in Tupperware containers at 20°C in a Panasonic refrigerated incubator in the dark. Animals were fed organic chicken liver once or twice a week and cleaned twice a week when not used for experiments. Animals were starved for at least 5 days before homogenization.
Preparation of planarian homogenates
To prepare homogenates, approximately 2 ml of suspended planarians were transferred to a 50 ml conical tube and placed on ice. All water was removed and replaced with 1 ml cold 1X Phosphate buffered saline containing 1% TritonX-100 (Sigma-Aldrich, St. Louis, MO). The worms were homogenized using a handheld electric homogenizer (Tissuemeiser, Fisher Scientific, Hampton, NH) until a homogeneous slurry was formed. The homogenate was incubated on ice for approximately 30 min and transferred to a pre-chilled 1.5 ml microcentrifuge tube to be centrifuged at 21,000 × g for 30 min at 4°C. The supernatant was removed and used for experiments. This clarified homogenate was stored at 4°C and used within one week of preparation.
Cholinesterase activity assays
Cholinesterase activity was measured using an Ellman assay (Ellman et al. 1961) wherein planarian homogenate was added to the Assay Buffer (0.01% BSA (Sigma-Aldrich), 0.3 mM 5,5’-dithio-bis-[2-nitrobenzoic acid] (DTNB, Sigma-Aldrich) in 0.1 M phosphate buffer, pH 7.4). Thiocholine substrates, acetylthiocholine (ATCh) or butyrylthiocholine (BTCh), both from Sigma-Aldrich, were added last. No background reaction of the homogenate with DTNB was observed under these conditions. Absorbance was immediately measured continuously for 1 min at 412 nm using a CARY 1E UV-Vis Spectrophotometer (Agilent Technologies, Santa Clara, CA). The slope of the absorbance was taken as the activity (min−1) of the sample. For all experiments, the planarian homogenate was diluted with 1% TritonX-100 in PBS to achieve an activity of approximately 0.2–0.4 min−1 when measured with 1 mM ATCh as substrate. All experiments were conducted at room temperature.
Detection of ATCh or BTCh catalysis in fixed worms was performed as previously described (Zheng et al. 2011).
Chemicals
Tetraisopropyl pyrophosphoramide (iso-OMPA) was purchased from Sigma-Aldrich and prepared in ethanol. BW284c51, ethopropazine, 2-PAM (pyridine-2-aldoxime), sodium fluoride (NaF) and donepezil were purchased from Sigma-Aldrich and prepared in deionized water. Edrophonium was purchased from Santa Cruz Biotechnology (Dallas, TX) and stocks were prepared in water. Tacrine hydrochloride (Spectrum Chemical, New Brunswick, NJ) stocks were prepared in phosphate buffer. RS194B was synthesized and purified as previously described (Radić et al. 2012). The OPs (chlorpyrifos oxon, diazinon oxon, dichlorvos, malaoxon, and paraoxon) and carbamylating agents (carbaryl, physostigmine (eserine), pyridostigmine, and neostigmine) were purchased from Sigma-Aldrich with the exception of chlorpyrifos oxon and diazinon oxon, which were purchased from Chem Service (West Chester, PA). Stocks were prepared in ethanol and further diluted in water or buffer, with the exception of physostigmine which was prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich). Solvent (ethanol or DMSO) content in inhibition or reactivation reactions was never above 1% in the samples and controls.
Reversible inhibition
To determine the effects of classic reversible inhibitors on planarian cholinesterase activity, diluted homogenate and inhibitor were added to Assay Buffer and incubated for 5 min at room temperature. Substrate (ATCh or BTCh) was then added and the absorbance measured. Percent activities are reported as the ratio of activity in the inhibited sample over the activity in a control sample incubated with buffer or appropriate solvent. Data are reported as the means ± standard deviation (SD) of at least two independent experiments, with activities measured in technical triplicates for each experiment. IC50 values were calculated by fitting with a four parameter logistic fit using the Standard Curve Analysis tool in SigmaPlot (Systat Software Inc., San Jose, CA). Kd’s were calculated from the IC50 values according to , where [S] is the substrate concentration.
Irreversible inhibition
The kinetics of inhibition with irreversible inhibitors reacting covalently with the cholinesterases (OPs and carbamylating agents) was determined by incubating the planarian homogenate with a 10% volume of inhibitor at room temperature (e.g. 10 μl 10X inhibitor to 90 μl homogenate). At the indicated time points, a 10 μl aliquot was taken from the inhibitor-homogenate mix and added to 980 μl Assay Buffer. Substrate was immediately added and the absorbance was measured. ATCh was used at a final concentration of 1 mM. Residual activity is reported as the percent activity of the average activity of a solvent control measured multiple times over the same inhibition time course. In SigmaPlot, the percent activity remaining over time was fit to the exponential decay formula to determine kobs (for example, see Fig. S1a). Where necessary, when inhibition was not complete at steady-state, a y0 parameter was added to provide a more accurate fit. For each inhibitor, the bimolecular rate constant, kr, was determined from the slope of the linear regression of kobs versus concentration (Fig. S1 and S2). For each inhibitor, at least 4 different concentrations were tested from at least 2 biologically independent samples.
Reactivation of OP inhibited cholinesterase
Homogenates were inhibited with diazinon oxon, chlorpyrifos oxon, or paraoxon to achieve at least 95% inhibition in 30 min or less. Inhibited homogenates in 0.1 ml volume were passed over a spin column (Sit et al. 2011) to separate the conjugated enzyme from excess inhibitor. Samples were further diluted 10-fold and reactivating quaternary oxime (2-PAM), zwitterionic oxime (RS194B), or NaF were added in the specified concentration. Aliquots of 0.01 ml were removed at various times and added to 1.0 ml of Assay Buffer and 1 mM ATCh and the activity immediately read. Determination of the rate of reactivation, kobs, were performed as described in (Kovarik et al. 2004).
Results
D. japonica shows ChE activity distinguishable from AChE and BChE.
Cholinesterase activity was measured in homogenates (Ellman et al. 1961) to compare hydrolysis of ATCh versus BTCh substrates over a range of substrate concentrations (Fig. 1a). Catalysis of the BTCh substrate occurred at approximately half the rate of ATCh. The Km’s were found to be 123± 6 and 59 ±4 μM (mean ± SE of 6 independent experiments) for the ATCh and BTCh substrates, respectively. Hydrolysis of ATCh shows marginal substrate inhibition at 100 mM substrate, whereas hydrolysis of BTCh is constant at concentrations of 1–100 mM. This is dissimilar to the characteristic marked substrate inhibition by ATCh (ACh) and substrate activation seen at high concentrations of BTCh (BCh) typically found in mammalian AChE and BChE, respectively (Augustinsson 1948; Radić et al. 1993). Staining of DjChE activity using ATCh and BTCh substrates after fixation of whole animals confirms that both activities are found in vivo and are localized to the CNS (Fig. 1b).
Fig. 1.
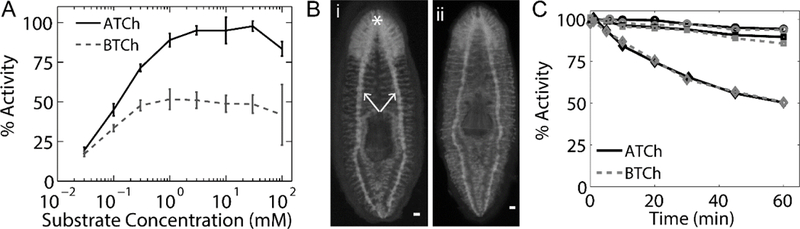
DjChE shows kinetic characteristics intermediate to mammalian AChE and BChE. a Activity of DjChE was determined using an Ellman assay over a range of ATCh (solid black line) and BTCh (dashed gray line) concentrations. Activity is reported as the percent of the maximum activity in that experiment using ATCh as a substrate. Error bars represent the standard deviation (SD) of 6 independent experiments. b Staining of DjChE activity in fixed D. japonica using ATCh (i) and BTCh (ii) substrates show activity is localized to the nervous system (* indicates the cephalic ganglion or brain and arrows indicate the ventral nerve cords) without clear spatial discrimination between the two activities. c Inhibition kinetics from 500 μM (circles), 1 mM (squares), and 5 mM (diamonds) iso-OMPA on DjChE activity using 1mM ATCh (black solid line) and BTCh (gray dashed line) as substrates. Activity is reported as the percent of the mean activity in the solvent control samples
AChE and BChE catalyze the hydrolysis of ATCh, whereas in mammals appreciable catalysis of the larger BTCh substrate molecule requires BChE (Taylor and Radić 1994). Therefore, to determine what extent of ATCh and BTCh are catalyzed by a possible BChE-like enzyme, activity was measured after inhibition with a bulky organophosphate anhydride, iso-OMPA, that at low concentrations inhibits mammalian BChE but not AChE (Radić et al. 1993; Vellom et al. 1993). We found that incubation with up to 1 mM iso-OMPA was unable to significantly inhibit either ATCh or BTCh hydrolysis (Figure 1c) and only slow inhibition was seen at 5 mM iso-OMPA. Differential inhibition for ATCh and BTCh catalysis was not evident. Hence, DjChE does not seem to carry classical BChE inhibition parameters. Notably, since we measure enzyme activity in planarian homogenates, we cannot distinguish whether DjChE activity is performed by a single enzyme or multiple enzymes with very similar catalytic parameters.
DjChE is far less sensitive to classic reversible inhibitors than human AChE
We measured the extent of inhibition on DjChE activity incurred by classic quaternary (BW284c51, edrophonium, and propidium) and amine reversible inhibitors (ethopropazine, donepezil, and tacrine) (Fig. 2). To determine if these inhibitors act competitively, as described for AChE of other species (Taylor and Radić 1994; Taylor et al. 1995), activity was compared using ATCh substrate at concentrations slightly below (0.1 mM) or above (1 mM) the Km. Because ethopropazine and BW284c51 are known to be specific inhibitors of mammalian BChE and AChE, respectively (Radić et al. 1993; Vellom et al. 1993; Taylor and Radić 1994), we also compared DjChE activity using 0.1 or 1 mM BTCh as substrate. In agreement with our results for iso-OMPA, differential inhibition was not evident between the ATCh and BTCh substrates when inhibiting with ethopropazine or BW284c51 (Fig. 2a-b).
Fig. 2.
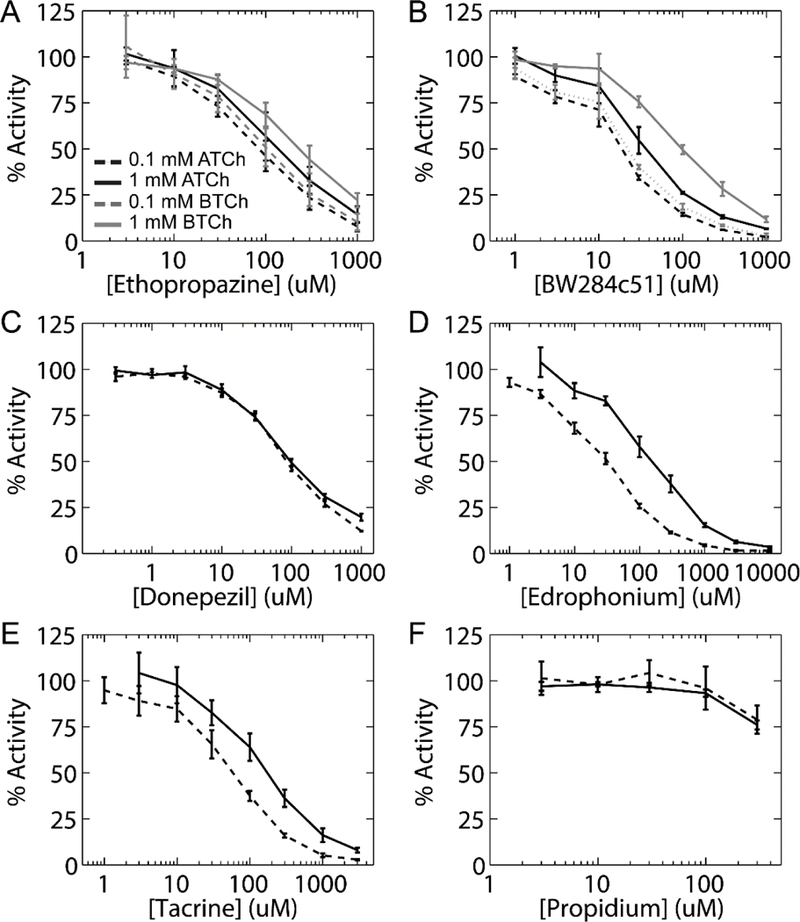
Inhibition by classic reversible quaternary and uncharged amine inhibitors. Panels show the percent of DjChE activity remaining in the samples after 5 min incubation with the reversible inhibitors ethopropazine (a), BW284c51 (b), donepezil (c), edrophonium (d), tacrine (e), and propidium (f) using 0.1 (dashed line) or 1 mM (solid line) ATCh (black) or BTCh (gray) substrates. Error bars indicate the SD of at least 2 independent experiments
DjChE ATCh hydrolytic activity was found to be resistant to inhibition by up to 300 μM propidium (Fig. 1f). The calculated IC50 values for these inhibitors at the various substrate concentrations are shown in Table 1. Corresponding Kd values are provided in Supplementary Table 1. Generally, DjChE was found to be far less sensitive to these reversible inhibitors than reported previously for human AChE (Supplementary Table 2) (Atack et al. 1989; Giacobini 2000; Giacobini 2001; Taylor P 2016).
Table 1.
IC50 (M) of reversible inhibitors for 0.1 and 1 mM ATCh and BTCh
| Substrate | ||||
|---|---|---|---|---|
| Inhibitor | 0.1 mM ATCh | 1 mM ATCh | 0.1 mM BTCh | 1 mM BTCh |
| Ethopropazine | 9.3± 3.1 × 10−5 | 1.3± 0.5 × 10−4 | 9.1± 2.1 × 10−5 | 2.2±0.7 × 10−4 |
| BW284c51 | 2.0± 0.3 × 10−5 | 3.2± 0.3 × 10−5 | 2.3± 0.3 × 10−5 | 8.9± 0.3 × 10−5 |
| Donepezil | 8.9± 0.6 ×10−5 | 6.9± 0.2 ×10−5 | NDa | NDa |
| Edrophonium | 3.1± 0.1 × 10−5 | 1.6±0.2 × 10−4 | NDa | NDa |
| Tacrine | 6.2± 0.6 × 10−5 | 1.4±0.1 × 10−4 | NDa | NDa |
| Propidium | >3.0 × 10−4 | >3.0 × 10−4 | NDa | NDa |
IC50 values given as the mean±SE of at least 2 independent experiments (shown in Fig. 2)
ND, not determined.
DjChE is inhibited by OPs and carbamylating agents
Progressive inhibition rates by the active oxon forms of various common OPs (diazinon oxon, chlorpyrifos oxon, dichlorvos, paraoxon, and malaoxon) were analyzed. Interestingly, bimolecular inhibition rate constants, kr, for these OPs differed by two orders of magnitude with DjChE being most sensitive to diazinon oxon and least sensitive to malaoxon (Table 2). Over the range of concentrations tested, the reaction rate was linear with concentration (Fig. S1), and limiting rates were not observed.
Table 2.
Rates of inhibition by OP oxons
| OP | kr (M−1min−1) |
|---|---|
| Diazinon oxon | 22±2 × 105 |
| Dichlorvos | 9.1± 0.9 × 105 |
| Chlorpyrifos oxon | 3.0± 0.3 × 105 |
| Paraoxon | 1.7± 0.1 × 105 |
| Malaoxon | 0.14± 0.01 × 105 |
kr values (bimolecular rate constants) were calculated from at least 5 different OP concentrations and reported as mean± 95% confidence intervals from at least 2 independent experiments (technical and biological replicates). Raw data are shown in Fig. S1
We similarly characterized the inhibition profiles for the carbamylating agents: carbaryl, neostigmine, physostigmine, and pyridostigmine. Carbamates, along with OPs, are used as insecticides due to their transient inhibition of AChE by covalent carbamylation of the active center serine. In contrast to OPs, carbamylation is comparably short-lived, since AChE can be reactivated by cleaving the carbamyl moiety through spontaneous hydrolysis within tens of minutes (Giacobini 2000; Taylor 2016). Similar to our results with OPs, inhibition constants varied over several orders of magnitude among these inhibitors, with neostigmine producing the fastest rates of inhibition and carbaryl the slowest (Table 3). Values are shown as bimolecular rate constants, since reaction rates were linear over the concentration range studied (Fig. S2).
Table 3.
Rates of inhibition by carbamylating agents
| Carbamylating agent | kr (M−1 min−1) |
|---|---|
| Neostigmine | 1.3±0.2 × 105 |
| Physostigmine | 0.32± 0.02 × 105 |
| Pyridostigmine | 0.10± 0.01 × 105 |
| Carbaryl | 0.00064± 0.00008 × 105 |
kr values (bimolecular rate constants) were calculated from at least 4 different carbamate concentrations and reported as mean± 95% confidence intervals from at least 2 independent experiments (technical and biological replicates). Raw data are shown in Fig. S2
Diethylphosphoryl DjChE formed by diazinon oxon is reactivated by the oximes, pralidoxime and RS194B
Since DjChE was found to be sensitive to phosphorylation and carbamylation by OPs and carbamates, respectively, we wondered whether reactivation of OP-inhibited DjChE could be enhanced by common oximes. To this end, we evaluated whether a member of the quaternary pyridinium aldoximes, pralidoxime (2-PAM) and a lead zwitterionic oxime (RS194B), would reactivate DjChE that had been inhibited completely by diazinon oxon (Fig. 3). Both oximes, at concentrations of 4 mM, were able to reactivate DjChE ATCh hydrolyzing activity. At this concentration, RS194B promoted reactivation significantly faster than 2-PAM. Under these conditions, no significant spontaneous reactivation was observed. As these rates are representative of reactivation of diethylphosphoryl DjChE, reactivation rates should be similar for other OPs forming the same conjugate (paraoxon and chlorpyrifos oxon).
Fig. 3.
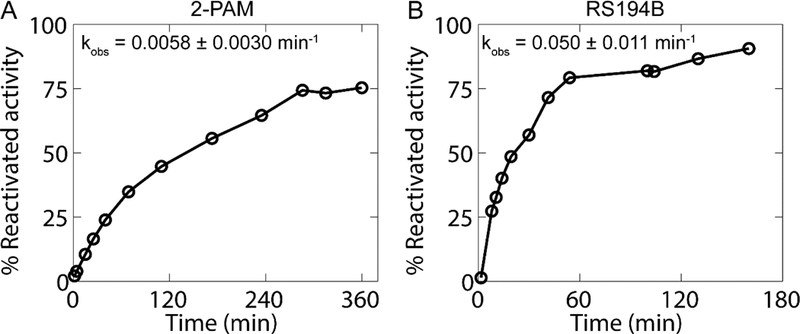
Oxime elicited reactivation of diazinon oxon inhibited DjChE activity using 4 mM 2-PAM (a) or RS194B (b). Percent reactivated activity is based on an uninhibited control measured several times over the course of reactivation. One representative experiment is shown. For each experiment, the kobs was calculated from the first order approach to full or steady-state reactivation and the mean kobs± SD of 2–3 experiments is shown
Reactivation of DjChE by NaF
Given the unique features of resistance to inhibition by the classic quaternary and cationic amines and rapid reactivation by a zwitterionic oxime observed with DjChE, we examined a largely hidden finding in cholinesterase research. In early studies on AChE reactivation, Heilbronn and colleagues (Albanus et al. 1965; Heilbronn 1965) found that fluoride anion will catalyze cholinesterase reactivation, albeit at a slow rate. We found that NaF could reactivate DjChE activity and that the rate of reactivation shows a linear dependence on fluoride concentration (Fig. 4). Moreover, reactivation was independent of the organophosphate inhibitor (diazinon oxon, paraoxon, and chlorpyrifos oxon), all of which form the diethylphosphoryl enzyme conjugate (Fig. S3). Interestingly, reactivation of DjChE was significantly more rapid with 10 mM NaF (mean kobs = 0.023 min−1) than with 4 mM 2-PAM (mean kobs =0.0058 min−1).
Fig. 4.
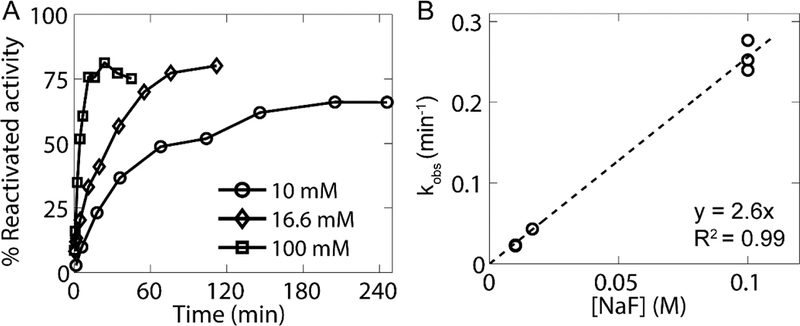
DjChE is efficiently reactivated by NaF. a Reactivation of diazinon oxon-inhibited DjChE after treatment with 10 (circles), 16.6 (diamonds), or 100 mM (squares) NaF. One representative experiment is shown for each concentration. Percent reactivated activity is based on an uninhibited control measured several times over the course of reactivation. b For each experiment, kobs was calculated from the first order approach to full or a steady-state of reactivation (see Materials and Methods). Since kobs appears to have a linear relationship with NaF concentration (dashed line), this represents a bimolecular reactivation where the binding site for F- shows no saturation at these concentrations.
Discussion
Kinetic characteristics of DjChE: catalysis and inhibition.
AChE can be distinguished from BChE in mammalian, avian, and most fish species on the basis of efficient catalysis of acetylcholine and propionyl choline, but marked reduction for butyrylcholine as noted by Augustinsson (Augustinsson 1948). However, in the case of DjChE activity, the relative difference between acetylcholine and butyrylcholine substrate catalysis, as measured with the thioesters, is only a factor of 2 (Fig.1a). This difference is much less than that reported for AChE from various species of Schistosoma, which showed approximately 5 times greater acetylcholine catalysis over butyrylcholine catalysis (Bentley et al. 2005). Moreover the bulky organophosphate, iso-OMPA, is an effective irreversible inhibitor of mammalian BChE, but as shown in Fig. 1c, does not inhibit DjChE activity up to 1 mM and only shows a slow progressive inhibition at 5 mM.
We note several similarities in substrate catalysis and inhibitor profiles for DjChE compared to cholinesterases from other invertebrate species, including Schistosoma (platyhelminths) (Bentley et al. 2005), nematodes (Johnson and Russell 1983; Combes et al. 2001), teleosts (Pezzementi et al. 2011), and jawless fish (Sanders et al. 1996). With the high turnover substrates, the acylcholine and acylthiocholine esters, mammalian AChEs exhibit substrate inhibition. For mammalian BChE, an enzyme that effectively catalyzes esters with a longer acyl chain length, higher concentrations of substrate leads to activation. Substrate inhibition is thought to be due to a second substrate molecule retarding the exchange of substrate and product in the space impacted gorge of AChE. In BChE, that possesses a larger gorge volume at its base (Sussman et al. 1991; Nicolet et al. 2003), the second substrate helps confer a gorge conformation facilitating the commitment to catalysis for initial substrate binding. By contrast, substrate inhibition or activation of DjChE is minimal and occurs at far higher concentrations (100 mM) (Fig.1a). This mirrors what has been found with other invertebrate ChEs, including the closely related Schistosoma blood fluke (Sanders et al. 1996; Bentley et al. 2005; Pezzementi et al. 2011), and, while common to many invertebrate and some vertebrate animal species, is sometimes referred to as an atypical cholinesterase (Pezzementi et al. 2011).
A discrete peripheral site has been proposed for AChE, based on allosteric actions of gallamine (Changeux 1966). Direct titration and subsequent characterization of the peripheral site was achieved with the quaternary fluorophore, propidium (Taylor and Lappi 1975). Extended bisquaternary molecules such as BW284c51 and the neutral molecule donepezil (Kryger et al. 1998) interact with the active center and partially occlude the peripheral site in AChE to varying degrees. The substantially diminished affinity found for DjChE for BW284c51, donepezil, and propidium (Fig. 2b, c, f) suggests this enzyme lacks or has an altered peripheral site. On the other hand, while the differences in binding energy may not be as dramatic, both edrophonium and tacrine show reduced binding affinities (greater Kd values) for DjChE (Fig. 2 d-e). Since both of the latter ligands reside at the base of the active center gorge and do not extend to the rim, it seems likely that some differences in binding determinants also reside near the base of the active center gorge. Interestingly, DjChE was notably less sensitive to propidium than Schistosoma AChE (Bentley et al. 2005), suggesting structural differences of the peripheral sites.
As expected for serine hydrolases, DjChE is inhibited by OPs and carbamylating agents representing families of pesticides widely used in home and garden as well as large scale agricultural use. Since, in both cases, inhibition is progressive forming a covalent conjugate with the target cholinesterase, potency is rank ordered in terms of the rate of inhibition. Since the carbamylating agents form a more labile conjugate, inhibition at lower concentrations will not carry the reaction to complete inhibition, rather to a steady state where inhibition and decarbamylation rates are equal. In all cases, analysis was carried out by manual reactant additions. Hence, rate measurements that rank order the covalent reactivity have been analyzed as bimolecular rate constants (kr), being linear with concentration (Tables 2 and 3, Fig. S1 and S2). Generally, DjChE was similarly sensitive to the various OPs as mammalian AChE, with bimolecular rate constants varying within approximately an order of magnitude of that reported for human or mouse AChE (Reiner and Radić 2000). Not surprisingly, DjChE showed greater sensitivity than mouse AChE to dichlorvos, the non-enzymatic byproduct of metrifonate, a commonly used schistosomiasis drug (Holmstedt et al. 1978). The most potent of the OPs was diazinon oxon. Consequently, it was employed as the lead inhibitor for the reactivation studies. Since chlorpyrifos oxon and paraoxon also form the diethylphosphoryl enzyme, reactivation should occur at the same rate for all of these inhibitors (Fig. S3).
The inhibitor data also suggest that if two or more cholinesterases were present in D. japonica, either their abundance based on catalytic properties is heavily weighted to one or their catalytic and inhibitor susceptibility properties do not vary sufficiently to distinguish two classes of activity. Thus, the most parsimonious explanation would be a single cholinesterase with hybrid properties between BChE and AChE. Analysis by Pezzementi and Chatonnet on the evolutionary history of cholinesterases has suggested that Platyhelminthes, including planarians and Schistosoma, contain the earliest true cholinesterases yet described (Pezzementi and Chatonnet 2010). Thus, the present study helps to elucidate the evolutionary origins of early cholinesterase structure and activity, which had previously only been described in Schistosoma. Interestingly, DjChE behaves more like a true hybrid AChE/BChE enzyme, whereas the Schistosoma cholinesterase has more AChE-like characteristics, suggesting potential evolutionary pressures leading to functional divergence in these closely related species.
Similar hybrid AChE/BChE enzymes have been cloned and characterized from hagfish (Sanders et al. 1996) and teleosts (Pezzementi et al. 2011), suggesting that early cholinesterase activity in lower vertebrates was accomplished by an enzyme with intermediate AChE and BChE properties, before the gene duplication event leading to the divergent AChE and BChE enzymes found in higher vertebrates (Chatonnet and Lockridge 1989). Based on parallels in catalytic properties with these other α,β−hydrolase-fold enzymes, we would predict the following sequence characteristics for DjChE: (a) classic catalytic triad resembling Ser200, Glu327 and His440 in the Torpedo californica sequence (Schumacher et al. 1986) and three disulfide bonded loops, (b) an acyl pocket corresponding to Phe295 and 297 that contains only a single aromatic residue, (c) a choline binding site dominated by Trp84 which serves a major role in the binding of quaternary inhibitors, (d) the absence or severe disruption of a peripheral anionic site defined by Trp286, Tyr72, and Tyr124, (e) a reduced number of aromatic side chains in the active center gorge compared with AChE, and (f) the absence of a clear distinction between AChE and BChE binding, where ethopropazine is selective for BChE. Future sequence identification and functional characterization of DjChE allow for a direct comparison with the published sequences from Schistosomes (Bentley et al. 2003), hagfish (Sanders et al. 1996), hemichordates (Pezzementi et al. 2015), and nematodes (Combes et al. 2001) to verify these proposed sequence features.
Reactivation of diazinon oxon-inhibited DjChE by common oximes and NaF
Examining the influence of the reactivation kinetics augmented by quaternary and zwitterionic oximes and F- anion on DjChE could yield new insights into the reactivation process. Moreover, the planarian’s capacity to regenerate makes the system well-suited to study not only toxic effects of OPs, but also the control of possible repair mechanisms in stem cells or neuronal precursor cells, necessitating an understanding of the reactivation process in this animal.
We found that diazinon oxon inhibited DjChE could be reactivated by the quaternary oxime 2-PAM, the zwitterion RS194B, and even fluoride ion, provided by NaF. When comparing the lowest concentration of NaF tested (10 mM), more complete and rapid reactivation occurred in the order of RS194B > NaF >2-PAM. These data are similar to studies with human AChE which have shown that RS194B is the most efficient reactivator, able to reactivate human AChE in vitro at a rate 2.5 times faster than equimolar 2-PAM (Radić et al. 2013). In mammals, RS194B, but not 2-PAM, crosses the blood-brain barrier and reactivates AChE in the central nervous system (Radić et al. 2012). RS194B can also cross the chorion membrane in zebrafish eggs (Schmidt et al. 2015). Hence, as a zwitterion, with a fraction of the compound existing as a neutral species at physiologic pH, RS194B appears to readily partition across a variety of biological membranes.
Similar to early studies by Heilbronn and colleagues (Albanus et al. 1965; Heilbronn 1965), we found that NaF could reactivate diethylphosphoryl DjChE. We cannot, however, directly compare our rates to the early studies with human AChE due to the use of different OP inhibitors (sarin vs diazinon oxon), resulting in reactivation of different cholinesterase conjugates. To date, studies have focused largely on oximes as the nucleophiles of choice and the use of quaternary ammonium cations to site direct the nucleophile. Surprisingly, we found that 10 mM NaF resulted in four times faster reactivation than 4 mM 2-PAM and appears to be more effective in reactivating DjChE than human AChE. Oximes in solution function as general bases in catalysis, but within the cholinesterase active center gorge, they may have unusual properties; the active center gorge may function to facilitate the oxime-oximate abstraction of a proton. Nucleophilicity of fluoride anion is to be expected, but the active center gorge structure reveals a strong dipole in the direction of the gorge base, facilitating the entry of a cationic substrate into the active center (Sussman et al. 1991). Dipole moments may differ among the cholinesterases, and this may also contribute to the diminished affinity of active center quaternary ligands, such as edrophonium. On the other hand, the small atomic size of fluoride may enhance its collisional access in the spatially constrained active center gorge that is further impacted by the conjugated OP. Finally, the potent reactivation of DjChE by fluoride anion and neutral oximes suggests an ancient and conserved reactivation mechanism already present in hybrid AChE/BChE enzymes of invertebrates. However, fluoride concentrations in the mM range are toxic. Accordingly, its value may not lie in its potential as a reactivator in the environment, but in providing mechanistic and kinetic insights for the design of new chemical landscapes for reactivation.
In conclusion, many of the hybrid AChE/BChE characteristics of DjChE seem to parallel those of cholinesterases found in other invertebrates and jawless fish species and may represent an evolutionarily ancient class of cholinesterase before the distinct separation of AChE and BChE activity in vertebrates (Chatonnet and Lockridge 1989; Pezzementi and Chatonnet 2010; Pezzementi et al. 2011). Although several aspects of DjChE kinetics, inhibitor binding, rates of reactivation, and thus structure seem to differ from mammalian AChE and BChE, the sensitivity of DjChE to OPs, and ability to reactivate in the presence of oximes and fluoride potentiate the use of planarians for toxicology studies on these harmful pesticides. As the peripheral site may play a role in aspects of OP toxicity (Kousba et al. 2004), the differences in the DjChE peripheral site, as compared to mammalian AChE, provides an opportunity to distinguish between these effects. The unique regenerative capabilities of planarians allows one not only to compare toxicity on adult and developing animals in parallel (Hagstrom et al. 2016), but also bears the potential to delineate how OP-induced neurological damage could be repaired.
Supplementary Material
Acknowledgments
This study was partially funded by the Burroughs Wellcome Fund CASI award (to EMSC); National Institutes of Health UO1 NS058046 and The Skaggs Family Foundation (to PT). DH was partially funded by the NIH Cell and Molecular Genetics Training Grant.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Albanus L, Heilbronn E, Sundwall A (1965) Antidote effect of sodium fluoride in organophosphorus anticholinesterase poisoning. Biochem Pharmacol 14:1375–1381. [Google Scholar]
- Atack JR, Yu Q-S, Soncrant TT, et al. (1989) Comparative inhibitory effects of various physostigmine analogs against acetyl- and butyrylcholinesterases. J Pharmacol Exp Ther 249:194–202. [PubMed] [Google Scholar]
- Augustinsson K-B (1948) Cholinesterases. Nature 162:194–195. doi: 10.1038/162680a0 [DOI] [PubMed] [Google Scholar]
- Bentley GN, Jones AK, Agnew A (2003) Mapping and sequencing of acetylcholinesterase genes from the platyhelminth blood fluke Schistosoma. Gene 314:103–112. doi: 10.1016/S0378-1119(03)00709-1 [DOI] [PubMed] [Google Scholar]
- Bentley GN, Jones AK, Agnew A (2005) Expression and comparative functional characterisation of recombinant acetylcholinesterase from three species of Schistosoma. Mol Biochem Parasitol 141:119–123. doi: 10.1016/j.molbiopara.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Changeux J-P (1966) Responses of Acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol 2:369–392. [PubMed] [Google Scholar]
- Chatonnet A, Lockridge O (1989) Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J 260:625–634. doi: 10.1111/j.1600-0730.1981.tb00784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Fedon Y, Toutant J-P, Arpagaus M (2001) Acetylcholinesterase genes in the nematode Caenorhabditis elegans. Int. Rev. Cytol. 209:207–239. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. doi: 10.1016/0006-2952(61)90145-9 [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, et al. (2009) Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect 117:1912–1918. doi: 10.1289/ehp.0900852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E (2001) Selective inhibitors of butyrylcholinesterase: a valid alternative for therapy of Alzheimer’s disease? Drugs Aging 18:891–898. [DOI] [PubMed] [Google Scholar]
- Giacobini E (2000) Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy In: Giacobini Ezio (ed) Cholinesterases and Cholinesterase Inhibitors. Martin Dunitz Ltd, London, pp 181–219 [Google Scholar]
- González-Alzaga B, Lacasaña M, Aguilar-Garduño C, et al. (2014) A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230:104–121. doi: 10.1016/j.toxlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L (2011) Pesticides industry sales and usage: 2006 and 2007 Market Estimates [Google Scholar]
- Hagstrom D, Cochet-Escartin O, Collins E-MS (2016) Planarian brain regeneration as a model system for developmental neurotoxicology. Regeneration 3:65–77. doi: 10.1002/reg2.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom D, Cochet-Escartin O, Zhang S, et al. (2015) Freshwater planarians as an alternative animal model for neurotoxicology. Toxicol Sci 147:270–285. doi: 10.1093/toxsci/kfv129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn E (1965) Action of fluoride on cholinesterase - II. In vitro reactivation of cholinesterases inhibited by organophosphorus compounds. Biochem Pharmacol 14:1363–1373. [DOI] [PubMed] [Google Scholar]
- Holmstedt B, Nordgren I, Sandoz M, Sundwall A (1978) Metrifonate. Summary of toxicological and pharmacological information available. Arch. Toxicol. 41:3–29. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Russell RL (1983) Multiple molecular forms of acetylcholinesterase in the nematode Caenorhabditis elegans. J Neurochem 41:30–46. [DOI] [PubMed] [Google Scholar]
- King AM, Aaron CK (2015) Organophosphate and carbamate poisoning. Emerg. Med. Clin. North Am. 33:133–151. [DOI] [PubMed] [Google Scholar]
- Kousba AA, Sultatos LG, Poet TS, Timchalk C (2004) Comparison of chlorpyrifos-oxon and paraoxon acetylcholinesterase inhibition dynamics: potential role of a peripheral binding site. Toxicol Sci 80:239–48. doi: 10.1093/toxsci/kfh163 [DOI] [PubMed] [Google Scholar]
- Kovarik Z, Radić Z, Berman HA, et al. (2004) Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry 43:3222–3229. doi: 10.1021/BI036191A [DOI] [PubMed] [Google Scholar]
- Kryger G, Silman I, Sussman JL (1998) Three-dimensional structure of a complex of E2020 with acetylcholinesterase from Torpedo californica. J Physiol 92:191–194. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, et al. (2013) Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology 39:158–168. doi: 10.1016/j.neuro.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet Y, Lockridge O, Masson P, et al. (2003) Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem 278:41141–41147. doi: 10.1074/jbc.M210241200 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K (2010) Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience 168:18–30. doi: 10.1016/j.neuroscience.2010.03.038 [DOI] [PubMed] [Google Scholar]
- Ohbu S, Yamashina A, Takasu N, et al. (1997) Sarin poisoning on Tokyo subway. South. Med. J. 90:587–593. [DOI] [PubMed] [Google Scholar]
- Okumura T, Takasu N, Ishimatsu S, et al. (1996) Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med 28:129–135. doi: 10.1016/S0196-0644(96)70052-5 [DOI] [PubMed] [Google Scholar]
- Pancetti F, Olmos C, Dagnino-Subiabre A, et al. (2007) Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J Toxicol Environ Heal Part B Crit Rev 10:623–630. doi: 10.1080/10937400701436445 [DOI] [PubMed] [Google Scholar]
- Pezzementi L, Chatonnet A (2010) Evolution of cholinesterases in the animal kingdom. Chem Biol Interact 187:27–33. doi: 10.1016/j.cbi.2010.03.043 [DOI] [PubMed] [Google Scholar]
- Pezzementi L, Geiss C, King W, et al. (2015) Molecular characterization of an acetylcholinesterase from the hemichordate Saccoglossus kowalevskii. Comp Biochem Physiol Part B 181:50–58. doi: 10.1016/j.cbpb.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Pezzementi L, Nachon F, Chatonnet A (2011) Evolution of acetylcholinesterase and butyrylcholinesterase in the vertebrates: An atypical butyrylcholinesterase from the medaka Oryzias latipes. PLoS One 6:e17396. doi: 10.1371/journal.pone.0017396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J (2005) Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol 19:433–46. doi: 10.1016/j.etap.2004.12.048 [DOI] [PubMed] [Google Scholar]
- Pope CN (1999) Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Heal Part B Crit Rev 2:161–181. doi: 10.1080/109374099281205 [DOI] [PubMed] [Google Scholar]
- Quinn DM (1987) Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev 87:955–979. doi: 10.1021/cr00081a005 [DOI] [Google Scholar]
- Radić Z, Pickering NA, Vellom DC, et al. (1993) Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 32:12074–12084. doi: 10.1021/bi00096a018 [DOI] [PubMed] [Google Scholar]
- Radić Z, Sit RK, Garcia E, et al. (2013) Mechanism of interaction of novel uncharged, centrally active reactivators with OP-hAChE conjugates. Chem Biol Interact 203:67–71. doi: 10.1016/j.cbi.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radić Z, Sit RK, Kovarik Z, et al. (2012) Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem 287:11798–11809. doi: 10.1074/jbc.M111.333732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner E, Radić Z (2000) Mechanism of action of cholinesterase inhibitors In: Giacobini E (ed) Cholinesterases and Cholinesterase Inhibitors. Martin Dunitz Ltd, London, pp 103–120 [Google Scholar]
- Rosenberry TL (1975) Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol 43:103–218. [DOI] [PubMed] [Google Scholar]
- Russom CL, LaLone CA, Villeneuve DL, Ankley GT (2014) Development of an adverse outcome pathway for acetylcholinesterase inhibition leading to acute mortality. Environ Toxicol Chem 33:2157–2169. doi: 10.1002/etc.2662 [DOI] [PubMed] [Google Scholar]
- Sanders M, Mathews B, Sutherland D, et al. (1996) Biochemical and molecular characterization of acetylcholinesterase from the hagfish Myxine glutinosa. Comp Biochem Physiol, Part B Biochem Mol Biol 115:97–109. doi: 10.1016/0305-0491(96)00088-0 [DOI] [PubMed] [Google Scholar]
- Schmidt HR, Radić Z, Taylor P, Fradinger EA (2015) Quaternary and tertiary aldoxime antidotes for organophosphate exposure in a zebrafish model system. Toxicol Appl Pharmacol 284:197–203. doi: 10.1016/j.taap.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Camp S, Maulet Y, et al. (1986) Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. Nature 319:407–409. doi: 10.1038/319407a0 [DOI] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, et al. (2014) Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 122:1103–9. doi: 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit RK, Radić Z, Gerardi V, et al. (2011) New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem 286:19422–19430. doi: 10.1074/jbc.M111.230656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman JL, Harel M, Frolow F, et al. (1991) Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science (80- ) 253:872–879. doi: 10.1126/science.1678899 [DOI] [PubMed] [Google Scholar]
- Taylor P (2016) Anticholinesterase agents In: Brunton Laurence L (ed) Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th edn. McGraw Hill, pp 239–254 [Google Scholar]
- Taylor P, Lappi S (1975) Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry 14:1989–1997. doi: 10.1021/bi00680a029 [DOI] [PubMed] [Google Scholar]
- Taylor P, Radić Z (1994) The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol 34:281–320. doi: 10.1146/annurev.pa.34.040194.001433 [DOI] [PubMed] [Google Scholar]
- Taylor P, Radić Z, Hosea NA, et al. (1995) Structural bases for the specificity of cholinesterase catalysis and inhibition. Toxicol Lett 82–83:453–458. doi: 10.1016/0378-4274(95)03575-3 [DOI] [PubMed] [Google Scholar]
- Vellom DC, Radić Z, Li Y, et al. (1993) Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry 32:12–17. doi: 10.1021/bi00052a003 [DOI] [PubMed] [Google Scholar]
- Zheng D-M, Xie H-Q, Wang A-T, Wu C-C (2011) The nerve system identificiation by histochemical localization of acetylcholinesterase in planarian Dugesia japonica. Chinese J Zool 45:68–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


