Abstract
Cellular senescence refers to a process induced by various types of stress that causes irreversible cell cycle arrest and distinct cellular alterations, including profound changes in gene expression, metabolism, and chromatin organization as well as activation/reinforcement of anti-apoptotic pathways and development of a pro-inflammatory secretome or senescence-associated secretory phenotype (SASP). However, because of challenges and technical limitations in identifying and characterizing senescent cells in living organisms, only recently have some of the diverse in vivo roles of these unique cells been discovered. New findings indicate that senescent cells and their SASP can have acute beneficial functions, such as in tissue regeneration and wound healing. However, in contrast, when senescent cells accumulate in excess chronically at sites of pathology or in old tissues they drive multiple age-associated chronic diseases. Senotherapeutics that selectively eliminate senescent cells (“senolytics”) or inhibit their detrimental SASP (“senomorphics”) have been developed and tested in aged preclinical models. These studies have established that targeting senescence is a powerful anti-aging strategy to improve “healthspan” – i.e., the healthy period of life free of chronic disease. The roles of senescence in mediating age-related bone loss have been a recent focus of rigorous investigation. Studies in mice and humans demonstrate that with aging, at least a subset of most cell types in the bone microenvironment become senescent and develop a heterogeneous SASP. Furthermore, age-related bone loss can be alleviated in old mice, with apparent advantages over anti-resorptive therapy, by reducing the senescent cell burden genetically or pharmacologically with the first class of senolytics or a senomorphic. Collectively, these findings point to targeting senescence as a transformational strategy to extend healthspan, therefore providing strong rationale for identifying and optimizing senotherapeutics to alleviate multiple chronic diseases of aging, including osteoporosis, and set the stage for translating senotherapeutics to humans, with clinical trials currently ongoing.
Keywords: Aging, Bone, Osteocyte, Osteoporosis, Disease Prevention
Introduction
Population growth, longer human lifespan, and unhealthy lifestyles will lead to a serious global problem of late-life chronic diseases, which will create a significant economic burden worldwide. Indeed, advanced chronological age is the greatest risk factor for most of the world’s chronic diseases, but our understanding of the fundamental biological mechanisms that drive aging has not kept pace. Characterized by progressive tissue and cellular functional decline over time, the aging process is a universal feature of virtually all biological organisms that affects multiple organ systems leading to the development of several degenerative pathologies. Recent mounting evidence, however, suggests that mammalian aging can perhaps be delayed by targeting fundamental aging mechanisms that contribute to a host of age-associated pathological conditions such as metabolic syndrome, frailty, cardiovascular disease, neurological disorders, macular degeneration, osteoarthritis, and osteoporosis as well as many others [1]. This concept of targeting fundamental aging mechanisms to improve “healthspan” – i.e., the period of life free of chronic disease, has emerged as a potentially transformational approach, that if successful, should be extended to the general population as a whole. One such modifiable basic aging mechanism that has gained considerable attention in bone and in essentially every other tissue is cellular senescence [2–5]. This review focuses on the biological roles of senescent cells, their in vivo characterization and identification, their causal roles in mediating chronic diseases, including age-related bone loss, and how their selective elimination may lead to new therapeutic approaches to treat osteoporosis and other chronic diseases of aging as group (instead of one at a time) to extend healthspan in the elderly population.
Hallmarks of senescent cells
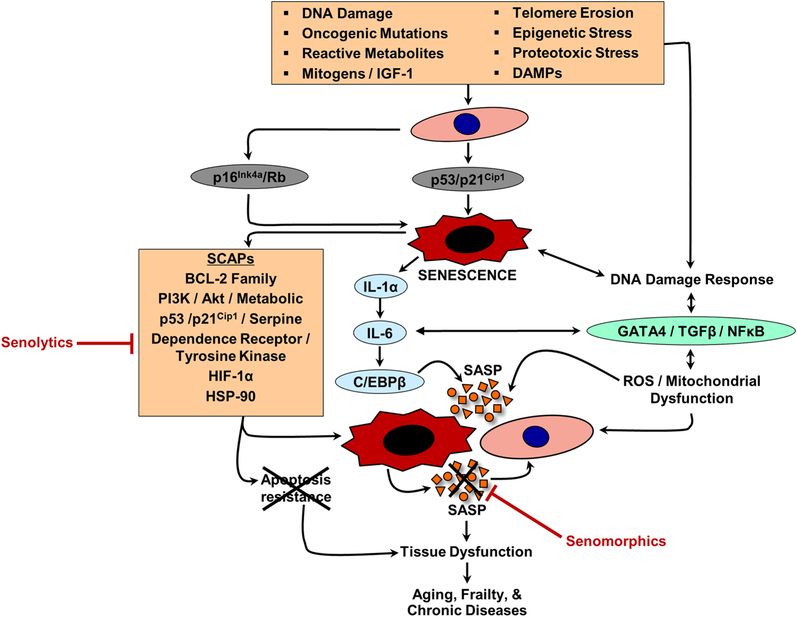
Cellular senescence refers to a process induced by various types of stress (e.g., oncogenic or metabolic insults) that causes essentially irreversible proliferative cell cycle arrest and distinctive cellular phenotypic alterations, including profound changes in gene expression, metabolism, and chromatin organization as well as activation/reinforcement of anti-apoptotic pathways and development of a complex pro-inflammatory secretome [2, 5]. Senescence is established and sustained at least by the p53/p21Cip1 (p53 is upstream of p21Cip1) and p16Ink4a/pRB tumor suppressor senescence effector pathways (Fig. 1) [2–5], although additional senescence pathways are likely to be discovered. Further, it should be noted that p16Ink4a expression has also been linked to quiescence [6]; thus, moderate and temporary p16Ink4a expression has beneficial effects in restraining unnecessary cell proliferation. By contrast, in response to, for example, severe stress, p16Ink4a expression becomes irreversible resulting in a permanent senescent cell growth arrest [2–5]. Much of the early work on the causes of cellular senescence was based on cell culture experiments whereby extensive serial passaging (replicative senescence) or exposure to various types of oncogenic or metabolic stress, such as radiation, oncogene activation, exposure to high glucose or bioactive lipids, chemotherapy, reactive oxygen species (ROS), and DNA damage, were shown to cause a cellular senescence growth arrest in vitro [3, 4]. Many of these and other stressors (Fig. 1) have since been hypothesized as in vivo inducers of senescence, including DNA damage, oncogenic mutations, reactive metabolites, mitogens, insulin-like growth factor 1 (IGF-1), telomere erosion, epigenetic stress, proteotoxic stress, and damage-associated molecular pattern proteins (DAMPs) [5]. Depending on the type of stress, senescence can take weeks to become fully established; nevertheless, despite their dysfunctional intra- and extra-cellular environments, these cells have heightened survival and metabolic activity, making them highly viable in culture for prolonged periods and resistant to apoptosis. Additional in vitro observations have revealed that senescent cells can also trigger senescence of their normal, neighboring counterparts; i.e., via the senescence-induced bystander effect [7, 8]. The cause of this phenomenon, regardless of the initial stress or trigger of senescence, is activation of a unique program in senescent cells that revs up their production and secretion of cytokines, chemokines, and extracellular matrix degrading proteins, termed the senescence-associated secretory phenotype (or SASP) [9–11]. Regulators and reinforcers of the SASP in senescent cells include IL-1α, IL-6, NFκB, C/EBPβ, ROS, or GATA4 (Fig. 1) [2–5]. Over time, the SASP creates a complex pro-inflammatory milieu that varies by senescent cell type [12–14] and depending on the setting can have diverse functions, both with beneficial or detrimental consequences [15–21]. Resistance to apoptosis in senescent cells is necessary to protect these cells from their own pro-apoptotic SASP. To achieve this protection, senescence cells upregulate several senescent cell anti-apoptotic pathways (SCAPs – summarized in Fig. 1) – e.g., B cell lymphoma 2 family inhibitors (BCL-2, BCL-XL, BCL-W), PI3K/Akt pathways, p53/p21Cip1/serpine pathways, dependence receptors/tyrosine kinases, HIF-1α, HSP-90 (reviewed in [5, 22]). These networks and other emerging SCAPs constitute the “Achilles’ heel” of senescent cells and led to the initial discovery and development of the first class of senolytic drugs [23], reviewed below (see – Senescent cells as therapeutic targets to delay aging and extend healthspan).
Fig. 1. Inducers and mediators of senescence, SCAPs, the SASP, and effects of senescent cells.
Cellular senescence refers to a process induced by various types of stress, including DNA damage, oncogenic mutations, reactive metabolites, mitogens, insulin-like growth factor 1 (IGF-1), telomere erosion, epigenetic stress, proteotoxic stress, and damage-associated molecular pattern proteins (DAMPs). A common feature of senescent cells is development of the senescence-associated secretory phenotype (SASP). Regulators and reinforcers of the SASP in senescent cells include IL-1α, IL-6, NFκB, C/EBPβ, ROS, or GATA4. “Senomorphics” block the detrimental effects of the SASP (depicted as red X on SASP factors). Resistance to apoptosis, via upregulation of senescent cell anti-apoptotic pathways (SCAPs), in senescent cells is necessary to protect these cells from their own pro-apoptotic SASP. SCAPs constitute the “Achilles’ heel” of senescent cells and thus are the targets of “senolytics” that selectively kill senescent cells by disabling the SCAPs (depicted as red X on apoptosis resistance). Adapted from [2, 5].
The multifaceted nature of cellular senescence in vivo has been rapidly evolving in recent years, but our understanding of the diverse biological functions of senescent cells remains far from complete. What is becoming clear, however, is that in addition to the well-established mechanism of tumor suppression to protect against unrestricted growth of damaged cells and prevent cancer [15, 16], senescent cells and their SASP have important roles in, for example, fine-tuning embryonic development [17, 18] and promoting tissue remodeling in response to injury [19–21]. In the latter context, the inflammation resulting from the SASP appears to serve as a signal to chemo-attract immune cells to sites of tissue damage in order to initiate the natural processes of wound healing and tissue regeneration.
In contrast to these acute beneficial biological functions, it has also become irrefutably clear that senescent cells accumulate in excess chronically at sites of pathology (e.g., in the setting of diabetes [24, 25]) and in a range of various old tissues [26] where they have been hypothesized to drive age-related tissue dysfunction and the accrual of chronic diseases in old age [2–5]. Whether this accumulation is due to an accelerated rate of senescent cell production with aging [26], the resistance of senescent cells to apoptosis [27], or the inability of the aged immune system to efficiently clear senescence cells [28] (or any combination thereof) is unclear. Regardless, despite the current challenges in identifying senescence-specific biomarkers and obstacles in quantifying senescent cells in vivo, combinations of tools that measure various senescent cell characteristics and features have convincingly demonstrated in humans and numerous other mammalian species that detrimental senescent cell populations accumulate excessively in multiple tissues with advancing chronological age [2–5].
These detection methods (summarized in Table 1) or indicators of senescence include, but are not limited to, high levels of p16Ink4a and/or p21Cip1 [2–5], senescence-associated distension of satellites (SADS; i.e., unraveled centromeres) [29, 30], telomere-associated foci (TAFs; i.e., sites of DNA damage within telomeres) [31], lipofuscin (GL13) [32], cytoplasmic chromatin fragments (CCFs) [33], and/or CENP-A [33, 34]) as well as loss of nuclear high-mobility group box 1 (HMGB1) [35] or reduced lamin B1 [36]. In addition, monitoring of senescent cell accumulation as well as tracking of senescent cell clearance can be accomplished in vivo using transgenic mice with senescent cell reporters, such as p16Ink4a luciferase (p16-LUC) [37] or in p16Ink4a trimodality reporter (p16–3MR) [21, 38], or p16INK4a-linked apoptosis through targeted activation of caspase (INK-ATTAC) [39] mice. Whereas the p16-LUC model [37] consists of a knock-in at the p16Ink4a locus to permit in vivo whole-body imaging of senescent cells using luciferase, p16–3MR [38] and INK-ATTAC [39] are transgenic mouse models that permit monitoring of p16Ink4a-positive senescent cells based on markers such as monomeric red fluorescent protein (mRFP) (p16–3MR) or enhanced green fluorescent protein (EGFP) (INK-ATTAC) as well as FLAG (INK-ATTAC) that allow for their in vivo monitoring/tracking (e.g., using real-time quantitative polymerase chain reaction [rt-qPCR] / immunohistochemistry [IHC] / immunofluorescence [IF]), sorting (fluorescence-activated cell sorting [FACS]), or that permit for their further characterization (e.g., proliferative capacity, changes in morphology, etc.). In addition to senescent cell monitoring, the p16–3MR [21, 38] and INKATTAC [39] mouse models also have similar, yet non-identical, transgenic senescent cell killing systems that are activated in an inducible manner upon administration of a unique “activator” molecule. For example, p16–3MR mice contain a truncated herpes simplex virus thymidine kinase (HSV-TK) that is driven by a truncated p16Ink4a promoter causing ganciclovir (GCV – an activator molecule that when administered to p16–3MR mice has a high affinity for HSV-TK but low affinity for the cellular TK) to be converted into a toxic metabolite, subsequently resulting in mitochondrial DNA (mtDNA) damage and caspase-dependent apoptosis of p16Ink4a-positive senescent cells [21, 38]. However, in regards to bone, given its relatively large molecular size, it will be important in future studies to determine whether GCV is capable of penetrating into bone to kill, for example, senescent osteocytes. On the other hand, in the INK-ATTAC mouse model [39], an FK506-binding protein (FKBP)-CASP8 fusion protein is driven by a 2,617-base pair fragment of the p16Ink4a promoter that is transcriptionally active in senescent cells and triggers their selective death upon administration of AP20187 (a small inducible synthetic “activator” molecule with no known off-target effects) to INK-ATTAC mice, by causing dimerization of the FKBPCASP8 fusion protein and subsequent caspase-mediated apoptosis of p16Ink4a-positive senescent cells (Fig. 2).
Table 1.
Detection methods of cellular senescence.
| Criteria | Kev Factors | Detection Method |
|---|---|---|
| Senescence effectors | pl6Ink4a, p21Cipl, transgenes (e.g.pl6-LUC, INK-ATTAC [EGFP, FLAG], 3MR [mRFP]) | FACS, rt-qPCR, Mass Cytometry, Western Blot, IHC/IF |
| SASP | Examples include: IL-6, IL-8, ILl-α, IL1-Jβ, MCP-1, Pai-1, Pai-2, MMPs, Activin A, TNFα, TGFβ, NFκβ, CEBPβ, GATA4, etc. | rt-qPCR (tissue or FACS cell populations), IHC/IF, Western blot, immunoassays, multiplex assays |
| Anti-apoptosis | SCAPs, Bcl-2, Bcl-xL, Bcl-w, etc. | rt-qPCR (tissue or FACS cell populations), IHC/IF |
| DNA damage | γH2AX, TAFs (co-localization of DNA damage with telomeric repeat sequences), phosphorylated p53 | IHC/IF, Western Blot |
| Cell cycle arrest | pl6Ink4a, p21Cipl, DNA synthesis rate | FACS, rt-qPCR, Mass Cytometry, Western Blot, IHC/IF, BrdU/EdU-incorporation assays |
| Lysosomal dysfunction | Lysosomal β-galactosidase quantity and activity at pH 6.0 | Senescence-associated β-galactosidase (SA-β-Gal) staining |
| Mitochondrial accumulation | Mito-trackers and shape (fusion/fission) | Membrane potential and electronic microscopy (EM) |
| Morphological alterations | Enlargement, flattening, granularity, karyomegaly, heterochromatinization, chromosomal missegregation, high CCFs | IHC/IF, FACS, SADS, SAHFs, CO-FISH |
| Other biomarkers | Presence of lipofuscin (GL13 staining); Loss of HMGB1 and decreased Lamin B1 | IHC/IF, rt-qPCR |
Legend: Given the lack of senescent-specific markers and the heterogeneity of senescent cells, multiple key factors and detection methods in combination are encouraged for the detection of senescent cells.
Key: LUC = luciferase; INK-ATTAC = P16Ink4a-linked apoptosis through targeted activation of caspase); 3MR = trimodality reporter; SASP = senescence-associated secretory phenotype; EGFP = enhance green fluorescent protein; mRFP = monomeric red fluorescent protein; FACS = fluorescence-activated cell sorting; rt-qPCR = real-time quantitative polymerase chain reaction; IHC = immunohistochemistry; IF = immunofluorescence; γH2AX = γH2A histone family member X; TAF = telomere-associated foci; SADS = senescence-associated distension of satellites; SAHFs = senescence-associated heterochromatic foci; CCFs = cytoplasmic chromatin fragments; CO-FISH = chromosome-orientation fluorescent in situ hybridization; HMGB1 = nuclear high-mobility group box 1.
Fig. 2. Clearance of p16Ink4a-positive senescent cells in INK-ATTAC mice delays aging-associated chronic diseases and improves healthspan.
Schematic of the INK-ATTAC construct showing the mechanism of apoptosis activation in p16Ink4a-positive senescent cells upon administration of AP20187 to INK-ATTAC mice. Adapted from [39].
Traditionally, and still commonly today in vitro, cellular senescence was measured in vivo in non-skeletal tissues, such as skin and adipose tissue, using senescence-associated β-galactosidase (SA-β-Gal) staining [40], although this approach is problematic in bone sections both due to a high background confounding and potentially false-positive staining in osteoclasts [41]. Moreover, the alternative of using an anti-β-gal antibody is not viable, since for senescence, it is not the absolute level of β-gal expression but rather the activity of the enzyme at pH 6.0 that identifies SA-β-gal-positive activity [40]. For these reasons, SA-β-Gal staining in bone should not be used as an approach to identify senescent cells in vivo, but can be useful as an in vitro indicator of, for example, bone cell senescence. In contrast, for in vivo detection of senescent cells, the use of multiple other senescence detection methods should be applied in combination (Table 1). Although this approach represents current best practices, the cellular senescence phenotype can be highly variable with mechanisms underlying the phenotype that are not widely conserved; thus, there is a still a great need for technological advancements and development of systematic approaches to identify universal hallmarks of senescent cells [42]. Given that senescent cell-to-cell variability exists [14] and the known heterogeneity of the SASP of different types of senescent cells [12, 13], it will be important in future studies to determine the efficiency by which senotherapeutics, including those that selectively eliminate senescent cells (“senolytics”) or those that inhibit the SASP (“senomorphics”), can target different types of senescent cells and their unique corresponding SASP.
Old bone
Bone is a complex tissue sculpted by coordinated actions of three major cell types: the relatively short-lived bone-forming osteoblasts and bone-resorbing osteoclasts as well as the terminally differentiated osteocytes, which are former osteoblasts embedded within the mineralized matrix. In addition, bone is rich in a heterogeneous population of marrow cells, including hematopoietic precursors capable of forming multinucleated osteoclasts as well as a very small population of pluripotent mesenchymal stem cells (MSCs), capable of not only becoming bone (i.e., by differentiating into osteoblasts and eventually osteocytes) but also other tissues such as cartilage and fat. Furthermore, recent studies have identified additional stem cell pools including a human skeletal stem cell (hSSC) that can differentiate into bone, cartilage or stroma (although not fat) and undergo, for example, local expansion in response skeletal injury [43] as well as a unique periosteal stem cell (PSC) that, among other biological functions, has an important role in mediating intramembranous bone formation [44]. Therefore, bone serves as a host to one of the largest progenitor pools in the body. This permits bone to be highly plastic and therefore capable of adapting to exercise and injury as well as many other stimuli – all of which depend on the complex interplay among the cells residing in the bone microenvironment.
Throughout life, the skeleton is continuously turned over via a self-renewal process termed bone “remodeling” whereby old damaged bone that has accumulated microfracture fatigue is resorbed by osteoclasts and under normal conditions is replaced with an equal amount of new bone by osteoblasts. These actions are coordinated by osteocytes – the “master regulators” of bone remodeling that coordinate the actions of osteoblasts and osteoclasts through, for example, secretion of endocrine and signaling factors and by acting as mechanosensory cells [45, 46]. With aging, however, these actions are disrupted leading to decreased bone formation (less new bone laid down) relative to resorption (more old bone removed), and ultimately negative bone balance. Over prolonged periods of time, these adverse events cause dramatic bone loss, resulting in osteoporosis – a very common, devastating skeletal fragility syndrome causing more than 9 million worldwide fractures annually [47]. In fact, an estimated one in three women and one in five men over the age of 50 will suffer a fracture resulting from osteoporosis in their remaining lifetime [48–50].
At the tissue level, skeletal aging is characterized by bone loss and the accumulation of bone marrow adipose tissue (BMAT). This is also apparent at the cellular level, where aging is associated with a concomitant reduction in bone formation and increase in marrow adipogenesis. Indeed, histologically measured mean wall thickness, a measure of the work done by osteoblasts in the basic multicellular unit (BMU), declines with advancing chronological age in both sexes [51]. The rate of bone formation depends on the level of availability of MSC progenitor pools and sufficient recruitment of MSCs from those pools as well as the activity of individual osteoblasts on bone surfaces [52]. While circulating levels of bone formation markers steadily decline with age in men [53, 54], they generally increase in older women because the menopause causes marked estrogen deficiency, which leads to the activation of more BMUs, and thus a higher bone turnover state, albeit with negative bone balance because at the cellular level bone formation is drastically reduced relative to osteoclastic bone resorption [55]. Therefore, aging results in a defect in bone formation in both women and men. Whether marrow fat exerts direct negative effects on bone formation is not completely clear, but is certainly probable since marrow adipocytes and osteoblasts share a common precursor – i.e., the MSC, and given their inverse relationship, age-related increases in marrow fat could be directly linked to decreases in osteoblasts and/or vice versa (reviewed in [56]).
As in humans, aging in both female and male mice is associated with loss of bone and increased BMAT, although in contrast to women, older female mice do not undergo menopause, but rather retain functional levels of estrogens despite becoming acyclic [57]. Nonetheless, both female and male mice develop several aspects of skeletal aging that mimic osteoporosis in humans, including reduced bone formation due to lower numbers of osteoblasts, as well as increased osteoclasts particularly on endocortical surfaces [58], leading to negative bone balance [57]. Like humans, defective bone formation with aging in mice may be due to a reduction in MSC progenitor pools, defective activation or differentiation of these progenitors, or alterations in allocation towards the adipogenic lineage, the net result of which is decreased bone formation and increased BMAT [59]. Thus, old age causes hallmarks of skeletal aging that are common to both mice and humans.
Although the mechanisms that cause these changes with aging, both acting directly on MSCs and indirectly on other cell types in the bone microenvironment, are not completely understood, it has be hypothesized that the accumulative age-related molecular and cellular damage from genomic instability, epigenetic alterations, telomere attrition, loss of proteostasis, mitochondrial dysfunction, deregulated nutrient sensing, altered intercellular communication, stem cell exhaustion, and cellular senescence – hallmarks of aging that cause functional decline in essentially every tissue throughout the body [1] – are responsible for causing age-related dysfunction in bone. Our current understanding has established that these hallmarks of aging, which are interconnected, linked, and overlap, manifest in old bone to varying degrees where they contribute to the bone loss that occurs with natural, chronological aging (reviewed recently in greater detail elsewhere [60]). Thus, greater effort is needed to understand how these fundamental mechanisms of skeletal aging cause age-related bone loss and the extent to which they can be manipulated to prevent or treat osteoporosis. But unfortunately, while we know that skeletal aging leads to dramatic increased fracture risk, the causes of age-related bone loss are far less clear than the consequences. By defining the causes, we can then identify novel therapeutic targets for prevention and treatment. Using this approach to date, based on past and recent mounting data, cellular senescence has emerged as a plausible candidate.
Cellular senescence in bone – Lessons learned from accelerated aging models
One of the primary hallmarks of aging that triggers cellular harm is genomic instability [61]. Indeed, damage to the genome, including DNA lesions and double-strand breaks, limits the ability of cells to repair the damage so in order to limit damage cells have developed ways to respond by activating specific programs resulting in at least one of two cellular fates: apoptosis or senescence [62]. Abnormalities in DNA repair mechanisms can result in the development of several human progeroid syndromes such as Bloom syndrome, Cockayne syndrome, Seckel syndrome, trichothiodystrophy (TTD), Werner syndrome, or xeroderma pigmentosum – each of which present with features of accelerated aging [63]. Furthermore, several of these progeroid syndromes caused by genomic instability are characterized by skeletal abnormalities, e.g., low bone density and an increased incidence of fractures, and have been modeled in, for example, transgenic mice that age prematurely and display low bone mass. Although use of such models has shed light on the underlying mechanisms linking genomic instability to bone loss in vivo, the often premature death of these animals in many instances limits their study with regards to the natural skeletal aging process. One perhaps exception to this, is in excision repair cross-complementary group 1 (Ercc1−/Δ; null on one allele, hypomorphic on the other) mutant mice as the ERCC1-XPF endonuclease is required for multiple DNA repair pathways; thus, these mice develop a well-characterized accelerated aging phenotype (by ~5–7 months of age) that models human XFE progeria [64]. With regards to bone, six-month-old progeroid Ercc1−/Δ mice have decreased bone mass relative to their wild-type littermates, resulting from reduced histologically assessed bone formation rates and enhanced bone resorption, mediated at least in part by increased NFκB signaling [65]. Interestingly, when bone marrow stromal cells (BMSCs) from Ercc1−/Δ mice were put into culture they displayed compromised osteoblast differentiation as well as increased markers of DNA damage (γH2AX) and cellular senescence (p16Ink4a). In addition, the conditioned medium (CM) derived from cultured BMSCs derived from Ercc1−/Δ mice was found to contain higher levels of multiple SASP factors, including IL-6 and TNFα, which is consistent with a pro-inflammatory bone microenvironment capable of supporting the higher levels of osteoclasts observed histologically in the bones of Ercc1−/Δ mice in vivo. Taken together, these results establish that DNA damage causes cellular senescence in bone and a low bone mass phenotype in progeroid Ercc1−/Δ mice through an NFκB-mediated mechanism [65]. The extent to which these findings are applicable to natural skeletal aging remains to be seen, but nonetheless they provide plausibility for the role of genomic instability in driving cellular senescence and age-related bone loss.
Another primary hallmark of aging that initiates cell damage in several premature aging syndromes and with normal, chronological aging is telomere attrition, which can lead to activation of the cellular senescence program in vivo [1]. The ability to maintain the length of telomeres, which are the protective ends of chromosomes, is coordinated by the specialized enzyme – telomerase – as well a complex of protective proteins [66]. Skeletal aging is accelerated in mice with deleted telomerase reverse transcriptase (Terc−/−) as these mice have reduced numbers of osteoblasts in combination with increased numbers of osteoclasts; the latter results from a pro-inflammatory bone microenvironment [67, 68]. Although the source of this inflammatory response is not known, it could come at least in part from senescent cells as MSCs derived from these animals with defects in telomere maintenance molecules not only display impaired osteoblast differentiation, but also increased p53/p21Cip1 signaling [69]. Thus, telomere attrition impairs osteoblast differentiation and perhaps contributes to skeletal aging due to increased cellular senescence of skeletal MSCs. However, similar to the direct role of genomic instability in mediating skeletal aging, the extent to which this phenomenon also occurs in bone with natural, chronological aging is still unclear at this time. Furthermore, since the majority of studies examining telomere length in bone rely on cultured cells or mouse models, and given the fundamental differences in mouse and human telomere biology [70], as well as the lack of data on tracking of MSCs with human aging, the relevance of telomere dynamics in aging human bone remains to be established. Notwithstanding, while some have proposed relatively slow osteoprogentior cycling [71], studies of human bone marrow stromal stem cells (BMSSCs) have postulated asymmetrical divisions to facilitate bone remodeling [72]. Collectively, these findings suggest that telomere attrition has a role for at least some subsets of cells (e.g., hematopoietic progenitors) in bone during the in vivo aging process.
Identification of senescent cells in the bone microenvironment with natural aging
While the studies in accelerated aging models with shortened lifespan and early onset of multiple comorbidities, including osteoporosis, provided evidence that senescent cells accumulate at the time and location of bone loss, for many years it was not known whether cellular senescence had a causal role in the bone loss that occurs in natural, chronologically aged animals or humans. To begin to address this, our group [73] asked the following: 1) Do senescent cells accumulate in the bone microenvironment with natural aging?; 2) What type(s) of cells within the bone microenvironment become senescent with natural aging?; and 3) Do any of these populations of various senescent cell types produce a SASP? Thus, to address these questions, we first measured in young (6-month) and old (24-month) C57BL/6 wild-type (WT) mice senescence and SASP markers in vivo using rt-qPCR in enriched populations of B cells (CD19+), T cells (CD3ε), myeloid cells (CD14+), osteoprogenitors (Lin-/Lepr+), osteoblasts (AP+/CD31/34/45/54-) and osteocytes (collagenase digested bones), all rapidly isolated from mouse bones/marrow using magnetic-activated cell sorting (MACS) (see Farr et al. [73] for validation of these cell isolation techniques). This study revealed that in both female and male mice, p16Ink4a expression increased significantly (by ~5–10 fold) with aging in B cells, T cells, myeloid cells, osteoprogenitors, osteoblasts, and osteocytes (Fig. 3). By contrast, p21Cip1 mRNA levels were significantly increased with aging predominantly in osteocyte-enriched cells isolated from males, whereas no age-related change was observed in females [73]. Thus, cellular senescence appears to be a global feature of natural aging throughout the body, including in bone, although it should be noted that additional senescent cell types in the bone microenvironment and their corresponding SASPs will likely be uncovered in the future given the rapidly evolving nature of the field. Nevertheless, based on current in vivo evidence it appears that in bone more high p16Ink4a-expressing senescent cells accumulate as compared to high p21Cip1-expressing senescent cells, although the latter may have more dominant roles in other settings. Furthermore, it is possible that p16Ink4a and p21Cip1 may drive senescence in different cell types [2]. For example, Kim and colleagues [74] in a recent study isolated a different population of osteoprogenitors, using Osx1-Cre-;TdRFP mice, and found that levels of p21Cip1, but not p16Ink4a, were elevated with aging, and that this osteoprogenitor population isolated from old mice also displayed characteristics of DNA damage and developed a SASP. Interestingly, in bone biopsies containing heterogeneous populations of various bone/marrow cell types, isolated from elderly and young women, our group found that expression of both p16Ink4a and p21Cip1 increased with aging, indicating that senescent cells accumulate at the time and location of age-related bone loss in humans, as they do in mice [73]. Therefore, in future studies, it will be important to determine the relative contributions of each senescence mediator, p16Ink4a versus p21Cip1, in contributing to senescence-driven bone loss.
Fig. 3. Senescent cells accumulate in the bone microenvironment with aging.
Expression of the senescence biomarker, p16Ink4a, increased in each of the mouse marrow/bone enriched cell populations examined (B- and T-cells, myeloid cells, osteoprogenitors, osteoblasts, and osteocytes – see [73] for details) ~5–10 fold with aging in both female and male mice, although the SASP has been shown to be predominantly produced by senescent osteoprogenitors [74] as well as senescent myeloid cells and senescent osteocytes [73]. It should be noted, however, that additional senescent cell types in the bone microenvironment and their corresponding SASPs will likely be uncovered in the future given the rapidly evolving nature of the field.
In order to determine which cell type(s) in young versus old WT mice develop the SASP with aging, our group used Gene Set Enrichment Analysis (GSEA) [75, 76] on each of the six separate cell populations isolated from mouse bone/marrow specified above, and examined the extent to which age-related changes in gene expression occurred in an a priori defined cluster of 36 established SASP factors [73]. This analysis showed that whereas relatively few SASP factors were altered significantly with aging in the early osteoblast lineage, in contrast mRNA expression of 26 of the 36 SASP factors increased with aging in osteocytes [73]. Furthermore, within the hematopoietic lineages, we found relatively few significant changes in the expression of these genes with aging in B- or T-cells [73]. However, several of the same SASP factors that were higher with aging in osteocytes were also significant upregulated in myeloid cells from old as compared to young mice (23 of the 36 SASP factors measured) [73]. Taken together with the findings from Kim and colleagues [74], the current collective evidence thus indicates (as summarized in Fig. 3) that with natural aging, a subset of cells of various lineages within the bone microenvironment undergo senescence, although senescent osteoprogenitors, senescent myeloid cells, and senescent osteocytes appear to be key producers of the SASP.
Do old osteocytes become senescent?
An important, unresolved question in the senescence field is whether terminally differentiated, post-mitotic non-dividing osteocytes can acquire features of senescence with aging. Previous studies have demonstrated that, with aging, non-proliferating adipocytes [24], hepatocytes [77, 78], as well as neurons in the brain [79] and retina [80] can acquire features of senescence. Consistent with these observations, data from our work [73] indicate that p16Ink4a expression increases with aging in osteocytes and that these cells develop a SASP; thus, demonstrating that old osteocytes acquire features of senescence. Further support for this came from our in vivo quantification of SADS [73], i.e., large-scale unraveling of pericentromeric satellite DNA [29], which revealed in bone cortices from old mice that ~11% of osteocytes become senescent with aging whereas only ~2% of osteocytes are senescent in young bone cortices (Fig. 4A–C). The finding of osteocytes in old bone with senescence features was later confirmed by Piemontese and colleagues [58] who also found increased mRNA levels of p16Ink4a and multiple SASP factors as well as indications of greater DNA damage in osteocyte-enriched bone samples from old as compared to young mice. Collectively, these data provide compelling evidence that at least a subset of osteocytes in old bone become senescent-like and acquire a SASP. Given that large-scale apoptosis of osteocytes in mice results in defective mechanotransduction and significant bone loss [81], senescence of damaged osteocytes may have evolved as a mechanism to preserve cellular integrity and function in bone; however, a paradoxical consequence of this adaptation is that after many years, senescent osteocytes in excess, as in the case of old age, may become culprits in accentuating osteoporotic disease progression through their pro-inflammatory SASP. Future studies are needed to rigorously explore this hypothesis.
Fig. 4. Osteocytes from old bone display SADS in vivo.
Senescence-associated distension of satellites (SADS, see arrows) in osteocytes from (A) young (6-month) versus (B) old (24-month) mice (100x). (C) Quantification of the percentage of senescent osteocytes in young (n=4) versus old (n=4) mice. Data are mean ± SEM. ***p < 0.001. Adapted from [73].
Senescent cells as therapeutic targets to delay aging and improve healthspan
Because with aging, the elevated senescent cell burden is associated with increased blood and tissue SASP factors, spread of senescence, metabolic and stem cell dysfunction, and multiple age-related diseases, including osteoporosis, senescent cells and their SASP have emerged has promising therapeutic targets to delay age-associated chronic diseases as a group and extend healthspan [2]. This is consistent with the “geroscience hypothesis,” which posits that “manipulation of aging will delay (in parallel) the appearance or severity of multiple chronic diseases because these diseases share the same underlying major risk factor – age itself [82].”
The first “proof of concept” evidence supporting the hypothesis that targeting senescent cells can alleviate age-related chronic diseases and their complications came from the Kirkland and van Deursen laboratories at Mayo Clinic. In their seminal 2011 study [39], a novel mouse model, INK-ATTAC (described above), was created in which senescent cells could be identified, isolated, and selectively killed. As noted earlier, the strategy involved coupling a fragment of the senescence-activated promoter, p16Ink4a, to EGFP/FLAG (permitting in vivo tracking of senescent cells) and to a drug-inducible suicide construct – ATTAC [83]), the CASP8/FKBP product of which becomes dimerized and activated upon the inducible administration of AP20187 (Fig. 2) [39]. Remarkably, in both progeroid and more recently in naturally aged INK-ATTAC mice, p16Inka-senescent cell elimination enhanced healthspan by having beneficial effects in multiple tissues [39, 84].
Given that this genetic approach to eliminating senescent cells is not applicable to humans, as an alternative strategy the Kirkland laboratory identified the first class of drugs to selectively kill senescent cells – “senolytics” [23]. Rather than only targeting p16Inka-positive cells, senolytics act by transiently disabling SCAPs (senescent cell anti-apoptotic pathways) that defend senescent cells against their own pro-apoptotic SASP (reviewed in [5, 22, 85]). Indeed, using a hypothesis-driven approach, Zhu and colleagues [23] discovered pro-survival SCAPs that are an “Achilles’ heel” of senescent cells (Fig. 5), and then identified the combination of dasatinib (D; an FDA-approved tyrosine kinase inhibitor [86]) and quercetin (Q; a flavanol present in many fruits and vegetables [87]) as the first senolytic compounds to disable SCAPs, thus causing senescent cells to become susceptible to their own SASP microenvironment and thereby undergo apoptosis. After establishing the senolytic nature of these compounds in vitro, the Kirkland laboratory and collaborators then demonstrated in vivo efficacy by periodically administering the combination of these drugs to Ercc1-/Δ mice, which extended healthspan by preventing multiple age-related symptoms of frailty and pathology [23]. In more recent studies, Xu et al. [88] demonstrated in naturally-aged mice, or in young mice “aged” by transplanting relatively small numbers of senescent cells to spread senescence into the host’s tissues and thereby recapitulate aging phenotypes, that periodic administration of senolytics D+Q improved various aspects of physical function, reduced symptoms of frailty, and increased remaining lifespan by 36%. In addition, even more recent work by Musi et al. [89] developed a tau transgenic mouse model that caused Tau-containing neurofibrillary tangle (NFT) accumulation characteristic among degenerative brain diseases, and demonstrated that periodic treatment of these animals with D+Q reduced total NFT density, neuron loss, and ventricular enlargement, thus pointing to senescent cells as potential novel targets for treating multiple brain diseases. Collectively, the findings from these studies have significantly broader implications insofar as repurposed FDA-approved drugs with senolytic activity as well as newer novel senolytics may relatively soon be readily translatable to elderly humans for enhancing remaining healthspan.
Fig. 5. Identification of a pro-survival signaling networks in senescent cells.
Pro-survival senescent cell apoptosis pathways (SCAPs) are an “Achilles’ heel” of senescent cells. The combination of dasatinib (D; an FDA-approved tyrosine kinase inhibitor) and quercetin (Q; a flavanol present in many fruits and vegetables) were identified as the first senolytic compounds to disable SCAPs, thus causing senescent cells to become susceptible to their own SASP microenvironment and thereby undergo apoptosis. Adapted from [23].
Causal role of senescent cells in mediating age-related bone loss
Based on the work establishing that senescent cells actively drive naturally occurring age-related tissues dysfunction in several non-skeletal tissues and our observations in mice and humans that markers of senescence increase with aging in cells of various lineages in the bone microenvironment [73], we hypothesized a causal role for senescent cells in mediating the bone loss that occurs during the natural aging process [90]. First, in order to determine when senescent cells begin to accumulate in bone and thus the timing to intervene, we performed rt-qPCR for p16Ink4a on bone samples isolated from WT mice at various timepoints throughout the adult lifespan (6, 12, 18, and 24 months of age – Fig. 6). In both sexes, a dramatic increase in p16Ink4a was observed at eighteen months of age, which coincided with age-related bone loss, thus indicating that senescent cells begin to accumulate in bone around this time [90]. Thus, our group then used multiple approaches [90] to target senescent cells (summarized in Fig. 7), including either a genetic (INK-ATTAC [39] – Fig. 7A) or pharmacological (with senolytics, D+Q [23] – Fig. 7B) means to eliminate senescent cells, and a senomorphic approach (with a JAK inhibitor, ruxolitinib [91] – Fig. 7C) to inhibit the SASP.
Fig. 6. Expression of the senescence biomarker, p16Ink4a, increases with natural, chronological aging in bone.
rt-qPCR analysis was performed on bones isolated from wild-type mice at various timepoints throughout the adult lifespan (6, 12, 18, and 24 months of age), which demonstrated that, in both sexes, p16Ink4a mRNA expression increases in bone around eighteen months of age, which coincides with age-related bone loss, thus indicating that senescent cells begin to accumulate in bone around this time. Data are mean ± SEM. ***p < 0.001. Adapted from [90].
Fig. 7. Targeting cellular senescence prevents age-related bone loss.
To address whether cellular senescence has a role in mediating age-related bone loss, our group used multiple approaches that have been described previously (Panel A – [39]; Panel B – [23]; Panel C – [91], including using the INKATTAC transgenic mouse model and the combination senolytics drugs – dasatinib (D) and quercetin (Q), which eliminate senescent cells in vivo, and found that both the genetic (Panel D) and pharmacological (Panel E) approaches prevented age-related bone loss in old mice. In addition, our group also performed studies where we inhibited the production of the pro-inflammatory secretome (i.e., SASP) of senescent cells using the JAK inhibitor, ruxolitinib, which also prevented bone loss in old mice (Panel F). Importantly, none of these interventions had any effects on bone parameters in young mice, indicating that these approaches are specific to targeting the senescent cells that accumulated in old age. *p < 0.05; **p < 0.01. Adapted from [90].
To begin to investigate the role of senescent cells in natural age-related bone loss, we randomized 20-month-old female INK-ATTAC mice to either vehicle or AP20187 treatment (biweekly) for four months [90]. Adipose and bone tissue samples isolated from AP20187- relative to vehicle-treated animals displayed by rt-qPCR significantly reduced mRNA levels of senescence marker, p16Ink4a, as well as EGFP, which encodes for the INK-ATTAC transgene, establishing that senescent cells were eliminated both systemically (e.g., in fat – as observed previously [39, 84]) and locally (in bone) [90]. The latter finding was confirmed by the demonstration of a significant reduction in senescent osteocytes (according to the SADS assay [29]) in AP20187- versus vehicle-treated animals [90].
Our group next examined the skeletal phenotype of female INK-ATTAC mice after global elimination of senescent cells, which revealed that AP20187 treatment prevented age-related trabecular bone loss at the spine (Fig. 7D), the loss of cortical bone at the femur, and improved bone strength at both sites [90]. Importantly, treatment of young INK-ATTAC mice with little to no senescent cell burden did not affect bone parameters, establishing that this strategy is specific to aging. Histomorphometric analyses revealed that eliminating senescent cells in old INK-ATTAC mice reduced osteoclastic bone resorption and increased bone formation; the latter effect was particularly evident on endocortical surfaces [90]. Consistent with these observations, in vitro studies demonstrated that the SASP promoted osteoclast-progenitor survival and inhibited osteoblast differentiation [90]. Interestingly, in contrast to effects of the SASP on inhibiting adipogenesis in peripheral and visceral fat depots [92], the SASP promotes adipogenesis of BMSCs [unpublished data], indicating that SASP factors produced and secreted by senescent cells may contribute to the alteration in lineage commitment of MSCs towards the adipocyte and away from the osteoblast lineage. Consistent with this premise, elimination of senescent cells in old INK-ATTAC mice prevented the excessive accumulation BMAT characteristic of skeletal aging [90].
Our group next performed pharmacological interventions in old male WT mice with either the combination of D+Q [23] to eliminate senescent cells (“senolytic” approach) or with the JAK inhibitor, ruxolitinib [91], to inhibit the pro-inflammatory secretome of senescent cells (“senomorphic” approach). Both approaches improved bone microarchitecture (Fig. 7E–F) and strength in old male WT mice, essentially phenocopying the genetic INK-ATTAC approach [90]. Collectively, these findings establish that senescent cells have a causal role in mediating age-related bone loss, and suggest that targeting senescent cells in humans may provide a novel therapeutic strategy to prevent or treat osteoporosis with potential advantages over conventional anti-resorptive therapy (reviewed in [93]). Furthermore, given that administration of the senomorphic, ruxolitinib, to old mice improves physical function [91] and that similarly elimination of senescent cells with senolytics, D+Q, does the same and increases lifespan in old mice [88], targeting senescent cells in humans may not only prevent bone loss but also alleviate several aspects of frailty, which collectively may confer a greater reduction in fracture risk in the elderly beyond pharmacological approaches that only target bone.
Summary, Perspectives, and Future Directions
To summarize, cellular senescence is a fundamental mechanism that presides at the nexus of age-related chronic disease [2]. Because of challenges and technical limitations in identifying and characterizing senescent cells in living organisms, only recently have some of the important diverse in vivo roles these unique cells been discovered. New findings indicate that senescent cells and their SASP can have acute beneficial functions, such as in tissue regeneration and wound healing. However, in contrast, when senescent cells accumulate in excess chronically at sites of pathology or in old tissues they drive multiple age-associated chronic diseases. Senotherapeutics including senolytics that selectively eliminate senescent cells or senomorphics that inhibit their detrimental SASP have been developed and tested in aged preclinical models. These studies have established that targeting senescence is a powerful anti-aging strategy to improve “healthspan” – i.e., the healthy period of life free of chronic disease. The roles of senescence in mediating age-related bone loss have been a recent focus of rigorous investigation.
Osteoporosis is an enormous, and growing, public health problem [94]. Indeed, up to 25% of elderly hip fracture patients will die within a year of this devastating event [95]. Due to ongoing challenges in osteoporosis treatment, guidelines for starting hormone replacement therapy as well as anti-resorptive therapy have been revised [96, 97]. Furthermore, fear of serious adverse side effects has significantly diminished patient compliance to a point that has become very concerning [98–100]. Thus, the gap between the onset of dramatic age-related bone loss and the initiation of therapy as well as adherence required to prevent or restore that loss has become increasingly problematic. In particular, elderly patients with diagnosed osteopenia or osteoporosis who are at significant risk, but have not yet experienced a fracture and thus do not qualify for anti-resorptive therapy, enter a precarious period of their lifetime when they are not receiving therapy to reduce their risk. Thus, given that this very common disease will inevitably impact almost everyone at some point in old age, there is an enormous unmet need to develop new approaches to prevent and treat osteoporosis. Sadly, in addition to the increasing treatment gap and concomitant reduced patient compliance to therapy, the current available drugs for elderly patients with osteoporosis are suboptimal for several reasons. Indeed, all currently approved drugs for osteoporosis either inhibit bone resorption with a marked, coupled reduction in bone formation (bisphosphonates, denosumab, estrogen) [101] or transiently stimulate bone formation with a coupled increase in bone resorption (PTH) [101]. Furthermore, although the sclerostin inhibitor, romosozumab, simultaneously increases bone formation and reduces resorption, at least transiently [102], recent concerns regarding an increase in cardiovascular events following treatment [103] may delay or perhaps even derail the development of this drug [104, 105]. Other emerging classes of novel drugs with anabolic actions in bone, such as the cathepsin K inhibitor – odanacatib, have also come close to FDA approval, but again an increased risk of stoke proved to be a terminal flaw [106]. Thus, there is a crucial need to develop novel approaches to treat osteoporosis in the elderly population.
The findings demonstrating that targeting senescent cells reduces bone resorption without a concomitant reduction in bone formation [90] and reduce frailty [88] represent hope that intermittent administration of senolytics to humans during periods of good health will delay bone loss, reduce fracture risk, and extend healthspan. Thus, in addition to potentially treating multiple aging co-morbidities by targeting a universal aging mechanism, also relevant to aging bone, this approach may, perhaps be the only available option in near future to suppress bone resorption without a “coupled” decrease in bone formation. Indeed, the approaches used for either eliminating senescent cells or suppressing their secretome represent perhaps the first therapeutic paradigm to treat osteoporosis by targeting not just bone-specific pathways, as with all currently available (or soon to be available) drugs, but rather by targeting a fundamental aging mechanism, cellular senescence, that is operative in all tissues, making it fundamentally different from all current therapies for osteoporosis that only benefit bone. Indeed, because selective elimination of senescent cells has proven to be a powerful anti-aging strategy to improve healthspan in old mice, including beneficial effects in several tissues such as adipose, skeletal muscle, eye, heart, liver, kidney, and cartilage [39, 78, 84, 88, 91, 92, 107–110] as well as in bone [90], these unique non-dividing cells are attractive therapeutic targets to delay chronic diseases of aging as a group and in the prevention or treatment of specific conditions, such as osteoporosis.
With regards to the future, to optimize senotherapeutic regimens for age-related bone loss we must understand the precise nature and mechanisms of effects of detrimental senescent cell populations on the natural aging process, including on the skeleton. Furthermore, in regards to elderly patients with osteoporosis, in order to identify and develop senotheapeutics that will be clinically impactful the correct senescent cell targeting strategy will need to be optimized for bone. In doing so, first and foremost the senotherapeutic regimen will need to be safe, with minimal adverse off-target effects on non-senescent cells. Furthermore, the benefits of any new promising senotherapeutic will need to be rigorously established in appropriate preclinical models of skeletal aging, and given that senescent cells can have beneficial roles in several biological processes that may extend to bone, the benefit/risk trade-off should be determined in multiple settings. One goal will be to identify the most critical SASP factors responsible for propagating age-related bone loss by spreading senescence and amplifying the SASP, as these factors potentially initiate and sustain positive feedback loops reinforcing the senescence program in old age within the bone microenvironment. Thus, an important area of focus will be examining the effects of SASP inhibitors (or “senomorphics) in addition to the JAKi – ruxolitinib (e.g., rapamycin, metformin, as well as other novel compounds) on age-related bone loss. That being said, because senescent cells do not divide, an advantage of using senolytic over senomorphic approaches, which require chronic administration, is that senolytics should not, in theory, need to be administered on a constant basis, but rather only intermittently with condensed exposure to eliminate senescent cell populations during periods of good health. Support for this approach comes from studies in old mice, with established bone loss, where only four treatments (separated monthly) with D+Q (drugs with half-lives of <6 hours, thus minimizing potential off-target effects) were effective in eliminating senescent cells in both adipose tissue and bone and significantly prevented age-related bone loss [90], thus indicating that these and similar novel senolytics hold promise for testing in near future clinical trials.
Another important future consideration will be the need to identify appropriate biomarkers for proper patient selection as targeting cellular senescence is not likely to be effective in individuals who do not present clinically with a high senescent cell burden as chronological age alone is not necessarily representative of an individual’s “biological” age, health status, or level of in vivo senescence. Therefore, an in vivo biomarker of senescence (or combination of biomarkers) in humans could be used to identify a minimal threshold or level of cellular senescence above which an individual is more likely to benefit from senotherapeutic therapy. By contrast, those individuals below this level of in vivo senescence will be less likely to benefit. However, translation of this concept to applications in elderly humans or those with specific diseases caused by a high senescence burden is currently limited by the lack of senescent biomarkers in human tissues as well as our incomplete understanding of the underlying biological mechanisms that cause senescence in vivo and the overall contribution of senescent cells in causing age-associated diseases in humans. These obvious gaps in biomarkers will undoubtedly be important areas of future research for translating senotherapeutics to applications in humans.
In conclusion, cellular senescence has emerged as a “targetable” fundamental aging mechanism to prevent or alleviate chronic diseases of aging, including osteoporosis. However, the existence of beneficial roles of senescent cells creates challenges in developing strategies to indiscriminately eliminate them altogether. Hence, whether this mechanism can be targeted to treat late-life disease in humans remains to be seen. Notwithstanding, several laboratories around the world have demonstrated in multiple model organisms, including mammals (mainly mice), that therapeutic approaches that selectively eliminate senescent cells or interfere with their detrimental secretome have the potential to delay aging with minimal side effects and help treat multiple chronic diseases as a group, including osteoporosis. This concept of targeting senescent cells to improve “healthspan” – i.e., the period of life free of chronic disease, has emerged as a potentially transformational approach that if successful should be extended to the general population as a whole. However, many challenges need to be overcome before this strategy can be applied to humans to delay or alleviate chronic diseases of aging or in the treatment of specific diseases that afflict essentially everyone late in life, such as osteoporosis.
Highlights.
Cellular senescence is process induced by various types of stress
Senescent cells develop a unique senescence-associated secretory phenotype (SASP)
With aging, at least a subset of most cell types in bone become senescent
Eliminating senescent cells or disrupting the SASP in old mice compresses morbidity
Targeting senescent cells in old mice prevents age-related bone loss and frailty
Acknowledgements
We thank our colleagues for helpful discussions and comments on the manuscript and apologize to investigators whose relevant work was omitted due to space limitations. The authors were supported by grants from the National Institutes of Health: K01 AR070241 (J.N.F), P01 AG004875 (S.K.) and R01s AG048792 and AR027065 (S.K.).
Funding: Dr. Farr was supported by NIH Grant K01 AR070241 and Career Development Awards from the Mayo Clinic Robert and Arlene Kogod Center on Aging, as well as the Richard F. Emslander Career Development Award in Endocrinology. Dr. Khosla was supported by NIH grants P01 AG004875, R01 AG048792, and R01 AR027065.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Farr and Dr. Khosla have no conflicts to disclose.
References
- [1].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153: 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation 2013;123: 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8: 729–740. [DOI] [PubMed] [Google Scholar]
- [4].Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nature reviews. Molecular cell biology 2014;15: 482–96. [DOI] [PubMed] [Google Scholar]
- [5].Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017;21: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matheu A, Maraver A, Collado M, Garcia-Cao I, Canamero M, Borras C, Flores JM, Klatt P, Vina J, Serrano M. Anti-aging activity of the Ink4/Arf locus. Aging cell 2009;8: 152–61. [DOI] [PubMed] [Google Scholar]
- [7].Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. A senescent cell bystander effect: senescence-induced senescence. Aging cell 2012;11: 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nelson G, Kucheryavenko O, Wordsworth J, von Zglinicki T. The senescent bystander effect is caused by ROS-activated NF-kappaB signalling. Mechanisms of ageing and development 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS biology 2008;6: 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology 2013;15: 978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual review of pathology 2010;5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Current biology : CB 2017;27: 2652–2660 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell metabolism 2016;23: 303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X, Unger MA, Vijg J, Melov S, Campisi J. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging cell 2017;16: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell 2007;130: 223–33. [DOI] [PubMed] [Google Scholar]
- [16].Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature reviews. Cancer 2010;10: 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell 2013;155: 1104–18. [DOI] [PubMed] [Google Scholar]
- [18].Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013;155: 1119–30. [DOI] [PubMed] [Google Scholar]
- [19].Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature cell biology 2010;12: 676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 2008;134: 657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell 2014;31: 722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The Clinical Potential of Senolytic Drugs. Journal of the American Geriatrics Society 2017;65: 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging cell 2015;14: 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature medicine 2009;15: 1082–7. [DOI] [PubMed] [Google Scholar]
- [25].Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. Journal of diabetes and its complications 2014;28: 604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation 2004;114: 1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang E Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer research 1995;55: 2284–92. [PubMed] [Google Scholar]
- [28].Wang J, Geiger H, Rudolph KL. Immunoaging induced by hematopoietic stem cell aging. Current opinion in immunology 2011;23: 532–6. [DOI] [PubMed] [Google Scholar]
- [29].Swanson EC, Manning B, Zhang H, Lawrence JB. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. The Journal of cell biology 2013;203: 929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Criscione SW, De Cecco M, Siranosian B, Zhang Y, Kreiling JA, Sedivy JM, Neretti N. Reorganization of chromosome architecture in replicative cellular senescence. Science advances 2016;2: e1500882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nature communications 2012;3: 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Munoz-Espin D, Kastrinakis NG, Pouli N, Marakos P, Townsend P, Serrano M, Bartek J, Gorgoulis VG. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging cell 2017;16: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jiang T, Shoshkes-Carmel M, Tanim K, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017;550: 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Giunta S, Funabiki H. Integrity of the human centromere DNA repeats is protected by CENP-A, CENP-C, and CENP-T. Proceedings of the National Academy of Sciences of the United States of America 2017;114: 1928–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. The Journal of cell biology 2013;201: 613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Molecular biology of the cell 2012;23: 2066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013;152: 340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Laberge RM, Adler D, DeMaria M, Mechtouf N, Teachenor R, Cardin GB, Desprez PY, Campisi J, Rodier F. Mitochondrial DNA damage induces apoptosis in senescent cells. Cell death & disease 2013;4: e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deuersen JM. Clearance of p16Ink4a-positive senescent cells delay aging-associated disorders. Nature 2011;479: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America 1995;92: 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Odgren PR, MacKay CA, Mason-Savas A, Yang M, Mailhot G, Birnbaum MJ. False-positive beta-galactosidase staining in osteoclasts by endogenous enzyme: studies in neonatal and month-old wild-type mice. Connective tissue research 2006;47: 229–34. [DOI] [PubMed] [Google Scholar]
- [42].Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends in cell biology 2018;28: 436–453. [DOI] [PubMed] [Google Scholar]
- [43].Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, Ransom RC, Reinisch A, Wearda T, Murphy M, Brewer RE, Koepke LS, Marecic O, Manjunath A, Seo EY, Leavitt T, Lu WJ, Nguyen A, Conley SD, Salhotra A, Ambrosi TH, Borrelli MR, Siebel T, Chan K, Schallmoser K, Seita J, Sahoo D, Goodnough H, Bishop J, Gardner M, Majeti R, Wan DC, Goodman S, Weissman IL, Chang HY, Longaker MT. Identification of the Human Skeletal Stem Cell. Cell 2018;175: 43–56 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang YS, Eiseman M, Shim JH, Hameed M, Healey JH, Bostrom MP, Landau DA, Greenblatt MB. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018;562: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bonewald LF. The amazing osteocyte. J Bone Miner Res 2011;26: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocrine reviews 2013;34: 658–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17: 1726–33. [DOI] [PubMed] [Google Scholar]
- [48].Melton LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis. J Bone Miner Res 1992;7: 1005–1010. [DOI] [PubMed] [Google Scholar]
- [49].Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res 1998;13: 1915–1923. [DOI] [PubMed] [Google Scholar]
- [50].Kanis JA, Johnell O, Oden A, Sernbo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 2000;11: 669–674. [DOI] [PubMed] [Google Scholar]
- [51].Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res 1978;26: 13–17. [DOI] [PubMed] [Google Scholar]
- [52].Parfitt AM. Bone remodeling in type I osteoporosis (letter to the editor). J Bone Miner Res 1991;6: 95–97. [DOI] [PubMed] [Google Scholar]
- [53].Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab 1998;83: 2266–2274. [DOI] [PubMed] [Google Scholar]
- [54].Fatayerji D, Eastell R. Age-related changes in bone turnover in men. J Bone Miner Res 1999;14: 1203–1210. [DOI] [PubMed] [Google Scholar]
- [55].Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23: 279–302. [DOI] [PubMed] [Google Scholar]
- [56].Paccou J, Penel G, Chauveau C, Cortet B, Hardouin P. Marrow adiposity and bone: Review of clinical implications. Bone 2019;118: 8–15. [DOI] [PubMed] [Google Scholar]
- [57].Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiological reviews 2017;97: 135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Piemontese M, Almeida M, Robling AG, Kim HN, Xiong J, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA, Jilka RL. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI insight 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. The lancet. Diabetes & endocrinology 2015;3: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Farr JN, Almeida M. The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2018;33: 1568–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hoeijmakers JH. DNA damage, aging, and cancer. The New England journal of medicine 2009;361: 1475–85. [DOI] [PubMed] [Google Scholar]
- [62].Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120: 513–22. [DOI] [PubMed] [Google Scholar]
- [63].Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nature reviews. Molecular cell biology 2010;11: 567–78. [DOI] [PubMed] [Google Scholar]
- [64].Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 2006;444: 1038–43. [DOI] [PubMed] [Google Scholar]
- [65].Chen Q, Liu K, Robinson AR, Clauson CL, Blair HC, Robbins PD, Niedernhofer LJ, Ouyang H. DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2013;28: 1214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Blasco MA. Telomere length, stem cells and aging. Nature chemical biology 2007;3: 640–9. [DOI] [PubMed] [Google Scholar]
- [67].Pignolo RJ, Suda RK, McMillan EA, Shen J, Lee SH, Choi Y, Wright AC, Johnson FB. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging cell 2008;7: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Saeed H, Abdallah BM, Ditzel N, Catala-Lehnen P, Qiu W, Amling M, Kassem M. Telomerase-deficient mice exhibit bone loss owing to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2011;26: 1494–505. [DOI] [PubMed] [Google Scholar]
- [69].Wang H, Chen Q, Lee SH, Choi Y, Johnson FB, Pignolo RJ. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging cell 2012;11: 704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature medicine 2000;6: 849–51. [DOI] [PubMed] [Google Scholar]
- [71].Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab 2010;21: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. Journal of cell science 2003;116: 1827–35. [DOI] [PubMed] [Google Scholar]
- [73].Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S. Identification of Senescent Cells in the Bone Microenvironment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2016;31: 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O’Brien CA, Jilka RL, Zhou D, Almeida M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging cell 2017;16: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide experssion profiles. Proc Natl Acad Sci USA 2005;102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Efron B, Tibshirani R. On testing the significance of sets of genes. Ann Appl Statist 2007;1: 107–129. [Google Scholar]
- [77].Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature communications 2014;2: 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nature communications 2017;8: 15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging cell 2012;11: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, Mallette FA, Sapieha P. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Science translational medicine 2016;8: 362ra144. [DOI] [PubMed] [Google Scholar]
- [81].Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 2007;5: 464–475. [DOI] [PubMed] [Google Scholar]
- [82].Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. GeroScience 2017;39: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Trujillo ME, Pajvani UB, Scherer PE. Apoptosis through targeted activation of caspase 8 (“ATTAC-mice”): novel mouse models of inducible and reversible tissue ablation. Cell cycle 2005;4: 1141–5. [DOI] [PubMed] [Google Scholar]
- [84].Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016;530: 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Experimental gerontology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yi JS, Huang Y, Kwaczala AT, Kuo IY, Ehrlich BE, Campbell SG, Giordano FJ, Bennett AM. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI insight 2016;1: e90220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].D’Andrea G Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015;106: 256–71. [DOI] [PubMed] [Google Scholar]
- [88].Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nature medicine 2018;24: 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME. Tau protein aggregation is associated with cellular senescence in the brain. Aging cell 2018: e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nature medicine 2017;23: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America 2015;112: E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015;4: e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Khosla S, Farr JN, Kirkland JL. Inhibiting Cellular Senescence: A New Therapeutic Paradigm for Age-Related Osteoporosis. The Journal of clinical endocrinology and metabolism 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2014;29: 2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007;22: 465–475. [DOI] [PubMed] [Google Scholar]
- [96].Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J. Long-term impact of the women’s health initiative on HRT. Arch Gynecol Obstet 2008;277: 219–224. [DOI] [PubMed] [Google Scholar]
- [97].Lewiecki EM, Binkley N. Evidence-based medicine, clinical practice guidelines, and common sense in the management of osteoporosis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2009;15: 573–9. [DOI] [PubMed] [Google Scholar]
- [98].Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2016;31: 1485–7. [DOI] [PubMed] [Google Scholar]
- [99].Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, Shane E. Addressing the Crisis in the Treatment of Osteoporosis: A Path Forward. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2016. [DOI] [PubMed] [Google Scholar]
- [100].Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. The lancet. Diabetes & endocrinology 2017;5: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Khosla S Odanacatib: location and timing are everything. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2012;27: 506–8. [DOI] [PubMed] [Google Scholar]
- [102].McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. The New England journal of medicine 2014;370: 412–20. [DOI] [PubMed] [Google Scholar]
- [103].Saag KG, Petersen J, Grauer A. Romosozumab versus Alendronate and Fracture Risk in Women with Osteoporosis. The New England journal of medicine 2018;378: 195–196. [DOI] [PubMed] [Google Scholar]
- [104].Rosen CJ. Romosozumab - Promising or Practice Changing? The New England journal of medicine 2017;377: 1479–1480. [DOI] [PubMed] [Google Scholar]
- [105].Khosla S Bone diseases: Romosozumab - on track or derailed? Nature reviews. Endocrinology 2017;13: 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mullard A Merck &Co. drops osteoporosis drug odanacatib. Nature reviews. Drug discovery 2016;15: 669. [DOI] [PubMed] [Google Scholar]
- [107].Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging cell 2016;15: 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. The journals of gerontology. Series A, Biological sciences and medical sciences 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016;354: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature medicine 2017;23: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]