Abstract
Background
Virtual reality and interactive video gaming have emerged as recent treatment approaches in stroke rehabilitation with commercial gaming consoles in particular, being rapidly adopted in clinical settings. This is an update of a Cochrane Review published first in 2011 and then again in 2015.
Objectives
Primary objective: to determine the efficacy of virtual reality compared with an alternative intervention or no intervention on upper limb function and activity.
Secondary objectives: to determine the efficacy of virtual reality compared with an alternative intervention or no intervention on: gait and balance, global motor function, cognitive function, activity limitation, participation restriction, quality of life, and adverse events.
Search methods
We searched the Cochrane Stroke Group Trials Register (April 2017), CENTRAL, MEDLINE, Embase, and seven additional databases. We also searched trials registries and reference lists.
Selection criteria
Randomised and quasi‐randomised trials of virtual reality ("an advanced form of human‐computer interface that allows the user to 'interact' with and become 'immersed' in a computer‐generated environment in a naturalistic fashion") in adults after stroke. The primary outcome of interest was upper limb function and activity. Secondary outcomes included gait and balance and global motor function.
Data collection and analysis
Two review authors independently selected trials based on pre‐defined inclusion criteria, extracted data, and assessed risk of bias. A third review author moderated disagreements when required. The review authors contacted investigators to obtain missing information.
Main results
We included 72 trials that involved 2470 participants. This review includes 35 new studies in addition to the studies included in the previous version of this review. Study sample sizes were generally small and interventions varied in terms of both the goals of treatment and the virtual reality devices used. The risk of bias present in many studies was unclear due to poor reporting. Thus, while there are a large number of randomised controlled trials, the evidence remains mostly low quality when rated using the GRADE system. Control groups usually received no intervention or therapy based on a standard‐care approach. Primary outcome: results were not statistically significant for upper limb function (standardised mean difference (SMD) 0.07, 95% confidence intervals (CI) ‐0.05 to 0.20, 22 studies, 1038 participants, low‐quality evidence) when comparing virtual reality to conventional therapy. However, when virtual reality was used in addition to usual care (providing a higher dose of therapy for those in the intervention group) there was a statistically significant difference between groups (SMD 0.49, 0.21 to 0.77, 10 studies, 210 participants, low‐quality evidence). Secondary outcomes: when compared to conventional therapy approaches there were no statistically significant effects for gait speed or balance. Results were statistically significant for the activities of daily living (ADL) outcome (SMD 0.25, 95% CI 0.06 to 0.43, 10 studies, 466 participants, moderate‐quality evidence); however, we were unable to pool results for cognitive function, participation restriction, or quality of life. Twenty‐three studies reported that they monitored for adverse events; across these studies there were few adverse events and those reported were relatively mild.
Authors' conclusions
We found evidence that the use of virtual reality and interactive video gaming was not more beneficial than conventional therapy approaches in improving upper limb function. Virtual reality may be beneficial in improving upper limb function and activities of daily living function when used as an adjunct to usual care (to increase overall therapy time). There was insufficient evidence to reach conclusions about the effect of virtual reality and interactive video gaming on gait speed, balance, participation, or quality of life. This review found that time since onset of stroke, severity of impairment, and the type of device (commercial or customised) were not strong influencers of outcome. There was a trend suggesting that higher dose (more than 15 hours of total intervention) was preferable as were customised virtual reality programs; however, these findings were not statistically significant.
Plain language summary
Virtual reality for stroke rehabilitation
Review question We wanted to compare the effects of virtual reality versus an alternative treatment or no treatment on recovery after stroke using arm function and other outcomes such as walking speed and independence in managing daily activities after stroke.
Background Many people after having a stroke have difficulty moving, thinking, and sensing. This often results in problems with everyday activities such as writing, walking, and driving. Virtual reality and interactive video gaming are types of therapy being provided to people after having a stroke. The therapy involves using computer‐based programs designed to simulate real life objects and events. Virtual reality and interactive video gaming may have some advantages over traditional therapy approaches as they can give people an opportunity to practise everyday activities that are not or cannot be practised within the hospital environment. Furthermore, there are several features of virtual reality programs that might mean that patients spend more time in therapy: for example, the activity might be more motivating.
Study characteristics We identified 72 studies involving 2470 people after stroke. A wide range of virtual reality programs were used, with most aimed to improve either arm function or walking ability. The evidence is current to April 2017.
Key results Twenty‐two trials tested whether the use of virtual reality compared with conventional therapy resulted in an improved ability to use one's arm and found that the use of virtual reality did not result in better function (low‐quality evidence). When virtual reality was used in addition to usual care or rehabilitation to increase the amount of time the person spent in therapy there were improvements in the functioning of the arm (low‐quality evidence). Six trials tested whether the use of virtual reality compared with conventional therapy resulted in improved walking speed. There was no evidence that virtual reality was more effective in this case (low‐quality evidence). Ten trials found that there was some evidence that virtual reality resulted in a slightly better ability to manage everyday activities such as showering and dressing (moderate‐quality evidence). However, these positive effects were found soon after the end of the treatment and it is not clear whether the effects are long lasting. Results should be interpreted with caution as, while there are a large number of studies, the studies are generally small and not of high quality. A small number of people using virtual reality reported pain, headaches, or dizziness. No serious adverse events were reported.
Quality of the evidence The quality of the evidence was generally of low or moderate quality. The quality of the evidence for each outcome was limited due to small numbers of study participants, inconsistent results across studies, and poor reporting of study details.
Summary of findings
Summary of findings for the main comparison. Virtual reality compared to conventional therapy for stroke rehabilitation.
| Virtual reality compared to conventional therapy for stroke rehabilitation | ||||||
|
Patient or population: people receiving stroke rehabilitation
Settings: hospital, clinic or home
Intervention: virtual reality Comparison: conventional therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Virtual reality | |||||
| Upper limb function | Same dose of conventional therapy | The mean upper limb function in the intervention groups was 0.07 standard deviations higher (‐0.05 to 0.20 higher) | 1038 (22 studies) | ⊕⊕⊝⊝ low1,2,3 | No statistically significant difference between groups | |
| Quality of life | Same dose of conventional therapy | No significant benefit found on total score of the SF‐36 | 300 (3 studies) |
⊕⊕⊝⊝ low1,2,4 |
Studies could not be pooled. None of the 3 studies found significant differences between groups in total score. 2 studies reported significant differences in domains of the SF36 | |
| Gait speed | Same dose of conventional therapy | The mean gait speed in the intervention groups was 0.09 metres per second faster (0.04 lower to 0.22 higher) | 139 (6 studies) | ⊕⊕⊝⊝ low1,3,4 | No statistically significant difference between groups | |
| ADL outcome | Same dose of conventional therapy | The mean ADL outcome in the intervention groups was 0.25 standard deviations higher (0.06 to 0.43 higher) | 466 (10 studies) | ⊕⊕⊕⊝ moderate1 | Small effect in favour of those receiving virtual reality intervention | |
| ADL: activities of daily living; CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1Risk of bias was unclear in a number of studies. 2Downgraded by 1 due to inconsistency in findings across studies. 3Surrogate outcome. 4Small total population size (< 400).
Summary of findings 2. Virtual reality plus usual care compared with usual care alone.
| Virtual reality intervention compared with usual care (thus provided as additional therapy) for stroke rehabilitation | ||||||
|
Patient or population: people receiving stroke rehabilitation Settings: hospital, clinic or home Intervention: virtual reality provided in addition to usual care Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Virtual reality (provided in addition to usual care) | |||||
| Upper limb function | Usual care | The SMD in the intervention groups was 0.49 standard deviations higher (0.21 to 0.77) | ‐ | 210 (10 studies) |
⊕⊕⊝⊝ low1,3,4 | Moderate effect in favour of providing virtual reality intervention in addition to usual care |
| Quality of life ‐ not measured in any of the studies | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in the studies |
| Gait speed | Usual care | The mean difference in the intervention groups was 0.08 metres per second faster (‐0.05 to 0.21) | ‐ | 57 (3 studies) |
⊕⊕⊝⊝ low1,3,4 | No statistically significant difference between groups |
| Global motor function | Usual care | The SMD in the intervention groups was 0.01 standard deviations higher (‐0.60 to 0.61) | ‐ | 43 (3 studies) |
⊕⊕⊝⊝ low1,3,4 | No statistically significant difference between groups |
| ADL outcome | Usual care | The SMD in the intervention groups was 0.44 standard deviations higher (0.11 to 0.76) | ‐ | 153 (8 studies) |
⊕⊕⊝⊝ low1,3,4 | Small to moderate effect in favour of virtual reality intervention |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1Risk of bias was unclear in a number of studies. 2Downgraded by 1 due to inconsistency in findings across studies. 3Surrogate outcome. 4Small total population size (< 400).
Background
Description of the condition
Stroke is one of the leading causes of death and disability and has been described as a worldwide epidemic (Feigin 2014; Go 2014). The effects of a stroke may include sensory, motor, and cognitive impairment as well as a reduced ability to perform self care and participate in social and community activities (Miller 2010). While most recovery is thought to be made in the first few weeks after stroke, patients may make improvements on functional tasks many months after having a stroke (Teasell 2014). Many stroke survivors report long‐term disability and reduced quality of life (Patel 2006; Sturm 2004).
Description of the intervention
Repetitive task training has been shown to be effective in some aspects of rehabilitation, such as improving walking distance and speed and improving upper limb function (French 2016; Veerbeek 2014). Virtual reality is a relatively recent approach that may enable simulated practice of functional tasks at a higher dosage than traditional therapies (Demain 2013; Fung 2012; Kwakkel 2004; Merians 2002). Virtual reality has been defined as the "use of interactive simulations created with computer hardware and software to present users with opportunities to engage in environments that appear and feel similar to real‐world objects and events" (Weiss 2006).
Virtual reality has previously been used in a variety of vocational training settings, such as flight simulation training for pilots (Lintern 1990) and procedural training for surgeons (Larsen 2009). Within health care, the intervention has been used to treat phobias, post‐traumatic stress disorder, and body image disorders (Jiandani 2014; Raghav 2016). Although its research in rehabilitation is becoming more prevalent as technology becomes more accessible and affordable, the use of virtual reality is not yet routinely used in clinical rehabilitation settings. However, gaming consoles are ubiquitous and so researchers and clinicians have turned to low‐cost commercial gaming systems as an alternative way of delivering virtual reality (Levac 2015). These systems, which were originally designed for recreation, are being adapted by clinicians for therapeutic purposes. In addition, interactive video games are specifically being designed for rehabilitation (Lange 2010; Lange 2012).
In virtual rehabilitation, virtual environments and objects provide the user with visual feedback, which may be presented though a head‐mounted device, projection system, or flat screen. Feedback may also be provided through the senses, for example, hearing, touch, movement, balance, and smell (Weiss 2006). The user interacts with the environment by a variety of mechanisms. These may be simple devices, such as a mouse or joystick, or more complex systems using cameras, sensors, or haptic (touch) feedback devices (Weiss 2006). Thus, depending on the intervention, the user's level of physical activity may range from relatively inactive (for example, sitting at a computer using a joystick), to highly active (for example, challenging, full‐body movements). Virtual reality relies on computer hardware and software that mediates the interaction between the user and the virtual environment (Gaggioli 2009).
Key concepts related to virtual reality are immersion and presence. Immersion refers to the extent to which the user perceives that they are in the virtual environment rather than the real world and is related to the design of the software and hardware (Gaggioli 2009; Weiss 2006). Virtual environments can range in their degree of immersion of the user. Systems that include projection onto a concave surface, head‐mounted display, or video capture in which the user is represented within the virtual environment are generally described as immersive, whereas a single screen projection or desktop display are considered low immersion.
Presence is the subjective experience of the user and is dependent on the characteristics of the virtual reality system, the virtual task, and the characteristics of the user. People are considered present when they report the feeling of being in the virtual world (Schuemie 2001).
How the intervention might work
Virtual reality may be advantageous as it offers several features, such as goal‐oriented tasks and repetition, shown to be important in neurological rehabilitation (Langhorne 2011; Veerbeek 2014). Animal research has shown that training in enriched environments results in better problem solving and performance of functional tasks than training in basic environments (Risedal 2002). Virtual reality may have the potential to provide an enriched environment in which people with stroke can problem solve and master new skills. Virtual tasks have been described as more interesting and enjoyable by children and adults, thereby encouraging higher numbers of repetitions (Lewis 2012).
Evidence of neuroplasticity as a result of training in virtual reality is modest; however, neuroimaging findings are guiding the development of virtual reality. Two studies have shown that functional improvements after virtual reality training were paralleled with a lateralisation of neural activation from the contralesional sensorimotor activation prior to training, to an ipsilesional representation after training (Jang 2005; You 2005). Tunik and colleagues have shown that when individuals post stroke were presented with discordant feedback, they activated the primary motor region (M1) to a greater extent than when feedback was not discordant (Tunik 2013). Notably, when discordant feedback corresponded to the affected and moving hand, the contralateral M1 region was recruited (Bagce 2012; Tunik 2013). Conversely, by having participants move the unaffected hand with virtual mirror feedback, the ipsilateral (affected) M1 region was recruited (despite the affected hand remaining static) (Saleh 2014). Their findings suggest that tailoring manipulation of the visual feedback in virtual reality to the needs of the patient may serve as a tool for rehabilitation.
One major advantage of virtual reality programs, which has been underutilised to date, is that they allow clinicians to be able to trial tasks that are unsafe to practise in the real world, such as crossing the street. In addition, some programs are designed to be used without supervision, also meaning that increased dosage of therapy can be provided without increased staffing levels.
Why it is important to do this review
As using technology becomes an integral part of daily living, virtual reality is likely to become even more widely used in clinical rehabilitation settings (Bohil 2011; Burridge 2010). It is important to evaluate the efficacy of virtual reality in order to guide future design and use. Furthermore, therapeutic interventions that increase the dose of task‐specific training without increasing staffing will be sought after.
There are now a number of systematic reviews examining the efficacy of virtual reality for stroke rehabilitation (Crosbie 2007; Darekar 2015; Lohse 2014; Moreira 2013; Saposnik 2011) and, more specifically, commercial gaming devices for upper limb stroke rehabilitation (Thomson 2014). Our initial review published in 2011 identified 19 studies and a number of ongoing studies. Our update published in 2015 resulted in the inclusion of more studies bringing the total to 37 studies. The area is rapidly expanding and therefore an update of our review was warranted.
Objectives
Primary objective
To determine the efficacy of virtual reality compared with an alternative intervention or no intervention on upper limb function and activity.
Secondary objectives
To determine the efficacy of virtual reality compared with an alternative intervention or no intervention on gait and balance, global motor function, cognitive function, activity limitation, participation restriction, quality of life, and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) and quasi‐randomised (e.g. allocation by birth date) controlled trials (QRCTs). We included one QRCT and the remaining studies were RCTs. Where the QRCT was included in a meta‐analysis we carried out a sensitivity analysis restricting analysis to truly randomised studies. We looked for studies that compared virtual reality with either an alternative intervention or no intervention. We did not include studies that compared two different types of virtual reality without an alternative group. We included trials that evaluated any intensity and duration of virtual reality that exceeded a single treatment session.
Types of participants
The study participants had a diagnosis of stroke, defined by the World Health Organization as "a syndrome of rapidly developing symptoms and signs of focal, and at times global, loss of cerebral function lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin" (WHO 1989), diagnosed by imaging or neurological examination. We included people who were 18 years and older with all types of stroke, all levels of severity, and at all stages post stroke, including those people with subarachnoid haemorrhage. We excluded studies of participants with mixed aetiology (for example, participants with acquired brain injury) unless data were available relating to the people with stroke only.
Types of interventions
We included studies using virtual reality interventions that met the following definition: "an advanced form of human‐computer interface that allows the user to 'interact' with and become 'immersed' in a computer‐generated environment in a naturalistic fashion" (Schultheis 2001).
We included studies using any form of non‐immersive or immersive virtual reality, and studies that used commercially available gaming consoles.
The comparison group received either an alternative intervention or no intervention. Given the broad range of alternative interventions, we considered these to include any activity designed to be therapeutic at the impairment, activity, or participation level that did not include the use of virtual reality.
Types of outcome measures
Primary outcomes
As one of the most common applications of virtual reality in stroke rehabilitation is upper limb rehabilitation, we selected the following primary outcome.
-
Upper limb function and activity:
arm function and activity: including assessments such as the Fugl Meyer, Motor Assessment Scale (upper limb), Action Research Arm Test, Wolf Motor Function Test, Box and Block Test, Jebsen Taylor Hand Function Test
hand function: grip strength
Secondary outcomes
-
Gait and balance:
lower limb activity: including assessments such as walking distance, walking speed, Community Walk Test, functional ambulation, Timed Up and Go Test;
balance and postural control: including assessments such as the Berg Balance Scale and forward reach test.
Global motor function: including assessments such as the Motor Assessment Scale.
Cognitive function: including assessments such as Trail Making Test, Useful Field of View Test.
Activity limitation: addressing activities of daily living and including assessments such as the Functional Independence Measure (FIM), Barthel Index, on‐road driving test.
Participation restriction and quality of life: including assessments such as the SF36, EQ5D, Stroke Impact Scale or other patient‐reported outcome measure.
Adverse events: including motion sickness, pain, injury, falls and death.
We included the primary outcome (upper limb function) and gait, global motor function, and quality of life in Table 1.
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We searched for relevant trials in all languages and arranged translation of trial reports published in languages other than English.
Electronic searches
The searches for studies in our previous reviews were conducted in March 2010 and November 2013. The search for this update was completed in May 2016 and then updated again in April 2017. Cochrane Stroke's Managing Editor searched the Group's Trials Register in April 2017 using the intervention codes 'computer‐aided therapy' and 'virtual reality therapy'.
In addition, we searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3, searched 1 April 2017) (Appendix 1); MEDLINE Ovid (1950 to April Week 1, 2017) (Appendix 2); Embase (1980 to Week 13, 2017) (Appendix 3); Ovid AMED (1985 to April 2017) (Appendix 4); CINAHL Ebsco (1982 to April Week 1, 2017) (Appendix 5); Ovid PsycINFO (1840 to April Week 1, 2017) (Appendix 6); PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy, www.psycbite.com/) (to 1 April 2017) and OTseeker (www.otseeker.com/) (to 1 April 2017). We also searched the engineering databases COMPENDEX (1970 to 1 April 2017) for studies from a non‐medical background.
The Cochrane Stroke Group Information Specialist developed our search strategies for MEDLINE (Ovid) and we adapted them for other databases with the assistance of an experienced medical librarian.
Searching other resources
In order to identify further published, unpublished and ongoing trials, we:
searched the following ongoing trials registers: Current Controlled Trials (www.isrctn.com), National Institute of Health Clinical Trials Database (www.clinicaltrials.gov) and Stroke Trials Registry (www.strokecenter.org/trials/) to 1 June 2016;
used the Cited Reference Search within Science Citation Index (SCI) and Social Science Citation Index (SSCI) to track relevant references for all included studies;
scanned the reference lists of all included studies;
searched Dissertation Abstracts via Proquest (1 June 2016);
scanned the abstracts of non‐English language studies if they were available in English;
searched the IEEE (Institute of Electrical and Electronic Engineers) electronic library (to 1 April 2017).
For the previous version of this review we carried out the following searches; however, we did not repeat these searches for this update.
We handsearched the proceedings of the International Workshop on Virtual Rehabilitation (2003 to 2005), Virtual Rehabilitation Conference (2007 to 2009), International Conference Series on Disability, Virtual Reality and Associated Technologies (2000 to 2008) and Cybertherapy (2003 to 2007).
We contacted 12 manufacturers of virtual reality equipment to ask for details of trials. We contacted the following manufacturers by telephone, email or postal mail: Nintendo, Sony, GestureTek, NeuroVR, Hocoma, Motek, Virtual Realities, Haptic Master, Microsoft Xbox, Essential Reality, SensAble, Novint and Cyberglove. Three of the manufacturers responded (Nintendo, Motek, and Novint); however, they were unable to provide details of studies eligible for inclusion in the review.
Data collection and analysis
Selection of studies
One review author (KL) performed the searches. Two of the authors (KL and BL) independently reviewed the titles and abstracts identified from the database searches to assess whether they met the pre‐defined inclusion criteria. The review authors obtained potentially relevant articles in full text and KL contacted study authors when more information was required. KL and BL then independently reviewed full‐text articles and correspondence with investigators to determine studies to be included in the review. JD made the final decision on studies that KL and BL disagreed on. We documented the reasons for the exclusion of studies. Where studies published in non‐English languages appeared relevant, we sought the full text of the study. In these cases, we arranged for someone fluent in the non‐English language to review the paper to ascertain whether the study met the inclusion criteria.
Data extraction and management
Two review authors (KL and SG, JD, GS or MC) independently extracted data using a pre‐designed data extraction form for each selected study. Data extracted included citation details, trial setting, inclusion and exclusion criteria, study population, participant flow, intervention details, outcome measures and results, and methodological quality. We resolved disagreements by discussion or by referral to a third review author (BL) as necessary. The review authors contacted study authors by email to request any missing information necessary for the review.
Assessment of risk of bias in included studies
Two review authors (KL and SG, JD, GS or MC) used Cochrane's 'Risk of bias' tool to independently assess the methodological quality of the included studies (Appendix 7; Higgins 2011a). The tool covers the domains of sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data and selective reporting. We classified items as 'low risk', 'high risk' or 'unclear risk' of bias. We omitted the domain that assesses the blinding of participants as we were of the opinion that this domain related to the nature of the intervention and not study quality. We contacted the authors of the included studies for more information where insufficient information was published to assess the risk of bias. We resolved disagreements with help from a third review author (BL).
We employed GRADE to interpret findings (Guyatt 2008) and used GRADEpro GDT to create 'Summary of findings' tables (GRADEpro GDT 2015). The tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparisons, the magnitude of effect of the intervention, and the sum of available data on the outcomes considered. When using GRADE, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Two review authors (KL and SG, JD, GS or MC) independently classified outcome measures in terms of the domain assessed (upper limb function, hand function, lower limb and gait activity, balance and postural control, global motor function, cognitive function, activity limitation, participation restriction, and quality of life). When a study presented more than one outcome measure for the same domain, we included the measure most frequently used across studies in the analysis. We planned to calculate risk ratios (RR) with 95% confidence intervals (CIs) for any dichotomous outcomes, if recorded. We calculated mean differences (MD) or standardised mean differences (SMD) for continuous outcomes as appropriate.
Unit of analysis issues
The unit of randomisation in these trials was the individual participant. We did not include any cluster‐randomised controlled trials. Seven of the studies were three‐armed trials. We used the approach of splitting the 'shared' group into two or more groups with smaller sample size and including two (reasonably independent) comparisons (as described in part 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions: Higgins 2011b). Lam 2006 compared virtual reality with an alternative intervention and no intervention. We used data in the analyses according to the comparison (i.e. we used the data comparing the virtual reality arm with the alternative intervention arm in one meta‐analysis and the data comparing virtual reality with no intervention in another meta‐analysis). Coupar 2012 compared a usual‐care group with a group that received additional 'low intensity' virtual reality intervention and a group that received additional 'high intensity' virtual reality intervention. We split the control group data enabling comparison of high intensity with usual care and low intensity with usual care. da Silva Cameirao 2011 compared a virtual reality intervention using a specialised program with a control group who either received gaming or conventional occupational therapy. Data were only provided for intervention (virtual reality) versus control (Wii or conventional therapy) and so were included in the meta‐analysis in this manner. Byl 2013 compared conventional therapy with unilateral and bilateral virtual reality intervention. We used the data from both intervention groups and split the control group. Zucconi 2012 compared a virtual reality intervention with feedback on performance with a virtual reality intervention without feedback and conventional therapy. We were only able to obtain data from the virtual reality with feedback on performance group versus the control group and so this is what was used in the analysis. A study published by Fan 2014 randomised people to an interactive video gaming group, a conventional occupational therapy group, and a recreational board game group; we were unable to obtain data from this study in a form suitable for meta‐analysis so provided a descriptive summary. Finally, Kong 2014 randomised participants to interactive video gaming, conventional therapy or usual care. We used data comparing the gaming, conventional therapy, and usual care in separate analyses.
Dealing with missing data
We contacted study authors to obtain any missing data and converted available data when possible (e.g. we converted gait speed reported as metres per minute to metres per second (Jaffe 2004)). We used the actual denominator of the participants contributing the data.
Assessment of heterogeneity
We pooled results to present an overall estimate of the treatment effect using a fixed‐effect model in the primary analysis. We assessed heterogeneity by visual inspection of the forest plot. We quantified inconsistency amongst studies using the I2 statistic (Higgins 2003), where we considered levels greater than 50% as substantial heterogeneity. We used a random‐effects model as part of a sensitivity analysis in the presence of heterogeneity (Deeks 2011).
Assessment of reporting biases
Our search of clinical trials registers assisted in reducing publication bias. We also investigated selective outcome reporting through the comparison of the methods section of papers with the results reported and contacting study authors to check whether additional outcomes had been collected. We inspected funnel plots for each of the analyses; however, interpretation was limited due to the small sample sizes.
Data synthesis
Where there were acceptable levels of heterogeneity, we pooled results. We used the fixed‐effect model with 95% CI using Review Manager 5 (RevMan 5) (RevMan 2014). We used a random‐effects model as part of a sensitivity analysis. Where meta‐analysis was not appropriate due to unacceptable heterogeneity, we have presented a narrative summary of study results. We pooled outcomes measured with different instruments using the SMD.
Subgroup analysis and investigation of heterogeneity
We attempted to perform subgroup analyses to determine whether outcomes varied according to age, severity of stroke, time since onset of stroke, dose of intervention (total hours of intervention) and type of intervention (highly specialised program designed for rehabilitation versus commercial gaming console). However, not all of these analyses were possible due to the homogeneity of trial participants. We were able to undertake subgroup analysis in some cases for:
dosage of intervention (for upper limb function we compared less than 15 hours' intervention with more than 15 hours' intervention and for lower limb function we compared less than 10 hours' intervention with more than 10 hours' intervention). We selected the doses of 10 and 15 hours based on examining the included studies and their characteristics and choosing a threshold that appeared to separate the studies approximately in half (to enable comparisons of higher‐ and lower‐dose treatments);
time since onset of stroke (less than or more than six months);
type of intervention (specialised program or commercial gaming console);
severity of impairment (upper limb).
Sensitivity analysis
We performed sensitivity analyses to determine whether there was a difference in using a fixed‐effect model versus a random‐effects model. We conducted sensitivity analyses where possible to explore the effects of the methodological quality of the included studies on overall effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified 168 studies from searching the Cochrane Stroke Group Trials Register and 11,664 references from the database searches totaling 11,832 references to studies. A search of the trials registries elicited a further 108 potentially relevant studies. From the 11,940 titles and abstracts retrieved, we sought 422 of the articles in full text for further review. We grouped articles reporting the same study. We removed articles that did not meet the inclusion criteria, such as studies that used interventions that were not considered virtual reality and non‐randomised controlled trials. We included a total of 72 studies. We have provided details on 34 excluded studies in the Characteristics of excluded studies table, which were closest to, but did not meet the inclusion criteria (Figure 1). We identified 14 studies awaiting classification, and 22 ongoing studies (Characteristics of ongoing studies).
1.
Study flow diagram
Included studies
We identified 72 RCTs with a total of 2470 participants, which met the inclusion criteria. Of the 72 included studies, we included 19 (with 565 participants) in the original version of this review, 18 new studies (with 454 participants) in the 2015 update, and 35 new studies (with 1451 participants) in this updated review.
Sample characteristics
All trials took place between 2004 and 2016. All but two were published in English (Galvao 2015; Xiang 2014). Over half (41; 57%) of the studies involved sample sizes of fewer than 25 participants and only 10 studies involved more than 50 participants (Adie 2017; Akinwuntan 2005; Kiper 2011; Klamroth‐Marganska 2014; Ko 2015; Kong 2014; Lam 2006; Linder 2015; Prange 2015; Saposnik 2016). A total of 2470 participants post stroke were included in the trials.
All studies, except for Ucar 2014, reported that they included both men and women. Although not always clearly reported, it appears that participants in the included studies were relatively young, with all studies reporting mean ages of 46 to 75 years.
Thirteen trials recruited participants within three months of stroke (Akinwuntan 2005; Coupar 2012; da Silva Cameirao 2011; Kwon 2012; Kong 2014; Low 2012; Mao 2015; Morone 2014; Piron 2007; Prange 2015; Saposnik 2010; Saposnik 2016; Xiang 2014); two trials recruited within six months of stroke (Adie 2017; Ko 2015); two trials recruited within 12 months (Kiper 2011; Yavuzer 2008); three trials recruited people more than two to three months post stroke (Levin 2012; McNulty 2015; Reinkensmeyer 2012); 31 trials recruited participants more than six months post stroke (Byl 2013; Crosbie 2008; da Silva Ribeiro 2015; Fan 2014; Givon 2016; Housman 2009; Hung 2014; Jaffe 2004; Jang 2005; Jung 2012; Kim 2009; Kim 2012a; Klamroth‐Marganska 2014; Lee 2013; Lee 2014a; Lee 2015a; Lee 2015b; Llorens 2015; Manlapaz 2010; Mirelman 2008; Nara 2015; Piron 2010; Sin 2013; Sucar 2009; Subramanian 2013; Thielbar 2014; Yang 2008; Ucar 2014; Yang 2011; You 2005; Zucconi 2012). Time since onset of stroke was not reported in the inclusion criteria for the remaining studies. The average recruitment time since stroke for each study is reported in the Characteristics of included studies table.
Several trials excluded people who were deemed medically unstable, though how this was determined was often unclear. Ten trials specified that people with a history of epilepsy or seizures would be excluded (Akinwuntan 2005; Fan 2014; Givon 2016; Kim 2012a; Mazer 2005; Saposnik 2010; Saposnik 2016; Sin 2013; Ucar 2014; Yin 2014). Most studies reported that people with significant cognitive impairment would be excluded; however, this criterion was often poorly defined. Several studies listed the presence of aphasia, apraxia, and visual impairment as exclusion criteria. One study excluded people with computer‐related phobias (Lam 2006). Studies involving upper limb training included participants with a range of function including those with severe functional impairment (Byl 2013; Coupar 2012; da Silva Cameirao 2011; Kiper 2011; Klamroth‐Marganska 2014; Levin 2012; Linder 2015; McNulty 2015; Reinkensmeyer 2012; Shin 2014; Sin 2013). All studies except Bower 2015 involving lower limb and gait training only involved participants that were able to walk independently.
Interventions
Intervention approaches
Five intervention approaches were used: activity retraining; upper limb training; lower limb, balance and gait training; global motor function training; and cognitive/perceptual training. Four trials involved activity retraining; Akinwuntan 2005 and Mazer 2005 examined automobile driving retraining; Jannink 2008 examined scooter driving retraining; and Lam 2006 tested retraining skills in using public transport. Thirty‐five trials involved upper limb training (Adie 2017; Byl 2013; Coupar 2012; Crosbie 2008; da Silva Cameirao 2011; Fan 2014; Galvao 2015; Housman 2009; Kim 2012a; Kiper 2011; Klamroth‐Marganska 2014; Kong 2014; Lee 2015b; Levin 2012; Linder 2015; Manlapaz 2010; Matsuo 2013; McNulty 2015; Prange 2015; Piron 2007; Piron 2009; Piron 2010; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Shin 2014; Shin 2015; Sin 2013; Standen 2011; Subramanian 2013; Sucar 2009; Thielbar 2014; Yavuzer 2008; Yin 2014; Zucconi 2012). Twenty‐three trials involved lower limb, balance and gait training (Barcala 2013; Bower 2015; Chow 2013; Han 2013; Hung 2014; Jaffe 2004; Jung 2012; Kim 2009; Ko 2015; Lee 2013; Lee 2014a; Lee 2015a; Llorens 2015; Mao 2015; Mirelman 2008; Morone 2014; Nara 2015; Rajaratnam 2013; Song 2015; Ucar 2014; Xiang 2014; Yang 2008; Yang 2011). Ten trials used virtual reality to improve global motor function (Cho 2012; da Silva Ribeiro 2015; Givon 2016; Jang 2005; Kim 2009; Kim 2011a; Kim 2011b; Kwon 2012; Low 2012; You 2005) and one trial used a visual‐perceptual retraining approach (Kang 2009).
Twenty‐two (31%) of the studies used commercially available gaming consoles: one study used the Playstation EyeToy (Yavuzer 2008), 15 studies used the Nintendo Wii (Barcala 2013; da Silva Ribeiro 2015; Fan 2014; Galvao 2015; Hung 2014; Kim 2012a; Kong 2014; Lee 2015a; Manlapaz 2010; Matsuo 2013; McNulty 2015; Morone 2014; Rajaratnam 2013; Saposnik 2010; Saposnik 2016) and four studies used the Microsoft Kinect (Chow 2013; Rajaratnam 2013; Sin 2013; Song 2015). Two studies used a mix of gaming consoles (Bower 2015; Givon 2016). Eight studies used GestureTek IREX, which is commercially available but more difficult to obtain and more expensive than off‐the‐shelf consoles (Cho 2012; Han 2013; Jang 2005; Kim 2009; Kim 2011a; Kim 2011b; Kwon 2012; You 2005). One study used the Armeo (Coupar 2012), one used the CAREN system (Subramanian 2013) and one used the Lokomat (Ucar 2014), which are also available for rehabilitation facilities to purchase. The remaining studies used customised virtual reality programs. The number of studies using commercially available gaming consoles increased from six in the previous version of this review to 22 in this update.
Setting
The majority of interventions were delivered in either an outpatient or inpatient setting, although five of the studies delivered the intervention in the participant's own home (Adie 2017; Linder 2015; McNulty 2015; Piron 2009; Standen 2011). Two of these studies used a telerehabilitation approach to deliver the intervention (Linder 2015; Piron 2009).
Amount of therapy provided
The total dose of therapy provided varied between studies. Fourteen studies provided less than five hours of total therapy (Barcala 2013; Bower 2015; Han 2013; Jannink 2008; Kim 2012a; Low 2012; Matsuo 2013; Morone 2014; Nara 2015; Shin 2014; Ucar 2014; Yang 2008; Yang 2011). Twenty‐five studies provided between six and 10 hours of therapy (Crosbie 2008; Fan 2014; Jaffe 2004; Jung 2012; Kang 2009; Kim 2009; Kim 2011a; Kim 2011b; Ko 2015; Kwon 2012; Lam 2006; Lee 2013; Lee 2014a; Lee 2015a; Lee 2015b; Levin 2012; Manlapaz 2010; Mao 2015; Prange 2015; Saposnik 2010; Saposnik 2016; Sin 2013; Subramanian 2013; Xiang 2014; Yavuzer 2008). A further 26 studies provided between 11 and 20 hours of therapy (Akinwuntan 2005; Byl 2013; Cho 2012; Chow 2013; da Silva Cameirao 2011; da Silva Ribeiro 2015; Galvao 2015; Hung 2014; Jang 2005; Kiper 2011; Kong 2014; Klamroth‐Marganska 2014; Llorens 2015; Mazer 2005; McNulty 2015; Mirelman 2008; Piron 2009; Piron 2010; Rajaratnam 2013; Shin 2015; Song 2015; Sucar 2009; Thielbar 2014; Yin 2014; You 2005; Zucconi 2012) and seven studies provided more than 21 hours of therapy (Adie 2017; Givon 2016; Housman 2009; Linder 2015; Piron 2007; Reinkensmeyer 2012; Standen 2011; ). The remaining study, Coupar 2012, had three arms; one of the arms received lower intensity therapy (four hours total) and another received higher intensity therapy (10 hours total).
Comparison interventions
Most of the trials compared virtual reality intervention with a comparable alternative intervention. The alternative intervention was often described as therapy using a conventional approach. One study allocated participants to either actively participating in the virtual reality intervention or watching others participate in the virtual reality intervention (Yavuzer 2008). Other studies of note compared virtual reality with recreational therapy (Saposnik 2016) and constraint‐induced movement therapy (McNulty 2015). Eighteen of the studies examined the effect of virtual reality when used alone (the control group received no intervention) or as an adjunct (the control group received usual care or rehabilitation) and thus there was a discrepancy in the dose of therapy received between the intervention and control groups (Barcala 2013; Bower 2015; Cho 2012; Jang 2005; Kim 2011a; Kim 2012a; Kong 2014; Kwon 2012; Lee 2013; Lee 2014a; Low 2012; Matsuo 2013; Mazer 2005; Shin 2014; Sin 2013; Standen 2011; Ucar 2014; You 2005). There were seven three‐armed trials with two comparison interventions (Byl 2013; Coupar 2012; da Silva Cameirao 2011; Fan 2014; Kong 2014; Lam 2006; Zucconi 2012).
Outcomes
As a result of the diverse intervention approaches, a wide range of outcome measures were used. Outcome measures for each of the predefined outcome categories are shown in Table 3. Due to the heterogeneity of outcome measures, we were unable to include all of them in the analyses. With regard to timing of outcome measurements, one study waited until five weeks after the end of the intervention to collect outcome measures (Jannink 2008). All remaining studies measured outcomes soon after the intervention was completed. For studies including further follow‐up, the time interval until follow‐up was generally at or less than three months (Coupar 2012; Crosbie 2008; da Silva Cameirao 2011; Fan 2014; Givon 2016; Hung 2014; Jaffe 2004; Kong 2014; Levin 2012; Matsuo 2013; Mirelman 2008; Morone 2014; Piron 2009; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Subramanian 2013; Thielbar 2014; Yang 2008; Yin 2014). Only five studies involved longer‐term follow‐up: four at six months (Adie 2017; Housman 2009; Klamroth‐Marganska 2014; McNulty 2015) and one at both six months and five years (Akinwuntan 2005).
1. Outcome measures used from the included trials.
| Author and year | Upper limb function | Hand function | Lower limb activity | Balance and postural control | Global motor function | Cognitive function | Activity limitation | Participation restriction and QOL |
| Adie 2017 | Action Research Arm Test, Motor Activity Log Arm Function Test |
Modified Rankin Scale | Stroke Impact Scale, EQ5D, Canadian Occupational Performance Measure |
|||||
| Akinwuntan 2005 | — | — | — | — | — | Useful Field of View test | On‐road driving test score, decision of fitness to drive | — |
| Barcala 2013 | — | — | Timed Up and Go | Berg Balance Scale, centre of pressure data, body symmetry data | — | — | Functional Independence Measure | — |
| Bower 2015 | 6‐minute walk test, step test | Functional reach | Motor Assessment Scale | Functional Independence Measure (transfers, mobility, stairs) | ||||
| Byl 2013 | Fugl Meyer UE Scale, Motor Proficiency Speed (abbreviated Wolf Motor Function test + Digital reaction time test) | Motor skill performance (Box and Block and tapper test) | — | — | — | — | Functional Independence (CAFE40) | |
| Cho 2012 | Wolf Motor Function Test | — | — | — | — | Motor Free Visual Perception Test | — | — |
| Chow 2013 | 10‐m walk test | Berg Balance Scale | Modified Barthel Index | |||||
| Crosbie 2008 | Action Research Arm Test, Upper Limb Motricity Index | — | — | — | — | — | — | — |
| da Silva Ribeiro 2015 | Fugl Meyer | Dynamic Gait Index | SF36 | |||||
| da Silva Cameirao 2011 | Fugl Meyer UE, Chedoke Arm and Hand Inventory | — | — | — | — | — | Barthel Index | — |
| Fan 2014 | Jebsen Taylor Hand Function Test | Stroke Impact Scale | ||||||
| Galvao 2015 | Fugl Meyer, Motor Activity Log | |||||||
| Givon 2016 | Action Research Arm Test | Grip strength | 10‐m walk test | |||||
| Han 2013 | Berg Balance Scale | Modified Barthel Index | ||||||
| Housman 2009 | Fugl Meyer UE Scale, Rancho Functional Test, Motor Activity Log (amount of use and quality of movement) |
Grip strength (kg) | — | — | — | — | — | ‐ |
| Hung 2014 | Timed Up and Go Test | Forward Reach Test | Falls Efficacy Scale International | |||||
| Jaffe 2004 | — | — | 6‐m walk test, Obstacle Test, 6‐minute walk test | Customised balance test designed by the researchers | — | — | — | — |
| Jang 2005 | Fugl Meyer UE Scale, Manual Function Test, Motor Activity Log (amount of use and quality of movement) | Box and Block Test | — | — | — | — | — | |
| Jannink 2008 | — | — | — | — | — | — | — | — |
| Jung 2012 | — | — | Timed Up and Go | — | — | — | — | — |
| Kang 2009 | — | — | — | — | — | Mini Mental State Examination | Modified Barthel Index | — |
| Kim 2009 | — | — | 10‐m walk test, GAIT‐RITE gait analysis system | Berg Balance Scale, balance performance monitor | Modified Motor Assessment Scale | — | — | — |
| Kim 2011a | Motricity Index | — | Motricity Index | — | — | Computerised neuropsychological test and Tower of London test | Korean Modified Barthel Index | — |
| Kim 2011b | — | — | — | — | — | Measures of spatial neglect (star cancellation, line bisection test, Catherine Bergego Scale) | Korean Modified Barthel Index | — |
| Kim 2012a | — | — | — | Postural assessment scale | Modified Motor Assessment Scale | — | Functional Independence Measure | — |
| Kiper 2011 | Fugl Meyer UE | — | — | — | — | — | Functional Independence Measure | — |
| Klamroth‐Marganska 2014 | Fugl Meyer UE, Wolf Motor Function Test, Motor Activity Log (quality of movement) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Stroke Impact Scale, Goal attainment scale |
| Ko 2015 | Timed Up and Go Test | Berg Balance Scale | ||||||
| Kong 2014 | Fugl Meyer, Action Research Arm Test | Functional Independence Measure | Stroke Impact Scale | |||||
| Kwon 2012 | Fugl Meyer UE, Manual Function Test | — | — | — | — | — | Korean Modified Barthel Index | — |
| Lam 2006 | — | — | — | — | — | — | — | — |
| Lee 2013 | Functional Reach Test | |||||||
| Lee 2014a | Timed Up and Go Test | Berg Balance Scale | ||||||
| Lee 2015a | Functional Reach Test | |||||||
| Lee 2015b | ||||||||
| Levin 2012 | Fugl Meyer UE Scale, Reach Performance Scale for Stroke, Box and Blocks Test, Wolf Motor Function Test, Motor Activity Log | |||||||
| Linder 2015 | Stroke Impact Scale | |||||||
| Llorens 2015 | Tinetti Performance Oriented Mobility Assessment, 10‐m walk test | Berg Balance Scale, Brunel Balance Assessment | ||||||
| Low 2012 | Fugl Meyer UE Scale, Action Research Arm Test | Gait speed | Berg Balance Scale | Functional Independence Measure | ||||
| Manlapaz 2010 | Fugl Meyer UE Scale | Motor Assessment Scale | ||||||
| Mao 2015 | Gait analysis (gaitlab assessment) | |||||||
| Matsuo 2013 | Fugl Meyer UE, Wolf Motor Function Test, Box and Block Test, Motor Activity Log | |||||||
| Mazer 2005 | — | — | — | — | — | — | DriveAble Testing Ltd Driver Evaluation | — |
| McNulty 2015 | Wolf Motor Function Test timed tasks and strength subtests, Motor Activity Log QOM scale, Fugl Meyer, Box and Block Test | |||||||
| Mirelman 2008 | — | — | Gait speed over 7‐metre walkway, 6‐minute walk test, Patient Activity Monitor | — | — | — | — | — |
| Morone 2014 | 10‐m walk test | Berg Balance Scale | Barthel Index | Functional Ambulation Category | ||||
| Nara 2015 | Static balance ability | |||||||
| Piron 2007 | Fugl Meyer UE Scale | — | — | — | — | — | Functional Independence Measure | — |
| Piron 2009 | Fugl Meyer UE Scale, Abilhand Scale | — | — | — | — | — | — | ‐ |
| Piron 2010 | Fugl Meyer UE Scale | — | — | — | — | — | Functional Independence Measure | — |
| Prange 2015 | Fugl Meyer UE, Stroke Upper Limb Capacity Sclae | |||||||
| Rajaratnam 2013 | — | — | Timed Up and Go | Berg Balance Scale, functional reach, centre of pressure | — | — | — | — |
| Reinkensmeyer 2012 | Fugl Meyer UE, Ranchos Functional Test for UE, Motor Activity Log, Box and Blocks Test | Grip strength | ||||||
| Saposnik 2010 | Abbreviated Wolf Motor Function Test | Box and Block Test, grip strength (kg) | — | — | — | — | — | Stroke Impact Scale (hand function, composite function, perception of recovery) |
| Saposnik 2016 | Abbreviated Wolf Motor Function Test, Box and Block Test | Grip strength | Functional Independence Measure, Barthel Index, Modified Rankin Scale | Stroke Impact Scale | ||||
| Shin 2014 | Fugl Meyer UE | — | — | — | — | — | Modified Barthel Index | — |
| Shin 2015 | Fugl Meyer UE | SF36 | ||||||
| Sin 2013 | Fugl Meyer UE, Box and Block Test | — | — | — | — | — | — | |
| Song 2015 | Timed Up and Go Test, 10‐minute walk test | Balance (Biofeedback system) | ||||||
| Standen 2011 | Wolf Motor Function Test, Motor Activity Log, Nine Hole Peg Test | — | — | — | — | Nottingham Extended Activities of Daily Living Scale | ||
| Subramanian 2013 | Fugl Meyer UE, Wolf Motor Function test, Reaching performance scale for stroke, Motor Activity Log | — | — | — | — | — | — | |
| Sucar 2009 | Fugl Meyer UE Scale, Upper Limb Motricity Index | — | — | — | — | — | — | — |
| Thielbar 2014 | Action Research Arm Test, Jebsen Taylor Hand Function Test, Fugl Meyer UE | Grip strength | ||||||
| Ucar 2014 | Timed walking speed test, Timed Up and Go | Mini Mental State Examination | Functional Ambulation Category | |||||
| Xiang 2014 | 10‐m walking speed, Fugl Meyer (LE) | Brunel Balance Assessment | ||||||
| Yang 2008 | — | — | Walking speed, Community Walk Test | — | — | — | — | Walking Ability Questionnaire, Activities Specific Balance Confidence Scale |
| Yang 2011 | — | — | Gait analysis data | Balance analysis data | — | — | — | — |
| Yavuzer 2008 | Brunnstrom Upper Extremity Stages | Brunnstrom Hand Stages | — | — | — | — | Functional Independence Measure self‐care section | — |
| Yin 2014 | Fugl Meyer, Action Research Arm Test, Motor Activity Log | Functional Independence Measure | ||||||
| You 2005 | — | — | Functional ambulation category | — | Modified Motor Assessment Scale | — | — | — |
| Zucconi 2012 | Fugl Meyer UE, Reaching performance scale | — | — | — | — | — | Functional Independence Measure | — |
fMRI: functional magnetic resonance imaging QOL: quality of life UE: upper extremity
Twenty‐four studies reported on the presence or absence of adverse events (Adie 2017; Bower 2015; Byl 2013; Coupar 2012; Crosbie 2008; Givon 2016; Housman 2009; Hung 2014; Jaffe 2004; Kiper 2011; Klamroth‐Marganska 2014; Levin 2012; Llorens 2015; McNulty 2015; Piron 2007; Piron 2010; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Shin 2015; Subramanian 2013; Sucar 2009; Yavuzer 2008; Yin 2014).
Excluded studies
We have provided details of 34 studies that we excluded. We listed studies as excluded if they were obtained in full text and required lengthy discussion between authors to confirm exclusion (Characteristics of excluded studies). Common reasons for exclusion were: studies compared different forms of virtual reality or the interaction between the virtual environment and the user was not genuine (for example, the person walked on a treadmill while viewing a virtual environment but there was no interaction between the user and environment and changes in speed of walking in the user did not impact on movement in the virtual world).
Risk of bias in included studies
Refer to Figure 2 and Figure 3.
2.
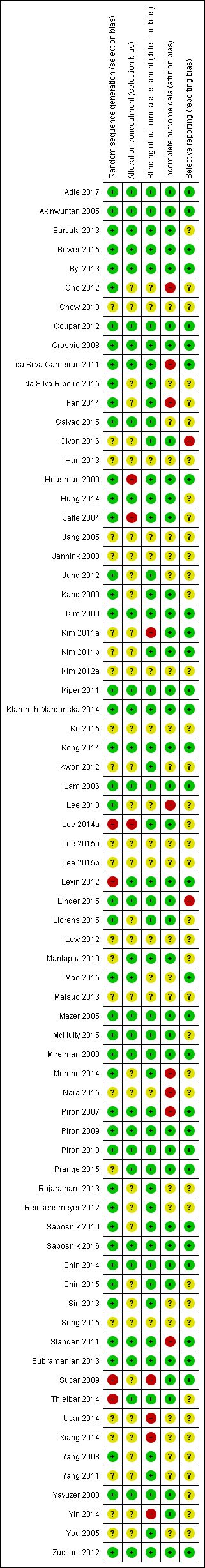
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
3.
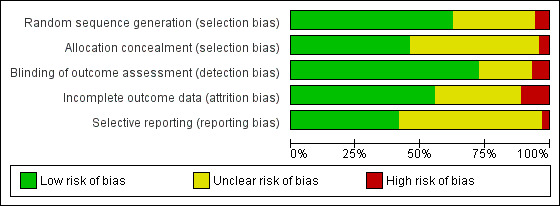
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
Not all included studies followed the CONSORT guidelines (Schulz 2010), in which case we contacted the corresponding authors for clarification of study methodology. If we did not obtain a response from a corresponding author we recorded the 'Risk of bias' criterion as 'unclear'.
Allocation
We assessed random sequence generation as being adequate in 63% of trials. Allocation concealment was reported as adequate in 46% of trials.
Blinding
Seventy‐two per cent of studies reported blinding of the outcome assessor. No trials were able to blind participants or personnel.
Incomplete outcome data
We deemed 56% of studies to be at low risk of bias in relation to incomplete outcome data. Dropouts from studies appeared generally balanced across groups.
Selective reporting
We judged that 43% of studies were free of selective reporting by comparing published results with trials register entries or protocol papers or through correspondence with study authors. It was unclear whether selective reporting was present in most other studies.
Effects of interventions
Primary outcome: upper limb function and activity
We present results for upper limb function and activity.
Virtual reality versus conventional therapy: effect on upper limb function post intervention
Results are presented for upper limb function and activity and hand function. All outcomes were taken within days of the end of the intervention program.
Comparison 1.1: Upper limb function and activity
Twenty‐two studies presented outcomes for upper limb function and activity in a form suitable for inclusion in the meta‐analysis (1038 participants) (Adie 2017; Byl 2013; Crosbie 2008; da Silva Cameirao 2011; da Silva Ribeiro 2015; Galvao 2015; Givon 2016; Housman 2009; Kiper 2011; Kong 2014; Levin 2012; Piron 2007; Piron 2009; Piron 2010; Prange 2015; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Subramanian 2013; Sucar 2009; Thielbar 2014; Zucconi 2012). The impact of virtual reality on upper limb function was not significant: standardised mean difference (SMD) 0.07, 95% confidence interval (CI) ‐0.05 to 0.20, low‐quality evidence (Analysis 1.1). Statistical heterogeneity was moderate (I2 = 43%).
1.1. Analysis.
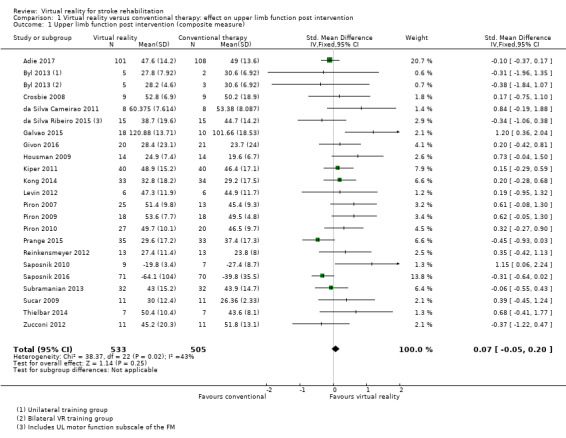
Comparison 1 Virtual reality versus conventional therapy: effect on upper limb function post intervention, Outcome 1 Upper limb function post intervention (composite measure).
We were unable to obtain data in a suitable format for pooling for three studies (Fan 2014; McNulty 2015; Shin 2015). Fan 2014 reported that there were no significant differences between groups on outcomes on the Jebsen Taylor Hand Function Test; McNulty 2015 reported no significant differences between virtual reality and constraint‐induced movement therapy on the Wolf Motor Function Test; and Shin 2015 reported no significant differences between groups on the Fugl Meyer Assessment.
Sensitivity analysis for comparison 1.1
Excluding those studies judged to be unclear or at high risk of bias in one or more categories left 10 studies (Adie 2017; Byl 2013; Crosbie 2008; Kiper 2011; Kong 2014; Piron 2009; Piron 2010; Saposnik 2016; Subramanian 2013; Zucconi 2012). The result was similar (SMD ‐0.02, 95% CI ‐0.17 to 0.13); however, statistical heterogeneity was lower (I2 = 7%). We conducted a sensitivity analysis involving use of a random‐effects model. The difference was minor: SMD 0.17 (95% CI ‐0.01 to 0.35).
Comparison 1.2: Upper limb function (Fugl Meyer Upper Extremity Scale)
Sixteen of the trials (with 599 participants) used the Fugl Meyer Upper Extremity (UE) Scale as an outcome measure (Byl 2013; da Silva Cameirao 2011; da Silva Ribeiro 2015; Galvao 2015; Housman 2009; Kiper 2011; Kong 2014; Levin 2012; Piron 2007; Piron 2009; Piron 2010; Prange 2015; Reinkensmeyer 2012; Subramanian 2013; Sucar 2009; Zucconi 2012). The impact of virtual reality as measured by the Fugl Meyer UE Scale showed a small significant effect: mean difference (MD) 2.85, 95% CI 1.06 to 4.65 (Analysis 1.2).
1.2. Analysis.
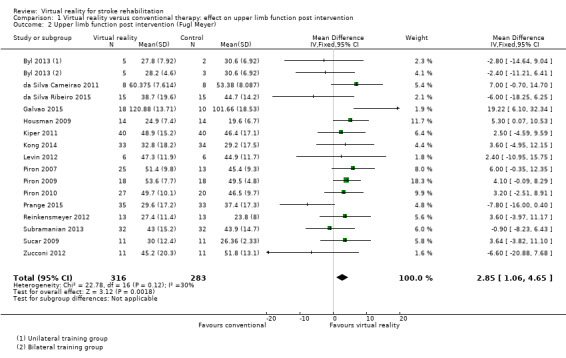
Comparison 1 Virtual reality versus conventional therapy: effect on upper limb function post intervention, Outcome 2 Upper limb function post intervention (Fugl Meyer).
Sensitivity analysis for comparison 1.2
When including only the seven trials deemed to be at low risk of bias in all categories in the analysis, the effect of virtual reality compared to conventional therapy on the Fugl Meyer was not significant (MD 2.01, 95% CI ‐0.46 to 4.47) (Byl 2013; Kiper 2011; Kong 2014; Piron 2009; Piron 2010; Subramanian 2013; Zucconi 2012).
Comparison 1.3: Hand function
Six trials measured the effect of virtual reality versus alternative therapy on grip strength (266 participants) (Givon 2016; Housman 2009; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Thielbar 2014). The impact of virtual reality compared to conventional therapy was not significant: SMD ‐0.02, 95% CI ‐0.27 to 0.22 (Analysis 1.3). Statistical heterogeneity was moderate (I2 = 44%).
1.3. Analysis.
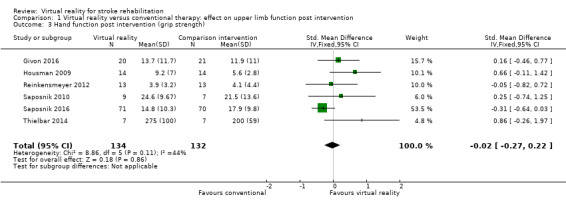
Comparison 1 Virtual reality versus conventional therapy: effect on upper limb function post intervention, Outcome 3 Hand function post intervention (grip strength).
Comparison 1.4: Amount of use of upper limb (self‐reported)
We pooled five studies (with 161 participants) to examine the effect on amount of use (self‐reported component of the Motor Activity Log) (Galvao 2015; Housman 2009; Levin 2012; Reinkensmeyer 2012; Subramanian 2013). There was no statistically significant difference between the groups receiving virtual reality and conventional therapy (SMD ‐0.11, 95% CI ‐0.42 to 0.21). Data from a further two studies could not be pooled; these studies both reported that there were greater improvements in the intervention group than the control group on the 'amount of use' scale (Jang 2005; Standen 2011). One study, which could not be included in the analysis due to unavailability of data in a suitable format for pooling, found no significant differences in outcome between virtual reality and constraint‐induced movement therapy (McNulty 2015).
Comparison 1.5: Upper limb function follow‐up
We pooled nine studies that reported follow‐up assessments of arm function taken between two weeks and three months after the end of intervention (Crosbie 2008; da Silva Cameirao 2011; Givon 2016; Kong 2014; Levin 2012; Piron 2009; Reinkensmeyer 2012; Saposnik 2016; Thielbar 2014). The difference between performance of the virtual reality and conventional therapy groups at this later follow‐up point was not significant (SMD 0.11, 95% CI ‐0.10 to 0.32). A further three studies measured outcomes six months after the end of intervention. Housman 2009 reported that participants in the virtual reality group had improved significantly more on the Fugl Meyer UE Scale at the six‐month follow‐up assessment than participants in the alternative treatment group (P = 0.045). Participants in the virtual reality group improved by 3.6 points (standard deviation (SD) 3.9) whereas participants in the alternative treatment group improved by 1.5 points (SD 2.7). However, the trial found no other significant differences between groups at six months on the other outcome measures used (Rancho Functional Test, grip strength and Motor Activity Log). In contrast, Adie 2017 reported no significant differences between groups on the Action Research Arm Test or Motor Activity Log at six‐month follow‐up and McNulty 2015 reported that at six months upper limb function was not significantly different between groups that had participated in Wii‐based movement therapy and those participating in modified constraint‐induced movement therapy.
Upper limb function: subgroup analyses
Comparison 2.1: Dose of treatment
We compared trials providing under 15 hours of intervention with trials providing 15 hours or more of intervention. Neither group had a statistically significant difference between virtual reality and alternative intervention. While trials providing less than 15 hours of intervention had a non‐significant effect (SMD ‐0.01, 95% CI ‐0.20 to 0.18), trials providing more than 15 hours of intervention showed a trend (although not statistically significant) in favour of the virtual reality intervention (SMD 0.13, 95% CI ‐0.03 to 0.29). The difference between groups was not statistically significant (Chi2 = 1.26, df = 1, P value = 0.26) (Analysis 2.1).
2.1. Analysis.
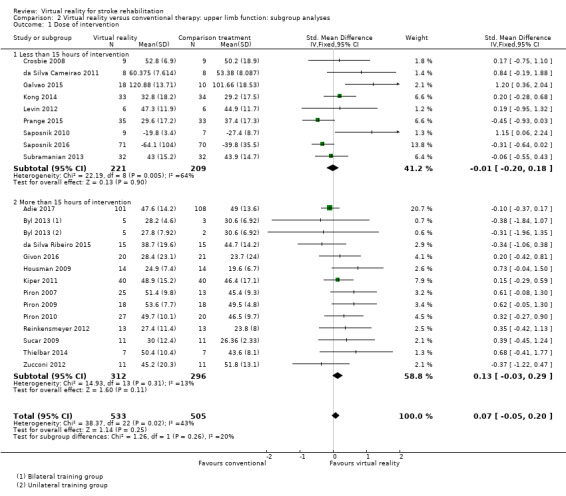
Comparison 2 Virtual reality versus conventional therapy: upper limb function: subgroup analyses, Outcome 1 Dose of intervention.
Comparison 2.2: Time since onset of stroke
We classified trials based on whether their participants were recruited within six months of stroke or more than six months post stroke. The group recruited within six months of stroke did not demonstrate a significant effect (SMD ‐0.06, 95% CI ‐0.23 to 0.11) nor did the group recruited after six months (SMD 0.19, 95% CI ‐0.02 to 0.39) although there was a trend towards the virtual reality intervention. The difference between groups bordered on significant (Chi2 = 3.36, df = 1, P value = 0.07) (Analysis 2.2).
2.2. Analysis.
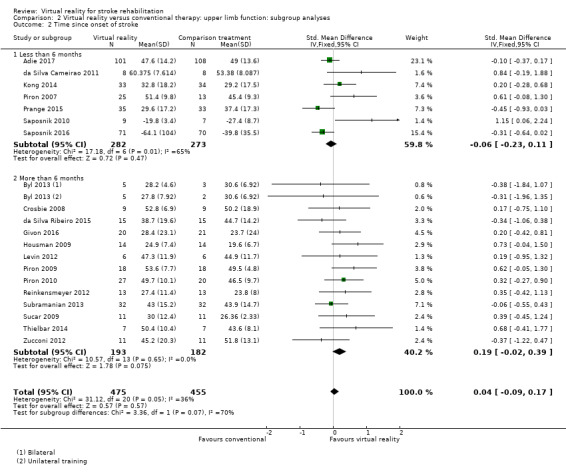
Comparison 2 Virtual reality versus conventional therapy: upper limb function: subgroup analyses, Outcome 2 Time since onset of stroke.
Comparison 2.3: Specialised virtual reality system or commercial gaming console
Studies utilising virtual reality programs specifically designed for rehabilitation settings demonstrated statistically significant benefits over alternative intervention (SMD 0.17, 95% CI 0.00 to 0.35). In contrast those involving off‐the‐shelf gaming programs were not found to be significant (SMD ‐0.02, 95% CI ‐0.20 to 0.15) (Analysis 2.3). However, the test for subgroup differences did not indicate significance (P value = 0.12).
2.3. Analysis.
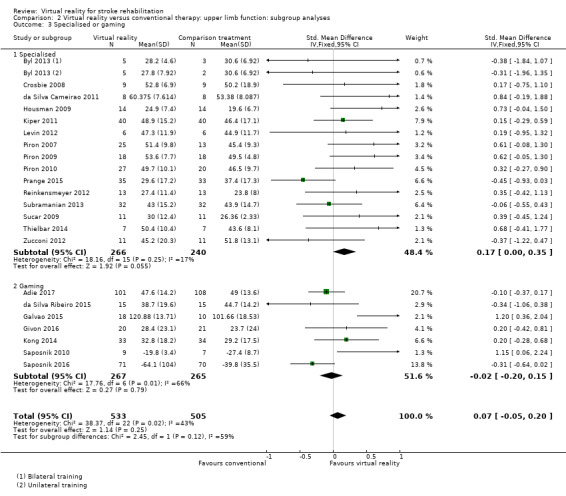
Comparison 2 Virtual reality versus conventional therapy: upper limb function: subgroup analyses, Outcome 3 Specialised or gaming.
Comparison 2.4: Severity of upper limb impairment
We compared outcomes for people with mild to moderate upper limb impairment and people with moderate to severe impairment. The group with mild to moderate impairment showed a non‐significant effect (SMD 0.10, 95% CI ‐0.06 to 0.25) as did the group with moderate to severe impairment (SMD 0.01, 95% CI ‐0.22 to 0.23) (Analysis 2.4).
2.4. Analysis.
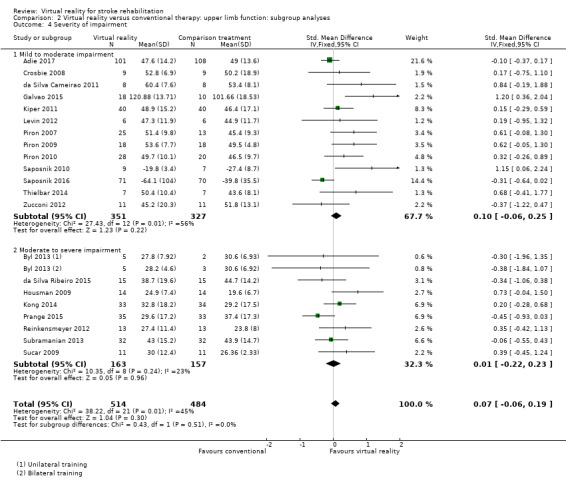
Comparison 2 Virtual reality versus conventional therapy: upper limb function: subgroup analyses, Outcome 4 Severity of impairment.
We did not undertake other planned subgroup analyses due to similarities in these studies in regard to the age of participants and frequency of intervention sessions.
Additional virtual reality intervention: effect on upper limb function post intervention
We examined the effects of virtual reality intervention when it was compared with no intervention and used to augment standard care (i.e. people in the virtual reality intervention group received additional therapy time relative to the control group).
Comparison 3.1: Upper limb function
Ten studies with a total of 210 participants presented outcomes for upper limb function (Cho 2012; Coupar 2012; Jang 2005; Kim 2011a; Kwon 2012; Manlapaz 2010; Shin 2014; Sin 2013; Standen 2011; Yavuzer 2008). There was a moderate significant effect that demonstrated that virtual reality intervention was more effective than no intervention: SMD 0.49, 95% CI 0.21 to 0.77, low‐quality evidence (Analysis 3.1). There was no statistical heterogeneity.
3.1. Analysis.
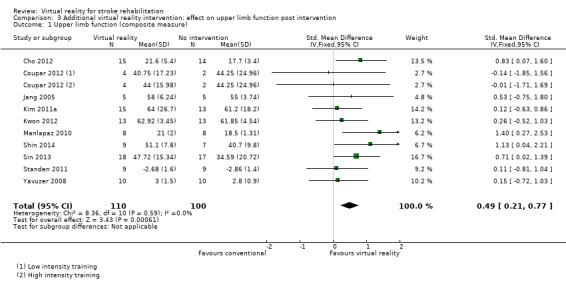
Comparison 3 Additional virtual reality intervention: effect on upper limb function post intervention, Outcome 1 Upper limb function (composite measure).
Two studies could not be included in the analysis due to our inability to obtain data in a suitable format for pooling (Low 2012; Yin 2014). Both studies reported that there were no significant differences between groups on Fugl Meyer score.
Sensitivity analysis
We excluded trials that we deemed to be at high risk of bias in one or more categories (Cho 2012; Kim 2011a; Standen 2011). The result was a slightly higher SMD than found in the original analysis (SMD 0.55, 95% CI 0.20 to 0.91).
Additional virtual reality intervention: effect on upper limb function post intervention: subgroup analyses
Comparison 4.1: Dose of treatment
We compared trials providing less than 15 hours of intervention with trials providing 15 hours or more of intervention. Pooling of seven trials with less than 15 hours of intervention had a significant effect on upper limb function (SMD 0.47, 95% CI 0.14 to 0.80) as did pooling of three trials providing more than 15 hours of intervention (SMD 0.54, 95% CI 0.00 to 1.07). The difference between groups was not significant (Chi2= 0.04, df = 1, P value = 0.83) (Analysis 4.1).
4.1. Analysis.
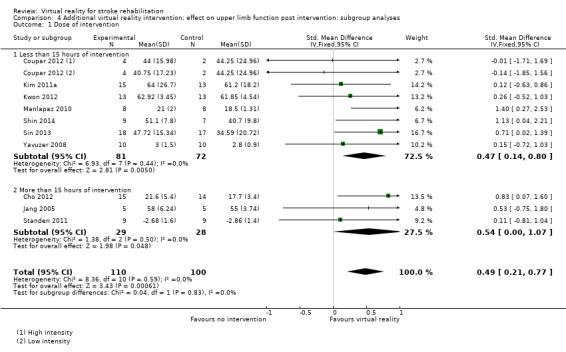
Comparison 4 Additional virtual reality intervention: effect on upper limb function post intervention: subgroup analyses, Outcome 1 Dose of intervention.
Comparison 4.2: Time since onset of stroke
We compared analysis of five trials recruiting participants within six months of stroke with four trials recruiting participants more than six months post stroke. Analysis of trials recruiting within six months did not reveal a significant effect (SMD 0.28, 95% CI ‐0.12 to 0.67) whereas those recruiting people in the chronic phase of stroke experienced statistically significant benefits (SMD 0.65, 95% CI 0.19 to 1.11). The difference between groups was not significant (P value = 0.23) (Analysis 4.2).
4.2. Analysis.
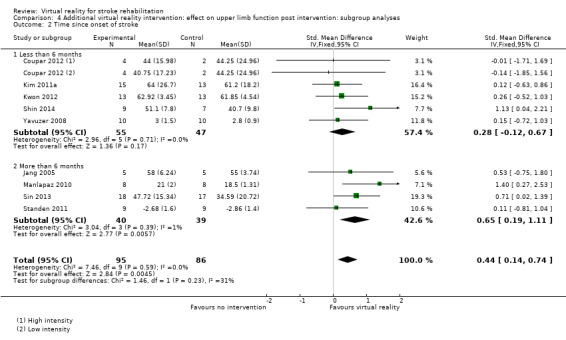
Comparison 4 Additional virtual reality intervention: effect on upper limb function post intervention: subgroup analyses, Outcome 2 Time since onset of stroke.
Comparison 4.3: Specialised virtual reality system or gaming console
We compared three trials evaluating the efficacy of gaming console use with seven trials evaluating the efficacy of virtual reality systems specifically designed for rehabilitation. Both types of virtual reality programs were found to be effective (when the virtual reality was used as an adjunct to treatment) and the difference between groups was not significant (Chi2 = 0.75, df = 1, P value = 0.39) (Analysis 4.3).
4.3. Analysis.
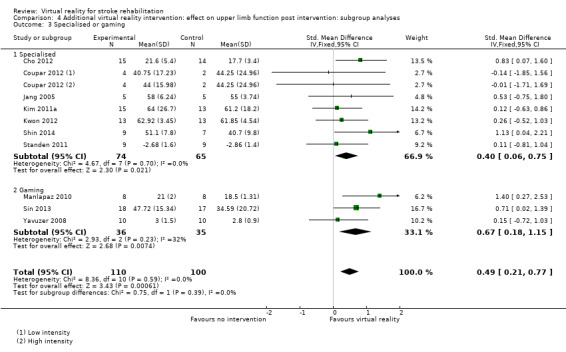
Comparison 4 Additional virtual reality intervention: effect on upper limb function post intervention: subgroup analyses, Outcome 3 Specialised or gaming.
Secondary outcomes
Virtual reality versus conventional therapy: effect on gait and balance: post intervention
Results are presented for gait speed. All outcomes are taken within days of the end of the intervention program and measured in metres per second. We were unable to include seven relevant studies; one of these studies, Barcala 2013, compared different doses of therapy, and six studies did not report data in a format that allowed pooling nor did the corresponding authors provide the data upon request (Hung 2014; Kim 2009; Morone 2014; Rajaratnam 2013; Ucar 2014; Yang 2011).
Comparison 5.1: Gait speed
Six studies provided data on gait speed (139 participants) (Givon 2016; Jaffe 2004; Llorens 2015; Mirelman 2008; Song 2015; Yang 2008). The effect of virtual reality on gait speed was not significant: MD 0.09, 95% CI ‐0.04 to 0.22, low‐quality evidence (Analysis 5.1). Low statistical heterogeneity was indicated (I2 = 10%). Jaffe 2004 examined the effect of virtual reality on comfortable walking speed and fast walking speed. We included the data relating to comfortable walking speed in the meta‐analysis. The effect on fast walking speed was found to be significantly greater in the virtual reality intervention group than the comparative group. One study, which could not be included in the analysis due to inability to obtain data in a suitable format for pooling, found no significant differences between groups on walking speed (Morone 2014). A second study, which could also not be pooled, reported that use of the Lokomat was significantly better than conventional therapy on walking speed (P = 0.007).
5.1. Analysis.
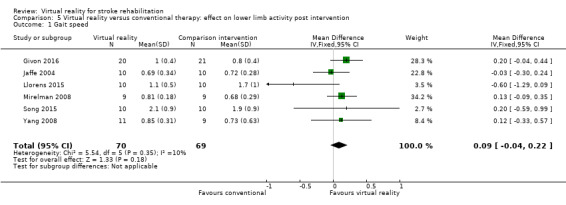
Comparison 5 Virtual reality versus conventional therapy: effect on lower limb activity post intervention, Outcome 1 Gait speed.
Comparison 5.2: Timed Up and Go test
We pooled three studies (89 participants, Hung 2014; Jung 2012; Song 2015) reporting data for the Timed Up and Go (TUG) test. There was no significant difference between those in the virtual reality and conventional therapy groups (MD ‐1.76, 95% CI ‐4.67 to 1.16) and statistical heterogeneity was high (I2 = 59%) (Analysis 5.2). One study could not be included in the analysis as standard deviations were not available (Ucar 2014). The study authors reported that those receiving therapy on the Lokomat had significantly better performance on the TUG test than those receiving conventional therapy (P = 0.035).
5.2. Analysis.
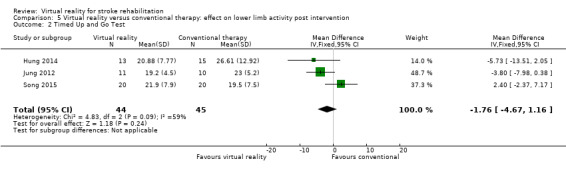
Comparison 5 Virtual reality versus conventional therapy: effect on lower limb activity post intervention, Outcome 2 Timed Up and Go Test.
Comparison 5.3: Balance
Three studies with 72 participants examined the effect of virtual reality intervention compared to conventional therapy on balance (Hung 2014; Lee 2014a; Llorens 2015). The effect was not statistically significant (SMD 0.39, 95% CI ‐0.09 to 0.86) (Analysis 5.3); heterogeneity was low. We could not include two studies in the analyses because we were unable to obtain the data required: Han 2013 found no significant differences between groups, whereas Morone 2014 reported that Wii Fit training was more effective than conventional balance therapy in improving performance on the Berg Balance Scale.
5.3. Analysis.
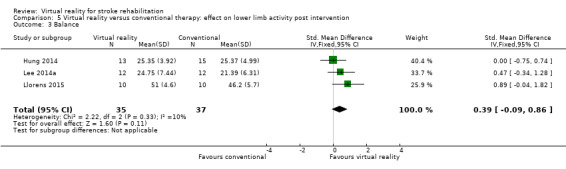
Comparison 5 Virtual reality versus conventional therapy: effect on lower limb activity post intervention, Outcome 3 Balance.
Gait and balance activity: subgroup analyses
Subgroup analyses comparing those receiving less than 10 hours' intervention with those receiving more than 10 hours' intervention did not suggest that this was an influential factor on gait speed outcome (Analysis 6.1).
6.1. Analysis.
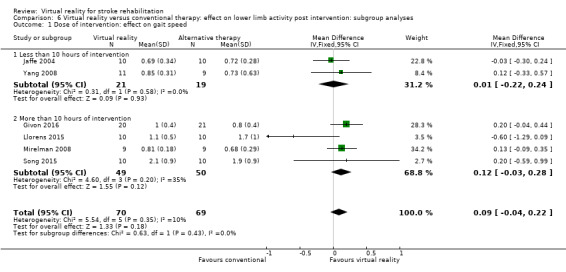
Comparison 6 Virtual reality versus conventional therapy: effect on lower limb activity post intervention: subgroup analyses, Outcome 1 Dose of intervention: effect on gait speed.
We did not undertake other planned subgroup analyses due to homogeneity with regard to the age of participants, severity of stroke, time since onset of stroke, frequency of intervention sessions, and type of virtual reality program.
Gait and balance activity: follow‐up
Only three trials measured the longer‐term effects (at three months) of virtual reality on gait speed. Hung 2014 and Mirelman 2008 both reported that initial benefits in the intervention group (relative to the control group) were still present at follow‐up, while Givon 2016 reported that initial differences between groups were not maintained.
Additional virtual reality intervention: effect on gait and balance post intervention
Comparison 7.1: Gait speed
Pooling of three studies with 57 participants utilising virtual reality intervention as an adjunct to usual care did not identify statistically significant benefits (SMD 0.08, 95% CI ‐0.05 to 0.21, low‐quality evidence) (Bower 2015; Lee 2014a; Xiang 2014). There was no statistical heterogeneity (Analysis 7.1). Two studies could not be included in the analysis due to our inability to obtain data in a suitable format for pooling (Chow 2013; Low 2012). Both papers (presented as conference abstracts only) reported no significant differences between groups in gait speed following intervention.
7.1. Analysis.
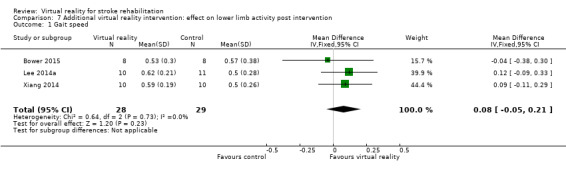
Comparison 7 Additional virtual reality intervention: effect on lower limb activity post intervention, Outcome 1 Gait speed.
Comparison 7.2: Timed Up and Go Test
Pooling of three studies with 93 participants identified a statistically significant difference between people after receiving additional intervention using virtual reality programs on the Timed Up and Go Test in contrast to those receiving usual care (MD ‐4.76, 95% CI ‐8.91 to ‐0.61) although statistical heterogeneity was present (I2 = 50%) (Analysis 7.2) (Barcala 2013; Ko 2015; Lee 2014a).
7.2. Analysis.
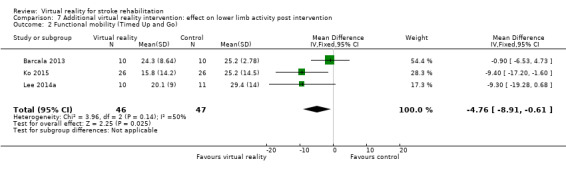
Comparison 7 Additional virtual reality intervention: effect on lower limb activity post intervention, Outcome 2 Functional mobility (Timed Up and Go).
Comparison 7.3: Balance
We pooled seven studies (with 173 participants) to examine the effect of providing virtual reality as an adjunct to usual care on balance (Barcala 2013; Bower 2015; Kim 2009; Ko 2015; Lee 2013; Lee 2014a; Xiang 2014). The effect was significant and the effect size was moderate (SMD 0.59, 95% CI 0.28 to 0.90, I2 = 32%, Analysis 7.3). Two studies could not be included in the analysis due to our inability to obtain data in a suitable format for pooling (Chow 2013; Low 2012). Both papers (presented as conference abstracts only) reported no differences between groups in outcome.
7.3. Analysis.
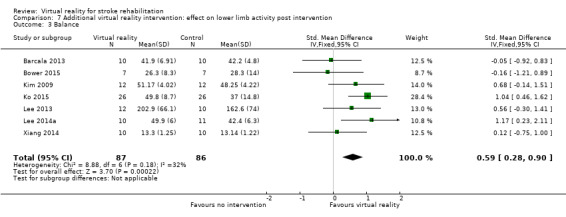
Comparison 7 Additional virtual reality intervention: effect on lower limb activity post intervention, Outcome 3 Balance.
Global motor function
Four studies reported outcomes for global motor function (using the Modified Motor Assessment scale). However, Kim 2009 compared virtual reality with an alternative intervention. We pooled three studies (with 43 participants) that examined the effect of virtual reality on global motor function when used in addition to usual care, thus increasing the therapy dose received by the intervention group (Bower 2015; Kim 2012a; You 2005). The effect on global motor function was not significant (SMD 0.01, 95% CI ‐0.60 to 0.61, low‐quality evidence) (Analysis 8.1).
8.1. Analysis.
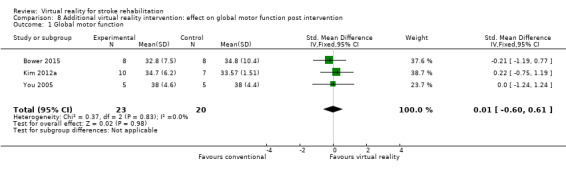
Comparison 8 Additional virtual reality intervention: effect on global motor function post intervention, Outcome 1 Global motor function.
Cognitive function
Insufficient trials included assessments of cognition to allow us to perform analysis for this outcome.
Activity limitation
Two studies reported outcomes of a driving evaluation. However, we were unable to pool results as Akinwuntan 2005 compared virtual reality intervention with an alternative intervention, and Mazer 2005 compared virtual reality intervention with no alternative intervention. Akinwuntan 2005 reported the results from the follow‐up assessments, which were completed at six months and five years post intervention. Six months post intervention they found that participants in the virtual reality intervention group had improved significantly more in their on‐road performance (measured by the Test Ride for Investigating Practical fitness to drive checklist) than participants in the alternative intervention group (P value = 0.005). Furthermore, 73% of the virtual reality group compared with 42% of the group that participated in driving‐related cognitive tasks were classified by driving assessors as 'fit to drive' at six months. At five years, there was no significant difference between the groups in regards to 'fitness to drive' or resumption of driving.
Virtual reality versus conventional therapy: effect on activity limitation
Comparison 9.1: Activities of daily living (ADL) outcome
We pooled 10 studies with 466 participants that examined the difference between virtual reality intervention and alternative intervention on ADL (Byl 2013; da Silva Cameirao 2011; Kang 2009; Kim 2011b; Kiper 2011; Kong 2014; Piron 2007; Piron 2010; Saposnik 2016; Zucconi 2012). There was a small, significant effect (SMD 0.25, 95% CI 0.06 to 0.43, moderate‐quality evidence) and presence of statistical heterogeneity (I2 = 22%) (Analysis 9.1). Two studies could not be included in the analysis due to our inability to obtain data in a suitable format for pooling (Han 2013; Morone 2014). Morone 2014 presented a graph indicating that those in the Nintendo Wii group had significantly better scores on the Barthel Index post intervention than those in the conventional therapy group, whereas Han 2013 reported no significant differences between groups.
9.1. Analysis.
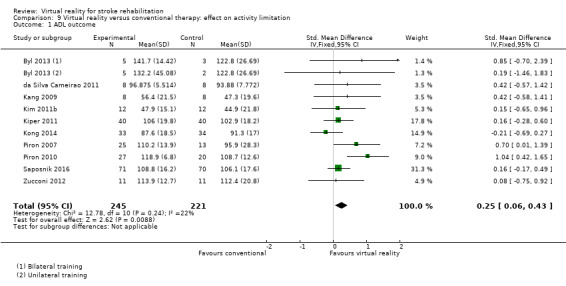
Comparison 9 Virtual reality versus conventional therapy: effect on activity limitation, Outcome 1 ADL outcome.
Sensitivity analysis
We explored the effects of methodological quality on the overall effect by excluding studies deemed to be at unclear or high risk of bias in one or more categories from the analysis (da Silva Cameirao 2011; Kang 2009; Kim 2011b; Piron 2007). The results were similar but the effect size was smaller and no longer statistically significant (SMD 0.20, 95% CI ‐0.01 to 0.40).
Additional virtual reality intervention: effect on activity limitation
Comparison 10.1: ADL outcome
Pooling of eight studies with 153 participants examined the effect of providing additional intervention using virtual reality on ADL outcome (Barcala 2013; Coupar 2012; Kim 2011a; Kim 2012a; Kwon 2012; Shin 2014; Standen 2011; Yavuzer 2008). The effect was statistically significant with a small to moderate effect size (SMD 0.44, 95% CI 0.11 to 0.76). There was no heterogeneity (Analysis 10.1). We conducted a sensitivity analysis based on risk of bias and only including the two studies deemed at low risk of bias in all categories. The result was still positive; however the confidence intervals were wide (SMD 0.92, 95% CI 0.04 to 1.81).
10.1. Analysis.
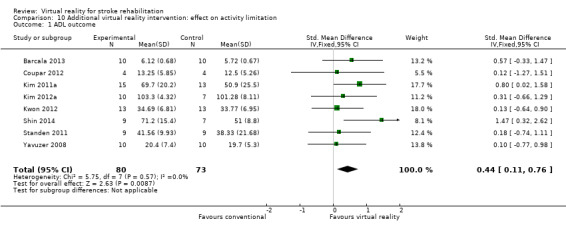
Comparison 10 Additional virtual reality intervention: effect on activity limitation, Outcome 1 ADL outcome.
We could not include three studies in the analysis due to our inability to obtain data in a suitable format for pooling (Chow 2013; Low 2012; Yin 2014); none of these studies reported a significant difference between groups on ADL outcome.
Participation restriction and quality of life
Heterogeneity between trials and outcome measures used meant that we did not perform any analysis for this outcome.
Six studies compared a virtual reality intervention with an alternative intervention and measured changes using either components or the full version of the Stroke Impact Scale (Adie 2017; Fan 2014; Kong 2014; Linder 2015; Saposnik 2010; Saposnik 2016). None of the six studies found a significant difference between the intervention and control group in score on the Stroke Impact Scale.
Three studies compared a virtual reality intervention with an alternative intervention and used a health‐related, quality‐of‐life measure. Adie 2017 reported that there was no difference between groups identified via the EQ5D tool. The other two studies reported differences between groups in some domains of the SF36; participants receiving conventional therapy in the study conducted by da Silva Ribeiro 2015 reported significantly higher scores on the physical‐functioning domain, whereas Shin 2015 reported that those in the virtual reality group reported significantly better scores in terms of role limitations due to physical problems.
Adverse events
Twenty‐three studies monitored and reported on adverse events. Nineteen studies reported no significant adverse events linked to study participation (Adie 2017; Byl 2013; Coupar 2012; Givon 2016; Housman 2009; Jaffe 2004; Kiper 2011; Levin 2012; Llorens 2015; McNulty 2015; Piron 2007; Piron 2010; Reinkensmeyer 2012; Saposnik 2010; Saposnik 2016; Shin 2015; Subramanian 2013; Yavuzer 2008; Yin 2014). Crosbie 2008 found that two people in the virtual reality group reported side effects of transient dizziness and headache, and Sucar 2009 found that three participants in the virtual reality group reported pain caused by the treatment in contrast to two participants in the conventional therapy group. Bower 2015 reported that several of the participants receiving the intervention had symptoms of pain and one participant reported dizziness; however, these were not thought to be related to the intervention, and Hung 2014 reported that three of the intervention group (out of 15) reported an increase in hypertonicity during treatment.
Discussion
Summary of main results
This review included 72 trials with 2470 participants. The main results are presented in Table 1 and Table 2.
Upper limb function and activity
Twenty‐two studies with 1033 participants compared a virtual reality intervention with conventional therapy and measured effects on upper limb function. These trials used a variety of different commercially available games or specialised virtual reality programs, and all interventions were delivered in a hospital or clinic setting, with the exception of one of these trials that used a home‐based telerehabilitation approach. More of the trials (13 studies) recruited participants more than six months after stroke, with remaining trials recruiting participants within the first six months of stroke.
Six trials compared a virtual reality intervention with conventional therapy and measured grip strength. Pooling of results indicated that there was no significant difference in the efficacy of the therapy approaches on upper limb function or grip strength.
We also examined the effect of a virtual reality intervention on upper limb function when the intervention was provided to augment the usual dose of therapy. Thus, the intervention group received more therapy time than the control group. Ten studies with 210 participants found a moderately significant effect in favour of the virtual reality intervention (low‐quality evidence). Eight of these studies involved the use of commercially available virtual reality programs and one of the studies provided the intervention in the home setting.
The addition of a virtual reality intervention to usual care resulted in improvements in upper limb function. However, the virtual reality intervention was not a more effective approach than conventional interventions. This finding is in contrast with the previous versions of this review where meta‐analysis revealed a small significant benefit associated with virtual reality intervention when compared with conventional therapy approaches (Laver 2011; Laver 2015). This review included more studies in which virtual reality was used as a way to increase the amount of therapy provided and thus provides more information about the effectiveness of virtual reality as a therapy to augment usual care.
Results of this review did not indicate the most effective time to utilise the intervention in recovery (i.e. whether it was more effective to use virtual reality in the earlier recovery phase or the chronic (more than six months) phase post stroke. It appeared that trials providing more than 15 hours of intervention resulted in greater benefits than those providing a smaller dose of virtual reality therapy. Comparison of the type of program (specialised system versus commercial gaming system) revealed no significant differences in effect although there was a trend suggesting that specialised systems may be more effective.
Secondary outcomes
Six trials with 139 participants measured gait speed and could be included in the analysis comparing virtual reality with alternative intervention. All six studies included people who were more than one year post stroke. There was insufficient evidence to draw conclusions on whether a virtual reality approach was more effective in improving gait speed than conventional therapy (low‐quality evidence). We were also unable to reach conclusions about the effects of virtual reality (compared to conventional therapy) on a more functional measure of mobility; performance on the Timed Up and Go Test. Four trials examined effect of virtual reality on global motor function (with three of these studies using the same virtual reality program). The effect on global motor function was not significant. There was a small effect on ADL when virtual reality was used instead of conventional therapy and a moderate effect on ADL when virtual reality was used to increase the dose of therapy and provided in addition to usual care (moderate‐quality evidence). We were unable to pool results for cognitive function, participation restriction, and quality of life studies. There were few adverse events reported across studies and those reported (transient dizziness, headache, pain) were relatively mild.
Heterogeneity of included studies
There was considerable clinical heterogeneity between the studies included in the review, particularly in regard to the variety of intervention approaches used to address a variety of different patient needs. Some of these interventions were very specific (for example, retraining participants to use the local public transport system) and therefore studies were not comparable in many circumstances. In addition, a wide variety of outcome measures were used; this also limited our ability to pool results. The use of meta‐analysis in cases where such heterogeneity is present can be considered controversial (Deeks 2011); however, we felt that meta‐analysis in this review was justified and we were careful only to pool studies that were relatively comparable in terms of participants, interventions, comparison, and outcome measures. Meta‐analysis of the individual studies enabled us to explore the overall treatment effect of the intervention when compared with an alternative, more traditional intervention or no intervention. Our sensitivity analyses suggested that there were no notable differences between using random‐effects and fixed‐effect models.
Overall completeness and applicability of evidence
Although we included 72 studies, the sample sizes of the included studies were generally small. There are now studies recruiting participants in both the earlier phases post stroke as well as the chronic phase. People with cognitive impairment, or communication or visual deficits were often excluded, thereby raising questions about how applicable this intervention is to a wide range of stroke survivors. Furthermore, the average age of participants in the included studies was relatively low, therefore, information about use with older stroke survivors is limited.
Researchers involved in future studies should provide more detail in their reporting, ensuring that they clearly describe their eligibility criteria, consent rate and the adherence and satisfaction of participants with the intervention. These details will be of interest to clinicians who will need to weigh up the cost of the virtual reality program with the potential benefits and the number of clients who may benefit from use.
Furthermore, the applicability of the intervention to stroke survivors needs further research in terms of which type of approach is best suited to the individual person and how acceptable the technology may be to stroke survivors. There are a number of studies suggesting that virtual reality training is motivating and enjoyable with some studies finding the intervention to be more engaging than usual therapy exercises (McNulty 2015; Webster 2014; Wingham 2015). Although there is a perception that people undergoing rehabilitation programs will find the technology difficult to use, the research suggests that a number of studies report the technology as acceptable and easy to use (Nawaz 2015) .
In contrast to our previous reviews, in which most of the virtual reality programs were specifically designed for rehabilitation purposes, this review has found a rise in the number of studies evaluating commercial gaming programs designed for the general population; yet it remains difficult to examine the effects of game‐based interventions as the goals of therapy and methods vary.
We did not conduct subgroup analyses to compare the effects of immersive and non‐immersive technologies as these types of analyses were not specified in our protocol or carried out in previous versions of this review. As the number of studies in the field expand it may be possible to determine more information about the types of virtual reality that are likely to be effective through this type of subgroup analysis.
Several trials reported on the presence or absence of adverse events. There were few events reported: the small number of events were mild and limited to dizziness, headache and pain.
Quality of the evidence
While we were able to include a relatively large number of studies in the review, sample sizes in the included studies were mostly small and larger, adequately powered studies are required to confirm initial findings. The risk of bias present in many studies was unclear due to poor reporting and lack of clarification from study authors. Approximately half of the studies reported adequate allocation concealment, and in five of the included studies assessors were not blind to allocation. Thus, while there are a large number of randomised controlled trials, the evidence remains 'moderate', 'low' or 'very low' quality when rated using the GRADE system.
Potential biases in the review process
Despite a comprehensive search strategy it is possible that we did not identify some studiesin the search process, for example, studies where there is no published abstract in English. Whilst in the previous version of this review we contacted manufacturers of virtual reality equipment and searched conference proceedings, we opted not to do so in this update, as this method was not previously effective in eliciting original studies. However, this does mean that unpublished data may not have been identified. Furthermore, although we contacted all corresponding authors of included studies and sent a follow‐up email to those that did not respond, few authors responded. This resulted in the study methodology of many trials being unclear and resulted in us being unable to include some data in the analyses. The process of two review authors independently reviewing abstracts and extracting data (with a third review author to moderate disagreements) enabled us to minimise bias. The search date of this review was April 2017. As this field is rapidly expanding there are likely to be more studies now eligible for inclusion.
Agreements and disagreements with other studies or reviews
Previous systematic reviews have argued that virtual reality appears promising (Cheok 2015; Corbetta 2015; Crosbie 2007; Li 2016; Lohse 2014; Moreira 2013; Saposnik 2011). This review is generally consistent with these reviews; however, due to the more recent and comprehensive search strategy we were able to identify a greater number of studies and conduct subgroup analyses. The various reviews have drawn different conclusions about the efficacy of virtual reality: most of the differences are due to different inclusion and exclusion criteria. For example, in this review we excluded studies where the interaction between the study participant and the virtual environment were mediated by the therapist rather than directly by the participant, such as when speed of movement through a virtual environment was controlled by the therapist during treadmill training. Other reviews did not make this distinction and included these types of studies. We were also careful to conduct separate analyses based on the treatment of the control group and the type and dose of therapy received.
In the previous version of this review, the main analysis examining effect on upper limb function included 12 studies and 397 participants and found that virtual reality intervention was more effective than conventional therapy (Laver 2015). There have been many studies published in the last couple of years and this updated version of the review included 22 studies with 1033 participants. The analysis for effect on upper limb function was not significant; this finding is a major change in the direction of results with practical implications for clinicians.
Authors' conclusions
Implications for practice.
We found that virtual reality therapy may not be more effective than conventional therapy but there is low‐quality evidence that virtual reality may be utilised to improve outcomes in the absence of other therapy interventions after stroke. We also found that virtual reality appears to be a safe intervention that is effective at improving arm function and activities of daily living (ADL) function following stroke. A greater improvement was seen at a higher dose but the association was not statistically significant. Gains made appear to be clinically significant with analyses showing reasonable effect sizes (that is, a moderate effect on upper limb function (standardised mean difference (SMD) 0.50, low‐quality evidence) and a small to moderate effect on ADL function (SMD 0.44), moderate‐quality evidence). However, at present, there is significant heterogeneity between studies. For example, there are only two studies that have examined the use of a virtual reality driving simulation program and thus it is unclear how effective virtual reality may be for driver rehabilitation after stroke. In addition, as virtual reality interventions may vary greatly (from inexpensive commercial gaming consoles to expensive customised programs), it is unclear which characteristics of the intervention are most important. Our analyses did not provide clear direction as to which virtual reality programs are superior to others.
The lack of adverse events, including motion sickness, nausea, headache, or pain suggests that these factors should not be of great concern to clinicians; however, this may vary depending on the characteristics of the person, the virtual reality hardware and software, and the task. Clinicians who currently have access to virtual reality programs should be reassured that their use as part of a comprehensive rehabilitation program appears reasonable, taking into account the patient's goals, abilities, and preferences.
Implications for research.
This updated version of the review revealed that 35 new randomised controlled trials (RCTs) were published over approximately two years. Despite the inclusion of some higher‐quality studies, the new RCTs mostly mirror those included in the previous review. Researchers in this field are strongly encouraged to conduct larger, adequately powered trials that can provide more definitive results.
Researchers and manufacturers designing new virtual reality programs for rehabilitation purposes should include the use of pilot studies assessing usability and validity as part of the development process. This is an important part of the development process and should be conducted with the intended users of the program.
Our review included only RCTs, resulting in the exclusion of observational studies that showed improvements in real‐world tasks based on virtual reality training. It is evident that the field is still developing and many studies are at feasibility and proof‐of‐concept levels. In addition, it is challenging to design a controlled trial comparing virtual reality to real‐world correlates. This is in part because virtual reality systems allow us to train in ways that are not possible in the real world. Future research needs to carefully examine what we control for when comparing real‐world with virtual reality‐based interventions and overcome, when possible, the challenge of making groups equivalent.
Ideally, studies should use common outcome measures. However, this is likely to be difficult due to the range of virtual reality interventions. Studies should measure whether effects are long lasting with outcome assessment more than three months after the end of the intervention. Researchers should also examine the impact of virtual reality on the person's motivation to participate in rehabilitation, engagement in therapy, and level of enjoyment.
Many of the studies included in this review did not report the number of participants screened against eligibility criteria. Future research trials should report these data as they provide useful information regarding the proportion of stroke survivors for whom virtual reality intervention may be appropriate.
The majority of studies to date have evaluated interventions that were designed to address motor impairments. There are few studies that include cognitive rehabilitation or studies that aim to make improvements at the levels of activity or participation. There is also currently insufficient evidence from RCTs to tell whether activity training in a virtual environment translates to activity performance in the real world.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2018 | Amended | Two copy‐editing errors corrected. |
History
Protocol first published: Issue 2, 2010 Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 31 July 2017 | New citation required and conclusions have changed | The conclusions of the review have changed. |
| 31 July 2017 | New search has been performed | We updated the searches to April 2017. We have added 35 new studies bringing the total number of included studies to 72, involving a total of 2470 participants. We have revised the review throughout. We re‐ran the searches in April 2017 and have added new studies to the 'studies awaiting classification' list. |
| 27 August 2014 | New citation required but conclusions have not changed | The conclusions of the review have not changed. |
| 27 August 2014 | New search has been performed | We updated the searches to November 2013. We have added 18 new studies, bringing the total number of included studies to 37, involving a total of 1019 participants. We have revised the review throughout. |
Acknowledgements
We thank Brenda Thomas for her help with the search strategies, and Hazel Fraser, Joshua Cheyne, Valentina Assi, Peter Langhorne, Maree Hackett, Rachel Stockley, and Tam Watson for their editorial review and recommendations.
We also thank Abiodun Akinwuntan, Jacqueline Crosbie, Sarah Housman and David Reikensmeyer, David Jaffe, Sung You, David Man, Barbara Mazer, Anat Mirelman, Andrea Turolla and Lamberto Piron, Gustavo Saposnik, Enrique Sucar, Penny Standen, Min Cheol Chang, Nancy Byl, Paul Vershure, Joon‐Ho Shin, Carla Zucconi, Pawel Kiper, Sandeep Subramanian, Mindy Levin, Le Li, Jen‐Wen Hung, Donald Manlapaz, Andreia Silva, Kelly Bower, Christoph Hollenstein, and Lisa Sheehy, who generously provided additional details and analyses from their trials to assist us with the review.
Appendices
Appendix 1. CENTRAL search strategy
#1. [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh “intracranial arteriovenous malformations”] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"]
#2. [mh ^"brain injuries"] or [mh ^"brain injury, chronic"]
#3. (stroke or cva or poststroke or "post‐stroke" or cerebrovasc* or cerebral next vasc*):ti,ab
#4.((cerebral* or cerebell* or brain* or vertebrobasilar) near/5 (isch*emi* or infarct* or thrombo* or emboli* or apoplexy*)):ti,ab
#5. ((brain* or cerebral* or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#6. [mh ^hemiplegia] or [mh paresis]
#7. (hemipleg* or hemipar* or paresis or paretic or brain next injur*):ti,ab
#8. [mh ^"gait disorders, neurologic"]
#9. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10. [mh ^”user‐computer interface”]
#11. [mh ^computers] or [mh microcomputers] or [mh ^”computer systems”] or [mh ^software]
#12. [mh ^”computer simulation”] or [mh ^”computer‐assisted instruction”] or [mh ^”therapy, computer‐assisted”]
#13. [mh ^”computer graphics”] or [mh ^”video games”] or [mh touch [mj]]
#14. (Virtual next reality* or “virtual‐reality” or VR):ti,ab
#15. (virtual near/3 (environment* or object* or world* or treatment* or system* or program* or rehabilitation* or therap* or driving or drive* or car or tunnel or vehicle)):ti,ab
#16. (computer near/3 (simulat* or graphic* or game* or interact*)):ti,ab
#17. (computer next assist* next (therap* or treat*)):ti,ab
#18. (computer next generat* next (environment* or object*)):ti,ab
#19. (video game* or video next gaming or gaming next console* or interactive next game or interactive next gaming or Nintendo next Wii or gaming next program*):ti,ab
#20. (haptics or haptic next device*):ti,ab
#21. (simulat* near/3 (environment* or object* or event* or events or driving or drive* or car or tunnel or vehicle)):ti,ab
#22. (user next computer next interface):ti,ab
#23. #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
#24. #9 and #23
Appendix 2. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid) and adapted it to search the other databases.
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/
2. brain injuries/ or brain injury, chronic/
3. (stroke$ or cva or poststroke or post‐stroke or cerebrovasc$ or cerebral vascular).tw.
4. ((cerebral or cerebellar or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy)).tw.
5. ((cerebral or brain or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed$)).tw.
6. exp hemiplegia/ or exp paresis/
7. (hempar$ or hemipleg$ or paresis or paretic or brain injur$).tw.
8. Gait Disorders, Neurologic/
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. user‐computer interface/
11. computers/ or exp microcomputers/ or computer systems/ or software/
12. computer simulation/ or computer‐assisted instruction/ or therapy, computer‐assisted/
13. computer graphics/ or video games/ or *touch/
14. (virtual reality$ or virtual‐reality$ or VR).tw.
15. (virtual adj3 (environment$ or object$ or world$ or treatment$ or system$ or program$ or rehabilitation$ or therap$ or driving or drive$ or car or tunnel or vehicle)).tw.
16. (computer adj3 (simulat$ or graphic$ or game$ or interact$)).tw.
17. (computer adj1 assist$ adj1 (therap$ or treat$)).tw.
18. (computer adj1 generat$ adj1 (environment$ or object$)).tw.
19. (video game$ or video gaming or gaming console$ or interactive game or interactive gaming or Nintendo Wii or gaming program$).tw.
20. (haptics or haptic device$).tw.
21. (simulat$ adj3 (environment$ or object$ or event$1 or driving or drive$ or car or tunnel or vehicle)).tw.
22. (user adj1 computer adj1 interface).tw.
23. or/10‐22
24. Randomized Controlled Trials as Topic/
25. random allocation/
26. Controlled Clinical Trials as Topic/
27. control groups/
28. clinical trials as topic/
29. double‐blind method/
30. single‐blind method/
31. Placebos/
32. placebo effect/
33. cross‐over studies/
34. Research Design/
35. randomized controlled trial.pt.
36. controlled clinical trial.pt.
37. clinical trial.pt.
38. (random$ or RCT or RCTs).tw.
39. (controlled adj5 (trial$ or stud$)).tw.
40. (clinical$ adj5 trial$).tw.
41. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
42. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
43. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
44. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
45. (cross‐over or cross over or crossover).tw.
46. (placebo$ or sham).tw.
47. trial.ti.
48. (assign$ or allocat$).tw.
49. or/24‐48
50. 9 and 23 and 49
51. limit 50 to ed=20100301‐20170401
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/ or stroke unit/ or stroke patient/
2. brain injury/ or acquired brain injury/
3. (stroke$ or cva or poststroke or post‐stroke or cerebrovasc$ or cerebral vascular).tw.
4. ((cerebral or cerebellar or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy)).tw.
5. ((cerebral or brain or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed$)).tw.
6. hemiparesis/ or hemiplegia/ or paresis/
7. (hempar$ or hemipleg$ or paresis or paretic or brain injur$).tw.
8. exp neurologic gait disorder/
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. virtual reality/ or computer interface/ or exp computer/ or computer program/ or computer simulation/ or computer assisted therapy/ or computer graphics/ or *touch/
11. (virtual reality$ or virtual‐reality$ or VR).tw.
12. (virtual adj3 (environment$ or object$ or world$ or treatment$ or system$ or program$ or rehabilitation$ or therap$ or driving or drive$ or car or tunnel or vehicle)).tw.
13. (computer adj3 (simulat$ or graphic$ or game$ or interact$)).tw.
14. (computer adj1 assist$ adj1 (therap$ or treat$)).tw.
15. (computer adj1 generat$ adj1 (environment$ or object$)).tw.
16. (video game$ or video gaming or gaming console$ or interactive game or interactive gaming or Nintendo Wii or gaming program$).tw.
17. (haptics or haptic device$).tw.
18. (simulat$ adj3 (environment$ or object$ or event$1 or driving or drive$ or car or tunnel or vehicle)).tw.
19. (user adj1 computer adj1 interface).tw.
20. or/10‐19
21. Randomized Controlled Trial/
22. Randomization/
23. Controlled Study/
24. control group/
25. clinical trial/
26. Crossover Procedure/
27. Double Blind Procedure/
28. Single Blind Procedure/ or triple blind procedure/
29. placebo/
30. "types of study"/
31. (random$ or RCT or RCTs).tw.
32. (controlled adj5 (trial$ or stud$)).tw.
33. (clinical$ adj5 trial$).tw.
34. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
35. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
36. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
37. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
38. (cross‐over or cross over or crossover).tw.
39. placebo$ or sham).tw.
40. trial.ti.
41. (assign$ or allocat$).tw.
42. or/21‐41
43. 9 and 20 and 42
44. limit 43 to DD=20131026‐20170401
Appendix 4. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ or brain injuries/
2. (stroke$ or cva or poststroke or post‐stroke or cerebrovasc$ or cerebral vascular).tw.
3. ((cerebral or cerebellar or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy)).tw.
4. ((cerebral or brain or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed$)).tw.
5. hemiplegia/ or gait disorders/
6. (hempar$ or hemipleg$ or paresis or paretic or brain injur$).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. virtual reality/ or computer systems/ or exp computers/ or internet/ or software/ or computer graphics/ or computer assisted instruction/ or computer simulation/ or therapy computer assisted/ or "play and playthings"/
9. (virtual reality$ or virtual‐reality$ or VR).tw.
10. (virtual adj3 (environment$ or object$ or world$ or treatment$ or system$ or program$ or rehabilitation$ or therap$ or driving or drive$ or car or tunnel or vehicle)).tw.
11. (computer adj3 (simulat$ or graphic$ or game$ or interact$)).tw.
12. (computer adj1 assist$ adj1 (therap$ or treat$)).tw.
13. (computer adj1 generat$ adj1 (environment$ or object$)).tw.
14. (video game$ or video gaming or gaming console$ or interactive game or interactive gaming or Nintendo Wii or gaming program$).tw.
15. (haptics or haptic device$).tw.
16. (simulat$ adj3 (environment$ or object$ or event$1 or driving or drive$ or car or tunnel or vehicle)).tw.
17. (user adj1 computer adj1 interface).tw.
18. or/8‐17
19. 7 and 18
20. limit 19 to UP=201310‐201704
Appendix 5. CINAHL search strategy
S55 S54 and EM 201310‐
S54 ‐S34 AND S53
S53 ‐S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S46 OR S47 OR S50 OR S51 OR S52
S52 ‐TI trial OR ( TI (RCT or RCTs) OR AB (RCT or RCTs) )
S51 ‐TI ( counterbalance* or multiple baseline* or ABAB design ) or AB ( counterbalance* or multiple baseline* or ABAB design )
S50 ‐S48 and S49
S49 ‐TI trial* or AB trial*
S48 ‐TI ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or intervention* or compar* or experiment* or preventive or therapeutic )
S47 ‐TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham )
S46 ‐S44 and S45
S45 ‐TI ( blind* or mask*) or AB ( blind* or mask* )
S44 ‐TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* )
S43 ‐TI random* or AB random*
S42 ‐(MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design")
S41 ‐(MH "Clinical Research") or (MH "Clinical Nursing Research")
S40 ‐(MH "Placebo Effect") or (MH "Placebos") or (MH "Meta Analysis")
S39 ‐(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials")
S38 ‐(MH "Control (Research)") or (MH "Control Group")
S37 ‐(MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies")
S36 ‐(MH "Random Assignment") or (MH "Random Sample+")
S35 ‐PT randomized controlled trial or clinical trial
S34 ‐S15 AND S33
S33 ‐S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32
S32 ‐TI (user N2 computer N2 interface) or AB (user N2 computer N2 interface)
S31 ‐TI (simulat* N3 (environment* or object* or event or events or driving or drive* or car or tunnel or vehicle)) or AB (simulat* N3 (environment* or object* or event or events or driving or drive* or car or tunnel or vehicle))
S30 ‐TI (haptics or haptic device*) or AB (haptics or haptic device*)
S29 ‐TI (video game* or video gaming or gaming console* or interactive game or interactive gaming or Nintendo Wii or gaming program*) or AB (video game* or video gaming or gaming console* or interactive game or interactive gaming or Nintendo Wii or gaming program*)
S28 ‐TI (computer generat* N3 (environment* or object*)) or AB (computer generat* N3 (environment* or object*))
S27 ‐TI (computer assist* N3 (therap* or treat*)) or AB (computer assist* N3 (therap* or treat*))
S26 ‐TI (computer N3 (simulat* or graphic* or game* or interact*)) or AB (computer N3 (simulat* or graphic* or game* or interact*))
S25 ‐TI (virtual N3 (environment* or object* or world* or treatment* or system* or program* or rehabilitation* or therap* or driving or drive* or car or tunnel or vehicle)) or AB (virtual N3 (environment* or object* or world* or treatment* or system* or program* or rehabilitation* or therap* or driving or drive* or car or tunnel or vehicle))
S24 ‐TI ( virtual reality* or virtual‐reality* or VR ) OR AB ( virtual reality* or virtual‐reality* or VR )
S23 ‐(MM "Touch")
S22 ‐(MH "Video Games")
S21 ‐(MH "Computer Graphics")
S20 ‐(MH "Microcomputers+")
S19 ‐(MH "Computer Systems") OR (MH "User‐Computer Interface+") OR (MH "Software+")
S18 ‐(MH "Computer Assisted Instruction")
S17 ‐(MH "Therapy, Computer Assisted")
S16 ‐(MH "Computer Simulation") OR (MH "Virtual Reality") OR (MH "Computing Methodologies") OR (MH "Computers and Computerization")
S15 ‐S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14
S14 ‐TI brain injur* OR AB brain inju*
S13 ‐(MH "Brain Injuries")
S12 ‐(MH "Gait Disorders, Neurologic+")
S11 ‐TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S10 ‐(MH "Hemiplegia")
S9 ‐S7 and S8
S8 ‐TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S7 ‐TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S6 ‐S4 and S5
S5 ‐TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S4 ‐TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S3 ‐TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S2 ‐(MH "Stroke Patients") OR (MH "Stroke Units")
S1 ‐(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
Appendix 6. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ or brain damage/
2. (stroke$ or cva or poststroke or post‐stroke or cerebrovasc$ or cerebral vascular).tw.
3. ((cerebral or cerebellar or brain$ or vertebrobasilar) adj5 (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy)).tw.
4. ((cerebral or brain or subarachnoid) adj5 (haemorrhage or hemorrhage or haematoma or hematoma or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hempar$ or hemipleg$ or paresis or paretic or brain injur$).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. virtual reality/ or role playing games/ or exp computer assisted instruction/ or computer assisted therapy/ or computer simulation/ or computer games/ or simulation games/ or computers/ or microcomputers/ or internet/ or computer applications/ or computer software/
9. (virtual reality$ or virtual‐reality$ or VR).tw.
10. (virtual adj3 (environment$ or object$ or world$ or treatment$ or system$ or program$ or rehabilitation$ or therap$ or driving or drive$ or car or tunnel or vehicle)).tw.
11. (computer adj3 (simulat$ or graphic$ or game$ or interact$)).tw.
12. (computer adj1 assist$ adj1 (therap$ or treat$)).tw.
13. (computer adj1 generat$ adj1 (environment$ or object$)).tw.
14. (video game$ or video gaming or gaming console$ or interactive game or interactive gaming or Nintendo Wii or gaming program$).tw.
15. (haptics or haptic device$).tw.
16. (simulat$ adj3 (environment$ or object$ or event$1 or driving or drive$ or car or tunnel or vehicle)).tw.
17. (user adj1 computer adj1 interface).tw.
18. or/8‐17
19. 7 and 18
20. limit 19 to yr=2013‐Current
Appendix 7. Cochrane 'Risk of bias' table
The Cochrane tool for assessing risk of bias (Higgins 2011a)
| Domain | Description | Review authors’ judgement |
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups |
Was the allocation sequence adequately generated? □ Yes □ No □ Unsure |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment |
Was allocation adequately concealed? □ Yes □ No □ Unsure |
|
Blinding of outcome assessors Assessments should be made for each main outcome (or class of outcomes) |
Describe all measures used, if any, to blind personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective |
Was knowledge of the allocated intervention adequately prevented during the study? Outcome assessors □ Yes □ No □ Unsure |
|
Incomplete outcome data Assessments should be made for each main outcome (or class of outcomes). |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors | Were incomplete outcome data adequately addressed? □ Yes □ No □ Unsure |
| Selective outcome reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found | Are reports of the study free of suggestion of selective outcome reporting? □ Yes □ No □ Unsure |
Data and analyses
Comparison 1. Virtual reality versus conventional therapy: effect on upper limb function post intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Upper limb function post intervention (composite measure) | 22 | 1038 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.05, 0.20] |
| 2 Upper limb function post intervention (Fugl Meyer) | 16 | 599 | Mean Difference (IV, Fixed, 95% CI) | 2.85 [1.06, 4.65] |
| 3 Hand function post intervention (grip strength) | 6 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.27, 0.22] |
| 4 Upper limb function post intervention: amount of use (subjective) | 5 | 161 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.21] |
| 5 Upper limb function at short term follow‐up (up to 3 months) | 9 | 366 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.10, 0.32] |
1.4. Analysis.
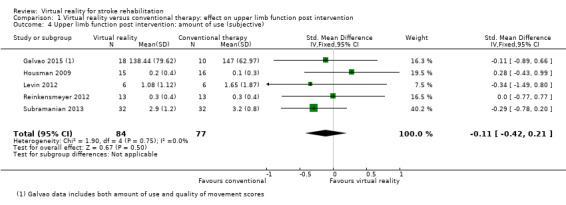
Comparison 1 Virtual reality versus conventional therapy: effect on upper limb function post intervention, Outcome 4 Upper limb function post intervention: amount of use (subjective).
1.5. Analysis.
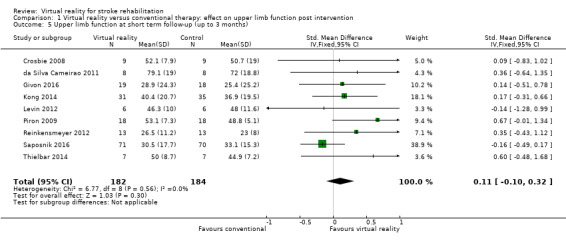
Comparison 1 Virtual reality versus conventional therapy: effect on upper limb function post intervention, Outcome 5 Upper limb function at short term follow‐up (up to 3 months).
Comparison 2. Virtual reality versus conventional therapy: upper limb function: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dose of intervention | 22 | 1038 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.05, 0.20] |
| 1.1 Less than 15 hours of intervention | 9 | 430 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.20, 0.18] |
| 1.2 More than 15 hours of intervention | 13 | 608 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.03, 0.29] |
| 2 Time since onset of stroke | 20 | 930 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.17] |
| 2.1 Less than 6 months | 7 | 555 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 2.2 More than 6 months | 13 | 375 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.02, 0.39] |
| 3 Specialised or gaming | 22 | 1038 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.05, 0.20] |
| 3.1 Specialised | 15 | 506 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.00, 0.35] |
| 3.2 Gaming | 7 | 532 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.20, 0.15] |
| 4 Severity of impairment | 21 | 998 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.06, 0.19] |
| 4.1 Mild to moderate impairment | 13 | 678 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.25] |
| 4.2 Moderate to severe impairment | 8 | 320 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.23] |
Comparison 3. Additional virtual reality intervention: effect on upper limb function post intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Upper limb function (composite measure) | 10 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.21, 0.77] |
Comparison 4. Additional virtual reality intervention: effect on upper limb function post intervention: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dose of intervention | 10 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.21, 0.77] |
| 1.1 Less than 15 hours of intervention | 7 | 153 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.14, 0.80] |
| 1.2 More than 15 hours of intervention | 3 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.00, 1.07] |
| 2 Time since onset of stroke | 9 | 181 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.14, 0.74] |
| 2.1 Less than 6 months | 5 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.12, 0.67] |
| 2.2 More than 6 months | 4 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.19, 1.11] |
| 3 Specialised or gaming | 10 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.21, 0.77] |
| 3.1 Specialised | 7 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.06, 0.75] |
| 3.2 Gaming | 3 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.18, 1.15] |
Comparison 5. Virtual reality versus conventional therapy: effect on lower limb activity post intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait speed | 6 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.22] |
| 2 Timed Up and Go Test | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.76 [‐4.67, 1.16] |
| 3 Balance | 3 | 72 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.09, 0.86] |
Comparison 6. Virtual reality versus conventional therapy: effect on lower limb activity post intervention: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dose of intervention: effect on gait speed | 6 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.22] |
| 1.1 Less than 10 hours of intervention | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.22, 0.24] |
| 1.2 More than 10 hours of intervention | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.03, 0.28] |
Comparison 7. Additional virtual reality intervention: effect on lower limb activity post intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait speed | 3 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.05, 0.21] |
| 2 Functional mobility (Timed Up and Go) | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐4.76 [‐8.91, ‐0.61] |
| 3 Balance | 7 | 173 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.28, 0.90] |
Comparison 8. Additional virtual reality intervention: effect on global motor function post intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global motor function | 3 | 43 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.60, 0.61] |
Comparison 9. Virtual reality versus conventional therapy: effect on activity limitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADL outcome | 10 | 466 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.06, 0.43] |
Comparison 10. Additional virtual reality intervention: effect on activity limitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADL outcome | 8 | 153 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.11, 0.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adie 2017.
| Methods | RCT | |
| Participants | Recruited from 10 stroke centres in the UK 235 participants: 117 intervention, 118 control Inclusion criteria: ischaemic or haemorrhagic stroke within the last 6 months, arm weakness owing to stroke, defined as MRC Scale power < 5 in any joint plane and able to manipulate the Wii™ remote control Exclusion criteria: severe comorbidity that could impair participation, symptomatic shoulder subluxation, or a pacemaker Mean (SD) age: intervention group 66.8 (14.6) years, control group 68.0 (11.9) years 56% men Stroke details: 89% ischaemic Timing post stroke: intervention group mean (SD) 57.3 (48.3) d, control group mean 56.3 (50.1) d |
|
| Interventions | VR intervention: therapists visited the participants home and installed the Wii and taught participants how to use it. Participants were given the choice of any of the Wii sports games. Performed in a seated position Control intervention: participant‐tailored arm exercises (based on the GRASP program) in a seated position Sessions: participants in both groups were instructed to warm up for 15 min and then perform the intervention for up to 45 min/d for 6 weeks |
|
| Outcomes | Outcomes were assessed at baseline, 6 weeks, and 6 months Action Research Arm Test Canadian Occupational Performance Measure Stroke Impact Scale Modified Rankin Scale EQ5D Motor Activity Log Arm Function Test (6 months) Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Web‐based service |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Clinical trial registration and accurate reporting |
Akinwuntan 2005.
| Methods | RCT | |
| Participants | Recruited from 1 rehabilitation unit in Belgium 83 participants: 42 intervention, 41 control Inclusion criteria: within 3 months of first stroke, actively driving before stroke, in possession of an active driver's licence Exclusion criteria: ≥ 75 years, history of epilepsy within previous 6 months, severe motor or sensory aphasia Mean (SD) age: intervention group 54 (12) years, control group 54 (11) years 81% men Stroke details: 77% ischaemic, 44% right hemiparesis Timing post stroke: intervention group mean (SD) 53 (6) d, control group 54 (6) d |
|
| Interventions | VR intervention: driving simulator in full‐sized, automatic gear transmission Ford Fiesta; a variety of 5 km driving scenarios were used including positioning on straight and curvy roads, stopping at crossings and avoiding pedestrians, overtaking and road sign recognition Control intervention: driving‐related cognitive tasks: these included route finding on a paper map, recognition of road signs, commercially available games including 'rush hour' and 'tantrix' Sessions were 60 min, 3 times a week for 5 weeks (15 h total) |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention and at 6 months with some participants followed up at 5 years Cognitive outcome measures: Useful Field of View Test Activity limitation outcome measures: on‐road driving test (using Test Ride for Investigating Practical Fitness to Drive checklist), decision of fitness to drive, Barthel Index (assessed at baseline and 5 years only) Other outcome measures: binocular acuity, kinetic vision, components of the Stroke Driver Screening Assessment Other outcome measures assessed at baseline and 5 years only: Hospital Anxiety and Depression Scale, number of km driven/year, number of self‐reported traffic tickets and accidents and driving status (actively driving or stopped driving) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised number generation |
| Allocation concealment (selection bias) | Low risk | Allocation managed by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A large amount of missing data due to the number of participants who withdrew (14% withdrew from their allocated intervention, 29% of participants were lost at 6‐month follow‐up); however, the authors completed an ITT analysis and found that dropout was random and balanced evenly across groups |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Barcala 2013.
| Methods | RCT | |
| Participants | Recruited from the physical therapy clinic at a university in Brazil 20 participants: 10 intervention, 10 control Inclusion criteria: people after stroke receiving weekly physical therapy sessions at the university; able to sit unsupported; able to understand the visual biofeedback; absence of osteoarticular deformities Exclusion criteria: unspecified comorbidities Mean (SD) age: intervention group 65.2 (12.5) years, control group 63.5 (14.5) years 45% men Stroke details: 65% right hemiparesis Timing post stroke: intervention group mean (SD) 12.3 (7.1) months, control group 15.2 (6.6 months) |
|
| Interventions | VR intervention: conventional physical therapy plus an additional 30 min of balance training with visual feedback using 3 of the Nintendo Wii Fit program games Control intervention: convention physical therapy (stretching, joint movement, muscle strengthening, balance training, training of functional activities) Sessions were twice/week over 5 weeks. Conventional therapy lasted 60 min; the intervention sessions were an additional 30 min (approximately 5 h duration of additional training in total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Gait outcomes: Timed Up and Go Test Balance outcomes: Berg Balance Scale, centre of pressure data, body symmetry Activity outcomes: Functional Independence Measure |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table at central office |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol |
Bower 2015.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation facility in Melbourne, Australia 16 participants: 8 intervention, 8 control Inclusion criteria: adults with stroke who were able to sit unsupported for longer than 10 seconds (Motor Assessment Scale ‐ Sitting Balance ≥ 2) Exclusion criteria: severe dysphasia, significant cognitive deficits (Mini‐Mental State Examination < 20), other medical conditions (e.g. progressive neurological condition, severe arthritis, unstable heart condition) impacting on their ability to participate in the study, or visual problems such that they were not able to adequately see the games when displayed on the television screen Mean (SD) age: intervention group 60.8 (16.1) years, control 60.9 (14.0) years 69% men Timing post stroke, median (IQR) intervention group 12.8 (3.9 to 137.8) weeks, control group 24.7 (5.8 to 51.1) weeks |
|
| Interventions | VR intervention: customised games developed for the research study. The system used a laptop, depth sensing camera and display on a television screen. The games were designed to encourage dynamic balance and upper limb activities and to be adaptable to users with different levels of balance, motor control and perceptual problems. Games included 'ball maze', 'fridge frenzy', 'tentacle dash' and 'bubble fish' Control intervention: usual care only (thus the VR therapy group received a greater overall dose of therapy) The intervention group completed eight 40‐min sessions over 4 weeks |
|
| Outcomes | Assessed at baseline and post intervention Lower limb function and activity: 6MWT, step test Balance: functional reach Global Motor Function: Motor Assessment Scale Functional Independence Measure (transfers, walking, stairs) Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | External management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low rate of withdrawals. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Registered clinical trial. All outcomes reported |
Byl 2013.
| Methods | RCT | |
| Participants | Recruited via the University of California, USA 15 participants completed the study: 5 intervention, 5 intervention, 5 control Inclusion criteria: stroke survivors > 6 months post stroke, 25‐75 years of age. Participants were independent in self care and independent in the community with minimal‐moderate voluntary function in the upper limb (Upper Limb Fugl Meyer score 16‐39). Participants needed to speak English or attend with an interpreter Exclusion criteria: people were excluded if they suffered from a neurological disease other than stroke, had co‐morbidities that would impact on participation, were in severe pain, were not mentally alert or had a skin condition that would prevent wearing the robotic orthosis Mean (SD) age: intervention group 65.2 (5.4) years, control group 54.2 (20.5) years Stroke details: 70% right hemiparesis Timing post stroke: intervention group 8.4 (4.2), control group 10.2 (5.0) months |
|
| Interventions | This trial had 3 arms: 2 of the intervention groups performed VR tasks; 1 of the VR groups performed bilateral tasks and the other group performed unilateral tasks VR intervention: the participant wore a robotic orthosis. Each session started with a motor‐control evaluation task and then followed with treatment in which participants performed repetitive movements while playing task‐specific games Control intervention: repetitive task practice involved reaching, grasping, object manipulation and self‐care activities. Dynamic orthoses were not included in training Sessions were 90 min for 12 treatment sessions (approximately 18 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function outcomes: Fugl Meyer, Motor Proficiency Speed (abbreviated Wolf Motor Function Test and Digital Reaction Time Test) Hand function outcomes: motor skill performance (Box and Block test and Tapper test) Activity limitation outcomes: functional independence (CAFE40) Quality of life outcomes: Stroke Impact Scale Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocated prospectively using a computer program |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reporting for all participants following intervention |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Cho 2012.
| Methods | RCT | |
| Participants | Recruited from a hospital in Korea 29 participants: 15 intervention, 14 control Inclusion criteria: no VR intervention in the previous 2 years, no surgery in the previous 2 months and no specific medical problems, including psychological problems Exclusion criteria: none described Mean (SD) age: intervention group 64 (7.1) years, control group 63.7 (8.8) years 62% men Stroke details: 41% hemiparesis Timing post stroke: not reported |
|
| Interventions | VR intervention: the Interactive Rehabilitation and Exercise System (IREX) was used for training. The participant performed 6 programs; each program was performed for 5 min Control intervention: no intervention Sessions were 60 min, 5 times/week for 4 weeks (approximately 20 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function outcomes: Wolf Motor Function Test Other outcomes: Motor Free Visual Perception Test |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random‐sampling numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals not clearly explained |
| Selective reporting (reporting bias) | Unclear risk | Protocol not publicly available |
Chow 2013.
| Methods | RCT | |
| Participants | Recruited from outpatient physiotherapy at the Hong Kong Buddhist Hospital 14 participants (size of each group not reported) Inclusion criteria: diagnosis of stroke Exclusion criteria: not reported Mean (SD) age: intervention group 69.14 years (2.73), control group 68.86 (8.25) years Stroke details: not reported Timing post stroke: not reported |
|
| Interventions | VR intervention: Xbox360 Kinect in addition to conventional physiotherapy training Control intervention: conventional physiotherapy training Sessions were 60 min, twice/week for 6 weeks |
|
| Outcomes | Outcomes recorded at baseline and post intervention Gait and balance function: 10 metre walk test, Berg Balance scale Activity limitation: Modified Barthel Index Other: Sensory organisation test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Not reported (conference abstract) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | Not reported (conference abstract) |
Coupar 2012.
| Methods | RCT | |
| Participants | Recruited from a stroke unit in Glasgow, UK 12 participants: 4 high‐intensity intervention, 4 low‐intensity intervention, 4 control Inclusion criteria: ≥ 18 years with a clinical diagnosis of stroke and grade 1‐4 on MRC scale of arm impairment. Medically stable and within 10 d post stroke. Able to give informed consent, understand and follow simple instruction and sitting balance sufficient to use the device safely Exclusion criteria: orthosis could not be fitted to the affected limb due to previous stroke or other condition, bone instability of affected upper limb, no functional use of affected upper limb due to previous stroke or other condition. Pronounced fixed contractures of affected upper limb, open skin lesions on affected upper limb; major sensory deficit of affected upper limb; shoulder instability or excessive pain; severe spasticity; severe spontaneous movements; confused or non‐co‐operative; isolation due to infection; visual, perceptual or cognitive problems precluding participation in study involvement or involvement in any other intervention study Mean (SD) age: high‐intensity intervention group 65 (14) years, low‐intensity 72 (10), control 59 (16) years 66% men Stroke details: 42% right hemiparesis Timing post stroke: high‐intensity intervention 8 (1) d, low‐intensity 9 (2), control 8 (3) |
|
| Interventions | VR intervention: Low‐intensity: standard care plus Armeo®Spring arm orthosis and VR games for arm rehabilitation used for 40 min/d, 3 d/week High‐intensity: standard care plus Armeo®Spring arm orthosis and VR games for arm rehabilitation used for 60 min/d, 5 d/week Games included catching rain drops, picking apples and cleaning a cooker Control intervention: standard care including standard physiotherapy and OT targeted at arm recovery Sessions were for 2 weeks or until discharge from the stroke unit (whichever was soonest) |
|
| Outcomes | Outcomes recorded at baseline, completion of intervention and 3 months following completion Upper limb function: Action Research Arm Test, Fugl Meyer UE Activity restriction: Barthel Index Other outcomes related to feasibility, acceptability, safety, arm pain, perceived exhaustion and adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few withdrawals and balanced across groups for reasons not clearly related to the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in thesis |
Crosbie 2008.
| Methods | RCT | |
| Participants | Recruited from 2 hospital stroke units and members of Stroke Association Clubs in Northern Ireland 18 participants: 9 intervention, 9 control Inclusion criteria: within 2 years of first stroke, medically stable, can follow 2‐stage commands, score of ≥ 25 on the upper limb Motricity Index Exclusion criteria: mental score < 7/10, neglect (star cancellation < 48/52), comorbid conditions impacting on rehabilitation potential, cardiac pacemaker, severe arm pain reported on visual analogue scale Mean (SD) age: intervention group 56 (15) years, control group 65 (7) years 55% men Stroke details: 39% right hemiparesis Timing post stroke: intervention group mean (SD) 10 (6) months, control group 12 (8) months |
|
| Interventions | VR intervention: the participant chose from a variety of activities involving reaching and grasping of virtual objects at a variety of heights, speeds and with varied number of targets; the participant wore a head‐mounted device and data glove Control intervention: therapy provided based on the Bobath approach Sessions were 35‐45 min, 3 times/week over 3 weeks (approximately 6 h total) |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention and at 6 weeks Upper limb function and activity outcomes: Action Research Arm Test, Upper Limb Motricity Index Adverse events were reported Other outcome measures: an exit questionnaire including questions about enjoyment and perception of improvement |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent colleague generated the sequence using a computer random number generator |
| Allocation concealment (selection bias) | Low risk | Group allocation cards were concealed in sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An ITT analysis was completed. Missing data points were dealt with using the simple mean imputation method |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
da Silva Cameirao 2011.
| Methods | RCT | |
| Participants | Recruited from a subacute rehabilitation unit in Spain 19 participants: 13 intervention, 6 control Inclusion criteria: recruited within 3 weeks of first stroke, severe‐moderate upper limb impairment, no moderate‐severe aphasia, no other cognitive deficits as assessed by the MMSE and aged ≤ 80 years Exclusion criteria: none specified Mean (SD) age: intervention group 63.7 (11.83) years, control group 59.4 (10.62) years, control group (Wii) 58 (14) years 47% men Stroke details: 37% right hemiparesis Timing post stroke: intervention group mean (SD) 11.5 (5.1) d, control group 16.8 (5.0) d, control group (Wii) 13 (4.7) d |
|
| Interventions | VR intervention: Rehabilitation Gaming System (RGS). The main elements of the system are the vision‐based analysis and tracking system that capture upper limb movements through colour detection, data gloves to capture finger flexion and a virtual environment where an avatar mimics the movements of the user Control intervention (OT): OT with emphasis on motor tasks similar to those in the RGS (i.e. object displacement, grasp and release) Control intervention (Wii): used the Wii gaming system. This intervention involved the gaming features but not the neuro‐scientific hypothesis regarding recovery Sessions were 20 min, 3 times/week for 12 weeks (approximately 12 h total). This was provided in addition to standard rehabilitation |
|
| Outcomes | Outcomes recorded at baseline, weeks 5, 12 and 24 Upper limb outcomes: Fugl Meyer, Chedoke Arm and Hand Activity Inventory Activity outcomes: Barthel Index Other outcomes: participant satisfaction |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program |
| Allocation concealment (selection bias) | Low risk | Managed externally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outliers excluded from the data analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
da Silva Ribeiro 2015.
| Methods | RCT | |
| Participants | Recruited from an outpatient setting in Sao Paolo, Brazil 30 participants: 15 intervention, 15 control Inclusion criteria: aged 18‐60 years with a diagnosis of stroke (based on neurologist assessment) and hemiparesis. Able to ambulate and hold the game controller without assistive devices. ≥ 6 months post stroke Exclusion criteria: associated disorders (such as hemineglect or pusher syndrome), intellectual disability that made it difficult to understand the games or a history of orthopaedic diseases that promoted dysfunction in the limbs or prevented the performance of the proposed activity Mean (SD) age: intervention group 53.7 (6.1) years, control group 52.8 (8.6) years 37% men Stroke details: 57% right hemiparesis Timing post stroke, mean (SD): intervention group 42.1 (26.9) months, control group 60.4 (44.) months |
|
| Interventions | VR intervention: Nintendo Wii projected onto the wall. After full body stretching for 10 min the participants spent 50 min using the Nintendo Wii. The tennis and hula hoop games were used during the 1st session and soccer and boxing used during the second weekly session. The difficulty level of the games was increased as participants progressed Control intervention: conventional physiotherapy including stretching, passive, active and resisted mobilisation activities, balance and gait activities and gripping activities Sessions were 60 min, twice/week for 2 months with a physiotherapist |
|
| Outcomes | Outcomes assessed post intervention Upper limb function and activity: Fugl Meyer Participation and quality of life: SF36 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number allocation (performed online) |
| Allocation concealment (selection bias) | Unclear risk | Used envelopes but unclear if opaque or not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Trial register not reported |
Fan 2014.
| Methods | RCT | |
| Participants | Recruited from a suburban hospital and affiliated nursing home in Taiwan 20 participants: allocated to 4 different treatment groups Inclusion criteria: aged 20‐85 years with evidence of a cerebrovascular accident (confirmed by CT or MRI). Onset for symptoms for ≥ 6 months and MMSE score of > 24. Able to produce active shoulder movements on the side of the hemiparesis (Fugl Meyer of ≥ 21). Visual analogue scale of < 4, Modified Ashworth Scale of ≤ 2 and no rehabilitation in the past 3 months Exclusion criteria: uncontrolled hypertension, unstable angina, history of seizure, artificial pacemaker and participation in other research Mean (SD) age: varied from 57‐67 years across the 4 intervention groups Stroke details: 90% ischaemic, 45% right hemiplegia Timing post stroke: ranged from an average of 1.8‐2.6 years across the 4 intervention groups |
|
| Interventions | VR intervention: used available games including the Nintendo Wii Sports Resort. Participants were supervised by a research staff member. The consoles and controller were not modified in the study. Participants were advised to take ≥ 5‐10‐min breaks between games Control intervention: OT involving Bobath and proprioceptive neuromuscular facilitation. Equipment included bean bags, target bags and cones Control intervention: leisure activities including mahjong, cards and checkers Control intervention: usual care Sessions were 60 min, 3 times/week for 3 weeks |
|
| Outcomes | Outcomes assessed at baseline, post intervention and 4 weeks after treatment Arm function: Jebsen Taylor Hand Function Test Stroke Impact Scale Intrinsic Motivation Inventory |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were a relatively high proportion of withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Trial registry not reported |
Galvao 2015.
| Methods | RCT | |
| Participants | Recruited from a physiotherapy clinic in Brazil 27 participants: intervention 17, control 10 Inclusion criteria: stroke, hemiparesis, aged 30‐70 years Exclusion criteria: failure to meet above criteria Mean (SD) age: 55.06 (11.52) years, control 60.8 (10.83) years |
|
| Interventions | VR intervention: exercises with the Nintendo Wii Control intervention: conventional therapy Sessions were 75 min for the Wii group and 60 min for the conventional therapy group and a total of 10 sessions were provided |
|
| Outcomes | Outcomes were assessed post intervention Fugl Meyer UL Motor Activity Log |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation program |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Detail not reported |
| Selective reporting (reporting bias) | Unclear risk | Detail not reported |
Givon 2016.
| Methods | RCT | |
| Participants |
NCT01304017 Recruited from the community in Israel 47 participants: 23 intervention, 24 control Inclusion criteria: community dwelling, aged 18‐8 years and sustained a stroke ≥ 6 months prior to the study. Able to walk ≥ 10 m (with or without aid) and had weakness of the UE and no significant cognitive deficits (score of ≥ 21 or more on the MMSE) Exclusion criteria: other neurological conditions or epilepsy Mean (SD) age: intervention group 56.7 (9.3) years, control group 62.0 (9.3) years 60% men Stroke details: 85% ischaemic, 57% right hemiparesis Timing post stroke: intervention group mean 3 (1.8) years, control group mean 2.6 (1.8) years |
|
| Interventions | VR intervention: interactive video games (Kinect, Sony Play Station Eyetoy 2, Sony Playstation 3 MOVE, Nintendo Wii Fit and SeeMe VR system) were set up in 3 workstations. Each session started with a 5‐min group warm up playing a Wii Fit walking game. Participants were then divided into workstations. They played games on 1 console then rotated to another console with a new partner. All games were played in pairs while standing. Partners either took turns or played simultaneously Control intervention: exercises and functional activities from existing community group programs such as the Fitness and Mobility Exercise Program, the GRASP program and task oriented intervention. The session started with a 5‐min group warm up and then participants were divided into pairs or triads to perform functional activities such as picking up and transferring objects from 1 side of the room to the other Sessions were 60 min, twice/week for 3 months. Intervention in both groups delivered by an occupational therapist |
|
| Outcomes | Outcomes measured at baseline, post intervention and 3 months' follow‐up (after the end of intervention) 10‐metre walk test Hand grip strength Action Research Arm Test Other outcome measures: number of steps walked per day Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detail not reported |
| Allocation concealment (selection bias) | Unclear risk | Detail not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropouts. ITT analysis conducted with the last observation carried forward method |
| Selective reporting (reporting bias) | High risk | Measures reported on the clinical trial registry were not reported in the paper |
Han 2013.
| Methods | RCT | |
| Participants | Recruited from a tertiary hospital in Korea 12 participants: 6 intervention, 6 control Inclusion criteria: impaired standing balance (Berg Balance Scale < 40) however can stand for ≥ 1 min Exclusion criteria: none reported Mean (SD) age: total sample 60.1 (17.6) years 50% men Stroke details: not reported Timing post stroke: not reported |
|
| Interventions | VR intervention: IREX system (games: Birds and Balls, Soccer, Conveyor, Drums, Sharkbait) Control intervention: balance training using tetrataxiometric posturography Sessions were 30 min/day, 3 d/week for 3 weeks |
|
| Outcomes | Outcomes assessed post intervention Balance: Berg Balance Scale Modified Barthel Index Tetraataxiometric posturography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Not reported (conference abstract) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | Not reported (conference abstract) |
Housman 2009.
| Methods | RCT | |
| Participants | Recruited from 1 rehabilitation institute in Chicago, USA 34 participants: 17 intervention, 17 control Inclusion criteria: single stroke ≥ 6 months ago, Fugl Meyer UE score 10‐30 Exclusion criteria: significant pain or instability of the shoulder, current participation in upper limb therapy program, severe cognitive dysfunction, aphasia, neglect, apraxia Mean (SD) age: intervention group 54 (12) years, control group 56 (13) years 64% men Stroke details: 61% ischaemic, 29% right hemiparesis Timing post stroke: intervention group mean (SD) 85 (96) months, control group 112 (129) months |
|
| Interventions | VR intervention: a custom‐designed software package ('Vu Therapy') provided activities including grocery shopping, cleaning a stove and playing basketball. The participant wore an arm orthosis (T‐WREX), which supports the weight of the arm allowing movement in the horizontal and vertical plane. Position sensors at each joint enable interaction with the virtual environment Control intervention: UE exercises including passive and active ranging, stretching, strengthening and using the arm in functional tasks Both groups involved 3 sessions of direct training followed by semi‐autonomous practice in the research clinic Sessions were 60 min, approximately 3 times/week for 6 weeks (approximately 24 h total) |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention and at 6 months Upper limb function and activity outcomes: Fugl Meyer UE Scale, Rancho Functional test UE, Reaching ROM (deficit) Hand function and activity: grip strength (dynamometer) Participation restriction and quality of life: Motor Activity Log (amount of use and quality of movement) Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a lottery system in which the supervising therapist (with an independent witness) drew a labelled tile from an opaque container. Randomisation occurred in blocks of 4 to ensure equal numbers in each group |
| Allocation concealment (selection bias) | High risk | Participants were allocated in strict sequential order of enrolment. However, with small blocks of 4 and the use of tiles it might have been possible to predict allocation in advance in some cases |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Rater was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of dropouts balanced across groups with similar reasons for dropout |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Hung 2014.
| Methods | RCT | |
| Participants | Recruited from an outpatient rehabilitation setting in Taiwan 30 participants: 15 intervention group, 15 control group Inclusion criteria: stroke with resulting hemiplegia ≥ 6 months prior to enrolment. Aged > 18 years and had a Berg Balance Scale score < 56. Able to understand verbal instructions and watch a television screen satisfactorily. Able to walk independently with or without a device for 10 m Exclusion criteria: bilateral lesions, receptive aphasis, significant visual field deficits or hemineglect and concomitant other neurological diagnoses or conditions that would prevent adherence to the exercise protocol Mean (SD) age: intervention group 55.38 (9.95) years, control group 53.40 (10.03) years 60% men Stroke details: 53% ischaemic, 37% right hemiparesis Timing post stroke: intervention group mean (SD) 21 (11.26) months, control group 15.93 (8.02) months |
|
| Interventions | VR intervention: Nintendo Wii Fit. 7 games (Table tilt, Ski Slalom, Soccer, Balance Bubble, Penguin Slide, Basic Step and Warrior) were selected. At each session the therapist supervised 2‐4 games for participants according to their ability, needs and favourites. A walker was placed in front of the balance board for safety. Control intervention: weight shift and balance exercises Sessions were twice/week for 12 weeks and were run by an occupational therapist |
|
| Outcomes | Outcomes assessed post intervention and at 3 months' follow‐up Tetrax Interactive Balance Systems Timed Up and Go Test Forward Reach Test Falls Efficacy Scale International Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition with clear rationale. Used data of actual number contributing |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or trial registration reported |
Jaffe 2004.
| Methods | RCT | |
| Participants | Recruited from community stroke association meetings in California, USA 20 participants: 10 intervention, 10 control Inclusion criteria: > 6 months post stroke with a diagnosis of hemiplegia secondary to single documented lesion, walked independently or with an aid and had an asymmetric gait pattern and short step‐length with either step (< 95th percentile of normal step length), scores representing average or minimally impaired in all Cognistat categories unless performance was markedly limited by aphasia making assessment of cognition difficult Exclusion criteria: neurological diagnoses of spinal cord injury, multiple sclerosis or brainstem lesion; any progressive critical or long‐term illness or unstable cardiovascular, orthopaedic, musculoskeletal or neurological condition that precluded exercise or was not controlled by medication or required oxygen during ambulation Mean (SD) age: intervention group 58 (11) years, control group 63 (8) years 60% men Stroke details: 50% right hemiparesis Timing post stroke: intervention group 4 years (SD 2), control group 4 years (SD 3) |
|
| Interventions | VR intervention: participants walked on a treadmill at a self‐selected walking speed and were secured by an overhead harness. The participant wore a head‐mounted display that showed real‐time video images of their feet walking and virtual objects. The participant was asked to step over the virtual objects and visual, vibrotactile and auditory feedback was provided during any collisions Control intervention: participants wore a gait belt and stepped over foam obstacles in a hallway. The sessions were videotaped and reviewed for collisions with the obstacles after the session was completed Sessions were approximately 60 min, for 6 sessions over 2 weeks (6 h total) |
|
| Outcomes | Outcomes recorded at baseline, post‐intervention and 2 weeks post‐intervention Lower limb function and activity outcomes: 6‐m walk test, obstacle test, 6MWT, the researcher's own balance test (adapted from others) that included natural stance, eyes closed, on toes, tandem stance, left and right leg stand Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An Excel spreadsheet was generated with a pre‐determined computerised randomisation sequence |
| Allocation concealment (selection bias) | High risk | The allocation in the spreadsheet was not visible due to black font and black background shading; however, there is the possibility that staff with access to the spreadsheet could have checked this |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing (according to personal correspondence with the researcher) |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ not privy to protocol |
Jang 2005.
| Methods | RCT | |
| Participants | Study took place in Korea 10 participants: 5 intervention, 5 control Inclusion criteria: > 6 months post first stroke, able to move the elbow against gravity Exclusion criteria: severe spasticity (Modified Ashworth Score of > 2) or tremor. Severe visual and cognitive impairments Mean (SD) age: intervention group 60 (8) years, control group 54 (12) years 60% men Stroke details: 60% ischaemic, 50% right hemiparesis Timing post stroke: intervention group 14 months, control group 13 months |
|
| Interventions | VR intervention: IREX VR system using a video capture system to capture the participant's whole body movement. The participant was able to view their body movements in real time on a screen in front of them immersed in a virtual environment. The games included soccer and moving objects from a conveyor belt and focused on reaching, lifting and grasping Control intervention: no intervention provided Sessions for the VR intervention group were 60 min, 5 times/week for 4 weeks (20 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb (arm) function and activity outcomes: Fugl Meyer UE Scale, Manual Function Test Upper limb (hand) function and activity outcomes: Box and Block Test Participation restriction and quality of life: Motor Activity Log (amount of use and quality of movement) Other outcomes: functional MRI (laterality index and activated voxels) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Jannink 2008.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation centre in the Netherlands 10 participants: 5 intervention, 5 control Inclusion criteria: not reported Exclusion criteria: not reported Mean (SD) age: intervention group 62 (3) years, control group 58 (13) years Timing post stroke: intervention group mean (SD) 89 d (31), control group 112 d (50) |
|
| Interventions | VR intervention: the participant sat on an electric scooter with customised interface and completed training in a traffic garden, residential area and a grocery store. The virtual environment was displayed using a head‐mounted device as well as a computer display. Training included 50% of the time using the VR simulation program and 50% training in the real world Control intervention: real‐world scooter training program Sessions were 30 min, twice/week for 5 weeks (5 h total) |
|
| Outcomes | Outcomes recorded at baseline and 5 weeks after training Other outcome measures: Functional Evaluation Rating Scale, Subjective Experience Questionnaire |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Jung 2012.
| Methods | RCT | |
| Participants | Recruited from outpatient community centre in Korea 21 participants: 11 intervention, 10 control Inclusion criteria: participants within 6 months after first stroke with a history of falling. Able to walk independently for > 30 min with no cognitive impairment, Brunnstrom Stage > 4 and no cardiovascular, orthopaedic or other neurological conditions that may interfere with study procedures Exclusion criteria: not reported Mean (SD) age: intervention group 60.5 (8.6) years, control group 63.6 (5.1) years 62% men Stroke details: 52% right‐sided hemiparesis Timing post stroke: intervention group mean (SD) 12.6 (3.3) months, control group 15.4 (4.7) months |
|
| Interventions | VR intervention: treadmill training while viewing a virtual scene through a head‐mounted device. The VR program simulated a park stroll Control intervention: treadmill training without the VR program Sessions were 30 min/d, 5 times a week for 3 weeks (approximately 7.5 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Gait outcomes: Timed Up and Go Test Other outcomes: Activity Specific Balance Confidence Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Kang 2009.
| Methods | RCT | |
| Participants | Study took place in Korea 16 participants: 8 intervention, 8 control Inclusion criteria: left hemiplegia after stroke, MMSE score of > 18/30 and Motor Free Visual Perception Test standard score < 109 Exclusion criteria: significant multiple small lacunar infarct, significantly decreased visual acuity or visual impairment from diabetic retinopathy or senile cataract, hearing difficulty or cranial nerve dysfunction Mean (SD) age: intervention group 60 (11) years, control group 63 (10) years Timing post stroke: intervention group mean (SD) 64 (37) d, control group 58 (30) d |
|
| Interventions | VR intervention: participants were seated and participated in visual spatial and motor tasks using their unaffected arm. Software recognised and displayed the movements of the hand through a camera and displayed the images on a computer screen Control intervention: training using the PSS CogRehab program Sessions were 30 min, 3 times/week for 4 weeks (6 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Cognitive outcome measures: MMSE Activity limitation outcomes: Modified Barthel Index Other outcome measures: motor free visual perception test, interest in performing the task |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation using block randomisation process. Envelopes were shuffled and the participant drew 1 after enrolment |
| Allocation concealment (selection bias) | Unclear risk | Whether the envelopes were opaque is unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any attrition and all outcome measures appear to be reported in full |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ not privy to protocol |
Kim 2009.
| Methods | RCT | |
| Participants | Study took place in Korea 24 participants: 12 intervention, 12 control Inclusion criteria: ≥ 1 year post stroke with plateau in motor recovery after conventional rehabilitation and the ability to stand for 30 min and walk indoors independently (approximately 30 m) Exclusion criteria: severe visual or cognitive impairment or musculoskeletal disorders that could interfere with tests Mean (SD) age: intervention group 52 (10) years, control group 52 (7) years 54% men Timing post stroke: intervention group mean (SD) 26 (10) months, control group 24 (9) months |
|
| Interventions | VR intervention: IREX VR system using a video capture system to capture the participant's whole body movement. The participant was able to view their body movements in real time on a screen in front of them immersed in a virtual environment. Games included stepping up/down, shark bait (capturing stars while avoiding eels and sharks by weight shift) and snowboarding. Participants were challenged by increasing resistance (e.g. adding weights) or increasing the speed. Control intervention: conventional physiotherapy designed to facilitate standing balance function during walking. Included practice of weight shift, muscle strengthening, functional reach or picking up objects Sessions for VR group: 30 min, 4 times/week for 4 weeks (8 h) of VR plus conventional physiotherapy 40 min, 4 times/week for 4 weeks (approximately 10.5 h) (approximately 18.5 h total) Sessions for control group: 40 min, 4 times/week for 4 weeks (approximately 10.5 h total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Lower limb function and activity outcomes: 10‐m walk test, GAIT‐RITE gait analysis system, Berg balance scale, Balance performance monitor Global motor function outcomes: modified Motor Assessment Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was generated using a lottery system |
| Allocation concealment (selection bias) | Low risk | Using sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to have any missing data |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Kim 2011a.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation hospital in Korea 28 participants: 15 intervention, 13 control Inclusion criteria: not stated Exclusion criteria: people with a MMSE‐K score of < 10; people presenting with severe cognitive impairment of aphasia and unable to understand instructions. People with poor sitting balance such that they could not sit on a chair with back and armrests. People with limited ROM of the neck due to orthopaedic problems, and people with loss of visual acuity such that they could not perceive content on a computer screen Mean (SD) age: intervention group 66.5 (11) years, control group 62 (15.8) years 39% men Stroke details: 39% right hemiparesis Timing post stroke: intervention group mean (SD) 18.2 (11.3) d, control group 24 (31.1) d |
|
| Interventions | VR intervention: IREX system (30 min 3 times/week) plus computer‐assisted cognitive rehabilitation (30 min twice/week) Control intervention: computer‐assisted rehabilitation (30 min 5 times/week) Sessions were 30 min, 5 times/week over 4 weeks (approximately 6 h of VR in total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Upper limb function outcomes: Motricity index Lower limb function outcomes: Motricity index Cognitive function: computerised neuropsychological test and Tower of London test Activity limitation outcome: Korean modified Barthel Index |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No other outcome data collected |
Kim 2011b.
| Methods | RCT | |
| Participants | Recruited from Department of Rehabilitation, Korea 24 participants: 12 intervention, 12 control Inclusion criteria: participants diagnosed with unilateral spatial neglect through the line bisection test or star cancellation test Exclusion criteria: severe cognitive impairment or aphasia; insufficient sitting balance to sit on a chair with a back and armrests; restricted neck movement, poor eyesight or unable to recognise objects on a screen Mean (SD) age: intervention group 62.3 (10.2) years, control group 67.2 (13.9) years 58% men Timing post stroke: intervention group 22.8 (7.6) d, control group 25.5 (18.5) d |
|
| Interventions | VR intervention: IREX Control intervention: conventional rehabilitation tasks such as visual tracking, reading and writing, drawing and puzzles Sessions were 30 min, 5 d/week for 3 weeks (approximately 7.5 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Activity limitation outcomes: Korean Modified Barthel Index Other outcomes: Star cancellation test, Line bisection test, Catherine Bergego Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No other outcome data collected |
Kim 2012a.
| Methods | RCT | |
| Participants | Recruited from an inpatient setting in Korea 20 participants: 10 intervention, 10 control Inclusion criteria: > 6 months post diagnosis of stroke. Score of ≥ 19/30 on the MMSE. Able to maintain upright posture without any assistance Exclusion criteria: orthopaedic surgery, history of arthritis, hand or upper limb pain, epilepsy, psychiatric illnesses Mean age: not reported Timing post stroke: intervention group mean (SD) 12.6 (7.12) months, control group 12.85 (6.06) months |
|
| Interventions | VR intervention: Nintendo Wii Sports (boxing and tennis) Control intervention: no intervention Sessions were 30 min, 3 times/week for 3 weeks |
|
| Outcomes | Outcomes recorded at baseline and post intervention Gait outcomes: postural assessment scale Global motor function outcomes: modified Motor Assessment Scale Activity limitation outcomes: Functional Independence Measure |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in adequate detail to make judgement |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Kiper 2011.
| Methods | RCT | |
| Participants | Recruited from an institute of rehabilitation, Italy 80 participants: 40 intervention, 40 control Inclusion criteria: diagnosis of stroke within 1 year of enrolment and score of > 24/30 on the MMSE Exclusion criteria: clinical evidence of cognitive impairment, apraxia, neglect, language disturbance, complete paralysis of the UE, upper limb sensory disorders or post‐traumatic injury, which prevented the execution of exercises Mean (SD) age: 64 (16.4) years 58% men Time since onset of stroke: mean (SD) 5.7 (3.5) months |
|
| Interventions | VR intervention: reinforced feedback in virtual environment (RFVE). Participants in the intervention group received 1 h of traditional rehabilitation and 1 h of RFVE. The RFVE involved sitting in front of a wall screen grasping a sensorised real object (ball, disc or cube) with the affected hand. The target objects were displayed on the wall screen. The physiotherapist created a sequence of virtual tasks that the participant had to perform on his workstation (e.g. pouring water from a glass, using a hammer) Control intervention: traditional neuromotor rehabilitation including postural control, exercises for hand pre‐configuration, manipulative and functional skills, proximal‐distal exercises Sessions were 1 h/d, 5 d/week for 4 weeks (approximately 20 h total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Upper limb function outcomes: Fugl Meyer Activity limitation outcomes: Functional Independence Measure Other outcomes: Modified Ashworth Scale (spasticity) Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Author confirmed no other outcomes collected |
Klamroth‐Marganska 2014.
| Methods | RCT | |
| Participants | 77 participants: 39 VR group, 38 control group Recruited from 4 clinical settings in Switzerland Main inclusion criteria: diagnosis of 1, first ever cerebrovascular accident verified by brain imaging (MRI or CT); chronic impairment after stroke (minimum 6 months); moderate‐severe arm paresis, as indicated by a score of 8‐38 on arm section of Fugl‐Meyer assessment (which has a maximum of 66 points); aged ≥ 18 years; able to sit in a chair without any additional support and without leaning on the back rest; passive ROM in the shoulder as assessed with the neutral zero method: anteversion/retroversion 80°/0°/20°, abduction/adduction 60°/0°/10°, inner and outer rotation 20°/0°/20°; passive ROM in the elbow as assessed with the neutral zero method; flexion/extension 100°/40°/40°; no excessive spasticity of the affected arm (modified Ashworth Scale ≤ 3); no serious medical or psychiatric disorder as assessed by their physician; no cybersickness (nausea when looking at a screen or playing computer games); no pacemaker or other implanted electric devices; bodyweight < 120 kg; no serious cognitive defects or aphasia Mean age (SD): intervention group 55 (13), control group 58 (14) years 60% men Timing post stroke: mean (SD) 52 (44) months intervention group, 40 (45) months control group |
|
| Interventions | VR intervention: during the robotic therapy with ARM in, each of 3 therapy modes (mobilisation, games, and training for ADL) had to be done for ≥ 10 min Control intervention: common neurorehabilitation treatment given to patients after stroke in outpatient facilities, namely OT or physiotherapy. Therapists were asked to give regular therapy, usually including mobilisation, games, ADL, or any combination of the 3. Their only restriction was not to use automated technical devices that might be available in therapy settings. For both groups, therapy was given 3 times/week in the centres for a period of 8 weeks (total 24 sessions) and sessions were ≥ 45 min |
|
| Outcomes | Outcomes assessed 3‐4 weeks before assignment, immediately before therapy (baseline), after 4 weeks of therapy, at the end of 8 weeks of therapy, and 16 weeks and 34 weeks after baseline Upper limb function: Fugl Meyer UE, Wolf Motor Function Test, Motor Activity Log (quality of movement) Quality of life and participation: Stroke Impact Scale, Goal attainment scale Adverse events reported |
|
| Notes | NCT00719433 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Tamper‐evident envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few withdrawals. ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Registered on clinical trial |
Ko 2015.
| Methods | RCT | |
| Participants | Recruited via a hospital in Korea 52 participants: 26 intervention, 26 control Inclusion criteria: 1865 years old and diagnosed with stroke within the last 6 months; able to walk > 10 m without or with assisting devices such as orthotics, a walker, or a cane; no symptoms with any lower motor neuron lesion and orthopedic diseases; a score > 24 points on the MMSE; and able to read the words on a monitor 60 cm away at eye level Exclusion criteria: failure to meet inclusion criteria Mean (SD) age: intervention group 48.1 (4.4) years, control group 45.3 (4.2) years 69% men |
|
| Interventions | VR intervention: the Space Balance 3D training system is equipped with 2 wireless force plates. 3 kinds of balance training were implemented using Space Balance 3D, which can be used for both training and testing. According to the participants’ movement, the real‐time tilting angle and foot plates are indicated on a computer screen. The participant moves to 'hit' a predetermined target. Intervention was provided in addition to conventional rehabilitation exercises Control intervention: conventional rehabilitation only Sessions were 30 min, 5 times/week for 3 weeks. The control group only participated in usual rehabilitation thus there was a difference between groups in the amount of therapy received |
|
| Outcomes | Outcomes assessed post intervention Balance: Berg Balance Scale Postural Assessment Scale for Stroke Patients Timed Up and Go Test |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol or clinical trial register not mentioned |
Kong 2014.
| Methods | RCT | |
| Participants | Recruited from inpatients in a tertiary rehabilitation setting in Singapore 105 participants Inclusion criteria: within first 6 weeks after stroke Exclusion criteria: none reported Mean (SD) age: 57.5 (9.8) years in the total sample Timing post stroke: mean 13.7 (8.9) d in the total sample |
|
| Interventions | VR intervention: Nintendo Wii gaming therapy in addition to standard conventional rehabilitation Control intervention: conventional therapy in addition to standard rehabilitation Control intervention: usual care Sessions were 4 times/week for 3 weeks |
|
| Outcomes | Outcomes assessed post intervention and at 4 and 8 weeks after the completion of intervention Upper limb: Fugl Meyer Assessment Upper limb: Action Research Arm Test Functional Independence Measure Stroke Impact Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Managed externally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. ITT analysis with baseline values used |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Kwon 2012.
| Methods | RCT | |
| Participants | Recruited from a hospital in Korea 26 participants: 13 intervention, 13 control Inclusion criteria: adults within 3 months of stroke with the capacity to understand and follow simple instructions. Able to grasp and release affected hand, with manual muscle test ≥ grade 3. Able to maintain standing or sitting position independently and no visual deficit Exclusion criteria: failure to meet above criteria Mean (SD) age: intervention group 57.15 (15.42) years, control group 57.92 (12.32) years Timing post stroke: intervention group mean (SD) 24.69 (15.59) d, control group 23.92 (20.70) d |
|
| Interventions | VR intervention: conventional therapy plus additional therapy time using IREX Control intervention: conventional therapy alone Sessions were 30 min, 5 d/week for 4 weeks |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function outcomes: Fugl Meyer, Manual Function Test Activity limitation outcomes: Korean Modified Barthel |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in adequate detail to make judgement |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Lam 2006.
| Methods | RCT | |
| Participants | Recruited from rehabilitation units in Hong Kong 58 participants: 20 VR, 16 video‐based program, 22 no treatment Inclusion criteria: 50‐85 years old, medically stable with no previous psychiatric history, able to follow simple instructions and write with a pen in Chinese or English, consistent volitional motor response, good visual tracking, discrimination ability and figure ground skills, sustained attention span of ≥ 10 min Exclusion criteria: computer‐related phobia or previous training in Mass Transit Railway Skills Mean (SD) age: VR group 71 (16) years, video‐based program group 71 (15) years, no treatment group 73 (10) years 31% men Timing post stroke: VR group mean (SD) 4 (4) years, video‐based program group 3 (3) years, no treatment group 5 (3) years |
|
| Interventions | VR intervention: a VR program designed to retrain skills using the Mass Transit Railway. Activities included crossing the road and using the facilities at the station Video based program intervention: a video‐based program included instruction, modelling, demonstration, role playing, coaching and feedback on using the Mass Transit Railway No treatment group: no treatment 10 sessions of unspecified duration were provided for the participants in the VR and video program group |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Other outcomes: behavioural rating scale, Mass Transit Railway Self Efficacy Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated into 2 groups using a statistical package random number generator tool |
| Allocation concealment (selection bias) | Low risk | Allocation was computer‐generated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Lee 2013.
| Methods | RCT | |
| Participants | Recruited from inpatients at a hospital in Seoul 22 Participants: 12 intervention group, 10 control group Inclusion criteria: > 6 months after stroke; could sit independently for ≥ 30 min, who had a MMSE‐K score of > 21 points, who had not participated in any balance training program during the previous 6 months, who had no orthopedic problems, such as a fracture, deformity, or severe osteoarthritis, and who were not taking any drugs for balance maintenance were included Exclusion criteria: failure to meet above criteria Mean (SD) age: intervention group 60.6 (8.8) years, control group 63.7 (4.7) years 27% men |
|
| Interventions | VR intervention: Visual Feedback Training (VFT) was performed individually in a dedicated room containing the required equipment. VFT was performed using BIORescue (RM INGENIERIE, Rodez, France) equipment, which consists of a computer, a monitor, and a force plate. This force plate detects the posture and movements made by participants and this information is transferred to the computer, and processed for display on the monitor. This system encourages adoption of the correct posture by providing visual feedback and allows for design of customised exercise programs based on pre‐test data. The system also allows different exercise times and intensities for selected games, and within‐session variable rest times. In the study, the participants sat 1 m‐1.5 m away from the monitor on a pressure platform. Four types of exercise were performed during each session. The first exercise was training for stability and weight shift by balancing the amount of water in a flask. The second was training for stability and weight shift by driving a vehicle. The third exercise was skiing, which involved shifting the body in the anterior, posterior, left, and right directions in three‐dimensional space; and the fourth exercise used a memory recall program, during which the participant had to remember 4 pictures and to match the picture Control intervention: general physical therapy Both groups received general physical therapy. In addition, those in the intervention group received additional 30‐min sessions, 5 d/week for 4 weeks |
|
| Outcomes | Outcomes assessed following intervention Static balance measured using the Good Balance System Balance: Functional Reach Test Visual perception: Motor Free Visual Perception Test |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some dropouts but details of this and method for dealing with this not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol or clinical trial register not mentioned |
Lee 2014a.
| Methods | RCT | |
| Participants | Recruited from a hospital in Korea 21 participants: 10 intervention group, 11 control group Inclusion criteria: > 6 months post stroke, not taking medication that can affect balance, MMSE score of < 24/30, no pain or disability associated with acute musculoskeletal conditions, sitting to sidelying with moderate assistance, sitting for > 10 s without support and standing without support for 1 min Exclusion criteria: Pusher syndrome Mean (SD) age: intervention group 47.9 (12) years, control group 54 (11.9) years 67% men Timing post stroke: intervention group mean (SD) 11.7 (4.5) months, control group mean 11.0 (4.7) months |
|
| Interventions | VR intervention: augmented reality had 3 stages and 16 scopes. The stages progressed from exercise programs in lying position to sitting to standing using a therapeutic ball or foothold. The VR included videos of postural control training for guiding the participants to perform ideal postural control motions. The head‐mounted device showed 2 views: the modelled movement was on one side and the actual movement on the other side. The participant could watch the modelled movement and listen to a recorded sound in order to compare the normal movement with his/her own movement. This was completed in addition to usual physiotherapy sessions Control intervention: no intervention except for usual physiotherapy sessions Sessions were 30 min/d for 4 weeks |
|
| Outcomes | Outcomes assessed post intervention Timed Up and Go Test Berg Balance Scale Gait (measured using the GAITRite system ‐ gait velocity, cadence, step length, and stride length) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Drawing lots |
| Allocation concealment (selection bias) | High risk | Participant selection from box (paper had either number 1 or 2 on) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of dropouts and ITT analysis performed (last observation carried forward) |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or clinical trial registry |
Lee 2015a.
| Methods | RCT | |
| Participants | Recruited from a hospital in Seoul 24 participants: 12 intervention, 12 control Inclusion criteria: stroke of > 6 months duration; a score of > 24 points on the MMSE‐K; ability to walk a distance of 10 m with or without an auxiliary device; no history of orthopedic conditions involving the lower limbs; ability to follow instructions and perform the exercise programs; and no visual or hearing impairment Exclusion criteria: failure to meet above criteria Mean (SD) age: intervention group 45.91 (12.28) years control group 49.16 (12.85) years 66% men |
|
| Interventions | VR intervention: Wii and Wii Balance Board provided by Nintendo (Kyoto, Japan) and the Wii Fit Plus software were used. The VR‐based program was selected depending on the participants' interests and motivation, and the levels of difficulty were decided based on information provided in previous studies regarding suitable levels for balance improvement. The program consisted of: (1) sitting posture, (2) the knee bend and the other leg knee extend, (3) tightrope walking, (4) penguin teeter‐totter seesaw, (5) balance skiing, (6) rolling marble board, and (7) balance Wii Control intervention: the duration of the task‐oriented training program was 30 min. Each task took 3 min to perform, and a 1‐min break was provided between tasks. Each of the warm‐up and cooldown phases lasted for 2 min. The level of difficulty and frequency for each task were gradually increased during the 6 weeks with the participants’ consent, starting with 3 sets (12 times/set) All the participants also received general exercise therapy for 60 min/d, 5 d/week for 6 weeks. They participated in the VR‐based training program or task‐oriented training for an additional 30 min/d, 3 d/week for 6 weeks. |
|
| Outcomes | Measured outcomes post intervention Balance: Functional reach test |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Lee 2015b.
| Methods | RCT | |
| Participants | Recruited from a general hospital in Korea 18 participants: 10 intervention, 8 control Inclusion criteria: diagnosed with stroke and hemiparesis; able to follow verbal instructions; ≥ 6 months post‐stroke diagnosed by a physician; able to communicate (i.e. MMSE language section score from 24‐30), and a Modified Ashworth Scale (MAS) score < 2 for the UE Exclusion criteria: diplegia or a visual field defect Mean (SD) age: intervention group 69.2 (5.5) years, control group 73.1 (8.9) years 45% men, 55% right hemiparesis Timing post stroke: intervention group mean (SD) 16.2 (6.5) months, control group 17 (6.5) months |
|
| Interventions | VR intervention: the VR‐based bilateral training (VRBT) involved a visual expression technique using animations and provided cognitive information for feedback. The animation consisted of symmetric and asymmetric upper‐extremity training as well as symmetric and asymmetric upper‐extremity training at 45° in a VR environment. The participants performed each movement for 4 min and then rested for 1 min to minimise fatigue. Depending on the severity of the deficits, the participant either grasped the handles or the affected hand was strapped to the handle. An UE instrument was used to control the inclination and width. A laptop, webcam, and monitor were used to create the VR environment Control intervention: the therapy program involved only bilateral UE exercises Both groups received conventional physical therapy: sessions were 30 min, 3 times/week for 6 weeks Both groups received additional therapy (either intervention or control) for 30 min, 3 times/week for 6 weeks |
|
| Outcomes | Outcomes were assessed post intervention Electroencephalography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
Levin 2012.
| Methods | RCT | |
| Participants | Recruited from an outpatient rehabilitation centre in Israel 12 participants: 6 intervention, 6 control Inclusion criteria: unilateral left‐ or right‐sided stroke > 3 months previously. No hemispatial neglect or uncorrected visual field deficits including hemianopia and could understand and follow instructions (no receptive aphasia, MMSE evaluation) Exclusion criteria: shoulder or arm pain, lack of endurance as judged by their treating physician Mean (SD) age: intervention group 58.1 (14.6) years, control group 59.8 (15.1) years 50% men Stroke details: 58% right hemiplegia Timing post stroke: intervention group mean 2.6 (1.2) years, control group mean 3.8 (0.9) years |
|
| Interventions | VR intervention: goal‐directed reaching tasks using the affected arm in a virtual environment (virtual supermarket, birds and balls, soccer, volleyball, VMall). Practice involved reaching but not grasp or manipulation. Task difficulty was matched to capabilities Control intervention: OT including exercises reaching for and holding cones, cups and other objects with and without external loading Sessions were 45 min for 9 sessions over a 3‐week period |
|
| Outcomes | Assessed post intervention and 4 weeks after the end of intervention Fugl Meyer Arm Scale Composite Spasticity Index Reach Performance Scale for Stroke Upper limb activity: box and blocks test Upper limb activity: Wolf Motor Function Test Motor Activity Log Adverse events |
|
| Notes | NCT01388400 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin toss |
| Allocation concealment (selection bias) | Low risk | As above ‐ coin toss |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of withdrawals |
| Selective reporting (reporting bias) | Low risk | Reported on clinical trial registry |
Linder 2015.
| Methods | RCT | |
| Participants | Recruited from outpatient services in the USA 99 participants: 51 intervention, 48 control Inclusion criteria: unilateral stroke within the previous 6 months with some voluntary UE movement (score of 11‐55 on the Fugl Meyer Assessment). Limited access to an organised stroke rehabilitation program and preserved cognitive function Exclusion criteria: lack of independence before the stroke (Modified Rankin Scale score of > 1) and injection to manage hypertonicity in the UE since stroke. Neglect (measured by > 3 errors on the star cancellation test), sensory loss score of ≥ 2 on the sensory item of the National Institutes of Health Stroke Scale and score of ≥ 3 on the Modified Ashworth Scale Mean (SD) age: intervention group 59.4 (13.6) years, control group 55.5 (12.6) years 65% men Stroke details: 49% right hemiplegia Timing post stroke: intervention group mean 117 (50.9) d, control group 125 (47) d |
|
| Interventions | VR intervention: Hand Mentor Pro Robot assisted device uses a pneumatic pump to facilitate active‐assisted movement of the wrist and fingers. The device consists of 3 components: a computer control box, an arm unit and data‐collection device and a communications module. The arm unit stabilises the forearm so that the user is able to isolate the wrist and finger movement with the assistance of the pneumatic pump and the computer control box provides targeted goals with corresponding visual and auditory feedback. Feedback from the session is displayed on the screen and stored (including time of use, attempted and successful repetitions, wrist angle and pneumatic pressure) Control intervention: UE home exercise program prescribed by a therapist from a pool of exercises and activities. Weekly telephone calls were made to progress the program. Each participant was given an exercise book with instructions Robotic sessions were 2 h/d, 5 d/week for 8 weeks within a 12‐week period Home exercise program was 1 h/d, 5 d/week for 8 weeks within a 12‐week period Sessions were conducted with a physiotherapist or occupational therapist |
|
| Outcomes | Outcomes assessed post intervention Stroke Impact Scale Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Notes | Disclosure: one author was Chairman of the Scientific Advisory Board and was previously a paid consultant for Kinetic Muscles. A second author was a paid consultant for Kinetic Muscles for this study NCT01144715 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program |
| Allocation concealment (selection bias) | Low risk | Computer‐based program |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted |
| Selective reporting (reporting bias) | High risk | Paper only reports 2 outcomes but others were described in the protocol |
Llorens 2015.
| Methods | RCT | |
| Participants | Recruited from an outpatient rehabilitation unit in Spain 20 participants: 10 intervention, 10 control Inclusion criteria: people with stroke attending a rehabilitation program. Had hemiparesis and were aged 40+ years but ≤ 70 years. Had a stroke > 6 months ago and had absence of cognitive impairment (MMSE of ≥ 24/30). Able to follow instructions and able to maintain stride‐standing position for 30 s without assistance from another person Exclusion criteria: severe dementia or aphasia (Mississippi Aphasia Screening Test < 45), visual or hearing impairment restricting ability to interact with the intervention, hemispatial neglect and ataxia or cerebellar symptoms Mean (SD) age: intervention group 58.3 (11.6) years, control group 55.0 (11.6) years 45% men Stroke details: 65% ischaemic Timing post stroke: intervention group mean 407 (232) d, control group mean 587 (222) d |
|
| Interventions | Intervention: 30 min conventional training plus 30 min of virtual rehabilitation. The set‐up consisted of a computer, audiovisual output system and motion tracking system. The output system consisted of a video display and audio system. The participant was immersed in a 3D environment; their feet were represented by 2 shoes that mimicked their movement in the real world. The objective of the task was to reach the items with 1 foot while maintaining the other foot within the circle. Conducted by a physiotherapist Control intervention: 1 h of conventional physiotherapy including balance exercises, task‐specific reaching, stepping and walking under different conditions. Conducted by a physiotherapist Sessions were 60 min, 5 times/week for 4 weeks |
|
| Outcomes | Outcomes assessed post intervention Berg Balance Scale Balance and gait subscales of the Tinetti Performance Oriented Mobility Assessment Brunel Balance Assessment 10 m walking test Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Concealed in envelopes. Not clear whether they were opaque or not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded therapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low withdrawals and analysis included only those contributing data |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
Low 2012.
| Methods | RCT | |
| Participants | 20 participants: 10 intervention, 10 control Inclusion criteria: diagnosis of stroke and medically stable Mean age 60.4 (13.3) years (total sample) 65% men Timing post stroke: 14.21 (5.5) d |
|
| Interventions | VR intervention: locally developed VR program Control intervention: usual care The VR group received an additional 30 min of daily VR therapy for 2 weeks |
|
| Outcomes | Fugl Meyer Motor Scale (upper limb) Action Research Arm Test Berg Balance Scale Functional Independence Measure Gait speed |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Not reported (conference abstract) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | Not reported (conference abstract) |
Manlapaz 2010.
| Methods | RCT | |
| Participants | Recruited from rehabilitation centres in Manila, Phillipines 16 participants: 8 intervention, 8 control Inclusion and exclusion criteria: not reported Mean age: 55.69 (9.88) for the total sample 69% men Timing post stroke: mean 38.56 (14.51) months |
|
| Interventions | VR intervention: Nintendo Wii Control intervention: not reported Intervention was provided twice/week for 6 weeks |
|
| Outcomes | Outcomes assessed post intervention Fugl Meyer Motor Assessment Scale Fast Fourier Transform (FFT) analysis |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that participants were randomised using the 'fishbowl' method |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Details not reported (conference abstract) |
Mao 2015.
| Methods | RCT | |
| Participants | Recruited from an inpatient hospital in China 23 participants: 11 intervention, 12 control Inclusion criteria: stroke (confirmed by CT or MRI), stable vital signs, aged 40‐78 years, able to walk independently for 10 m, unilateral hemipareses for < 3 months resulting from first stroke and residual gait impairment (reduced walking speed) and adequate mental and physical capacity to attempt the tasks as instructed Exclusion criteria: history of recent deep vein thrombosis of the lower limbs, other neurological or orthopedic pathology, or serious visual deficits Mean (SD) age: intervention group 58.18 (11.15) years, control group 63.09 (11.51) years 78% men Timing post stroke: intervention group mean 48.91 (17.01) d, control group mean 48.91 (17.92) d |
|
| Interventions | VR intervention: a series of videos (e.g. climbing a mountain, crossing a street) was shown on screen and synced with treadmill velocity. The participant wore a harness to support body weight Control intervention: individualised walking training on the ground according to neurodevelopmental therapy Both of the groups received training of 20‐40 min/d, 5 d/week, for 3 weeks |
|
| Outcomes | Outcomes assessed post intervention Motion analysis system (Vicon) to measure pelvic tilt, obliquity and rotation |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation program |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described in sufficient detail to make judgement |
| Selective reporting (reporting bias) | Low risk | Registered on clinical trial and all measures reported |
Matsuo 2013.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation inpatient unit in Japan 28 participants No further details reported |
|
| Interventions | VR intervention: 10 sessions of upper limb exercises via a Nintendo Wii over 2 weeks in addition to conventional rehabilitation Control intervention: conventional rehabilitation |
|
| Outcomes | Outcomes assessed post intervention and 2 weeks after the end of intervention Fugl Meyer Assessment of Upper Limb Motor Function Wolf Motor Function Test Box and Block Test Motor Activity Log |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Details not reported (conference abstract) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported (conference abstract) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not reported (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | Details not reported (conference abstract) |
Mazer 2005.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation hospital in Quebec, 2 driving evaluation centres in Montreal and from a private driving evaluation clinic 39 participants: 20 intervention, 19 control Inclusion criteria (for stroke participants): people with a diagnosis of stroke that did not pass the driving tests at a recognised driving evaluation service. Had licence to drive and were driving prior to the stroke and desire to return to driving Exclusion criteria: medical condition precluding driving (for example, hemianopia, seizures), received their driving evaluation > 2 years post diagnosis, unable to communicate in English or French, inadequate communication of basic verbal instructions or judged as dangerous by the therapist in the on‐road evaluation Mean (SD) age: intervention group 68 (14) years, control group 69 (9) years Stroke details: 31% right hemiparesis Timing post stroke: intervention group mean (SD) 1.4 (1) years, control group 1.7 (1) years |
|
| Interventions | VR intervention: driving simulator. Simulator is a car frame with 3 large screens providing a large field of view. Participants were progressed through 4 increasingly complex scenarios. In level 1, participants were familiarised with the simulator and controls; level 2 involved a simulated road circuit without traffic; level 3 focused on performing different driving manoeuvres and level 4 involved a variety of traffic conditions (for example, rain, wind, reduced visibility, pedestrians). Instant feedback was provided by the simulator when errors were made Control intervention: no intervention provided Sessions were 60 min, twice/week for 8 weeks (16 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention (or after 8 weeks for the control group) Activity limitation outcomes: DriveAble Testing Ltd Driver Evaluation |
|
| Notes | Note that this study also recruited 6 participants with traumatic brain injury. However, data for participants with stroke were able to be separated. This review reports on the stroke data only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer program to generate |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants (5 control group, 2 simulator group) did not complete the outcome evaluation and were therefore considered to have dropped out from the study. Analysis was completed based on the actual number of participants contributing data. ITT analyses were conducted |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
McNulty 2015.
| Methods | RCT | |
| Participants | Recruited from hospitals in Australia 41 participants: 21 intervention group, 20 control group Inclusion criteria: ischaemic lesion or haemorrhagic stroke with upper limb motor impairment; 2‐48 months post stroke; ≥ 10° active movement at the shoulder, elbow, wrist and ≥ 2 digits; English speaking and ≥ 18 years Exclusion criteria: MMSE score of < 24/30; peripheral neuropathy significantly affecting sensorimotor function; unstable blood pressure; and formal upper limb therapy during the trial. Mean (SD) age: intervention group 59.9 (13.8) years, control group 56.1 (17) years 76% men Stroke details: 79% ischaemic Timing post stroke: intervention group mean (SD) 11.0 (3.1) months, control group 6.5 (2.1) months |
|
| Interventions | VR intervention: Nintendo Wii Sports (golf, boxing, baseball, bowling and tennis) with the controller used in the person's more affected hand. Rather than playing each game, specific drills were introduced and varied. For people with poor grip strength, a self‐adhesive wrap was applied. Therapy was performed in standing position wherever possible Control intervention: modified constraint‐induced movement therapy: participants wore the mitt on the less affected hand for up to 90% of waking hours. Therapy included shaping practice tailored to each person's motor function with increasing task complexity, strength, dexterity, movement distance and speed. Training tasks included everyday activities using the more affected arm for 15‐20 min of continuous activity Therapy for both groups was delivered in the research institute or the person's home by a trained therapist. Dose was matched Sessions were 60 min on 10 consecutive weekdays augmented by progressively increasing home practice |
|
| Outcomes | Outcomes assessed post intervention and at 6 months Upper limb outcomes: Wolf Motor Function Test timed tasks Motor Activity Log Quality of Movement Scale Fugl Meyer assessment Wolf Motor Function Test, maximal strength and submaximal strength Active and passive ROM Modified Ashworth Scale Box and Block Test Self‐perceived improvement and participant satisfaction questionnaire Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed in numbered, opaque envelopes prior to trial commencement by a person not involved with assessments or therapy and opened by the therapist after baseline assessments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded therapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Transparent reporting and ITT analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Could not find reference to study protocol or trial registration |
Mirelman 2008.
| Methods | RCT | |
| Participants | Study took place in New Jersey, USA 18 participants: 9 intervention, 9 control Inclusion criteria: chronic hemiparesis after stroke with residual gait deficits, partial antigravity dorsiflexion, able to walk 15 metres without the assistance of another person, sufficient communication and cognitive ability to participate Exclusion criteria: motion sickness and receiving concurrent therapy Mean (SD) age: intervention group 62 (10) years, control group 61 (8) years 83% men Stroke details: 44% right hemiparesis Timing post stroke: intervention group mean (SD) 38 (25) months, control group 58 (26) months |
|
| Interventions | VR intervention: Rutgers ankle rehabilitation system (a 6‐degree‐of‐freedom platform force‐feedback system) that allows participants to exercise the lower extremity by navigating through a virtual environment displayed on a desktop computer. Participants executed the exercises by using the foot movements to navigate a plane or a boat through a virtual environment that consisted of a series of targets Control intervention: Rutgers ankle rehabilitation system without the virtual environment. Participants were instructed by the therapist on which direction to move their foot and were paced by a metronome cueing them to complete a comparable number of repetitions Sessions were 60 min, 3 times/week for 4 weeks (12 h total) |
|
| Outcomes | Outcomes recorded at baseline, post intervention and at 3 months Lower limb function and activity outcomes: gait speed over 7‐m walkway, 6MWT, Patient Activity Monitor (distance walked, number of steps/d, average speed, step length, top speed) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed based on the table of numbers method (generated by a computer) |
| Allocation concealment (selection bias) | Low risk | Allocation was done by an external person to the project and held in a database spreadsheet on a computer in his office which was password protected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in the robotic‐VR group was lost to follow‐up because of personal reasons. 1 outlier was identified in the robotic‐VR group following the descriptive analysis of the endurance test (6MWT), the values presented for this individual were 2 SD from the mean therefore he was excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Morone 2014.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation unit in Italy 50 participants: 25 intervention, 25 control Inclusion criteria: hemiparesis in the subacute phase (< 3 months from onset), with moderate gait deficits (FAC ≥ 2) caused by a first ever stroke and aged 18‐85 years Exclusion criteria: motor or cognitive sequale from prior cardiovascular accidents, other chronic disabling pathologies, orthopaedic injuries that could impair locomotion, spasticity that limited lower extremity ROM to < 80%, sacral skin lesions, MMSE score < 24/30 and hemispatial neglect, attention or memory deficit Mean (SD) age: intervention group 58.36 (9.62) years, control group 61.96 (10.31) years Stroke details: 58% right hemiparesis Timing post stroke: intervention group mean (SD) 61 (36.47) d, control group mean (SD) 41.65 (36.89) d |
|
| Interventions | VR intervention: balance therapy using the Nintendo Wii Fit. During the intervention, 3 games were carried out in order to train balance, co‐ordination and endurance under the supervision of a physiotherapist: hula hoop, bubble blower and sky slalom Control intervention: balance therapy focusing on trunk stabilisation, weight transfer to the paretic leg and exercise with Freeman board for balance and proprioception Sessions for the VR and control interventions were 20 min, 3 times/week for 4 weeks. This was in addition to usual physical therapy which was 40 min, twice/d |
|
| Outcomes | Outcomes assessed post intervention and 1 month after the end of intervention Berg Balance Scale 10 mwalk test at a self‐selected speed Functional Ambulatory Category Barthel Index |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Multiple withdrawals and unbalanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol or trial registration not reported |
Nara 2015.
| Methods | RCT | |
| Participants | Recruited in Korea 20 participants: 10 intervention group, 10 control group Inclusion criteria: history of stroke onset of > 6 months prior to the study; ability to walk without using a walking aid for a minimum of 15 m; MMSE score of > 24/30; able to comprehend and follow simple instructions Exclusion criteria: other neurological condition, orthopaedic disease or visual impairment Participant details not reported |
|
| Interventions | VR intervention: community‐based VR scene exposure combined with treadmill training. A VR video was displayed on a screen 3 m in front of the treadmill using a video projector. The VR video comprised images of community ambulation, such as walking on sidewalks, level walking, slope walking and walking over obstacles. 5 min of treadmill training was followed by 2 min rest to minimise fatigue Control intervention: muscle strengthening, balance training, indoor and outdoor gait training Both groups had conventional physical therapy for 60 min/d, 5 d/week for 4 weeks The VR and control intervention was an additional 30 min/d, 3 d/week for 4 weeks |
|
| Outcomes | Outcomes assessed post intervention Static balance ability (postural sway path length and speed at the center of pressure) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded participants with low participation rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Piron 2007.
| Methods | RCT | |
| Participants | Study took place in Italy 38 participants: 25 intervention, 13 control Inclusion criteria: mild‐intermediate arm motor impairment due to ischaemic stroke in the MCA territory within the past 3 months Exclusion criteria: cognitive impairment, neglect, apraxia, aphasia interfering with comprehension Mean (SD) age: intervention group 62 (9) years, control group 61 (7) years 66% men Timing post stroke: intervention group mean (SD) 2.5 (1.5) months, control group 2.6 (1.6) months |
|
| Interventions | VR intervention: magnetic receivers were positioned on the participant's arm. As the participant grasped and moved real objects, software created a virtual environment, which displayed virtual handling and target objects, for example an envelope and a mailbox, a hammer and a nail, a glass and a carafe. While performing the virtual tasks such as putting the envelope in the mailbox the participant moves the real envelope and sees on screen the trajectory of the corresponding virtual objects toward the virtual mailbox. Participants could see not only their own movement but also the correct trajectory that they had to execute, pre‐recorded by the therapist. This allowed participants to easily perceive motion errors and adjust them during the task Control intervention: 'conventional' rehabilitation focused on the upper limb Sessions were 60 min, 5 times/week for 5‐7 weeks (approximately 25‐35 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function and activity outcomes (arm): Fugl Meyer UE Scale Activity limitation outcomes: Functional Independence Measure Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with the study author reports the use of a simple computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 3 dropouts from the control group and the analysis was per‐protocol |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Piron 2009.
| Methods | RCT | |
| Participants | Study took place in Italy 36 participants: 18 intervention, 18 control Inclusion criteria: single ischaemic stroke in the MCA region with mild to intermediate arm motor impairment (Fugl Meyer UE score 30‐55) Exclusion criteria: clinical evidence of cognitive impairment, apraxia (< 62 points on the 'De Renzi' test), neglect or language disturbance interfering with verbal comprehension (> 40 errors on the Token test) Mean (SD) age: intervention group 66 (8) years, control group 64 (8) years 58% men Stroke details: 44% right hemiparesis Timing post stroke: intervention group mean (SD) 15 (7) months, control group 12 (4) months |
|
| Interventions | VR intervention: the telerehabilitation program used 1 computer workstation at the participant's home and 1 at the rehabilitation hospital. The system used a 3D motion tracking system to record arm movements through a magnetic receiver into a virtual image. The participant moved a real object following the trajectory of a virtual object displayed on the screen in accordance with the requested virtual task. 5 virtual tasks comprising simple arm movements were devised for training Control intervention: specific exercises for the upper limb with progressive complexity. Started with control of isolated movements without postural control, then postural control including touching different targets and manipulating objects Sessions were 60 min, 5 times/week for 4 weeks (20 h total) |
|
| Outcomes | Outcomes recorded at baseline, post intervention and at 1 month Upper limb function and activity outcomes (arm): Fugl Meyer UE Scale Participation restriction and quality of life outcomes: Abilhand scale Other outcome measures: Modified Ashworth Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with the study author reports the use of a simple computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Piron 2010.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation hospital in Rome, Italy 50 participants: 27 intervention, 23 control Inclusion criteria: single ischaemic stroke in the MCA territory > 6 months ago demonstrated by CT or MRI, received conventional physiotherapy early after stroke, mild‐intermediate motor impairments of the arm (score of 20‐60 on the Fugl Meyer UE Scale) Exclusion criteria: clinical history or evidence of cognitive impairments, neglect, apraxia or aphasia interfering with verbal comprehension Mean (SD) age: intervention group 59 (8) years, control group 62 (10) years 58% men Stroke details: 58% right hemiparesis Timing post stroke: intervention group mean 15 (13) months, control group 15 (12) months |
|
| Interventions | VR intervention: participants were asked to perform motor tasks with real objects (for example an envelope or a glass), which were displayed as tasks within the virtual environment (for example putting an envelope in the mailbox, breaking eggs, moving a glass over a table, placing a ball in a basket). A 3D magnetic receiver was used to record the motions. Participants were asked to emulate the tasks as per the therapist's pre‐recorded movement Control intervention: participants were asked to perform specific exercises for the arm, for example touching different targets, manipulating objects and following trajectories on a plan Sessions were 60 min, 5 times/week for 4 weeks (20 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function and activity outcomes (arm): Fugl Meyer UE Scale Activity limitation outcomes: Functional Independence Measure Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with the study author reports the use of a simple computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was completed. In the case of missing data the authors used a 'best, worst and likely' approach to data imputation. There was a small amount of attrition and the reasons for this were reported. |
| Selective reporting (reporting bias) | Low risk | No other outcomes were collected |
Prange 2015.
| Methods | RCT | |
| Participants | Recruited from an inpatient rehabilitation centre in the Netherlands 70 participants: 37 intervention, 33 control Inclusion criteria: first stroke 1‐12 weeks ago, medically stable, display limited arm function but have active control of the elbow/shoulder of ≥ 15°, be free from other conditions or pain, be able to follow instructions and understand (and see) the visual game display Exclusion criteria: treated with botulinum toxin and/or electrical stimulation to improve arm function before or during participation Mean (SD) age: intervention group 60.3 (9.7) years, 58 (11.4) years Stroke details: 78% ischaemic, 60% right hemiparesis Timing post stroke: intervention group mean 7.3 (3.4) years, control group mean 6.8 (3.1) years |
|
| Interventions | VR intervention: training using a customised arm support program. Training consisted of playing games with the affected arm, supported by the device, working toward maximising movement ability with as little arm support as possible. The training involved mostly shoulder and elbow movements with exercises structured according to categorisation of the games for increasing difficulty (1D, 2D and 3D) Conventional therapy: standard set of exercises to reflect usual physiotherapy and OT Sessions were 30 min, 3 times/week for 6 weeks |
|
| Outcomes | Outcomes assessed post intervention Fugl‐Meyer assessment UE Maximal reach distance Stroke Upper Limb Capacity Scale (SULCS) Visual Analogue Scale for arm pain Intrinsic Motivation Inventory post training |
|
| Notes | NTR2539 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 withdrawals and both withdrew due to inadvertent concurrent treatment |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per trial registration |
Rajaratnam 2013.
| Methods | RCT | |
| Participants | Recruited from a community rehabilitation hospital in Singapore 19 participants: 10 intervention, 9 control Inclusion criteria: recent first stroke with moderate or moderate‐severe disability (Modified Rankin Scale Grade 3 or 4) Participants were haemodynamically stable and had a MMSE score of > 23 Exclusion criteria: terminal illness, uncontrolled hypertension and angina and severe spatial neglect or visual impairments Mean (SD) age: intervention group 58.67 (8.62) years, control group 65.33 (9.59) years 37% men Stroke details: 42% right hemiparesis Timing post stroke: intervention group mean (SD) 14.7 (7.5) d, control group 15.2 (6.3) d |
|
| Interventions | VR intervention: used either a Nintendo Wii Fit or Microsoft Kinect program during rehabilitation. The Nintendo Wii Fit was performed in standing and the Kinect was performed in sitting and standing. Sessions involved 40 min of conventional therapy and 20 min of VR Control intervention: conventional therapy (not described). Sessions involved 60 min of conventional therapy Sessions were 60 min for 15 sessions (approximately 15 h) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Gait outcomes: Timed Up and Go Test Balance function: Berg Balance Scale, Functional Reach Test, centre of pressure |
|
| Notes | Activity limitation outcomes: Modified Barthel Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to ascertain |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Reinkensmeyer 2012.
| Methods | RCT | |
| Participants | Recruited from local hospitals and stroke support groups in Orange County, California 26 participants: 13 intervention, 13 control Inclusion criteria: single stroke and ≥ 3 months post stroke; moderate‐severe weakness in their affected upper limbs, defined by the upper limb Fugl Meyer Motor Scale (score of 10‐35/66) Exclusion criteria: significant pain, instability or subluxation of the affected shoulder, severe elbow or wrist contractures, concurrent severe medical problems, cognitive dysfunction to the extent that would interfere with therapy participation, visual deficits, severe neglect or apraxia and current enrolment in ongoing upper limb therapy Mean (SD) age: intervention group 60 (10) years, control group 61 (13) years Stroke details: 50% ischaemic, 31% haemorrhagic, 19% unknown Timing post stroke: intervention group mean (SD) 65 (47) months, control group 67 (56) months |
|
| Interventions | VR intervention: Pneu‐WREX is a robotic device (4‐degree‐of‐freedom robot based on a passive arm support (WREX)). It is a lightweight exoskeleton that allows a wide ROM of the arm in a 3D space. The degrees of freedom are elbow flexion/extension, shoulder abduction/adduction, shoulder flexion/extension and shoulder forward/backward translation.The device can provide assistance as needed for a patient to actively participate and to be able to perform 3D tasks. Hand training through grasp and release is incorporated through a grip sensor that measures the pressure of a water‐filled cylinder bladder that the user holds, to detect even trace finger movement. A software package called Vu Therapy allowed for interface between the hardware and software. Tasks included grocery shopping, cleaning a window, playing basketball and driving a car. Auditory and visual feedback and a game score were provided to maintain attention and interest Control intervention: conventional exercises including ROM and task‐oriented movements Sessions were 60 min, 3 times/week for 8‐9 weeks (total = 24) for both groups |
|
| Outcomes | Outcomes assessed post intervention and 3 months following the end of intervention Arm Motor section of the Fugl Meyer Scale Rancho Functional Test for the hemiplegic UE Motor Activity Log Box and Blocks Test Grip strength (Jamar) Adverse events reported |
|
| Notes | Disclosure reported that the lead author has a financial interest in Hocoma, a company that makes robotic therapy devices | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in detail |
| Selective reporting (reporting bias) | Unclear risk | Unable to ascertain (does not mention protocol or trial registration) |
Saposnik 2010.
| Methods | RCT | |
| Participants | Recruited from a subacute rehabilitation facility in Toronto, Canada 22 participants: 11 intervention, 11 control Inclusion criteria: 18‐85 years with first time ischaemic or haemorrhagic stroke within the last 6 months, Chedoke McMaster scale (UE) score of > 3 in the arm or hand Exclusion criteria: unable to follow instructions, pre‐stroke Modified Rankin Score of ≥ 2, medically unstable or with uncontrolled hypertension, severe illness with life expectancy of < 3 months, unstable angina, recent MI (within 3 months), history of seizures or epilepsy, participating in another clinical trial involving an investigational drug or physical therapy, any condition that might put the patient at risk (for example, known shoulder subluxation) Mean age: intervention group 55 years, control group 67 years 64% men Stroke details: 45% right hemiparesis Timing post stroke: intervention group mean (SD) 27 (16) d, control group 23 (9) d |
|
| Interventions | VR intervention: participants used the Nintendo Wii gaming console playing 'Wii sports' and 'Cooking Mama' Control intervention: leisure activities including cards, bingo and Jenga Sessions were 60 min for 8 sessions (8 h total) |
|
| Outcomes | Outcomes recorded at baseline, post intervention and at 1 month Upper limb function and activity outcomes (arm): abbreviated version of the Wolf Motor Function Test Upper limb function and activity outcomes (hand): Box and Block test, Grip strength (kg) Participation restriction and quality of life: Stroke Impact Scale (hand function, composite function, perception of recovery) Adverse events reported Other outcomes: therapy time |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using a basic computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some attrition was reported. Outcomes were calculated based on the number of participants and there was no reporting of imputation of data. ITT analysis was completed |
| Selective reporting (reporting bias) | Low risk | Reports on all measures reported in the study protocol paper |
Saposnik 2016.
| Methods | RCT | |
| Participants | Recruited from rehabilitation units in 4 countries: Canada, Argentina, Peru, Thailand 141 participants: 71 intervention group, 70 control group Inclusion criteria: 18‐85 years with first time ischaemic stroke within 3 months of enrolment and with mild to moderate motor disability (Chedoke McMaster Stroke Assessment stage > 3) Exclusion criteria: no disability in the UE (arm components of the Chedoke McMaster scale = 7), were unable to follow instructions, pre‐stroke Modified Rankin score of ≥ 2, medically unstable or uncontrolled hypertension; severe illness with a life expectancy of < 3 months, unstable angina or MI within 3 months, history of seizures or epilepsy (except for febrile seizures of childhood); participating in another clinical trial involving an investigational drug or physical therapy or had any condition that might put the patient at risk (e.g. known shoulder subluxation) Mean (SD) age: intervention group 62 (13) years, control group 62 (12) years Stroke details: 100% ischaemic; right hemiparesis 47% Timing post stroke: intervention group mean 27 d, control group mean 24.5 d |
|
| Interventions | VR intervention: Nintendo Wii Sports and Game Party 3. Progression through the intervention allowed participants to choose some specific activities within those games (last 3 min of the intervention) based on their capabilities and interest with the goals of enhancing flexibility, ROM, strength and co‐ordination of the affected arm Control intervention: recreational therapy with progression through activities such as cards, bingo, Jenga or a ball game Administered 1:1 by a rehabilitation therapist Sessions were 60 min, 5 times/week for 2 weeks |
|
| Outcomes | Outcomes were recorded at 2 weeks (post intervention) and 4 weeks Abbreviated Wolf Motor Function Test Box and Block Test Quality of life after stroke ‐ Stroke Impact Scale Functional Independence Measure, Barthel Index, Modified Rankin Scale Grip strength (dynamometer) Hand function ‐ Stroke Impact Scale Adverse events reported |
|
| Notes | NCT01406912 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated assignment |
| Allocation concealment (selection bias) | Low risk | Assignment at the point enrolment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted. Details of withdrawals reported transparently |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Shin 2014.
| Methods | RCT | |
| Participants | Recruited from 2 rehabilitation units and the neurorehabilitation ward of a hospital in Korea 16 participants: 9 intervention, 7 control Inclusion criteria: hemiparetic upper limb dysfunction due to first‐ever stroke, mild‐to‐severe deficits of the paretic UE (2‐4 on the MRC Scale and 2‐5 on the Brunnstrom Stage of motor recovery) Exclusion criteria: pre‐existing arm impairment, any painful condition affecting the upper limbs, difficulty in sitting for ≥ 20 min, severe cognitive impairment (MMSE score < 10 points) and severe aphasia Mean (SD) age: intervention group 46.6 (5.8) years, control group 52.0 (11.9) years 50% men Stroke details: 38% right lesion Timing post stroke: intervention group mean (SD) 76.6 (28.5) d, control group 67.1 (45.3) d |
|
| Interventions | VR intervention: RehabMaster™. The participant sits in a chair in front of a monitor. The therapist can control the program and level of difficulty. Rehabilitation games were designed to combine rehabilitation exercises with gaming elements. The 4 games suggested were goalkeeper, bug hunter, underwater fire and rollercoaster Control intervention: conventional OT Sessions were 20 min of OT. The intervention group received an additional 20 min of VR. The duration of intervention was 10 sessions over 2 weeks |
|
| Outcomes | Outcomes recorded at baseline and post intervention Upper limb function outcomes: Fugl Meyer Activity limitation outcomes; Modified Barthel Index Other outcomes: passive ROM of the upper limb, MRC Score |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported except for the SF36 measure, which will be reported in a subsequent publication |
Shin 2015.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation hospital in Seoul, Korea 35 participants: 18 intervention, 17 control Inclusion criteria: aged ≥ 18 years with chronic hemiparetic upper limb dysfunction, secondary to a first ever stroke. MRC Scale scores of 2‐4 (inclusive) and a Brunnstrom motor recovery stage for the proximal UE of 2‐5 inclusive Exclusion criteria: severe cognitive impairment or aphasia, pre‐existing mental illness or arm impairment, difficulty in sitting for ≥ 30 min and/or uncontrolled medical illness Mean (SD) age: intervention group 53.3 (11.8) years, control group 54.6 (13.4) years 69% men Stroke details: 50% right hemiparesis Timing post stroke: intervention group mean (SD) 202 (89), control group 165 (87) d |
|
| Interventions | VR intervention: game‐based VR using 10 min of rehabilitation training and 20 min of rehabilitation games selected by an occupational therapist to encourage active arm and trunk movements. Participants sat in a chair in front of the monitor and depth sensor and moved according to the training protocol. The difficulty was set by manipulating the ROM or speed of the activity or by manipulating the number, size, location, speed or trajectories of the targets Control intervention: conventional OT including exercises, table top activities and training for ADL Sessions for the VR group were 30 min of VR plus 30 min of conventional OT, 5 d/ week for 4 weeks Sessions for the control group were 60 min of OT, 5 d/week for 4 weeks |
|
| Outcomes | Outcomes assessed post intervention Korean SF36 Korean Hamilton Depression Rating Scale Fugl Meyer Assessment UE Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minor loss to follow‐up. Method of dealing with this in the analysis is not reported |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
Sin 2013.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation hospital in Korea 35 participants: 18 intervention, 17 control Inclusion criteria: > 6 months post stroke, no problems with auditory or visual functioning, active ROM of the shoulder, elbow, wrist and fingers of > 10°, ability to walk > 10 m independently not taking any medication that could influence balance or gait and no severe cognitive disorders (MMSE score of > 16/30) Exclusion criteria: uncontrolled blood pressure or angina, history of seizure, any intervention other than conventional therapy, or refusal to use a video game Mean (SD) age: intervention group 71.78 (9.42) years, control group 75.59 (5.55) years 43% men Stroke details: 66% right hemiparesis Timing post stroke: intervention group mean (SD) 7.22 (1.21) months, control group 8.47 (2.98) months |
|
| Interventions | VR intervention: use of Xbox Kinect for 30 min followed by conventional OT for 30 min. Kinect programs that required use of the UEs were selected Control intervention: conventional OT, which focused on retraining UE and hand function and ADL Sessions were performed 3 times/week for 6 weeks |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb outcomes: Fugl Meyer UE, Box and Block test Other outcomes: UE Active ROM |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | To be determined |
| Selective reporting (reporting bias) | Unclear risk | To be determined |
Song 2015.
| Methods | RCT | |
| Participants | Recruited from a hospital in South Korea 40 participants: 20 intervention group, 20 control group Inclusion criteria: no visual field deficit, no abnormality in the vestibular organs, no orthopaedic disease, an unrestricted ROM, able to understand and perform the exercise as instructed by the researcher and a score of ≥ 24 on the MMSE‐K Exclusion criteria: none reported Mean (SD) age: intervention group mean (SD) 51.37 (40.6) years, control group 50.10 (7.83) years 55% men Stroke details: 48% right hemiparesis Timing post stroke: intervention group 14.75 (6.06) months, 14.30 (3.40) months |
|
| Interventions | VR intervention: Xbox Kinect including Kinect Sport, Kinect Sport Season 2, Kinect Adventure, Kinect Gunstringer. Mostly sports programs such as bowling, skiing, golf, ground walking, walking over obstacles and climbing stairs were used for training Control intervention: ergometer bicycle training using a Motomed Viva 2. The Motomed provides detailed feedback, software‐controlled therapy programs and motivation and training games Sessions for both interventions were 30 min, 5 d/week for 8 weeks |
|
| Outcomes | Outcomes assessed post intervention Balance (biofeedback analysis system) Timed Up and Go Test 10 Minute Walk Test |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detail not reported |
| Allocation concealment (selection bias) | Unclear risk | Detail not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Detail not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Detail not reported |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
Standen 2011.
| Methods | RCT | |
| Participants | Study took place in the UK 27 participants: 17 intervention, 10 control Inclusion criteria: ≥ 18 years, no longer receiving any other intensive rehabilitation and still had residual upper limb dysfunction Exclusion criteria: failure to meet above criteria Mean (SD) age: intervention group 59 (12.03) years, control group 63 (14.6) years 59% men Timing post stroke: intervention group mean (SD) 38 (41.28) weeks, control group 24 (36.26) weeks |
|
| Interventions | VR intervention: virtual glove which translates the position of the hand into gameplay. Participants were instructed to use the program at home Control intervention: usual care (no specific intervention) Sessions were 20 min, 3 times/d for 8 weeks (approximately 52 h) |
|
| Outcomes | Outcomes recorded at baseline, 4 weeks and post intervention (8 weeks) Upper limb function outcome: Wolf Motor Function Test, Nine Hole Peg Test Other: Motor Activity Log Activity outcomes: Nottingham Extended ADL Scale (NEADL) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Managed externally |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of dropouts in the intervention group |
| Selective reporting (reporting bias) | Low risk | Unpublished data obtained via personal communication |
Subramanian 2013.
| Methods | RCT | |
| Participants | Study took place in Canada 32 participants: 16 intervention, 16 control Inclusion criteria: aged 40‐80 years, sustained single ischaemic or haemorrhagic stroke 6‐60 months previously, scored 3‐6 on the Chedoke McMaster Stroke Assessment arm subscale and had no other neurologic or neuromuscular/orthopaedic problems affecting the upper limb and trunk Exclusion criteria: brainstem or cerebellar lesions, comprehension difficulties and marked apraxia, attention or visual field deficits Mean (SD) age: intervention group 62 (9.7) years, control group 60 (11) years 72% men Stroke details: 47% right hemiparesis Timing post stroke: intervention group mean (SD) 3.7 (2.2) years, control group 3.0 (1.9) years |
|
| Interventions | VR intervention: a 3D virtual environment (CAREN system) simulated a supermarket scene. Participants had to reach for objects in the virtual environment. Training was high in intensity with 72 trials of reaching in each session Control intervention: pointing at targets in a physical environment Sessions were 45 min for 12 d spaced over 4 weeks |
|
| Outcomes | Outcomes were recorded at baseline, post intervention and 3 months following intervention Upper limb outcomes: Fugl Meyer, Reaching Performance Scale for Stroke, Wolf Motor Function Test Adverse events reported Other outcomes: Motor Activity Log‐AS Other outcomes: Motivation Task Evaluation Questionnaire Other outcomes: kinematic data |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Managed by external personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All completed the assessments. Small number of intervention dropouts and balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per entry on clinical trial registry |
Sucar 2009.
| Methods | Quasi RCT | |
| Participants | Recruited from the National Institute of Neurology in Mexico City, Mexico 22 participants: 11 intervention, 11 control Inclusion criteria: ≥ 6 months after stroke Exclusion criteria: none reported Mean age: intervention group 51 years, control group 52 years Timing post stroke: intervention group 22 months, control group 26 months |
|
| Interventions | VR intervention: participants used a 'Gesture Therapy' program designed by the researchers. Movements of the participant's upper limbs are tracked by a camera and the person interacts with on‐screen games. Games included shopping in the supermarket, making breakfast, playing basketball, cleaning, painting and driving Control intervention: a variety of exercises guided by the therapist using equipment such as cones and balls Sessions were 60 min, 3 times/week for 5 weeks (15 h total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Upper limb function and activity outcomes (arm): Fugl Meyer UE scale, Motricity Index Adverse events reported Other outcomes: level of interest, competence, effort, pressure and utility of the intervention |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation based on odd or even numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Low risk | No additional outcomes were collected |
Thielbar 2014.
| Methods | RCT | |
| Participants | Recruited from an outpatient clinic in the USA 14 participants: 7 intervention, 7 control Inclusion criteria: chronic hemiparesis resulting from a single stroke ≥ 6 months prior with mild‐moderate hand impairment as evidenced by a score of 5 or 6 on the Hand subsection of the Chedoke McMaster Stroke Assessment scale. Limitations with fine motor control but able to perform 2 of 3 specified hand movements Exclusion criteria: receiving outpatient physical or OT, biomechanical limitations which limited passive digit extension to 20° of finger flexion; had received botulinum toxin < 6 months prior to enrolment; cognitive deficits limiting simple 1‐step commands or significant UE pain Mean (SD) age: intervention group 54 (7) years, control group 59 (6) years Stroke details: right hemiparesis 43% Timing post stroke: intervention group 46.6 (32.5) months, control group 47.9 (47.4) months |
|
| Interventions | VR intervention: trained with the actuated virtual keyboard (AVK) system to practice movements of different combinations. Participants wore a PneuGlove and pressed virtual keys. Visual displays guided the user as did the therapist. Each key played a unique tone which would play whenever the key was struck Control intervention: high‐intensity task‐oriented OT centred on fine motor control, dexterity, in‐hand manipulation and isolated finger movements. Examples of activities included practise of buttoning, typing, tying knots, writing and using tools Both group had sessions of 60 min, 3 times a week for 6 weeks |
|
| Outcomes | Outcomes assessed post intervention and 1 month after the end of intervention Action Research Arm Test Jebsen Taylor Hand Function Test Fugl Meyer (UE) Grip strength (Jamar dynamometer) Other: Kinematic actuation |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Drawing lots |
| Allocation concealment (selection bias) | Low risk | Drawing lots |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded therapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
Ucar 2014.
| Methods | RCT | |
| Participants | Recruited from an outpatient unit in Turkey 22 participants: 11 intervention, 11 control Inclusion criteria: adult male (> 18 years), capability to ambulate 10 m without personal assistance and not receiving any other physical therapy Exclusion criteria: body weight > 135 kg, FAC score < 3; unable to walk consistently or independently within the community, cognitive deficits, cardiac disease, spasticity of the lower limbs preventing them from robotic walking, traumatic stroke, intracranial space occupying lesion‐induced strokes and seizures Mean age: intervention group 56.2 years, control group 61.5 years 100% men Stroke details not reported |
|
| Interventions | VR intervention: robotic (Lokomat) training with a computer monitor placed in front of the participants. It provided them with biofeedback of their performance Control intervention: conventional physiotherapy in the home environment. Home exercise focused on gait and body weight support on the paretic leg. Also included active assisted exercises, leg strengthening and balance training Both groups received 30‐min sessions, 5 d/week for 2 weeks |
|
| Outcomes | Outcomes assessed post intervention 10 m Timed Walking Speed Test Timed Up and Go Test MMSE Hospital Anxiety and Depression Scale Functional Ambulation Category |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detail not reported |
| Allocation concealment (selection bias) | Unclear risk | Detail not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Detail not reported in enough detail to make a judgement |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
Xiang 2014.
| Methods | RCT | |
| Participants | Recruited from a hospital in China 20 participants: 10 intervention, 10 control Inclusion criteria: aged 40‐80 years within 3 months of first onset of stroke. Abnormal 10 m walking time but could walk > 10 m with no more than the assistance of 1 person Exclusion criteria: cerebellum/brainstem infarct; impairment in all 4 limbs, reduced consciousness, respiratory or heart failure, Parkinson's Disease, recent MI, recent leg fracture, recent deep vein thrombosis, recent stroke with gait disorder Mean (SD) age: intervention group 57.1 (10.43) years, control group 62.2 (10.21) years 70% men Stroke details: 45% right hemiparesis Timing post stroke: intervention group mean (SD) 44.4 (14.78) d, control group 40.80 (16.52) d |
|
| Interventions | VR intervention: VR enhanced body weight supported treadmill training Control intervention: muscle strength training, stretching and balance exercises Both groups participated in 15 sessions of conventional therapy; the VR intervention group received an additional 20‐40 min of training at each session |
|
| Outcomes | Outcomes assessed post intervention 10 m walking speed Fugl Meyer (LE) Brunel Balance Assessment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detail not reported |
| Allocation concealment (selection bias) | Unclear risk | Detail not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in sufficient detail to make judgement |
| Selective reporting (reporting bias) | Unclear risk | Unable to find protocol or trial registration |
Yang 2008.
| Methods | RCT | |
| Participants | Study took place in Taiwan 24 participants: 12 intervention, 12 control Inclusion criteria: hemiparesis resulting from a single stroke occurring > 6 months earlier, limited household walker, unlimited household walker or most‐limited community walker by functional walking category, not presently receiving any rehabilitation services, no visual field deficit or hemianopia, stable medical condition to allow participation in the testing protocol and intervention, ability to understand instructions and follow commands Exclusion criteria: any comorbidity or disability other than stroke that would preclude gait training, uncontrolled health condition for which exercise was contraindicated, neurological or orthopaedic disease that might interfere with the study Mean (SD) age: intervention group 55 (12) years, control group 61 (9) years 50% men Stroke details: 45% right hemiparesis Timing post stroke: intervention group mean (SD) 6 (4) years, control group 6 (10) years |
|
| Interventions | VR intervention: the participant walked on a treadmill as virtual environments were displayed on a screen in front of the person with a wide field of view. Speed and incline of the treadmill was able to be varied in conjunction with scenery changes. Leg movements were tracked by an electromagnetic system to detect collisions with virtual objects. The virtual environment was designed to simulate a typical community in Taipei. Scenarios consisted of lane walking, street crossing, negotiating obstacles and strolling through the park Control intervention: treadmill training. While walking on the treadmill the participant was asked to execute different tasks. The tasks included lifting the legs to simulate stepping over obstacles, uphill and downhill walking and fast walking Sessions were 20 min, 3 times/week for 3 weeks (3 h total) |
|
| Outcomes | Outcomes recorded at baseline, post intervention and at 1 month Lower limb function and activity outcomes: walking speed (m/s), community walk test Participation restriction and quality of life: walking ability questionnaire, Activities Specific Balance Confidence Scale |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent person picked 1 of the sealed envelopes before the start of the intervention |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether envelopes were opaque |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Yang 2011.
| Methods | RCT | |
| Participants | Recruited from a hospital in Taiwan 14 participants: 7 intervention, 7 control Inclusion criteria: hemiplegia resulting from a stroke > 6 months ago. Able to understand the treadmill exercises Exclusion criteria: inability to walk independently (without using an assistive device), abnormal neuro‐opthalmologic findings after examination and visual acuity problems after correction Mean (SD) age: intervention group 56.3 (10.2) years, control group 65.7 (5.9) years Stroke details: 36% right hemiparesis Timing post stroke: intervention group mean (SD) 17 (8.6) months, control group 16.3 (10.4) months |
|
| Interventions | VR intervention: standard OT and physiotherapy program plus VR treadmill training. The treadmill was co‐ordinated with the interactive scenes so that a stepping switch turned the scenes left or right as if the person was turning a corner. Participants had to make 16 turns/session Control intervention: treadmill training facing a window Sessions were 20 min, 3 times/week for 3 weeks (approximately 3 h total) |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Gait outcomes: bilateral limb loading symmetric index, paretic limb stance time, number of steps of the paretic limb, contact areas of the paretic foot during quiet stance, sit‐to‐stand transfer and level walking Balance outcomes: centre of pressure |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient detail reported to tell |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Yavuzer 2008.
| Methods | RCT | |
| Participants | Recruited from an inpatient rehabilitation centre in Turkey 20 participants: 10 intervention, 10 control Inclusion criteria: first episode of unilateral stroke with hemiparesis during the previous 12 months, score of 1‐4 on the Brunnstrom stages for the UE, able to understand and follow simple verbal instructions, no severe cognitive disorders that would interfere with the study's purpose (MMSE score of > 16/30) Mean (SD) age: intervention group 58 (10) years, control group 64 (11) years 45% men Stroke details: 45% right hemiparesis Timing post stroke: intervention group mean (SD) 3 (3) months, control group 5 (1) months |
|
| Interventions | VR intervention: active use of the Playstation EyeToy games involving use of the upper limbs Control intervention: watched the Playstation EyeToy games but did not get physically involved Sessions were 30 min, 5 times/week for 4 weeks (10 h total) Sessions were in addition to the conventional rehabilitation programme that both groups were participating in, which involved approximately 60 min of therapy for the upper limb |
|
| Outcomes | Outcomes recorded at baseline and post‐intervention Upper limb function and activity outcome measures (arm function): Brunnstrom UE stages Upper limb function and activity outcome measures (hand function): Brunnstrom hand stages Activity limitation outcome measures: Functional Independence Measure self care component Adverse events reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated using a computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | An independent doctor operated the random number program |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any attrition and all outcome measures appear to have been reported in full |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Yin 2014.
| Methods | RCT | |
| Participants | Recruited from an inpatient rehabilitation unit in Singapore 23 participants: 11 intervention, 12 control Inclusion criteria: medically stable to participate in active rehabilitation, > 21 years old, able to stand unsupported for 30 s, Fugl Meyer Assessment for the UE score of < 62 and MMSE score of > 20 Exclusion criteria: epilepsy, photophobia or known side effects from watching digital media, were pregnant, had implanted electronic devices including pacemakers or defibrillators, joint pain that could limit participation, severe visual deficits and presented with a spasticity score of > 2 in the affected limb quantified by the Modified Ashworth Scale Median age: intervention group 62 years, control group 56 years 70% men Stroke details: 35% right hemiparesis Timing post stroke: intervention group median 15 d, control group median 14 d |
|
| Interventions | VR intervention: the VR system comprised a hand‐held remote controller detected with a base movement sensor, laptop computer, customised rehabilitation gaming software and a 80 centimetre, liquid crystal display screen. The tasks were highly repetitive but functional tasks in an enriched motivating environment, with customisable but challenging difficulty levels.The virtual environment consisted of a local supermarket setting to increase familiarity and engagement of participants. Participants were instructed to pick a virtual fruit from a shelf and release it into a virtual basket as many times as possible within a 2‐min trial. This reaching practice was carried out standing, simulating real‐life Control intervention: conventional rehabilitation training The experimental group received 30 min of non‐immersive VR training for 9 weekdays within 2 weeks (5 d/week) in addition to conventional therapy. The control group received only conventional therapy. The total dose provided was comparable (17 h intervention vs 15.5 h control) |
|
| Outcomes | Outcomes assessed post intervention and at 4 weeks Fugl Meyer Assessment Action Research Arm Test Motor Activity Log Functional Independence Measure Adverse events reproted |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clear |
| Allocation concealment (selection bias) | Unclear risk | Allocation was concealed using opaque envelopes. Not clear if sealed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropout |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
You 2005.
| Methods | RCT | |
| Participants | Study took place in Korea 10 participants: 5 intervention, 5 control Inclusion criteria: ≥ 1 year after first stroke, plateau in the maximum motor recovery after conventional neurorehabilitation, > 60° extension at the knee Exclusion criteria: severe spasticity (modified Ashworth scale > 2) or tremor, severe visual and cognitive impairment Mean age: intervention group 55 years, control group 55 years 70% men Stroke details: 30% right hemiparesis Timing post stroke: intervention group 18 months, control group 19 months |
|
| Interventions | VR intervention: IREX VR system using a video capture system to capture the participant's whole body movement. The participant is able to view their body movements in real time on a screen in front of them immersed in a virtual environment. Games included stepping up/down, 'shark bait' and snowboarding Control intervention: no intervention provided Sessions for the VR group were 60 min, 5 times/week for 4 weeks (20 h total) |
|
| Outcomes | Outcomes recorded at baseline and post intervention Lower limb function and activity outcomes: Functional Ambulation Category Global motor function: modified Motor Assessment Scale Imaging studies: functional MRI ‐ laterality index |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Zucconi 2012.
| Methods | RCT (3 arms) | |
| Participants | Recruited from a neurorehabilitation ward in Italy 33 participants: 11 intervention, 11 control, 11 control Inclusion criteria: stroke in the MCA territory ≥ 6 months before enrolment, absence of ideomotor apraxia, neglect and aphasia interfering with verbal comprehension Exclusion criteria: apraxia, neglect and language disturbances Median (IQR) age: intervention group 60 (57.25‐76) years, control group 60 (49‐74.25) years, control group 64.5 (54.50‐69) years 39% men Timing post stroke: intervention group median (IQR) 10.05 (4.05‐17.90) months, control group 8.75 (2.75‐24.95) months, control group 5.05 (1.75‐17.90) months |
|
| Interventions | VR intervention (Ever teacher group): Reinforced Feedback in Virtual Environment (RFVE). Participants were asked to manipulate sensorised objects (ball, plastic cup or cylinder). Specific feedback was provided (like a virtual teacher) to encourage the participant to emulate the correct movement VR intervention (No teacher group): VR intervention but with no feedback Control intervention: conventional rehabilitation programme Sessions were 60 min, 5 times/week for 4 weeks |
|
| Outcomes | Outcomes recorded at baseline and post intervention Upper limb outcomes: Fugl Meyer UE, Reaching performance scale Other outcomes: Modified Ashworth Scale, kinematics Activity outcomes: Functional Independence Measure |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | No other outcomes collected |
6MWT: 6‐minute walk test ADL: activities of daily living CT: computerised tomography ITT: intention‐to‐treat IQR: interquartile range MCA: middle cerebral artery MI: myocardial infarction MMSE(‐K): Mini Mental State Examination( ‐ Korean) MRC: Medical Research Council MRI: magnetic resonance imaging OT: occupational therapy RCT: randomised controlled trial ROM: range of motion SD: standard deviation UE: upper extremity VR: virtual reality
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdollahi 2014 | Cross‐over design |
| Bower 2014 | Both the intervention and control group receive VR |
| Braun 2016 | Did not meet the definition of VR intervention |
| Broeren 2008 | Study design: not a RCT |
| Cameirao 2012 | Compares different types of VR |
| Cho 2013 | Did not meet the definition of VR (no real 'interaction' between the person and the virtual environment) |
| Cho 2015 | Both intervention and control group received VR |
| Chortis 2008 | Study design: not a RCT |
| Cikaljo 2012 | Study design: not a RCT |
| Der‐Yeghiaian 2009 | Study design: not a RCT |
| Edmans 2009 | Study design: not a RCT |
| Fischer 2007 | Compares different types of VR |
| Fritz 2013 | Not considered to be properly randomised or quasi‐randomised |
| Gnajaraj 2007 | Did not meet the definition of a VR intervention |
| Hollenstein 2011 | Cross‐over design |
| In 2012 | Did not meet the definition of a VR intervention |
| Katz 2005 | Study design: not all participants were randomised |
| Kim 2012b | Did not meet the definition of a VR intervention |
| Kim 2015a | It did not appear that the participant had control over the interaction with the virtual environment. We emailed the study authors to clarify this but there was no response |
| Kim 2015b | Not clear that the VR is synced with real interaction between the person and the system |
| Krebs 2008 | Study design: participants were not randomly allocated to groups |
| Lee 2014b | Compared assymetric training with symmetric training. Both groups had VR |
| Llorens 2014 | Outlines two studies: both included participants with acquired brain injury and did not report the results for different diagnoses separately |
| Masiero 2014 | Not considered VR intervention matching the definition in this review |
| McEwen 2014 | Compares groups VR in standing with VR in sitting |
| Rand 2014 | Secondary analysis of a subgroup of participants from a larger study |
| Rutz‐LaPitz 2011 | Cross‐over design |
| Shin 2010 | Study design: participants were not randomly allocated to groups |
| Song 2010 | Unable to obtain further information to confirm inclusion criteria or obtain basic study data |
| Turolla 2013 | Not randomised |
| Viana 2014 | Examines VR with or without transcranial direct current stimulation |
| Wolf 2015 | Did not meet definition of VR used in this review |
| Yom 2015 | There is not genuine interaction between the participant and the virtual environment |
| Yoo 2015 | Not VR intervention |
RCT: randomised controlled trial VR: virtual reality
Characteristics of studies awaiting assessment [ordered by study ID]
Almeida 2014.
| Methods | RCT |
| Participants | People post stroke |
| Interventions | Physical therapy associated with VR therapy |
| Outcomes | Berg Balance Scale |
| Notes | Conference abstract. Appears to be preliminary results for an ongoing trial. Study authors did not respond to queries regarding study |
Connor 2016.
| Methods | RCT |
| Participants | People with stroke ≥ 6 months earlier |
| Interventions | Intervention group: 18 individualised training sessions using the YouGrabber over 12 weeks Control group: usual rehabilitation within the gym |
| Outcomes | Interviews, other outcome measures not described |
| Notes |
de Paula Oliveira 2015.
| Methods | RCT |
| Participants | People in the chronic phase post stroke |
| Interventions | Nintendo Wii Fit |
| Outcomes | Fugl Meyer‐Lower Extremity, QOL |
| Notes | Conference paper. States preliminary results. Study authors did not respond to queries regarding study |
Faria 2016.
| Methods | RCT |
| Participants | Individuals within 6 months of stroke |
| Interventions | VR: VR motor‐cognitive task group performed a VR motor and cognitive attention/memory task customised to each user in terms of the positive content Control: standard rehabilitation group performed conventional motor and cognitive rehabilitation tasks |
| Outcomes | Primary outcome: Fugl Meyer |
| Notes | NCT02539914. Co‐investigator AL Faria |
In 2016.
| Methods | RCT |
| Participants | People in the chronic phase post stroke |
| Interventions | VR: VR reflection therapy in addition to usual rehabilitation Control group: conventional rehabilitation and placebo VR |
| Outcomes | Berg Balance Scale, Functional Reach test, Timed Up and Go Test |
| Notes |
Lee 2015c.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | Treadmill training‐based, real‐walk simulation |
| Outcomes | Motor‐Free Visual Perception Test, Berg Balance Scale |
| Notes | Conference abstract only and unable to source further study details |
Lee 2016a.
| Methods | RCT |
| Participants | People in the chronic phase of stroke |
| Interventions | VR‐based rehabilitation group Group‐based rehabilitation group |
| Outcomes | Fugl Meyer‐Upper Extremity, manual function test, Box and Block Test, Modified Barthel Index, SF‐12 |
| Notes | ISRCTN04144761 |
Lee 2016b.
| Methods | RCT |
| Participants | People following stroke |
| Interventions | VR group received additional 30 min of therapy utilising canoe‐based game Control group received conventional rehabilitation program |
| Outcomes | Trunk postural stability, balance and upper limb motor function |
| Notes |
Lin 2015.
| Methods | RCT |
| Participants | People in the chronic phase post stroke |
| Interventions | Computer‐aided interlimb force coupling training task with visual feedback |
| Outcomes | Barthel Index, Fugl Meyer Assessment, Motor Assessment Score, Wolf Motor Function Test |
| Notes | Contacted authors to clarify details of intervention and whether this met our criteria for inclusion but received no response |
Marshall 2016.
| Methods | Quasi RCT |
| Participants | People after stroke with aphasia |
| Interventions | Intervention: daily language stimulation sessions in 'EVA Park' with a support worker Control group: waitlist control group |
| Outcomes | Communication ADL, Verbal fluency task, Word finding in conversation (POWERS), narrative production, Communication Confidence Rating Scale for Aphasia, Friendship Scale |
| Notes |
Nijenhuis 2017.
| Methods | RCT |
| Participants | People in the chronic phase following stroke |
| Interventions | Intervention: self‐administered, home‐based arm and hand training using either a passive or dynamic wrist and hand orthosis combined with computerised gaming exercises Control: prescribed conventional exercises from a book |
| Outcomes | Action Research Arm Test, Intrinsic Motivation Inventory, Fugl Meyer Assessment, Motor Activity Log, Stroke Impact Scale, grip strength |
| Notes |
Simsek 2016.
| Methods | RCT |
| Participants | Adults following stroke |
| Interventions | Intervention: Nintendo Wii for upper limb and balance Control: Bobath NDT |
| Outcomes | Functional Independence Measure, Nottingham Health Profile |
| Notes |
Turkbey 2017.
| Methods | RCT |
| Participants | People following stroke |
| Interventions | Additional therapy using the Xbox Kinect Control group received usual therapy |
| Outcomes | Feasibility and safety (treatment attendance, patient feedback, adverse events, Borg Scale) |
| Notes |
Zondervan 2016.
| Methods | RCT |
| Participants | People with chronic stroke |
| Interventions | Participants were allocated to 3 weeks of home‐based MusicGlove therapy or conventional tabletop exercises |
| Outcomes | Primary outcome: Box and Blocks test |
| Notes |
NDT: neurodevelopmental treatment OT: occupational therapy QOL: quality of life RCT: randomised controlled trial VR: virtual reality
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000427673.
| Trial name or title | 'FIND Technology': investigating the feasibility, efficacy and safety of controller‐free interactive digital rehabilitation technology in an inpatient stroke population: study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | Inpatient stroke population |
| Interventions | Intervention group receive Jintronix JRS Wave in addition to their individualised targeted therapy Control group receive repetitive exercises in addition to their individualised targeted therapy |
| Outcomes | Activity (measured using accelerometer), Modified Motor Assessment Scale (upper extremity component), sitting balance, standing balance, dynamic balance, mobility |
| Starting date | April 2014 |
| Contact information | Dr Marie‐Louise Bird birdm@utas.edu.au |
| Notes | ACTRN12614000427673 |
Deutsch 2010.
| Trial name or title | Interactive video gaming compared with optimal standard of care to improve balance and mobility |
| Methods | Single‐blind pilot RCT |
| Participants | Individuals post stroke (> 6 months), able to walk ≥ 50 m, follow instructions |
| Interventions | VR intervention: Wii‐based balance and mobility training Control: optimal standard of care Dosing 3 h/week for 4 weeks |
| Outcomes | Gait variables (gait rite), 6‐Minute Walk Test, Dynamic Gait Index, Timed Up and Go, Activities Balance Questionnaire, Canadian Occupational Performance Measure, Postural Control |
| Starting date | Commenced Summer 2008 |
| Contact information | Professor Judith Deutsch: deutsch@umdnj.edu |
| Notes | Data collection completed with results to be presented at upcoming conferences |
Duff 2013.
| Trial name or title | The optimal dosage of the rehabilitation gaming system: the impact of a longer period of VR‐based and standard OT on upper limb recovery in the acute phase of stroke |
| Methods | RCT |
| Participants | People after acute stroke |
| Interventions | VR intervention: rehabilitation gaming system Control: OT |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Professor Armin Duff armin.duff@gmail.com |
| Notes | — |
Dunsky 2014.
| Trial name or title | Dual‐task training using virtual reality: influence on walking and balance in individuals post‐stroke |
| Methods | RCT |
| Participants | > 1 year following stroke |
| Interventions | VR intervention: 'SeeMe' video capture system Control intervention: unclear |
| Outcomes | Primary outcome: gait speed |
| Starting date | Unclear |
| Contact information | Dr Ayelet Dunsky ayelet@wincol.ac.il |
| Notes | — |
Kairy 2015.
| Trial name or title | Using a virtual reality gaming system to supplement upper extremity rehabilitation post stroke |
| Methods | RCT |
| Participants | People following stroke with upper extremity impairment |
| Interventions | Intervention group: upper extremity VR Control group: usual care |
| Outcomes | Fugl Meyer, Box and Blocks Test, Stroke Impact Scale |
| Starting date | Unknown |
| Contact information | Professor Dahlia Kairy dahlia.kairy@umontreal.ca |
| Notes | — |
Kairy 2016.
| Trial name or title | Maximizing post‐stroke upper limb rehabilitation using a novel telerehabilitation interactive virtual reality system in the patient's home: study protocol of a randomized clinical trial |
| Methods | RCT |
| Participants | People following stroke with upper extremity impairment |
| Interventions | Intervention: telerehabilitation VR (Jintronix system) Control: continuation of exercises or GRASP program |
| Outcomes | Primary outcome: Fugl Meyer |
| Starting date | Unknown |
| Contact information | Professor Dahlia Kairy dahlia.kairy@umontreal.ca |
| Notes | — |
Karatas 2014.
| Trial name or title | Wii‐based rehabilitation in stroke |
| Methods | RCT |
| Participants | Individuals post stroke |
| Interventions | VR intervention: traditional balance rehabilitation plus Nintendo Wii Fit Control intervention: traditional balance rehabilitation |
| Outcomes | Berg Balance Scale, Functional Reach Test, postural assessment scale for stroke patients Timed Up and Go Test (TUG) and static balance index |
| Starting date | Unknown |
| Contact information | Professor Gülçin Kaymak Karataş: gulcink@gazi.edu.tr |
| Notes | — |
Kiper 2014.
| Trial name or title | Reinforced feedback in virtual environment for rehabilitation of upper extremity dysfunction after stroke: preliminary data from a randomized controlled trial |
| Methods | RCT |
| Participants | People ≥ 1 year post stroke |
| Interventions | Intervention: reinforced feedback in virtual environment Control: traditional rehabilitation |
| Outcomes | Primary outcome: Fugl Meyer‐upper extremity |
| Starting date | Unsure |
| Contact information | Dr Pawel Kiper pawel.kiper@ospedalesancamillo.net |
| Notes | — |
Kizony 2013.
| Trial name or title | Evaluation of a tele‐health system for upper extremity stroke rehabilitation |
| Methods | RCT |
| Participants | People following stroke |
| Interventions | Intervention: quasi‐home‐based tele‐motion‐rehabilitation (TMR) program using the Gertner System Control: self‐training upper extremity home exercise group |
| Outcomes | Not reported in conference abstract |
| Starting date | — |
| Contact information | racheli.kizony@gmail.com |
| Notes | — |
NCT01365858.
| Trial name or title | Virtual action planning in stroke: a control rehabilitation study |
| Methods | RCT |
| Participants | Individuals with stroke |
| Interventions | VR intervention: rehabilitation using the 'Virtual Action Planning supermarket' Control intervention: conventional rehabilitation |
| Outcomes | Primary outcome: ability to perform shopping test in real supermarket |
| Starting date | May 2011 |
| Contact information | Professor Pierre‐Alain Joseph: pierre‐alain.joseph@chu‐bordeaux.fr |
| Notes | Date accessed December 2013 |
NCT01806883.
| Trial name or title | Evaluation of the effects of rehabilitation using the 'Wii' on upper limb kinematics in chronic stroke patients |
| Methods | RCT |
| Participants | Post‐stroke hemiparetic patients (≥ 6 months post stroke) |
| Interventions | VR: Nintendo Wii based therapy Control: traditional physiotherapy |
| Outcomes | Primary outcome: degree of elbow extension during an active reaching task |
| Starting date | — |
| Contact information | Dr Djamel Bensmail djamel.bensmail@rpc.aphp.fr |
| Notes | NCT01806883 |
NCT02013999.
| Trial name or title | The development of upper extremity rehabilitation program using virtual reality for the stroke patients |
| Methods | RCT |
| Participants | Individuals with stroke |
| Interventions | VR intervention Control intervention: standard OT |
| Outcomes | Primary outcome: Fugl Meyer Upper Extremity Scale |
| Starting date | October 2013 |
| Contact information | Professor Nam‐Jong Paik, Department of Rehabilitation Medicine, Seoul National University Email: njpaik@snu.ac.kr |
| Notes | Date accessed December 2013 |
NCT02079103.
| Trial name or title | VIrtual Reality Training for Upper Extremity after Stroke (VIRTUES) |
| Methods | RCT |
| Participants | 1‐12 weeks post stroke |
| Interventions | VR intervention: VR training using the YouGrabber® for participants with impaired arm motor function after stroke. The YouGrabber exercises focus on intensity, repetitions, and motivating tasks ,and are adapted to the patient's motor abilities Control: participants receive supervised self‐training exercises with focus on functional tasks adapted to their motor abilities |
| Outcomes | Primary outcome: Action Research Arm Test |
| Starting date | Unclear |
| Contact information | Dr Iris Brunner Iris.Brunner@igs.uib.no |
| Notes | NCT02079103 |
NCT02553993.
| Trial name or title | Comparing the cognition effects of two exergame systems and traditional weight shifting training in patients with chronic stroke: a pilot randomized comparison trial |
| Methods | RCT |
| Participants | People in the chronic phase after stroke |
| Interventions | Intervention arm 1: Wii Fit Intervention arm 2: Tetrax biofeedback Control: conventional weight shifting |
| Outcomes | Primary outcome measure: Cognitive Abilities Screening Instrument Scale Chinese version |
| Starting date | 2015 |
| Contact information | Dr Jen Wen Hung hungjw@yahoo.com.tw |
| Notes | NCT02553993 |
NCT02592759.
| Trial name or title | Effects of upper extremity rehabilitation using Smart Glove in stroke patients |
| Methods | RCT |
| Participants | People following stroke |
| Interventions | Intervention: participants will be treated with conventional OT for 30 min and smart glove treatment for 30 min. 5 treatments/week will be conducted for a total of 2 weeks Control: participants will be treated with conventional OT for 30 min and upper extremity rehabilitation homework which means the self‐training at bedside, for 30 min. 5 treatments/week will be conducted for a total of 2 weeks |
| Outcomes | Primary outcome measure: Fugl Meyer UE |
| Starting date | Unclear |
| Contact information | A/Prof Han Gil Seo hangil_seo@snuh.org |
| Notes | NCT02592759 |
NCT02688413.
| Trial name or title | Evaluating the MindMotionPRO for early post‐stroke upper‐limb rehabilitation (MOVE‐Rehab) |
| Methods | RCT |
| Participants | 1‐6 weeks following first stroke |
| Interventions | VR intervention: MindMotionPRO exercises in addition to standard practice for upper limb rehabilitation Control intervention: self‐directed prescribed exercises |
| Outcomes | Primary outcome: dose of therapy |
| Starting date | 2016 |
| Contact information | — |
| Notes | NCT02688413 |
NCT02857803.
| Trial name or title | A randomised controlled trial comparing the impact of virtual reality, paper and pencil and conventional methods on stroke rehabilitation |
| Methods | RCT |
| Participants | Post stroke |
| Interventions | VR: Reh@City Paper and Pencil Conventional therapy All provided for 30 min, 3 times/week until 12 sessions |
| Outcomes | Montreal Cognitive Assessment, Stroke Impact Scale, Positive and Negative Affect Scale |
| Starting date | August 2016 |
| Contact information | Ana Lúcia Faria, ana.faria@m‐iti.org |
| Notes | NCT02857803 |
NTR2247.
| Trial name or title | Effect of virtual reality training on reach after stroke |
| Methods | RCT |
| Participants | Individuals in the chronic phase post stroke |
| Interventions | VR intervention: reach training using a VR program Control intervention: reach training in a traditional therapy setting |
| Outcomes | Primary outcomes: Action Research Arm test, Fugl‐Meyer assessment, Intrinsic Motivation Inventory |
| Starting date | April 2010 |
| Contact information | Dr Kottink: a.hutten@rrd.nl |
| Notes | Date accessed December 2013 |
Piemonte 2014.
| Trial name or title | Effects of training in a virtual environment in chronic stroke patients |
| Methods | RCT |
| Participants | People in the chronic phase after stroke |
| Interventions | VR intervention: Nintendo Wii Fit Plus balance and mobility games Control intervention: conventional balance and mobility training |
| Outcomes | Balance, cognition and functional assessments |
| Starting date | Unknown |
| Contact information | Dr Maria Piemonte: elisapp@usp.br |
| Notes | — |
Rand 2015.
| Trial name or title | Home‐based self training using video games: preliminary data from a randomised controlled trial |
| Methods | RCT |
| Participants | People following stroke 6‐36 months earlier |
| Interventions | Intervention: video game self‐training group using PS2 EyeToy, PS3 MOVE or Xbox Kinect Control: self‐training program |
| Outcomes | Box and Block Test, ARAT, Functional Reach Test |
| Starting date | — |
| Contact information | drand@post.tau.ac.il |
| Notes | — |
Schuster‐Amft 2014.
| Trial name or title | Using mixed methods to evaluate efficacy and user expectations of a virtual reality based training system for upper limb recovery in patients after stroke: a study protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | People after stroke |
| Interventions | Intervention: 16 YouGrabber training sessions Control: 16 conventional therapy sessions |
| Outcomes | Primary outcome: Box and Block Test |
| Starting date | Unclear |
| Contact information | c.schuster@reha‐rhf.ch |
| Notes | NCT01774669 |
Sheehy 2016.
| Trial name or title | Virtual reality exercise for stroke rehabilitation in inpatients who are unable to stand |
| Methods | RCT |
| Participants | Stroke inpatients unable to stand |
| Interventions | VR: each participant will engage in 10‐12 sessions of 30‐50 min each of VR training (VRT) using Jintronix Rehabilitation Software and 3‐dimensional motion capture technology. A camera captures the movements of the participant and allows him or her to control an avatar, which interacts with the game. Exercises challenge sitting balance control, reaching and shifting the base of support; for example, controlling a ball as it rolls down a maze or reaching to put dishes away in a virtual kitchen. The difficulty of the games is monitored to maintain a challenge to sitting balance. The participant sits on a CONFORMat pressure mat which continuously monitors his or her centre of pressure to ensure that the participant is adequately challenged during the VRT Control: each participant will engage in 10‐12 sessions of 30‐50 min each of VRT using Jintronix Rehabilitation Software and 3‐dimensional motion capture technology. A camera captures the movements of the participant and allows him or her to control an avatar, which interacts with the game. Control group exercises require limited hand and arm movements; for example, using an arm to move a fish along a simple pathway or using the arms to pop balloons without reaching. Control group participants are strapped into their chair to minimise trunk movement. The participant sits on a CONFORMat pressure mat which continuously monitors his or her centre during the VRT |
| Outcomes | Primary outcome: change in the Function in Sitting Test |
| Starting date | 2014 |
| Contact information | Dr Lisa Sheehy LSheehy@bruyere.org |
| Notes | — |
RCT: randomised controlled trial VR: virtual reality
Differences between protocol and review
The protocol stated that we would handsearch conference proceedings and contact manufacturers of virtual reality equipment. We conducted these searches for the 2010 review. However, they were not successful in identifying additional studies for inclusion and therefore were not repeated in the updates. We also did not search INSPEC as stated in the protocol due to changes in access.
The protocol stated that we would assess trials for risk of bias related to blinding of participants and personnel. We assessed blinding of participants and personnel in the 2010 review. As expected, we deemed all the studies included in the 2010 review to be at high risk of bias. As blinding is not possible in most cases we decided to omit this domain of the 'Risk of bias' assessment tool in this update of the review.
The protocol listed three primary outcomes. This review identified upper limb function and activity as being the primary outcome and considered all other outcomes as secondary outcomes. We selected upper limb function and activity as the primary outcome as one of the most common applications of virtual reality in stroke rehabilitation is upper limb rehabilitation.
The protocol stated that we would look at imaging outcomes. We have removed this in this update as imaging is not considered an outcome that is of relevance to patients as it does not necessarily translate to changes in function.
Contributions of authors
Kate Laver is the guarantor of the review. She was involved in conceiving, designing, and co‐ordinating the review; designing the search strategies; undertaking the searches; screening the search results; organising retrieval of papers; screening retrieved papers against the inclusion criteria; appraising the quality of the papers; extracting data from the papers; writing to study authors for additional information; managing and entering data into Review Manager 5; analysing and interpreting the data; and writing the review.
Belinda Lange was involved in screening the search results; organising retrieval of papers; screening retrieved papers against the inclusion criteria; analysing and interpreting the data; and writing the review.
Stacey George was involved in conceiving and designing the review; extracting data; analysing and interpreting the data; and writing the review.
Judith Deutsch was involved in designing the review; screening retrieved papers against inclusion criteria; extracting data; appraising the quality of papers; analysing and interpreting the data; and writing the review.
Gustavo Saposnik was involved in extracting data; appraising the quality of papers; analysing and interpreting the data; and writing the review.
Maria Crotty was involved in conceiving and designing the review; extracting data; appraising the quality of papers; analysing and interpreting the data; and writing the review.
Declarations of interest
Kate Laver: none known.
Belinda Lange: none known.
Stacey George: none known.
Judith Deutsch conducts research on virtual reality for stroke rehabilitation. This research is funded by various sources and presented at scientific and professional meetings. She is co‐owner of a company that develops virtual reality for rehabilitation.
Gustavo Saposnik is the first author on two of the studies included in the review. He was not involved in assessment of these studies for inclusion or risk of bias and did not extract data for these studies. He is supported by the Distinguished Clinician‐Scientist Award given by the Heart and Stroke Foundation of Canada following an open peer‐reviewed competition.
Maria Crotty: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Adie 2017 {published data only}
- Adie K, Schofield C, Berrow M, Wingham J, Humfryes J, Pritchard C, et al. Does the use of Nintendo Wii Sports improve arm function? Trial of Wii in stroke: a randomized controlled trial and economic analysis. Clinical Rehabilitation 2017;31(2):173‐85. [DOI] [PubMed] [Google Scholar]
Akinwuntan 2005 {published data only}
- Akinwuntan A, Devos H, Verheyden G, Baten G, Kiekens C, Feys H, et al. Retraining moderately impaired stroke survivors in driving‐related visual attention skills. Topics in Stroke Rehabilitation 2010;17(5):328‐36. [DOI] [PubMed] [Google Scholar]
- Akinwuntan AE, Weerdt W, Feys H, Pauwels J, Baten G, Arno P, et al. Effect of simulator training on driving after stroke. Neurology 2005;65(6):843‐50. [DOI] [PubMed] [Google Scholar]
- Devos H, Akinwuntan AE, Nieuwboer A, Ringoot I, Berghen K, Tant M, et al. Effect of simulator training on fitness to drive after stroke: a 5‐year follow up of a randomised controlled trial. Neurorehabilitation and Neural Repair 2010;24(9):843‐50. [DOI] [PubMed] [Google Scholar]
- Devos H, Akinwuntan AE, Nieuwboer A, Tant M, Truijen S, Wit L, et al. Comparison of the effect of two driving retraining programs on on‐road performance after stroke. Neurorehabilitation and Neural Repair 2009;23(7):699‐705. [DOI] [PubMed] [Google Scholar]
Barcala 2013 {published data only}
- Barcala L, Grecco LAC, Colella F, Lucareli PRG, Salgado ASI, Oliveira CS. Visual biofeedback balance training using Wii Fit after stroke: a randomized controlled trial. Journal of Physical Therapy Science 2013;25(8):1027‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2015 {published data only}
- Bower K, Louie J, Landesrocha Y, Seedy P, Gorelik A, Bernhardt J. Clinical feasibility of interactive motion‐controlled games for stroke rehabilitation. Journal of Neuroengineering and Rehabilitation 2015;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byl 2013 {published data only}
- Byl N, Abrams G, Pitsch E, Fedulow I, Kim H, Simkins M, et al. Chronic stroke survivors achieve comparable outcomes following virtual task specific repetitive training guided by a wearable robotic orthosis (UL‐EXO7) and actual task specific repetitive training guided by a physical therapist. Journal of Hand Therapy 2013;26(4):343‐51. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho K, Yu J, Jung J. Effects of virtual reality based rehabilitation on upper extremity function and visual perception in stroke patients: a randomized control trial. Journal of Physical Therapy Science 2012;24:1205‐8. [Google Scholar]
Chow 2013 {published data only}
- Chow TK, Chan CM, Tong JMC. Effectiveness of virtual reality in balance training in stroke rehabilitation: a pilot study. Cerebrovascular Diseases 2013;36:17‐8. [Google Scholar]
Coupar 2012 {published data only}
- Coupar F. Exploring Upper Limb Interventions After Stroke [PhD thesis]. Glasgow, UK: University of Glasgow, 2012. [Google Scholar]
Crosbie 2008 {published data only}
- Crosbie J. Virtual Reality in the Rehabilitation of the Upper Limb Following Stroke [PhD Thesis]. UK: University of Ulster, 2008. [Google Scholar]
- Crosbie J, Lennon S, McGoldrick M, McNeil M, McDonough S. Virtual reality in the rehabilitation of the arm after hemiplegic stroke: a randomized controlled pilot study. Clinical Rehabilitation 2012;26(9):798‐806. [DOI] [PubMed] [Google Scholar]
da Silva Cameirao 2011 {published and unpublished data}
- Silva Cameirao M, Badia S, Duarte E, Verschure P. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the Rehabilitation Gaming System. Restorative Neurology and Neuroscience 2011;29(5):287‐98. [DOI] [PubMed] [Google Scholar]
da Silva Ribeiro 2015 {published data only}
- Fonseca EP, Silva Ribeiro N, Pinto EB. Therapeutic effect of virtual reality on post‐stroke patients: randomized clinical trial. Journal of Stroke and Cerebrovascular Diseases 2017;26(1):94‐100. [DOI] [PubMed] [Google Scholar]
- Silva Ribeiro NM, Ferraz DD, Pedreira E, Pinheiro I, Silva Pinto AC, Neto MG, et al. Virtual rehabilitation via Nintendo Wii and conventional physical therapy effectively treat post‐stroke hemiparetic patients. Topics in Stroke Rehabilitation 2015;22(4):299‐305. [DOI] [PubMed] [Google Scholar]
Fan 2014 {published data only}
- Fan SC, Su FC, Chen SS, Hou WH, Sun JS, Chen KH, et al. Improved intrinsic motivation and muscle activation patterns in reaching task using virtual reality training for stroke rehabilitation: a pilot randomized control trial. Journal of Medical and Biological Engineering 2014;34(4):399‐407. [Google Scholar]
Galvao 2015 {published data only}
- Galvao MLC, Gouvea PM, Ocamato GN, Silva AT, dos Reis LM, Kosour C, et al. Virtual reality effect on upper limb motor function paretic in post stroke. Revista Neurociencias 2015;23(4):493‐8. [Google Scholar]
Givon 2016 {published data only}
- Givon N, Zeilig G, Weingarden H, Rand D. Video‐games used in a group setting is feasible and effective to improve indicators of physical activity in individuals with chronic stroke: a randomized controlled trial. Clinical Rehabilitation 2016;30(4):383‐92. [DOI] [PubMed] [Google Scholar]
Han 2013 {published data only}
- Han JY. The effect of virtual reality program on stroke patients with impaired standing balance. PM & R: the Journal of Injury, Function, and Rehabilitation 2013;5(9 Suppl 1):S237‐8. [Google Scholar]
Housman 2009 {published data only}
- Housman SJ, Scott KM, Reikensmeyer DJ. A randomized controlled trial of gravity‐supported, computer‐enhanced arm exercise for individuals with severe hemiparesis. Neurorehabilitation and Neural Repair 2009;23(5):505‐14. [DOI] [PubMed] [Google Scholar]
Hung 2014 {published and unpublished data}
- Hung JW, Chou CX, Hsieh YW, Wu WC, Yu MY, Chen PC, et al. Randomized comparison trial of balance training by using exergaming and conventional weight‐shift therapy in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation 2014;95(9):1629‐37. [DOI] [PubMed] [Google Scholar]
Jaffe 2004 {published data only}
- Jaffe DL, Brown DA, Pierson‐Carey CD, Buckley EL, Lew HL. Stepping over obstacles to improve walking in individuals with poststroke hemiplegia. Journal of Rehabilitation Research and Development 2004;41(3A):283‐92. [DOI] [PubMed] [Google Scholar]
Jang 2005 {published data only}
- Jang SH, You SH, Hallett M, Cho YW, Park CM, Cho SH, et al. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter‐blind preliminary study. Archives of Physical Medicine and Rehabilitation 2005;86:2218‐23. [DOI] [PubMed] [Google Scholar]
Jannink 2008 {published data only}
- Jannink MJA, Erren‐Wolters CV, Kort AC, Kooij H. An electric scooter simulation program for training the driving skills of stroke patients with mobility problems: a pilot study. Cyberpsychology and Behavior 2008;11(6):751‐4. [DOI] [PubMed] [Google Scholar]
Jung 2012 {published data only}
- Jung J, Yu J, Kang H. Effects of virtual reality treadmill training on balance and balance self‐efficacy in stroke patients with a history of falling. Journal of Physical Therapy Science 2012;24(11):1133‐6. [Google Scholar]
Kang 2009 {published data only}
- Kang SH, Kim DK, Seo KM, Choi KN, Yoo JY, Sung SY, et al. A computerized visual perception rehabilitation programme with interactive computer interface using motion tracking technology ‐ a randomized controlled, single‐blinded, pilot clinical trial study. Clinical Rehabilitation 2009;23:434‐44. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim JH, Jang SH, Kim CS, Jung JH, You JH. Use of virtual reality to enhance balance and ambulation in chronic stroke: a double‐blind, randomized controlled study. American Journal of Physical Medicine and Rehabilitation 2009;88:693‐701. [DOI] [PubMed] [Google Scholar]
Kim 2011a {published data only}
- Kim BR, Chun MH, Kim LS, Park JY. Effect of virtual reality on cognition in stroke patients. Annals of Rehabilitation Medicine 2011;35:450‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2011b {published data only}
- Kim YM, Chun MH, Yun GJ, Song YJ, Young HE. The effect of virtual reality training on unilateral spatial neglect in stroke patients. Annals of Rehabilitation Medicine 2011;35:309‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012a {published data only}
- Kim E, Kang J, Park J, Jung B. Clinical feasibility of interactive commercial Nintendo gaming for chronic stroke rehabilitation. Journal of Physical Therapy Science 2012;24(9):901‐3. [Google Scholar]
Kiper 2011 {published and unpublished data}
- Kiper P, Piron L, Turolla A, Stozek J, Tonin P. The effectiveness of reinforced feedback in virtual environment in the first 12 months after stroke. Neurologia i Neurochirurgia Polska 2011;45(5):436‐44. [DOI] [PubMed] [Google Scholar]
Klamroth‐Marganska 2014 {published data only}
- Klamroth‐Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, et al. Three‐dimensional, task‐specific robot therapy of the arm after stroke: a multicentre, parallel‐group randomised trial. Lancet Neurology 2014;13(2):159‐66. [DOI] [PubMed] [Google Scholar]
Ko 2015 {published data only}
- Ko YJ, Ha HG, Bae YH, Lee WH. Effect of space balance 3D training using visual feedback on balance and mobility in acute stroke patients. Journal of Physical Therapy Science 2015;27(5):1593‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2014 {published data only}
- Kong KH. Efficacy of a virtual reality commercial gaming device in upper limb recovery after stroke: a randomized, controlled study. Topics in Stroke Rehabilitation 2016;23(5):333‐40. [DOI] [PubMed] [Google Scholar]
- Kong KH. Efficacy of computer gaming in upper limb recovery after stroke: a randomized, controlled study. Cerebrovascular Diseases 2014;38:109. [Google Scholar]
- Loh YJ. Effectiveness of Nintendo Wii gaming in facilitating upper limb recovery after stroke: a randomised, controlled study. 5th Singapore Health and Biomedical Congress 2014. 2014; Vol. 43 (Suppl), issue 9:S147.
Kwon 2012 {published data only}
- Kwon J, Park M, Yoon I, Park S. Effects of virtual reality on upper extremity function and activities of daily living performance in acute stroke: a double‐blind randomized clinical trial. Neurorehabilitation 2012;31(4):379‐85. [DOI] [PubMed] [Google Scholar]
Lam 2006 {published data only}
- Lam YS, Man DWK, Tam SF, Weiss PL. Virtual reality training for stroke rehabilitation. Neurorehabilitation 2006;21:245‐53. [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee SW, Shin DC, Song CH. The effects of visual feedback training on sitting balance ability and visual perception of patients with chronic stroke. Journal of Physical Therapy Science 2013;25(5):635‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014a {published data only}
- Lee CH, Kim Y, Lee BH. Augmented reality‐based postural control training improves gait function in patients with stroke: randomized controlled trial. Hong Kong Physiotherapy Journal 2014;32(2):51‐7. [Google Scholar]
Lee 2015a {published data only}
- Lee HY, Kim YL, Lee SM. Effects of virtual reality based training and task oriented training on balance performance in stroke patients. Journal of Physical Therapy Science 2015;27(6):1883‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015b {published data only}
- Lee S, Kim Y, Lee B. Effect of virtual reality‐based bilateral upper extremity training on upper extremity function after stroke: a randomized controlled clinical trial. Occupational Therapy International 2016;23(4):357‐68. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim YM, Lee BH. Effects of virtual reality‐based bilateral upper‐extremity training on brain activity in post‐stroke patients. Journal of Physical Therapy Science 2015;27(7):2285‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 2012 {published data only}
- Levin MF, Snir O, Liebermann DG, Weingarden H, Weiss PL. Virtual reality versus conventional treatment of reaching ability in chronic stroke: clinical feasibility study. Neurology and Therapy 2012;1(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2015 {published data only}
- Linder SM, Rosenfeldt AB, Bay RC, Sahu K, Wolf SL, Alberts JL. Improving quality of life and depression after stroke through telerehabilitation. American Journal of Occupational Therapy 2015;69:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llorens 2015 {published data only}
- Llorens R, Gil‐Gomez JA, Alcaniz M, Colomer C, Noe E. Improvement in balance using a virtual‐reality based stepping exercise: a randomized controlled trial involving individuals with chronic stroke. Clinical Rehabilitation 2015;29(3):261‐8. [DOI] [PubMed] [Google Scholar]
Low 2012 {published data only}
- Low AY, Ng YS, Chan Y, Tan DML, Bok CW, Fook Chong SMC, et al. Effect of virtual reality rehabilitation as an adjunct to conventional therapy in people with sub‐acute stroke: a randomised controlled pilot trial. Proceedings of Singapore Healthcare 2012;21:S357. [Google Scholar]
Manlapaz 2010 {published and unpublished data}
- Manlapaz D, Silverio A, Navarro JA, Regacho M, Ang M, Canaberal C, et al. Effectiveness of using Nintendo Wii in rehabilitation of chronic stroke patients with upper limb hemiparesis. Physiotherapy 2011;97:eS746‐eS747. [Google Scholar]
- Manlapaz D, Silverio L, Navarro J, Ang M, Regacho M, Canaberal K, et al. Effectiveness of using Nintendo Wii in rehabilitation of chronic stroke patients with upper limb hemiparesis. Hong Kong Physiotherapy Journal 2010;28:25. [Google Scholar]
Mao 2015 {published and unpublished data}
- Mao Y, Chen P, Li L, Li L, Huang D. Changes of pelvis control with subacute stroke: a comparison of body‐weight support treadmill training coupled virtual reality system and over‐ground training. Technology and Health Care 2015;23:S355‐S364. [DOI] [PubMed] [Google Scholar]
Matsuo 2013 {published data only}
- Matsuo A, Takahara T, Hiraoka N, Hiyamizu M, Maeoka H, Okada Y. Effectiveness of interactive video gaming system in stroke rehabilitation. Cerebrovascular Diseases 2013;35(Suppl 3):779. [Google Scholar]
Mazer 2005 {published and unpublished data}
- Mazer B, Gelinas I, Duquette J, Vanier M, Rainville C, Chilingaryan G. A randomized clinical trial to determine effectiveness of driving simulator retraining on the driving performance of clients with neurological impairment. British Journal of Occupational Therapy 2015;78(6):369‐76. [Google Scholar]
- Mazer B, Gelinas I, Vanier M, Duquette J, Rainville C, Hanley J. Effectiveness of retraining using a driving simulator on the driving performance of clients with a neurological impairment. Neurorehabilitation and Neural Repair 2005;19:383. [Google Scholar]
McNulty 2015 {published data only}
- McNulty PA, Thompson‐Butel AG, Faux S, Lin G, Katrak P, Harris LR, et al. The efficacy of Wii‐based movement therapy for upper limb rehabilitation in the chronic poststroke period: a randomized controlled trial. International Journal of Stroke 2015;10:1253‐60. [DOI] [PubMed] [Google Scholar]
- Trinh T, Scheuer SE, Thompson‐Butel AG, Shiner CT, McNulty PA. Cardiovascular fitness is improved post‐stroke with upper limb Wii‐based movement therapy but not dose matched constraint therapy. Topics in Stroke Rehabilitation 2016;23(3):208‐16. [DOI] [PubMed] [Google Scholar]
Mirelman 2008 {published and unpublished data}
- Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot‐virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke 2008;40:169‐74. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Pattriti B, Bonato P, Deutsch J. Effects of virtual reality training on gait biomechanics of individuals post‐stroke. Gait and Posture 2010;31(4):433‐7. [DOI] [PubMed] [Google Scholar]
Morone 2014 {published data only}
- Morone G, Tramontano M, Iosa M, Shofany J, Iemma A, Musicco M, et al. The efficacy of balance training with video game‐based therapy in subacute stroke patients: a randomized controlled trial. BioMed Research International 2014;DOI: 10.1155/2014/580861:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nara 2015 {published data only}
- Nara K, Yuhyun P, Byoung‐Hee L. Effects of community‐based virtual reality treadmill training on balance ability in patients with chronic stroke. Journal of Physical Therapy Science 2015;27(3):655‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piron 2007 {published data only}
- Piron L, Tombolini P, Turolla A, Zucconi C, Agostini M, Dam M, et al. Reinforced feedback in virtual environment facilitates the arm motor recovery in patients after a recent stroke. International Workshop of Virtual Rehabilitation. 2007:121‐3.
Piron 2009 {published data only}
- Piron L, Turolla A, Agostini M, Zucconi C, Cortese F, Zampolini M, et al. Exercises for paretic upper limb after stroke: a combined virtual‐reality and telemedicine approach. Journal of Rehabilitation Medicine 2009;41:1016‐20. [DOI] [PubMed] [Google Scholar]
Piron 2010 {published data only}
- Piron L, Turolla A, Agostini M, Zucconi C, Ventura L, Tonin P, et al. Motor learning principles for rehabilitation: a pilot randomized controlled study in poststroke patients. Neurorehabilitation and Neural Repair 2010;24(6):501‐8. [DOI] [PubMed] [Google Scholar]
Prange 2015 {published data only}
- Prange GB, Kottink AI, Buurke JH, Eckhardt MM, Keulen‐Rouweler BJ, Ribbers GM, et al. The effect of arm support combined with rehabilitation games on upper‐extremity function in subacute stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(2):174‐82. [DOI] [PubMed] [Google Scholar]
Rajaratnam 2013 {published data only}
- Rajaratnam BS, Gui Kaien J, Lee Jialin K, Sweesin K, Sim Fenru S, Enting L, et al. Does the inclusion of virtual reality games within conventional rehabilitation enhance balance retraining after a recent episode of stroke?. Rehabilitation Research and Practice 2013; Vol. 2013:649561. [DOI] [PMC free article] [PubMed]
Reinkensmeyer 2012 {published data only}
- Reinkensmeyer DJ, Wolbrecht ET, Chan V, Chou C, Cramer SC, Bobrow JE. Comparison of three dimensional, assist‐as‐needed robotic arm/hand movement training provided with Pneu‐WREX to conventional tabletop therapy after chronic stroke. American Journal of Physical and Medical Rehabilitation 2012;91(11):S232‐S241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saposnik 2010 {published and unpublished data}
- Saposnik G, Teasell R, Mamdani M, Hall J, McIlroy W, Cheung D, Stroke Outcome Research Canada (SORCan) Working Group. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke 2010;41:1477‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saposnik 2016 {published data only}
- Saposnik G, Cohen LG, Mamdani M, Pooyania S, Ploughman M, Cheung D, et al. Efficacy and safety of non‐immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre, single blind, controlled trial. Lancet Neurology 2016;15(10):1019‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2014 {published data only}
- Shin JH, Ryu H, Jang SH. A task‐specific interactive game‐based virtual reality rehabilitation system for patients with stroke: a usability test and two clinical experiments. Journal of NeuroEngineering and Rehabilitation 2014;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2015 {published data only}
- Shin JH, Kim MY, Lee JY, Jeon YJ, Kim S, Lee S, et al. Effects of virtual reality‐based rehabilitation on distal upper extremity function and health related quality of life: a single blinded, randomized controlled trial. Journal of Neuroengineering and Rehabilitation 2016;13(17):doi:10.1186/s12984‐016‐0125‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Park SB, Jang SH. Effects of game‐based virtual reality on health‐related quality of life in chronic stroke patients: a randomized, controlled study. Computers in Biology and Medicine 2015;63:92‐8. [DOI] [PubMed] [Google Scholar]
Sin 2013 {published data only}
- Sin HH, Lee GC. Additional virtual reality training using Xbox Kinect in stroke survivors with hemiplegia. American Journal of Physical Medicine and Rehabilitation 2013;92:871‐80. [DOI] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song GB, Park EC. Effect of virtual reality games on stroke patients' balance, gait, depression, and interpersonal relationships. Journal of Physical Therapy Science 2015;27(7):2057‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Standen 2011 {unpublished data only}
- Standen P, Brown D, Battersby S, Walker M, Connell L, Richardson A, et al. A study to evaluate a low cost virtual reality system for home based rehabilitation of the upper limb following stroke. International Journal on Disability and Human Development 2011;10(4):337‐41. [Google Scholar]
- Standen PJ, Threapleton K, Richardson A, Connell L, Brown DJ, Battersby S, et al. A low cost virtual reality system for home based rehabilitation of the arm following stroke: a randomised controlled feasibility trial. Clinical Rehabilitation 2017;31(3):340‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramanian 2013 {published and unpublished data}
- Subramanian S, Lourenco C, Chilingaryan G, Sveistrup H, Levin M. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabilitation and Neural Repair 2013;27(1):13‐23. [DOI] [PubMed] [Google Scholar]
Sucar 2009 {published data only}
- Sucar LE, Leder R, Hernandez J, Sanchez I, Azcarate G. Clinical evaluation of a low‐cost alternative for stroke rehabilitation. IEEE 11th International Conference on Rehabilitation Robotics. 2009:863‐6.
Thielbar 2014 {published data only}
- Thielbar KO, Lord TJ, Fischer HC, Lazzaro EC, Barth KC, Stoykov ME, et al. Training finger individuation with a mechatronic‐virtual reality system leads to improved fine motor control post‐stroke. Journal of NeuroEngineering and Rehabilitation 2014;11(690):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ucar 2014 {published data only}
- Ucar D, Paker N, Bugdayci D. Lokomat: a therapeutic chance for patients with chronic hemiplegia. NeuroRehabilitation 2014;34(3):447‐53. [DOI] [PubMed] [Google Scholar]
Xiang 2014 {published data only}
- Xiang X, Yu‐rong M, Jiang‐li Z, Li L, Guang‐qing X, Dong‐feng H. Virtual reality enhanced body weight supported treadmill training improved lower limb motor function in patients with cerebral infarction. Chinese Journal of Tissue Engineering Research 2014;18(7):1143‐8. [Google Scholar]
Yang 2008 {published data only}
- Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality‐based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait and Posture 2008;28:201‐6. [DOI] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang S, Hwang WH, Tsai YC, Liu FK, Hsieh LF, Chern JS. Improving balance skills in patients who had stroke through virtual reality treadmill training. American Journal of Physical Medicine and Rehabilitation 2011;90:969‐78. [DOI] [PubMed] [Google Scholar]
Yavuzer 2008 {published data only}
- Yavuzer G, Senel A, Atay MBG, Stam HJ. 'Playstation EyeToy games' improve upper extremity‐related motor functioning in subacute stroke: a randomized controlled clinical trial. European Journal of Physical and Rehabilitation Medicine 2008;44:237‐44. [PubMed] [Google Scholar]
Yin 2014 {published data only}
- Yin CW, Sien NY, Ying LA, Chung SFM, Leng DTM. Virtual reality for upper extremity rehabilitation in early stroke: a pilot randomized controlled trial. Clinical Rehabilitation 2014;28(11):1107‐14. [DOI] [PubMed] [Google Scholar]
You 2005 {published data only}
- You SH, Jang SH, Kim YH, Hallett M, Ahn SH, Kwon YH, et al. Virtual reality‐induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter‐blind randomized study. Stroke 2005;36:1166‐71. [DOI] [PubMed] [Google Scholar]
Zucconi 2012 {published and unpublished data}
- Zucconi C, Valt V, Agostini M, Turolla A, Tonin P, Piron L. Assessment of a virtual teacher feedback for the recovery of the upper limb after stroke. Neurorehabilitation and Neural Repair 2012;26(4):407. [Google Scholar]
References to studies excluded from this review
Abdollahi 2014 {published data only}
- Abdollahi F, Case Lazarro ED, Listenberger M, Kenyon RV, Kovic M, Bogey RA. Error augmentation enhancing arm recovery in individuals with chronic stroke: randomized crossover design. Neurorehabilitation and Neural Repair 2014;28(2):120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2014 {published data only}
- Bower KJ, Clark RA, McGinley JL, Martin CL, Miller KJ. Clinical feasibility of the Nintendo WiiTM for balance training post‐stroke: a phase II randomized controlled trial in an inpatient setting. Clinical Rehabilitation 2014;28(9):912‐23. [DOI] [PubMed] [Google Scholar]
Braun 2016 {published data only}
- Braun T, Marks D, Thiel C, Zietz D, Zutter D, Gruneberg C. Effects of additional, dynamic supported standing practice on functional recovery in patients with sub‐acute stroke: a randomized pilot and feasibility trial. Clinical Rehabilitation 2016;30(4):374‐82. [DOI] [PubMed] [Google Scholar]
Broeren 2008 {published data only}
- Broeren J, Claesson L, Goude D, Rydmark M, Sunnerhagen K. Virtual rehabilitation in an activity centre for community‐dwelling persons with stroke. Cerebrovascular Diseases 2008;26:289‐96. [DOI] [PubMed] [Google Scholar]
Cameirao 2012 {published data only}
- Cameirao M, Badia S, Duarte E, Frisoli A, Verschure P. The combined impact of virtual reality neurorehabilitation and its interfaces on upper extremity functional recovery in patients with chronic stroke. Stroke 2012;43(10):2720‐8. [DOI] [PubMed] [Google Scholar]
Cho 2013 {published data only}
- Cho KH, Lee WH. Virtual walking training program using a real‐world video recording for patients with chronic stroke. American Journal of Physical Medicine and Rehabilitation 2013;92:371‐84. [DOI] [PubMed] [Google Scholar]
Cho 2015 {published data only}
- Cho KH, Kim MK, Lee HJ, Lee WH. Virtual reality training with cognitive load improves walking function in chronic stroke patients. Tohoku Journal of Experimental Medicine 2015;236(4):273‐80. [DOI] [PubMed] [Google Scholar]
Chortis 2008 {published data only}
- Chortis A, Standen PJ, Walker M. Virtual reality system for upper extremity rehabilitation of chronic stroke patients living in the community. International Conference on Disability, Virtual Reality & Associated Technologies (ICDVRAT). 2008:221‐8.
Cikaljo 2012 {published data only}
- Cikaljo I, Rudolf M, Goljar N, Burger H, Matjacic Z. Telerehabilitation using virtual reality task can improve balance in patients with stroke. Disability and Rehabilitation 2012;34(1):13‐8. [DOI] [PubMed] [Google Scholar]
Der‐Yeghiaian 2009 {published data only}
- Der‐Yeghiaian L, Sharp K, See J, Abidi N, Mai K, Cramer S. Robotic therapy after stroke and the influence of baseline motor status. International Stroke Conference Poster Presentations. 2009:e169.
Edmans 2009 {published data only}
- Edmans J, Gladman J, Hilton D, Walker M, Sunderland A, Cobb S, et al. Clinical evaluation of a non‐immersive virtual environment in stroke rehabilitation. Clinical Rehabilitation 2009;23:106‐16. [DOI] [PubMed] [Google Scholar]
Fischer 2007 {published data only}
- Fischer HC, Stubblefield K, Kline T, Luo X, Kenyon RV, Kamper DG. Hand rehabilitation following stroke: a pilot study of assisted finger extension training in a virtual environment. Topics in Stroke Rehabilitation 2007;14(1):1‐12. [DOI] [PubMed] [Google Scholar]
Fritz 2013 {published data only}
- Fritz S, Peters D, Merlo A, Donley J. Active video‐gaming effects on balance and mobility in individuals with chronic stroke: a randomized controlled trial. Topics in Stroke Rehabilitation 2013;20(3):218‐25. [DOI] [PubMed] [Google Scholar]
Gnajaraj 2007 {published data only}
- Gnajaraj J, Chowdry H, Kumar S. Influence of virtual reality environment on the recovery after stroke. Archives of Physical Medicine and Rehabilitation 2007;88:E3. [Google Scholar]
Hollenstein 2011 {published data only}
- Hollenstein C, Cabri J. Supplementary therapy with computer‐assisted training system compared to ergotherapeutic arm group therapy [Zusatztherapie mit computerunterstütztem Trainingssystem im Vergleich zu ergotherapeutischer Armgruppentherapie]. NeuroRehabilitation 2011;3.01:40‐2. [Google Scholar]
In 2012 {published data only}
- In T, Jung K, Lee S, Song C. Virtual reality reflection therapy improves motor recovery and motor function in the upper extremities of people with chronic stroke. Journal of Physical Therapy Science 2012;24(4):339‐43. [Google Scholar]
Katz 2005 {published data only}
- Katz N, Ring H, Naveh Y, Kizony R, Feintuch U, Weiss PL. Interactive virtual environment training for safe street crossing of right hemisphere stroke patients with unilateral spatial neglect. Disability and Rehabilitation 2005;27(20):1235‐43. [DOI] [PubMed] [Google Scholar]
Kim 2012b {published data only}
- Kim IC, Lee BH. Effects of augmented reality with functional electric stimulation on muscle strength, balance and gait of stroke patients. Journal of Physical Therapy Science 2012;24(8):755‐62. [Google Scholar]
Kim 2015a {published data only}
- Kim HS, Choi WJ, Lee K, Song CH. Virtual dual‐task treadmill training using video recording for gait of chronic stroke survivors: a randomized controlled trial. Journal of Physical Therapy Science 2015;27(12):3693‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim N, Park Y, Lee BH. Effects of community‐based virtual reality treadmill training on balance ability in patients with chronic stroke. Journal of Physical Therapy Science 2015;27(3):655‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2008 {published data only}
- Krebs HI, Mernoff S, Fasoli SE, Hughes R, Stein J, Hogan N. A comparison of functional and impairment‐based robotic training in severe to moderate chronic stroke: a pilot study. NeuroRehabilitation 2008;23:81‐7. [PMC free article] [PubMed] [Google Scholar]
Lee 2014b {published data only}
- Lee D, Lee M, Lee K, Song C. Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: a randomized controlled trial. Journal of Stroke and Cerebrovascular Diseases 2014;23(6):1319‐26. [DOI] [PubMed] [Google Scholar]
Llorens 2014 {published data only}
- Llorens R, Albiol S, Gil‐Gomez J, Alcaniz M, Colomer C, Noe E. Balance rehabilitation using custom‐made Wii Balance Board exercises: clinical effectiveness and maintenance of gains in an acquired brain injury population. International Journal on Disability and Human Development 2014;13(3):327‐332. [Google Scholar]
Masiero 2014 {published data only}
- Masiero S, Armani M, Ferlini G, Rosati G, Rossi A. Randomized trial of a robotic assistive device for the upper extremity during early inpatient stroke rehabilitation. Neurorehabilitation and Neural Repair 2014;28(4):377‐86. [DOI] [PubMed] [Google Scholar]
McEwen 2014 {published data only}
Rand 2014 {published data only}
- Rand D, Givon N, Weingarden H, Nota A, Zeilig G. Eliciting upper extremity purposeful movements using video games: a comparison with traditional therapy for stroke rehabilitation. Neurorehabilitation and Neural Repair 2014;28(8):733‐9. [DOI] [PubMed] [Google Scholar]
Rutz‐LaPitz 2011 {published data only}
- Rutz‐LaPitz L, Hollenstein C, Baumann Y, Kaufeler R, Gosoniu N. Effectiveness of armeo versus task‐oriented arm group as augmentation to upper extremity conventional therapy in acute rehabilitation post stroke. Physiotherapy 2011;97:eS1072‐3. [Google Scholar]
Shin 2010 {published data only}
- Shin WS, Lee DY, Lee SW. The effects of rehabilitation exercise using a home video game (PS2) on gait ability of chronic stroke patients. Journal of the Korea Academia‐Industrial Cooperation Society 2010;11(1):368‐74. [Google Scholar]
Song 2010 {published data only}
- Song CH, Seo SM, Lee GC. Video game‐based exercise for upper extremity function rehabilitation of chronic stroke patients: results from a randomized, controlled, single‐blind trial. International Journal of Stroke 2010;5:304. [Google Scholar]
Turolla 2013 {published data only}
- Turolla A, Dam M, Ventura L, Tonin P, Agostini M, Zucconi C. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. Journal of NeuroEngineering and Rehabilitation 2013;10(85):doi: 10.1186/1743‐0003‐10‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viana 2014 {published data only}
- Viana RT, Laurentino GE, Souza RJ, Fonseca JB, Silva Filho EM, Dias SN. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: a pilot randomized controlled trial. Neurorehabilitation 2014;34:437‐46. [DOI] [PubMed] [Google Scholar]
Wolf 2015 {published data only}
- Wolf S, Sahu K, Bay C, Buchanan S, Reiss A, Linder S, et al. The HAAPI (Home Arm Assistance Progression Initiative) Trial. Neurorehabilitation & Neural Repair 2015;29(10):958‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yom 2015 {published data only}
- Yom C, Cho HY, Lee B. Effects of virtual reality‐based ankle exercise on the dynamic balance, muscle tone, and gait of stroke patients. Journal of Physical Therapy Science 2015;27(3):845‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoo 2015 {published data only}
- Yoo C, Yong M, Chung J, Yang Y. Effect of computerized cognitive rehabilitation program on cognitive function and activities of living in stroke patients. Journal of Physical Therapy Science 2015;27(8):2487‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Almeida 2014 {published data only}
- Almeida J, Castro P, Dos Santoa Moreira C, Battistella LR. Development of a protocol for treatment of post stroke patients with virtual reality: preliminary results. PM & R : the Journal of Injury, Function, and Rehabilitation 2014;6(8 Suppl 2):S110. [Google Scholar]
- Do Amaral Santos PL, Castro PCG, Dos Santos Moreira C, Battistella LR. Retaining the effects of virtual reality therapy on posture control of patients with stroke: preliminary results. PM & R : the Journal of Injury, Function, and Rehabilitation 2014;6(8 Suppl 2):S110. [Google Scholar]
Connor 2016 {published data only}
- Connor D, Stockley R, Moss S, Allsop L, Edge W. Using virtual reality for upper limb recovery post stroke: a pilot study. Cerebrovascular Diseases 2016;41:49. [Google Scholar]
de Paula Oliveira 2015 {published data only}
- Oliveira TDP, Miranda CS, Gouvea J, Perez DB, Marques AP, Piemonte MEP. Improvement of balance and gait in patients with stroke after training based on Nintendo Wii fitTM games: randomized controlled trial. Physiotherapy 2015;101:eS1207. [Google Scholar]
- Paula Oliveira T, Miranda CS, Gouva JXM, Perez DB, Marques AP, Piemonte MEP. Balance training in virtual reality in patients with chronic sequels of stroke: effects on ICF domains, preliminary data. 3rd Workshop on ICTs for improving Patients Rehabilitation Research Techniques, REHAB 2015, 1‐2 October 2015. 2015.
Faria 2016 {published data only}
- Faria AL, Andrade A, Soares L, Badia SB. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. Journal of NeuroEngineering and Rehabilitation 2016;13(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
In 2016 {published data only}
- In T, Lee K, Song C. Virtual reality reflection therapy improves balance and gait in patients with chronic stroke: randomized controlled trials. Medical Science Monitor 2016;22:4046‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015c {published data only}
- Lee HJ, Kim MK, Lee KB, Lee WH. The effects of treadmill training using real‐walk simulation in stroke patients. Physiotherapy 2015;May:eS846. [Google Scholar]
Lee 2016a {published data only}
- Lee M, Son J, Kim J, Pyun SB, Eun SD, Yoon B. Comparison of individualized virtual reality‐ and group‐based rehabilitation in older adults with chronic stroke in community settings: a pilot randomized controlled trial. European Journal of Integrative Medicine 2016;8(5):738‐46. [Google Scholar]
Lee 2016b {published data only}
- Lee MM, Shin DC, Song CH. Canoe game‐based virtual reality training to improve trunk postural stability, balance, and upper limb motor function in subacute stroke patients: a randomized controlled pilot study. Journal of Physical Therapy Science 2016;28(7):2019‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2015 {published data only}
- Lin CH, Chou LW, Luo HJ, Tsai PY, Lieu FK, Chiang AL, et al. Effects of computer‐aided interlimb force coupling training on paretic hand and arm motor control following chronic stroke: a randomized controlled trial. PLoS ONE 2015; Vol. 10, issue 7. [DOI] [PMC free article] [PubMed]
Marshall 2016 {published data only}
- Marshall J, Booth T, Devane N, Galliers J, Greenwood H, Hilari K, et al. Evaluating the benefits of aphasia intervention delivered in virtual reality: results of a quasi‐randomised study. PLoS ONE 2016; Vol. DOI: 10.1371/journal.pone.0160381. [DOI] [PMC free article] [PubMed]
Nijenhuis 2017 {published data only}
- Nijenhuis SM, Prange‐Lasonder GB, Stienen AHA, Rietman JS, Buurke JH. Effects of training with a passive hand orthosis and games at home in chronic stroke: a pilot randomised controlled trial. Clinical Rehabilitation 2017;31(2):207‐16. [DOI] [PubMed] [Google Scholar]
Simsek 2016 {published data only}
- Simsek T, Cekok K. The effects of Nintendo WiiTM‐based balance and upper extremity training on activities of daily living and quality of life in patients with sub‐acute stroke: a randomized controlled study. International Journal of Neuroscience 2016;126(12):1061‐70. [DOI] [PubMed] [Google Scholar]
Turkbey 2017 {published data only}
- Turkbey T, Kutlay S, Gok H. Clinical feasibility of Xbox Kinect training for stroke rehabilitation: a single blind randomized controlled pilot study. Journal of Rehabilitation Medicine 2017;49(1):22‐9. [DOI] [PubMed] [Google Scholar]
Zondervan 2016 {published data only}
- Zondervan D, Friedman N, Chang E, Zhao X, Augburger R, Reinkensmeyer D, et al. Home‐based hand rehabilitation after chronic stroke: randomized, controlled single‐blind trial comparing the music glove with a conventional exercise program. Journal of Rehabilitation Research and Development 2016;53(4):457‐72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12614000427673 {published data only}
- Bird ML, Cannell J, Callisaya ML, Moles E, Rathjen A, Lane K, et al. "FIND Technology": investigating the feasibility, efficacy and safety of controller‐free interactive digital rehabilitation technology in an inpatient stroke population: study protocol for a randomized controlled trial. Trials 2016;17(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deutsch 2010 {published data only}
- Deutsch J. Interactive video gaming compared to optimal standard of care to improve balance and mobility. Personal communication 2010.
Duff 2013 {published data only}
- Duff A, Nirme J, Rubio B, Duarte E, Cuxart A, Rodriguez S, et al. The optimal dosage of the rehabilitation gaming system: the longer impact of a longer period of virtual reality based and standard occupational training on upper limb recovery in the acute phase of stroke. Cerebrovascular Diseases 2013;35:146. [Google Scholar]
Dunsky 2014 {published data only}
- Dunsky AD, Hutzler YH, Fishbein PF. Dual task training using virtual reality: influence on walking and balance in individuals post‐stroke. European Geriatric Medicine 2014;5:S165. [Google Scholar]
Kairy 2015 {published data only}
- Kairy D, Poissant L, Higgins J, Hernandez A, Archambault PS, Norouzi‐Gheidari N. Using a virtual reality gaming system to supplement upper extremity rehabilitation post stroke. Archives of Physical Medicine and Rehabilitation 2015;96:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kairy 2016 {published data only}
- Kairy D, Veras M, Archambault P, Hernandez A, Higgins J, Levin M, et al. Maximizing post‐stroke upper limb rehabilitation using a novel telerehabilitation interactive virtual reality system in the patient's home: study protocol of a randomized clinical trial. Contemporary Clinical Trials 2016;47:49‐53. [DOI] [PubMed] [Google Scholar]
Karatas 2014 {published data only}
- Karatas GK, Karasu AU, Balevi E. Wii‐based balance rehabilitation is effective in stroke: a randomized controlled study. Neurorehabilitation and Neural Repair 2012;26:767. [Google Scholar]
Kiper 2014 {published data only}
- Kiper P, Agostini M, Luque‐Moreno C, Tonin P, Turolla A. Reinforced feedback in virtual environment for rehabilitation of upper extremity dysfunction after stroke: preliminary data from a randomized controlled trial. BioMed Research International 2014. [DOI] [PMC free article] [PubMed]
Kizony 2013 {published data only}
- Kizony R, Weiss PL, Feldman Y, Shani M, Elion O, Kizony R, et al. Evaluation of a tele‐health system for upper extremity stroke rehabilitation. 2013 10th International Conference on Virtual Rehabilitation, ICVR 2013, 26‐29 August 2013. 2013.
NCT01365858 {published and unpublished data}
- NCT01365858. Virtual action planning in stroke: a control rehabilitation study. clinicaltrials.gov/ct2/show/NCT01365858 (accessed December 2013). [NCT01365858]
NCT01806883 {published data only}
- Bensmail B. Evaluation of the Effects of Rehabilitation Using the "Wii" on Upper Limb Kinematics in Chronic Stroke Patients. clinicaltrials.gov 2013.
NCT02013999 {published data only}
- NCT02013999. The development of upper extremity rehabilitation program using virtual reality for the stroke patients. clinicaltrials.gov/ct2/show/NCT02013999 (accessed December 2013). [NCT02013999]
NCT02079103 {published data only}
- Brunner I, Skouen J, Hofstad H, Strand L, Becker F, Sanders AM, et al. Virtual reality training for upper extremity in subacute stroke (VIRTUES): study protocol for a randomized controlled multicenter trial. BMC Neurology 2014; Vol. 14, issue 1. [DOI] [PMC free article] [PubMed]
NCT02553993 {published data only}
- Hung, JW. Comparing the Cognitive Effects of Two Exergame Training and Traditional Training in Patients With Chronic Stroke. ClinicalTrials.gov.
NCT02592759 {published data only}
- Gil Seo H. Effects of Upper Extremity Rehabilitation Using Smart Glove in Stroke Patients. ClinicalTrials.gov.
NCT02688413 {published data only}
- Caso V. Study Evaluating the MindMotionPRO for Early Post‐stroke Upper‐limb Rehabilitation (MOVE‐Rehab). ClinicalTrials.gov.
NCT02857803 {unpublished data only}
- Faria AL, Bermudez i Badia S. Development and evaluation of a web‐based cognitive task generator for personalized cognitive training: a proof of concept study with stroke patients. REHAB 2015: 3rd workshop on ICTs for improving patients research techniques. 2015:1‐4.
- Faria AL, Pinho M, Bermudez i Badia S. Personalizing cognitive rehabilitation through a web‐based Task Generator: an evaluation study with stroke patients. International Neuropsychological Society 2016 Mid year meeting. 2016; Vol. 2016.
- Faria Al, Vourvopoulos A, Cameirao MS, Fernandes JC, Bermudez i Badia S. An integrative virtual reality cognitive‐motor intervention approach in stroke rehabilitation: a pilot study. 10th International Conference, Disability, Virtual Reality and Associated Technologies (ICDVRAT). 2014.
- NCT02857803. A randomised controlled trial comparing the impact of virtual reality, paper and pencil and conventional methods on stroke rehabilitation. ClinicalTrials.gov 2016.
- Vourvopoulos A, Faria AL, Ponnam K, Bermudez i Badia S. RehabCity: design and validation of a cognitive assessment and rehabilitation tool through gamified simulations of activities of daily living. 11th Advances in Computer Entertainment Technology Conference. November 2014.
NTR2247 {published data only}
- NTR2247. Effect of virtual reality training on reach after stroke. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2247 (accessed December 2013). [NTR2247]
Piemonte 2014 {published data only}
- Piemonte MEP, Oliveira TP, Miranda C, Muzzi J, Perez DB. Motor‐cognitive intervention based on Nintendo Wii Fit games to improve balance and cognitive functions in patients with stroke: a randomized controlled trial. Personal communication (to be presented at the Neurorehabilitation Congress) 2014.
Rand 2015 {published data only}
- Rand D, Yacoby A, Weiss R, Reif S, Malka R, Weingarden H, et al. Home‐based self‐training using video‐games: preliminary data from a randomised controlled trial. Virtual Rehabilitation Proceedings (ICVR), 2015. 2015:86‐91.
Schuster‐Amft 2014 {published data only}
- Schuster‐Amft C, Eng K, Thalers I, Lehmann I, Signer S, McCaskey MA, et al. Evaluating efficacy and users' expectations of a virtual reality training system: a multicenter randomized controlled trial. Annals of physical and rehabilitation medicine 2014;57:e85. [Google Scholar]
Sheehy 2016 {published data only}
- Sheehy L, Taillon‐Hobson A, Sveistrup H, Bilodeau M, Fergusson D, Levac D, et al. Does the addition of virtual reality to a standard program of inpatient rehabilitation improve sitting balance ability and function after stroke? Protocol for a single‐blind randomized controlled trial. BMC Neurology 2016; Vol. 16, issue 42. [DOI] [PMC free article] [PubMed]
Additional references
Bagce 2012
- Bagce HF, Saleh S, Adamovich SV, Tunik E. Visuomotor gait distortion alters online motor performance and enhances primary motor cortex excitability in patients with stroke. Neuromodulation 2012;15(4):361‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohil 2011
- Bohil CJ, Alicea B, Biocca FA. Virtual reality in neuroscience research and therapy. Nature Reviews Neuroscience 2011;12:752‐62. [DOI] [PubMed] [Google Scholar]
Burridge 2010
- Burridge JJ, Hughes AM. Potential for new technologies in clinical practice. Current Opinion in Neurology 2010;23:671‐7. [DOI] [PubMed] [Google Scholar]
Cheok 2015
- Cheok G, Tan D, Low A, Hewitt J. Is Nintendo Wii an effective intervention for individuals with stroke? A systematic review and meta‐analysis. Journal of the American Medical Directors Association 2015;16(11):923‐32. [DOI] [PubMed] [Google Scholar]
Corbetta 2015
- Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: a systematic review. Journal of Physiotherapy 2015;61(3):117‐24. [DOI] [PubMed] [Google Scholar]
Crosbie 2007
- Crosbie J, Lennon S, Basford J, McDonough S. Virtual reality in stroke rehabilitation: still more virtual than real. Disability and Rehabilitation 2007;29(14):1139‐46. [DOI] [PubMed] [Google Scholar]
Darekar 2015
- Darekar A, McFadyen BJ, Lamontagne A, Fung J. Efficacy of virtual reality‐based intervention on balance and mobility disorders post‐stroke: a scoping review. Journal of Neuroengineering in Rehabilitation 2015;10(12):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Demain 2013
- Demain S, Burridge J, Ellis‐Hill C. Assistive technologies after stroke: self management or fending for yourself? A focus group study. BMC Health Services Research 2013;13:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
French 2016
- French B, Thomas L, Coupe J, McMahon N, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD006073.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fung 2012
- Fung V, Ho A, Shaffer J, Chung E, Gomez M. Use of Nintendo Wii fit in the rehabilitation of outpatients following total knee replacement: a preliminary randomised controlled trial. Physiotherapy 2012;98:183‐8. [DOI] [PubMed] [Google Scholar]
Gaggioli 2009
- Gaggioli A, Keshner E, Weiss PL, Riva G. Advanced Technologies in Rehabilitation. United States: IOS Press, 2009. [Google Scholar]
Go 2014
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014;129:e28‐e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jiandani 2014
- Jiandani N, Nair SR, Shukla H. Efficacy of virtual reality exposure therapy in the management of symptoms associated with post traumatic stress disorder. Value in Health 2014;17(7):A572. [DOI] [PubMed] [Google Scholar]
Kwakkel 2004
- Kwakkel G, Peppen R, Wagenaar R, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke. A meta‐analysis. Stroke 2004;35:1‐11. [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange B, Flynn S, Proffitt R, Chang C, Rizzo A. Development of an interactive game‐based rehabilitation tool for dynamic balance training. Topics in Stroke Rehabilitation 2010;17(5):345‐52. [DOI] [PubMed] [Google Scholar]
Lange 2012
- Lange B, Koenig S, Chang CY, McConnell E, Suma E, Bolas M, et al. Designing informed game‐based rehabilitation tasks leveraging advances in virtual reality. Disability and Rehabilitation 2012;34(22):1863‐70. [DOI] [PubMed] [Google Scholar]
Langhorne 2011
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377(9778):1693‐702. [DOI] [PubMed] [Google Scholar]
Larsen 2009
- Larsen C, Sorensen J, Grantcharov T, Dalsgaard T, Schouenborg L, Ottosen C, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 2009;338:b1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levac 2015
- Levac D, Espy D, Fox E, Pradhan S, Deutsch J. "Kinect‐ing" with clinicians: a knowledge translation resource to support decision making about video game use in rehabilitation. Physical Therapy 2015;95(3):426‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012
- Lewis GN, Rosie JA. Virtual reality games for movement rehabilitation in neurological conditions: how do we meet the needs and expectations of the users?. Disability and Rehabilitation 2012;34(22):1880‐6. [DOI] [PubMed] [Google Scholar]
Li 2016
- Li Z, Han XG, Sheng J, Ma SJ. Virtual reality for improving balance in patients after stroke: a systematic review and meta‐analysis. Clinical Rehabilitation 2016;30(5):432‐40. [DOI] [PubMed] [Google Scholar]
Lintern 1990
- Lintern G, Roscoe S, Koonce J, Segal L. Display principles, control dynamics and environmental factors in pilot training and transfer. Human Factors 1990;32:299‐317. [Google Scholar]
Lohse 2014
- Lohse K, Hilderman CGE, Cheung KL, Tatla S, Loos HFM. Virtual reality therapy for adults post‐stroke: a systematic review and meta‐analysis exploring virtual environments and commercial games in therapy. PLoS ONE 2014;9(3):e93318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merians 2002
- Merians A, Jack D, Boian R, Tremaine M, Burdea G, Adamovich S, et al. Virtual reality augmented rehabilitation for patients following stroke. Physical Therapy 2002;82(9):898‐915. [PubMed] [Google Scholar]
Miller 2010
- Miller E, Murray L, Richards L, Zorowitz R, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke 2010;41:2402‐48. [DOI] [PubMed] [Google Scholar]
Moreira 2013
- Moreira MC, Amorim Lima AM, Ferraz KM, Benedetti Rodrigues MA. Use of virtual reality in gait recovery among post stroke patients ‐ a systematic literature review. Disability and Rehabilitation Assistive Technology 2013;8(5):357‐62. [DOI] [PubMed] [Google Scholar]
Nawaz 2015
- Nawaz A, Skjaeret N, Helbostad J, Vereijken B, Boulton E, Svanaes D. Usability and acceptability of balance exergames in older adults: a scoping review. Health Informatics Journal 2015;22(4):911‐31. [DOI] [PubMed] [Google Scholar]
Patel 2006
- Patel M, Tilling K, Lawrence E, Rudd A, Wolfe C, McKevitt C. Relationships between long‐term stroke disability, handicap and health‐related quality of life. Age and Ageing 2006;35:273‐9. [DOI] [PubMed] [Google Scholar]
Raghav 2016
- Raghav K, Wijk AJ, Abdullah F, Islam MN, Bernatchez M, Jongh A. Efficacy of virtual reality exposure therapy for treatment of dental phobia: a randomized control trial. BMC Oral Health 2016; Vol. 16, issue 25. [DOI] [PMC free article] [PubMed]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Risedal 2002
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson B. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Research Bulletin 2002;58:315‐21. [DOI] [PubMed] [Google Scholar]
Saleh 2014
- Saleh S, Adamovich SV, Tunik E. Mirrored feedback in chronic stroke: recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehabilitation and Neural Repair 2014;28(4):344‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saposnik 2011
- Saposnik G, Levin M, Stroke Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: a meta‐analysis and implications for clinicians. Stroke 2011;42(5):1380‐6. [DOI] [PubMed] [Google Scholar]
Schuemie 2001
- Schuemie M, Straaten P, Krijn M, Mast C. Research on presence in virtual reality: a survey. CyberPsychology & Behavior 2001;4(2):183‐201. [DOI] [PubMed] [Google Scholar]
Schultheis 2001
- Schultheis M, Rizzo A. The application of virtual reality technology in rehabilitation. Rehabilitation Psychology 2001;46:296‐311. [Google Scholar]
Schulz 2010
- Schulz K, Altman D, Moher D for the CONSORT group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturm 2004
- Sturm J, Donnan G, Dewey H, Macdonnell R, Gilligan A, Srikanth A, et al. Quality of life after stroke: the North East Melbourne stroke incidence study (NEMESIS). Stroke 2004;35:2340‐5. [DOI] [PubMed] [Google Scholar]
Teasell 2014
- Teasell RW, Murie‐Fernandez M, McIntyre A, Mehta S. Rethinking the continuum of stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 2014;95(4):595‐6. [DOI] [PubMed] [Google Scholar]
Thomson 2014
- Thomson K, Pollock A, Bugge C, Brady M. Commercial gaming devices for stroke upper limb rehabilitation: a systematic review. International Journal of Stroke 2014;9(4):479‐88. [DOI] [PubMed] [Google Scholar]
Tunik 2013
- Tunik E, Saleh S, Adamovich SV. Visuomotor discordance during visually guided hand movement in virtual reality modulates sensorimotor cortical activity in healthy and hemiparetic subjects. IEEE Transactions in Neural System Rehabilitation Engineering 2013;21(2):198‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veerbeek 2014
- Veerbeek JM, Wegen E, Peppen R, Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta‐analysis. PLoS ONE 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2014
- Webster D, Celik O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. Journal of NeuroEngineering and Rehabilitation 2014;11(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2006
- Weiss P, Kizony R, Feintuch U, Katz N. Virtual reality in neurorehabilitation. In: Selzer M, Cohen L, Gage F, Clarke S, Duncan P editor(s). Textbook of Neural Repair and Rehabilitation. Cambridge University Press, 2006:182‐97. [Google Scholar]
WHO 1989
- World Health Organization Task Force on Stroke and Other Cerebrovascular Disorders. Recommendations on stroke prevention, diagnosis, and therapy: report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407‐31. [DOI] [PubMed] [Google Scholar]
Wingham 2015
- Wingham J, Adie K, Turner D, Schofield C, Pritchard C. Participant and caregiver experience of the Nintendo Wii SportsTM after stroke: qualitative study of the trial of WiiTM in stroke (TWIST). Clinical Rehabilitation 2015;29(3):295‐305. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Laver 2010
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2011
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008349.pub2] [DOI] [PubMed] [Google Scholar]
Laver 2015
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD008349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


