SYNOPSIS
Contemporary trials and meta-analyses have evaluated the role of thrombolytics in hemodynamically stable patients with acute pulmonary embolism (PE) and evidence of right ventricular dysfunction and myocardial injury (intermediate-risk PE). The most important findings of these studies are that thrombolytic therapy may prevent hemodynamic deterioration and even all-cause mortality but increases major (including intracranial and fatal) bleeding. These benefits and harms are finely balanced, with no convincing net benefit from thrombolytic therapy among unselected patients. Among patients with intermediate-high risk PE, additional prognostic factors (e.g., syncope, elevated lactate, concomitant deep vein thrombosis, severe respiratory insufficiency) or subtle hemodynamic changes (e.g. increasing heart rate or persistent downtrend of systolic blood pressure) might alter the risk-benefit assessment in favor of thrombolytic therapy before the development of frank hemodynamic instability.
Keywords: Pulmonary embolism, submassive, intermediate-risk, thrombolysis, reperfusion, prognosis
INTRODUCTION
Pulmonary embolism (PE) remains a worldwide major health issue (1). PE is the most common cause of vascular death after myocardial infarction and stroke, and is the leading preventable cause of death in hospitalized patients (2). Although contemporary observational data indicate significant reductions in all-cause and PE-related mortality over time (3, 4), the overall short-term mortality rate continues to remain significant, and many non-fatal long-term complications may arise. Guidelines recommend risk stratification of patients with acute symptomatic PE (5, 6). Early prognostication allows clinicians to better determine the level of care (e.g. intensive care versus step-down, regular floor, or outpatient treatment) and associated ancillary therapies.
In this perspective, we provide an overview of the current definition of intermediate-risk PE, followed by a discussion of the available treatments for these patients, focusing on thrombolytic therapy. We also include recently completed and ongoing clinical studies for the treatment of patients with intermediate-risk PE. Finally, we provide a practical clinical algorithm that integrates risk stratification and management alternatives.
DEFINITION OF INTERMEDIATE-RISK PULMONARY EMBOLISM
The definition of intermediate-risk (or submassive) PE has evolved over time (Table 1). The classic definition of intermediate-risk PE is the presence of either right ventricular (RV) dysfunction or myocardial injury in acute PE without systemic hypotension (systolic blood pressure ≥ 90 mm Hg) (6). However, observational studies have suggested that concomitant use of blood biomarkers and imaging markers of RV dysfunction improve the prognostic value over use of either alone (7–10). Scridon et al enrolled 141 patients with acute PE and found that those with echocardiographic RV enlargement and elevated troponin levels had a 30-day all-cause mortality of 38% (7). In a study of 124 stable and unstable patients with acute PE, the combination of echocardiography and troponin T had improved prognostic value compared with each test alone (8). Accordingly, the European Society of Cardiology (ESC) guidelines define intermediate-high risk patients with acute symptomatic PE as those who are hemodynamically stable, and have myocardial injury and RV dysfunction (5).
Table 1.
Definitions used for stratification of pulmonary embolism
| Definition | Major studies using the definition | Comment | |
|---|---|---|---|
| Massive PE or ESC High | Persistent systolic hypotension (systolic blood pressure <90mmHg) or cardiogenic shock | Almost all studies | Initial appropriate management, including adequate use of intravenous fluids should be attempted before hypotension is attributed to acute PE |
| Submassive PE | Presence of RV dysfunction evidence by increased RV/LV ratio on CT or echocardiography | TOPCOAT ULTIMA AINEP INOPE |
Some studies have raised concerns about the prognostic utility of some of the echocardiographic factors, in isolation. |
| Submassive PE | Defined by echo or CT plus biomarkers | PEITHO | Mortality rate within the first 30 days after randomization of “only” 3.2% in the placebo group |
| Moderate PE | Defined by imaging findings | MOPPET | Needs further validation on impact on prognosis |
| ESC Intermediate-High | Absence of hypotension, positive PESI or sPESI, but presence of RV dysfunction plus myocardial injury | – | Needs validation in a management study or RCT |
| ESC Intermediate-Low | Absence of hypotension, positive PESI or sPESI, but presence of RV dysfunction or myocardial injury or none | – | The difference in the risk of death in patients at intermediate-high and intermediate-low risk is not pronounced (46) |
Abbreviations: PE, Pulmonary embolism; ESC, European Society of Cardiology; RV, right ventricle; LV, left ventricle; CT, computed tomography; PESI, Pulmonary Embolism Severity Index; sPESI, simplified PESI; RCT, randomized controlled trial.
AGGRESSIVE TREATMENT OF INTERMEDIATE-RISK PULMONARY EMBOLISM
Thrombolytic therapy provides more rapid lysis of PE and more rapid restoration of pulmonary perfusion, with associated reduction in pulmonary artery pressure and resistance, and improvement in RV function than anticoagulation alone (11). In the past decade, a number of randomized controlled trials and meta-analyses have contributed to substantially clarify the optimal management of intermediate-risk PE (Table 2). These studies have focused on the use of full-dose systemic thrombolysis, low-dose systemic thrombolysis, and pharmacomechanical catheter-directed therapy.
Table 2.
Major randomized controlled trials for the treatment of intermediate-risk pulmonary embolism
| Study | Definition of intermediate-risk PE | Number of patients | Interventiona | Controla | Age, Mean (range or SD), y | Follow-up, d | Male, N° (%) | Primary outcome | Effect | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Meyer et al (13), 2014 | RV dysfunction (by echo or CT) and elevated cTnI or T | 1005 | Tenecteplase (30–50 mg) |
Placebo | 66.2 (15.3) | 30 | 473 (47%) | All-cause mortality or hemodynamic collapse within 7 days after randomization | OR 0.44 (95% CI, 0.23–0.87; P =0.02) in favor of tenecteplase | No change in mortality; significant increase in bleeding |
| Kline et al (12), 2014 | Echocardiographic RV dysfunction, or elevated cTnI or T, or elevated BNP or NT-proBNP | 83 | Tenecteplase | Placebo | 55.4 (14) | 5 | 49 (59.0) | Composite of (1) death, circulatory shock requiring vasopressor support, need for intubation, or serious treatment-related adverse bleeding outcomes within 5 days posttreatment; and (2) VTE recurrence, poor functional capacity, or poor physical health-related quality of life within 90 days. | 37% placebo-treated and 15% tenecteplase-treated patients had at least one adverse outcome (P =0.02) | The trial was terminated prematurely |
| Sharifi et al (16), 2012 | Moderate PE (defined by radiologic thrombus burden) and ≥2 of the following: chest pain, tachypnea, tachycardia, dyspnea, cough, oxygen desaturation, or elevated jugular venous pressure | 121 | t-PA (50 mg) |
Placebo | Intervention: 58 (9) Control: 59 (10) |
840 | 55 (45.5) | Incidence of pulmonary hypertension, and the composite of pulmonary hypertension and PE recurrence. | Pulmonary hypertension: 16 vs. 57%, P <0.001 Composite: 16 vs. 63%, P <0.001 |
Unusual high rates of pulmonary hypertension, as a surrogate endpoint |
| Kucher et al (22), 2014 | Echocardiographic RV/LV ratio >1.0 | 59 | rt-PA (10 to 20 mg through PA catheter) |
Placebo | 63 (14) | 90 | 28 (47.5) | RV/LV ratio at 24 hours vs. baseline | CDT group: 1.28 vs. 0.99; P <0.001. Heparin group: 1.20 vs. 1.17; P =0.31). |
Not powered for clinical outcomes |
Patients also received standard anticoagulation.
Abbreviations: RV, right ventricle; CT, computed tomography; cTnI, cardiac troponin I; OR, odds ratio; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; VTE, venous thromboembolism; PE, pulmonary embolism; t-PA, tissue plasminogen activator; LV, left ventricle; CDT, catheter-directed thrombolysis.
Full-dose systemic thrombolysis
The Tenecteplase or Placebo: Cardiopulmonary Outcomes At Three Months (TOPCOAT) study sought to determine the efficacy and safety of tenecteplase in normotensive patients with acute symptomatic PE and RV strain (determined by echocardiography or biomarkers) (12). These patients were randomized to receive heparin plus weight-based tenecteplase or heparin plus placebo. The primary composite outcome included 5-day survival to hospital discharge without shock, intubation, or major hemorrhage; 90-day rate of normal RV function, 6-minute walk distance >330 m, no dyspnea at rest, and no recurrent PE or deep vein thrombosis (DVT). The study was terminated early due to logistical constraints for the principal investigator, and after enrolling 83 patients. Despite being underpowered, the primary endpoint occurred significantly less frequently in patients randomized to thrombolysis compared with the low-molecular-weight heparin (LMWH) (15% vs. 37%, P = 0.017) (12).
The Pulmonary Embolism Thrombolysis Trial (PEITHO) was a randomized, double blind trial that compared tenecteplase plus heparin with placebo plus heparin in normotensive patients with intermediate-risk PE (13). Eligible patients had RV dysfunction/enlargement on echocardiography or computed tomography (CT), as well as myocardial injury as indicated by a positive test for cardiac troponin I or troponin T. The primary outcome was death or hemodynamic collapse within 7 days after randomization. The main safety outcomes were major extracranial bleeding and ischemic or hemorrhagic stroke within 7 days after randomization. The results of the trial showed that thrombolytic therapy prevented hemodynamic decompensation (1.6% vs. 5.0%, P = 0.002) but increased the risk of major bleeding (11.5% vs. 2.4%, P < 0.001) and hemorrhagic stroke (2.0 vs. 0.2%, P = 0.003). Interestingly, there was a trend toward greater major extracranial bleeding incidence in patients > 75 years of age than in those ≤ 75 years of age with tenecteplase versus placebo treatment.
Since publication of these studies, a number of average effect meta-analyses have compared systematic thrombolytic therapy plus anticoagulation with anticoagulation alone in patients with acute PE. Marti et al. evaluated 15 trials comprising 2,057 patients with acute PE (14). After exclusion of studies including high-risk PE, thrombolytic therapy was not associated with a significant reduction of overall mortality (odds ratio [OR], 0.64; 95% confidence interval [CI], 0.35-1.17). Chatterjee et al. identified 16 trials comprising 2,115 patients and performed subset analyses in the 1,775 patients with intermediate-risk PE (15). In the latter subgroup, thrombolysis was associated with lower mortality risk compared with standard anticoagulation (OR, 0.48; 95% CI, 0.25-0.92). In both meta-analyses, thrombolysis was associated with higher rates of major bleeding and intracranial hemorrhage compared with anticoagulation. In summary, these data indicate that the benefits of full-dose systemic thrombolytic therapy in unselected normotensive patients with acute PE are largely offset by the increase in bleeding complications. To our knowledge, a patient-level meta-analysis from these trials does not exist, and pooled results per key clinical subgroups (e.g. younger patients) remain unknown.
Low-dose systemic thrombolysis
It has been hypothesized that a lower dose of a systemically administered thrombolytic drug might be effective in PE, with the additional benefit of enhancing its safety profile. The Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) study was a prospective, controlled, randomized, single-center open study that randomized 121 patients with moderate PE to receive either 50 mg of tissue plasminogen activator with anticoagulation or anticoagulation alone (16). The primary outcomes were the development of pulmonary hypertension (i.e., pulmonary artery systolic pressures ≥ 40 mmHg) measured by echocardiography and a composite of pulmonary hypertension and recurrent PE at intermediate-term follow-up. In this trial, pulmonary hypertension developed less commonly in the thrombolysis group (16% vs. 57%, P < 0.001). The study was not powered, however, for clinical endpoints and the difference for the rates of recurrent pulmonary emboli (0% vs. 5%; P = 0.08) or death (1.6% vs. 5%; P = 0.30) did not reach statistical significance. There were no bleeding complications with the low dose thrombolysis.
A recent network meta-analysis evaluated 4 trials (17–20) (298 patients) that compared low dose to standard dose thrombolysis (21). In this meta-analysis, there was no statistically significant difference in overall mortality (OR 0.96, 95% CI, 0.23-4.17), or major bleeding, although low dose showed a nonsignificant trend towards reduced major bleeding events (OR 0.44, 95% CI, 0.15-1.28), which needs to be confirmed in larger studies. Lack of statistical power might account for the nonsignificant results, as suggested by the wider confidence intervals.
Pharmacomechanical catheter-directed therapy
Catheter-directed thrombolysis (CDT) has the potential to offer the benefits of systemic thrombolysis while minimizing bleeding risk attributable to a lower dose of the thrombolytic agent. Further, some forms of CDT also use ultrasound assistance, which is hypothesized to improve the clot resolution in the pulmonary vasculature. The ULTrasound accelerated thrombolysIs of pulMonAry embolism (ULTIMA) trial was the first randomized catheter intervention study for patients with acute PE, and enrolled patients with acute symptomatic PE with embolus located in at least one main or proximal lower lobe pulmonary artery and an RV/left ventricle (LV) ratio ≥ 1 per bedside echocardiography (22). This multicenter trial investigated whether ultrasound-assisted CDT was superior to anticoagulation alone in the reversal of RV dilatation in intermediate-risk PE patients. The primary outcome was the difference in the RV/LV ratio from baseline to 24 hours. In the interventional group (30 patients), the RV/LV ratio was reduced from 1.28 ± 0.19 at baseline to 0.99 ± 0.17 at 24 h (P < 0.001), while in the control group (29 patients) no significant decrease of the RV/LV ratio was observed at 24 h (1.20 ± 0.14 vs. 1.17 ± 0.20; P = 0.31). In both study groups, bleeding complications were rare, with three (10%) minor bleedings in the interventional group and one (3%) in the control group (P = 0.61). There was no major bleeding. The sample size was too small to demonstrate a change in mortality. The SEATTLE II study was a prospective, single-arm, multicenter study to assess the efficacy and safety of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis to reverse RV dysfunction in a total of 150 patients with CT-confirmed PE, symptoms for 14 days or less, and a RV/LV diameter ratio of at least 0.9 (23). Ultrasound-facilitated, catheter-directed, low-dose fibrinolysis decreased RV/LV diameter ratio (1.55 vs. 1.13; P < 0.0001), reduced pulmonary hypertension (51.4 mm Hg vs. 36.9 mm Hg; P < 0.0001), decreased anatomic thrombus burden (modified Miller Index score, 22.5 vs. 15.8; P < 0.0001), and minimized major bleeding complications (16 moderate and 1 major bleedings in 15 patients) in patients with acute massive and submassive PE.
Indirect evidence suggests a low major bleeding rate following ultrasound-assisted thrombolysis (rate of major bleeding complications, 3.6%; 95% CI, 1.4%-7.2%) compared to full-dose systemic thrombolytic therapy (24), but likely higher than that seen with anticoagulation alone. Clinical outcome studies are warranted to confirm a favorable risk to benefit ratio.
CDT has several variants (Table 3). The balance of the available data suggests that ultrasound-assisted thrombolysis does not increase thrombolytic efficacy when added to traditional CDT, and does not reduce the thrombolytic dose or shorten infusion times (25, 26).
Table 3.
Catheter-directed therapies
| Technique | Description |
|---|---|
| Thrombolysis | Catheter in main PA, thrombolytic infused. Often combined with mechanical or ultrasound fragmentation to increase surface area of thrombus exposed to thrombolytic. |
| Fragmentation | Breaking up large, central clot with catheter device; device rotated by operator. Fragments migrate distally. Often combined with local thrombolysis. |
| Embolectomy | Catheter directed to thrombus and manual suction used to remove thrombus. |
| Balloon Angioplasty | Compression of embolus. Often combined with local thrombolysis. |
| Percutaneous Thrombectomy | Clot pulverized and removed via catheter by rotation of device or hydrodynamic vortex. |
Abbreviations: PA, pulmonary artery.
Ongoing studies
DS-1040B is an inhibitor of the activated form of thrombin-activatable fibrinolysis inhibitor (TAFIa). In a thrombus, TAFIa removes lysine residues at the carboxy terminal of fibrin degradation products, which prevents effective binding of plasminogen and tissue plasminogen activator (t-PA), resulting in impaired thrombolysis (27). A phase 1b, double-blind, placebo-controlled, randomized, single-ascending dose, multi-center study is assessing the safety, efficacy, tolerability, pharmacokinetics, and pharmacodynamics of DS-1040B in subjects with acute submassive PE (NCT02923115).
ThE Recombinant hUman Prourokinase to Treat acute pulmonary Embolism (ERUPTE) trial will randomize patients with massive or submassive PE to low-dose (40 mg) recombinant human prourokinase or to alteplase (100 mg if weight ≥ 65 Kg, 1.5 mg/Kg if weight < 65 Kg) (NCT03108833). The primary outcome will be the change in the CT-assessed Qanadii score from baseline to 48 hours.
The primary aim of the Efficacy and safety of half dose alteplase added to heparin in patients with moderate pulmonary embolism (MONALYSE) open-label trial is to evaluate whether mid dose (safe dose) of alteplase, in addition to standard treatment with LMWH, is effective to reduce RV dysfunction, pulmonary hypertension and recurrent PE within the first 7 and 30 days after randomization of patients with intermediate-risk PE (NCT02604238).
Close monitoring of intermediate-risk pulmonary embolism
Patients with intermediate-risk PE could benefit from monitoring for deterioration. An important finding of the PEITHO trial was that “rescue thrombolytic therapy” appeared to be of benefit in patients who developed cardiovascular collapse after initially being treated with anticoagulant therapy alone. Of the 499 patients who received placebo in this trial, 25 (5.0%) experienced hemodynamic decompensation 1.79 ± 1.60 days after randomization. Persistent hypotension or a drop in blood pressure was recorded in 18 patients, vasopressors were administered to 14 patients, and 5 patients in the placebo group required cardiopulmonary resuscitation. Twenty-three of these patients received open-label thrombolysis, and only 2 of them died (success of “rescue thrombolysis”, 91%).
Pulmonary Embolism Response Teams
Given the relative frequency of PE, and complexity of decision making for advanced therapies for PE including for those with intermediate-risk PE, multiple groups have recently developed Pulmonary Embolism Response Teams (PERT) (28), which coordinate the management and interventions for these critical and complicated patients. A recent study showed that 91% of the PERT activations in the Weill Cornell Medical College came form patients with submassive (intermediate-risk) PE (28). Future studies will address whether PERTs improve clinical outcomes and are cost-effective.
ADDITIONAL/ALTERNATIVE TREATMENT STRATEGIES
Additional treatment strategies for patients with acute PE may include use of inferior vena cava (IVC) filters (29). The overall body of evidence for efficacy and safety of IVC filters for prevention of PE is slim (30). Temporary use of IVC filters in patients receiving thrombolytic therapy appears interesting, but is not yet supported by solid data. Such treatment should not be used in unselected patients with intermediate-risk PE. It might, however, be considered in a minority of high-risk patients on a case-by-case basis after multidisciplinary discussions (see above for Pulmonary Embolism Response Teams).
The inflammatory response associated with acute PE contributes to the development of RV dysfunction (31–33). Nonsteroidal anti-inflammatory drugs (NSAIDs) might facilitate the reversal of PE-associated RV dysfunction (34). Jimenez et al randomly assigned 34 normotensive patients who had acute PE associated with echocardiographic RV dysfunction and normal systemic blood pressure to receive intravenous (IV) diclofenac (two doses of 75 mg in the first 24 hours after diagnosis) or IV placebo (NCT01590342). All patients received standard anticoagulation with subcutaneous LMWH and an oral vitamin K antagonist. The study stopped prematurely due to slow recruitment. The intention-to-treat analysis showed persistent RV dysfunction at 48 hours in 59% (95% CI, 33-82%) of the diclofenac group and in 76% (95% CI, 50-93%) of the placebo group, a difference that did not reach statistical significance. Similar proportions (35%) of patients in the diclofenac and placebo groups had persistent RV dysfunction at 7 days. Major bleeding occurred in none of patients in the diclofenac group and in 5.9% of patient in the placebo group.
Interest has arisen in use of pulmonary arterial vasodilators (e.g., inhaled nitric oxide, oral phosphodiesterase inhibitors) in the treatment of acute PE (35, 36). Vasodilator drugs could affect hypoxic vasoconstriction, platelet activation, and release of vasoactive mediators (e.g., endothelin thromboxane). Theoretically, vasodilator treatment would lower the pulmonary artery pressure and unload the RV. Since PE causes acute RV overload by both mechanical obstruction and pulmonary vasospasm, Kline et al. tested if adjunctive inhaled nitric oxide (NO) gas causes would improve RV function and viability in acute PE (NCT 01939301). They conducted a four hospital, randomized, double blind, placebo-controlled trial (37). Seventy eight eligible normotensive patients acute PE and RV dysfunction received either oxygen plus 50 parts per million nitrogen (placebo) or oxygen plus 50 parts per million NO for 24 hours. At 24 hours, 5 of the 38 (13%) patients treated with placebo and 9 of the 38 (24%) patients treated with NO reached the primary composite endpoint (P = 0.375), which required a normal RV on echocardiography and a plasma troponin T concentration <14 pg/mL. The secondary endpoint required a blood brain natriuretic peptide concentration < 90 pg/mL and a Borg dyspnea score <=2, and it was reached in 34% with placebo and 13% of the NO (P = 0.11).
REDEFINITION OF INTERMEDIATE-RISK PULMONARY EMBOLISM
Among PE patients without hypotension, it is still not possible to confidently identify those who will derive net benefit from thrombolytic therapy. One of the important findings of the PEITHO trial was that the combination of RV dysfunction and myocardial injury might be insufficient for identifying normotensive patients with acute PE at a high-risk for short-term PE-related complications (mortality rate within the first 30 days after randomization of 3.2% in the placebo group) (13). For this reason, several prognostic scoring systems (e.g., PROTECT multimarker index, FAST score, and Bova score) exist for identification of patients with PE who have an intermediate-high risk for short-term PE-related adverse events. The PROTECT study derived (n = 848) and validated (n = 529) a multimarker prognostication that consisted of a clinical prognostic rule (i.e., simplified Pulmonary Embolism Severity Index [sPESI], brain natriuretic peptide (BNP), cardiac troponin I (cTnI), and complete compression ultrasound testing for concomitant DVT for hemodynamically stable patients diagnosed with acute symptomatic PE in the Emergency Department (38). A 30-day complicated course was defined as death from any cause, hemodynamic collapse (need for cardiopulmonary resuscitation, systolic blood pressure < 90 mm Hg for at least 15 min, need for cathecolamine administration or need for thrombolysis), and/or adjudicated recurrent PE. The positive predictive value of the PROTECT score for the prediction of a complicated course was 25.8% (95% CI, 10.4-41.2%) in the derivation cohort and 21.2% (95% CI, 9.0-38.9%) in the validation cohort.
The FAST score includes heart-type fatty acid binding protein (H-FABP), heart rate, and syncope (39). A prospective cohort study that enrolled 271 normotensive patients with acute PE assessed the validity of the FAST score for accurately identifying intermediate-high risk PE patients. In this study, a FAST score ≥ 3 points had a positive predictive value of 22% (95% CI, 14-33%) for 30-day complications (i.e., all-cause death or at least one of the following major complications: (i) need for administration of vasopressors to maintain adequate tissue perfusion and prevent or treat cardiogenic shock; (ii) mechanical ventilation; or (iii) cardiopulmonary resuscitation).
The Bova score consisted of heart rate ≥ 110 beats per minute, systolic blood pressure 90-100 mmHg, RV dysfunction, and elevated cardiac troponin (40). The model identified three stages (I, II, and III) with 30-day PE-related complication rates of 4.2%, 10.8%, and 29.2%, respectively. A recent study demonstrated that the Bova score shows good reproducibility and evidence of validity for identification of intermediate-high risk patients with acute symptomatic PE (41).
A recent study compared the performance of a modified (i.e., using high-sensitivity troponin T instead of H-FABP) FAST score and the Bova score for risk stratification of 388 consecutive normotensive patients with acute symptomatic PE (42). The primary endpoint was defined as PE-related death, need for mechanical ventilation, cardiopulmonary resuscitation, or the administration of vasopressors during the first month after the diagnosis of acute PE. While the modified FAST score identified a significantly higher proportion of patients (111/388, 28.6%; 95% CI, 24.2-33.4%) in the intermediate-high risk class compared to the Bova score (63/388, 16.2%; 95% CI, 12.7-20.3%), the positive predictive values with regard to the primary endpoint were similar (FAST score, 19%; 95% CI, 13-27%; Bova score 19%; 95% CI, 11-30%). Ideally, future studies should enroll patients with these refined criteria to determine the net benefit in select subgroups. In the interim, subgroup analyses of the published large trials (e.g. PEITHO) or pooled patient-level analyses from these studies can clarify which of the existing scores providers better discrimination about intermediate-risk patients who derive benefit from thrombolytic therapy.
A PRACTICAL TREATMENT APPROACH (Figure)
Figure 1. A management approach to patients with intermediate-risk* pulmonary embolism.
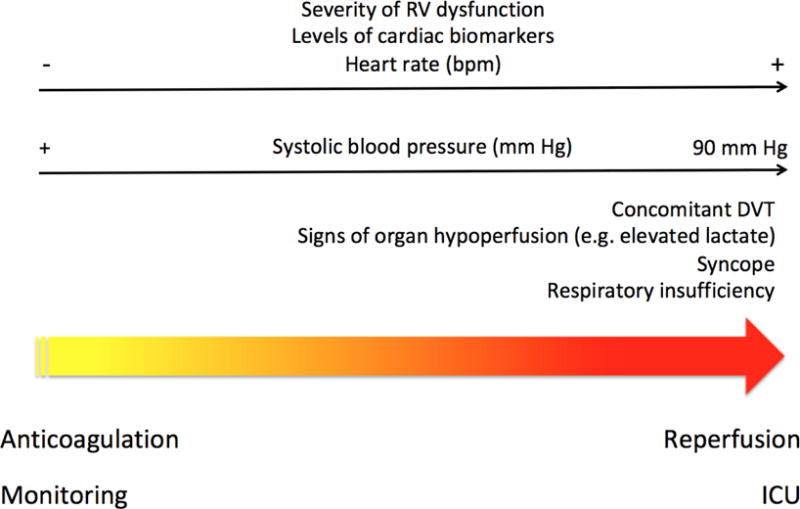
*Defined as the presence of both right ventricular dysfunction and myocardial injury.
Accumulation of factors indicating poor prognosis (right side of the Figure) may alter the risk-benefit assessment in favor of thrombolytic therapy before the development of hemodynamic instability.
Abbreviations: RV, right ventricle; bpm, beats per minute; DVT, deep vein thrombosis; ICU, intensive care unit.
In hemodynamically stable patients with acute symptomatic PE, presence of syncope, tachycardia, mild hypotension (which remains ≥ 90 mm Hg), an increase in jugular venous pressure, severe respiratory insufficiency (arterial oxyhemoglobin saturation < 90%), or early signs of shock (among others) may prompt the order of echocardiography and cardiac biomarkers of myocardial injury (i.e., cTnI or T). We suggest the use of echocardiography over CT to assess RV function in normotensive patients with acute symptomatic PE (43). Patients who have myocardial injury and RV dysfunction should receive standard anticoagulation, and be monitored for short-term deterioration, which might prompt activation of PERT for rapid and individualized care. In these patients, evidence of severe RV dysfunction and myocardial injury, accumulation of prognostic factors (e.g., syncope [39], concomitant DVT [44], elevated lactate levels [45], severe respiratory insufficiency) indicating poor prognosis from PE, or subtle hemodynamic changes (e.g. increasing heart rate or persistent downtrend of systolic blood pressure) might alter the risk-benefit assessment in favor of thrombolytic therapy before the development of frank hemodynamic instability (Table 4). For patients who require thrombolytic therapy and do not have a high risk of bleeding, full-dose systemic thrombolytic therapy should be preferred over low-dose systemic thrombolytic therapy or CDT.
Table 4.
Pulmonary embolism markers of severity
| Markers | Comment | |
|---|---|---|
| Clinical prognostic scores | PESI (47) | Identification of low-risk PE. Validated in a RCT (48) |
| Simplified PESI (49) | Identification of low-risk PE. Not validated in a RCT | |
| Hestia criteria (50) | Identification of low-risk PE. Validated in a management study (48) | |
| RIETE score (51) | Identification of low-risk PE. Not validated in a RCT | |
| Bova score (40) | Identification of intermediate-risk PE. Not validated in a RCT |
|
| FAST score (39) | Identification of intermediate-risk PE. Not validated in a RCT |
|
| Right ventricular enlargement/dysfunction | ECG (52) Echocardiogram (53) Computed tomography (54) BNP or NT-proBNP (55) Adrenomodullin (56) |
Identification of intermediate-risk PE. Limited usefulness, in isolation. |
| Clot burden | Concomitant deep vein thrombosis (44) D-dimer (57) CT-assessed thrombus load (58) |
Identification of intermediate-risk PE. Limited usefulness, in isolation. |
| Myocardial injury | cTnI or cTnT (59) hsTnT (60) H-FABP (61) |
Identification of intermediate-risk PE. Limited usefulness, in isolation. |
| Kidney injury | Cystatin C (62) | Lacks large validation. Not well defined role for identification of intermediate-risk PE |
| Organ hypoperfusion | Lactate (45) Copeptin (63) |
Lacks large validation. Not well defined role for identification of intermediate-risk PE |
Abbreviations: PESI, Pulmonary Embolism Severity Index; PE, pulmonary embolism; RCT, randomized controlled trial; RIETE, Registro Informatizado de la Enfermedad Tromboembólica; ECG, electrocardiogram; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; CT, computed tomography; cTnI, cardiac troponin I, cTnT, cardiac troponin T; hsTnT, high-sensitivity troponin T; H-FABP, hear-type fatty acid binding protein.
CONCLUSIONS
Management of patients with intermediate-risk PE is complicated by the various definitions, paucity of data for certain interventions, and uncertain guideline recommendations. While evidence suggests that most patients with intermediate-risk PE who receive standard anticoagulation and monitoring have an excellent short-term prognosis, the more severity of vital signs and markers of RV dysfunction and myocardial injury, factors indicating poor prognosis from PE, or early deterioration on standard anticoagulation might alter the risk-benefit assessment in favor of thrombolytic therapy before the development of hemodynamic instability. PERTs may provide rapid multidisciplinary assessment and optimal treatment of intermediate-risk PE patients.
KEY POINTS.
Intermediate-risk pulmonary embolism (PE) is defined by hemodynamic stability, but the presence of right ventricular dysfunction, myocardial injury, or both.
Most patients with intermediate-risk PE who receive standard anticoagulation and monitoring have an excellent short-term prognosis.
Accumulation of factors indicating worse outcomes from PE or early deterioration on standard anticoagulation might alter the risk-benefit assessment in favor of thrombolytic therapy before the development of hemodynamic instability.
Acknowledgments
B.B. is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, through grant number T32 HL007854. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. B.B. reports that he serves as an expert (on behalf of the plaintiff) for litigation related to IVC filters. The content of the current manuscript is not directly related to that litigation.
Footnotes
Disclosure statements: None
Declaration of interests
D.J. has nothing to disclose.
References
- 1.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez D, de Miguel J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol. 2016;67:162–170. doi: 10.1016/j.jacc.2015.10.060. [DOI] [PubMed] [Google Scholar]
- 4.Minges KE, Bikdeli B, Wang Y, Kim N, Curtis JP, Desai MM, Krumholz HM. National Trends in Pulmonary Embolism Hospitalization Rates and Outcomes for Adults Aged ≥65 Years in the United States (1999 to 2010) Am J Cardiol. 2015;116:1436–1442. doi: 10.1016/j.amjcard.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Torbicki A, Agnelli G, et al. Authors/Task Force Members 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS) Eur Heart J. 2014;35:3033–3073. [Google Scholar]
- 6.Jaff MR, McMurtry MS, Archer SL, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis and chronic thromboembolic pulmonary hypertension. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 7.Scridon T, Scridon C, Skali H, et al. Prognostic significance of troponin elevation and right ventricular enlargement in acute pulmonary embolism. Am J Cardiol. 2005;96:303–305. doi: 10.1016/j.amjcard.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 8.Binder L, Pieske B, Olschewski M, et al. N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation. 2005;112:1573–1579. doi: 10.1161/CIRCULATIONAHA.105.552216. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez D, Aujesky D, Moores L, et al. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66:75–81. doi: 10.1136/thx.2010.150656. [DOI] [PubMed] [Google Scholar]
- 10.Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J. 2003;24:1651–1656. doi: 10.1016/s0195-668x(03)00394-4. [DOI] [PubMed] [Google Scholar]
- 11.Tapson VF. Thrombolytic therapy in acute pulmonary embolism. Curr Opin Cardiol. 2012;27:585–591. doi: 10.1097/HCO.0b013e328358308c. [DOI] [PubMed] [Google Scholar]
- 12.Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, Diercks DB, Klinger JR, Hernandez J. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12:459–468. doi: 10.1111/jth.12521. [DOI] [PubMed] [Google Scholar]
- 13.Meyer G, Vicaut E, Danays T, et al. PEITHO Investigators Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 14.Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, Meyer G, Perrier A. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36:605–614. doi: 10.1093/eurheartj/ehu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M, “MOPETT” Investigators Moderate pulmonary embolism treated with thrombolytics (from the ‘MOPETT’ trial) Am J Cardiol. 2013;111:273–277. doi: 10.1016/j.amjcard.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Goldhaber SZ, Agnelli G, Levine MN. Reduced dose bolus alteplase vs conventional alteplase infusion for pulmonary embolism thrombolysis. An international multicenter randomized trial. The Bolus Alteplase Pulmonary Embolism Group. Chest. 1994;106:718–724. doi: 10.1378/chest.106.3.718. [DOI] [PubMed] [Google Scholar]
- 18.Sors H, Pacouret G, Azarian R, Meyer G, Charbonnier B, Simonneau G. Hemodynamic effects of bolus vs 2-h infusion of alteplase in acute massive pulmonary embolism. A randomized controlled multicenter trial. Chest. 1994;106:712–717. doi: 10.1378/chest.106.3.712. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Zhai Z, Yang Y, Wu Q, Cheng Z, Liang L, Dai H, Huang K, Lu W, Zhang Z, Cheng X, Shen YH, China Venous Thromboembolism (VTE) Study Group Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137:254–262. doi: 10.1378/chest.09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelsamad AA, El-Morsi AS, Mansour AE. Efficacy and safety of high dose versus low dose streptokinase for treatment of submassive pulmonary embolism. The Egyptian Heart Journal. 2011;63:67–72. [Google Scholar]
- 21.Jimenez D, Martin-Saborido C, Muriel A, Zamora J, Morillo R, Barrios D, Klok FA, Huisman MV, Tapson V, Yusen RD. Efficacy and safety outcomes of recanalization procedures in patients with acute symptomatic pulmonary embolism: systematic review and network meta-analysis. Thorax. 2017 doi: 10.1136/thoraxjnl-2017-210040. (press) [DOI] [PubMed] [Google Scholar]
- 22.Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Müller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Härtel D, Grünwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 23.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial od ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism. J Am Coll Cardiol Intv. 2015;8:1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur Heart J. 2014;35:758–764. doi: 10.1093/eurheartj/ehu029. [DOI] [PubMed] [Google Scholar]
- 25.Engelberger RP, Spirk D, Willenberg T, Alatri A, Do DD, Baumgartner I, Kucher N. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002027. [DOI] [PubMed] [Google Scholar]
- 26.Liang NL, Avgerinos ED, Marone LK, Singh MJ, Makaroun MS, Chaer RA. Equivalent outcomes between ultrasound-assisted thrombolysis and standard catheter-directed thrombolysis for the treatment of acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2015;3:120–121. doi: 10.1016/j.jvsv.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 27.DS-1040b Global Investigator’s Brochure Version 4.0. 2016 Jan 14; [Google Scholar]
- 28.Sista AK, Friedman OA, Dou E, Denvir B, Askin G, Stern J, Estes J, Salemi A, Winokur RS, Horowitz JM. A Pulmonary Embolism Response Team’s initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med. 2017 Sep 1; doi: 10.1177/1358863X17730430. 1358863X17730430. [DOI] [PubMed] [Google Scholar]
- 29.Bikdeli B, Bikdeli B. Updates on advanced therapies for acute pulmonary embolism. Int J Cardiovasc Pract. 2016;1:47–50. [Google Scholar]
- 30.Bikdeli B, Chatterjee S, Desai N, Kirtane AJ, Desai M, Bracken MH, Spencer FA, Monreal M, Goldhaber SZ, Krumholz HM. Inferior vena caval filters to prevent pulmonary embolism: systematic review and meta-analysis of efficacy and safety. J Am Coll Cardiol. 2017;70:1587–1597. doi: 10.1016/j.jacc.2017.07.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts JA, Gellar MA, Obraztsova M, et al. Role of inflammation in right ventricular damage and repair following experimental pulmonary embolism in rats. Int J Exp Pathol. 2008;89:389–399. doi: 10.1111/j.1365-2613.2008.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41:296–307. doi: 10.1016/j.yjmcc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Watts J, Marchick MR, Kline JA. Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension. J Cardiac Fail. 2010;16:250–259. doi: 10.1016/j.cardfail.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Watts JA, Gellar MA, Stuart LK, et al. Proinflammatory events in right ventricular damage during pulmonary embolism: effects of treatment with ketorolac in rats. J Cardiovasc Pharmacol. 2009;54:246–252. doi: 10.1097/FJC.0b013e3181b2b699. [DOI] [PubMed] [Google Scholar]
- 35.Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med. 1997;23:1089–1092. doi: 10.1007/s001340050461. [DOI] [PubMed] [Google Scholar]
- 36.Szold O, Khoury W, Biderman P, Klausner JM, Halpern P, Weinbroum AA. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung. 2006;184:1–5. doi: 10.1007/s00408-005-2550-7. [DOI] [PubMed] [Google Scholar]
- 37.Kline JA, Hall CL, Jones AE, Puskarich MA, Mastouri RA, Lahm T. Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: The iNOPE trial. Am Heart J. 2017;186:100–110. doi: 10.1016/j.ahj.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez D, Kopecna D, Tapson V, et al. on behalf of the Protect investigators Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189:718–726. doi: 10.1164/rccm.201311-2040OC. [DOI] [PubMed] [Google Scholar]
- 39.Dellas C, Tschepe M, Seeber V, et al. A novel H-FABP and fast prognostic score for risk assessment of normotensive pulmonary embolism. Thromb Haemost. 2014;111:996–1003. doi: 10.1160/TH13-08-0663. [DOI] [PubMed] [Google Scholar]
- 40.Bova C, Sanchez O, Prandoni P, Lankeit M, Konstantinides S, Vanni S, Jiménez D. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014;44:694–703. doi: 10.1183/09031936.00006114. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148:211–218. doi: 10.1378/chest.14-2551. [DOI] [PubMed] [Google Scholar]
- 42.Hobhom L, Hellenkamp K, Hasenfuß G, Münzel T, Konstantinides S, Lankeit M. Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur Respir J. 2016;47:1170–1178. doi: 10.1183/13993003.01605-2015. [DOI] [PubMed] [Google Scholar]
- 43.Barrios D, Morillo R, Lobo JL, Nieto R, Jaureguizar A, Portillo AK, Barbero E, Fernandez-Golfin C, Yusen RD, Jiménez D, PROTECT investigators Assessment of right ventricular function in acute pulmonary embolism. Am Heart J. 2017;185:123–129. doi: 10.1016/j.ahj.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Jiménez D, Aujesky D, Díaz G, Monreal M, Otero R, Martí D, Marín E, Aracil E, Sueiro A, Yusen RD, RIETE Investigators Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010;181:983–991. doi: 10.1164/rccm.200908-1204OC. [DOI] [PubMed] [Google Scholar]
- 45.Vanni S, Nazerian P, Bova C, Bondi E, Morello F, Pepe G, Paladini B, Liedl G, Cangioli E, Grifoni S, Jiménez D. Comparison of clinical scores for identification of patients with pulmonary embolism at intermediate-high risk of adverse clinical outcome: the prognostic role of plasma lactate. Int Emerg Med. 2017;12:657–665. doi: 10.1007/s11739-016-1487-6. [DOI] [PubMed] [Google Scholar]
- 46.Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F, Vanni S, Nitti C, Kamphuisen P, Vedovati MC, De Natale MG, Konstantinides S. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48:780–786. doi: 10.1183/13993003.00024-2016. [DOI] [PubMed] [Google Scholar]
- 47.Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, Renaud B, Verhamme P, Stone RA, Legall C, Sanchez O, Pugh NA, N’gako A, Cornuz J, Hugli O, Beer HJ, Perrier A, Fine MJ, Yealy DM. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378:41–48. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]
- 49.Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD, RIETE Investigators Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 50.Zondag W, Mos IC, Creemers-Schild D, Hoogerbrugge AD, Dekkers OM, Dolsma J, Eijsvogel M, Faber LM, Hofstee HM, Hovens MM, Jonkers GJ, van Kralingen KW, Kruip MJ, Vlasveld T, de Vreede MJ, Huisman MV, Hestia Study Investigators Outpatient treatment in patients with acute pulmonary embolism: the Hestia study. J Thromb Haemost. 2011;9:1500–1507. doi: 10.1111/j.1538-7836.2011.04388.x. [DOI] [PubMed] [Google Scholar]
- 51.Maestre A, Trujillo-Santos J, Riera-Mestre A, Jiménez D, Di Micco P, Bascuñana J, Vela JR, Peris L, Malfante PC, Monreal M, RIETE Investigators Identification of Low-Risk Patients with Acute Symptomatic Pulmonary Embolism for Outpatient Therapy. Ann Am Thorac Soc. 2015;12:1122–1129. doi: 10.1513/AnnalsATS.201504-202OC. [DOI] [PubMed] [Google Scholar]
- 52.Qaddoura A, Digby GC, Kabali C, Kukla P, Zhan ZQ, Baranchuk AM. The value of electrocardiography in prognosticating clinical deterioration and mortality in acute pulmonary embolism: A systematic review and meta-analysis. Clin Cardiol. 2017 Jun 19; doi: 10.1002/clc.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with hemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–1577. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 54.Trujillo-Santos J, den Exter PL, Gomez V, Del Castillo H, Moreno C, van der Hulle T, Huisman MV, Monreal M, Yusen RD, Jimenez D. Computed tomography-assessed right ventricular dysfunction and risk stratification of patients with acute non-massive pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11:1823–1832. doi: 10.1111/jth.12393. [DOI] [PubMed] [Google Scholar]
- 55.Pieralli F, Olivotto I, Vanni S, Conti A, Camaiti A, Targioni G, Grifoni S, Berni G. Usefulness of bedside testing for brain natriuretic peptide to identify right ventricular dysfunction and outcome in normotensive patients with acute pulmonary embolism. Am J Cardiol. 2006;97:1386–1390. doi: 10.1016/j.amjcard.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 56.Pedowska-Włoszek J, Kostrubiec M, Kurnicka K, Ciurzynski M, Palczewski P, Pruszczyk P. Midregional proadrenomedullin (MR-proADM) in the risk stratification of patients with acute pulmonary embolism. Thromb Res. 2013;132:506–510. doi: 10.1016/j.thromres.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Lobo JL, Zorrilla V, Aizpuru F, Grau E, Jiménez D, Palareti G, Monreal M, RIETE Investigators D-dimer levels and 15-day outcome in acute pulmonary embolism. Findings from the RIETE Registry. J Thromb Haemost. 2009;7:1795–1801. doi: 10.1111/j.1538-7836.2009.03576.x. [DOI] [PubMed] [Google Scholar]
- 58.Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, Fetzer DT, Saul M, Roberts MS, Bae KT. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283–293. doi: 10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 60.Lankeit M, Jiménez D, Kostrubiec M, Dellas C, Hasenfuss G, Pruszczyk P, Konstantinides S. Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation. 2011;124:2716–2724. doi: 10.1161/CIRCULATIONAHA.111.051177. [DOI] [PubMed] [Google Scholar]
- 61.Bajaj A, Rathor P, Sehgal V, Shetty A, Kabak B, Hosur S. Risk stratification in acute pulmonary embolism with heart-type fatty acid-binding protein: A meta-analysis. J Crit Care. 2015;30:1151.e1–7. doi: 10.1016/j.jcrc.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Kostrubiec M, Łabyk A, Pedowska-Włoszek J, Dzikowska-Diduch O, Wojciechowski A, Garlińska M, Ciurzyński M, Pruszczyk P. Neutrophil gelatinase-associated lipocalin, cystatin C and eGFR indicate acute kidney injury and predict prognosis of patients with acute pulmonary embolism. Heart. 2012;98:1221–1228. doi: 10.1136/heartjnl-2012-301884. [DOI] [PubMed] [Google Scholar]
- 63.Hellenkamp K, Schwung J, Rossmann H, Kaeberich A, Wachter R, Hasenfuß G, Konstantinides S, Lankeit M. Risk stratification of normotensive pulmonary embolism: prognostic impact of copeptin. Eur Respir J. 2015;46:1701–1710. doi: 10.1183/13993003.00857-2015. [DOI] [PubMed] [Google Scholar]


