Abstract
Background
Methotrexate, a folate antagonist, is an immunosuppressant drug that is effective for treating several inflammatory disorders including Crohn's disease. Ulcerative colitis, a related chronic inflammatory bowel disease, can be challenging to treat. T his updated systematic review summarizes the current evidence on the use of methotrexate for induction maintenance of remission in ulcerative colitis.
Objectives
The objectives of this review were to assess the efficacy and safety of methotrexate for maintenance of remission in patients with ulcerative colitis.
Search methods
We searched MEDLINE, EMBASE, CENTRAL and the Cochrane IBD/FBD group specialized trials register from inception to June 26, 2014. Study references and review papers were also searched for additional trials. Abstracts from major gastroenterological meetings were searched to identify research published in abstract form only.
Selection criteria
Randomized controlled trials in which methotrexate was compared to placebo or an active comparator in patients with quiescent ulcerative were considered for inclusion.
Data collection and analysis
Two authors independently extracted data and assessed the risk of bias for each study. The primary outcome was the occurrence of clinical or endoscopic relapse as defined by the primary studies. Secondary outcomes included frequency and nature of adverse events, change of disease activity score and steroid‐sparing effect. We calculated the risk ratio and corresponding 95% confidence interval for dichotomous outcomes. Data were analyzed on an intention‐to‐treat basis. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria.
Main results
Three trials (165 patients) fulfilled the inclusion criteria. One study compared oral methotrexate (12.5 mg/week) to placebo, another compared oral methotrexate (15 mg/week) to 6‐mercaptopurine (6‐MP, 1.5 mg/kg/day) or 5‐aminosalicylic acid (5‐ASA, 3 g/day) and the other compared methotrexate (15 mg/week) in combination sulfasalazine (3 g/day) to sulfasalazine. The placebo‐controlled study was rated as low risk of bias. The study comparing methotrexate to 6‐MP and 5‐ASA was rated as high risk of bias and the study assessing methotrexate and sulfasalazine was rated as unclear risk of bias for sequence generation, allocation concealment and blinding. The placebo‐controlled study found no statistically significant differences in the proportion of patients who maintained remission. At nine months, 36% (5/14) of methotrexate patients maintained remission compared to 54% (10/18) of placebo patients (RR 0.64, 95% CI 0.28 to 1.45). A GRADE analysis indicated that the overall quality of the evidence for this outcome was low due to very sparse data (15 events). The study comparing combination therapy to sulfasalazine found no statistically significant difference in the proportion of patients who maintained remission. At 12 months, 100% (14/14) of patients in the combination group maintained remission compared to 75% (9/12) of sulfasalazine patients (RR 1.32, 95% CI 0.94 to 0.86), A GRADE analysis indicated that the overall quality of the evidence for this outcome was very low due to unknown risk of bias and very sparse data (23 events). There were no statistically significant differences in maintenance of remission rates between methotrexate and 6‐MP or between methotrexate and 5‐ASA. At 76 weeks, 14% (1/7) of methotrexate patients maintained remission compared to 64% (7/11) of 6‐MP patients (RR 0.22, 95% CI 0.03 to 1.45) and 0% (0/2) of 5‐ASA patients (RR 1.13, 95% CI 0.06 to 20.71). A GRADE analysis indicated that the overall quality of the evidence from this study was very low due to high risk of bias and very sparse data. Adverse events reported in these studies included transient leucopenia, migraine, nausea and dyspepsia, mild alopecia, mild increase in aspartate aminotransferase levels, peritoneal abscess, hypoalbuminemia, severe rash and atypical pneumonia
Authors' conclusions
The results for efficacy and safety outcomes between methotrexate and placebo, methotrexate and sulfasalazine, methotrexate and 6‐mercaptopurine and methotrexate and 5‐aminosalicylic acid were uncertain. Whether a higher dose or parenteral administration of methotrexate would be effective in quiescent ulcerative colitis is unknown. At present there is no evidence supporting the use of methotrexate for maintenance of remission in ulcerative colitis. More studies are needed to determine the efficacy and safety of methotrexate maintenance therapy in patients with quiescent ulcerative colitis. Large scale methodologically rigorous randomized controlled trials are needed. These studies should investigate higher doses of methotrexate (e.g. 15 to 25 mg/week) and parenteral administration.
Plain language summary
Methotrexate for keeping ulcerative colitis inactive
Review Question
We reviewed the evidence about the effects and safety of methotrexate for maintaining remission in patients with ulcerative colitis, with the medical literature up to June 26, 2014.
Background
What is ulcerative colitis?
Ulcerative colitis is a chronic inflammatory bowel disease characterized by recurrent episodes of active disease, which commonly affect the rectum or colon or both. Patients with active disease may experience abdominal cramping, urgency to pass stools, and bloody diarrhea. When the symptoms stop, patients are considered to be in remission.
What is methotrexate?
Methotrexate is a medication that reduces the body's immune responses and may reduce inflammation associated with ulcerative colitis.
Study Characteristics
The researchers identified three studies that included a total of 165 patients. One study (67 patients) compared oral methotrexate (12.5 mg/week) to placebo (e.g. a sugar pill or fake medicine), one study (26 patients) compared oral methotrexate (15 mg/week) and sulfasalazine (3 g/day) to sulfasalazine alone, and one study (72 patients in total; of which, 34 had ulcerative colitis ) compared oral methotrexate (15 mg/week) to 6‐mercaptopurine (1.5 mg/kg/day) or 5‐aminosalicylic acid (3 g/day). Two studies were judged to be of very low quality and the placebo‐controlled study was judged to be of high quality.
Key Results
There was no difference between the methotrexate and placebo treatment groups for the number of people who maintained remission at nine months. This suggests that, when used at this low dose (12.5 mg/week) methotrexate does not maintain remission in patients with inactive ulcerative colitis. However, this result is uncertain due to the small number of people who were assessed
There was no difference between the combination therapy (methotrexate plus sulfasalazine) and sulfasalazine treatment groups for the number of people who maintained remission at 12 months. This result is uncertain due to poor study design and the low number of participants.
The other, small study showed no differences between methotrexate and the other treatments (6‐mercaptopurine and 5‐aminosalicylic acid) in the proportion of participants who were able to maintain remission. These results are uncertain due to poor study design and the low number of participants.
The side effects reported in the studies included leucopenia (a decrease in the number of white blood cells), migraine, rash, nausea and dyspepsia (indigestion), mild alopecia (hair loss), mild increase in levels of an enzyme found in the liver (aspartate aminotransferase), a collection of pus in the abdominal tissue (peritoneal abscess), abnormally low levels of the protein albumin in the blood (hypoalbuminemia), and pneumonia.
At present, the results from medical trials do not support the use of low dose oral methotrexate (12.5 mg to 15 mg/week) for maintenance of remission in people with inactive ulcerative colitis. It is not known whether a higher dose of oral methotrexate, or giving methotrexate by a different route (e.g. by injection), would be effective for maintenance of remission in people with inactive ulcerative colitis.
In future, researchers should consider organizing a study with a larger number of participants who receive a higher dose of methotrexate (15 to 25 mg/week). Future studies should also investigate methotrexate given by injection. The results of such studies may resolve the uncertainty surrounding the use of methotrexate as maintenance therapy in people with inactive ulcerative colitis.
Summary of findings
Background
Description of the condition
Ulcerative colitis is a chronic inflammatory disease of the colon. Typical symptoms include bloody diarrhea, abdominal cramps and urgency to pass stool. Some patients do not respond to first line therapy which usually consists of oral or topical 5‐aminosalicylic acid. Thus additional treatments are needed.
Description of the intervention
Methotrexate, a folate antagonist with potent anti‐inflammatory effects, was first used for the treatment of rheumatoid arthritis in 1951 (Schnabel 1994).
How the intervention might work
Thiopurines including azathioprine and 6‐mercaptopurine have been the traditional first‐line immunosuppressive agents in both Crohn’s disease and ulcerative colitis. However, two multi‐center randomized controlled trials demonstrated efficacy of parenteral methotrexate for induction and maintenance of remission in steroid‐dependant Crohn's disease (Feagan 1995; Feagan 2000). In uncontrolled studies, other investigators have reported efficacy with both subcutaneous and oral administration of methotrexate in thiopurine‐refractory or intolerant pediatric Crohn's patients (Turner 2007).
Why it is important to do this review
The efficacy of methotrexate for induction and maintenance of remission in ulcerative colitis has yet to be defined. A recent Cochrane review found methotrexate to be ineffective compared to placebo or active comparators for induction of remission in ulcerative colitis (Chande 2014). Methotrexate may be of value for maintenance of remission in ulcerative colitis. This systematic review is an update of a previously published Cochrane review (El‐Matary 2009).
Objectives
The primary objectives were to systematically assess the efficacy and safety of methotrexate for maintenance of remission in ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Only randomized controlled trials (RCTs) comparing methotrexate to placebo or any other active intervention were considered for inclusion in this review. Cohort studies, case series and studies with other non‐experimental designs were not considered for inclusion.
Types of participants
Participants, of any age who were diagnosed with ulcerative colitis as defined by clinical and endoscopic criteria and were in remission (as defined by any activity index) when started or continued on methotrexate were considered for inclusion.
Types of interventions
Studies were considered if participants in the treatment group received oral, subcutaneous (SC) or intramuscular (IM) methotrexate at any dose and the comparison group received either a placebo or any other active intervention. Any duration of follow‐up and any co‐intervention (provided it was given to both groups) were allowed.
Types of outcome measures
Primary outcomes
The primary outcome measure was the occurrence of clinical or endoscopic relapse as defined by the primary studies.
Secondary outcomes
Secondary outcomes included:
1) Frequency and nature of adverse events;
2) Change of disease activity score; and
3) Steroid‐sparing effect.
Search methods for identification of studies
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, from inception to June 26, 2014 to identify applicable studies. We searched the Cochrane IBD/FBD Group Specialized Register to identify studies published in abstract form, The clinical trial database (www.clinicaltrials.gov) was searched to identify ongoing studies. Review papers on ulcerative colitis, and references from identified studies papers were also searched in an effort to identify all randomized trials studying methotrexate use in patients with ulcerative colitis. The search strategies are reported in Appendix 1. No language restrictions or filters were used for the searches.
Data collection and analysis
Selection of studies
Initially, one author (YW) screened all of the articles retrieved through the database searches described above. All studies were first screened on the basis of title, and then on the basis of abstract. During this screening process, studies were classified into three categories: 'exclude' for studies in which it was clear from either the title or abstract that the study did not address the research question or was not an RCT, 'include' for RCT studies that addressed the research question, and 'unsure' for studies for which there was uncertainty. Studies which were denoted 'include' or 'unsure' were considered potentially relevant, and were further examined in the second phase of the selection process. In the second phase of study selection, two independent investigators (YW and JKM) assessed potentially relevant studies based on the inclusion criteria described above. The full‐text of the articles was retrieved in order to decide whether these criteria were met. Disagreement was resolved by consensus. The authors were not blinded to the name of authors and institutions, journal of publication, or study results during study selection or at any other stage of the review.
Data extraction and management
Data were extracted by one author (YW), confirmed by a second independent investigator (JKM) and entered into Review Manager (RevMan 5.3.5). The following data were extracted: study methods (study design, method of randomization, withdrawals and loss‐to‐follow‐up), study participants (sample size, age, and gender), intervention (methotrexate), dose, route, duration of treatment and adverse events), control (agent, dose) and outcomes (types of outcomes, timing of outcome measurements, reported outcomes).
Assessment of risk of bias in included studies
Two independent investigators (YW and JKM) assessed study quality using the Cochrane risk of bias tool (Higgins 2011).
Factors that were assessed included:
Sequence generation (i.e. was the allocation sequence adequately generated?);
Allocation sequence concealment (i.e. was allocation adequately concealed?);
Blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
Incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
Selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
Other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?).
A judgment of 'Yes' indicated low risk of bias, 'No' indicated high risk of bias and 'Unclear' indicated unclear or unknown risk of bias. Differences in rating were resolved through discussion and mutual consensus.
The overall quality of the evidence was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Outcome data are rated as being of high, moderate, low or very low quality evidence. Data from randomized controlled trials begin as high quality but can be downgraded based on the following criteria:
Risk of bias in the included trials;
Indirect evidence;
Inconsistent findings (including unexplained heterogeneity);
Imprecision (i.e. sparse data and/or wide confidence interval); and
Reporting bias.
Measures of treatment effect
For dichotomous outcomes we calculated the risk ratio (RR) and corresponding 95% confidence intervals (95% CI). For continuous variables, data were to be reported as the mean difference (MD) and corresponding 9%% CI.
Dealing with missing data
Authors of the selected studies were contacted to obtain missing data. All data were analyzed on an intention‐to‐treat basis whereby all study withdrawals were treated as treatment failures.
Assessment of heterogeneity
If more than one study was included in a pooled analysis, we planned to assess heterogeneity by visually inspecting forest plots and by calculating the Chi2 (a P value of 0.10 or less would be considered statistically significant) and I 2 statistics (Higgins 2003).
Assessment of reporting biases
For future updates if 10 or more studies are included in a pooled analysis, we will construct a funnel plot to assess potential publication bias.
Data synthesis
Data were entered into Review Manager (Cochrane Collaboration, Version 5.3.5). For future updates of this review we will combine data from individual trials for meta‐analysis when the interventions, patient groups and outcomes are sufficiently similar (to be determined by consensus). The pooled RR and 95% CI will be calculated for dichotomous outcomes. For continuous outcomes the pooled MD and corresponding 95% CI will be calculated. We will calculate the standardized mean difference (SMD) and 95% CI when different scales have been used to measure the same underlying construct. A fixed‐effect model will be used to pool data unless significant heterogeneity exists between the studies. A random‐effects model will be employed if heterogeneity exits (I2 50 to 75%). We will not pool data for meta‐analysis if a high degree of heterogeneity (I2 ≥ 75%) is detected.
Sensitivity analysis
If future updates of this review include pooled analyses Planned sensitivity analyses included calculating a random effects model and assessing the effect of methodological quality on the pooled estimate.
Results
Description of studies
Results of the search
The literature search conducted on 26 June 2014 identified 2770 records. Eight additional studies were identified through searching of conference abstracts. After duplicates were removed, a total of 2483 studies remained for review of titles and abstracts. Thirty‐six studies were selected for full text review (see Figure 1). Thirty‐three reports of 28 studies were excluded. Three studies met the pre‐defined inclusion criteria and were included in the review (Mate‐Jimenez 2000; Onuk 1996; Oren 1996). Two large scale studies are currently underway (NCT00498589; NCT01393405). However, the results of these studies are currently unavailable.
1.
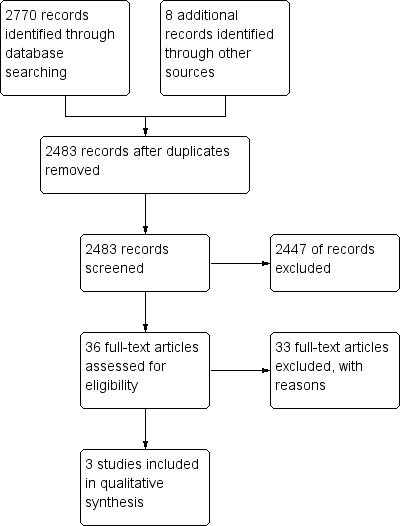
Study flow diagram.
Included studies
Mate‐Jimenez 2000 recruited 72 steroid‐dependent IBD patients (34 with ulcerative colitis and 38 with Crohn's disease). The trial was 106 weeks in length (30 weeks of induction phase and 76 weeks of maintenance phase). The patients were randomly assigned in a 2:2:1 ratio to receive 1.5 mg/kg/day of oral 6‐mercaptopurine (6‐MP, n = 14), 15 mg/week of oral methotrexate (n = 12) or 3 g/day of oral 5‐aminosalicylic acid (5‐ASA, n = 8).
Onuk 1996 recruited 26 patients with quiescent ulcerative colitis who were receiving maintenance therapy with sulfasalazine. Patients were randomized to receive either oral methotrexate (15 mg/week) plus oral sulfasalazine (3 g/day, n = 14) or sulfasalazine alone (n = 12) for 12 months.
Oren 1996 recruited 67 patients with active ulcerative colitis (Mayo Clinic score > 7). Concurrent use of steroids or 5‐ASA was allowed. No other immunosuppressive agent was allowed during the study or during the 3 months preceding randomization. Patients were randomly assigned to oral methotrexate 12.5 mg/week (n = 30) or placebo (n = 37) for 9 months of treatment.
Excluded studies
Reasons for exclusion are reported in the Characteristics of excluded studies table. The results of the excluded studies are described in additional Table 5.
1. Results of excluded studies.
| ID | Description | Results |
| Adedokun 2013 | PURSUIT‐SC induction study randomized UC patients to PBO/PBO (n=331), GLM 100mg/50mg (n=72), GLM 200mg/100mg (n=331), and GLM 400mg/200mg (n=331) at week 0 and 2. PURSUIT maintenance study enrolled 1228 patients, but only those who responded to GLM induction (n=464) were randomized (PBO, GLM 50mg or GLM 100 mg Q4W for 52 weeks) and included in the primary analysis. | During induction and maintenance, there was a positive Exposure‐Response relationship between SGC and efficacy outcomes regardless of immunomodulator (IM) use. Concomitant IM use did not appear to affect efficacy outcomes. Antibodies to GLM (ATG) incidence was lower in patients receiving IMs vs those who were not (1.5% vs 3.5%, overall 2.8%). |
| Aloi 2010 | A retrospective chart review of 38 pediatric UC patients treated with methotrexate (n=18 azathioprine unresponsiveness, n =10 azathioprine intolerance, n=4 spondyloarthropathy). Pediatric Ulcerative Colitis Activity Index (PUCAI) and use of corticosteroids were the main outcomes evaluated at baseline and at 3, 6 and 12 months. | Response or remission was achieved in 72%, 63% and 50% of patients at 3, 6 and 12 months respectively. Mean PUCAI were 49.5 ± 23.3 at baseline and 32.9 ± 21.9, 29.5 ± 21.8 and 29.4 ± 19.9 at 3, 6 and 12 months respectively (P = 0.03). 13 of the 16 patients on corticosteroids, had their steroid discontinued by 6 months. At the end of the study, 11 patients needed short course of corticosteroids for disease relapse. |
| Baron 1993 | Open‐label 18 week study in which 19 (11 CD and 8 UC) patients with refractory IBD were given 15 mg/week methotrexate (starting at a low dose then increased gradually) | In the UC group, only 3 patients had a partial response. None of the UC patients has complete remission. However, there was a significant reduction in the daily prednisone dose. Adverse events were minor |
| Cummings 2005 | Retrospective chart review that evaluated 50 patients with UC (8 had rheumatoid arthritis as well and analyzed separately) who were intolerant or resistant to azathioprine. The mean dose of methotrexate was 19.9 mg/week for a median duration of 30 weeks and was given orally | Remission occurred in 42% of patients. Colitis remained in remission in 7/8 patients with concomitant rheumatoid arthritis. Side effects occurred in 23% but only 10% needed to stop methotrexate due to adverse effects |
| Dejica 1998 | A 20 week non‐randomized, open‐label study evaluate the effectiveness of weekly 25 mg methotrexate intramuscular injection (with 1 mg/day folic acid supplementation) in patients (n=22) with chronic active ulcerative colitis, refractory to steroids ± sulfasalazine for at least 3 months. | Fifteen patients (68%) had significant clinical improvement (Mayo Clinic score decreased from 8.2 to 4.7; P<0.001). Eleven patients achieved clinical remission (Mayo Clinic score ≤ 3), and 5/11 achieved histological remission. Under continuous sulfasalazine administration, 8/11 patients (73%) remained in remission after 6 months from cessation of methotrexate therapy. Adverse events (n=5) were not severe enough to warrant treatment interruptions. |
| Egan 1999 | A 16 week‐randomized single‐blinded study comparing weekly SC 15 mg to 25 mg of methotrexate in 32 steroid‐dependant patients (10 with UC and 22 with CD) | Only 6 patients improved at the end of the induction period. Overall the dose escalation did not result in significant difference in treatment effect or toxicity |
| Egan 2000 | Case series of 5 steroid‐refractory patients (3 with UC and 2 with CD) who failed methotrexate 25g/week SC for 16 weeks and had oral cyclosporine 3mg/kg/day added to methotrexate for another 16 weeks | Improvement of 3 patients with UC. One patient developed hypertension |
| Fraser 2002 | Retrospective chart review of 70 patients (48 with CD and 22 with UC) who were given methotrexate for a mean duration of 17.1 months at a mean maintenance dose of 20mg/week (oral in 62 patients and IM in 8) | Remission was achieved in 62% of patients who completed more than 3 months of treatment. The chance of maintaining remission (if treatment continues) at 12, 24 and 36 months were 90%, 73% and 51% respectively. Treatment was equally effective in UC and CD |
| Fraser 2003a | 16 week non‐randomized, open label study included 8 patients with moderate to severe refractory UC confirmed by sigmoidoscopy. They were treated with 25 mg IM MTX (and folic acid) and severity of disease was assessed by Mayo Clinic score (at recruitment, median 9, range 7‐11) | Six of eight patients completed 16 week treatment. Median Mayo Clinical score 8 (range 6‐11). One patient withdrew due to sever exacerbation and 1 withdrew because failure to improve. Treatment with 25mg MTX IM for 16 weeks was ineffective in this small group of patients with refractory UC. |
| Ghiselli 2011 | Retrospective study in 17 patients who were less than 16 years old and affected by steroid‐dependent or steroid‐resistant UC, They were treated with IM methotrexate (20 mg/m2/week). Clinical remission ‐ discontinuation of steroids and achievement of S0 stage of Montreal classification after 3, 6 and 12 months. endoscopic remission ‐ level 0 of Baron score at the same timing of clinical score calculation. | After 3 months, MTX induced clinical and endoscopic remission in 82% and 71% of UC patients, respectively. Of the nine UC patients continued MTX for > 3 months, 56% maintained clinical and endoscopic remission after 6 months; and 33% at 1 year. Adverse events occurred in one patient (acute pancreatitis) and led to discontinuation of treatment. |
| Gibson 2006 | Retrospective chart review that evaluated 65 patients with IBD (20 UC and 45 CD). Dose of methotrexate varied from 10‐25 mg/week | 63% achieved remission and treatment continued for a median of 11 months |
| González‐Lama 2012 | Retrospective chart review of 77 IBD patients (80% Crohn's disease) treated with methotrexate in eight hospitals of Madrid, Spain | Initially, 82% of patients responded (28% in remission). Forty percent of the patients lost response at a mean of 57 weeks after starting methotrexate. Adverse events included GI symptoms (n=10), myelotoxicity (n=4), and abnormal LFT (n=10), which let to withdrawal of treatments in 4 patients. |
| Hayes 2014 | Restrospective analysis of UC patients treated with infliximab (IFX, n=85), comparing duration of efficacy, and serum IFX and serum IFX and antibody‐to‐IFX (ATI) levels between those receiving IFX as monotherapy (n=46) and in combination with an immunosuppressant (n=38). | Conconmitant immunosuppressant use was associated with associated with increased duration of IFX therapy (90% vs.61% remained on therapy at 1 year, P =0.025), and less frequent ATI formation (4.5% vs. 33.3%, P=0.031). The majority of patients received purine analogues (e.g. azathioprine or mercaptopurine), only 3 out of 46 patients in the combination group received methotrexate. |
| Herrlinger 2005 | Pharmacogenetics study of 102 IBD patients treated with MTX and 202 patients with Crohn's disease, 205 patients with ulcerative colitis and 189 healthy volunteers to assess allele frequencies in the disease and healthy populations. Four polymorphisms (G80A in the RCF1 gene, G452T in GGH gene, and C677T and A1298C in MTHFR gene) were genotyped for genotype‐phenotype association with respect to efficacy and toxicity of MTX therapy. | No significant differences in the allele frequencies between CD, UC, and healthy controls were detected. Side effects of MTX in IBD are more likely to associate with a SNP (MTHFR 1298C) in the MTHFR gene (21% vs. 6.3%, P<0.05), but response cannot be predicted by any of the investigated SNPs. |
| Houben 1994 | Retrospective chart review of 15 patients (13 with CD and 2 with UC) who were given 25 mg/week IM for 12 weeks followed by tapering oral dose | Symptomatic improvement occurred in 12/15 patients with reduction of prednisone dose after 3 months |
| Kariyawasam 2013 | A multicentre, longitudinal cohort study of 1118 Crohn's disease patients (14530 patient years of follow‐up) and 1171 ulcerative colitis patients (18035 patient years of follow‐up). Patient demographics, disease characteristics according to Montreal classification, drug and surgical treatments were reviewed. Comparisons were made between 3 times periods of pre 1990, 1990‐2000, and post 2000. | In Crohn's disease cohort, cumulative probability of commencing immunomodulators was significantly reduced over the time periods (mean time to commence IM use, 15.9, 6.0 and 1.3, P<0.0001). Long‐term steroid (LTS) use (P<0.0001), surgical resection and recurrent resection (P=0.002) also decreased significantly. In ulcerative colitis cohort, time to introduction of IM significantly decreased over successive decades (P<0.0001) and was associated with significant reduction in the use of LTS (P<0.0001) and surgical resection rate (P=0.033). |
| Katsanos 2012 | A retrospective study of effectiveness and safety electronic files of 543 IBD patients (58.6% UC patients) treated from 1981‐2010, of which, 48 patients were treated with methotrexate (33 CD, 14 UC, 1 undetermined) | Methotrexate was effective in 57% of patients with IBD and 27% of patients with fistulizing disease. Infection was the commonest adverse event. |
| Kozarek 1989 | An open‐label observational study that recruited 21 patients with refractory IBD (14 CD and 7 UC) who were given 25 mg/week of methotrexate IM for 12 weeks | Five patients with UC had clinical improvement with successful reduction of the concurrent steroid dose. None had endoscopic healing. Complications were reported in 7 out of the 21 patients and included transient diarrhea, leucopenia, elevated AST, brittle nails and atypical pneumonitis |
| Kozarek 1992 | Retrospective chart review where 86 patients (30 with UC) with refractory IBD were given 25 mg/week of parenteral methotrexate. The responders at 12 weeks were continued on oral methotrexate (7.5‐15 mg/week) | 70% of patients with UC showed some response but only 40 % continued to have response at a mean follow‐up of 59 weeks |
| Mañosa 2011 | Retrospective chart review of 40 patients with UC from 8 Spanish IBD referral hospital, who received MTX for steroid dependency (70%) or steroid refractoriness (27%). Therapeutic success was defined as the absence of UC symptoms, complete steroid withdrawal and no requirement of rescue therapies within the first 6 months after starting MTX. | Forty‐five percent of patients met criteria for therapeutic success. The cumulative probability of maintaining steroid‐free clinical remission was 60%, 48%, and 35% at 6, 12, 24 months after starting MTX, respectively. Eleven patients had adverse events and 8 required MTX discontinuation. |
| Nathan 2008 | Retrospective chart review of 68 patients with IBD (45 with CD and 23 with UC) who received methotrexate because of intolerance or resistance to purine analogues. UC patients received a mean does of 23mg/week methotrexate SC. The duration of treatment was not clear | In UC group, 11 patients achieved remission and 3 had some symptomatic improvement. Adverse events developed in 26% of patients and included nausea, leucopenia, abnormal LFTs, and diarrhea |
| Paoluzi 2002 | Open‐label study in which 10 patients with UC‐ who were intolerant or resistant to azathioprine‐ were given methotrexate at a dose of 12.5mg/week IM | Six patients achieved complete remission and remained in remission at the end of long‐term treatment (duration was not specified). Four improved at 6 months after induction and two of them remained well |
| Rook 2005 | Non‐randomized, open‐label study evaluating the use of methotrexate in patients with steroid dependant chronic active ulcerative colitis intolerant of thiopurines. They were treated with subcutaneous methotrexate 25 mg weekly for 12 weeks followed by oral methotrexate 15 mg/week thereafter, with standard folic acid therapy. | Six of eight patients were in clinical remission and able to fully withdraw steroid therapy. There were no relapse after a mean follow‐up period of 10 months. Two patients who failed to respond went on to receive colectomy. Methotrexate was well‐tolerated with one incidence of dose reduction due to nausea. |
| Siveke 2003 | Case series of 4 patients with UC who started on methotrexate after stopping azathioprine for various reasons. Methotrexate was given at a dose of 25 mg/week IM in 3 patients while the 4th patient had a starting dose of 15 mg which was then increased to 25 mg/week | Three patients remained in remission after 2‐3 years of treatment with methotrexate. The fourth patient had an elevated ALT concentration that lead to discontinuation of the drug |
| Soon 2004 | Retrospective chart review of 6 patients with UC and 66 patients with CD who received methotrexate at a mean dose of 18.2 mg/week (IM in 8 patients and oral for the rest) for different indications. Fifteen patients were treated with both methotrexate and azathioprine simultaneously | All the six patients with UC completed at least 6 months of treatment. Only 3 patients had a clinical response. None of the patients with UC had adverse events while 25% of patients with CD had one or more adverse event |
| Wahed 2009 | Retrospective chart review of 131 patients with IBD (CD, n=99; UC, n=32) who were treated with MTX to examine the efficacy and safety profile of methotrexate in patients who are either intolerant or non‐responsive to AZA/MP. Clinical response (defined as steroid withdrawal, normalization of previously raised CRP or physician's clinical assessment of improvement) was assessed at 6 months. | In Crohn's disease, clinical response occurred in 18 of 29 patients (62%) refractory to AZA/MP and 42 of 70 patients (60%) intolerant to AZA/MP. In UC, clinical response occurred in 7 of 9 patients (78%) refractory to AZA/MP and 15 of 23 (65%) intolerant to thiopurines. Methotrexate was well tolerated in a majority of patients. |
| Willot 2011 | A retrospective chart review of 93 pediatric IBD patients (75 CD, 5 UC, and 13 IC) from a single center, who received MTX treatment. Remission was defined as discontinuation of steroids and Harvey‐Bradshaw Index <4 for CD patients, PUCAI < 10 for UC or IC patients. | Among the 79 patients assessed for effectiveness of MTX, clinical remission was observed in 29, 37, 25, and 16% of CD patients (n=63) and 18, 25, 13, and 7% of patients with UC or IC (n=16), respectively, 3, 6, 12, and 24 months after initiation of MTX. Forty‐six patients (49%) experienced side effects but only 13 (14%) required discontinuation. |
| Zadvornova 2010 | Retrospective chart review of 104 UC patients who initiated on IFX maintenance therapy (57% on combination therapy with immunomodulator and 43% on IFX alone). Combined immunomodulator therapy included AZA, 6‐MP, or MTX. The primary outcome was occurrence of IFX discontinuation and/or colectomy. | The primary outcome occurred in 18 patients in the combination group (31%) and 18 patients in the IFX alone group (40%, P=0.31). Ten patients (17%) in the combination group and 8 patients (18%) in the IFX alone group underwent colectomy (P=0.91). Combined immunomodulator therapy was not predictive of IFX discontinuation or colectomy (HR 1.22, 95% CI 0.54‐2.76). |
Risk of bias in included studies
The risk of bias results are summarized in Figure 2. Mate‐Jimenez 2000 was an open‐label study and was rated as high risk of bias for blinding. In addition, Mate‐Jimenez 2000 did not report the methods used for randomization or allocation concealment and these items were rated as unclear risk of bias. Oren 1996 used adequate methods of randomization, blinding, and allocation concealment and was rated as low risk of bias for these items. Onuk 1996 was an abstract publication and methods used for randomization, allocation concealment and blinding were not described. These items were rated as unclear risk of bias. All three included studies were rated as low risk of bias for incomplete outcome data (Mate‐Jimenez 2000; Onuk 1996; Oren 1996). No other issues were found with the trials and they were rated as low risk of bias for the other bias item (Mate‐Jimenez 2000; Onuk 1996; Oren 1996).
2.
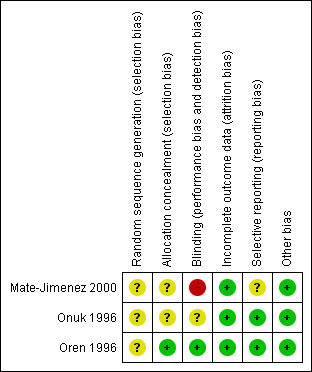
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Methotrexate compared to placebo for maintenance of remission in ulcerative colitis.
| Methotrexate compared to placebo for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: patients with quiescent ulcerative colitis Settings: Outpatient Intervention: Methotrexate Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Methotrexate | |||||
| Maintenance of remission Follow‐up: mean 36 weeks | 556 per 10001 | 356 per 1000 (156 to 806) | RR 0.64 (0.28 to 1.45) | 32 (1 study) | ⊕⊕⊝⊝ low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of study 2 Downgraded two levels due to very sparse data (15 events)
Summary of findings 2. Methotrexate compared to 5‐ASA for maintenance of remission in ulcerative colitis.
| Methotrexate compared to 5‐ASA for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: patients with quiescent ulcerative colitis Settings: Outpatient Intervention: Methotrexate Comparison: 5‐ASA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐ASA | Methotrexate | |||||
| Maintenance of remission Follow‐up: mean 76 weeks | 0 per 10001 | 0 per 1000 (0 to 0) | RR 1.12 (0.06 to 20.71) | 9 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of study 2 Downgraded one level due to high risk of bias 3 Downgraded two levels due to very sparse data (1 event)
Summary of findings 3. Methotrexate compared to 6‐MP for maintenance of remission in ulcerative colitis.
| Methotrexate compared to 6‐MP for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: patients with maintenance of remission in ulcerative colitis Settings: Outpatient Intervention: Methotrexate Comparison: 6‐MP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 6‐MP | Methotrexate | |||||
| Maintenance of Remission Follow‐up: mean 76 weeks | 636 per 10001 | 140 per 1000 (19 to 923) | RR 0.22 (0.03 to 1.45) | 18 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of study 2 Downgraded one level due to high risk of bias 3 Downgraded two levels due to very sparse data (8 events)
Summary of findings 4. Methotrexate + sulfasalazine compared to sulfasalazine.
| Methorexate + SFZN compared to SFZN alone for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: patients with maintenance of remission in ulcerative colitis Settings: Outpatient Intervention: Methorexate + SFZN Comparison: SFZN alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SFZN alone | Methorexate + SFZN | |||||
| Maintenance of Remission Follow‐up: mean 52 weeks | 750 per 10001 | 990 per 1000 (705 to 1000) | RR 1.32 (0.94 to 1.86) | 26 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of study 2 Downgraded one level due to unclear risk of bias for random sequence generation, allocation concealment and blinding 3 Downgraded one level due to very sparse data (23 events)
Methotrexate versus placebo
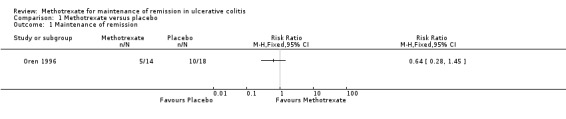
There was no statistically significant difference in the proportion of patients who maintained remission in the Oren 1996 study. Thirty‐six per cent (5/14) of methotrexate patients maintained remission compared to 54% (10/18) of placebo patients (RR 0.64; 95% CI 0.28 to 1.45; P = 0.29 ; See Figure 3). A GRADE analysis indicated that the quality of evidence is low due to very sparse data (See Table 1 ). The mean monthly steroid dose was not significantly different between the two groups. There were no statistically significant differences in the Mayo Clinic score between the two groups at baseline or during the study. Three patients were withdrawn from the study because of adverse events; two from the methotrexate group (transient leucopenia and migraine) and one from placebo group (severe rash).
3.

Forest plot of comparison: 1 Methotrexate versus Placebo, outcome: 1.1 Maintenance of Remission.
Methotrexate versus active comparators (5‐ASA or 6‐MP)
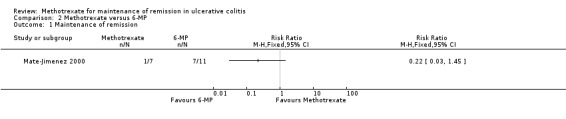
Mate‐Jimenez 2000 looked at the efficacy of methotrexate in patients with ulcerative colitis (N = 34) for both induction of remission and maintenance of remission. Patients in remission and off steroids at the end of 30 weeks were then entered into a 76 week maintenance phase. There were no statistically significant differences in the proportion of patients who maintained remission. Only one of seven (14%) patients in the methotrexate group maintained remission at the end of study compared to 7/11 (64%) patients in 6‐MP group (RR 0.22, 95% CI 0.03 to 1.45; See Figure 4). No patients in the 5‐ASA group (0/2) maintained remission (RR 1.13, 95% CI 0.06 to 20.71, see Figure 5). However, the results of this study should be interpreted with caution due to the small sample size and poor methodological quality. GRADE analyses found the overall quality of the evidence was very low due to very sparse data and high risk of bias (see Table 2; Table 3). Adverse events experienced by methotrexate patients included nausea and dyspepsia, mild alopecia, mild increase in aspartate aminotransferase levels, peritoneal abscess, hypoalbuminemia, severe rash and atypical pneumonia.
4.

Forest plot of comparison: 2 Methotrexate versus 6‐MP, outcome: 2.1 Maintenance of remission.
5.

Forest plot of comparison: 3 Methotrexate versus 5‐ASA, outcome: 3.1 Maintenance of remission.
Methothrexate plus sulfasalazine versus sulfasalazine
Onuk 1996 studied the efficacy of methotrexate in quiescent ulcerative colitis patients receiving sulfasalazine as maintenance therapy. There was no statistically significant difference in the proportion of patients who maintained remission at 52 weeks. One hundred per cent (14/14) of patients in the combination group maintained remission compared to 75% (9/12) of patients in the sulfasalazine alone group (RR 1.32, 95% CI 0.94 to 0.86, see Figure 6). A GRADE analysis indicated that the quality of evidence is very low due to very sparse data and unknown high risk of bias (see Table 4). Onuk 1996 reported that patient compliance with therapy was excellent and that no patients were withdrawn in either group because of drug‐related intolerance or adverse events.
6.

Forest plot of comparison: 4 Methorexate + SFZN versus SFZN alone, outcome: 4.1 Maintenance of Remission.
Discussion
Methotrexate is an analogue of dihydrofolic acid that inhibits dihydrofolate reductase and folate dependant enzymes which are vital for the de novo synthesis of purines and pyrimidines, formation of polyamines, and transmethylation of DNA, RNA, phospholipids, and proteins (Te 2000). Interestingly these effects take place with high dose methotrexate rather than low dose as low dose methotrexate has no cytotoxic or antiproliferative effects but is instead an immunosuppressive (Herrliger 2005; Bianchi Porro 2007).
Over the last 20 years, methotrexate has become recognized as a potent anti‐inflammatory drug and is currently used in a spectrum of inflammatory conditions including rheumatoid arthritis, psoriasis, collagen vascular diseases and inflammatory bowel disease (Bianchi Porro 2007).
Traditionally, induction of remission in ulcerative colitis is achieved using corticosteroids or 5‐ASA preparations (Schroder 2003). The latter can also be used for maintenance of remission. However, some patients with ulcerative colitis may be steroid‐dependent or resistant in which case immunosuppressives may be considered (Fraser 2003b). Colectomy is reserved for patients who are refractory to medical management.
The efficacy of methotrexate for treating steroid‐dependent and steroid‐resistant Crohn's disease has been evaluated in a number of studies and parenteral methotrexate is effective for inducing and maintaining remission in Crohn's disease (Feagan 1995; Feagan 2000; Turner 2007; McDonald 2014).
In contrast, a recent Cochrane review included only two randomized, controlled trials that examined the efficacy of low dose (12.5 to 15 mg/week) oral methotrexate in chronic ulcerative colitis (Chande 2014). This systematic review concluded that methotrexate provided no benefit for inducing remission in active ulcerative colitis. However, the dose and the route of methotrexate used were different from those commonly used for the treatment of active Crohn's disease(25 mg/week; Feagan 1995).
In the Oren 1996 study patients were followed up for nine months. Patients who entered remission were followed until they relapsed. Oral methotrexate (12.5 mg/week) provided no benefit over placebo for maintenance of remission in quiescent ulcerative colitis. The results of the GRADE analysis indicate that overall quality of the evidence supporting this outcome was low due to sparse data. Although the low dose selected used in this study was proven effective in other inflammatory conditions (rheumatoid arthritis), one can argue the severity of inflammation is different in UC. Methotrexate is known to have antiproliferative effects only when given in higher doses (Herrliger 2005). This action may be needed to induce and maintain remission in refractory IBD. Feagan 2000 found that parenteral methotrexate at a dose of 15 mg/week was effective for maintaining remission in patients with Crohn’s disease who entered remission after treatment with methotrexate. Whether or not a higher dose or parenteral administration of methotrexate would be more effective for maintenance of remission in ulcerative colitis patients is unknown.
Two smaller studies (Mate‐Jimenez 2000; Onuk 1996) assessed the effectiveness of methotrexate in comparison with active controls. There were no statistically significant differences in clinical remission rates. GRADE analyses found the quality of the evidence to be very low due to sparse data and risk of bias. Thus, no conclusions regarding efficacy can be drawn from these two studies.
Adverse events were poorly reported on in the three included studies and no conclusions can be drawn regarding the safety of methotrexate maintenance therapy in patients with quiescent ulcerative colitis. Adverse events reported in the placebo‐controlled study included two study withdrawals in the methotrexate group due to transient leucopenia and migraine and one withdrawal in the placebo group due to severe rash (Oren 1996). It was unclear if these withdrawals occurred during induction or maintenance therapy. Oren 1996 did not report on the proportion of patients who experienced any adverse event or serious adverse events. Adverse events experienced by methotrexate patients in the Mate‐Jimenez 2000 study included nausea and dyspepsia, mild alopecia, mild increase in aspartate aminotransferase levels, peritoneal abscess, hypoalbuminemia, severe rash and atypical pneumonia. Mate‐Jimenez 2000 reported on the proportion of patients who withdrew due to adverse effects but it was not clear whether these patients had ulcerative colitis or Crohn's disease or if the withdrawals occurred during the induction or maintenance phases of the study. Mate‐Jimenez 2000 did not report on the proportion of patients who experienced any adverse event or serious adverse events. Onuk 1996 reported that patient compliance was excellent and that no patients were withdrawn because of treatment‐related intolerance or adverse events. The Onuk 1996 did not report on the proportion of patients who experienced any adverse event or serious adverse events.
Authors' conclusions
Implications for practice.
The results for efficacy and safety outcomes between methotrexate and placebo, methotrexate and sulfasalazine, methotrexate and 6‐mercaptopurine and methotrexate and 5‐aminosalicylic acid were uncertain. Whether a higher dose or parenteral administration of methotrexate would be effective in quiescent ulcerative colitis is unknown. At present there is no evidence supporting the use of methotrexate for maintenance of remission in ulcerative colitis.
Implications for research.
More studies are needed to determine the efficacy and safety of methotrexate maintenance therapy in patients with quiescent ulcerative colitis. Large scale methodologically rigorous randomized controlled trials are needed. These studies should investigate higher doses of methotrexate (e.g. 15 to 25 mg/week) and parenteral administration.
What's new
| Date | Event | Description |
|---|---|---|
| 26 June 2014 | New citation required and conclusions have changed | Substantively updated review with new conclusions and authors |
| 26 June 2014 | New search has been performed | New literature search was performed to update the review |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Search strategies
MEDLINE search strategy
1. ulcerative colitis.mp. or exp ulcerative colitis/
2. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
3. 1 or 2
4. methotrexate.mp. or exp methotrexate derivative/ or exp methotrexate/ or exp methotrexate gamma aspartic acid/ or exp methotrexate polyglutamate/
5. 3 and 4
EMBASE search strategy
1. ulcerative colitis.mp. or exp ulcerative colitis/
2. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
3. 1 or 2
4. methotrexate.mp. or exp methotrexate derivative/ or exp methotrexate/ or exp methotrexate gamma aspartic acid/ or exp methotrexate polyglutamate/
5. 3 and 4
CENTRAL search strategy
1. ulcerative colitis
2. methotrexate
3. 1 and 2
SR‐IBD
colitis AND methotrexate
Data and analyses
Comparison 1. Methotrexate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Methotrexate versus placebo, Outcome 1 Maintenance of remission.
Comparison 2. Methotrexate versus 6‐MP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Methotrexate versus 6‐MP, Outcome 1 Maintenance of remission.
Comparison 3. Methotrexate versus 5‐ASA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
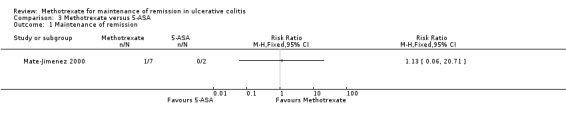
Comparison 3 Methotrexate versus 5‐ASA, Outcome 1 Maintenance of remission.
Comparison 4. Methotrexate + sulfasalazine versus sulfasalazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
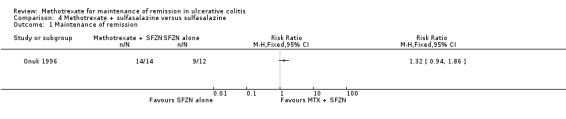
Comparison 4 Methotrexate + sulfasalazine versus sulfasalazine, Outcome 1 Maintenance of remission.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mate‐Jimenez 2000.
| Methods | Randomized (in 2:2:1 ratio to methotrexate, 6‐MP, and 5‐ASA), single‐center, controlled clinical trial | |
| Participants | Radiological or endoscopic diagnosis of CD or UC and steroid dependent (N = 72) Steroid dependent was defined as those patients whose prednisone could not be lowered to 20 mg/day without presenting inflammatory activity determined by a Mayo ClinicScore of 7 or more or having presented more than two episodes in the last 6 months or more than 3 in the last 12 months None of the patients had received 6‐MP or methotrexate prior to entry Numbers for ulcerative colitis participants: Methotrexate n = 12, 6‐mercaptopurine n = 14, 5‐aminosalicylic acid n = 8 | |
| Interventions | Oral methotrexate 15 mg/wk or 6‐mercaptopurine 1.5 mg/kg/day or 5‐aminosalicylic acid 3 g/day for 30 weeks For 2 weeks after randomization no attempt was made to decrease prednisone dose, thereafter prednisone was decreased by 8 mg/week Prednisone was reduced if the condition of the patient remained stable or improved and discontinued if clinical remission was achieved Methotrexate was reduced to 10 mg/week and the 6‐mercaptopurine dose to 1 mg/kg/day if clinical remission was achieved Patients in the 5‐aminosalicylic acid group continued to receive 3 g/day after achieving remission and stopping prednisone |
|
| Outcomes | For maintenance remission study: relapse within 76 weeks (defined as CD Activity index >150 for patients with CD, and 7 or more in the Mayo Clinc Score for patients with UC) or severe side effects, such as bone marrow suppression and serum concentration increases to twice the upper limit of normal for aminotransferases. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in published study |
| Allocation concealment (selection bias) | Unclear risk | Not described in published study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned in published study Authors assumed the study was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/72 patients dropped out in the first 30 weeks of the trial (worst outcome assumed) |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were reported Some post hoc outcomes were also reported |
| Other bias | Low risk | The study appears to be free of other biases |
Onuk 1996.
| Methods | Randomized controlled trial | |
| Participants | Patients diagnosed with ulcerative colitis using the appropriate combinations of clinical, endoscopic, histological and radiological criteria. All patients were maintained in full remission on oral SFZN for at least 2 months at study entry (N = 26). | |
| Interventions | Twenty‐six patients were randomly assigned to receive either MTX (15 mg per week, orally) plus oral sulfasalazine (3 g per day, orally) (n = 14) or sulfasalazine alone (n = 12) for 12 months | |
| Outcomes | Relapse was defined as endoscopic inflammatory score and histological activity index were grade 2 or higher, or if symptoms were present. | |
| Notes | Abstract publication, further efforts to locate the full article publication were not successful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were randomly assigned to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the published abstract |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the published abstract |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in the study were accounted for |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcome |
| Other bias | Low risk | The study appears to be free of other biases |
Oren 1996.
| Methods | Randomized, double‐blinded controlled trial | |
| Participants | Patients (N = 67) with definite, chronic active ulcerative colitis (Mayo clinic score of > or = 7 at entry) Chronicity was defined as steroid therapy at > or = 7.5 mg/day for at least 4 months of the proceeding year Ulcerative colitis was diagnosed by clinical, radiographic, endoscopic, and pathological criteria | |
| Interventions | Oral methotrexate (n=30; 12.5 mg/wk ‐ 2.5 mg/day) or identical placebo (n=37) for 9 months | |
| Outcomes | Remission defined as a Mayo Clinic score of < 3 and off steroids Time to first remission Relapse defined as an increase in the Mayo Clinic score of > 3 and/or reintroduction of steroids at a dose of > 300 mg/month |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The manuscript does not describe the method used for randomization |
| Allocation concealment (selection bias) | Low risk | Adequate: Prepackaged coded sets (equal number of methotrexate or placebo tablets) were delivered to each centre. When the sets were used subsequent randomization was performed by a central pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The centralized pharmacy and unblinded observer were only ones with access to the code |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were more dropouts in the placebo group (n=9) than in the methotrexate group (n=2). Three patients withdrew from the study due to adverse events (1 from the placebo group and 2 from the methotrexate group). Attrition bias does not appear to be a serious problem in the study as the trial was negative and ITT analyses were used |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adedokun 2013 | RCT of golimumab without separate presentation of methotrexate data |
| Aloi 2010 | Retrospective chart review |
| Baron 1993 | Open label design (Not RCT) |
| Cummings 2005 | Retrospective chart review |
| Dejica 1998 | Non‐randomized, open label clinical trial |
| Egan 1999 | Single blinded trail comparing 2 different doses of methotrexate in inducing remission |
| Egan 2000 | Case series |
| Fraser 2002 | Retrospective study |
| Fraser 2003a | Open label trial on UC induction |
| Ghiselli 2011 | Retrospective chart review |
| Gibson 2006 | Retrospective chart review |
| González‐Lama 2012 | Retrospective chart review |
| Hayes 2014 | Retrospective chart review |
| Herrlinger 2005 | Case‐controlled, pharmacogenetic study of methotrexate in IBD |
| Houben 1994 | Retrospective chart review |
| Kariyawasam 2013 | Prospective cohort study |
| Katsanos 2012 | Retrospective chart review |
| Kozarek 1989 | Non‐randomized open‐label trial |
| Kozarek 1992 | Retrospective chart review |
| Mañosa 2011 | Retrospective chart review |
| Nathan 2008 | Retrospective chart review |
| Paoluzi 2002 | Open‐label trial |
| Rook 2005 | Non‐randomized, uncontrolled, open label trial |
| Siveke 2003 | Case series |
| Soon 2004 | Retrospective chart review |
| Wahed 2009 | Retrospective chart review |
| Willot 2011 | Retrospective chart review |
| Zadvornova 2010 | Retrospective chart review |
Characteristics of ongoing studies [ordered by study ID]
NCT00498589.
| Trial name or title | A controlled, randomized, double‐blinded, multicenter study, comparing methotrexate to placebo in steroid‐refractory ulcerative colitis (METEOR) |
| Methods | A randomized, double‐blinded controlled multicenter study |
| Participants | Patients with steroid‐refractory UC (n=110) |
| Interventions | Methotrexate (25 mg/week by intramuscular injection, n=55) versus placebo (n=55) (Phase II) |
| Outcomes | Steroid free remission at 16 and 24 weeks |
| Starting date | September 2007 |
| Contact information | Franck Carbonnel, Tel: 00 33 3 81 66 82 53, Email: fcarbonnel@chu‐besancon.fr |
| Notes | NCT00498589; study is ongoing |
NCT01393405.
| Trial name or title | Randomized, double blind, prospective trial investigating the efficacy of methotrexate in induction and maintenance of steroid free remission in ulcerative colitis (MEthotrexate Response In Treatment of UC ‐ MERIT‐UC) |
| Methods | double‐blind, placebo controlled, randomized, multicenter, parallel group trial |
| Participants | Active ulcerative colitis (n=220) |
| Interventions | Methotrexate: induction period (week 1‐16) (open label): 25 mg MTX subcutaneous (sq) once weekly + steroid taper + 1 mg folic acid daily; maintenance period (week 17‐48) (randomization):25 mg MTX sq once weekly + 1 mg folic acid daily + 2.4 g mesalamine Placebo: sq once weekly + 1 mg folic acid daily + 2.4 g mesalamine |
| Outcomes | Primary outcome: relapse free survival; Secondary outcome: mucosal healing and relapse of disease Aims of the study: i) the safety and tolerability MTX over 48 weeks; ii) the relapse‐free survival of MTX maintenance therapy compared to placebo over 32 weeks; iii) the efficacy of MTX to induce steroid free remission over 16 weeks; iv) the evaluation of clinical and pharmacogenomic models to predict the response to MTX therapy in patients with UC |
| Starting date | February 2012 |
| Contact information | Hans Herfarth, Tel: 919‐966‐6806, Email: hherf@med.unc.edu |
| Notes | NCT01393405; study is ongoing, estimated completion date is June 2016 |
Sources of support
Internal sources
None, Other.
External sources
New Source of support, Other.
-
None, Other.
None
Declarations of interest
YW: None known
JKM: None known
BV: None known
AMG: Anne Marie Griffiths has received fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbvie; lecture fee(s) from: Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the submitted work.
WE: Dr El‐Matary received a research support from Janssen, Canada and served as an advisory board member for Janssen and AbbVie Canada. All of these financial activities are outside the submitted work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Mate‐Jimenez 2000 {published data only}
- Mate‐Jimenez J, Hermida C, Cantero‐Perona J, Moreno‐Otero R. 6‐mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12(11):1227‐33. [PUBMED: 11111780] [DOI] [PubMed] [Google Scholar]
Onuk 1996 {published data only}
- Onuk MD, Kavmakoğlu S, Demir K, Çakaloğlu Y, Boztaş G, Munga, Z, et al. Low‐dose weekly methotrexate therapy in remission maintenance in ulcerative colitis. Gut 1996;39(Suppl 3):A75. [Google Scholar]
Oren 1996 {published data only}
- Oren R, Arber N, Odes S, Moshkowitz M, Keter D, Pomeranz I, et al. Methotrexate in chronic active ulcerative colitis: a double‐blind, randomized, Israeli multicenter trial. Gastroenterology 1996;110(5):1416‐21. [PUBMED: 8613046] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adedokun 2013 {published data only}
- Adedokun O, Xu Z, Marano C, Strauss R, Zhang H, Johanns J, et al. Effects of immunomodulators on the pharmacokinetics and efficacy of golimumab in patients with moderately to severely active ulcerative colitis: Results from phase 2/3 pursuit‐SC induction and maintenance studies. American Journal of Gastroenterology 2013;108:S517. [Google Scholar]
Aloi 2010 {published data only}
- Aloi M, Conte F, Cavallari N, Iacono O, Viola F, Civitelli F, et al. Role of methotrexate in pediatric ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 2009;48:S111‐2. [Google Scholar]
- Aloi M, Nardo G, Conte F, Mazzeo L, Cavallari N, Nuti F, et al. Methotrexate in paediatric ulcerative colitis: a retrospective survey at a single tertiary referral centre. Alimentary Pharmacology and Therapeutics 2010;32(8):1017‐22. [PUBMED: 20937047] [DOI] [PubMed] [Google Scholar]
Baron 1993 {published data only}
- Baron TH, Truss CD, Elson CO. Low‐dose oral methotrexate in refractory inflammatory bowel disease. Digestive Diseases and Sciences 1993;38(10):1851‐6. [PUBMED: 8404406] [DOI] [PubMed] [Google Scholar]
Cummings 2005 {published data only}
- Cummings JR, Herrlinger KR, Travis SP, Gorard DA, McIntyre AS, Jewell DP. Oral methotrexate in ulcerative colitis. Alimentary Pharmacology and Therapeutics 2005;21(4):385‐9. [PUBMED: 15709988] [DOI] [PubMed] [Google Scholar]
Dejica 1998 {published data only}
- Dejica D, Porr PJ. Long‐term parenteral therapy with methotrexate in refractory ulcerative colitis: preliminary results. Romanian Journal of Gastroenterology 1998;7(3):175‐8. [Google Scholar]
Egan 1999 {published data only}
- Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, et al. A randomized dose‐response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn's disease and ulcerative colitis. Alimentary Pharmacology and Therapeutics 1999;13(12):1597‐604. [PUBMED: 10594394] [DOI] [PubMed] [Google Scholar]
- Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, et al. A randomized, single‐blind, pharmacokinetic and dose response study of subcutaneous methotrexate, 15 and 25 mg/week, for refractory ulcerative colitis and Crohn's disease. Gastroenterology 1998;114(4 Pt 2):A227. [Google Scholar]
Egan 2000 {published data only}
- Egan LJ, Tremaine WJ, Mays DC, Lipsky JJ, Sandborn WJ. Clinical outcome and pharmacokinetics after addition of low‐dose cyclosporine to methotrexate: a case study of five patients with treatment‐resistant inflammatory bowel disease. Inflammatory Bowel Diseases 2000;6(4):286‐9. [PUBMED: 11149561] [DOI] [PubMed] [Google Scholar]
Fraser 2002 {published data only}
- Fraser AG, Morton D, McGovern D, Travis S, Jewell DP. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2002;16(4):693‐7. [PUBMED: 11929386] [DOI] [PubMed] [Google Scholar]
Fraser 2003a {published data only}
- Fraser GM, Ben‐Bassat O, Segal N, Fishman‐Mor M, Niv Y. Parenteral methotrexate is not effective treatment for refractory ulcerative colitis. Gastroenterology 2003;125(4 Suppl 1):A525. [Google Scholar]
Ghiselli 2011 {published data only}
- Ghiselli A, Calzolari C, Perazzo P, Soriani P, Madia C, Vincenzi F, et al. Use of methotrexate in children and young adults affected by ulcerative colitis: a retrospective study. American Journal of Gastroenterology 2011;106:S459. [Google Scholar]
Gibson 2006 {published data only}
- Gibson P, Nathan D, John I. Subcutaneous methotrexate: a safe and effective therapy in IBD. Gastroenterology 2006;130(4 Suppl 2):A661. [Google Scholar]
González‐Lama 2012 {published data only}
- González‐Lama Y. Efficacy and safety of methotrexate therapy in inflammatory bowel disease. The Madrid experience. Gastroenterology 2009;136(5 Suppl 1):A662. [Google Scholar]
- González‐Lama Y, Taxonera C, López‐Sanromán A, Pérez‐Calle JL, Bermejo F, Pajares R, et al. Methotrexate in inflammatory bowel disease: a multicenter retrospective study focused on long‐term efficacy and safety. The Madrid experience. European Journal of Gastroenterology and Hepatology 2012;24(9):1086‐91. [PUBMED: 22713509] [DOI] [PubMed] [Google Scholar]
Hayes 2014 {published data only}
- Hayes MJ, Sakuraba A, Stein AC, Hanauer SB. A comparison of efficacy, pharmacokinetics and immunogenicity in patients with ulcerative colitis receiving infliximab monotherapy versus combination therapy. Gastroenterology 2013;144(5 Suppl 1):S430. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Stein AC, Sakuraba A. Comparison of efficacy, pharmacokinetics, and immunogenicity between infliximab mono‐ versus combination therapy in ulcerative colitis. Journal of Gastroenterology and Hepatology 2014 Jun;29(6):1177‐85. [PUBMED: 24955449] [DOI] [PubMed] [Google Scholar]
Herrlinger 2005 {published data only}
- Herrlinger KR, Cummings JR, Barnardo MC, Schwab M, Ahmad T, Jewell DP. The pharmacogenetics of methotrexate in inflammatory bowel disease. Pharmacogenetics and Genomics 2005;15(10):705‐11. [PUBMED: 16141796] [DOI] [PubMed] [Google Scholar]
Houben 1994 {published data only}
- Houben MH, Wijk HJ, Driessen WM, Spreeuwel JP. Methotrexate as possible treatment in refractory chronic inflammatory intestinal disease [Methotrexaat als mogelijke behandeling bij refractaire chronische inflammatoire darmziekte]. Nederlands Tijdschrift Voor Geneeskunde 1994;138(51):2552‐6. [PUBMED: 7830804] [PubMed] [Google Scholar]
Kariyawasam 2013 {published data only}
- Kariyawasam V, Huang T, Lunney P, Middleton K, Wang R, Selinger C, et al. Early treatment with immunomodulators is associated with change in the natural history of inflammatory bowel disease ‐ multicentre longitudinal cohort study ‐ Sydney, Australia. Journal of Crohn's and Colitis 2013;7:S272. [Google Scholar]
Katsanos 2012 {published data only}
- Katsanos KH, Sigounas DE, Strogyli K, Tatsioni A, Panagiotou E, Vagias I, et al. Methotrexate use in inflammatory bowel disease: a referral centre's 30 years of experience. Journal of Crohn's and Colitis 2012;6:S98. [Google Scholar]
Kozarek 1989 {published data only}
- Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Annals of Internal Medicine 1989;110(5):353‐6. [PUBMED: 2492786] [DOI] [PubMed] [Google Scholar]
Kozarek 1992 {published data only}
- Kozarek RA, Patterson OJ, Gelfand MD, Ball TJ, Botoman VA. Long‐term use of methotrexate in inflammatory bowel disease. Gastroenterology 1992;102(Suppl):A648. [Google Scholar]
Mañosa 2011 {published data only}
- Mañosa M, García V, Castro L, García‐Bosch O, Chaparro M, Barreiro‐de Acosta M, et al. Methotrexate in ulcerative colitis: a Spanish multicentric study on clinical use and efficacy. Journal of Crohn's and Colitis 2011 Oct;5(5):397‐401. [PUBMED: 21939912] [DOI] [PubMed] [Google Scholar]
Nathan 2008 {published data only}
- Nathan DM, Iser JH, Gibson PR. A single center experience of methotrexate in the treatment of Crohn's disease and ulcerative colitis: a case for subcutaneous administration. Journal of Gastroenterology and Hepatology 2008;23(6):954‐8. [PUBMED: 17559377] [DOI] [PubMed] [Google Scholar]
Paoluzi 2002 {published data only}
- Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, et al. Azathioprine or methotrexate in the treatment of patients with steroid‐dependent or steroid‐resistant ulcerative colitis: results of an open‐label study on efficacy and tolerability in inducing and maintaining remission. Alimentary Pharmacology and Therapeutics 2002;16(10):1751‐9. [PUBMED: 12269968] [DOI] [PubMed] [Google Scholar]
Rook 2005 {published data only}
- Rook L, Kelly SM. Rescue methotrexate therapy in chronic active ulcerative colitis patients intolerant of thiopurines. Gut 2005;54:A92. [Google Scholar]
Siveke 2003 {published data only}
- Siveke JT, Folwaczny C. Methotrexate in ulcerative colitis. Alimentary Pharmacology and Therapeutics 2003;17(3):479‐80. [PUBMED: 12562464] [DOI] [PubMed] [Google Scholar]
Soon 2004 {published data only}
- Soon SY, Ansari A, Yaneza M, Raoof S, Hirst J, Sanderson JD. Experience with the use of low‐dose methotrexate for inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2004;16(9):921‐6. [PUBMED: 15316419] [DOI] [PubMed] [Google Scholar]
Wahed 2009 {published data only}
- Wahed M, Louis‐Auguste JR, Baxter LM, Limdi JK, McCartney SA, Lindsay JO, et al. Efficacy of methotrexate in Crohn's disease and ulcerative colitis patients unresponsive or intolerant to azathioprine /mercaptopurine. Alimentary Pharmacology and Therapeutics 2009;30(6):614‐20. [PUBMED: 19552632] [DOI] [PubMed] [Google Scholar]
Willot 2011 {published data only}
- Willot S, Noble A, Deslandres C. Methotrexate in the treatment of inflammatory bowel disease: An 8‐year retrospective study on 91 patients treated in a Canadian pediatric IBD centre. Canadian Journal of Gastroenterology 2009;23:Conference: Canadian Digestive Diseases Week, Banff, AB Canada. [Google Scholar]
- Willot S, Noble A, Deslandres C. Methotrexate in the treatment of inflammatory bowel disease: an 8‐year retrospective study in a Canadian pediatric IBD center. Inflammatory Bowel Diseases 2011;17(22):2521‐6. [PUBMED: 21337668] [DOI] [PubMed] [Google Scholar]
Zadvornova 2010 {published data only}
- Zadvornova Y, Ananthakrishnan AN, Stein DJ, Skaros S, Johnson K, Naik AS, et al. Infliximab monotherapy vs. combined infliximab‐immunomodulator therapy in ulcerative colitis. Gastroenterology 2010;138(5 Suppl 1):S690. [Google Scholar]
References to ongoing studies
NCT00498589 {unpublished data only}
- NCT00498589. A controlled, randomized, double‐blind, multicenter study, comparing methotrexate vs placebo in corticosteroid‐dependent ulcerative colitis. https://www.clinicaltrials.gov/ct2/show/NCT00498589 (accessed 21 July 2015).
NCT01393405 {unpublished data only}
- NCT01393405. Randomized, double blind, prospective trial investigating the efficacy of methotrexate in induction and maintenance of steroid free remission in ulcerative colitis (MEthotrexate Response In Treatment of UC ‐ MERIT‐UC). https://www.clinicaltrials.gov/ct2/show/NCT01393405 (accessed 21 July 2015).
Additional references
Bianchi Porro 2007
- Bianchi Porro G, Cassinotti A, Ferrara E, Maconi G, Ardizzone S. Review article: the management of steroid dependency in ulcerative colitis. Alimentary Pharmacology and Therapeutics 2007;26(6):779‐94. [PUBMED: 17767462] [DOI] [PubMed] [Google Scholar]
Chande 2014
- Chande N, Wang Y, MacDonald JK, MacDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD006618.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 1995
- Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, et al. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. New England Journal of Medicine 1995;332(5):292‐7. [PUBMED: 7816064] [DOI] [PubMed] [Google Scholar]
Feagan 2000
- Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. New England Journal of Medicine 2000;342(22):1627‐32. [PUBMED: 10833208] [DOI] [PubMed] [Google Scholar]
Fraser 2003b
- Fraser AG. Methotrexate: first‐line or second‐line immunomodulator?. European Journal of Gastroenterology and Hepatology 2003;15(3):225‐31. [PUBMED: 12610315] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrliger 2005
- Herrlinger KR, Cummings JR, Barnardo MC, Schwab M, Ahmad T, Jewell DP. The pharmacogenetics of methotrexate in inflammatory bowel disease. Pharmacogenetics and Genomics 2005;15(10):705‐11. [PUBMED: 16141796] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
McDonald 2014
- McDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD003459.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnabel 1994
- Schnabel A, Gross WL. Low‐dose methotrexate in rheumatic disease ‐‐ efficacy, side effects, and risk factors for side effects. Seminars in Arthritis and Rheumatism 1994;23(5):310‐27. [PUBMED: 8036521] [DOI] [PubMed] [Google Scholar]
Schroder 2003
- Schroder O, Stein J. Low dose methotrexate in inflammatory bowel disease: current status and future directions. American Journal of Gastroenterology 2003;98(3):530‐7. [PUBMED: 12650783] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Te 2000
- Te HS, Schiano TD, Kuan SF, Hanauer SB, Conjeevaram HS, Baker AL. Hepatic effects of long‐term methotrexate use in the treatment of inflammatory bowel disease. American Journal of Gastroenterology 2000;95(11):3150‐6. [PUBMED: 11095334] [DOI] [PubMed] [Google Scholar]
Turner 2007
- Turner D, Grossman AB, Rosh J, Kugathasan S, Gilman AR, Baladassano R, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn disease. American Journal of Gastroenterology 2007;102(12):2804‐12. [PUBMED: 18042110] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
El‐Matary 2009
- El‐Matary W, Vandermeer B, Griffiths AM. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007560.pub2] [DOI] [PubMed] [Google Scholar]


