Abstract
Background
Clinical guidelines differ regarding their recommended blood glucose targets for patients with type 1 diabetes and recent studies on patients with type 2 diabetes suggest that aiming at very low targets can increase the risk of mortality.
Objectives
To assess the effects of intensive versus conventional glycaemic targets in patients with type 1 diabetes in terms of long‐term complications and determine whether very low, near normoglycaemic values are of additional benefit.
Search methods
A systematic literature search was performed in the databases The Cochrane Library, MEDLINE and EMBASE. The date of the last search was December 2012 for all databases.
Selection criteria
We included all randomised controlled trials (RCTs) that had defined different glycaemic targets in the treatment arms, studied patients with type 1 diabetes, and had a follow‐up duration of at least one year.
Data collection and analysis
Two review authors independently extracted data, assessed studies for risk of bias, with differences resolved by consensus. Overall study quality was evaluated by the 'Grading of Recommendations Assessment, Development, and Evaluation' (GRADE) system. Random‐effects models were used for the main analyses and the results are presented as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes.
Main results
We identified 12 trials that fulfilled the inclusion criteria, including a total of 2230 patients. The patient populations varied widely across studies with one study only including children, one study only including patients after a kidney transplant, one study with newly diagnosed adult patients, and several studies where patients had retinopathy or microalbuminuria at baseline. The mean follow‐up duration across studies varied between one and 6.5 years. The majority of the studies were carried out in the 1980s and all trials took place in Europe or North America. Due to the nature of the intervention, none of the studies could be carried out in a blinded fashion so that the risk of performance bias, especially for subjective outcomes such as hypoglycaemia, was present in all of the studies. Fifty per cent of the studies were judged to have a high risk of bias in at least one other category.
Under intensive glucose control, the risk of developing microvascular complications was reduced compared to conventional treatment for a) retinopathy: 23/371 (6.2%) versus 92/397 (23.2%); RR 0.27 (95% CI 0.18 to 0.42); P < 0.00001; 768 participants; 2 trials; high quality evidence; b) nephropathy: 119/732 (16.3%) versus 211/743 (28.4%); RR 0.56 (95% CI 0.46 to 0.68); P < 0.00001; 1475 participants; 3 trials; moderate quality evidence; c) neuropathy: 29/586 (4.9%) versus 86/617 (13.9%); RR 0.35 (95% CI 0.23 to 0.53); P < 0.00001; 1203 participants; 3 trials; high quality evidence. Regarding the progression of these complications after manifestation, the effect was weaker (retinopathy) or possibly not existent (nephropathy: RR 0.79 (95% CI 0.37 to 1.70); P = 0.55; 179 participants with microalbuminuria; 3 trials; very low quality evidence); no adequate data were available regarding the progression of neuropathy. For retinopathy, intensive glucose control reduced the risk of progression in studies with a follow‐up duration of at least two years (85/366 (23.2%) versus 154/398 (38.7%); RR 0.61 (95% CI 0.49 to 0.76); P < 0.0001; 764 participants; 2 trials; moderate quality evidence), while we found evidence for an initial worsening of retinopathy after only one year of intensive glucose control (17/49 (34.7%) versus 7/47 (14.9%); RR 2.32 (95% CI 1.16 to 4.63); P = 0.02; 96 participants; 2 trials; low quality evidence).
Major macrovascular outcomes (stroke and myocardial infarction) occurred very rarely, and no firm evidence could be established regarding these outcome measures (low quality evidence).
We found that intensive glucose control increased the risk for severe hypoglycaemia, however the results were heterogeneous and only the 'Diabetes Complications Clinical Trial' (DCCT) showed a clear increase in severe hypoglycaemic episodes under intensive treatment. A subgroup analysis according to the baseline haemoglobin A1c (HbA1c) of participants in the trials (low quality evidence) suggests that the risk of hypoglycaemia is possibly only increased for patients who started with relatively low HbA1c values (< 9.0%). Several of the included studies also showed a greater weight gain under intensive glucose control, and the risk of ketoacidosis was only increased in studies using insulin pumps in the intensive treatment group (very low quality evidence).
Overall, all‐cause mortality was very low in all studies (moderate quality evidence) except in one study investigating renal allograft as treatment for end‐stage diabetic nephropathy. Health‐related quality of life was only reported in the DCCT trial, showing no statistically significant differences between the intervention and comparator groups (moderate quality evidence). In addition, only the DCCT published data on costs, indicating that intensive glucose therapy control was highly cost‐effective considering the reduction of potential diabetes complications (moderate quality evidence).
Authors' conclusions
Tight blood sugar control reduces the risk of developing microvascular diabetes complications. The evidence of benefit is mainly from studies in younger patients at early stages of the disease. Benefits need to be weighed against risks including severe hypoglycaemia, and patient training is an important aspect in practice. The effects of tight blood sugar control seem to become weaker once complications have been manifested. However, further research is needed on this issue. Furthermore, there is a lack of evidence from RCTs on the effects of tight blood sugar control in older patient populations or patients with macrovascular disease. There is no firm evidence for specific blood glucose targets and treatment goals need to be individualised taking into account age, disease progression, macrovascular risk, as well as the patient's lifestyle and disease management capabilities.
Plain language summary
Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus
Review question
The primary objective of this review was to assess the positive and negative outcomes of tighter blood glucose control ('intensive' glucose control) compared to less intense treatment targets ('conventional' glucose control) in individuals with type 1 diabetes.
Background
Treatment of type 1 diabetes consists of life‐long blood sugar control through insulin replacement. It is generally agreed that achieving 'good' blood sugar control while avoiding episodes of very low blood sugars (severe hypoglycaemia) should be the primary treatment goal for individuals with type 1 diabetes. However, clinical guidelines differ regarding their recommended blood glucose targets.
Study characteristics
We identified 12 relevant studies, which included a total of 2230 participants. The participant populations varied widely across studies regarding age, disease duration, and existing diabetes complications. The mean follow‐up duration across studies varied between one and 6.5 years. The majority of the studies were carried out in the 1980s and all studies took place in Europe or North America.
Key results
We found that intensive glucose control was highly effective in reducing the risk of developing microvascular diabetes complications, such as retinopathy (eye disease), nephropathy kidney disease), and neuropathy (nerve disease). For developing retinopathy, 63 per 1000 people with intensive glucose control compared to 232 per 1000 people with conventional glucose control experienced this diabetes complication. For developing nephropathy, 159 per 1000 people with intensive glucose control compared to 284 per 1000 people with conventional glucose control experienced this diabetes complication. For developing neuropathy, 49 per 1000 people with intensive glucose control compared to 139 per 1000 people with conventional glucose control experienced this diabetes complication.
A weaker effect was found on the progression of retinopathy, while we could not find clear evidence of benefit of tight blood sugar control on the progression of nephropathy once participants had developed microalbuminuria (the kidney leaking small amounts of the protein albumin into the urine); no adequate data were available regarding the progression of neuropathy.
Major macrovascular outcomes (such as stroke and myocardial infarction) occurred very rarely; therefore we could not draw firm conclusions from the studies included in this review.
We found that intensive glucose control can increase the risk of severe hypoglycaemia, however the results varied across studies and only one big study showed a clear increase in severe hypoglycaemic episodes under intensive treatment. An analysis according to haemoglobin A1c (HbA1c) levels (a long‐term measure of glucose control) at the start of the study suggests that the risk of hypoglycaemia with intensive glucose control is possibly only increased for people who started the study with relatively low HbA1c values (less than 9.0%).
There were very few data on health‐related quality of life, death from any cause, and costs. Overall, mortality was very low in almost all studies. The effects of intensive glucose control on health‐related quality of life were unclear and were consistent with benefit or harm. One study reported that intensive glucose control could be highly cost‐effective when considering the potential reduction of diabetes complications in the future.
Tight blood sugar control reduced the risk of developing microvascular diabetes complications. The main benefits identified in this review came from studies in younger individuals who were at early stages of the disease. Appropriate patient training is important with these interventions in order to avoid the risk of severe hypoglycaemia. The effects of tight blood sugar control seem to become weaker once complications occur. However, further research is needed on this issue. Furthermore, there is a lack of evidence from randomised controlled trials on the effects of tight blood sugar control on older patient populations or individuals with macrovascular disease. There is currently no firm evidence for specific blood glucose targets, therefore treatment goals need to be individualised, taking into account age, disease progression, macrovascular risk, as well as people's lifestyle and disease management capabilities.
Quality of the evidence
For the majority of outcomes we evaluated the overall quality of evidence as moderate or low (analysed by the 'Grading of Recommendations Assessment, Development, and Evaluation' (GRADE) system).
Currentness of data
This evidence is up to date as of December 2012.
Summary of findings
Summary of findings for the main comparison. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus.
| Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus | ||||||
|
Patient or population: persons with type 1 diabetes mellitus Settings: outpatient clinics in North America and Europe Intervention: intensive glucose control Comparison: conventional glucose control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intensive treatment | |||||
|
Macrovascular complications Follow‐up: 1 ‐ 6.5 years |
See comment | ⊕⊕⊝⊝ lowa | Macrovascular outcomes were not considered as primary outcomes in any of the included studies and most studies did not report this outcome; myocardial infarctions and strokes were very rare | |||
| Microvascular complications | ||||||
| Manifestation of retinopathy Follow‐up: 5 ‐ 6.5 years | 232 per 1000 | 63 per 1000 (42 to 97) | RR 0.27 (0.18 to 0.42) | 768 (2) | ⊕⊕⊕⊕ highb | |
| Progression of retinopathy Follow‐up duration ≥ 2 years; follow‐up: 5 ‐ 6.5 years | 387 per 1000 | 236 per 1000 (190 to 294) | RR 0.61 (0.49 to 0.76) | 764 (2) | ⊕⊕⊕⊝ moderatec | |
| Progression of retinopathy Follow‐up duration < 2 years; follow‐up: 1 year | 149 per 1000 | 346 per 1000 (173 to 690) | RR 2.32 (1.16 to 4.63) | 96 (2) | ⊕⊕⊝⊝ lowd | |
| Manifestation of nephropathy Follow‐up: 3.5 ‐ 6.5 years | 284 per 1000 | 159 per 1000 (131 to 193) | RR 0.56 (0.46 to 0.68) | 1475 (3) | ⊕⊕⊕⊝ moderatee | |
| Progression of nephropathy Follow‐up: 5 ‐ 6.5 years | 14 per 1000 | 11 per 1000 (5 to 24) |
RR 0.79 (0.37 t0 1.70) |
179 (3) | ⊕⊝⊝⊝ very lowf | |
| Manifestation of neuropathy Follow‐up: 5 ‐ 6.5 years | 139 per 1000 | 49 per 1000 (32 to 74) | RR 0.35 (0.23 to 0.53) | 1203 (3) | ⊕⊕⊕⊕ highg | |
| Progression of neuropathy | See comment | Not adequately investigated | ||||
| Adverse events | ||||||
|
Severe hypoglycaemia, baseline HbA1c < 9.0 Follow‐up: 1.5 ‐ 6.5 years |
351 per 1000 | 590 per 1000 (453 to 769) | RR 1.68 (1.29 to 2.19) | 1583 (3) | 1a. ⊕⊕⊝⊝ lowh | |
|
Severe hypoglycaemia, baseline HbA1c ≥9.0 Follow‐up: 1 ‐ 5 years |
104 per 1000 | 108 per 1000 (68 to 170) | RR 1.04 (0.66 to 1.64) | 525 (8) | 1b. ⊕⊕⊝⊝ lowh | |
|
Ketoacidosis Follow‐up: 1 ‐ 2 years |
21 per 1000 |
95 per 1000 (50 to 866) |
OR 4.93 (1.18 to 20.60) |
96 (3) | 2. ⊕⊝⊝⊝ very lowi | In studies using insulin pumps |
|
Health‐related quality of life Follow‐up: 6.5 years |
See comment | 1441 (2) | ⊕⊕⊕⊝ moderatej | Only the DCCT reported on this outcome using several instruments (Diabetes‐Quality of Life Measure (DQHL), Symptom‐Checklist‐90R, Medical Outcome Study 36‐Item Short Form (SF‐36)); none of these measures showed a statistically significant difference between the intervention and comparator groups | ||
|
All‐cause mortality Follow up: 1 ‐ 6.5 years |
14 per 1000 |
13 per 1000 (13 to 60) |
OR 1.02 (0.48 to 2.19) | 2039 (10) | ⊕⊕⊕⊝ moderatek | Overall, the mortality rate was very low in all studies except MDCCT 1994, investigating renal allograft as treatment for end‐stage diabetic nephropathy |
|
Costs Follow up: 1 ‐ 6.5 years |
See comment | 1441 (2) | ⊕⊕⊕⊝ moderatej | Only the DCCT reported on this outcome; intensive treatment using multiple injections was calculated to cost 4014 US$/year, intensive treatment using CSII 5784 US$/year and conventional treatment 1666 US$/year taking into account resources used for therapy and handling side‐effects; considering the reduction of future diabetes complications, intensive therapy was found to be highly cost‐effective | ||
| **The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CSII: continuous subcutaneous insulin infusion; DCCT: 'Diabetes Complications Clinical Trial'; HbA1c: glycosylated haemoglobin A1c; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The basis for the assumed risk is the number of events in the control groups
aDowngraded by two levels owing to outcome measures either not being addressed as primary endpoints or reported in included studies and few events bNot downgraded because of few participants due to large effect size (RR < 0.5) cDowngraded by one level owing to substantial diversity in outcome measures definition dDowngraded by two levels owing to few participants and substantial diversity in outcome measures definition eDowngraded by one level owing to indirectness (surrogate outcome measures) fDowngraded by three levels owing to few participants, indirectness (surrogate outcome measures) and imprecise results (confidence intervals include null effect and appreciable benefit or harm) gLarge effect size hDowngraded by two levels owing to risk of bias in outcome definition and observational nature of subgroup analyses iDowngraded by three levels owing to imprecision (wide confidence intervals), low number of participants and observational nature of subgroup analyses jDowngraded by one level because only one study group (DCCT) investigated this outcome in two studies kDowngraded by one level owing to imprecise results (confidence intervals include null effect and appreciable benefit or harm)
Background
Description of the condition
Type 1 diabetes can be acquired at any age and accounts for about 5% to 10% of all diabetes mellitus cases (Daneman 2006). It is a metabolic disease caused by a cellular‐mediated autoimmune destruction of pancreatic β cells which results in a deficiency of insulin secretion. What causes the pathological autoimmune response is not yet fully understood but includes genetic susceptibility in combination with an environmental trigger (Field 1997; Maahs 2010; van der Werf 2007). The incidence of type 1 diabetes varies geographically, being highest in Northern Europe where it can be higher than 30 cases per 100,000 per year (Karvonen 1993). Over the years a worldwide increase in incidence has been observed, the reasons for which are not yet clear (Onkamo 1999; Pitkaniemi 2004).
To date, no cure has been found and treatment consists of life‐long blood sugar control through insulin replacement. Long‐term complications include neuropathy, nephropathy, retinopathy, and cardiovascular disease.
Description of the intervention
Since high blood glucose is associated with long‐term complications (Nordwall 2009), many efforts are made to reduce blood glucose to as low as possible. Different types of approaches could be taken when aiming for a low glucose target. For example, one could change to a different insulin regimen, which might be more effective in lowering blood sugar levels than another regimen, or one could switch to a different type of insulin, which could potentially be more successful than other types. In general, all these efforts should be nested in patient counselling and education efforts, which are further factors helping to achieve good glycaemic control (Aschner 2010). The primary research question for this review was to assess the effects of different blood glucose treatment targets and determine whether very low, near normoglycaemic values are of additional benefit. To answer this question, ideally only studies targeting different glycaemic levels but using identical insulin regimens in the treatment groups (for example multiple daily injections in both groups) should be considered. However, in previous studies with type 1 diabetic patients, for example in the 'Diabetes Control and Complications Trial' (DCCT) (DCCT 1993), the term 'intensive' therapy has often implied much more than just a lower glucose treatment target. In fact, intensive treatment usually refers to a multi‐factorial intervention with an intensified treatment regimen and additional factors such as patient education, individual counselling and increased frequency of blood glucose monitoring compared to the 'conventional' treatment. The results of these studies can only be attributed to a combination of factors rather than the effects of different glycaemic targets alone.
Since the results of the DCCT became known, 'intensive insulin therapy' has become the standard therapy that is recommended by most clinical guidelines for patients with type 1 diabetes. In addition, most clinical guideline recommendations (see Table 2) take their evidence from the results (based on the achieved glycosylated haemoglobin A1c (HbA1c) level) of the DCCT, in which the HbA1c treatment target of the intervention group was 6.05%. This target, however, was reached by less than 5% of the patients. On average, the HbA1c in this group could be reduced by 1.8% from 9.1% at baseline to a mean level of 7.1% throughout the randomised follow‐up period in the intervention group (DCCT 1993; DCCT 1995). The results showed a substantial reduction in the risk of developing microvascular complications during the follow‐up period in the intervention group compared to the control group. The results regarding macrovascular complications were less clear. Although the number of macrovascular events was higher under conventional treatment than intensive treatment, the overall number of events was small so that the power of the study might not have been high enough for the effect to reach statistical significance (DCCT 1995a). A recently published meta‐analysis (Stettler 2006), which combined the effects of eight randomised trials, came to the conclusion that an improvement of glycaemic control reduces the risk of macrovascular complications. In addition, in the long‐term follow‐up observation of the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) trial a substantial reduction in cardiovascular disease was shown in the former intensive treatment group compared to the former conventional treatment group (Nathan 2005), which further supports the assumption that intensive glucose control not only reduces microvascular but macrovascular complications as well.
1. Glycaemic targets for type 1 diabetes mellitus in different treatment guidelines.
| Country | Guideline | Year | HbA1c |
| Canada | Canadian Diabetes Association (Canadian 2008) | 2008 | ≤ 7.0% |
| Germany | Deutsche Diabetes Gesellschaft (Martin 2007) | 2007 | < 7.0% |
| UK | National Institute for Health and Clinical Excellence (NICE) (NICE 2010) | 2010 | < 7.5% (in case of increased arterial disease risk: < 6.5%) |
| USA | American Association of Clinical Endocrinologists (AACE) (Rodbard 2007) | 2007 | ≤ 6.5% |
| USA | American Diabetes Association (ADA) (ADA 2010) | 2010 | < 7.0% |
HbA1c: glycosylated haemoglobin A1c
Those studies aiming for the same glucose targets in both groups (or if no targets were specified) will be excluded from this review because any effect must be attributed to the treatment regimen and cannot have been caused by setting different treatment targets.
It is generally agreed that achieving 'good' glycaemic control with concurrent avoidance of hypoglycaemic episodes should be the primary treatment goal for type 1 diabetic patients. It is not yet completely clear how 'good' glycaemic control should be defined. Should it be the goal to get as close as possible to the HbA1c level of a healthy person, or could a higher level be a better target to achieve optimal long‐term outcomes when all the benefits and risks associated with tight blood glucose control are considered?
Adverse effects of the intervention
For patients with type 2 diabetes, a recent study has raised concerns that aiming for very strict glycaemic targets could potentially cause more harm than benefit. The ACCORD study (Gerstein 2007; Gerstein 2008), which had an HbA1c target of less than 6.0% in the intervention group, had to be discontinued due to an increase in mortality in this group. This effect, however, was not found by other similar trials recently published such as the ADVANCE or the VADT trial (Abraira 1997; Duckworth 2009). The reason for this increase in mortality in some studies but not others has not yet been clearly understood, but it is suggested that patient circumstances such as age, cardiovascular risk factors, type of antiglycaemic agents and duration of diabetes could potentially affect the balance of risks and benefits of tight blood sugar control. In addition, as shown in previously published meta‐analyses, none of the trials examining different treatment targets could demonstrate a clear superiority of lower glucose target levels regarding micro‐ or macrovascular complications. Only for non‐fatal myocardial infarction, a small but clinically non‐relevant reduction in the intervention group was observed (Montori 2009; Turnbull 2009; Yudkin 2010).
Whether similar concerns could apply to tight blood sugar control in individuals with type 1 diabetes is not yet clear. An observational analysis of the HbA1c values within the intervention group of the DCCT in relation to the reduction of risk regarding the development of microvascular complications could not identify a threshold or turning point over which a higher HbA1c level would be associated with an increase in risk (DCCT 1995b; DCCT 1996). However, this analysis has not been performed with regard to macrovascular outcomes. Furthermore, it is problematic to assign causality to an observational association between HbA1c and risks. From this association one can not necessarily conclude that an intervention that causes a reduction of the HbA1c would show a similar effect.
Observational studies show that blood sugar control varies widely among type 1 diabetic patients (Calvert 2009; Mortensen 1997; Thomsett 1999). While some of this variation can be attributed to behavioural factors, there are also biological influences that make control easier for some patients compared to others. For example, hormonal changes during puberty are thought to be one factor contributing to the particularly poor control observed in adolescents (Amiel 1986). The results of the DCCT clearly show that even in highly motivated selected patients treated under optimal conditions, close‐to‐normal HbA1c levels are extremely difficult to reach. The HbA1c target in the DCCT intervention group was less than 6.05%, which is, according to the DCCT‐HbA1c standard, close to the upper end of the range of a non‐diabetic person (HbA1c approximately 4% to 6%). In the EDIC cohort, the observational continuation of the DCCT, the mean HbA1c increased to 7.8% and several observational studies on the general population in Europe show that the percentage of patients with type 1 diabetes who reach an HbA1c lower than 7.5% is less than 50% (Calvert 2009; Mortensen 1997; Nordrheinische Gemeinsame Einrichtung 2008). Considering the difficulty of achieving recommended treatment targets, it is even more important to not only look at the benefits of an intervention aiming at strict metabolic control but to also carefully consider all possible adverse effects.
Hypoglycaemic episodes are a relatively common problem in type 1 diabetic patients. The event rates for severe hypoglycaemic episodes in type 1 diabetic patients reported in various studies lie between 62 and 320 events per 100 patient‐years, whereby a severe episode is defined as one requiring the assistance of another person (Desouza 2010). To what extent frequent hypoglycaemia can have negative long‐term effects is not yet fully understood. Recent epidemiological studies have suggested a link between hypoglycaemia and cardiovascular risk (Desouza 2003; Gill 2009). Other studies have found an association in type 2 diabetic persons between hypoglycaemia and cognitive dysfunction (Whitmer 2009). Furthermore, physiological counter‐regulation mechanisms triggered by low blood sugar levels can hinder achieving stable blood glucose control and it has been shown that frequent hypoglycaemic episodes can lead to hypoglycaemia unawareness (Cryer 2008; Zoungas 2010).
Several trials have shown that intensive glucose control increases the risk of hypoglycaemic episodes (DCCT 1993; Shalitin 2008). In the DCCT, the incidence of severe hypoglycaemia was 68% in the intervention group compared to 35% in the control group (DCCT 1993); however, no increase in risk of cardiovascular events or other clinical outcomes associated with this higher frequency of hypoglycaemic episodes has been reported. Another reported adverse effect of tight blood sugar control is weight gain (Conway 2010). Also in the DCCT, the risk of weight gain was increased under intensive treatment resulting in 12.7% of overweight cases compared to 9.3% of cases under conventional treatment (DCCT 1993).
Furthermore, intensive insulin therapy is associated with an increased insulin dose compared to conventional insulin therapy (DCCT 1993). In animal studies, exogenous hyperinsulinaemia resulted in a thickening of arterial walls, raising concerns that higher insulin use might increase the risk of atherosclerosis. However, in human studies the effects of exposure to high levels of insulin on cardiovascular disease remains controversial (Muis 2005).
Potential effects of tight blood sugar control on patients’ quality of life should not be ignored. For many patients achieving close to normal HbA1c levels might only be possible by adhering to a strict treatment plan, which might involve major restrictions on the patient’s lifestyle, for example through adhering to a strict diet, frequent blood glucose measurements and insulin injections or the careful documentation of blood measurements, insulin doses and food intake, which can be very time‐consuming (Davidson 2004). Not being able to achieve ambitious treatment targets could also have an effect on the emotional well‐being of the patient by creating a feeling of failure or by raising fear about possible future health complications (Herzer 2010; Ingerski 2010; McGrady 2009).
Why it is important to do this review
Recent studies on people with type 2 diabetes suggest that the effects of tight blood glucose control on cardiovascular risk is more complex than originally assumed and might depend on a variety of factors such as age, diabetes duration, gender and cardiovascular risk factors (Desouza 2010). To date it is not clear whether the situation could be similar in type 1 diabetes. A meta‐analysis from 2006 has found a decrease in long‐term clinical outcomes associated with strict glycaemic control (Stettler 2006). However, in this analysis little attention was paid to differences regarding age, study duration and diabetes duration. Furthermore, this meta‐analysis analysed the risk of macrovascular disease but did not consider any other outcomes. It also did not study adverse effects of tight blood sugar control, such as hypoglycaemia, weight gain or a potential burden on the quality of life. In contrast to our review, Stettler et al (2006) did not focus on the effects of different treatment targets. They included all trials that compared regimens with the aim of improving glycaemic control compared to conventional treatment. Whether different treatment targets were set for the intensive and conventional treatment was not an inclusion criterion.
Since the completion of the DCCT in 1993 intensive insulin therapy as well as other treatment innovations, such as new insulin analogues (Siebenhofer 2006), have become widely available to many type 1 diabetic patients. In addition, there have been improvements regarding the treatment of co‐morbidities such as hypertension. These factors have a significant impact on the clinical course of type 1 diabetes so that patients’ prospects today are much better than what they have been in the past (Nathan 2009). This, however, also implies that in the future many more type 1 diabetic patients might reach old age and it will become an important question whether the treatment goals, which are predominantly based on relatively young patients, can be applied to an older age group. An analysis of different age subgroups as part of this meta‐analysis could potentially provide further insight.
The recommended glycaemic target for type 1 diabetic patients varies between less than or equal to 6.5% and less than 7.5% in different clinical guidelines (see Table 2). Considering that more than 50% of type 1 diabetic patients do not achieve the highest target of less than 7.5%, as well as evidence from studies on type 2 diabetes that strict glucose control could potentially lead to an increased mortality risk, the balance of all harms and benefits related to interventions aimed at lowering glycaemic levels should be carefully evaluated. To date the risks of strict glycaemic control in type 1 diabetic patients are not fully understood and might differ depending on factors such as duration of diabetes, age, hypoglycaemic unawareness, baseline HbA1c levels and cardiovascular risk factors. Therefore, a thorough evaluation of the potential benefits and harms that depend on these factors is important.
At the heart of this review lies the question, considering that with current treatment very few type 1 diabetic patients achieve glycaemic levels close to those of a healthy person, should the optimal treatment always consist of aiming for a lower HbA1c; or could, depending on different patient factors, a higher glycaemic level be considered optimal when taking into account all harms and benefits?
Objectives
The primary objective of this review was to assess the effects of intensive versus conventional glycaemic targets in patients with type 1 diabetes in terms of long‐term complications and determine whether very low, near normoglycaemic values are of additional benefit.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials with a parallel group design comparing different glycaemic treatment targets in people with type 1 diabetes that assess any of the outcome measures of interest for this review and have a follow‐up period of at least one year.
Types of participants
Females and males of any age with type 1 diabetes mellitus were considered. The diagnosis should be based on clearly described criteria, which should be consistent with worldwide accepted standards at the time of study initiation (for example ADA 1999; ADA 2010; Alberti 1998).
Types of interventions
All included trials should (prior to patient allocation) have a predefined more intensive treatment target in the intervention group in comparison with the control group. Ideally, studies with the same treatment regimens in both treatment groups were planned to be included in the review. For studies using different treatment regimens (for example multiple daily injections versus conventional therapy), inclusion was accepted if a difference in glycaemic target could be clearly identified. Trials aiming for the same treatment targets or unspecified treatment targets in the different groups, although achieving differences in glycosylated haemoglobin A1c (HbA1c) at follow‐up, were excluded from this review. Either HbA1c (or equivalent, such as total glycosylated haemoglobin) target levels or target levels measured by fasting blood or plasma glucose or postprandial blood or plasma glucose had to be presented to fulfil the criteria for inclusion.
Types of outcome measures
Primary outcomes
Macrovascular complications (nonfatal and fatal myocardial infarction, stroke).
Microvascular complications (manifestation and progression of retinopathy, nephropathy, neuropathy, and endstage renal disease).
Severe hypoglycaemic episodes.
Secondary outcomes
Health‐related quality of life.
Adverse events (e.g. hypoglycaemic episodes, ketoacidosis, weight gain).
All‐cause mortality.
Costs.
Timing of outcome measurement
If possible, outcomes were assessed as short‐term (less than two years) and long‐term (two years or more) measurements.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date.
The Cochrane Library (2012, Issue 12).
MEDLINE (until December 2012).
EMBASE (until December 2012).
We also searched the following trial registers: ClinicalTrials.gov (www.clinicaltrials.gov/), Current Controlled Trials metaRegister (http://www.controlled‐trials.com/mrct/), the European (EU) Clinical Trials register (www.clinicaltrialsregister.eu/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
For detailed search strategies see Appendix 1. In the case where we detected new studies for inclusion we would have evaluated these and incorporated the findings in our review before submission of the final review draft (Beller 2013).
If additional key words of relevance had been detected during any of the electronic or other searches, we would have modified the electronic search strategies to incorporate these terms.
We placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists in identified studies.
Searching other resources
We tried to identify additional studies by searching the reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (BFand KH, or MS and KH) independently scanned the abstract, title or both sections of every record retrieved to determine the studies to be assessed further. A third person (TS) resolved any differences in opinion. If resolving disagreement was not possible, we planned to add the article to those 'awaiting classification' and we planned to contact the study authors for clarification. We present a PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow chart of study selection (Figure 1) (Liberati 2009).
Studies were selected based on the following criteria.
The study was a randomised controlled trial.
The target population was patients with type 1 diabetes.
The study intervention aimed to achieve an improvement in glycaemic control.
Different glycaemic targets were specified for the intervention and comparator groups.
Outcome measures of interest to our review were recorded as part of the study.
All publications identified by the search strategy were first analysed based on the title and abstract. If the abstract and title did not provide sufficient information, the full‐text article was obtained. All potentially relevant articles were investigated as full text. Two review authors (BF and KH, or MS and KH) independently assessed studies according to the selection criteria. Where differences in opinion existed, they were resolved by a third party (AS). If resolving disagreement was not possible, the article was added to those 'awaiting assessment' and we contacted study authors for clarification.
The selection process was plotted in a flow diagram (Figure 1) in accordance with the PRISMA statement (Liberati 2009).
1.
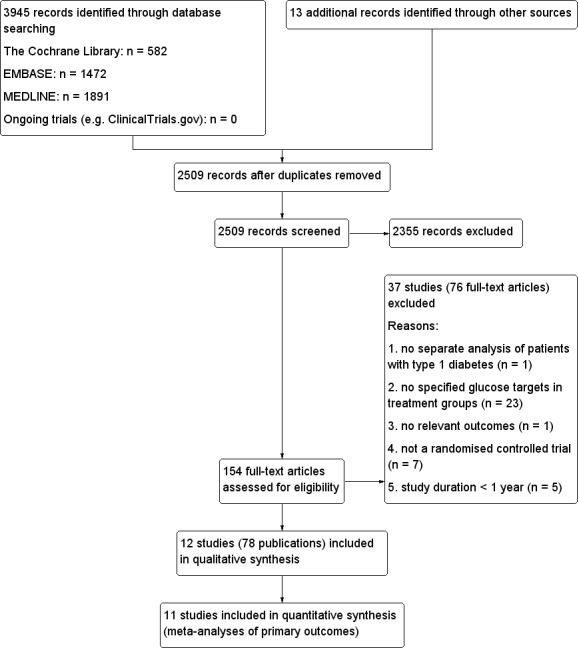
Study flow diagram.
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (BF, MS) independently abstracted relevant population and intervention characteristics using standard data extraction templates, with any disagreements resolved by discussion, or, if required, by a third author (AS) (for details see Characteristics of included studies; Table 3; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12).
2. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and comparator | Screened / eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] |
| (1) Bucharest‐Düsseldorf 1984 | I: intensive therapy (group B) | 200 | 100 | 100 | 100 | 98 | 98.0 |
| C: basic (group C) | 100 | 100 | 100 | 92 | 92.0 | ||
| total: | 200 | 200 | 200 | 190 | 95.0 | ||
| (2) DCCT1 (primary prevention) 1993 | I: intensive therapy | ‐ | 348 | 348 | 348 | ‐ | ‐ |
| C: conventional Therapy | 378 | 378 | 378 | ‐ | ‐ | ||
| total: | 726 | 726 | 726 | ‐a | ‐ | ||
| (3) DCCT2 (secondary intervention) 1993 | I: intensive therapy | ‐ | 363 | 363 | 363 | ‐ | ‐ |
| C: conventional therapy | 352 | 352 | 352 | ‐ | ‐ | ||
| total: | 715 | 715 | 715 | ‐a | ‐a | ||
| (4) Linn 1996 | I: intensive therapy | 49 | 23 | ‐ | ‐ | 23 | ‐ |
| C: conventional therapy | 19 | ‐ | ‐ | 19 | ‐‐ | ||
| total: | 49b | ‐ | ‐ | 42 | 85.7 | ||
| (5) MSCG 1995 | I: intensive therapy | ‐ | 36 | 36 | 36 | 31 | 86.1 |
| C: conventional therapy | 34 | 34 | 34 | 31 | 91.2 | ||
| total: | 70 | 70 | 70 | 62 | 88.6 | ||
| (6) MDCCT 1994 | I: intensive therapy | 98 | 52 | ‐ | ‐ | 25 | 48.1 |
| C: conventional therapy | 47 | ‐ | ‐ | 23 | 48.9 | ||
| total: | 99 | ‐ | ‐ | 48 | 48.5 | ||
| (7) Holman 1983 | I: intensive therapy (Group A) | 82 | 36 | 36 | 36 | 35 | 97.2 |
| C: conventional therapy (Group U) | 38 | 38 | 38 | 34 | 89.5 | ||
| total: | 74 | 74 | 74 | 69 | 93.2 | ||
| (8) Oslo 1987 | I1: intensive 1: multiple injections | 45 | 15 | 15 | 15 | 13 | 86.7 |
| I2: intensive 2: continuous insulin infusion | 15 | 15 | 15 | 13 | 86.7 | ||
| C: conventional | 15 | 15 | 15 | 10 | 66.7 | ||
| total: | 45 | 45 | 45 | 36 | 80 | ||
| (9) Steno 1 1983 | I: intensive therapy | 38 | 15 | 15 | 15 | 15 | 100 |
| C: conventional therapy | 15 | 15 | 15 | 15 | 100 | ||
| total: | 30 | 30 | 30 | 30 | 100 | ||
| (10) Steno 2 1986 | I: intensive therapy | 49 | 18 | 18 | 18 | 18 | 100 |
| C: conventional therapy | 18 | 18 | 18 | 18 | 100 | ||
| total: | 36 | 36 | 36 | 36 | 100 | ||
| (11) Verrillo 1988 | I: intensive therapy | 54 | 22 | 22 | 22 | 18 | 81.8 |
| C: conventional therapy | 22 | 22 | 22 | 20 | 90.9 | ||
| total: | 44 | 44 | 44 | 38 | 86.4 | ||
| (12) Wysocki 2003 | I: intensive therapy | 142 | 72 | 72 | 72 | ‐ | ‐ |
| C: conventional therapy | 70 | 70 | 70 | ‐ | ‐ | ||
| total: | 142 | 142 | 142 | ‐ | ‐ | ||
| Grand total | All interventions | 1115c | |||||
| All comparators | 1108c | ||||||
| All interventions and comparators | 2230c | ||||||
aIn the DCCT1 and DCCT2 combined, 1433 (99.4%) of 1441 patients finished the study bForty‐nine participants were randomised, authors only included data of 42 participants completing five years cNumbers do not match exactly because of 'b'
C: comparator; I: intervention
We sent an e‐mail request to authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 13 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the study authors of the article, if required.
Dealing with duplicate publications
When we found several articles related to the same trial, they were evaluated together to extract the maximum amount of information. In the case of an unresolvable conflict between two articles, we contacted the study authors.
Repeated observations
In the case of repeated observations on the same participants, we used the outcome assessed after the longest follow‐up period.
Assessment of risk of bias in included studies
Two review authors (BF, MS) assessed each trial independently. Disagreements were resolved by consensus, or in consultation with a third party. In cases of disagreement, the rest of the group was consulted and a judgement was made based on consensus.
We assessed risk of bias using the Cochrane Collaboration’s tool (Higgins 2011a; Higgins 2011b). We used the following bias criteria:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment;
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias.
We judged the risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and used individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We present a 'Risk of bias' graph (Figure 2) and 'Risk of bias summary' figure (Figure 3).
2.
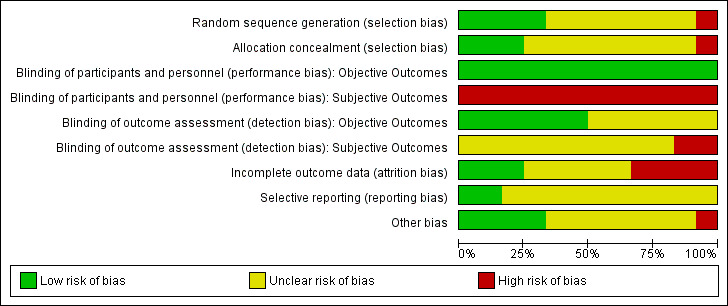
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
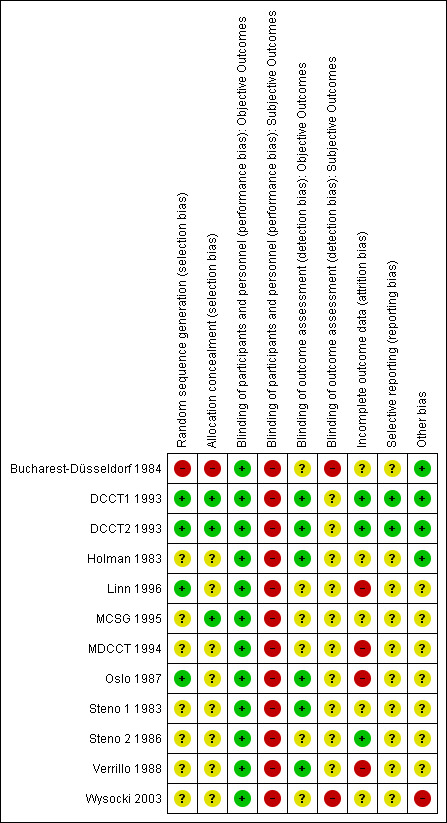
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
Health‐related quality of life.
Adverse events (e.g. hypoglycaemic episodes, ketoacidosis, weight gain).
We defined the following outcomes as objective outcomes.
Macrovascular complications (nonfatal and fatal myocardial infarction, stroke).
Microvascular complications (manifestation and progression of retinopathy, nephropathy, neuropathy, and endstage renal disease).
Severe hypoglycaemic episodes (depending on specific outcome definition).
All‐cause mortality.
Costs.
The overall quality of evidence for each outcome was assessed using the GRADE approach (Guyatt 2008; Higgins 2011a) and summarised in the Table 1.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We planned to express continuous data as mean differences (MD) with 95% CIs.
Data analysis was performed with Review Manager 5.2. All assessed outcomes were binary and were described by relative risks with 95% CI. Primarily, DerSimonian and Laird’s random‐effects model was used. Sensitivity analyses were performed using ORs and fixed‐effect models. In the case of rare events the fixed‐effect method of Peto was used for the main analysis.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from study authors, if feasible, and carefully performed evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT), as‐treated and per protocol (PP) populations. We investigated attrition rates, for example dropouts, losses to follow up and withdrawals, and critically appraised issues of missing data and imputation methods (for example last observation carried forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity we did not report study results as meta‐analytically pooled effect estimates. We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity employing the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% and more indicates a considerable level of inconsistency (Higgins 2011a).
Had we found heterogeneity we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Age.
Gender.
Cardiovascular risk factors.
Hypoglycaemia unawareness.
Duration of disease.
Primary versus secondary prevention.
Duration of follow‐up.
Assessment of reporting biases
We planned to use funnel plots to assess small study effects in the case where we included 10 or more studies for a given outcome. Due to several explanations for funnel plot asymmetry, we interpreted the results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we primarily summarised low risk of bias data by means of a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects (Higgins 2009). In addition, we performed statistical analyses according to the statistical guidelines contained in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses of the primary outcome parameters (see above) to investigate potential causes of heterogeneity.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors (when applicable) on effect sizes.
Restricting the analysis to published studies.
Restricting the analysis by taking into account risk of bias, as specified in the section Assessment of risk of bias in included studies.
Restricting the analysis to very long or large studies to establish the extent to which they dominated the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), country.
We also tested the robustness of the results by repeating the analysis using different measures of effect size (RR, OR etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the sections Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
The database search and handsearching of reference lists of reviews and included studies resulted in 2509 records, after the removal of duplicates. A total of 2355 of these records could be excluded based on the abstracts alone. After screening the full texts of the remaining 154 articles, 12 randomised controlled trials described in 78 publications fulfilled the inclusion criteria (Figure 1). All of the included articles were published in English.
Searching the registers of ongoing trials did not provide any additional studies.
The database search for relevant meta‐analyses and reviews provided 493 abstracts, which after further screening resulted in 22 relevant meta‐analyses and reviews. Looking through the references of these reviews provided additional articles that were relevant to already identified trials but did not result in the identification of any new trials. The sources found by searching the secondary literature are included in the additional 13 other sources in Figure 1.
Except for the DCCT trials, for which the protocol is published online, we could not retrieve any study protocols, although a request for a copy of the study protocol was included in all author requests.
The inter‐rater agreement expressed as Cohen’s Kappa was 80% for the full‐text screening.
Inconsistent or missing information
We tried to contact all study authors to request additional information or clarify inconsistencies we might have found across or within publications. Apart from one study (Verrillo 1988), contact information could be found for all studies (for more information on the status of the author requests see Appendix 13). One study that was identified in the literature search (Hershey 1999) seemed to be a substudy of another study included in this review (Wysocki 2003). Unfortunately, we were not able to receive a confirmation from the authors on this issue. For that reason, we decided not to include the article as a separate study in this review. For some of the studies, results on adverse events could be obtained from a previously published meta‐analysis (Egger 1997).
Included studies
A detailed description of the characteristics of the included studies is presented elsewhere (see sections Characteristics of included studies and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12). The following is a succinct overview.
Participants
A total of 2230 patients participated in the 12 included studies. The patient characteristics varied widely across trials. One study only included patients newly diagnosed with type 1 diabetes (Linn 1996); six other studies (DCCT1 1993; DCCT2 1993; Oslo 1987; Steno 2 1986; Verrillo 1988; Wysocki 2003) required a minimum disease duration, ranging from one year to 15 years. Overall, the mean disease duration across studies ranged from 0 to 22 years. There was also heterogeneity across studies regarding patient age: one study was conducted in children only, with a mean age of 12 years. The mean age at baseline across the other 11 studies was 29 years with a range of 26 to 42 years in the intervention group, and 28 years with a range of 26 to 43 years in the control group. Overall, 46% of the patients in the intervention group and 43% of the patients in the control group were females. The mean baseline HbA1c was 9.5% (range 8.2% to 12.4%) and 9.3% (range 8.1% to 13.1%) in the intervention and control groups, respectively. One study did not provide data on the HbA1c at baseline (MDCCT 1994). Finally, the patient populations across studies also varied substantially due to different inclusion criteria. Two studies only included patients with microalbuminuria at baseline (MCSG 1995; Steno 2 1986). Four studies were carried out in patients with background retinopathy (DCCT2 1993; Holman 1983; Steno 1 1983; Verrillo 1988) and one study only included patients who had received a kidney transplant (MDCCT 1994).
Interventions
The glycaemic targets varied between trials. Six trials specified an HbA1c target value while the other trials only defined pre‐ or postprandial blood glucose target values. In the intervention groups, the HbA1c targets varied between < 6.05% to ≤ 7.5%; preprandial glucose targets ranged from < 5.0 mmol/L (90 mg/dL) to < 8.3 mmol/L (150 mg/dL); and postprandial targets varied between < 8.3 mmol/L (150 mg/dL) to < 10 mmol/L (180 mg/dL). For the control group, blood glucose target values were frequently not specified (DCCT1 1993; DCCT2 1993; Holman 1983; Linn 1996; MCSG 1995); instead, the treatment goal was expressed as avoiding symptoms associated with glucosuria, hyperglycaemia as well as severe hypoglycaemia. Furthermore, some of the trials set targets for the amount of glucose in the urine. Only two of the trials had defined HbA1c targets in the control group: < 14.0% (which at a later time during the study was changed to < 12.0%) in the MDCCT 1994, and ≤ 8.0% in Wysocki 2003; three studies (Oslo 1987; Verrillo 1988; Wysocki 2003) had defined preprandial targets (< 7.0 mmol/L (126 mg/dL) to < 12.0 mmol/L (216 mg/dL); and three studies (Steno 1 1983; Steno 2 1986; Wysocki 2003) had defined postprandial targets (< 10 mmol/L (180 mg/dL) to < 15 mmol/L (270 mg/dL)).
In all of the studies the intervention and control treatments differed by more than just the glycaemic targets. While intervention patients usually used multiple daily injections (MI) or continuous subcutaneous insulin infusion (CSII), control patients applied one to three insulin injections per day, usually using mixed insulin preparations. In two studies, patients in the intervention group were only using insulin pumps (Steno 1 1983; Steno 2 1986); four studies only used insulin injections (Bucharest‐Düsseldorf 1984; Holman 1983; Linn 1996; Verrillo 1988); the Oslo 1987 study had two treatment arms, one for patients using CSII and one for patients using MI; and the remaining studies allowed patients to choose the type of insulin therapy (DCCT1 1993; DCCT2 1993; MCSG 1995; MDCCT 1994; Wysocki 2003).
Furthermore, frequent self‐monitoring of blood glucose was part of all intervention groups, while it was less encouraged or even absent in the control groups. Since intensified insulin therapy also requires more education and support, the frequency of contact with nurses or doctors of patients in the intervention groups was higher compared to patients in the control groups. In some studies, patients in the control group were not supposed to adjust their insulin dose themselves and had to adhere to a strict diet. For more information on the interventions see Appendix 2.
Excluded studies
Overall, 37 studies (76 articles) were excluded for reasons such as not being a randomised trial, no specified glucose targets as part of the intervention, trial duration being shorter than one year, having no separate analysis of patients with type 1 diabetes or having no relevant outcomes. For further details, see Characteristics of excluded studies.
Risk of bias in included studies
For details on the risk of bias of included studies see Characteristics of included studies.
For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies see Figure 2 and Figure 3.
We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures.
Allocation
The generation of the random sequence for allocation was considered adequate in four studies (DCCT1 1993; DCCT2 1993; Linn 1996; Oslo 1987). For the Bucharest‐Düsseldorf 1984 study the risk of selection bias was considered high since a group randomisation procedure was used, that is blocks of 100 patients were randomised to one of three treatment arms. We considered excluding the study because of this lack of randomisation but because of the large number of participants we decided to cautiously include it. However, for all meta‐analyses that included the Bucharest‐Düsseldorf 1984 study we carried out a sensitivity analysis for which the study was excluded. For the remaining eight studies, it was mentioned that patients were randomly assigned to a treatment group but the description was not detailed enough to allow a judgement on whether the sequence was generated adequately.
Allocation concealment was considered appropriate in three studies (DCCT1 1993; DCCT2 1993; MCSG 1995). In all other studies not enough information was provided to allow a judgement.
Blinding
Due to the nature of the intervention, patients or study personnel were not blinded in any of the trials. Therefore, for subjective outcomes all studies were judged to have a high risk of performance bias. For objective outcomes, however, we considered the risk of detection bias as low if the outcome assessment occurred in a blinded manner; this was the case in six of the studies (DCCT1 1993; DCCT2 1993; Holman 1983; Oslo 1987; Steno 1 1983; Verrillo 1988). For the six remaining studies blinding of outcome assessment was insufficiently described.
Incomplete outcome data
The risk of bias due to incomplete outcome data was considered low in three studies (DCCT1 1993; DCCT2 1993; Steno 2 1986). Four studies were judged to have a high risk of bias because the amount of missing data was large or not appropriately handled, or both (Linn 1996; MDCCT 1994; Oslo 1987; Verrillo 1988). For the remaining five studies the risk of bias was unclear.
Selective reporting
Reporting bias was difficult to evaluate since the study protocol was only available for the DCCT. For all studies we had the impression that some data were available that were not fully reported on, but often that was likely to have been done for the sake of keeping manuscripts short and not necessarily to selectively not report on insignificant results. Therefore, the risk of bias due to selective reporting was judged unclear for all studies apart from the low risk of bias for the DCCT1 1993 and DCCT2 1993 for which the study protocol was available.
Other potential sources of bias
One study was judged as having a high risk of bias in this category (Wysocki 2003) for two reasons: first, all publications on this study seemed to be partial reports of a larger study, the objectives of which were never clearly described; second, there was a large baseline difference regarding gender. For seven studies, other potential risks of bias were considered unclear either due to some inconsistencies in the reporting of results across or within publications or because the reporting was too sparse to allow a judgement. Four studies were judged as having a low risk of bias in this category (Bucharest‐Düsseldorf 1984; DCCT1 1993; DCCT2 1993; Holman 1983).
Overall risk of bias
We considered the overall risk of bias of a study to be high if it obtained a ‘high risk’ rating in at least two of the categories (selection, performance, detection, attrition, selective reporting or other bias). According to this definition, six of the 12 studies (Bucharest‐Düsseldorf 1984; Linn 1996; MDCCT 1994; Oslo 1987; Verrillo 1988; Wysocki 2003) were considered to have a high risk of bias.
Publication bias
For all analysed outcomes, we planned to explore the risk of publication bias by inspection of funnel plots. For most outcomes, however, the number of included studies was too low to obtain useful information from these plots. For the outcome including more studies (severe hypoglycaemia), the funnel plot looked inconspicuous.
Effects of interventions
See: Table 1
The following outcomes reflect the results of comparing intensive glucose control versus conventional glucose control.
Primary outcomes
Macrovascular complications
Macrovascular outcomes were not considered as primary outcomes in any of the included studies. Most studies did not report these outcomes and in those that did, events were rare. Only the DCCT reported on strokes, and no strokes were recorded in either of the cohorts (primary prevention and secondary intervention) during the whole follow‐up period. For two other studies, the reporting on mortality allowed us to conclude that no fatal strokes had occurred (Bucharest‐Düsseldorf 1984; Holman 1983). Also, myocardial infarctions were very rare. The DCCT reported four definite nonfatal myocardial infarctions in the intensive treatment group (primary prevention and secondary intervention combined) compared to no events in the control group. In addition, there was one fatal major cardiovascular event in each treatment arm. Holman 1983 reported one fatal myocardial infarction in the control group compared to no events in the treatment group. From the reporting on mortality in the Bucharest‐Düsseldorf 1984 study, it was evident that no fatal myocardial infarctions had occurred during follow‐up.
Microvascular complications
Retinopathy
Overall, nine of the 12 studies reported some results on retinopathy (DCCT1 1993; DCCT2 1993; Holman 1983; Linn 1996; MCSG 1995; Oslo 1987; Steno 1 1983; Steno 2 1986; Verrillo 1988). Two of the studies, in which all patients were free of retinopathy at baseline, reported on the manifestation of retinopathy (DCCT1 1993; Linn 1996), and four studies included only patients with baseline retinopathy and therefore presented results on the progression of retinopathy (DCCT2 1993; Holman 1983; Steno 1 1983; Verrillo 1988). The other three studies were likely to have included patients with and without retinopathy at baseline and they did not provide separate results according to baseline retinopathy status (MCSG 1995; Oslo 1987; Steno 2 1986). Furthermore, the MCSG 1995 only reported that the changes in retinopathy were similar for the two treatment groups, and for the Steno 2 1986 study the only result presented was the number of patients requiring laser treatment. The results of these two studies were not considered in any meta‐analyses.
A meta‐analysis of all trials providing information on retinopathy as a binary outcome (DCCT1 1993; DCCT2 1993; Holman 1983; Linn 1996; Oslo 1987; Steno 1 1983; Verrillo 1988), irrespective of primary or secondary prevention, follow‐up duration or the exact outcome definition, resulted in a substantial amount of heterogeneity (I2 = 79%, P < 0.0001) (Analysis 1.1). All further analyses were carried out separately for primary prevention (that is manifestation of retinopathy) and secondary intervention (that is progression of retinopathy) as defined in the protocol for this review.
1.1. Analysis.
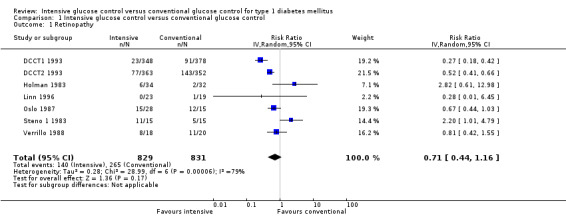
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 1 Retinopathy.
Manifestation of retinopathy
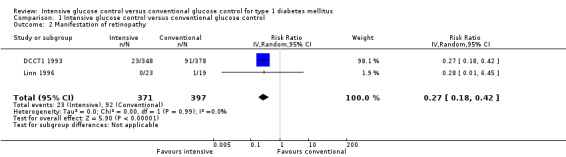
Manifestation of retinopathy was the primary endpoint in the primary prevention cohort of the DCCT (DCCT1 1993). The results showed a statistically significant effect favouring intensive blood glucose control (relative risk based on proportional hazards model: 0.24 (95% CI 0.15 to 0.38)) (DCCT 1993). This effect remained significant even if other definitions of the outcome were used (DCCT 1995c). For the meta‐analysis, we used the risk ratio (RR) calculated from the number of patients who developed retinopathy during follow‐up instead of the results obtained from the proportional hazards analysis reported in the publications (RR 0.27( 95% CI 0.18 to 0.42); P < 0.00001; I2 = 0%; 768 participants; 2 trials; Analysis 1.2). The study by Linn 1996 added little additional information to the result of the DCCT. The study was small and retinopathy was not the primary outcome of the study. No patient in the intervention group (n = 22) and one patient in the control group (n = 19) developed retinopathy during five years of follow‐up. Both included studies had an overall low risk of bias for this outcome.
1.2. Analysis.
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 2 Manifestation of retinopathy.
Due to the low number of studies, we did not carry out any of the planned subgroup or sensitivity analyses. However, subgroup analyses previously published on the data of the DCCT showed a stronger risk reduction in patients with a disease duration of less than 2.5 years (DCCT 1995c). Other analyses showed a similar effects for different subgroups according to age (adolescents and adults), gender and baseline HbA1c (DCCT 1994; DCCT 1995c).
Progression of retinopathy
The four trials that studied the progression of retinopathy in patients with baseline retinopathy included a total number of 860 patients with 263 patients showing a deterioration of retinopathy during study follow‐up.
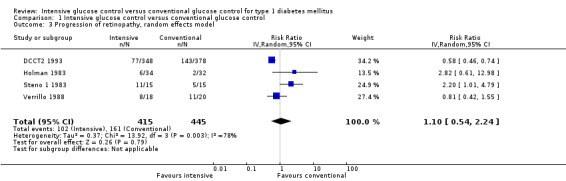
The Steno 1 1983 study had originally been planned for one year but was then extended for another year. We initially included the study results after one year in our analysis. However, in the analysis after two years of follow‐up, which excluded the data of one patient who decided to switch treatment group after the first year, the effect of the intervention was the opposite of what is was after one year of follow‐up. For that reason we also repeated the meta‐analysis including the results of the Steno study after two years. The result of the first analysis showed no statistically significant differences between intervention groups (RR 1.10 (95% CI 0.54 to 2.24); P = 0.79; I2 = 78%; 860 participants; 4 trials; Analysis 1.3). The second analysis including the results of the Steno 1 1983 study after two years demonstrated a statistically significant effect in favour of intensive glucose control (RR 0.68 (95% CI 0.47 to 0.99); P = 0.04; I2 = 37%; 859 participants; 4 trials; Analysis 1.4). All included studies had an overall low risk of bias for this outcome.
1.3. Analysis.
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 3 Progression of retinopathy, random effects model.
1.4. Analysis.
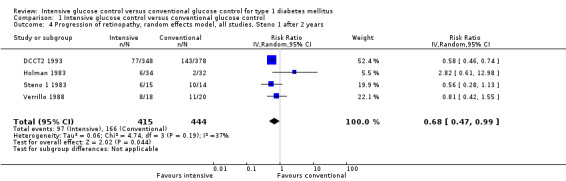
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 4 Progression of retinopathy, random effects model, all studies, Steno 1 after 2 years.
An additional analysis stratifying the trials according to duration of follow‐up (≥ 2 years versus < 2 years), including the Steno 1 1983 study as originally planned (with one year of follow‐up), eliminated statistical heterogeneity and showed a reduced risk of retinopathy progression in the intensive treatment group for longer follow‐up periods (RR 0.61 (95% CI 0.49 to 0.76); P < 0.0001; I2 = 0%; 764 participants; 2 trials; Analysis 1.5.1) but an increased risk for studies with a short follow‐up period (RR 2.32 (95% CI 1.16 to 4.63); P = 0.02; I2 = 0%; 96 participants; 2 trials; Analysis 1.5.2). This deterioration of retinopathy in the first year after beginning intensive therapy has also been reported in both cohorts of the DCCT (DCCT 1995) and the Oslo 1987 study (Dahl‐Jorgensen 1985).
1.5. Analysis.
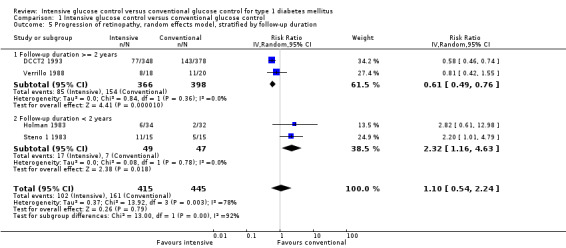
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 5 Progression of retinopathy, random effects model, stratified by follow‐up duration.
Apart from follow‐up duration, heterogeneity could have been introduced by various other differences between trials: all trials were set in different countries and there were baseline differences regarding age, disease duration, and HbA1c. Furthermore, the definition of progression of retinopathy was different for each trial. The DCCT used the Early Treatment of Diabetic Retinopathy Study (ETDRS) scale (25 steps) and defined progression of retinopathy as a change of at least three steps from baseline sustained for at least six months. In the study by Holman 1983, retinopathy was primarily measured on a continuous scale using a study‐specific retinopathy index. Additionally, the number of patients who had formed new vessels was reported, which was used in this meta‐analysis as a dichotomous outcome for retinopathy progression. In the Steno 1 1983 study, fundus photographs and fluorescein angiograms were evaluated as to whether they showed a deterioration compared to baseline measurements. The results were presented separately for the fundus photographs and fluorescein angiograms; in our meta‐analysis the results of the fluorescein angiograms were used but the results based on fundus photographs were very similar. It was not clear, however, whether the patients that showed a deterioration in the fundus photographs were the same patients that showed a deterioration in the fluorescein angiograms. For the reporting of results after two years of follow‐up, fundus photographs and fluorescein angiograms were assessed on six‐ and four‐rank scales and then combined in a retinal morphology index, for which the number of patients showing a deterioration was presented. In Verrillo 1988, retinopathy was evaluated on a five‐grade scale based on fundus photographs, fluorescein angiograms and ophthalmoscopy.
Due to the low number of studies, an exploration of the effects of these differences in trial characteristics and baseline variables was not possible. Furthermore, we did not carry out any subgroup analyses since, apart from the secondary prevention group of the DCCT (DCCT2 1993), no data on patient subgroups were available. Subgroup analyses on the DCCT2 1993 have been published and show similar results in patient groups differing by age (adolescents and adults), gender, or baseline HbA1c (DCCT 1994; DCCT 1995).
Sensitivity analyses using a fixed‐effect model instead of a random‐effects model, or odds ratios instead of risk ratios, led to similar results (Analysis 1.6; Analysis 1.7).
1.6. Analysis.
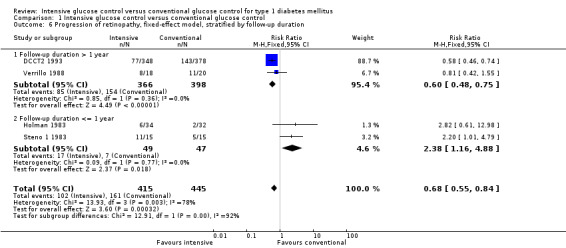
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 6 Progression of retinopathy, fixed‐effect model, stratified by follow‐up duration.
1.7. Analysis.
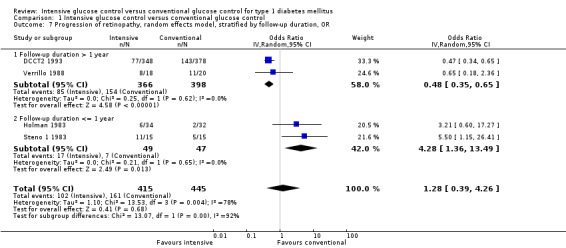
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 7 Progression of retinopathy, random effects model, stratified by follow‐up duration, OR.
Nephropathy
Results on nephropathy were reported in nine of the 12 trials (DCCT1 1993; DCCT2 1993; Holman 1983; Linn 1996; MCSG 1995; MDCCT 1994; Oslo 1987; Steno 1 1983; Steno 2 1986). For three of the studies, nephropathy was the primary outcome of the trial (MCSG 1995, MDCCT 1994, Steno 2 1986). The MDCCT 1994 followed a special sample of patients having received a kidney transplant and used renal biopsy samples to investigate the development of nephropathy in the newly implanted kidney. The primary outcome was the renal glomerular mesangial expansion, assessed from the biopsy samples with electron microscopy. Mesangial expansion has been shown to be a glomerular lesion that is highly correlated with the manifestation of diabetic nephropathy (Mauer 1984; Osterby 1988). The results showed a more than two‐fold mesangial expansion in the conventional treatment group compared to the patients under intensive glucose control. Since this study was very different in terms of patient population and the outcome measure, which was only presented on a continuous scale, we did not try to combine these results with those of other studies in a meta‐analysis. The other two studies, which focused on nephropathy as a primary outcome, observed the development of clinical albuminuria in patients with microalbuminuria at baseline.
As specified in the protocol, we carried out separate analyses for the outcomes manifestation of nephropathy and progression of nephropathy. A meta‐analysis by Wang 1993 reported results on nephropathy within the Steno 1 1983 study. However, the cited publication could not be obtained and we have not yet received a response from the study authors. Furthermore, we would assume that the results were not separated according to the manifestation and progression of nephropathy. The results of Steno 1 1983 were therefore not included in this analysis. Also, Holman 1983 reported on renal function at baseline and follow‐up. Presented measures were the mean plasma creatinine levels and creatinine clearance. The results showed significantly higher plasma creatinine levels and a stronger deterioration in creatinine clearance (plasma creatinine: 91.0 (SD 17.8) versus 103.8 (SD 19.7) µmol/L; creatinine clearance: 99.1 (SD 29.6) versus 82.9 (SD 26.0) ml/min) in the conventional treatment group compared to the intensive group after two years of follow‐up. However, also in this study, no distinction was made between patients who already showed signs of nephropathy at baseline and those who did not. Furthermore, urinary albumin excretion was not reported, which made the results difficult to compare to the other studies. For these reasons, Holman 1983 was not included in any of the meta‐analyses presented below.
Manifestation of nephropathy
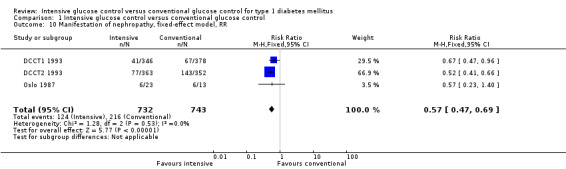
Apart from the MDCCT 1994, which studied the manifestation of nephropathy in transplanted kidneys, four other studies reported on this outcome. The study by Linn 1996 only included newly diagnosed patients and reported on nephropathy as a secondary outcome; by the end of the five‐year follow‐up the urinary albumin excretion rate was higher in the conventional treatment group than in the intensive treatment group (19.4 (SD 10) versus 11.2 (SD 10) mg/24 h, P < 0.05). It was not reported whether any of the patients in the two groups had developed microalbuminuria. For both DCCT cohorts (DCCT1 1993; DCCT2 1993), the publications included results on the subgroup of patients without microalbuminuria at baseline who developed microalbuminuria during follow‐up. Similar results could be extracted from the Oslo 1987 study since mean urinary albumin excretion rates at baseline and during follow‐up were presented for individual patients. We excluded all patients who had a urinary albumin excretion rate above 30 mg/24h at baseline and defined manifestation of nephropathy as an increment in mean urinary albumin excretion to above 30 mg/24h, which was shown by one patient under conventional and one patient under intensive treatment (MI and CSII combined). The meta‐analysis showed a statistically significant effect in favour of the intensive treatment group (RR 0.56 (95% CI 0.46 to 0.68); P < 0.00001; I2 = 0%; 1475 participants; 3 trials; Analysis 1.8). All included studies had an overall low risk of bias for this outcome.
1.8. Analysis.
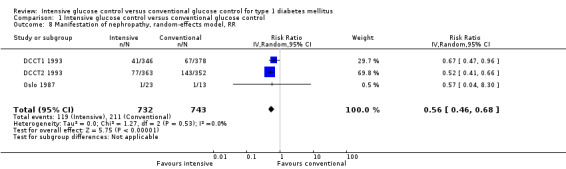
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 8 Manifestation of nephropathy, random‐effects model, RR.
Using a different outcome definition for the Oslo 1987 study, in which we counted all patients who showed any increment in mean urinary albumin excretion from baseline to end of follow‐up, resulted in the same effect (Analysis 1.9).
1.9. Analysis.
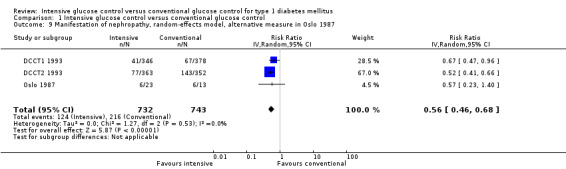
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 9 Manifestation of nephropathy, random‐effects model, alternative measure in Oslo 1987.
Sensitivity analyses were carried out using odds ratios instead of risk ratios and applying a fixed‐effect model instead of a random‐effects model. Similar results were obtained in all analyses (Analysis 1.10; Analysis 1.11).
1.10. Analysis.
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 10 Manifestation of nephropathy, fixed‐effect model, RR.
1.11. Analysis.
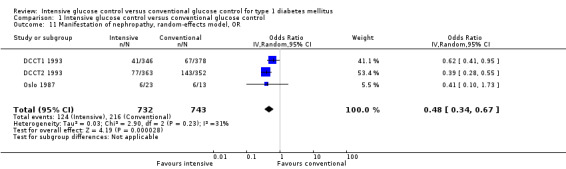
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 11 Manifestation of nephropathy, random‐effects model, OR.
Subgroup data were only available for the DCCT, for which analyses have already been published: subgroups defined by various baseline characteristics such as age, HbA1c level or diabetes duration showed similarly beneficial effects of intensive therapy over conventional treatment (DCCT 1995d). The subgroup analysis by gender showed a significantly weaker effect for women than for men. However, this gender difference disappeared if women were excluded after the onset of pregnancy or if a stricter outcome definition requiring two consecutive annual measurements of microalbuminuria was used.
Progression of nephropathy
Analysis 1.12 shows the results of a meta‐analysis on the three studies that reported on the progression from microalbuminuria to clinical albuminuria (DCCT2 1993; MCSG 1995; Steno 2 1986). In the DCCT2 1993, the analysis applied only to a subset of 72 patients who had microalbuminuria at baseline. The combined RR was 0.79 (95% CI 0.37 to 1.70; P = 0.55; I2 = 19%; 179 participants), finding no statistically significant reduction in the risk of nephropathy progression in patients with microalbuminuria. Thirty‐three of the 179 participants (18%) developed clinical albuminuria. All included studies had an overall low risk of bias for this outcome.
1.12. Analysis.
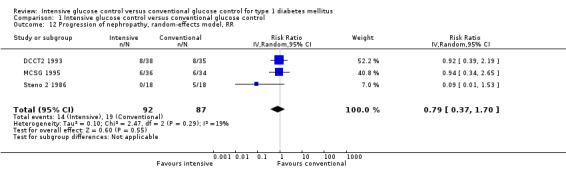
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 12 Progression of nephropathy, random‐effects model, RR.
Data for subgroup analyses were not available. Sensitivity analyses using odds ratios instead of risk ratios and applying a fixed‐effect model instead of a random‐effects model led to similar results (Analysis 1.13; Analysis 1.14). Other sensitivity analyses that were originally planned in the protocol (see Methods) were not carried out due to the low number of studies included.
1.13. Analysis.
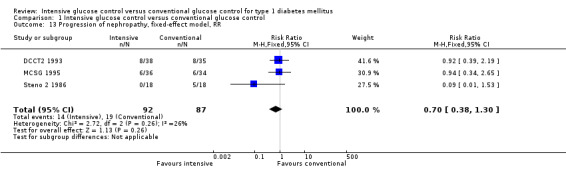
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 13 Progression of nephropathy, fixed‐effect model, RR.
1.14. Analysis.
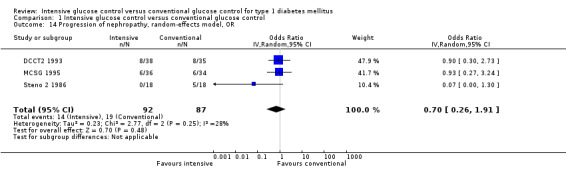
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 14 Progression of nephropathy, random‐effects model, OR.
Endstage renal disease
Results on endstage renal disease were only mentioned in one of the studies (MCSG 1995), most likely because in the patient populations studied none or only very few of the patients reached this outcome within the follow‐up period. The MCSG 1995 reported one case of renal failure in the intensive treatment group.
Neuropathy
Data on neuropathy were reported in six of the included studies (DCCT1 1993; DCCT2 1993; Holman 1983; Linn 1996; Oslo 1987; Steno 1 1983). Both the Oslo 1987 study and Holman 1983, did not provide any definitions for the manifestation or progression of neuropathy. They only provided continuous measurements of nerve conduction velocity (Oslo 1987) or vibration sensory thresholds (Holman 1983) at baseline and after follow‐up. Holman 1983 found that the vibration sensory threshold improved in the intensive treatment group but deteriorated under conventional treatment. Results for neuropathy in the Oslo 1987 study were published after two years of follow‐up and showed that the motor nerve conduction had improved under intensive treatment but deteriorated in the conventional treatment group. Significant group differences were only observed between the conventional treatment group and the intensive group receiving CSII, but not in the MI group. In the Steno 1 1983 study, peripheral and autonomic neuropathy were assessed by vibration sense and beat‐to‐beat variations during five consecutive deep inspirations. Even though these outcomes were measured at baseline no results were reported for the originally planned one‐year follow‐up. Only results for two years of follow‐up have been published, showing no statistically significant differences between the two treatment groups on either type of neuropathy.
Manifestation of neuropathy
Data on the manifestation of neuropathy were available in three studies (DCCT1 1993; DCCT2 1993; Linn 1996). In the DCCT (DCCT1 1993 and DCCT2 1993), confirmed clinical neuropathy was defined as an abnormal neurologic examination combined with either abnormal nerve conduction in at least two peripheral nerves or abnormal autonomic nerve testing. Results were presented for the subgroups of patients who showed no neuropathy at baseline, which applied to 25 patients in the primary prevention cohort (DCCT1 1993) and 67 patients in the secondary intervention cohort (DCCT2 1993). Since the neurologic examination was only done at baseline and after five years, only 76% of the full study cohort could be taken into account for this analysis and the results were reported after five years of follow‐up for all patients. In the study by Linn 1996, neuropathy was diagnosed if at least three of the following were positive according to the San Antonio consensus statement: clinical symptoms, signs, quantitative sensory testing, and peroneal motor nerve conduction velocity.
Overall, 115 patients out of 1203 patients developed neuropathy during a follow‐up period of five years. The risk of developing neuropathy under intensive glucose control was statistically significantly lower compared to conventional treatment (RR 0.35 (95% CI 0.23 to 0.53); P < 0.00001; I2 = 0%; 1203 participants; 3 trials; Analysis 1.15). All included studies had an overall low risk of bias for this outcome.
1.15. Analysis.
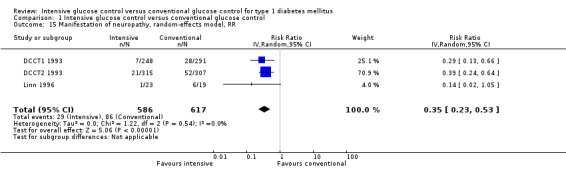
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 15 Manifestation of neuropathy, random‐effects model, RR.
Similar results were obtained when using odds ratios instead of risk ratios, or a fixed‐effect model instead of a random‐effects model (Analysis 1.16; Analysis 1.17).
1.16. Analysis.
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 16 Manifestation of neuropathy, fixed‐effect model, RR.
1.17. Analysis.
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 17 Manifestation of neuropathy, random‐effects model, OR.
Progression of neuropathy
None of the studies provided detailed analyses on the progression of neuropathy. In the DCCT, five‐year results were available for 84 of the 92 patients with baseline confirmed clinical neuropathy, only 41 of these received the same diagnosis after five years. To what extent neuropathy was reversible or had progressed was not investigated due to the low number of patients.
Severe hypoglycaemic episodes
Results on severe hypoglycaemic episodes were available for all of the included studies. However, there was substantial variation regarding the definition of severe hypoglycaemia across studies: in three studies severe hypoglycaemia was defined as an episode requiring hospital admission (Holman 1983; Steno 1 1983; Verrillo 1988), whereas in other studies the need for medical intervention (Steno 2 1986) or assistance from another person was sufficient for a hypoglycaemic episode to be categorized as severe (DCCT1 1993; DCCT2 1993; MCSG 1995; MDCCT 1994; Wysocki 2003). In two studies a severe hypoglycaemic episode was defined by the loss of consciousness (Bucharest‐Düsseldorf 1984; Oslo 1987), and in Linn 1996 severe hypoglycaemia was reported but not defined.
In all studies severe hypoglycaemia was either reported as the number of patients with at least one episode or as a rate. All but one study (MDCCT 1994) reported the number of patients with at least one episode. The MDCCT 1994 reported a much higher incidence of severe hypoglycaemic events in the intensive treatment group compared to the control group (1.7 episodes per patient‐year versus < 0.1 episodes per patient‐year). It was also reported that there were 26 hospital visits due to severe hypoglycaemia in the intervention group and three visits in the control group. Even though the number of patient‐years in the two groups that was used for the calculation of the incidence rates was not given, it is difficult to understand how such a low rate in the control group could be achieved.
In the remaining 11 studies, 834 out of 2108 patients experienced at least one severe hypoglycaemic episode during follow‐up. A meta‐analysis of these studies provided a RR of 1.50 (95% CI 1.17 to 1.91; P = 0.001; I2 = 52%; 2108 participants; 11 trials; Analysis 1.18 ). Analysis 1.19 and Analysis 1.20 show the combined results of only those studies that had defined severe hypoglycaemia as an episode requiring the assistance of another person (RR 1.64 (95% CI 1.27 to 2.12); P = 0.0002; I2 = 71%; 1653 participants; 4 trials) or those studies that used a definition that required either coma or hospital admission (RR 1.67 (95% CI 1.09 to 2.55); P = 0.02; I2 = 63%; 1818 participants; 7 trials). However, heterogeneity was substantial in both analyses so that the pooled effect measures of these analyses should be interpreted with caution. The DCCT was included in both analyses since results on both outcomes were provided. Irrespective of the outcome definition, the analysis showed a higher risk of severe hypoglycaemia in the intensive treatment group.
1.18. Analysis.
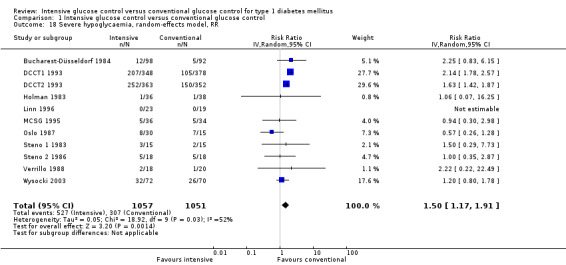
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 18 Severe hypoglycaemia, random‐effects model, RR.
1.19. Analysis.
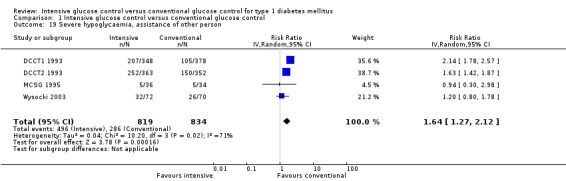
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 19 Severe hypoglycaemia, assistance of other person.
1.20. Analysis.
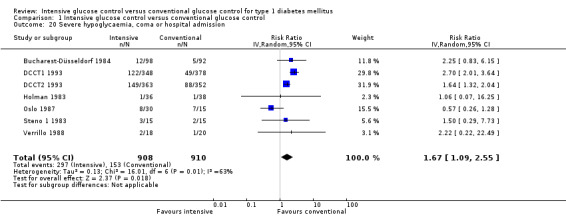
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 20 Severe hypoglycaemia, coma or hospital admission.
Results were dominated by the DCCT cohort. Leaving out the DCCT resulted in no statistically significant difference between the two treatment groups (Analysis 1.21).
1.21. Analysis.
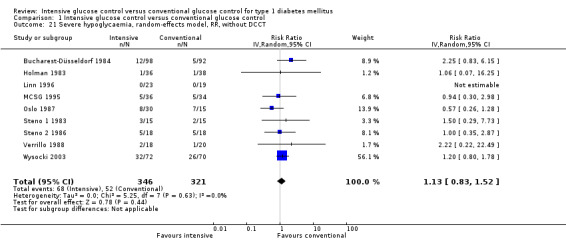
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 21 Severe hypoglycaemia, random‐effects model, RR, without DCCT.
We also checked whether the effect of intensive treatment on severe hypoglycaemia could be influenced by the baseline HbA1c level. Figure 4 shows that the HbA1c at baseline was correlated with the rate of severe hypoglycaemia across studies, which varied between 0 and 66 episodes per patient‐year and between 0 and 50 episodes per patient‐year in the treatment and control groups, respectively.
4.
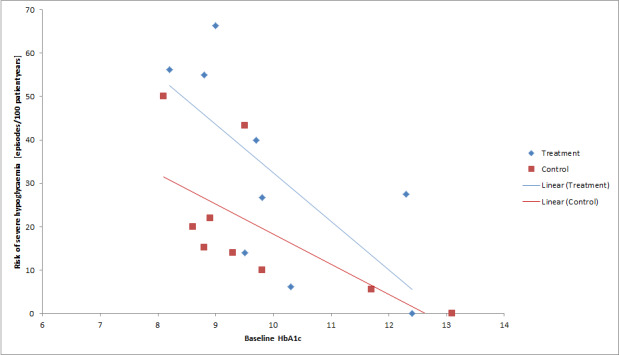
Relationship between baseline HbA1c and risk of severe hypoglycaemia.
A meta‐analysis stratified the studies according to whether the studies demonstrated a baseline HbA1c equal to or greater than 9.0% or below 9.0%. It showed an increased risk for the intensive treatment groups in studies with participants starting at lower HbA1c values (RR 1.68 (95% CI 1.29 to 2.19); P = 0.001; I2 = 78%; 1583 participants; 3 trials; Analysis 1.22.1), but no statistically significant effect for studies that had a high baseline HbA1c (RR 1.04 (95% CI 0.66 to 1.64); P = 0.86; I2 = 0%; 525 participants; 8 trials; Analysis 1.22.2). Re‐analysis with the studies showing an overall low risk of bias for this outcome did not substantially change the effect estimates.
1.22. Analysis.
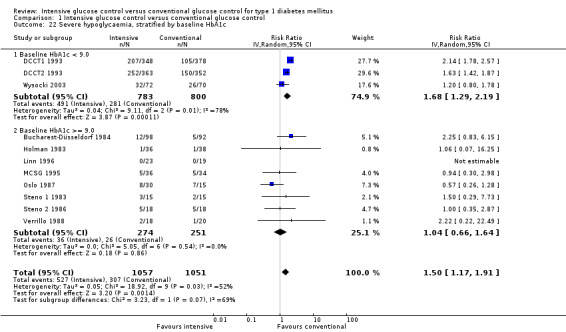
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 22 Severe hypoglycaemia, stratified by baseline HbA1c.
However, since it was basically the two DCCT cohorts (both studies with an overall low risk of bias) that contributed to the effect for low baseline HbA1c levels, it was difficult to establish based on these data whether it was really the baseline HbA1c that was responsible for this difference or whether it was due to other characteristics of the DCCT. The high amount of heterogeneity in the subgroup analysis of studies with an HbA1c < 9.0% was due to the different effect sizes in DCCT1 1993 and DCCT2 1993; both studies showed a significantly increased risk of severe hypoglycaemia under intensive treatment compared to conventional treatment but the effect was stronger in the primary prevention cohort (DCCT1 1993).
Since the Bucharest‐Düsseldorf 1984 study did not use an appropriate randomisation procedure, we evaluated all analyses without this study. This did not have any impact on the interpretation of the overall analysis (Analysis 1.23), the analysis without the DCCT cohort (Analysis 1.24) or the analysis stratified by baseline HbA1c (Analysis 1.25). In the subgroup analysis of only the studies that provided data on hypoglycaemic episodes associated with coma or hospital admission, removal of the study pushed the confidence interval over the significance boundary so that the effect appeared to be marginally non‐significant without the Bucharest‐Düsseldorf 1984 study (RR 1.58 (95% CI 0.98 to 2.56); P = 0.06; I2 = 68%; 1628 participants; 6 trials; Analysis 1.26).
1.23. Analysis.
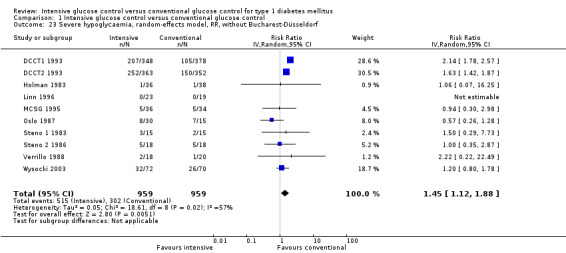
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 23 Severe hypoglycaemia, random‐effects model, RR, without Bucharest‐Düsseldorf.
1.24. Analysis.
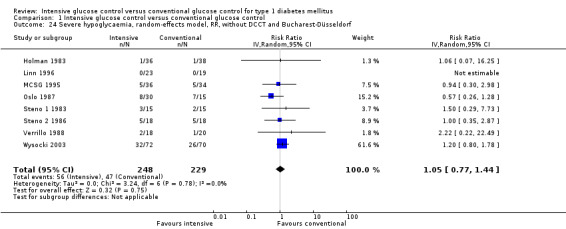
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 24 Severe hypoglycaemia, random‐effects model, RR, without DCCT and Bucharest‐Düsseldorf.
1.25. Analysis.
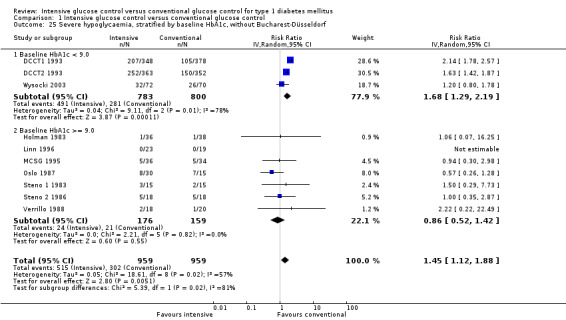
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 25 Severe hypoglycaemia, stratified by baseline HbA1c, without Bucharest‐Düsseldorf.
1.26. Analysis.
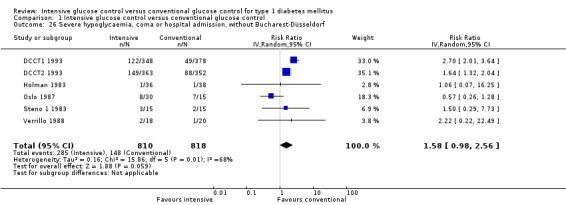
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 26 Severe hypoglycaemia, coma or hospital admission, without Bucharest‐Düsseldorf.
Repeating the stratified analysis using a fixed‐effect model instead of a random‐effects model, or using odds ratios instead of risk ratios gave comparable results (Analysis 1.27; Analysis 1.28).
1.27. Analysis.
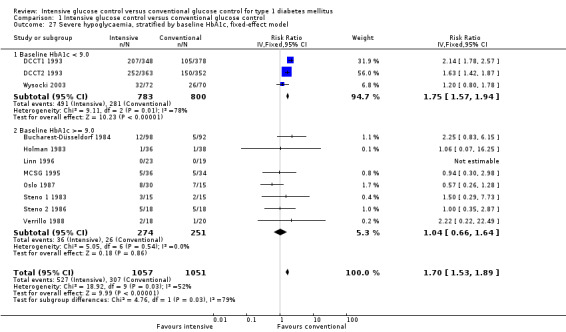
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 27 Severe hypoglycaemia, stratified by baseline HbA1c, fixed‐effect model.
1.28. Analysis.
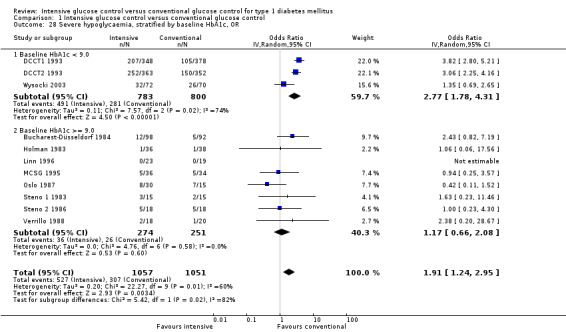
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 28 Severe hypoglycaemia, stratified by baseline HbA1c, OR.
Secondary outcomes
Health‐related quality of life
Only the DCCT reported on health‐related quality of life. Several measures related to health‐related quality of life were assessed (Diabetes‐Quality of Life Measure (DQHL), Symptom‐Checklist‐90R, Medical Outcome Study 36‐Item Short Form (SF‐36)) but none of the evaluations showed a statistically significant difference between the intervention and comparator groups.
Adverse events
Overall hypoglycaemia
Several studies only reported on severe hypoglycaemia, but eight of the 12 studies also attempted to assess milder forms of hypoglycaemia (DCCT1 1993; DCCT2 1993; Linn 1996; MDCCT 1994; Oslo 1987; Steno 1 1983; Steno 2 1986; Verrillo 1988). In Steno 1 1983 and Steno 2 1986, overall hypoglycaemia was measured as the percentage of blood glucose readings below 2.5 mmol/L (45 mg/dL) during a few test nights in hospital. In both studies, patients under intensive treatment showed a higher percentage of low blood glucose measurements but the differences were not statistically significant. In Linn 1996, the blood glucose readings on patients’ blood glucose meters were analysed to assess the frequency of blood measurements below 3.5 mmol/L (63 mg/dL). The intensive treatment group showed a statistically significant higher percentage of low blood glucose values compared to patients in the conventional treatment arm. In the Oslo 1987 study patients’ blood measurement records were analysed to compare the frequency of values below 2.5 mmol/L. Furthermore, patients recorded any subjectively experienced hypoglycaemic episode and were asked to report them at every hospital visit. While the frequency of reported symptomatic hypoglycaemia was similar in all of the three study arms, patients using CSII showed a statistically significant higher percentage of blood glucose measurements below 2.5 mmol/L compared to the intervention arm using MI as well as the conventional treatment arm. In the DCCT, patients reported on any hypoglycaemic events at quarterly visits (severe episodes were reported immediately) and symptoms associated with these episodes were recorded. As for severe hypoglycaemic episodes, symptomatic hypoglycaemia was statistically significantly more frequent under intensive than under conventional treatment. Verrillo 1988 reported no statistically significant differences regarding mild, self‐treated hypoglycaemic episodes between the two treatment arms, while in the MDCCT 1994 intensively treated patients showed a statistically significant higher frequency of moderate hypoglycaemic episodes, which were defined as episodes associated with severe symptoms but preserved capability for self‐treatment.
Ketoacidosis
Nine studies provided data on the number of patients who experienced at least one ketoacidotic episode during follow‐up (Bucharest‐Düsseldorf 1984; DCCT1 1993; DCCT2 1993; Holman 1983; MCSG 1995; Oslo 1987; Steno 1 1983; Steno 2 1986; Verrillo 1988).
Most of the studies showed a tendency favouring the control treatment, however the overall effect using Peto’s odds ratios did not reach statistical significance (OR 1.33 (95% CI 0.95 to 1.86); P = 0.10; I2 = 0%; 1924 participants; 9 trials; Analysis 1.29).
1.29. Analysis.
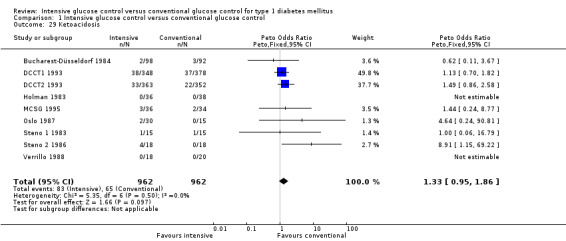
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 29 Ketoacidosis.
In an analysis separating studies according to the type of insulin therapy used in the intervention group (insulin pump, insulin injections or both) we found a statistically significantly higher risk of ketoacidosis in those studies using insulin pumps (CSII arm of Oslo 1987 study; Steno 1 1983; Steno 2 1986) (OR 4.93 (95% CI 1.18 to 20.60); P = 0.03; I2 = 0%; 96 participants; 3 trials; Analysis 1.30). All three studies had an overall low risk of bias for this outcome.
1.30. Analysis.
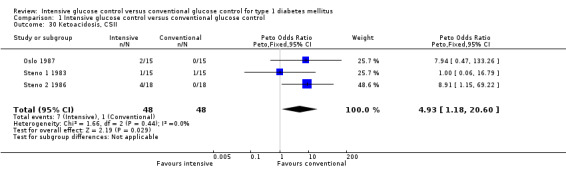
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 30 Ketoacidosis, CSII.
We found no statistically significant difference for those studies using insulin injections (Bucharest‐Düsseldorf 1984; Holman 1983, MI arm of Oslo 1987; Verrillo 1988) (OR 0.62 (95% CI 0.11 to 3.67); P =0.60; 332 participants; 3 trials; Analysis 1.31) and no statistically significant difference (OR 1.28 (95% CI 0.90 to 1.82); P = 0.17; I2 = 0%; 135 participants; 3 trials; Analysis 1.32) in the studies that allowed patients to choose the type of insulin therapy (DCCT1 1993; DCCT2 1993; MCSG 1995). The Oslo 1987 study originally had two intervention arms, one using multiple injections (MI) and one using CSII. Therefore, the study was included in Analysis 1.30 and Analysis 1.31 using the data of the relevant treatment arm.
1.31. Analysis.
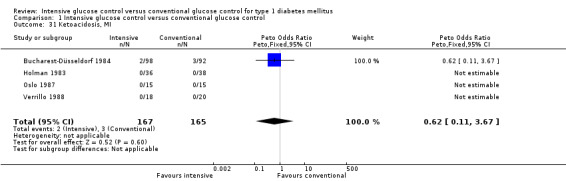
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 31 Ketoacidosis, MI.
1.32. Analysis.
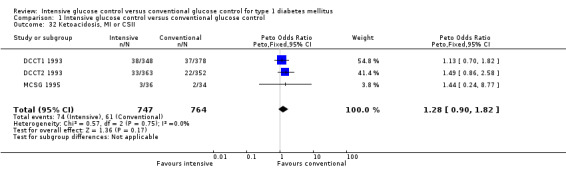
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 32 Ketoacidosis, MI or CSII.
We carried out a sensitivity analysis calculating the odds ratio using the Mantel‐Haenszel odds ratio with a ‘treatment arm’ continuity correction and the method of Liu 2012. The odds ratio for the meta‐analysis including all studies was similar to Peto’s OR (OR 1.29 (95% CI 0.93 to 1.78) and 1.28 (95% CI 0.92 to 1.79)).
Weight gain
Four studies provided results on weight gain under intensive or conventional glucose control (Bucharest‐Düsseldorf 1984; DCCT (DCCT1 1993 and DCCT2 1993 combined); Linn 1996; Oslo 1987). All of the studies showed at least a tendency for a higher weight gain in the intensive treatment group compared to the control group. In the DCCT (primary prevention and secondary intervention combined), for patients in the intensive treatment group the risk of becoming overweight was statistically significantly increased compared to the control treatment group (RR 1.73 (95% CI 1.43 to 2.09)). Statistically significant differences between treatment and control were also found regarding the BMI or body weight at the end of follow‐up in the Bucharest‐Düsseldorf 1984 and the Oslo 1987 studies. In the Oslo 1987 study, only patients undergoing intensive therapy using MIs showed this effect while patients on pump therapy did not exhibit higher body weights compared to the control group. Linn 1996 observed a trend towards more weight gain in the intervention group but the effect did not reach statistical significance.
All‐cause mortality
In 10 of the included studies mortality was either directly reported or could be deduced from the information provided. Two studies were not included in the analysis (Linn 1996; Wysocki 2003) because the information provided by the study authors was insufficient. Since these two studies included children and adult patients newly diagnosed with type 1 diabetes it was likely that the number of deaths was zero and therefore not reported.
Overall, the mortality rate was very low in all studies but the MDCCT 1994 in which 13% and 17% of the patients died in the intensive and conventional treatment groups, respectively, during follow‐up. There were 15 deaths in 1020 patients under intensive treatment and 14 deaths in 1019 patients under conventional treatment. A meta‐analysis using Peto’s odds ratio showed no difference between the two treatment arms (OR 1.02 (95% CI 0.48 to 2.19); P = 0.95; I2 = 0%; 2039 participants; 10 trials; Analysis 1.33). Since Peto’s odds ratio can be biased in situations with event rates higher than 1% and with imbalanced intervention and control groups (Diamond 2007; Sweeting 2004) we carried out a sensitivity analysis using two other methods: a fixed‐effect model Mantel‐Haenszel odds ratio using a ‘treatment arm’ continuity correction for zero cells as described in Sweeting 2004, and a recently published method which allows the inclusion of zero cells without continuity correction (Liu 2012). The results obtained with these methods (OR 1.02 (95% CI 0.49 to 2.16); OR 1.05 (95% CI 0.46 to 2.45)) were similar to the Peto’s odds ratio. Exclusion of the Bucharest‐Düsseldorf 1984 study did not substantially change the effect estimate. All other studies showed an overall low risk of bias for this outcome.
1.33. Analysis.
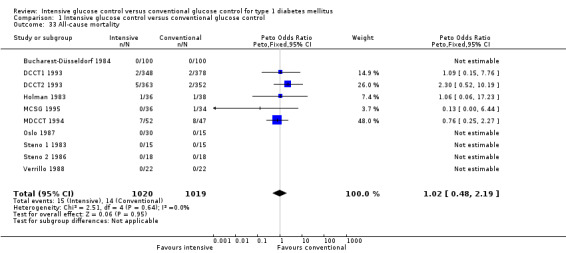
Comparison 1 Intensive glucose control versus conventional glucose control, Outcome 33 All‐cause mortality.
Costs
Results on the cost of treatment were only reported in the DCCT (overall low risk of bias for this outcome). Intensive treatment using MIs was calculated to cost USD 4014 per year, intensive treatment using CSII USD 5784 per year and conventional treatment USD 1666 per year taking into account resources used for therapy and handling side effects (hypoglycaemia, weight gain). The cost difference between intensive and conventional treatment was largely due to the higher frequency of outpatient visits and the increased blood glucose self‐monitoring in the intensive treatment group. Also the costs for treating side effects were three times as high in the intervention group compared to the control group. However, overall treatment of side effects only constituted 5% of the costs. The higher costs of insulin pump therapy (CSII) compared to MI were entirely due to the higher costs of pump supplies. Considering the reduction of future diabetes complications, intensive therapy was found to be highly cost‐effective (DCCT 1996a; Meltzer 2000).
Discussion
Summary of main results
Our results show that under intensive glucose control the risk of developing microvascular complications (retinopathy, nephropathy and neuropathy) is significantly reduced compared to conventional treatment. Regarding the progression of these complications after manifestation, the effect is weaker (retinopathy) or possibly non‐existent (nephropathy).
Based on the trials included in this review, we cannot provide results regarding the development of macrovascular complications since the incidence of major macrovascular events (strokes and myocardial infarctions) was too low.
We found that intensive glucose control can be associated with a higher risk of adverse events, such as severe hypoglycaemic episodes, ketoacidosis and weight gain. The results regarding severe hypoglycaemia showed some heterogeneity, particularly in both DCCT cohorts. This heterogeneity could potentially be explained by study differences regarding the baseline HbA1c. Our results suggest that the risk of severe hypoglycaemia is particularly relevant for patients with lower HbA1c levels (< 9.0%) who aim for more intensive glucose targets.
We found that the risk of ketoacidosis was only increased in those studies that used insulin pumps in the intervention group. Therefore, ketoacidosis seems to be an adverse event of insulin pump therapy but not necessarily of intensive glucose control.
Weight gain was not consistently assessed across the different trials but in those trials that presented results patients in the intervention group consistently showed a higher amount of weight gain. Whether this weight gain could be considered an adverse effect in the sense that it leads to patients being overweight was only analysed in the DCCT, in which the percentage of overweight patients increased under intensive treatment.
Other outcomes assessed were health‐related quality of life, costs and all‐cause mortality. The former two were only assessed in the DCCT, showing no statistically significant effect regarding health‐related quality of life and higher costs associated with intensive treatment, which was however considered cost‐effective when taking into account the reduction in later diabetes complications. Mortality rates were similar in the treatment and control arms although overall the mortality rates were very low.
Overall completeness and applicability of evidence
Our results are based on studies identified through an extensive and systematic literature search, including articles in all languages. We included patients of all ages independent of existing co‐morbidities or diabetes complications at baseline. We also searched trial registers to find potentially relevant but not yet published studies.
The included studies covered a wide spectrum of patient characteristics. There was one study that only included children (Wysocki 2003). Some studies included only patients with background retinopathy, other studies looked at a patient population only of individuals with microalbuminuria and one study was carried out on patients who had received a kidney transplant. While the inclusion of all these different studies allowed us to cover a wide range of patients, as represented in the general population of patients with type 1 diabetes, our results should be interpreted with caution. First of all, the number of studies was too small to carry out extensive subgroup analyses that would allow us to investigate to what extent our results can be applied to all patients or only certain subgroups of patients. Secondly, apart from one study (Wysocki 2003) all studies have been completed more than 15 years ago. Therefore, we have to consider to what extent these results can be applied to the patient population today. Since then, therapy for patients with type 1 diabetes has changed substantially through the introduction of insulin analogues (Home 2012; Monami 2009; Siebenhofer 2006), improved insulin pumps (Tamborlane 2001; Valla 2010), as well as well as a stronger focus on patient training and management including the development of systematic disease management programs (Pimouguet 2011). We do not know how these changes could affect the results observed in the studies included in this review. Finally, most of the studies excluded patients above a certain age or with very long disease durations. Looking at the characteristics of the included trials, we can see that we do not have any evidence for old people with type 1 diabetes, nor for those who have lived with the disease for a long time nor those who received a late diagnosis. The exclusion of these patients might also be a reason for the lack of results on macrovascular complications, so that any evidence we have on this topic today is purely based on observational studies.
The glycaemic targets that were defined in the included studies varied substantially from trial to trial, with definitions sometimes using pre‐ or postprandial blood glucose and sometimes HbA1c values. Based on the available data, it is not possible to draw any conclusions regarding specific glycaemic targets or how quickly patients should try to reach a specific target given their current glycaemic control. Appendix 12 shows the average HbA1c levels achieved in the treatment and control groups. While the treatment group always achieved better glycaemic control compared to the control group, it is worth noting that in none of the studies did patients manage to achieve glycaemic levels close to the normal range.
Quality of the evidence
Due to the nature of the intervention, none of the studies could be carried out in a blinded fashion so that the risk of performance bias, especially for subjective outcomes such as hypoglycaemia, was present in all of the studies. Half (50%) of the studies were judged to have a high risk of bias in at least one other category. The Bucharest‐Düsseldorf 1984 was judged to have a high risk of bias because it used what the study authors referred to as a group randomisation procedure in which patients were allocated to one of the three treatment arms corresponding to the time period when they visited the hospital. Four studies were considered at a high risk of bias due to the handling of missing data (Linn 1996; MDCCT 1994; Oslo 1987; Verrillo 1988), and one study was considered to be at a high risk of bias because the information across publications was often inconsistent and the primary objective of the trial remained unclear (Wysocki 2003). Overall, the evaluation of the bias risks was hampered by the lack of information provided in many of the publications, so that we frequently had to judge the risk of bias as unclear.
A major limitation of this review is that the intervention of interest (different glycaemic targets) was confounded by the type of treatment used in the two study arms. There was not a single study that only compared different glycaemic targets while keeping all other aspects of treatment constant. Therefore, our results cannot be fully attributed to the difference in treatment targets alone but might also be due to other differences in the treatment arms, such as type of insulin regimen, intensity of support through nurses and doctors, and more frequent blood glucose monitoring.
The quality of the evidence provided in this review might further be limited by the heterogeneity among included trials due to baseline differences between patient groups, different lengths of follow‐up, and also due to a high variability regarding the definition of outcomes. We explored this heterogeneity through subgroup and sensitivity analyses but only to an extent considered adequate given the low number of trials included. The subgroups that were analysed sometimes only included two studies; therefore these results should be considered explorative and only seen in the context of the literature.
Several outcomes, such as adverse events or mortality, were not assessed as the primary outcome in any of the trials. Therefore, reporting on these outcomes was frequently incomplete and the studies were not sufficiently powered to find effects on these outcomes. We tried to avoid outcome reporting bias by asking authors for additional data on these outcomes. However, since the studies included in this review were carried out many years ago many author requests remained unanswered or the study authors told us that it would not be feasible for them to access the original data of the trial (for more information on author requests see Appendix 13).
Diabetes complications are long‐term complications that often only develop after many years of the disease. RCTs with a follow‐up duration of just a few years can therefore only capture a small window out of the full time course for the development of these complications. If an intervention needs to be introduced at an early stage of the disease to be effective, as seems to be the case with intensive glucose control, it becomes almost impossible to study the effects on long‐term outcomes such as mortality or endstage renal disease within an RCT.
Some of the included studies investigated the introduction of intensive treatment in patients who had already developed complications, but for some outcomes the amount of data in this patient population is still insufficient to draw reliable conclusions. None of the studies have focused on the progression of neuropathy, and for the progression of nephropathy the amount of data available in these studies is insufficient to clearly establish whether intensive therapy can still slow down further progression or becomes ineffective once a certain stage of the disease has been reached.
Potential biases in the review process
Many of the trials included in this review were relatively small, and we often observed a high amount of heterogeneity among these trials. Smaller trials should not bias the outcomes of a meta‐analysis if methodological quality is high but particular caution should be applied to heterogenous results (Cappelleri 1996; Farkouh 2008; Kjaergard 2001), and frequently small trials are found to have methodological shortcomings (Rerkasem 2010; Zhang 2013). In our review, the amount of information regarding the design and methods of the trial was much higher for the large DCCT compared to the other smaller trials, where it was often difficult to judge the risks of bias due to insufficient information. We tried to clarify these issues through author requests but often received no further information. For that reason, and because the number of studies was low in general, we usually did not carry out sensitivity analyses based on excluding studies with a high risk of bias. The only exception was sensitivity analyses in which we excluded the Bucharest‐Düsseldorf 1984 study, which we considered to clearly have a high risk of bias due to an inappropriate randomisation procedure. Furthermore, for most of our meta‐analyses the results were dominated by the results of the DCCT cohorts; of all included studies the DCCT studies were judged to have the lowest risk of bias.
For several analyses there was a high level of heterogeneity if all of the studies were combined. In these cases we tried to explore the reasons for heterogeneity by carrying out subgroup and sensitivity analyses. Furthermore, we repeated analyses using fixed‐effect models instead of random‐effect models, and odds ratios instead of risk ratios.
For most of the outcomes analysed in this review, outcome definitions varied across studies. In the case of retinopathy, every study used a different kind of retinopathy index and particular caution should be applied to the results for Steno 1 1983 and Holman 1983. The Steno 1 1983 study used an improved retinopathy index combining the results of fluorescein angiography and fundus photography after two years of follow‐up. However, for the results after one year, which was the prospectively planned length of follow‐up, we only had separate gradings for the two measures. While both measures showed similar results overall, we do not know whether the results were also consistent on an individual patient level. In the study by Holman 1983, retinopathy was primarily reported as a continuous measure, however the researchers did additionally report the number of patients with newly formed vessels, which we used as a dichotomous outcome in the meta‐analysis. These two studies were combined in the subgroup analysis of studies with short follow‐up for the progression of retinopathy. Our uncertainty regarding the result of this subgroup analysis is reflected in the Table 1, where the quality of the evidence was judged as low. Also, regarding nephropathy, neuropathy and hypoglycaemia outcomes were not defined according to exactly the same criteria (for details see Appendix 8 and Appendix 9). However, subgroup analyses in which we grouped studies according to their outcome definition generally did not show a strong impact on the results. Nevertheless, these different outcome definitions are likely to have introduced variability and since the number of studies was low studying the impact of these different definitions in subgroup analyses was difficult. Especially regarding hypoglycaemic episodes, we would expect definitions that depend on the subjective judgement of patients or staff, such as definitions based on the need for assistance from another person, symptoms associated with hypoglycaemia or even the number of self‐measured low blood glucose measurements (as it is the patient who chooses when to measure), to be at a high risk of bias.
Agreements and disagreements with other studies or reviews
In this review we summarised for the first time the evidence from all randomised controlled trials that have explicitly specified different levels of glucose control in the intervention and control groups for patients with type 1 diabetes mellitus. There have been several other reviews that have looked at intensive versus conventional insulin therapy (Callaghan 2012; Egger 1997; Lawson 1999; Mattila 2010; Stettler 2006; Wang 1993). While to some extent these reviews included the same studies as our review, our review is more specific as it excluded several studies that had not specified glycaemic targets.
Regarding the results on microvascular complications, our results are generally consistent with other reviews. Wang 1993 had carried out meta‐analyses on retinopathy and nephropathy outcomes, before the results of the DCCT were available. The meta‐analyses did not distinguish between manifestation and progression of these microvascular diseases and did not differentiate between measures of deterioration of retinopathy or nephropathy. For retinopathy, only half of the eight studies that were analysed fulfilled the inclusion criteria for our review. Studies were analysed separately according to the length of treatment (less than two years or two years or more) and a significant effect favouring intensive treatment was found for studies with a long follow‐up, whereas the opposite trend (although not statistically significant) was found for short studies. The meta‐analysis on nephropathy included seven studies, three of which were also included in our review, and showed a significant effect. Our results on the manifestation of neuropathy are consistent with a recent meta‐analysis by Callaghan 2012, as well as observational studies showing a strong association between metabolic control and development of neuropathy (Larsen 2003; Tesfaye 2005).
Our result on the progression of retinopathy, showing that intensive glucose control still shows a beneficial but smaller effect, is consistent with findings from epidemiological studies (Klein 1998; Lovestam‐Adrian 2001; Porta 2001) showing that metabolic control and blood pressure are the main risk factors associated with the development as well as progression of retinopathy.
For the effect of intensive glucose control on the progression of nephropathy and neuropathy, there is a lack of evidence from RCTs. Epidemiological data suggest that even at advanced stages intensive glucose control can slow or sometimes even reverse progression. However, results on this issue are inconsistent (Boulton 2004; Fowler 2008; Vinik 2003).
There is evidence for the importance of several other factors for the progression of nephropathy complications. It was observed that even with long periods of high glucose levels only up to 40% of the patients developed nephropathy, which seems to be partly due to genetic differences that make a subset of patients more susceptible to the disease (Krolewski 1985; Quinn 1996). Especially at later stages of nephropathy, other factors such as blood pressure control, low levels of low density lipoprotein (LDL)‐cholesterol and a protein‐restricted diet might become more important in slowing disease progression compared to blood sugar control alone (Alaveras 1997; Collins 2003; Fried 2001; Hansen 2002; Mogensen 2003; Pedrini 1996).
In our review, there were not enough data on the development of endstage renal disease. The observational follow‐up of the original DCCT patients showed a significantly lower incidence of endstage renal disease in patients who received intensive treatment at early stages of the disease compared to those who received conventional treatment (DCCT/EDIC 2011).
Our meta‐analysis cannot provide any insight into the effects of intensive blood glucose control on major macrovascular outcomes such as myocardial infarction and stroke. Since the studies mostly included young patients at relatively early stages of the disease, the event rates were too low to provide enough data for analysis. The meta‐analysis by Stettler 2006 combined all cardiac and peripheral vascular events and found a reduced incidence of macrovascular events in patients under intensive treatment. However, one of the studies included in that meta‐analysis was excluded from our review because we could not identify different glycaemic targets in the intervention and control group (SDIS 1993). Furthermore, for the two Steno studies (Steno 1 1983; Steno 2 1986) the results referred to follow‐up durations of five and eight years, which suggests that the data used were not based on just the randomised follow‐up periods. However, intensive treatment was also associated with improved outcomes regarding cardiovascular disease in the EDIC study, which followed the patients originally enrolled in the DCCT, and showed a significantly reduced risk of cardiovascular disease in those patients that were originally assigned to intensive treatment compared to those patients that were enrolled in the conventional treatment arm (Nathan 2005). However, it is important to keep in mind that the DCCT population consisted of relatively young and non‐obese patients without hypertension or hypercholesterolaemia at baseline, as these had been defined as exclusion criteria.
Inconsistent results regarding the risk of coronary artery disease are obtained from other observational studies, finding no relationship with glycaemic control. This discrepancy of results can be explained by a lower percentage of patients with albuminuria in the DCCT/EDIC cohort compared to other epidemiological studies. For patients with renal disease, improvement of glycaemic control might be of little benefit while traditional factors such as insulin resistance, blood pressure and cholesterol levels play a stronger role in predicting cardiovascular risk (Orchard 2003; Soedamah‐Muthu 2004; Wajchenberg 2008).
Our review is mostly consistent with other meta‐analyses regarding the occurrence of adverse effects (Egger 1997; Wang 1993). The meta‐analysis by Egger 1997, which again included several studies that did not fulfil the inclusion criteria of our review, showed a significantly increased risk of severe hypoglycaemic episodes for patients under intensive treatment compared to patients under conventional treatment. As in this review, the studies also showed a significant amount of heterogeneity which the authors explored by meta‐regression, showing a significant interaction between the intervention and HbA1c reduction. In our meta‐analysis the effect seemed to be dependent on the baseline HbA1c level. Since all RCTs on intensified glucose control have been carried out a long time ago, it is difficult to assess to what extent modern disease management and patient training programs could prevent the occurrence of severe hypoglycaemia under intensive treatment. Other studies looking at the effect of patient training and the risk of severe hypoglycaemia in intensively treated patients suggest that it is possible to achieve tight glycaemic control without increasing the risk of severe hypoglycaemia (Berger 1995; Sämann 2005).
As in our review, in other reviews (Egger 1997; Wang 1993) the risk of ketoacidosis was only increased in studies using insulin pumps in the intensive treatment arm. Since this effect was only observed under CSII treatment, we do not consider ketoacidosis an adverse effect of intensive treatment per se but rather a potential adverse effect of insulin pump therapy. However, recent meta‐analyses comparing MI with CSII indicate that this effect might only apply to older studies (Misso 2010; Yeh 2012).
While Egger 1997 also found the overall mortality rates to be similar in both treatment arms, they did observe a higher proportion of deaths that were likely to be caused by acute complications associated with insulin therapy in the intensive treatment arm. However, we question whether this result can be attributed to intensive treatment targets. More than half of the studies included in the review by Egger 1997 compared insulin pump therapy to conventional treatment, and many of those studies had not specified different blood glucose targets for the two treatment arms. Overall, there were only seven deaths due to acute metabolic complications, five of them due to ketoacidosis and two were sudden deaths. The causative role of hypoglycaemia in the occurrence of sudden death is still not fully understood (Tu 2010; Weston 1999) and, as described above, ketoacidosis might be a risk of insulin pump therapy as practised in old studies. Furthermore, we believe that the number of deaths reported in Egger 1997 for Steno 1 1983 and Steno 2 1986 refer to longer follow‐up periods than the randomised follow‐up durations of one and two years, respectively. In general, the RCTs performed on intensive glucose control do not provide sufficient data to obtain reliable estimates on the mortality risks in the intervention and control arms. The results of the EDIC study on mortality are yet to be published but investigators have already mentioned that an increased risk of mortality as observed in the ACCORD study on type 2 diabetes (Gerstein 2007; Gerstein 2008) cannot be seen (http://www.medscape.com/viewarticle/806768). However, the EDIC is likely to be the wrong study to provide an answer to the question of whether intensive therapy could be associated with an increased mortality risk, as described in some studies on type 2 diabetes. Such a study would need to investigate the introduction of (more) intensive therapy in an older population of patients with type 1 diabetes, where a large proportion of patients would show cardiovascular risk factors and may have already developed several other diabetic complications. There is currently a lack of data on this kind of patient population.
Some observational data are available on old patients with type 1 diabetes who have lived with diabetes for more than 50 years (Sun 2011). Interestingly, in this patient cohort no association can be found between diabetic complications and the current or longitudinal HbA1c over the last 15 years. This further supports the idea that tight glycaemic control, while clearly being effective in a young and relatively healthy patient population, might not show the same effects in other patient groups.
Authors' conclusions
Implications for practice.
Based on the studies in this review, there is no firm evidence on any specific treatment target. Treatment targets in current guidelines vary between an HbA1c of 6.5% and 7.5% and it is unclear how these targets were established based on the evidence available. The evidence we have presented in this systematic review supports targeting tight (close to normal) glucose levels in young people at relatively early stages of the disease. Our results indicate that for this patient group intensive therapy leads to a reduced risk regarding the development of microvascular complications. The decision to implement these interventions needs to consider managing the risk of hypoglycaemia, highlighting the need for appropriate patient training and support. With the progression of microvascular diabetic complications, intensive treatment becomes less effective but can still slow the progression of retinopathy and possibly also other microvascular complications. Good blood glucose control might still be important, especially since there are likely to be many patients who exhibit some complications but not others. Overall, however, in the case of nephropathy the treatment of symptoms associated with these complications as well as the control of other risk factors, for example high blood pressure, diet and cholesterol levels, might become more important for the further progression of the diabetic complications. There is a general lack of evidence regarding the effects of intensive treatment in type 1 diabetes patients who are older or those who have already developed diabetic complications. We do not know the risks or benefits associated with introducing intensive treatment in older patients or patients with cardiovascular disease.
Overall, no firm evidence exists regarding HbA1c thresholds for all type 1 diabetes patients; it seems necessary to set treatment goals at the individual patient level depending on age, disease progression, macrovascular risk, ability to avoid hypoglycaemic episodes, as well as psychological factors such as burden of tight blood glucose control on health‐related quality of life, fear of hypoglycaemia, or the mental capabilities of the patient to successfully manage the various components necessary for tight blood glucose control.
Implications for research.
Further research is especially needed on whether intensive glucose control should be recommended for patients with type 1 diabetes who are of older age, at advanced stages of the disease, or with cardiovascular disease. The existing results based on randomised trials do not provide adequate data to give insights for such patient populations. Studies on patients with type 2 diabetes showed that especially for the subgroups of patients with long‐lasting diabetes or cardiovascular disease, tight blood glucose control does not show any benefits and might be associated with a higher mortality risk (Gerstein 2007; Gerstein 2008; Nicholas 2013). Comparable subgroups of patients with type 1 diabetes have not been studied. Studying this subgroup of patients is of particular importance considering that due to improvements in diabetes management as well as the treatment of diabetes complications, the life expectancy of patients with type 1 diabetes has increased substantially (Miller 2012) and will lead to a larger population of older patients with type 1 diabetes for whom we currently have no evidence‐based treatment guidelines.
What's new
| Date | Event | Description |
|---|---|---|
| 20 June 2016 | Amended | This review is the same as the previously published version (CD009122.pub2). The only change is that we have corrected a mistake in the PLS. In the 2nd sentence the correct word must be "neuropathy" instead of "nephropathy". |
Acknowledgements
The review authors would like to thank Karla Bergerhoff, the Trials Search Co‐ordinator of the Cochrane Metabolic and Endocrine Disorders Group for her assistance in developing the search strategy. We would like to thank Thomas Selmitsch for help with the abstract screening and Nick Vanneman, Bigit Schorsch, Dania Gruber and Antonia Zengerer for help with acquiring trial publications. Thanks to Erika Kojima, Peter Sawicki and Miramontes Villarreal for their help with screening articles published in Japanese, Polish and Spanish and to Matthew Fullerton for proof‐reading the manuscript.
This review was funded by the German Federal Ministry of Education and Research (BMBF).
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Diabetes mellitus, type 1 explode all trees #2 (IDDM in All Text or T1DM in All Text or T1D in All Text) #3 ( ("insulin* depend*" in All Text or "insulindepend*" in All Text) and not ("non insulin* depend*" in All Text or "non insulindepend*" in All Text) ) #4 ("typ? 1 diabet*" in All Text or "typ?1 diabet*" in All Text or "typ? I diabet*" in All Text or "typ?I diabet*" in All Text) #5 (child* in All Text near/2 diabet* in All Text) #6 (acidos* in All Text near/2 diabet* in All Text) #7 (labil* in All Text near/2 diabet* in All Text) #8 (britt* in All Text near/2 diabet* in All Text) #9 (keto* in All Text near/2 diabet* in All Text) #10 (juvenil* in All Text near/2 diabet* in All Text) #11 (autoimmun* in All Text near/2 diabet* in All Text) #12 (auto in All Text and (immun* in All Text near/2 diabet* in All Text) ) #13 (sudden in All Text and (onset in All Text near/2 diabet* in All Text) ) #14 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 (intensiv* in All Text near/3 control* in All Text) #16 (conventional* in All Text near/3 control* in All Text) #17 (regular in All Text near/3 control* in All Text) #18 (tight* in All Text near/3 control* in All Text) #19 (usual in All Text near/3 control* in All Text) #20 (routin* in All Text near/3 control* in All Text) #21 (standard* in All Text near/3 control* in All Text) #22 (intensiv in All Text near/3 therap* in All Text) #23 (conventional* in All Text near/3 therap* in All Text) #24 (regular in All Text near/3 therap* in All Text) #25 (tight* in All Text near/3 therap* in All Text) #26 (usual in All Text near/3 therap* in All Text) #27 (routin* in All Text near/3 therap* in All Text) #28 (standard* in All Text near/3 therap* in All Text) #29 (intensiv* in All Text near/3 treatment* in All Text) #30 (conventional* in All Text near/3 treatment* in All Text) #31 (regular in All Text near/3 treatment* in All Text) #32 (tight* in All Text near/3 treatment* in All Text) #33 (usual in All Text near/3 treatment* in All Text) #34 (routin* in All Text near/3 treatment* in All Text) #35 (standard* in All Text near/3 treatment* in All Text) #36 (intensiv* in All Text near/3 intervention* in All Text) #37 (conventional in All Text near/3 intervention* in All Text) #38 (regular in All Text near/3 intervention* in All Text) #39 (tight* in All Text near/3 intervention* in All Text) #40 (usual in All Text near/3 intervention* in All Text) #41 (routin* in All Text near/3 intervention* in All Text) #42 (standard* in All Text near/3 intervention* in All Text) #43 (intensiv* in All Text near/3 management* in All Text) #44 (conventional* in All Text near/3 management* in All Text) #45 (regular in All Text near/3 management* in All Text) #46 (tight* in All Text near/3 management* in All Text) #47 (usual in All Text near/3 management* in All Text) #48 (routin* in All Text near/3 management* in All Text) #49 (standard* in All Text near/3 management* in All Text) #50 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30) #51 (#31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49) #52 (#50 or #51) #53 (#14 and #52) |
| MEDLINE |
| 1 exp Diabetes Mellitus, Type 1/ 2 exp Diabetic Ketoacidosis/ 3 (IDDM or T1DM or T1D).tw,ot. 4 (("insulin* depend*" or "insulin?depend*") not ("non‐insulin* depend*" or "non insulindepend*")).tw,ot. 5 ("typ? 1 diabet*" or "typ? I diabet*" or "typ?1 diabet*" or "typ?I diabet*").tw,ot. 6 ((acidos* or juvenil* or child* or keto* or labil* or britt*) adj2 diabet*).tw,ot. 7 ((auto‐immun* or autoimmun* or sudden onset) adj2 diabet*).tw,ot. 8 (insulin* defic* adj2 absolut*).tw,ot. 9 or/1‐8 10 exp Diabetes Insipidus/ 11 diabet* insipidus.tw,ot. 12 10 or 11 13 9 not 12 14 ((intensiv* or conventional* or regular or tight* or usual or routin* or standard) adj3 (control* or therap* or treatment* or intervention* or management*)).tw,ot. 15 13 and 14 16 randomised controlled trial.pt. 17 controlled clinical trial.pt. 18 randomi?ed.ab. 19 placebo.ab. 20 drug therapy.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 or/16‐23 25 Meta‐analysis.pt. 26 exp Technology Assessment, Biomedical/ 27 exp Meta‐analysis/ 28 exp Meta‐analysis as topic/ 29 hta.tw,ot. 30 (health technology adj6 assessment$).tw,ot. 31 (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 32 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 33 or/25‐32 34 24 or 33 35 (comment or editorial or historical‐article).pt. 36 34 not 35 37 15 and 36 38 (animals not (animals and humans)).sh. 39 37 not 38 40 39 and 24 [Medline results for RCTs] 41 39 and 33 [Medline results for SRs] |
| EMBASE |
| 1 exp insulin dependent diabetes mellitus/ 2 exp diabetic ketoacidosis/ 3 (IDDM or T1DM or T1D).tw,ot. 4 (("insulin* depend*" or "insulin?depend*") not ("non insulin* depend*" or "non insulin?depend*")).tw,ot. 5 (("typ? 1" or "typ? I" or "typ?1" or "typ?I") adj2 diabet*).tw,ot. 6 ((acidos* or juvenil* or child* or keto* or labil* or britt*) adj2 diabet*).tw,ot. 7 ((auto‐immun* or autoimmun* or sudden onset) adj2 diabet*).tw,ot. 8 (insulin* defic* adj2 absolut*).tw,ot. 9 or/1‐8 10 exp diabetes insipidus/ 11 diabet* insipidus.tw,ot. 12 10 or 11 13 9 not 12 14 ((intensiv* or conventional* or regular or tight* or usual or routin* or standard*) adj3 (control* or therap* or treatment* or intervention* or management*)).tw,ot. 15 13 and 14 16 exp Randomized Controlled Trial/ 17 exp Controlled Clinical Trial/ 18 exp Clinical Trial/ 19 exp Comparative Study/ 20 exp Drug comparison/ 21 exp Randomization/ 22 exp Crossover procedure/ 23 exp Double blind procedure/ 24 exp Single blind procedure/ 25 exp Placebo/ 26 exp Prospective Study/ 27 ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 28 (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 29 ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 30 (cross over or crossover).ab,ti. 31 or/16‐30 32 exp meta analysis/ 33 (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 34 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 35 exp Literature/ 36 exp Biomedical Technology Assessment/ 37 hta.tw,ot. 38 (health technology adj6 assessment$).tw,ot. 39 or/32‐38 40 31 or 39 41 (comment or editorial or historical‐article).pt. 42 40 not 41 43 15 and 42 44 limit 43 to human |
Appendix 2. Description of interventions
|
Characteristic Study ID |
Intensive glucose control [route, frequency, total dose/day] | Conventional glucose control [route, frequency, total dose/day] |
| Bucharest‐Düsseldorf 1984 |
|
|
| DCCT 1&2 (primary prevention and secondary intervention) 1993 |
|
|
| Holman 1983 |
|
|
| MCSG 1995 |
|
|
| MDCCT 1994 |
|
|
| Linn 1996 |
|
|
| Oslo 1987 |
|
|
| Steno 1 1983 |
|
|
| Steno 2 1986 |
|
|
| Wysocki 2003 4 |
|
|
| Verrillo 1988 |
|
|
|
Footnotes "‐" denotes not reported aSince 1980 the aim was to avoid HbA1c values ≥ 12% bApproximately one third of the patients were treated with two injections of a mixture of isophane insulin and crystalline regular insulin daily for extended periods to improve glycaemic control cDuring the last five years of the study most patients switched to an insulin regimen using human insulin dGlycaemic targets were relaxed for children who experienced ≥ 2 severe hypoglycaemic episodes within 6 months HbA1c: glycated haemoglobin concentration; NPH: neutral protamine Hagedorn | ||
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Participating population | Study period [year to year] | Country | Setting | Ethnic groups [%] | Duration of disease [mean/range years (SD), or as reported] |
| Bucharest‐ Düsseldorf 1984 | I: intensive therapy | 1 yra | Insulin‐dependent diabetic patients | 1984‐1986 | Romania | Diabetes unit of the Bucharest University Hospital | ‐ | Patients with newly diagnosed diabetes: 13 Duration of diabetes: 6b |
| C: basic therapy | ‐ | Patients with newly diagnosed diabetes: 10 Duration of diabetes: 5b |
||||||
| all: | ‐ | 5 (‐) / ‐ | ||||||
| DCCT1 1993 (primary prevention) | I: intensive therapy | 6.5 (3‐9cyr) | Insulin‐dependent diabetic patients without retinopathy at baseline | 1983‐1993 | USA and Canada | Outpatient treatment in 29 clinics | White: 96 | 3 (1) |
| C: conventional therapy | White: 96 | 3 (1) | ||||||
| all: | 3(1) | |||||||
| DCCT2 1993 (secondary prevention) | I: intensive therapy | 6.5 (3‐9cyr) | Insulin‐dependent diabetic patients with very‐mild‐to‐ moderate non‐ proliferative retinopathy | 1983‐1993 | USA and Canada | Outpatient treatment in 29 clinics | White: 97 | 9 (4) |
| C: conventional therapy | White: 97 | 9 (4) | ||||||
| all: | 9(4) | |||||||
| Holman 1983 | I: intensive therapy | 2 yr | Insulin‐dependent diabetic patients | ‐ | England | Diabetic clinics at Oxford and Aylesbury | ‐ | 18 (5) 9‐29 |
| C: conventional therapy | ‐ | 19 (7) 1‐39 | ||||||
| all: | 19 (6) 1‐39 | |||||||
| Linn 1996 | I: intensive therapy | 5 yr | Newly diagnosed insulin‐dependent diabetic patients | Starting year: 1988 | Germany | Medical Clinic III, Justus Liebig Univ, Giessen, Germany | ‐ | Newly diagnosed diabetes type 1 |
| C: conventional therapy | ||||||||
| all: | ||||||||
| MCSG 1995 | I: intensive therapy | 2‐8 yr (median 5 yr) |
European insulin dependent diabetic patients with microalbuminuria | 1984‐1993 | England, Wales | Nine hospital based specialist diabetes centres |
‐ | 21 (‐) 6‐35 |
| C: conventional therapy | ‐ | 18 (‐) 7‐34 | ||||||
| all: | 20 (‐) 6‐35 | |||||||
| MDCCT 1994 | I: intensive therapy | 5 yr | Patients with insulin‐dependent type 1 diabetes who had received a renal allograft as treatment for end‐stage diabetic nephropathy | 1978‐?d | USA | University of Minnesota Hospital and Clinic and the Clinical Research Center and Hennepin County Medical Center, Minneapolis |
White: 100 | 23 (6) 14‐39 |
| C: conventional therapy | 21 (5) 14‐30 | |||||||
| all: | 22 (6) 14‐39 | |||||||
| Oslo 1987 | I: intensive therapy, MI | 43 mo (7‐48) | C peptide negative insulin dependent diabetes patients | ‐ | Norway | Patients from various outpatient clinics in the Oslo area |
‐ | 154 (81‐250 mo) |
| I: intensive therapy, CSII | 47 mo (24‐48) | ‐ | 153 (77‐280 mo) | |||||
| C: conventional therapy | 45 mo (26‐48) | ‐ | 152 (81‐240 mo) | |||||
| all: | ‐ | 153 (77‐280 mo) | ||||||
| Steno 1 1983 | I: intensive therapy | 1 yre | Insulin‐dependent diabetic patients with background retinopathy | ‐ | Denmark | Steno Memorial Hospital, outpatient clinic |
‐ | 19f (11‐ 23) |
| C: conventional therapy | ‐ | 19f (9‐27 | ||||||
| all: | 19f (9‐27) | |||||||
| Steno 2 1986 | Intensive therapy | 2 yr | Insulin‐dependent diabetic patients with micro‐ albuminuria |
‐ | Denmark | Steno Memorial Hospital, outpatient clinic |
‐ | 15a (10‐26) |
| C: conventional therapy | ‐ | 15a (5‐26) | ||||||
| all: | ‐ | 15a (5‐26) | ||||||
| Verrillo 1988 | I: intensive therapy | 5 yr | Patients with insulin‐dependent diabetes mellitus and background retinopathy | ‐ | Italy | Outpatient clinic in Naples | ‐ | 19 (5) |
| C: conventional therapy | ‐ | 21 (6) | ||||||
| all: | ‐ | 20 (6) | ||||||
| Wysocki 2003 g | I: intensive therapy | 18 mo | School‐aged children with diabetes type 1 | 1997‐2001 | USA | Nemours Children Clinic, Florida St. Louis Children’s hospital, Missouri |
White: 80 African: American: 16 Hispanic: 2 Other: 2 | 5 (3) |
| C: conventional therapy | White: 91 African: American: 7 Hispanic: 0 Other: 2 | 5 (3) | ||||||
| all: | White: 85 African: American: 13 Hispanic: 1 Other: 2 | 5 (3) | ||||||
|
Footnotes "‐" denotes not reported aTwo of the study arms were followed for two years, with one group switching to another treatment after one year; therefore, for this review only the first year of follow‐up was used bMedian of those patients who were not newly diagnosed cNumbers apply to the full DCCT population including primary prevention and secondary intervention cohorts dAt least 1992 eStudy was extended by one more year, but patients were allowed to switch groups; although only one patient decided to change treatment, we included the results after one year fMedian gInconsistent baseline data provided across different publications (Wysocki 2003) BMI: body mass index; C: comparator; CSII: continuous subcutaneous insulin infusion; DCCT: 'Diabetes Control and Complications Trial'; HbA1c: glycosylated haemoglobin A1c; I: intervention; MI: multiple daily injections; mo: month; SD: standard deviation; yr: year | ||||||||
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Sex [female %] | Age [mean (SD)/ range years, or as reported] | HbA1 / HbA1c [mean % (SD or range] | BMI [mean kg/m2 (SD)] | Co‐medications/Co‐interventions | Co‐morbidities |
| Bucharest‐ Düsseldorf 1984 | I: intensive therapy | 43 | 26 (10) | 12.3 (0.2)h | 21.8 (0.3)h | ‐ | ‐ |
| C: basic therapy | 46 | 26 (10) | 11.7 (0.2)h | 21.5 (0.2)h | ‐ | ‐ | |
| all: | 45 | 26 (10) | ‐ | ‐ | |||
| DCCT1 1993 (primary prevention) | I: intensive therapy | 51 | 27 (7) | 8.8 (1.6) | Male: 24 (3) Female: 23 (3) |
‐ | Clinical neuropathy: 4.9% Autonomic neuropathy: 2.6% |
| C: conventional therapy | 46 | 26 (8) | 8.8 (1.7) | Male: 23 (3) Female: 23 (3) |
‐ | Clinical neuropathy: 2.1% Autonomic neuropathy: 2.4% |
|
| all: | 48 | 26 (7) | 8.8 (1.7) | Male: 24 (3) Female: 23 (3) |
‐ | Clinical neuropathy: 3.5% Autonomic neuropathy: 2.5% |
|
| DCCT2 1993 (secondary prevention) | I: intensive therapy | 47 | 27 (7) | 9.0 (1.5) | Male: 23 (3) Female: 24 (3) |
‐ | Clinical neuropathy: 9.4% Autonomic neuropathy: 5.3% Retinopathy: Microaneurysms only: 67% Mild: 18% Moderate: 15% |
| C: conventional therapy | 46 | 27 (7) | 8.9 (1.5) | Male: 24 (3) Female: 23 (3) |
‐ | Clinical neuropathy: 9.4% Autonomic neuropathy: 8.3% Retinopathy: Microaneurysms only: 58% Mild: 23% Moderate: 19% |
|
| all: | 46 | 27(7) | 9.0 (1.5) | Male: 24 (3) Female: 24 (3) |
‐ | Clinical neuropathy: 9.4% Autonomic neuropathy: 6.8% Retinopathy: Microaneurysms only: 53% Mild: 20% Moderate: 17% |
|
| Holman 1983 | I: intensive therapy | 42 | 42 (12) / 24‐60 | 11.7 (1.6) | 25 (4) / 20 ‐29) | 1 patient received antihypertensive therapy | ‐ |
| C: conventional therapy | 32 | 43 (13) / 21‐60 | 11.8 (2.1) | 25 (2) / 21‐29 | 2 patients received antihypertensive therapy | ‐ | |
| all: | 37 | 43 (12) / 21‐60 | 11.8 (1.9) | 25 (3) / 20‐29 | 3 patients received antihypertensive therapy | ‐ | |
| Linn 1996 | I: intensive therapy | 43 | 27 (8) | 12.4 (5.5) | 23 (1)a | ‐ | ‐ |
| C: conventional therapy | 47 | 29 (8) | 13.1 (6.2) | 24 (4)a | ‐ | ‐ | |
| all: | 45 | 28 (8) | 12.7 (5.8) | 23 (3) | ‐ | ‐ | |
| MCSG 1995 | I: intensive therapy | 25 | 37 / 19‐59 | 10.3 (1.9b) | 26 / 18‐40 | ‐ | Retinopathy: 11 patients |
| C: conventional therapy | 29 | 37 / 17‐58 | 9.8 (1.6b) | 26 / 19‐34 | ‐ | Retinopathy: 12 patients | |
| all: | 27 | 37 / 17‐59 | 10.1 (‐) | 26 / 18‐40 | ‐ | Retinopathy: 23 patients | |
| MDCCT 1994 | I: intensive therapy | 21 | 35 (6) / 21‐50 | ‐ | 28 (8) / 19‐57c | Immunosupression regimens; most patients received medication for hypertension | Most patients had hypertension |
| C: conventional therapy | 28 | 36 (8) / 21‐58 | ‐ | 26 (4) / 21‐49c | |||
| all: | 24 | 35 (7) / 21‐58 | ‐ | 27 (6) / 19‐57c | |||
| Oslo 1987 | I1: intensive therapy, MI | 53 | 26 / 19‐42 | 9.4 (0.4d) | 72 (10)e | None | Retinopathy grade > 1: 12 patients |
| I2: intensive therapy, CSII | 53 | 26 / 18‐32 | 10.1 (0.4d) | 69 (9)e | None | Retinopathy grade > 1: 10 patients | |
| C: conventional therapy | 53 | 26 / 18‐36 | 9.5 (0.4d) | 71 (9)e | None | Retinopathy grade > 1: 12 patients | |
| all: | 53 | 26 / 18‐42 | 9.7 (1.5) | 70 (9)e | None | 34 patients with simplex retinopathy | |
| Steno 1 1983 | I: intensive therapy | 53 | 36 / 21‐51f | 9.7 (7.4‐12.1)g | 106 / 84‐123f,h | 1 patient had well‐ regulated hypertension treated with 25mg hydroflumethizide daily. Otherwise no co‐medication | Intermittent proteinuria: 5 patients |
| C: conventional therapy | 40 | 32 / 24‐26f | 8.6 (6.0‐10.4)g | 100 / 79‐123f,h | No co‐medication | Intermittent proteinuria: 5 patients | |
| all: | 47 | 21‐519 | 6.0‐12.1i | 79‐123h,i | Intermittent proteinuria: 10 patients | ||
| Steno 2 1986 | I: intensive therapy | 39 | 32 / 18‐48f | 9.5 (6.6‐13.6)f | ‐ | ‐ | Retinopathy simplex: 12 patients Retinopathy, proliferative: 1 |
| C: conventional therapy | 44 | 29 / 18‐47f | 9.3 (7.0‐11.7)f | ‐ | ‐ | Retinopathy simplex: 11 patients Retinopathy, proliferative: 1 |
|
| all: | 42 | 18‐48i | 7.0‐11.7i | ‐ | ‐ | Retinopathy simplex: 23 patients Retinopathy, proliferative: 2 |
|
| Verrillo 1988 | I: intensive therapy | 45 | 37 (10) | 10.8 (1.4) | 26 (4) | ‐ | ‐ |
| C: conventional therapy | 45 | 38 (9) | 11.1 (1.8) | 26 (4) | ‐ | ‐ | |
| all: | 45 | 38 (9) | 11.0 (1.6) | 26 (4) | ‐ | ‐ | |
| Wysocki 2003 g | I: intensive therapy | 55 | 12 (3) | 8.2 (1.1) | ‐ | ‐ | ‐ |
| C: conventional therapy | 35 | 12 (3) | 8.1 (0.9) | ‐ | ‐ | ‐ | |
| all: | 44 | 12 (3) | 8.1 (1.0) | ‐ | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported aValues read from figure 1 in Linn 1996, measured ˜ 6 months after baseline bIn the publication described as standard error of the mean, but the standard deviation appears more plausible cOnly includes patients who completed the study dProbably standard error of the mean eMean body weight in kg fMedian/range gMean/range hValues denote % of ideal body weight iRange BMI: body mass index; CSII: continuous subcutaneous insulin infusion; HbA1c: glycosylated haemoglobin A1c; MI: multiple daily injections; SD: standard deviation | |||||||
Appendix 5. Matrix of study endpoints (publications)
|
Characteristic Study ID |
Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa |
| Bucharest‐Düsseldorf 1984 | Review's primary outcomes reported in publication | ||
| Myocardial infarction (fatal/ non‐fatal)c | Throughout study period | ||
| Stroke (fatal, non‐fatal)c | Throughout study period | ||
| Retinopathy (Manifestation / Progression mixed) | x | ||
| Neuropathy (Manifestation/ Progression) | x | ||
| Nephropathy (Manifestation/ Progression) | x | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes | x | ||
| Ketoacidosis (O) | Throughout study period | ||
| Weight gain (O) | 0, 6, 12 mo | ||
| All‐cause mortality (O) | Throughout study period | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), diabetes‐related knowledge (O), compliance (O), frequency of metabolic self‐monitoring (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
|
DCCT 1993 (DCCT1 & DCCT2) |
Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) (O) | 1‐9 y (yearly), endpoint (3.5‐9 y) | ||
| Stroke (fatal, non‐fatal) (O) | 1‐9 y (yearly), endpoint (3.5‐9 y) | ||
| Retinopathy (Manifestation / Progression) (P)e | |||
| Neuropathy (Manifestation/ Progression) (O) | 0, 5 y | ||
| Nephropathy (Manifestation/ Progression) (O) | 0, then yearly until endpoint (3.5‐9 y) | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | Throughout study period | ||
| Ketoacidosis (O) | Throughout study period | ||
| Weight gain (O) | Yearly until endpoint | ||
| All‐cause mortality (O) | Throughout study period | ||
| Health‐related quality of life (O) | 0, then yearly until endpoint (3.5‐9 y) | ||
| Costs (O) | Throughout study period | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Home blood glucose profiles (O), Triglycerides (O), Total cholesterol (O), HDL cholesterol (O), Resting ECG (O), Neurobehavioral assessment (O), Psychological symptoms (O), Diet history (O), insulin dose (O), Significant ventricular arrhythmia (O), Congestive heart failure (O), Transient ischaemic attack (O), Hypertension (O), Severe lipid abnormality (O), Adherence (O) | |||
| Subgroups reported in publication | |||
| Age, gender, diabetes duration, different cardiovascular risk factors, HbA1c baseline level | |||
| Holman 1983 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal)c | 2 y | ||
| Stroke (fatal, non‐fatal)c | 2 y | ||
| Retinopathy (Manifestation/ Progression) (O) | 0,1, 2 y | ||
| Neuropathy (Manifestation and progression mixed) (O) | 0,1,2 y | ||
| Nephropathy (Manifestation and progression mixed) (O) | 0,1,2 y | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | 2 y | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes | x | ||
| Ketoacidosis | x | ||
| Weight gain | 0, then every 4 mo for up to 2 y | ||
| All‐cause mortality | 2 y | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| Hba1c (O), Blood pressure (O), Insulin dose (O), Cardiac ischaemia (O), LDL and HDL Cholersterol (O), Triglycerides, N‐acetylglucosaminidase (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
| Linn 1996 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation and Progression mixed) (O) | 0,1,2,3,4,5 y | ||
| Neuropathy (Manifestation/ Progression) (O) | 0,1,2,3,4,5 y | ||
| Nephropathy (Manifestation/ Progression) (O) | 0,1,2,3,4,5 y | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes | Throughout study period | ||
| Ketoacidosis (O) | x | ||
| Weight gain | x | ||
| All‐cause mortality | x | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| Glucagon‐stimulated C‐peptide (O), arginine‐stimulated insulin secretion (O), insulin sensitivity (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
| MCSG 1995 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation/ Progression) (O) | 0, then every 6 mo until endpoint (max = 8 y) | ||
| Neuropathy (Manifestation/ Progression) (O) | x | ||
| Nephropathy (Manifestation/ Progression) (P) | 0, then every 6 mo until endpoint (max = 8 y) | ||
| Endstage renal disease (O) | within first 2 y, endpoint (max = 8 y) | ||
| Hypoglycaemic episodes, severe (O) | 0, then every 6 mo until endpoint (max = 8 y) | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes, all | x | ||
| Ketoacidosis | 0, then every 6 mo until endpoint (max = 8 y) | ||
| Weight gain | x | ||
| All‐cause mortality | 0, then every 6 mo until endpoint (max = 8 y) | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Blood pressure (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
| MDCCT 1994 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation / Progression) | x | ||
| Neuropathy (Manifestation/ Progression) | x | ||
| Nephropathy (Manifestation / Progression) (P) | 0, 5 yd | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | Throughout study period | ||
| Ketoacidosis | x | ||
| Weight gain (O) | |||
| All‐cause mortality (O) | Throughout study period | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Blood pressure (O), different morphometric measures of the kidney biopsy samples (P), insulin dose (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
| Oslo 1987 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation and Progression mixed) (O) | ‐2,0,3,6,12,24,41 mo | ||
| Neuropathy (Manifestation/ Progression) (O) | 0, 2 y | ||
| Nephropathy (Manifestation/ Progression) (O) | ‐2 mo, 1st year, 2nd year, 3rd & 4th year | ||
| Endstage renal disease (O) | 0, 6‐8 mo | ||
| Hypoglycaemic episodes, severe (O) | During first two years | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | Monthly during first year, bi‐monthly after that | ||
| Ketoacidosis (O) | During first two years | ||
| Weight gain (O) | 2 y | ||
| All‐cause mortality (O) | Throughout study period | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Insulin antibodies (O), Blood glucose profiles (O), Diet (O), Insulin requirement (O) | |||
| Subgroups reported in publication | |||
| Two intervention groups: multiple injections and continuous subcutaneous insulin infusion | |||
| Steno 1 1983 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation/ Progression) (P) | 0,6,12 mo | ||
| Neuropathy (Manifestation and Progression mixed) (O) | 0,6,12 mo | ||
| Nephropathy (Manifestation/ Progression) | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | 4,8, and 12 mo | ||
| Ketoacidosis (O) | Throughout study period | ||
| Weight gain | x | ||
| All‐cause mortalityc | Throughout study period | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Fasting and 1.5h postprandial blood glucose (O) | |||
| Subgroups reported in publication | |||
| Steno 2 1986 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation and Progression mixed) (O) | 0,6,12,24 mo | ||
| Neuropathy (Manifestation/ Progression) | x | ||
| Nephropathy (Manifestation/ Progression) (P) | Every other month until endpoint | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | Throughout study period | ||
| Ketoacidosis (O) | Throughout study period | ||
| Weight gain | x | ||
| All‐cause mortalityc | Throughout study period | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), Peripheral vascular disease (O), blood pressure (O), | |||
| Subgroups reported in publication | |||
| ‐ | |||
| Verrillo 1988 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation / Progression) (P) | 0, 1, 3, 5 y | ||
| Neuropathy (Manifestation/ Progression) | x | ||
| Nephropathy (Manifestation/ Progression) | x | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes (O) | Throughout study period | ||
| Ketoacidosis | x | ||
| Weight gain | x | ||
| All‐cause mortality | x | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), plasma glucose profile (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
| Wysocki 2003 | Review's primary outcomes reported in publication | Endpoint not reported in publication | Time of measurementa |
| Myocardial infarction (fatal/ non‐fatal) | x | ||
| Stroke (fatal, non‐fatal) | x | ||
| Retinopathy (Manifestation / Progression mixed) | x | ||
| Neuropathy (Manifestation/ Progression) | x | ||
| Nephropathy (Manifestation/ Progression) | x | ||
| Endstage renal disease | x | ||
| Hypoglycaemic episodes, severe (O) | Throughout study period | ||
| Review's secondary outcomes reported in publication | |||
| Adverse events, serious | x | ||
| Adverse events, all | x | ||
| Hypoglycaemic episodes | x | ||
| Ketoacidosis | x | ||
| Weight gain (O) | Quarterly | ||
| All‐cause mortality | x | ||
| Health‐related quality of life | x | ||
| Costs | x | ||
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | |||
| HbA1c (O), intelligence quotient (O), hospitalizations (O), emergency room admissions (O), height (O), Tanner stage of pubertal development (O), autonomy/maturity ratio (O) | |||
| Subgroups reported in publication | |||
| ‐ | |||
|
aUnderlined data denote times of measurement for primary and secondary review outcomes, if measured and reported in the results section of the publication (other times represent planned but not reported points in time) b(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the publication cNot explicitly reported, but could be deduced dDeveopment of diabetic renal lesions in transplanted kidneys eThe primary endpoint of the DCCT1 was the manifestation of retinopathy, the primary endpoint of the DCCT2 was progression of retinopathy DCCT: 'Diabetes Control and Complications Trial'; FBG: fasting blood glucose; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; mo: months; PPG (postprandial glucose), y: years | |||
Appendix 6. Matrix of study endpoints (trial documents)
| Characteristic / Study ID (trial identifier) | Endpoint | Time of measurement a |
| DCCT 1993 (DCCT1 & DCCT2) | Retinopathy, Manifestation and Progression (P)d | 0 + every six months |
| HbA1c (O) | N/A | |
| Home blood glucose profiles (O) | N/A | |
| Nephropathy (S)e | 0 + yearly | |
| Autonomic Neuropathy (S) | 0 + every other year | |
| Peripheral Neuropathy (S) | 0, 5 y, study termination | |
| Peripheral vascular disease (O) | N/A | |
| Triglycerides (O) | N/A | |
| Total cholesterol (O) | N/A | |
| HDL cholesterol (O) | N/A | |
| Resting ECG (O) | N/A | |
| Neurobehavioral assessment (O) | N/A | |
| Psychological symptoms (O) | N/A | |
| Quality of life (O) | 0 + yearly | |
| Diet history (O) | N/A | |
| Adverse events (P)f | Throughout study period | |
| Myocardial infarction (S) | Throughout study period | |
| Significant ventricular arrhythmia (O) | N/A | |
| Congestive heart failure (O) | N/A | |
| Definitive cerebrovascular accident (O) | Throughout study period | |
| Transient ischaemic attack (O) | N/A | |
| Hypertension (O) | N/A | |
| Severe lipid abnormality (O) | N/A | |
| Adherence (O) | N/A | |
|
Footnotes aUnderlined data denote times of measurement for primary and secondary review outcomes, if measured and reported in the results section of the publication (other times represent planned but not reported points in time) bEndpoint in bold/italic = review primary/secondary outcome c(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the report dMeasures included visual acuity, intraocular pressure, slit lamp once a year, stereo fundus photography every six months and stereo fluorescein angiography, which was only performed in the primary prevention group at baseline, after 5 years and after 9 years eMeasures include tests for microalbuminuria, creatinine clearance, serum creatinine, serum albumin fIncluded death, severe hypoglycaemia, ketoacidosis, weight gain, inability to maintain normal growth and development, inability to maintain psychological well‐being, cerebral dysfunction ECG: electrocardiogram; HbA1c: glycosylated haemoglobin A1c; HDL: high density lipoprotein; mo: months; N/A: not applicable | ||
Appendix 7. Examination of outcome reporting bias
| Study ID | Outcome | Clear that outcome was measured and analyseda [trial report states that outcome was analysed but only reports that result was not significant] | Clear that outcome was measured and analysedb [trial report states that outcome was analysed but no results reported] | Clear that outcome was measuredc [clear that outcome was measured but not necessarily analysed (judgement says likely to have been analysed but not reported because of non‐significant results)] | Unclear whether the outcome was measuredd [not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results] |
| Bucharest‐Düsseldorf 1984 | Symptomatic hypoglycaemia | x | |||
| DCCT 1993 (DCCT 1 & DCCT 2) | Weight gain | x | |||
| DCCT 1993 (DCCT 1 & DCCT 2) | Adverse events | x | |||
| Holman 1983 | Autonomic neuropathy | x | |||
| Linn 1996 | Retinopathy | x | |||
| MCSG 1995 | Adverse events | x | |||
| MDCCT 1994 | Overall hypoglycaemia | x | |||
| Oslo 1987 | Neuropathy (autonomic and peripheral)e | x | |||
| Steno 1 1983 | Weight gain | x | |||
| Steno 2 1986 | Adverse events | x | |||
| Mortality | x | ||||
| Verrillo 1988 | Mild hypoglycaemia | x | |||
| Wysocki 2003 | Adverse events | x | |||
| Mortality | x | ||||
| Weight gain | x | ||||
|
Footnotes 'High risk of bias' categories for outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) study classification system for missing or incomplete outcome reporting in reports of randomised trials (Kirkham 2010) aClassification 'A' (table 2, Kirkham 2010) bClassification 'D' (table 2, Kirkham 2010) cClassification 'E' (table 2, Kirkham 2010) dClassification 'G' (table 2, Kirkham 2010) eResults on neuropathy were reported in the 2‐year extension study (Lauritzen 1985) including baseline measurements. We therefore assume that results might have also been available at 1 year | |||||
Appendix 8. Definition of endpoint measurement (I)
|
Characteristic Study ID |
Myocardial infarction | Stroke | Retinopathy | Neuropathy | Nephropathy |
| Bucharest‐Düsseldorf 1984 | N/A | N/A | N/A | N/A | N/A |
| DCCT1&2 1993 | ‐ | ‐ | Grading according to ETDRS protocol (25‐step scale)a: Manifestation: change of at least three steps from baseline sustained for at least six months in primary prevention group;b Progression: change of at least three steps from baseline sustained for at least six months in secondary intervention groupc |
Confirmed clinical neuropathy: abnormal neurologic examinationa + either abnormal nerve conduction in at least two peripheral nerves or abnormal autonomic‐nerve testing | Microalbuminuria: albumin excretion rate ≥ 40 mg/24h Clinical albuminuria: albumin excretion rate ≥ 300 mg/24hd |
| Holman 1983 | Fatal: ‐ Non‐fatal: N/A |
Fatal: N/A Non‐fatal: N/A |
Retinal colour photography, fluorescein angiography; retinopathy index (RI); RI = (log10 ma + 0.38 log10 cws + 0.21 √hm + 0.17 √ex + 4.8) ma: number of microaneurysms, cws: number of cotton‐wool spots, hm: areas of haemorrhage, ex: number of exudates |
Autonomic neuropathy: lying/standing 30/15 ratio; peripheral neuropathy: vibration sensory threshold |
Plasma creatinine and creatinine clearance |
| Linn 1996 | N/A | N/A | Screening according to the recommendations of the Early Treatment Diabetic Retinopathy Study Group and the St. Vincent Declaration | At least three of the following: clinical symptoms, signs, quantitative sensory testing, and peroneal motor nerve conduction velocity (following the San Antonio Consensus Statement) | Urinary albumin excretion was used as a screening test for diabetic nephropathy. |
| MCSG 1995 | Fatal: N/A Non‐fatal: N/A |
Fatal: N/A Non‐fatal: N/A |
Retinal appearances graded according to the scoring system used in the WHO multinational study of vascular disease in diabetese | N/A | Progression: change from microalbuminuria to clinical albuminuria |
| MDCCT 1994 | N/A | N/A | N/A | N/A | Manifestation of nephropathy: renal glomerular mesangial expansion, determined by electron microscopy of renal biopsy samples No definition regarding the minimum level of expansion constituting nephropathy |
| Oslo 1987 | N/A | N/A | Color fundus photography: Counting of microaneurysms and haemorrhages as “red spots”; Fluorescein angiography: rating “better”, “worse” or “unchanged” |
Measurement of motor nerve conduction velocities; no definition of which velocities constitute neuropathy | Measurement of urinary albumin excretion |
| Steno 1 1983 | Fatal:N/A Non‐fatal: N/A |
Fatal: N/A Non‐fatal: N/A |
Color fundus photography, fluorescein angiography Retinal morphology: blind rating by two independent ophthalmologists: deterioration, no change, improvement Retinal function: macular recovery time and oscillatory potential |
‐ | N/A |
| Steno 2 1986 | N/A | N/A | Exam with ophthalmoscope | N/A | Progression: clinical diabetic nephropathy: albumin excretion rate > 300mg/24h (200 ug/min) in two of three 24h specimens, Also measurement of glomerular filtration rate and serum creatinine |
| Verillo 1988 | N/A | N/A | Grading of fluorescein angiograms, fundal photographs and ophthalmoscopy results: grade 0‐5 (no retinopathy – proliferative retinopathy) | N/A | N/A |
| Wysocki 2003 | N/A | N/A | N/A | N/A | N/A |
|
Footnotes "‐" denotes not reported aDefined by at least two of the following: symptoms consistent with peripheral neuropathy, abnormal sensory examination findings, or absent or decreased deep tendon reflexes bIn DCCT 1995a the primary outcome for retinopathy manifestation is defined as the presence of at least one microaneurysm in either eye at two consecutive 6‐monthly gradings cIn DCCT 1995a, the primary outcome for retinopathy progression was defined as a three‐step change without the requirement of presence in two consecutive 6‐monthly gradings dIn DCCT 1995b, an additional more advanced level of microalbuminuria was defined at >100 mg/24h eJarret RJ, Keen H, Grabauskas V. The WHO multinational study of vascular disease in diabetes; general description. Diabetes Care 1979;2: 175‐86. ETDRS : Early Treatment Diabetic Retinopathy Study; N/A: not applicable, PVD: peripheral vascular disease | |||||
Appendix 9. Definition of endpoint measurement (II)
|
Characteristic Study ID |
End‐stage renal disease | Health‐related quality of life | Hypoglycaemia | Ketoacidosis | Other adverse events | Costs |
| Bucharest‐Düsseldorf 1984 | N/A | N/A |
All: ‐ Severe: loss of consciousness, either treated by intravenous glucose administration or glucagon injection |
Hyperglycaemic ketotic metabolic decompensation with clinical signs of ketoacidosis, arterial pH < 7.3, and hospital treatment | BMI at endpoint and baseline | N/A |
| DCCT1&2 1993 | N/A | DQOL, scale form 0 (lowest)‐100 (highest), was developed for the DCCT, SCL‐90R, SF‐36 |
Severe: requiring assistance of another person + blood glucose < 50 mg/dL or prompt recovery after oral carbohydrate or intravenous glucagon or glucose | Four criteria had to be satisfied: blood glucose > 250 mg/dL, presence of large/moderate ketones in urine or serum, at least one of the following: arterial blood pH < 7.30, venous blood pH < 7.25, serum bicarbonate < 15 mEq/L, treatment within a healthcare facility |
Weight gain: Overweight: Men: BMI ≥ 27.8 kg/m2, Women: BMI ≥ 27.3 kg/m2 Major weight gain: BMI increase by more than 5 kg/m2 |
Product of resources used and unit costs of those resources. Time away from usual activities (e.g. time lost from work) was not included |
| Linn 1996 | N/A | N/A |
All: blood glucose value < 3.5 mmol/L Severe: ‐ |
N/A | Weight gain: expressed as an increase in body mass index | N/A |
| Holman 1983 | N/A | N/A |
All: N/A Severe: requiring hospital admission |
N/A | Weight gain: ‐ | N/A |
| MCSG 1995 | Measured through glomerular filtration rate, but no threshold specified | N/A |
All: N/A Severe: assistance of another person required |
‐ | Weight gain: ‐ | N/A |
| MDCCT 1994 | N/A | N/A |
Severe: episode of behavioural change requiring the help of others for treatment Moderately severe: severe hyperglycaemic symptoms but with preserved capability for self‐treatment |
N/A | ‐ | N/A |
| Oslo 1987 | N/A | N/A |
All: symptomatic and home measured blood glucose values < 2.5 mmol/L (45 mg/dL) Severe: hypoglycaemic coma |
‐ | Body weight in kg | N/A |
| Steno 1 1983 | N/A | N/A |
All: blood glucose value < 2.5 mmol/L (45 mg/dL), assessed during test nights in hospital Severe: requiring hospital admission |
‐ | N/A | N/A |
| Steno 2 1986 | N/A | N/A |
All: blood glucose value < 2.5 mmol/L (45 mg/dL) Severe: requiring medical intervention |
‐ | N/A | N/A |
| Verillo 1988 | N/A | N/A |
All: self‐treated Severe: requiring hospital admission |
N/A | N/A | N/A |
| Wysocki 2003 | N/A | N/A |
All: N/A Severe: coma or seizure, or an episode requiring administration of intravenous glucagon, dextrose or assistance from another person; Documented by parents |
N/A | N/A | N/A |
|
Footnotes "‐" denotes not reported BMI: body mass index; DQOL: diabetes quality of life questionnaire; N/A: not applicable | ||||||
Appendix 10. Adverse events (I)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Randomised / Safety [N]a | Deaths [N] | Deaths [%] | All adverse events [N] | All adverse events [%] | Severe/serious adverse events [N] | Severe/serious adverse events [%] | Left study due to adverse events [n] | Left study due to adverse events [%] |
| Bucharest‐Düsseldorf 1984 | I: intensive therapy ‐ B | 100 | 0 | 0.0 | ||||||
| C1: basic ‐ C | 100 | 0 | 0.0 | |||||||
| C2: conventional ‐ A | 100 | 4 | 4.0 | |||||||
| all: | 300 | 4 | 1.3 | |||||||
| DCCT1 1993 | I: intensive therapy | 348 | 2 | 0.6 | ||||||
| C: conventional therapy | 378 | 2 | 0.5 | |||||||
| all: | 726 | 4 | 0.6 | |||||||
| DCCT2 1993 | I: intensive therapy | 363 | 5 | 1.4 | ||||||
| C: conventional therapy | 352 | 2 | 0.6 | |||||||
| all: | 715 | 7 | 1.0 | |||||||
| Holman 1983 | I: intensive therapy | 36 | 1 | 2.8 | 0 | |||||
| C: conventional therapy | 38 | 1 | 2.6 | 1 | 2.6 | |||||
| all: | 74 | 2 | 2.7 | 1 | 1.4 | |||||
| Linn 1996 | I: intensive therapy | 23 | ||||||||
| C: conventional therapy | 19 | |||||||||
| all: | 42 | |||||||||
| MSCG 1995 | I: intensive therapy | 36 | 0 | 0.0 | ||||||
| C: conventional therapy | 34 | 1 | 2.9 | |||||||
| all: | 70 | 1 | 1.4 | 0 | ||||||
| MDCCT 1994 | I: intensive therapy | 52 | 7 | 13.5 | ||||||
| C: conventional therapy | 47 | 8 | 17.0 | |||||||
| all: | 99 | 15 | 15.2 | |||||||
| Oslo 1987 | I1: intensive therapy, multiple injections | 15 | 0 | 0.0 | ||||||
| I2: intensive therapy, continuous insulin infusion | 15 | 0 | 0.0 | |||||||
| C: conventional therapy | 15 | 0 | 0.0 | |||||||
| all: | 45 | 0 | ||||||||
| Steno 1 1983 | I: intensive therapy | 15 | 0 | 0.0 | ||||||
| C: conventional therapy | 15 | 0 | 0.0 | |||||||
| all: | 30 | 0 | 0.0 | |||||||
| Steno 2 1986 | I: intensive therapy | 18 | 0 | 0.0 | ||||||
| C: conventional therapy | 18 | 0 | 0.0 | |||||||
| all: | 36 | 0 | 0.0 | |||||||
| Verrillo 1988 | I: intensive therapy | 22 | 0 | 0.0 | ||||||
| C: conventional therapy | 22 | 0 | 0.0 | |||||||
| all: | 44 | 0 | 0.0 | |||||||
| Wysocki 2003 | I: intensive therapy | 72 | ||||||||
| C: conventional therapy | 70 | |||||||||
| all: | 142 | |||||||||
|
Footnotes "‐" denotes not reported aThe number of patients provided here might not be the number of patients randomised if a different number of patients was relevant for the analysis of adverse events C: comparator; I: intervention | ||||||||||
Appendix 11. Adverse events (II)
| Characteristic Study ID | Intervention(s) and comparator(s) | [n] Randomised / Safetya | All hypoglycaemic episodes [n] | All hypoglycaemic episodes [%] | Severe / serious hypoglycaemic episodes [n] | Severe / serious hypoglycaemic episodes [%] | Ketoacidotic episodes [n] | Ketoacidotic episodes [%] | Weight gain [mean] | Weight gain [SD] |
| Bucharest‐Düsseldorf 1984 | I: intensive therapy | 98 | 12 | 12.2 | 2 | 2.0 | ||||
| C1: basic ‐ C | 92 | 5 | 5.4 | 3 | 3.3 | |||||
| C2: conventional ‐ A | 97 | 6 | 6.2 | 13 | 13.4 | |||||
| all: | 287 | 23 | 8.0 | 18 | 6.3 | |||||
| DCCT1 1993 | I: intensive therapy | 348 | 207 | 59.5 | 38 | 10.9 | ||||
| C: conventional therapy | 378 | 105 | 27.8 | 37 | 9.8 | |||||
| all: | 726 | 312 | 43.0 | 75 | 10.3 | |||||
| DCCT2 1993 | I: intensive therapy | 363 | 252 | 69.4 | 33 | 9.1 | ||||
| C: conventional therapy | 352 | 150 | 42.6 | 22 | 6.3 | |||||
| all: | 715 | 402 | 56.2 | 55 | 7.7 | |||||
| Holman 1983 | I: intensive therapy | 36 | 1 | 2.8 | 0 | 0.0 | ||||
| C: conventional therapy | 38 | 1 | 2.6 | 0 | 0.0 | |||||
| all: | 74 | 2 | 2.7 | 0 | 0.0 | |||||
| Linn 1996 | I: intensive therapy | 23 | 0 | 0.0 | ||||||
| C: conventional therapy | 19 | 0 | 0.0 | |||||||
| all: | 42 | 0 | 0.0 | |||||||
| MCSG 1995 | I: intensive therapy | 36 | 5 | 13.9 | 3 | |||||
| C: conventional therapy | 34 | 5 | 14.7 | 2 | ||||||
| all: | 70 | 10 | 14.3 | 5 | ||||||
| MDCCT 1994 | I: intensive therapy | 52 | ||||||||
| C: conventional therapy | 47 | |||||||||
| all: | 99 | |||||||||
| Oslo 1987 | I1: intensive therapy: multiple injections | 15 | 6 | 40.0 | 0 | 0.0 | 3.4 | 1.2 | ||
| I2: intensive therapy: continuous insulin infusion | 15 | 2 | 13.3 | 2 | 13.3 | 1.9 | 1.1 | |||
| C: conventional | 15 | 7 | 46.7 | 0 | 0.0 | ‐0.6 | 1.2 | |||
| all: | 45 | 15 | 33.3 | 2 | 4.4 | |||||
| Steno 1 1983 | I: intensive therapy | 15 | 6 | 40.0 | 3 | 20.0 | 1 | 6.7 | ||
| C: conventional therapy | 15 | 4 | 26.7 | 2 | 13.3 | 1 | 6.7 | |||
| all: | 30 | 10 | 33.3 | 5 | 16.7 | 2 | 6.7 | |||
| Steno 2 1986 | I: intensive therapy | 18 | 5 | 27.8 | 4 | 22.2 | ||||
| C: conventional therapy | 18 | 5 | 27.8 | 0 | 0.0 | |||||
| all: | 36 | 10 | 27.8 | 4 | 11.1 | |||||
| Verrillo 1988 | I: intensive therapy | 18 | 2 | 11.1 | 0 | |||||
| C: conventional therapy | 20 | 1 | 5.0 | 0 | ||||||
| all: | 38 | 3 | 7.9 | 0 | ||||||
| Wisocki 2003 | I: intensive therapy | 72 | 32 | 44.4 | ||||||
| C: conventional therapy | 70 | 26 | 37.1 | |||||||
| all: | 142 | 58 | 40.8 | |||||||
|
Footnotes "‐" denotes not reported aThe number of patients provided here might not be the number of patients randomised if a different number of patients was relevant for the analysis of adverse events C: comparator; I: intervention | ||||||||||
Appendix 12. Glycosylated haemoglobin A1c (HbA1c) measurements during the study
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Baseline HbA1c [mean % (SD or range)] | End of study HbA1c [mean % (SD) or range] | Change in HbA1c [mean % (SD)] | Between group HbA1c difference [mean % (CI/SD)] |
| Bucharest‐ Düsseldorf 1984 | I: intensive therapy | 12.3 (0.2 SE) | 9.3a | ‐ | ‐ |
| C: basic therapy | 11.7 (0.2 SE) | 11.3a | |||
| DCCT1 1993 (primary prevention) | I: intensive therapy | 8.8 (1.6) | 7.2b | ‐ | ‐ |
| C: conventional therapy | 8.8 (1.7) | 9.2b | |||
| DCCT2 1993 (secondary prevention) | I: intensive therapy | 9.0 (1.5) | 7.2b | ‐ | ‐ |
| C: conventional therapy | 8.9 (1.5) | 9.2b | |||
| Holman 1983 | I: intensive therapy | 11.7 (1.6) | 10.5c (1.4) | ‐ | ‐ |
| C: conventional therapy | 11.8 (2.1) | 11.4c (1.5) | |||
| Linn 1996 | I: intensive therapy | 12.4 (5.5) | 6.3 (1.9) | ‐ | ‐ |
| C: conventional therapy | 13.1 (6.2) | 8.1 (2.1) | |||
| MCSG 1995 | I: intensive therapy | 10.3 (1.9d) | ‐ | 0.0e | ‐ |
| C: conventional therapy | 9.8 (1.6d) | ‐ | + 0.2e | ||
| MDCCT 1994 | I: intensive therapy | ‐ | 0.09f | ‐ | 0.117 (0.013)g |
| C: conventional therapy | ‐ | 0.11f | |||
| Oslo 1987 | I1: intensive therapy, MI | 9.4 (0.4 SEh) | 9.1 (0.4 SE)h | ‐ | ‐ |
| I2: intensive therapy, CSII | 10.1 (0.4 SEh) | 8.7 (0.3 SE)h | |||
| C: conventional therapy | 9.5 (0.4 SEh) | 10.2 (0.5 SE)h | |||
| Steno 1 1983 | I: intensive therapy | 9.7 (7.4‐12.1) | 6.7 (5.6‐8.0)i | ‐ | ‐ |
| C: conventional therapy | 8.6 (6.0‐10.4) | 8.3 (6.3‐10.7)i | |||
| Steno 2 1986 | I: intensive therapy | 9.5 (6.6‐13.6) | 7.2 (5.9‐8.8)j | ‐ | ‐ |
| C: conventional therapy | 9.3 (7.0‐11.7) | 8.6 (7.2‐13.3)j | |||
| Verrillo 1988 | I: intensive therapy | 10.8 (1.4) | 7.9 | ‐ | ‐ |
| C: conventional therapy | 11.1 (1.8) | 8.7 | |||
| Wysocki 2003 g | I: intensive therapy | 8.2 (1.1) | 7.8 (0.9) | ‐ | ‐ |
| C: conventional therapy | 8.1 (0.9) | 8.6 (1.1) | |||
|
Footnotes "‐" denotes not reported aRead from figure 1 of the publication bRead from figure 1, panel A of the publication for both primary and secondary prevention groups at 6.5 years (DCCT 1 and 2) cMean of all values during the two years dIn the publication described as standard error of the mean, but the standard deviation appears more plausible eRead from figure of the publication (mean cumulative absolute changes in HbA1c concentration after 5 years) fRead from figure at year 5 (HbA1 values for each full or partial patient year) gStandard (maximized, 0.096 (SD 0.016); P < 0.001) hHbA1 values iMean month 3‐12 jMedian of mean HbA1c from the third month of study CI: confidence interval; CSII: continuous subcutaneous insulin infusion; DCCT: 'Diabetes Control and Complications Trial'; I: intervention; MI: multiple daily injections; SD: standard deviation; SE: standard error of the mean | |||||
Appendix 13. Survey of authors' reactions to provide information on trials
| Study ID | Study author contacted | Study author replied | Current status |
| Bucharest‐Düsseldorf 1984 | 20/6/2013 | 22/6/2013 | Provided more information on study period, but original study data not accessible anymore |
| DCCT1&2 1993 | 20/6/2013 | 22/6/2013: will send data | Still waiting for data |
| Holman 1983 | 21/6/2013 | No | |
| Linn 1996 | 20/6/2013 | No | |
| MCSG 1995 | 21/6/2013 | No | |
| MDCCT 1994 | 21/6/2013 | No | |
| Oslo 1987 | 21/6/2013 | 04/07/2013: will send data | Still waiting for data |
| Steno 1 1983 | 24/6/2013 | No | |
| Steno 2 1986 | 24/6/2013 | 25/06/2013: will send data | Still waiting for data |
| Verrillo 1988 | No contact information | ||
| Wysocki 2003 | 12/6/2013: email to Tamara Hershey to check whether Hershey et al 1999
is based on subgroup of Wysocki et al. 2003 22/6/2013 contacted Neil White again 24/6/2013 contacted Tim Wysocki |
12/6/2013 | 12/6/2013 Email forwarded to Neil White ‐> no response |
Data and analyses
Comparison 1. Intensive glucose control versus conventional glucose control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Retinopathy | 7 | 1660 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.44, 1.16] |
| 2 Manifestation of retinopathy | 2 | 768 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.18, 0.42] |
| 3 Progression of retinopathy, random effects model | 4 | 860 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.54, 2.24] |
| 4 Progression of retinopathy, random effects model, all studies, Steno 1 after 2 years | 4 | 859 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.47, 0.99] |
| 5 Progression of retinopathy, random effects model, stratified by follow‐up duration | 4 | 860 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.54, 2.24] |
| 5.1 Follow‐up duration >= 2 years | 2 | 764 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.49, 0.76] |
| 5.2 Follow‐up duration < 2 years | 2 | 96 | Risk Ratio (IV, Random, 95% CI) | 2.32 [1.16, 4.63] |
| 6 Progression of retinopathy, fixed‐effect model, stratified by follow‐up duration | 4 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.84] |
| 6.1 Follow‐up duration > 1 year | 2 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.75] |
| 6.2 Follow‐up duration <= 1 year | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.16, 4.88] |
| 7 Progression of retinopathy, random effects model, stratified by follow‐up duration, OR | 4 | 860 | Odds Ratio (IV, Random, 95% CI) | 1.28 [0.39, 4.26] |
| 7.1 Follow‐up duration > 1 year | 2 | 764 | Odds Ratio (IV, Random, 95% CI) | 0.48 [0.35, 0.65] |
| 7.2 Follow‐up duration <= 1 year | 2 | 96 | Odds Ratio (IV, Random, 95% CI) | 4.28 [1.36, 13.49] |
| 8 Manifestation of nephropathy, random‐effects model, RR | 3 | 1475 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.46, 0.68] |
| 9 Manifestation of nephropathy, random‐effects model, alternative measure in Oslo 1987 | 3 | 1475 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.46, 0.68] |
| 10 Manifestation of nephropathy, fixed‐effect model, RR | 3 | 1475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.47, 0.69] |
| 11 Manifestation of nephropathy, random‐effects model, OR | 3 | 1475 | Odds Ratio (IV, Random, 95% CI) | 0.48 [0.34, 0.67] |
| 12 Progression of nephropathy, random‐effects model, RR | 3 | 179 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.37, 1.70] |
| 13 Progression of nephropathy, fixed‐effect model, RR | 3 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.38, 1.30] |
| 14 Progression of nephropathy, random‐effects model, OR | 3 | 179 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.26, 1.91] |
| 15 Manifestation of neuropathy, random‐effects model, RR | 3 | 1203 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 16 Manifestation of neuropathy, fixed‐effect model, RR | 3 | 1203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.23, 0.51] |
| 17 Manifestation of neuropathy, random‐effects model, OR | 3 | 1203 | Odds Ratio (IV, Random, 95% CI) | 0.31 [0.20, 0.48] |
| 18 Severe hypoglycaemia, random‐effects model, RR | 11 | 2108 | Risk Ratio (IV, Random, 95% CI) | 1.50 [1.17, 1.91] |
| 19 Severe hypoglycaemia, assistance of other person | 4 | 1653 | Risk Ratio (IV, Random, 95% CI) | 1.64 [1.27, 2.12] |
| 20 Severe hypoglycaemia, coma or hospital admission | 7 | 1818 | Risk Ratio (IV, Random, 95% CI) | 1.67 [1.09, 2.55] |
| 21 Severe hypoglycaemia, random‐effects model, RR, without DCCT | 9 | 667 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.83, 1.52] |
| 22 Severe hypoglycaemia, stratified by baseline HbA1c | 11 | 2108 | Risk Ratio (IV, Random, 95% CI) | 1.50 [1.17, 1.91] |
| 22.1 Baseline HbA1c < 9.0 | 3 | 1583 | Risk Ratio (IV, Random, 95% CI) | 1.68 [1.29, 2.19] |
| 22.2 Baseline HbA1c >= 9.0 | 8 | 525 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.66, 1.64] |
| 23 Severe hypoglycaemia, random‐effects model, RR, without Bucharest‐Düsseldorf | 10 | 1918 | Risk Ratio (IV, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 24 Severe hypoglycaemia, random‐effects model, RR, without DCCT and Bucharest‐Düsseldorf | 8 | 477 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.77, 1.44] |
| 25 Severe hypoglycaemia, stratified by baseline HbA1c, without Bucharest‐Düsseldorf | 10 | 1918 | Risk Ratio (IV, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 25.1 Baseline HbA1c < 9.0 | 3 | 1583 | Risk Ratio (IV, Random, 95% CI) | 1.68 [1.29, 2.19] |
| 25.2 Baseline HbA1c >= 9.0 | 7 | 335 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.52, 1.42] |
| 26 Severe hypoglycaemia, coma or hospital admission, without Bucharest‐Düsseldorf | 6 | 1628 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.98, 2.56] |
| 27 Severe hypoglycaemia, stratified by baseline HbA1c, fixed‐effect model | 11 | 2108 | Risk Ratio (IV, Fixed, 95% CI) | 1.70 [1.53, 1.89] |
| 27.1 Baseline HbA1c < 9.0 | 3 | 1583 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [1.57, 1.94] |
| 27.2 Baseline HbA1c >= 9.0 | 8 | 525 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.66, 1.64] |
| 28 Severe hypoglycaemia, stratified by baseline HbA1c, OR | 11 | 2108 | Odds Ratio (IV, Random, 95% CI) | 1.91 [1.24, 2.95] |
| 28.1 Baseline HbA1c < 9.0 | 3 | 1583 | Odds Ratio (IV, Random, 95% CI) | 2.77 [1.78, 4.31] |
| 28.2 Baseline HbA1c >= 9.0 | 8 | 525 | Odds Ratio (IV, Random, 95% CI) | 1.17 [0.66, 2.08] |
| 29 Ketoacidosis | 9 | 1924 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.95, 1.86] |
| 30 Ketoacidosis, CSII | 3 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.93 [1.18, 20.60] |
| 31 Ketoacidosis, MI | 4 | 332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.11, 3.67] |
| 32 Ketoacidosis, MI or CSII | 3 | 1511 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.90, 1.82] |
| 33 All‐cause mortality | 10 | 2039 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.48, 2.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bucharest‐Düsseldorf 1984.
| Methods |
Randomised controlled clinical trial (RCT) Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: patients hospitalised due to metabolic decompensation or initiation of insulin treatment, age: 15‐40 years Exclusion criteria: admission primarily because of severe acute or chronic disorders unrelated to diabetes, mental retardation or psychiatric diseases, clinically overt diabetic nephropathy (urinary protein excretion exceeding 0.5 g/day and/or raised serum creatinine levels), proliferative retinopathy or blindness, severe foot complications Diagnostic criteria: patients with type 1 diabetes mellitus (ketosis‐prone) |
|
| Interventions |
Number of study centres: 1 Treatment before studya |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c, incidence rates of ketoacidosis, hospitalisation rates, frequency of severe hypoglycaemia | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial (insulin and syringes were provided by various pharmaceutical companies) Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "It has been questioned whether aiming at near‐normoglycaemia by intensified insulin treatment regimens is feasible and safe for the majority of patients with insulin‐dependent diabetes" | |
| Notes | aThree different treatment groups were studied, two groups were followed‐up for two years, but for this review only the first year is relevant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from publication: "Patients were group randomised to 3 different treatment regimens. The first consecutive 100 patients meeting the eligibility criteria (group A) continues the standard treatment….The second 100 patients (group B)….The last 100 patients (group C)". Comment: inappropriate sequence generation |
| Allocation concealment (selection bias) | High risk | Comment: not described and inappropriate sequence generation |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Comment: no blinding of participants and personnel, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Comment: no blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | High risk | Quote from publication: "Severe hypoglycaemia and ketoacidosis were assessed by a standardised interview and by a review of patients records" Comment: likely not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: analysis excluded patients who dropped out, however dropout rate was low |
| Selective reporting (reporting bias) | Unclear risk | Comment: data presentation seems complete, but no study protocol available |
| Other bias | Low risk | Comment: no other risks of bias found |
DCCT1 1993.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: age: 13‐39 years, IDDM for 1‐5 years, urinary albumin excretion < 40 mg/24h Exclusion criteria: hypertension, hypercholesterolaemia, severe diabetic complications or medical conditions, retinopathy (as detected by seven‐field stereoscopic fundus photography) Diagnostic criteria: insulin dependence, as evidenced by deficient C‐peptide secretion |
|
| Interventions |
Number of study centres: 29 Treatment before study: ‐ |
|
| Outcomes | Outcomes reported in abstract of publication: retinopathy, microalbuminuria, nephropathy, neuropathy, severe hypoglycaemia | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: yes ("In June 1993, after an average follow‐up of 6.5 years (range, 3 to 9), the independent data monitoring committee determined that the study results warranted terminating the trial") |
|
| Publication details |
Language of publication: English Funding: non‐commercial and commercial (various corporate sponsors, see DCCT 1987) Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "Will intensive therapy prevent the development of diabetic retinopathy in patients with no retinopathy (primary prevention), and will intensive therapy affect the progression of early retinopathy (secondary intervention)? Although retinopathy was the principal study outcome, we also studied renal, neurologic, cardiovascular, and neuropsychological outcomes and the adverse effects of the two treatment regimens" | |
| Notes | IDDM: insulin‐dependent diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from protocol: "For large samples, the Urn procedure minimizes the potential for selection bias, i.e., minimizes the potential that the clinics may influence the assignment of treatments to subjects by guessing which treatment will be assigned next. The Urn procedure, however, does not guarantee equal numbers of subjects in each treatment group. Rather, the probability of a substantial imbalance in the numbers assigned to each treatment is virtually eliminated by this procedure. Within either the primary prevention or secondary intervention trial, the probability that more than 370 of the 700 subjects would be assigned to either group is only 0.0073. The exact number of subjects to be randomised to either group is unknown because the exact number of subjects to be recruited within each clinic‐retinopathy stratum is unknown." Quote from publication: "Randomization was stratified according to the primary‐prevention and secondary‐intervention cohorts at each centre". Comment: urn randomizations procedures (Wei 1988) |
| Allocation concealment (selection bias) | Low risk | Quote from protocol: "The list of random assignments will be kept confidential and accessible only to the Coordinating Center staff at the time of randomizations. Randomization into one of the two treatment groups will be accomplished by a telephone call to the Coordinating Center after all criteria for entry into the study have been satisfied and documented at the Coordinating Center. At the time of randomizations, the next treatment assignment for that subjects clinic‐retinopathy stratum is communicated by telephone to the treatment centre staff, with written verification to follow." Comment: allocation concealment considered appropriate |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "The two treatment regimens will, of necessity, be conducted in an unmasked manner.” ; “With the exception of HbA1c, all centrally determined outcome measurements will ordinarily be masked from the investigator responsible for the treatment regimens and from the subjects.“ Comment: treatment assignment not blinded, risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "The two treatment regimens will, of necessity, be conducted in an unmasked manner.” ; “With the exception of HbA1c, all centrally determined outcome measurements will ordinarily be masked from the investigator responsible for the treatment regimens and from the subjects.“ Comment: treatment assignment not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: “The Morbidity and Mortality Classification Committee classified deaths and cardiovascular events. Coding was performed without knowledge of treatment assignment, according to pre‐established criteria" Comment: objective outcomes were assessed in a blinded manner |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: due to the open design of the trial it is likely that it was not possible to have a blinded assessment of all subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Each subject will then be included in the assigned treatment group in all statistical analyses regardless of the eventual therapeutic course. Thus subjects who fail to comply with or who are unable to complete the assigned treatment regimen will nevertheless be included in the originally assigned group for statistical analyses" Comment: ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no reason to assume selective reporting found |
| Other bias | Low risk | Comment: no other risks of bias found |
DCCT2 1993.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: age: 13‐39 years, IDDM for 1‐15 years, urinary albumin excretion < 200 mg/24h, very mild‐to‐moderate nonproliferative retinopathy Exclusion criteria: hypertension, hypercholesterolaemia, severe diabetic complications or medical conditions Diagnostic criteria: insulin dependence, as evidenced by deficient C‐peptide secretion |
|
| Interventions |
Number of study centres: 29 Treatment before study: |
|
| Outcomes | Outcomes reported in abstract of publication: retinopathy, microalbuminuria, nephropathy, neuropathy, severe hypoglycaemia | |
| Study details |
Run‐in period: Study terminated before regular end: yes ("In June 1993, after an average follow‐up of 6.5 years (range, 3 to 9), the independent data monitoring committee determined that the study results warranted terminating the trial") |
|
| Publication details |
Language of publication: English Funding: non‐commercial and commercial (various corporate sponsors, see DCCT 1987) Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "Will intensive therapy prevent the development of diabetic retinopathy in patients with no retinopathy (primary prevention), and will intensive therapy affect the progression of early retinopathy (secondary intervention)? Although retinopathy was the principal study outcome, we also studied renal, neurologic, cardiovascular, and neuropsychological outcomes and the adverse effects of the two treatment regimens" | |
| Notes | IDDM: insulin‐dependent diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from protocol: "For large samples, the Urn procedure minimizes the potential for selection bias, i.e., minimizes the potential that the clinics may influence the assignment of treatments to subjects by guessing which treatment will be assigned next. The Urn procedure, however, does not guarantee equal numbers of subjects in each treatment group. Rather, the probability of a substantial imbalance in the numbers assigned to each treatment is virtually eliminated by this procedure. Within either the primary prevention or secondary intervention trial, the probability that more than 370 of the 700 subjects would be assigned to either group is only 0.0073. The exact number of subjects to be randomised to either group is unknown because the exact number of subjects to be recruited within each clinic‐retinopathy stratum is unknown." Quote from publication: "Randomization was stratified according to the primary‐prevention and secondary‐intervention cohorts at each centre". Comment: urn randomizations procedures (Wei 1988) |
| Allocation concealment (selection bias) | Low risk | Quote from protocol: "The Coordinating Center disclosed the random assignment of each patient to the clinic via telephone at the time of randomizations".The list of random assignments will be kept confidential and accessible only to the Coordinating Center staff at the time of randomizations. Randomization into one of the two treatment groups will be accomplished by a telephone call to the Coordinating Center after all criteria for entry into the study have been satisfied and documented at the Coordinating Center. At the time of randomizations, the next treatment assignment for that subjects clinic‐retinopathy stratum is communicated by telephone to the treatment centre staff, with written verification to follow." Comment: allocation concealment considered appropriate |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "The two treatment regimens will, of necessity, be conducted in an unmasked manner.” ; “With the exception of HbA1c, all centrally determined outcome measurements will ordinarily be masked from the investigator responsible for the treatment regimens and from the subjects.“ Comment: treatment assignment not blinded, risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "The two treatment regimens will, of necessity, be conducted in an unmasked manner.” ; “With the exception of HbA1c, all centrally determined outcome measurements will ordinarily be masked from the investigator responsible for the treatment regimens and from the subjects.“ Comment: treatment assignment not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: “The Morbidity and Mortality Classification Committee classified deaths and cardiovascular events. Coding was performed without knowledge of treatment assignment, according to pre‐established criteria". Comment: objective outcomes were assessed in a blinded manner |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: due to the open design of the trial it is likely that it was not possible to have a blinded assessment of all subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Each subject will then be included in the assigned treatment group in all statistical analyses regardless of the eventual therapeutic course. Thus subjects who fail to comply with or who are unable to complete the assigned treatment regimen will nevertheless be included in the originally assigned group for statistical analyses" Comment: ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no reason to assume selective reporting found |
| Other bias | Low risk | Comment: no other risks of bias found |
Holman 1983.
| Methods |
Randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: type 1 diabetes with background retinopathy Exclusion criteria: age > 60, proliferative retinopathy, renal impairment (creatinine > 175 µmol/L), more than one significant cardiovascular event (or one in the previous year), other major disease Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: diabetic clinics in Oxford and Aylesbury Treatment before study: conventional care |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c, renal and sensory‐nerve function, low‐density‐lipoprotein‐cholesterol and whole‐blood low‐shear viscosity, rate of progression of retinopathy | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "The randomised prospective study of insulin‐dependent diabetic patients with background retinopathy aimed to determine the degree to which near‐normal glycaemia can be achieved in an unselected clinic population with two injections per day and whether the progress of diabetic complications could be retarded" | |
| Notes | HbA1c: glycosylated haemoglobin level | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to two treatment groups by means of sealed envelopes with stratification for body‐weight and blood pressure" Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to two treatment groups by means of sealed envelopes with stratification for body‐weight and blood pressure" Comment: allocation concealment not adequately described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "All A patients were intensively educated in the care of their diabetes; the U group continued their usual therapy and attended the routine diabetic clinic" Comment: neither participants nor personnel blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "All A patients were intensively educated in the care of their diabetes; the U group continued their usual therapy and attended the routine diabetic clinic" Comment: neither participants nor personnel blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: "All readings were made by the same research nurse who was aware of the patient´s group but had no record of previous measurements. Ophthalmoscopy…was undertaken by an ophthalmologist without knowledge of the patients group.” Comment: blinded assessment of primary outcome |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Quote from publication: "All readings were made by the same research nurse who was aware of the patient´s group but had no record of previous measurements" Comment: assessment not blinded, but some measures were taken to avoid bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Data are shown for all patients where available. The initial comparison of groups would be unchanged if the 5 patients who did not complete two years in the study were excluded, with the exception of the vibration sensory threshold, in which case the changes over 2 years were assessed in relation to possible confounding variables by analysis of covariance" Comment: complete case analysis, missingness likely not random, but number of missing values not very large |
| Selective reporting (reporting bias) | Unclear risk | Comment: there is insufficient information to assess whether a risk of selective outcome reporting is present |
| Other bias | Low risk | Comment: no other sources of bias became apparent |
Linn 1996.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: newly diagnosed type 1 diabetes, adults Exclusion criteria: ‐ Diagnostic criteria: IDDM defined on the basis of insulin dependency according to WHO 1985 |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ |
|
| Outcomes | Outcomes reported in abstract of publication: glucagon‐stimulated C‐peptide, microalbuminuria, retinopathy, neuropathy, HbA1c, hypoglycaemia frequency, insulin sensitivity | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: ‐ Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "In this study, intensive insulin treatment was initiated in newly diagnosed adult patients to determine if it preserved endogenous insulin secretion longer than conventional therapy" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "…randomizations was performed with the use of computer‐selected random numbers" Comment: considered adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "The I group contacted the diabetes educator by visit or telephone once per month to review and adjust the regimens". Comment: neither participants nor personnel blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "The I group contacted the diabetes educator by visit or telephone once per month to review and adjust the regimens". Comment: neither participants nor personnel blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "Forty‐two of 49 randomised patients completed the 5 years, and only their data were included" Comment: no reasons given for the withdrawals, analysis not ITT |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to make judgement |
| Other bias | Unclear risk | Comment: no other risks of bias found, but amount of information considered insufficient to make judgement |
MCSG 1995.
| Methods |
Randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: patients with insulin‐dependent diabetes mellitus (IDDM); age: 16 ‐ 60 years; microalbuminuria (albumin excretion >30 and <200μg/min); onset of diabetes before the age of 39; sitting blood pressure below 160/95 mm Hg Exclusion criteria: arterial hypertension; albuminuria by dipstick test, antihypertensive treatment, clinical evidence of cardiovascular, peripheral vascular, or renal disease Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: nine hospital based specialist diabetes centres Treatment before study: conventional care |
|
| Outcomes | Outcomes reported in abstract of publication: development of clinical albuminuria (defined as albumin excretion greater than 200 μg/min on at least two consecutive occasions, and rate of change of albumin excretion), HbA1c, blood pressure | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "To study the effect of intensive therapy of diabetes on the progression to clinical albuminuria in insulin dependent diabetic patients with microalbuminuria" | |
| Notes | IDDM: insulin‐dependent diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were stratified by age and sex and randomised to either intensive therapy or conventional therapy by a centralised procedure" Comment: method used for the allocation sequence generation was not exactly described |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Patients were stratified by age and sex and randomised to either intensive therapy or conventional therapy by a centralised procedure" Comment: “Centralised procedure” is likely adequate |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "Clear glycaemic targets were set in the intensive therapy group and they adjusted their treatment regimen in consultation with the investigation team" Comment: patients and investigators were not blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "Clear glycaemic targets were set in the intensive therapy group and they adjusted their treatment regimen in consultation with the investigation team" Comment: patients and investigators were not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Quote from publication: "A blood sample was drawn for measurement of glycated haemoglobin concentration; these measurements were done in four participating centres that regularly exchanged quality control samples and cross validated results" Comment: not described if the outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Quote from publication: "At each visit a medical history was taken, including a record of severe episodes of hypoglycaemia or ketoacidosis…" Comment: not described if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not described; withdrawals in both groups are reported, but the reasons are not reported separately for each group |
| Selective reporting (reporting bias) | Unclear risk | Comment: there is insufficient information to assess whether a risk of selective outcome reporting is present |
| Other bias | Unclear risk | Comment: some information presented in the paper was inconsistent |
MDCCT 1994.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratioa : 1:1(1978), 2:1 (in favour of intensive therapy, 1978‐1984), 2:1 (in favour of control treatment, after 1984) Superiority design |
|
| Participants |
Inclusion criteria: received a renal allograft as treatment for end‐stage diabetic nephropathy Exclusion criteria: ‐ Diagnostic criteria: insulin‐dependent type 1 diabetes |
|
| Interventions |
Number of study centres: 1 Treatment before study: ‐ |
|
| Outcomes | Outcomes reported in abstract of publication: haemoglobin A1 level, renal glomerular mesangial expansion, volume fraction of mesangial matrix per glomerulus, increase in arteriolar hyalinosis, widening of the glomerular basement membrane, increase of volume fraction of the total mesangium, incidence of severe hypoglycaemic episodes, cognitive function | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication English Funding: partially commercial Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "To determine whether optimised glycaemic control in type I diabetic recipients of renal allografts will prevent or delay diabetic renal lesions in the allograft" | |
| Notes | aInitially patients were randomised before transplantation, after 2 years, patients were randomised three months after transplantation to exclude patients whose grafts were rejected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The subjects were recruited from the large population of type I diabetics with uremia who sought kidney transplantation at the University of Minnesota…About 300 patients were invited to join the study…The remaining 99 were originally randomised equally between the two treatment groups (before transplantation). Two years later, we began randomizations 3 months after transplantation…and to exclude patients whose grafts were rejected during this high‐risk period. Between 1978 and 1984, randomizations was 2:1 in favour of the maximized group, since we hypothesized that this group would have more withdrawals from the trial…To balance the size of the two groups, in 1985 we began 2:1 randomizations in favour of the standard group" Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "The patients in the maximized group were contacted frequently by the study dietitian" Comment: neither participants nor personnel blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "The patients in the maximized group were contacted frequently by the study dietitian" Comment: neither participants nor personnel blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "Conclusions of our study may be tempered somewhat by the relatively high proportion of patients for whom data could not be evaluated". “The rate of voluntary withdrawal tended to be higher in the maximized than the standard group.” Comment: high number of dropouts; analysis of drop‐outs suggests non‐randomness of missing data; complete case analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: results on hypoglycaemia insufficiently reported |
| Other bias | Unclear risk | Comment: several analyses and publications before the end of the trial; some inconsistencies in the reporting of results |
Oslo 1987.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio:1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: age: 18‐45 years, diabetes duration > 7 years, but < 30 years, diabetes diagnosis before age = 30, negative for C‐peptide Exclusion criteria: clinical signs of nephropathy (serum creatinine ≤ 150 µmol/L), systemic hypertension (diastolic blood pressure ≤ 100 mm Hg), history of neuropathy, proliferative retinopathy, pregnant, medication other than insulin (apart from contraceptives) Diagnostic criteria: C‐peptide negative insulin dependent diabetes |
|
| Interventions |
Number of study centres: 1 Treatment before study: two daily insulin injections of mixed insulin |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c, hypoglycaemic coma, ketoacidosis, cutaneous infections at injection site, insulin antibodies, retinopathy, urinary albumin excretion, glomerular hyperfiltration, sensory and motor nerve conduction velocity | |
| Study details |
Run‐in period: 2 months Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: partially commercial Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "To study the influence of long‐term near‐normoglycaemia on early stages of microangiopathy and neuropathy in young insulin dependent diabetic patients" | |
| Notes | For several outcomes results have only be reported after 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "To ensure comparable treatment groups a block randomizations procedure was chosen. The patients were randomised into three groups by a computer programme making the best possible distribution of basic characteristics in the following priority: Age, duration of disease, sex, initial HbA1c value and retinopathy grading" Comment: considered adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Comment: not blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: "To avoid observer bias, all pictures were coded and evaluated in a masked manner by the ophthalmologist" Comment: blinded outcome assessment of retinopathy, unclear for other outcomes |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "Data collected until change of treatment was used for statistical analysis" Comment: About 35% of the patients in the control group and 15% of patients in the treatment groups changed the treatment arm at some point during the study; it is not clear across the different publications of the Oslo study how these data were handled. Since the proportion of patients changing treatment was substantial, risk of bias was considered high |
| Selective reporting (reporting bias) | Unclear risk | Comment: data were analysed for many different times of follow‐up |
| Other bias | Unclear risk | Comment: reporting insufficient to assess the risk of other biases |
Steno 1 1983.
| Methods |
Randomised controlled clinical trial (RCT) Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: type 1 diabetes with background retinopathy, postprandial C‐peptide ≤ 0.2 nmol/L, serum creatine ≤ 150 μmol/L, age 18‐51 years, diabetes onset before age 30, diabetes duration < 35 years Exclusion criteria: ‐ Diagnostic criteria: type 1 diabetes |
|
| Interventions |
Number of study centres: 1 Treatment before study: 1‐3 insulin injections |
|
| Outcomes | Outcomes reported in abstract of publication: mean blood glucose, HbA1c, retinal morphology, retinal function, proliferative retinopathy | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "The aim of the study was to evaluate the effect of near‐normal glycaemic control on retinopathy" | |
| Notes | HbA1c: glycosylated haemoglobin level; IDDM: insulin‐dependent diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to unchanged conventional treatment or to continuous subcutaneous insulin infusion". Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "Patients were randomised to unchanged conventional treatment or to continuous subcutaneous insulin infusion ". Comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Comment: patients and investigators were not blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Comment: patients and investigators were not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: "At the end of the study all fundus photographs were mixed and read in a ‘blind’ fashion by two ophthalmologists who had to agree whether the photographs showed deterioration, no change, or improvement" Comment: blinded outcome assessment of retinopathy, unclear for other outcomes |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: all 30 patients were included in the analysis, but it is not clear whether there were missing data and how they were treated |
| Selective reporting (reporting bias) | Unclear risk | Comment: there is insufficient information to assess whether a risk of selective outcome reporting is present |
| Other bias | Unclear risk | Comment: HbA1c baseline difference between treatment groups, inconsistencies regarding number of enrolled patients across publications |
Steno 2 1986.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: 18‐50 years, postprandial C‐peptide level < 0.2 nmol/L, diabetes duration: 5 ‐ 26 years, supine systolic blood pressure < 160 mm Hg, diastolic blood pressure < 95 mm Hg, consistently negative albustix reaction on 24h urine, raised urinary albumin excretion (30 to 300 mg/24h) in two of three tests in 3‐month period (incipient diabetic nephropathy) Exclusion criteria: history of renal disease, active proliferative retinopathy, laser treatment, psychiatric disorders, medication other than oral contraceptives, unable to sense hypoglycaemia Diagnostic criteria: insulin‐dependent diabetes |
|
| Interventions |
Number of study centres: 1 Treatment before study: subcutaneous depot injections of intermediate‐acting insulin preparations, often mixed with short‐acting insulin, two to three times daily |
|
| Outcomes | Outcomes reported in abstract of publication: glycosylated haemoglobin, manifestation of clinical diabetic nephropathy | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "to evaluate the effect…of strict metabolic control on kidney function in patients with microalbuminuria, using serial analysis of albumin excretion before and during the study period.” | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The 36 patients were matched in pairs according to urinary albumin level, degree of metabolic control, and sex and were assigned randomly to either continuous insulin infusion or unchanged conventional treatment" Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Comment: neither participants or personnel blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all 36 patients were included in the analysis, likely no dropouts, although this was not explicitly stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available, but no evidence for selective reporting found in the manuscript. Adverse events were likely collected but not reported on |
| Other bias | Unclear risk | Comment: inconsistencies regarding number of enrolled patients across publications |
Verrillo 1988.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1.1 Superiority design |
|
| Participants |
Inclusion criteria: age: 18‐50 years; diabetes for 15‐30 years, supine systolic blood pressure under 150 mm Hg and a supine diastolic blood pressure under 95 mm Hg, no evidence of ischaemic heart disease according to Minnesota code, urinary protein excretion below 0.5 g/day; background retinopathy Exclusion criteria: ‐ Diagnostic criteria: no residual endogenous insulin secretory capacity defined as a plasma C‐peptide concentration below 0.1 pmol per mL in the postabsorptive state, and 6 min after the intravenous injection of 1 mg glucagon |
|
| Interventions |
Number of study centres: ‐ Treatment before study: subcutaneous injections of intermediate‐acting insulin preparations, often mixed with short acting insulin, not more than twice daily |
|
| Outcomes | Outcomes reported in abstract of publication: plasma glucose profile, glycosylated haemoglobin, retinal morphology, retinopathy | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: ‐ Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "…to evaluate (a) the degree of glycaemic control which can be achieved and maintained in patients with IDDM by using a more intensive insulin regimen employing long‐acting insulin as basal cover and soluble insulin at mealtimes, and (b) what is the effect of this treatment on the rate of deterioration of already established retinopathy” | |
| Notes | IDDM: insulin dependent diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Originally, the patients were identified in a screening for retinopathy by ophthalmoscopy through dilated pupils in our outpatient clinic. Of the 54 consecutive insulin‐treated diabetic patients with background retinopathy, 44 agreed to take part in the study. They were randomly allocated to one of the treatment regimens – UCT or ICT. Block randomizations was performed to ensure comparable groups". Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "The UCT patients attended to the routine diabetic clinic; ICT patients were seen in the outpatient clinic every four weeks for the first year and then every eight weeks" Comment: not blinded, , but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Low risk | Quote from publication: "Colour photographs and angiograms were evaluated blindly by a senior ophthalmologist, the identity of the patient and number of examination being masked" Comment: outcomes assessment blinded |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "Subsequent to randomizations…., six patients were lost to follow‐up" Comment: drop‐outs not considered in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: incomplete reporting on some outcomes (e.g. mild hypoglycaemia) |
| Other bias | Unclear risk | Comment: no other risks of bias found, but reporting insufficient to make judgement |
Wysocki 2003.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: school‐aged children (6‐15 years), type 1 diabetes for at least 2 years or for 1 year with a negligible stimulated C‐peptide level, reside in a family situation, telephone service at home, plan to continue treatment at the enrolling centre throughout the study Exclusion criteria: other chronic medical conditions (except well‐controlled Hashimoto thyroiditis or well‐controlled asthma), inpatient psychiatric treatment within the previous six months, caregivers not literate in English, caregivers treated for psychosis, major depression, bipolar disorder or substance abuse in the prior 6 months Diagnostic criteria: type 1 diabetes mellitus |
|
| Interventions |
Number of study centres: 2 Treatment before study: ‐ Titration period: 18 months |
|
| Outcomes | Outcomes reported in abstract of publication: severe hypoglycaemia, HbA1c, decline in IQ | |
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: peer‐reviewed journal/full article |
|
| Stated aim of study | Quote from publication: "The objective of this study was to determine whether severe hypoglycaemia in children with type 1 diabetes is associated with cognitive decline over 18 months”; “The primary purpose of the trial was to identify variables that predict benefit from the two regimens" | |
| Notes | IQ: intelligence quotient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Randomization was stratified according to the patient´s age and HbA1c and was performed by the trial coordinator at the other centre" Comment: not adequately described |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: "Randomization was stratified according to the patient´s age and HbA1c and was performed by the trial coordinator at the other centre" Comment: not adequately described |
| Blinding of participants and personnel (performance bias) Objective Outcomes | Low risk | Quote from publication: "Intensive therapy (IT) patients received approximately four times more contacts with nurses, dietitians, and psychologists than those in the usual care (UC) group" Comment: neither participants nor personnel blinded, but risk of bias considered low for objective outcomes |
| Blinding of participants and personnel (performance bias) Subjective Outcomes | High risk | Quote from publication: "Intensive therapy (IT) patients received approximately four times more contacts with nurses, dietitians, and psychologists than those in the usual care (UC) group"Comment: neither participants nor personnel blinded |
| Blinding of outcome assessment (detection bias) Objective Outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective Outcomes | High risk | Quote from publication: "…Parents documented this information immediately after any apparent severe hypoglycaemia episode. Parents telephoned the study nurse during the next business day to review each such episode to verify that it met the DCCT criteria" Comment: likely not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: it is not clearly described whether there were any dropouts or missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study likely investigated other outcomes, which were not mentioned in this study. Also, no reference is given to other articles on this study or a study protocol. It is not quite clear what the primary aim of the overall study was |
| Other bias | High risk | Comment: gender shows a large baseline difference, all articles seem partial reports of a larger study, which is not well referenced; inconsistent baseline data reporting across different publications |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azar 1999 | No relevant outcomes |
| Bangstad 1992 | No specified glucose targets in treatment groups |
| Barr 2001 | Not randomised controlled trial |
| Beck‐Nielsen 1990 | No specified glucose targets in treatment groups |
| Biesenbach 1988 | No specified glucose targets in treatment groups |
| Bougneres 1993 | No specified glucose targets in treatment groups |
| Christensen 1987 | No specified glucose targets in treatment groups |
| Christiansen 1987 | No specified glucose targets in treatment groups |
| Ciavarella 1985 | No specified glucose targets in treatment groups |
| Crepaldi 1989 | No specified glucose targets in treatment groups |
| de Beaufort 1989 | No specified glucose targets in treatment groups |
| Ditzel 1987 | Study duration < 1 year |
| Dzien 1988 | Not randomised controlled trial |
| Edelmann 1987 | No specified glucose targets in treatment groups |
| Eschwege 1979 | No specified glucose targets in treatment groups |
| Franklin 2006 | No specified glucose targets in treatment groups |
| Goicolea 1987 | No specified glucose targets in treatment groups |
| Itoh 1990 | No separate analysis of patients with type 1 diabetes |
| Kaufman 2005 | Not randomised controlled trial |
| Kordella 2005 | Not randomised controlled trial |
| Kritz 1983 | Not randomised controlled trial |
| KROC 1988 | Study duration < 1 year |
| Levy 1984 | Study duration < 1 year |
| Malmberg 1997 | No specified glucose targets in treatment groups |
| Montanya 1997 | No specified glucose targets in treatment groups |
| Nosadini 1988 | No specified glucose targets in treatment groups |
| Perlman 1984 | No specified glucose targets in treatment groups |
| Podgorski 1987 | Study duration < 1 year |
| Rodger 1988 | Study duration < 1 year |
| Rosenstock 1988 | Not randomised controlled trial |
| Saito 1996 | Not randomised controlled trial |
| SDIS 1993 | No specified glucose targets in treatment groups |
| Shah 1989 | No specified glucose targets in treatment groups |
| Skare 1986 | No specified glucose targets in treatment groups |
| Tubner 1996 | No specified glucose targets in treatment groups |
| Weinrauch 2009 | No specified glucose targets in treatment groups |
| Wiseman 1985 | No specified glucose targets in treatment groups |
Differences between protocol and review
There are no major differences between the protocol and the review. Some of the planned sensitivity and subgroup analyses were not possible due to the low number of studies. For rare events, we used the fixed‐effect method of Peto for the main analysis, which we had not specified in the protocol.
Contributions of authors
Birgit Fullerton (BF): protocol development, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, manuscript draft and review of manuscript.
Klaus Jeitler (KJ): protocol development, search strategy development, acquiring trial reports, data interpretation and review of manuscript.
Mirjam Seitz (MS): trial selection, data extraction and review of manuscript.
Karl Horvath (KH): trial selection, data interpretation and review of manuscript.
Andrea Berghold (AB): protocol development, data analysis, data interpretation and review of manuscript.
Andrea Siebenhofer (AS): protocol development, search strategy development, acquiring trial reports, trial selection, data interpretation and review of manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Federal Ministry of Education and Research (BMBF), Germany.
financial support
Declarations of interest
Birgit Fullerton: none known.
Klaus Jeitler: participation in the preparation of a report for the Institute for Quality and Efficiency in Health Care on the benefits of long‐term blood glucose lowering to near‐normal levels in patients with type 2 diabetes mellitus (Nutzenbewertung einer langfristigen normnahen Blutzuckersenkung bei Patienten mit Diabetes mellitus Typ 2).
Mirjam Seitz: none known.
Karl Horvath: has received payments (congress fees, travel expenses and accomodation) from Roche, Novartis, Sanofi Aventis and Novo Nordisk to attend annual meetings of the Austrian Diabetes Association; has received payments (congress fees, travel expenses and accomodation) from Novartis and Aventis to attend annual meetings of the EASD; has received financial compensation as a speaker from Novartis, Eli Lilly, Medtronic, The Styrian Health Insurance Company and The Styrian Medical Association; participation in the preparation of a report for the Institute for Quality and Efficiency in Health Care on the benefits of long‐term blood glucose lowering to near‐normal levels in patients with type 2 diabetes mellitus (Nutzenbewertung einer langfristigen normnahen Blutzuckersenkung bei Patienten mit Diabetes mellitus Typ 2).
Andrea Berghold: none known.
Andrea Siebenhofer: participation in the preparation of a report for the Institute for Quality and Efficiency in Health Care on the benefits of long‐term blood glucose lowering to near‐normal levels in patients with type 2 diabetes mellitus (Nutzenbewertung einer langfristigen normnahen Blutzuckersenkung bei Patienten mit Diabetes mellitus Typ 2).
Edited (no change to conclusions)
References
References to studies included in this review
Bucharest‐Düsseldorf 1984 {published and unpublished data}
- Mühlhauser I, Bruckner I, Berger M, Cheta D, Jorgens V, Ionescu‐Tirgoviste C, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin‐dependent) diabetes. The Bucharest‐Dusseldorf study. Diabetologia 1987;30(9):681‐90. [DOI] [PubMed] [Google Scholar]
DCCT1 1993 {published data only (unpublished sought but not used)}
- Albers JW, Herman WH, Pop‐Busui R, Martin CL, Cleary P, Waberski B. Subclinical neuropathy among diabetes control and complications trial participants without diagnosable neuropathy at trial completion: Possible predictors of incident neuropathy?. Diabetes Care 2007;30(10):2613‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers JW, Kenny DJ, Brown M, Greene D, Cleary PA, Lachin JM, et al. Effect of intensive diabetes treatment on nerve conduction the diabetes control and complications trial. Annals of Neurology 1995;38(6):869‐80. [DOI] [PubMed] [Google Scholar]
- Carter RE, Lackland DT, Cleary PA, Yim E, Lopes‐Virella MF, Gilbert GE, et al. Intensive treatment of diabetes is associated with a reduced rate of peripheral arterial calcification in the diabetes control and complications trial. Diabetes Care 2007;30(10):2646‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH, Eastman RC. The effects of treatment on the direct costs of diabetes. Diabetes Care 1998;21(Suppl. 3):C19‐C24. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: "Double diabetes" in the Diabetes Control and Complications Trial. Diabetes Care 2007;30(3):707‐12. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Atkin SL. Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clinical Chemistry 2007;53(5):897‐901. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007;50(12):2553‐61. [DOI] [PubMed] [Google Scholar]
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial‐‐revisited. Diabetes 2008;57(4):995‐1001. [DOI] [PubMed] [Google Scholar]
- Meltzer D, Egleston B, Stoffel D, Dasbach E. Effect of future costs on cost‐effectiveness of medical interventions among young adults: the example of intensive therapy for type 1 diabetes mellitus. Medical Care 2000;38(6):679‐85. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long‐term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48(4):870‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen G, Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care 2008;31(10):1933‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England Journal of Medicine 22.12.2005;353(25):2643‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Rutledge BN, Cleary PA, Lachin JM, Crow RS. The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2007;30(8):2107‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. [Erratum appears in JAMA 1998 Nov 4;280(17):1484]. JAMA 1998;280(2):140‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, et al. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the Diabetes Control and Complications Trial. Circulation 2005;111(19):2446‐53. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Cox MS, Buzzard IM, Cleary PA. Reproducibility of a comprehensive diet history in the Diabetes Control and Complications Trial. The DCCT Research Group. Journal of the American Dietetic Association 1994;94(12):1392‐7. [DOI] [PubMed] [Google Scholar]
- Service FJ, O'Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. [Erratum appears in Diabetologia 2002 Jun;45(6):936]. Diabetologia 2001;44(10):1215‐20. [DOI] [PubMed] [Google Scholar]
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelyzn M, et al. Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(3):361‐76. [DOI] [PubMed] [Google Scholar]
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelyzn M, et al. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin‐dependent diabetes mellitus: The Diabetes Control and Complications Trial. Archives of Ophthalmology 1995;113(1):36‐51. [DOI] [PubMed] [Google Scholar]
- Szucs TD, Smala AM, Fischer T. [Costs of intensive insulin therapy in type 1 diabetes mellitus. Experiences from the DCCT study]. [German]. Fortschritte der Medizin 1998;116(31):34‐8. [PubMed] [Google Scholar]
- The DCCT Research Group. Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(11):1415‐27. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT). Update. Diabetes Care 1990;13(4):427‐33. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): Results of feasibility study. The DCCT Research Group. Diabetes Care 1987 Jan‐Feb;10(1):1‐19. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 1987;10(1):1‐19. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. [Erratum appears in Arch Ophthalmol 1998 Nov;116(11):1469]. Archives of Ophthalmology 1998;116(7):874‐86. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. American Journal of Cardiology 1995;75(14):894‐903. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Journal of Pediatrics 1994;125(2):177‐88. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive therapy on residual beta‐cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1998;128(7):517‐23. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney International 1995;47(6):1703‐20. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 2000;23(8):1084‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. Effects of age, duration and treatment of insulin‐dependent diabetes mellitus on residual beta‐cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. The Journal of Clinical Endocrinology and Metabolism 1987;65(1):30‐6. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial: The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1996;124(4):379‐88. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. The DCCT Research Group. American Journal of Medicine 1991;90(4):450‐9. [PubMed] [Google Scholar]
- The DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1997;46(2):271‐86. [PubMed] [Google Scholar]
- The DCCT Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care 2001;24(10):1711‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. Influence of intensive diabetes treatment on quality‐of‐life outcomes in the Diabetes Control and Complications Trial. Diabetes Care 1996;19(3):195‐203. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group.[Erratum appears in JAMA 1997 Jul 2;278(1):25]. JAMA 1996;276(17):1409‐15. [PubMed] [Google Scholar]
- The DCCT Research Group. Pregnancy outcomes in the Diabetes Control and Complications Trial. American Journal of Obstetrics and Gynecology 1996;174(4):1343‐53. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology 1995;102(4):647‐61. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Protocol for the Diabetes Control and Complications Trial. 1987 http://www2.bsc.gwu.edu/bsc/docs/dcctprot.pdf (last accessed: 29.07.2013).
- The DCCT Research Group. Resource utilization and costs of care in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(11):1468‐78. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes 1986;35(5):530‐45. [PubMed] [Google Scholar]
- The DCCT Research Group. The absence of a glycemic threshold for the development of long‐term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45(10):1289‐98. [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41(4):416‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1995;122(8):561‐8. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44(8):968‐83. [PubMed] [Google Scholar]
- Wing RR, Cleary PA. Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care 1988;11(7):567‐73. [DOI] [PubMed] [Google Scholar]
- Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Archives of Internal Medicine 2008;168(17):1867‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
DCCT2 1993 {published data only}
- Albers JW, Herman WH, Pop‐Busui R, Martin CL, Cleary P, Waberski B. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: Possible predictors of incident neuropathy?. Diabetes Care 2007;30(10):2613‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers JW, Kenny DJ, Brown M, Greene D, Cleary PA, Lachin JM, et al. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Annals of Neurology 1995;38(6):869‐80. [DOI] [PubMed] [Google Scholar]
- Carter RE, Lackland DT, Cleary PA, Yim E, Lopes‐Virella MF, Gilbert GE, et al. Intensive treatment of diabetes is associated with a reduced rate of peripheral arterial calcification in the Diabetes Control and Complications Trial. Diabetes Care 2007;30(10):2646‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman WH, Eastman RC. The effects of treatment on the direct costs of diabetes. Diabetes Care 1998;21 Suppl 3:C19‐C21. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: "Double diabetes" in the Diabetes Control and Complications Trial. Diabetes Care 2007;30(3):707‐12. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Atkin SL. Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clinical Chemistry 2007;53(5):897‐901. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia 2007;50(12):2553‐61. [DOI] [PubMed] [Google Scholar]
- Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial‐‐revisited. Diabetes 2008;57(4):995‐1001. [DOI] [PubMed] [Google Scholar]
- Meltzer D, Egleston B, Stoffel D, Dasbach E. Effect of future costs on cost‐effectiveness of medical interventions among young adults: the example of intensive therapy for type 1 diabetes mellitus. Medical Care 2000;38(6):679‐85. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long‐term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48(4):870‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musen G, Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care 2008;31(10):1933‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England Journal of Medicine 22.12.2005;353(25):2643‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Rutledge BN, Cleary PA, Lachin JM, Crow RS. The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2007;30(8):2107‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial.[Erratum appears in JAMA 1998 Nov 4;280(17):1484]. JAMA 1998;280(2):140‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, et al. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the Diabetes Control and Complications Trial. Circulation 2005;111(19):2446‐53. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Cox MS, Buzzard IM, Cleary PA. Reproducibility of a comprehensive diet history in the Diabetes Control and Complications Trial. The DCCT Research Group. Journal of the American Dietetic Association 1994;94(12):1392‐7. [DOI] [PubMed] [Google Scholar]
- Service FJ, O'Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. [Erratum appears in Diabetologia 2002 Jun;45(6):936]. Diabetologia 2001;44(10):1215‐20. [DOI] [PubMed] [Google Scholar]
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelyzn M, et al. Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(3):361‐76. [DOI] [PubMed] [Google Scholar]
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelyzn M, et al. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin‐dependent diabetes mellitus: The Diabetes Control and Complications Trial. Archives of Ophthalmology 1995;113(1):36‐51. [DOI] [PubMed] [Google Scholar]
- Szucs TD, Smala AM, Fischer T. [Costs of intensive insulin therapy in type 1 diabetes mellitus. Experiences from the DCCT study]. [German]. Fortschritte der Medizin 1998;116(31):34‐8. [PubMed] [Google Scholar]
- The DCCT Research Group. Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(11):1415‐27. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT). Update. Diabetes Care 1990;13(4):427‐33. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): Results of feasibility study. Diabetes Care 1987;10(1):1‐19. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): Results of feasibility study. The DCCT Research Group. Diabetes Care 1987 Jan‐Feb;10(1):1‐19. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. [Erratum appears in Arch Ophthalmol 1998 Nov;116(11):1469]. Archives of Ophthalmology 1998;116(7):874‐86. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. American Journal of Cardiology 1995;75(14):894‐903. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Journal of Pediatrics 1994;125(2):177‐88. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive therapy on residual beta‐cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1998;128(7):517‐23. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney International 1995;47(6):1703‐20. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effect of pregnancy on microvascular complications in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 2000;23(8):1084‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. Effects of age, duration and treatment of insulin‐dependent diabetes mellitus on residual beta‐cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. The Journal of Clinical Endocrinology and Metabolism 1987;65(1):30‐6. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial: The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1996;124(4):379‐88. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. The DCCT Research Group. The American Journal of Medicine 1991;90(4):450‐9. [PubMed] [Google Scholar]
- The DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1997;46(2):271‐86. [PubMed] [Google Scholar]
- The DCCT Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care 2001;24(10):1711‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. Influence of intensive diabetes treatment on quality‐of‐life outcomes in the Diabetes Control and Complications Trial. Diabetes Care 1996;19(3):195‐203. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. [Erratum appears in JAMA 1997 Jul 2;278(1):25]. JAMA 1996;276(17):1409‐15. [PubMed] [Google Scholar]
- The DCCT Research Group. Pregnancy outcomes in the Diabetes Control and Complications Trial. American Journal of Obstetrics and Gynecology 1996;174(4):1343‐53. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology 1995;102(4):647‐61. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. Protocol for the Diabetes Control and Complications Trial. 1987 http://www2.bsc.gwu.edu/bsc/docs/dcctprot.pdf (last accessed: 29.07.2013).
- The DCCT Research Group. Resource utilization and costs of care in the Diabetes Control and Complications Trial. Diabetes Care 1995;18(11):1468‐78. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes 1986;35(5):530‐45. [PubMed] [Google Scholar]
- The DCCT Research Group. The absence of a glycemic threshold for the development of long‐term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45(10):1289‐98. [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41(4):416‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Annals of Internal Medicine 1995;122(8):561‐8. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44(8):968‐83. [PubMed] [Google Scholar]
- Wing RR, Cleary PA. Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care 1988;11(7):567‐73. [DOI] [PubMed] [Google Scholar]
- Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Archives of Internal Medicine 2008;168(17):1867‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holman 1983 {published data only}
- Holman RR, Dornan TL, Mayon‐White V, Howard‐Williams J, Orde‐Peckar C, Jenkins L, et al. Prevention of deterioration of renal and sensory‐nerve function by more intensive management of insulin‐dependent diabetic patients. A two‐year randomised prospective study. Lancet 1983;1(8318):204‐8. [DOI] [PubMed] [Google Scholar]
Linn 1996 {published data only}
- Linn T, Ortac K, Laube H, Federlin K. Intensive therapy in adult insulin‐dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism: Clinical and Experimental 1996;45(12):1508‐13. [DOI] [PubMed] [Google Scholar]
MCSG 1995 {published data only}
- Viberti GC. Intensive therapy and progression to clinical albuminuria in patients with insulin dependent diabetes mellitus and microalbuminuria. BMJ 1995;311(7011):973‐7. [PMC free article] [PubMed] [Google Scholar]
MDCCT 1994 {published data only}
- Barbosa J, Connett J, Fryd D, Sutherland D, Rao V, Anderson R, et al. The Minnesota Diabetes Complications Clinical Trial. The first three years. Acta Diabetologica Latina 1983;20(2):165‐71. [DOI] [PubMed] [Google Scholar]
- Barbosa J, Johnson S. Severe hypoglycemia during maximized insulin treatment of diabetes in a randomized clinical trial. Diabetes Care 1983;6(1):62‐3. [DOI] [PubMed] [Google Scholar]
- Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM. Effect of glycemic control on early diabetic renal lesions. A 5‐year randomized controlled clinical trial of insulin‐dependent diabetic kidney transplant recipients. JAMA 1994;272(8):600‐6. [PubMed] [Google Scholar]
- Fryd DS, Migliori R, Simmons RL, Chavers B, Dunn D, Payne W, et al. Renal transplantation at the University of Minnesota during the 1980s. Clinical Transplants 1987;02:167‐81. [PubMed] [Google Scholar]
- Hung J, Menth L, Thompson MJ, Saner B, Rao KV, Barbosa J. The Minnesota Diabetes Complications Clinical Trial cognitive functions under long‐term maximized and standard metabolic controls. Diabète & Métabolisme 1984;10(1):48‐51. [PubMed] [Google Scholar]
Oslo 1987 {published data only}
- Amthor KF, Dahl‐Jorgensen K, Berg TJ, Skard Heier M, Sandvik L, Aagenaes O, et al. The effect of 8 years of strict glycaemic control on peripheral nerve function in IDDM patients: The Oslo study. Diabetologia 1994;37(6):579‐84. [DOI] [PubMed] [Google Scholar]
- Brinchmann‐Hansen O, Dahl‐Jorgensen K, Hanssen KF, Sandvik L. Effects of intensified insulin treatment on various lesions of diabetic retinopathy. American Journal of Ophthalmology 1985;100(5):644‐53. [DOI] [PubMed] [Google Scholar]
- Brinchmann‐Hansen O, Dahl‐Jorgensen K, Hanssen KF, Sandvik L. Oscillatory potentials, macular recovery time, and diabetic retinopathy through 3 years of intensified insulin treatment. Ophthalmology 1988;95(10):1358‐66. [DOI] [PubMed] [Google Scholar]
- Brinchmann‐Hansen O, Dahl‐Jørgensen K, Hanssen KF, Sandvik L. The response of diabetic retinopathy to 41 months of multiple insulin injections, insulin pumps, and conventional insulin therapy. Archives of Ophthalmology 1988;106(9):1242‐6. [DOI] [PubMed] [Google Scholar]
- Brinchmann‐Hansen O, Dahl‐Jørgensen K, Sandvik L, Hanssen KF. Blood glucose concentrations and progression of diabetic retinopathy: the seven year results of the Oslo study. BMJ (Clinical research ed.) 1992;304(6818):19‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl‐Jorgensen K. Near‐normoglycemia and late diabetic complications. The Oslo Study. Acta Endocrinologica. Supplementum 1987;284:1‐38. [PubMed] [Google Scholar]
- Dahl‐Jorgensen K, Brinchmann‐Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. British Medical Journal (Clinical Research Ed.) 1986;293(6556):1195‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl‐Jorgensen K, Brinchmann‐Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. British Medical Journal (Clinical Research Ed.) 1985;290(6471):811‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl‐Jorgensen K, Hanssen KF, Kierulf P, Bjoro T, Sandvik L, Aagenaes O. Reduction of urinary albumin excretion after 4 years of continuous subcutaneous insulin infusion in insulin‐dependent diabetes mellitus. The Oslo study. Acta Endocrinologica 1988;117(1):19‐25. [DOI] [PubMed] [Google Scholar]
Steno 1 1983 {published data only}
- Deckert T, Lauritzen T, Parvig HH, Christiansen JS, Steno Study Group. Effect of two years of strict metabolic functions in long term insulin‐dependent diabetes. Diabetic Nephropathy 1984;3:6‐10. [Google Scholar]
- Feldt‐Rasmussen B, Mathiesen ER, Jensen T, Lauritzen T, Deckert T. Effect of improved metabolic control on loss of kidney function in type 1 (insulin‐dependent) diabetic patients: an update of the Steno studies. Diabetologia 1991;34(3):164‐70. [DOI] [PubMed] [Google Scholar]
- Lauritzen T, Frost‐Larsen K, Larsen HW, Deckert T. Effect of 1 year of near‐normal blood glucose levels on retinopathy in insulin‐dependent diabetics. Lancet 1983;1(8318):200‐4. [DOI] [PubMed] [Google Scholar]
- Lauritzen T, Frost‐Larsen K, Larsen HW, Deckert T. Two‐year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes 1985;34 Suppl 3:74‐9. [DOI] [PubMed] [Google Scholar]
Steno 2 1986 {published data only}
- Feldt‐Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin‐dependent diabetes. Lancet 1986;2(8519):1300‐4. [DOI] [PubMed] [Google Scholar]
- Feldt‐Rasmussen B, Mathiesen ER, Hegedüs L, Deckert T. Kidney function during 12 months of strict metabolic control in insulin‐dependent diabetic patients with incipient nephropathy. The New England Journal of Medicine 1986;Volume(11):665‐70. [DOI] [PubMed] [Google Scholar]
- Feldt‐Rasmussen B, Mathiesen ER, Jensen T, Lauritzen T, Deckert T. Effect of improved metabolic control on loss of kidney function in type 1 (insulin‐dependent) diabetic patients: an update of the Steno studies. Diabetologia 1991;34(3):164‐70. [DOI] [PubMed] [Google Scholar]
Verrillo 1988 {published data only}
- Verrillo A, Teresa A, Martino C, Verrillo L, Chiara G. Long‐term correction of hyperglycemia and progression of retinopathy in insulin dependent diabetes. A five‐year randomized prospective study. Diabetes Research 1988;8(2):71‐6. [PubMed] [Google Scholar]
Wysocki 2003 {published data only}
- Hershey T, Bhargava N, Sadler M, White NH, Craft S. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care 1999;22(8):1318‐24. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Wilkinson K, Sadler M, Mauras N, et al. Self‐care autonomy and outcomes of intensive therapy or usual care in youth with type 1 diabetes. Journal of Pediatric Psychology 2006;31(10):1036‐45. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, et al. Absence of adverse effects of severe hypoglycemia on cognitive function in school‐aged children with diabetes over 18 months. Diabetes Care 2003;26(4):1100‐5. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Wilkinson K, Sadler M, Mauras N, White NH. Self‐management competence as a predictor of outcomes of intensive therapy or usual care in youth with type 1 diabetes. Diabetes Care 2003;26(7):2043‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Azar 1999 {published data only}
- Azar ST, Kanaan N. Intensive insulin therapy compared with conventional insulin therapy does not reduce depressive symptoms in parents of children with type 1 diabetes. Diabetes Care 1999;22(8):1372‐3. [DOI] [PubMed] [Google Scholar]
Bangstad 1992 {published data only}
- Bangstad HJ, Kofoed‐Enevoldsen A, Dahl‐Jørgensen K, Hanssen KF. Glomerular charge selectivity and the influence of improved blood glucose control in type 1 (insulin‐dependent) diabetic patients with microalbuminuria. Diabetologia 1992;35(12):1165‐9. [DOI] [PubMed] [Google Scholar]
Barr 2001 {published data only}
- Barr CC. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy, by The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. New England Journal of Medicine 2000;342:381‐9. Survey of Ophthalmology 2001;45(5):459‐60. [DOI] [PubMed] [Google Scholar]
Beck‐Nielsen 1990 {published data only}
- Beck‐Nielsen H, Olesen T, Mogensen CE, Richelsen B, Olsen HW, Ehlers N, et al. Effect of near normoglycemia for 5 years on progression of early diabetic retinopathy and renal involvement. Diabetes Research (Edinburgh, Scotland) 1990;15(4):185‐90. [PubMed] [Google Scholar]
Biesenbach 1988 {published data only}
- Biesenbach G, Grafinger P, Kaiser W, Stuby U, Zazgornik J. [Metabolic control in labile type I diabetes with conventional insulin therapy, basal bolus insulin therapy using pens and continuous subcutaneous insulin infusion with the pump]. [German]. Medizinische Klinik 1988;83(12):398‐401. [PubMed] [Google Scholar]
Bougneres 1993 {published data only}
- Bougneres PF, Landais P, Mairesse AM, Jais JP, Jos J, Peyraud J, et al. Improvement of diabetic control and acceptability of a three‐injection insulin regimen in diabetic adolescents. A multicenter controlled study. Diabetes Care 1993;16(1):94‐102. [DOI] [PubMed] [Google Scholar]
Christensen 1987 {published data only}
- Christensen CK, Christiansen JS, Schmitz A, Christensen T, Hermansen K, Mogensen CE. Effect of continuous subcutaneous insulin infusion on kidney function and size in IDDM patients: a 2 year controlled study. The Journal of Diabetic Complications 1987;1(3):91‐5. [DOI] [PubMed] [Google Scholar]
Christiansen 1987 {published data only}
- Christiansen JS, Ingerslev J, Bernvil SS, Christensen CK, Hermansen K, Schmitz A. Near normoglycemia for 1 year has no effect on platelet reactivity, factor VIII, and von Willebrand factor in insulin‐dependent diabetes mellitus: a controlled trial. The Journal of Diabetic Complications 1987;1(3):100‐6. [DOI] [PubMed] [Google Scholar]
Ciavarella 1985 {published data only}
- Ciavarella A, Vannini P, Flammini M, Bacci L, Forlani G, Borgnino LC. Effect of long‐term near‐normoglycemia on the progression of diabetic nephropathy. Diabète & Métabolisme 1985;11(1):3‐8. [PubMed] [Google Scholar]
Crepaldi 1989 {published data only}
- The Italian National Research Council Study Group on Diabetic Retinopathy. The effect of continuous insulin infusion as compared with conventional insulin therapy in the evolution of diabetic retinal ischaemia. Two years report. Diabetes Nutrition & Metabolism ‐ Clinical & Experimental 1989;2(3):209‐18. [Google Scholar]
de Beaufort 1989 {published data only}
- Beaufort CE, Houtzagers CMGJ, Bruining GJ, Aarsen RSR, Boer NC, Grose WFA, et al. Continuous subcutaneous insulin infusion (CSII) versus conventional injection therapy in newly diagnosed diabetic children: Two‐year follow‐up of a randomized, prospective trial. Diabetic Medicine 1989;6(9):766‐71. [DOI] [PubMed] [Google Scholar]
Ditzel 1987 {published data only}
- Ditzel J, Clemmensen NK. [A comparative study of intensive conventional insulin treatment and a multiple insulin‐injection regimen with NovoPen]. [Danish]. Ugeskrift for Laeger 1987;149(20):1335‐8. [PubMed] [Google Scholar]
Dzien 1988 {published data only}
- Dzien A, Drexel H, Hopferwieser T, Patsch JR, Braunsteiner H. [Effects of intensified insulin therapy on fat metabolism in type 1 diabetes mellitus]. [German]. Deutsche Medizinische Wochenschrift 1988;113(43):1669‐72. [DOI] [PubMed] [Google Scholar]
Edelmann 1987 {published data only}
- Edelmann E, Walter H, Biermann E, Schleicher E, Bachmann W, Mehnert H. Sustained normoglycemia and remission phase in newly diagnosed type I diabetic subjects. Comparison between continuous subcutaneous insulin infusion and conventional therapy during a one year follow‐up. Hormone and Metabolic Research 1987;19(9):419‐21. [DOI] [PubMed] [Google Scholar]
Eschwege 1979 {published data only}
- Eschwege E, Job D, Guyot‐Argenton C, Aubry J P, Tchobroutsky G. Delayed progression of diabetic retinopathy by divided insulin administration: a further follow‐up. Diabetologia 1979;16(1):13‐5. [DOI] [PubMed] [Google Scholar]
Franklin 2006 {published data only}
- Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text‐messaging system to support young people with diabetes. Diabetic Medicine 2006;23(12):1332‐8. [DOI] [PubMed] [Google Scholar]
Goicolea 1987 {published data only}
- Goicolea Opacua I, Hernandez Colau I, Cortazar Galarza A, Vazquez Garcia JA. [Comparison of metabolic control between the continuous subcutaneous insulin infusion pump and augmented conventional treatment. Effects after 12 months]. [Spanish]. Medicina Clinica 1987;88(16):617‐20. [PubMed] [Google Scholar]
Itoh 1990 {published data only}
- Itoh H, Namba M, Kono N, Tarui S. [Multiple insulin injection therapy as intensive insulin therapy]. [Japanese]. Nippon Rinsho 1990;48:1015‐21. [PubMed] [Google Scholar]
Kaufman 2005 {published data only}
- Kaufman FR. Intensive management of type 1 diabetes in young children. Lancet 2005;365(9461):737‐8. [DOI] [PubMed] [Google Scholar]
Kordella 2005 {published data only}
- Kordella T. Tight control can protect your heart. Diabetes Forecast 2005;58(10):44‐5. [PubMed] [Google Scholar]
Kritz 1983 {published data only}
- Kritz H, Hagmuller G, Lovett R, Irsigler K. Implanted constant basal rate insulin infusion devices for Type 1 (insulin‐dependent) diabetic patients. Diabetologia 1983;25(2):78‐81. [DOI] [PubMed] [Google Scholar]
KROC 1988 {published data only}
- The Kroc Collaborative Study Group. Diabetic retinopathy after two years of intensified insulin treatment. Follow‐up of the Kroc Collaborative Study. JAMA 1988;260(1):37‐41. [DOI] [PubMed] [Google Scholar]
Levy 1984 {published data only}
- Levy I, Bergua M, Esmatjes E, Halperin I, Figuerola D. [Unstable type I diabetes mellitus: comparative study between intensive conventional treatment and continuous subcutaneous infusion of insulin]. [Spanish]. Medicina Clinica 1984;83(13):525‐8. [PubMed] [Google Scholar]
Malmberg 1997 {published data only}
- Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenstrom A, Wedel H. Mortality prediction in diabetic patients with myocardial infarction: experiences from the DIGAMI study.[Erratum appears in Cariovasc Res 1997 Dec;36(3):460]. Cardiovascular Research 1997;34(1):248‐53. [DOI] [PubMed] [Google Scholar]
Montanya 1997 {published data only}
- Montanya E, Fernandez‐Castaner M, Soler J. Improved metabolic control preserved beta‐cell function two years after diagnosis of insulin‐dependent diabetes mellitus. Diabetes & Metabolism 1997;23(4):314‐9. [PubMed] [Google Scholar]
Nosadini 1988 {published data only}
- Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic‐ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1988;1(4):289‐96. [Google Scholar]
Perlman 1984 {published data only}
- Perlman K, Ehrlich RM, Filler RM, Albisser AM. Sustained normoglycemia in newly diagnosed type I diabetic subjects. Short‐term effects and one‐year follow‐up. Diabetes 1984;33(10):995‐1001. [DOI] [PubMed] [Google Scholar]
Podgorski 1987 {published data only}
- Podgorski G, Taton J, Czech A, Laz R, Szalkiewicz G. [Comparison of the effectiveness of conventional and intensive insulin therapy in the treatment of diabetes mellitus type 1 in pregnant women or those planning pregnancy]. [Polish]. Polski Tygodnik Lekarski 1987;42(50):1571‐6. [PubMed] [Google Scholar]
Rodger 1988 {published data only}
- Rodger NW, Behme MT, Dupre J, Chamberlain MJ. Metabolic effects of continuous subcutaneous insulin infusion: evidence that a rise and fall of portal vein insulin concentration with each major meal facilitates post‐absorptive glycemic control. Clinical and Investigative Medicine. Médecine Clinique et Experimentale 1988;11(3):167‐86. [PubMed] [Google Scholar]
Rosenstock 1988 {published data only}
- Rosenstock J, Challis P, Strowig S, Raskin P. Improved diabetes control reduces skeletal muscle capillary basement membrane width in insulin‐dependent diabetes mellitus. Diabetes Research & Clinical Practice 1988;4(3):167‐75. [DOI] [PubMed] [Google Scholar]
Saito 1996 {published data only}
- Saito Y, Ohmi G, Tano Y, Kinoshita S. Lens changes during rapid tightening of metabolic control in diabetes. Lancet 1996;347(9017):1764. [DOI] [PubMed] [Google Scholar]
SDIS 1993 {published data only}
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. The New England Journal of Medicine 1993;329(5):304‐9. [DOI] [PubMed] [Google Scholar]
Shah 1989 {published data only}
- Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin‐dependent diabetes mellitus. The New England Journal of Medicine 1989;320(9):550‐4. [DOI] [PubMed] [Google Scholar]
Skare 1986 {published data only}
- Skare S, Dahl‐Jorgensen K, Kriz V, Hanssen KF. Plasma somatostatin and plasma glucagon in long‐term IDDM without residual B‐cell function. No effect of different long‐term metabolic control. Scandinavian Journal of Clinical and Laboratory Investigation 1986;46(7):635‐8. [DOI] [PubMed] [Google Scholar]
Tubner 1996 {published data only}
- Tubner RC, Cull CA, Holman RR. Frequency of hypoglycemic episodes during intensive therapy with human insulin. Diabetes Care 1996;19(2):181‐2. [PubMed] [Google Scholar]
Weinrauch 2009 {published data only}
- Weinrauch LA, Bayliss G, Gleason RE, Lee AT, D'Elia JA. Utilization of an abbreviated diabetes impact management scale to assess change in subjective disability during a trial of pulsatile insulin delivery demonstrates benefit. Metabolism: Clinical and Experimental 2009;58(4):488‐91. [DOI] [PubMed] [Google Scholar]
Wiseman 1985 {published data only}
- Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin‐dependent diabetes. The New England Journal of Medicine 1985;312(10):617‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Abraira 1997
- Abraira C, Colwell J, Nuttall F, Sawin CT, Henderson W, Comstock JP, et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Archives of Internal Medicine 1997;157(2):181‐8. [PubMed] [Google Scholar]
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Standards of medical care in diabetes ‐ 2010. Diabetes Care 2010;33 Suppl 1:S11‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alaveras 1997
- Alaveras AE, Thomas SM, Sagriotis A, Viberti GC. Promoters of progression of diabetic nephropathy: the relative roles of blood glucose and blood pressure control. Nephrology, Dialysis, Transplantation 1997;12 Suppl 2:71‐4. [PubMed] [Google Scholar]
Alberti 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Amiel 1986
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. The New England Journal of Medicine 1986;315(4):215‐9. [DOI] [PubMed] [Google Scholar]
Aschner 2010
- Aschner P, Horton E, Leiter LA, Munro N, Skyler JS. Practical steps to improving the management of type 1 diabetes: recommendations from the Global Partnership for Effective Diabetes Management. International Journal of Clinical Practice 2010;64(3):305‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 1995
- Berger M, Mühlhauser I. Implementation of intensified insulin therapy: a European perspective. Diabetic Medicine 1995;12(3):201‐8. [DOI] [PubMed] [Google Scholar]
Boulton 2004
- Boulton AJM, Malik RA, Arezzo JC, Sosenko JM. Diabetic Somatic Neuropathies. Diabetes Care 2004;27(6):1458‐86. [DOI] [PubMed] [Google Scholar]
Callaghan 2012
- Callaghan BC, Little AA, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD007543.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvert 2009
- Calvert M, Shankar A, McManus RJ, Lester H, Freemantle N. Effect of the quality and outcomes framework on diabetes care in the United Kingdom: retrospective cohort study. BMJ (Clinical Research Ed.) 2009;338:b1870. [PUBMED: 19474024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canadian 2008
- Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2008; Vol. 32:Suppl 1.
Cappelleri 1996
- Cappelleri JC, Ioannidis JP, Schmid CH, Ferranti SD, Aubert M, Chalmers TC, et al. Large trials vs meta‐analysis of smaller trials: how do their results compare?. JAMA 1996;276(16):1332‐8. [PubMed] [Google Scholar]
Collins 2003
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet 2003;361(9374):2005‐16. [DOI] [PubMed] [Google Scholar]
Conway 2010
- Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabetic Medicine 2010;27(4):398‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cryer 2008
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57(12):3169‐76. [PUBMED: 19033403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahl‐Jorgensen 1985
- Dahl‐Jorgensen K, Brinchmann‐Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. BMJ (Clinical Research Ed.) 1985;290(6471):811‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daneman 2006
- Daneman D. Type 1 diabetes. Lancet 2006;367(9513):847‐58. [DOI] [PubMed] [Google Scholar]
Davidson 2004
- Davidson M, Penney ED, Muller B, Grey M. Stressors and self‐care challenges faced by adolescents living with type 1 diabetes. Applied Nursing Research 2004;17(2):72‐80. [DOI] [PubMed] [Google Scholar]
DCCT 1987
- The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): Results of feasibility study. Diabetes Care 1987 Jan‐Feb;10(1):1‐19. [DOI] [PubMed] [Google Scholar]
DCCT 1993
- The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
DCCT 1994
- The DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Journal of Pediatrics 1994;125(2):177‐88. [DOI] [PubMed] [Google Scholar]
DCCT 1995
- The DCCT Research Group. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial. Archives of Ophthalmology 1995;113(1):36‐51. [DOI] [PubMed] [Google Scholar]
DCCT 1995a
- The DCCT Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. American Journal of Cardiology 1995;75(14):894‐903. [DOI] [PubMed] [Google Scholar]
DCCT 1995b
- The Diabetes Control and Complications Trial (DCCT) Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44(8):968‐83. [PUBMED: 7622004] [PubMed] [Google Scholar]
DCCT 1995c
- The DCCT Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology 1995;102(4):647‐61. [DOI] [PubMed] [Google Scholar]
DCCT 1995d
- The DCCT Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney International 1995;47(6):1703‐20. [DOI] [PubMed] [Google Scholar]
DCCT 1996
- The Diabetes Control and Complications Trial (DCCT) Research Group. The absence of a glycemic threshold for the development of long‐term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45(10):1289‐98. [PUBMED: 8826962] [PubMed] [Google Scholar]
DCCT 1996a
- The DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group.[Erratum appears in JAMA 1997 Jul 2;278(1):25]. JAMA 1996;276(17):1409‐15. [PubMed] [Google Scholar]
DCCT/EDIC 2011
- The DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. The New England Journal of Medicine 2011;365(25):2366‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desouza 2003
- Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003;26(5):1485‐9. [DOI] [PubMed] [Google Scholar]
Desouza 2010
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33(6):1389‐94. [PUBMED: 20508232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Annals of Internal Medicine 2007;147(8):578‐81. [DOI] [PubMed] [Google Scholar]
Duckworth 2009
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England Journal of Medicine 2009;360(2):129‐39. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin‐dependent diabetes mellitus: A meta‐analysis. Diabetic Medicine 1997;14(11):919‐28. [DOI] [PubMed] [Google Scholar]
Farkouh 2008
- Farkouh ME, Fuster V. Meta‐analysis of small trials: proceed with caution. Nature Clinical Practice. Nephrology 2008;4(3):115. [PubMed] [Google Scholar]
Field 1997
- Field LL, Tobias R. Unravelling a complex trait: the genetics of insulin‐dependent diabetes mellitus. Clinical and Investigative Medicine 1997;20(1):41‐9. [PubMed] [Google Scholar]
Fowler 2008
- Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes 2008;26(2):77‐82. [Google Scholar]
Fried 2001
- Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta‐analysis. Kidney International 2001;59(1):260‐9. [DOI] [PubMed] [Google Scholar]
Gerstein 2007
- Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. American Journal of Cardiology 2007;99(12A):34i‐43i. [DOI] [PubMed] [Google Scholar]
Gerstein 2008
- Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England Journal of Medicine 2008;358(24):2545‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 2009
- Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes‐‐the 'dead in bed' syndrome revisited. Diabetologia 2009;52(1):42‐5. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2002
- Hansen HP, Tauber‐Lassen E, Jensen BR, Parving H‐H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney International 2002;62(1):220‐8. [DOI] [PubMed] [Google Scholar]
Hershey 1999
- Hershey T, Bhargava N, Sadler M, White NH, Craft S. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care 1999;8:1318‐24. [DOI] [PubMed] [Google Scholar]
Herzer 2010
- Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. Journal of Pediatric Psychology 2010;35(4):415‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Home 2012
- Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes, Obesity & Metabolism 2012;14(9):780‐8. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingerski 2010
- Ingerski LM, Laffel L, Drotar D, Repaske D, Hood KK. Correlates of glycemic control and quality of life outcomes in adolescents with type 1 diabetes. Pediatric Diabetes 2010;11(8):563‐71. [DOI] [PubMed] [Google Scholar]
Karvonen 1993
- Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin‐dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia 1993;36(10):883‐92. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Klein 1998
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14‐year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105(10):1801‐15. [DOI] [PubMed] [Google Scholar]
Krolewski 1985
- Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. The American Journal of Medicine 1985;78(5):785‐94. [DOI] [PubMed] [Google Scholar]
Larsen 2003
- Larsen JR, Sjoholm H, Hanssen KF, Sandvik L, Berg TJ, Dahl‐Jorgensen K. Optimal blood glucose control during 18 years preserves peripheral nerve function in patients with 30 years' duration of type 1 diabetes. Diabetes Care 2003;26(8):2400‐4. [DOI] [PubMed] [Google Scholar]
Lawson 1999
- Lawson ML, Gerstein HC, Tsui E, Zinman B. Effect of intensive therapy on early macrovascular disease in young individuals with type 1 diabetes: a systematic review and meta‐analysis (Structured abstract). Diabetes Care 1999;Suppl 2:B35‐9. [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2012
- Liu D. Combining information for heterogeneous studies and rare events studies. Ph.D. Thesis, Rutgers University. New Brunswick, NJ. 2012. New Brunswick, NJ: Rutgers University, http://mss3.libraries.rutgers.edu/dlr/showfed.php?pid=rutgers‐lib:37435 (last accessed 19.06.2013).
Lovestam‐Adrian 2001
- Lovestam‐Adrian M, Agardh CD, Torffvit O, Agardh E. Diabetic retinopathy, visual acuity, and medical risk indicators: a continuous 10‐year follow‐up study in Type 1 diabetic patients under routine care. Journal of Diabetes and its Complications 2001;15(6):287‐94. [DOI] [PubMed] [Google Scholar]
Maahs 2010
- Maahs DM, West NA, Lawrence JM, Mayer‐Davis EJ. Epidemiology of type 1 diabetes. Endocrinology and Metabolism Clinics of North America 2010;39(3):481‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2007
- Martin S, Dreyer M, Kiess W, Ldecke H‐J, Mller UA, Schatz H, et al. Evidenzbasierte Leitlinie der DDG ‐ Therapie des Diabetes mellitus Typ 1. Deutsche Diabetes Gesellschaft 2007.
Mattila 2010
- Mattila TK, Boer A. Influence of intensive versus conventional glucose control on microvascular and macrovascular complications in type 1 and 2 diabetes mellitus. Drugs 2010;70(17):2229‐45. [DOI] [PubMed] [Google Scholar]
Mauer 1984
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural‐functional relationships in diabetic nephropathy. Journal of Clinical Investigation 1984;74(4):1143‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrady 2009
- McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: mediational role of blood glucose monitoring. Diabetes Care 2009;32(5):804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 2000
- Meltzer D, Egleston B, Stoffel D, Dasbach E. Effect of future costs on cost‐effectiveness of medical interventions among young adults: the example of intensive therapy for type 1 diabetes mellitus. Medical care 2000;38(6):679‐85. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study Cohort. Diabetes 2012;61(11):2987‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Misso 2010
- Misso ML, Egberts KJ, Page M, O'Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. The Cochrane Database of Systematic Reviews 2010;20(1):CD005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mogensen 2003
- Mogensen CE. Microalbuminuria and hypertension with focus on type 1 and type 2 diabetes. Journal of Internal Medicine 2003;254(1):45‐66. [DOI] [PubMed] [Google Scholar]
Monami 2009
- Monami M, Marchionni N, Mannucci E. Long‐acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta‐analysis. Diabetes, Obesity & Metabolism 2009;11(4):372‐8. [DOI] [PubMed] [Google Scholar]
Montori 2009
- Montori VM, Fernandez‐Balsells M. Glycemic control in type 2 diabetes: time for an evidence‐based about‐face?. Annals of Internal Medicine 2009;150(11):803‐8. [DOI] [PubMed] [Google Scholar]
Mortensen 1997
- Mortensen HB, Hougaard P. Comparison of metabolic control in a cross‐sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabetes Care. 1997;20(5):714‐20. [DOI] [PubMed] [Google Scholar]
Muis 2005
- Muis MJ, Bots ML, Grobbee DE, Stolk RP. Insulin treatment and cardiovascular disease; friend or foe? A point of view. Diabetic Medicine 2005;22(2):118‐26. [DOI] [PubMed] [Google Scholar]
Nathan 2005
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine 2005;353(25):2643‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2009
- Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, et al. Modern‐day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983‐2005). Archives of Internal Medicine 2009;169(14):1307‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- CG15 Type 1 diabetes in children, young people and adults: NICE guideline. National Institute for Clinical Excellence 2010.
Nicholas 2013
- Nicholas J, Charlton J, Dregan A, Gulliford MC. Recent HbA1c values and mortality risk in type 2 diabetes. Population‐Based Case‐Control Study. PLoS One 2013;8(7):e68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nordrheinische Gemeinsame Einrichtung 2008
- DMP Diabetes mellitus type 1 [DMP Diabetes mellitus Typ 1]. Qualitätssicherungsbericht: Disease‐Management‐Programme in Nordrhein 2008.
Nordwall 2009
- Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains crucial in prevention of late diabetic complications‐‐the Linkoping Diabetes Complications Study. Pediatric Diabetes 2009;10(3):168‐76. [DOI] [PubMed] [Google Scholar]
Onkamo 1999
- Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes‐‐the analysis of the data on published incidence trends. Diabetologia 1999;42(12):1395‐403. [DOI] [PubMed] [Google Scholar]
Orchard 2003
- Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY‐Z, Smithline KL, et al. Insulin resistance‐related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10‐year follow‐up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26(5):1374‐9. [DOI] [PubMed] [Google Scholar]
Osterby 1988
- Osterby R, Parving HH, Nyberg G, Hommel E, Jørgensen HE, Løkkegaard H, et al. A strong correlation between glomerular filtration rate and filtration surface in diabetic nephropathy. Diabetologia 1988;31(5):265‐70. [DOI] [PubMed] [Google Scholar]
Pedrini 1996
- Pedrini MT. The Effect of Dietary Protein Restriction on the Progression of Diabetic and Nondiabetic Renal Diseases: A Meta‐Analysis. Annals of Internal Medicine 1996;124(7):627. [DOI] [PubMed] [Google Scholar]
Pimouguet 2011
- Pimouguet C, Goff M, Thiebaut R, Dartigues JF, Helmer C. Effectiveness of disease‐management programs for improving diabetes care: a meta‐analysis. Canadian Medical Association Journal 2011;183(2):E115‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkaniemi 2004
- Pitkaniemi J, Onkamo P, Tuomilehto J, Arjas E. Increasing incidence of Type 1 diabetes‐‐role for genes?. BMC Genetics 2004;5:5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porta 2001
- Porta M, Sjoelie AK, Chaturvedi N, Stevens L, Rottiers R, Veglio M, et al. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia 2001;44(12):2203‐9. [DOI] [PubMed] [Google Scholar]
Quinn 1996
- Quinn M, Angelico MC, Warram JH, Krolewski AS. Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 1996;39(8):940‐5. [DOI] [PubMed] [Google Scholar]
Rerkasem 2010
- Rerkasem K, Rothwell PM. Meta‐analysis of small randomized controlled trials in surgery may be unreliable. The British Journal of Surgery 2010;97(4):466‐9. [DOI] [PubMed] [Google Scholar]
Rodbard 2007
- Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocrine Practice 2007;13 Suppl 1:1‐68. [DOI] [PubMed] [Google Scholar]
Shalitin 2008
- Shalitin S, Phillip M. Hypoglycemia in type 1 diabetes: a still unresolved problem in the era of insulin analogs and pump therapy. Diabetes Care 2008;31 Suppl 2:S121‐4. [DOI] [PubMed] [Google Scholar]
Siebenhofer 2006
- Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database of Systematic Reviews 2006;19(2):CD003287. [DOI] [PubMed] [Google Scholar]
Soedamah‐Muthu 2004
- Soedamah‐Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, et al. Risk Factors for Coronary Heart Disease in Type 1 Diabetic Patients in Europe: The EURODIAB Prospective Complications Study. Diabetes Care 2004;27(2):530‐7. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stettler 2006
- Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta‐analysis of randomized trials. American Heart Journal 2006;152(1):27‐38. [DOI] [PubMed] [Google Scholar]
Sun 2011
- Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: The Joslin 50‐Year Medalist Study. Diabetes Care 2011;34(4):968‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Sämann 2005
- Sämann A, Mühlhauser I, Bender R, Kloos CH, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005;48(10):1965‐70. [DOI] [PubMed] [Google Scholar]
Tamborlane 2001
- Tamborlane WV, Bonfig W, Boland E. Recent advances in treatment of youth with Type 1 diabetes: better care through technology. Diabetic Medicine 2001;18(11):864‐70. [DOI] [PubMed] [Google Scholar]
Tesfaye 2005
- Tesfaye S, Chaturvedi N, Eaton SEM, Ward JD, Manes C, Ionescu‐Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. The New England Journal of Medicine 2005;352(4):341‐50. [DOI] [PubMed] [Google Scholar]
Thomsett 1999
- Thomsett M, Shield G, Batch J, Cotterill A. How well are we doing? Metabolic control in patients with diabetes. Journal of Paediatrics and Child Health 1999;35(5):479‐82. [DOI] [PubMed] [Google Scholar]
Tu 2010
- Tu E, Twigg SM, Semsarian C. Sudden death in type 1 diabetes: the mystery of the 'dead in bed' syndrome. International Journal of Cardiology 2010;138(1):91‐3. [DOI] [PubMed] [Google Scholar]
Turnbull 2009
- Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52(11):2288‐98. [DOI] [PubMed] [Google Scholar]
Valla 2010
- Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Experimental Diabetes Research 2010:178372. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Werf 2007
- Werf N, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential triggers of type 1 diabetes. Diabetes/Metabolism Research and Reviews 2007;23(3):169‐83. [DOI] [PubMed] [Google Scholar]
Vinik 2003
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26(5):1553‐79. [DOI] [PubMed] [Google Scholar]
Wajchenberg 2008
- Wajchenberg BL, Feitosa AC, Rassi N, Lerario AC, Betti RT. Glycemia and cardiovascular disease in type 1 diabetes mellitus. Endocrine Practice 2008;14(7):912‐23. [DOI] [PubMed] [Google Scholar]
Wang 1993
- Wang PH, Lau J, Chalmers TC. Meta‐analysis of effects of intensive blood‐glucose control on late complications of type I diabetes. Lancet 1993;341(8856):1306‐9. [DOI] [PubMed] [Google Scholar]
Wei 1988
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Controlled Clinical Trials 1988;9(4):345‐64. [DOI] [PubMed] [Google Scholar]
Weston 1999
- Weston PJ, Gill GV. Is undetected autonomic dysfunction responsible for sudden death in Type 1 diabetes mellitus? The 'dead in bed' syndrome revisited. Diabetic Medicine 1999;16(8):626‐31. [DOI] [PubMed] [Google Scholar]
Whitmer 2009
- Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301(15):1565‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1985
- World Health Organization Study Group. Diabetes mellitus. WHO Technical Report Series 1985;727:17‐9. [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeh 2012
- Yeh H‐C, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta‐analysis. Annals of Internal Medicine 2012;157(5):336‐47. [DOI] [PubMed] [Google Scholar]
Yudkin 2010
- Yudkin JS, Richter B, Gale EA. Intensified glucose lowering in type 2 diabetes: time for a reappraisal. Diabetologia 2010;53(10):2079‐85. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta‐analyses: a meta‐epidemiological study. Critical Care 2013;17(1):R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoungas 2010
- Zoungas S, Patel A, Chalmers J, Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. The New England Journal of Medicine 2010;363(15):1410‐8. [DOI] [PubMed] [Google Scholar]


