Abstract
Background
Bisphosphonates are specific inhibitors of osteoclastic activity and are used in the treatment of patients with multiple myeloma (MM). While bisphosphonates are shown to be effective in reducing vertebral fractures and pain, their role in improving overall survival (OS) remains unclear. This is an update of a Cochrane review first published in 2002 and previously updated in 2010 and 2012.
Objectives
To assess the evidence related to benefits and harms associated with use of various types of bisphosphonates (aminobisphosphonates versus non‐aminobisphosphonates) in the management of patients with MM. Our primary objective was to determine whether adding bisphosphonates to standard therapy in MM improves OS and progression‐free survival (PFS), and decreases skeletal‐related morbidity. Our secondary objectives were to determine the effects of bisphosphonates on pain, quality of life, incidence of hypercalcemia, incidence of bisphosphonate‐related gastrointestinal toxicities, osteonecrosis of jaw (ONJ) and hypocalcemia.
Search methods
We searched MEDLINE, Embase (September 2011 to July 2017) and the CENTRAL (2017, Issue 7) to identify all randomized controlled trial (RCT) in MM up to July 2017 using a combination of text and MeSH terms.
Selection criteria
Any randomized controlled trial (RCT) comparing bisphosphonates versus placebo/no treatment/bisphosphonates and observational studies or case reports examining bisphosphonate‐related ONJ in patients with MM were eligible for inclusion.
Data collection and analysis
Two review authors extracted the data. Data were pooled and reported as hazard ratio (HR) or risk ratio (RR) using a random‐effects model. We used meta‐regression to explore statistical heterogeneity. Network meta‐analysis using Bayesian approach was conducted.
Main results
In this update, we included four new studies (601 participants), resulting in a total of 24 included studies.
Twenty RCTs compared bisphosphonates with either placebo or no treatment and four RCTs involved another bisphosphonate as a comparator. The 24 included RCTs enrolled 7293 participants. Pooled results showed that there was moderate‐quality evidence of a reduction in mortality with on OS from 41% to 31%, but the confidence interval is consistent with a larger reduction and small increase in mortality compared with placebo or no treatment (HR 0.90, 95% CI 0.76 to 1.07; 14 studies; 2706 participants). There was substantial heterogeneity among the included RCTs (I2 = 65%) for OS. To explain this heterogeneity we performed a meta‐regression assessing the relationship between bisphosphonate potency and improvement in OS, which found an OS benefit with zoledronate but limited evidence of an effect on PFS. This provided a further rationale for performing a network meta‐analyses of the various types of bisphosphonates that were not compared head‐to‐head in RCTs. Results from network meta‐analyses showed evidence of a benefit for OS with zoledronate compared with etidronate (HR 0.56, 95% CI 0.29 to 0.87) and placebo (HR 0.67, 95% CI 0.46 to 0.91). However, there was no evidence for a difference between zoledronate and other bisphosphonates.
The effect of bisphosphonates on disease progression (PFS) is uncertain. Based on the HR of 0.75 (95% CI 0.57 to 1.00; seven studies; 908 participants), 47% participants would experience disease progression without treatment compared with between 30% and 47% with bisphosphonates (low‐quality evidence). There is probably a similar risk of non‐vertebral fractures between treatment groups (RR 1.03, 95% CI 0.68 to 1.56; six studies; 1389 participants; moderate‐quality evidence). Pooled analysis demonstrated evidence for a difference favoring bisphosphonates compared with placebo or no treatment on prevention of pathological vertebral fractures (RR 0.74, 95% CI 0.62 to 0.89; seven studies; 1116 participants; moderate‐quality evidence) and skeletal‐related events (SREs) (RR 0.74, 95% CI 0.63 to 0.88; 10 studies; 2141 participants; moderate‐quality evidence). The evidence for less pain with bisphosphonates was of very low quality (RR 0.75, 95% CI 0.60 to 0.95; eight studies; 1281 participants).
Bisphosphonates may increase ONJ compared with placebo but the confidence interval is very wide (RR 4.61, 95% CI 0.99 to 21.35; P = 0.05; six studies; 1284 participants; low‐quality evidence). The results from the network meta‐analysis did not show any evidence for a difference in the incidence of ONJ (eight RCTs, 3746 participants) between bisphosphonates. Data from nine observational studies (1400 participants) reported an incidence of 5% to 51% with combination of pamidronate and zoledronate, 3% to 11% with zoledronate alone, and 0% to 18% with pamidronate alone.
The pooled results showed no evidence for a difference in increase in frequency of gastrointestinal symptoms with the use of bisphosphonates compared with placebo or no treatment (RR 1.23, 95% CI 0.95 to 1.59; seven studies; 1829 participants; low‐quality evidence).The pooled results showed no evidence for a difference in increase in frequency of hypocalcemia with the use of bisphosphonates compared with placebo or no treatment (RR 2.19, 95% CI 0.49 to 9.74; three studies; 1090 participants; low‐quality evidence). The results from network meta‐analysis did not show any evidence for differences in the incidence of hypocalcemia, renal dysfunction and gastrointestinal toxicity between the bisphosphonates used.
Authors' conclusions
Use of bisphosphonates in participants with MM reduces pathological vertebral fractures, SREs and pain. Bisphosphonates were associated with an increased risk of developing ONJ. For every 1000 participants treated with bisphosphonates, about one patient will suffer from the ONJ. We found no evidence of superiority of any specific aminobisphosphonate (zoledronate, pamidronate or ibandronate) or non‐aminobisphosphonate (etidronate or clodronate) for any outcome. However, zoledronate was found to be better than placebo and first‐generation bisposphonate (etidronate) in pooled direct and indirect analyses for improving OS and other outcomes such as vertebral fractures. Direct head‐to‐head trials of the second‐generation bisphosphonates are needed to settle the issue if zoledronate is truly the most efficacious bisphosphonate currently used in practice.
Plain language summary
Bisphosphonates in multiple myeloma
Review question: What is the effect of bisphosphonates if added to the existing treatments for multiple myeloma?
Background: Multiple myeloma (also known as myeloma or plasma cell myeloma) is a B‐cell malignancy or, more precisely, plasma cell neoplasm. This cancer grows inside or outside of bones. The bone damage, or osteolytic lesions, may lead to fractures of the long bones or compression fractures in the spine. The mechanism of bone destruction appears to be related to increased bone resorption by cells called osteoclasts. Bisphosphonates are drugs that can inhibit bone resorption by reducing the number and activity of osteoclasts.
Search date: The evidence is current to July 2017.
Study characteristics: This is an updated review of 24 trials enrolling 7293 participants. Twenty randomized controlled trials compared bisphosphonates with either placebo or no treatment and four randomized controlled trials involved another bisphosphonate as a comparator.
Key results: Use of bisphosphonates in participants with multiple myeloma did not improve overall survival or disease progression‐free survival. Use of bisphosphonates in participants with multiple myeloma reduces overall fractures, fractures of the vertebra but not the non‐vertebral fractures. Bisphosphonates also alleviates pain without many side effects except a significant increase in reduced blood flow to bones of the jaw resulting in decay of the bone also called osteonecrosis. Overall, for every 1000 participants treated with bisphosphonates, about one patient will suffer from the osteonecrosis of the jaw. Zoledronate was found to be better than etidronate and placebo, but not superior to pamidronate or clodronate for improving overall survival and other outcomes such as fractures in general or specifically fractures of vertebra. There was no evidence of superiority of any specific aminobisphosphonate (zoledronate, pamidronate or ibandronate) or non‐aminobisphosphonate (etidronate or clodronate) for any outcome.
Quality of evidence: The overall quality of evidence ranged from moderate to very low indicating the need for more research on this issue and specifically randomized controlled trials comparing different bisphosphonates directly instead of no treatment or placebo.
Summary of findings
Summary of findings for the main comparison. Summary of findings (direct comparisons).
| Bisphosphonates in multiple myeloma | |||||
|
Patient or population: patients with multiple myeloma
Intervention: bisphosphonates Control: no treatment/placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Bisphosphonates | ||||
| Overall survival## | Medium‐risk population# | HR 0.90 (0.76 to 1.07) | 2706 (14 studies) | ⊕⊕⊕⊝ moderate1,2,3 | |
| 410 per 1000 | 378 per 1000 (330 to 431) | ||||
| Progression‐free survival### | Medium‐risk population# | HR 0.75 (0.57 to 1.00) | 908 (7 studies) | ⊕⊕⊝⊝ low1,4,11 | |
| 470 per 1000 | 379 per 1000 (304 to 470) | ||||
| Vertebral fractures | Medium‐risk population# | RR 0.74 (0.62 to 0.89) | 1116 (7 studies) | ⊕⊕⊕⊝ moderate1,5 | |
| 360 per 1000 | 266 per 1000 (223 to 320) | ||||
| Non‐vertebral fractures | Medium‐risk population# | RR 1.03 (0.68 to 1.56) | 1389 (6 studies) | ⊕⊕⊕⊝ moderate1,6 | |
| 140 per 1000 | 144 per 1000 (95 to 218) | ||||
| Skeletal‐related events | Medium‐risk population# | RR 0.74 (0.63 to 0.88) | 2141 (10 studies) | ⊕⊕⊕⊝ moderate1,7 | |
| 400 per 1000 | 296 per 1000 (252 to 352) | ||||
| Pain | Medium‐risk population | RR 0.75 (0.60 to 0.95) | 1281 (8 studies) | ⊕⊝⊝⊝ very low8,9 | |
| 540 per 1000 | 410 per 1000 (329 to 508) | ||||
| Osteonecrosis of jaw | Medium‐risk population# |
RR 4.61 (0.99 to 21.35) |
1284 (6 studies) | ⊕⊕⊝⊝ low10,11 |
|
| NE | 0 per 1000 (0 to 2) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; NE: not estimable due to rarity of events in the control arm | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 A total of 20 RCTs were included in the direct meta‐analysis. Only 35% (7/20) of trials had adequate allocation concealment. Only 20% (4/20) of trials reported methods of randomization. Similarly, 15% (3/20) of trials reported blinding procedures and personnel who were blinded to the intervention assignment. However, sensitivity analyses based on the methodological quality domains did not change the estimates. Hence, the assessment of studies’ limitations may represent the poor quality of reporting rather than true biased estimates. 2 Downgraded the quality of evidence for the outcome of overall survival (OS) by one for the observed inconsistency (I2 = 65%). However we noticed that this heterogeneity in the pooled estimate is driven by studies by Aviles and colleagues (Aviles 2007; Aviles 2013); when we removed these RCTs heterogeneity disappeared. 3 Note that overall mortality data denotes the mortality rates, i.e. the number of events refers to the number of deaths. 4 Downgraded the quality of evidence by one level due to the potential for publication bias.The progression‐free survival data were extractable from only 35% (7/20) of studies eligible for direct meta‐analysis. 5 Downgraded the quality of evidence by one level due to the potential for publication bias. Data related to patients with vertebral fractures were extractable from only 35% (7/20) of studies eligible for direct meta‐analysis. 6 Downgraded the quality of evidence by one level due to the potential for publication bias. Data related to patients with non‐vertebral fractures were extractable from only 30% (6/20) of studies eligible for direct meta‐analysis. 7 Downgraded the quality of evidence by one level due to the potential for publication bias. Skeletal‐related events data were extractable from 50% (10/20) of studies. 8 Downgraded the quality of evidence by one level due to variation in assessment instruments.There was significant variation in the assessment methods used to measure pain.
9 Downgraded the quality of evidence by one level due to variation in assessment of pain based on blinding of the assessors. Only 15% (3/20) of trials reported blinding procedures and personnel who were blinded to the intervention assignment. Moreover, we found that RCTs with double‐blinding showed no significant benefit of bisphosphonates over placebo for amelioration of pain, while non‐blinded RCTs favored bisphosphonates over placebo for pain relief. We also downgraded the quality of evidence by one level due to imprecision.
10 Downgraded the quality of evidence by one level due to the potential for publication bias.The Osteonecrosis of jaw data were extractable from 30% (6/20) of studies eligible for direct meta‐analysis.
11 Downgraded the quality of evidence by one level due to imprecision.All included RCTs and also the pooled estimate have wide confidence intervals.
# The moderate control risk was calculated via GRADEpro software based on average risk in the control arm of the included studies.
## We have calculated and presented overall mortality instead of OS. The expected events represent a median timeline of 5 years.
### PFS events represent death or progress or relapse. The expected events represent a median timeline of 5 years.
Background
Description of the condition
Multiple myeloma (MM) is characterized by neoplastic proliferation of plasma cells, mainly contained within the bone marrow. It is a debilitating malignancy that is a part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance to plasma cell leukemia (Anderson 2015; Tricot 2000). MM can present outside the bone marrow as a solitary plasmacytoma or extramedullary plasmacytoma. MM is more prevalent after the age of 40 years. A diagnosis of symptomatic myeloma requires the presence of monoclonal protein (M‐protein) in serum, urine, or both; bone marrow clonal plasma cells (> 10%) or plasmacytoma; and related organ or tissue impairment) (Anderson 2015; Greipp 2005). Ninety‐seven per cent of people with MM have M‐protein present in serum, urine, or both. A diagnosis of asymptomatic myeloma (also known as smoldering myeloma) requires the presence of M‐protein in serum of 30 g/L or more and bone marrow clonal plasma cells of 10%, and no related organ or tissue impairment or symptoms (Anderson 2015). A mnemonic for end‐organ damage, which is the hallmark of MM, is CRAB, for hypercalcemia, renal insufficiency,anemia and lytic bone lesions. The most common symptoms of MM are those related to anemia, renal dysfunction, infections and bone lesions. In the majority of patients, slow and steady progressive bone damage (osteolytic lesions) caused by myeloma may lead to fractures of the long bones or compression fractures in the spine. Bone pain is often a symptom of this disease, especially in the form of severe back pain.
Description of the intervention
Bisphosphonates are used in the management of MM as supportive therapy to inhibit progression of osteoclastic activity and affect skeletal‐related morbidity and mortality secondary to this process. Several randomized trials (Description of studies) have been conducted investigating the use of bisphosphonates in MM. Etidronate was the first bisphosphonate tested in a clinical setting, but with no apparent benefit (Belch 1991). Pamidronate, a second‐generation bisphosphonate, demonstrated a significant clinical effect on the rate of skeletal‐related events (SREs) and pain control in a double‐blind, placebo‐included randomized controlled trial (RCT) (Berenson 1998). This study also suggested a trend toward an increase in survival with pamidronate in a subgroup of participants. Similarly, RCTs comparing zoledronate with no therapy showed a survival benefit with zoledronate (Aviles 2007; Aviles 2013), and more recently an RCT comparing zoledronate with clodronate showed survival benefit with zoledronate as well (Morgan 2010), indicating the need for the updating of previous reviews (Mhaskar 2010; Mhaskar 2012). In another RCT, the proportion of participants who experienced a progression of lytic lesions was smaller in the clodronate‐treated group than in the placebo group (Lahtinen 1992). However, no significant effect on survival was seen. In a German open‐label study, there was a trend toward reduction in the number of new bone lesions in the clodronate‐treated group (Heim 1995). Again, no significant effect on survival was seen. Moreover, our previous systematic reviews published in 2010 and 2012 (Mhaskar 2010; Mhaskar 2012) found that adding bisphosphonates to the treatment of myeloma reduces pathological vertebral fractures and pain but—from the published evidence available then—not mortality.
All bisphosphonates are poorly absorbed after oral administration, but effective plasma levels can be achieved with clodronate. Aminobisphosphonates such as pamidronate have caused gastrointestinal (GI) ulceration when given orally (Lufkin 1994). The other adverse effects associated with the use of bisphosphonates typically consist of renal functional impairment, myalgias and hypocalcemia. Osteonecrosis of the jaw (ONJ) has been described as a serious new complication associated with bisphosphonates (Bagan 2006; Durie 2005; Ruggiero 2004). Bisphosphonate‐associated ONJ has been described in various malignancies, including MM, breast cancer and prostate cancer, and can be a debilitating problem associated with significant morbidity.
How the intervention might work
Bisphosphonates are specific inhibitors of osteoclastic activity (Berenson 1998b). In addition, some studies in vitro suggest an additional antitumor effect of bisphosphonates (Aparicio 1998; Shipman 1997). Therefore, there exists a pharmacological rationale for the use of these agents in MM. Bisphosphonates are a heterogeneous group of molecules that resemble pyrophosphates that are used in technical chemistry for calcium binding. The bisphosphonate core structure is formed by two phosphonate groups attached to a single carbon atom (the so called P‐C‐P structure). In contrast to pyrophosphates, bisphosphonates are stable in biological environments. There are many types of bisphosphonates. Alendronate, risedronate, ibandronate, pamidronate and zoledronate, termed aminobisphosphonates (Figure 1), are bisphosphonates containing nitrogen in one of the side chains. These nitrogen‐containing bisphosphonates inhibit the mevalonate pathway (the main target being farnesyl diphosphate synthase). Clodronate, etidronate and tiludronate, termed non‐aminobisphosphonates (Figure 1), do not contain nitrogen and are incorporated into hydrolytically stable analogs of adenosine triphosphate. Both events cause impairment of osteoclast cell function and, ultimately, lead to osteoclast apoptosis (Brown 2004). The pathogenesis of osteoclast bone resorption may also be understood to be the result of abnormal cytokine signaling between malignant plasma cells, osteoclasts and osteoblasts. Increased levels of RANK ligand produced by myeloma cells and marrow stromal cells, coupled with suppression of soluble osteoprotegerin, favors osteoclast bone resorption (Cassidy 2006). Other cytokines such as interleukin‐6 further support an excess of osteoclast activity (Cassidy 2006). In summary, bisphosphonates are broadly classified into two categories (amino‐ and non‐aminobisphosphonates) based on their chemical structure and molecular mechanism of action. Aminobisphosphonates are considered to be more potent than non‐aminobisphosphonates. Based on in vitro data, zoledronate is considered the most potent and etidronate the least potent among bisphosphonates (Drake 2008; Dunford 2001) (Table 2).
1.

Bisphosphonate chemical structures
1. Bisphosphonate potency.
| Type of bisphosphonates | Bisphosphontes | Relative potency |
| Nonaminobisphosphonates | Etidronate | 1 |
| Clodronate | 10 | |
| Tiludronate | 10 | |
| Aminobisphosphonates | Pamidronate | 100 |
| Alendronate | 500 | |
| Ibandronate | 1,000 | |
| Risendronate | 2,000 | |
| Zoledronate | 10,000 |
Based on information from (Drake 2008; Dunford 2001).
Why it is important to do this review
This is an update of our previous systematic review addressing the uncertainty regarding the role of bisphosphonates in the management of MM (Mhaskar 2012). While we found no direct effect of bisphosphonates on overall survival (OS) compared with placebo or no treatment, there was statistically significant heterogeneity for the outcome of OS among the included RCTs (Mhaskar 2012). However, since our last review, a new study claiming OS benefit (Aviles 2013) and additional data from a study (Morgan 2010) that was included in our 2012 systematic review have been published. This created the impetus to update our previous review to assess the evidence related to benefits and harms associated with the use of various types of bisphosphonates (aminobisphosphonates versus non‐aminobisphosphonates) in the management of patients with MM.
Objectives
Our primary objective was to determine whether adding bisphosphonates to standard therapy in MM decreases skeletal‐related morbidity (pathological fractures) and improves overall survival (OS) and progression‐free survival (PFS).
Our secondary objectives were to determine the effects of bisphosphonates on pain, quality of life, incidence of hypercalcemia, incidence of bisphosphonate‐related GI toxicities, ONJ and hypocalcemia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in which interventions consisted of bisphosphonates versus placebo or no treatment or other bisphosphonates in patients with MM.
We excluded studies that used other agents to affect skeletal‐related morbidity or mortality (e.g. fluoride), duplicate reports and those studies that reported subgroup analyses from larger RCTs. In the case of duplicate reports, we extracted data from the articles published at later dates. We also excluded studies that included participants with an underlying disease other than MM and studies that reported insufficient data, as well as studies with fewer than 10 participants.
We also included observational studies and case reports regarding bisphosphonate‐related osteonecrosis of the jaw (ONJ).
Types of participants
Participants with the diagnosis of MM as defined by the researchers in each study. We found no uniform criteria for the diagnosis (Alexanian 1994; Anderson 2015) among the studies selected for this systematic review. However, all studies required biopsy‐proven myeloma as the diagnostic criterion and bone involvement that met criteria for administration of bisphosphonates according to the studies' investigators.
Types of interventions
Experimental group: treatment included any bisphosphonate
Control group: no therapy, placebo or other bisphosphonates
Types of outcome measures
We extracted data on the following outcomes.
Primary outcomes
Overall survival (OS) (measured as mortality)
Progression‐free survival (PFS)
Skeletal‐related events (SRE): number of participants experiencing pathological fractures (vertebral and non‐vertebral), total skeletal‐related events (SREs) (as defined by individual authors; these included vertebral fractures, non‐vertebral fractures and osteolytic lesions)
Secondary outcomes
Number of participants with pain relief (as defined by individual authors)
Incidence of hypercalcemia (defined as ≥ 2.65 mmol/L)
Adverse events (grade III/IV)
Quality of life (as defined by individual authors)
Search methods for identification of studies
This is an update of the review published in 2012 (Mhaskar 2012). We searched the electronic databases from September 01 2011 onwards up to 17 July 2017.
Electronic searches
We identified all RCTs in MM in the following databases:
MEDLINE (2011/09/01 to July 2017) (see Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2017, Issue 7) (see Appendix 2);
ClinicalTrials.gov (search date: July 2017) (see Appendix 3);
Embase (2011/09/01 to July 2017) (see Appendix 4).
We also identified observational studies and case reports regarding bisphosphonate‐related ONJ in the following database:
MEDLINE (2011/09/01 to July 2017) (see Appendix 1).
Searching other resources
We searched the American Society of Hematology (date of search 5 February 2016) EudraCT, ISRCTN (date of search 24 May, 2016) and WHO registry (date of search 21 September, 2017).
Contacting authors: Where a study contained unclear information, we contacted the authors to ensure accuracy. This occurred in one instance, but the email listed on the publication was not a valid email address. Hence we were not able to contact the author (Aviles 2013).
Data collection and analysis
Selection of studies
Review authors RM and AK independently scanned the retrieved titles and abstracts of all studies for their eligibility for inclusion in this systematic review. If a decision on inclusion was not made on the basis of the review of the title and abstract, we obtained the full text of the article to assess eligibility. Disagreements in the selection of studies were resolved by consensus (Higgins 2011a). At every stage of searching and screening, the overall numbers of studies identified, excluded and included with reasons, were documented according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (Liberati 2009). We used this information to create a flow diagram (Figure 2).
Data extraction and management
For this update two review authors (RM and AK) extracted all data and resolved disagreements by consensus. After the extraction, a third review author (BM) rechecked all data. The outcomes extracted are listed above. Data regarding the methods of trial conduct and design were also extracted. Specifically, we extracted data regarding methods of allocation concealment, method of randomization, adequacy of blinding procedures (who was blinded: participants, investigators, data analysts, etc), description of withdrawals and dropouts, and method of data analysis (intention‐to‐treat (ITT)/per protocol). To determine whether the analysis was performed according to the ITT principle, data were extracted and matched on the numbers of participants randomized and analyzed. If the numbers of participants randomized and analyzed were the same, we considered the analysis as ITT.
We also extracted details regarding drug, dose, average length of treatment, length of follow‐up, number of randomized participants, number of participants excluded from the analysis, OS and PFS, presence of pain, level of calcium and adverse events. Unfortunately, we were not able to extract all data from all papers (see Table 3; ).
2. Type and content of reporting in RCTs on bisphosphonates in myeloma.
| Study ID |
Adverse events (gastrointestinal symptoms) |
Adverse events (hypocalcemia) |
Adverse events (serum creatinine) |
Adverse events (osteonecrosis of the jaw) |
| Belch 1991 | No | No | No | No |
| Berenson 1998a | Yes | Yes | No | No |
| Brincker 1998 | Yes | No | No | No |
| Delmas 1982 | No | No | No | No |
| Daragon 1993 | Yes | No | Yes | No |
| Heim 1995 | No | No | No | No |
| Lahtinen 1992 | Yes | No | Yes | No |
| McCloskey 2001 | Yes | Yes | No | No |
| Terpos 2000 | Yes | No | No | No |
| Terpos 2003 | No | Yes | No | No |
| Kraj 2000 | No | No | No | No |
| Attal 2006 | No | No | No | Yes |
| Musto 2003 | No | No | No | Yes |
| Musto 2008 | No | No | No | Yes |
| Aviles 2007 | No | No | No | No |
| Menssen 2002 | No | No | No | No |
| Leng 2002 | No | No | No | No |
| Morgan 2010 | Yes | No | No | Yes |
| Rosen 2003 | No | No | No | No |
| Gimsing 2010 | No | No | No | Yes |
| Aviles 2013 | No | No | No | Yes |
| Sezer 2010 | Yes | No | No | Yes |
| Zhang 2012 | No | No | No | No |
| Garcia‐Sanz 2015 | No | No | No | Yes |
Assessment of risk of bias in included studies
Two review authors (RM and AK) independently assessed all eligible studies for their risk of bias (assessment of methodological quality) using methods suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We used the extracted data to assess the methodological quality (risk of bias and random error) of each trial. The review authors judged each quality domain based on the following three‐point scale.
'Yes' (low risk of bias: plausible bias unlikely to seriously alter the results if all criteria were met)
'No' (high risk of bias: plausible bias that seriously weakens confidence in the results if one or more criteria were not met)
'Unclear' (uncertain risk of bias: plausible bias that raises some doubt about the results if one or more criteria were assessed as unclear)
The risk of bias domain included the following: selection bias, performance bias, detection bias and other bias.
The quality items related to risk of random error included details about the power of the study (beta error), predetermination of alpha error and a priori estimation of sample size.
Measures of treatment effect
For time‐to‐event outcome: data were summarized as hazard ratio (HR) and 95% confidence intervals (CIs).
For dichotomous outcome: data were measured as risk ratio (RR) with 95% CIs.
For continuous outcome: data were summarized as mean difference and standard deviation.
Unit of analysis issues
The unit of analysis was a study from which we extracted the aggregate data as follows: for dichotomous variables, the number of participants in the intervention arm and the number of participants in the control arm. For continuous outcomes, we extracted the mean, standard deviation, and the number of participants in the intervention and control arm. For time‐to‐event outcomes, we extracted log HR and the standard error of log HR.
Dealing with missing data
We did not conduct analyses for the missing data.
Assessment of heterogeneity
We calculated the Chi2 and I2 statistics to test for heterogeneity. We assessed the degree of heterogeneity among trials and between subgroups using the I² statistic. We used the following guide to interpret the I² statistic: I² = 0% to 40%(heterogeneity that might not be important), I² = 30% to 60% (moderate heterogeneity), I² = 50% to 90% (substantial heterogeneity), I² = 75% to 100% (considerable heterogeneity) (Deeks 2011).
Assessment of reporting biases
We investigated the possibility of publication bias using the funnel plot method of Begg and Mazumdar (Begg 1994) and Egger and colleagues (Egger 2001) as outlined in chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
Data synthesis
Direct comparison of treatment effects (bisphosphonates versus placebo or no treatment)
We summarized dichotomous data using RR and pooled these data using a random‐effects model in Review Manager 5.3 (RevMan) (RevMan 5.3). In cases of time‐to‐event data, for each included RCT, we calculated the observed minus expected events and variance from the reported mortality estimates to calculate HR. In cases where the authors did not report the mortality estimates, we extracted data from papers using methods described by Tierney and colleagues (Tierney 2007). We pooled the time‐to‐event data using the random‐effects model (DerSimonian 1986) in Revman 5.3 (RevMan 5.3) to calculate overall HRs. We also calculated the number of participants who needed to be treated to avoid one adverse outcome and number of participants who were treated to cause an additional harm (Laupacis 1988) to express treatment benefits and harms, respectively, in the context of the estimated absolute risks in the control arms. All data are reported with 95% CIs.
Indirect comparison of treatment effects
For this update, we used the Bayesian method outlined by Lu and colleagues (Lu 2004) to perform the indirect meta‐analyses.
We used the Bayesian methods under random‐effects multiple treatment comparisons (MTC) for indirect comparisons (Lu 2004; Higgins 1996). The random‐effects model assumes homogeneous between‐studies variance. We derived posterior estimates for Bayesian methods using Gibbs sampling via Markov chain Monte Carlo simulation in WinBUGS (version 1.4). All means were given a vague prior distribution (normal distribution with mean 0 and sufficiently large variance). We report the HR or RR estimates and credibility intervals based on Bayesian methods (Table 4). In the presence of loops, the consistency of the network was assessed using methods described by Dias and colleagues (Dias 2010). We followed guidelines suggested by Salanti and colleagues (Salanti 2011) for graphical presentations and numerical summaries of the multiple treatment meta‐analysis. For each comparison, we also derived ranking probability based on SUCRA (Surface Under the Cumulative Ranking Curve) (Chaimani 2013). A formal assessment of transitivity was not done. The assumption of transitivity was difficult to assess due to the lack of closed loops in the networks for different outcomes. However, since the distribution of treatment effects in direct comparison was in agreement with the results of the network meta‐analysis, we had no reason to believe the transitivity principle was violated (Salanti 2014). Additionally, small studies received less weight in our network meta‐analysis.
3. Indirect comparisons.
| MTC method (REM) | ||||||||
| Outcome | Treatment1 | Treatment2 | NRCTs | Patients | HR/RR | 95% LCRL | 95% UCRL | Quality of the evidence (GRADE) |
| OS | PL | CLO | 16 | 5260 | 1.19 | 0.88 | 1.63 | ⊕⊕⊕⊝ moderate |
| OS | ETI | CLO | 16 | 5260 | 1.48 | 0.96 | 2.51 | ⊕⊕⊕⊝ moderate |
| OS | IBAN | CLO | 16 | 5260 | 1.34 | 0.60 | 2.62 | ⊕⊕⊕⊝ moderate |
| OS | PAM 90 mg | CLO | 16 | 5260 | 1.04 | 0.64 | 1.64 | ⊕⊕⊕⊝ moderate |
| OS | ZOL | CLO | 16 | 5260 | 0.78 | 0.52 | 1.14 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | CLO | 16 | 5260 | 1.04 | 0.48 | 2.09 | ⊕⊕⊝⊝ low* |
| OS | ETI | PL | 16 | 5260 | 1.25 | 0.88 | 1.95 | ⊕⊕⊝⊝ low* |
| OS | IBAN | PL | 16 | 5260 | 1.13 | 0.54 | 2.06 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | PL | 16 | 5260 | 0.87 | 0.60 | 1.23 | ⊕⊕⊕⊝ moderate |
| OS | ZOL | PL | 16 | 5260 | 0.67 | 0.46 | 0.91 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | PL | 16 | 5260 | 0.87 | 0.44 | 1.64 | ⊕⊕⊝⊝ low* |
| OS | IBAN | ETI | 16 | 5260 | 0.94 | 0.37 | 1.80 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | ETI | 16 | 5260 | 0.73 | 0.38 | 1.14 | ⊕⊕⊝⊝ low* |
| OS | ZOL | ETI | 16 | 5260 | 0.56 | 0.29 | 0.87 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | ETI | 16 | 5260 | 0.72 | 0.30 | 1.40 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | IBAN | 16 | 5260 | 0.87 | 0.39 | 1.74 | ⊕⊕⊝⊝ low* |
| OS | ZOL | IBAN | 16 | 5260 | 0.67 | 0.29 | 1.31 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | IBAN | 16 | 5260 | 0.87 | 0.32 | 2.06 | ⊕⊕⊝⊝ low* |
| OS | ZOL | PAM 90 mg | 16 | 5260 | 0.79 | 0.46 | 1.26 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | PAM 90 mg | 16 | 5260 | 1.00 | 0.57 | 1.74 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | ZOL | 16 | 5260 | 1.35 | 0.62 | 2.76 | ⊕⊕⊝⊝ low* |
| PFS | PL | PAM 90 mg | 9 | 3472 | 0.84 | 0.30 | 1.88 | ⊕⊝⊝⊝ very low *^ |
| PFS | ZOL | PAM 90 mg | 9 | 3472 | 0.59 | 0.20 | 1.39 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | PAM 90 mg | 9 | 3472 | 0.66 | 0.16 | 1.71 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | PAM 90 mg | 9 | 3472 | 1.04 | 0.38 | 2.16 | ⊕⊝⊝⊝ very low *^ |
| PFS | ZOL | PL | 9 | 3472 | 0.70 | 0.46 | 1.03 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | PL | 9 | 3472 | 0.77 | 0.30 | 1.47 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | PL | 9 | 3472 | 1.55 | 0.34 | 4.29 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | ZOL | 9 | 3472 | 1.10 | 0.45 | 1.95 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | ZOL | 9 | 3472 | 2.30 | 0.45 | 6.78 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | CLO | 9 | 3472 | 2.38 | 0.43 | 8.15 | ⊕⊝⊝⊝ very low *^ |
| SREs | PL | CLO | 13 | 5727 | 1.27 | 0.81 | 1.84 | ⊕⊕⊝⊝ low* |
| SREs | ETI | CLO | 13 | 5727 | 1.01 | 0.37 | 2.20 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | CLO | 13 | 5727 | 0.90 | 0.51 | 1.38 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | CLO | 13 | 5727 | 1.37 | 0.68 | 2.55 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | CLO | 13 | 5727 | 0.72 | 0.41 | 1.02 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | CLO | 13 | 5727 | 0.89 | 0.44 | 1.62 | ⊕⊕⊝⊝ low* |
| SREs | ETI | PL | 13 | 5727 | 0.79 | 0.33 | 1.61 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | PL | 13 | 5727 | 0.71 | 0.49 | 0.96 | ⊕⊕⊕⊝ moderate |
| SREs | IBAN | PL | 13 | 5727 | 1.08 | 0.60 | 1.86 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | PL | 13 | 5727 | 0.57 | 0.37 | 0.76 | ⊕⊕⊕⊝ moderate |
| SREs | PAM 30 mg | PL | 13 | 5727 | 0.71 | 0.38 | 1.23 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | ETI | 13 | 5727 | 1.06 | 0.40 | 2.25 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | ETI | 13 | 5727 | 1.61 | 0.55 | 3.79 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | ETI | 13 | 5727 | 0.84 | 0.31 | 1.76 | ⊕⊕⊝⊝ low* |
| SREs | PAM30mg | ETI | 13 | 5727 | 1.06 | 0.35 | 2.57 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | PAM 90 mg | 13 | 5727 | 1.56 | 0.80 | 2.90 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | PAM 90 mg | 13 | 5727 | 0.81 | 0.52 | 1.14 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | PAM 90 mg | 13 | 5727 | 1.00 | 0.60 | 1.70 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | IBAN | 13 | 5727 | 0.56 | 0.26 | 0.98 | ⊕⊕⊕⊝ moderate |
| SREs | PAM 90 mg | IBAN | 13 | 5727 | 0.70 | 0.29 | 1.44 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | ZOL | 13 | 5727 | 1.28 | 0.68 | 2.51 | ⊕⊕⊝⊝ low* |
| Pain | ETI | CLO | 8 | 1281 | 2.15 | 0.22 | 9.56 | ⊕⊝⊝⊝ very low *^ |
| Pain | IBAN | CLO | 8 | 1281 | 4.13 | 0.57 | 16.99 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | CLO | 8 | 1281 | 1.76 | 0.57 | 16.99 | ⊕⊝⊝⊝ very low *^ |
| Pain | IBAN | ETI | 8 | 1281 | 4.07 | 0.23 | 19.62 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | ETI | 8 | 1281 | 1.75 | 0.11 | 7.64 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | IBAN | 8 | 1281 | 0.75 | 0.06 | 3 | ⊕⊝⊝⊝ very low *^ |
| Vertebral fractures | PL | CLO | 8 | 3076 | 1.50 | 0.87 | 2.62 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | IBAN | CLO | 8 | 3076 | 1.76 | 0.56 | 4.45 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | PAM 90 mg | CLO | 8 | 3076 | 1.07 | 0.45 | 2.07 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | CLO | 8 | 3076 | 0.59 | 0.22 | 1.17 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | IBAN | PL | 8 | 3076 | 1.16 | 0.41 | 2.56 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | PAM90mg | PL | 8 | 3076 | 0.72 | 0.35 | 1.18 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | PL | 8 | 3076 | 0.42 | 0.12 | 0.94 | ⊕⊕⊕⊝ moderate |
| Vertebral fractures | PAM90mg | IBAN | 8 | 3076 | 0.76 | 0.21 | 1.91 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | IBAN | 8 | 3076 | 0.45 | 0.08 | 1.29 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | PAM 90 mg | 8 | 3076 | 0.64 | 0.17 | 1.68 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | PL | CLO | 7 | 3349 | 1.47 | 0.65 | 3.10 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | IBAN | CLO | 7 | 3349 | 2.13 | 0.44 | 7.20 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | PAM 90 mg | CLO | 7 | 3349 | 3.17 | 0.52 | 10.88 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | ZOL | CLO | 7 | 3349 | 0.82 | 0.24 | 2.32 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | IBAN | PL | 7 | 3349 | 1.46 | 0.40 | 3.98 | ⊕⊕⊝⊝ low* |
| Non vertebral fractures | PAM 90 mg | PL | 7 | 3349 | 2.01 | 0.46 | 6.32 | ⊕⊕⊝⊝ low* |
| Non vertebral fractures | ZOL | PL | 7 | 3349 | 0.66 | 0.13 | 2.30 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | PAM 90 mg | IBAN | 7 | 3349 | 1.98 | 0.25 | 7.66 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | ZOL | IBAN | 7 | 3349 | 0.64 | 0.07 | 2.82 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | ZOL | PAM 90 mg | 7 | 3349 | 0.49 | 0.04 | 2.14 | ⊕⊕⊝⊝ low* |
| Hypercalcemia | PL | CLO | 11 | 4146 | 1.64 | 0.71 | 3.58 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ETI | CLO | 11 | 4146 | 2.59 | 0.51 | 8.80 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | CLO | 11 | 4146 | 1.27 | 0.20 | 4.53 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM90mg | CLO | 11 | 4146 | 1.14 | 0.32 | 3.05 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | CLO | 11 | 4146 | 1.04 | 0.32 | 2.47 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ETI | PL | 11 | 4146 | 1.55 | 0.40 | 4.27 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | PL | 11 | 4146 | 0.76 | 0.16 | 2.27 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | PL | 11 | 4146 | 0.70 | 0.26 | 1.40 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | PL | 11 | 4146 | 0.73 | 0.16 | 1.92 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | ETI | 11 | 4146 | 0.68 | 0.08 | 2.53 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | ETI | 11 | 4146 | 0.62 | 0.11 | 1.92 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | ETI | 11 | 4146 | 0.65 | 0.08 | 2.28 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | IBAN | 11 | 4146 | 1.42 | 0.22 | 4.79 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | IBAN | 11 | 4146 | 1.50 | 0.15 | 5.74 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | PAM 90 mg | 11 | 4146 | 1.23 | 0.21 | 4.05 | ⊕⊝⊝⊝ very low *$ |
| GIToxicity | PL | CLO | 8 | 3789 | 0.87 | 0.45 | 1.49 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ETI | CLO | 8 | 3789 | 1.14 | 0.01 | 7.59 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | CLO | 8 | 3789 | 1.18 | 0.45 | 2.49 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | CLO | 8 | 3789 | 0.86 | 0.35 | 1.74 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ETI | PL | 8 | 3789 | 1.32 | 0.01 | 8.73 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | PL | 8 | 3789 | 1.36 | 0.69 | 2.39 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | PL | 8 | 3789 | 1.07 | 0.38 | 2.39 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | ETI | 8 | 3789 | 15.96 | 0.14 | 102.19 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | ETI | 8 | 3789 | 12.63 | 0.10 | 81.10 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | PAM 90 mg | 8 | 3789 | 0.86 | 0.24 | 2.27 | ⊕⊕⊝⊝ low* $$ |
| ONJ | PL | PAM 90 mg | 8 | 3746 | 1.10 | 0.04 | 5.99 | ⊕⊝⊝⊝ very low *^ |
| ONJ | ZOL | PAM 90 mg | 8 | 3746 | 6.19 | 0.09 | 38.16 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | PAM 90 mg | 8 | 3746 | 0.77 | 0.01 | 4.94 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | PAM 90 mg | 8 | 3746 | 0.44 | 0.05 | 1.83 | ⊕⊝⊝⊝ very low *^ |
| ONJ | ZOL | PL | 8 | 3746 | 5.70 | 0.72 | 21.26 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | PL | 8 | 3746 | 0.71 | 0.04 | 3.43 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | PL | 8 | 3746 | 2.19 | 0.03 | 13.55 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | ZOL | 8 | 3746 | 0.13 | 0.02 | 0.44 | ⊕⊝⊝⊝ very low ** |
| ONJ | PAM 30 mg | ZOL | 8 | 3746 | 0.77 | 0.00 | 5.09 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | CLO | 8 | 3746 | 11.14 | 0.04 | 76.15 | ⊕⊝⊝⊝ very low *^ |
REM: Random effects model, for multiple treatment comparison method: sigma˜Unif(0,1); ONJ: osteonecrosis of the jaw; PL: Placebo; #RCTS: Number of randomized controlled trials; LCRL: Lower credibility limit; UCRL: Upper credibility limit; OS: Overall survival; PFS: Progression‐free survival; SREs: Skeletal‐related events; HR: Hazard ratio; RR: Risk ratio; ETI: Etidronate; CLO: Clodronate; PAM 90 mg: Pamidronate 90 mg: PAM 30 mg: Pamidronate 30 mg; IBAN: Ibandronate: ZOL: Zoledronate; *Randomized controlled trial with direct (head‐to‐head) comparison of zoledronate versus clodronate (Morgan 2010).* Imprecision; ^ Contributing direct evidence of low quality;$ For the contributing direct evidence, the pooled estimate along with individual studies have wide confidence intervals. Therefore, we downgraded the quality of evidence by two levels resulting in low quality evidence. $$ For the contributing direct evidence, individual studies have wide confidence intervals. Therefore, we downgraded the quality of evidence by one level resulting in moderate quality evidence.**The results from head to head RCT comparing zoledronate with clodronate showed no difference in risk of ONJ (Morgan 2010). However, the results from network meta‐analysis showed an increased risk of ONJ with zoledronate over clodronate which is indicative of incoherence.
We performed and reported the work according to PRISMA guidelines (Cornell 2015; Liberati 2009). We created a 'Summary of findings' table using the GRADE software for direct comparisons (Balshem 2011; GRADEpro 2008; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e) and network meta‐analysis (Puhan 2014) separately.
Subgroup analysis and investigation of heterogeneity
Apart from sensitivity analyses, we conducted subgroup analyses based on duration of treatment. We assessed the differences between the subgroups using the test of heterogeneity between subgroups in RevMan 5.3 (RevMan 5.3). In the presence of relevant heterogeneity, we used meta‐regression to estimate the extent to which covariates explain the treatment effects (Thompson 2002). Meta‐regression was performed with the Knapp‐Hartung modification, which is more sensitive to false‐positive rates than the normal approximation estimates (Higgins 2004) using STATA statistical analysis software (STATA V10.1). Meta‐regression was performed using only one covariate of bisphosphonate potency and has been reported (not adjusted for any other variables). We used potency as a covariate and expressed the association as a slope of (1‐Hazard Ratio) per 1000 unit increase in potency.
Sensitivity analysis
We conducted sensitivity analyses according to methodological quality dimensions to assess the existence of a potential bias in our results (Jüni 2001). In particular, we focused on those dimensions that have been empirically linked to bias and random error on all outcomes.
Results
Description of studies
Results of the search
For this update, we searched the electronic databases from 1 September 2011 onwards until 17 July, 2017. The initial search identified 994 citations. We excluded 769 studies and reviewed the full text of 15 studies. Our initial search for observational studies reporting osteonecrosis of the jaw (ONJ) identified 39 citations. We excluded 35 citations and included four case reports/case series reporting ONJ (Gabbert 2015; Gander 2014; Watters 2013; Wickham 2013).
Of these, seven studies were found to be ineligible due to following reasons: two were non‐randomized studies (Chiang 2013; Teoh 2012), one was a cost‐effectiveness study (Delea 2012), three studies addressed the role of denosumab (Henry 2014; Lipton 2012; Vadhan‐Raj 2012), and in one study participants in both arms received the same dose of zoledronate with a hypothesis that treatment with thalidomide and zoledronate would prolong the time to progression to MM over alone (Witzig 2013). We identified four publications related to the study by Morgan and colleagues (Morgan 2010), which was included in the previous version. We included additional data from these four publications in this update and we added the citations of these publications under Morgan 2010. In summary, we identified and included three new RCTs (Aviles 2013; Sezer 2010; Zhang 2012) in this review (Figure 2). The study by Zhang and colleagues was published in English and Chinese. We reviewed both publications. However, none of the publications included outcomes that are relevant for our systematic review. One additional study was identified through personal communication with the Cochrane Hematological Malignancies group (Garcia‐Sanz 2015) and was not retrieved through the formal search.
2.

Study flowchart
We also found one ongoing open‐label multi‐center international RCT comparing zoledronic acid for four years versus stopping treatment with zoledronic acid after two years (Lund 2014).
Included studies
In this update, we included four new studies (Aviles 2013; Garcia‐Sanz 2015; Sezer 2010; Zhang 2012) that were not part of the previous published version (Mhaskar 2012), resulting in 20 RCTs comparing bisphosphonates with either placebo or no treatment and four RCTs with a different bisphosphonate as a comparator.
Two trials reported the effects of etidronate compared with placebo or no treatment (Belch 1991; Daragon 1993); seven trials reported the effects of pamidronate compared with placebo or no treatment (Attal 2006; Berenson 1998a; Brincker 1998; Kraj 2000; Leng 2002; Musto 2003; Terpos 2000); five trials reported the effects of clodronate compared with placebo or no treatment (Delmas 1982; Heim 1995; Lahtinen 1992; McCloskey 2001; Zhang 2012) and one trial described the effects of ibandronate compared with placebo (Menssen 2002). Five trials compared the effects of zoledronate versus no therapy in myeloma (Aviles 2007; Aviles 2013; Garcia‐Sanz 2015; Musto 2008; Sezer 2010).
We also included the following RCTs comparing pamidronate versus ibandronate (Terpos 2003), zoledronate versus pamidronate (Morgan 2010), 30 mg of pamidronate versus 90 mg of pamidronate (Gimsing 2010) and zoledronate versus pamidronate (Rosen 2003). In this update, we included four additional publications related to the study by Morgan and colleagues (Morgan 2010).
In total we included 24 RCTs in this systematic review (see details in Characteristics of included studies).
Excluded studies
The search conducted for our previous review excluded 16 trials (see details in Characteristics of excluded studies). One trial studied antitumor and bone metabolism effects and reported no outcomes of interest (Martin 2002). One was a duplicate report (Kraj 2000a); seven trials were not randomized (Ali 2001; Barlogie 2008; Bergner 2007; Morris 2001; Spencer 2008; Tassinari 2007; Vogel 2004). One trial with nine enrolled participants was too small to be included (Kraj 2002). Three studies had used combination therapy (Caparrotti 2003; Ciepluch 2002; Tosi 2006a). We also excluded two phase II RCTs that tested denosumab (Fizazi 2009; Vij 2009) and one prognostic study (Terpos 2010).
For this update, we excluded a further seven studies; two non‐randomized studies (Chiang 2013; Teoh 2012), one cost‐effectiveness study (Delea 2012), three studies addressing the role of denosumab (Henry 2014; Lipton 2012; Vadhan‐Raj 2012), and one study in which patients in both the arms received the same dose of zoledronate with a hypothesis that treatment with thalidomide and zoledronate would prolong the time to progression to MM over zoledronate alone (Witzig 2013).
In total, in this update we excluded 23 studies (see details in Characteristics of excluded studies).
Risk of bias in included studies
We have presented the results of the 'Risk of bias' assessment in Figure 3. The study by Sezer and colleagues was published as meeting abstract (Sezer 2010) and lacked the details needed for us to assess the methodological quality of this study. Hence we have extracted data from www.clinicaltrials.gov related to this study (Sezer 2010).
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Twenty‐nine per cent (7/24) of trials reported the method of generating the randomization sequence and are deemed as having a low risk of selection bias. Sixteen studies had a high risk of selection bias and one study had an unclear risk of selection bias.
Thirty‐seven percent (9/24) of trials had adequate allocation concealment and are deemed as having a low risk of selection bias. Fourteen studies had a high risk of selection bias and one study had an unclear risk of selection bias.
Blinding
Thirty‐seven per cent (9/24) of studies were reported as double‐blinded and were deemed to have a low risk of performance bias. However, of these nine studies only four reported blinding procedures and details on specific personnel who were blinded to the intervention assignment and therefore to have a low risk of detection bias.Twelve per cent (3/24) of studies were open‐label (Aviles 2013; Garcia‐Sanz 2015; Morgan 2010).
Incomplete outcome data
Withdrawals and dropouts (attrition bias) were described in 62% (15/24) of trials and deemed to have a low risk of attrition bias. Nine studies were deemed to have a high risk of attrition bias. Sixty‐two per cent (15/24) of trials analyzed the data according to the ITT principle and were considered to have a low risk of attrition bias.
Selective reporting
We assessed the included studies for completeness of reporting for both benefits as well as treatment‐related harms. All included studies reported the benefits and harms of the interventions in the way specified in the methods section of the trial publications. It is important to note that we did not have access to trial protocols, and hence could not assess the trial publications for selective reporting of outcomes. Overall, the risk of reporting bias was low in the included studies.
Other potential sources of bias
The expected difference in the primary outcomes was prespecified in 37% (9/24) of RCTs. Type I and type II errors were reported in 33% (8/24) of RCTs. A priori sample size calculations were reported in 33% (8/24) of RCTs. (see Figure 3 and Characteristics of included studies for details). We have categorized the elements extracted to calculate the risk of random error (pre‐specification of alpha and beta errors and sample size calculation) as "other bias" in the 'risk of bias' table (Figure 3). The funnel plots for the primary outcome of overall mortality did not suggest publication bias.
Effects of interventions
See: Table 1
Altogether 24 RCTs enrolled 3680 participants in the bisphosphonates treatment group and 3621 in the control group. The study by Zhang et al did not report data on any of the outcomes of interest for this SR (Zhang 2012). Data on overall survival (OS)/mortality were reported in 67% (16/24) of RCTs, and 37% (9/24) of RCTs reported PFS estimates. Data on vertebral fractures were reported in 353 (8/24) of RCTs, and 29% (7/24) of RCTs reported non‐vertebral fracture data. Data on SREs were reported in 58% (14/24) of RCTs. Data on pain amelioration were reported in 37% (9/24) of RCTs. Data on hypercalcemia were reported in 50% (12/24) of RCTs. Data on GI toxicities were reported in 29% (7/24) of RCTs, and data on renal dysfunction were reported in only 12% (3/24) of RCTs. Data on hypocalcemia were reported in 12% (3/24) of RCTs. Calcium data were extractable in the continuous format from only two RCTs. However, effects of bisphosphonates on calcium were reported in the dichotomous (number of participants with hypocalcemia or hypercalcemia) format in most of the studies. Hence, we performed an meta‐analysis using the dichotomous data. Data on the quality of life were not reported at all.
Results of direct comparison of treatment effects (bisphosphonates versus placebo or no treatment)
Efficacy of bisphosphonates (benefits)
(see also: Table 1)
1) Effect on overall survival (OS)
Data were extractable from 14 RCTs. These studies enrolled 2706 participants. The pooled results showed no evidence for a difference in improvement of OS with the use of bisphosphonates compared with placebo or no treatment. The pooled hazard ratio (HR) for the outcome of OS was 0.90 (95% confidence interval (CI) 0.76 to 1.07; P = 0.24) (Analysis 1.1). There was substantial heterogeneity among included trials (I2 = 65%; P = 0.0004) (see 'Assessment of bias: sensitivity analysis' section below for explanation of heterogeneity). The overall quality of evidence for OS was moderate (Table 1).
1.1. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 1 Mortality.
2) Effect on progression‐free survival (PFS)
Data were extractable from seven RCTs. These studies enrolled 908 participants. Use of bisphosphonates showed no evidence for a difference in improvement in PFS compared with placebo or no treatment. The pooled HR for PFS was 0.75 (95% CI 0.57 to 1.00; P = 0.05) (Analysis 1.2). There was no heterogeneity among trials reporting PFS estimates (I2 = 41%; P = 0.12). The overall quality of evidence for PFS was low (Table 1).
1.2. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 2 Progression‐free survival.
3) Effect on the number of participants with vertebral fractures
Data were extractable from seven RCTs. These studies enrolled 1116 participants. The pooled results showed evidence for a difference in improvement in reducing vertebral fractures with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of vertebral fractures was 0.74 (95% CI 0.62 to 0.89; P = 0.001) (Analysis 1.3). There was no heterogeneity among included RCTs (I2 = 7%; P = 0.38). The overall quality of evidence for vertebral fractures was moderate (Table 1).
1.3. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 3 Vertebral fractures.
4) Effect on the number of participants with non‐vertebral fractures
Data were extractable from six RCTs. These studies enrolled 1389 participants. The pooled results showed no evidence for a difference in reducing non‐vertebral fractures with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of vertebral fractures was 1.03 (95% CI 0.68 to 1.56; P = 0.90) (Analysis 1.4). We noted moderate heterogeneity among the included trials (I2 = 54%; P = 0.07). The overall quality of evidence for non‐vertebral ractures was moderate (Table 1).
1.4. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 4 Non‐vertebral fractures.
5) Effect on the total skeletal‐related events (SREs)
Data were extractable from 10 RCTs. These studies enrolled 2141 participants. The pooled results showed evidence for a difference in reducing SREs with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of SREs was 0.74 (95% CI 0.63 to 0.88; P = 0.0005) (Analysis 1.5). We noted moderate heterogeneity among included RCTs (I2 = 48%; P = 0.04). The overall quality of evidence for SREs was moderate (Table 1).
1.5. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 5 Total skeletal‐related events.
6) Effect on pain
Data were extractable from eight RCTs. These studies enrolled 1281 participants. The pooled results showed evidence for a difference in amelioration of pain with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of pain was 0.75 (95% CI 0.60 to 0.95; P = 0.01) (Analysis 1.6). There was substantial heterogeneity among included RCTs (I2 = 63%; P = 0.008) (see 'Assessment of bias: sensitivity analysis' section below for explanation of heterogeneity). The overall quality of evidence for pain was very low (Table 1).
1.6. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 6 Pain.
7) Effect on the incidence of hypercalcemia (≥ 2.65 mmol/L)
Data were extractable from 10 RCTs. These studies enrolled 2174 participants. The pooled results showed no evidence for a difference in reducing hypercalcemia with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of hypercalcemia was 0.78 (95% CI 0.56 to 1.09; P = 0.14) (Analysis 1.7).There was no heterogeneity among included RCTs (I2 = 21%; P = 0.25).
1.7. Analysis.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 7 Incidence of hypercalcemia.
Treatment‐related harms
The bisphosphonate‐related harms that were extractable among eligible studies were gastrointestinal (GI) symptoms, hypocalcemia, renal dysfunction and ONJ. No bisphosphonate‐related mortality was reported in any of the studies eligible for the analysis.
1) Osteonecrosis of the jaw (ONJ)
Data were extractable from six RCTs. These studies enrolled 1284 participants. The pooled results showed evidence for a difference in frequency of ONJ with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of ONJ was 4.61 (95% CI 0.99 to 21.35; P = 0.05) (Analysis 2.1). There was no statistically significant heterogeneity among included RCTs (I2 = 0%; P = 0.95). The overall quality of evidence for ONJ was low (Table 1).
2.1. Analysis.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 1 Osteonecosis of jaw.
Two RCTs with bisphosphonate as the comparator also reported estimates of ONJ. In the RCT by Morgan et al (Morgan 2010), zoledronate was associated with higher rates of ONJ (35/983 (4%)) than was clodronate (3/979 (< 1%)). In the RCT by Gimsing et al, ONJ was reported in 2 of 252 participants receiving 30 mg of pamidronate compared with 8 of 250 participants receiving 90 mg of pamidronate (Gimsing 2010). Even though only 5 RCTs reported ONJ, a growing number of ONJ case reports and observational studies evaluating ONJ have been published in recent years (Table 5; Table 6; Table 7). We analyzed 9 observational trials that evaluated 1400 participants regarding ONJ. The highest frequencies of ONJ were seen in studies that used a combination of pamidronate and zoledronate (range 5% to 51%). Zoledronate was associated with ONJ in 3% to 11% of cases. Pamidronate related frequencies of ONJ ranged from 0% to 18%. Overall, for every 1,000 participants treated with bisphosphonates, about one patient will suffer from the ONJ.
4. Included ONJ studies.
| Study | Study design | Type of bisphosphonate | Total number of patients | Number of patients with ONJ | Route, dose, frequency | Treatment duration | ONJ frequency |
| Badros 2006 | Retrospective study | Pamidronate | 17 | 3 | Not reported | Not reported | 17.65% |
| Zoledronate | 34 | 2 | 5.88% | ||||
| Pamidronate + zoledronate | 33 | 17 | 51.51% | ||||
| Berenson 2011 | Retrospective study | Zoledronate | 300 | 14 | Not clear | Median: 18 months Range: 1‐121 months | 5% |
| Calvo‐Villas 2006 | Not clear | Zoledronate | 64 | 7 | Not reported | Not clear | 9% |
| Cetiner 2009 | Prospective study | Zoledronate | 32 | 5 | 15 minute infusion of 4 mg IV zoledronate once a month | Mean duration: 26.5 months, SD 18.7 months | 15% |
| Corso 2007 | Retrospective study | Pamidronate | 20 | 0 | Not clear | 23 months | 0% |
| Zoledronate | 37 | 5 | Not clear | 28 months | 11.9% | ||
| Pamidronate + zoledronate | 42 | 2 | Not clear | 47 months | 4.55% | ||
| Dimopoulos 2006 | Pamidronate | 93 | 7 | Not reported | 39 months ONJ patients (11‐76) vs 28 (4.5‐123) months without ONJ | 7.5% | |
| Zoledronate | 33 | 1 | 3% | ||||
| Pamidronate + zoledronate | 66 | 6 | 9.1% | ||||
| Ibandronate | 1 | 0 | 0% | ||||
| Ibandronate + zoledronate | 4 | 1 | 25% | ||||
| Clodronate + zoledronate | 1 | 0 | 0% | ||||
| Alendronate + zoledronate | 1 | 0 | 0% | ||||
| Garcia‐Garay 2006 | Retrospective study | Pamidronate | 49 | 1 | 90 mg monthly | 28 months | 2% |
| Zoledronate | 64 | 6 | 4 mg monthly | 12 months (7‐28) | 9.3% | ||
| Pamidronate + zoledronate | 30 | 7 | 43.5 months (24‐59) | 23.3% | |||
| Tosi 2006b | Retrospective study | Zoledronate | 225 | 6 | Not reported | 10 months (4‐35) | 2.7% |
| Zervas 2006 | Retrospective study from 1991, prospective from 2001‐2006 | Pamidronate | 78 | 1 | 90 mg | 24 months (4‐120) | 1.28% |
| Pamidronate | 91 | 6 | 4 mg 4‐6 weeks | 6.59% | |||
| Pamidronate + zoledronate | 85 | 21 | 24.71% |
ONJ: Osteonecrosis of the jaw; SD: standard deviation; IV: intravenous.
5. Excluded ONJ studies.
| Study_ID | Reason for exclusion |
| Bujanda 2007 | No multiple myeloma patients with ONJ |
| Hoff 2006 | No extractable data for multiple myeloma patients (abstract) |
| Kut 2004 | American Society of Hematology 2004 (abstract no 4933): Approximately 600 multiple myeloma patients. Teported frequency: 7 patients. Excluded due to imprecise reporting (e.g. approximately 600 multiple myeloma patients) |
ONJ: Osteonecrosis of the jaw.
6. ONJ case reports/case series: data stratified by bisphosphonate type.
A: Alendronate; C: Clodronate; I: Ibandronate; P: Pamidronate; R: Risedronate; Z: Zoledronate; MM: multiple myeloma; U: Unknown.
2) Gastrointestinal symptoms
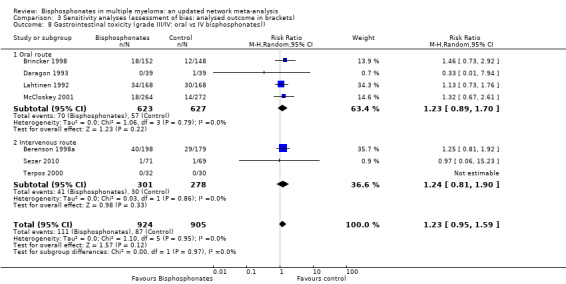
Data were extractable from seven RCTs. These studies enrolled 1829 participants. The pooled results showed no evidence for a difference in frequency of GI symptoms with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of GI symptoms was 1.23 (95% CI 0.95 to 1.59; P = 0.12) (Analysis 2.2). There was no significant statistical heterogeneity among included RCTs (I2 = 0%; P = 0.95).
2.2. Analysis.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 2 Gastrointestinal toxicity (grade III/IV).
One RCT with bisphosphonate as the comparator also reported estimates of GI symptoms (Morgan 2010). In the RCT by Morgan and colleagues, 24 of 981 participants enrolled in the zoledronate arm had GI symptoms, and 30 of 979 participants receiving clodronate had GI symptoms (Morgan 2010).
The most common adverse events with oral bisphosphonates are upper GI toxicities, such as gastritis (Van Holten‐Verzantvoort 1993) and diarrhea (Atula 2003). The intravenous infusions can be associated with injection site reaction and acute systematic inflammatory reactions (Tanvetyanon 2006). Different authors have used various methods to assess GI symptoms. Our first choice was to use the overall number of participants with GI symptoms. When this number was not available, we used the most common symptoms; in the majority of cases, it was abdominal pain. However, in some studies, nausea or vomiting was a more prevalent symptom. For our analysis, we pooled all GI symptoms together.
3) Hypocalcemia
Data were extractable from three RCTs. These studies enrolled 1090 participants. The pooled results showed no evidence for a difference in frequency of hypocalcemia with use of bisphosphonates compared with placebo or no treatment. The pooled RR for the outcome of hypocalcemia was 2.19 (95% CI 0.49 to 9.74; P = 0.30) (Analysis 2.3). There was no statistically significant heterogeneity among included RCTs (I2 = 0%; P = 0.88).
2.3. Analysis.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 3 Hypocalcaemia.
One RCT with bisphosphonate as the comparator also reported estimates of hypocalcemia (Terpos 2003). In the RCT by Terpos and colleagues, none of the 23 participants enrolled in the pamidronate arm had hypocalcemia, while two of 19 participants receiving ibandronate did (Terpos 2003). All bisphosphonates can cause hypocalcemia, regardless of the method of administration, although this only occurs infrequently as a clinically symptomatic problem. Effects of bisphosphonates on calcium were reported in the dichotomous (number of participants with hypocalcemia) rather than in the continuous format in most of the studies, leading to loss of available information.
4) Renal dysfunction
Data were extractable from two RCTs (Daragon 1993; Lahtinen 1992) in the form of serum creatinine estimates. These studies enrolled 414 participants. The pooled results showed no evidence for a difference in the frequency of elevated serum creatinine with the use of bisphosphonates compared with placebo or no treatment. The pooled mean difference for the outcome of renal dysfunction was −0.36 (95% CI −9.75 to 9.03; P = 0.94) (Analysis 2.4). There was no significant statistical heterogeneity among included RCTs (I2 = 18%; P = 0.27).
2.4. Analysis.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 4 Renal dysfunction.
One RCT (Garcia‐Sanz 2015), reported estimates of renal dysfunction where two of 51 participants enrolled in the zoledronate arm and two of 49 participants in the control arm reported renal dysfunction. One RCT with bisphosphonate as the comparator also reported estimates of renal failure (Morgan 2010); 57 of 983 participants enrolled in the zoledronate arm had renal failure, while 60 of 979 participants receiving clodronate had renal failure (Morgan 2010).
Renal dysfunction is a particularly problematic adverse event that can also occur after infusion of intravenous bisphosphonates. The US Food and Drug Administration reported that 72 participants had renal failure following zoledronate therapy (Chang 2003). As a result, the product labels for pamidronate and zoledronate were amended to include additional nephrotoxicity warnings. However, the true incidence of this adverse event remains unknown.
Quality of life: None of the included studies reported quality of life.
Assessment of bias: sensitivity analysis
Sensitivity analyses were performed to assess the robustness of our findings and explore possible reasons for heterogeneity.
Sensitivity analysis according to methodological quality of reporting
All trials were evaluated according to methodological quality dimensions to assess the existence of potential inaccuracies in our results (Jüni 2001). In particular, we focused on dimensions that have been empirically linked to bias and random error. We preformed sensitivity analyses according to adequacy of allocation concealment (Schulz 1995), blinding, ITT analysis, description of withdrawals and dropouts, and pre specification of type I and II errors for all outcomes (see Characteristics of included studies). The results did not change for any outcome except in case of pain. We found that RCTs with double‐blinding showed no significant benefit of bisphosphonates over placebo for amelioration of pain (RR 0.83, 95% CI 0.69 to 1.00), while non‐blinded RCTs favored bisphosphonates over placebo for pain relief (RR 0.28, 95% CI 0.12 to 0.67; test of interaction: P = 0.005). Similarly, RCTs with ITT analysis showed no significant benefit of bisphosphonates over placebo for amelioration of pain (RR 0.93, 95% CI 0.75 to 1.14), while RCTs with per protocol analysis favored bisphosphonates over placebo for pain relief (RR 0.54, 95% CI 0.33 to 0.89) (test of interaction: P = 0.04).
Examples based on the outcome of vertebral fractures and corresponding figures are provided to give a visual impression of results of the sensitivity analyses (Data and analyses). Only two out of seven studies that reported the rate of vertebral fractures had adequate allocation concealment but sensitivity analysis performed according to this criterion indicated no change in the results. Three of seven trials reporting vertebral fractures were performed according to ITT analysis, and these trials showed a smaller treatment effect, which may be due to the exclusion of the largest trial from the analysis (Berenson 1998a). Five of seven studies reporting vertebral fractures were double‐blind, but the results were unchanged in this subgroup analysis. Only one of seven studies reporting vertebral fractures described the randomization method, but the results were also unchanged in this subgroup analysis. Furthermore, only two of seven studies reporting vertebral fractures clearly noted the withdrawals and dropouts, but the results remained unchanged in this subgroup analysis. Only Lahtinen 1992 prespecified the type I and II errors out of seven studies reporting vertebral fractures, but the results were unchanged in this subgroup analysis.
Sensitivity analysis according to bisphosphonate potency
The potential cause of heterogeneity was explored by meta‐regression. We investigated whether potency of bisphosphonates contributed to the decrease in mortality according to bisphosphonate potency as a covariate (on the log scale) (Figure 4). The Knapp‐Hartung meta‐regression showed that HR decreases by 7% per 1000 unit increase in bisphosphonate potency (HR 0.94, 95% CI 0.90 to 0.98) given on the log scale. The bisphosphonate potency explained 91.34% of between‐study variance (the between‐study variance dropped from 0.035 to 0.009). Meta‐regression results indicated that the beneficial effect of bisphosphonates on survival in participants with MM may be a function of drug potency, with zoledronate being the most potent bisphosphonate (Figure 4).
4.

Bisphosphonate potency metaregression for overall survival. HR: Hazard ratio.
We also conducted meta‐regression for PFS. However, meta‐regression results indicated that the effect of a bisphosphonate on restricting disease progression in participants with MM did not differ by bisphosphonate potency (HR 0.94, 95%CI 0.77 to 1.14).
Sensitivity analysis according to treatment duration
We conducted additional sensitivity analyses based on duration of treatment (indefinite versus 0 to 24 months). The results were unchanged for all outcomes except for non‐vertebral fractures. The only RCT (McCloskey 2001) with an indefinite duration of treatment with clodronate showed a statistically significant benefit in favor of clodronate (RR 0.53, 95% CI 0.29 to 0.97), while four RCTs with 0 to 24 months of treatment duration showed no benefit for reduction of non‐vertebral fractures (RR 1.25, 95% CI 0.94 to 1.66) (test of interaction: P = 0.01).
Sensitivity analysis according to route of administration for gastrointestinal toxicity outcome
We conducted additional sensitivity analyses based on route of administration (oral versus intravenous) for GI toxicity outcome (Analysis 3.8). The results were unchanged for this outcome (test of interaction: P = 0.97).
3.8. Analysis.
Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)).
Results of network meta‐analysis of treatment effects
Indirect comparisons or network meta‐analysis was conducted for all the outcomes. Data for only the key outcomes are shown here.
Efficacy of bisphosphonates (benefits)
1) Effect on overall survival (OS)
Overall survival/mortality data were available from 16 RCTs involving 5260 participants. A total of 21 indirect comparisons were possible (Figure 5). The network meta‐analysis by MTC method (16 RCTs, 5260 participants) showed the superiority of zoledronate over placebo (HR 0.67, 95% CI 0.46 to 0.91) and etidronate (HR 0.56, 95% CI 0.29 to 0.87) (Table 4). The results of the MTC method are in agreement with the direct comparison of zoledronate versus placebo in two RCTs (Aviles 2007; Aviles 2013).
5.

Randomized controlled trial (RCT) network for overall survival (OS), progression free survival (PFS) and skeletal related events (SREs).
2) Effect on progression‐free survival (PFS)
Data on PFS were available from nine RCTs involving 3472 participants. A total of 10 indirect comparisons were possible (Figure 5). The network meta‐analysis by MTC method (Nine RCTs, 3472 participants) did not show the superiority of any particular bisphosphonate regimen over others (Table 4). MTC analysis did not show a benefit with zoledronate over clodronate ( nine RCTs, 3472 participants; HR 1.10, 95% CI 0.46 to 1.95) (Table 4).
3) Effect on the number of participants with vertebral fractures
Data on participants with vertebral fractures were available from eight RCTs involving 3076 participants. A total of six indirect comparisons were possible (Figure 5). The head‐to‐head (direct) comparison of zoledronate with clodronate in the RCT Morgan 2010 showed a statistically significant benefit with zoledronate over clodronate (one RCT, 1960 participants; RR 0.62, 95% CI 0.44 to 0.87). The MTC method did not show a benefit with zoledronate over clodronate (eight RCTs, 3076 participants; HR 0.59, 95% CI 0.22 to 1.17) (Table 4). The results of MTC analysis showed that zoledronate was superior (eight RCTs, 3076 participants; RR 0.42, 95% CI 0.12 to 0.94) to placebo (Table 4). For all other comparisons the network meta‐analysis by MTC method (eight RCTs, 3076 participants) did not show the superiority of any particular bisphosphonate regimen over others (Table 4).
4) Effect on the total skeletal‐related events
Data on participants with SREs were available from 13 RCTs involving 5727 participants. A total of 21 indirect comparisons were possible (Figure 5). Indirect comparison by MTC showed the superiority of zoledronate over placebo (RR 0.57, 95% CI 0.37 to 0.76), zoledronate over ibandronate (RR 0.56, 95% CI 0.26 to 0.98) and pamidronate 90 mg over placebo (RR 0.71, 95% CI 0.49 to 0.96). For all other comparisons the network meta‐analysis by MTC method (13 RCTs, 5727 participants) did not show the superiority of any particular bisphosphonate regimen over others (Table 4).
5) Effect on pain
Data on the effect of bisphosphonates on pain control were available from eight RCTs involving 1281 participants. A total of six indirect comparisons were possible. The network meta‐analysis by MTC method (eight RCTs, 1281 participants) did not show superiority of any particular bisphosphonate regimen over others (Table 4). MTC analysis did not show a benefit with clodronate over ibandronate (eight RCTs, 1281 participants; RR 4.13, 95% CI 0.57 to 16.99) (Table 4).
6) Effect on hypercalcemia
Data on the effect of bisphosphonates on hypercalcemia were available from 11 RCTs involving 4146 participants. Indirect comparisons by the MTC method (11 RCTs, 4146 participants) did not show superiority of any particular bisphosphonate regimen over others (Table 4).
Treatment‐related harms
We also conducted network meta‐analysis by the MTC method for the outcomes of hypocalcemia, renal dysfunction, GI toxicity and ONJ. Results of network meta‐analysis by the MTC method were consistent with the results from direct comparisons. Network meta‐analysis by the MTC method did not show any differences in the incidence of hypocalcemia, renal dysfunction, and GI toxicity among the bisphosphonates used.
Effect on osteonecrosis of the jaw (ONJ)
Data on the effect of bisphosphonates on ONJ were available from eight RCTs involving 3746 participants. Indirect comparisons by the MTC method show an increased risk of ONJ with zoledronate over clodronate (RR 0.13, 95% CI 0.02 to 0.44). For all other comparisons network meta‐analysis by the MTC method did not show any differences in the incidence of ONJ (Table 4).
Since our network has only one triangle loop, its consistency was assessed using a simple method described by Bucher and colleagues (Bucher 1997). The estimates of consistency indicate that the network was consistent for OS (95% CI −0.86 to 0.07) and PFS (95% CI −0.38 to 0.33). We also ranked each treatment according to the probability that it is best for the outcomes of OS, PFS, SREs, hypercalcemia, GI toxicity and ONJ (Figure 6). We also calculated the surface under the cumulative ranking (SUCRA) line for each treatment, which is equal to 1 when the treatment is certain to be the best and 0 when it is certain to be the worst (Figure 6). As both rankogram SUCRA show, zoledronate consistently ranked as the best treatment, followed by pamidronate and clodronate. Since the choice of prior for between‐studies variance can lead to drastic variation in results (Lambert 2005), especially in a small number of studies, we had already performed an extensive sensitivity analysis in our previous systematic reviews (Mhaskar 2010; Mhaskar 2012), in which we assessed a total of five priors (gamma, uniform, Pareto, logistic and half‐normal) based on their application in the literature (Scurrah 2000; Thompson 1997). Gamma and logistic priors exhibited poor convergence. Generally, as the number of studies decreased, the selection of prior dominated the estimates. We noted that the credibility intervals became wider for the less informative priors.
6.
A: Ranking probabilities of competing bisphosphonates. The size of each bar corresponds to the probability of each treatment to be at a specific rank. OS: Overall survival; PFS: Progression‐free survival; SRE: Skeletal‐related events; Osteonecrosis; GI: Gastrointestinal toxicity; Hyper: Hypercalcemia.
B: Surface under the cumulative ranking curve (SUCRA) plots for each treatment. The outcomes are listed on the horizontal axis. SUCRA for each outcome are on the vertical axis.
Discussion
Summary of main results
Key benefits with use of bisphosphonates:
The potential beneficial effect of bisphosphonates on survival is the most intriguing question, as anti‐tumor effects against myeloma cells were seen both in vitro (Aparicio 1998; Shipman 1997) and in vivo (Dhodapkar 1998). The direct comparisons in this meta‐analysis demonstrate no evidence for a difference related to survival with bisphosphonate compared to placebo. However, evidence for a difference in overall survival (OS) with bisphosphonates compared with placebo/other bisphosphonate was seen in three RCTs (Aviles 2007; Aviles 2013; Morgan 2010). The two studies by Aviles and colleagues comparing zoledronate with placebo showed evidence for a difference with zoledronate in improving OS compared with placebo (Aviles 2007; Aviles 2013). The study by Morgan and colleagues showed a clinically significant anti‐MM effect of zoledronic acid compared with clodronate on OS (Morgan 2010). However, the results from our network meta‐analysis of 15 randomized controlled trials (RCTs) and 4866 participants showed no evidence for a difference with zoledronate over any other amino‐bisphosphonates or clodronate for OS. Similarly, direct comparisons and network meta‐analysis did not show superiority of any specific bisphosphonate over placebo or no treatment and other bisphosphonates in improving progression‐free survival (PFS).
The results of this meta‐analysis show a reduction in total skeletal‐related events (SREs) and vertebral fractures, a significant advantage for the participants treated with bisphosphonates. Our results show evidence for a difference with bisphosphonates compared with placebo for the prevention of pathological fractures, most likely due to the effect on preventing pathological vertebral collapses. Interestingly, there was no evidence for a difference with bisphosphonates compared with placebo on reduction of non‐vertebral fractures. Bisphosphonates inhibit osteoclastic bone resorption and thereby increase the degree of bone mineralization. Animal models focusing on trabecular bones have also shown that the mechanical properties of the bone (torsion stiffness and elasticity) are improved with the use of bisphosphonates. A comparative study of trabecular and cortical bones in beagle dogs demonstrated that the effect of pamidronate to improve torsion stiffness and elasticity is restricted to trabecular bones, whereas no change was seen in cortical bones (Acito 1994). Similar observations have been made in postmenopausal women treated with pamidronate (Fromm 1991). These studies support our findings that the vertebral fractures are clearly reduced and there was no significant effect on non‐vertebral fractures. However, it must be taken into consideration that several studies did not report these events in sufficient detail.
Key harms with use of bisphosphonates:
There was evidence for a difference with bisphosphonates related to increased risk for osteonecrosis of the jaw (ONJ) in participants using bisphosphonates compared with placebo or no treatment, which was not seen in the previous meta‐analysis (Mhaskar 2012). RCTs showed that, on average, for every 1000 participants treated with bisphosphonates, about one patient will suffer from the ONJ. Only eight RCTs reported ONJ, and the network meta‐analysis showed that no specific bisphosphonate was associated with an increased incidence of ONJ compared with other bisphosphonates. However, this in part could be a reflection of rare events and thus RCTs with more power are needed for more precise estimates. We also identified 61 observational studies (including case reports) reporting 676 cases of ONJ. The observational studies estimated ONJ to be 0% to 51%, which is in a stark contrast with the estimates obtained from RCTs. The reason for this discrepancy is probably due to the combined effect of inherent limitations associated with observational studies and different eligibility criteria in RCTs versus non‐RCTs. No other significant adverse effects associated with the administration of bisphosphonates were identified in the included RCTs.
Overall completeness and applicability of evidence
Physicians’ choices regarding type of bisphosphonates for the management of people with multiple myeloma should ideally be based on evidence from comparative trials. There are only four head‐to‐head comparative studies of bisphosphonates (Gimsing 2010; Morgan 2010; Rosen 2003; Terpos 2003). Unfortunately, the data from Rosen 2003 were not extractable for participants with MM for outcomes other than SREs, and Terpos and colleagues (Terpos 2003) report data addressing only two outcomes (hypercalcemia and hypocalcemia) of interest for this systematic review. Terpos 2003 concluded that a monthly dose of 90 mg of pamidronate is more effective than 4 mg of ibandronate in reducing osteoclast activity, bone resorption and possibly tumour burden in MM. The RCT by Morgan and colleagues compared the effects of zoledronate versus clodronate in participants with MM. The results of this head‐to‐head comparison showed that treatment with zoledronate was associated with a significant reduction in the proportion of participants with SREs (27% versus 35% with clodronate) and vertebral fractures (5% versus 9% with clodronate), and slightly increased median PFS by two months. But most remarkably, the median OS was significantly improved by 5.5 months with zoledronate compared with clodronate (Morgan 2010; Rajkumar 2010). Our meta‐regression analysis showed a significant trend (P = 0.007) based on the bisphosphonate potency (7% reduction in mortality for each 1000 unit increase in bisphosphonate potency), with zoledronate being the most potent bisphosphonate (Figure 4). This would imply that zoledronate indeed is the most effective bisphosphonate. However, no such effect was seen in a meta‐regression for PFS raising the issue of the mechanism of improving survival, if PFS is not affected, it does not appear to be plausible to improve OS. In addition, our network meta‐analyses of 15 RCTs and 5160 participants indicated that zoledronate was not superior to any other bisphosphonate for any outcomes except etidronate. Nevertheless, we should note that in the context of MTC uncertainty analysis, zoledronate ranked as the best treatment, followed by clodronate and pamidronate. However, we noted no difference when pamidronate was compared with clodronate or zoledronate for all outcomes. These findings underscore the need for comparative evaluation of the newer potent amino‐bisphosphonates, especially zoledronate versus pamidronate, in an RCT to enable appropriate healthcare decision making. Also, future studies should investigate bisphosphonate treatments as a palliative treatment by measuring its influence on quality‐of‐life outcomes.
Quality of the evidence
We assessed the potential risk of bias of the included trials according to the previously described quality domains; these are represented in Figure 3. The majority of included trials suffered from a high risk of selection bias. The majority of the included studies reported analyses according to the principle of intention to‐treat (ITT). The majority of included trials had a high risk of performance and detection bias. Twelve per cent (3/24) of studies were open‐label (Aviles 2013; Garcia‐Sanz 2015; Morgan 2010).
Overall, the quality of evidence according to the GRADE criteria was moderate to low. The study by Zhang and colleagues was published in both English and Mandarin language. We have reviewed and have included both of these publications in this systematic review (Zhang 2012). Nonetheless, we noticed that the publication in the Mandarin language referred to this study as a randomized study but the English language publication does not explicitly mention any details regarding randomization. However, it is important to note that, this study did not report any of the outcomes of interest for this systematic review. We also noticed a discrepancy between the www.clinicaltrials.gov record and the publication of the study by Aviles and colleagues (Aviles 2013). Specifically, on the www.clinicaltrials.gov this study has been labeled as an observational case‐control type of study and the publication refers to this study as a randomized control trial.
One criterion for high‐quality reporting is that the data should be reported in a form that allow them to be extracted and used in a quantitative research synthesis (i.e. meta‐analysis). In most of the studies included in this systematic review, treatment‐related morbidities were not reported as events per patient and thus could not be used in the meta‐analysis. That is, treatment‐related morbidities were reported using statements that did not allow us to distinguish between specific adverse events occurring in multiple participants or multiple events occurring in a single patient.
Potential biases in the review process
We did not find any methodological issues in the preparation of the review that could put it at risk for bias. We identified one ongoing study comparing zoledronic acid with no treatment. No findings were available as the trial is still recruiting participants (Lund 2014). Sezer 2010 was published as a meeting abstract only. We were able to obtain some data form www.clinicaltrials.gov record for this study. Nonetheless, for reasons unknown to us, this study was not published as a full manuscript, and hence we were not able to access the complete data and findings from this study. Indeed, we were not able to extract data on all outcomes from all the studies (Table 3). Furthermore, one RCT (Garcia‐Sanz 2015) did not come up in the formal search and was identified by the Cochrane Hematological Malignancies Group which, we suspect, was due to inadequate cataloguing. The funnel plot for assessment of publication bias showed asymmetry only for the outcome of pain, indicating the possibility of outcome reporting bias or publication bias (Figure 7). However, the findings are also consistent with heterogeneity in interventions or the tendency for the smaller studies to show larger treatment effects (Sterne 2001). Similarly, the observed between‐trial heterogeneity makes interpretation of funnel plots difficult and increases the false‐positive rate of the tests. Given the comprehensiveness of our search efforts and the fact that most of the trials testing bisphosphonates were small and underpowered, the small‐study effect is the most likely explanation for the asymmetry seen in the funnel plots (Sterne 2001).
7.

Funnel plot of comparison: 1 Bisphosphonates vs. control (efficacy), outcome: 1.6 Pain.
Agreements and disagreements with other studies or reviews
A Cochrane systematic review examined relief of pain secondary to bone metastases by using bisphosphonates in 30 identified RCTs (Wong 2002). The review concluded that the evidence is insufficient to recommend bisphosphonates for immediate pain‐relief effect. This finding contradicts our results, as well as those of earlier reviews examining the role of bisphosphonates in myeloma patients (Mhaskar 2010; Mhaskar 2012), indicating a likely beneficial effect of bisphosphonates on pain reduction. The discrepancy between the results of our systematic review and the systematic review by Wong and colleagues lies in the decision about which studies should be included and methods of pain reporting as extensively discussed in our previous reviews (Mhaskar 2010; Mhaskar 2012). Although there was a beneficial effect of bisphosphonates on the number of patients with myeloma reporting bone pain in this review, these data must be treated with caution because of the lack of uniformity in data reporting and the presence of statistically significant heterogeneity. The variation in the methods of reporting pain and the quality of included RCTs contributed to the statistically significant heterogeneity observed in pain estimates.
Authors' conclusions
Implications for practice.
Adding bisphosphonates to the treatment of multiple myeloma (MM) reduces vertebral fractures and probably pain. We found no evidence for a difference for overall survival (OS) and progression‐free survival (PFS) with bisphosphonates compared with placebo. The benefits of bisphosphonates should be considered, keeping in mind the risk of developing osteonecrosis of the jaw (ONJ). Zoledronate appears to be superior to etidronate (non‐aminobisphosphonate) and placebo for the outcome of OS. However, whether zoledronate is superior to pamidronate and other aminobisphosphonates remains to be determined.
Implications for research.
Our findings underscore the urgent need for a randomized controlled trial (RCT) with a head‐to‐head comparison of zoledronate and pamidronate for their efficacy and safety in patients with MM. In addition, future studies should investigate bisphosphonate treatments as a palliative treatment by measuring its influence on quality‐of‐life outcomes. There is also a need for studies addressing cost‐effectiveness and adverse events, especially ONJ, of bisphosphonate therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2017 | New citation required but conclusions have not changed | Update of previous version. Four new studies added. Conclusions remain unchanged. |
| 31 July 2017 | New search has been performed | Update of previous systematic review published in 2012 |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 19 June 2012 | Amended | Affiliation JR corrected |
| 6 February 2012 | New citation required and conclusions have changed | Revised conclusions |
| 20 October 2011 | Feedback has been incorporated | Amendments done |
| 15 October 2011 | New search has been performed | New search |
Acknowledgements
We want to thank Dr Terpos and Dr McCloskey for verification of extracted data from their papers, as well as Dr Ruiz‐Erenchun, Hoffmann‐La Roche Ltd, Basel, Switzerland, who provided additional data from the ibandronate trial. Dr Poglód provided additional information on the trials of his group. We also want to thank the Cochrane peer‐reviewers and editors for critical reading of our review and helpful feedback. We thank Dr Thomas A. Trikalinos for his suggestions regarding meta‐regression. We also thank the Cochrane Hematological Malignancies Group for their assistance in identifying the RCT by Garcia‐Sanz and colleagues.
Appendices
Appendix 1. MEDLINE search strategy
(((“Multiple Myeloma”[MeSH] OR “Plasmacytoma”[MeSH] OR multiple myeloma OR plasmacytoma OR plasmacytom* OR myelom*) AND (bisphosphonates OR pamidronate OR zoledronate OR etidronate OR ibandronate OR clodronate OR “Clodronic Acid”[MeSH] OR “pamidronate ”[Substance Name] OR “Etidronic Acid”[MeSH] OR “zoledronic acid ”[Substance Name] OR “ibandronic acid ”[Substance Name]))) AND ((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading]) AND ((“2011/09/01”[EDat] : “3000”[EDat]) AND (Humans[MeSH])) Limits:Publication Date from 2011/09/01 (((((((("pamidronate "[Substance Name] OR "Etidronic Acid"[MeSH]) OR "ibandronic acid "[Substance Name]) OR "Clodronic Acid"[MeSH]) OR "zoledronic acid "[Substance Name]) ) OR "Alendronate"[MeSH]) OR "risedronic acid "[Substance Name]) OR "tiludronic acid "[Substance Name]) AND "Multiple Myeloma"[MeSH] Limits:Publication Date from 2011/09/01, Humans
2) Search strategy aimed at identifying observational studies and ONJ case reports.
(“MultipleMyeloma”[MeSH]AND(“pamidronate ”[SubstanceName]OR“EtidronicAcid”[MeSH])OR“ibandronic acid ”[Substance Name]) OR “Clodronic Acid”[MeSH]) OR “zoledronic acid ”[Substance Name]) ) OR “Alendronate”[MeSH]) OR “risedronic acid ”[Substance Name]) OR “tiludronic acid ”[Substance Name]) AND (“Osteonecrosis ”[MeSH] OR “Jaw Diseases”[MeSH])
Limits: Publication Date from 2011/09/01, Humans
Appendix 2. Cochrane Library search strategy
"bisphosphonate and myeloma"
Appendix 3. www.clinicaltrials.gov search strategy
"bisphosphonates and multiple myeloma"
Appendix 4. Embase search strategy
'bone'/exp OR bone AND ('neoplasms'/exp OR neoplasms) OR 'bone'/exp OR bone AND ('neoplasm'/exp OR neoplasm) OR multiple AND ('myeloma'/exp OR myeloma) OR 'neoplasm'/exp OR neoplasm AND ('metastasis'/exp OR metastasis) OR 'neoplasms'/exp OR neoplasms AND ('alendronate'/exp OR alendronate) OR 'clodronate'/exp OR clodronate OR 'etidronate'/exp OR etidronate OR 'risedronate'/exp OR risedronate OR 'ibandronate'/exp OR ibandronate OR 'pamidronate'/exp OR pamidronate OR 'tiludronate'/exp OR tiludronate OR 'zoledronate'/exp OR zoledronate OR diphosphonates. AND mp OR 'bisphosphonate'/exp OR bisphosphonate AND adj AND agent OR derivative.mp.tw. AND random.tw. OR clinical AND trial.mp. OR exp AND ('health'/exp OR health) AND care AND quality Date limits used: 09012011 onwards (till 07132017)
Data and analyses
Comparison 1. Bisphosphonates vs. control (efficacy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 14 | 2706 | Hazard Ratio (Random, 95% CI) | 0.90 [0.76, 1.07] |
| 1.1 Etidronate | 2 | 244 | Hazard Ratio (Random, 95% CI) | 1.24 [0.86, 1.80] |
| 1.2 Clodronate | 3 | 885 | Hazard Ratio (Random, 95% CI) | 0.93 [0.66, 1.29] |
| 1.3 Pamidronate | 5 | 977 | Hazard Ratio (Random, 95% CI) | 0.85 [0.67, 1.07] |
| 1.4 Ibandronate | 1 | 198 | Hazard Ratio (Random, 95% CI) | 1.07 [0.69, 1.64] |
| 1.5 Zoledronate | 3 | 402 | Hazard Ratio (Random, 95% CI) | 0.57 [0.43, 0.75] |
| 2 Progression‐free survival | 7 | 908 | Hazard Ratio (Random, 95% CI) | 0.75 [0.57, 1.00] |
| 2.1 Clodronate | 1 | 26 | Hazard Ratio (Random, 95% CI) | 0.63 [0.17, 2.34] |
| 2.2 Pamidronate | 1 | 177 | Hazard Ratio (Random, 95% CI) | 1.24 [0.66, 2.33] |
| 2.3 Zoledronate | 5 | 705 | Hazard Ratio (Random, 95% CI) | 0.70 [0.52, 0.95] |
| 3 Vertebral fractures | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 3.1 Clodronate | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.89] |
| 3.2 Pamidronate | 3 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.20] |
| 3.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.81] |
| 4 Non‐vertebral fractures | 6 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.68, 1.56] |
| 4.1 Clodronate | 3 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.31] |
| 4.2 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.95, 2.87] |
| 4.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.79, 1.98] |
| 5 Total skeletal‐related events | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] |
| 5.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.39] |
| 5.2 Clodronate | 1 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.89] |
| 5.3 Pamidronate | 3 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.59, 0.91] |
| 5.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 5.5 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.89] |
| 6 Pain | 8 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.95] |
| 6.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.32] |
| 6.2 Clodronate | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.91] |
| 6.3 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 6.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.86, 1.17] |
| 7 Incidence of hypercalcemia | 10 | 2174 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.56, 1.09] |
| 7.1 Etidronate | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.73, 2.38] |
| 7.2 Clodronate | 3 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.31] |
| 7.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.33] |
| 7.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.42] |
| 7.5 Zoledronate | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.91] |
Comparison 2. Bisphosphonates vs. control (adverse effects).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Osteonecosis of jaw | 6 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 4.61 [0.99, 21.35] |
| 1.1 Pamidronate | 2 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.13, 74.69] |
| 1.2 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [0.91, 29.90] |
| 2 Gastrointestinal toxicity (grade III/IV) | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| 2.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.94] |
| 2.2 Clodronate | 2 | 872 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.72] |
| 2.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.90, 1.88] |
| 2.4 Zoledronate | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.23] |
| 3 Hypocalcaemia | 3 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.49, 9.74] |
| 3.1 Clodronate | 1 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.38, 11.16] |
| 3.2 Pamidronate | 2 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.11, 66.19] |
| 4 Renal dysfunction | 2 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐9.75, 9.03] |
Comparison 3. Sensitivity analyses (assessment of bias: analysed outcome in brackets).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allocation concealment (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 1.1 Adeqaute concealment of allocation | 2 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.82] |
| 1.2 Inadequate concealment of allocation | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 2 Blinding (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 2.1 Double‐blind | 5 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.85] |
| 2.2 Not blinded | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.08, 3.72] |
| 3 Randomization method (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 3.1 Randomization method is described | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.85] |
| 3.2 Randomization method is NOT described | 6 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 4 Type of data analysis (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 4.1 Intention‐to‐treat analysis | 3 | 463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 4.2 Per protocol analysis | 4 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.89] |
| 5 Description of withdrawals and drop outs (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 5.1 Withdrawals and dropouts well described | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.55, 0.82] |
| 5.2 Withdrawals and dropouts NOT described | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 6 Alpha error (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 6.1 Alpha error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 6.2 Alpha error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 7 Beta error (vertebral fractures) | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 7.1 Beta error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 7.2 Beta error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)) | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| 8.1 Oral route | 4 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.70] |
| 8.2 Intervenous route | 3 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.81, 1.90] |
3.1. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 1 Allocation concealment (vertebral fractures).
3.2. Analysis.
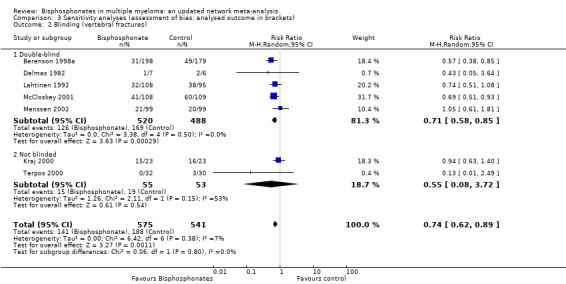
Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 2 Blinding (vertebral fractures).
3.3. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 3 Randomization method (vertebral fractures).
3.4. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 4 Type of data analysis (vertebral fractures).
3.5. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 5 Description of withdrawals and drop outs (vertebral fractures).
3.6. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 6 Alpha error (vertebral fractures).
3.7. Analysis.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 7 Beta error (vertebral fractures).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Attal 2006.
| Methods | Study design: Parallel, not double‐blind Study length: not reported Study conducted during: not reported (study started in 1999) |
|
| Participants | Bisphosphonates: enrolled 196, analyzed 196.
Bisphosphonates + thalidomide: enrolled 201, analyzed 201.
Placebo: enrolled 200, analyzed 200. Sex (M/F): Bisphosphonates: 109/87 Bisphosphonates + thalidomide: 112/89 Placebo: 110/90 Age: mean(SD): Bisphosphonates: 59 (8) Bisphosphonates + thalidomide: 58 (8) Placebo: 59 (8) Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Pamidronate: 90 mg IV, every 4 weeks; Indefinitely. Pamidronate and thalidomide: 400 mg orally, dose reduction to a minimum dose of 50 mg was allowed for treatment‐related toxicity. |
|
| Outcomes | Total skeletal‐related events; total mortality; response rates; ONJ. | |
| Notes | SRE: bone lesion requiring a specific therapy (chemotherapy, irradiation or surgery). Funding: Supported by a major grant from the Programme Hospitalier de Recherche Clinique and by the Swiss Group for Clinical Cancer Research (SAKK). COI statement included: The authors declare no competing financial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Aviles 2007.
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled study Study length: not reported Study conducted during: Mar 1999 ‐ Dec 2001 |
|
| Participants | Bisphosphonates: enrolled 46, analyzed 46.
Control: enrolled 48, analyzed 48. Sex (M/F): Bisphosphonates: 26/20 Control: 23/25 Mean age (range): Bisphosphonates: 67.3 (43‐75) Control: 65.4 (39‐75) Inclusion criteria: Stage (Durie 2005): III Osteolytic lesion: At least one Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Zoledronate: 4 mg IV, every 4 weeks; indefinitely. Control: no zoledronate. |
|
| Outcomes | Total mortality; PFS. | |
| Notes | SRE: appearance of a new lytic lesion (excluding skull), after patient began zoledronate or progression of previous bone lesion according to criteria of Union Internationale Centre le Cancer. Funding: Not reported. COI statement included: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Aviles 2013.
| Methods | Study design: Parallel, open‐label; not placebo‐controlled study Study length: not reported Study conducted during: Jun 2002 – Dec 2007 |
|
| Participants | Bisphosphonates: enrolled 151, analyzed 151.
Control: No zoledronate: enrolled 157, analyzed 157. Sex (M/F): Bisphosphonates: 71/80 Control: 85/72 Mean age (range): Bisphosphonates: 56.4 (29‐65) Control: 57.8 (33‐65) Inclusion criteria: Stage (Durie 2005): IIB‐ IIIB Osteolytic lesion: NS Creatinine: creatinine clearance > 30 mL/min Calcium: NS Other criteria: Adult patients at least 18 years but less than 65 years of age with untreated symptomatic multiple myeloma and measurable paraprotein in serum and urine, Eastern Cooperative Oncology Group performance status 0–2, and adequate renal (no end‐stage renal failure and creatinine clearance > 30 mL/min), hematologic (platelet count > 50×109/L, neutrophil count > 0.75×109/L), and liver function were eligible |
|
| Interventions | Zoledronate: IV; 4 mg (or dose‐adjusted based on creatinine clearance) once every 28 days for 24 months. Control: no zoledronate. |
|
| Outcomes | SRE; overall survival *$; progression free survival *$; | |
| Notes | PFS is defined as time from the start of high‐dose induction therapy (or time from the start of next treatment) to time of progression, relapse, or death. The primary endpoints were PFS and OS at 10 years, and the secondary endpoints included overall response, rates of complete response and very good partial response, and safety. Funding: This study was supported entirely with resources from the Mexican Institute of Social Security. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation, but the study was not sponsored by Novartis or by any other pharmaceutical company. ProEd Communications, Inc., provided medical editorial assistance with this manuscript. COI statement included: Aside from support from Novartis Pharmaceuticals Corporation for medical editorial assistance, all authors declare that they have no financial conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Belch 1991.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Nov 1983 – Feb 1987 (enrollment in the high dose arm was stopped in Jun 1984) |
|
| Participants | Bisphosphonates: enrolled 98, analyzed 92.
Control: enrolled 78, analyzed 74. Sex (M/F): Bisphosphonates: 60/32 Control: 44/30 Mean age (SD/range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: < 3 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Etidronate: capsules (20 mg/kg for 28 days every other 28 days: but this arm was discontinued); enrollment took place for: 5 mg/kg until death or discontinuation. Placebo: identical appearance. | |
| Outcomes | Vertebral index; total mortality*; pain; calcium.*** | |
| Notes | SRE: bone progression (appearances of new lesions or worsening of existing ones)$; mortality* (from the date of randomization); calcium reported as a dichotomous variable. Funding: Supported by Norwich Eaton Pharmaceuticals Inc. and The National Cancer Institute of Canada. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Berenson 1998a.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Aug 1990 – Jun 1993 |
|
| Participants | Bisphosphonates: enrolled 205, analyzed 198.
Placebo: enrolled 187, analyzed 179. Sex (M/F): Bisphosphonates: 108/88 Control: 109/72 Mean age (SD): Bisphosphonates: 64 (10) Control: 63 (10) Inclusion criteria: Stage (Durie 2005): III only Osteolytic lesion: at least one Creatinine: < 5 mg/dL Calcium: NS Other criteria: No bone specific treatment prior to entry |
|
| Interventions | Pamidronate: 90 mg in 500 mL of 5% dextrose in water, every 4 weeks for 24 months. Control: identical placebo in 5% dextrose. | |
| Outcomes | SRE (total); vertebral fractures; non‐vertebral fractures; total mortality (#); calcium***; pain; adverse events. | |
| Notes | SRE: pathologic fracture or radiation treatment/surgery on bone or spinal cord compression. Pain control assessment: Bone pain reported by authors at 29 months. Funding: Supported by a grant from the Pharmaceuticals Division, Ciba–Geigy Corporation, Summit, N.J. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Brincker 1998.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Sept 1990 – Jul 1995 |
|
| Participants | Bisphosphonates: enrolled 152, analyzed 152.
Placebo: enrolled 148, analyzed 148. Sex (M/F): Bisphosphonates: 83/69 Control: 109/72 Mean age (SD): Bisphosphonates: 69 (NR) Control: 69 (NR) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: < 2.8 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Pamidronate: 75 mg capsules orally bid (total 300mg /day); for at least 2 years. Control: identical placebo. | |
| Outcomes | Total mortality*$; SRE; pain; calcium(&); adverse events | |
| Notes | SRE: bone fracture other than vertebral or surgery or increase in number of osteolytic lesions + vertebral collapse.
Pain reported as the number of events, not as the number of patients experiencing pain. Funding: not reported. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Daragon 1993.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Jan 1985 – Jun 1990 |
|
| Participants | Bisphosphonates: enrolled 49, analyzed 39.
Placebo: enrolled 45, analyzed 39. Sex (M/F): Bisphosphonates: 22/27 Control: 22/23 Mean age (SD): Bisphosphonates: 65.6 (9.8) Control: 66.9 (9) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: < 2.8 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Etidronate: 10 mg/kg orally daily with lunch; for 4 months. Control: identical placebo. | |
| Outcomes | Total mortality *$ ;SRE (total); total fractures; vertebral fractures; non‐vertebral fractures; vertebral index; total mortality; pain; calcium; adverse events. | |
| Notes | SRE: new extraspinal osteolytic bone lesions or fractures or vertebral index; total mortality: total number of deaths reported in the text.
Pain recorded as the number of patients taking class 2 and 3 narcoanalgesics at four months. Funding: Supported in part by Nativelle, Issy‐les‐Moulineaux, France and by INSERM ; etudes biochimiques et therpaeuteques du myeloma multiple, by association pour la researche contre le cancer and by Ligue nationale de lute contre le cancer. COI statement included: not reported. Pain control assessment: Analgesic use at 4 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Delmas 1982.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: not reported |
|
| Participants | Bisphosphonates: enrolled 7, analyzed 7.
Placebo: enrolled 6, analyzed 6. Sex (M/F): not reported Mean age (SD): Not reported Inclusion criteria: Stage (Durie 2005): NS Osteolytic lesion: NS Creatinine: < 1.8 mg/dL Calcium: Normal or elevated Other criteria: none |
|
| Interventions | Clodronate: 1600 mg/day orally; for 24 months. Control: identical placebo. | |
| Outcomes | SRE; total fractures; vertebral fracture; non‐vertebral fractures; total mortality; pain; calcium; adverse events. | |
| Notes | SRE: new osteolytic lesions or fractures or vertebral index ($);
vertebral fractures for control group not reported;
total mortality reported for clodronate group only;
adverse events stated only (data could not be extracted). Pain control assessment: Pain index at 12 months. Funding: Clodronate: Dichloromthylene diphosphonate (CI2MDP) was provided by Procter and Gamble Inc, USA. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Garcia‐Sanz 2015.
| Methods | Study design: Parallel, open‐label; not blinded, not placebo‐controlled phase IV study Study length: not reported Study conducted during: Jun 2010 – Jul 2012 |
|
| Participants | Bisphosphonates: enrolled 51, analyzed 51. The original plan was to enroll 96 patients per‐group. After enrollment of 75 patients an interim analysis was performed which suggested a beneficial effect with zoledronate. No treatment: 49 enrolled, analyzed 49. Sex (M/F): Bisphosphonates: 30/21 Control: 23/26 Mean age (range): 68 (40 – 87) Inclusion criteria: Stage (Durie 2005): Asymptomatic patients; stage not specified Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: Confirmed biochemical relapse of MM after an initial response, without symptoms derived from the disease |
|
| Interventions | Zoledronic acid: 4 mg in a 15‐minute intravenous infusion every 4 weeks, for a total of 12 doses, plus standard supportive care. No treatment: supportive care only. |
|
| Outcomes | Time to new treatment, overall survival, response rate, time to clinical symptoms, skeletal‐related events, time to a skeletal‐related event, hypercalcemia, osteonecrosis of jaw and renal dysfunction. | |
| Notes | SRE: bone fracture (vertebral and non‐vertebral), requirement for bone radiotherapy, requirement for bone surgery, or hypercalcemia. Funding: supported by an unrestricted grant from Novartis Farmaceutica S.A., Barcelona, Spain and sponsored by GEM/PETHEMA. Part of the work was also supported by grants PS09/01450 and PI12/02311 from the Spanish “Institutode Salud Carlos III (ISCIII)” and Fondo Europeo de DesarrolloRegional (FEDER), the Spanish Ministry of Economy andCompetitiveness and the European Regional Development Fund(ERDF) “Una manera de hacer Europa” (Innocampus; CEI‐2010‐1‐0010), the grant RD12/0036/0069 from “Red Temáticade Investigación Cooperativa en Cáncer (RTICC), and grant GCB‐120981SAN from the “Asociación Española Contra elCáncer (AECC)”. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The process for early stoppage is clearly described |
| Other bias | High risk | It is unclear if the interim analysis was pre‐planned or post‐hoc |
| Intention to treat Analysis | Low risk | Benefits data on accrued patients are analyzed according to ITT principle. |
Gimsing 2010.
| Methods | Study design: Parallel, double‐blind, comparing 30 mg versus 90 mg pamidronate Study length: median follow‐up: 3.4 years (range: 1.1 – 5.7) Study conducted during: Jan 2001 – Aug 2005 |
|
| Participants | Pamidornate 30 mg: enrolled 252, analyzed 198.
Pamidronate 90 mg: enrolled 252, analyzed 179. No treatment: 49 enrolled, analyzed 49. Sex (M/F): Pamidronate 90 mg: 149/101 Pamidronate 30 mg: 155/97 Mean age (range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NS Creatinine: < 400 µmol/L Calcium: NS Other criteria: No prior treatment with bisphosphonates |
|
| Interventions | Pamidronate: 90 mg in 500 mL of 5% dextrose in water, every 4 weeks for at least 36 months. Control: 30 mg in 500 mL of 5% dextrose in water, every 4 weeks for at least 36 months. | |
| Outcomes | SRE (total); vertebral fractures; non‐vertebral fractures; total mortality (#); calcium***; pain; adverse events. | |
| Notes | SRE: pathologic fracture or radiation treatment/surgery on bone or spinal cord compression. Funding: Nordic Cancer Union and Novartis Healthcare. COI statement included: “PG has received grant support from Janssen‐Cilag, a speaker’s bureau from Celgene, and fee as chairman of the data monitoring committee for BioInvent. AW has received grant support from Janssen‐Cilag, fees for consultancy from Janssen‐Cilag and Pharmion, and payment for advisory board participation from Novartis. HH‐H has received speakers fees. The Nordic Myeloma Study Group has received grant support from Janssen‐Cilag, Celgene, Amgen, and Nordpharma. All other authors declared no conflicts of interest.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Heim 1995.
| Methods | Study design: Parallel, placebo‐controlled study Study length: not reported Study conducted during: not reported (stay started in 1989) |
|
| Participants | Total: 170; 13 withdrawn after treatment. premature termination in additional 75.
Bisphosphonates: analyzed: 39.
Placebo: analyzed: 32. Sex (M/F): not available for the entire study cohort Mean age (range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: < 2.5 mg/dL Calcium: NS Other criteria: none |
|
| Interventions | Clodronate: 1600 mg/day orally; for 12 months. Control: no treatment. | |
| Outcomes | SRE; pain; total fractures; calcium; adverse events. | |
| Notes | SRE: bone progression ($); effect on pain characterized as the number of patients without pain or no need for therapy. Pain control assessment: Analgesic use OR presence of pain at 9 months. Funding: not reported. COI statement included: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Kraj 2000.
| Methods | Study design: Parallel, not double‐blind, placebo‐controlled study Study length: not reported Study conducted during: not reported (study started in 1989) |
|
| Participants | Bisphosphonates: analyzed 23; Placebo: analyzed 23. Sex (M/F): Bisphosphonates: 10/13 Control: 16/7 Mean age (SD): Bisphosphonates: 60 (10) Control: 66 (9) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: unclear Calcium: NS Other criteria: none |
|
| Interventions | Pamidronate 60 mg IV, every 4 weeks; indefinitely. control: no treatment. | |
| Outcomes | Total mortality, vertebral fractures. | |
| Notes | Funding: not reported COI statement included: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Lahtinen 1992.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: 1986 and 1989 |
|
| Participants | Bisphosphonates (clodronate): enrolled 168, analyzed 168.
Placebo: enrolled 168, analyzed 168. Sex (M/F): Bisphosphonates (clodronate): 84/84 Placebo: 82/86 Age: mean(SD): Bisphosphonates: Not reported Placebo: Not reported Inclusion criteria: Stage (Durie 2005): NR Osteolytic lesion: NR Creatinine: NS Calcium: NS Other criteria: No prior use of bisphosphonates and capacity to tolerate systemic chemotherapy |
|
| Interventions | Clodronate 2400 mg capsules orally daily. Identical placebo. Duration 24 months. | |
| Outcomes | SRE (total); total mortality; vertebral fractures; non‐vertebral fractures; calcium** | |
| Notes | Total mortality reported as a total number of deaths. Pain control assessment: Pain index at 12 months. Funding: Supported by Huhtamaki Oy, Leiras, Turku and the Finnish Cancer Foundation. COI statement included: NR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Leng 2002.
| Methods | Study design: Parallel, not double‐blind; placebo‐controlled study Study length: NR Study conducted during: NR |
|
| Participants | Bisphosphonates (pamidronate): enrolled 16, analyzed 16.
Placebo: enrolled 18, analyzed 18. Sex (M/F): Bisphosphonates: NR Placebo: NR Age: mean(SD): Bisphosphonates: NR Placebo: NR Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: Verbal rating scale > II |
|
| Interventions | Pamidronate 90 mg daily IV. Identical placebo. Duration indefinite. | |
| Outcomes | Pain (continuous data). | |
| Notes | Pain control assessment: Visual analogue scale. Funding: NR. COI statement included: NR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
McCloskey 2001.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: NR Study conducted during: 1986 to 1992 |
|
| Participants | Bisphosphonates: enrolled/analyzed 264.
Placebo: enrolled/analyzed 272. Sex (M/F): Bisphosphonates: 1.33 ratio Placebo: 1.43 ratio Age: Median (Inter‐quartile range) Bisphosphonates: 62 (55‐67) Placebo: 63 (57‐68) Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: at least one Creatinine: any Calcium: normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Clodronate 1600 mg orally daily. Identical placebo. Duration indefinite or progression. | |
| Outcomes | Total mortality*; SRE; total fractures; vertebral fractures; non‐vertebral fracture; pain; calcium.*** | |
| Notes | SRE: event‐free survival (pathological fractures or hypercalcemia), calculated from survival curves; outcome on calcium also reported as a dichotomous variable on the number of patients with hypercalcemia; pain calculated as the number of patients with maximal pain over 24 months. Pain control assessment: Severe pain at 24 months. Funding: Drug provided by Leiras Oy, Finland and MRC grant. COI statement included: NR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Menssen 2002.
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: NR Study conducted during: 1994 to 1996 |
|
| Participants | Bisphosphonates: enrolled 107, analyzed 99.
Placebo: enrolled 107, analyzed 99. Sex (M/F): Bisphosphonates: 53/46 Placebo: 51/48 Age: Mean (SD) Bisphosphonates: 62.9 (NR) Placebo: 63.4 (NR) Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: at least one Creatinine: ≤ 3 mg/dL Calcium: normal Other criteria: No bone specific treatment prior to entry |
|
| Interventions | Ibandronate 2 mg IV every month. Identical placebo, duration 24 months. Duration: 12 to 24 months. | |
| Outcomes | SRE (total)/year; mortality;* vertebral fractures (!); non‐vertebral fractures (!); hypercalcemia (!); pain (!). | |
| Notes | SRE: pathological fractures or vertebral fractures, hypercalcemia, severe bone pain, and bone radiotherapy or surgery.
Pain control assessment: Opiate usage. Funding: Roche Diagnostics GmbH, Mannheim, Germany. COI statement included: NR. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Morgan 2010.
| Methods | Study design: Parallel, open‐label; comparing zoledronate versus clodronate Study length: NR Study conducted during: 1994 to 1996 |
|
| Participants | Zoledronate: analyzed 981.
Clodronate: analyzed 979 Sex (M/F): Bisphosphonates: 53/46 Placebo: 51/48 Age: Mean (SD) Bisphosphonates: NR Placebo: NR Inclusion criteria: Stage (Durie 2005): I‐III (International Staging System) Osteolytic lesion: NS Creatinine: <5.65 mg/dL Calcium: NS Other criteria: No previous or concurrent active malignancies, No acute renal failure (serum creatinine > 500 µmol/L and unresponsive to 72 hours of rehydration |
|
| Interventions | Zoledronate 4 mg IV every 3‐4 weeks. Clodronate 1600 mg orally daily. Duration: until progression. | |
| Outcomes | Mortality; SREs; complete response; vertebral fractures, other fractures; hypercalcemia; renal failure; very good partial response; treatment‐related toxicities. | |
| Notes | SRE: vertebral fractures, other fractures, spinal cord compression, need for radiation or surgery to bone lesions, and new osteolytic bone lesions were recorded until disease progression. Complete response: negative immunofixation (100% M‐protein reduction) very good partial response: at least 90% M‐protein reduction with positive immunofixation. Funding: UK MRC, unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech for trial coordination and the laboratory studies. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. COI statement included: "F.E. Davies has honoraria from Speakers Bureau of Celgene and is a consultant/advisory board member of Celgene and Novartis. G. Cook is a consultant/advisory board member of and has honoraria from Speakers Bureau of Celgene. R.G. Owen has honoraria from Speakers Bureau of Celgene and Ortho Biotech, United Kingdom. G.H. Jackson has honoraria from Speakers Bureau of Celgene and is a consultant/advisory board member of Celgene and J&J. No potential conflicts of interest were disclosed by the other authors." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Musto 2003.
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1996 to 2001 |
|
| Participants | Bisphosphonates: analyzed 45.
Control: analyzed 45. Sex (M/F): Bisphosphonates: 26/19 Control: 24/21 Age: Median (range) Bisphosphonates: 67 (47‐79) Placebo: 68 (45‐80) Inclusion criteria: Stage (Durie 2005): I‐II Osteolytic lesion: Any Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Pamidronate 60 mg IV, every month. Control: NS. Duration: 1 year or until progression. | |
| Outcomes | Total skeletal‐related events; PFS, adverse events. | |
| Notes | SRE: single/multiple osteolytic lesions, pathological fractures and/or hypercalcemia. Funding: NR COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Musto 2008.
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1996 to 2001 |
|
| Participants | Bisphosphonates: enrolled 81, analyzed 81.
Control: enrolled 82, analyzed 82. Sex (M/F): Bisphosphonates: 43/38 Control: 47/35 Age: Median (range) Bisphosphonates: 66 (41‐82) Control: 67 (42‐84) Inclusion criteria: Stage (Durie 2005): I Osteolytic lesion: Any Creatinine: < 1.2 mg/dL Calcium: < 10 mg/dL Other criteria: No cytotoxic chemotherapy prior to entry |
|
| Interventions | Zoledronate 4 mg IV, every month. Control: observation. Duration: 1 year or until progression. | |
| Outcomes | SRE (total); PFS; ONJ. | |
| Notes | SRE: single/multiple osteolytic lesions, pathological fractures and/or hypercalcemia. The trial was prematurely stopped due to ONJ case in patient receiving zoledronate. Funding: NR. COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods used for concealment of allocation are described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Rosen 2003.
| Methods | Study design: Parallel, double‐blinded; double dummy; stratified; not placebo‐controlled Study length: 25 months Study conducted during: 1998 to 2000 |
|
| Participants | Zoledronate: enrolled 564, analyzed 561
Pamidronate: enrolled 558, analyzed 555 Sex (M/F): Zoledronate: NR Pamidronate: NR Age: Mean (SD) Zoledronate: 58 (NR) Pamidronate: 57 (NR) Inclusion criteria: Stage (Durie 2005): III Osteolytic lesion: at least one Creatinine: <= 3 mg/dL Calcium: <= 12 mg/dL Other criteria: Serum bilirubin ≤ 2.5 mg/dL. No prior treatment with bisphosphonates within 12 months of the screening visit |
|
| Interventions | Zoledronate 4 mg IV, every 4 weeks. Pamidronate 90 mg IV, every 4 weeks. Duration: 24 months. | |
| Outcomes | SREs | |
| Notes | SREs were defined as pathologic fracture, spinal cord compression, radiation therapy to bone, and surgery to bone.
Data for MM and breast carcinoma patients were reported in combined manner for all outcomes except SREs. Funding: Novartis Pharmaceuticals Corporation. COI statement included: "Dr. Seaman, Dr. Chen, and Dr. Reitsma are employed by Novartis Pharmaceuticals and may own stock in the company; Dr. Coleman has received honoraria from Novartis; and Dr. Hussein has received a research grant from Novartis." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods used for concealment of allocation are described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Sezer 2010.
| Methods | Study design: Parallel; open‐label; placebo‐controlled Study length: 48 months Study conducted during: 2000 to 2008 |
|
| Participants | Bisphosphonates: enrolled72; analyzed 71.
Control: enrolled71; analyzed 69. Sex (M/F): Zoledronate: 30/42 Control: 40/31 Age: Mean (SD) Zoledronate: 58.8 (12.02) Control: 62.1 (10.79) Inclusion criteria: Stage (Durie 2005): Asymptomatic patients stage I Osteolytic lesion: at least one Creatinine: NS Calcium: NS Other criteria: Patients with evidence of paraprotein in the serum or urine and bone marrow infiltration with plasma cells which represent more than 10% of the nucleated cells. |
|
| Interventions | Zoledronate 4 mg IV (or dose‐adjusted based on creatinine clearance) monthly. Control: observation. Duration: until progression or 48 months whichever comes first. | |
| Outcomes | Days of PFS; SREs (defined as: pathologic fracture, initiation of radiotherapy or surgery on bone, spinal cord compression or hypercalcemia); adverse events. | |
| Notes | PFS was defined as time from date of randomization to death from any cause or one of the following events:
The trial was stopped early due to slow recruitment. Funding: Novartis Pharmaceuticals Corporation. COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Unclear risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study is not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts are described. |
| Other bias | Unclear risk | Alpha and beta errors are not prespecified. However, a total sample size of 220 patients was specified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Terpos 2000.
| Methods | Study design: Parallel; not double‐blind; not placebo‐controlled Study length: NR Study conducted during: NR |
|
| Participants | Bisphosphonates: enrolled/analyzed 32.
Control: enrolled/analyzed 30. Sex (M/F): Pamidronate: 18/14 Control: 14/16 Age: Median (range) Pamidronate: 68 (55‐78) Control: 66 (46‐78) Inclusion criteria: Stage (Durie 2005): Stage I‐III Osteolytic lesion: NS Creatinine: <5 mg/dL Calcium: NS Other criteria: No prior treatment with any kind of bisphosphonate within 3 months before enrollment or calcitonine or mithramycin within 2 weeks before enrollment , or treatment with corticosteroids for any reason except part of the patient's chemotherapeutic regimen. |
|
| Interventions | Pamidronate: 90 mg IV monthly. Control: observation. Duration: 14 months | |
| Outcomes | Total mortality;* total fractures; vertebral fractures; non‐vertebral fracture; pain; hypercalcemia; abdominal pain. | |
| Notes | Data provided by the authors of the article. Pain control assessment: Opiate usage Funding: NR COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
Terpos 2003.
| Methods | Study design: Parallel; Not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1999 to 2001 |
|
| Participants | Pamidronate: enrolled 23, analyzed 23.
Ibandronate: enrolled 21, analyzed 20. Sex (M/F): Pamidronate: 12/11 Ibandronate: 12/9 Age: Median (range) Pamidronate: 66 (55‐78) Ibandronate: 65.5 (60‐77) Inclusion criteria: Stage (Durie 2005): Stage II‐III Osteolytic lesion: at least one Creatinine: >4 mg/dL Calcium: NS Other criteria: No prior treatment with any kind of bisphosphonate within 3 months before enrollment or calcitonine or mithramycin within 2 weeks before enrollment , or treatment with corticosteroids for any reason except part of the patient's chemotherapeutic regimen. |
|
| Interventions | Pamidronate: 90 mg IV monthly. Ibandronate: 4 mg IV monthly. Duration: 4 months. | |
| Outcomes | Hypocalcemia, hypercalcemia.**** | |
| Notes | Funding: NR. COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
Zhang 2012.
| Methods | Study design: Parallel; not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 2004 to 2009 |
|
| Participants | Bisphosphonates: enrolled/analyzed 33.
Control: enrolled/analyzed 20. Sex (M/F): Clodronate: 23/10 Control: 13/7 Age: Mean (SD) Clodronatee: 59.9 (8.1) Control: 58.5 (8.2) Inclusion criteria: Stage (Durie 2005): Stage II‐III Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: NS |
|
| Interventions | Clodronate: During the intermittent period of chemotherapy, clodronate injection 300 mg (Bonefos®, Bayer Schering Pharma, Leverkusen, Germany) was administered for 5 days in 250 mL glucose injection through slow intravenous drip; after that, Bonefos® capsules were administered orally at 1600 mg every day in the morning. Control: chemotherapy. Duration: NS. | |
| Outcomes | Bone metabolic markers | |
| Notes | Other notes: This study did not report any outcome of interest for this systematic review. Funding: NR. COI statement included: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study is not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
COI: conflict of interest; ITT: intention‐to‐treat; IV: intravenous; MM: multiple myeloma; ONJ: osteonecrosis of the jaw; OS: overall survival; PFS:progression‐free survival; SD: standard deviation; SRE: skeletal‐related events.
* mortality data obtained from authors; *$ mortality data derived using the Tierney method # total number of deaths reported in Berenson 1996 $ defined by reviewers **hypercalcemia defined as > 2.65 mmol/L &hypercalcemia defined as > 2.75 mmol/L ***hypercalcemia defined as > 3.00 mmol/L
**** hypercalcemia defined as presence of symptoms or serum calcium concentration, corrected for the serum albumin concentration, of at least 12.0 mg/dL or 3.0 mmol/L ! Data obtained from (author Fontana et al) and data from previous publication (abstract) were used
‐‐‐‐‐‐‐‐‐‐ The most common adverse effect that was reported was related to gastrointestinal symptoms (abdominal pain, diarrhea, pancreatitis). The number of patients with highest number of gastrointestinal symptoms was recorded and combined in the final analysis (since often it was not clear whether the same patients had one or more gastrointestinal symptoms). Effects on other organs (blood, kidney, liver, etc) were sporadically reported, and therefore not systematically extracted. However, the narrative summary was presented in the review. __________ Effect on pain was non uniformly described. Data were extractable from 8 trials. (Study by Brincker et al reported data as the number of pain episodes instead of the number of patients with pain. Paper by Belch et al did not report data in an extractable form.) Study by McCloskey et al reported effect on back pain only, while other studies reported effect on 'pain' without specifying site of pain. The study by Lahtinen et al also reported pain according to its severity. However, we extracted data on the number of patients with pain on bisphosphonates vs. placebo, except in the study by McCloskey et al, where the effect on pain refers to patients without 'marked improvement in back pain'.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2001 | Nonrandomized study |
| Barlogie 2008 | Nonrandomized study |
| Bergner 2007 | Nonrandomized study |
| Caparrotti 2003 | Nonrandomized and a combination therapy |
| Chiang 2013 | Observational phase IV study |
| Ciepluch 2002 | Nonrandomized and a combination therapy |
| Delea 2012 | Cost‐effectiveness study |
| Fizazi 2009 | Phase II denosumab randomized controlled trial |
| Henry 2014 | Denosumab randomized controlled trial |
| Kraj 2000a | Duplicate publication |
| Kraj 2002 | Enrolled only 9 patients. Only one pathological fracture was reported among 6 patients enrolled in zoledronate arm and one pathological fracture among 3 patients enrolled in pamidronate arm. |
| Lipton 2012 | Study addressing data from denosumab randomized controlled trials |
| Martin 2002 | No data of interest |
| Morris 2001 | Nonrandomized and a combination therapy |
| Spencer 2008 | Nonrandomized and a combination therapy |
| Tassinari 2007 | Observational study |
| Teoh 2012 | Nonrandomized study |
| Terpos 2010 | Prognostic study |
| Tosi 2006a | Combination therapy |
| Vadhan‐Raj 2012 | Denosumab randomized controlled trial |
| Vij 2009 | Phase II denosumab randomized controlled trial |
| Vogel 2004 | Nonrandomized study |
| Witzig 2013 | Patients in both the study arms received the same dose of zoledronate |
Characteristics of ongoing studies [ordered by study ID]
Lund 2014.
| Trial name or title | Prolonged protection from bone disease in multiple myeloma (Magnolia) |
| Methods | An open‐label phase 3 multicenter international randomised trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Active Comparator: zoledronic acid (treatment with zoledronic acid for 4 years) Placebo Comparator: no treatment (treatment with zoledronic acid withheld after two years) |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2015 |
| Contact information | Name: Thomas Lund, MD Ph.D. Phone: +45 21450256; Email: thomas.lund1@rsyd.dk |
| Notes |
Differences between protocol and review
Compared with the last version of this systematic review, we employed only the Bayesian method for the network meta‐analysis. That is, we have not used the frequentist method for performing adjusted indirect comparisons as described by Bucher, Glenny and Caldwell and colleagues. According to the revised guidelines for network meta analysis; we used the Bayesian methods under random‐effects multiple treatment comparisons (MTC) for indirect comparisons/network meta analysis (Higgins 1996; Lu 2004). Also, as per the peer reviewer's suggestions; we conducted additional sensitivity analyses based on the route of administration (oral versus intravenous) for gastrointestinal toxicity outcome (Analysis 3.8), which was not part of the protocol.
We also identified observational studies and case reports regarding bisphosphonate‐related ONJ in the MEDLINE database
Contributions of authors
For this update:
RM handsearched journals and references, extracted and analyzed data, wrote the initial and final drafts and participated in all phases of the project.
BD oversaw and co‐ordinated the group activity, maintained contact with Cochrane, provided vital content and methodological inputs, and edited the review.
AK extracted data, contacted a researcher regarding unpublished data, and maintained contact with Cochrane.
BM conducted the indirect comparisons using Bayesian methods.
BD, RM and AK discussed methodological problems.
All co‐authors interpreted data, provided constructive critiques and agreed on the final version of the paper.
Sources of support
Internal sources
Center for Evidence‐based Medicine,The University of South Florida, USA.
Department of Internal Medicine, University of Bonn, Germany.
External sources
Leukämie‐Initiative Bonn e.v., Germany.
Cochrane Haematological Malignancies Group (CHMG), Germany.
Declarations of interest
RM: None known.
AK: None known.
BM: None known.
BD: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Attal 2006 {published data only}
- Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood 2006;108(10):3289‐94. [PUBMED: 16873668] [DOI] [PubMed] [Google Scholar]
Aviles 2007 {published data only}
- Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta‐Guzman J. Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Medical Oncology (Northwood, London, England) 2007;24(2):227‐30. [PUBMED: 17848748] [DOI] [PubMed] [Google Scholar]
Aviles 2013 {published data only}
- Aviles A, Neri N, Huerta‐Guzman J, Nambo MJ. Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high‐dose chemotherapy and stem‐cell transplantation. Current oncology (Toronto, Ont.) 2013; Vol. 20, issue 1:e13‐20. [DOI: 10.3747/co.20.1055; CN‐00912202] [DOI] [PMC free article] [PubMed]
Belch 1991 {published data only}
- Belch AR, Bergsagel DE, Wilson K. Effect of daily etidronate on the osteolysis of multiple myeloma. Journal of Clinical Oncology 1991;9:1397‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berenson 1998a {published data only}
- Berenson JR, Lichtenstein A, Porter L. Long‐term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Journal of Clinical Oncology 1998;16:593‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. New England Journal of Medicine 1996;334:488‐93. [DOI] [PubMed] [Google Scholar]
Brincker 1998 {published data only}
- Abildgaard N, Rungby J, Glerup H, Brixen K, Kassem M, Brincker H, et al. Long‐term oral pamidronate treatment inhibits osteoclastic bone resorption and bone turnover without affecting osteoblastic function in multiple myeloma. European Journal of Hematology 1998;61:128‐34. [DOI] [PubMed] [Google Scholar]
- Brincker JW, Abildgaard N. Failure of oral pamidronate to reduce skeletal morbidity in multiple myeloma: a double‐blind placebo‐controlled trial. British Journal of Haematology 1998;101:280‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daragon 1993 {published data only}
- Daragon A, Humez C, Michot CXLL. Treatment of multiple myeloma with etidronate results of a multicentre double‐blind study. European Journal of Medicine 1993;2:449‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Delmas 1982 {published data only}
- Delmas PD, Charhon S, Chapuy MC, Vignon E, Briancon D, Edouard C, et al. Long‐term effects of dichloromethylene diphosphonate (CI2MDP) on skeletal lesions in multiple myeloma. Metabolic Bone Disease and Related Research 1982;4:163‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Sanz 2015 {published data only}
- Garcia‐Sanz R, Oriol A, Moreno MJ, Rubia J, Payer AR, Hernandez MT, et al. Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: results of the randomized AZABACHE Spanish trial. Haematologica 2015;100(9):1207‐13. [PUBMED: 26069291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gimsing 2010 {published data only}
- Gimsing P, Carlson K, Turesson I, Fayers P, Waage A, Vangsted A, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double‐blind, randomised controlled trial. Lancet Oncology 2010;11(10):973‐82. [PUBMED: 20863761] [DOI] [PubMed] [Google Scholar]
Heim 1995 {published and unpublished data}
- Clemens MR, Fessele K, Heim ME. Multiple myeloma: effect of daily dichloromethylene bisphosphonate on skeletal Multiple myeloma: effect of daily dichloromethylene bisphosphonate on skeletal complications. Annals of Hematology 1993;66:141‐6. [DOI] [PubMed] [Google Scholar]
- Heim ME, Clemens MR, Queißer W, Pecherstorfer M, Boewer C, Herold M, et al. Prospective randomized trial of dichloromethilene bisphosphonate (clodronate) in patients with multiple myeloma. Onkologie 1995;18:439‐48. [Google Scholar]
Kraj 2000 {published data only}
- Kraj M, Poglód R, Pawlikowsky J, Maj S. The effect of long‐term pamidronate treatment on skeletal morbidity in advanced multiple myeloma. Acta Haematologica Polonica 2000;31:379‐89. [Google Scholar]
- Kraj M, Póglod R, Pawlikowski J, Maj S, Nasiloska B. Effect of pamidronate on skeletal morbidity in myelomatosis. Part 1: The results of the 12 months of pamidronate therapy. Acta Poloniae Pharmaceutica 2000;57 (suppl 1):113‐6. [PubMed] [Google Scholar]
Lahtinen 1992 {published data only}
- Lahtinen R, Laakso M, Palva I. Randomized, placebo‐controlled multicentre trial of clodronate in multiple myeloma. Lancet 1992;340:1049‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leng 2002 {published data only}
- Leng Y, Chen SL, Shi HZ. Effects of pamidronate disodium (Bonin) combined with chemotherapy on bone pain in multiple myeloma. Hang tian yi xue yu yi xue gong cheng [Space Medicine & Medical Engineering] 2002;15(5):377‐8. [PUBMED: 12449148] [PubMed] [Google Scholar]
McCloskey 2001 {published and unpublished data}
- McCloskey EV, Dunn JA, Kanis JA, MacLennan ICM, Drayson MT. Long‐term follow‐up of a prospective, double‐blind, placebo‐controlled randomised trial of clodronate in multiple myeloma. British Journal of Haematology 2001;113:1035‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McCloskey EV, MacLennan IC, Drayson MT, Chapman C, Dunn J, Kanis JA. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. British Journal of Haematology 1998;100:317‐25. [DOI] [PubMed] [Google Scholar]
Menssen 2002 {published data only}
- Fontana A, Herrmann Z, Menssen HD, Sakalova A, Boewer C, Facon T, et al. Effects of intravenous ibandronate therapy on skeletal related events (SRE) and survival in patients with advanced multiple myeloma. Blood 1998;92(Suppl 1):106a. [DOI] [PubMed] [Google Scholar]
- Menssen HD, Sakalová A, Fontana A, Herrmann Z, Boewer C, Facon T, et al. Effects of long‐term intravenous ibandronate therapy on skeletal‐related events, survival and bone resorption in patients with advanced multiple myeloma. Journal of Clinical Oncology 2002;20 (9):2353‐9. [DOI] [PubMed] [Google Scholar]
Morgan 2010 {published data only}
- Jackson GH, Morgan GJ, Davies FE, Wu P, Gregory WM, Bell SE, et al. Osteonecrosis of the jaw and renal safety in patients with newly diagnosed multiple myeloma: Medical Research Council Myeloma IX Study results. British Journal of Haematology 2014;166(1):109‐17. [PUBMED: 24673708] [DOI] [PubMed] [Google Scholar]
- Larocca A, Child JA, Cook G, Jackson GH, Russell N, Szubert A, et al. The impact of response on bone‐directed therapy in patients with multiple myeloma. Blood 2013;122(17):2974‐7. [PUBMED: 23974194] [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, et al. Long‐term follow‐up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clinical Cancer Research : an official journal of the American Association for Cancer Research 2013;19(21):6030‐8. [PUBMED: 23995858] [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First‐line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 2010;376(9757):1989‐99. [PUBMED: 21131037] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GJ, Jackson GH, Davies F, Wu P, Gregory W, Bell SE, et al. Efficacy and side‐effect profile of long‐term bisphosphonate therapy in patients (pts) with multiple myeloma (MM): MRC myeloma IX study results. Journal of Clinical Oncology 2012; Vol. 30, issue 15 SUPPL. 1. [CN‐01028214]
Musto 2003 {published data only}
- D'arena G, Gobbi PG, Broglia C, Sacchi S, Quarta G, Baldini L, et al. Pamidronate versus observation in asymptomatic myeloma: final results with long‐term follow‐up of a randomized study. Leukemia & Lymphoma 2011;1:1‐5. [PUBMED: 21299465] [DOI] [PubMed] [Google Scholar]
- Musto P, Falcone A, Sanpaolo G, Bodenizza C, Cascavilla N, Melillo L, et al. Pamidronate reduces skeletal events but does not improve progression‐free survival in early‐stage untreated myeloma: results of a randomized trial. Leukemia & Lymphoma 2003;44(9):1545‐8. [PUBMED: 14565658] [DOI] [PubMed] [Google Scholar]
Musto 2008 {published data only}
- Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 2008;113(7):1588‐95. [PUBMED: 18683218] [DOI] [PubMed] [Google Scholar]
Rosen 2003 {published data only}
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal‐related events in patients with osteolytic metastases: a double‐blind, randomized dose‐response study. Cancer 2001;91(7):1191‐200. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer 2003;98(8):1735‐44. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double‐blind, comparative trial. Cancer 2001;7(5):377‐87. [PubMed] [Google Scholar]
- Rosen LS, Gordon DH, Dugan W Jr, Major P, Eisenberg PD, Provencher L, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36‐43. [DOI] [PubMed] [Google Scholar]
Sezer 2010 {published data only}
- Sezer O, Jakob C, Aldaoud A, Schmidt K, Schwarzer A, Maintz C, et al. Zoledronic acid therapy versus control in patients with multiple myeloma in stage I (Durie & Salmon): results of a phase III study of the DSMM and OSHO. 15th Congress of the European Hematology Association Abstr 0361. Barcelona, Spain, Jun 10–13, 2010.
Terpos 2000 {published and unpublished data}
- Terpos E, Palermos J, Tsionos K, Anargyrou K, Viniou N, Papassavas P, et al. Effect of pamidronate administration on markers of bone turnover and disease activity in multiple myeloma. European Journal of Haematology 2000;65:331‐6. [DOI] [PubMed] [Google Scholar]
Terpos 2003 {published data only (unpublished sought but not used)}
- Terpos E, Viniou N, Fuente J, Meletis J, Voskaridou E, Karkantaris C, et al. Pamidronate is superior to ibandronate in decreasing bone resorption, interleukin‐6and b2‐microglobulin in multiple myeloma. European Journal of Haematology 2003;70:34‐42. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang X, Chang C, Zhao Y, Wu L, Zhang Z, Li X. The effect of the combination of bisphosphonates and conventional chemotherapy on bone metabolic markers in multiple myeloma patients. Hematology (Amsterdam, Netherlands) 2012;17(5):255‐60. [PUBMED: 22971530] [DOI] [PubMed] [Google Scholar]
- Zhang X, Chang CK, Zhang Z, Zhao YS, Xiao C, Li X. [Influence of bisphosphonate combined with chemotherapy on bone mineral density of patients with multiple myeloma]. Zhongguo shi yan xue ye xue za zhi / Zhongguo bing li sheng li xue hui [Journal of experimental hematology / Chinese Association of Pathophysiology] 2012;20(5):1135‐8. [PUBMED: 23114134] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2001 {published data only}
- Ali SM, Esteva FJ, Hortobagyi G, Harvey H, Seaman J, Knight R, et al. Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. Journal of Clinical Oncology 2001;19(14):3434‐7. [DOI] [PubMed] [Google Scholar]
Barlogie 2008 {published data only}
- Barlogie B, Rhee F, Shaughnessy JD Jr, Epstein J, Yaccoby S, Pineda‐Roman M, et al. Seven‐year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood 2008;112(8):3122‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergner 2007 {published data only}
- Bergner R, Henrich DM, Hoffmann M, Honecker A, Mikus G, Nauth B, et al. Renal safety and pharmacokinetics of ibandronate in multiple myeloma patients with or without impaired renal function. Journal of Clinical Pharmacology 2007;47(8):942‐50. [DOI] [PubMed] [Google Scholar]
Caparrotti 2003 {published data only}
- Caparrotti G, Catalano L, Feo C, Vallone R, Pagnini D, Rotoli B. Perspective study on pamidronate in stage I multiple myeloma. Hematology Journal 2003;4(6):459‐60. [DOI] [PubMed] [Google Scholar]
Chiang 2013 {published data only}
- Chiang PH, Wang HC, Lai YL, Chen SC, Yen‐Hwa W, Kok CK, et al. Zoledronic acid treatment for cancerous bone metastases: a phase IV study in Taiwan. Journal of Cancer Research and Therapeutics 2013;9(4):653‐9. [PUBMED: 24518712] [DOI] [PubMed] [Google Scholar]
Ciepluch 2002 {published data only}
- Ciepluch H, Baran W, Hellmann A. Combination of pamidronate and thalidomide in the therapy of treatment‐resistant multiple myeloma. Medical Science Monitor 2002;8(4):PI31‐PI36. [PubMed] [Google Scholar]
Delea 2012 {published data only}
- Delea TE, Rotter J, Taylor M, Chandiwana D, Bains M, Ouagari K, et al. Cost‐effectiveness of zoledronic acid vs clodronic acid for newly‐diagnosed multiple myeloma from the United Kingdom healthcare system perspective. Journal of Medical Economics 2012;15(3):454‐64. [PUBMED: 22316275] [DOI] [PubMed] [Google Scholar]
Fizazi 2009 {published data only}
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27(10):1564‐71. [PUBMED: 19237632] [DOI] [PubMed] [Google Scholar]
Henry 2014 {published data only}
- Henry D, Vadhan‐Raj S, Hirsh V, Moos R, Hungria V, Costa L, et al. Delaying skeletal‐related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Supportive Care in Cancer : official journal of the Multinational Association of Supportive Care in Cancer 2014;22(3):679‐87. [PUBMED: 24162260] [DOI] [PubMed] [Google Scholar]
Kraj 2000a {published data only}
- Kraj M, Póglod R, Pawlikowski J, Maj S, Nasilowska B. Effect of pamidronate on skeletal morbidity in myelomatosis. Part 1. The results of the first 12 months of pamidronate therapy. Acta Poloniae Pharmaceutica 2000;57(suppl 1):113‐6. [PubMed] [Google Scholar]
Kraj 2002 {published data only}
- Kraj M, Poglod R, Maj S, Pawlikowski J, Sokolowska U, Szczepanik J. Comparative evaluation of safety and efficacy of pamidronate and zoledronic acid in multiple myeloma patients (single center experience). Acta Poloniae Pharmaceutica 2002;59(6):478‐82. [PUBMED: 12669777] [PubMed] [Google Scholar]
Lipton 2012 {published data only}
- Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal‐related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. European Journal of Cancer (Oxford, England : 1990) 2012;48(16):3082‐92. [PUBMED: 22975218] [DOI] [PubMed] [Google Scholar]
Martin 2002 {published data only}
- Martín A, García‐Sanz R, Hernández J, Bladé J, Suquía B, Fernández‐Calvo J, et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti‐tumour effect. British Journal of Haematology 2002;118(1):239‐42. [DOI] [PubMed] [Google Scholar]
Morris 2001 {published data only}
- Morris TC, Ranaghan L, Morrison J. Phase II trial of clarithromycin and pamidronate therapy in myeloma. Medical Oncology 2001;18(1):79‐84. [DOI] [PubMed] [Google Scholar]
Spencer 2008 {published data only}
- Spencer A, Roberts A, Kennedy N, Ravera C, Cremers S, Bilic S, et al. Renal safety of zoledronic acid with thalidomide in patients with myeloma: a pharmacokinetic and safety sub‐study. BMC Clinical Pharmacology 2008;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tassinari 2007 {published data only}
- Tassinari D, Poggi B, Nicoletti S, Fantini M, Tamburini E, Possenti C, et al. Zoledronic acid treatment at home: safety data from an observational prospective trial. Journal of Palliative Medicine 2007;10(2):352‐8. [DOI] [PubMed] [Google Scholar]
Teoh 2012 {published data only}
- Teoh G, Chen Y, Kim K, Srivastava A, Pai VR, Yoon SS, et al. Lower dose dexamethasone/thalidomide and zoledronic acid every 3 weeks in previously untreated multiple myeloma. Clinical Lymphoma, Myeloma & Leukemia 2012;12(2):118‐26. [PUBMED: 22206804] [DOI] [PubMed] [Google Scholar]
Terpos 2010 {published data only}
- Terpos E, Berenson J, Cook RJ, Lipton A, Coleman RE. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia 2010;24(5):1043‐9. [PUBMED: 20376081] [DOI] [PubMed] [Google Scholar]
Tosi 2006a {published data only}
- Tosi P, Zamagni E, Cellini C, Parente R, Cangini D, Tacchetti P, et al. First‐line therapy with thalidomide, dexamethasone and zoledronic acid decreases bone resorption markers in patients with multiple myeloma. European Journal of Haematology 2006;76(5):399‐404. [DOI] [PubMed] [Google Scholar]
Vadhan‐Raj 2012 {published data only}
- Vadhan‐Raj S, Moos R, Fallowfield LJ, Patrick DL, Goldwasser F, Cleeland CS, et al. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Annals of Oncology : official journal of the European Society for Medical Oncology / ESMO 2012;23(12):3045‐51. [PUBMED: 22851406] [DOI] [PubMed] [Google Scholar]
Vij 2009 {published data only}
- Vij R, Horvath N, Spencer A, Taylor K, Vadhan‐Raj S, Vescio R, et al. An open‐label, phase 2 trial of denosumab in the treatment of relapsed or plateau‐phase multiple myeloma. American Journal of Hematology 2009;84(10):650‐6. [PUBMED: 19714603] [DOI] [PubMed] [Google Scholar]
Vogel 2004 {published data only}
- Vogel CL, Yanagihara RH, Wood AJ, Schnell FM, Henderson C, Kaplan BH, et al. Safety and pain palliation of zoledronic acid in patients with breast cancer, prostate cancer, or multiple myeloma who previously received bisphosphonate therapy. Oncologist 2004;9(6):687‐95. [DOI] [PubMed] [Google Scholar]
Witzig 2013 {published data only}
- Witzig TE, Laumann KM, Lacy MQ, Hayman SR, Dispenzieri A, Kumar S, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 2013;27(1):220‐5. [PUBMED: 22902362] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, Mandrekar S, Detweiler‐Short K, Q Lacy M, Laumann K, Dispenzieri A, et al. A phase III randomized trial of thalidomide (THAL) plus zoledronic acid (ZLD) versus zoledronic acid alone in patients with early stage multiple myeloma (MC0289). Blood 2010; Vol. 116, issue 21. [CN‐01032934]
References to ongoing studies
Lund 2014 {published data only}
- Thomas Lund. Magnolia study prolonged protection from bone disease in multiple myeloma. An open label phase 3 multicenter international randomised trial. https://clinicaltrials.gov/ct2/show/NCT02286830.
Additional references
Acito 1994
- Acito AJ, Kasra M, Lee JM, Grynpas MD. Effect of intermittent administration of pamidronate on the mechanical proprieties of canine cortical and trabecular bone. Journal of Orthopedic Research 1994;12:742‐6. [DOI] [PubMed] [Google Scholar]
Alexanian 1994
- Alexanian R, Dimopulos M. The treatment of multiple myeloma. New England Journal of Medicine 1994;330:484‐9. [DOI] [PubMed] [Google Scholar]
Anderson 2015
- Anderson KC, Alsina M, Atanackovic D, Biermann JS, Chandler JC, Costello C, et al. Multiple Myeloma, Version 2.2016: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN 2015;13(11):1398‐435. [PUBMED: 26553768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aparicio 1998
- Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 1998;12:220‐9. [DOI] [PubMed] [Google Scholar]
Atula 2003
- Atula S, Powles T, Paterson A, McCloskey E, Nevalainen J, Kanis J. Extended safety profile of oral clodronate after long term use in primary breast cancer patients. Drug Safety 2003;26:661‐71. [DOI] [PubMed] [Google Scholar]
Badros 2006
- Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. Journal of Clinical Oncology 2006;24(6):945‐52. [DOI] [PubMed] [Google Scholar]
Bagan 2006
- Bagan JV, Jimenez Y, Murillo J, Hernandez S, Poveda R, Sanchis JM, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases [Letter]. Oral Oncology 2006;42:327‐9. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: Rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Battley 2006
- Battley J, Jayathissa S, Seneviratne E. Jaw osteonecrosis associated with bisphosphonates. New Zealand Medical Journal 2006;119(1246):U2341. [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Berenson 1998b
- Berenson JR. The efficacy of pamidronate disodium in the treatment of osteolytic lesions and bone pain in multiple myeloma. Reviews in Contemporary Pharmacotherapy 1998;9:195‐203. [Google Scholar]
Berenson 2011
- Berenson JR, Yellin O, Crowley J, Makary A, Gravenor DS, Yang HH, et al. Prognostic factors and jaw and renal complications among multiple myeloma patients treated with zoledronic acid. American Journal of Hematology 2011;86(1):25‐30. [PUBMED: 21120861] [DOI] [PubMed] [Google Scholar]
Braun 2006
- Braun E, Iacono VJ. Bisphosphonates: case report of non surgical periodontal therapy and osteochemonecrosis. International Journal of Periodontics and Restorative Dentistry 2006;26(4):315‐9. [PubMed] [Google Scholar]
Broglia 2006
- Broglia C, Merlati G, Valentino F, Benatti C, Gobbi P, Ascari E, et al. Avascular jaw osteonecrosis associated with bisphosphonate therapy. Recenti Progressi in Medicina (Roma) 2006;97(3):140‐4. [PubMed] [Google Scholar]
Brown 2004
- Brown JE, Neville‐Webbe H, Coleman RE. The role of bisphosphonates in breast and prostate cancers. Endocrine Related Cancer 2004;11:207‐24. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [PUBMED: 9250266] [DOI] [PubMed] [Google Scholar]
Bujanda 2007
- Bujanda AD, Sarmiento BU, Suarez CMA, Morales AJ. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Annals of Oncology 2007;18(3):556‐60. [DOI] [PubMed] [Google Scholar]
Calvo‐Villas 2006
- Calvo‐Villas JM, Tapia Torres M, Govantes Rodríguez J, Carreter de Granda E, Sicilia Guillén F. Osteonecrosis of the jaw in patients with multiple myeloma during and after treatment with zoledronic acid. Medicina Clinicia (Barcelona) 2006;127(15):576‐9. [DOI] [PubMed] [Google Scholar]
Capalbo 2006
- Capalbo S, Delia M, Diomede D, Dargenio M, Chiefa A, Favia G, et al. Jaw osteonecrosis associated with use of bisphosphonates and chemotherapy: paradoxical complication of treatment of bone lesions in multiple myeloma patients. International Journal of Hematology 2006;83(5):439‐42. [DOI] [PubMed] [Google Scholar]
Carneiro 2006
- Carneiro E, Vibhute P, Montazem A, Som PM. Bisphosphonate‐associated mandibular osteonecrosis. American Journal of Neuroradiology 2006;27(5):1096‐7. [PMC free article] [PubMed] [Google Scholar]
Carter 2005
- Carter G, Goss AN, Doecke C. Bisphosphonates and avascular necrosis of the jaw: a possible association. Medical Journal of Australia (Sydney) 2005;182:413‐5. [DOI] [PubMed] [Google Scholar]
Cassidy 2006
- Cassidy J, Bisset D, Spence RAJ, Payne M, editors. Oxford Handbook of Oncology. New York: Oxford University Press, 2006:504‐5. [Google Scholar]
Cetiner 2009
- Cetiner S, Sucak GT, Kahraman SA, Aki SZ, Kocakahyaoglu B, Gultekin SE, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. Journal of Bone and Mineral Metabolism 2009;27(4):435‐43. [PUBMED: 19240969] [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLOS One 2013;8(10):e76654. [PUBMED: 24098547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2003
- Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. New England Journal of Medicine 2003;349:1676‐9. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke BM, Boyette J, Vural E, Suen JY, Anaissie EJ, Stack BC Jr. Bisphosphonates and jaw osteonecrosis: the UAMS experience. Otolaryngology‐‐Head and Neck Surgery 2007;136(3):396‐400. [DOI] [PubMed] [Google Scholar]
Cornell 2015
Corso 2007
- Corso A, Varettoni M, Zappasodi P, Klersy C, Mangiacavalli S, Pica G, et al. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia 2007;21(7):1545‐8. [DOI] [PubMed] [Google Scholar]
Curi 2007
- Curi MM, Cossolin GS, Koga DH, Araújo SR, Feher O, dos Santos MO, et al. Treatment of avascular osteonecrosis of the mandible in cancer patients with a history of bisphosphonate therapy by combining bone resection and autologous platelet‐rich plasma: report of 3 cases. Journal of Oral Maxillofacial Surgery 2007;65(2):349‐55. [DOI] [PubMed] [Google Scholar]
Dannemann 2007
- Dannemann C, Graetz KW, Riener MO, Zwahlen RA. Jaw osteonecrosis related to bisphosphonate therapy: a severe secondary disorder. Bone 2007;40(4):828‐34. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Dhodapkar 1998
- Dhodapkar MV, Singh J, Mehta J, Fassas A, Desikan KR, Perlman M, et al. Anti‐myeloma activity of pamidronate in vivo. British Journal of Haematology 1998;103:530‐2. [DOI] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [PUBMED: 20213715] [DOI] [PubMed] [Google Scholar]
Diego 2007
- Diego R, D'Orto O, Pagani D, Agazzi A, Marzano U, Derada Troletti G, et al. Bisphosphonate‐associated osteonecrosis of the jaws: a therapeutic dilemma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;103(3):e1‐5. [DOI] [PubMed] [Google Scholar]
Dimitrakopoulos 2006
- Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonate‐induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. International Journal of Oral and Maxillofacillary Surgery 2006;35(7):588‐93. [DOI] [PubMed] [Google Scholar]
Dimopoulos 2006
- Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 2006;91:968‐71. [PubMed] [Google Scholar]
Drake 2008
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proceedings 2008;83(9):1032‐45. [PUBMED: 18775204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunford 2001
- Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure‐activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen‐containing bisphosphonates. Journal of Pharmacology and Experimental Therapeutics 2001;296(2):235‐42. [PUBMED: 11160603] [PubMed] [Google Scholar]
Durie 2005
Egger 2001
- Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd edition. London: BMJ, 2001. [Google Scholar]
Elad 2006
- Elad S, Yarom N, Hamed W, Ayalon S, Yahalom R, Regev E. Osteomylelitis and necrosis of the jaw in patients treated with bisphosphonates: a comparative study focused on multiple myeloma. Clinical and Laboratory Haematology 2006;28(6):393‐8. [DOI] [PubMed] [Google Scholar]
Ficarra 2005
- Ficarra G, Beninati F, Rubino I, Vannucchi A, Longo G, Tonelli P, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. Journal of Clinical Periodontology 2005;32:1123‐8. [DOI] [PubMed] [Google Scholar]
Fortuna 2012
- Fortuna G, Ruoppo E, Pollio A, Aria M, Adamo D, Leuci S, et al. Multiple myeloma vs. breast cancer patients with bisphosphonates‐related osteonecrosis of the jaws: a comparative analysis of response to treatment and predictors of outcome. Journal of Oral Pathology & Medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 2012;41(3):222‐8. [PUBMED: 22092390] [DOI] [PubMed] [Google Scholar]
Fromm 1991
- Fromm GA, Vega E, Plantalech L, Galich AM, Mautalen CA. Differential action of pamidronate on trabecular and cortical bone in women with involutional osteoporosis. Osteoporosis International 1991;1:126‐33. [DOI] [PubMed] [Google Scholar]
Gabbert 2015
- Gabbert TI, Hoffmeister B, Felsenberg D. Risk factors influencing the duration of treatment with bisphosphonates until occurrence of an osteonecrosis of the jaw in 963 cancer patients. Journal of Cancer Research and Clinical Oncology 2015;141(4):749‐58. [PUBMED: 25319961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gander 2014
- Gander T, Obwegeser JA, Zemann W, Gratz KW, Jacobsen C. Malignancy mimicking bisphosphonate‐associated osteonecrosis of the jaw: a case series and literature review. Oral Surgery, Oral Medicine, oral pathology and Oral Radiology 2014;117(1):32‐6. [PUBMED: 24332325] [DOI] [PubMed] [Google Scholar]
Garcia‐Garay 2006
- Garcia‐Garay C, Gonzalez‐Garcia C, Juliana Majado M, Santos T, Borrego D. Osteonecrosis of the jaw in multiple myeloma patients: experience of two hospitals. Blood 2006;108(11):Abstract 5086. [Google Scholar]
GRADEpro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Greipp 2005
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. Journal of Clinical oOncology : official journal of the American Society of Clinical Oncology 2005;23(15):3412‐20. [PUBMED: 15809451] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Hansen 2006
- Hansen T, Kunkel M, Weber A, Kirkpatrick CJ. Osteonecrosis of the jaws in patients treated with bisphosphonates: histomorphologic analysis in comparison with infected osteoradionecrosis. Journal of Oral Pathology & Medicine 2006;35(3):155‐60. [DOI] [PubMed] [Google Scholar]
Hay 2006
- Hay KD, Bishop PA. Association of osteonecrosis of the jaws and bisphosphonate pharmacotherapy: dental implications. New Zealand Dental Journal 2006;102(1):4‐9. [PubMed] [Google Scholar]
Herbozo 2007
- Herbozo PJ, Briones DL, Ferres AJ, Torrealba RL. Severe spontaneous cases of bisphosphonate‐related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery 2007;65(8):1650‐4. [DOI] [PubMed] [Google Scholar]
Higgins 1996
Higgins 2004
- Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine 2004;23(11):1663‐82. [PUBMED: 15160401] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
- Jonathan AC Sterne, Matthias Egger and David Moher (editors) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Hoff 2006
- Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Osteonecrosis of the jaw in patients receiving intravenous bisphosphonate therapy. Journal of Clinical Oncology 2006;24:8528a. [Google Scholar]
Junquera 2009
- Junquera L, Gallego L, Cuesta P, Pelaz A, Vicente JC. Clinical experiences with bisphosphonate‐associated osteonecrosis of the jaws: analysis of 21 cases. American Journal of Otolaryngology 2009;30(6):390‐5. [PUBMED: 19880027] [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kademani 2006
- Kademani D, Koka S, Lacy MQ, Rajkumar SV. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clinic Proceedings 2006;81(8):1100‐3. [DOI] [PubMed] [Google Scholar]
Kamoh 2012
- Kamoh AK, Ogle O. Bisphosphonate‐related osteonecrosis of the jaws‐‐a case report. Compendium of Continuing Education in Dentistry (Jamesburg, N.J. : 1995) 2012;33(5):e74‐7. [PUBMED: 23268588] [PubMed] [Google Scholar]
Katz 2005
- Katz H. Endodontic implications of bisphosphonate‐associated osteonecrosis of the jaws: a report of three cases. Journal of Endodontics 2005;31:831‐4. [DOI] [PubMed] [Google Scholar]
Khamaisi 2006
- Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I, et al. Possible association between diabetes and bisphosphonate‐related jaw osteonecrosis. Journal of Clinical Endocrinology and Metabolism 2007;92(3):1172‐5. [DOI] [PubMed] [Google Scholar]
Kumar 2007
- Kumar V, Pass B, Guttenberg SA, Ludlow J, Emery RW, Tyndall DA, et al. Bisphosphonate‐related osteonecrosis of the jaws: a report of three cases demonstrating variability in outcomes and morbidity. Journal of the American Dental Association 2007;138(5):602‐9. [DOI] [PubMed] [Google Scholar]
Kut 2004
- Kut V, Mehta J, Tariman J, et al. Osteonecrosis of the jaw in myeloma patients receiving pamidronate or zoledronate. Blood 2004;104:Abstract 4933. [Google Scholar]
Lambert 2005
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Statistics in Medicine 2005;24(15):2401‐28. [PUBMED: 16015676] [DOI] [PubMed] [Google Scholar]
Laupacis 1988
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318:1728‐33. [DOI] [PubMed] [Google Scholar]
Lazarovici 2009
- Lazarovici TS, Yahalom R, Taicher S, Elad S, Hardan I, Yarom N. Bisphosphonate‐related osteonecrosis of the jaws: a single‐center study of 101 patients. Journal of Oral and Maxillofacial Surgery 2009;67(4):850‐5. [PUBMED: 19304045] [DOI] [PubMed] [Google Scholar]
Lenz 2005
- Lenz JH, Steiner‐Krammer B, Schmidt W, Fietkau R, Mueller PC, Gundlach KK. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates?. Jornal of Craniomaxillofacillary Surgery 2005;33:395‐403. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [PUBMED: 19631507] [DOI] [PubMed] [Google Scholar]
Lu 2004
Lufkin 1994
- Lufkin EG, Argueta R, Whitaker MD, Cameron AL, Wong VH, Egan KS, et al. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporosis International 1994;4:320‐2. [DOI] [PubMed] [Google Scholar]
Lugassy 2004
- Lugassy G, Shaham R, Nemets A, Ben‐Dor D, Nahlieli O. Severe osteomyelitis of the jaw in long‐term survivors of multiple myeloma: a new clinical entity. American Journal of Medicine 2004;117:440‐1. [DOI] [PubMed] [Google Scholar]
Magopoulos 2007
- Magopoulos C, Karakinaris G, Telioudis Z, Vahtsevanos K, Dimitrakopoulos I, Antoniadis K, et al. Osteonecrosis of the jaws due to bisphosphonate use: a review of 60 cases and treatment proposals. American Journal of Otolaryngology 2007;28(3):158‐63. [DOI] [PubMed] [Google Scholar]
Marunick 2005
- Marunick M, Miller R, Gordon S. Adverse oral sequelae to bisphosphonate administration. Journal of Michigan Dental Association 2005;87:44‐9. [PubMed] [Google Scholar]
Melo 2005
- Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. Journal of the American Dental Association 2005;136(12):1675‐81. [DOI] [PubMed] [Google Scholar]
Merigo 2006
- Merigo E, Manfredi M, Meleti M, Guidotti R, Ripasarti A, Zanzucchi E, et al. Bone necrosis of the jaws associated with bisphosphonate treatment: a report of twenty‐nine cases. Acta Bio‐medica: Atenei Parmensis 2006;77(2):109‐17. [PubMed] [Google Scholar]
Migliorati 2005
- Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate‐associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer 2005;104:83‐93. [DOI] [PubMed] [Google Scholar]
Montazeri 2007
- Montazeri AH, Erskine JG, McQuaker IG. Oral sodium clodronate induced osteonecrosis of the jaw in a patient with myeloma. European Journal of Haematology 2007;79(1):69‐71. [DOI] [PubMed] [Google Scholar]
Mortensen 2007
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: Presentation of seven cases and literature review. Laryngoscope 2007;117(1):30‐4. [DOI] [PubMed] [Google Scholar]
Murad 2007
- Murad OM, Arora S, Farag AF, Guber HA. Bisphosphonates and osteonecrosis of the jaw: a retrospective study. Endocrine Practice 2007;13(3):232‐8. [DOI] [PubMed] [Google Scholar]
Pastor‐Zuazaga 2006
- Pastor‐Zuazaga D, Garatea‐Crelgo J, Martino‐Gorbea R, Etayo‐Pérez A, Sebastián‐López C. Osteonecrosis of the jaws and bisphosphonates: report of three cases. Medicina Oral, Patología Oral y Cirugía Bucal 2006;11(1):76‐9. [PubMed] [Google Scholar]
Phal 2007
- Phal PM, Myall RW, Assael LA, Weissman JL. Imaging findings of bisphosphonate‐associated osteonecrosis of the jaws. American Journal of Neuroradiology 2007;28(6):1139‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pires 2005
- Pires FR, Miranda A, Cardoso ES, Cardoso AS, Fregnani ER, Pereira CM, et al. Oral avascular bone necrosis associated with chemotherapy and bisphosphonate therapy. Oral Diseases 2005;11:365‐9. [DOI] [PubMed] [Google Scholar]
Polizzotto 2006
- Polizzotto MN, Cousins V, Schwarer AP. Bisphosphonate‐associated osteonecrosis of the auditory canal. British Journal of Haematology 2006;132(1):114. [DOI] [PubMed] [Google Scholar]
Pozzi 2007
- Pozzi S, Marcheselli R, Sacchi S, Baldini L, Angrilli F, Pennese E, et al. Bisphosphonate‐associated osteonecrosis of the jaw: a review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leukemia & Lymphoma 2007;48(1):56‐64. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical research ed.) 2014;349:g5630. [PUBMED: 25252733] [DOI] [PubMed] [Google Scholar]
Purcell 2005
- Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Medical Journal of Australia 2005;182:417‐8. [DOI] [PubMed] [Google Scholar]
Rajkumar 2010
- Rajkumar SV. Zoledronic acid in myeloma: MRC Myeloma IX. Lancet 2010;376(9757):1965‐6. [PUBMED: 21131042] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Ruggiero 2004
- Ruggiero SL, Meherotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral and Maxillofacillary Surgery 2004;62:527‐34. [DOI] [PubMed] [Google Scholar]
Saad 2012
- Saad F, Brown JE, Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active‐controlled phase III trials in cancer patients with bone metastases. Annals of Oncology : official journal of the European Society for Medical Oncology / ESMO 2012;23(5):1341‐7. [PUBMED: 21986094] [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [PUBMED: 20688472] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLOS One 2014;9(7):e99682. [PUBMED: 24992266] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salesi 2006
- Salesi N, Pistilli R, Marcelli V, Govoni FA, Bozza F, Bossone G, et al. Bisphosphonates and oral cavity avascular bone necrosis: a review of twelve cases. Anticancer Research 2006;26(4B):3111‐5. [PubMed] [Google Scholar]
Saussez 2009
- Saussez S, Javadian R, Hupin C, Magremanne M, Chantrain G, Loeb I, et al. Bisphosphonate‐related osteonecrosis of the jaw and its associated risk factors: a Belgian case series. Laryngoscope 2009;119(2):323‐9. [PUBMED: 19172621] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias, dimension of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Scurrah 2000
- Scurrah KJ, Palmer LJ, Burton PR. Variance components analysis for pedigree‐based censored survival data using generalized linear mixed models (GLMMs) and Gibbs sampling in BUGS. Genetic Epidemiology 2000;19(2):127‐48. [PUBMED: 10962474] [DOI] [PubMed] [Google Scholar]
Senel 2007
- Senel FC, Saracoglu Tekin U, Durmus A, Bagis B. Severe osteomyelitis of the mandible associated with the use of non‐nitrogen‐containing bisphosphonate (disodium clodronate): report of a case. Journal of Oral and Maxillofacillary Surgery 2007;65(3):562‐5. [DOI] [PubMed] [Google Scholar]
Shipman 1997
- Shipman CM, Rogers MJ, Apperley JF. Bisphosphonates induce apoptosis in human myeloma cells: a novel anti‐tumour activity. British Journal of Haematology 1997;98:665‐72. [DOI] [PubMed] [Google Scholar]
Sitters 2005
- Sitters MA, Caldwell CS. Bisphosphonates, dental care and osteonecrosis of the jaws. Texas Dental Journal 2005;122:968‐72. [PubMed] [Google Scholar]
STATA V10.1 [Computer program]
- StataCorp LP. STATA. Version 10.1. College Station: StataCorp LP, 2008.
Sterne 2001
- Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith S, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd edition. London: BMJ Books, 2001:189‐208. [Google Scholar]
Tanvetyanon 2006
- Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Annals of Oncology 2006;17:897‐907. [DOI] [PubMed] [Google Scholar]
Tennis 2012
- Tennis P, Rothman KJ, Bohn RL, Tan H, Zavras A, Laskarides C, et al. Incidence of osteonecrosis of the jaw among users of bisphosphonates with selected cancers or osteoporosis. Pharmacoepidemiology and Drug Safety 2012;21(8):810‐7. [PUBMED: 22711458] [DOI] [PubMed] [Google Scholar]
Then 2012
- Then C, Horauf N, Otto S, Pautke C, Tresckow E, Rohnisch T, et al. Incidence and risk factors of bisphosphonate‐related osteonecrosis of the jaw in multiple myeloma patients having undergone autologous stem cell transplantation. Onkologie 2012;35(11):658‐64. [PUBMED: 23147542] [DOI] [PubMed] [Google Scholar]
Thompson 1997
- Thompson SG, Smith TC, Sharp SJ. Investigating underlying risk as a source of heterogeneity in meta‐analysis. Statistics in Medicine 1997;16(23):2741‐58. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [PUBMED: 12111920] [DOI] [PubMed] [Google Scholar]
Thumbigere‐Math 2012
- Thumbigere‐Math V, Tu L, Huckabay S, Dudek AZ, Lunos S, Basi DL, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. American Journal of Clinical Oncology 2012;35(4):386‐92. [PUBMED: 22561331] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tosi 2006b
- Tosi P, Zamagni E, Cangini D, Tacchetti P, Raimondo F, Catalano L, et al. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide‐dexamethasone. Blood 2006;108(12):3951‐2. [DOI] [PubMed] [Google Scholar]
Treister 2006
- Treister N, Woo SB. Images in clinical medicine. Bisphosphonate‐associated osteonecrosis of the jaw. New England Journal of Medicine 2006;355(22):2348. [DOI] [PubMed] [Google Scholar]
Tricot 2000
- Tricot G. Multiple myeloma and other plasma cell disorders. In: Hoffman R, Benz EJ, Shattil SJ, et al. editor(s). Hematology: Basic Principles and Practice. 3rd Edition. Philadelphia: Churchill Livingstone, 2000. [Google Scholar]
Van Holten‐Verzantvoort 1993
- Holten‐Verzantvoort AT, Kroon HM, Bijvoet OL, Cleton FJ, Beex LV, Blijham G, et al. Palliative pamidronate treatment in patients with bone metastases from breast cancer. Journal of Clinical Oncology 1993;11:491‐8. [DOI] [PubMed] [Google Scholar]
Vannucchi 2005
- Vannucchi AM, Ficarra G, Antonioli E, Bosi A. Osteonecrosis of the jaw associated with zoledronate therapy in a patient with multiple myeloma. British Journal of Haematology 2005;28:738. [DOI] [PubMed] [Google Scholar]
Vescovi 2012
- Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S. Bisphosphonates‐related osteonecrosis of the jaws: a concise review of the literature and a report of a single‐centre experience with 151 patients. Journal of Oral Pathology & Medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 2012;41(3):214‐21. [PUBMED: 21958312] [DOI] [PubMed] [Google Scholar]
Walter 2007
- Walter C, Grőtz KA, Kunkel M, Al‐Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007;15(2):197‐202. [DOI] [PubMed] [Google Scholar]
Watters 2013
- Watters AL, Hansen HJ, Williams T, Chou JF, Riedel E, Halpern J, et al. Intravenous bisphosphonate‐related osteonecrosis of the jaw: long‐term follow‐up of 109 patients. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2013;115(2):192‐200. [PUBMED: 23036797] [DOI] [PubMed] [Google Scholar]
Wickham 2013
- Wickham N, Crawford A, Carney AS, Goss AN. Bisphosphonate‐associated osteonecrosis of the external auditory canal. Journal of Laryngology and Otology 2013;127 Suppl 2:S51‐3. [PUBMED: 23673152] [DOI] [PubMed] [Google Scholar]
Wong 2002
- Wong RKS, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002068; PUBMED: 12076438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wutzl 2006
- Wutzl A, Eisenmenger G, Hoffmann M, Czerny C, Moser D, Pietschmann P, et al. Osteonecrosis of the jaws and bisphosphonate treatment in cancer patients. Wiener Klinische Wochenschrift 2006;118(15‐16):473‐8. [DOI] [PubMed] [Google Scholar]
Yeo 2005
- Yeo AC, Lye KW, Poon CY. Bisphosphonate‐related osteonecrosis of the jaws. Singapore Dental Journal 2005;27:36‐40. [PubMed] [Google Scholar]
Zarychanski 2006
- Zarychanski R, Elphee E, Walton P, Johnston J. Osteonecrosis of the jaw associated with pamidronate therapy. American Journal of Hematology 2006;81:73‐5. [DOI] [PubMed] [Google Scholar]
Zervas 2006
- Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single‐centre experience in 303 patients. British Journal of Haematology 2006;134(6):620‐3. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Djulbegovic 2002
- Djulbegovic B, Wheatley K, Ross J, Clark O, Bos G, Goldschmidt H, et al. Bisphosphonates in multiple myeloma. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003188; PUBMED: 12137679] [DOI] [PubMed] [Google Scholar]
Mhaskar 2010
- Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, et al. Bisphosphonates in multiple myeloma. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD003188.pub2; PUBMED: 20238320] [DOI] [PubMed] [Google Scholar]
Mhaskar 2012
- Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, et al. Bisphosphonates in multiple myeloma: a network meta‐analysis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003188.pub3] [DOI] [PubMed] [Google Scholar]



