Abstract
Background
Childhood cancer survivors are at a higher risk of developing health conditions such as osteoporosis, and cardiovascular disease than their peers. Health‐promoting behaviour, such as consuming a healthy diet, could lessen the impact of these chronic issues, yet the prevalence rate of health‐protecting behaviour amongst survivors of childhood cancer is similar to that of the general population. Targeted nutritional interventions may prevent or reduce the incidence of these chronic diseases.
Objectives
The primary aim of this review was to assess the efficacy of a range of nutritional interventions designed to improve the nutritional intake of childhood cancer survivors, as compared to a control group of childhood cancer survivors who did not receive the intervention. Secondary objectives were to assess metabolic and cardiovascular risk factors, measures of weight and body fat distribution, behavioural change, changes in knowledge regarding disease risk and nutritional intake, participants' views of the intervention, measures of health status and quality of life, measures of harm associated with the process or outcomes of the intervention, and cost‐effectiveness of the intervention
Search methods
We searched the electronic databases of the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 3), MEDLINE/PubMed (from 1945 to April 2013), and Embase/Ovid (from 1980 to April 2013). We ran the search again in August 2015; we have not yet fully assessed these results, but we have identified one ongoing trial. We conducted additional searching of ongoing trial registers ‐ the International Standard Randomised Controlled Trial Number register and the National Institutes of Health register (both screened in the first half of 2013) ‐ reference lists of relevant articles and reviews, and conference proceedings of the International Society for Paediatric Oncology and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (both 2008 to 2012).
Selection criteria
We included all randomised controlled trials (RCTs) that compared the effects of a nutritional intervention with a control group which did not receive the intervention in this review. Participants were childhood cancer survivors of any age, diagnosed with any type of cancer when less than 18 years of age. Participating childhood cancer survivors had completed their treatment with curative intent prior to the intervention.
Data collection and analysis
Two review authors independently selected and extracted data from each identified study, using a standardised form. We assessed the validity of each identified study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. We used the GRADE criteria to assess the quality of each trial.
Main results
Three RCTs were eligible for review. A total of 616 participants were included in the analysis. One study included participants who had been treated for acute lymphoblastic leukaemia (ALL) (275 participants). Two studies included participants who had all forms of paediatric malignancies (266 and 75 participants). All participants were less than 21 years of age at study entry. The follow‐up ranged from one month to 36 months from the initial assessment. All intended outcomes were not evaluated by each included study. All studies looked at different interventions, and so we were unable to pool results. We could not rule out the presence of bias in any of the studies.
There was no clear evidence of a difference in calcium intake at one month between those who received the single, half‐day, group‐based education that focused on bone health, and those who received standard care (mean difference (MD) 111.60, 95% confidence interval (CI) ‐258.97 to 482.17; P = 0.56, low quality evidence). A regression analysis, adjusting for baseline calcium intake and changes in knowledge and self‐efficacy, showed a significantly greater calcium intake for the intervention as compared with the control group at the one‐month follow‐up (beta coefficient 4.92, 95% CI 0.33 to 9.52; P = 0.04). There was statistically significant higher, self‐reported milk consumption (MD 0.43, 95% CI 0.07 to 0.79; P = 0.02, low quality evidence), number of days on calcium supplementation (MD 11.42, 95% CI 7.11 to 15.73; P < 0.00001, low quality evidence), and use of any calcium supplementation (risk ratio (RR) 3.35, 95% CI 1.86 to 6.04; P < 0.0001, low quality evidence), with those who received this single, face‐to‐face, group‐based, health behaviour session.
There was no clear evidence of a difference in bone density Z‐scores measured with a dual‐energy X‐ray absorptiometry (DEXA) scan at 36 months follow‐up (MD ‐0.05, 95% CI ‐0.26 to 0.16; P = 0.64, moderate quality evidence) between those who received calcium and vitamin D supplementation combined with nutrition education and those who received nutrition education alone. There was also no clear evidence of a difference in bone mineral density between the intervention and the control group at the 12‐month (median difference ‐0.17, P = 0.99) and 24‐month follow‐up (median difference ‐0.04, P = 0.54).
A single multi‐component health behaviour change intervention, focusing on general healthy eating principles, with two telephone follow‐ups brought about a 0.17 lower score on the four‐point Likert scale of self‐reported junk food intake compared with the control group (MD ‐0.17, 95% CI ‐0.33 to ‐0.01; P = 0.04, low quality evidence); this result was statistically significant. There was no clear evidence of a difference between the groups in the self‐reported use of nutrition as a health protective behaviour (MD ‐0.05, 95% CI ‐0.24 to 0.14; P = 0.60, low quality evidence).
Authors' conclusions
Due to a paucity of studies, and the heterogeneity of the studies included in this review, we are unable to draw conclusions regarding the effectiveness of nutritional interventions for use with childhood cancer survivors. Although there is low quality evidence for the improvement in health behaviours using health behaviour change interventions, there remains no evidence as to whether this translates into an improvement in dietary intake. There was also no evidence that the studies reduced the risk of cardiovascular and metabolic disorders in childhood cancer survivors, although no evidence of effect is not the same as evidence of no effect. This review highlights the need for further well designed trials to be implemented in this population.
Plain language summary
Nutritional interventions for survivors of childhood cancer
Background
Survivors of childhood cancer are at a higher risk of chronic health conditions such as, osteoporosis, metabolic syndrome (including obesity and type II diabetes), and cardiovascular disease. These diseases have the potential to be reduced or prevented with targeted nutritional interventions.
Objective
This review looks at three randomised controlled trials that studied the effects of interventions designed to improve the dietary intake of children who have completed treatment for cancer.
Study Characteristics
The three studies included 616 participants who had completed their therapy for childhood cancer. All of the participants were less than 21 years of age at study entry. The interventions ranged from the promotion of health behaviours to vitamin and mineral supplementation. The follow‐up ranged from one month to 36 months from the initial assessment.
Key results
There was low quality evidence that those who received a health behaviour intervention decreased their self‐reported intake of “junk food”. They also increased their intake of dairy foods, as well as increasing their calcium supplementation. The interventions did not appear to translate to an improvement in their dietary intake, body composition, or bone mineral density.
Quality of the evidence
The results from this review do not provide enough evidence regarding the effectiveness of nutritional interventions for childhood cancer survivors. There was low quality evidence overall. Further well designed research is needed in this area.
Background
Description of the condition
In the last thirty years, detection and treatment methods for childhood cancer have improved to such an extent that up to 80% of paediatric patients now survive their cancer (Cox 2009; Jemal 2009). This has resulted in a growing number of child cancer survivors and an increased clinical and research interest in the survivorship issues as a consequence of treatment, in particular treatment‐related morbidity and quality of life (Cox 2009). Childhood cancer survivors have a relative risk of developing a chronic condition of 3.3 and a relative risk of a severe or life‐threatening condition of 8.2 when compared with their siblings (Oeffinger 2006). Female sex and older age at diagnosis are independent risk factors for developing chronic conditions (Oeffinger 2009). These chronic health conditions include (but are not limited to) secondary cancers, endocrine disorders, renal dysfunction, and severe musculoskeletal problems (Dickerman 2007; Diller 2009; Ness 2007; Oeffinger 2006). However, it may be many years before patients display these conditions which tend to worsen over time (Oeffinger 2006).
There is now much focus in the literature on the importance of long‐term monitoring of these patients (Friedman 2006; Hudson 2009; Landier 2006), and increasing recognition of the need for both secondary and tertiary interventions that may lessen the burden of these chronic conditions (Oeffinger 2009; Steinberger 2012; Stolley 2010). It may be possible to reduce the incidence of these chronic conditions with focused prevention strategies (Nathan 2009; Oeffinger 2006), aiming for quality of life similar to peers (Skinner 2006). Specific chronic health conditions of long‐term survivors that have the potential to be managed by lifestyle factors include osteoporosis, metabolic syndrome, endocrine disorders, and cardiovascular disease (Nathan 2009). An individual's risk of these conditions varies depending on factors such as disease and treatment type, age, and sex. For example, survivors of acute lymphoblastic leukaemia (ALL) who were treated with radiotherapy are at a greater risk of obesity, whereas those who received treatment for brain tumours are at risk of inadequate growth hormone (Hudson 2009). Those who received chemotherapy agents such as anthracycline are at risk of cardiovascular disease (Mulrooney 2009).
Description of the intervention
Despite the fact that health‐promoting behaviour, such as consuming a healthy diet or maintaining adequate physical activity, could lessen the impact of these chronic issues (Stolley 2009), the prevalence of health‐protecting behaviour in adults who have survived childhood cancer is similar to that of the general population (Mulhern 1995; Nathan 2009). There is a strong association in the general population between inadequate physical activity combined with a diet high in saturated fat and sugar and low in fruit and vegetable intake, and symptoms associated with the metabolic syndrome (Pereira 2009). This is of concern, since many adult survivors of childhood cancer do not meet guidelines for fruit and vegetable intake, consume excessive fat, and have an inadequate calcium intake (Demark‐Wahnefried 2005; Robien 2008). These poor eating habits appear to be manifesting themselves early after treatment completion. Young childhood cancer survivors have been shown to have an excessive energy intake and an inadequate calcium and folate intake (Cohen 2012). Long‐term survivors report barriers to consuming a healthy diet that include taste preferences for higher fat foods and the lack of availability of healthier foods (Arroyave 2008). They may also be unaware of their risk of chronic disease (Nathan 2009), lessening the motivation to change their lifestyle. As childhood cancer survivors are already at a higher risk of long‐term metabolic complications as a result of their cancer therapy, poor nutritional intake may be exacerbating this risk.
Interventions may need to be age‐specific and differ between the older and younger childhood cancer survivor cohorts. Interventions may also need to target specific conditions and high risk groups or may target the general paediatric population. For example, childhood cancer survivors treated for ALL using cranial irradiation are at a higher risk for obesity and subsequently metabolic syndrome (Oeffinger 2008), and therefore, they could be targeted with specific nutritional interventions to reduce obesity rates. In contrast, patients treated with anthracycline are at risk of cardiovascular sequelae (Oeffinger 2008), and therefore, interventions may target not only weight reduction but also aim to reduce cardiovascular risk (Siviero‐Miachon 2008). Strategies to manage these chronic conditions may involve prevention interventions for younger cancer survivors or treatment interventions for older cancer survivors. Due to these variations in risk, a “one‐size fits all” approach may not be indicated.
How the intervention might work
There is clear evidence that lifestyle changes, including improved diet and physical activity, are effective in the prevention or reduction of metabolic and cardiovascular risk factors in the general adult population (Lakka 2007). A range of nutritional interventions have been reported to be effective in preventing or reducing risk factors associated with the metabolic syndrome. These include: low glycaemic index/high protein diets, increased fruit, vegetable and fibre intake, reduced salt diets and a Mediterranean‐style diet (Brunner 2009; Tota‐Maharaj 2010). A recent Cochrane review assessing nutritional interventions for reducing or preventing cardiovascular risk found that interventions were more likely to be effective in participants who were told of their higher risk of disease (Brunner 2009).
In the general paediatric population, little research has focused on the prevention of metabolic syndrome. Rather, there is a focus on prevention and treatment of childhood obesity. The literature suggests that family‐targeted behavioural lifestyle interventions, using a combination of nutrition, physical activity, and behavioural components are effective for bringing about change in overweight children (Oude Luttikhuis 2009). There does not appear to be research focusing on the efficacy of specific types of nutritional interventions. As the mechanisms for the increased incidence of these chronic diseases may be different in the general population to the oncology population, the results and recommendations from these studies may not be able to be extrapolated to childhood cancer survivors. Interventions focusing on older and adult survivors of childhood cancer may not be appropriate for the younger survivors.
Why it is important to do this review
As this is a new area of study, there are minimal data in the literature with regard to the most effective nutritional interventions available to reduce the incidence of chronic disease after childhood cancer, despite the ongoing focus on long‐term follow‐up of these patients. The purpose of this Cochrane review was to assess the literature regarding nutritional interventions developed for childhood cancer survivors, to facilitate the production of best‐evidence management guidelines.
Objectives
The primary aim of this review was to assess the efficacy of a range of nutritional interventions designed to improve the nutritional intake of childhood cancer survivors, as compared to a control group of childhood cancer survivors who did not receive the intervention. Secondary objectives were to assess metabolic and cardiovascular risk factors, measures of weight and body fat distribution, behavioural change, changes in knowledge regarding disease risk and nutritional intake, participants' views of the intervention, measures of health status and quality of life, measures of harm associated with the process or outcomes of the intervention, and cost‐ effectiveness of the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) that studied the effects of nutritional interventions in this review. There was no limit to length of the intervention, type of intervention, or length of follow‐up.
Types of participants
Studies that involved childhood cancer survivors of any age, who were diagnosed with any type of cancer when less than 18 years of age were eligible for the review. Participating childhood cancer survivors had completed their treatment with curative intent prior to the intervention. We also included studies including parents and/or carers of this participant group if the parents/caregivers were involved in the intervention or reported on the participant outcomes. Treatment included chemotherapy and/or radiotherapy. We excluded studies which included participants with a comorbidity that may have affected eating, such as autism (Emond 2010), developmental delay (Kuhn 2004), and Down’s syndrome (Lewis 2004).
Types of interventions
Strategies
We included interventions that included educational and counselling strategies, health promotion or behavioural interventions with either individual or family‐based interventions in this review.
Topics
We captured nutritional interventions involving cancer survivors, with or without their family members. We excluded physical activity interventions for cancer survivors and nutritional interventions for childhood cancer patients receiving active treatment as these have been targeted by alternate Cochrane reviews (Braam 2013a; Jones 2010).
Settings
We did not impose any restriction on the settings for the interventions; settings may have included community, home‐based or hospital‐based interventions.
Delivery
All methods of delivery of the intervention were eligible, including face‐to‐face, telephone and online interventions. There were no restrictions regarding the interventionist. That is, eligible interventions were those that were delivered by specialist and non‐specialist medical and allied health professionals, as well as by other non‐health professionals.
Types of comparison
We included studies which compared nutrition interventions to a non‐intervention control group that received usual care or another intervention.
Types of outcome measures
Primary outcomes
A change in nutritional intake which was measured by one or more of the following.
Weighed food diaries.
Self‐reported food diaries.
Single or multiple 24‐hour recalls.
Food frequency questionnaires.
The nutrients may include but are not limited to:
energy;
protein;
fat;
carbohydrate;
calcium;
iron;
folate;
vitamin(s);
mineral(s).
Secondary outcomes
Metabolic risk factors, i.e. glucose and insulin metabolism.
Cardiovascular risk factors, i.e. resting blood pressure, blood lipids, and cholesterol.
Measures of weight and body fat distribution, i.e. body mass index (BMI), Dual‐energy X‐ray Absorptiometry (DEXA) and weight/height percentiles.
Behavioural change, i.e. changes in nutritional intake.
Changes in knowledge regarding disease risk and nutritional intake.
Participant views of the intervention.
Measures of health status and quality of life.
Measures of harm associated with the process or outcomes of the intervention.
Cost‐effectiveness of the intervention.
Search methods for identification of studies
See: Cochrane Childhood Cancer methods used in reviews (Module CCG 2014).
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 3), MEDLINE/PubMed (from 1945 to 6 April 2013), and Embase/Ovid (from 1980 to 6 April 2013). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3). We did run the search again in August 2015; we have not yet fully assessed these results, but we will fully incorporate them in the review at the next update.
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE/PubMED, Embase/OVID, either published or unpublished, by searching the reference lists of relevant articles and review articles. We handsearched the conference proceedings of the International Society for Paediatric Oncology (SIOP) (from 2008 to 2012) and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (2008 to 2012). We scanned the ISRCTN register (www.isrctn.com) and the register of the National Institute of Health (NIH) (clinicaltrials.gov) for ongoing trials in the first half of 2013. We did not impose language restrictions on the search.
Data collection and analysis
Selection of studies
Two review authors (JC, CW), worked independently, screening all the titles and abstracts resulting from the searches and excluded articles that were clearly irrelevant. The same review authors retrieved full‐text copies of all relevant articles and using the defined eligibility criteria, determined their eligibility for inclusion. We resolved any disagreement between review authors on classification of an article between the review authors. Third party arbitration was not necessary. There was a need for clarification of detail of one trial. One of the review authors (JC) contacted the study authors from Rai 2008 to obtain clarification for a complete assessment of the trial’s relevance for the review. The reasons for exclusion of any study considered for review are summarised below (Characteristics of excluded studies).
Data extraction and management
Two review authors (JC and CW) independently extracted data, using a standardised form, from each article. For each trial, they extracted the following data.
Characteristics of the studies, including the study sponsors and the authors’ affiliations, study design, risk of bias items, duration of study, loss to follow‐up, and compliance.
Characteristics of study population, including country where participants enrolled, inclusion and exclusion criteria, number randomised in each arm, information on the control group, demographic characteristics, type of cancer, age at diagnosis, cancer treatment, time since diagnosis, and time beyond active treatment.
Characteristics of the intervention, including type of nutritional intervention, details of the intervention, frequency, duration, intensity, number of sessions, intervention format (i.e. individual or group, professionally led or not, home‐ or facility‐based), description of control intervention, adherence and contaminations, as well as cointerventions (i.e. physical activity, medication use).
Characteristics of the outcomes, as stated previously.
We entered and combined the trial data using Review Manager 5 (RevMan 2014). One review author entered the data into RevMan 5 (JC), and another review author worked independently to verify the data entry (CW). We resolved any disagreement between review authors on classification of an article between the review authors. Third party arbitration was not necessary.
Assessment of risk of bias in included studies
Two independent review authors (JC, CW) assessed the validity of each study using the risk of bias items, as described in the module of Cochrane Childhood Cancer (Module CCG 2014), which are based on the risk of bias domains from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We reported the following criteria for each trial: adequate sequence generation and allocation concealment (selection bias), masking or blinding of personnel, participants, and outcome assessors (performance or detection bias), incomplete data (attrition bias), and selective outcome reporting (reporting bias). We also assessed baseline imbalance (gender, ethnicity, diagnosis, age, and health behaviour or nutritional intake) and differential diagnostic activity as other potential sources of bias.
We assessed these issues as 'low risk of bias', 'high risk of bias', or 'unclear'. We resolved any disagreement between review authors. Third party arbitration was not necessary.
Measures of treatment effect
For continuous outcomes, we assessed the mean difference between groups. For dichotomous outcomes, we assessed relative risk.
Unit of analysis issues
We aimed to include cluster‐randomised, cross‐over, and repeated measures trials in this analysis, though none of the eligible studies used these methodologies.
Dealing with missing data
It was necessary to contact the authors of the Rai 2008 study to gather further detail on the nutrition intervention.
We performed intention‐to‐treat analysis for all studies.
Assessment of heterogeneity
As we were unable to pool any of the data due to the different outcome measures and interventions between the trials, we were unable to assess heterogeneity using the I2 analysis.
Assessment of reporting biases
We had planned to assess reporting bias by constructing funnel plots. However, as there were less than 10 studies included in this review, the power of the tests was too low to distinguish chance from real asymmetry (Higgins 2011), and so we did not carry this out.
Data synthesis
We entered the data of the included studies into Review Manager 5 (RevMan 2014). We performed data analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As we were unable to pool the data for a meta‐analysis, we provided a narrative summary of the trial findings according to the review objectives. For data that was provided as medians and ranges, we converted the mean difference to mean and standard deviation based on the methodology of Hozo 2005.
GRADE
Two independent review authors used the GRADE system to rate the overall quality of the evidence for each of the following outcomes (Guyatt 2008a; Guyatt 2008b): calcium intake, bone mineral density, use of nutrition as health protective behaviour, junk food intake, milk consumption, and calcium supplementation. The GRADE approach defines the quality of a body of evidence as 'high', 'moderate', 'low' or 'very low'. (Higgins 2011). Factors that may have resulted in a decrease in the quality of evidence included: 1) study limitations; 2) inconsistency; 3) indirectness; 4) imprecision; and 5) publication bias.
Subgroup analysis and investigation of heterogeneity
We had planned to perform subgroup analyses based on the following categories: 1) age at intervention (< 13 years; 13 to 18 years; > 18 years); 2) forms of intervention (face‐to‐face; phone etc); 3) duration of intervention; 4) childhood cancer type; and 5) type of treatment received. Due to insufficient trials and lack of data in the included studies, we were unable to conduct such analyses.
Sensitivity analysis
As pooling of the results was not possible, we were unable to use sensitivity analyses to explore the impact of the inclusion of studies with a high risk of bias and studies with an unclear risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We identified a total of 3607 studies from running the search through three electronic databases: CENTRAL, MEDLINE/PubMED, and Embase/OVID in April 2013. We identified an additional study from searching the ongoing trial registries. We did not identify any studies upon screening reference lists of relevant articles and reviews. We did not identify any studies from the conference proceedings from the International Pediatric Oncology Society (SIOP) or the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer. Initial screening of the title and abstracts of each study allowed the exclusion of 3598 publications. We obtained ten full‐text articles, of which three studies, described in 6 full‐text articles, met the inclusion criteria. Three studies did not meet the inclusion criteria and we classified one of the studies as ongoing. In August 2015 we identified an additional study, which we assessed in full‐text and classified as an ongoing study (see Figure 1).
1.
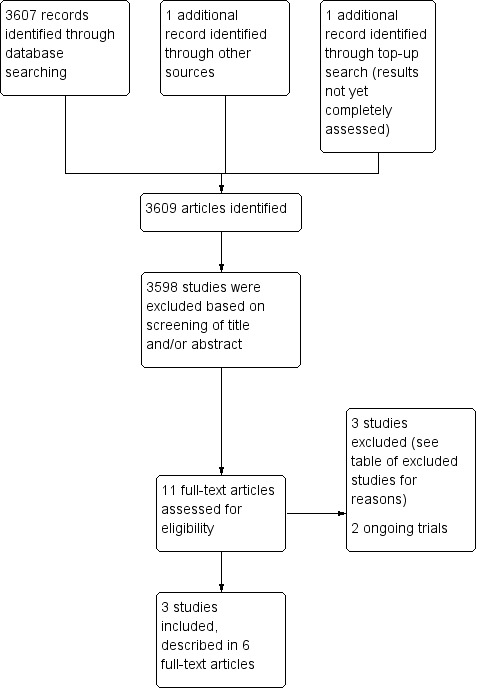
Study flow diagram.
Included studies
We included three studies in this review. All three studies were randomised controlled trials (RCTs). For further details on the studies see Characteristics of included studies.
Participants
A total of 616 participants from the three studies were included in the analysis. One of the studies included participants who had been treated for ALL ( Rai 2008). Cox 2005 and Mays 2011 included participants with all forms of paediatric cancer.
The number of participants in each study varied. The smallest study included a total of 38 participants in the intervention and 37 in the control group (Mays 2011). It was unclear whether any participants were lost to follow‐up. The Cox 2005 study included a total of 266 participants (131 in the intervention and 135 in the control group). Four and one participant(s), respectively were lost to follow‐up. The largest study included a total of 275 participants (141 in the intervention and 134 in the control group) (Rai 2008). Ninety‐four participants (45 in the intervention and 49 in the control group) did not complete the study.
The ages of the participants varied among the three studies. Two studies recruited adolescent childhood cancer survivors (ages 11 to 21 years (Mays 2011) and 12 to 18 years (Cox 2005)). The third study included childhood cancer survivors of all ages up to 18 years (Rai 2008). None of the included studies had participants older than 21 years at study entry. The participants were a mean of seven years (Rai 2008), and a mean of 15 years since their cancer treatment had been completed (Cox 2005). Information on time since diagnosis was not clear for the study of Mays 2011.
Intervention
The timing of the interventions after the childhood cancer therapy varied among the studies. Mays 2012 included participants within two years of diagnosis, Cox 2005 included patients who had completed treatment at least two years prior, and Rai 2008 included participants who had completed treatment at least five years prior. The intervention and timing also varied among the three studies included in this analysis. Two of the studies included interventions that consisted of an initial, single, face‐to‐face health education session focusing on health behaviour change (Cox 2005; Mays 2011). One of these studies focused on general health behaviours (Cox 2005), such as reducing junk food intake. The individual education session was provided by a clinician or nurse practitioner during a routine visit to the hospital. These participants were giving education reinforcement, via the telephone, at three and six months after the intervention. The other intervention focused on bone health, calcium, and dairy intake, and the final assessment was done one month after the intervention (Mays 2011). The education session was provided in a group setting by a registered Dietitian.
The final study had a 36‐month follow‐up, with the focus of the intervention being on bone health (Rai 2008). The intervention consisted of calcium and vitamin D supplementation. Nutrition education was provided at baseline and every six months for 24 months. At baseline and 12 months postbaseline, the education was given face‐to‐face by a registered dietitian. At six months and 18 months the nutrition education was in the form of mailed information. For further information on these studies, see Characteristics of included studies.
The study of Cox 2005 also included a cointervention of changing the health behaviour practices of smoking cessation, sun protection, and exercise. This study did not have any contraindications. The studies of Mays 2011 and Rai 2008 did not include any cointerventions or contraindications.
Control
Of the three studies included in this review, the control groups of two of those studies received standard care (Cox 2005; Mays 2011). The standard care between these groups did vary. The standard care of the control group for the study of Cox 2005 included late‐effects screening and education on their risk factors which was provided during routine clinic visits. The standard care of the control group for the Mays 2011 study was no education on nutrition related risk factors. The control group of the final study received an identical nutrition education component as the intervention group in combination with placebo tablets (Rai 2008).
Outcomes
The primary outcomes of the studies in this review, were dietary/nutrient intake. The secondary outcomes measured by the included studies were body composition (bone mineral density) and health behaviours. The control group measurements were assessed at the same time points as the intervention groups for all three of the studies. The time points for the outcome measures differed between the studies. The study of Mays 2011 measured their outcomes (milk consumption frequency, calcium supplementation, dietary calcium intake) at baseline and one‐month postintervention. The study of Cox 2005 measured their outcomes (frequency of nutrition as a health protective behaviour, frequency of junk food consumption as a health risk behaviour) at baseline and 12 months postintervention. The final study of Rai 2008 measured their outcomes (bone mineral density) at baseline, 12 months, 24 months and 36 months postintervention.
The other secondary outcomes were not addressed in any of the three included studies. These secondary outcomes were: metabolic risk factors, cardiovascular risk factors, changes in knowledge, participant views of the intervention, health status and quality of life, measures of harm, or cost‐effectiveness of the intervention. All three studies had different methodologies and different outcomes being measured, and for this reason we were unable to pool the data. For further information on these studies see Characteristics of included studies and Data and analyses.
Excluded studies
We analysed the full‐text publications of three studies but subsequently excluded them. Mays 2012 was a validation study and did not include an intervention. Nathan 2009 was a review of the literature and the results of a smoking cessation intervention. The final study included participants on maintenance therapy who had not completed their cancer therapy (Moyer‐Mileur 2009). For information on the excluded studies, see Characteristics of excluded studies.
Ongoing studies
We could not include two studies in this review as the data collection is ongoing (NCT01473342; Stern 2015). For further information about these studies, see Characteristics of ongoing studies.
Risk of bias in included studies
See the risk of bias section in Characteristics of included studies and Figure 2 and Figure 3 for detailed information on the risk of bias assessment.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
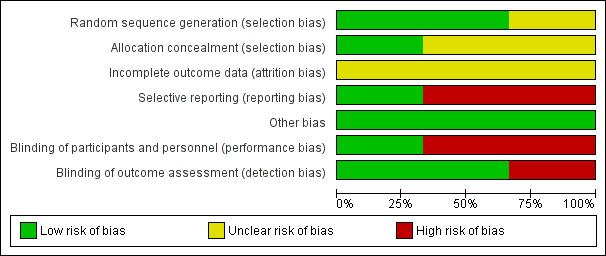
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Two of the studies described an adequate random sequence generation and we assessed them at low risk of bias (Cox 2005; Rai 2008). In the study by Rai 2008, randomisation was completed by the pharmacy after participants had been stratified into sex, race, age, and bone mineral density. The Cox 2005 study used a randomisation procedure that was stratified by gender and age. We assessed the final study as unclear in the use of random sequence generation (Mays 2011). Mays 2011 reported that the participants were randomised, but no further information was provided on the procedure. We assessed two of the studies as having an unclear allocation concealment as there was no mention of the procedures used in the study methodologies (Cox 2005; Mays 2011). The Cox 2005 study referred to the methodology used by another author, though the methods used were still not clear. The Rai 2008 study used a well described randomisation procedure and we assessed this as having a low risk of allocation concealment.
Blinding
Performance bias
Due to the nature of the interventions, blinding of personnel or participants was impossible with two of the three studies (Cox 2005; Mays 2011); we assessed both studies as having a high risk of performance bias. In the final study (Rai 2008), participants and personnel were blinded to the intervention, as participants were given a vitamin supplement or a placebo; we assessed this study as having a low risk of performance bias.
Detection Bias
Although personnel cannot be blinded when delivering nutrition interventions such as these, it is possible for detection bias to be minimised by blinding the outcome assessment. The Cox 2005 study did not provide any information regarding blinding of the outcome assessment and the outcome was subjective (a self‐reported outcome) and therefore we assessed the blinding of outcome assessment as high risk. In the remaining two studies (Mays 2011; Rai 2008), we assessed detection bias as low risk because the assessors were blinded to the study groups.
Incomplete outcome data
Two of the three studies reported dropouts during the study (Cox 2005; Rai 2008). No further information was provided on how the missing data were handled and we assessed these studies as having an unclear risk of attrition bias. Although the third study had a short follow‐up time of one month and was less likely to have dropouts, no information was provided on study attrition; we assessed this study as having an unclear risk (Mays 2011).
Selective reporting
We assessed Mays 2011 as having a low risk of reporting bias. This study reported data at baseline and follow‐up on all outcomes cited in the protocol or methodology section. Cox 2005 presented the results of a secondary analysis, not mentioned in the original protocol (Hudson 2002) and Rai 2008 did not publish all outcomes that were reported on the clinical trials registry. We assessed these two studies to be at high risk of reporting bias.
Other potential sources of bias
We assessed all studies for baseline imbalances and differential diagnostic activity as other potential sources of bias. In regards to baseline imbalances, there was no significant difference between the baseline data between the intervention and the control group for all studies (Cox 2005; Mays 2011; Rai 2008). We assessed all three studies as being at low risk for baseline imbalances.
We classified all three studies at low risk of bias for differential diagnostic activity because the studies performed the same assessments in the intervention and the control group at all time points (Cox 2005; Mays 2011; Rai 2008).
Effects of interventions
The three studies included in this review focused on different outcomes. We were unable to pool the data and the findings reported were from individual studies only.
Primary Outcome
Change in nutritional intake
Calcium intake was the only nutrient that was assessed across any of the studies (Mays 2011). Use of a single, group‐based behaviour change intervention showed no statistically significant difference in the calcium intake (as measured by a 24‐hour recall) between the intervention (n = 38) and control group (n = 37) at the one‐month follow‐up (mean difference (MD) 111.60, 95% confidence interval (CI) ‐258.97 to 482.17; P = 0.56, low quality evidence) (Mays 2011; Figure 4). We downgraded the quality of the evidence for study limitations and imprecision (Table 1). After regression analysis, adjusting for baseline calcium intake and changes in knowledge and self‐efficacy, there was a significantly greater calcium intake for the intervention as compared with the control group at the one‐month follow‐up (beta coefficient 4.92, 95% CI 0.33 to 9.52; P = 0.04) Mays 2011.
4.
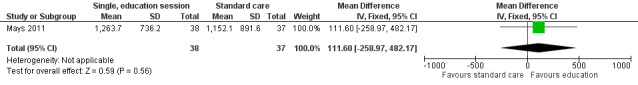
Forest plot of comparison: 1 Comparison of a single, group behaviour intervention, with standard care, outcome: 1.1 Change in nutritional intake (calcium).
1. GRADE Asssessment.
| Outcomes No. studies (No. participants) | Study limitations | Inconsistency | Indirectness | Imprecision | Publication bias | GRADE assessment |
| Calcium intake 1 (75) |
Serious limitations (‐1): lack of details on randomisation procedure, and lack of blinding of participants and personnel | n.a. | n.a. | Imprecision (‐1): only one small study | n.a. | ++, Low quality |
| Bone mineral density 1 (275) |
No serious limitations | n.a. | n.a. | Imprecision (‐1): only one study | n.a. | +++, Moderate quality |
| Use of nutrition as health protective behaviour 1 (266) |
Serious limitations (‐1): lack of blinding of participants, personnel, and outcome assessors | n.a. | n.a. | Imprecision (‐1): only one study | n.a. | ++, Low quality |
| Junk food intake 1 (266) |
Serious limitations (‐1): lack of blinding of participants, personnel, and outcome assessors | n.a. | n.a. | Imprecision (‐1): only one study | n.a. | ++, Low quality |
| Milk consumption 1 (75) |
Serious limitations (‐1): lack of details on randomisation procedure, and lack of blinding of participants and personnel | n.a. | n.a. | Imprecision (‐1): only one small study | n.a. | ++, Low quality |
| Calcium supplementation 1 (75) |
Serious limitations (‐1): lack of details on randomisation procedure, and lack of blinding of participants and personnel | n.a. | n.a. | Imprecision (‐1): only one small study | n.a. | ++, Low quality |
n.a. = not applicable
Secondary Outcome
1. Metabolic risk factors
This outcome was not assessed in any of the included studies.
2. Cardiovascular risk factors
This outcome was not assessed in any of the included studies.
3. Measures of weight and body fat distribution
Body composition was used as an outcome measure in one study (Rai 2008). The data were provided as medians and ranges. We converted the data to mean and standard deviation based on the methodology of Hozo 2005. There was no statistically significant difference in bone mineral density (measured with a DEXA scan) at the 36‐month follow‐up (MD ‐0.05, 95% CI ‐0.26 to 0.16; P = 0.64, moderate quality evidence) between those who received the calcium and vitamin D supplementation in conjunction with nutrition education (n = 141) and those participants who received nutrition education alone (n = 134) (Rai 2008; Figure 5). We downgraded the quality of the evidence due to imprecision (Table 1). There was no statistically significant difference in bone mineral density between the intervention and the control group at the 12‐month (median difference ‐0.17, P = 0.99) and 24‐month follow‐up (median difference ‐0.04, P = 0.54).
5.
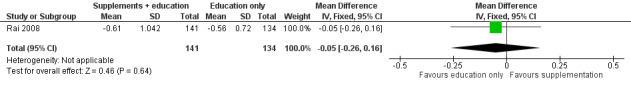
Forest plot of comparison: 2 Comparison of calcium and vitamin D supplementation and nutrition education with nutrition education alone, outcome: 2.1 Body composition (bone mineral density).
4. Behavioural Change
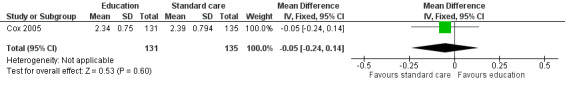
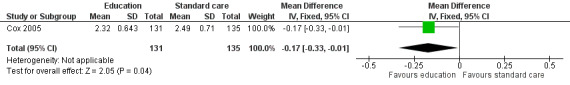
The behaviour change outcome was assessed in two studies. In the first study, health behaviour was measured using single questions on a four‐point Likert scale (Cox 2005). The participants were asked how often they engaged in health practising behaviours and rated this from 1 = never to 4 = always. A single, face‐to‐face, multi‐component health behaviour change intervention with two telephone follow‐ups brought about no statistically significant difference in the use of nutrition as a health protective behaviour (n = 131) compared with those who received standard care (n = 135) (MD ‐0.05, 95% CI ‐0.24 to 0.14; P = 0.60, low quality evidence) (Cox 2005; Figure 6). We downgraded the quality of the evidence due to study limitations and imprecision (Table 1). The same intervention brought about a statistically significant reduction in self‐reported junk food intake (measured on a four‐point Likert scale: 1 = never to 4 = always) in the intervention (n = 131) compared with the control group (n = 135) (MD ‐0.17, 95% CI ‐0.33 to ‐0.01; P = 0.04, low quality evidence) (Figure 7). We downgraded the quality of the evidence due to study limitations and imprecision (Table 1).
6.
Forest plot of comparison: 3 Comparison of a 12 month, face‐to‐face and telephone health behaviour intervention with standard care, outcome: 3.1 Behavioural change (nutrition)
7.
Forest plot of comparison: 3 Comparison of a 12 month, face‐to‐face and telephone health behaviour intervention with standard care, outcome: 3.2 Behavioural change (junk food)
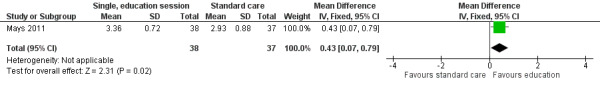
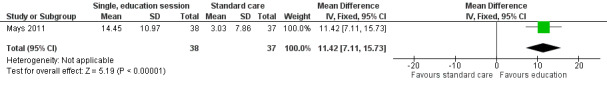
A single, face‐to‐face, group‐based health behaviour session focusing on bone health brought about a statistically significant increase in the intervention group’s self‐reported milk consumption (measured in number of days) (MD 0.43, 95% CI 0.07 to 0.79; P = 0.02, low quality evidence) as compared with those who received standard care (Mays 2011; Figure 8). We downgraded thequality of the evidence due to study limitations and imprecision (Table 1). The intervention was also effective in increasing the participants days on calcium supplementation (MD 11.42, 95% CI 7.11 to 15.73; P < 0.00001) (Figure 9). There was a statistically significant increase in calcium supplementation in the group that received the education sessions compared with those who received standard care (risk ratio (RR) 3.35, 95% CI 1.86 to 6.04; P < 0.0001, low quality evidence) (Figure 10). We downgraded the quality of the evidence due to study limitations and imprecision (Table 1). A total of 31 participants took some form of calcium supplementation after the intervention and nine participants took some form of calcium supplementation in the standard care group.
8.
Forest plot of comparison: 1 Comparison of a single, group behaviour intervention, with standard care, outcome: 1.2 Behavioural change (milk consumption).
9.
Forest plot of comparison: 1 Comparison of a single, group behaviour intervention, with standard care, outcome: 1.3 Behavioural change (days of calcium supplementation).
10.
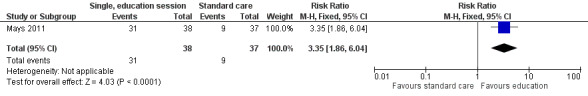
Forest plot of comparison: 1 Comparison of a single, group behaviour intervention, with standard care, outcome: 1.4 Behavioural change (any calcium supplementation).
5. Changes in knowledge regarding disease risk and nutritional intake
This outcome was not assessed in any of the included studies.
6. Participant views of the intervention
This outcome was not assessed in any of the included studies.
7. Measures of health status and quality of life
This outcome was not assessed in any of the included studies.
8. Measures of harm associated with the process or outcomes of the intervention
This outcome was not assessed in any of the included studies.
9. Cost‐effectiveness of the intervention
This outcome was not assessed in any of the included studies.
Discussion
Summary of main results
Childhood cancer survivors are at higher risk of health conditions such as osteoporosis, metabolic syndrome, endocrine disorders, and cardiovascular disease than their peers (Nathan 2009). Targeted nutritional interventions may prevent (Steinberger 2012; Stolley 2010), or reduce the incidence of these chronic diseases (Nathan 2009; Oeffinger 2006). This systematic review included three trials that have studied the efficacy of a nutritional intervention, in a randomised manner, in childhood cancer survivors (Cox 2005; Mays 2011; Rai 2008). These studies utilised differing methodologies, and as a consequence, we were unable to pool results.
The interventions that appeared to bring about a significant positive change were those that focused on health behaviour change. A single, group‐based health behaviour education session significantly increased self‐reported milk intake (mean difference (MD) 0.43, 95% confidence interval (CI) 0.07 to 0.79; P = 0.02), use of calcium supplementation (risk ratio (RR) 3.35, 95% CI 1.86 to 6.04; P < 0.0001), and the number of days on calcium supplementation (MD 11.42, 95% CI 7.11 to 15.73; P < 0.00001) as compared with standard care (Mays 2011). The intervention did not improve calcium intake (MD 111.60, 95% CI ‐258.97 to 482.17; P = 0.56), though a regression analysis, adjusting for baseline calcium intake and changes in knowledge and self‐efficacy, found a significantly greater calcium intake for the intervention as compared with the control group at the one month follow‐up (beta coefficient 4.92, 95% CI 0.33 to 9.52; P = 0.04). This study had a short follow‐up time of one month and the effect of the intervention long‐term was not assessed.
A face‐to‐face, multi‐component health behaviour session with two telephone follow‐ups with education reinforcement, over a 12‐month period, reduced self‐reported junk food intake (MD ‐0.17, 95% CI ‐0.33 to ‐0.01; P = 0.04) but did not improve childhood cancer survivors’ use of nutrition as a health‐protecting behaviour (MD ‐0.05, 95% CI ‐0.24 to 0.14; P = 0.60) as compared with standard care (Cox 2005).
The Rai 2008 study was the only study to assess the efficacy of nutritional supplementation on childhood cancer survivors’ body composition. This study was a randomised, double‐blind randomised controlled trial (RCT) of calcium and vitamin D supplementation versus placebo. Both the intervention and control group received nutrition education by a registered dietitian. There was no statistically significant difference on bone mineral density as measured by dual‐energy X‐ray absorptiometry (DEXA) between the intervention and the control group at the 36‐month follow‐up (MD ‐0.05, 95% CI ‐0.26 to 0.16; P = 0.64). There was also no statistically significant difference in bone mineral density between the intervention and the control group at the 12‐month (median difference ‐0.17, P = 0.99) and 24‐month follow‐up (median difference ‐0.04, P = 0.54).
Overall completeness and applicability of evidence
This review does not provide evidence that the nutritional interventions used in these studies improved dietary intake or body composition in childhood cancer survivors. The Mays 2011 study was the only included study that assessed the primary outcome of a change in nutritional intake. Mays 2011 found no statistically significant improvement in calcium intake with a single, group‐based, education session. Although a regression analysis, adjusting for baseline calcium intake and changes in knowledge and self‐efficacy, found a significantly greater calcium intake for the intervention as compared with the control group at the one month follow‐up.The study had a short follow‐up time of one month and long‐term compliance with the nutritional changes were not assessed. There was a modest, positive effect for health behaviour change interventions on improving self‐reported health behaviours such as junk food consumption (Cox 2005), and milk intake (Mays 2011). As the results of the Cox 2005 study were based on a secondary analysis, these results do need to be interpreted with caution. Although no statistically significant differences were found for many of the outcomes, this could be the result of low power in the studies. It should be noted that no evidence of effect is not the same as evidence of no effect.
The following outcomes were not assessed in any of the included studies: metabolic and cardiovascular markers, changes in knowledge, participant views of the intervention, health status and quality of life, measures of harm, or the cost‐effectiveness of the intervention.
The two studies that did show a positive change in health behaviours may not be applicable in all settings. The intervention required an initial face‐to‐face information session. This type of intervention may not be possible for survivors of childhood cancer who come from geographically diverse regions who may not travel to the primary care centre for long‐term follow‐up. An efficacy of interventions utilising computer and other technologies may need to be assessed. The ongoing study that was not included in this review is assessing the use of Smartphone applications and other virtual technologies to provide nutritional education for survivors of childhood cancer (NCT01473342). This type of intervention may be more applicable across a variety of clinical settings.
Many of this systematic review’s predetermined outcomes (e.g. metabolic risk factors, cardiovascular risk factors, changes in knowledge, and measures of harm) were not assessed in the included studies. Only one of the studies assessed the primary outcome of dietary intake. Although two of the interventions found a significant positive change in health behaviours, there is no evidence to suggest that this translates to the prevention of risk factors such as cardiovascular disease, metabolic syndrome, or obesity. Future interventions should consider assessing outcomes such as body composition and blood lipids in combination with dietary intake and changes in health behaviours.
All three of the captured studies were from paediatric oncology units in the USA. The findings therefore may not be generalisable to childhood cancer survivors from other countries, especially low‐income countries.
Quality of the evidence
By applying the GRADE criteria (Guyatt 2008a; Guyatt 2008b), the quality of findings varied between moderate (bone mineral density) and low (all other outcomes). We downgraded all outcomes one level for imprecision. Due to lack of blinding of participants, personnel, and outcome assessors, we further downgraded the quality of evidence for the outcomes ‘self‐reported nutrition’ and ‘junk food’. Due to lack of details regarding the randomisation procedure and lack of blinding of participants and personnel’, we also downgraded the outcomes ‘calcium intake’, ‘milk consumption’, and ‘calcium supplementation’ to low quality.
The Cox 2005 study had a high risk of reporting bias (results were from a secondary analysis), performance bias (inadequate blinding of personnel) and detection bias (inadequate blinding of outcome assessors). The Cox 2005 study had unclear selection bias and attrition bias. Results from this study therefore need to be interpreted with caution. The Rai 2008 study was the only study that we assessed as having a low risk of performance bias, as both the participants and personnel were blinded; the other two studies were at high risk of performance bias. Although it is difficult to blind participants to the intervention due to the nature of many nutritional trials, two studies blinded the assessors (Mays 2011; Rai 2008). Adequate allocation concealment would be possible for all nutritional intervention trials, though the Rai 2008 study was the only study that we assessed at low risk of selection bias; Mays 2011 had an unclear risk. The Mays 2011 study had a low risk of reporting bias and the other studies were at high risk of reporting bias. We assessed all three studies as unclear in their attrition bias and at low risk of other bias(Cox 2005; Mays 2011; Rai 2008). The studies had minimal baseline imbalance and no differential diagnostic activity.
Potential biases in the review process
We developed the search strategies for the electronic databases (CENTRAL, MEDLINE/PubMED, Embase/OVID) in collaboration with Cochrane Childhood Cancer. We undertook additional searching of clinical trial databases, reference lists, and proceedings from conferences. Although it is always possible that we have not identified all studies, an earlier published review did not identify any different additional interventions prior to 2010 (Stolley 2010).
Agreements and disagreements with other studies or reviews
Only one other review paper was identified in the literature systematically reviewing diet (and exercise) in childhood cancer survivors (Stolley 2010). This review included studies that focused on diet in childhood cancer survivors, though the majority of these were observational studies and unable to be included in the current review. They identified one nutritional intervention in childhood cancer survivors which was also included in our review (Cox 2005). Stolley 2010 concluded that the literature on the dietary intake of childhood cancer survivors is methodologically weak. There were very limited intervention studies and use of control groups in the observational studies was rare. Stolley 2010, highlights the minimal use of validated methods of dietary assessment. Since the Stolley 2010 review was published, the three trials included in this review have been completed, though the use of validated dietary methods remains poor.
Authors' conclusions
Implications for practice.
Due to a paucity of research and the heterogeneity of the studies included in this review, the review authors are unable to draw conclusions regarding the effectiveness of nutritional interventions for childhood cancer survivors. Although there is weak evidence for the improvement in health behaviours using health behaviour change interventions, there remains no evidence as to whether this translates into an improvement in dietary intake.
It is important to note that no evidence of effect is not the same as evidence of no effect. Many outcomes were not assessed in the included studies. We are unable to conclude whether nutritional interventions can reduce the risk of long‐term conditions, such as cardiovascular disease and metabolic syndrome in survivors of childhood cancer.
Implications for research.
This review highlights the need for further intervention trials to be implemented in this population. More robust research methodology is required to determine whether dietary changes can occur in survivors of childhood cancer and whether these can reduce their risk of long‐term health issues. The use of a randomised design with blinding of personnel to the outcome measures is possible with this type of nutritional intervention and is recommended in future studies. It is also suggested that future studies utilise validated measures of dietary intake. Objective measures of body composition, cardiovascular, and metabolic risk should also be included as outcome measures in these studies.
Acknowledgements
The authors would like to acknowledge the editorial base of Cochrane Childhood Cancer for their advice and support. The authors would also like to thank Susan Kaste for providing additional data for the study of Rai 2008 and Jodie Bartle for input into the initial protocol. We also thank Dr A Spinola‐Castro and an undisclosed person who kindly agreed to peer review our manuscript. The editorial base of Cochrane Childhood Cancer is funded by Stichting Kinderen Kankervrij (KiKa), the Netherlands.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. ForPopulation the following text words were used:
(infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR young adult OR young adults OR young adult*)
AND (post treatment OR off treatment OR treatment complet* OR treatment termin* OR follow up OR follow‐up OR followup OR survivor OR survivors OR Long‐Term Survivors OR Long Term Survivors OR Long‐Term survivor OR survivo* OR surviving)
2. For Nutrition the following text words were used:
patient education OR practice guideline OR practice guidelines OR dietary guideline OR dietary guidelines OR practice guideline* OR dietary guideline* OR diet OR diets OR diet* OR diets* OR dietetic OR dietetics OR diet therapy OR health diet OR healthy food OR health promoting behaviour OR health promoting behaviour OR (diet* AND intervent*) OR (diet* AND advic*) OR diet* AND counsel* OR (diet* AND therap*) OR (diet* AND treatment*) OR (diet* AND educat*) OR (nutriti* AND intervent*) OR (nutriti* AND advice*) OR (nutriti* AND counsel*) OR (nutriti* AND therap*) OR (nutriti* AND treatment*) OR (nutriti* AND educat*) OR (nutriti* AND support) OR supportive therapy
3. ForOutcome the following text words were used:
food OR foods OR food* OR foods* OR food intake OR eating OR ingestion OR nutrition OR nutrition* OR (health* AND diet*) OR (health* AND food*) OR energy intake OR caloric intake OR kilojoule OR kilojoules OR calorie OR calori* OR caloric restriction OR vitamin OR vitamins OR vitamin* OR minerals OR minerals* OR mineral OR mineral* OR micro‐nutrient OR micro‐nutrients OR macro‐nutrient OR macro‐nutrients OR nutrient OR nutrients OR calcium OR folate OR folic acid OR iron OR ferric OR ferrous OR protein OR proteins OR fat intake OR fat reduced OR dietary fat restriction OR low fat OR low calorie OR low energy OR reduced energy OR calorie controlled OR fatty foods OR high fat OR fruit OR fruits OR vegetable OR vegetables OR dietary composition OR carbohydrate intake OR obesity OR obese OR adiposity OR body weight OR overweight OR body mass index OR BMI OR body mass OR body fat distribution OR body composition OR “bioelectrical impedance analysis” OR health behavior OR health behaviors OR health behaviour OR health behaviours OR health behaviour* OR health behaviour* OR health promotion OR behaviour change OR behavior change OR behaviour change* OR behavior change* OR health behaviour change OR health behavior change OR helath behaviour change* OR health behavior change* OR life style OR life style* OR weight gain OR weight gains OR weight gain* OR body weight OR weight loss OR weight change OR weight changes OR weight change* OR overnutrition OR overeating OR hyperphagia OR Metabolic syndrome OR Waist hip ratio OR Waist height ratio OR Skinfold thickness OR Skinfold thicknesses OR Skinfold thickness* OR DEXA OR Diabetes OR type 2 diabetes OR glucose metabolism OR insulin metabolism OR insulin resistance OR hyperinsulinemia OR hyperinsulinaemia OR cardiomyopathy OR myocardial Infarction OR fat metabolism OR cardiovascular risk factor OR cardiovascular risk factors OR cardiovascular risk factor* OR cardiovascular disease OR cardiovascular diseases OR blood pressure OR hypertension OR blood lipid OR blood lipids OR blood lipid* OR hyperlipidemia OR hyperlipidaemia OR dyslipidemia OR dyslipidaemia OR cholesterol metabolism OR hypercholesterolemia OR osteoporosis OR bone mineral density OR dual energy x‐ray absorptiometry OR malnutrition OR undernutrition OR Nutritional Deficiency OR Nutritional Deficiencies OR ideal body weight OR body image OR eating disorder OR eating disorders OR eating disorder* OR disordered eating OR fussy eating OR food refusal OR quality of life OR QoL
4. ForCancer the following text words were used:
cancer OR oncology OR oncolog* OR neoplasms OR neoplas* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR cancer* OR malignan* OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo* OR bone marrow transplantation OR bone marrow transplant* OR leukemia OR leukaemia OR lymphoma
The search was performed in title, abstract or keywords Final search 1 and 2 and 3 and 4
[* = zero to many characters]
Appendix 2. Search strategy for MEDLINE (PubMed)
1. For Population the following MeSH headings and text words were used:
(infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn OR young adult[mh] OR adult[mh] OR young adult)
AND (post treatment OR off treatment OR treatment complet* OR treatment termin* OR follow up OR follow‐up OR followup OR survivor OR survivors OR Long‐Term Survivors OR Long Term Survivors OR Long‐Term survivor OR Survivor, Long‐Term OR Survivors, Long‐Term OR survivo* OR surviving)
2. For Nutrition the following MeSH headings and text words were used:
patient education OR practice guideline OR practice guidelines OR dietary guideline OR dietary guidelines OR practice guideline* OR dietary guideline* OR diet OR diets OR diet* OR diets* OR dietetic OR dietetics OR diet therapy OR health diet OR healthy food OR health promoting behaviour OR health promoting behaviour OR (diet* AND intervent*) OR (diet* AND advic*) OR diet* AND counsel* OR (diet* AND therap*) OR (diet* AND treatment*) OR (diet* AND educat*) OR (nutriti* AND intervent*) OR (nutriti* AND advice*) OR (nutriti* AND counsel*) OR (nutriti* AND therap*) OR (nutriti* AND treatment*) OR (nutriti* AND educat*) OR (nutriti* AND support) OR supportive therapy
3. For Outcome the following MeSH headings and text words were used:
food OR foods OR food* OR foods* OR food intake OR eating OR ingestion OR nutrition OR nutrition* OR (health* AND diet*) OR (health* AND food*) OR energy intake OR caloric intake OR kilojoule OR kilojoules OR calorie OR calori* OR caloric restriction OR vitamin OR vitamins OR vitamin* OR minerals OR minerals* OR mineral OR mineral* OR micro‐nutrient OR micro‐nutrients OR macro‐nutrient OR macro‐nutrients OR nutrient OR nutrients OR calcium OR folate OR folic acid OR iron OR ferric OR ferrous OR protein OR proteins OR fat intake OR fat reduced OR dietary fat restriction OR low fat OR low calorie OR low energy OR reduced energy OR calorie controlled OR fatty foods OR high fat OR fruit OR fruits OR vegetable OR vegetables OR dietary composition OR carbohydrate intake OR obesity OR obese OR adiposity OR body weight OR overweight OR body mass index OR BMI OR body mass OR body fat distribution OR body composition OR “bioelectrical impedance analysis” OR health behavior OR health behaviors OR health behaviour OR health behaviours OR health behaviour* OR health behaviour* OR health promotion OR behaviour change OR behavior change OR behaviour change* OR behavior change* OR health behaviour change OR health behavior change OR health behaviour change* OR health behavior change* OR life style OR life style* OR weight gain OR weight gains OR weight gain* OR body weight OR weight loss OR weight change OR weight changes OR weight change* OR overnutrition OR overeating OR hyperphagia OR Metabolic syndrome OR Waist hip ratio OR Waist height ratio OR Skinfold thickness OR Skinfold thicknesses OR Skinfold thickness* OR DEXA OR Diabetes OR type 2 diabetes OR glucose metabolism OR insulin metabolism OR insulin resistance OR hyperinsulinemia OR hyperinsulinaemia OR cardiomyopathy OR myocardial Infarction OR fat metabolism OR cardiovascular risk factor OR cardiovascular risk factors OR cardiovascular risk factor* OR cardiovascular disease OR cardiovascular diseases OR blood pressure OR hypertension OR blood lipid OR blood lipids OR blood lipid* OR hyperlipidemia OR hyperlipidaemia OR dyslipidemia OR dyslipidaemia OR cholesterol metabolism OR hypercholesterolemia OR osteoporosis OR bone mineral density OR dual energy x‐ray absorptiometry OR malnutrition OR undernutrition OR Nutritional Deficiency OR Nutritional Deficiencies OR ideal body weight OR body image OR eating disorder OR eating disorders OR eating disorder* OR disordered eating OR fussy eating OR food refusal OR quality of life OR QoL
4. For Cancer the following MeSH headings and text words were used:
cancer OR oncology OR oncolog* OR neoplasms OR neoplas* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR cancer* OR malignan* OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo* OR bone marrow transplantation OR bone marrow transplant* OR leukemia OR leukaemia OR lymphoma
5. For RCTs and CCTs the following MeSH headings and text words were used:
(randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) AND (humans[mh]
Final search 1 and 2 and 3 and 4 and 5
[pt = publication type; tiab = title, abstract; sh = subheading; mh = MeSH term; * = zero to many characters; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 3. Search strategy for EMBASE (OVID)
1. For Popuation the following Emtree terms and text words were used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$ or young adult or young adults or young adult$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
AND
1. (survivor or survivors or (long adj term survivor) or (long adj term survivors) or survivo$).mp. 2. survivor/ or cancer survivor/ 3. survivi$.mp. 4. (post treatment or off treatment).mp. 5. (treatment complet* or treatment termin*).mp. 6. (follow up or followup or follow‐up).mp. or exp follow up/ 7. or/1‐6
2. ForNutrition the following Emtree terms and text words were used:
1. patient education.mp. or exp patient education/ 2. (practice guideline or practice guidelines or practice guideline$).mp. 3. exp practice guideline/ 4. (dietary guideline or dietary guidelines or dietary guideline$).mp. 5. exp DIET/ or diet.mp. 6. (diets or diet$ or diets$ or dietetic or dietetics).mp. 7. diet therapy.mp. or exp diet therapy/ 8. (health diet or healthy food).mp. or exp health food/ 9. exp health behavior/ 10. (health promoting behaviour or health promoting behavior).mp. 11. (diet$ and intervent$).mp. 12. (diet$ and advic$).mp. 13. (diet$ and counsel$).mp. 14. (diet$ and therap$).mp. 15. (diet$ and treatment$).mp. 16. (diet$ and educat$).mp. 17. (nutriti$ and intervent$).mp. 18. (nutriti$ and advice$).mp. 19. (nutriti$ and counsel$).mp. 20. (nutriti$ and therap$).mp. 21. (nutriti$ and treatment$).mp. 22. (nutriti$ and educat$).mp. 23. (nutriti$ and support).mp. 24. supportive therapy.mp. 25. or/1‐24
3. For Outcome the following Emtree terms and text words were used:
1. (food or foods or food* or foods* or food intake).mp. 2. exp FOOD INTAKE/ or exp FOOD/ 3. eating.mp. or exp EATING/ 4. ingestion.mp. or exp INGESTION/ 5. exp NUTRITION/ 6. (nutrition or nutrition$).mp. 7. (health$ and diet$).mp. 8. (health$ and food$).mp. 9. (energy intake or carbohydrate intake or caloric intake).mp. or exp caloric intake/ 10. (kilojoule or kilojoules or calorie or calori$ or caloric restriction).mp. 11. vitamin/ 12. (vitamin or vitamins or vitamin$).mp. 13. exp MINERAL/ 14. (minerals or minerals$ or mineral or mineral$).mp. 15. exp trace element/ 16. (micro‐nutrient or micro‐nutrients).mp. 17. exp MACRONUTRIENT/ 18. (macro‐nutrient or macro‐nutrients or nutrient or nutrients).mp. 19. (calcium or 7440‐70‐2).mp. 20. (folate or folic acid or 59‐30‐3).mp. 21. (iron or 7439‐89‐6 or ferric or ferrous).mp. 22. protein/ 23. (protein or proteins).mp. 24. exp low fat diet/ 25. (fat reduced or dietary fat restriction or low fat or fat intake).mp. 26. (low calorie or low energy or reduced energy or calorie controlled).mp. 27. (fatty foods or high fat).mp. 28. (fruit or fruits or vegetable or vegetables).mp. 29. exp dietary intake/ or dietary composition.mp. 30. exp OBESITY/ 31. (obesity or obese).mp. 32. adiposity.mp. 33. body weight.mp. or exp body weight/ 34. overweight.mp. 35. exp body mass/ 36. (body mass index or BMI or body mass).mp. 37. body fat distribution.mp. or exp body fat distribution/ 38. bioelectrical impedance analysis.mp. 39. body composition.mp. or exp body composition/ 40. exp health behavior/ 41. (health behavior or health behaviors or health behaviour or health behaviours or health behaviour$ or health behaviour$).mp. 42. health/ 43. (health knowledge or health attitude$).mp. 44. health promotion.mp. or exp health promotion/ 45. exp behavior change/ 46. (behaviour change or behavior change or behaviour change$ or behavior change$ or health behaviour change or health behavior change or health behaviour change$ or health behavior change$).mp. 47. exp lifestyle/ 48. (life style or life style$ or lifestyle or lifestyle$).mp. 49. (weight gain or weight gains or weight gain$).mp. 50. exp weight gain/ 51. exp weight reduction/ 52. (weight loss or weight change or weight changes or weight change$).mp. 53. exp OVERNUTRITION/ 54. exp HYPERPHAGIA/ 55. (overnutrition or overeating or hyperphagia).mp. 56. Metabolic syndrome.mp. or metabolic syntrome X/ 57. Waist hip ratio.mp. or exp waist hip ratio/ 58. Waist height ratio.mp. 59. exp skinfold thickness/ 60. (Skinfold thickness or Skinfold thicknesses or Skinfold thickness$).mp. 61. DEXA.mp. or exp dual energy X ray absorptiometry/ 62. (Diabetes or type 2 diabetes).mp. or exp diabetes mellitus/ 63. glucose metabolism.mp. or exp glucose metabolism/ 64. insulin metabolism.mp. or exp insulin metabolism/ 65. exp hyperinsulinemia/ or (hyperinsulinemia or hyperinsulinaemia).mp. 66. exp CARDIOMYOPATHY/ or cardiomyopathy.mp. 67. myocardial Infarction.mp. or exp heart infarction/ 68. fat metabolism.mp. or exp lipid metabolism/ 69. exp cardiovascular risk/ 70. (cardiovascular risk factor or cardiovascular risk factors or cardiovascular risk factor$).mp. 71. exp cardiovascular disease/ or (cardiovascular disease or cardiovascular diseases).mp. 72. blood pressure.mp. or exp blood pressure/ 73. exp hypertension/ or hypertension.mp. 74. exp lipid blood level/ 75. (blood lipid or blood lipids or blood lipid$).mp. 76. cholesterol metabolism.mp. or exp cholesterol metabolism/ 77. exp hypercholesterolemia/ or hypercholesterolemia.mp. 78. exp hyperlipidemia/ or (hyperlipidemia or hyperlipidaemia).mp. 79. exp dyslipidemia/ or (dyslipidemia or dyslipidaemia).mp. 80. osteoporosis/co, dt, rt, si, th [Complication, Drug Therapy, Radiotherapy, Side Effect, Therapy] 81. Osteoporosis.mp. 82. bone mineral density.mp. or exp bone density/ 83. malnutrition.mp. or exp MALNUTRITION/ 84. undernutrition.mp. 85. exp nutritional deficiency/ 86. (Nutritional Deficiency or Nutritional Deficiencies).mp. 87. ideal body weight.mp. or exp body weight/ 88. body image.mp. or exp body image/ 89. exp eating disorder/ 90. (eating disorder or eating disorders or eating disorder$ or disordered eating or fussy eating).mp. 91. exp food refusal/ or food refusal.mp. 92. exp "quality of life"/ or (quality of life or QoL).mp. 93. or/1‐92
4. ForCancer the following Emtree terms and text words were used:
1. (cancer or cancers or cancer$).mp. 2. (oncology or oncolog$).mp. or exp oncology/ 3. (neoplasm or neoplasms or neoplasm$).mp. or exp neoplasm/ 4. (carcinoma or carcinom$).mp. or exp carcinoma/ 5. (tumor or tumour or tumor$ or tumour$ or tumors or tumours).mp. or exp tumor/ 6. (malignan$ or malignant).mp. 7. (hematooncological or hemato oncological or hemato‐oncological or hematologic neoplasms or hematolo$).mp. or exp hematologic malignancy/ 8. (leukemia or leukaemia).mp. or exp LEUKEMIA/ 9. lymphoma.mp. or exp LYMPHOMA/ 10. or/1‐9
5. For RCTs and CCTs the following Emtree terms and text words were used:
1. Randomized Controlled Trial/ 2. Controlled Clinical Trial/ 3. randomized.ti,ab. 4. placebo.ti,ab. 5. randomly.ti,ab. 6. trial.ti,ab. 7. groups.ti,ab. 8. drug therapy.sh. 9. or/1‐8 10. Human/ 11. 9 and 10
Final search 1 AND 2 AND 3 AND 4 AND 5
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; sh = subject heading; ti,ab = title or abstract; / = Emtree term; $= zero to many characters; co = complication; dt = drug therapy; rt = radiotherapy; si = side effect; th = therapy; RCT = randomized controlled trial; CCT = controlled clinical trial]
Data and analyses
Comparison 1. Comparison of a single, group behaviour intervention, with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in nutritional intake (calcium) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 111.60 [‐258.97, 482.17] |
| 2 Behavioural change (milk consumption) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.07, 0.79] |
| 3 Behavioural change (days of calcium supplementation) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 11.42 [7.11, 15.73] |
| 4 Behavioural change (any calcium supplementation) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.86, 6.04] |
1.1. Analysis.
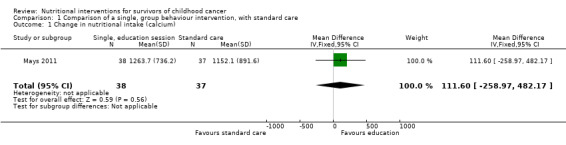
Comparison 1 Comparison of a single, group behaviour intervention, with standard care, Outcome 1 Change in nutritional intake (calcium).
1.2. Analysis.
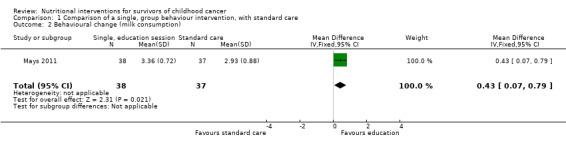
Comparison 1 Comparison of a single, group behaviour intervention, with standard care, Outcome 2 Behavioural change (milk consumption).
1.3. Analysis.
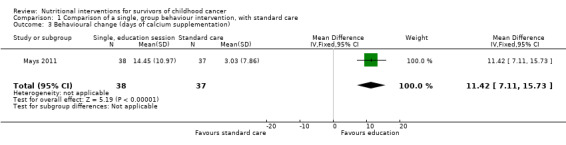
Comparison 1 Comparison of a single, group behaviour intervention, with standard care, Outcome 3 Behavioural change (days of calcium supplementation).
1.4. Analysis.
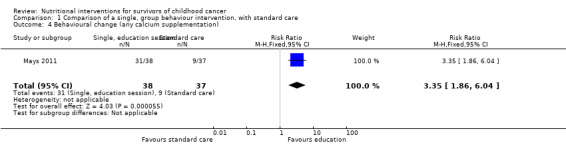
Comparison 1 Comparison of a single, group behaviour intervention, with standard care, Outcome 4 Behavioural change (any calcium supplementation).
Comparison 2. Comparison of calcium and vitamin D supplementation combined with nutrition education with nutrition education only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body composition (bone mineral density) | 1 | 275 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.16] |
2.1. Analysis.
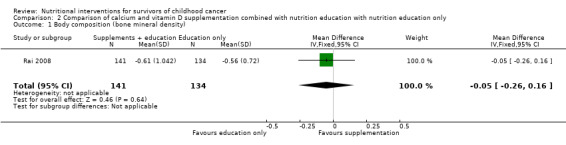
Comparison 2 Comparison of calcium and vitamin D supplementation combined with nutrition education with nutrition education only, Outcome 1 Body composition (bone mineral density).
Comparison 3. Comparison of a 12‐month, face‐to‐face and telephone health behaviour intervention with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Behavioural change (nutrition) | 1 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.24, 0.14] |
| 2 Behavioural change (junk food) | 1 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.33, ‐0.01] |
3.1. Analysis.
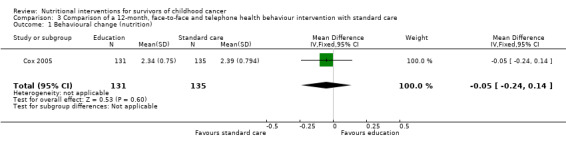
Comparison 3 Comparison of a 12‐month, face‐to‐face and telephone health behaviour intervention with standard care, Outcome 1 Behavioural change (nutrition).
3.2. Analysis.
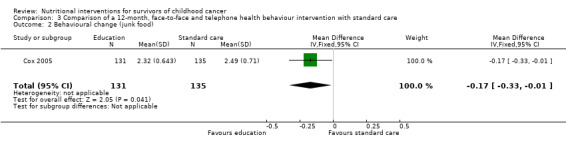
Comparison 3 Comparison of a 12‐month, face‐to‐face and telephone health behaviour intervention with standard care, Outcome 2 Behavioural change (junk food).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cox 2005.
| Methods | Design: Parallel RCT Setting: Single site paediatric oncology unit, USA |
|
| Participants |
Number Intervention: n = 131 (4 lost to follow‐up) Control: n = 135 (1 lost to follow‐up) Age at study entry Group:12‐18 years Intervention (mean ± SD): 15.09 ± 1.90 years Control (mean ± SD): 14.96 ± 1.97 years Sex Intervention: 57 males: 74 females Control: 61 males: 74 females Diagnosis Leukaemia/lymphoma: Intervention: 73 Control: 72 Solid tumour: Intervention: 58 Control: 63 Treatment Information not available Age at diagnosis Information not available Time since diagnosis Intervention (mean ± SD): 15.09 (1.90) years Control (mean ± SD): 10.31 (2.94) years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention The intervention consisted of standard care plus a single multi‐behavioural intervention provided by a clinical physician or nurse practitioner during a routine visit to the long‐term follow‐up clinic. The multi‐behavioural intervention consisted of:
Telephone reinforcement of the education was provided at 3 and 6 months after their initial clinic visit Cointerventions: Changing the health behaviour practices of smoking cessation, sun protection and exercise Contraindications: None Control Group Standard care consists of:
Cointerventions Other health behaviour practices were targeted during the intervention. These included; smoking cessation, sun protection and exercise |
|
| Outcomes | The outcomes were measured at baseline and 12 months postintervention for both the intervention and control groups Outcome measure: Behavioural change 1) Frequency of nutrition as a health protective behaviour 2) Frequency of junk food consumption as a health risk behaviour |
|
| Notes |
Study sponsors Oncology Nursing Society Foundation (2003‐2005) American Lebanese Syrian Associated Charities (ALSAC) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " The randomisation was stratified by gender and age because of the clinical impression that risk perception could carry by gender or age" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Although randomisation was performed using the procedure as set out by Zelen 1974, it was unclear which actual randomisation technique was used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The study reported that five participants (four in the intervention group and one in the control group) were lost to follow‐up. There was no discussion on how this data were handled. We were unable to assess how this would influence the outcome or whether this would have a clinically relevant effect |
| Selective reporting (reporting bias) | High risk | Comment: This study presented a secondary analysis of data. This analysis was not in the original publication of the results |
| Other bias | Low risk | Comment: Minimal baseline imbalance: At baseline, there was no significant difference between the intervention and the control group for demographic and other reported characteristics No differential diagnostic activity: All assessments were performed at baseline and follow‐up for both the intervention and the control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: This study does not discuss whether participants or personnel were blinded. Due to the nature of the study and the form of the intervention, it would be impossible for the participants and personnel to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: The outcome is subjective (a self‐reported outcome) and the participants are not blinded |
Mays 2011.
| Methods | Design: Parallel RCT Setting: Two sites, Paediatric oncology units, USA |
|
| Participants |
Number Intervention: 38 Control: 37 No information on attrition was available Age at study entry Group:11‐21 years Intervention (mean ± SD): 14.2 ± 2.0 years Control (mean ± SD): 14.2 ± 2.8 years Sex Intervention: 17 males: 21 females Control: 19 males: 18 females Diagnosis Intervention: 21 leukaemia: 17 others Control: 18 leukaemia: 19 others Treatment Information not available Age at diagnosis Information not available Time since treatment completion Information not available Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention The intervention consisted of a single half‐day, group workshop in addition to standard care. The workshop was given by a registered dietitian. The workshop included an interactive behavioural session and focused on risk reducing health promotion behaviours. The workshop had a focus on bone health Cointerventions: None Contrainidications: None Control group The control group received standard care and were offered the intervention at the conclusion of the study |
|
| Outcomes | The outcomes were measured at baseline and 1 month postintervention for both the intervention and control groups. These outcomes were:
A) change in nutritional intake: 1) dietary calcium intake measured with 24‐hour recall B) behaviour change: 1) milk consumption frequency 2) use of calcium supplementation |
|
| Notes |
Study sponsors American Cancer Society Lance Armstrong Foundation National Cancer Institute (CA091831) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The paper states that the participants were randomly allocated but no further information on the methodology was provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: The paper states that the participants were randomly allocated but no further information on the methodology was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There was no information provided on participant attrition |
| Selective reporting (reporting bias) | Low risk | Comment: This study reported data at baseline and follow‐up on all outcomes cited in the protocol or methodology section |
| Other bias | Low risk | Comment: Minimal baseline imbalance: At baseline, there was no significant difference between the intervention and the control group for demographic and other reported characteristics No differential diagnostic activity: All assessments were performed at baseline and follow‐up for both the intervention and the control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comments: This study does not discuss whether participants or personnel were blinded. Due to the nature of the study and the form of the intervention, it would be impossible for the participants and personnel to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All telephone interviews were administered by a trained research assistant who was masked to the trial condition" |
Rai 2008.
| Methods | Design: Parallel RCT Setting: Single site, paediatric oncology unit, USA |
|
| Participants |
Number Intervention: n = 141 (45 dropouts) Control: n = 134 (49 dropouts) Age at study entry Intervention (mean; range): 16.6 (9.4 to 35.3) years Control (mean; range): 17.2 (9.4 to 33.5) years Sex Intervention: 78 males: 63 females Control: 78 males: 56 females Diagnosis ALL Treatment Radiation: Intervention: 53 Control: 34 Chemotherapy: Intervention: 141 Control: 134 Age at diagnosis Intervention (mean; range): 4.7 (0.7 to 17.4) years Control (mean; range): 4.6 (1.0 to 16.39) years Time since treatment completion Intervention (mean; range): 7.1 (5.0 to 18.2) years Control (mean; range): 7.2 (4.6 to 19.1) years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention This study was a 24‐month nutrition and supplementation intervention. The intervention group received nutrition education sessions every 6 months. At baseline and 12 months these were given face‐to‐face by a registered dietitian, and at 6 months and 18 months these were given in the form of mailed information. The education included information such as:
The intervention group was also be given 24 months of calcium and vitamin D supplementation which were taken daily Cointerventions: None Contraindications: None Control group The control group received education sessions identical to the intervention group. They also received placebo tablets instead of calcium and vitamin D supplements |
|
| Outcomes | The outcomes were measured at baseline, 12 months, 24 months and 36 months postintervention for both the intervention and control groups Outcome measure: body composition 1) Bone mineral density |
|
| Notes |
Study sponsors National Institutes of Health; Grant number: P30 CA‐21765 Center of Excellence grant from the State of Tennessee Le Bonheur Foundation (Memphis TN) American Lebanese Syrian Associated Charities (ALSAC) NIH; Grant numbers: R21 HD059292; GM 92666 Grant sponsor Gabrielle’s Angel Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The participants were stratified when randomised into sex, race, age and BMD Z‐score |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the St Jude pharmacy had access to the randomisation system, which is maintained by the Department of Biostatistics at St Jude" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There were a large number of dropouts in both the intervention (45) and control groups (49). It is unclear how this data were treated |
| Selective reporting (reporting bias) | High risk | Comment: This study did not publish all outcomes that were reported on the clinical trials registry |
| Other bias | Low risk |
Minimal baseline imbalance: At baseline, there was no significant difference between the intervention and the control group for demographic and other reported characteristics No differential diagnostic activity: All assessments were performed at baseline and follow‐up for both the intervention and the control group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Both the participants and the research personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Both the participants and the research personnel were blinded |
ALL: acute lymphoblastic leukaemia BMD: bone mineral density RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mays 2012 | This was a validation study and did not include an intervention |
| Moyer‐Mileur 2009 | The study included participants on maintenance therapy |
| Nathan 2009 | This study contains a review of the literature and only reported on a smoking cessation intervention in childhood cancer survivors |
Characteristics of ongoing studies [ordered by study ID]
NCT01473342.
| Trial name or title | Mila Blooms intervention study: an App for promoting physical activity and health diet among adolescent survivors of chilhood cancer |
| Methods | Design: RCT (own controls) Setting: Single site paediatric oncology unit, USA |
| Participants |
Number Estimated enrolment: 30 Age 12‐19 years Sex Both genders Diagnosis ALL Inclusion criteria
Exclusion criteria
|
| Interventions | Control phase: All participants will take part in the 8‐week control phase where they will complete initial data collection Intervention: The intervention will commence after the 8‐week control phased has finished and will run for 9 weeks. The participants will receive a smart phone which contains the Mila Blooms gaming app. The app consists of:
Participants will also receive weekly coaching telephone calls to help with goal‐setting and compliance |
| Outcomes | Healthy diet Physical activity Prevent weight gain |
| Starting date | April 2011 |
| Contact information | Sharnail Bazemore: sharnail.bazemore@duke.edu |
| Notes |
Stern 2015.
| Trial name or title | NOURISH‐T |
| Methods | Design: Feasability study RCT Setting: Two paediatric oncology units, USA |
| Participants |
Number Estimated enrolment: 110 Age Caregivers of 5‐12 year olds Sex Both genders Diagnosis All childhood cancer survivors between 6 months to 4 years off treatment |
| Interventions |
Intervention 6‐week, 10 session study 6 sessions delivered face‐to‐face 4 sessions delivered via the telephone Focus on behavioural change, healthy eating, and physical activity Control Enhanced usual care 1 x wellness session + written education materials on healthy eating |
| Outcomes | Feasibility Dietary intake QoL BMI Physical activity |
| Starting date | Unknown |
| Contact information | Marilyn Stern: mstern1@usf.edu |
| Notes |
BMI: body mass index QoL: quality of life RCT: randomised controlled trial
Differences between protocol and review
There has been a change to the author team
Contributions of authors
JC and CW were the main contributors to the data extraction, management, and assessment of bias. RC reviewed the manuscript.
Sources of support
Internal sources
There are no internal sources of support, Other.
External sources
There are no external sources of support, Other.
Declarations of interest
Jennifer Cohen: None known.
Claire Wakefield: None Known.
Richard Cohn: None Known.
New
References
References to studies included in this review
Cox 2005 {published data only}
- Cox CL, McLaughlin RA, Rai SN, Steen BD, Hudson MM. Adolescent survivors: A secondary analysis of a clinical trial targeting behavior change. Pediatric Blood and Cancer 2005;45:144‐54. [DOI] [PubMed] [Google Scholar]
Mays 2011 {published data only}
- Mays D, Donze Black J, Mosher RB, Heinly A, Shad AT, Tercyak KP. Efficacy of the survivor health and resilience education (SHARE) program to improve bone health behaviors among adolescent survivors of childhood cancer. Annals of Behavioral Medicine 2011;42:91‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rai 2008 {published data only}
- Hudson MM, Tyc VL, Jayawardene DA, Gattuso J, Quargnenti A, Greenwald C, et al. Feasibility of implementing health promotion interventions to improve health‐related quality of life. International Journal of Cancer 1999;Suppl 12:138‐42. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Tyc VL, Srivastava DK, Gattuso J, Quargnenti A, Crom DB, et al. Multi‐component behavioral intervention to promote health protective behaviors in childhood cancer survivors: The protect study. Medical and Pediatric Oncology 2002;39:2‐11. [DOI] [PubMed] [Google Scholar]
- Kaste SC, Qi A, Surprise H, Lovorn E, Boyett J, Ferry Jr RJ, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatric Blood and Cancer 2014;61:885‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SN, Hudson MM, McCammon E, Carbone L, Tylavsky F, Smith K, et al. Implementing an intervention to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia: BONEII, a prospective placebo‐controlled double‐blind randomized interventional longitudinal study design. Contemporary Clinical Trials 2008;29:711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Mays 2012 {published data only}
- Mays D, Gerfen E, Mosher RB, Shad AT, Tercyak KP. Validation of a milk consumption stage of change algorithm among adolescent survivors of childhood cancer. Journal of Nutrition Education and Behavior 2012;44(5):464‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyer‐Mileur 2009 {published data only}
- Moyer‐Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard‐risk acute lymphoblastic leukemia during maintenance therapy. Journal of Pediatric Hematology Oncology 2009;31:259‐66. [DOI] [PubMed] [Google Scholar]
Nathan 2009 {published data only}
- Nathan PC, Ford JS, Henderson TO, Hudson MM, Emmons KM, Casillas JN, et al. Health behaviors, medical care, and interventions to promote healthy living in childhood cancer survivor study cohort. Journal of Clinical Oncology 2009;27(14):2363‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01473342 {published data only}
- NCT01473342. Mila Blooms intervention study: an app for promoting physical activity and health diet among adolescent survivors of childhood cancer. https://clinicaltrials.gov/ct2/show/NCT01473342 (accessed 2013). [DOI] [PMC free article] [PubMed]
Stern 2015 {published data only}
- Stern M, Ewing L, Davila E, Thompson AL, Hale G, Mazzeo S. Design and rationale for NOURISH‐T: A randomized control trial targeting parents of overweight children off treatment. Contemporary Clinical Trials 2015;41:227‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arroyave 2008
- Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviours. Oncology Nursing Forum 2008;35(1):121‐30. [DOI] [PubMed] [Google Scholar]
Braam 2013a
- Braam KI, Torre P, Takken T, Veening MA, Dulmen‐den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008796.pub2; CD008796] [DOI] [PubMed] [Google Scholar]
Brunner 2009
- Brunner E, Rees K, Ward K, Burke M, Thorogood M. Dietary advice for reducing cardiovascular risk. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002128.pub3] [DOI] [PubMed] [Google Scholar]
Cohen 2012
- Cohen J, Wakefield CE, Fleming CA, Gawthorne R, Tapsell LC, Cohn RJ. Dietary intake after treatment in child cancer survivors. Pediatric Blood and Cancer 2012;58(5):752‐5. [DOI] [PubMed] [Google Scholar]
Cox 2009
- Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: results from the Childhood Cancer Survivor Study. Cancer 2009;115(3):642‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried 2005
- Demark‐Wahnefried W, Werner C, Clipp EC, Guill AB, Bonner M, Jones LW, et al. Survivors of childhood cancer and their guardians. Cancer 2005;103(10):2171‐80. [DOI] [PubMed] [Google Scholar]
Dickerman 2007
- Dickerman JD. The late effects of childhood cancer therapy. Pediatrics 2007;119(3):554‐68. [DOI] [PubMed] [Google Scholar]
Diller 2009
- Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin‐Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. Journal of Clinical Oncology 2009;27(14):2339‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emond 2010
- Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics 2010;126(2):e337‐42. [DOI] [PubMed] [Google Scholar]
Friedman 2006
- Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatric Blood and Cancer 2006;46:159‐68. [DOI] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Visit GE, Liberati A, et al. Going from evidence to recommendations. BMJ 2008;336(7652):1049‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudson 2002
- Hudson MM, Tyc VL, Srivastava DK, Gattuso J, Quargnenti A, Crom DB, et al. Multi‐component behavioral intervention to promote health protective behaviors in childhood cancer survivors: The Protect Study. Pediatric Blood & Cancer 2002;39(1):2‐11. [DOI] [PubMed] [Google Scholar]
Hudson 2009
- Hudson MM, Mulrooney DA, Bowers DC, Sklar CA, Green DM, Donaldson SS, et al. High‐risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk‐based surveillance. Journal of Clinical Oncology 2009;27(14):2405‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jemal 2009
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M J. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians 2009;59(4):225‐49. [DOI] [PubMed] [Google Scholar]
Jones 2010
- Jones L, Watling RM, Wilkins S, Pizer B. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD003298.pub2] [DOI] [PubMed] [Google Scholar]
Kuhn 2004
- Kuhn DE, Matson JL. Assessment of feeding and mealtime behavior problems in persons with mental retardation. Behavior Modification 2004;28(5):638‐48. [DOI] [PubMed] [Google Scholar]
Lakka 2007
- Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Applied Physiology, Nutrition, and Metabolism 2007;32(1):76‐88. [DOI] [PubMed] [Google Scholar]
Landier 2006
- Landier W, Wallace WHB, Hudson MM. Long‐term follow‐up of pediatric cancer survivors: education, surveillance, and screening. Pediatric Blood and Cancer 2006;46:149‐58. [DOI] [PubMed] [Google Scholar]
Lewis 2004
- Lewis E, Kritzinger A. Parental experiences of feeding problems in their infants with Down syndrome. Down Dyndrome Research and Practice 2004;9(2):45‐52. [DOI] [PubMed] [Google Scholar]
Module CCG 2014
- Kremer LCM, Leclercq E, Dalen EC. Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2014, Issue 11. Art. No.: CHILDCA.
Mulhern 1995
- Mulhern RK, Tyc VL, Phipps S, Crom DB, Barclay D, Greenwald C, et al. Health‐related behaviours of survivors of childhood cancer. Medical and Pediatric Oncology 1995;27(14):159‐65. [DOI] [PubMed] [Google Scholar]
Mulrooney 2009
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ness 2007
- Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annual Review of Public Health 2007;28:279‐302. [DOI] [PubMed] [Google Scholar]
Oeffinger 2006
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine 2006;355(15):1572‐82. [DOI] [PubMed] [Google Scholar]
Oeffinger 2008
- Oeffinger KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased risk of cardiovascular disease?. Pediatric Blood and Cancer 2008;50(Suppl 2):462‐7. [DOI] [PubMed] [Google Scholar]
Oeffinger 2009
- Oeffinger KC, Hudson MM, Landier W. Survivorship: childhood cancer survivors. Primary Care 2009;36(4):743‐80. [DOI] [PubMed] [Google Scholar]
Oude Luttikhuis 2009
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001872.pub2] [DOI] [PubMed] [Google Scholar]
Pereira 2009
- Pereira MA, Kottke TE, Jordan C, O'Connor PJ, Pronk NP, Carreon R. Preventing and managing cardiometabolic risk: the logic for intervention. International Journal of Enviromental Research and Public Health 2009;6:2568‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robien 2008
- Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology/Oncology 2008;30(11):815‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siviero‐Miachon 2008
- Siviero‐Miachon AA, Spinola‐Castro AM, Guerra‐Junior G. Detection of metabolic syndrome features among childhood cancer survivors: a target to prevent disease. Vascular Health and Risk Management 2008;4(4):825‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2006
- Skinner R, Wallace WHB, Levitt GA. Long‐term follow‐up of people who have survived cancer during childhood. Lancet Oncology 2006;7(6):489‐98. [DOI] [PubMed] [Google Scholar]
Steinberger 2012
- Steinberger J, Sinaiko AR, Kelly AS, Leisenring WM, Steffen LM, Goodman P, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. Journal of Pediatrics 2012;160(3):494‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolley 2009
- Stolley MR, Sharp LK, Arroyo C, Ruffin C, Restrepo J, Campbell R. Design and recruitment of the Chicago Healthy Living Study: a study of health behaviours in a diverse cohort of adult childhood cancer survivors. Cancer 2009;115(Suppl 18):4385‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolley 2010
- Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Annals of Behavioral Medicine 2010;39(3):232‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tota‐Maharaj 2010
- Tota‐Maharaj R, Defilippis AP, Blumenthal RS, Blaha MJ. A practical approach to the metabolic syndrome: review of current concepts and management. Current Opinion in Cardiology 2010;25:502‐12. [DOI] [PubMed] [Google Scholar]
Zelen 1974
- Zelen M. The randomization and stratification of patients to clinical trials. Journal of Chronic Disease 1974;27:365‐75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cohen 2012a
- Cohen JE, Wakefield CE, Bartle J, Cohn RJ. Nutritional interventions for survivors of childhood cancer. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009678] [DOI] [PMC free article] [PubMed] [Google Scholar]


